Abstract
Background.
Use of human leukocyte antigen (HLA)-mismatched donors could enable more patients with ethnically diverse backgrounds to receive allogeneic hematopoietic cell transplantation (HCT) in the United States. However, real-world trends and outcomes following mismatched donor HCT for diverse patients remain largely undefined.
Objective.
To determine whether mismatched donor platforms have increased access to allogeneic HCT for ethnically diverse patients, particularly through the application of novel graft-versus-host disease (GvHD) prophylaxis regimens, and if outcomes for diverse patients were comparable to those of non-Hispanic White patients.
Design.
Observational cross-sectional study using real-world data from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. All patients receiving their first allogeneic HCT in the U.S. from 2009–2020 with focus on transplants performed in 2020 were included. Data from patients receiving allogeneic HCT using bone marrow, peripheral blood or cord blood from HLA-matched or mismatched related and unrelated donors was analyzed. Specifically, relative proportions of allogeneic HCT were generated as percent of total for donor type and for patient age, disease indication, GvHD prophylaxis, and race and ethnicity. Causes of death were summarized using frequencies, and the Kaplan-Meier estimator was used for estimating overall survival.
Results.
Compared to matched related donor and matched unrelated donor HCT, more ethnically diverse patients received mismatched unrelated donor, haploidentical donor, and cord blood HCT. Matched unrelated donor remains the most common donor type, but use of haploidentical donors has increased significantly over the last 5 years. Paralleling the increase in haploidentical HCT is the increased use of post-transplant cyclophosphamide (PTCy) as GvHD prophylaxis. Relative to older transplant eras, the most contemporary transplant era associates with the highest survival rates following allogeneic HCT irrespective of patient race and ethnicity. However, disease relapse remains the primary cause of death for both adult and pediatric allogeneic HCT recipients by donor type and across all patient race and ethnicity groups.
Conclusions.
Ethnically diverse patients are undergoing allogeneic HCTs at higher rates largely through the use of alternative donor platforms incorporating PTCy. Maintaining access to potential life-saving allogeneic HCT using alternative donors and novel GvHD prophylaxis strategies and improving HCT outcomes, particularly disease relapse, are urgent clinical needs.
Keywords: Bone marrow transplantation, cord blood, death, ethnicity, graft versus host disease, haploidentical, hematopoietic cell transplantation, infection, related donor, unrelated donor, peripheral blood, race, relapse, survival
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is the definitive cure for many malignant and non-malignant hematologic diseases. Yet the patient journey to allogeneic HCT can be difficult, particularly for ethnically diverse (ED) patients, who can experience several patient-, physician- and healthcare system-related barriers to HCT1. A human leukocyte antigen (HLA)-matched sibling donor (MSD) currently remains the preferred donor type and yields the best allogeneic HCT outcomes among all donor types2, but is available only for approximately 13–51% of patients3. Therefore, the majority of patients requiring allogeneic HCT receive a graft from an alternative donor source, most commonly from a matched unrelated donor (MUD)4,5. However, unrelated donor (URD) availability is not universal especially for diverse patients, as the likelihood of finding a MUD is largely determined by racial and ethnic representation in donor registries6. Furthermore, as the United States (U.S.) is becoming more racially and ethnically diverse7, finding a MUD for a patient in need will likely become more challenging.
To expand the options for all patients who could benefit from allogeneic HCT, alternative donor types have been used, including cord blood (CB), haploidentical donor, and mismatched unrelated donor (MMUD). Success of these alternative donor platforms requires crossing the HLA barrier, as donor-recipient HLA disparity underlies graft-versus-host disease (GvHD) and graft failure, significant causes of transplant-related morbidity and mortality8. Historically, combination methotrexate and calcineurin inhibitors have been used as GvHD prophylaxis for MUD bone marrow (BM) and peripheral blood (PB) HCT. Post-transplant cyclophosphamide (PTCy) has been used successfully as GvHD prophylaxis in the haploidentical transplant setting9 and has recently been applied for other allogeneic donor HCT platforms, including MSD, MUD and MMUD10.
The Center for International Blood and Marrow Transplant Research (CIBMTR) has been collecting detailed demographic and transplant-specific clinical and outcome data for HCT and other cellular therapies for 50 years. Since 2005, the CIBMTR has held a federal contract to deliver an annual transplant center-specific one-year survival report. Thus, reporting all allogeneic HCT in the US to CIBMTR is mandated by Human Research and Services Administration (HRSA) as part of the C.W. Bill Young Cell Transplantation Program. As a result, the CIBMTR is uniquely positioned to study trends in allogeneic HCT activity, including use of alternative donors, types of GvHD prophylaxis, and patient outcomes. The CIBMTR produces a comprehensive set of summary slides each year showing allogeneic HCT trends and outcomes5. In this publication, our focus is restricted to allogeneic HCT recipients in the U.S. to determine if the use of alternative donor platforms increased access to allogeneic HCT for ethnically diverse patients, particularly through the application of novel GvHD prophylaxis regimens, and if outcomes among diverse patients were comparable to those of non-Hispanic White (NHW) patients.
Methods
Center for International Blood and Marrow Transplant Research Cellular Therapy Outcomes Registry
The CIBMTR is a research collaboration between the National Marrow Donor Program and the Medical College of Wisconsin. The CIBMTR has information for more than 625,000 recipients from more than 330 participating centers.
Transplant centers submit data to the CIBMTR using a web-based data collection system, FormsNet. HCT patients have core or “essential” data completed using Transplant Essential Data (TED) forms or detailed disease- and treatment-related data using Comprehensive Report Forms. Patient assignment to specific data tracks is done upon submission of the initial pre-HCT TED form using a weighted randomization algorithm. The algorithm is designed to produce a representative cohort of current practice with adequate numbers of patients with rare conditions undergoing HCT or receiving emerging HCT strategies.
Data are collected at pre-HCT and 100 days, 6 months, 1 year, annually for the subsequent 6 years post-HCT, and then biannually. Details including data collection forms, forms instruction manual, data quality and audit guide, and data management guide as well as the CIBMTR Manual of Operations are available online at https://www.cibmtr.org.
The current publication is based on the 2020 transplant activity representing all first allogeneic HCTs registered with the CIBMTR in the U.S.5 Prior years are included to analyze trends.
Statistics
Patient-, disease-, and transplant-related factors describe all patients with counts and percent of total for categorical variables. The Kaplan-Meier estimator was used for estimating the overall survival with 95% confidence intervals, and estimations of survival and comparisons across survival curves were not adjusted for potentially important contributing factors. Cause of death was summarized using frequencies.
Results
Trends in allogeneic hematopoietic cell transplantation
Annual numbers of transplant recipients in the U.S. were compiled according to the number of first transplants registered with the CIBMTR (Table 1). In 2020, 8,326 allogeneic hematopoietic cell transplants (HCT) were reported, down from 8,740 in 2019 (4.7% decrease) (Supplemental Figure 1). Over the last decade, relative proportions of patients by race and ethnicity receiving allogeneic HCT have remained fairly constant with the exception being decreases in the proportions of Non-Hispanic White (NHW) patients, while absolute numbers of ethnically diverse patients receiving allogeneic HCT have increased (Figure 1, panel A). In 2010, 75% (n=4914) NHW, 13% (n=852) Hispanic, and 8% (n=524) non-Hispanic Black or African American patients received allogeneic HCT. In 2020, 69% (n=5432) NHW, 15% (n=1169) Hispanic, and 9% (n=740) non-Hispanic Black or African American patients received allogeneic HCT.
Table 1.
Characteristics of Domestic First Allogeneic Transplant Recipients (2009–2020)
| Characteristic | 2009–2019 | 2020 | |
|---|---|---|---|
| No. of patients | 85464 | 8326 | |
| Age in years at HCT - no. (%) | |||
| Median (min-max) | 51 (0–100) | 54 (0–82) | |
| <10 | 8273 (10) | 657 (8) | |
| 10–17 | 5425 (6) | 533 (6) | |
| 18–29 | 8796 (10) | 761 (9) | |
| 30–39 | 7578 (9) | 750 (9) | |
| 40–49 | 11106 (13) | 931 (11) | |
| 50–59 | 19241 (23) | 1538 (18) | |
| 60–69 | 20913 (24) | 2306 (28) | |
| 70+ | 4132 (5) | 850 (10) | |
| Race - no. (%) | |||
| White | 69185 (81) | 6472 (78) | |
| Black or African-American | 7608 (9) | 790 (9) | |
| Asian | 3668 (4) | 356 (4) | |
| Native Hawaiian or other Pacific Islander | 266 (0) | 23 (0) | |
| American Indian or Alaska Native | 430 (1) | 43 (1) | |
| More than one race | 635 (1) | 76 (1) | |
| Not reported | 3672 (4) | 566 (7) | |
| Ethnicity - no. (%) | |||
| Hispanic or Latino | 10814 (13) | 1169 (14) | |
| Not Hispanic or Latino | 71994 (84) | 6749 (81) | |
| Non-resident of the U.S. | 843 (1) | 75 (1) | |
| Not reported | 1813 (2) | 333 (4) | |
| Race and Ethnicity - no. (%) | |||
| Hispanic | 10814 (13) | 1169 (14) | |
| Non-Hispanic White | 59408 (70) | 5432 (65) | |
| Non-Hispanic Black or African American | 7158 (8) | 740 (9) | |
| Non-Hispanic Asian | 3538 (4) | 342 (4) | |
| Non-Hispanic Others | 1899 (2) | 177 (2) | |
| Not reported | 2647 (3) | 466 (6) | |
| Donor type - no. (%) | |||
| Matched related donor | 26190 (31) | 1808 (22) | |
| Matched unrelated donor | 33073 (39) | 3566 (43) | |
| Haploidentical donor | 10122 (12) | 1970 (24) | |
| Mismatched unrelated donor | 7492 (9) | 550 (7) | |
| Cord Blood | 7612 (9) | 381 (5) | |
| Multiple donor | 200 (0) | 22 (0) | |
| Twins | 343 (0) | 14 (0) | |
| Unknown Match Unrelated donor | 429 (1) | 2 (0) | |
| Not reported | 3 (0) | 13 (0) | |
| Graft type - no. (%) | |||
| BM | 19030 (22) | 1454 (17) | |
| PB | 58822 (69) | 6491 (78) | |
| CB | 7612 (9) | 381 (5) | |
| Disease group - no. (%) | |||
| AML | 31671 (37) | 3216 (39) | |
| ALL | 12652 (15) | 1299 (16) | |
| MDS | 10999 (13) | 1170 (14) | |
| MPN | 3789 (4) | 623 (7) | |
| NHL/HL | 9974 (12) | 709 (9) | |
| Aplastic Anemia | 2947 (3) | 340 (4) | |
| CML | 2569 (3) | 250 (3) | |
| Myeloma/PCD | 2039 (2) | 87 (1) | |
| Other Leukemia | 2292 (3) | 107 (1) | |
| Other malignancy | 125 (0) | 4 (0) | |
| Other Non-Malignant Disease | 6306 (7) | 496 (6) | |
| Not reported | 101 (0) | 25 (0) | |
| Disease status at time of HCT (AML/ALL) - no. (%) | |||
| CR1 | 26511 (60) | 3105 (69) | |
| CR2+ | 9630 (22) | 928 (21) | |
| Relapse/Never in CR | 7161 (16) | 369 (8) | |
| Not reported | 1021 (2) | 113 (2) | |
| N/A, other disease | 41141 | 3811 | |
| MDS disease status - no. (%) | |||
| Early disease | 2441 (22) | 239 (21) | |
| Advanced disease | 6351 (58) | 784 (67) | |
| Not reported | 2207 (20) | 147 (12) | |
| N/A, other diseases | 74472 | 7159 | |
| GVHD Prophylaxis - no. (%) | |||
| Ex vivo graft manipulation | 3414 (4) | 111 (1) | |
| PTCy ± others | 12326 (14) | 3214 (39) | |
| CNI ± others | 67453 (79) | 4686 (56) | |
| Other | 1294 (2) | 149 (2) | |
| Not reported | 473 (1) | 156 (2) | |
| Conditioning regimen for AML/ALL/MDS/MPN - no. (%) | |||
| MAC Bu/Cy ± others | 7884 (13) | 563 (9) | |
| MAC Bu/Flu ± others | 11753 (20) | 1276 (20) | |
| MAC TBI ± others | 13751 (23) | 1154 (18) | |
| MAC others | 2690 (5) | 227 (4) | |
| RIC/NMA Bu/Flu ± others | 5991 (10) | 512 (8) | |
| RIC/NMA Flu/Mel ± others | 7947 (13) | 1119 (18) | |
| RIC/NMA TBI ± others | 7918 (13) | 1371 (22) | |
| RIC/NMA other | 1125 (1) | 67 (1) | |
| Not reported | 53 (0) | 23 (0) | |
| N/A, other diseases | 26352 | 2014 | |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BM, bone marrow; Bu, busulfan; CB, cord blood; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CNI, calcineurin inhibitor; CR, complete response; Cy, cyclophosphamide; Flu, fludarabine; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; HL, Hodgkin lymphoma; Mel, melphalan; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasms; MAC, myeloablative conditioning; N/A, not available; NHL, non-Hodgkin lymphoma; No., number; NMA, non-myeloablative; PB, peripheral blood; PCD, plasma cell disorder; PTCy, post-transplant cyclophosphamide; RIC, reduced intensity conditioning; TBI, total body irradiation.
Figure 1. Relative proportion of allogeneic hematopoietic cell transplants (HCTs) in the United States by recipient race and ethnicity, disease indications, and donor types.
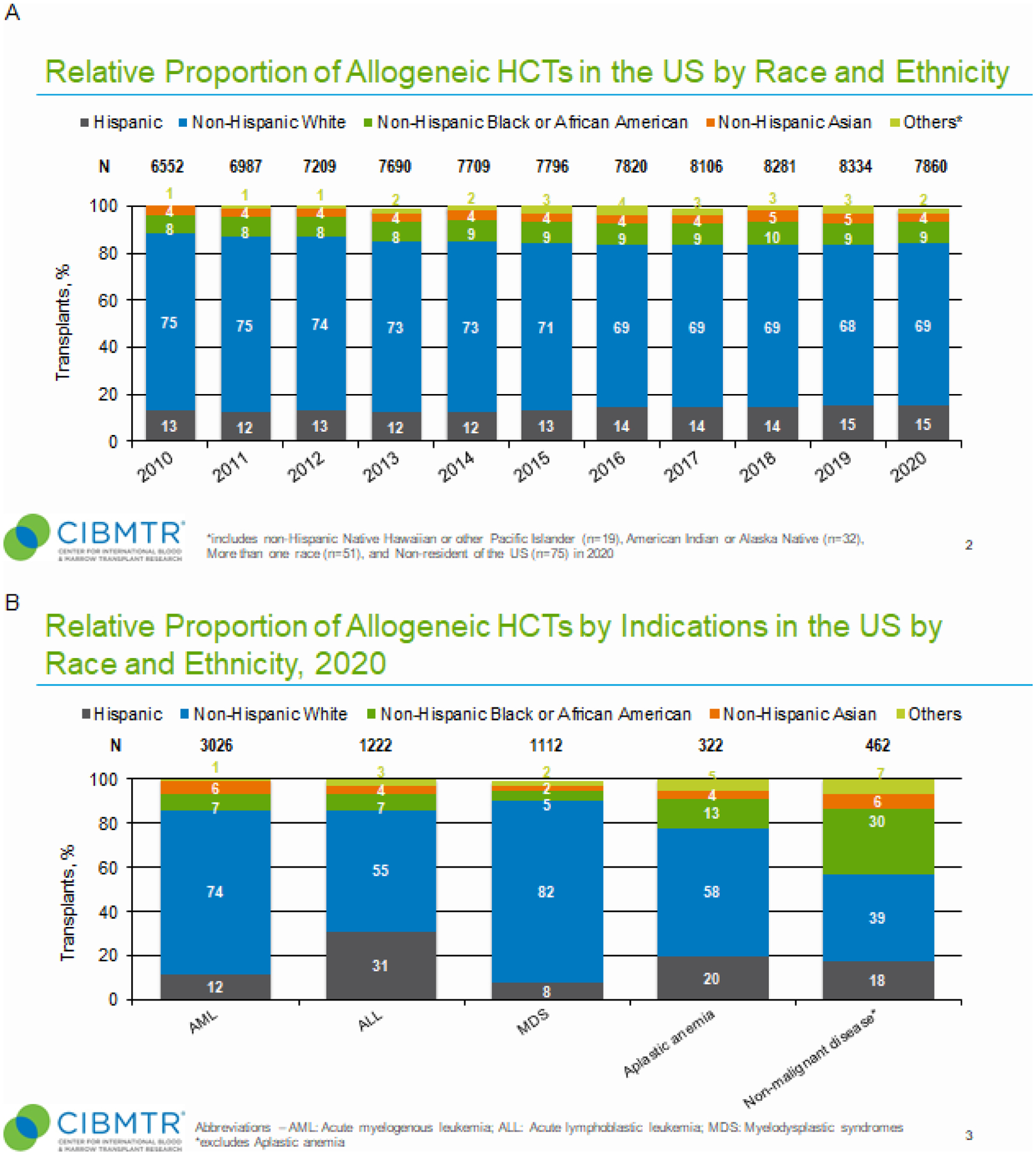
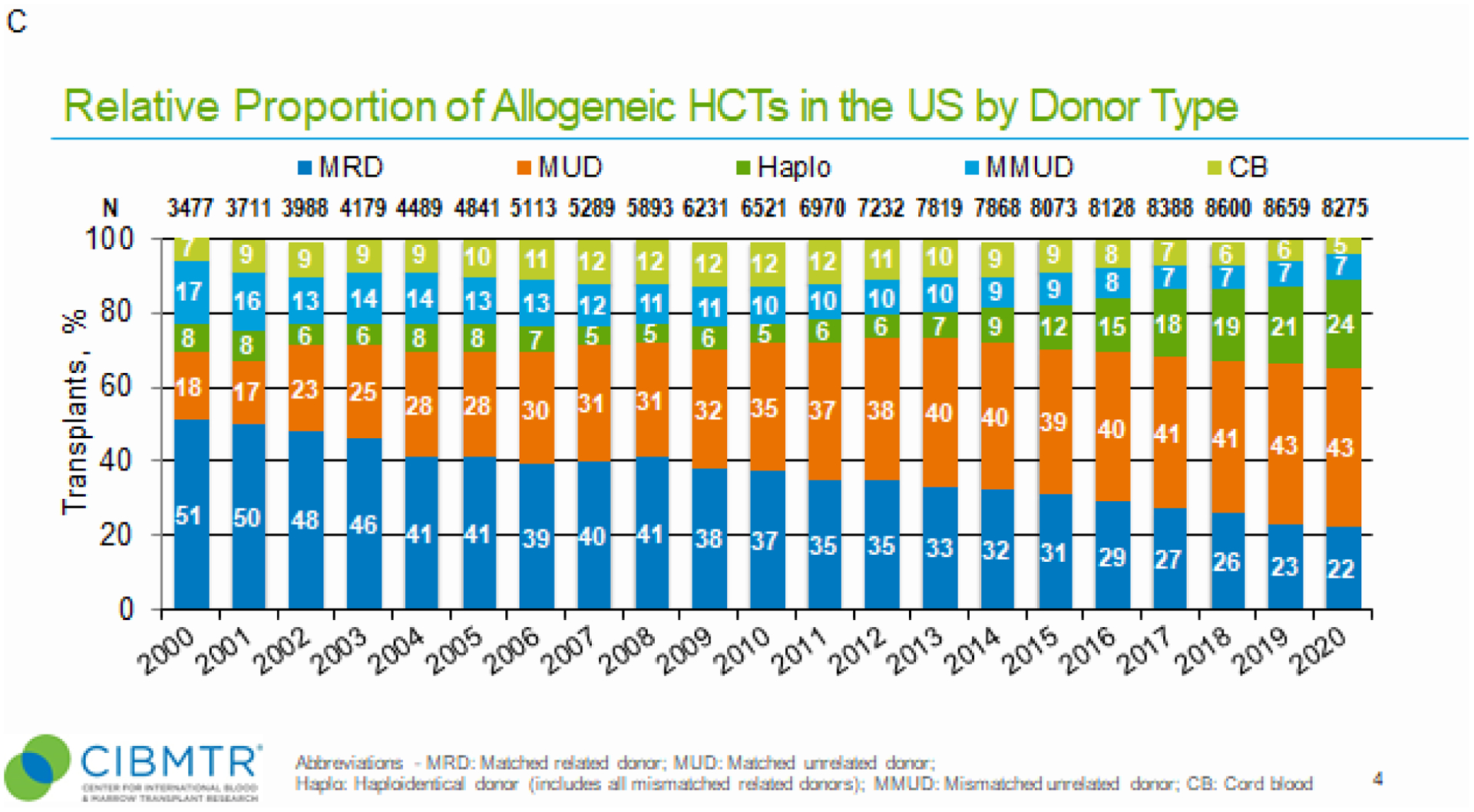
Panel A, Relative proportion of allogeneic HCTs in the U.S. by recipient race and ethnicity (2010–2020). For each year, proportions of transplants by indicated race and ethnicity group is shown as percentage of absolute number of transplants performed (N) for that year. “Other” race and ethnicity group includes non-Hispanic Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, more than one race, and non-resident of the U.S. Panel B, Relative proportion of allogeneic HCTs by disease indications in the U.S. by race and ethnicity in 2020. For each year, proportions of transplants by indicated disease indication is shown as percentage of absolute number of transplants performed (N) for that year. Non-malignant diseases category excludes aplastic anemia. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes. Panel C, Relative proportion of allogeneic HCT in the U.S. by donor type (2010–2020). For each year, proportions of transplants by indicated donor type is shown as percentage of absolute number of transplants performed (N) for that year. CB, cord blood; Haplo, haploidentical donor; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
In 2020, the most frequent disease indications for allogeneic HCT were acute myeloid leukemia (AML, n=3,216), ALL (n=1,299), and myelodysplastic syndrome (MDS, n=1,170) (Table 1). Relative proportions of diverse patients across these disease indications were similar with the exception of ALL, which had the largest proportion of Hispanic recipients (31%). Notably, aplastic anemia (13%) and non-malignant disease (30%) had higher proportions of non-Hispanic Black or African Americans (B/AA) relative to AML (7%), ALL (7%), and MDS (5%) (Figure 1, panel B).
Donor types and graft-versus-host disease prophylaxis
Since 2000, use of MUD has steadily increased, while use of MRD has decreased (Figure 1, panel C). In 2020, MUD and MRD represented 43% and 22% of donor types, respectively. Use of haploidentical donors has increased, representing 6% of donor types in 2013 to 24% of donor types in 2020. MMUD has remained nearly constant for the last decade. In contrast, CB has decreased during this time frame.
MUD remains the most common donor type irrespective of recipient age and has increased in relative proportion with advancing recipient age (Supplemental Figure 2). Notably, recipients aged ≥65y are second only to recipients aged 40–64y as the most frequent age group receiving allogeneic HCT (10,281 and 22,939 transplants, respectively). Pediatric recipients represent 18% (7,623) of total allogeneic HCT (50,123) in the last 6 years. CB donors are most commonly used in pediatrics (age<18y), and haploidentical donors are similarly represented across age groups.
Use of alternative donor types (haploidentical, MMUD, CB) may provide greater opportunity for ethnically diverse patients to receive allogeneic HCT. Therefore, we determined how donor types used in allogeneic HCT differed by race and ethnicity (R&E) and how donor type trends changed across two transplant periods, 2009–2014 and 2015–2020 (Figure 2). Overall, NHW patients received the most allogeneic HCT irrespective of donor type and across both transplant periods. For HLA-matched donors, more diverse groups received allogeneic HCT using a MRD than a MUD, which was consistent across both transplant periods (Figure 2). Alternative donor types associated with the highest relative proportions of diverse patients receiving allogeneic HCT. Specifically, both haploidentical donor and MMUD HCT had greater proportions of diverse patients than MUD HCT (Figure 2). CB also had a high proportion of diverse groups (Figure 2). While relative proportions of NHW and ethnically diverse patients receiving CB remained nearly constant between transplant periods, proportions of NHW patients receiving haploidentical and MMUD transplants decreased (57% to 52% and 67% to 69%, respectively). In contrast to NHW patients, relative proportions of Hispanic patients receiving haploidentical and MMUD transplants increased (13% to 19% and 15% to 18%, respectively) between transplant periods.
Figure 2. Relative proportion of allogeneic HCTs in the United States by recipient race and ethnicity for two transplant eras, 2009–2014 and 2015–2020.
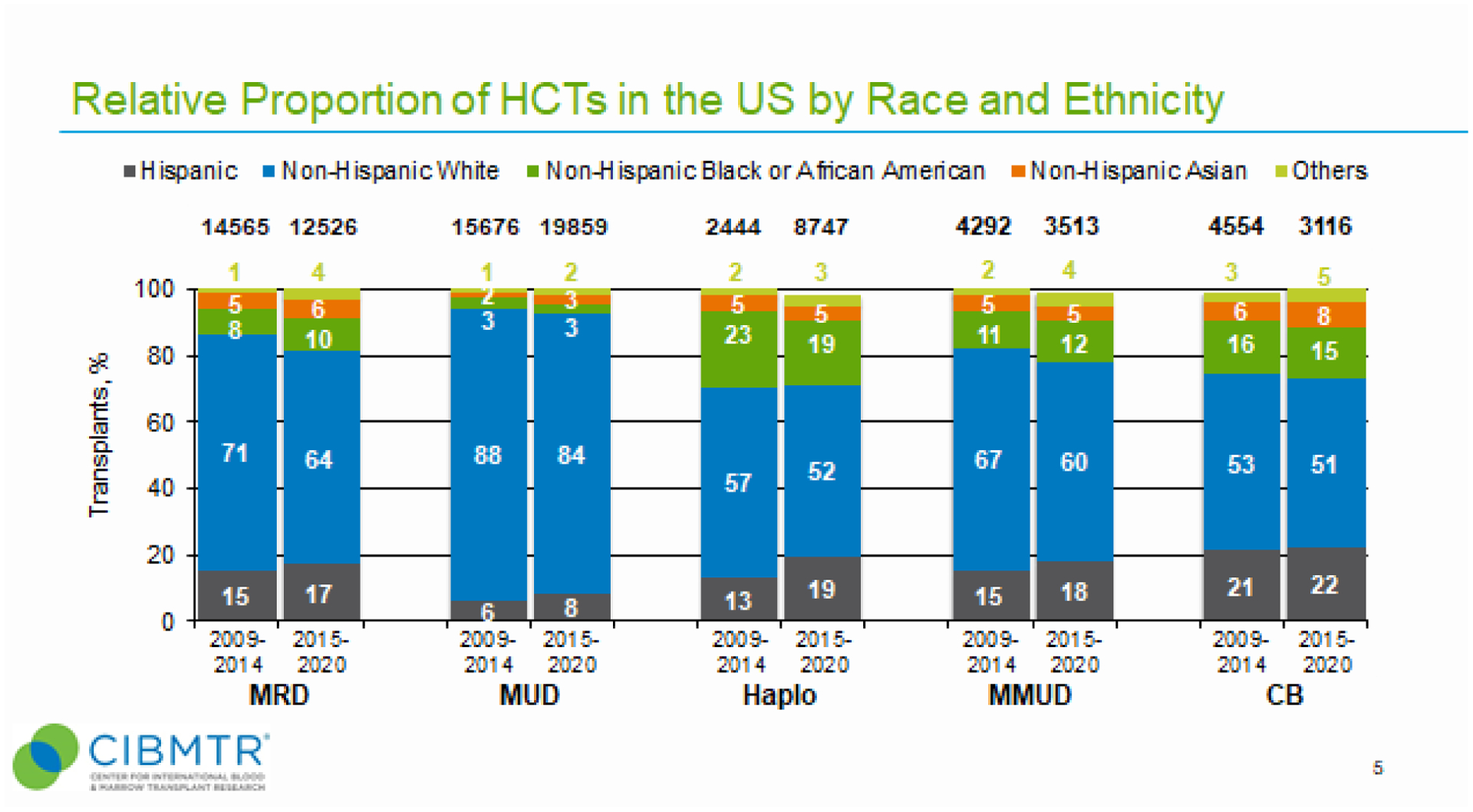
For each transplant era, proportions of transplants by indicated donor type is shown as percentage of absolute number of transplants performed (N) for that year. “Other” patient race and ethnicity group includes non-Hispanic Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, more than one race, and non-resident of the U.S.
Given the use of alternative donor platforms, we sought to define trends in GvHD prophylaxis (Figure 3). Overall, CNI-based GvHD prophylaxis was used primarily in the HLA-matched donor setting (Figure 3, panel A), whereas post-transplant cyclophosphamide (PTCy) was used primarily in the HLA-mismatched donor setting (Figure 3, panel B). As graft source differs by recipient age (BM in pediatrics and PB in adults) (Supplemental Figure 3, panels A and B, respectively), we also wanted to determine if recipient age influenced the choice of GvHD prophylaxes. Despite age-related differences in allograft types, calcineurin inhibitor (CNI)-based GvHD prophylaxis predominated in the HLA-matched setting for both pediatric and adult allogeneic HCT (Supplemental Figure 4, panels A-D). For pediatric patients, ex vivo graft manipulation was used more in the MUD than MRD settings, but overall much less than CNI-based GvHD prophylaxis in either setting (Supplemental Figure 4, panels A and C). For adult recipients, use of PTCy increased in the HLA-matched donor settings (Supplemental Figure 4, panel E). While CNI-based GvHD prophylaxis in haploidentical HCT dramatically decreased, use of PTCy increased nearly to exclusivity by 2020 (Supplemental Figure 4, panel E). Use of PTCy also increased for MMUD HCT from 8% in 2015 to 55% of MMUD transplants in 2020 (Supplemental Figure 4, panel F).
Figure 3. Relative proportion of matched and mismatched donor HCT in the U.S. by graft-versus-host disease (GvHD) prophylaxis (2010–2020).
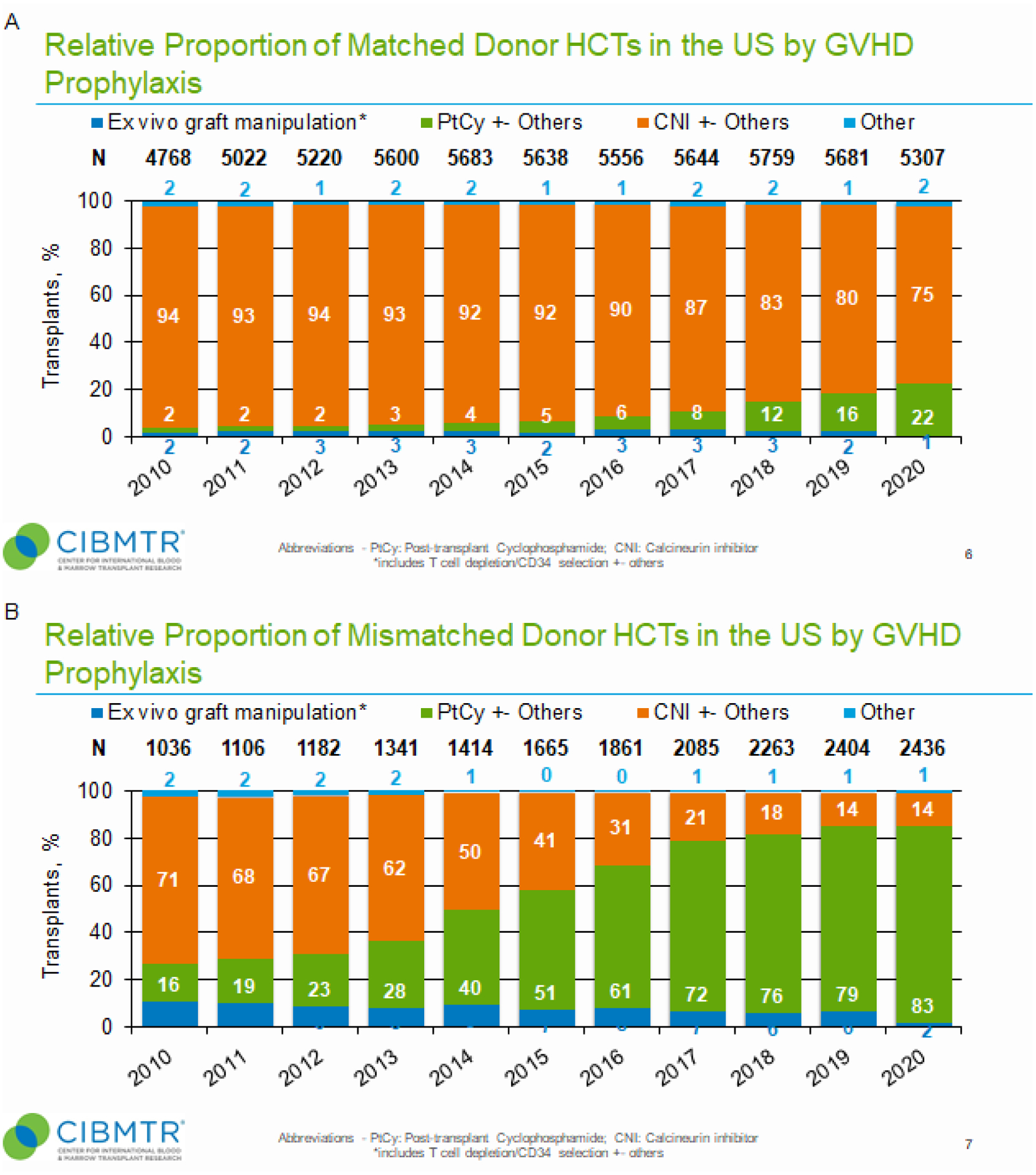
Panel A, Relative proportion of matched donor HCTs in the U.S. by GvHD prophylaxis. For each year, proportions of transplants by indicated GvHD prophylaxis is shown as percentage of absolute number of transplants performed (N) for that year. Panel B, Relative proportion of mismatched donor HCTs in the U.S. by GvHD prophylaxis. Ex vivo graft manipulation includes T-cell depletion/CD34 selection as well as other techniques. CNI, calcineurin inhibitor; PTCy, post-transplant cyclophosphamide.
Lastly, with a notable decline in the number of allogeneic HCT performed in 2020, we wanted to determine if frequencies of graft types and GvHD prophylaxis also differed from those in previous years (2009–2019) (Table 1). Specifically, less BM (17% vs. 22%) and more PB (78% vs. 69%) were used as allografts in 2020 than in the previous decade, respectively. Also, less CNI-based (56% vs. 79%) and more PTCy-based (39% vs. 14%) GvHD prophylaxes were used in 2020 than in the previous decade, respectively.
Trends in survival and causes of death after allogeneic HCT
We next examined trends in survival probability by transplant era, race and ethnicity, and age. For the most frequent disease indications for allogeneic HCT (ALL, AML, and MDS), trends in survival were highest during the most recent contemporary transplant era (2016–2019) (Figure 4). Favorable survival rates for patients with hematologic malignancies associating with the most contemporary transplant era was noted for all race and ethnic patient groups (Supplemental Figures 5, 6 and 7) and in both pediatric and adult patients (data not shown).
Figure 4. Trends in recipient overall survival after allogeneic HCT for hematologic malignancies by transplant era.
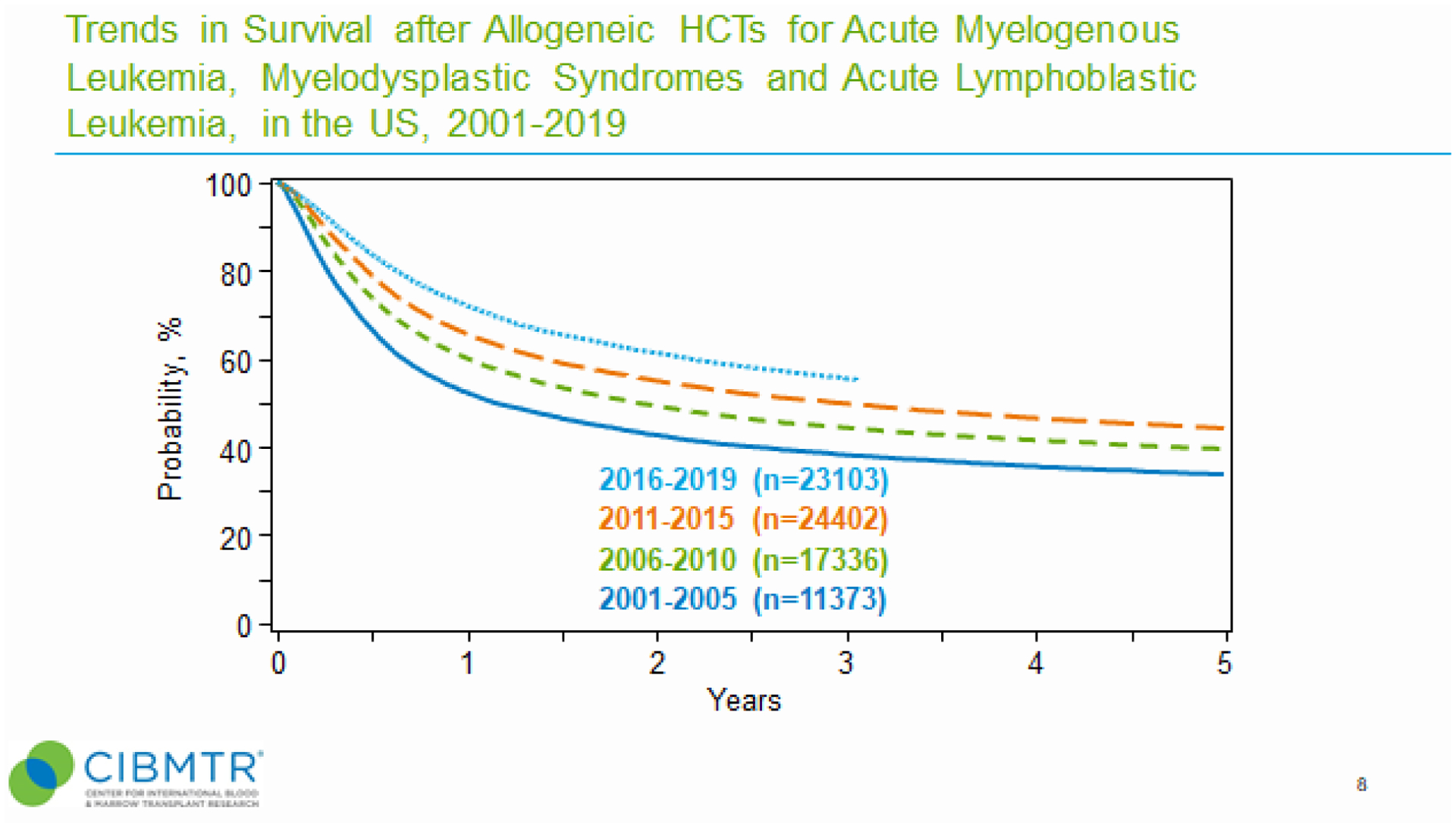
Kaplan-Meier curves combining survival data for AML, ALL and MDS is shown for each indicated transplant era.
Having observed similar survival probability among R&E groups, we also wanted to compare trends in reported causes of death (COD) by age and donor type across patient R&E groups (Supplemental Table 1). Primary disease was the most common COD following allogeneic HCT for adults and pediatrics across all patient groups. In pediatrics, organ failure was the second most frequent reported COD after allogeneic HCT. In contrast to adults, pediatric allogeneic HCT recipients had lower trends in GvHD as a reported COD. Comparing trends in COD by donor types, primary disease was again the most frequent reported COD across all patient R&E groups and donor types (Supplemental Table 1). Among patient R&E groups, GvHD was generally reported more frequently as COD following MUD and MMUD HCT versus MRD and haploidentical donor HCT. General trends for reported COD were similar across R&E groups with one notable exception being a higher reported frequency of GvHD as a COD for Black/African American patients following MUD HCT.
Discussion
Analyzing real-world data in allogeneic HCT in the CIBMTR registry provides an accurate assessment of practice trends in allogeneic HCT in the U.S. Five key findings resulted from our analyses. First, in 2020, total numbers of allogeneic HCTs decreased for the first time since 20005. Specifically, ~5% less allogeneic HCTs were performed in 2020 relative to 2019, likely reflecting the first full year of Coronavirus Disease 2019 (COVID-19), which was declared a pandemic by the World Health Organization (WHO) on March 11, 202011. COVID-19 caused many transplant centers to defer all but the most urgent procedures and impeded unrelated donor confirmatory typing and clearance as well as product acquisition and transport12. Secondary impacts of the pandemic including staff and supply shortages still remain13. Immunotherapy, including cellular therapy like chimeric antigen receptor (CAR) T-cells, may also have contributed to the decrease in allogeneic HCT for malignant diseases like diffuse large B-cell lymphoma14 and precursor B-cell acute lymphoblastic leukemia (ALL)15. The decrease in allogeneic HCT in 2020 was also associated with increased frequencies of PB allografts and PTCy-based GvHD prophylaxis relative to the previous decade (2009–2019), likely reflecting mandates for using cryopreservation early in the pandemic16. Compared to 2020, numbers of allogeneic HCT have risen by 2% in 2021 (8498) but remain lower than in 2019 (8740) and 2018 (8678). Second, use of haploidentical donors and post-transplant cyclophosphamide (PTCy) as GvHD prophylaxis have increased in frequency over the last decade. While the use of PTCy has increased with all donor types, its use is most marked in the HLA-mismatched setting. Third, although allogeneic HCT has been performed in more NHW patients, relative proportions of ethnically diverse patients receiving haploidentical and MMUD have increased over the last decade. Fourth, CB also associates with diverse patient backgrounds similar to haploidentical and MMUD, but its use as a graft source relative to bone marrow and peripheral blood has decreased over the last decade. Fifth, patients in all R&E groups experienced increases in survival in the more contemporary transplant eras, and primary disease was the main cause of death by age and donor type. Together, these data suggest that more ethnically diverse patients are able to receive allogeneic HCT largely due to the use of alternative donor platforms and PTCy as GvHD prophylaxis. While not shown in this manuscript, the annual center-specific analysis shows race and ethnicity are not significant factors associated with survival when survival is adjusted for all appropriate variables, including donor type and GvHD prophylaxis17–19.
Allogeneic transplant indications including ALL, aplastic anemia and non-malignant disorders like hemoglobinopathies differentially affect patients based upon their R&E, suggesting a persistent unmet need in that allogeneic HCT should be offered more to patients with diverse backgrounds20. Haploidentical, MMUD and UCB have been used for similar relative proportions of diverse patients, which are higher in and all associated with greater R&E diversity than MUD. Novel GvHD prophylaxis strategies like PTCy and abatacept have successfully been used in the HLA-mismatched setting, enabling more diverse patients to receive allogenic HCT. In the NMDP-sponsored 15-MMUD phase II study (NCT02793544) incorporating PTCy in combination with sirolimus and mycophenolate mofetil (MMF) as GvHD prophylaxis and MMUD BM, 48% of total enrolled patients were ED10. In the City of Hope experience using MMUD PBSC and combination PTCy, MMF and tacrolimus as GvHD as GvHD prophylaxis (NCT03128359), 61% of total enrolled patients were ethnically diverse21. Lastly, in a post-hoc analysis of the phase II trial Abatacept 2 (ABA2, NCT01743131) incorporating abatacept in combination with calcineurin inhibitor and MMF22, 30.2% patients receiving this combination as GvHD prophylaxis were diverse patients23. Together, these studies suggest that more ethnically diverse patients with hematologic malignancies can receive transplants if HLA mismatch donors are used.
Historical data shows that patients receiving HLA-mismatch donor types have inferior survival to those using HLA-matched donors8. However, whether patients receiving HLA-mismatched HCT using novel GvHD prophylaxis achieve similar post-transplant outcomes as patients receiving HLA-matched HCT remains to be determined. As no randomized control trials have been performed in this setting. Furthermore, study results suggest that access barriers beyond securing an available donor continue to impede ethnically diverse patients from receiving allogeneic HCT within the U.S.24 and internationally25. Therefore, a holistic approach is needed not only incorporating patient support services including education, counseling and financial assistance, but also addressing fundamental issues like poverty and racial inequity by key stakeholders across the HCT ecosystem such as transplant centers, payors, industry and federal institutions26.
Access barriers based upon R&E may result in outcome disparities by not enabling such patients to achieve best outcomes by receiving standard-of-care therapy for high-risk malignant disease. In addition, R&E could impact outcomes by influencing care received post-HCT. In a previous CIBMTR study, Baker et al. found that Blacks receiving myeloablative MUD HCT for ALL, AML, MDS and chronic myeloid leukemia had worse OS, disease free survival (DFS), and higher transplant-related mortality (TRM) than Whites27. In the MUD HCT setting, worse survival associating with race may reflect not only underlying HLA biology28, but also social determinants of health that may have biologic ramifications29. Likewise, biology of underlying malignant disease also contributes to post-transplant survival. AML in Black/African American (B/AA) adult and pediatric patients is associated with high-risk cytogenetic profiles and subsequent poor survival30,31. Therefore, B/AA patients with newly diagnosed AML may require faster donor identification and referral to transplant centers in order to improve and achieve best survival outcomes32.
The study has several limitations, largely reflecting its data being from a retrospective, observational registry. First, CIBMTR only collects data on those patients who receive HCT so the denominator of patients requiring but not receiving HCT cannot be addressed. In addition, R&E and minimal other social determinants of health were reported by transplant centers and may not accurately reflect persons of mixed race, given mixed races were grouped as “other” rather than defined by patient self-reporting. Second, the data are presented as trends. Notwithstanding, findings from this real-world evidence, observational database provide the foundation to investigate further the influence of R&E on post-transplant outcomes and use of alternative donor platforms to provide allogeneic transplant to more ethnically diverse patients in need.
In summary, these results show that ethnically diverse patients are receiving higher numbers of allogeneic HCT largely through the use of alternative donor platforms incorporating PTCy. Improving HCT outcomes using alternative donors and novel GvHD prophylaxis strategies is an urgent need for the field.
Supplementary Material
Financial Disclosure Statement
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Sanofi; Sanofi-Aventis U.S. Inc.; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
Conflicts of Interest
Dr. Jeffery Auletta reports Employee, National Marrow Donor Program and Advisory Committee for AscellaHealth.
Dr. Steven Devine reports providing paid advise to the following companies - Orca Bio, Vor, Magenta Inc. He states that this money is paid to NMDP, not to him directly.
Kathryn Flynn reports research grants from NIH and Novartis, consulting for ReFocus, Inhibikase Therapeutics, and Pfizer.
Dr. Mehdi Hamadani reports Compensation: Research Support/Funding: Takeda Pharmaceutical Company; ADC Therapeutics; Spectrum Pharmaceuticals; Astellas Pharma. Research Funding to Institution: Janssen R&D, Celgene Corporation, Merck, MedImmune, Seattle Genetics, Millennium Pharmaceuticals. Consultancy: Incyte Corporation (2021); ADC Therapeutics (2021); Pharmacyclics (2018), Omeros (2021), Verastem (2019), TeneoBio (2019), MorphoSys (2021), Kite (2021), Genmab (2021), SeaGen (2021), Gamida Cell (2021), Novartis (2021), Legend Biotech (2021). Speaker’s Bureau: Sanofi Genzyme (2021), AstraZeneca (2021), BeiGene (2021), ADC Therapeutics (2022). DMC: Myeloid Therapeutics, Inc.
Dr. Stephanie Lee reports: Compensation: Consultant: Mallinckrodt, Equillium. Spouse: Consultant: Almirall, Rain Therapeutics, EMD Serono/Pfizer Honoraria: Wolters Kluwer
Payments: Research funding: Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, Takeda. Provision of study medication: Janssen. Spouse: Research funding: BMS, EMD Serono
Relationships: Non-profit leadership: Board of Directors, National Marrow Donor Program. Spouse - Non-profit leadership: Secretary/Treasurer, Society for Investigative Dermatology Proprietary Interests: Spouse - Patents: Immunotherapy for Merkel Cell Carcinoma.
Dr. Marcelo Pasquini reports compensation from Bristol Myers Squibb for consulting.
Dr. Rachel Phelan reports compensation - advisory board from both Blue Bird Bio and Bioline Rx; research funding from Amgen.
Dr. Marcie Riches reports: Compensation: IQVIA Biotech, employee (as of 1/10/22).
Dr. Douglas Rizzo reports: Compensation: I participate in Optum Stem Cell Expert Panel to provide input /information about the center specific survival analysis based on my expertise. Compensation for this effort (~$1000) is directed to MCW rather than to me personally.
Dr. Bronwen Shaw reports consulting to Orcabio and Mallinkrodt.
Footnotes
Data Use Statement
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy EA, Ferguson SS, Omondi NA, et al. The National Marrow Donor Program’s symposium on patient advocacy in cellular transplantation therapy: addressing barriers to hematopoietic cell transplantation. Biol Blood Marrow Transplant 2010;16:147–56. [DOI] [PubMed] [Google Scholar]
- 2.Shouval R, Fein JA, Labopin M, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol 2019;6:e573–e84. [DOI] [PubMed] [Google Scholar]
- 3.Besse K, Maiers M, Confer D, Albrecht M. On Modeling Human Leukocyte Antigen-Identical Sibling Match Probability for Allogeneic Hematopoietic Cell Transplantation: Estimating the Need for an Unrelated Donor Source. Biol Blood Marrow Transplant 2016;22:410–7. [DOI] [PubMed] [Google Scholar]
- 4.Holtan SG, Versluis J, Weisdorf DJ, Cornelissen JJ. Optimizing Donor Choice and GVHD Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. J Clin Oncol 2021;39:373–85. [DOI] [PubMed] [Google Scholar]
- 5.Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2021.
- 6.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 2014;371:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2020 U.S. Population more racially and ethnically diverse than measured in 2010. 2022. (Accessed March 25, 2022, 2022, at https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html.)
- 8.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 2007;110:4576–83. [DOI] [PubMed] [Google Scholar]
- 9.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008;14:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol 2021;39:1971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Organization WH. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020.
- 12.Algwaiz G, Aljurf M, Koh M, et al. Real-World Issues and Potential Solutions in Hematopoietic Cell Transplantation during the COVID-19 Pandemic: Perspectives from the Worldwide Network for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol Blood Marrow Transplant 2020;26:2181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo J, Auletta JJ, Hartley DM, et al. Secondary Impact of the Coronavirus Disease 19 Pandemic on Patients and the Cellular Therapy Healthcare Ecosystem. Transplant Cell Ther 2022;28:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson CA, Locke FL, Ma L, et al. Real-World Evidence of Axicabtagene Ciloleucel for the Treatment of Large B Cell Lymphoma in the United States. Transplant Cell Ther 2022;28:581 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 2020;4:5414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auletta JJ, Novakovich JL, Stritesky GL, et al. Meeting the Demand for Unrelated Donors in the Midst of the COVID-19 Pandemic: Rapid Adaptations by the National Marrow Donor Program and Its Network Partners Ensured a Safe Supply of Donor Products. Transplant Cell Ther 2021;27:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Transplant and Survival Statistics on Related Sites. CIBMTR, 2022. at https://www.cibmtr.org/ReferenceCenter/SlidesReports/USStats/Pages/index.aspx.)
- 18.Transplant Center Search. 2022. at https://bethematch.org/tcdirectory/search/.)
- 19.Transplant Survival Rates. 2022. at https://bloodstemcell.hrsa.gov/data/transplant-survival-report.)
- 20.Jabo B, Morgan JW, Martinez ME, Ghamsary M, Wieduwilt MJ. Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One 2017;12:e0174760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Malki MM, Tsai NC, Palmer J, et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv 2021;5:2650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins B, Qayed M, McCracken C, et al. Phase II Trial of Costimulation Blockade With Abatacept for Prevention of Acute GVHD. J Clin Oncol 2021;39:1865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qayed M, Watkins B, Gillespie S, et al. Abatacept for GVHD prophylaxis can reduce racial disparities by abrogating the impact of mismatching in unrelated donor stem cell transplantation. Blood Adv 2022;6:746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong S, Majhail NS. Increasing access to allotransplants in the United States: the impact of race, geography, and socioeconomics. Hematology Am Soc Hematol Educ Program 2021;2021:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha V, Fatobene G, Niederwieser D, Brazilian Society of Bone Marrow T, the Worldwide Network for B, Marrow T. Increasing access to allogeneic hematopoietic cell transplant: an international perspective. Hematology Am Soc Hematol Educ Program 2021;2021:264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auletta JJ, Sandmaier BM, Jensen E, et al. The ASTCT-NMDP ACCESS Initiative: A Collaboration to Address and Sustain Equal Outcomes for All Across the Hematopoietic Cell Transplantation and Cellular Therapy Ecosystem. Transplant Cell Ther 2022. [DOI] [PubMed] [Google Scholar]
- 27.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009;15:1543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishima Y, Morishima S, Stevenson P, et al. Race and Survival in Unrelated Hematopoietic Cell Transplantation. Transplant Cell Ther 2022;28:357 e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight JM, Rizzo JD, Logan BR, et al. Low Socioeconomic Status, Adverse Gene Expression Profiles, and Clinical Outcomes in Hematopoietic Stem Cell Transplant Recipients. Clin Cancer Res 2016;22:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatnagar B, Kohlschmidt J, Mrozek K, et al. Poor Survival and Differential Impact of Genetic Features of Black Patients with Acute Myeloid Leukemia. Cancer Discov 2021;11:626–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conneely SE, McAtee CL, Gupta R, Lubega J, Scheurer ME, Rau RE. Association of race and ethnicity with clinical phenotype, genetics, and survival in pediatric acute myeloid leukemia. Blood Adv 2021;5:4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagel JM, Othus M, Garcia-Manero G, et al. Rapid Donor Identification Improves Survival in High-Risk First-Remission Patients With Acute Myeloid Leukemia. JCO Oncol Pract 2020;16:e464–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


