Abstract
Objective
Famotidine has been proposed as a promising candidate for the treatment of coronavirus disease 2019 (COVID-19). However, there is limited research on the association of famotidine with the poor prognosis of COVID-19.
Methods
The Korean nationwide cohort included 6,556 patients who tested positive on RT-PCR for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The poor COVID-19-related outcomes were defined on the basis of having encountered the composite outcome of high oxygen therapy, intensive care unit admission, administration of mechanical ventilation, or death. In addition, we performed exposure-driven propensity score matching for no H2-blocker use versus current famotidine use, and other H2-blocker use versus current famotidine use.
Results
4,785 (73.0%) patients did not use a H2-blocker, 393 (6.0%) patients were currently used famotidine, and 1,292 (19.7%) patients currently used H2-blocker other than famotidine. In multivariable analysis after matching (no H2-blocker use versus current famotidine use), there was no significant association between current famotidine use and composite outcomes (adjusted odd ratios [aOR]: 1.30, 95% confidence interval [CI]: 0.55–3.06). On the other hand, another matched cohort (other H2-blocker use versus current famotidine use), demonstrated a positive association between current famotidine use and composite outcomes (aOR: 3.56, 95% CI: 1.03–12.28)
Conclusions
Our study results did not support the potential of famotidine as a therapeutic agent for COVID-19. A rather unexpected result could be observed in the comparisons between current famotidine use and other H2-blocker use; it was observed that current famotidine use increased the risk of poor COVID-19 related outcomes. Further studies are needed to clearly prove the causal relationship with several H2-blockers, including famotidine.
Keywords: COVID-19, Famotidine, Intensive care unit, Mechanical ventilation, Mortality
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, is the greatest global health threat in the 21st century [1]. Even though most patients with COVID-19 have a good prognosis, a significant proportion of patients have a poor prognosis [2,3]. These patients may require mechanical ventilation, admission to an intensive care unit, or extended hospitalization, and some patients may have fatal outcomes [2,4]. Recent studies have identified elderly patients, and those with underlying medical conditions such as hypertension, diabetes mellitus, heart failure, atrial fibrillation, fatty liver diseases, dementia, epilepsy, insulin resistance, cancer, lung disease, chronic kidney disease, and cardiovascular disease are at higher risk of developing severe medical complications of COVID-19 [5]. Furthermore, a patient's prognosis may depend on underlying health conditions and medications the patient has taken.
Many drugs have been proposed as a potential drug against COVID-19 and SARS-CoV-2, including ACE inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), hydroxychloroquine, metformin, and famotidine. Among them, famotidine has been identified as a potential repurposed drug candidate for COVID-19. It has been shown to bind to the SARS-CoV-2 papain-like protease with high affinity and inhibit its enzymatic activity, which is critical for viral replication and immune evasion [6].
Famotidine is a histamine H2-receptor antagonist (H2-blocker) that inhibits gastric acid secretion and treats gastroesophageal reflux disease (GERD) and gastritis (Fig. S1) [7]. In addition to its use in treating GERD and gastritis, Famotidine has also been found to have immunomodulatory effects by reducing the levels of pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha [8]. Elevated levels of these cytokines have been associated with severe COVID-19 and poor clinical outcomes. Due to reducing the inflammatory response [9], it has been proposed as a potential inhibitor of 3-chymotrypsin-like protease in computational analysis and has been investigated as a potential treatment for COVID-19 [10].
The propensity score-matched retrospective, single-center cohort studies found that famotidine use significantly reduces the risks of mortality and intubation in patients with COVID-19 [11,12]. However, a retrospective multi-center study and meta-analysis showed no correlation between famotidine use and poor COVID-19 related outcomes [[13], [14], [15], [16], [17]].
Several retrospective analyses of famotidine use in COVID-19 have reported conflicting results due to several factors [13,16,[18], [19], [20]]. One probable reason for the lack of consistency in these studies is that famotidine's mechanism of action in COVID-19 is not fully postulated. While some studies have suggested that famotidine may benefit COVID-19 outcomes by modulating the interferon response and reducing inflammation, others have not found significant differences in outcomes between patients who received famotidine and those who did not. Another probable reason for the inconsistency in results is the differences in study design and patient populations (Table S1). Retrospective studies rely on data that has already been collected, which can limit the ability to control for confounding factors and can lead to selection bias. Additionally, the patient populations in these studies may vary in terms of age, country, comorbidities, and disease severity, which can also influence the effectiveness of famotidine.
Here, we hypothesized that the risk of poor outcomes from COVID-19 would decrease in current famotidine users. The purpose of this study was to investigate the association between famotidine use and poor prognosis of COVID-19 in a nationwide population-based cohort.
2. Methods
2.1. Study design and participants
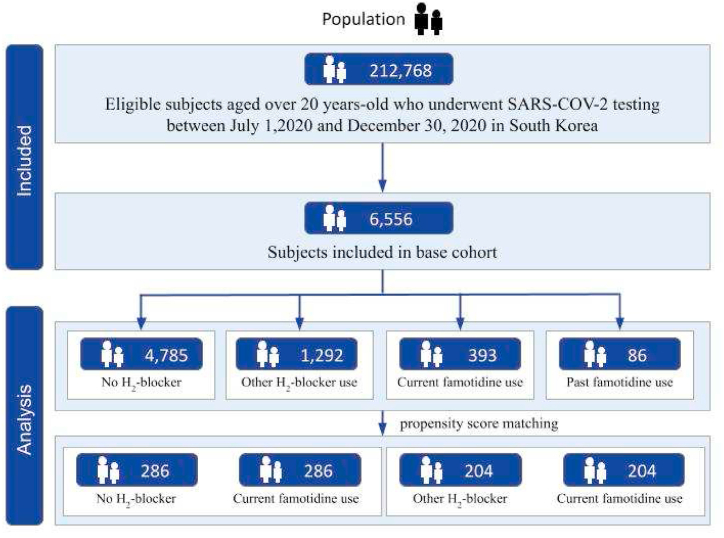
Our study is a retrospective and observational study using a nationwide South Korea COVID-19 dataset. The National Health Insurance Service (NHIS), a single-payer national health insurance system in South Korea, has established a population-based cohort named National Health Information Database (NHID). This dataset includes health care information on demographics, hospital visits, diagnoses (via International Statistical Classification of Diseases and Related Health Problems [ICD]-10 codes), medical procedures, prescriptions, and mortality of the entire Korean population. In addition, the Korea Disease Control and Prevention Agency (KCDA) and NHIS released a nationwide COVID-19 dataset that encompasses from July 1, 2020 to December 31, 2020 [2,4]. The Korea national COVID-19 dataset contained confirmed patients diagnosed based on of RT-PCR analysis taken from nasal and pharyngeal swabs. The RT-PCR assay kit has been certified by the KCDC according to the World Health Organization guideline [21]. We combined the NHIS claims-based data from January 1, 2017 to June 30, 2020, and extracted information on the age, sex, and region of residence from the eligible nationwide dataset. A total of 6556 patients among 212,768 were undergone SARS-COV-2 testing during the above period and were included in this study (Fig. 1).
Fig. 1.
Flow chart of study population selection.
The date when the patient tested positive for COVID-19 was defined as the index date. We divided patients into four groups based on the prescription of H2-blocker in an outpatient clinic or during hospitalization at the time of confirmation of COVID-19. Patient who were prescribed famotidine before and after July 1, 2020, were classified as the past famotidine use group and the current famotidine use group, respectively. Patients who received H2-blocker other than famotidine or did not receive any H2-blocker between January 1, 2020 and December 31, 2020 were categorized as the other H2-blocker use group and the no H2-blocker group.
This study was approved by the Institutional Review Board of Seoul Hospital, Ewha Womans University College of Medicine (2020-10-021); since this was a retrospective analysis based on a fully anonymized dataset, the requirement for informed consent was waived.
2.2. Study outcomes
The poor prognosis of COVID-19 were composite outcomes of administration of high oxygen therapy, admission to intensive care unit, or administration of mechanical ventilation or death [21]. Administration of high oxygen therapy was established with claim codes, including intubation and/or mechanical ventilation (M5850, M5857, M5858, and M5860).
2.3. Covariates
We acquired information regarding sex, age at COVID-19 diagnosis, and region of residence. Comorbidities were defined using medical claims according to ICD-10 codes and medication prescription in the NHIS data before a RT-PCR test for SARS-CoV-2. Hypertension (I10-15), diabetes mellitus (E11–E14), cardiovascular disease (I21) and cerebrovascular disease (I60-63, and I69) were ascertained using ICD-10 codes [[21], [22], [23]]. Hypertension and diabetes mellitus were defined as having a diagnostic code for each disease and a claim code for antihypertensive or antidiabetic drugs at the same time [24]. Patients were classified as having cardiovascular disease or cerebrovascular disease if they had at least two outpatient visits or at least one admission with the relevant diagnostic codes with performed brain computed tomography/magnetic resonance image in case of cerebrovascular disease [25]. Patients were classified as having chronic obstructive pulmonary disease (COPD) or asthma if they had a diagnostic code (COPD, J45; asthma, J46) as the primary diagnosis two or more times [26]. Chronic kidney disease was defined by the presence of the relevant diagnostic codes (N03, N05, N165, N18–9, N250, I12-3, Z490, Z491-2, Z940, Z992, E102, E112, E132, E142, or T861) [27]. A study of diagnostic accuracy using ICD-10 codes for comorbidities was conducted and the results were 85.0%–94.1% [28,29].
2.4. Statistical analysis
Our study performed a comparative analysis of poor COVID-19 related outcomes using propensity score matching (PSM) with calipers less than 0.001 [30]. PSM stabilization is a statistical technique that helps to balance the distribution of confounding factors between the treatment and control groups, which can reduce the potential for bias and improve the accuracy of the estimated treatment effect. Without PSM stabilization, it may be difficult to draw accurate conclusions about the effectiveness of a treatment, as the estimated treatment effect may be confounded by differences in baseline characteristics between the treatment and control groups. In doing so, PSM was performed using a greedy nearest-neighbour algorithm to compare the current famotidine use group with the no H2-blocker group and other H2-blocker use group using a 1:1 ratio. A standardized mean difference (SMD) of less than 0.1 indicated suitability [31].
Logistic regression analysis was performed to investigate poor COVID-19 related outcomes depending on the use of famotidine. This is adjusted for sex, age, region of residence, hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease, asthma, COPD, chronic kidney disease, and Charlson comorbidity index, and previous use of systemic steroid, proton pump inhibitor (PPI), aspirin, and metformin in cohorts balanced after PSM. For the sensitivity analysis, further analysis was performed by separating the high oxygen therapy, composite outcomes of admission to intensive care unit, administration of mechanical ventilation, or death. The results were demonstrated with odds ratio (OR) and 95% confidence interval (CI). An analysis of covariance (ANCOVA) model with factors for sex, age, region of residence, hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease, asthma, COPD, chronic kidney disease, and Charlson comorbidity index, and previous use of systemic steroid, PPI, aspirin, and metformin was adjusted to assess the heterogeneity of famotidine use on the length of hospitalization across groups. Statistical analyses were executed using SAS version 9.4 (SAS Inc., Cary, NC, USA) [[32], [33], [34]]. Two-sided P-values less than 0.05 were considered significant.
3. Results
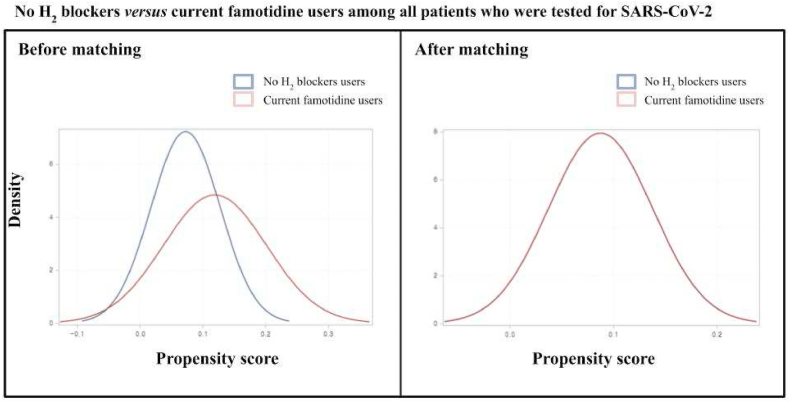
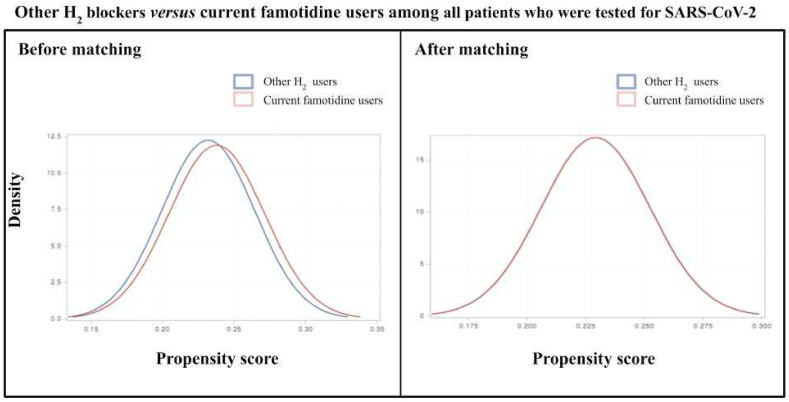
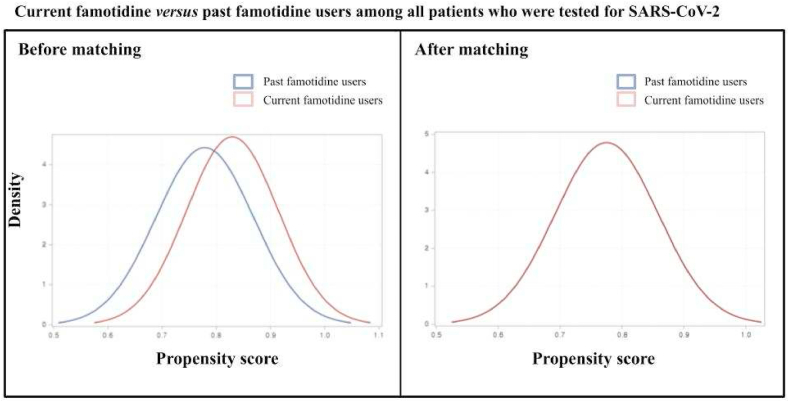
There were 6,556 participants who successfully met the inclusion criteria. This included those aged older than 18 years from the Korean nationwide COVID-19 cohort who tested positive for SARS-CoV-2 between July 1 and December 31, 2020. In the entire cohort, 4,785 (73.0%) patients were not used with H2-blocker, 393 (6.0%) patients were currently used with famotidine, 86 (1.3%) were previously used with famotidine, and 1,292 (19.7%) patients were currently used with H2-blocker other than famotidine. The baseline characteristics of the entire cohort are displayed in Table 1 and Fig. 2. After applying PSM, we included 286 patients that do not have previous experience with the H2-blocker and 286 patients that are currently treated with famotidine; 204 patients used with other H2-blocker and 204 patients that are currently using with famotidine were also appropriately matched (Table 2 and Fig. 3). No major imbalances in the demographics and clinical characteristics were observed for all variables when evaluated using the SMD within groups in the PSM cohorts (Table 2).
Table 1.
Demographic and clinical characteristics of patients with confirmed positive of SARS-CoV-2 in the Korean nationwide cohort.
| Characteristic | Entire cohort | No H2-blocker | Current famotidine use | Past famotidine use | Other H2-blocker use |
|---|---|---|---|---|---|
| Total | 6556 | 4785 | 393 | 86 | 1292 |
| Age, years (SD) | 46.98 (±19.0) | 45.44 (±18.8) | 52.32 (±18.7) | 47.83 (±20.9) | 50.99 (±18.9) |
| Sex | |||||
| Female | 3867 (59.0) | 2682 (56.1) | 270 (68.7) | 52 (60.5) | 863 (66.8) |
| Region of residence | |||||
| Rural | 3147 (48.0) | 2367 (49.5) | 164 (41.7) | 36 (41.9) | 580 (45.0) |
| Urban | 3409 (52.0) | 2418 (50.5) | 229 (58.3) | 50 (58.1) | 712 (55.1) |
| History of diabetes mellitus | 846 (12.9) | 524 (11.0) | 74 (18.8) | 14 (16.3) | 234 (18.1) |
| History of cardiovascular disease | 457 (7.0) | 263 (5.5) | 47 (12.0) | 7 (8.1) | 140 (10.8) |
| History of cerebrovascular disease | 420 (6.4) | 272 (5.7) | 35 (8.9) | 10 (11.6) | 103 (8.0) |
| History of COPD | 310 (4.7) | 185 (3.9) | 30 (7.6) | 4 (4.7) | 91 (7.0) |
| History of asthma | 615 (9.4) | 338 (7.1) | 61 (15.5) | 6 (7.0) | 210 (16.3) |
| History of hypertension | 1468 (22.4) | 945 (19.8) | 112 (28.5) | 25 (29.1) | 386 (29.9) |
| History of chronic kidney disease | 236 (3.6) | 150 (3.1) | 23 (5.9) | 3 (3.5) | 60 (4.6) |
| Charlson comorbidity index | |||||
| 0 | 4399 (67.1) | 3431 (71.7) | 211 (53.7) | 46 (53.5) | 711 (55.0) |
| 1 | 704 (10.7) | 439 (9.2) | 58 (14.8) | 15 (17.4) | 192 (14.9) |
| ≥2 | 1453 (22.2) | 915 (19.1) | 124 (31.6) | 25 (29.1) | 389 (30.1) |
| Previous use of systemic steroid | 1801 (27.5) | 1030 (21.5) | 179 (45.6) | 26 (30.2) | 566 (43.8) |
| Previous use of PPI | 845 (12.9) | 489 (10.2) | 85 (21.6) | 16 (18.6) | 255 (19.7) |
| Previous use of aspirin | 279 (4.3) | 166 (3.5) | 28 (7.1) | 6 (7.0) | 79 (6.1) |
| Previous use of metformin | 490 (7.5) | 315 (6.6) | 46 (11.7) | 6 (7.0) | 123 (9.5) |
Data were presented as number (%).
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; COPD, chronic obstructive pulmonary disease; PPI, proton pump inhibitor.
Fig. 2.
No H2 blockers versus current famotidine users among all patients who were tested for SARS-CoV-2. After matching, the blue and red lines are nearly overlapping, as observed. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Baseline characteristics of propensity-score matching cohort among no H2-blocker versus current famotidine use group, or other H2-blocker versus current famotidine use group, current famotidine use group versus past famotidine use group in patients with positive of SARS-CoV2 infection test.
| No H2-blocker vs. Current famotidine use |
SMD | Other H2-blocker use vs. Current famotidine use |
SMD | Current famotidine use vs. Past famotidine use |
SMD | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No H2-blocker | Current famotidine use | Other H2-blocker use | Current famotidine use | Current famotidine use | Past famotidine use | |||
| Total | 286 | 286 | 204 | 204 | 15 | 15 | |||
| Age, years (SD) | 49.3 (±19.4) | 49.0 (±18.5) | −0.014 | 45.5 (±17.7) | 46.0 (±18.0) | 0.022 | 33.3 (±14.1) | 32.7 (±16.4) | 0.030 |
| Sex | 0.058 | 0.021 | −0.140 | ||||||
| Male | 92 (32.2) | 100 (35.0) | 62 (30.4) | 64 (31.4) | 3 (20.3) | 4 (26.7) | |||
| Female | 194 (67.8) | 186 (65.0) | 142 (69.6) | 140 (68.6) | 12 (80.0) | 11 (73.3) | |||
| Region of residence | −0.014 | −0.040 | −0.270 | ||||||
| Rural | 122 (42.7) | 124 (43.4) | 91 (44.6) | 95 (46.6) | 8 (53.3) | 6 (40.0) | |||
| Urban | 164 (57.3) | 162 (56.6) | 113 (55.4) | 109 (53.4) | 7 (46.7) | 9 (60.0) | |||
| History of diabetes mellitus | 32 (11.2) | 31 (10.8) | 0.010 | 23 (11.3) | 19 (9.3) | 0.051 | 0 (0.0) | 0 (0.0) | <0.001 |
| History of cardiovascular disease | 19 (6.6) | 21 (7.3) | −0.025 | 13 (6.4) | 10 (4.9) | 0.046 | 0 (0.0) | 0 (0.0) | <0.001 |
| History of cerebrovascular disease | 19 (6.6) | 21 (7.3) | −0.027 | 7 (3.4) | 9 (4.4) | −0.035 | 0 (0.0) | 0 (0.0) | <0.001 |
| History of COPD | 18 (6.3) | 14 (4.9) | 0.060 | 5 (2.5) | 9 (4.4) | −0.075 | 0 (0.0) | 0 (0.0) | <0.001 |
| History of asthma | 23 (8.0) | 28 (9.8) | −0.056 | 19 (9.3) | 23 (11.3) | −0.054 | 0 (0.0) | 0 (0.0) | <0.001 |
| History of hypertension | 68 (23.8) | 61 (21.3) | 0.058 | 35 (17.2) | 37 (18.1) | −0.022 | 0 (0.0) | 0 (0.0) | <0.001 |
| History of chronic kidney disease | 11 (3.9) | 13 (4.6) | −0.034 | 4 (2.0) | 4 (2.0) | 0.000 | 0 (0.0) | 0 (0.0) | <0.001 |
| Charlson comorbidity index | −0.035 | 0.007 | 0.131 | ||||||
| 0 | 188 (65.7) | 181 (63.3) | 142 (69.6) | 141 (69.1) | 0 (0.0) | 0 (0.0) | |||
| 1 | 32 (11.2) | 41 (14.3) | 20 (9.8) | 28 (13.7) | 0 (0.0) | 0 (0.0) | |||
| ≥2 | 66 (23.1) | 64 (22.4) | 42 (20.6) | 35 (17.2) | 2 (13.3) | 0 (0.0) | |||
| Previous use of systemic steroid | 95 (33.2) | 96 (33.6) | −0.008 | 89 (43.6) | 87 (42.7) | 0.020 | 5 (33.3) | 5 (33.3) | <0.001 |
| Previous use of PPI | 39 (13.6) | 41 (14.3) | −0.019 | 24 (11.8) | 32 (15.7) | −0.097 | 0 (0.0) | 0 (0.0) | <0.001 |
| Previous use of aspirin | 9 (3.2) | 12 (4.2) | −0.047 | 9 (4.4) | 8 (3.9) | 0.020 | 0 (0.0) | 0 (0.0) | <0.001 |
| Previous use of metformin | 22 (7.7) | 18 (6.3) | 0.049 | 9 (4.4) | 9 (4.4) | 0.000 | 0 (0.0) | 0 (0.0) | <0.001 |
| Composite outcomesa | |||||||||
| Crude | 1.00 (reference) | 1.20 [0.61:2.39] | 1.00 (reference) | 2.43 [0.92:6.46] | 1.00 (reference) | NA | |||
| Minimally adjusted ORb | 1.00 (reference) | 1.31 [0.60:2.88] | 1.00 (reference) | 2.70 [0.92:7.93] | 1.00 (reference) | NA | |||
| Fully adjusted ORc | 1.00 (reference) | 1.30 [0.55:3.061] | 1.00 (reference) | 3.56 [1.03:12.28] | 1.00 (reference) | NA | |||
| Oxygen therapy | |||||||||
| Crude | 1.00 (reference) | 1.15 [0.72:1.82] | 1.00 (reference) | 1.57 [0.81:3.05] | 1.00 (reference) | NA | |||
| Minimally adjusted OR | 1.00 (reference) | 1.27 [0.75:2.15] | 1.00 (reference) | 1.61 [0.78:3.33] | 1.00 (reference) | NA | |||
| Fully adjusted OR | 1.00 (reference) | 1.30 [0.74:2.28] | 1.00 (reference) | 2.09 [0.94:4.61] | 1.00 (reference) | NA | |||
| Composite outcomesd | |||||||||
| Crude | 1.00 (reference) | 1.17 [0.75:1.84] | 1.00 (reference) | 1.49 [0.80:2.77] | 1.00 (reference) | NA | |||
| Minimally adjusted OR | 1.00 (reference) | 1.29 [0.77:2.17] | 1.00 (reference) | 1.53 [0.77:3.06] | 1.00 (reference) | NA | |||
| Fully adjusted OR | 1.00 (reference) | 1.29 [0.74:2.24] | 1.00 (reference) | 2.07 [0.96:4.47] | 1.00 (reference) | NA | |||
Data were presented as number (%).
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SMD, standardized mean difference; SD, standard deviation; COPD, chronic obstructive pulmonary disease; PPI, proton pump inhibitor; OD, odds ratio.
An SMD <0.1 indicates no major imbalance.
Numbers in bold indicate significant differences (p < 0.05).
Composite outcomes of administration of high oxygen therapy, admission of intensive care unit, mechanical ventilation or death.
Minimally adjusted: adjustment for age and sex.
Fully adjusted: adjustment for age, sex, region of residence, history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, COPD, hypertension, and chronic kidney disease, and Charlson comorbidity index, previous use of steroid, aspirin, metformin, PPI.
Composite outcomes of admission of intensive care unit, mechanical ventilation or death.
Fig. 3.
Other H2 blockers versus current famotidine users among all patients who were tested for SARS-CoV-2. After matching, the blue and red lines are nearly overlapping, as observed. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the matched cohort with 572 patients with COVID-19 (no H2-blocker versus current famotidine use), there was no significant correlation between current famotidine use and composite outcomes of administration of high oxygen therapy, admission to intensive care unit, administration of mechanical ventilation, or death (crude OR: 1.20, 95% CI: 0.61–2.39), administration of high oxygen therapy (crude OR: 1.15, 95% CI: 0.72–1.82), or composite outcomes of admission to intensive care unit, administration of mechanical ventilation or death (crude OR: 1.17, 95% CI: 0.75–1.84). In the other matched cohort with 408 patients with COVID-19 (other H2-blocker use versus current famotidine use), there was also no significant correlation between current famotidine use and composite outcomes of administration of high oxygen therapy, admission to intensive care unit, administration of mechanical ventilation or death (crude OR: 2.43, 95% CI: 0.92–6.46), administration of high oxygen therapy (crude OR: 1.57, 95% CI: 0.81–3.05), or composite outcomes of admission of intensive care unit, administration of mechanical ventilation or death (crude OR: 1.49, 95% CI: 0.80–2.77) (Fig. 4).
Fig. 4.
Current famotidine versus past famotidine users among all patients who were tested for SARS-CoV-2. After matching, the blue and red lines are nearly overlapping, as observed. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Adjustments for age and sex (minimally adjusted) in both PSM cohorts did not change the result. Adjustments for age, sex, region of residence, history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, COPD, hypertension, chronic kidney disease, Charlson comorbidity index, and previous administration of steroids, aspirin, metformin, and PPI in both PSM cohort did not affect the associations between the current famotidine use and composite outcomes of admission of intensive care unit, administration of mechanical ventilation, or death (fully adjusted OR: 1.29, 95% CI: 0.74–2.24) and administration of oxygen therapy (fully adjusted OR: 1.30, 95% CI: 0.74–2.28) as compared to no H2-blocker group. However, we did identify a significant positive association between current famotidine use and composite outcomes of administration of high oxygen therapy, admission to the intensive care unit, administration of mechanical ventilation, or death (fully adjusted OR: 3.56, 95% CI: 1.03–12.28) as compared to other H2-blocker use group. Although not significant, similar trends were observed between current famotidine use and high oxygen therapy (fully adjusted OR: 2.09, 95% CI: 0.94–4.61), and between the current famotidine use and composite outcomes of admission to the intensive care unit, administration of mechanical ventilation, or death (fully adjusted OR: 2.07, 95% CI: 0.96–4.47). There was no significant difference in the length of hospitalization before and after adjustment in either PSM cohort (no H2-blocker versus current famotidine use, mean difference: −1.15 days, 95% CI: −3.81 to 0.96, other H2-blocker use versus current famotidine use, mean difference: −1.07 days, 95% CI: −3.66 to 1.52) (Table 3).
Table 3.
Propensity-score-matching subgroup analyses for difference in length of stay at hospitalization according to famotidine use using analysis-of-covariance model.
| Length of stay at hospitalization, day | No H2-blocker vs. Current famotidine use |
Other H2-blocker use vs. Current famotidine use |
Current famotidine use vs. Past famotidine use |
|---|---|---|---|
| Mean difference (95% CI) | Mean difference (95% CI) | Mean difference (95% CI) | |
| Crude | −1.42 (−3.81:0.96) | −0.91 (−3.52:1.70) | −6.20 (−14.30: 1.87) |
| Minimally adjusteda | −1.31 (−3.61:0.99) | −0.97 (−3.56:1.62) | −6.02 (−13.80:1.76) |
| Fully adjustedb | −1.15 (−3.41:1.10) | −1.07 (−3.66:1.52) | −4.39 (−12.20:3.46) |
CI, confidence interval.
Numbers in bold indicate significant differences (p < 0.05).
Minimally adjusted: adjustment for age and sex.
Fully adjusted: adjustment for age, sex, region of residence, history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, COPD, hypertension, and chronic kidney disease, and Charlson comorbidity index, previous use of steroid, aspirin, metformin, PPI.
4. Discussion
The key findings of this study are that famotidine use does not decrease the risk of poor COVID-19 related outcomes. A rather unexpected result could be observed in the comparisons between current famotidine use and other H2-blocker use; it was observed that current famotidine use increased the risk of poor COVID-19 related outcomes. This might be because there are differences between H2 blockers in terms of potency, duration of action, and pharmacokinetics, which can influence their effectiveness in treating different conditions. Famotidine has a longer half-life and is more potent than other H2 blockers, such as cimetidine or ranitidine. This may make famotidine more effective in reducing gastric acid production and treating GERD and gastritis. However, these differences in pharmacokinetics may not necessarily translate to differences in their effectiveness against COVID-19. Mechanistically, it is possible that the differences between H2 blockers are due to their ability to modulate the immune response.
After famotidine emerged as an option for the treatment of COVID-19,7 research on the correlation between famotidine use and clinical outcomes was conducted in various centers. Although some retrospective, single-center, PSM studies showed famotidine treatment significantly reduced the risk of poor COVID-19 related outcomes in patients with COVID-19, retrospective multicenter studies and a meta-analysis showed no correlation between famotidine use and poor COVID-19 related outcomes [[11], [12], [13], [14], [15], [16], [17]]. In the most recent randomized clinical trial of patients with COVID-19 with mild to moderate symptoms, patients in the high-dose famotidine use group had to earlier resolution of symptoms and inflammation than other patients. This result suggested that famotidine could be used to prevent the progression of COVID-19 [18]. However, most of the previous studies conducted in the western countries, and although there were some multi-center studies, no studies have examined a nationwide population in East Asia. Therefore, our research is significant because our results are consistent with previous studies. It also gives additional information regarding the no association of famotidine use with the decrease of poor prognosis of COVID-19 in the general population of East Asia.
There was no difference in poor COVID-19 related outcomes between COVID-19 patients who did and did not receive famotidine. However, logistic regression analysis between the current famotidine use and other H2-blocker use group showed a significant association between current famotidine use and increased poor COVID-19 related outcomes. COVID-19 is associated with a spectrum of severities, from asymptomatic disease to death. Various potential candidate for COVID-19 treatments have been proposed, and pharmaceutical companies in various countries have been competitively developing therapeutic agents. Some proven effective therapeutic agents in practice have been developed [35], and some that have been proven safe are undergoing phase Ⅲ clinical trials to determine their effectiveness. Representative examples include hydroxychloroquine, an antimalarial drug; metformin, an antidiabetic drug; and famotidine, an antiulcer drug. Famotidine has relatively few side effects, so it can be purchased over-the-counter in South Korea, and this H2-blocker is already being used by many patients. Since famotidine has been proposed as a treatment for COVID-19, in patients with severe initial symptoms or worsening of pneumonia, it is highly likely that doctors newly prescribed famotidine to patients who needed H2-blocker for the first time or changed the currently prescribed H2-blocker to famotidine. Thus, by administering famotidine over other H2-blocker to patients with severe or worsening symptoms, we observed no difference in poor COVID-19 related outcomes in the current famotidine use and no H2-blocker group. However, current famotidine use is associated with poor COVID-19 related outcomes when compared with other H2-blocker use.
Previous studies have shown that famotidine acts as an antagonist or inverse-agonist of histamine-mediated mast cell activation leading to acute bronchoconstriction and inflammation due to SARS-CoV-2 infection [36,37]. Another study showed that famotidine could inhibit histamine-induced expression of toll-like receptor 3 (TLR3) in SARS-CoV-2 infected cells and reduce TLR3-dependent signaling [38]. Famotidine has the above-mentioned anti-inflammatory effect and inhibits 3-chymotrypsin-like protease, an important protein for viral replication [7]. The steady-state concentration varies depending on the dose of famotidine, and the half maximal inhibitory concentration (IC50) must be reached for a gastric acid inhibitory through the inhibiting H2-receptor. Although the IC50 of famotidine for GERD has been standardized (20 mg BID or 20 mg TID), the IC50 to properly inhibit SARS-CoV-2 infection is unknown [36]. A preceding case series study and randomized clinical trial reported that high-dose of oral famotidine improved the prognosis in COVID-19 patients [18,39], suggesting that the IC50 level of famotidine for inhibiting SARS-CoV-2 infection is higher than that of treating GERD. Therefore, propensity score matching with large cohort studies using higher doses than those used for GERD are needed in patients with severe COVID-19 symptoms.
There are some limitations to this study. First, it is difficult to generalize our results across ethnicities because our dataset consisted of only Koreans. Second, detailed information on each patient's daily and total doses of famotidine could not be acquired because the dataset did not include detailed drug dose information. Therefore, the analysis could not be performed depending on the famotidine dose. Third, only patients who had a SARS-CoV-2 test were included in our study, and patients who took the test were not assigned randomly, which might have caused a selection bias, although Korea government performed nationwide and complementary SARS-CoV-2 test. Fourth, in this study we used prescription data from the hospitals' claim based data and could not verify if the prescriptions were actually filled. Besides, famotidine can be sold at pharmacies but records of over-the-counter-famotidine sales did not include in our data. However, most of famotidine is sold when the prescription is written. Finally, although some drugs (steroids, aspirin, metformin, and PPIs) were considered as confounders in the logistic regression analysis, we did not include all drugs such as ACEIs/ARBs and histamines known to affect the prognosis of COVID-19 [40,41].
5. Conclusion
Our study did not support the hypothesis that famotidine use reduces the risk of poor COVID-19 related outcomes in the general population in Asian countries. Further study including large sample size randomized clinical trials should be needed to confirm whether famotidine use can reduce poor COVID-19 related outcomes, particularly in COVID-19 positive patients with moderate to severe COVID-19 related complications.
Author contribution statement
Rosie Kwon, Hyung Jun Kim,Seung Won Lee,: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lee Smith, Ai Koyanagi: Contributed reagents, materials, analysis tools or data.
Jae Il Shin, Tae-Jin Song, Dong Keon Yon: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1F1A1048113 to T-JS), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C0233), and a grant (21153MFDS601) from Ministry of Food and Drug Safety in 2023. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate healthcare professionals dedicated to treating patients with COVID-19 in Korea, the Ministry of Health and Welfare, the Health Insurance Review & Assessment Service of Korea and the National Health Insurance Service of Korea for sharing invaluable national cohorts in a prompt manner.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16171.
Contributor Information
Jae Il Shin, Email: shinji@yuhs.ac.
Tae-Jin Song, Email: knstar@ewha.ac.kr.
Dong Keon Yon, Email: yonkkang@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kim S.Y., Yeniova A.Ö. Global, regional, and national incidence and mortality of COVID-19 in 237 countries and territories, January 2022: a systematic analysis for World Health Organization COVID-19 Dashboard. Life Cycle. 2022;2:e10. doi: 10.54724/lc.2022.e10. [DOI] [Google Scholar]
- 2.Lee S.W., et al. Association between mental illness and COVID-19 in South Korea: a post-hoc analysis. Lancet Psychiatr. 2021;8:271–272. doi: 10.1016/s2215-0366(21)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha Y., Jung W., Seo M., Rahmati M. The emerging pandemic recent: SARS-CoV-2. Life Cycle. 2023;3:e2. doi: 10.54724/lc.2023.e2. [DOI] [Google Scholar]
- 4.Lee S.W., et al. Proton pump inhibitors and the risk of severe COVID-19: a post-hoc analysis from the Korean nationwide cohort. Gut. 2021;70:2013–2015. doi: 10.1136/gutjnl-2020-323672. [DOI] [PubMed] [Google Scholar]
- 5.Xu J., et al. A meta-analysis on the risk factors adjusted association between cardiovascular disease and COVID-19 severity. BMC Publ. Health. 2021;21:1533. doi: 10.1186/s12889-021-11051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin D., et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rommasi F., Nasiri M.J., Mirsaeidi M. Immunomodulatory agents for COVID-19 treatment: possible mechanism of action and immunopathology features. Mol. Cell. Biochem. 2022;477:711–726. doi: 10.1007/s11010-021-04325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z., Patel N., Vemparala P., Krishnamurthy M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci. Rep. 2022;12:5553. doi: 10.1038/s41598-022-09639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis G., et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial. Lanc. Reg. Heal. Am. 2022;6 doi: 10.1016/j.lana.2021.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mather J.F., Seip R.L., McKay R.G. Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am. J. Gastroenterol. 2020;115:1617–1623. doi: 10.14309/ajg.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedberg D.E., et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020;159:1129–1131.e1123. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoaibi A., Fortin S.P., Weinstein R., Berlin J.A., Ryan P. Comparative effectiveness of famotidine in hospitalized COVID-19 patients. Am. J. Gastroenterol. 2021;116:692–699. doi: 10.14309/ajg.0000000000001153. [DOI] [PubMed] [Google Scholar]
- 14.Sun C., et al. Does famotidine reduce the risk of progression to severe disease, death, and intubation for COVID-19 patients? A systemic review and meta-analysis. Dig. Dis. Sci. 2021;66:3929–3937. doi: 10.1007/s10620-021-06872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kow C.S., Abdul Sattar Burud I., Hasan S.S. Use of famotidine and risk of severe course of illness in patients with COVID-19: a meta-analysis. Mayo Clin. Proc. 2021;96:1365–1367. doi: 10.1016/j.mayocp.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeramaneni S., et al. Famotidine use is not associated with 30-day mortality: a coarsened exact match study in 7158 hospitalized patients with coronavirus disease 2019 from a large healthcare system. Gastroenterology. 2021;160:919–921.e913. doi: 10.1053/j.gastro.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuno T., So M., Takahashi M., Egorova N.N. The association between famotidine and in-hospital mortality of patients with COVID-19. J. Med. Virol. 2021 doi: 10.1002/jmv.27375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan C.M., et al. Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial. Gut. 2022;71:879–888. doi: 10.1136/gutjnl-2022-326952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahwani S., et al. Efficacy of oral famotidine in patients hospitalized with severe acute respiratory syndrome coronavirus 2. Cureus. 2022;14 doi: 10.7759/cureus.22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mura C., et al. Real-world evidence for improved outcomes with histamine antagonists and aspirin in 22,560 COVID-19 patients. Signal Transduct. Targeted Ther. 2021;6:267. doi: 10.1038/s41392-021-00689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.W., et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.W., et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: a nationwide cohort study. Br. J. Sports Med. 2022;56:901–912. doi: 10.1136/bjsports-2021-104203. [DOI] [PubMed] [Google Scholar]
- 23.Yoo I.K., Marshall D.C., Cho J.Y., Yoo H.W., Lee S.W. N-Nitrosodimethylamine-contaminated ranitidine and risk of cancer in South Korea: a nationwide cohort study. Life Cycle. 2021;1:e1. doi: 10.54724/lc.2021.e1. [DOI] [Google Scholar]
- 24.Woo M.H., Lee H.S., Kim J. Effect of pioglitazone in acute ischemic stroke patients with diabetes mellitus: a nested case-control study. Cardiovasc. Diabetol. 2019;18:67. doi: 10.1186/s12933-019-0874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son M.K., Lim N.K., Park H.Y. Trend of prevalence of atrial fibrillation and use of oral anticoagulation therapy in patients with atrial fibrillation in South Korea (2002-2013) J. Epidemiol. 2018;28:81–87. doi: 10.2188/jea.JE20160149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S.W., et al. Estimating COVID-19 infection and severity risks in patients with chronic rhinosinusitis: a Korean nationwide cohort study. J. Allergy Clin. Immunol. 2021;9:2262–2271.e2262. doi: 10.1016/j.jaip.2021.03.044. In practice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.J., Kim M.J., Kim Y.J., Kim W.Y. Short and long-term mortality trends for cancer patients with septic shock stratified by cancer type from 2009 to 2017: a population-based cohort study. Cancers. 2021;13 doi: 10.3390/cancers13040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S.W., et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatr. 2020;7:1025–1031. doi: 10.1016/s2215-0366(20)30421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Y., Woo H.G., Park J., Lee J.S., Song T.J. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: a nationwide population-based cohort study. Eur. J. Prev. Cardiol. 2020;27:1835–1845. doi: 10.1177/2047487319886018. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.W., Acharya K.P. Propensity score matching for causal inference and reducing the confounding effects: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e18. doi: 10.54724/lc.2022.e18. [DOI] [Google Scholar]
- 31.Shin Y.H., et al. Autoimmune inflammatory rheumatic diseases and COVID-19 outcomes in South Korea: a nationwide cohort study. Lancet Rheumat/ 2021;3:e698–e706. doi: 10.1016/s2665-9913(21)00151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S.W. Methods for testing statistical differences between groups in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e1. doi: 10.54724/lc.2022.e1. [DOI] [Google Scholar]
- 33.Lee S.W. Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e3. doi: 10.54724/lc.2022.e3. [DOI] [Google Scholar]
- 34.Kwon R., et al. National trends in physical activity among adolescents in South Korea before and during the COVID-19 pandemic, 2009-2021. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28456. [DOI] [PubMed] [Google Scholar]
- 35.Wen W., et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann. Med. 2022;54:516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malone R.W., et al. COVID-19: famotidine, histamine, mast cells, and mechanisms. Res. Sq. 2020 doi: 10.21203/rs.3.rs-30934/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kritas S.K., et al. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34:9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee R., et al. Famotidine inhibits toll-like receptor 3-mediated inflammatory signaling in SARS-CoV-2 infection. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janowitz T., et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69:1592–1597. doi: 10.1136/gutjnl-2020-321852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogan Ii R.B., et al. Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients. Pulm. Pharmacol. Ther. 2020;63 doi: 10.1016/j.pupt.2020.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H.W., Yoon C.H., Jang E.J., Lee C.H. Renin-angiotensin system blocker and outcomes of COVID-19: a systematic review and meta-analysis. Thorax. 2021;76:479–486. doi: 10.1136/thoraxjnl-2020-215322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.