Key Points
Question
To what extent does prenatal folate status modify associations between early pregnancy perfluoroalkyl and polyfluoroalkyl substance (PFAS) exposure and birth outcomes?
Findings
In this cohort study of 1400 mother-singleton pairs in the US, higher early pregnancy plasma PFAS concentrations were associated with lower birth weight and gestational age and a higher risk of low birth weight. These findings were observed only among mothers in the lowest quartile of prenatal folate status measured by dietary intake or plasma biomarker.
Meaning
The findings of this study suggest that PFAS exposure may be associated with adverse birth outcomes only among mothers with low folate status in early pregnancy.
Abstract
Importance
Prenatal perfluoroalkyl and polyfluoroalkyl substances (PFAS) have been linked to adverse birth outcomes. Previous research showed that higher folate concentrations are associated with lower blood PFAS concentrations in adolescents and adults. Further studies are needed to explore whether prenatal folate status mitigates PFAS-related adverse birth outcomes.
Objective
To examine whether prenatal folate status modifies the negative associations between pregnancy PFAS concentrations, birth weight, and gestational age previously observed in a US cohort.
Design, Setting, and Participants
In a prospective design, a prebirth cohort of mothers or pregnant women was recruited between April 1999 and November 2002, in Project Viva, a study conducted in eastern Massachusetts. Statistical analyses were performed from May 24 and October 25, 2022.
Exposure
Plasma concentrations of 6 PFAS compounds were measured in early pregnancy (median gestational week, 9.6). Folate status was assessed through a food frequency questionnaire and measured in plasma samples collected in early pregnancy.
Main Outcomes and Measures
Birth weight and gestational age, abstracted from delivery records; birth weight z score, standardized by gestational age and infant sex; low birth weight, defined as birth weight less than 2500 g; and preterm birth, defined as birth at less than 37 completed gestational weeks.
Results
The cohort included a total of 1400 mother-singleton pairs. The mean (SD) age of the mothers was 32.21 (4.89) years. Most of the mothers were White (73.2%) and had a college degree or higher (69.1%). Early pregnancy plasma perfluorooctanoic acid concentration was associated with lower birth weight and birth weight z score only among mothers whose dietary folate intake (birth weight: β, −89.13 g; 95% CI, −166.84 to −11.42 g; birth weight z score: −0.13; 95% CI, −0.26 to −0.003) or plasma folate concentration (birth weight: −87.03 g; 95% CI, −180.11 to 6.05 g; birth weight z score: −0.14; 95% CI, −0.30 to 0.02) were below the 25th percentile (dietary: 660 μg/d, plasma: 14 ng/mL). No associations were found among mothers in the higher folate level groups, although the tests for heterogeneity did not reject the null. Associations between plasma perfluorooctane sulfonic acid and perfluorononanoate (PFNA) concentrations and lower birth weight, and between PFNA and earlier gestational age were noted only among mothers whose prenatal dietary folate intake or plasma folate concentration was in the lowest quartile range. No associations were found among mothers in higher folate status quartile groups.
Conclusions and Relevance
In this large, US prebirth cohort, early pregnancy exposure to select PFAS compounds was associated with adverse birth outcomes only among mothers below the 25th percentile of prenatal dietary or plasma folate levels.
This cohort study examines the outcomes associated with folate status and exposure to perfluoroalkyl and polyfluoroalkyl substances in women who are pregnant.
Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a class of synthetic chemicals with diverse commercial and industrial applications, such as nonstick cookware, cosmetics, fire-fighting foams, and food packaging.1 The PFAS are extremely persistent in the environment and have been detected in populations worldwide.2,3,4,5,6 Exposure to PFAS has been associated with adverse reproductive end points and other poor health outcomes.7,8,9
Prenatal PFAS exposure has been linked to lower birth weight, shorter gestational age, and preterm birth in epidemiological studies,10,11,12,13,14,15 including the Project Viva, a large, prospective prebirth cohort in the US.11 Despite adverse birth outcomes associated with prenatal PFAS exposure, we are not aware of any study that has assessed potential modifiable factors that may reduce harmful outcomes of PFAS exposure in vulnerable populations. Previous studies reported inverse associations between folate and PFAS concentrations in a representative US population,16 and protective effects of folate on PFAS–lower antibody associations in children.17 Folic acid supplementation has been universally recommended to prevent neural tube defects.18 Folate plays a critical role in DNA synthesis and methylation.19 Folate and PFAS may share common transporters in the human body,20,21,22,23,24,25,26,27,28 thus resulting in potential biological interactions between folate and PFAS. This study aimed to examine whether prenatal folate status modifies the association between PFAS exposure and singleton birth outcomes.
Methods
Population
Mother-singleton pairs were recruited at their first prenatal visit between April 1999 and November 2002, in Project Viva, a large prebirth cohort in the US. Data analysis was performed from May 24 to October 25, 2022. Details on the Project Viva study design and population are described elsewhere.29 The institutional review board of Harvard Pilgrim Health Care approved the study protocol. Participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
In brief, sociodemographic characteristics, reproductive history, early pregnancy diet, and nutrient supplementation were assessed by questionnaires and interviews. Participants provided a nonfasting blood sample at recruitment (median gestational week, 9.6; IQR, 8.7-10.9).
There were 1400 mother-singleton pairs with plasma PFAS measurement, dietary folate intake, and birth outcome data. Among these participants, a subpopulation of 898 mothers had plasma folate concentrations quantified. We compared the study population with the original Project Viva cohort. The participant inclusion diagram can be found in eFigure 1 in Supplement 1.
PFAS Measurements
The nonfasting blood samples obtained in early pregnancy were centrifuged and stored in non–PFAS-containing cryovial tubes in liquid nitrogen freezers (≤−130 °C). Plasma samples were sent to the Division of Laboratory Sciences at the Centers for Disease Control and Prevention for quantification of 8 PFAS compounds, including perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid, perfluorononanoate (PFNA), 2-(N-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA), 2-(N-methyl-perfluorooctane sulfonamido) acetate (MeFOSAA), perfluorodecanoic acid (PFDeA), and perfluorooctane sulfonamide (PFOSA), using online solid-phase extraction coupled to high-performance liquid chromatography isotope dilution tandem mass spectrometry.30,31 Limits of detection were 0.2 ng/mL for PFOS and 0.1 ng/mL for all other PFAS compounds. Concentrations below the limit of detection were imputed with the limit of detection divided by the square root of 2.32,33 Detection rates for PFDeA (47.52%) and PFOSA (10.93%) were low and were excluded because imputing values may incur bias.33 All other PFAS were detected in more than 99% of the samples.
Folate Measurements
A semiquantitative food frequency questionnaire was used to assess diet from the last menstrual period of the index pregnancy to the time of study recruitment.34,35 An interview was conducted at recruitment to assess supplement intake for the 3-month period before the date of the index pregnancy detection. Pregnancy dietary folate equivalent (DFE) intake (micrograms per day) included both natural food folate and folic acid from fortification and supplement intake and was estimated by multiplying the frequency of intake of standard portion sizes of each food item or supplement times its folate or folic acid content, according to the Harvard nutrient composition database.35 To account for the variations in folate intake by total energy consumption, we adjusted the DFE intake for total energy intake via the residual method.36
Baseline plasma samples for a subpopulation were sent to Boston Children’s Hospital’s Clinical and Epidemiological Research Laboratory for folate quantification (to convert from nanograms per milliliter to millimoles per liter, multiply by 2.266) by an electrochemiluminescence binding assay (Elecsys Folate III, Roche Diagnostic). The assay is approved by the US Food and Drug Administration and has high reliability for plasma folate measurement (day-to-day imprecision <3%).
Birth Outcome Measurements
Date of birth and birth weight in grams were abstracted from medical records. Gestational age was calculated using the date of the last menstrual period and corrected by ultrasonography (16-20 weeks) if the 2 estimates differed by more than 10 days (9%).11 We computed birth weight z score standardized by gestational age and infant sex, using a US reference.37 Preterm birth was defined as birth less than 37 completed gestational weeks (259 days) and low birth weight as less than 2500 g.
Covariates
Data on maternal characteristics were collected by questionnaires and interviews at recruitment. Demographic and socioeconomic characteristics included maternal age, maternal and paternal educational level, self-reported maternal race and ethnicity, and annual household income. Maternal prepregnancy body mass index was calculated by self-reported weight in kilograms divided by the square of self-reported height in meters. Smoking status was grouped as ever smokers, former smokers, and early pregnancy smokers. Reproductive history included parity and infertility history defined as having tried for more than 12 months (if age <35 years) or 6 months (if age ≥35 years) to achieve the index pregnancy or having a diagnosis of infertility or claims for infertility treatments and/or prescriptions in medical records.38,39 Index pregnancy was self-reported as planned or unplanned. Breastfeeding history was estimated using parity and breastfeeding of the index birth as previously described.11 We calculated the Alternative Healthy Eating Index for Pregnancy score as a measure of diet quality in early pregnancy.40,41
Statistical Analysis
Primary Analyses
The study population was grouped by folate quartiles of DFE intake or plasma folate concentrations. We conducted descriptive analyses on population characteristics and PFAS distributions by folate groups. Spearman correlation coefficients of 6 PFAS concentrations, DFE intake, and plasma folate concentration were calculated.
Plasma PFAS concentrations were log-2 transformed to reduce the influence of outliers and facilitate comparison with previous studies.12,14,42 Multivariable linear regression models were used to assess differences in the continuous birth outcomes—gestational age (days), birth weight (grams), and birth weight z score, per doubling of plasma PFAS concentrations—by DFE intake or plasma folate concentration groups. Adjusted covariates were selected a priori using a directed acyclic graph and included maternal age, educational level, race and ethnicity, body mass index, smoking history, parity, breastfeeding history, infertility, planned pregnancy, infant sex, gestational age at recruitment, and Alternative Healthy Eating Index for Pregnancy score as well as paternal educational level and annual household income (eFigure 2 in Supplement 1).11,43,44
Quantile-based g computation (QGC) was used to assess the joint association of the total PFAS mixture with birth outcomes by folate groups.45 Quantile-based g computation estimated the differences in continuous birth outcomes per quartile increase in total PFAS mixture concentrations (lowest quartile as reference), adjusting for the above-mentioned covariates.
The associational estimates in each folate group were obtained from regression models with the interaction term of folate group × individual PFAS concentration. Likelihood ratio tests were used to test statistical significance of interaction terms in models with meaningful associations.
Supplemental Analyses
The 2 binary outcomes—preterm birth and low birth weight—were examined using logistic regressions (eMethods in Supplement 1). We additionally restricted DFE analyses to the subpopulation who had both DFE and plasma folate measurements to have comparable samples across both types of folate measures. We further adjusted for early pregnancy fish consumption since it is an important source of PFAS and associated with birth outcomes.46 Generalized additive models were used to explore nonlinear modification by folate (as DFE or plasma folate concentration) in individual PFAS and birth outcome associations (eMethods in Supplement 1).
All tests were 2-sided at an α = .05 significance threshold, except for the likelihood ratio tests of interaction terms (P < .20). All analyses were conducted in R, version 4.0.3 (R Foundation for Statistical Computing). In the linear and logistic regressions, we used multiple imputation with chained equations (MICE) to impute missing covariate values (missingness rates, 0.07%-6.93%) (eFigure 1 in Supplement 1) and obtained pooled regression coefficients with the MICE package (version 3.14.0). We used qgcompint (version 0.6.6) for quantile-based g computation models. Because of the difficulties in applying MICE in mixture models, missing values were imputed with the mean (continuous covariates) or the mode (categorical or binary covariates) in the mixture models and results by imputation methods were compared.
Results
Population
In the overall population of 1400 mothers or pregnant women, the mean (SD) age was 32.21 (4.89) years. Most mothers identified as White (1025 [73.2%]), had a college degree or higher (968 [69.1%]), had an annual household income greater than $70 000 (820 [58.6%]), planned for the pregnancy (879 [62.8%]), and reported periconception folic acid supplement use (957 [68.4%]) (Table 1). The incidence of preterm birth was 7.1% (n = 99) and the incidence of low birth weight was 4.6% (n = 64). Characteristics of the subpopulation were similar to those of the study population (Table 2). A comparison of the study population with the original Project Viva cohort can be found in eTable 1 and the eResults in Supplement 1.
Table 1. Characteristics of the Study Population in Project Viva.
| Characteristic | No. (%) | P valuea | ||||
|---|---|---|---|---|---|---|
| Total population (N = 1400) | First quartile (n = 350) | Second quartile (n = 350) | Third quartile (n = 350) | Fourth quartile (n = 350) | ||
| Dietary folate intake, range, μg/d | 128.7-3497.2 | 128.7-660.4 | 660.4-936.2 | 936.2-1187.3 | 1187.3-3497.2 | NA |
| Maternal age, mean (SD), y | 32.21 (4.89) | 30.67 (5.85) | 32.20 (4.93) | 32.82 (4.30) | 33.15 (3.89) | <.001 |
| Prepregnancy BMI, mean (SD) | 24.78 (5.39) | 25.99 (6.31) | 24.66 (5.51) | 24.31 (4.64) | 24.16 (4.74) | <.001 |
| AHEI-P score, mean (SD)b | 60.46 (10.25) | 55.67 (9.74) | 58.53 (9.38) | 63.09 (9.96) | 64.56 (9.39) | <.001 |
| Maternal race and ethnicityc | ||||||
| Asian | 68 (4.9) | 22 (6.3) | 16 (4.6) | 16 (4.6) | 14 (4.0) | <.001 |
| Black | 167 (11.9) | 83 (23.7) | 40 (11.4) | 23 (6.6) | 21 (6.0) | |
| Hispanic | 87 (6.2) | 38 (10.9) | 17 (4.9) | 22 (6.3) | 10 (2.9) | |
| White | 1025 (73.2) | 183 (52.3) | 265 (75.7) | 285 (81.4) | 292 (83.4) | |
| Other | 53 (3.8) | 24 (6.9) | 12 (3.4) | 4 (1.1) | 13 (3.7) | |
| Maternal education ≥ college degree | 968 (69.1) | 173 (49.4) | 246 (70.3) | 261 (74.6) | 288 (82.3) | <.001 |
| Paternal education ≥ college degreed | 888 (63.4) | 144 (41.1) | 213 (60.9) | 257 (73.4) | 274 (78.3) | <.001 |
| Annual household income >$70 000e | 820 (58.6) | 149 (42.6) | 201 (57.4) | 222 (63.4) | 248 (70.9) | <.001 |
| Prenatal smokingf | ||||||
| Never | 932 (66.6) | 228 (65.1) | 225 (64.3) | 233 (66.6) | 246 (70.3) | <.001 |
| Former | 296 (21.1) | 53 (15.1) | 82 (23.4) | 81 (23.1) | 80 (22.9) | |
| Early pregnancy | 168 (12.0) | 68 (19.4) | 43 (12.3) | 35 (10.0) | 22 (6.3) | |
| Nulliparous | 696 (49.7) | 133 (38.0) | 165 (47.1) | 208 (59.4) | 190 (54.3) | <.001 |
| Planned pregnancyg | 879 (62.8) | 124 (35.4) | 215 (61.4) | 255 (72.9) | 285 (81.4) | <.001 |
| Infertility | 306 (21.9) | 40 (11.4) | 73 (20.9) | 82 (23.4) | 111 (31.7) | <.001 |
| Periconception folic acid supplement useh | 957 (68.4) | 79 (22.6) | 230 (65.7) | 305 (87.1) | 343 (98.0) | <.001 |
| Ever breastfeedingi | 516 (36.9) | 147 (42.0) | 127 (36.3) | 112 (32.0) | 130 (37.1) | <.001 |
| Female infant | 685 (48.9) | 175 (50.0) | 170 (48.6) | 166 (47.4) | 174 (49.7) | .90 |
| Gestational age, mean (SD), wk | 39.49 (1.86) | 39.30 (2.14) | 39.56 (1.76) | 39.72 (1.50) | 39.36 (1.95) | .01 |
| Birth weight, mean (SD), g | 3486.7 (572.8) | 3427.6 (637.6) | 3503.6 (571.3) | 3522.4 (493.6) | 3493.1 (577.6) | .14 |
| Birth weight z score, mean (SD)j | 0.21 (0.95) | 0.14 (1.03) | 0.22 (0.96) | 0.20 (0.90) | 0.27 (0.90) | .38 |
| Preterm birth | 99 (7.1) | 33 (9.4) | 22 (6.3) | 15 (4.3) | 29 (8.3) | .04 |
| Low birth weight | 64 (4.6) | 21 (6.0) | 16 (4.6) | 9 (2.6) | 18 (5.1) | .16 |
Abbreviations: AHEI-P, Alternative Healthy Eating Index in Pregnancy; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
P values obtained from χ2 tests for categorical variables and t tests for continuous variables across folate groups.
Higher AHEI-P score indicates better diet quality.
Race and ethnicity was self-reported in the questionnaire. American Indian or Alaska Native and other were collapsed into a single category to balance sample size.
Missingness: 97 (6.93%) for paternal educational level.
Missingness: 96 (6.86%) for household income.
Missingness: 4 (0.29%) for prenatal smoking.
Missingness: 27 (1.93%) for planned pregnancy.
Periconception folic acid supplement use was defined by any use of prenatal vitamin or folic acid supplement during gestational weeks 0 to 4. Missingness: 1.
Missingness: 78 (5.57%) for ever breastfeeding.
Birth weight z score standardized by gestational age and sex, using a US national reference.
Table 2. Characteristics of the Subpopulation in Project Viva.
| Characteristic | No. (%) | P valuea | ||||
|---|---|---|---|---|---|---|
| Subpopulation (n = 898) | First quartile (n = 225) | Second quartile (n = 225) | Third quartile (n = 224) | Fourth quartile (n = 224) | ||
| Plasma folate concentration range, ng/mL | 7.08-359.80 | 7.08-14.01 | 14.01-18.92 | 18.92-28.48 | 28.48-359.80 | |
| Dietary folate intake range, μg/d | 128.7-2874.3 | 128.7-676.1 | 676.1-941.9 | 941.9-1194.3 | 1194.3-2874.3 | |
| Maternal age, mean (SD), y | 32.68 (4.62) | 32.25 (5.57) | 32.91 (4.23) | 32.71 (4.46) | 32.86 (4.06) | .42 |
| Prepregnancy BMI, mean (SD) | 24.76 (5.25) | 25.37 (6.13) | 24.39 (5.21) | 24.71 (5.14) | 24.59 (4.35) | .22 |
| AHEI-P score, mean (SD)b | 61.10 (10.28) | 59.91 (10.93) | 60.72 (10.19) | 61.96 (10.03) | 61.81 (9.86) | .11 |
| Maternal race and ethnicityc | ||||||
| Asian | 39 (4.3) | 15 (6.7) | 8 (3.6) | 8 (3.6) | 8 (3.6) | .05 |
| Black | 86 (9.6) | 28 (12.4) | 25 (11.1) | 22 (9.8) | 11 (4.9) | |
| Hispanic | 57 (6.3) | 18 (8.0) | 11 (4.9) | 14 (6.2) | 14 (6.2) | |
| White | 678 (75.5) | 150 (66.7) | 173 (76.9) | 175 (78.1) | 180 (80.4) | |
| Other | 38 (4.2) | 14 (6.2) | 8 (3.6) | 5 (2.2) | 11 (4.9) | |
| Maternal education ≥ college degree | 663 (73.8) | 154 (68.4) | 164 (72.9) | 170 (75.9) | 175 (78.1) | .11 |
| Paternal education ≥ college degreed | 596 (66.4) | 131 (58.2) | 151 (67.1) | 155 (69.2) | 159 (71.0) | .006 |
| Annual household income >$70 000 | 580 (64.6) | 138 (61.3) | 145 (64.4) | 156 (69.6) | 141 (62.9) | .07 |
| Prenatal smoking | ||||||
| Never | 611 (68.0) | 151 (67.1) | 152 (67.6) | 147 (65.6) | 161 (71.9) | .19 |
| Former | 194 (21.6) | 45 (20.0) | 50 (22.2) | 60 (26.8) | 39 (17.4) | |
| Early pregnancy | 93 (10.4) | 29 (12.9) | 23 (10.2) | 17 (7.6) | 24 (10.7) | |
| Nulliparous | 431 (48.0) | 103 (45.8) | 99 (44.0) | 113 (50.4) | 116 (51.8) | .29 |
| Planned pregnancye | 580 (64.6) | 142 (63.1) | 142 (63.1) | 140 (62.5) | 156 (69.6) | .27 |
| Infertility | 210 (23.4) | 51 (22.7) | 55 (24.4) | 50 (22.3) | 54 (24.1) | .94 |
| Periconception folic acid supplement usef | 629 (70.0) | 132 (58.7) | 152 (67.6) | 169 (75.4) | 176 (78.6) | <.001 |
| Ever breastfeedingg | 381 (42.4) | 103 (45.8) | 100 (44.4) | 92 (41.1) | 86 (38.4) | .22 |
| Female infant | 437 (48.7) | 113 (50.2) | 108 (48.0) | 115 (51.3) | 101 (45.1) | .56 |
| Gestational age, mean (SD), wk | 39.55 (1.79) | 39.40 (1.88) | 39.49 (1.84) | 39.50 (2.01) | 39.81 (1.35) | .09 |
| Birth weight, mean (SD), g | 3519.5 (559.2) | 3451.6 (585.6) | 3499.5 (573.0) | 3513.2 (592.6) | 3613.9 (467.1) | .02 |
| Birth weight z score, mean (SD)h | 0.26 (0.95) | 0.17 (0.99) | 0.22 (0.93) | 0.27 (0.95) | 0.37 (0.90) | .14 |
| Preterm birth | 56 (6.2) | 24 (10.7) | 12 (5.3) | 15 (6.7) | 5 (2.2) | .003 |
| Low birth weight | 34 (3.8) | 14 (6.2) | 10 (4.4) | 9 (4.0) | 1 (0.4) | .01 |
Abbreviations: AHEI-P, Alternative Healthy Eating Index in Pregnancy; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
SI conversion: To convert plasma folate to millimoles per liter, multiply by 2.266.
P values obtained from χ2 tests for categorical variables and t tests for continuous variables across folate groups.
Higher AHEI-P score means better diet quality.
Race and ethnicity were self-reported in the questionnaire. American Indian or Alaskan Native and other were collapsed into a single category to balance sample size.
Missingness: 53 (5.90%) for paternal education.
Missingness: 14 (1.56%) for planned pregnancy.
Periconception folic acid supplement use was defined by any use of prenatal vitamin or folic acid supplement during gestational weeks 0 to 4. Missingness: 1.
Missingness: 17 (1.89%) for ever breastfeeding.
Birth weight z score standardized by gestational age and sex, using a US national reference.
Across DFE groups, mothers in the lowest quartile were more racially and ethnically diverse, less educated, reported lower annual household income, were more likely to smoke during pregnancy, and were less likely to be nulliparous, plan for this pregnancy, have infertility, and use periconception folic acid supplements, compared with the other 3 DFE quartile groups (Table 1). However, population characteristics were similar across quartile groups by pregnancy plasma folate concentrations, except that the lowest plasma quartile group had a higher proportion of mothers who were Black and of nonusers of periconception folic acid supplement than the other 3 groups (Table 2).
PFAS and Folate Distributions
The 6 PFAS compounds were detected in more than 99% of the samples. Distributions of plasma concentrations of the 6 PFAS compounds were similar in the study population and the subpopulation with plasma folate measurements and were similar across folate groups (DFE or plasma) (eTable 2 and eTable 3 in Supplement 1). The median pregnancy DFE intake was 936.2 (IQR, 660.4-1187.3) μg/d, and the median plasma folate concentration was 18.92 (IQR, 14.01-28.48) ng/mL.
Correlation coefficients for the 6 PFAS compounds ranged between 0.18 (PFNA and EtFOSAA) and 0.74 (PFOA and PFOS). Dietary folate intake had a low correlation (r = 0.24) with plasma folate (eFigure 3 in Supplement 1). Negative correlations with DFE were noted for MeFOSAA (r = −0.10) and EtFOSAA (r = −0.12), and no other correlations were found (eFigure 3 in Supplement 1).
Associations of PFAS With Birth Outcomes Across Folate Groups
Early pregnancy plasma PFOA, PFOS, and PFNA concentrations were associated with lower birth weight only among mothers in the lowest quartile of plasma folate concentrations (PFOA: −87.03 g; 95% CI, −180.11 to 6.05 g; PFOS: −100.23 g; 95% CI, −199.06 to −1.40 g; PFNA: −91.34 g; 95% CI, −186.84 to 4.15 g), and no associations were found for the other plasma folate quartile groups (PFOA: P = .54, PFOS: P = .60, PFNA: P = .20 for heterogeneity) (Figure 1). Higher plasma PFOA concentrations were associated with lower birth weight among mothers with the lowest DFE intake (β, −89.13 g; 95% CI, −166.84 to −11.42 g), but not among the other 3 DFE quartile groups (P = .49 for heterogeneity) (Figure 1). No associations with birth weight were found for the remaining PFAS compounds or PFAS mixture among either the DFE or plasma folate groups (eTable 4 in Supplement 1).
Figure 1. Associations of Early Pregnancy Plasma Polyfluoroalkyl and Polyfluoroalkyl Substances Concentrations With Birth Weight by Quartiles of Early Pregnancy Dietary Folate Intake (DFE) or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva.
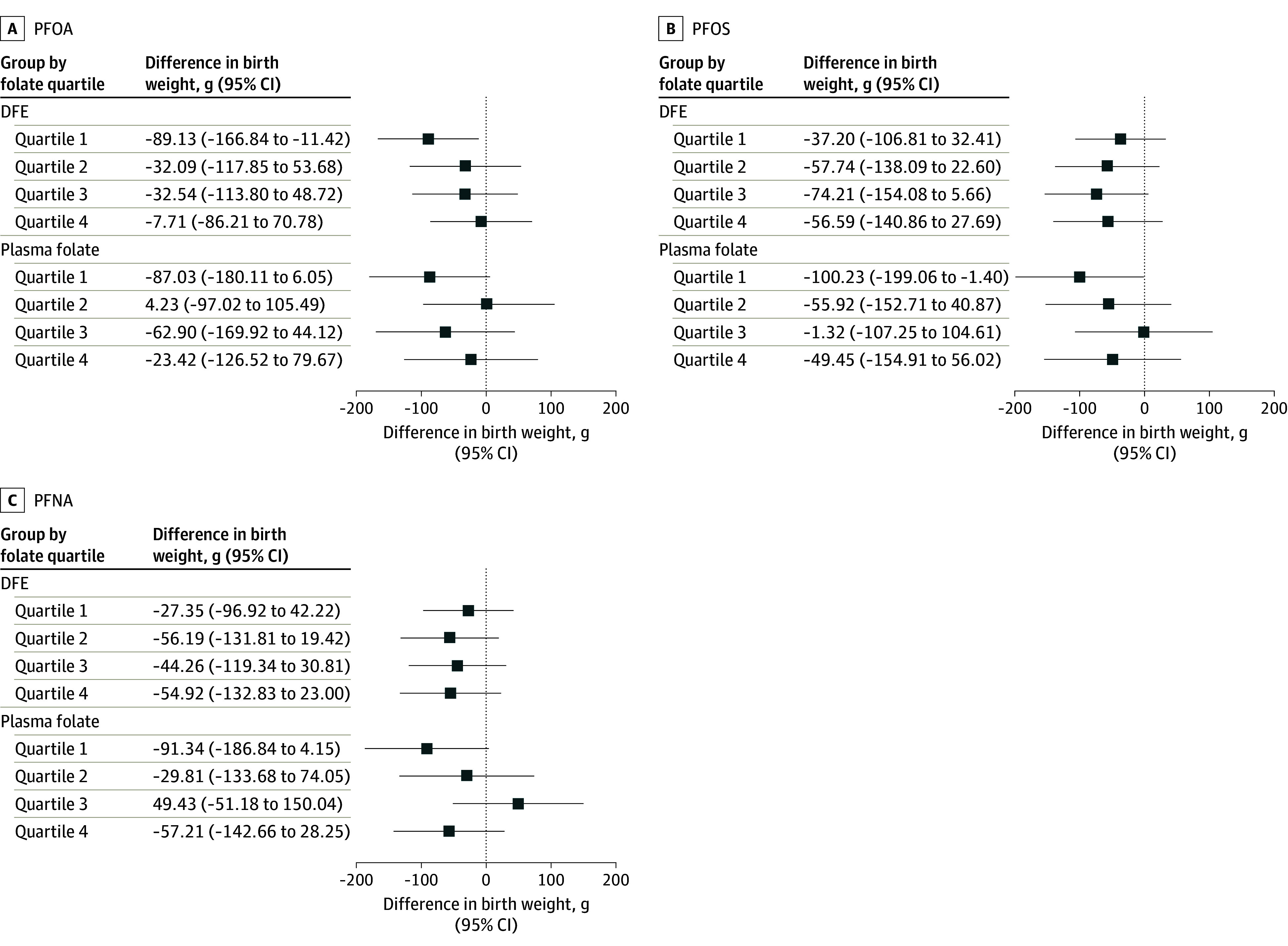
Models were adjusted for maternal age (continuous), educational level (≥college graduate vs not college graduate), race and ethnicity (Asian, Black, Hispanic, White, other), prepregnancy body mass index (continuous), smoking history (never, former, early pregnancy), nulliparous (yes vs no), breastfeeding history (yes vs no), paternal educational level (≥college graduate vs not college graduate), annual household income (>$70 000 vs ≤$70 000), infertility (yes vs no), planned pregnancy (yes vs no), infant sex (male vs female), gestational age at recruitment (continuous), and Alternative Healthy Eating Index in Pregnancy score in early pregnancy (continuous). PFNA indicates perfluorononanoate; PFOA, perfluorooctanoic acid; and PFOS, perfluorooctane sulfonic acid.
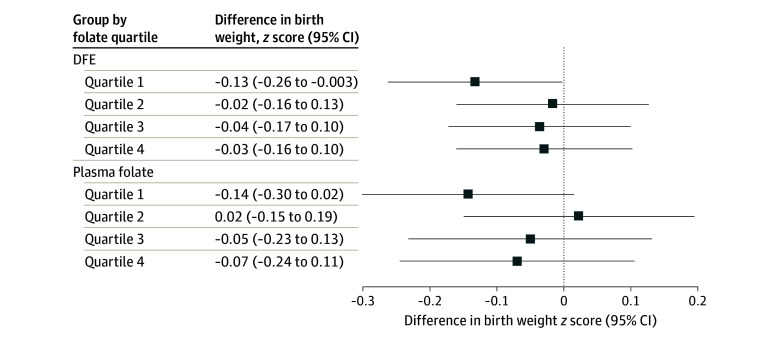
Consistent with birth weight findings, plasma PFOA concentration was associated with a lower birth weight z score among the lowest DFE quartile (β, −0.13; 95% CI, −0.26 to −0.003) and the lowest plasma folate quartile groups (β, −0.14; 95% CI, −0.30 to 0.02), but not in the higher quartile groups (DFE: P = .57, plasma folate: P = .55 for heterogeneity) (Figure 2). The remaining PFAS compounds or PFAS mixture were not associated with birth weight z scores (eTable 5 in Supplement 1).
Figure 2. Associations of Early Pregnancy Plasma Perfluorooctanoic Acid Concentrations With Birth Weight z Score (Standardized by Gestational Age and Sex) by Quartiles of Early Pregnancy Dietary Folate Equivalent (DFE) Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva.
Models were adjusted for maternal age (continuous), educational level (≥college graduate vs not college graduate), race and ethnicity (Asian, Black, Hispanic, White, other), prepregnancy body mass index (continuous), smoking history (never, former, early pregnancy), nulliparous (yes vs no), breastfeeding history (yes vs no), paternal educational level (≥college graduate vs not college graduate), annual household income (>$70 000 vs ≤$70 000), infertility (yes vs no), planned pregnancy (yes vs no), infant sex (male vs female), gestational age at recruitment (continuous), and Alternative Healthy Eating Index in Pregnancy score in early pregnancy (continuous).
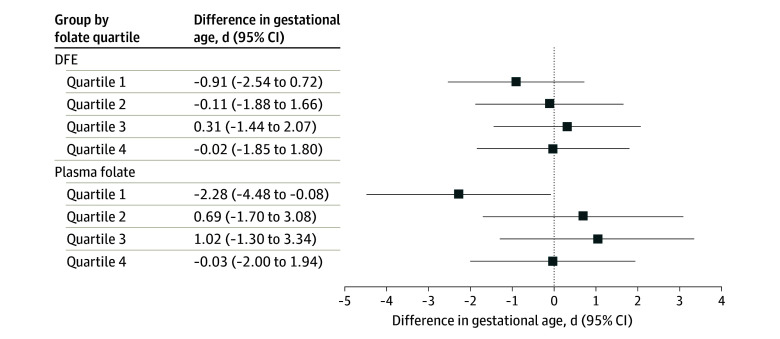
Similarly, PFNA concentration was associated with lower gestational age among mothers in the lowest plasma folate quartile (β, −2.28 days; 95% CI, −4.48 to −0.08 days), while no associations were found for higher quartiles (P = .13 for heterogeneity) (Figure 3). No associations with gestational age were found for the remaining PFAS compounds or PFAS mixture in any of the other folate groups examined (eTable 6 in Supplement 1).
Figure 3. Associations of Early Pregnancy Plasma Perfluorononanoate Concentrations With Gestational Age by Quartiles of Early Pregnancy Dietary Folate Equivalent (DFE) Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva.
Models were adjusted for maternal age (continuous), educational level (≥college graduate vs not college graduate), race and ethnicity (Asian, Black, Hispanic, White, other), prepregnancy body mass index (continuous), smoking history (never, former, early pregnancy), nulliparous (yes vs no), breastfeeding history (yes vs no), paternal educational level (≥college graduate vs not college graduate), annual household income (>$70 000 vs ≤$70 000), infertility (yes vs no), planned pregnancy (yes vs no), infant sex (male vs female), gestational age at recruitment (continuous), and Alternative Healthy Eating Index in Pregnancy score in early pregnancy (continuous).
Supplemental Analyses
Plasma PFOS concentration was associated with a higher risk of low birth weight among mothers in the lowest plasma folate quartile (odds ratio, 2.46; 95% CI, 1.05-5.80; P = .15 for heterogeneity) (eTable 7 in Supplement 1). There was no association between PFAS concentrations and preterm birth in any of the folate groups examined (eTable 7 and eTable 8 in Supplement 1).
Restricting the DFE analyses to the subpopulation in which we quantified plasma folate concentrations did not materially change the results from the total study population (eTable 9 in Supplement 1). Further adjusting for early pregnancy fish consumption only slightly attenuated the estimates (eTable 10 and eTable 11 in Supplement 1). The generalized additive models showed consistent modification by folate status with findings from primary analyses (eFigures 4-9 and eResults 2 in Supplement 1). Results obtained from single-value imputation were similar to those using MICE (eTables 12-16 in Supplement 1).
Discussion
In this large, prospective prebirth cohort, we found that early pregnancy exposure to select PFAS compounds was associated with lower birth weight, birth weight z score, and gestational age, and higher risk of low birth weight only among mothers whose prenatal dietary folate intake or plasma folate concentrations were in the lowest quartile range (cutoff: <660 μg/d for dietary folate intake, <14 ng/mL for plasma folate), but not among mothers with higher dietary folate intake or plasma folate concentrations in early pregnancy. No consistent associations were found between PFAS exposure and risk of preterm birth or between PFAS mixture exposure and any of the birth outcomes in any of the folate groups.
Our findings of early pregnancy PFAS exposure and lower birth weight, lower gestational age, and higher risk of low birth weight among women with the lowest folate status are consistent with findings from several birth cohorts.42,47,48,49,50,51,52 A previous study with 1645 mothers from Project Viva reported inverse associations between early pregnancy plasma PFNA concentration and birth weight z score and gestational age in the total population, but the study did not examine birth weight as an outcome.11 In the present study, the associations between PFNA and lower gestational age were only seen in the lowest quartile group of plasma folate concentrations. A lower birth weight z score was associated with PFOA instead of PFNA only among mothers whose early pregnancy folate status was in the lowest quartile range. We additionally found associations of prenatal PFOA, PFOS, and PFNA concentrations with lower birth weight, and of PFOS with higher risk of low birth weight only in mothers with the lowest folate status. The early pregnancy plasma PFAS concentrations in our study population were similar to those in women of reproductive age in the general US population during similar time periods.53
To our knowledge, this is the first study to examine prenatal folate status as a modifiable factor for the associations between prenatal PFAS exposure and birth outcomes. We examined folate as both dietary intake and as a plasma biomarker, representing complementary folate assessments. The early pregnancy dietary folate intake crudely reflected folate intake from the last menstrual period up to the first trimester,54 which may not be representative of folate status over the entire pregnancy due to changes in food preferences in very early pregnancy. In contrast, plasma folate concentrations measure the internal dose of folate in circulation within a short time frame (days) before the time of sample collection in early pregnancy,55 when 86% of the Project Viva population reported using prenatal vitamin or folic acid supplements. Mothers in the lowest dietary folate quartile generally had lower socioeconomic status (SES) compared with mothers with higher folate status. However, SES in general was similar across quartile groups by plasma folate concentrations. Drawing similar conclusions from the 2 different folate measurements showing the PFAS associations with adverse birth outcomes were only found in mothers whose dietary folate intake or plasma folate levels were lower than the 25th percentiles supported our interpretation that these findings were not primarily due to residual confounding by SES. Furthermore, models were adjusted for parental educational level and household income. These results support our hypothesis that higher prenatal folate status could reduce or counteract PFAS exposure in birth outcomes. A recent study reported that higher PFAS concentrations were associated with lower antibody levels to rubella and mumps among adolescents with lower red blood cell folate levels but not in adolescents with higher folate levels in the US general population.17 In addition to neural tube defect prevention, these cumulative findings suggest that adequate folate status may counteract PFAS adverse effects and possibly the adverse effects associated with other environmental pollutants, as reported in experimental research.56,57,58
The mechanisms by which folate interacts with PFAS in birth outcomes are unclear and need further experimental investigation. Folate plays an important role in DNA synthesis and methylation, both critical to fetal growth.59,60 Sufficient folate supply in pregnancy has been associated with a decreased risk of low birth weight and small for gestational age.61,62 Evidence also shows that both folate and PFAS are substrates for several shared transporters in the human placenta, specifically the organic anion transporter family22,63; solute carrier family, such as folate receptor α; and the adenosine triphosphate–binding cassette family, including the breast cancer resistance protein and P-glycoprotein.20,21,64,65,66 Folate might compete with PFAS on shared transporters and thus reduce the placental transport of PFAS to the fetus. Furthermore, prenatal PFAS exposure is related to epigenetic changes in cord blood.67 Sufficient folate status might counteract PFAS-related epigenetic changes through its critical methyl-donor role in DNA methylation, thus mitigating the deleterious health effects.68,69,70
Strengths and Limitations
This study examined prenatal folate as a modifiable factor for the associations between PFAS exposure and birth outcomes. The first strength of the study is its relatively large sample size, permitting stratified analyses by folate status. Second, PFAS concentrations were measured in plasma samples collected in early pregnancy, precluding potential confounding by hemodynamic changes occurring in late pregnancy.11 Third, dietary intake and plasma folate concentrations were both used to facilitate comparison and interpretation.
The study has limitations. Although both dietary and plasma folate status were evaluated, data on red blood cell folate, which reflects medium- to long-term folate intake, were not available.55 Mothers in Project Viva were folate replete; the 25th percentiles of the sample had dietary folate intake and plasma folate concentration above the recommended levels for neural tube defect prevention (dietary folate intake: 660 μg/d in Project Viva vs 600 μg/d in recommendations; plasma folate concentration: 14 ng/mL in Project Viva vs 11.26 ng/mL in recommendations).18,71 Further studies are needed to replicate the present findings in other populations with lower prenatal folate intake. Multiple comparisons were conducted that could result in inflated type I error. However, tests were based on prior hypotheses and consistent patterns of heterogeneity by folate status were identified that were unlikely due to chance. Dietary folate intake measured by food frequency questionnaires could have nondifferential measurement errors. However, food frequency questionnaires perform well in measuring the relative dietary intake (ie, ranks) in the population.54 While covariate adjustment included overall diet quality, residual confounding by diet cannot be ruled out. Because of the observational nature of this cohort study, causal effects of folate status on PFAS-related birth outcomes cannot be inferred. Selection bias is also possible given the differences in SES factors in the study sample with the original population in the Project Viva cohort. However, differences were subtle and several SES factors were included as covariates in the models. In addition, because of the complexity of the data analyses, we did not further examine sex-specific heterogeneities in the infants or nonlinear associations.
Conclusions
In this prebirth cohort study, higher early pregnancy PFAS concentrations were associated with lower birth weight, birth weight z score, and gestational age, and higher risk of low birth weight only among mothers whose early pregnancy dietary folate intake or plasma folate levels were below the 25th percentiles. Findings suggest that mothers with the lowest folate status were more susceptible to PFAS-related adverse birth outcomes. Folate might be used as a PFAS prevention measure during preconception and pregnancy. If confirmed in other settings, the findings may have important implications for identifying vulnerable populations and implementing intervention studies.
eTable 1. Characteristics Across the Original Project Viva Cohort (N=2128), the Study Population (N=1400), and the Subpopulation (n=898)
eTable 2. Distributions of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances in the Study Population and by Quartiles of Early Pregnancy Dietary Folate Intake Among Mother-Singleton Pairs in Project Viva
eTable 3. Distributions of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances in the Study Population and by Quartiles of Early Pregnancy Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 4. Differences (95% CI) in Birthweight (Grams) per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 5. Differences (95% CI) in Birthweight z Score per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 6. Differences (95% CI) in Gestational Age per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 7. Odds Ratios (95% CI) for Low Birthweight per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 8. Odds Ratios (95% CI) for Preterm Birth per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 9. Associations Between Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Birth Outcomes Across Quartile Groups by Early Pregnancy Dietary Folate Intake in the Subpopulation
eTable 10. Associations Between Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Birth Outcomes Across Quartile Groups by Early Pregnancy Dietary Folate Intake, Further Adjusting for Average Fish Consumption in Early Pregnancy
eTable 11. Associations Between Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Birth Outcomes Across Quartile Groups by Early Pregnancy Plasma Folate Concentrations, Further Adjusting for Average Fish Consumption in Early Pregnancy
eTable 12. Differences (95% CI) in Birthweight (Grams) per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 13. Differences (95% CI) in Birthweight z Score per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 14. Differences (95% CI) in Gestational Age (Days) per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 15. Odds Ratios (95% CI) for Low Birthweight per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 16. Odds Ratios (95% CI) for Preterm Birth per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eFigure 1. Participants’ Inclusion Diagram of Mother-Singleton Pairs in Project Viva
eFigure 2. Directed Acyclic Graph Under the Null Hypothesis of PFAS-Birth Outcome Associations
eFigure 3. Spearman Correlation Coefficient Matrix for Early Pregnancy Dietary Folate Intake and Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Folate Among Mother-Singleton Pairs in Project Viva
eFigure 4. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight Across Early Pregnancy Dietary Folate Equivalent Intake
eFigure 5. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight Across Early Pregnancy Plasma Folate Concentrations
eFigure 6. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight z Score Across Early Pregnancy Dietary Folate Equivalent Intake
eFigure 7. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight z Score Across Early Pregnancy Plasma Folate Concentrations
eFigure 8. Changes in Log (Odds Ratio) for Low Birthweight in Relation to Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Early Pregnancy Plasma Folate Concentrations
eFigure 9. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Gestational Age Across Early Pregnancy Plasma Folate Concentrations
eMethods. Details on Analyses and Models
eResults. Details on Comparisons and Models
Data Sharing Statement
References
- 1.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019;29(2):131-147. doi: 10.1038/s41370-018-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glynn A, Benskin J, Lignell S, et al. Temporal trends of perfluoroalkyl substances in pooled serum samples from first-time mothers in Uppsala 1997-2014. September 7, 2015. Accessed August 26, 2022. https://www.diva-portal.org/smash/get/diva2:856854/FULLTEXT01.pdf
- 3.Bjerregaard-Olesen C, Bach CC, Long M, et al. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008-2013. Environ Int. 2016;91:14-21. doi: 10.1016/j.envint.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Land M, De Wit CA, Bignert A, et al. What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? a systematic review. Environ Evid. 2018;7(1):1-32. doi: 10.1186/s13750-017-0114-y [DOI] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Fourth National Report on Human Exposure to Environmental Chemicals. February 2015. Accessed August 26, 2022. https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_feb2015.pdf
- 6.Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the US. population: 1999-2008. Environ Sci Technol. 2011;45(19):8037-8045. doi: 10.1021/es1043613 [DOI] [PubMed] [Google Scholar]
- 7.Fenton SE, Ducatman A, Boobis A, et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. 2021;40(3):606-630. doi: 10.1002/etc.4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeWitt JC, Blossom SJ, Schaider LA. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol. 2019;29(2):148-156. doi: 10.1038/s41370-018-0097-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake BE, Fenton SE. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: a review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology. 2020;443:152565. doi: 10.1016/j.tox.2020.152565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apelberg BJ, Witter FR, Herbstman JB, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115(11):1670-1676. doi: 10.1289/ehp.10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagiv SK, Rifas-Shiman SL, Fleisch AF, et al. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in project viva: confounded by pregnancy hemodynamics? Am J Epidemiol. 2018;187(4):793-802. doi: 10.1093/aje/kwx332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environ Health Perspect. 2013;121(10):1207-1213. doi: 10.1289/ehp.1206372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starling AP, Adgate JL, Hamman RF, et al. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the healthy start study. Environ Health Perspect. 2017;125(6):067016. doi: 10.1289/EHP641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health. 2018;15(9):1832. doi: 10.3390/ijerph15091832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardener H, Sun Q, Grandjean P. PFAS concentration during pregnancy in relation to cardiometabolic health and birth outcomes. Environ Res. 2021;192:110287. doi: 10.1016/j.envres.2020.110287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Mustieles V, Wang Y, et al. Dietary intake and blood concentrations of folate and folic acid in relation to serum per- and polyfluoroalkyl substances (PFAS) concentrations. Presented at: International Society for Environmental Epidemiology (ISEE) Annual Meeting. 2021. Accessed April 28, 2023. https://ehp.niehs.nih.gov/doi/10.1289/isee.2021.P-618
- 17.Zhang Y, Mustieles V, Wang YX, et al. Red blood cell folate modifies the association between serum per- and polyfluoroalkyl substances and antibody concentrations in US adolescents. Environ Sci Technol. 2023;57(6):2445-2456. doi: 10.1021/acs.est.2c07152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . Folic acid recommendations. Accessed July 12, 2022. https://www.cdc.gov/ncbddd/folicacid/recommendations.html
- 19.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3(1):21-38. doi: 10.3945/an.111.000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Sun X, Xu J, Tan H, Zeng EY, Chen D. Transplacental transfer of environmental chemicals: roles of molecular descriptors and placental transporters. Environ Sci Technol. 2021;55(1):519-528. doi: 10.1021/acs.est.0c06778 [DOI] [PubMed] [Google Scholar]
- 21.Shulpekova Y, Nechaev V, Kardasheva S, et al. The concept of folic acid in health and disease. Molecules. 2021;26(12):3731. doi: 10.3390/molecules26123731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kummu M, Sieppi E, Koponen J, et al. Organic anion transporter 4 (OAT 4) modifies placental transfer of perfluorinated alkyl acids PFOS and PFOA in human placental ex vivo perfusion system. Placenta. 2015;36(10):1185-1191. doi: 10.1016/j.placenta.2015.07.119 [DOI] [PubMed] [Google Scholar]
- 23.Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403-456. doi: 10.1016/S0083-6729(03)01012-4 [DOI] [PubMed] [Google Scholar]
- 24.Ducatman A, Luster M, Fletcher T. Perfluoroalkyl substance excretion: effects of organic anion-inhibiting and resin-binding drugs in a community setting. Environ Toxicol Pharmacol. 2021;85:103650. doi: 10.1016/j.etap.2021.103650 [DOI] [PubMed] [Google Scholar]
- 25.Worley RR, Yang X, Fisher J. Physiologically based pharmacokinetic modeling of human exposure to perfluorooctanoic acid suggests historical non drinking-water exposures are important for predicting current serum concentrations. Toxicol Appl Pharmacol. 2017;330:9-21. doi: 10.1016/j.taap.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang CH, Glover KP, Han X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci. 2010;117(2):294-302. doi: 10.1093/toxsci/kfq219 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol. 2013;47(18):10619-10627. doi: 10.1021/es401905e [DOI] [PubMed] [Google Scholar]
- 28.Bangma J, Guillette TC, Bommarito PA, et al. Understanding the dynamics of physiological changes, protein expression, and PFAS in wildlife. Environ Int. 2022;159:107037. doi: 10.1016/j.envint.2021.107037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44(1):37-48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuklenyik P, Baker SE, Bishop AM, Morales-A P, Calafat AM. On-line solid phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry approach to quantify N,N-diethyl-m-toluamide and oxidative metabolites in urine. Anal Chim Acta. 2013;787:267-273. doi: 10.1016/j.aca.2013.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218(15):2133-2137. doi: 10.1016/j.chroma.2010.10.051 [DOI] [PubMed] [Google Scholar]
- 32.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46-51. doi: 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- 33.Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691-1696. doi: 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14(10):754-762. doi: 10.1016/j.annepidem.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 35.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 36.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4)(suppl):1220S-1228S. doi: 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 37.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soria-Contreras DC, Oken E, Tellez-Rojo MM, Rifas-Shiman SL, Perng W, Chavarro JE. History of infertility and long-term weight, body composition, and blood pressure among women in Project Viva. Ann Epidemiol. 2022;74:43-50. doi: 10.1016/j.annepidem.2022.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Practice Committee of the American Society for Reproductive Medicine . Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2020;113(3):533-535. doi: 10.1016/j.fertnstert.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 40.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261-1271. doi: 10.1093/ajcn/76.6.1261 [DOI] [PubMed] [Google Scholar]
- 41.Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109(6):1004-1011. doi: 10.1016/j.jada.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int. 2017;108:278-284. doi: 10.1016/j.envint.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 43.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: a prospective investigation among US women. Environ Health Perspect. 2018;126(3):037001. doi: 10.1289/EHP2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soria-Contreras DC, Perng W, Rifas-Shiman SL, Hivert MF, Oken E, Chavarro JE. History of infertility and pregnancy outcomes in Project Viva: a prospective study. BMC Pregnancy Childbirth. 2022;22(1):549. doi: 10.1186/s12884-022-04885-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4):47004. doi: 10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seshasayee SM, Rifas-Shiman SL, Chavarro JE, et al. Dietary patterns and PFAS plasma concentrations in childhood: Project Viva, USA. Environ Int. 2021;151:106415. doi: 10.1016/j.envint.2021.106415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environ Health Perspect. 2013;121(10):1207-1213. doi: 10.1289/ehp.1206372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health. 2018;15(9):1832. doi: 10.3390/ijerph15091832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao X, Ni W, Zhu S, et al. Per- and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: a systematic review and meta-analysis. Environ Res. 2021;201:111632. doi: 10.1016/j.envres.2021.111632 [DOI] [PubMed] [Google Scholar]
- 50.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677-1682. doi: 10.1289/ehp.10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall SM, Zhang S, Hoffman K, Miranda ML, Stapleton HM. Concentrations of per- and polyfluoroalkyl substances (PFAS) in human placental tissues and associations with birth outcomes. Chemosphere. 2022;295:133873. doi: 10.1016/j.chemosphere.2022.133873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitworth KW, Haug LS, Baird DD, et al. Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2012;175(12):1209-1216. doi: 10.1093/aje/kwr459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect. 2007;115(11):1596-1602. doi: 10.1289/ehp.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willett W. Nutritional Epidemiology. Oxford University Press; 2012. doi: 10.1093/acprof:oso/9780199754038.001.0001 [DOI] [Google Scholar]
- 55.Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of nutrition for development—folate review. J Nutr. 2015;145(7):1636S-1680S. doi: 10.3945/jn.114.206599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Academies of Sciences . Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. National Academies Press; 2022. [PubMed] [Google Scholar]
- 57.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A–induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056-13061. doi: 10.1073/pnas.0703739104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y, Zhang C, Gao XB, et al. Folic acid protects against arsenic-mediated embryo toxicity by up-regulating the expression of Dvr1. Sci Rep. 2015;5(1):16093. doi: 10.1038/srep16093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83(5):993-1016. doi: 10.1093/ajcn/83.5.993 [DOI] [PubMed] [Google Scholar]
- 60.Bailey LB, Gregory JF III. Folate metabolism and requirements. J Nutr. 1999;129(4):779-782. doi: 10.1093/jn/129.4.779 [DOI] [PubMed] [Google Scholar]
- 61.Iyengar L, Rajalakshmi K. Effect of folic acid supplement on birth weights of infants. Am J Obstet Gynecol. 1975;122(3):332-336. doi: 10.1016/0002-9378(75)90179-9 [DOI] [PubMed] [Google Scholar]
- 62.Rolschau J, Date J, Kristoffersen K. Folic acid supplement and intrauterine growth. Acta Obstet Gynecol Scand. 1979;58(4):343-346. doi: 10.3109/00016347909154593 [DOI] [PubMed] [Google Scholar]
- 63.Lu Y, Meng L, Ma D, et al. The occurrence of PFAS in human placenta and their binding abilities to human serum albumin and organic anion transporter 4. Environ Pollut. 2021;273:116460. doi: 10.1016/j.envpol.2021.116460 [DOI] [PubMed] [Google Scholar]
- 64.Dankers AC, Roelofs MJ, Piersma AH, et al. Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro. Toxicol Sci. 2013;136(2):382-391. doi: 10.1093/toxsci/kft198 [DOI] [PubMed] [Google Scholar]
- 65.Li J, Cai D, Chu C, et al. Transplacental transfer of per- and polyfluoroalkyl substances (PFASs): differences between preterm and full-term deliveries and associations with placental transporter mRNA expression. Environ Sci Technol. 2020;54(8):5062-5070. doi: 10.1021/acs.est.0c00829 [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Cheng X, Lei B, Zhang G, Bi Y, Yu Y. A review of the transplacental transfer of persistent halogenated organic pollutants: transfer characteristics, influential factors, and mechanisms. Environ Int. 2021;146:106224. doi: 10.1016/j.envint.2020.106224 [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Eliot MN, Papandonatos GD, et al. Gestational perfluoroalkyl substance exposure and DNA methylation at birth and 12 years of age: a longitudinal epigenome-wide association study. Environ Health Perspect. 2022;130(3):37005. doi: 10.1289/EHP10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20(1):63-68. doi: 10.1016/j.nut.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 69.Pennisi E. Environmental epigenomics meeting: supplements restore gene function via methylation. Science. 2005;310(5755):1761. doi: 10.1126/science.310.5755.1761 [DOI] [PubMed] [Google Scholar]
- 70.Sinclair KD, Allegrucci C, Singh R, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;104(49):19351-19356. doi: 10.1073/pnas.0707258104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen MY, Rose CE, Qi YP, et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr. 2019;109(5):1452-1461. doi: 10.1093/ajcn/nqz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics Across the Original Project Viva Cohort (N=2128), the Study Population (N=1400), and the Subpopulation (n=898)
eTable 2. Distributions of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances in the Study Population and by Quartiles of Early Pregnancy Dietary Folate Intake Among Mother-Singleton Pairs in Project Viva
eTable 3. Distributions of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances in the Study Population and by Quartiles of Early Pregnancy Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 4. Differences (95% CI) in Birthweight (Grams) per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 5. Differences (95% CI) in Birthweight z Score per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 6. Differences (95% CI) in Gestational Age per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 7. Odds Ratios (95% CI) for Low Birthweight per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 8. Odds Ratios (95% CI) for Preterm Birth per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva
eTable 9. Associations Between Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Birth Outcomes Across Quartile Groups by Early Pregnancy Dietary Folate Intake in the Subpopulation
eTable 10. Associations Between Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Birth Outcomes Across Quartile Groups by Early Pregnancy Dietary Folate Intake, Further Adjusting for Average Fish Consumption in Early Pregnancy
eTable 11. Associations Between Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Birth Outcomes Across Quartile Groups by Early Pregnancy Plasma Folate Concentrations, Further Adjusting for Average Fish Consumption in Early Pregnancy
eTable 12. Differences (95% CI) in Birthweight (Grams) per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 13. Differences (95% CI) in Birthweight z Score per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 14. Differences (95% CI) in Gestational Age (Days) per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 15. Odds Ratios (95% CI) for Low Birthweight per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eTable 16. Odds Ratios (95% CI) for Preterm Birth per Doubling of Early Pregnancy Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Quartile Groups by Early Pregnancy Dietary Folate Intake or Plasma Folate Concentrations Among Mother-Singleton Pairs in Project Viva, Using Single-Value Imputation to Account for Missingness
eFigure 1. Participants’ Inclusion Diagram of Mother-Singleton Pairs in Project Viva
eFigure 2. Directed Acyclic Graph Under the Null Hypothesis of PFAS-Birth Outcome Associations
eFigure 3. Spearman Correlation Coefficient Matrix for Early Pregnancy Dietary Folate Intake and Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Folate Among Mother-Singleton Pairs in Project Viva
eFigure 4. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight Across Early Pregnancy Dietary Folate Equivalent Intake
eFigure 5. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight Across Early Pregnancy Plasma Folate Concentrations
eFigure 6. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight z Score Across Early Pregnancy Dietary Folate Equivalent Intake
eFigure 7. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Birthweight z Score Across Early Pregnancy Plasma Folate Concentrations
eFigure 8. Changes in Log (Odds Ratio) for Low Birthweight in Relation to Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances Across Early Pregnancy Plasma Folate Concentrations
eFigure 9. Changes in the Beta Coefficients for the Relationships Between Log-2 Transformed Plasma Concentrations of Per- and Polyfluoroalkyl Substances With Gestational Age Across Early Pregnancy Plasma Folate Concentrations
eMethods. Details on Analyses and Models
eResults. Details on Comparisons and Models
Data Sharing Statement