Abstract
Background
A rapid and reliable diagnostic test is needed to reduce mortality through early diagnosis of invasive aspergillosis (IA) in patients with hematological malignancies.
Objective
To evaluate the efficacy of serum and bronchoalveolar lavage (BAL) Aspergillus galactomannan lateral flow assay (GM-LFA) in IA diagnosis and determine the correlation of GM-LFA with GM enzyme immunoassay (GM-EIA) in patients with hematological malignancies.
Methods
In this prospective multicenter study, we used serum and BAL fluid samples from patients with hematological malignancies and suspected IA and performed GM-LFA and GM-EIA. According to the EORTC/MSGERC criteria, patients were grouped as proven (n = 6), probable (n = 22), possible IA (n = 55), or no IA (n = 88). The performance of serum GM-LFA at 0.5 optical density index (ODI) and area under the curve (AUC) were calculated. Spearman’s correlation analysis and kappa statistics were performed to determine the agreement between the tests.
Results
GM-LFA showed an AUC of 0.832 in proven/probable IA (sensitivity [SEN], specificity [SPE], negative predictive value [NPV], and diagnostic accuracy were 75%, 100%, 92.6%, and 93.9%, respectively, at a 0.5 ODI) versus that in no IA. A moderate positive correlation was noted between the GM-LFA and GM-EIA scores (p = 0.01). The observed agreement between the tests at 0.5 ODI was almost perfect (p < 0.001). After excluding patients who received mold-active antifungal prophylaxis or treatment, the SEN, SPE, NPV, and diagnostic accuracy for proven/probable IA were 76.2%, 100%, 93.3%, and 94.5%, respectively.
Conclusions
Serum GM-LFA demonstrated high discriminatory power and good diagnostic performance for IA in patients with hematological malignancies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11046-023-00749-7.
Keywords: Enzyme immunoassay, Galactomannan, Hematological malignancy, Invasive aspergillosis, Lateral flow assay
Introductıon
Invasive aspergillosis (IA) is a significant cause of morbidity and mortality in patients with hematological malignancies [1, 2]. Early diagnosis and treatment are of great importance; however, sterile tissue biopsy and culture, the gold standard methods for IA, are time-consuming and inappropriate for early diagnosis and treatment. The most common serological test for diagnosing IA is the galactomannan enzyme immunoassay (GM-EIA) in serum and bronchoalveolar lavage (BAL), with sensitivity (SEN) and specificity (SPE) of 77% and 87%, respectively [3]. However, this test has some disadvantages, such as long working time; it is not designed to study a single sample, and it can be run 1–2 times a week in most centers; it is expensive and requires an experienced laboratory technician.
Two immunochromatographic tests have recently been developed for IA diagnosis: the Aspergillus lateral flow device, which detects antigenic mannoproteins, and the GM-based Aspergillus GM lateral flow assay (GM-LFA). IMMY sõna Aspergillus LFA is a sandwich test system that detects Aspergillus GM in serum and BAL samples. It uses a mixture of two monoclonal antibodies (MAb) to detect GM. The first is the ME-A5 MAb, which binds to a GM epitope similar to the EB-A2 used in Platelia GM-EIA. The second is an undisclosed MAb that can detect other GM epitopes not captured by GM-EIA [4, 5]. Aspergillus GM-LFA is a potential candidate for a rapid, cheap, and easy-to-perform diagnostic test, with advantages such as having a simple working method and providing results in less than an hour.
A number of studies have been conducted to determine the efficacy of GM-LFA in patients with hematological malignancies [5–12]. In this prospective multicenter study, we aimed to evaluate the efficacy of serum Aspergillus GM-LFA in diagnosing IA and the correlation between GM-LFA and GM-EIA in serum and BAL samples from patients with hematological malignancies. We also performed a literature review evaluating the prior studies looking at the efficacy of Aspergillus GM-LFA and comparing these findings with those in our study.
Patıents and Methods
Patient Groups and Data Collection
In this prospective multicenter validation study, we aimed to evaluate the performance of Aspergillus GM-LFA in the diagnosis of IA in patients with hematological malignancies. Between September 2019 and December 2021, patients with hematological malignancies or recipients of allogeneic or autologous hematopoietic stem cell transplantation (HSCT) were hospitalized at four centers in Turkey (Marmara University Faculty of Medicine Hospital, Bursa Uludag Faculty of Medicine Hospital, Kayseri Erciyes Faculty of Medicine Hospital, and Antalya Medstar Hospital). The included patients had at least one of the following IA host factors previously defined in the revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSGERC) [13]: (i) hematological malignancy with active chemotherapy or refractory disease, (ii) recent history of neutropenia after chemotherapy (> 10 days, < 500 neutrophils/mm3), (iii) recipient of allogeneic HSCT, (iv) prolonged use of corticosteroid in the last 60 days, ≥ 0.3 mg/kg for ≥ 3 weeks, (v) treatment with recognized T- or B-cell immunosuppressants during the past 90 days. Patients aged < 18 years, those with only BAL samples, and those receiving secondary antifungal prophylaxis were excluded. This prospective study was approved by the Marmara University Clinical Research Ethics Committee (protocol number: 09.2019.796). The patients or their relatives provided informed consent before beginning of the study.
The patients were followed-up for infection and fever during hospitalization. Thoracic and/or sinus computed tomography (CT) was performed in patients with neutropenic fever, which exceeded 96 h despite the use of broad-spectrum antibiotics, or those with respiratory symptoms/signs and sinusitis. Patients suspected of IA and whose symptoms could not be explained by any other etiological factor with micro/macro nodule, cavity, air-crescent, or consolidation findings on chest tomography were included in the case group. Among hospitalized hematology patients, those who did not develop an infection and did not have clinical or radiological findings suggestive of IA were included in the control group (no IA). GM-EIA (Platelia™ Aspergillus EIA, Bio-Rad Laboratories, France) was routinely performed twice weekly on the serum samples of patients who did not receive mold-active antifungal prophylaxis or treatment. Furthermore, both GM-EIA and GM-LFA were performed using the same serum sample when IA was suspected in the case group. In the control group, GM-LFA was performed in addition to GM-EIA, which was routinely performed. Bronchoscopic evaluation was performed in patients who were suitable for bronchoscopy. Fungal cultures, GM-EIA, and GM-LFA were performed using BAL fluid samples. The day when the serum GM-LFA test was performed was considered as day 0. Patients’ condition was classified as proven, probable, or possible IA, according to the revised EORTC/MSGERC criteria [13].
Data evaluation and analysis were performed in triplicates. In the first analysis, the performance of GM-LFA and the correlation of GM-LFA with GM-EIA were evaluated using serum samples from all patients. In the second analysis, patients receiving mold-active antifungal therapy or prophylaxis on day 0 were excluded to observe the effect of antifungal therapy or prophylaxis on the performance of GM-LFA. The third analysis was performed to examine the correlation between GM-LFA and GM-EIA in the BAL samples.
Aspergillus GM-LFA
The Aspergillus GM-LFA (IMMY, Norman, OK, USA) was performed according to the manufacturer’s instructions [14]. First, 300 μL of serum or BAL was mixed with 100 μL of pretreatment buffer, vortexed, heated at 120 °C for 6 min, and centrifuged at 14,000 × g for 5 min. Then 80 μL of the pretreated sample was mixed with 40 μL of Aspergillus GM-LFA running buffer, and a test strip was placed into the sample for 30 min before reading. Optic density index (ODI) results were then obtained by placing the strips in an Aspergillus GM-LFA cube reader calibrated according to the manufacturer’s instructions. The Cube Reader used an LED at 525 nm to read the results of Aspergillus GM-LFA. Index values of ≥ 0.50 were considered positive, while < 0.50 were regarded as negative.
Statistical Analysis
Data were analyzed using SPSS Statistics for Windows, version 26.0 (IBM, Armonk, NY, USA). Descriptive statistics are presented as frequencies and percentages for qualitative categorical variables and as median, minimum (min) – maximum (max) value and interquartile range (IQR) for non-normally distributed quantitative variables. Pearson’s chi-squared test was used in 2 × 2 tables for qualitative categorical variables. Fisher’s exact probability test was used if the expected frequency in the cells was > 20%. For non-normally distributed quantitative data, we used the Mann–Whitney U-test to compare two independent groups and the Kruskal–Wallis test to compare more than two groups. A t-test was used to compare two independent groups of normally distributed quantitative data.
The SEN, SPE, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy for the diagnosis of proven/probable IA were determined for serum Aspergillus GM-LFA at a cut-off point of 0.5. Receiver operating characteristic (ROC) analysis was performed by comparing the performance of GM-LFA for proven/probable IA and no IA in serum samples, and the area under the curve (AUC) at the 95% confidence interval (95% CI) are presented. The Youden index (sensitivity + specificity-1) was used to determine the cut-off point with the highest accuracy. Quantitative agreement between GM-LFA and GM-EIA was determined using Spearman’s correlation. Spearman’s correlation coefficient (ρ) of 0.40–0.69 indicated moderate correlation, 0.70–0.89 indicated strong correlation, and 0.90–1.00 indicated very strong correlation [15]. Qualitative agreement (test positivity at 0.5 ODI) between the two tests was demonstrated by generating observed agreement (accuracy) and a kappa statistic, with values of 0.41–0.6 indicating moderate agreement, 0.61–0.8 indicating substantial agreement, and 0.81–1.00 indicating almost perfect agreement between tests [16]. P < 0.05 values were considered statistically significant.
Results
Patient Characteristics
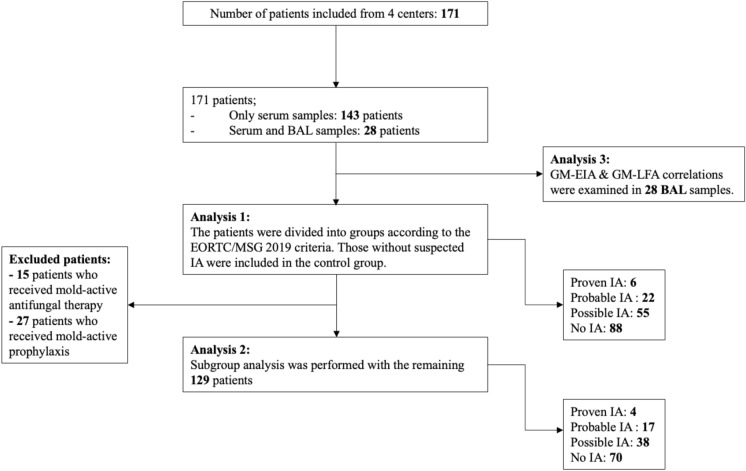
Serum samples were obtained from 171 patients with hematological malignancies, and additional BAL samples were obtained from 28 patients. The median age of the patients was 54 years (range 18–91 years), and 100 of them were male. The underlying hematological malignancies were acute myeloid leukemia in 62 patients, non-Hodgkin lymphoma in 35 patients, acute lymphoblastic leukemia in 25 patients, multiple myeloma in 20 patients, and other hematological diseases in 29 patients. The most common comorbidities in patients were hypertension (15.8%), cardiovascular disease (12.3%), and diabetes mellitus (9.4%); three patients had chronic pulmonary disease, and one patient had a human immunodeficiency virus infection. According to the EORTC/MSGERC criteria, the number of proven, probable, and possible IA cases was 6, 22, and 55, respectively, and 88 patients were classified as no IA. Of the patients with proven IA, diagnosis was made based on sinonasal tissue culture in five cases and lung tissue biopsy in one case. Patient characteristics are summarized in Table 1. The diagnostic classification of the patients, diagnostic efficiency of GM-LFA, and test results are shown in Fig. 1. A detailed overview of all proven and probable IA cases is provided in Supplementary Table 1. Antifungal prophylaxis use was 50.3% (15.8% mold active, 34.5% mold inactive) in all study patients when they were included in the study. No significant difference was noted in prophylactic antifungal use among the proven/probable IA, possible IA, and no IA groups (p = 0.24). Nine of the patients in the proven/probable IA group received mold-inactive antifungal prophylaxis, while only one received mold-active antifungal prophylaxis and was diagnosed with possible IA on day 12. At the time of enrollment, 15 patients had received mold-active antifungal therapy for fungemia (n = 2, echinocandin), possible IFI (n = 5, polyene or mold-active azole), or empirical therapy (n = 8, mold-active azole, polyene or echinocandin). The median duration of neutropenia was 6 days (Supplementary Table 2). The most common radiological findings on thoracic CT for proven/probable and possible IA cases were nodules (72.3%), consolidation (69.9%), and ground glass opacities (61.4%). The 30-day crude mortality rate in all patients was 19.9% (50% in proven IA, 31.8% in probable IA, 27.3% in possible IA, and 10.2% in no IA cases).
Table 1.
Demographics of study participants and IA status according to the EORTC/MSGERC criteria
| Overall | Proven IA | Probable IA | Possible IA | No IA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 171 | N = 6 | % | N = 22 | % | N = 55 | % | N = 88 | % | |||
| Median age, y (min–max, IQR) | 54 (18–91, 27) | % | 47 (37–63, 23) | 52 (19–73, 29) | 57 (18–77, 27) | 54 (18–91, 27) | |||||
| Men | 100 | 58.5 | 3 | 50.0 | 15 | 68.2 | 28 | 50.9 | 54 | 61.4 | |
| Hematological malignancies | |||||||||||
| AML | 62 | 36.3 | 1 | 16.7 | 9 | 40.9 | 30 | 54.5 | 22 | 25.0 | |
| NHL | 35 | 20.5 | 2 | 33.3 | 4 | 18.2 | 4 | 7.3 | 25 | 28.4 | |
| ALL | 25 | 14.6 | 1 | 16.7 | 3 | 13.6 | 7 | 12.7 | 14 | 15.9 | |
| MM | 20 | 11.7 | 0 | 0 | 2 | 9.1 | 4 | 7.3 | 14 | 15.9 | |
| HL | 13 | 7.6 | 1 | 16.7 | 0 | 0 | 4 | 7.3 | 8 | 9.1 | |
| MDS | 4 | 2.3 | 0 | 0 | 0 | 0 | 3 | 5.5 | 1 | 1.1 | |
| Others | 12 | 7.0 | 1 | 16.7 | 4 | 18.2 | 3 | 5.5 | 4 | 4.5 | |
| HSCT | |||||||||||
| Overall | 29 | 17.0 | 0 | 0 | 3 | 13.6 | 13 | 23.6 | 13 | 14.8 | |
| Autologous | 17 | 9.9 | 0 | 0 | 2 | 9 | 8 | 14.5 | 7 | 8.0 | |
| Allogeneic | 12 | 7.0 | 0 | 0 | 1 | 4.5 | 5 | 9.0 | 6 | 6.8 | |
| GVHD | 4 | 2.3 | 0 | 0 | 0 | 0 | 1 | 1.8 | 3 | 3.4 | |
AML: acute myeloid leukemia, ALL: acute lymphoblastic leukemia, EORTC/MSGERC: European Organization for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium, GVHD: graft versus host disease, HL: Hodgkin lymphoma, HSCT: hematopoietic stem cell transplantation, IA: invasive aspergillosis, IQR: interquartile range, Max: maximum, MDS: myelodysplastic syndrome, Min: minimum, MM: multiple myeloma, NHL: non-Hodgkin lymphoma
Fig. 1.
Study flow diagram BAL: Bronchoalveolar lavage, EIA: Enzyme immunoassay, GM: Galactomannan, IA: Invasive aspergillosis, LFA: Lateral flow assay
Aspergillus GM-LFA and GM-EIA ODI Values According to Diagnostic Classification
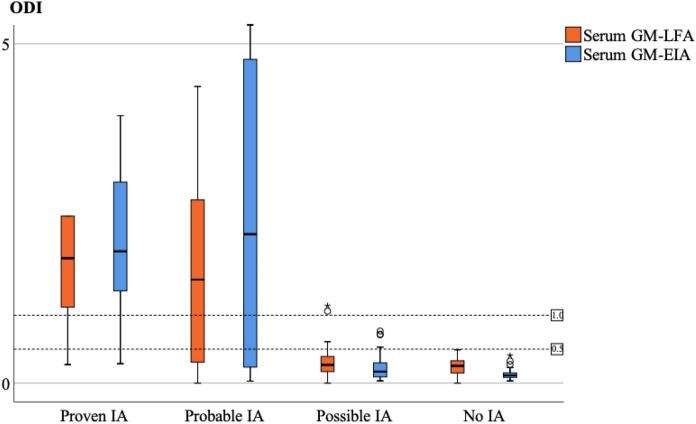
The serum GM-LFA and GM-EIA ODI was significantly higher in in proven/probable IA cases than in both possible and no IA cases (p < 0.001) (Fig. 2). The median ODI of GM-LFA for the proven, probable, possible, and no IA samples was 1.84, 1.52, 0.27, and 0.25, respectively (Table 2). The median ODI of serum GM-LFA was 0.26 in those with serum GM-EIA < 0.5 ODI (n = 146) and that of serum GM-LFA was 1.69 in those with serum GM-EIA ≥ 0.5 ODI (n = 25).
Fig. 2.
Clustered box plot of serum GM-LFA and GM-EIA ODIs EIA: Enzyme immunoassay, GM: Galactomannan, IA: Invasive aspergillosis, LFA: Lateral flow assay, ODI: Optical density index
Table 2.
GM-LFA and GM-EIA index values by diagnosis type
| GM ODI | Proven IA | Probable IA | Possible IA | No IA | ||||
|---|---|---|---|---|---|---|---|---|
| LFA | EIA | LFA | EIA | LFA | EIA | LFA | EIA | |
| Min | 0.27 | 0.28 | 0.0 | 0.03 | 0.0 | 0.03 | 0.0 | 0.03 |
| 25th percentile | 0.90 | 1.09 | 0.28 | 0.23 | 0.17 | 0.086 | 0.15 | 0.083 |
| Median | 1.84 | 1.94 | 1.52 | 2.19 | 0.27 | 0.16 | 0.25 | 0.115 |
| 75th percentile | 5.6 | 3.2 | 2.78 | 4.87 | 0.4 | 0.30 | 0.33 | 0.15 |
| Max | 15.03 | 3.94 | 15.2 | 22.4 | 1.14 | 0.76 | 0.49 | 0.40 |
| IQR | 4.69 | 2.1 | 2.49 | 4.6 | 0.23 | 0.21 | 0.18 | 0.069 |
EIA: enzyme immunoassay, GM: galactomannan, IA: invasive aspergillosis, IQR: interquartile range, LFA: lateral flow assay, Max: maximum, Min: minimum, ODI: optical density index
Clinical Performance of the GM-LFA (Analysis 1)
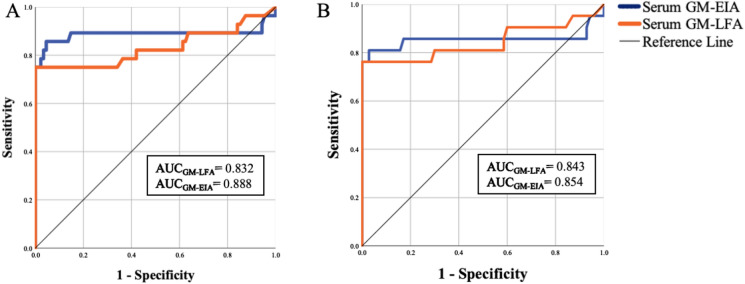
The SEN, SPE, NPV, and diagnostic accuracy of GM-LFA for proven IA versus no IA were 83.3%, 100%, 98.9%, and 98.9%, respectively, and for proven/probable IA versus no IA were 75%, 100%, 92.6%, and 93.9%, respectively, at a GM positivity threshold of ≥ 0.5 (95% CI). ROC curve analysis revealed an AUC of 0.930 (95% CI 0.802–1.0) for differentiation between proven and no IA and an AUC of 0.832 (95% CI 0.716–0.949) for differentiation between proven/probable and no IA (p < 0.001). GM-EIA showed an AUC of 0.888 (95% CI 0.778–0.998) for differentiating between proven/probable and no IA (p < 0.001). ROC analysis identified an optimal positivity threshold of 0.725 for GM-LFA, with SEN and SPE being 75% and 100%, respectively. SEN and SPE were not changed by lowering the threshold to 0.5 (Fig. 3A). In the analysis of 19 proven/probable cases enrolled at one center, the mean time to obtain GM-EIA results from the laboratory was 1.63 days; however, the GM-LFA test results were available on the same day (p = 0.001).
Fig. 3.
ROC curve of the GM-LFA when testing serum samples A. Proven/Probable IA vs. No IA in all patients, B. Proven/Probable IA vs. No IA in patients without the use of mold-active antifungals on day 0. ROC: Receiver operator characteristic, GM: Galactomannan, LFA: Lateral flow assay, IA: Invasive aspergillosis
Sample Test Result Concordance Between GM-LFA and GM-EIA
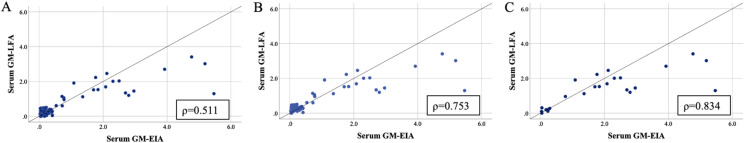
The overall qualitative observed sample agreement between GM-LFA and GM-EIA at 0.5 ODI was 99.4%, generating a kappa statistic of 0.977 (95% CI, SE ± 0.023, p < 0.001), indicating almost perfect agreement. The qualitative observed agreement between GM-LFA and GM-EIA for samples obtained from patients with proven/probable IA and those with proven/probable/possible IA was 100% (95% CI κ = 1.0) and 98.7% (95% CI κ = 0.972 ± 0.028), respectively. The overall quantitative correlation between the ODI calculated by GM-LFA and by GM-EIA was moderate (Spearman’s ρ = 0.511, p = 0.01) (Fig. 4A). The correlation between GM ODI of samples obtained from patients with proven/probable/possible IA and those with proven/probable IA was strong (Spearman’s ρ = 0.753 and ρ = 0.834, respectively, p = 0.01) (Figs. 4B, C). No significant correlation was noted between the ODI for samples from patients with no IA (Spearman’s ρ = 0.104 p = 0.33).
Fig. 4.
Linear correlation between GM ODI values generated by the GM-LFA and GM-EIA when testing serum samples A. Overall patients, B. Proven/Probable/Possible IA, C. Proven/Probable IA GM: Galactomannan, ODI: Optical density index, LFA: Lateral flow assay, EIA: Enzyme immunoassay, IA: Invasive aspergillosis
Clinical Performance of GM-LFA in Patients not Receiving Mold-Active Antifungal Prophylaxis or Treatment (Analysis 2)
A total of 15 patients who received mold-active antifungal treatment and 27 who received mold-active antifungal prophylaxis on day 0 were excluded. The remaining patients were evaluated in the subgroup analysis. According to the revised EORTC/MSGERC criteria, there were 4 proven, 17 probable, 38 possible IA, and 70 non-IA patients. In the proven, probable, possible IA, and no IA groups, the median GM-LFA ODI was 2.34, 1.52, 0.3, and 0.24, respectively, and the median GM-EIA ODI was 1.94, 1.83, 0.15, and 0.11, respectively (Supplementary Table 3 and Fig. 1). In all four cases, the GM-LFA ODI was > 0.5 ODI. The SEN, SPE, NPV, and diagnostic accuracy for proven/probable IA versus no IA were 76.2%, 100%, 93.3%, and 94.5%, respectively, at a GM positivity threshold of ≥ 0.5 (95% CI). GM-LFA and GM-EIA showed AUC of 0.843 (95% CI 0.711–0.974) and 0.854 (95% CI 0.712–0.997) for differentiating between proven/probable and no IA (p < 0.001) (Fig. 3B).
Evaluation of Correlation Between GM-LFA and GM-EIA in Bronchoalveolar Lavage Samples (Analysis 3)
GM-LFA and GM-EIA were compared using BAL samples obtained from 28 patients (2 proven, 9 probable, and 17 possible IA cases). The ODIs of GM-LFA and GM-EIA studied using the BAL fluid samples obtained from patients with proven and probable IA cases were significantly higher than those from patients with possible IA (p < 0.001). In the proven/probable and possible IA groups, the median ODI of GM-LFA was 1.24 and 0.26, respectively, and the median ODI of GM-EIA was 2.88 and 0.185, respectively (Supplementary Fig. 2 and Table 4).
The overall qualitative observed sample agreement between the BAL GM-LFA and GM-EIA at 0.5 ODI was 82.14%, generating a kappa statistic of 0.653 (95% CI, SE ± 0.132, p < 0.001), representing substantial agreement. In 90.9% (10/11) of the proven and probable cases, both GM-EIA and GM-LFA showed positive results at an ODI of 0.5; both the tests showed negative result in one case. The overall quantitative correlation between the ODI calculated by GM-LFA and GM-EIA was strong (Spearman ρ = 0.777 p = 0.01).
Discussion
In our prospective multicenter study, the effectiveness of GM-LFA in diagnosing IA and the correlation of GM-EIA with GM-LFA were evaluated. GM-LFA was found to have high efficiency and high IA discrimination power. Considering the categorical correlation between GM-LFA and GM-EIA at an ODI cut-off of 0.5, an almost perfect agreement was found. In a previous single-center, prospective study evaluating the performance of serum GM-LFA in patients with hematological malignancies, the SEN, SPE, NPV, and PPV of GM-LFA were 49%, 95%, 90%, and 69%, respectively, for proven/probable IA versus controls [6]. In a previous retrospective cohort study, the SEN and SPE of serum GM-LFA at 0.5 ODI were 85% and 72%, respectively, in patients with hematological malignancies [11]. In a previous retrospective case–control study in which 82% of cases involved patients with hematological malignancies, the SEN, SPE, PPV, and NPV of serum GM-LFA at 0.5 ODI were found as 96.9%, 98%, 93.9%, and 99%, respectively, and the qualitative agreement between GM-LFA and GM-EIA was 89% [5]. Another multicenter retrospective study that analyzed BAL samples from 235 hematology patients, including 75 with probable or proven IPA, found SEN of 83% and SPE of 87% [10]. Studies evaluating the performance of serum and BAL GM-LFA in diagnosing IA in patients with hematological malignancies are summarized in Table 3. In our study, serum GM-LFA and GM-EIA showed positive finding above 1.0 ODI in five of the six proven IA cases, and the SEN was 83.3% at 0.5 ODI. One patient with proven IA with GM-LFA- and GM-EIA-negative findings for serum samples had GM-LFA and GM-EIA-positive findings for BAL samples (> 1.0), and Aspergillus flavus was detected in a lung biopsy culture, and the patient received empirical L-AmB treatment for over a week on day 0. When the proven and probable cases were evaluated together, the SEN of GM-LFA decreased to 75% (PPV, 100%; NPV, 92.6%). Six patients with probable IA had serum GM-LFA and GM-EIA levels less than 0.5; they were diagnosed with probable IA based on microbiological findings from respiratory samples. In the possible IA group, the GM-LFA score of five patients was above 0.5 ODI (0.51–1.14), while the GM-EIA score was 0.53–0.76 in four of them. Since no other microbiological data supported the diagnosis of probable IA in these four patients, they were accepted as possible IA cases according to the EORTC/MSGERC criteria. In one possible IA case, the serum GM-LFA score was 0.51, and the GM-EIA score was 0.2.
Table 3.
Main characteristics of studies evaluating GM-LFA performance for the diagnosis of IA in patients with hematological malignancies
| Study | Patients | Diagnostic standard | Sample source | Sample processing | Sample size | Proven and Probable IA | Sensitivity | Specificity | Antifungal prophylaxis/ therapy |
|---|---|---|---|---|---|---|---|---|---|
| Jenks et al.[8] | HM | 2008 modified EORTC/MSG | BAL | Retrospective | 24 | 9 | 89% | 88% | Yes, mold-active (42% of patients) |
| Jenks et al.[9] | HM, SOT, ICU | 2019 EORTC/MSG | BAL | Retrospective | 295 (HM:104) | 58 (HM:35) | Overall: 89%HM: 89% | Overall: 44%HM: 54% | Yes, mold active (29% of patients with HM) |
| Hoenigl et al.[11] | HM, CPD, SOT, various underlying diseases | 2019 EORTC/MSG and 2020 ECMM/ISHAM (CAPA) | Serum | Retrospective | 122 (HM:55) | 28* (HM:20*) | Overall: 79% HM: 85% | Overall: 80% HM: 72% | Yes, mold active (38% of all patients) |
| Mercier et al.[6] | HD | 2019 EORTC/MSG | Serum | Prospective | 239 | 41 | 49% | 95% | Yes, fluconazole (all patients) |
| Mercier et al.[10] | HD | 2008 modified EORTC/MSG | BAL | Retrospective | 235 | 75 | 83% | 87% | Yes, mold-active (6.4% of patients) |
| Serin et al.[7] | HM | 2019 EORTC/MSG | Serum | Prospective | 87 | 65 | 90.9% | 90.8% | NA for prophylaxis; no therapy |
| Arkell et al.[12] | HM, RT, various underlying diseases | 2019 EORTC/MSG | BAL | Prospective | 92 (HM:49) | 15 (HM:10) | Overall: 67% HM: 70% | Overall: 82% HM: 70% | Yes, mold active (47% of all patients) |
| White et al.[5] | HM, various underlying diseases | 2019 EORTC/MSG | Serum | Retrospective | 135 cases, 179 samples (HM:82.2% of patients) | 27 cases | Overall: 96.9% | Overall: 98% | NA |
| Linder et al.[19] | HM, SOT, various underlying diseases | 2019 EORTC/MSG | BAL | Retrospective | 40 (HM:9) | 20 (HM:5) | Overall: 40% | Overall: 80% | Yes, mold active (only one patient) |
BAL: bronchoalveolar lavage, CAPA: COVID-19-associated pulmonary aspergillosis, CPD: chronic pulmonary diseases, ECMM/ISHAM: European Confederation of Medical Mycology/International Society for Human and Animal Mycoses, EORTC/MSG: European Organization for Research and Treatment of Cancer/Mycoses Study Group, HD: hematological diseases, HM: hematological malignancy, IA: invasive aspergillosis, ICU: intensive care unit, RT: renal transplantation, SOT: solid organ transplant.
*Proven or probable IA/CAPA
The SEN of serum GM-EIA may decrease under mold-active antifungal treatment or prophylaxis, as reported in a previous study [17]. On the other hand, GM-EIA may also be helpful in the diagnosis of breakthrough IA in patients receiving mold-active treatment or prophylaxis [18]. In the subgroup analysis performed by excluding patients receiving mold-active antifungal treatment or prophylaxis in our study, the SEN and NPV of GM-LFA were 76.2% and 93.3%, respectively (Supplementary Tables 5–6), indicating that the performance of GM-LFA was not affected by antifungal prophylaxis or treatment.
GM-EIA uses a mouse monoclonal antibody (EB-A2) to detect GM antigens, and GM-LFA uses a mixture of two monoclonal antibodies [5, 19]. Considering the quantitative correlation between GM-LFA and GM-EIA in our study, a moderate positive correlation was noted for all patients (Spearman’s ρ = 0.511, p = 0.01) and a strong correlation was noted for the proven/probable IA cases (Spearman’s ρ = 0.834 p = 0.01). Mercier et al. reported a weak correlation between the two tests for serum samples (adjusted R2 0.446, p < 0.001), whereas White et al. reported a moderate correlation (Spearman ρ = 0.64 p < 0.0001) [5, 6].
Neutrophil count is another important factor affecting the serum GM levels and the SEN of GM-EIA. In a study investigating the correlation between neutrophil count and serum GM-EIA antigen level in patients with hematological malignancies diagnosed with proven/probable IA, patients with a neutrophil count < 100/mm3 had significantly higher serum GM levels than patients with neutrophil count ≥ 100/mm3 [20]. In our study, 62 (36.3%) of all patients (18 cases of proven/probable IA) were neutropenic at day 0 (< 500/µL). In the proven/probable IA group, there was no significant difference between the mean GM-LFA ODI of neutropenic and non-neutropenic patients (p = 0.96), nor was there any significant difference between the mean GM-EIA of these patient groups (p = 0.44). (Supplementary Table 7). Among patients with proven/probable IA, those with and without neutropenia had GM-LFA SEN of 77.8% and 70.0%, respectively, and both these groups had SPE of 100% at 0.5 ODI. In the subgroup analysis of 19 proven/probable cases, GM-LFA provided results on day 0, whereas GM-EIA provided results on days 0–4, suggesting that GM-LFA is an important test for the rapid diagnosis of aspergillosis.
This study has some limitations. One is that BAL samples were obtained from only 28 patients. The limitation of bronchoscopic procedures during the COVID-19 pandemic and the difficulty in obtaining tissue biopsy samples from patients with hematological malignancies have resulted in case classification to be made mostly according to the GM-EIA results. For the condition to be classified as probable IA according to the EORTC/MSG criteria, the GM-EIA ODI must be ≥ 1. In our study, the GM-EIA ODI was 0.5–1 in four cases (all with positive GM-LFA levels). The condition in these cases was considered as possible IA, as no other supporting mycological evidence existed.
In conclusion, GM-LFA is a promising, easy-to-perform, and rapid diagnostic test compared with GM-EIA for the diagnosis of IA in patients with hematologic malignancies. It has a high NPV, high diagnostic accuracy, and excellent agreement with the GM-EIA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge Neutec Pharmaceutical Turkey for donating the lateral flow assay kits for this study.
Author contributions
O.A. and Z.O. designed the study. O.A., R.S., E.H.A., Z.T.Y. and A.F.Y. collected the patients’ data. B.D., B.E., H.N.K and M.M.G. performed the LFA procedure. O.A. and Z.O. analyzed the data. O.A. and Z.O. wrote the paper with input from all authors. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest
All authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Ethics approval and patient consent statement
This prospective study was approved by the Marmara University Clinical Research Ethics Committee (protocol number: 09.2019.796). The patients or their relatives provided informed consent before beginning of the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lass-Flörl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses. 2009;52(3):197–205. doi: 10.1111/j.1439-0507.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- 2.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45(9):1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 3.Leeflang MMG, Debets-Ossenkopp YJ, Wang J, et al. (2015) Galactomannan detection for invasive aspergillosis in immunocompromised patients. The Cochrane database of systematic reviews 12: CD007394. [DOI] [PMC free article] [PubMed]
- 4.Heldt S, Prattes J, Eigl S, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Infect. 2018;77(3):235–241. doi: 10.1016/j.jinf.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White PL, Price JS, Posso R, Cutlan-Vaughan M, Vale L, Backx M. Evaluation of the performance of the IMMY sona Aspergillus galactomannan lateral flow assay when testing serum to aid in diagnosis of invasive aspergillosis. J Clin Microbiol. 2020;58(6):e00053–e120. doi: 10.1128/JCM.00053-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercier T, Guldentops E, Lagrou K, Maertens J. Prospective evaluation of the turbidimetric β-D-glucan assay and 2 lateral flow assays on serum in invasive aspergillosis. Clin Infect Dis. 2021;72(9):1577–1584. doi: 10.1093/cid/ciaa295. [DOI] [PubMed] [Google Scholar]
- 7.Serin I, Dogu MH. Serum Aspergillus galactomannan lateral flow assay for the diagnosis of invasive aspergillosis: a single-centre study. Mycoses. 2021;64(6):678–683. doi: 10.1111/myc.13265. [DOI] [PubMed] [Google Scholar]
- 8.Jenks JD, Mehta SR, Taplitz R, Law N, Reed SL, Hoenigl M. Bronchoalveolar lavage Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test for diagnosis of invasive pulmonary Aspergillosis in patients with hematological malignancies. J Infect. 2019;78(3):249–259. doi: 10.1016/j.jinf.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Jenks JD, Prattes J, Frank J, et al. Performance of the bronchoalveolar lavage fluid Aspergillus galactomannan lateral flow assay with cube reader for diagnosis of invasive pulmonary aspergillosis: a multicenter cohort study. Clin Infect Dis. 2021;73(7):e1737–e1744. doi: 10.1093/cid/ciaa1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercier T, Dunbar A, De Kort E, et al. Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: a comparative multicenter study. Med Mycol. 2020;58(4):444–452. doi: 10.1093/mmy/myz079. [DOI] [PubMed] [Google Scholar]
- 11.Hoenigl M, Egger M, Boyer J, Schulz E, Prattes J, Jenks JD. Serum lateral flow assay with digital reader for the diagnosis of invasive pulmonary aspergillosis: a two-centre mixed cohort study. Mycoses. 2021;64(10):1197–1202. doi: 10.1111/myc.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arkell P, Mahboobani S, Wilson R, et al. Bronchoalveolar lavage fluid IMMY Sona Aspergillus lateral-flow assay for the diagnosis of invasive pulmonary aspergillosis: a prospective, real life evaluation. Med Mycol. 2021;59(4):404–408. doi: 10.1093/mmy/myaa113. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The package insert of sōna Aspergillus galactomannan LFA (IMMY N, Oklahoma, USA) for the detection of Aspergillus galactomannan, 2021. (https://www.immy.com/asp). Accessed February 1, 2023.
- 15.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 17.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40(12):1762–1769. doi: 10.1086/429921. [DOI] [PubMed] [Google Scholar]
- 18.Hoenigl M, Seeber K, Koidl C, et al. Sensitivity of galactomannan enzyme immunoassay for diagnosing breakthrough invasive aspergillosis under antifungal prophylaxis and empirical therapy. Mycoses. 2013;56(4):471–476. doi: 10.1111/myc.12060. [DOI] [PubMed] [Google Scholar]
- 19.Linder KA, Kauffman CA, Miceli MH. Performance of Aspergillus galactomannan lateral flow assay on bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis. J Fungi. 2020;6(4):297. doi: 10.3390/jof6040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordonnier C, Botterel F, Ben Amor R, et al. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin Microbiol Infect. 2009;15(1):81–86. doi: 10.1111/j.1469-0691.2008.02122.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.