Summary
Throughout the developing GC response, B cell survival and fate choices made at the single cell level are dependent on signals received largely through interactions with other cells, often with cognate T cells. The type of signals that a given B cell can encounter is dictated by its location within tissue microarchitecture. The focus of this review is on the initiation and evolution of the GC response at the earliest time points. Here we review the key factors influencing the progression of GC B cell differentiation that are both stage and context dependent. Finally, we describe the coevolution of niches within and surrounding the GC that influence the outcome of the GC response.
Keywords: B cells, stromal cells, cytokines, transcription factors, cell differentiation, lineage commitment/specification
1. Introduction
Effective immune responses to pathogens and vaccines critically depend on the generation of high affinity antibodies. The steady increase in antibody affinity during adaptive immune responses, known as affinity maturation, requires the formation of an organized cluster of proliferating B and T lymphocytes in germinal centers (GC). Germinal center responses are initiated within secondary lymphoid tissues, that are inherently organized, where antigen-inexperienced T and B cells are separated into T cell zones and B cell follicles. CD4+ T cells expressing T cell receptors (TCR) specific for newly arriving antigen are activated to both proliferate and differentiate through interactions with dendritic cells (DCs) that have internalized antigen and presented proteolytically degraded fragments on surface MHC II molecules. At the same time, antigen-specific B cells become activated after encountering antigen in its intact, unprocessed form and experiencing B cell receptor (BCR) ligation. B cells that have internalized antigen bound to its specific BCR can then also present processed components in the context of surface MHC II. A series of coordinate interactions between activated T and B cells specific for the same (cognate) antigen are the foundation for B cell activation throughout the GC response, both during the initial pre-GC phase and within the mature GC.
The GCs that emerge within B cell follicles of secondary lymphoid tissue are highly organized structures, within which GC B cells compete to acquire additional antigen and engage follicular helper T cells. With contemporary thinking, GC B cells expressing BCR with higher affinities are selected to further proliferate, while less fit B cells expressing BCRs with weaker affinities may not receive sufficient survival or instructive signals and undergo apoptotic cell death. In addition to survival, T/B interactions also drive differentiation of GC B cells into one of two lineages that are fundamental to durable immunity; long-lived high-affinity plasma cells and memory B cells1–4. In this way, GCs mold, amplify and add persistence to the most effective B cells of the immune response.
Throughout the developing GC response, B cell survival and fate choices made at the single cell level are dependent on signals received largely through interactions with other cells, often with cognate T cells. The type of signals that a given B cell can encounter is dictated by its location within tissue microarchitecture. A major component of B cell activation and differentiation in the GC response involves sequential changesin migration patterns that move the cell from one specialized zone to the next. The focus of this review is on the initiation and evolution of the GC response at the earliest time points. We’ll review the key factors influencing the progression of GC B cell differentiation that are both stage and context dependent. The review will finish with a discussion of the niches within and surrounding the GC that may influence the outcome of the GC response.
2. Landmarks and unique features of established GCs
2.1. Tissue compartments of mature GCs
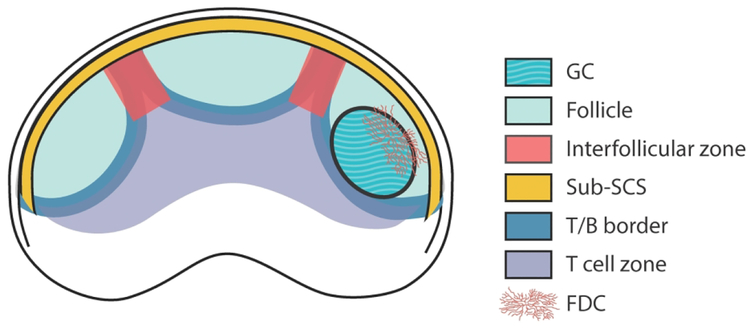
To better appreciate the similarities and disparities of the initial GC response, we begin with an overview of the fundamentals of fully mature GCs. As the newly established GC expands, non-responding B cells are peripherally displaced and surround the emerging GC, forming what is known as the follicular mantle. Mature GCs have a complex architecture that is comprised of two prominent subdomains, the light zone (LZ) and dark zone (DZ), originally named for their relative appearance in hematoxylin/eosin-stained tissue sections5–9. The light zone (LZ) is distinguished by the presence of the fine processes of a stromal cell subset, follicular dendritic cells (FDC), which entirely comprise the stromal cell matrix in this region and form a reticular network that GC B cells infiltrate8. Within primary follicles FDCs are a key source of CXCL13, a chemokine that is requisite for the correct organization of GCs into compartmentalized DZ and LZs10. FDCs retain deposits of activated complement components and antigen–antibody complexes via the low-affinity Fc receptor for IgG (FcγRIIb) and the complement receptors CD21 and CD3511–15. FDCs can serve as long-term depots of undegraded Ag 11, 13, 15–17, and for this reason they have classically been considered an important site of antigen presentation to B cells. Intrafollicular CD4+ T helper cells known as T follicular helpers (Tfh) are predominantly found within the LZ, sometimes in great numbers, but are also present throughout the follicle mantle5, 18, 19. Tfh are critical to the formation and maintenance of a productive GC, largely due to their provision of IL-21, IL-4 and CD4020–26. At the peak of the response, Tfh can be distinguished from other cell subsets by a high level of expression of the molecules PD-1, ICOS, and the chemokine receptor CXCR527–30.
The DZ contains a higher density of proliferating cells and harbors another stromal cell type that produces CXCL12, the ligand for CXCR4, and lacks the distinctive CD35 expression of FDCs31, 32. The majority of DZ resident B cells, also referred to as centroblasts, express lower levels of a variety of surface markers, including BCRs, CD40, and CD86, but a higher level of Bcl6 and CXCR4 compared to their LZ counterparts32–34. Many LZ resident GC B cells, aka centrocytes, are smaller in size and are typically CD86hi, CD83hi, and CXCR4lo33. Although these phenotypic differences have come to be viewed as surrogate markers of GC B cell location, it is important to note that B cells residing within each zone are far from homogeneous. GCs are consistently oriented within B cell follicles (Fig 1). The LZ is reproducibly more proximal to the subcapsular sinus of lymph nodes or the marginal sinus enveloping the white pulp of spleens. The dark zone balloons beneath the FDC network, extending toward the border of the follicle with the T cell zone.
Figure 1.
Lymph node tissue compartments influencing the developmental stages of germinal center B cells.
2.2. Selection of higher affinity variants, inter-zonal migration and cyclic reprogramming
During their extensive clonal expansion, B cells with higher affinity B-cell receptor (BCR) variants are selectively enriched35–38. GC B cells express high levels of activation induced cytidine deaminase (AID) that drives a unique process of somatic hypermutation (SHM) of immunoglobulin (Ig) genes that can introduce amino acid substitutions in the antibodies produced39, 40. Because SHM is introduced during DNA replication, it was thought to occur in the DZ where mitotic cells are more evident, especially in tonsils32, 33, 41, 42. Selection for higher affinity B cells has been proposed to result from competition for the acquisition of antigen6, 43, 44. As FDCs are the most evident site of antigen accumulation within follicles, and have the ability to promote the survival of GC B cells that are prone to apoptosis, the selection process is thought to occur within the LZ compartment45–47. Early thinking on GC B cell selection envisioned that a key element involved the crosslinking of BCRs while in contact with antigen-laden FDCs48–50. However, a recent study by Luo et al indicates that some aspects of canonical BCR signaling are rewired in Bcl6hi GC B cells, such that BCR crosslinking per se cannot fully instruct the extensive self-renewal observed within mature GCs51, 52. Other studies strongly support a model in which positive selection requires GC B cells to form immunological synapses with Tfh cells53–55 and reviewed in56, 57. B cells with higher affinity acquire more protein antigen, resulting in increased surface presentation of MHC II-antigen complexes, and a greater capacity to stimulate Tfh cells with a cognate specificity33, 58–62. It is important to note that BCR signaling during antigen acquisition and Tfh cell engagement need not be mutually exclusive and could be synergistic. In support of this, the combination of BCR and CD40 agonism more robustly invokes cMYC expression and further cell division then either alone52. Other models for affinity maturation postulated forms of negative selection, which could operate in concert with positive selection48, 63–66.
To reconcile a perceived need for sequential events to occur in spatially separated regions of the GC, the “cyclic re-entry” model was proposed67, 68. With a contemporary view of this model, DZ resident B cells that have exited cell cycle lose the transcriptional profile typical of centroblasts and with lower CXCR4 levels subsequently migrate to the LZ10, 32, 33, 69. Intravital imaging studies of GC B cell migration patterns are consistent with infrequent movement between compartments59, 70–72. Within the LZ, the recent immigrants compete for access to antigen, potentially experience BCR crosslinking during antigen acquisition, and then compete for contacts with Tfh cells that selectively provide factors such as CD40L, IL-21 and IL-452, 73, 74. FoxO1, cMyc and AP4 are among the transcription factors known to play a critical role in the transition of such positively selected centrocytes to the centroblast transcriptional program, although expression of these transcription factors (TF) can be short-lived34, 75–78. Thus, the LZ is currently seen as the compartment in which both self-renewal and differentiation toward long-lived effector lineages are initiated, the latter evidenced by the expression of antibody secreting cell (ASC) associated TFs by B cells within the LZ harboring the majority of Tfh cells55, 79–82. Self-renewing GC B cells undergo a centrocyte to centroblast transition that is characterized by a reciprocal increase in CXCR4, a decrease in costimulatory molecules, initiation of cell division and their return to the DZ to complete cell division there33. Within the DZ, centroblasts reduce their surface BCR and recycle existing MHC II complexes, and in this way have an opportunity to better express their newly mutated BCR that can be more fully tested when antigen is next encountered83, 84.
The bulk of research on GCs has interrogated mature GCs. However recent studies have implications on its initiation and development. The focus of the remainder of this review is on the progression of the GC response at the earliest time points, both of the responding B cells and within the evolving stromal compartments that coordinate the context of their cellular interactions.
3. T-B cell contacts during GC initiation
Exploration of the earliest stages of GC development had long been hampered by the seemingly inscrutable developmental steps that preceded the appearance of the classical surface markers of GC B cells. Markers typically ascribed to GC B cells include high levels of CD95 (Fas), the GL7 epitope, a loss of IgD and an increased ability to bind peanut agglutinin (PNA)85–87. B cells within established murine GCs have uniformly low levels of CD38 in mice, while curiously exactly the opposite pattern is seen with human GC B cells88. However, it is important to note that during the early phases of an immune response, some of these markers are also expressed by developing plasmablasts89, 90. A further challenge to the study of early B cell activation events is the downregulation of surface BCR after crosslinking, making antigen specificity hard to confirm in some cases. A superior indicator of this lineage commitment emerged when the requisite role of Bcl6 was demonstrated, empowering investigations into the early steps in this pathway that were previously enigmatic. Expression of the transcriptional repressor Bcl6 is required for both Tfh and GC B cell differentiation, a process that involves shifts in the transcription of other influential TFs91–95. In order to more easily identify and track the behavior of antigen specific T and B cells, we and others have developed strains of mice that ubiquitously express fluorescent proteins such as GFP or RFP and harbor transgenic or knockin BCR or TCR alleles. With the transfer of such fluorescently tagged cells, specific cells can be interrogated while remaining agnostic on all other phenotypic aspects during their activation and as they undergo significant change during subsequent differentiation.
The creation of a productive GC involves step-wise changes to the transcriptional profiles and phenotype of responding lymphocytes, often including shifts in their chemotactic tendencies. Prior to activation, antigen-naïve B and T cells are segregated into B cell follicles and T cell zones (Fig 1). This is the result of migration patterns not dictated by structural or anatomical limits, but rather by localized expression of chemotactic agents drawing in B and T cells to their respective zones based on their expression of receptors for those agents. By virtue of B cell expression of CXCR5, they are attracted to its ligand chemokine CXCL13 that is produced by FDCs centrally positioned in follicles96, 97 and by Marginal Reticular Cells (MRC) that line the subcaspular sinus (SCS) of LNs and splenic marginal sinus (SMS)98–100. T cell localization to the T cell zone is mediated by their expression of CCR7, the receptor for CCL19/21101, 102. An additional chemoattractant within lymphoid tissue includes the compound EBI2L that is enzymatically modified by stroma lining the SCS and SMS. EBI2L is also found at the cortical sinuses descending through the interfollicular (IF) regions, at the border between T cell zones and follicles (T-B border), as well as at the bridging channels positioned between follicles in spleens (Fig 1 and103–109). The combinatorial expression of even just these 3 chemoattractants by stromal cell populations establishes multiple distinct architectural domains within which lymphocytes with similar tropisms can aggregate (Fig 1).
With an influx of inflammatory material via lymph to lymph nodes, or via blood in the spleen, B and T cells specific for any newly arriving antigens become activated and adjust their migratory propensities. Within lymph nodes, naïve B cells within follicles first encounter their cognate antigen delivered via lymphatic vessels, cortical sinuses or at the surface of SCS lining macrophages110–116. Exposure to pathogen associated molecular motifs and BCR crosslinking result in increased levels of CCR7, CD86 and MHC II, as well as receptivity to CD40 signaling117, 118. With this increase in CCR7, activated B cells rapidly relocate to the periphery of the follicle119–121 and are retained in part by further expression of EBI2103, 104. Activated CD4+ T cells also adjust their chemotactic propensities by an increase of their expression of CXCR5 and EBI296, 122. As a result, recently activated CD4+ T cells and B cells are similarly CXCR5+ CCR7+ and EBI2+ and both migrate to and congregate at the T-B border and interfollicular regions within LNs or the analogous bridging channels within spleens. After engaging T cells that share their cognate specificity, activated B cells commit to one of several potential fates; they either re-enter the follicle and commit to the formation of a new GC, or they differentiate outside of GCs into low-affinity short-lived antibody secreting cells (ASC), early memory B cells, or other effector B cell lineages123–125. It is at these locations that elevated Bcl6 protein levels are first apparent in both responding B and T cells126, 127. Once Bcl6 mediated transcriptional repression is fully enforced in nascent GC B cells and Tfh cells, they lose expression of chemokine receptors such as EBI2 and CCR7 that retained them at the follicle periphery, resulting in revised chemotactic patterns that instead drive them to the follicle interior shortly thereafter108, 127–130.
Importantly, the requirements for commitment to the intra-follicular GC pathway differ for T and B cells. It is clear that T cell differentiation to the perifollicular Tfh state can be driven initially by their interactions with DCs 131. Bcl6 expression by a subset of responding T cells is detectable very early, within one-two days of antigen encounter126, 132, 133. Interestingly, typical Tfh markers CXCR5 and PD-1 are quickly upregulated on many responding T cells, but after three days of interactions with B cells at the follicle periphery are then only maintained on a population that ultimately gains entry to the follicle and continues to interact with B cells126, 132–135. In contrast, full activation of B cells to commit to the GC pathway depends on those B cells interacting with cognate T cells.
Over the course of several days, cognate B and T cells form stable immunological synapses, but repeatedly engage new partners during this protracted and formative time period, all the while remaining at the follicle periphery. Bcl6 expression by B cells is not detectable until after the emergence of Bcl6+ Th cells. Thus B cell commitment to the GC pathway, reentry to the follicle and the coalescence to form a proliferative foci there requires several days of ongoing serial interactions with perifollicular Tfh. Interestingly, Tfh entry into the follicle proceeds that of GC B cells126, suggesting that the availability of Tfh-support within the follicle is a prerequisite for the survival of newly arriving GC B cells. Early, preGC cognate interactions that occur at the follicle periphery are therefore critical to establishing the GC and determining immune outcome.
4. Progressive GC B cell differentiation has distinctly regulated stages
4.1. Germinal center B cells are developmentally delayed
Shortly after BCR signaling in vitro and in vivo, activated B cells are well poised to engage Th cells and respond to CD40 ligation117. However, many cell divisions are required before Bcl6 protein expression becomes evident in responding B cells26. This is true even under conditions in which activation and cell contacts are occurring with rapidity. The delay in the appearance of GC B cells had been a long-standing paradox. Multiple models have proposed mechanisms that may influence the differentiation of activated B cells to either the GC or ASC lineages. In vitro studies have suggested that B cell fate decisions could be stochastic and B cell intrinsic136–138. Alternatively, the initial BCR signal strength139, 140 or competition for antigen and its presentation to cognate Th cells141 may be deciding factors. However, the delay in GC B cell development could also be influenced by other potential constraints. For example, Tfh cells require multiple days to complete their effector differentiation, but once complete might be capable of instructing GC B cell differentiation expeditiously. A teleologic explanation for the delay in GC B cell formation might instead relate to the need for multiple lineage fates to be created from every antigen-specific B cell after its initial activation. Perhaps multiple rounds of proliferation are key to ensuring that an investment in the germinal center response only occurs after the production of early memory and short-term ASC B cells. Here we review the results of recent studies that suggest additional mechanisms and provide some insights and into these aspects of B cell differentiation.
4.2. Impact of CD40 signaling
B cell lineage commitment is governed by opposing transcriptional networks that can be mutually antagonistic. Bcl6 acts as a transcriptional repressor of the TFs IRF4 and BLIMP1, and whereas it is essential for GC B cell formation93, 127, 142–144, when over-expressed it can prevent ASC formation145–147. Reciprocally, high levels of IRF4 or induction of BLIMP1 represses transcription of Bcl6 but facilitates ASC differentiation148, 149. To further confound our understanding of transcriptional regulation, a transient and low expression of IRF4 promotes the transcription of Bcl6 and is required for proper GC B cell progression150, despite the fact that it directly binds to and represses the Bcl6 promoter when at higher levels92, 151. Thus, IRF4 is required for both ASC and GC development, although ASC associated genes are promoted when IRF4 is at sustained or high levels of expression79, 150, 152–155.
Tfh cells express the surface co-stimulatory molecule CD40L and supply the cytokines IL-4 and IL-21 to GC B cells, all of which are critical to the formation of fully mature and productive GCs20–26. The combined expression of IL-4 and IL-21 is unique to Tfh cells, however all Th cell populations defined to date are thought to express CD40L, and very early in an immune response156, 157. Signaling via CD40 ligation invokes the non-canonical NFkB pathway and uniquely the nuclear translocation of heterodimeric RelB/p52158–160. Although CD40 signaling is required for GC B cell development1, 161, their formation can also be antagonized by chronic CD40 signaling that instead promotes ASC development151, 162–165. ASC formation during chronic CD40 signaling is in part mediated by IRF4, a TF whose nuclear translocation is promoted by CD40 signaling151, 166. These seemingly conflicting roles of CD40 pose a conundrum: CD40 and CD40L are required for GC B cell formation, but continued CD40 signaling discourages it.
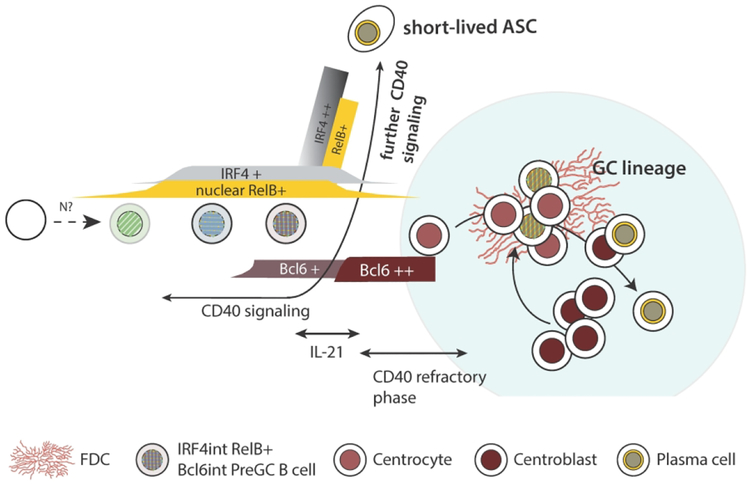
Some insight into the paradoxical roles of CD40 was garnered by our recent work that examined the temporal relationship between CD40 signaling and TF shifts during GC B cell differentiation26. In this study, B cells were first observed to express intermediate levels of Bcl6 within the interfollicular regions of LNs. Intriguingly, these B cells also were found at their first emergence to co-express RelB and IRF4 at intermediate levels, factors known to be mutually antagonistic to Bcl692, 151. Consistent with the presence of nuclear RelB and IRF4, CD40 signaling was required for this very first step in Bcl6 protein production. At this early stage, Bcl6int GC precursors retained some plasticity - the infusion of either anti-CD40 or additional antigen in vivo at this incomplete stage of development could still divert them towards Bcl6lo IRF4hi ASCs26. However it is important to note that Bcl6lo IRF4hi ASC can be seen prior to the detection of Bcl6int pre-GC B cells26, an observation that is consistent with the co-existence of an alternative pathway for extra-follicular ASC formation that is CD40 dependent but does not involve Bcl6.
Although CD40 signaling may establish a transcriptional landscape that is initially compatible with intermediate expression levels of Bcl6, the presence of RelB and the antagonistic TF it promotes, IRF4, is incompatible with a complete repression of Bcl6 target genes within preGC B cells26. Consistent with this, preGC B cells display a partial GC B cell phenotype (CD38int, PNAint)26. Bcl6 is a member of the BTB-POZ zinc finger family of TFs that forms a homodimer capable of binding the corepressors SMRT, NCOR and BCOR167, 168. Bcl6 controls GC B cell differentiation by regulating cell cycle genes, repressing terminal differentiation factors, and suppressing some signaling pathways, including some aspects of B cell receptor signaling91, 169. Repressed target genes in dark zone GC B cells in mice include cd38, irf4 and prdm1 (encoding BLIMP1)92, 143, 144. Notably, the extent of repression of potential Bcl6 target genes is heavily influenced by co-repressors that are themselves independently regulated91, 147, 170, 171. Therefore, Bcl6 expression per se does not ensure target repression. It is unclear whether the ineffective repression of Bcl6 target genes in GC B cell precursors is the result of subthreshold levels of Bcl6, or alternatively the absence of other co-repressors that would otherwise enable effective transcriptional repression170, 172, 173.
Although CD40 signaling is required for this first step in preGC B cell development, CD40 signaling must cease at the immediately subsequent stage to allow for that incomplete stage of differentiation to further progress26. The intermediate differentiative stage of preGC B cells is characterized by mutually antagonistic TFs (IRF4int Bcl6int) and is followed shortly thereafter by the appearance of mature GC B cells that are Bcl6hi with low levels of IRF4 and RelB and a more classical phenotype (CD38lo PNAhi)26. Interestingly, the shift from the immature preGC B cell state to the more mature Bcl6hi state involves both transcriptional and post-transcriptional regulation26, the latter a form of regulation that can be mediated by microRNAs162, 174, 175. Importantly, disrupting T-B interactions or inhibiting CD40 signaling in vivo during this early transitional stage encourages GC B cell maturation. However, when CD40 agonism is prolonged or additional soluble antigen is introduced, GC precursors are instead expanded and shunted away from the intra-follicular GC pathway26, 165. Based on these results, a model of initial GC B cell differentiation has been proposed to be a multi-staged process completed by a transient diminution in the receipt of T cell help within its immediate microenvironment (Fig 2). Here it is envisioned that GC B cell differentiation is a two-stage process involving first a T-cell contact and CD40-dependent phase that generates RelB+ IRF4+ Bcl6int GC precursors and second, a maturation phase immediately following that leads to the Bcl6hi intrafollicular state that is prevented by further CD40 signaling. Thus the impact of CD40 signaling may be stage specific in that it promotes GC B cell precursor formation, but diverts precursor progression once formed. It is intriguing that the shifting transcriptional profiles of GC B cell precursors are staged over the course of multiple cell divisions. More research is required to understand how the sequential introduction and subsequent removal of TFs is advantageous to the initial formation of GC B cells.
Figure 2.
Temporal and context dependent shifts in transcription factor composition during GC B cell development.
It is unclear whether a transient abstinence from CD40L stimulation plays a similar role in established GCs. The transition between the classical centrocyte phenotype to that of an idealized centroblast may involve progressive changes as well. LZ B cells experiencing CD40L stimulation during engagement with Tfh cells gain cMyc expression, which subsequently promotes expression of AP4, a TF that is maintained within B cells by IL-21 signaling78. Subsequent loss of AP4 requires further cell division(s) with the DZ, a process that is needed to eventually exit cell cycle78. The potential impact of additional CD40 signaling by DZ resident B cells, and whether it could occur in the absence of IL-21, is unclear. Since there are far fewer Tfh cells within the DZ than the contiguous LZ, this compartment offers a relative dearth of T cell derived CD40L. The sheltered spaces within the DZ compartment appear to provide other advantages as well to newly transitioning centroblasts to GC dark zones. A study by Bannard et al indicates that GC B cells unable to escape the Tfh-rich environment of the LZ have a competitive disadvantage over time32, suggesting that in the long run the cyclic movement between zones ultimately empowers a selective advantage in a way that is not yet understood. Although some analogies can be made between the initial development of GC B cells at the follicle periphery and the cyclic reprogramming of mature GCs, there are two major differences – 1) newly arriving Bcl6hi centrocytes that are seeking antigen and Tfh help are already experiencing some level of transcriptional repression by Bcl6, and 2) a reservoir of antigen is accessible within the FDC network. More research is needed to untangle this complex series of events.
How might preGC B cells transiently avoid CD40 signaling and resolve their mixed state of mutually antagonistic TFs? One means by which RelB+ pre-GC B cells could lose that status is by an asymmetric cell division (ACD) that results in one daughter receiving less RelB. Consistent with this, we have observed an additional cell division of preGC B cells correlates with reduced RelB levels and a rise in Bcl626. In this scenario, preGC B cells could undergo an ACD in which Bcl6 is unequally partitioned to one of the emerging daughters and RelB to the other, resulting in two distinct cell fates. ACDs involving an unequal distribution of Bcl6 has been reported among GC B cells, although its role in GC maintenance has been questioned176, 177. Alternatively, engagement with perifollicular cognate T cells could be attenuated by a sub-threshold level of antigen presentation. Multiple cell divisions could lead to a reduced capacity to present antigen, particularly under conditions when antigen is limiting within that local microenvironment. This would be consistent with previous reports of progressively shorter T-B interaction lengths with non-infectious immunogens59, 126, 127. In further support of this, conditions that instead create persistent pathogen-derived antigen promote extrafollicular ASCs and impair GC responses178. Moreover, antigen that has been internalized after BCR capping has been reported to be asymmetrically distributed between daughters after B cell division, resulting in two daughters that differ in their ability to engage cognate T cells179, 180. However, once GC B cells have migrated into the follicle interior, they again have access to a rich source of antigen within the FDC network.
4.3. Role of IL-21 and IL-4 in GC B cell maturation and intrafollicular development.
Similar to CD40L, the Tfh-derived cytokines IL-4 and IL-21 play distinct roles at discreet phases of GC B cell development. Although many Tfh cells found within mature GCs can produce both IL-4 and IL-21, the cytokine expression profiles of Tfh cells can shift during their development and can differ at distinct locations within lymphoid tissue21–23, 181, 182. Earlier in adaptive immune responses, peri-follicular Tfh predominantly express IL-21 and less IL-423, 182, while the reverse is true within the distal LZ of fully mature GCs where Tfh cells are more likely to produce IL-4 than IL-2123. CXCR5+ iNK T cells positioned at follicular boundaries are also capable of providing IL-21 and/or IL-4 and promoting the GC lineage development of B cells that are presenting glycolipid antigens, but the resulting GCs are short-lived and unproductive without the added participation of intrafollicular Tfh cells responding to protein antigens183–186. Importantly, the delivery of IL-4 and/or IL-21 by iNK T cells is insufficient for the formation of sustainable GCs when CD40 signaling is absent185. Other innate cell types including eosinophils and basophils are also known to produce IL-4 and can function as antigen presenting cells to Th2 cells187–190, but they have not been observed within intrafollicular GCs188 and it is unclear whether such innate cell types have the ability to provide IL-4 in a directed fashion discreetly to antigen-specific B cells.
Both IL-4 and IL-21 are critical to GC B cell formation. Although B cells that are unable to signal via IL-21R or IL-4Rs can still develop GCs in vivo, the stunted GCs that do form are significantly reduced in size182, 191–194. In vitro, both IL-21 and IL-4 can enhance the transcription and/or translation of Bcl6 in B cells195, 196. Both cytokines are also known to promote ASC formation22, 197–201. In the case of IL-21, an inability to signal via its receptor has a direct effect on B cells, resulting in lower Bcl6 levels among the more limited number of GC B cells that form182, 191, 192.
IL-4R and IL-21R complexes activate distinct signaling pathways. Like other cytokine receptors employing the common gamma chain, both IL-21R and IL-4R signal via the Janus kinase molecules JAK1 and JAK3202–205. However, these cytokine receptors activate different signal transducer and activator of transcription (STAT) molecules. Whereas IL-21 predominantly activates STAT3 and STAT1, and to a lesser extent STAT5,206–209, IL-4 and IL-13 uniquely and requisitely activate STAT6,210, 211.
The results of a recent study we performed suggest that B cell signaling by these cytokines may influence GC B cells at different stages182. These experiments employed the cell transfer of antigen-specific B cells that were either deficient in IL-21R, STAT6, or both in order to assess the impact of IL-21 and IL-4/IL-13 to B cells intrinsically. We found that the initial transition from the peri-follicular preGC B cell state to the intra follicular stage is reduced in IL-21R−/− B cells, but unaffected in STAT6−/− B cells, suggesting that IL-21 has a greater influence on differentiation at the time of preGC B cell re-entry to the follicle interior182. Importantly, in the absence of both IL-21 and IL-4 signaling pathways, Bcl6+ B cells can still form, however they fail to establish intrafollicular GCs182. These findings support the idea that GC B cell development undergoes a step-wise progression in which the initial steps toward this lineage can occur in the absence of IL-4 and IL-21, but is dependent upon CD40L. The Bcl6-expressing B cells that form without IL-21R or STAT6 signaling can persist transiently in vivo, but their expansion is limited, indicating that GC B cell development can be arrested at this stage. This could functionally provide a key checkpoint in this lineage pathway that would allow for the initial creation of GC precursors, without committing to their furthered proliferation. In this way, a diverse repertoire could be potentiated without a concomitant clonal expansion of antigen reactive B cells for which there are not yet cognate Tfh cells capable of sustaining a mature GC.
The results of this study further suggest that proper GC form and function within follicles is highly reliant on both cytokines182. IL-21 and IL-4 play non-redundant roles in the subsequent maturation and self-renewal of GC B cells within follicles. Smaller numbers of intra-follicular GC B cells form in the absence of either of these signaling pathways, but with aberrant phenotypic features182. When GCs mature in the absence of either IL-21 or STAT6 signaling, the efficiency in the transition between the centroblast and centrocyte states is compromised and self-renewal is reduced182, 197. IL-21R−/− B cells can form CXCR4hi centroblasts but they overexpress some features that are more typical of centrocytes such as CD40 and CD86182. Moreover, IL-21R−/− B cells are predominantly located within the FDC network, further suggesting that IL-21 signaling is required to properly instruct the centocyte to centroblast transition. By contrast, IL-4 dependent signaling appears to have a greater contribution to the proper formation of the centrocyte profile. Although STAT6−/− GC B cells have a broadly normal spatial distribution within their smaller GCs, albeit with a smaller percentage of centrocytes, the CXCR4lo centrocytes that form aberrantly underexpress CD86. Together, these results suggest that newly generated centroblasts formed without prior IL-4 exposure might be compromised in their ability to recapitulate the complete centrocyte program. This is consistent with a study by Turqueti-Neves and co-workers that examined the response of IL-4 deficient mice to helminth infection and observed a preponderance of DZ phenotype GC B cells that could be corrected by the introduction of IL-4 sufficient cells in BM chimeras194. Together, these intriguing studies raise more questions than they answer. Can these signals be received independently during sequential cell contacts with Tfh cells that differ in their cytokine profile? Are DZ centroblasts engaging Th cells within that compartment? Although more work is needed to address these and other questions, it is clear that the programing of GC B cells capable of responding to both cytokines is different from that obtained with one but not the other.
5. Establishment of intra-follicular GCs and the coevolution of follicular stromal cell niches.
The cellular composition of tissue compartments effectively creates micro-climates that can be quite different. The cell types residing in each compartment, together with the cytokines and chemokines they secrete, likely shape the nature of lymphocyte activation, proliferation and differentiation10, 110. Differences in local accessory cells ensures that B cell experiences within the molecular milieu of one compartment will be different from those experienced in another compartment. For example, the composition of the stromal cell population, their activation status, and any molecular products could potentially create a microenvironment that fosters modes of B cell growth or effector lineages that are distinct from those occurring elsewhere. Although stromal communities may delineate the boundaries of neighborhoods defined by characteristic chemokine compositions, they are themselves capable of change under inflammatory conditions212. Here we review the progression of events that occur within the follicle as Tfh and GC B cells arrive, stromal cell populations evolve, and the unique compartments of the mature GC are established.
5.1. Early intra-follicular events and FDC maturation
Follicular dendritic cells are derived from a stromal cell subset that uniquely differentiates within B cell follicles and are the primary source of CXCL13 in this zone of primary lymphoid tissues97, 213, 214. Within lymphoid tissue of naïve mice, FDCs can be identified as uniquely expressing the long variant of the complement receptor CR1 (CD35) within follicles14. They are centrally and sparsely distributed within primary B cell follicles and are substantially less dendritic then is typically observed within mature GCs (Fig 3a,b). Immunization promotes the furthered development of FDCs from perivascular associated stromal cells, which then protrude long dendritic extensions forming an extensive network of dendrites9. Immunization can also elicit the formation of FDC-like cells from the marginal reticular cells (MRC) that descend from the SCS of LNs or marginal sinus lining cells of spleens, and hence contribute to the CD35+ CXCL13-producing dendritic network closer to the outer follicle sinus98, 99. Both a numerical increase and furthered differentiation occurs with these MRC-derived FDCs, known to uniquely produce RANK-L within the follicle99.
Figure 3.
Stages of GC maturation and associated tissue niches. (A) Schematic representation of the stages of germinal center (GC) maturation. (B - E) Immunofluorescence staining of popliteal lymph node (B) and spleen (C-E) from C57BL/6 mice that received an adoptive transfer of OVA-specific T cells and antigen-specific B cells enriched by immunomagnetic purification (StemCell Technologies). T cells specific for OVA were obtained from spleens of OT-II mice while either B1-8+ Jκ-/- or MD4 mice served as donors for anti-NP or anti-HEL specific B cells respectively. Recipients were immunized after transfer with 50 μg of either NP-OVA/CFA in the footpad (lymph node, panels B), NP-OVA/alum injected i.p. (spleen, panels C, E), or HEL-OVA/alum (spleen, panel D). (B) Popliteal lymph node histology time-course demonstrating GC maturation after immunization. Stages of maturation represented include the unimmunized follicle (d0/naïve), maturation of the FDC network and light zone expansion (day 4), centroblast increase, zonal segregation and follicle swelling (day 5), and a mature GC (day 10). Sections were stained to highlight CD4+ T cells (blue), IgD+ non-responding B cells within the follicular mantel (green), and CD35+ FDC network (red). (C) Histology showing a maturing GC (day 6) stained to highlight IgD+ naïve B cells (grayscale), PNA+ GC cells (green), and the distribution of sonic hedgehog (SHH, red) in relation to the FDC network stained with CD35 (blue). (D) Representative histology of splenic GCsdemonstrating initial differentiative events shortly after the arrival of Ag-specific B cells to the follicle interior(day 3). Sections were stained to highlight GFP+ Ag-specific B cells (green), CD35 FDCs (blue), Bcl6 (red) toidentify GC committed B cells, and IgMa to assess the level of cytosolic immunoglobulin. Arrows point to Bcells within the FDC network that are cIg hi (consistent with ASC effector lineage), Bcl6hi (GC lineage), andBcl6neg cIg lo (of an undefined alternative fate). (E) Histology showing GFP+ Ag-specific B cell interactionwith dendritic cells (DC) at the T-B border (day 6). Section were stained for CD4+ T cells (blue), B220+ Bcells (red), GFP+ Ag-specific B cells (green) and CD11c+ DC cells (grayscale). Higher magnification imagesof the T-B border demonstrate the ability of GC B cells, DCs and T cells at the T zone border to directly engage each other.
After Tfh and preGC B cells have further differentiated at the interfollicular zone and T-B border, they are observed to migrate directly from that region toward the follicle interior, with the migration of disengaged cognate T cells occurring independently and preceding that of GC B cells126. Responsiveness to CXCL13, downregulation of EBI2 and CCR7 all promote this initial intra-follicular movement10, 103, 104, 129. Once within the follicle, Bcl6hi B cells are primarily found in the outer follicle, the upper follicle more distal to the T cell zone, and appear there typically 3–4 days post immunization in non-infectious experimental systems126, 127.
FDCs undergo a form of activation after immunization that is influenced by TLRs and NF-kB signaling pathways, as well as immune complex binding215–218. This activation results in an increase in the expression of numerous proteins that can play a role in GC B biology, including Fc receptor FcγRIIb, complement receptors CD21/35, ICAM1 and VCAM1215, 217–219. FcγRIIb together with the complement receptors CD21/CD35 mediates the bulk of the antigen retention by the binding to proteins in immune complexes or tagged with activated complement components220. After immunization, FDCs also increase their production of Mfge8 that will promote the phagocytosis of emerging apoptotic B cells by tingible body macrophages7, 221. FDC activation and dendrite extension is similar in timing to the arrival of Tfh and GC B cells to the follicle interior. Notably, Tfh cells when first arriving to the follicle interior within LNs can first be found primarily in contact with the centrally located and underdeveloped CD35+ FDCs, but are also found in the follicular space above nearer the SCS (Fig 3b and126, 127). Although coincident, there is only in vitro evidence to suggest that Tfh cells can influence FDC activation222; there is no evidence to date in vivo. However, the TLR dependent activation of FDCs suggests that some aspects of their furthered differentiation can take place after exposure to adjuvant alone216.
As the GC matures, FDC network differences become more apparent. ICAM, FCγRIIb expression223 and sonic hedgehog (SHH) expression (Fig 3c and224) by FDCs is more evident within the GC LZ, and in particular at the LZ-DZ interface, than is observed with the FDCs within the FM more proximal to the SCS. Given the positioning of MRC-derived FDCs closer to the SCS of LNs, it may be that their contribution to the FDC network found within the FM is larger99. The functional differences between these FDC zones suggest that the nature of the cellular interactions with lymphocytes within these distinct domains could differ. For example, SHH found within GC LZs is known to influence self-renewal and cell-fate determination in other tissue compartments, including as hematopoietic niches, hair follicles and thymocyte selection225–228. In support of the idea that SHH could influence LZ centrocytes, it has been reported that a subset of GC B cells express both of the required components of the Hedgehog (Hh) receptor, Ptc and Smo224. Interestingly, expression of Smo can be increased with CD40 or BCR signaling224, suggesting that successful engagement with stimulatory antigen and/or Tfh cells would empower hedgehog signaling. Hedgehog signaling in GC B cells promotes their viability in vitro and reduces Fas-induced apoptosis224, providing further evidence that within the GC LZ both FDC-derived and Tfh-derived factors can promote protection from the apoptosis prone state of GC B cells215, 229.
5.2. B cell differentiation within the newly formed LZ
Studies that have examined the formation of BM-resident long-lived plasma cells have observed that the plasma cells that persist are predominantly generated after the peak of the GC response125, 230. However, there is evidence of B cell differentiation toward this effector lineage within the FDC network, even at this very early stage. In addition to Bcl6+ GC B cells, antigen specific B cells with large amounts of cytosolic immunoglobulin (cIg) can be seen centrally placed in follicles and adjacent to FDCs (Fig 3d,126). It is unclear whether the interactions with accessory cells within the still evolving LZ microenvironment are sufficient to instruct the transcriptional program that is needed for longevity in plasma cells231.
At this same place and time, within the emerging GC LZ, responding B cells lacking both Bcl6 and cIg can also be observed, suggestive of an alternative lineage fate such as a GC-derived memory B cell. Although the lineage fate of such Bcl6neg antigen-specific B cells remains unclear, and whether they have the capacity to persist long-term is unknown, these observations nevertheless suggest that not all GC B cell progeny are committed to Bcl6 expression and self-renewal in this arena even during this stage of LZ expansion. Although Tfh cells are already in residence at this point in time, it is intriguing that the first events are not entirely devoted to centroblast formation. Given that the production of long-lived memory B cells is the predominant effector cell output of younger GCs that persists125, 232, it is tempting to speculate that the unique conditions of this stage may contribute to that lineage-fate preference. There are multiple differences within newly formed GCs (days 3–5 with most immunization regimes): 1) FDCs have more recently undergone activation, 2) antigen deposits may be less likely initially to be in complexes with high-affinity antibody, possibly enabling more processes reliant on activated complement220, 233, 3) newly arrived Tfh cells are more prone to produce IL-21 than IL-4 than at later stages in GC maturation23, and 4) LZ resident B cells have just transitioned from the peri-follicular preGC program and could therefore theoretically not have yet obtained the centrocyte program or higher affinities that are typical of a late time point55, 234.
5.3. B cell expansion and effector differentiation at peripheral sinuses
When colonization of the FDC network by GC B cells has just begun, B cell differentiation toward other non-GC lineages is also apparent by histology at other peripheral arenas. Strikingly, at this very early stage of the immune response, expanded populations of antigen-specific B cells form immediately under the SCS of LNs126, 127 and at splenic marginal sinuses235, 236. This phenomena is short lived and quickly diminishes as the GC response progresses. During this early phase, responding B cells at the SCS were seen to exit follicles via the sub-capsular lumen of LNs during intravital imaging, but importantly, the reverse was not true – antigen-specific B cells near the SCS were not observed to enter the follicle via that route, strongly suggesting that proliferative B cells at the SCS do not contribute to the GC reaction126. In this study, Bcl6 protein was not apparent within B cells at that location as assessed histologically. This is in contrast to the results of another histologic study that discerned a temporal relationship of first a predominance of Ki67+ GL7+ B cells adjacent to the marginal sinus, followed by an appearance of aggregated GL7+ B cells in follicle interiors. Here GL7 expression was presumed to be a marker uniquely expressed by GC destined B cells. Although GL7 expression appears restricted to B cells within GCs at mature timepoints87, it is important to note that it is also known to be expressed by LPS stimulated B cells in vitro, including by B cells forming ASC89 as well as by IgM+ early memory B cells237. In the Coffey et al study, Bcl6 levels were assessed by qRT PCR of mRNA235. However, Bcl6 can be transcribed with little translation to protein in some cell types162. Thus the difference in these studies vis a vis GC B cell formation might therefore relate to the well known post-transcriptional regulation of Bcl6238–240
There is some evidence of ASC development among activated B cells at LN SCS. Some SCS-adjacent B cells have copious amounts of clg light chain, consistent with commitment to a ASC lineage126. However in another study, B cells adjacent to splenic marginal sinus lacked the CD138 expression that is characteristic of late stage plasma(blast) cell formation and had not undergone class switch recombination to IgG235. Together the results of these studies remain consistent with the idea that a subset of sinus lining B cells are in the process of differentiating to IgM ASCs. In addition to the SCS, ASC cell formation can also be seen at the T-B border, and among the remaining cognate B and T cells within the interfollicular region126, 241. Consistent with this, prdm1-expressing ASC B cells have also been observed to move from deeper peri-follicular locations toward medullary sinuses90. The lineage fates of the non-ASC B cells at these alternative locations remains unclear, but given the absence of Bcl6 protein and prior reports of very early IgM+ memory B cells that are GC-independent237, it is likely that a subset is destined to become early memory B cells or alternative B cell effectors123, 125.
5.4. Zonal segregation and DZ evolution
Depending on the immunogen and mode of immunization, the emergence of a discernable DZ may not occur for another day or two. This zonal segregation requires, by definition, the presence of GC B cells outside of the FDC network, but as described below there are several other unique features of the DZ compartment that evolve during the course of the response. One key element of the centroblast program is the increased expression of CXCR4, which enables centroblast migration to CXL12 producing stroma initially found most proximal to the basement of the follicle, near the T-B border10, 33, 242. These CXCL12-producing reticular cells (CRC) also have a reticular morphology, although the dendritic extensions lack the high levels of CD35 expression and complement binding that are distinctive for FDCs31, 32, 242. Interestingly, in some but not all GCs, there are DZ areas that are not filled by a CRC network242. The DZ stromal cells without CRC features are as yet uncharacterized, but are found closer to the DZ/LZ interface and at this position could potentially shelter GC B cells from the influences of both CRC and FDCs. In this regard, the distribution of freshly minted centroblasts is intriguing. The emerging centroblasts typically form both a collection immediately under the FDC network as well as a loose column that stretches toward the T-zone border (Fig 3b,141, 182). This is consistent with chemotaxis toward CXCL12 as the primary drive for movement out of the FDC zone10.
CRC can differ phenotypically and morphologically within the DZ, perhaps relating to differences in either their derivation or mode of activation. Although fibroblastic reticular cells (FRC) similar to T zone FRC are only peripherally present at the edge of the B cell follicle in naïve LNs, after immunization the expanding GC swells the follicle, causing both IgD+ and GC B cells to shift past the large cortical lymphatic sinuses present at the naïve border and into the T zone FRC rich area100, 105, 106. Results presented by Mionnet et al are consistent with the idea that at least some DZ stroma may derive from T zone FRC cells100. In this way, new microenvironments are created by evolving stromal cells thereby forming a novel venue for events that might not have otherwise been possible, in this case an avenue bringing DZ GC B cells in close contact with the T zone border. Like other T zone FRC, the stromal network newly infiltrated by the expanding follicle also exhibits a collagen-dense core typical of FRC-ensheathed lymph conduits243, 244, a feature that is less apparent among the more centrally positioned CRC within mature DZs32, 242. Given that CRCs are also found within primary non-reactive B cell follicles242, it seems possible that CXCL12-expressing stroma within DZ compartments can derive from more than one cell type.
In addition to the blasting centroblasts, GC DZs also harbor follicular T cells as well as DCs (Fig 3b). Not all follicular T cells are located in the LZ. They can also be found within the center of DZs as well as at the outer edges of DZs adjacent to the FM, albeit in lower densities (Fig 3b,21, 245). Although little is known about how these CD4+ T cells and DC subsets might differ from their counterparts at the T-B border and GC LZ, there is evidence to suggest that Tfh cells producing IFNγ or IL-21 can localize to the DZ outer edge within the follicle21, 23. Follicular regulatory T cells (Tfr) have also been observed within DZs245. Such DZ resident T cells are in a unique position to influence centroblast behavior, either favorably as they might engage Tfh cells within the LZ, or in the case of DZ Tfr cells influencing centroblast self-renewal or differentiation to long-lived memory or plasma cells246–248.
5.5. Follicular Mantle
As the GC expands, GC B cells form a large egg-shaped aggregate that displaces non-responding B cells, causing them to form the follicular mantle. Intravital imaging has demonstrated that non-responding B cells are not excluded by a physical barrier and can move freely through GCs70, 72. Although early stages of GC development show extensive admixing with IgD+ non-cognate B cells (Fig 3b), this is uncommon within mature GCs. The follicular mantle harbors more than just non-responding B cells. In addition to the sinus-proximal FDCs described above, a distinct subset of Tfh cells can also be found within FMs. The follicular mantle surrounding mature GCs harbors Tfh cells that are phenotypically and functionally distinct, with a lower expression of SAP and IL-422, 122. After the resolution of a primary response, both memory B cells and memory Tfh cells can be found within FMs, a positioning that is influenced by their EBI2 expression122, 249, 250.
Theoretically, this compartment could influence the differentiative state of antigen-specific B cells that are leaving the GC via this route. As cells within this arena are less likely to be in active cell cycle, this environment may be more conducive to gaining a quiescent state, sheltered from the cellular interactions that promote a centroblast program and their self-renewal. It is important to note that this is not the predominant route of exit for the prdm1-expresing ASCs leaving GCs, which largely leave via the GC outer zone through the DZ base due to their down-regulation of CXCR5 and increased responsiveness to CXCL1282, 90, 251–253. However, the FM could be the preferred exit route of GC-derived memory B cells, as has been shown for Tfh memory cells during secondary responses122. CCR6 expression has been identified as a distinctive feature of memory B cells254, 255, including those observed to be completing their differentiation within GC LZs256. A ligand for CCR6, CCL20, is known to be produced by some epithelial cell types257 and importantly by lymphatic endothelial cells lining the subcapsular sinus of LNs near B cell follicles, the latter shown to be key to the localization of IL7Ra+ CCR6hi innate-like lymphocytes258. FM non-responding B cells are also CCR6hi, in sharp contrast to CCR6lo GC B cells. However naive B cells lack responsiveness to CCL20 despite expressing the receptor for this chemokine, unlike CCR6hi memory B cells which chemotax well to CCL20255. Although memory B cells have been reported to be positioned within lymphoid tissues at marginal zones, perifollicular zones and some follicles after the resolution of response255, 259, 260, two studies have reported a predominant FM location for Ki67-memory B cells in spleens and LNs249, 250. Although the above studies support the idea that memory B cells primarily exit via follicle adjacent sinuses, it is worth noting that CCR6hi memory B cells are also responsive to CXCL12255.
5.6. The unique niche of the DZ-T zone border in mature GCs
The follicular mantle is thin to non-existent at the true base of the DZ in mature GCs. In cross sections of mature GCs that are central and en face, IgD+ B cells can be infrequent or absent at the base of the DZ. This DZ-T zone interface is commonly observed in splenic and LN GCs when canvased in their entirety via serial sections (Fig 3e, Haberman lab unpublished data and32, 82, 242, 261). The DZ-T zone interface can be quite extensive in larger GCs and could theoretically allow DZ GC B cells to interact with cell types that are not present in other GC compartments.
This arena of mature GCs is poorly understood but clearly has the potential to be an influential venue for self-renewing centroblasts as well as GC-derived long-term plasma cells. Maturing plasmablasts can be found at this location82, 251, 262, and in a recent study found to be in association with CD157hi podoplanin+ fibroblastic reticular cells as early as 6 days post immunization241. Similar to the CRC cells within the DZ, the reported CD157hi FRC at the DZ base of young GCs also express CXCL12, but appear to lack dendritic extensions into the DZ241, 242. An intriguing analysis of LN stromal cells by single-cell RNAseq further suggests that FRC at the T zone interface with B cell follicles are heterogenous, and may include both CCL19lo and CCL19hi subsets, as well as activated subsets responding to inflammation109.
There are several features to this unique niche that have the potential to influence centroblasts and other DZ resident cell types at this DZ-T zone border. Here there are opportunities for direct contact with peri-follicular Th cell and DC subsets (Fig 3e), as well as Tregs245. There is also the potential for fresh antigen delivery via incoming local lymph within adjacent sinuses106, 243, 263. Scavenging APCs could theoretically participate in antigen acquisition from local sinuses, similar to DCs probing lymphatic sinuses or FRC junctures244, 264, and provide antigen processing and presentation to peri-follicular Tfh cell present at the DZ-T border. In this regard it is interesting to note that some DCs have a specialized capacity to retain and present antigens in native form without degradation, theoretically permitting BCR crosslinking of centroblasts in direct contact with DCs presenting incoming lymph-borne antigen115, 265. Thus at this niche, DZ GC B cells may have access to antigen to process and present, as well as APCs and T cell help, which in toto could be sufficient for any of the proposed mechanisms for GC B cell selection33, 43, 45, 59. Consistent with this niche playing a role in DZ B cell dynamics, movement to and from the DZ-T zone border has been observed by intravital imaging70. Future work in this arena is very much needed to gain insight into this poorly understood niche.
Conclusion
GC development is a complex series of events that requires that cognate B and T cells periodically engage during discreet stages in their differentiation and at distinct locations within tissue. While GC B cell development begins at the periphery of B cell follicles, this line of differentiation is progressive and is characterized by step-wise changes in transcription and phenotype over the course of multiple cell divisions. GC B cell development can be paused at an intermediate stage of differentiation when conditions are not quite right, permitting the refinement of the immune response when antigen is in excess, or when the differentiation of regional activated T cells has not sufficiently progressed. Under conditions when recently activated CD40L+ CD4+ T cells are present, but IL-21 and IL-4 are lacking, preGC B cells will transiently persist at perifollicular locations. This is in part regulated by a stage specific effect of CD40 signaling; while an early phase in GC B cell development is dependent on CD40 signaling, the subsequent phase is refractory to CD40 agonism and likely involves a transient abstention from T cell help at the periphery of B cell follicles. When IL-21 or IL-4 signaling also occurs, then GC B cell maturation is allowed to progress to the next stage that empowers follicular entry, but only if CD40 agonism is transiently avoided. The migration of Tfh cells into the follicle interior before GC B cell arrival might therefore help at multiple stages and locations by: 1) vacating the perifollicular sites and thereby reducing the chance of CD40L stimulation, and 2) entering into the follicle in advance, immediately ensuring that centrocyte and centroblast instruction occurs properly and sustainably, events that are reliant on both IL-21 and IL-4. Thus early B and T cognate interactions at the follicle periphery change in quality as they co-evolve, critically influencing when and how intrafollicular GCs are established.
All these early events are temporally and spatially choreographed by the serial movements of cognate B and T cells to different and discreet tissue locales. The stroma at these tissue destinations are themselves influenced by the evolving immune response in their arena. Together these interactions help to ensure that activated B cells receive the correct signals for their stage of GC B cell differentiation and coordinate the timing and extent of that differentiation. Finally, we conclude that it is advantageous for GC B cell differentiation to be staged and slowly progressive in that this creates checkpoints that allow for the initial creation of GC precursors, without committing to an energy intensive, and potentially misdirected and unproductive germinal center.
Acknowledgements:
We thank Jaymin Patel for skillful technical assistance with IF histology. This work was supported by National Institutes of Health NIAID grants R01AI080850 and R21AI101704 and NIAMS Rheumatic Diseases Research Core Centers grant P30AR053495–07. TZ was supported by a fellowship by the Canadian Institutes of Health Research. SMK is funded by an Operating Grant from the Canadian Institutes of Health Research and was the recipient of the Garrett Herman endMS Research and Training Network Career Development Award from the Multiple Sclerosis Society of Canada.
Bibliography
- 1.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E and Noelle RJ. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994; 180: 157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linton PJ, Lo D, Lai L, Thorbecke GJ and Klinman NR. Among naive precursor cell subpopulations only progenitors of memory B cells originate germinal centers. Eur J Immunol. 1992; 22: 1293–7. [DOI] [PubMed] [Google Scholar]
- 3.Linton PL, Decker DJ and Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989; 59: 1049–59. [DOI] [PubMed] [Google Scholar]
- 4.Tarlinton DM and Smith KG. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol Today. 2000; 21: 436–41. [DOI] [PubMed] [Google Scholar]
- 5.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994; 12: 117–39. [DOI] [PubMed] [Google Scholar]
- 6.Liu YJ, Johnson GD, Gordon J and MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992; 13: 17–21. [DOI] [PubMed] [Google Scholar]
- 7.Camacho SA, Kosco-Vilbois MH and Berek C. The dynamic structure of the germinal center. Immunol Today. 1998; 19: 511–4. [DOI] [PubMed] [Google Scholar]
- 8.Liu YJ, Grouard G, de Bouteiller O and Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol. 1996; 166: 139–79. [DOI] [PubMed] [Google Scholar]
- 9.Krautler NJ, Kana V, Kranich J, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012; 150: 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen CD, Ansel KM, Low C, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004; 5: 943–52. [DOI] [PubMed] [Google Scholar]
- 11.Szakal AK, Kosco MH and Tew JG. Microanatomy of lymphoid tissue during humoral immune responses: structure function relationships. Annu Rev Immunol. 1989; 7: 91–109. [DOI] [PubMed] [Google Scholar]
- 12.Qin D, Wu J, Vora KA, et al. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J Immunol. 2000; 164: 6268–75. [DOI] [PubMed] [Google Scholar]
- 13.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998; 16: 545–68. [DOI] [PubMed] [Google Scholar]
- 14.Liu YJ, Xu J, de Bouteiller O, et al. Follicular dendritic cells specifically express the long CR2/CD21 isoform. J Exp Med. 1997; 185: 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heesters BA, Chatterjee P, Kim YA, et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013; 38: 1164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandel TE, Phipps RP, Abbot A and Tew JG. The follicular dendritic cell: long term antigen retention during immunity. Immunol Rev. 1980; 53: 29–59. [DOI] [PubMed] [Google Scholar]
- 17.Klaus GG, Humphrey JH, Kunkl A and Dongworth DW. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980; 53: 3–28. [DOI] [PubMed] [Google Scholar]
- 18.King C, Tangye SG and Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008; 26: 741–66. [DOI] [PubMed] [Google Scholar]
- 19.Arnold CN, Campbell DJ, Lipp M and Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol. 2007; 37: 100–9. [DOI] [PubMed] [Google Scholar]
- 20.Foy TM, Aruffo A, Bajorath J, Buhlmann JE and Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996; 14: 591–617. [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt RL, Liang HE and Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunology. 2009; 10: 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf I, Kageyama R, Monticelli L, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol. 2010; 185: 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein JS, Herman EI, Lainez B, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016; 17: 1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramiscal RR and Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev. 2013; 252: 146–55. [DOI] [PubMed] [Google Scholar]
- 25.Crotty S A brief history of T cell help to B cells. Nat Rev Immunol. 2015; 15: 185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang TT, Gonzalez DG, Cote CM, et al. Germinal center B cell development has distinctly regulated stages completed by disengagement from T cell help. elife. 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P and Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000; 192: 1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L and Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001; 193: 1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000; 192: 1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu D, Batten M, Mackay CR and King C. Lineage specification and heterogeneity of T follicular helper cells. Curr Opin Immunol. 2009; 21: 619–25. [DOI] [PubMed] [Google Scholar]
- 31.Hardie DL, Johnson GD, Khan M and MacLennan IC. Quantitative analysis of molecules which distinguish functional compartments within germinal centers. Eur J Immunol. 1993; 23: 997–1004. [DOI] [PubMed] [Google Scholar]
- 32.Bannard O, Horton RM, Allen CD, An J, Nagasawa T and Cyster JG. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013; 39: 912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Victora GD, Schwickert TA, Fooksman DR, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010; 143: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calado DP, Sasaki Y, Godinho SA, et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol. 2012; 13: 1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacob J, Kassir R and Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991; 173: 1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob J, Kelsoe G, Rajewsky K and Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991; 354: 389–92. [DOI] [PubMed] [Google Scholar]
- 37.Dal Porto JM, Haberman AM, Kelsoe G and Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med. 2002; 195: 1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarlinton D Germinal centers: form and function. Curr Opin Immunol. 1998; 10: 245–51. [DOI] [PubMed] [Google Scholar]
- 39.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y and Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000; 102: 553–63. [DOI] [PubMed] [Google Scholar]
- 40.Cattoretti G, Buttner M, Shaknovich R, Kremmer E, Alobeid B and Niedobitek G. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood. 2006; 107: 3967–75. [DOI] [PubMed] [Google Scholar]
- 41.Rogerson B, Hackett J Jr., Peters A, Haasch D and Storb U. Mutation pattern of immunoglobulin transgenes is compatible with a model of somatic hypermutation in which targeting of the mutator is linked to the direction of DNA replication. Embo J. 1991; 10: 4331- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuberger MS, Di Noia JM, Beale RC, Williams GT, Yang Z and Rada C. Somatic hypermutation at A.T pairs: polymerase error versus dUTP incorporation. Nat Rev Immunol. 2005; 5: 171–8. [DOI] [PubMed] [Google Scholar]
- 43.Haberman AM and Shlomchik MJ. Reassessing the function of immune-complex retention by follicular dendritic cells. Nat Rev Immunol. 2003; 3: 757–64. [DOI] [PubMed] [Google Scholar]
- 44.Hauser AE, Kerfoot SM and Haberman AM. Cellular choreography in the germinal center: new visions from in vivo imaging. Seminars in ImmunoPathology. 2010; 32: 239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J and MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989; 342: 929–31. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Qin D, Burton GF, Szakal AK and Tew JG. Follicular dendritic cell-derived antigen and accessory activity in initiation of memory IgG responses in vitro. J Immunol. 1996; 157: 3404–11. [PubMed] [Google Scholar]
- 47.Yoon SO, Zhang X, Berner P, Blom B and Choi YS. Notch ligands expressed by follicular dendritic cells protect germinal center B cells from apoptosis. J Immunol. 2009; 183: 352–8. [DOI] [PubMed] [Google Scholar]
- 48.Liu YJ, de Bouteiller O and Fugier-Vivier I. Mechanisms of selection and differentiation in germinal centers. Curr Opin Immunol. 1997; 9: 256–62. [DOI] [PubMed] [Google Scholar]
- 49.Kosco MH, Pflugfelder E and Gray D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J Immunol. 1992; 148: 2331–9. [PubMed] [Google Scholar]
- 50.Qin D, Wu J, Carroll MC, Burton GF, Szakal AK and Tew JG. Evidence for an important interaction between a complement-derived CD21 ligand on follicular dendritic cells and CD21 on B cells in the initiation of IgG responses. J Immunol. 1998; 161: 4549–54. [PubMed] [Google Scholar]
- 51.Khalil AM, Cambier JC and Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012; 336: 1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo W, Weisel F and Shlomchik MJ. B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells. Immunity. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casamayor-Palleja M, Gulbranson-Judge A and MacLennan IC. T cells in the selection of germinal center B cells. Chem Immunol. 1997; 67: 27–44. [DOI] [PubMed] [Google Scholar]
- 54.Casamayor-Palleja M, Khan M and MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995; 181: 1293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ise W, Fujii K, Shiroguchi K, et al. T Follicular Helper Cell-Germinal Center B Cell Interaction Strength Regulates Entry into Plasma Cell or Recycling Germinal Center Cell Fate. Immunity. 2018; 48: 702–15 e4. [DOI] [PubMed] [Google Scholar]
- 56.Mesin L, Ersching J and Victora GD. Germinal Center B Cell Dynamics. Immunity. 2016; 45: 471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papa I and Vinuesa CG. Synaptic Interactions in Germinal Centers. Front Immunol. 2018; 9: 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T and Lane PJ. Co-stimulation and selection for T-cell help for germinal centres: the role of CD28 and OX40. Immunol Today. 2000; 21: 333–7. [DOI] [PubMed] [Google Scholar]
- 59.Allen CD, Okada T, Tang HL and Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007; 315: 528–31. [DOI] [PubMed] [Google Scholar]
- 60.Meyer-Hermann ME, Maini PK and Iber D. An analysis of B cell selection mechanisms in germinal centers. Math Med Biol. 2006; 23: 255–77. [DOI] [PubMed] [Google Scholar]
- 61.Liu D, Xu H, Shih C, et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2014. [DOI] [PubMed] [Google Scholar]
- 62.Gitlin AD, Shulman Z and Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014; 509: 637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hennino A, Berard M, Krammer PH and Defrance T. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis. J Exp Med. 2001; 193: 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi Y, Ohta H and Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001; 14: 181–92. [DOI] [PubMed] [Google Scholar]
- 65.Han S, Zheng B, Dal Porto J and Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995; 182: 1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hao Z, Duncan GS, Seagal J, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008; 29: 615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kepler TB and Perelson AS. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol Today. 1993; 14: 412–5. [DOI] [PubMed] [Google Scholar]
- 68.Oprea M and Perelson AS. Somatic mutation leads to efficient affinity maturation when centrocytes recycle back to centroblasts. J Immunol. 1997; 158: 5155–62. [PubMed] [Google Scholar]
- 69.Okada T and Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006; 18: 278–85. [DOI] [PubMed] [Google Scholar]
- 70.Hauser AE, Junt T, Mempel TR, et al. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007; 26: 655–67. [DOI] [PubMed] [Google Scholar]
- 71.Hauser AE, Shlomchik MJ and Haberman AM. In vivo imaging studies shed light on germinal-centre development. Nat Rev Immunol. 2007; 7: 499–504. [DOI] [PubMed] [Google Scholar]
- 72.Schwickert TA, Lindquist RL, Shakhar G, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007; 446: 83–7. [DOI] [PubMed] [Google Scholar]
- 73.Goodnow CC, Vinuesa CG, Randall KL, Mackay F and Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010; 11: 681–8. [DOI] [PubMed] [Google Scholar]
- 74.Bannard O and Cyster JG. Germinal centers: programmed for affinity maturation and antibody diversification. Curr Opin Immunol. 2017; 45: 21–30. [DOI] [PubMed] [Google Scholar]
- 75.Dominguez-Sola D, Victora GD, Ying CY, et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol. 2012; 13: 1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sander S, Chu VT, Yasuda T, et al. PI3 Kinase and FOXO1 Transcription Factor Activity Differentially Control B Cells in the Germinal Center Light and Dark Zones. Immunity. 2015; 43: 1075–86. [DOI] [PubMed] [Google Scholar]
- 77.Dominguez-Sola D, Kung J, Holmes AB, et al. The FOXO1 Transcription Factor Instructs the Germinal Center Dark Zone Program. Immunity. 2015; 43: 1064–74. [DOI] [PubMed] [Google Scholar]
- 78.Chou C, Verbaro DJ, Tonc E, et al. The Transcription Factor AP4 Mediates Resolution of Chronic Viral Infection through Amplification of Germinal Center B Cell Responses. Immunity. 2016; 45: 570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cattoretti G, Shaknovich R, Smith PM, Jack HM, Murty VV and Alobeid B. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol. 2006; 177: 6930–9. [DOI] [PubMed] [Google Scholar]
- 80.Cattoretti G, Angelin-Duclos C, Shaknovich R, Zhou H, Wang D and Alobeid B. PRDM1/Blimp-1 is expressed in human B-lymphocytes committed to the plasma cell lineage. J Pathol. 2005; 206: 76–86. [DOI] [PubMed] [Google Scholar]
- 81.Liu YJ and Banchereau J. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin Immunol. 1997; 9: 235–40. [DOI] [PubMed] [Google Scholar]
- 82.Krautler NJ, Suan D, Butt D, et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med. 2017; 214: 1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MacLennan IC, Liu YJ and Johnson GD. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol Rev. 1992; 126: 143–61. [DOI] [PubMed] [Google Scholar]
- 84.Bannard O, McGowan SJ, Ersching J, et al. Ubiquitin-mediated fluctuations in MHC class II facilitate efficient germinal center B cell responses. J Exp Med. 2016; 213: 993–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J and Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996; 183: 971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith KG, Nossal GJ and Tarlinton DM. FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc Natl Acad Sci U S A. 1995; 92: 11628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cervenak L, Magyar A, Boja R and Laszlo G. Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol Lett. 2001; 78: 89–96. [DOI] [PubMed] [Google Scholar]
- 88.Oliver AM, Martin F and Kearney JF. Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells. J Immunol. 1997; 158: 1108–15. [PubMed] [Google Scholar]
- 89.Laszlo G, Hathcock KS, Dickler HB and Hodes RJ. Characterization of a novel cell-surface molecule expressed on subpopulations of activated T and B cells. J Immunol. 1993; 150: 5252–62. [PubMed] [Google Scholar]
- 90.Fooksman DR, Schwickert TA, Victora GD, Dustin ML, Nussenzweig MC and Skokos D. Development and migration of plasma cells in the mouse lymph node. Immunity. 2010; 33: 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bunting KL and Melnick AM. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Current Opinion in Immunology. 2013; 25: 339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Basso K, Saito M, Sumazin P, et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood. 2010; 115: 975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dent AL, Shaffer AL, Yu X, Allman D and Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997; 276: 589–92. [DOI] [PubMed] [Google Scholar]
- 94.Ye BH, Cattoretti G, Shen Q, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997; 16: 161–70. [DOI] [PubMed] [Google Scholar]
- 95.Choi YS, Yang JA and Crotty S. Dynamic regulation of BcI6 in follicular helper CD4 T (Tfh) cells. Current Opinion in Immunology. 2013; 25: 366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG and Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999; 190: 1123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cyster JG, Ansel KM, Reif K, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000; 176: 181–93. [DOI] [PubMed] [Google Scholar]
- 98.Katakai T, Suto H, Sugai M, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008; 181: 6189–200. [DOI] [PubMed] [Google Scholar]
- 99.Jarjour M, Jorquera A, Mondor I, et al. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med. 2014; 211: 1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mionnet C, Mondor I, Jorquera A, et al. Identification of a new stromal cell type involved in the regulation of inflamed B cell follicles. PLoS Biol. 2013; 11: e1001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999; 99: 23–33. [DOI] [PubMed] [Google Scholar]
- 102.Luther SA, Tang HL, Hyman PL, Farr AG and Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000; 97: 12694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gatto D, Paus D, Basten A, Mackay CR and Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009; 31: 259–69. [DOI] [PubMed] [Google Scholar]
- 104.Pereira JP, Kelly LM, Xu Y and Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009; 460: 1122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sinha RK, Park C, Hwang IY, Davis MD and Kehrl JH. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 2009; 30: 434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grigorova IL, Panteleev M and Cyster JG. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc Natl Acad Sci U S A. 2010; 107: 20447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yi T and Cyster JG. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. elife. 2013; 2: e00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelly LM, Pereira JP, Yi T, Xu Y and Cyster JG. EBI2 guides serial movements of activated B cells and ligand activity is detectable in lymphoid and nonlymphoid tissues. J Immunol. 2011; 187: 3026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodda LB, Lu E, Bennett ML, et al. Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity. 2018; 48: 1014–28 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Batista FD and Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009; 9: 15–27. [DOI] [PubMed] [Google Scholar]
- 111.Harwood NE and Batista FD. The antigen expressway: follicular conduits carry antigen to B cells. Immunity. 2009; 30: 177–9. [DOI] [PubMed] [Google Scholar]
- 112.Heesters BA, van der Poel CE, Das A and Carroll MC. Antigen Presentation to B Cells. Trends Immunol. 2016; 37: 844–54. [DOI] [PubMed] [Google Scholar]
- 113.Junt T, Moseman EA, Iannacone M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007; 450: 110–4. [DOI] [PubMed] [Google Scholar]
- 114.Carrasco YR and Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007; 27: 160–71. [DOI] [PubMed] [Google Scholar]
- 115.Qi H, Egen JG, Huang AY and Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006; 312: 1672–6. [DOI] [PubMed] [Google Scholar]
- 116.Wykes M, Pombo A, Jenkins C and MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998; 161: 1313–9. [PubMed] [Google Scholar]
- 117.Damdinsuren B, Zhang Y, Khalil A, et al. Single round of antigen receptor signaling programs naive B cells to receive T cell help. Immunity. 2010; 32: 355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turner JS, Marthi M, Benet ZL and Grigorova I. Transiently antigen-primed B cells return to naive-like state in absence of T-cell help. Nat Commun. 2017; 8: 15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ and Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998; 281: 96–9. [DOI] [PubMed] [Google Scholar]
- 120.Okada T, Miller MJ, Parker I, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005; 3: e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reif K, Ekland EH, Ohl L, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002; 416: 94–9. [DOI] [PubMed] [Google Scholar]
- 122.Suan D, Nguyen A, Moran I, et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity. 2015; 42: 704–18. [DOI] [PubMed] [Google Scholar]
- 123.Lund FE and Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010; 10: 236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi W, Liao Y, Willis SN, et al. Transcriptional profiling of mouse B cell terminal differentiation defines a signature for antibody-secreting plasma cells. Nat Immunol. 2015; 16: 663–73. [DOI] [PubMed] [Google Scholar]
- 125.Weisel F and Shlomchik M. Memory B Cells of Mice and Humans. Annu Rev Immunol. 2017; 35: 255–84. [DOI] [PubMed] [Google Scholar]
- 126.Kerfoot SM, Yaari G, Patel JR, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011; 34: 947–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitano M, Moriyama S, Ando Y, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011; 34: 961–72. [DOI] [PubMed] [Google Scholar]
- 128.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP and Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000; 13: 199–212. [DOI] [PubMed] [Google Scholar]
- 129.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N and Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007; 179: 5099–108. [DOI] [PubMed] [Google Scholar]
- 130.Pereira JP, Kelly LM and Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010; 22: 413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goenka R, Barnett LG, Silver JS, et al. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011; 187: 1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011; 34: 932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baumjohann D, Okada T and Ansel KM. Cutting Edge: Distinct waves of BCL6 expression during T follicular helper cell development. J Immunol. 2011; 187: 2089–92. [DOI] [PubMed] [Google Scholar]
- 134.Deenick EK, Chan A, Ma CS, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010; 33: 241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Poholek AC, Hansen K, Hernandez SG, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010; 185: 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG and Hodgkin PD. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol. 2004; 5: 55–63. [DOI] [PubMed] [Google Scholar]
- 137.Hasbold J, Lyons AB, Kehry MR and Hodgkin PD. Cell division number regulates IgG1 and IgE switching of B cells following stimulation by CD40 ligand and IL-4. Eur J Immunol. 1998; 28: 1040–51. [DOI] [PubMed] [Google Scholar]
- 138.Hodgkin PD, Lee JH and Lyons AB. B cell differentiation and isotype switching is related to division cycle number. J Exp Med. 1996; 184: 277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paus D, Phan TG, Chan TD, Gardam S, Basten A and Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006; 203: 1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.O’Connor BP, Vogel LA, Zhang W, et al. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. J Immunol. 2006; 177: 7723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schwickert TA, Victora GD, Fooksman DR, et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011; 208: 1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang CX, Gonzalez DG, Cote CM, et al. The BCL6 RD2 Domain Governs Commitment of Activated B Cells to Form Germinal Centers. Cell Rep. 2014; 8: 1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ci W, Polo JM, Cerchietti L, et al. The BCL6 transcriptional program features repression of multiple oncogenes in primary B cells and is deregulated in DLBCL. Blood. 2009; 113: 5536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009; 325: 1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM and Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004; 173: 1158–65. [DOI] [PubMed] [Google Scholar]
- 146.Crotty S, Johnston RJ and Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010; 11: 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Basso K and Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev. 2012; 247: 172–83. [DOI] [PubMed] [Google Scholar]
- 148.Shaffer AL, Lin KI, Kuo TC, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002; 17: 51–62. [DOI] [PubMed] [Google Scholar]
- 149.Nutt SL, Hodgkin PD, Tarlinton DM and Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015; 15: 160–71. [DOI] [PubMed] [Google Scholar]
- 150.Ochiai K, Maienschein-Cline M, Simonetti G, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013; 38: 918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saito M, Gao J, Basso K, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007; 12: 280–92. [DOI] [PubMed] [Google Scholar]
- 152.Kwon H, Thierry-Mieg D, Thierry-Mieg J, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009; 31: 941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Klein U, Casola S, Cattoretti G, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006; 7: 773–82. [DOI] [PubMed] [Google Scholar]
- 154.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM and Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006; 25: 225–36. [DOI] [PubMed] [Google Scholar]
- 155.Willis SN, Good-Jacobson KL, Curtis J, et al. Transcription factor IRF4 regulates germinal center cell formation through a B cell-intrinsic mechanism. J Immunol. 2014; 192: 3200–6. [DOI] [PubMed] [Google Scholar]
- 156.Koguchi Y, Buenafe AC, Thauland TJ, et al. Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD4+ 8-thymocytes and invariant NKT cells but not in Treg cells. PLoS One. 2012; 7: e31296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rothoeft T, Balkow S, Krummen M, et al. Structure and duration of contact between dendritic cells and T cells are controlled by T cell activation state. Eur J Immunol. 2006; 36: 3105–17. [DOI] [PubMed] [Google Scholar]
- 158.Fusco AJ, Savinova OV, Talwar R, Kearns JD, Hoffmann A and Ghosh G. Stabilization of RelB requires multidomain interactions with p100/p52. J Biol Chem. 2008; 283: 12324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vallabhapurapu S and Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009; 27: 693–733. [DOI] [PubMed] [Google Scholar]
- 160.Homig-Holzel C, Hojer C, Rastelli J, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappa B pathway and promotes lymphomagenesis. Journal of Experimental Medicine. 2008; 205: 1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu J, Foy TM, Laman JD, et al. Mice deficient for the CD40 ligand. Immunity. 1994; 1: 423–31. [DOI] [PubMed] [Google Scholar]
- 162.Allman D, Jain A, Dent A, et al. BCL-6 expression during B-cell activation. Blood. 1996; 87: 5257–68. [PubMed] [Google Scholar]
- 163.Bolduc Long AE, Stapler D, et al. Constitutive CD40L expression on B cells prematurely terminates germinal center response and leads to augmented plasma cell production in T cell areas (vol 185, pg 220, 2010). Journal of Immunology. 2010; 185: 2631-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kishi Y, Aiba Y, Higuchi T, et al. Augmented antibody response with premature germinal center regression in CD40L transgenic mice. J Immunol. 2010; 185: 211–9. [DOI] [PubMed] [Google Scholar]
- 165.Erickson LD, Durell BG, Vogel LA, et al. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J Clin Invest. 2002; 109: 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grumont RJ and Gerondakis S. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J Exp Med. 2000; 191: 1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ghetu AF, Corcoran CM, Cerchietti L, Bardwell VJ, Melnick A and Prive GG. Structure of a BCOR corepressor peptide in complex with the BCL6 BTB domain dimer. Molecular Cell. 2008; 29: 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ahmad KF, Melnick A, Lax S, et al. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Molecular Cell. 2003; 12: 1551–64. [DOI] [PubMed] [Google Scholar]
- 169.Basso K and Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010; 105: 193–210. [DOI] [PubMed] [Google Scholar]
- 170.Hatzi K, Jiang YW, Huang CX, et al. A Hybrid Mechanism of Action for BCL6 in B Cells Defined by Formation of Functionally Distinct Complexes at Enhancers and Promoters. Cell Rep. 2013; 4: 578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hatzi K, Nance JP, Kroenke MA, et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015; 212: 539–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beguelin W, Popovic R, Teater M, et al. EZH2 Is Required for Germinal Center Formation and Somatic EZH2 Mutations Promote Lymphoid Transformation. Cancer Cell. 2013; 23: 677–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Velichutina I, Shaknovich R, Geng H, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood. 2010; 116: 5247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Belver L, Papavasiliou FN and Ramiro AR. MicroRNA control of lymphocyte differentiation and function. Curr Opin Immunol. 2011; 23: 368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.O’Connell RM, Rao DS, Chaudhuri AA and Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010; 10: 111–22. [DOI] [PubMed] [Google Scholar]
- 176.Barnett BE, Ciocca ML, Goenka R, et al. Asymmetric B cell division in the germinal center reaction. Science. 2012; 335: 342–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hawkins ED, Oliaro J, Kallies A, et al. Regulation of asymmetric cell division and polarity by Scribble is not required for humoral immunity. Nat Commun. 2013; 4: 1801. [DOI] [PubMed] [Google Scholar]
- 178.Di Niro R, Lee SJ, Vander Heiden JA, et al. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity. 2015; 43: 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thaunat O, Granja AG, Barral P, et al. Asymmetric segregation of polarized antigen on B cell division shapes presentation capacity. Science. 2012; 335: 475–9. [DOI] [PubMed] [Google Scholar]
- 180.Thaunat O and Batista FD. Daughter B cells are not created equal: asymmetric segregation of antigen during B cell division. Cell Cycle. 2012; 11: 2219–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Luthje K, Kallies A, Shimohakamada Y, et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature Immunology. 2012; 13: 491–U93. [DOI] [PubMed] [Google Scholar]
- 182.Gonzalez DG, Cote CM, Patel J, et al. Non-redundant roles of IL-21 and IL-4 in the phased initiation of germinal center B cells and subsequent self-renewal transitions Journal of Immunology. 2018; 201: 3569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chang PP, Barral P, Fitch J, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2012; 13: 35–43. [DOI] [PubMed] [Google Scholar]
- 184.King IL, Fortier A, Tighe M, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2012; 13: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tonti E, Fedeli M, Napolitano A, et al. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol. 2012; 188: 3217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gaya M, Barral P, Burbage M, et al. Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells. Cell. 2018; 172: 517–33 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Voehringer D, Reese TA, Huang X, Shinkai K and Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006; 203: 1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sokol CL, Barton GM, Farr AG and Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008; 9: 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009; 10: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gessner A, Mohrs K and Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005; 174: 1063–72. [DOI] [PubMed] [Google Scholar]
- 191.Linterman MA, Beaton L, Yu D, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010; 207: 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zotos D, Coquet JM, Zhang Y, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010; 207: 365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cunningham AF, Serre K, Toellner KM, et al. Pinpointing IL-4-independent acquisition and IL-4-influenced maintenance of Th2 activity by CD4 T cells. European Journal of Immunology. 2004; 34: 686–94. [DOI] [PubMed] [Google Scholar]
- 194.Turqueti-Neves A, Otte M, Prazeres da Costa O, et al. B-cell-intrinsic STAT6 signaling controls germinal center formation. Eur J Immunol. 2014; 44: 2130–8. [DOI] [PubMed] [Google Scholar]
- 195.Chevrier S, Kratina T, Emslie D, Tarlinton DM and Corcoran LM. IL4 and IL21 cooperate to induce the high Bcl6 protein level required for germinal center formation. Immunol Cell Biol. 2017; 95: 925–32. [DOI] [PubMed] [Google Scholar]
- 196.Arguni E, Arima M, Tsuruoka N, Sakamoto A, Hatano M and Tokuhisa T. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol. 2006; 18: 1079–89. [DOI] [PubMed] [Google Scholar]
- 197.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002; 298: 1630–4. [DOI] [PubMed] [Google Scholar]
- 198.Ozaki K, Spolski R, Ettinger R, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004; 173: 5361–71. [DOI] [PubMed] [Google Scholar]
- 199.Kuchen S, Robbins R, Sims GP, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4(+) T cell-B cell collaboration. Journal of Immunology. 2007; 179: 5886–96. [DOI] [PubMed] [Google Scholar]
- 200.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007; 179: 8180–90. [DOI] [PubMed] [Google Scholar]
- 201.Defrance T, Vanbervliet B, Pene J and Banchereau J. Human Recombinant Il-4 Induces Activated Lymphocytes-B to Produce IgG and IgM. Journal of Immunology. 1988; 141: 2000–5. [PubMed] [Google Scholar]
- 202.Russell SM, Keegan AD, Harada N, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993; 262: 1880–3. [DOI] [PubMed] [Google Scholar]
- 203.Habib T, Senadheera S, Weinberg K and Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002; 41: 8725–31. [DOI] [PubMed] [Google Scholar]
- 204.Spolski R and Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014; 13: 379–95. [DOI] [PubMed] [Google Scholar]
- 205.Asao H, Okuyama C, Kumaki S, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001; 167: 1–5. [DOI] [PubMed] [Google Scholar]
- 206.Zeng R, Spolski R, Casas E, Zhu W, Levy DE and Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007; 109: 4135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Avery DT, Deenick EK, Ma CS, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. Journal of Experimental Medicine. 2010; 207: 155–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deenick EK, Avery DT, Chan A, et al. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J Exp Med. 2013; 210: 2739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mehta DS, Wurster AL and Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 2004; 202: 84–95. [DOI] [PubMed] [Google Scholar]
- 210.Quelle FW, Shimoda K, Thierfelder W, et al. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995; 15: 3336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Paul WE. History of interleukin-4. Cytokine. 2015; 75: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dasoveanu DC, Shipman WD, Chia JJ, Chyou S and Lu TT. Regulation of Lymph Node Vascular-Stromal Compartment by Dendritic Cells. Trends Immunol. 2016; 37: 764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kapasi ZF, Qin D, Kerr WG, et al. Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol. 1998; 160: 1078–84. [PubMed] [Google Scholar]
- 214.Balogh P, Aydar Y, Tew JG and Szakal AK. Ontogeny of the follicular dendritic cell phenotype and function in the postnatal murine spleen. Cell Immunol. 2001; 214: 45–53. [DOI] [PubMed] [Google Scholar]
- 215.Garin A, Meyer-Hermann M, Contie M, et al. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity. 2010; 33: 84–95. [DOI] [PubMed] [Google Scholar]
- 216.El Shikh ME, El Sayed RM, Wu Y, Szakal AK and Tew JG. TLR4 on follicular dendritic cells: an activation pathway that promotes accessory activity. J Immunol. 2007; 179: 4444–50. [DOI] [PubMed] [Google Scholar]
- 217.Victoratos P, Lagnel J, Tzima S, et al. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity. 2006; 24: 65–77. [DOI] [PubMed] [Google Scholar]
- 218.El Shikh ME, El Sayed R, Szakal AK and Tew JG. Follicular dendritic cell (FDC)-FcgammaRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur J Immunol. 2006; 36: 2715–24. [DOI] [PubMed] [Google Scholar]
- 219.Balogh P, Aydar Y, Tew JG and Szakal AK. Appearance and phenotype of murine follicular dendritic cells expressing VCAM-1. Anat Rec. 2002; 268: 160–8. [DOI] [PubMed] [Google Scholar]
- 220.Heesters BA, Myers RC and Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. 2014; 14: 495–504. [DOI] [PubMed] [Google Scholar]
- 221.Kranich J, Krautler NJ, Heinen E, et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008; 205: 1293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim HS, Zhang X and Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994; 153: 2951–61. [PubMed] [Google Scholar]
- 223.Yoshida K, van den Berg TK and Dijkstra CD. Two functionally different follicular dendritic cells in secondary lymphoid follicles of mouse spleen, as revealed by CR1/2 and FcR gamma II-mediated immune-complex trapping. Immunology. 1993; 80: 34–9. [PMC free article] [PubMed] [Google Scholar]
- 224.Sacedon R, Diez B, Nunez V, et al. Sonic hedgehog is produced by follicular dendritic cells and protects germinal center B cells from apoptosis. J Immunol. 2005; 174: 1456–61. [DOI] [PubMed] [Google Scholar]
- 225.Crompton T, Outram SV and Hager-Theodorides AL. Sonic hedgehog signalling in T-cell development and activation. Nat Rev Immunol. 2007; 7: 726–35. [DOI] [PubMed] [Google Scholar]
- 226.Trowbridge JJ, Scott MP and Bhatia M. Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci U S A. 2006; 103: 14134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Campbell C, Risueno RM, Salati S, Guezguez B and Bhatia M. Signal control of hematopoietic stem cell fate: Wnt, Notch, and Hedgehog as the usual suspects. Curr Opin Hematol. 2008; 15: 319–25. [DOI] [PubMed] [Google Scholar]
- 228.St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998; 8: 1058–68. [DOI] [PubMed] [Google Scholar]
- 229.Allen CD and Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008; 20: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Smith KG, Light A, Nossal GJ and Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. Embo J. 1997; 16: 2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peperzak V, Vikstrom I, Walker J, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013; 14: 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Weisel FJ, Zuccarino-Catania GV, Chikina M and Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity. 2016; 44: 116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang Y, Garcia-Ibanez L and Toellner KM. Regulation of germinal center B-cell differentiation. Immunol Rev. 2016; 270: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shinnakasu R, Inoue T, Kometani K, et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol. 2016; 17: 861–9. [DOI] [PubMed] [Google Scholar]
- 235.Coffey F, Alabyev B and Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009; 30: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nikbakht N, Shen S and Manser T. Cutting edge: Macrophages are required for localization of antigen-activated B cells to the follicular perimeter and the subsequent germinal center response. J Immunol. 2013; 190: 4923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Taylor JJ, Pape KA and Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012; 209: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Duan S, Cermak L, Pagan JK, et al. FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature. 2012; 481: 90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006; 9: 435–43. [DOI] [PubMed] [Google Scholar]
- 240.Takahashi H, Kanno T, Nakayamada S, et al. TGF-beta and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol. 2012; 13: 587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang Y, Tech L, George LA, et al. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J Exp Med. 2018; 215: 1227–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rodda LB, Bannard O, Ludewig B, Nagasawa T and Cyster JG. Phenotypic and Morphological Properties of Germinal Center Dark Zone Cxcl12-Expressing Reticular Cells. J Immunol. 2015; 195: 4781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gretz JE, Anderson AO and Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997; 156: 11–24. [DOI] [PubMed] [Google Scholar]
- 244.Sixt M, Kanazawa N, Selg M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005; 22: 19–29. [DOI] [PubMed] [Google Scholar]
- 245.Sayin I, Radtke AJ, Vella LA, et al. Spatial distribution and function of T follicular regulatory cells in human lymph nodes. J Exp Med. 2018; 215: 1531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lim HW, Hillsamer P and Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004; 114: 1640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lim HW, Hillsamer P, Banham AH and Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005; 175: 4180–3. [DOI] [PubMed] [Google Scholar]
- 248.Sage PT, Ron-Harel N, Juneja VR, et al. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016; 17: 1436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009; 10: 1292–9. [DOI] [PubMed] [Google Scholar]
- 250.Moran I, Nguyen A, Khoo WH, et al. Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nat Commun. 2018; 9: 3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R and Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014; 85: 15–24. [DOI] [PubMed] [Google Scholar]
- 252.Hauser AE, Debes GF, Arce S, et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002; 169: 1277–82. [DOI] [PubMed] [Google Scholar]
- 253.Hargreaves DC, Hyman PL, Lu TT, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001; 194: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bhattacharya D, Cheah MT, Franco CB, et al. Transcriptional profiling of antigen-dependent murine B cell differentiation and memory formation. J Immunol. 2007; 179: 6808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Elgueta R, Marks E, Nowak E, et al. CCR6-dependent positioning of memory B cells is essential for their ability to mount a recall response to antigen. J Immunol. 2015; 194: 505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Suan D, Krautler NJ, Maag JLV, et al. CCR6 Defines Memory B Cell Precursors in Mouse and Human Germinal Centers, Revealing Light-Zone Location and Predominant Low Antigen Affinity. Immunity. 2017; 47: 1142–53 e4. [DOI] [PubMed] [Google Scholar]
- 257.Piqueras B, Connolly J, Freitas H, Palucka AK and Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006; 107: 2613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang Y, Roth TL, Gray EE, et al. Migratory and adhesive cues controlling innate-like lymphocyte surveillance of the pathogen-exposed surface of the lymph node. elife. 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liu YJ, Oldfield S and MacLennan IC. Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur J Immunol. 1988; 18: 355–62. [DOI] [PubMed] [Google Scholar]
- 260.Anderson SM, Tomayko MM, Ahuja A, Haberman AM and Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007; 204: 2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liu YJ, Zhang J, Lane PJ, Chan EY and MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991; 21: 2951–62. [DOI] [PubMed] [Google Scholar]
- 262.Meyer-Hermann M, Mohr E, Pelletier N, Zhang Y, Victora GD and Toellner KM. A theory of germinal center B cell selection, division, and exit. Cell Rep. 2012; 2: 162–74. [DOI] [PubMed] [Google Scholar]
- 263.Gonzalez SF, Degn SE, Pitcher LA, Woodruff M, Heesters BA and Carroll MC. Trafficking of B cell antigen in lymph nodes. Annu Rev Immunol. 2011; 29: 215–33. [DOI] [PubMed] [Google Scholar]
- 264.Gerner MY, Torabi-Parizi P and Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015; 42: 172–85. [DOI] [PubMed] [Google Scholar]
- 265.Bergtold A, Desai DD, Gavhane A and Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005; 23: 503–14. [DOI] [PubMed] [Google Scholar]