Abstract
Background
Epidural analgesia is often used for pain relief during labour and childbirth, and involves administration of local anaesthetics (LA) into the epidural space resulting in sensory blockade of the abdomen, pelvis, and perineum. Epidural opioids are often co‐administered to improve analgesia. Administration of epidural medications can be accomplished by basal infusion (BI) or automated mandatory bolus (AMB). With BI, medications are administered continuously, while AMB involves injecting medications at set time intervals. Patient‐controlled epidural analgesia (PCEA) on top of AMB or BI enables patients to initiate additional boluses of epidural medications.
The superior method of delivering epidural medications would result in lower incidence of pain requiring anaesthesiologist intervention (breakthrough pain). Also, it should be associated with lower incidence of epidural‐related adverse effects including caesarean delivery, instrumental delivery (use of forceps or vacuum devices), prolonged duration of labour analgesia, and LA consumption. However, clear evidence of the superiority of one technique over the other is lacking. Also, differences in the initiation of epidural analgesia such as combined spinal‐epidural (CSE) (medications given into the intrathecal space in addition to the epidural space) compared to epidural only, and medications used (types and doses of LA or opioids) may not have been accounted for in previous reviews.
Our prior systematic review suggested that AMB reduces the incidence of breakthrough pain compared to BI with no significant difference in the incidence of caesarean delivery or instrumental delivery, duration of labour analgesia, and LA consumption. However, several studies comparing AMB and BI have been performed since then, and inclusion of their data may improve the precision of our effect estimates.
Objectives
To assess the benefits and harms of AMB versus BI for maintaining labour epidural analgesia in women at term.
Search methods
We searched CENTRAL, Wiley Cochrane Library), MEDLINE, (National Library of Medicine), Embase(Elseiver), Web of Science (Clarivate), the WHO‐ICTRP (World Health Organization) and ClinicalTrials.gov (National Library of Medicine) on 31 December 2022. Additionally, we screened the reference lists of relevant trials and reviews for eligible citations, and we contacted authors of included studies to identify unpublished research and ongoing trials.
Selection criteria
We included all randomised controlled studies that compared bolus dosing AMB with continuous BI during epidural analgesia. We excluded studies of women in preterm labour, with multiple pregnancies, with fetal malposition, intrathecal catheters, those that did not use automated delivery of medications, and those where AMB and BI were combined.
Data collection and analysis
We used standard methodology for systematic review and meta‐analysis described by Cochrane. Primary outcomes included: incidence of breakthrough pain requiring anaesthesiologist intervention; incidence of caesarean delivery; and incidence of instrumental delivery. Secondly, we assessed the duration of labour; hourly LA consumption in bupivacaine equivalents, maternal satisfaction after fetal delivery, and neonatal Apgar scores.
The following subgroup analyses were chosen a priori: epidural alone versus CSE technique; regimens that used PCEA versus those that did not; and nulliparous versus combination of nulli‐ and multi‐parous women.
We used the GRADE system to assess the certainty of evidence associated with our outcome measures.
Main results
We included 18 studies of 4590 women, of which 13 enrolled healthy nulliparous women and five included healthy nulli‐ and multiparous women. All studies excluded women with preterm or complicated pregnancies. Techniques used to initiate epidural analgesia differed between the studies: seven used combined spinal epidural, 10 used epidural, and one used dural puncture epidural (DPE). There was also variation in analgesics used. Eight studies utilised ropivacaine with fentanyl, three used ropivacaine with sufentanil, two utilised levobupivacaine with sufentanil, one used levobupivacaine with fentanyl, and four utilised bupivacaine with fentanyl. Most of the studies were assessed to have low risk of randomisation, blinding, attrition, and reporting biases, except for allocation concealment where eight studies were assessed to have uncertain risk and three with high risk.
Our results showed that AMB was associated with lower incidence of breakthrough pain compared to BI (risk ratio (RR) 0.71; 95% confidence interval (CI) 0.55 to 0.91; I2 = 57%) (16 studies, 1528 participants), and lower hourly LA consumption in bupivacaine equivalents (mean difference (MD) ‐0.84 mg/h; 95% CI ‐1.29 to ‐0.38, I2 = 87%) (16 studies, 1642 participants), both with moderate certainty. AMB was associated with an estimated reduction in breakthrough pain incidence of 29.1% (incidence 202 per 1000, 95% CI 157 to 259), and was therefore considered clinically significant.
The incidence of caesarean delivery (RR 0.85; 95% CI 0.69 to 1.06; I2 = 0%) (16 studies, 1735 participants) and instrumental delivery (RR 0.85; 95% CI 0.71 to 1.01; I2 = 0%) (17 studies, 4550 participants) were not significantly, both with moderate certainty. There was no significant difference in duration of labour analgesia (MD ‐8.81 min; 95% CI ‐19.38 to 1.77; I2 = 50%) (17 studies, 4544 participants) with moderate certainty. Due to differences in the methods and timing of outcome measurements, we did not pool data for maternal satisfaction and Apgar scores. Results reported narratively suggest AMB may be associated with increased maternal satisfaction (eight studies reported increased satisfaction and six reported no difference), and all studies showed no difference in Apgar scores.
WIth the exception of epidural alone versus CSE which found significant subgroup differences in LA consumption between AMB and BI, no significant differences were detected in the remaining subgroup analyses.
Authors' conclusions
Overall, AMB is associated with lower incidence of breakthrough pain, reduced LA consumption, and may improve maternal satisfaction. There were no significant differences between AMB and BI in the incidence of caesarean delivery, instrumental delivery, duration of labour analgesia, and Apgar scores. Larger studies assessing the incidence of caesarean and instrumental delivery are required.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Analgesia, Epidural; Analgesia, Epidural/adverse effects; Analgesics; Analgesics, Opioid; Breakthrough Pain; Breakthrough Pain/etiology; Levobupivacaine; Ropivacaine; Sufentanil; United States
Plain language summary
Do automated mandatory boluses of epidural medications provide superior labour pain relief than basal infusion?
Key messages
‐ When used to maintain epidural pain relief during labour, automated mandatory boluses are associated with lower incidence of pain requiring clinical intervention and medication consumption, compared to basal infusion.
‐ Both automated mandatory boluses and basal infusion are comparable in their associated incidence of caesarean delivery, instrumental delivery, and duration of labour epidural.
What are the methods of maintaining epidural pain relief during labour?
Epidurals are often used to provide pain relief during labour, and involve administration of local anaesthetic medications into the epidural space around the spinal column. Broadly, medications can be delivered via two techniques: basal infusion (BI) and automated mandatory boluses (AMB). With BI, medications are administered without interruption over an extended period of time, whereas AMB involves administration of medications at set time intervals with each dose delivered within a short period of time.
The superior method of delivering epidural medications would result in effective pain relief and low incidence of experiencing pain that requires anaesthesiologist intervention (also termed breakthrough pain). Also, it would be associated with lower incidence of epidural‐related adverse effects including caesarean delivery, instrumental delivery (use of forceps or vacuum device to assist delivery), prolonged duration of labour pain relief, and increased local anaesthetic consumption.
What did we want to find out?
Prior studies have reported contradicting data regarding which method (AMB compared to BI) provides superior pain relief during labour, and previous systematic reviews are outdated as there have been several new studies published on this topic. Inclusion of their data may improve the precision of our results regarding the effectiveness and potential adverse effects of AMB versus BI for maintenance of epidural pain relief during labour.
Hence, we aimed to compare AMB with BI in terms of:
‐ incidence of breakthrough pain (pain occurring during labour epidural requiring anaesthesiologist intervention)
‐ incidence of caesarean delivery
‐ incidence of instrumental delivery
Additionally, we compared AMB with BI in terms of duration of epidural analgesia and local anaesthetic consumption.
What did we do?
We searched for studies that compared AMB with BI for labour epidural pain relief. We compared and summarised the results of these studies, and rated our confidence in the evidence based on factors such as study methods and sizes.
What did we find?
Our review included 18 studies involving 4590 women at term with uncomplicated pregnancies. Overall, we found that AMB was associated with lower incidence of breakthrough pain and lower local anaesthetic consumption compared to BI, but both methods were comparable regarding the incidence of caesarean delivery, instrumental delivery, and duration of labour epidural.
What are the limitations of the evidence?
We have moderate confidence in the evidence, but it was limited by two main factors. First, there were differences between the studies in their respective methods, which includes differences in the types of medications used, stage of labour at which the epidural procedures were performed, and use of concurrent forms of pain relief in addition to labour epidural. These differences between the included studies could have contributed to the observed differences between AMB and BI. Second, some of our results were based on data obtained from a small number of women, which may have limited the precision of our findings.
How up to date is this evidence?
This review updates our previous review, and the evidence is up to date to 31 December 2022.
Summary of findings
Summary of findings 1. Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour.
| Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour | |||||
| Patient or population: term, pregnant women (nulliparous, or combination of nulli‐ and muliparous) requesting for labour epidural analgesia Setting: labour ward Intervention: programmed intermittent boluses (after initiation with combined spinal‐epidural, or epidural alone) Comparison: continuous infusion (after initiation with combined spinal‐epidural, or epidural alone) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with basal infusion | Risk with automated mandatory boluses | ||||
| Breakthrough pain assessed with: need for anaesthetic intervention during labour epidural analgesia | Study population | RR 0.71 (0.55 to 0.91) | 1528 (16 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 285 per 1000 | 202 per 1000 (157 to 259) |
||||
| Caesarean delivery during labour epidural analgesia | Study population | RR 0.85 (0.69 to 1.06) | 1735 (16 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 173 per 1000 | 147 per 1000 (120 to 184) |
||||
| Instrumental delivery during labour epidural analgesia | Study population | RR 0.85 (0.71 to 1.01) | 4550 (17 RCTs) | ⊕⊕⊕⊝ Moderateb |
|
| 95 per 1000 | 81 per 1000 (68 to 96) |
||||
| Duration of labour analgesia in minutes | The mean duration of labour in min ranged from 186.3 to 689.9 min | MD 8.81 min lower (19.38 lower to 1.77 higher) | — | 4544 (17 RCTs) | ⊕⊕⊕⊝ Moderateb |
| Local anaesthetic consumption per hour (mg/hr)c during labour epidural analgesia | The mean local anaesthetic consumption per hour ranged from 3.0 mg to 16.2 mg | MD 0.84 mg/h lower (1.29 lower to 0.38 lower) | — | 1642 (16 RCTs) | ⊕⊕⊕⊝ Moderatec,d |
| Maternal satisfaction following fetal delivery | Eight studies (five reported dichotomous data, three reported ordinal data) reported increased maternal satisfaction with automated mandatory boluses compared to basal infusion, while six studies found no difference between the groups. | 14 RCTs | ‐ | ||
| Apgar scores at 1‐ and 5‐minutes following fetal delivery | None of the studies reported any significant difference in Apgar scores | 14 RCTs | ‐ | ||
| *The risk in the intervention group (AMB) (and its 95% confidence interval) is based on the assumed risk in the comparison group (BI) and the relative effect of the intervention (and its 95% CI). Assumed comparator risks for dichotomous outcomes were derived from the median outcome incidence in patients receiving basal infusion within the studies included in this systematic review. AMB: automated mandatory bolus; CI: confidence interval; MD: mean difference; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
a Downgraded one level due to high statistical heterogeneity for this outcome, i.e. I2 = 57%.
b Downgraded one level due to imprecision, i.e. the wide range from upper to lower confidence limits and the 95% CI overlaps no effect.
c Converted into bupivacaine equivalents to account for variation in the type of local anaesthetic utilised.
d Downgraded one level due to high statistical inconsistency for this outcome, i.e. I2 = 87%.
Background
Description of the condition
Many women find labour and childbirth to be an extremely painful experience. Provision of pain relief (analgesia) during labour depends on each individual woman's needs and wishes, and requires consideration of medication effectiveness, risk of adverse effects, drug transfer to the fetus, and personal preferences. Modalities used to provide pain relief during labour include epidural analgesia, systemic opioids, nitrous oxide, and non‐pharmacological methods (Jones 2012).
Contemporary epidural analgesia involves the administration of dilute local anaesthetic solutions such as bupivacaine and ropivacaine into the epidural space, resulting in sensory blockade of the lower abdomen, pelvis, and perineum. Opioids including fentanyl and sufentanil are often co‐administered together with local anaesthetic into the epidural space to supplement and improve the analgesic effects. However, there is significant variability in current practice, which involves a variety of local anaesthetics (such as ropivacaine or bupivacaine) and opioids (including fentanyl or sufentanil) used to achieve labour epidural analgesia, at varying doses (Anim‐Somuah 2018; Tan 2019). Epidural analgesia can also be initiated through several techniques, including combined spinal‐epidural (CSE: medications given into the intrathecal space via a spinal needle in addition to epidural space), dural puncture epidural (DPE: puncture of the dura with a spinal needle without administration of intrathecal medications, followed by delivery of medications into the epidural space), and the standard epidural technique (administration of medications into the epidural space only) (Anim‐Somuah 2018; Tan 2019).
Description of the intervention
Broadly, the delivery of medications into the epidural space can be accomplished via two techniques: basal infusion (BI) and automated mandatory bolus (AMB). BI (also known as continuous epidural infusion, CEI) involves administration of medications without interruption over an extended period of time. Although the analgesic efficacy of BI is well established, it has been associated with higher local anaesthetic consumption and motor blockade, which may impair maternal ability to bear down during the second stage of labour and increase the incidence of instrumental delivery and fetal complications such as shoulder dystocia (Thornton 2001).
Conversely, AMB (also known as programmed intermittent epidural bolus, PIEB) involves the administration of medications into the epidural space at set time intervals with each dose delivered within a short period of time (Wong 2006). AMB delivers medications into the epidural space at higher flow rates compared to BI, which may improve medication spread and distribution within the epidural space. Several studies have reported lower local anaesthetic consumption, decreased motor blockade, reduced incidence of instrumental deliveries, and improved patient satisfaction with AMB compared to BI (Capogna 2011; Fettes 2006; Leo 2010; George 2013; Wong 2006).
The addition of patient‐controlled epidural analgesia (PCEA) on top of BI or AMB techniques enables patient‐initiated boluses of local anaesthetic to treat labour pain. Compared to BI alone, the addition of PCEA has been shown to reduce breakthrough pain, decrease local anaesthetic consumption without compromising analgesic efficacy, and improve patient satisfaction (Loubert 2011).
How the intervention might work
Cadaveric and experimental models have demonstrated that AMB resulted in wider and more uniform spread within the epidural space compared to BI (Kaynar 1999; Hogan 2002). For instance, Kaynar and Shankar compared the spread of contrast agent within the epidural space when administered via AMB or BI techniques though a multi‐orifice epidural catheter, and showed that AMB resulted in a wider and more uniform spread of contrast agent, while BI was associated with limited spread that was exclusively through the proximal orifice of the epidural catheter (Kaynar 1999). Furthermore, Hogan discovered that fluid spread within the epidural space occurred in a highly non‐uniform manner through multiple small channels (Hogan 2002). Thus, it was hypothesised that higher injectate flow rates associated with the AMB technique enhances local anaesthetic spread by engaging the other catheter orifices and channels within the epidural space, which may in turn result in reduced local anaesthetic consumption, decreased motor blockade, and improved analgesic efficacy (Riazanova 2019).
Why it is important to do this review
The AMB technique for labour analgesia necessitates the use of more sophisticated drug delivery pumps that may not be commonly available. In addition, the transition to pumps that are capable of AMB may incur the need for additional provider training and increase healthcare‐related costs.
Furthermore, available evidence regarding the benefits of AMB over BI is conflicting. Although several studies reported improved analgesia, reduction in the incidence of breakthrough pain (pain requiring anaesthesiologist intervention, despite receiving epidural analgesia), and less motor blockade with the AMB technique (Chua 2004; Lim 2005; Fettes 2006; Wong 2006; Capogna 2011; Sia 2013; Ferrer 2017), others suggest there was no significant difference compared to BI (Salim 2005; Sia 2007; Leo 2010; Lim 2010; Capogna 2011). Given the lack of clear evidence of clinical superiority of either AMB or BI techniques, our previous version of this systematic review (Sng 2018) was performed to provide a comprehensive summary of evidence comparing AMB versus BI for labour analgesia. We considered relevant anaesthetic, obstetric and fetal outcomes including the incidence of breakthrough pain, caesarean delivery, instrumental delivery, local anaesthetic consumption, and duration of labour analgesia. Our findings suggested, with moderate‐certainty, that AMB was associated with lower incidence of breakthrough pain, without significant change in mean duration of labour analgesia or hourly local anaesthetic consumption. Also, AMB was not associated with significant change in the incidence of caesarean delivery or instrumental delivery compared to BI, with low certainty.
However, since the publication of the previous version of this review (Sng 2018), several studies examining the effectiveness of AMB and BI for labour analgesia have been performed, and updating our pooled results may improve the precision of our effect estimates. By evaluating important clinical outcomes associated with AMB or BI, we aim to justify adoption of the superior epidural delivery method for labour analgesia, which may in turn improve analgesic effectiveness and maternal and fetal outcomes.
Objectives
To assess the benefits and harms of automated mandatory bolus (AMB) versus basal infusion (BI) for maintaining labour epidural analgesia in women at term.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomised controlled trials (RCTs) that compared automated mandatory bolus (AMB) with basal infusion (BI) for the maintenance of labour epidural analgesia, irrespective of language, publication date, or publication type.
We excluded non‐randomised studies such as cohort studies due to their increased risk of bias, and cross‐over studies as this methodology was not appropriate to evaluate interventions administered at specific time points.
Types of participants
We included studies involving term, pregnant women who requested for labour epidural analgesia. Studies in which a subset of participants met our eligibility criteria were included if these participants comprised at least 65% of the study population, and only data relevant to the eligible participants were analysed.
We excluded studies of women in preterm labour, with multiple pregnancies, or with fetal malposition such as breech presentations.
Types of interventions
We included studies that compared AMB with BI to maintain epidural labour analgesia. AMB was defined as automated, intermittent bolus administration of local anaesthetic into the epidural space at set time intervals. Conversely, BI was defined as continuous administration of local anaesthetic into the epidural space without interruption. All forms and doses of local anaesthetics with the addition of opioids administered during labour epidural analgesia were included. Studies that utilised patient‐controlled epidural analgesia (PCEA) were included, as long as the intervention groups compared AMB with BI.
We excluded interventions involving intrathecal or spinal catheters, those that did not use automated delivery or which utilised manual delivery of local anaesthetics to maintain labour analgesia, and interventions where AMB and BI were combined.
Types of outcome measures
Outcomes were dichotomous (breakthrough pain, caesarean delivery, and instrumental delivery), continuous (duration of labour analgesia, and local anaesthetic consumption), or ordinal (maternal satisfaction and Apgar score).
Outcomes were measured from the start of labour analgesia to immediately after childbirth, as reported by the individual studies. Outcomes were not used as eligibility criteria for study selection.
Primary outcomes
Incidence of breakthrough pain, defined as pain during labour epidural analgesia requiring anaesthetic intervention (dichotomous)
Incidence of caesarean delivery (dichotomous)
Incidence of instrumental delivery, defined as the use of forceps or vacuum‐assisted delivery (dichotomous)
The minimally important risk difference in incidence of breakthrough pain was set at 5%. The minimally important risk difference of caesarean delivery and instrumental delivery was set at 1%.
Methodological differences in the measurement of the outcomes were resolved by contacting the original authors, or reported narratively in our review.
Secondary outcomes
Duration of labour analgesia, defined as the start of epidural analgesia to discontinuation of local anaesthetic administration (continuous)
Local anaesthetic consumption per hour during labour epidural analgesia (continuous)
Maternal satisfaction after fetal delivery (ordinal)
Apgar scores (ordinal) at 1‐ and 5‐minutes after fetal delivery, measured by Apgar score scale
Methodological differences in the measurement of the outcomes were resolved by contacting the original authors, or reported narratively in our review.
Search methods for identification of studies
Electronic searches
Following the Cochrane guidelines for searching and identification of relevant studies (Lefebvre 2021), the databases of Cochrane Central Register of Controlled Trials (CENTRAL, Wiley Cochrane Library); MEDLINE (National Library of Medicine); Embase (Elseiver), Web of Science (Clarivate), the WHO‐ICTRP (World Health Organization) and ClinicalTrials.gov (National Library of Medicine) were searched from inception to 31 December 2020, with our search strategies detailed in Appendix 1. Updated searches were performed from 1 January 2021 to 31 December 2021, and 1 January 2022 to 31 December 2022. Collections used for the databases were: CENTRAL ‐ all, MEDLINE ‐ Ovid Medline (R) 1946‐2022, Embase ‐ Biomedica, Web of Science ‐ Core Collection, WHO‐ICTRP ‐ all, ClinicalTrials.gov ‐ all.
No language restrictions were placed on our searches. We used free‐text terms in all databases and subject headings in combination when thesauri were a component of a database.
Searching other resources
We reviewed the 'Related articles' feature of PubMed for all eligible trials and reviews. We screened the reference lists of all eligible trials, reviews, and systematic reviews for potentially eligible studies. We also contacted authors of included studies in this field in order to identify unpublished research and trials still underway. Reference lists of the included articles were screened for potentially relevant articles.
Data collection and analysis
A minimum of two review authors (HST, ZYZ, YYQ, FJS) independently collected and verified data on a standardised data collection form that was pilot‐tested prior to use (see Appendix 1), with a third review author (BLS) available to arbitrate any disagreements through discussion.
Selection of studies
Titles, abstracts, or records identified by our search criteria (Criteria for considering studies for this review) were uploaded into Covidence, a systematic review screening tool (Covidence). A minimum of two review authors (HST, ZYZ, YYQ, FJS) independently reviewed each title and abstract, followed by an examination of the full‐text documents to identify studies meeting the inclusion criteria. Conflicts were resolved by discussion, or with arbitration by a third review author (BLS).
Data extraction and management
A minimum of two review authors (HST, ZYZ, YYQ, FJS) independently extracted the data using a standardised form that was pilot‐tested prior to use (see Appendix 1). We extracted information pertaining to the study design, method of randomisation, use of allocation concealment, blinding of caregivers and outcome assessors, reporting of the study setting and participants, inclusion and exclusion criteria, sample size, interventions, outcomes, and loss to follow‐up. Two review authors (HST, ZYZ) entered and checked the data independently, and a third review author (BLS) resolved disagreements. The included studies were checked for errata, comments and retractions. The outcomes of included studies were compared with the ones reported in ClinicalTrials.gov protocols.
Non‐English studies were professionally translated prior to data collection.
Assessment of risk of bias in included studies
A minimum of two review authors (HST, ZYZ, YYQ, FJS, RS) independently assessed trial quality and risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and a third review author (BLS) resolved any disagreements.
Based on the Cochrane risk of bias tool in Review Manager Web (RevMan Web), we considered the following domains (Higgins 2011): random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Appendix 2 presents the details on the Cochrane risk of bias tool and criteria for judgement.
We graded each of the above dimensions of trial quality as being at low, high or unclear risk of bias. In this review, stratified analysis based on study quality was not performed given the lack of included studies with high risk of bias.
Measures of treatment effect
Dichotomous data
Dichotomous data were presented as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
Continuous data were summarised as mean difference (MD) with corresponding 95% CIs.
Unit of analysis issues
The woman was the unit of analysis in all of the studies. In the case of multi‐arm studies, only the relevant groups were to be included. In the event that data from multi‐arm studies was used in single meta‐analysis, we divided the number of participants in the control group by the number of arms.
Dealing with missing data
Wherever possible, we contacted the authors of the original articles for missing data via the provided contact information in the original paper.
Assessment of heterogeneity
We evaluated clinical heterogeneity by qualitatively appraising differences in study characteristics such as participants, interventions, outcomes assessed, and study methodology. Quantitative pooling of the data was first justified by a consensus of clinical judgement of sufficient clinical homogeneity. We informally evaluated and investigated the degree of statistical heterogeneity by visual inspection of forest plots and more formally by using the Tau2, I2, and Chi2 statistics. We regarded heterogeneity as considerable if the I2 value was greater than 75%, substantial if the I2 value was between 50% and 75%, moderate if the I2 value was between 30% and 50%, and low if the I2 value was less than 30%. In future updates of this review outcomes with substantial or considerable heterogeneity (I2 greater than 50%) will be further evaluated for sources of heterogeneity and if found, subgroup analysis or meta‐regression analysis will be considered.
Assessment of reporting biases
We checked the methodology and study protocols of the primary studies where available. We assessed publication bias and other small‐study effects in a qualitative manner using a funnel plot.
Funnel plot asymmetry was tested using weighted linear regression of effect estimates on their standard error (SE) if more than 10 trials were included in an analysis (Egger 1997).
Data synthesis
Statistical analyses were performed using RevMan Web. Data synthesis of dichotomous outcomes was performed using the Mantel‐Haenszel method, with the results presented as RRs and 95%CIs. The inverse variance method was used for continuous outcomes, and reported as MD with 95%CI. We analysed maternal satisfaction as a continuous outcome, even if measured on an ordinal scale. Some studies administered ropivacaine or levobupivacaine local anaesthetics in place of bupivacaine. For such studies we assumed 60% potency of bupivacaine based on a similar systematic review and meta‐analysis (George 2013), and the means and standard deviations (SDs) in our results were multiplied by 0.6.
In the case of data presented in the included studies as median and range, we attempted to obtain data in the form of mean and standard deviation (SD) from the respective authors. If this was not possible, we converted the median and range to mean and SD using the formula by Hozo 2005. Data presented as 95%CI were converted to SD (Cochrane Handbook 7.7.3.2 Obtaining standard deviations from standard errors and confidence intervals for group means).
We expected both clinical and statistical heterogeneity, and therefore we used the the random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were chosen a priori based on prior evidence of association with the outcomes in this review.
Epidural technique: epidural alone versus combined spinal‐epidural technique (the dural‐puncture epidural technique was not included). Rationale: prior evidence suggests that combined spinal‐epidural technique may reduce breakthrough pain and local anaesthetic consumption (Tan 2019).
PCEA: regimens that used PCEA versus those that did not. Rationale: prior studies demonstrated that PCEA use was associated with reduced local anaesthetic consumption and breakthrough pain, while other studies reported that PCEA increased local anaesthetic consumption without improving analgesia (Tan 2019).
Nulliparous versus combination of nulli‐ and multi‐parous women. Rationale: nulliparity has been associated with increased risk of breakthrough pain in several studies (Tan 2019; Tan 2021).
Subgroup differences were analysed by testing for heterogeneity across subgroup results (Borenstein 2013).
In addition, outcomes with substantial or considerable heterogeneity (I2 greater than 50%) were evaluated for sources of heterogeneity and if found, subgroup analysis or meta‐regression analysis were considered.
Sensitivity analysis
We did not perform sensitivity analyses on the quality of the studies because the quality of the studies was consistent across the different studies (Risk of bias in included studies). We will consider performing sensitivity analyses in future updates of this review if required.
Sensitivity analysis for trial quality involves analysis based on the rating of selection bias and attrition bias. We excluded studies of poor quality from the analysis (those rated as unclear or high risk of bias) in order to assess for any substantive difference to the overall result. The sensitivity analysis for compliance were based on trials where women did not receive their allocated treatment, combination therapy, or intervention, or if they received an additional form of analgesia to the one allocated. If required, these sensitivity analyses will be performed on the primary outcomes only.
Summary of findings and assessment of the certainty of the evidence
We used the principles of the GRADE system in order to assess the certainty of evidence associated with the following specific outcomes (Guyatt 2008).
Incidence of breakthrough pain requiring anaesthesiologist intervention during labour epidural analgesia
Incidence of caesarean delivery during labour epidural analgesia
Incidence of instrumental delivery during labour epidural analgesia
Duration of labour analgesia
Hourly dose of local anaesthetic during labour epidural analgesia
Maternal satisfaction following fetal delivery
Apgar score at 1‐ and 5‐minutes following fetal delivery
We constructed a summary of findings table comparing programmed AMB versus BI using GRADEpro software (GRADEpro GDT 2015). The GRADE approach is a transparent and structured system of assessing the certainty of evidence based on the confidence that an estimate of effect reflects the true value. Evidence from randomised trials were assigned high certainty, but can be downgraded based on risk of bias, inconsistency of results, indirectness of evidence, imprecision, or publication bias.
In this review, the risk of bias was considered present if there was a high risk of lack of allocation concealment or assessor blinding, significant loss to follow‐up, or selective reporting that may affect interpretation of results. The GRADE level was downgraded one level for inconsistency if I2 50% to 90%, and two levels if I2 >90%. Imprecision was considered present if the upper or lower 95% CI extended from the line of equality by >5%. Publication bias was considered significant with P < 0.05 in the Egger’s test.
Assumed comparator risks for dichotomous outcomes were derived from the median outcome incidence in patients receiving basal infusion within the studies included in this systematic review.
Results
Description of studies
Please refer to Characteristics of included studies for further details and a summary of study characteristics.
Results of the search
A total of 7704 studies were identified by our search criteria. After the removal of 3524 duplicates, titles and abstracts of 4180 studies were screened to remove clearly irrelevant studies. Subsequently, remaining 66 articles were assessed for eligibility, of which 29 were non‐full text and/or duplicates, 19 full ‐text articles were deemed non‐eligible and excluded, and 18 articles were included in our systematic review.
In addition, through screening the references of relevant studies and systematic reviews, another five studies were identified that were potentially eligible for inclusion (Fang 2016; Ji 2016; Wang 2016; Wang 2017; Zhao 2013), but we were unable to obtain full‐text copies and therefore enlisted the assistance of a medical librarian. Pending full‐text review, these five studies were considered 'awaiting classification'.
Non‐English studies (if any) were translated by an external translator.
The disposition of the identified citations is detailed in the PRISMA diagram (Figure 1).
1.
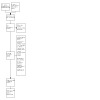
Study flow diagram.
Included studies
We included 18 studies involving 4590 participants (Capogna 2011; Chalekar 2021; Chua 2004; Fan 2019; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006), of which 13 enrolled healthy, term, nulliparous women (Capogna 2011; Chalekar 2021; Chua 2004; Fan 2019; Fettes 2006; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Sia 2007; Sia 2013; Song 2020), while five studies enrolled healthy nulli‐ or multiparous women at term (Ferrer 2017; Fidkowski 2019; Haidl 2020; Ojo 2020; Wong 2006). All studies excluded women with complicated pregnancies.
Out of 18 studies, 16 reported breakthrough pain, caesarean delivery and local anaesthetic consumption, while 17 reported instrumental delivery and duration of labour analgesia.
The technique used to initiate epidural analgesia differed between the studies. Seven studies used combined spinal‐epidural (CSE) (Chua 2004; Leo 2010; Lim 2005; Lim 2010; Sia 2007; Sia 2013; Wong 2006), with two of these studies administering only intrathecal opioid (fentanyl in both) without any intrathecal local anaesthetic (Chua 2004; Lim 2005). Epidural catheter without any intrathecal injection was used in ten studies (Capogna 2011; Chalekar 2021; Fan 2019; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Lin 2016; Morau 2019; Ojo 2020), while one study (Song 2020) performed a dural puncture epidural (DPE) for both : automated mandatory bolus (AMB) and basal infusion (BI) groups.
There was also variation in the choice of analgesics and dosages used. Eight studies utilised ropivacaine with fentanyl (Chua 2004; Chalekar 2021; Fettes 2006; Leo 2010; Lim 2010; Ojo 2020; Sia 2007; Sia 2013), three used ropivacaine with sufentanil (Fan 2019; Lin 2016; Song 2020), two utilised levobupivacaine with sufentanil (Capogna 2011; Morau 2019), one used levobupivacaine with fentanyl (Lim 2005), and four studies utilised bupivacaine with fentanyl (Ferrer 2017; Fidkowski 2019; Haidl 2020; Wong 2006).
Please refer to Characteristics of included studies for additional details.
Excluded studies
Out of the 66 studies assessed for eligibility, 48 were excluded for the following reasons.
Nineteen studies full‐text reviewed and excluded:
12 studies ‐ did not include the use of automated mandatory boluses of local anaesthetic (Boutros 1999; Feng 2014; Garg 2022; Lamont 1989; Mukherjee 2013; Patkar 2015; Priyadarshini 2022; Roofthooft 2021; Shidhaye 2010; Skrablin 2011; Smedstad 1988; Vilaplana 1995);
3 studies ‐ not randomised controlled trials (Delgado 2018; Liu 2020; Rodriguez Gonzalez 2019);
3 studies ‐ varying local anaesthetic concentrations and volumes administered to the intervention groups (Nunes 2014; Nunes 2016; Salim 2005);
1 study ‐ did not administer opioids for epidural analgesia (Riazanova 2019).
Non‐articles and/or duplicates:
26 studies ‐ trial registration or conference abstracts of studies included in this review;
3 studies ‐ duplicates of included studies.
Please refer to Characteristics of excluded studies for additional details.
Studies awaiting classification
An additional five citations were identified from screening the references of relevant studies and systematic reviews (Fang 2016; Ji 2016; Wang 2016; Wang 2017; Zhao 2013). However, we were unable to obtain full‐text copies and therefore enlisted the assistance of a medical librarian. Pending full‐text review, these five citations were considered 'awaiting classification'.
Please refer to Studies awaiting classification for additional details.
Ongoing studies
No ongoing studies were identified.
Risk of bias in included studies
Please refer to Figure 2 and Figure 3 for additional details.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Twelve studies utilised computer‐generated random numbers for randomisation (Capogna 2011; Fan 2019; Ferrer 2017; Fettes 2006; Haidl 2020; Leo 2010; Lim 2005; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006) and one study utilised shuffling of sealed envelopes as their randomisation method (Fidkowski 2019); these studies were considered to be at low risk for selection bias. However, four studies did not describe the method of randomisation (Chalekar 2021; Chua 2004; Lim 2010; Lin 2016), and one study stated that women were randomised in blocks of four and six but no further details were provided (Morau 2019). We considered the risk of selection bias to be unclear in these studies.
Sealed opaque envelopes were used for allocation in seven studies (Capogna 2011; Chua 2004; Leo 2010; Lim 2010; Ojo 2020; Sia 2007; Sia 2013), and were considered at low risk of selection bias. Seven studies (Chalekar 2021; Fan 2019; Fettes 2006; Fidkowski 2019; Haidl 2020; Lin 2016; Wong 2006) stated that allocation concealment were performed using envelopes, but did not specify if the envelopes were sealed or if they were opaque; these studies were considered at unclear risk for selection bias. One study (Ferrer 2017) stated that the participants, caregivers and outcome assessors were not aware of the treatment allocation but did not specify how this was achieved, and was assessed to be at unclear risk for selection bias. Three studies (Lim 2005; Morau 2019; Song 2020) did not specify if allocation concealment was performed, and were considered at high risk of selection bias.
Blinding
Blinding of participants and outcome assessors were performed in 13 studies (Capogna 2011; Fan 2019; Ferrer 2017; Haidl 2020; Leo 2010; Lim 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006), and were considered to be at low risk of performance bias. Four studies (Chalekar 2021; Chua 2004; Fettes 2006; Lim 2005) did not specify if the participants were blinded, and were assessed to be at unclear risk for performance bias. Fidkowski 2019 stated that participants were blinded, but anaesthesia providers were not blinded and was considered to be at high risk of performance bias.
In assessment of detection bias, studies in which participants were not blinded were considered high risk for detection bias, as several outcomes were patient‐reported. Overall, 13 studies described blinding of both participants and outcome assessors (Capogna 2011; Fan 2019; Ferrer 2017; Haidl 2020; Leo 2010; Lim 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006) and were considered low risk for detection bias. Four studies (Chalekar 2021; Chua 2004; Fettes 2006; Lim 2005) did not specify if participants were blinded, and one study (Fidkowski 2019) did not specify who performed the outcome assessment; these studies were considered at unclear risk of detection bias.
Incomplete outcome data
With the exception of two studies that excluded over 20% of the cohort from analysis and was assessed to be at high risk of attrition bias (Fidkowski 2019; Ojo 2020), the risk of attrition bias was considered low in the remaining studies as all outcome measures were reported, without significant missing data or loss to follow up.
Intention‐to‐treat analyses were performed in all studies, with the exception of one study that used a per protocol analysis, although this affected only two participants (Fidkowski 2019).
Selective reporting
Outcome measures were pre‐specified and reported in all included studies, and were therefore considered to be at low risk for reporting bias. The outcomes of all included studies matched their ClinicalTrials.gov protocols.
Other potential sources of bias
No other sources of significant bias was noted in all included studies.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
1. Incidence of breakthrough pain
Breakthrough pain was reported by 16 studies (1528 women) (Capogna 2011; Chalekar 2021; Chua 2004; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Leo 2010; Lim 2005; Lim 2010; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006). Based on the pooled results, maintenance of labour epidural analgesia using automated mandatory bolus (AMB) was associated with reduced incidence of breakthrough pain (risk ratio (RR) 0.71; 95% confidence (CI) 0.55 to 0.91) compared to basal infusion (BI), although substantial heterogeneity was present (I2 = 57%) (Analysis 1.1). Based on an assumed comparator incidence of 285 per 1000 with BI, AMB was associated with an estimated reduction in breakthrough pain incidence of 29.1% (incidence 202 per 1000, 95%CI 157 to 259), and was therefore considered clinically significant. Due to substantial heterogeneity, this result was assessed as moderate in certainty.
1.1. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 1: Breakthrough pain
Epidural alone versus combined spinal‐epidural technique
Labour analgesia was initiated using combined‐spinal epidural (CSE) in seven studies (Chua 2004; Leo 2010; Lim 2005; Lim 2010; Sia 2007; Sia 2013; Wong 2006), while eight studies used epidural alone (Capogna 2011; Chalekar 2021; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Morau 2019; Ojo 2020). One study used dural puncture epidural, and was not included in this subgroup analysis (Song 2020). In subgroup analysis of women who received CSE versus those who received epidural only, no significant difference was found between the subgroups in terms of the incidence of breakthrough pain (test for subgroup differences: Chi2=0.01, df=1, P = 0.94, I2 = 0%) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 2: Breakthrough pain (epidural vs CSE)
Regimens that used PCEA versus those that did not
Patient controlled epidural analgesia (PCEA) was utilised in nine studies (Capogna 2011; Haidl 2020; Leo 2010; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006), while seven studies did not use PCEA (Chalekar 2021; Chua 2004; Ferrer 2017; Fettes 2006; Fidkowski 2019; Lim 2005; Lim 2010). In subgroup analysis of women who received PCEA versus those who did not, no significant difference was found between the subgroups in terms of the incidence of breakthrough pain (test for subgroup differences: Chi2= 0.32, df =1, P = 0.57, I2 = 0%) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 3: Breakthrough pain (PCEA vs no PCEA)
Nulliparous versus combination of nulli‐ and multi‐parous women
Out of 16 studies that reported breakthrough pain, 11 enrolled nulliparous women only (Capogna 2011; Chalekar 2021; Chua 2004; Fettes 2006; Leo 2010; Lim 2005; Lim 2010; Morau 2019; Sia 2007; Sia 2013; Song 2020), while five enrolled both nulliparous and multiparous women (Ferrer 2017; Fidkowski 2019; Haidl 2020; Ojo 2020; Wong 2006). In subgroup analysis of nulliparous women versus a combination of nulliparous and multiparous women, no significant difference was found between the subgroups in terms of the incidence of breakthrough pain (test for subgroup differences: Chi2 = 0.05, df = 1, P = 0.83, I 2 = 0%) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 4: Breakthrough pain (nulliparous vs nulliparous + multiparous)
2. Incidence of caesarean delivery
The incidence of caesarean delivery was reported in 16 studies involving 1735 women (Capogna 2011; Chalekar 2021; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006). The pooled results showed that the use of AMB to maintain labour analgesia were not associated with significant change in the incidence of caesarean delivery compared to BI (RR 0.85; 95% CI 0.69 to 1.06) (Analysis 1.7). Although this result was associated with low heterogeneity (I2 = 0%), the overall certainty of evidence was considered moderate due to imprecision. Based on an assumed comparator incidence of 173 per 1000 with BI, AMB was associated with an estimated reduction in caesarean delivery incidence of 15.0% (incidence 147 per 1000, 95%CI 120 to 184), and was therefore considered clinically significant.
1.7. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 7: Caesarean delivery (PCEA vs no PCEA)
Epidural alone versus combined spinal‐epidural technique
Labour analgesia was initiated using CSE in six studies (Leo 2010; Lim 2005; Lim 2010; Sia 2007; Sia 2013; Wong 2006), while nine studies used epidural alone (Capogna 2011; Chalekar 2021; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Lin 2016; Morau 2019; Ojo 2020). One study used dural puncture epidural, and was not included in this subgroup analysis (Song 2020). In subgroup analysis of women who received epidural only versus those who received CSE, no significant difference was found between the subgroups in terms of the incidence of caesarean delivery (test for subgroup differences: Chi2 = 2.00, df = 1, P = 0.16, I2 = 50.1%) (Analysis 1.6).
1.6. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 6: Caesarean delivery (epidural vs CSE)
Regimens that used PCEA versus those that did not
PCEA was utilised in ten studies (Capogna 2011; Haidl 2020; Leo 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006), while six studies did not use PCEA (Chalekar 2021; Ferrer 2017; Fettes 2006; Fidkowski 2019; Lim 2005; Lim 2010). In subgroup analysis of women who received PCEA versus those who did not, no significant difference was found between the subgroups in terms of the incidence of caesarean delivery (test for subgroup differences: Chi2 = 0.88, df = 1, P = 0.35, I2 = 0%) (Analysis 1.7).
Nulliparous versus combination of nulli‐ and multi‐parous women
Out of 16 studies that reported caesarean delivery, 11 enrolled nulliparous women (Capogna 2011; Chalekar 2021; Fettes 2006; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Sia 2007; Sia 2013; Song 2020) while five enrolled both nulliparous and multiparous women (Ferrer 2017; Fidkowski 2019; Haidl 2020; Ojo 2020; Wong 2006). In subgroup analysis of nulliparous women versus a combination of nulli‐ and multiparous women, no significant difference was found between the subgroups in terms of the incidence of caesarean delivery (test for subgroup differences: Chi2=0.43, df =1, P = 0.51, I2 = 0%) (Analysis 1.8).
1.8. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 8: Caesarean delivery (nulliparous vs nulliparous + multiparous)
3. Incidence of instrumental delivery
The incidence of instrumental delivery was reported in 17 studies that enrolled 4550 women (Capogna 2011; Chalekar 2021; Fan 2019; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006). The use of AMB or BI was not associated with significant change in the incidence of instrumental delivery (RR 0.85; 95% CI 0.71 to 1.01) (Analysis 1.9), with low heterogeneity present (I2 = 0%) (Analysis 1.9). We assessed the certainty of evidence as moderate, due to the imprecision. Based on an assumed comparator incidence of 95 per 1000 with BI, AMB was associated with an estimated reduction in instrumental delivery incidence of 14.7% (incidence 81 per 1000, 95%CI 68 to 96), and was therefore considered clinically significant.
1.9. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 9: Instrumental delivery
Epidural alone versus combined spinal‐epidural technique
Labour analgesia was initiated using CSE in six studies (Leo 2010; Lim 2005; Lim 2010; Sia 2007; Sia 2013; Wong 2006), while ten studies used epidural alone (Capogna 2011; Chalekar 2021; Fan 2019; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Lin 2016; Morau 2019; Ojo 2020). One study used dural puncture epidural, and was not included in this subgroup analysis (Song 2020). In subgroup analysis of women who received epidural alone versus those who received CSE, no significant difference was found between the subgroups in terms of the incidence of instrumental delivery (test for subgroup differences: Chi2=1.63, df=1, P = 0.20, I2 = 38.5%) (Analysis 1.10).
1.10. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 10: Instrumental delivery (epidural vs CSE)
Regimens that used PCEA versus those that did not
PCEA was utilised in 11 studies (Capogna 2011; Fan 2019; Haidl 2020; Leo 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006), while six studies did not use PCEA (Chalekar 2021; Ferrer 2017; Fettes 2006; Fidkowski 2019; Lim 2005; Lim 2010). In subgroup analysis of women who received PCEA versus those who did not, no significant difference was found between the subgroups in terms of the incidence of instrumental delivery (test for subgroup differences: Chi2 = 0.89, df = 1, P = 0.34, I2 = 0%) (Analysis 1.11).
1.11. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 11: Instrumental delivery (PCEA vs No PCEA)
Nulliparous versus combination of nulli‐ and multi‐parous women
Of 17 studies, 12 enrolled only nulliparous women (Capogna 2011; Chalekar 2021; Fan 2019; Fettes 2006; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Sia 2007; Sia 2013; Song 2020), while five studies enrolled both nulliparous and multiparous women (Ferrer 2017; Fidkowski 2019; Haidl 2020; Ojo 2020; Wong 2006). In subgroup analysis of nulliparous women versus a combination of nulli‐ and multiparous women, no significant difference was found between the subgroups in terms of the incidence of instrumental delivery (test for subgroup differences: Chi2=0.74, df = 1, P = 0.39, I2 = 0%) (Analysis 1.12).
1.12. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 12: Instrumental delivery (nulliparous vs nulliparous + multiparous)
Secondary outcomes
1. Duration of labour analgesia in minutes
The duration of labour analgesia was reported in 17 studies involving 4544 women (Capogna 2011; Chalekar 2021; Fan 2019; Ferrer 2017; Fettes 2006; Fidkowski 2019; Haidl 2020; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006). The use of AMB or BI was not associated with a significant difference in the duration of labour analgesia (mean difference (MD) ‐8.81 min; 95% CI ‐19.38 to 1.77), with moderate heterogeneity (I2 = 50%) (Analysis 1.13). Due to the presence of imprecision, the certainty of evidence for this outcome was assessed as moderate.
1.13. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 13: Duration of labour analgesia in minutes
Epidural alone versus combined spinal‐epidural technique
One study used dural puncture epidural, and was not included in this subgroup analysis (Song 2020). In subgroup analysis of women who received epidural alone versus those who received CSE, no significant difference was found between the subgroups in terms of duration of labour analgesia (test for subgroup differences: Chi2 = 2.89, df = 1, P = 0.09, I2 = 65.4%) (Analysis 1.14).
1.14. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 14: Duration of labour analgesia in minutes (epidural vs CSE)
Regimens that used PCEA versus those that did not
In subgroup analysis of women who received PCEA versus those who did not, no significant difference was found between the subgroups in terms of duration of labour analgesia (test for subgroup differences: Chi2=0.64, df=1, P = 0.42, I2 = 0%) (Analysis 1.15).
1.15. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 15: Duration of labour analgesia in minutes (PCEA vs no PCEA)
Nulliparous versus combination of nulli‐ and multi‐parous women
In subgroup analysis of nulliparous women versus a combination of nulli‐ and multiparous women, no significant difference was found between the subgroups in terms of duration of labour analgesia (test for subgroup differences: Chi2 = 0.86, df = 1, P = 0.35, I2 = 0%) (Analysis 1.16).
1.16. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 16: Duration of labour analgesia in minutes (nulliparous vs nulliparous + multiparous)
2. Local anaesthetic consumption in milligrams per hour
Hourly consumption of local anaesthetics (LA) was reported by 16 studies (1642 women) (Capogna 2011; Chalekar 2021; Chua 2004; Ferrer 2017; Fettes 2006; Haidl 2020; Leo 2010; Lim 2005; Lim 2010; Lin 2016; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006). The pooled results demonstrate that AMB was associated with lower LA consumption compared to BI (MD ‐0.84 mg/h; 95% CI ‐1.29 to ‐0.38) (Analysis 1.17). Due to the presence of considerable heterogeneity (I2 = 87%), the certainty of this result was considered moderate.
1.17. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 17: LA consumption per hour
Epidural alone versus combined spinal‐epidural technique
One study used dural puncture epidural, and was not included in this subgroup analysis (Song 2020). There was a significant subgroup difference between epidural alone versus CSE, in terms of LA consumption with AMB and BI (test for subgroup differences: Chi2=5.75, df=1, P = 0.02, I2 = 82.5%). The use of AMB following initiation of labour analgesia with epidural alone was associated with significantly lower LA consumption compared to BI (MD ‐1.22 mg/h; 95% CI ‐1.75 to ‐0.69), although no significant difference was found between AMB and BI with CSE (MD ‐0.36 mg/h; 95% CI ‐0.82 to 0.10) (Analysis 1.18).
1.18. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 18: LA consumption per hour (epidural vs CSE)
Regimens that used PCEA versus those that did not
In subgroup analysis of women who received PCEA versus those who did not, no significant difference was found between the subgroups in terms of LA consumption (test for subgroup differences: Chi2 = 0.01, df =1, P = 0.91, I2 = 0%) (Analysis 1.19).
1.19. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 19: LA consumption per hour (PCEA vs no PCEA)
Nulliparous versus combination of nulli‐ and multi‐parous women
In subgroup analysis of nulliparous women versus a combination of nulli‐ and multiparous women, no significant difference was found between the subgroups in terms of LA consumption (test for subgroup differences: Chi2 = 1.72, df = 1, P = 0.19, I2 = 41.7%) (Analysis 1.20).
1.20. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 20: LA consumption per hour (nulliparous vs nulliparous + multiparous)
3. Maternal satisfaction
Maternal satisfaction scores were reported in 14 studies (Chalekar 2021; Fan 2019; Ferrer 2017; Fidkowski 2019; Haidl 2020; Leo 2010; Lim 2005; Lim 2010; Morau 2019; Ojo 2020; Sia 2007; Sia 2013; Song 2020; Wong 2006). Most studies assessed maternal satisfaction using a Likert scale or visual analogue scale (VAS). The results are described narratively, due to the inter‐study heterogeneity in the methods used for evaluating maternal satisfaction. Out of 14 studies, six reported increased maternal satisfaction with AMB compared to BI (Fan 2019; Leo 2010; Lim 2005; Lim 2010; Sia 2013; Wong 2006), while six studies found no difference in maternal satisfaction between AMB and BI (Chalekar 2021; Ferrer 2017; Haidl 2020; Morau 2019; Ojo 2020; Sia 2007). Using a Likert scale (0: unsatisfied; 1: satisfied; 2: very satisfied) Fidkowski 2019 reported that 95.4% of women receiving AMB had satisfaction scores of 1 or 2 compared to 94.1% of women who received BI. Song 2020 reported that the use of AMB was associated with median satisfaction scores of 97.5/100 (assessed using VAS, 0: not satisfied; 100: very satisfied), compared to BI (median satisfaction score = 92.5/100). In summary, a total of eight studies (five reported dichotomous data, and three reported ordinal data) reported increased maternal satisfaction with AMB than BI, while six reported no difference between the groups.
4. Apgar scores
Fourteen studies reported Apgar scores. One study reporting Apgar scores at 1, 5, and 10 minutes (Ferrer 2017), four studies reporting Apgar scores at 5 minutes (Leo 2010; Lim 2005; Lim 2010; Sia 2013), eight studies reporting Apgar scores at both 1 and 5 minutes (Chalekar 2021; Fan 2019; Fettes 2006; Lin 2016; Morau 2019; Ojo 2020; Salim 2005; Song 2020), and one study reporting Apgar scores greater than seven at 5 minutes (Sia 2007). In view of the inter‐study heterogeneity in reporting Apgar scores, the results were described narratively. None of the 14 studies reported any significant difference in Apgar scores associated with the use of AMB or BI.
Discussion
Summary of main results
Cumulative data from 18 studies showed that the use of automated mandatory bolus (AMB) for maintenance of labour analgesia was associated with significantly lower incidence of breakthrough pain compared to basal infusion (BI), with moderate certainty. There was no significant difference in the incidence of caesarean delivery or instrumental delivery between AMB and BI, with moderate certainty for both outcomes.
Additionally, we found that AMB was associated with significantly lower hourly local anaesthetic consumption in bupivacaine equivalents, with moderate certainty. The use of AMB or BI was not associated with significant difference in the duration of labour analgesia, with moderate certainty. Most of the included studies reported that AMB may be associated with increased maternal satisfaction, and was not associated with significant difference in Apgar scores compared to BI.
Please refer to the Table 1 for additional details.
Overall completeness and applicability of evidence
Overall, the included studies were of sufficient scope, utilised clinical methodology, and evaluated relevant outcome measures that addressed the objectives of this review.
The initiation of epidural analgesia in the included studies reflects contemporary practice; at present, combined spinal‐epidural (CSE) and epidural are the most commonly used techniques for initiation of labour analgesia. In our review, seven of the included studies utilised CSE, ten used epidural, and only one study (Song 2020) used dural puncture epidural (DPE), which is not as commonly employed compared to CSE or epidural. In addition, most of the studies utilised patient controlled epidural analgesia (PCEA), which is also commonly used in contemporary practice.
Most of the included studies used ropivacaine with fentanyl (eight studies) and bupivacaine with fentanyl (four studies), while few studies used ropivacaine with sufentanil (three studies), levobupivacaine with sufentanil (two studies), and levobupivacaine with fentanyl (one study). There was variation in the concentrations of local anaesthetic (LA) and opioids utilised in the included studies, but variations in the types and concentrations of epidural medications are reflective of contemporary practice.
The majority of included studies enrolled nulliparous women (13 studies), while five included both nulliparous and multiparous women. Of note, women with preterm or complicated pregnancies were excluded from all studies.
Hence, potential biases may arise from clinical heterogeneity between the studies included in this review, such as variation in the LAs or supplemental opioids used, as well as method of initiation of labour analgesia. Additionally, the labour stage at which neuraxial analgesia was initiated, the use of concurrent or prior forms of analgesia, and augmentation of labour with oxytocin may influence our outcome measures (Tan 2019; Tan 2021).
Quality of the evidence
Overall, the majority of included studies (13 studies) were assessed to be at low risk of bias relating to random sequence generation, with the exception of five studies that were considered to be at unclear risk as they did not specify the method of randomisation.
Similarly, seven studies were at low risk of bias relating to allocation concealment, while another seven studies stated that allocation concealment was performed using envelopes, but did not state if these envelopes were sealed or opaque and were hence considered to be at unclear risk. Of note, three studies were assessed to be at high risk of bias as they did not specify if allocation concealment was performed.
The majority of studies (13 studies) were at low risk of performance bias and detection bias, with the exception of four studies judged to be at unclear risk as they did not specify if the participants were blinded. One study (Fidkowski 2019) blinded the participants but not anaesthesia providers, and was considered to be at high risk of performance bias, and unclear risk of detection bias as the outcome assessor was not stated.
Only two studies had high risk of attrition bias (Fidkowski 2019; Ojo 2020). All of the studies had a low risk of reporting or other biases.
The GRADE certainty of evidence was assessed to be moderate for the incidence of breakthrough pain, caesarean delivery, instrumental delivery, duration of labour, and hourly local anaesthetic consumption, mainly due to potential imprecision or heterogeneity.
Potential biases in the review process
Statistical heterogeneity may be present despite our pre‐planned subgroup analyses. However, these subgroups were selected after careful consideration of clinically‐meaningful sub‐populations, instead of based on anticipated statistical heterogeneity.
Our highly‐sensitive search strategy was extended beyond CENTRAL, Embase, Web of Science, and Pubmed to include trial registries (clinicaltrials.gov and www.who.int/ictrp/en), and the reference lists of relevant studies in order to reduce the risk of publication bias and omission of unpublished studies. Of note, we identified five citations that were potentially relevant, but as full‐text copies were unavailable, these citations were therefore listed as 'awaiting classification'.
Finally, Alex Sia is an author of six of the studies that are included in this review (Chua 2004; Leo 2010; Lim 2005; Lim 2010; Sia 2007; Sia 2013). In this review, he was not involved in study selection, data entry, or data analysis, however, he coordinated the review, was an author in the previous version of this review (Sng 2018) that laid the foundation for the current study, and contributed to the writing and rechecking of the final manuscript prior to submission.
No significant bias in funding sources were noted in the included studies.
Agreements and disagreements with other studies or reviews
Our results showed good agreement with that of a recent review and meta‐analysis by Hussain 2020. Similar results were reported in the incidence of breakthrough pain (decreased with automated mandatory bolus, AMB), mode of delivery (no difference), and local anaesthetic consumption (decreased with AMB). However, Hussain 2020 reported that AMB was associated with shortened labour duration, while our overall pooled result showed no significant difference. Furthermore, the review by Hussain 2020 included five studies that were not included in our review due to the unavailability of full‐text copies (see Studies awaiting classification).
Authors' conclusions
Implications for practice.
This systematic review found moderate‐certainty evidence that the use of automated mandatory bolus (AMB) for maintenance of labour analgesia was associated with a decrease in the incidence of breakthrough pain requiring anaesthetic intervention as compared with basal infusion (BI). There is also moderate‐certainty evidence that AMB is associated with reduced hourly local anaesthetic consumption, compared to BI. However, both AMB and BI were associated with comparable incidence of caesarean or instrumental delivery, with moderate certainty. There was no significant difference in duration of labour analgesia between AMB and BI, with moderate certainty. Finally, AMB may be associated with increased maternal satisfaction, but without change in Apgar scores compared to BI.
Implications for research.
The certainty of evidence pertaining to the incidence of caesarean and instrumental delivery were mainly limited by imprecision due to the limited number of events resulting in wide confidence intervals. Hence, larger studies assessing these outcomes are required. Furthermore, the majority of studies did not examine the effects of AMB or BI on motor blockade, which may have implications on the incidence of caesarean or instrumental delivery. Additional well‐designed and adequately powered studies utilising standardised definitions for motor block such as the modified Bromage score are required to better delineate this outcome.
The labour stage at which neuraxial analgesia was initiated, the use of concurrent or prior forms of analgesia, and augmentation of labour with oxytocin may influence our outcome measures, but were not adequately controlled in available studies. Furthermore, patient‐centric outcomes, such as maternal satisfaction or cost‐effectiveness analysis, could also be considered given the recent advancements in pump technology.
What's new
| Date | Event | Description |
|---|---|---|
| 5 June 2023 | New citation required and conclusions have changed | Updated review with new citations |
| 5 June 2023 | New search has been performed | Updated search performed |
History
Protocol first published: Issue 10, 2014 Review first published: Issue 5, 2018
| Date | Event | Description |
|---|---|---|
| 17 October 2014 | Amended | Acknowledgement section updated |
Acknowledgements
Editorial contributions
Cochrane Acute and Emergency Care supported the review authors in the development of this systematic review. The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Zarko Alfirevic, University of Liverpool.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Marwah Anas El‐Wegoud, Cochrane Central Editorial Service.
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service.
Copy Editor (copy editing and production): Heather Maxwell.
Peer‐reviewers (provided comments and recommended an editorial decision)
Lisa Leffert, MD Yale University Medical School (clinical review), Dr Neel Desai, Guy's and St Thomas' NHS Foundation Trust (clinical review), Liz Bickerdike, Cochrane Evidence Production and Methods Directorate (methods review), Yuan Chi, Cochrane Campbell Global Ageing Partnership (search review). Two additional peer reviewers provided clinical peer review and consumer peer review (a professor of anesthesiology, and a member of the Cochrane Pregnancy and Childbirth Consumer Panel and the Cochrane Consumer Network respectively) but chose not to be publicly acknowledged.
Appendices
Appendix 1. Data collection form
Data collection form
| Review title or ID |
| Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report that data are extracted from) |
|
|
Report ID (ID for this paper/abstract/report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
2. Study eligibility
| Study characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomised controlled trial | |||||
| Controlled clinical trial (quasi‐randomised trial) |
||||||
| Participants | Healthy parturients requesting for epidural analgesia during labour | |||||
| Types of intervention | Automated mandatory bolus Basal infusion |
|||||
| Types of outcome measures | 1. Risk of breakthrough pain with need for anaesthetic intervention (dichotomous) 2. Risk of caesarean delivery (dichotomous) 3. Risk of instrumental delivery (dichotomous) 4. Duration of labour (continuous) 5. Total dose of local anaesthetic per hour (continuous) 6. Maternal satisfaction (continuous) 7. Apgar scores (continuous) |
|||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
|
Informed consent obtained |
Yes No Unclear |
||
| Notes: | |||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Aim of study | |||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, cluster/groups or body parts) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval needed/obtained for study | Yes No Unclear |
||
| Notes: | |||
5. Risk of bias assessment
See Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions
| Domain |
Risk of bias |
Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: All |
||||
| (if required) |
Outcome group: |
||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: All |
||||
| (if required) |
Outcome group: |
||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
| Other bias | |||||
| Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomized (or total pop. at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/ethnicity | ||
| Severity of illness | ||
| Co‐morbidities | ||
| Other treatment received(additional to study intervention) | ||
| Other relevant sociodemographics | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention Group 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomized to group (specify whether no. people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
| Co‐interventions | ||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
| Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcome
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, cluster/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
Continuous outcome
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, cluster/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||||||
| Reanalysis possible? | Yes No Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Other outcome
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | Yes No Unclear | |
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear | |
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear | |
| Notes: | ||
11. Other information
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
Appendix 2. Cochrane 'Risk of bias' tool and criteria for judgment
1. Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
3. Blinding
3.1 Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
3.2 Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we had about other possible sources of bias.
7. Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the CochraneHandbook for Systematic Reviews of Interventions (Higgins 2011). With reference to items 1 to 6 above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Appendix 3. Search strategies
CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Analgesia, Patient‐Controlled] explode all trees
#2 (bolus* or ((basal or continuous) near/2 infusion*) or PCEA or AMB or BCI or intermittent or (variable next frequency) or patientcontrolled or (patient next controlled)):ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Analgesia, Epidural] explode all trees
#5 MeSH descriptor: [Anesthesia, Epidural] explode all trees
#6 MeSH descriptor: [Infusions, Spinal] explode all trees
#7 MeSH descriptor: [Injections, Spinal] explode all trees
#8 MeSH descriptor: [Analgesia, Obstetrical] explode all trees
#9 MeSH descriptor: [Anesthesia, Obstetrical] explode all trees
#10 MeSH descriptor: [Anesthesia, Spinal] explode all trees
#11 MeSH descriptor: [Bupivacaine] explode all trees
#12 MeSH descriptor: [Ropivacaine] explode all trees
#13 MeSH descriptor: [Mepivacaine] explode all trees
#14 MeSH descriptor: [Fentanyl] explode all trees
#15 MeSH descriptor: [Sufentanil] explode all trees
#16 (epidural* or peridural* or extradural* or (spinal near/3 (infusion* or injection* or anaesth* or anesth*)) or ((anaesth* or anesth* or analg*) near/2 obstet*) or (pain near/3 relief) or bupivacain* or ropivacain* or mepivacain* or fentanyl or sufentanil):ti,ab,kw
#17 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 MeSH descriptor: [Labor, Obstetric] explode all trees
#19 MeSH descriptor: [Labor Pain] explode all trees
#20 MeSH descriptor: [Delivery, Obstetric] explode all trees
#21 MeSH descriptor: [Parturition] explode all trees
#22 (labor or labour or parturient* or parturition or childbirth* or (child next birth*) or obstet* or deliver*):ti,ab,kw
#23 #18 or #19 or #20 or #21 or #22
#24 #3 and #17 and #23
#25 #24 in Trials
OVID MEDLINE search strategy
1 Analgesia, Patient‐Controlled/
2 (bolus* or ((basal or continuous) adj1 infusion*) or PCEA or AMB or BCI or intermittent or variable frequency or patientcontrolled or patient controlled).mp.
3 1 or 2
4 Analgesia, Epidural/
5 exp Anesthesia, Epidural/
6 Infusions, Spinal/
7 exp Injections, Spinal/
8 analgesia, obstetrical/
9 anesthesia, obstetrical/
10 Anesthesia, Spinal/
11 exp Bupivacaine/
12 Ropivacaine/
13 Mepivacaine/
14 exp Fentanyl/
15 sufentanil/
16 (epidural* or peridural* or extradural* or (spinal adj3 (infusion* or injection* or an?esth*)) or ((an?esth* or analg*) adj2 obstet*) or (pain adj3 relief) or bupivacain* or ropivacain* or mepivacain* or fentanyl or sufentanil).mp.
17 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
18 exp Labor, Obstetric/
19 Labor Pain/
20 exp Delivery,Obstetric/
21 exp Parturition/
22 (labo?r or parturient* or parturition or childbirth* or child birth* or obstet* or deliver*).mp.
23 18 or 19 or 20 or 21 or 22
24 3 and 17 and 23
25 ((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (exp animals/ not humans.sh.)
26 24 and 25
OVID Embase search strategy
1 continuous infusion/
2 patient controlled analgesia/
3 automation/
4 bolus injection/
5 (bolus* or ((basal or continuous) adj1 infusion*) or PCEA or AMB or BCI or intermittent or variable frequency or patientcontrolled or patient controlled).mp.
6 1 or 2 or 3 or 4 or 5
7 exp epidural anesthesia/
8 epidural analgesia/
9 exp intraspinal drug administration/
10 obstetric anesthesia/
11 obstetric analgesia/
12 bupivacaine/
13 ropivacaine/
14 mepivacaine/
15 fentanyl/
16 sufentanil/
17 (epidural* or peridural* or extradural* or (spinal adj3 (infusion* or injection* or an?esth*)) or ((an?esth* or analg*) adj2 obstet*) or (pain adj3 relief) or bupivacain* or ropivacain* or mepivacain* or fentanyl or sufentanil).mp.
18 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 labor/
20 labor pain/
21 exp obstetric delivery/
22 birth/
23 (labo?r or parturient* or parturition or childbirth* or child birth* or obstet* or deliver*).mp.
24 19 or 20 or 21 or 22 or 23
25 6 and 18 and 24
26 (randomized controlled trial/ or controlled clinical study/ or random$.ti,ab. or randomization/ or intermethod comparison/ or placebo.ti,ab. or (compare or compared or comparison).ti. or ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. or (open adj label).ti,ab. or ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. or double blind procedure/ or parallel group$1.ti,ab. or (crossover or cross over).ti,ab. or ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. or (assigned or allocated).ti,ab. or (controlled adj7 (study or design or trial)).ti,ab. or (volunteer or volunteers).ti,ab. or human experiment/ or trial.ti.) not (((random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)) or (cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)) or (((case adj control$) and random$) not randomi?ed controlled).ti,ab. or (Systematic review not (trial or study)).ti. or (nonrandom$ not random$).ti,ab. or Random field$.ti,ab. or (random cluster adj3 sampl$).ti,ab. or ((review.ab. and review.pt.) not trial.ti.) or (we searched.ab. and (review.ti. or review.pt.)) or update review.ab. or (databases adj4 searched).ab. or ((rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/) or (Animal experiment/ not (human experiment/ or human/)))
27 25 and 26
Web of Science
#1 TS=(bolus* or ((basal or continuous) near/2 infusion*) or PCEA or AMB or BCI or intermittent or (variable next frequency) or patientcontrolled or (next controlled) )
Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#2 TS=(epidural* or peridural* or extradural* or (spinal near/3 (infusion* or injection* or anaesth* or anesth*) ) or ((anaesth* or anesth* or analg*) near/2 obstet*) or (pain near/3 relief) or bupivacain* or ropivacain* or mepivacain* or fentanyl or sufentanil)
Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#3 TS=(labor or labour or parturient* or parturition or childbirth* or (child next birth*) or obstet* or deliver*)
Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
#4 #4 AND #3 AND #2 AND #1
Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
WHO‐ICTRP
1 (basal infusion OR analgesia OR bolus) AND labor
ClinTrials.gov
1 labor [DISEASE] AND ( "basal infusion" OR bolus ) [TREATMENT] AND EXACT NOT "Male" [GENDER]
Data and analyses
Comparison 1. Automated mandatory bolus vs basal infusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Breakthrough pain | 16 | 1528 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.55, 0.91] |
| 1.2 Breakthrough pain (epidural vs CSE) | 15 | 1450 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.56, 0.94] |
| 1.2.1 Epidural | 8 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.52, 1.01] |
| 1.2.2 CSE | 7 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.44, 1.13] |
| 1.3 Breakthrough pain (PCEA vs no PCEA) | 16 | 1528 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.55, 0.91] |
| 1.3.1 PCEA | 9 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 1.3.2 No PCEA | 7 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.98] |
| 1.4 Breakthrough pain (nulliparous vs nulliparous + multiparous) | 16 | 1528 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.55, 0.91] |
| 1.4.1 Nulliparous | 11 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.49, 1.01] |
| 1.4.2 Nulliparous + multiparous | 5 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.49, 0.93] |
| 1.5 Caesarean delivery | 16 | 1735 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.06] |
| 1.6 Caesarean delivery (epidural vs CSE) | 15 | 1657 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.06] |
| 1.6.1 Epidural | 9 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.60, 1.00] |
| 1.6.2 CSE | 6 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.59] |
| 1.7 Caesarean delivery (PCEA vs no PCEA) | 16 | 1735 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.06] |
| 1.7.1 PCEA | 10 | 1320 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.18] |
| 1.7.2 No PCEA | 6 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.07] |
| 1.8 Caesarean delivery (nulliparous vs nulliparous + multiparous) | 16 | 1735 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.06] |
| 1.8.1 Nulliparous | 11 | 1134 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.17] |
| 1.8.2 Nulliparous + multiparous | 5 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.54, 1.11] |
| 1.9 Instrumental delivery | 17 | 4550 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
| 1.10 Instrumental delivery (epidural vs CSE) | 16 | 4472 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 1.01] |
| 1.10.1 Epidural | 10 | 4030 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.06] |
| 1.10.2 CSE | 6 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 1.11 Instrumental delivery (PCEA vs No PCEA) | 17 | 4550 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
| 1.11.1 No PCEA | 6 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.66, 1.67] |
| 1.11.2 PCEA | 11 | 4135 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 0.99] |
| 1.12 Instrumental delivery (nulliparous vs nulliparous + multiparous) | 17 | 4550 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
| 1.12.1 Nulliparous | 12 | 3949 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 1.00] |
| 1.12.2 Nulliparous + multiparous | 5 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.58] |
| 1.13 Duration of labour analgesia in minutes | 17 | 4544 | Mean Difference (IV, Random, 95% CI) | ‐8.81 [‐19.38, 1.77] |
| 1.14 Duration of labour analgesia in minutes (epidural vs CSE) | 16 | 4473 | Mean Difference (IV, Random, 95% CI) | ‐7.68 [‐18.08, 2.71] |
| 1.14.1 Epidural | 10 | 4031 | Mean Difference (IV, Random, 95% CI) | ‐4.31 [‐15.87, 7.24] |
| 1.14.2 CSE | 6 | 442 | Mean Difference (IV, Random, 95% CI) | ‐31.62 [‐60.92, ‐2.32] |
| 1.15 Duration of labour analgesia in minutes (PCEA vs no PCEA) | 17 | 4544 | Mean Difference (IV, Random, 95% CI) | ‐8.81 [‐19.38, 1.77] |
| 1.15.1 PCEA | 11 | 4129 | Mean Difference (IV, Random, 95% CI) | ‐8.51 [‐18.92, 1.91] |
| 1.15.2 No PCEA | 6 | 415 | Mean Difference (IV, Random, 95% CI) | ‐26.52 [‐69.42, 16.39] |
| 1.16 Duration of labour analgesia in minutes (nulliparous vs nulliparous + multiparous) | 17 | 4544 | Mean Difference (IV, Random, 95% CI) | ‐8.81 [‐19.38, 1.77] |
| 1.16.1 Nulliparous | 12 | 3943 | Mean Difference (IV, Random, 95% CI) | ‐10.72 [‐19.97, ‐1.48] |
| 1.16.2 Nulliparous + multiparous | 5 | 601 | Mean Difference (IV, Random, 95% CI) | 14.65 [‐38.13, 67.43] |
| 1.17 LA consumption per hour | 16 | 1642 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.29, ‐0.38] |
| 1.18 LA consumption per hour (epidural vs CSE) | 15 | 1564 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.33, ‐0.36] |
| 1.18.1 Epidural | 8 | 1080 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.75, ‐0.69] |
| 1.18.2 CSE | 7 | 484 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.82, 0.10] |
| 1.19 LA consumption per hour (PCEA vs no PCEA) | 16 | 1642 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.29, ‐0.38] |
| 1.19.1 PCEA | 10 | 1262 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.19, ‐0.71] |
| 1.19.2 No PCEA | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.85, 0.06] |
| 1.20 LA consumption per hour (nulliparous vs nulliparous + multiparous) | 16 | 1642 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.29, ‐0.38] |
| 1.20.1 Nulliparous | 12 | 1118 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.14, ‐0.19] |
| 1.20.2 Nulliparous + multiparous | 4 | 524 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐3.67, ‐0.11] |
1.5. Analysis.

Comparison 1: Automated mandatory bolus vs basal infusion, Outcome 5: Caesarean delivery
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Capogna 2011.
| Study characteristics | ||
| Methods | Prospective randomised double‐blind controlled study | |
| Participants | Setting: recruited from Citta di Roma Hospital, Roma, Italy Sample size: N = 150 (N completers = 145) Participants: age 27 ± 5 years (BI) and 29 ± 5 years (AMB) Inclusion criteria: healthy, nulliparous, term women with singleton, vertex pregnancies in spontaneous labour if cervical dilation was < 4 cm and if her baseline pain score, assessed at the peak of the contraction, was > 50 mm on a 100 mm visual analogue pain scale (VAPS) 5 women in the continuous epidural infusion group excluded: 4 reported VAPS > 10 mm 30 min after the epidural injection and one unintentional epidural catheter dislodgement during labour |
|
| Interventions | AMB (n = 75): 0.0625% levobupivacaine with sufentanil 0.5 µg/mL, 10 mL every hour, beginning 60 min after the administration of the initial epidural loading dose. PCEA pump was programmed to deliver 5 mL patient‐activated boluses of levobupivacaine 0.125% with a lockout interval of 10 min and a per hour maximum volume of 15 mL BI (n = 70): 0.0625% levobupivacaine with sufentanil 0.5 µg/mL, 10 mL/h, beginning immediately after the administration of the initial epidural loading dose. PCEA pump was programmed to deliver 5 mL patient‐activated boluses of levobupivacaine 0.125% with a lockout interval of 10 min, and a per hour maximum volume of 15 mL |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Total dose of LA (levobupivacaine) |
|
| Notes | Study dates: April 2009 to July 2010 Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "During epidural catheter placement, a sequentially numbered, opaque envelope containing the group assignment (computer‐generated random‐number sequence) was opened by an unblinded researcher who set up the 2 epidural pumps according to group allocation." |
| Allocation concealment (selection bias) | Low risk | Quote:"During epidural catheter placement, a sequentially numbered, opaque envelope containing the group assignment (computer‐generated random‐number sequence) was opened by an unblinded researcher who set up the 2 epidural pumps according to group allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"The subjects and other study personnel were blinded to group assignment and all the observations and assessments were performed by a researcher blinded to the mode of drug administration." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"The subjects and other study personnel were blinded to group assignment and all the observations and assessments were performed by a researcher blinded to the mode of drug administration." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusions reported; 5 out of 150 participants dropped out; however, this dropout rate is not significant (3%). |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size of 70 participants in each group had a power of at least 80% for a 2‐sided Chi2 test of association between maintenance technique and incidence of motor block, with a significance level set to 0.05. |
Chalekar 2021.
| Study characteristics | ||
| Methods | Prospective randomised double‐blind controlled study | |
| Participants | Setting: Obstetrics and Gynaecology Department at Fortis Hospital, Bannerghatta road, Bengaluru, Karnataka, India from June 2014 to June 2015 Sample size: N = 60 Inclusion criteria: American Society of Anesthesiologists (ASA) II parturients, aged 18‐35 years, admitted with term gestation for safe confinement in active labour were included. Also primiparturients with singleton pregnancy, term gestation, cephalic presentation in active first stage of labour willing for epidural analgesia, cervical dilatation >3 cm and <5 cm, aged 18‐35 years, height >145 cm, and Body Mass Index (BMI) 18‐25 kg/m2. Exclusion criteria: Parturients who were unwilling, had medical disorders and pregnancy associated disorders, spine abnormalities and local skin infections, coagulopathies, preterm gestation, non reassuring non stress test, pregnant women with preterm labour or false labour pains, parturients in whom epidural analgesia was inadequate even after 45 minutes of initial bolus, parturients who experience unilateral block, parturients with blood tap during epidural and those with accidental dural puncture. |
|
| Interventions | Group I (PIEB) (n=30) parturients received 8 mL of 0.15% ropivacaine with fentanyl 2 µg/mL hourly. Group C (CEI) (n=30) parturients received same solution as continuous infusion immediately. |
|
| Outcomes | Primary outcome: LA consumption Secondary outcomes: 1. Level of sensory block 2. Motor block 3. Breakthrough pain 4. Duration of epidural analgesia 5. Maternal satisfaction 6. Mode of delivery 7. Second stage of labour 8. APGAR scores 9. Contraction stress test 10. Pain scores |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment using sealed envelopes, but method of randomisation was not stated: Quote:"The parturients were then randomly assigned, using sealed envelope method..." |
| Allocation concealment (selection bias) | Unclear risk | Allocation via sealed envelopes, but it was not stated that the envelopes were opaque: Quote:"The parturients were then randomly assigned, using sealed envelope method..." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not stated if participants were blinded. Quote:"Observations were made by an assessor ‘blind’ to the mode of drug administration. The attending anaesthesiologist was informed whenever pain recurred (VAS ≥4) and additional top‐ups of the study drug were given" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated if participants were blinded. Quote:"Observations were made by an assessor ‘blind’ to the mode of drug administration. The attending anaesthesiologist was informed whenever pain recurred (VAS ≥4) and additional top‐ups of the study drug were given" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusions |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported |
| Other bias | Low risk | Sample size calculation provided |
Chua 2004.
| Study characteristics | ||
| Methods | Parallel, randomiSed controlled trial | |
| Participants | Setting: recruited from Singapore General Hospital, Singapore Sample size: N = 42 Participants: age not provided Inclusion criteria: ASA physical status I nulliparous women in early spontaneous labour pain with at least one contraction every 5 min who had requested neuraxial block |
|
| Interventions | AMB (n = 21): ropivacaine 0.1% plus fentanyl 2 µg/mL for maintaining epidural analgesia The initial 5 mL bolus was administered 30 min after time 0, followed by 5 mL boluses every hour thereafter. As the highest rate of delivery afforded by the pump was 100 mL/h, each epidural bolus was delivered over 3 min. BI (n = 21): ropivacaine 0.1% plus fentanyl 2 µg/mL for maintaining epidural analgesia. A rate of 5 mL/h was initiated 1 min after time 0 by using a Terumo syringe pump |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention LA consumption per hour |
|
| Notes | Study dates not stated Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated: Quote: "The parturients were then randomly assigned by the blind opaque envelope technique to receive either epidural CIB (n = 21) or CEI (n = 21)" |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed opaque envelopes: Quote:"The parturients were then randomly assigned by the blind opaque envelope technique to receive either epidural CIB (n = 21) or CEI (n = 21)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Epidurals were performed by the principal investigator: Quote:"All blocks performed by the principal investigator (S.M.H.C.)" Blinding of epidural pump settings to participants were not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated if participants were blinded. Quote:"All data was collected by an anaesthesiologist who was not involved in instituting the block" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Sample size was computed to detect a 30‐min difference (α = 0.05, ß = 0.2) in the duration of analgesia |
Fan 2019.
| Study characteristics | ||
| Methods | Prospective randomiSed double‐blind controlled study | |
| Participants | Setting: Affiliated Obstetrics and Gynecology Hospital of Nanjing Medical University, China from October 2012 to December 2017 Sample size: N=3000 Inclusion criteria: singleton, spontaneous labor, participants who requesting epidural labor analgesia, age from 20 to 45 years, gestation week from 37 to 41, nulliparous, cervical dilation from 1 to 3 cm. Exclusion criteria: contraindications for epidural analgesia, a baseline temperature of ≥ 37.5 °C, allergic to opioids and/or local anaesthetics, failed to perform epidural catheterization, organic dysfunction, those who were not willing to or could not finish the whole study at any time, unable to perform analgesia evaluation, using or used in the past 14 days of the monoamine oxidase inhibitors, alcohol addicts or narcotic dependent patients, subjects with a non vertex presentation or scheduled induction of labor, multiple pregnancy, ASA physical status of 3 or higher, height less than 150 cm or more than 170 cm, morbid obesity (BMI more than 35), high‐risk pregnancy (gestational diabetes mellitus, gestational hypertension, placenta previa, placental abruption, preeclampsia). |
|
| Interventions | Group PIEB (n=1454) parturients received 10 mL of 0.08% ropivacaine with sufentanil 0.4 µg/mL hourly. Group CEI (n=1411) parturients received 10 mL of 0.08% ropivacaine with sufentanil 0.4 µg/mL hourly. |
|
| Outcomes | Primary outcome: incidence of maternal fever (>38C) Secondary outcomes 1. Sensory levels to cold 2. Pain scores 3. Motor block 4. Maternal satisfaction 5. Duration of epidural analgesia 6. Mode of delivery 7. APGAR scores 8. Number of epidural boluses 9. Medication consumption |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Random numbers were generated by computer, and then they were sequentially sealed in the envelopes for grouping." |
| Allocation concealment (selection bias) | Unclear risk | Allocation via sealed envelopes, but it was not stated that the envelopes were opaque: Quote:"...sequentially sealed in the envelopes for grouping..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded: Quote:"study investigators who observed, assessed, and collected the clinical data, obstetricians, midwifes, and the participants were blinded to group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote: "study investigators who observed, assessed, and collected the clinical data, obstetricians, midwifes, and the participants were blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate of 4.5%: CEI: 89/1500 and PIEB: 46/1500 lost to follow‐up (4.5%) |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported |
| Other bias | Low risk | Sample size calculation provided |
Ferrer 2017.
| Study characteristics | ||
| Methods | Prospective, randomised, controlled, single‐blind, parallel study | |
| Participants | Setting: recruited from Hospital Universitario Fundación Santa Fe de Bogotá (Colombia) Sample size: N = 132 (N completers = 128) Participants: age 32.3 ± 3.8 years (BI) and 31.6 ± 5.1 years (AMB) Inclusion criteria: labouring term women aged between 18 and 45 years requiring epidural analgesia Two women in each group were excluded from analysis as they delivered within 60 min of epidural initiation |
|
| Interventions | AMB (n = 64): initial loading dose of 10 mL of 0.1% bupivacaine (2 mL of 0.5% bupivacaine plus 50 μg/mL of fentanyl in 7 mL of 0.9% normal saline), then a 10 mL bolus of a mixture of 0.1% bupivacaine plus 2 μg/mL of fentanyl in 0.9% normal saline every hour starting 1 hour after the initial loading dose BI (n = 64): initial loading dose of 10 mL of 0.1% bupivacaine (2 mL of 0.5% bupivacaine plus 50 μg/mL of fentanyl in 7 mL of 0.9% normal saline) then a 10 mL/h infusion of a mixture of 0.1% bupivacaine plus 2 μg/mL of fentanyl in 0.9% normal saline every hour starting immediately after the loading dose |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour analgesia Total LA dose Maternal satisfaction Apgar scores |
|
| Notes | Study dates not stated No funding sources No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Next, patients were randomized according to a computer generated sequence to analgesia with either PIEB or CEI with a 1:1 allocation ratio." |
| Allocation concealment (selection bias) | Unclear risk | Participants, caregivers and outcome assessors were not aware of the next treatment allocation: Quote:"neither the patient nor the attending anesthesiologist nor the outcome assessor knew the randomization sequence." However, the method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and personnel were blinded: Quote:"Neither the patient nor the attending anesthesiologist nor the outcome assessor knew the randomization sequence." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded: Quote:"Neither the patient nor the attending anesthesiologist nor the outcome assessor knew the randomization sequence." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (4 out of 132, 3%) |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size of 132 participants (66 per group) with 10% attrition would give the study a power of > 0.8 to detect a 10% reduction in the difference of means of breakthrough pain between the 2 groups |
Fettes 2006.
| Study characteristics | ||
| Methods | Parallel, randomised controlled trial | |
| Participants | Setting: recruited from Ninewells Hospital and Medical School, Dundee, UK Sample size: N = 47 (N completers = 40) Participants: age 25.8 ± 6.3 years (AMB) and 27.1 ± 4.5 years (BI) Inclusion criteria: ASA I–II primigravid participants with uncomplicated, full‐term (> 37 weeks) pregnancy 7 women were excluded after epidural catheter placement: 3 because of inadequate analgesia at 45 min; and 1 each because of patchy block, epidural filter disconnection, catheter occlusion and study protocol violation |
|
| Interventions | AMB (n = 20): ropivacaine 2 mg/mL with fentanyl 2 mg/mL. Hourly boluses, delivered at 2 mL/min, were started 30 min after time zero BI (n = 20): ropivacaine 2 mg/mL with fentanyl 2 mg/mL. Infusion was started immediately at a constant rate of 10 mL/h |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Total LA dose Apgar scores |
|
| Notes | Study dates not stated Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated numbers: Quote:"Patients were then randomized (computer generated numbers inserted into opaque envelopes) to receive either a continuous infusion (control group) or intermittent administration (study group)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment by opaque envelopes, but it was not stated if envelopes were sealed: Quote:"Patients were then randomized (computer generated numbers inserted into opaque envelopes) to receive either a continuous infusion (control group) or intermittent administration (study group)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants was not stated. Clinical staff were blinded: Quote:"participants were nursed in the sitting position by staff that were unaware of the treatment used." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of participants was not stated. The outcome assessor was blinded: Quote:"Observations were made by an assessor 'blind' (the pump was covered) to the mode of drug administration" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition; all randomised patients completed the study. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size of 40 participants (20 per group) would give the study a power of > 0.9 to detect a statistically significant difference in visual analogue pain scores |
Fidkowski 2019.
| Study characteristics | ||
| Methods | Prospective randomised single‐blind controlled study | |
| Participants | Setting: recruited from Henry Ford Hospital, Detrot, MI, USA Sample size: N = 150 Participants: age 27.2 ± 5.5 years (BI) and 24.9 ± 4.5 years (AMB) Inclusion criteria: English speaking women at term gestation with uncomplicated pregnancies 5 women in the continuous epidural infusion group excluded: 4 reported VAPS > 10 mm 30 min after the epidural injection and one unintentional epidural catheter dislodgement during labour |
|
| Interventions | AMB (n = 43): 0.125% bupivacaine + fentanyl 2 µg/mL at 10 mL every 60 minutes following a 5 mL loading dose. First bolus administered immediately upon connection of the epidural pump. Breakthrough pain managed with a physician‐administered epidural bolus. BI (n = 34): 0.125% bupivacaine + fentanyl 2 µg/mL at 10 mL/h infusion following a 5 mL loading dose. Breakthrough pain managed with a physician‐administered epidural bolus. |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Maternal satisfaction |
|
| Notes | Excluded 3rd arm of study that received 5 mL boluses every 30 minutes. Study dates: May 2015 to July 2017 Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by shuffling of opaque envelopes: quote: "Prior to enrolling the first patient, group assignments with instructions for epidural pump settings were placed in 150 opaque envelopes, 50 for each group. These envelopes were mixed and randomly placed in a container. At the time of randomization, the anesthesia provider randomly selected one of these opaque concealed envelopes to determine group randomization." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment via opaque envelopes, but it was not stated if the envelopes were sealed: Quote:"group assignments with instructions for epidural pump settings were placed in 150 opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"The patients, obstetrical staff, and nursing staff remained blinded to the epidural pump settings and group assignment throughout the study. The anesthesia providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although patients, obstetrical staff, and nursing staff were blinded, the anaesthesia providers were not blinded and the article did not state who collected outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition: AMB: 9 of 50 participants randomised excluded from analysis, 2 participants from BI group received AMB and were analysed under AMB BI: 13 of 50 participants randomised excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other bias. Sample size calculation: 50 participants in each group was needed to detect an effect size of 0.056 change in pain score with an alpha less than 0.05 and a power greater than 80% |
Haidl 2020.
| Study characteristics | ||
| Methods | Prospective randomised controlled study | |
| Participants | Setting: recruited from Akershus University Hospital, Norway Sample size: N = 151 Participants: age 29.8 ± 4.3 years (BI) and 30.4 ± 4.1 years (AMB) Inclusion criteria: Adult women, ASA<3, singleton term pregnancy (gestation >37 weeks), maximum of one previous delivery. Exclusion/ criteria: Poor communication skills in Norwegian or English, height <150cm, pre‐eclampsia, contraindication to epidural. 1 women in BI group excluded after randomisation ‐ withdrew consent. |
|
| Interventions | AMB (n = 75): 0.1% bupivacaine + fentanyl 2 µg/mL at 5 mL every 60 minutes following a 10 mL loading dose. First bolus administered 15 minutes after loading dose. PCEA (5 mL, lockout 20 minutes) allowed, breakthrough pain despite PCEA managed with a physician‐administered epidural bolus. BI (n = 75): 0.1% bupivacaine + fentanyl 2 µg/mL at 5 mL/h infusion following a 10 mL loading dose. Infusion started 15 minutes after loading dose. PCEA (5 mL, lockout 20 minutes) allowed, breakthrough pain despite PCEA managed with a physician‐administered epidural bolus. |
|
| Outcomes | LA consumption Number of participants needing physician intervention Duration of treatment Mode of delivery Overall satisfaction Motor block |
|
| Notes | Study dates: March 2017 to September 2018 Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer generated algorithm: Quote:"The group assignment was determined by a computer generated algorithm" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment via sealed envelopes, but it was not stated if envelopes were opaque: Quote:"...and kept in individual sealed envelopes until patients were included." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded, but the anaesthetist was not blinded: Quote:"All participants and study personnel assessing patients were blinded to the intervention. The anesthetist including the patient, and starting the treatment, was not blinded to the intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote:"All participants and study personnel assessing patients were blinded to the intervention." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition: only 1 patient was excluded after randomisation |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Leo 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Setting: recruited from KK Women's and Children's Hospital, Singapore Sample size: N = 62 Participants: age not provided Inclusion criteria: healthy ASA I nulliparous parturients with term (> 36 weeks of gestation), singleton fetuses in the vertex presentation, who were in early labour (cervical dilation < 5 cm) and requested labour epidural analgesia |
|
| Interventions | AMB (n = 31): 0.1% ropivacaine + fentanyl 2 µg/mL. PCEA algorithm initiated immediately after completion of CSE. Participants in this group received automated mandatory boluses (AMB) of 5 mL every hour instead of a basal infusion. The first AMB dose was delivered 30 min from CSE and epidural catheter placement and every hour subsequently if no PCEA demands were made. If the participant had made a successful PCEA self‐bolus, the next AMB bolus would be delivered 30 min after the last successful PCEA self‐bolus and every hour thereafter. The lockout period for both PCEA and AMB boluses was 10 min. If a PCEA demand was made within 10 min of an AMB dose, no further bolus would be given. This would be recorded as an unsuccessful PCEA attempt. PCEA bolus was set at 5 mL and maximal hourly limit at 20 mL/h (inclusive of basal infusion and automated boluses) BI (n = 31): 0.1% ropivacaine + fentanyl 2 μg/mL. PCEA with basal infusion 5 mL/h initiated immediately after intrathecal drug administration and epidural catheter placement. PCEA bolus was set at 5 mL, lockout interval at 10 min and maximal dose at 20 mL/h |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Total LA/hour (time weighted hourly consumption of ropivacaine) Maternal satisfaction Apgar scores |
|
| Notes | Study dates not stated Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer generated random numbers: Quote:"Parturients were randomly allocated into two groups using sealed opaque envelopes and computer‐generated random number tables by an independent assistant, who then programmed the epidural drug delivery system according to group assignment." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by sealed opaque envelopes: Quote:"Parturients were randomly allocated into two groups using sealed opaque envelopes..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pumps were programmed by a blinded assistant. The patients were blinded. Quote:"...independent assistant, who then programmed the epidural drug delivery system according to group assignment." Quote:"Neither the parturients nor the anesthesiologists who monitored and collected post‐block data were aware of group assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote:"Neither the parturients nor the anesthesiologists who monitored and collected post‐block data were aware of group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size of 30 participants in each group was calculated to detect a 30% reduction in the incidence of breakthrough pain requiring physician top‐up for participants in the PCEA + AMB arm compared with those in the PCEA + BI arm (α = 0.05, ß = 0.2) |
Lim 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Setting: recruited from KK Women's and Children's Hospital, Singapore Participants: age 30 ± 6 years (AMB) and 31 ± 5 years (BI) Sample size: N = 60 Inclusion criteria: ASA I nulliparous labouring parturients at term who requested neuraxial analgesia in established labour with cervical dilatation less than or equal to 5 cm and with baseline pain scores more than or equal to 50 (on a 0–100 visual analogue scale (VAS): 0 = no pain, 100 = worst pain imaginable) |
|
| Interventions | AMB (n = 30): 5 mL epidural boluses of levobupivacaine 0.1% with fentanyl 2 µg/mL every 30 min. This was initiated 15 min after the intrathecal component was given BI (n = 30): levobupivacaine 0.1% with fentanyl 2 µg/mL at a rate of 10 mL/h as a continuous infusion delivered by a syringe pump. The epidural infusion was initiated in the next minute after the intrathecal component was given |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Total LA/hour Maternal satisfaction Apgar scores |
|
| Notes | Study dates not stated Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a computer‐generated table: Quote:"The parturients were randomized using a computer generated table into two groups." |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was:Quote:"double‐blinded", but did not specifically state if participants and clinical personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of participants were not stated. Outcome assessors were blinded: Quote:"An anesthetist, who was not involved in performing the block and blinded to the mode of drug delivery, collected the following data..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size was computed to detect a 40% reduction of incidence of breakthrough pain |
Lim 2010.
| Study characteristics | ||
| Methods | Randomised double‐blinded controlled clinical trial | |
| Participants | Setting: recruited from KK Women's and Children's Hospital, Singapore Sample size: N = 51 (N completers = 50) Participants: age not provided Inclusion criteria: healthy nulliparous parturients with cephalic presentation at > 36 weeks gestation in early, spontaneous labour (cervical dilation < 5 cm) 1 woman from CEI group excluded as epidural catheter was blocked and re‐sited 2 hours after initiation of CSE |
|
| Interventions | AMB (n = 25): 2.5 mL epidural boluses of 0.1% ropivacaine with fentanyl 2 µg/mL, infused over a 2‐minute period, every 15 min. The first bolus was given 7.5 min after the intrathecal injection BI (n = 25): 0.1% ropivacaine with fentanyl 2 µg/mL at 10 mL/hour, delivered by syringe pump and initiated immediately after the intrathecal injection |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Time‐weighted consumption of LA Maternal satisfaction Apgar scores |
|
| Notes | Study dates: 18 February to 19 March 2007 Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not stated: Quote:"This was a randomised, double‐blind controlled clinical trial. We allocated the participants to the AIB and CEI groups using a sealed opaque envelope, which was opened after recruitment by the anaesthetist who was to perform the epidural." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment via sealed opaque envelopes: Quote:"We allocated the participants to the AIB and CEI groups using a sealed opaque envelope..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinical team were blinded: Quote:"The parturient was blinded to the group allocation. All baseline data were gathered by the attending nurse/midwife, who was also blinded to participant study group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote:"The parturient was blinded to the group allocation. All baseline data were gathered by the attending nurse/midwife, who was also blinded to participant study group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition; 1 participant out of 51 did not complete the study |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size was computed to detect a 30% difference in the incidence of breakthrough pain |
Lin 2016.
| Study characteristics | ||
| Methods | Randomised double‐blinded controlled clinical trial | |
| Participants | Setting: recruited from First Affiliated Hospital of Guangxi Medical University, Nanning, People's Republic of China Sample size: N = 200 (N completers = 197) Participants: age 27.45 ± 4.61 (AMB) and 28.16 ± 4.679 (CI) Inclusion criteria: healthy nulliparous women in early spontaneous labor (> 37 weeks' gestation) having at least one uterine contraction every 5 min and who had requested neuraxial block 3 women were excluded because of unplanned epidural catheter removal |
|
| Interventions | AMB (n = 98): test dose of 4 mL of 1% lignocaine then 10 mL of 0.15% ropivacaine loading dose, then maintenance with 0.1% ropivacaine with sufentanil 0.3 µg/mL at 5 mL bolus per hour plus PCEA of 5 mL with 20 min lockout period, maximum 15 mL/h BI (n = 99): test dose of 4 mL of 1% lignocaine then 10 mL of 0.15% ropivacaine loading dose, then maintenance with 0.1% ropivacaine with sufentanil 0.3 µg/mL at 5 mL/h infusion plus PCEA of 5 mL with 20 min lockout period, maximum 15 mL/h |
|
| Outcomes | Rate of caesarean delivery Rate of instrumental delivery Duration of labour LA used Apgar scores at 1 and 5 min |
|
| Notes | Study dates: not provided Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated: Quote:"...parturients were enrolled in our study and randomly allocated using a sealed envelope technique to receive..." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment with sealed envelopes, but it was not stated if envelopes were opaque: Quote:"...parturients were enrolled in our study and randomly allocated using a sealed envelope technique to receive..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant and monitoring team were blinded: Quote:"The subjects and other study personnel were blinded to the group assignment and all the observations and assessments were performed by a researcher blinded to the mode of drug administration." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: "...Quote:all the observations and assessments were performed by a researcher blinded to the mode of drug administration." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition: 3 out of 200 participants were excluded from the analysis; however, this dropout rate is minimal (1.5%). |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation not explained |
Morau 2019.
| Study characteristics | ||
| Methods | Prospective, controlled, randomised, triple‐blind | |
| Participants | Inclusion criteria: nulliparous term women, aged 18 to 44 years, with a healthy singleton pregnancy and a foetal vertex position, in spontaneous labour with cervical dilation of 4 cm or less and a pain score more than 4 on a 0 to 10 verbal numeric pain scale | |
| Interventions | AMB (n = 124): 0.1% levobupivacaine with sufentanil 0.36 µg/mL 8 mL bolus every hour beginning 60 minutes after loading dose of 15 mlLof 0.1% levobupivacaine and 10 µg of sufentanil. PCEA with patient requested boluses of 8 mL, with a 10 min refractory period and a maximum hourly dose of 24 mL. BI (n = 125): 0.1% levobupivacaine with sufentanil 0.36 µg/mL at 8 mL/h infusion after loading dose of 15 mL of 0.1% levobupivacaine and 10 µg of sufentanil. PCEA with patient requested boluses of 8 mL, with a 10 min refractory period and a maximum hourly dose of 24 mL. |
|
| Outcomes | Number of anaesthesiologist intervention due to breakthrough pain Rate of caesarean delivery (then excluded from analysis) Rate of instrumental delivery Duration of labour Time‐weighted consumption of LA Maternal satisfaction Apgar scores |
|
| Notes | Study dates: January 2014 to June 2016 Funding sources: Research grant from the French Society of Anesthesiology and Intensive Care (SFAR), 75016 Paris. This study was funded by Smiths Medical France, 94656 Rungis Cedex. The study sponsors had no role in study design or in the writing of the report. Conflict of interests declared: Lead author reports receiving payment for the development of educational support on post partum haemorrhage from LFB (Laboratoire Francais des Biotechnologies) in 2017 and reimbursement of meeting fees and travel expenses from Smith Medical France, LFB and Grunenthal in 2015 and 2016. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated: Quote: "Patients were randomised by blocks of 4 and 6, and randomisation was stratified by centre." |
| Allocation concealment (selection bias) | High risk | No allocation concealment was performed: Quote: "The anaesthesia nurse allocated the patient to a group according to the randomisation list..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinical team were blinded: Quote: "The anaesthesiologist, the obstetrician and the patient were all blind to the group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The anaesthesiologist, the obstetrician and the patient were blinded to the group assignment: quote: "The anaesthesiologist, the obstetrician and the patient were all blind to the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition: 25 of 149 (16.7%) participants in AMB group excluded from analysis and 24 of 149 (16.1%) participants in BI group excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size of 300 would allow the detection of an absolute difference of 15% in the rate of the primary outcome, with a power of 80% and an alpha risk of 5%. |
Ojo 2020.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind controlled trial | |
| Participants | Inclusion criteria: American Society of Anesthesiologists physical status II and III women >18 years of age, at gestational age >36 weeks, with singleton pregnancies, vertex presentation, in labour, desiring epidural labour analgesia at cervical dilation between 2 cm and 7 cm and reporting a verbal pain score >5 | |
| Interventions | AMB (n = 61): Initial loading dose of 20 mL 0.1% ropivacaine with 2 µg/mL fentanyl then 6 mL programmed intermittent epidural boluses every 45 minutes with the first bolus administered 30 minutes after epidural initiation BI (n = 59): Initial loading dose of 20 mL 0.1% ropivacaine with 2 µg/mL fentanyl then 8 mL/h infusion beginning immediately after the loading dose |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Time‐weighted consumption of LA Maternal satisfaction Apgar scores |
|
| Notes | Study dates: November 2016 to November 2017 Funding sources not declared Conflicts of interest declared: A. S. Habib is a senior editor for Anesthesia and Analgesia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated assignment: Quote: "After obtaining informed consent, subjects were allocated to a study arm (continuous epidural infusion or programmed intermittent epidural boluses) by computer‐generated random assignment placed in sequentially numbered sealed opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by sequentially numbered sealed opaque envelopes: Quote: "...placed in sequentially numbered sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patient, anaesthesia provider and outcome assessor were blinded: Quote: "The patient, anesthesia provider, and outcome assessor were blinded to the randomization assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote: "The patient, anesthesia provider, and outcome assessor were blinded to the randomization assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 30% of study population excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size of 44 women per group had 91% power to identify a difference in LA consumption based on pilot study |
Sia 2007.
| Study characteristics | ||
| Methods | Parallel, randomised controlled trial | |
| Participants | Setting: recruited from KK Women's and Children's Hospital, Singapore Sample size: n = 42 Participants: age not provided Inclusion criteria: healthy (ASA I), nulliparous parturients with cephalic presentation at ≥ 36 weeks of gestation who were in early spontaneous labour (cervical dilation ≤ 5 cm) and who had requested neuraxial blocks for analgesia and had a VAPS of > 3 cm |
|
| Interventions | AMB (n = 21): 0.1% ropivacaine + fentanyl 2 µg/mL. PCEA + automated mandatory boluses (based on an empirical algorithm, maximal dose per hour = 20 mL), initiated the minute after time 0. In this group, apart from PCEA boluses of 5 mL per demand, the parturients received mandatory boluses of 5 mL/h with the first AMB dose delivered 30 min after the initiation of the pump and every hour after that if no PCEA demands were made. The lockout period for both PCEA and AMB boluses was 10 min. If a PCEA demand was made within 10 min of an AMB dose, no further bolus would be given. This would be recorded as an unsuccessful PCEA attempt. Provided that no further PCEA demands were made, the next AMB bolus would then be delivered 1 h after the last AMB. If there had been a successful PCEA bolus, the next AMB bolus would be delivered one hour after the last successful PCEA bolus BI (n = 21): 0.1% ropivacaine + fentanyl 2 µg/mL. PCEA + basal continuous infusion (BCI 5 mL/h, PCEA bolus of 5 mL, lockout interval = 10 min, maximal dose per hour = 20 mL), initiated the minute after time 0 |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Time weighted ropivacaine consumed per hour Maternal satisfaction Apgar scores |
|
| Notes | Study dates not stated Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer generated number: Quote: "The parturients were randomized using a sealed opaque envelope containing a computer generated number..." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by sealed opaque envelopes: Quote: "The parturients were randomized using a sealed opaque envelope containing a computer generated number..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinical staff were blinded: Quote: "Neither the parturients nor the investigators who monitored and collected data were aware of the patient group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote: "Neither the parturients nor the investigators who monitored and collected data were aware of the patient group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition: no dropouts |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size determined (α = 0.05, ß = 0.2) to detect a 20% reduction in the time weighted epidural ropivacaine consumption for PCEA‐AMB compared with PCEA‐BCI |
Sia 2013.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial | |
| Participants | Setting: recruited from KK Women's and Children's Hospital, Singapore Sample size: N = 102 Participants: age not provided Inclusion criteria: healthy (ASA 1) nulliparous parturients at term (> 36 weeks gestation) with a singleton fetus, who were in early labour (cervical dilation < 5 cm) and who had requested labour epidural analgesia with VAS > 3 cm |
|
| Interventions | AMB (n = 51): 0.1% ropivacaine + fentanyl 2 µg/mL. Automated bolus group: a PCEA algorithm was used, initiated immediately after the completion of CSE. The pump was designed to administer automated boluses of 5 mL in addition to the patient‐controlled boluses. The frequency of such automated boluses was dependent on the history of the participant's analgesic requirement over the past hour. The first automated bolus was programmed to be delivered 60 min from time 0 and every hour thereafter if no PCEA patient‐bolus was made (1 automated bolus of 5 mL every hour). At the first activation of a PCEA patient‐bolus, the timer would be reset with the subsequent automated bolus delivered 30 min following the PCEA patient‐bolus, and every hour thereafter if no further PCEA patient bolus was made (1 automated bolus of 5 mL every hour). If there was a second PCEA patient bolus in that same hour after the initial bolus, the time interval between 2 automated boluses would be shortened to 30 min (2 automated boluses of 5 mL every hour). If there was a third PCEA patient‐bolus within that hour, the automated bolus would be delivered at 20‐min intervals (3 automated boluses of 5 mL every hour). A fourth PCEA patient‐bolus within the same hour would further shorten the time interval between 2 automated boluses to 15 min (4 automated boluses of 5 mL every hour). On the other hand, if there were no patient‐bolus for 60 min, the frequency of automated boluses would step down in the reverse fashion. The lockout period for both PCEA and automated boluses was 10 min. If a PCEA demand was made within 10 min of an automated bolus, no patient bolus would be given and this would be recorded as an unsuccessful PCEA attempt. The PCEA demand bolus was set at 5 mL with a maximum hourly limit of 20 mL/h (inclusive of automated boluses). BI (n = 51): 0.1% ropivacaine + fentanyl 2 µg/mL. Infusion group: PCEA with basal infusion 5 mL/h initiated immediately following intrathecal drug administration (noted as time 0). The PCEA demand bolus was set at 5 mL, lockout interval at 10 min and maximum dose at 20 mL/h (inclusive of background infusion) |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour Total LA/hour (time‐weighted mean hourly consumption of ropivacaine) Maternal satisfaction Apgar scores |
|
| Notes | Study dates not stated Funding sources: no external funding No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated random number tables: Quote: "The parturients were randomly allocated into two groups using sealed opaque envelopes and computer generated random number tables" |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by sealed opaque envelopes: Quote: "The parturients were randomly allocated into two groups using sealed opaque envelopes and computer generated random number tables" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and monitoring clinical team were blinded: Quote: "The parturients were subsequently monitored by a second anaesthetist who was not involved in performing the block. Neither the parturient nor the anaesthetist who recorded the post‐block data was aware of the group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote: "Neither the parturient nor the anaesthetist who recorded the post‐block data was aware of the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition: no dropouts |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size of 49 participants in each group was required to detect an 80% reduction in the incidence of breakthrough pain requiring physician top‐up (α = 0.05, ß = 0.2) |
Song 2020.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind, controlled trial | |
| Participants | Setting: Shanghai First Maternity and Infant Hospital, Shanghai, China Sample Size: N = 120 (N completers = 116) Participants: age = 28.8 ± 3.17 years (EP + CEI), 29.9 ± 2.89 years (DPE + CEI), and 29.1 ± 3.06 (DPE + PIEB) Inclusion Criteria: healthy nulliparous women classified as American Society of Anesthesiologists class II who had a singleton vertex presentation at 37–42 weeks’ gestation in active labor with a cervical dilation <5 cm as well as a baseline pain score >50 mm on a 100 mm visual analogue scale (VAS) at the time of request for epidural analgesia were recruited. Exclusion Criteria: Exclusion criteria included age <20 or >40 years, morbid obesity, pregnancy‐related diseases (i.e. gestational diabetes, gestational hypertension, and preeclampsia), history of drug abuse, contraindications to neuraxial blocks, conditions that increase the risk of a cesarean delivery (ie. placenta previa, history of uterine anomaly, or surgery), and known fetal abnormalities. Participants were also excluded from the analysis in the event of an inadvertent dural puncture using the epidural needle, when cerebrospinal fluid (CSF) could not be confirmed with the spinal needle while performing the dural puncture, or if a delivery occurred within 1 hour after epidural catheter placement. |
|
| Interventions | 3 mL of 1.5% lidocaine with epinephrine 15 μg was administered as the test dose. Labor analgesia was initiated with 10 mL of 0.1% ropivacaine with 0.3 μg/mL of sufentanil over 2 minutes. Epidural infusions consisted of 0.1% ropivacaine with 0.3 μg/mL of sufentanil. 1. EP (conventional epidural) + CEI (n = 38): the epidural pump was programmed to deliver at a constant rate of 8 mL/h, beginning immediately after initiation. A patient‐controlled epidural analgesia (PCEA) bolus of 5 mL with a 20‐minute lockout was programmed in all pumps. 2. DPE (dural puncture epidural) + CEI (n = 40): the epidural pump was programmed to deliver at a constant rate of 8 mL/h, beginning immediately after initiation. A patient‐controlled epidural analgesia (PCEA) bolus of 5 mL with a 20‐minute lockout was programmed in all pumps. 3. DPE (dural puncture epidural) + PIEB (n = 38): the pump was programmed to administer the first bolus of 8 mL 1 hour after initiation and every hour afterward. A patient‐controlled epidural analgesia (PCEA) bolus of 5 mL with a 20‐minute lockout was programmed in all pumps. |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour analgesia Ropivacaine hour consumption Maternal satisfaction Apgar scores |
|
| Notes | Study dates: Dec 2017 to Jun 2018 Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via computer generated random number sequence: Quote: "Eligible women were randomized via a computer generated random number sequence" |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants and clinical staff were blinded to the group allocations: Quote: "The anesthesiologist completed the neuraxial protocol and connected the epidural pump, and then the observer was asked to enter the room and began the evaluation 2 minutes after zero time. The participants and the outcome assessor were blinded to the group allocations." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote: "The participants and the outcome assessor were blinded to the group allocations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition: four participants were excluded after randomisation; however, this exclusion rate is not significant (3%). |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size of 120 participants (40 per group) with less than expected (10%) drop out rate would give the study a power of more than 80% to detect a difference in the survival curves between groups. |
Wong 2006.
| Study characteristics | ||
| Methods | Parallel, randomised controlled trial | |
| Participants | Setting: recruited from Northwestern University, Chicago, Ilinois, USA Sample size: N = 158 (N completers = 126) Participants' age not provided Inclusion criteria: healthy, parous (at least one previous vaginal delivery), term women with singleton, vertex pregnancies, scheduled for induction of labour 11 women from the PIEB group and 9 women from the CEI group were excluded for having delivered within 90 min of intrathecal analgesia. 10 women from the PIEB group were excluded for exceeded pump occlusion limits. 2 women were excluded for VAS > 10 mm 10 min after intrathecal injection |
|
| Interventions | AMB (n = 63): PIEB pump delivered a 6‐mL bolus at a rate of 400 mL/h every 30 min beginning 45 min after administration of the intrathecal dose The PCEA pump was programmed to deliver 5 mL patient‐activated boluses with a lockout interval of 10 min and a per hour maximum of 15 mL. The participant was instructed on the use of the PCEA pump and was told to push the button whenever she felt uncomfortable. If the parturient felt she had inadequate analgesia after having activated the PCEA bolus twice in a 20‐min period an anaesthesiologist administered manual boluses of bupivacaine 1.25 mg/mL (5 mL to 15 mL) until the VAS was < 10 mm BI (n = 63): the CEI pump delivered a continuous infusion at 12 mL/h beginning 15 min after the intrathecal dose The PCEA pump was programmed to deliver 5 mL patient‐activated boluses with a lockout interval of 10 min and a per hour maximum of 15 mL. The participant was instructed on the use of the PCEA pump and was told to push the button whenever she felt uncomfortable. If the parturient felt she had inadequate analgesia after having activated the PCEA bolus twice in a 20‐min period an anaesthesiologist administered manual boluses of bupivacaine 1.25 mg/mL (5 to 15 mL) until the VAS was < 10 mm |
|
| Outcomes | Rate of breakthrough pain with need for anaesthetic intervention Rate of caesarean delivery Rate of instrumental delivery Duration of labour LA per hour Maternal satisfaction |
|
| Notes | Study dates: June 2003 to April 2005 Funding sources not declared No conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated random number sequence: Quote: "A sequentially numbered opaque envelope containing the group assignment (computer generated random number sequence) was opened by the unblinded anesthesia researcher at the time of randomization." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment by sequentially numbered opaque envelopes, but it was not stated that envelopes were sealed: Quote: "A sequentially numbered opaque envelope containing the group assignment (computer generated random number sequence)..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and other personnel were blinded: Quote: "The subject and other study personnel were blinded as to group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded: Quote: "The subject and other study personnel were blinded as to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition: no dropouts |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes reported based on published protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. Sample size calculation: sample size would be required to avoid a type II error at 0.05 and power of 0.80. 30 additional participants were included in the randomisation to allow for anticipated exclusion of participants from data analysis |
AMB: automated mandatory bolus; ASA: American Society of Anesthesiologists; BCI: basal continuous infusion; BI: basal infusion; BMI: body mass index; CEI: continuous epidural infusion; CSE: combined spinal‐epidural; DPE: dural puncture epidural; LA: local anaesthetic; PCEA: patient controlled epidural analgesia; PIEB: programmed intermittent epidural boluses; VAPS: visual analogue pain scale; VAS: visual analogue scale..
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boutros 1999 | Study did not use automated bolus, instead manual bolus was administered |
| Delgado 2018 | Not a randomised controlled study |
| Feng 2014 | Study did not use automated bolus, instead manual bolus was administered |
| Garg 2022 | Study did not use automated bolus, instead manual bolus was administered |
| Lamont 1989 | Study did not use automated bolus, instead manual bolus was administered |
| Liu 2020 | This is not a randomised controlled study |
| Mukherjee 2013 | Study did not use automated bolus, instead manual bolus was administered |
| Nunes 2014 | Intervention groups not comparable in study as given different volumes and concentrations of LA |
| Nunes 2016 | Intervention groups not comparable in study as given different volumes and concentrations of LA |
| Patkar 2015 | Study did not use automated bolus, instead manual bolus was administered |
| Priyadarshini 2022 | Study did not use automated bolus, instead manual bolus was administered |
| Riazanova 2019 | LA without opioids used in both groups ‐ this differs from contemporary clinical practice |
| Rodriguez Gonzalez 2019 | This is not a randomised controlled study |
| Roofthooft 2021 | Study did not use automated bolus, instead manual bolus was administered |
| Salim 2005 | The intervention groups received different analgesic medications. |
| Shidhaye 2010 | Study did not use automated bolus, instead manual bolus was administered |
| Skrablin 2011 | Study did not use automated bolus, instead manual bolus was administered |
| Smedstad 1988 | Study did not use automated bolus, instead manual bolus was administered |
| Vilaplana 1995 | Study did not use automated bolus, instead manual bolus was administered |
LA: local anaesthetic.
Characteristics of studies awaiting classification [ordered by study ID]
Fang 2016.
| Methods | Awaiting full‐text review |
| Participants | Awaiting full ‐ext review |
| Interventions | Awaiting full‐text review |
| Outcomes | Awaiting full‐text review |
| Notes |
Ji 2016.
| Methods | Awaiting full‐text review |
| Participants | Awaiting full‐text review |
| Interventions | Awaiting full ‐ext review |
| Outcomes | Awaiting full‐text review |
| Notes |
Wang 2016.
| Methods | Awaiting full‐text review |
| Participants | Awaiting full‐text review |
| Interventions | Awaiting full‐text review |
| Outcomes | Awaiting full‐text review |
| Notes |
Wang 2017.
| Methods | Awaiting full‐text review |
| Participants | Awaiting full‐text review |
| Interventions | Awaiting full‐text review |
| Outcomes | Awaiting full‐text review |
| Notes |
Zhao 2013.
| Methods | Awaiting full‐text review |
| Participants | Awaiting full‐text review |
| Interventions | Awaiting full‐text review |
| Outcomes | Awaiting full‐text review |
| Notes |
Differences between protocol and review
No differences between protocol and review
Contributions of authors
Conceiving the review: Hon Sen Tan (HST), Yanzhi Zeng (ZYZ), Yueyue Qi (YYQ), Fahad Javaid Siddiqui (FJS), Rehena Sultana (RS), Chin Wen Tan (CWT), Alex T Sia (AS), Ban Leong Sng (BLS).
Co‐ordinating the review: HST, ZYZ, YYQ, FJS, CWT, SBL, AS
Undertaking manual searches: HST, FJS
Screening search results: HST, ZYZ, YYQ, SBL
Organizing retrieval of papers: HST, ZYZ, YYQ, FJS, SBL
Screening retrieved papers against inclusion criteria: HST, ZYZ, YYQ, FJS, SBL
Appraising quality of papers: HST, ZYZ, YYQ, FJS, RS, SBL
Abstracting data from papers: HST, ZYZ, YYQ, FJS, SBL
Writing to authors of papers for additional information: HST
Providing additional data about papers: HST
Obtaining and screening data on unpublished studies: HST, ZYZ, YYQ, FJS, SBL
Data management for the review: HST, ZYZ, YYQ, FJS, RS, SBL
Entering data into Review Manager 5 (RevMan 5): HST, ZYZ, YYQ, SBL
RevMan 5 statistical data: HST, ZYZ, YYQ, FJS, RS, SBL
Other statistical analysis not using RevMan: HST, ZYZ, FJS, RS
Interpretation of data: HST, ZYZ, YYQ, FJS, RS, CWT, SBL
Statistical inferences: HST, ZYZ, YYQ, FJS, RS, SBL
Writing the review: HST, ZYZ, YYQ, FJS, RS, CWT, SBL, AS
Securing funding for the review: CWT, SBL, AS
Performing previous work that was the foundation of the present study: SBL, AS
Guarantor for the review (one author): HST
Person responsible for reading and checking review before submission: HST, SBL, FJS, AS
Sources of support
Internal sources
-
KK Women's and Children's Hospital, Singapore
Research time utilised
External sources
-
Duke‐NUS Graduate Medical School, Singapore
Research time utilised
-
Singapore Clinical Research Institute, Singapore
Research time utilised
Declarations of interest
1. Hon Sen TAN: no declarations of interest
2. Yanzhi ZENG: no declarations of interest
3. Rehena SULTANA: no declarations of interest
4. Yueyue QI: no declarations of interest
5. Chin Wen TAN: no declarations of interest
6. Alex T SIA: author of six of the studies that are included in this review (Chua 2004; Leo 2010; Lim 2005; Lim 2010; Sia 2007; Sia 2013). The variable frequency automated mandatory bolus technique described in Sia 2013 prompted the filing of a patent for the technique. In this review, he was not involved in study selection, data entry, or data analysis, however, he coordinated the review, was an author in the previous version of the review (Sng 2018) that laid the foundation for the current study, and contributed to the writing and rechecking of the final manuscript prior to submission.
7. Ban Leong SNG: no declarations of interest
8. Fahad J SIDDIQUI: no declarations of interest
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Capogna 2011 {published data only}
- Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesthesia and Analgesia 2011;113(4):826-31. [DOI: 10.1213/ANE.0b013e31822827b8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chalekar 2021 {published data only}
- Chalekar R, Patil B, Kagalkar N, Keshava Reddy K. Comparison of intermittent bolus versus continuous infusion of epidural labour analgesia by 0.15% ropivacaine and fentanyl: a randomised clinical study. Journal of Clinical and Diagnostic Research 2021;15(11):UC05-09. [Google Scholar]
Chua 2004 {published data only}
- Chua SM, Sia AT. Automated intermittent epidural boluses improve analgesia induced by intrathecal fentanyl during labour. Canadian Journal of Anaesthesia 2004;51(6):581-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fan 2019 {published data only}
- Fan Y, Hou W, Feng S, Mao P, Wang X, Jiang J, et al. Programmed intermittent epidural bolus decreases the incidenceof intra‑partum fever for labor analgesia in primiparous women:a randomized controlled study. Archives of Gynecology and Obstetrics 2019;300:1551-7. [DOI] [PubMed] [Google Scholar]
Ferrer 2017 {published data only}
- Ferrer LE, Romemro DJ, Vásquez OI, Matute EC, Van de Velde M. Effect of programmed intermittent epidural boluses and continuous epidural infusion on labor analgesia and obstetric outcomes: a randomized controlled trial. Archives of Gynecology and Obstetrics 2017;296(5):915-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fettes 2006 {published data only}
- Fettes PD, Moore CS, Whiteside JB, McLeod GA, Wildsmith JA. Intermittent vs continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. British Journal of Anaesthesia 2006;97(3):359-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fidkowski 2019 {published data only}
- Fidkowski CW, Shah S, Alsaden MR. Programmed intermittent epidural bolus as compared to continuous epidural infusion for the maintenance of labor analgesia: a prospective randomized single-blinded controlled trial. Korean Journal of Anesthesiology 2019;72(5):472-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haidl 2020 {published data only}
- Haidl F, Arne Rosseland L, Rørvik AM, Dahl V. Programmed intermittent boluses vs continuous epidural infusion in labor using an adrenaline containing solution: a randomized trial. Acta Anaesthesiologica Scandinavica 2020;64(10):1505-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leo 2010 {published data only}
- Leo S, Ocampo CE, Lim Y, Sia AT. A randomized comparison of automated intermittent mandatory boluses with a basal infusion in combination with patient-controlled epidural analgesia for labor and delivery. International Journal of Obstetric Anaesthesia 2010;19(4):357-64. [DOI: 10.1016/j.ijoa.2010.07.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lim 2005 {published data only}
- Lim Y, Sia AT, Ocampo C. Automated regular boluses for epidural analgesia: a comparison with continuous infusion. International Journal of Obstetric Anaesthesia 2005;14(4):305-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lim 2010 {published data only}
- Lim Y, Chakravarty S, Ocampo CE, Sia AT. Comparison of automated intermittent low volume bolus with continuous infusion for labour epidural analgesia. Anaesthesia and Intensive Care 2010;38(5):894-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2016 {published data only}
- Lin Y, Li Q, Liu J, Yang R, Liu J. Comparison of continuous epidural infusion and programmed intermittent epidural bolus in labor analgesia. Therapeutics and Clinical Risk Management 2016;12:1107-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morau 2019 {published data only}
- Morau E, Jaillet M, Storme B, Nogue N, Bonnin M, Chassard D, et al. Does programmed intermittent epidural bolus improve childbirth conditions of nulliparous women compared with patient-controlled epidural analgesia? European Journal of Anaesthsiology 2019;36(10):755-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ojo 2020 {published data only}
- Ojo OA, Mehdiratta JE, Gamez BH, Hunting J, Habib AS. Comparison of programmed intermittent epidural boluses with continuous epidural infusion for the maintenance of labor analgesia: a randomized, controlled, double-blind study. Anesthesia and Analgesia 2020;130(2):426-35. [DOI] [PubMed] [Google Scholar]
Sia 2007 {published data only}
- Sia AT, Lim Y, Ocampo C. A comparison of a basal infusion with automated mandatory boluses in parturient-controlled epidural analgesia during labor. Anesthesia and Analgesia 2007;104(3):673-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sia 2013 {published data only}
- Sia AT, Leo S, Ocampo CE. A randomised comparison of variable-frequency automated mandatory boluses with a basal infusion for patient-controlled epidural analgesia during labour and delivery. Anaesthesia 2013;68(3):267-75. [DOI: 10.1111/anae.12093] [PMID: ] [DOI] [PubMed] [Google Scholar]
Song 2020 {published data only}
- Song Y, Du W, Zhou S, Zhou Y, Yu Y, Xu Z, et al. Effect of dural puncture epidural technique combined with programmed intermittent epidural bolus on labor analgesia onset and maintenance: a randomized controlled trial. Anesthesia and Analgesia 2021;132(4):971-8. [DOI: 10.1213/ANE.0000000000004768] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong CA, Ratliff JT, Sullivan JT, Scavone BM, Toledo P, McCarthy RJ. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesthesia and Analgesia 2006;102(3):904-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Boutros 1999 {published data only}
- Boutros A, Blary S, Bronchard R, Bonnet F. Comparison of intermittent epidural bolus, continuous epidural infusion and patient controlled-epidural analgesia during labor. International Journal of Obstetric Anesthesia 1999;8(4):236-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Roofthooft E, Barbe A, Schildermans S, Cromheecke S, Devroe S, Fieuws S, et al. Programmed intermittent epidural bolus vs. patient-controlled epidural analgesia for maintenance of labour analgesia: a two-centre, double-blind, randomised study. Anaesthesia 2020;75:1635-42. [DOI] [PubMed] [Google Scholar]
Delgado 2018 {published data only}
- Delgado C, Ciliberto C, Bollag L, Sedensky M, Landau R. Continuous epidural infusion versus programmed intermittent epidural bolus for labor analgesia: optimal configuration of parameters to reduce physician-administered top-ups. Current Medical Research and Opinion 2018;34(4):649-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Feng 2014 {published data only}
- Feng SW, Xu SQ, Ma L, Li CJ, Wang X, Yuan HM, et al. Regular intermittent bolus provides similar incidence of maternal fever compared with continuous infusion during epidural labor analgesia. Saudi Medical Journal 2014;35(10):1237-42. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Garg 2022 {published data only}
- Garg LK, Bindra TK, Kumar P, Mohi MK, Kaur S. Comparison of intermittent bolus and continuous infusion of 0.1% levobupivacaine with fentanyl for epidural labor analgesia. Asian Journal of Pharmaceutical and Clinical Research 2022;15(6):119-21. [DOI: ] [Google Scholar]
Lamont 1989 {published data only}
- Lamont RF, Pinney D, Rodgers P, Bryant TN. Continuous vs intermittent epidural analgesia. A randomised trial to observe obstetric outcome. Anaesthesia 1989;44(11):893-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2020 {published data only}
- Liu X, Zhang H, Zhang HJ, Guo MZ, Gao YC, Du CY. Intermittent epidural bolus versus continuous epidural infusions for labor analgesia: A meta-analysis of randomized controlled trials. PLOS One 2020;15(6):e0234353. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukherjee 2013 {published data only}
- Mukherjee S, Das S, Mukhopadhyay S, Basu SR. Comparison between continuous epidural infusion and intermittent epidural bolus of bupivacaine fentanyl combination for labour analgesia. Scholars Journal of Applied Medical Sciences 2013;1:953-8. [Google Scholar]
Nunes 2014 {published data only}
- Nunes J, Nunes S, Veiga M, Rodrigues R, Seifert I. Programmed intermittent boluses: are we improving epidural labour analgesia? European Journal of Anaesthesiology 2014;31:182-3. [Google Scholar]
Nunes 2016 {published data only}
- Nunes J, Nunes S, Veiga M, Cortez M, Seifert I. A prospective, randomized, blinded-endpoint, controlled study - continuous epidural infusion versus programmed intermittent epidural bolus in labor analgesia. Brazilian Journal of Anesthesiology 2016;66(5):439-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patkar 2015 {published data only}
- Patkar CS, Vora K, Patel H, Shah V, Modi MP, Parikh G. A comparison of continuous infusion and intermittent bolus administration of 0.1% ropivacaine with 0.0002% fentanyl for epidural labor analgesia. Journal of Anaesthesiology Clinical Pharmacology 2015;31(2):234-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Priyadarshini 2022 {published data only}
- Priyadarshini P, Verma R, Singh P, Gautam S, Singh D, Kohli, M, et al. Comparison of continuous infusion of ropivacaine and fentanyl with intermittent bolus doses of ropivacaine and fentanyl for epidural labor analgesia: a randomized open-label study. Cureus journal of Medical science 2022;14(8):e28243. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riazanova 2019 {published data only}
- Riazanova OV, Alexandrovich YS, Guseva YV, Ioscovich AM. A randomized comparison of low dose ropivacaine programmed intermittent epidural bolus with continuous epidural infusion for labour analgesia. Romanian Journal of Anaesthesia and Intensive Care 2019;26(1):25-30. [DOI: 10.2478/rjaic-2019-0004] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez Gonzalez 2019 {published data only}
- Rodriguez Gonzalez IP, Espinosa Dominguez E, Quesada Garcia C, Rodriguez Chimeno A, Borges R. Comparison between different epidural analgesia modalities for labor [Comparativa entre diferentes modalidades de analgesia epidural para trabajo de parto]. Revista Española de Anestesiología y Reanimación 2019;66(8):417-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roofthooft 2021 {published data only}
- Roofthooft E, Barbe A, Schildermans J. Programmed intermittent epidural bolus vs. patient-controlled epidural analgesia for maintenance of labour analgesia: a two-centre, double-blind, randomised study. Anaesthesia 2021;76(4):567. [DOI] [PubMed] [Google Scholar]
Salim 2005 {published data only}
- Salim R, Nachum Z, Moscovici R, Lavee M, Shalev E. Continuous compared with intermittent epidural infusion on progress of labor and patient satisfaction. Obstetrics and Gynaecology 2005;106(2):301-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shidhaye 2010 {published data only}
- Shidhaye RV, Badhe V, Divekar D, Dhulkhed V, Thorat P, Shidhaye R. A randomized clinical trial to compare small frequent boluses technique with that of traditional intermittent top-ups and continuous epidural infusion, for maintenance of epidural labour analgesia. Sri Lankan Journal of Anaesthesiology 2010;18(2):75-80. [Google Scholar]
Skrablin 2011 {published data only}
- Skrablin S, Grgic O, Mihaljevic S, Blajic J. Comparison of intermittent and continuous epidural analgesia on delivery and progression of labour. Journal of Obstetrics and Gynaecology 2011;31(2):134-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Smedstad 1988 {published data only}
- Smedstad KG, Morison DH. A comparative study of continuous and intermittent epidural analgesia for labour and delivery. Canadian Journal of Anaesthesia 1988;35(3):234-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vilaplana 1995 {published data only}
- VVilaplana J, Borrás R, Robert M, García-Jiménez R, Busquets C, Villalonga A. Comparative study of continuous epidural analgesia versus intermittent, patient-controlled, epidural analgesia during labor [Estudio comparativo de la analgesia epidural continua frente a la intermitente, controlada por la paciente, en el trabajo de parto]. Revista Española de Anestesiología y Reanimación 1995;42(7):269-73. [PMID: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fang 2016 {published data only}
- Fang X, Xie L, Chen X. Clinical efficacy of programmed intermittent epidural bolus and continuous epidural infusion for labor analgesia. Journal of Clinical Anesthesia 2016;32:757-60. [Google Scholar]
Ji 2016 {published data only}
- Ji J, Xu Z, Jin C, Liu Z. A randomized comparison between intermittent bolus and continuous infusion in combination with patient-controlled epidural analgesia in labor. Shanghai Medical Journal 2016;39:461-5. [Google Scholar]
Wang 2016 {published data only}
- Wang Z, Shiqin X, Feng S, Qian R, Shen X. Efficacy of programmed intermittent epidural bolus for labor analgesia in parturients and the effect on neonates. Chinese Journal of Anesthesiology 2016;36:1134-7. [Google Scholar]
Wang 2017 {published data only}
- Wang Z, Feng S, Shiqin X, Zhang P, Wang N, Shen X. Comparison of programmed intermittent epidural bolus with continuous epidural infusion at different time intervals for epidural labor analgesia. Journal of Clinical Anesthesia 2017;33:755-9. [Google Scholar]
Zhao 2013 {published data only}
- Zhao J, Wu J, Li S, Wan X. Comparison of automated mandatory bolus and continuous epidural infusion in parturient-controlled epidural analgesia during labor. Shanghai Medical Journal 2013;36:504-6. [Google Scholar]
Additional references
Anim‐Somuah 2018
- Anim-Somuah M, Smyth RMD, Cyna AM, Cuthbert A. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD000331. [DOI: 10.1002/14651858.CD000331.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2013
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prevention Science 2013;14(2):134-43. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 31 December 2022. Veritas Health Innovation, Melbourne, Australia..
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2013
- Geroge RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesthesia and Analgesia 2013;116(1):133-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Version accessed prior to 31 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336(7651):995-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hogan 2002
- Hogan Q. Distribution of solution in the epidural space: examination by cryomicrotome section. Regional Anaesthesia and Pain Medicine 2002;27(2):150-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 20 April 2005;5(13):1-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussain 2020
- Hussain N, Lagnese CM, Hayes B, Kumar N, Weaver TE, Essandoh MK, et al. Comparative analgesic efficacy and safety of intermittent local anaesthetic epidural bolus for labour: a systematic review and meta-analysis. British Journal of Anaesthesia 2020;125(4):560-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD009234. [DOI: 10.1002/14651858.CD009234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaynar 1999
- Kaynar AM, Shankar KB. Epidural infusion: continuous or bolus? Anesthesia and Analgesia 1999;89(2):534. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 Updated February 2021.
Loubert 2011
- Loubert C, Hinova A, Fernando R. Update on modern neuraxial analgesia in labour: a review of the literature of the last 5 years. Anaesthesia 2011;66(3):191-212. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 2019
- Tan HS, Sng BL, Sia AT. Reducing breakthrough pain during labour epiduralanalgesia: an update. Current opinion Anesthesiology 2019;32:307-14. [DOI] [PubMed] [Google Scholar]
Tan 2021
- Tan HS, Liu N, Sultana R, Han NL, Tan CW, Zhang J, et al. Prediction of breakthrough pain during labour neuraxial analgesia: comparison of machine learning and multivariable regression approaches. International Journal of Obstetric Anbesthesia 2021;45:99-110. [DOI] [PubMed] [Google Scholar]
Thornton 2001
- Thornton J, Capogna G. Reducing likelihood of instrumental delivery with epidural anaesthesia. Lancet 2001;358(9275):2. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sng 2014
- Sng BL, Zeng Y, Leong WL, Oh TT, Siddiqui FJ, Assam PN, et al. Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD011344. [DOI: 10.1002/14651858.CD011344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sng 2018
- Sng BL, Zeng Y, Souza NNA, Leong WL, Oh TT, Siddiqui FJ, Assam PN, Han NLR, Chan ESY, Sia AT. Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD011344. [DOI: 10.1002/14651858.CD011344.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


