Abstract
Background
Autism spectrum disorder (autism) is a neurodevelopmental condition characterised by impairments in social communication and interaction, plus restricted, repetitive patterns of behaviour and interests. Whilst some people embrace autism as part of their identity, others struggle with their difficulties, and some seek treatment. There are no current interventions that result in complete reduction of autism features.
Acetylcholine is a neurotransmitter for the cholinergic system and has a role in attention, novelty seeking, and memory. Low levels of acetylcholine have been investigated as a potential contributor to autism symptomatology. Donepezil, galantamine, and rivastigmine (commonly referred to as acetylcholinesterase inhibitors) all inhibit acetylcholinesterase, and have slightly different modes of action and biological availability, so their effectiveness and side‐effect profiles may vary. The effect of various acetylcholinesterase inhibitor on core autism features across the lifespan, and possible adverse effects, have not been thoroughly investigated.
Objectives
To evaluate the efficacy and harms of acetylcholinesterase inhibitors for people with the core features (social interaction, communication, and restrictive and repetitive behaviours) of autism.
To assess the effects of acetylcholinesterase inhibitors on non‐core features of autism.
Search methods
In November 2022, we searched CENTRAL, MEDLINE, Embase, eight other databases, and two trials registers. We also searched the reference lists of included studies and relevant reviews, and contacted authors of relevant studies.
Selection criteria
Randomised controlled trials (RCTs), comparing acetylcholinesterase inhibitors (e.g. galantamine, donepezil, or rivastigmine) of varying doses, delivered orally or via transdermal patch, either as monotherapy or adjunct therapy, with placebo. People of any age, with a clinical diagnosis of autism were eligible for inclusion.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our primary outcomes were core features of autism and adverse effects. Secondary outcomes were language, irritability, hyperactivity, and general health and function. We used GRADE to assess certainty of evidence.
Main results
We included two RCTs (74 participants). One study was conducted in Iran, the second in the USA, although exact location in the USA is unclear.
Galantamine plus risperidone versus placebo plus risperidone
One study compared the effects of galantamine plus risperidone to placebo plus risperidone (40 participants, aged 4 years to 12 years). Primary and secondary outcomes of interest were measured postintervention, using subscales of the Aberrant Behavior Checklist (score 0 to 3; higher scores = greater impairment). Very low‐certainty evidence showed there was little to no difference between the two groups postintervention for social communication (mean difference (MD) ‐2.75, 95% confidence interval (CI) ‐5.88 to 0.38), and restricted and repetitive behaviour (MD ‐0.55, 95% CI ‐3.47 to 2.37). Overall autism features were not assessed. Adverse events may be higher in the galantamine plus risperidone group (75%) compared with the placebo plus risperidone group (35%): odds ratio 5.57, 95% CI 1.42 to 21.86, low‐certainty evidence. No serious adverse events were reported. Low‐certainty evidence showed a small difference in irritability (MD ‐3.50, 95% CI ‐6.39 to ‐0.61), with the galantamine plus risperidone group showing a greater decline on the irritability subscale than the placebo group postintervention. There was no evidence of a difference between the groups in hyperactivity postintervention (MD ‐5.20, 95% CI ‐10.51 to 0.11). General health and function were not assessed.
Donepezil versus placebo
One study compared donepezil to placebo (34 participants aged 8 years to 17 years). Primary outcomes of interest were measured postintervention, using subscales of the Modified Version of The Real Life Rating Scale (scored 0 to 3; higher scores = greater impairment). Very low‐certainty evidence showed no evidence of group differences immediately postintervention in overall autism features (MD 0.07, 95% CI ‐0.19 to 0.33), or in the autism symptom domains of social communication (MD ‐0.02, 95% CI ‐0.34 to 0.30), and restricted and repetitive behaviours (MD 0.04, 95% CI ‐0.27 to 0.35). Significant adverse events leading to study withdrawal in at least one participant was implied in the data analysis section, but not explicitly reported. The evidence is very uncertain about the effect of donepezil, compared to placebo, on the secondary outcomes of interest, including irritability (MD 1.08, 95% CI ‐0.41 to 2.57), hyperactivity (MD 2.60, 95% CI 0.50 to 4.70), and general health and function (MD 0.03, 95% CI ‐0.48 to 0.54) postintervention.
Across all analyses within this comparison, we judged the evidence to be very low‐certainty due to high risk of bias, and very serious imprecision (results based on one small study with wide confidence intervals). The study narratively reported adverse events for the study as a whole, rather than by treatment group.
Authors' conclusions
Evidence about the effectiveness of acetylcholinesterase inhibitors as a medication to improve outcomes for autistic adults is lacking, and for autistic children is very uncertain.
There is a need for more evidence of improvement in outcomes of relevance to clinical care, autistic people, and their families. There are a number of ongoing studies involving acetylcholinesterase inhibitors, and future updates of this review may add to the current evidence.
Keywords: Adolescent; Child; Child, Preschool; Humans; Acetylcholine; Autism Spectrum Disorder; Autism Spectrum Disorder/drug therapy; Cholinesterase Inhibitors; Cholinesterase Inhibitors/adverse effects; Donepezil; Donepezil/adverse effects; Galantamine; Galantamine/adverse effects; Risperidone; Risperidone/adverse effects; Rivastigmine; Rivastigmine/adverse effects
Plain language summary
Do acetylcholinesterase inhibitors reduce the core impairments associated with autism spectrum disorder
Key messages
At present, it is unclear whether acetylcholinesterase inhibitors are effective in treating the core features of autism. This is because very few studies have been carried out. The two we found were small, and did not report the outcomes very well. However, there are a number of ongoing studies in this area.
What is autism spectrum disorder?
Autism spectrum disorder (autism) is a condition that affects the way a person thinks, feels, interacts with others, and experiences their environment. The core behaviours of autism include reduced sharing of interests, difficulties with nonverbal communication (for example, little eye contact), difficulties forming and maintaining relationships and restricted, or repetitive behaviours or interests. These behaviours can vary in severity, but they still interfere with the person's daily functioning and participation in activities. Medicines, called acetylcholinesterase inhibitors (chemicals that block the normal breakdown of nerve transmitters), have been used to treat Alzheimer's disease (for example, donepezil and galantamine). Recently, it has been suggested that they may also decrease some of the difficulties seen in people with autism.
What did we want to find out?
Do acetylcholinesterase inhibitors, alone or with other medicines, reduce the core features of autism (social communication and interaction, restrictive and repetitive behaviours) compared with a placebo (a dummy pill). We also wanted to find out if acetylcholinesterase inhibitors were associated with any unwanted side effects.
What did we do?
We searched for studies that explored whether there were any differences in autistic people's behaviours after they had taken acetylcholinesterase inhibitors compared to people who had taken placebo pills. We compared and summarised the results of the studies, and rated our confidence in the evidence, based on things, such how big and well carried out the studies were.
What did we find?
We found two relevant studies. One study looked at whether an acetylcholinesterase inhibitor called galantamine, combined with a drug called risperidone (traditionally used to treat psychosis), was better than a placebo combined with risperidone. The study included 40 children, aged between 4 and 12 years, with a diagnosis of autism. it took place over 10 weeks in Iran. A second study compared an acetylcholinesterase inhibitor called donepezil with placebo. It included 34 children, aged between 8 and 17 years, with a diagnosis of autism. It was carried out in the USA. All studies involved children attending outpatient clinics.
What are the main results of the review?
It is unclear if acetylcholinesterase inhibitors make any difference to the core features of autism in children or adults.
Galantamine may cause little to no difference in social interaction and communication skills and irritability levels in children with autism after 10 weeks of treatment. There is no evidence that galantamine reduces difficulties associated with restricted or repetitive behaviours or hyperactivity. Galantamine may also cause some side effects, including nervousness, drowsiness, increased appetite, and tremor.
There is no evidence that donepezil reduces difficulties associated with the core features of autism or associated secondary problems. We were unable to find out whether it causes unwanted side effects in children with autism, because the study did not report the results completely.
What are the limitations of the evidence?
We have little confidence in the evidence for using this medicine to treat problems associated with autism, because the evidence comes from only two small studies that were not very well carried out or reported. Each study looked at a different type of acetylcholinesterase inhibitor and delivered the medicine differently. They did not provide information about all the issues we were interested in, and they measured the side effects differently.
If more studies are included in future reviews, these results may change.
How up‐to‐date is this review?
The evidence in this review is current to November 2022.
Summary of findings
Summary of findings 1. Galantamine plus risperidone compared to placebo plus risperidone for treatment of the core symptoms of autism spectrum disorder.
| Galantamine plus risperidone compared to placebo plus risperidone for treatment of the core symptoms of autism spectrum disorder | ||||||
| Patient or population: children and adolescents with a diagnosis of autism spectrum disorder Setting: outpatient and/or community health setting Intervention: galantamine plus risperidone Comparison: placebo plus risperidone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo plus risperidone | Risk with galantamine plus risperidone | |||||
| Mean score | Mean score | |||||
|
Overall autism features Not assessed |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Social communication
(Lethargy/Social withdrawal subscale of the ABC‐C; 0 to 64; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo + risperidone group was 8.35 |
The mean score in the galantamine + risperidone group was 2.75 lower (5.88 lower to 0.38 higher) |
‐ | 40 (1 RCT) | ⊕⊝⊝⊝ Very Lowa,b | |
|
Repetitive and restricted behaviour
(Stereotypical Behaviour subscale of the ABC‐C; 0 to 28; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo + risperidone group was 4.5 |
The mean score in the galantamine + risperidone group was 0.55 lower (3.47 lower to 2.37 higher) | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ Very Lowa,b | |
|
Adverse events
(Side Effects Checklist) follow‐up: immediately postintervention |
Study population | OR 5.57 (1.42 to 21.86) | 40 (1 RCT) | ⊕⊕⊝⊝ Lowa | OR > 1 means an increased number of adverse events in the galantamine plus risperidone group. | |
| 350 per 1000 | 749 per 1000 (433 to 922) | |||||
|
Irritability (Irritability subscale of the ABC‐C; 0 to 60; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo + risperidone group was 8.8 | The mean score in the galantamine + risperidone group was 3.50 lower (6.39 lower to 0.61 lower) | ‐ | 40 (1 RCT) |
⊕⊕⊝⊝ Lowa | |
|
Hyperactivity (Hyperactivity subscale of the ABC‐C; 0 to 64; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo + risperidone group was 16.05 | The mean score in the galantamine + risperidone group was 5.20 lower (10.51 lower to 0.11 higher) | 40 (1 RCT) |
⊕⊕⊝⊝ Lowa | ||
|
General health and function Not assessed |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
ABC‐C: Aberrant Behavior Checklist ‐ Community; CI: confidence interval; OR: odds ratio; RCT: randomised control trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
adowngraded by two levels due to imprecision relating to the small sample size, and only one study contributing to this outcome bdowngraded by one level due to indirectness because the subscales from the ABC‐C are not considered to adequately measure the core features of autism
Summary of findings 2. Donepezil compared to placebo for treatment of core symptoms of autism spectrum disorder.
| Donepezil compared to placebo for treatment of core symptoms of autism spectrum disorder | |||||
|
Patient or population: children and adolescents with a diagnosis of autism Setting: outpatient clinics and/or community settings Intervention: donepezil 5 mg/day at 5 weeks, 10 mg/day at 10 weeks Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks * (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with donepezil | ||||
|
Overall autism features (total score of RLRS; raw score means; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo group was 0.32 | The mean score in the donepezil group was 0.07 higher (0.19 lower to 0.33 higher) | 34 (1) |
⊕⊝⊝⊝ Very Lowa,b |
|
|
Social communication (Social Relationships subscale of RLRS; raw score means; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo group was 0.17 | The mean score in the donepezil group was 0.02 lower (0.34 lower to 0.30 higher) | 34 (1) |
⊕⊝⊝⊝ Very Lowa,b |
|
|
Repetitive and restricted behaviour (Sensory Motor subscale of RLRS; raw score means; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo group was 0.42 | The mean score in the donepezil group was 0.04 higher (0.27 lower to 0.35 higher) |
34 (1) |
⊕⊝⊝⊝ Very Lowa,b |
|
|
Adverse events (Side Effects Checklist) follow‐up: immediately postintervention |
No results reported. One participant from the placebo group withdrew early due to concerns with increased aggression and agitation. The majority of participants reporting side effects at baseline experienced a decrease in those side effects during the trial. They reported reduced trouble sleeping, appetite, and depression. Others reported a slight increase in diarrhoea, headache, and fatigue. | 34 (1) |
⊕⊝⊝⊝ Very Lowa,b |
‐ | |
|
Irritability (Child Behaviour Checklist Oppositional Defiant Disorder Subscale; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo group was 2.53 | The mean score in the donepezil group was 1.08 higher (0.41 lower to 2.57 higher) | 34 (1) |
⊕⊝⊝⊝ Very Lowa,b |
|
|
Hyperactivity (Child Behaviour Checklist Attention problems Subscale; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo group was 5.07 | The mean score in the donepezil group was 2.60 higher (0.50 higher to 4.70 higher) | 34 (1) |
⊕⊝⊝⊝ Very Lowa,b |
|
|
General health and function (Severity of Illness subscale of Clinical Global Impression Scale; lower = better) follow‐up: immediately postintervention |
The mean score in the placebo group was 3.47 | The mean score in the donepezil group was 0.03higher (0.48 lower to 0.54 higher) | 34 (1) |
⊕⊝⊝⊝ Very Lowa,b |
|
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| CI: confidence interval; OR: odds ratio; RCT: randomised control trial; RLRS: the Real Life Rating Scale | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | |||||
adowngraded by one level for risk of bias, due to high or unclear risk of bias across all but one domain bdowngraded by two levels due to imprecision relating to the small sample size, and only one study contributing to this outcome
Background
Description of the condition
Autism spectrum disorder (autism) is a diverse, neurodevelopmental condition characterised by impairments in social communication and interaction, plus restricted, repetitive patterns of behaviour and interests. The term autism, as a distinct developmental disorder, was first included in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐III (APA 1980)); however, the diagnostic characteristics and terms have evolved over time. The term Autism Spectrum Disorder replaced previous classifications used in earlier versions of the DSM, such as infantile autism (APA 1980), and autistic disorder (APA 1987; APA 1994). Conditions previously classified as pervasive developmental disorder–not otherwise specified (PDD‐NOS), other pervasive developmental disorders, pervasive developmental disorder–unspecified, Asperger syndrome or disorder, and atypical autism are encompassed by the term Autism Spectrum Disorder under the current classification system of the DSM‐5 (APA 2013). Similarly, the 11th revision of the International Classification of Disease (ICD‐11) has replaced all related diagnoses with the term Autism Spectrum Disorder (WHO 2018).
There is wide variability in the severity and manifestation of behaviours in autism (Shattuck 2007; Van Wijngaarden‐Cremers 2014). Core features are generally characterised by persistent deficits in social interaction, social communication, forming and maintaining relationships, and understanding social cues from other people (APA 2013; Shattuck 2007). Other core features of autism include restricted, repetitive patterns and behaviours, such as preoccupations or special interests, rigid adherence to routines, hypo‐ or hyper‐reactivity to or interest in sensory stimuli, and stereotypical behaviours (APA 2013). Co‐occurring behaviours commonly associated with autism include anxiety, language impairments, attention deficit hyperactivity disorder (ADHD), intellectual disability, irritability, and aggression; but these features do not occur in all autistic people and are not required to make a diagnosis (Lai 2014).
Autism is not obvious at birth, but the features of autism become apparent during infancy and toddlerhood, when there is atypical development in social communication skills. Studies have found that the first signs of autism can emerge between 6 and 18 months of age (Tanner 2020). Infants later diagnosed with autism present with reduced eye contact, social smiling, and time spent examining a social scene (Chawarska 2013; Ozonoff 2010). Children who have an intelligence quotient (IQ) within the average range and less profound autism tend to be diagnosed later, as a result of more subtle features (May 2018; Mazurek 2014). More recent research into the presentation of autism in girls also suggests that this cohort tends to be diagnosed later (Begeer 2013). Although the DSM‐5 requires the presence of difficulties early in life, it does not state a specific age for the onset of symptoms.
Autism should be diagnosed using a comprehensive assessment by an appropriately trained clinician. It should incorporate an interview with the parent or caregiver, assessment of the person, gathering information from other settings, such as childcare or school, and a medical examination. The assessor requires knowledge of typical speech and language development, social skill development, and attainment of developmental abilities. A diagnostic tool can be used as part of each assessment. Diagnostic tools include; the Autism Diagnostic Interview‐Revised (ADI‐R (Rutter 2003a)), the Autism Diagnostic Observation Schedule (ADOS (Lord 1999)), and the Childhood Autism Rating Scale (CARS (Schopler 1994)), among others.
The prevalence of autism has been increasing over past decades, in part as a result of changes in diagnostic criteria, but also because of increased awareness. Recent studies from America have reported the prevalence as 18.5 per 1000 (Maenner 2020). In a review of the global prevalence of autism, studies since 2000 had a prevalence of 1 to 189 per 10,000 (Elsabbagh 2012). Prevalence in the male population is around four times higher than in the female population (APA 2013), although more recent evidence suggests the ratio is closer to 3:1 (Loomes 2017).
Adults with autism have variable outcomes, which are associated with their characteristics and the severity of these characteristics. Outcomes range from supported living with no participation in employment, to living independently and working in a profession for which they are trained. Two‐thirds of adults with autism are employed at least some of the time, in settings ranging from supported employment to competitive workplaces (Henninger 2013; Roux 2013; Taylor 2015).
Autism is now thought to be a group of neurodevelopmental conditions with distinct aetiology (Genovese 2020; Happé 2006; Lai 2014). As yet, there remains no conclusive pathophysiological process explaining all characteristics that are associated with autism, and not all autistic people will respond to the same treatments. As a result, it is important to look at characteristics that vary, in order to determine if there are specific subgroups (e.g. age, severity of characteristics, IQ, and any co‐occuring conditions) that may have a greater or lesser response to various treatments.
Description of the intervention
Therapies for autism spectrum disorders (autism)
Autism is a heterogeneous condition with no current interventions that result in complete resolution of autistic features, and no one treatment or intervention that is suitable for all people (Kumar 2012; Lai 2014).
Developmental, behavioural, and educational interventions involve improving social communication and decreasing challenging behaviours, thereby maximising a person's opportunities to learn and participate in activities (NICE 2021). There are many different approaches to developmental, behavioural, and educational interventions, which should be tailored to each child’s strengths and areas of need, ability and age, and be delivered in the most appropriate setting for the intervention. Applied behavioural analysis (ABA) is a well researched intervention programme proven to be effective for some autistic children in a research setting. The principals of ABA now form the basis of many early intervention programmes, which usually also include approaches to improve communication, manage the environment, and understand each child’s behaviour in terms of its triggers and function (NICE 2021). In general, comprehensive intervention programmes are thought ideal for preschool‐aged children, whether these are named or eclectic programmes (Reichow 2018; Weitlauf 2014). More targeted approaches for school‐aged children and adults are still being explored (Fletcher‐Watson 2014; Ke 2018; Reichow 2012).
Although interventions to date have been predominantly psychosocial, pharmacological interventions are often used as adjunctive therapy to target specific unwanted behaviours that are predominantly non‐core features of autism (Farmer 2013). There has been increasing interest in exploring the potential effectiveness of specific pharmacological interventions that might target specific abnormal neuropathological pathways. These include: selective serotonin reuptake inhibitors (SSRI; e.g. fluoxetine, citalopram) for anxiety and restricted and repetitive behaviours (Kumar 2012; Lai 2014; Livingstone 2015; Williams 2013); anti‐psychotics (e.g. risperidone, aripiprazole) for aggressive behaviour and self‐harm (Hirsh 2016; Jesner 2007; Kumar 2012; Lai 2014); stimulants (e.g. methylphenidate) for hyperactivity (Kumar 2012; Lai 2014; Sturman 2017); anti‐epileptic agents (e.g. lamotrigine, sodium valproate) for mood stabilisation (Limbu 2022); and glutamate receptor‐related medications, such as memantine, for stereotyped behaviours and social communication (Karahmadi 2018). In view of uncertainties and inconsistent findings, the British Association for Psychopharmacology co‐ordinated the development of consensus guidelines to review and make recommendations for the management of autism with a focus on drug treatments, noting some medications have evidence for associated symptoms (e.g. ADHD medications and antipsychotics for irritability, melatonin for sleep problems), but others do not (e.g. SSRs for repetitive behaviours; see Howes 2018).
Another pharmacological intervention that has been used in recent years to alleviate some of the symptoms of autism are acetylcholinesterase inhibitors, such as donepezil and galantamine. Although acetylcholinesterase inhibitors are widely used to treat Alzheimer's disease (Rossignol 2014), and dementia (Niederhofer 2003), in order to improve cognition and behaviour, interest is growing in their role in targeting symptoms of autism. For example, the acetylcholinesterase inhibitor donepezil is hypothesised to play a role in improving expressive and receptive speech in autistic children, as well as improving sleep (Chez 2003). A non‐Cochrane systematic review of medications approved for Alzheimer’s disease in autism indicated that findings from four studies investigating galantamine were preliminarily positive, with reasonable evidence for improvements to both core and associated autism symptoms (Rossignol 2014). In contrast, a recent systematic review and network meta‐analysis of pharmacological and dietary‐supplement treatments for autism included acetylcholinesterase inhibitors, and found no evidence of a treatment effect on core features of autism for donepezil in children (Siafis 2022). This review will focus on acetylcholinesterase inhibitors, including donepezil and galantamine, and their role in autism.
How the intervention might work
Acetylcholine and the cholinergic system
Acetylcholine is the neurotransmitter for the cholinergic system and acts on both muscarinic and nicotinic cholinergic receptors, with muscarinic receptors being more abundant than nicotinic receptors in the central nervous system. Acetylcholine is active in the basal forebrain projections into cortex and limbic structures and therefore, has a role in attention, novelty seeking, and memory (Lee 2014; Yoo 2007). This has resulted in low levels of acetylcholine being investigated as a potential contributor to autism symptomatology (Deutsch 2020). Post‐mortem studies using samples from brain banks in the UK and the USA have shown decreased cholinergic activity with lower binding to the nicotinic and muscarinic receptors in those with autism, in conjunction with normal levels of choline acetyltransferase activity (Lee 2002; Perry 2001). In a study of black and tan brachyury (BTBR) mice, considered a reliable model for autism, increasing acetylcholine levels through the administration of one acetylcholinesterase inhibitor, donepezil, decreased cognitive rigidity and increased sociability in a dose‐dependent manner. However, there was no improvement in repetitive behaviour (Karvat 2014).
Acetylcholinesterase inhibitors
Donepezil, galantamine, and rivastigmine all inhibit acetylcholinesterase, which increases acetylcholine in the central nervous system, providing increased levels of acetylcholine to act on the available muscarinic and nicotinic receptors. Donepezil reaches its peak concentration three to five hours after administration, has a half life of 70 to 80 hours and reaches a steady state within 14 to 22 days (Jann 2002). It is metabolised by the liver and therefore, its concentration is increased by medications, such as cimetidine and ketoconazole (Jann 2002). Studies in autistic children have used doses of 1.25 mg to 10 mg (Handen 2010; Buckley 2011). Galantamine reaches its peak concentration 0.5 to two hours after administration and has a half life of five to seven hours. Galantamine is metabolised by the liver and its concentration is also increased by ketoconazole (Jann 2002). In a study in autistic children, doses ranged from 2 mg daily to 12 mg twice daily (Nicolson 2006). Specifically, galantamine also enhances the action of acetylcholine on nicotinic receptors (Jann 2002). Rivastigime can be administered orally and via transdermal patch. In oral administration, it reaches its peak concentration 0.8 to 1.7 hours after administration, and has a half life of 0.3 to three hours (Jann 2002). Rivastigime is not metabolised by the liver and therefore, has no identified drug interactions. Another acetylcholinesterase inhibitor called tacrine has not been used in studies for the treatment of autism to date, and therefore, it is unclear what dosage would be suitable. The main identified side effects for all acetylcholinesterase inhibitors are gastrointestinal, including nausea, vomiting, and diarrhoea. They also negate the effects of anticholinergic agents, such as oxybutynin or trihexyphenidyl.
Why it is important to do this review
All acetylcholinesterase inhibitors have slightly different modes of action and biological availability, so their effectiveness and side effect profiles may vary. As a result, it is important to analyse the differences between the respective acetylcholinesterase inhibitors, as well as any adverse effects. Various doses have also been used in different studies of autistic people, and further exploration is needed to assess whether dosage affects the efficacy of any given acetylcholinesterase inhibitors.
To date, there are no Cochrane Reviews that examine the role of acetylcholinesterase inhibitors in autism across the lifespan. Findings to date have been inconsistent. One review conducted in 2014 highlighted that at that time, the use of acetylcholinesterase inhibitors in autism was an emerging area of research interest (Rossignol 2014). This review indicated that studies investigating galantamine showed positive effects on both core and associated autism symptoms, however, findings from studies exploring the other three medications were more inconsistent, making recommendations for use difficult at that time. A more recent review and network meta‐analysis investigating pharmacological and dietary‐supplement treatments for autism included 143 RCTs, including acetylcholinesterase inhibitors as experimental medications, to treat core autism features, associated noncore features, and side effects (Siafis 2022). Only two RCTs of donepezil were included in this review, with preliminary findings suggesting the medication had little effect on core autism features. Larger clinical studies and a review of trials with more rigorous randomised, controlled methodology is needed to help define the role for some of these medications in the treatment of autistic people.
A broad array of treatments is available for autistic people, with unproven interventions rapidly adopted in practice (Matson 2013). Therefore, it is timely to reassess the current available evidence for the use of acetylcholinesterase inhibitors, which can inform whether they are effective, for whom, at what dosage, and whether there are any adverse effects. A systematic review will also inform whether additional studies should be conducted, or if methodological modifications to future studies are indicated, and therefore, contribute to more accurate and conclusive evidence regarding the effectiveness and harms of acetylcholinesterase inhibitors for autistic people.
Objectives
To evaluate the efficacy and harms of acetylcholinesterase inhibitors for people with the core features (social communication difficulties, and restrictive and repetitive behaviours) of autism spectrum disorder.
Secondary objective
To assess the effects of acetylcholinesterase inhibitors on non‐core features of autism spectrum disorder.
Methods
Criteria for considering studies for this review
Types of studies
All variants of randomised controlled trials (RCTs), including individually randomised, parallel‐group, cross‐over, and cluster trials.
We did not include studies in which assignment to treatment condition was decided through a deterministic method, such as alternate days of the week, or case record number, and we excluded non‐randomised trial designs. We did not apply any language restrictions.
Types of participants
Studies of children, adolescents, and adults with a diagnosis of autism spectrum disorder (autism); we applied no age limits. Studies included people with a clinical diagnosis of autism, made by an established classification system, or a tool that was validated against an established classification system. Autism spectrum disorder encompassed the following conditions: autistic disorder, Asperger's disorder, atypical autism, pervasive developmental disorder–not otherwise specified (PDD‐NOS), and diagnoses that use similar but slightly different terms like Asperger's syndrome. Studies could have included children with autism and other neurodevelopmental or psychiatric comorbid conditions, such as attention deficit hyperactivity disorder (ADHD), or an anxiety disorder.
Eligible classification systems used for autism diagnosis included the DSM‐5 (APA 2013), DSM‐IV (APA 1994), and the 10th edition of the ICD (ICD‐10 (WHO 1993)). Tools validated against a suitable diagnostic classification system included the Childhood Autism Rating Scale (CARS (Schopler 1994)); Autism Diagnostic Interview‐Revised (ADI‐R (Rutter 2003a)); Autism Diagnostic Observation Schedule (ADOS), including more recent versions (Lord 1999; Lord 2012); the Developmental, Dimensional and Diagnostic Interview (3di (Skuse 2004)); and the Diagnostic Interview for Social and Communication Disorders (DISCO (Wing 2006)).
Rett syndrome is no longer considered an autism spectrum disorder and is routinely excluded from autism studies. Childhood degenerative condition is not a separate diagnosis in DSM‐5, but is included as part of autism spectrum disorder. Childhood degenerative condition was not an exclusion criterion; however, we did not encounter any studies that treated people with childhood degenerative condition with an eligible intervention.
Types of interventions
All studies comparing acetylcholinesterase inhibitors (AChEI; e.g. galantamine, donepezil, or rivastigmine) of varying doses, administered orally or via a transdermal patch, as a single agent for autism, to placebo. The acetylcholinesterase inhibitor could also be taken as adjunct to other medication, or in addition to other therapy, as long as the placebo group also received the same types of medication and therapies.
We excluded studies comparing acetylcholinesterase inhibitors to other interventions.
Types of outcome measures
Outcomes were measured during and immediately post‐intervention using standardised assessments, parent questionnaires and rating scales, and behavioural observations.
Primary outcomes
Core features of autism spectrum disorder, that is, social communication and stereotypy or restricted, repetitive patterns of behaviour, interests or activities. Outcomes needed to be measured by standardised diagnostic assessment instruments, including, but not limited to, the Childhood Autism Rating Scale (CARS), the Autism Diagnostic Interview‐Revised (ADI‐R; Lord 1994), the Autism Diagnostic Observation Schedule (ADOS), or the Diagnostic Interview for Social and Communication Disorders (DISCO). Assessment tools for social communication and repetitive and restricted behaviours, included the Social Communication Questionnaire (Rutter 2003b), and the Repetitive Behavior Scale‐Revised (RBS‐R (Bodfish 2000)).
-
Adverse events, including risk or presence of side effects directly attributed to the use of AChEIs including:
gastrointestinal (e.g. nausea, vomiting, diarrhoea);
urinary incontinence;
fatigue;
sedation; and
sleep disruption.
We extracted both systematically and non‐systematically reported adverse events. As non‐systematic reporting of adverse events tends to underestimate how frequently they occur (see Chapter 5 of the Cochrane Handbook for Systematic Reviews of Interventions; Li 2022), we recorded the collection method, precise definitions of adverse effect outcomes, and their intensity for each study, where possible.
Secondary outcomes
Secondary outcomes, included changes in any non‐core symptoms commonly associated with ASD, were assessed using standardised measures, including but not limited to:
Hyperactivity, irritability, aggression and/or disruptive behaviour, measured by the Connors 3 Manual (Conners 2008), the Behaviour Assessment Scale for Children, 3rd Edition (BASC‐3 (Reynolds 2015)), or the irritability, agitation subscale of the Aberrant Behaviour Checklist (ABC (Aman 1986));
Mood (e.g. symptoms of depression or anxiety, or both), measured by the Spence Children's Anxiety Scale (SCAS (Spence 1997)), or the Center for Epidemiologic Studies Depression Scale (CES‐D (Radloff 1977));
Cognition (e.g. attention and memory), measured by the Behaviour Rating Inventory of Executive Function (BRIEF (Baron 2000));
Self‐injury, measured by the critical items within the Vineland Adaptive Behaviour Scales (VABS (Sparrow 1989));
Quality of life for autistic people and their carers, measured by scales, such as the Pediatric Quality of Life Inventory (PedsQL (Varni 2001)); and
General health and function at home and school, measured using the VABS (Sparrow 1989).
Search methods for identification of studies
Our search strategy combined two concepts (population and intervention) plus a filter to identify RCTs when appropriate. The intervention section included search terms for the following medications: donepezil (Aricept), galantamine (Razadyne), rivastigmine (Exelon), and Tacrine (Cognex).
Electronic searches
We searched the following databases and trials registers up to November 2022.
Cochrane Central Register of Controlled Trials (CENTRAL, 2022, Issue 10), in the Cochrane Library (searched 29 November 2022)
MEDLINE(R) Ovid (1946 to November Week 3 2022)
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid via MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 1 November 2021)
MEDLINE Epub Ahead of Print Ovid via MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 29 November 2022)
Embase Ovid (1974 to 29 November 2022)
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 2 November 2021)
APA PsycINFO Ovid (1967 to November Week 3 2022)
Cochrane Database of Systematic Reviews (CDSR; 2022, Issue 11), in the Cochrane Library (searched 29 November 2022)
Epistemonikos (www.epistemonikos.org; searched 30 November 2022)
Web of Science Core Collection, Clarivate (Science Citation Index ‐ Expanded; Social Sciences Citation Index; Conference Proceedings Citation Index ‐ Science; Conference Proceedings Citation Index ‐ Social Science & Humanities; Emerging Sources Citation Index; 1970 to 30 November 2022)
Proquest Dissertations & Theses A&I (searched 30 November 2022)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; trialsearch.who.int/; searched 30 November 2022)
ClinicalTrials.gov (clinicaltrials.gov/; searched 30 November 2022)
We used the search strategies in Appendix 1. The MEDLINE strategy included the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE, as described in Chapter 4 of the Cochrane Handbook (Lefebvre 2022). We put no limits on publication date, publication status, or language.
Searching other resources
We searched the reference lists of included studies and relevant reviews identified by the electronic searches, and contacted the first author of all included studies and known experts in the field of developmental paediatrics and child psychiatry, to ask if they could provide details of any additional, relevant studies not already identified. We identified one study from a reference list, and obtained the relevant full text report through a Google search (Handen 2010).
We also contacted companies that manufacture acetylcholinesterase inhibitors, in an attempt to identify unpublished literature. On 30 August 2022, we searched MEDLINE, Embase, and Retraction Watch to identify retraction notices for the included studies; we did not identify any retracted studies.
Data collection and analysis
A summary of methods that we intended to use, as stipulated in our protocol (Cox 2021), but did not undertake due to limited included studies in this review, can be found in Appendix 2.
Selection of studies
Two review authors (AU and GC) independently checked the titles and abstracts of all records located by the electronic search using the specialised systematic review data management software, Covidence, and excluded those that did not meet the inclusion criteria. If a record appeared to meet the inclusion criteria, or additional information was needed to confirm this, we retrieved the full‐text report, and assessed it for eligibility. Cohen’s kappa coefficient between authors determining the eligibility of studies for inclusion was high (0.89). Any disagreement pertaining to whether a study met the inclusion criteria was resolved by a third review author, not involved in initial screening, acting as arbiter (KW or RH). We presented the results of our selection process in a PRISMA diagram (Moher 2009). We listed relevant excluded studies, along with the specific reason for their exclusion, in the Characteristics of excluded studies tables.
Data extraction and management
We extracted data into a pre‐designed data extraction form, piloted prior to use. Two review authors, with data extraction roles shared between three authors (GC, AU, or JW), independently extracted data from each included study. We extracted the following data.
study design, sample size, inclusion and exclusion criteria, duration of follow‐up
participant characteristics
intervention (generic and trade names) characteristics, including dose, administration type, duration of treatment and type of control used
primary and secondary outcomes and the measurement tools used
results
type and source of financial support
publication status from study reports
potential conflicts of interest
stated/declared conflicts of interest
Whenever possible, we use results from an intention‐to‐treat (ITT) analysis. To ensure consistency across review authors, we conducted calibration exercises before starting the review and data extraction process. We compared extracted data to ensure accuracy. A third review author (KW or RH), who did not extract data, acted as arbiter to resolve any discrepancies. We used Review Manager 5 (RevMan 5) software and RevMan Web for data organisation, management, and analysis, and to compute graphic representations of potential bias within and across studies (Review Manager 2020; RevMan Web 2023).
Assessment of risk of bias in included studies
Using the data extraction form, two review authors independently assessed each study, with the role shared between three authors (GC, AU and KW). Risk of bias was assessed using RoB 1 and the criteria outlined in Chapter 8 of the Cochrane Handbook, without blinding to authorship or source, across the domains listed below (Higgins 2017).
Random sequence generation: was the allocation sequence and randomisation adequate?
Allocation concealment: was allocation adequately concealed?
Blinding of participants and personnel: were participants, their families, and personnel adequately blinded to receiving the allocated intervention?
Blinding of outcome assessment: was knowledge of the allocated intervention adequately concealed during the study?
Incomplete outcome data: were incomplete outcome data adequately addressed in the analyses?
Selective outcome reporting: were all planned outcomes reported as specified in the protocol or methods section of the study?
Other sources of bias: was the study free of any other potential sources of bias, such as stopping early, extreme baseline imbalance, funding of the study, and conflicts of interest of the study authors and investigators.
For each domain, we assigned ratings of low, unclear, or high risk of bias. We gave a low risk of bias judgement if there was an agreement that any plausible bias was unlikely to have altered the results of a study; we reached an unclear risk of bias judgement if plausible bias raised some doubt about the results of a study; and we gave a high risk of bias judgement if plausible bias was agreed to seriously weaken confidence in the results of a study. For a study to be rated as being at low risk of bias overall, it needed to receive a low risk of bias judgement across all domains; for a rating of unclear risk of bias overall, judgements of unclear or low risks of bias needed to be present for one or more key domains; and for a high risk of bias to be present, a high risk of bias judgement must have been present for one or more key domains.
We compared the assessments for inconsistencies and resolved differences in interpretation by discussion and consensus. Any persisting disagreements were resolved by a third review author (KW), acting as arbiter.
Measures of treatment effect
Dichotomous data
We analysed dichotomous outcomes by calculating the odds ratio (OR) and corresponding 95% confidence intervals (CIs). We calculated the OR using RevMan 5, as described in Chapter 10 of the Cochrane Handbook (Deeks 2022; Review Manager 2020; RevMan Web 2023). If other forms of effect measures (e.g. standardised mean difference (SMD)) were provided, we used the available information to compute the OR using the formula provided in Chapter 10 of the Cochrane Handbook (Deeks 2022).
Continuous data
For continuous outcomes, we calculated mean differences (MD) and corresponding 95% CIs. If the scales used in studies were different, but the outcomes they measured were conceptually similar, we calculated the SMD, as recommended in Chapter 10 of the Cochrane Handbook (Deeks 2022).
As recommended in Deeks 2022, we focused on final values unless change scores were used in the studies. If data were not reported, or we were unable to extract them, we contacted trial authors.
Unit of analysis issues
We did not encounter any unit of analysis issues.
Dealing with missing data
We contacted the original study authors for clarification about data. We did not impute any missing data, and only analysed the data available in published reports and protocols located in our search.
Assessment of heterogeneity
We explored clinical heterogeneity by inspecting studies for variability in participants, interventions, and outcomes, and methodological heterogeneity by inspecting studies for variability in study design and risk of bias. Due to the different interventions and outcomes reported by the included studies, we were unable to assess statistical heterogeneity.
Assessment of reporting biases
We attempted to locate the protocol for the included studies, and compared all outcomes reported against those specified in their protocols.
Data synthesis
We were unable to perform a meta‐analysis because the two included studies reported on different interventions and outcomes. Instead, we provided a narrative description of the individual study results. However, both studies reported data suitable for analysis, and these we entered into RevMan Web. We calculated an odds ratio for the dichotomous outcome, using Revman Web and the formula given in Chapter 10 of the Cochrane Handbook (Deeks 2022; RevMan Web 2023).
Subgroup analysis and investigation of heterogeneity
Due to the limited number of studies, and associated variation in intervention and outcomes reported, we were unable to carry out subgroup analyses or investigate heterogeneity.
Sensitivity analysis
Due to the limited number of studies, and associated variation in intervention and outcomes reported, we were unable to perform a sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We generated summary of findings tables using GRADEpro GDT and RevMan Web, for the following comparisons (GRADEpro GDT; Review Manager 2020).
galantamine plus risperidone versus placebo plus risperidone
donepezil versus placebo
We reported the following outcomes in the table, measured post‐intervention.
overall ASD features
social communication
restricted and repetitive behaviours
adverse events
irritability
hyperactivity
general health and function
We included our secondary variables, irritability, hyperactivity, and general health and function, in the summary of findings table as the autism community identified them as important indicators of progress and outcomes (McConachie 2018).
We followed recommendations in Chapter 14 of the Cochrane Handbook (Schünemann 2022). Using the GRADE approach, we assessed the effect of risk of bias, directness, consistency, precision, and publication bias on the overall certainty of evidence; we assessed it as high, moderate, low, or very low. Risk of bias assesses the overall risk of bias of included studies that provided data for each outcome; directness assesses how well the included studies address the review question; consistency assesses how well unexplained heterogeneity has been accounted for in the study results; precision assesses the statistical precision of the results; and publication bias assesses transparency of publication and risk of publication bias among the studies that contribute to the outcome.
We rated evidence from RCTs initially as high‐certainty, and downgraded the certainty ratings depending on the presence of the aforementioned criteria, up to a maximum of three levels. We detailed our reasons for downgrading the certainty of the evidence for each outcome in the footnotes of the summary of findings table (Schünemann 2020).
Two review authors independently conducted the assessments (GC and AU), with a third (KW) acting as arbiter in the case of disagreements. In instances where adequate data were not available to conduct an assessment, we discussed the outcomes in the Results and Discussion sections of the review.
Results
Description of studies
Two studies met the eligibility criteria for this review, and are described in detail in the Characteristics of included studies tables (Ghaleiha 2014; Handen 2010).
Results of the search
The searches for this review were run up to November 2022. A total of 680 records were retrieved, 679 from the database searches, and one from a reference list. There were 356 records remaining after duplicates were removed. The title and abstracts of 356 records were screened in Covidence, and 324 irrelevant records were excluded. We retrieved the full‐text reports for the remaining 32 records. From these, we excluded 17 studies (from 22 reports), for the reasons reported in the Characteristics of excluded studies table. We found three studies (from three reports) for which results are not yet available (see Characteristics of ongoing studies). One study is awaiting classification due to insufficient information; we contacted the authors, but have yet to receive further information (see Characteristics of studies awaiting classification). We included two studies (from six reports); see Characteristics of included studies. The flow of study selection is shown in Figure 1.
1.
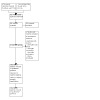
PRISMA flow chart for study selection
Included studies
Study design
Both included studies were individual participant, parallel‐group, randomised controlled trials (RCTs).
Location and funding
The galantamine study by Ghaleiha 2014 was conducted in Iran, with participants recruited from the children's outpatient clinic of the Roozbeh Hospital, a psychiatric academic hospital affiliated with Tehran University of Medical Science. The donepezil study by Handen 2010 was conducted in the USA. The authors were affiliated with the University of Pittsburgh and the Children's Hospital of Pittsburgh. Funding for the study was supported by an NIMH grant, and financial gifts from Pfizer and Eisai Pharmaceutical, who also provided the medication and placebo for the study.
Participants
The participants ranged from 4 years (Ghaleiha 2014) to 17 years (Handen 2010).
Ghaleiha 2014 initially recruited 48 male and female participants between the ages of 4 years and 12 years. All participants met the criteria for a diagnosis of autism spectrum disorder (ASD) according to DSM IV‐TR criteria (APA 2000). An expert child psychiatrist confirmed the historical diagnosis of ASD using the Autism Diagnostic Interview‐Revised (ADI‐R (Lord 1994)), and observing the child's behaviours. All participants scored > 12 on the Aberrant Behaviour Checklist‐Community (ABC‐C (Aman 1986)).
Handen 2010 recruited 34 participants between the ages of 8 years and 17 years, who met research diagnostic criteria for ASD (autistic disorder, pervasive developmental disorder–not otherwise specified (PDD–NOS), Asperger’s disorder), based on both the Autism Diagnostic Interview–Revised (ADI‐R; Rutter 2003a) and the Autism Diagnostic Observation Schedule (ADOS (Lord 1999)), and had impairments in executive function.
Interventions
Ghaleiha 2014 started galantamine at 2 mg/day, increasing the dose weekly by 2 mg to a maximum dose of 12 mg/day to 24 mg/day, depending on the body weight of the child, their tolerance to the medication, and the clinical indication. Risperidone was started at 0.5 mg/day, and increased weekly by 0.5 mg/day to 2 mg/day, depending on the body weight of the child.
In the donepezil study, dosing began at 2.5 mg/day and was increased to 5.0 mg/day for a one‐week period (Handen 2010). Participants were re‐evaluated after four weeks at the 5 mg/day dose. Doses were subsequently increased to 7.5 mg/day for a one‐week period and then titrated to 10.0 mg/day for the final four weeks of the double‐blind trial. A second re‐evaluation was conducted following four weeks on the 10 mg/day dose. Participants were also seen at weeks one and six to assess safety and to determine if the person was able to have his/her medication titrated to the next dose level. If reported side effects were determined to be interfering with functioning or well‐being, the medication was reduced to the previous highest tolerated dose and maintained at that level for the remainder of the study.
Comparators
Galantamine and placebo capsules were identical in coat, shape, size, texture, colour, taste and odour, and were packed in identical containers. The prescribed dosage and administration was not reported (Ghaleiha 2014).
Placebo and donepezil medication were packed in opaque capsules. Dosage and administration instructions were not reported (Handen 2010).
Outcomes
Primary outcomes
Core features of ASD
Core features of ASD were described as outcomes of interest in the two included studies.
Ghaleiha 2014 measured outcomes reported by parents using the ABC‐C at baseline, week 5 (mid‐treatment), and week 10 (postintervention). They used the lethargy/social withdrawal subscale to measure social communication impairment; and the stereotyped behaviour subscale to measure restricted, repetitive behaviour.
Handen 2010 measured outcomes reported by parent using a modified version of The Real Life Rating Scale (RLRS (Freeman 1986)), administered at baseline, week 5, and week 10. They used the total score to measure overall ASD features; the social relationships subscale to measure social communication; and the sensory motor subscale to measure restricted, repetitive behaviour.
Adverse events
Ghaleiha 2014 administered a 25‐item adverse events checklist one week after medication administration, with weekly follow‐up. Handen 2010 asked parents to complete a 20‐item checklist of the most common side effects for donepezil at each visit, and to rate these on a six‐point Likert scale (0 = not present; 1 or 2 = mild; 3 or 4 = moderate; and 5 or 6 = severe).
Secondary outcomes
Hyperactivity, irritability, aggression or disruptive behaviour, or both
Ghaleiha 2014 measured hyperactivity or noncompliance, and irritability with the ABC‐C at baseline, week 5, and week 10.
Handen 2010 measured attention, rule breaking, and aggression with subscales of the Child Behavior Checklist (CBCL (Achenbach 2001)), administered at baseline, week 5, and week 10. They also used an externalising problems total score.
Mood
Handen 2010 measured outcomes associated with mood with a range of scales at baseline, week 5, and week 10: the affectual responses subscale of the RLRS; and the anxiety, withdrawn, somatic complaints, social problems, and thought problems subscales of the CBCL. They also used an internalising problems total score from the CBCL.
Cognition
Handen 2010 reported on cognition at baseline, week 5, and week 10 in an affiliated published report.
General health and function
Handen 2010 administered the severity of illness and global improvement subscale from the Clinical Global Impression Scale (CGIS (Guy 1976)), at baseline, week 5, and week 10.
Neither study measured our other secondary outcomes: self‐injury, and quality of life for people with ASD and their carers.
Excluded studies
We excluded 17 studies (in 22 records) after full‐text review: seven studies were not RCTs, three studies had the wrong participants, four had the wrong interventions, one had co‐interventions in one study arm only, two studies were terminated early. See Characteristics of excluded studies for details.
Studies awaiting classification
One study is awaiting classification, as it was only described in a letter to the editor, and was lacking methodology detail required to confirm eligibility (Niederhofer 2002). See Characteristics of studies awaiting classification table.
Ongoing studies
Three relevant studies are currently ongoing (IRCT2017041333406N1; NCT00252603; Rahman 2018). Two are based in the USA and evaluate the efficacy of galantamine with various outcome measures, including core features of ASD in children (NCT00252603; Rahman 2018). One study protocol, published in 2005, and registered as completed in 2007, still has no publicly available results (NCT00252603). The third RCT, based in Iran, describes a 12‐week trial to assess the efficacy of donepezil as an adjunct to risperidone in treatment of core features of autism in children.
We contacted the authors for all three studies on 19 March 2021; however, did not receive a response. Further details of these studies are provided in the Characteristics of ongoing studies tables.
Risk of bias in included studies
See Figure 2, the risk of bias graph showing the proportion of studies with each judgement, and Figure 3 for the risk of bias summary. For further details on the risk of bias in each included study, please see the risk of bias tables in the Characteristics of included studies tables.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
We judged Ghaleiha 2014 to be at low risk of bias across all domains, and Handen 2010 at low risk for performance bias, high risk of reporting bias and other bias, and unclear on the remaining domains.
Allocation
Random sequence generation
We judged random sequence generation at low risk in one study, in which a computer generated random sequence was generated using computer software by an individual not otherwise involved in the study (Ghaleiha 2014). We judged random sequence generation as unclear in Handen 2010, since they reported but did not describe their process of randomisation.
Allocation concealment
Allocation concealment was considered adequate and at low risk in Ghaleiha 2014, in which independent personnel and opaque envelopes were used to ensure blinding of participants and research staff, which was maintained. We judged allocation concealment as unclear in Handen 2010, as they reported but did not describe their process, except to state that their randomisation was conducted by the study pharmacist.
Blinding
Performance bias
We judged both studies at low risk of performance bias, since both explicitly reported blinding of participants, their parents, referring physicians, research investigators, and the person who administered the medication, since both the active and placebo medications were similar, and similarly packaged.
Blinding of outcome assessment
We judged Ghaleiha 2014 to be at low risk of blinding of the outcome assessor, since they reported that the parents and study investigators either remained uncertain about their assigned trial group, or there was no significant difference in their treatment guesses. We judged Handen 2010 as unclear risk of bias, as they did not adequately describe their methodology.
Incomplete outcome data
Ghaleiha 2014 reported that eight participants withdrew their consent: the placebo group retained 20/23 participants (86%), and the galantamine group retained 20/25 participants (80%). All dropouts occurred before first follow‐up. We rated this study at low risk of attrition bias due to relatively low and equal levels of attrition.
Handen 2010 did not explicitly state dropout rates but implied that three participants withdrew due to side effects. They used an intention‐to‐treat approach, by carrying forward the last observation for dropouts. We were unclear if this methodology prevented attrition bias due to uneven withdrawal.
Selective reporting
Ghaleiha 2014 registered their protocol and listed outcomes consistent with this publication; we rated this study at low risk of reporting bias.
We judged Handen 2010 at high risk of selective reporting bias, since it was unregistered, and did not report comparison data between groups for adverse events.
Other potential sources of bias
We considered other potential sources of bias as low for Ghaleiha 2014: the investigators declared no conflict of interest; the study was funded by a grant to the Tehran University, and the authors stipulated that the funding organisation had no role in any aspect of the design, conduct, or reporting of the study.
Although Handen 2010 denied any potential conflict of interest, the authors were partly supported by pharmaceutical companies, so we considered the study at high risk of other potential sources of bias.
Effects of interventions
Comparison 1: Galantamine plus risperidone versus placebo plus risperidone
Only one study investigated this comparison (Ghaleiha 2014; see Table 1). Overall features of autism and secondary outcomes, including mood, cognition and function, were not assessed.
Primary outcomes
Core features of Autism Spectrum Disorder
There may be little to no difference in social communication impairment immediately postintervention (mean difference (MD) ‐2.75, 95% confidence interval (CI) ‐5.88 to 0.38; 1 study, 40 participants; very low‐certainty evidence; Analysis 1.1), and possibly no difference in restricted and repetitive behaviour immediately postintervention, but the evidence is very uncertain (MD ‐0.55, 95% CI ‐3.47 to 2.37; 1 study, 40 participants; very‐low‐certainty evidence; Analysis 1.2). We downgraded the certainty for both outcomes due to very serious imprecision (results based on one small study), and indirectness (outcome measures did not adequately measure our outcomes of interest).
1.1. Analysis.

Comparison 1: Galantamine plus risperidone versus placebo plus risperidone, Outcome 1: Social interaction impairment postintervention
1.2. Analysis.

Comparison 1: Galantamine plus risperidone versus placebo plus risperidone, Outcome 2: Repetitive and restricted behaviour postintervention
Adverse events
Ghaleiha 2014 reported on the frequency of adverse events rather than the number of participants experiencing adverse events. They reported six different types of adverse events out of a possible 25 included in the adverse event checklist. A total of 15 adverse events were reported in the treatment group, compared with 7 adverse events reported in the comparison group. There may be no meaningful group difference in the frequency across all adverse event subtypes (odds ratio (OR) 5.57, 95% CI 1.42 to 21.86; 1 study, 40 participants; low‐certainty evidence; Analysis 1.3). Nervousness and increased appetite were the most frequent adverse events observed. Participants treated with galantamine experienced a weight gain of 0.75 (1.29) kg; those in the placebo group gained 0.19 (0.52) kg (MD 0.56, 95% CI ‐0.05 to 1.17; 1 study, 40 participants; low‐certainty evidence; Analysis 1.4). We downgraded the certainty by two levels due to imprecision (results based on one small study, with wide confidence intervals).
1.3. Analysis.

Comparison 1: Galantamine plus risperidone versus placebo plus risperidone, Outcome 3: Adverse events
1.4. Analysis.

Comparison 1: Galantamine plus risperidone versus placebo plus risperidone, Outcome 4: Adverse events; weight gain
Secondary outcomes
There might be a small difference between the two groups immediately postintervention in irritability, the galantamine group showed a greater decline in the irritability subscale than the placebo group, measured postintervention (MD ‐3.50, 95% CI ‐6.39 to ‐0.61; 1 study; 40 participants; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Galantamine plus risperidone versus placebo plus risperidone, Outcome 5: Irritability postintervention
There was no clear evidence of a difference in hyperactivity immediately postintervention (MD ‐5.20, 95% CI ‐10.51 to 0.11; 1 study, 40 participants; low‐certainty evidence; Analysis 1.6). We downgraded the certainty by two levels due to imprecision (results based on one small study).
1.6. Analysis.

Comparison 1: Galantamine plus risperidone versus placebo plus risperidone, Outcome 6: Hyperactivity postintervention
Comparison 2: Donepezil versus placebo
This comparison was explored in one study with 34 participants (Handen 2010; See Table 2).
Primary outcomes
Core features of Autism Spectrum Disorder
There was no clear evidence of a difference immediately postintervention in overall autism features (MD 0.07, 95% CI ‐0.19 to 0.33; 1 study; 34 participants; very low‐certainty evidence; Analysis 2.1), or in specific domains of autism: social communication impairment (MD ‐0.02, 95% CI ‐0.34 to 0.30; 1 study; 34 participants; very low‐certainty evidence; Analysis 2.2), and restricted and repetitive behaviours (MD 0.04, 95% CI ‐0.27 to 0.35; 1 study; 34 participants; very low‐certainty evidence; Analysis 2.3). We downgraded the certainty by one level due to risk of bias (high/unclear risk of bias across all but one domain), and by two levels due to very serious imprecision (results based on one small study with wide confidence intervals).
2.1. Analysis.

Comparison 2: Donepezil versus placebo, Outcome 1: Real‐life Rating Scale (RLRS) total score postintervention
2.2. Analysis.

Comparison 2: Donepezil versus placebo, Outcome 2: Social communication impairment postintervention
2.3. Analysis.

Comparison 2: Donepezil versus placebo, Outcome 3: Restricted and repetitive behaviours postintervention
Adverse events
No adverse events were reported. Significant adverse events leading to study withdrawal in at least one participant was implied in the data analysis section but not explicitly reported. This report stands in contrast to the information from the clinical study registry, which suggests no adverse events were experienced in either the placebo or treatment group (NCT00047697).
Secondary outcomes
The evidence is very uncertain about the effect of donepezil, compared to placebo, across all analyses immediately postintervention: irritability/oppositionality (MD 1.08, 95% CI ‐0.41 to 2.57; 1 study; 34 participants; very low‐certainty evidence; Analysis 2.4), and hyperactivity (MD 2.60, 95% CI 0.50 to 4.70; 1 study, 34 participants; very low‐certainty evidence; Analysis 2.5).
2.4. Analysis.

Comparison 2: Donepezil versus placebo, Outcome 4: Behavioural difficulties postintervention
2.5. Analysis.

Comparison 2: Donepezil versus placebo, Outcome 5: Attention and hyperactivity difficulties postintervention
There was no clear evidence of a difference for general health and function (MD 0.03, 95% CI ‐0.48 to 0.54; 1 study; 34 participants; very low‐certainty evidence; Analysis 2.6). Results also reported findings no group differences immediately post‐intervention for mood, anxiety, a range of executive functioning tests, and somatic complaints (data not shown).
2.6. Analysis.

Comparison 2: Donepezil versus placebo, Outcome 6: General health and function postintervention
We downgraded the certainty by one level due to risk of bias (high/unclear risk of bias across all but one domain), and two levels due to very serious imprecision (results based on one small study with wide confidence intervals).
Discussion
Summary of main results
We included two randomised controlled trials (RCTs), but were unable to conclude with certainty whether acetylcholinesterase inhibitors are effective in alleviating impairment associated with the core features of autism spectrum disorder (autism) in children or adults.
One RCT (40 participants) evaluated the effects of galantamine compared with placebo as an adjunct to risperidone. They found little to no difference between groups in the social communication skills of autistic children, no evidence of a difference for repetitive behaviour and hyperactivity, and little to no difference in their levels of irritability, following 10 weeks of treatment. We judged the evidence base for the outcomes associated with core features of autism as having very low‐certainty; outcomes associated with secondary features were supported by low‐certainty evidence. Galantamine may cause side effects, including nervousness, drowsiness, increased appetite, and tremor. This study did not assess overall autism features or general health and function (Ghaleiha 2014).
A second RCT compared donepezil to placebo in autistic children over a 10‐week period. The results identified no clear evidence of group differences across the core symptoms of autism, and all measures of behaviour and general health and function. We were not able to determine whether donepezil caused unwanted side effects in autistic children, due to the inconsistent reporting of data from the included study (Handen 2010).
Overall completeness and applicability of evidence
We found no studies that reported on the effectiveness of acetylcholinesterase inhibitors in adults. We intended to include studies of autistic people and other neurodevelopmental or psychiatric comorbid conditions, such as attention deficit hyperactivity disorder or an anxiety disorder, but did not encounter any studies with this population who were receiving an eligible intervention. For children aged 4 years to 17 years, only two relevant RCTs have been completed, only one of which was at an overall low risk of bias. There was considerable variation between studies in participants, interventions, study design, and methodological quality. The measures used were not considered to adequately assess our outcomes of interest. Adverse events were reported in only one study. As such, there is insufficient evidence upon which to base clinical care for all age groups.
Quality of the evidence
Our primary outcome associated with overall autism features was assessed in the donepezil comparison only. Using the GRADE approach, we considered the certainty of evidence associated with this outcome to be very low (Schünemann 2022). We downgraded by one level due to the high or unclear risk of bias across all but one domain; and two levels due to imprecision associated with a single, small study with wide confidence intervals.
For outcomes associated with core features of autism, including social communication and restricted, repetitive behaviours, our certainty of the evidence was very low in both comparisons, downgraded by two levels due to concerns of imprecision associated with a single, small study with wide confidence intervals contributing to each comparison. We downgraded by one more level due to indirectness in the galantamine comparison, since the subscales from the ABC‐C are not considered to adequately measure the core features of autism; and one more level in the donepezil comparison due to the high and unclear risks of bias across all but one domain in the included study.
We judged the certainty of evidence for adverse events outcomes as low in the galantamine comparison, downgraded by two levels for imprecision, because only one small study contributed to the outcome. We were unable to determine whether donepezil causes unwanted side effects in autistic children.
We assessed the certainty of evidence for non‐core features of autism as low for the galantamine comparison, downgrading for outcomes associated with irritability and hyperactivity by two levels due to imprecision, because only one small study contributed to the outcome. We downgraded the evidence for the same outcomes in the donepezil comparison one level due to high risk of bias, and two levels due to imprecision, because only one small study contributed to the outcome, leaving very low‐certainty evidence.
General health and function were not assessed.
Potential biases in the review process
We carefully managed potential conflicts by ensuring we declared any conflicts, as per Cochrane guidelines (see Declarations of interest). We included studies of all languages, and where relevant, had these translated to ensure we included all eligible studies. Whilst our attempts to obtain relevant information from study authors was unsuccessful, we were able to access information from clinical trial directories. It is unlikely that published or ongoing studies were missed due to the nature of the medication‐based search and the breadth of databases used.
Agreements and disagreements with other studies or reviews
A systematic review and network meta‐analysis of RCTs investigating all pharmacological and dietary‐supplement treatments for autism was recently published (Siafis 2022). Donepezil was one of the 41 drugs and 17 dietary supplements included, drawing from 125 RCTs (N = 7450 participants) in children and adolescents, and 18 RCTs (N = 1104) in adults. Siafis included placebo‐controlled and head‐to‐head RCTs. Both blinded and open‐label RCTs were eligible. Two published studies using donepezil for autism symptoms, met their inclusion criteria, with a combined total of 94 participants (Gabis 2019; Handen 2010). Gabis 2019 investigated donepezil with choline as an adjunct medication versus placebo. We excluded this study from our review because participants in the placebo group were not given the same adjunct medication as the treatment group. Nonetheless, results were consistent with our findings, with no evidence of group differences in overall autism features or social communication impairments. Restricted and repetitive behaviours were not explicitly assessed.
Authors' conclusions
Implications for practice.
Evidence about the effectiveness of acetylcholinesterase inhibitors as a medication to improve outcomes for autistic adults is lacking, and for autistic children is very uncertain.
There is a need for more evidence of improvement in outcomes of relevance to clinical care for autistic people and their families. There are a number of ongoing studies involving acetylcholinesterase inhibitors, and future updates of this review may add to the current evidence.
Implications for research.
There is a lack of research for acetylcholinesterase inhibitors in autistic people of all ages, and no studies of autistic adults. Co‐design with people with lived experience of autism using agreed measures of autism outcomes in clinical trials is needed, with consideration to different age groups and abilities. If people living with autism, or their carers, or professionals involved in their care value exploring this approach as a potential intervention, studies are needed that use methodologically rigorous designs. Optimal trial methods, including larger sample sizes to enable subgroup analyses, participants with a broad range of characteristics (e.g. IQ, verbal ability, and age), consistent data, and close monitoring of adverse events and outcomes that are important to clinicians, families and autistic people should be used. Consideration of the likely impacts of acetylcholinesterase inhibitors on brain function, and whether core features of autism are the right outcome measure will be important, given medications are usually used to improve associated difficulties, such as irritability, attention, mood, and anxiety.
History
Protocol first published: Issue 1, 2021
Acknowledgements
We are very grateful for the contributions, advice and support received from Cochrane Developmental, Psychococial and Learning Problems, including the Editors, Information Specialist, and Statistician.
We would like to acknowledge Jade Woon who contributed to earlier versions of this review.
Editorial and peer‐reviewer contributions:
Cochrane Developmental, Psychosocial and Learning Problems supported the authors in the development of this intervention review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Geraldine Macdonald, University of Bristol Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joanne Duffield, Queen's University Belfast Deputy Managing Editor (conducted editorial policy checks and supported editorial team): Sarah Davies, University of Bristol Copy Editor (copy editing and production): Victoria Pennick, Copy‐edit Support Group Peer‐reviewers (provided comments and recommended an editorial decision): Dr Marinos Kyriakopoulos, Assistant Professor in Child and Adolescent Psychiatry, National and Kapodistrian University of Athens, Greece; Consultant Child and Adolescent Psychiatrist, Maudsley Hospital, UK; and Visiting Senior Lecturer, Institute of Psychiatry, Psychology and Neuroscience, King's College London, UK (clinical/content review); Spyridon Siafis, Section of Evidence‐Based Medicine in Psychiatry and Psychotherapy, School of Medicine, Technical University of Munich, Munich, Germany (clinical/content review); Brian Duncan, USA (consumer review); Dr Helen McAneney, University College Dublin, Ireland, and Ulster University, Northern Ireland (methods review); and Margaret Anderston, Queen's University Belfast, Northern Ireland (search review).
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
Searched 7 December 2020 (31 records) Searched 2 November 2021 (no new records) Searched 29 November 2022 (1 new record)
#1 [mh "child development disorders, pervasive"] #2 [mh ^"Developmental Disabilities"] #3 [mh ^"Neurodevelopmental disorders"] #4 pervasive NEXT development* NEXT disorder* #5 (pervasive Near/3 child*) #6 ( PDDs or PDD‐NOS or ASD or ASDs) #7 autis* #8 asperger* #9 kanner* 1 #10 "childhood schizophrenia" #11 {OR #1‐#10} #12 [mh acetylcholine] #13 acetylc*olin* #14 [mh ^"Cholinesterase Inhibitors"] #15 (Cholinesterase Near/3 inhibit*) #16 (AChE Near/3 inhibit*) #17 cognex #18 [mh Donepezil] #19 Donepezil #20 [mh Galantamine ] #21 Galantamine #22 Galanthamine #23 [mh Rivastigmine] #24 (Rivastigmine or Rivastig*mine) #25 Razadyne #26 Tacrine #27 Aricept #28 GalantaMind #29 Reminyl #30 E2020 #31 Exelon #32 Nivalin #33 (anti next cholinesterase or anticholinesterase) #34 (Anticholesterase or Anti next cholesterase) #35 {or #12‐#34} #36 #11 and #35 in Trials
MEDLINE Ovid
Searched 7 December 2020 (134 records) Searched 2 November 2021 (6 new records) Searched 30 November 2022 (3 new records)
1 exp child development disorders, pervasive/ 2 Developmental Disabilities/ 3 Neurodevelopmental disorders/ 4 pervasive development$ disorder$.tw,kf. 5 (pervasive adj3 child$).tw,kf. 6 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 7 autis$.tw,kf. 8 asperger$.tw,kf. 9 kanner$.tw,kf. 10 childhood schizophrenia.tw,kf. 11 or/1‐10 12 acetylcholine/ 13 acetylc?olin$.mp. 14 Cholinesterase Inhibitors/ 15 (Cholinesterase adj3 inhibit$).tw,kf. 16 (AChE adj3 inhibit$).tw,kf. 17 Cognex.mp. 18 Donepezil/ 19 Donepezil.mp. 20 Galantamine/ 21 Galantamine.mp. 22 Galanthamine.mp. 23 Rivastigmine/ 24 Rivastig?mine.mp. 25 Razadyne.mp. 26 Tacrine.mp. 27 Aricept.mp. 28 GalantaMind.mp. 29 Reminyl.mp. 30 E2020.mp. 31 Exelon.mp. 32 Nivalin.mp. 33 (anti‐cholinesterase or anticholinesterase).mp. 34 (Anticholesterase or Anti‐cholesterase).mp. 35 or/12‐34 36 randomized controlled trial.pt. 37 controlled clinical trial.pt. 38 randomi#ed.ab. 39 placebo$.ab. 40 drug therapy.fs. 41 randomly.ab. 42 trial.ab. 43 groups.ab. 44 or/36‐43 45 exp animals/ not humans.sh. 46 44 not 45 47 11 and 35 and 46
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid via Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R)
Searched 7 December 2020 (36 records) Searched 2 November 2021 (33 records) Searched 30 November 2022 (30 records)
1 autis$.tw,kf. 2 asperger$.tw,kf. 3 kanner$.tw,kf. 4 childhood schizophrenia.tw,kf. 5 pervasive development$ disorder$.tw,kf. 6 (pervasive adj3 child$).tw,kf. 7 Neurodevelopmental disorder$.tw,kf. 8 Developmental Disabilit$.tw,kf. 9 or/1‐8 10 acetylc?olin$.mp. 11 (Cholinesterase adj3 inhibit$).tw,kf. 12 Cognex.mp. 13 Donepezil.mp. 14 Rivastigmine.mp. 15 Razadyne.mp. 16 Tacrine.mp. 17 Aricept.mp. 18 GalantaMind.mp. 19 Reminyl.mp. 20 E2020.mp. 21 Exelon.mp. 22 Nivalin.mp. 23 (anti‐cholinesterase or anticholinesterase).mp. 24 (Anticholesterase or Anti‐cholesterase).mp. 25 or/10‐24 26 9 and 25 27 limit 26 to ("in data review" or in process or "pubmed not medline")
MEDLINE Epub Ahead of Print Ovid via Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R)
Searched 7 December 2020 (4 records) Searched 2 November 2021 (3 new records) Searched 30 November 2022 (3 new records)
1 autis$.tw,kf. 2 asperger$.tw,kf. 3 kanner$.tw,kf. 4 childhood schizophrenia.tw,kf. 5 pervasive development$ disorder$.tw,kf. 6 (pervasive adj3 child$).tw,kf. 7 Neurodevelopmental disorder$.tw,kf. 8 Developmental Disabilit$.tw,kf. 9 or/1‐8 10 acetylc?olin$.mp. 11 (Cholinesterase adj3 inhibit$).tw,kf. 12 Cognex.mp. 13 Donepezil.mp. 14 Rivastigmine.mp. 15 Razadyne.mp. 16 Tacrine.mp. 17 Aricept.mp. 18 GalantaMind.mp. 19 Reminyl.mp. 20 E2020.mp. 21 Exelon.mp. 22 Nivalin.mp. 23 (anti‐cholinesterase or anticholinesterase).mp. 24 (Anticholesterase or Anti‐cholesterase).mp. 25 or/10‐24 26 9 and 25 27 limit 26 to publisher
Embase Ovid
Searched 7 December 2020 (45 records) Searched 2 November 2021 (6 new records) Searched 30 November 2022 (2 new records)
1 autism/ 2 asperger syndrome/ 3 developmental disorder/ 4 Neurodevelopmental disorder$.tw,kw. 5 "pervasive developmental disorder not otherwise specified"/ 6 childhood disintegrative disorder/ 7 pervasive development$ disorder$.tw,kw. 8 (pervasive adj3 child$).tw,kw. 9 (PDD‐NOS or PDDs or ASD or ASDs).tw,kw. 10 autis$.tw,kw. 11 asperger$.tw,kw. 12 kanner$.tw,kw. 13 childhood schizophren$.tw,kw. 14 (pervasive adj3 child$).tw,kw. 15 or/1‐14 16 acetylcholine/ 17 acetylc?olin$.mp. 18 cholinesterase inhibitor/ 19 (Cholinesterase adj3 inhibit$).tw,kw. 20 Cognex.mp. 21 (AChE adj3 inhibit$).tw,kw. 22 donepezil/ or donepezil plus memantine/ 23 Donepezil.mp. 24 galantamine/ 25 (Galantamine or Galanthamine).mp. 26 (Rivastigmine or Rivastigamine).mp. 27 Razadyne.mp. 28 tacrine/ 29 Tacrine.mp. 30 Aricept.mp. 31 GalantaMind.mp. 32 Reminyl.mp. 33 E2020.mp. 34 Exelon.mp. 35 Nivalin.mp. 36 (anti‐cholinesterase or anticholinesterase).mp. 37 (Anticholesterase or Anti‐cholesterase).mp. 38 (Anticholesterase or Anti‐cholesterase).mp. 39 or/16‐38 40 15 and 39 41 Randomized controlled trial/ 42 Controlled clinical study/ 43 random$.ti,ab. 44 randomization/ 45 intermethod comparison/ 46 placebo.ti,ab. 47 (compare or compared or comparison).ti. 48 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 49 (open adj label).ti,ab. 50 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 51 double blind procedure/ 52 parallel group$1.ti,ab. 53 (crossover or cross over).ti,ab. 54 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 55 (assigned or allocated).ti,ab. 56 (controlled adj7 (study or design or trial)).ti,ab. 57 (volunteer or volunteers).ti,ab. 58 human experiment/ 59 trial.ti. 60 or/41‐59 61 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 62 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 63 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 64 (Systematic review not (trial or study)).ti. 65 (nonrandom$ not random$).ti,ab. 66 "Random field$".ti,ab. 67 (random cluster adj3 sampl$).ti,ab. 68 (review.ab. and review.pt.) not trial.ti. 69 "we searched".ab. and (review.ti. or review.pt.) 70 "update review".ab. 71 (databases adj4 searched).ab. 72 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 73 Animal experiment/ not (human experiment/ or human/) 74 or/61‐73 75 60 not 74 76 40 and 75
CINAHL EBSCOhost
Searched 7 December 2020 (33 records) Searched 2 November 2021 (1 new record) Searched 30 November 2022 (1 new record)
S1 (MH "Child Development Disorders, Pervasive+") S2 (MH "Developmental Disabilities") S3 (MH "Mental Disorders Diagnosed in Childhood") S4 (autis* or ASD or ASDs) S5 Asperger* S6 Kanner* S7 childhood schizophren* S8 pervasive N3 child* S9 (pervasive development* disorder* or PDDs or PDD‐NOS) S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S11 (MH "Acetylcholine") S12 acetylc*olin* S13 (MH "Cholinesterase Inhibitors") S14 (Cholinesterase N3 inhibit*) S15 (AChE N3 inhibit*) S16 (MH "Donepezil") S17 Donepezil S18 (MH "Galanthamine") S19 Galantamine or Galanthamine S20 (MH "Rivastigmine") S21 (Rivastigmine or Rivastigamine) S22 Razadyne S23 (MH "Tacrine") S24 Tacrine or Cognex S25 "Aricept" S26 "GalantaMind" S27 Reminyl S28 E2020 S29 Exelon S30 Nivalin S31 (anti‐cholinesterase or anticholinesterase) S32 (Anticholesterase or Anti‐cholesterase) S33 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 S34 S10 AND S33 S35 MH randomized controlled trials S36 MH double‐blind studies S37 MH single‐blind studies S38 MH random assignment S39 MH pretest‐posttest design S40 MH cluster sample S41 TI (randomised OR randomized) S42 AB (random*) S43 TI (trial) S44 MH (sample size) AND AB (assigned OR allocated OR control) S45 MH (placebos) S46 PT (randomized controlled trial) S47 AB (control W5 group) S48 MH (crossover design) OR MH (comparative studies) S49 AB (cluster W3 RCT) S50 MH animals+ S51 MH (animal studies) S52 TI (animal model*) S53 S50 OR S51 OR S52 S54 MH (human) S55 S53 NOT S54 S56 S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 S57 S56 NOT S55 S58 S34 AND S57
APA PsycINFO Ovid
Searched 7 December 2020 (67 records) Searched 2 November 2021 (1 new record) Searched 30 November 2022 (1 new record)
1 exp pervasive developmental disorders/ 2 Developmental disabilities/ 3 Neurodevelopmental Disorders/ 4 pervasive development$ disorder$.tw. 5 (pervasive adj3 child$).tw. 6 autis$.tw. 7 asperger$.tw. 8 Kanner$.tw. 9 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 10 childhood schizophreni$.tw. 11 or/1‐10 12 acetylcholine/ 13 acetylc?olin$.mp. 14 cholinesterase inhibitors/ 15 (Cholinesterase adj3 inhibit$).tw. 16 (acetylc?olin$ adj3 inhibit$).tw. 17 (AChE adj3 inhibit$).tw. 18 Cognex.mp. 19 Donepezil.mp. 20 Galanthamine/ 21 (Galanthamine or Galantamine).mp. 22 Rivastigmine.mp. 23 Razadyne.mp. 24 Tacrine.mp. 25 Aricept.mp. 26 GalantaMind.mp. 27 Reminyl.mp. 28 E2020.mp. 29 Exelon.mp. 30 Nivalin.mp. 31 (anti‐cholinesterase or anticholinesterase).mp. 32 (Anticholesterase or Anti‐cholesterase).mp. 33 or/12‐32 34 randomized controlled trials/ 35 randomized clinical trials/ 36 clinical trials/ 37 exp treatment effectiveness evaluation/ 38 placebo/ 39 random$.tw. 40 (placebo or treatment as usual or tau).ab. 41 (control$ adj3 (study or studies or trial$ or group$)).tw. 42 ((singl$ or doubl$ or tripl$ or trebl$) adj3 (blind$ or mask$)).tw. 43 (allocat$ or assign$).tw. 44 (crossover or cross‐over).tw. 45 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 46 or/34‐45 47 11 and 33 and 46
Cochrane Database of Systematic Reviews
Searched 7 December 2020 (no records) Searched 2 November 2021 (1 new record) Searched 29 November 2022 (no new records)
#1 [mh "child development disorders, pervasive"] #2 [mh ^"Developmental Disabilities"] #3 [mh ^"Neurodevelopmental disorders"] #4 (pervasive NEXT development* NEXT disorder):TI,AB,KW #5 (pervasive Near/3 child*):TI,AB,KW #6 ( PDDs or PDD‐NOS or ASD or ASDs):TI,AB,KW #7 autis*:TI,AB,KW #8 asperger*:TI,AB,KW #9 kanner*:TI,AB,KW #10 "childhood schizophrenia":TI,AB,KW #11 {OR #1‐#10} #12 [mh acetylcholine] #13 acetylc*olin*:TI,AB,KW #14 [mh ^"Cholinesterase Inhibitors"] #15 (Cholinesterase Near/3 inhibit*):TI,AB,KW #16 (AChE Near/3 inhibit*):TI,AB,KW #17 Cognex:TI,AB,KW #18 [mh Donepezil] #19 Donepezil:TI,AB,KW #20 [mh Galantamine ] #21 Galantamine:TI,AB,KW #22 Galanthamine:TI,AB,KW #23 [mh Rivastigmine] #24 (Rivastigmine or Rivastig*mine):TI,AB,KW #25 Razadyne:TI,AB,KW #26 Tacrine:TI,AB,KW #27 Aricept:TI,AB,KW #28 GalantaMind:TI,AB,KW #29 Reminyl:TI,AB,KW #30 E2020:TI,AB,KW #31 Exelon:TI,AB,KW #32 Nivalin:TI,AB,KW #33 (anti next cholinesterase or anticholinesterase):TI,AB,KW #34 (Anticholesterase or Anti next cholesterase):TI,AB,KW #35 {or #12‐#34} #36 #11 and #35
Epistemonikos
Searched 7 December 2020 (14 records) Searched 2 November 2021 (no new records) Searched 30 November 2022 (no new records)
(title:((acetylcholine OR "Cholinesterase inhibitor" OR Aricept OR Donepezil OR E2020 OR Exelon OR Galantamine OR Galanthamine OR GalantaMind OR Nivalin OR Reminyl OR Rivastigmine OR Rivastigamine OR Razadyne OR Tacrine )) OR abstract:((acetylcholine OR "Cholinesterase inhibitor" OR Aricept OR Cognex OR Donepezil OR E2020 OR Exelon OR Galantamine OR Galanthamine OR GalantaMind OR Nivalin OR Reminyl OR Rivastigmine OR Rivastigamine OR Razadyne OR Tacrine ))) AND (title:(autis* OR asperger* OR ASD OR ASDs OR PDDs OR PDD‐NOS OR "pervasive developmental disorder") OR abstract:(autis* OR asperger* OR ASD OR ASDs OR PDDs OR PDD‐NOS OR "pervasive developmental disorder"))
Web of Science Core Collection, Clarivate (Science Citation Index ‐ Expanded; Social Sciences Citation Index; Conference Proceedings Citation Index ‐ Science; Conference Proceedings Citation Index ‐ Social Science & Humanities; Emerging Sources Citation Index)
Searched 7 December 2020 (118 records) Searched 2 November 2021 (12 new records) Searched 30 November 2022 (7 new records)
# 12 #11 AND #10 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 11 (random* or trial* or control* or placebo* or TAU or "treatment as usual" or group* ) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 10#9 AND #5 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 9#8 OR #7 OR #6 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All year # 8TS= (anticholinesterase or "anti cholinesterase" or anticholesterase or "anti cholinesterase") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All year # 7 TS=(Aricept or Cognex or Donepezil or E2020 or Exelon or Galantamine or Galanthamine or GalantaMind or Nivalin or Reminyl or Rivastigmine or Rivastigamine or Razadyne or Tacrine ) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 6 TS=(acetylc*olin* or Cholinesterase N/3 inhibit* or AChE N/3 inhibit*) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years #5 #4 OR #3 OR #2 OR #1 #4 TS=("childhood schizophren*") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years #3 TS=(pervasive N/3 child*) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years #2 TS=("pervasive development* disorder*") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years #1 TS=(autis* or asperger* or ASD or ASDs or PDDs or PDD‐NOS)
Proquest Dissertations & Theses A&I
Searched 7 December 2020 (2 records) Searched 2 November 2021 (no new records) Searched 30 November 2022 (1 new record)
noft(acetylcholine OR "Cholinesterase inhibitor" OR Aricept OR Cognex OR Donepezil OR E2020 OR Exelon OR Galantamine OR Galanthamine OR GalantaMind OR Nivalin OR Reminyl OR Rivastigmine OR Rivastigamine OR Razadyne OR Tacrine) AND noft((autis* OR asperger* OR ASD OR ASDs OR PDDs OR PDD‐NOS OR "pervasive developmental disorder")) AND noft(RANDOM* OR PLACEBO* OR TRIAL* OR rct or control* or group*)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
Searched 7 December 2020 (11 records) Searched 2 November 2021 (no new records) Searched 30 November 2022 (1 new record)
autism AND acetylcholine OR autism AND "Cholinesterase inhibitor" OR autism AND Aricept OR autism AND Donepezil OR autism AND E2020 OR autism AND Exelon OR autism AND Galantamine OR autism AND Galanthamine OR autism AND GalantaMind OR autism AND Nivalin OR autism AND Reminyl OR autism AND Rivastigmine OR autism AND Rivastigamine OR autism AND Razadyne OR autism AND Tacrine
ClinicalTrials.gov
Searched 7 December 2020 (8 records) Searched 30 November 2022 (1 new record) Searched 30 November 2022 (no new records)
Autism OR Asperger OR PDD‐NOS OR ASD OR Pervasive development Disorder | acetylcholine OR "Cholinesterase inhibitor" OR Aricept OR Donepezil OR E2020 OR Exelon OR Galantamine OR Galanthamine OR GalantaMind OR Nivalin OR Reminyl OR Rivastigmine OR Rivastigamine OR Razadyne OR Tacrine
Appendix 2. Unused methods
| Method | Approach |
| Types of outcome measures |
Time points For our primary outcomes, we intended to assess outcomes at any time post‐intervention (i.e. completion of treatment), and to subdivide the treatment indices a priori into early response (between one and four weeks post‐intervention); acute response (between five and 12 weeks post‐intervention), and later response over 13 weeks. |
|
Multiple measures If a single study had reported data for the same outcome more than once within either the early, acute, or later response time frame, we would have included the data point at the longest follow‐up within a time period. If multiple studies had reported more than one observation for each time frame that was suitable for meta‐analysis, we would have inputted the data from the time point most similarly reported across studies. | |
|
Multiple informants We intended to consider quantitative and qualitative data from standardised assessments, parent and teacher questionnaires, and rating scales, and behavioural observation. We intended to prioritise parent‐report measures over teacher measures followed by self‐report measures, and to analyse data from these outcomes separately. If a study presented two measures for a single outcome, we would have prioritised standardised measures over non‐standardised measures, and if a study presented two standardised measures for an outcome, we would have selected the most commonly used measure across all studies. | |
| Measures of treatment effects |
Continuous data If studies used different scales to measure the same outcomes, we would have calculated the standardised mean difference (SMD) with 95% confidence intervals (CIs). |
|
Dichotomous data Had other forms of effect size been provided in the included studies, we would have used the available information to compute the odds ratio (OR) using the formulae given in Chapter 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020). | |
|
Multiple outcomes We planned to calculate the average SMD across outcomes if multiple, interchangeable measures of the same construct were reported at the same point of time. | |
| Unit of analysis |
Cross‐over trials In the case of data from a cross‐over trial, we would have used the available data from the first phase only, up to the point of cross‐over, to avoid any problems associated with any carry‐over effects from the first phase to the second phase of the study. If repeated observations on participants had been reported, we would have extracted the time points closest to 4 weeks, 12 weeks, 6 months, and at the end of the study, in keeping with the end of our three time categories: early, acute, and late. If a study had more than two treatment groups, we would have presented the additional treatment arms. When the additional treatment arms were not relevant, we would not have taken them into account for analyses, summary of findings, or implications of the review. We would have acknowledged heterogeneity in the randomisation unit and performed a sensitivity analysis (Sensitivity analysis). We would have incorporated cross‐over trials into the meta‐analysis using results from paired analyses (Higgins 2022a). |
|
Multiple interventions We would have included all intervention groups within a study that matched the inclusion criteria. In order to overcome any unit‐of‐analysis errors for a study that could contribute multiple, correlated, comparisons, we would have performed single pair‐wise comparisons. We would have combined groups relevant for inclusion to create a single pair‐wise comparison. | |
| Dealing with missing data | If the study authors had provided us with missing data, we would have conducted a meta‐analysis according to intention‐to‐treat (ITT) principles, keeping the participants in the treatment group to which they were originally randomised, regardless of the treatment they actually received (Higgins 2022b). In addition, we would have assessed the sensitivity of any primary meta‐analysis to missing data (Deeks 2022). |
| Assessment of heterogeneity | We intended to compare the distribution of important participant characteristics (e.g. children versus adults) or study design features (e.g. cross‐over versus parallel design) with intervention characteristics (e.g. drug and dose). We would have used the Chi² test (where a P value < 0.10 would have been interpreted as statistically significant) and the I² statistic (the proportion of variation in point estimates due to heterogeneity of studies rather than sampling error); to assess the extent to which these clinical and methodological variations would have given rise to statistical heterogeneity (variability in intervention effects (Higgins 2003)). As recommended in Deeks 2022, we would have considered I² values as follows:
We would have used Tau² to estimate the between‐study variance in a random‐effects meta‐analysis (DerSimonian 1986). In addition to assessing the postulated efficacy of acetylcholine inhibitors, it is important to investigate whether its use has an impact on, and correlates with the severity of behaviours (for instance, people with verbal abilities versus those with no verbal skills; people with an associated low intelligence quotient (IQ)); the age of participants (children versus adults); different dosage and frequency of administration (once a day versus multiple daily dosing); and duration of treatment. |
| Assessment of reporting bias | If we had located 10 or more studies, we would have investigated publication bias and small‐study effects using funnel plots and statistical tests to assess asymmetry (i.e. rank correlation between standardised intervention effect and its standard error, linear regression of intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (Begg 1994; Egger 1997)). |
| Data synthesis | If data had been available for any treatment comparison from at least two included studies that were sufficiently homogeneous in terms of design and comparator, we would have performed standard pairwise meta‐analyses on the results. We would have conducted a meta‐analysis using a random‐effects model, according to the DerSimonian and Laird method (DerSimonian 1986). This approach assumes that different studies are estimating different, yet related intervention effects. To account for concerns regarding the influence of small‐study effects on the results of a meta‐analysis in which there was between‐study heterogeneity (I² > 0), we would have compared the fixed‐effect and random‐effects estimates of the intervention effect. A fixed‐effect meta‐analysis uses the inverse‐variance method, as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). The inverse‐variance method gives larger studies that have smaller standard errors more weight than smaller studies that have larger standard errors, in order to minimise the imprecision (uncertainty) of the pooled effect estimate. Had the results been similar, suggesting that any small‐study effects had little effect on the intervention estimate, we would have presented the results from the random‐effects model. Had the random‐effects model showed larger intervention effects, it might be that the intervention was more effective in the smaller studies, which would represent more weight in a random‐effects analysis (Deeks 2022). When conducting a meta‐analysis to combine the results of cross‐over trials, we would have used inverse variance methods (Higgins 2022a). If we had obtained a mixture of change scores and end of intervention scores, we would have used the (non‐standardised) MD, inverse‐variance method in RevMan 5 (Review Manager 2020), as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). We would use SMD with caution, given the assumption that between study variation in SDs reflects only differences in measurement scales and not differences in the reliability of outcome measures or variability among study populations. |
| Subgroup analysis and investigation of heterogeneity | Subgroup analysis facilitates exploration of whether an observed effect of an intervention is consistent across different groups of participants. We intended to undertake subgroup analyses to explore the possibility of differential responses to acetylcholinesterase inhibitors based on the following between‐study differences: Participant characteristics including:
Treatment variability including:
We would have followed the recommendations in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions and conducted subgroup analyses where at least 10 observations were available for each characteristic analysed (Deeks 2022). |
| Sensitivity analysis | We intended to undertake sensitivity analyses to explore the impact of the risk of bias on the overall result, that is, studies rated as high risk of bias for:
In the event of a cross‐over trial, where there were additional treatment arms that were not relevant, we would have acknowledged heterogeneity in the randomisation unit and performed a sensitivity analysis. |
Data and analyses
Comparison 1. Galantamine plus risperidone versus placebo plus risperidone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Social interaction impairment postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Repetitive and restricted behaviour postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Adverse events | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Adverse events; weight gain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Irritability postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Hyperactivity postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Donepezil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Real‐life Rating Scale (RLRS) total score postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Social communication impairment postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 Restricted and repetitive behaviours postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 Behavioural difficulties postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Attention and hyperactivity difficulties postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6 General health and function postintervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ghaleiha 2014.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group study Unit of allocation: individual Setting and method of recruitment: single‐centre clinical trial conducted at Roozbeh Hospital, a psychiatric academic hospital affiliated with Tehran University of Medical Sciences. All patients recruited from the children’s outpatient clinic of this hospital. Patients were referred by paediatricians, family physicians and parents from different parts of Tehran and different regions of Iran; therefore, there was no regional restriction for participation. Start date: April 2012 End date: January 2013 Duration of participation: 10 weeks | |
| Participants |
Inclusion criteria
Exclusion criteria
Total number randomised: out of 65 screened, 48 were randomised (galantamine + risperidone (treatment) = 25; placebo + risperidone (control) = 23); 40 completed Baseline imbalances: no significant difference between baseline scores of the two study groups on the different ABC‐C subscales Withdrawals and exclusions: 8 dropouts (treatment = 5; control = 3), due to withdrawal of consent. This occurred prior to the first baseline assessment; therefore, intention to treat analysis could not be applied to these participants. Mean age: treatment = 6.85 years (standard deviation (SD) 1.98); control = 5.9 years (SD 1.38) Sex: treatment = 20 participants (17 male); control = 20 participants (18 male) Race/ethnicity: not documented (patients referred by paediatricians, family physicians and parents from different parts of Tehran and different regions of Iran) Comorbidities: intellectual disability (treatment: mild = 6, moderate = 2; control: mild = 4, moderate = 4); epilepsy (treatment = 1; control = 2) Other relevant sociodemographics: drug history (treatment: valproic acid = 1; control valproate = 1; carbamazepine = 1 ; vigabatrin = 1; phenobarbital = 1 Hirschsprung’s disease = 1) |
|
| Interventions |
Treatment (n = 20): galantamine + risperidone The initial dosage of galantamine was 2 mg/day, which increased to 2 mg twice per day in the first week. If tolerated and clinically indicated, the daily dose of galantamine was increased weekly, in increments of 2 mg. The maximum dose of galantamine was determined based on the patients’ body weight: 12 mg/day for patients weighing less than 20 kg, 16 mg/day for patients weighing 20–30 kg, 20 mg/day for patients weighting 30–40 kg and 24 mg/day for patients weighing 40 kg. If aiming to reach the maximum dose of 24 mg/day, the last dose increment was done in 4 mg, to reach such a dosage by week 10. Other than the trial drugs, participants were not allowed to receive any concomitant medication during the course of the study. All patients were drug‐free at least 6 weeks prior to the study. Patients did not receive any behaviour therapy during the trial. The starting dose of risperidone was 0.5 mg/day, with subsequent weekly dose increases in increments of 0.5 mg. The dose of risperidone was titrated up to 1 mg/day for children weighing less than 20 kg and 2 mg/day for those with a body weight equal to or greater than 20 kg. Duration: 10 weeks Timing: daily Compliance: Medication adherence was assessed through checking with parents and conducting a pill count at each visit. Control (n =20): placebo + risperidone Placebo capsules and their ingredients were made to appear identical to galantamine capsules in coat, shape, size, texture, colour, taste and odour. The study drugs were packed in identical containers and were dispensed by an investigational drug pharmacist. The starting dose of risperidone was 0.5 mg/ day, with subsequent weekly dose increases, in increments of 0.5 mg. The dose of risperidone was titrated up to 1 mg/day for children weighing less than 20 kg and 2 mg/day for those with a body weight equal to or greater than 20 kg. Duration: 10 weeks Timing: daily Compliance: Medication adherence was assessed through checking with parents and conducting a pill count at each visit. |
|
| Outcomes |
Primary outcomes All primary outcomes were measured using subscales of the Aberrant Behavior Checklist‐Community (ABC‐C; Aman 1986).
The following subscales within the ABC‐C were used as measures of core features of Autism Spectrum Disorder (ASD) as follows:
Secondary outcomes
|
|
| Notes | Funding source(s): work was supported by Tehran University of Medical Sciences (grant number 13216 to SA). The funding organisation had no role in the design or conduct of the study; nor in the collection, analysis and interpretation of the data; nor in the preparation, review or approval of the manuscript; nor in the decision to submit the paper for publication. Declarations of interest(s): The authors declare no conflict of interest. Comment(s): contacted on 22 March 2021 at s.akhond@neda.net. Requested information on whether the authors knew of any additional trials of interest but received no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The random allocation method was used to randomly and equally assign the participants to either the galantamine or the placebo group, in a 1:1 ratio. Randomization codes were generated by Excel software by an independent person who was not involved elsewhere in the research project” (p 679) |
| Allocation concealment (selection bias) | Low risk | Quote: “Assignments were kept in sequentially‐numbered, sealed, opaque envelopes and were opened sequentially, only after the participant details were written on the envelope. Aluminium foil inside the envelope rendered the envelope impermeable to intense light. Separate persons were responsible for rating and random allocation of the patients” (p 679) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The patients, their parents and the physicians who referred them were all blind to the treatment assignments, so were the research investigators and the person who administered the medications. Placebo capsules and their ingredients were made to be identical to galantamine capsules in coat, shape, size, texture, colour, taste and odour. The study drugs were packed in identical containers and were dispensed by an investigational drug pharmacist. By study completion, the parents and investigators filled out a questionnaire that aimed to assess the effectiveness of our blinding strategy” (p 679) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “In most cases (more than one‐half), the parents and the investigators were uncertain about the assigned trial group. In other cases, there was no significant difference between the rates of placebo or active treatment guesses” (p 680) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Overall, eight patients (placebo = 3; galantamine = 5) dropped out from the trial, due to withdrawal of consent. Because all of the dropouts occurred before the first post‐baseline visit, we could not apply the intention‐to‐treat analysis and the related data were omitted” (p 680) |
| Selective reporting (reporting bias) | Low risk | Comment: all data stipulated to be reported in the protocol were reported |
| Other bias | Low risk | Comment: no other potential sources of bias were identified |
Handen 2010.
| Study characteristics | ||
| Methods | Study design: double‐blind, placebo‐controlled, parallel‐group design Unit of allocation: randomisation was conducted by the study pharmacist and the sample was stratified based upon Tanner stage (stages 1 and 2 and 4 and 5) and gender Setting and method of recruitment: unclear Start date: unclear End date: unclear Duration of participation: 10 weeks (with a further 10 weeks open label for placebo group) | |
| Participants |
Inclusion criteria
Exclusion criteria: unclear Total number randomised: 34 (donepezil (treatment) = 18; placebo (control) = 16), with 31 able to complete protocol as designed Baseline imbalances: no statistically significant between‐group differences found for age, gender, IQ, or ADOS scores Withdrawals and exclusions: 3 withdrawals (2 unable to tolerate 10 mg/day and maintained 5 mg/day; 1 terminated due to increased aggression and irritability) Mean age: treatment = 11 years 6 months; control = 11 years 8 months Sex: treatment = 18 participants (17 boys); control = 16 participants (14 boys) Race/ethnicity: treatment = 17 Caucasian, 1 Other; control = 14 Caucasian, 1 African American, 1 Other Comorbidities: of the 34 participants, 11 were prescribed a single concomitant medication during the trial; 2 participants were prescribed atomoxetine, 5 participants an SSRI, and 4 participants stimulants. All concomitant medication trials were initiated before participant enrolment and all medication doses were maintained during the donepezil trial. Other relevant sociodemographics: unclear |
|
| Interventions |
Treatment (N = 18): donepezil Dosing began at 2.5 mg/day and was increased to 5.0 mg/day after a 1‐week period. Participants were re‐evaluated after 4 weeks at the 5 mg/day dose. Doses were subsequently increased to 7.5 mg/day for a 1‐week period and then titrated to 10.0 mg/day for the final 4 weeks of the double‐blind trial. A second re‐evaluation was conducted following 4 weeks on the 10 mg/day dose. In addition to the re‐evaluation visits, participants were seen at weeks 1 and 6 to assess safety and to determine if the person was able to have his/her medication titrated to the next dose level (these assessments were conducted by the author, AYH, as well as by other psychiatrists associated with the study). If side effects were reported that were determined to be interfering with a participant's functioning or well‐being, the medication dose was reduced to the previous highest tolerated dose and maintained at that level for the remainder of the study. If problems persisted, a final re‐evaluation was completed at that time and the medication discontinued. Families were asked to complete daily logs, track when medication was given, and to note if there were any problems, to assess compliance with the medication regimen. Concomitant medications (e.g. aspirin, cold medicine) were also tracked on the daily log. Families were required to return the medication bottles at each visit to conduct a pill count. The hospital research pharmacy packed all medication. At the conclusion of the 10‐week re‐evaluation, all participants who had been placed on placebo were offered a 10‐week open‐label trial (involving the same titration schedule as used in the double‐blind study). Participants participating in the open‐label study were re‐evaluated at week 5 (5 mg/day dose) and week 10 (10 mg/ day dose). Duration: 10 weeks Timing: daily Compliance: daily logs completed; return medication for pill count at each visit Control (N = 16): pill placebo; administered in the same daily regimen as the treatment medication Duration: 10 weeks Timing: daily Compliance: daily logs completed; return medication for pill count at each visit |
|
| Outcomes |
Primary outcomes All primary outcomes of interest were measured using subscales within the modified version of The Real Life Rating Scale (RLRS (Freeman 1986; Ritvo 2013)).
The following subscales within RLRS were used as measures of core features of Autism Spectrum Disorder.
Secondary outcomes Secondary outcomes were measured using subscales of the RLRS (method of measurement described above), the Child Behavior Checklist (CBCL (Achenbach 2001)) and the Clinical Global Impression Scale (CGI (Guy 1976)).
Specifically, the following secondary outcomes were measured using the following subscales
|
|
| Notes |
Funding source(s): research funding for authors Handen and Hardan
Declarations of interest(s)
Comment(s)
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Comment: the process of randomisation was unclear Quote: "Following a one‐week baseline, subjects were randomly assigned to either active medication or placebo. The study pharmacist conducted the randomization for each subject based upon Tanner stage (stages 1 and2 and 4 and 5) and gender" (p 128) |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomisation was reported to be completed by a pharmacist at an assumed distance to the trial team. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Comment: allocation was completed by a pharmacist, at an assumed distance to the trial team. Quote: "Medication was packed by the hospital research pharmacy, with both active medication and placebo packed in opaque capsules" (p 128) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Comment: the report did not state whether the person doing the analysis remained blinded Quote: "One subjected terminated due to an increase in aggression and irritability. Two other subjects were unable to tolerate the 10 mg/day dose and were maintained on a 5 mg/day dose" (p129). Comment: those who withdrew due to side effects had results carried forward. The report did not state which personnel was responsible for this and whether blinding was maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Comment: intention‐to‐treat (ITT) was stated (see quote below). However, the N for the ITT analyses was not reported; the report stated that some did not complete (3/34). It is not clear if other participants had missing data for any reason. Quote: "For subjects who did not complete the entire 10‐week protocol due to the appearance of significant side effects, an intention to treatment approach was employed by the last observation being carried forward" (p129). |
| Selective reporting (reporting bias) | High risk | Comment: the trial outcomes were reported in several records; only behavioural outcomes were reported in the primary record. |
| Other bias | High risk |
Comment: the trial was partly supported by pharmaceutical companies. Quote: "This study was supported by an NIMHS grant... as well as by a gift by Pfizer and Eisai Pharmaceutical Companies (who also provided the medication and placebo for this trial)" (p132‐3) |
DSM IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; IQ: intelligence quotient; SSRI: selective serotonin reuptake inhibitor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnold 2012 | Ineligible intervention (mecamylamine) |
| Chez 2003 | Ineligible study design (used deterministic method to allocate to condition) |
| Gabis 2019 | Ineligible intervention (active comparator) |
| NCT01887132 | Study terminated |
| Sahu 2013 | Ineligible participants (fragile x syndrome with no diagnosis of autism) |
Characteristics of studies awaiting classification [ordered by study ID]
Niederhofer 2002.
| Methods | Design: placebo controlled, double‐blind cross‐over randomised controlled trial Unit of randomisation: not reported Duration: not reported |
| Participants | Location/Setting: outpatient clinic, location not reported Sample size: 20 Number of withdrawals/dropouts: not reported Sex: all boys Mean age: 7.4 (SD 3.2) Inclusion criteria:
Exclusion Criteria: |
| Interventions | Treatment (N = not reported): galantamine dosage not reported Control (N = not reported): placebo Administration: not reported |
| Outcomes | Primary outcomes: combined parent and teacher scores on subscales of Aberrant Behaviour Checklist Timing of outcome assessment: unspecified time during treatment |
| Notes | Funding sources: not reported Declarations of interest: not reported Title registration link: none Comment(s): none |
N: number
Characteristics of ongoing studies [ordered by study ID]
IRCT2017041333406N1.
| Study name | Public title: The efficacy of augmentation donepezil to risperidone in treatment of autism spectrum disorders Scientific title: Assessment the efficacy of augmentation donepezil to risperidone in treatment of autism spectrum disorders |
| Methods | Design: randomised controlled trial Unit of randomisation: parallel Duration: 12 weeks |
| Participants |
Location/Setting: Iran
Sample size: 66 (target)
Number of withdrawals/dropouts: not reported
Sex: not reported
Mean age: not reported
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment (N = 33): 2 mg risperidone plus donepezil (titrating from 2.5 mg to 10 mg) Control (N = 33): 2 mg risperidone plus placebo Administration: not reported |
| Outcomes |
Primary outcomes
Timing of outcome assessment: baseline, 6 weeks and 12 weeks |
| Starting date | Start date: not reported. Registered on 20 September 2017 |
| Contact information | Name: Neda Ghanei Name of organization/entity: Mashhad University of Medical Sciences Country: Iran (Islamic Republic of) Phone: + 98 51 3711 2721 Email address: ghanein931@mums.ac.ir |
| Notes | Funding source(s): Vice Chancellor for Research of Mashhad University of Medical Sciences Declarations of interest(s): not available Trial registration link:en.irct.ir/trial/25769?revision=25769 Comment(s): contacted on 19 March 2021 to request any results of the study and whether it was still recruiting but no response received |
NCT00252603.
| Study name | Galantamine versus placebo in childhood autism |
| Methods | Design: double‐blind, randomised control trial Unit of randomisation: parallel Duration: 12 weeks |
| Participants |
Location/setting: not reported
Sample size: 20
Number of withdrawals/dropouts: not reported
Sex: not reported
Mean age: not reported
Inclusion criteria
Exclusion criteria
|
| Interventions |
Treatment (N = not reported): galantamine
Control (N = not reported): placebo
Administration: Participants will be assigned by chance to receive either the active medication (Galantamine) or placebo (sugar pill) for 12 weeks. The parent/child and investigator will be blinded to treatment allocation. The child has a 50% chance of being assigned to receive placebo or the active medication, galantamine, during the study. The child is seen weekly by the study psychiatrist for the first 4 weeks of the 12‐week study, and every other week for the remaining weeks of the study. During these visits the study psychiatrist will ask the parent for feedback on his/her child's condition and any changes that may be related to the medication, including possible side effects, and will check the child's condition. The psychiatrist will also record his/her weight. |
| Outcomes |
|
| Starting date | April 2004 |
| Contact information | Name: Sherie Novotny, MD Name of organization/entity: Rutgers, The State University of New Jersey Country: USA Phone: not reported Email address: not reported |
| Notes | Funding sources: sponsored by the University of Medicine and Dentistry of New Jersey in collaboration with the National Alliance for Autism Research Declarations of interest: not reported Trial registration link:clinicaltrials.gov/ct2/show/NCT00252603 Comment(s): none |
Rahman 2018.
| Study name | Alpha‐7 nicotinic acetylcholine receptor positive allosteric modulator galantamine in autism spectrum disorder |
| Methods | Design: randomised, three‐arm, single dose challenge Unit of randomisation: not reported Duration: not reported |
| Participants |
Location/Setting: unclear
Sample size: 18 children with ASD
Number of withdrawals/dropouts: not reported
Sex: not reported
Mean age: not reported
Inclusion criteria
Exclusion criteria: not reported |
| Interventions | Treatment (N = not reported): 12 mg galantamine or 24 mg galantamine Control (N = not reported): placebo Administration: not reported |
| Outcomes | Primary outcomes: Target engagement, assessed using a P50 inhibitory paradigm, with additional assessments (RBANS, ABC‐I, RBS‐R, ASR‐R, CGI‐I) performed for correlation with electrophysiologic findings. No further information about this outcome variable was reported. Timing of outcome assessment: unclear |
| Starting date | Unclear |
| Contact information | Name: Asif Rahman Name of organisation/entity: Albert Einstein College of medicine and Montefiore Medical Center Country: USA Phone: not available Email address: not available |
| Notes | Funding sources: not reported Declarations of interest: not reported Title registration link: none Comment(s): none |
ABC(‐I): Aberrant Behavioral Checklist, Irritability Subscale; ADI‐R: Autism Diagnostic Interview Revised; ADOS‐2: Autism Diagnostic Observation Schedule Second Edition; ADOS‐G: Autism Diagnostic Observation Schedule‐Generic; ASD: Autism Spectrum Disorder; ASR‐R: Adult Self Report ‐ Revised; CGI‐I: Clinical Global Impression‐Improvement; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; IQ: Intelligence Quotient; n: number; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status Update; RBS‐R: Repetitive Behaviors Scale‐Revised.
Differences between protocol and review
We were not able to use all of our preplanned methods (Cox 2021). Please see Appendix 2 for a summary of methods outlined in the protocol that were unused in the review. In addition to the primary outcome measures identified in our protocol, we added 'overall autism features' to incorporate all relevant subdomains of outcome measures in our included studies. We also included secondary outcomes: irritability, hyperactivity, and general health and function in the summary of findings table, as these factors have been identified as important indicators of progress and outcomes by the autism community (McConachie 2018).
Contributions of authors
All authors contributed to the development of this review and provided input based on their expertise.
Alexandra Ure has overall responsibility and is the guarantor for this review. She contributed to the design of review, co‐ordination of the review; search and selection of studies to include in the review; collection of data for the review; assessment of the risk of bias in the included studies; analysis of data; assessment of the certainty in the body of evidence; interpretation of data, and writing of the review.
Georgina Cox contributed to the design of review, co‐ordination of the review, search and selection of studies to include in the review, assessment of the risk of bias in the included studies, analysis of data, assessment of the certainty in the body of evidence, interpretation of data, and writing of the review.
Richard Haslam contributed to interpretation of data, and writing of the review.
Katrina Williams contributed to conception of the review, design of the review, assessment of the risk of bias in the included studies, analysis of data, assessment of the certainty in the body of evidence; interpretation of data, and writing of the review.
Sources of support
Internal sources
-
Monash Health, Australia
Salary for AU and KW
-
Department of Paediatrics, Monash University, Australia
Salary for AU and KW
-
Royal Children's Hospital, Australia
Salary for GC, JW, and RH
External sources
-
none to declare, Other
none
Declarations of interest
Alexandra Ure (AU) is a Senior Psychologist with Monash Children's Hospital, where she is involved in child developmental assessments and the supervision of staff and students. She is also a Senior Research Fellow with Monash University, with an interest in complex neurodevelopmental disorders, including autism spectrum disorder. AU declares a Clinical Sciences Fellowship from Monash University for her work to embed research into new clinical service, involving assessments of children presenting with neurodevelopmental concerns and their families; paid to Monash University.
Georgina Cox (GC) is an Editor with Cochrane Developmental, Psychosocial and Learning Problems (DPLP) and Cochrane Common Mental Disorders. GC was not involved in the editorial process for this review.
Richard Haslam is the Director of Mental Health at the Royal Children's Hospital, Melbourne (salaried position). He has declared that he has no conflicts of interest.
Katrina Williams (KW) reports a grant from the National Health and Medical Research Council (NHMRC) for a phase III trial of cannabidiol for severe behaviour disorders in children with an intellectual disability, with or without autism, on which she is Chief Investigator (1 January 2020 to 31 May 2023); paid to institution, but KW benefited from this payment, or had access to the funds. KW also reports contracts with Epsilon Healthcare (formerly THC Global Group Ltd; ongoing since 1 January 2020), to develop an interventional product and placebo for children, which is being provided by the Victorian Government for a Medical Research Future Fund funded phase III multisite trial; and with Tilray (from 28 November 2018 to 27 November 2019) for the investigation drug and placebo for a pilot trial of cannabidiol for severe behaviour problems in children with intellectual disability, with or without autism; both paid to the institution. KW reports a grant from the NHMRC for an ongoing prognosis study about predictors of autism outcome, which will also publish diagnostic stability outcomes, and which could be included in an update of this systematic review; paid to the institution. Lastly, KW reports that she is an Editor with DPLP; however, she was not involved in the editorial process for this review.
New
References
References to studies included in this review
Ghaleiha 2014 {published data only}IRCT201204081556N40
- Ghaleiha A, Ghyasvand M, Mohammadi M-R, Farokhnia M, Yadegari N, Tabrizi M, et al. Galantamine efficacy and tolerability as an augmentative therapy in autistic children: a randomized, double-blind, placebo-controlled trial. Journal of Psychopharmacology 2014;28(7):677-85. [DOI: 10.1177/0269881113508830] [DOI] [PubMed] [Google Scholar]
- IRCT201204081556N40. Galantamine in the treatment of autism [Galantamine added to risperidone in the treatment of autism: a double blind and placebo controlled trial]. en.irct.ir/trial/890?revision=890 (first received 14 April 2012).
Handen 2010 {published data only}
- Handen BJ, Johnson CR, McAuliffe-Bellin S, Hardan A. Safety and efficacy of donepezil in children and adolescents with autism: behavioral measures. International Public Health Journal 2010;2(1 - Special Issue):125-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handen BL, Johnson CR, McAuliffe-Bellin S, Murray PJ, Hardan AY. Safety and efficacy of donepezil in children and adolescents with autism: neuropsychological measures. Journal of Child & Adolescent Psychopharmacology 2011;21(1):43-50. [DOI: 10.1089/cap.2010.0024] [PMCID: PMC3037196] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Johnson C, Handen BL. 161. Preliminary findings from a double-blind placebo-controlled study of donepezil in pervasive developmental disorders. Biological Psychiatry 2006;59(Suppl 8):50S. [DOI: 10.1016/j.biopsych.2006.03.005] [DOI] [Google Scholar]
- NCT00047697. Donepezil HCl & cognitive deficits in autism [Donepezil HCl: treating cognitive deficits in autism]. clinicaltrials.gov/show/NCT00047697 (first received 11 October 2002).
References to studies excluded from this review
Arnold 2012 {published data only}
- Arnold LE, Aman MG, Hollway J, Hurt E, Bates B, Li X, et al. Placebo-controlled pilot trial of mecamylamine for treatment of autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology 2012;22(3):198-205. [DOI: 10.1089/cap.2011.0056] [PMCID: PMC3417385] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chez 2003 {published data only}
- Chez MG, Buchanan TM, Becker M, Kessler J, Aimonovitch MC, Mrazek SR. Donepezil hydrochloride: a double-blind study in autistic children. Journal of Pediatric Neurology 2003;1(2):83-8. [DOI: 10.1055/s-0035-1557175] [URL: tinyurl.com/694xrxmv] [DOI] [Google Scholar]
- Chez MG, Tremb RJ, Nowinski CV, Field-Chez M. Double-blinded placebo-controlled aricept study in children with autistic spectrum disorder. Annals of Neurology 2001;50(Suppl 3):S60-1. [DOI: 10.1002/ana.1189] [DOI] [Google Scholar]
Gabis 2019 {published data only}
- Gabis LV, Ben-Hur R, Shefer S, Jokel A, Shalom DB. Improvement of language in children with autism with combined donepezil and choline treatment. Journal of Molecular Neuroscience 2019;69(2):224‐34. [DOI: 10.1007/s12031-019-01351-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01098383. Treatment with acetyl-choline esterase inhibitors in children with autism spectrum disorders [Treatment with acetyl-choline esterase inhibitors in children with autism]. clinicaltrials.gov/show/NCT01098383 (first received 11 March 2010).
NCT01887132 {published data only}
- NCT01887132. A trial of the drug donepezil for sleep enhancement and behavioral change in children with autism [A randomized controlled trial of donepezil for REM enhancement and behavioral change in autism]. clinicaltrials.gov/ct2/show/NCT01887132 (first received 22 June 2013).
Sahu 2013 {published data only}
- Sahu JK, Gulati S, Sapra S, Arya R, Chauhan S, Chowdhury MR, et al. Effectiveness and safety of donepezil in boys with fragile x syndrome: a double-blind, randomized, controlled pilot study. Journal of Child Neurology 2013;28(5):570‐5. [DOI: 10.1177/0883073812449381] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Niederhofer 2002 {published data only}
- Niederhofer H, Staffen W, Mair A. Galantamine may be effective in treating autistic disorder. BMJ 2002;325(7377):1422. [DOI: 10.1136/bmj.325.7377.1422/a] [PMCID: PMC1124870] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
IRCT2017041333406N1 {published data only}IRCT2017041333406N1
- IRCT2017041333406N1. The efficacy of augmentation donepezil to risperidon in treatment of autism spectrum disorders [Assessment the efficacy of augmentation donepezil to risperidon in treatment of autism spectrum disorders]. en.irct.ir/trial/25769 (first received 20 September 2017).
NCT00252603 {published data only}
- NCT00252603. Galantamine versus placebo in childhood autism. clinicaltrials.gov/ct2/show/NCT00252603 (first received 9 November 2005).
Rahman 2018 {published data only}
- Rahman A, Freedman R, Hollander E. S59. Alpha-7 nicotinic acetylcholine receptor positive allosteric modulator galantamine in autism spectrum disorder. Biological Psychiatry 2018;83(Suppl 9):S369‐70. [DOI: 10.1016/j.biopsych.2018.02.950] [DOI] [Google Scholar]
Additional references
Achenbach 2001
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles: An Integrated System of Multi-informant Assessment. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families, 2001. [Google Scholar]
Aman 1986
- Aman MG, Singh NN. Aberrant Behavior Checklist: Manual. East Aurora (NY): Slosson Educational Publications, 1986. [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Arlington, VA: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd Revision edition. Arlington, VA: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Arlington, VA: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). Vol. 4th, Text Revision. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
Arnold 2000
- Arnold LE, Aman MG, Martin A, Collier-Crespin A, Vitello B, Tierney E, et al. Assessment in multisite randomized clinical trials (RCTs) of patients with autistic disorder: the Autism RUPP Network. Journal of Autism and Developmental Disordorders 2000;30:99-111. [DOI] [PubMed] [Google Scholar]
Baron 2000
- Baron IS. Behavior rating inventory of executive function. Child Neuropsychology 2000;6(3):235–8. [DOI: 10.1076/chin.6.3.235.3152] [PMID: ] [DOI] [PubMed] [Google Scholar]
Begeer 2013
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, et al. Sex differences in the timing of identification among children and adults with autism spectrum disorder. Journal of Autism and Developmental Disorders 2013;43(5):1151-6. [DOI: 10.1007/s10803-012-1656-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088–101. [DOI: 10.2307/2533446] [DOI] [PubMed] [Google Scholar]
Bodfish 2000
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders 2000;30(3):237-43. [DOI: 10.1023/a:1005596502855] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brugha 2015
- Brugha TS, Doos L, Tempier A, Einfeld S, Howlin P. Outcome measures in intervention trials for adults with autism spectrum disorders; a systematic review of assessments of core autism features and associated emotional and behavioural problems. International Journal of Methods in Psychiatric Research 2015;24(2):99–115. [DOI: 10.1002/mpr.1466] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckley 2011
- Buckley AW, Sassower K, Rodriguez AJ, Jennison K, Wingert K, Buckley J, et al. An open label trial of donepezil for enhancement of rapid eye movement sleep in young children with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology 2011;21(4):353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chawarska 2013
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with ASD. Biological Psychiatry 2013;74(3):195-203. [DOI: 10.1016/j.biopsych.2012.11.022] [PMC3646074] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conners 2008
- Conners CK. Conners Manual. 3rd edition. Toronto (ON): Multi-Health Systems, 2008. [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 7 December 2020. Melbourne, Australia: Veritas Health Innovation, 2020. Available at covidence.org.
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Delis 1994
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Children’s Version Manual. San Antonio (TX): Psychological Corporation, 1994. [Google Scholar]
Delis 2001
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System Technical Manual. San Antonio (TX): Psychological Corporation, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI: 10.1016/0197-2456(86)90046-2] [DOI] [PubMed] [Google Scholar]
Deutsch 2020
- Deutsch SI, Burket JA. An evolving therapeutic rationale for targeting the α7 nicotinic acetylcholine receptor in autism spectrum disorder. Current Topics in Behavioral Neurosciences 2020;45:167-208. [DOI: 10.1007/7854_2020_136] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith G D, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsabbagh 2012
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Research 2012;5(3):160-79. [DOI: 10.1002/aur.239] [PMC3763210] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farmer 2013
- Farmer C, Thurm A, Grant P. Pharmacotherapy for the core symptoms in autistic disorder: current status of the research. Drugs 2013;73(4):303-14. [DOI: 10.1007/s40265-013-0021-7] [PMC3632301] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher‐Watson 2014
- Fletcher-Watson S, McConnell F, Manola E, McConachie H. Interventions based on the Theory of Mind cognitive model for autism spectrum disorder (ASD). Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD008785. [DOI: 10.1002/14651858.CD008785.pub2] [PMC6923148] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 1986
- Freeman BJ, Ritvo ER, Yokota A, Ritvo A. A scale for rating symptoms of patients with the syndrome of autism in real life settings. Journal of the American Academy for Child and Adolescent Psychiatry 1986;25(1):130-6. [DOI] [PubMed] [Google Scholar]
Gabis 2019
- Gabis LV, Ben-Hur R, Shefer S et al. Improvement of language in children with autism with combined donepezil and choline treatment. Journal of Molecular Neuroscience 2019;69(2):224–34. [DOI] [PubMed] [Google Scholar]
Genovese 2020
- Genovese A, Butler MG. Clinical assessment, genetics, and treatment approaches in autism spectrum disorder (ASD). International Journal of Molecular Sciences 2020;21(13):4726. [DOI: 10.3390/ijms21134726] [PMC7369758] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 7 December 2020. Available at gradepro.org.
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): National Institute of Mental Health, 1976. [Google Scholar]
Happé 2006
- Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature Neuroscience 2006;9(10):1218-20. [DOI: 10.1038/nn1770] [PMID: ] [DOI] [PubMed] [Google Scholar]
Henninger 2013
- Henninger NA, Taylor JL. Outcomes in adults with autistic spectrum disorder: a historical perspective. Autism 2013;17(1):103-16. [DOI: 10.1177/1362361312441266] [PMC3769945] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2. (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022a
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Higgins 2022b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hirsh 2016
- Hirsh LE, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD009043. [DOI: 10.1002/14651858.CD009043.pub3] [PMC7120220] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howes 2018
- Howes OD, Rogdaki M, Findon JL, Wichers RH, Charman T, King BH, et al. Autism spectrum disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. Journal of Psychopharmacology 2018;32(1):3-29. [DOI: 10.1177/0269881117741766] [PMCID: PMC5805024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jann 2002
- Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clinical Pharmacokinetics 2002;41(10):719-39. [DOI: 10.2165/00003088-200241100-00003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jesner 2007
- Jesner OS, Aref-Adib M, Coren E. Risperidone for autism spectrum disorder. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD005040. [DOI: 10.1002/14651858.CD005040.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karahmadi 2018
- Karahmadi M, Tarrahi M, Vatankhah AS, Omranifard V, Farzaneh B. Efficacy of memantine as adjunct therapy for autism spectrum disorder in children aged < 14 years. Advanced Biomedical Research 2018;7:131. [DOI: 10.4103/abr.abr_100_18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karvat 2014
- Karvat G, Kimchi T. Acetylcholine elevations relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 2014;39(4):831-40. [DOI: 10.1038/npp.2013.274] [PMC3924518] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ke 2018
- Ke F, Whalon K, Yun J. Social skill interventions for youth and adults with autism spectrum disorder: a systematic review. Review of Educational Research 2018;88(1):3-42. [DOI: 10.3102/0034654317740334] [DOI] [Google Scholar]
Kumar 2012
- Kumar B, Prakash A, Sewal RK, Medhi B, Modi M. Drug therapy in autism: a present and future perspective. Pharmacological Reports 2012;64(6):1291-304. [DOI: 10.1016/s1734-1140(12)70927-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lai 2014
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014;383(9920):896-910. [DOI: 10.1016/S0140-6736(13)61539-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Le Couteur 2003
- Le Couteur A, Lord C, Rutter M. Autism Diagnostic Interview-Revised. Los Angeles (CA): Western Psychological Services, 2003. [Google Scholar]
Lee 2002
- Lee M, Martin-Ruiz C, Graham A, Court J, Jaros E, Perry R, et al. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 2002;125(Pt 7):1483-95. [DOI: 10.1093/brain/awf160] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee YJ, Oh SH, Park C, Hong M, Lee AR, Yoo HJ, et al. Advanced pharmacotherapy evidenced by pathogenesis of autism spectrum disorder. Clinical Psychopharmacology and Neuroscience 2014;12(1):19-30. [DOI: 10.9758/cpn.2014.12.1.19] [PMC4022762] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from: www.training.cochrane.org/handbook.
Li 2022
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Limbu 2022
- Limbu B, Deb S, Roy M, Lee R, Roy A, Taiwo O. Randomised controlled trials of mood stabilisers for people with autism spectrum disorder: systematic review and meta-analysis. BJPsych Open 2022;8(2):e52. [DOI: 10.1192/bjo.2022.18] [PMCID: PMC8935918] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingstone 2015
- Livingstone N, Macdonald G, Williams K, Caldwell DM, Baker LB, Hazell P. Pharmacological intervention for irritability, aggression, and self-injury in autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD011769. [DOI: 10.1002/14651858.CD011769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loomes 2017
- Loomes R, Hull L, Mandy WP. What Is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(6):466-74. [DOI: 10.1016/j.jaac.2017.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lord 1994
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659-85. [DOI: 10.1007/BF02172145] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lord 1999
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observational Schedule. Los Angeles (CA): Western Psychological Services, 1999. [Google Scholar]
Lord 2012
- Lord C, Luyster RJ, Gotham K, Guthrie W. ADOS-2: Autism Diagnostic Observation Schedule Manual. Part II: Toddler Module. 2nd edition. Torrance (CA): Western Psychological Services, 2012. [Google Scholar]
Maenner 2020
- Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2016. Morbidity and Mortality Weekly Report. Surveillance Summaries 2020;69(4):1-12. [DOI: 10.15585/mmwr.ss6904a1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matson 2013
- Matson JL, Adams HL, Williams LW, Rieske RD. Why are there so many unsubstantiated treatments in autism? Research in Autism Spectrum Disorder 2013;7:466-474. [Google Scholar]
May 2018
- May T, Williams K. Brief report: gender and age of diagnosis time trends in children with autism using Australian medicare data. Journal of Autism and Developmental Disorders 2018;48(12):4056-62. [DOI: 10.1007/s10803-018-3609-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mazurek 2014
- Mazurek MO, Handen BL, Wodka EL, Nowinski L, Butter E, Engelhardt CR. Age at first autism spectrum diagnosis: the role of birth cohort, demographic factors and clinical features. Journal of Developmental Behavioral Pediatrics 2014;35(9):561-9. [DOI: 10.1097/DBP.0000000000000097] [PMID: ] [DOI] [PubMed] [Google Scholar]
McConachie 2018
- McConachie H, Livingstone N, Morris C, Beresford B, Le Couteur A, Gringras P, et al. Parents suggest which indicators of progress and outcomes should be measured in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders 2018;48(4):1041–51. [DOI: 10.1007/s10803-017-3282-2] [PMCID: PMC5861173] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00047697
- NCT00047697. Donepezil HCl & cognitive deficits in autism [Donepezil HCl: treating cognitive deficits in autism]. clinicaltrials.gov/ct2/show/NCT00047697 (first received 16 October 2017).
NICE 2021
- National Institute for Health and Care Excellence (NICE). Autism spectrum disorder in under 19s: support and management. NICE clinical guideline ‒ CG170; first published August 2013; updated June 2021. www.nice.org.uk/Guidance/CG170 (accessed 23 November 2022).
Nicolson 2006
- Nicolson R, Craven-Thuss B, Smith J. A prospective, open-label trial of galantamine in autistic disorder. Journal of Child and Adolescent Psychopharmacology 2006;16(5):621-9. [DOI] [PubMed] [Google Scholar]
Niederhofer 2003
- Niederhofer H. Acetylcholinesterase-inhibitors in the treatment of autistic disorders. Expert Review of Neurotherapeutics 2003;3(4):409-12. [DOI: 10.1586/14737175.3.4.409] [19810924] [DOI] [PubMed] [Google Scholar]
Ozonoff 2010
- Ozonoff S, Iosif A-M, Baguio F, Cook IC, Moore Hill M, Hutman T, et al. A prospective study of the emergence of early behavioural signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry 2010;49(3):256-66.e1-2. [DOI: 10.1016/j.jaac.2009.11.009] [PMC2923050] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandolfi 2014
- Pandolfi V, Magyar CI, Norris M. Validity study of the CBCL 6–18 for the assessment of emotional problems in youth with ASD. Journal of Mental Health Research in Intellectual Disabilities 2014;7(4):306-22. [DOI: 10.1080/19315864.2014.930547] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2001
- Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, et al. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. American Journal of Psychiatry 2001;158(7):1058-66. [DOI: 10.1176/appi.ajp.158.7.1058] [PMID: ] [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385-401. [DOI: 10.1177/014662167700100306] [DOI] [Google Scholar]
Reichow 2012
- Reichow B, Steiner AM, Volkmar F. Social skills groups for people aged 6 to 21 with autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD008511. [DOI: 10.1002/14651858.CD008511.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reichow 2018
- Reichow B, Hume K, Barton EE, Boyd BA. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD009260. [DOI: 10.1002/14651858.CD009260.pub3] [PMC6494600] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web 2023 [Computer program]
- Review Manager (RevMan). Version 5.2. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Reynolds 2015
- Reynolds CR, Kamphaus RW. Behavior Assessment for Children. 3rd edition. Bloomington (MN): Pearson, 2015. [Google Scholar]
Ritvo 2013
- Ritvo E. Real-life Rating Scale. In: Volkmar FR, editors(s). Encyclopedia of Autism Spectrum Disorders. New York: Springer, 2013. [DOI: 10.1007/978-1-4419-1698-3_1580] [DOI] [Google Scholar]
Rossignol 2014
- Rossignol D, Frye RE. The use of medications approved for Alzheimer's disease in autism spectrum disorder: a systematic review. Frontiers in Pediatrics 2014;2:87. [DOI: 10.3389/fped.2014.00087] [PMC4141213] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roux 2013
- Roux AM, Shattuck PT, Cooper BP, Anderson KA, Wagner M, Narendorf SC. Postsecondary employment experiences among young adults with an autistic spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2013;52(9):931-9. [DOI: 10.1016/j.jaac.2013.05.019] [PMC3753691] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutter 2003a
- Rutter M, Le Couteur A, Lord C. ADI-R: Autism Diagnostic Interview-Revised. Los Angeles (CA): Western Psychological Services, 2003. [Google Scholar]
Rutter 2003b
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire Manual. Los Angeles (CA): Western Psychological Services, 2003. [Google Scholar]
Schopler 1994
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Los Angeles (CA): Western Psychological Services, 1994. [Google Scholar]
Schünemann 2020
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI: 10.1016/j.jclinepi.2019.12.020] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sevin 1991
- Sevin JA, Matson JL, Coe DA, Fee VE, Sevin BM. A comparison and evaluation of three commonly used autism scales. Journal of Autism and Developmental Disorders 1991;21:417–32. [DOI] [PubMed] [Google Scholar]
Siafis 2022
- Siafis S, Çıray O, Wu H, Schneider-Thoma J, Bighelli I, Krause M, et al. Pharmacological and dietary-supplement treatments for autism spectrum disorder: a systematic review and network meta-analysis. Molecular Autism 2022;13(1):10. [DOI: 10.1186/s13229-022-00488-4] [PMCID: PMC8896153] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skuse 2004
- Skuse D, Warrington R, Bishop D, Chowdhury U, Lau J, Mandy W, et al. The developmental, dimensional and diagnostic interview (3di): a novel computerized assessment for autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(5):548-58. [DOI: 10.1097/00004583-200405000-00008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sparrow 1989
- Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, editors(s). Major Psychological Assessment Instruments. Vol. 2. Needham Heights (MA): Allyn & Bacon, 1989:199-231. [Google Scholar]
Spence 1997
- Spence SH. Structure of anxiety symptoms among children: a confirmatory factor-analytic study. Journal of Abnormal Psychology 1997;106(2):280-97. [DOI: 10.1037//0021-843x.106.2.280] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sturman 2017
- Sturman N, Deckx L, Van Driel ML. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD011144. [DOI: 10.1002/14651858.CD011144.pub2] [PMC6486133] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanner 2020
- Tanner A, Dounavi K. The emergence of autism symptoms prior to 18 months of age: a systematic literature review. Journal of Autism and Developmental Disorders 2020 Jul 30 [Epub ahead of print]. [DOI: 10.1007/s10803-020-04618-w] [PMID: ] [DOI] [PMC free article] [PubMed]
Taylor 2015
- Taylor JL, Henninger NA, Mailich MR. Longitudinal patterns of employment and postsecondary education for adults with autism and average-range IQ. Autism 2015;19(7):785-93. [DOI: 10.1177/1362361315585643] [PMC4581899] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Varni 2001
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care 2001;39(8):800-12. [DOI: 10.1097/00005650-200108000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weitlauf 2014
- Weitlauf AS, McPheeters ML, Peters B, Sathe N, Travis R, Aiello R, et al. Therapies for Children with Autism Spectrum Disorder: Behavioral Interventions Update. Rockville (MD): Agency for Healthcare Research and Quality (US), 2014. [NBK241444] [PMID: ] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. apps.who.int/iris/handle/10665/37108 1993.
WHO 2018
- World Health Organization. ICD-11: International Classification of Diseases for Mortality and Morbidity Statistics. Eleventh Revision. icd.who.int/icd11refguide/en/index.html (accessed 8 December 2020).
Williams 2013
- Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004677. [DOI: 10.1002/14651858.CD004677.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wing 2006
- Wing L. Diagnostic Interview for Social and Communication Disorders. 11th edition. Bromley: Centre for Social and Communication Disorders, 2006. [Google Scholar]
Yoo 2007
- Yoo JH, Valdovinos MG, Williams DC. Relevance of donepezil in enhancing learning and memory in special populations: a review of the literature. Journal of Autism and Developmental Disorders 2007;37(10):1883-901. [DOI: 10.1007/s10803-006-0322-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cox 2021
- Cox GR, Williams K, Woon JM, Haslam R, Ure AM. Acetylcholinesterase inhibitors for autistic spectrum disorders. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013851. [DOI: 10.1002/14651858.CD013851] [DOI] [PMC free article] [PubMed] [Google Scholar]


