Abstract
Three pathologic processes are characteristic of Alzheimer disease (AD): β-amyloid, hyperphosphorylated tau, and neurodegeneration. Our understanding of AD is undergoing a transformation due to our ability to measure biomarkers of these processes across different stages of cognitive impairment. There is growing interest in using AD biomarker tests in care and research and, with this, a growing need for guidance on how to return these sensitive results to patients and participants. Here, we propose a 5-step approach informed by clinical and research experience designing and implementing AD biomarker disclosure processes, extant evidence describing how individuals react to AD biomarker information, ethics, law, and the literature on breaking bad news. The clinician should (1) determine the appropriateness of AD biomarker testing and return of results for the particular patient or research participant. If testing is appropriate, the next steps are to (2) provide pretest education and seek consent for testing from the individual and their support person, (3) administer testing, (4) return the results to the individual and their support person, and (5) follow-up to promote the recipient's well-being.
Until quite recently, many clinicians refrained from disclosing diagnoses of dementia to patients. This reflected a desire to protect patients from adverse psychological effects, such as “depressive illness, suicide, or catastrophic reaction.”1 Yet, studies did not support this practice. Most patients wished to be fully informed, and if they were informed, learning their dementia diagnosis generally did not cause the harms feared.2 These data, together with the ability to prescribe treatments such as acetylcholinesterase inhibitors and to provide long-term care services and supports, led to a change in practice. Patients ought to be told a diagnosis of dementia, and disclosure protocols have since been developed.
There are parallels between the history of dementia disclosure and the current state of Alzheimer disease (AD) biomarker disclosure. Questions about the appropriateness of biomarker disclosure are giving way to a recognition that disclosure is generally safe and that many individuals want to know their results. Disease-modifying treatments will soon available, making biomarker information more medically actionable and also spurring widespread availability of biomarker testing. All this has resulted in growing interest in disclosing AD biomarkers in both clinical and research contexts while also highlighting the need for guidance on how to disclose them with due scientific and ethical care.
This article outlines a 5-step disclosure process: assessing the appropriateness of testing for a particular individual and, if it is appropriate, delivering pretest education and seeking consent; administering testing; returning results; and providing for follow-up. In developing this disclosure process, we have drawn on our own experience designing and implementing AD biomarker disclosure processes, as well as others' disclosure recommendations; available evidence describing how individuals react to learning AD biomarker results; legal and ethical considerations salient to AD biomarkers; and insights from the literature on breaking bad news to patients.
Overview of AD Biomarkers
Three pathologic processes define AD: deposition of β-amyloid (Aβ) fibrillar plaques, accumulation of hyperphosphorylated tau-based neurofibrillary tangles, and neuronal and synaptic degeneration or neurodegeneration.3 Biomarkers for these 3 processes can be measured using various modalities, including PET, CSF assays, and MRI. These modalities differ in important ways. Measures of molecular pathology can indicate the pathology's presence or absence or its regional distributions and overall load. Biomarkers of neurodegeneration are not specific to AD, although their regional distribution can suggest AD.
AD biomarkers can be applied across different stages of cognitive impairment. They can explain whether AD is the likely cause of a person's dementia or mild cognitive impairment (MCI). Biomarkers can also provide risk stratification: their presence increases the likelihood of progression from MCI to dementia. Because AD neuropathology can be present years or even decades before the onset of measurable cognitive impairment, biomarkers can be measured in people who have cognitive complaints but continue to perform normally on cognitive testing—that is, those with subjective cognitive impairment—as well as in those who are unimpaired and asymptomatic. For these persons, biomarker tests inform their risk of future cognitive impairment caused by AD; presently, however, we lack a precise understanding of what AD biomarkers mean for the individual.
To date, access to AD biomarker testing has, outside of research, been limited. The confluence of reasons for this includes the burdens of testing for individuals, the specialized resources needed to conduct testing or the expertise needed to interpret results, and the substantial expense coupled with a lack of insurance coverage.3 Recent advances in blood-based AD biomarker tests will likely overcome many of these barriers. Some blood-based tests are already commercially available, although there are ongoing discussions about appropriate use.4 When disease-modifying therapies for AD become widely available, biomarker testing will likely be used to inform identification of individuals for whom therapy is indicated and so will be covered by insurers. Lowered barriers to testing and increased medical actionability of results will speed uptake of AD biomarker testing, including in clinical settings beyond specialized memory centers.
Overview of AD Biomarker Disclosure
The prospect of widespread AD biomarker testing—and, with it, widespread disclosure of results—lends importance to the project of understanding disclosure's effects and of developing best-practices.
AD biomarker results, typically Aβ PET scan results, have been disclosed in research settings—both in clinical trials where their presence is an eligibility criterion and in longitudinal cohort studies.5 Researchers have developed protocols for disclosing Aβ PET scan results to either cognitively unimpaired or cognitively impaired individuals.6-8 These protocols consistently incorporate practices intended to ensure participant understanding, communicate results effectively, and promote participant safety.
Trials and studies incorporating AD biomarker disclosure have offered opportunities to discover disclosure's effects on older adults and their family members.9-11 A limitation of this research is that participants in studies of disclosure—like participants in AD research more broadly—are not representative. There is an urgent need to study disclosure in broader populations.
Studies have addressed several concerns that loomed over disclosure.12-16 With adequate pretest and posttest education, individuals generally recall and understand the meaning of their AD biomarker results.17,18 Furthermore, education and psychological screening allow for safe delivery of these results. Individuals who learn they have “elevated” Aβ, indicative of AD neuropathology, are no more likely than those receiving a “not elevated” result to experience clinically significant anxiety, depression, or suicidality.10,19
Learning AD biomarker results does, however, have an emotional effect. Cognitively unimpaired persons who learn they have “elevated” Aβ have increased scores on the Impact of Events Scale (IES), a measure of distress caused by traumatic events.10 By comparison, cognitively unimpaired individuals who learn a “not elevated” result almost uniformly express relief.9,20 Some cognitively impaired individuals experience emotional distress after learning they have “elevated” Aβ, as reflected in increased IES scores.19 Persons with subjective cognitive impairment or MCI who receive “not elevated” results may express frustration because they lack an explanation for their cognitive complaints; others experience relief, given the reduced likelihood of symptom progression.9
Cognitively unimpaired adults use AD biomarker results to shape future plans and may adopt new behaviors to promote brain health.6 Individuals with MCI or mild-to-moderate dementia may benefit from a conclusion of their diagnostic odyssey. AD biomarker testing may avert delayed and missed diagnoses as well as imprecise medical management. Even at the dementia stage, etiologic misdiagnosis is common.21 AD biomarker results can thus provide valuable diagnostic and prognostic information and assist cognitively impaired adults and their families in future planning.22
Family members' emotional reactions to AD biomarker results typically parallel the reactions of the older adult who undergoes testing, and family members use the results to inform their own future plans.11,20 For instance, some anticipate and prepare for caregiving responsibilities.11 Clinicians use biomarkers to more confidently deliver diagnoses, reduce additional or unnecessary testing, change diagnoses when AD biomarkers conflict with initial clinical impressions, and instruct treatment choices.23-25
Commonalities across published disclosure protocols and empirical insights into the effects of AD biomarker disclosure, as well as recognition of ethical and legal challenges with particular salience to AD biomarkers, informed the development of our 5-step AD biomarker disclosure process, which we turn to next.
AD Biomarker Disclosure Process
Several preconditions and assumptions frame this process, which is summarized in the Figure. First, a knowledgeable clinician will disclose AD biomarker results. At minimum, this clinician should have facility discussing cognitive impairment, disease staging, and AD biomarkers, and also confidence interpreting and contextualizing biomarker results.26
Figure. The 5-Step AD Biomarker Disclosure Process.
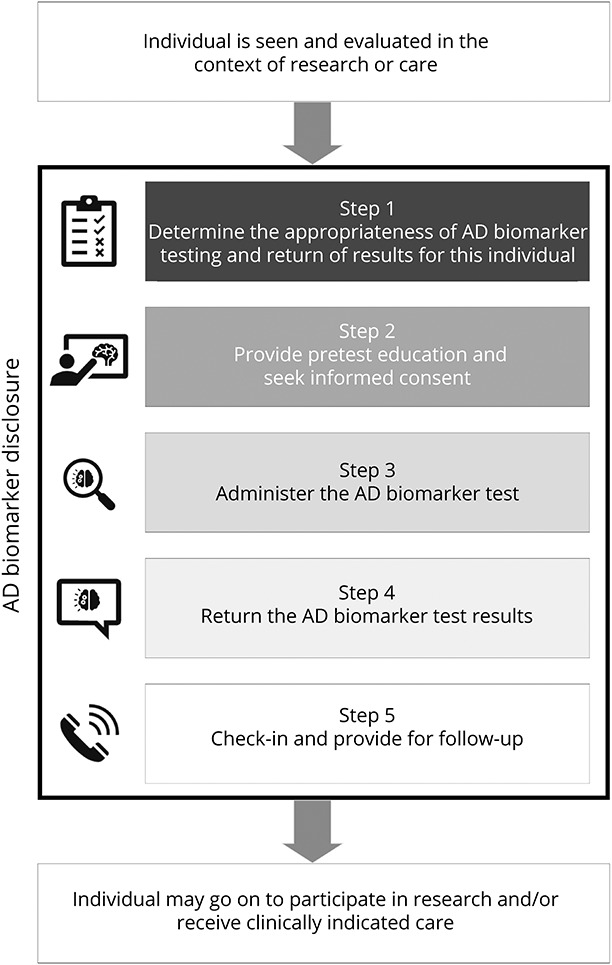
This disclosure process is to be led by a knowledgeable clinician and embedded within the individual's ongoing participation in research or receipt of care, which is understood to include cognitive testing and capacity assessment. AD = Alzheimer disease.
Second, AD biomarker disclosure is just 1 aspect of an individual's participation in research or receipt of care. This process therefore assumes that cognitive testing—and any necessary clinical assessment—has already been completed when a clinician contemplates biomarker testing. Adjustments to the disclosure process are warranted to account for an individual's cognitive stage.
A closely related consideration is whether the individual has decision-making capacity.27 Capacity is not the same as cognition; however, the presence of cognitive impairment suggests that capacity may also be impaired and should prompt a capacity assessment. If the clinician determines that the individual lacks decision-making capacity, a surrogate decision maker should be prospectively identified and included throughout the disclosure process.
Finally, the clinician should recommend that a family member, close friend, or other member of an individual's support network (hereafter, “support person”) be included in the AD biomarker disclosure process.28 A support person's inclusion is likely to enhance the diagnostic process, support decision making, and facilitate the steps after disclosure. Nevertheless, some individuals, particularly those who are cognitively unimpaired or have MCI, may decline because they fear stigma or a loss of privacy.29
Step 1: Determine Appropriateness of AD Biomarker Testing and Return of Results for This Individual
The ordering clinician must determine whether it is appropriate to offer AD biomarker testing to any given individual. Determination of appropriateness inherently has a “case-by-case” quality because features of the specific test, the clinical or research context, and the individual's circumstances will vary and merit consideration.
Cognitively Unimpaired, With or Without Cognitive Complaints
Biomarker testing is not indicated in the clinical care of cognitively unimpaired individuals.30 This position will evolve with the approval of a disease-modifying therapy that makes a diagnosis of preclinical AD—that is, when AD biomarkers are present in individuals who have no measurable cognitive impairment—medically actionable and with a greater understanding of the implications of these results for individuals.
For now, disclosure of AD biomarkers to cognitively unimpaired individuals should be limited to research, the design of which allows for discoveries about the effect of learning the result. In interventional studies that assign treatment to persons who are biomarker-positive, disclosure of biomarker results allows for an efficient study. It also protects biomarker-negative individuals from the risks of study participation and contributes to knowledge about the experience of being biomarker-positive.15 In longitudinal cohort studies, such as those tracking the progression of disease using biomarkers, disclosures should be accompanied by assessments of the effects on individuals and their support persons.
Cognitively Impaired: MCI or Dementia
For individuals with MCI or dementia who desire to know their AD biomarker results, disclosure can permissibly be offered in both clinical and research contexts. Even when biomarker disclosure is undertaken as a clinical activity to inform diagnosis or care, there may be value in conducting research to understand how individuals react to and use their results or in referring them to other relevant research opportunities.
After considering the individual's cognitive stage and the potential utility of AD biomarker testing and before recommending the test, the clinician should assess the individual's psychological readiness to receive the result. This assessment includes an evaluation of the individual's (and, as appropriate, their support person's) current thinking around their condition. The clinician should consider depression, suicidality, and presence of recent or ongoing significant life stressors.6 Individuals may be candidates for AD biomarker testing even if they are experiencing depression or anxiety. In fact, they may be particularly good candidates if these psychiatric symptoms are judged to be the consequence of a neurodegenerative disease.31 If serious psychiatric symptoms are identified, the clinician should offer care as necessary and exercise clinical judgment as to whether to delay or abandon biomarker testing.
Step 2: Provide Pretest Education and Seek Informed Consent
The aim of pretest education is to ensure individuals understand the strengths and limitations of the proposed AD biomarker test: what it can and—as important—what it cannot do. For example, can it offer prognostic information about the timing or rate of decline? The discussion of strengths and limitations will differ depending on which AD biomarkers are being tested and on the testing modality. Education should address possible results and their implications for care or research.
Furthermore, education should address the results' implications for the individual's life, broadly construed. These include their utility for future planning but also their potential, if shared, to precipitate stigma or discrimination. Individuals, particularly cognitively unimpaired individuals, ought to be told about and understand the risks—such as the possibility for discrimination by insurers, employers, or continuing care communities—and the gaps in existing legal protections for AD biomarker results.32,33 Prospective research participants should understand whether these results will be part of their medical record (or kept in a research record) and the consequences of a loss of confidentiality.34
Table 1 provides exemplary prompts for introducing these topics and for assessing individuals' understanding (their knowledge of facts) and appreciation (how they apply these facts to themselves). These are suggestions and should be tailored accordingly.
Table 1.
Pretest Education
The pretest education process culminates in the clinician seeking the individual's voluntary informed consent—or informed refusal—for biomarker testing. If the individual lacks capacity, the clinician must identify their surrogate decision maker; seek that surrogate's permission; and, if appropriate, seek the individual's assent or dissent.35
In select instances, an individual may previously have consented to and undergone AD biomarker testing in the course of research—for example, as part of a longitudinal cohort study—but their result went unreturned.30 The clinician-investigator may now be contemplating return of results. Step 1 and Step 2 should still be followed before proceeding to Step 4. In such cases, it is important to frame the result as “a snapshot in time” and note it may have since changed.
Step 3: Administer the AD Biomarker Test
It may be desirable to perform the test on a different day than the pretest education and consent. This grants individuals time to reflect and, potentially, change their mind.23 A pause may be more beneficial for some individuals than others and so, like the appropriateness of testing, should be determined case-by-case. For example, an individual with MCI—given the prognostic implications and, perhaps, greater emotional significance of biomarker results—may have more need for a pause than an individual with dementia.
Individuals should know when to expect their result. It may be useful to schedule a return-of-results visit at the time of testing. In the United States, the 21st Century Cures Act requires, among other things, that clinical test results should rapidly be made available to patients.36 Clinicians should be aware that individuals who undergo biomarker testing in a clinical context might have access to AD biomarker results through their electronic medical record before the return-of-results visit. If this is a possibility, the clinician ought to discuss with the individual and support person at the time of testing how they want to first learn the result (i.e., self-mediated through the electronic medical record or clinician-mediated in a visit).
Step 4: Return the AD Biomarker Results
The time between testing and the return of AD biomarker results allows for additional reflection. Therefore, before returning results, it is important for the clinician to ascertain the individual's continued desire to learn them.
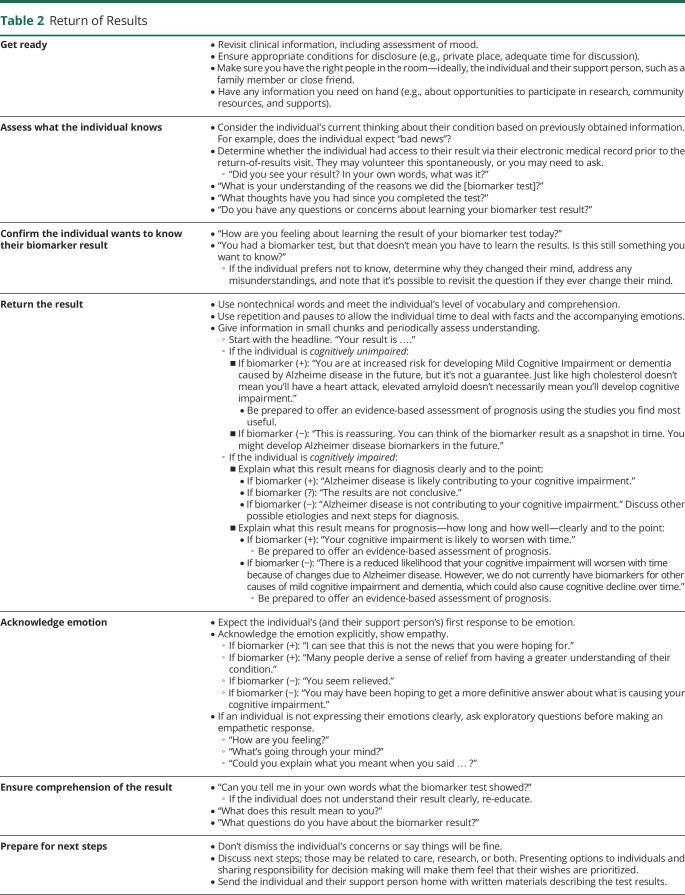
After sharing the “headline,” the clinician should explain the result's meaning and discuss next steps with the individual and their support person. Table 2 includes prompts for returning AD biomarker results, which may feel familiar to clinicians who have experience disclosing other sensitive health information.
Table 2.
Return of Results
Some AD biomarker results will be perceived by individuals and support persons as “bad news.” How bad news is discussed can affect an individual's comprehension, satisfaction, and their subsequent psychological adjustment.37 Therefore, the prompts reflect established methods for breaking bad news. There are 4 essential goals of disclosing bad news: (1) gather information from the patient, (2) provide information in accordance with the patient's needs and desires, (3) support the patient, and (4) form a treatment plan with the patient's input and cooperation.37
As noted above, some individuals may have seen their AD biomarker result before the return-of-results visit through their electronic medical record. (Or, they may have learned the result in a different context and are now seeking the clinician's opinion.38) If the individual has previously learned their result, the clinician can ask the individual to relay it in their own words; what they say will inform the subsequent dialogue.
Cognitively Unimpaired, With or Without Cognitive Complaints
Individuals who are cognitively unimpaired or who have subjective cognitive complaints will be interested in learning their risk for developing cognitive impairment caused by AD. The AD field is still developing risk prediction tools and does not have any widely accepted tools akin to what is available, for example, for heart disease or hip fracture. Nevertheless, studies offer some risk-prediction data. Clinicians should identify the studies most relevant to their participant population and be prepared to discuss them. Emphasizing uncertainty—for instance, that AD biomarkers cannot speak to onset, rate of progression, or prognosis—is important when returning results to cognitively unimpaired individuals.
Because AD biomarker testing for this population should only occur in a research context, the discussion should also address how, if at all, the result affects their participation in research—for example, their eligibility to participate in a clinical trial.
Cognitively Impaired: MCI or Dementia
Whether AD biomarker disclosure occurs in the course of care or research, it is important to discuss the result's implications for clinical care with cognitively impaired individuals.
If the biomarker results—consistent with the broader clinical picture—suggest impairment is likely caused by AD, there should be a discussion of what additional changes in cognition and function might be expected moving forward, as well as a timeline for these changes. A diagnosis of definite AD should also address the potential for additional pathologies to be present in the brain and contribute to cognitive symptoms. This is a time to discuss medical interventions and caregiving needs; these conversations will, of course, be shaped by the availability of disease-modifying drugs.
For other individuals, the results may indicate that their cognitive impairment is likely not caused by AD. In such cases, the clinician can provide information about other potential etiologies and discuss next steps in diagnosis or care.
If the individual is not already enrolled in a study or trial, the clinician might share information about clinical trials or longitudinal cohort studies for which the individual's biomarker profile makes them eligible.
For biomarker-positive individuals, 2 additional topics are relevant, regardless of the individual's cognitive stage. First, clinicians might highlight the importance of life planning—such as identifying people to provide assistance in making decisions, drafting advance directives or wills, and reviewing plans for paying for long-term care services and supports. Clinicians can refer individuals and their support persons to a social worker, if available; alternatively, they might encourage individuals to seek out reputable resources and professional advice. Second, clinicians should discuss pursuing a healthy lifestyle and avoiding unproven treatments. AD is deeply feared, and desperation can render persons with AD biomarkers vulnerable to trying interventions that lack evidence of efficacy or that are potentially harmful.39
Step 5: Check-in and Provide for Follow-up
Individuals and their support persons may need additional time to reflect on the information they have received. Therefore, after the disclosure of AD biomarker results, it is desirable that the clinician check-in with the individual, their support person, or both to confirm their understanding of the results, ascertain how they are feeling, and ask if they have any questions. This is an opportunity to clarify or provide further information and to address any concerns. The necessity of this check-in will depend on the result and how the disclosure process unfolded for the individual's or support person's understanding and emotions. For instance, biomarker-positive persons who are cognitively unimpaired but now understand themselves to be at heightened risk for impairment may benefit from a check-in call or visit. By contrast, persons with well-characterized dementia who expected and accepted their result may simply need an invitation to reach out with questions.
In addition, as the disclosure process concludes, there ought to be a clear plan for postdisclosure follow-up. The precise nature of follow-up, and whether that includes research or care, will depend on the individual's AD biomarker result, cognitive stage, and preferences. For cognitively impaired individuals, follow-up might include the provision of medical care and identification of community-based services and supports to address unmet needs.40 For cognitively unimpaired individuals, it might include participation in an interventional study or a study of disclosure's effects. Follow-up may be handled by the disclosing clinician, by other members of the care team, or by additional professionals as appropriate.
Conclusion
Over time, disclosure practices change, often reflecting a move away from paternalism and withholding toward respecting autonomy through truth-telling. Although an MCI or dementia diagnosis can be highly emotionally charged, practice has evolved, and individuals are now understood to have a right to know these diagnoses. As AD biomarkers become more clinically valuable, individuals will understandably want and reasonably expect to receive these results, too, despite their emotional charge. Guidance is needed to ensure that AD biomarker information is delivered in a manner that is scientifically and ethically informed. To that end, we have proposed a 5-step process that can be used in research or care and that seeks to ensure individuals understand the test, their results, and the implications for their lives. We anticipate that as the care and research contexts evolve, so too will this process.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- IES
Impact of Events Scale
- MCI
mild cognitive impairment
Appendix. Authors
Footnotes
Editorial, page 993
Study Funding
This work was supported by the NIA (P30-AG-072979). E.A. Largent was supported by the NIA (K01-AG064123) and a Greenwall Faculty Scholar Award.
Disclosure
E.A. Largent has no disclosures relevant to the manuscript. J.D. Grill reports research support from Eli Lilly, Genentech, Biogen, Eisai, NIA (P30AG066519, U24 AG057437), the Alzheimer's Association, and BrightFocus Foundation; he has provided consulting to SiteRx, Cogniciti, and Flint Rehab. K. O'Brien and K. Harkins report no disclosures relevant to the manuscript. D. Wolk has consulted for GE Healthcare, Eli Lilly, and Qynapse; he serves on the DSMB for Functional Neuromodulation; he has been a site-PI for a study by Biogen. J. Karlawish is a site investigator for Eli Lilly, Biogen, and Eisai. Go to Neurology.org/N for full disclosures.
References
- 1.Pinner G. Truth-telling and the diagnosis of dementia. Br J Psychiatry. 2000;176(6):514-515. doi: 10.1192/bjp.176.6.514. [DOI] [PubMed] [Google Scholar]
- 2.Pinner G, Bouman WP. What should we tell people about dementia? Adv Psychiatr Treat. 2003;9(5):335-341. doi: 10.1192/apt.9.5.335. [DOI] [Google Scholar]
- 3.Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18(12):2669-2686. doi: 10.1002/alz.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts JS, Ferber R, Blacker D, Rumbaugh M, Grill JD, for the Advisory Group on Risk Evidence Education for Dementia (AGREED). Disclosure of individual research results at federally funded Alzheimer's Disease Research Centers. Alzheimers Dement (NY). 2021;7(1):e12213. doi: 10.1002/trc2.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson CM, Chin NA, Johnson SC, Gleason CE, Clark LR. Disclosure of preclinical Alzheimer's disease biomarker results in research and clinical settings: why, how, and what we still need to know. Alzheimers Dement (Amst). 2021;13(1):e12150. doi: 10.1002/dad2.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingler JH, Butters MA, Gentry AL, et al. Development of a standardized approach to disclosing amyloid imaging research results in mild cognitive impairment. J Alzheimers Dis. 2016;52(1):17-24. doi: 10.3233/JAD-150985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harkins K, Sankar P, Sperling R, et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7:26. doi: 10.1186/s13195-015-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Largent EA, Harkins K, van Dyck CH, Hachey S, Sankar P, Karlawish J. Cognitively unimpaired adults' reactions to disclosure of amyloid PET scan results. PLoS One. 2020;15(2):e0229137. doi: 10.1371/journal.pone.0229137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill JD, Raman R, Ernstrom K, et al. Short-term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol. 2020;77(12):1504-1513. doi: 10.1001/jamaneurol.2020.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Largent EA, Abera M, Harkins K, et al. Family members' perspectives on learning cognitively unimpaired older adults' amyloid-β PET scan results. J Am Geriatr Soc. 2021;69(11):3203-3211. doi: 10.1111/jgs.17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caselli RJ, Marchant GE, Hunt KS, et al. Predictive testing for Alzheimer's disease: suicidal ideation in healthy participants. Alzheimer Dis Assoc Disord. 2015;29(3):252-254. doi: 10.1097/WAD.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grill JD, Johnson DK, Burns JM. Should we disclose amyloid imaging results to cognitively normal individuals? Neurodegener Dis Manag. 2013;3(1):43-51. doi: 10.2217/nmt.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JS, Dunn LB, Rabinovici GD. Amyloid imaging, risk disclosure and Alzheimer's disease: ethical and practical issues. Neurodegener Dis Manag. 2013;3(3):219-229. doi: 10.2217/nmt.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SYH, Karlawish J, Berkman BE. Ethics of genetic and biomarker test disclosures in neurodegenerative disease prevention trials. Neurology. 2015;84(14):1488-1494. doi: 10.1212/WNL.0000000000001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingler JH, Klunk WE. Disclosure of amyloid imaging results to research participants: has the time come? Alzhiemers Dement. 2013;9(6):741-744.e2. doi: 10.1016/j.jalz.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozersky J, Sankar P, Harkins K, Hachey S, Karlawish J. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol. 2018;75(1):44-50. doi: 10.1001/jamaneurol.2017.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderschaeghe G, Schaeverbeke J, Bruffaerts R, Vandenberghe R, Dierickx K. Amnestic MCI patients' experiences after disclosure of their amyloid PET result in a research context. Alzheimers Res Ther. 2017;9(1):92. doi: 10.1186/s13195-017-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingler JH, Sereika SM, Butters MA, et al. A randomized controlled trial of amyloid positron emission tomography results disclosure in mild cognitive impairment. Alzheimers Dement. 2020;16(9):1330-1337. doi: 10.1002/alz.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grill JD, Cox CG, Kremen S, et al. Patient and caregiver reactions to clinical amyloid imaging. Alzheimers Dement. 2017;13(8):924-932. doi: 10.1016/j.jalz.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grill JD, Apostolova LG, Bullain S, et al. Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther. 2017;9(1):35. doi: 10.1186/s13195-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gooblar J, Carpenter BD, Coats MA, Morris JC, Snider BJ. The influence of cerebrospinal fluid (CSF) biomarkers on clinical dementia evaluations. Alzheimers Dement. 2015;11(5):533. doi: 10.1016/j.jalz.2014.04.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286. doi: 10.1001/jama.2019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perini G, Rodriguez-Vieitez E, Kadir A, Sala A, Savitcheva I, Nordberg A. Clinical impact of 18F-FDG-PET among memory clinic patients with uncertain diagnosis. Eur J Nucl Med Mol Imaging. 2021;48(2):612-622. doi: 10.1007/s00259-020-04969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9(1):E1-E16. doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiong W, Tsou AY, Simmons Z, Bonnie RJ, Russell JA, on behalf of the Ethics, Law, and Humanities Committee (a joint committee of the American Academy of Neurology, American Neurological Association, and Child Neurology Society). Ethical considerations in dementia diagnosis and care: AAN position statement. Neurology. 2021;97(2):80-89. doi: 10.1212/WNL.0000000000012079. [DOI] [PubMed] [Google Scholar]
- 28.Largent EA, Karlawish J, Grill JD. Study partners: essential collaborators in discovering treatments for Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):101. doi: 10.1186/s13195-018-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Schaar J, Visser LNC, Bouwman FH, et al. Considerations regarding a diagnosis of Alzheimer's disease before dementia: a systematic review. Alzheimers Res Ther. 2022;14(1):31. doi: 10.1186/s13195-022-00971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill JD, Karlawish J. Disclosing Alzheimer disease biomarker results to research participants. JAMA Neurol. 2022;79(7):645. doi: 10.1001/jamaneurol.2022.1307. [DOI] [PubMed] [Google Scholar]
- 31.Creese B, Ismail Z. Mild behavioral impairment: measurement and clinical correlates of a novel marker of preclinical Alzheimer's disease. Alzheimers Res Ther. 2022;14(1):2. doi: 10.1186/s13195-021-00949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arias JJ, Tyler AM, Oster BJ, Karlawish J. The proactive patient: long-term care insurance discrimination risks of Alzheimer's disease biomarkers. J Law Med Ethics. 2018;46(2):485-498. doi: 10.1177/1073110518782955. [DOI] [PubMed] [Google Scholar]
- 33.Largent EA, Stites SD, Harkins K, Karlawish J. “That would be dreadful”: the ethical, legal, and social challenges of sharing your Alzheimer's disease biomarker and genetic testing results with others. J Law Biosci. 2021;8(1):lsab004. doi: 10.1093/jlb/lsab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arias JJ, Karlawish J. Confidentiality in preclinical Alzheimer disease studies: when research and medical records meet. Neurology. 2014;82(8):725-729. doi: 10.1212/WNL.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black BS, Rabins PV, Sugarman J, Karlawish JH. Seeking assent and respecting dissent in dementia research. Am J Geriatr Psychiatry. 2010;18(1):77-85. doi: 10.1097/JGP.0b013e3181bd1de2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Largent EA, Bradbury AR. Bringing Alzheimer disease testing and results disclosure into the 21st century cures act. JAMA Neurol. 2022;79(3):219-220. doi: 10.1001/jamaneurol.2021.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES—a six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302-311. doi: 10.1634/theoncologist.5-4-302. [DOI] [PubMed] [Google Scholar]
- 38.Largent EA, Wexler A, Karlawish J. The future is p-tau—anticipating direct-to-consumer Alzheimer disease blood tests. JAMA Neurol. 2021;78(4):379-380. doi: 10.1001/jamaneurol.2020.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellmuth J, Rabinovici GD, Miller BL. The rise of pseudomedicine for dementia and brain health. JAMA. 2019;321(6):543. doi: 10.1001/jama.2018.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner P, Karnieli‐Miller O, Eidelman S. Current knowledge and future directions about the disclosure of dementia: a systematic review of the first decade of the 21st century. Alzheimers Dement. 2013;9(2):e74-e88. doi: 10.1016/j.jalz.2012.02.006. [DOI] [PubMed] [Google Scholar]