Abstract
Psychiatric disorders share neurobiology and frequently co-occur. This neurobiological and clinical overlap highlights opportunities for transdiagnostic treatments. In this study, we used coordinate and lesion network mapping to test for a shared brain network across psychiatric disorders. In our meta-analysis of 193 studies, atrophy coordinates across six psychiatric disorders mapped to a common brain network defined by positive connectivity to anterior cingulate and insula, and by negative connectivity to posterior parietal and lateral occipital cortex. This network was robust to leave-one-diagnosis-out cross-validation and specific to atrophy coordinates from psychiatric versus neurodegenerative disorders (72 studies). In 194 patients with penetrating head trauma, lesion damage to this network correlated with the number of post-lesion psychiatric diagnoses. Neurosurgical ablation targets for psychiatric illness (four targets) also aligned with the network. This convergent brain network for psychiatric illness may partially explain high rates of psychiatric comorbidity and could highlight neuromodulation targets for patients with more than one psychiatric disorder.
Introduction
Psychiatric disorders are often studied individually1. However, up to half of patients who meet criteria for one psychiatric disorder also meet criteria for another2–7. These patients are difficult to diagnose and treat6,8–13. Relative to those with one disorder, patients with two or more have worse treatment outcomes, more functional impairment, and a greater risk of premature death9,13–21.
High rates of psychiatric comorbidity are often attributed to symptom heterogeneity within diagnoses and symptom overlap between diagnoses 22,23. The “p factor” intends to capture this shared variation across diagnoses, accounting for comorbidity and severity of psychopathology5,24–27. Genomic28–34 and epidemiological35,36 evidence further suggest that psychiatric comorbidity reflect a shared risk architecture. For example, any psychiatric disorder significantly increases the absolute risk of developing later psychiatric disorders35,36. In this context, it is unsurprising that many psychotherapies and medications reduce symptoms of various psychiatric disorders37–40.
Psychiatric comorbidity is an important consideration for neuromodulation treatments like transcranial magnetic stimulation (TMS) that often target one psychiatric disorder at a time. Targeting methods are increasingly focused on specific symptoms within a diagnosis41–44, a strategy that may be difficult to scale and optimize in the setting of high psychiatric comorbidity. By contrast, neurosurgical ablation of single treatment targets has shown effectiveness across psychiatric disorders45–47, suggesting that transdiagnostic targets are feasible.
Neuroimaging has revealed important insights into the neurobiological basis of psychiatric illness 48–57. However, prior work has critical limitations that we seek to address. First, studies often attempt to map abnormalities to common brain regions50,58 rather than to a common brain network. Natural heterogeneity and noise prevent some abnormalities from consistently mapping to the same brain region across studies. However, these same abnormalities map to different brain regions within the same brain network. Network-level analyses account for such a possibility, thereby increasing the statistical power to detect commonalities across studies56,57,59. Second, prior studies rarely test specificity by comparing psychiatric disorders to other brain disorders. Finally, the causal interpretation of neuroimaging findings is ambiguous when studies focus on correlates of illness. Neuroimaging correlates might cause, compensate for, or be coincidentally related to psychiatric disorders. The correct interpretation of these correlates is essential for developing effective neuromodulation targets60,61.
In this study, we used morphometric and brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.
Results
Significant, Sensitive, and Specific Transdiagnostic Network
Traditional ALE meta-analysis of psychiatric coordinates (Dataset 1) identified gray matter decreases in bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, superior temporal gyrus, and parietal operculum, consistent with prior work50 (Figure 1A). These regions survived cluster-wise multiple comparisons correction (Figure 1A Significant Results). However, fewer than 35% of studies contributed to any one cluster (Figure 1A Sensitivity), and no cluster was specific to psychiatric (Dataset 1) versus neurodegenerative coordinates (Dataset 2) (Figure 1A Specificity, two-sample t-test, nothing survives multiple comparisons correction).
Figure 1.
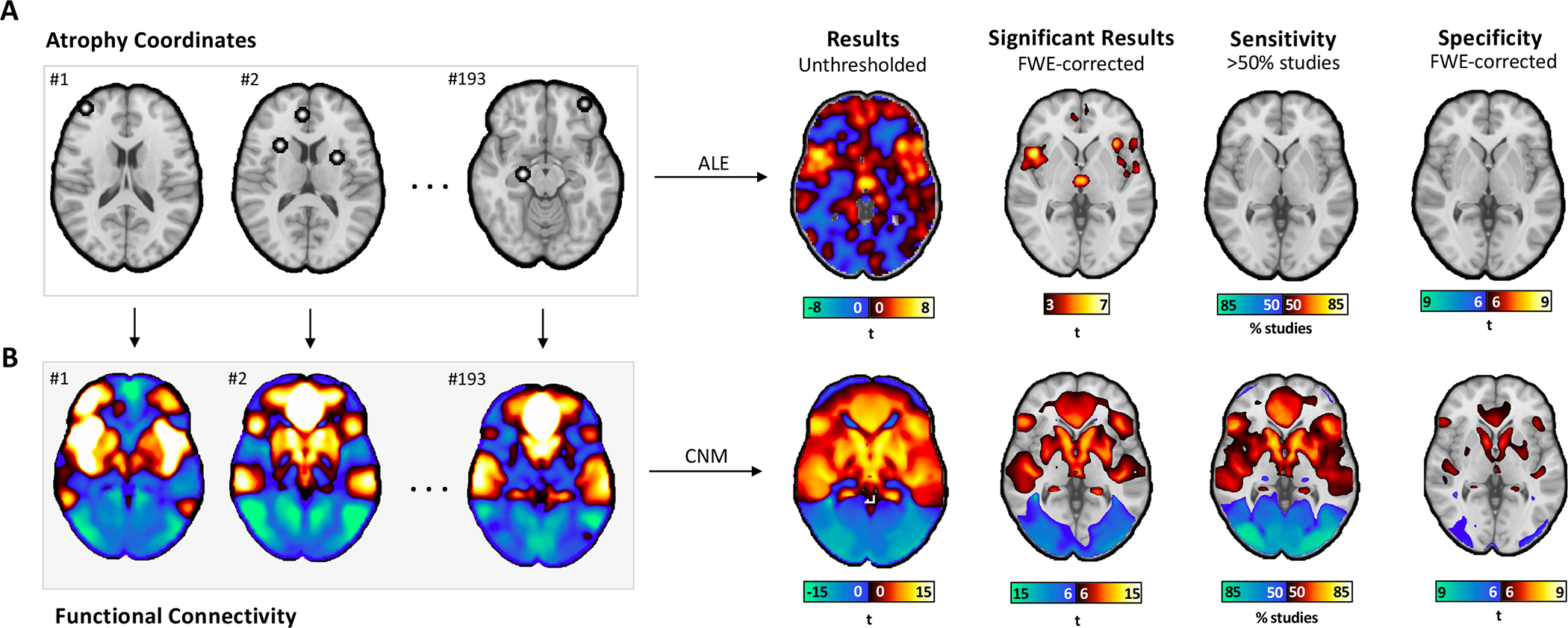
Mapping atrophy coordinates in psychiatric illness to networks rather than regions. (A) Atrophy coordinates from 15892 individuals in 193 VBM studies were analyzed via ALE. The results of this regional analysis aligned with those published previously (Significant Results). However, none of the ALE clusters had more than half of studies contributing to them (Sensitivity), and none of the ALE clusters were specific to psychiatric disorders versus neurodegenerative disorders (Specificity, two-sample t-test, no voxels survived multiple comparisons correction). (B) The same atrophy coordinates were analyzed via coordinate network mapping (CNM), a network-based analysis. Functional connectivity between atrophy coordinates in each study and the rest of the brain was computed using a normative connectome (n=1000). Positive functional connectivity is shown in warm colors, and negative functional connectivity (i.e., anticorrelations) are shown in cool colors. Relative to ALE results, CNM results were statistically stronger (Significant Results, one-sample t-test, voxels displayed survived multiple comparisons correction), explained more variance (Sensitivity), and survived comparison to neurodegenerative disorders (Specificity, two-sample t-test, voxels displayed survived multiple comparisons correction).
Coordinate network mapping identified results that were more statistically robust than those from the ALE meta-analysis (Figure 1B Significant Results, one-sample t-test, voxels survive multiple comparisons correction). Psychiatric atrophy coordinates from 85% of studies were functionally connected to the same network of brain regions (Figure 1B Sensitivity). This network was defined by positive connectivity to bilateral insula, anterior cingulate cortex, posterior cingulate, and left frontal pole, and by negative connectivity to right inferior temporal gyrus, posterior parietal cortex, bilateral lateral occipital cortex (superior division), brainstem, and cerebellum (Supplementary Figure 1, Supplementary Table 1). The topography of this transdiagnostic network was independent of statistical threshold (Supplementary Figure 2) and specific to psychiatric (Dataset 1) versus neurodegenerative disorders (Dataset 2)(Figure 1B Specificity, two-sample t-test, voxels survive multiple comparisons correction). The strongest peak of this transdiagnostic network was in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus (MNI Coordinates: −22, −70, 64).
All Psychiatric Diagnoses Independently Contribute
Leave-one-diagnosis-out analyses (Dataset 1) assessed whether any single diagnosis was disproportionately influencing our results. With ALE meta-analysis, dropping one diagnosis repeatedly changed the map topography, resulting in low spatial correlations with the comprehensive ALE map from all diagnoses (spatial r = 0.344–0.453, Supplemental Table 2). In contrast, coordinate network mapping results were robust to leave-one-out analyses (spatial r = 0.980–0.998) and significantly more robust than the ALE maps (p<0.001). These results did not change when we excluded negatively correlated regions from the coordinate network mapping results (Supplementary Table 2).
Network Damage Correlates with Psychiatric Comorbidity
We overlayed lesions from an independent dataset (Dataset 3; Figure 2A) onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with the number of post-lesion psychiatric diagnoses (i.e., psychiatric comorbidity). Each lesion was associated with a post-lesion SCID score (i.e., number of psychiatric diagnoses).
Figure 2.
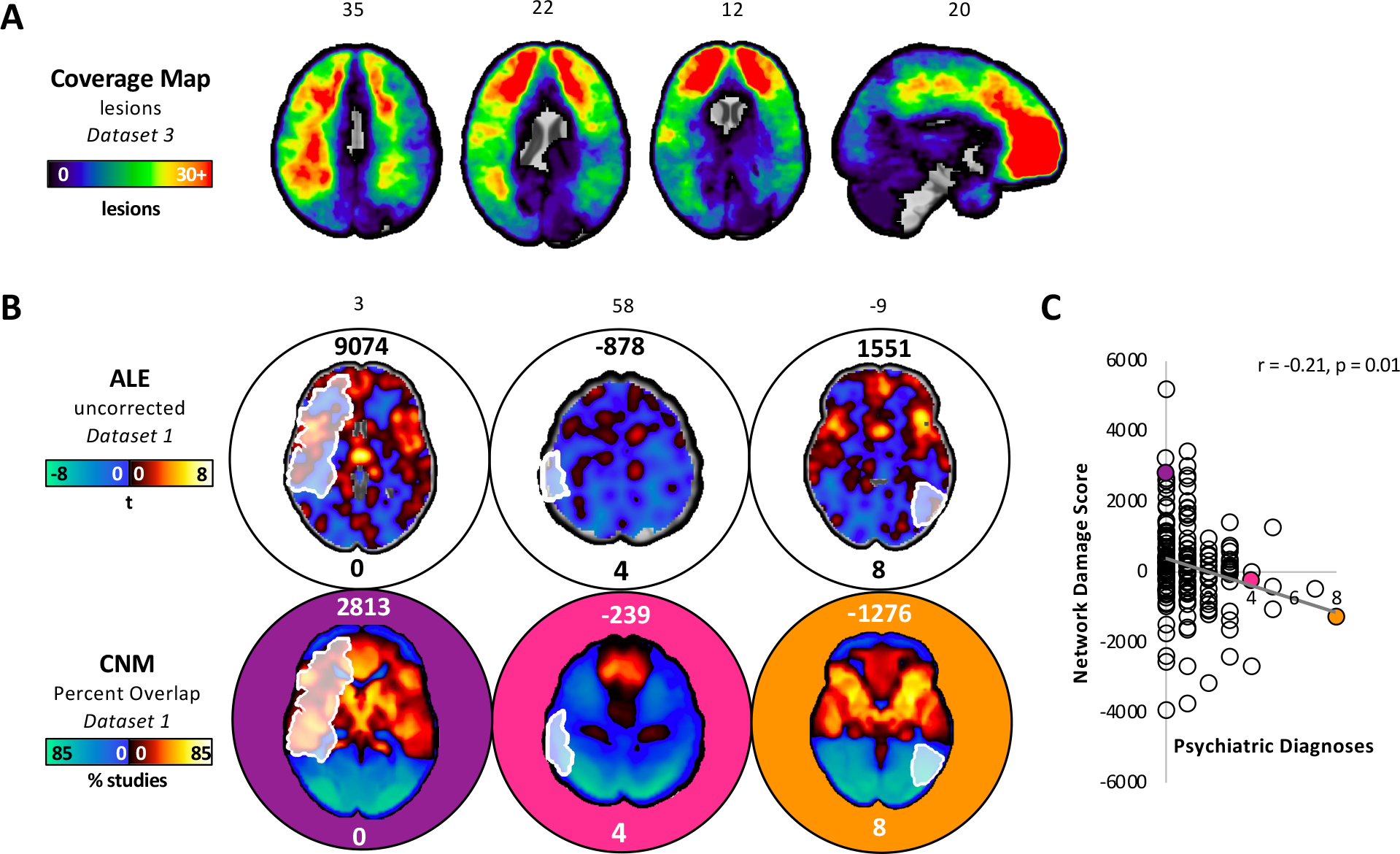
Network mapping results align better with lesion-induced psychiatric diagnoses than traditional ALE. (A) A coverage map depicting the number of lesions in an independent dataset (dataset 3) that intersected each voxel in the brain. (B) The top panel shows three lesions overlayed onto the ALE map. The number above each brain slice represents the network damage score, or the sum of the voxel values circumscribed by each lesion. The number below each brain slice represents the number of psychiatric diagnoses associated with that lesion. We found no evidence of a correlation between network damage and the number of post-lesion psychiatric diagnoses (Pearson r=0.02, p=0.766). The bottom panel shows the same three lesions overlayed onto the transdiagnostic network from coordinate network mapping (CNM). (C) There was a correlation between network damage and the number of post-lesion psychiatric diagnoses (Pearson r=−0.21, p=0.01). Lesions with positive network damage scores on the transdiagnostic network were correlated with lower psychiatric comorbidity. By contrast, lesions with negative network damage scores on the transdiagnostic network were correlated with higher psychiatric comorbidity. A multiple regression model showed that the transdiagnostic network, but not the ALE map, independently predicted the number of post-lesion psychiatric diagnoses (p = 0.003 versus 0.1, respectively).
We found no evidence of a correlation between psychiatric comorbidity and damage to the ALE map (Pearson r=0.02, p=0.766; Figure 2B). By contrast, there was a statistically significant correlation between psychiatric comorbidity and lesion damage to the transdiagnostic network (Figure 2B and Figure 2C; Pearson r=−0.21, p=0.01), which was independent of statistical threshold (Supplemental Figure 3). A multiple regression model showed that the transdiagnostic network, but not the ALE map, independently predicted the number of post-lesion psychiatric diagnoses (p = 0.003 versus 0.1, respectively).
Replication in an Independent Lesion Dataset
The same lesion dataset (Dataset 3) was used to generate a data-driven lesion network showing connections that co-vary with the number of psychiatric diagnoses (Figure 3A and Figure 3B). The topography of this lesion network was similar when we controlled for lesion size (spatial r=0.96). Despite being derived from an independent dataset, the topography of this lesion network was similar to the coordinate-based transdiagnostic network (r=0.65) and more similar than expected by chance (p=0.02 with 10,000 permutations: Supplemental Figure 4). The lesion network was also significantly more similar to the transdiagnostic network than it was to the ALE map (r=0.65 versus r=0.11, p=0.02).
Figure 3.
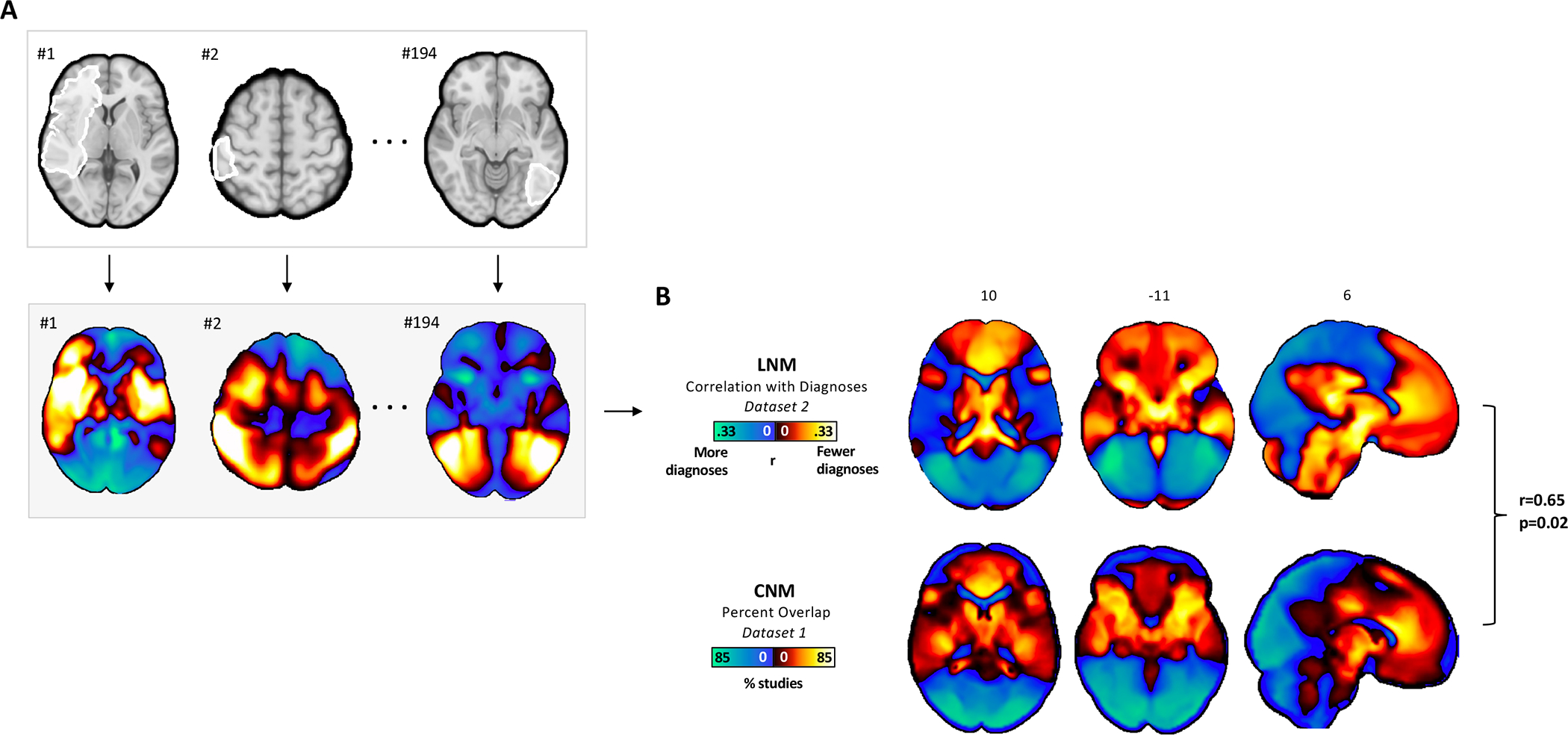
Convergent network topography across atrophy and brain lesions associated with psychiatric illness. (A) Brain lesions from an independent dataset (Dataset 3) were analyzed via lesion network mapping, a network-based analysis. Functional connectivity between each brain lesion and the rest of the brain was computed using a normative connectome. (B) The lesion network map shown reflects the correlation between each brain voxel and post-lesion psychiatric diagnoses. This lesion network map was compared to the coordinate network map (Dataset 2) via permutation testing. Briefly, the lesion network map was recomputed 10,000 times after random assignment of psychiatric diagnoses to functional connectivity profiles. Each of the 10,000 recomputed lesion network maps was compared to the coordinate network map via spatial correlation. The real lesion network map showed a higher spatial correlation to the coordinate network map than randomly permuted lesion network maps in greater than 98% of the instances (r=0.65, p=0.02). The lesion network was also significantly more similar to the transdiagnostic network than it was to the ALE map (r=0.65 versus r=0.11, p=0.02).
Neurosurgical Ablation Targets the Network
All four published neurosurgical ablative targets for psychiatric disorders (Dataset 4) intersected the transdiagnostic network (Figure 4). One-sample t-tests showed anterior capsulotomy (p<0.001), anterior cingulotomy (p<0.001), subcaudate tractotomy (p<0.001), and limbic leucotomy (p<0.001) damage the regions positively connected to psychiatric coordinates. Two-sample t-tests showed that these findings were all specific to psychiatric versus neurodegenerative coordinates (anterior capsulotomy p=0.044, anterior cingulotomy p=0.007, subcaudate tractotomy p = 0.002, and limbic leucotomy p<0.001).
Figure 4.
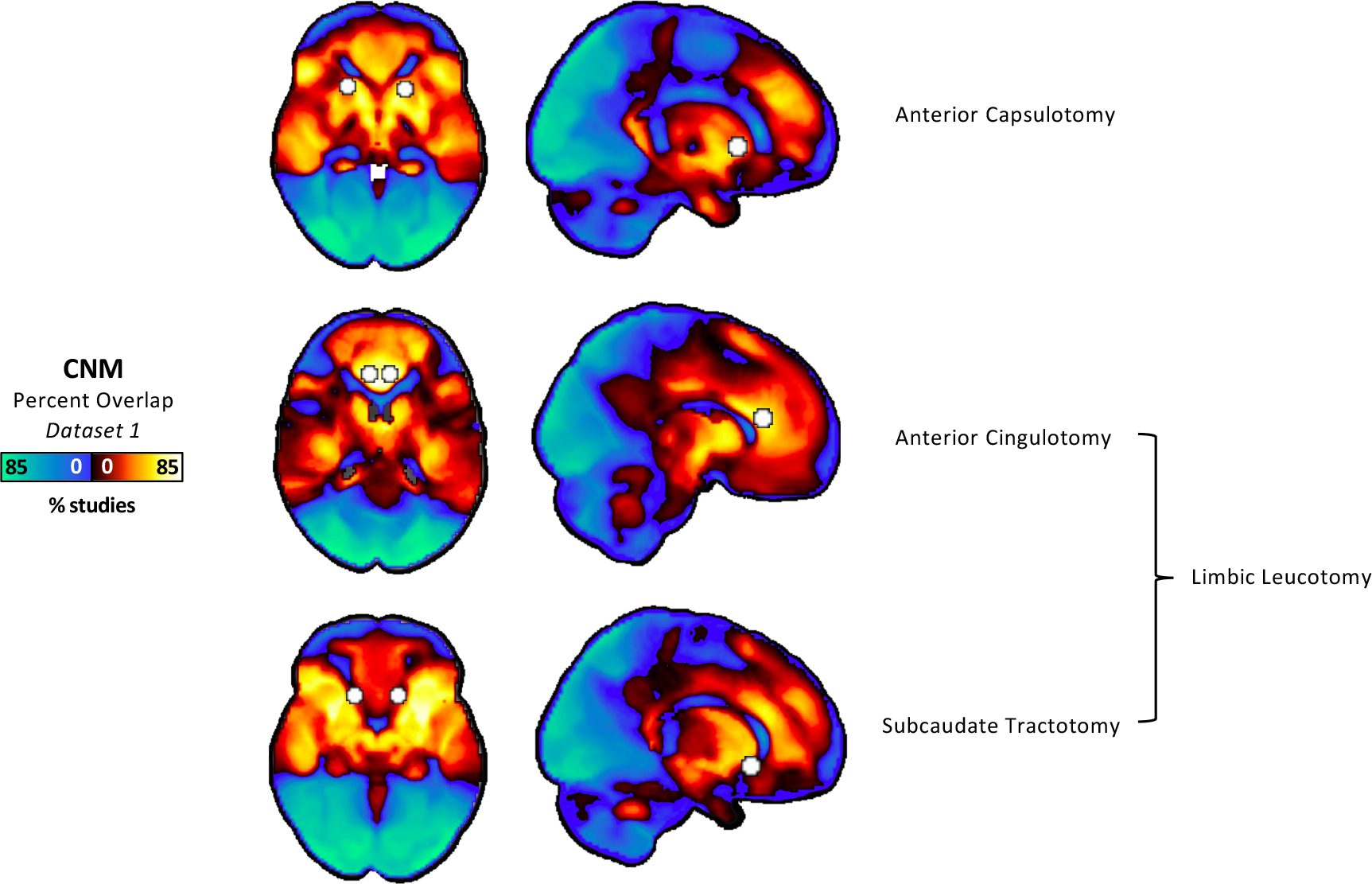
Alignment between neurosurgical ablation targets for psychiatric disorders and our coordinate-based transdiagnostic network. Spheres were placed at published coordinates for anterior capsulotomy, anterior cingulotomy, subcaudate tractotomy, and limbic leucotomy. These spheres were overlayed onto the transdiagnostic network from CNM. All targets hit the regions with positive connectivity to atrophy coordinates, which aligns with prior analyses showing that atrophy in these regions correlates with lower psychiatric comorbidity. One-sample t-tests showed anterior capsulotomy (p<0.001), anterior cingulotomy (p<0.001), subcaudate tractotomy (p<0.001), and limbic leucotomy (p<0.001) damage the regions positively connected to psychiatric coordinates. Two-sample t-tests showed that these findings were all specific to psychiatric versus neurodegenerative coordinates (anterior capsulotomy p=0.044, anterior cingulotomy p=0.007, subcaudate tractotomy p = 0.002, and limbic leucotomy p<0.001).
Spatial Correlation with Canonical Brain Networks
Canonical brain networks most similar to our transdiagnostic network were the visual (spatial r= 0.48), dorsal attention (spatial r=0.32), and default mode (spatial r=−0.34) networks (Supplemental Table 3). However, none of these canonical networks aligned with our transdiagnostic network as well as our data-driven lesion network for psychiatric comorbidity (spatial r=0.65).
Discussion
Psychiatric disorders are typically studied individually despite sharing neurobiology and frequently co-occurring. This neurobiological and clinical overlap highlights opportunities for transdiagnostic treatments that target a shared brain network. In this study, we used morphometric and brain lesion datasets coupled with a wiring diagram of the human brain to derive this convergent brain network for psychiatric illness.
There are five main findings. First, atrophy coordinates across psychiatric disorders mapped better to a common brain network than they did to common brain regions. This transdiagnostic network included positive connectivity to insula, anterior cingulate, posterior cingulate, and left frontal pole as well as negative connectivity to posterior parietal cortex, lateral occipital cortex, brainstem, and dorsal attention regions of the cerebellum62. Second, this network was robust to leave-one-diagnosis out analyses. Third, this network was specific to psychiatric versus neurodegenerative disorders. Fourth, lesion-induced damage to the network correlated with the number of post-lesion psychiatric diagnoses. Finally, this network aligned with neurosurgical ablation targets for psychiatric disorders, suggesting possible therapeutic relevance and generating testable hypotheses for neuromodulation studies.
Our study builds on prior work leveraging neuroimaging, genetic, and phenotypic data to identify commonalities across psychiatric diagnoses6,26,50,51,63–72. An example of this prior work is the ALE meta-analysis by Goodkind et al.50 showing convergent gray matter loss in bilateral insula and anterior cingulate. We reproduced these ALE results, but we also found limitations in terms of sensitivity, specificity, robustness, and correlation with lesion-induced effects. We addressed these limitations by analyzing the same dataset with coordinate network mapping and by leveraging additional datasets. Our findings are consistent with recent work suggesting that coordinate network mapping can identify convergent findings across neuroimaging studies in ways that complement ALE meta-analyses53,54,57. More broadly, our findings suggest that atrophy coordinates across psychiatric diagnoses map better to a brain network than they do to an individual brain region.
Our transdiagnostic network includes positive peaks in the bilateral insula, anterior cingulate, posterior cingulate, and left frontal pole. As expected, many of these positive peaks are consistent with Goodkind et al. and with an ENGIMA consortium study that examined structural variance across six major psychiatric disorders48,49. Our positive peaks are commonly associated with psychiatric illnesses, with hypothesized roles in salience detection, emotion regulation, self-referential processes, and executive function48–50,73–76.
Our transdiagnostic network also includes negative peaks in the posterior parietal cortex, occipital cortex, brainstem, and dorsal attention regions of the cerebellum62. In fact, the strongest overall peak in our network was a negative peak near the intraparietal sulcus in Brodmann Area 7. Our negative peaks are not traditionally associated with psychiatric illnesses. Instead, they are associated with non-specific processes such as visual processing and multisensory integration77–86. However, data-driven transdiagnostic research has increasingly highlighted occipitoparietal regions as well as cerebellum63,72,80–83,87. For example, a data-driven analysis of connectome-wide functional connectivity found an association between higher “p factor” scores and connectivity abnormalities between visual association cortex and frontoparietal networks implicated in executive control and self-referential processes 63. Similarly, an integrated analysis of structural connectomes and single nucleotide polymorphisms highlighted occipital cortex and its links to default mode and cognitive control networks in a “vulnerability network” for psychiatric illness64. There is also evidence from data-driven multimodal neuroimaging that higher “p factor” scores are associated with structural changes within cerebello-thalamo-cortical circuit as well as visual association cortex, although the reproducibility of the cerebellum finding has been slightly less consistent 71,72,87.
Taken together, the positive and negative peaks that emerged from our analyses represent a transdiagnostic network that might be implicated in selective attention and multisensory processing, both of which are important for cognitive control 63,72,80–83,87. This transdiagnostic network cuts across canonical brain networks. There is precedent for looking beyond single canonical brain networks to explain transdiagnostic psychopathology64. For example, the triple network model proposes that general psychopathology is associated with an imbalance between multiple networks (i.e., CEN, DMN, and salience network)88. Similarly, the “vulnerability network” for psychiatric illness mentioned above overlapped with but was not fully encapsulated by canonical networks64. In our study, no single canonical network showed more spatial similarity to our transdiagnostic network than the network generated from an independent lesion dataset (Dataset 3).
In the end, our study answers important questions about if and where psychiatric neuroimaging findings converge, but it does not address why or how these locations contribute to psychiatric illness.
Our transdiagnostic network was derived from gray matter atrophy across psychiatric disorders. Gray matter atrophy is challenging to interpret, especially in psychiatric disorders for which there are few sources of causal information. Even studies that show reversal of gray matter atrophy with successful treatment provide limited causal insights, as morphometric changes could still be correlates or epiphenomena of illness or treatment 57,89–91. An important strength of our study is that we tested correlative findings from a morphometric dataset with brain lesions associated with psychiatric illness in an independent dataset (dataset 3). These tests examined the correlation between lesion location and the number of post-lesion psychiatric diagnoses quantified via SCID. It is possible that some participants had psychiatric illnesses that preceded the brain lesion or the SCID. Similarly, some participants may have had psychiatric illnesses that were causally unrelated to the brain lesions. However, such cases would bias us against the present findings59,92. Our lesion location results were statistically significant despite these sources of noise, highlighting the possibility that the true effect size is larger than what we observed.
Despite its limitations, mapping brain lesions is an important strategy for beginning to address the causality gap in psychiatric neuroimaging60,61,93. Our results highlight the importance of causal sources, as lesion-induced effects on our transdiagnostic network were opposite of what one may have predicted if atrophy was causally linked with greater psychiatric illness or higher psychiatric comorbidity. In our study, atrophy in anterior cingulate and bilateral insula (as well as regions positively connected to them) correlated with transdiagnostic psychiatric illness. This finding alone provides limited insight into how such atrophy should be interpreted. However, brain lesions that intersect anterior cingulate and bilateral insula (as well as regions positively connected to them) correlated with lower psychiatric comorbidity, aligning with neurosurgical ablation targets for psychiatric disorders. Taken together, our results suggest that transdiagnostic gray matter atrophy in anterior cingulate and bilateral insula are not causally related to psychiatric illness. Instead, this atrophy may be a consequence of or a compensation for psychiatric illness. This interpretation is difficult to contextualize because no prior studies leveraged brain lesions to assess transdiagnostic circuitry or comorbidity. The field of epilepsy offers some precedent for interpreting volumetric changes as an effect of a disorder rather than a cause of it. In patients with epilepsy, gray matter atrophy and cortical thinning may be prevented or diminished with resective surgery94–96. Similar models have been proposed for psychiatric disorders, but more data are needed97–99.
Our preliminary results may have therapeutic relevance for neuromodulation. Historically, lesion-based treatments have often targeted the same brain region for different psychiatric diagnoses45–47. Our transdiagnostic network aligns with these lesion-based targets and identifies testable targets for future trials that consider psychiatric comorbidity. For example, our peak near the intraparietal sulcus could be targeted with excitatory TMS in patients with multiple psychiatric disorders. This trial would be justified by mounting evidence that brain lesions can provide the causal insights necessary for treatment target derivation100–102. Our results may also have relevance for medications and psychotherapies that are effective for multiple psychiatric illnesses, but it is challenging to measure their focal effects on brain networks.
There are several limitations to consider. First, this study was retrospective rather than prospective. We intentionally used data from published meta-analyses to minimize selection bias, so future studies are needed to prospectively test our findings. Second, our morphometric dataset (Dataset 1) had no accompanying metadata on demographics, illness severity or duration, medication use, or biopsychosocial variables that might vary across studies and influence morphometric changes. However, these variables should increase the probability of a false negative. Our transdiagnostic network survived multiple statistical tests across multiple datasets despite these sources of noise, highlighting the possibility that the true effect size may be larger than what we observed. Third, we only had access to a single lesion dataset that quantified the number of post-lesion psychiatric diagnoses at a single timepoint (Dataset 3). It is possible that some participants had psychiatric illnesses that preceded the brain lesion or was unrelated to the brain lesion. However, such cases would bias us against the present findings. Future work replicating our findings in additional independent lesion datasets is needed. Fourth, coordinate and lesion network mapping were performed with a normative connectome. Prior work suggests that disorder-specific connectomes do not change results43,103, but future studies could explore this possibility further. Fifth, our study highlights similarities across psychiatric diagnoses, but it does not address differences between them.
In summary, atrophy coordinates across psychiatric disorders mapped to common brain network that was sensitive, specific, robust, and aligned with lesion-induced effects. This network may help explain high rates of psychiatric comorbidity and could highlight neuromodulation targets for patients with psychiatric comorbidities.
Methods
Dataset Overview
We analyzed four independent published datasets in full to minimize selection bias. In each dataset, participants provided informed consent to data collection or the institutional review board approved retrospective analysis of symptom and imaging data.
Dataset 1 was sourced from a published activation likelihood estimation (ALE) meta-analysis of whole-brain voxel-based morphometry studies comparing patients with psychiatric disorders to healthy controls50. The authors excluded neurodevelopmental disorders, personality disorders, patients with neurological comorbidities, and disorders assessed in fewer than 10 studies. These criteria yielded a sample size of 15892 individuals from 193 studies50 covering six diagnostic categories (i.e., schizophrenia, bipolar disorder, depression, addiction, obsessive-compulsive disorder, and anxiety). Each of the 193 studies reported MNI coordinates at which patients with psychiatric disorders had more atrophy than controls.
Dataset 2 was sourced from published neuroimaging studies in patients with Alzheimer’s disease, behavioral variant frontotemporal dementia, corticobasal syndrome, and progressive non-fluent aphasia54. Each of the 72 studies reported a series of coordinates at which patients with neurodegenerative disorders had more atrophy than controls.
Dataset 3 was sourced from the Vietnam Head Injury Study, a multi-decade prospective follow-up study of veterans with and without penetrating head injuries104,105 . Data from 194 veterans who had completed the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID) after a penetrating head injury were analyzed. For each participant, penetrating lesions were localized using head CT and post-lesion psychiatric diagnoses were quantified via SCID. The lesion masks used in this study were identical to those used in prior studies106,107. These lesion masks were created by manual segmentation, spatial normalization to Montreal Neurological Institute (MNI) 152 atlas space, and binarization such that voxels inside the lesion were assigned a value of “1” and voxels outside the lesion were assigned a value of “0”107.
Dataset 4 was sourced from published neurosurgical ablation coordinates for depression, all of which have also been used for multiple psychiatric disorders46.
Activation Likelihood Estimation
First, we mapped atrophy coordinates to common brain regions by replicating the Goodkind et. al.50 results using Dataset 1 and GingerALE108 (10,000 permutations, cluster-forming threshold at voxel-level p < 0.005, cluster-level I-corrected threshold p < 0.05). We assessed sensitivity by quantifying the number of studies contributing to each significant cluster. Next, we assessed specificity by comparing ALE maps for psychiatric disorders (Dataset 1) versus neurodegenerative disorders (Dataset 2) using the Contrast Analysis function in GingerALE (10,000 permutations), which searches for statistically significant differences in convergence between two datasets. We also generated an unthresholded ALE map for analyses requiring whole-brain coverage.
Coordinate Network Mapping
Next, we mapped atrophy coordinates to a common brain network. This network was identified in accordance with previously published methods using custom Python code54. Study-level atrophy maps were created with spherical seeds (4mm radius) centered at each coordinate associated with greater atrophy in patients with psychiatric disorders versus controls. A normative connectome of healthy controls (n=1000)109 was used to generate resting-state functional connectivity maps for each study-level atrophy map (Figure 1B). Resting-state functional connectivity data were processed in accordance with prior work109. A composite t-map was generated from these study-level maps using a voxel-wise one-sample t-test, with Bonferroni correction resulting in a threshold t-value of 5.66 (p<0.05/285,903 voxels = 1.76×10−7). Sensitivity was assessed by combining thresholded (t>5) study-level maps into a composite map depicting the percentage of studies overlapping at each brain voxel. We will refer to this coordinate network overlap map as the “transdiagnostic network.”
To ensure the transdiagnostic network was not dependent on our threshold choice, we repeated our analysis at higher thresholds as in prior work (t>7, t>10)54. We assessed specificity by comparing the resting state functional connectivity maps from psychiatric coordinates (Dataset 1) to similar maps from neurodegenerative coordinates (Dataset 2) using a voxel-wise two-sample t-test and Permutation Analysis of Linear Models (PALM) in FSL110,111, correcting for multiple comparisons using threshold-free cluster enhancement with a voxel-based FWE-corrected p<0.05. Note that this specificity map was generated using unthresholded functional connectivity maps, and thus is independent of the statistical threshold used to generate the overlap map.
Leave-One-Diagnosis-Out Analyses
We assessed the impact of each psychiatric diagnosis on our results by repeating ALE and coordinate network mapping in serial fashion, each time with one psychiatric diagnosis omitted (Dataset 1). Spatial correlation was used to compare each leave-one-diagnosis-out map to the comprehensive ALE map and transdiagnostic network101,107. Two-sample t-tests were used to determine whether coordinate network mapping was more robust than ALE meta-analysis for leave-one-diagnosis out analyses (p < 0.05).
Lesion Network Mapping
We used an independent lesion dataset (Dataset 3) to inform the causal interpretation of the ALE map and our transdiagnostic network (Dataset 1). First, we hypothesized that damage to our transdiagnostic network, but not the ALE map, would correlate with psychiatric comorbidity. To test this hypothesis, we overlayed each lesion onto the ALE map and the transdiagnostic network. The sum of lesion-circumscribed voxel values is considered the “damage score” 107,112. We assessed the correlation between damage score and the number of post-lesion psychiatric diagnoses while controlling for lesion size, GAF, and outliers.
Second, we hypothesized that a network derived from lesions associated with psychiatric disorders (Dataset 3) would align better with the transdiagnostic network than it did with the ALE map (Dataset 1). To test this hypothesis, we computed the whole-brain connectivity of each lesion using a normative connectome. Connections that co-varied with the number of psychiatric diagnoses were identified, generating a lesion network for psychiatric comorbidity. We used permutation testing101 with custom Matlab code to assess the similarity between this lesion network and the ALE map and the transdiagnostic network. Briefly, we recomputed the lesion network 10,000 times by randomly assigning SCID scores to connectivity profiles. Each randomly generated map was compared to the ALE map and the transdiagnostic network via spatial correlation. Significance was defined as greater spatial correlation between real versus randomly permuted maps in at least 95% of instances (p<0.05).
Neurosurgical Ablation Alignment
We tested whether neurosurgical ablation targets for psychiatric disorders aligned better with the transdiagnostic network than they did with the ALE map. We placed 10mm spheres at published target coordinates for anterior capsulotomy, anterior cingulotomy, subcaudate tractotomy, and limbic leucotomy 46. For each sphere, we calculated the “damage score” by summing the lesion-circumscribed voxel values on the ALE map and the transdiagnostic network 107,112. Significance (p < 0.05) was assessed in Excel by comparing this damage score versus zero (via one-sample t-test). We also compared the damage score of each simulated lesion versus the damage score on the neurodegenerative network (via two-sample t-test).
Canonical Network Comparison
We compared the transdiagnostic network to canonical Yeo networks via spatial correlation. This exploratory analysis, run using custom Python code, tested the extent to which the transdiagnostic network was distinct from existing canonical networks.
Supplementary Material
Acknowledgments
We would like to acknowledge the authors of Schoene-Bake et al. 2010 who kindly provided us with simulated lesions from their work. We would also like to thank William Drew and Jing Li for their technical support and Patrick Flynn for his administrative support.
The authors received no specific funding for this work. JJT: Harvard Medical School (Dupont Warren Fellowship Award, Livingston Award), Brain and Behavior Research Foundation Young Investigator Grant (31081), Sidney R. Baer, Jr. Foundation, Baszucki Brain Research Fund, and the NIH (K23MH129829, R01MH113929). CL: none. DT: NIMH T32 fellowship (T32MH020004), Harvard Medical School (Dupont Warren Fellowship Award), MAF: none. FLWVJS: Epilepsy Society (846534). JJ: Brain and Behavior Research Foundation Young Investigator Grant (29441). MG: none. JG: none. AE: none. SS: NIH (K23MH121657, R21MH126271), Brain and Behavior Research Foundation Young Investigator Grant, Neuronetics investigator-initiated grant, Baszucki Brain Research Fund, and Department of Veterans Affairs (I01CX002293). MDF: funded by the Nancy Lurie Marks Foundation, the Kaye Family Research Endowment, Baszucki Brain Research Fund, and the NIH (R01MH113929, R21MH126271, R56AG069086, R01MH115949, and R01AG060987)
Footnotes
Competing Interests
JJT: none. CL: none, DT: none. MAF: none. FLWVJS: none. JJ: none. MG: none. JG: none. AE: salary and equity from Alto Neuroscience, and equity from Mindstrong Health and Akili Interactive. SS: Owner of intellectual property involving the use of brain connectivity to target TMS, scientific consultant for Magnus Medical, investigator-initiated research funding from Neuronetics and Brainsway, speaking fees from Brainsway and Otsuka (for PsychU.org), shareholder in Brainsway (publicly traded) and Magnus Medical (not publicly traded). None of these entities were directly involved in the present work. MDF: Scientific consultant for Magnus Medical, owns independent intellectual property involving the use of functional connectivity to target TMS. This intellectual property was not used in the present manuscript.
Code Availability
GingerALE is publicly available. The custom Matlab and Python code used in this study is available: https://github.com/nimlab/NHB_Taylor2023.
Data Availability
This paper used de-identified data from multiple datasets collected by different investigators at different institutions. Datasets 1 (doi: 10.1001/jamapsychiatry.2014.2206), 2 (doi:10.1093/brain/awy292), and 4 (doi:10.1038/npp.2010.132) are publicly available peer-reviewed publications. Inquiries regarding the Vietnam Head Injury Study (Dataset 3) can be directed to Jordan Grafman, Ph.D. (jgrafman@northwestern.edu). The one-sample t-test transdiagnostic network is available: https://github.com/nimlab/NHB_Taylor2023.
References
- 1.Barch DM What Does It Mean to Be Transdiagnostic and How Would We Know? Am J Psychiatry 177, 370–372, doi: 10.1176/appi.ajp.2020.20030243 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Newman DL, Moffitt TE, Caspi A & Silva PA Comorbid mental disorders: implications for treatment and sample selection. J Abnorm Psychol 107, 305–311, doi: 10.1037//0021-843x.107.2.305 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR & Walters EE Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62, 617–627, doi: 10.1001/archpsyc.62.6.617 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein-Piekarski AN, Williams LM & Humphreys K A trans-diagnostic review of anxiety disorder comorbidity and the impact of multiple exclusion criteria on studying clinical outcomes in anxiety disorders. Transl Psychiatry 6, e847, doi: 10.1038/tp.2016.108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspi A & Moffitt TE All for One and One for All: Mental Disorders in One Dimension. Am J Psychiatry 175, 831–844, doi: 10.1176/appi.ajp.2018.17121383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grisanzio KA et al. Transdiagnostic Symptom Clusters and Associations With Brain, Behavior, and Daily Function in Mood, Anxiety, and Trauma Disorders. JAMA Psychiatry 75, 201–209, doi: 10.1001/jamapsychiatry.2017.3951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspi A et al. Longitudinal Assessment of Mental Health Disorders and Comorbidities Across 4 Decades Among Participants in the Dunedin Birth Cohort Study. JAMA Netw Open 3, e203221, doi: 10.1001/jamanetworkopen.2020.3221 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman SE The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol 6, 155–179, doi: 10.1146/annurev.clinpsy.3.022806.091532 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51, 8–19, doi: 10.1001/archpsyc.1994.03950010008002 (1994). [DOI] [PubMed] [Google Scholar]
- 10.McGrath JJ et al. Comorbidity within mental disorders: a comprehensive analysis based on 145 990 survey respondents from 27 countries. Epidemiol Psychiatr Sci 29, e153, doi: 10.1017/S2045796020000633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plana-Ripoll O et al. Nature and prevalence of combinations of mental disorders and their association with excess mortality in a population-based cohort study. World Psychiatry 19, 339–349, doi: 10.1002/wps.20802 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plana-Ripoll O et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet 394, 1827–1835, doi: 10.1016/S0140-6736(19)32316-5 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Fridell M et al. Prediction of psychiatric comorbidity on premature death in a cohort of patients with substance use disorders: a 42-year follow-up. BMC Psychiatry 19, 150, doi: 10.1186/s12888-019-2098-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trivedi MH et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163, 28–40, doi: 10.1176/appi.ajp.163.1.28 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Saravay SM & Lavin M Psychiatric comorbidity and length of stay in the general hospital. A critical review of outcome studies. Psychosomatics 35, 233–252, doi: 10.1016/S0033-3182(94)71772-2 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Mojtabai R & Olfson M National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch Gen Psychiatry 67, 26–36, doi: 10.1001/archgenpsychiatry.2009.175 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Sareen J et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry 62, 1249–1257, doi: 10.1001/archpsyc.62.11.1249 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Stein DJ Comorbidity in generalized anxiety disorder: impact and implications. J Clin Psychiatry 62 Suppl 11, 29–34; discussion 35–26 (2001). [PubMed] [Google Scholar]
- 19.Wittchen HU, Zhao S, Kessler RC & Eaton WW DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Arch Gen Psychiatry 51, 355–364, doi: 10.1001/archpsyc.1994.03950050015002 (1994). [DOI] [PubMed] [Google Scholar]
- 20.Cacciola JS, Alterman AI, Rutherford MJ, McKay JR & Mulvaney FD The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend 61, 271–280, doi: 10.1016/s0376-8716(00)00148-4 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Souery D et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry 68, 1062–1070, doi: 10.4088/jcp.v68n0713 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Maj M “Psychiatric comorbidity”: an artefact of current diagnostic systems? Br J Psychiatry 186, 182–184, doi: 10.1192/bjp.186.3.182 (2005). [DOI] [PubMed] [Google Scholar]
- 23.van Loo HM, Romeijn JW, de Jonge P & Schoevers RA Psychiatric comorbidity and causal disease models. Prev Med 57, 748–752, doi: 10.1016/j.ypmed.2012.10.018 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Caspi A et al. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clin Psychol Sci 2, 119–137, doi: 10.1177/2167702613497473 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore TM et al. Development of a computerized adaptive screening tool for overall psychopathology (“p”). J Psychiatr Res 116, 26–33, doi: 10.1016/j.jpsychires.2019.05.028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprooten E, Franke B & Greven CU The P-factor and its genomic and neural equivalents: an integrated perspective. Mol Psychiatry 27, 38–48, doi: 10.1038/s41380-021-01031-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried EI, Greene AL & Eaton NR The p factor is the sum of its parts, for now. World Psychiatry 20, 69–70, doi: 10.1002/wps.20814 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J et al. Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am J Psychiatry 167, 1254–1263, doi: 10.1176/appi.ajp.2010.09091335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross-Disorder Group of the Psychiatric Genomics, C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381, 1371–1379, doi: 10.1016/S0140-6736(12)62129-1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brainstorm C et al. Analysis of shared heritability in common disorders of the brain. Science 360, doi: 10.1126/science.aap8757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross-Disorder Group of the Psychiatric Genomics, C. et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 45, 984–994, doi: 10.1038/ng.2711 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders SJ et al. Whole genome sequencing in psychiatric disorders: the WGSPD consortium. Nat Neurosci 20, 1661–1668, doi: 10.1038/s41593-017-0017-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeffding LK et al. Risk of Psychiatric Disorders Among Individuals With the 22q11.2 Deletion or Duplication: A Danish Nationwide, Register-Based Study. JAMA Psychiatry 74, 282–290, doi: 10.1001/jamapsychiatry.2016.3939 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address, p. m. h. e. & Cross-Disorder Group of the Psychiatric Genomics, C. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell 179, 1469–1482 e1411, doi: 10.1016/j.cell.2019.11.020 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyman SE New Evidence for Shared Risk Architecture of Mental Disorders. JAMA Psychiatry 76, 235–236, doi: 10.1001/jamapsychiatry.2018.4269 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Plana-Ripoll O et al. Exploring Comorbidity Within Mental Disorders Among a Danish National Population. JAMA Psychiatry 76, 259–270, doi: 10.1001/jamapsychiatry.2018.3658 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahey BB et al. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol 121, 971–977, doi: 10.1037/a0028355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark LA, Watson D & Reynolds S Diagnosis and classification of psychopathology: challenges to the current system and future directions. Annu Rev Psychol 46, 121–153, doi: 10.1146/annurev.ps.46.020195.001005 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID & Zald DH A hierarchical causal taxonomy of psychopathology across the life span. Psychol Bull 143, 142–186, doi: 10.1037/bul0000069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krueger RF & Eaton NR Transdiagnostic factors of mental disorders. World Psychiatry 14, 27–29, doi: 10.1002/wps.20175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzgerald PB Targeting repetitive transcranial magnetic stimulation in depression: do we really know what we are stimulating and how best to do it? Brain Stimul 14, 730–736, doi: 10.1016/j.brs.2021.04.018 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Siddiqi SH, Weigand A, Pascual-Leone A & Fox MD Identification of Personalized Transcranial Magnetic Stimulation Targets Based on Subgenual Cingulate Connectivity: An Independent Replication. Biol Psychiatry, doi: 10.1016/j.biopsych.2021.02.015 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Siddiqi SH et al. Distinct Symptom-Specific Treatment Targets for Circuit-Based Neuromodulation. Am J Psychiatry 177, 435–446, doi: 10.1176/appi.ajp.2019.19090915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cash RFH et al. Using Brain Imaging to Improve Spatial Targeting of Transcranial Magnetic Stimulation for Depression. Biol Psychiatry, doi: 10.1016/j.biopsych.2020.05.033 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Stelten BM, Noblesse LH, Ackermans L, Temel Y & Visser-Vandewalle V The neurosurgical treatment of addiction. Neurosurg Focus 25, E5, doi: 10.3171/FOC/2008/25/7/E5 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Schoene-Bake JC et al. Tractographic analysis of historical lesion surgery for depression. Neuropsychopharmacology 35, 2553–2563, doi: 10.1038/npp.2010.132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel SR, Aronson JP, Sheth SA & Eskandar EN Lesion procedures in psychiatric neurosurgery. World Neurosurg 80, S31 e39–16, doi: 10.1016/j.wneu.2012.11.038 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Opel N et al. Cross-Disorder Analysis of Brain Structural Abnormalities in Six Major Psychiatric Disorders: A Secondary Analysis of Mega- and Meta-analytical Findings From the ENIGMA Consortium. Biol Psychiatry 88, 678–686, doi: 10.1016/j.biopsych.2020.04.027 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Eckstrand KL Shared Versus Disorder-Specific Brain Morphometric Features of Major Psychiatric Disorders in Adulthood. Biol Psychiatry 88, e41–e43, doi: 10.1016/j.biopsych.2020.07.015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodkind M et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315, doi: 10.1001/jamapsychiatry.2014.2206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McTeague LM et al. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am J Psychiatry 174, 676–685, doi: 10.1176/appi.ajp.2017.16040400 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitelman SA Transdiagnostic neuroimaging in psychiatry: A review. Psychiatry Res 277, 23–38, doi: 10.1016/j.psychres.2019.01.026 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Zhukovsky P et al. Coordinate-Based Network Mapping of Brain Structure in Major Depressive Disorder in Younger and Older Adults: A Systematic Review and Meta-Analysis. Am J Psychiatry, appiajp202121010088, doi: 10.1176/appi.ajp.2021.21010088 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Darby RR, Joutsa J & Fox MD Network localization of heterogeneous neuroimaging findings. Brain 142, 70–79, doi: 10.1093/brain/awy292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weil RS, Hsu JK, Darby RR, Soussand L & Fox MD Neuroimaging in Parkinson’s disease dementia: connecting the dots. Brain Commun 1, fcz006, doi: 10.1093/braincomms/fcz006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fox MD Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med 379, 2237–2245, doi: 10.1056/NEJMra1706158 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Taylor JJ, Siddiqi SH & Fox MD Coordinate Network Mapping: An Emerging Approach for Morphometric Meta-Analysis. Am J Psychiatry 178, 1080–1081, doi: 10.1176/appi.ajp.2021.21100987 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eickhoff SB et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30, 2907–2926, doi: 10.1002/hbm.20718 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siddiqi SH, Kording KP, Parvizi J & Fox MD Causal mapping of human brain function. Nat Rev Neurosci 23, 361–375, doi: 10.1038/s41583-022-00583-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Etkin A Addressing the Causality Gap in Human Psychiatric Neuroscience. JAMA Psychiatry, doi: 10.1001/jamapsychiatry.2017.3610 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Siddiqi SH, Kording NP, Parvizi J & Fox MD Causal mapping of human brain function. Nature Reviews Neuroscience in press (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buckner RL, Krienen FM, Castellanos A, Diaz JC & Yeo BT The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106, 2322–2345, doi: 10.1152/jn.00339.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elliott ML, Romer A, Knodt AR & Hariri AR A Connectome-wide Functional Signature of Transdiagnostic Risk for Mental Illness. Biol Psychiatry 84, 452–459, doi: 10.1016/j.biopsych.2018.03.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taquet M et al. A structural brain network of genetic vulnerability to psychiatric illness. Mol Psychiatry 26, 2089–2100, doi: 10.1038/s41380-020-0723-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheffield JM et al. Transdiagnostic Associations Between Functional Brain Network Integrity and Cognition. JAMA Psychiatry 74, 605–613, doi: 10.1001/jamapsychiatry.2017.0669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barch DM The Neural Correlates of Transdiagnostic Dimensions of Psychopathology. Am J Psychiatry 174, 613–615, doi: 10.1176/appi.ajp.2017.17030289 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Sharma A et al. Common Dimensional Reward Deficits Across Mood and Psychotic Disorders: A Connectome-Wide Association Study. Am J Psychiatry 174, 657–666, doi: 10.1176/appi.ajp.2016.16070774 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McTeague LM, Goodkind MS & Etkin A Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res 83, 37–46, doi: 10.1016/j.jpsychires.2016.08.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romer AL et al. Pervasively Thinner Neocortex as a Transdiagnostic Feature of General Psychopathology. Am J Psychiatry 178, 174–182, doi: 10.1176/appi.ajp.2020.19090934 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McTeague LM et al. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. Am J Psychiatry 177, 411–421, doi: 10.1176/appi.ajp.2019.18111271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romer AL et al. Replicability of structural brain alterations associated with general psychopathology: evidence from a population-representative birth cohort. Mol Psychiatry 26, 3839–3846, doi: 10.1038/s41380-019-0621-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romer AL et al. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol Psychiatry 23, 1084–1090, doi: 10.1038/mp.2017.57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamilton JP et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 169, 693–703, doi: 10.1176/appi.ajp.2012.11071105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gursel DA, Avram M, Sorg C, Brandl F & Koch K Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev 87, 151–160, doi: 10.1016/j.neubiorev.2018.01.016 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Patel R, Spreng RN, Shin LM & Girard TA Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36, 2130–2142, doi: 10.1016/j.neubiorev.2012.06.003 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Peters SK, Dunlop K & Downar J Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci 10, 104, doi: 10.3389/fnsys.2016.00104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rushworth MF, Paus T & Sipila PK Attention systems and the organization of the human parietal cortex. J Neurosci 21, 5262–5271 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnsten AF & Rubia K Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry 51, 356–367, doi: 10.1016/j.jaac.2012.01.008 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Lee KH et al. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage 29, 578–586, doi: 10.1016/j.neuroimage.2005.07.036 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Moran J & Desimone R Selective attention gates visual processing in the extrastriate cortex. Science 229, 782–784, doi: 10.1126/science.4023713 (1985). [DOI] [PubMed] [Google Scholar]
- 81.Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J & Fischer C Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex 15, 654–662, doi: 10.1093/cercor/bhh167 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Karten A, Pantazatos SP, Khalil D, Zhang X & Hirsch J Dynamic coupling between the lateral occipital-cortex, default-mode, and frontoparietal networks during bistable perception. Brain Connect 3, 286–293, doi: 10.1089/brain.2012.0119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grill-Spector K, Kourtzi Z & Kanwisher N The lateral occipital complex and its role in object recognition. Vision Res 41, 1409–1422, doi: 10.1016/s0042-6989(01)00073-6 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Plewan T, Weidner R, Eickhoff SB & Fink GR Ventral and dorsal stream interactions during the perception of the Muller-Lyer illusion: evidence derived from fMRI and dynamic causal modeling. J Cogn Neurosci 24, 2015–2029, doi: 10.1162/jocn_a_00258 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Vander Wyk BC et al. Cortical integration of audio-visual speech and non-speech stimuli. Brain Cogn 74, 97–106, doi: 10.1016/j.bandc.2010.07.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naumer MJ et al. Visuohaptic convergence in a corticocerebellar network. Eur J Neurosci 31, 1730–1736, doi: 10.1111/j.1460-9568.2010.07208.x (2010). [DOI] [PubMed] [Google Scholar]
- 87.Moberget T et al. Cerebellar Gray Matter Volume Is Associated With Cognitive Function and Psychopathology in Adolescence. Biol Psychiatry 86, 65–75, doi: 10.1016/j.biopsych.2019.01.019 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Menon V Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15, 483–506, doi: 10.1016/j.tics.2011.08.003 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Arnone D et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry 18, 1265–1272, doi: 10.1038/mp.2012.150 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Rashidi-Ranjbar N et al. Frontal-executive and corticolimbic structural brain circuitry in older people with remitted depression, mild cognitive impairment, Alzheimer’s dementia, and normal cognition. Neuropsychopharmacology 45, 1567–1578, doi: 10.1038/s41386-020-0715-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nuninga JO, Mandl RCW & Sommer IEC Clinical Relevance of Brain Changes After Electroconvulsive Therapy: Is There Really No Link at All? Biol Psychiatry 89, e13–e14, doi: 10.1016/j.biopsych.2020.04.030 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Marinescu IE, Lawlor PN & Kording KP Quasi-experimental causality in neuroscience and behavioural research. Nat Hum Behav 2, 891–898, doi: 10.1038/s41562-018-0466-5 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Etkin A A Reckoning and Research Agenda for Neuroimaging in Psychiatry. Am J Psychiatry 176, 507–511, doi: 10.1176/appi.ajp.2019.19050521 (2019). [DOI] [PubMed] [Google Scholar]
- 94.Lariviere S et al. Network-based atrophy modeling in the common epilepsies: A worldwide ENIGMA study. Sci Adv 6, doi: 10.1126/sciadv.abc6457 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whelan CD et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 141, 391–408, doi: 10.1093/brain/awx341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galovic M et al. Resective surgery prevents progressive cortical thinning in temporal lobe epilepsy. Brain 143, 3262–3272, doi: 10.1093/brain/awaa284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keramatian K, Su W, Saraf G, Chakrabarty T & Yatham LN Preservation of Gray Matter Volume in Early Stage of Bipolar Disorder: A Case for Early Intervention: Preservation du volume de matiere grise au stade precoce du trouble bipolaire: un cas pour intervention precoce. Can J Psychiatry 66, 139–146, doi: 10.1177/0706743720927827 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moylan S, Maes M, Wray NR & Berk M The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18, 595–606, doi: 10.1038/mp.2012.33 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Fineberg NA et al. Early intervention for obsessive compulsive disorder: An expert consensus statement. Eur Neuropsychopharmacol 29, 549–565, doi: 10.1016/j.euroneuro.2019.02.002 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Ganos C et al. A neural network for tics: insights from causal brain lesions and deep brain stimulation. Brain, doi: 10.1093/brain/awac009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siddiqi SH et al. Convergent causal mapping of human neuropsychiatric symptoms using brain stimulation and brain lesions. Nature Human Behavior, in press. (2021). [Google Scholar]
- 102.Reich MM et al. A brain network for deep brain stimulation induced cognitive decline in Parkinson’s disease. Brain, doi: 10.1093/brain/awac012 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weigand A et al. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol Psychiatry 84, 28–37, doi: 10.1016/j.biopsych.2017.10.028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grafman J, Salazar AM, Weingartner H & Amin D Face memory and discrimination: an analysis of the persistent effects of penetrating brain wounds. Int J Neurosci 29, 125–139, doi: 10.3109/00207458608985643 (1986). [DOI] [PubMed] [Google Scholar]
- 105.Raymont V, Salazar AM, Krueger F & Grafman J “Studying injured minds” - the Vietnam head injury study and 40 years of brain injury research. Front Neurol 2, 15, doi: 10.3389/fneur.2011.00015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koenigs M et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci 11, 232–237, doi: 10.1038/nn2032 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Padmanabhan JL et al. A Human Depression Circuit Derived From Focal Brain Lesions. Biol Psychiatry, doi: 10.1016/j.biopsych.2019.07.023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eickhoff SB, Bzdok D, Laird AR, Kurth F & Fox PT Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361, doi: 10.1016/j.neuroimage.2011.09.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yeo BT et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165, doi: 10.1152/jn.00338.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW & Smith SM Fsl. Neuroimage 62, 782–790, doi: 10.1016/j.neuroimage.2011.09.015 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Winkler AM, Ridgway GR, Webster MA, Smith SM & Nichols TE Permutation inference for the general linear model. Neuroimage 92, 381–397, doi: 10.1016/j.neuroimage.2014.01.060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferguson MA et al. A human memory circuit derived from brain lesions causing amnesia. Nat Commun 10, 3497, doi: 10.1038/s41467-019-11353-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper used de-identified data from multiple datasets collected by different investigators at different institutions. Datasets 1 (doi: 10.1001/jamapsychiatry.2014.2206), 2 (doi:10.1093/brain/awy292), and 4 (doi:10.1038/npp.2010.132) are publicly available peer-reviewed publications. Inquiries regarding the Vietnam Head Injury Study (Dataset 3) can be directed to Jordan Grafman, Ph.D. (jgrafman@northwestern.edu). The one-sample t-test transdiagnostic network is available: https://github.com/nimlab/NHB_Taylor2023.


