Abstract
Introduction
Postoperative delirium (POD) is a common cognitive disturbance in elderly individuals that is characterised by acute and fluctuating impairments in attention and awareness. Remimazolam tosylate is a novel, ultrashort-acting benzodiazepine, and there is limited evidence of its correlation with the incidence of early POD. The aim of this study is to evaluate the incidence of POD after anaesthesia induction and maintenance with remimazolam tosylate or propofol in elderly patients undergoing major non-cardiac surgery.
Methods and analysis
This is a single-centre, randomised controlled trial. 636 elderly patients undergoing major non-cardiac surgery will be enrolled and randomised at a 1:1 ratio to receive total intravenous anaesthesia with either remimazolam tosylate or propofol. The primary outcome is the incidence of POD within 5 days after surgery. Delirium will be assessed twice daily by the 3 min Diagnostic Interview for the Confusion Assessment Method or the Confusion Assessment Method for the intensive care unit (ICU) for ICU patients. Secondary outcomes are the onset and duration of delirium, cognitive function at discharge and within 1-year postoperatively, postoperative analgesia within 5 days, chronic pain at 3 months, quality of recovery and postoperative inflammatory biomarker levels.
Ethics and dissemination
The study was approved by the institutional ethics committee of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences (approval No. 22/520–3722). Written informed consent will be obtained from each patient before enrolment. The results of this trial will be presented at scientific conferences and in peer-reviewed scientific journals.
Trial registration number
ChiCTR2300067368.
Keywords: Adult anaesthesia, MENTAL HEALTH, Adult neurology
Strengths and limitations of this study.
A randomised controlled trial design will be used in this study to assess the incidence of postoperative delirium (POD) in elderly patients undergoing major non-cardiac surgery.
The study will include a longer-term follow-up and assess multiple dimensions of postoperative cognition, quality of recovery and inflammation levels.
Against the background of a high incidence of malignancies worldwide, our study will reflect the POD incidence of a homogenous population with tumours, except for brain tumours.
Although investigators cannot be blinded, the anaesthesia process and intraoperative data may be affected, a large sample size and strict randomisation may also minimise the confounding effect.
Introduction
Postoperative delirium (POD), characterised by an acute change in mental status, commonly occurs after major surgery.1 Although the incidence of POD in patients undergoing general surgery is 2–3%,2 it increases with age. Previous studies have shown that the incidence of POD in elderly patients is between 5.7% and 71%,3–5 often leading to longer hospitalisations, higher costs, worse functional recovery and even higher mortality.6 7
Although the incidence of POD is closely associated with the duration of anaesthesia, the relationship between POD and general anaesthetic drugs remains inconclusive. Midazolam and propofol are most commonly used for sedating patients. Propofol, which is commonly used in total intravenous anaesthesia, has the advantages of a rapid effect, no muscle twitching during induction, no adrenocortical inhibition and rapid recovery.8 However, the disadvantages of propofol include potential respiratory depression, hypotension, injection pain and hypertriglyceridaemia.9–11 Additionally, the interaction between propofol and muscarinic acetylcholine receptors may be related to postoperative cognitive impairment.12 Our previous meta-analysis study involving 1404 elderly patients with lung cancer revealed that propofol had a more significant adverse effect on cognitive function than sevoflurane.13 Therefore, the identification of alternative sedatives in general anaesthesia has become an urgent priority.
Remimazolam is a novel, water-soluble, ultrashort-acting benzodiazepine that inhibits the central nervous system by acting on γ-aminobutyric acid receptors, thereby exerting sedative and amnestic effects.14 It has the characteristics of high clearance, a small distribution volume, a short half-life, a rapid onset of action and inactivation with a terminal half-life of 0.75 hours.15 Unlike propofol, remimazolam is metabolised by tissue esterases and eliminated by first-order pharmacokinetics.15 Even high-dose bolus injections, prolonged infusion and administration in subjects with hepatic or renal impairment are unlikely to result in accumulation and extended effects.16 17 Moreover, the sedative effect of remimazolam can be rapidly reversed by flumazenil, which increases the controllability.18 A previous study on the use of remimazolam in general anaesthesia, based on a population pharmacokinetic (popPK) analysis of plasma concentration-time data from 11 Phase 1–3 clinical trials, as well as a population pharmacokinetic-pharmacodynamic (popPK-PD) analysis of concentration-bispectral index (BIS) data from eight trials, revealed that 6 mg/kg/hours of remimazolam until loss of consciousness followed by 1 mg/kg/hours during the period of general anaesthesia and 0.25 mg/kg/hours for postoperative sedation for up to 24 hours is safe and optimal, regardless of subjects’ American Society of Anesthesiologists (ASA) class or sensitivity.19 Moreover, available evidence suggests that the long-term use of oral benzodiazepines is associated with cognitive decline,20 whereas single intraoperative doses do not increase the incidence of POD.21 Although the rapid elimination of remimazolam might theoretically prevent POD,22 the use of continuous intravenous infusion in total intravenous anaesthesia remains uncertain.
Remimazolam tosylate was approved for general anaesthesia in adult patients in China on 9 November 2021. Existing studies have adequately assessed its safety and efficacy,23–25 while evidence of its effect on POD is limited. Accordingly, we designed a randomised, controlled trial to evaluate whether remimazolam tosylate or propofol can reduce the incidence of POD in older patients undergoing major non-cardiac surgery.
Methods and analysis
Objective
The primary objective of this study is to compare the incidence of POD in elderly patients undergoing major non-cardiac surgery who receive remimazolam tosylate with that in those who receive propofol. The secondary objectives of the study are to evaluate the onset and duration of delirium, cognitive function within 1-year postoperatively, quality of recovery, acute and chronic postoperative pain and postoperative inflammatory biomarker levels.
Study design
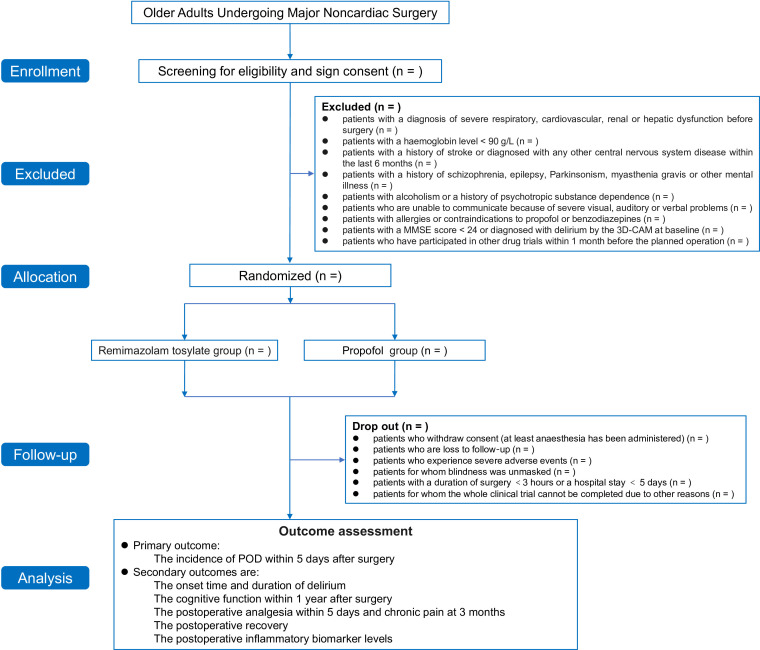
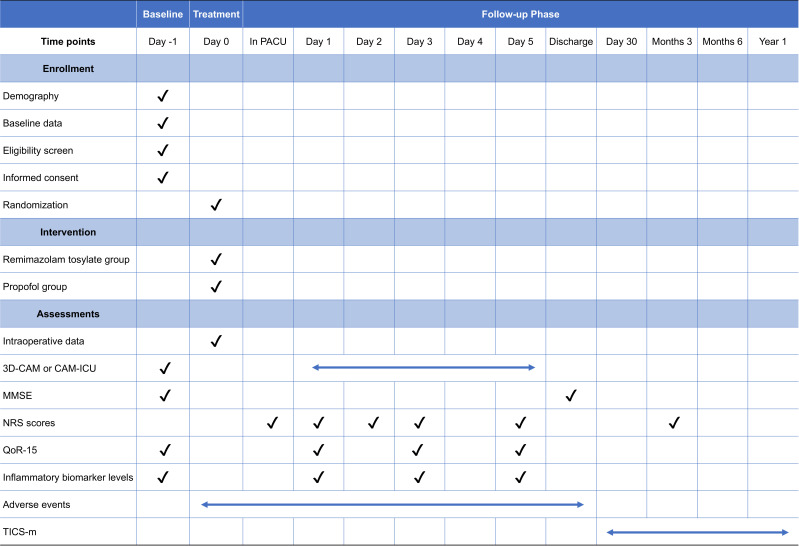
This is a single-centre, randomised, controlled study (figure 1). Participants will be recruited from the Cancer Hospital of the Chinese Academy of Medical Sciences, which is a tertiary academic hospital in China. The study was approved by the institutional ethics committee of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences (approval No. 22/520–3722) and has been registered in the Clinical Trial Registry. Written informed consent will be obtained from each patient before enrolment (online supplemental appendix). Eligible patients will be enrolled and randomly assigned to either of the two study groups. The protocol is reported according to the Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines (figure 2).26
Figure 1.
Study flowchart. ASA, American Society of Anesthesiologists; MMSE, Mini-Mental State Examination; POD, postoperative delirium; 3D-CAM: 3-min Diagnostic Interview for the Confusion Assessment Method.
Figure 2.
Standard Protocol Items: Recommendations for Interventional Trials diagram for enrolment, interventions and assessments. MMSE, Mini-Mental State Examination; NRS, Numerical Rating Scale; PACUs, postanaesthetic care units; QoR-15, Quality of Recovery-15; TICS-m, Telephone Interview for Cognitive Status-Modified; 3D-CAM, 3-min Diagnostic Interview for the Confusion Assessment Method.
bmjopen-2023-071912supp001.pdf (106.1KB, pdf)
Participants
We will recruit 636 elderly patients undergoing major non-cardiac surgery according to the inclusion and exclusion criteria. We plan to enrol the first patient on 1 March 2023, and finish recruiting on 28 February 2025.
Inclusion criteria
The inclusion criteria are as follows: (1) male or female patients between 65 and 90 years of age; (2) patients with an ASA physical status score ≤3; (3) patients scheduled to undergo major non-cardiac surgery with an expected duration of at least 3 hours and an anticipated hospital stay of at least 5 days; and (4) patients with a body mass index (BMI) between 18 and 27.9 kg/m2.
Exclusion criteria
The exclusion criteria are as follows: (1) patients with a diagnosis of severe respiratory, cardiovascular, renal or hepatic dysfunction before surgery; (2) patients with a haemoglobin level <90 g/L; (3) patients with a history of stroke or a diagnosis of any other central nervous system disease within the last 6 months; (4) patients with a history of schizophrenia, epilepsy, Parkinsonism, myasthenia gravis or other mental illness; (5) patients with alcoholism or a history of psychotropic substance dependence; (6) patients who are unable to communicate because of severe visual, auditory or verbal problems; (7) patients with allergies or contraindications to propofol or benzodiazepines; (8) patients with a Mini-Mental State Examination (MMSE) score <24 or diagnosed with delirium by the Confusion Assessment Method (CAM) or the 3 min Diagnostic Interview for the Confusion Assessment Method (3D-CAM) at baseline; and (9) patients who have participated in other drug trials within 1 month before the planned operation.
Dropout criteria
The dropout criteria are as follows: (1) patients who withdraw consent (at least after anaesthesia has been administered); (2) patients who are lost to follow‐up; (3) patients who experience severe adverse events; (4) patients for whom blinding will be unsuccessful; (5) patients with a duration of surgery <3 hours or a hospital stay <5 days; and (6) patients for whom the whole clinical trial cannot be completed due to other reasons. The detailed reasons for cases of dropout will be recorded in the case report forms (CRFs).
Rejection criteria
The rejection criteria are as follows: (1) patients who withdraw consent (before anaesthesia); (2) patients for whom surgery is delayed or cancelled; (3) patients whose case operation violates the protocol; and (4) patients with no study records or incomplete records due to other reasons. The detailed reasons for rejection will be recorded in the CRFs.
Randomisation and masking
Randomisation at a 1:1 ratio will be achieved using SAS V.9.2 software (SAS Institute, USA), and participants will be enrolled and randomly assigned to either of the two study groups by an independent statistician. Treatment allocations will be sealed in opaque envelopes, and anaesthesiologists will not be informed of the anaesthesia strategy until the envelopes are opened before the induction of anaesthesia. Intraoperative data will be collected by unblinded anaesthesiologists. Investigators who are responsible for identifying potential participants according to the inclusion and exclusion criteria, performing follow-up in the postanaesthetic care units (PACUs) and the ward and collecting data on laboratory testing outcomes will all be blinded to randomisation throughout the study period. In case of an emergency (such as severe adverse events), unblinding will be permissible.
Intraoperative monitoring and anaesthesia management
Standard intraoperative monitoring will include pulse oxygen saturation, non-invasive arterial blood pressure, ECG and end-tidal carbon dioxide partial pressure (EtCO2) monitoring. Invasive blood pressure and central venous pressure will be monitored when considered necessary. Bispectral analysis (BIS, Philips, Amsterdam, The Netherlands) will be used to monitor anaesthetic depth, and neuromuscular monitoring (JS-100, Slgo Medical Technology, Beijing, China) will be applied to confirm the degree of neuromuscular block (NMB).
Before anaesthesia induction, patients will undergo preoxygenation at a fraction of inspiration O2 of 100% for 5 min with a face mask. Anaesthesia will be induced with the investigational product (remimazolam tosylate or propofol), sufentanil (0.3–0.5 µg/kg) and rocuronium (0.6 mg/kg). Intubation will be performed when the train-of-four count (TOFC) reaches 0.
During anaesthesia, mechanical ventilation will be performed with a tidal volume of 6–8 mL/kg (predicted body weight (PBW), PBW=50.0+0.905×((height in cm)−152.4) for men and PBW=45.4+0.905×((height in cm)−152.4) for women).27 The respiratory rate will be adjusted as required to maintain an EtCO2 between 35 and 45 mm Hg, with an inspiratory to expiratory ratio of 1:2. Anaesthesia will be maintained with the investigational product, and the infusion rate will be adjusted to maintain the patient’s BIS value between 40 and 60. Remifentanil will be constantly infused at a rate of 0.1–0.2 µg/kg/min, sufentanil will be added as needed, and vasoactive drugs will be administered to maintain an arterial blood pressure within 25% of the baseline value. Rocuronium will be administered and adjusted to achieve deep NMB (defined as a TOFC=0 and a post-tetanic count=1–2). The investigational products and remifentanil will be stopped during skin suture, and 10 mL of 0.5% ropivacaine will be injected subcutaneously at the incision site for postoperative analgesia. The NMB will be reversed with sugammadex (2–4) mg/kg according to the instructions.28 After the operation, patients will be transferred to the PACUs or intensive care unit (ICU) for further monitoring.
Patient-controlled intravenous analgesia (PCIA) consisting of 2 µg/kg of sufentanil at a total volume of 100 mL will be used for postoperative pain control, the background dose will be 2 mL/hours, the single dose will be 0.5 mL and the locked time will be 10 min. The PCIA pump will be removed after 48 hours.
Interventions
Participants will be randomly allocated to either the remimazolam tosylate group (R group) or the propofol group (P group).
Remimazolam tosylate group (R group)
Induction of general anaesthesia: remimazolam tosylate (0.2–0.3 mg/kg) will be intravenously injected; maintenance of general anaesthesia: remimazolam tosylate (1–2 mg/kg/hours) will be continuously infused. If awakening is not observed 15 min after remimazolam tosylate administration is stopped, then 0.2 mg of flumazenil will be given. If necessary, a repeated dose of 0.1 mg will be allowed.
Propofol group (P group)
Induction of general anaesthesia: propofol (2–3 mg/kg) will be intravenously injected; maintenance of general anaesthesia: propofol (4–10 mg/kg/hours) will be continuously infused.
Outcomes
The primary outcome will be the incidence of POD within 5 days after surgery. The secondary outcomes will be as follows: onset time and duration of delirium, cognitive function at discharge, cognitive function of 30-day survivors, cognitive function of 1-year survivors, postoperative analgesia within 5 days, postoperative chronic pain at 3 months, postoperative recovery (including quality of recovery, the occurrence of complications within 30 days and postoperative hospitalisation) and postoperative inflammatory biomarker levels.
Data collection and follow-up
Baseline data will include demographic data (age, sex, level of education and BMI), baseline mental assessment, preoperative diagnosis and tumour staging, smoking and alcohol history, diagnoses of comorbidities, preoperative medication use, surgical history and relevant laboratory test results.
Intraoperative data will include patients’ vital signs, mechanical ventilation index, types and doses of anaesthetic drugs, durations of surgery and anaesthesia, types and volumes of fluids infused and blood transfusion status.
Patients will be followed-up twice daily (08:00 to 10:00 and 18:00 to 20:00) for five postoperative days and on the day of discharge (08:00 to 10:00). Patients will be interviewed via telephone at 30 days, 3 months, 6 months and 1 year after surgery. The collected data will include delirium and cognitive function evaluations, postoperative analgesia, Quality of Recovery-15 (QoR-15) scale scores and the occurrence of complications. If the patients are diagnosed with complications, the onset, duration and evidence will be documented.
Delirium assessment
The diagnosis and evaluation of POD will be overseen by a collaborating neuropsychologist (Dr Zhang Xiao of National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing, China). He will be responsible for training staff and arbitrating difficult cases. All researchers responsible for POD assessment will be required to receive training and obtain approval before commencing assessments. Once weekly, the cases that are difficult to evaluate will be referred to the collaborating neuropsychologist and discussed.
Delirium within 5 days after surgery will be assessed at baseline and twice daily by the 3D-CAM or the Confusion Assessment Method for the ICU (CAM-ICU) for ICU patients.29 30 Because delirium often presents at night and subsyndromal delirium increases the difficulty of diagnosis, abnormal situations (eg, inappropriate behaviour, confusion, hallucinations) will be recorded in detail by nurses or clinicians and reported to the collaborating neuropsychologist.
Cognitive function evaluation
Cognitive function will be evaluated at baseline, discharge, 30 days, 3 months, 6 months and 1 year postoperatively. The MMSE will be performed at baseline to identify undiagnosed cognitive impairment and at discharge to explore the association with POD (a difference between preoperative and postoperative MMSE scores ≥2).30 31 The Telephone Interview for Cognitive Status-Modified (TICS-m) will be administered by telephone after discharge.32 Total TICS-m scores range from 0 to 48, and higher scores indicate better cognitive function.
Assessments of postoperative analgesia
Postoperative pain will be evaluated by the Numerical Rating Scale (NRS), with scores ranging from 0 to 10 (0=no pain, 10=worst pain imaginable), at 1 hour, postoperative day 1, postoperative day 2, postoperative day 3, postoperative day 5 and 3 months postoperatively. The occurrence of three distinct types of pain will be recorded: superficial wound pain, pain at rest and pain during coughing. Forty-eight hours after surgery, the PCIA pump will be discontinued. The cumulative sufentanil consumption, rescue analgesia use and occurrence of nausea and vomiting will be recorded. During hospitalisation, if the NRS score is >3, flurbiprofen axetil (100 mg) will be administered intravenously for rescue analgesia; if the score is ≥7 for more than 24 hours, this situation will be defined as an adverse event, and all details will be recorded in the CRF.
Quality of recovery evaluation
Quality of recovery will be evaluated by the QoR-15 scale at postoperative day 1, postoperative day 3 and postoperative day 5.33 It evaluates five dimensions: pain, physical comfort, physical independence, psychological support and emotional state.
Inflammatory biomarker assays
Postoperative inflammatory levels will be evaluated by the white blood cell (WBC) count and serum C-reactive protein (CRP) level. Peripheral venous blood samples will be taken at four time points: before the operation, and at postoperative day 1, postoperative day 3 and postoperative day 5. Blood samples will be analysed at the Cancer Hospital clinical laboratory using a Sysmex XN-9100 to detect the WBC count and a Beckman-AU5800 to detect the CRP level.
Adverse events
All adverse events observed by the investigators or reported spontaneously by the subjects will be recorded. Adverse events related to the study intervention and other factors may occur during the study, including (but not limited to) hypertension, hypotension, tachycardia, bradycardia and respiratory depression. Serious adverse events refer to any unpredictable serious medical events such as persistent dysfunction, disability, life-threatening events or even death. The investigators will record any adverse events during the study and address them in accordance with the regulations. The recorded information will include the diagnosis, occurrence time, remission time, severity, relationship with the intervention, treatment and outcomes of adverse events. All serious adverse events will be reported to the Ethics Committee within 24 hours. Other adverse events will be reported in the final report. Patients with any adverse consequences directly caused by the research will be compensated in accordance with the law.
Statistical analysis
Sample size
In a previous study, the incidence of POD after total intravenous anaesthesia with propofol in elderly patients was 33%,34 and after usual anaesthetic care, it was 23%.35 We assume that the incidence of POD in the remimazolam tosylate group will be 23%, which is consistent with usual anaesthetic care. With a significance level of 0.05 and a power of 80%, we estimated that 289 patients are needed in each group. Considering a loss-to-follow-up rate of approximately 10%, 318 patients will be needed in each group. The sample size was calculated with PASS V.16.0 software (StataCorp, College Station, Texas, USA).
Analyses
All statistical analyses will be performed with SPSS V.25.0 (IBM SPSS, USA) and the R software package by statisticians in the Office of Cancer Screening of the National Cancer Center. Since propofol and remimazolam tosylate will be administered according to the instructions and their safety has been confirmed by previous studies,16 25 interim analysis is not planned.
Analysis sets
A full analysis set will include all eligible patients randomly assigned to either group with at least one follow-up evaluation. The per-protocol analysis set will include all patients meeting the eligibility criteria of the study with at least one follow-up evaluation after randomisation and with a treatment compliance rate of at least 80%. The safety set will involve all randomised patients with at least one safety assessment after randomisation.
Baseline data
The statistical description will be provided for baseline data, and the baseline balance will be assessed by the absolute standardised difference (ASD). Imbalance is defined as an ASD value greater than 0.2.
Outcome analysis
The primary outcome (the incidence of POD within 5 days after surgery) will be compared with the χ2 test. The difference and the 95% CI of the difference will be calculated. The Kaplan-Meier method combined with log-rank analysis will be used to determine the relationship between the incidence of POD and the time after major non-cardiac surgery.
For secondary outcomes, quantitative data will be compared using Student’s t-test or the Mann-Whitney rank test. Categorical variables will be analysed with χ2 or Fisher exact tests. Two-tailed p values<0.05 will indicate statistical significance.
Data management
Before recruitment, a password-protected electronic record will be established to store patient details. Their data will be encoded and identified by specific identification numbers that will be assigned. We will designate a trained investigator who is independent of the study to collect the research data and complete the case reports. The informed consent forms and the research data will be recorded in the corresponding electronic medical records, and the signed paper documents will be stored in a locked room in the hospital. Protected data sets will be backed up periodically (every 3 months) and synchronously audited by an independent data administrator. Only this administrator will be able to obtain the key for the identification codes during the study. The lead investigator will record and protect the data according to the guidelines at the end of the study. When recruitment and follow-up are completed, the data will be collected, entered and reviewed, all data queries will be completed and the data set will be locked. To help researchers understand and repeat the results, all data will be archived for 10 years after the study is completed. The details of the patients’ identities will not be reported in any publication.
Patient and public involvement
None.
Ethics and dissemination
The study was approved by the institutional ethics committee of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences (approval No. 22/520–3722) and has been registered in the Clinical Trial Registry. This study will be conducted in accordance with the Helsinki declaration. Written informed consent will be obtained from each patient before enrolment (online supplemental appendix). The results of this trial will be presented at scientific conferences and in peer-reviewed scientific journals.
Discussion
Various risk factors related to POD have been reported previously. First, older age is the most common predisposing factor. A randomised study reported that the incidence of postoperative cognitive dysfunction in the first week after major non-cardiac surgery was 29% and 23% in patients aged over 70 years and in those aged between 60 and 69 years, respectively,36 compared with an incidence of 2–3% for general morbidity.37 Moreover, frailty, poor vision and hearing, multiple comorbidities, etc, in older patients have also been associated with an increased risk.38–40 Second, surgery, anaesthesia, pain, etc, are also precipitating factors.40 Major surgery is usually accompanied by a longer operative time, more intense postoperative pain and a greater risk of complications, all of which increase the risk of POD.41–43 In a study of 1232 enrolled elderly patients undergoing major surgery, including cardiac surgery, with a median duration of anaesthesia of 264 min, the incidence of POD was 23.0–26.0%.35 Among elderly patients, especially those who underwent cardiac surgery, the incidence was 10–28%.4 However, in elderly patients (≥65 years old) who underwent hip fracture surgery with a median duration of anaesthesia of 120 min, the incidence of POD was only 5.1–6.2%,3 regardless of whether general or regional anaesthesia was adopted. These results suggest that elderly patients undergoing major surgery with a long anaesthesia duration are more prone to POD; hence, we included these susceptibility and predisposing factors in the inclusion criteria of our study, enrolling patients undergoing prolonged major surgical procedures for malignant tumours. In addition, the well-predefined risk factor for postoperative pain cannot be neglected for such a long duration of major surgery. First, most patients suffer from postoperative pain within 48 hours postoperatively; hence, PCIA is routinely used for acute pain relief in our cancer centre. Second, we will also perform an analgesia follow-up within 5 days. If the postoperative pain score is >3, flurbiprofen axetil (100 mg) or opioids, if necessary, will be administered intravenously for rescue analgesia, and the administration and analgesic effects will also be recorded. Third, as each patient has a different level of pain tolerance, if a patient suffers from continuous postoperative pain with an analgesia score ≥7 for more than 24 hours, the case will be defined as an adverse event and compared in the two groups by statistical analysis. Although postoperative pain cannot be completely avoided after major surgeries, which might produce confounding effects in our intervention, a large sample size and strict randomisation will help to balance and minimise this bias between the groups. Moreover, patients with brain tumours will be excluded to avoid the confounding effect of trauma interference on the incidence of POD in this study.
Delirium is a cognitive disturbance characterised by acute and fluctuating impairments in attention and awareness.37 The CAM is the most widely used diagnostic algorithm and examines four features: an acute change in mental status with a fluctuating course (feature 1), inattention (feature 2), disorganised thinking (feature 3) and an altered level of consciousness (feature 4). Delirium will be diagnosed if the CAM indicates the presence of features 1 and 2 and either feature 3 or 4. The 3D-CAM and CAM-ICU are further optimisations of the CAM with wide acceptability worldwide because they are less time consuming and offer higher sensitivity and specificity.29 Hence, both algorithms have been chosen for diagnosing delirium in our study. Moreover, of all delirium patients, only 25% present with hyperactive delirium, and the remaining patients are diagnosed with hypoactive delirium.44 For these relatively ‘quiet’ delirium patients, clinicians often face difficulties in diagnosis due to atypical symptoms. Hence, in this study, these cases will be diagnosed by collaborating neuropsychologists once weekly to increase diagnostic reliability. Moreover, a growing body of literature shows that POD is closely associated with long-term cognitive function.45–47 A study including 103 participants who underwent valve replacement or coronary artery bypass grafting confirmed the relationship between cognitive impairment within 1 year after surgery and delirium.48 In this study, cognitive changes will be followed-up through telephone questionnaires to assess the effect of remimazolam tosylate on long-term cognitive function.
The adoption of effective anaesthesia strategies to prevent POD is essential in older patients. Researchers hope to find a breakthrough in anaesthetic drugs to prevent POD. For commonly used inhaled and intravenous general anaesthetic drugs, some studies have shown that volatile anaesthetics have a greater impact on cognitive function than propofol;49 some have also shown that cognitive function after sevoflurane anaesthesia was superior to that after propofol anaesthesia in patients with impaired cerebral oxygenation,50 while others have suggested that there is no significant difference between the two anaesthesia strategies.51 In addition, there is still some evidence revealing that low-dose dexmedetomidine significantly decreases the risk of POD in elderly patients admitted to the ICU after non-cardiac surgery,52 while recent studies with large samples have shown that intraoperative dexmedetomidine does not prevent POD,53–55 regardless of whether it is used for cardiac or non-cardiac surgery. Additionally, researchers hope to develop drugs that can reduce the risk of POD through anti-inflammation, analgesia and sympathetic arousal suppression mechanisms. Some studies have shown that acetaminophen combined with propofol or dexmedetomidine reduces the incidence of POD.4
This carefully designed, single-centre, randomised, controlled trial will be implemented in strict accordance with the protocol. However, there are some limitations in this study. First, pain and the administration of long-acting opioids may be associated with POD, inducing confounding effects in our results. Although there may be a discrepancy in the total opioid dosage between the two groups, a similar dose of hourly drug input volume for either group will be guaranteed. In addition, since the anaesthesiologists cannot be blinded to the interventions, this will not be a double-blinded clinical trial. In this study, the researchers will be blinded to the group allocation and/or the study hypothesis to reduce investigator bias. Moreover, the confounding effect may also be minimised by the large sample size and strict randomisation in this study.
Total intravenous anaesthesia with remimazolam tosylate causes minimal haemodynamic and respiratory depression in elderly patients. Compared with propofol, remimazolam tosylate may have an equivalent anaesthetic effect and a higher safety profile for elderly patients undergoing hip replacement, gastrointestinal endoscopy, bronchoscopy and general anaesthesia.56–58 Moreover, previous literature has also proven remimazolam tosylate’s availability as a general anaesthetic for continuous infusions during procedures longer than 2 hours.59 However, research on the effect of remimazolam tosylate on the incidence of POD is very limited. Only one study showed that 0.1 mg/kg of remimazolam tosylate did not affect cognition recovery in elderly patients who underwent upper gastrointestinal endoscopy.60 At present, another similar study enrolling cardiac surgery patients is in progress.61 The subjects in this study will be a homogenous population with malignant tumours except for brain tumours. Unlike benign surgical approaches, lymph node dissection is the most challenging step during the entire process of removing tumours, leading to increased surgical trauma, stress and subsequent postoperative pain, prolonged anaesthesia duration and major surgical complications. Under the background of a high incidence of malignancies worldwide, our study will reflect the POD incidence of a homogenous population with tumours except for brain tumours in real-world practice. Additionally, this will be the first study with a large sample to compare remimazolam tosylate with propofol in terms of the incidence of POD among elderly patients undergoing major non-cardiac surgery.
Supplementary Material
Acknowledgments
We gratefully acknowledge the patients for their time and willingness to participate in the trial, FW (statistician in the Office of Cancer Screening of National Cancer Center) for performing the statistical analysis, and XiaoZ (Department of Psychiatry, Peking University Sixth Hospital, National Psychiatric Clinical Research Center) for his help in performing cognitive disorder assessments and training.
Footnotes
H-xL, B-lL and T-hW contributed equally.
Contributors: H-xL, TY and HZ helped to design this clinical trial. H-xL and B-lL helped with drafting the paper. XinZ and H-yL will be responsible for the acquisition of data. FW, BM and Y-lS will perform the statistical analysis. XiaoZ and XX will be responsible for conducting the postoperative cognitive survey. T-hW helped with the English language revision. All the authors contributed to the development of the trial. TY performed a critical revision of the manuscript. All the authors read and approved the final manuscript.
Funding: This study is funded by the following foundations: The Special Research Fund for Central Universities, Peking Union Medical College (3332021099); The Beijing Hope Run Special Fund of Cancer Foundation of China (LC2021A18); and The CAMS Innovation Fund for Medical Sciences (CIFMS, 2022-I2M-C&T-B-061). The study funders will not play any role in designing the study, collecting, managing, analysing or interpreting the data or writing the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and Surgery-2018. Anesthesiology 2018;129:872–9. 10.1097/ALN.0000000000002334 [DOI] [PubMed] [Google Scholar]
- 2.Ha A, Krasnow RE, Mossanen M, et al. A contemporary population-based analysis of the incidence, cost, and outcomes of postoperative delirium following major urologic cancer surgeries. Urol Oncol 2018;36:341. 10.1016/j.urolonc.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 3.Li T, Li J, Yuan L, et al. Effect of regional vs general anesthesia on incidence of postoperative delirium in older patients undergoing hip fracture surgery: the RAGA randomized trial. JAMA 2022;327:50. 10.1001/jama.2021.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA 2019;321:686. 10.1001/jama.2019.0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watne LO, Idland A-V, Fekkes D, et al. Increased CSF levels of aromatic amino acids in hip fracture patients with delirium suggests higher Monoaminergic activity. BMC Geriatr 2016;16:149. 10.1186/s12877-016-0324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA 2012;308:73–81. 10.1001/jama.2012.6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011;112:1202–11. 10.1213/ANE.0b013e3182147f6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahinovic MM, Struys MMRF, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet 2018;57:1539–58. 10.1007/s40262-018-0672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalota L, Kalira V, George E, et al. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ 2011;342:bmj.d1110. 10.1136/bmj.d1110 [DOI] [PubMed] [Google Scholar]
- 10.Schick A, Driver B, Moore JC, et al. Randomized clinical trial comparing procedural amnesia and respiratory depression between moderate and deep sedation with propofol in the emergency Department. Acad Emerg Med 2019;26:364–74. 10.1111/acem.13548 [DOI] [PubMed] [Google Scholar]
- 11.Krajčová A, Waldauf P, Anděl M, et al. Propofol infusion syndrome: a structured review of experimental studies and 153 published case reports. Crit Care 2015;19:398. 10.1186/s13054-015-1112-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown KE, Mirrakhimov AE, Yeddula K, et al. Propofol and the risk of delirium: exploring the anticholinergic properties of propofol. Med Hypotheses 2013;81:536–9. 10.1016/j.mehy.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Zhang G, Ai B, et al. A systematic review: comparative analysis of the effects of propofol and sevoflurane on postoperative cognitive function in elderly patients with lung cancer. BMC Cancer 2019;19. 10.1186/s12885-019-6426-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee A, Shirley M. Remimazolam: A review in procedural sedation. Drugs 2021;81:1193–201. 10.1007/s40265-021-01544-8 [DOI] [PubMed] [Google Scholar]
- 15.Antonik LJ, Goldwater DR, Kilpatrick GJ, et al. A Placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of Remimazolam (CNS 7056): part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg 2012;115:274–83. 10.1213/ANE.0b013e31823f0c28 [DOI] [PubMed] [Google Scholar]
- 16.Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of Remimazolam in subjects with hepatic or renal impairment. Br J Anaesth 2021;127:415–23. 10.1016/j.bja.2021.05.027 [DOI] [PubMed] [Google Scholar]
- 17.Wesolowski AM, Zaccagnino MP, Malapero RJ, et al. Remimazolam: pharmacologic considerations and clinical role in Anesthesiology. Pharmacotherapy 2016;36:1021–7. 10.1002/phar.1806 [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Sang N, Song K, et al. Psychomotor recovery following Remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clinical Therapeutics 2020;42:614–24. 10.1016/j.clinthera.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for Remimazolam in the induction and maintenance of General anesthesia in healthy subjects and in surgical subjects. J Clin Anesth 2020;66:109899. 10.1016/j.jclinane.2020.109899 [DOI] [PubMed] [Google Scholar]
- 20.Picton JD, Marino AB, Nealy KL. Benzodiazepine use and cognitive decline in the elderly. Am J Health Syst Pharm 2018;75:e6–12. 10.2146/ajhp160381 [DOI] [PubMed] [Google Scholar]
- 21.Wang ML, Min J, Sands LP, et al. Midazolam premedication immediately before surgery is not associated with early postoperative delirium. Anesth Analg 2021;133:765–71. 10.1213/ANE.0000000000005482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol 2020;33:506–11. 10.1097/ACO.0000000000000877 [DOI] [PubMed] [Google Scholar]
- 23.Choi JY, Lee HS, Kim JY, et al. Comparison of Remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J Clin Anesth 2022;82:110955. 10.1016/j.jclinane.2022.110955 [DOI] [PubMed] [Google Scholar]
- 24.Chae D, Kim H-C, Song Y, et al. Pharmacodynamic analysis of intravenous bolus Remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth 2022;129:831. 10.1016/j.bja.2022.08.010 [DOI] [PubMed] [Google Scholar]
- 25.Dai G, Pei L, Duan F, et al. Safety and efficacy of Remimazolam compared with propofol in induction of General anesthesia. Minerva Anestesiol 2021;87:1073–9. 10.23736/S0375-9393.21.15517-8 [DOI] [PubMed] [Google Scholar]
- 26.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linares-Perdomo O, East TD, Brower R, et al. Standardizing predicted body weight equations for mechanical ventilation tidal volume settings. Chest 2015;148:73–8. 10.1378/chest.14-2843 [DOI] [PubMed] [Google Scholar]
- 28.Brull SJ, Kopman AF. Current status of neuromuscular reversal and monitoring: challenges and opportunities. Anesthesiology 2017;126:173–90. 10.1097/ALN.0000000000001409 [DOI] [PubMed] [Google Scholar]
- 29.Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 2014;161:554. 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evered LA, Chan MTV, Han R, et al. Anaesthetic depth and delirium after major surgery: a randomised clinical trial. British Journal of Anaesthesia 2021;127:704–12. 10.1016/j.bja.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu B, Sun D, Yang L, et al. The effects of Neostigmine on postoperative cognitive function and inflammatory factors in elderly patients - a randomized trial. BMC Geriatr 2020;20:387. 10.1186/s12877-020-01793-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry 2003;18:318–24. 10.1002/gps.830 [DOI] [PubMed] [Google Scholar]
- 33.Stark PA, Myles PS, Burke JA. Development and Psychometric evaluation of a postoperative quality of recovery score: the Qor-15. Anesthesiology 2013;118:1332–40. 10.1097/ALN.0b013e318289b84b [DOI] [PubMed] [Google Scholar]
- 34.Mei X, Zheng H-L, Li C, et al. The effects of propofol and sevoflurane on postoperative delirium in older patients: A randomized clinical trial study. J Alzheimers Dis 2020;76:1627–36. 10.3233/JAD-200322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA 2019;321:473. 10.1001/jama.2018.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laalou FZ, Carre AC, Forestier C, et al. Pathophysiology of post-operative cognitive dysfunction: Current hypotheses. J Chir (Paris) 2008;145:323–30. 10.1016/s0021-7697(08)74310-2 [DOI] [PubMed] [Google Scholar]
- 37.Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth 2020;125:492–504. 10.1016/j.bja.2020.06.063 [DOI] [PubMed] [Google Scholar]
- 38.Gracie TJ, Caufield-Noll C, Wang NY, et al. The Association of preoperative Frailty and postoperative delirium: A meta-analysis. Anesth Analg 2021;133:314–23. 10.1213/ANE.0000000000005609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witlox J, Eurelings LSM, de Jonghe JFM, et al. Delirium in elderly patients and the risk of Postdischarge mortality, Institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443–51. 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. The Lancet 2014;383:911–22. 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang G, Wang Z, Wang D, et al. A systematic review and meta-analysis of the correlation between operation time and postoperative delirium in total hip Arthroplasty. Ann Palliat Med 2021;10:10459–66. 10.21037/apm-21-2190 [DOI] [PubMed] [Google Scholar]
- 42.O’Gara BP, Gao L, Marcantonio ER, et al. Pain, and cognition: Modifiable targets for optimal perioperative brain health. Anesthesiology 2021;135:1132–52. 10.1097/ALN.0000000000004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swarbrick CJ, Partridge JSL. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anaesthesia 2022;77 Suppl 1:92–101. 10.1111/anae.15607 [DOI] [PubMed] [Google Scholar]
- 44.Mattison MLP. Ann intern MED. 2020;173:ITC49-ITC64. 10.7326/AITC202010060 [DOI] [PubMed] [Google Scholar]
- 45.Wilcox ME, Girard TD, Hough CL. Delirium and long term cognition in critically ill patients. BMJ 2021;373:1007. 10.1136/bmj.n1007 [DOI] [PubMed] [Google Scholar]
- 46.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement 2016;12:766–75. 10.1016/j.jalz.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devore EE, Fong TG, Marcantonio ER, et al. Prediction of long-term cognitive decline following postoperative delirium in older adults. J Gerontol A Biol Sci Med Sci 2017;72:1697–702. 10.1093/gerona/glx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive Trajectories after postoperative delirium. N Engl J Med 2012;367:30–9. 10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Shan G-J, Zhang Y-X, et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed Neurocognitive recovery in older adults. Br J Anaesth 2018;121:595–604. 10.1016/j.bja.2018.05.059 [DOI] [PubMed] [Google Scholar]
- 50.Egawa J, Inoue S, Nishiwada T, et al. Effects of anesthetics on early postoperative cognitive outcome and intraoperative cerebral oxygen balance in patients undergoing lung surgery: a randomized clinical trial. Can J Anaesth 2016;63:1161–9. 10.1007/s12630-016-0700-4 [DOI] [PubMed] [Google Scholar]
- 51.Geng Y-J, Wu Q-H, Zhang R-Q. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following Laparoscopic Cholecystectomy in elderly patients: A randomized controlled trial. J Clin Anesth 2017;38:165–71. 10.1016/j.jclinane.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 52.Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. The Lancet 2016;388:1893–902. 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]
- 53.Momeni M, Khalifa C, Lemaire G, et al. Propofol plus low-dose dexmedetomidine infusion and postoperative delirium in older patients undergoing cardiac surgery. Br J Anaesth 2021;126:665–73. 10.1016/j.bja.2020.10.041 [DOI] [PubMed] [Google Scholar]
- 54.Deiner S, Luo X, Lin H-M, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: A randomized clinical trial. JAMA Surg 2017;152:e171505. 10.1001/jamasurg.2017.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turan A, Duncan A, Leung S, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet 2020;396:177–85. 10.1016/S0140-6736(20)30631-0 [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Wang X, Zhang Q, et al. Application effects of Remimazolam and propofol on elderly patients undergoing hip replacement. BMC Anesthesiol 2022;22. 10.1186/s12871-022-01641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo J, Qian Y, Zhang X, et al. Remimazolam Tosilate compared with propofol for gastrointestinal Endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol 2022;22. 10.1186/s12871-022-01713-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of Remimazolam compared with placebo and midazolam for moderate sedation during Bronchoscopy. Chest 2019;155:137–46. 10.1016/j.chest.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 59.Doi M, Morita K, Takeda J, et al. Efficacy and safety of Remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth 2020;34:543–53. 10.1007/s00540-020-02788-6 [DOI] [PubMed] [Google Scholar]
- 60.Tan Y, Ouyang W, Tang Y, et al. Effect of Remimazolam Tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal Endoscopy. J Gastroenterol Hepatol 2022;37:576–83. 10.1111/jgh.15761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang M, Liu X, Yang D, et al. Effect of Remimazolam Besylate compared with propofol on the incidence of delirium after cardiac surgery: study protocol for a randomized trial. Trials 2021;22:717. 10.1186/s13063-021-05691-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-071912supp001.pdf (106.1KB, pdf)