This randomized clinical trial investigates if low-dose atropine, 0.01% or 0.02%, slows the progression of myopia in children.
Key Points
Question
Does low-dose atropine in concentrations of 0.01% and/or 0.02% slow the progression of myopia?
Finding
In this randomized clinical trial including 573 participants in the safety set and 489 participants in the modified intention-to-treat set over 3 years of therapy, 0.02% atropine vs placebo did not significantly increase the proportion of participants’ eyes responding to therapy; however, 0.01% atropine increased the responder portion and reduced both myopia progression and axial elongation vs placebo over 3 years of therapy.
Meaning
Trial results show that the efficacy and safety observed may be supportive of low dose atropine as a pharmacological treatment option for myopia progression in children.
Abstract
Importance
The global prevalence of myopia is predicted to approach 50% by 2050, increasing the risk of visual impairment later in life. No pharmacologic therapy is approved for treating childhood myopia progression.
Objective
To assess the safety and efficacy of NVK002 (Vyluma), a novel, preservative-free, 0.01% and 0.02% low-dose atropine formulation for treating myopia progression.
Design, Setting, and Participants
This was a double-masked, placebo-controlled, parallel-group, randomized phase 3 clinical trial conducted from November 20, 2017, through August 22, 2022, of placebo vs low-dose atropine, 0.01% and 0.02% (2:2:3 ratio). Participants were recruited from 26 clinical sites in North America and 5 countries in Europe. Enrolled participants were 3 to 16 years of age with −0.50 diopter (D) to −6.00 D spherical equivalent refractive error (SER) and no worse than −1.50 D astigmatism.
Interventions
Once-daily placebo, low-dose atropine, 0.01%, or low-dose atropine, 0.02%, eye drops for 36 months.
Main Outcomes and Measures
The primary, prespecified end point was the proportion of participants’ eyes responding to 0.02% atropine vs placebo therapy (<0.50 D myopia progression at 36 months [responder analysis]). Secondary efficacy end points included responder analysis for atropine, 0.01%, and mean change from baseline in SER and axial length at month 36 in a modified intention-to-treat population (mITT; participants 6-10 years of age at baseline). Safety measurements for treated participants (3-16 years of age) were reported.
Results
A total of 576 participants were randomly assigned to treatment groups. Of these, 573 participants (99.5%; mean [SD] age, 8.9 [2.0] years; 315 female [54.7%]) received trial treatment (3 participants who were randomized did not receive trial drug) and were included in the safety set. The 489 participants (84.9%) who were 6 to 10 years of age at randomization composed the mITT set. At month 36, compared with placebo, low-dose atropine, 0.02%, did not significantly increase the responder proportion (odds ratio [OR], 1.77; 95% CI, 0.50-6.26; P = .37) or slow mean SER progression (least squares mean [LSM] difference, 0.10 D; 95% CI, −0.02 D to 0.22 D; P = .10) but did slow mean axial elongation (LSM difference, −0.08 mm; 95% CI, −0.13 mm to −0.02 mm; P = .005); however, at month 36, compared with placebo, low-dose atropine, 0.01%, significantly increased the responder proportion (OR, 4.54; 95% CI, 1.15-17.97; P = .03), slowed mean SER progression (LSM difference, 0.24 D; 95% CI, 0.11 D-0.37 D; P < .001), and slowed axial elongation (LSM difference, −0.13 mm; 95% CI, −0.19 mm to −0.07 mm; P < .001). There were no serious ocular adverse events and few serious nonocular events; none was judged as associated with atropine.
Conclusions and Relevance
This randomized clinical trial found that 0.02% atropine did not significantly increase the proportion of participants’ eyes responding to therapy but suggested efficacy for 0.01% atropine across all 3 main end points compared with placebo. The efficacy and safety observed suggest that low-dose atropine may provide a treatment option for childhood myopia progression.
Trial Registration
ClinicalTrials.gov Identifier: NCT03350620
Introduction
The global prevalence of myopia was estimated at 30% to 34% in 2020 and is predicted to increase to approximately 50% by 20501; 938 million people are projected to develop myopia with refractive error worse than −6 diopters (D) by 2050 with an increased risk of major sequelae.2 Myopia usually presents at 6 to 12 years of age.3 Early-onset myopia increases the risk of high myopia4,5,6 and complications including glaucoma, cataracts, retinal detachment, and myopic maculopathy.7,8 Vision correction alone will not alter myopia progression.9 Multifocal contact lenses and orthokeratology are effective in slowing axial elongation and myopia progression,10,11,12,13,14,15 however, no pharmaceutical products are currently approved in the US or Europe to treat myopia progression.
Atropine may block receptors throughout the eye to limit choroidal and scleral thinning and slow eye elongation.16,17 Low-dose atropine, at concentrations up to 0.05%, has recently emerged as a pharmacologic option to control myopia progression in children.18,19,20,21,22,23 The dose-ranging Atropine Treatment of Myopia 2 (ATOM2) trial supported the use of atropine eye drops, including a concentration as low as 0.01% in Asian children.18 The Low-Concentration Atropine for Myopia Progression (LAMP) study23 along with other randomized clinical studies of low-dose atropine19,21,22 demonstrated the efficacy of atropine, 0.01%, in slowing myopia progression with fewer visual adverse effects, compared with higher concentrations of atropine.
Currently, compounded low-dose atropine is used off label in the US. These preparations are not of pharmaceutical grade quality and contain preservatives.24,25,26,27 Preservative-free formulations can avoid potential associated toxicities including dry eye and chronic corneal irritation.28 NVK002 (Vyluma) is a novel, preservative-free, pharmaceutical-grade atropine formulation under development for the treatment of myopia progression.
Here, we report the 3-year results of the Childhood Atropine for Myopia Progression (CHAMP) phase 3 randomized clinical trial that evaluated the efficacy and safety of low-dose atropine at concentrations of 0.01% and 0.02% vs placebo to treat myopia progression in children.
Methods
Trial Design and Oversight
CHAMP was a 3-arm, parallel, randomized, multicenter, double-masked, placebo-controlled, phase 3 trial conducted across 27 clinical sites in North America and 5 countries in Europe. The protocol and the statistical analysis plan (SAP) were developed by the sponsor and are available in Supplement 1 and Supplement 2, respectively. The trial was approved by central and/or local institutional review boards or ethics committees for each clinical site. The trial was conducted in accordance with the Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. Before randomization, written informed consent was obtained from parents or legal guardians, and assent was obtained from participants. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
The trial included 2 stages (eFigure 1 in Supplement 3). Stage 1 was the 36-month efficacy and safety phase presented herein. Participants were randomly assigned in a 2:2:3 ratio to receive placebo (vehicle), atropine, 0.01%, or atropine, 0.02%, respectively, dosed 1 time per day in each eye at bedtime. The randomization was stratified by age at randomization (<9 years and ≥9 years) and by refractive error (spherical equivalent refractive error [SER] −0.50 to −3.00 D and SER −3.01 to −6.00 D).
The low-dose atropine used in the trial was a preservative-free formulation in the concentrations 0.01% and 0.02% manufactured under Good Manufacturing Practices. It was packaged in sterile, single-use ampules, formulated with standard topical ophthalmic excipients, and was stable at room temperature (eMethods in Supplement 3). Placebo trial medication was of identical formulation but without atropine. Trial sites used Interactive Response Technology (Suvoda), a computer-based randomization and distribution software, to randomly assign participants to treatment groups, and masked trial medication was dispensed accordingly. The randomization list, a permuted block design, was created by Design and Analysis of Trials Associates. The sponsor (Vyluma), site personnel, and trial participants were masked to the identity of treatment throughout the trial.
Trial Participants
Key inclusion criteria included an age of 3 to 17 years or younger (inclusive) at the time of enrollment, myopic SER of −0.50 to −6.00 D in each eye, astigmatism of no more than −1.50 D in each eye, and anisometropic SER of less than 1.50 D. The primary objective was to evaluate myopia progression in participants who were 6 to 10 years of age at randomization. Enrollment of participants from the age range of 11 to 16 years was discontinued after 66 participants. The protocol specified that after enrollment of 50 participants older than 11 years, enrollment could be closed to avoid overenrollment of a subpopulation least likely to benefit. Participants self-identified with the following race and ethnicity categories: American Indian or Alaskan Native, Asian, Black or African American, Hispanic or Latino, Native Hawaiian or Other Pacific Islander, White, and other (which included races not shown in the previous list as well as missing race and ethnicity information). Key exclusion criteria included history of any ocular disease or surgery that might confound trial results; this included prior myopia control treatment including orthokeratology, multifocal contact lenses, or progressive-addition spectacle lenses and chronic use of topical or systemic antimuscarinic/anticholinergic medications. A complete list of inclusion and exclusion criteria is provided in the trial protocol in Supplement 1.
Trial End Points and Assessments
The primary efficacy end point was the proportion of participants’ eyes that showed less than 0.50 D myopia progression from baseline (responder analysis) at month 36 for atropine, 0.02%, vs placebo. Secondary end points were defined as the responder analysis for atropine, 0.01%, and change from baseline for SER and axial length for both doses at month 36.
Cycloplegic SER and axial length were measured every 6 months. For each evaluation, 3 to 5 measurements were averaged. The SER for each eye was normalized to a vertex distance of 0 mm (the corneal plane) according to the calculation specified in the SAP. Safety assessments included best-corrected distance visual acuity, photopic pupil size, slitlamp with fundus examination, intraocular pressure every 6 months, and recording of adverse events (AEs) at each visit. AEs were coded using the Medical Dictionary for Regulatory Activities, version 25. In cases of COVID-19–related site closures, virtual visits were conducted by phone, assessing AEs and compliance (Supplement 1).
Statistical Analysis
Primary and secondary efficacy analyses were performed in the modified intention-to-treat (mITT) data set, which included all participants aged 6 to 10 years at baseline. The mITT set was predefined as the primary analysis population because this age group has been widely studied in the literature. Safety analyses included all participants who were administered at least 1 dose of trial medication. The ITT data set included all participants aged 3 to 16 years of age who were randomly assigned to treatment groups.
At a 2-sided 5% significance level, 136 participants in the atropine, 0.02%, group and 91 participants in the placebo group were estimated to provide 95% power to detect the difference between treatment responder proportions of 0.25 and 0.07. A sample size of 91 in the atropine, 0.01%, group and 91 in the placebo group were estimated to provide 90% power to detect the same treatment difference. To account for dropouts, we planned to randomly assign at least 436 participants into the 6- to 10-year age groups (the primary efficacy population) and at least 483 participants total.
The primary and all secondary end points comprised a fixed sequence set of end points to be tested in order. The responder analysis used a mixed-effects model based on the binomial distribution using a logit function, with progression as the dependent variable, and participant, treatment, visit, eye, baseline age group (<9 or ≥9 years), and SER group (−0.50 to −3.00 D or −3.01 to −6.00 D) as independent variables, including a treatment-by-visit interaction. Random intercept for participants and eye-within participant were included using variance components and compound symmetry covariance structures. The least squares mean (LSM) change from baseline in SER or axial length was also analyzed using a mixed-effects model (SAP in Supplement 2). All analyses were performed assuming data missing at random, with no imputation for missing data. P values were 2-sided and not adjusted for multiple analyses. A P value < .05 was considered statistically significant for the primary outcome. Further details are provided in the SAP in Supplement 2 with analyses using SAS software, version 9.4 (SAS Institute).
Results
Trial Population
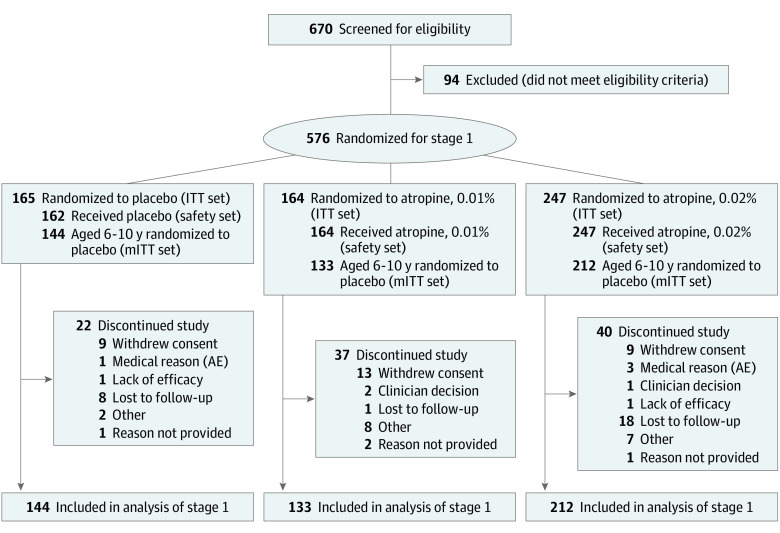
From November 20, 2017, through August 22, 2022, 576 participants were randomly assigned to treatment groups. Of these, 573 participants (99.5%; mean [SD] age, 8.9 [2.0] years; 261 male [45.5%]; 312 female [54.5%]) received trial treatment (3 participants who were randomized did not dose with trial drug) and were included in the safety set. Participants self-identified with the following race and ethnicity groups: 7 American Indian or Alaskan Native (1.2%), 109 Asian (19%), 81 Black or African American (14.1%), 155 Hispanic or Latino (27.1%), 4 Native Hawaiian or Other Pacific Islander (0.7%), 308 White (53.8%), and 64 other (11.2%). The 489 participants (84.9%) who were 6 to 10 years of age at randomization composed the mITT set. Of the 576 randomly assigned participants, 477 (82.8%) completed all 3 years of stage 1, whereas 99 (17.2%) discontinued the trial; this proportion was within the estimated dropout rate of 27%. The main reasons reported for trial discontinuation were loss to follow-up (42 [7.3%]) and withdrawal of consent (31 [5.4%]) (Figure 1). Baseline characteristics were generally balanced among the 3 treatment groups (Table 1; eTable 1 in Supplement 3).
Figure 1. Participant Disposition.
AE indicates adverse event; ITT, intention to treat; mITT, modified intention to treat.
Table 1. Baseline Demographic and Clinical Characteristics (Intention-to-Treat Set).
| Characteristic | Placebo (n = 165) | Atropine, 0.01% (n = 164) | Atropine, 0.02% (n = 247) |
|---|---|---|---|
| Age, mean (SD), y | 8.8 (1.8) | 9.0 (2.1) | 9.0 (2.1) |
| < 9 y, No. (%) | 63 (38.2) | 64 (39.0) | 94 (38.1) |
| ≥ 9 y, No. (%) | 102 (61.8) | 100 (61.0) | 153 (61.9) |
| Sex, No. (%) | |||
| Female | 94 (57.0) | 85 (51.8) | 136 (55.1) |
| Male | 71 (43.0) | 79 (48.2) | 111 (44.9) |
| Ethnicity, No. (%) | |||
| Hispanic or Latino | 47 (28.5) | 44 (26.8) | 64 (25.9) |
| Not Hispanic | 112 (67.9) | 114 (69.5) | 171 (69.2) |
| Race, No. (%) | |||
| American Indian or Alaskan Native | 1 (0.6) | 3 (1.8) | 3 (1.2) |
| Asian | 26 (15.8) | 29 (17.7) | 54 (21.9) |
| Black or African American | 27 (16.4) | 23 (14.0) | 31 (12.6) |
| Native Hawaiian or Other Pacific Islander | 3 (1.8) | 1 (0.6) | 0 |
| White | 93 (56.4) | 84 (51.2) | 132 (53.4) |
| Other (including missing)a | 15 (9.1) | 24 (14.6) | 27 (10.9) |
| Refractive error range, No. (%) | |||
| Less myopic (SER: −0.50 to −3.00 D) | 111 (67.3) | 110 (67.1) | 164 (66.4) |
| More myopic (SER: −3.01 to −6.00 D) | 54 (32.7) | 54 (32.9) | 83 (33.6) |
Abbreviations: D, diopter; SER, spherical equivalent refractive error.
Other includes races other than those listed in the table and missing race and ethnicity.
Efficacy
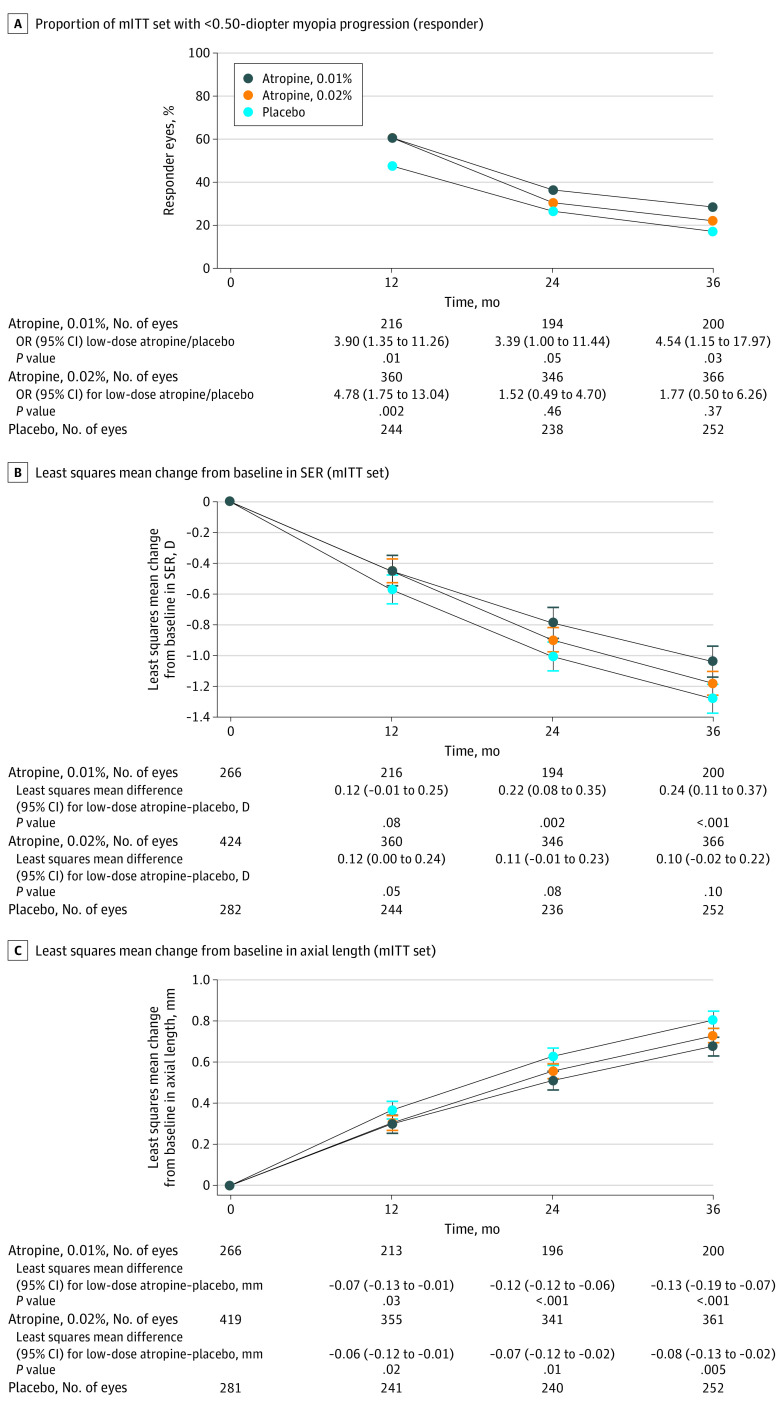
The proportion of responder eyes was 17.5% (44 of 252) in the placebo group, 28.5% (57 of 200) in the atropine, 0.01%, group, and 22.1% (81 of 366) in the atropine, 0.02%, group (Table 2). The primary outcome of the difference vs placebo was not significant for atropine, 0.02% (OR, 1.77; 95% CI, 0.50-6.26; P = .37) at 3 years. The secondary outcome for atropine, 0.01%, had an odds ratio (OR) of 4.54 (95% CI, 1.15-17.97; P = .03). Atropine, 0.01%, was associated with significantly higher responder rates than placebo at all time points, whereas atropine, 0.02%, was associated with a higher responder rate vs placebo only at month 12 (OR, 4.78; 95% CI, 1.75-13.04; P = .002) (Figure 2A; eTable 2 in Supplement 3). Post hoc analysis comparing atropine, 0.01% and 0.02%, showed an OR of 2.6 (95% CI, 0.77-8.57; P = .13) for the responder analysis comparing 0.01% with 0.02% (eTable 3 in Supplement 3). Post hoc evaluation of confounding therapies indicated that exclusion of data associated with confounding therapies led to an increased treatment effect size (eTable 4 in Supplement 3) noted subsequently in the Discussion.
Table 2. Efficacy Measures (Modified Intention-to-Treat Set).
| End point | Placebo (n = 144) | Atropine, 0.01% (n = 133) | Atropine, 0.02% (n = 212) |
|---|---|---|---|
| Responder analysis: <0.50 D myopia progression at month 36 | |||
| No. of eyes | 252 | 200 | 366 |
| No. (%) of responder eyes | 44 (17.5) | 57 (28.5) | 81 (22.1) |
| Odds ratio for low-dose atropine/placebo (95% CI) | NA | 4.54 (1.15 to 17.96) | 1.77 (0.50 to 6.26) |
| P value | NA | .03 | .37 |
| Cycloplegic refraction: mean change from baseline in SER | |||
| Baseline | |||
| No. of eyes | 282 | 266 | 424 |
| Mean SER (SD), D | −2.45 (1.13) | −2.41 (1.17) | −2.42 (1.17) |
| Median (IQR) | −2.38 (1.66) | −2.20 (1.53) | −2.26 (1.57) |
| Month 36 | |||
| No. of eyes | 252 | 200 | 366 |
| Mean SER (SD), D | −3.72 (1.42) | −3.41 (1.49) | −3.61 (1.53) |
| Median (IQR) | −3.73 (2.05) | −3.28 (2.02) | −3.51 (2.10) |
| Least squares mean change (95% CI) from baseline at month 36, D | −1.28 (−1.37 to −1.19) | −1.04 (−1.14 to −0.94) | −1.18 (−1.26 to −1.10) |
| Least squares mean difference (95% CI) between low-dose atropine and placebo, D | NA | 0.24 (0.11 to 0.37) | 0.10 (−0.02 to 0.22) |
| P value | NA | <.001 | .10 |
| Biometric measure: mean change from baseline in axial length | |||
| Baseline | |||
| No. of eyes | 281 | 266 | 419 |
| Mean axial length (SD), mm | 24.33 (0.84) | 24.37 (0.81) | 24.30 (0.87) |
| Median (IQR) | 24.40 (1.20) | 24.39(1.09) | 24.29 (1.14) |
| Month 36 | |||
| No. of eyes | 252 | 200 | 366 |
| Mean axial length (SD), mm | 25.09 (0.88) | 25.12 (0.87) | 25.01 (0.98) |
| Median (IQR) | 25.13 (1.29) | 25.09 (1.27) | 25.01 (1.26) |
| Least squares mean change (95% CI) from baseline at month 36, mm | 0.81 (0.76 to 0.85) | 0.68 (0.63 to 0.72) | 0.73 (0.69 to 0.76) |
| Least squares mean difference (95% CI) between low-dose atropine and placebo, mm | NA | −0.13 (−0.19 to −0.07) | −0.08 (−0.13 to −0.02) |
| P value | NA | <.001 | .005 |
Abbreviations: D, diopter; NA, not applicable; SER, spherical equivalent refractive error.
Figure 2. Change in Ophthalmic Parameters Over Time.
A, Proportion with less than 0.50-diopter (D) myopia progression (responder) (modified intention-to-treat [mITT] set). P value for odds ratio (OR) (low-dose atropine/placebo). B, Least squares mean change from baseline in spherical equivalent refractive error (SER; mITT set). P value for least squares mean difference (low-dose atropine − placebo). C, Least squares mean change from baseline in axial length (mITT set). P value for least squares mean difference (low-dose atropine − placebo).
The change in SER from baseline (LSM) at month 36 was −1.28 D (95% CI, −1.37 to −1.19 D) in the placebo group, −1.04 D (95% CI, −1.14 to −0.94 D) in the atropine, 0.01%, group, and −1.18 D (95% CI, −1.26 to −1.10 D) in the atropine, 0.02%, group (Table 2). The LSM difference for atropine, 0.01% vs placebo was 0.24 D (95% CI, 0.11-0.37 D; P < .001). There was a smaller treatment effect for atropine, 0.02% vs placebo (LSM difference, 0.10 D; 95% CI, −0.02 to 0.22 D; P = .10). Although the primary end point was defined at 36 months, compared with placebo, atropine, 0.01%, also reduced mean SER progression at month 24 (LSM change, 0.22 D; 95% CI, 0.08-0.35 D; P = .002), and atropine, 0.02%, reduced mean SER progression at month 12 (LSM change, 0.12 D; 95% CI, 0-0.24 D; P = .05) (Figure 2B; eTable 2 in Supplement 3).
Regarding axial length, the LSM change from baseline at month 36 was 0.81 mm (95% CI, 0.76-0.85 mm) in the placebo group, 0.68 mm (95% CI, 0.63-0.72 mm) in the atropine, 0.01%, group, and 0.73 mm (95% CI, 0.69-0.76 mm) in the atropine, 0.02%, group (Table 2). The LSM difference for atropine, 0.01%, vs placebo was −0.13 mm (95% CI, −0.19 to −0.07 mm; P < .001) and for atropine, 0.02%, vs placebo was −0.08 mm (−0.13 to −0.02 mm; P = .005). Both doses also reduced axial length elongation compared with placebo at month 12 (atropine, 0.01%, LSM difference, −0.07 mm; 95% CI, −0.13 to −0.01 mm; P = .03; atropine, 0.02%, LSM difference, −0.06 mm; 95% CI, −0.12 to −0.01 mm; P = .02) and month 24 (atropine, 0.01%, LSM difference, −0.12 mm; 95% CI, −0.18 to −0.06 mm; P < .001; atropine, 0.02%, LSM difference, −0.07 mm; 95% CI, −0.12 to −0.02 mm; P = .01) (Figure 2C; eTable 2 in Supplement 3). The SER change from baseline and axial length change from baseline were correlated (placebo, Pearson r = −0.79; 95% CI, −0.83 to −0.74; P < .001; atropine, 0.01%, Pearson r = −0.74; 95% CI, −0.80 to −0.68; P < .001; atropine, 0.02%, Pearson r = −0.85; 95% CI, −0.88 to −0.82; P < .001) (eFigure 2 in Supplement 3).
Prespecified subgroup analyses for baseline characteristics, analyzed for both responder rate and SER change from baseline, were consistent with overall outcomes, generally showing a positive treatment effect vs placebo across all subgroups. No treatment-by-subgroup interaction was identified (eFigure 3 in Supplement 3).
Prespecified analyses of the efficacy of atropine, 0.01% and 0.02% vs placebo across all 3 end points in the ITT population (eTable 5 in Supplement 3) showed similar results as in the mITT population (Table 2; eTable 2 in Supplement 3). Compliance with dosing (as measured by the return of used and unused ampules) in the ITT set was high (eTable 6 in Supplement 3).
Safety
Both low-dose atropine concentrations were safe and well tolerated. In the safety analysis, there was no increase in treatment-emergent AEs (TEAEs) associated with nonocular antimuscarinic function with low-dose atropine treatments (0% in atropine, 0.01%; 0.8% in atropine, 0.02%) vs placebo (2.5%), and there were no serious ocular TEAEs. TEAEs were defined as any new or worsening of an existing AE that occurred or worsened between the first dose date and the last dose date of stage 1. Serious nonocular TEAEs were reported in 13 participants (2.3%), including 4 in the placebo group (2.5%), 1 in the atropine, 0.01%, group (0.6%), and 8 in the atropine, 0.02%, group (3.2%). One serious TEAE, a seizure, occurred in a participant taking placebo with prior history of seizures (Table 3; eTable 7 in Supplement 3).
Table 3. Treatment-Emergent Adverse Events (Safety Set).
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Placebo (n = 162) | Atropine, 0.01% (n = 164) | Atropine, 0.02% (n = 247) | Total (n = 573) | |
| Any treatment-emergent adverse event, No. (%) | 116 (71.6) | 98 (59.8) | 163 (66.0) | 377 (65.8) |
| Serious ocular treatment-emergent adverse event, No. (%) | 0 | 0 | 0 | 0 |
| Serious nonocular treatment-emergent adverse event, No. (%) | 4 (2.5) | 1 (0.6) | 8 (3.2) | 13 (2.3) |
| Any treatment-emergent adverse event leading to permanent discontinuation of trial drug, No. (%) | 10 (6.2) | 0 | 5 (2.0) | 15 (2.5) |
| Treatment-emergent adverse events (partial list), No. (%)a | ||||
| Photophobia | 5 (3.1) | 4 (2.4) | 11 (4.5) | 20 (3.5) |
| Allergic conjunctivitis | 5 (3.1) | 3 (1.8) | 11 (4.5) | 19 (3.3) |
| Eye irritation | 6 (3.7) | 1 (0.6) | 2 (0.8) | 9 (1.6) |
| Mydriasis (enlarged pupil) | 0 | 2 (1.2) | 4 (1.6) | 6 (1.0) |
| Blurred vision | 0 | 2 (1.2) | 4 (1.6) | 6 (1.0) |
| Eyelid swelling | 0 | 3 (1.8) | 1 (0.4) | 4 (0.7) |
| Eyelid irritation | 1 (0.6) | 0 | 2 (0.8) | 3 (0.5) |
| Elevated heart rate | 1 (0.6) | 0 | 2 (0.8) | 3 (0.5) |
| Seizure | 1 (0.6) | 0 | 0 | 1 (0.2) |
eTable 1 in Supplement 3 contains a complete list.
The incidence of any TEAE was similar between placebo (116 of 162 participants [71.6%]), atropine, 0.01% (98 of 164 participants [59.8%]), and atropine, 0.02% (163 of 247 participants [66.0%]). The most common ocular TEAEs were photophobia, allergic conjunctivitis, eye irritation, mydriasis, and blurred vision (Table 3; eTable 7 in Supplement 3).
Discussion
The CHAMP trial demonstrated that atropine, 0.02%, did not significantly increase the proportion of participants’ eyes responding to therapy compared with placebo and did not significantly decrease myopia progression at month 36. However, atropine, 0.02%, did show a significant reduction in axial length elongation at month 36 in the modified ITT population.
Importantly, the trial also demonstrated that atropine, 0.01%, slowed myopia progression compared with placebo across all efficacy measures (eg, increasing the number of responders, slowing SER progression, and reducing axial length elongation) in participants aged 6 to 10 years.
The treatment effect observed with atropine, 0.01%, is clinically meaningful as reflected in the increased number of responders to therapy compared with placebo (28.5% vs 17.5%; a difference of 11%; 95% CI, 3%-18.5%). Although no reference exists to show that this difference is clinically meaningful, the predefined threshold for a responder eye was myopia progression of less than 0.50-D SER from baseline at 3 years. This is a stringent criterion for defining myopia control29 and indicative of clinically negligible progression or stable myopia.30 Compared with placebo, atropine, 0.01%, resulted in a greater proportion of responders at months 12, 24, and 36 and reduced mean SER progression at months 24 and 36 compared with placebo.
A previous review has shown that the anatomical end point of change in axial length correlates with refractive changes,29 which was also shown in our analysis (eFigure 2 in Supplement 3). Because of its objective nature, axial length elongation is emerging as an important end point for monitoring myopic progression.29 Biometric measurements demonstrated that both atropine, 0.01% and 0.02%, slowed axial elongation, compared with placebo at all time points measured over 3 years. The clear efficacy of atropine, 0.01%, in slowing myopia progression and axial elongation may lead to less frequent or delayed changes in glasses, progression to less severe correction, and potential reduction of long-term sequelae, which could lead to vision loss later in life such as myopic maculopathy.31
CHAMP was a 3-year, placebo-controlled, phase 3 clinical trial of atropine, 0.01% and 0.02%, conducted in children with myopia. Previous studies showing the benefit of low-dose atropine19,21,22,23,32 were conducted at a single site or multiple sites in Asia. The ATOM2 study32 provided evidence of dose-dependent effects in the range of 0.01% to 0.5% but did not include a placebo group. The other 4 studies19,21,22,23 were placebo-controlled for 1 or 2 years, with treatment effects generally consistent with atropine, 0.01%, over the same period. The Western Australia-ATOM trial used a single site and showed a statistically significant benefit for atropine, 0.01%, after 1 year but not at the 2-year time point. The authors ascribe this result to a loss of fast myopia progressors in the placebo group during the second year of the trial.20
The CHAMP trial results support the possibility that atropine, 0.01%, eye drops as applied in this trial may be a safe and effective pharmacologic option for individuals similar to those enrolled in this trial. AEs potentially associated with topical atropine included photophobia or blurred vision, although the incidence of these AEs were low compared with placebo in this trial (Table 3; eTable 7 in Supplement 3).
Post hoc analyses were conducted to address the unexpected results found for the 2 doses of atropine. Although atropine, 0.02%, slowed axial elongation compared with placebo, there was no significant increase in responder analysis or SER progression compared with placebo and a numerically smaller treatment effect compared with atropine, 0.01%. In a post hoc analysis comparing the 2 active doses, we observed an OR of 2.6 (95% CI, 0.77-8.57; P = .13) for the responder analysis comparing atropine, 0.01% with 0.02% (eTable 3 in Supplement 3), suggesting that the 2 doses were not different in treatment effect for the responder analysis in CHAMP. Other studies of atropine, 0.01% and 0.02% (or 0.025%), showed small, nonsignificant differences between the lower and higher concentrations in SER change from baseline.23,33
To further understand the results of atropine, 0.02%, another post hoc analysis was conducted, which excluded the data after treatment cessation for participants who discontinued trial medication but stayed in the trial through month 36, while switching to other myopia therapies (ie, orthokeratology, multifocal contact lenses, or compounded atropine) (eTable 4 in Supplement 3). This analysis showed an increased difference between atropine, 0.02%, and placebo in the proportion of responders (OR increased from 1.8 [95% CI, 0.50-6.26]; P = .37 in the mITT set to an OR of 2.5 [95% CI, 0.71-8.7]; P = .16 in this post hoc analysis) and in the mean difference of SER change from baseline (LSM difference increased from 0.10 D [95% CI, −0.02 to 0.22]; P = .10 to 0.15 D [95% CI, 0.03-0.26]; P = .01). There was an important confounding effect due to the inclusion of the data from these participants in the mITT set after they stopped trial medication and went on to other treatments, which likely contributed to the atropine, 0.02%, results as reported.
In evaluating our results, a higher-than-expected placebo responder rate was observed (17% vs the assumed 7% in the protocol). However, on review of more recently available data, the observed placebo myopia progression rate in CHAMP appears within the range of the progression rate observed in control arms of other myopia therapy studies (eTable 8 in Supplement 3).11,20,34,35 Importantly when comparing the treatment effect of atropine, 0.01%, from the CHAMP study with other published trials studying 0.01%, comparable treatment effects were seen.36
The CHAMP trial demonstrated that both atropine concentrations, 0.01% and 0.02%, exhibited a safety profile notable for minimal ocular AEs and no increase in nonocular antimuscarinic AEs compared with placebo. Other potential therapies for the treatment of myopia progression include defocus-incorporated multiple-segment lenses, multifocal contact lenses, and orthokeratology. Atropine is generally considered suitable in younger children, when compared with CLs or other devices.37,38,39 The future myopia treatment paradigm may involve initiation of therapy earlier in the disease progression and may include a combination of low-dose atropine and device-based therapies.40,41,42,43,44
Strengths and Limitations
A strength of this trial was the placebo-controlled design over 3 years of treatment in a US and European population. Trial limitations include potential bias introduced by those participants who discontinued trial medication and switched to confounding treatments and the relatively lower representation of participants in the range of 3 to 5 years and 11 to 17 years of age. Another limitation is that the prespecified, primary end point of 0.02% responder analysis did not meet statistical significance. As detailed in the SAP in Supplement 2, the subsequent secondary end points should be considered not formally statistically significant at the .05 level. However, the consistency of the atropine, 0.01%, effects across all end points and the qualitative similarity of treatment effect between 0.01% and 0.02% support the evaluation of 0.01% end points in totality.
Conclusions
Results from this multicenter, randomized clinical trial of 6- to 10-year-old participants demonstrated that a preservative-free formulation of atropine, 0.02%, did not significantly increase the proportion of participants’ eyes responding to therapy compared with placebo, but that atropine, 0.01%, increased the proportion of eyes with less than 0.50-D myopia progression after 3 years of treatment. This dose also reduced both SER progression and axial elongation vs placebo over the same period.
From a risk/benefit perspective, the efficacy and safety observed suggests that low-dose atropine, 0.01%, may provide a treatment option for children aged 3 to 17 years with myopia progression, which may lead to less frequent or delayed change in glasses, progression to less severe correction, and potentially reduce long-term sequelae, which could lead to vision loss later in life, such as myopic maculopathy.31 In the population studied, low-dose atropine could provide an important early treatment option for young children.
Trial Protocol
Statistical Analysis Plan
eMethods.
eFigure 1. Trial Design
eFigure 2. Correlation Between Axial Length Change From Baseline and SER Change From Baseline at Month 36 for the 3 Treatment Groups (mITT Set)
eFigure 3. Subgroup and Subgroup-By-Treatment Interaction Analyses (mITT Set)
eTable 1. Baseline Age and Asian/Non-Asian Distribution (ITT Set)
eTable 2. Efficacy Measures by Visit (mITT Set)
eTable 3. Post Hoc Analysis Comparing NVK002 0.01% vs 0.02% (mITT Set)
eTable 4. Post Hoc Analyses on Primary and Secondary End Points (mITT Month 36), Excluding Post–Late Dose Data From 23 Participants Who Discontinued Trial Treatment, Stayed in Trial, and Switched to Orthokeratology or Multifocal Contact Lens (MFCL) or Compounded Atropine
eTable 5. Efficacy Measures by Visit (ITT Set)
eTable 6. Compliance to Trial Medication (ITT Set)
eTable 7. Complete List of Treatment-Emergent Adverse Events (Safety Set)
eTable 8 Myopia Progression Rate and Less Than 0.5 D Responder Proportion in Placebo Arms of Randomized Pharmacologic Trials or Single-Vision Lens Arms of Randomized Lens Trials in the Literature With a Trial Duration of at Least 1 Year
Nonauthor Collaborators. CHAMP Trial Group Investigators.
Data Sharing Statement
References
- 1.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 2.Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modeling. Br J Ophthalmol. 2018;102(7):855-862. doi: 10.1136/bjophthalmol-2017-311266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolffsohn JS, Flitcroft DI, Gifford KL, et al. IMI: myopia control reports overview and introduction. Invest Ophthalmol Vis Sci. 2019;60(3):M1-M19. doi: 10.1167/iovs.18-25980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL III, Holden BA. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012;89(1):27-32. doi: 10.1097/OPX.0b013e3182357f79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pärssinen O, Kauppinen M, Viljanen A. The progression of myopia from its onset at age 8-12 to adulthood and the influence of heredity and external factors on myopic progression: a 23-year follow-up study. Acta Ophthalmol. 2014;92(8):730-739. doi: 10.1111/aos.12387 [DOI] [PubMed] [Google Scholar]
- 6.Sankaridurg P, Tahhan N, Kandel H, et al. IMI impact of myopia. Invest Ophthalmol Vis Sci. 2021;62(5):2. doi: 10.1167/iovs.62.5.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The risks and benefits of myopia control. Ophthalmology. 2021;128(11):1561-1579. doi: 10.1016/j.ophtha.2021.04.032 [DOI] [PubMed] [Google Scholar]
- 8.Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. doi: 10.1167/iovs.61.4.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sankaridurg P. Contact lenses to slow progression of myopia. Clin Exp Optom. 2017;100(5):432-437. doi: 10.1111/cxo.12584 [DOI] [PubMed] [Google Scholar]
- 10.Bao J, Huang Y, Li X, et al. Spectacle lenses with aspherical lenslets for myopia control vs single-vision spectacle lenses: a randomized clinical trial. JAMA Ophthalmol. 2022;140(5):472-478. doi: 10.1001/jamaophthalmol.2022.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of MiSight Lenses for myopia control. Optom Vis Sci. 2019;96(8):556-567. doi: 10.1097/OPX.0000000000001410 [DOI] [PubMed] [Google Scholar]
- 12.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30(1):71-80. doi: 10.1080/02713680590907256 [DOI] [PubMed] [Google Scholar]
- 13.Lam CS, Tang WC, Lee PH, et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: results of a 3-year follow-up study. Br J Ophthalmol. 2022;106(8):1110-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rappon J, Chung C, Young G, et al. Control of myopia using diffusion optics spectacle lenses: 12-month results of a randomized controlled, efficacy and safety study (CYPRESS). Br J Ophthalmol. Published online September 1, 2022. doi: 10.1136/bjo-2021-321005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walline JJ, Lindsley KB, Vedula SS, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020;1(1):CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upadhyay A, Beuerman RW. Biological mechanisms of atropine control of myopia. Eye Contact Lens. 2020;46(3):129-135. doi: 10.1097/ICL.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu PC, Chuang MN, Choi J, et al. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond). 2019;33(1):3-13. doi: 10.1038/s41433-018-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. doi: 10.1016/j.ophtha.2011.07.031 [DOI] [PubMed] [Google Scholar]
- 19.Hieda O, Hiraoka T, Fujikado T, et al. ; ATOM-J. Study Group . Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. 2021;65(3):315-325. doi: 10.1007/s10384-021-00822-y [DOI] [PubMed] [Google Scholar]
- 20.Lee SS, Lingham G, Blaszkowska M, et al. Low-concentration atropine eyedrops for myopia control in a multiracial cohort of Australian children: a randomized clinical trial. Clin Exp Ophthalmol. 2022;50(9):1001-1012. doi: 10.1111/ceo.14148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena R, Dhiman R, Gupta V, et al. Atropine for the treatment of childhood myopia in India: multicentric randomized trial. Ophthalmology. 2021;128(9):1367-1369. doi: 10.1016/j.ophtha.2021.01.026 [DOI] [PubMed] [Google Scholar]
- 22.Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178-1184. doi: 10.1001/jamaophthalmol.2020.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113-124. doi: 10.1016/j.ophtha.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 24.Gudeman J, Jozwiakowski M, Chollet J, Randell M. Potential risks of pharmacy compounding. Drugs R D. 2013;13(1):1-8. doi: 10.1007/s40268-013-0005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guharoy R, Noviasky J, Haydar Z, Fakih MG, Hartman C. Compounding pharmacy conundrum: “we cannot live without them but we cannot live with them” according to the present paradigm. Chest. 2013;143(4):896-900. doi: 10.1378/chest.13-0212 [DOI] [PubMed] [Google Scholar]
- 26.Richdale K, Tomiyama ES, Novack GD, Bullimore MA. Compounding of low-concentration atropine for myopia control. Eye Contact Lens. 2022;48(12):489-492. doi: 10.1097/ICL.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration . Compounding and the FDA: questions and answers. Accessed July 12, 2022. https://www.fda.gov/drugs/human-drug-compounding/compounding-and-fda-questions-and-answers
- 28.Gifford KL, Richdale K, Kang P, et al. IMI: clinical management guidelines report. Invest Ophthalmol Vis Sci. 2019;60(3):M184-M203. doi: 10.1167/iovs.18-25977 [DOI] [PubMed] [Google Scholar]
- 29.Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83:100923. doi: 10.1016/j.preteyeres.2020.100923 [DOI] [PubMed] [Google Scholar]
- 30.COMET Group . Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Invest Ophthalmol Vis Sci. 2013;54(13):7871-7884. doi: 10.1167/iovs.13-12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci. 2019;96(6):463-465. doi: 10.1097/OPX.0000000000001367 [DOI] [PubMed] [Google Scholar]
- 32.Chia A, Lu QS, Tan D. Five-year clinical trial on Atropine for the Treatment of Myopia 2: myopia control with atropine 0.01% Eyedrops. Ophthalmology. 2016;123(2):391-399. doi: 10.1016/j.ophtha.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 33.Cui C, Li X, Lyu Y, et al. Safety and efficacy of 0.02% and 0.01% atropine on controlling myopia progression: a 2-year clinical trial. Sci Rep. 2021;11(1):22267. doi: 10.1038/s41598-021-01708-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses vs single-vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44(4):1492-1500. doi: 10.1167/iovs.02-0816 [DOI] [PubMed] [Google Scholar]
- 35.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD; US Pirenzepine Study Group . Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122(11):1667-1674. doi: 10.1001/archopht.122.11.1667 [DOI] [PubMed] [Google Scholar]
- 36.Hemmati HD, Fong T, Lang E, Chandler S. Efficacy of NVK002 low-dose atropine for treatment of pediatric myopia progression over 24 months: comparison of CHAMP vs other published trials. IOVS. 2023;64(8):4962. [Google Scholar]
- 37.Bullimore MA, Sinnott LT, Jones-Jordan LA. The risk of microbial keratitis with overnight corneal reshaping lenses. Optom Vis Sci. 2013;90(9):937-944. doi: 10.1097/OPX.0b013e31829cac92 [DOI] [PubMed] [Google Scholar]
- 38.Chalmers RL, McNally JJ, Chamberlain P, Keay L. Adverse event rates in the retrospective cohort study of safety of paediatric soft contact lens wear: the ReCSS study. Ophthalmic Physiol Opt. 2021;41(1):84-92. doi: 10.1111/opo.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalmers RL, Wagner H, Mitchell GL, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci. 2011;52(9):6690-6696. doi: 10.1167/iovs.10-7018 [DOI] [PubMed] [Google Scholar]
- 40.Erdinest N, London N, Levinger N, Morad Y. Myopia control with combination low-dose atropine and peripheral defocus soft contact lenses: a case series. Case Rep Ophthalmol. 2021;12(2):548-554. doi: 10.1159/000515568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan L, Wei CC, Chen CS, et al. The synergistic effects of orthokeratology and atropine in slowing the progression of myopia. J Clin Med. 2018;7(9):259. doi: 10.3390/jcm7090259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang N, Bai J, Liu L. Low concentration atropine combined with orthokeratology in the treatment of axial elongation in children with myopia: a meta-analysis. Eur J Ophthalmol. 2022;32(1):221-228. doi: 10.1177/1120672121998903 [DOI] [PubMed] [Google Scholar]
- 43.Yu S, Du L, Ji N, et al. Combination of orthokeratology lens with 0.01% atropine in slowing axial elongation in children with myopia: a randomized double-blinded clinical trial. BMC Ophthalmol. 2022;22(1):438. doi: 10.1186/s12886-022-02635-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan Y, Zhu C, Liu M, et al. Efficacy of combined orthokeratology and 0.01% atropine for myopia control: the study protocol for a randomized, controlled, double-blind, and multicenter trial. Trials. 2021;22(1):863. doi: 10.1186/s13063-021-05825-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods.
eFigure 1. Trial Design
eFigure 2. Correlation Between Axial Length Change From Baseline and SER Change From Baseline at Month 36 for the 3 Treatment Groups (mITT Set)
eFigure 3. Subgroup and Subgroup-By-Treatment Interaction Analyses (mITT Set)
eTable 1. Baseline Age and Asian/Non-Asian Distribution (ITT Set)
eTable 2. Efficacy Measures by Visit (mITT Set)
eTable 3. Post Hoc Analysis Comparing NVK002 0.01% vs 0.02% (mITT Set)
eTable 4. Post Hoc Analyses on Primary and Secondary End Points (mITT Month 36), Excluding Post–Late Dose Data From 23 Participants Who Discontinued Trial Treatment, Stayed in Trial, and Switched to Orthokeratology or Multifocal Contact Lens (MFCL) or Compounded Atropine
eTable 5. Efficacy Measures by Visit (ITT Set)
eTable 6. Compliance to Trial Medication (ITT Set)
eTable 7. Complete List of Treatment-Emergent Adverse Events (Safety Set)
eTable 8 Myopia Progression Rate and Less Than 0.5 D Responder Proportion in Placebo Arms of Randomized Pharmacologic Trials or Single-Vision Lens Arms of Randomized Lens Trials in the Literature With a Trial Duration of at Least 1 Year
Nonauthor Collaborators. CHAMP Trial Group Investigators.
Data Sharing Statement