Abstract
The most serious complication in the treatment of hemophilia A (HA) is the development of factor (F)VIII inhibitors or antidrug antibodies (ADA) occurring in 25-35% of patients with severe HA. The immunological mechanisms underlying the development of ADA against FVIII products have not been completely understood yet. Immunological danger signals associated with events such as infection or surgery have been suggested to play a critical role. In previous studies, we demonstrated that plasma-derived (pd)FVIII but not recombinant (r)FVIII can activate human monocyte-derived dendritic cells (DC) in a danger signal-dependent manner, which subsequently mediate the proliferation of autologous CD4+ T cells. In this study, we investigated the ability of plasma components, naturally present in pdFVIII products, to mediate T-cell responses. In fact, we show that addition of plasma to rFVIII plus lipopolysaccharide (LPS)-stimulated DC induces proliferation of autologous CD4+ T cells. Interestingly, although DC pulsed with LPS plus plasma induce T-cell proliferation upon co-culture, the addition of FVIII significantly increases the number of proliferating as well as FVIII-specific CD4+ T cells. Total proliferating CD4+ T cells and FVIII-specific subsets were identified mainly as central memory T cells. Experiments using blocking antibodies and receptor antagonists revealed that the complement proteins C3a and, to a lesser extent, C5a are critically involved in these LPS-mediated T-cell responses. Collectively, our results indicate that complement proteins are potent drivers of T-cell responses to FVIII. Data presented provide a model how event-related substitution of FVIII in HA patients might contribute to inhibitor development.
Introduction
Hemophilia A (HA) is a hereditary bleeding disorder characterized by the complete absence or functional deficiency of the coagulation factor VIII (FVIII). In order to restore normal hemostasis, HA patients are treated with infusions of either plasma-derived (pd) or recombinant (r)FVIII products. A serious complication upon administration is the development of anti-FVIII antibodies, so called inhibitors, which can substantially limit treatment efficacy.1 The reported inhibitor incidence in treated patients with severe HA is approximately 25-35%.2 The risk for inhibitor development is higher in those HA patients completely lacking endogenous FVIII when compared to those suffering from mild or moderate HA with only a mutated or truncated FVIII protein.3 However, this genetic predisposition does not necessarily predict the risk of inhibitor development of an
individual patient. Patients with minor FVIII variations can develop inhibitors and patients with large deletions or a complete FVIII protein deficiency can remain free from inhibitor development. Consequently, it has been demonstrated that other factors such as ethnicity, HLA genotype, polymorphisms in immune regulatory genes, and/or the intensity and context of FVIII-treatment contribute to inhibitor development.4 An additional risk factor in the development of inhibitors is provided by the presence of immunological danger signals, in particular during intensive FVIII-treatment episodes. It has been observed that avoiding FVIII-treatment during acute infectious diseases or vaccinations, events associated with an enhanced presence of danger signals, limits the risk of inhibitor formation.5 In line with this, we have previously shown that in the presence of the danger signal lipopolysaccharide (LPS), human dendritic cells (DC) can be synergistically activated by pdFVIII but not rFVIII products in vitro.6 Furthermore, we have demonstrated that only pd but not rFVIII plus LPS-stimulated DC induce proliferation of co-cultured autologous T cells.7 As a medicinal product derived from human plasma, pdFVIII products co-deliver other blood-derived proteins. In fact, within pdFVIII products, a maximum of 2% is made up by FVIII while ~98% consist of (other) plasma proteins.8 Some of the plasma components such as proteins of the complement system, may promote immune responses. Of note, plasma components can either be delivered by pdFVIII products as in in vitro experimental settings or are ubiquitously present in pdFVIII- or rFVIII-treated patients per se.
The complement system is an ancient and evolutionary conserved arm of innate immunity. However, its diverse role in regulating adaptive immune responses has been underestimated for a long time. Complement serves as a natural adjuvant for B-cell activation and antibody production and has modulating effects on T-cell immunity.9 Particularly, the complement components C3a and C5a, which interact with their respective receptors C3aR and C5aR on antigen presenting cells (APC) and T cells, were shown to alter T-cell responses both directly and indirectly via APC.10,11 Furthermore, there is a body of evidence pinpointing towards a substantial crosstalk between complement and Toll-like receptor (TLR) signaling pathways resulting in synergistic interactions.12 For example, the TLR4 ligand LPS has been shown to activate complement which can lead to differential LPS-induced inflammatory processes.13 In line with this, excessive complement activation can contribute to destructive inflammatory conditions present in sepsis, allergy, transplant rejection, or autoimmunity.14 However, experimental data investigating the impact of plasma components and LPS on FVIII inhibitor development are largely missing.
In our current study, we investigated whether plasma, and in particular the complement proteins C3a and C5a, contribute to FVIII plus LPS-mediated T-cell responses. In addition, we explore which cellular and molecular mechanisms are involved in this T-cell activation elicited by both pd and rFVIII, depending on the experimental setting. In order to further shed light on these mechanisms, we quantified and characterized proliferating and FVIII-specific CD4+ T cells and their subsets.
Methods
Ethics statement
The research was approved by the Ethics Committee of the Medical Faculty of the Goethe University Frankfurt, Germany (trade number 70/15) and is in accordance with the Declaration of Helsinki of 1975, revised in 2008. All volunteers have given written consent to the study.
FVIII products
One pdFVIII (corresponding to FVIII1 in Miller et al. 2015 and 20186,7) and one full-length rFVIII product produced in a baby hamster kidney cell line were used throughout the study. DC were incubated with 1 IU/mL of FVIII products. The generation of plasma is described in the Online Supplementary Appendix.
Quantification of C3a and C5a
In order to quantify C3a/C3adesArg and C5a/C5adesArg, OptEIA enzyme-linked immunsorbant assay (ELISA) kit from BD Biosciences (San Diego, USA) was used in accordance with the manufacturer's instructions.
T-cell proliferation assay
Proliferation assays were performed as previously described.7 In experiments performed using autologous, FVIII-deficient (Siemens Healthcare Diagnostics GmbH), and heat-treated plasma, cells seeded in different ratios (see the Online Supplementary Appendix for details) were pooled for analysis.
Complement blocking
Blocking of complement receptors was performed by incubating DC for 1 hour with the indicated concentrations of a specific C3aR antagonist (C3aRA) or C5aR antagonist (C5aRA; both from Calbiochem, now Sigma-Aldrich; dissolved in dimethyl sulfoxide [DMSO]) prior to treatment with pdFVIII plus LPS. As a control, DC were incubated with DMSO only (Sigma-Aldrich). Complement proteins were blocked by treating DC with pdFVIII plus LPS in the presence of the following neutralizing monoclonal antibodies: anti-C3/C3a/C3adesArg (clone K13/16), anti-C5/C5a/C5adesArg (clone G25/2, both isotype IgG1, BioLegend, Fell, Germany), eculizumab (anti-C5, isotype IgG2/4, Alexion, Paris, France), or the C3-targeting peptide compstatin (Tocris Bioscience, Bristol, UK). An IgG1 isotype control antibody (clone MOPC-21, BioLegend) and ovalbumin (OVA)323-339 (ISQAVHAAHAEINEAGR, AnaSpec, Fremont, USA) served as controls.
Flow cytometry
For cell proliferation analyses by flow cytometry, T cells were collected by up- and down-pipetting. Complement receptors on DC and T cells were stained for 20 minutes (min) at 4°C using anti-human C3aR-APC (clone hC3aRZ8, BioLegend) and anti-C5aR-PE (clone D53-1473, BD Pharmingen, San Diego, USA) at their optimal concentrations. As isotype control, IgG2b-APC (clone 27-35) and IgG1-PE (clone MOPC 21, both from BD Pharmingen) were used. T-cell surface markers were stained for 20 min at 4°C using the following antibodies: anti-CD3-AmCyan (clone SK7, BD Pharmingen), anti-CD4-PacBlue, anti-CD4-APC (both clone RPA-T4, BD Pharmingen), anti-CD45RA-PerCP (clone HI100, BioLegend), anti-CD45RO-AF700 (clone IV N31, BioLegend), and anti-CCR7-PE-Cy7 (clone 3C12, BD Pharmingen). For the detection of FVIII-specific CD4+ T cells, cells were harvested, incubated for 20 min with polyglobin (Bayer Vital, Leverkusen, Germany), and stained for 2 hours with HLA-DRB1*11:01 ProT2589-608 (ENIQRFLPNPAGVQLEDPEF) tetramer-PE (Proimmune, Oxford, UK). Subsequently, cells were stained with antibodies for 20 min at 4°C, washed, and fixed with 1% paraformaldehyde. All fluorescence-activated cell sorting (FACS) analyses were performed using LSR II and LSR Fortessa flow cytometers. Data were analyzed with the BD FACSDiva software version 8.0.1 (BD Biosciences) or FlowJo software version 7.6.5 or 10.0.8 (Tree Star, Ashland, USA).
Statistical analysis and calculations
Statistical analyses are further described in the Online Supplementary Appendix.
Results
Addition of human plasma to recombinant FVIII plus lipopolysaccharide-treated dendritic cells increases T-cell proliferation
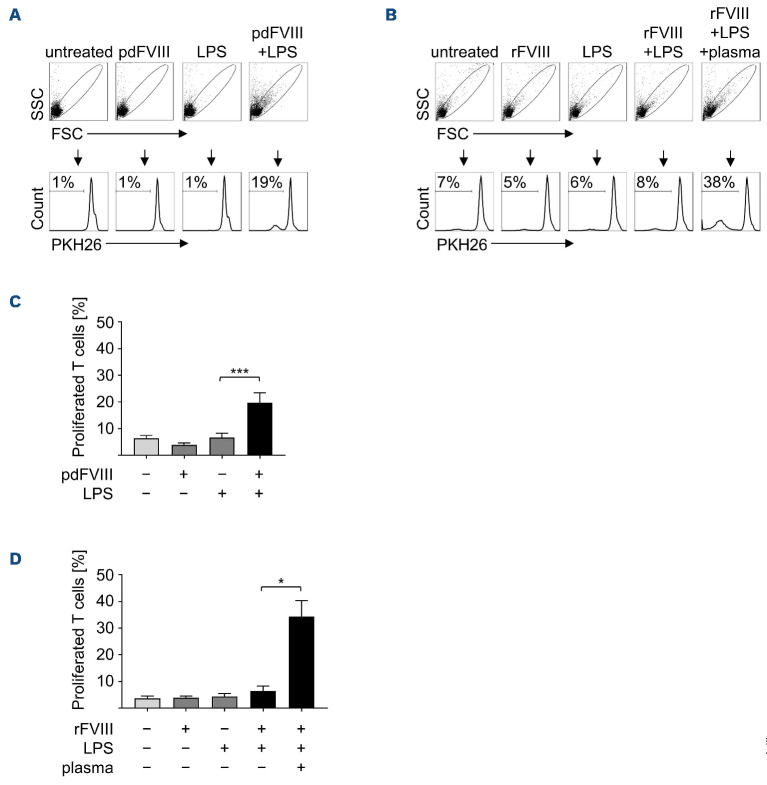
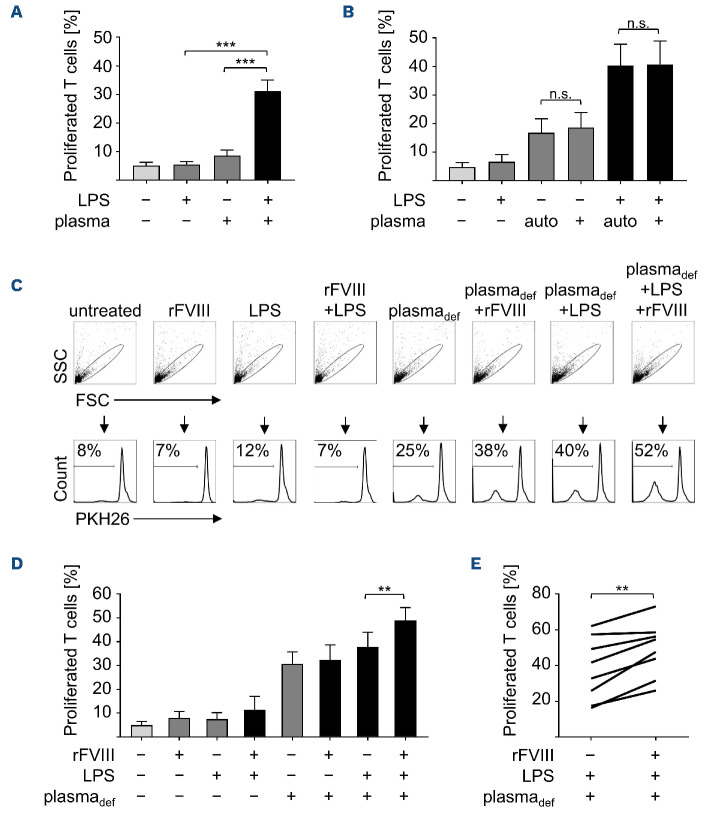
We have previously shown that pd but not rFVIII-treated DC synergistically mediate T-cell proliferation, when applied along with LPS.7 This is reproduced in Figure 1 where DC were treated either with a pd or rFVIII product, LPS, or with FVIII plus LPS. Subsequently, PKH26-labeled auto-logous T cells were added for 9 days and T-cell proliferation was analyzed as a measure of PKH26 dilution. In order to investigate if plasma components contribute to the immunogenicity of pdFVIII products, plasma was added to rFVIII plus LPS-treated DC. If not stated otherwise, the plasma used throughout this study was collected and pooled from ten individual donors. With the exception of this exogenously added plasma, all experiments throughout this study were performed in serumfree media. As shown in Figure 1B, D, addition of plasma to rFVIII plus LPS-treated DC indeed induced proliferation of co-cultivated autologous T cells. These data indicate that plasma components which are co-delivered by pd but not rFVIII products cause the synergistically enhanced induction of T-cell proliferation and thus the immunogenicity of pdFVIII products observed in vitro. This might also hold true for the in vivo situation, where plasma components are ubiquitously present within an FVIII-treated HA patient.
Figure 1.
Addition of human plasma to recombinant FVIII plus lipopolysaccharide-treated dendritic cells induces increased T-cell proliferation. Dendritic cells (DC) were treated for 24 hours with (A) plasma-derived (pd) FVIII or (B) rFVIII (each with 1 IU/mL), lipopolysaccharide (LPS) (0.1 mg/mL), or with FVIII plus LPS. In addition, DC were treated with rFVIII plus LPS in presence of plasma (2.5 mL/mL). Untreated DC served as control. PKH26-labeled autologous T cells were added and at day 9 of co-culture, cells were harvested and percentages of proliferated T cells were analyzed by fluorescence-activated cell sorting (FACS). Flow cytometry plots of 1 representative donor are shown in (A) and (B). Data of several donors are summarized from at least 3 independent experiments for (C) pdFVIII (n=14) and (D) rFVIII treatment (n=7-10). Statistical significance was determined using the Wilcoxon matched-pairs signed rank test. Error bars indicate the means ± standard error of the mean. *P<0.05; ***P<0.001.
Proliferating T cells are FVIII-specific and predominantly central memory cells
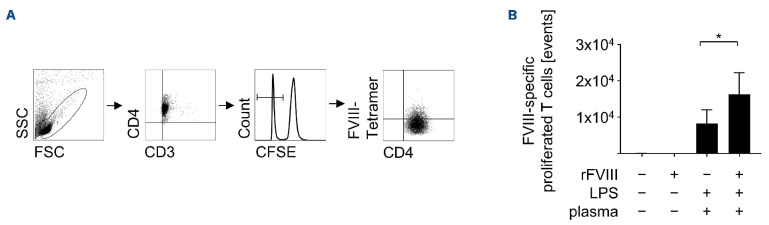
In order to verify that co-stimulation with LPS plus plasma indeed amplifies T-cell responses towards FVIII, we determined FVIII-specific T cells within proliferating CD4+ T cells. For this purpose, DC were stimulated with LPS plus plasma in the presence or absence of rFVIII, cocultivated with autologous T cells, and proliferated CD4+ FVIII-specific T cells were analyzed using a FVIII-specific tetramer. As shown in Figure 2B, DC pulsed with rFVIII alone did not mediate expansion of FVIII-specific T cells, while LPS plus plasma-stimulated DC mediated some expansion of FVIII-specific T cells. Of note, stimulating DC with LPS plus plasma in the presence of rFVIII significantly increased FVIII-specific T-cell counts within the total number of proliferating CD4+ T cells (Figure 2B). These data strongly indicate that addition of an antigen such as FVIII to LPS plus plasma-pulsed DC directs the induced T-cell response towards this given antigen. In order to further dissect T-cell subsets proliferating
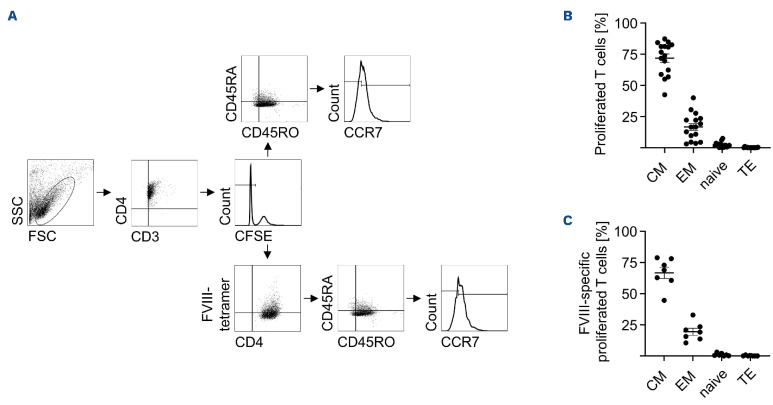
upon co-culture with LPS plus plasma and rFVIII-stimulated DC, we classified proliferating T cells as published by Thome et al.15 According to this classification, naïve T cells are CD3+CD4+CD45RO-CD45RA+CCR7+ while effector T cells are CD3+CD4+CD45RO-CD45RA+CCR7-. Furthermore, central memory (CM) T cells are characterized as being CD3+CD4+CD45RO+CD45RA-CCR7+ and effector memory (EM) T cells as being CD3+CD4+CD45RO+CD45RA-CCR7-.15
All four subsets were analyzed within the proliferating T-cell population. As shown in Figure 3B, C, both T-cell populations, total proliferating CD3+CD4+ T cells as well as proliferating CD3+CD4+ FVIII-specific T cells, consisted of approximately 72% or 67% CM and 17% or 19% EM T cells, respectively, while naïve or effector T cells were clearly underrepresented with a maximum of approximately 2%. Thus, in the presence of the antigen rFVIII, LPS plus plasma-stimulated DC predominantly induce the expansion of antigen-specific CM CD4+ T cells.
C3a and C5a are present in plasma-derived FVIII products
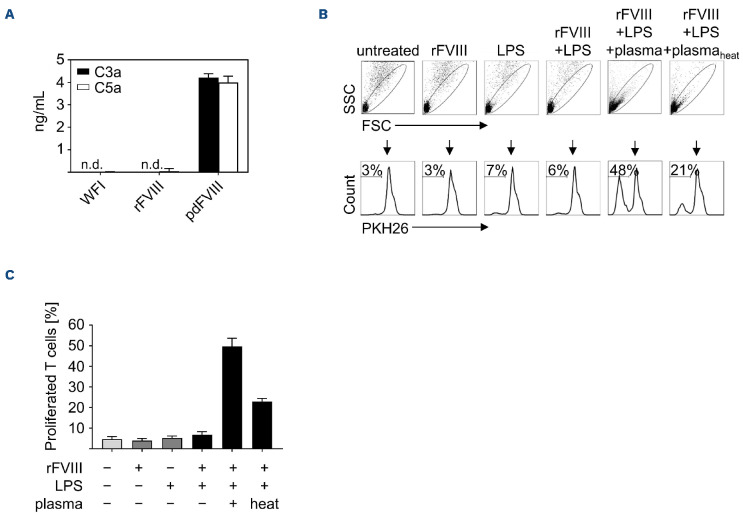
It has been shown before that C3a is present in various pdFVIII products.16 In order to investigate whether C3a and/or C5a are present in FVIII products used in our study, we determined C3a and C5a levels within these products by ELISA. As shown in Figure 4A, both C3a and C5a were detectable in the pdFVIII product, whereas both were absent in the solvent (water for injection [WFI]) and the rFVIII product. Heat treatment of plasma is a rather crude but commonly applied laboratory method to inactivate complement.17 As shown in Figure 4B, C, T-cell proliferation was strongly reduced when DC were stimulated with rFVIII plus LPS in the presence of heat-treated plasma when compared to untreated plasma. Collectively, these data provide a first hint that complement components C3a and/or C5a might be involved in the induction of T-cell proliferation under the above-mentioned conditions.
Figure 2.
Recombinant FVIII co-stimulation with lipopolysaccharide plus plasma induces the proliferation of FVIII-specific T cells. Dendritic cells (DC) from HLA-DRB1*11-positive donors were stimulated with recombinant (r)FVIII (1 IU/mL), lipopolysaccharide (LPS) (0.1 mg/mL), and plasma (2.5 mL/mL). Control DC were left untreated. After 24 hours, carboxyfluorescein succinimidyl ester (CFSE)-labeled autologous T cells were co-cultured with DC for 9 days. On day 9, T cells were harvested and stained with HLA-matched FVIII-specific tetramer and anti-CD3 and anti-CD4 antibodies for fluorescence-activated cell sorting (FACS) analyses. (A) Gating strategy for 1 representative donor is shown. In order to analyze only living T cells, gates were set in the forward/side scatter (FSC/SSC) and further on CD3+CD4+ T cells. T-cell proliferation was assessed by measuring the decrease of CFSE fluorescence intensity in the histogram. Then, CFSE- cells were defined in the dot plot as CD4+tetramer+ FVIII-specific T cells. (B) The proliferated FVIII-specific T cells are shown as absolute numbers in events. Data in (B) were obtained from 5 independent experiments (n=6-11). Statistical significance was determined using the Wilcoxon matched-pairs signed rank test. Error bars indicate the means ± standard error of the mean. *P<0.05.
C3a promotes pdFVIII plus lipopolysaccharide-mediated T-cell proliferation
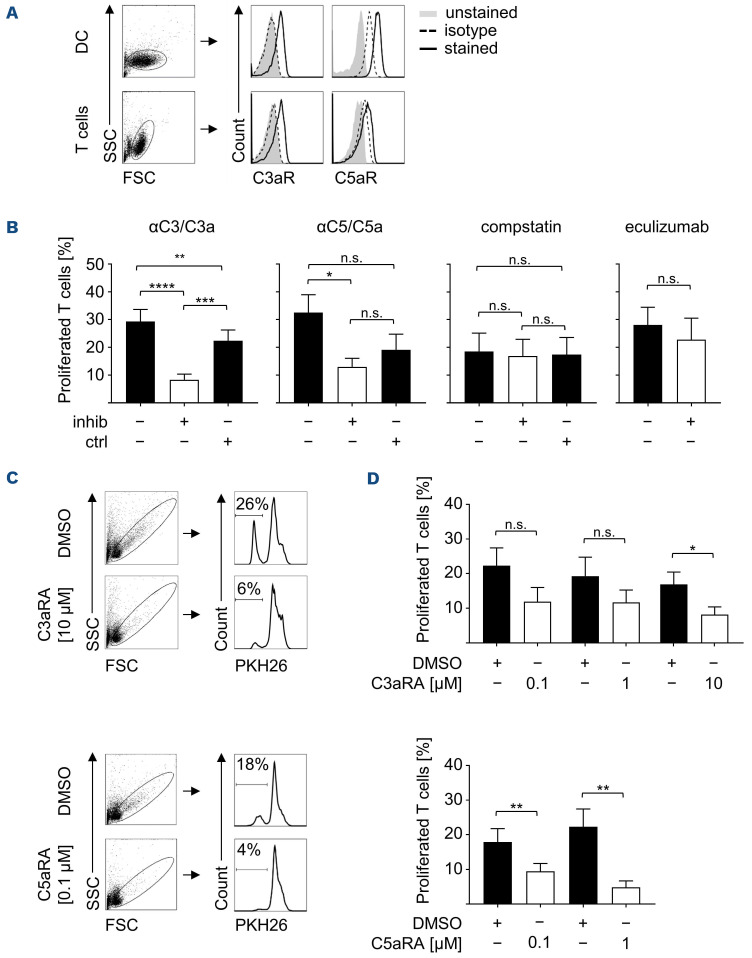
The expression of C3aR and C5aR is not limited to cells of the myeloid lineage,18 but has been reported for lymphocytes as well.11,19 Expression of the respective receptors on cells used within our study is the prerequisite for the suggested (direct) action of C3a and/or C5a on these cells. As expected, FACS analyses confirmed the expression of C3aR and C5aR on the surface of in vitro-differentiated DC and freshly isolated T cells (Figure 5A). However, the expression levels of C3aR and C5aR were found to be donor-specific (data not shown) as reported before.20-22
In order to investigate the role of C3a and/or C5a in synergistically enhanced T-cell responses observed upon coculture with pdFVIII plus LPS-treated DC, we blocked these complement components. For blocking, we used a monoclonal antibody (mAb) binding an epitope shared by both C3 and C3a, and C5 and C5a, respectively. As shown in Figure 5B, T-cell proliferation mediated by pdFVIII plus LPS-treated DC was significantly reduced upon blocking of C3/C3a. Blocking C5/C5a by a specific mAb also significantly reduced T-cell proliferation. However, also applying the isotype control mAb had an inhibitory effect. Using compstatin, a specific peptide inhibitor of C3, did not reduce T-cell proliferation indicating that T-cell proliferation mediated by pdFVIII plus LPS-treated DC indeed requires C3a but not its uncleaved precursor C3 (Figure 5B). Along this line, no reduction of T-cell proliferation was observed upon blocking C5 using the therapeutic mAb eculizumab (Figure 5B). Eculizumab is a chimeric IgG2/4 construct; hence, a corresponding isotype control was not available to us.
In order to confirm the role of C3a and/or C5a in T-cell proliferation mediated by pdFVIII plus LPS-stimulated DC, we made use of C3aR and C5aR antagonists (C3aRA and C5aRA).10,23 As shown in Figure 5C and D (upper panels), blocking C3aR significantly reduced T-cell proliferation when compared to the solvent control (DMSO). Similarly, C5aR blocking resulted in significantly lower T-cell proliferation rates when compared to controls (Figure 5C, D lower panels). Of note, using the indicated concentrations of C3aRA and C5aRA had no effect on T-cell viability but using C5aRA at 10 mM reduced T-cell viability (data not shown) which is in line with Lalli et al. showing that some C5aR signaling is necessary for maintaining murine T-cell survival.24 For this reason, we omitted data for C5aRA treatment at 10 mM.
Figure 3.
Recombinant FVIII co-stimulation with lipopolysaccharide plus plasma mainly induces proliferation of central memory T cells. Dendritic cells (DC) from HLA-DRB1*11-positive donors were treated with recombinant (r)FVIII (1 IU/mL), lipopolysaccharide (LPS) (0.1 mg/mL), and plasma (2.5 mL/mL). After 24 hours, carboxyfluorescein succinimidyl ester (CFSE)-labeled autologous CD4+ T cells were added. On day 9 of co-culture, T cells were harvested and stained with HLA-matched FVIII-specific tetramer and an antibody mix containing anti-CD3, anti-CD4, anti-CD45RA, anti-CD45RO, and anti-CCR7 antibodies. The CD4+ T cells were defined as 4 subsets: central memory (CM) T cells (CD45RO+CD45RA-CCR7+), effector memory (EM) T cells (CD45RO+CD45RA-CCR7-), naïve T cells (CD45RO-CD45RA+CCR7+), and effector T cells (TE) (CD45RO-CD45RA+CCR7-). (A) Data from 1 representative donor are shown. (B) Percentage distribution of CD4+ T-cell subtypes from proliferated CD4+ T cells (n=17, 5 donors from 7 independent experiments) and (C) from proliferated CD4+ FVIII-specific T cells (n=7, 6 donors from 2 independent experiments) are summarized. Error bars indicate the mean ± standard error of the mean. SSC: side scatter; FSC: forward scatter.
Collectively, data presented here indicate that pdFVIII products co-deliver C3a and C5a peptides of which particularly C3a is critically involved in the induction of synergistically enhanced T-cell responses upon co-cultivation with pdFVIII plus LPS-stimulated DC.
The antigen recombinant FVIII increases lipopolysaccharide- plus plasma-mediated T-cell proliferation
Adding plasma to rFVIII plus LPS-treated DC increases the proliferation of co-cultivated, autologous T cells (Figure 1C). However, enhanced T-cell proliferation was also observed upon co-culture with DC treated with LPS plus plasma in the absence of FVIII as an antigenic component (Figure 2B; Figure 6A). These observations raised two questions: first, since the plasma used in our study is of allogenic origin, non-self determinants are present and might contribute to DC-mediated T-cell proliferation; second, whether FVIII as an antigenic component contributes to T-cell proliferation at all. In order to answer the first question, we collected plasma from ten individual donors which was used to stimulate cells from each corresponding donor. As a control, the pooled plasma was used throughout the study. As shown in Figure 6B, there was no difference in T-cell proliferation no matter whether the plasma pool or autologous plasma was used in the absence or presence of LPS, indicating that mechanisms independent of self/non-self discrimination are involved. In order to investigate, whether the presence of the antigen FVIII contributes to T-cell proliferation, we treated DC with LPS and FVIII-deficient plasma in the presence or absence of rFVIII, and analyzed proliferation of co-cultured T cells. As shown in Figure 6C, D, percentages of proliferated T cells were significantly increased when rFVIII was added. This increase in proliferating T cells in the presence of rFVIII was consistent for all individual donors tested (Figure 6E). These data show that rFVIII increases T-cell proliferative responses and suggests that if an antigen such as FVIII is present, plasma plus LPS-treated DC mediate adaptive immune responses towards this given antigen.
Figure 4.
C3a and C5a are present in plasma-derived FVIII products. (A) Plasma-derived (pd) or recombinant (r)FVIII products (5 IU each) were analyzed for C3a (black bars) and C5a (white bars) by enzymelinked immunosorabant assay (ELISA) with water for injection (WFI) as solvent control. Error bars indicate standard deviations from triplicate or quadruplicate ELISA measurements. (B) Dendritic cells (DC) were treated for 24 hours with rFVIII (1 IU/mL), lipopolysaccharide (LPS) (0.1 mg/mL), or with rFVIII plus LPS in the presence or absence of plasma or heat-treated plasma (both at 2.5 mL/mL). Control DC were left untreated. PKH26-labeled autologous T cells were added and 9 days after co-culture, cells were harvested and percentages of proliferated T cells were analyzed by fluorescence-activated cell sorting. Data of 1 representative donor are shown. (C) Summarized results of several donors from 2 independent experiments are given (n=4). Error bars indicate the means ± standard error of the mean. SSC: side scatter; FSC: forward scatter.
Figure 5.
C3a promotes plasma-derived FVIII plus lipopolysaccharide-mediated T-cell responses. (A) Untreated dendritic cells (DC) and freshly isolated T cells were stained with antibodies directed against C3aR and C5aR (solid lines), respective isotype controls (dashed lines), or were left unstained (grey-shaded curves), and analyzed by fluorescence-activated cell sorting (FACS). Overlay histograms of 1 representative donor are shown. (B) DC were treated for 24 hours with plasma-derived (pd)FVIII (1 IU/mL) plus lipopolysaccharide (LPS) (0.1 mg/mL) in the presence or absence of the following complement inhibitors (inhib) or controls (ctrl): anti-C3/C3a/C3adesArg (αC3/C3a, n=16), anti-C5/C5a/C5adesArg (αC5/C5a, n=10), isotype control antibody (at 2 or 5 mg/mL), C3-targeting peptide inhibitor compstatin (n=7), control peptide (OVA323-339, both at 1 mg/mL), or anti-C5 (eculizumab, at 2 mg/mL, n=11). On day 9 of co-culture with PKH26-labeled autologous T cells, T-cell proliferation was assessed by FACS. Shown are the percentages of proliferated T cells from at least 3 independent experiments. (C) and (D) DC were pretreated for 1 hour with antagonists for C3aR (C3aRA) or C5aR (C5aRA) at the indicated concentrations followed by treatment with pdFVIII (1 IU/mL) plus LPS (0.1 mg/mL). Control DC were pretreated with dimethyl sulfoxide (DMSO) prior to stimulation with pdFVIII plus LPS. 24 hours later, PKH26-labeled auto-logous T cells were added and co-cultured for 9 days before the percentages of proliferated PKH26-labeled T cells were analyzed by flow cytometry. (C) FACS plots of 1 representative donor are given for C3aRA applied at 10 mM and C5aRA at 0.1 mM. (D) Summarized data of several donors (n=8) from 3 independent experiments are shown. Statistical significance was determined using the Wilcoxon matched-pairs signed rank test. Error bars indicate the means ± standard error of the mean.*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; ns: not significant. SSC: side scatter; FSC: forward scatter.
Discussion
We have previously shown that pd but not rFVIII products plus LPS synergistically activate DC and that these DC subsequently mediate enhanced proliferation of auto-logous T cells in vitro.6,7 Here we show that DC pulsed with rFVIII plus LPS mediate synergistic T-cell activation when human plasma is added (Figure 1; Figure 6). Until now, it is still under debate whether pd or rFVIII products are more immunogenic. There are studies claiming higher inhibitor rates for one or the other type of product as well as those showing similar risks for both.4 In a randomized trial (SIPPET), the incidence of FVIII inhibitors was assessed among patients treated with pd or rFVIII. Here, Peyvandi et al.25 found that patients treated with pdFVIII had a lower incidence of inhibitors than those treated with rFVIII. Subsequently, they suggested that rFVIII products might be more immunogenic.
Figure 6.
Addition of the antigen FVIII increases T-cell proliferation. (A) Dendritic cells (DC) were either left untreated or treated with lipopolysaccharide (LPS) (0.1 mg/mL), plasma (2.5 mg/mL), or plasma plus LPS. 24 hours later, PKH26-labeled autologous T cells were added and co-cultured for 9 days before fluorescence-activated cell sorting (FACS) analysis. T-cell proliferation is indicated as percentages of PKH26-labeled T cells from 7 independent experiments (n=18). (B) DC were left untreated or treated for 24 hours with LPS (0.1 mg/mL), plasma of either autologous (auto) or foreign origin (each at 25 µmL/mL), or with LPS plus the indicated plasma followed by co-cultivation with PKH26-labeled autologous T cells for 9 days. The percentages of proliferated T cells were analyzed for 10 donors by flow cytometry. (C) Treatment of DC for 24 hours with rFVIII (1 IU/mL), LPS (0.1 mg/mL), or FVIII-deficient plasma (plasmadef, 2.5 mL/mL), each alone or in combination as indicated. Untreated DC served as control. PKH26-labeled autologous T cells were added and after 9 days, T-cell proliferation was analyzed by FACS. Representative data for 8 donors analyzed are shown. (D) Summarized data from 8 donors and 3 experiments. (E) Comparison of the percentages of proliferated T cells obtained in the presence or absence of rFVIII. Statistical significance was determined using the Wilcoxon matched-pairs signed rank test. Error bars indicate the means ± standard error of the mean. **P<0.01; ***P<0.001; ns: not significant. SSC: side scatter; FSC: forward scatter.
We hypothesize that plasma components, which are either co-delivered by pd but not rFVIII products within in vitro experiments, or which are ubiquitously present in FVIII-treated patients per se, contribute to the observed T-cell activation. This explains why we observe synergistic DC and T-cell activation in vitro only in the presence of pdFVIII or plasma (this study and6,7), while in HA patients both pd and rFVIII products can induce the generation of inhibitors. Of note, pdFVIII products consist of approximately 2% FVIII while 98% are made up by plasma proteins others than FVIII.26
Interestingly, it has been shown that plasmapheresis, the most common method to obtain plasma for fractionation of pdFVIII, can induce complement activation resulting in the formation of C3a and C5a27,28 and consequently, we and others detected C3a and C5a in various pdFVIII products (Figure 4, data not shown, and 16). Complement components are broadly recognized for their pro-inflammatory and immunomodulatory effects. Accordingly, they can even be involved in autoimmunity (summarized in29-31). Assembling this information, we suggested a role for the complement components C3a and C5a in FVIII-specific T-cell activation and indeed, we identified C3a and, to a lesser extent, C5a as mediators of enhanced proliferation of total CD3+CD4+ and FVIII-specific T cells (Figure 5).
In our experimental setting, C3a and C5a act in concert with LPS in order to mediate T-cell activation (Figure 5). Stimulating T cells with DC pulsed with LPS or plasma alone minimally induces T-cell proliferation while DC pulsed with both LPS plus plasma clearly induce T-cell proliferation (Figure 6A). Interestingly, it has been assumed for decades that within LPS-stimulated peripheral blood mononuclear cells, T-cell proliferative responses can be enhanced if human serum is added.32 In line with this, Morrison et al. showed that LPS can activate the complement system via the classical or the alternative pathway13 and Zhang et al. observed an enhancement of TLR-mediated inflammatory responses by complement components.33 Additionally, the expression of C3a and C5a receptors on human DC can be increased when cells are treated with LPS.34 Hence, it is most likely that the TLR and the complement pathway crosstalk in order to mediate T-cell proliferative responses when T cells are co-cultured with pdFVIII plus LPS-pulsed or rFVIII plus LPS plus plasma-pulsed DC.
Whether these direct effects of complement are at the DC or T-cell level or whether C3a and C5a have an impact on both cell types cannot be dissected within our experimental setting. However, we have previously shown a synergistic effect of pdFVIII plus LPS directly on human DC which upregulated co-stimulatory molecules and enhanced the expression of pro-inflammatory cytokines,6,7 suggesting an effect on the level of DC for our recent analyses as well. This is in line with data showing that stimulation of human DC with C3a and C5a increases the expression of HLA-DR and CD86 as well as the expression of pro-inflammatory cytokines.35 Of note, Gutzmer et al. also observed the upregulation of co-stimulatory molecules CD83 and CD86 on human DC when stimulated with recombinant C3a. However, these C3a-stimulated DC did not mediate enhanced proliferative responses of auto-logous T cells towards the tetanus toxoid antigen.21 Thus, stimulation of DC with complement alone does not seem to be sufficient to mediate full-blown T-cell responses which is reflected also by our data showing that DC pulsed with pdFVIII or plasma alone do not mediate T-cell proliferation but addition of LPS is needed (Figure 1; Figure 6).
Of note, a direct effect of C3a and/or C5a on T cells might also contribute to pdFVIII- or plasma-mediated T-cell proliferation since also T cells express C3aR and C5aR (Figure 5 and 11,19) and expand upon co-culture with DC-derived C3a or C5a.10
It has been reported before that complement component C3 can mediate antigen uptake by APC36 and that C3 and its cleavage product C3b can enhance FVIII endocytosis by DC in vitro.37 Whether these mechanisms are also of importance in our experimental setting has not been elucidated yet.
Under the experimental conditions chosen here, a source of complement is the pdFVIII product or the exogenously added plasma. However, it has been repeatedly shown that also immune cells themselves including DC and T cells can produce and secrete complement components such as C3a and C5a.10 In particular, co-stimulatory interactions of cognate APC:T cell partners can generate C3a and C5a locally and moreover, upregulate C3aR and C5aR.38,39 In vivo, plasma, complement components, and danger signals are provided by the FVIII-treated patients themselves. Especially in episodes of massive bleeding, injuries, or surgeries, complement can be activated and danger signals can be present. Interestingly, it has been shown that event-related FVIII substitution, which is required in exactly these aforementioned situations, is associated with enhanced inhibitor development.4,40,41
As can be deduced from our experiments using plasma plus LPS to induce T-cell proliferation (Figure 6) and from our analyses detecting FVIII-specific T cells (Figure 2), a substantial number of T cells proliferate under these experimental conditions that have a specificity different from FVIII. It is a well-known in vitro phenomenon that bystander cells, in particular CD4+ memory T cells, can be activated independently of antigen by cytokines secreted by T cells that have been activated antigen-dependently.42-44 However, our data clearly indicate that adding FVIII as antigen significantly enhances total T-cell proliferation and expansion of FVIII-specific T cells (Figure 2; Figure 6). Whether this FVIII-specific T-cell activation/proliferation translates into antibody formation is still an open question. However, development of FVIII-specific antibodies is not restricted to HA patients. Natural FVIII-specific autoantibodies are found in 19% of healthy subjects45,46 and FVIII-specific CD4+ T cells are found in healthy donors and show a naïve and CM phenotype.47
Collectively, our data suggest a model in which danger signals such as LPS and the complement components C3a and/or C5a act in concert to synergistically enhance CD4+ T- cell responses towards a given antigen, present at this particular time point. In case of FVIII-substituted HA patients, specifically upon an event-related FVIII substitution, these danger signals plus complement-mediated T-cell responses are (largely or to a certain amount) directed towards the infused FVIII. Whether this mechanism of enhancing CD4+ T-cell responses is specific for FVIII or holds true for a number of (self)antigens will be a matter of future investigations.
Supplementary Material
Acknowledgments
We thank Stefanie Kronhart and Dorothea Kreuz for excellent technical support, Peter Crauwels for providing an isotype control, Kay-Martin Hanschmann for statistical analyses, and Ger van Zandbergen and Andreas Hunfeld for fruitful discussion. We are thankful to Gerrit Praefcke for critically reading the manuscript.
Funding Statement
Funding: This study was supported by a scholarship from the Stiftung Polytechnische Gesellschaft (Frankfurt, Germany) (to JH).
References
- 1.Graw J, Brackmann H-H, Oldenburg J, Schneppenheim R, Spannagl M, Schwaab R. Haemophilia A: from mutation analysis to new therapies. Nat Rev Genet. 2005;6(6):488-501. [DOI] [PubMed] [Google Scholar]
- 2.Male C, Andersson NG, Rafowicz A, et al. Inhibitor incidence in an unselected cohort of previously untreated patients with severe haemophilia B: a PedNet study. Haematologica. 2021;106(1):123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oldenburg J, Schröder J, Brackmann HH, Müller-Reible C, Schwaab R, Tuddenham E. Environmental and genetic factors influencing inhibitor development. Semin Hematol. 2004;41(1 Suppl 1):S82-88. [DOI] [PubMed] [Google Scholar]
- 4.Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046-4055. [DOI] [PubMed] [Google Scholar]
- 5.Kurnik K, Bidlingmaier C, Engl W, Chehadeh H, Reipert B, Auerswald G. New early prophylaxis regimen that avoids immunological danger signals can reduce FVIII inhibitor development. Haemophilia. 2010;16(2):256-262. [DOI] [PubMed] [Google Scholar]
- 6.Miller L, Weissmüller S, Ringler E, et al. Danger signaldependent activation of human dendritic cells by plasma-derived factor VIII products. Thromb Haemost. 2015;114(2):268-276. [DOI] [PubMed] [Google Scholar]
- 7.Miller L, Ringler E, Kistner KM, Waibler Z. Human dendritic cells synergistically activated by FVIII plus LPS induce activation of autologous CD4+ T cells. Thromb Haemost. 2018;118(4):688-699. [DOI] [PubMed] [Google Scholar]
- 8.Timperio AM, Gevi F, Grazzini G, Vaglio S, Zolla L. Comparison among plasma-derived clotting factor VIII by using monodimensional gel electrophoresis and mass spectrometry. Blood Transfus. 2010;8(Suppl 3):S98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981-986. [DOI] [PubMed] [Google Scholar]
- 10.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant. 2013;13(10):2530-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liszewski MK, Kolev M, Le Friec G, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Lambris JD. Crosstalk pathways between Tolllike receptors and the complement system. Trends Immunol. 2010;31(4):154-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison DC, Kline LF. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977;118(1):362-368. [PubMed] [Google Scholar]
- 14.Klos A, Tenner AJ, Johswich K-O, Ager RR, Reis ES, Köhl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46(14):2753-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thome JJC, Yudanin N, Ohmura Y, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159(4):814-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodde MF, Kehrel BE. Markers of blood cell activation and complement activation in factor VIII and von Willebrand factor concentrates. Transfus Med Hemother. 2010;37(4):175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soltis RD, Hasz D, Morris MJ, Wilson ID. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology. 1979;36(1):37-45. [PMC free article] [PubMed] [Google Scholar]
- 18.Dustin ML. Complement receptors in myeloid cell adhesion and phagocytosis. Microbiol Spectr. 2016;4(6):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbore G, West EE, Spolski R, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science. 2016;352(6292):aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nataf S, Davoust N, Ames RS, Barnum SR. Human T cells express the C5a receptor and are chemoattracted to C5a. J Immunol. 1999;162(7):4018-4023. [PubMed] [Google Scholar]
- 21.Gutzmer R, Lisewski M, Zwirner J, et al. Human monocyte-derived dendritic cells are chemoattracted to C3a after up-regulation of the C3a receptor with interferons. Immunology. 2004;111(4):435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werfel T, Kirchhoff K, Wittmann M, et al. Activated human T lymphocytes express a functional C3a receptor. J Immunol. 2000;165(11):6599-6605. [DOI] [PubMed] [Google Scholar]
- 23.Camous L, Roumenina L, Bigot S, et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117(4):1340-1349. [DOI] [PubMed] [Google Scholar]
- 24.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112(5):1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054-2064. [DOI] [PubMed] [Google Scholar]
- 26.Basilico F, Nardini I, Mori F, et al. Characterization of factor VIII pharmaceutical preparations by means of MudPIT proteomic approach. J Pharm Biomed Anal. 2010;53(1):50-57. [DOI] [PubMed] [Google Scholar]
- 27.Sonntag J, Emeis M, Vornwald A, Strauss E, Maier RF. Complement activation during plasma production depends on the apheresis technique. Transfus Med. 1998;8(3):205-208. [DOI] [PubMed] [Google Scholar]
- 28.Burnouf T, Eber M, Kientz D, Cazenave J-P, Burkhardt T. Assessment of complement activation during membranebased plasmapheresis procedures. J Clin Apher. 2004;19(3):142-147. [DOI] [PubMed] [Google Scholar]
- 29.Kemper C, Köhl J. Novel roles for complement receptors in T cell regulation and beyond. Mol Immunol. 2013;56(3):181-190. [DOI] [PubMed] [Google Scholar]
- 30.Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol. 2015;194(8):3542-3548. [DOI] [PubMed] [Google Scholar]
- 31.Karasu E, Demmelmaier J, Kellermann S, et al. Complement C5a induces pro-inflammatory microvesicle shedding in severely injured patients. Front Immunol. 2020;11:1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RA, Gartner S, Kaplan HS. Stimulation of mitogenic responses in human peripheral blood lymphocytes by lipopolysaccharide: serum and T helper cell requirements. J Immunol. 1978;121(6):2160-2164. [PubMed] [Google Scholar]
- 33.Zhang X, Kimura Y, Fang C, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110(1):228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li K, Fazekasova H, Wang N, et al. Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol. 2011;48(9-10):1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li K, Fazekasova H, Wang N, et al. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology. 2012;217(1):65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacquier-Sarlin MR, Gabert FM, Villiers MB, Colomb MG. Modulation of antigen processing and presentation by covalently linked complement C3b fragment. Immunology. 1995;84(1):164-170. [PMC free article] [PubMed] [Google Scholar]
- 37.Rayes J, Ing M, Delignat S, et al. Complement C3 is a novel modulator of the anti-factor VIII immune response. Haematologica. 2018;103(2):351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strainic MG, Liu J, Huang D, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188(2):183-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouw SC, van der Bom JG, van den Marijke Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648-4654. [DOI] [PubMed] [Google Scholar]
- 41.Eckhardt CL, van der Bom JG, van der Naald M, Peters M, Kamphuisen PW, Fijnvandraat K. Surgery and inhibitor development in hemophilia A: a systematic review. J Thromb Haemost. 2011;9(10):1948-1958. [DOI] [PubMed] [Google Scholar]
- 42.Bangs SC, Baban D, Cattan HJ, Li CK-F, McMichael AJ, Xu X-N. Human CD4+ memory T cells are preferential targets for bystander activation and apoptosis. J Immunol. 2009;182(4):1962-1971. [DOI] [PubMed] [Google Scholar]
- 43.Di Genova G, Savelyeva N, Suchacki A, Thirdborough SM, Stevenson FK. Bystander stimulation of activated CD4+ T cells of unrelated specificity following a booster vaccination with tetanus toxoid. Eur J Immunol. 2010;40(4):976-985. [DOI] [PubMed] [Google Scholar]
- 44.Boyman O. Bystander activation of CD4+ T cells. Eur J Immunol. 2010;40(4):936-939. [DOI] [PubMed] [Google Scholar]
- 45.Whelan SFJ, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121(6):1039-1048. [DOI] [PubMed] [Google Scholar]
- 46.Lacroix-Desmazes S, Misra N, Bayry J, Mohanty D, Kaveri SV, Kazatchkine MD. Autoantibodies to factor VIII. Autoimmun Rev. 2002;1(1-2):105-110. [DOI] [PubMed] [Google Scholar]
- 47.Meunier S, Menier C, Marcon E, Lacroix-Desmazes S, Maillère B. CD4 T cells specific for factor VIII are present at high frequency in healthy donors and comprise naïve and memory cells. Blood Adv. 2017;1(21):1842-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.