Abstract
Makorin ring finger protein 3 (MKRN3) is an important neuroendocrine player in the control of pubertal timing and upstream inhibitor of gonadotropin-releasing hormone secretion. In mice, expression of Mkrn3 in the hypothalamic arcuate and anteroventral periventricular nucleus is high early in life and declines before the onset of puberty. Therefore, we aimed to explore if the persistence of hypothalamic Mkrn3 expression peripubertally would result in delayed puberty. Female mice that received neonatal bilateral intracerebroventricular injections of a recombinant adeno-associated virus expressing Mkrn3 had delayed vaginal opening and first estrus compared with animals injected with control virus. Subsequent estrous cycles and fertility were normal. Interestingly, male mice treated similarly did not exhibit delayed puberty onset. Kiss1, Tac2, and Pdyn mRNA levels were increased in the mediobasal hypothalamus in females at postnatal day 28, whereas kisspeptin and neurokinin B protein levels in the arcuate nucleus were decreased, following Mkrn3 overexpression, compared to controls. Cumulatively, these data suggest that Mkrn3 may directly or indirectly target neuropeptides of Kiss1 neurons to degradation pathways. This mouse model suggests that MKRN3 may be a potential contributor to delayed onset of puberty, in addition to its well-established roles in central precocious puberty and the timing of menarche.
Keywords: MKRN3, puberty, hypothalamus, kisspeptin, neurokinin B
The gene encoding makorin ring finger protein 3 (MKRN3), located within the maternally imprinted Prader–Willi syndrome region on chromosome 15q11.2, was reported as the first gene in which loss-of-function mutations are associated with central precocious puberty (CPP) due to premature activation of the hypothalamic–pituitary–gonadal (HPG) axis and is now the most common known genetic cause of CPP (1, 2). In rodents, hypothalamic expression of Mkrn3 in the arcuate nucleus is high early in life and declines before the onset of puberty, remaining low into adulthood (2, 3). This expression pattern, together with the identification of loss-of-function mutations in children with CPP, supports a role for MKRN3 as an inhibitor of gonadotropin-releasing hormone (GnRH) secretion. The protein structure of MKRN3 predicts E3 ubiquitin ligase activity as well as RNA binding; MKRN3 has recently been demonstrated to exhibit auto-ubiquitination and ubiquitination of target proteins in pathways involved in pubertal timing, insulin signaling, RNA metabolism and transport, and cell–cell adhesion (4–6).
The genes encoding kisspeptin and its receptor, important regulators of GnRH secretion in the HPG axis, have previously been described in association with pubertal disorders in humans. Gain-of-function mutations in KISS1 and KISS1R have been associated with CPP, while loss-of-function mutations in these genes have been associated with congenital hypogonadotropic hypogonadism (CHH) (7–12). Similarly, loss-of-function mutations in TAC3 and TACR3, encoding the tachykinin, neurokinin B, and its receptor, which are coexpressed in Kiss1 neurons in the arcuate nucleus, have also been associated with CHH (13). Loss-of-function mutations in delta like non-canonical Notch ligand 1 (DLK1) are the most recent monogenic etiology of CPP (14–16). However, mutations in MKRN3 far outweigh other known genetic causes, yet the potential role of genetic variants in MKRN3 in delayed pubertal onset in humans and mice has not been previously explored.
MKRN3 belongs to a family of makorin proteins, which have been described in both invertebrate and vertebrate animals, showing a high degree of evolutionary conservation (17). Curiously, Caenorhabditis elegans has a single makorin, lep-2, an ortholog of vertebrate Mkrn3, yet lep-2–deficient mutants demonstrate an opposite phenotype, such that lep-2 deficiency results in delayed maturation from the juvenile to adult transition (18, 19). Interestingly, to explore if MKRN3 has inhibitory effects on maturation in this model, as supported by children with loss-of-function mutations of MKRN3 resulting in loss of inhibitory restraint on the HPG axis; overexpression of human MKRN3 in C. elegans was performed and led to delayed sexual maturation. This suggests a strong conservation of MKRN3 function and suggests that overexpression of MKRN3 or gain-of-function mutations may play a role in delayed puberty (18). Therefore, in this study, we aimed to generate a novel mouse model with neonatal hypothalamic overexpression of Mkrn3. The purpose of this study was to test the hypothesis that MKRN3 may also have the ability to delay pubertal onset, and to develop a novel model to explore the mechanism of action of Mkrn3.
Materials and Methods
Animals
Wild-type C57BL/6J mice were provided by Charles River Laboratories (Massachusetts). The animal protocol, including all experiments, were reviewed and approved by Brigham and Women’s Hospital Institutional Animal Care and Use Committee and animals were housed at the Brigham and Women’s Hospital Center for Comparative Medicine. Mice were maintained in a 12-hour light, 12-hour dark cycle and were fed a standard rodent diet and had access to water ad libitum.
In Vitro Validation of AAV-CMV-Mkrn3-IRES-EGFP
Recombinant adeno-associated viruses were obtained from a commercial vendor (VectorBuilder, Chicago, IL) for viral packaging and quantification, including the custom Adeno-Associated Virus-Cytomegalovirus-Makorin ring finger protein 3-Internal Ribosome Entry Site-Enhanced Green Fluorescence Protein (referred to herein as AAV-Mkrn3; serotype 2/9) at a titer of 6.25 × 1011 genome copies (GC)/mL and AAV-CMV-EGFP at a titer of 1.6 × 1011 GC/mL. Viruses were transduced into immortalized mouse Kiss1 neuron-derived cells, KTaR-1 (RRID:CVCL_VS93) and KTaV-3 (RRID:CVCL_VS94), derived from the mouse arcuate and anteroventral periventricular nucleus (AVPV), respectively, to confirm Mkrn3 and EGFP expression (20). Cells were transduced at 30% to 50% confluence using a multiplicity of infection of 1 × 104.5 GC/cell and total RNA was isolated (RNEasy Micro Kit, Qiagen) 72 hours later. A 0.5-µg bolus of RNA was DNase-treated (RQ1 RNase-free DNase, Promega) and reverse transcribed (iScript Reverse Transcription Supermix for RT-qPCR, Bio-Rad Laboratories, Inc.) followed by quantitative real-time polymerase chain reaction.
Intracerebroventricular Injections
Intracerebroventricular (ICV) injections were performed following previously published procedures (21, 22). Briefly, neonatal male and female mice at postnatal day (PND) 0-1 were anesthetized using cryoanesthesia on a small aluminum plate. The injection site was determined as 2/5 of the distance from the eye to the lambda suture, approximately 1 mm lateral to the midline (21). Bilateral injections using a Hamilton syringe attached to a 26-gauge needle fitted with polyethylene tubing were performed using 2 µL per lateral ventricle of AAV-Mkrn3 or AAV-EGFP at a depth of 2 mm. A rest period of 1 minute was performed for each injection before removing the needle. After injections were completed, the mice were returned to the cage with their mother and monitored for recovery from cryoanesthesia.
Reproductive Phenotyping
Prepubertal littermates were monitored beginning at PND21 for pubertal and reproductive phenotyping. For female mice, markers of pubertal onset were evaluated by daily assessment for vaginal opening and, subsequently, for first estrus (23, 24). Estrous cyclicity was monitored by vaginal cytology using a pipette that flushed 10 µL of sterile saline into the vagina and vaginal fluid was then collected on a glass slide. Cytology samples were stained by hematoxylin (Sigma-Aldrich) and eosin (Sigma-Aldrich) and the estrous cycle phase was characterized by light microscopy (25). Cycle length was defined from diestrus to the beginning of the following diestrus phase (23). For male mice, pubertal onset was assessed by preputial separation (24). For both sexes, body weight was measured weekly from PND21 to PND60 as well as on the day of vaginal opening or preputial separation.
Fertility assessment
A subset of mice (n = 6 females for each virus) were mated with a wild-type male breeding partner for 3 months, and time to first litter, litter size, and number of litters were measured.
Hormone assays
Blood samples for luteinizing hormone (LH) measurements were obtained by making a single excision at the tip of the tail using a razor at PND28, when 80% of AAV-EGFP females had achieved vaginal opening. Four microliters of whole blood was collected from the tail tip with a pipette. The whole blood was immediately diluted in 116 µL of 0.05% PBST (phosphate-buffered saline [Boston BioProducts] containing Tween-20 [Sigma-Aldrich]), vortexed, and frozen on dry ice. Samples were stored at −80°C until analysis by LH enzyme-linked immunosorbent assay as previously described (26).
RNA Extraction
Tissues from the mediobasal hypothalamus (MBH) and preoptic area (POA) were collected from AAV-Mkrn3 and AAV-EGFP injected mice (n = 5 per group) at PND28 for females and PND60 for males and stored at −80°C. Briefly, the MBH was dissected by 1 rostral cut along the posterior border of the optic chiasm, 1 caudal cut along the border of the mammillary bodies, and 2 lateral cuts along the hypothalamic sulci. The POA was dissected via 2 oblique cuts on the lateral edge of the optic chiasm to the point anterior to decussation of the optic nerves and a transverse cut behind the optic chiasm. The dissection depth was approximately 2 mm (27–29). Total RNA was isolated (RNEasy Micro Kit, Qiagen) from collected tissue samples. One microgram of RNA was DNase treated (RQ1 RNase-free DNase, Promega) and reverse transcribed (iScript Reverse Transcription Supermix for RT-qPCR, BioRad Laboratories, Inc.).
Quantitative Real-time Polymerase Chain Reaction
Quantitative real-time Polymerase Chain Reaction assays were performed on a QuantStudio3 software (Thermo Fisher Scientific). The cycling conditions were as follows: 2-minute incubation at 95°C, 45 amplification cycles (95°C for 30 seconds, 60°C for 30 seconds, and 45 seconds at 75°C), with fluorescence detection at the end of each cycle. The mRNA of the genes of interest were detected using SYBR green mix (Bio-Rad Laboratories, Inc.) according to the manufacturer’s instructions. Data were normalized using Hprt (NM_013556.2) as an internal control. Primer sequences are shown elsewhere (Table S1 (30)).
Immunohistochemistry
Animals were terminally anesthetized with ketamine/xylazine in saline (0.9% NaCl) cocktail and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde (diluted to 0.01 M phosphate buffer, pH 7.4, Boston BioProducts) on PND28. Whole brains were collected and stored in 4% paraformaldehyde solution overnight and then transferred to 20% sucrose (American Bioanalytical, Inc.) solution in 0.1 M phosphate buffer (Sigma Aldrich). After sucrose infiltration, whole brains were transferred to an Optimal Cutting Temperature-filled mold (Fisher Healthcare Tissue-Plus) and submerged in 2-methylbutane (Fisher Chemical) for 2 minutes, then stored at −80°C. Tissue was cut into 35 µm coronal sections using a cryostat (CM3050 S, Leica) and separated into 4 parallel series across the length of the brain. Sections were stored at 4°C in PBS (diluted to 0.01 M phosphate buffered saline, Boston BioProducts) and 0.01% sodium azide (Sigma-Aldrich) until immunohistochemistry was performed. A single series of sections per mouse was used in the histological studies.
For kisspeptin immunohistochemistry studies, antigen retrieval was performed by incubating sodium citrate buffer (10 mM sodium citrate [American Bioanalytical] and 0.05% Tween-20 [Fisher Scientific] diluted in water) and incubating the brain sections at 90°C for 20 minutes followed by 40 minutes of cooling at room temperature. Brain sections were washed vigorously with PBS and blocked in 2% normal donkey serum (D9663, Sigma-Aldrich) and in PBST (0.01 M PBS with 0.3% Triton X-100 [Fisher BioReagants]) for 2 hours at room temperature, followed by incubation in blocking solution containing PBST with 1% normal donkey serum containing antikisspeptin-10 antibody (AC566, RRID:AB_2314709, National Institute for Agricultural Research, Food, and Environment [Institut National De Recherche Pour L’agriculture, L’alimentation Et L’environnement], Nouzilly, France) at a dilution of 1:2000 for 48 hours at 4°C (27). Sections were then extensively washed in PBS and incubated with donkey antirabbit Alexa Fluor 594 secondary antibody (Thermo Fisher Scientific Cat# A-21207, RRID:AB_141637, 1:500) for 2 hours at room temperature. After washing, sections were mounted on gelatin-coated Superfrost glass slides.
For neurokinin B and GnRH immunohistochemistry studies, antigen retrieval was not performed and brain sections were incubated in blocking solution as described above with primary antiserum (rabbit anti-neurokinin B, Novus Cat# NB300-201, RRID:AB_10000783, 1:1000 for 48 hours; rabbit anti-GnRH, ImmunoStar Cat# 20075, RRID:AB_572248, 1:1000 for 24 hours). The kisspeptin, neurokinin B, and GnRH antibodies have all been previously validated using knockout animals as negative controls (31–33).
For MKRN3 immunohistochemistry studies, blocking was performed using PBST with 2% normal goat serum (NGS G9023, Sigma-Aldrich), and then slides were incubated with primary antiserum (Sigma-Aldrich Cat# HPA029494, RRID:AB_10603873, 1:500) using 1% NGS/PBST overnight at 4°C followed by incubation with goat antirabbit Dylight 488 secondary antibody (Thermo Fisher Scientific Cat# 35553, RRID:AB_1965947, 1:500) in 1% NGS/PBST for 2 hours at room temperature. The antibody has been validated in an Mkrn3-deficient mouse model.
Fluorescence images for kisspeptin, neurokinin B, and GnRH immunohistochemistry were captured with a Leica TCS SP8 STED confocal microscope.
Image Analysis
Total kisspeptin and neurokinin B immunoreactivity (voxel count) were quantified on 2 anatomically matched sections of the anterior and posterior areas of the arcuate nucleus. Quantification of GnRH-immunoreactive cell bodies was also performed on 2 anatomically matched sections (medial septum and rostral preoptic area) (34). A set of confocal images from the plane was obtained in the image so that the X- and Y-axes remained the same but the Z-axis differed to form a 3-dimensional image. Images were collected in a set of 30 serial image planes (ie, Z-stack) at a z step distance of 0.83 µM. Gray density was quantified by Image J (NIH).
Data Analysis
Data were analyzed using Prism statistics software (GraphPad, Inc., San Diego, CA). All data are presented as the mean ± SEM. Results were analyzed by unpaired t test for the comparison of mean differences between 2 groups. Multiple comparisons were analyzed by 1- or 2-way analysis of variance (ANOVA). Differences were considered statistically significant when P < .05.
Results
In Vivo and In Vitro Validation of Mkrn3 Hypothalamic Overexpression
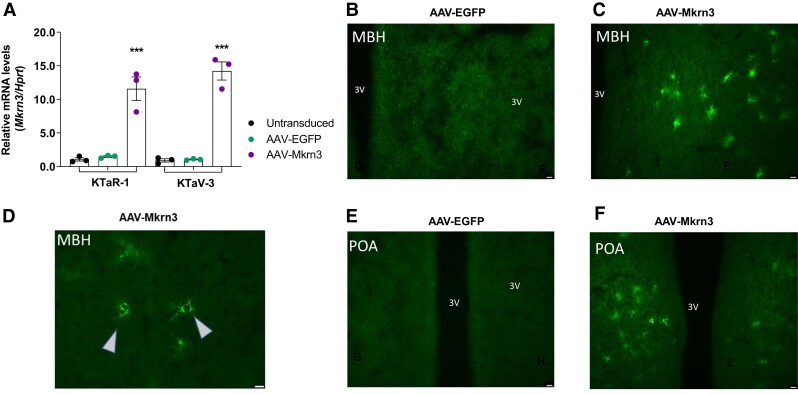
In immortalized Kiss1 cell lines derived from the mouse arcuate nucleus (KTaR-1) and AVPV (KTaV-3), Mkrn3 mRNA was significantly increased when these cells were transduced with AAV-Mkrn3, but not in cells transduced with AAV-EGFP or in untransduced cells (Fig. 1A; P < .0001) (20). Neonatal bilateral ICV injection has been shown to lead to widespread neuronal transduction, which was necessary in order to achieve our goal of having adequate levels of hypothalamic Mkrn3 in the peripubertal period, as endogenous hypothalamic Mkrn3 expression declines before the onset of puberty in female and male mice (2, 3, 21, 22, 35). Mkrn3 protein was not detected in the MBH or POA in AAV-EGFP female mice at PND28 (Fig. 1B and 1E), whereas Mkrn3 protein was present in these areas in AAV-Mkrn3 female mice (Fig. 1C, 1D and 1F).
Figure 1.
In vivo and in vitro validation of AAVs. (A) Cell lines (n = 3 wells for each cell line and experimental condition) derived from Kiss1 neurons from the mouse arcuate nucleus (KTaR-1) and the anteroventral periventricular nucleus (KTaV-3) demonstrate significantly increased Mkrn3 mRNA levels (P < .0001 by 1-way ANOVA, Tukey post hoc) in cells transduced with AAV-Mkrn3 (purple circles) compared with cells transduced with AAV-EGFP (green circles) or untransduced cells (black circles). Representative hypothalamic sections of (B) mediobasal hypothalamus (MBH) and (E) preoptic area at PND28 from AAV-EGFP female mice, in which Mkrn3 protein was not detected. (C, D) Mkrn3 protein detection, levels and distribution from the MBH of a representative AAV-Mkrn3 female mouse at PND28, shown at 2 different magnifications. Arrows denote examples of neuronal cell bodies demonstrating immunofluorescence. (F) Similarly, MKRN3 protein detection, levels and distribution from the POA at PND28 of a representative AAV-Mkrn3 female mouse. Scale bar 100 μm (A-E).
Pubertal Assessment of Female and Male AAV-Mkrn3 Injected mice
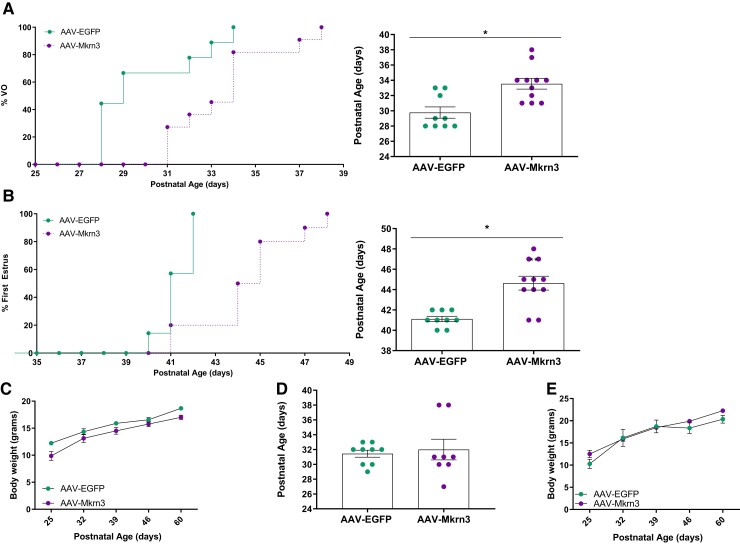
We hypothesized that female but not male mice may exhibit a phenotype of delayed puberty, given the human and mouse phenotypes of earlier pubertal onset in females with paternally inherited loss-of-function mutations or deletions in MKRN3 (1, 2). To assess the timing of puberty in AAV-Mkrn3 and AAV-EGFP injected female mice, we first monitored vaginal opening, a marker of pubertal onset reflecting the actions of circulating estradiol. AAV-Mkrn3 females showed a significant delay in age of vaginal opening compared to AAV-EGFP controls (Fig. 2A; 33.5 ± 0.7 days vs 29.8 ± 0.7 days, respectively, P < .05). Subsequently, age of first estrus was assessed and was also found to be significantly delayed in AAV-Mkrn3 females compared to controls (Fig. 2B; mean age 44.4 ± 0.7 days vs 41.2 ± 0.2 days, respectively, P < .05). Body weight from PND25-60 did not differ between AAV-Mkrn3 and AAV-EGFP female mice (Fig. 2C; 2-way ANOVA F(1,18) = 2.98, P = .10), indicating that lower body weights do not account for the phenotype of delayed pubertal onset in the female mice.
Figure 2.
Neonatal bilateral ICV injection of AAV-Mkrn3 leads to delayed pubertal onset in female mice. (A) Percentage of female mice with VO with increasing postnatal age is shown (left panel) for mice injected with AAV-Mkrn3 (purple circles, n = 11) or AAV-EGFP (green circles, n = 9); mean age of VO is delayed in AAV-Mkrn3 female mice (purple) compared to AAV-EGFP (green) controls (right panel; *P < .05, unpaired t-test). (B) Similarly, percentage of these 2 groups of female mice with first estrus with increasing postnatal age is shown (left panel) and mean age of first estrus is delayed in AAV-Mkrn3 female mice (n = 11) compared with AAV-EGFP controls (n = 9) (right panel; **P < .05, unpaired t-test). (C) Mean body weight of female mice measured at weaning (PND 25) to PND 60 did not differ (AAV-Mkrn3, n = 11, purple circles, AAV-EGFP n = 9, green circles, 2-way ANOVA F(1,18) = 2.986, P = .10). (D) In male mice, the mean age of preputial separation was not significantly different between AAV-Mkrn3 injected (n = 8) compared with AAV-EGFP injected (n = 9) mice (P = .76, unpaired t-test). (E) Mean body weight from PND 25 to 60 also did not differ in male mice (AAV-Mkrn3 n = 8, purple circles, AAV-EGFP, n = 9, green circles, 2-way ANOVA F(1,14) = 0.09, P = .76). Data are shown as mean ± SEM. VO; vaginal opening; PND: postnatal day.
In contrast to the females, male littermates injected with AAV-Mkrn3 did not exhibit a delay in preputial separation, a marker of pubertal onset reflecting androgen exposure, compared to AAV-EGFP injected males (Fig. 2D) (24). There was no difference in body weight between AAV-Mkrn3 and AAV-EGFP male mice (Fig. 2E; 2-way ANOVA, F(1,14) = 0.09, P = .76).
Postpubertal Assessment of Reproductive Function and Fertility of Female AAV-Mkrn3 Injected Mice
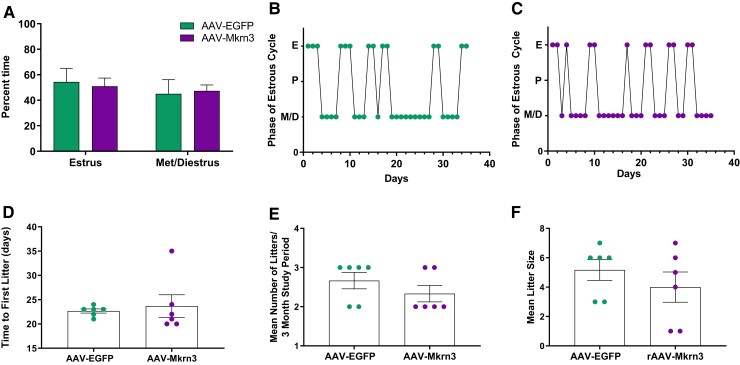
After puberty, we further assessed activity of the HPG axis in female mice by monitoring vaginal cytology to assess the patterns of estrous cyclicity for 4 to 5 weeks following first estrus. The total time spent in estrus compared with metestrus/diestrus did not differ between the 2 groups (Fig. 3A). Both AAV-Mkrn3 and AAV-EGFP female mice exhibited normal estrous cycles (representative mice in Fig. 3B and 3C; mean cycle length 4.6 days ± 0.5 days vs 5.8 days ± 1.3, respectively, P = .36).
Figure 3.
Estrous cyclicity and fertility are normal in AAV-Mkrn3 injected female mice. (A) Following puberty, estrous cycles were monitored following first estrus for 4 to 5 weeks until the fertility study was initiated at 10-12 weeks of age. Percent time spent in estrus compared with metestrus/diestrus did not differ between AAV-Mkrn3 (n = 11) and AAV-EGFP (n = 9) injected mice (P = .58, 2-way ANOVA). Fertility was assessed over a 3-month study period by mating 1:1 with a wild-type male (n = 6 per group). (D) Time to first litter, (E) mean number of litters, and (F) mean litter size did not differ significantly between the 2 groups (P = .68, P = .51, and P = .37, respectively, unpaired t-test).
To assess any potential effects on fertility, female mice were subsequently singly housed with a sexually experienced wild-type male partner for a 3-month study period. AAV-Mkrn3 female mice exhibited a mean time similar to the first litter compared with AAV-EGFP mice (Fig. 3D). Similarly, the mean number of litters over the 3-month study period (Fig. 3E) and the average number of pups per litter (Fig. 3F) did not significantly differ between AAV-Mkrn3 and AAV-EGFP injected female mice. Therefore, although AAV-Mkrn3 female mice had delayed vaginal opening and first estrus, subsequent estrous cyclicity and fertility were found to be normal. Mean basal LH levels were analyzed on PND28, when 80% of AAV-EGFP injected females had achieved vaginal opening, and were not significantly different (AAV-Mkrn3; 0.33 ± 0.06 ng/mL vs AAV-EGFP; 0.48 ng/mL±0.06). The fertility in male mice was not studied given they did not exhibit a pubertal phenotype.
Impact of Mkrn3 Overexpression on Reproductive Neuropeptide mRNA Levels in the MBH and POA of the Hypothalamus
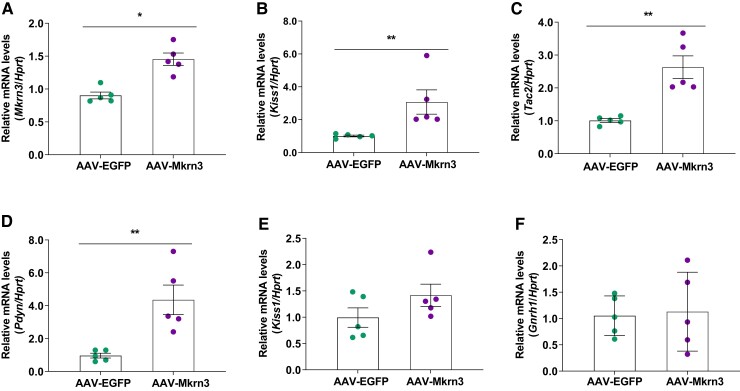
To understand the mechanism of action by which Mkrn3 overexpression in the central nervous system affects the HPG axis, subsequent analyses were performed, with a focus in female mice, since they, but not male mice, exhibited a pubertal phenotype. Expression of Mkrn3 and other hypothalamic regulators of pubertal initiation were measured during the peripubertal period (ie, PND28), an age at which 80% of AAV-EGFP control mice (n = 9) but none of the AAV-Mkrn3 mice (n = 11) had achieved vaginal opening. Consistent with the validations in the prior experiments (Fig. 1), Mkrn3 mRNA levels were significantly higher in AAV-Mkrn3 mice than in AAV-EGFP mice (P < .05; Fig. 4A) in the MBH, which encompasses the arcuate nucleus.
Figure 4.
Hypothalamic mRNA levels in female mice on PND28 as measured by RT-qPCR. In the mediobasal hypothalamus, (A) Mkrn3, (B) Kiss1, (C) Tac2, and (D) Pdyn mRNA levels were all significantly higher in AAV-Mkrn3 mice (purple circles) than in AAV-EGFP mice (green circles). Kiss1 (E; P = .16) and Gnrh1 (F; P = .48) mRNA levels in the preoptic area were not significantly different between the AAV-Mkrn3 and AAV-EGFP mice at PND28. Data are shown as mean ± SEM (n = 5 per group), normalized to the housekeeping gene Hprt. *P < .05; **P < .01; unpaired t-tests.
Mkrn3 has been shown to be coexpressed with Kiss1 in neurons in the mouse arcuate nucleus and MKRN3 has been demonstrated to repress human KISS1 and TAC3 promoter activity (36). Therefore, hypothalamic mRNA levels of the corresponding mouse genes encoding the neuropeptides in the KNDy (kisspeptin, neurokinin B, dynorphin) neurons, Kiss1, Tac2, and Pdyn, respectively, were measured. Interestingly, relative mRNA levels of Kiss1 (Fig. 4B), Tac2 (Fig. 4C), and Pdyn (Fig. 4D) were significantly higher in the MBH in the AAV-Mkrn3 mice than in AAV-EGFP mice (P < .05). In the POA, where the sexually dimorphic Kiss1 neurons are located, Kiss1 (Fig. 4E) and Gnrh1 mRNA (Fig. 4F) levels did not differ between groups.
The existence of compensatory mechanisms among the tachykinin ligand receptor systems (eg, neurokinin A and neurokinin B) has been demonstrated recently (37). We measured Tac1 and Tacr1 mRNA levels in the MBH of AAV-Mkrn3 and AAV-EGFP female mice at PND28 but there were no differences between the 2 groups (Fig. S1A (38)). In addition, as Mkrn3 belongs to a family of evolutionary conserved Makorin proteins, the possibility of a compensatory change in Mkrn1 or Mkrn2 mRNA levels was explored, but no differences were found (Fig. S1A (38)) (17). Additionally, mRNA levels of 2 other imprinted genes implicated in age of menarche, Dlk1 and Kcnk9, were evaluated but did not differ in the setting of Mkrn3 overexpression (Fig. S1A (38)) (14, 36). There were also no differences between female AAV-Mkrn3 mice compared with AAV-EGFP mice in Lhb or Gnrhr mRNA levels in the pituitary (Fig. S1B (38)).
To validate that the lack of a pubertal phenotype in AAV-Mkrn3 injected male mice (Fig. 1D) compared with controls was not due to a lack of Mkrn3 mRNA overexpression in the MBH, Mkrn3 mRNA levels (Fig. S2A (38)) were measured and confirmed to be significantly higher in male AAV-Mkrn3 mice at PND60 than in male AAV-EGFP mice (P < .001). Interestingly, mRNA levels of Kiss1 (Fig. S2B (38)) and Tac2 (Fig. S2C (38)) but not Pdyn (Fig. S2D (38)) were significantly higher in the MBH of the AAV-Mkrn3 male mice than in AAV-EGFP males (P < .05).
Effects of Mkrn3 Overexpression on Hypothalamic Kisspeptin, Neurokinin B, and GnRH Protein Levels
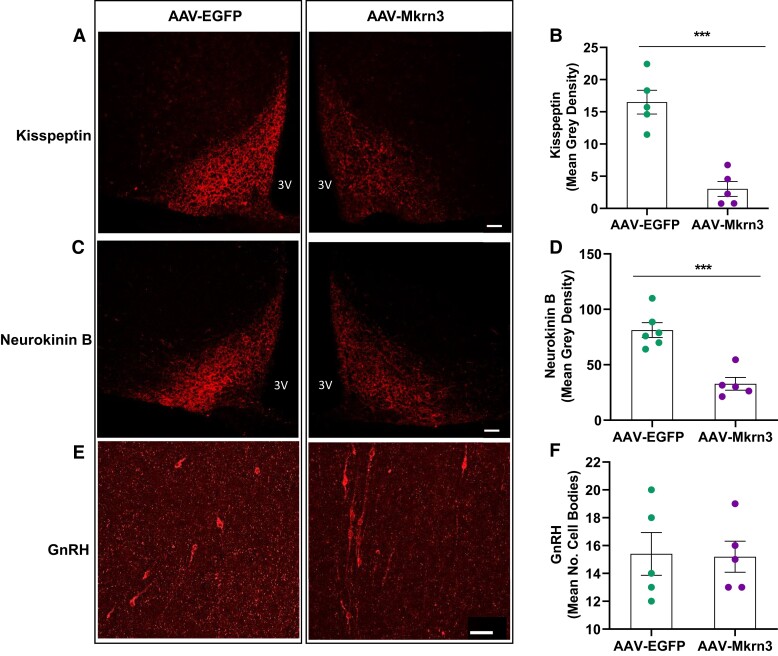
As described earlier, female AAV-Mkrn3 mice had higher Kiss1, Tac2, and Pdyn mRNA levels in the MBH at PND28 than female AAV-EGFP control mice. These findings were surprising in the context of the delayed puberty phenotype. MKRN3 has a RING finger domain characteristic of E3 ubiquitin ligases and has recently been demonstrated to be involved in protein ubiquitination (4, 6, 27, 39). These findings raised the possibility that MKRN3 may be involved in protein degradation of the KNDy neuron neuropeptides, thereby potentially resulting in a compensatory increase in the corresponding mRNA levels. To explore this hypothesis, arcuate nucleus neurokinin B and kisspeptin protein levels were assessed by immunohistochemistry at PND28 in female AAV-Mkrn3 mice and compared with AAV-EGFP controls. Kisspeptin protein levels in the arcuate nucleus were significantly lower (Fig. 5A and 5B) in the AAV-Mkrn3 mice (mean gray density 16.5 ± 1.8) than in AAV-EGFP controls (3.0 ± 1.6, P < .001). Similarly, neurokinin B protein levels in the arcuate nucleus were also significantly lower (Fig. 5C and 5D) in female AAV-Mkrn3 mice (mean gray density 81.2 ± 6.7) than in AAV-EGFP mice (32.8 ± 5.7, P < .001). The mean number of GnRH cell bodies in the POA was not significantly different between the AAV-Mkrn3 and AAV-EGFP mice (Fig. 5E and 5F; AAV-Mkrn3 15.4 ± 1.5 vs AAV-EGFP 15.2 ± 1.1; P = .91).
Figure 5.
Immunohistochemistry shows reduced kisspeptin and neurokinin B protein levels in the arcuate nucleus at PND28 in female AAV-Mkrn3 mice compared with AAV-EGFP mice, whereas the number of GnRH cell bodies in the preoptic area were unchanged. (A) Representative photomicrograph of kisspeptin immunohistochemistry in the arcuate nucleus of AAV-EGFP (left panel) and AAV-Mkrn3 (right panel) female mice at PND28. Scale bar 50 μm. (B) Mean gray density (n = 5 per group) was significantly lower for the AAV-Mkrn3 than for the control AAV-EGFP mice (P < .001, unpaired t-test). (C) Representative photomicrograph of neurokinin B immunohistochemistry in the arcuate nucleus of AAV-EGFP (left) and AAV-Mkrn3 (right) female mice. Scale bar 50 μm. (D) Mean gray density (n = 5 per group) was significantly lower for AAV-Mkrn3 than for AAV-EGFP mice (P < .001, unpaired t-test). (E) Representative photomicrograph of GnRH immunohistochemistry in the preoptic area of AAV-EGFP (left) and AAV-Mkrn3 (right) female mice. Scale bar 50 μm. (F) Mean number of cell bodies for GnRH did not statistically differ between the 2 groups (P = .98).
Discussion
Pubertal onset is believed to result from a shift of inhibitory pathways restraining neuroendocrine factors involved in puberty onset to a predominance of excitatory inputs, resulting in reactivation of GnRH secretion following a period of quiescence in childhood. MKRN3 was the first inhibitory factor in which mutations were identified in humans in association with CPP and has been hypothesized to act at the level or upstream of kisspeptin, GnRH or potentially other intermediate neurons (2). This study presents a novel mouse model of delayed puberty and further advances the understanding of actions of MKRN3, a key neuroendocrine player in pubertal timing (1, 27). The rationale for this study was based, in part, on human and/or mouse models of KISS1/Kiss1 and KISS1R/Kiss1r (formerly GPR54) mutations or deletions, which exhibited phenotypes of CPP in the setting of gain-of-function mutations as well as CHH, which can present with delayed puberty, in the setting of loss-of-function mutations (7–12, 32). Therefore, the aim of this study was to explore the hypothesis that Mkrn3 may also have a role in causing delayed pubertal onset and/or hypogonadotropic hypogonadism, via gain-of-function mutations, in addition to its established role in causing CPP.
The novel mouse model described in this report exhibits a significant delay in vaginal opening and first estrus in female mice, with subsequent normal fertility, but no change in pubertal timing as measured by preputial separation in male mice. In parallel, in a mouse model of paternally inherited Mkrn3 deficiency, a statistically significant advance in vaginal opening and first estrus in female mice was observed (39). Estrous cyclicity in female mice following attainment of first estrus was not reported. In Mkrn3-deficient male mice, an advancement in preputial separation was also observed (39).
We found a sex difference in the response to Mkrn3 overexpression; however, further investigation is needed to fully understand the effects of MKRN3 on both sexes. Preputial separation, a commonly employed marker of pubertal onset in male mice and indicator of sufficient androgen exposure, is more limited than monitoring vaginal cytology in addition to vaginal opening in female mice (23, 24). In wild-type mice, in contrast to the sexually dimorphic kisspeptin neurons of the rodent AVPV/periventricular nuclei, Mkrn3 mRNA levels are similar between males and females in the MBH and AVPV both at PND12, when hypothalamic Mkrn3 expression is high, and at PND30, when low (40, 41). Our reported sex difference in phenotype is analogous to reports in humans, in which girls with loss-of-function mutations in MKRN3 exhibit an earlier age of pubertal onset than affected boys (1, 42). However, a recent meta-analysis of affected individuals with CPP due to MKRN3 mutations reported that while cumulatively more girls have been described with mutations in this gene, boys with CPP are statistically more likely to harbor a mutation in MKRN3 (1).
In children, self-limited delayed puberty (ie, constitutional delay of growth and puberty) remains the most common etiology of delayed puberty in both girls and boys (43). The mouse model reported here suggests that genetic variants resulting in increased MKRN3 expression could be an unrecognized cause of self-limited delayed puberty in children, and/or potentially delayed puberty in association with a CHH phenotype. In 1 study of men with CHH, circulating MKRN3 levels were measured and did not differ from those in healthy men with normal HPG axis function (44). However, the source of peripheral circulating MKRN3 has not yet been determined, and circulating levels may not reflect levels in the central nervous system. Additionally, serum MKRN3 assays have not yet been validated in patients with frameshift or nonsense MKRN3 mutations, which would be expected to result in the loss of MKRN3 protein.
Our findings in this study revealed no impact of Mkrn3 overexpression in the central nervous system of mice on body weight at the ages and metabolic conditions tested; therefore, the phenotype of delayed puberty in female mice cannot be attributed to a lower body weight. A reduction in body weight in the setting of Mkrn3 overexpression might have been expected, as patients with Prader–Willi syndrome, who typically have a deletion encompassing MKRN3, can develop obesity and hyperphagia; however, they have the loss of expression of multiple genes in addition to MKRN3 (45). Given that Mkrn3 mRNA is expressed in wild-type mice in the arcuate and ventromedial nuclei (ie, hypothalamic nuclei that receive metabolic cues such as insulin, ghrelin, and leptin) at PND10, it could be speculated that deficiency of Mkrn3 may contribute to a metabolic phenotype (27). A Mkrn3-deficient mouse model demonstrated an increase in body weight in the juvenile/peripubertal period, followed by decreased body weight in adulthood, compared with age-matched controls, although this differs from the human phenotype of children with CPP due to mutations in MKRN3, who do not appear to exhibit a metabolic phenotype (39).
In the setting of Mkrn3 overexpression, this study brings novel insight into the potential for Mkrn3 to lead to delayed puberty and provides a new potential mechanism of action for Mkrn3, demonstrating that Mkrn3 overexpression resulted in reduced hypothalamic kisspeptin and neurokinin B protein levels. Nonetheless, it is not yet known if the decrease in kisspeptin and neurokinin B levels is a direct vs. indirect effect of Mkrn3 action. MKRN3 has recently been shown to be expressed in mouse Kiss1 neurons in the arcuate nucleus (27). The observed decreases in neurokinin B and kisspeptin protein levels may be due to direct effects of Mkrn3 on both neurokinin B and kisspeptin protein production or degradation. Alternatively, the decrease in kisspeptin protein may be indirect, resulting from the reduced neurokinin B protein levels, as neurokinin B acts autosynaptically on KNDy neurons to stimulate Kiss1 gene expression and, thereby, kisspeptin protein synthesis (46, 47). However, this explanation is less likely, given the observed increase in Kiss1 mRNA levels in the mediobasal hypothalamus in this Mkrn3 overexpression mouse model. Indeed, the observed increase in Kiss1 and Tac2 mRNA levels in the MBH suggest a level of action of Mkrn3 in the KNDy neuron at the Kiss1 and Tac2 posttranscriptional level, reducing kisspeptin and neurokinin B protein levels while increasing Kiss1 and Tac2 mRNA levels. The observed increase in Kiss1, Tac2 and Pdyn mRNA levels in the MBH may be indirect, due to reduced activity of the HPG axis in association with lower neurokinin B and kisspeptin protein levels and hence likely reduced release from KNDy neurons, ultimately resulting in reduced production of gonadal sex steroids and decreased negative feedback by sex steroids at the arcuate nucleus (40). As MKRN3 has been demonstrated to inhibit the human promoters of KISS1 and TAC3 in vitro, it is also possible that Mkrn3 is repressing the Kiss1 and Tac2 promoters in the current model; however, this inhibition appears to have been insufficient to overcome the effects of a putative decrease in negative feedback from sex steroids (27). The transient delay in pubertal onset in female mice in our model with subsequent normal estrous cycles and fertility may be due to other compensatory factors and/or related to the small percentage of KNDy neurons that are required to induce puberty onset and maintain GnRH pulses in rodents (48, 49).
While speculative, the observed effects on kisspeptin and neurokinin B protein levels in the arcuate nucleus may be mediated by ubiquitination and/or RNA binding activity of MKRN3 (27). The protein structure of MKRN3 includes a Makorin-type Cys-His motif, 3 C3H1 zinc fingers suggestive of RNA binding activity, and a C3HC4 RING finger domain characteristic of E3 ubiquitin ligases (50). Among its targets are poly-(A)-binding proteins, which when ubiquitinated by MKRN3 result in attenuation of binding to poly(A) tail of GNRH1 mRNA, resulting in disrupted formation of its translation initiation complex (6). Interestingly, MKRN3 has also been recently reported to regulate poly-(A)-binding protein C1 ubiquitination in non–small cell lung cancer (51). MKRN3 has also been shown to ubiquitinate methyl-DNA binding protein 3 (MBD3), leading to epigenetic silencing of GNRH1 transcription, although we did not detect any differences in Gnrh1 mRNA levels in our Mkrn3 overexpression model (39). A reduction of polyubiquitination of neural pentraxin-1 precursor in the hypothalamus has also been shown, following ICV injection of an Mkrn3 vector harboring a mutated RING domain, compared with wild-type Mkrn3 (4). Additionally, Abreu et al have reported that pathogenic human mutations in the RING finger domain of MKRN3, identified in patients with CPP, resulted in reduced ubiquitin ligase activity (27).
MKRN3 likely has multiple protein targets, as suggested by protein–protein interaction studies performed in HEK-293 T cells lines by 2 different groups, which have identified more than 100 interacting proteins, including some involved in pubertal timing and age of menarche (eg, LIN28B), insulin signaling, RNA metabolism, stability and transport, ubiquitin-mediated proteolysis, zinc finger protein interaction, and cell–cell adhesion (5, 6). Interestingly, MKRN3 was also found to interact with the deubiquitinase encoded by the gene OTUD4, and has been implicated in CHH in association with ataxia and dementia (52). In vitro studies performed in biallelic MKRN3 knockout human pluripotent stem cell lines differentiated into GNRH1-expressing neurons suggested MKRN3 to be dispensable for GnRH neuronal differentiation and GNRH1 expression (5). In contrast, a mouse model of Mkrn3 deficiency has reported increased levels of hypothalamic Gnrh1 mRNA and serum GNRH1 concentrations in male and female mice at PND 15 compared with controls (39).
While the findings of this study are novel, there are several considerations of the model. Given that hypothalamic Mkrn3 mRNA declines in the peripubertal period, it was necessary to inject mice on PND1 to obtain and maintain high levels of Mkrn3 expression peri-pubertally (2, 21, 22, 27, 41). More targeted localization of viral delivery, such as into the arcuate nucleus, cannot be achieved by stereotaxic injection in a mouse at this early age. Therefore, it cannot be ascertained if the effects observed on kisspeptin and neurokinin B protein levels in the arcuate nucleus are the result of direct effects of MKRN3 in the KNDy neurons, or indirect actions via effects in other neurons. Double label staining was also not performed in this model to quantify how many KNDy neurons were infected with the AAVs. Additionally, the transient delay in puberty observed in female mice in this study rather than a more prolonged maintenance of a prepubertal phenotype may have been impacted by the use of viral transduction rather than a transgenic mouse model with a sustained overexpression of Mkrn3. It is possible that in a transgenic model of Mkrn3 overexpression, females might exhibit a more marked and more sustained pubertal and reproductive phenotype (eg, long-term hypogonadism, subfertility) and that males could potentially also exhibit a reproductive phenotype—although it is also conceivable that even in a state of sustained overexpression of Mkrn3, its inhibitory effects on puberty onset could be overcome by progressively increasing excitatory inputs. In this study, measurement of protein levels of neuropeptides known to be involved in regulation of the HPG axis were only pursued in female mice, given that only the females exhibited a reproductive phenotype; however, it is possible that Mkrn3 overexpression also impacts neurokinin B and kisspeptin protein in the arcuate nucleus of males in a similar manner to that observed in females and remains an area for further exploration.
In summary, hypothalamic overexpression of Mkrn3 delays pubertal onset in female mice, manifested by delayed vaginal opening and first estrus in association with a decrease in kisspeptin and neurokinin B protein levels in the arcuate nucleus. These findings suggest that a central response to Mkrn3 may not be restricted by developmental windows and allows for the possibility that MKRN3 expression could be modulated as a future therapeutic target for CPP and other reproductive disorders. Additionally, the findings of this study raise the possibility that children with delayed puberty may have yet unrecognized genetic variants in MKRN3.
Acknowledgments
The authors acknowledge Han Kyeol Kim for assistance with animal husbandry, Dr. A. F. Parlow at the National Hormone and Peptide Program for providing detection polyclonal antibody for LH assays (rabbit LH antiserum, AFP240580Rb) and the National Institute for Agricultural Research, Food, and Environment (Institut National De Recherche Pour L’agriculture, L’alimentation Et L’environnement) via Dr. Isabelle Franceschini for providing kisspeptin antibody.
Abbreviations
- ANOVA
analysis of variance
- AVPV
anteroventral periventricular nucleus
- CHH
congenital hypogonadotropic hypogonadism
- CPP
central precocious puberty
- DLK1
delta like non-canonical Notch ligand 1
- GC
genome copies
- GnRH
gonadotropin-releasing hormone
- HPG
hypothalamic–pituitary–gonadal
- ICV
intracerebroventricular
- KNDy
kisspeptin, neurokinin B, dynorphin
- LH
luteinizing hormone
- MBH
mediobasal hypothalamus
- MKRN3
makorin ring finger protein 3
- NGS
normal goat serum
- PBST
phosphate-buffered saline containing Tween-20
- PND
postnatal day
- POA
preoptic area
Contributor Information
Stephanie A Roberts, Division of Endocrinology, Boston Children’s Hospital, Boston, MA 02115, USA; Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA.
Lydie Naulé, Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Soukayna Chouman, Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Tatyana Johnson, Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Marciana Johnson, Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Rona S Carroll, Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA.
Victor M Navarro, Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA.
Ursula B Kaiser, Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA.
Funding
Funding support includes National Institute of Health K08HD100595-01A1, K12HD051959, NIH Loan Repayment Program, Pediatric Endocrine Society Clinical Scholar Award, and Boston Children’s Hospital Faculty Development Award (S.A.R.), R01HD082314, R37HD019938, and R21HD098684 (U.B.K.), and R01HD090151 and R01HD099084 (V.M.N.).
Disclosures
The authors have no disclosures.
Data Availability
The data generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Valadares LP, Meireles CG, De Toledo IP, et al. MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc. 2019;3(5):979‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467‐2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLOS Biol. 2019;17(11):e3000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu H, Kong X, Chen F. Mkrn3 functions as a novel ubiquitin E3 ligase to inhibit Nptx1 during puberty initiation. Oncotarget. 2017;8(49):85102‐85109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yellapragada V, Liu X, Lund C, et al. MKRN3 interacts with several proteins implicated in puberty timing but does not influence GNRH1 expression. Front Endocrinol. 2019;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li C, Han T, Li Q, et al. MKRN3-mediated ubiquitination of Poly(A)-binding proteins modulates the stability and translation of GNRH1 mRNA in mammalian puberty. Nucleic Acids Res. 2021;49(7):3796‐3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silveira LG, Noel SD, Silveira-Neto AP, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95(5):2276‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teles MG, Bianco SDC, Brito VN, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629‐635. [DOI] [PubMed] [Google Scholar]
- 10. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614‐1627. [DOI] [PubMed] [Google Scholar]
- 11. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972‐10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nimri R, Lebenthal Y, Lazar L, et al. A novel loss-of-function mutation in GPR54/KISS1R leads to hypogonadotropic hypogonadism in a highly consanguineous family. J Clin Endocrinol Metab. 2011;96(3):E536‐E545. [DOI] [PubMed] [Google Scholar]
- 13. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dauber A, Cunha-Silva M, Macedo DB, et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab. 2017;102(5):1557‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomes LG, Cunha-Silva M, Crespo RP, et al. DLK1 is a novel link between reproduction and metabolism. J Clin Endocrinol Metab. 2019;104(6):2112‐2120. [DOI] [PubMed] [Google Scholar]
- 16. Montenegro L, Labarta JI, Piovesan M, et al. Novel genetic and biochemical findings of DLK1 in children with central precocious puberty: a Brazilian-Spanish study. J Clin Endocrinol Metab. 2020;105(10):dgaa461. [DOI] [PubMed] [Google Scholar]
- 17. Naulé L, Kaiser UB. Evolutionary conservation of MKRN3 and other Makorins and their roles in puberty initiation and endocrine functions. Semin Reprod Med. 2019;37(4):166‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawson H, Vuong E, Miller RM, Kiontke K, Fitch DH, Portman DS. The Makorin lep-2 and the lncRNA lep-5 regulate lin-28 to schedule sexual maturation of the C. elegans nervous system. eLife. 2019;8:e43660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrera RA, Kiontke K, Fitch DHA. Makorin ortholog LEP-2 regulates LIN-28 stability to promote the juvenile-to-adult transition in Caenorhabditis elegans. Dev Camb Engl. 2016;143(5):799‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobs DC, Veitch RE, Chappell PE. Evaluation of immortalized AVPV- and arcuate-specific neuronal kisspeptin cell lines to elucidate potential mechanisms of estrogen responsiveness and temporal gene expression in females. Endocrinology. 2016;157(9):3410‐3419. [DOI] [PubMed] [Google Scholar]
- 21. Kim JY, Grunke SD, Levites Y, Golde TE, Jankowsky JL. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J Vis Exp. 2014;91:51863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JY, Grunke SD, Jankowsky JL. Widespread neuronal transduction of the rodent CNS via neonatal viral injection. In: Manfredsson FP, ed. Gene Therapy for Neurological Disorders: Methods and Protocols. Methods in Molecular Biology. Springer; 2016:239‐250. [DOI] [PubMed] [Google Scholar]
- 23. Caligioni C. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix:Appendix-4I. doi:10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffmann HM. Determination of reproductive competence by confirming pubertal onset and performing a fertility assay in mice and rats. J Vis Exp. 2018;140:58352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7(4):e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939‐4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020;130(8):4486‐4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leon S, Talbi R, McCarthy EA, et al. Sex-specific pubertal and metabolic regulation of Kiss1 neurons via Nhlh2. eLife. 2021;10:e69765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castellano JM, Wright H, Ojeda SR, Lomniczi A. An alternative transcription start site yields estrogen-unresponsive Kiss1 mRNA transcripts in the hypothalamus of prepubertal female rats. Neuroendocrinology. 2014;99(2):94‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roberts SA, Naule L, Chouman S, et al. Primer sequences for RT-PCR. Deposited June 5, 2022. Figshare. Doi: 10.6084/m9.figshare.19723618.v1 [DOI]
- 31. Franceschini I, Yeo SH, Beltramo M, et al. Immunohistochemical evidence for the presence of various kisspeptin isoforms in the mammalian brain. J Neuroendocrinol. 2013;25(9):839‐851. [DOI] [PubMed] [Google Scholar]
- 32. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927‐4936. [DOI] [PubMed] [Google Scholar]
- 33. True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naulé L, Robert V, Parmentier C, et al. Delayed pubertal onset and prepubertal Kiss1 expression in female mice lacking central oestrogen receptor beta. Hum Mol Genet. 2015;24(25):7326‐7338. [DOI] [PubMed] [Google Scholar]
- 35. Chakrabarty P, Rosario A, Cruz P, et al. Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS One. 2013;8(6):e67680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perry JR, Day F, Elks CE, et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514(7520):92‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. León S, Fergani C, Talbi R, et al. Tachykinin signaling is required for induction of the preovulatory luteinizing hormone surge and normal luteinizing hormone pulses. Neuroendocrinology. 2021;111(6):542‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts SA, Naulé L, Chouman S, et al. Hypothalamic mRNA levels by RT-PCR. Deposited June 5, 2022. Figshare. Doi: 10.6084/m9.figshare.19723585.v1 [DOI]
- 39. Li C, Lu W, Yang L, et al. MKRN3 regulates the epigenetic switch of mammalian puberty via ubiquitination of MBD3. Natl Sci Rev. 2020;7(3):671‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817‐5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roberts SA, Abreu AP, Navarro VM, et al. The peripubertal decline in Makorin ring finger protein 3 expression is independent of leptin action. J Endocr Soc.2020;4(7):bvaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seraphim CE, Canton APM, Montenegro L, et al. Genotype-phenotype correlations in central precocious puberty caused by MKRN3 mutations. J Clin Endocrinol Metab. 2021;106(4):1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jonsdottir-Lewis E, Feld A, Ciarlo R, Denhoff E, Feldman HA, Chan YM. Timing of pubertal onset in girls and boys with constitutional delay. J Clin Endocrinol Metab. 2021;106(9):dgab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Varimo T, Hero M, Känsäkoski J, Vaaralahti K, Matikainen N, Raivio T. Circulating makorin ring-finger protein-3 (MKRN3) levels in healthy men and in men with hypogonadotropic hypogonadism. Clin Endocrinol (Oxf). 2016;84(1):151‐152. [DOI] [PubMed] [Google Scholar]
- 45. Butler MG, Miller JL, Forster JL. Prader-Willi syndrome – clinical genetics, diagnosis and treatment approaches: an update. Curr Pediatr Rev. 2019;15(4):207‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Navarro VM. Tachykinin signaling in the control of puberty onset. Curr Opin Endocr Metab Res. 2020;14:92‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci Off J Soc Neurosci. 2009;29(38):11859‐11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Popa SM, Moriyama RM, Caligioni CS, et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154(8):2784‐2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagae M, Uenoyama Y, Okamoto S, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci U S A. 2021;118(5):e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jong MT, Gray TA, Ji Y, et al. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8(5):783‐793. [DOI] [PubMed] [Google Scholar]
- 51. Li K, Zheng X, Tang H, et al. E3 ligase MKRN3 is a tumor suppressor regulating PABPC1 ubiquitination in non-small cell lung cancer. J Exp Med. 2021;218(8):e20210151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Margolin DH, Kousi M, Chan YM, et al. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N Engl J Med. 2013;368(21):1992‐2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.