Abstract
Objective:
We evaluated whether relationships between area deprivation (ADI), body mass index (BMI) and brain structure (e.g., cortical thickness, subcortical volume) during pre-adolescence supported the immunologic model of self-regulation failure (NI) and/or neuronal stress (NS) theories of overeating. The NI theory proposes that ADI causes structural alteration in the brain due to the neuroinflammatory effects of overeating unhealthy foods. The NS theory proposes that ADI-related stress negatively impacts brain structure, which causes stress-related overeating and subsequent obesity.
Methods:
Data were gathered from the Adolescent Brain Cognitive DevelopmentSM Study® (9–12-years-old; n=3,087, 51% male). Linear mixed-effects models identified brain regions that were associated with both ADI and BMI; longitudinal associations were evaluated with mediation models. The NI model included ADI and BMI at 9/10-years-old and brain data at 11/12-years-old. The NS model included ADI and brain data at 9/10-years-old and BMI at 11/12-years-old.
Results:
BMI at 9/10-years-old partially mediated the relationship between ADI and Ventral DC volume at 11/12-years-old. Additionally, the Ventral DC at 9/10-years-old partially mediated the relationship between ADI and BMI at 11/12-years-old, even in youth who at baseline, were of a healthy weight. Results were unchanged when controlling for differences in brain structure and weight across the two-years.
Conclusion:
Greater area deprivation may indicate fewer access to resources that support healthy development, like nutritious food and nonstressful environments. Our findings provide evidence in support of the NI and NS theories of overeating, specifically, with greater ADI influencing health outcomes of obesity via brain structure alterations.
Keywords: pediatric obesity, neighborhood deprivation, brain structure, adolescence
Introduction:
Environmental resources, such as good nutrition and safety, are critical for optimal development and decreased access or opportunities for resources in the environment can negatively impact health outcomes (Hurley et al., 2016; Rosales et al., 2009). Therefore, it is not surprising that neighborhood (i.e., area) deprivation has been related to negative health and developmental outcomes, like increased risk for childhood obesity (Papas et al., 2007; Sharifi et al., 2016), decreased cognitive performance, and altered brain structure and function (Brito & Noble, 2014; Cockerham et al., 2017; Noble et al., 2015; Smith & Pollak, 2020; Twaits & Alwan, 2020; Vargas et al., 2020). Recently, cross-sectional studies in youth have shown correlations between obesity, area deprivation, and structural variation (Dennis et al., 2022; Hall et al., 2021) but how area deprivation contributes to long-term obesity/brain relationships is unknown. Further, there may be different mechanisms that explain how resource deprivation facilitates and maintains overeating and subsequent obesity that are not yet well understood.
Area deprivation is known to contribute to an increase in obesogenic behaviors. For example, deprived neighborhoods often have an abundance of unhealthy food and less access to health promoting behaviors (e.g., green spaces, walkability) and greater childhood obesity rates (Papas et al., 2007). In animal studies, repeated intake of unhealthy food is associated with neuroinflammation and brain changes that are a result of neural atrophy (C. A. Mullins et al., 2020). Because area deprivation is related to both obesity and brain variation in youth (Dennis et al., 2022), it is possible that youth who live in deprived neighborhoods may have a stronger relationship between brain structure variation and obesity due to neuroinflammation from overeating unhealthy foods. Cross-sectionally, studies have shown that area deprivation moderates the relationship between childhood obesity and cortical thickness in regions associated with inhibitory control and executive function (Hall et al., 2021). However, but how area deprivation contributes to change over time in brain structure is unknown. Therefore, this study will test the theory that decreased access to resources in the neighborhood is related to increase in body mass index (BMI) which then results in changes in brain structure later on; a theory known as the immunologic model of self-regulation failure (NI) (Shields et al., 2017).
Although area deprivation is associated with decreased access to healthy foods, living in deprived neighborhoods is also associated with more stressors (e.g., neighborhood safety, affordability) and altered cortisol levels (Barrington et al., 2014). Chronic stress can induce overeating in humans and animals (Razzoli et al., 2017) by triggering the hypothalamus to induce cravings for palatable food, and food intake is proposed to downregulate negative effects of stress in the amygdala and nucleus accumbens (Razzoli & Bartolomucci, 2016). However, stress is also linked to structural and functional changes in the brain (Barrington et al., 2014) in regions associated with cognitive control and executive functioning, learning, and memory (Smith & Pollak, 2020; Vargas et al., 2020), which may alter signaling to the hypothalamus and influence dysregulated food intake. Accordingly, this suggests that area deprivation may lead to changes in brain structure that facilitate overeating and increased BMI over time, but this theory has not been tested. Therefore, an additional aim of this study was to test the hypothesis that area deprivation is associated with differences in brain structure prior to weight gain later in development, a theory known as the neuronal stress of overeating (NS).
Area (i.e., neighborhood) deprivation can be measured using the area deprivation index score (ADI). ADI is a measure of access to resources and opportunities in each neighborhood (Kind & Buckingham, 2018) and incorporates aspects of the living environment such as physical infrastructure (e.g., living spaces, walkability, grocery stores, and medical resources), and social factors (e.g., education, affordability, income-to-needs, safety) that work together to influence lifestyles. Area deprivation is thought to be a critical social determinant of health for child obesity. Childhood obesity continues to rise and disproportionately affects groups that have been historically economically and socially marginalized (Ogden et al., 2020). Moreover, area deprivation is in part the result of systemic inequities in the US and also disproportionately negatively impacts some more than others (Singh et al., 2017). Thus, understanding how area deprivation contributes to facilitating and maintaining childhood obesity within the neuroinflammation and neuronal stress frameworks of overeating may provide optimal insight for health policy and intervention in communities that have been disproportionately affected.
In this report, using a longitudinal mediation framework, we assess the validity of two competing (or complimentary) theoretical models that may explain the relationship between area deprivation, obesity, and brain structure in peripubertal youth. In the first model, we tested the immunological model of self-regulation failure which posits that having a higher body mass index (BMI) at 9/10-years-old, possibly due to decreased access to healthy foods (i.e., area deprivation) mediates the relationship between area deprivation and brain structure two-years later (e.g., 11/12-years-old; Fig 1A). Support for this hypothesis comes from findings that access to good food is related to greater BMI (Papas et al., 2007), and that greater BMI is related to brain structure variation (Adise, Allgaier, et al., 2021), but no studies have assessed these relationships in tandem. In the second model, we tested the neuronal stress theory of overeating, which hypothesizes that structural variation at 9/10-years-old, possibly due to the stress-related effects of living in an area deprived of resources, mediates the relationship between area deprivation at 9/10-years-old and BMI at 11/12-years-old by triggering stress-induced overeating (Fig 1B). Data were curated from the Adolescent Brain Cognitive Development Study (ABCD Study®). Although the ABCD Study® collects various MRI modalities, we focused on cortical thickness and subcortical volume, as prefrontal cortical thinning has cross-sectionally showed a relationship with ADI and BMI at 9/10-years-old (Hall et al., 2021), and much of food intake is regulated by subcortical regions (Berthoud, 2012). As some indices of area deprivation can be modified to reduce social inequities leading to health disparities, understanding how it relates to obesity development and maintenance may be crucial for health policy and intervention.
Figure 1.
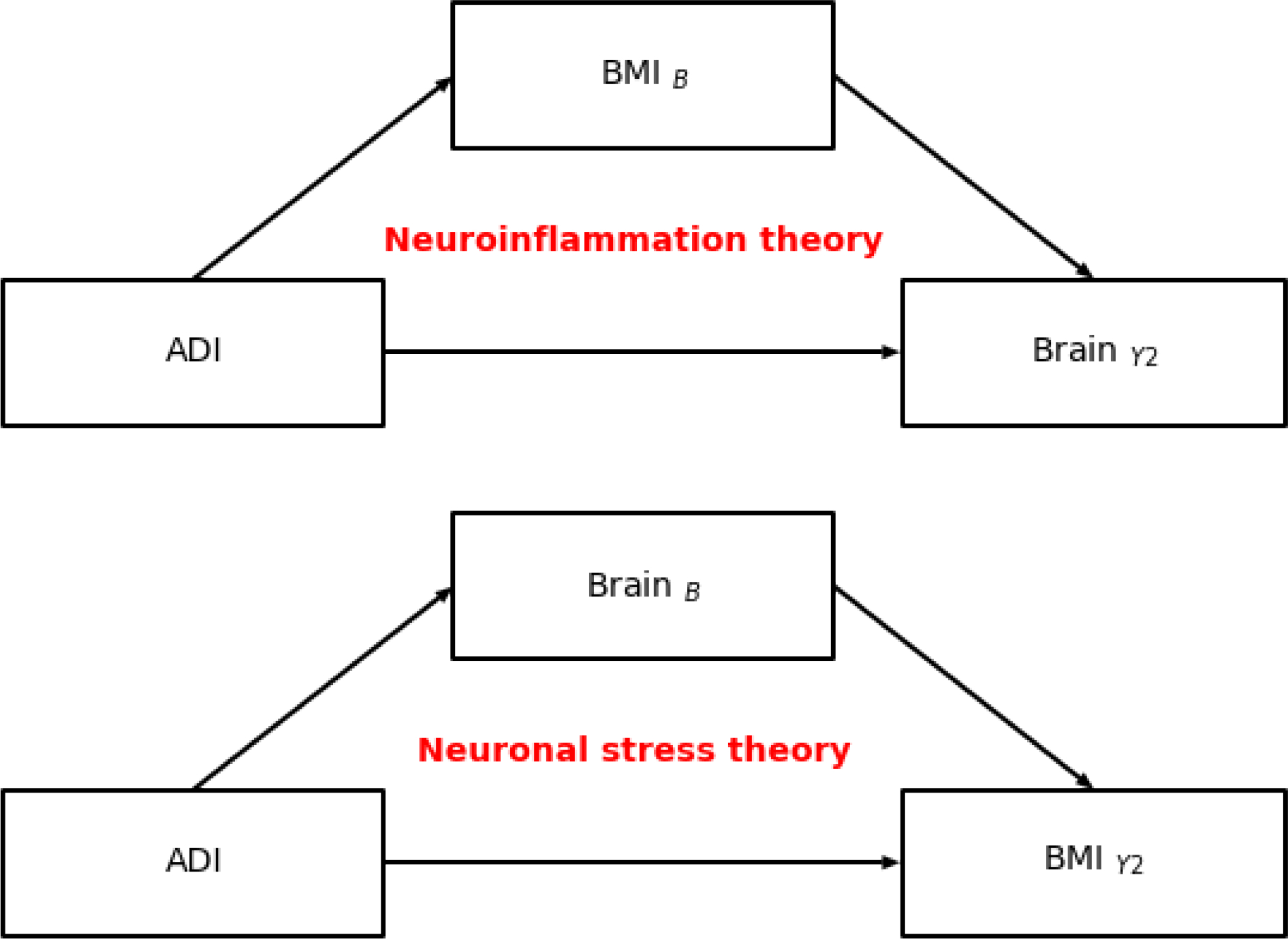
A schematic overview of the neuroinflammation (ie., immunologic model of self-regulation failure) and neuronal stress theories of overeating. B = baseline (aged 9/10-years-old). Y2 = two-year follow up (aged 11/12-years-old).
Methods:
Study design:
The ABCD Study® is a 21-site 10-year longitudinal cohort study (Jernigan et al., 2018). The current manuscript presents data from the 4.0 release (nbaseline=11,878; nyear2=10,415) with neuroimaging and anthropometric data (acquired at baseline and the two-year follow-up). All data were collected prior to the onset of the coronavirus pandemic. Caregivers and youth provided written consent. A centralized institutional review was approved by the University of California San Diego. Details for each measure are listed in the Supplemental Materials (SM).
Exclusion criteria:
A full list of exclusion criteria determined by the ABCD Study® are listed elsewhere (Garavan et al., 2018), but additional exclusion criteria (see SM) were applied to obtain a sample that was acceptable for these analyses and hypotheses (e.g., BMI measurement error, failed MRI preprocessing). The final sample consisted of 3,087 youth (Table 1).
Table1.
Participant characteristics. AIAN/NHPI=American Indian, Alaskan Native/Native Hawaiian and Pacific Islanders. HS=high school; GED=Generalized education diploma. BA=Bachelor’s degree. SD=standard deviation.
| Variable | Mean | SD |
|---|---|---|
|
| ||
| Age | ||
| Baseline | 119.1 | 7.2 |
| Y2 | 142.9 | 7.5 |
| Puberty | ||
| Baseline | 2 | 0.8 |
| Y2 | 2.7 | 1.0 |
| BMI | ||
| Baseline | 19.1 | 3.7 |
| Y2 | 21 | 4.5 |
| ADI score | 37.3 | 26.2 |
| n | % | |
|
|
||
| Sex | ||
| Male | 1473 | 51.3 |
| Female | 1399 | 48.7 |
| Race | ||
| White | 1998 | 69.6 |
| Black | 336 | 11.7 |
| Asian | 69 | 2.4 |
| AIAN/NHPI | 28 | 1.0 |
| Other | 140 | 4.9 |
| Multi-race | 301 | 10.5 |
| Ethnicity | ||
| Hispanic | 598 | 20.8 |
| Non-Hispanic | 2274 | 79.2 |
| Education | ||
| <HS | 101 | 3.5 |
| HS/GED | 212 | 7.4 |
| Some College | 723 | 25.2 |
| BA degree | 764 | 26.6 |
| Postgraduate degree | 1072 | 37.3 |
| Baseline Weight Class | ||
| Healthy Weight | 1899 | 66.1 |
| Overweight | 489 | 17.0 |
| Obese | 484 | 16.8 |
| Y2 Weight Class | ||
| Healthy Weight | 1810 | 63.0 |
| Overweight | 522 | 18.2 |
| Obese | 540 | 18.8 |
Anthropometrics.
Height and weight assessments were gathered by a trained researcher and converted into BMI (kg/m2).
Demographic variables, puberty.
The caregiver reported the child’s race, ethnicity, date of birth, sex at birth, and caregiver education. Puberty was assessed using the caregiver and youth self-report sex-specific questionnaires (Petersen et al., 1988).
Area Deprivation Index (ADI):
ADI is a neighborhood area deprivation composite score based on 17 factors (e.g., income, education, housing) from the American Community Survey. Higher values indicate more disadvantage (see (Fan et al., 2021))
Neuroimaging Measures.
Image acquisition and preprocessing.
Exact details of the ABCD Study® MRI data acquisition and analyses are published elsewhere (Hagler et al., 2019). In brief, MRI data were collected with 29 scanners. Youth underwent a T1- and T2-weighted MRI, diffusion tensor imaging, resting state MRI, and three functional MRI scans. The current manuscript focuses on the T1-weighted structural MRI data. Cortical data were surface projected and parcellated with Freesurfer using the Desikan Atlas (# of cortical regions of interest [ROIs]=68, volumetric subcortical ROIs=16). ROI estimates (e.g., mean cortical thinning, total gray matter volumes) were averaged across hemispheres (e.g., left, right), for a total of 34 cortical and 8 subcortical ROI estimates.
Statistics:
Linear Mixed-Effects Modeling:
Multicollinearity issues were assessed by a variance inflation factor, and winsorization was implemented to normalize outliers. Brain regions that were significantly related to both BMI and ADI were determined by implementing linear random mixed effects models with the Python package statsmodel (Seabold & Perktold, 2010). The models included corrections for sex, puberty (at baseline), highest household education, race, and ethnicity. Independent continuous variables were standardized, and categorical variables were effects coded. Intracranial volume and age were not included due to multicollinearity issues. Random effects accounted for variability across scanners. Models 1–4 (below) were corrected using the Benjamini-Hochberg approach. Only significant (and corrected) ROIs that were related to BMI and ADI were used for further analyses.
Neuroinflammation models:
Model 1: ROIY2 ~ BMIB + puberty + sex + Race + Ethnicity + education + handedness + (1|Scanner ID)
Model 2: ROIY2 ~ ADIB+ puberty + sex + Race + Ethnicity + education + handedness + (1|Scanner ID)
Neuronal stress models:
Model 3: BMIY2 ~ ROIB + puberty + sex + Race + Ethnicity + education + handedness + (1|Scanner ID)
Model 4: ADIB ~ ROIB + puberty + sex + Race + Ethnicity + education + handedness + (1|Scanner ID)
Mediation analyses:
Longitudinal mediation analyses were conducted with Pyprocessmacro (https://github.com/QuentinAndre/pyprocessmacro). Covariates included were as follows: puberty, sex, race, ethnicity, caregiver education, handedness, and MRI serial number (i.e., scanner ID). Continuous variables were mean centered and categorical variables were dummy coded. Significance of the indirect effect was analyzed by bootstrapped bias-corrected 95% confidence intervals (CI; 10,000 samples). To ensure that our models were adequately predicting temporal associations, we also ran additional analyses in which a difference score was calculated for each brain region and BMI variable and entered into the model as covariates.
Results.
Demographics:
Among the 3,087 youth, 51.3% were female, 69.6% were White, and 79.2% were not Hispanic (Table S1). Most youth had at least one parent with a bachelor’s degree or higher (63.9%). The sociodemographic composition of the sample analyzed reflected the composition of the larger ABCD Study® sample (Garavan et al., 2018), while the rates of overweight and obese youth were comparable to national estimates at baseline (66.1% healthy weight) and at the two-year follow-up (63% healthy weight) (Ogden et al., 2020). There was a wide range of ADIs (Fig 2A). Pearson’s correlation showed that ADI was positively related to BMI at baseline and the two-year follow-up (r=0.17, p<0.001). Partial correlations controlled for the covariates of interest (e.g., sex, race, education) showed that ADI was positively related to BMI at baseline (r=0.07, p<0.001, Fig 2B) and the two-year follow-up (r=0.06, p<0.001, Fig 2C).
Figure 2.
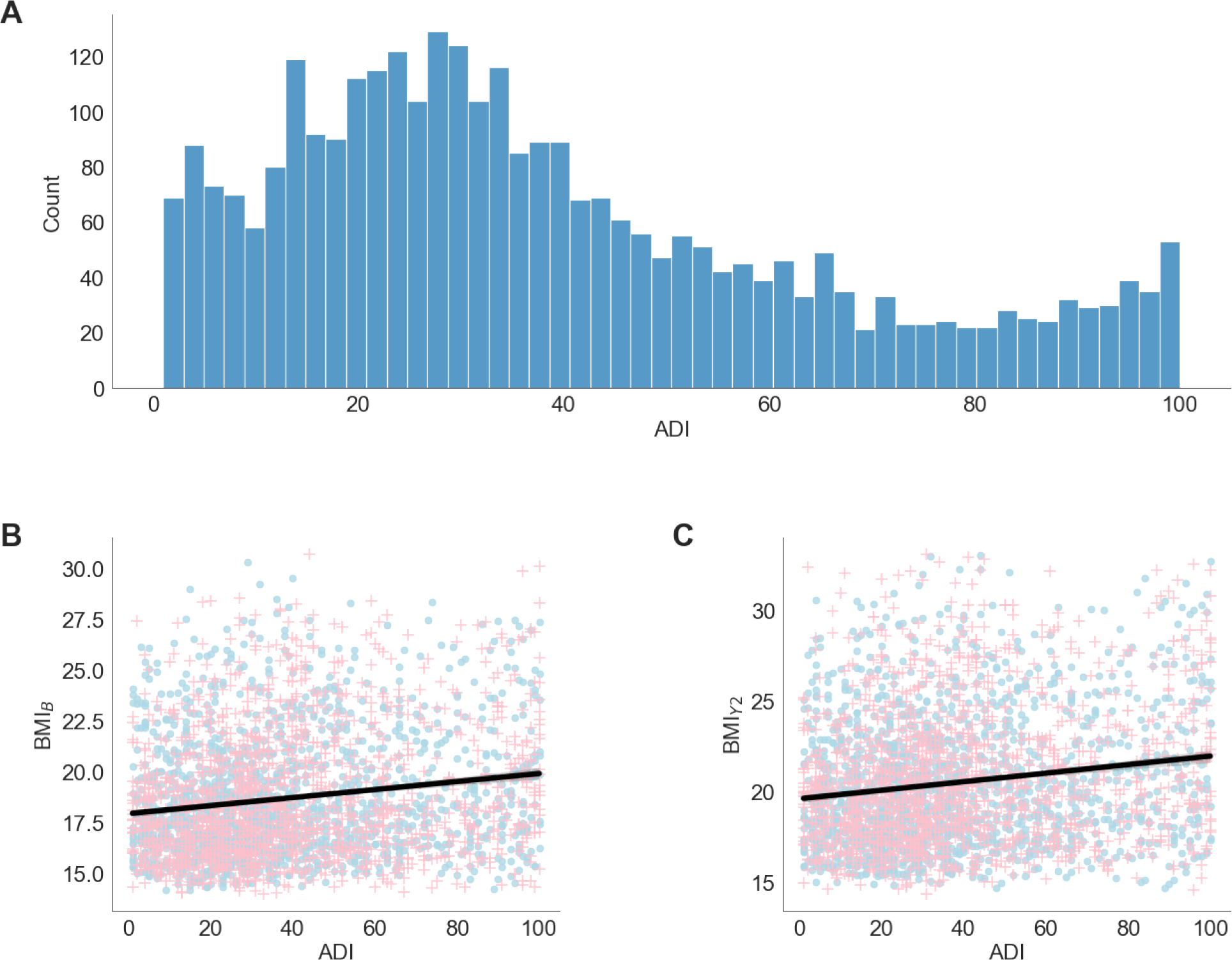
A) Distribution of area deprivation index (ADI; higher values = greater neighborhood deprivation). B and C) The relationship between BMI and ADI at baseline, 9/10 years-old (B) and the two-year follow up (Y2), 11/12 years-old colored by sex assigned at birth (blue circle =male; pink + =female). The black line indicates the regression fit collapsed across sex (r=0.17, p<0.001 for both time points).
Immunologic model of self-regulation failure (NI):
At 11/12-years-old, only the Ventral diencephalon (Ventral DC) was significantly retrospectively associated with ADI and BMI at 9/10-years-old (see Tables S3–S4). BMI at 9/10-years-old partially mediated the associations of ADI at 9/10-years-old on Ventral DC subcortical volume at 11/12-years-old (β=0.1, 95% Bootstrapped 95% CI = [0.04,0.19] Fig 3A). ADI was positively associated with BMI at 9/10-years-old (a path, β=0.01, p<0.001) but negatively associated with brain structure at 11/12-years-old (C path, total direct effect, β=−1.4, p<0.001). BMI was positively associated with volume of the Ventral DC at 11/12-years-old (b path, β=9.5, p<0.001). Controlling for a difference in subcortical volume and BMI from baseline (9/10-years-old) to the two-year follow-up (11/12-years-old) did not change the results (Fig 3B). In healthy weight youth, BMI did not mediate the relationship between ADI at 9/10-years-old and the Ventral DC at 11/12-years-old.
Figure 3.
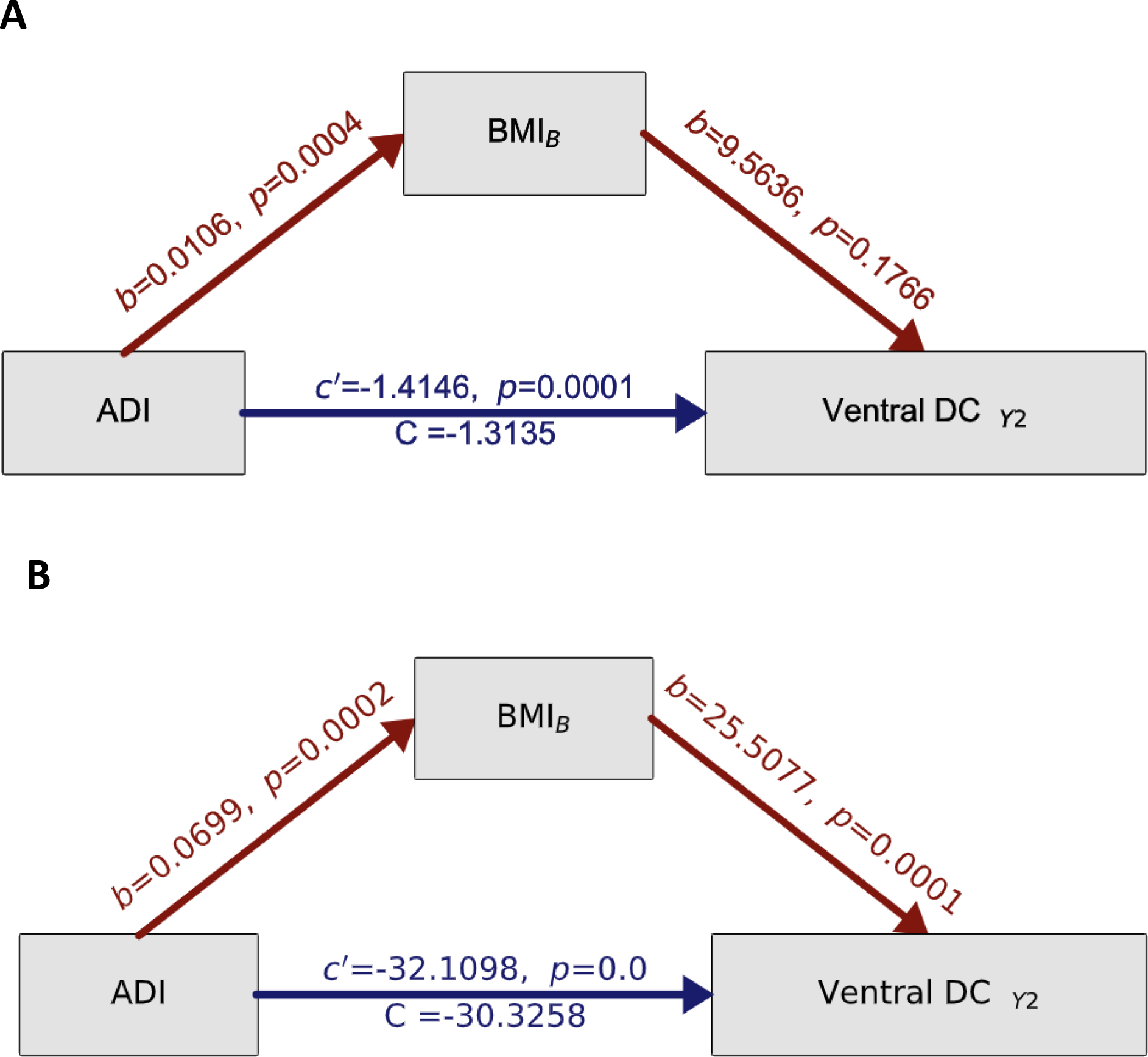
Testing the causal pathway of the Immunologic model of self-regulation failure. Mediating effects of BMI at 9/10-years-old on ADI and brain at 11/12-years-old. A) Mediation models where the colored arrows reflect the strength (and direction) of the indirect effects, while controlling for sex, age, race, ethnicity, education, handedness and scanner ID. Total effects are represented by c’, direct effects of ADI are represented by C while a and b values refer to the association of ADI on BMI at 9/10-years-old and BMI at 9/10-years-old on brain at 11/12-years-old, respectively. All a, b, c, and c’ values are unstandardized regression coefficients. Significance testing was carried out by bias-corrected bootstrapping (n=10,000) 95% confidence intervals. B) Mediation analysis results controlled for a difference in BMI and Ventral DC subcortical volume as well as sex, age, race, ethnicity, education, handedness, and scanner ID. B=baseline (aged 9/10-years-old). Y2=two-year follow-up (aged 11/12-years-old).
Neural stress theory of overeating (NS):
At 9/10-years-old, only the Ventral DC was associated with ADI at 9/10-years-old and BMI at 11/12-years-old (Tables S5–S6). The Ventral DC at 9/10-years-old partially mediated the association between ADI at 9/10-years-old and on BMI at 11/12-years-old (β=−0.0009, 95% Bootstrapped CI = [−0.0018, −0.0004]. Positive and negative brain structure mediators are displayed in Fig 4A. ADI was positively associated with BMI at 11/12-years-old (c path, β=0.1, p=0.004, Total direct effect, Fig 4B) but smaller subcortical volumes of the Ventral DC at 9/10-years-old (a path, β=−1.2, p<0.001, Fig 4B). Cortical thinning and larger subcortical volume of the Ventral DC at 9/10-years-old were associated with greater BMI at 11/12-years-old (b path, β=0.0008, p<0.001, Fig 4B). Controlling for differences in subcortical volume and BMI from baseline (9/10-years-old) to the two-year follow-up (11/12-years-old) did not change the results (Fig 4B), nor did removing youth with overweight/obesity at baseline (see Supplemental Materials Results and Figure S1 and Table S7–S8).
Figure 4.
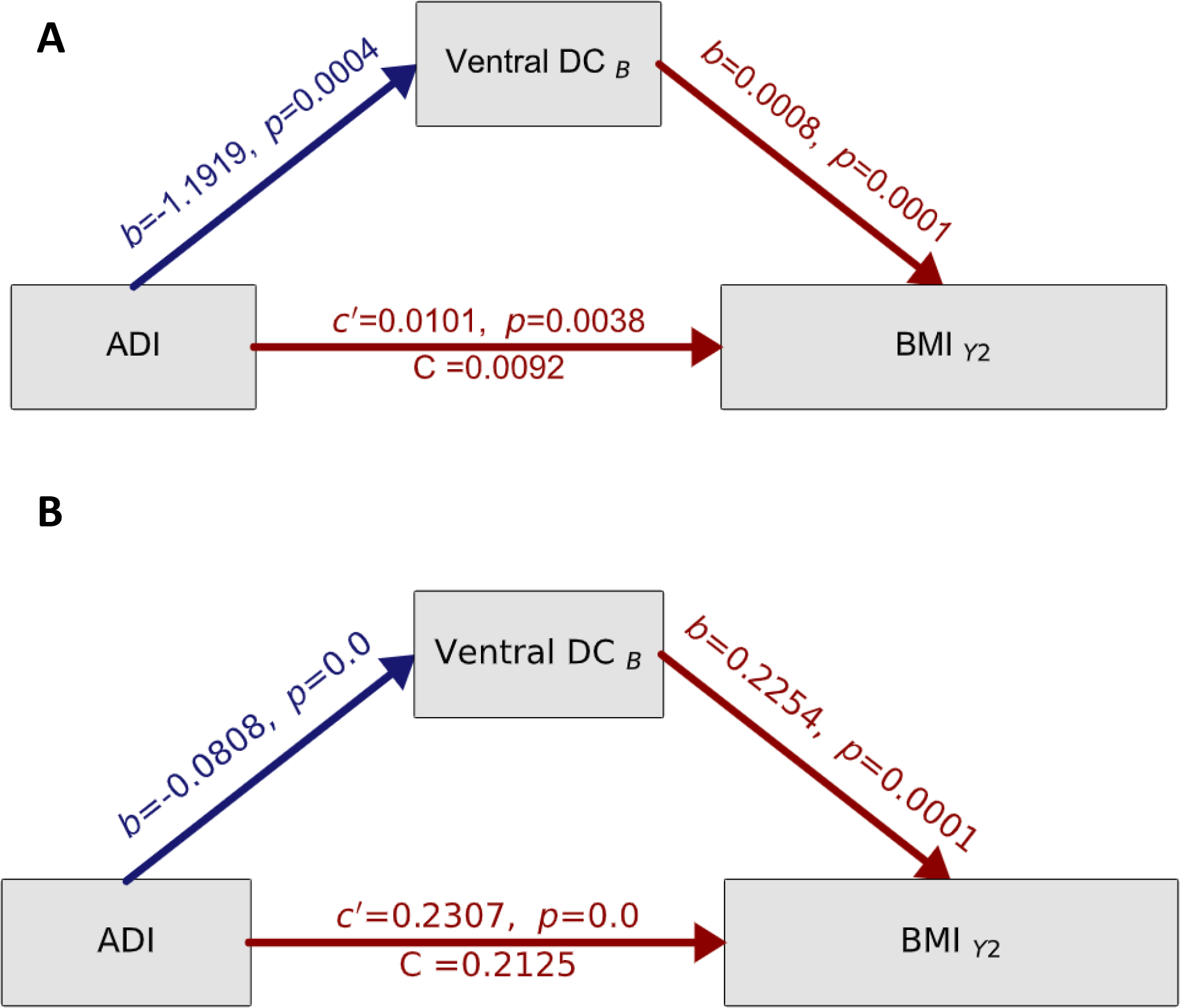
Testing the causal pathway of the neuronal stress theory of overeating. A) Mediation models where the colored arrows reflect the strength (and direction) of the indirect effects, while controlling for sex, age, race, ethnicity, education, handedness, and scanner ID. Total effects are represented by c’, direct effects of ADI are represented by C while a and b values refer to the association of ADI on brain structure at 9/10-years-old and brain structure at 9/10-years-old on BMI at 11/12-years-old, respectively. All a, b, c, and c’ values are unstandardized regression coefficients. Significance testing was carried out by bias-corrected bootstrapping (n=10,000) 95% confidence intervals. B) The results of the mediation analyses while controlling for a difference in BMI and Ventral DC subcortical volume as well as sex, age, race, ethnicity, education, handedness, and scanner ID. B=baseline (aged 9/10-years-old). Y2=two-year follow-up (aged 11/12-years-old).
Discussion:
The current study was the first to evaluate if two competing (and complimentary) theories explain how resource deprivation may facilitate and maintain obesity. Here, we found support for the immunologic model of self-regulation failure (NI) which posits that the relationship between ADI and BMI at (9/10) may stem from a lack of neighborhood resource access, like healthy food options, and that the relationship between BMI at 9/10 and brain structural variation at 11/12 may be a result of neuroinflammation (C. A. Mullins et al., 2020), consistent with the immunologic model. We also found evidence to support the neuronal stress theory of overeating, which posits that a lack of resources may explain may alter brain structure and promote greater BMI at 11/12 due to possible effects of ADI on appetite regulation networks (T. S. Mullins et al., 2020). Our results provided increased support for this theory in healthy weight youth. Together, the overall pattern of findings highlights the implications of greater area deprivation for potential child health disparities in obesity and alterations in brain structure. Greater area deprivation is a proxy for fewer neighborhood and environmental resources that are in part a result of systemic inequities. Therefore, these findings add to our understanding of how the social determinants of health may influence childhood obesity (Dixon et al., 2021). We note that testing these models starting at age 9/10 does not account for pre-existing conditions that may contribute to differences in brain structure. However, testing this longitudinal mediation framework allows for insight into how area deprivation may contribute to or facilitate obesity from 9/10 onward. To our knowledge, this is the first study to test two parallel and competing theoretical models explaining relationships between area deprivation, brain development, and childhood obesity.
The Ventral diencephalon (Ventral DC) emerged as an important region that may explain how area deprivation is associated with both childhood obesity and brain structure. The Ventral DC is an area that incorporates grey and white matter structures, such as the hypothalamus, mamillary bodies, medial geniculate nucleus, red nucleus, and parts of the basal ganglia (e.g., substantia nigra and subthalamic nuclei) (Herrero et al., 2002); these regions are involved in food intake signaling and dopamine secretion (Gantz et al., 2018). The immunologic model of self-regulation failure suggested that greater ADI may influence Ventral DC volume at 11/12-years-old from the indirect associations on BMI at 9/10-years-old. From the immunologic model of self-regulation failure, this pattern of results suggests that overconsumption of unhealthy foods (which is more common in deprived neighborhoods) may explain these relationships. Overconsumption of unhealthy foods may alter food intake neurons and increase neuroinflammation as well as trigger an overexpression of dopamine (a reward response neurotransmitter) (C. A. Mullins et al., 2020). Although ABCD did not measure dopamine expression per se, other studies have observed a relationship between overexpression of dopamine in this region, hypersensitivity to rewards, and obesity (Kroemer & Small, 2016; Stice & Yokum, 2016)) and overeating in youth (Adise et al., 2018). However, it may be that larger volumes of the Ventral DC at 11/12-years-old occur due to an adaptive response based on neuroinflammation deficits in other brain regions (de Groot et al., 2017). Taken together, this suggests a relationship between area deprivation, obesity, and brain structure within the neuroinflammation model, but more research is needed to understand the exact mechanism of neuroinflammation, area deprivation, brain structure, and obesity.
When testing the neuronal stress theory of overeating, at 9/10-years-old, greater ADI was associated with reduced subcortical volume of the Ventral DC (Gearhardt et al., 2014; Rolls, 2015) but larger BMI at 11/12-years-old. Other studies have shown that area deprivation is associated with smaller volumes (Brito & Noble, 2014; Noble et al., 2015), possibly due to neuronal atrophy from overstimulation of the hypothalamic response to the chronic stress of living in an area deprived of resources (Miller & Spencer, 2014). Smaller volumes are thought to decrease dopamine availability (C. A. Mullins et al., 2020), which may trigger overeating as an attempt to (1) normalize reward processing (Stice & Yokum, 2016), (2) downregulate stress (Razzoli & Bartolomucci, 2016), or (3) reduced dopamine availability may also increase BMI by reducing spontaneous exercise (Friend et al., 2017). However, the ABCD Study® did not assess dopamine expression, so future research is needed to evaluate this hypothesis in youth.
It is also important to note that within the neuronal stress theory of overeating, we observed a positive correlation between Ventral DC volume and BMI at 11/12-years-old, suggesting that the relationship between ADI and brain structure at 9/10-years-old and BMI at 11/12-years-old is complex. One reason to explain this may be related to the bidirectional effects of stress-induced overeating: Animal models show that chronic stress triggers the hypothalamus to increase food intake, but, because stress also increases energy expenditure, stress-induced overeating does not always lead to weight gain (Razzoli & Bartolomucci, 2016). However, one study in adults showed that obesity was associated with reduced subcortical volumes (Marqués-Iturria et al., 2013). Although these findings do support that area deprivation may relate to brain structure and BMI possibly due to the stressful impacts of living in a deprived neighborhood, future research is needed to tease apart these mechanisms.
Moreover, in a sample of youth, who at baseline were of a healthy weight, we found support for the neuronal stress model of overeating, but not the immunologic model of self-regulation failure. Thus, this provides additional support for the neuronal stress model, which suggests that perhaps the stressful impacts of living in a deprived neighborhood may facilitate unhealthy food intake patterns, leading to greater obesity. It was surprising to find no evidence for the immunologic model of self-regulation failure in healthy weight youth at baseline. However, it is possible that the effects of neuroinflammation are only evident in heavier youth and/or take a longer time to develop in healthy weight individuals. Thus, more research is needed to understand the long-term associations of neuroinflammation, ADI, and weight gain.
We note that the ventral DC showed bidirectional associations with ADI and BMI. The Ventral DC volume was positively associated with BMI but negatively with ADI. One interpretation could be that even though area deprivation is associated with smaller cortical volumes, amongst these youth, those who are heavier have larger subcortical volume of the Ventral DC than those who are leaner. This theory would be consistent with the extant literature showing that BMI is related to larger subcortical volumes (Opel et al., 2021), but also that not all youth who live in deprived neighborhoods have overweight/obesity. Thus, this could be one reason to explain why area deprivation shows bidirectional effects of Ventral DC volume within the context of BMI. Future research is needed to further investigate this.
Interestingly, brain regions associated with inhibitory control showed no effects of ADI or BMI for either theory to explain the associations of area deprivation, brain development, and obesity. This is contrary to another ABCD Study®, in which ADI moderated the strength of the relationship between BMI at 9/10-years-old and cortical thinning at 9/10-years-old in inhibitory control regions such as the inferior frontal and medial frontal cortex (Hall et al., 2021). However, cross-sectional studies provide little insight into temporal stability and causal inferences, particularly in a sample that is undergoing brain maturation in reward and inhibitory control regions (Shulman et al., 2016). Our present findings may be indicative of a developmental effect (Shaw et al., 2006), in which area deprivation accelerates maturation of brain regions associated with reward processing and occur faster than those involved in inhibitory control (Luna et al., 2004; Mills et al., 2014). Thus, within this age range, greater area deprivation may accelerate reward sensitivity that leads to increased food intake while an immature inhibitory control system lacks the maturity to suppress urges to overeat. Accelerated development of reward sensitivity and food intake signaling may have downstream effects on cognition, and future releases of the ABCD Study® will provide insight into how ADI and the brain change in respect to obesity over time, particularly within this developmental window.
Our findings reinforce how the human-made landscape of the communities in which we live influence child health beyond individual sociodemographics and economics. Importantly, the relationships between area deprivation, brain structure, and BMI were independent of both sociodemographic (e.g., race) and socioeconomic (e.g., education). As ADI considers several aspects of social and environmental factors, it may be a better measure to capture how social determinants of health relate to poorer health outcomes. Therefore, our results serve as a reference to study the longitudinal associations of the brain and obesity development over time.
Strengths and limitations:
The findings in this manuscript offer a unique contribution to the literature by demonstrating how lack of resources in the environment can differentially affect obesity via two different theorized causal frameworks. However, there were a few limitations. Our methodological framework was setup in a temporal fashion to test causal pathways. Yet, caution should still be exercised when interpreting results. The first MRI available was at 9/10 years old, so it is possible that other pre-existing conditions may explain these relationships. The ABCD Study® did not collect markers of oxidative stress nor inflammation, therefore we are limited regarding the inferences that can be made. In addition, other potential mediators that were not assessed or measured may partially or equally explain our results, such as gene by environment interactions. It is also not known how these observations may change after pubertal onset and with normative brain maturation that occurs over the course of development. Related to this, we were not able to assess longitudinal change within these causal frameworks as additional data collections are needed to have temporal specificity and longitudinal change in the same model. However, future releases of MRI data will allow for these investigations. Although ADI is a better measure of deprivation than family income or parental education, it may be subject to bias. For example, environmental stressors in urban versus rural areas may be different, and it is hard to tease apart these differences within ADI. Additional environmental data, such as access to grocery stores and objective cortisol measures, may help to clarify some of the fine-grained associations observed in this study. Future releases of the ABCD Study® may be able to offer insight into more fine-grained mechanisms, but this current study serves as a reference point.
Conclusions:
These findings highlight the crucial role of area deprivation for obesity and brain development during pre- and early adolescence. Our findings show that that ADI may relate to health and development from a neuroinflammatory and neuronal stress framework. Without proper intervention from a public health policy perspective, our findings support the notion that communities that have been disproportionately affected by greater neighborhood deprivation will continue to be at risk for deleterious health outcomes associated with a lack of environmental resources. This dovetails previous research stating how important neighborhood resources are for optimal health. The neighborhood environment factor that can be modified by improving resource access (e.g., adding health food options, increasing green space, creating safe environments, increasing access to affordable health care), which can greatly influence health outcomes. Thus, public health policy may wish to advocate for more funds to improve resources in deprived areas to decrease negative health outcomes.
Supplementary Material
Funding acknowledgements:
Data were obtained from the Adolescent Brain Cognitive DevelopmentSM Study® (https://abcdstudy.org/), held in the NIMH Data Archive (from https://doi.org/10.15154/1503209.). A full list of supporters is available at https://abcdstudy.org/federal-partners/. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or other ABCD Study® consortium members.
Footnotes
We have no known conflict of interest to disclose.
References:
- Adise S, Allgaier N, Laurent J, Hahn S, Chaarani B, Owens M, Yuan D, Nyugen P, Mackey S, Potter A, & Garavan HP (2021). Multimodal brain predictors of current weight and weight gain in children enrolled in the ABCD study ®. Developmental Cognitive Neuroscience, 49(December 2020), 100948. 10.1016/j.dcn.2021.100948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adise S, Geier CF, Roberts NJ, White CN, & Keller KL (2018). Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite, 128(June), 167–179. 10.1016/j.appet.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adise S, White CN, Roberts NJ, Geier CF, & Keller KL (2021). Children’s inhibitory control abilities in the presence of rewards are related to weight status and eating in the absence of hunger. Appetite, 167, 105610. 10.1016/j.appet.2021.105610 [DOI] [PubMed] [Google Scholar]
- Barrington WE, Stafford M, Hamer M, Beresford SAA, Koepsell T, & Steptoe A (2014). Neighborhood socioeconomic deprivation, perceived neighborhood factors, and cortisol responses to induced stress among healthy adults. Health and Place, 27, 120–126. 10.1016/j.healthplace.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R (2012). The neurobiology of food intake in an obesogenic environment. Proceedings of the Nutrition Society, 71(04), 478–487. 10.1017/S0029665112000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8(SEP), 1–12. 10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, & Savage CR (2010). Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity, 34(10), 1494–1500. 10.1038/ijo.2010.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Lasselin J, & Capuron L (2014). Neuropsychiatric comorbidity in obesity: Role of inflammatory processes. Frontiers in Endocrinology, 5(MAY), 1–9. 10.3389/fendo.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Luheshi G, & Layé S (2015). Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. In Frontiers in Neuroscience (Vol. 9, Issue JUL). Frontiers Research Foundation. 10.3389/fnins.2015.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerham WC, Hamby BW, & Oates GR (2017). The Social Determinants of Chronic Disease. In American Journal of Preventive Medicine (Vol. 52, Issue 1, pp. S5–S12). Elsevier Inc. 10.1016/j.amepre.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot CJ, van den Akker ELT, Rings EHHM, Delemarre-van de Waal HA, & van der Grond J (2017). Brain structure, executive function and appetitive traits in adolescent obesity. Pediatric Obesity, 12(4), e33–e36. 10.1111/ijpo.12149 [DOI] [PubMed] [Google Scholar]
- Dennis E, Manza P, & Volkow ND (2022). Socioeconomic status, BMI, and brain development in children. Translational Psychiatry, 12(1), 1–10. 10.1038/s41398-022-01779-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon BN, Ugwoaba UA, Brockmann AN, & Ross KM (2021). Associations between the built environment and dietary intake, physical activity, and obesity: A scoping review of reviews. Obesity Reviews, 22(4). 10.1111/obr.13171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CC, Marshall A, Smolker H, Gonzalez MR, Tapert SF, Barch DM, Sowell E, Dowling GJ, Cardenas-Iniguez C, Ross J, Thompson WK, & Herting MM (2021). Adolescent Brain Cognitive Development (ABCD) study Linked External Data (LED): Protocol and Practices for Geocoding and Assignment of Environmental Data. Developmental Cognitive Neuroscience, 52, 101030. 10.1016/j.dcn.2021.101030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Devarakonda K, O’Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR, Liow JS, Guo J, Rane SG, Rubinstein M, Alvarez VA, Hall KD, & Kravitz AV (2017). Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metabolism, 25(2), 312–321. 10.1016/j.cmet.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, Ford CP, Morikawa H, & Williams JT (2018). The Evolving Understanding of Dopamine Neurons in the Substantia Nigra and Ventral Tegmental Area. Annual Review of Physiology, 80, 219–241. 10.1146/annurev-physiol-021317-121615 [DOI] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, & Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience, 32(April), 16–22. 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Stice E, Harris JL, & Brownell KD (2014). Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience, 9(7), 932–938. 10.1093/scan/nst059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Dickstein DP, MacNamara AE, Phan KL, O’Brien S, Le Grange D, Fisher JO, & Keedy S (2018). A pilot study of neural correlates of loss of control eating in children with overweight/obesity: Probing intermittent access to food as a means of eliciting disinhibited eating. Journal of Pediatric Psychology, 43(8), 846–855. 10.1093/jpepsy/jsy009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnani M, Birken C, & Hamilton J (2015). Childhood Obesity: Causes, Consequences, and Management. Pediatric Clinics of North America, 62(4), 821–840. 10.1016/j.pcl.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Hatton SN, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP, Watts R, Bjork JM, Garavan HP, Hilmer L, Pung CJ, Sicat CS, Kuperman J, Bartsch H, Xue F, … Dale AM (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage, 202(August). 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Best JR, Beaton EA, Sakib MN, & Danckert J (2021). Morphology of the prefrontal cortex predicts body composition in early adolescence: cognitive mediators and environmental moderators in the ABCD Study. Social Cognitive and Affective Neuroscience, August, 1–12. 10.1093/scan/nsab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson MA, & Pitt MB (2021). Three Areas Where Our Growth Chart Conversations Fall Short—Room to Grow. JAMA Pediatrics. 10.1001/jamapediatrics.2021.4330 [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, & Navarro JM (2002). Functional anatomy of thalamus and basal ganglia. Child’s Nervous System, 18(8), 386–404. 10.1007/s00381-002-0604-1 [DOI] [PubMed] [Google Scholar]
- Hurley KM, Yousafzai AK, & Lopez-boo F (2016). Early Child Development and Nutrition : A Review of the Bene fi ts and Challenges of. Advances in Nutrition, 7(2), 357–363. 10.3945/an.115.010363.by [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown SA, & Dowling GJ (2018). The Adolescent Brain Cognitive Development Study. Journal of Research on Adolescence, 28(1), 154–156. 10.1111/jora.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kind AJH, & Buckingham WR (2018). Making Neighborhood-Disadvantage Metrics Accessible — The Neighborhood Atlas. New England Journal of Medicine, 378(26), 2456–2458. 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, & Small DM (2016). Fuel not fun: Reinterpreting attenuated brain responses to reward in obesity. Physiology and Behavior, 162, 1–9. 10.1016/j.physbeh.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, & Guo SS (2002). 2000 CDC Growth Charts for the United States: Methods and Development. In National Center for Health Statistics. Vital Health Stat (Vol. 11, Issue 246). 10.1590/S1516-35982002000600018 [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban T. a, Lazar N. a, & Sweeney J. a. (2004). Maturation of cognitive processes from late childhood to adulthood. Child Development, 75(5), 1357–1372. 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Marqués-Iturria I, Pueyo R, Garolera M, Segura B, Junqué C, García-García I, José Sender-Palacios M, Vernet-Vernet M, Narberhaus A, Ariza M, & Jurado MÁ (2013). Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Research - Neuroimaging, 214(2), 109–115. 10.1016/j.pscychresns.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Miller AA, & Spencer SJ (2014). Obesity and neuroinflammation: A pathway to cognitive impairment. Brain, Behavior, and Immunity, 42, 10–21. 10.1016/j.bbi.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Clasen LS, Giedd JN, & Blakemore S-J (2014). The Developmental Mismatch in Structural Brain Maturation during Adolescence. Developmental Neuroscience, 36(3–4), 147–160. 10.1159/000362328 [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Blasco G, Puig J, Biarnés C, Rivero M, Gich J, Fernández-Aranda F, Garre-Olmo J, Ramió-Torrentà L, Alberich-Bayarri, García-Castro F, Pedraza S, Ricart W, & Fernández-Real JM (2017). Neuroinflammation in obesity: Circulating lipopolysaccharide-binding protein associates with brain structure and cognitive performance. International Journal of Obesity, 41(11), 1627–1635. 10.1038/ijo.2017.162 [DOI] [PubMed] [Google Scholar]
- Mullins CA, Gannaban RB, Khan MS, Shah H, Siddik MAB, Hegde VK, Hemachandra Reddy P, & Shin AC (2020). Neural underpinnings of obesity: The role of oxidative stress and inflammation in the brain. In Antioxidants (Vol. 9, Issue 10, pp. 1–21). MDPI AG. 10.3390/antiox9101018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins TS, Campbell EM, & Hogeveen J (2020). Neighborhood Deprivation Shapes Motivational-Neurocircuit Recruitment in Children. Psychological Science, 31(7), 881–889. 10.1177/0956797620929299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, & Jansen A (2006). Why obese children cannot resist food: The role of impulsivity. Eating Behaviors, 7(4), 315–322. 10.1016/j.eatbeh.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, van Zijl P, … Sowell ER (2015). Family Income, Parental Education and Brain Structure in Children and Adolescents and for the Pediatric Imaging, Neurocognition, and Genetics Study. Nat Neurosci, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, & Hales CM (2020). Trends in obesity prevalence by race and hispanic origin - 1999–2000 to 2017–2018. In JAMA - Journal of the American Medical Association (Vol. 324, Issue 12, pp. 1208–1210). American Medical Association. 10.1001/jama.2020.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel N, Narr KL, Abbott C, Argyelan M, Espinoza R, Emsell L, Bouckaert F, Sienaert P, Vandenbulcke M, Nordanskog P, Repple J, Kavakbasi E, Jorgensen MB, Paulson OB, Hanson LG, Dols A, van Exel E, Oudega ML, Takamiya A, … Redlich R (2021). Elevated body weight modulates subcortical volume change and associated clinical response following electroconvulsive therapy. Journal of Psychiatry and Neuroscience, 46(4), E418–E426. 10.1503/jpn.200176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CE, Sheth C, Marshall AT, Adise S, Baker FC, Chang L, Clark DB, Coronado C, Dagher RK, Diaz V, Dowling GJ, Gonzalez MR, Haist F, Herting MM, Huber RS, Jernigan TL, LeBlanc K, Lee K, Lisdahl KM, … Yurgelun-Todd D (2021). A Comprehensive Overview of the Physical Health of the Adolescent Brain Cognitive Development Study Cohort at Baseline. Frontiers in Pediatrics, 9. 10.3389/fped.2021.734184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, & Klassen AC (2007). The built environment and obesity. Epidemiologic Reviews, 29(1), 129–143. 10.1093/epirev/mxm009 [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Razzoli M, & Bartolomucci A (2016). The Dichotomous Effect of Chronic Stress on Obesity. Trends in Endocrinology & Metabolism, 27(7), 504–515. 10.1016/j.tem.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Pearson C, Crow S, & Bartolomucci A (2017). Stress, overeating, and obesity: Insights from human studies and preclinical models. Neuroscience & Biobehavioral Reviews, 76(4), 154–162. 10.1016/j.neubiorev.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2015). Taste, olfactory, and food reward value processing in the brain. Progress in Neurobiology, 127–128, 64–90. 10.1016/j.pneurobio.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Rosales FJ, Reznick JS, & Zeisel SH (2009). Understanding the role of nutrition in the brain and behavioral development of toddlers and preschool children: Identifying and addressing methodological barriers. Nutritional Neuroscience, 12(5), 190–202. 10.1179/147683009X423454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold S, & Perktold J (2010). Statsmodels: Econometric and Statistical Modeling with Python. Proceedings of the 9th Python in Science Conference, Scipy, 92–96. 10.25080/majora-92bf1922-011 [DOI] [Google Scholar]
- Sharifi M, Sequist TD, Rifas-Shiman SL, Melly SJ, Duncan DT, Horan CM, Smith RL, Marshall R, & Taveras EM (2016). The role of neighborhood characteristics and the built environment in understanding racial/ethnic disparities in childhood obesity. Preventive Medicine, 91, 103–109. 10.1016/j.ypmed.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, & Giedd J (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440(7084), 676–679. 10.1038/nature04513 [DOI] [PubMed] [Google Scholar]
- Shields GS, Moons WG, & Slavich GM (2017). Inflammation, Self-Regulation, and Health: An Immunologic Model of Self-Regulatory Failure. Perspectives on Psychological Science, 12(4), 588–612. 10.1177/1745691616689091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, & Steinberg L (2016). The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17, 103–117. 10.1016/j.dcn.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Daus G, Allender M, Ramey C, Martin E, Perry C, Reyes A, & Vedamuthu I (2017). Social Determinants of Health in the United States: Addressing Major Health Inequality Trends for the Nation, 1935–2016. International Journal of MCH and AIDS (IJMA), 6(2), 139–164. 10.21106/ijma.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, & Pollak SD (2020). Early life stress and development: potential mechanisms for adverse outcomes. Journal of Neurodevelopmental Disorders, 12(1), 1–15. 10.1186/s11689-020-09337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, & Yokum S (2016). Neural vulnerability factors that increase risk for future weight gain. Psychological Bulletin, 142(5), 447–471. 10.1037/bul0000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaits A, & Alwan NA (2020). The association between area-based deprivation and change in body-mass index over time in primary school children: a population-based cohort study in Hampshire, UK. International Journal of Obesity, 44(3), 628–636. 10.1038/s41366-019-0418-9 [DOI] [PubMed] [Google Scholar]
- Vargas T, Damme KSF, & Mittal VA (2020). Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. NeuroImage, 220. 10.1016/j.neuroimage.2020.117086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeken S, Braet C, Claus L, Nederkoorn C, & Oosterlaan J (2009). Childhood Obesity and Impulsivity: An investigation with performance-based measures. Behaviour Change, 26(3), 153–167. 10.1375/bech.26.3.153 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


