Significance
Here, we describe a previously unidentified structural class of lantibiotics (named salivaricin 10) produced in the oral cavity and contains a phosphorylated amino acid (pThr) in the mature form of the peptide, which is atypical for lanthionine-containing peptides. We have found the pThr4 residue to be essential for the induction of important yet unreported proimmune functions, including enhancing neutrophil recruitment and phagocytosis and promoting antiinflammatory macrophages. The discovery of salivaricin 10 opens the question of the abundance of phosphorylated lantibiotics in other human microbiomes and their role in promoting health beyond antimicrobial capacity.
Keywords: lantibiotics, phosphorylated lanthipeptides, oral microbiome, immunomodulation, antibiofilm
Abstract
Lantibiotics are ribosomally synthesized and posttranslationally modified peptides (RiPPs) that are produced by bacteria. Interest in this group of natural products is increasing rapidly as alternatives to conventional antibiotics. Some human microbiome–derived commensals produce lantibiotics to impair pathogens’ colonization and promote healthy microbiomes. Streptococcus salivarius is one of the first commensal microbes to colonize the human oral cavity and gastrointestinal tract, and its biosynthesis of RiPPs, called salivaricins, has been shown to inhibit the growth of oral pathogens. Herein, we report on a phosphorylated class of three related RiPPs, collectively referred to as salivaricin 10, that exhibit proimmune activity and targeted antimicrobial properties against known oral pathogens and multispecies biofilms. Strikingly, the immunomodulatory activities observed include upregulation of neutrophil-mediated phagocytosis, promotion of antiinflammatory M2 macrophage polarization, and stimulation of neutrophil chemotaxis—these activities have been attributed to the phosphorylation site identified on the N-terminal region of the peptides. Salivaricin 10 peptides were determined to be produced by S. salivarius strains found in healthy human subjects, and their dual bactericidal/antibiofilm and immunoregulatory activity may provide new means to effectively target infectious pathogens while maintaining important oral microbiota.
The human oral microbiota, also known as the oralome, consists of diverse composition of bacteria (1–3), fungi (4, 5), viruses (6, 7), archaea (8, 9), and protozoa (10) that inhabit a dynamic and diverse set of ecological niches that coexist in the oral cavity of the host. Commensal microorganisms have evolved the means to cohabitate as polyspecies biofilms that can be found on the surfaces of the buccal mucosa, gingiva, teeth, palate, and tonsils located within the confines of the mouth (11, 12). When enteric microorganisms infiltrate oral biofilms, a diverse pool of commensal bacteria is capable of mounting a robust antimicrobial response to inhibit the colonization of harmful pathogens (13–15) and, through chemical signaling, can communicate with host tissues of the buccal mucosa to elicit an appropriate immune response at the epithelial interface (16, 17).
Streptococcus salivarius is a pioneer colonizer of the oral cavity and gastrointestinal tract in humans (18) and is known to promote the establishment of immune homeostasis and management of host inflammatory responses by inhibiting the activation of the nuclear factor kappa B (NF-κβ) pathway (19). In addition to immunoregulation, some strains of S. salivarius secrete bacteriocins with antimicrobial activity against other species; these compounds are called salivaricins. Salivaricins are ribosomally synthesized and posttranslationally modified peptides (RiPPs) (20), mostly belonging to the lantibiotic class II of lanthipeptides (21). Recent sequencing efforts have expanded the number of known lanthipeptides and have led to the discovery that their functions extend beyond antimicrobial activities to include antifungal (22), antiviral (23, 24), antinociceptive (25), and antiallodynic functions (26) and promoters of host immunomodulation (27, 28).
Lantibiotics are RiPPs produced through a process that involves the dehydration of selected serine (Ser) and threonine (Thr) residues and the intramolecular addition of cysteine thiols to the resulting unsaturated amino acids to produce lanthionine (Lan) and methylLan rings, respectively (29, 30). Dehydration during biosynthesis of class II lanthipeptides is accompanied by the phosphorylation of Thr and Ser residues by the Lan dehydratase enzyme, followed by phosphate elimination with antistereoselectivity. As such, the final active forms of all known lanthipeptides described to date are not phosphorylated (31, 32).
Here, we report the discovery of a phosphorylated form of lanthipeptides with potent antimicrobial and antibiofilm activities produced by the oral commensal S. salivarius. These peptides, collectively named salivaricin 10, can prime human neutrophils and macrophages to enhance phagocytosis and induce antiinflammatory responses in vitro and in vivo, providing multilevel protection against infectious diseases. Salivaricin 10 appears to broadly inhibit upper respiratory tract pathogens while maintaining the survival of key commensal Streptococci in oral biofilms, a highly desirable property for a potential therapeutic. Surprisingly, and in contrast to all known lanthipeptides, the active forms of these peptides are phosphorylated, and this modification is essential for their activity. Altogether, our work expands the definition of lanthipeptides to include phosphorylated species and uncovers a potential therapeutic candidate for oral infections.
Results
Salivaricin 10 RiPPs Are Selective Antimicrobials against Oral Pathogens.
Whole-genome sequencing of S. salivarius SALI-10 revealed a single lanthipeptide encoding operon (srn) on a 164 kb megaplasmid [(Fig. 1); National Center for Biotechnology Information (NCBI) accession number CP090008]. The guanine-cytosine content of the srn operon was comparable to the megaplasmidPSaLI10 as a whole (33.17% and 34.8%, respectively), indicating that the srn operon may be native to S. salivarius SALI-10. The srn operon includes genes encoding four putative lantibiotic prepropeptides with an N-terminal leader peptide (srnA/1-4), putative self-immunity system composed of an adenosine triphosphate-binding cassette and a transmembrane protein (srnXY), a histidine kinase (srnK) and response regulator (srnR), a class II Lan synthetase enzyme (srnM-10), and a lanthipeptide transporter (srnT) (Fig. 1). The PSaLI10 megaplasmid from S. salivarius SALI-10 was compared to other megaplasmids found in lantibiotic-producing S. salivarius strains using BLAST analysis. The results showed that the srn genes in PSaLI10 have less than 50% similarity with the sal genes, which are part of a conserved salivaricin A locus found in all other compared strains except S. salivarius SALI-10 (SI Appendix, Fig. S1). To test whether the megaplasmid of S. salivarius SALI-10 is responsible for its antimicrobial activity, we performed megaplasmid curing and found that the strain lacking the megaplasmid (SALI-10-PSaLI10) lost the strong antimicrobial activity noted in the wild-type strain (Fig. 2A). Ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) revealed three distinct peaks from S. salivarius SALI-10 WT extracts that were absent in the megaplasmid-cured S. salivarius SALI-10-PSaLI10 strain (Fig. 2B). This may indicate that out of the four potential peptides encoded in the megaplasmid, only three posttranslationally modified/protease-processed peptides are expressed in the mature form (SrnA1, SrnA2, and SrnA4), or the fourth peptide (SrnA3) was produced but not identified under the current detection and isolation methods we used.
Fig. 1.
Genetic map of pSALI-10 megaplasmid encoding for the production of salivaricin 10 peptides in strain S. salivarius SALI-10. BLAST was performed against other S. salivarius megaplasmids encoding other salivaricin-family lantibiotics operons (strains K12, M18, NU10, and YU10). Rapid genome-wide mining was used to detect putative secondary metabolite biosynthesis gene clusters in S. salivarius SALI-10 which identified only one potential lanthipeptide operon (srn). The srn operon includes characteristic class II LanM dehydratase, response regulator and histidine kinase, putative self-immunity genes, and ABC transporter. Putative lanthipeptide precursors detected by genome mining are shown in the Top Left, the blue sequence is the putative leader peptide, and the black sequence is the putative mature peptide as predicted by antiSMASH. Residues that may be involved in lanthionine/β-methyllanthionine ring formation are colored as follows: serine and threonine residues are in red, and cysteine residues are underlined in purple.
Fig. 2.
Salivaricin 10 production by wild-type S. salivarius and megaplasmid-cured mutant. (A) Bioactivity assay with the S. salivarius SALI-10 wild type, the plasmid lacking strain (−PSaLI10) was tested as a negative control, and it lacks the megaplasmid harboring the srn locus responsible for producing salivaricin 10 peptides. Both SALI-10 and SALI-10−PSaLI10 strains were spotted on the surface of Tryptic Soy Agar plates and were incubated for 18 h before overlaying with M. luteus ATCC10240 as a sensitive bacterial target. An inhibition zone of no target bacteria growth is considered as antibacterial action. (B) Colony mass spectrometry profile of salivaricin 10 producer strain SALI-10 (red) and plasmid-negative variant (green). Cell extracts were compared by reversed-phase UPLC-QTOF-MS analysis. The three peptides composing salivaricin 10 (peaks 1, 2, and 3) are indicated in a box. (C) The absorption spectrum of salivaricin 10 peptides. All the three compounds have a prominent absorption at 280 nm—which corresponds to the absorption of an aromatic ring—indicating the presence of tryptophan residue on the peptide since the gene sequence is known, and thus the UV absorption profile observed matches the predicted profile from the expected product.
After detecting the three peptides in the cell extract, we purified secreted SrnA1, SrnA2, and SrnA4 peptides from the cell-free supernatant of S. salivarius SALI-10 agar cultures using freezing and thawing method followed by chloroform extraction, solid phase extraction, and C18 reversed-phase chromatography. UPLC-QTOF-MS analysis showed that the masses of the three peptides from the cell extract are identical to those derived from the cell-free supernatant (SI Appendix, Figs. S2–S4). The combined peptides fraction exhibited potent antimicrobial activity against a wide range of gram-positive bacteria, including opportunistic pathogens such as multidrug-resistant (MDR) Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, vancomycin-resistant Enterococcus faecium, and MDR Enterococcus faecalis clinical isolates (SI Appendix, Table S1). Importantly, salivaricin 10 also possessed antibacterial activity against selected gram-negative disease–associated bacteria, including Porphyromonas gingivalis, Tannerella forsythia, and Neisseria gonorrhoeae. Bacterial species not inhibited by salivaricin 10 under the current test conditions were Streptococcus mutans UA159 and Lactobacillus rhamnosus ATCC 11981. The antimicrobial activity of salivaricin 10 against gram-negative bacteria is gripping, especially since gram-negative bacteria have an outer membrane acting as an excellent shielding barrier to prevent lanthipeptides from reaching the inner cytoplasmic membrane (33). Minimal inhibitory concentration (MIC) values of salivaricin 10 were in the range of 0.125 to 64 μg/mL for gram-positive bacteria and 32 to 64 μg/mL for gram-negative bacteria, demonstrating high potency (SI Appendix, Table S1). To address whether the observed inhibitory activity is bactericidal or bacteriostatic, we tested salivaricin 10 in a time-killing assay against either S. pyogenes or E. faecalis. E. faecalis is known to produce membrane vesicles that contribute to antimicrobial resistance and host immune evasion (34), and we show that salivaricin 10 rapidly reduced the number of E. faecalis cells within 2 h of exposure with logarithmic-killing kinetics and complete killing observed after 24 h exposure. S. pyogenes cells were killed entirely after 4 h of exposure without any cellular regrowth at the 24-h time point (SI Appendix, Fig. S5). These results indicate potent bactericidal activity of salivaricin 10 against key bacterial pathogens.
As the next step in our investigation of salivaricin 10, we asked whether it is bioactive against saliva-derived multispecies biofilms, and whether it exhibits selectivity in its antimicrobial activity in this context. Biofilms were formed ex vivo using cell-free saliva as a medium, and total salivary cells pooled from six healthy subjects as an inoculum. S. salivarius isolates of the six healthy subjects did not harbor the biosynthetic gene cluster of salivaricin 10 (srn) (SI Appendix, Fig. S6). Preformed multispecies biofilms grown anaerobically for 24 h were treated with either nisin A (50 µg/mL) or two concentrations of salivaricin 10 (25 and 50 µg/mL) for 30 min. The biofilms were then washed to remove tested compounds and scraped to make a total cell suspension which was inoculated into a brain–heart infusion (BHI) broth to allow surviving cells to grow overnight. 16S rRNA gene amplicon sequencing was used to profile the bacterial composition of viable cells that survived the peptides’ bactericidal action and were able to regrow again posttreatment compared to viable cells derived from the PBS treatment as a negative control (Fig. 3 A–C), BioProject: PRJNA924352 (35). The rarefaction curve, which corresponds to species richness, was relatively decreased in nisin A- and salivaricin 10-treated samples compared to phosphate-buffered saline (PBS) (SI Appendix, Fig. S7). We evaluated the effects of nisin A and salivaricin 10 on the oral biofilm diversity using alpha diversity metrics. Nisin A and salivaricin 10 reduced the richness and evenness of oral biofilm operational taxonomic units (OTUs), as reflected by a decrease in Shannon and Simpson diversity. Compared to PBS, nisin A at 50 µg/mL caused a greater reduction in Shannon and Simpson diversity than that of salivaricin 10 at 50 or 25 µg/mL (Fig. 3D). Beta diversity analysis using principal coordinate analysis and Bray–Curtis distances demonstrated clustering by treatment, with significant differences among the control, nisin A, and salivaricin 10 groups; however, no significant changes (P > 0.05) were observed in the beta diversity when comparing individual groups to each other (SI Appendix, Fig. S8). The most abundant genera in the multispecies biofilm were Streptococcus and Veillonella (Fig. 3B), and salivaricin 10 treatment did not affect their relative abundances at the genus level (SI Appendix, Tables S6 and S8). In contrast, nisin A treatment increased the relative abundance of Streptococcus genus (SI Appendix, Tables S4). Both salivaricin 10 and nisin A treatments significantly reduced the relative abundance of Bifidobacterium, Schaalia, Actinomyces, Alloscardovia, and Gemella genera (SI Appendix, Tables S4, S6, and S8). We identified the OTUs at the species level (SI Appendix, Figs. S9 and S10) using the Human Oral Microbiome Database v3. Due to limitations in 16S rRNA gene sequencing, some closely related oral bacteria may not be distinguishable. When two species exhibit equal identity, we denoted them using a slash call. In cases where three or more species have equal identity, we grouped them together as previously mentioned (36) (SI Appendix, Table S3). In the control group, the four most abundant OTUs were Veillonella dispar/atypica, Streptococcus parasanguinis group, Streptococcus oralis group, and S. salivarius group. Salivaricin 10 treatment reduced the levels of S. oralis group, maintained the levels of S. parasanguinis group, and increased the levels of S. salivarius group and Haemophilus species compared to PBS (Fig. 3E and SI Appendix, Tables S7 and S9). Nisin A treatment increased the relative abundance of S. salivarius group but inhibited the growth of S. oralis groups and S. parasanguinis group (Fig. 3E and SI Appendix, Tables S5). Notably, S. parasanguinis is a beneficial oral commensal associated with oral health and lower proportions of cariogenic S. mutans (37). It has also been shown to inhibit the growth of periodontal pathogens (38). Salivaricin 10 treatment also inhibited the pathogen S. agalactiae and the opportunistic cariogenic Bifidobacterium dentium (Fig. 3E). Altogether, nisin A disrupted the composition of the oral microbiome–derived biofilm, including beneficial microbes like S. parasanguinis group, due to its broader spectrum of antimicrobial activity. To directly observe the effects of salivaricin 10 on biofilm architecture and the spatial distribution of its antimicrobial activity in this context, we used confocal microscopy and Live/Dead staining. Both salivaricin 10 and nisin A control treatments caused significant damage to the biofilm architecture, and both antimicrobial agents appeared to penetrate the saliva-derived multispecies biofilms to effectively kill bacterial cells (dead cells labeled in red) within the biofilm structure (Fig. 3F), resulting in 6 times the ratio of dead/live cells in comparison to the PBS-treated control biofilm (Fig. 3G). The higher concentration of salivaricin 10 appeared to reduce the overall biofilm thickness (Fig. 3H), a previously reported phenomenon for nisin A (39). Interestingly, the heterogeneity of bacterial distribution (roughness) measured by the green signal intensity of each pixel in confocal microscopy images showed a twofold decrease upon treatment with salivaricin 10 compared to PBS (Fig. 3I), which indicates less variation in the distribution of bacteria within biofilms due to membrane-associated structural damages. Our data suggest that salivaricin 10 consists of a group of naturally occurring antimicrobial peptides in the oral cavity that can induce strong antimicrobial activities against multispecies oral biofilms while maintaining important oral microbes including Veillonella, Haemophilus, S. salivarius group, and S. parasanguinis group for a balanced oral microbiome. We expect such modulation to enable better immune response and promote lower levels of oral inflammation compared to broad-spectrum antimicrobial agents that may disrupt microbes that are potentially harmful and other nonharmful microbiota alike.
Fig. 3.

Antibiofilm activity of salivaricin 10. (A) Collected salivary samples from healthy subjects were cultured in 24-well imaging plates to grow oral biofilms. The biofilms were treated with either PBS, nisin A, or salivaricin 10 followed by washing steps as mentioned in the method section. The biofilms were then subcultured and incubated overnight, before DNA extraction and 16S rRNA gene sequencing using Illumina MiSeq as mentioned in the method section. In a duplicate experiment, live/dead molecular probes were used to assess the antimicrobial activity of salivaricin 10 against preformed saliva-derived multispecies biofilms. Live cells are stained with Syto 9 (green), while dead (permeabilized) cells are labeled with PI (red). (B) Total relative abundance of genera. (C) Low-abundant genera (<1%). Green genera names are those increased after salivaricin 10 treatment. Red genera names are reduced after salivaricin 10 treatment. (D) α-diversity (Shannon and Simpson indexes) of the multispecies biofilms with different antimicrobial treatments. Tukey multiple comparisons of means ANOVA were used to compare the α-diversity. (E) Absolute abundance showing maintenance, increase, or reduction of representative taxa. Significance was calculated via multiple linear regression using MaAsLin2. See SI Appendix for a full analysis of all affected taxa. N (nisin A treatment at 50 µg/mL), S50 (salivaricin 10 treatment at 50 µg/mL), S25 (salivaricin 10 treatment at 25 µg/mL). (F) Sum projections of the biofilm z-stack images taken by confocal microscopy are represented. A green signal indicates viable live cells (Syto 9), a red signal indicates damaged/dead cells (propidium iodide). (G) The ratio of green signal to red signal was measured using ImageJ. (H) The thickness of the biofilm was measured after z-stack images from the lower part of the biofilm to the highest part of the biofilm (highest signal intensity). (I) The heterogeneity of bacterial distribution (roughness) was measured by the green signal intensity of each pixel using ImageJ. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Salivaricin 10 RiPPs Exhibit Proimmune Functions.
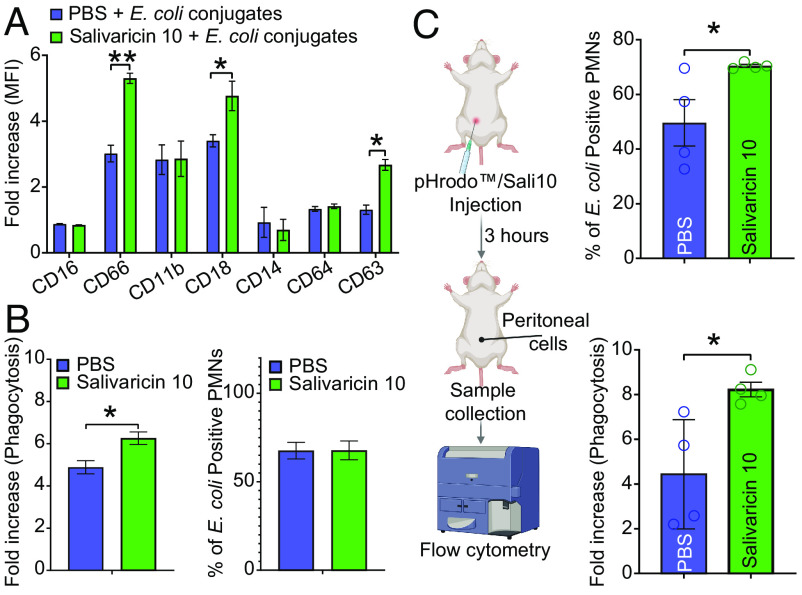
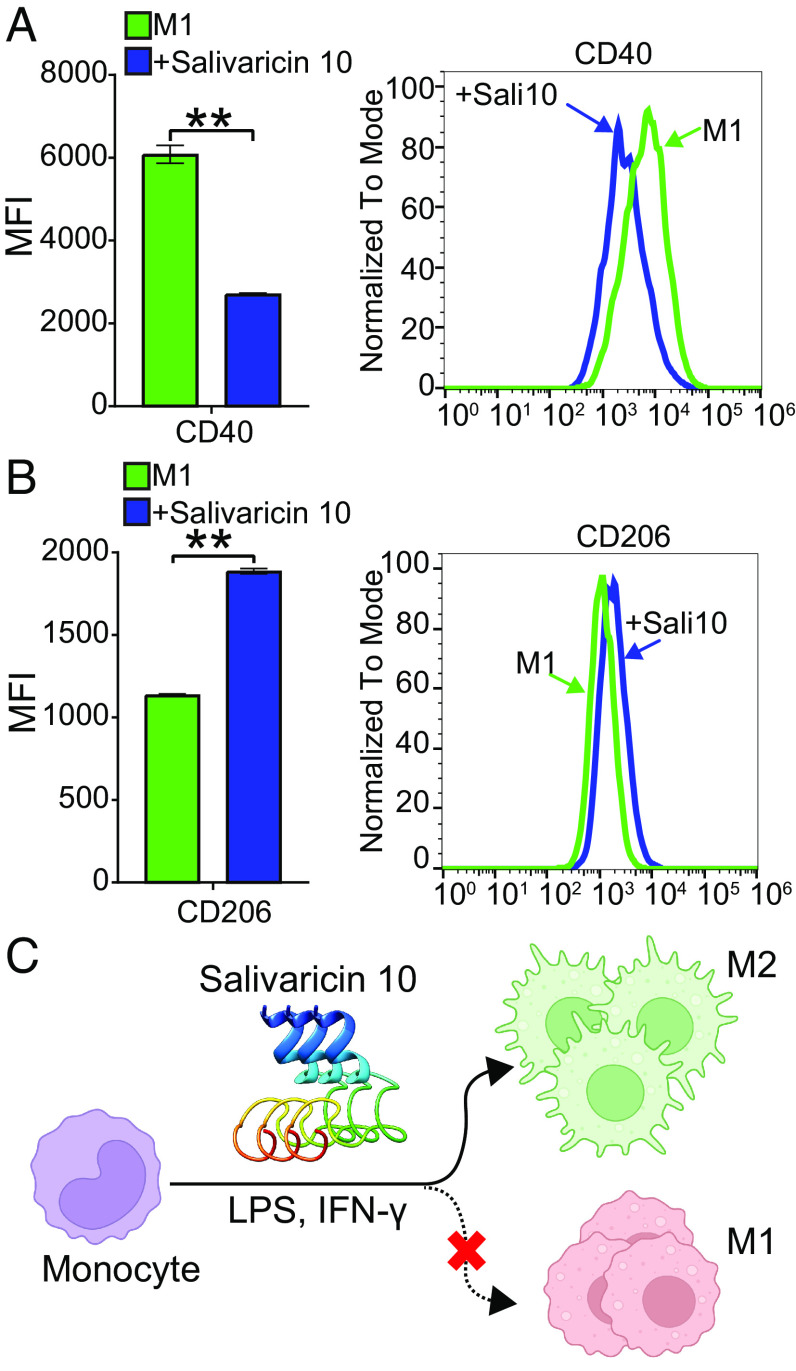
Neutrophil priming by salivaricin 10 indicates that it may have a potent immunomodulatory role that enhances the first line of defense against microbial pathogens. Our data suggest that salivaricin 10 has a unique immunomodulatory mechanism of action and can prime neutrophils in a similar manner to commensal microbes by increasing surface expression of key cluster of differentiation (CD) markers involved in neutrophil function as well as by enhancing phagocytosis. The activation state of neutrophils is characterized by expression levels of cell surface CD markers in the oral cavity depending on specific bacterial–host cell interactions. We assessed the surface expression of specific activation CD markers on human whole-blood neutrophils. A proinflammatory phenotype is characterized by high levels of CD63, CD64, and CD66 surface expression (40, 41). In a previous study, we observed that oral pathogens could induce the surface expression of CD63 but not CD66, while commensal bacteria can induce the expression of both (42). Upregulation of CD66 may promote neutrophil adhesion to extracellular matrix proteins or promote nonopsonic phagocytosis of select bacteria as is observed with opacity protein–positive bacteria (43). Similar to what was previously shown with commensal bacteria activation of CD markers, the salivaricin 10 peptides up-regulated the surface expression of CD63 and CD66 in human neutrophils (Fig. 4A). CD18 was also shown to be up-regulated and has antiinflammatory activity (44). The induction of the phagocytic state was measured using pHrodo (pH-sensitive dye conjugated to heat-killed E. coli) with either PBS or salivaricin 10. Neutrophils phagocytosed the heat-killed E. coli more efficiently in the presence of salivaricin 10 (Fig. 4B). Although we used purified peptide preparations for this assay (a combined fraction containing SrnA1, SrnA2, and SrnA4), we tested neutrophil phagocytosis using crude peptide preparations from the wild-type strain of S. salivarius SALI-10 and its plasmid-cured version to ensure that no other microbial elements contributed to the observed immunomodulation activity. We confirmed that the crude peptide preparation from the wild-type strain, but not from the plasmid-cured strain, significantly induced neutrophil phagocytosis (SI Appendix, Fig. S11C). We further investigated the immunomodulatory role of salivaricin 10 in a mouse model. The pHrodo E. coli BioParticle conjugates were injected intraperitoneally together with either PBS or salivaricin 10, and phagocytosis of E. coli by recruited neutrophils was assessed after 3 h. Concordant with our in vitro assays, salivaricin 10 administration increased the phagocytic activity per neutrophil and increased the number of positive phagocytic neutrophils in mice compared to PBS-treated control animals (P < 0.05) (Fig. 4C). This finding indicates that salivaricin 10 can enhance the phagocytic activity of neutrophils in a modeled acute infection site. We further investigated whether salivaricin 10 can promote an M2 proinflammatory resolution phenotype in monocytes. Oral commensal Streptococci stimulate an M2 response when incubated with the THP-1 myelomonocytic human cell line (M0 cells) (45). We used THP-1 cells and polarized them in vitro to a proinflammatory, M1 phenotype. Interestingly, the addition of salivaricin 10 in the cell culture media reversed the polarization and promoted a proresolution M2 phenotype (suppressed CD40 and induced CD206 surface expression) (Fig. 5). We further performed chemotaxis assays with isolated human neutrophils and monitored neutrophil migration speed after the addition of increasing concentrations of salivaricin 10. N-formyl-methionyl-leucyl-phenylalanine (fMLP, 1 µM) was used as a positive control (46), and it induced chemotaxis of neutrophils at a speed of ~11 µm/min. Salivaricin 10 also showed similar neutrophil chemotaxis at all salivaricin 10 concentrations (5, 10, 20, and 40 µg/mL). Notably, at 10 µg/mL, salivaricin 10 induced twice the level of chemotaxis compared to fMLP (Fig. 6A). Moreover, the crude peptide preparation from S. salivarius SALI-10 wild-type strain, but not the one from plasmid-cured strain, induced neutrophil chemotaxis significantly at 5 µg/mL (SI Appendix, Fig. S11D). These studies show that salivaricin 10 plays critical immunomodulatory roles in the innate immune response and salivaricin 10 peptides are the first reported RiPPs with neutrophil chemoattractant function.
Fig. 4.
Salivaricin 10 augments neutrophils priming in human blood and induces phagocytosis in vitro and in vivo. pHrodo E. coli BioParticles conjugates were added to human blood in the presence or absence of salivaricin 10, and the expression levels of specific CD markers were monitored using flow cytometry in neutrophils (A). The percentage of the phagocytic cells and fold increase in phagocytosis was measured in neutrophils (B). (C) Salivaricin 10 and pHrodo were injected concomitantly into the peritoneal cavity of mice. The ability of salivaricin 10 to increase the percentage of phagocytic neutrophils and the total signal of phagocytosis in neutrophils compared to PBS was measured. *P < 0.05, **P < 0.01.
Fig. 5.
Salivaricin 10 suppresses M1 proinflammatory macrophages and promotes monocytes toward M2 proresolution phenotype. Thp1 monocytes were differentiated with LPS + INF-γ, in the presence or absence of salivaricin 10. (A) CD40 was used as a biomarker for M1 macrophages and was shown to be suppressed by salivaricin 10. (B) CD206 was used as a biomarker for M2 macrophages and was shown to be induced by salivaricin 10. The representative flow spectrum of each CD marker is shown to the Right. Green line: cells stimulated with LPS + INF-γ (M1), blue line: cells stimulated with IFN, LPS, and then salivaricin 10 to alter polarization. (MFI): mean fluorescence intensity. (C) Proposed effect of salivaricin 10 on reversing monocyte polarization from M1 to M2 phenotypes.
Fig. 6.
The immunomodulatory effects of the phosphorylated N-terminal tail of salivaricin 10. (A) Chemotaxis assay of isolated human neutrophils. Salivaricin 10 was used as a chemoattractant, and the neutrophil movement speed was monitored. (B) Phosphorylated peptide [p(1-8) Sali10] and nonphosphorylated peptide [np(1-8) Sali10] were chemically synthesized and tested in the neutrophil chemotaxis assay at a peptide concentration of 40 µg/mL. The data show that phosphate removal resulted in a drastic reduction in neutrophil chemotaxis (P < 0.0001), fMLP was used as chemotaxis positive control. (C) Gene expression data show that phosphorylation is important to down-regulate CXCL10 gene associated with M1 macrophages. However, authentic salivaricin 10 showed stronger suppression. (D) p(1-8) Sali10 up-regulated the gene expression of TGM2 associated with M2 antiinflammatory macrophages. However, there was no statistically significant difference in the TGM2 gene expression compared with cells treated with the nonphosphorylated analog. The concentration of peptides used in the gene expression assay was 40 µg/mL. *P < 0.05, **P < 0.01, ***P < 0.001. ****P < 0.0001.
Salivaricin 10 RiPPs Have a Posttranslational Modification (PTM) Phosphorylation Site That Is Responsible for the Proimmune Functions.
The three distinct peptides were separated and purified using RP-HPLC and have monoisotopic masses of 3669.60 Da, 3551.58 Da, and 3535.58 Da, respectively (Fig. 7 A, C, and E). The peptides all had a higher molecular weight than the predicted mass according to the peptide sequences deduced from the genomic information. High-resolution mass spectrometry (HRMS) revealed that the two major peptides (SrnA1 and SrnA2) are phosphorylated; interestingly, nonphosphorylated variants were not observed, and each peptide contains primarily two or three dehydrations, with no more than three per peptide (SI Appendix, Tables S10–S17). The chemical formula for each peptide containing three dehydrations and one phosphorylation is provided in SI Appendix, Table S18. Two-dimensional NMR spectroscopy and accurate-mass tandem mass spectrometry (MS/MS)–based analyses showed that the Thr at position 4 is the site for phosphorylation (Fig. 7 B, D, F, and G). Tryptic digestion of SrnA1 and SrnA2 followed by LC-MS/MS (liquidchromatography-tandem mass spectrometry) analyses supported the assignments of the Me(Lan) rings (SI Appendix, Table S19). Structural analysis on SrnA4 confirmed the phosphorylation at Thr4, similar to SrnA1 and SrnA2. However, more than three dehydrations were confirmed on SrnA4, including the Ser3 residue, which is dehydrated to form Dha. Importantly, rings B and C are mainly not formed in SrnA4 since fragmentations within the predicted ring spanning regions were observed (Fig. 7F and SI Appendix, Fig. S12). For a detailed characterization of the structure of the peptides, refer to SI Appendix file. The discovery of a phosphorylated Lan-containing antimicrobial peptide defines a previously unidentified class of lanthipeptide/lantibiotics with a unique biological activity particularly in its capacity to modulate critical host immune responses. The structural feature of the N-terminal phosphorylation found in all the isolated salivaricin 10 peptides suggested that this feature may be important for the observed immunomodulatory activity.
Fig. 7.
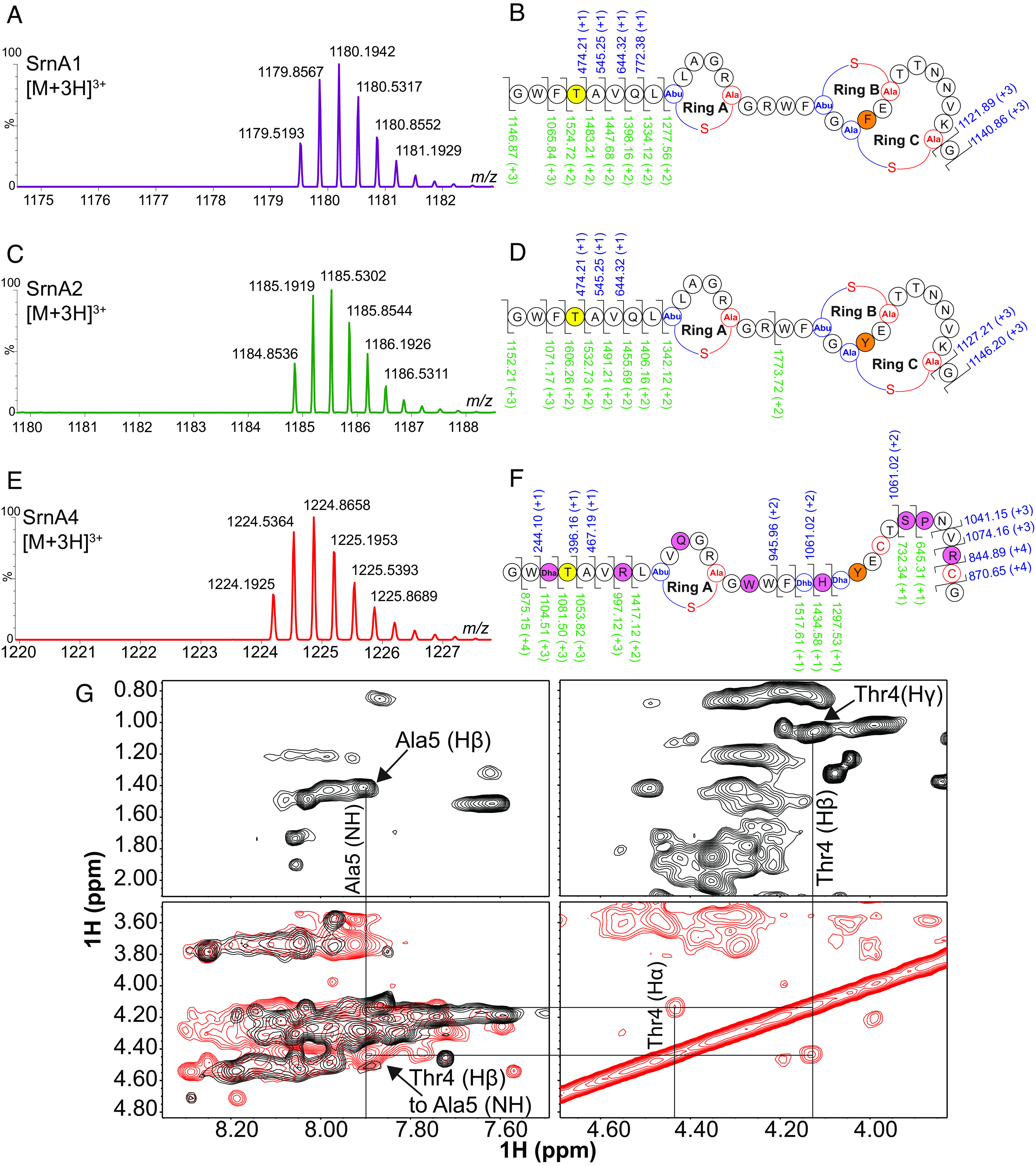
Salivaricin 10 consists of three lanthipeptides that are phosphorylated in their mature form. (A, C, and E) MS analysis showing characteristic triply charged ions of SrnA1, SrnA2, and SrnA4, respectively, identified directly from S. salivarius SALI-10 cell extract. (B, D, and F) High Resolution-MS/MS analysis of the SrnA1, SrnA2, and SrnA4 peptides show three dehydrations. The ions with a signal/noise (s/n) greater than 5.0 were used for assignments, and all but one ion for each peptide had less than 10 ppm mass difference from expected and observed masses (SI Appendix, Tables S8–S10). Phosphorylated Thr4 residue is highlighted in yellow. b and y ions are labeled in blue and green, respectively. (G) Representative NMR data illustrating the assignment of a threonine at position 4. A sequential walk from the alpha and beta protons on the phosphorylated threonine to the amide spine system of alanine at position 5. The alpha and beta protons for Thr4 are shifted downfield due to the presence of phosphate. NOESY spectra and TOCSY spectra are shown in red and black, respectively.
To test whether the phosphorylated N-terminal tail of SrnA1 and SrnA2 is critical for the observed immunomodulatory activity, we synthesized the eight N-terminal unbridged residues NH2-GWFTAVQL-COOH, with the phosphorylated threonine at position 4 (pThr4). This short peptide analog, named p(1–8) Sali10, induced chemotaxis by neutrophils with very similar activity to the full-length salivaricin 10 (Fig. 6B), supporting the importance of the N-terminal tail in sensitizing neutrophils. The chemotaxis of neutrophils treated with a nonphosphorylated analog of the N-terminal tail [np(1–8) Sali10] was significantly reduced, suggesting that the phosphate group at position 4 (pThr4) is crucial for the observed immunomodulatory activity. We also show that both full-length salivaricin 10 and p(1–8) Sali10, but not the nonphosphorylated np(1–8) Sali10, down-regulated the gene expression of the C–X–C motif ligand-10 gene (CXCL10), which is one of the main biomarkers of M1 proinflammatory macrophages (Fig. 6C). Additionally, crude peptide preparation from the S. salivarius SALI-10 WT strain, but not the plasmid-cured strain, showed to suppress the expression of CXCL10 gene, confirming the specific immunomodulation activity assigned to salivaricin 10 (SI Appendix, Fig. S11E). Both full-length salivaricin 10 and p(1–8) Sali10 peptides, but not np(1–8) Sali10, up-regulated the gene expression of TGM2, M2 antiinflammatory macrophage biomarker, (47) significantly compared to M1 (control cells) (Fig. 6D). Neither p(1–8) Sali10 nor np(1–8) Sali10 showed effects on the neutrophils’ CD marker expression (SI Appendix, Fig. S13), unlike full-length salivaricin 10, suggesting that the full length or a component of the full peptides is essential for stimulating these other immunomodulatory activities. The data provide strong evidence that the N-terminal 8 amino acids of salivaricin 10, particularly the unique phosphorylated Thr residue (pThr4), are important for the immunomodulatory properties of this naturally occurring oral lanthipeptide.
Salivaricin 10 RiPPs Are Unique to the Oral Cavity.
The srn operon is almost exclusively related to oral streptococcal strains, and it appears to be capable of producing a combination of lanthipeptides modified by one class II bifunctional dehydratase/cyclase enzyme (SrnM). To understand how similar the S. salivarius SrnM is to other members of this enzyme family, we constructed a sequence similarity network (SSN) in different bacterial phyla (Fig. 8A). We observed a distinct oral Streptococci cluster separate from other genes found in other bacterial groups. The oral Streptococci cluster contains srnM-like genes belonging to S. salivarius, S. mitis, S. oralis, S. suis, and S. pneumoniae and shares the highest sequence similarity to the srnM-10 gene of S. salivarius SALI-10 (SI Appendix, Fig. S14A). The closest linkage to the oral Streptococci cluster exhibited only 30% sequence similarity and included bacteria related to other members of Firmicutes, suggesting that streptococcal SrnM-10 is distinct among Lan synthetases, supporting the evolution of the unique immunomodulatory and antibacterial properties of the SrnA lanthipeptides (Fig. 8A). We further separated the clusters of the generated SSN by applying 50% sequence similarity and 500 amino acid sequence length thresholds which resulted in 110 clusters (SrnM cluster is identified as C5) and 105 singletons (Fig. 8B and SI Appendix, Fig. S14B). We used chemically guided functional profiling (CGFP) to analyze the abundance of SNN clusters including the SrnM-10 enzyme (cluster C5) against 380 high-quality metagenomes within the human microbiome project (HMP) (see SI Appendix for the method), including the body site of the nares, buccal mucosa, supragingival plaque, tongue, stool, and posterior fornix as mentioned previously (48). The SrnM cluster (C5) was primarily found on the buccal mucosa, supragingival plaque, and tongue dorsum (Fig. 8 C and D and Dataset S1), with no hits within other body sites, suggesting that this enzyme and its operon are unique to bacteria colonizing the oral cavity.
Fig. 8.
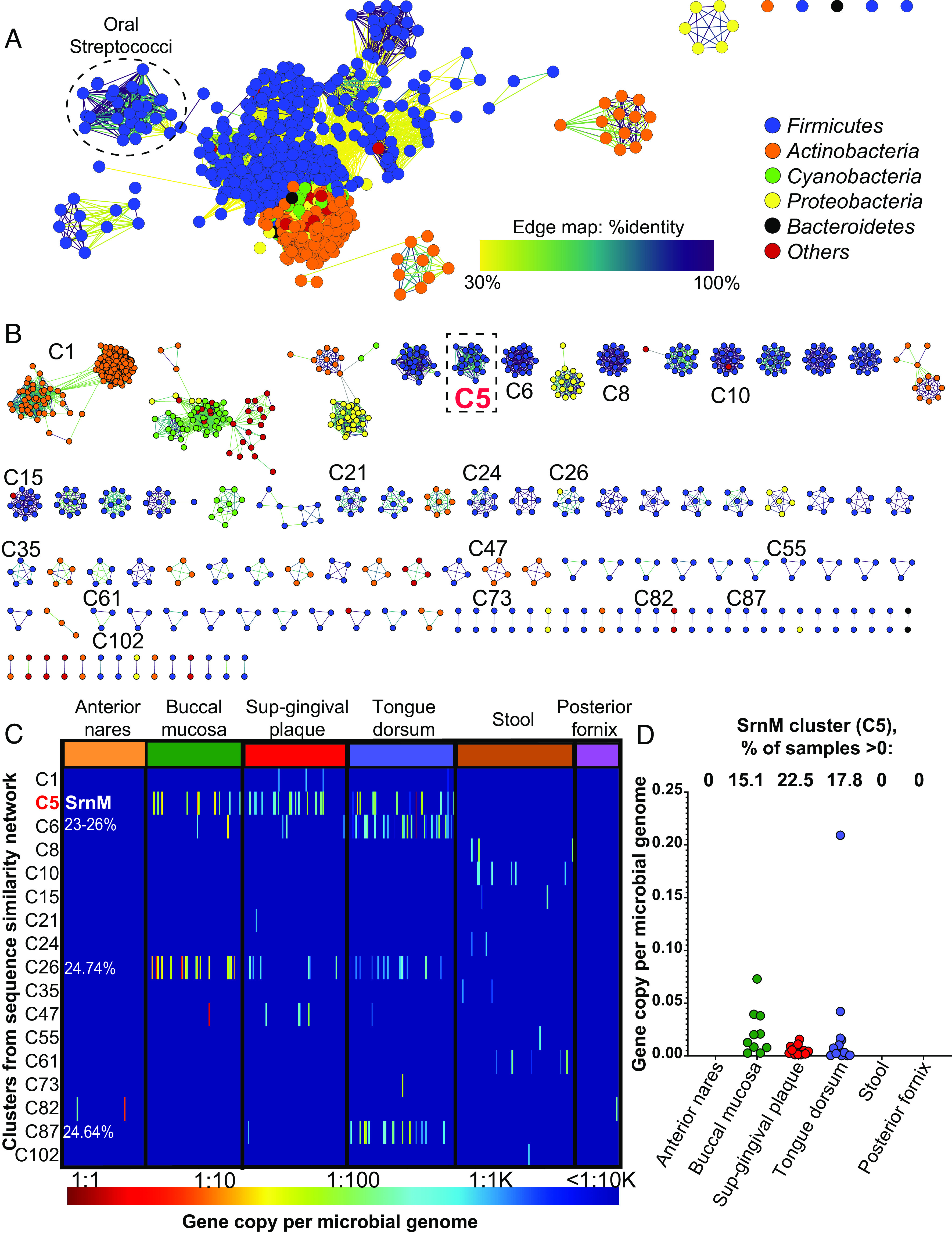
The SrnM-10 enzyme that makes salivaricin 10 lanthipeptides is unique to the oral cavity. (A) Sequence similarity network (SSN) of the lanthionine dehydratase enzyme (SrnM-10) of strain S. salivarius SALI-10 in different bacterial phyla generated using the Enzyme Similarity Tool-Enzyme Function Initiative (EST-EFI, https://efi.igb.illinois.edu/efi-est/) and visualized in Cytoscape (v.3.9.1) with an alignment score threshold of 120 (∼30% sequence identity). Each node represents one protein sequence, each edge represents a BLASTP hit. (B) The same SSN in (A) was modified by applying 50% sequence identity and 500 aa sequence length thresholds, which resulted in the generation of 110 clusters and 105 singletons (SI Appendix, Fig S14). The C5 cluster (in dashed box) is identified as SrnM cluster. (C) Heatmap showing the abundance of 17 clusters (from B) identified in the 380 high-quality metagenomic sequences of microbiomes from healthy human subjects. The bar below the heatmap defines the color key for the “gene copies per microbial genome”. C6, C26, and C87 clusters contain sequences that are less than 25% similar to SrnM. (D) Boxplots showing per site abundance of SrnM (C5 cluster) across six body sites.
Discussion
In this study, we isolated salivaricin 10, a combination of phosphorylated lanthipeptides with antibacterial, antibiofilm, and immunomodulatory properties produced by certain S. salivarius strains from the human oral cavity. Other unique PTMs have been identified in a few other lanthipeptide systems, including C-terminal oxidative decarboxylation (49) and N-terminal lactate group formation (50). In this manuscript, we identified a unique PTM in lanthipeptides that is important for immunomodulatory bioactivity. The PTM is an unusual phosphorylation site within the N-terminal linear peptide sequence that has a role in the immunomodulation characteristics including neutrophil chemoattraction and modulation of macrophage polarization.
Salivaricin 10 RiPPs belong to a previously unidentified structural and functional class of phosphorylated lanthipeptides naturally produced by bacteria. Most salivaricins are type AII lanthipeptides that undergo dehydrations and cyclizations by one bifunctional synthetase enzyme (20). This is a typical and well-documented reaction in this class of lanthipeptides that involves the formation of Me(Lan) rings (51). Future studies are warranted to determine the precision of phosphorylation at Thr4. However, salivaricin 10 synthetase (SrnM-10) is likely responsible for the phosphorylation of the Thr4 residue. Previous work on LctM, a type AII lanthipeptide dehydratase that produces lacticin 481, revealed distinct residues contributing to the phosphorylation step vs. the elimination of the phosphate that accompanies lanthipeptide ring formation (52). Moreover, the crystal structure of CylM (another type AII lanthipeptide synthetase) revealed that phosphorylation and phosphate elimination likely occur in the same active site and specific residues such as Arg506 and Thr512 are important for phosphate elimination (53). These Arg and Thr residues are also present in SrnM-10 and hence the observation of phosphorylation but not elimination at Thr4 is intriguing and warrants further investigation.
A recent study utilizing genome mining found that a deep-ocean species “Ca. Eudoremicrobium malaspinii” encodes a precursor peptide modified by a sole maturase (embM). In this maturase, the authors found a functional domain homologous to the dehydration domain of lanthipeptide synthetases. Gene synthesis, in vitro heterologous expression, and in vitro enzyme assays of this RiPP biosynthetic cluster led to the production of a linear peptide reported to contain up to two phosphates. The peptide was reported to not contain any of the typical lanthipeptide PTMs, i.e., dehydrated amino acids or formation of Me(Lan) structures (54). The authors also reported that the phosphorylation of this linear peptide is associated with mammalian neutrophil elastase inhibition. The HRMS data in our current manuscript demonstrated that the isolated matured and modified SrnA1, SrnA2, and SrnA4 lanthipeptides did not have any variability in the phosphorylation of the N-terminal region of the leader peptide. The observation supports that the PTM is an essential component of the peptide’s bioactivity, and this observation is supported by the observed immunomodulatory activity of the peptides.
In addition to the interesting structural features of the N-terminal phosphorylation site, HRMS data also revealed that there is variability in the number of dehydrations introduced into the SrnA1, SrnA2, and SrnA4 peptides. More specifically, some of the SrnA1 and SrnA2 variants were revealed to be missing either 1 or 2 of their predicted dehydrations, and hence the corresponding rings. Similarly, SrnA4 was also shown to be missing these PTMs, but the fragmentation patterns observed in the MS/MS data of SrnA4 suggest that rings B and/or C are more often absent than what was observed for the SrnA1 and SrnA2 peptides. Ring B of salivaricin 10 has the lipid II-binding motif that is found in many class II lantibiotics. This motif was first demonstrated in mersacidin and since then in many other lanthipeptides including lacticin 481 (55). The Glu residue of the lipid II–binding motif is critical for the antimicrobial activity of class AII lantibiotics (55) and it is also conserved in ring B of salivaricin 10 peptides.
MS analyses of SrnA1 and SrnA2 peptides showed that ring A is essentially intact, while there was a detected product lacking ring C formation. These irregularities would strongly suggest that the Lan rings predicted in salivaricin 10 are not being consistently generated within the mature peptide. Whether the decreased efficiency in Lan ring formation is related to the inefficiency in phosphate elimination at Thr4 position will need to be evaluated in future studies. However, the evolution of the system to produce phosphorylated products of all the three SrnA peptides with high efficiency does support the notion that a lanthipeptide system has evolved proimmune function, while balancing its antibacterial properties to support health of the human oral cavity. We note that production of a set of lanthipeptides that differ in the number of dehydrations in the native producer organism is not uncommon and is also observed for nisin (56) and geobacillin I (57).
Genes homologous to salivaricin 10 structural genes srnA1, srnA2, and srnA4 were previously found in an S. pneumoniae strain P174 with limited antimicrobial activity. In that study, the authors showed that deletion of pldA1 (homolog of srnA1), pldA2 (homolog of srnA2), or pldA3 (homolog of srnA3, the gene encodes for SrnA3 peptide that was not detectably produced by S. salivarius SALI-10 in our study) eliminated the antimicrobial activity of the strain, while pldA4 (homolog of srnA4) is dispensable for inhibition (58). The authors did not isolate the products for direct analysis in their study; thus, direct comparison on their observations cannot be made. The in-frame deletions made in their study for each homolog may have interfered with the transcription of the operon or interfered with the translation of the peptide products. In our study, each of the peptides (SrnA1, SrnA2, and SrnA4) of salivaricin 10 exhibited antimicrobial activities. Relative concentrations of each purified peptide were determined by area under the peak and the sample concentrations for each peptide were matched. Using the same concentration of each peptide, the isolated peptide fractions (SrnA1, SrnA2, and SrnA4) had the same MIC value against Micrococcus luteus ATCC10240 (SI Appendix, Fig. S15). The three peptides appear to have redundancy in their antimicrobial and immunomodulatory functions. Previous attempts to isolate the peptides from the S. pneumoniae strain P174 found that the two main peptides produced (PldA3 and PldA1) are truncated from the first eight N-terminal amino acids, and their roles (inhibitory and signaling activities) are in direct contrast to what was previously deduced using information from the individual peptide deletions (59). The proteolytic cleavage of the bioactive product suggests that the biosynthesis gene cluster has recently been integrated into the bacterium and does not form a completely functional product or that the immunomodulatory N-terminal region of the peptide has deleterious activity toward S. pneumoniae. This is intriguing as we have shown in the current manuscript that the acyclic phosphorylated N-terminal sequence of the salivaricin 10 peptides is an important component of the unique immunomodulatory functions. Such differences in the biosynthesis and PTMs between S. salivarius and S. pneumoniae are likely to result in different biological functions. For this class of peptides, it appears that the biosynthetic cluster can be present in different genomes reported in the GenBank database; however, apparently, there are critical structural differences in the final produced products at the species and strain levels.
While S. salivarius SALI-10 described in this study is the only reported bacterial strain to produce and modify the three peptides successfully, S. salivarius JH was reported previously to produce only one peptide (salivaricin E) sharing the same amino acid sequence as SrnA2 of salivaricin 10. Salivaricin E was reported to possess antimicrobial activity against S. mutans and to have a molecular weight of 3,565.9 Da (with four dehydrations) (60), and this mass is 112 Da higher than the predicted mass of 3,454 Da for the successfully translated peptide with four dehydrated residues (60). Noteworthy, pair-wise sequence alignment of the SrnM-10 Lan synthetases of strains SALI-10 and JH showed four mutations; whether these mutations contribute to the diversity of the salivaricin peptides produced is yet to be determined (SI Appendix, Fig. S16).
Very few lanthipeptides have been tested against multispecies biofilms. While the prototype lanthipeptide nisin A demonstrated potent killing against preformed saliva-derived multispecies oral biofilms (39), a potential concern is that the broad-spectrum activity of nisin A could eliminate many beneficial oral microbiota. Nisin A is not indigenous to the oral cavity, and its producer strains, Lactococcus lactis, are of bovine origin and cannot form persistent populations in the mouth (61). Nisin homologs were also shown previously to be produced in the human gut (62), and thus nisin-like peptides might be more relevant to the gut microbiome. Comparatively, salivaricin 10 is exclusively produced in the human oral cavity by strains of the oral pioneer colonizer S. salivarius.
Salivaricin 10 peptides were successfully able to penetrate oral biofilms and kill select disease-associated bacteria while sparing other beneficial microbes. These studies show that using an orally derived antimicrobial product from a well-established oral cavity commensal bacterium is superior to the broad activity of an antibiotic that had not evolved in the oral cavity.
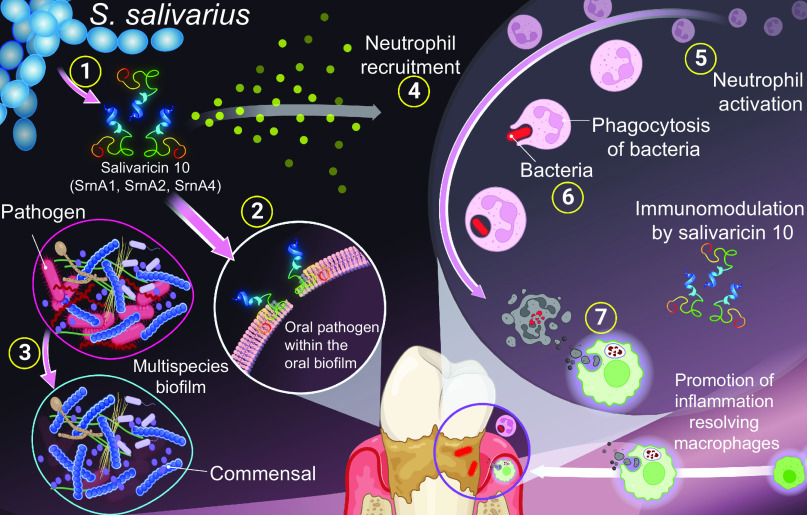
In addition to the salivaricin 10 peptides’ direct antimicrobial activities, the peptides promote host defense by directly enhancing the phagocytic activity of neutrophils. Salivaricin 10 also appears to promote a resolution of inflammation by biasing macrophages toward M2 polarization. Salivaricin 10 may complement host immunity by directly killing oral pathogens that have host immunity evasion functions (63, 64) and acting as a potent neutrophil chemoattractant and enhancing the recruitment of phagocytic cells and targeted pathogen killing (Fig. 9). Such bacterial peptide–host immune cell communication was recently observed in the nasal microbiome for the commensal Staphylococcus lugdunensis that produces lugdunin, a thiazolidine-containing cyclic peptide (65). There is an increasing interest in developing antibiotics that also function as neutrophil chemoattractants to increase antimicrobial efficacy. For example, formulated neutrophil-chemoattractant peptides linked to the antibiotic vancomycin were shown to exhibit dual antibacterial and immunomodulatory properties against Staphylococcus aureus (66).
Fig. 9.
Proposed model of salivaricin 10–mediated oral protection. Salivaricin 10 has multifunctional characteristics: First, when it is produced by the oral commensal S. salivarius (1), it directly penetrates oral biofilms and inhibits the growth of disease-associated bacteria by disrupting the bacterial cytoplasmic membrane integrity (killed cells were labeled with propidium iodide, in antibiofilm assay) (2). This antibacterial action maintains indigenous oral microbiota that are beneficial to oral health while reducing the number of disease-associated bacteria (3). Second, salivaricin 10 is a neutrophil chemoattractant (4) that cooperates with the innate immune cells by priming neutrophils (5), inducing phagocytosis (6), and promoting the inflammation-resolving macrophages (M2 phenotype) that clear cellular debris to prevent further inflammatory responses (7). This figure was created using BioRender.com.
In summary, the results of this study show that phosphorylated lanthipeptides from the commensal oral microbiome can confer multilevel protection to the host against bacterial infections. Salivaricin 10 peptides can act as antibiotics and antibiofilm agents and induce immunomodulatory effects by communicating with innate immune cells that regulate antiinflammatory and proresolution responses. Phosphorylation of salivaricin 10 is required to induce effective neutrophil chemotaxis and for its regulation of inflammation resolution through M2 macrophage differentiation. The phosphorylated mature form of the salivaricin 10 peptides sets them apart from all other lanthipeptides studied to date. Our study sets the stage for future development of salivaricin 10 as an oral antimicrobial therapeutic that has multiple distinct bioactivities, i.e., penetrating biofilms, killing pathogens while sparing other important health-associated bacteria, and stimulating inflammation and its resolution.
Materials and Methods
Detailed procedures for isolation and screening of lantibiotic-producing bacterial strains, discovery of strain S. salivarius SALI-10, whole-genome sequencing, lantibiotic production and purification, antimicrobial activity, 16S rRNA gene sequencing of the multispecies biofilms, monocyte culture and polarization, and gene expression studies are provided in SI Appendix.
Isolation of S. salivarius from the oral cavity of healthy subjects was approved by the Office of Research Ethics, University of Toronto (protocol number 39217), and research subjects signed informed consent before enrollment and sample collection.
Identification and Characterization of Salivaricin 10 Peptides.
Initial mass determination of all peptide products prepared either from cell extracts or semi-purified cell-free peptide preparation, obtained from agar cultures (SI Appendix, Method), was performed using UPLC-ESI-QTOFMS with a Xevo G2-XS MS (Waters, Mississauga, ON, Canada). The raw data were processed using a MassLynx software system (Waters, Mississauga, ON, Canada). The LC-MS/MS system used on the HPLC-purified peptides from the cell-free supernatant obtained from agar cultures was comprised of a Shimadzu Nexera X2 MP Ultra-high-Performance Liquid Chromatography system (Kyoto, Japan) coupled to a Thermo Fisher Scientific high-resolution Orbitrap Fusion™ Tribrid™ MS. Operational control of the LC-MS/MS system was performed with the following software packages from Thermo Fisher Scientific: Xcalibur for data acquisition, FreeStyle for chromatographic peak and MS/MS spectral analysis, and TraceFinder for peak integration. A parallel-reaction monitoring (PRM) scan mode was used to specifically target the Salivaricin 10a-10c peptides produced by S. salivarius SALI-10 for MS1 and MS2 spectral analysis—see SI Appendix, Table S23 to view the experimental conditions used to perform the PRM experiment. NMR analysis was performed as described previously (67). Approximately 1 mg SrnA1 (Phe-variant) and SrnA2 (Tyr-variant) was each dissolved in 600 µL deuterated dimethyl sulfoxide (DMSO-d6), and the NMR data were collected on a Bruker Advance III-HD spectrometer operating at a proton frequency of 850 MHz. The 1H resonances were assigned according to standard methods (68) using correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY) (69), and nuclear overhauser effect spectroscopy (NOESY) (70) experiments. NMR experiments were conducted at 25 °C. The TOCSY experiment was acquired with a 60-ms mixing time using the Bruker DIPSI-2 spinlock sequence. The NOESY experiment was acquired with 400- and 500-ms mixing times. Phase-sensitive indirect detection for NOESY, TOCSY, and COSY experiments was achieved using the standard Bruker pulse sequences. Peaks were assigned using NMRView software (71).
Antimicrobial Activity against Preformed Biofilms (Saliva-Derived De Novo Model).
Saliva-derived biofilms were established in 24-well glass-bottomed SensoPlates (Greiner Bio-One, Monroe, NC, USA) as described previously (39). The formed biofilms were then challenged with either salivaricin 10 at 25 or 50 µg/mL or nisin A at 50 µg/mL for 30 min, and PBS was used as a negative control. The peptides were removed by pipette, and the biofilms were washed twice with PBS. The biofilm cells were then labeled with Filmtracer LIVE/DEAD Biofilm Viability Kit (Invitrogen, USA), following the manufacturer's instructions. The stains were removed, and the biofilms were washed twice with PBS before fixation using 1.6% paraformaldehyde (PFA). PFA was removed by PBS washing, and then Fluoromount-G Mounting Medium (Invitrogen, USA) was added to thin coverslips before flipping over each biofilm sample. The samples were left to mount overnight in the dark at room temperature. Microscopy and imaging were carried out as follows: All biofilm images were acquired by a Zeiss LSM 800 with the Plan Apochromat objectives featuring Zeiss 20× (Plan Apochromat; 20×/0.8). 3D biofilm images were constructed by ZEN software (Zeiss). The mean intensity of pixels for green (Syto-9; live) and red (PI; dead) signals was measured within biofilm areas of each image, with the slices containing the highest respective signal intensity using custom Fiji (ImageJ) (72). The thickness of biofilms was defined using a green channel (the distance between the lowest signal intensity from the bottom of biofilms to the highest signal intensity from the top of biofilms). The roughness calculation plugin from custom Fiji (ImageJ) was used to measure the heterogeneity of bacterial distribution based on green channel (Syto-9; live). The inputs were z-stacks in which the pixel values represent the distance to the surface, and the average of roughness was reported as arithmetic mean deviation (RA). All quantifications and statistical calculations are based on 10 to 15 images and 5 to 10 stacks for each triplicate.
A duplicate of the above biofilms was processed differently. After treatment with salivaricin 10 (50 µg/mL or 25 µg/mL), or nisin A (50 µg/mL), or PBS, the biofilms were washed as mentioned above. Then, they were scrapped off the wells and suspended in 100 µL PBS. These biofilm suspensions were used to inoculate 10 mL BHI broth (Difco) before incubation at 37 °C overnight in anaerobic conditions to enable the growth of unaffected bacterial species that survived the bactericidal action of the peptides. Inoculum derived from PBS-treated biofilm was used as a negative control to compare bacterial growth rates. The cultures were adjusted to OD600 = 1.0 and then centrifuged at 4,000 × g for 15 min, and the supernatant was discarded. DNA extraction was performed on the cell pellets using DNeasy PowerSoil Pro Kit (Qiagen) according to the manufacturer's instructions. The extracted DNA was subjected to 16S rRNA gene sequencing.
In Vivo Measurement of Salivaricin 10’s Impact on Phagocytosis.
Mouse studies were performed in accordance with all relevant ethical regulations and were approved by the University of Toronto Animal Care Committee and the Research Ethics Board (Protocol #20010664). Peritonitis was induced by intraperitoneal injection of pHrodo-Red E. coli BioParticles as described previously (73) followed by a 100-µL injection of salivaricin 10 (50 µg total dose) in PBS using the same method. All mice were 8- to 16-wk-old C57BL/6 males and were killed by CO2 inhalation before injections and cell collection. After 3 h, cells were retrieved from the peritoneal cavity by lavage with 3 mL cold PBS. Cell counts were obtained using Beckman Coulter Z2 Cell and Particle Counter, and the counts were normalized to the volume recovered. Sample preparation for flow cytometry analysis was carried out as described above for in vitro experiments.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
R.C.H. from MG Lab is the recipient of CIHR (Canadian Institutes of Health Research) Frederick Banting and Charles Best Canada Graduate Scholarship under the supervision of A.B. and M.G. The Texas Children’s Hospital Department of Pathology provides salary support to Texas Children’s Microbiome Center-Metabolomics Lab staff and purchased the reagents, consumables, and durable supplies related to the LC-MS/MS equipment described. The research of B.H. is supported by a foundation grant from the CIHR (#375597) and support from the John Evans Leadership funds (#36050 and #38861) and innovation funds (“Fibrosis Network, #36349”) from the Canada Foundation for Innovation and the Ontario Research Fund. We thank D. Rajshankar at the Collaborative Advanced Microscopy Labs of Dentistry (Faculty of Dentistry, University of Toronto) for training and help with image acquisition and data analysis. We thank Dr. Cameron Stewart for his excellent MS technical support and Danielle Lebowitz for her lab support in DNA extraction and peptide production. We thank Dr. Tony Mazzulli for providing the pathogenic clinical isolates used as targets in the MIC test. We thank Dr. Jennifer Spinler and Julia Copeland for their comments on the 16S rRNA gene sequencing data.
Author contributions
A.B., L.S., and M.G. designed research; A.B., L.S., M.O., M.W., R.C.H., C.M., N.F., C.S., F.Y., S.Z., R.O., T.D.H., S.J.H., and A.M.H. performed research; A.B., L.S., B.H., and M.G. contributed new reagents/analytic tools; A.B., L.S., M.O., M.W., C.S., F.Y., R.O., T.D.H., S.J.H., A.M.H., B.H., and M.G. analyzed data; and A.B., L.S., T.D.H., A.S., and M.G. wrote the paper.
Competing interests
A.B. and M.G., University of Toronto, submitted a provisional patent application for salivaricin 10. The other authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Abdelahhad Barbour, Email: abdelahhad.barbour@utoronto.ca.
Leif Smith, Email: jsmith@bio.tamu.edu.
Michael Glogauer, Email: michael.glogauer@dentistry.utoronto.ca.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix and Dataset S1 files. Raw read sequences of the 16S rRNA genes were deposited at Sequence Read Archive (SRA, NCBI), BioProject: PRJNA924352 (35).
Supporting Information
References
- 1.Caselli E., et al. , Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 20, 120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao L., et al. , Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 9, 488–500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., et al. , Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 99, 883–893 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Ghannoum M. A., et al. , Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 6, e1000713 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niimi M., Firth N. A., Cannon R. D., Antifungal drug resistance of oral fungi. Odontology 98, 15–25 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Abeles S. R., et al. , Human oral viruses are personal, persistent and gender-consistent. ISME J. 8, 1753–1767 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang G., Bushman F. D., The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 19, 514–527 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepp P. W., et al. , Methanogenic Archaea and human periodontal disease. Proc. Natl. Acad. Sci. U.S.A. 101, 6176–6181 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang C., Schmitz R. A., Archaea associated with human surfaces: Not to be underestimated. FEMS Microbiol. Rev. 39, 631–648 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Yaseen A., et al. , Oral colonization by entamoeba gingivalis and trichomonas tenax: A PCR-based study in health, gingivitis, and periodontitis. Front. Cell. Infect. Microbiol. 11, 782805 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamont R. J., Koo H., Hajishengallis G., The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilian M., et al. , The oral microbiome–An update for oral healthcare professionals. Br. Dent. J. 221, 657–666 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Khan R., Petersen F. C., Shekhar S., Commensal bacteria: An emerging player in defense against respiratory pathogens. Front. Immunol. 10, 1203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seal B. S., et al. , Microbial-derived products as potential new antimicrobials. Vet. Res. 49, 66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C., et al. , Mining and biosynthesis of bioactive lanthipeptides from microorganisms. Front. Bioeng. Biotechnol. 9, 692466 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radaic A., Kapila Y. L., The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 19, 1335–1360 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelaez-Prestel H. F., Sanchez-Trincado J. L., Lafuente E. M., Reche P. A., Immune tolerance in the oral mucosa. Int J. Mol. Sci. 22, 12149 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulyanto R. M., Thompson Z. A., Beall C. J., Leys E. J., Griffen A. L., The predominant oral microbiota is acquired early in an organized pattern. Sci. Rep. 9, 10550 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaci G., et al. , Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl. Environ. Microbiol. 80, 928–934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbour A., Wescombe P., Smith L., Evolution of lantibiotic Salivaricins: New weapons to fight infectious diseases. Trends Microbiol. 28, 578–593 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Arnison P. G., et al. , Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr K. I., et al. , Pinensins: The first antifungal lantibiotics. Angew. Chem. Int. Ed Engl. 54, 11254–11258 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Fu Y., Jaarsma A. H., Kuipers O. P., Antiviral activities and applications of ribosomally synthesized and post-translationally modified peptides (RiPPs). Cell Mol. Life Sci 78, 3921–3940 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prochnow H., et al. , Labyrinthopeptins exert broad-spectrum antiviral activity through lipid-binding-mediated virolysis. J. Virol. 94, e01471-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iorio M., et al. , A glycosylated, labionin-containing lanthipeptide with marked antinociceptive activity. ACS Chem. Biol. 9, 398–404 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Meindl K., et al. , Labyrinthopeptins: A new class of carbacyclic lantibiotics. Angew. Chem. Int. Ed Engl. 49, 1151–1154 (2010). [DOI] [PubMed] [Google Scholar]
- 27.van Staden A. D. P., van Zyl W. F., Trindade M., Dicks L. M. T., Smith C., Therapeutic application of lantibiotics and other lanthipeptides: Old and new findings. Appl. Environ. Microbiol. 87, e0018621 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kindrachuk J., et al. , Manipulation of innate immunity by a bacterial secreted peptide: Lantibiotic nisin Z is selectively immunomodulatory. Innate Immun. 19, 315–327 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee C., Paul M., Xie L., van der Donk W. A., Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105, 633–684 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Repka L. M., Chekan J. R., Nair S. K., van der Donk W. A., Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem. Rev. 117, 5457–5520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagedroste M., Reiners J., Knospe C. V., Smits S. H. J., Schmitt L., A structural view on the maturation of lanthipeptides. Front. Microbiol. 11, 1183 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayikpoe R. S., van der Donk W. A., “Chapter Eighteen–Peptide backbone modifications in lanthipeptides” in Methods in Enzymology, Petersson E. J., Ed. (Academic Press, 2021), vol. 656, pp. 573–621. [DOI] [PubMed] [Google Scholar]
- 33.Helander I. M., Mattila-Sandholm T., Permeability barrier of the gram-negative bacterial outer membrane with special reference to nisin. Int. J. Food Microbiol. 60, 153–161 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Afonina I., et al. , The composition and function of Enterococcus faecalis membrane vesicles. MicroLife 2, uqab002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbour A., Glogauer M., 16S rRNA sequence of saliva-derived multispecies biofilm. NCBI Sequence Read Archive (SRA). http://www.ncbi.nlm.nih.gov/bioproject/PRJNA924352. Deposited 16 January 2023.
- 36.Butler C. A., et al. , Breastmilk influences development and composition of the oral microbiome. J. Oral. Microbiol. 14, 2096287 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abranches J., et al. , Biology of oral streptococci. Microbiol. Spectr. 6, 6.5.11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrero E. R., et al. , Antimicrobial effects of commensal oral species are regulated by environmental factors. J. Dent. 47, 23–33 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Shin J. M., et al. , Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front. Microbiol. 6, 617 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fine N., et al. , Periodontal inflammation primes the systemic innate immune response. J. Dent. Res. 100, 318–325 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Fine N., et al. , Distinct oral neutrophil subsets define health and periodontal disease states. J. Dent. Res. 95, 931–938 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Oveisi M., et al. , Novel assay to characterize neutrophil responses to oral biofilms. Infect. Immun. 87, e00790-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen T., Gotschlich E. C., CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl. Acad. Sci. U.S.A. 93, 14851–14856 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kragstrup T. W., et al. , Altered levels of soluble CD18 may associate immune mechanisms with outcome in sepsis. Clin. Exp. Immunol. 190, 258–267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang C. B., Alimova Y., Ebersole J. L., Macrophage polarization in response to oral commensals and pathogens. Pathog. Dis. 74, ftw011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang E. R., Liu S., Wu L. F., Altschuler S. J., Cobb M. H., Chemoattractant concentration-dependent tuning of ERK signaling dynamics in migrating neutrophils. Sci. Signal 9, ra122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez F. O., et al. , Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: Similarities and differences. Blood 121, e57–e69 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Levin B. J., et al. , A prominent glycyl radical enzyme in human gut microbiomes metabolizes trans-4-hydroxy-l-proline. Science 355, eaai8386 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kupke T., Kempter C., Gnau V., Jung G., Gotz F., Mass spectroscopic analysis of a novel enzymatic reaction. Oxidative decarboxylation of the lantibiotic precursor peptide EpiA catalyzed by the flavoprotein EpiD. J. Biol. Chem. 269, 5653–5659 (1994). [PubMed] [Google Scholar]
- 50.Velasquez J. E., Zhang X., van der Donk W. A., Biosynthesis of the antimicrobial peptide epilancin 15X and its N-terminal lactate. Chem. Biol. 18, 857–867 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller L. M., Chatterjee C., van der Donk W. A., Kelleher N. L., The dehydratase activity of lacticin 481 synthetase is highly processive. J. Am. Chem. Soc. 128, 1420–1421 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You Y. O., Levengood M. R., Ihnken L. A., Knowlton A. K., van der Donk W. A., Lacticin 481 synthetase as a general serine/threonine kinase. ACS Chem. Biol. 4, 379–385 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong S. H., et al. , The enterococcal cytolysin synthetase has an unanticipated lipid kinase fold. Elife 4, e07607 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paoli L., et al. , Biosynthetic potential of the global ocean microbiome. Nature 607, 111–118 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ongpipattanakul C., et al. , Mechanism of action of ribosomally synthesized and post-translationally modified peptides. Chem. Rev. 122, 14722–14814 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Connor M., et al. , Nisin M: A bioengineered Nisin A variant that retains full induction capacity but has significantly reduced antimicrobial activity. Appl. Environ. Microbiol. 86, e00984-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garg N., Tang W., Goto Y., Nair S. K., van der Donk W. A., Lantibiotics from Geobacillus thermodenitrificans. Proc. Natl. Acad. Sci. U.S.A. 109, 5241–5246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maricic N., Anderson E. S., Opipari A. E., Yu E. A., Dawid S., Characterization of a multipeptide lantibiotic locus in Streptococcus pneumoniae. mBio 7, e01656-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maricic N., “Genetic and structural characterization of pneumolancidin, a broad spectrum inhibitory lantibiotic, produced by Streptococcus pneumoniae”, Doctoral thesis, University of Michigan, University of Michigan Library, Michigan, USA; (2016). [Google Scholar]
- 60.Walker G. V., Heng N. C. K., Carne A., Tagg J. R., Wescombe P. A., Salivaricin E and abundant dextranase activity may contribute to the anti-cariogenic potential of the probiotic candidate Streptococcus salivarius JH. Microbiology (Reading) 162, 476–486 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Snel J., et al. , Competitive selection of lactic acid bacteria that persist in the human oral cavity. Appl. Environ. Microbiol. 77, 8445–8450 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S. G., et al. , Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature 572, 665–669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolar S. L., et al. , Group b streptococcus evades host immunity by degrading hyaluronan. Cell Host Microbe 18, 694–704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finlay B. B., McFadden G., Anti-immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell 124, 767–782 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Bitschar K., et al. , Lugdunin amplifies innate immune responses in the skin in synergy with host- and microbiota-derived factors. Nat. Commun. 10, 2730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Payne J. A. E., et al. , Antibiotic-chemoattractants enhance neutrophil clearance of Staphylococcus aureus. Nat. Commun. 12, 6157 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravichandran A., Gu G., Escano J., Lu S. E., Smith L., The presence of two cyclase thioesterases expands the conformational freedom of the cyclic Peptide occidiofungin. J. Nat. Prod. 76, 150–156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wüthrich K. J. E. N., NMR with proteins and nucleic acids. Europhys. News 17, 11–13 (1986). [Google Scholar]
- 69.Braunschweiler L., Ernst R. R., Coherence transfer by isotropic mixing: Application to proton correlation spectroscopy. J. Magn. Reson. 1969, 521–528 (1983). [Google Scholar]
- 70.Kumar A., Ernst R. R., Wuthrich K., A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem. Biophys. Res. Commun. 95, 1–6 (1980). [DOI] [PubMed] [Google Scholar]
- 71.Johnson B. A., Blevins R. A., View N. M. R., A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 (1994). [DOI] [PubMed] [Google Scholar]
- 72.Schindelin J., et al. , Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fine N., et al. , Primed PMNs in healthy mouse and human circulation are first responders during acute inflammation. Blood Adv. 3, 1622–1637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All study data are included in the article and/or SI Appendix and Dataset S1 files. Raw read sequences of the 16S rRNA genes were deposited at Sequence Read Archive (SRA, NCBI), BioProject: PRJNA924352 (35).