Abstract
Background:
Despite the excellent clinical efficacy of oral propranolol in the management of infantile haemangiomas (IHs), there is a need to further evaluate other beta blockers that may be equally efficacious but result in lesser adverse effects. We compared the efficacy and short-term safety of atenolol, a hydrophilic cardio-selective beta blocker, with propranolol, in the treatment of IHs.
Materials and Methods:
Sixty patients with complicated and/or cosmetically significant IHs were randomised into two groups, oral propranolol group (2 mg/kg/day) and the oral atenolol (1 mg/kg/day) group, respectively, for 9 months. Patients were assessed clinically, by the use of Doppler ultrasonography (USG) and measurement of serum hypoxia-inducible factor 1 alpha (HIF-1α).
Results:
Twenty-two of 30 patients achieved complete clearance in the propranolol group (0.73; 95% CI = 0.54 to 0.87) compared with 13 of 25 patients in the atenolol group (0.52; 95% CI = 0.31 to 0.72). The mean time to achieve Physician Global Assessment Score 5 (PGA5) (25.00 ± 8.87 weeks) was significantly lesser in the propranolol group versus the atenolol group (31.69 ± 7.01 weeks; log-rank = 0.04). The two groups were comparable in terms of adverse effect profile, degree of volume reduction in USG and reduction in HIF-1α levels.
Conclusions:
Propranolol (2 mg/kg/day) is better than atenolol (1 mg/kg/day) in inducing complete clinical clearance of IH although the results need to be reproduced in larger studies.
KEY WORDS: Atenolol, infantile haemangioma, propranolol
Introduction
Infantile haemangiomas (IHs) are the most common soft-tissue tumours of infancy, occurring in about 4–10% of all infants.[1] Since the serendipitous finding of the usefulness of propranolol in IHs in 2008,[2] it has become the first line of management of IHs. However, being a lipophilic, non-selective beta-adrenergic blocker, it is associated with side effects, such as hypotension, hypoglycaemia, bronchial hyper-reactivity and sleep disturbances.[2] Moreover, in the early developmental stages of life, the exact magnitude of central nervous system (CNS) effects resulting from propranolol use is currently not known. The impairment in psychomotor function, memory, sleep quality and mood have been reported.[3]
Atenolol, a hydrophilic cardio-selective beta blocker, has thus been proposed to be used to address the side effects caused by the lipophilicity and non-selective action of propranolol. Atenolol also provides the ease of once-daily dosing as opposed to twice- or thrice-daily dosing of propranolol.[4] Hence, once we decide to use a beta blocker in the treatment of haemangiomas, it seems judicious to use the one that is safest. Through this study, we aimed to compare the effectiveness and safety of atenolol with that of propranolol in the treatment of IH.
Materials and Methods
Study design: A prospective, observer-blinded, parallel-group, randomised controlled study.
Study place: Departments of Dermatology, Venereology and Leprology and Pediatric Surgery, at Post Graduate Institute of Medical Education and Research, Chandigarh, India. A prior approval from the Institutional Research and Ethics Committee was obtained.
Inclusion and exclusion criteria: Infants of either sex diagnosed with problematic IHs, defined as potentially disfiguring IH; functionally threatening IHs near the eyes, nose, natural orifices, limbs and genitalia; ulcerated IHs; segmental IHs; multiple IHs; and rapidly progressive IHs with an unpredictable future course were included. Infants with heart disease, broncho-obstructive disease, premature infants with a corrected age of less than 40 weeks, known hypoglycaemia, diabetes mellitus, blood pressure (BP) abnormalities, liver failure visceral haemangiomas and those with posterior fossa anomalies, haemangioma, arterial anomalies, cardiac anomalies and eye anomalies (PHACES) syndrome were excluded.
Sample size: Taking the odds of the primary outcome in Group A (Propranolol) pA as 90% based on previous studies,[5,6] the minimal acceptable efficacy in group B (Atenolol) pB as 70%, keeping the power of the test as 80%, α level as 5%, the matching ratio as 1, and accounting for 10% drop outs, sample size obtained was 68 per group. Due to the limited study duration of one and a half years, a feasible sample size of at least 60 was decided upon.
Baseline clinical assessment
Children satisfying the inclusion and exclusion criteria were recruited into the study after obtaining written informed consent from their parents. Upon recruitment, each lesion was evaluated clinically for size, colour, consistency and the phase of evolution. Photographs were taken along with the Haemangioma Activity Score (HAS).[7] HAS uses the colour and depth of the lesion along with the presence of ulceration as clinical indicators of activity. Baseline ultrasonography (USG) was performed before the start of treatment. Heart rate, BP, oxygen saturation, electrocardiogram, respiratory rate and random blood sugar were recorded to rule out contraindications. All patients were admitted for 24 hours at the initiation of treatment to monitor heart rate, BP, blood sugar, oxygen saturation and respiratory rate.
Randomisation and treatment protocol
Upon inclusion, patients were randomised into two groups; group A and group B, using a computer-generated sequence (block randomisation). Group A received propranolol, given at a starting dose of 1 mg/kg/day (as crushed tablets) in two divided doses and increased to 2 mg/kg/day after 24 hours, if tolerated well. Group B received atenolol, given at a starting dose of 0.5 mg/kg/day as crushed tablets as a once-daily dosage and increased to 1 mg/kg/day after 24 hours, if tolerated well.
Follow-up and evaluation: Serial photography, along with the Physician Global Assessment (PGA) and HAS, was calculated at monthly intervals. PGA score includes grading of response in the perceived percentage change in the lesions from baseline (5 ≥90% improvement or complete clearance; 4 = excellent improvement (75–90% decrease); 3 = good improvement (50–74% decrease); 2 = minimal improvement (25–49% decrease); 1 = poor improvement (1–24% decrease); 0 = failure (no difference or regrowth)). USG was performed at baseline and the completion of therapy to objectively assess the size and depth of haemangioma using a linear L12-5 probe (Philips iU22, Philips, Eindhoven, Netherlands). The length, breadth, depth and echogenicity, colour flow, vessel density and peak systolic velocity were noted pre- and post-treatment.
Parents were asked to report all perceivable adverse events and specifically asked about the occurrence of sleep disturbances, hypoglycaemia, hypotension, respiratory distress and gastrointestinal upset. Drugs were stopped after 9 months of treatment (primary endpoint).
Laboratory evaluation
Serum hypoxia-inducible factor 1α (HIF-1α) was measured by enzyme-linked immunosorbent assay (ELISA) at baseline and 9 months. The mean absorbance was calculated for standards, controls and samples; the average zero standard optical density was subtracted from the optical densities of samples and standards. The standard curve was plotted using SigmaPlot software. The minimal detectable limit of HIF-1α was determined to be 61 pg/mL.
Outcome measures: The number of patients achieving complete clinical clearance of lesion (defined as PGA score of 5) in the two groups was taken as the primary outcome variable, while the time taken to do so, mean decrease in HAS, frequency of adverse effects, number of patients achieving complete clearance of vascularity and fall in HIF-1α between the groups were taken as secondary outcome measures.
Statistical analysis
The statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, Version 15.0 Windows). All quantitative variables were estimated using measures of central location (mean, median) and measures of dispersion (standard deviation and standard error). For normally distributed data, means were compared using Student's t-test for groups. For skewed data, the Mann–Whitney test was applied to the group. Qualitative or categorical variables were described as frequencies and proportions. Proportions were compared using the Chi-square or Fisher's exact test whichever was applicable. The proportion of patients who achieved Physician Global Assessment Score 5 (PGA5) in the two groups was evaluated by 95% CI using the Clopper–Pearson method. All statistical tests were two-sided and performed at a significance of α = 0.05.
Results
Seventy-five consecutive patients with IHs were screened, and 60 patients satisfying the inclusion and exclusion criteria were included in the study. Of the 15 patients who were excluded, 11 had small lesions on cosmetically non-significant sites, managed with topical timolol. The parents of the remaining four patients did not consent to participate in the study. During the one and a half years of the study period, five patients were lost to follow-up: one from group A and four from group B. Three patients could not be contacted, and the other two cited distance from the hospital as a reason for the inability to follow-up [Figure 1]. The last observed values of these patients were carried forward as the final outcome in the intention-to-treat (ITT) analysis, while they were omitted in the per-protocol (PP) analysis.
Figure 1.
Flow chart of the study
Baseline demographic and lesional data
Table 1 summarises the baseline demographics of the study cohort and IHs in the two groups. Both groups did not differ significantly from each other in any of the demographic variables. Sixty-eight were present in the head and neck region, 15.6% in the trunk, 9.4% in the upper limbs, 6.2% in the lower limbs and none in the perineal region in the atenolol group, while in the propranolol group the site-specific frequencies of IH were 51.3%, 23.1%, 10.3%, 7.7% and 4.2%, respectively.
Table 1.
Baseline demographic characteristics and lesional characteristics of IHs in group A and group B
| Group A (propranolol) (N=31) | Group B (atenolol) (N=29) | Significance (P) | |
|---|---|---|---|
| Age (mean) in months | 5.2±2.88 (1–12) | 4.4±2.51 (2–11) | 0.263 |
| Gender (F: M) | 2.1:1 | 1.07:1 | 0.206 |
| Birthweight (mean) in grams | 2609±671 (900–4500) | 2640±686 (700–3700) | 0.860 |
| Birthweight<2500 g (%) | 10 (32) | 9 (31) | 0.919 |
| Preterm delivery (%) | 6 (19) | 4 (13) | 0.563 |
| Delivery by caesarean section (%) | 12 (39) | 8 (28) | 0.361 |
| Risk factor for possible hypoxia | 13 (42) | 6 (21) | 0.077 |
|
| |||
| Baseline lesional characteristics of IHs | |||
|
| |||
| Group A (propranolol) (N=31) | Group B (atenolol) (N=29) | Significance (P) | |
|
| |||
| Age at presentation in days (mean) | 17.5±24.3 (0–120) | 12.48±15.89 (0–60) | 0.347 |
| Redness/lesion present at birth | 21 (67) | 12 (41) | 0.122 |
| >1 lesion in | 6 (19) | 8 (28) | 0.743 |
| Depth | |||
| Superficial | 12 (39) | 16 (55) | 0.426 |
| Mixed | 18 (58) | 12 (41) | |
| Deep | 1 (3) | 1 (4) | |
| Segmental IH | 3 (7) | 3 (10) | 0.931 |
| Stage | |||
| Progressive | 27 (87) | 23 (79) | 0.419 |
| Plateau | 4 (13) | 6 (21) | |
| Local complications | |||
| Ulceration | 8 (26) | 6 (21) | 0.618 |
| Bleeding | 3 (10) | 1 (3) | |
| Crusting | 2 (6) | 1 (3) | |
| Mean area of IH (cm2) | 7.92±4.88 (1–20) | 7.37±6.92 (1–24) | 0.727 |
| Mean baseline HAS | 4.66±1.16 (2–6.5) | 4.99±0.83 (2.6–6.5) | 0.203 |
| Mean baseline HIF-1α (ng/mL) (N=20 each) | 1.73±1.42 (0–6.12) | 2.18±1.60 (0–5.41) | 0.360 |
|
| |||
| Baseline ultrasonographic characteristics | |||
|
| |||
| Group A (propranolol) (N=21) | Group B (atenolol) (N=19) | Significance (P) | |
|
| |||
| Mean volume of lesions (cm3) | 3.46±3.09 (0.73–12.48) | 3.6±4.19 (0.10–14.3) | 0.799 |
| Vessel density/cm2 | |||
| <3 | 6 (28.5) | 3 (15.8) | 0.907 |
| 3–5 | 6 (28.5) | 6 (31.6) | |
| >5 | 9 (43) | 10 (52.6) | |
| Calcification | Absent | Absent | |
| Peak arterial velocity (cm/s) | 20.79±15.73 | 15.50±4.29 | 0.167 |
IH=infantile haemangioma, HAS=Haemangioma Activity Score, HIF-1α = hypoxia-inducible factor 1 alpha
USG was performed in 23 patients in group A and 22 patients in group B. No lesion could be detected on USG in two patients in group A and three patients in group B owing to their superficial nature. The parents of the remaining patients did not consent to the procedure/administration of sedatives before the procedure. The volume of lesions was calculated using the ellipsoid formula (volume = 0.52 × length × breadth × depth), and the mean volume was comparable in both groups.
Response to treatment
Primary outcome variables
As per ITT analysis, 22 of 31 patients achieved complete clearance (PGA5 or >90% improvement) in the propranolol group (0.70; 95% CI = 0.51 to 0.85) compared with 13 of 29 patients in the atenolol group (0.45; 95% CI = 0.26 to 0.64). The observed difference between the success rates of the two groups was 26% in favour of the propranolol group [95% CI = −0.503 to − 0.02; P = 0.04; Table 2].
Table 2.
Response to treatment in the two groups and summary of side effects in the two groups
| Primary outcome variable | |||||
|---|---|---|---|---|---|
|
| |||||
| Group A propranolol) | Group B (atenolol) | Significance (P) | 95% confidence interval | ||
|
| |||||
| Lower | Upper | ||||
| PGA5 attained within 9 months by ITT population (N=31 and 29, respectively) | 22 (71) | 13 (45) | 0.040 | ‒ 0.503 | ‒ 0.02 |
| PGA5 attained within 9 months by PP population (N=30 and 25, respectively) | 22 (73%) | 13 (52%) | 0.101 | ‒ 0.465 | 0.038 |
| Secondary outcome variables | |||||
| Mean time taken to achieve PGA5 (weeks) | 25.00±8.87 (12–36) | 31.69±7.01 (12–36) | 0.044 | ‒ 11.598 | ‒ 0.513 |
| ΔHAS over 9 months in the ITT population | 4.06±1.53 (1–6) | 3.53±1.44 (0–6) | 0.176 | ‒ 0.243 | 1.297 |
| ΔHAS over 9 months in the PP population | 4.14±1.51 (1–6) | 3.97±0.93 (1.6–6) | 0.612 | ‒ 0.499 | 0.839 |
|
| |||||
| Changes in ultrasonographic parameters | |||||
|
| |||||
| Group A (propranolol) | Group B (atenolol) | P | 95% confidence interval | ||
|
| |||||
| Lower | Upper | ||||
|
| |||||
| Change in volume over 9 months (N=19 and 17, respectively) | 2.07±2.04 (0.24–7.70) | 2.09±2.72 (0.08–9.20) | 0.736 | ‒ 1.831 | 1.305 |
| Clearance of vascularity (N=26 and 23, respectively | 20 (76.9%) | 15 (65.2%) | 0.365 | ||
| Calcification | None | None | |||
| Fall in HIF in 9 months (N=17 and 18, respectively) | 0.79±1.39 (−2.53 to 3.08) | 1.24±2.3 (−4.87 to 4.32) | 0.499 | ‒ 1.782 | 0.886 |
| Summary of side effects in the two groups. | |||||
| Side effects | 19 (61) | 19 (65) | 0.734 | ‒ 0.204 | 0.284 |
| >1 side effect | 4 (13) | 5 (17) | 0.638 | ‒ 0.140 | 0.220 |
| Increased frequency of stools | 12 (39) | 10 (34) | 0.734 | ‒ 0.193 | 0.293 |
| Fall in blood pressure from baseline | 4 (13) | 6 (21) | 0.419 | ‒ 0.109 | 0.269 |
| Sleep disturbance | 3 (10) | 1 (3) | 0.334 | ‒ 0.055 | 0.195 |
| Excessive crying | 2 (6) | 2 (7) | 0.945 | ‒ 0.114 | 0.134 |
| Increased appetite | 1 | 0 | |||
| Telogen effluvium | 0 | 1 | |||
| Decreased weight gain | 1 | 1 | |||
| Constipation | 0 | 1 | |||
PGA5=Physician Global Assessment Score 5, ITT=intention to treat, PP=per protocol, ΔHAS=change in Haemangioma Activity Score
Secondary outcome variables
Using the Kaplan–Meier analysis, the mean time to achieve PGA5 (25.00 ± 8.87 weeks) was significantly lesser in group A as compared to group B [31.69 ± 7.01 weeks; log-rank = 0.04; Figure 2]. The two groups did not differ significantly in terms of achieving PGA3 at 9 months, mean time to achieve PGA3 and mean reduction in HAS [Table 2]. However, the fall in HAS was significant within groups [P < 0.05; Figures 3 and 4]. None of the patients in either group experienced any serious adverse effects requiring additional intervention or discontinuation of treatment [Table 2].
Figure 2.

Survival time-to-event analysis (Kaplan–Meier curve) of patients achieving PGA5 over 36 weeks
Figure 3.
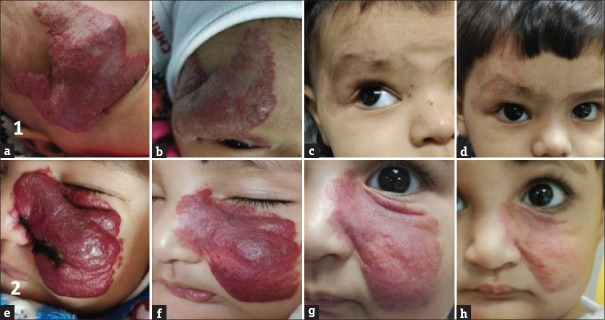
Infantile haemangiomas. (a-d) Clinical photographs of a patient in group A (propranolol) at baseline (HAS = 5), 3 months (HAS = 3), 6 months (HAS = 0.5) and 9 months (HAS = 0.5), respectively. (e-h) Clinical photographs of a patient in group B (atenolol) at baseline (HAS = 6), 3 months (HAS = 3), 6 months (HAS = 3) and 9 months (HAS = 1), respectively. HAS = Hemangioma Activity Score
Figure 4.
Infantile haemangiomas. (a-d) Clinical photographs of a patient in group A (propranolol) at baseline (HAS = 5.5), 3 months (HAS = 4), 6 months (HAS = 0) and 9 months (HAS = 0), respectively. (e-h) Clinical photographs of a patient in group B (atenolol) at baseline (HAS = 6), 3 months (HAS = 4), 6 months (HAS = 3) and 9 months (HAS = 1.3), respectively. HAS = Haemangioma Activity Score
Change in USG features
Both groups showed a significant fall in mean volume of lesions following 9 months of therapy [Table 2]. Five patients in group A and four patients in group B, whose baseline USG was not performed, consented to it at the end of treatment of lesional USG. There was no significant difference in the degree of fall in mean volume between the two groups. Twenty patients in group A and 15 patients in group B showed complete vascular clearance. All patients who did not show complete clearance of vascularity showed a decrease in vessel density from baseline.
Change in HIF-1α levels
Of 60 patients, 40 (20 patients from each group) consented to a blood test at the start of treatment. However, paired data were only available for 17 patients in group A and 18 patients in group B, at the end of the study. The two groups showed a significant fall in mean HIF-1α levels from baseline.
To determine the factors affecting the outcome of PGA5, univariate analysis was performed. None of the pretreatment characteristics proved to be a statistically significant factor in determining the outcome.
Discussion
Despite the excellent efficacy of oral propranolol in IHs, the possibility of other alternative beta blockers that may be equally efficacious and safer has not been adequately explored. The present study set out to bridge this knowledge gap and evaluate the effectiveness of one such beta blocker, atenolol in the treatment of IHs.
Our results reaffirmed the clinical effectiveness of oral propranolol in the treatment of IH with a response rate of 93.5% and complete clearance in 71% of patients. Many recent studies by Zhang et al., Malik et al. and de Graaf et al. have reported a response rate of 95–97% with propranolol.[6,8,9] The present study also confirmed the effectiveness of atenolol in the management of IHs, however, with comparatively less effectiveness than propranolol. These results are in contrast to other studies by Abarzua-Araya et al.,[4] De Graaf et al.[9] and Dakoutro et al.,[10] who found the effectiveness to be similar between the two drugs. These differences can be explained based on the differences in factors, such as smaller sample size,[4] longer duration of atenolol therapy[9] and higher dose of atenolol.[10] Zhao et al. noted an ’excellent’ response (>80% clearance) in 35.3% of a series of 133 patients after a mean duration of 4.9 months and diarrhoea was the only reported side effect (3% of patients).[11] Our results also differ from the trial by Ji et al., which has a much larger sample size.[12] However, in contrast to their trial in which the primary outcome is based on ’any response’, our primary outcome is ’inducing PGA5 or complete clinical remission’, which is a more stringent outcome. Second, the authors also noted a difference in the mean duration of treatment in the two groups in favour of propranolol.[12] Chen et al., in a systematic review, concluded that propranolol had better rates of complete clearance as compared to atenolol (85.4% vs 73.3%). None of the pre-treatment characteristics proved to be a statistically significant factor in determining the final outcome of the present study.[13] Castaneda et al. noted that patients who had been started on therapy before five months of age had a significantly better response.[14] Similarly, another study reported that deep lesions required a longer treatment duration.[15] Some studies have also pointed to the segmental distribution of lesions as contributing factor to poor outcome and relapse.[16,17]
The major concern regarding the use of propranolol in infants has been the occurrence of rare, but serious side effects. Recent meta-analyses show that the common non-life-threatening complications of propranolol are disturbed sleep, cool and mottled extremities, diarrhoea and gastrointestinal disturbance. The serious side effects include asymptomatic hypotension, hypoglycaemia, asymptomatic bradycardia, bronchial hyper-reactivity and hyperkalaemia.[18,19,20] The occurrence of this has been reportedly lower among patients treated with atenolol.[13,18] Seebauer et al. proposed that the R(+) enantiomers of widely used beta blockers could be repurposed to increase the efficiency of current IH treatment and lower adverse associated side effects.[21] In the present study, both groups had comparable side effect profile, with the most common side effects being self limiting diarrhoea and self limiting asymptomatic hypotension. No life threatening side effects were noted in either group. 10% of patients on propranolol had sleep. In the present study, 10% of patients on propranolol had sleep disturbances in the initial month of starting therapy. Interestingly, one patient in the atenolol group also had similar complaints. Theoretically, atenolol, being hydrophilic, would not cross the blood–brain barrier and hence would avoid the sleep disturbances seen with propranolol and the possible long-term effects on cognitive development. Atenolol also provides the ease of once-daily dosing. Despite the results of the present study, it may still be the preferred drug in infants with a contraindication to non-selective beta blockers, such as bronchial hyper-reactivity. Atenolol has been safely used in infants for cardiological indications, such as supraventricular tachycardia without significant adverse effects.[22]
In the study by Dubois et al., Doppler sonography recorded a vessel density >5/cm2 in 65 (93%) of 70 haemangiomas.[23] However, less than half of the lesions showed this feature in the present study. There was no significant difference in the degree of fall in mean volume between the two groups (P = 0.736, 95% CI - 1.83 to 1.305). Shi et al. found that, although treatment with propranolol almost completely cleared the superficial portion of some IHs, blood flow signals were still detected in the subcutaneous regions of the lesions on USG.[24]
HIF-1α has been reported to be increased in IH and has been indicated to be involved in the regulation of angiogenic factors, such as vascular endothelial growth factor A (VEGF-A) and matrix metalloproteinase 9 (MMP-9).[25] Li et al. found that propranolol decreased the levels of HIF-1α in the serum and urine of IH patients.[26] There are no similar studies on the effect of atenolol on the same, so far. Despite the beta-2 adrenergic receptor being the proposed site of action, atenolol being a beta-1 selective antagonist still resulted in a response. This warrants a reconsideration of the said mechanism of action.
Despite our best efforts, our study had certain limitations, such as the relatively low sample size. The parents of the patients were not blinded to the treatment given. The study was not powered to make conclusions about adverse effects and does not provide information on the long-term adverse effects of the two drugs due to the 9-month follow-up period.
Conclusions
Based on the observations from the present study, we deduce that propranolol (2 mg/kg/day) was significantly better than atenolol (1 mg/kg/day) in achieving complete clinical response in IH. Future studies may be aimed at a dose-ranging efficacy of atenolol in IH and may reveal a more favourable response to atenolol at higher doses of 2 mg/kg/day.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We acknowledge the help of Dr. Amanjot Kaur Arora in the initial preparation of study protocol.
References
- 1.Frieden IJ, Haggstrom AN, Drolet BA, Mancini AJ, Friedlander SF, Boon L, et al. Infantile hemangiomas: Current knowledge, future directions.Proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383–406. doi: 10.1111/j.1525-1470.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 2.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 3.Langley A, Pope E. Propranolol and central nervous system function: Potential implications for paediatric patients with infantile haemangiomas. Br J Dermatol. 2015;172:13–23. doi: 10.1111/bjd.13379. [DOI] [PubMed] [Google Scholar]
- 4.Ábarzúa-Araya A, Navarrete-Dechent CP, Heusser F, Retamal J, Zegpi-Trueba MS. Atenolol versus propranolol for the treatment of infantile hemangiomas: A randomized controlled study. J Am Acad Dermatol. 2014;70:1045–9. doi: 10.1016/j.jaad.2014.01.905. [DOI] [PubMed] [Google Scholar]
- 5.Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735–46. doi: 10.1056/NEJMoa1404710. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Wu HW, Yuan W, Zheng JW. Propranolol therapy for infantile hemangioma: Our experience. Drug Des Devel Ther. 2017;11:1401–8. doi: 10.2147/DDDT.S134808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janmohamed SR, de Waard-van der Spek FB, Madern GC, de Laat PC, Hop WCJ, Oranje AP. Scoring the proliferative activity of haemangioma of infancy: The haemangioma activity score (HAS) Clin Exp Dermatol. 2011;36:715–23. doi: 10.1111/j.1365-2230.2011.04080.x. [DOI] [PubMed] [Google Scholar]
- 8.Malik MA, Menon P, Rao KLN, Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: A randomized controlled study. J Pediatr Surg. 2013;48:2453–9. doi: 10.1016/j.jpedsurg.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 9.de Graaf M, Raphael MF, Breugem CC, Knol MJ, Bruijnzeel-Koomen CAFM, Kon M, et al. Treatment of infantile haemangiomas with atenolol: Comparison with a historical propranolol group. J Plast Reconstr Aesthet Surg. 2013;66:1732–40. doi: 10.1016/j.bjps.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Dakoutrou M, Alexopoulos A, Miligkos M, Georgiadou E, Kanaka-Gantenbein C, Kakourou T. Atenolol treatment for severe infantile hemangiomas: Comparison with a propranolol group of our centre. J Eur Acad Dermatol Venereol. 2019;33:e199–200. doi: 10.1111/jdv.15464. [DOI] [PubMed] [Google Scholar]
- 11.Zhao ZL, Liu C, Wang QZ, Wu HW, Zheng JW. Oral atenolol treatment for infantile hemangiomas: Clinical analysis of 133 consecutive patients. Ann Transl Med. 2021;9:116. doi: 10.21037/atm-20-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji Y, Chen S, Yang K, Zhang X, Zhou J, Li L, et al. Efficacy and safety of propranolol vs atenolol in infants with problematic infantile hemangiomas: A randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2021;147:599–607. doi: 10.1001/jamaoto.2021.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen T, Gudipudi R, Nguyen SA, Carroll W, Clemmens C. Should propranolol remain the gold standard for treatment of infantile hemangioma.A systematic review and meta-analysis of propranolol versus atenolol? Ann Otol Rhinol Laryngol. 2023;132:332–40. doi: 10.1177/00034894221089758. [DOI] [PubMed] [Google Scholar]
- 14.Castaneda S, Garcia E, De la Cruz H, Ramirez O, Melendez S, Sanchez-Palacio J. Therapeutic effect of propranolol in mexican patients with infantile hemangioma. Drugs Real World Outcomes. 2016;3:25–31. doi: 10.1007/s40801-015-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlastarakos PV, Papacharalampous GX, Chrysostomou M, Tavoulari EF, Delidis A, Protopapas D, et al. Propranolol is an effective treatment for airway haemangiomas: A critical analysis and meta-analysis of published interventional studies. Acta Otorhinolaryngol Ital. 2012;32:213–21. [PMC free article] [PubMed] [Google Scholar]
- 16.Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: Clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567–76. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- 17.Ahogo CK, Ezzedine K, Prey S, Colona V, Diallo A, Boralevi F, et al. Factors associated with the relapse of infantile haemangiomas in children treated with oral propranolol. Br J Dermatol. 2013;169:1252–6. doi: 10.1111/bjd.12432. [DOI] [PubMed] [Google Scholar]
- 18.Pattanshetti SA, Mahalmani VM, Sarma P, Kaur H, Ali MM, Malik MA, et al. Oral atenolol versus propranolol in the treatment of infantile hemangioma: A systematic review and meta-analysis. J Indian Assoc Pediatr Surg. 2022;27:279–86. doi: 10.4103/jiaps.jiaps_3_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Léaute-Labrèze C, Boccara O, Degrugillier-Chopinet C, Mazereeuw-Hautier J, Prey S, Lebbé G, et al. Safety of oral propranolol for the treatment of infantile hemangioma: A systematic review. Pediatrics. 2016;138:e20160353. doi: 10.1542/peds.2016-0353. [DOI] [PubMed] [Google Scholar]
- 20.Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics. 2013;131:128–40. doi: 10.1542/peds.2012-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seebauer CT, Graus MS, Huang L, McCann A, Wylie-Sears J, Fontaine F, et al. Non-beta blocker enantiomers of propranolol and atenolol inhibit vasculogenesis in infantile hemangioma. J Clin Invest. 2022;132:e151109. doi: 10.1172/JCI151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta AV, Subrahmanyam AB, Anand R. Long-term efficacy and safety of atenolol for supraventricular tachycardia in children. Pediatr Cardiol. 1996;17:231–6. doi: 10.1007/BF02524799. [DOI] [PubMed] [Google Scholar]
- 23.Dubois J, Patriquin HB, Garel L, Powell J, Filiatrault D, David M, et al. Soft-tissue hemangiomas in infants and children: Diagnosis using Doppler sonography. AJR Am J Roentgenol. 1998;171:247–52. doi: 10.2214/ajr.171.1.9648798. [DOI] [PubMed] [Google Scholar]
- 24.Shi H, Song H, Wang J, Xia L, Yang J, Shang Y, et al. Ultrasound in assessing the efficacy of propranolol therapy for infantile hemangiomas. Ultrasound Med Biol. 2014;40:2622–9. doi: 10.1016/j.ultrasmedbio.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Chim H, Armijo BS, Miller E, Gliniak C, Serret MA, Gosain AK. Propranolol induces regression of hemangioma cells through HIF-1α-mediated inhibition of VEGF-A. Ann Surg. 2012;256:146–56. doi: 10.1097/SLA.0b013e318254ce7a. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Guo Z, Gao Y, Pan W. Propranolol represses infantile hemangioma cell growth through the β2-adrenergic receptor in a HIF-1α-dependent manner. Oncol Rep. 2015;33:3099–107. doi: 10.3892/or.2015.3911. [DOI] [PubMed] [Google Scholar]