Abstract
Background:
Diesel exhaust is a complex mixture, including polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (nitro-PAHs), many of which are potent mutagens and possible bladder carcinogens. To explore the association between diesel exposure and bladder carcinogenesis, we examined the relationship between exposure and somatic mutations and mutational signatures in bladder tumors.
Methods:
Targeted sequencing was conducted in bladder tumors from the New England Bladder Cancer Study. Using data on 797 cases and 1,418 controls, two-stage polytomous logistic regression was used to evaluate etiologic heterogeneity between bladder cancer subtypes and quantitative, lifetime estimates of respirable elemental carbon (REC), a surrogate for diesel exposure. Poisson regression was used to evaluate associations between REC and mutational signatures.
Results:
We observed significant heterogeneity in the diesel-bladder cancer risk relationship, with a strong positive association among cases with high-grade, non-muscle invasive TP53-mutated tumors compared to controls (Odds Ratio(OR)Top Tertile vs.Unexposed=4.8, 95% Confidence Interval(CI)=2.2,10.5; p-trend<0.001; p-heterogeneity=0.002). In muscle-invasive tumors, we observed a positive association between diesel exposure and the nitro-PAH signatures of 1,6-dintropyrene (RR=1.93, 95% CI=1.28,2.92) and 3-nitrobenzoic acid (RR=1.97, 95% CI=1.33,2.92).
Conclusion:
The relationship between diesel exhaust and bladder cancer was heterogeneous based on the presence of TP53 mutations in tumors, further supporting the link between PAH exposure and TP53 mutations in carcinogenesis. Future studies that can identify nitro-PAH signatures in exposed tumors are warranted to add human data supporting the link between diesel and bladder cancer.
Impact:
This study provides additional insight into the etiology and possible mechanisms related to diesel exhaust-induced bladder cancer.
Keywords: Bladder cancer, TP53, diesel, next-generation sequencing, nitrated polycyclic aromatic hydrocarbons
Introduction
Bladder cancer is the tenth most common cancer worldwide [1]. Certain occupational exposures, as well as increasing age, male sex, and a history of cigarette smoking, are established risk factors for bladder cancer [2]. Diesel exhaust, a notable occupational and environmental exposure, was classified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer (IARC) in 2012 based on sufficient positive evidence for lung cancer and limited positive evidence for bladder cancer [3]. Diesel exhaust is a complex mixture of varying compounds, including polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (nitro-PAHs), many of which are potent mutagens and possible bladder carcinogens [2, 3]. Associations between diesel exhaust and bladder cancer risk have largely been reported from studies where diesel exhaust exposure was inferred for specific occupations (including truck drivers, mechanics, railroad workers, and operators of heavy equipment) [4]. More recent studies that quantified cumulative exposure to diesel exhaust have found a 60% increased risk of bladder cancer in subjects with high levels of exposure [5, 6], including our previous work that combined data from two large population-based case-control studies [6]. We aim to build on our previous observations by evaluating possible mechanisms by which diesel exhaust might lead to bladder cancer.
In this study, we evaluated the associations between exposure to diesel exhaust and somatic mutations observed in bladder tumors based on data from two large studies of bladder cancer. Studies such as these are needed to establish biologic plausibility that diesel exhaust causes bladder cancer in humans.
Materials and Methods
Study Population
The New England Bladder Cancer Study (NEBCS) is a population-based case-control study that included 1,213 cases and 1,418 controls. Cases in the NEBCS were patients with histologically confirmed bladder cancer newly diagnosed between 2001 and 2004 among residents of Maine, New Hampshire, and Vermont, ages 30 to 79 years. Sixty-five percent of all eligible cases, identified through hospital pathology departments and hospital or state cancer registries, were interviewed by trained interviewers after providing written informed consent. A standardized histopathology review to assign stage and grade was carried out by a study pathologist. Tumors were staged according to the tumor, node, and metastases (TNM) criteria and graded according to the 1973 World Health Organization (WHO) criteria. The study protocol was approved by all appropriate institutional review boards. All cases from Maine and Vermont were considered (n=797) in the current analysis, with sequencing data available for 322 cases. The study protocol was approved by the institutional review board of the National Cancer Institute (in accordance with the Belmont Report).
Targeted sequencing of 44 genes frequently mutated in bladder cancer (Supplemental Table 1) was conducted in 322 formalin-fixed paraffin-embedded (FFPE) bladder tumors from NEBCS patients enrolled in Maine and Vermont who returned pathology consent forms, completed the interview, and had DNA (tumor and normal) available for the current analysis. DNA from FFPE tumors was isolated using the phenol-based AutoGenprep 245T Animal Tissue DNA Extraction Kit (Autogen). Normal DNA was extracted, using the Autopure protocol (QIAGEN), from exfoliated buccal cells collected from mouthwash samples.
Ampliseq Designer was used to design a panel of amplicons to cover the coding region and splice site regions of each exon in the 44 targeted genes. For each patient, 30 ng of tumor and germline DNA was used for amplification, library construction, and sequencing (performed on an Ion Torrent S5). Additional details pertaining to the sequencing, variant calling and quality control have been previously published [7]. Variants with a quality score ≥50 and allele fraction ≥10% were called and included in the current analysis.
In the NEBCS, lifetime occupational histories combined with exposure-oriented questions were used to estimate cumulative exposure to respirable elemental carbon (REC), a primary surrogate for diesel exhaust [8]. A detailed description of the assessment of diesel exhaust exposure and risk associated with bladder cancer have been previously published [6]. An industrial hygienist blindly assigned probability, intensity, and frequency of diesel exposure for each job based on an extensive review of the diesel exhaust occupational health literature and detailed questionnaire responses from the subjects on their work activities [9]. The cumulative exposure calculation was limited to participants with jobs where the estimated proportion of workers exposed to diesel exhaust was ≥50%. This metric was calculated by summing the product of intensity, frequency, and duration of exposure over all jobs. In addition to frequency (hours/week) and duration (years), participants were also queried about location (indoor/outdoor), activities performed, products/services made/provided, equipment/chemicals handled, and whether they worked near engines or smelled engine exhaust.
We also used information from 412 muscle-invasive bladder cancers included in The Cancer Genome Atlas (TCGA) project (https://tcga-data.nci.nih.gov/). Publicly available, whole-exome sequencing data was used to identify tumor mutations [10]. In TCGA, patients reported occupational information such as job title of usual occupation (“occupation in which the patient was employed for the majority of their working years”), industry of usual occupation, and chemical exposures (“any chemical exposure the patient had during their working years in their primary occupation”). These data were used to create a dichotomized variable (no/yes) for exposure to diesel exhaust, based on expert knowledge of jobs with either moderate or high probability of occupational diesel exposure as previously described [11].
Statistical Analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using two-stage polytomous regression [12] to evaluate etiologic heterogeneity and measure the association between cumulative REC and risk of bladder cancer subtypes defined by the presence/absence of specific gene mutations. Additional subtype models were also considered using tumor characteristics such as stage (non-muscle invasive vs. muscle-invasive) and grade (low vs. high). We used the R package SignatureEstimation [13] to extract the four major single-base substitution signatures observed in bladder tumors: COSMIC signature 1, APOBEC-mediated signatures 2 and 13, and 5*/ERCC2 signature [10, 14], as well as four mutational signatures for nitro-PAHs: 1,6-dinitropyrene (1,6-DNP), 1,8-dinitropyrene (1,8-DNP), 3-nitrobenzoic acid (3-NBA), and 6-nitrochysene [15]. These nitro-PAH signatures were selected because the nitro-PAHs are considered specific markers of diesel exhaust exposure[3], they are potent mutagens which are considered to be the main contributors to the genotoxicity of diesel exhaust[16, 17], they have been shown to result in robust single base substitution signatures in experimental models[15], and studies of populations exposed to diesel have shown the presence of nitrated PAHs excreted in urine (and thus bioavailable to the bladder)[18–20]. Poisson regression was used to evaluate the relationship between cumulative REC and these mutational signatures. Case-control analyses to identify risk heterogeneity were conducted only in the NEBCS and case-case analyses with mutational signatures were conducted in both the NEBCS and TCGA datasets.
Diesel exhaust exposure was dichotomized (no/yes) in both NEBCS and TCGA and was also evaluated continuously (cumulative REC per 100 μg/m3) or categorically (based on the cumulative REC distribution in exposed cases) in the NEBCS. We used the Wald test for linear trends, treating the median value for each category (including unexposed) among control subjects as continuous. In these models, we adjusted for covariates that changed parameter estimates by more than 10%. Multivariate analyses for binary outcomes (absence or presence of gene mutation) were performed using R package TOP [12] and analyses for mutational signatures were performed using SAS 9.3 (SAS Institute Inc., Cary NC).
Data availability
The data from the current study are available from the corresponding author on reasonable request.
Results
Characteristics of cases and controls from the NEBCS and cases from TCGA are shown in Table 1. A total of 15 cases were excluded from NEBCS and 29 from TCGA analyses because of variant quality-control. An additional 33 were excluded from NEBCS (9 controls and 24 cases) due to missing information on occupational histories. Cases with and without tumor sequencing data in the NEBCS were similar with regard to age, sex, race, smoking status, stage/grade, and diesel exposure (Supplemental Table 2). In the population-based NEBCS, 260 (84.5%) tumors were non-muscle invasive and 47 (15.5%) were muscle-invasive, while all 383 (100%) of the tumors from TCGA were muscle-invasive.
Table 1:
Select characteristics among cases and controls in the New England Bladder Cancer Study and cases in The Cancer Genome Atlas.
| New England Bladder Cancer Study (NEBCS) | The Cancer Genome Atlas (TCGA) | ||
|---|---|---|---|
| Characteristic | Cases* N=307 |
Controls N=1,418 |
Cases* N=383 |
| Age; N (%) | |||
| <55 | 52 (17.0) | 255 (18.0) | 45 (11.8) |
| 55–64 | 87 (28.3) | 336 (23.7) | 107 (27.9) |
| 65–74 | 109 (35.5) | 544 (38.3) | 127 (33.2) |
| 75+ | 59 (19.2) | 283 (20.0) | 104 (27.2) |
| Sex; N (%) | |||
| Female | 74 (24.1) | 379 (26.7) | 94 (24.5) |
| Male | 233 (75.9) | 1,039 (73.3) | 289 (75.5) |
| Race; N (%) | |||
| White | 279 (90.9) | 1,313 (92.6) | 301 (78.6) |
| Non-White | 28 (9.1) | 105 (7.4) | 58 (17.8) |
| Smoking; N (%) | |||
| Occasional smokers | 4 (1.3) | 40 (2.8) | N/A |
| Never smokers | 46 (15.0) | 472 (33.3) | 95 (24.8) |
| Former smokers | 154 (50.2) | 699 (49.3) | 187 (48.8) |
| Current smokers | 103 (33.5) | 206 (14.5) | 83 (21.7) |
| Stage/Grade; N (%) | |||
| Ta, Low-Grade | 139 (45.3) | N/A | 0 (0.0) |
| Ta, High-Grade | 60 (19.5) | N/A | 0 (0.0) |
| T1, High-Grade | 61 (19.9) | N/A | 0 (0.0) |
| T2+ (MI) | 47 (15.3) | N/A | 383 (100.0) |
| Diesel Exposure; N (%) | |||
| Unexposed | 126 (41.0) | 639 (45.1) | 184 (48.0) |
| Possibly exposed | 37 (12.1) | 165 (11.6) | N/A |
| Exposed | 141 (45.9) | 605 (42.7) | 56 (14.6) |
| Missing | 3 (1.0) | 9 (0.6) | 143 (37.4) |
Excludes cases that were dropped after quality control.
N/A=Not Applicable or data not collected/available.
We observed significant heterogeneity in the relationship between diesel exhaust and risk of bladder cancer by TP53 mutation status in the NEBCS (p-heterogeneity=0.013) (Table 2, Supplemental Table 3). The highest tertile (T) of REC exposure from diesel exhaust was associated with bladder cancer risk only when comparing patients with TP53-mutated tumors with controls (compared to unexposed: ORT1=1.36, 95% CI=0.66, 2.78; ORT2=1.30, 95% CI=0.63, 2.69; ORT3=2.84, 95% CI=1.40, 5.74; p-trend=0.003). Among patients with wild-type TP53 tumors, there was no apparent association between cumulative REC exposure and bladder cancer (p-trend=0.603). Occurrence of somatic mutations in other genes was not associated with cumulative REC. Similar to the results from the NEBCS in Table 3, there was no association between diesel exposure and TP53 mutation in muscle-invasive cases in TCGA (case-case OREver=0.76, 95% CI=0.40, 1.46).
Table 2:
Odds ratios for cumulative respirable elemental carbon (REC) and risk of bladder cancer by TP53 tumor subtype from two-stage polytomous logistic regression in the New England Bladder Cancer Study.
| No TP53 mutation | TP53 mutation | ||||
|---|---|---|---|---|---|
| Cumulative REC ** (μg/m3-yrs) | Controls | Cases | Odds Ratio* (95% CI) |
Cases | Odds Ratio* (95% CI) |
| Case-case analysis | |||||
| Unexposed | - | 98 | Ref | 28 | Ref |
| 0 – 8.6 | - | 44 | Ref | 12 | 1.28 (0.55, 3.01) |
| >8.6 – 73.5 | - | 36 | Ref | 13 | 1.52 (0.65, 3.59) |
| >73.5 | - | 24 | Ref | 12 | 3.20 (1.28, 7.99) |
| p-trend | 0.015 | ||||
| Case-control analysis | |||||
| Unexposed | 639 | 98 | Ref | 28 | Ref |
| 0 – 8.6 | 255 | 44 | 1.06 (0.76, 1.48) | 12 | 1.36 (0.66, 2.78) |
| >8.6 – 73.5 | 211 | 36 | 0.86 (0.60, 1.23) | 13 | 1.30 (0.63, 2.69) |
| 013 | 139 | 24 | 0.89 (0.56, 1.40) | 12 | 2.84 (1.40, 5.74) |
| p-trend=0.603 | p-trend=0.003 | ||||
| p-heterogeneity = 0.013 | |||||
| Continuous REC (per 100 μg/m3-yrs) | 1244 | 202 | 1.05 (0.95, 1.15) | 65 | 1.11 (0.97, 1.28) |
Adjusted for age, race/ethnicity, sex, smoking status, and employment in a non-diesel related high-risk occupation.
Cut points for cumulative REC (tertiles) are based on TP53-mutated cases.
Note: counts do not add up to total due to exclusion of those possibly exposed to diesel from the reference group.
Table 3:
Odds ratios from two-stage polytomous logistic regression for cumulative respirable elemental carbon (REC) and risk of bladder cancer using alternate subtype classifications (TP53, pathologic stage, and grade according to 1973 WHO criteria) in the New England Bladder Cancer Study.
| Subtype** | |||||
|---|---|---|---|---|---|
| NMI/low-grade/TP53 (−) N=138 |
NMI/high-grade/TP53 (−) N=72 |
NMI/high-grade/TP53 (+) N=42 |
MI/high-grade/TP53(+) N=28 |
p-heterogeneity | |
| Odds Ratio* (95% CI) |
Odds Ratio* (95% CI) |
Odds Ratio* (95% CI) |
Odds Ratio* (95% CI) |
||
| Continuous REC (per 100 μg/m3-yrs) | 1.08 (0.99, 1.18) | 0.98 (0.81, 1.19) | 1.14 (1.00, 1.29) | 1.07 (0.91, 1.26) | |
| Cumulative REC (μg/m3-yrs) | |||||
| Unexposed | Ref | Ref | Ref | Ref | |
| 0 – 8.6 | 1.12 (0.78, 1.62) | 1.02 (0.62, 1.68) | 2.34 (1.09, 5.04) | 0.92 (0.44, 1.95) | |
| >8.6 – 73.5 | 0.90 (0.60, 1.35) | 0.80 (0.48, 1.35) | 2.02 (0.90, 4.53) | 1.13 (0.54, 2.39) | |
| >73.5 | 1.12 (0.72, 1.76) | 0.78 (0.40, 1.54) | 4.79 (2.19, 10.51) | 1.26 (0.54, 2.89) | |
| p-trend=0.627 | p-trend=0.492 | p-trend<0.001 | p-trend=0.604 | 0.002 | |
Adjusted for age, race/ethnicity, sex, smoking status, and employment in a non-diesel related high-risk occupation.
Carcinoma in situ is included in NMI/high-grade. Only subtypes with sufficient sample size are listed.
NMI=Non-muscle invasive, MI=Muscle invasive.
Subtype analyses incorporating TP53 mutation status and additional clinical features (stage: non-muscle invasive, muscle-invasive and grade: low-grade, high-grade) also indicate etiologic heterogeneity in the relationship between diesel exhaust and bladder cancer (p-het=0.002) (Table 3). The observed association between increasing cumulative REC tertiles was strongest among non-muscle invasive, high-grade tumors with a TP53 mutation (compared to unexposed: ORT1=2.34, 95% CI=1.09, 5.04; ORT2=2.02, 95% CI=0.90, 4.53; ORT3=4.79, 95% CI=2.19, 10.51; p-trend<0.001). In other subtypes, there was no association between diesel exposure and bladder cancer risk. Consistent with the overall mutational profile of bladder tumors, the majority of mutations observed in TP53 were of APOBEC-type and multivariable analysis did not reveal any specific TP53 mutation type to be associated with exposure (Supplemental Table 4).
We observed several positive associations between nitro-PAH mutational signatures and exposure to diesel exhaust in muscle-invasive bladder cancer in both the NEBCS and TCGA datasets (Table 4). In pooled analyses adjusted for study, we saw the strongest associations for 1,6-DNP (OR=1.93, 95% CI=1.28, 2.92) and 3-NBA (OR=1.97, 95% CI=1.33, 2.92). No associations were found in non-muscle invasive cases from the NEBCS. Exposure-response relationships were limited to the NEBCS, but also suggested an increasing trend between cumulative REC and 1,6-DNP signature mutations (ORT1: 2.12, 95% CI=0.57, 7.93; ORT2=4.02, 95% CI=1.46, 11.03; ORT3=4.92, 95% CI=1.03, 23.56; p-trend 0.05; Supplemental Table 5). Risk estimates did not change significantly after being adjusted for smoking status (Supplemental Table 6) or other risk factors. Plots of the nitro-PAH mutational signatures (and the four major single-base substitution signatures observed in bladder tumors) in the NEBCS are presented in Figure 1. Cosine similarities between the mutational signatures induced by the nitro-PAHs, those reported in the COSMIC database (Version 2: https://cancer.sanger.ac.uk/signatures/signatures_v2/), and those observed in bladder tumors are reported in Supplemental Table 7.
Table 4:
Association between any diesel exposure and polycyclic aromatic hydrocarbon mutation signatures in muscle invasive bladder cancer cases from the New England Bladder Cancer Study (NEBCS) and The Cancer Genome Atlas (TCGA).
| 1,8-DNP | 1,6-DNP | 3-NBA | 6-nitrochrysene | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N MI Cases |
Risk Ratio* (95% CI) |
p-value | Risk Ratio* (95% CI) |
p-value | Risk Ratio* (95% CI) |
p-value | Risk Ratio* (95% CI) |
p-value | |
| Pooled | |||||||||
| Unexposed | 213 | Ref | Ref | Ref | Ref | ||||
| Any Diesel Exposure | 74 | 1.50 (1.03, 2.20) | 0.036 | 1.93 (1.28, 2.92) | 0.002 | 1.97 (1.33, 2.92) | 0.001 | 1.73 (1.00, 3.00) | 0.051 |
| TCGA | |||||||||
| Unexposed | 184 | Ref | Ref | Ref | Ref | ||||
| Any Diesel Exposure | 56 | 1.37 (0.89, 2.10) | 0.153 | 2.11 (1.32, 3.39) | 0.002 | 2.11 (1.38, 3.23) | 0.001 | 2.20 (1.23, 3.96) | 0.008 |
| NEBCS (MI) | |||||||||
| Unexposed | 29 | Ref | Ref | Ref | Ref | ||||
| Any Diesel Exposure | 18 | 4.38 (1.54, 12.44) | 0.006 | 3.34 (1.38, 8.11) | 0.008 | 2.99 (1.09, 8.20) | 0.033 | 0.70 (0.14, 3.47) | 0.66 |
Adjusted for presence of TP53 mutation. Pooled estimates adjusted for study.
Figure 1. Plots of signature profiles in the New England Bladder Cancer Study.
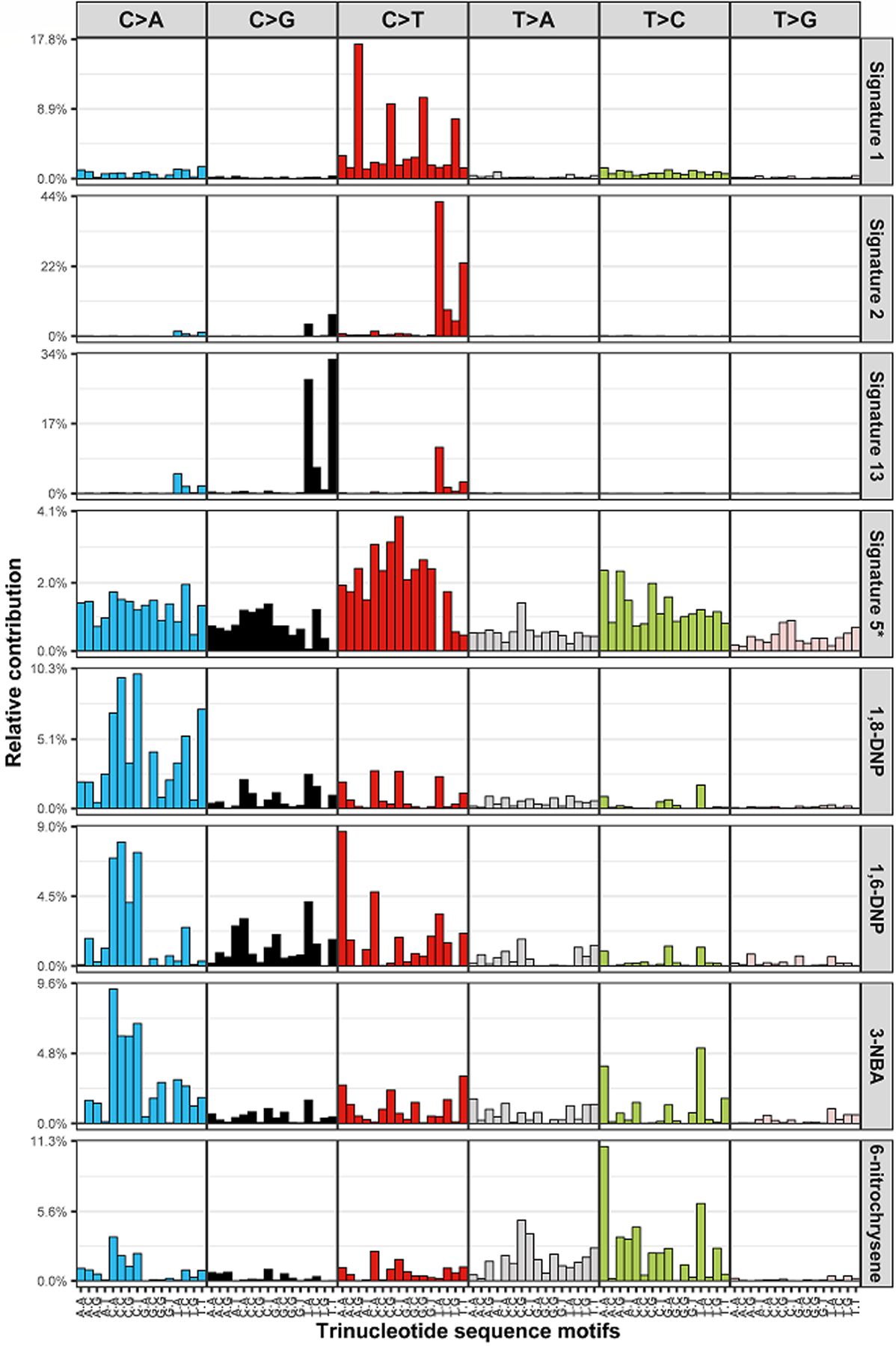
They include the four major mutational signatures (COSMIC signature 1, APOBEC-mediated signatures 2 and 13, and 5*/ERCC2 signature) and the four mutational signatures for nitro-PAHs (1,6-dinitropyrene (1,6-DNP), 1,8-dinitropyrene (1,8-DNP), 3-nitrobenzoic acid (3-NBA), and 6-nitrochysene). The x-axis denotes the 5’ and 3’ nucleotides for each substitution type (e.g., A[T>C]C, thymine to cytosine substitution with 5’ adenine and 3’ cytosine).
Positive associations between quartiles of cumulative REC and mutational signature 1, characterized by C>T mutations at CpG dinucleotides (Table 5) were also apparent. Compared to unexposed cases, exposure to REC nearly doubled the risk of mutations characterized by signature 1 (RRQuartile 1 or Q1=1.86, 95% CI=1.06, 3.26; RRQ2=1.64, 95% CI=0.90, 3.00; RRQ3=1.97, 95% CI=1.08, 3.60; RRQ4=2.31, 95% CI=1.31, 4.06; p-trend=0.042), adjusting by age at diagnosis and other confounding factors. Ever exposure to diesel exhaust was also associated with an increasing proportion of signature 1 mutations in the TCGA dataset (RR=1.46, 95% CI=1.37, 1.56); the pooled estimate for ever exposure to diesel exhaust in NEBCS and TCGA was RR=1.47, 95% CI=1.38, 1.57. There was no relationship between cumulative REC and any of the other single-base substitution signatures.
Table 5:
Associations between cumulative respirable elemental carbon (REC) and single-base substitution signatures in the New England Bladder Cancer Study.
| Sig 1 Mutations C>T at CpG dinucleotides |
APOBEC-Sig 2 Mutations C>T in TC[A/T] motifs |
APOBEC-Sig 13 Mutations C>G in TC[A/T] motifs |
ERCC2-Sig 5* Mutations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N Cases | Risk Ratio* (95% CI) |
p-value | Risk Ratio* (95% CI) |
p-value | Risk Ratio* (95% CI) |
p-value | Risk Ratio* (95% CI) |
p-value | |
| Cumulative REC** (μg/m3-yrs) | |||||||||
| Unexposed | 163 | Ref | Ref | Ref | Ref | ||||
| 0 – 2.1 | 35 | 1.86 (1.06, 3.26) | 0.031 | 0.82 (0.59, 2.25) | 0.249 | 0.66 (0.38, 1.15) | 0.139 | 1.51 (1.14, 2.00) | 0.004 |
| >2.1 – 14 | 35 | 1.64 (0.90, 3.00) | 0.106 | 1.14 (0.83, 1.57) | 0.426 | 0.95 (0.57, 1.61) | 0.862 | 0.87 (0.62, 1.24) | 0.450 |
| >14 – 80 | 36 | 1.97 (1.08, 3.60) | 0.027 | 0.89 (0.63, 1.27) | 0.527 | 1.10 (0.65, 1.86) | 0.720 | 1.09 (0.79, 1.49) | 0.616 |
| >80 | 35 | 2.31 (1.31, 4.06) | 0.004 | 1.07 (0.78, 1.46) | 0.688 | 0.76 (0.43, 1.33) | 0.331 | 1.01 (0.74, 1.38) | 0.938 |
| p-trend | 0.042 | 0.542 | 0.538 | 0.534 | |||||
Adjusted for age, race/ethnicity, sex, smoking status/duration, employment in a non-diesel related high-risk occupation, ERCC2 mutations, and tumor cellularity.
Cut points for cumulative REC (quartiles) are based on distribution of cases.
Discussion
In the current analysis, we observed an increased risk of TP53-mutated tumors among cases with increasing exposure to cumulative REC, suggesting that diesel exhaust may lead to somatic TP53 mutations in bladder tumors. Additionally, associations between diesel exhaust exposure and nitro-PAH mutational signatures in muscle-invasive disease observed here further implicate diesel exhaust in the development of bladder carcinogenesis. The observed associations between diesel exhaust and mutagenic activity at CpG dinucleotides also suggests a possible link with epigenetic mechanisms.
Epidemiologic evidence has linked diesel exhaust to bladder cancer [4–6, 21], but research on the mechanisms by which diesel may lead to bladder cancer remains limited. Recently, experimental studies of cells exposed to environmental carcinogens have provided insights into the mechanisms of carcinogenesis by identifying specific mutational patterns across the whole genome that are induced by exposure. This includes specific mutational signatures attributed to PAHs and nitro-PAHs (genotoxic constituents of diesel exhaust), such as 1,8-DNP, 1,6-DNP and 3-NBA [15]. Our study found associations between these mutational signatures and diesel exhaust exposure in muscle-invasive disease, providing further evidence for the carcinogenicity of diesel exhaust in bladder tumors. Although we saw positive associations for all four of the nitro-PAH mutational signatures that we examined, 1,6-DNP and 3-NBA had the most consistent associations with diesel exposure across the two studies. Based on the quantitative estimates of cumulative REC, a significant exposure-response trend was only observed for 1,6-DNP (p-trend=0.045) and a potential borderline trend was observed for 1,8-DNP (p-trend=0.164). However, these finding was based on a small number of muscle-invasive cases in the NEBCS, and more studies that have quantitative data on level of diesel exposure are needed to further explore these observations. Urinary biomonitoring studies have demonstrated the presence of excreted PAH/nitro-PAH metabolites after exposure to diesel exhaust [3, 22]. Experimental studies have demonstrated PAH carcinogenicity by formation of DNA adducts, reactive oxygen species, and DNA breaks in cells treated with 3-NBA [23–25] and 1,6-DNP [26]. Thus, these potent mutagens may be important contributors to diesel-induced bladder cancer.
PAHs have also been proposed to induce mutations in important genes such as TP53 through the formation of adducts [3, 27, 28]. The link between PAH exposure and TP53 mutations has been demonstrated in lung cancer [28–30], and may be similar for bladder cancer. TP53 is a tumor suppressor gene important in cell cycle regulation and is commonly mutated or inactivated in muscle-invasive bladder cancers [31, 32]. Employment in a high-risk occupation for bladder cancer has been previously associated with TP53 inactivation [7, 33]. Consistent with our recent report [6], this analysis reveals an association between quantitative cumulative diesel exhaust and bladder cancer risk, but additionally finds significant etiologic heterogeneity by TP53 mutation status. A suggested p53-dependent mechanism for diesel exposure has been previously discussed in the literature [34], and the importance of cell cycle regulators in bladder tumors has been noted [3, 10, 35]. TP53 mutations in the DNA-binding domain may prevent or inhibit the protective responses to cellular stress and DNA damage such as cell cycle arrest, DNA repair, or programmed cell death [36, 37]. One experimental study suggests that cell line differences in p53 status may partly explain the increased levels of DNA adducts [25]. Thus, TP53 mutations may be one mechanistic pathway in which diesel exhaust exposure induces bladder cancer.
In our case-control analyses, we found that the association between diesel exhaust and TP53-mutated tumors was particularly strong among high-grade non-muscle invasive (NMI) bladder cancers. Although TP53 mutations are more common in muscle-invasive bladder cancers, mutations are still present in 10–20% of non-invasive disease and are more prevalent in high-grade tumors [38, 39]. TP53 mutations could play a role in the overexpression of p53 protein that has been observed in higher grades of NMI bladder cancers [40–42]. Additionally, studies of NMI bladder cancers have found higher likelihood of progression among tumors with TP53 mutations, suggesting the acquisition of TP53 mutation as one possible mechanism of tumor progression to invasiveness [36, 42–45]. Thus, the heterogeneity across stage/grade subtypes found in our study may suggest that diesel exhaust has a promoting role both in the initiation and progression of bladder cancer.
We also observed a positive association between cumulative REC and COSMIC mutational signature 1 in both the NEBCS and TCGA datasets. This signature is characterized by C>T base changes at CpG dinucleotides and reflects the natural age-dependent degradation of 5-methylcytosine [46, 47]. However, associations persisted after adjustment for age was considered. Data also suggest that some of the most frequent TP53 mutations found in a variety of tumor types have a strong link to the activity of mutational signature 1 [48]. And in fact, in our data, we observed a significant positive association between signature 1 and mutated TP53 in NEBCS tumors (OR=1.49, 95% CI=1.05, 2.11). Thus, the observed associations between signature 1 and diesel exhaust exposure may be indirectly associated with TP53 mutations. Models adjusting for the presence of TP53 mutation, however, did not impact the positive results for signature 1; thus, it is possible that alternative mechanisms are also at play. Some controlled human exposure studies have shown diesel exhaust can affect DNA methylation [49–51]. Assessing the impact of diesel exhaust exposure at CpG dinucleotides and any resultant transcriptional changes in bladder tumors may be valuable considering the wealth of data linking particulate exposure to altered DNA methylation [52].
This study’s strengths include expert assessment of diesel exhaust exposure with additional quantitative estimates of cumulative REC in the NEBCS. The NEBCS is a population-based set of cases while TCGA is a muscle-invasive-only case series. However, a standardized pathologic review with genomic characterization of tumors and bladder cancer risk factor information is available for both studies; this combination of features is uncommon but hugely advantageous to further understand the role of diesel exhaust exposure in bladder carcinogenesis. Limitations of these analyses include the limited exposure characterization for diesel exhaust in TCGA and the small size of the targeted sequencing panel in the NEBCS. While we were able to reliably characterize previously reported mutational signatures, some misclassification of the contribution of individual mutations to each signature is also possible when using only a 44-gene targeted panel in the NEBCS. The NEBCS study population, however, is a representative sample of the New England population and allowed for risk (case-control) analyses using quantitative data for diesel exposure. Nonetheless, our analyses of heterogeneity by subtype and mutational signatures sometimes resulted in small numbers.
In conclusion, our study provides additional insight into the etiology and possible mechanisms related to diesel exhaust-induced bladder cancer. Our findings also suggest diesel exhaust is a risk factor specifically for aggressive bladder cancer subtypes. Future confirmatory research and experimental studies are needed to continue exploring the mounting evidence linking diesel exhaust exposure and bladder cancer risk.
Supplementary Material
Funding:
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (ZIA CP010125-24).
Footnotes
Declarations: The authors declare no potential conflicts of interest.
References
- [1].Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of Bladder Cancer. Med Sci (Basel: ). 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Silverman DT, Koutros S, Figueroa JD, Prokunina-Olsson L, Rothman N. Bladder Cancer. In: Thun MJ, Linet MS, Cerhan C, Haiman C, Schottenfelf D, editors. Schottenfeld and Fraumeni Cancer Epidemiology and Prevention. 4th ed. New York: Oxford University Press; 2018. p. 977–96. [Google Scholar]
- [3].IARC. Diesel and gasoline engine exhausts and some nitroarenes. IARC Monogr Eval Carcinog Risks Hum. 2014;105:9–699. [PMC free article] [PubMed] [Google Scholar]
- [4].Boffetta P, Silverman DT. A Meta-Analysis of Bladder Cancer and Diesel Exhuast Exposure. Epidemiology. 2001;12:125–30. [DOI] [PubMed] [Google Scholar]
- [5].Latifovic L, Villeneuve PJ, Parent ME, Johnson KC, Kachuri L, Canadian Cancer Registries Epidemiology G, et al. Bladder cancer and occupational exposure to diesel and gasoline engine emissions among Canadian men. Cancer Med. 2015;4:1948–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koutros S, Kogevinas M, Friesen MC, Stewart PA, Baris D, Karagas MR, et al. Diesel exhaust and bladder cancer risk by pathologic stage and grade subtypes. Environ Int. 2020;135:105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Koutros S, Rao N, Moore LE, Nickerson ML, Lee D, Zhu B, et al. Targeted deep sequencing of bladder tumors reveals novel associations between cancer gene mutations and mutational signatures with major risk factors. Clinical Cancer Research. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Birch ME, Cary RA. Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust. Aerosol Science and Technology. 1996;25:221–41. [DOI] [PubMed] [Google Scholar]
- [9].Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: a literature review. J Expo Sci Environ Epidemiol. 2009;19:443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171:540–56 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wilcox AN, Silverman DT, Friesen MC, Locke SJ, Russ DE, Hyun N, et al. Smoking status, usual adult occupation, and risk of recurrent urothelial bladder carcinoma: data from The Cancer Genome Atlas (TCGA) Project. Cancer Causes Control. 2016;27:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang H, Zhao N, Ahearn TU, Wheeler W, Garcia-Closas M, Chatterjee N. A mixed-model approach for powerful testing of genetic associations with cancer risk incorporating tumor characteristics. Biostatistics. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang X, Wojtowicz D, Przytycka TM. Detecting presence of mutational signatures in cancer with confidence. Bioinformatics. 2018;34:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim J, Mouw KW, Polak P, Braunstein LZ, Kamburov A, Kwiatkowski DJ, et al. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat Genet. 2016;48:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kucab JE, Zou X, Morganella S, Joel M, Nanda AS, Nagy E, et al. A Compendium of Mutational Signatures of Environmental Agents. Cell. 2019;177:821–36 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Purohit V, Basu AK. Mutagenicity of nitroaromatic compounds. Chem Res Toxicol. 2000;13:673–92. [DOI] [PubMed] [Google Scholar]
- [17].Scheepers PT, Martens MH, Velders DD, Fijneman P, van Kerkhoven M, Noordhoek J, et al. 1-Nitropyrene as a marker for the mutagenicity of diesel exhaust-derived particulate matter in workplace atmospheres. Environ Mol Mutagen. 1995;25:134–47. [DOI] [PubMed] [Google Scholar]
- [18].Du M, Mullins BJ, Franklin P, Musk AW, Elliot NSJ, Sodhi-Berry N, et al. Measurement of urinary 1-aminopyrene and 1-hydroxypyrene as biomarkers of exposure to diesel particulate matter in gold miners. Sci Total Environ. 2019;685:723–8. [DOI] [PubMed] [Google Scholar]
- [19].Laumbach R, Tong J, Zhang L, Ohman-Strickland P, Stern A, Fiedler N, et al. Quantification of 1-aminopyrene in human urine after a controlled exposure to diesel exhaust. J Environ Monit. 2009;11:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang Z, Lin Y, Wang S, Liu X, Cullinan P, Chung KF, et al. Urinary Amino-Polycyclic Aromatic Hydrocarbons in Urban Residents: Finding a Biomarker for Residential Exposure to Diesel Traffic. Environ Sci Technol. 2021;55:10569–77. [DOI] [PubMed] [Google Scholar]
- [21].Silverman DT, Hoover RN, Albert S, Graff KM. Occupation and Cancer of the Lower Urinary Tract in Detroit. Journal of the National Cancer Institute. 1983;70:237–45. [PubMed] [Google Scholar]
- [22].Seidel A, Dahmann D, Krekeler H, Jacob J. Biomonitoring of polycyclic aromatic compounds in the urine of mining workers occupationally exposed to diesel exhaust. Int J Hyg Environ Health. 2002;204:333–8. [DOI] [PubMed] [Google Scholar]
- [23].vom Brocke J, Krais A, Whibley C, Hollstein MC, Schmeiser HH. The carcinogenic air pollutant 3-nitrobenzanthrone induces GC to TA transversion mutations in human p53 sequences. Mutagenesis. 2009;24:17–23. [DOI] [PubMed] [Google Scholar]
- [24].Landvik NE, Arlt VM, Nagy E, Solhaug A, Tekpli X, Schmeiser HH, et al. 3-Nitrobenzanthrone and 3-aminobenzanthrone induce DNA damage and cell signalling in Hepa1c1c7 cells. Mutat Res. 2010;684:11–23. [DOI] [PubMed] [Google Scholar]
- [25].Reshetnikova G, Sidorenko VS, Whyard T, Lukin M, Waltzer W, Takamura-Enye T, et al. Genotoxic and cytotoxic effects of the environmental pollutant 3-nitrobenzanthrone on bladder cancer cells. Exp Cell Res. 2016;349:101–8. [DOI] [PubMed] [Google Scholar]
- [26].Djuric Z, Potter DW, Culp SJ, Luongo DA, Beland FA. Formation of DNA adducts and oxidative DNA damage in rats treated with 1–6,dinitropyrene. Cancer Letters. 1993:51–6. [DOI] [PubMed] [Google Scholar]
- [27].Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci. 2015;145:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shen Y, Troxel AB, Vendantam S, Penning TM, Field J. Comparison of p53 mutations induced by PAH o-quinones with those caused by anti-benzo[a]pyrene diol epoxide in vitro: role of reactive oxygen and biological selection. Chem Res Toxicol. 2006;19:1441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–917. [PubMed] [Google Scholar]
- [30].Denissenko MF, Pao A, Tang M, Pfieifer G. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–2. [DOI] [PubMed] [Google Scholar]
- [31].Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15:25–41. [DOI] [PubMed] [Google Scholar]
- [32].Ouerhani S, Rouissi K, Kourda N, Marrakchi R, Bougatef K, Riadh Ben Slama M, et al. Combined analysis of smoking, TP53, and FGFR3 mutations in Tunisian patients with invasive and superficial high-grade bladder tumors. Cancer Invest. 2009;27:998–1007. [DOI] [PubMed] [Google Scholar]
- [33].Kelsey KT, Hirao T, Hirao S, Devi-Ashok T, Nelson HH, Andrew A, et al. TP53 alterations and patterns of carcinogen exposure in a U.S. population-based study of bladder cancer. Int J Cancer. 2005;117:370–5. [DOI] [PubMed] [Google Scholar]
- [34].Yun YP, Lee JY, Ahn EK, Lee KH, Yoon HK, Lim Y. Diesel exhaust particles induce apoptosis via p53 and Mdm2 in J774A.1 macrophage cell line. Toxicol In Vitro. 2009;23:21–8. [DOI] [PubMed] [Google Scholar]
- [35].Landvik NE, Gorria M, Arlt VM, Asare N, Solhaug A, Lagadic-Gossmann D, et al. Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: possible implications for their mutagenic and carcinogenic effects. Toxicology. 2007;231:159–74. [DOI] [PubMed] [Google Scholar]
- [36].Ecke TH, Sachs MD, Lenk SV, Loening SA, Schlechte HH. TP53 gene mutations as an independent marker for urinary bladder cancer progression. Int J Mol. 2008;21:655–61. [PubMed] [Google Scholar]
- [37].Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lindskrog SV, Prip FF, Lamy P, Taber A, Groeneveld CS, Birkenkamp-Demtroder K, et al. An integrated multi-omics analysis identifies clinically relevant molecular subtypes of non-muscle-invasive bladder cancer. medRxiv [Preprint]. 2020. [Google Scholar]
- [39].Pietzak EJ, Bagrodia A, Cha EK, Drill EN, Iyer G, Isharwal S, et al. Next-generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur Urol. 2017;72:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lenz P, Pfeiffer R, Baris D, Schned AR, Takikita M, Poscablo MC, et al. Cell-cycle control in urothelial carcinoma: large-scale tissue array analysis of tumor tissue from Maine and Vermont. Cancer Epidemiol Biomarkers Prev. 2012;21:1555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Duenas M, Perez-Figueroa A, Oliveira C, Suarez-Cabrera C, Sousa A, Oliveira P, et al. Gene Expression Analyses in Non Muscle Invasive Bladder Cancer Reveals a Role for Alternative Splicing and Tp53 Status. Sci Rep. 2019;9:10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hegazy R, Kamel M, Salem EA, Salem NA, Fawzy A, Sakr A, et al. The prognostic significance of p53, p63 and her2 expression in non-muscle-invasive bladder cancer in relation to treatment with bacille Calmette-Guerin. Arab J Urol. 2015;13:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bellmunt J, Kim J, Reardon B, Perera-Bel J, Orsola A, Rodriguez-Vida A, et al. Genomic Predictors of Good Outcome, Recurrence, or Progression in High-Grade T1 Non-Muscle-Invasive Bladder Cancer. Cancer Res. 2020;80:4476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Du J, Wang SH, Yang Q, Chen QQ, Yao X. p53 status correlates with the risk of progression in stage T1 bladder cancer: a meta-analysis. World J Surg Oncol. 2016;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Smith ND, Rubenstein JN, Eggener SE, Kozlowski JM. The p53 tumor suppressor gene and nuclear protein: basic science review and relevance in the management of bladder cancer. J Urol. 2003;169:1219–28. [DOI] [PubMed] [Google Scholar]
- [46].Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Alexandrov LB, Jones PH, Wedge DC, Sale JE, Campbell PJ, Nik-Zainal S, et al. Clock-like mutational processes in human somatic cells. Nat Genet. 2015;47:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Giacomelli AO, Yang X, Lintner RE, McFarland JM, Duby M, Kim J, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet. 2018;50:1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Clifford RL, Jones MJ, MacIsaac JL, McEwen LM, Goodman SJ, Mostafavi S, et al. Inhalation of diesel exhaust and allergen alters human bronchial epithelium DNA methylation. J Allergy Clin Immunol. 2017;139:112–21. [DOI] [PubMed] [Google Scholar]
- [50].Jiang R, Jones MJ, Sava F, Kobor MS, Carlsten C. Short-term diesel exhaust inhalation in a controlled human crossover study is associated with changes in DNA methylation of circulating mononuclear cells in asthmatics. Particle and Fibre Toxicology. 2014;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang X, Li J, He Z, Duan H, Gao W, Wang H, et al. Associations between DNA methylation in DNA damage response-related genes and cytokinesis-block micronucleus cytome index in diesel engine exhaust-exposed workers. Arch Toxicol. 2016;90:1997–2008. [DOI] [PubMed] [Google Scholar]
- [52].IARC. Outdoor Air Pollution. IARC Monogr Eval Carcinog Risks Hum. 2016;109. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from the current study are available from the corresponding author on reasonable request.


