Abstract
CONTEXT
The Modified Checklist for Autism in Toddlers, Revised with Follow-up (M-CHAT-R/F) is used worldwide to screen for autism spectrum disorder (ASD).
OBJECTIVE
To calculate psychometric properties of the M-CHAT-R/F for subsequent diagnosis of ASD.
DATA SOURCES
Systematic searches of Medline, Embase, SCOPUS, and Trip Pro databases from January 2014 to November 2021.
STUDY SELECTION
Studies were included if they (1) used the M-CHAT-R/F (2) applied standard scoring protocol, (3) used a diagnostic assessment for ASD, and (4) reported at least 1 psychometric property of the M-CHAT-R/F.
DATA EXTRACTION
Two independent reviewers completed screening, full-text review, data extraction, and quality assessment, following Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. A random-effects model was used to derive pooled estimates and assess for between-study heterogeneity.
RESULTS
Of 667 studies identified, 15 with 18 distinct samples from 10 countries (49 841 children) were used in the meta-analysis. Pooled positive predictive value (PPV), was 57.7% (95% confidence interval [CI] 48.6–66.8, τ2 = 0.031). PPV was higher among high-risk (75.6% [95% CI 66.0–85.2]) than low-risk samples (51.2% [95% CI 43.0–59.5]). Pooled negative predictive value was 72.5% (95% CI 62.5–82.4 τ2 = 0.031), sensitivity was 82.6% (95% CI 76.2–88.9) and specificity 45.7% (95% CI 25.0–66.4).
LIMITATIONS
Negative predictive value, sensitivity, and specificity were calculated based on small sample sizes because of limited or no evaluation of screen-negative children.
CONCLUSIONS
These results support use of the M-CHAT-R/F as a screening tool for ASD. Caregiver counseling regarding likelihood of an ASD diagnosis after positive screen should acknowledge the moderate PPV.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social communication, and restricted, repetitive patterns of behavior.1 The prevalence rate of ASD is estimated to be 1.0% globally (median value, range 0.01% to 4.4% in a recent systematic review of studies since 2012) and 2.3% in the United States.2,3 Behavioral intervention initiated before 24 months of age has been shown to improve cognitive and adaptive functioning more than intervention initiated later in life.4–8 One of the focal points of research has been on developing effective screening tools to make the diagnosis of ASD at the youngest possible age, in tandem with the drive toward earlier initiation of appropriate therapeutic and educational services. In line with these priorities, the American Academy of Pediatrics advocates for routine ASD screening in children ages 18 and 24 months9 given the superior effectiveness of early behavioral therapy and the accuracy and diagnostic stability of ASD diagnosis as early as 18 months.10–12
Among the available screening tools, the Modified Checklist for Autism in Toddlers, Revised with Follow-Up (M-CHAT-R/F) has been one of the most studied and implemented screening tools.13,14 It has been translated into 58 different languages and dialects. It has been recommended as a screening tool by several national guidelines, especially for children at high-risk of autism, based on its high sensitivity in the original sample and the ease of administration.9,15,16 The M-CHAT-R/F is a 2-stage parent-report screening tool for ASD17 designed for use in children ages 16 to 30 months. In the norming sample, sensitivity was reported to be 0.83 to 0.85, specificity 0.95 to 0.99 and positive predictive value (PPV) was 0.465.17 The M-CHAT-R/F reported a lower false positive rate compared with its predecessor, the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F)18 and has replaced the older version as the preferrable screening instrument.
Given the widespread use of the M-CHAT-R/F as a screening instrument for ASD around the globe, it is imperative to understand its psychometric properties beyond the original validation study and to determine if the M-CHAT-R/F is sensitive and specific in other samples, including non-English speaking samples worldwide using translated versions of the M-CHAT-R/F. The PPV and the negative predictive value (NPV) are both of importance in a screening tool for ASD given that false positive and negative screening test results could be highly detrimental for children and their families. Previous systematic reviews to date have focused on the original Modified Checklist for Autism in Toddlers (M-CHAT) questionnaire or other screening tools,19–21 not the M-CHAT-R/F. A recent meta-analysis evaluated the original M-CHAT and the M-CHAT-R/F without distinguishing between the 2 versions.22 That review has limited applicability for evaluating the M-CHAT-R/F, even though this version is the preferred screening instrument in the present era. Hence, we performed a systematic review and meta-analysis to evaluate the utility of the M-CHAT-R/F exclusively to screen for ASD in young children. We intentionally screened studies irrespective of language or country of use. We planned to report PPV and NPV in addition to sensitivity and specificity because of the importance of these psychometric values for interpreting the results of screening. In primary analyses, to maintain equipoise and increase the accuracy of sensitivity, specificity, and NPV, we required that screen-negative children received a diagnostic evaluation rather than assumed they were condition-free.
Methods
Search Strategy
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines23 from January 2014, date of the publication of the instrument, to 19th November 2021 across Medline, Embase, SCOPUS, and Trip Pro database by a health sciences librarian (Supplemental Table 3). We reviewed papers written in Spanish, French, and Russian during the initial search, though none met criteria for full text review.
Study Inclusion and Exclusion Criteria
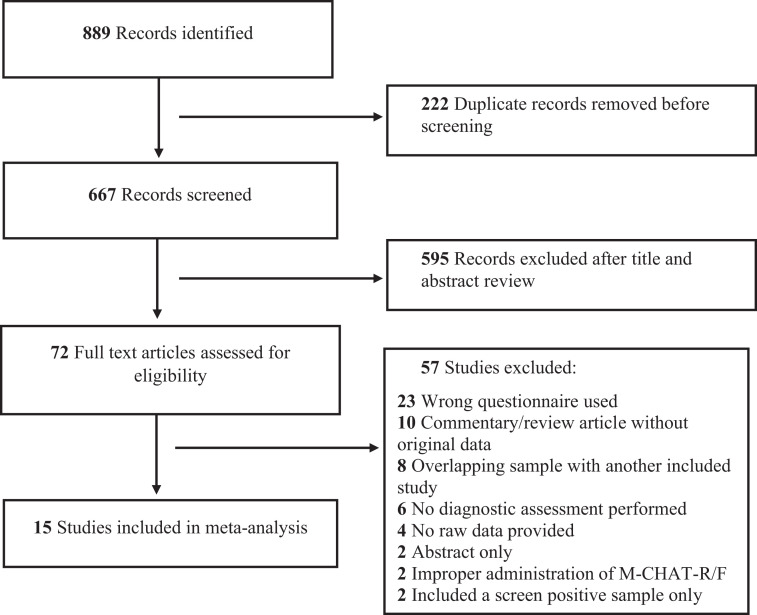
Inclusion criteria were: (1) an original sample of children ≤ 48 months of age from a low-risk (ie, community) sample or high-risk (ie, children of siblings with ASD and premature infants) sample, (2) use of the 20-question M-CHAT-R/F questionnaire and clarifying interview and standard scoring protocol, (3) report of at least 1 psychometric property, and use of a diagnostic evaluation for diagnosis of ASD. Studies that reported only rates of the positive questionnaire screens without data from clarifying interviews were excluded. Studies with samples that overlapped other included studies in the meta-analysis were excluded (Fig 1). Two researchers (R.A. and V.K.M.) independently screened titles and abstracts of studies identified from the initial search. Discrepancies in decisions were discussed with a third researcher (H.M.F.) and consensus was reached following group discussion. Included articles then underwent full text review by the same independent process with all conflicts resolved by consensus following group discussion. We contacted corresponding authors of 8 studies via e-mail for additional information to clarify if the study met inclusion criteria; we requested raw data on screening numbers in 5 cases and details of the administration process of the M-CHAT-R/F in 3 cases. We received responses from 5 corresponding authors.
FIGURE 1.
PRISMA diagram for study selection
Data Extraction
Data collected included: demographics, risk profile of children studied, country of administration, socioeconomic status, administrator and interviewer of the M-CHAT-R/F questionnaire, language used and translation process, diagnostic measure of ASD, results of screening and diagnostic assessments, and number of individuals lost to follow-up at any stage within each study.
Study Quality Assessment
The quality of studies in the final sample was evaluated based on the US Preventive Services Task Force framework (Table 1).24 Ratings of “good,” “fair,” and “poor,” were assigned based on sample size, validity of diagnostic evaluation, diagnostic evaluation applied to at least a proportion of the screen-negative participants, and administration of the screening and diagnostic evaluations by different personnel. When study attributes fell across 2 quality categories, the lower category was assigned. This rule was applied to 3 studies, which were ultimately rated “fair.” Quality ratings of each study were assigned independently by 2 researchers (R.A. and V.K.M.) and discrepancies were resolved following discussion with a third researcher (H.M.F.).
TABLE 1.
Quality Rating Definition
| Quality | Characteristics |
|---|---|
| Good | Evaluates M-CHAT-R/F questionnaire with standard scoring protocol |
| Reliable reference standard used as per DSM-5 criteria | |
| Independent assessor for reference standard and screening questionnaire | |
| Reliability of M-CHAT-R/F assessed | |
| Handles indeterminate results in a reasonable manner or has few such results | |
| Includes large number (greater than 1000) of subjects | |
| Fair | Evaluates M-CHAT-R/F questionnaire with standard scoring protocol |
| Uses reasonable although not best reference standard | |
| Independent assessor for reference standard and screening questionnaire | |
| Includes moderate number (500 to 1000) of subjects | |
| Poor | Has a major flaw, such as: |
| - uses inappropriate reference standard | |
| - improperly administers screening test | |
| - biased ascertainment of reference standard | |
| - has very small (<500) number of subjects |
Data Analysis
We applied a rigorous approach to the M-CHAT-R/F scoring criteria; to be counted as a screen-positive, the child must have been classified either as high-risk (score ≥ 8) or scored above the cut-off after the follow-up interview (post interview score ≥ 2). For each distinct study sample, PPV was estimated as the proportion of participants with a positive screen who were confirmed to have ASD on the diagnostic evaluation. In the primary analyses, for each distinct study sample in which all, or a random sample, of participants who screened negative were evaluated by the diagnostic evaluation, the NPV was estimated as the proportion of participants with a negative screen who were confirmed not to have ASD. If all evaluated participants with a given screening outcome had the same diagnostic outcome (eg, all negative screens were deemed negative by the diagnostic evaluation), a continuity correction of 0.5 was added to the numerator and 1.0 to the denominator of the proportion. The variance of each PPV and NPV estimate was computed as p(1-p)/n, where p is the proportion, and n is the denominator of the proportion; binomial confidence intervals were estimated as specified by Fleiss (1981).25 Pooled estimates of overall PPV and for subgroups (risk-status, United States versus non-United States country and good or fair quality studies only), and NPV of selected study samples, were estimated using random effects models according to the method of DerSimonian and Laird.26 Pooled estimates of sensitivity and specificity were similarly calculated.
In the primary analysis, screen-negative participants who did not have a diagnostic evaluation were assumed to be of unknown ASD status. We conducted secondary analyses for NPV and specificity, assuming that all screen-negative participants without a diagnostic evaluation did not have ASD (ie, true negatives).
The SAS macro METAANAL27 was used to compute estimates and associated statistics: SE, Q, and I2 (ie, τ2), and forest plots were created. A meta-regression of PPV versus mean participant age was performed using a generalized linear mixed model specifying a binomial outcome with identity link and a random intercept for study sample. All analyses were performed with SAS Enterprise Guide version 7.15; a graph of PPV versus mean participant age was created with Excel.
Results
Our search yielded 667 nonduplicate records. A total of 72 full-text articles were reviewed. A total of 15 studies,17,28–41 comprised of 18 distinct samples, with a total of 49 841 participants met full inclusion criteria. Two studies did not meet inclusion criteria as they did not administer the follow-up interview after an initial positive screen.
Study Characteristics
Table 2 shows the characteristics of the study sample from included studies (published between 2014 and 2021). The majority were of children 16 to 30 months of age; 2 studies33,37 included 14-month-old children and 3 studies included children up to 36 months30,33,34 (mean age of sample 23.3 months). Two studies reported results of screening of more narrow age-range subcohorts and their results were analyzed as 2 discrete samples.33,37 There was a good representation of international studies; 9 of 15 studies were conducted in countries other than the United States, including 4 in Asia,29,31,34,38 3 in Europe,30,32,33 and 1 each in Africa28 and South America.39 The mean age of the sample from studies conducted in the United States was 22.3 months as compared with 23.9 months for that from other countries. All international studies used the M-CHAT-R/F in languages other than English; forward and backward translation completion was specifically mentioned in 6 of the 9 studies.
TABLE 2.
Characteristics of Included Studies
| Source and Country | Total Number | Age Range (Months) | Mean Age (SD)b | Males (%) | Type of Sample | Initial Interviewer | Follow up Interviewer | Language | Diagnostic Evaluation Method | Quality Indicator | Screen- Negative Evaluated |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Guo et al31 2019; China | 7928 | 16–30 | 22.7 (4.1) | 55.6 | Community | Healthcare | NS | Mandarine | Clinical evaluation | Good | Someg |
| Jonsdottir et al32 2021; Iceland | 1586 | NS | 31.7 (1.7) | 50.5 | Community | Healthcare | Research personnel | Icelandishe | ADOS | Good | Someg |
| Magan-Maganto et al33 2020; Spain | 6625 | 14–36 | 14–22 mo: 18.2 (0.7); 23–36 mo: 24.5 (1.2) | 51.2 | Community | Healthcare | Research personnel, Healthcare | Spanishe | ADOS | Good | Someg |
| Oner et al34 2020; Turkey | 6712 | 16–36 | 26.8 (5.8) | 51.5 | Community | Healthcare | Healthcare | Turkishe | ADOS | Good | Someh |
| Robins et al17 2014; US | 16071 | 16–30.9 | 21.0 (3.3) | 50.12 | Community | Healthcare | Research personnel | English | ADOS | Good | Someh |
| Wieckowski et al37a 2021; US | 4281 | 14.0–21.9 | NA | 51.2 (15 mo); 49.3 (18 mo) | Community | Healthcare | NS | English | ADOS | Good | Someg |
| Bradbury et al35 2020; US | 187 | 16–30 | 21.2 (4.1) | 58.4 | High-riskc | Healthcare | Research personnel | English | ADOS | Fair | Someg |
| Brennan et al30 2016; Albania | 2594 | 16–36 | 24 (2.8) | 50.1 | Community | Healthcare | Research personnel | Albaniane | ADOS | Fair | None |
| Christopher et al40 2021; US | 290 | 18–48 | 32.7 (7.7) | 79.6 | High-risk | NS | NS | English | ADOS | Fair | All |
| McNally Keehn et al41 2021; US | 605 | 18–48 | 30.4 (6.5) | NS | Community | Healthcare | Healthcare | English | Clinical evaluation | Fair | Somei |
| Tsai et al38 2019; Taiwan | 317 | 16–32 | 24.3 (4.4) | 52.7 | Community, High-riskc | Parent-completed via mail | NS | Mandarine | Clinical evaluation | Fair | All |
| Weitlauf et al36 2015; US | 74 | 16–21 | 18.0 (NS) | 63 | High-riskc | NS | Research personnel | English | ADOS | Fair | Somei |
| Coelho-Medeiros et al39 2019; Chile | 120 | 16–30 | 22.47 (4.22) | 64.2 | Community high-riskc | Healthcare | Healthcare provider | Spanishf | ADOS | Poor | Someh |
| Manzouri et al29 2019; Iran | 1504 | 16–30 | 20.26 (3.74) | 49.8 | Community | Healthcare | Healthcare provider | Persiand | Clinical evaluation | Poor | None |
| Sangare et al28 2019; Mali | 947 | NS | NS | NS | Community | Research | Research personnel | Frenchf | Clinical evaluation | Poor | Somei |
Healthcare provider refers to personnel at healthcare facility including but not limited to physicians and nurses. ADOS, Autism Diagnostic Observation Schedule; NS, not specified.
Distinct sample of 15- and 18-mo children screened with M-CHAT-R/F and rescreened at older ages; only results of initial screen included in analysis.
Ages and standard deviations rounded to the nearest tenth.
High risk samples include: siblings of children with ASD and children referred for developmental concerns for ASD.
Forward translated.
Forward and backward translated.
Translation not specified.
Evaluated based on screening tool results, physician concern, or referral for diagnostic assessment.
Evaluated based on random selection.
Evaluation reason not specified.
Most of the studies were conducted solely on community samples (10 of 15). Two were conducted solely on a high-risk sample of siblings of children previously diagnosed with ASD.35,36 One study was conducted solely on children referred for concerns of ASD40 and 2 studies included a mixture of a low-risk community sample and a high-risk sample of children with developmental concerns referred for further evaluation.38,39 Among the studies which included both a community and a high-risk sample, 1 did not report findings for each sample separately,38 hence it was not included in the risk-based subgroup analysis. No other high-risk samples were included (eg, extreme premature infants). Thirteen of 15 studies applied a diagnostic evaluation for ASD on at least a portion of screen-negative patients, although this step was applied to the entire sample or a completely random screen-negative sample in only 5 studies. The remainder applied the diagnostic assessment on screen-negative children who had other concerns for an eventual ASD diagnosis (such as provider clinical concerns or failure of another screening test). Across all 15 included studies, only 780 screen-negative children had a diagnostic evaluation (1.67% of the total screen-negative sample). The majority of the studies (12 of 15) had a quality rating of “good” or “fair”; 3 studies had a “poor” rating. The reasons for a poor rating included lack of information on diagnostic assessments used, small sample size (<500 children), and same personnel administering both the M-CHAT-R/F and diagnostic assessment.
Data on family socioeconomic status or parental education was not reported uniformly across studies, and thus was not adequate for analysis within the meta-analysis. There was significant loss to follow-up of screen-positive children who did not have subsequent evaluations in each study (range 3.2% to 71.0%, mean 28.8%, median 32.3%). None of the studies compared children who were lost to follow-up and those who received diagnostic assessment. Further, a portion of children in each study did not complete the full 2-stage screening process, with indeterminate screening results following a failed first stage (mean 25.7%, median 18.6%).
Overall Findings From All Studies
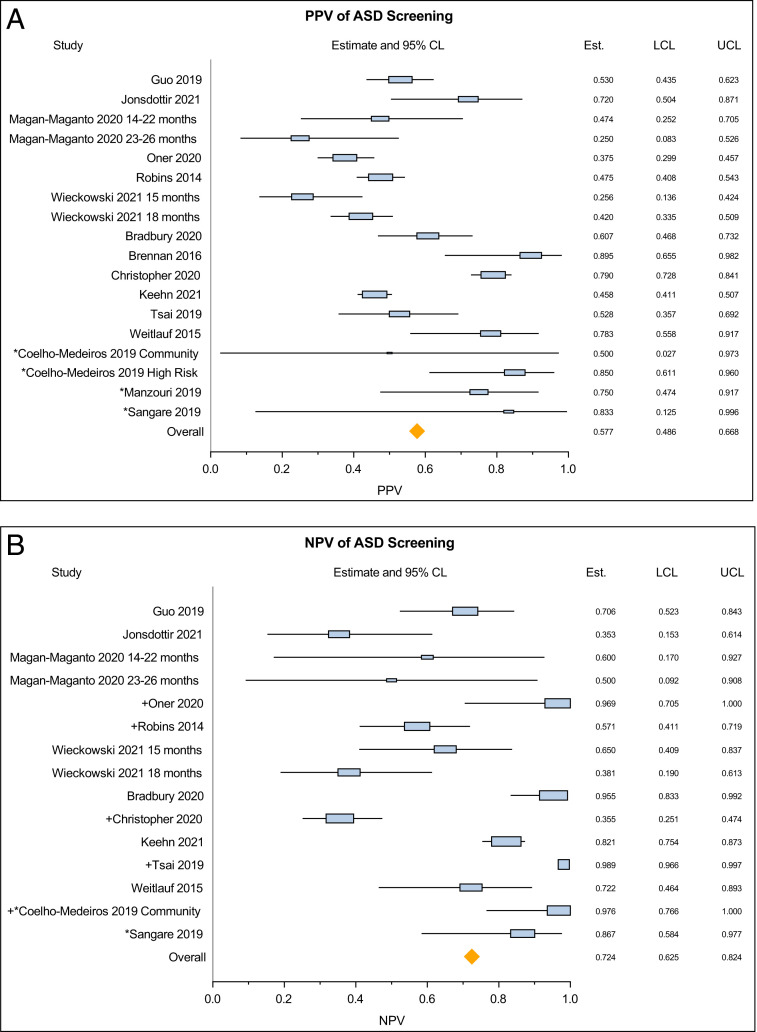
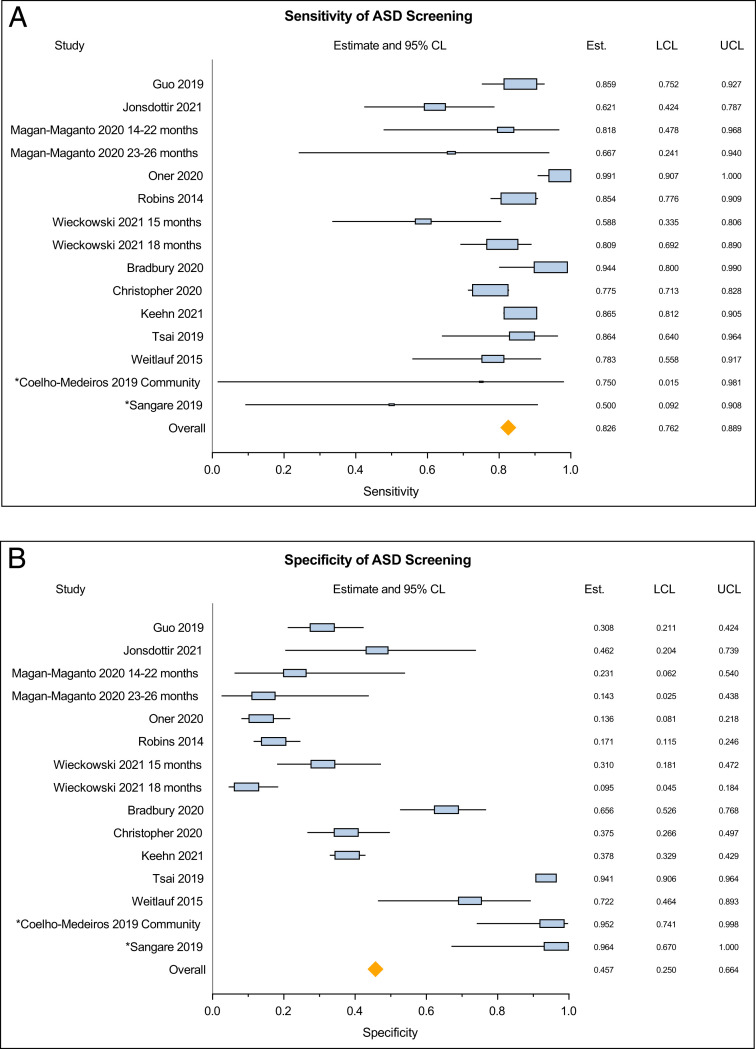
The meta-analysis of 15 studies covering 18 distinct samples showed a pooled PPV of 57.7% (95% CI 48.6–66.8) with significant between-study heterogeneity (Q = 203.54, P < .0001, τ2 = 0.031) (Fig 2A). The pooled NPV derived from the primary analysis of screen-negative children who had a diagnostic evaluation (from 13 studies) was 72.5% (95% CI 62.5–82.4), with significant between-study heterogeneity (Q = 280.27, P < .0001, τ2 = 0.031) (Fig 2B). The pooled NPV based on the 5 studies that evaluated a random sample of screen-negative children was 78.0% (95% CI 59.5–96.5, Q = 160.25, P < .0001). In the secondary analysis (assuming that screen-negative participants who did not have a diagnostic evaluation were true negatives) the pooled NPV was 99.7% (95% CI 99.6–99.9). The pooled sensitivity was 82.6% (95% CI 76.2–88.9), with significant between-study heterogeneity (Q = 106.49, P < .0001, τ2 = 0.010). Pooled specificity in the primary analysis was 45.7% (95% CI 25.0–66.4), with significant between-study heterogeneity (Q = 1532.70, P < .0001, τ2 = 0.163) (Figs 3, A and B). Pooled specificity in the secondary analysis was 97.1% (95% CI 96.4–97.9).
FIGURE 2.
(A) Pooled estimates of positive predictive value of the M-CHAT-R/F questionnaire – all studies. (B) Pooled estimates of negative predictive value of the M-CHAT-R/F questionnaire. Studies are listed based on quality from “good” to “poor.” *, Denotes studies related as “poor” quality. +, Denotes studies that assessed a random sample of screen-negative children.
FIGURE 3.
(A) Pooled estimates of sensitivity of the M-CHAT-R/F questionnaire. (B) Pooled estimates of specificity of the M-CHAT-R/F questionnaire.
A meta-regression for PPV versus mean age of sample estimated an increase of 0.012 (SE 0.010) in PPV per month of mean age, but this trend was not statistically significant (P = .23) (Supplemental Fig 5). A funnel plot depicting number of screen-positive cases evaluated versus PPV was symmetrical suggesting low possibility of publication bias (Supplemental Fig 6). We calculated a pooled PPV for the presence of any developmental disorder (eg, global developmental delay or language delay) from 8 studies that provided these data. The PPV was higher at 89.0% (95% CI 82.9–94.6), with significant between-study heterogeneity (Q = 60.21, P < .0001). Because of inconsistent and/or absent reporting of these data across studies, including for screen-negative children, we could not derive sensitivity, specificity, or NPV for the presence of any developmental disorder.
Additional Findings From Subgroup Analyses
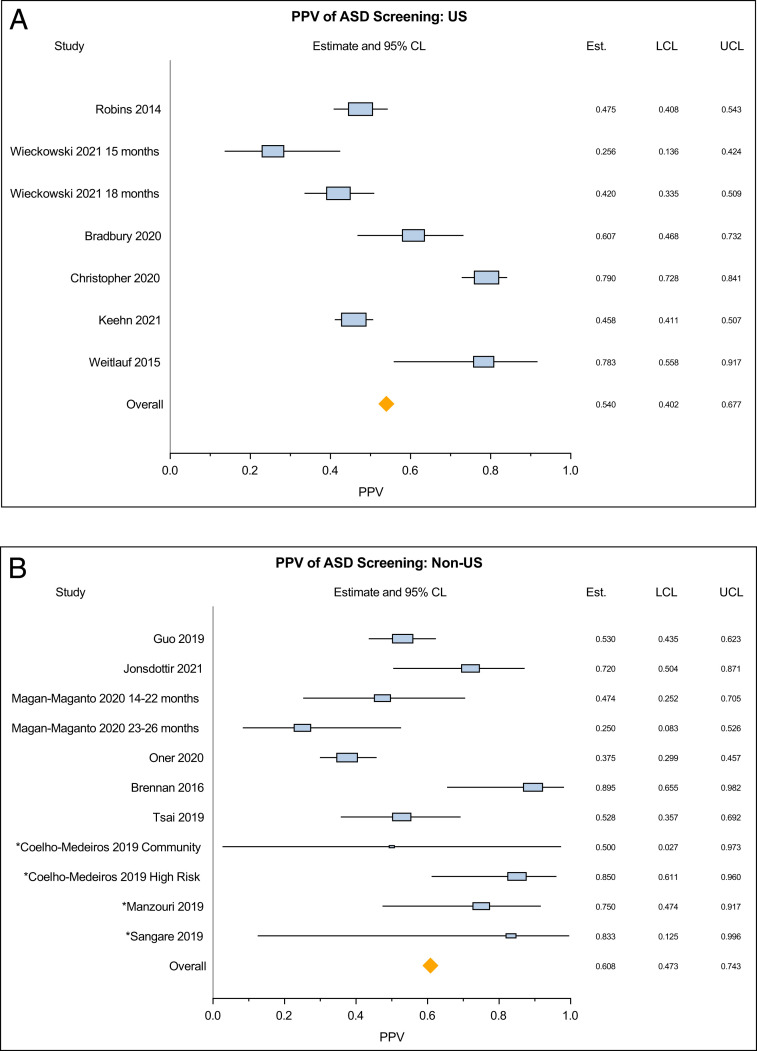
Subgroup analysis was conducted based on risk status and country of origin of the study (United States versus non-United States), as planned a priori. PPV of the high-risk sample based on 4 studies was 75.6% (95% CI 66.0–85.2) with no significant between-study heterogeneity (Q = 57.75, P = .051, τ2 = 0.006) (Supplemental Fig 7). In contrast, PPV for the low-risk sample was 51.2% (95% CI 43.0–59.5, Q = 76.28, P < .0001, τ2 = 0.016). Pooled PPV of studies conducted in the United States was lower than PPV of those conducted in other countries (PPV 54.0% [95% CI 40.2–67.7] versus 60.8% [95% CI 47.3–74.3]) (Fig 4). There was significant between-study heterogeneity in both of these subgroups.
FIGURE 4.
Pooled estimates of positive predictive value of the M-CHAT-R/F questionnaire – stratified by country of origin of studies (United States versus non-United States).
Discussion
This study is a meta-analysis of the specific performance of the M-CHAT-R/F as a screening tool for ASD from a global sample that employed the tool in various languages.
The findings of our meta-analysis, from 10 different countries and using 8 different languages, including English, showed an overall pooled sensitivity of the M-CHAT-R/F at 82.5%. Sensitivity is above the recommended 70% to 80% expected of a good screening tool.42 Thus, the proportion of young children with ASD who are missed by screening with the M-CHAT-R/F is small. Some children with ASD who have subtle social communication difficulties may not be detected by screening before 36 months of age.
Our results reveal a pooled estimated PPV of 57.7% for the subsequent diagnosis of ASD following a positive screen. In a low-risk community sample, for whom the tool is most appropriately used, the chance of an eventual ASD diagnosis following a positive M-CHAT-R/F screen decreases to 51.2%. This finding is similar to that reported in the original validation study of the M-CHAT-R/F of 46.5%17 and to that of the original M-CHAT.21 It is also in line with the findings of the US Preventive Services Task Force that looked at both the M-CHAT and M-CHAT-R/F.43 The pooled estimate of the PPV of the M-CHAT-R/F in relation to the presence of any developmental disorder, such as global developmental delay, increases to 89.0%, in keeping with previous studies.17,33,36
Real-world performance of a screening tool must consider PPV and NPV. The pooled PPV estimate that we derived is in the moderate range at 57.5%. The PPV for ASD is relevant when counseling parents of children with a positive screen with respect to the likelihood of an eventual ASD diagnosis; only approximately half of those children with a positive screen will ultimately receive the diagnosis of ASD; the other half may have no or another developmental disorder. Many factors affect PPV. PPV is related to the population prevalence of the condition; lower prevalence is associated with lower PPV, regardless of sensitivity.44 Given the prevalence rate of ASD at 1% to 2.3%, even a screening tool with 99% sensitivity would result in a PPV of 67% at best.44 In addition, given that the M-CHAT-R/F is a parent-reported screening tool, lack of parental awareness of expected social-communication milestones may lead to false responses on the M-CHAT-R/F, compromising PPV. PPV rates would also be influenced by screen-positive children who were lost to follow-up; these children may have sought diagnostic evaluation elsewhere, lowering the reported PPV by the study. The moderate PPV emphasizes the need for further evaluations for the presence of ASD and/or any other developmental conditions rather than reliance on the results of this screening tool for diagnosis. Repeated screening of the same child at multiple timepoints, such as at 18 and 24 months as recommended by the American Academy of Pediatrics, may improve the accuracy of ASD screening,37 though this recommendation bears further evaluation. A positive screening result may also indicate a developmental disorder other than ASD. The diagnostic evaluation after a positive screening test should consider a range of diagnostic possibilities in addition to ASD.
Our finding of a higher PPV for studies originating from non-United States countries compared with those within the United States is surprising. This finding could have been related to the varying prevalence of ASD within each country given the variation within global prevalence rates.2,45 Further, when using the questionnaire in different languages, the translation process could have unintentionally altered the phrasing of the questions or interpretation of the questions could have been influenced by culture and local contexts, such that the nature of a positive screen is different from that when used in English. Another possibility could also be related to the older mean age of the sample from non-United States studies as compared with those from the United States, with the PPV of the M-CHAT-R/F being better among older children. However, the meta-regression found that the increase in PPV with age of sample was not statistically significant. It is also possible that within each country, a selection bias for being screened is at play such that children who are at higher risk for ASD are inadvertently more likely to participate in research studies on screening. We also note that of 9 international studies that were included, only 4 had “good” quality ratings, 2 had “fair,” and 3 had “poor” quality ratings. The studies that received lower ratings had serious methodological flaws, including small sample sizes or the same personnel administering both the M-CHAT-R/F and the diagnostic evaluation. Hence, the PPV rate could potentially have been artificially inflated because of study-related biases. However, in the overall sample, elimination of studies rated as “poor” quality did not significantly alter the pooled PPV.
The pooled NPV of 72.5% in the primary analyses indicates the need for a screening tool for ASD with a smaller proportion of false negative screens. We recognize that the estimates for NPV, sensitivity, and specificity were based on the small proportion of screen-negative children who had a diagnostic evaluation. When we assumed that all nonevaluated screen-negative children did not have ASD, the NPV unsurprisingly increased to 99.7%. However, we are concerned about the appropriateness of assuming that nonevaluated screen-negative children were condition-free. The assumption would lead to significant overestimation of the true NPV, even if the pooled estimate based on data of screen-negative children who had a diagnostic evaluation may be an underestimate based on the small sample size. This difference in analytic approach explains why 3 of the studies17,31,32 included, reported a higher NPV in their manuscript as compared with what we used in our meta-analysis. Conversely, 4 of the studies28,36,38,40 used the same analytical method as we did in the primary analysis and these studies had reported NPV values that were similar (lower) to those that we derived. This limitation regarding calculation of NPV is inherent in almost all studies examining screening tools in developmental disorders because of the large numbers of children who have negative screening tests, the financial costs involved in testing screen-negative children, and the long time-frame within which the disorder could potentially develop.44,46–48 The other unanticipated limitation was that even though the majority (13 of 15) of the final included studies applied the diagnostic assessment to a sample of screen-negative patients, the majority of these patients had bias toward an eventual positive diagnosis (ie, it was a not a randomly selected sample). This bias likely contributed to the widely varying estimates of NPV across studies. Cultural factors influencing parental reporting of developmental milestones may also affect NPV rates. For example, parents may over-report the presence of developmental skills, leading to false negative screens because of cultural stigma associated with developmental delays. Lack of understanding of the screening questions because of language or translation-related difficulties may also lead to false responses. Overall, the true NPV is likely to lie somewhere in between 72.5% and 78.0% and closer to 78.0%, which was estimated based on a less-biased, random sample.
Future studies of screening measures for ASD or other developmental disorders should evaluate a larger and random sample of children who screen-negative to ensure that a nonbiased subsample has the same reference test applied as screen-positive children. This improvement would be important to allow reliable interpretation of the tool’s performance, especially in terms of NPV, sensitivity, and specificity. It would also be ideal to have more detailed reporting with respect to the presence of other developmental disorders apart from ASD, if any, following diagnostic evaluations of both screen-positive and screen-negative children. This calculation would allow greater inference of the utility of the M-CHAT-R/F for the presence of general developmental disorders apart from ASD. The substantial number of children who were screen-positive but were lost to follow-up and those who did not complete the full 2 stage screening also add caution to the overall estimates derived in our meta-analysis. The reason for these high attrition rates within each study context were not fully discussed in the studies. However, possible reasons include that the families of children who screened positive were able to access services for ASD without a diagnostic evaluation or alternatively, that caregivers were reluctant to continue follow-up evaluations following an initial positive screen for ASD for cultural or other reasons. This selective sample loss and the individual study’s handling of this sample could have distorted estimates of PPV across studies. For example, a previous study that examined a previous version of the M-CHAT in a real-world setting assumed that all screen-positive children who were lost to follow-up did not have ASD, affecting its PPV estimate.49
Strengths and Limitations
Strengths of this meta-analysis include the intentional focus solely on the M-CHAT-R/F, the preferred screening tool for ASD screening in many healthcare settings. By calculating pooled sensitivity, specificity, and NPV based on screen-negative children who received a diagnostic evaluation, the estimates provided here are likely accurate, and not an over-estimation of the true values. The diverse nature of the samples allowed us to capture the performance of the M-CHAT-R/F in countries and languages that the questionnaire was not originally developed or normed. We were also able to obtain relevant data from studies that employed the M-CHAT-R/F for purposes other than the detection of ASD but included data pertinent to ASD screening.
Limitations include the lack of a manual search of conference papers and proceedings, although we tried to circumvent this limitation by reviewing the references of all included studies to minimize potentially missed ones. Although we did not intentionally exclude any papers, it is possible that those without an abstract or keywords in English were not be identified in the systematic search. Lastly, we were not able to analyze results based on family socioeconomic status and demographic variables; as a result, findings may not be generalizable across all populations of children within the studied age ranges.
Conclusions
This meta-analysis supports the use of the M-CHAT-R/F as a screening questionnaire for ASD, given favorable sensitivity and acceptable PPV in the context of a low prevalence condition. A positive screen with the M-CHAT-R/F is predictive of the diagnosis of ASD in approximately 50% of children and a predictive diagnosis of any developmental disorder in about 90%. The PPV should be considered in the clinical application of the M-CHAT-R/F, particularly when counseling caregivers with respect to the presence of ASD. Future research should evaluate a substantial, random sample of children who screen-negative to increase the accuracy of estimates of NPV, sensitivity, and specificity. Studies examining the performance of the M-CHAT-R/F with outcome data from longitudinal follow-up of a cohort of screen-positive and screen-negative children will improve our assessment of the M-CHAT-R/F as a screening tool for ASD.
Supplementary Material
Acknowledgments
We thank Bruce T Abbott, MLS, the health sciences librarian who conducted the systematic search; and Robin Hansen, MD for critically reviewing the manuscript before submission.
Glossary
- ASD
autism spectrum disorder
- M-CHAT-R/F
Modified Checklist for Autism in Toddlers, Revised with Follow-Up
- NPV
negative predictive value
- PPV
positive predictive value
Footnotes
Dr Aishworiya conceptualized the study, critically screened and appraised potential studies, performed data extraction, and wrote the first draft and subsequent versions of the manuscript; Dr Ma critically screened and appraised potential studies, performed data extraction, and contributed to writing the manuscript and subsequent revisions; Dr Stewart conducted data analysis and contributed to writing the manuscript; Dr Hagerman contributed to the manuscript and critically reviewed revised versions; Dr Feldman conceptualized the study, resolved conflicts during the screening and appraisal process, and (Continued) critically reviewed and revised versions of the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FUNDING: The project described was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Partial salary support was received for VKM from T77MC25733 and P50HD103526, and for HMF from T77MC09796 from the Maternal Child Health Bureau Grant, Health Resources and Services Administration.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no conflicts of interest relevant to this article to disclose.
References
- 1. Association D-AP . Diagnostic and Statistical Manual of Mental Disorders. Arlington, TX: American Psychiatric Publishing; 2013 [Google Scholar]
- 2. Zeidan J, Fombonne E, Scorah J, et al. Global prevalence of autism: a systematic review update. Autism Res. 2022;15(5):778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maenner MJ, Shaw KA, Bakian AV, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021;70(11):1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben Itzchak E, Zachor DA. Who benefits from early intervention in autism spectrum disorders? Res Autism Spectr Disord. 2011;5(1):345–350 [Google Scholar]
- 5. Virues-Ortega J, Rodríguez V, Yu CT. Prediction of treatment outcomes and longitudinal analysis in children with autism undergoing intensive behavioral intervention. Int J Clin Health Psychol. 2013;13(2):91–100 [Google Scholar]
- 6. Granpeesheh D, Dixon DR, Tarbox J, %Kaplan AM, Wilke AE. The effects of age and treatment intensity on behavioral intervention outcomes for children with autism spectrum disorders. Res Autism Spectr Disord. 2009;3(4):1014–1022 [Google Scholar]
- 7. Cicchetti D. Developmental Psychopathology, Developmental Neuroscience. New York, NY: John Wiley and Sons; 2016 [Google Scholar]
- 8. Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20(3):775–803 [DOI] [PubMed] [Google Scholar]
- 9. Hyman SL, Levy SE, Myers SM. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. [DOI] [PubMed] [Google Scholar]
- 10. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790–800 [DOI] [PubMed] [Google Scholar]
- 11. Pierce K, Gazestani VH, Bacon E, et al. Evaluation of the diagnostic stability of the early autism spectrum disorder phenotype in the general population starting at 12 months. JAMA Pediatr. 2019;173(6):578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zwaigenbaum L, Bauman ML, Choueiri R, et al. Early intervention for children with autism spectrum disorder under 3 years of age: recommendations for practice and research. Pediatrics. 2015;136 Suppl 1(Suppl 1):S60–S81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marlow M, Servili C, Tomlinson M. A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: recommendations for use in low- and middle- income countries. Autism Res. 2019;12(2):176–199 [DOI] [PubMed] [Google Scholar]
- 14. Campbell K, Carpenter KL, Espinosa S, Hashemi J, Qiu Q, Tepper M, et al. Use of a digital modified checklist for autism in toddlers–revised with follow-up to improve quality of screening for autism. J Pediatr. 2017;183:133–139. e131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuentes J, Hervás A, Howlin P; ESCAP ASD Working Party . ESCAP practice guidance for autism: a summary of evidence-based recommendations for diagnosis and treatment. Eur Child Adolesc Psychiatry. 2021;30(6):961–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robins DL, Casagrande K, Barton M, Chen C-MA, Dumont-Mathieu T, Fein D. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics. 2014;133(1):37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chlebowski C, Robins DL, Barton ML, Fein D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics. 2013;131(4): e1121–e1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrocchi S, Levante A, Lecciso F. Systematic review of level 1 and level 2 screening tools for autism spectrum disorders in toddlers. Brain Sci. 2020;10(3):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy SE, Wolfe A, Coury D, et al. Screening tools for autism spectrum disorder in primary care: a systematic evidence review. Pediatrics. 2020;145(Suppl 1):S47–S59 [DOI] [PubMed] [Google Scholar]
- 21. Yuen T, Penner M, Carter MT, Szatmari P, Ungar WJ. Assessing the accuracy of the modified checklist for autism in toddlers: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(11):1093–1100 [DOI] [PubMed] [Google Scholar]
- 22. Wieckowski AT, Williams LN, Rando J, Lyall K, Robins DL. Sensitivity and specificity of the modified checklist for autism in toddlers (Original and Revised): a systematic review and meta-analysis. JAMA Pediatr. 2023;e225975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Preventative Services Task Force . Procedure Manual. Rockville, MD: US Preventative Services Task Force; 2008 [Google Scholar]
- 25. Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. New York, NY: John Wiley and Sons; 2013 [Google Scholar]
- 26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 27. Hertzmark E, Spiegelman D. The SAS METAANAL Macro. Boston, MA: Channing Laboratory; 2012:24 [Google Scholar]
- 28. Sangare M, Toure HB, Toure A, et al. Validation of two parent-reported autism spectrum disorders screening tools M-CHAT-R and SCQ in Bamako, Mali. eNeurologicalSci. 2019;15:100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manzouri L, Yousefian S, Keshtkari A, Hashemi N. Advanced parental age and risk of positive autism spectrum disorders screening. Int J Prev Med. 2019;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brennan L, Fein D, Como A, Rathwell IC, Chen CM. Use of the Modified Checklist for Autism, Revised with Follow Up-Albanian to screen for ASD in Albania. J Autism Dev Disord. 2016;46(11):3392–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo C, Luo M, Wang X, et al. Reliability and validity of the Chinese version of Modified Checklist for Autism in Toddlers, Revised, with Follow-Up (M-CHAT-R/F). J Autism Dev Disord. 2019;49(1):185–196 [DOI] [PubMed] [Google Scholar]
- 32. Jonsdottir SL, Saemundsen E, Jonsson BG, Rafnsson V. Validation of the Modified Checklist for Autism in Toddlers, Revised with Follow-up in a population sample of 30-month-old children in Iceland: a prospective approach. J Autism Dev Disord. 2022;52(4):1507–1522 [DOI] [PubMed] [Google Scholar]
- 33. Magán-Maganto M, Canal-Bedia R, Hernández-Fabián A, et al. Spanish cultural validation of the Modified Checklist for Autism in Toddlers, Revised. J Autism Dev Disord. 2020;50(7):2412–2423 [DOI] [PubMed] [Google Scholar]
- 34. Oner O, Munir KM. Modified Checklist for Autism in Toddlers Revised (MCHAT-R/F) in an urban metropolitan sample of young children in Turkey. J Autism Dev Disord. 2020;50(9):3312–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bradbury K, Robins DL, Barton M, et al. Screening for autism spectrum disorder in high-risk younger siblings. J Dev Behav Pediatr. 2020;41(8):596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weitlauf AS, Vehorn AC, Stone WL, Fein D, Warren ZE. Using the M-CHAT-R/F to identify developmental concerns in a high-risk 18-month-old sibling sample. J Dev Behav Pediatr. 2015;36(7):497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wieckowski AT, Hamner T, Nanovic S, et al. Early and repeated screening detects autism spectrum disorder. J Pediatr. 2021;234:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsai JM, Lu L, Jeng SF, et al. Validation of the modified checklist for autism in toddlers, revised with follow-up in Taiwanese toddlers. Res Dev Disabil. 2019;85:205–216 [DOI] [PubMed] [Google Scholar]
- 39. Coelho-Medeiros ME, Bronstein J, Aedo K, et al. M-CHAT-R/F Validation as a screening tool for early detection in children with autism spectrum disorder. Rev Chil Pediatr. 2019;90(5):492–499 [DOI] [PubMed] [Google Scholar]
- 40. Christopher K, Bishop S, Carpenter LA, Warren Z, Kanne S. The implications of parent-reported emotional and behavioral problems on the Modified Checklist for Autism in Toddlers. J Autism Dev Disord. 2021;51(3):884–891 [DOI] [PubMed] [Google Scholar]
- 41. McNally Keehn R, Tang Q, Swigonski N, %Ciccarelli M. Associations among referral concerns, screening results, and diagnostic outcomes of young children assessed in a statewide Early Autism Evaluation Network. J Pediatr. 2021;233:74–81.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee . Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006;118(1):405–420 [DOI] [PubMed] [Google Scholar]
- 43. McPheeters ML, Weitlauf A, Vehorn A, et al. U.S. Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews. Screening for Autism Spectrum Disorder in Young Children: A Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016 [PubMed] [Google Scholar]
- 44. McCarty P, Frye RE. Early Eetection and Diagnosis of Autism Spectrum Disorder: Why is it so Difficult? Seminars in Pediatric Neurology. New York, NY: Elsevier; 2020:100831. [DOI] [PubMed] [Google Scholar]
- 45. Fombonne E. The Rising Prevalence of Autism. New York, NY: Wiley Online Library; 2018:717–720 [DOI] [PubMed] [Google Scholar]
- 46. Glascoe FP, Foster EM, Wolraich ML. An economic analysis of developmental detection methods. Pediatrics. 1997;99(6):830–837 [DOI] [PubMed] [Google Scholar]
- 47. Randall M, Egberts KJ, Samtani A, Scholten RJ, Hooft L, Livingstone N, et al. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst Rev. 2018;7(7):CD009044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robins DL. How do we determine the utility of screening tools? Autism. 2020;24(2):271–273 [DOI] [PubMed] [Google Scholar]
- 49. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4):e20183963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.