Abstract
Iberian ibex (Capra pyrenaica) is an ecologically and economically relevant medium-sized emblematic mountain ungulate. Diseases participate in the population dynamics of the species as a regulating agent, but can also threaten the conservation and viability of vulnerable population units. Moreover, Iberian ibex can also be a carrier or even a reservoir of pathogens shared with domestic animals and/or humans, being therefore a concern for livestock and public health. The objective of this review is to compile the currently available knowledge on (1) diseases of Iberian ibex, presented according to their relevance on the health and demography of free-ranging populations; (2) diseases subjected to heath surveillance plans; (3) other diseases reported in the species; and (4) diseases with particular relevance in captive Iberian ibex populations. The systematic review of all the information on diseases affecting the species unveils unpublished reports, scientific communications in meetings, and scientific articles, allowing the first comprehensive compilation of Iberian ibex diseases. This review identifies the gaps in knowledge regarding pathogenesis, immune response, diagnostic methods, treatment, and management of diseases in Iberian ibex, providing a base for future research. Moreover, this challenges wildlife and livestock disease and wildlife population managers to assess the priorities and policies currently implemented in Iberian ibex health surveillance and monitoring and disease management.
Keywords: Disease, Epidemiology, Iberian ibex, Management, Pathogens, Sarcoptic mange
Introduction
Disease can be defined as damage or deterioration affecting normal functions, including responses to extrinsic and intrinsic factors such as nutrition, climate, toxic agents, micro- and macroparasites, and congenital defects, alone or combined (Wobeser 1994). Wildlife can share diseases with humans, known as zoonoses, as well as with domestic animals. Thus, wildlife diseases are relevant for human and domestic livestock health, since wildlife can act as a disease reservoir, but they are also a matter of concern for wildlife conservation, particularly in endangered species or populations (Gortázar et al. 2007, 2016; Cunningham et al. 2017).
Iberian ibex (Capra pyrenaica) is a medium-sized mountain ungulate, formerly endemic to the Iberian Peninsula but recently introduced in the northern Pyrenees in France (Manceau et al. 1999; Granados et al. 2001; Pérez et al. 2002; Acevedo and Cassinello 2009; Alados and Escós 2017; Garnier 2021). Although two of the four subspecies originally described are extinct, most of the Iberian ibex evolutionary significant units and populations are currently expanding and spreading or at least stable (Granados et al. 2001; Pérez et al. 2002; Acevedo and Cassinello 2009; Alados and Escós 2017). However, some ibex populations are vulnerable to risk factors that threaten their viability and conservation, including habitat alteration and fragmentation, inadequate management, overabundance, low genetic diversity, competition with domestic and wild ungulates, human disturbance, and diseases (Manceau et al. 1999; Pérez et al. 2002; Acevedo and Cassinello 2009). On the other hand, the expansion of this species increases the risk of direct or indirect transmission of pathogens with other wild and domestic animals and/or humans (Gortázar et al. 2007, 2016; Lawson et al. 2021).
Research and reviews on wildlife diseases, conservation, and management are required (Illarietti et al. 2023). The objective of this review is to compile the currently available knowledge on diseases of Iberian ibex. The diseases are presented in order of relevance, dealing first with those with proven disease, epizootic, and/or mortality effects on free-ranging Iberian ibex populations (“Infectious diseases causing disease and mortality in Iberian ibex populations” section) and then the diseases included in the Spanish national wildlife health surveillance program (Lawson et al. 2021; Gobierno de España et al. 2022; “Diseases under targeted surveillance in the Iberian ibex” section), followed by other diseases investigated and/or reported in Iberian ibex but with little or unknown effects in population health and demography (“Other diseases” section). Finally, the diseases favored by captivity are specifically mentioned, since captive Iberian ibex populations are subjected to particular conditions and health risks (Granados et al. 1996b; Espinosa et al. 2017c).
Infectious diseases causing symptomatology and mortality with demographic impact in Iberian ibex populations
Sarcoptic mange
Sarcoptic mange is considered an emerging or re-emerging zoonotic disease, caused by the skin-burrowing mite Sarcoptes scabiei. This mite is considered a single species with host taxon-specific varieties, which are morphologically undistinguishable although they have high host specificity and low capacity of cross-infection (Fain 1968, 1978; Pence et al. 1975; Arlian et al. 1984b; Walton et al. 1999; Zahler et al. 1999; Pence and Ueckermann 2002; Rasero et al. 2010). Additionally, within each taxon, geographic differences can also be found (Berrilli et al. 2002).
Despite such specificity, interspecific transmission of S. scabiei has been reported within the same taxon among domestic and wild species both for ungulates and carnivores (Kutzer 1966; Samuel 1981; Ibrahim and Abu-Samra 1987; León-Vizcaíno et al. 1992; Lavín et al. 2000a; Menzano et al. 2007; Valldeperes et al. 2021). Cross-transmission related to predation and scavenging with the establishment of specific predator/scavenger-prey cycles has also been documented (Gakuya et al. 2011; Oleaga et al. 2011; Matsuyama et al. 2019). Being a zoonotic disease, cross-transmission between humans and different animal species also occurs, either directly or mediated through domestic animals, especially pets (Arlian et al. 1984b; Morsy et al. 1994; Mitra et al. 1995; Menzano et al. 2004; Walton and Currie 2007; Rentería Solís et al. 2014; Pisano et al. 2019; Moroni et al. 2022b). Nevertheless, such interspecific transmissions among hosts from different taxa are usually self-limited both in the individual host and in the population and thus do not lead to outbreaks or demographic consequences. On the other hand, although they usually act as dead-end hosts, these temporal host species could be relevant as parasite reservoirs for the original natural host species (Menzano et al. 2004; Rossi et al. 2007).
Sarcoptic mange is an emerging panzootic in wildlife, reported in up to 148 domestic and wild animal mammal species, including humans, causing wildlife population declines and livestock economic losses (Pence and Ueckermann 2002; Walton et al. 2004; Escobar et al. 2022). Among the wild host species, the effect of sarcoptic mange has been dramatic when affecting mountain caprine populations in Eurasia, being considered a health emergency at the wild/domestic caprine interface in Europe (Rossi et al. 2019; Pérez et al. 2021). Particularly, sarcoptic mange has been described in most of the Iberian ibex populations, frequently associated with dramatic demographic declines (Fandos 1991; León-Vizcaíno et al. 1999; Valldeperes et al. 2018a, b).
Etiology
Sarcoptes scabiei has a monoxenous life cycle, developing all the stages in a single host. Once fertilized, females burrow galleries in the host skin and lay up to 50 eggs during their 4–6 weeks lifetime, at a rhythm of 3–4 eggs per day (Walton and Currie 2007; Arlian and Morgan 2017). After three to eight days, hexapodial larvae exit the eggs and burrow into short galleries known as pouches. There they molt consecutively to protonymphs in 3 to 4 days, to tritonymphs in 2 to 3 days, and finally into adults after 2 or 3 days more (Arlian and Vyszenski-Moher 1988; Bornstein et al. 2001). Apparently, less than 1% of the eggs complete this cycle to become adults (Mellanby 1944).
Survival of S. scabiei off the host is limited because the mites cannot maintain their water balance. Mite survival increases with lower temperatures and higher humidity, although rising temperatures up to 35° enhance mite mobility (Mellanby et al. 1942; Arlian et al. 1989). Congelation at − 25 °C and 50% of relative humidity for 90 min killed 100% of S. scabiei var. canis mites (Arlian et al. 1984a). The lifespan of adult females and nymphs doubles that of male mites (Arlian et al. 1989).
Symptomatology, pathogenesis, and immune response
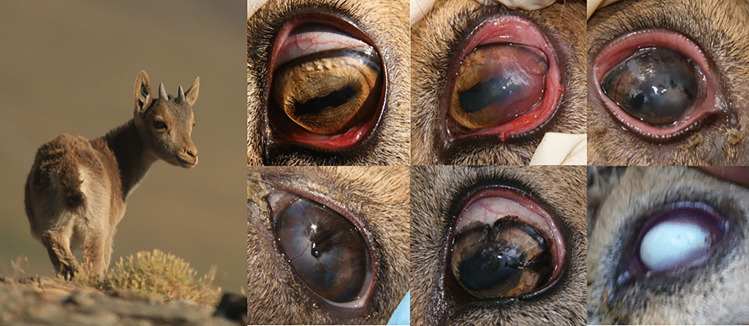
Clinical signs vary depending on the host species and the individual immune status (Pence and Ueckermann 2002), but sarcoptic mange consistently induces skin local inflammatory response directly related to the severity of the disease and the number of mites (Petersen et al. 2004). Clinical signs include pruritus, seborrhoea, erythema, papules, and alopecia, progressing to hyperkeratosis, skin thickening, and crusts when the disease becomes chronic, followed by secondary bacterial infections (Bornstein et al. 2001; Nakagawa et al. 2009; Niedringhaus et al. 2019; Swe et al. 2014; Espinosa et al. 2017d). These secondary bacterial infections are favored by the immunosuppressing action of the mite and the scratching lesions (Mika et al. 2012; Swe et al. 2014), originating deep recurring pyodermas that can evolve to septicaemia (McCarthy et al. 2004; Nakagawa et al. 2009). In Iberian ibex, five categories regarding the extension of body surface affected by lesions compatible with sarcoptic mange have been defined, namely, 0, without apparent lesions; grade I, skin surface affected ≤ 25%; grade II, skin surface affected between 25 and 50%; grade III, skin surface affected between 50 and 75%; and grade IV, skin surface affected > 75% (Pérez et al. 2011a; Ráez-Bravo et al. 2015) (Fig. 1). However, S. scabiei infestation has further multisystemic consequences beyond the local skin lesions (Espinosa et al. 2020), including a reduction in body condition, hematological and serum biochemical disorders (Perez et al. 2015), increase in oxidative stress with a decrease in the antioxidant status (Saleh et al. 2011; Espinosa et al. 2017b; Naesborg-Nielsen et al. 2022), and/or inflammatory acute phase protein response (Ráez-Bravo et al. 2015; Pastor et al. 2019), with amyloid deposits in parenchymal organs (Espinosa et al. 2017d, 2020). All these changes contribute to the pathogenesis of the disease, reducing survival and/or hampering the recovery of ibexes in chronic stages of sarcoptic mange. The intense pruritus and continuous scratching related to the massive release of histamine and other antigenic compounds impair feeding and resting, causing constant stress and energetic imbalance (Bates 1997). This negative energy balance is further worsened by heat loss due to general hair loss (Cross et al. 2016; Simpson et al. 2016; Süld et al. 2017) and feeding impairment by severe lesions in the lips making food apprehension difficult (Abu-Samra et al. 1981; León-Vizcaíno et al. 1999), leading overall to weight loss independently of the availability of resources, which is more severe in males (Carvalho et al. 2015; López-Olvera et al. 2015). The progressive decline of physical condition also affects reproduction (Davies 1995; Sarasa et al. 2011; Espinosa et al. 2017a), skeletal development, and body and horn growth (Serrano et al. 2007; Pérez et al. 2011b).
Fig. 1.

Iberian ibexes affected by sarcoptic mange with skin lesions of different severity. a Female ibex with mild sarcoptic mange (grade I). b Scratching mangy adult male collared for monitoring (left) accompanied by a healthy young male. c Young male ibex with severe sarcoptic mange (grade III–IV). d Young male ibex died because of sarcoptic mange with 100% of the body surface affected (grade IV)
As the pathogenic effects of the mites, host immune response against sarcoptic mange is also both local and systemic. Systemically, S. scabiei infestation induces antibody production, namely, immunoglobulins E and G, although this systemic immune humoral response is correlated with the extension of the lesions, acting as an indicator of inflammation rather than being protective (Lastras et al. 2000; Rodríguez-Cadenas et al. 2010; Pérez et al. 2015; Ráez-Bravo et al. 2016; Naesborg-Nielsen et al. 2022). Similarly, the acute-phase protein response is also correlated with the severity of sarcoptic mange in Iberian ibex (Ráez-Bravo et al. 2015). Although the effects of sarcoptic mange in male Iberian ibex are more severe (López-Olvera et al. 2015), the systemic humoral immune response is more intense in females, either naïve or in reinfestations (Sarasa et al. 2010). Thus, although previous exposition to the mite may induce a certain resistance to reinfection, with a faster and more intense systemic immune response (Van Neste 1986; Arlian et al. 1994a, b; Arlian et al. 1996, 1995; Taringan 2014), in Iberian ibex, this seems to be true for males rather than for females (Sarasa et al. 2010). The systemic cellular immune response consists of an increase in leukocyte count, including an increase in T lymphocytes, neutrophils, and eosinophils in the blood (Pérez et al. 2015).
In the skin, sarcoptic mange induces a local immune cellular response with macrophages and T lymphocytes and, to a lesser extent, B lymphocytes and plasma cells, overall resembling a type I and/or type IV hypersensitivity immune response (Lalli et al. 2004; Salvadori et al. 2016; Bhat et al. 2017; Martínez et al. 2020; Naesborg-Nielsen et al. 2022). The predominant cellular type and the immune response pathway and genes activated by the mite seem to determine the clinical outcome of scabietic humans and animals. Consequently, in wildlife species, the local immune cellular response can determine the probability of individual survival and the population demographic impact. Overall, the individuals developing a less intense but more efficient local skin inflammatory cellular response have more probability of developing mild clinical mange, preventing the progression to severer disease (Bhat et al. 2017; Ráez-Bravo 2019). Immunosuppressant effects by the mite through the downregulation of the immune response pathways and genes to facilitate the colonization of the skin have been suggested (Lalli et al. 2004; Mika et al. 2012; Ráez-Bravo 2019). The trade-off between the immunosuppressing action of the mite and the local and systemic immune response of the hosts determines the distribution and abundance of mites in the affected skin, which is not homogeneous (Castro et al. 2016; Arlian and Morgan 2017); the number of mites carried by a host; the proportion of skin surface affected; the severity of the disease; and, consequently, the clinical outcome (Pérez et al. 2011a; Ráez-Bravo 2019).
Diagnosis
There is not yet a diagnostic method for sarcoptic mange with the ideal sensitivity and specificity (Chandler and Fuller 2019; Pérez et al. 2021). While visual diagnosis is the most basic, extended, and long-term used method for sarcoptic mange monitoring on the field and has therefore become the reference (Pérez et al. 2011a) (Fig. 1), both false negatives and false positives occur (Valldeperes et al. 2019; Pérez et al. 2021). Such limitations led to the recommendation of completing visual diagnosis on the field with the detection of mites and/or eggs in skin scrapings or biopsies digested with potassium hydroxide (Pérez et al. 2011a; Valldeperes et al. 2019), unveiling an 87% sensitivity and a 61% specificity for visual diagnosis on the field (Valldeperes et al. 2019). However, this method requires capturing and handling the ibex or retrieving the carcass for sampling, and while 100% specific, it can also produce false negatives (Rambozzi et al. 2004; Walton and Currie 2007; Walter et al. 2011).
Further advances in sarcoptic mange diagnosis have been proposed to overcome limitations and improve sensitivity, specificity, and/or applicability on the field, including (1) molecular techniques to detect genetic material of S. scabiei through polymerase chain reaction (PCR) (Mounsey et al. 2012; Alasaad et al. 2015), which allows phylogenetic analyses (Ueda et al. 2019; Moroni et al. 2021); (2) detection of the immunoglobulin G generated by the host species after contact with S. scabiei (Puigdemont et al. 2002; Rambozzi et al. 2004; Bornstein et al. 2006; Casais et al. 2007; Oleaga et al. 2008; Millán et al. 2012; Haas et al. 2015a; Ráez-Bravo et al. 2016), which allows retrospective epidemiologic studies (Haas et al. 2018); (3) camera-trapping, useful for detecting mange in naïve or suspected areas reducing surveillance effort but unreliable to assess prevalence and with the same probability of false negatives and positives as visual diagnosis (Oleaga et al. 2011; Haas et al. 2015b; Brewster et al. 2017; Carricondo Sánchez et al. 2017; Saito and Sonoda 2017); (4) thermal imaging, based in the increased body heat radiation of mangy individuals due to hair loss and skin inflammation, with low sensitivity beyond a distance of 100 m and therefore mostly useful only with handled individuals (Cross et al. 2016; Arenas et al. 2002; Granados et al. 2011), where other reliable specific and sensitive alternatives exist; and (5) trained dogs, which have successfully detected dead chamois or severely affected by sarcoptic mange in the Alps, apparently with high sensitivity and 100% specificity (Alasaad et al. 2012). However, none of these diagnostic methodologies have been either validated or standardized yet.
Finally, the non-specific increases of acute-phase proteins and oxidative stress induced by sarcoptic mange have been proposed to diagnose and monitor sarcoptic mange in wildlife (Rahman et al. 2010; Ráez-Bravo et al. 2015; Espinosa et al. 2017b). However, these indicators can also increase for other disorders (Pastor et al. 2019) and therefore cannot be considered diagnostic for an individual, although they can be helpful to monitor wildlife populations where sarcoptic mange is the only or the main disease circulating.
Epidemiology
Direct contact between infested and naïve hosts is the main transmission route of S. scabiei. The individuals with chronic lesions and higher percentages of affected skin carrying more mites are the main source of infestation for their conspecifics (Arlian and Vyszensky-Moher 1988; Pérez et al. 2011a). Nevertheless, indirect transmission through fomites also exists. Sarcoptic mange epidemiology is further determined by both intrinsic factors, such as host sex, age, body condition, and social behavior (González-Candela and León-Vizcaíno 1999; Rahbari et al. 2009; Carvalho et al. 2015; López-Olvera et al. 2015), and extrinsic factors, such as temperature, relative humidity, and seasonality (Pérez et al. 1997a; Rahbari et al. 2009; Pérez et al. 2011a, 2017; Iacopelli et al. 2020; Martín-Monedero et al. 2022; Pérez et al. 2022).
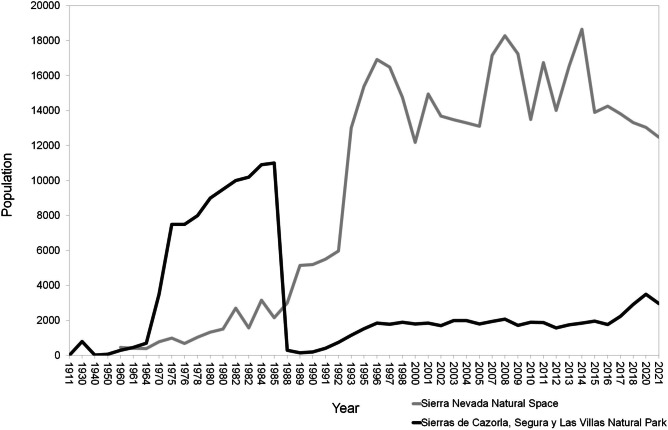
Beyond these particular determinants, in naïve free-ranging populations of wild mountain ungulates, the entry of S. scabiei produces an epizootic of disease that spreads to affect the whole population in a variable number of years (Fernández-Morán et al. 1997; Pérez et al. 1997a; León-Vizcaíno et al. 1999; Rossi et al. 2007; Turchetto et al. 2014; Valldeperes et al. 2018a, b; Obber et al. 2022; Sosa et al. 2022). Such outbreaks are usually associated with variable mortality rates until sarcoptic mange becomes enzootic and the population eventually starts recovering. Nevertheless, the affected populations do not always reach the densities existing before the epizootics, which are also conditioned by other factors such as population abundance and genetic variability before the outbreak, interspecific competition, culling management strategy, and other population, environment, and management factors (Fandos 1991; Loison et al. 1996; Pérez et al. 1997a, 2021, 2022; León-Vizcaíno et al. 1999; González-Quirós et al. 2002a, b; González-Candela et al. 2004; Rossi et al. 2007; Nores and González-Quirós 2009; Espinosa et al. 2020; Granados et al. 2020) (Fig. 2).
Fig. 2.
Different population trends in two Iberian ibex populations affected by sarcoptic mange, namely, Sierras de Cazorla, Segura y Las Villas Natural Park population (deep gray) and Sierra Nevada Natural Space population (light gray), before and after the outbreak in 1985-1986 and 1992-1993, respectively
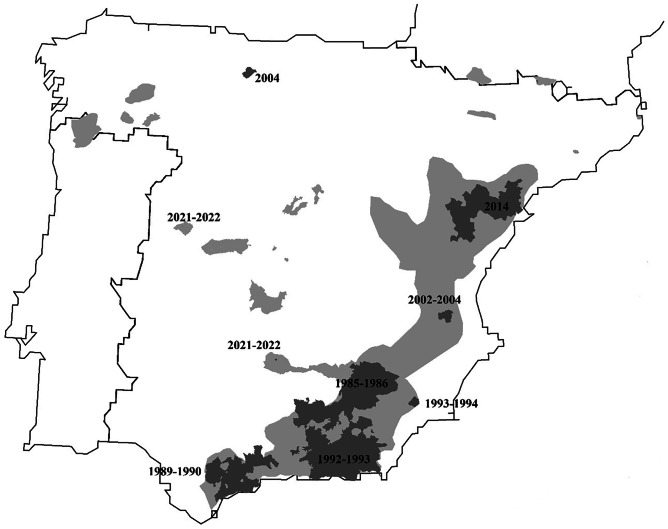
This eco-epidemiological transition from epizootic outbreak to endemic disease participating as a regulation factor in population dynamics has occurred in most of the Iberian ibex populations affected by sarcoptic mange (Fig. 3; Fandos 1991; Pérez et al. 1992; Pérez et al. 1994; Palomares and Ruiz-Martínez et al. 1993; Ruiz Martínez et al. 1996; Pérez et al. 1997a; Sánchez 1998; León-Vizcaino et al. 1999; Sánchez Isarria et al. 2008a, b; Prada and Herrero 2013; Mentaberre et al. 2015; Valldeperes et al. 2018a, b). The initial outbreaks provoked variable mortalities, reaching up to 90% (Fandos 1991; Pérez et al. 1997a; León-Vizcaíno et al. 1999), but afterward, the disease remained enzootic in all the affected populations, with prevalences even below 1% and, correspondingly, associated low mortality (Ruiz-Martínez et al. 1993). These enzootic situations, however, can be intercalated with sporadic outbreaks with associated mortality below 20% (Gil Collado 1960).
Fig. 3.
Distribution of Iberian ibex populations not affected (light grey) and affected (dark grey) by sarcoptic mange at a municipality scale, indicating the year(s) of the first description or initial outbreak of sarcoptic mange in each affected population
Such eco-epidemiological transition can only be explained by the apparition of mange-resistant hosts during the epizootic phase (Guberti and Zamboni 2000; Turchetto et al. 2014; Pérez et al. 2022), as demonstrated, among other evidences, by (1) the transition of sarcoptic mange in the affected populations from epizootic to enzootic, with decreasing prevalences reaching values down to 1% (Fandos 1991; Pérez et al. 1997a, 2021, 2022; León-Vizcaíno et al. 1999; Valldeperes et al. 2018a, b; Granados et al. 2020); (2) the differential clinical outcome of free-ranging mangy ibexes, with recovery and long-term survival of the resistant individuals (over 649 days), as compared to critical and rapidly lethal clinical evolution in the non-resistant ones (around 90 days) (León-Vizcaíno et al. 1999; Alasaad et al. 2013) (Fig. 4); and (3) the identification of the same clinical trends and outcomes (total recover, partial recover, and terminal) in Iberian ibexes experimentally infested with S. scabiei (Ráez-Bravo et al. 2015, 2016; Espinosa et al. 2017d, 2017b; Ráez-Bravo 2019; Valldeperes et al. 2019).
Fig. 4.

Male Iberian ibex captured and GPS-collared in Sierra Nevada Natural Space and monitored for more than 2 years. The disease evolved through different infestation stages, and finally, the ibex healed and completely recovered from sarcoptic mange spontaneously without treatment. Similar trends were observed in a number of individuals
Management of sarcoptic mange in Iberian ibex populations
The management of sarcoptic mange in wild Caprinae populations has been recently revised (Espinosa et al. 2020; Pérez et al. 2021). Briefly, potential measures include (1) preventing the entry of a disease in a naïve population; (2) eradicating a disease already present in a population; (3) controlling a disease present in a population, maintaining low prevalence but without eradicating it; or (4) not acting (Wobeser 1994, 2002; Espinosa et al. 2020). Any wildlife disease management, however, must be integrated with a comprehensive holistic management of the ecosystem (Aguirre et al. 1995).
Since domestic livestock is the origin of most of the outbreaks of sarcoptic mange in Iberian ibex (Lavín et al. 2000a; Moroni et al. 2021), controlling sarcoptic mange in sympatric small ruminant flocks seems therefore a key to control the entry of S. scabiei in naïve Iberian ibex populations.
Eradication of S. scabiei is deemed impossible and unfeasible even in human populations, where known treatments exist and all the individuals can be treated (Falk 1982; Arlian 1989). However, Iberian ibex depopulation has been proposed to eradicate sarcoptic mange in this species (Arenas, et al. 2002, 2009a, b), although it is against all odds and the minimum essential requirements of an integrated ecosystem management of wildlife diseases (Aguirre et al. 1995; Espinosa et al. 2020). Moreover, even if complete Iberian ibex depopulation was achieved, other sympatric mammal hosts, either domestic or wild, could maintain the mite, acting as a reservoir until an eventual repopulation (natural or anthropic) by Iberian ibex.
Ivermectin is an antiparasitic macrocyclic lactone traditionally used to control sarcoptic mange in domestic animals (Ibrahim and Abu-Samra 1987; Geary 2005), which has also been parenterally used to deworm wildlife species and boost the immune system (López-Olvera et al. 2006). The successful individual treatment of sarcoptic mange in captive Iberian ibexes with parenteral injections of ivermectin (Pérez et al. 1996c; León-Vizcaíno et al. 2001; Sarasa et al. 2010) has led to the empiric mass release of ivermectin-medicated feed in natural protected areas in eastern Spain inhabited by Iberian ibex populations affected by sarcoptic mange (Sánchez-Isarria et al. 2008a, b; Valldeperes et al. 2022a), being even legally authorized in different regions within Spain, such as Andalucía (southern Spain) and Aragon (northern Spain) (Junta de Andalucía 2021; Gobierno de Aragón 2022).
However, oral administration of macrocyclic lactones to free-ranging wildlife populations through medicated feed is mostly empiric and entails unanswered concerns regarding its usefulness, efficacy, and potential risks to the target species, other wildlife, the environment, and even human public health (Rowe et al. 2019; Espinosa et al. 2020; Moroni et al. 2020; Valldeperes et al. 2022b). On-field administration of in-feed ivermectin-induced mean plasma concentrations below the therapeutic threshold was reported for other parasites and only in a proportion of Iberian ibexes, which is insufficient to decrease S. scabiei transmission (Nolan et al. 1985; Pound et al. 1996; Davey et al. 2010; Valldeperes et al. 2022a). Moreover, ivermectin plasma concentration decreased below 20% in 72 h after experimental administration in Iberian ibexes (Moroni et al. 2022a), further demonstrating the lack of efficacy of this measure. On the other hand, massive administration of topic macrocyclic lactones can lead to overdosing (Old et al. 2021; Mounsey et al. 2022). Moreover, such inadequate dosage and massive release of antiparasitic drugs in the environment increase the probability of the mite generating resistance against the treatment (Tabashnik 1994; Vermunt 1996; Currie et al. 2004; Bliss et al. 2008; Terada et al. 2010; Andriantjoanirina et al. 2014), which adds to the detrimental environmental consequences for coprophagous arthropods and other non-target invertebrates (Sommer et al. 1992; Herd et al. 1996; Verdú et al. 2015, 2018).
Conversely, monitoring and exceptional individual culling because of animal welfare only in severely affected ibexes have been implemented as a conservative and minimally interventionist management measure in Iberian ibex populations affected by sarcoptic mange (Ruiz-Martínez et al. 1996; Espinosa et al. 2020; Moroni et al. 2020). Such an approach eases the eco-epidemiological transition of mange from epizootic to enzootic, through the selection and survival of resistant host and mutual adaption of host and parasite (Granados et al. 2011). Besides, the simultaneous monitoring of population and health status (Cardoso et al. 2021; Barroso et al. 2022) allows for assessing, determining, and monitoring the prevalence and population trends over time (Espinosa et al. 2020; Granados et al. 2020; Pérez et al. 2021).
Infectious keratoconjunctivitis
Etiology
Infectious keratoconjunctivitis is a disease affecting the eyes of domestic and wild small ruminants. The causal role of keratoconjunctivitis has been attributed to a number of bacteria, including Corynebacterium pyogenes, Escherichia coli, Chlamydia psittaci, Ch. abortus, Moraxella bovoculi, Moraxella (Branhamella) ovis (formerly named Neisseria ovis), Listeria monocytogenes, Mycoplasma other than M. conjunctivae (including M. agalactiae), Staphylococcus aureus, or Rickettsia-like spp. (Bijlenga et al. 1983; Blanco et al. 1982; Egwu et al. 1989; Dagnall 1994a, b; Rodríguez et al. 1996; González-Candela et al. 2007; Verbisck-Bucker et al. 2008; Verbisck et al. 2010; Holzwarth et al. 2011; Arnal et al. 2013; Osman et al. 2013; Dickey et al. 2016). However, evidences have finally identified that infectious keratoconjunctivitis is related to the infection by virulent strains of M. conjunctivae (Nicolet and Freundt 1975; Giacometti et al. 1998, 1999; Zimmermann et al. 2008), although M. conjunctivae and M. agalactiae have been pointed out indistinctively as etiological agents in the past since both were phylogenetically classified together (Rodríguez et al. 1996; González-Candela et al. 2007, León-Vizcaíno et al. 2008c; Verbisck-Bucker et al. 2008; Verbisck et al. 2010). Initially, M. conjunctivae infection was believed to always cause clinical disease in wild mountain ungulate populations (Giacometti et al. 1998, 1999, 2002a, b), but later studies showed that M. conjunctivae can be carried out asymptomatically by these species (Ryser-Degiorgis et al. 2009; Mavrot et al. 2012; Fernández-Aguilar et al. 2017a, b). Nevertheless, the role of Chlamydiaceae in infectious keratoconjunctivitis of wild free-ranging mountain ungulate populations is still in discussion (Holzwarth et al. 2011; Arnal et al. 2013; Dias-Alves et al. 2021).
Symptomatology
Clinical signs of infectious keratoconjunctivitis include blepharospasm, conjunctivitis, epiphora, varying degrees of corneal opacity and ulceration, ocular damage, and inflammation, which cause visual impairment and blindness (Mayer et al. 1997). The disease is usually transient, and the infection commonly courses to spontaneous clinical recovery, although clinical signs may progress to staphyloma and corneal perforation (Giacometti et al. 1998; Fernández-Aguilar et al. 2017a, b, c) (Fig. 5).
Fig. 5.
Free-ranging Iberian ibex kid from Sierra Nevada Natural Space with mild conjunctivitis in the right eye consistent with infectious keratoconjunctivitis. Different clinical stages of infectious keratoconjunctivitis caused by Mycoplasma conjunctivae in Iberian ibexes kept in captivity in Sierra Nevada (Espinosa et al. 2017c; Fernández-Aguilar et al. 2017b)
Diagnosis
In the past, the study of infectious keratoconjunctivitis was limited by the performance of traditional culture techniques, not only in wildlife but also in domestic animals, due to the difficulty to culture and isolate M. conjunctivae (Nicolet and Freundt 1975; Giacometti et al. 1998, 1999). However, the development of molecular techniques, namely, PCR, allowing the identification, characterization, and sequencing of M. conjunctivae has allowed not only to certify the central role of this pathogen in the etiology of infectious keratoconjunctivitis, but also to diagnose this disease in infected individuals and to understand the relationship between M. conjunctivae strains and disease (Vilei et al. 2007; Fernández-Aguilar. 2017a, b, c; Yang et al. 2022). Additionally, serological tests detecting antibodies both in serum and in lachrymal against M. conjunctivae allow retrospective epidemiological studies in wild free-ranging mountain ungulates populations (Belloy et al. 2001).
Epidemiology
Mycoplasma conjunctivae is transmitted through direct contact and by flies, which act as mechanical vectors (Degiorgis et al. 1999; Giacometti et al. 2002a; Fernández-Aguilar et al. 2019), allowing infectious keratoconjunctivitis to spread at a speed estimated over 15 km/year (Degiorgis et al. 2000). This disease is characterized by a relatively high morbidity with a low mortality ranging from 5% up to 27%, mostly due to starvation or falls in the steep mountain environment inhabited by wild caprines during the blindness stage of the disease (Loison et al. 1996; Degiorgis et al. 2000; Giacometti et al. 2002a). Mountain ungulate populations can be demographically affected both by keratoconjunctivitis epidemics and the associated culling management to try to reduce density to decrease M. conjunctivae transmission, but populations usually recover in 5 years after the outbreak (Gauthier 1991; Loison et al. 1996). Clinical infectious keratoconjunctivitis follows a seasonal pattern, with more cases in summer due to the increase of susceptible host densities, because of the birth of naïve kids and the higher abundance of insect vectors of M. conjunctivae. Although the morbidity is lower in winter, the relative mortality is higher due to the more adverse environmental conditions (Rossi et al. 2019). Small domestic ruminants, mainly sheep, were traditionally assumed as the main reservoir of M. conjunctivae, and wild mountain caprines were considered spillover hosts that could not maintain infectious keratoconjunctivitis without the participation of domestic hosts (Giacometti et al. 2002a, b; Fernández-Aguilar et al. 2013, 2017c; Rossi et al. 2019). However, separate independent epidemiological cycles in domestic and wild ruminants and the capability of wild mountain ungulate populations to maintain M. conjunctivae circulation without the participation of domestic hosts have been demonstrated later (Ryser-Degiorgis et al. 2009; Fernández-Aguilar et al. 2017a, b).
Infectious keratoconjunctivitis in Iberian ibex populations
In Iberian ibex, M. conjunctivae has been reported in association with sporadic cases of keratoconjunctivitis (Antón et al. 1999; Cubero et al. 2002; Arnal et al. 2009; Revilla Calavia 2012), outbreaks in free-ranging and captive populations (León-Vizcaíno et al. 2008c; Fernández-Aguilar et al. 2017b), and endemicity in free-ranging populations (Ramírez-Rosales 2018) (Fig. 5, Table 1). While prevalences can be low in unaffected Iberian ibex populations, without the presence of M. conjunctivae in asymptomatic individuals (León-Vizcaíno et al. 2008c; Revilla Calavia 2012; Fernández-Aguilar et al. 2017b), they can rise during epizootics and reach even higher enzootic values after the epizootic, turning to asymptomatic as in domestic small ruminant flocks (Fernández-Aguilar et al. 2013, 2017b, c; Ramírez-Rosales 2018) (Table 1). The 1-year evolution from an outbreak with severe clinical signs to an asymptomatic endemic status in a captive Iberian ibex population (Fernández-Aguilar et al. 2017b) suggests that at least certain outbreaks can be related to the exposure of populations to new virulent strains of M. conjunctivae, until the relationship between the pathogen and the host ibex population evolves to an endemic balance situation. This finding is consistent with the higher prevalence of M. conjunctivae described in young individuals, which later achieve to eliminate or control the infection as they develop an efficient immune response with increasing age and exposure to the infectious agent (Giacometti et al. 2002a, b; González-Candela et al. 2006, 2007; León-Vizcaíno et al. 2008c; Fernández-Aguilar et al. 2017a, b, c).
Table 1.
Prevalences and 95% central confidence intervals reported for Mycoplasma conjunctivae, Corynebacterium pseudotuberculosis, Oestrus caucasicus, and Babesia ovis in Iberian ibex
| Reference | Prevalence | 95% central CI (%) | Sample | Technique | Comments |
|---|---|---|---|---|---|
| Infectious keratoconjunctivitis (Mycoplasma conjunctivae) | |||||
| Antón et al. (1999) | 0.4% | Culture | No further data available in the reference | ||
| Cubero et al. (2002) | 0.4% (2/450) | 0.0–1.1 | Serum | CFT | |
| González-Candela et al. (2006, 2007) | 14.3% (46/321) | 10.5–18.2 | Conjunctive/ear swabs | Culture | Mycoplasma agalactiae |
| León Vizcaíno et al. (2008c) | 0.8% (2/259) | 0.0–1.8 | Conjunctive swabs | Culture | |
| 0.48% (2/415) | 0.0–1.1 | Serum | ELISA | ||
| Arnal et al. (2009) | 27.8% (5/18) | 7.1–48.5 | Conjunctive swabs | PCR | All the ibexes sampled had clinical signs |
| Revilla Calavia (2012) | 3.6% (7/196) | 1.0–6.2 | Conjunctive swabs | PCR | 29/196 of the ibexes sampled had clinical signs |
| 24.1% (7/29) | 8.6–39.7 | All the ibexes sampled had clinical signs | |||
| 0.0% (0/167) | 0.0–0.0 | None of the ibexes sampled had clinical signs | |||
| Fernández-Aguilar et al. (2017b) | 35.4% (17/48) | 23.4–49.6 | Conjunctive swabs | PCR | Epizootic in a captive population |
| 8.7% (12/46) | 3.4–20.3 | Post-epizootic decrease in a captive population | |||
| 4.3% (2/46) | 1.2–14.5 | Post-epizootic decrease in a captive population | |||
| 75.3% (52/69) | 64.0–84.0 | Asymptomatic enzootic in a captive population | |||
| 68.7% (33/48) | 54.7–80.0 | Serum | ELISA | Epizootic in a captive population | |
| 54.3% (25/46) | 40.2–67.8 | Post-epizootic decrease in a captive population | |||
| 46.5% (20/43) | 32.5–61.1 | Post-epizootic decrease in a captive population | |||
| 38.5% (10/26) | 23.4–59.3 | Asymptomatic enzootic in a captive population | |||
| Ramírez-Rosales (2018) | 17.7% (26/147) | 11.9–24.8 | Conjunctive swabs | PCR | Enzootic in a free-ranging population |
| Caseous lymphadenitis (Corynebacterium pseudotuberculosis) | |||||
| González-Candela et al. (2006) | 3.1% (10/321) | 1.2–5.0 | Conjunctive, nasal, and vaginal swabs | Culture | Corynebacterium sp. |
| León Vizcaíno et al. (2008b) | 2.9% (1/35) | 0.0–8.4 | Serum | ELISA | |
| Colom-Cadena et al. (2014) | 0.0% (0/18) | 0.0–0.0 | Inspection | Lesions | Initial entry at an enclosure from the wild |
| 68.8% (11/16) | 46.0–91.5 | Epizootic after less than 1 year in captivity | |||
| 5.6% (1/18) | 0.0–16.1 | Serum | ELISA | Initial entry at an enclosure from the wild | |
| 85.7% (12/14) | 67.4–100.0 | Epizootic after less than 1 year in captivity | |||
| Varela-Castro et al. (2017) | 18.9% (68/360) | 14.8–22.9 | Serum | ELISA | Enzootic in a free-ranging population |
| Oestrus caucasicus | |||||
| Pérez et al. (1996a, b) | 73.9% (133/180) | 67.5–80.3 | Head | Sinus inspection | |
| Pérez et al. (1997b) | 67.1% (271/404) | 62.5–71.7 | Head | Sinus inspection | |
| Antón et al. (2002) | 59.0% (343/581) | 55.0–63.0 | Head | Sinus inspection | |
| Calero-Bernal et al. (2020) | 9.5% (4/42) | 0.6–18.4 | Serum | ELISA | Antibodies against Oestrus sp. |
| Babesia ovis | |||||
| Ferrer et al. (1998) | 32.6% (155/475) | 28.4–36.8 | Serum | IFAT | |
| Antón et al. (2002) | 59.8% (73/122) | 51.1–68.5 | Serum | IFAT | |
| 0.0% (0/110) | 0.0–0.0 | Blood smear | Microscopic inspection | ||
| Calero-Bernal et al. (2020) | 43.4% (56/129) | 34.9–52.0 | Serum | IFAT | |
CFT complement fixation test, CI confidence interval, ELISA enzyme-linked immunoassay, IFAT indirect fluorescent antibody test, PCR polymerase chain reaction
Management of infectious keratoconjunctivitis
Targeted health surveillance for M. conjunctivae in transhumant domestic small ruminant flocks (Lawson et al. 2021) should help to prevent the introduction of new virulent strains of M. conjunctivae in Iberian ibex populations, although this will not prevent the circulation of the strains already endemically maintained in the wildlife populations (Fernández-Aguilar et al. 2013, 2017a, c; Rossi et al. 2019).
Caseous lymphadenitis
Etiology
Caseous lymphadenitis is a chronic infectious disease caused by the bacterium Corynebacterium pseudotuberculosis, affecting domestic and wild small ruminants (Dorella et al. 2006; Domenis et al. 2018).
Pathogenesis and symptomatology
This disease is characterized by caseous lesions most frequently affecting the superficial lymph nodes, which can open through fistulae through the skin and leak their necrotic-purulent content (Fig. 6). The lesions may also affect parenchymal organs, and then, the disease can often be fatal, with progressive cachexia, weakness, and death (Baird and Fontaine 2007; Domenis et al. 2018).
Fig. 6.
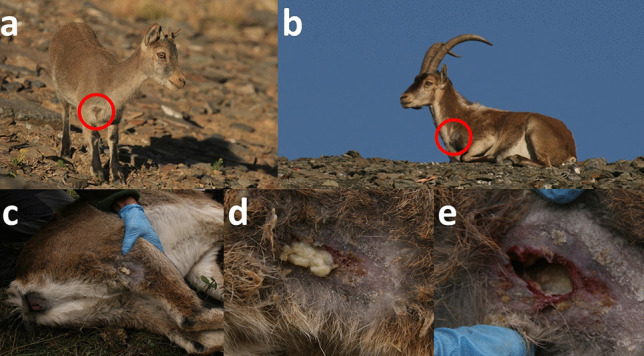
Free-ranging Iberian ibex kid (a) and adult male (b) from Sierra Nevada with apparently draining prescapular lymph node fistulae, compatible with Corynebacterium pseudotuberculosis infection. c–e Fistulized popliteal lymph node of a female Iberian ibex kept in captivity at the Ports de Tortosa i Beseit National Game Reserve enclosure. The presence of C. pseudotuberculosis was confirmed through bacterial culture and microbiological characterization (Colom-Cadena et al. 2014; Espinosa et al. 2017c)
Diagnosis
The diagnosis is based on compatible clinical signs and lesions and is further confirmed by bacteriological culture and identification or PCR from the purulent exudates (Colom-Cadena et al. 2014; Espinosa et al. 2017c). Antibodies against C. pseudotuberculosis can also be investigated through ELISA tests, allowing retrospective epidemiological studies at the population scale (Solanet et al. 2011; Colom-Cadena et al. 2014; Varela-Castro et al. 2017).
Caseous lymphadenitis in Iberian ibex
Corynebacterium pseudotuberculosis has been reported in Iberian ibex, both endemically in free-ranging populations and associated with disease outbreaks with mortality due to visceral forms in captive populations (González-Candela et al. 2006; León-Vizcaíno et al. 2008b; Colom-Cadena et al. 2014; Varela-Castro et al. 2017) (Fig. 6, Table 1), but there is a lack of knowledge on the potential demographic effects of this disease on the populations of this species. Although domestic livestock has been suspected as the origin of caseous lymphadenitis in the affected Iberian ibex populations, once the interspecific transmission has occurred, C. pseudotuberculosis can circulate and probably be maintained by Iberian ibex alone, both in captive and free-ranging populations (Colom-Cadena et al. 2014; Varela-Castro et al. 2017). Captivity seems to enhance the transmission of C. pseudotuberculosis and the severity of the clinical signs due to increased pathogen circulation, increased risk of penetrating wounds caused by the facilities or in intraspecific fights, and immunosuppression induced by prolonged captivity-related stress (Colom-Cadena et al. 2014; Espinosa et al. 2017c). Although endemic caseous lymphadenitis probably has little relevance in Iberian ibex free-ranging populations, it can hinder the management of captive populations or in intensive game estates aimed at conservation actions or game resource exploitation, respectively (Granados et al. 1996b; Colom-Cadena et al. 2014; Espinosa et al. 2017c).
Management of caseous lymphadenitis in Iberian ibex populations
In captivity, individual treatment of the affected ibexes through aspiration, cleaning, and disinfection of the purulent material and fistulae and administration of systemic antibiotic, combined with autovaccination using the strains isolated in the population, have been recommended and have apparently successfully controlled outbreaks (Colom-Cadena et al. 2014; Espinosa et al. 2017c). Since the epidemiological knowledge of C. pseudotuberculosis in Iberian ibex is still limited, no specific management recommendations exist for the management of caseous lymphadenitis in free-ranging Iberian ibex populations, apart from identifying the epidemiological status of the population and the potential domestic and wild reservoirs participating in the circulation and maintenance of the pathogen (Varela-Castro et al. 2017).
Parasitic diseases
Most of the parasites identified in Iberian ibex are shared with domestic livestock (Rossi et al. 1994; Pérez et al. 1996c) and are not related to clinical disease (Calero-Bernal et al. 2020). However, some parasites are exclusive or at least more prevalent in wild ungulates and specifically in Iberian ibex than in domestic animals. This seems to be the case for the larvae of the Diptera Oestrus spp., with prevalences above 87% in the Iberian ibex population in Sierra Nevada, usually parasitizing nasal, sinus, and horn cavities (Ruiz-Martínez et al. 1992; Granados et al. 1996a; Pérez et al. 1996b, 1997b; Antón et al. 2002) (Table 1). Another parasite apparently common and frequently reported in Iberian ibex populations is the intracellular protozoan Babesia ovis. Not only a contact with this parasite has been reported in Iberian ibex through serology (Ferrer et al. 1998; Calero-Bernal et al. 2020) (Table 1), but mortality related to babesiosis has also been described in a Mediterranean Iberian ibex population (Marco et al. 2000). In this same population, the gastrointestinal nematode Haemonchus contortus also was reported to cause mortality in Iberian ibex, apparently related to a combination of high host population density with environmental conditions that favored the parasite (Lavín et al. 1997).
Diseases under targeted surveillance in the Iberian ibex
Apart from the sarcoptic mange, the Spanish national wildlife health surveillance program includes brucellosis among the diseases subjected to targeted health surveillance (Lawson et al. 2021; Gobierno de España et al. 2022). Additionally, to move Iberian ibex among game estates and/or natural areas, the Spanish legislation requires performing specific diagnostic tests for tuberculosis and bluetongue, as well as for sarcoptic mange and brucellosis as aforementioned (Gobierno de España et al. 2009). Although an impact of brucellosis, tuberculosis, and bluetongue on Iberian ibex population dynamics has not been reported, brucellosis and tuberculosis are zoonotic diseases shared with livestock subjected to eradication programs (Gobierno de España et al. 1996; Gobierno de España 2003), and bluetongue is a notifiable animal disease (Gobierno de España et al. 2014), which motivates the targeted surveillance of these three diseases for Iberian ibex movements in Spain (Gobierno de España et al. 2009; Lawson et al. 2021).
Brucellosis
Brucellosis is a zoonotic disease caused by the bacterium Brucella melitensis and B. abortus, which can affect domestic and wild animals and humans (Corbel 1997; Seleem et al. 2010). Despite being included in the targeted health surveillance of Iberian ibex (Gobierno de España et al. 2009, 2022), a single clinical case with isolation of B. melitensis biovar 1 (Muñoz et al. 2010) has been reported, and the seroprevalences detected in this species range from 0 to 2.6% depending on the diagnostic test used. This suggests that Iberian ibex is not a reservoir for brucellosis but a spillover accidental host (Antón et al. 1999; León-Vizcaíno et al. 2008a; Muñoz et al. 2010; Astorga-Márquez et al. 2014; Junta de Andalucía 2015; Calero-Bernal et al. 2020; Gómez-Guillamón et al. 2020) (Table 2). Nevertheless, the isolation of B. melitensis in the closely related Alpine ibex (Capra ibex) was first reported almost 25 years ago (Ferroglio et al. 1998), and this species has been described as the first wild ungulate species maintaining and possibly acting as a reservoir for brucellosis in the last decade, causing serious concern because of spillback to domestic cattle (Hars et al. 2013, 2015; Rossi et al. 2019). Since the causes for the new reservoir role for brucellosis in this species and the management of this disease are still unclear and generate concern and conflict (Lambert et al. 2020, 2021, 2022), keeping brucellosis surveillance and preventing the entry of this disease in Iberian ibex populations are warranted.
Table 2.
Prevalences and 95% central confidence intervals reported for brucellosis, tuberculosis, and bluetongue in Iberian ibex
| Reference | Prevalence | 95% central CI (%) | Sample | Technique |
|---|---|---|---|---|
| Brucellosis (Brucella melitensis and B. abortus) | ||||
| Antón et al. (1999)* | 1.0% | Culture | ||
| Cubero et al. (2002) | 0.9% (4/450) | 0.0–1.8 | Serum | RBT, CFT |
| León Vizcaíno et al. (2008a) | 1.0% (6/598) | 0.2–1.8 | Serum, lymph node, spleen, vaginal fluid | Culture, PCR, RBT, CFT, ELISA |
| Muñoz et al. (2010) | 0.3% (3/1086) | 0.0–0.6 | Serum | ELISA |
| Revilla Calavia (2012) | 0.0% (0/1474) | 0.0–0.0 | Serum | ELISA |
| Astorga Márquez et al. (2014) | 0.0% (0/214) | 0.0–0.0 | Serum | RBT, CFT |
| Junta de Andalucía (2015) | 2.6% (15/571) | 1.3–3.9 | Serum | RBT |
| 0.3% (2/585) | 0.0–0.8 | CFT | ||
| Calero-Bernal et al. (2020) | 0.0% (0/50) | 0.0–0.0 | Serum | RBT, CFT, AGIDT |
| 0.0% (0/50) | 0.0–0.0 | Genital mucosa | Culture | |
| Gómez-Guillamón et al. (2020) | 2.5% (2/549) | 1.2–3.8 | Serum | RBT |
| 0.4% (2/564) | 0.0–0.8 | CFT | ||
| Tuberculosis (Mycobacterium tuberculosis complex) | ||||
| Cubero et al. (2002) | 0.0% (0/175) | 0.0–0.0 | Lung, lymph node | Immunofluorescence |
| León Vizcaíno et al. (2008b) | 0.0% (0/35) | 0.0–0.0 | Carcass | ELISA, lesions, culture |
| Mentaberre et al. (2010) | 0.0% (0/205) | 0.0–0.0 | Carcass | Lesions |
| Revilla Calavia (2012) | 1.5% (10/648) | 0.6–2.5 | Serum | ELISA |
| 0.0% (0/150) | 0.0–0.0 | Carcass | Necropsy, culture, Ziehl–Neelsen | |
| García-Bocanegra et al. (2012a) | 0.0% (0/460) | 0.0–0.0 | Serum | ELISA |
| Mentaberre et al. (2014) | 0.0% (0/355) | 0.0–0.0 | Carcass | Lesions |
| Ruiz-Rodríguez (2018) | 14.6% (35/239) | 10.2–19.1 | Serum | ELISA |
| Calero-Bernal et al. (2020) | 6.0% (3/50) | 0.0–12.6 | Serum | ELISA |
| 0.0% (0/50) | 0.0–0.0 | Lung, mediastinal lymph nodes | Culture | |
| González-Insa (2021) | 7.9% (3/38) | 0.0–16.5 | Serum | ELISA |
| Bluetongue virus | ||||
| García et al. (2009) | 10.8% (9/83) | 0.0–32.4 | Serum | ELISA |
| Lorca-Oró et al. (2011) | 4.0% (31/770) | 2.6–5.4 | Serum | ELISA |
| 0.0% (0/380) | 0.0–0.0 | Blood | PCR | |
| 2.9% (1/34) | 0.0–8.6 | Spleen | PCR | |
| Revilla Calavia (2012) | 0.3% (2/722) | 0.0–0.7 | Serum | ELISA |
| Astorga Márquez et al. (2014) | 1.9% (4/214) | 0.1–3.7 | Serum | ELISA |
| Lorca-Oró et al. (2014) | 11.1% (4/36) | 0.8–21.4 | Serum | ELISA |
| Junta de Andalucía (2015) | 3.4% (19/559) | 1.9–4.9 | Serum | ELISA |
| Gómez-Guillamón et al. (2020) | 3.3% (18/538) | 1.8–4.9 | Serum | ELISA |
AGID agar gel immunodiffusion test, CFT complement fixation test, ELISA enzyme-linked immunoassay, PCR polymerase chain reaction, RBT Rose Bengal test
*No further data available in this reference
Tuberculosis
Tuberculosis is another zoonosis in the wildlife-livestock-human interface, caused by bacteria of the Mycobacterium tuberculosis complex (MTC) (Desta et al. 2022). A single clinical case of apparent clinical tuberculosis attributed to Mycobacterium bovis affecting the lung and liver in a female Iberian ibex has been reported out of a sample size of 450 (0.2%), although the confirmation through microbiological culture diagnostic techniques was not specified. This same study did not achieve to identify M. bovis through immunofluorescence in the lungs and lymph nodes of 175 Iberian ibexes (Cubero et al. 2002). Antibodies against MTC have been detected in Iberian ibex with seroprevalences up to 14.6% (Revilla Calavia 2012; Ruiz-Rodríguez 2018; Calero-Bernal et al. 2020) (Table 2). However, clinical cases of tuberculosis-like lesions confirmed through microbiological culture have not been described in the available literature or official reports, not even in areas with a known circulation of MTC in the wildlife-livestock interface and with apparent high infection pressure (León Vizcaíno et al. 2008b; Mentaberre et al. 2010, 2014; García-Bocanegra et al. 2012a; Revilla Calavia 2012) (Table 2). Hence, resistance against tuberculosis, either behavioral, immunological, and/or ecological, has been suggested for Iberian ibex (Mentaberre et al. 2010). Experimental studies including the challenge of Iberian ibex with MTC under controlled conditions should be carried out in order to elucidate whether this apparent resistance of Iberian ibex exists or not and whether it is related to ecological, behavioral, and/or immunological factors in case of existing. The results of such research should consequently justify the need to continue targeted surveillance of tuberculosis in this species or, conversely, allow the elimination of tuberculosis from the list of diseases to monitor and control in Iberian ibex.
Bluetongue
Bluetongue is a disease caused by different serotypes of orbivirus, mainly transmitted by vector midges of the genus Culicoides (Mellor and Wittmann 2002; Mertens et al. 2004). This disease can affect domestic and wild ruminants and camelids and is a notifiable disease included in the health surveillance and eradication programs of the European Union (Council Directive 2000/75/EC). Consequently, bluetongue has been added to the diseases under surveillance previously to Iberian ibex translocations according to the specific Spanish legislation (Gobierno de España et al. 2009). Experimental infection has demonstrated that Iberian ibex is susceptible to bluetongue virus infection, albeit subclinically (Lorca-Oró et al. 2012), and seroprevalences against bluetongue virus ranging from 3.4 to 11.1% have been reported in Iberian ibex free-ranging populations without associated clinical signs (García et al. 2009; Lorca-Oró et al. 2011; Astorga-Márquez et al. 2014; Lorca-Oró et al. 2014; Junta de Andalucía 2015; Gómez-Guillamón et al. 2020) (Table 2). Nevertheless, in 2018 the circulation of bluetongue virus serotype 4 in domestic ruminants was associated with an outbreak of mortality in sympatric Iberian ibexes (Gómez-Guillamón et al. 2021). The change in the epidemiological consideration of Iberian ibex with regard to bluetongue virus from susceptible asymptomatic host to clinical host where mortality can occur warrants further monitoring and targeting this disease in the health surveillance of this species.
In summary, except for sarcoptic mange, no significant epidemiological impact or demographic effect has been reported up to date for the remaining diseases under targeted health surveillance in Iberian ibex (brucellosis, tuberculosis, and bluetongue). Hence, the reason to monitor and survey these three diseases in Iberian ibex is rather their relevance in domestic livestock, where they are included in eradication programs, and the risk for zoonosis and transmission among domestic animals and wildlife from a One Health approach (Gortázar et al. 2007, 2016; Espinosa et al. 2017c; Rossi et al. 2019).
Other diseases
Diseases not included in the targeted health surveillance of Iberian ibex and without known demographic effects have nonetheless been investigated in the populations of this species.
Paratuberculosis
Paratuberculosis is an intestinal chronic wasting disease affecting ruminants, caused by the facultative intracellular pathogen Mycobacterium avium subspecies paratuberculosis (MAP) (Windsor 2015). MAP is considered endemic in Spain (Fanelli et al. 2020), where antibodies against MAP have been detected in wild ungulates (Reyes-García et al. 2008; López-Olvera et al. 2009; Falconi et al. 2010). Although the low prevalences generally found suggest that wild ungulates are not a reservoir for MAP (Carta et al. 2012, 2013), paratuberculosis outbreaks with associated mortality have been reported in naïve wild ungulate populations including Alpine ibex, but not in Iberian ibex (Marco et al. 2002; Ferroglio et al. 2000). Antibodies against MAP have been reported in this species with prevalences ranging from 0% up to 4.4% (Revilla Calavia 2012; Astorga-Márquez et al. 2014; Pizzato 2017; Ruiz-Rodríguez 2018; Calero-Bernal et al. 2020; Gómez-Guillamón et al. 2020) (Table 3). The spatial analyses of the seroprevalences obtained have pointed out domestic livestock as a risk factor for positivity in Iberian ibex, suggesting that this species is rather a spillover or dead-end host than a reservoir for MAP (Pizzato 2017; Ruiz-Rodríguez 2018). However, the detection of MAP by PCR in the ileocecal valve of 22% of the Iberian ibexes analyzed compared to the lower seroprevalence of 4.4% in the same population (Pizzato 2017) suggests that MAP carriage in Iberian ibexes could be underestimated when assessed through serological studies. Apart from the higher sensitivity of ileocecal valve PCR to diagnose MAP infection as compared to serum ELISA analysis in Iberian ibex (Pizzato 2017), these results raise a concern about a potential hidden role of this species in the epidemiology of MAP.
Table 3.
Prevalences and 95% central confidence intervals reported for paratuberculosis, Crimean-Congo hemorrhagic fever virus, and abortive agents in Iberian ibex
| Reference | Prevalence | 95% central CI (%) | Sample | Technique | Comments |
|---|---|---|---|---|---|
| Paratuberculosis (Mycobacterium avium subsp. paratuberculosis) | |||||
| Cubero et al. (2002) | 0.0% (0/5) | 0.0–0.0 | Intestine and lymph node | IF | |
| Revilla Calavia (2012) | 1.1% (7/652) | 0.3–1.9 | Serum | ELISA | |
| Astorga Márquez et al. (2014) | 1.9% (4/214) | 0.1–3.7 | Serum | ELISA | |
| Pizzato (2017) | 9.2% (2/46) | 6.2–12.3 | Serum | ELISA | |
| 22.0% (20/91) | 13.5–30.5 | Ileocecal valve | PCR | ||
| Ruiz-Rodríguez (2018) | 4.4% (12/271) | 2.0–6.9 | Serum | ELISA | |
| Calero-Bernal et al. (2020) | 0.0% (0/50) | 0.0–0.0 | Serum | ELISA | |
| 0.0% (0/50) | 0.0–0.0 | Ileocecal valve | Culture | ||
| Gómez-Guillamón et al. (2020) | 0.5% (3/564) | 0.0–1.1 | Serum | ELISA | |
| González-Insa (2021) | 2.6% (1/38) | 0.0–7.7 | Serum | ELISA | |
| Crimean-Congo hemorrhagic fever virus (CCHFV) | |||||
| Espunyes et al. (2021) | 78.6% (66/84) | 69.8–87.3 | Serum | ELISA | An Iberian ibex free-ranging population had a 100% prevalence of antibodies against CCHFV |
| Carrera-Faja et al. (2022) | 96.0% (121/126) | 92.6–99.4 | Serum | ELISA | |
| Abortive agents | |||||
| Chlamydophila spp. | |||||
| Cubero et al. (2002) | 13.3% (60/450) | 10.2–16.5 | Serum | CFT | Chlamydia psittacci |
| León Vizcaíno et al. (2008d) | 13.4% (67/500) | 10.4–16.4 | Serum | ELISA | Chlamydophila abortus |
| Astorga Márquez et al. (2014) | 0.0% (0/214) | 0.0–0.0 | Serum | ELISA | Chlamydophila abortus |
| Junta de Andalucía (2015) | 0.0% (0/58) | 0.0–0.0 | Serum | ELISA | Chlamydia abortus |
| Varela-Castro et al. (2018) | 30.0% (39/130) | 22.1–37.9 | Serum | ELISA | |
| 9.8% (11/112) | 4.3–15.3 | Spleen/Submandibular LN | PCR | ||
| Calero-Bernal et al. (2020) | 4.0% (2/50) | 0.0–9.4 | Serum | CFT | Chlamydia sp. |
| González-Insa (2021) | 0.0% (0/38) | 0.0–0.0 | Serum | ELISA | Chlamydophila abortus |
| Q fever (Coxiella burnetii) | |||||
| León Vizcaíno et al. (2008d) | 5.4% (27/500) | 3.4–7.4 | Serum | CFT | |
| Astorga Márquez et al. (2014) | 12.6% (27/214) | 8.2–17.1 | Serum | ELISA | |
| Ruiz-Rodríguez (2018) | 13.4% (34/254) | 9.2–17.6 | Serum | ELISA | |
| Varela-Castro et al. (2018) | 30.0% (39/130) | 10.0–29.8 | Serum | ELISA | |
| 9.8% (11/112) | 7.6–27.25 | Spleen/Lymph node | PCR | ||
| Calero-Bernal et al. (2020) | 10.0% (5/50) | 1.7–18.3 | Serum | ELISA | |
| González-Insa (2021) | 2.6% (1/38) | 0.0–7.7 | Serum | ELISA | |
| Leptospira pomona | |||||
| León Vizcaíno et al. (2008d) | 0.0% (0/450) | 0.0–0.0 | Serum | MAT | |
| Contagious agalactia (Mycoplasma agalactiae, M. mycoides subsp. mycoides, and other mycoplasmas) | |||||
| Cubero et al. (2002) | 2.0% (9/450) | 0.7–3.3 | Serum | CFT | M. agalactiae |
| González-Candela et al. (2007) | 18.4% (59/321) | 14.1–22.6 | Conjunctive/ear swabs | Culture | All mycoplasmas |
| 14.3% (46/321) | 10.5–18.2 | M. agalactiae | |||
| 5.9% (19/321) | 3.3–8.5 | M. arginini | |||
| 0.3% (1/321) | 0.0–0.9 | M. mycoides mycoides | |||
| 6.2% (20/321) | 3.6–8.9 | Unidentified mycoplasmas | |||
| Verbisck-Bucker et al. (2008) | 11.2% (46/411) | 8.1–14.2 | Conjunctive/ear swabs | Culture | M. agalactiae |
| Verbisck et al. (2010) | 14.3% (5/35) | 2.7–25.9 | Conjunctive/ear swabs | Culture | M. agalactiae |
| 85.7% (30/35) | 74.1–97.3 | Serum | ELISA | ||
| Astorga Márquez et al. (2014) | 0.0% (0/214) | 0.0–0.0 | Serum | CFT | M. mycoides mycoides |
| 0.9% (2/214) | 0.0–2.2 | Serum | ELISA | M. agalactiae | |
| Junta de Andalucía (2015) | 5.8% (32/550) | 3.9–7.8 | Serum | ELISA | M. agalactiae |
| Gómez-Guillamón et al. (2020) | 5.7% (30/529) | 3.7–7.6 | Serum | ELISA | M. agalactiae |
| Neospora caninum | |||||
| Almería et al. (2007) | 0.0% (0/3) | 0.0–0.0 | Serum | ELISA | |
| León Vizcaíno et al. (2008d) | 1.0% (5/485) | 0.1–1.9 | Serum | ELISA | |
| Calero-Bernal et al. (2020) | 13.0% (14/108) | 6.6–19.3 | Serum | ELISA | |
| García-Bocanegra et al. (2012b) | 5.6% (30/531) | 3.7–7.6 | Serum | ELISA | |
| 5.1% (27/531) | 3.2–7.0 | Serum | IFAT | ||
| Cano-Manuel (2017) | 4.8% (7/147) | 1.3–8.2 | Serum | ELISA | |
| 0.0% (0/147) | 0.0–0.0 | Serum | WB | ||
| Border disease (pestivirus) | |||||
| León Vizcaíno et al. (2008d) | 8.4% (42/500) | 6.0–10.8 | Serum | ELISA | |
| Astorga Márquez et al. (2014) | 2.3% (5/214) | 0.3–4.4 | Serum | ELISA | |
| Junta de Andalucía (2015) | 11.4% (62/545) | 8.7–14.0 | Serum | ELISA | |
| Gómez-Guillamón et al. (2020) | 11.0% (58/525) | 8.4–13.7 | Serum | ELISA | |
| González-Insa (2021) | 0.0% (0/38) | 0.0–0.0 | Serum | ELISA | |
| Salmonella abortusovis | |||||
| Cubero et al. (2002) | 0.2% (1/450) | 0.0–0.7 | Serum | WAT | S. abortusovis serogroup B |
| León Vizcaíno et al. (2008d) | 0.8% (4/500) | 0.0–1.6 | Serum | MAT | |
| Junta de Andalucía (2015) | 0.4% (1/231) | 0.0–1.3 | Serum | ELISA | Salmonella sp. |
| Toxoplasma gondii | |||||
| León Vizcaíno et al. (2008d) | 18.2% (91/500) | 14.8–21.6 | Serum | ELISA | |
| García-Bocanegra et al. (2012b) | 27.5% (146/531) | 23.7–31.3 | Serum | MAT | |
| Cano-Manuel (2017) | 11.6% (17/147) | 6.4–16.7 | Serum | ELISA | |
| 0.0% (0/147) | 0.0–0.0 | Serum | WB | ||
| Calero-Bernal et al. (2020) | 2.9% (4/137) | 0.1–5.7 | Serum | ELISA | |
| Almería et al. (2021) | 13.9% (14/101) | 7.4–20.6 | Serum | MAT | |
| Schmallenberg virus | |||||
| Jiménez-Ruiz et al. (2021) | 19.9% (49/246) | 14.9–24.9 | Serum | ELISA | |
| González-Insa (2021) | 5.3% (2/38) | 0.0–12.4 | Serum | ELISA | |
CFT complement fixation test, ELISA enzyme-linked immunoassay, IF immunofluorescence, IFAT indirect fluorescent antibody test, LN lymph node, MAT modified agglutination test, PCR polymerase chain reaction, WAT Wright’s agglutination test, WB western blot
Crimean-Congo hemorrhagic fever
Crimean-Congo hemorrhagic fever is a disease caused by a Crimean-Congo hemorrhagic fever virus (CCHFV), a tick-transmitted orthonairovirus. It is a zoonotic disease that can cause potentially lethal systemic hemorrhagic disease in humans, with a mortality rate ranging between 3 and 30% of the humans infected (Ergonul 2012; Bente et al. 2013). Domestic and wild animals can be subclinically infected but are infectious for ticks feeding from them (Spengler et al. 2016). Autochthonous human clinical cases with associated mortality have been recently reported in the Iberian Peninsula (Negredo et al. 2017, 2021a, b), although it is yet unclear whether the virus was already present or its distribution area has lately spread (Spengler and Bente 2017). Ticks belonging to the genus Hyalomma spp. were traditionally considered the main vector of CCHFV (Spengler et al. 2016), but different CCHFV strains have been recently identified in Spain in multiple tick species of different genera obtained from the environment and domestic and wild animals (Moraga-Fernández et al. 2021; Sánchez-Seco et al. 2022), widening the epidemiological scenario of CCHFV transmission. Seroprevalences of antibodies against CCHFV reaching up to 100% have been detected in Iberian ibex Mediterranean populations, suggesting that CCHFV is endemic in the region at least since 2010, with a clustered distribution associated with high densities of bovine livestock (Espunyes et al. 2021; Carrera-Faja et al. 2022) (Table 3). Although CCHFV has not been detected in Iberian ibex nor in ticks carried by this species, the high seroprevalences reported warrant further research on the potential role of this species as a CCHFV reservoir and/or mechanical spreader of CCHFV-infected ticks, to assess the potential zoonotic risk.
Abortive agents
A number of abortive agents can cause congenital perinatal mortality in wild ruminants. In Iberian ibex, the presence and/or contact with the abortive agents Chlamydophila spp., Coxiella burnetii (etiological agent of Q fever), Leptospira pomona, Mycoplasma agalactiae, M. arginine and M. mycoides subspecies mycoides (etiological agents of contagious agalactia), Neospora caninum, pestivirus (etiological agent of border disease), Salmonella abortusovis, Schmallenberg virus, and Toxoplasma gondii has been investigated and/or reported (Almería et al. 2007; González-Candela et al. 2007; León-Vizcaíno et al. 2008d; García-Bocanegra et al. 2012b; Astorga-Márquez et al. 2014; Junta de Andalucía 2015; Cano-Manuel 2017; Ruiz-Rodríguez 2018; Varela-Castro et al. 2018; Calero-Bernal et al. 2020; Gómez-Guillamón et al. 2020; Almería et al. 2021; González-Insa 2021; Jiménez-Ruiz et al. 2021) (Table 3). Although undoubtedly all these agents can affect population dynamics by decreasing fertility, birth rate, and kid survival, the demographic impact of these pathogens has not been studied nor reported in Iberian ibex populations. Moreover, if Iberian ibex populations are capable of maintaining the circulation of these pathogens as a reservoir, this may also suppose a risk and concern for sympatric domestic livestock.
The clinical outcomes and prevalences reported vary from failure to detect and/or identify the agent to high prevalences, so no consistent trend can be identified for abortive pathogens in Iberian ibex (Table 3). The prevalences reported probably depend on the particular agent and population studied; the objective of the diagnosis (contact through antibodies or identification of the etiological agent), the specific diagnostic methodology used, the specific ecosystem and environment inhabited by each ibex population studied, and the contact with and pressure by domestic livestock, among other factors. It is therefore difficult to establish a role as a reservoir or spillover host for Iberian ibex with regard to these pathogens, as well as the aforementioned effect on population demography and dynamics. However, since these diseases are shared with domestic livestock and some of them have zoonotic potential, increasing health surveillance at the interface between domestic and wild animals, including Iberian ibex, is warranted, as well as performing further specific studies aimed at elucidating the demographic effect of these infections in Iberian ibex population dynamics and the potential risk for domestic livestock.
Bacterial and viral pneumoniae
Besides abortive agents, pneumoniae play a relevant and long-known role in population dynamics of wild mountain ungulates, either through yearly mortality of young animals during their first winter or through mortality outbreaks in adult individuals, usually linked to the exposition to new pathogens carried by domestic animals and/or changes in environmental or climatological conditions (Foreyt and Jessup 1982; Black et al. 1988; Gonzalez and Crampe 2001; Rudolph et al. 2007; Besser et al. 2008; Ytrehus et al. 2008; Posautz et al. 2014; Kock et al. 2018). There is an extended number of potential pathogens identified in pneumonia outbreaks in wildlife, including among other bacteria such as Bibersteinia trehalosi (formerly known as Pasteurella trehalosi), Corynebacterium pyogenes (most likely opportunistic), Mannheimia glucosida, Mannheimia haemolytica, Moraxella bovis, Mycoplasma ovipneumoniae, Pasteurella multocida, other hemolytic Pasteurellaceae or Mannheimia spp. or viruses as parainfluenza-3 virus (pneumovirus), and syncytial respiratory virus (respirovirus) (Foreyt and Jessup 1982; Black et al. 1988; Lavín et al. 2000b; Rudolph et al. 2007; Besser et al. 2008; Ytrehus et al. 2008; Dassanayake et al. 2010, 2013; Posautz et al. 2014; Kock et al. 2018). However, despite the long time elapsed since the causal relationship among pathogens, disease, mortality, and demographic effects has been established, there is still controversy about the relationship among the different pathogens identified and what combinations or action sequences cause disease and mortality and which do not (Dassanayake et al. 2010, 2013). Although the impact of pneumoniae on the population dynamics of other mountain ungulate species mainly by affecting recruitment is well known, this disease has not been thoroughly investigated in Iberian ibex, whose populations occupy different habitats and environments ranging from sea level to more than 3400 m above sea level (Pérez et al. 2002) and can therefore be challenged by pathogens causing pneumonia in different environmental conditions and seasons. A serological study was carried out in Iberian ibexes from southern Spain, revealing 8.1% and 1.3% seroprevalence against P. multocida A and P. multocida D, respectively (Cubero et al. 2002). Since pneumonia can cause mass mortalities leading to wiping out completely the affected populations and even leading almost to the extinction of common and abundant species (Ytrehus et al. 2008; Kock et al. 2018), further research on the etiology, pathogenesis, and demographic effects of these respiratory processes in wild mountain ungulates and particularly in Iberian ibex is required, in order to be able to prevent future disease and mortality outbreaks and improve their management if they occur, as well as to take into account their demographic effect for population management. In captive populations, stress-related immunosuppression, overcrowding and deficient ventilation, and changes in diet favor pneumonic and septicaemic outbreaks by M. haemolytica, P. multocida and B. trehalosi, and paramyxovirus (Espinosa et al. 2017c).
Other infectious bacterial and viral diseases
Other pathogens have been punctually described in Iberian ibex without associated widespread distribution in the investigated population, disease outbreak, mortality, demographic effect, and/or potential risk for domestic livestock or humans (Antón et al. 1999, 2002; González-Candela et al. 2006; Revilla Calavia 2012; Calero-Bernal et al. 2020). Thus, low prevalence (1.25%) associated with domestic livestock has been described against Escherichia coli O157:H7, a potentially zoonotic bacterium (Navarro-González et al. 2015). Staphylococcus spp. has been cultivated from Iberian ibex, even with a 100% prevalence (González-Candela et al. 2006; Calero-Bernal et al. 2020), although no clear connection with pathology and population impact could be established. A new bacterium, Streptococcus caprae spp. nov., with phenotypic, biochemical, and phylogenetic differences with other Streptococcus species has been described in Iberian ibex (Vela et al. 2016). Antibodies against the Maedi-Visna virus were detected in a serosurvey in two out of 38 Iberian ibexes analyzed, accounting for a 5.3% prevalence (González-Insa 2021).
Other non-lethal parasites
Apart from the parasites described above, widespread in Iberian ibex populations and/or related to disease and mortality outbreaks (S. scabiei, O. caucasicus, B. ovis, and H. contortus, see “Sarcoptic mange” and “Parasitic diseases”), more than 60 parasite species have been described in the Iberian ibex, including ectoparasites, gastrointestinal and pulmonary nematodes (with prevalences over 80%), coccidia, cestodes, trematodes, and protozoans (Rossi et al. 1994; Cano et al. 1996; Castellà et al. 1996; Pérez et al. 1996a, 2003, 2006; Antón et al. 1999, 2002; Granados et al. 2001; Refoyo et al. 2016; Calero-Bernal et al. 2020; Carrau et al. 2021a, b) (Table 4). More recently, intense tick infestations have been reported, which could increase in the future if global change provides conditions favoring their life cycle. Such intense tick burdens can affect the health and body condition of the host both per se through blood loss and by acting as vectors and/or amplifiers of other pathogens (Varela-Castro et al. 2018). The parasitic fauna of Iberian ibex forms a complex ecosystem where the different parasite species interact among them, with the host, and the environment, maintaining a dynamic balance that can lead to disease in the individual or even cause population epizootics as a result of disruptions in the system (Pérez et al. 2006).
Table 4.
Parasites reported in Iberian ibex
| Parasite | Prevalence | 95% central CI (%) | Reference |
|---|---|---|---|
| Abomasum | |||
| Haemonchus contortus | 50.0% (7/14) | 23.8 – 76.2 | Rossi et al. (1994) |
| 14.3% (2/14) | 0.0 – 32.6 | Carrau et al. (2021a) | |
| Marshallagia dentispicularis | 28.6% (4/14) | 4.9 – 52.2 | Carrau et al. (2021a) |
| Marshallagia marshalli | 35.7% (5/14) | 10.6 – 60.8 | Rossi et al. (1994) |
| 86.7% (13/15) | 69.5 – 100.0 | Pérez et al. (1996a) | |
| 85.5% (94/110) | 78.9 – 92.0 | Antón et al. (2002), Pérez et al. (2006) | |
| 78.5% (62/79) | 69.4 – 87.5 | Pérez et al. (2003) | |
| 35.7% (5/14) | 10.6 – 60.8 | Carrau et al. (2021a) | |
| Marshallagia occidentalis | 14.3% (2/14) | 0.0 – 32.6 | Rossi et al. (1994) |
| 7.3% (8/110) | 2.4 – 12.1 | Antón et al. (2002), Pérez et al. (2006) | |
| 22.8% (18/79) | 13.5 – 32.0 | Pérez et al. (2003) | |
| Nematodirus abnormalis | 0.9% (1/110) | 0.0 – 2.7 | Antón et al. (2002), Pérez et al. (2006) |
| 3.8% (3/79) | 0.0 – 8.0 | Pérez et al. (2003) | |
| Nematodirus davtiani | 20.0% (3/15) | 0.0 – 40.2 | Pérez et al. (1996a) |
| 21.8% (24/110) | 14.1 – 29.5 | Antón et al. (2002), Pérez et al. (2006) | |
| 21.5% (17/79) | 12.5 – 30.6 | Pérez et al. (2003) | |
| Nematodirus oiratianus | 35.5% (39/110) | 26.5 – 44.4 | Antón et al. (2002), Pérez et al. (2006) |
| 2.5% (2/79) | 0.0 – 6.0 | Pérez et al. (2003) | |
| Ostertagia circumcincta | 100.0% (14/14) | 100.0 – 100.0 | Rossi et al. (1994) |
| Ostertagia leptospicularis | 14.3% (2/14) | 0.0 – 32.6 | Carrau et al. (2021a) |
| Ostertagia lyrate | 0.9% (1/110) | 0.0 – 2.7 | Antón et al. (2002), Pérez et al. (2006) |
| 1.3% (1/79) | 0.0 – 3.7 | Pérez et al. (2003) | |
| Ostertagia occidentalis | 7.1% (1/14) | 0.0 – 20.6 | Carrau et al. (2021a) |
| 0stertagia ostertagi | 80.0% (12/15) | 59.8 – 100.0 | Pérez et al. (1996a) |
| 14.5% (16/110) | 8.0 – 21.1 | Antón et al. (2002), Pérez et al. (2006) | |
| 20.3% (16/79) | 11.4 – 29.1 | Pérez et al. (2003) | |
| 0.0% (0/14) | 0.0 – 0.0 | Carrau et al. (2021a) | |
| Ostertagia trifurcata/pinnata | 64.3% (9/14) | 39.2 – 89.4 | Rossi et al. (1994) |
| Spiculopteragia asymmetrica | 42.9% (6/14) | 16.9 – 68.8 | Carrau et al. (2021a) |
| Spiculopteragia quadrispiculata | 21.4% (3/14) | 0.0 – 42.9 | Carrau et al. (2021a) |
| Teladorsagia circumcincta | 85.5% (94/110) | 78.9 – 92.0 | Antón et al. (2002), Pérez et al. (2006) |
| 97.5% (77/79) | 94.0 – 100.0 | Pérez et al. (2003) | |
| 92.9% (13/14) | 79.4 – 100.0 | Carrau et al. (2021a) | |
| Teladorsagia davtiani | 0.9% (1/110) | 0.0 – 2.7 | Antón et al. (2002), Pérez et al. (2006) |
| 1.3% (1/79) | 0.0 – 3.7 | Pérez et al. (2003) | |
| 0.0% (0/14) | Carrau et al. (2021a) | ||
| Teladorsagia trifurcata | 14.5% (16/110) | 8.0 – 21.1 | Antón et al. (2002), Pérez et al. (2006) |
| 46.8% (37/79) | 35.8 – 57.8 | Pérez et al. (2003) | |
| 71.4% (10/14) | 47.8 – 95.1 | Carrau et al. (2021a) | |
| Trichostrongylus axei | 35.7% (5/14) | 10.6 – 60.8 | Rossi et al. (1994) |
| 0.9% (1/110) | 0.0 – 2.7 | Antón et al. (2002), Pérez et al. (2006) | |
| 2.5% (2/79) | 0.0 – 6.0 | Pérez et al. (2003) | |
| 35.7% (5/14) | 10.6 – 60.8 | Carrau et al. (2021a) | |
| Trichostrongylus vitrinus | 1.8% (2/110) | 0.0 – 4.3 | Antón et al. (2002), Pérez et al. (2006) |
| 2.5% (2/79) | 0.0 – 6.0 | Pérez et al. (2003) | |
| Small intestine | |||
| Avitellina centripunctatta | 0.2% (1/489) | 0.0 – 0.6 | Antón et al. (2002), Pérez et al. (2006) |
| Marshallagia marshalli | 20.9% (23/110) | 13.3 – 28.5 | Antón et al. (2002), Pérez et al. (2006) |
| 14.4% (12/83) | 6.9 – 22.0 | Pérez et al. (2003) | |
| Marshallagia occidentalis | 7.3% (8/110) | 2.4 – 12.1 | Antón et al. (2002), Pérez et al. (2006) |
| 3.6% (3/83) | 0.0 – 7.6 | Pérez et al. (2003) | |
| Nematodirus abnormalis | 28.6% (4/14) | 4.9 – 52.2 | Rossi et al. (1994) |
| 100.0% (15/15) | 100.0 – 100.0 | Pérez et al. (1996a) | |
| 54.5% (60/110) | 45.2 – 63.9 | Antón et al. (2002), Pérez et al. (2006) | |
| 42.2% (35/83) | 7.9 – 76.4 | Pérez et al. (2003) | |
| 82.4% (14/17) | 64.2 – 100.0 | Carrau et al. (2021a) | |
| Nematodirus davtiani | 42.9% (6/14) | 16.9 – 68.8 | Rossi et al. (1994) |
| 57.3% (63/110) | 48.0 – 66.5 | Antón et al. (2002), Pérez et al. (2006) | |
| 44.6% (37/83) | 33.9 – 55.3 | Pérez et al. (2003) | |
| Nematodirus filicollis | 100.0% (15/15) | 100.0 – 100.0 | Pérez et al. (1996a) |
| 1.8% (2/110) | 0.0 – 4.3 | Antón et al. (2002), Pérez et al. (2006) | |
| 1.2% (1/83) | 0.0 – 3.6 | Pérez et al. (2003) | |
| 11.8% (2/17) | 0.0 – 27.1 | Carrau et al. (2021a) | |
| Nematodirus oiratianus | 56.4% (62/110) | 47.1 – 65.6 | Antón et al. (2002), Pérez et al. (2006) |
| 47.0% (39/83) | 36.3 – 57.7 | Pérez et al. (2003) | |
| Nematodirus spathiger | 7.1% (1/14) | 0.0 – 20.6 | Rossi et al. (1994) |
| 2.7% (3/110) | 0.0 – 5.8 | Antón et al. (2002), Pérez et al. (2006) | |
| 2.4% (2/83) | 0.0 – 5.7 | Pérez et al. (2003) | |
| 47.1% (8/17) | 23.3 – 70.8 | Carrau et al. (2021a) | |
| Ostertagia ostertagi | 4.5% (5/110) | 0.7 – 8.4 | Antón et al. (2002), Pérez et al. (2006) |
| 3.6% (3/83) | 0.0 – 7.6 | Pérez et al. (2003) | |
| Teladorsagia circumcincta | 47.3% (52/110) | 37.9 – 56.6 | Antón et al. (2002), Pérez et al. (2006) |
| 31.3% (26/83) | 21.3 – 41.3 | Pérez et al. (2003) | |
| Teladorsagia trifurcata | 9.1% (10/110) | 3.7 – 14.5 | Antón et al. (2002), Pérez et al. (2006) |
| 7.2% (6/83) | 1.7 – 12.8 | Pérez et al. (2003) | |
| Trichostrongylus capricola | 85.7% (12/14) | 67.4 – 100.0 | Rossi et al. (1994) |
| 2.7% (3/110) | 0.0 – 5.8 | Antón et al. (2002), Pérez et al. (2006) | |
| 1.2% (1/83) | 0.0 – 3.6 | Pérez et al. (2003) | |
| 70.6% (12/17) | 48.9 – 92.2 | Carrau et al. (2021a) | |
| Trichostrongylus colubriformis | 50.0% (7/14) | 23.8 – 76.2 | Rossi et al. (1994) |
| 21.6% (8/37) | 8.4 – 34.9 | Pérez et al. (1996a) | |
| 0.0% (0/17) | 0.0 – 0.0 | Carrau et al. (2021a) | |
| Trichostrongylus vitrinus | 21.4% (3/14) | 0.0 – 42.9 | Rossi et al. (1994) |
| 4.5% (5/110) | 0.7 – 8.4 | Antón et al. (2002), Pérez et al. (2006) | |
| 3.6% (3/83) | 0.0 – 7.6 | Pérez et al. (2003) | |
| 70.6% (12/17) | 48.9 – 92.2 | Carrau et al. (2021a) | |
| Large intestine | |||
| Chabertia ovina | 52.9% (9/17) | 29.2 – 76.7 | Carrau et al. (2021a) |
| Oesophagostomum venulosum | 36.3% (4/11) | 7.9 – 64.8 | Rossi et al. (1994) |
| 70.6% (12/17) | 48.9 – 92.2 | Carrau et al. (2021a) | |
| Skrjabinema ovis | 29.4% (5/17) | 7.8 – 51.1 | Carrau et al. (2021a) |
| Skrjabinema sp. | 27.3% (3/11) | 1.0 – 53.6 | Rossi et al. (1994) |
| Trichuris ovis | 9.1% (1/11) | 0.0 – 26.1 | Rossi et al. (1994) |
| 5.9% (1/17) | 0.0 – 17.1 | Carrau et al. (2021a) | |
| Faeces | |||
| Eimeria aspheronica | 0.5% (2/379) | 0.0 – 1.3 | Antón et al. (2002), Pérez et al. (2006) |
| Eimeria arloingi | 73.9% (280/379) | 69.5 – 78.3 | Antón et al. (2002), Pérez et al. (2006) |
| Eimeria caprina | 5% (19/379) | 2.8 – 7.2 | Antón et al. (2002), Pérez et al. (2006) |
| Eimeria capraovina | 5% (19/379) | 2.8 – 7.2 | Antón et al. (2002), Pérez et al. (2006) |
| Eimeria christenseni | 10.0% (38/379) | 7.0 – 13.1 | Antón et al. (2002), Pérez et al. (2006) |
| 86.4% (19/22) | 72.0 – 100.0 | Pérez et al. (1996a) | |
| Eimeria folchijevi | 2.9% (11/379) | 1.2 – 4.6 | Antón et al. (2002), Pérez et al. (2006) |
| Eimeria hirci | 1.1% (4/379) | 0.0 – 2.1 | Antón et al. (2002), Pérez et al. (2006) |
| Eimeria ninakohlykimovae | 59.0% (13/22) | 38.5 – 79.6 | Pérez et al. (1996a) |
| 6.1% (23/379) | 3.7 – 8.5 | Antón et al. (2002), Pérez et al. (2006) | |
| Eimeria parva | 63.6% (14/22) | 43.5 – 83.7 | Pérez et al. (1996a) |
| Eimeria sp. | 0.5% (2/379) | 0.0 – 1.3 | Antón et al. (2002), Pérez et al. (2006) |
| 97.7% (127/130) | 95.1 – 100.0 | Calero-Bernal et al. (2020) | |
| Moniezia expansa | 5.4% (2/37) | 0.0 – 12.7 | Pérez et al. (1996a) |
| 8.0% (39/489) | 5.6 – 10.4 | Antón et al. (2002), Pérez et al. (2006) | |
| Moniezia benedeni | 7.8% (38/489) | 5.4 – 10.1 | Antón et al. (2002), Pérez et al. (2006) |
| Moniezia sp. | 10.0% (13/130) | 4.8 – 15.2 | Calero-Bernal et al. (2020) |
| Bunostomum sp. | 2.5% (1/40) | 0.0 – 7.3 | Refoyo et al. (2016) |
| Cooperia sp. | 2.5% (1/40) | 0.0 – 7.3 | Refoyo et al. (2016) |
| Cystocaulus ocreatus | 16.3%* | Cano et al. (1996) | |
| Dictyocaulus filaria | 37.5%* | Cano et al. (1996) | |
| 1.5% (2/130) | 0.0 – 3.7 | Calero-Bernal et al. (2020) | |
| Dictyocaulus sp. | 7.5% (3/40) | 0.0 – 15.7 | Refoyo et al. (2016) |
| Muellerius capillaris | 66.0%* | Cano et al. (1996) | |
| 97.3% (110/113) | 94.4 – 100.0 | Calero-Bernal et al. (2020) | |
| Muellerius sp. | 62.5% (25/40) | 47.5 – 77.5 | Refoyo et al. (2016) |
| Nematodirus/Marshallagia sp. | 22.5% (9/40) | 9.6 – 35.4 | Refoyo et al. (2016) |
| Nematodirus sp. | 50.0% (65/130) | 41.4 – 58.6 | Calero-Bernal et al. (2020) |
| Ostertagia sp. | 7.5% (3/40) | 0.0 – 15.7 | Refoyo et al. (2016) |
| Protostrongylus sp. | 4.5% (1/22) | 0.0 – 13.2 | Pérez et al. (1996a) |
| Protostrongylus rufescens* | 21.4% | Cano et al. (1996) | |
| Skrjabinema sp. | 42.5% (17/40) | 27.2 – 57.8 | Refoyo et al. (2016) |
| Strongyloides sp. | 17.5% (7/40) | 5.7 – 29.3 | Refoyo et al. (2016) |
| Strongylidae | 93.1% (121/130) | 88.7 – 97.4 | Calero-Bernal et al. (2020) |
| Teladorsagia sp. | 10.0% (4/40) | 0.7 – 19.3 | Refoyo et al. (2016) |
| Toxocara vitulorum | 0.8% (1/130) | 0.0 – 2.3 | Calero-Bernal et al. (2020) |
| Trichuris sp. | 2.4% (9/379) | 0.8 – 3.9 | Antón et al. (2002), Pérez et al. (2006) |
| 2.5% (1/40) | 0.0 – 7.3 | Refoyo et al. (2016) | |
| 0.8% (1/130) | 0.0 – 2.3 | Calero-Bernal et al. (2020) | |
| Dicrocoelium dendriticum | 0.5% (2/379) | 0.0 – 1.3 | Antón et al. (2002), Pérez et al. (2006) |
| 20.8% (30/144) | 14.2 – 27.5 | Calero-Bernal et al. (2020) | |
| Dicrocoelium sp. | 2.5% (1/40) | 0.0 – 7.3 | Refoyo et al. (2016) |
| Fasciola hepatica | 1.3% (5/379) | 0.2 – 2.5 | Antón et al. (2002), Pérez et al. (2006) |
| Paramphistomum sp. | 0.5% (2/379) | 0.0 – 1.3 | Antón et al. (2002), Pérez et al. (2006) |
| 9.1% (2/22) | 0.0 – 21.1 | Pérez et al. (1996a) | |
| Liver | |||
| Fasciola hepatica | 6.7% (1/15) | 0.0 – 19.3 | Pérez et al. (1996a) |
| 1.7% (8/478) | 0.5 – 2.8 | Antón et al. (2002), Pérez et al. (2006) | |
| Lungs | |||
| Cystocaulus ocreatus | 20.0% (3/15) | 0.0 – 40.2 | Pérez et al. (1996a) |
| 32.1% (267/831) | 29.0 – 35.3 | Antón et al. (2002), Pérez et al. (2006) | |
| 26.3% (5/19) | 6.5 – 46.1 | Carrau et al. (2021b) | |
| Dictyocaulus filaria | 1.2% (10/831) | 0.5 – 1.9 | Antón et al. (2002), Pérez et al. (2006) |
| 0.0% (0/19) | 0.0 – 0.0 | Carrau et al. (2021b) | |
| Echinococcus granulosus | 0.2% (1/479) | 0.0 – 0.6 | Antón et al. (2002), Pérez et al. (2006) |
| Muellerius capillaris | 26.7% (4/15) | 4.3 – 49.0 | Pérez et al. (1996a) |
| 74.2% (617/831) | 71.3 – 77.2 | Antón et al. (2002), Pérez et al. (2006) | |
| 84.2% (16/19) | 67.8 – 100.0 | Carrau et al. (2021b) | |
| Neostrongylus linearis | 78.9% (15/19) | 60.6 – 97.3 | Carrau et al. (2021b) |
| Neostrongylus sp. | 31.3% (260/831) | 28.1 – 34.4 | Antón et al. (2002), Pérez et al. (2006) |
| Protostrongylus sp. | 35.3% (293/831) | 32.0 – 38.5 | Antón et al. (2002), Pérez et al. (2006) |
| 57.9% (11/19) | 35.7 – 80.1 | Carrau et al. (2021b) | |
| Diaphragm | |||
| Sarcocystis sp. | 80% (24/30) | 65.7 – 94.3 | Pérez et al. (1996a) |
| 27.4% (118/430) | 23.2 – 31.7 | Antón et al. (2002), Pérez et al. (2006) | |
| 84.0% (42/50) | 73.8 – 94.2 | Calero-Bernal et al. (2020) | |
| Peritoneum | |||
| Avitellina sp. | 6.7% (1/15) | 0.0 – 19.3 | Pérez et al. (1996a) |
| Taenia hydatigena | 27.1% (130/479) | 23.2 – 31.1 | Antón et al. (2002), Pérez et al. (2006) |
| 6.9% (10/144) | 2.8 – 11.1 | Calero-Bernal et al. (2020) | |
| Brain | |||
| Multiceps multiceps | 1.1% (2/180) | 0.0 – 2.6 | Pérez et al. (1996a) |
| Taenia multiceps | 0.3% (2/582) | 0.0 – 0.8 | Antón et al. (2002), Pérez et al. (2006) |
| Skin | |||
| Dermacentor marginatus | ** | Castellà et al. (1996) | |
| 2.1% (10/475) | 0.8 – 3.4 | Antón et al. (2002), Pérez et al. (2006) | |
| Dermacentor reticulatus | 0.6% (3/475) | 0.0 – 1.3 | Antón et al. (2002), Pérez et al. (2006) |
| Haemaphysalis sulcata | Castellà et al. (1996) | ||
| 32.2% (153/475) | 28.0 – 36.4 | Antón et al. (2002), Pérez et al. (2006) | |
| 10.9% (15/138) | 5.7 – 16.1 | Calero-Bernal et al. (2020) | |
| Haemaphysalis punctata | ** | Castellà et al. (1996) | |
| Hyalomma beneditai | 18.9% (36/190) | 13.4 – 24.5 | Pérez et al. (1996a) |
| Hyalomma marginatum marginatum | 5.1% (7/138) | 1.4 – 8.7 | Calero-Bernal et al. (2020) |
| Ixodes ricinus | 20.0% (38/190) | 14.3 – 25.7 | Pérez et al. (1996a) |
| 4.2% (20/475) | 2.4 – 6.0 | Antón et al. (2002), Pérez et al. (2006) | |
| 10.9% (15/138) | 5.7 – 16.1 | Calero-Bernal et al. (2020) | |
| Rhipicephalus bursa | ** | Castellà et al. (1996) | |
| 42.7% (203/475) | 38.3 – 47.2 | Antón et al. (2002), Pérez et al. (2006) | |
| 52.2% (72/138) | 43.8 – 60.5 | Calero-Bernal et al. (2020) | |
| Rhipicephalus pusilus | 15.8% (30/190) | 10.6 – 21.0 | Pérez et al. (1996a) |
| Bovicola crassipes | 14.2% (27/190) | 9.2 – 19.2 | Pérez et al. (1996a) |
| 20.2% (96/475) | 16.6 – 23.8 | Antón et al. (2002), Pérez et al. (2006) | |
| Linnognathus sp. | 1.1% (2/190) | 0.0 – 2.5 | Pérez et al. (1996a) |
| Linognathus stenopsis | 1.1% (5/475) | 0.1 – 2.0 | Antón et al. (2002), Pérez et al. (2006) |
| Psoroptes sp. | 0.6% (3/475) | 0.0 – 1.3 | Antón et al. (2002), Pérez et al. (2006) |
| Trombicula sp. | 1.6% (3/190) | 0.0 – 3.4 | Pérez et al. (1996a) |
| 0.4% (2/475) | 0.0 – 1.0 | Antón et al. (2002), Pérez et al. (2006) | |
| Straelensia cynotis | One case report | Cevidanes et al. (2019) | |
| Blood | |||
| Anaplasma ovis | 4.5% (5/110) | 0.7 – 8.4 | Antón et al. (2002), Pérez et al. (2006) |
| Eperytrozoon ovis | 1.8% (2/110) | 0.0 – 4.3 | Antón et al. (2002), Pérez et al. (2006) |
| Ehrlichia phagocytophila | 0.9% (1/110) | 0.0 – 2.7 | Antón et al. (2002), Pérez et al. (2006) |
| Theileria ovis | 1.8% (2/110) | 0.0 – 4.3 | Antón et al. (2002), Pérez et al. (2006) |
Non-infectious diseases
Dental and skull abnormalities have been reported in Iberian ibex, both in current populations and in paleontological remnants (Vigal and Machodorm 1987; Gómez-Olivencia et al. 2011). Although anecdotal and with little impact on the population dynamics of the species, tumors have been reported in Spanish ibex. Thyroid carcinoma, cutaneous horn, pheochromocytoma, intestinal leiomyoma, and ruminal papilloma have been described in Iberian ibexes after necropsy during regular wildlife health surveillance (Arnal et al. 2006). Two gastrointestinal tumors were found in two free-ranging Iberian ibexes apparently healthy otherwise (Velarde et al. 2008), and disseminated melanoma concurrent with pheochromocytoma severely affected an old male to euthanasia (Arnal and Fernández de Luco 2017).
Diseases related to or favored by captivity
Captive Iberian ibex populations are kept in enclosures or at high density in fenced estates either for conservation purposes or for exploitation as game resource (Granados et al. 1996b; Espinosa et al. 2017c). The higher host densities associated with these conditions can favor pathogen circulation, generating a more intense infection pressure that can explain the higher prevalence and severity and even the different epidemiological nature of disease outbreaks in captive Iberian ibex populations as compared to free-ranging populations, as described for caseous lymphadenitis in the “Caseous lymphadenitis” section and for pneumonia in the “Bacterial and viral pneumoniae” section (Colom-Cadena et al. 2014; Espinosa et al. 2017c; Varela-Castro et al. 2017). Moreover, other diseases mentioned below have not been described or do not cause symptomatology in free-ranging Iberian ibex populations but have been detected and caused disease only in captive populations (Espinosa et al. 2017c).
Enterotoxemia or “pulpy kidney disease” is a disease caused by the epsilon toxins of the gram-positive bacterium Clostridium perfringens type D. The disease is normally related to qualitative and/or quantitative changes in diet leading to intestinal dysbiosis, favoring bacterium encapsulation and toxin production (Uzal and Kelly 1996; García et al. 2013; Espinosa et al. 2017c). Although such diet changes can also happen seasonally or because of habitat modifications in free-ranging Iberian ibex populations, this is more frequent and sudden in captive populations where diet is managed by humans, increasing the probability and severity of clostridiosis (Espinosa et al. 2017c). Quarantine and vaccination (at the entry to captivity and annually), a fiber-rich diet without sudden changes, and avoiding stress are the recommended prophylactic measures. If disease occurs in a captive Iberian ibex population, treatment should include antibiotics and the aforementioned fiber-rich diet (Uzal and Kelly 1996; García et al. 2013; Espinosa et al. 2017c).
Contagious ecthyma is a disease caused by the orf virus, included in the genus Parapoxvirus. This disease affects both domestic and wild small ruminants and is characterized by proliferative dermatitis with papules and pustules, usually affecting the mouth, lips, and nose, although the lesions can extend to the oral cavity, esophagus, abomasum, skin of the face, hooves, flanks, scrotum, perianal region, preputium, vulva, and mamma (Fig. 7). The clinical signs are more severe in kids, and the infection can complicate with secondary bacterial infections leading to death, although it is normally self-limiting. It is considered a professional zoonosis, although the clinical course is also benign and self-limiting in humans (Spyrou and Valiakos 2015). The stress associated with captivity seems to increase the morbidity and the clinical severity of the disease (Junta de Andalucía 2015). However, antibodies against this virus were detected in the sera of 11 out of 450 (2.4%) Iberian ibexes investigated in southern Spain (Cubero et al. 2002), indicating that the virus probably circulates asymptomatically or subclinically in free-ranging populations. Occasional clinical cases (Camacho et al. 2017) and a disease outbreak affecting both adults and kids with at least one case of associated mortality (Estruch et al. 2020) have been described in free-ranging Iberian ibex populations. Management of contagious ecthyma outbreaks in captive populations includes isolation and treatment with antiseptics and antibiotics of the affected individuals, while prophylaxis is based on facility and equipment disinfection, since there is not an effective prophylactic vaccine (Spyrou and Valiakos 2015; Espinosa et al. 2017c).
Fig. 7.
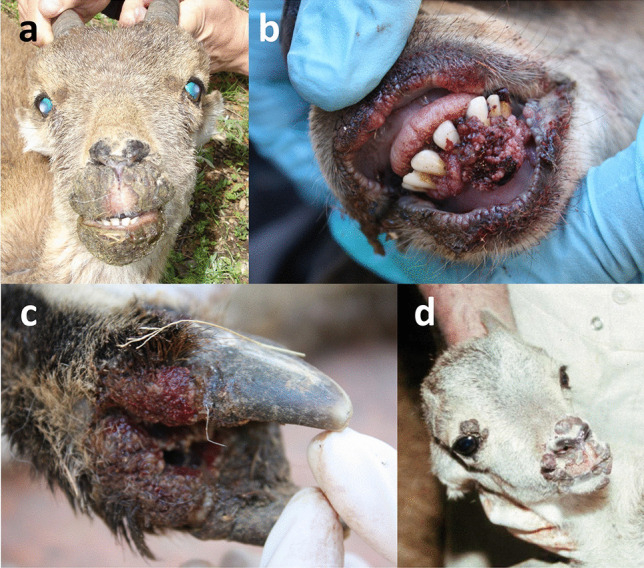
Adult Iberian ibex female (a–c) and kid (d) with crusted skin lesions affecting the commissures of the mouth, peripheral lip epidermis (a, d), oral mucosa (b), and interdigital area (c), consistent with severe proliferative, pustular, and ulcerative dermatitis compatible with orf virus infection (contagious ecthyma)
Footrot or infectious pododermatitis is a disease affecting the hooves of domestic and wild ungulates, with the involvement of bacteria such as Dichelobacter (Bacteroides) nodosus, Fusobacterium necrophorum, Arcanobacterium (Trueperella) pyogenes, and Treponema spp. (Lavín et al. 2004; Frosth et al. 2015; Espinosa et al. 2017c; Rzewuska et al. 2019). Although footrot has not been reported in free-living Iberian ibex, in captive populations, this disease is favored by the increase in concentration and the higher survival of these bacteria in moist soils with abundant organic contents (Muzafar et al. 2015), as well as by hoof wounds related to overgrowth because of lower wear and softening due to humid conditions (Espinosa et al. 2017c). Treatment of outbreaks in captive Iberian ibex populations should include hoof trimming with the removal of dead tissue, systemic antibiotics, local footbaths with disinfectants, and soil management to prevent moisture and accumulation of organic matter. Prophylactic vaccination can be considered if prevalence is high and/or outbreaks are recurrent (Frosth et al. 2015; Muzafar et al. 2015; Espinosa et al. 2017c).
Finally, A. (T.) pyogenes and/or Staphylococcus aureus subsp. anaerobius can cause suppurative lesions. These bacteria are normally present in the skin and mucosae and can enter the body through wounds, producing multiple abscesses in the inner organs such as the lymph nodes, lungs, liver, kidneys, and/or central nervous system, among others, which can cause death (Espinosa et al. 2017c; Rzewuska et al. 2019). Although this is a sporadic disease in free-ranging populations, captivity conditions can increase skin trauma due to wounds caused by the facility, increased intraspecific fights, or increased moisture, favoring the casuistic for pyogenic infection (Espinosa et al. 2017c). Quarantine isolation with surgical debriding and antibiotic treatment can be effective in initial stages, but in advanced cases, prognosis is poor. Facility design and careful handling to avoid injuries combined with early healing and disinfection of wounds are likely the best prophylactic options (Espinosa et al. 2017c).
Future research on Iberian ibex diseases
The comparison of the existent knowledge on sarcoptic mange and other diseases in Iberian ibex identifies the gaps to complete through future research. The knowledge on pathogenesis, host immune response, and pathogen-host relationship for sarcoptic mange overcomes the research performed in these aspects for all the other diseases together. While the performance, usefulness on the field and with handled ibexes, specificity, and sensitivity of diagnostic methods have been assessed for sarcoptic mange (Arenas et al. 2002; Granados et al. 2011; Alasaad et al. 2012, 2015; Mounsey et al. 2012; Ráez-Bravo et al. 2016; Valldeperes et al. 2019; Pérez et al. 2021), such knowledge is limited if existing at all for the other diseases reported in Iberian ibex. Therefore, research on the pathogenesis, epidemiology, treatment, and management of diseases in Iberian ibex populations can be hampered by diagnostic limitations. Further research should be aimed at developing, testing, validating, improving, and innovating diagnostic methods, particularly non-invasive and telemetric methods which could be applied in free-ranging individuals and populations. Despite research on sarcoptic mange predominates the investigations of Iberian ibex diseases, the scientific and manager community is far from agreeing on the best management of the disease, so further research on the efficacy and applicability of the treatment and population management of sarcoptic mange in Iberian ibex should continue to be a priority. Such research should include prophylactic measures, not only for sarcoptic mange but also for the rest of Iberian ibex diseases. Finally, the study and detection of coinfection patterns, studying the interaction among different pathogens and the host, should be key to understand disease epidemiology in Iberian ibex, as it is for sympatric wild ungulates (Alasaad et al. 2008; González-Candela et al. 2009; García-Bocanegra et al. 2012b).
Conclusions
This review compiles the current knowledge available on the diseases of Iberian ibex, which has allowed the detection of gaps in knowledge with regard to the diseases that may affect this species and their management. Overall, the information presented draws the major guidelines for the future of disease research in this species and the consequent management applications, including the diseases to monitor and the best management measures.
While sarcoptic mange is the main health threat to naïve Iberian ibex populations, associated with morbidity and mortality with demographic impact, most of the affected populations recover from the initial outbreaks and achieve an enzootic status, where the disease no longer hampers population viability. Thus, applying empiric measures to manage this disease without scientific validation may not only be useless or even counterproductive, but can also negatively affect the evolution from epidemic outbreak to enzootic status by eliminating potentially resistant individuals. Moreover, apart from being inefficient, massive on-field administration of antiparasitic drugs (namely, macrocyclic lactones) intended for individual parenteral use is a major animal welfare, environmental, and health concern. Since domestic livestock has been identified as the origin of sarcoptic mange outbreaks in Iberian ibex, surveillance and control of this disease in small ruminant livestock sympatric with Iberian ibex are probably more efficient management options.
The review of other diseases reported in Iberian ibex raises doubts about the rationale for the diseases included in the health surveillance of this species, such as tuberculosis or brucellosis, since Iberian ibex seems not to be a reservoir for these diseases and even rarely if ever affected by them. Instead, other diseases that might be relevant in the health surveillance of Iberian ibex populations deserve further research and monitoring to elucidate their potential effects in the population dynamics of the species.
Acknowledgements
The authors are grateful to Fundación Artemisan (https://fundacionartemisan.com/), which promotes environmental conservation and sustainable game management through research and communication. Fundación Artemisan promoted a holistic-approach study on Iberian ibex in Spain, which gathered together the authors and originated the idea for this article. This review benefitted from the knowledge generated through the research grants project 173/2009/M/00, 03/15/M/00, 861_11_M_00, and 2016/00014/M, funded by the Consejería de Medio Ambiente de la Junta de Andalucía, and CGL2012-40043-C02-01, CGL2012-40043-C02-02, and CGL2016-80543-P, funded by the Spanish Ministerio de Economía y Competitividad. The authors’ research activities are partially supported by the Plan Andaluz de Investigación (RNM-118 group). MV was supported by a FI-GENCAT Fellowship (2020_FI_B2_00049, co-financed by Agència de Gestió d’Ajuts Universitaris i de Recerca of the Generalitat de Catalunya and the European Social Fund). GM is a Serra Húnter Fellow.
Author contribution
MV, PF, RCSE, ARB, FJCML, JESP, JMP, and JEG compiled the information and drafted the sarcoptic mange section of the manuscript; PP, JRLO, SL, GM, ST, JEST, and RV gathered information on other diseases; JRLO drafted the section on other diseases, elaborated all the tables, and prepared Figs. 5 and 6; JEG prepared Fig. 2; JEG and JRLO prepared Figs. 1 and 4; JEG and MV prepared Fig. 3; JEG, JESP, and JRLO prepared Fig. 7. All the authors critically revised the manuscript and made significant contributions to the text and tables.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona. Part of the authors benefitted of the support of the Consejería de Medio Ambiente of the Junta de Andalucía (Spain) to the group RNN 118 through the grants 173/2009/M/00; 03/15/M/00; 861_11_M_00, 2016/00014/M. This review benefitted from funding of the Spanish Ministerio de Economía y Competitividad through the grants CGL2012-40043-C02-01, CGL2012-40043-C02-02, and CGL2016-80543-P. Marta Valldeperes was supported by the predoctoral grant 2020_FI_B2_00049, funded by the Agència de Gestió d'Ajuts Universitaris i de Recerca of the Generalitat de Catalunya (Spain) and the European Social Fund.
Data Availability
All the data used to generate this review are publicly available. The authors will share information on data location upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Samra MT, Hago BED, Aziz MA, Awad FW. Sarcoptic mange in sheep in the Sudan. Ann Trop Med Parasitol. 1981;75(6):639–645. doi: 10.1080/00034983.1981.11687495. [DOI] [PubMed] [Google Scholar]
- Acevedo P, Cassinello J. Biology, ecology and status of Iberian ibex Capra pyrenaica: a critical review and research prospectus. Mamm Rev. 2009;39(1):17–32. doi: 10.1111/j.1365-2907.2008.00138.x. [DOI] [Google Scholar]
- Aguirre AA, Starkey EE, Hansen DE. Wildlife diseases in national park ecosystems. Wildl Soc Bull. 1995;23:415–419. [Google Scholar]
- Alados CL, Escós J (2017) Cabra montés – Capra pyrenaica. In: Salvador A, Cassinello J (eds) Enciclopedia Virtual de los Vertebrados Españoles. Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org. Accessed 17 Dec 2022
- Alasaad S, Granados JE, Cano-Manuel FJ, Meana A, Zhu XQ, Pérez JM. Epidemiology of fasciolosis affecting Iberian ibex (Capra pyrenaica) in southern Spain. Parasitol Res. 2008;102(4):751–755. doi: 10.1007/s00436-007-0830-2. [DOI] [PubMed] [Google Scholar]
- Alasaad S, Permunian R, Gakuya F, Mutinda M, Soriguer RC, Rossi L. Sarcoptic-mange detector dogs used to identify infected animals during outbreaks in wildlife. BMC Vet Res. 2012;8:110. doi: 10.1186/1746-6148-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasaad S, Granados JE, Fandos P, Cano-Manuel FJ, Soriguer RC, Pérez JM. The use of radio-collars for monitoring wildlife diseases: a case study from Iberian ibex affected by Sarcoptes scabiei in Sierra Nevada. Spain Parasites Vectors. 2013;6:242. doi: 10.1186/1756-3305-6-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasaad S, Min AM, Pasquetti M, Alagaili AN, D’Amelio S, Berrilli F, Obanda V, Gebely MA, Soriguer RC, Rossi L. Universal conventional and real-time PCR diagnosis tools for Sarcoptes scabiei. Parasites Vectors. 2015;8(1):58. doi: 10.1186/s13071-015-1204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almería S, Vidal D, Ferrer D, Pabón M, Fernández-de-Mera MI, Ruiz-Fons F, Alzaga V, Marco I, Calvete C, Lavin S, Gortazar C, López-Gatius F, Dubey JP. Seroprevalence of Neospora caninum in non-carnivorous wildlife from Spain. Vet Parasitol. 2007;143(1):21–28. doi: 10.1016/j.vetpar.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Almería S, Cano-Terriza D, Prieto P, Dubey JP, Jiménez-Martín D, Castro-Scholten S, Paniagua J, García-Bocanegra I (2021) Seroprevalence and risk factors of Toxoplasma gondii infection in wild ungulates that cohabit in a natural park with human–animal interaction in the Mediterranean ecosystem. Zoonoses Public Health 68(3):263–270. 10.1111/zph.12821 [DOI] [PubMed]
- Andriantsoanirina V, Izri A, Botterel F, Foulet F, Chosidow O, Durand R. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20(2):2013–2015. doi: 10.1111/1469-0691.12334. [DOI] [PubMed] [Google Scholar]
- Antón JM, Arenas AJ, Astorga RJ, Boticario D, Buzzi A, Carrasco PA, Chirosa M, Cubero MJ, Delibes-Senna JR, De Martin P, Fandos P, Ferroglio E, Gaina C, Gallego L, García J, Gil JM, González FJ, González M, Granados JE, Habela MÁ, León L, Linde M, Márquez FJ, Meana A, Meneguz PG, Morales A, Moreno PA, Palmquist P, Peña J, Pérez MC, Pérez JM, Pérez J, Rambozzi L, Riquelme JA, Rodolfi M, Rodríguez JJ, Rossi L, Ruiz Martínez I, Sangenís J, Serrano E, Soriguer RC, Weykam S (1999) Seguimiento y control de la sarna sarcóptica que afecta a las poblaciones de cabra montés (Capra pyrenaica hispanica) existentes en Andalucía. Unpublished report, 278 pp. https://www.researchgate.net/publication/255997536_Seguimiento_y_control_de_la_Sarna_sarcoptica_que_afecta_a_las_poblaciones_de_Cabra_montes_Capra_pyrenaica_hispanica_existentes_en_Andalucia
- Antón JM, Boticario D, Granados JE, Habela MA, Márquez FJ, Meana A, Meneguz PG, Peña J, Pérez J, Pérez JM, Pérez MC, Rossi L, Ruiz-Martínez I, Sangenís J. Estudio de las enfermedades parasitarias de la cabra montés. In: Pérez JM, editor. Distribución, genética y status sanitario de las poblaciones andaluzas de cabra montés. Jaén, Spain: Universidad de Jaén; 2002. pp. 116–153. [Google Scholar]
- Arenas AJ, Gómez F, Salas R, Carrasco P, Borge C, Maldonado A, O´Brien DJ, Martínez-Moreno FJ, An evaluation of the application of infrared thermal imaging to the tele-diagnosis of sarcoptic mange in the Spanish ibex (Capra pyrenaica) Vet Parasitol. 2002;109(1–2):111–117. doi: 10.1016/s0304-4017(02)00248-0. [DOI] [PubMed] [Google Scholar]
- Arenas A, García I, Gómez F, Salas R, Rojas A, Sánchez-Vera M, Arenas AJ (2009a) Determinación de los puntos calientes en el control de la epidemia de sarna sarcóptica en una población de cabra montés (Capra pyrenaica) en libertad. V World Conference on Mountain Ungulates, Granada, Spain, p. 295
- Arenas A, Gómez F, Salas R, García I, Carbonero A, Borge A, Perea A, Arenas AJ (2009b) Efectividad de la caza selectiva como medida de control frente a la sarna sarcóptica en una población de cabra montés (Capra pyrenaica) en libertad. V World Conference on Mountain Ungulates, Granada, Spain, p. 299
- Arlian LG, Runyan RA, Achar S, Estes SA (1984a) Survival and infestivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol 11(2):210–215. 10.1016/s0190-9622(84)70151-4 [DOI] [PubMed]
- Arlian LG, Runyan RA, Estes SA. Cross infestivity of Sarcoptes scabiei. J Am Acad Dermatol. 1984;10(6):979–986. doi: 10.1016/s0190-9622(84)80318-7. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Vyszenski-Moher DL (1988) Life cycle of Sarcoptes scabiei var. canis. J Parasitol 74(3):427–430 [PubMed]
- Arlian LG. Biology, host relations and epidemiology of Sarcoptes scabiei. Ann Rev Entomol. 1989;34:139–161. doi: 10.1146/annurev.en.34.010189.001035. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Vyszenski-Moher DL, Pole MJ (1989) Survival of adults and development stages of Sarcoptes scabiei var. canis when off the host. Exp Appl Acarol 6(3):181–187. 10.1007/BF01193978 [DOI] [PubMed]
- Arlian LG, Morgan MS, Vyszenski-Moher DL, Stemmer BL. Sarcoptes scabiei: the circulating antibody response and induced immunity to scabies. Exp Parasitol. 1994;78(1):37–50. doi: 10.1006/expr.1994.1004. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Rapp CM, Vyszenski-Moher DL, Morgan MS. Sarcoptes scabiei: histopathological changes associated with acquisition and expression of host immunity to scabies. Exp Parasitol. 1994;78(1):51–63. doi: 10.1006/expr.1994.1005. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Rapp CM, Morgan MS. Resistance and immune response in scabies-infested hosts immunized with Dermatophagoides mites. Am J Trop Med Hyg. 1995;52(6):539–545. doi: 10.4269/ajtmh.1995.52.539. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL. The development of protective immunity in canine scabies. Vet Parasitol. 1996;62:133–142. doi: 10.1016/0304-4017(95)00854-3. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasites Vectors. 2017;10:297. doi: 10.1186/s13071-017-2234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal MC, Fernández de Luco D (2017) Melanoma diseminado y feocromocitoma en un macho montés Capra pyrenaica hispanica. 35èmes Rencontres du Groupe d’Étude sur l’Eco-pathologie de la Faune Sauvage de Montagne (GEEFSM). Cofrentes, Valencia (Spain), June 2017 1st-4th, Abstracts
- Arnal MC, Fernández de Luco D, Ferroglio E, Formisano F, Gortázar C, Höfle U, Martín MP, Meneguz PG, Revilla M, Rossi L, Vicente J (2006) Neoplasias en rumiantes silvestres. 24émes Rencontres du Groupe d’Étude sur l’Eco-pathologie de la Faune Sauvage de Montagne (GEEFSM). Roquetes, Tarragona (Spain), June 9th-11th 2006, Abstracts
- Arnal M, Herrero J, de la Fe C, Revilla M, Prada C, Martínez-Durán D, Gómez-Martín A, Fernández-Arberas O, Amores J, Contreras A, García-Serrano A, de Luco DF (2013) Dynamics of an infectious keratoconjunctivitis outbreak by Mycoplasma conjunctivae on Pyrenean chamois Rupicapra p. pyrenaica. PLoS One 8(4):e61887. 10.1371/journal.pone.0061887 [DOI] [PMC free article] [PubMed]
- Arnal MC, Revilla M, Corrales JC, Martínez D, Sánchez A, Herrero J, Contreras A, Fernández de Luco D (2009) Queratoconjuntivitis infecciosa por M. conjunctivae en sarrio (Rupicapra p. pyrenaica) y en cabra montés (Capra pyrenaica) en Aragón. XXI Reunión de la Sociedad Española de Anatomía Patológica Veterinaria. Lugo, Spain. June 2009 24th to 26th, p. 61
- Astorga Márquez RJ, Carvajal A, Maldonado A, Gordon SV, Salas R, Gómez-Guillamón F, Sánchez-Baro A, López-Sebastián A, Santiago-Moreno J. Influence of cohabitation between domestic goat (Capra aegagrus hircus) and Iberian ibex (Capra pyrenaica hispanica) on seroprevalence of infectious diseases. Eur J Wildl Res. 2014;60:387–390. doi: 10.1007/s10344-013-0785-9. [DOI] [Google Scholar]
- Baird GJ, Fontaine MC. Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J Comp Pathol. 2007;137:179–210. doi: 10.1016/j.jcpa.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Barroso P, Relimpio D, Zearra JA, Cerón JJ, Palencia P, Cardoso B, Ferreras E, Escobar M, Cáceres G, López-Olvera JR, Gortázar C (2022) Using integrated wildlife monitoring to prevent future pandemics through one health approach. One Health 16:100479. 10.1016/j.onehlt.2022.100479 [DOI] [PMC free article] [PubMed]
- Bates P (1997) The pathogenesis and ageing of sheep scab lesions-part 2. State Veterinary Journal, United Kingdom
- Belloy L, Giacometti M, Abdo EA, Nicolet J, Rawinklera MK, Anovskyc MJ, Bruderer U, Frey JF, Abdo EM, Nicolet J, Krawinkler M, Janovsky M, Bruderer U, Frey JF. Detection of specific Mycoplasma conjunctivae antibodies in the sera of sheep with infectious keratoconjunctivitis. Vet Res. 2001;32:155–164. doi: 10.1051/vetres:2001118. [DOI] [PubMed] [Google Scholar]
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Berrilli F, D’Amelio S, Rossi L. Ribosomal and mitochondrial DNA sequence variation in Sarcoptes mites from different hosts and geographical regions. Parasitol Res. 2002;88(8):772–777. doi: 10.1007/s00436-002-0655-y. [DOI] [PubMed] [Google Scholar]
- Besser TE, Cassirer EF, Potter KA, VanderSchalie J, Fischer A, Knowles DP, Herndon DR, Rurangirwa FR, Weiser GC, Srikumaran S. Association of Mycoplasma ovipneumoniae infection with population-limiting respiratory disease in free-ranging Rocky Mountain bighorn sheep (Ovis canadensis canadensis) J Clin Microbiol. 2008;46(2):423–430. doi: 10.1128/JCM.01931-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat SA, Mounsey KE, Liu X, Walton SF. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasites Vectors. 2017;10:385. doi: 10.1186/s13071-017-2320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlenga G, Tektoff J, Richard Y, Joubert L, Gastellu J, Prave M, Dumont O, Favrier C, Oudar J. Isolement de 5 souches de “Rickettsia” des lésions de kérato-conjonctivite du chamois ou du bouquetin et du mouton d’alpage. Sci Vétérinaire Médecine Comparée. 1983;85:6–281. [Google Scholar]
- Black SR, Barker IK, Mehren KG, Crawshaw GJ, Rosendal S, Ruhnke L, Thorsen J, Carman PS. An epizootic of Mycoplasma ovipneumoniae infection in captive Dall’s sheep (Ovis dalli dalli) J Wildl Dis. 1988;24(4):627–635. doi: 10.7589/0090-3558-24.4.627. [DOI] [PubMed] [Google Scholar]
- Blanco LA, Marcotegui Jaso MA, Frutos Palacios I, Esperanza Martin P, Saez de Ocariz C. Querato-conjuntivitis clamidial en el rebeco Rupicapra rupicapra ssp. Pyrenaica INIA Ser Ganad. 1982;17:79–85. [Google Scholar]
- Bliss DH, Moore RD, Kvasnicka WG (2008) Parasite resistance in US cattle. Proceedings of the 41st Annual Conference of the American Association of Bovine Practitioners, Charlotte, NC, USA, P- 109
- Bornstein ST, Mörner T, Samuel WM (2001) Sarcoptes scabiei and sarcoptic mange. In: Samuel WM, Pybus MJ, Kocan AA (eds) Parasitic Diseases of Wild Mammals, 2nd ed. Manson Publishing/The Veterinary Press, London, pp 107–119
- Bornstein S, Frössling J, Näslund K, Zakrisson G, Mörner T. Evaluation of a serological test (indirect ELISA) for the diagnosis of sarcoptic mange in red foxes (Vulpes vulpes) Vet Dermatol. 2006;17(6):411–416. doi: 10.1111/j.1365-3164.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- Brewster K, Henke SE, Hilton C, Ortega-S A., Jr Use of remote cameras to monitor the potential prevalence of sarcoptic mange in Southern Texas, USA. J Wildl Dis. 2017;53:377–381. doi: 10.7589/2016-08-180. [DOI] [PubMed] [Google Scholar]
- Calero-Bernal R, García-Moreno A, González-Barrio D, Nieto-Rodríguez JM, Fernández-Llario P, Hermoso de Mendoza J, Habela MÁ. Vigilancia de enfermedades infecciosas de la cabra montés (Capra pyrenaica victoriae) en el oeste de España: implicaciones para la salud y la conservación. Galemys. 2020;32:13–20. doi: 10.7325/Galemys.2020.A2. [DOI] [Google Scholar]
- Camacho L, Gómez-Guillamón F, Risalde MA, González D, Zorrilla I, García-Bocanegra I (2017) Primer caso de ectima contagioso en cabra montés (Capra pyrenaica hispanica) en libertad. 35émes Rencontres du G.E.E.F.S.M. Cofrentes, Valencia, Spain, June 1st-4th, 2017
- Cano E, Notario A, Cano J, Marín JL, Montero F, Montero E (1996) Nematodosis broncopulmonares en Capra pyrenaica hispanica de Sierra Nevada. I Parasitología. Primeras Jornadas sobre ecopatología de mamíferos silvestres. Bellaterra, Spain, p. 41
- Cano-Manuel Herrero A (2017) Study of Toxoplasma gondii and Neospora caninum in the Spanish ibex (Capra pyrenaica) population of Sierra Nevada National Park (Andalucía, S.E. España). Trabajo de Fin de Máster. Máster Universitario en Gestión de la Fauna Silvestre, Universidad de Murcia, Spain
- Cardoso B, García-Bocanegra I, Acevedo P, Cáceres G, Alves PC, Gortázar C. Stepping up from wildlife disease surveillance to integrated wildlife monitoring in Europe. Res Vet Sci. 2021;144:149–156. doi: 10.1016/j.rvsc.2021.11.003. [DOI] [PubMed] [Google Scholar]
- Carrau T, Martínez-Carrasco C, Garijo MM, Alonso F, Vizcaíno LL, Herrera-Russert J, Tizzani P, Ruiz de Ybáñez R. Epidemiological approach to nematode polyparasitism occurring in a sympatric wild ruminant multi-host scenario. J Helminthol. 2021;95:e29. doi: 10.1017/S0022149X21000183. [DOI] [PubMed] [Google Scholar]
- Carrau T, Martínez-Carrasco C, Garijo MM, Alonso F, Ruiz de Ybáñez R, Tizzani P. Multivariate abundance analysis of multi-host/multi-parasite lungworms in a sympatric wild ruminant population. Diversity. 2021;13(6):227. doi: 10.3390/d13060227. [DOI] [Google Scholar]
- Carrera-Faja L, Cardells J, Pailler-García L, Lizana V, Alfaro-Deval G, Espunyes J, Napp S, Cabezón Ó. Evidence of prolonged Crimean-Congo hemorrhagic fever virus endemicity by retrospective serosurvey. Eastern Spain Emerg Infect Dis. 2022;28(5):1031–1034. doi: 10.3201/eid2805.212335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carricondo-Sánchez D, Odden M, Linell JDC, Odden J (2017) The range of the mange: spatiotemporal patterns of sarcoptic mange in red foxes (Vulpes vulpes) as revealed by camera trapping. PLoS One 12:e0176200. 10.1371/journal.pone.0176200 [DOI] [PMC free article] [PubMed]
- Carta T, Martin-Hernando MP, Boadella M, Fernández-de-Mera IG, Balseiro A, Sevilla IA, Vicente J, Maio E, Vieira-Pinto M, Alvarez J, Pérez-de-la-Lastra JM, Garrido J, Gortázar C. No evidence that wild red deer (Cervus elaphus) on the Iberian Peninsula are a reservoir of Mycobacterium avium subspecies paratuberculosis infection. Vet J. 2012;192(3):544–546. doi: 10.1016/j.tvjl.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Carta T, Álvarez J, Pérez de la Lastra JM, Gortázar C. Wildlife and paratuberculosis: a review. Res Vet Sci. 2013;94(2):191–197. doi: 10.1016/j.rvsc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Carvalho J, Granados JE, López-Olvera JR, Cano-Manuel FJ, Pérez JM, Fandos P, Soriguer RC, Velarde R, Fonseca C, Ráez-Bravo A, Espinosa J, Pettorelli N, Serrano E. Sarcoptic mange breaks up bottom-up regulation of body condition in a large herbivore population. Parasites Vectors. 2015;8(1):572. doi: 10.1186/s13071-015-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R, Prieto M, Balseiro A, Solano P, Parra F, Alonso JMM. Identification and heterologous expression of a Sarcoptes scabiei cDNA encoding a structural antigen with immunodiagnostic potential. Vet Res. 2007;38(3):435–450. doi: 10.1051/vetres:2007007. [DOI] [PubMed] [Google Scholar]
- Castellà J, Marco I, Lavín S, Ortuño A (1996) Los ixódidos de la cabra montés y del muflón en los Puertos de Tortosa y Beceite. Primeras Jornadas sobre ecopatología de mamíferos silvestres. Bellatera, Spain, p. 56
- Castro I, de la Fuente A, Fandos P, Cano-Manuel F, Granados JE, Soriguer RC, Pérez JM. On the population biology of Sarcoptes scabiei infesting Iberian ibex (Capra pyrenaica) Int J Acarol. 2016;42(1):7–11. doi: 10.1080/01647954.2015.1109710. [DOI] [Google Scholar]
- Cevidanes A, Pérez JM, Mentaberre G, Lavín S, Velarde R. First report of Straelensia cynotis Fain and Le Net, 2000 (Trombidiformes: Leeuwenhoekiidae) parasitizing Capra pyrenaica (Artiodactyla: Bovidae) with histopathological analysis. Int J Acarol. 2019;45(4):214–216. doi: 10.1080/01647954.2019.1586994. [DOI] [Google Scholar]
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatol. 2019;235(2):79–80. doi: 10.1159/000495290. [DOI] [PubMed] [Google Scholar]
- Colom-Cadena A, Velarde R, Salinas J, Borge C, García-Bocanegra I, Serrano E, Gassó D, Bach E, Casas-Díaz E, López-Olvera JR, Lavín S, León-Vizcaíno L, Mentaberre G. Management of a caseous lymphadenitis outbreak in a new Iberian ibex (Capra pyrenaica) stock reservoir. Acta Vet Scand. 2014;56:83. doi: 10.1186/s13028-014-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3(2):213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council Directive 2000/75/EC of 20 November 2000, laying down specific provisions for the control and eradication of bluetongue. Official Journal of the European Communities, 22.12.2000, L327/74-L327/83. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32000L0075f&from=en
- Cross PC, Almberg ES, Haase CG, Hudson PJ, Maloney SK, Metz MC, Munn AJ, Nugent P, Putzeys O, Stahler DR, Stewart AC, Smith DW. Energetic costs of mange in wolves estimated from infrared thermography. Ecology. 2016;97(8):1938–1948. doi: 10.1890/15-1346.1. [DOI] [PubMed] [Google Scholar]
- Cubero MJ, González M, León L (2002) Enfermedades infecciosas de las poblaciones de cabra montés In: Pérez-Jiménez JM (ed) Distribución, genética y estatus sanitario de las poblaciones andaluzas de cabra montés. Junta de Andalucía, Granada, Spain, pp 197–254
- Cunningham AA, Daszak P, Wood JLN. One Health, emerging infectious diseases and wildlife: two decades of progress? Phil Trans R Soc B. 2017;372(1725):20160167. doi: 10.1098/rstb.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie BJ, Harumal P, McKinnon M, Walton SF. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis. 2004;39(1):e8–12. doi: 10.1086/421776. [DOI] [PubMed] [Google Scholar]
- Dagnall GJR. An investigation of colonization of the conjunctival sac of sheep by bacteria and mycoplasmas. Epidemiol Infect. 1994;112:561–567. doi: 10.1017/s0950268800051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnall GJR. The role of Branhamella ovis, Mycoplasma conjunctivae and Chlamydia psittaci in conjunctivitis of sheep. Br Vet J. 1994;150:65–71. doi: 10.1016/S0007-1935(05)80097-1. [DOI] [PubMed] [Google Scholar]
- Dassanayake RP, Shanthalingam S, Herndon CN, Subramaniam R, Lawrence PK, Bavananthasivam J, Cassirer EF, Haldorson GJ, Foreyt WJ, Rurangirwa FR, Knowles DP, Besser TE, Srikumaran S. Mycoplasma ovipneumoniae can predispose bighorn sheep to fatal Mannheimia haemolytica pneumonia. Vet Microbiol. 2010;145:354–359. doi: 10.1016/j.vetmic.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Dassanayake RP, Shanthalingam S, Subramaniam R, Herndon CN, Bavananthasivam J, Haldorson GJ, Foreyt WJ, Evermann JF, Herrmann-Hoesing LM, Knowles DP, Srikumaran S. Role of Bibersteinia trehalosi, respiratory syncytial virus, and parainfluenza-3 virus in bighorn sheep pneumonia. Vet Microbiol. 2013;162:166–172. doi: 10.1016/j.vetmic.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Davey RB, Pound JM, Miller JA, Klavons JA. Therapeutic and persistent efficacy of a long-acting (LA) formulation of ivermectin against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and sera concentration through time in treated cattle. Vet Parasitol. 2010;169(1–2):149–156. doi: 10.1016/j.vetpar.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Davies PR. Sarcoptic mange and production performance of swine: a review of the literature and studies of associations between mite infestation, growth rate and measures of mange severity in growing pigs. Vet Parasitol. 1995;60(3):249–264. doi: 10.1016/0304-4017(95)00795-3. [DOI] [PubMed] [Google Scholar]
- Degiorgis MP, Obrecht E, Ryser A, Giacometti M. The possible role of eye-frequenting flies in the transmission of Mycoplasma conjunctivae. Mitt Schweiz Entomol Ges. 1999;72:189–194. doi: 10.5169/seals-402751. [DOI] [Google Scholar]
- Degiorgis MP, Frey J, Nicolet J, Abdo EM, Fatzer R, Schlatter Y, Reist S, Janovsky M, Giacometti M. An outbreak of infectious keratoconjunctivitis in Alpine chamois (Rupicapra r. rupicapra) in Simmental-Gruyères. Switzerland Schweiz Arch Tierheilkd. 2000;142:520–527. [Google Scholar]
- Desta GB, Jena PK, Hassen HM, Behera MR (2022) Factors associated with zoonosis and reverse zoonosis of Mycobacterium tuberculosis and Mycobacterium bovis in Ethiopia: a systematic review and meta-analysis. Int J Health Sci 6(S5):1630–1653. 10.53730/ijhs.v6nS5.8922
- Dias-Alves A, Cabezón Ó, Borel N, López-Olvera JR, Mentaberre G, Lavín S, Fernández-Aguilar X. Molecular detection and identification of Chlamydiaceae in the eyes of wild and domestic ruminant hosts from northern Spain. Pathogens. 2021;10(3):383. doi: 10.3390/pathogens10030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey AM, Loy JD, Bono JL, Smith TP, Apley MD, Lubbers BV, DeDonder KD, Capik SF, Larson RL, White BJ, Blom J, Chitko-McKown CG, Clawson ML. Large genomic differences between Moraxella bovoculi isolates acquired from the eyes of cattle with infectious bovine keratoconjunctivitis versus the deep nasopharynx of asymptomatic cattle. Vet Res. 2016;47:31. doi: 10.1186/s13567-016-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenis L, Spedicato R, Pepe E, Orusa R, Robetto S. Caseous lymphadenitis caused by Corynebacterium pseudotuberculosis in Alpine chamois (Rupicapra r. rupicapra): a review of 98 cases. J Comp Pathol. 2018;161:11–19. doi: 10.1016/j.jcpa.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Dorella FA, Pacheco LG, Oliveira SC, Miyoshi A, Azevedo V. Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet Res. 2006;37:201–218. doi: 10.1051/vetres:2005056. [DOI] [PubMed] [Google Scholar]
- Egwu G, Faull W, Bradbury J, Clarkson M. Ovine infectious keratoconjunctivitis: a microbiological study of clinically unaffected and affected sheep’s eyes with special reference to Mycoplasma conjunctivae. Vet Rec. 1989;125:253–256. doi: 10.1136/vr.125.10.253. [DOI] [PubMed] [Google Scholar]
- Ergonul O. Crimean-Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol. 2012;2:215–220. doi: 10.1016/j.coviro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Escobar LE, Carver S, Cross PC, Rossi L, Almberg ES, Yabsley MJ, Niedringhaus KD, Van Wick P, Dominguez-Villegas E, Gakuya F, Xie Y, Angelone S, Gortázar C, Astorga F. Sarcoptic mange: an emerging panzootic in wildlife. Transbound Emerg Dis. 2022;69(3):927–942. doi: 10.1111/tbed.14082. [DOI] [PubMed] [Google Scholar]
- Espinosa J, Granados JE, Cano-Manuel FJ, López-Olvera JR, Ráez-Bravo A, Romero D, Soriguer RC, Pérez JM, Fandos P. Sarcoptes scabiei alters follicular dynamics in female Iberian ibex through a reduction in body weight. Vet Parasitol. 2017;243:151–156. doi: 10.1016/j.vetpar.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Espinosa J, Pérez JM, López-Olvera JR, Ráez-Bravo A, Cano-Manuel FJ, Fandos P, Soriguer RC, Granados JE, Romero D. Evaluation of oxidant/antioxidant balance in Iberian ibex (Capra pyrenaica) experimentally infested with Sarcoptes scabiei. Vet Parasitol. 2017;242:63–70. doi: 10.1016/j.vetpar.2017.05.027. [DOI] [PubMed] [Google Scholar]
- Espinosa J, López-Olvera JR, Cano-Manuel FJ, Fandos P, Pérez JM, López-Graells C, Ráez-Bravo A, Mentaberre G, Romero D, Soriguer RC, Granados JE. Guidelines for managing captive Iberian ibex herds for conservation purposes. J Nat Conserv. 2017;40:24–32. doi: 10.1016/j.jnc.2017.09.002. [DOI] [Google Scholar]
- Espinosa J, Ráez-Bravo A, López-Olvera JR, Pérez JM, Lavín S, Tvarijonaviciute A, Cano-Manuel FJ, Fandos P, Soriguer RC, Granados JE, Romero D, Velarde R. Histopathology, microbiology and the inflammatory process associated with Sarcoptes scabiei infection in the Iberian ibex. Capra Pyrenaica Parasites Vectors. 2017;10:596. doi: 10.1186/s13071-017-2542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa J, Pérez JM, Raéz–Bravo A, Fandos P, Cano–Manuel FJ, Soriguer RC, López–Olvera JR, Granados JE (2020) Recommendations for the management of sarcoptic mange in free–ranging Iberian ibex populations. Animal Biodivers Conserv 43:137–149, 10.32800/abc.2020.43.0137
- Espunyes J, Cabezón O, Pailler-García L, Dias-Alves A, Lobato-Bailón L, Marco I, P. Ribas M, Encinosa-Guzmán PE, Valldeperes M, Napp S, Hotspot of Crimean-Congo hemorrhagic fever virus seropositivity in wildlife, northeastern Spain. Emerg Infect Dis. 2021;27(9):2480–2484. doi: 10.3201/eid2709.211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch J, Serrano E, Valldeperes M, López O, Lavín S, Ruiz-Olmo J, Velarde R (2020) Primer brote de mortalidad asociado a ectima contagioso en cabra montés. XI Reunión de Ungulados Silvestres Ibéricos, Madrid (Spain), October 23rd-24th 2020, p. 30
- Fain A. Etude de la variabilité de Sarcoptes scabiei avec une révision des Sarcoptidae. Acta Zool Pathol Antverp. 1968;47:1–196. [Google Scholar]
- Fain A. Epidemiological problems of scabies. Int J Dermatol. 1978;17(1):20–30. doi: 10.1111/j.1365-4362.1978.tb06040.x. [DOI] [PubMed] [Google Scholar]
- Falconi C, Oleaga Á, López-Olvera JR, Casais R, Prieto M, Gortázar C. Prevalence of antibodies against selected agents shared between Cantabrian chamois (Rupicapra pyrenaica parva) and domestic goats. Eur J Wildl Res. 2010;56:319–325. doi: 10.1007/s10344-009-0322-z. [DOI] [Google Scholar]
- Falk ES. Scabies. Some aspects of its relationship to the immune mechanisms: University of Tromso, Norway; 1982. p. 22. [Google Scholar]
- Fandos P (1991) La cabra montés (Capra pyrenaica) en el Parque Natural de las Sierras de Cazorla, Segura y Las Villas. ICONA – CSIC, Madrid, 176 pp.
- Fanelli A, Buonavoglia D, Pleite CMC, Tizzani P (2020) Paratuberculosis at European scale: an overview from 2010 to 2017. Vet Ital 56(1):13–21. 10.12834/VetIt.1829.9692.3 [DOI] [PubMed]
- Fernández-Aguilar X, Cabezón O, Marco I, Mentaberre G, Frey J, Lavín S, López-Olvera JR. Mycoplasma conjunctivae in domestic small ruminants from high mountain habitats in northern Spain. BMC Vet Res. 2013;9:253. doi: 10.1186/1746-6148-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Aguilar X, Cabezón Ó, Frey J, Velarde R, Serrano E, Colom-Cadena A, Gelormini G, Marco I, Mentaberre G, Lavín S, López-Olvera JR (2017a) Long-term dynamics of Mycoplasma conjunctivae at the livestock-wildlife interface in the Pyrenees. PLoS One 12 (10):e0186069. 10.1371/journal.pone.0186069 [DOI] [PMC free article] [PubMed]
- Fernández-Aguilar X, Cabezón Ó, Granados JE, Frey J, Serrano E, Velarde R, Cano-Manuel FJ, Mentaberre G, Ráez-Bravo A, Fandos P, López-Olvera JR. Post epizootic persistence of asymptomatic Mycoplasma conjunctivae infection in Iberian ibex. Appl Environ Microbiol. 2017;83(15):e00690–e717. doi: 10.1128/AEM.00690-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Aguilar X, Rossi L, Cabezón Ó, Giorgino A, Victoriano-Llopis I, Frey J, López-Olvera JR. Infectious keratoconjunctivitis and occurrence of Mycoplasma conjunctivae and Chlamydiaceae in small domestic ruminants from the Central Karakoram. Pakistan Vet Rec. 2017;181(9):237. doi: 10.1136/vr.103948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Aguilar X, López-Olvera JR, Puig-Ribas M, Begovoeva M, Velarde R, Cardells J, Cabezón Ó. Mycoplasma conjunctivae in insect vectors and anatomic locations related to transmission and persistence. Vet Microbiol. 2019;228(1):7–11. doi: 10.1016/j.vetmic.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Fernández Morán J, Gómez S, Ballesteros F, Quirós P, Benito JL, Feliu C, Nieto JM. Epizootiology of sarcoptic mange in a population of Cantabrian chamois (Rupicapra pyrenaica parva) in northwestern Spain. Vet Parasitol. 1997;73:163–171. doi: 10.1016/s0304-4017(97)00061-7. [DOI] [PubMed] [Google Scholar]
- Ferrer D, Castellà J, Gutiérrez JF, Lavín S, Marco I. Seroprevalence of Babesia ovis in Spanish ibex (Capra pyrenaica) in Catalonia, northeastern Spain. Vet Parasitol. 1998;75:93–98. doi: 10.1016/s0304-4017(97)00199-4. [DOI] [PubMed] [Google Scholar]
- Ferroglio E, Tolari F, BoIIo E, Bassano B. Isolation of Brucella melitensis from Alpine ibex. J Wildl Dis. 1998;34(2):400–402. doi: 10.7589/0090-3558-34.2.400. [DOI] [PubMed] [Google Scholar]
- Ferroglio E, Nebbia P, Robino P, Rossi L, Rosati S (2000) Mycobacterium paratuberculosis infection in two free-ranging Alpine ibex. Rev Sci Tech OIE 19(3):859–862. 10.20506/rst.19.3.1260 [DOI] [PubMed]
- Foreyt WJ, Jessup DA. Fatal pneumonia of bighorn sheep following association with domestic sheep. J Wildl Dis. 1982;18(2):163–168. doi: 10.7589/0090-3558-18.2.163. [DOI] [PubMed] [Google Scholar]
- Frosth S, König U, Nyman AK, Pringle M, Aspán A (2015) Characterisation of Dichelobacter nodosus and detection of Fusobacterium necrophorum and Treponema spp. in sheep with different clinical manifestations of footrot. Vet Microbiol 179(1–2):82–90. 10.1016/j.vetmic.2015.02.034 [DOI] [PubMed]
- Gakuya F, Rossi L, Ombui J, Maingi N, Muchemi G, Ogara W, Soriguer RC, Alasaad S. The curse of the prey: Sarcoptes mite molecular analysis reveals potential prey-to-predator parasitic infestation in wild animals from Masai Mara. Kenya Parasites Vectors. 2011;4:193. doi: 10.1186/1756-3305-4-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García I, Napp S, Casal J, Perea A, Allepuz A, Alba A, Carbonero A, Arenas A. Bluetongue epidemiology in wild ruminants from southern Spain. Eur J Wildl Res. 2009;55(2):173–178. doi: 10.1007/s10344-008-0231-6. [DOI] [Google Scholar]
- Garcia JP, Anderson M, Blanchard P, Mete A, Uzal FA. The pathology of enterotoxemia by Clostridium perfringens type C in calves. J Vet Diagn Investig. 2013;25(3):438–442. doi: 10.1177/1040638713483467. [DOI] [PubMed] [Google Scholar]
- García-Bocanegra I, Pérez de Val B, Arenas-Montes A, Paniagua J, Boadella M, Gortázar C, Arenas A (2012a) Seroprevalence and risk factors associated to Mycobacterium bovis in wild artiodactyl species from southern Spain, 2006–2010. PLoS ONE 7(4):e34908. 10.1371/journal.pone.0034908 [DOI] [PMC free article] [PubMed]
- García-Bocanegra I, Cabezón Ó, Pabón M, Gómez-Guillamón F, Arenas A, Alcaide E, Salas-Vega R, Dubey JP, Almería S. Prevalence of Toxoplasma gondii and Neospora caninum antibodies in Spanish ibex (Capra pyrenaica hispanica) Vet J. 2012;191:257–260. doi: 10.1016/j.tvjl.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Garnier A (2021) Conservation of ibex species (Capra ibex et Capra pyrenaica) in France: contribution of monitoring data from reintroduced and settled population. Biodiversity and Ecology. Université Paul Sabatier - Toulouse III, France. https://theses.hal.science/tel-03560035/
- Gauthier D (1991) La kerato-conjonctivite infectieuse du chamois. Etude épidémiologique dans le département de la Savoie, 1983–1990. DVM Thesis, Université Lyon, France.
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21(11):530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Giacometti M, Janovsky M, Belloy L, Frey J (2002a) Infectious keratoconjunctivitis of ibex, chamois and other Caprinae. Rev Sci Tech OIE 21:335–345. 10.20506/rst.21.2.1338 [DOI] [PubMed]
- Giacometti M, Janovsky M, Jenny H, Nicolet J, Belloy L, Goldschmidt-Clermont E, Frey J. Mycoplasma conjunctivae infection is not maintained in alpine chamois in eastern Switzerland. J Wildl Dis. 2002;38:297–304. doi: 10.7589/0090-3558-38.2.297. [DOI] [PubMed] [Google Scholar]
- Giacometti M, Nicolet J, Frey J, Krawinkler M, Meier W, Welle M, Johansson KE, Degiorgis MP. Susceptibility of alpine ibex to conjunctivitis caused by inoculation of a sheep-strain of Mycoplasma conjunctivae. Vet Microbiol. 1998;61:279–288. doi: 10.1016/s0378-1135(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Giacometti M, Nicolet J, Johansson KE, Naglic T, Degiorgis MP, Frey J. Detection and identification of Mycoplasma conjunctivae in infectious keratoconjunctivitis by PCR based on the 16S rRNA gene. Zentralbl Veterinarmed B. 1999;46(3):173–180. doi: 10.1046/j.1439-0450.1999.00218.x. [DOI] [PubMed] [Google Scholar]
- Gil Collado J (1960) Insectos y ácaros de los animales domésticos. Ed Salvat, Barcelona, Spain, 555 pp.
- Gobierno de Aragón (2022) Resolución de 29 de junio de 2022, del Director de Calidad y Seguridad Alimentaria, por la que se establece el protocolo de actuación ante el brote de sarna sarcóptica en cabra montés (Capra pyrenaica) en Aragón. https://www.aragon.es/documents/20127/3979473/Resolucion_SARNA_20220203.pdf/b5b223f6-01d8-1cc9-fc05-cce32a55788d?t=1644243287483
- Gobierno de España (2003) Ley 8/2003, de 24 de abril, de sanidad animal. Boletín Oficial del Estado 99, BOE-A-2003–8510. https://boe.es/buscar/pdf/2003/BOE-A-2003-8510-consolidado.pdf
- Gobierno de España, Ministerio de Agricultura, Pesca y Alimentación (1996) Real Decreto 2611/1996. de 20 de diciembre, por el que se regulan los programas nacionales de erradicaci6n de enfermedades de 105 animales. Boletín Oficial del Estado 307, 38115–38133. https://www.boe.es/boe/dias/1996/12/21/pdfs/A38115-38133.pdf
- Gobierno de España, Ministerio de Medio Ambiente, y Medio Rural y Marino (2009) Real Decreto 1082/2009, de 3 de julio, por el que se establecen los requisitos de sanidad animal para el movimiento de animales de explotaciones cinegéticas, de acuicultura continental y de núcleos zoológicos, así como de animales de fauna silvestre. Boletín Oficial del Estado 177, BOE-A-2009–12206, https://www.boe.es/buscar/pdf/2009/BOE-A-2009-12206-consolidado.pdf
- Gobierno de España, Ministerio de Agricultura, Alimentación y Medio Ambiente (2014) Real Decreto 526/2014, de 20 de junio, por el que se establece la lista de las enfermedades de los animales de declaración obligatoria y se regula su notificación. Boletín Oficial del Estado 167, BOE-A-2014–7291. https://www.boe.es/buscar/pdf/2014/BOE-A-2014-7291-consolidado.pdf
- Gobierno de España, Ministerio de Agricultura, Pesca y Alimentación (2022) Plan Nacional de Vigilancia Sanitaria en Fauna Silvestre. https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/pnvfs2022_tcm30-437517.pdf
- Gómez-Guillamón E, Díaz-Cao JM, Camacho-Sillero L, Cano-Terriza D, Alcaide EM, Cabezón Ó, Arenas A, García-Bocanegra I. Spatiotemporal monitoring of selected pathogens in Iberian ibex (Capra pyrenaica) Transbound Emerg Dis. 2020;67(5):2259–2265. doi: 10.1111/tbed.13576. [DOI] [PubMed] [Google Scholar]
- Gómez-Guillamón F, Caballero-Gómez J, Agüero M, Camacho-Sillero L, Risalde MÁ, Zorrilla I, Villalba R, Rivero-Juárez A, García-Bocanegra I. Re-emergence of bluetongue virus serotype 4 in Iberian ibex (Capra pyrenaica) and sympatric livestock in Spain, 2018–2019. Transbound Emerg Dis. 2021;68(2):458–466. doi: 10.1111/tbed.13696. [DOI] [PubMed] [Google Scholar]
- Gómez-Olivencia A, Arceredillo D, Ríos-Garaizar J, Garate D, Iriarte E, San Pedro Z. Dental anomalies in the mandible of Capra pyrenaica: presence of two permanent fourth premolars in a Pleistocene wild goat from Arlanpe Cave (Bizkaia, Northern Spain) Int J Osteoarchaeol. 2011;23:737–745. doi: 10.1002/oa.1295. [DOI] [Google Scholar]
- Gonzalez G, Crampe JP. Mortality patterns in a protected population of isards (Rupicapra pyrenaica) Can J Zool. 2001;79:2072–2079. doi: 10.1139/cjz-79-11-2072. [DOI] [Google Scholar]
- González-Candela M, León-Vizcaíno L. Sarna sarcóptica en la población de arrui (Ammotragus lervia) del Parque Regional de Sierra Espuña. Murcia Galemys. 1999;11(2):43–58. [Google Scholar]
- González-Candela M, León-Vizcaíno L, Cubero-Pablo MJ. Population effects of sarcoptic mange in Barbary sheep (Ammotragus lervia) from Sierra Espuña Regional Park. Spain J Wildl Dis. 2004;40(3):456–465. doi: 10.7589/0090-3558-40.3.456. [DOI] [PubMed] [Google Scholar]
- González-Candela M, Cubero M, Martín-Atance P, León L. Potential pathogens carried by Spanish ibex (Capra pyrenaica hispanica) in southern Spain. J Wildl Dis. 2006;42(2):325–334. doi: 10.7589/0090-3558-42.2.325. [DOI] [PubMed] [Google Scholar]
- González-Candela M, Serrano E, Martínez-Carrasco C, Martín-Atance P, Cubero MJ, Alonso F, León L. Coinfection is an important factor in epidemiological studies: the first serosurvey of the aoudad (Ammotragus lervia) Eur J Clin Microbiol Infect Dis. 2009;28:481–489. doi: 10.1007/s10096-008-0654-8. [DOI] [PubMed] [Google Scholar]
- González-Candela M, Verbisck-Bucker G, Martín-Atance P, Cubero-Pablo MJ, León-Vizcaíno L. Mycoplasmas isolated from Spanish ibex (Capra pyrenaica hispanica): frequency and risk factors. Vet Rec. 2007;161(5):167–168. doi: 10.1136/vr.161.5.167. [DOI] [PubMed] [Google Scholar]
- González-Insa N (2021) Estudio seroepidemiológico de distintas enfermedades compartidas entre rumiantes domésticos y silvestres del Parque Natural de Cazorla, Segura y las Villas. Master Thesis. Máster Universitario en Gestión de la Fauna Silvestre, Universidad de Murcia, Murcia, Spain
- González-Quirós P, Silva Manzano P, Solano Rodríguez S. Demographic patterns during an epizootic of sarcoptic mange in a Cantabrian chamois (Rupicapra pyrenaica parva) population. Pirineos. 2002;157:199–200. doi: 10.3989/pirineos.2002.v157.71. [DOI] [Google Scholar]
- González-Quirós P, Silva Manzano P, Solano Rodríguez S. Population evolution of Cantabrian chamois (Rupicapra pyrenaica parva) with sarcoptic mange (Sarcoptes scabiei) in centre-eastern Asturias (northwest Spain) Pirineos. 2002;157:201–210. doi: 10.3989/pirineos.2002.v157.72. [DOI] [Google Scholar]
- Gortázar C, Ferroglio E, Höfle U, Frölich K, Vicente J. Diseases shared between wildlife and livestock: a European perspective. Eur J Wildl Res. 2007;53:241–256. doi: 10.1007/s10344-007-0098-y. [DOI] [Google Scholar]
- Gortázar C, Ruiz-Fons JF, Höfle U. Infections shared with wildlife: an updated perspective. Eur J Wildl Res. 2016;62:511–525. doi: 10.1007/s10344-016-1033-x. [DOI] [Google Scholar]
- Granados JE, Pérez JM, Chirosa M, Ruiz-Martínez I (1996a) Aspectos de la biología de la pupación de Oestrus caucasicus Grunin (Diptera: Oestridae). VII Congreso Ibérico de Entomología, Santiago de Compostela, Spain, 203 pp.
- Granados JE, Ruiz-Martínez I, Pérez P-A, JM, Experiencias de mantenimiento de cabra montés en cautividad. Bulletin D'informations Sur La Pathologie Des Animaux Sauvages (BIPAS) 1996;15:59–67. [Google Scholar]
- Granados JE, Pérez JM, Márquez FJ, Serrano E, Soriguer RC, Fandos P. La cabra montés (Capra pyrenaica, Schinz 1838) Galemys. 2001;13(1):3–37. [Google Scholar]
- Granados JE, Cano-Manuel FJ, Fandos P, Pérez JM, Alasaad S, Sarasa M, Collantes-Fuentes S, Castro I, Palanco L, García-Sánchez S, González D, Soriguer RC (2011) Uso de la termografía para determinar el grado de infestación por Sarcoptes scabiei en ejemplares de cabra montés (Capra pyrenaica) en el P.N. de Sierra Nevada. 29èmes Rencontres du Groupe d’Etudes sur l’Ecopathologie de la Faune Sauvage de Montagne, Nerja (Spain), October 6th-9th, 2011.
- Granados JE, Ros-Candeira A, Pérez-Luque AJ, Moreno-Llorca R, Cano-Manuel FJ, Fandos P, Soriguer RC, Espinosa Cerrato J, Pérez Jiménez JM, Ramos B, Zamora R. Long-term monitoring of the Iberian ibex population in the Sierra Nevada of the southeast Iberian Peninsula. Scientific Data. 2020;7:203. doi: 10.1038/s41597-020-0544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guberti V, Zamboni L. Can the host resistance hypothesis explain the cyclic patterns observed in Sarcoptes scabiei in chamois (Rupicapra rupicapra)? Parassitologia. 2000;42(Supplement 1):72. [Google Scholar]
- Haas C, Rossi S, Meier R, Ryser-Degiorgis MP. Evaluation of a commercial ELISA for the detection of antibodies to Sarcoptes scabiei in wild boar (Sus scrofa) J Wildl Dis. 2015;51(3):729–733. doi: 10.7589/2014-09-222. [DOI] [PubMed] [Google Scholar]
- Haas C, Origgi FC, Akdesir E, Batista-Linhares M, Giovannini S, Mavrot F, Casaubon J, Ryser-Degiorgis MP (2015b) First detection of sarcoptic mange in free-ranging wild boar (Sus scrofa) in Switzerland. Schweiz Arch Tierheilkd 157:269–275. 10.17236/sat00020 [DOI] [PubMed]
- Haas C, Origgi FC, Rossi S, López-Olvera JR, Rossi L, Castillo-Contreras R, Malmsten A, Dalin AM, Orusa R, Robetto S, Pignata L, Lavín S, Ryser Degiorgis MP. Serological survey in wild boar (Sus scrofa) in Switzerland and other European countries: Sarcoptes scabiei may be more widely distributed than previously thought. BMC Vet Res. 2018;14:117. doi: 10.1186/s12917-018-1430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hars J, Rautureau S, Jaÿ M, Game Y, Gauthier D, Herbaux JP, Le Horgne JM, Maucci E, Pasquier JJ, Vaniscotte A, Mick V, Garin-Bastuji B. Un foyer de brucellose chez les ongulés sauvages du massif du Bargy en Haute-Savoie. Bulletin Epidémiologique Santé Anim Alim Anses-DGAL. 2013;60:2–7. [Google Scholar]
- Hars J, Rautureau S, Vaniscotte A, Herbaux JP, Pasquier D, Depecker A, Le Bourg V, Game Y, Toïgo C, Mick V, Garin-Bastuji B. La brucellose des bouquetins du massif du Bargy (Haute-Savoie): où en est-on en 2015 ? Bulletin Epidémiologique Santé Anim Alim Anses-DGAL. 2015;70:14–18. [Google Scholar]
- Herd RP, Sams RA, Ashcraft SM. Persistence of ivermectin in plasma and faeces following treatment of cows with ivermectin sustained-release, pour-on or injectable formulations. Int J Parasitol. 1996;26:1087–1093. doi: 10.1016/S0020-7519(96)80007-5. [DOI] [PubMed] [Google Scholar]
- Holzwarth N, Pospischil A, Mavrot F, Vilei EM, Hilbe M, Zlinszky K, Regenscheit N, Pewsner M, Thoma R, Borel N (2011) Occurrence of Chlamydiaceae, Mycoplasma conjunctivae, and pestiviruses in Alpine Chamois (Rupicapra r. rupicapra) of Grisons, Switzerland) J Vet Diagn Invest 23:333. 10.1177/104063871102300223 [DOI] [PubMed]
- Iacopelli F, Fanelli A, Tizzani P, Berriatua E, Prieto P, Martínez-Carrasco C, León L, Rossi L, Candela MG. Spatio-temporal patterns of sarcoptic mange in red deer and Iberian ibex in a multi-host natural park. Res Vet Sci. 2020;128:224–229. doi: 10.1016/j.rvsc.2019.11.014. [DOI] [PubMed] [Google Scholar]
- Ibrahim KEE, Abu-Samra MT. Experimental transmission of a goat strain of Sarcoptes scabiei to desert sheep and its treatment with ivermectin. Vet Parasitol. 1987;26(1–2):157–164. doi: 10.1016/0304-4017(87)90085-9. [DOI] [PubMed] [Google Scholar]
- Illarieti T, Acevedo P, Alves P, Jung TS, Kierdorf H, Lach G, López-Olvera J, Putman R, Scandura M, Vallinoto M, Gortázar C. 18 years of the European Journal of Wildlife Research: profile and prospects. Eur J Wildl Res. 2023;69:8. doi: 10.1007/s10344-022-01635-1. [DOI] [Google Scholar]
- Jiménez-Ruiz S, Risalde MA, Acevedo P, Arnal MC, Gómez-Guillamón F, Prieto P, Gens MJ, Cano-Terriza D, Fernández de Luco D, Vicente J, García-Bocanegra I. Serosurveillance of Schmallenberg virus in wild ruminants in Spain. Transbound Emerg Dis. 2021;68(2):347–354. doi: 10.1111/tbed.13680. [DOI] [PubMed] [Google Scholar]
- Junta de Andalucía (2015) Informe Programa de Vigilancia Epidemiológica de cabra montés (Capra pyrenaica hispanica). Temporadas de caza 2009/2012 a 2012/2015. https://www.juntadeandalucia.es/medioambiente/portal/areas-tematicas/biodiversidad-y-vegetacion/fauna-amenazada/seguimiento-de-fauna/programa-de-vigilancia-epidemiologica-de-la-fauna-silvestre-en-andalucia
- Junta de Andalucía (2021) Resolución de 25 de mayo de 2021, de la Dirección General de Medio Natural, Biodiversidad y Espacios Protegidos, por la que se declara Área de Emergencia Cinegética temporal por sarna sarcóptica en cabra montés, en varios términos municipales de las provincias de Almería, Cádiz, Granada, Jaén, Málaga y Sevilla. Boletín Oficial de la Junta de Andalucía 103:139–146. https://www.juntadeandalucia.es/boja/2021/103/38
- Kock RA, Orynbayev M, Robinson S, Zuther S, Singh NJ, Beauvais W, Morgan ER, Kerimbayev A, Khomenko S, Martineau HM, Rystaeva R, Omarova Z, Wolfs S, Hawotte F, Radoux J, Milner-Gulland EJ (2018) Saigas on the brink: multidisciplinary analysis of the factors influencing mass mortality events. Sci Adv 4(1):eaao2314. 10.1126/sciadv.aao2314 [DOI] [PMC free article] [PubMed]
- Kutzer E. Zur epidemiologie der Sarcoptesräude. Angew Parasitol. 1966;7:241–248. [Google Scholar]
- Lalli PN, Morgan MS, Arlian LG. Skewed Th1/Th2 immune response to Sarcoptes scabiei. J Parasitol. 2004;90(4):711–714. doi: 10.1645/GE-214R. [DOI] [PubMed] [Google Scholar]
- Lambert S, Gilot-Fromont E, Toïgo C, Marchand P, Petit E, Garin-Bastuji B, Gauthier D, Gaillard JM, Rossi S, Thébault A (2020) An individual-based model to assess the spatial and individual heterogeneity of Brucella melitensis transmission in Alpine ibex. Ecol Model 425:109009. 10.1016/j.ecolmodel.2020.109009
- Lambert S, Thébault A, Rossi S, Marchand P, Petit E, Toïgo C, Gilot-Fromont E. Targeted strategies for the management of wildlife diseases: the case of brucellosis in Alpine ibex. Vet Res. 2021;52:116. doi: 10.1186/s13567-021-00984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Gilot-Fromont E, Toïgo C, Marchand P, Petit E, Rossi S, Thébault A (2022) Combining seroprevalence and capture-mark-recapture data to estimate the force of infection of brucellosis in a managed population of Alpine ibex. Epidemics 38:100542. 10.1016/j.epidem.2022.100542 [DOI] [PubMed]
- Lastras ME, Pastor J, Marco I, Ruiz M, Viñas L, Lavín S. Effects of sarcoptic mange on serum proteins and immunoglobulin G levels in chamois (Rupicapra pyrenaica) and Spanish ibex (Capra pyrenaica) Vet Parasitol. 2000;88(3):313–319. doi: 10.1016/s0304-4017(99)00221-6. [DOI] [PubMed] [Google Scholar]
- Lavín S, Marco I, Rossi L, Meneguz PG, Viñas L. Haemonchosis in Spanish ibex. J Wildl Dis. 1997;33(3):656–659. doi: 10.7589/0090-3558-33.3.656. [DOI] [PubMed] [Google Scholar]
- Lavín S, Ruiz-Bascarán M, Marco I, Fondevila MD, Ramis AJ. Experimental infection of chamois (Rupicapra pyrenaica parva) with Sarcoptes scabiei derived from naturally infected goats. Zoonoses Public Health. 2000;47(9):693–699. doi: 10.1046/j.1439-0450.2000.00406.x. [DOI] [PubMed] [Google Scholar]
- Lavín S, Lastras ME, Marco I, Cabañes FX. Report of a case of bronchopneumonia associated with Moraxella bovis isolation in a chamois (Rupicapra pyrenaica) J Vet Med B. 2000;47:225–227. doi: 10.1046/j.1439-0450.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- Lavín S, Ruiz-Bascarán M, Marco I, Abarca ML, Crespo MJ, Franch J. Foot infections associated with Arcanobacterium pyogenes in free-living fallow deer (Dama dama) J Wildl Dis. 2004;40(3):607–611. doi: 10.7589/0090-3558-40.3.607. [DOI] [PubMed] [Google Scholar]
- Lawson B, Neimanis A, Lavazza A, López-Olvera JR, Tavernier P, Billinis C, Duff P, Mladenov DT, Rijks JM, Savić S, Wibbelt G, Ryser-Degiorgis MP, Kuiken T. How to start up a national wildlife health surveillance programme. Animals. 2021;11:2543. doi: 10.3390/ani11092543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Vizcaíno L, Astorga R, Escós J, Alonso F, Alados C, Contreras A, Cubero MJ (1992) Epidemiología de la sarna sarcóptica en el Parque Natural de las Sierras de Cazorla, Segura y Las Villas. In: Proceedings of the International Congress on the Genus Capra in Europe. Sevilla: Junta Rectora del Parque Natural Sierra de las Nieves, Consejería de Medio Ambiente, Junta de Andalucía, Spain, pp. 95–99.
- León-Vizcaíno L, Ruíz de Ybáñez MR, Cubero MJ, Ortíz JM, Espinosa J, Pérez L, Simón MA, Alonso F. Sarcoptic mange in Spanish ibex from Spain. J Wildl Dis. 1999;35(4):647–659. doi: 10.7589/0090-3558-35.4.647. [DOI] [PubMed] [Google Scholar]
- León-Vizcaíno L, Cubero MJ, González-Capitel E, Simón MA, Pérez L, Ruiz de Ybáñez MR, Ortiz JM, González-Candela M, Alonso F. Experimental ivermectin treatment of sarcoptic mange and establishment of a mange-free population of Spanish ibex. J Wildl Dis. 2001;37:775–785. doi: 10.7589/0090-3558-37.4.775. [DOI] [PubMed] [Google Scholar]
- León Vizcaíno L, Garrido Abellán F, González Candela M, Pérez Béjar L, Cubero Pablo MJ, Perales Flores A, Pacheco I, Martín Atance P (2008a) Ineficacia de los rumiantes silvestres como reservorios de Brucella en los ambientes naturales de las sierras béticas. XXXIII Jornadas Científicas SEOC 1, 331–334. https://www.juntadeandalucia.es/export/drupaljda/1337161080ovinotecnia_baja.pdf
- León Vizcaíno L, Garrido Abellán F, González Candela M, Cubero Pablo MJ, Martín Atance P (2008b) Infecciones de evolución crónica comunes entre rumiantes domésticos y silvestres en las sierras béticas. XXXIII Jornadas Científicas SEOC 1, 335–338. https://www.juntadeandalucia.es/export/drupaljda/1337161080ovinotecnia_baja.pdf
- León Vizcaíno L, González Candela M, Verbisk-Bucker G, Perales Flores A, Cubero Pablo MJ, Martín Atance P, Garrido Abellán F (2008c) Estudios en masa sobre infecciones que causan queratoconjuntivitis entre rumiantes domésticos y silvestres en las sierras béticas XXXIII Jornadas Científicas SEOC 1, 339–342. https://www.juntadeandalucia.es/export/drupaljda/1337161080ovinotecnia_baja.pdf
- León Vizcaíno L, Alonso de Vega F, Garrido Abellán F, González Candela M, Martínez Carrasco-Pleite C, Pérez Béjar L, Cubero Pablo MJ, Ruiz de Ybáñez Carnero R, Arenas Casas A (2008d) Estudio en masa sobre infecciones que causan mortalidad perinatal congénita entre rumiantes domésticos y silvestres en las sierras béticas. XXXIII Jornadas Científicas SEOC 1, 325–330. https://www.juntadeandalucia.es/export/drupaljda/1337161080ovinotecnia_baja.pdf
- Loison A, Gaillard J-M, Jullien J. Demographic patterns after an epizootic of keratoconjunctivitis in a chamois population. J Wildl Manage. 1996;60(3):517–527. doi: 10.2307/3802069. [DOI] [Google Scholar]
- López-Olvera JR, Höfle U, Vicente J, Fernández-de-Mera IG, Gortázar C. Effects of parasitic helminths and ivermectin treatment on clinical parameters in the European wild boar (Sus scrofa) Parasitol Res. 2006;98(6):582–587. doi: 10.1007/s00436-005-0099-2. [DOI] [PubMed] [Google Scholar]
- López-Olvera JR, Vidal D, Vicente J, Pérez M, Luján L, Gortázar C. Serological survey of selected infectious diseases in mouflon (Ovis aries musimon) from south-central Spain. Eur J Wildl Res. 2009;55:75–79. doi: 10.1007/s10344-008-0215-6. [DOI] [Google Scholar]
- López-Olvera JR, Serrano E, Armenteros A, Pérez JM, Fandos P, Carvalho J, Velarde R, Cano-Manuel FJ, Ráez-Bravo A, Espinosa J, Soriguer RC, Granados JE. Sex-biased severity of sarcoptic mange at the same biological cost in a sexually dimorphic ungulate. Parasites Vectors. 2015;8(1):583. doi: 10.1186/s13071-015-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca-Oró C, López-Olvera JR, Ruiz-Fons F, Acevedo P, García- Bocanegra I, Oleaga Á, Gortázar C, Pujols J (2014) Long-term dynamics of bluetongue virus in wild ruminants: relationship with outbreaks in livestock in Spain, 2006–2011. PLoS One 9(6):e0100027. 10.1371/journal.pone.0100027 [DOI] [PMC free article] [PubMed]
- Lorca-Oró C, Pujols J, Arenas A, Gómez-Guillamón F, Zorrilla I, Domingo M, Arenas-Montes A, Ruano MJ, García-Bocanegra I. Epidemiological surveillance of bluetongue virus serotypes 1, 4 and 8 in Spanish ibex (Capra pyrenaica hispanica) in southern Spain. Vet Microbiol. 2011;149(1–2):230–235. doi: 10.1016/j.vetmic.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Lorca-Oró C, Pujols J, García-Bocanegra I, Mentaberre G, Granados JE, Solanes D, Fandos P, Galindo I, Domingo M, Lavín S, López-Olvera JR (2012) Protection of Spanish ibex (Capra pyrenaica) against bluetongue virus serotypes 1 and 8 in a subclinical experimental infection. PLoS ONE 7(5):e36380. 10.1371/journal.pone.0036380 [DOI] [PMC free article] [PubMed]
- Manceau V, Crampe JP, Boursot P, Taberlet P. Identification of evolutionary significant units in the Spanish wild goat, Capra pyrenaica (Mammalia, Artiodactyla) Anim Conserv. 1999;2:33–39. doi: 10.1111/j.1469-1795.1999.tb00046.x. [DOI] [Google Scholar]
- Marco I, Velarde R, Castellà J, Ferrer D, Lavín S. Presumptive Babesia ovis infection in a Spanish ibex (Capra pyrenaica) Vet Parasitol. 2000;87:217–221. doi: 10.1016/s0304-4017(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Marco I, Ruiz M, Juste R, Garrido JM, Lavin S. Paratuberculosis in free-ranging fallow deer in Spain. J Wildl Dis. 2002;38(3):629–632. doi: 10.7589/0090-3558-38.3.629. [DOI] [PubMed] [Google Scholar]
- Martín-Monedero I, Fandos-Paris P, Macías-García H, Fandos-Guzmán G, García-Lara R, Ramos-Losada B, López-Olvera JR, Soriguer-Escofet RC, Pérez-Jiménez JM, Aleix-Mata G, Cano-Manuel León FJ, Granados-Torres JE (2022) Análisis de la influencia del clima en la epizootia de sarna sarcóptica que afecta a la población de cabra montés de Sierra Nevada. 39º Encuentro GEEFSM, Groupe d’Étude sur l’Écopathologie de la Faune Sauvage de Montagne – . Libro de resúmenes. Córdoba, Spain: UCOPress Editorial Universidad de Córdoba; 2022. p. 19. [Google Scholar]
- Martínez IZ, Oleaga Á, Sojo I, García-Iglesias MJ, Pérez-Martínez C, García Marín JF, Balseiro A. Immunohistochemical assessment of immune response in the dermis of Sarcoptes scabiei-infested wild carnivores (wolf and fox) and ruminants (chamois and red deer) Animals. 2020;10(7):1146. doi: 10.3390/ani10071146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama R, Yabusaki T, Senjyu N, Okano T, Baba M, Tsuji-Matsukane T, Yokoyama M, Kido N, Kadosaka T, Kato T, Suzuki M, Asano M. Possible transmission of Sarcoptes scabiei between herbivorous Japanese serows and omnivorous Caniformia in Japan: a cryptic transmission and persistence? Parasites Vectors. 2019;12:389. doi: 10.1186/s13071-019-3630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrot FM, Vilei E, Marreros N, Signer C, Ryser-Degiorgis MP. Occurrence, quantification, and genotyping of Mycoplasma conjunctivae in wild Caprinae with and without infectious keratoconjunctivitis. J Wildl Dis. 2012;48:619–631. doi: 10.7589/0090-3558-48.3.619. [DOI] [PubMed] [Google Scholar]
- Mayer D, Degiorgis MP, Meier W, Nicolet J, Giacometti M. Lesions associated with infectious keratoconjunctivitis in Alpine ibex. J Wildl Dis. 1997;33:413–419. doi: 10.7589/0090-3558-33.3.413. [DOI] [PubMed] [Google Scholar]
- McCarthy JS, Kemp DJ, Walton SF, Currie BJ. Scabies: more than just an irritation. Postgrad Med J. 2004;80(945):382–387. doi: 10.1136/pgmj.2003.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitol. 1944;35(4):197–206. doi: 10.1017/S0031182000021612. [DOI] [Google Scholar]
- Mellanby K, Johnson CG, Bartley WC, Brown P (1942) Experiments on the survival and behaviour of the itch mite, Sarcoptes scabiei DeG. var. hominis. Bull Entomol Res 33(4):267–271. 10.1017/S0007485300026584
- Mellor PS, Wittmann EJ. Bluetongue virus in the Mediterranean Basin 1998–2001. Vet J. 2002;164:20–37. doi: 10.1053/tvjl.2002.0713. [DOI] [PubMed] [Google Scholar]
- Mentaberre G, Serrano E, Velarde R, Marco I, Lavín S. Absence of TB in Iberian ibex (Capra pyrenaica) in a high-risk area. Vet Rec. 2010;166:700. doi: 10.1136/vr.c2552. [DOI] [PubMed] [Google Scholar]
- Mentaberre G, Romero B, de Juan L, Navarro-González N, Velarde R, Mateos A, Marco I, Olivé-Boix X, Domínguez L, Lavín S, Serrano E (2014) Long-term assessment of wild boar harvesting and cattle removal for bovine tuberculosis control in free ranging populations. PLoS ONE 9(2):e88824. 10.1371/journal.pone.0088824 [DOI] [PMC free article] [PubMed]
- Mentaberre G, Velarde R, García-Readigòs MA, Serrano E, Jovaní J, Olivé-Boix X, López-Olvera JR, Castillo R, Ruiz-Olmo J, Lavín S 2015. Nuevo brote de sarna sarcóptica en la cabra montés (Capra pyrenaica). In: 33èmes Rencontres du Groupe d'Étude sur l'Eco-pathologie de la Faune Sauvage de Montagne 20 R. García-González et al. (GEEFSM). 21–24 May 2015, Balme, Valli di Lanzo, Italy, p. 15
- Menzano A, Rambozzi L, Rossi L. Outbreak of scabies in human beings, acquired from chamois (Rupicapra rupicapra) Vet Rec. 2004;155(18):568. doi: 10.1136/vr.155.18.568. [DOI] [PubMed] [Google Scholar]
- Menzano A, Rambozzi L, Rossi L. A severe episode of wildlife derived scabies in domestic goats in Italy. Small Rumin Res. 2007;70(2):154–158. doi: 10.1016/j.smallrumres.2006.02.010. [DOI] [Google Scholar]
- Mertens PP, Diprose J, Maan S, Singh KP, Attoui H, Samuel AR. Bluetongue virus replication, molecular and structural biology. Vet Ital. 2004;40(4):426–437. [PubMed] [Google Scholar]
- Mika A, Reynolds SL, Mohlin FC, Willis C, Swe PM, Pickering DA, McMillan D, Sriprakash KS, Kemp DJ, Fischer K (2012) Novel scabies mite serpins inhibit the three pathways of the human complement system. PLoS ONE 7(7):e40489. 10.1371/journal.pone.0040489 [DOI] [PMC free article] [PubMed]
- Millán J, Casáis R, Delibes-Mateos M, Calvete C, Rouco C, Castro F, Colomar V, Casas-Díaz E, Ramírez E, Moreno S, Prieto JM, Villafuerte R. Widespread exposure to Sarcoptes scabiei in wild European rabbits (Oryctolagus cuniculus) in Spain. Vet Parasitol. 2012;183(3):323–329. doi: 10.1016/j.vetpar.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Mitra M, Mahanta SK, Sen S, Ghosh C, Hati AK. Transmission of Sarcoptes scabiei from animal to man and its control. J Indian Med Assoc. 1995;93(4):142–143. [PubMed] [Google Scholar]
- Moraga-Fernández A, Ruiz-Fons F, Habela MÁ, Royo-Hernández L, Calero-Bernal R, Gortazar C, de la Fuente J, Fernández de Mera IG. Detection of new Crimean-Congo haemorrhagic fever virus genotypes in ticks feeding on deer and wild boar. Spain Transbound Emerg Dis. 2021;68(3):993–1000. doi: 10.1111/tbed.13756. [DOI] [PubMed] [Google Scholar]
- Moroni B, Valldeperes M, Serrano E, López-Olvera JR, Lavín S, Rossi L. Comment on: “The treatment of sarcoptic mange in wildlife: a systematics review”. Parasites Vectors. 2020;13:471. doi: 10.1186/s13071-020-04347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni B, Angelone S, Pérez JM, Molinar Min AR, Pasquetti M, Tizzani P, López-Olvera JR, Valldeperes M, Granados JE, Lavín S, Mentaberre G, Camacho-Sillero L, Martínez-Carrasco C, Oleaga A, Candela M, Meneguz PG, Rossi L. Sarcoptic mange in wild ruminants in Spain: solving the epidemiological enigma using microsatellite markers. Parasites Vectors. 2021;14:171. doi: 10.1186/s13071-021-04673-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni B, Granados Torres JE, López-Olvera JR, Espinosa Cerrato J, Ráez Bravo A, Metaberre G, Fandos P, Pazzi M, Romagnoli M, Gardini G, Rossi L, Valldeperes M, Serrano E, Ramos B, Odore R (2022a) Ivermectin plasma concentration in Iberian ibex (Capra pyrenaica) following oral administration: a pilot study. Front Vet Sci 9:830157. 10.3389/fvets.2022.830157 [DOI] [PMC free article] [PubMed]
- Moroni B, Rossi L, Bernigaud C, Guillot J. Zoonotic episodes of scabies: a global overview. Pathogens. 2022;11:213. doi: 10.3390/pathogens11020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy TA, Bakr ME, Ahmed MM, Kotb MM. Human scabies acquired from a pet puppy. J Egypt Soc Parasitol. 1994;24(2):305–308. [PubMed] [Google Scholar]
- Mounsey K, Harvey RJ, Wilkinson V, Takano K, Old J, Stannard H, Wicker L, Phalen D, Carver S. Drug dose and animal welfare: important considerations in the treatment of wildlife. Parasitol Res. 2022;121:1065–1071. doi: 10.1007/s00436-022-07460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounsey KE, Willis C, Burgess ST, Holt DC, McCarthy J, Fischer K. Quantitative PCRbased genome size estimation of the astigmatid mites Sarcoptes scabiei, Psoroptes ovis and Dermatophagoides pterronyssinus. Parasites Vectors. 2012;5:3. doi: 10.1186/1756-3305-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz PM, Boadella M, Arnal M, de Miguel MJ, Revilla M, Martínez D, Vicente J, Acevedo P, Oleaga Á, Ruiz-Fons F, Marín CM, Prieto JM, de la Fuente J, Barral M, Barberán M, Fernández de Luco D, Blasco JM, Gortázar C. Spatial distribution and risk factors of brucellosis in Iberian wild ungulates. BMC Infect Dis. 2010;10:46. doi: 10.1186/1471-2334-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzafar M, Calvo-Bado LA, Green LE, Smith EM, Russell CL, Grogono-Thomas R, Wellington EMH. The role of the environment in transmission of Dichelobacter nodosus between ewes and their lambs. Vet Microbiol. 2015;179:53–59. doi: 10.1016/j.vetmic.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Næsborg-Nielsen C, Wilkinson V, Mejia-Pacheco N, Carver S. Evidence underscoring immunological and clinical pathological changes associated with Sarcoptes scabiei infection: synthesis and meta-analysis. BMC Infect Dis. 2022;22:658. doi: 10.1186/s12879-022-07635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa TLDR, Takai Y, Kubo M, Sakai H, Masegi T, Yanai T. A pathological study of sepsis associated with sarcoptic mange in raccoon dogs (Nyctereutes procyonoides) in Japan. J Comp Pathol. 2009;141(2–3):177–181. doi: 10.1016/j.jcpa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Navarro-González N, Porrero MC, Mentaberre G, Serrano E, Mateos A, Cabal A, Domínguez L, Lavín S. Escherichia coli O157:H7 in wild boars (Sus scrofa) and Iberian ibex (Capra pyrenaica) sharing pastures with free-ranging livestock in a natural environment in Spain. Vet Q. 2015;35(2):102–106. doi: 10.1080/01652176.2015.1023404. [DOI] [PubMed] [Google Scholar]
- Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, Bermejo Lope.z E, Menárguez J, Fernández-Cruz A, Sánchez-Artola B, Keough-Delgado E, Ramírez de Arellano E, Lasala F, Milla J, Fraile JL, Ordobás Gavín M, Martinez de la Gándara A, López Perez L, Diaz-Diaz D, López-García MA, Delgado-Jimenez P, Martín-Quirós A, Trigo E, Figueira JC, Manzanares J, Rodriguez-Baena E, Garcia-Comas L, Rodríguez-Fraga O, García-Arenzana N, Fernández-Díaz MV, Cornejo VM, Emmerich P, Schmidt-Chanasit J, Arribas JR, for the Crimean Congo Hemorrhagic Fever@Madrid Working Group Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med. 2017;377:154–161. doi: 10.1056/NEJMoa1615162. [DOI] [PubMed] [Google Scholar]
- Negredo A, Sánchez-Arroyo R, Díez-Fuertes F, de Ory F, Budiño MA, Vázquez A, Garcinuño A, Hernández L, de la Hoz GC, Gutiérrez-Arroyo A, Grande C. Sánchez-Seco P (2021a) Fatal case of Crimean-Congo hemorrhagic fever caused by reassortant virus, Spain. Emerg Infect Dis. 2018;27(4):1211–1215. doi: 10.3201/eid2704.203462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negredo A, Sánchez-Ledesma M, Llorente F, Pérez-Olmeda M, Belhassen-García M, González-Calle D, Sánchez-Seco MP. Jiménez-Clavero MA (2021b) Retrospective identification of early autochthonous case of Crimean-Congo hemorrhagic fever, Spain. Emerg Infect Dis. 2013;27(6):1754–1756. doi: 10.3201/eid2706.204643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet J, Freundt EA. Isolation of Mycoplasma conjunctivae from chamois and sheep affected with kerato-conjunctivitis. Zoonoses Public Health. 1975;22(4):302–307. doi: 10.1111/j.1439-0450.1975.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Niedringhaus KD, Brown JD, Sweeley KM, Yabsley MJ. A review of sarcoptic mange in North American wildlife. Int J Parasitol Parasites Wildl. 2019;9:285–297. doi: 10.1016/j.ijppaw.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan J, Schnitzerling HJ, Bird P. The use of ivermectin to cleanse tick infested cattle. Aust Vet J. 1985;62(11):386–388. doi: 10.1111/j.1751-0813.1985.tb14219.x. [DOI] [PubMed] [Google Scholar]
- Nores C, González-Quirós P (2009) Demographic changes caused by sarcoptic mange in the Cantabrian chamois (Rupicapra pyrenaica parva) in Asturias (North of Spain). In: Pérez-Barbería FJ, Palacios B (eds) El rebeco cantábrico Rupicapra pyrenaica parva. Conservación y gestión de sus poblaciones. Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid, Spain, pp. 338–359.
- Obber F, Celva R, Libanora M, Da Rold G, Dellamaria D, Partel P, Ferraro E, Calabrese MS, Morpurgo L, Pisano SRR, Citterio CV, Cassini R. Description of a sarcoptic mange outbreak in Alpine chamois using an enhanced surveillance approach. Animals. 2022;12:2077. doi: 10.3390/ani12162077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old JM, Skelton CJA, Stannard HJ. The use of Cydectin® by wildlife carers to treat sarcoptic mange in free-ranging bare-nosed wombats (Vombatus ursinus) Parasitol Res. 2021;120(3):1077–1090. doi: 10.1007/s00436-020-07012-8. [DOI] [PubMed] [Google Scholar]
- Oleaga A, Casais R, González Quirós P, Prieto M, Gortázar C. Sarcoptic mange in red deer from Spain: improved surveillance or disease emergence? Vet Parasitol. 2008;154:103–113. doi: 10.1016/j.vetpar.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Oleaga A, Casais R, Balseiro A, Espí A, Llaneza L, Hartasánchez A, Gortázar C. New techniques for an old disease: sarcoptic mange in the Iberian wolf. Vet Parasitol. 2011;181:255–266. doi: 10.1016/j.vetpar.2011.04.036. [DOI] [PubMed] [Google Scholar]
- Osman KM, Ali HA, ElJakee JA, Galal HM. Prevalence of Chlamydophila psittaci infections in the eyes of cattle, buffaloes, sheep and goats in contact with a human population. Transbound Emerg Dis. 2013;60:245–251. doi: 10.1111/j.1865-1682.2012.01337.x. [DOI] [PubMed] [Google Scholar]
- Palomares F, Ruiz-Martínez I. Status and conservation perspectives for the Spanish ibex population (Capra pyrenaica Schinz, 1838) of Sierra Mágina Natural Park. Z Jagdwiss. 1993;39:87–94. doi: 10.1007/BF02285321. [DOI] [Google Scholar]
- Pastor J, Bach E, Ráez-Bravo A, López-Olvera JR, Tvarijonaviciute A, Granados JE, Espinosa J, Pérez JM, Lavín S, Cuenca R. Method validation, reference values and characterization of acute phase proteins response to experimentally induced inflammation and bluetongue virus infection in Iberian ibex. Vet Clin Pathol. 2019;48(4):695–701. doi: 10.1111/vcp.12802. [DOI] [PubMed] [Google Scholar]
- Pence DB, Casto SD, Samuel WM. Variation in the chaetotaxy and denticulation of Sarcoptes scabiei (Acarina: Sarcoptidae) from wild canids. Acarologia. 1975;17(1):160–165. [PubMed] [Google Scholar]
- Pence DB, Ueckermann E. Sarcoptic manage in wildlife. Rev Sci Tech OIE. 2002;21(2):385–398. doi: 10.20506/rst.21.2.1335. [DOI] [PubMed] [Google Scholar]
- Pérez JM, Ruiz Martínez I, Palomares F (1992) Impacto de la sarna sarcóptica sobre la cabra montés (Capra pyrenaica) del Parque Natural de Sierra Mágina (Jaén). Datos sobre prevalencia y mortalidad. Actas del Congreso Internacional del género Capra en Europa. Junta Rectora del Parque Natural de la Sierra de las Nieves, Málaga, Spain, pp. 239–241.
- Pérez JM, Granados JE, Soriguer RC. Population dynamic of the Spanish ibex Capra pyrenaica in Sierra Nevada Natural Park (southern Spain) Acta Teriol (warsz) 1994;39:289–294. doi: 10.4098/AT.arch.94-32. [DOI] [Google Scholar]
- Pérez JM, Granados JE, Gómez F, Pérez MC, Ruiz-Martínez I. Las parasitosis de la cabra montés de Sierra Nevada (Granada). 1st International Conference Sierra Nevada, Conservación y Desarrollo Sostenible, March 20th to 22nd 1996. Granada, Spain. 1996;3:31–45. [Google Scholar]
- Pérez JM, Granados JE, Ruiz SRC, I, Prevalence and seasonality of Oestrus caucasicus Grunin, 1948 (Diptera: Oestridae) parasitizing the Spanish ibex, Capra pyrenaica (Mammalia: Artiodactyla) J Parasitol. 1996;82:233–236. doi: 10.2307/3284152. [DOI] [PubMed] [Google Scholar]
- Pérez JM, Ruiz I, Chirosa M. Management of sarcoptic mange and host populations. The case of the Spanish ibex from Sierra Nevada. Bulletin D'information Sur La Pathologie Des Animaux Sauvages (BIPAS) 1996;15:69–76. [Google Scholar]
- Pérez JM, Ruiz-Martínez I, Granados JE, Soriguer RC, Fandos P. The dynamics of sarcoptic mange in the ibex population of Sierra Nevada in Spain–influence of climatic factors. J Wildl Res. 1997;2(1):86–89. [Google Scholar]
- Pérez JM, Granados JE, Bueno L, Moreno V. Las miasis en los ungulados silvestres. Ovis. 1997;49:89–106. [Google Scholar]
- Pérez JM, Granados JE, Soriguer RC, Fandos P, Márquez FJ, Crampe JP. Distribution, status and conservation problems of the Spanish ibex, Capra pyrenaica (Mammalia: Artiodactyla) Mamm Rev. 2002;32:26–39. doi: 10.1046/j.1365-2907.2002.00097.x. [DOI] [Google Scholar]
- Pérez JM, Granados JE, Pérez MC, Márquez FJ, Ferroglio E, Rossi L. A survey of the gastrointestinal nematodes of Spanish ibex (Capra pyrenaica) in a high mountain habitat. J Parasitol. 2003;89:315–318. doi: 10.1645/0022-3395(2003)089[0315:ASOTGN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pérez JM, Meneguz PG, Dematteis A, Rossi L, Serrano E. Parasites and conservation biology: the ‘ibex-ecosystem’. Biodivers Conserv. 2006;15:2033–2047. doi: 10.1007/s10531-005-0773-9. [DOI] [Google Scholar]
- Pérez JM, Granados JE, Sarasa M, Serrano E. Usefulness of estimated surface area of damaged skin as a proxy of mite load in the monitoring of sarcoptic mange in free-ranging populations of Iberian wild goat. Capra Pyrenaica Vet Parasitol. 2011;176(2):258–264. doi: 10.1016/j.vetpar.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Pérez JM, Serrano E, Gónzález-Candela M, León-Vizcaíno L, Barberá CG, de Simón MA, Fandos P, Granados JE, Soriguer RC, Festa-Bianchet M. Reduced horn size in two wild trophy-hunted species of Caprinae. Wildl Biol. 2011;17:102–112. doi: 10.2981/09-102. [DOI] [Google Scholar]
- Pérez JM, Serrano E, Soriguer RC, González FJ, Sarasa M, Granados JE, Cano-Manuel FJ, Cuenca R, Fandos P. Distinguishing disease effects from environmental effects in a mountain ungulate: seasonal variation in body weight, hematology, and serum chemistry among Iberian ibex (Capra pyrenaica) affected by sarcoptic mange. J Wildl Dis. 2015;51(1):148–156. doi: 10.7589/2014-01-008. [DOI] [PubMed] [Google Scholar]
- Pérez JM, Castro I, Granados JE, Cano-Manuel FJ, Fandos P, Espinosa J, Soriguer RC. Does Sarcoptes scabiei synchronize its breeding cycle with that of the Iberian ibex, Capra pyrenaica? Int J Acarol. 2017;43(3):199–203. doi: 10.1080/01647954.2016.1272634. [DOI] [Google Scholar]
- Pérez JM, Granados JE, Espinosa J, Ráez-Bravo A, López-Olvera JR, Rossi L, Meneguz PG, Angelone S, Fandos P, Soriguer RC. Biology and management of sarcoptic mange in wild Caprinae populations. Mamm Rev. 2021;51:82–94. doi: 10.1111/mam.12213. [DOI] [Google Scholar]
- Pérez JM, López‐Montoya AJ, Cano‐Manuel FJ, Soriguer RC, Fandos P, Granados JE (2022) Development of resistance to sarcoptic mange in ibex. J Wildl Manage 86(5):e22224. 10.1002/jwmg.22224
- Petersen HH, Nielsen JP, Heegaard PMH. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35(2):163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Pisano S, Ryser-Degiorgis M, Rossi L, Peano A, Keckeis K. Roosje P (2019) Sarcoptic mange of fox origin in multiple farm animals and scabies in humans, Switzerland. Emerg Infect Dis. 2018;25(6):1235–1238. doi: 10.3201/eid2506.181891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzato F (2017) Paratuberculosis: prevalence and epidemiological role of Iberian ibex (Capra pyrenaica). Master Thesis. Máster Universitario en Gestión de la Fauna Silvestre, Universidad de Murcia, Murcia, Spain
- Posautz A, Loncaric I, Kübber-Heiss A, Knoll A, Walzer C. Acute die-off of chamois (Rupicapra rupicapra) in the Eastern Austrian Alps due to bacterial bronchopneumonia with Pasteurellaceae. J Wildl Dis. 2014;50(3):616–620. doi: 10.7589/2013-04-090. [DOI] [PubMed] [Google Scholar]
- Pound JM, Miller JA, George JE, Oehler DD, Harmel DE. Systemic treatment of white-tailed deer with ivermectin-medicated bait to control free-living populations of lone star ticks (Acari:Ixodidae) J Med Entomol. 1996;33(3):385–394. doi: 10.1093/jmedent/33.3.385. [DOI] [PubMed] [Google Scholar]
- Prada C, Herrero J. Viabilidad del hábitat y del reforzamiento poblacional de Capra pyrenaica en la Reserva Nacional de Caza de Os Ancares. Pirineos. 2013;168:59–76. doi: 10.3989/Pirineos.2013.168004. [DOI] [Google Scholar]
- Puigdemont A, Brazís P, Fondati S, Ferrer L (2002) Diagnóstico serológico de la sarna sarcóptica en el perro. Consult Difusión Vet 89:71–73. https://saludanimal.leti.com/es/diagnostico-serologico-de-la-sarna-sarcoptica-en-el-perro_1204.pdf
- Ráez-Bravo A (2019) Pathophysiology of sarcoptic mange in Iberian ibex. PhD Thesis, Universitat Autònoma de Barcelona, Bellaterra, Barcelona, Spain, 138 pp.
- Ráez-Bravo A, Granados JE, Cerón JJ, Cano-Manuel FJ, Fandos P, Pérez JM, Espinosa J, Soriguer RC, López-Olvera JR. Acute phase proteins increase with sarcoptic mange status and severity in Iberian ibex (Capra pyrenaica, Schinz 1838) Parasitol Res. 2015;114(11):4005–4010. doi: 10.1007/s00436-015-4628-3. [DOI] [PubMed] [Google Scholar]
- Ráez-Bravo A, Granados JE, Serrano E, Dellamaria D, Casais R, Rossi L, Puigdemont A, Cano-Manuel FJ, Fandos P, Pérez JM, Espinosa J, Soriguer RC, Citterio C, López-Olvera JR. Evaluation of three enzyme linked immunosorbent assays for sarcoptic mange diagnosis and assessment in the Iberian ibex. Capra Pyrenaica Parasites Vectors. 2016;9(1):558. doi: 10.1186/s13071-016-1843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari S, Nabian S, Bahonar AR. Some observations on sheep sarcoptic mange in Tehran province. Iran Trop Anim Health Prod. 2009;41(3):397–401. doi: 10.1007/s11250-008-9203-9. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Lecchi C, Fraquelli C, Sartorelli P, Ceciliani F. Acute phase protein response in Alpine ibex with sarcoptic mange. Vet Parasitol. 2010;168(3):293–298. doi: 10.1016/j.vetpar.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Rambozzi L, Menzano A, Lavín S, Rossi L (2004) Biotin-avidin amplified ELISA for detection of antibodies to Sarcoptes scabiei in chamois (Rupicapra spp.). Vet Res 35(6):701–708. 10.1051/vetres:2004039 [DOI] [PubMed]
- Ramírez-Rosales E (2018) Dynamics of Mycoplasma conjunctivae at the wildlife-livestock interface in the Natural Space of Sierra Nevada (Spain). Master Thesis. Máster Universitario en Gestión de la Fauna Silvestre, Universidad de Murcia, Murcia, Spain.
- Rasero R, Rossi L, Soglia D, Maione S, Sacchi P, Rambozzi L, Sartore S, Soriguer RC, Spalenza V, Alasaad S. Host taxon-derived Sarcoptes mite in European wild animals revealed by microsatellite markers. Biol Conserv. 2010;143(5):1269–1277. doi: 10.1016/j.biocon.2010.03.001. [DOI] [Google Scholar]
- Refoyo P, Olmedo C, Barba M, Muñoz B (2016) Parasite load in the Iberian ibex, Capra pyrenaica victoriae. Arxius de Miscel·lània Zoològica 14:108–113. 10.32800/amz.2016.14.0108
- Rentería-Solís Z, Min AM, Alasaad S, Müller K, Michler FU, Schmäschke R, Wittstatt U, Rossi L, Wibbelt G. Genetic epidemiology and pathology of raccoon-derived Sarcoptes mites from urban areas of Germany. Med Vet Entomol. 2014;28(Suppl. 1):98–103. doi: 10.1111/mve.12079. [DOI] [PubMed] [Google Scholar]
- Revilla Calavia M (2012) Estudio sanitario de la cabra montés Capra pyrenaica hispanica en Aragón. PhD Thesis, Universidad de Zaragoza, Zaragoza, Spain.
- Reyes-García R, Pérez-de-la-Lastra JM, Vicente J, Ruiz-Fons F, Garrido JM, Gortázar C. Large-scale ELISA testing of Spanish red deer for paratuberculosis. Vet Immunol Immunopathol. 2008;124(1–2):75–81. doi: 10.1016/j.vetimm.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cadenas F, Carbajal-González MT, Fregeneda-Grandes JM, Aller-Gancedo JM, Rojo-Vázquez FA (2010) Clinical evaluation and antibody responses in sheep after primary and secondary experimental challenges with the mange mite Sarcoptes scabiei var. ovis. Vet Immunol Immunopathol 133(2):109–116. 10.1016/j.vetimm.2009.07.004 [DOI] [PubMed]
- Rodríguez JL, Poveda JB, Rodríguez F, Espinosa de los Monteros A, Ramírez AS, Fernández A, Ovine infectious keratoconjunctivitis caused by Mycoplasma agalactiae. Small Rum Res. 1996;22(1):93–96. doi: 10.1016/0921-4488(96)00857-7. [DOI] [Google Scholar]
- Rossi L, Marco I, Meneguz PG (1994) Nematodes of the digestive tract of Capra pyrenaica: preliminary contribution. In: Catalino MA, Zulueta J, Meneguz PG, Rossi L. Actas del Congreso Internacional del género Capra en Europa. Junta Rectora del Parque Natural de la Sierra de las Nieves, Málaga, Spain, pp. 249–251.
- Rossi L, Fraquelli C, Vesco U, Permunian R, Sommavilla GM, Carmignola G, Da Pozzo R, Meneguz PG. Descriptive epidemiology of a scabies epidemic in chamois in the Dolomite Alps. Italy Eur J Wildl Res. 2007;53(2):131–141. doi: 10.1007/s10344-006-0067-x. [DOI] [Google Scholar]
- Rossi L, Tizzani P, Rambozzi L, Moroni B, Meneguz PG. Sanitary emergencies at the wild/domestic caprines interface in Europe. Animals. 2019;9:922. doi: 10.3390/ani9110922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML, Whiteley PL, Carver S. The treatment of sarcoptic mange in wildlife: a systematic review. Parasites Vectors. 2019;12:99. doi: 10.1186/s13071-019-3340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KM, Hunter DL, Rimler RB, Cassirer EF, Foreyt WJ, DeLong WJ, Weiser GC, Ward ACS. Microorganisms associated with a pneumonic epizootic in Rocky Mountain bighorn sheep (Ovis canadensis canadensis) J Zoo Wildl Med. 2007;38(4):548–558. doi: 10.1638/2006-0027R.1. [DOI] [PubMed] [Google Scholar]
- Ruiz Martínez I, Lasso de la Vega B, Pérez Jiménez JM. Datos preliminares sobre la prevalencia de la oestrosis en la cabra montés del parque natural de la Sierra de las Nieves. Spain: Málaga; 1992. pp. 253–255. [Google Scholar]
- Ruiz Martínez I, Madero A, Chirosa M. La sarna podría regular las poblaciones de cabra montés. Quercus. 1993;86:20–22. [Google Scholar]
- Ruiz Martínez I, Pérez JM, Chirosa M, Norman C, Soriguer RC, Fandos P (1996) Plan de manejo y gestión de la cabra montés (Capra pyrenaica) ante la epizootia de sarcoptidosis en el Parque Natural de Sierra Nevada (Granada, España). 1st International Conference Sierra Nevada, Conservación y Desarrollo Sostenible, Granada, Spain, March 20th to 22nd 1996, volume 3, pp. 47–68.
- Ruiz-Rodríguez C (2018) Seroprevalencia y factores de riesgo de tuberculosis, paratuberculosis y fiebre Q en la cabra montés (Capra pyrenaica) del Espacio Natural de Sierra Nevada y en ganado ovino y caprino simpátrico. Master Thesis. Máster Universitario en Gestión de la Fauna Silvestre, Universidad de Murcia, Murcia, Spain.
- Ryser-Degiorgis MP, Bischof DF, Marreros N, Willisch C, Signer C, Filli F, Brosi G, Frey J, Vilei EM. Detection of Mycoplasma conjunctivae in the eyes of healthy, free-ranging Alpine ibex: possible involvement of Alpine ibex as carriers for the main causing agent of infectious keratoconjunctivitis in wild Caprinae. Vet Microbiol. 2009;134:368–374. doi: 10.1016/j.vetmic.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Rzewuska M, Kwiecień E, Chrobak-Chmiel D, Kizerwetter-Świda M, Stefańska I, Gieryńska M. Pathogenicity and virulence of Trueperella pyogenes: a review. Int J Mol Sci. 2019;20(11):2737. doi: 10.3390/ijms20112737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito MU, Sonoda Y. Symptomatic racoon dogs and sarcoptic mange along an urban gradient. EcoHealth. 2017;14:318–328. doi: 10.1007/s10393-017-1233-1. [DOI] [PubMed] [Google Scholar]
- Saleh MA, Mahran OM, Bassam Al-Salahy M. Circulating oxidative stress status in dromedary camels infested with sarcoptic mange. Vet Res Commun. 2011;35:35–45. doi: 10.1007/s11259-010-9450-x. [DOI] [PubMed] [Google Scholar]
- Salvadori C, Rocchigiani G, Lazzarotti C, Formenti N, Trogu T, Lanfranchi P, Zanardello C, Citterio C, Poli A (2016) Histological lesions and cellular response in the skin of Alpine chamois (Rupicapra r. rupicapra) spontaneously affected by sarcoptic mange. BioMed Res Int, Article ID 3575468, 8 pages. 10.1155/2016/3575468 [DOI] [PMC free article] [PubMed]
- Samuel WM. Attempted experimental transfer of sarcoptic mange (Sarcoptes scabiei, Acarina: Sarcoptidae) among red fox, coyote, wolf and dog. J Wildl Dis. 1981;17(3):343–347. doi: 10.7589/0090-3558-17.3.343. [DOI] [PubMed] [Google Scholar]
- Sánchez MA (1998) Plan de Conservación de la cabra montés en la Región de Murcia. Dirección General del Medio Natural. Consejería de Medio Ambiente, Agricultura y Agua de Murcia, Murcia, Spain.
- Sánchez-Isarria MA, Hermoso J, Theureau de la Peña J, Casanova G, Burgui JM, Sanchís G, Arévalo P, Sánchez R (2008a) Metodología empleada en la estrategia del control de la sarna sarcóptica en la cabra montés de la Reserva Valenciana de Caza de la Muela de Cortes entre los años 2002–2007 (Valencia). In: Granados JE, Cano-Manuel FJ, Fandos P, Cadenas de Llano R (eds) Tendencias actuales en el Estudio y Conservación de los caprinos europeos. Junta de Andalucía, Granada, Spain, pp. 255–268.
- Sánchez-Isarria MA, Hermoso J, Theureau de la Peña J, Casanova G, Burgui JM, Sanchís G, Arévalo P, Sánchez R (2008b) La ivermectina como tratamiento de la sarna sarcóptica en el medio natural: vías de aplicación. In: Granados JE, Cano-Manuel FJ, Fandos P, Cadenas de Llano R (eds) Tendencias actuales en el Estudio y Conservación de los caprinos europeos. Junta de Andalucía, Granada, Spain, pp. 269–276.
- Sánchez-Seco MP, Sierra MJ, Estrada-Peña A, Valcárcel F, Molina R, Ramírez de Arellano E, Olmeda AS, García San Miguel L, Jiménez M, Romero LJ, Negredo A, Group for CCHFv Research Widespread detection of multiple strains of Crimean-Congo hemorrhagic fever virus in ticks. Spain Emerg Infect Dis. 2022;28(2):394–402. doi: 10.3201/eid2802.211308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasa M, Rambozzi L, Rossi L, Meneguz PG, Serrano E, Granados JE, González FJ, Fandos P, Soriguer RC, Gonzalez G, Joachim J, Pérez JM. Sarcoptes scabiei: specific immune response to sarcoptic mange in the Iberian ibex Capra pyrenaica depends on previous exposure and sex. Exp Parasitol. 2010;124(3):265–271. doi: 10.1016/j.exppara.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Sarasa M, Serrano E, Soriguer RC, Granados JE, Fandos P, Gonzalez G, Joachim J, Pérez JM. Negative effect of the arthropod parasite, Sarcoptes scabiei, on testes mass in Iberian ibex. Capra Pyrenaica Vet Parasitol. 2011;175(3):306–312. doi: 10.1016/j.vetpar.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140:392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Serrano E, Granados JE, Pérez JM. Sarcoptic mange and metapodial development in growing male Iberian ibex (Capra pyrenaica) Vet Parasitol. 2007;144(3):375–379. doi: 10.1016/j.vetpar.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Simpson K, Johnson CN, Carver S (2016) Sarcoptes scabiei: the mange mite with mighty effects on the common wombat (Vombatus ursinus). PLoS ONE 11(3):e0149749. 10.1371/journal.pone.0149749 [DOI] [PMC free article] [PubMed]
- Solanet JJ, Malena R, Estein SM, Belchior SE, Paolicchi FA, Tract AB. Desarrollo de una prueba de ELISA para detectar anticuerpos en carneros vacunados o infectados con Corynebacterium pseudotuberculosis. Rev Argent Microbiol. 2011;43(9):17. doi: 10.1590/S0325-75412011000100003. [DOI] [PubMed] [Google Scholar]
- Sommer C, Steffansen B, Nielsen BO, Grønvold J, Jensen KMV, Jespersen JB, Springborg J, Nansen P. Ivermectin excreted in cattle dung after subcutaneous injection or pour-on treatment: concentrations and impact on dung fauna. Bull Entomol Res. 1992;82:257–264. doi: 10.1017/S0007485300051804. [DOI] [Google Scholar]
- Sosa FE, Bertoni EA, Micheloud JF, Medina Vallejo DMN, Olmos LH, Florin-Christensen M, Romero SR. Occurrence of sarcoptic mange in free-ranging vicuñas (Vicugna vicugna) of the Andean high plateau region of Argentina. Parasitol Res. 2022;121:1587–1595. doi: 10.1007/s00436-022-07506-7. [DOI] [PubMed] [Google Scholar]
- Spengler JR, Bergeron É, Rollin PE (2016) Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis 10 :e0004210. 10.1371/journal.pntd.0004210 [DOI] [PMC free article] [PubMed]
- Spengler JR, Bente DA. Crimean-Congo hemorrhagic fever in Spain — new arrival or silent resident? N Engl J Med. 2017;377(2):106–108. doi: 10.1056/NEJMp1707436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyrou V, Valiakos G. Orf virus infection in sheep or goats. Vet Microbiol. 2015;181(1–2):178–182. doi: 10.1016/j.vetmic.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Süld K, Tammeleht E, Valdmann H, Saarma U. Severe impact of sarcoptic mange on the movements and space use for one of its most important vector species, the raccoon dog. Vet Parasitol. 2017;247:67–70. doi: 10.1016/j.vetpar.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Swe PM, Zakrzewski M, Kelly A, Krause L, Fischer K (2014) Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PLOS Negl Trop Dis 8(5):e2897 10.1371/journal.pntd.0002897 [DOI] [PMC free article] [PubMed]
- Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Ann Rev Entomol. 1994;39:47–49. doi: 10.1146/annurev.en.39.010194.000403. [DOI] [Google Scholar]
- Tarigan S. Antibody response in naïve and sensitised goats infested by Sarcoptes scabiei. Indonesian Journal of Animal and Veterinary Sciences. 2014;9(4):258–265. [Google Scholar]
- Terada Y, Murayama N, Ikemura H, Morita T, Nagata M. Sarcoptes scabiei var canis refractory to ivermectin treatment in two dogs. Vet Dermatol. 2010;21:608–612. doi: 10.1111/j.1365-3164.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- Turchetto S, Obber F, Permunian R, Vendrami S, Lorenzetto M, Ferré N, Stancampiano L, Rossi L, Citterio CV (2014= Spatial and temporal explorative analysis of sarcoptic mange in Alpine chamois (Rupicapra r. rupicapra). Hystrix 25(1):25–30. 10.4404/hystrix-25.1-9460
- Ueda T, Tarui H, Kido N, Imaizumi K, Hikosaka K, Abe T, Minegishi D, Tada Y, Nakagawa M, Tanaka S, Omiya T, Morikaku K, Kawahara M, Kikuchi-Ueda T, Akuta T, Ono Y. The complete mitochondrial genome of Sarcoptes scabiei var. nyctereutis from the Japanese raccoon dog: prediction and detection of two transfer RNAs (tRNA-A and tRNA-Y) Genomics. 2019;111:1183–1191. doi: 10.1111/mve.12079. [DOI] [PubMed] [Google Scholar]
- Uzal FA, Kelly WR. Enterotoxaemia in goats. Vet Res Commun. 1996;20:481–492. doi: 10.1007/BF00396291. [DOI] [PubMed] [Google Scholar]
- Valldeperes M, Granados JE, Gregorio M, Prieto P, Escribano F, Cardells J, Foj R, Puigdemont A, Fandos P, Ráez-Bravo A, Espinosa J, Pérez JM, Soriguer R, López-Olvera JR (2018a) Serological survey of sarcoptic mange in Mediterranean Iberian ibex (Capra pyrenaica) populations. In: 13th conference of the European Wildlife Disease Association, August 27th to September 1st 2018a, Larissa, Greece, p. 18071
- Valldeperes M, Granados JE, Mentaberre G, Prieto P, Escribano F, Cardells J, Foj R, Puigdemont A, Fandos P, Ráez-Bravo A, Espinosa J, Pérez JM, Lizana V, Soriguer R, López-Olvera JR (2018b) Estudio serológico de la sarna sarcóptica en las poblaciones mediterráneas de cabra montés (Capra pyrenaica). IX meeting of the Red de Ungulados Silvestres Ibéricos, October 4th to 6th, 2018b, Capileira, Spain.
- Valldeperes M, Granados JE, Pérez JM, Castro I, Ráez-Bravo A, Fandos P, López-Olvera JR, Serrano E, Mentaberre G. How sensitive and specific is the visual diagnosis of sarcoptic mange in free-ranging Iberian ibexes? Parasites Vectors. 2019;12:405. doi: 10.1186/s13071-019-3665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valldeperes M, Moroni B, Rossi L, López-Olvera JR, Velarde R, Molinar Min AR, Mentaberre G, Serrano E, Angelone S, Lavín S, Granados JE. First report of interspecific transmission of sarcoptic mange from Iberian ibex to wild boar. Parasites Vectors. 2021;14:481. doi: 10.1186/s13071-021-04979-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valldeperes M, Rossi L, López-Olvera JR, Moroni B, Gardini G, Odore R, Granados-Torres JE, Mentaberre G (2022a) Uso de pienso medicado con ivermectina para el control de la sarna sarcóptica en una población de cabra montés (Capra pyrenaica) de vida libre. 39èmes Rencontres du Groupe d’Étude sur l’Écopathologie de la Faune Sauvage de Montagne (GEEFSM). Libro de resúmenes. UCOPress, Editorial Universidad de Córdoba, Córdoba, Spain, p. 15.
- Valldeperes M, Rossi L, Mentaberre G, Moroni B, Gardini G, Odore R, Granados-Torres JE, López-Olvera JR (2022b) Un comensal inesperado: detección de ivermectina en jabalíes (Sus scrofa) simpátricos con una población de cabra montés (Capra pyrenaica) afectada por sarna sarcóptica y tratada con pienso medicado. 39èmes Rencontres du Groupe d’Étude sur l’Écopathologie de la Faune Sauvage de Montagne (GEEFSM). Libro de resúmenes. UCOPress, Editorial Universidad de Córdoba, Córdoba, Spain, p. 44.
- Van Neste DJJ. Immunology of scabies. Parasitol Today. 1986;2:194–196. doi: 10.1016/0169-4758(86)90192-4. [DOI] [PubMed] [Google Scholar]
- Varela-Castro L, Lara-Vergara J, Ortega N, Salinas J, Colom-Cadena A, Lavín S, Tizzani P, Velarde R, Serrano E, Mentaberre G. Endemic caseous lymphadenitis in a wild Caprinae population. Vet Rec. 2017;180(16):405. doi: 10.1136/vr.103925. [DOI] [PubMed] [Google Scholar]
- Varela-Castro L, Zuddas C, Ortega N, Serrano E, Salinas J, Castellà J, Castillo-Contreras R, Carvalho J, Lavín S, Mentaberre G. On the possible role of ticks in the eco-epidemiology of Coxiella burnetii in a Mediterranean ecosystem. Ticks Tick-Borne Dis. 2018;9:687–694. doi: 10.1016/j.ttbdis.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Vela AI, Mentaberre G, Lavín S, Domínguez L, Fernánndez-Garayzábal JF. Streptococcus caprae sp. nov., isolated from Iberian ibex (Capra pyrenaica hispanica) Int J Syst Evol Microbiol. 2016;66:196–200. doi: 10.1099/ijsem.0.000697. [DOI] [PubMed] [Google Scholar]
- Velarde R, Mentaberre G, Sánchez J, Marco I, Lavín S. KIT-positive gastrointestinal stromal tumours in two Spanish ibex (Capra pyrenaica hispanica) Vet J. 2008;177:445–447. doi: 10.1016/j.tvjl.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Verbisck-Bucker G, González-Candela M, Galián J, Cubero-Pablo MJ, Martín-Atance P, León-Vizcaíno L. Epidemiology of Mycoplasma agalactiae infection in free-ranging Spanish ibex (Capra pyrenaica) in Andalusia, southern Spain. J Wildl Dis. 2008;44(2):369–380. doi: 10.7589/0090-3558-44.2.369. [DOI] [PubMed] [Google Scholar]
- Verbisck G, González-Candela M, Cubero MJ, León L, Serrano E, Perales A. Mycoplasma agalactiae in Iberian ibex (Capra pyrenaica) in Spain. Vet Rec. 2010;167(11):425–426. doi: 10.1136/vr.c4908. [DOI] [PubMed] [Google Scholar]
- Verdú JR, Cortez V, Ortiz AJ, González-Rodríguez E, Martínez-Pinna J, Lumarte JP, Lobo JM, Numa C, Sánchez-Piñero F. Low doses of ivermectin cause sensory and locomotor disorders in dung beetles. Sci Rep. 2015;5:13912. doi: 10.1038/srep13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdú JR, Cortez V, Martínez-Pinna J, Ortiz AJ, Lumaret JP, Lobo JM, Sánchez Piñero F, Numa C. First assessment of the comparative toxicity of ivermectin and moxidectin in adult dung beetles: sub-lethal consequences. Sci Rep. 2018;8:14885. doi: 10.1038/s41598-018-33241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JJ, West DM, Pomroy WE. Inefficacy of moxidectin and doramectin against ivermectin-resistant Cooperia spp. of cattle in New Zealand. N Z Vet J. 1996;44:188–193. doi: 10.1080/00480169.1996.35971. [DOI] [PubMed] [Google Scholar]
- Vigal CR, Machordom A. Dental and skull anomalies in the Spanish wild goat, Capra pyrenaica Schinz, 1838. Zeitschrift Für Saugetierkunde. 1987;52:38–50. [Google Scholar]
- Vilei EM, Bonvin-Klotz L, Zimmermann L, Ryser-Degiorgis MP, Giacometti M, Frey J. Validation and diagnostic efficacy of a TaqMan real-time PCR for the detection of Mycoplasma conjunctivae in the eyes of infected Caprinae. J Microbiol Methods. 2007;70:384–386. doi: 10.1016/j.mimet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Walter B, Heukelbach J, Fengler G, Worth C, Hengge U, Feldmeier H. Comparison of dermoscopy, skin scraping, and the adhesive tape test for the diagnosis of scabies in a resource-poor setting. Arch Dermatol. 2011;147(4):468–473. doi: 10.1001/archdermatol.2011.51. [DOI] [PubMed] [Google Scholar]
- Walton SF, Choy JL, Bonson A, Valle A, McBroom J, Taplin D, Arlian L, Mathews JD, Currie B, Kemp DJ. Genetically distinct dog-derived and human-derived Sarcoptes scabiei in scabies-endemic communities in northern Australia. Am J Trop Med Hyg. 1999;61(4):542–547. doi: 10.4269/ajtmh.1999.61.542. [DOI] [PubMed] [Google Scholar]
- Walton SF, Holt DC, Currie BJ, Kemp DJ. Scabies: new future for a neglected disease. Adv Parasitol. 2004;57:309–376. doi: 10.1016/S0065-308X(04)57005-7. [DOI] [PubMed] [Google Scholar]
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20(2):268–279. doi: 10.1128/CMR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor PA. Paratuberculosis in sheep and goats. Vet Microbiol. 2015;181(1–2):161–169. doi: 10.1016/j.vetmic.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Wobeser GA. Investigation and management of disease in wild mammals. New York-London: Plenum Press; 1994. p. 265. [Google Scholar]
- Wobeser GA (2002) Disease management strategies for wildlife. Rev Sci Tech OIE 21(1):159–178. 10.20506/rst.21.1.1326 [DOI] [PubMed]
- Yang Y, Li Y, Dao X, Fei L, Yang F (2022) Molecular detection and isolation of Mycoplasma conjunctivae from goats with infectious keratoconjunctivitis in Sichuan province, southwest China. The Thai Journal of Veterinary Medicine 52(3): 601–606. https://he01.tci-thaijo.org/index.php/tjvm/article/view/258657
- Ytrehus B, Bretten T, Bergsjø B, Isaksen K. Fatal pneumonia epizootic in musk ox (Ovibos moschatus) in a period of extraordinary weather conditions. EcoHealth. 2008;5:213–223. doi: 10.1007/s10393-008-0166-0. [DOI] [PubMed] [Google Scholar]
- Zahler M, Essig A, Gothe R, Rinder H. Molecular analyses suggest monospecificity of the genus Sarcoptes (Acari: Sarcoptidae) Int J Parasitol. 1999;29(5):759–766. doi: 10.1016/s0020-7519(99)00034-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann L, Jambresic S, Giacometti M, Frey J. Specificity of Mycoplasma conjunctivae strains for alpine chamois Rupicapra r. rupicapra. Wildl Biol. 2008;14:118–124. doi: 10.2981/0909-6396(2008)14[118:SOMCSF]2.0.CO;2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used to generate this review are publicly available. The authors will share information on data location upon request.