Abstract
Arsenate tolerance is conferred by suppression of the high-affinity phosphate/arsenate uptake system, which greatly reduces arsenate influx in a number of higher plant species. Despite this suppressed uptake, arsenate-tolerant plants can still accumulate high levels of As over their lifetime, suggesting that constitutive detoxification mechanisms may be required. Phytochelatins are thiol-rich peptides, whose production is induced by a range of metals and metalloids including arsenate. This study provides evidence for the role of phytochelatins in the detoxification of arsenate in arsenate-tolerant Holcus lanatus. Elevated levels of phytochelatin were measured in plants with a range of tolerance to arsenate at equivalent levels of arsenate stress, measured as inhibition of root growth. The results suggest that arsenate tolerance in H. lanatus requires both adaptive suppression of the high-affinity phosphate uptake system and constitutive phytochelatin production.
Arsenate is an analog of phosphate, competing for the same uptake carriers in the root plasmalemma (Meharg and Macnair, 1992b). Arsenate tolerance has been identified in a number of plant species (Meharg, 1994; Sharples et al., 2000b). Tolerance in grasses results via suppression of the high-affinity phosphate/arsenate uptake system (Meharg and Macnair, 1992b). This suppression reduces arsenate influx to a level at which it is thought that the plant can detoxify it, presumably by constitutive mechanisms (Meharg, 1994). The theory is based on the fact that arsenate tolerance is achieved by a single gene, which codes for the suppressed phosphate/arsenate transport (Meharg et al., 1992; Meharg and Macnair, 1992b).
Despite this clear understanding of the processes controlling decreased arsenate uptake, tolerant grasses still assimilate As, albeit at much lower rates compared with non-tolerants. Nevertheless, assimilation over the life history of plants growing on contaminated soil can result in very high As concentrations, e.g. 3,470 μg g−1 As in Agrostis tenuis and 560 μg g−1 As in Holcus lanatus (Porter and Peterson, 1975). It is postulated that arsenate is transformed within plant cells to other less phytotoxic As species (Meharg, 1994). Metabolism to other As species has been observed in phytoplankton and macroalgae where arsenate is converted to arsenite, dimethylarsinic acid (DMA), and monomethylarsinic acid (MMA) (Phillips, 1990). These methylated forms of As are then metabolized to organophospholipids and arsenosugars (Phillips, 1990). Studies of terrestrial plants have only revealed the presence of arsenate and arsenite (Meharg, 1994; Van den Broeck et al., 1998), but a recent study on a range of terrestrial plants reported low concentrations of methylated As species, including MMA and DMA. However, the majority of the As was still present as the inorganic forms arsenate and arsenite, which are more phytotoxic (Koch et al., 2000).
Three studies recently have described the formation of As-phytochelatin complexes on exposure to arsenate, in arsenate-sensitive Silene vulgaris plants (Sneller et al., 1999), cell suspension cultures of Rauvolfia serpentina (Schmöger et al., 2000), and root cultures of Rubia tinctorum (Maitani et al., 1996). Phytochelatins (PCs) are thiol (SH)-rich peptides (common structure [γ − glu − cys]ngly in which n = 2−11 [e.g. PC2, PC3, PC4]) whose production is induced by a range of heavy metals including Cd, As, Cu, and Zn (Grill et al., 1985). Previous studies of PC production in response to Cd and Cu have illustrated that although PCs are involved in the detoxification process, they are not responsible for metal tolerance (De Knecht et al., 1992; Schat and Kalff, 1992). However, the processes that govern arsenate tolerance have already been shown to be significantly different to those of other metals (Meharg, 1994). A role for PCs in the detoxification of arsenate was first suggested because of their induction by arsenate (Grill et al., 1987). This hypothesis was supported by evidence of the formation of As-SH complexes both in vivo and in vitro (Jocelyn, 1972; Scott et al., 1993). The gene encoding PC synthase (the enzyme responsible for the production of PCs from glutathione [GSH]) has recently been identified in Arabidopsis (Clemens et al., 1999; Ha et al., 1999; Vatamaniuk et al., 1999), Triticum aestivum, and Schizosaccharomyces pombe (Clemens et al., 1999; Ha et al., 1999). It has also been shown that a mutant Arabidopsis lacking the ability to synthesize PCs, was much more sensitive to arsenate than the wild type (Ha et al., 1999). Since then, the production of As-PC complexes has been unequivocally demonstrated, through purification of PCs by electrospray ionization mass spectroscopy (ESI-MS) (Schmöger et al., 2000) and x-ray absorption spectroscopy (Pickering et al., 2000). This study investigated whether As metabolism (methylation) or complexation by PCs was responsible for the enhanced ability of arsenate-tolerant plants to detoxify and accumulate arsenate.
RESULTS
Effect of Increasing Arsenate Exposure on Root Growth and Arsenate Uptake
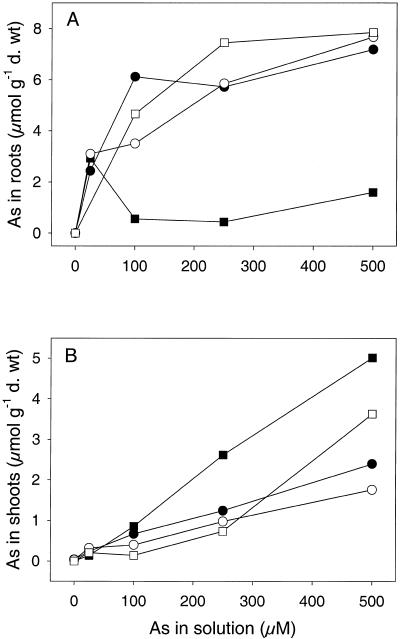
Inhibition of root growth was an accurate indicator of metal toxicity with large differences in As EC50 (effective concentration that inhibits root growth by 50%) between tolerant, intermediate, and non-tolerant clones (Table I). The most tolerant clone (M1) had an EC50 greater than 1,000 μm As, whereas the EC50 of the least tolerant clone (NM2) was 3 μm As (Table I). For three of the clones, root As concentrations were higher than in the shoots at all external concentrations (Fig. 1). The exception was non-tolerant clone (NM2) where root As remained low, yet shoot As continued to increase with increasing external arsenate (Fig. 1). NM2, the least tolerant clone, was expected to show elevated uptake, but because of the large concentration range used for this experiment, the growth of NM2 was completely inhibited above 25 μm As. Therefore, As concentrations in roots and shoots of NM2 above this exposure level were measured on plants that were no longer growing.
Table I.
Arsenate EC50 values and estimated PC production at EC50 concentration of H. lanatus clones from an uncontaminated (NM) and a contaminated (M) site
| Code | AsEC50 | Estimated PC |
|---|---|---|
| μm | μmol GSH equivalent g−1 dry wt | |
| NM1 | 40 | 4.5 |
| NM2 | 3 | 1.5 |
| M1 | >1,000 | 28.5 |
| M2 | 800 | 23.9 |
PC production was estimated from the dose response curves in Figure 2.
Figure 1.
Concentration of As in the roots (A) and shoots (B) of four H. lanatus clones from an As contaminated (M) and an uncontaminated (NM) site. Plants were exposed to a range of arsenate concentrations for 7 d. Data represent the mean ±se (n = 3). NM1, ●; NM2, ▪; M1, ○; M2, □.
Assay of Acid-Soluble Thiols
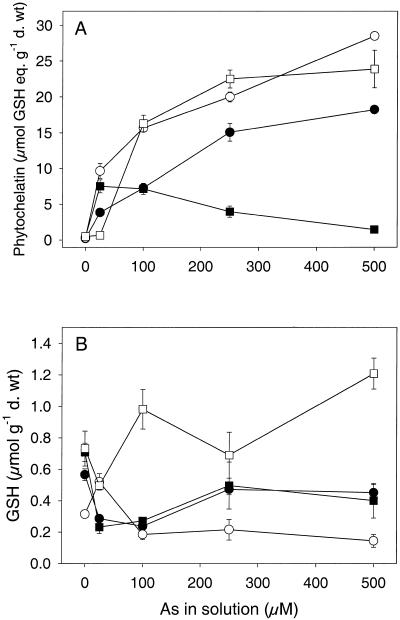
Total PC production increased with external arsenate concentration for the two tolerant clones (M1 and M2) and the intermediate tolerant clone (NM1) (Fig. 2). The exception was the non-tolerant clone (NM2), where PC concentrations decreased above 25 μm As due to the death of the plants and corresponded with a decrease in As concentrations in NM2 roots (Fig. 1). Arsenate-tolerant (M1 and M2) plants produced higher concentrations of PC than the non-tolerant plant (NM2) at equal external concentrations, the intermediate tolerant plant showing intermediate PC production (Fig. 2). To gain an estimate of PC production at an equivalent level of stress in the four clones, curves were fitted to the total PC data (Sigmaplot 2000, SPSS, Chicago) to estimate the concentration of total PC at the arsenate EC50 concentrations (Table I). From this calculation, PC production was shown to be 15- to 20-fold higher in the tolerant clones (M1 and M2) than the non-tolerant clone (NM2) under equivalent stress (Table I). GSH concentrations were low compared with PC concentrations and there was no significant difference between GSH concentrations in the tolerant and non-tolerant clones (Fig. 2; P > 0.05; data not shown). However, GSH concentrations in the two non-tolerant clones were very similar with initial depletion of GSH levels at low arsenate concentrations but some recovery at higher arsenate concentrations. This discrepancy could be due to the death of the plants above 25 μm As, as normal cell functioning would be severely disrupted before the plants died. GSH levels in the two tolerant clones were very different with depleted GSH levels in M1 and increased levels in M2.
Figure 2.
Concentrations of total PC (A) and GSH (B; equivalent μmol GSH equivalent. g−1 dry weight) in four H. lanatus clones from an As contaminated (M) and an uncontaminated (NM) site. Plants were exposed to a range of arsenate concentrations for 7 d. Data represent the mean ± se (n = 3). NM1, ●; NM2, ▪; M1, ○; M2, □.
As Analysis of EC50 Samples
As concentrations in the roots were significantly (P < 0.05) higher in the tolerant clone (M2) compared with the non-tolerant and intermediate tolerant clones at the EC50 exposure concentrations (Table II). In addition M3 had significantly higher root As concentrations than NM1 and NM3 but significantly lower root As than tolerant M2. This was not expected but may reflect the fact that this clone was from a contaminated site. Relative to the exposure concentrations, accumulation from the solution to the root was 10- to 60-fold lower in the tolerant clone (M2) compared with the remaining clones (NM1, NM3, and M3) (Table II). The arsenate exposure of the tolerant clone (M2) was 160 times higher than the non-tolerant clone (NM3), and yet the corresponding concentration in the roots was only three times higher in the tolerant (M2) compared with the non-tolerant clone (NM3) (Table II).
Table II.
As, total PC, and GSH concentrations in roots of H. lanatus clones of varying sensitivity exposed to their own As EC50 concentration
| Clone | As EC50 (μm) | As Root Conc | As Accumulation (Root/Solution) | Total PC | GSH | Ratio PC-SH/As | Ratio PC2:PC3:PC4 |
|---|---|---|---|---|---|---|---|
| μmol g−1 dry wt | μmol GSH equivalent g−1 dry wt | μmol g−1 dry wt | |||||
| NM1 | 40 | 3.8 ± 0.3a | 0.10 | 3.9 ± 0.7b | 0.08 ± 0.01a | 1.04 | 7:6:1 |
| NM3 | 5 | 3.1 ± 0.4a | 0.62 | 0.8 ± 0.1a | 0.01 ± 0.001b | 0.25 | 2:4:1 |
| M2 | 800 | 8.7 ± 0.9b | 0.01 | 15.4 ± 1.3c | 0.16 ± 0.04a | 1.77 | 12:6:1 |
| M3 | 15 | 5.9 ± 0.4c | 0.40 | 6.0 ± 0.5b | 0.14 ± 0.03a | 101 | 5:5:1 |
As accumulation from solution to root (As content of control root samples was < 0.003 μmol g−1 dry wt). Data represent the mean ± se (n = 4).
Values followed by the same letter do not differ at P < 0.05.
As Speciation by Liquid Chromatography- Mass Spectrometry
No MMA, DMA, or tetramethylarsonium ion (TMA) were detected in root or shoot samples of H. lanatus from the As-Cu contaminated site using liquid chromatography-mass spectrometry (LC-MS) ESI.
Identification of Acid-Soluble Thiols by HPLC
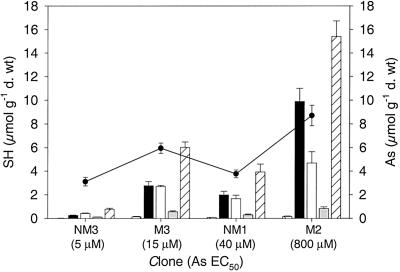
Total PC concentrations were significantly higher (P < 0.001) in the tolerant clone (M2) than in the remaining three clones at the EC50 concentrations (Fig. 3, Table II). This might imply that tolerant plants have a greater capacity for PC production than non-tolerant plants at equivalent levels of stress. The ratio of PC-SH/As was calculated to determine the number of SH groups available for coordination of As. PC concentrations were calculated in μmol GSH equivalent, this therefore accounts for the fact that different PC species, PC2, PC3, and PC4 contain 2, 3, and 4 SH groups, respectively. The ratio of PC-SH/As differed with plant tolerance (Table II). The most tolerant clone (M2) had the highest PC-SH/As ratio of 1.77, whereas the non-tolerant clone (NM3) had the lowest PC-SH/As ratio of 0.25. Following this trend it was expected that of the two intermediate clones, NM1 would have a higher total PC content than M3 due to its higher As EC50 value. However, this was not the case, as the total PC value corresponded with As root concentrations, not EC50 values, resulting in both clones having a PC-SH/As ratio of 1.0 (Table II). GSH concentrations were higher in the arsenate-treated plants than the control plants for all four clones (data not shown). Furthermore, the non-tolerant clone (NM3) had a significantly lower GSH concentration than the remaining clones, although overall GSH concentrations were low in relation to PC levels (P = 0.001; Table II).
Figure 3.
Concentrations of arsenate induced PCs (PC2, PC3, PC4), total PC, and GSH (μmol GSH equivalent g−1 dry weight) in roots of four H. lanatus clones exposed to their own arsenate EC50 concentration for 7 d. Data represent the mean ±se (n = 4). GSH (cross hashed), PC2 (▪), PC3 (□), PC4 (░⃞), total PC (left hashed), As root concentration (●).
The production of different chain length PCs varied between the four clones (Fig. 3). In the non-tolerant clone (NM3), PC3 was the dominant form, although overall total PC-SH concentrations were low (Fig. 3, Table II). In the intermediate clones (NM1, M3), PC2 and PC3 concentrations were approximately equal as indicated by the PC ratios, however, in the arsenate-tolerant clone (M2) PC2 was dominant (Fig. 3, Table II). PC4 remained at low concentrations in all four clones exposed to arsenate.
DISCUSSION
Over a range of arsenate concentrations, tolerant H. lanatus clones produced higher concentrations of PC than non-tolerant clones at equal external concentrations. When these data were modeled it was revealed that at the arsenate EC50 concentrations, estimated PC production was 15- to 20-fold higher in tolerant compared with non-tolerant clones. A second set of clones subsequently were exposed to their own arsenate EC50 concentrations, and the results were confirmed with a tolerant H. lanatus clone producing significantly higher PC concentrations than non-tolerant and intermediate tolerant clones. These results were in contrast to previous findings where PC production at Cd and Cu EC50 concentrations was equal at equivalent levels of stress (De Knecht et al., 1992; Schat and Kalff, 1992).
A difference in the distribution of PCs over chain length classes, in tolerant and non-tolerant plants, was also observed in response to arsenate. Previous studies of arsenate induced PCs have reported PC2 as the dominant species over a range of arsenate concentrations (Sneller et al., 1999; Schmöger et al., 2000). In this study PC2 was dominant in the non-tolerant clone, whereas PC3 was dominant in the most tolerant clone. The speciation in arsenate-tolerant and non-tolerant plants has not been examined previously, however, in studies of Cd and Zn tolerance, no difference in the PC2:PC3:PC4 ratio was reported between tolerant and non-tolerant plants (De Knecht et al., 1992; Harmens et al., 1993).
The variation in tolerance of the clones investigated is very clear with a 300-fold difference in EC50 values. This significant difference in tolerance is due to suppression of the high-affinity phosphate/arsenate uptake system, reducing arsenate influx to a much lower rate and this has been shown conclusively in H. lanatus clones from the populations studied here (Meharg and Macnair, 1992b). However, arsenate-tolerant plants do assimilate arsenate to higher concentrations than non-tolerant plants over their life-history, indicating that they must also have a successful internal detoxification mechanism.
The findings presented here indicate a different role for PCs in arsenate tolerance compared with Cd and Cu tolerance. For Cd and Cu, PCs provide a constitutive detoxification mechanism that is the same in tolerant and non-tolerant plants (De Knecht et al., 1992; Schat and Kalff, 1992). However, elevated PC production in arsenate-tolerant plants might imply that this response is adaptive, providing an additional mechanism of arsenate tolerance along-side suppression of arsenate uptake. This could be explained by inherently increased PC synthesis capacity (Vatamaniuk et al., 1999), but there is strong evidence against this conclusion. First, the adaptation of suppressed arsenate uptake is under single gene control (Meharg et al., 1992). Suppressed influx was shown to cosegregate with tolerance, demonstrating that the gene coding for tolerance also coded for suppression of the arsenate uptake system (Meharg and Macnair, 1992a). If elevated PC production was an adaptive response, this would imply that this same gene for suppression also coded for differential PC production, or would at least suggest that these genes were linked, this is unlikely. However, as there is a degree of quantitative variation among tolerant plants, it is possible that the gene for PC synthase is acting as a hypostatic modifying factor to the tolerance allele (Macnair et al., 1992). Second, arsenate-tolerant plants have reduced rates of influx compared with non-tolerant plants (Meharg and Macnair, 1992b), and as PC responses have been shown to be related to the level of tolerance, they are also likely to be related to arsenate influx. Rates of influx were not measured in this study, however, a large number of clones from the same H. lanatus populations have been characterized for their arsenate tolerance and influx rates by Meharg and coworkers (Meharg and Macnair, 1992a, 1992b). Therefore, conclusions made in this study are based on previous knowledge of the influx rates of arsenate-tolerant and non-tolerant H. lanatus.
In these experiments, As and PC concentrations were measured after 7 d. The rapid rate of arsenate influx in non-tolerant plants initially would result in a high toxic burden in the cells of these plants. PCs would be induced, but the toxicity of arsenate could lead to disruption of the transport process and also disruption of PC production. Furthermore, arsenate/phosphate transport is under feedback regulation by phosphate and potentially by arsenate in non-tolerant plants (Meharg and Macnair, 1992b; Sharples et al., 2000a). This regulation could alter arsenate assimilation rates over the 7-d experiment. In contrast, arsenate influx into tolerant plants would be significantly lower, resulting in lower and more constant toxic burdens, thus allowing arsenate transport and PC production to continue unaffected.
An additional factor that might explain the differential PC production in response to arsenate and Cd and Cu is the different stability of the As-PC complex. The stability of As-PC complexes is different from that of Cd or Cu-PC complexes at vacuolar pH. There are two lines of evidence for this. First, As-PC complexes destabilize in alkaline buffer but not in weakly acidic buffer (Schmöger et al., 2000). Second, a comparison of derivitization of arsenate induced PCs using (a) post-column DTNB derivitization at acid pH and (b) pre-column mBBr derivitization at pH 8.2 (Sneller et al., 2000) showed that As-PC complexes only dissociated under alkaline conditions. Cd-PC complexes are transported across the tonoplast into root vacuoles where they eventually dissociate due to the acidic vacuolar pH (Vogeli-Lange and Wagner, 1990; Salt and Rauser, 1995; Johanning and Strasdeit, 1998). From there, it is suggested that they either degrade or are shuttled back into the cytoplasm. If As-PC complexes are also transported into the root vacuole, then under the acidic conditions present, they might remain stable preventing re-oxidation of As (III) and allowing accumulation of high concentrations of As-PC complexes in arsenate-tolerant plants. In non-tolerant plants this would also be possible, however, as described above, the differential influx and feedback regulation would prevent the formation of high levels of As-PC complexes due to the toxic effect of arsenate in non-tolerant plants.
This differential stability of As-PC complexes could also affect the production of longer chain PCs (Hayashi et al., 1991) and might explain why PC2 is the dominant form in the arsenate-tolerant clone. In PC biosynthesis, shorter chain PCs act as substrates for longer chain PCs (Hayashi et al., 1991). The strong binding of As to PC2 could, therefore, result in a shortage of substrate for the production of PC3. Sneller et al. (1999) reported that in arsenate-sensitive S. vulgaris PC3 and PC4 only started to form 24 to 48 h after exposure. This is in contrast to studies of Cd exposure where PC3 concentrations exceeded PC2 concentrations after only 2 h (Grill et al., 1987). The ratio of PC-SH/As also differed with plant tolerance (ranging from 0.25–1.77) with the tolerant clone (M2) having the highest PC-SH/As ratio. In a study of reconstituted As-PC complexes, three SH groups from two PC2 molecules were shown to coordinate one As ion, leaving one SH un-coordinated (Schmöger et al., 2000), whereas in arsenate-sensitive plants the ratio of PC-SH/As was 4 ± 1.5 (Sneller et al., 1999). The results reported here indicate that not all the As is being complexed by PC in the H. lanatus clones with least complexation in the non-tolerant clones.
This study has shown that H. lanatus is not converting arsenate or arsenite into organic As species. Other studies of As speciation, in a wide range of terrestrial plants and vegetables, reported that As was present either totally or predominantly as arsenate and arsenite (Helgesen and Larsen, 1988; Van den Broeck et al., 1998; Koch et al., 2000). The detection of free arsenate and arsenite species does not preclude the presence of PC in these plants as the HPLC-ICP MS techniques used in speciation did not set out to look for PC complexes, and as noted above, these complexes are constitutive under a range of conditions. However, this finding is important as inorganic As species are more phytotoxic than organic species (Cullen and Reimer, 1989). The lack of evidence for As methylation and the incomplete complexation of As in H. lanatus by PCs suggests that compartmentalization of As in a non-PC coordinated form may also be occurring in terrestrial plants.
So what is the role of PC complexation in arsenate tolerance? Arsenate tolerance is a result of suppression of the high-affinity phosphate uptake system, and this has been demonstrated in a range of terrestrial higher plants (Meharg, 1994). However, tolerant plants are still capable of enhanced accumulation and storage of arsenate compared with non-tolerant plants. Evidence presented here indicates that the adaptation of grasses to withstand high levels of arsenate relies on constitutive production of As-PC complexes. Furthermore, elevated levels of PC in arsenate-tolerant plants are due to differential influx in tolerant and non-tolerant plants resulting in differential toxicity.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seed of Holcus lanatus was collected from an uncontaminated site (University of Exeter, Devon; code NM) and an As-Cu contaminated site (Gawton United mine, Devon Grid ref. SX452688; code M). Clonal plants were grown from seed of each population, and plants were maintained in a glasshouse and grown in John Innes compost No. 2. Tillers of clonal plants from both populations were screened for arsenate tolerance using a standard rooting test over 7 d (Wilkins, 1957). Clones with a range of tolerance to arsenate (non-tolerant, tolerant, and intermediate tolerant) were selected from each population where possible, as measured by inhibition of root-length. Detailed dose-response experiments over 7 d were then conducted to determine their EC50 (effective concentration that inhibits root length by 50%).
Dose Response Exposure Experiment
Two clones were selected from each of the populations for the dose-response experiment based on their EC50 values (Table I). NM1 and NM2 from the uncontaminated site were intermediate tolerant and non-tolerant, respectively, whereas M1 and M2, from the contaminated site, were arsenate tolerant. Un-rooted tillers of these H. lanatus clones were placed in a phosphate free nutrient solution containing 0.2 mm Ca(NO3)2 + 0.2 mm KNO3 + 0.1 mm MgSO4 (pH 5.5, adjusted with HCl). Tillers were then grown for 10 d prior to arsenate exposure in a growth room with a 16-h photoperiod, and a day/night temperature of 21°C/17°C. Rooted tillers were exposed to a range of arsenate concentrations and grown for 7 d under the conditions described above. Treatments were: basal nutrient solution amended with 0, 10, 50 100, 500, and 1,000 μm As added as Na2HAsO4 (pH 5.5, adjusted with HCl). For each treatment, 12 tillers were placed in three 180-mL polystyrene cups containing 170 mL of control or arsenate-amended solution with three replicate groups of pots per treatment. Longest root-length was measured at the beginning and end of the exposure period. After 7 d all plant material was rinsed in deionized water and blotted. From each replicate group, root material was separated into two sub-samples. One subsample was frozen in liquid nitrogen, freeze-dried, and stored under vacuum at 20°C for analysis of glutathione (GSH) and PC complexes. The second subsample was rinsed in ice-cold phosphate buffer and blotted (1 mm Na2HPO4 + 10 mm MES [2-(N-morpholino)ethanesulfonic acid] + 0.5 mm Ca(NO3)2) to ensure desorption of arsenate from the roots free space (Asher and Reay, 1979). This subsample and all shoot material were oven dried at 70°C for 48 h and analyzed for As as described later.
EC50 Exposure Experiment
A second set of clones were chosen for the EC50 experiment based on their arsenate EC50 values (Table II). The clones used were different from those used in the dose-response experiment due to repeated problems with leaf rust. NM2 was replaced with NM3 a clone with very similar EC50, M1 (tolerant) was replaced with M3 (intermediate) so we could compare the PC production in intermediate-tolerant clones from a contaminated and uncontaminated site. NM1 and NM3 from the uncontaminated site were intermediate tolerant and non-tolerant, respectively. M2 was a tolerant clone from the contaminated site, whereas M3 was an intermediate tolerant clone from the contaminated site. Tillers of these clones were rooted in basal nutrient solution for 10 d as described above. They were then exposed to 0 μm arsenate or their own arsenate EC50 concentration (Table II) and grown for 7 d. Longest root-length was measured at the beginning and end of the exposure period. For each treatment, 20 tillers were placed in five 180-mL polystyrene cups containing 170 mL of control or arsenate-amended solution with four replicate groups of pots per treatment. After 7 d all plant material was rinsed in ice-cold phosphate buffer (see above) and blotted. Root material within each replicate group was frozen in liquid nitrogen, freeze-dried, and stored (see above) for analysis of As, GSH, and arsenate-induced PCs.
Extraction and Assay of Acid-Soluble Thiols
Total acid soluble thiols (TAST) in the dose-response samples were assayed according to De Vos et al. (1992). Extraction was carried out by grinding 10 to 20 mg of freeze-dried root material (using a mortar, pestle, and quartz sand) in 2 mL 5% (w/v) sulfosalicylic acid with 6.3 mm diethylenetriaminepentaacetic acid (DTPA) (pH < 1) at 0°C. After centrifugation at 10,000g for 15 min (4°C) the supernatants were immediately assayed. The concentration of TAST was determined using Ellman's reagent (Ellman, 1959). Supernatant (300 μL) was mixed with 630 μL of 0.5 m K2HPO4 and the absorbance measured after 2 min at 412 nm (30°C). After addition of 25 μL of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) solution (10 mm DTNB, 0.143 m K2HPO4, 6.3 mm DTPA, pH 7.5) the absorbance was remeasured after 2 min. The increase in absorbance was corrected for the absorbance of DTNB. Values were calculated using the molecular extinction coefficient λ412 = 13,600 m−1 cm−1 (Harmens et al., 1993). Total glutathione (GSH + GSSG) and oxidized glutathione (GSSG) were determined by the GSSG recycling method (Anderson, 1985) using GSSG as a standard. The concentration of PC was calculated as: PC = TAST − (TG − GSSG). The recovery and oxidation of acid-soluble thiols were determined using GSSG as an external and internal standard.
Identification of Acid-Soluble Thiols by HPLC
Root material from the EC50 experiment was used for HPLC analysis of GSH and arsenate-induced PCs. GSH and different chain-length PCs (PC2, PC3, PC4) were separated out using this method. Freeze-dried root material (20 mg) was ground (using a mortar, pestle, and quartz sand) in 2 mL of 6.3 mm DTPA with 0.1% (v/v) trifluoroacetic acid at 0°C. N-acetyl Cys was added during grinding as an internal standard. After centrifugation at 10,000g for 15 min (4°C) the supernatant was filtered through a Costar Spin-X centrifuge tube with a nylon filter (0.22 μm). The derivitization procedure was that of Sneller et al. (2000): 450 μL of 200 mm 4-(2-hydroxyethel)-piperazine-1-propanesulphonic acid buffer pH 8.2, containing 6.3 mm DTPA was mixed with 10 μL of 25 mm monobromobimane. To this mixture, 250 μL of the filtered supernatant was added, and derivitization was carried out for 30 min at 45°C in a covered water bath. The reaction was stopped by the addition of 300 μL of 1 m methansulphonic acid. Samples were stored in the dark at 4°C until HPLC analysis.
GSH and arsenate-induced PCs were separated on a Nova-Pak C18 column (6 nm, 4 μm, 3.9 × 300 mm, catalogue no. 11695, Waters, Milford, MA) at 37°C. Before injection the column was equilibrated in 12% (v/v) methanol and 88% (v/v) water both containing 0.1% (v/v) trifluoroacetic acid. The sample was injected and eluted with a slightly concave gradient of 12% to 25% (v/v) methanol for 15 min and then a linear gradient from 25% to 50% (v/v) methanol (v/v) from 15 to 50 min. Fluorescence was monitored using a Waters 474 fluorescence detector. The volume of derivitized sample injected was 6 to 25 μL depending on the concentration of PC. Reduced glutathione (GSH) was used as an external standard. PC concentrations were calculated as μmol GSH equivalent g−1 dry weight and corrected for derivitization efficiency (Sneller et al., 2000).
Total As Analysis
Total As was extracted by digesting 100 to 300 mg dry root or shoot material in 5 mL of Aqua Regia (1:4, v/v, HCl:HNO3) at reflux temperature for 2 h, after which the sample was filtered and the volume adjusted to 25 mL with 12.5% (v/v) HNO3. Total As concentrations were determined using inductively coupled plasma-optical emission spectroscopy (ICP-OES, Jobin-Yvon JY38+).
Mass Spectroscopy Analysis
Samples of H. lanatus roots and shoots collected from the As-Cu contaminated mine site were analyzed for the following methylated As species: MMA, DMA, and TMA. Shoot samples were freeze-fractured in liquid nitrogen and extracted using methanol and water (1:1, v/v). Analysis by LC-MS with ESI was conducted on methanol extracts (model HP1100 series LC-MS ESI, Hewlett-Packard, Bracknell, UK). Chromatographic and mass spectrometer conditions were those outlined by Inoue et al. (1999). An Altech (UK) SCX 5-m, 250-mm-length, 4.6-mm-i.d. cation exchange column was chosen to conduct separation with a mobile phase of HNO3 (8 mm)/NH4 NO3 (5 mm). The column was maintained at a temperature of 30°C and a flow rate of 0.4 mL min−1. Injection volume was 25 μL. The MSD was run in positive mode with 5.0-V ion energy. The quadrapole temperature was 99°C and the gas temperature 300°C with a drying gas flow rate of 10 L min−1 and a nebulization pressure of 45 pounds per square inch. Retention times and mass-spectral fingerprints of MMA, DMA, and TMA standards were used to identify these compounds in plant tissues.
Statistical Analysis
Total As, PC, and GSH concentration data were analyzed using one-way analysis of variance. Means were compared using Tukeys multiple comparison test (Minitab v. 13.1, Minitab, State College, PA).
ACKNOWLEDGMENTS
The authors would like to thank Natural Environment Research Council and COST Action 837 (Short term scientific missions program) for funding Jeanette Hartley-Whitaker. We would also like to thank the staff in the Department of Ecology and Ecotoxicology of Plants, Vrije Universiteit, Amsterdam for helping with the PC analysis.
Footnotes
This work was supported by the Natural Environment Research Council, U.K. and by COST Action 837 Short Term Scientific Mission (to J.H.W.). .
LITERATURE CITED
- Anderson ME. Tissue glutathione. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985. pp. 317–323. [Google Scholar]
- Asher CJ, Reay PF. Arsenic uptake by barley seedlings. Aust J Plant Physiol. 1979;6:459–466. [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeasts. EMBO J. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89:713–764. [Google Scholar]
- De Knecht JA, Koevoets PLM, Verkleij JAC, Ernst WHO. Evidence against a role for phytochelatins in naturally selected increased cadmium tolerance in Silene vulgaris(Moench) Garcke. New Phytol. 1992;122:681–688. [Google Scholar]
- De Vos CHR, Vonk MJ, Vooijs R, Schat H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992;98:853–858. doi: 10.1104/pp.98.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker E-L, Zenk MH. Phytochelatins: the principal heavy metal complexing peptides of higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker E-L, Zenk MH. Phytochelatins: a class of heavy metal binding peptides of plants, are functionally analagous to metallothioneins. Proc Natl Acad Sci USA. 1987;84:439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S-B, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsborough PB, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1163. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmens H, Den Hartog PR, ten Bookum WM, Verkleij JAC. Increased zinc tolerance in Silene vulgaris(Moench) Garcke is not due to increased production of phytochelatins. Plant Physiol. 1993;103:1305–1309. doi: 10.1104/pp.103.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Nakagawa CW, Mutoh N, Isobe M, Goto T. Two pathways in the biosynthesis of cadystins (γ-EC) nG in the cell-free system of the fission yeast. Biochem Cell Biol. 1991;69:115–121. doi: 10.1139/o91-018. [DOI] [PubMed] [Google Scholar]
- Helgesen H, Larsen EH. Bioavailability and speciation of arsenic in carrots grown in contaminated soil. Analyst. 1988;123:791–796. doi: 10.1039/a708056e. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Date Y, Sakai T, Shimizu N, Yoshida K, Chen H, Kuroda K, Endo G. Identification and quantification by LC-MS and LC-ICP MS of arsenic species in urine of rats chronically exposed to dimethylarsinic acid (DMAA) Appl Organomet Chem. 1999;13:81–88. [Google Scholar]
- Jocelyn PC. Biochemistry of the SH group: the occurrence, chemical properties, metabolism and biological function of thiols and disulphides. London: Academic Press; 1972. [Google Scholar]
- Johanning J, Strasdeit H. A coordination-chemical basis for the biological function of the phytochelatins. Angew Chemie Int Ed. 1998;37:2464–2466. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2464::AID-ANIE2464>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Koch I, Wang L, Ollson CA, Cullen WR, Reimer KJ. The predominance of inorganic arsenic species in plants from Yellowknife, Northwest Territories, Canada. Environ Sci Technol. 2000;34:22–26. [Google Scholar]
- Macnair MR, Cumbes QJ, Meharg AA. The genetics of arsenate tolerance in Yorkshire Fog, Holcus lanatusL. Heredity. 1992;69:325–335. [Google Scholar]
- Maitani T, Kubota H, Sato K, Yamada T. The composition of metals bound to class III metallothionein (phytochelatin and its desglycyl peptide) induced by various metals in root culture of Rubia tinctorum. Plant Physiol. 1996;110:1145–1150. doi: 10.1104/pp.110.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharg AA. Integrated tolerance mechanisms - constitutive and adaptive plant - responses to elevated metal concentrations in the environment. Plant Cell Environ. 1994;17:989–993. [Google Scholar]
- Meharg AA, Cumbes QJ, Macnair MR. The genetics of arsenate tolerance in Yorkshire fog, Holcus lanatusL. Heredity. 1992;69:325–335. doi: 10.1111/j.1558-5646.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Macnair MR. Genetic correlation between arsenate tolerance and the rate of influx of arsenate and phosphate in Holcus lanatusL. Heredity. 1992a;69:336–341. [Google Scholar]
- Meharg AA, Macnair MR. Suppression of the high-affinity phosphate-uptake system: a mechanism of arsenate tolerance in Holcus lanatusL. J Exp Bot. 1992b;43:519–524. [Google Scholar]
- Phillips DJH. Arsenic in aquatic organisms: a review emphasising chemical speciation. Aquat Toxicol. 1990;16:151–186. [Google Scholar]
- Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE. Reduction and coordination of arsenic in Indian Mustard. Plant Physiol. 2000;122:1171–1177. doi: 10.1104/pp.122.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter EK, Peterson PJ. Arsenic accumulation by plants on mine waste (United Kingdom) Environ Poll. 1975;4:365–371. [Google Scholar]
- Salt DE, Rauser WE. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat H, Kalff MMA. Are phytochelatins involved in differential metal tolerance or do they merely reflect metal-imposed strain? Plant Physiol. 1992;99:1475–1480. doi: 10.1104/pp.99.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmöger MEV, Oven M, Grill E. Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 2000;122:793–802. doi: 10.1104/pp.122.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N, Hatlelid KM, MacKenzie NE, Carter DE. Reactions of arsenic (III) and arsenic (V) species with glutathione. Chem Res Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- Sharples JM, Meharg AA, Chambers SM, Cairney JWG. Mechanism of arsenate resistance in the ericoid mycorrhizal fungus Hymenoscyphus ericae. Plant Physiol. 2000a;124:1327–1334. doi: 10.1104/pp.124.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples JM, Meharg AA, Chambers SM, Cairney JWG. Evolution: symbiotic solution to arsenic contamination. Nature. 2000b;404:951–952. doi: 10.1038/35010193. [DOI] [PubMed] [Google Scholar]
- Sneller FEC, Van Heerwaarden LM, Koevoets PLM, Vooijs R, Schat H, Verkleij JAC. Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman's reagent and monobromobimane. J Agric Food Chem. 2000;48:4014–4019. doi: 10.1021/jf9903105. [DOI] [PubMed] [Google Scholar]
- Sneller FEC, Van Heerwaarden LM, Kraaijeveld-Smit FJL, Ten Bookum WM, Koevoets PLM, Schat H, Verkleij JAC. Toxicity of arsenate in Silene vulgaris, accumulation and degradation of arsenate-induced phytochelatins. New Phytol. 1999;144:223–232. [Google Scholar]
- Van den Broeck K, Vendecasteele C, Geuns JMC. Speciation by liquid chromatography-inductively coupled plasma-mass spectrometry of arsenic in mung bean seedlings used as a bio-indicator for the arsenic contamination. Anal Chim Acta. 1998;361:101–111. [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y-P, Rea PA. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitroreconstitution. Proc Natl Acad Sci USA. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli-Lange R, Wagner GJ. Subcellular-localization of cadmium and cadmium-binding peptides in tobacco leaves: implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990;92:1086–1093. doi: 10.1104/pp.92.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins DA. A technique for the measurement of lead tolerance in plants. Nature. 1957;180:37–38. [Google Scholar]