Highlights
-
•
Individuals with SCD showed smaller amygdala volumes but not hippocampus volumes.
-
•
Amygdala volumes might contribute to an objective identification of SCD patients.
-
•
Associations to memory change in SCD were weak for amygdala and hippocampus volumes.
-
•
Hippocampus volumes showed stronger associations to memory changes in MCI.
Keywords: Amygdala, Hippocampus, 7T MRI, Alzheimer’s disease, SCD, Memory
Abstract
Introduction
The hippocampus is the most prominent single region of interest (ROI) for the diagnosis and prediction of Alzheimer’s disease (AD). However, its suitability in the earliest stages of cognitive decline, i.e., subjective cognitive decline (SCD), remains uncertain which warrants the pursuit of alternative or complementary regions. The amygdala might be a promising candidate, given its implication in memory as well as other psychiatric disorders, e.g. depression and anxiety, which are prevalent in SCD. In this 7 tesla (T) magnetic resonance imaging (MRI) study, we aimed to compare the contribution of volumetric measurements of the hippocampus, the amygdala, and their respective subfields, for early diagnosis and prediction in an AD-related study population.
Methods
Participants from a longitudinal study were grouped into SCD (n = 29), mild cognitive impairment (MCI, n = 23), AD (n = 22) and healthy control (HC, n = 31). All participants underwent 7T MRI at baseline and extensive neuropsychological testing at up to three visits (baseline n = 105, 1-year n = 78, 3-year n = 39). Analysis of covariance (ANCOVA) was used to assess group differences of baseline volumes of the amygdala and the hippocampus and their subfields. Linear mixed models were used to estimate the effects of baseline volumes on yearly changes of a z-scaled memory score. All models were adjusted to age, sex and education.
Results
Compared to the HC group, individuals with SCD showed smaller amygdala ROI volumes (range across subfields −11% to −1%), but not hippocampus ROI volumes (-2% to 1%) except for the hippocampus-amygdala-transition-area (-7%). However, cross-sectional associations between baseline memory and volumes were smaller for amygdala ROIs (std. ß [95% CI] ranging between 0.16 [0.08; 0.25] and 0.46 [0.31; 0.60]) than hippocampus ROIs (between 0.32 [0.19; 0.44] and 0.53 [0.40; 0.67]). Further, the association of baseline volumes with yearly memory change in the HC and SCD groups was similarly weak for amygdala ROIs and hippocampus ROIs. In the MCI group, volumes of amygdala ROIs were associated with a relevant yearly memory decline [95% CI] ranging between −0.12 [−0.24; 0.00] and −0.26 [−0.42; −0.09] for individuals with 20% smaller volumes than the HC group. However, effects were stronger for hippocampus ROIs with a corresponding yearly memory decline ranging between −0.21 [−0.35; −0.07] and −0.31 [−0.50; −0.13].
Conclusion
Volumes of amygdala ROIs, as determined by 7T MRI, might contribute to objectively and non-invasively identify patients with SCD, and thus aid early diagnosis and treatment of individuals at risk to develop dementia due to AD, however associations with other psychiatric disorders should be evaluated in further studies. The amygdala’s value in the prediction of longitudinal memory changes in the SCD group remains questionable. Primarily in patients with MCI, memory decline over 3 years appears to be more strongly associated with volumes of hippocampus ROIs than amygdala ROIs.
1. Introduction
Individuals with subjective cognitive decline (SCD) and patients with mild cognitive impairment (MCI) are known to be at elevated risk to develop dementia due to Alzheimer’s disease (AD) (Mitchell et al., 2014). The early identification of SCD and MCI and the detection of those individuals that eventually will experience severe cognitive decline is crucial to apply preventive and early therapeutic strategies. Current diagnostic tools for the accurate prediction of cognitive decline due to AD, such as collection of cerebrospinal fluid or positron emission tomography, are highly invasive. Alternative or complementary approaches are therefore needed.
A diagnostic tool that has been explored in numerous studies is magnetic resonance imaging (MRI). The most prominent single region targeted so far is the hippocampus, a key region for memory functions that shows major atrophy in MCI and AD (Shi et al., 2009). Several studies have pointed to a diagnostic and predictive value of volumetric measurements of the hippocampus in the context of AD. Baseline volumes of the whole hippocampus are suggested to predict future memory decline in patients with MCI (Kovacevic et al., 2009, Wei et al., 2018) and AD-like patterns of hippocampus atrophy can already be found in individuals with SCD (Perrotin et al., 2015, Wang et al., 2020).
The measurement of hippocampus volumes is nevertheless not sufficient to serve as a stand-alone diagnostic tool for individuals with SCD and MCI. A review from Lombardi et al. (2020) reported that the accuracy of whole hippocampus volume for early diagnosis of AD in people with MCI was heterogeneous with sensitivities ranging between 0.28 and 1.00 and specificities between 0.43 and 0.94. Although previous studies found reduced hippocampus volumes in individuals with SCD (Hafkemeijer et al., 2013, Striepens et al., 2010, Yue et al., 2018), others were not able to reproduce these findings (Fan et al., 2018, Ryu et al., 2017). The lack of consensus in volumetric measurements of the hippocampus especially, but not exclusively, in SCD participants warrants the pursuit of a more suitable region.
The amygdala is strongly connected with the hippocampus and similarly affected by early degeneration in the course of AD pathology (Brady and Mufson, 1990). Besides memory formation, the amygdala seems to play a major role in psychiatric conditions such as depression and anxiety which are prevalent in SCD (LeDoux, 2007). The amygdala could therefore be relevant to identify individuals with SCD who, by definition, experience persistent decline in memory function and associated rumination and worries (Jessen et al., 2020). Significantly smaller volumes of the whole amygdala compared to cognitively healthy control participants (HC) have been reported for individuals with MCI (Nunes et al., 2010, Poulin et al., 2011, Yue et al., 2018). Moreover, amygdala volume was shown to be associated with memory decline over time in longitudinal studies of MCI cohorts (Kovacevic et al., 2009, Wei et al., 2018). Similar to the hippocampus, studies examining amygdala volumes in SCD cohorts reached inconsistent results. While some studies reported reduced amygdala volumes in individuals with SCD (Kim et al., 2013, Schultz et al., 2015, Striepens et al., 2010, Yue et al., 2018) others did not (Hafkemeijer et al., 2013, Rogne et al., 2016).
The lack of consensus concerning volumetric measurements of the hippocampus and the amygdala in individuals with SCD might be due to the variety of methodological approaches including uncertainties in cognitive measures, distinct criteria for the inclusion of SCD participants, and most importantly insufficient MRI resolution. It has been shown that only MRI at high resolution can provide volumetric measurements of small brain regions with satisfactory accuracy (Rhindress et al., 2015). Low field strengths might have restrained the exploitation of structural MRI of the amygdala, the hippocampus and particularly their subfields.
In this study, we therefore performed T1-weighted MRI at 7 tesla (T) and extensive neuropsychological testing in a well-characterized cohort ranging from individuals with SCD, patients with MCI or dementia due to suspected AD, and HC participants. Ultrahigh field MRI allowed us to examine the amygdala down to its subfields and we hypothesized that their volumes have considerable contributions for 1) the identification of patients with (subjective) memory impairment and 2) the association with memory decline. Volumes of the hippocampus, its subfields and a control region, the precentral gyrus, were analyzed in parallel for comparison.
2. Methods
2.1. Study design
This study used data from the projects 15HLT04 NeuroMET (NeuroMET, 2016) and 18HLT09 NeuroMET2 (NeuroMET2, 2019) which both aimed to improve diagnosis and management of neurodegenerative diseases through high quality and standardized methods. By the time of the present analysis, the study population was tested at baseline and at up to 2 follow-up visits (1 and 3 years after baseline). All visits comprised a standardized medical interview, neurological examination, saliva collection and extensive neuropsychological testing performed at the Charité university hospital, Berlin, Germany. Further, participants underwent blood draw and 7T MRI and magnetic resonance spectroscopy at baseline and some follow-up visits.. The study was approved by the ethics committee of the Charité university hospital (EA1/197/16 and EA2/121/19), and was conducted in accordance with the declaration of Helsinki.
2.2. Participants
All participants (n = 126) that were available from the NeuroMET longitudinal study by March 2022 were included in the present analysis. They were stratified into SCD, MCI, AD or HC. Besides severe or untreated medical, neurological or psychiatric diseases which could potentially interfere with cognition, exclusion criteria comprised history of drug or alcohol abuse and eating disorder. All participants were native German speakers and gave informed written consent before participation in the study. All participants were right-handed, except for one individual who was ambidextrous and three who were left-handed.
For the present analyses, we further excluded participants of the study population with severe depression, participants who dropped out because of a Parkinson’s diagnosis after baseline visits, participants with missing values for the memory composite score, and missing/low-quality structural MRI at baseline so that neither hippocampus nor amygdala volumes could be quantified. After exclusion, the study population consisted of 105 participants.
In the AD group, the main reason for dropping out at follow-ups was the lack of ability to consent (n = 12 of 15 dropouts). Across the whole study population, the main reason for dropping out was a lack of interest in participation (n = 22 of a total of 38 dropouts). Other reasons were new severe diseases (n = 3) and death (n = 1).
2.3. Memory assessment
We combined four memory-related sub-scores from commonly used neuropsychological tests to one single score of memory performance, which has previously been described (van de Rest et al., 2008). Three of the sub-scores derived from the German version of the Rey Auditory Verbal Learning Test (AVLT, German “Verbaler Merk- und Lernfähigkeitstest”, Helmstaedter et al. (2001)): AVLT learning (sum of trial 1 to 5), AVLT delayed recall (sum of trial 7), AVLT recognition (difference between correctly and falsely recognized positive items). As a fourth sub-score, the sum of correctly answered backward sequences of the Digit Span test (Wechsler, 1981) was included. All sub-scores were z-transformed centered around the mean of the HC group, summed up and divided by 4, which resulted in a z-scaled score for memory performance.
2.4. 7T MRI
We acquired T1-weighted structural cerebral images on a 7T whole-body scanner (Magnetom 7T, Siemens Healthineers, Erlangen, Germany) using a 1TX/32RX head coil (NOVA Medical, Wilmington, USA). The scan protocol for the Magnetization-Prepared 2 Rapid Acquisition Gradient Echoes (MP2RAGE) sequence (Marques et al., 2010) included the following parameters: TR = 5000 ms, TE = 2.51 ms, TI1 = 900 ms und TI2 = 2700 ms, bandwidth 250 Hz/Pixel, α1 = 7°, α2 = 5°, FoV 240 × 240 mm2, parallel acquisition with GRAPPA 2 (32 reference lines), resulting in 240 sagittal slices with a resolution of 0.75 × 0.75 × 0.75 mm3 and an acquisition time of 11:17 ms. This rather long scan time was chosen in order to allow for high quality images with submillimeter resolution, which in turn enabled the detection of subtle differences in ROI volumes. A denoised reconstruction was created according to O'Brien et al. (2014).
2.5. (Pre-)processing of MRI data
Best segmentation results were achieved when a composed T1 weighted image served as input for the in-house workflow based on NiPype (Gorgolewski et al., 2011). The complete (pre-)processing pipeline can be found on Github (Dell'Orco, 2022). Briefly, we combined the original and the denoised images to improve contrasts: the brain tissue was extracted from the bias field corrected original T1 weighted image and the skull and background from its denoised reconstruction. The resulting image was used for cortical reconstruction and volumetric segmentation of the whole brain and hippocampus and amygdala subfields by the cross-sectional pipeline of the open source FreeSurfer 7.1.1 image analysis suite (Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, USA, http://surfer.nmr.mgh.harvard.edu/, Fischl (2012)). For this study, we selected the following regions of interest (ROIs): whole amygdala, lateral nucleus, basal nucleus, accessory basal nucleus, anterior amygdaloid area (AAA), central nucleus, medial nucleus, cortical nucleus, corticoamygdaloid transition area (CATA), paralaminar nucleus, whole hippocampus, cornu ammonis (CA) regions 1, CA3, CA4, molecular layer, subiculum, granule cell and molecular layer of the dentate gyrus (GC-ML-DG), hippocampus-amygdala-transition-area (HATA) and the precentral gyrus (motor cortex) as a control region. All FreeSurfer segmentations were visually inspected and incorrectly segmented ROIs were excluded from further analysis. All ROI volumes were adjusted to the individual total intracranial volumes (TIV) and normalized to the mean TIV of the HC group (mean TIV) according to the formula
adjusted volume = raw volume-b×(TIV-mean TIV),
where the coefficient b represents the slope of regression between the ROI volume and the TIV. This approach for volume adjustment was shown to be effective previously (Voevodskaya et al., 2014).
2.6. Statistics
Statistical analyses were performed using R v4.0.2 (R-Core-Team, 2020), the lme4 (Bates et al., 2015), emmeans (Lenth, 2021), tidyverse (Hadley Wickham, 2019) and mice (van Buuren and Groothuis-Oudshoorn, 2011) packages. The full reproducible code is available elsewhere (Göschel, 2023). No adjustment for multiple testing between ROIs was applied except for Tukey post-hoc tests after group comparisons as indicated below. Therefore, p-values have to be interpreted with caution. Interpretation of results is primarily based on effect estimates and corresponding 95% confidence intervals (CI). For all models assessing the relationships between ROI volume and memory performance, we transformed volume measures to a percentage scale where 10 corresponded to the mean ROI volume of the HC group; 9 corresponded to 90% of the mean of the HC group, and so forth. This allowed us to compare the effects between ROIs independently of the distribution of this study sample. Therefore, all reported std. ß coefficients can be interpreted as the difference of memory performance for participants with 10% larger volumes. Figure A 2 visualizes the −10% and −20% marks in this AD-related cohort.
2.6.1. Cross-sectional analyses
Group differences for demographical data were calculated by analyses of variance (ANOVA) for continuous variables and Pearson’s χ2-Test for nominal data. Age, sex and education adjusted means for memory at each time point were extracted from the linear mixed model described in the longitudinal analyses 2.6.2 and tested by post-hoc pairwise comparisons. Age, sex and education adjusted analyses of covariance (ANCOVA) were calculated to assess group differences for baseline ROI volumes. Tukey post-hoc pairwise comparisons of group means were computed. Using linear regression models adjusted to age, sex and education, we explored the relationship between each baseline ROI volume (independent variable) and memory at baseline (dependent variable).
2.6.2. Longitudinal analyses
Linear mixed models (random intercept models) adjusted for age, sex and education were calculated. First, memory (dependent variable) was modeled longitudinally including an interaction for time and group (time*group: independent variable). Second, two models to explore the association of each baseline ROI volume on memory changes (dependent variable) over time were computed for the total study sample (including an interaction of ROI_volume*time; model 1) and a more complex model with additional estimating group specific effects (including an interaction of group*time*ROI_volume; model 2):
-
1.
Model 1 (whole study population) memoryij = β0 + u0i + β1*ROI_volumei*timeij + β2*agei + β3*sexi + β4*educationi + εij
-
2.
Model 2 (group-wise) memory ij = β0 + u0i + β1*ROI_volumei*timeij*groupi + β2*agei + β3*sexi + β4*educationi + εij
with β0: intercept, u0i: residual for intercept for participant i, memoryij: memory of participant i at time j, ROI_volumei: volume of ROI of participant i at baseline, timeij: time point of measure at time j for participant i, agei: age of participant i at baseline, educationi: education of participant i at baseline, sexi: sex of participant i, group: group of participants i, εij: residual for participant i at time j. We refrained from drawing inferences from the results concerning the AD group because of the large amount of missing data not at random, i.e. the majority of patients dropped out at follow-up visits due to severe advance in cognitive decline (informative missing, missing not at random, MNAR). Data from the AD group was however included for completeness. Analyses for a multiple imputation model including methods for handling MNAR are shown in the supplemental material. Memory decline greater than −0.1 standard deviations (SD) per year was considered as a relevant effect in this study. Assuming a simplified linear progression of memory decline, this corresponds to a conversion to MCI, i.e. memory impairment of 1.0 SD below the norm (Jessen et al., 2014), after at most 10 years.
3. Results
3.1. Cross-sectional analyses
3.1.1. Participant’s characteristics
Data from a total of 105 participants were analyzed. Table 1 presents baseline demographic details of the study sample. There were fewer female participants in the MCI group than in the remaining groups. Participants of the AD group were on average older and the SCD group younger than the rest of the study population. Participants of the SCD group reported on average higher and those of the AD group lower education. Further, there were more APOE ε4 carriers in the MCI and AD group compared to the other groups.
Table 1.
Participant’s characteristics at baseline.
|
HC |
SCD |
MCI |
AD |
p | |
|---|---|---|---|---|---|
| N = 31 | N = 29 | N = 23 | N = 22 | ||
| Females N (%) | 17 (55%) | 17 (59%) | 7 (30%) | 10 (46%) | 0.189a |
| Age [years] | 71 (8) | 69 (6) | 71 (6) | 75 (6) | 0.036b |
| Education [years] | 15 (3) | 16 (3) | 15 (3) | 14 (2) | 0.035b |
| APOE ε4 | |||||
| N carrier/non-carrier | 4/22 | 7/13 | 10/9 | 13/8 | 0.006a |
| (% carrier) | 15% | 35% | 53% | 62% |
Values are reported as mean (SD). Group differences for (a) categorical variables were tested with Chi2 test and (b) for continuous variables with ANOVA. APOE ε4 status was not available for all participants as indicated. Abbreviations: AD = Alzheimer’s disease, APOE ε4 = Apolipoprotein E ε4 allele, HC = Healthy control, MCI = Mild cognitive impairment, SCD = Subjective cognitive decline.
3.1.2. Memory
Number of participants, time between visits and adjusted means of memory scores [95% CI] are shown in Table 2. At all three time points, memory differed between groups with highest scores in HC and lowest scores in the AD group. At all visits, cross-sectional post-hoc comparisons derived from multiple regression models showed substantial differences between all groups except between HC and SCD. Group differences concerning memory impairment became more pronounced in the 1- and 3-years follow-ups.
Table 2.
Memory performance and number of participants at baseline and follow-up visits.
| HC | SCD | MCI | AD | |
|---|---|---|---|---|
| Baseline | ||||
| N | 31 | 29 | 23 | 22 |
| Memory | 0.04 [-0.18; 0.26] | −0.06 [-0.30; 0.17] | −1.31 [-1.57; −1.05] | −2.28 [-2.56; −2.01] |
| 1-year follow-up | ||||
| N | 23 | 21 | 17 | 17 |
| Time [days] | 369 (13) | 392 (38) | 386 (30) | 370 (11) |
| Memory | 0.08 [-0.13; 0.29] | −0.10 [-0.33; 0.12] | −1.37 [-1.62; −1.12] | −2.58 [-2.85; −2.31] |
| 3-years follow-up | ||||
| N | 16 | 11 | 6 | 6 |
| Time [days] | 1117 (63) | 1129 (71) | 1112 (54) | 1019 (166) |
| Memory | 0.15 [-0.12; 0.41] | −0.19 [-0.49; 0.11] | −1.50 [-1.87; −1.13] | −3.18 [-3.57; −2.79] |
Z-scaled memory is reported as age, sex and education adjusted means [95% CI] which were exported from a linear mixed model including 105 participants and a total of 222 observations. Time from baseline is reported as mean (SD) in days. Abbreviations: AD = Alzheimer’s disease, CI = Confidence interval, HC = Healthy control, MCI = Mild cognitive impairment, SCD = Subjective cognitive decline.
3.1.3. Volumes
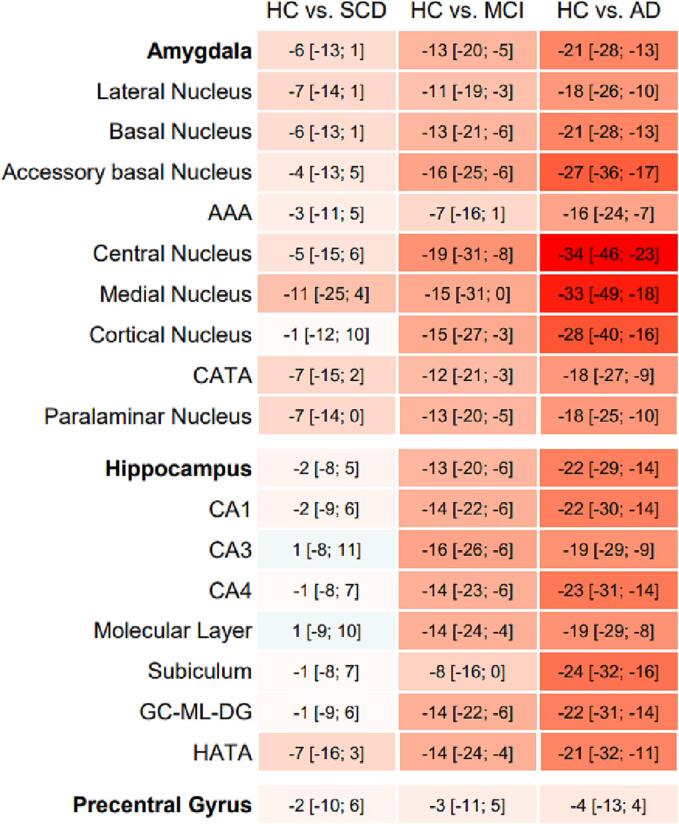
After visual inspection of all segmentations, data for whole amygdala (n = 104), amygdala subfields (n = 104), whole hippocampus (n = 104), hippocampus subfields (n = 103) and precentral gyrus (n = 101) were included in statistical analyses. Without exception, age, sex and education adjusted mean volumes of all amygdala ROIs were substantially smaller in SCD compared to HC, in MCI compared to SCD and in AD compared to MCI (table A 1). Percentages of group comparisons are visualized in Fig. 1. When compared to the HC group, we identified relevant volume differences in the SCD stage for several amygdala ROIs, e.g. the medial nucleus (adjusted mean difference [95 %CI] = -11% [−25%; 4%], p = 0.210), the CATA (-7% [−15%; 2%], p = 0.172), the lateral nucleus (-7% [−14%; 1%], p = 0.077) and the paralaminar nucleus (-7% [−14%; 0%], p = 0.055), but only for one hippocampus ROI, the HATA (-7% [−16%; 3%], p = 0.253). Amygdala ROIs further showed an average volume difference of −7% [-16%; 1%] (AAA) to −19% [-31%; −8%] (central nucleus) in the MCI group, and −16% [-24%; −7%] (AAA) to −34% [-46%; –23%] (central nucleus) in the AD group compared to HC. Overall, the group differences for the volumes of the hippocampus ROIs were not as pronounced as for the amygdala ROIs. As expected, no relevant group differences were found for the control region, the precentral gyrus.
Fig. 1.
Estimated mean group differences [95 %CI] of ROI volumes in percent (%). Values were estimated adjusting for the covariates age, sex and education in ANCOVA models which corrected for multiple group comparison (Tukey). Percentages are highlighted in red for a relative smaller and blue for relative bigger volume when compared to the HC group. Abbreviations: AAA = anterior amygdaloid area, AD = Alzheimer’s disease, CATA = corticoamygdaloid transition area, CA = cornu ammonis region, GC-ML-DG = granule cell and molecular layer of the dentate gyrus, HATA = hippocampus-amygdala-transition-area, HC = healthy control, MCI = mild cognitive impairment, SCD = subjective cognitive decline. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1.4. Association of baseline memory and ROI volumes
Next, we rescaled all volumes to their percentage difference [95% CI] to the HC average. By doing so, we aimed to determine those ROIs whose atrophy have stronger associations with memory performance in the cross-sectional sample (all estimates are reported in table A 2). In linear regression models across the whole study population, participants with a 10% smaller amygdala ROI volume showed worse baseline memory by −0.16 [-0.08; −0.25] (medial nucleus) to −0.46 [-0.31; −0.60] (basal nucleus). Compared to amygdala ROIs, hippocampus ROIs showed stronger associations between volume and baseline memory performance. Participants with −10% volumes of hippocampus ROIs showed worse memory by −0.32 [-0.19; −0.44] (CA3) to −0.53 [-0.40; −0.67] (whole hippocampus). As expected, the association between memory and the control region, the precentral gyrus, was weak with −0.17 [-0.37; 0.03] worse memory performance per −10% smaller volumes.
3.2. Longitudinal models
3.2.1. Change of memory performance over the course of 3 years
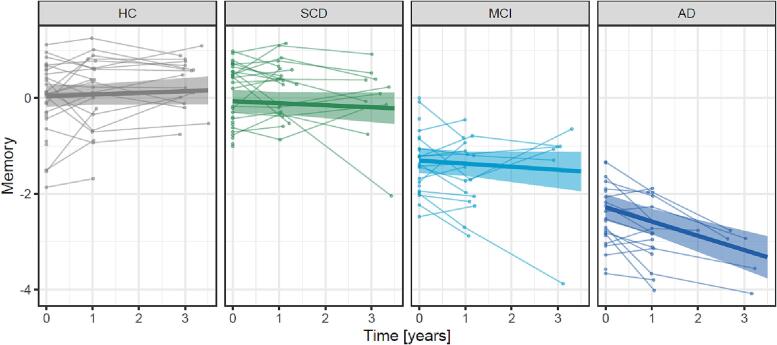
The number of participants and the average time between the assessments are shown in Table 2. In a linear mixed model, a substantial yearly decline in memory performance was found only for individuals of the AD group (ß [95 %CI] = -0.30 SD [-0.42; −0.17], p < 0.001) but not for the HC (0.03 SD [-0.04; 0.11], p = 0.375), SCD (-0.04 SD [-0.13; 0.05], p = 0.341) or MCI (-0.06 SD [-0.18; 0.05], p = 0.273) groups (Fig. 2).
Fig. 2.
Change of memory performance during three years. Memory was assessed by a z-scaled memory composite score for 105 participants at baseline, and when available at one (n = 78) and three years (n = 39) after baseline. The thin lines link individual memory performances at each available visit. The linear fits are presented with 95% CI (shaded areas) estimated by a linear mixed model adjusted for age, sex and education. Abbreviations: AD = Alzheimer’s disease, CI = confidence interval, HC = healthy control, MCI = mild cognitive impairment, SCD = subjective cognitive decline.
3.2.2. Associations between ROI volumes and memory performance over time
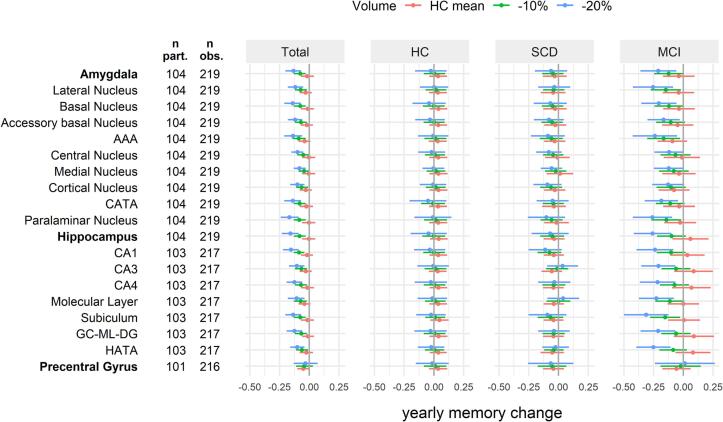
Models 1 included data across all groups, in order to assess whether baseline amygdala and hippocampus ROI volumes affected the yearly change in memory performance, accounting for baseline age, sex and education. All ROIs showed similar small interaction effects of volume on yearly memory change (0.03 < std. ß < 0.08, table A 3). In order to put these interaction effects into context, we provide values of yearly memory decline [95% CI] estimated for discrete baseline ROI volumes (HC mean, −10%, −20%; Fig. 3). On average, participants with HC mean volumes and −10% volumes had no relevant memory changes (<0.09 SD per year). Participants with −20% amygdala ROI volumes showed yearly memory changes between −0.08 [-0.13; −0.03] (medial nucleus) and −0.17 [-0.24; −0.10] (paralaminar nucleus). Effects were similar for hippocampus ROI volumes of −20% with yearly memory changes between −0.10 [-0.16; −0.04] (HATA) and −0.16 [-0.23; −0.09] (whole hippocampus). While both amygdala and hippocampus ROIs showed similar associations in models 1, baseline volumes of the control region, the precentral gyrus, showed no relevant association to yearly change of memory performance across the whole study population (yearly memory change for −10% volume = -0.04 [-0.11; 0.03], for −20% = −0.03 [-0.13; 0.07]).
Fig. 3.
Total (model 1) and group-wise (model 2) estimated yearly change of z-scaled memory performance depending on the baseline volume of the ROIs. Adjusted marginal effect estimates [95 %CI] were calculated for specific values to ease interpretation: baseline mean volume of the HC group, −10% and −20% volume. Number of participants (n part.) in the models ranged between 101 and 104. Number of baseline and follow-up observations (n obs.) ranged between 216 and 219. Estimates for the AD group are not shown due to the high number of missing values not at random (MNAR) for follow-up memory assessment. Linear mixed models included age, sex and education as covariates. Abbreviations: AAA = anterior amygdaloidarea, AD = Alzheimer’s disease, CA = cornu ammonis, CATA = corticoamygdaloid transition area, CI = confidence interval, GC-ML-DG = granule cell and molecular layer of the dentate gyrus, HATA = hippocampus-amygdala-transition-area, HC = healthy control, MCI = mild cognitive impairment, obs. = observations, part. = participants, ROI = region of interest, SCD = subjective cognitive decline.
Models 2 extended on models 1 by introducing the interaction group * time * ROI_volume to evaluate group-wise estimates of yearly change of memory performance by baseline ROI volume. Results are visualized in Fig. 3 (values in table A 4). In the HC group, 10% and 20% smaller baseline volumes than the HC mean were not associated with any relevant memory change (-0.04 < yearly memory change < 0.05). In the SCD group, we found a relevant association only for individuals with 20% smaller volumes of the CA1 region and the paralaminar nucleus with yearly memory changes of −0.11 [-0.25; 0.03] and −0.10 [-0.26; 0.05], respectively. In the MCI group, a relevant yearly memory change was associated with various amygdala ROIs with the strongest effects for the paralaminar nucleus (for −10% volume = -0.14 [-0.27; −0.02], for −20% = −0.26 [-0.42; −0.09]). Stronger associations were however found for several hippocampus ROIs, with strongest effects for the subiculum (for −10% volume = -0.15 [-0.28; −0.03], for −20% = −0.31 [-0.50; −0.13]). The precentral gyrus had no relevant group-wise effect on the yearly change of memory performance in neither HC, SCD nor MCI (-0.07 < yearly memory change < 0.04).
4. Discussion
In a study population of 105 participants with either SCD, MCI, AD, or cognitively healthy individuals, we examined associations between memory performance and 7T MRI volumes of amygdala ROIs in comparison with hippocampus ROIs. In a first cross-sectional analysis, we found smaller volumes in the SCD group for the amygdala ROIs but not the hippocampus ROIs except for the hippocampus-amygdala-transition-area. In longitudinal analyses across the total study population, however, we found that associations between memory changes and volumes of amygdala and hippocampus ROIs were of similar magnitude. Further group-wise analyses showed that these effects were mainly relevant in participants of the MCI but not HC and SCD group. Further, the association in MCI was stronger for hippocampus ROIs when compared to amygdala ROIs.
Previous studies have reported AD-related atrophy of the whole hippocampus and hippocampus subfields of similar dimensions as in the present study (de Flores et al., 2015). However, only few studies have examined amygdala volumes in AD-related cohorts. Previous literature showed that the whole amygdala is smaller in individuals with MCI (Nunes et al., 2010, Rogne et al., 2016, Yue et al., 2018) and AD (Basso et al., 2006, Poulin et al., 2011). These volume differences were generally of similar or even stronger magnitude than for the whole hippocampus, which is in line with the results of the present study. However, there is no consensus about a potential volume reduction of the whole amygdala in individuals with SCD. In this high-resolution 7T MRI study, volumes of the whole amygdala were smaller in SCD than in HC, which agrees with some previous 1.5 to 3 T studies (Kim et al., 2013, Schultz et al., 2015, Striepens et al., 2010, Yue et al., 2018) but not all (Hafkemeijer et al., 2013, Rogne et al., 2016). Relevant atrophy of amygdala volume in earliest stages of AD is reasonable because the amygdala is affected early by distribution of AD pathology, i.e. amyloid and phosphorylated tau deposition with associated volume loss (Brady and Mufson, 1990). The heterogeneity of previous findings is likely due to inconsistencies in the SCD stratification. Distinct atrophy patterns have been described for individuals with SCD recruited at the clinic versus those recruited in the community (Pini and Wennberg, 2021) emphasizing the importance of a well-characterized SCD group. In this study, SCD participants reported persistent self-perceived cognitive decline for over 6 months and associated worries that would motivate the individual to seek medical help, analogue to SCD plus criteria proposed by Jessen et al. (2020). Baseline memory performance of the SCD group in this study did not substantially differ from the HC group which further confirms the correct stratification of SCD participants. Addressing the shortcomings concerning MRI resolution and SCD stratification, our data support literature reporting smaller volumes of the whole amygdala in individuals with MCI, AD and strikingly SCD, when compared to HC.
We further expanded on the analyses of amygdala volumes by segmenting the amygdala down to its subfields. Literature on amygdala subfields in AD-related cohorts is scarce. Although not entirely comparable, shape analyses which deduced atrophy of the nuclei from the external shape of the amygdala showed AD-related volume reduction predominantly in the basolateral complex (Qiu et al., 2009, Tang et al., 2014). The basolateral complex consists of the large amygdala subfields, i.e. lateral, basal and accessory basal nuclei, regions that were reported to be reciprocally connected to the hippocampus and frontotemporal cortical regions (Poulin et al., 2011). In this study, the regions of the basolateral complex showed group differences to a similar extent as the whole amygdala. Strikingly larger effects were however found for the central nucleus (in MCI and AD) and the medial nucleus (in SCD and AD), both of which might suffer from higher measurement uncertainties due to their small size. The particular potential of the central and medial nucleus needs to be confirmed in independent studies. When compared to the hippocampus subfields, however, amygdala subfields showed group differences of similar or even greater magnitude. This is of especial relevance in the SCD group where smaller volumes were detected for several amygdala ROIs but neither for the whole hippocampus nor the hippocampus subfields, except for the HATA region, which connects the hippocampus with the amygdala. Thus, our cross-sectional group-level analyses suggested that volumes of amygdala subfields (especially the central and medial nucleus) could be more relevant in the identification of individuals with MCI, AD and particularly SCD, than volumes of the whole amygdala and hippocampus ROIs.
Previous studies reported substantial associations between volumes of the whole amygdala and a continuous measure of memory performance in several AD-related cohorts (Basso et al., 2006, Mizuno et al., 2000, Mori et al., 1997). We expanded on these findings by showing that most amygdala subfields reached associations with memory performance of similar magnitude as the whole amygdala. However, it needs to be pointed out that the central and medial nucleus showed weakest associations, which is remarkable considering the previously discussed group-differences in this study. At odds with our expectations, the association between memory performance and volumes of the hippocampus ROIs tended to be stronger than the association between memory performance and the amygdala ROIs, reaching strongest associations for the whole hippocampus. This lack of strength in association between volumes of the amygdala ROIs and baseline memory performance despite promising group differences might indicate that smaller amygdala volumes are less related to memory impairment but rather to other cognitive domains (e.g. executive function) or psychiatric dysfunctions (e.g. depression, anxiety) which are prevalent in SCD, MCI and AD. Additionally, a possible reverse causality in participants with SCD must be considered, i.e. cognitively healthy individuals with smaller amygdala volumes due to non-AD-related reasons may express more worries and therefore be stratified as SCD. The inconsistencies between larger group differences in amygdala volumes despite weaker associations to memory performance have to be investigated in further studies including other cognitive domains and psychiatric variables.
In order to draw inferences on the relevance of amygdala ROIs for cognitive decline, longitudinal analyses are essential. Previous studies showed that smaller whole amygdala volumes can predict a relevant longitudinal change in cognitive performance (den Heijer et al., 2006, Kovacevic et al., 2009, Li et al., 2022). Kovacevic et al. (2009) showed that smaller whole amygdala volumes predicted a decline in MMSE scores after 6 months in an MCI cohort, with associations that were at least as strong as those seen for hippocampus volume. Another recent study including participants with SCD, MCI and HC, used a novel machine-learning approach to predict 1-year changes in global cognitive outcomes by brain volumes such as the bilateral amygdala and hippocampus (Li et al., 2022). They showed that the volume of the left amygdala was more predictive for cognitive outcomes than the left or right hippocampus. In the present study, we included memory data for an extended timeframe (up to 2 follow-up visits in up to 3 years). We found stronger predictive effects for volumes of the whole hippocampus than the whole amygdala, which is not in line with the studies described above. When analyzing amygdala and hippocampus subfields, the whole hippocampus remained the strongest predictor in our study, while Li et al. (2022) found strongest effects in the right and left fimbria of the hippocampus. In further group-wise analyses, we found that the association was predominantly driven by participants with MCI with generally stronger effects for hippocampus ROIs than amygdala ROIs. In the HC and SCD groups, no ROI was predictive for relevant changes in memory, except for the hippocampus subfield CA1 and the amygdala subfield paralaminar nucleus. However, the effects were only marginally relevant in estimations for participants with −20% smaller volumes, a volume size that was rarely observed in the HC and SCD groups. Overall, these results suggest that the potential of amygdala and hippocampus ROIs in the prediction of memory decline might be limited to patients with MCI and is stronger for hippocampus ROIs than amygdala ROIs. In line with the cross-sectional results as discussed above, these findings may be due to a stronger association of amygdala volumes with other cognitive or psychiatric processes related to early AD, and less with memory performance.
Several limitations should be noted when interpreting our findings. First, we refrained from drawing inferences from the results concerning the AD group as the amount of missing follow-up data due to severe AD and associated incapability to consent for participation (missing not at random) would have likely led to an underestimation of the effects, the main limitation of this study. An approach to impute memory changes in AD patients is shown in the supplements, however, we concluded that the robust estimation was not reliably possible on the basis of this small sample. Second, as SCD is an early stage of AD, which might start 15 years before symptom onset, the longitudinal effects especially for the amygdala may be underestimated, as they might not be captured by a study duration of only 3 years. Further longitudinal studies over an extended time span should confirm our results. Third, participants were stratified primarily by their clinical manifestations, because CSF biomarkers for AD-pathology were available only for a subset of participants (MCI n = 13, AD n = 18). Therefore, pathological features were considered for stratification when available, but not included in statistical analyses.
4.1. Conclusion
This 7T MRI study found that the assessment of volumes of the amygdala and its subfields, especially the central and medial nucleus, might aid in the identification of individuals with SCD, a potential contribution to early diagnosis and treatment of individuals at risk to develop Alzheimer’s disease. However, an advantage over hippocampus ROIs for the prediction of memory decline was not observed in patients with neither SCD nor MCI, leading to the assumption that amygdala volume might rather be associated with other cognitive or psychiatric dysfunctions. Due to the larger size and the convenient location of the hippocampus, surrounded by cerebrospinal fluid, it is easier to delineate and segment as the amygdala, making it not only a more reliable but also more accessible target for the prediction of memory decline. Therefore, this study does not support assessing amygdala over hippocampus ROIs to detect individuals at risk for memory decline.
Data and code availability statement
Due to data protection regulations and the risk of identification of brain anatomy, individual MRI data are available from the authors for scientific purposes only and under a data sharing agreement. Other data will be available on https://zenodo.org/communities/neuromet after the project end of NeuroMET2. The code for the MRI (pre-)processing pipeline is available in a public repository (Dell'Orco, 2022). The R code for statistics is available under (Göschel, 2023).
CRediT authorship contribution statement
Laura Göschel: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization, Project administration, Funding acquisition. Lea Kurz: Methodology, Formal analysis, Data curation, Writing – original draft. Andrea Dell'Orco: Software. Theresa Köbe: Writing – review & editing, Supervision. Peter Körtvélyessy: Writing – review & editing. Ariane Fillmer: Investigation, Writing – review & editing, Supervision, Funding acquisition. Semiha Aydin: Investigation. Layla Tabea Riemann: Investigation. Hui Wang: Data curation. Bernd Ittermann: Writing – review & editing, Supervision. Ulrike Grittner: Formal analysis, Writing – review & editing, Supervision. Agnes Flöel: Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the EMPIR programme, co-financed by the Participating States and from the European Union's Horizon 2020 research and innovation programme, under grant numbers 15HLT04 NeuroMET and 18HLT09 NeuroMET2, and by the German Research Foundation (327654276-SFB 1315). The authors thank the NeuroMET and NeuroMET2 consortia for their cooperation, Dr. med. Jens Bohlken and Sonja Fabian for their effort in recruiting and classification of participants, Almut Dünnebeil for cognitive assessments and the participants and their families for their time and interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103439.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Basso M., Yang J., Warren L., MacAvoy M.G., Varma P., Bronen R.A., van Dyck C.H. Volumetry of amygdala and hippocampus and memory performance in Alzheimer's disease. Psychiatry Res. 2006;146(3):251–261. doi: 10.1016/j.pscychresns.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Brady D.R., Mufson E.J. Amygdaloid pathology in Alzheimer's disease: Qualitative and quantitative analysis. Dementia. 1990;1:5–17. [Google Scholar]
- de Flores R., La Joie R., Chetelat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience. 2015;309:29–50. doi: 10.1016/j.neuroscience.2015.08.033. [DOI] [PubMed] [Google Scholar]
- Dell'Orco, A., 2022. 0rC0/NeuroMet_MP2rage_pypes: v0.1.1-beta. Zenodo.
- den Heijer T., Geerlings M.I., Hoebeek F.E., Hofman A., Koudstaal P.J., Breteler M.M. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch. Gen. Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Fan L.-Y., Lai Y.-M., Chen T.-F., Hsu Y.-C., Chen P.-Y., Huang K.-Z., Cheng T.-W., Tseng W.-Y., Hua M.-S., Chen Y.-F., Chiu M.-J. Diminution of context association memory structure in subjects with subjective cognitive decline. Hum. Brain Mapp. 2018;39(6):2549–2562. doi: 10.1002/hbm.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinf. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göschel L. Zenodo; 2023. HippieAtrophy/R-Code-Amygdala_2023: V1.1. [Google Scholar]
- Hafkemeijer A., Altmann-Schneider I., Oleksik A.M., van de Wiel L., Middelkoop H.A.M., van Buchem M.A., van der Grond J., Rombouts S.A.R.B. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3(4):353–362. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C., Lendt M., Lux S. Hogrefe; Goettingen: 2001. Verbaler Lern- und Merkfähigkeitstest. [Google Scholar]
- Jessen F., Wolfsgruber S., Wiese B., Bickel H., Mösch E., Kaduszkiewicz H., Pentzek M., Riedel‐Heller S.G., Luck T., Fuchs A., Weyerer S., Werle J., van den Bussche H., Scherer M., Maier W., Wagner M. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2014;10(1):76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., Buckley R.F., van der Flier W.M., Han Y., Molinuevo J.L., Rabin L., Rentz D.M., Rodriguez-Gomez O., Saykin A.J., Sikkes S.A.M., Smart C.M., Wolfsgruber S., Wagner M. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271–278. doi: 10.1016/S1474-4422(19)30368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.-J., Seo S.W., Kim G.H., Kim S.T., Lee J.-M., Qiu A., Na D.L. Less depressive symptoms are associated with smaller hippocampus in subjective memory impairment. Arch. Gerontol. Geriatr. 2013;57(1):110–115. doi: 10.1016/j.archger.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Kovacevic S., Rafii M.S., Brewer J.B., Alzheimer's Disease Neuroimaging I. High-throughput, fully automated volumetry for prediction of MMSE and CDR decline in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2009;23:139–145. doi: 10.1097/WAD.0b013e318192e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr. Biol. 2007;17(20):R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lenth, R.V., 2021. emmeans: Estimated Marginal Means, aka Least-Squares Means.
- Li A., Yue L., Xiao S., Liu M. Cognitive Function Assessment and Prediction for Subjective Cognitive Decline and Mild Cognitive Impairment. Brain Imaging Behav. 2022;16(2):645–658. doi: 10.1007/s11682-021-00545-1. [DOI] [PubMed] [Google Scholar]
- Lombardi G., Crescioli G., Cavedo E., Lucenteforte E., Casazza G., Bellatorre A.G., Lista C., Costantino G., Frisoni G., Virgili G., Filippini G. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2020;3:Cd009628. doi: 10.1002/14651858.CD009628.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques J.P., Kober T., Krueger G., van der Zwaag W., Van de Moortele P.-F., Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271–1281. doi: 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Mitchell A.J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr. Scand. 2014;130(6):439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Wakai M., Takeda A., Sobue G. Medial temporal atrophy and memory impairment in early stage of Alzheimer's disease: an MRI volumetric and memory assessment study. J. Neurol.Sci. 2000;173(1):18–24. doi: 10.1016/s0022-510x(99)00289-0. [DOI] [PubMed] [Google Scholar]
- Mori E., Yoneda Y., Yamashita H., Hirono N., Ikeda M., Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer's disease: an MRI volumetric study. J. Neurol. Neurosurg. Psychiatry. 1997;63(2):214–221. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NeuroMET, 2016. Publishable Report for 15HLT04 NeuroMET; Innovative measurements for improved diagnosis and management of neurodegenerative diseases. Available: https://www.lgcgroup.com/media/2090/15hlt04_final_publishable_report.pdf.
- NeuroMET2, 2019. Publishable Summary for 18HLT09 NeuroMET2; Metrology and innovation for early diagnosis and accurate stratification of patients with neurodegenerative diseases. Available: https://www.lgcgroup.com/our-programmes/empir-neuromet/neuromet2/.
- Nunes T., Fragata I., Ribeiro F., Palma T., Maroco J., Cannas J., Secca M., Menezes C., Carmo I., Cunha G., Branco M.C., Guerreiro M., de Mendonça A., Lovell M.A. The outcome of elderly patients with cognitive complaints but normal neuropsychological tests. J. Alzheimers Dis. 2010;19(1):137–145. doi: 10.3233/JAD-2010-1210. [DOI] [PubMed] [Google Scholar]
- O'Brien K.R., Kober T., Hagmann P., Maeder P., Marques J., Lazeyras F., Krueger G., Roche A., Margulies D. Robust T1-weighted structural brain imaging and morphometry at 7T using MP2RAGE. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotin A., de Flores R., Lamberton F., Poisnel G., La Joie R., de la Sayette V., Mézenge F., Tomadesso C., Landeau B., Desgranges B., Chételat G., Tales A., Jessen F., Butler C., Wilcock G., Phillips J., Bayer T. Hippocampal Subfield Volumetry and 3D Surface Mapping in Subjective Cognitive Decline. J. Alzheimers Dis. 2015;48(s1):S141–S150. doi: 10.3233/JAD-150087. [DOI] [PubMed] [Google Scholar]
- Pini L., Wennberg A.M. Structural imaging outcomes in subjective cognitive decline: Community vs. clinical-based samples. Exp. Gerontol. 2021;145 doi: 10.1016/j.exger.2020.111216. [DOI] [PubMed] [Google Scholar]
- Poulin S.P., Dautoff R., Morris J.C., Barrett L.F., Dickerson B.C. Amygdala atrophy is prominent in early Alzheimer's disease and relates to symptom severity. Psychiatry Res. 2011;194(1):7–13. doi: 10.1016/j.pscychresns.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Fennema-Notestine C., Dale A.M., Miller M.I. Regional shape abnormalities in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2009;45(3):656–661. doi: 10.1016/j.neuroimage.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R-Core-Team, 2020. R: A Language and Environment for Statistical Computing.
- Rhindress K., Ikuta T., Wellington R., Malhotra A.K., Szeszko P.R. Delineation of hippocampal subregions using T1-weighted magnetic resonance images at 3 Tesla. Brain Struct. Funct. 2015;220:3259–3272. doi: 10.1007/s00429-014-0854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogne S., Vangberg T., Eldevik P., Wikran G., Mathiesen E.B., Schirmer H. Magnetic Resonance Volumetry: Prediction of Subjective Memory Complaints and Mild Cognitive Impairment, and Associations with Genetic and Cardiovascular Risk Factors. Dement Geriatr Cogn Dis Extra. 2016;6:529–540. doi: 10.1159/000450885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S.Y., Lim E.Y., Na S., Shim Y.S., Cho J.H., Yoon B., Hong Y.J., Yang D.W. Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int. Psychogeriatr. 2017;29(5):785–792. doi: 10.1017/S1041610216002349. [DOI] [PubMed] [Google Scholar]
- Schultz S.A., Oh J.M., Koscik R.L., Dowling N.M., Gallagher C.L., Carlsson C.M., Bendlin B.B., LaRue A., Hermann B.P., Rowley H.A., Asthana S., Sager M.A., Johnson S.C., Okonkwo O.C. Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers Dement (Amst) 2015;1:33–40. doi: 10.1016/j.dadm.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Liu B., Zhou Y., Yu C., Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Striepens N., Scheef L., Wind A., Popp J., Spottke A., Cooper-Mahkorn D., Suliman H., Wagner M., Schild H.H., Jessen F. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement. Geriatr. Cogn. Disord. 2010;29(1):75–81. doi: 10.1159/000264630. [DOI] [PubMed] [Google Scholar]
- Tang X., Holland D., Dale A.M., Younes L., Miller M.I. Shape abnormalities of subcortical and ventricular structures in mild cognitive impairment and Alzheimer's disease: detecting, quantifying, and predicting. Hum. Brain Mapp. 2014;35(8):3701–3725. doi: 10.1002/hbm.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S., Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011;45:1–67. [Google Scholar]
- van de Rest O., Geleijnse J.M., Kok F.J., van Staveren W.A., Dullemeijer C., OldeRikkert M.G.M., Beekman A.T.F., de Groot C.P.G.M. Effect of fish oil on cognitive performance in older subjects. A randomized, controlled trial. 2008;71(6):430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- Voevodskaya O., Simmons A., Nordenskjöld R., Kullberg J., Ahlström H., Lind L., Wahlund L.O., Larsson E.M., Westman E. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front. Aging Neurosci. 2014;6:264. doi: 10.3389/fnagi.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Huang W., Su L., Xing Y., Jessen F., Sun Y., Shu N., Han Y. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol. Neurodegener. 2020;15:1–27. doi: 10.1186/s13024-020-00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; New York: 1981. Manual for the Wechsler Adult Intelligence Scale, Revised (WAIS- R) [Google Scholar]
- Wei H., Kong M., Zhang C., Guan L., Ba M. The structural MRI markers and cognitive decline in prodromal Alzheimer's disease: a 2-year longitudinal study. Quant. Imaging Med. Surg. 2018;8(10):1004–1019. doi: 10.21037/qims.2018.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., Grolemund G., Hayes A., Henry L., Hester J., Kuhn M., Pedersen T., Miller E., Bache S., Müller K., Ooms J., Robinson D., Seidel D., Spinu V., Takahashi K., Vaughan D., Wilke C., Woo K., Yutani H. Welcome to the tidyverse. Journal of Open Source Software. 2019;4(43):1686. [Google Scholar]
- Yue L., Wang T., Wang J., Li G., Wang J., Li X., Li W., Hu M., Xiao S. Asymmetry of Hippocampus and Amygdala Defect in Subjective Cognitive Decline Among the Community Dwelling Chinese. Front. Psych. 2018;9:226. doi: 10.3389/fpsyt.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to data protection regulations and the risk of identification of brain anatomy, individual MRI data are available from the authors for scientific purposes only and under a data sharing agreement. Other data will be available on https://zenodo.org/communities/neuromet after the project end of NeuroMET2. The code for the MRI (pre-)processing pipeline is available in a public repository (Dell'Orco, 2022). The R code for statistics is available under (Göschel, 2023).