Abstract
After allogeneic hematopoietic stem cell transplantation (HSCT), the emergence of circulating cytomegalovirus (CMV)-specific T cells correlates with protection from CMV reactivation, an important risk factor for non-relapse mortality. However, functional assays measuring CMV-specific cells are time-consuming and often inaccurate at early time-points. We report the results of a prospective single-center, non-interventional study that identified the enumeration of Dextramer-positive CMV-specific lymphocytes as a reliable and early predictor of viral reactivation. We longitudinally monitored 75 consecutive patients for 1 year after allogeneic HSCT (n=630 samples). The presence of ≥0.5 CMV-specific CD8+ cells/mL at day +45 was an independent protective factor from subsequent clinically relevant reactivation in univariate (P<0.01) and multivariate (P<0.05) analyses. Dextramer quantification correlated with functional assays measuring interferon-γ production, and allowed earlier identification of high-risk patients. In mismatched transplants, the comparative analysis of lymphocytes restricted by shared, donor- and host-specific HLA revealed the dominant role of thymic-independent CMV-specific reconstitution. Shared and donor-restricted CMV-specific T cells reconstituted with similar kinetics in recipients of CMV-seropositive donors, while donor-restricted T-cell reconstitution from CMV-seronegative grafts was impaired, indicating that in primary immunological responses the emergence of viral-specific T cells is largely sustained by antigen encounter on host infected cells rather than by cross-priming/presentation by non-infected donor-derived antigen-presenting cells. Multiparametric flow cytometry and high-dimensional analysis showed that shared-restricted CMV-specific lymphocytes display a more differentiated phenotype and increased persistence than donor-restricted counterparts. In this study, monitoring CMV-specific cells by Dextramer assay after allogeneic HSCT shed light on mechanisms of immune reconstitution and enabled risk stratification of patients, which could improve the clinical management of post-transplant CMV reactivations.
Introduction
The outcome of allogeneic hematopoietic stem cell transplantation (HSCT), a therapeutic option for several hematologic diseases,1 has improved dramatically in recent years.2 However, cytomegalovirus (CMV) reactivation is still one of the most important complications of allogeneic HSCT.
CMV serological status of the donor and recipient represents a well-recognized risk factor for CMV disease after allogeneic HSCT.3 Its impact on survival remains controversial, dampened by the introduction of new detection methods, highly effective prophylactic (e.g., letermovir) and/or preemptive strategies, but amplified by the growing use of new transplant platforms such as post-transplant cyclophosphamide,4,5 associated with a high incidence of viral infections, including CMV.6,7 Post-transplant immune reconstitution profoundly influences the risk of CMV reactivation and survival.8,9 Immune reconstruction occurs through different mechanisms, including thymus-independent expansion of naïve and antigen-experienced T cells present in the graft, and differentiation of donor-derived hematopoietic precursors educated in the host thymus.10 The relative contribution of these mechanisms in protecting patients from CMV still needs to be fully elucidated. Functional CMV-specific immune reconstruction, evaluated by either by interferon (IFN)-γ enzymelinked immunospot (ELISpot),11,12 flow cytometry13,14 or QuantiFERON-CMV,15,16 correlates with a lower rate of CMV reactivation. Nonetheless, IFN-γ ELISpot and cytokine detection by flow cytometry are cumbersome and time-consuming techniques. QuantiFERON-CMV on whole blood can be more easily implemented by routine diagnostic laboratories but a large percentage (38%) of results are “indeterminate”.17 Circulating CMV-specific T cells can also be enumerated directly by using fluorescent-labeled multimers of MHC-peptide complexes.18-20 Gratama et al. rigorously demonstrated that the recovery of CMV-specific T cells, evaluated by multimer staining, within 65 days after allogeneic HSCT is associated with a reduced incidence of CMV reactivation.19 Recently, more powerful tools have been developed, consisting of multiple MHC-peptide complexes and fluorochromes covalently bound to a dextran polymer backbone (i.e., Dextramer reagents). The higher number of MHC-complexes increases the binding avidity of these reagents compared to tetramers, allowing more specific and sensitive monitoring of CMV-specific CD8+ T cells early after allogeneic HSCT.21 The availability of a large repertoire of Dextramer reagents allows the evaluation of patients with different human leukocyte antigen (HLA) haplotypes.22
Recent European Guidelines for CMV management suggest monitoring IFN-γ-producing CMV-specific T lymphocytes to better identify patients at highest risk of developing viral reactivation,23 but no consensus has yet been found on the best setting for this monitoring.24 The inclusion of CMV-specific T-cell responses in the risk stratification and clinical decision-making of patients undergoing allogeneic HSCT needs further validation in prospective clinical trials.
We report herein the results of a prospective, singlecenter, non-interventional study to ascertain the potential of CMV-specific T-cell reconstitution assessed by Dextramer assay in conferring protection against CMV-related clinically relevant events (CRE) after allogeneic HSCT with post-transplant cyclophosphamide. Furthermore, taking advantage of the possibility afforded by flow cytometry to quantify and characterize CMV-specific T cells restricted for different HLA alleles, we describe the differential contribution of CD8+ T lymphocytes restricted for either shared or donor-specific HLA to the viral-specific immune reconstitution after HLA-mismatched HSCT.
Methods
Allogeneic hematopoietic stem cell transplantation procedures
The majority of patients received a treosulfan-based conditioning regimen with post-transplant cyclophosphamide (50 mg/kg/day), on days +3 and +4, and sirolimus.25,26 Patients received antiviral prophylaxis with acyclovir, according to institutional guidelines. Until 100 days after HSCT, CMV was monitored weekly in whole blood by realtime quantitative polymerase chain reaction assay. CRE were defined as viral disease or viremia requiring preemptive treatment,27 and CMV end-organ disease was classified according to reported criteria.28 Anti-viral therapy was started after the first detection of CMV DNAemia above the cutoff (10,000 IU/mL). Furthermore, in the case of detectable CMV DNAemia below the cutoff value, even at low levels (<100 IU/mL), the analysis was repeated every 2-3 days until negativity or positivity above the threshold. All patients were treated according to institutional programs upon written informed consent for transplant procedures, use of medical records and immunological studies, within the non-interventional “CMVMON study”, approved by Ospedale San Raffaele Institutional Ethical Committee on 09/03/2017.
Dextramer staining on fresh blood
CMV-specific CD8+ T cells were quantified using a Dextramer CMV kit (Immudex) according to the manufacturer’s instructions. High HLA resolution was required to identify the correct Dextramer reagent to be used. More details, including the limit of detection and the analytical sensitivity of the assay, are provided in the Online Supplementary Materials.
Flow cytometry analysis on frozen peripheral blood mononuclear cells
Peripheral blood mononuclear cells were obtained from whole blood by density gradient centrifugation (Lymphoprep, Sentinel diagnostics) and frozen. After thawing, cells were stained with a 20-color panel. High-dimensional analysis was performed using the application cytoChain.29
Interferon-γ ELISpot
Frequencies of CMV-specific T cells were assessed on frozen peripheral blood mononuclear cells by IFN-γ ELISpot (Lophius). Specific spot-forming cells (sfc) were counted by the ImmunoCapture 7.0 software (TLC ELISpot Reader). Results were expressed as CMV-specific sfc/µL.30
QuantiFERON-CMV
Peripheral blood (3 mL) was collected into QuantiFERONCMV (Qiagen) tubes and analyzed according to the manufacturer’s instruction. Data and results were analyzed using REVELATION DSX® software.
Statistical analysis
Statistical analyses were performed by GraphPad Prism 9 and Jmp 13 software. Comparisons between two groups were carried out by the Mann-Whitney test. For the comparison of more than two groups, in the presence of one variable, a Kruskal-Wallis test was used followed by the Dunn multiple comparison test. In the presence of two variables, the comparison between groups was carried out by two-way analysis of variance followed by the Sidak (for related factors) or uncorrected Fisher LSD (for not related factors) multiple comparison tests. The Shapiro-Wilk test was employed to check for normality. Multivariate analysis was performed using logistic regression. For the comparison of cumulative incidences, the Gray test was used. The time-event analysis was performed using a logrank test and Wilcoxon survival test. More details on the Methods are included in the Online Supplementary Materials.
Results
Characteristics of the patients and allogeneic transplants
Between December 13, 2017 and February 23, 2019, 84 consecutive adult patients who underwent allogeneic HSCT in our center were screened for inclusion in the study (Figure 1A). HLA class-I alleles were not covered by the Dextramer CMV Kit in only nine patients (10.7%), who were therefore excluded.
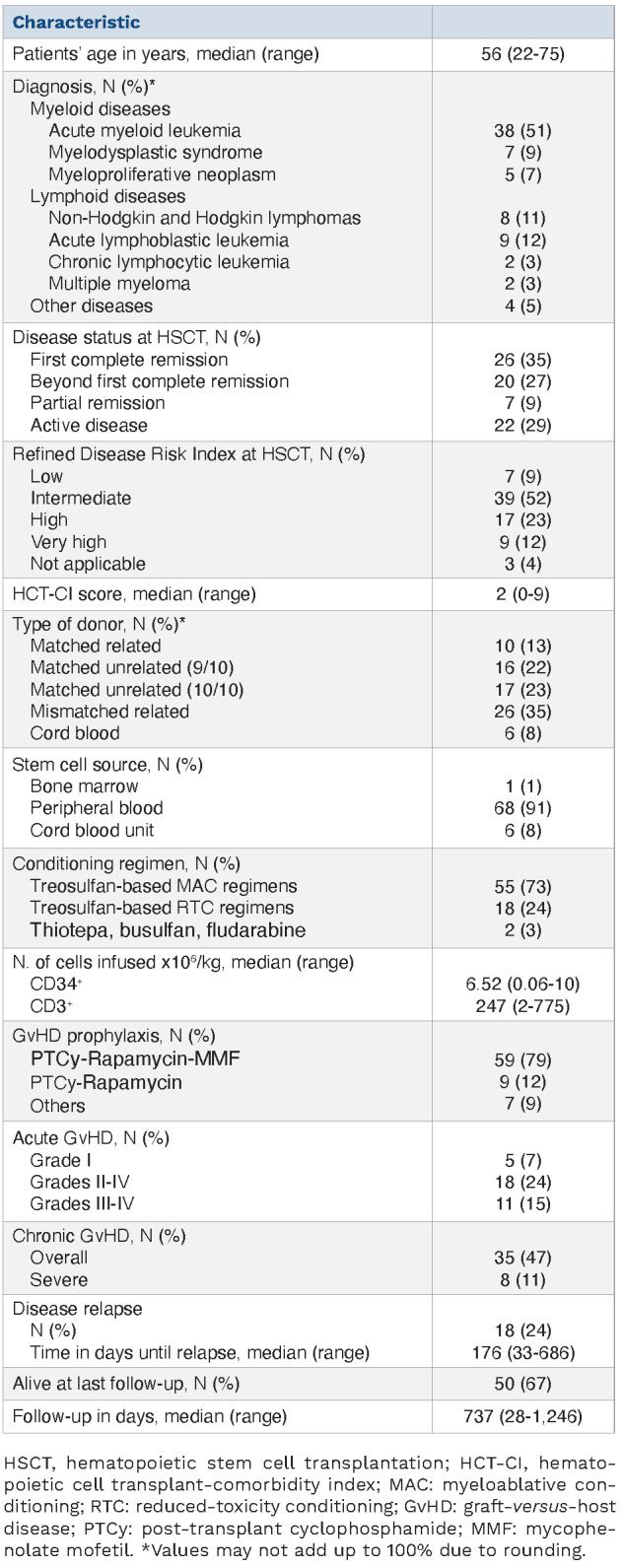
The characteristics of the patients and their transplants are shown in Table 1. Most patients were affected by myeloid malignancies (acute myeloid leukemia, 51%; myelodysplastic syndrome, 9%). At HSCT, 38% of patients were not in complete remission. According to the Disease Risk Index, patients were stratified in low-intermediate (61%), high (23%), and very high (12%) risk; three patients affected by benign disorders were not classifiable. Conditioning was myeloablative in most of cases (76%). The sources of the stem cells for transplantation were matched-unrelated donors (n=33), mismatched-related donors (n= 26), matched-related donors (n=10) and cord blood units (n=6).
Engraftment was obtained in 72 patients within a median of 24 and 22 days after HSCT for neutrophils and platelets, respectively. There were two deaths within the first 30 days, before engraftment, and one graft failure. Eighteen patients developed grades II-IV acute graft-versus-host disease (GvHD; 24%), and 11 patients reported grades III-IV acute GvHD (15%). Relapse occurred in 18 patients (24%). At last follow-up 50 patients were alive. Causes of death were disease (n=11) or non-relapse events (n=14).
Management of clinically relevant events and immunomonitoring
CRE occurred in 39 patients (52%) at a median time of 68 days (range, 0-208) (Table 2). These patients received preemptive treatment, mainly consisting of foscarnet, ganciclovir or valganciclovir. Subsequent viral reactivations occurred in 7% of patients. Moreover, 11 patients developed end-organ CMV disease (3 cases of pneumonia and 8 of colitis/gastroenteritis), and were treated with foscarnet (n=3) or ganciclovir (n=8).
A total of 630 samples, comprising the donor apheresis and peripheral blood harvested before lymphodepletion and at eight time-points after HSCT for up to 1 year, were analyzed using the Dextramer CMV Kit (Figure 1B, Online Supplementary Figure S1), which allows the evaluation of seven HLA alleles covering ~95% of the European population (Online Supplementary Table S1). In these samples we evaluated CMV-specific CD8+ T cells restricted for HLA molecules shared (S) between the donor and the host, and for donor-specific (D) and host-specific (H) HLA. In about half the patients (47.9%) CMV-specific T cells restricted for only one HLA allele could be evaluated with the Dextramer CMV Kit, whereas for the remaining patients we could evaluate two (37.0%), three (13.7%) or four (1.4%) HLA. No differences were observed in CMV-specific T lymphocyte counts detected in patients with different numbers of evaluable restrictions (Online Supplementary Figure S2A), whether D-specific or S (Online Supplementary Figure S2B). CMV-specific T cells from 21 patients who received their transplant from HLA-mismatched donors and were evaluable for either D- or H-specific HLA restrictions were deeply characterized by polychromatic flow cytometry after thawing at +30, +60, +90, +180 and +365 days (n=84 samples). CMV-specific T-cell responses were also quantified by quantiFERON-CMV on fresh peripheral blood samples (n=47, based on the availability of the diagnostic kit) and by IFN-γ ELISpot on frozen peripheral blood mononuclear cells (n=113 samples) at +30, +60, +90 and +180 days after HSCT (Figure 1A).
Figure 1.
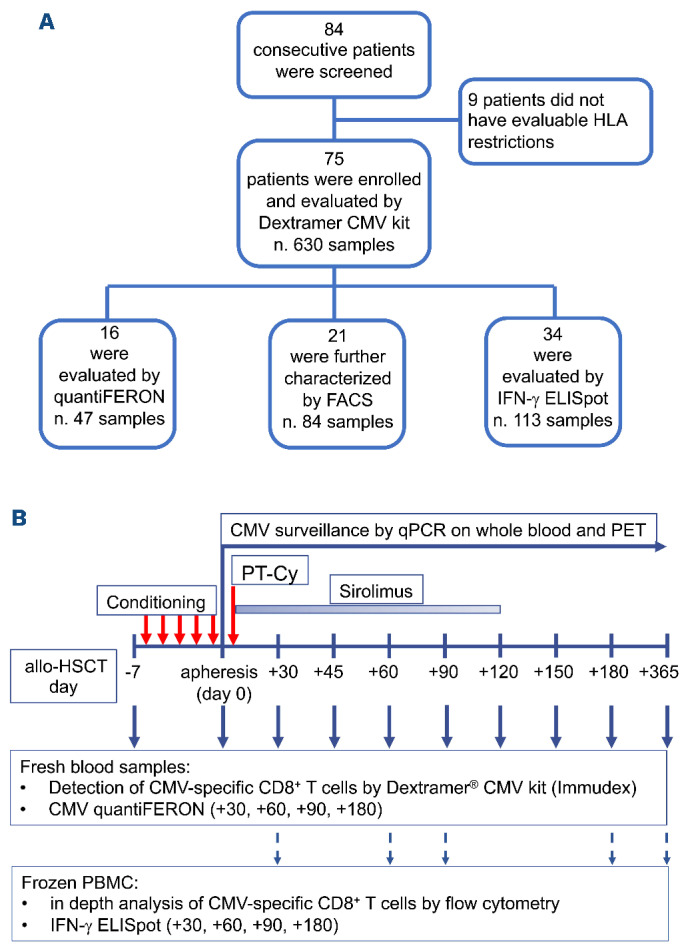
Study design and enrollment flow chart. (A) Diagram showing the numbers of patients and samples included in each analysis. Eighty-four consecutive patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) were screened for inclusion in the study. Nine patients were excluded because no HLA class-I allele of either the patient or the donor was evaluable for assessment by the Dextramer CMV Kit. For the remaining 75 patients, in 630 longitudinal fresh samples CMV-specific CD8+ T cells were enumerated by the Dextramer CMV Kit and 47 fresh samples from 16 patients were analyzed by QuantiFERON-CMV. Eighty-four frozen samples from the 21 patients in whom donor- or host-specific restrictions could be evaluated were characterized by an extensive flow cytometry panel. IFN-γ ELISpot was also used to detect CMV-specific T cells in all the samples analyzed by QuantiFERON-CMV and/or in-depth flow cytometry. (B) Design of the study. Peripheral blood samples were taken before conditioning and at the indicated timepoints (solid blue arrows) after HSCT and used for the Dextramer CMV Kit and QuantiFERON-CMV assays. Donors’ apheresis samples were analyzed on the day of infusion. At selected time-points (dotted blue arrows) peripheral blood mononuclear cells were also frozen for subsequent flow cytometry analysis and IFN-γ ELISpot assay. HLA: human leukocyte antigen; CMV: cytomegalovirus; FACS: fluorescence activated cell sorting; IFN: interferon; ELISpot: enzyme-linked immunospot; qPCR: quantitative polymerase chain reaction; PET: pre-emptive antiviral therapy; PT-Cy: post-transplant cyclophosphamide; allo-HSCT: allogeneic hematopoietic stem cell transplantation; PBMC: peripheral blood mononuclear cells.
Early reconstitution of cytomegalovirus-specific CD8+ T cells is associated with reduced subsequent clinically relevant events
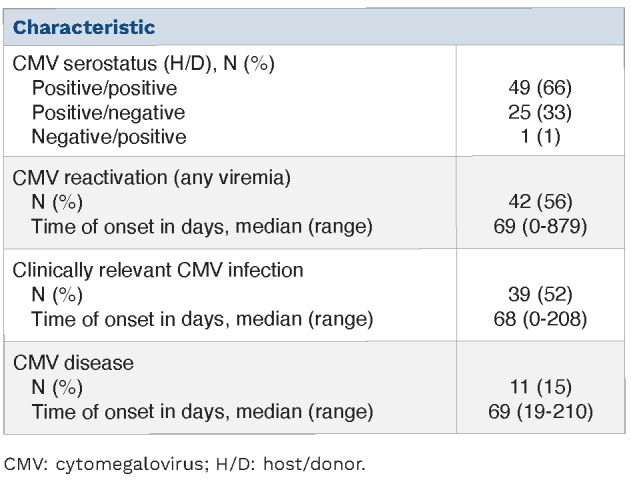
The majority of CRE occurred in the first 120 days after HSCT. Within the same timeframe CMV-specific T cells increased in patients, reaching the highest level at 150 days (mean=45.0 CMV-specific T cells/mL) and then remaining stable up to day 365 (Figure 2A). Notably, donor CMV-seronegative status was associated with an increased incidence of CRE (P=0.0414) (Online Supplementary Figure S3A). Despite this, counts of CMV-specific CD8+ T cells infused with the graft were comparable in patients who did or did not experience post-transplant CRE (Online Supplementary Figure S3B). This finding was confirmed even considering only patients receiving transplants from seropositive donors, the only grafts with detectable CMV-specific cells (Online Supplementary Figure S3B). These data indicate that the infusion of CMV-specific primed T cells is necessary but not sufficient for early protection from CRE, and that early post-HSCT factors (e.g., prophylaxis and treatment for GvHD) may impinge on in vivo CMV-specific T-cell expansion. Indeed, among post-transplant variables, the occurrence of acute GvHD (P=0.0116) (Online Supplementary Figure S3C), particularly those cases requiring administration of systemic steroids (grade II-IV, P=0.0019) (Online Supplementary Figure S3D), was associated with an increased incidence of CRE. After HSCT, patients showed fast reconstitution of CD3+, CD8+ T lymphocytes and NK cells, and delayed reconstitution of B cells, CD4+ and regulatory T lymphocytes (Online Supplementary Figure S4A), as previously described in allogeneic HSCT with post-transplant cyclophosphamide and sirolimus.25
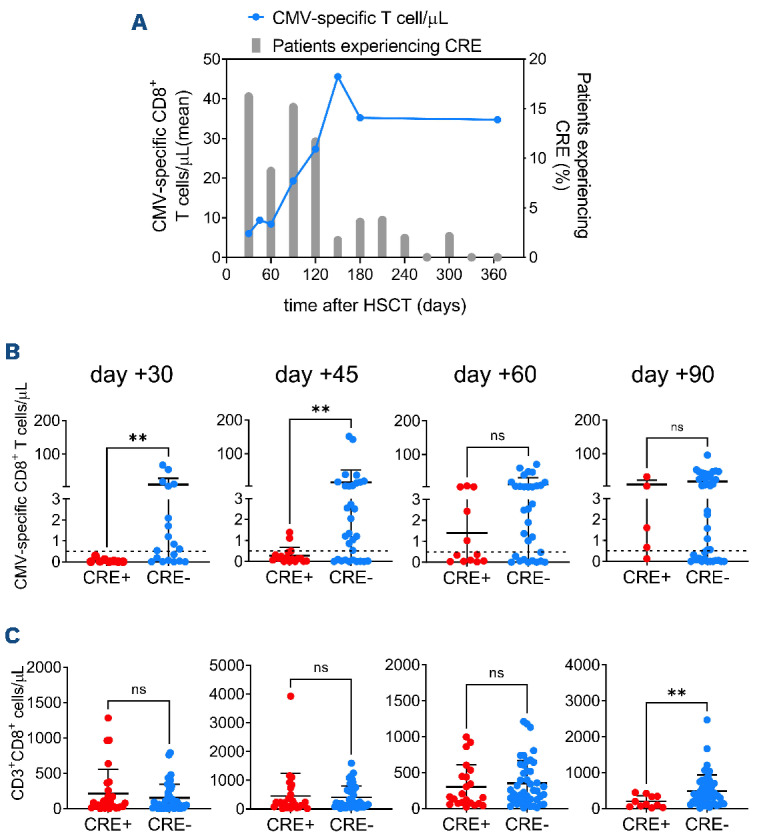
Interestingly, at 30 days (P=0.0088) and 45 days (P=0.0084) after HSCT, CMV-specific CD8+ T-cell counts were higher in patients who did not experience CRE after these time-points than in patients with subsequent CRE, indicating a protective role for early CMV-specific immunity against succeeding viral reactivations (Figure 2B, Online Supplementary Table S2). This correlation was not found at day +30 for any other immune subset evaluated (Online Supplementary Figure S4B). High amounts of total CD8+ T cells were associated with reduced subsequent CRE only at 90 days (Figure 2C). No association between CMV-specific CD8+ T cell counts and subsequent CRE was found from 60 to 180 days after HSCT (Figure 2B, Online Supplementary Figure S5A). Receiver operating characteristic (ROC) analysis identified the threshold of 0.5 CMV-specific CD8+ T-cells/μL at day 45 after HSCT as the best predictor of subsequent CRE (P=0.0094, sensitivity=88.24%, specificity=67.74%) (Figure 3A, Online Supplementary Table S3). Reaching this threshold was confirmed as an independent protective factor from CRE in both competitive risk analysis with non-relapse mortality (P=0.02) (Figure 3B) and in a multivariate analysis including acute GvHD and host/donor serostatus (P=0.04) (Figure 3C). CMV-specific CD8+ T-cell counts in patients’ peripheral blood prior to HSCT did not correlate with CRE risk (Online Supplementary Figure S5B). Interestingly, reduced risk of CMV disease was associated with the presence of CMV-specific T lymphocytes in pre-HSCT host peripheral blood but not at any post-HSCT timepoint (Online Supplementary Figure S5B, C and data not shown), suggesting a role in the protection against tissue CMV-mediated damage for the recently described peripheral host T cells remaining after HSCT.31
Table 1.
Characteristics of the patients (N=75) and their transplants.
Overall, these data highlight the importance of an early evaluation of CMV-specific T cells as a reliable predictor of CRE.
Quantification of cytomegalovirus-specific T cells by Dextramer staining correlates with functional assays
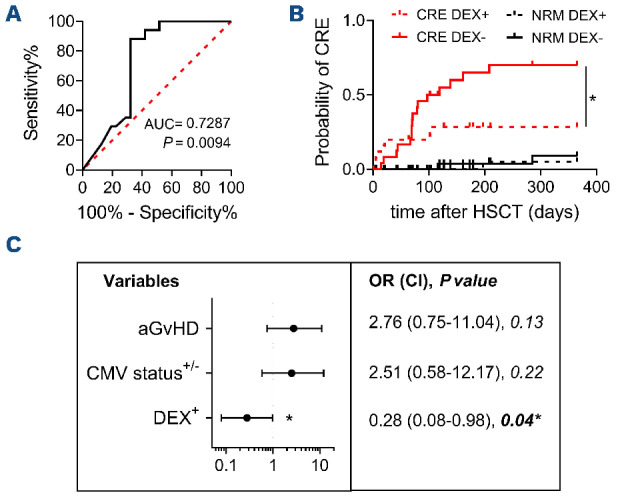
To further investigate the predictive value of CMV-specific T-cell quantification, QuantiFERON-CMV15,32 and IFN-γ ELISpot functional assays were also performed on a selected subset of patients (Figure 1A). IFN-γ ELISpot at +30 days failed to predict subsequent CRE (Online Supplementary Figure S6A-F). At later timepoints absolute counts of CMV-specific sfc/μL were lower in patients who experienced subsequent CRE than in patients able to control the virus (P=0.0249 for 60 days; P=0.0402 for 90 days after HSCT) (Online Supplementary Figure S6B). ROC analysis performed on data collected at +60 and +90 days identified 1.75 CMV-specific sfc/μL as the threshold to discriminate patients at higher risk of viral reactivations (P=0.0018, sensitivity=92.86%, specificity=67.67%) (Online Supplementary Figure S6G). Dextramer staining showed 81.94% agreement with IFN-γ ELISpot (n=59/72 concordant results) (Figure 4A, Online Supplementary Table S4) and 88.89% agreement with QuantiFERON-CMV (n=24/27) (Figure 4B, Online Supplementary Table S4). The two functional assays showed a concordance of 81.82% (n=27/33) (Online Supplementary Table S4). Linear regression analysis confirmed the agreement between CMV-specific T-cell counts evaluated by Dextramer assay and those obtained by either IFN-γ ELISpot (P<0.0001) (Figure 4C) or QuantiFERON-CMV (P=0.0007) (Figure 4D). Collectively, these correlations indicate that quantification of CMV-specific T lymphocytes by Dextramer staining represents a strong surrogate biomarker of functional viral-specific T-cell responses, able to predict protection from viral reactivations earlier and more rapidly than functional assays.
Table 2.
Cytomegalovirus reactivation and disease after hematopoietic stem cell transplantation.
Cytomegalovirus-specific T cells restricted by shared, donor, and host HLA molecules reconstitute with different kinetics and have different impacts on the incidence of clinically relevant events
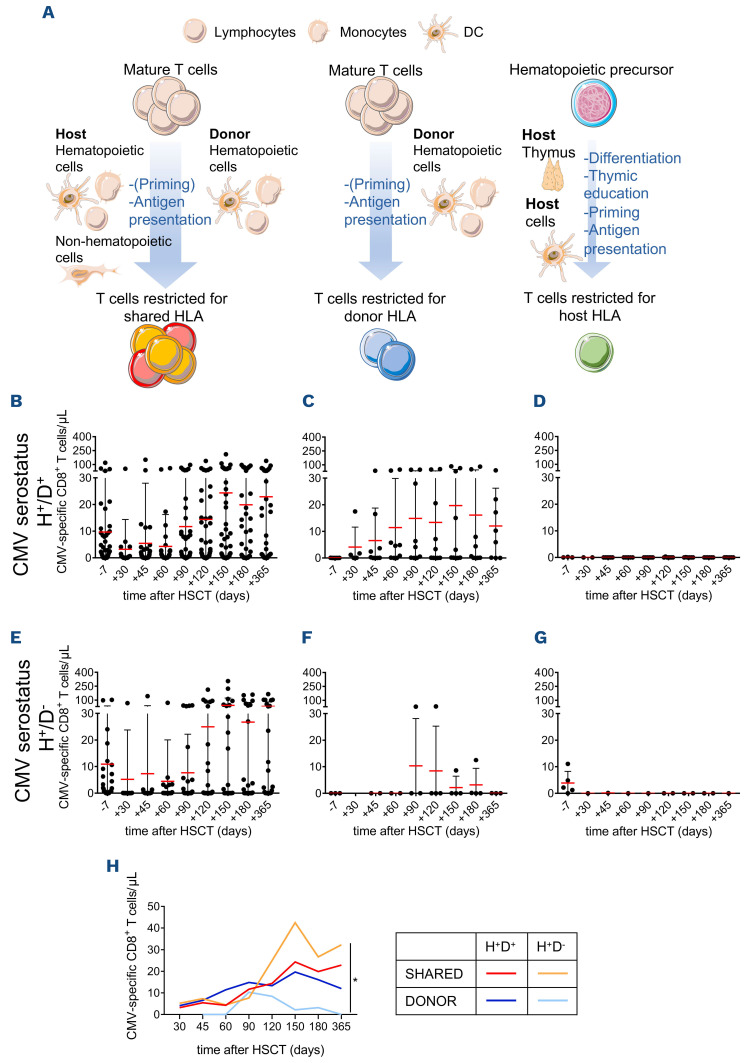
After HSCT, the expansion of memory T cells restricted for S HLA might be sustained by either host cells or donorderived hematopoietic cells, whereas only the latter express D-specific HLA and therefore activate D-restricted CMV-specific T lymphocytes. Being absent in the donor T-cell repertoire, H-restricted T cells require maturation in the host thymus and priming by host antigen-presenting cells (APC) (Figure 5A). To characterize these different dynamics, we separately analyzed CMV-specific CD8+ T cells restricted for S (n=64 patients), D (n=19 patients) or H (n=13 patients) HLA molecules. The kinetics of immune reconstruction were evaluated in CMV-seropositive patients who received their grafts from either seropositive (Figure 5B-D) or seronegative (Figure 5E-G) donors. When the donor is seronegative, D-restricted CMV-specific T cells can be primed and stimulated in the host only by donor-derived hematopoietic cells, whereas S-restricted lymphocytes can be primed by either D-derived or residual host APC and can be further stimulated by any infected cell. We found that D-restricted reconstitution was impaired in the case of seronegative donors, differently from S-restricted immune reconstruction (P<0.001 for time, P=0.04 for restriction type) (Figure 5E, F, H), indicating that in primary immunological responses the emergence of viral-specific T cells is largely sustained by the antigen encounter on host infected non-hematopoietic or residual tissue-resident myeloid cells rather than by cross-priming/presentation by non-infected donor-derived APC. Accordingly, S- and D-restricted T lymphocytes reconstituted with similar magnitudes and kinetics when the donor was seropositive (Figure 5B, C, H), indicating an equal ability of infected donor hematopoietic cells and host cells in presenting viral antigens, thus sustaining secondary immune responses. H-restricted T cells were not detected in post-HSCT samples for up to 1 year (Figure 5D, G). H-restricted T lymphocytes were absent also at 2 or 3 years after HSCT in the patients evaluable at those time-points (n=4 and n=2, respectively) or in three other younger patients (aged 20-29) evaluated for up to 4 years after haploidentical HSCT (data not shown).
Figure 2.
Early reconstitution of cytomegalovirus-specific immunity is associated with reduced subsequent clinically relevant events. (A) Longitudinal evaluation of cytomegalovirus (CMV)-specific immunity by Dextramer staining (blue line, mean value of CMV-specific CD8+ T cells/mL in all patients) and of the percentage of evaluable patients experiencing clinically relevant events (CRE; gray bars) at each time-point after hematopoietic stem cell transplantation (HSCT). (B) Absolute numbers of CMV-specific CD8+ T lymphocytes at the indicated time-points after HSCT in patients experiencing or not subsequent CRE. Continuous lines, mean ± standard deviation. The dotted lines indicate the level of 0.5 CMV-specific T cell/mL. (C) Absolute numbers of total CD8+ T lymphocytes at the indicated time-points after HSCT in patients experiencing or not subsequent CRE. Continuous lines, mean ± standard deviation. The analyses in (B) and (C) were performed with the MannWhitney test: **P<0.01; ns: not significant.
Figure 3.
The presence of ≥0.5 cytomegalovirus-specific CD8+ T cells/mL at 45 days afer hematopoietic stem cell transplantation protects against subsequent clinically relevant events. (A) Receiver operating characteristic analysis for the identification of a protective amount of cytomegalovirus (CMV)-specific CD8+ T cells/mL at 45 days after hematopoietic stem cell transplantation (HSCT). (B) Competitive risk analysis for the risks of clinically relevant events and non-relapse mortality in patients having or not at least 0.5 CMV-specific CD8+ T cells/mL at 45 days after HSCT. (C) Left panel: forest plot showing CRE risk according to the occurrence of acute graft-versus-host disease, host/donor CMV serostatus or the presence of at least 0.5 CMV-specific CD8+ T cells/mL at 45 days after HSCT. Right panel: results of the statistical analysis. The analysis in (B) was performed with the Gray test and that in (C) by logistic regression: *P<0.05. AUC: area under the curve; DEX: Dextramer; aGvHD: acute graft-versus-host disease; OR: odds ratio; CI: confidence interval.
High-dimensional analysis of cytomegalovirus-specific CD8+ T lymphocytes reveals earlier maturation in T cells restricted by donor-specific HLA molecules
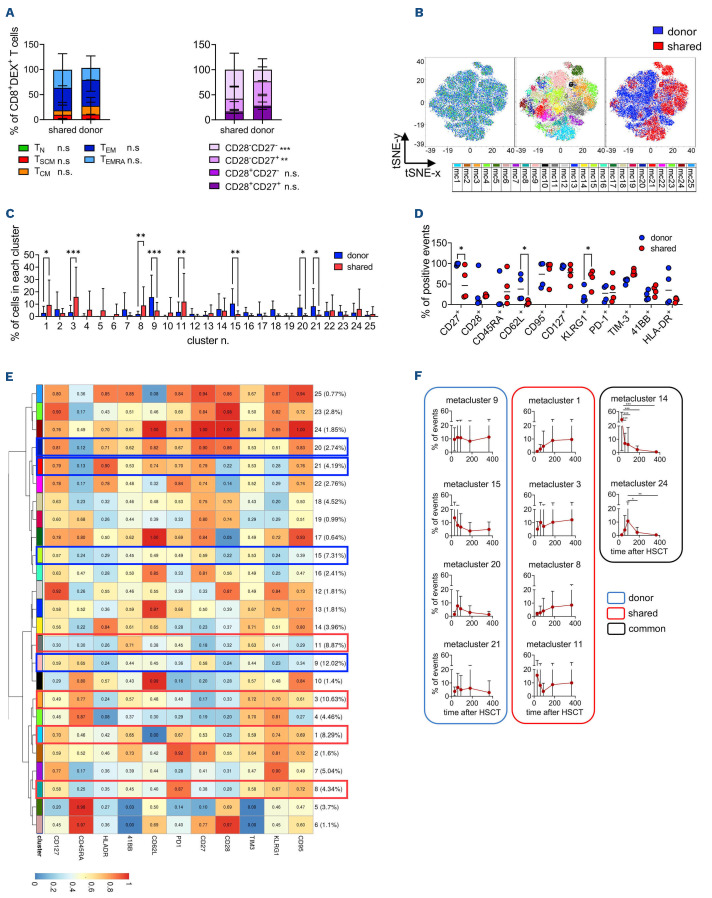
Divergent differentiation, activation or exhaustion of CMV-specific T cells might account for the different dynamics we observed in T lymphocytes restricted for S or D HLA. To shed light on these potential mechanisms, the phenotype of CMV-specific CD8+ T lymphocytes was investigated by multiparametric flow cytometry with additional Dextramer reagents in 21 patients for whom either a S or a D-specific HLA restriction could be separately evaluated by Dextramer analysis. Overall, 68 CMV-specific T-cell subpopulations (n=15 patients) were detected at different time-points. A representative gating strategy is shown in Online Supplementary Figure S7. CMV-specific CD8+ T-cell counts in frozen samples correlated with those evaluated in fresh samples (P<0.001) (Online Supplementary Figure S8A). By manual gating we found that CMV-specific CD8+ T cells contained more TEM (P=0.002) and fewer TN (P=0.003), TCM (P=0.002) and early-differentiated CD28+CD27+ cells (P<0.001) compared to bulk CD8+ T lymphocytes (Online Supplementary Figure S8B). CMV-specific CD8+ T cells restricted by D HLA molecules displayed a less differentiated phenotype compared to S-restricted ones (P<0.001 for CD28–CD27–; P=0.002 for CD28–CD27+) (Figure 6A).
To identify S and D-specific signatures, we performed high-dimensional analysis and clustering by cytoChain29 on CMV-specific CD8+ T-cell populations with at least 65 events (n=55). The results revealed a clear segregation of the metaclusters depicting S- and D-restricted CMV-specific CD8+ T cells (Figure 6B). Interestingly, this segregation was also maintained in T cells derived from the same samples but differentially restricted for D or S alleles (Online Supplementary Figure S8C). In detail, metaclusters n. 9 (P<0.001), 15 (P=0.002), 20 (P=0.02) and 21 (P=0.01) were enriched in D-restricted T lymphocytes (Figure 6C), and were characterized by enhanced expression of early-differentiation markers (CD62L, P=0.046; CD27, P=0.014) and by lower KLRG1 expression (P=0.026) compared to those enriched in S-restricted T cells (metacluster n.1, P=0.01; 3, P<0.001; 8, P=0.009; and 11, P=0.002) (Figure 6C-E, Online Supplementary Figure S8D). Furthermore, HLA-DR expression was high in metaclusters n. 20 and 21, indicating recent activation of D-restricted lymphocytes. S-restricted metaclusters showed a trend to reduced CD127 expression, confirming a late differentiated phenotype. No differences were detected in the expression of inhibitory receptors, such as PD-1 and TIM-3, nor in 4-1BB or CD28 between D- and S-specific metaclusters (Figure 6D). Longitudinal analysis revealed variation across time of two metaclusters. Metacluster n. 14 was enriched at +30 days compared to other time-points (P<0.001), and was composed of activated TCM cells. Metacluster n. 24, which comprises early differentiated activated TSCM/TCM lymphocytes, peaked at +90 days after HSCT compared to later time-points (P=0.03 for day +180, P=0.007 for day +365) (Figure 6E, F), in line with an early triggering of CMV-specific cells by CMV reactivation events. Consistently, we also found early peaks of metaclusters expressing high CD28 and low PD-1 (n. 6 and n. 12), features that have been associated with higher anti-CMV activity33,34 (Online Supplementary Figure S8E). Interestingly the majority of metaclusters that accumulated in time (n. 1, 3, 8 [all S-specific], and 9 [D-specific]) displayed a TEM/TEMRA phenotype, in part characterized by the expression of CD27 (metaclusters n. 1 and n.9) but not CD28, a phenotype typical of memory inflation, often observed in chronic infections (Figure 6E, F).35
Figure 4.
Quantification of cytomegalovirus-specific T cells by Dextramer staining correlates with functional assays. (A, B) Concordance between cytomegalovirus (CMV)-specific T-cell quantification by the Dextramer assay (positivity threshold 0.5 cells/mL) and either IFN-γ ELISpot for CMV (A, positivity threshold 1.75 sport-forming cells/mL after CMV antigen stimulation) or QuantiFERON-CMV (B, positivity threshold 0.2 IU/mL in the CMV antigen tube). (C, D) Linear regression analysis between CMV-specific CD8+ T cells quantified by the Dextramer assay and the presence of functional IFN-γ producing CMV-specific T lymphocytes detected by either IFN-γ ELISpot (C) or QuantiFERON-CMV (D). CMVag: cytomegalovirus antigen; sfc: spot-forming cells; IFN: interferon.
Kinetics of D-restricted metaclusters showed the rise of metacluster n. 9, depicting CD27+ TEM/TEMRA, and a stable curve for metacluster n. 21, composed of activated TCM cells, while metaclusters n. 15 and n. 20 contracted rapidly (Figure 6E, F).
Overall, these results suggest that although D- and S-restricted CMV-specific T lymphocytes are triggered simultaneously, early after transplantation, the quality of this triggering is different, leading to the brisk accumulation of a pool of early memory D-restricted cells, while S-restricted cells expand more robustly and persist longer.
Discussion
CMV-specific T cells protect patients not only from CMV reactivations but also from severe infections overall,30 thus representing a good surrogate for general immune competence after allogeneic HSCT. There is, therefore, a growing interest in the use of immunomonitoring to adjust treatment according to individual risk. In this prospective, observational study, we performed a detailed longitudinal analysis of CMV-specific immune reconstruction and its dynamics.
Looking for a highly sensitive assay we relied on MHC-multimer staining with Dextramer reagents to enumerate CMV-specific T cells in whole blood by flow cytometry. Allogeneic HSCT is the ideal context for MHC multimerbased immunomonitoring, since the HLA type of donor-host couples is known. We observed an inverse correlation between CMV-specific T-cell counts at +30/+45 days after allogeneic HSCT and the risk of experiencing subsequent CRE. Most of the previously tested biomarkers of CMV-specific immunity predicted relevant clinical events at later time-points (tetramer staining at day +65;19 QuantiFERON-CMV at day +90;15 and IFN-γ ELISpot at day +10012), thus reflecting the advantage of Dextramer staining in the management of allogeneic HSCT patients. One of the main concerns related to MHC multimer-based immunomonitoring is the possibility of evaluating limited HLA restrictions. Strikingly, the large repertoire of Dextramer reagents allowed us to evaluate nearly 90% of enrolled patients. The majority of patients in our cohort were Caucasian (76 out of 84; 90.5%), followed by six Hispanics (7.1%) and two Asians (2.4%). Interestingly, five out of six Hispanic patients and one out of two Asian patients could be enrolled in our study.
Figure 5.
Cytomegalovirus-specific T cells restricted by shared, donor or host HLA molecules reconstitute with different kinetics and differentially affect the incidence of clinically relevant events. (A) Immune reconstruction after hematopoietic stem cell transplantation (HSCT) with T lymphocytes restricted for shared, donor- or host-specific HLA molecules require priming/activation by different antigen-presenting cells or infected cells. (B-G) Longitudinal reconstitution of cytomegalovirus (CMV)-specific CD8+ T lymphocytes evaluated by Dextramer assay at the indicated time-points before and after HSCT is shown separately for CMV-seropositive recipients infused with cells from either seropositive (panels B-D) or seronegative (panels E-G) donors. Absolute counts of T cells restricted for shared (B and E), donor-specific (C and F) or host-specific (D and G) HLA alleles are reported. Lines, mean ± standard deviation. (H) Means of longitudinal reconstitution of CMV-specific CD8+ T lymphocytes restricted for either shared or donor-specific HLA alleles in CMV-seropositive patients receiving grafts from either seropositive or seronegative donors, as indicated in the graph legend. The analysis in (H) was by two-way analysis of variance. *P<0.05. DC: dendritic cell; HLA: human leukocyte antigen; H; host; D: donor These data highlight the feasibility of this technique in the Caucasian population, and possibly in other ethnicities. It is largely recognized that both CD4+ and CD8+ CMV-specific T cells contribute to anti-viral immune surveillance.36,37 Thus, Dextramer reagents evaluating only CD8+ T cells can theoretically underestimate CMV-specific T-cell immunity. In addition, MHC-multimer staining does not measure T-cell function. To verify the impact of these potential limitations, we compared different techniques for the evaluation of CMV-specific immunity. The enumeration of CMV-specific T cells by Dextramer staining showed strong concordance with functional assays, proving a good surrogate biomarker of functional immunity. Despite evaluating both CD4+ and CD8+ T-cell responses, IFN-γ ELISpot predicted the clinical outcome at later time-points (day +60/+90) than did the Dextramer-binding assay (day +30/45), underlining the higher sensitivity of the latter and confirming previous results obtained in different allogeneic HSCT transplant platforms.30,38 The Dextramer CMV kit is already available as an in vitro diagnostic test, is characterized by reliable and reproducible performances, requires a smaller amount (1 mL) of peripheral blood and should be preferred when studying individuals with evaluable HLA. As an alternative assay for patients lacking evaluable HLA types, we propose IFN-γ ELISpot, which is not dependent on pre-defined HLA alleles and which predicted a lower risk of developing CMV reactivations when the threshold of 1.75 specific sfc/μL was reached at 60/90 days after HSCT.
Flow cytometry performed after Dextramer staining also allows single-cell multiparametric characterization of CMV-specific lymphocytes. We exploited this feature to shed light on the kinetics and weight of viral-specific responses that emerge during post-transplant immune reconstruction through different mechanisms. Our data suggest that up to 1 year after HSCT the CMV-specific immune reconstruction mainly relies on mature T cells infused within the graft, which could be antigen-experien ced or naïve depending on the donor’s CMV serostatus and can recognize CMV epitopes restricted by S or D-specific HLA. In the same time-frame, thymic instruction of donor-derived progenitors is impaired. H-restricted subpopulations of CMV- and flu-specific T cells have been detected in adult patients 6-14 years after HSCT;39 the absence of the thymic contribution to CMV-specific immune reconstruction in our cohort (median age, 56 years) could be due to age-related involution of the host thymus or to thymic damage caused by conditioning regimens or by subclinical GvHD.40 For this reason, in adult patients, we suggest that Dextramers restricted by D or S HLA alleles should be preferentially used, rather than H HLA alleles. In recipients of grafts from CMV-seropositive donors, we observed similar kinetics in S- and D-restricted CMV-specific T cells, pointing to an equal ability of donor hematopoietic cells and host cells in presenting viral antigens thus sustaining secondary immune responses to CMV. Interestingly, in recipients of CMV-seronegative donors, D-restricted CMV-specific cells appeared with delayed kinetics and at a reduced frequency compared to S-restricted cells. This observation suggests that the emergence of viral-specific T cells in primary responses is largely sustained by infected non-hematopoietic cells, presenting only epitopes on shared HLA alleles. Considering the wide tropism of CMV,41 we may assume that the amount and duration of antigenic stimulation may favor S-restricted T cells. An alternative explanation for this observation could be early priming by residual infected host APC,42 able to prime S-restricted T cells from seronegative donors, thus providing them an initial advantage and impairing the contribution of the latecoming D-restricted cells.43 The more extensive evaluation of S- and D-restricted CMV-specific T cells in patients receiving transplants from CMV-seronegative donors and that experience delayed CMV reactivation in the absence of host APC, such as in the context of prophylaxis with letermovir,27 could allow these hypotheses to be discriminated. The memory phenotype of CMV-specific T lymphocytes, a critical parameter for protective CMV-specific immunity after HSCT,44,45 indicates that CMV-specific cells restricted for D or S HLA alleles contribute differentially to memory subpopulations. D-restricted T lymphocytes display an earlier memory phenotype and lower persistence than S-restricted cells. In contrast, S-restricted cells expand progressively, peaking at 150 days, when CMV reactivations contract, and remain stable thereafter. Our study indicates the benefits of evaluating CMV-specific immunity by Dextramer assay and allowed us to identify a threshold of CMV-specific T cells which stratifies patient’s risk of CRE in the context of allogeneic HSCT with post-transplant cyclophosphamide. Letermovir has recently been approved for the prophylaxis of CMV infection in seropositive transplant recipients27 and is associated with reduced non-relapse mortality by preventing or delaying CRE.46 However, late CMV reactivations occur,27,47 and in the few patients experiencing CMV DNAemia during letermovir prophylaxis the currently used assays cannot discriminate between non-infective DNA from abortively infected cells and infective virions.48 Impaired polyclonal T-cell reconstitution and CMV-specific immunity have recently been reported in patients receiving prophylaxis with letermovir.49,50 In this context, enumeration of CMV-specific T cells might allow identification of the patients who need prophylaxis to be prolonged beyond day 100.
In conclusion, monitoring CMV-specific T-cell counts in peripheral blood by Dextramer assay may become an important biomarker-driven strategy to facilitate risk stratification and to optimize anti-viral therapy, minimizing drug exposure, thus improving the clinical management of patients undergoing allogeneic HSCT.
Figure 6.
Unsupervised analysis of CD8+Dextramer+ T lymphocytes reveals earlier maturation in donor-restricted T cells. (A) Manual gating analysis for the distribution of differentiation T-cell subsets in CD8+Dextramer+ T lymphocytes restricted for shared or donor-specific HLA. Differentiation T-cell subsets were defined according to the expression profile of CD45RA, CD62L and CD95 markers (left panel) or to the combination of CD28 and CD27 (right panel). TN (T-naïve): CD45RA+CD62L+CD95–; TSCM (T stem cell memory): CD45RA+CD62L+CD95+; TCM (T central memory): CD45RA–CD62L+; TEM (T effector memory): CD45RA–CD62L–; TEMRA (T effector memory RA): CD45RA+CD62L–. (B) tSNE map (left panel) and Flow-SOM metacluster overlay (middle panel) of CD8+Dextramer+ T cells from all the concatenated samples. The right panel shows the different distribution of Dextramer+ T lymphocytes restricted for donor-specific (blue) or shared (red) HLA molecules. (C) Frequency of metaclusters expressed as the percentage of cells in each metacluster for each type of HLA restriction among total CD8+Dextramer+ T lymphocytes. Shared subpopulations (n=29), donor-specific subpopulations (n=26). (D) Percentage of events positive for the indicated markers in the metaclusters enriched in CD8+Dextramer+ T cells restricted for donor-specific (blue, metaclusters n. 9, 15, 20 and 21) or shared (red, metaclusters n. 1, 3, 8, and 11) HLA alleles. (E) Heatmap for the 25 Flow-SOM metaclusters; the ratio of fluorescence intensity of each marker with respect to the maximum is indicated in the color-legend. Blue and red rectangles highlight the clusters significantly enriched in CD8+Dextramer+ T cells restricted for donor-specific or shared alleles, respectively. (F) The frequency of each metacluster over time expressed as the percentage of events in each metacluster among total CD8+Dextramer+ T lymphocytes. Among the cytomegalovirus (CMV)-specific T-cell populations evaluated, three were obtained at 30 days after hematopoietic stem cell transplantation, 11 at day +60, 16 at day +90, 12 at day +180 and 13 at day +365. Red, blue and black contours highlight metaclusters enriched in shared or donor-restricted CMV-specific T cells or common metaclusters, respectively. The analyses in (A), (C) and (D) were conducted using two-way analysis of variance. *P<0.05; **P<0.01; ***P<0.001. DEX: Dextramer; tSNE: t-distributed stochastic neighbor embedding; HSCT: hematopoietic stem cell transplantation.
Supplementary Material
Acknowledgments
The authors would like to thank San Rafaele URC (Clinical Trial Ofce), the participating patients and their families, as well as all nurses and data managers who contributed to this study.
Funding Statement
Funding: This work was partially supported by Immudex. Funding was also provided by the Associazione Italiana per la Ricerca sul Cancro (AIRC-IG 18458 and AIRC 5 per Mille 22737), the Italian Ministry of Education, University and Research (PRIN 2017WC8499), Alliance Against Cancer (Ricerca Corrente CAR T project: RCR-2019-23669115), EHA, Cellnex (ACT4Covid) to CB; the Italian Ministry of Health (RF-COVID-19) to CB and FC; and the Italian Ministry of Health (GR-2016-02364847) to ER.
References
- 1.Passweg JR, Baldomero H, Chabannon C, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56(7):1651-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penack O, Peczynski C, Mohty M, et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4(24):6283-6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25(1):151-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47(1-3):65-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mussetti A, Greco R, Peccatori J, Corradini P. Post-transplant cyclophosphamide, a promising anti-graft versus host disease prophylaxis: where do we stand? Expert Rev Hematol. 2017;10(5):479-492. [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith SR, Abid MB, Auletta JJ, et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: a CIBMTR analysis. Blood. 2021;137(23):3291-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oltolini C, Greco R, Galli L, et al. Infections after allogenic transplant with post-transplant cyclophosphamide: impact of donor HLA matching. Biol Blood Marrow Transplant. 2020;26(6):1179-1188. [DOI] [PubMed] [Google Scholar]
- 8.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83(7):1971-1979. [PubMed] [Google Scholar]
- 9.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861-3868. [DOI] [PubMed] [Google Scholar]
- 10.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012;19(4):324-335. [DOI] [PubMed] [Google Scholar]
- 11.El Haddad L, Ariza-Heredia E, Shah DP, et al. The ability of a cytomegalovirus ELISPOT assay to predict outcome of low-level CMV reactivation in hematopoietic cell transplant recipients. J Infect Dis. 2019;219(6):898-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner-Drouet E, Teschner D, Wolschke C, et al. Standardized monitoring of cytomegalovirus-specific immunity can improve risk stratification of recurrent cytomegalovirus reactivation after hematopoietic stem cell transplantation. Haematologica. 2021;106(2):363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avetisyan G, Aschan J, Hagglund H, Ringden O, Ljungman P. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant. 2007;40(9):865-869. [DOI] [PubMed] [Google Scholar]
- 14.Navarro D, Amat P, de la Camara R, et al. Efficacy and safety of a preemptive antiviral therapy strategy based on combined virological and immunological monitoring for active cytomegalovirus infection in allogeneic stem cell transplant recipients. Open Forum Infect Dis. 2016;3(2):ofw107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong MK, Cameron PU, Slavin M, et al. Identifying cytomegalovirus complications using the Quantiferon-CMV assay after allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2017;215(11):1684-1694. [DOI] [PubMed] [Google Scholar]
- 16.Krawczyk A, Ackermann J, Goitowski B, et al. Assessing the risk of CMV reactivation and reconstitution of antiviral immune response post bone marrow transplantation by the QuantiFERON-CMV-assay and real time PCR. J Clin Virol. 2018;99-100:61-66. [DOI] [PubMed] [Google Scholar]
- 17.Tey SK, Kennedy GA, Cromer D, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV(R) assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One. 2013;8(10):e74744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97(5):1232-1240. [DOI] [PubMed] [Google Scholar]
- 19.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655-1662. [DOI] [PubMed] [Google Scholar]
- 20.Varanasi PR, Ogonek J, Luther S, et al. Cytomegalovirus-specific CD8+ T-cells are associated with a reduced incidence of early relapse after allogeneic stem cell transplantation. PLoS One. 2019;14(3):e0213739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato R, Tamaki H, Ikegame K, et al. Early detection of cytomegalovirus-specific cytotoxic T lymphocytes against cytomegalovirus antigenemia in human leukocyte antigen haploidentical hematopoietic stem cell transplantation. Ann Hematol. 2015;94(10):1707-1715. [DOI] [PubMed] [Google Scholar]
- 22.Tario JD Jr, Chen GL, Hahn TE, et al. Dextramer reagents are effective tools for quantifying CMV antigen-specific T cells from peripheral blood samples. Cytometry B Clin Cytom. 2015;88(1):6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(8):e260-e272. [DOI] [PubMed] [Google Scholar]
- 24.Greco R, Ciceri F, Noviello M, et al. Immune monitoring in allogeneic hematopoietic stem cell transplant recipients: a survey from the EBMT-CTIWP. Bone Marrow Transplant. 2018;53(9):1201-1205. [DOI] [PubMed] [Google Scholar]
- 25.Cieri N, Greco R, Crucitti L, et al. Post-transplantation cyclophosphamide and sirolimus after haploidentical hematopoietic stem cell transplantation using a treosulfanbased myeloablative conditioning and peripheral blood stem cells. Biol Blood Marrow Transplant. 2015;21(8):1506-1514. [DOI] [PubMed] [Google Scholar]
- 26.Greco R, Lorentino F, Morelli M, et al. Posttransplantation cyclophosphamide and sirolimus for prevention of GVHD after HLA-matched PBSC transplantation. Blood. 2016;128(11):1528-1531. [DOI] [PubMed] [Google Scholar]
- 27.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433-2444. [DOI] [PubMed] [Google Scholar]
- 28.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87-91. [DOI] [PubMed] [Google Scholar]
- 29.Manfredi F, Abbati D, Cianciotti BC, et al. Flow cytometry data mining by cytoChain identifies determinants of exhaustion and stemness in TCR-engineered T cells. Eur J Immunol. 2021;51(8):1992-2005. [DOI] [PubMed] [Google Scholar]
- 30.Noviello M, Forcina A, Veronica V, et al. Early recovery of CMV immunity after HLA-haploidentical hematopoietic stem cell transplantation as a surrogate biomarker for a reduced risk of severe infections overall. Bone Marrow Transplant. 2015;50(9):1262-1264. [DOI] [PubMed] [Google Scholar]
- 31.Divito SJ, Aasebo AT, Matos TR, et al. Peripheral host T cells survive hematopoietic stem cell transplantation and promote graft-versus-host disease. J Clin Invest. 2020;130(9):4624-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantisan S, Lara R, Montejo M, et al. Pretransplant interferongamma secretion by CMV-specific CD8+ T cells informs the risk of CMV replication after transplantation. Am J Transplant. 2013;13(3):738-745. [DOI] [PubMed] [Google Scholar]
- 33.Zielinski M, Tarasewicz A, Zielinska H, et al. CD28 positive, cytomegalovirus specific cytotoxic T lymphocytes as a novel biomarker associated with cytomegalovirus viremia in kidney allorecipients. J Clin Virol. 2016;83:17-25. [DOI] [PubMed] [Google Scholar]
- 34.Pei XY, Zhao XY, Chang YJ, et al. Cytomegalovirus-specific T-cell transfer for refractory cytomegalovirus infection after haploidentical stem cell transplantation: the quantitative and qualitative immune recovery for cytomegalovirus. J Infect Dis. 2017;216(8):945-956. [DOI] [PubMed] [Google Scholar]
- 35.Snyder CM, Cho KS, Bonnett EL, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29(4):650-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabanti E, Bruno F, Lilleri D, et al. Human cytomegalovirus (HCMV)-specific CD4+ and CD8+ T cells are both required for prevention of HCMV disease in seropositive solid-organ transplant recipients. PLoS One. 2014;9(8):e106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson SE, Sedikides GX, Mason GM, Okecha G, Wills MR. Human cytomegalovirus (HCMV)-specific CD4(+) T cells are polyfunctional and can respond to HCMV-infected dendritic cells in vitro. J Virol. 2017;91(6):e02128-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemaly RF, El Haddad L, Winston DJ, et al. Cytomegalovirus (CMV) cell-mediated immunity and CMV infection after allogeneic hematopoietic cell transplantation: the REACT study. Clin Infect Dis. 2020;71(9):2365-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira G, Ruggiero E, Stanghellini MT, et al. Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci Transl Med. 2015;7(317):317ra198. [DOI] [PubMed] [Google Scholar]
- 40.Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21(5):277-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63-83. [DOI] [PubMed] [Google Scholar]
- 42.Collin MP, Hart DN, Jackson GH, et al. The fate of human Langerhans cells in hematopoietic stem cell transplantation. J Exp Med. 2006;203(1):27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177(2):777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Chang YJ, Yan CH, et al. Poor CMV-specific CD8+ T central memory subset recovery at early stage post-HSCT associates with refractory and recurrent CMV reactivation. J Infect. 2016;73(3):261-270. [DOI] [PubMed] [Google Scholar]
- 45.La Rosa C, Longmate J, Lingaraju CR, et al. Rapid acquisition of cytomegalovirus-specific T cells with a differentiated phenotype, in nonviremic hematopoietic stem transplant recipients vaccinated with CMVPepVax. Biol Blood Marrow Transplant. 2019;25(4):771-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2020;70(8):1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill JA, Zamora D, Xie H, et al. Delayed-onset cytomegalovirus infection is frequent after discontinuing letermovir in cord blood transplant recipients. Blood Adv. 2021;5(16):3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassaniti I, Colombo AA, Bernasconi P, et al. Positive HCMV DNAemia in stem cell recipients undergoing letermovir prophylaxis is expression of abortive infection. Am J Transplant. 2021;21(4):1622-1628. [DOI] [PubMed] [Google Scholar]
- 49.Sperotto A, Candoni A, Gottardi M, et al. Cytomegalovirus prophylaxis versus pre-emptive strategy: different CD4(+) and CD8(+) T cell reconstitution after allogeneic hematopoietic stem cell transplantation. Transplant Cell Ther. 2021;27(6):518.e1-518.e4. [DOI] [PubMed] [Google Scholar]
- 50.Zamora D, Duke ER, Xie H, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138(1):34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.