Abstract
Screening for early esophageal adenocarcinoma (EAC), including screening for its precursor Barrett’s esophagus (BE), has the potential to reduce EAC-related mortality and morbidity. This literature review aimed to explore professionals’ views on the justification for EAC screening. A systematic search of Ovid Medline, EMBASE, and PsycInfo, from January 1, 2000 to September 22, 2022, identified 5 original studies and 63 expert opinion articles reporting professionals’ perspectives on EAC screening. Included articles were qualitatively analyzed using the framework method, which was deductively led by modernized screening principles. The analyses showed that many professionals are optimistic about technological advancements in BE detection and treatment. However, views on whether the societal burden of EAC merits screening were contradictory. In addition, knowledge of the long-term benefits and risks of EAC screening is still considered insufficient. There is no consensus on who to screen, how often to screen, which screening test to use, and how to manage non-dysplastic BE. Professionals further point out the need to develop technology that facilitates automated test sample processing and public education strategies that avoid causing disproportionately high cancer worry and social stigma. In conclusion, modernized screening principles are currently insufficiently fulfilled to justify widespread screening for EAC. Results from future clinical screening trials and risk prediction modeling studies may shift professionals’ thoughts regarding justification for EAC screening.
Keywords: Early detection of cancer; Esophageal neoplasms; Barrett esophagus; Health knowledge, attitudes, practice
1. Introduction
Since the 1990s, an increasing incidence of esophageal adenocarcinoma (EAC) has been observed in Western countries. The overall 5-year survival rate remains less than 20% due to detection at an advanced stage (Peters et al., 2019, GBD Oesophageal Cancer Collaborators, 2020). For this reason, it has been suggested to screen high-risk individuals for the presence of Barrett’s esophagus (BE), which is the precursor of EAC (Peters et al., 2019). Those with BE can be followed up to detect incident dysplasia and early EAC (Fitzgerald et al., 2014, Shaheen et al., 2022, Qumseya et al., 2019). Subsequent endoscopic resection of early EAC or eradication of detected dysplasia may ultimately reduce the incidence, morbidity and mortality associated with EAC (Shaheen et al., 2009, Codipilly et al., 2018). High-definition upper endoscopy (EGD) combined with pathological assessment of biopsies is considered the gold standard for identification of BE, dysplasia, and EAC, although this test is considered too invasive and costly to screen large populations.
Recently, alternative screening tests more suitable to screen larger populations are being developed. First, transnasal endoscopy (TNE) is performed with an ultrathin endoscope inserted through the nose and performed without sedation. TNE can accurately diagnose BE, but there is no consensus on whether the small biopsies are sufficient to identify dysplasia (Huibertse et al., 2022). Second, the Cytosponge-TFF3, EsoCheck, and other non-endoscopic cell-collection devices comprise either an encapsulated sponge or a balloon attached to a thread (Ross-Innes et al., 2015, Moinova et al., 2018). Biomarker panels can be applied to on the collected cells to identify BE. Third, an ‘electronic nose’ device can be used to measure exhaled volatile organic compounds, byproducts of (patho)physiologic processes in cells, which has shown promising diagnostic accuracy for detecting BE (Peters et al., 2020).
Screening is more than applying a screening test. Assessment of the justification for screening policies is conventionally based on principles first described by Wilson and Jungner (World Health Organization, 1966). Previous studies that used these principles to evaluate EAC screening (performed in 2002 and 2005) concluded that endoscopic screening should not be endorsed, mainly due to lacking evidence that screening is beneficial and the lack of a well-characterized target population (Dellon and Shaheen, 2005, Shaheen et al., 2002).
Views on the justification for screening may vary over time and change with new evidence. For example, the increasing incidence of EAC may have changed professionals’ views on the burden of the disease – and thus the perceived likelihood that screening could be beneficial. Furthermore, the development of novel screening tests may have changed views on the performance of available test options (Fitzgerald et al., 2020). Table 1 illustrates that screening recommendations in guidelines are also dynamic (Fitzgerald et al., 2014, Shaheen et al., 2022, Playford, 2006, Wang and Sampliner, 2008, Shaheen et al., 2016, Spechler et al., 2011). The British Society of Gastroenterology (BSG) and the American College of Gastroenterology (ACG) have switched from not recommending screening in the 2000s to endorsing screening for a high-risk population in the past decade. The most recent ACG guideline is the first to endorse the application of non-endoscopic cell-collection devices for ‘once-in-a-lifetime’ examination of high-risk individuals (Shaheen et al., 2022). However, international guidelines are not consistent: the 2019 guideline by the American Society for Gastrointestinal Endoscopy (ASGE) and 2011 guideline by the American Gastroenterological Association (AGA) emphasize the lack of randomized clinical trials (RCTs) that support BE screening (Qumseya et al., 2019, Spechler et al., 2011).
Table 1.
Former and current Gastroenterology and Endoscopy Society guidelines for BE screening.
| Society | Year | Screening Recommendation | Screening test | Screening population |
|---|---|---|---|---|
| Former | ||||
| British Society of Gastroenterology (Playford, 2006) | 2006 | Not recommended | Endoscopy | – |
| American College of Gastroenterology (Wang and Sampliner, 2008) | 2008 | Insufficient evidence | Endoscopy | – |
| American College of Gastroenterology (Shaheen et al., 2016) | 2016 | Consider | Endoscopy | Men > 5 y GERD, or with more than weekly symptoms + ≥2 risk factors: >50 y, central obesity, Caucasian, smoking, first-degree relative with BE or EAC |
| Current | ||||
| British Society of Gastroenterology (Fitzgerald et al., 2014) | 2014 | Consider | Endoscopy | GERD with ≥ 3 risk factors: >50 y, Caucasian, male gender, obesity, family history |
| American Gastroenterological Association (Spechler et al., 2011) | 2011 | Insufficient evidence | Endoscopy | – |
| American Society for Gastrointestinal Endoscopy (Qumseya et al., 2019) | 2019 | Insufficient evidence. However, if performed, suggested to target an at-risk population. | Endoscopy | Individuals with a family history of EAC or BE (high risk) OR GERD with ≥ 1 risk factors (moderate risk): >50 y, male gender, Caucasian, smoking, obesity |
| American College of Gastroenterology (Shaheen et al., 2022) | 2022 | Single examination suggested | Endoscopy or non-endoscopic capsule sponge device combined with a biomarker | Chronic GERD with ≥ 3 risk factors: male sex, age > 50 y, White race, tobacco smoking, obesity, a first-degree relative with BE or EAC |
GERD, gastro-esophageal reflux; BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma.
Systematic re-evaluation of the fulfillment of screening principles will help guide complex decisions surrounding the implementation of novel screening tests and identify knowledge gaps. The objectives of this literature review were to systematically explore and analyze health care professionals’ views on the justification for EAC screening and how these views developed over time.
2. Review methods
2.1. Systematic literature search
Typical of a newly developing area, original research exploring professionals’ views on EAC screening is scarce. We, therefore, searched for both original studies and expert opinion articles, such as editorials, letters, and narrative reviews from which the author’s perspective on EAC screening could be extracted. Three electronic databases, Ovid Medline/PubMed, Ovid EMBASE and PsychInfo, were searched for the period January 1, 2000 – September 22, 2022. The search strategies were database-specific and developed in consultation with an experienced medical information specialist. We included a combination of subject headings and free-text terms for “Barrett’s esophagus”, “esophageal neoplasm or adenocarcinoma”, “mass screening”, “early detection of cancer”, “physician practice patterns”, “facilitator”, “barrier”, “implication”, “ethics”, “legislation and jurisprudence”, “cultural factor”, “social factor” and “qualitative research” in the title and abstract (Supplementary Table 1). The reference lists of all included studies were reviewed. Two researchers (JS and MG) independently screened each identified article for eligibility. An article was excluded if: 1) it was a systematic review or cost-effectiveness analysis, 2) it only reported perceptions of the public, or 3) it only addressed issues important for developing countries.
2.2. Definitions
EAC screening was defined as offering screen-eligible individuals a test aimed at detection of BE and/or BE-related neoplasia, with the intention to follow-up identified BE patients and treat dysplasia and early cancer once diagnosed. Screen-eligibility was deliberately not further specified to guarantee an encompassing overview.
The term screening program is inconsistently used. In this review, we adhere to the European Council’s definitions of screening program status (Ponti et al., 2017). EAC screening should currently be classified as non-program screening, defined as any examination for early detection of cancer performed in a clinical context. The screening policy requires some degree of public responsibility, organization, and supervision to receive a program status. It would therefore need to be publicly documented and funded, for example by the National Health Service (in the UK).
2.3. Data analysis
Data from original studies were summarized in an evidence table. We utilized the framework method to qualitatively analyze expert opinion articles, which entails a systematic method of selecting and organizing text based on key themes (Ritchie et al., 2013). The framework analysis was deductively led by a modernized version of Wilson and Junger’s principles that were previously identified through a systematic literature review and a Delphi consensus process (Dobrow et al., 2018). The following key themes are incorporated in the modernized set of screening principles: 1) epidemiology of the condition, 2) natural history of the condition, 3) target population, 4) screening test performance, 5) interpretation of test results, 6) post-screening test options, 7) infrastructure, 8) coordination and integration, 9) acceptability and ethics, 10) benefits and harms, 11) economic evaluation, and 12) quality. With these themes in mind, we extracted relevant text fragments from expert opinion articles. In parallel, the extracted text was inductively and iteratively coded to capture concepts relevant for EAC screening across included articles. The codes and accompanying text fragments, authors, and publication years were subsequently mapped back on the twelve screening principles to create the framework matrix (accessible via https://doi.org/10.17026/dans-x67-9bzx). Two independent researchers (JS and MG) performed each step, with discrepancies resolved through discussion. We managed qualitative data using ATLAS.ti version 8.4.20.
To illustrate if professionals’ views on EAC screening varied over time, we mapped their screening recommendations by calendar year. We used a two-stage process including extraction of the text fragment containing a recommendation from each article (independently, by JS and MG), followed by a classification of the recommendation in five predefined categories (independently and blinded to the author and publication year, by YP and PS). The following categories were applied: ++, recommending; +, motivation; +/−, neutral position; −, serious doubt; −/−, recommending against. We used SPSS (version 25; IBM Corporation, Armonk, NY) to calculate Cohen’s kappa values to assess inter-rater agreement.
2.4. Quality appraisal
The data relevant to this review were expected to be mainly drawn from narrative texts that reflected the author’s perspective on EAC screening. Thus, traditional quality assessment of included studies was not considered appropriate.
3. Results
3.1. Included articles
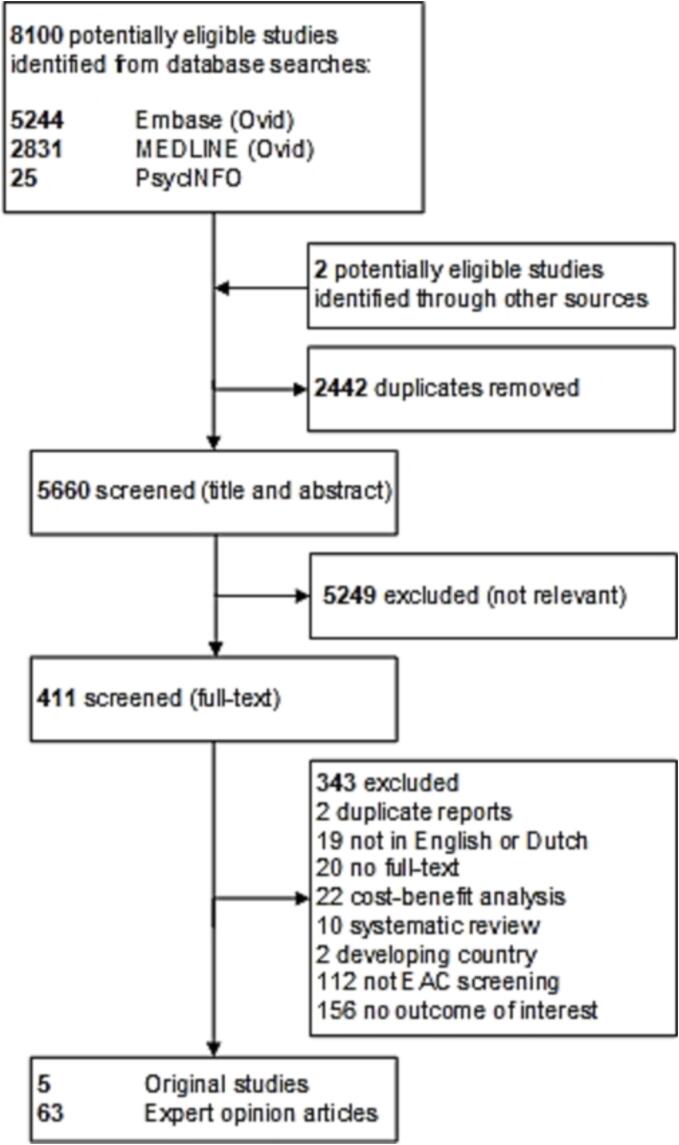
Of the 8100 references identified in the literature search, 411 were included after title and abstract assessment (by JS and MG, moderate inter-rater agreement κ = 0.51; SE = 0.026) (Fig. 1). After full-text assessment, 68 articles were selected (by JS and MG, substantial inter-rater agreement κ = 0.73; SE = 0.043); including 5 (7%) original studies and 63 (93%) expert opinion articles (Supplementary Table 2). All original studies were performed in the US (Table 2). Corresponding authors of expert opinion articles were from the US (68%), the UK (17%), other European countries (13%), and Australia (2%).
Fig. 1.
Study selection.
Table 2.
Original studies reporting professionals’ views on BE/EAC screening.
| Reference | Country | Study design | Test | Professional types and sample size | Key findings relevant to this review |
|---|---|---|---|---|---|
| Boolchand et al., 2006 | US | Cross-sectional survey | EGD | PCPs (n = 271) Internists (n = 215)Other (n = 56) |
|
| Chey et al., 2005 | US | Cross-sectional survey | EGD | PCPs (n = 1046) |
|
| Kolb et al., 2022 | US | Cross-sectional survey | EGD | GIs (n = 120) PCPs (n = 195) |
|
| Lin et al., 2002 | US | Cross-sectional survey | EGD | GIs (n = 162) |
|
| Rubenstein et al., 2008 | US | Cross-sectional survey | EGD | GIs (n = 224) |
|
Conventional upper endoscopy, EGD; PCP, primary care provider; GI, gastroenterologist; GERD, gastro-esophageal reflux; BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; IQR, interquartile range; CRC, colorectal cancer; OR, odds ratio.
3.2. Screening approach
A variety of potential EAC screening approaches were described in included articles:
3.2.1. Targeted screening | clinician’s judgement vs. systematic approach
Targeted screening is only offered to individuals at increased risk for EAC, such as individuals with gastro-esophageal reflux disease (GERD) or other risk factors for BE/EAC. Several articles suggest that such high-risk individuals could undergo a screening test that is either self-initiated or initiated during a regular office visit, thus depending on individual clinician’s judgment of eligibility (Boolchand et al., 2006, Chey et al., 2005, Kolb et al., 2022, Lin et al., 2002, Rubenstein et al., 2008, Adams et al., 2014, Atkinson and Chak, 2010, Craanen and Kuipers, 2001, Crockett et al., 2010, Cross, 2011, Falk, 2019, Gerson, 2011). Some articles describe a scenario in which a health care organization systematically invites a group of at-risk individuals to undergo screening (di Pietro et al., 2018, Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018, Vaughan and Fitzgerald, 2015, Yusuf and Fitzgerald, 2021).
3.2.2. Population screening | combining with colorectal cancer screening
Population screening is offered to everyone in a predefined age group, regardless of other risk factors (Patel and Gyawali, 2019, Bretthauer and Kalager, 2013, Graham et al., 2016, Malagelada, 2011). A minority of professionals have suggested integrating EAC screening into established colorectal cancer population screening programs (Graham and Tan, 2020, Desai et al., 2021). One article suggested to combine this strategy with ablation of all Barrett’s mucosa along with tailored acid-suppressive–antireflux therapy to prevent recurrence (Graham and Tan, 2020).
3.3. Findings from original studies
Characteristics and findings from original studies are summarized in Table 2. All included original studies were surveys conducted in the US, addressed EAC screening with conventional upper endoscopy, and included clinicians (primary care providers [PCPs], gastroenterologists, or internists). In these surveys, 16%–98% of clinicians screened patients with GERD (Boolchand et al., 2006, Chey et al., 2005, Lin et al., 2002, Rubenstein et al., 2008), 71%–72% believed that screening for BE is effective for early detection of EAC, and 38%–56% believed that EAC screening is cost-effective (Kolb et al., 2022, Lin et al., 2002). Two surveys reported that clinicians’ belief in EAC screening benefits is low compared with colorectal cancer screening (Kolb et al., 2022, Rubenstein et al., 2008). Reported drivers for EAC screening included: ‘patient has risk factors’, ‘patient request’, ‘prior malpractice suits’, and ‘procedure reimbursement’ (Boolchand et al., 2006, Chey et al., 2005, Kolb et al., 2022, Lin et al., 2002, Rubenstein et al., 2008). Reported barriers included: ‘poor patient acceptance’, ‘insufficient evidence’, ‘risk of complications’, ‘difficulty identifying at-risk patients’, ‘lack of knowledge of guidelines’, ‘ineffective treatment’, ‘not my responsibility’, ‘competing concerns’, ‘insufficient clinic time’, ‘unsure about insurance coverage’, and ‘patients don’t understand’ (Boolchand et al., 2006, Kolb et al., 2022).
3.4. Qualitative analysis of expert opinion pieces
A summary of the framework analysis of perceived fulfilment of screening principles is provided below and in Table 3 (fulfillment of disease/condition and program/system principles) and Table 4 (fulfillment of test/intervention principles, stratified by screening test).
Table 3.
Perceived fulfilment of screening principles within the disease/condition and program/system domains (Dobrow et al., 2018).
| Domain | Screening principle | 2000–2010 | 2010–2022 | What is needed |
|---|---|---|---|---|
| Disease/condition principles | 1. Epidemiology of the disease or condition | +/− | +/− |
|
| 2. Natural history of disease or condition and detectable preclinical stage | – | +/− |
|
|
| 3. Target population for screening | – | +/− |
|
|
| Program/system principles | 7. Screening infrastructure* | – | +/− |
|
| 8. Screening coordination and integration | – | – |
|
|
| 9. Screening acceptability and ethics | +/− | +/− |
|
|
| 10. Screening benefits and harms | – | – |
|
|
| 11. Economic evaluation | +/− | + |
|
|
| 12. Quality and performance management | – | – |
|
BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; RCT, randomized controlled trial.
* The test/intervention principles (4, 5 and 6) are shown in Table 4.
Table 4.
Perceived fulfilment of screening principles within the test/intervention domains (Dobrow et al., 2018), stratified by screening tests.
|
Conventional upper endoscopy |
Transnasal endoscopy |
Non-endoscopic cell-collection devices |
Analysis of circulating and exhaled biomarkers |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Screening principle | Currently known limitations | Currently known limitations | Currently known limitations | Currently known limitations | |||||
| Test/intervention principles | 4. Screening test performance characteristics | +/− |
|
+/− |
|
+/− |
|
+ |
|
|
| 5. Interpretation of screening test results | + |
|
+ |
|
+ |
|
+/− |
|
||
| 6. Post screening test options | Diagnostic follow-up | − |
|
− |
|
− |
|
− |
|
|
| Treatment | ++ |
|
++ |
|
++ |
|
++ |
|
3.4.1. Principle one | epidemiology of the disease or condition
Most professionals view EAC as an important public health problem due to its exponentially rising incidence (range: 300%–700% increase in recent decades) (Blevins and Iyer, 2017, Crockett et al., 2010, di Pietro et al., 2015, Eisen et al., 2004, Falk, 2019, Fitzgerald, 2005, Gopal et al., 2004, Kolb and Wani, 2021, Lieberman and Sampliner, 2001, Malagelada, 2011, O’Donovan and Fitzgerald, 2018, Otaki and Iyer, 2018, Sami and Iyer, 2018, Sharma and Sidorenko, 2005, Spechler, 2004), late stage disease presentation (>50% presenting with stage II disease or higher) (Cook and Thrift, 2021, Crockett et al., 2010, Desai et al., 2021, di Pietro and Fitzgerald, 2013, Gopal et al., 2004, Katzka and Fitzgerald, 2020, Kolb and Wani, 2021, Lieberman and Sampliner, 2001, O’Donovan and Fitzgerald, 2018, Vaughan and Fitzgerald, 2015), and poor outcome (range: 5-year survival rate of 15–20%) (Blevins and Iyer, 2017, Cook and Thrift, 2021, di Pietro et al., 2015, Enslin and Kaul, 2020, Falk, 2019, Kolb and Wani, 2021, O’Donovan and Fitzgerald, 2018, Otaki and Iyer, 2018). However, EAC is also seen as a minor public health problem compared to other cancer types due to its relatively low absolute population risk (Blevins and Iyer, 2017, Bretthauer and Kalager, 2013, Dellon and Shaheen, 2005, Eisen et al., 2004, Fitzgerald, 2005, Kamboj et al., 2021, Lao-Sirieix and Fitzgerald, 2012, Malagelada, 2011, O’Donovan and Fitzgerald, 2018, Spechler, 2004, Spechler et al., 2018).
Some professionals see BE as an important health problem because of the increased relative risk of developing EAC (range: 30- to 125-fold greater lifetime risk) (Dellon and Shaheen, 2005, Amadi and Gatenby, 2017, Gopal et al., 2004). Others argue against this because the absolute risk for developing EAC in BE is small (range: annual risk of 0.12%–0.5%), and BE patients are more likely to die from other causes than EAC (Eisen et al., 2004, Esserman et al., 2014, Frei et al., 2021, Kamboj et al., 2021, Knox, 2011, Kuipers, 2011, Otaki and Iyer, 2018, Patel and Gyawali, 2019, Sami and Iyer, 2018, Shaheen and Palmer, 2009, Spechler, 2004). Thus, judgment on the importance of BE and EAC is equivocal.
3.4.2. Principle two | natural history of disease or condition
Most professionals adhere to the following pathological sequence theory: intestinal metaplasia (IM) typical for BE developing under the influence of GERD, then developing into low-grade dysplasia (LGD) or high-grade dysplasia (HGD) in BE, and finally developing into early EAC or even advanced EAC (Dellon and Shaheen, 2005, di Pietro and Fitzgerald, 2013, Gopal et al., 2004, Kamboj et al., 2021, Patel and Gyawali, 2019, Wani and Sharma, 2006, Malagelada, 2011, Desai et al., 2021, Fitzgerald, 2005). Based on this, they see BE as a detectable preclinical phase of EAC (Amadi and Gatenby, 2017, Blevins and Iyer, 2017, Crockett et al., 2010, Dellon and Shaheen, 2005, Graham et al., 2016, Kuipers, 2011, Lao-Sirieix and Fitzgerald, 2012, Tan and di Pietro, 2022). However, some professionals perceive the pathological sequence theory as a reiterated paradigm unsupported by direct evidence (Gopal et al., 2004, Lieberman and Sampliner, 2001, Cook and Thrift, 2021). Contradicting this theory is the lack of concomitant detectable IM in surgical/biopsy specimens in a proportion of cases with EAC (range: 0%–77%) (Cook and Thrift, 2021, Dellon and Shaheen, 2005, di Pietro et al., 2015, Katzka and Fitzgerald, 2020, Vaughan and Fitzgerald, 2015). Some researchers suggest the possibility of two EAC phenotypes, one arising in a background of IM and another phenotype without the necessity of IM, (Kamboj et al., 2021, Katzka and Fitzgerald, 2020, Cook and Thrift, 2021) or that pre-existent IM is overgrown by EAC (di Pietro et al., 2015, Katzka and Fitzgerald, 2020, O’Donovan and Fitzgerald, 2018). The uncertainty about distinct EAC phenotypes limits screening and surveillance strategies that depend on BE detection (Katzka and Fitzgerald, 2020). Professionals further note that the natural history of LGD and indefinite dysplasia (IND) is insufficiently understood due to variation in reported rates for progression to HGD or EAC, or even regression to IM (Amadi and Gatenby, 2017, Eisen et al., 2004, Lieberman and Sampliner, 2001, Patel and Gyawali, 2019).
3.4.3. Principle three | target population for screening
There is no consensus on the target population for EAC screening (Amadi and Gatenby, 2017, Frei et al., 2021, Tan et al., 2021, Crockett et al., 2010, Blevins and Iyer, 2017, Cook and Thrift, 2021). Professionals state that targeting GERD patients alone results in significant miss rates, because a large proportion of EAC patients (range: 40%–57%) have no history of GERD symptoms before diagnosis (Dellon and Shaheen, 2005, Shaheen et al., 2002, Amadi and Gatenby, 2017, di Pietro et al., 2015, Eisen et al., 2004, Lieberman and Sampliner, 2001, Patel and Gyawali, 2019, Rubenstein and Thrift, 2015, Atkinson and Chak, 2010, Crockett et al., 2010, de Jonge et al., 2014, Sharma and Sidorenko, 2005, Graham and Tan, 2020, Desai et al., 2021, Spechler, 2004, Shaheen and Palmer, 2009, Knox, 2011, Katzka and Fitzgerald, 2020, Kolb and Wani, 2021, Spechler et al., 2018, Tan et al., 2021, Tan and di Pietro, 2022). They think that failure to report GERD may result from experiencing atypical symptoms (e.g., cough) (Gopal et al., 2004, Patel and Gyawali, 2019, Sharma and Smith, 2020), the esophageal hyposensitivity associated with BE despite high reflux burden (Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018, Patel and Gyawali, 2019, Rubenstein and Thrift, 2015), or not seeking help (and using over-the-counter medication) (di Pietro et al., 2015, Kamboj et al., 2021, di Pietro et al., 2018). Risk-assessment tools combining the presence of GERD with additional risk factors are seen as potentially valuable instruments to identify the at-risk population more precisely (Blevins and Iyer, 2017, Cook and Thrift, 2021, Falk, 2019, Fitzgerald, 2005, Frei et al., 2021, Graham and Tan, 2020, Kamboj et al., 2021, Kolb and Wani, 2021, O’Donovan and Fitzgerald, 2018, Tan et al., 2021, Vaughan and Fitzgerald, 2015, Yusuf and Fitzgerald, 2021). Professionals note the need to determine the target age range for screening (de Jonge et al., 2014), and, in the case of implementing a risk-assessment tool, to determine the risk threshold (Yusuf and Fitzgerald, 2021, Blevins and Iyer, 2017), evaluate acceptance among physicians and individuals (Tan and di Pietro, 2022), and incorporate family history, comorbidity, and life expectancy (Kamboj et al., 2021, Otaki and Iyer, 2018, Tan and di Pietro, 2022, Blevins and Iyer, 2017, Enslin and Kaul, 2020). There is no consensus on the benefits of screening females and non-Caucasian ethnicities (Otaki and Iyer, 2018, Rajendra, 2015, Tan et al., 2021, Tan and di Pietro, 2022).
Published ideas for retrieving personal risk information include: 1) targeting acid suppression therapy users through electronic patient files in primary care (O’Donovan and Fitzgerald, 2018), 2) applying a risk calculator on these files (Vaughan and Fitzgerald, 2015), or 3) applying web-based self-assessment risk calculators in the general population (Yusuf and Fitzgerald, 2021).
3.4.4. Principle four | screening test performance characteristics
Conventional EGD is not considered an option for widespread screening due to its high cost, risk of adverse events, and use limited to secondary care (Atkinson and Chak, 2010, Crockett et al., 2010, Cross, 2011, Dellon and Shaheen, 2005, Kamboj et al., 2021, Kuipers, 2011, Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018, Sami and Iyer, 2018, Spechler, 2004, Yusuf and Fitzgerald, 2021).
The fact that TNE can be performed outside the hospital, which allows higher throughput compared with sedated endoscopy, is viewed as an advantage (Craanen and Kuipers, 2001, Crockett et al., 2010, O’Donovan and Fitzgerald, 2018, Patel and Gyawali, 2019, Spechler et al., 2018, Tan and di Pietro, 2022). However, uptake by physicians and the public is low, likely due to the requirement of professional expertise and expensive equipment (less applicable in primary care), small biopsy size, and incongruent patient tolerability and preference (di Pietro et al., 2015, di Pietro et al., 2018, Frei et al., 2021, Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018, Sami and Iyer, 2018, Tan et al., 2021, Tan and di Pietro, 2022).
Video capsule endoscopy is not viewed as a suitable technique for screening. This is due to the time-intensive need for image interpretation, reduced image quality, risk of insufficient esophageal imaging or capsule retention, and inability to take biopsies (di Pietro et al., 2015, Michalak et al., 2009, Sami and Iyer, 2018, Spechler et al., 2018, Lao-Sirieix and Fitzgerald, 2012, Yusuf and Fitzgerald, 2021).
Non-endoscopic cell-collection devices, such as the Cytosponge-TFF3 or EsoCheck, are considered as safe, simple, tolerable and affordable alternatives to endoscopy that may be suitable for primary care and automated reading (di Pietro et al., 2015, Frei et al., 2021, Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018, Spechler et al., 2018, Tan and di Pietro, 2022, Yusuf and Fitzgerald, 2021). Some professionals see these tests as a potential solution for sampling bias in endoscopy (Amadi and Gatenby, 2017). Professionals indicated that there is room for improvement in test uptake and in the addition of risk stratification biomarkers (di Pietro et al., 2015, Frei et al., 2021, Kolb and Wani, 2021, Tan et al., 2021, Lao-Sirieix and Fitzgerald, 2012, Yusuf and Fitzgerald, 2021). Potential barriers are the need for confirmatory endoscopy, poor DNA yield in some cases, limited test sensitivity, and some individuals’ inability to swallow these devices (di Pietro et al., 2015, Lao-Sirieix and Fitzgerald, 2012, Sami and Iyer, 2018, Sharma and Smith, 2020, Yusuf and Fitzgerald, 2021).
Analysis of circulating and exhaled biomarkers is seen as ideal for screening in terms of safety, tolerability, cost, and applicability in primary care (Amadi and Gatenby, 2017, Konda and Souza, 2019, Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018, Sami and Iyer, 2018, Tan et al., 2021, Yusuf and Fitzgerald, 2021). However, professionals note that external validation and reproducibility of conducted studies is challenging (Frei et al., 2021, Tan et al., 2021, Tan and di Pietro, 2022), and they view the need for confirmatory endoscopy as disadvantageous (O’Donovan and Fitzgerald, 2018, Spechler et al., 2018, Yusuf and Fitzgerald, 2021).
3.4.5. Principle five | interpretation of screening test results
In the case of non-endoscopic cell-collection devices, it is noted that decreasing the number of equivocal test results and defining a follow-up strategy for patients with low-confidence results is needed to achieve this principle (Kolb and Wani, 2021).
Regarding conventional EGD, professionals are concerned that interobserver variability (endoscopic and pathologic) may result in widespread false-positive BE diagnosis. Perceived causes include accidental biopsy of IM at the gastro-esophageal junction (Dellon and Shaheen, 2005, Kolb and Wani, 2021, Crockett et al., 2010, Falk, 2019, Gerson, 2011, de Jonge et al., 2014, Graham et al., 2016), or reluctance to accept the 1 cm threshold for BE diagnosis (Kamboj et al., 2021, Falk, 2019). Additionally, histopathological interobserver variability may affect LGD diagnosis and the distinction between HGD and early EAC, especially when erosive esophagitis is present (Dellon and Shaheen, 2005, Patel and Gyawali, 2019, Wani and Sharma, 2006, Craanen and Kuipers, 2001, Crockett et al., 2010, Sharma and Sidorenko, 2005, Malagelada, 2011, Spechler, 2004, Blevins and Iyer, 2017, Shaheen and Palmer, 2009). Misclassification of BE and dysplasia may lead to oversurveillance and overtreatment (Crockett et al., 2010). Assessment by a specialized gastrointestinal pathologist is perceived as helpful in preventing misclassification (Patel and Gyawali, 2019, Falk, 2019).
3.4.6. Principle six | post screening test options
There is no consensus on whether once-only or periodic screening has the best benefit-harm ratio. Professionals reason that BE developing after the age of 50 years is unlikely to progress to EAC within the remainder of the patient’s life (Gopal et al., 2004, Falk, 2019). They therefore suggest that, if screening aims to detect BE, once-only screening may be appropriate. However, it would be optimal if this initial test would, next to proving the presence or absence of BE, also allow for stratification in low- and high-risk for progression groups (Frei et al., 2021). Screening aimed at detecting dysplasia and early EAC may replace endoscopic surveillance and should be offered periodically (Kolb and Wani, 2021).
Regarding endoscopic follow-up, the effectiveness of current surveillance programs is considered questionable (di Pietro et al., 2015, Eisen et al., 2004, Frei et al., 2021, Malagelada, 2011, O’Donovan and Fitzgerald, 2018, Sharma and Sidorenko, 2005, Vaughan and Fitzgerald, 2015). Professionals highlight the lack of RCTs reporting EAC-specific mortality reduction following BE surveillance (di Pietro and Fitzgerald, 2013, Gopal et al., 2004, Otaki and Iyer, 2018, Atkinson and Chak, 2010, Knox, 2011, Bretthauer et al., 2016), and the many biases in observational surveillance studies (di Pietro and Fitzgerald, 2013, Shaheen et al., 2002, Spechler, 2004, Spechler et al., 2018).
Regarding treatment options, professionals agree that evidence sufficiently supports the effectiveness of endoscopic therapy for treatment of early-stage EAC (stage T1a) and prevention of progression of dysplastic BE (Blevins and Iyer, 2017, di Pietro et al., 2015, di Pietro et al., 2018, di Pietro and Fitzgerald, 2013, Kamboj et al., 2021, Kolb and Wani, 2021, Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018, Otaki and Iyer, 2018, Patel and Gyawali, 2019, Sami and Iyer, 2018, Tan and di Pietro, 2022, Vaughan and Fitzgerald, 2015). This is in contrast to the situation before 2010, when esophagectomy was the only treatment option for both HGD and EAC (Lieberman and Sampliner, 2001, Shaheen and Palmer, 2009, Knox, 2011).
3.4.7. Principle seven | infrastructure
Professionals’ main concern is that systematically inviting individuals for EAC screening would drain medical resources, i.e., trained physicians/assistants for triage and performing the screening test, equipment, and, depending on the test used, pathology services (Cook and Thrift, 2021, Craanen and Kuipers, 2001, Crockett et al., 2010, de Jonge et al., 2014, Eisen et al., 2004, Gopal et al., 2004, Katzka and Fitzgerald, 2020, Lao-Sirieix and Fitzgerald, 2012, Vaughan and Fitzgerald, 2015). Additionally, downstream confirmatory endoscopy, surveillance, and treatment services would require experienced and advanced endoscopists, expert pathologists, anesthetists, endoscopy and recovery rooms, nurses, planners, and equipment (Cook and Thrift, 2021, Craanen and Kuipers, 2001, Crockett et al., 2010, de Jonge et al., 2014, Eisen et al., 2004, Gopal et al., 2004, Katzka and Fitzgerald, 2020, Lao-Sirieix and Fitzgerald, 2012, Vaughan and Fitzgerald, 2015). Professionals suggest that technological developments might facilitate initial screening test logistics (e.g., sample-tracking), remote pathologist reporting (e.g., slide-scanner technology and machine learning assistance), and communication (e.g., electronic reporting systems that include recommendations for management) (Frei et al., 2021, O’Donovan and Fitzgerald, 2018, Tan and di Pietro, 2022). They note that these reporting systems must ensure patient confidentiality and data protection (O’Donovan and Fitzgerald, 2018). Depending on the screening test, mass transportation and storage of specimens may also be needed (O’Donovan and Fitzgerald, 2018).
3.4.8. Principle eight | coordination and integration
According to some professionals, a formal system is needed to inform, invite, counsel, test, and manage the treatment of screening participants (Bretthauer et al., 2016, Ilbawi and Anderson, 2015). Although several countries already have screening organizations in place, it is unclear whether these organizations should be responsible for potential implementation and monitoring of EAC screening as this depends on the target population and country-specific legislation (Kolb and Wani, 2021). Furthermore, some authors of included articles assumed that PCPs would take ownership of selecting, counseling and, in the case of non-endoscopic cell-collection devices, testing participants (Cross, 2011, Kamboj et al., 2021, Smith et al., 2015). However, it is not evident that all PCPs will support EAC screening (Boolchand et al., 2006).
3.4.9. Principle nine | ethics and risk communication
Professionals have identified several ethical concerns, including potential insurance discrimination following BE diagnosis (particularly in the US) (Crockett et al., 2010, de Jonge et al., 2014, Dellon and Shaheen, 2005, Falk, 2019, Gerson, 2011, Kuipers, 2011, Shaheen and Palmer, 2009, Sharma and Sidorenko, 2005), and false reassurance for individuals due to false-negative test results (Craanen and Kuipers, 2001, Lao-Sirieix and Fitzgerald, 2012). Other identified ethical implications concern the attitude of health care providers. The idea that screening can be harmful is counterintuitive, which may be dangerous if screening is causing more harm than good (Bretthauer and Kalager, 2013, Esserman et al., 2014). Providers may also be driven to perform screening for reasons other than belief in effectiveness, such as a fee-for-service model, fear of liability claims, the expectation that risk communication requires more time than esophageal examination, patient request, fear of missing cancer, or frustration about the inability to reduce EAC mortality (Adams et al., 2014, Crockett et al., 2010, Esserman et al., 2014, Shaheen, 2011, Zakko et al., 2017). Furthermore, inviting individuals based on sex may be seen as sexist (Shaheen, 2011).
Moreover, professionals report that education about EAC risk may lead to cancer worry (Eisen et al., 2004, de Jonge et al., 2014, Lao-Sirieix and Fitzgerald, 2012, Sharma and Sidorenko, 2005, Graham et al., 2016, Esserman et al., 2014). This may increase if individuals undertake an online search, which is likely to suggest an unreasonable high cancer risk (Crockett et al., 2010, de Jonge et al., 2014, Gerson, 2011, Kuipers, 2011). It is therefore recommend to train physicians in careful risk communication at all stages of the screening process (Graham et al., 2016, Cook and Thrift, 2021, Mehta and Asch, 2014). Professionals further suggest reframing non-dysplastic BE as an alteration of tissue, and framing LGD and HGD in BE as precancerous (Esserman et al., 2014, Mehta and Asch, 2014).
3.4.10. Principle ten | benefits and harms
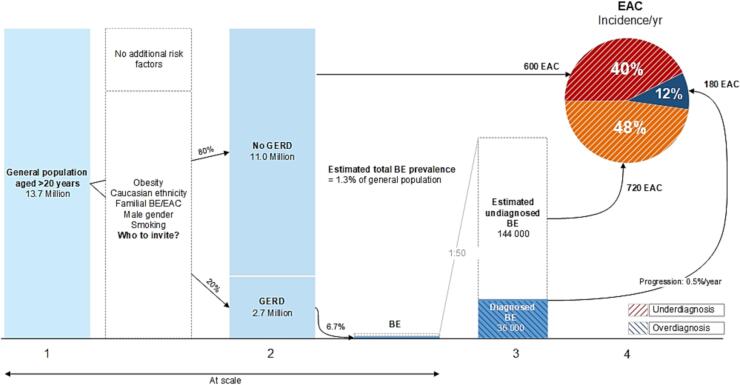
According to professionals, this principle is not fulfilled due to the lack of RCTs on benefits and harms of EAC screening (Bretthauer and Kalager, 2013, Craanen and Kuipers, 2001, Crockett et al., 2010, Eisen et al., 2004, Gerson, 2011, Kolb and Wani, 2021, Kuipers, 2011, Malagelada, 2011, Michalak et al., 2009, Otaki and Iyer, 2018, Rajendra, 2015, Reid, 2017, Sami and Iyer, 2018, Shaheen et al., 2009, Sharma and Sidorenko, 2005, Wani and Sharma, 2006, Zakko et al., 2017). Perceived potential benefits of screening include the increased opportunity for early diagnosis and curative treatment (di Pietro et al., 2015, Sami and Iyer, 2018, Spechler, 2004, Shaheen and Palmer, 2009), the theoretical ability to decrease EAC-related mortality (di Pietro et al., 2015, Falk, 2002, Gopal et al., 2004, Lao-Sirieix and Fitzgerald, 2012, Michalak et al., 2009, O’Donovan and Fitzgerald, 2018, Patel and Gyawali, 2019, Sami and Iyer, 2018, Spechler, 2004), reassurance for participants following a negative test result (Spechler, 2004), and the potential quality-of-life (QoL) benefit following early treatment compared with esophagectomy for advanced EAC (Otaki and Iyer, 2018). Reported potential harms of EAC screening include iatrogenic injury (Crockett et al., 2010, Cross, 2011, de Jonge et al., 2014, Dellon and Shaheen, 2005, Eisen et al., 2004, Enslin and Kaul, 2020, Kamboj et al., 2021, Knox, 2011, Kuipers, 2011, Malagelada, 2011, O’Donovan and Fitzgerald, 2018, Sami and Iyer, 2018, Spechler, 2004, Spechler et al., 2018), psychological distress (Kolb and Wani, 2021, Craanen and Kuipers, 2001, Lao-Sirieix and Fitzgerald, 2012, Sharma and Sidorenko, 2005, Bretthauer and Kalager, 2013, Gerson and Triadafilopoulos, 2002), false-positive or -negative test results (Kolb and Wani, 2021, Lieberman and Sampliner, 2001, Craanen and Kuipers, 2001, Lao-Sirieix and Fitzgerald, 2012, Sharma and Sidorenko, 2005, Bretthauer and Kalager, 2013), and decreased QoL due to a BE diagnosis (Bretthauer and Kalager, 2013, Crockett et al., 2010, de Jonge et al., 2014, Eisen et al., 2004, Kuipers, 2011). Professionals are concerned that overdiagnosis, detecting conditions that may never cause symptoms during a lifetime, will expose the target population to additional harm (Bretthauer et al., 2016, Bretthauer and Kalager, 2013, Craanen and Kuipers, 2001, Ilbawi and Anderson, 2015, Lambert, 2012, Reid, 2017, Sharma and Sidorenko, 2005, Vaughan and Fitzgerald, 2015). There is also concern that underdiagnosis, missing cancers that then present at advanced stages in the clinic, may diminish screening benefit (Fig. 2) (Reid, 2017, Sami and Iyer, 2018).
Fig. 2.
Under- and overdiagnosis in the current esophageal adenocarcinoma screening and surveillance paradigm. Legend: 1. Dutch population in 2021. 2. Based on systematic literature review, GERD symptoms are prevalent in 20% of the general population (El-Serag et al., 2014). 3. Calculated from estimated EAC cases (see number 4) and a BE to EAC progression rate of 0.5%/year (Peters et al., 2019). Prior BE diagnosis: 36 000 × 0.5%/year = 180 EAC/year. Estimated undiagnosed BE: 144 000 × 0.5%/year = 720 EAC/year. The calculated total BE prevalence of 180 000 (1.3% of the general population; 6.7% of the population with GERD) is in line with a meta-analysis reporting global BE prevalence of 0.96% and prevalence of BE in GERD of 6.7% (Marques de Sa et al., 2020). 4. There were 2500 esophageal cancer cases in 2021 in the Netherlands, of which approximately 1500 (61%) were adenocarcinomas (Vegt et al., 2022). Based on a meta-analysis, an estimated 180 cases (12%) had prior BE diagnosis (Tan et al., 2020). Approximately 600 (40%) had no prior BE diagnosis and no history of GERD symptoms (Chak et al., 2006). The remaining 720 cases (48%) had history of GERD symptoms without prior BE diagnosis.
3.4.11. Principle eleven | economic evaluation
EAC screening is expected to require significant financial resources (Bretthauer et al., 2016, Cook, 2013, Craanen and Kuipers, 2001, di Pietro and Fitzgerald, 2013, Falk, 2019, Frei et al., 2021, Gopal et al., 2004, Kamboj et al., 2021, Katzka and Fitzgerald, 2020, Knox, 2011, Kuipers, 2011, Lao-Sirieix and Fitzgerald, 2012, Otaki and Iyer, 2018, Patel and Gyawali, 2019, Sami and Iyer, 2018, Sharma and Sidorenko, 2005, Sharma and Smith, 2020, Spechler, 2004). On the other hand, costs associated with the care of a patient with advanced esophageal cancer, such as surgery, chemotherapy, radiotherapy, hospitalizations, and cancer nursing, may be avoided (Gopal et al., 2004, Sharma and Sidorenko, 2005, Sharma and Smith, 2020). Professionals further warn for unrealistic assumptions in cost-effectiveness studies that address endoscopic screening (which show incremental cost-effectiveness ratios ranging from $10,000 to $24,000 per quality-adjusted life year), such as overestimated cancer progression rates, optimistic assumptions of participation rates and performance of screening tests, and neglecting downstream surveillance and treatment costs (Atkinson and Chak, 2010, Dellon and Shaheen, 2005, di Pietro et al., 2015, Falk, 2002, Otaki and Iyer, 2018, Spechler, 2004).
3.4.12. Principle twelve | quality and performance management
The expected increase in the quality of BE management following the introduction of a potential screening program is viewed as a beneficial side-effect (Bretthauer and Kalager, 2013, Ilbawi and Anderson, 2015). Professionals further highlight the need for a system that monitors program quality and mortality if screening is implemented nationally (Bretthauer et al., 2016, Bretthauer and Kalager, 2013, Ilbawi and Anderson, 2015, Lao-Sirieix and Fitzgerald, 2012, O’Donovan and Fitzgerald, 2018).
3.5. Screening recommendations over time
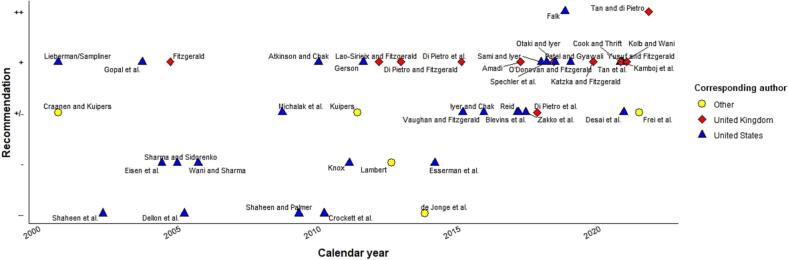
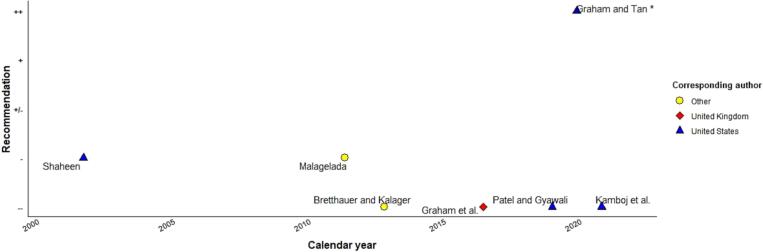
Screening recommendations were extracted from 49 expert opinion articles (text fragments and blinded categorization results are available in Supplementary Table 3). In Fig. 3, professionals’ recommendations regarding targeted EAC screening are mapped by calendar year (substantial inter-rater agreement κw = 0.71; SE = 0.062), showing that their attitudes towards targeted screening appear increasingly supportive. In contrast, Fig. 4 depicting professionals’ attitude toward population screening for EAC is mostly negative (almost perfect inter-rater agreement κw = 0.87; SE = 0.144).
Fig. 3.
Professionals’ recommendations regarding targeted screening for esophageal adenocarcinoma. Legend: ++, recommending; +, motivation; +/−, neutral position; −, serious doubt; −/−, recommending against.
Fig. 4.
Professionals’ recommendations regarding population screening for esophageal adenocarcinoma. Legend: ++, recommending; +, motivation; +/−, neutral position; −, serious doubt; −/−, recommending against.
4. Discussion
Our findings imply a discrepancy between professionals’ increased support for targeted EAC screening and the continued perceived unfulfillment of most screening principles. It appears that professionals’ motivation is mainly driven by the recent introduction of novel less-invasive screening modalities and the possibility to safely and effectively treat screen-detected neoplasia. However, examination of professionals’ perceptions reveals two major bottlenecks preventing full acceptance of EAC screening: the unmet need to precisely identify a target population with a high burden of EAC and the lack of favorable data on screening benefits and harms. We further identified several surmountable implications, such as the need to determine how often to screen, which screening test to use, how to manage non-dysplastic BE, how to inform the public, and how to adequately invest human and financial resources (see Table 3 for future research directions).
Although most professionals agree that the burden of EAC does not merit population screening, their belief in the possibility of identifying subpopulations at risk of developing EAC appears to have grown over the last decade. The increased interest in screening for relatively low-incident cancers aligns with the ‘new EU approach on cancer screening’ released by the European Health Union, September 2022 (European Commision, 2022). This new approach aims to increase the number of screenings, covering more target groups and more cancers. Similar to the findings presented in this review, the EU proposal refers to the promise of risk-assessment tools to optimize risk-based cancer screening. Implementation of these tools will rely on discriminatory accuracy, transparency and validation of the model, provider and public acceptance, and definition of criteria for sufficient evidence to declare a model as ‘valid’ for the selection of individuals at high risk for EAC (Shaheen et al., 2016, Eddy et al., 2012).
Our analysis further revealed that randomized studies on screening benefits and harms are crucial to establish professionals’ full support for EAC screening. Despite numerous studies on the feasibility, safety, acceptability, and efficacy of several BE screening devices, only two studies have, to the best of our knowledge, addressed the effectiveness of BE screening. One multicenter, pragmatic, randomized, controlled trial showed that the Cytosponge-TFF3 technology detected ten times more cases of BE compared with usual care (Fitzgerald et al., 2020). Another targeted screening trial, which is expected to finish in 2035, aims to determine the extent to which the Cytosponge-TFF3 can reduce mortality from EAC (BEST4).
Evaluation of the long-term effect of screening requires sufficient follow-up time because the impact of screening on mortality reduction takes at least ten years to become evident. This is challenging because rapidly developing technologies (risk-assessment tools, screening modalities, artificial intelligence models to assess collected samples, and biomarker panels to risk stratify them) may diminish the relevance of screening trials that are started now. In addition, variation in these elements and screen intervals, start- and stop ages, and public participation can change the benefit-harm trade-offs (Albhert et al., 2017). For similar reasons, breast and bowel cancer experts recommend hybrid effectiveness-implementation research combined with modeling studies to evaluate the long-term population outcomes of risk-stratified cancer screening (Pashayan et al., 2020, Green et al., 2019). It is advisable that researchers intending to set up an EAC screening trial collaborate with policy makers to determine if such outcome measures would deliver sufficient proof of effectiveness.
The strengths of this literature review include the rigorous qualitative analysis of expert opinion articles and the application of a theoretical framework. Our method is also highly transparent since the included articles, i.e., the data supporting the findings, are publicly available and free from privacy restrictions. However, the following limitations should be taken into consideration. First, original peer-reviewed studies reporting professionals’ views on EAC screening were limited to the US, mainly published before 2010, and only addressed the use of endoscopy for this purpose. Surveys conducted in other contexts may yield different estimates of clinicians’ belief in (cost-) effectiveness and drivers/barriers to screening. Second, the opinions of professionals summarized in this review are not static and will likely shift when results of new studies become available. Similarly, few included articles contain reflections on the most recent innovations and epidemiologic data. Third, this review does not provide a complete overview of cost-effectiveness studies and screening or risk-stratification technologies. We refer to articles containing the authors’ reflections on novel technologies rather than the original studies reporting their performance. Fourth, the distinction between recommendation categories in Fig. 3, Fig. 4 was a matter of interpretation. Categorization was done independently by two researchers that were blinded for author and publication year of the extracted recommendations to minimize subjectivity.
4.1. Conclusion
Although professionals’ motivation to conduct targeted EAC screening appears to have increased over the past decade, relevant screening principles remain insufficiently fulfilled until now. In particular, the identification of an appropriate screening policy and evidence that screening reduces EAC-related mortality are still considered inadequate.
5. Grant support
This study was funded by the Netherlands Organization for Health Research and Development (ZonMw) under grant 555004206.
6. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
7. Data transparency statement
Study data are available on https://doi.org/10.17026/dans-x67-9bzx.
Author contributions
YP, LR, MB and PS were involved in conception of the work and acquisition of funding. JS, YP, LR, MB and PS designed the study and wrote the protocol. JS and MG did the study search, study selection, data extraction, and data analysis. YP, LR, MB and PS supervised all the steps in the review process. All authors contributed to interpretation of the findings and revision of the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JS, YP, LR, MG, and MB have no conflicts of interest or financial ties related to this work to disclose. PS is receiving unrestricted research grants from Pentax (Japan), Norgine (UK), Motus GI (USA), MicroTech (China) and The eNose Company (Netherlands) and is in the advisory board of Motus GI (US) and Boston Scientific (US).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2023.102264.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
We have shared the URL to the data.
References
- BEST 4: A Platform Trial to determine whether Cytosponge-biomarker technology reduces mortality from oesophageal cancer. https://fundingawards.nihr.ac.uk/award/NIHR135565
- Adams M.A., Parikh P.D., Miller K., Rubenstein J.H. Medical professional liability claims related to esophageal cancer screening. JAMA. 2014;312(13):1348–1349. doi: 10.1001/jama.2014.7960. [DOI] [PubMed] [Google Scholar]
- Albhert T., Kiasuwa R., van den Bulcke M. 2017. EUROPEAN guide on quality improvement in comprehensive cancer control.https://cancercontrol.eu/archived/uploads/images/Guide/pdf/CanCon_Guide_FINAL_Web.pdf [Google Scholar]
- Amadi C., Gatenby P. Barrett’s oesophagus: current controversies. World J. Gastroenterol. 2017;23(28):5051–5067. doi: 10.3748/wjg.v23.i28.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M., Chak A. Screening for Barrett’s esophagus. Techn. Gastrointest. Endosc. 2010;12(2):62–66. doi: 10.1016/j.tgie.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins C.H., Iyer P.G. Who deserves endoscopic screening for esophageal neoplasia? Gastrointest. Endosc. Clin. N. Am. 2017;27(3):365–378. doi: 10.1016/j.giec.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Boolchand V., Faulx A., Das A., Zyzanski S., Isenberg G., Cooper G., Sivak M.V., Chak A. Primary care physician attitudes toward endoscopic screening for GERD symptoms and unsedated esophagoscopy. Gastrointest. Endosc. 2006;63(2):228–233. doi: 10.1016/j.gie.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Bretthauer M., Kalager M. Principles, effectiveness and caveats in screening for cancer. Brit. J. Surg. 2013;100(1):55–65. doi: 10.1002/bjs.8995. [DOI] [PubMed] [Google Scholar]
- Bretthauer M., Kalager M., Adami H.O. Do’s and don’ts in evaluation of endoscopic screening for gastrointestinal cancers. Endoscopy. 2016;48(1):75–80. doi: 10.1055/s-0034-1393094. [DOI] [PubMed] [Google Scholar]
- Chak A., Faulx A., Eng C., Grady W., Kinnard M., Ochs-Balcom H., Falk G. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer. 2006;107(9):2160–2166. doi: 10.1002/cncr.22245. [DOI] [PubMed] [Google Scholar]
- Chey W.D., Inadomi J.M., Booher A.M., Sharma V.K., Fendrick A.M., Howden C.W. Primary-care physicians’ perceptions and practices on the management of GERD: results of a national survey. Am. J. Gastroenterol. 2005;100(6):1237–1242. doi: 10.1111/j.1572-0241.2005.41364.x. [DOI] [PubMed] [Google Scholar]
- Codipilly D.C., Chandar A.K., Singh S., Wani S., Shaheen N.J., Inadomi J.M., Chak A., Iyer P.G. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154(8):2068–2086.e5. doi: 10.1053/j.gastro.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.B. Optimization and expansion of predictive models for barrett’s esophagus and esophageal adenocarcinoma: Could a life-course exposure history be beneficial? Am. J. Gastroenterol. 2013;108(6):923–925. doi: 10.1038/ajg.2013.83. [DOI] [PubMed] [Google Scholar]
- Cook M.B., Thrift A.P. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma: Implications for Screening and Surveillance. Gastrointest. Endosc. Clin. N. Am. 2021;31(1):1–26. doi: 10.1016/j.giec.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craanen M.E., Kuipers E.J. Advantages and disadvantages of population screening for cancer and surveillance of at-risk groups. Best Pract. Res. Clin. Gastroenterol. 2001;15(2):211–226. doi: 10.1053/bega.2000.0170. [DOI] [PubMed] [Google Scholar]
- Crockett S.D., Barritt I.A.S., Shaheen N.J. A 52-year-old man with heartburn: should he undergo screening for Barrett’s esophagus? Clin. Gastroenterol. Hepatol. 2010;8(7):565–571. doi: 10.1016/j.cgh.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. Barrett esophagus: a practical challenge for primary care. Nurse Pract. 2011;36(11):18–21. doi: 10.1097/01.NPR.0000406492.84414.c0. [DOI] [PubMed] [Google Scholar]
- de Jonge P.J., van Blankenstein M., Grady W.M., Kuipers E.J. Barrett’s oesophagus: epidemiology, cancer risk and implications for management. Gut. 2014;63(1):191–202. doi: 10.1136/gutjnl-2013-305490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon E.S., Shaheen N.J. Does screening for Barrett’s esophagus and adenocarcinoma of the esophagus prolong survival? J. Clin. Oncol. 2005;23(20):4478–4482. doi: 10.1200/JCO.2005.19.059. [DOI] [PubMed] [Google Scholar]
- Desai M., Hamade N., Sharma P. Screening for Barrett’s esophagus: challenges in identifying the population at risk. Gastrointestinal Endosc. 2021;93(2):420–421. doi: 10.1016/j.gie.2020.11.002. [DOI] [PubMed] [Google Scholar]
- di Pietro M., Fitzgerald R.C. Screening and risk stratification for Barrett’s esophagus: how to limit the clinical impact of the increasing incidence of esophageal adenocarcinoma. Gastroenterol. Clin. North Am. 2013;42(1):155–173. doi: 10.1016/j.gtc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- di Pietro M., Chan D., Fitzgerald R.C., Wang K.K. Screening for Barrett’s Esophagus. Gastroenterology. 2015;148(5):912–923. doi: 10.1053/j.gastro.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pietro M., Canto M.I., Fitzgerald R.C. Endoscopic management of early adenocarcinoma and squamous cell carcinoma of the esophagus: screening, diagnosis, and therapy. Gastroenterology. 2018;154(2):421–436. doi: 10.1053/j.gastro.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrow M.J., Hagens V., Chafe R., Sullivan T., Rabeneck L. Consolidated principles for screening based on a systematic review and consensus process. CMAJ. 2018;190(14):E422–E429. doi: 10.1503/cmaj.171154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy D.M., Hollingworth W., Caro J.J., Tsevat J., McDonald K.M., Wong J.B. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices Task Force–7. Value Health. 2012;15(6):843–850. doi: 10.1016/j.jval.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Eisen G.M., Lieberman D., Fennerty M.B., Sonnenberg A. Screening and surveillance in Barrett’s esophagus: a call to action. Clin. Gastroenterol. Hepatol. 2004;2(10):861–864. doi: 10.1016/s1542-3565(04)00388-x. [DOI] [PubMed] [Google Scholar]
- El-Serag H.B., Sweet S., Winchester C.C., Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enslin S., Kaul V. Barrett’s esophagus management in the elderly: principles and best practice. Curr. Gastroenterol. Rep. 2020;22(8):37. doi: 10.1007/s11894-020-00774-2. [DOI] [PubMed] [Google Scholar]
- Esserman L.J., Thompson I.M., Reid B., Nelson P., Ransohoff D.F., Welch H.G., Hwang S., Berry D.A., Kinzler K.W., Black W.C., Bissell M., Parnes H., Srivastava S. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15(6) doi: 10.1016/S1470-2045(13)70598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commision, 2022. Proposal for a Council Recommendation (CR) on Strengthening prevention through early detection: A new approach on cancer screening replacing CR 2003/878/EC (europa.eu).
- Falk G.W. Barrett’s esophagus. Gastroenterology. 2002;122(6):1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- Falk G.W. Screening and surveillance of Barrett’s esophagus: where are we now and what does the future hold? Am. J. Gastroenterol. 2019;114(1):64–70. doi: 10.1038/s41395-018-0374-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R.C. Genetics and prevention of oesophageal adenocarcinoma. Recent Results Cancer Res. 2005;166:35–46. doi: 10.1007/3-540-26980-0_3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R.C., di Pietro M., Ragunath K., Ang Y., Kang J.-Y., Watson P., Trudgill N., Patel P., Kaye P.V., Sanders S., O’Donovan M., Bird-Lieberman E., Bhandari P., Jankowski J.A., Attwood S., Parsons S.L., Loft D., Lagergren J., Moayyedi P., Lyratzopoulos G., de Caestecker J. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R.C., di Pietro M., O'Donovan M., et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396(10247):333–344. doi: 10.1016/S0140-6736(20)31099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei N.F., Bergman J., Pouw R.E. Screening for Barrett’s esophagus: ready for prime time or still hard to swallow? Gastrointest. Endosc. 2021;94(3):506–508. doi: 10.1016/j.gie.2021.06.004. [DOI] [PubMed] [Google Scholar]
- GBD Oesophageal Cancer Collaborators The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5(6):582–597. doi: 10.1016/S2468-1253(20)30007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson L.B. Are we ready for gender-based guidelines for Barrett’s esophagus screening? Gastroenterology. 2011;141(6):2271–2273. doi: 10.1053/j.gastro.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Gerson L.B., Triadafilopoulos G. Screening for esophageal adenocarcinoma: an evidence-based approach. Am. J. Med. 2002;113(6):499–505. doi: 10.1016/s0002-9343(02)01234-2. [DOI] [PubMed] [Google Scholar]
- Gopal D.V., Reichelderfer M., Gaumnitz E.A., Harter J., Jobe B.A. Barrett’s esophagus: is screening and surveillance justified? Dis. Manag. Health Outcomes. 2004;12(6):353–361. doi: 10.2165/00115677-200412060-00002. [DOI] [Google Scholar]
- Graham D., Lipman G., Sehgal V., Lovat L.B. Monitoring the premalignant potential of Barrett’s oesophagus. Front. Gastroenterol. 2016;7(4):316–322. doi: 10.1136/flgastro-2016-100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.Y., Tan M.C. No Barrett’s-no cancer: a proposed new paradigm for prevention of esophageal adenocarcinoma. J. Clin. Gastroenterol. 2020;54(2):136–143. doi: 10.1097/MCG.0000000000001298. [DOI] [PubMed] [Google Scholar]
- Green B.B., Coronado G.D., Schwartz M., Coury J., Baldwin L.M. Using a continuum of hybrid effectiveness-implementation studies to put research-tested colorectal screening interventions into practice. Implement Sci. 2019;14(1):53. doi: 10.1186/s13012-019-0903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibertse L.J., Peters Y., Westendorp D., Siersema P.D. Unsedated transnasal endoscopy for the detection of Barrett’s esophagus: systematic review and meta-analysis. Dis. Esophagus. 2022 doi: 10.1093/dote/doac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilbawi A.M., Anderson B.O. Cancer in global health: How do prevention and early detection strategies relate? Sci. Transl. Med. 2015;7(278) doi: 10.1126/scitranslmed.3008853. [DOI] [PubMed] [Google Scholar]
- Kamboj A.K., Katzka D.A., Iyer P.G. Endoscopic screening for barrett’s esophagus and esophageal adenocarcinoma: rationale, candidates, and challenges. Gastrointest. Endosc. Clin. N. Am. 2021;31(1):27–41. doi: 10.1016/j.giec.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzka D.A., Fitzgerald R.C. Time to challenge current strategies for detection of Barrett’s esophagus and esophageal adenocarcinoma. Dig. Dis. Sci. 2020;65(1):18–21. doi: 10.1007/s10620-019-05965-0. [DOI] [PubMed] [Google Scholar]
- Knox M.A. Should we screen patients for Barrett’s esophagus? Am. Fam. Phys. 2011;83(10):1148–1150. [PubMed] [Google Scholar]
- Kolb J.M., Wani S. A paradigm shift in screening for Barrett’s esophagus: the BEST is yet to come. Gastroenterology. 2021;160(1):467–469. doi: 10.1053/j.gastro.2020.11.024. [DOI] [PubMed] [Google Scholar]
- Kolb J.M., Chen M., Tavakkoli A., Singal A.G., Vajravelu R.K., Wani S., Hochheimer C.J., Tarter W., Gallegos J., O’Hara J., Devireddy S., Golubski B., Chang K.J., Samarasena J., Scott F.I., Falk G.W. Understanding compliance, practice patterns, and barriers among gastroenterologists and primary care providers is crucial for developing strategies to improve screening for Barrett’s esophagus. Gastroenterology. 2022;162(6):1568–1573.e4. doi: 10.1053/j.gastro.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konda V.J.A., Souza R.F. Barrett’s esophagus and esophageal carcinoma: can biomarkers guide clinical practice? Curr. Gastroenterol. Rep. 2019;21(4):14. doi: 10.1007/s11894-019-0685-6. [DOI] [PubMed] [Google Scholar]
- Kuipers E.J. Barrett Esophagus and life expectancy: implications for screening? Gastroenterol. Hepatol. 2011;7(10):689–691. [PMC free article] [PubMed] [Google Scholar]
- Lambert R. Endoscopy in screening for digestive cancer. World J. Gastrointest. Endosc. 2012;4(12):518–525. doi: 10.4253/wjge.v4.i12.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao-Sirieix P., Fitzgerald R.C. Screening for oesophageal cancer. Nat. Rev. Clin. Oncol. 2012;9(5):278–287. doi: 10.1038/nrclinonc.2012.35. [DOI] [PubMed] [Google Scholar]
- Lieberman D.A., Sampliner R.E. How far to go? screening and surveillance in Barrett’s esophagus. Am. J. Manag. Care. 2001;7(1 SUPPL):S19–S26. [PubMed] [Google Scholar]
- Lin O.S., Mannava S., Hwang K.L., Triadafilopoulos G. Reasons for current practices in managing Barrett's esophagus. Dis. Esophagus. 2002;15(1):39–45. doi: 10.1046/j.1442-2050.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Malagelada J.-R. Diseases of the Digestive Tract: is prevention possible and feasible? Dig. Dis. 2011;29(2):255–263. doi: 10.1159/000323931. [DOI] [PubMed] [Google Scholar]
- Marques de Sa I., Marcos P., Sharma P., Dinis-Ribeiro M. The global prevalence of Barrett’s esophagus: a systematic review of the published literature. United European Gastroenterol. J. 2020;8(9):1086–1105. doi: 10.1177/2050640620939376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S.J., Asch D.A. How to help gastroenterology patients help themselves: leveraging insights from behavioral economics. Clin. Gastroenterol. Hepatol. 2014;12(5):711–714. doi: 10.1016/j.cgh.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Michalak J., Bansal A., Sharma P. Screening and surveillance of Barrett’s esophagus. Curr. Gastroenterol. Rep. 2009;11(3):195–201. doi: 10.1007/s11894-009-0031-5. [DOI] [PubMed] [Google Scholar]
- Moinova H.R., LaFramboise T., Lutterbaugh J.D., Chandar A.K., Dumot J., Faulx A., Brock W., De la Cruz Cabrera O., Guda K., Barnholtz-Sloan J.S., Iyer P.G., Canto M.I., Wang J.S., Shaheen N.J., Thota P.N., Willis J.E., Chak A., Markowitz S.D. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 2018;10(424) doi: 10.1126/scitranslmed.aao5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan M., Fitzgerald R.C. Screening for Barrett’s esophagus: are new high-volume methods feasible? Dig. Dis. Sci. 2018;63(8):2105–2114. doi: 10.1007/s10620-018-5192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaki F., Iyer P.G. Point-counterpoint: screening and surveillance for Barrett’s esophagus, is it worthwhile? Dig. Dis. Sci. 2018;63(8):2081–2093. doi: 10.1007/s10620-018-5065-9. [DOI] [PubMed] [Google Scholar]
- Pashayan N., Antoniou A.C., Ivanus U., Esserman L.J., Easton D.F., French D., Sroczynski G., Hall P., Cuzick J., Evans D.G., Simard J., Garcia-Closas M., Schmutzler R., Wegwarth O., Pharoah P., Moorthie S., De Montgolfier S., Baron C., Herceg Z., Turnbull C., Balleyguier C., Rossi P.G., Wesseling J., Ritchie D., Tischkowitz M., Broeders M., Reisel D., Metspalu A., Callender T., de Koning H., Devilee P., Delaloge S., Schmidt M.K., Widschwendter M. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat. Rev. Clin. Oncol. 2020;17(11):687–705. doi: 10.1038/s41571-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Gyawali C.P. Screening for Barrett's esophagus: balancing clinical value and cost-effectiveness. J. Neurogastroenterol. Motil. 2019;25(2):181–188. doi: 10.5056/jnm18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters Y., Al-Kaabi A., Shaheen N.J., et al. Barrett oesophagus. Nat. Rev. Dis. Primers. 2019;5(1):35. doi: 10.1038/s41572-019-0086-z. [DOI] [PubMed] [Google Scholar]
- Peters Y., Honing J., Kievit W., Kestens C., Pestman W., Nagtegaal I.D., van der Post R.S., Siersema P.D. Incidence of progression of persistent nondysplastic Barrett’s esophagus to malignancy. Clin. Gastroenterol. Hepatol. 2019;17(5):869–877.e5. doi: 10.1016/j.cgh.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Peters Y., Schrauwen R.W.M., Tan A.C., Bogers S.K., de Jong B., Siersema P.D. Detection of Barrett’s oesophagus through exhaled breath using an electronic nose device. Gut. 2020;69(7):1169–1172. doi: 10.1136/gutjnl-2019-320273. [DOI] [PubMed] [Google Scholar]
- Playford R.J. New British Society of Gastroenterology guidelines for the diagnosis and management of Barrett’s oesophagus. Gut. 2006;55(4):442. doi: 10.1136/gut.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti A., Anttila A., Ronco G., Senore C., Basu P., Segnan N., Tomatis M., Zaklj M.P., Dillner J., Fernan M., Elfstrom K.M., Lonnberg S., Soerjomataram I., Sankaranaryanan R., Vale D. Cancer screening in the European Union. 2017. https://health.ec.europa.eu/system/files/2017-05/2017_cancerscreening_2ndreportimplementation_en_0.pdf. [Google Scholar]
- Qumseya B., Sultan S., Bain P., Jamil L., Jacobson B., Anandasabapathy S., Agrawal D., Buxbaum J.L., Fishman D.S., Gurudu S.R., Jue T.L., Kripalani S., Lee J.K., Khashab M.A., Naveed M., Thosani N.C., Yang J., DeWitt J., Wani S. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019;90(3):335–359.e2. doi: 10.1016/j.gie.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Rajendra S. Barrett’s oesophagus: can meaningful screening and surveillance guidelines be formulated based on new data and rejigging the old paradigm? Best Pract. Res.: Clin. Gastroenterol. 2015;29(1):65–75. doi: 10.1016/j.bpg.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Reid B.J. Genomics, endoscopy, and control of gastroesophageal cancers: a perspective. Cell. Mol. Gastroenterol. Hepatol. 2017;3(3):359–366. doi: 10.1016/j.jcmgh.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J., Lewis J., Nicholls C.M., Ormston R. Qualitative research practice: a guide for social science students and researchers. Sage. 2013 [Google Scholar]
- Ross-Innes C.S., Debiram-Beecham I., O’Donovan M., Walker E., Varghese S., Lao-Sirieix P., Lovat L., Griffin M., Ragunath K., Haidry R., Sami S.S., Kaye P., Novelli M., Disep B., Ostler R., Aigret B., North B.V., Bhandari P., Haycock A., Morris D., Attwood S., Dhar A., Rees C., Rutter M.D.D., Sasieni P.D., Fitzgerald R.C., Franco E.L. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med. 2015;12(1) doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J.H., Saini S.D., Kuhn L., McMahon L., Sharma P., Pardi D.S., Schoenfeld P. Influence of malpractice history on the practice of screening and surveillance for Barrett’s esophagus. Am. J. Gastroenterol. 2008;103(4):842–849. doi: 10.1111/j.1572-0241.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein J.H., Thrift A.P. Risk factors and populations at risk: selection of patients for screening for Barrett’s oesophagus. Best Pract. Res. Clin. Gastroenterol. 2015;29(1):41–50. doi: 10.1016/j.bpg.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Sami S.S., Iyer P.G. Recent advances in screening for Barrett’s esophagus. Curr. Treat Opt. Gastroenterol. 2018;16(1):1–14. doi: 10.1007/s11938-018-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen N.J. Should women with heartburn undergo screening upper endoscopy for prevention of cancer. Am. J. Gastroenterol. 2011;106(2):261–263. doi: 10.1038/ajg.2010.471. [DOI] [PubMed] [Google Scholar]
- Shaheen N.J., Palmer L.B. Improving screening practices for Barrett’s esophagus. Surg. Oncol. Clin. N. Am. 2009;18(3):423–437. doi: 10.1016/j.soc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Shaheen N.J., Provenzale D., Sandler R.S. Upper endoscopy as a screening and surveillance tool in esophageal adenocarcinoma: a review of the evidence. Am. J. Gastroenterol. 2002;97(6):1319–1327. doi: 10.1111/j.1572-0241.2002.05767.x. [DOI] [PubMed] [Google Scholar]
- Shaheen N.J., Sharma P., Overholt B.F., et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N. Engl. J. Med. 2009;360(22):2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- Shaheen N.J., Falk G.W., Iyer P.G., Gerson L.B., American College of Gastroenterology ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen N.J., Falk G.W., Iyer P.G., Souza R.F., Yadlapati R.H., Sauer B.G., Wani S. Diagnosis and management of Barrett’s esophagus: an updated ACG guideline. Am. J. Gastroenterol. 2022;117(4):559–587. doi: 10.14309/ajg.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Sidorenko E.I. Are screening and surveillance for Barrett’s oesophagus really worthwhile? Gut. 2005;54(SUPPL. 1):i27–i32. doi: 10.1136/gut.2004.041566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Smith M.S. New screening methods for Barrett esophagus. Gastroenterol. Hepatol. 2020;16(11):590–593. [PMC free article] [PubMed] [Google Scholar]
- Smith R.A., Manassaram-Baptiste D., Brooks D., Doroshenk M., Fedewa S., Saslow D., Brawley O.W., Wender R. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2015;65(1):30–54. doi: 10.3322/caac.21261. [DOI] [PubMed] [Google Scholar]
- Spechler S.J. Should patients with GERD be screened once at least for Barrett’s epithelium? A balancing view: To screen or not to screen: scoping out the issues. Am. J. Gastroenterol. 2004;99(12):2295–2296. doi: 10.1111/j.1572-0241.2004.41295_3.x. [DOI] [PubMed] [Google Scholar]
- Spechler S.J., Sharma P., Souza R.F., Inadomi J.M., Shaheen N.J. American gastroenterological association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):e18–e52. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spechler S.J., Katzka D.A., Fitzgerald R.C. New screening techniques in Barrett’s esophagus: great ideas or great practice? Gastroenterology. 2018;154(6):1594–1601. doi: 10.1053/j.gastro.2018.03.031. [DOI] [PubMed] [Google Scholar]
- Tan W.K., di Pietro M. Barrett’s oesophagus: today’s mistake and tomorrow’s wisdom in screening and prevention. Visc. Med. 2022;38(3):161–167. doi: 10.1159/000522015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M.C., Mansour N., White D.L., Sisson A., El-Serag H.B., Thrift A.P. Systematic review with meta-analysis: prevalence of prior and concurrent Barrett’s oesophagus in oesophageal adenocarcinoma patients. Aliment Pharmacol. Ther. 2020;52(1):20–36. doi: 10.1111/apt.15760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W.K., Sharma A.N., Chak A., Fitzgerald R.C. Progress in screening for Barrett’s esophagus: beyond standard upper endoscopy. Gastrointest. Endosc. Clin. N. Am. 2021;31(1):43–58. doi: 10.1016/j.giec.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Vaughan T.L., Fitzgerald R.C. Precision prevention of oesophageal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015;12(4):243–248. doi: 10.1038/nrgastro.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegt F., Gommers J.J.J., Groenewoud H., Siersema P.D., Verbeek A.L.M., Peters Y., Broeders M.J.M. Trends and projections in the incidence of oesophageal cancer in the Netherlands: an age-period-cohort analysis from 1989 to 2041. Int. J. Cancer. 2022;150(3):420–430. doi: 10.1002/ijc.33836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.K., Sampliner R.E. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am. J. Gastroenterol. 2008;103(3):788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- Wani S., Sharma P. The rationale for screening and surveillance of Barrett's metaplasia. Best Pract. Res. Clin. Gastroenterol. 2006;20(5):829–842. doi: 10.1016/j.bpg.2006.03.009. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Wilson J.M.G., Jungner G. The principles and practice of screening for disease. World Health Organization; 1966. https://apps.who.int/iris/handle/10665/208882. [Google Scholar]
- Yusuf A., Fitzgerald R.C. Screening for Barrett’s oesophagus: are we ready for it? Curr. Treat Opt. Gastro. 2021;19(2):321–336. doi: 10.1007/s11938-021-00342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakko L., Visrodia K., Wang K.K., Iyer P.G. The effect of bias on estimation of improved survival after diagnosis of Barrett’s esophagus. Am. J. Gastroenterol. 2017;112(8):1265–1266. doi: 10.1038/ajg.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have shared the URL to the data.