Summary
Background
Anti-PD-1 and PD-L1 antibodies (mAbs) are approved immunotherapy agents to treat metastatic non-small cell lung cancer (NSCLC) patients. Only a minority of patients responds to these treatments and biomarkers predicting response are currently lacking.
Methods
Immunoscore-Immune-Checkpoint (Immunoscore-IC), an in vitro diagnostic test, was used on 471 routine single FFPE-slides, and the duplex-immunohistochemistry CD8 and PD-L1 staining was quantified using digital-pathology. Analytical validation was performed on two independent cohorts of 206 NSCLC patients. Quantitative parameters related to cell location, number, proximity and clustering were analysed. The Immunoscore-IC was applied on a first cohort of metastatic NSCLC patients (n = 133), treated with anti-PD1 or anti-PD-L1 mAbs. Another independent cohort (n = 132) served as validation.
Findings
Anti-PDL1 clone (HDX3) has similar characteristics as anti-PD-L1 clones (22C3, SP263). Densities of PD-L1+ cells, CD8+ cells and distances between CD8+ and PD-L1+ cells were quantified and the Immunoscore-IC classification was computed. Using univariate Cox model, 5 histological dichotomised variables (CD8 free of PD-L1+ cells, CD8 clusters, CD8 cells in proximity of PD-L1 cells, CD8 density and PD-L1 cells in proximity of CD8 cells) were significantly associated with Progression-Free Survival (PFS) (all P < 0.0001). Immunoscore-IC classification improved the discriminating power of prognostic model, which included clinical variables and pathologist PD-L1 assessment. In two categories, the Immunoscore-IC risk-score was significantly associated with patients’ PFS (HR = 0.39, 95% CI (0.26–0.59), P < 0.0001) and Overall Survival (OS) (HR = 0.42, 95% CI (0.27–0.65), P < 0.0001) in the training-set. Further increased hazard ratios (HR) were found when stratifying patients into three-category Immunoscore-IC (IS-IC). All patients with Low-IS-IC progressed in less than 18 months, whereas PFS at 36 months were 34% and 33% of High-IS-IC patients in the training and validation sets, respectively.
Interpretation
Immunoscore-IC is a powerful tool to predict the efficacy of immune-checkpoint inhibitors (ICIs) in patients with NSCLC.
Funding
Veracyte, INSERM, Labex Immuno-Oncology, Transcan ERAnet European project, ARC, SIRIC, CARPEM, Ligue Contre le Cancer, ANR, QNRF, INCa France, Louis Jeantet Prize Foundation.
Keywords: Digital pathology, Immunoscore-IC, Prognostic marker, Non-small cell lung cancer, Immunotherapy
Research in context.
Evidence before this study
In cancer physiopathology, immune infiltration of tumours is closely related to clinical outcomes. Tumour-infiltrating immune cells can serve as biomarkers to predict survival in different cancer types and can be the deciding factor for the first-line therapy (i.e., chemotherapy and/or immunotherapy). In 2020, the European Society of Medical Oncology introduced the Immunoscore in its Clinical Practice Guidelines. Immunoscore measures the density of two populations of immune cells involved in response to cancer (CD3+ and CD8+ T lymphocytes) in the core and at the margin of the tumour, therefore allowing researchers and clinicians to account for immune infiltration. A low infiltration in T lymphocytes is associated with a high risk of relapse whereas a high infiltration of the same population is linked with a low risk of relapse. This is the first test available in clinics that appears to be a promising biomarker for diagnosis, treatment and follow-up of localised colon cancer. PD-L1+ cells and CD8+ cells have been proposed as biomarkers to predict response to Immune-Checkpoint Inhibitors (ICI) immunotherapy, and PD-L1 immunohistochemistry represents FDA-approved companion diagnostic test, especially for Non-Small Cell Lung Cancer (NSCLC). However, PD-L1 expression alone remains poorly predictive of the efficacy of ICIs in lung cancer since only a small proportion of patients with NSCLC highly expressing PD-L1 shows good response to ICI. Moreover, some patients with PD-L1 negative tumours derive a major benefit from treatment with ICI.
Added value of this study
There is an imperious need in developing simple and efficient tools that would allow clinicians to determine whether NSCLC patients could respond to anti PD1/PD-L1 immunotherapy (Nivolumab, Pembrolizumab, Cemiplimab, Durvalumab, etc.). In this study, we investigate the clinical implications of the Immunoscore Immune-Checkpoint (IC) in 2 independent cohorts of NSCLC patients. Selection criteria include patients treated with checkpoint immunotherapy as single agent (anti-PD-1, anti-PD-L1). This assay consists in a dual-staining of both CD8+ and PD-L1+ cells and a quantification of their densities and spatial distances, using digital pathology tools. Herein, we showcase its potential to identify patients with high-risk clinical features, to predict a recurrence and to decipher the benefits of ICI immunotherapy for certain NSCLC patients. Additional benefits of Immunoscore IC would be to select patients with the highest chances of response to ICI, with the best cost/benefit ratio, while avoiding adverse events for non-responding patients and proposing them enrolment into clinical trials with combination immunotherapy.
Implications of all the available evidence
Immunoscore-IC can be of great prognostic value and guide patient selection for ICI therapy. Immunoscore-IC has a predictive value superior to that of the currently used MSI status or PD-L1 solo-staining and could guide clinicians to choose appropriate treatment for NSCLC patients.
Introduction
According to the World Health Organisation (WHO), non-small cell lung cancer (NSCLC) is the most frequent form of thoracic cancer, accounting for 20% of all cancer-associated mortality worldwide (https://gco.iarc.fr/today). The treatment of this devastating disease changed with the development of targeted therapies for patients with adenocarcinoma bearing specific mutations and translocations in EGFR, ALK, RAS, BRAF, HER2, NTRK genes.1 The emergence of immunotherapeutic drugs using monoclonal antibodies (mAbs) targeting PD-1 and PD-L1, also called immune checkpoint inhibitors (ICIs), completely changed the therapeutic management of patients. Moreover, the association of chemotherapy with anti-PD-(L)1 treatments was proven more beneficial than chemotherapy alone in either adenocarcinoma or squamous NSCLC.2,3 For locally advanced disease, radio-chemotherapy and durvalumab (anti-PD-L1 mAb) as adjuvant therapy also demonstrated greater longevity than radio-chemotherapy alone.4, 5, 6 These types of treatments have now been approved as the standard-of-care (SOC) for patients with advanced NSCLC.7 However, not all patients benefit from ICIs, thus highlighting the importance to develop valuable biomarkers for better patient selection.
PD-L1 labelling using classical immunohistochemistry (IHC) is the SOC biomarker for NSCLC patient selection.8 PD-L1 tumour proportion score (TPS) above 50% could isolate a population of patients in which immunotherapy alone, using pembrolizumab, is better than chemotherapy in first-line treatment.9 In contrast, for other patients, chemo-immunotherapy using platinum-based chemotherapy and anti-PD-1 is the preferred treatment.10 Various cut-offs of PD-L1 expression and various antibodies, which determine PD-L1 expression at the surface of tumour cells (TC) and/or immune cells, could be used.11 However, this biomarker remains incapable to explain the complexity of anti-tumoral immune response and unable to isolate responder or resistant patients with good specificity.
The understanding of in situ immune response underlines that additional factors could be essential to predict responders to anti-PD-1/PDL1 treatments. Genomic parameters such as tumour mutational burden became an emerging biomarker.12 Furthermore, cytotoxic T-cells and PD-L1 status were associated with response to ICIs therapy4, 5, 6,13,14; adding CD8 infiltration analysis to PD-L1 status that significantly improved outcome prediction.13,15,16 Multiplex immunohistochemistry (IHC) and digital pathology (DP) have emerged as powerful tools to quantify tumour infiltration by immune cells and their interactions with other tumour micro-environment components.13,17,18 Immunoscore-Immune-Checkpoint (IS-IC) is a dual-staining IHC assay of PD-L1+ and CD8+ cells, on a single slide prepared from FFPE tissue. After digitisation, the samples are analysed with a DP tool to account for PD-L1+ and CD8+ cells. Data collected include quantitative variables on PD-L1/CD8 densities and proximity between these cell populations.
This study reports the first evaluation of Immunoscore-IC in two independent NSCLC cohorts treated with anti-PD-1/PD-L1 mAbs. Herein, we unravel the predictive power of Immunoscore-IC to classify patients according to clinical response and survival.
Methods
Patients
265 NSCLC patients were statistically analysed, after quality control of Immunoscore-IC biomarker and clinical data assessment. Quality control included biomarker quality control (anapathological invalidation—no tumour, patient identification—removal of duplication) and clinical data quality control. These 265 patients include a training set of 133 patients (Training cohort) and a validation set of 132 patients (Validation cohort). The Training cohort comprises patients from the CGFL (“Centre Georges Francois Leclerc”, Dijon, France) and the Caen hospitals. The Validation cohort corresponds to patients from the AP-HM center (“Assistance Publique des Hôpitaux de Marseille”).
Immunohistochemistry staining with anti-PD-L1 antibodies (222C3, SP263, HDX3)
Immunohistochemistry for PD-L1 was performed on 4-μm thick whole sections. Staining with mAb 22C3 (PD-L1 IHC 22C3, pharmDx; Agilent Technologies, Carpinteria, CA, USA), mAb SP263 (Roche Diagnostics, Meylan, France) and mAb HDX3 (Veracyte SAS, Marseille, France) were performed according to the instructions of the manufacturers.
Immunoscore-IC test
Immunoscore-IC (Veracyte SAS, Marseille, France) is designed to measure the densities of PD-L1+ and CD8+ cells as well as the proximity between these cells on a single tissue section with image analysis tools.
Immunohistochemistry-based staining was performed on Benchmark XT instrument (Roche-Ventana) as follows: standard deparaffinisation, Cell Conditioning 1 for 54 min, anti-PD-L1 (clone HDX3, Veracyte) 1-h incubation at 37 °C, anti-CD8 (clone HDX1, Veracyte) 1-h incubation at 37 °C, and Hematoxylin II 8-min counterstaining. Anti-PD-L1 and anti-CD8 antibodies were revealed with OptiView DAB IHC Detection Kit and UltraView Universal Alkaline Phosphatase Red Detection Kit respectively. Every stained slide was scanned with a high-resolution scanner (NanoZoomer XR, Hamamatsu) to obtain 20× digital images. Whole slide images were analysed by DP using HALO software (Indica labs, Corrales, NM, USA) for the detection of the tissue section, definition of the tumour core, identification and quantification of stained cells within the tumour core. Cell coordinates and phenotypes were exported to analyse their spatial distribution.
Main computed quantitative and spatial variables were CD8+ and PD-L1+ cell density, cell proximity, and cell clustering. The cut off distance used to compute proximity and cluster indexes was arbitrarily set to 20 μm.
Immunoscore-IC DP analysis
The Immunoscore-IC was built using a LASSO Cox-based algorithm on the training cohort, taking as input the Immunoscore-IC variables dichotomised into low (−1)/high (+1). Five parameters were selected based on their association with PFS (Supplementary Fig. S2). For each parameter, the Cox model returned an odd ratio indicating the contribution of the variable in predicting PFS. A risk-score was computed incorporating the prognostic information of the selected markers. This score was then dichotomised into two- or three-category Immunoscore-IC ("Low", "Intermediate" or "High") based on the association with patients’ PFS: the low-risk group, characterised by high values of Immunoscore-IC markers, was defined as Immunoscore-IC High, whereas the high-risk group, with low markers density values, was defined as an Immunoscore-IC Low. The Immunoscore-IC was then calculated for the validation set using the parameters (variables, coefficients and cut-offs) identified on the training set (Supplementary Figs. S5 and S6).
Ethics committee approval
The study was approved by an ethical review board (#0912082). Informed Consent Statement was obtained from all subjects involved in the study. Patient declaration form has been also provided.
Statistical analysis
The association between Immunoscore-IC markers and patients’ progression-free survival (PFS) were analysed using univariate Cox proportional hazard model. The dichotomisation of markers was performed using R package “maxstat”.
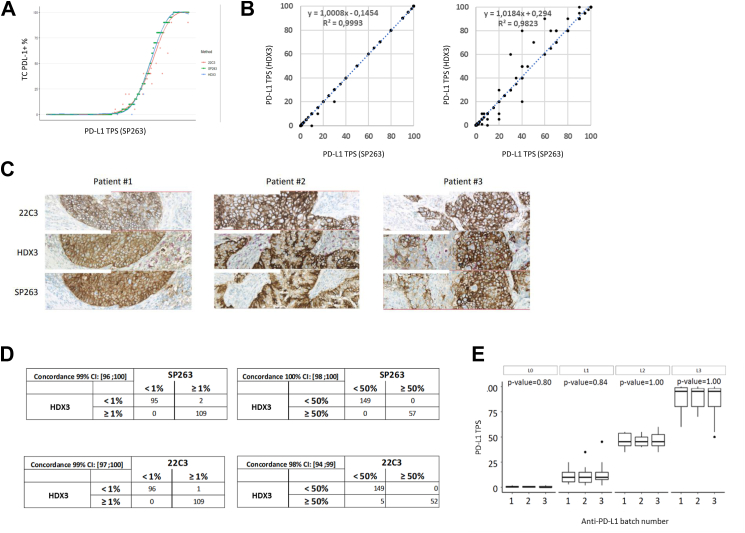
The Immunoscore-IC risk-score was calculated using LASSO Cox based algorithm implemented in the “glmnet” R package. Heatmap, showing the levels of markers by groups, was created using R/Bioconductor package “ComplexHeatmap”. Boxplots with statistical significance were generated using the “ggplot2” and “ggpubr” R packages. Fisher’s exact test, χ2 test and Wilcoxon test were used to assess the associations between Immunoscore-IC status and clinical variables. Statistical analyses were done using R version 3.6.3. In Fig. 1B, linear regression was applied and Pearson correlation was calculated to summarise the goodness of fit. Data in Fig. 1A were fitted with a general additive model (GAM) with a smooth on the predictor variable at the 8th dimension. As illustrated in Supplementary Fig. S6, we assessed the distribution of all the variables measured by the Immunoscore-IC test. As normal distribution can’t be assumed for almost all the variables, we applied overall nonparametric methods, which are less sensitive to deviations from normality, and can be more robust in the presence of skewed or irregularly shaped data. Kaplan–Meier curves for PFS and OS rates show that patients treated with ICIs have similar outcomes when only accounting for PD-L1 TPS by pathologists (Supplementary Fig. S3). The high degree of correlation between the tests is mirrored by the concordance between HDX3, SP263 and 22C3 on the same set of 206 commercial samples at the clinically relevant cut-off points (PD-L1 TPS 1% and 50%) [as shown in Fig. 1D].
Fig. 1.
Anti-PDL1 clone HDX3 has a similar staining profile as approved anti-PD-L1 clones (22C3, SP263). (A) 206 whole-slide samples from NSCLC patients were analysed for PD-L1 TPS: (Blue dot) HDX3, (orange dot) SP263, (Cyan dot) 22C3. (B) Correlation between the PD-L1 clones HDX3, 22C3 and SP263 for the quantification of PD-L1 TPS. (C) Representative images of PD-L1 staining of tissue sections from 3 different patients. (D) Contingency tables showing the agreement between HDX3 and 22C3 or SP263 for the assessment of PD-L1 TPS. (E) Evaluation of the impact of batch-to-batch variability for HDX3/PD-L1 TPS across 4 levels (L0–L3): level 0: non-stained tumour; level 1: weakly stained tumour; level 2: moderately stained tumour; and level 3: strongly stained tumour.
Role of the funders
This work is financially supported by Veracyte, INSERM, LabEx Immuno-oncology, Transcan ERAnet European project, ARC, SIRIC, CARPEM, Ligue contre le Cancer, ANR, QNRF, INCa France, Louis Jeantet Prize Foundation. Funders do not have contributions in the study design, data collection, data analyses, interpretation, writing the manuscript or in the decision to submit it for publication.
Results
HDX3 PD-L1 TPS vs SP263 and 22C3 PD-L1 TPS
We compared the staining performance of a new anti-PD-L1 monoclonal antibody (clone HDX3), with anti-PD-L1 antibodies from Agilent (22C3) and Roche-Ventana (SP263).
Consecutive tissue section from formalin-fixed paraffin-embedded (FFPE) tissue blocks, mounted on glass slides, were stained with 3 anti-PD-L1 mAb: 22C3 (Top-layer images), HDX3 (middle-layer images) or SP263 (bottom-layer images) (Fig. 1C). On sections taken from 3 NSCLC patients, HDX3 mAb showed similar staining patterns and intensities to those obtained with 22C3 and SP263 mAb.
Those three anti-PD-L1 antibodies were used to stain sections prepared from FFPE tissue blocks of a cohort of 206 NSCLC patients. The results were reported as PD-L1 tumour proportion score (TPS), i.e. the percentage of viable tumour cells showing partial or complete membrane staining (≥1+) relative to all viable tumour cells present in the sample. PD-L1 TPS assessed with HDX3, SP263 and 22C3 antibodies were reported by two pathologists, on a cohort ranging from 0 to 100% PD-L1 TPS (Fig. 1). Similar distribution patterns of PD-L1 TPS were observed with the three antibodies across the 206 evaluated samples (Fig. 1A) and coefficients of correlation “R2” of 0.99 and 0.93 between HDX3 vs SP263 and 22C3 were found, respectively (Fig. 1B).
That high degree of correlation between the tests is mirrored by the concordance between HDX3, SP263 and 22C3 on the same set of 206 commercial samples at the clinically relevant cut-off points (PD-L1 TPS 1% and 50%). Results showed a high degree of overall agreement HDX3 vs 22C3 (99%, [97%–100%] CI) and SP263 (99%, [96%–100%] CI) for TPS 1% and HDX3 vs 22C3 (98%, [94%–99%] CI) and vs SP263 (100%, [98%–100%] CI) for PD-L1 TPS 50%, respectively (Fig. 1D).
Assessment of Immunoscore-IC sources of variability and multi-centric validation
The anti-PD-L1 HDX3 clone was used in combination with HDX1, an anti-CD8 clone from Veracyte Inc. to develop the Immunoscore-IC test, a dual staining protocol designed to detect PD-L1+ and CD8+ cells. PDL1 TPS precision obtained with Immunoscore-IC was measured according to CLSI LA28-A2 standard on 11 NSCLC tumour grouped in four classes representative of distinct PD-L1 tumour expression levels (classes L0–L3, Fig. 1E). 30 consecutive slides per sample were stained following the Immunoscore-IC procedure across 14 IHC runs on 2 Benchmark XT instruments with 3 batches of antibodies and revelation kits. Stained sections were randomised prior to PD-L1 TPS assessment by a pathologist. None of the tested variables had an impact on PD-L1 result according to Fisher’s exact test. Fig. 1E shows an example of the distribution of PD-L1 TPS obtained with 3 primary antibody batches across 4 levels of expression.
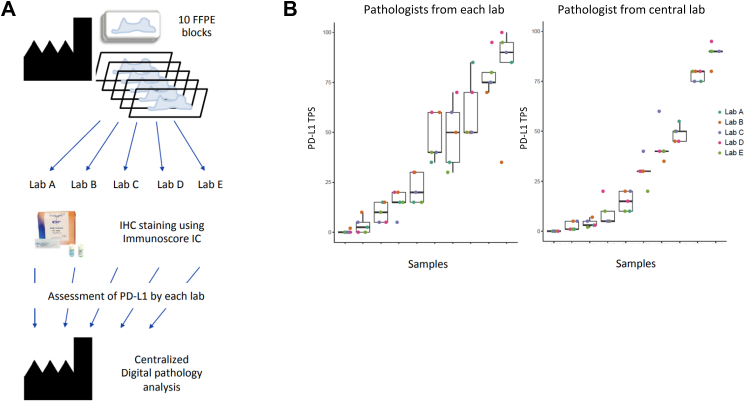
Next, the robustness of the Immunoscore-IC was assessed following a multicentric validation. 10 FFPE tissue blocks from NSCLC patients were sent to five different laboratories with Immunoscore-IC kits at their disposal. Following dual staining of CD8 and PL-L1, pathologists at each site quantified the PD-L1 TPS. Data and stained sections were returned to Veracyte laboratories for a second quantification before statistical analysis (Fig. 2A). Based on the TPS, reproducible results were obtained using the Immunoscore-IC assay across 5 independent laboratories. Noteworthily, distribution plots show that lab-to-lab variability is mainly observable between readers. This variability is not issued by the automated staining procedure or the well-controlled reagents (Fig. 2B).
Fig. 2.
Multicentric comparison of HDX3 staining on NSCLC samples. (A) Schematic representation of the study design to assess HDX3 reproducibility across five different laboratories and their pathologists before a centralised analysis. (B) Dot-and-box histograms of PD-L1 TPS for each centre. Dots can be overlapping for cases of low PD-L1 expression.
Immunoscore-IC staining protocol and test
As PD-L1 biological function is to inhibit PD1-expressing lymphocytes through interaction of both proteins, lymphocyte quantification and localisation seem important for anti-PD-1/L1 ICI efficacy. Immunoscore-IC test provides a dual-staining protocol of PD-L1 and CD8, associated to a dedicated DP tool hat allows quantification, localisation and assessment of proximity between stained cells.
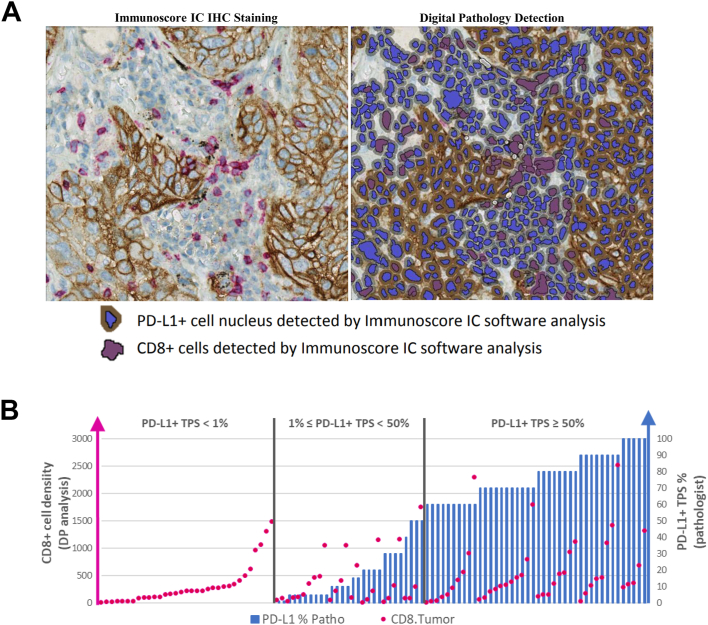
Since PD-L1+ cells present the ability to inhibit cytotoxic T lymphocytes expressing PD-1, DP tool allows the quantification of all PD-L1+ cells, including non-tumoral cells. In addition, it detects, localises and quantifies CD8+ cells in the pre-defined Region Of Interest (ROI) which is the core of the tumour (CT). The density of both cell types in the ROI are expressed in cells per squared millimetre (cells/mm2). The left panel of Fig. 3A shows NSCLC tissue where PD-L1+ brown and CD8+ red staining are detected on the plasma membrane (Fig. 3A, right panel).
Fig. 3.
Immunoscore-IC is both an IHC assay and a digital pathology tool to help clinicians categorise patients for potential ICIs therapy. (A) Representative IHC staining of a lung tissue section from a NSCLC patient before (left) and after (right) DP detection. (B) Distribution of PD-L1+ TC% (blue bars) and CD8+ cells density (cells/mm2, red dots) across 103 NSCLC patient samples.
Immunoscore-IC dual IHC staining was performed on a training cohort of 133 metastatic NSCLC tumour samples (Table 1). Patients’ samples were ordered by increasing PD-L1 TPS and then by density of CD8+ cells for matching PD-L1 TPS (Fig. 3B). At every level of PD-L1 TPS, CD8+ cell density varied greatly between samples, suggesting that CD8+ and PD-L1+ cell densities could improve patients’ stratification.
Table 1.
Main characteristics of the training and validation cohorts.
| Cohorts | Training | Validation | FisherTestPv |
|---|---|---|---|
| Characteristic | 133 | 132 | |
| Sex | 1.0000 | ||
| Male | 90 | 89 | |
| Female | 43 | 43 | |
| Age_at_diag | 65 (45–84) | 61 [32, 84] | |
| Unknown | 9 | 1 | |
| Immunotherapy PD1/PDL1 | 0.3463 | ||
| PD1 based | 132 | 116 | |
| PDL1 based | 1 | 16 | |
| Immunotherapy monotherapy/combo | 1.0000 | ||
| Monotherapy | 129 | 129 | |
| Combo therapy | 4 | 3 | |
| Immunotherapy monotherapy/combo | 0.0000 | ||
| Nivolumab-based | 99 | 113 | |
| Pembrolizumab-based | 33 | 3 | |
| Others | 1 | 16 | |
| Response | 0.0814 | ||
| SD | 28 | 46 | |
| PD | 76 | 63 | |
| PR | 18 | 18 | |
| CR | 9 | 4 | |
| Unknown | 2 | 1 | |
| Pfs_event | 112 | 118 | |
| Pfs_time | 4.5 (0–41) | 3 [0–67] | |
| Unknown | 1 | ||
| OS_event | 96 | 105 | 1.0000 |
| Unknown | 2 | 3 | |
| OS_time | 12.5 (0–41) | 11 [0–95] | |
| Unknown | 2 | 7 |
133 NSCLC patients were included in a training cohort. An independent cohort of 132 NSCLC patients from a different care centre was used as validation. Nivolumab was the most frequently prescribed treatment, followed by pembrolizumab. The majority of patients received monotherapy as standard of care.
Anti-PD-(L)1 immunotherapies target the molecular interaction between PD-L1 present at the surface of tumour or immune cells and PD1 expressed by various lymphoid cells, including cytotoxic T-cells. This suggests that their efficacy depends on the proximity between PD-L1+ and cytotoxic T lymphocytes. We hypothesised that, in addition to cell densities within the tumour, a proximity index between PD-L1+ and CD8+ cells could enhance the predictive value of Immunoscore-IC. Examples of variability in the colocalisation of CD8+ and PD-L1+ cells are showed in Supplementary Fig. S1A.
In order to assess the proximity between CD8+ and PD-L1+ cells, an Immunoscore-IC DP module was developed. This module can (1) measure a proximity index based on the density of PD-L1+ cells with at least one CD8+ cell within a radius of 20 μm around the centroid of the considered cell (Supplementary Fig. S1B), (2) measure the density of CD8+ cells with at least one PD-L1+ cell within 20 μm, (3) account for the clustering of CD8+ cells (CD8+ surrounded by at least one CD8+ cell within 20 μm), (4) evaluate the clustering of PD-L1+ cells. The widespread values obtained suggest that proximity indexes could stratify NSCLC patients based on the density of PD-L1+ and CD8+ cells and their proximity within the tumour (Supplementary Fig. S1C).
LASSO model construction based on clinical data and IS-IC
Having laid-down the analytical validation and the performances of the Immunoscore-IC, we quantified tumour samples from two independent cohorts of 265 NSCLC patients treated with anti-PD-(L)1 immunotherapy. Clinical characteristics are shown in Table 1. A training-set including 133 patients (Training cohort) from two different care-centers, and a validation-set including 132 patients from a third care center were analysed. For both cohorts, 68% of patients were men, with a similar median age of 65 and 61 for the Training and Validation cohorts, respectively. Response rates, survival, mono- or combo-immunotherapy rates, and PD1-based vs PD-L1-based immunotherapy rates were not significantly different in both cohorts. Nivolumab was the most commonly used immunotherapy in both cohorts, and Training cohort received more frequently Pembrolizumab than the Validation cohort (Table 1).
In both training and validation sets, a significant univariate association with PFS was observed for most Immunoscore-IC parameters in CT, using continuous variables. This by using univariate Cox model score tests of P < 0.05 with hazard ratios (HR) ranging from 0.6 (0.44–0.82) to 0.85 (0.72–0.99) for the training set and 0.62 (0.48–0.81) to 0.8 (0.66–0.98) for the validation set. With dichotomisation, the Immunoscore-IC parameters remained highly significant in a univariate analysis for PFS (Supplementary Fig. S2). In contrast, PD-L1 TPS alone could not predict PFS nor Overall Survival (OS) consistently (at the exception of PFS in the validation set). Kaplan–Meier curves for PFS and OS rates show that patients treated with ICIs have similar outcomes when only accounting for PD-L1 TPS by pathologists (Supplementary Fig. S3).
We applied a LASSO method to select parameters and coefficients to define a score stratifying patients into two- (Low, High) or three-category Immunoscore-IC (Low, Intermediate or High). Immunoscore-IC model includes five parameters: CD8+ density, PD-L1+ density and three parameters related to the spatial distribution of these cells (Supplementary Fig. S4).
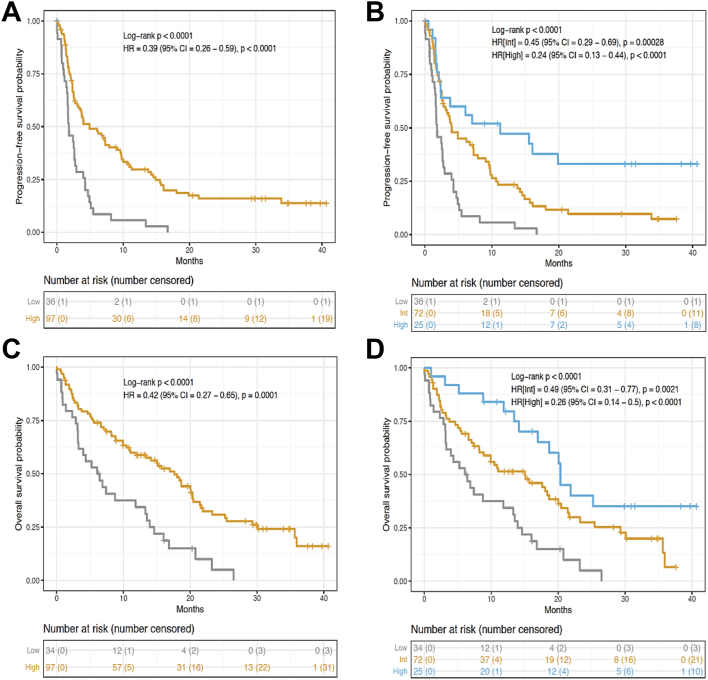
In the training set, analysing the two-category Immunoscore-IC allowed the identification of patients with distinct clinical outcome for PFS and OS (Fig. 4A–C). 97 (73%) patients had a high Immunoscore-IC whereas 36 (27%) patients had a low Immunoscore-IC. Patients with a low Immunoscore-IC had the highest-risk of recurrence. At 24 months, PFS was seen in (0%) of patients with low Immunoscore-IC and in 12 (16%) patients with a high Immunoscore-IC (unadjusted [HR] = 0.39, 95% CI (0.26–0.59), P < 0.0001, Fig. 4A). OS at 24 months was recorded for 1 (5%) patient with a low Immunoscore-IC and 23 (31%) patients with a high Immunoscore-IC (unadjusted [HR] = 0.42, 95% CI, P < 0.0001, Fig. 4C). Moreover, PFS and OS at 36 months highlight patients with the highest Immunoscore-IC as long-term survivors, whereas 100% of low Immunoscore-IC patients relapsed before 18 months and died before 30 months following ICIs therapy (Fig. 4A–C).
Fig. 4.
Immunoscore-IC is associated with improved PFS and OS of patients in the training set. Kaplan–Meier curves describing PFS (A, B) and OS (C, D) of patients in the two- or three-category Immunoscore-IC.
In the three-category Immunoscore-IC, 25 (19%) patients had a high Immunoscore-IC, 72 (54%) patients had an intermediate Immunoscore-IC and 36 (27%) patients had a low Immunoscore-IC (Fig. 4B–D). PFS at 24 months was seen in (0%) patients with low Immunoscore-IC, in 5 (10%) patients with an intermediate Immunoscore-IC and in 7 (34%) patients with a high Immunoscore-IC (unadjusted [HR_High] = 0.24, 95% CI (0.13–0.44), P < 0.0001, Fig. 4B). OS at 24 months was recorded for 1 (5%) patient with a low Immunoscore-IC, 12 (27%) patients with an intermediate Immunoscore-IC and 9 (40%) patients with a high Immunoscore-IC (unadjusted [HR_High] = 0.26, 95% CI (0.14–0.5), P < 0.0001, Fig. 4D). PFS and OS curves showed that patients with a high Immunoscore-IC have the best outcome compared to the low Immunoscore-IC group of patients (Fig. 4B–D).
LASSO model validation on an independent cohort of NSCLC patients
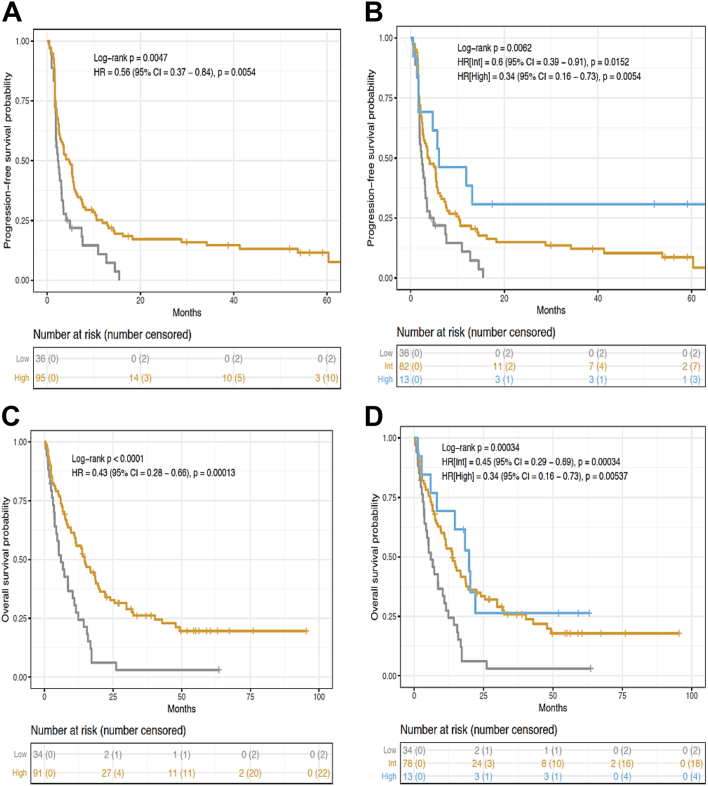
Comparable results were found between Immunoscore-IC and patient’s survival in an independent validation cohort when investigating two- or three-category Immunoscore-IC patients for PFS and OS (Fig. 5). In the two-category Immunoscore-IC, 95 (73%) patients had a high Immunoscore-IC whereas 36 (27%) patients had a low Immunoscore-IC. Patients with a low Immunoscore-IC had the highest risk of recurrence. Indeed, PFS at 24 months was seen in (0%) of patients with low Immunoscore-IC and in 14 (17%) patients with a high-IS-IC (unadjusted [HR] = 0.56, 95% CI (0.37–0.84), P < 0.0054, Fig. 5A). Moreover, PFS curve is reaching a plateau for high Immunoscore-IC patients at 24 months and OS at 24 months was recorded for 2 (6%) patients with a low-IS-IC and 28 (34%) patients with a high-IS-IC (unadjusted [HR] = 0.43, 95% CI (0.28–0.66), P < 0.0001, Fig. 5A and C).
Fig. 5.
Immunoscore-IC is linked with improved PFS and OS of patients in the validation set. Kaplan–Meier curves describing PFS (A, B) and OS (C, D) of patients in the two- or three-category Immunoscore-IC.
In the three-category Immunoscore-IC, 13 (10%) patients had a high Immunoscore-IC, 82 (63%) patients had an intermediate Immunoscore-IC and 36 (27%) patients had a low Immunoscore-IC (Fig. 5B–D). PFS at 24 months was seen in (0%) of patients with low Immunoscore-IC, in 11 (15%) patients with an intermediate Immunoscore-IC and in 3 (31%) patients with a high Immunoscore-IC (unadjusted [HR_High] = 0.34, 95% CI (0.16–0.73), P < 0.0054, Fig. 5B). PFS curve reached a plateau at 24 months for intermediate and high Immunoscore-IC patients. Of note, OS at 24 months was recorded for 2 (6%) patients with a low Immunoscore-IC, 24 (35%) patients with an intermediate Immunoscore-IC and 3 (26%) patients with a high Immunoscore-IC (unadjusted [HR_High] = 0.34, 95% CI (0.16–0.73), P = 0.005, Fig. 5D). Similarly to the two-category Immunoscore-IC, PFS and OS at 36 months show that patients with a high Immunoscore-IC have a better outcome compared to the low Immunoscore-IC group of patients.
Discussion
Investigations demonstrated that particular immune subpopulations infiltrating tumours, like cytotoxic T cells, were significantly associated with the survival of the patients.19 The cancer immune contexture of solid tumours could be a dominant determinant of clinical outcome, also associated with immunotherapy response.13 The consensus Immunoscore is the first worldwide standardised consensus assay to define cold and hot immune tumours by quantifying CD3 and CD8 T-cells.13,20 Its clinical utility was reinforced by demonstrating its predictive value in response to chemotherapy in colon cancer patients.20, 21, 22 The effectiveness of immune modulation strategies depends on the existence of a proper pre-existing immunity.23, 24, 25, 26, 27 Hallmarks of successful anti-cancer immunotherapy and ways to treat hot and cold immune tumours have been proposed.14 Despite anti-PD-(L)1 IC therapy approval for NSCLC patients, a sizeable proportion of patients still do not respond to it.2 Thus, in the era of precision medicine and combination immunotherapy, biomarkers for patient selection are highly desirable.14 To this date, IHC solo-staining for PD-L1 was the biomarker of choice for selecting lung cancer patients for anti-PD-(L)1 therapy in phase 3 clinical trials.2,28 Nevertheless, it is reported here that PD-L1 is an imperfect biomarker. Indeed, PD-L1 expression alone remains poorly predictive of the ICIs’ efficacy in lung cancer, since only a small proportion of NSCLC patients highly expressing PD-L1 undergo a good response to ICI. Moreover, tumour mutation burden (TMB) or the combination of TMB and high-PD-L1 expression outperform PD-L1 alone as predictive biomarkers.29 PD-L1+ patients do not always respond to ICIs therapy.30 Therefore, additional enrichment for response in the PD-L1+ population may be needed to assess if PD-L1 is expressed in an adaptive (adaptive negative feedback-loop) rather than a constitutive (oncogenic induction) manner.
On the one hand, our findings are aligned with a previous human study linking PD-1+ and CD8+ lymphocytes to a positive response to ICIs.31 On the other hand, the visual evaluation of PD-L1 as a predictive biomarker for cancer immunotherapy is controversial. This is due to the subjective semi-quantitative evaluation of a simple stain reported by a pathologist, with impact on the interobserver variability and diagnostic accuracy of PD-L1 immunostaining.32 Herein, our results show that (1) anti-PD-L1 HDX3 clone performed similarly to commercial anti-PD-L1 targeting mAbs (22C3, SP263), (2) Immunoscore-IC DP tools were as strong as the pathologist’s evaluation to decipher PD-L1+ and CD8+ cells and (3) Immunoscore-IC test and SP263, 22C3 mAbs present a strong concordance.33 Immunoscore-IC is a potent predictive marker of response to anti-PD-1/PD-L1 immunotherapy. The positive impact of ICIs therapy is far greater for high Immunoscore-IC patients than it is for low Immunoscore-IC patients. Indeed, all patients (100%) with a low Immunoscore-IC relapsed in less than 18 months, in contrast to 34% and 33% of high Immunoscore-IC patients who did not relapse for more than 36 months in the training and validation cohorts, respectively. Immunoscore-IC is a fast and simple standardised assay run on a single FFPE slide, which is often a matter of contention when managing patient’s care for NSCLC. Hence, Immunoscore-IC (1) is a potent quantitative and predictive marker of response to anti-PD-1/PD-L1 immunotherapy, (2) allows the identification of responder and non-responder NSCLC patients for ICIs therapy, (3) was shown to be highly standardised and reproducible and (4) has a predictive value superior to the currently used PD-L1 solo-staining and could guide clinicians to choose between ICIs therapy or chemotherapy.34
However, this study has several limitations. Moving forward, it would be of interest to validate the predictive value of the Immunoscore-IC. Even if two independent cohorts showed equivalent results regarding the Immunoscore-IC predictive performance, patients may be heterogeneous. These results should be validated on larger cohorts of NSCLC patients within randomised clinical trials, as well as in other cancer types.35
Moreover, in order to standardise the assays, additional efforts are needed to reveal the cell type expressing PDL-1, since preclinical data suggests that PD-L1 expression in tumour and immune cells can modulate T-cell function in the TM.36 Furthermore, the implementation of using digital slides would be essential, as reported by The College of American Pathologists and the Digital Pathology Association guidelines.37 In the era of personalised medicine, immunotherapy with anti-PD-(L)1 mAbs represents a relevant clinical option for patients with advanced stage NSCLC.38
Innovative characterisation of the TME with a focus on multidimensional, spatially resolved interactions at a cellular level will provide critical mechanistic insights into therapeutic responses and potentially identify improved biomarkers for patient selection. Whole-slide image scanning and DP of several markers have paved the way for the development of immune contexture signatures as well as its implementation in hospital-hubs.13 Besides, pathologists are less reluctant to the idea of signing-out reports based on digital slides, especially when comparative studies have been published and showed solid data on safety and feasibility.39
ICIs therapy is potentially highly effective in specific groups of patients. Delivering robust predictive signatures can allow a better patient stratification and better clinical decision-making in cancer treatment. Indeed, immune-related adverse-events associated with anti-PD-(L)1 treatment in NSCLC patients were studied in a meta-analysis and showed that the overall incidence of these events was 22% for all grades and 4% for high-grade (grade ≥3) NSCLC.40 Furthermore, Immunoscore-IC was also used in metastatic colorectal cancer patients treated with immunotherapy. The combination of the anti-PD-L1 atezolizumab with first-line FOLFOXIRI plus bevacizumab was significantly improving the outcome of metastatic colorectal cancer patients only in the group of Immunoscore-IC high tumours.41 Our preliminary analyses also demonstrated the predictive power of Immunoscore-IC in another combination immunotherapy (Chemotherapy + anti-VEGF + anti-PDL1) in first line metastatic colorectal cancer. Immunoscore-IC significantly predicted responder to this combination immunotherapy. It is likely that Immunoscore-IC will be a relevant and informative test to multiple combination immunotherapy with anti-PD1/L1, such as anti-CTL4 + anti-PD1/L1. However, further studies are ongoing and are essentially needed to further investigate the predictive power of IS-IC for other immunotherapy or chemo-immunotherapy combinations.
Immunoscore-IC can minimise treatment costs by excluding potential non-responding patients. It could also serve pharmaceutical companies and academic clinical-centers to select the right patients, thus improving the success rate of clinical trials and allowing unresponsive patients to enter immunotherapy combination trials.
In conclusion, the recent success of immune-based cancer therapy and digital imaging are changing the pathology practice. Immunoscore-IC using spatial quantitative analysis of CD8 and PD-L1 markers is predictive of the efficacy of anti-PD-(L)1 immunotherapy in NSCLC.
Contributors
Study concept and design: (JG, JF). Acquisition of data: (JG, JF, FM, TS, IB, AC). Statistical analysis (AK). Interpretation of data: (JG, JF, FM, FB, FG, AM, NB, JA, LG, PT). Drafting of the manuscript: (JG, FG, AM). Drafting of the figures: (AK, FM, JG). Critical revision of the manuscript: (JG, JF, AK, JA, AM, EM, YO, FB, FG, LG, PT). Technical support: (FM, CL, NB, JA, TS). Material support: (FB, FG, LG, PT, ALL, JDF, AI). Clinical data: (FB, FG, LG, PT). Study supervision: (JG). Data verification: (FG and JG). All authors read and approved the final version of the manuscript.
Data sharing statement
Detailed extracted data on all included studies are available immediately following publication upon request to the corresponding author. Proposals should be directed by email to the corresponding author JG, at jerome.galon@crc.jussieu.fr.
Declaration of interests
JG has patents associated with the immune prognostic biomarkers. JG is co-founder of HalioDx, a Veracyte company. Immunoscore® a registered trademark owned by the National Institute of Health and Medical Research (INSERM) and licenced to Veracyte.
Acknowledgements
The work was supported by Veracyte and by grants from INSERM, the Labex Immuno-Oncology, the Transcan ERAnet European project, Association pour la Recherche contre le Cancer (ARC), Site de Recherche intégrée sur le Cancer (SIRIC), CAncer Research for PErsonalised Medicine (CARPEM), La Ligue contre le Cancer, Agence Nationale de la Recherche (ANR Grant TERMM ANR-20-CE92-0001), The Qatar National Research Fund (QNRF) grant number NPRP11S-0121-180351, Institut National Du Cancer, France (INCa), the Louis Jeantet Prize Foundation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104633.
Appendix A. Supplementary data
References
- 1.Imyanitov E.N., Iyevleva A.G., Levchenko E.V. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103194. [DOI] [PubMed] [Google Scholar]
- 2.Akinboro O., Larkins E., Pai-Scherf L.H., et al. FDA approval summary: pembrolizumab, atezolizumab, and cemiplimab-rwlc as single agents for first-line treatment of advanced/metastatic PD-L1 high NSCLC. Clin Cancer Res. 2022;28(11):2221–2228. doi: 10.1158/1078-0432.CCR-21-3844. [DOI] [PubMed] [Google Scholar]
- 3.García-González J., Ruiz-Bañobre J., Afonso-Afonso F.J., et al. PD-(L)1 inhibitors in combination with chemotherapy as first-line treatment for non-small-cell lung cancer: a pairwise meta-analysis. J Clin Med. 2020;9(7):2093. doi: 10.3390/jcm9072093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirasawa M., Yoshida T., Imabayashi T., et al. Baseline PD-L1 expression and tumour-infiltrated lymphocyte status predict the efficacy of durvalumab consolidation therapy after chemoradiotherapy in unresectable locally advanced patients with non-small-cell lung cancer. Eur J Cancer. 2022;162:1–10. doi: 10.1016/j.ejca.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Tufman A., Neumann J., Manapov F., et al. Prognostic and predictive value of PD-L1 expression and tumour infiltrating lymphocytes (TiLs) in locally advanced NSCLC treated with simultaneous radiochemotherapy in the randomized, multicenter, phase III German Intergroup lung Trial (GILT) Lung Cancer. 2021;160:17–27. doi: 10.1016/j.lungcan.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Yoneda K., Kuwata T., Kanayama M., et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer. 2019;121(6):490–496. doi: 10.1038/s41416-019-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong W., Zhao Y., Du H., Guo X. Current status of immune checkpoint inhibitor immunotherapy for lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew M., Safyan R.A., Shu C.A. PD-L1 as a biomarker in NSCLC: challenges and future directions. Ann Transl Med. 2017;5(18):375. doi: 10.21037/atm.2017.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 10.Hofman P. The power of immunotherapy plus platinum-based chemotherapy for locally advanced or early stage non-small cell lung cancer. Ann Transl Med. 2020;8(5):151. doi: 10.21037/atm.2020.01.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto D., Yamashita D., Fukuoka J., et al. Comparison of PD-L1 assays in non-small cell lung cancer: 22C3 pharmDx and SP263. Anticancer Res. 2018;38(12):6891–6895. doi: 10.21873/anticanres.13065. [DOI] [PubMed] [Google Scholar]
- 12.Chan T.A., Yarchoan M., Jaffee E., et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruni D., Angell H.K., Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 14.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 15.Fumet J.D., Richard C., Ledys F., et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018;119(8):950–960. doi: 10.1038/s41416-018-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng M.W., Ngiow S.F., Ribas A., Smyth M.J. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu S., Stein J.E., Rimm D.L., et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195–1204. doi: 10.1001/jamaoncol.2019.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele K.E., Tan T.H., Korn R., et al. Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J Immunother Cancer. 2018;6(1):20. doi: 10.1186/s40425-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindea G., Mlecnik B., Fridman W.H., Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33(4):335–340. doi: 10.1007/s00281-011-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagès F., André T., Taieb J., et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31(7):921–929. doi: 10.1016/j.annonc.2020.03.310. [DOI] [PubMed] [Google Scholar]
- 21.Mlecnik B., Bifulco C., Bindea G., et al. Multicenter International Society for Immunotherapy of Cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020;38(31):3638–3651. doi: 10.1200/JCO.19.03205. JCO1903205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galon J., Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52(1):55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Bindea G., Mlecnik B., Angell H.K., Galon J. The immune landscape of human tumors: implications for cancer immunotherapy. Oncoimmunology. 2014;3(1) doi: 10.4161/onci.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buque A., Bloy N., Aranda F., et al. Trial watch: immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology. 2015;4(4) doi: 10.1080/2162402X.2015.1008814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galluzzi L., Vacchelli E., Fridman W.H., et al. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2012;1(1):28–37. doi: 10.4161/onci.1.1.17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vacchelli E., Eggermont A., Galon J., et al. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2(1) doi: 10.4161/onci.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vacchelli E., Senovilla L., Eggermont A., et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2013;2(3) doi: 10.4161/onci.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sezer A., Kilickap S., Gümüş M., et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 29.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi M., Jiao D., Xu H., et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thommen D.S., Koelzer V.H., Herzig P., et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butter R., Hondelink L.M., van Elswijk L., et al. The impact of a pathologist's personality on the interobserver variability and diagnostic accuracy of predictive PD-L1 immunohistochemistry in lung cancer. Lung Cancer. 2022;166:143–149. doi: 10.1016/j.lungcan.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Adam J., Le Stang N., Rouquette I., et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018;29(4):953–958. doi: 10.1093/annonc/mdy014. [DOI] [PubMed] [Google Scholar]
- 34.El-Deiry W.S., Goldberg R.M., Lenz H.J., et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J Clin. 2019;69(4):305–343. doi: 10.3322/caac.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau J., Cheung J., Navarro A., et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017;8 doi: 10.1038/ncomms14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantanowitz L., Sinard J.H., Henricks W.H., et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710–1722. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi G., Russo A., Tagliamento M., et al. Precision medicine for NSCLC in the era of immunotherapy: new biomarkers to select the most suitable treatment or the most suitable patient. Cancers. 2020;12(5):1125. doi: 10.3390/cancers12051125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahn S.W., Plass M., Moinfar F. Digital pathology: advantages, limitations and emerging perspectives. J Clin Med. 2020;9(11):3697. doi: 10.3390/jcm9113697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X., Roudi R., Dai T., et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19(1):558. doi: 10.1186/s12885-019-5701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antoniotti C., Rossini D., Pietrantonio F., et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23(7):876–887. doi: 10.1016/S1470-2045(22)00274-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.