Abstract
Positron emission tomography (PET) constitutes a functional imaging technique that is harnessed to probe biological processes in vivo. PET imaging has been used to diagnose and monitor the progression of diseases, as well as to facilitate drug development efforts at both preclinical and clinical stages. The wide applications and rapid development of PET have ultimately led to an increasing demand for new methods in radiochemistry, with the aim to expand the scope of synthons amenable for radiolabeling. In this work, we provide an overview of commonly used chemical transformations for the syntheses of PET tracers in all aspects of radiochemistry, thereby highlighting recent breakthrough discoveries and contemporary challenges in the field. We discuss the use of biologicals for PET imaging and highlight general examples of successful probe discoveries for molecular imaging with PET – with a particular focus on translational and scalable radiochemistry concepts that have been entered to clinical use.
Subject terms: Medicinal chemistry, Radionuclide imaging
Positron emission tomography is widely used to diagnose and monitor different disease states and interest in the technique has led to the demand for the development of new method for radiolabelling. Here the authors review the recent progress in the development of new PET probes.
Introduction
Translational molecular imaging has facilitated the diagnosis and management of numerous pathologies and has become an integral part of daily clinical routine—with significant implications in oncology, cardiology, and neurology1–3. In contrast to structural and anatomical imaging, molecular imaging provides functional information via the interaction between a targeted probe and the biological system. In nuclear medicine, the molecular probe constitutes a radionuclide-bearing radiopharmaceutical, frequently referred to as a tracer. From an imaging point of view, the field of nuclear medicine is composed of two mechanistically distinct, albeit somewhat comparable imaging techniques, namely positron emission tomography (PET) and single-photon emission computed tomography (SPECT). While the former imaging modality exploits coincidence detection of colinear annihilation photons, the latter is based on the detection of single gamma rays. Accordingly, although PET and SPECT probes are both generated via the incorporation of a radionuclide within a biomolecule, PET radionuclides emit positrons while SPECT radionuclides are γ-emitters. PET and SPECT are non-invasive techniques that provide information on a molecular and cellular level, which makes them particularly valuable for the detection of biochemical changes at an early stage of disease development, often before clinical symptoms are evident4.
Positron Emission Tomography (PET)
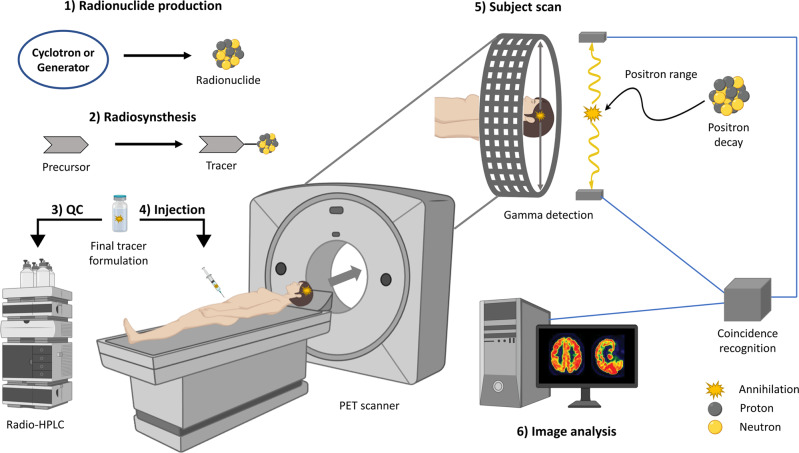
Given the excellent sensitivity and the recent technical advances made in accurate image quantification, PET has been widely used for disease staging and drug development purposes4–7. Notably, only trace amounts of the radioactive probe are typically necessary to obtain high-quality images. Due to this remarkable sensitivity, PET radioligands have the capability to probe biological processes without eliciting pharmacological activity8. The principle of PET imaging is illustrated in Fig. 1. In the initial step, PET radionuclides are typically generated via nuclear reactions within a cyclotron or by specific decay mechanisms from a generator. The resulting radionuclides are incorporated through a variety of available radiochemical reactions into molecules, thus yielding the desired tracers. Quality control (QC) measures, according to legal requirements and monographs in the respective Pharmacopoeias (e.g., US and European Pharmacopoeia), are undertaken to ensure proper radiopharmaceutical quality for intravenous injection, before the tracer is administered to the patient. The patient is subsequently scanned in a round-shaped scanner, where quantification of the radioactive signal provides information on the annihilation site, and thereby, the tracer localization at different organs. Importantly, the emitted positron travels a certain distance (positron range), depending on its energy, and undergoes inelastic collisions until the kinetic energy is so low that the timely overlap with an electron and annihilation is possible. Annihilation typically converts the combined mass into electromagnetic energy that can be in the form of two gamma rays released in opposite directions. Spatial image resolution is inherently limited by the positron range of a given PET radionuclide. Coincidences of photons are detected by the full-ring array of detectors of the PET camera and annihilation sources are reconstructed. Indeed, through distinct algorithms, PET data can be reconstructed into the spatial distribution of a given probe. Major challenges in contemporary PET image reconstruction include the correction for scattering and random coincidence events as well as distinct signal attenuation through different body tissues. As such, PET imaging is routinely combined with computed tomography for anatomical orientation and attenuation correction9.
Fig. 1. Principle of positron emission tomography (PET) imaging.
1) Radionuclide generation. 2) Tracer synthesis. 3) Quality control (QC). 4) Intravenous tracer injection. 5) PET scan: positron decay, annihilation, and coincidence detection. 6) Image analysis and data quantification. Created with BioRender.com.
Among the various existing positron-emitting nuclides (Table 1), fluorine-18 (18F) is the most widely used radionuclide for PET due to its unique advantages over other PET nuclides. These advantages include (1) the low positron range of <1 mm10, which allows the generation of high spatial resolution images, (2) the clear positron emission profile comprising 97% positron emission and 3% electron capture (EC), and (3) the optimal physical half-live of 109.8 min that enables off-site use in satellite facilities without a cyclotron11. Notwithstanding the favorable physical properties of 18F, fluorine atom is typically not a constituent of most biomolecules and hence, frequently introduced via bioisosteric substitution of a hydrogen atom or hydroxyl functionality5. These substitutions are not associated with significant steric effects; however, electron-withdrawing fluorine atoms may substantially affect pharmacokinetic and pharmacodynamic properties of PET radioligands12,13. Given that carbon atoms are encountered in virtually every biomolecule, carbon-11 (11C) labeling is frequently considered a useful alternative to circumvent the need for bioisosteric substitution, despite the relatively short half-life and higher positron range as compared to 18F. Indeed, remarkable advances in 11C-labeling strategies have enabled the labeling of several functional groups, thereby substantially expanding the chemical space of substrates amenable to PET. Besides fluorine-18 and carbon-11, there is a number of other commonly used PET radionuclides (Table 1). In this review, we will summarize commonly used radiolabeling strategies in PET radiochemistry, thereby focusing on recent advances8,14 with fluorine-18, carbon-11, nitrogen-13, oxygen-15, and other PET radiohalogens, as well as chelator-based radiometal chemistry. Breakthrough examples of PET ligands with clinical relevance will be discussed. Further, we will elucidate how advances in bioconjugation methods in radiochemistry led to novel opportunities for the use of biologicals in molecular imaging with PET.
Table 1.
| Isotope | Half-life | Productiona | Mode of decay | Common source |
|---|---|---|---|---|
| 18F | 109.8 min | 18O(p,n)18F | β+ (97%), EC (3%) | Cyclotron |
| 11C | 20.4 min | 14N(p,α)11C | β+ (100%) | Cyclotron |
| 13N | 10 min | 16O(p,α)13N | β+ (100%) | Cyclotron |
| 15O | 2 min | 15N(p,n)15O | β+ (100%) | Cyclotron |
| 124I | 4.2 d | 124Te(p,n)124I | β+ (23%), EC (77%) | Cyclotron |
| 44Sc | 4.0 h | 44Ti/44Sc | β+ (94%), EC (6%) | Generatorb |
| 64Cu | 12.7 h | 64Ni(p,n)64Cu | β+ (17%), EC (44%), β- (39%) | Cyclotronc |
| 68Ga | 67.7 min | 68Ge/68Ga | β+ (89%), EC (11%) | Generatord |
| 82Rb | 1.3 min | 82Sr/82Rb | β+ (95%), EC (5%) | Generator |
| 86Y | 14.7 h | 86Sr(p,n)86Y | β+ (32%), EC (68%) | Cyclotron |
| 89Zr | 78.4 h | 89Y(p,n)89Zr | β+ (23%), EC (77%) | Cyclotron |
PET tracers as diagnostic biomarker and PK/PD tool in drug discovery
Over the past decades, PET imaging has substantially gained attention and is increasingly used not only as a preclinical and clinical research tool but also in routine clinical diagnosis. As such, PET is ideally suited for visualizing molecular and cellular events that occur early in the course of a disease or following therapeutic intervention9. Further, depending on the applied tracer, PET can be used to gain prognostic information, offering a valuable risk-stratification tool in clinical practice, as it is currently performed with various probes in atherosclerotic inflammation imaging2,15.
In addition to its role in disease diagnosis and therapy monitoring, PET has been established as a vital tool that is being used at various stages of modern drug development. As such, PET can be applied for target validation studies by assessing the target protein expression and localization at different disease stages6. Further, lead compound optimization is typically conducted by in vivo assessment of pharmacokinetic and pharmacodynamic drug properties. Proof of target engagement, brain penetration, and potential differences between patients and healthy individuals are of particular interest in PET studies. If amenable to labeling with a PET nuclide, drug candidates can be directly radiolabeled and injected into living organisms, thereby yielding information on drug pharmacokinetics and metabolic stability through blood sampling or post-mortem analysis. This approach is limited by the costly, and in some cases challenging, development of a suitable radiosynthesis for every drug candidate to be tested. As an alternative, promising compounds in development can be challenged by competitive target engagement studies against an established PET probe that shares the same binding site. The advantage of the latter method is that multiple compounds can be effectively screened against one single probe, thereby addressing the following questions: (1) Were the right patients selected to test a given hypothesis and which patient populations are most likely to benefit from this trial (patient stratification)? In many cases, obtaining histological evidence from biopsies is not feasible, whereas a non-invasive assessment of target abundancy in the organ of interest by PET may substantially improve patient selection. (2) Does the drug reach the desired target site and what exposure can be achieved (target occupancy at a given dose)? In addition, while histological assessment may be hampered by protein degradation outside of the human body, and only covers a limited area of the target tissue, PET provides real-time information, allowing whole-body imaging. Obtaining such information at early stage is of paramount value to improving clinical trials and establishing an efficient drug development process. Other applications include the use of PET as accompanying tests for precision therapies, whereas PET probes are frequently employed as basic and clinical research tools to study disease pathophysiology. Indeed, considering the quantitative nature of PET, non-invasive studies to elucidate disease mechanisms and pathophysiology are feasible not only in animal models but also in human subjects, as shown by various recent clinical studies16–19. The translational value of PET is further corroborated by the ability to directly monitor disease progression on a molecular level, thereby providing a unique functional readout that is complementary to structural and morphological information typically detected by conventional imaging modalities.
Translational Radiochemistry
The successful clinical translation of a PET tracer depends not only on its biological characteristics but also on the implementation of practical radiolabeling solutions that allow fully automated tracer synthesis under required quality assurance levels (e.g., GMP, cGRPP). Indeed, the selection of PET nuclide and radiosynthetic approach merits careful considerations since synthetic conditions deemed appropriate for research applications may not be suitable to deliver pharmaceutical-grade tracers. Potential issues may arise from the lack of precursor stability at ambient temperature, the use of metal catalysts that may exert toxicity if injected into humans, synthetic steps that require manual intervention, volatile solvents with volume reproducibility issues, radioactive and non-radioactive byproducts that co-elute with the tracer, lengthy multistep syntheses that do not yield sufficient radioactivity or insufficient molar radioactivity levels at the end of synthesis, and the lack of tracer stability in the final formulation. The concept translational radiochemistry is introduced herein to emphasize the unique characteristics of PET chemistry aimed at human translation and routine radiopharmaceutical production. To qualify any novel radiochemical method from benchtop discovery as translational radiochemistry, we evaluate it not only by its radiochemical yield and molar activity, but also by the feasibility and translatability of automation or remotely-controlled radiosynthesis in a hot cell, reproducibility and regulatory compliance in a clinical production/manufacturing environment (e.g., cGMP process) with the operation, participation, and approval of a diverse team, including cyclotron engineers, radiochemists, QC/QA analysts, formulation specialists, radiopharmacists, and nuclear radiologists. As such, translational radiochemistry is refined as scalable, robust, and reproducible PET chemistry that consistently provides high-quality radiopharmaceuticals, meeting a predefined set of specifications for human use, which is critical to protect patients from unnecessary toxicities and ensure the best possible diagnostic care.
PET chemistry of carbon-11, nitrogen-13 and oxygen-15
Carbon-11
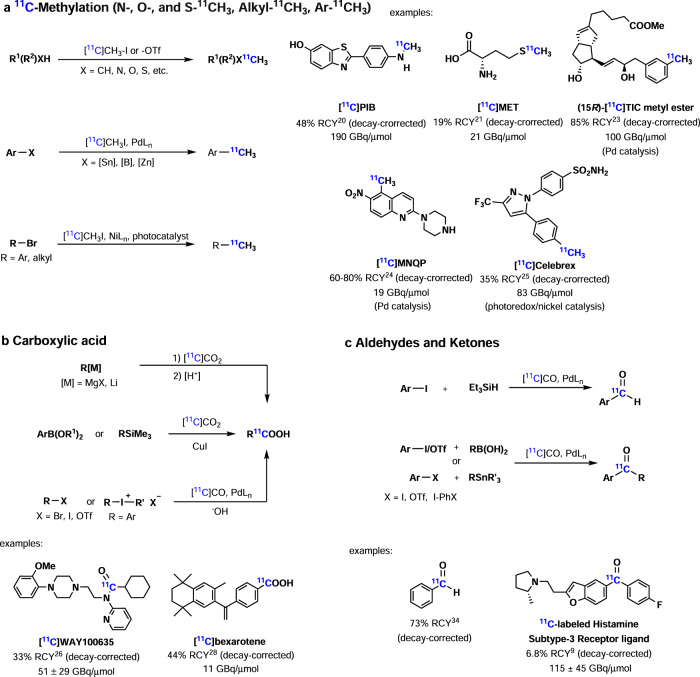
Given that carbon atoms are encountered in the vast majority of biologically active molecules, increasing efforts have been devoted to advancing carbon-11 (11C, half-life t1/2 = 20 min) labeling strategies, thus yielding a plethora of [11C]methyl-, [11C]carbonyl-, [11C]cyano-, or 11C-trifluoromethyl-labeled PET radiopharmaceuticals. The most frequently used 11C-labeling methods are 11C-methylation of amines, alcohols, thiols, carboxylates, or amides with [11C]CH3I or more reactive [11C]CH3OTf to provide N-, O-, S-, or in some cases C(sp2)-[11C]methyl-substituted products (Fig. 2a). Due to the commercial availability of modules that allow reliable production of [11C]CH3I and [11C]CH3OTf, as well as the simple labeling conditions, methylation with [11C]CH3I or [11C]CH3OTf is generally considered useful for automated synthesis not only for preclinical applications, but also in a GMP setting. A large number of PET tracers, including the clinically used [11C]PIB20 (a beta-amyloid radioligand) and [11C]MET21 (a tracer for amino acid transporters), were prepared using this method (Fig. 2a). In addition, Billard and co-workers reported the direct 11C-methylation of amines with [11C]CO2 under reductive conditions (ZnCl2, IPr, [3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene], and PhSiH322. Notably, Palladium-mediated cross-coupling reactions with [11C]CH3I, including Stille coupling, Suzuki coupling, and Negishi coupling, provide access to 11C-methylated aromatics, such as (15R)-[11C]TIC methyl ester23 (a prostacyclin receptor radioligand) and [11C]MNQP24 (a serotonin transporter radioligand). Recently, MacMillan and co-workers developed the metallaphotoredox 11C-methylation of alkyl and aryl bromides with [11C]CH3I25. Both alkyl and aryl bromides can be 11C-methylated by photoredox/nickel dual catalysis under mild conditions.
Fig. 2. Carbon-11 chemistry.
a 11C-methylation with [11C]CH3I or [11C]CH3OTf (N-, O-, S-, C-11C-methylation). b Synthesis of [11C]carboxylic acids. c Synthesis of [11C]aldehydes and [11C]ketones.
When there is no suitable methyl group for 11C-labeling in the target molecule, alternative 11C-labeling strategies are employed, such as 11C-carbonylation. Carbonyl groups are commonly found in biologically active molecules, such as carboxylic acids, aldehydes, ketones, esters, amides, carbamates, and ureas. For [11C]carboxylic acids (Fig. 2b), one classic preparation method is the fixation of [11C]CO2 with strong basic Grignard or organolithium reagents. A representative example is the preparation of [carbonyl-11C]WAY10063526 (a radioligand for imaging 5-HT1A receptor), which has been used for clinical research. The high reactivity of these organometallic reagents limits the potential scope of the method, and these organometallic reagents readily absorb (12C)CO2 from the atmosphere, thereby leading to low molar activity of the tracer. Transition metal-catalyzed coupling reactions in the presence of [11C]CO and [11C]CO2 were efficiently employed to introduce [11C]carbonyl groups into various organic compounds. Indeed, Riss and co-workers reported a Cu-mediated carboxylation of boronic acid esters with [11C]CO2 in 201127, whereas Vasdev, Liang and co-workers used this method in the synthesis of [11C]bexarotene (a retinoid X receptor agonist), with which PET/MR imaging was successfully performed in nonhuman primates28. Recently, Gee, Bongarzone and co-workers developed a Cu-mediated carboxylation of trialkoxysilanes and trimethylsilane derivatives with [11C]CO2 to afford [11C]carboxylic acids29. In addition, Karimi and co-workers reported on the Pd-mediated carboxylation of aryl halides/triflates and benzyl halides using [11C]CO in the presence of tetrabutylammonium hydroxide or trimethylphenyl-ammonium hydroxide, respectively30. These reactions were conducted in a micro-autoclave at 180–190 °C for 5 min using high pressure (35 MPa). Similarly, Telu and co-workers reported a novel Pd-mediated carbonylation of aryl(mesityl)iodonium salts with [11C]CO as an efficient approach to obtain [11C]arylcarboxylic acids in generally good to high yields (up to 71%)31. Recently, Lundgren, Rotstein, and co-workers reported the carbon isotope exchange of carboxylic acids via decarboxylation/11C-carboxylation enabled by organic photoredox catalysis32. This reaction provides a mild and rapid method for direct carboxylate exchange, but the molar activity is low. For example, [11C]Fenoprofen could be prepared through this method in 9.5% decay-corrected RCY (radiochemical yield33, the amount of activity in the product expressed as the percentage (%) of starting activity) with a molar activity of 0.029 GBq/μmol.
For [11C]aldehyde and [11C]ketone (Fig. 2c), Dahl and co-workers described a Pd-mediated 11C-carbonylation protocol of aryl halides or aryl triflates with [11C]CO using xantphos as the supporting catalyst ligand to afford [11C]aldehyde, [11C]ketone, [11C]carboxylic acid, [11C]ester, and [11C]amides34. Notably, [11C]formaldehyde proved to be a useful building block in the synthesis of 11C-labeled compounds via electrophilic aromatic substitution, Mannich-type condensations or cyclization reactions. In 2008, Hooker and co-workers reported a simple, accessible, and high-yielding (up to 89% radiochemical conversion (RCC)) method for the production of [11C]formaldehyde by converting [11C]methyl iodide to [11C]formaldehyde via trimethylamine N-oxide under mild conditions35. There are also other methods to access [11C]ketones, such as Pd-mediated 11C-carbonylative cross-coupling of alkyl/aryl iodides or diaryliodonium salts with organostannanes, aryl iodide or aryl triflates with boronic acids8.
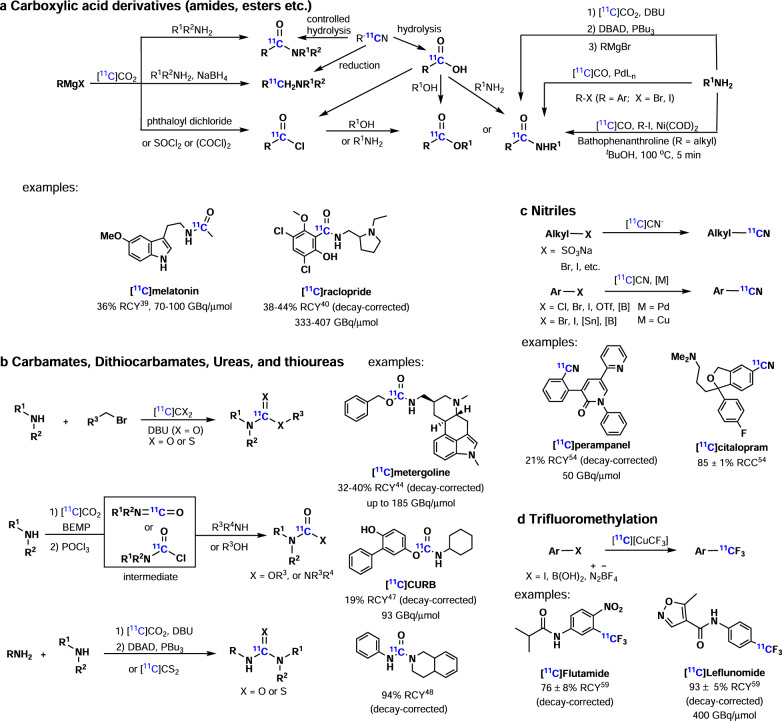
[11C]carboxylic acid derivatives (Fig. 3a), such as [11C]amides, [11C]esters, [11C]amines, and [11C] acyl chlorides, can be easily obtained from [11C]carboxylic acids or [11C]nitriles. Among carboxylic acid derivatives, amides and esters are commonly encountered in biologically active molecules. Accordingly, several methods have been developed for their syntheses. Traditionally, [11C]amides could be obtained from [11C]carboxymagnesium halides and amine, and [11C]amides and [11C]esters could be prepared from [11C]acyl chlorides36–38. In 2017, Gee, Bongarzone and co-workers reported the rapid one-pot synthesis of [11C]amides from primary or activated aromatic amines with [11C]CO2 in the presence of Mitsunobu reagents via an isocyanate intermediate, followed by the addition of Grignard reagents39. The respective [11C]amides were generated in moderate RCYs, including [11C]melatonin (a hormone of the pineal gland), which was obtained in 36% RCY and with a molar activity of 70–100 GBq/µmol. Transition metal-mediated carbonylation cross-coupling reactions with [11C]CO provide an alternative pathway to afford a variety of 11C-labeled compounds. The main challenge is the low solubility of [11C]CO in organic solvents. Indeed, several strategies have been introduced to overcome this limitation, including [11C]CO recirculation, high-pressure reactors, continuous-flow microreactors, xenon as carrier gas (the high solubility of xenon gas in organic solvents facilitates the transfer of [11C]CO), [11C]CO-releasing molecules (such as silacarboxylic acids), solvent-soluble adducts of [11C]CO (such as BH3·[11C]CO), and the addition of XantPhos—a hindered bidentate phosphine ligand, found to facilitate the 11C-carbonylation process. Among these improved strategies, Skrydstrup, Antoni and co-workers developed pre-generated Pd-aryl oxidative addition complexes as stoichiometric reagents in carbonylation reactions with [11C]CO to produce structurally challenging, yet pharmaceutically relevant compounds, including [11C]raclopride (a dopamine D2/D3 receptor antagonist), which was obtained in 38–44% RCY (decay-corrected) and with a molar activity of 333–407 GBq/μmol40. The use of pre-generated Pd-aryl complexes were an excellent solution to utilize the oxidative addition process in 11C-carbonylation of structurally challenging substrates. The Pd-mediated 11C-carbonylation reactions provide an alternative access to PET tracers, but the electrophiles used in this reaction were limited to aryl, alkenyl, methyl, or benzyl halides and triflates without β-hydride to avoid the competing β-hydride elimination. In 2016, Rahman and co-workers overcame the limitation and reported the nickel-mediated 11C-aminocarbonylation of non-activated alkyl iodides containing β-hydrogen with [11C]CO at ambient pressure41. Ni(COD)2 and bathophenanthroline were used as pre-catalyst and ligand, respectively. Several aliphatic [11C]amides were furnished via this method in moderate to good yields. Transition metal-mediated 11C-carbonylation was also used to prepare [11C]esters (cf. recent 11C-carbonylation review42); however, the reaction scope was often limited to substrates lacking β-elimination pathway. One approach to extend the scope of accessible labeled esters entails photoinitiated free radical reactions by Långström and co-workers in the synthesis of [11C]carboxylic esters from a variety of primary, secondary, and tertiary alkyl iodides and [11C]CO43.
Fig. 3. Carbon-11 Chemistry part 2.
a Synthesis of [11C]carboxylic acid derivatives ([11C]amides, [11C]esters etc). b Synthesis of [11C]carbamates, [11C]dithiocarbamates, [11C]ureas, and [11C]thioureas. c Synthesis of [11C]nitriles. d 11C-Labeling with [11C]CF3 group.
Due to their in vivo stability, [11C]carbamates, [11C]dithiocarbamates, [11C]ureas, and [11C]thioureas (Fig. 3b) are attractive functional groups for PET imaging ligands. In 2009, Hooker and co-workers reported a one-pot, synthesis of [11C]carbamates from amines and alkyl halides with [11C]CO2, employing DBU as a [11C]CO2 trapping base44. Several [11C]carbamates were synthesized via this method, including [11C]metergoline (antagonist of the serotonin (5HT) receptor), which was obtained in 32–40% RCY (decay-corrected) and a molar activity of up to 185 GBq/μmol. Similarly, primary amines react with [11C]CS2 very rapidly at room temperature to form the dithiocarbamate salts, followed by alkylation with alkyl halides to generate the corresponding [11C]thiocarbamates45. Of note, Miller and co-workers reported the synthesis of [11C]thioureas from rapid reactions between amines and [11C]CS246. Wilson and co-workers reported the synthesis of [11C]carbamates and [11C]ureas with BEMP (tert-butylimino-2-diethylamino−1,3-dimethylperhydro-1,3,2-diazaphosphorine) as an effective [11C]CO2 trapping reagent and with POCl3 as the dehydrating or chlorinating reagent via the intermediate [11C]isocyanate47. A range of unsymmetrical [11C]carbamates and [11C]ureas were prepared in this one-pot, rapid procedure, including [11C]CURB (potent inhibitor of the enzyme fatty acid amide hydrolase), which was obtained in 19% RCY (decay-corrected) and a molar activity of 93 GBq/μmol. Similarly, the synthesis of unsymmetrical [11C]carbamates was also accomplished by Gee and co-workers via [11C]CO2 trapping with DBU in the presence of aliphatic and aromatic amines that reacted with Mitsunobu reagents48. Further, Rh-mediated 11C-carbonylation of azides or diazo compounds with [11C]CO and amines or alcohols via [11C]isocyanate intermediates provided another access to [11C]ureas or [11C]carbamates49. [11C]phosgene has also been used in the synthesis of [11C]ureas and [11C]carbonates (cf. recent [11C]phosgene review50). For example, a series of radiolabeled monoacylglycerol lipase inhibitors [11C]MAGL-051951, [11C]SAR12730352, and [11C]TZPU52 were obtained from [11C]COCl2 in 6–20% RCYs with high molar activity. However, the preparation of [11C]COCl2 often requires special apparatus through the use of chlorine gas and [11C]CCl4 intermediate. Recently, Jakobsson, Pike and co-workers reported [11C]carbonyl difluoride ([11C]COF2) as a novel [11C]carbonyl group transfer agent53. [11C]COF2 was prepared in quantitative yields by passing [11C]CO gas through a AgF2 column at room temperature and then it was employed directly in the synthesis of a wide range of [11C]heterocycles, including [11C](S)-CGP-12177 (a β-adrenoceptor radioligand) and [11C]DMO (a radiotracer for determining tissue pH in vivo).
As nitriles are often found in biologically active molecules and are also used in the synthesis of carboxylic acids, amides, amines, and related derivatives, several 11C-cyanation methods have been developed (Fig. 3c). As such, aliphatic [11C]nitriles can be obtained by nucleophilic substitution with [11C]cyanide ions. The transition-metal-mediated aromatic 11C-cyanation reaction is an attractive approach to prepare aryl [11C]nitriles and has been investigated for more than 20 years. The limitation of these reactions often entails harsh reaction conditions, such as high temperatures, relatively long reaction times, and the use of inorganic bases, which limits the substrate scope. In 2015, Hooker, Buchwald and co-workers reported a rapid Pd-mediated 11C-cyanation of aryl halides or triflates with [11C]HCN and biaryl phosphine as a ligand at room temperature54. The sterically hindered biaryl phosphine ligands facilitated a rapid transmetalation with [11C]HCN and reductive elimination of aryl nitriles, whereby the reactions were completed in 1 min. A range of aryl [11C]nitriles was prepared via this method, including several drugs, such as [11C]perampanel (antiepileptic drug) obtained in 21% RCY (decay-corrected) with a molar activity of 50 GBq/μmol, and [11C]citalopram (antidepressant) obtained in 85% RCC. Recently, Hosoya, Zhang and co-workers developed a Pd-mediated 11C-cyanation of (hetero)arylboronic acids or esters with [11C]NH4CN/NH3 in high RCYs55. This method demonstrated excellent functional group tolerance and wide substrate scope. In addition, Cu-mediated 11C-cyanation provided an alternative access to aryl [11C]nitriles. Initially, Ponchant and co-workers developed the Cu-mediated 11C-cyanation of aryl halides, where [11C]cyanide ions were trapped by copper at high temperatures (180 °C)56. In 2017, Liang, Vasdev and co-workers developed the first Cu-mediated 11C-cyanation of aryl boronic acids in aqueous solutions57. Indeed, a broad range of arylboronic acids was labeled in 9–70% RCYs via this method. In the following year, Scott, Sanford, and co-workers further extended Cu-mediated 11C-cyanations to arylstannanes and other arylboron precursors with Cu(OTf)258.
Trifluoromethyl (CF3) groups can be found in many drugs and potential PET radiotracers. An efficient method to incorporate 11C-labeled trifluoromethyl group ([11C]CF3) with high molar activity is necessary (Fig. 3d). In 2017, Pike and co-workers reported the synthesis of [CF3-11C]trifluoromethylarenes from arylboronic acids, aryl iodides and aryldiazonium salts with [11C]CuCF359. [11C]CuCF3 was prepared from [11C]fluoroform with copper(I) bromide and potassium tert-butoxide, while [11C]fluoroform was generated from cyclotron-produced [11C]methane passing over heated (270 °C) cobalt(III) fluoride (CoF3) in good yield. As [11C]methane can be generated by cyclotron with a high molar activity, a range of [CF3-11C]trifluoromethylarenes could be synthesized via this method with a high molar activity (200−500 GBq/μmol), such as flutamide (an antiandrogen drug) obtained in 76% RCY (decay-corrected) and leflunomide (an antirheumatic drug) obtained in 93% RCY (decay-corrected) with a molar activity of 400 GBq/μmol. Therefore, [CF3-11C]-trifluoromethylation with [11C]CuCF3 from [11C]fluoroform represents an important breakthrough for labeling PET tracers with trifluoromethyl groups with high molar activity.
Nitrogen-13 and Oxygen-15
Nitrogen-13 (13N, half-life t1/2 = 10 min) and Oxygen-15 (15O, half-life t1/2 = 2 min) are short-lived PET radionuclides, rendering them unsuitable for multiple-step radiosynthesis in most scenarios8. Of note, [13N]NH3 is the most important 13N-labeled tracer and it could be produced via the 16O(p,α)13N nuclear reaction60. Currently, [13N]NH3 has been widely used in clinical PET imaging to identify myocardial perfusion defects and assess coronary flow reserve in patients with suspected coronary artery disease. In addition, [13N]NH3 serves as a building block for further 13N-transformations, such as efficient enzymatic preparations of 13N-labeled amino acids from [13N]NH3. [15O]H2O is produced via the 14N(d,n)15O nuclear reaction61, and [15O]H2O is the most widely used 15O-labeled PET tracer and constitutes the current gold standard for cerebral blood flow measurements.
PET chemistry of radiohalogens
Fluorine-18 chemistry
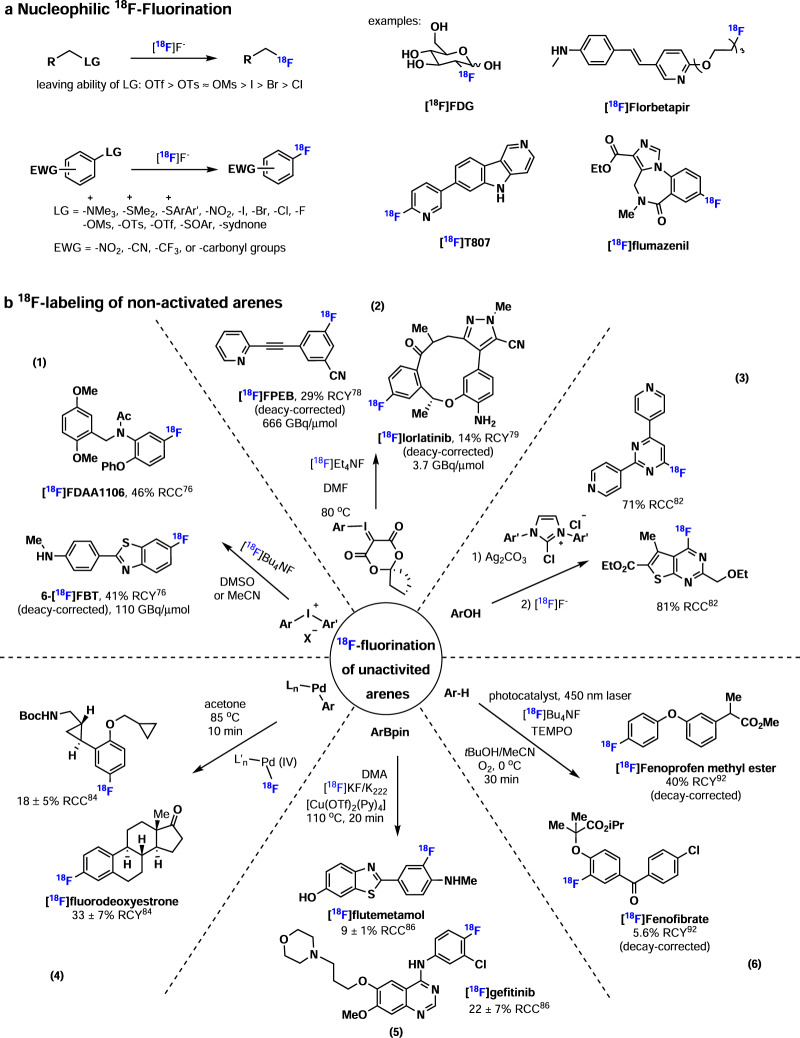
As [18F]fluoride can be produced in almost all cyclotrons62, [18F]fluoride ion is the most common F-18 source to radiochemists, and most 18F-labeling methods start from [18F]fluoride. Indeed, the most frequently used method for the preparation of alkyl [18F]fluorides is aliphatic nucleophilic substitutions involving the displacement of a leaving group (-OTf, -OTs, -OMs, or halides etc.) with [18F]fluoride (Fig. 4a), where two important examples are [18F]FDG and [18F]florbetapir (a tracer for β-amyloid). Generally, nucleophilic 18F-fluorination is conducted in polar aprotic solvents, such as MeCN, DMF, or DMSO, to pursue high 18F-incorporation in a relatively short reaction time (usually ≤30 min). In a counterintuitive manner, Chi and co-workers reported an effective nucleophilic 18F-fluorination of aliphatic mesylates in ionic liquid and protic tert-butyl alcohol63. These reactions showed an increased 18F-fluorination rate and a decreased formation of undesired elimination byproducts. 18F-Fluorination of alcohols commonly involves two steps, including the modification of the hydroxyl group (-OH) to a leaving group (-OTs, -OMs, or -ONs) and the subsequent 18F-fluorination. In 2015, Doyle and co-workers reported PyFluor (2-pyridinesulfonyl fluoride) as a deoxyfluorination reagent for alcohols64. The 18F-deoxyfluorination of protected carbohydrates was achieved with [18F]PyFluor in 15% RCC. In addition, O’Hagan and co-workers reported the enzymatic 18F-fluorination of SAM [(S)-adenosyl-L-methionine], and the following [18F]-5′-FDA ([18F]5′-fluoro-5′-deoxy-adenosine) was obtained in an RCY of up to 95%65. Transition metal-mediated aliphatic 18F-fluorination provides mild conditions to access alkyl [18F]fluorides. As such, Gouverneur, and Nguyen and co-workers successfully developed Pd- and Ir-mediated allyl 18F-fluorination reactions66–68. Further, Groves, Hooker and co-workers reported the Mn-mediated 18F-fluorination of benzylic C−H and non-activated secondary and tertiary C−H bonds69,70. Doyle and co-workers developed the Co-mediated enantioselective aliphatic 18F-fluorination of epoxides71.
Fig. 4. Fluorine-18 chemistry.
a Nucleophilic 18F-Fluorination. b 18F-labeling of non-activated arenes.
Generally, C(sp2)-18F bonds have higher stability than C(sp3)-18F bonds towards in vivo defluorination, and aryl [18F]fluorides are commonly found in a wide range of PET tracers. The most classic method to prepare aryl [18F]fluorides is nucleophilic aromatic substitution (SNAr) with [18F]fluoride, involving the displacement of an appropriate leaving group (-N+Me3, - sulfonium salt, -NO2, -halides, etc.) with [18F]fluoride (Fig. 4a). Ideally, these precursors contain an electron-withdrawing functionality (-NO2, -CN, -CF3 or -C=O etc.) as an activating group in the ortho or para position to the leaving group to facilitate SNAr reactions. Recently, several novel precursors for SNAr reactions with [18F]fluoride have been developed, including triarylsulfonium salts72,73 and N-arylsydnones74.
A main challenge in aromatic 18F-fluorination is the poor reactivity of non-activated or electron-rich arenes, and efforts have been made to address this challenge (Fig. 4b). In 1995, Pike and co-workers reported the 18F-fluorination of diaryliodonium salts under metal-free conditions (Figs. 4b-1)75. In the 18F-fluorination of unsymmetrical diaryliodonium salts, 18F-fluorination preferably occurs in the relatively electron-deficient aryl group. In addition, aryl groups bearing electron-withdrawing ortho substituents are more amenable to radiofluorination. Several electron-rich partner aryl groups were incorporated into unsymmetrical diaryliodonium salts to achieve regioselective 18F-fluorination, including 2-thienyl, 4-methoxyphenyl, 3-methoxyphenyl, 4-methylphenyl and 2,4,6-trimethoxyphenyl. Electron-deficient, -neutral or -rich [18F]fluoroarenes were successfully prepared via this method, including [18F]FDAA110676 (a radioligand for translocator protein), obtained in 46% RCC, and 6-[18F]FBT76 (a radioligand for β-amyloid), obtained in 41% RCY (decay-corrected) with a molar activity of 110 GBq/µmol. In 2014, Liang, Vasdev and co-workers reported the spirocyclic iodonium ylides (SCIDY) as excellent precursors in 18F-fluorination of non-activated arenes (Fig. 4b-2)77. In contrast to diaryliodonium salts, aryliodonium ylides have no counterion and therefore allow convenient purification via normal phase liquid chromatography. In addition, iodonium ylides with a spirocyclic auxiliary are crystalline solids with high stability. A wide range of aryl [18F]fluorides was prepared via 18F-fluorination of spirocyclic iodonium ylides, including the clinically validated PET tracers [18F]FPEB (a tracer for metabotropic glutamate receptor subtype 5), obtained in 29% RCY (decay-corrected) with a molar activity of 666 GBq/µmol78, and [18F]lorlatinib (a ROS1/ALK inhibitor), obtained in 14% RCY (decay-corrected) with a molar activity of 3.7 GBq/µmol79. Notably, Liang, Vasdev and co-workers further developed the second generation of iodonium ylides with a SPIAd (spiroadamantyl-1,3-dioxane-4,6-dione) auxiliary80. SPIAd ylide is more sterically hindered and has excellent stability under labeling conditions. A wide range of heterocycles and drug fragments were 18F-labeled with SPIAd ylides, including the clinically validated [18F]mFBG and [18F]FDPA, the latter of which was obtained in 23% RCY (decay-corrected) with a molar activity of 529 GBq/μmol81. In 2016, Ritter and co-workers reported the 18F-fluorination of phenols involving a concerted nucleophilic aromatic substitution (CSNAr) mechanism, which was different from the classic SNAr reactions via a Meisenheimer intermediate (Fig. 4b-3)82. This reaction was highly effective with electron-deficient phenols. Further, Ritter and co-workers extended the 18F-fluorination of phenols to electron-rich phenols activated by a ruthenium complex83.
Transition metal-mediated 18F-fluorination with an enhanced activity provides a mild approach to radiofluorinate non-activated arenes. In 2011, Ritter, Hooker, and co-workers developed a Pd-mediated aromatic 18F-labeling method using a palladium(IV) [18F]fluoride complex as the electrophilic fluorination reagent (Fig. 4b-4)84. In particular, 18F-fluorination involved reductive elimination of the C−18F bond from a palladium(IV) aryl [18F]fluoride complex intermediate. Subsequently, Ritter and co-workers described a nickel-mediated oxidative radiofluorination approach with a hypervalent iodine oxidant at room temperature, requiring <1 min reaction time85. In 2014, Gouverneur and co-workers described a Cu-mediated 18F-fluorination of aryl boronic esters (aryl-BPin) (Fig. 4b-5)86. As aryl-BPin reagents have high stability to air and moisture, they are considered excellent precursors for 18F-fluorination. Indeed, the latter approach was used to radiofluorinate a large range of electron-neutral, -rich, and -poor arenes, including 18F-gefitinib, obtained in 22% RCC (an inhibitor of the epidermal growth factor receptor−tyrosine kinase), and [18F]flutemetamol (a β-amyloid-targeted probe) obtained in 9% RCC, and clinically validated [18F]FDOPA obtained in 10% RCY (decay-corrected) with a molar activity of 76 GBq/μmol87. Subsequently, Scott, Sanford and co-workers also reported Cu-mediated 18F-fluorination of aryl boronic esters (aryl-BPin) and aryl boronic acids88. Additionally, diaryliodonium salts89 and aryl stannanes90 were found to be useful precursors for Cu-mediated 18F-fluorination, and a range of clinically validated PET tracers was prepared via these methods, such as [18F]SDM-8 (a PET tracer for imaging synaptic density) was obtained from 18F-labeling of aryl stannane precursor in 35% RCY (decay-corrected) with a molar activity of 241.7 GBq/μmol91. Recently, Li, Nicewicz and co-workers developed the aryl C−H 18F-fluorination via organic photoredox catalysis (Fig. 4b-6)92. An organic acridinium-based photocatalyst was used, along with TEMPO as a redox co-mediator. Various electron-rich aromatics were 18F-labeled via this method, including [18F]fenoprofen methyl ester, obtained in 39% RCC, and [18F]fenofibrate (cholesterol-lowering drug), obtained in 5.6% RCY (decay-corrected). In addition, they extended this method to site-selective radiofluorination of C(sp2)–O bond93 and C(sp2)–halide bond94. A broad range of readily available aryl halide and ether precursors could be used in the synthesis of aryl [18F]fluoride via site-selective [18F]fluorination under mild photoredox conditions.
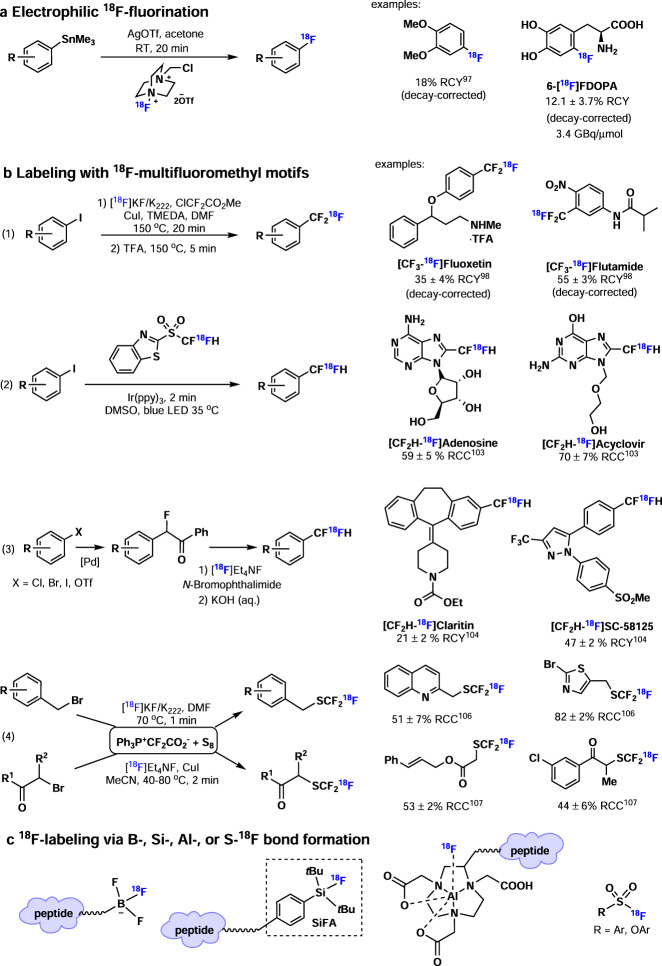
As an electrophilic 18F-fluorination reagent, [18F]F2 can be used as an alternative F-18 source (Fig. 5a). With non-radioactive F2 applied as a carrier gas, the production of [18F]F2 typically yields low molar activities (0.04 to 0.4 GBq/μmol), which limits its widespread use. To overcome this limitation, an improved method was developed, harnessing the gas phase 19F/18F isotopic exchange between [18F]CH3F and [19F]F2 using an electrochemical chamber, and yielding [18F]F2 molar activities of up to 55 GBq/μmol95. However, this method has proven too delicate for widespread use. Poor chemo- and regio-selectivity constitutes another main challenge in 18F-fluorination with [18F]F2 because of its high reactivity. Several mild electrophilic 18F-fluorination reagents have been synthesized from [18F]F2, including [18F]NFSI (N-fluorobenzenesulfonimide) and [18F]Selectfluor. Indeed, [18F]NFSI was synthesized and successfully employed for the enantioselective enamine-mediated 18F-fluorination of aldehydes by Gouverneur and co-workers96. Similarly, [18F]Selectfluor was developed by Gouverneur, Luthra, Solin and co-workers and utilized for the 18F-fluorination of silyl enol ether and electron-rich arylstannanes97—including the synthesis of 6-[18F]FDOPA. However, these approaches still yield tracers with low molar activities, and further work has to be conducted to extend this method for clinically relevant tracer production.
Fig. 5. Fluorine-18 chemistry part 2.
a Electrophilic 18F-fluorination. b Labeling with 18F-multifluoromethyl motifs. c 18F-labeling via B-, Si-, Al-, or S-18F bond formation.
As drugs with multifluoromethyl groups, such as -CF3, -CF2H, and -SCF3, are increasingly being developed, there is a continuous demand for novel methods to 18F-label these multifluoromethyl groups (Fig. 5b). In the early days, halide/18F exchange reactions under harsh reactions were the main approach to 18F-label multifluoromethyl groups. In 2013, Gouverneur and co-workers reported a Cu-mediated 18F-trifluoromethylation of aryl iodides with chlorodifuoroacetate and [18F]fluoride, generating [18F]CuCF3 in situ (Fig. 5b-1)98. Various [18F]trifluoromethyl arenes were successfully prepared via this method, including [CF3-18F]-fluoxetine (antidepressant) and [CF3-18F]flutamide (anti-androgen). In addition, Riss and co-workers reported Cu-mediated 18F-trifluoromethylation using CHF2I to generate [18F]CuCF399. Furthermore, several methods were developed to generate [18F]CuCF3 in a stepwise strategy. [18F]HCF3 was generated from [18F]fluoride and CHF2I100 or difluoromethyl sulfonium salt101 in the first step, then [18F]HCF3 was converted to [18F]CuCF3, which was subsequently used in 18F-trifluoromethylation. Recently, Gouverneur and co-workers further described the radical 18F-trifluoromethylation of unmodified peptides at tryptophan or tyrosine residues using [18F]CF3SO2NH4 that was prepared from [18F]fluoride, PDFA (difluorocarbene), and SO2102. The difluoromethyl group (−CF2H) is also of great importance in biologically active compounds and several methods have been developed to synthesize [18F]difluoromethyl arenes. In 2019, Genicot, Luxen and co-workers reported the 18F-difluoromethylation of heteroaromatics with [18F]difluoromethyl benzothiazole sulfone via organic photoredox catalysis under mild conditions (Fig. 5b-2)103. Representative examples include [CF2H-18F]adenosine and [CF2H-18F]acyclovir, obtained in 59% RCC and 70% RCC, respectively. In addition, Ritter, Vasdev, Liang and co-workers report a practical one-pot method for the synthesis of 18F-difluoromethyl arenes from [18F]fluoride (Fig. 5b-3)104. At the beginning, a benzoyl auxiliary was built on aryl chloride, bromide, iodide or triflate. Then the labeling procedure entails the following steps: C-H bromination, in situ Br/18F exchange, and benzophenone cleavage to generate a range of highly functionalized [18F]difluoromethyl arenes, including [CF2H-18F]Claritin, and [CF2H-18F]SC-58125 obtained in 21% and 47% RCY, respectively. Initially, [18F]trifluoromethylthiol arenes were prepared via halide/18F exchange, whereby silver(I) was used to promote this process. Recently, Gouverneur and co-workers reported the 18F-labeling of unmodified peptides at the cysteine residue via [18F]CF3 group transfer using the [18F]Umemoto reagent105. In 2015, Liang, Xiao and co-workers reported the 18F-trifluoromethylthiolation of alkyl halides via transfer of -SC[18F]F3, generated from difluorocarbene (PDFA), sulfur, and [18F]fluoride under neutral conditions (Fig. 5b-4)106. Of note, [18F]CF3S− was successfully afforded by reacting [18F]CF3− with sulfur. They further extended the scope of 18F-trifluoromethylthiolation reactions to α-bromo carbonyl compounds (Fig. 5b-4)107. Intriguingly, mechanistic studies unveiled that thiocarbonyl fluoride (S=CF2) was generated via sulfuration of difluorocarbene with sulfur (S8), followed by the reaction of S=CF2 with [18F]fluoride to give [18F]CF3S−. The addition of Cu(I) promoted the reactions.
In addition, a number of 18F-labeling methods via B-, Si-, Al-, S-18F bond formation were developed in the past decade and reviewed recently (Fig. 5c)8,108,109. As B-, Si-, and Al-18F bonds have high bond dissociation energies, the 18F-labeling process is efficient in an aqueous solution, which is highly suited for labeling biomolecules for PET imaging studies. Although low pH and/or elevated temperatures are still needed for efficient 18F-labeling, a recent advance has been made to achieve Al-18F efficient labeling at physiological temperatures110. Compared to the C-18F formation via SN2- or SNAr-type reaction, SIV-18F formation has a lower kinetic barrier, thus enabling 18F-labeling at room temperature as evidenced in the work of aryl [18F]fluorosulfates by Wu, Yang, Sharpless and co-workers, including a PARP1-targeted probe for tumor imaging111.
Furthermore, a stepwise and indirect 18F-labeling method via the link 18F-labeled prosthetic group to the target molecule is an attractive approach for labeling well-functionalized molecules109. A range of 18F-labeled prosthetic groups have been developed, such as 2-[18F]fluoroethyl tosylate112, and utilized in the synthesis of PET tracers, such as [18F]FET and [18F]FMeNER-d2 (deuterium atoms are incorporated to enhance the stability of the tracer). More details about the 18F-labeled prosthetic groups and strategies to increase the metabolic stability of radiotracers can be found in some excellent reviews113–115.
Related radiohalogens (Bromine-76 and Iodine-124 chemistry)
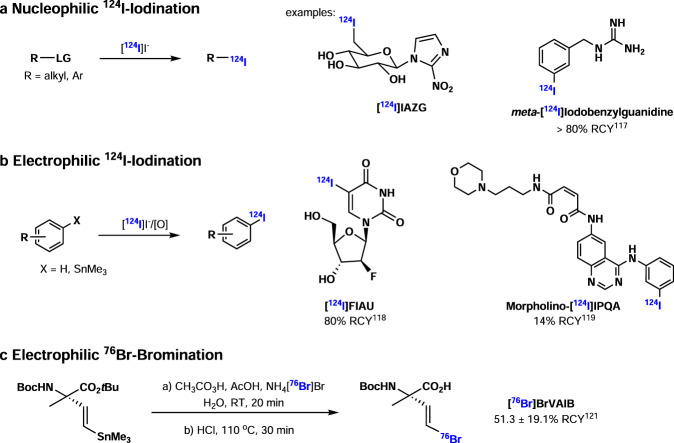
Iodine-124 is usually produced via 124Te(p,n)124I nuclear reaction in the cyclotron and Iodine-124 (124I, t1/2 = 4.2 d) has a relatively long half-life of 4.2 days, which is attractive for investigation of enduring biological processes in vivo. Although the maximum positron energy of 2.14 MeV and the positron intensity of 25% make iodine-124 not an ideal positron emitter, iodine-124 is still a clinically relevant long-lived, non-radiometal nuclide, which enables quantitative PET imaging over several days. The well-established iodine chemistry provides an excellent platform to incorporate 124I into organic compounds. Generally, nucleophilic 124I-iodination reactions can be used to synthesize 124I-labeled alkyl or aryl compounds via SN2 or SNAr mechanism (Fig. 6). For example, the hypoxia imaging agent [124I]IAZA could be generated via a nucleophilic substitution reaction with sodium [124I]iodide116. In recent years, copper (I) salts were found to be an accelerant for halogen-124I exchange in reactions between [124I]iodide with bromo- or iodo-aryls. As an example, meta-[124I]iodobenzylguanidine (MIBG), a norepinephrine transporter (NET) imaging tracer, could be synthesized via copper-mediated nucleophilic halogen exchange reaction with sodium [124I]iodide in a yield over 80%117. Another useful method to synthesize aryl [124I]iodides is electrophilic 124I-iodination with [124I]iodide in oxidative conditions. [124I]Iodide can be easily oxidized in situ to positive iodine species which serve as an electrophile in 124I-iodination of aromatics or aromatic stannyl precursors. A range of 124I-labeled compounds was generated via this method, including [124I]FIAU (2′-fluoro-2′-deoxy-1-β-D-arabinofuranosyl-5-[124I]iodouracil, a herpes virus thymidine kinase (HSV1-tk) PET tracer) obtained in 80% RCY118, and morpholino-[124I]IPQA (EGFR kinase PET tracer) obtained in 14% RCY119. In addition, electrophilic 124I-iodination has also been used in labeling biomolecules, like peptides, antibodies, and proteins, and the presence of tyrosine, histidine, or an aromatic moiety is needed to complete such transformation. Notably, if the pH exceeds 8.5, the 124I-iodination of histidine is preferred120.
Fig. 6. I-124/Br-76 chemistry.
a Nucleophilic 124I-iodination. b Electrophilic 124I-iodination. c Electrophilic 76Br-bromination.
Bromine-76 is often produced via 76Se(p,n)76Br nuclear reaction in the cyclotron and bromine-76 (76Br, t1/2 = 16.2 h) has a medium half-life of 16.2 h, longer than fluorine-18 (t1/2 = 109.8 min) and shorter than iodine-124 (t1/2 = 4.2 d). Bromine-76 has a high positron energy (4 MeV) and its positron emission intensity is 57%. Similar to 124I-iodination reactions, bromine-76 could also be introduced into organic molecules via nucleophilic 76Br-bromination with [76Br]bromide or electrophilic 76Br-bromination with [76Br]bromide under oxidative conditions. In general, [76Br]bromide is more difficult to oxidize than [124I]iodide, so harsher conditions are required for oxidative 76Br-bromination. For example, Lapi and co-workers reported the synthesis (S)-amino-2-methyl-4-[76Br]bromo-3-(E)-butenoic Acid (BrVAIB) via oxidative 76Br-bromination of the corresponding tin precursor in the present of peracetic acid in 51% RCY, and this tracer was evaluated in PET imaging of mice with brain tumor121. Recently, Zhou and co-workers reported copper-mediated 76Br-bromination of aryl boron precursors, and a 76Br-labeled derivative of Olaparib (an inhibitor of PARP-1 (poly (ADP-ribose) polymerase-1)) was synthesized in 99% RCC via this method122.
Clinical examples of small molecule-based PET pharmaceuticals
In this chapter, we will discuss selected examples of PET radioligands that have obtained US Food and Drug Administration (FDA) approval, thereby elaborating on their clinical indications as well as their impact on patient management in clinical routine (Fig. 7). While PET radiopharmaceuticals are of paramount value in both diagnostic imaging and drug discovery123, the majority of contemporary FDA-approved small molecule PET radiopharmaceuticals are labeled with fluorine-18, which is attributed to its optimal decay properties, including a relatively clean positron decay, a short positron range as well as a physical half-life that allows satellite distribution5. Since the first human PET studies in the 1970s, [18F]FDG has been the cornerstone of PET imaging. Indeed, as an imaging biomarker for glucose metabolism, [18F]FDG has been widely used for patient diagnosis, disease staging, and therapy monitoring of various pathologies in oncology, neurology, and cardiology.
Fig. 7. Selected FDA-approved small molecule-based PET radiopharmaceuticals.
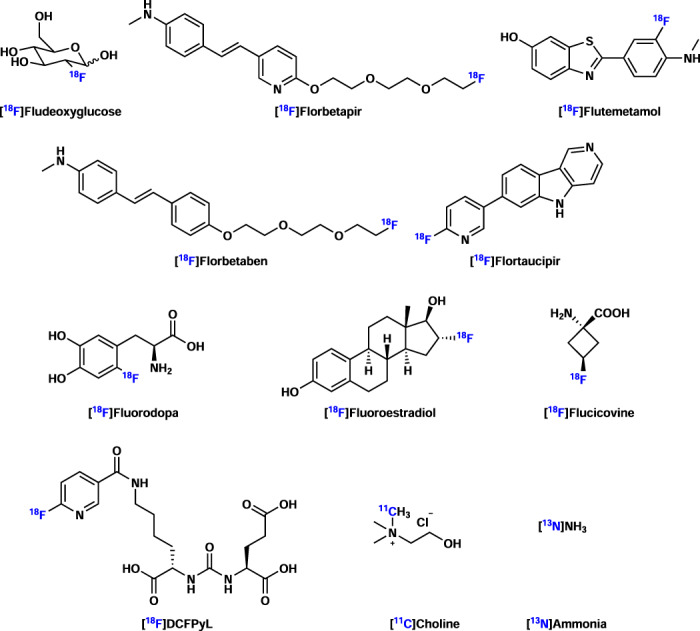
A number of small molecules labeled with F-18, C-11, and N-13 have been approved by FDA and used as PET radiopharmaceuticals.
Although [18F]FDG remains the most widely used PET tracer in the clinic, there is a continuous demand for more specific probes that are highly selective for a given biological target. As such, over the past several decades, PET has provided unique and powerful insights into normal brain function as well as various neurological disorders and neurodegenerative diseases via probes that accumulate at the sites of misfolded protein aggregates. For instance, Alzheimer’s disease (AD) constitutes a progressive neurodegenerative disease that is typically characterized by extracellular amyloid-beta (Aβ) plaques and intracellular neurofibrillary tangles that are formed by misfolded tau aggregates124. Notably, the ground-breaking discovery of Aβ-targeted PET ligand, [11C]PIB, and its successful application for the early diagnosis of AD fueled the development of radiofluorinated analogs, [18F]florbetapir, [18F]flutemetamol, and [18F]florbetaben, all of which obtained FDA approval for the diagnosis of AD between 2012 and 2014. It should be noted that Aβ-targeted PET has substantially improved the diagnostic accuracy (the presence or absence of Aβ in the living brain) of AD in clinical routine, exhibiting a remarkably high predictive value. Similarly, a series of tau-targeted PET tracers have been developed. Among them, [18F]flortaucipir was the first PET tracer approved by the FDA for imaging aggregated tau neurofibrillary tangles in 2020125. It is anticipated that tau pathology can be detected early in the disease course, thus enabling more clinical findings by tau-targeted PET compared to Aβ-targeted PET in the course of AD progression. Parkinson’s disease (PD) is a long-term degenerative disease that mainly affects the motor system. [18F]Fluorodopa ([18F]FDOPA) was approved by FDA for imaging dopaminergic nerve terminals in the striatum of patients with suspected Parkinsonian syndrome in 2019126.
In cardiology, PET imaging is a powerful tool for in vivo evaluation of myocardial function. Among other PET probes, [13N]NH3 was approved by the FDA for rest/stress myocardial perfusion imaging (MPI) to detect ischemic heart disease. The strength of PET-MPI lies in the ability to measure absolute myocardial blood flow at rest and following pharmacologically induced stress, which allows the assessment of perfusion defects in larger coronary arteries, as well as the calculation of coronary flow reserve—an indicator of microvascular function. Notably, PET has been established as the clinical reference standard for the quantification of myocardial perfusion127.
In oncology, non-invasive tumor imaging provides crucial advantages in the assessment of tumor progression and regression. In 2020, [18F]fluoroestradiol was approved by the FDA for imaging estrogen receptor (ER)-positive breast cancer lesions128. Prostate cancer is common cancer among men, and PET imaging is a powerful tool for monitoring the progression or recurrence of prostate cancer129. Indeed, [11C]choline was the first FDA-approved PET tracer that was employed for the detection of recurrent prostate cancer. Similarly, [18F]fluciclovine, an 18F-labeled analog of L-leucine, was approved by the FDA for imaging prostate cancer recurrence. Although these probes proved to be useful in prostate cancer patients, their accumulation reflected the increased amino acid turnover of tumor cells; however, their mechanism of uptake was not related to tumor-specific markers. In contrast, the more recently FDA-approved prostate-specific membrane antigen (PSMA)-targeted PET radioligand, [18F]DCFPyL (Pylarify®), proved to be highly selective for PSMA-positive tumor cells and as such, provide high accuracy for the detection of PSMA-positive lesions in men with prostate cancer.
PET chemistry of radiometals
PET Radiometals
Besides organic PET radionuclides, radiometals provide new possibilities to radiolabel targeted vectors for PET which typically consists of biological molecules (e.g., peptides, proteins, and antibodies). Metal-based radiopharmaceuticals (Fig. 8a) often include a radiometal (M*) bound to a chelator (C), which is attached to a targeting vector (V) through a linker (L) in the format of M*-C-L-V. Compared to other PET radionuclides, radiometals generally have a straightforward labeling process that relies on coordination chemistry. Further, the relatively mild labeling conditions make radiometals suitable for labeling complex biological molecules. Notably, PET radiometals have a wide range of physical half-lives, ranging from hours to several days, such as gallium-68 (68Ga, t1/2 = 67.7 min), copper-64 (64Cu, t1/2 = 12.7 h), and zirconium-89 (89Zr, t1/2 = 78.4 h), which provides a broad radionuclide scope with suitable physical half-life to match the intended application. As such, short-lived radiometals can be used for vectors with fast pharmacokinetics and long-lived radiometals to visualize long-lasting biological processes.
Fig. 8. Radiometal chemistry for PET.
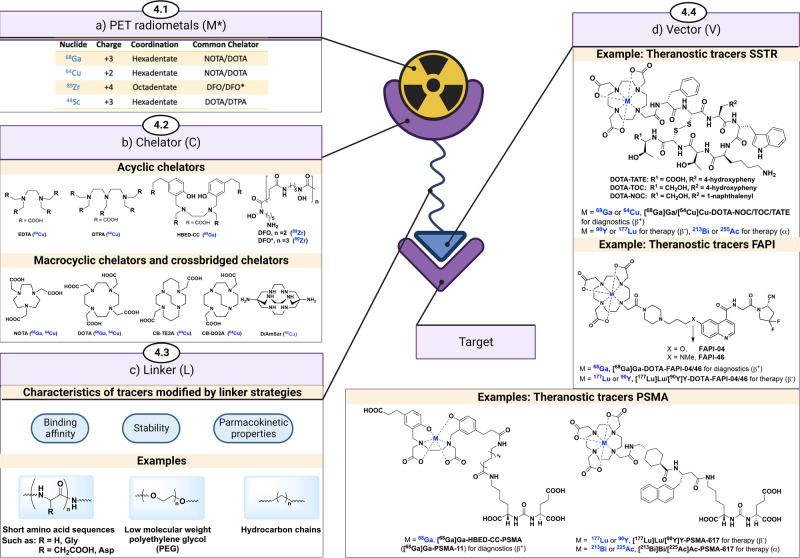
a Schematic diagram for radiometal-based PET radiopharmaceuticals (M*-C-L-TV). b Chelators in radiometal-based PET pharmaceuticals (acyclic chelators, cyclic chelators, and cross-bridged cyclic chelators). c Likers in metal-based radiopharmaceuticals (short sequences of amino acids, polyethylene glycols (PEG), and hydrocarbon chains). d Clinical examples of radiometal-based PET pharmaceuticals. Created with BioRender.com.
An increasingly utilized feature of PET imaging is radiotheranostics. When a radiometal-based tracer is used in PET imaging for diagnosis, the following radiotherapy could be conducted by changing M* from a positron emitter (44Sc, 64Cu, 68Ga, or 89Zr) to an alpha emitter (225Ac, 212Pb, or 213Bi)130,131 or beta emitter (177Lu, 90Y, or 67Cu) for radiotherapy132. Theranostic pairs are also feasible without radiometals, for example, with Iodine isotopes133, or between [18F]MFBG134 and [211At]MABG135, but the inherent potential in the theranostic approach with radiometals and chelators is more modular. Of note, contemporary concepts and potential future developments in radiotheranostics have been recently reviewed by Weissleder and co-workers133. We will focus on the commonly used radiometals in PET, including 68Ga, 64Cu, and 89Zr. Other radiometals are discussed elsewhere136,137.
68Ga (t1/2 = 67.7 min) can be produced by commercially available generators (68Ge/68Ga) and is easily accessible to radiochemists. Owing to the long half-life of the parent isotope 68Ge (t1/2 = 271 days), 68Ge/68Ga generators can typically be used within one year. The different chemical properties between 68Ga and 68Ge make the separation possible. In the past ten years, several excellent 68Ga-based radiopharmaceuticals have been developed for clinical use. The accessibility and potential of these radiopharmaceuticals make 68Ga attractive and promising in PET. It should be noted, however, that the limited capacity and supply of GMP-grade 68Ge/68Ga generators has prompted the search for improved ways to generate gallium-68 via cyclotron. As an important improvement and supplement to the generator method (usually at ≤100 mCi capacity), the cyclotron-based method could produce 68Ga in high quantity up to multi Ci levels, which provides strong support for the successful use of 68Ga in the clinic, especially for a larger patient population138. 64Cu can be produced by different methods via a reactor, an accelerator or a cyclotron. Highly enriched 64Ni is used in the 64Ni(p,n)64Cu nuclear reaction within a cyclotron to produce carrier-free 64Cu in high purity, yield, and molar activity139. Unlike other PET radiometals, [64Cu]Cu2+ is not redox-inert under physiological conditions. Indeed, [64Cu]Cu2+ can be reduced to [64Cu]Cu+ under reducing conditions in vivo, which represents a practical consideration during PET tracer design and/or chelator selection (vide infra). In certain cases, the redox dynamics of copper offer some advantages. For example, this redox behavior is the mode of action for [64Cu]Cu-ATSM140, a hypoxia imaging tracer. In hypoxic regions, Cu(II) is reduced and subsequently trapped, which can be visualized via PET. Of note, the smaller positron range of 64Cu compared to 68Ga renders tracers based on 64Cu applicable for pathologies with higher spatial requirements, such as cardiovascular diseases.
89Zr is typically produced via the 89Y(p,n)89Zr nuclear reaction in a cyclotron. With a relatively long half-life (t1/2 = 78.4 h), 89Zr can be transported to nuclear medicine facilities without an on-site cyclotron and can be used in antibody-based PET imaging (immuno-PET). Recently, 44Sc has gained attention as a promising PET radionuclide due to its clean β+-decay (94%) and suitable physical half-life (t1/2 = 4 h). 44Sc can be produced either via 44Ca(p,n)44Sc in a cyclotron or by 44Ti/44Sc generators.
Chelators
Contrary to the labeling with other PET radionuclides involving covalent bond formation (e.g., 18F-C bond), labeling with radiometals requires coordination with chelators (Fig. 8b). Even if certain chelators prefer some radiometals over others, no chelator is fully metal-specific and will bind other non-radioactive metals, if present during radiolabelling. Chelators are essential to ensure (1) fast complexation of the radiometal at low temperatures, low substrate concentrations, and physiological pH, and (2) thermodynamic and kinetic stabilization of radiometals by forming inert complexes. In general, chelators can be classified in three types, acyclic chelators, cyclic chelators, and cross-bridged chelators.
Acyclic chelators provide fast complexation kinetics at room temperature and physiological pH. Nonetheless, acyclic chelator-metal complexes, in general, exhibit less favorable stability than the cyclic ones. The earliest chelators for radiometals were acyclic, such as the polycarboxylate chelators EDTA and DTPA. Although these chelators exhibited fast complexation kinetics at room temperature, the complexes were plagued by their low stability in vivo. Further, EDTA and DTPA have low selectivity for cations with a similar valence; however, they bind M3+ with a higher affinity than M2+. The acyclic chelator HBED-CC is unique in its use of two lipophilic phenolic groups for [68Ga]Ga3+ coordination141. The [68Ga]Ga-HBED-CC complex consists of two negatively charged carboxyl groups, two neutral nitrogen atoms and two negatively charged phenolic oxygens, creating a complex with one negative charge and with a more hydrophobic character (from the benzyl rings of the phenolic groups), as compared to other acyclic chelators. The acyclic chelator desferoxamine B (DFO)142 has been developed and recognized as a gold standard chelator for Zr4+ in the past decade. DFO is an iron-binding siderophore from the bacteria Streptomyces pilosus, which was approved by FDA for iron-overload disease, using the six oxygens of three hydroxamate groups to bind Zr4+ in a [89Zr][Zr(DFO)(H2O)2]+ complex. Escorcia and co-workers recently showed that [89Zr]Zr-DFO-onartuzumab, a monoclonal antibody targeting the Met receptor tyrosine kinase, can be used to predict the therapeutic response to targeted radioligand therapy in a rodent model of pancreatic cancer that was resistant to Met kinase inhibitors143. With the long biological and physical half-lives of [89Zr]Zr-DFO-mAbs (mAb, monoclonal antibody), the chelation stability of [89Zr]Zr-DFO-mAb needs improvement. Hence, there is an ongoing effort to obtain 89Zr chelates with improved stability. Notably, Mindt, Gasser, and co-workers developed a novel chelator DFO* by adding one more hydroxamate donor to DFO, and the resulting two more coordination sites of DFO* largely improved the stability of [89Zr]Zr-DFO* complexes144.
To improve the stability of M*-C complexes, cyclic chelators have been designed to strengthen the binding of radiometals. Cyclic chelators have more rigid chemical structures, in which the donor atoms are in a more optimal position to chelate certain radiometals. Cyclic complexes are generally more stable than acyclic complexes. Although many chelators react with radiometals at low temperatures, albeit at lower radiochemical yields, higher temperatures (40–100 °C) are typically needed for rapid radiometal binding. A few exceptions can also be found in the cases of [64Cu]Cu-NOTA, [64Cu]Cu-DiAmSar, and [68Ga]Ga-PCTA136,137. The workhorse cyclic chelator is DOTA, with many commercially available DOTA derivatives that can be easily conjugated to peptides on the solid phase. A large number of trivalent radiometals bind sufficiently tight to DOTA, including [68Ga]Ga3+ and [44Sc]Sc3+ as positron emitters for PET, [177Lu]Lu3+ and [90Y]Y3+ as beta emitters for therapy, as well as [225Ac]Ac3+ and [213Bi]Bi3+ as alpha emitters for therapy. Thus, DOTA is an excellent chelator for radiopharmaceuticals and/or the chelator of choice during the design because it allows the use of trivalent imaging radiometals (e.g., [68Ga]Ga3+), followed by treatment with therapeutic radiometals (e.g., [177Lu]Lu3+, [225Ac]Ac3+). With a smaller ring structure, NOTA is a more stable chelator than DOTA with [68Ga]Ga3+ and [64Cu]Cu2+. Further, NOTA (with one pendant arm in use for coupling to the linker/vector) is still the chelator of choice for binding Al[18F]F, despite elevated temperature (90–110 °C) being needed, allowing chelator-mediated 18F-labeling of proteins and peptides without formation of a covalent 18F-C bond. It is worthy of note that recently, related to Al[18F]F labeling, novel scaffolds HBED-CC and RESCA1, as acyclic chelators, have shown promise for Al[18F]F chelation at room temperature110,145, which is advantageous for biologics that are sensitive to elevated temperatures (>37 °C).
Cross-bridged cyclic chelators (Fig. 8b) have been developed to provide additional rigidity over their cyclic counterparts of similar ring dimensions, in most cases cross-bridging between N atoms and reducing the number of carboxyl groups available for radiometal binding. In general, the complexation process requires higher temperatures than cyclic chelators. [64Cu]Cu-CB-DO2A and [64Cu]Cu-CB-TE2A were found to have superior in vivo stabilities to DOTA and TETA. Cross-bridged chelators are widely used with [64Cu]Cu2+, possibly owing to the aforementioned redox potential of [64Cu]Cu2+, requiring more stable metal-chelate complexes to ensure continued binding, even on reduction. In addition, DiAmSar is a sarcophagine type chelator that can be labeled with [64Cu]Cu2+ at room temperature. The DiAmSar chelator shows an even higher in vivo stability than most other cross-bridge cyclic chelators146. Another sarcophagine analog, MeCOSAR147, was used in the synthesis of [64Cu]Cu-SARTATE in the clinical trial (NCT04438304) for imaging patients with known or suspected neuroendocrine tumors. Other chelators for radiometal labeling include, but are not limited to, DOTA variants, NOTA variants, TE2A variants, PCTA, macropa, HOPO, sulfur-containing chelators (e.g., ECD), DATA analogs, DTPA analogs (e.g., CHX-A”-DTPA), octapa and etc. A comprehensive discussion of these chelators is beyond the scope of the present review and can be found elsewhere136,146,148.
Linkers
Linkers are important for radiometal-based radiopharmaceuticals, as a direct coupling of the chelator and vector may result in the reduction of receptor affinity. To address this problem, a linker can be introduced between the chelator and the vector. Further, the linker can be utilized to fine-tune the physical properties of the tracer to affect in vivo behavior. An ideal linker does not negatively affect the vector affinity towards the target (sometimes even increases the binding affinity) but can be used to improve the pharmacokinetic properties of the tracer. While linkers have not been studied to a greater extent for radiopharmaceuticals, and the reports are not conclusive, the lessons we learned from antibody-drug-conjugates demonstrated that the appropriate linker depends highly on vector and chelator149.
Properties of linkers are critical because a suitable linker may improve radiotracer pharmacokinetics properties. There are several commonly used linkers, such as short sequences of amino acids, polyethylene glycols (PEG), and hydrocarbon chains (Fig. 8c). Simple hydrocarbon chains tend to increase the lipophilicity of radiotracer with an unfavorably slow clearance via the hepatobiliary pathway to follow. In contrast, linkers of amino acid sequences tend to increase the hydrophilicity of radiotracer with primary excretion via the renal-urinary pathway as a consequence. Generally, a radiotracer needs to achieve a high target accumulation and a high target-to-background ratio in the shortest possible time. This means that the radiotracer needs to reach the target and be cleared from non-target tissues quickly, while still matching the binding kinetics of the vector. The introduction of PEG linkers to the radiotracers was found to increase receptor-mediated uptake and renal-urinary clearance, decrease non-specific binding, and achieve a high target-to-background ratio, in most cases. As an example, the introduction of PEG linker in [64Cu]Cu-DOTA-PEGn-AVP04-50 could significantly decrease kidney uptake, and increase tumor uptake, compared to the non-PEGylated counterpart150. And for [64Cu]Cu-NOTA-PEGn-trastuzumab151, the clearance of PEGylated-radiotracer was three-fold faster while maintaining comparable tumor uptake compared to its non-PEG counterpart. In addition, some functional moieties, including albumin binders, can be introduced to the linker to improve the drug pharmacokinetics. For example, Evans Blue has been introduced to the linker to help the drugs’ binding to albumin, extend the half-life, and decrease renal clearance152. In all, the interplay of vector, linker, and radiometal-chelator determines in vivo imaging properties of the tracer. While the radiometal, chelator and vector are often pre-determined, the linker is easier to modify at late stage, making it an ideal component for fine-tuning and achieving optimal physicochemical properties of radiometal-based pharmaceuticals.
Clinical examples of radiometal-based PET pharmaceuticals
Although 99mTc-labeled radiopharmaceuticals have historically dominated the landscape of radiometal-based clinical applications, the enormous potential of radiometals in nuclear medicine goes well beyond these conventional probes. Indeed, the clinical use of gallium-68 has experienced remarkable growth with recent examples of successful phase III clinical trials—particularly in oncology133,153. The representative examples include somatostatin receptors (SSTRs)-targeted probes in neuroendocrine tumors and PSMA-targeted probes in prostate cancer (Fig. 8d). Even though there are many reports on antibodies labeled with 89Zr or 64Cu, some of which are currently in clinical trials, none of these has been FDA approved yet.
SSTRs are receptors for somatostatin, a small neuropeptide associated with neural signaling. Overexpression of SSTRs on the cell membrane is a unique feature of neuroendocrine tumors, which is a promising target for imaging diagnosis and radiotherapy of neuroendocrine tumors. In the past decade, a range of somatostatin analogs have been developed for SSTRs PET imaging and targeted radiotherapy of neuroendocrine tumors, and the commonly used tracers include [68Ga]Ga-DOTA-TATE, [68Ga]Ga-DOTA-TOC, and [68Ga]Ga-DOTA-NOC154. In addition, DOTA-TATE/TOC/NOC was further used in targeted radiotherapy of neuroendocrine tumors after labeling with alpha emitter (213Bi) and beta emitter (177Lu or 90Y). Notably, [68Ga]Ga-DOTA-TATE (NETSPOTTM), [177Lu]Lu-DOTA-TATE (Lutathera®), and [68Ga]Ga-DOTA-TOC were approved by the FDA in 2016, 2018, and 2019, respectively155.
PSMA has a very low expression in normal prostate cells, but a high expression in prostate cancer cells, and the expression level is directly proportional to prostate cancer progression. Hence, PSMA is a promising target for imaging diagnostics and targeted radionuclide therapy for prostate cancer and its metastases. Currently, various PET tracers have been developed for the diagnosis and treatment of prostate cancer, such as [68Ga]Ga-PSMA-HBED-CC (also known as [68Ga]Ga-PSMA-11)156,157, and [18F]PMSA-1007158. In addition to [18F]DCFPyL (Pylarify®; cf. section 3.3, FDA approved 18F radiopharmaceuticals), [68Ga]Ga-PSMA-11 is another widely used radiotracer in clinical research and has been approved by the FDA in December 2020 for the imaging of PSMA-positive lesions in men with prostate cancer159. [68Ga]Ga-PSMA-11 exhibited high stability and a high uptake in prostate cancer cells. Another ligand PSMA-617 was developed for radionuclide therapy with 177Lu (t1/2 = 6.7 d). Indeed, [177Lu]PSMA-617 was granted FDA breakthrough designation in castration-resistant prostate cancer after both primary clinical endpoints of overall survival and radiographic progression-free survival were met in the VISION trial (NCT03511664) and received FDA approval in March 2022160,161.
Fibroblast activation protein (FAP) is highly expressed in more than 90% of epithelial tumors, which makes FAP a promising target for diagnosis and therapy of various types of cancers. Notably, the FAP-targeted probes, [68Ga]Ga-DOTA-FAPI-04 and [68Ga]Ga-DOTA-FAPI-46, were introduced as suitable radioligands for imaging different cancers and demonstrated high tumor uptake and fast clearance from the normal tissue, resulting high image quality162,163. Further, the universal DOTA chelator, in principle, warrants the use of DOTA-FAPI-04 and DOTA-FAPI-46 for theranostics. Although [68Ga]Ga-DOTA-FAPI-04 and [68Ga]Ga-DOTA-FAPI-46 have not yet been approved by the FDA, the results obtained so far have been promising.
More recent examples of radiometal-based PET pharmaceuticals that were advanced to human research include the angiotensin-converting enzyme 2 (ACE2)-targeted probes, [64Cu]HZ20, and [68Ga]HZ20164. Notably, ACE2 constitutes a key viral gateway that facilitates SARS-CoV-2 entry into host cells165–167. As such, it was hypothesized that non-invasive quantification on ACE2 abundancy in the lungs and other organs holds promise to deliver prognostic value and support the development of ACE2-targeted therapeutic intervention164. [64Cu]HZ20 and [68Ga]HZ20 exhibited nanomolar potency and showed favorable pharmacokinetics in a fist-in-human study with 20 healthy volunteers. Future studies with [64Cu]HZ20 and [68Ga]HZ20 will shed light on the diagnostic and prognostic value of ACE2-targeted PET in COVID-19 patients. Recently, [89Zr]Zr-Atezolizumab has been used as a promising PET tracer for assessing clinical response to PD-L1 blockade in cancers168. PET imaging with [89Zr]Zr-Atezolizumab before and after the therapy might be of great usefulness for determining PDL-1 expression in tumors.
Bioconjugation in PET tracer development
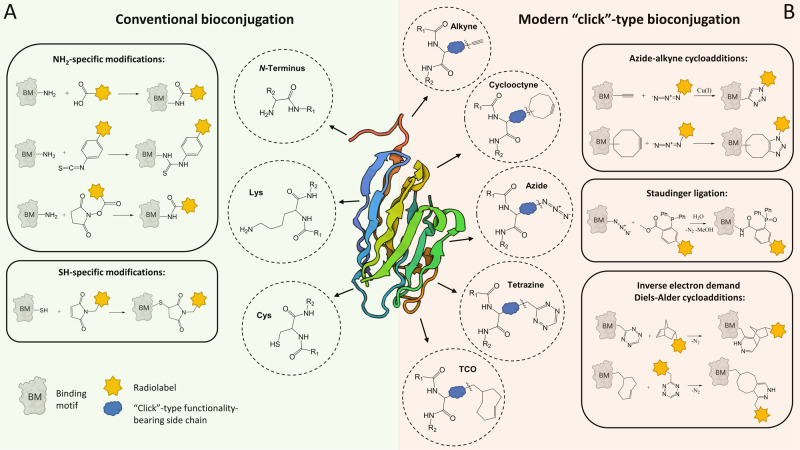
Herein, we refer to bioconjugation as a general chemical process to form a covalent bond between two molecules, of which at least one is a biomolecule (can be found in biological systems). Of note, biomolecules such as antibodies, antibody fragments and peptides have become a crucial component of modern drug development. The continuously growing number of FDA approvals for therapeutic biomolecules has prompted enthusiasm to further exploit the use of biomolecules for diagnostic purposes169,170. While PET radiometals have traditionally been used in combination with antibodies, novel radiochemical tools have widened the radiolabeling scope, particularly with regard to non-metal radionuclides171,172. Bioconjugation reactions can be subdivided into conventional methods, that use primary amine with N-hydroxysuccinimides (NHS), isothiocyanates and anhydrides as well as thiol modifications with maleimides, and modern click-type bioconjugation methods, which are typically based on orthogonal reactions including, but not limited to, azide-alkyne cycloadditions, Staudinger ligations and inverse electron demand Diels-Alder cycloadditions (Fig. 9)173. Orthogonal reactions are highly selective between a pair of chemical functionalities and can proceed with high yields in aqueous media and at ambient temperature174,175. The employed chemical functionalities are typically not present in nature, which allows specific reactions in the presence of naturally occurring functional groups. When orthogonal reactions are inert to biological moieties, they are termed bioorthogonal. Indeed, bioorthogonal reactions constitute the cornerstone of pretargeted imaging, where two components, namely the functionalized targeting vector and the radioisotope-bearing prosthetic group, are administered separately and react in vivo176. In this chapter, we will discuss conventional and modern click-type bioconjugation methods, thereby highlighting recent advances in orthogonal PET chemistry for fast and efficient in vitro/in vivo applications.
Fig. 9. Conventional vs. modern (Click-type) bioconjugation chemistry.
A While peptides and proteins were traditionally labeled via NH2- and thiol-specific modification with N-hydroxysuccinimides (NHS), isothiocyanates, or maleimides, respectively, B modern (Click-type) bioconjugation strategies involve fast and high-yielding orthogonal radiochemistry with non-naturally occurring chemical functionalities. Created with BioRender.com.
Conventional bioconjugation
Conventional bioconjugation methods harness the reactivity of amines and thiols, which are present in natural amino acids such as lysine and cysteine residues as well as the N-terminal residues (Fig. 9a). Traditionally, the radiolabeling of proteins was accomplished by reacting their amine or thiol functionalities to chelator-bearing reactive electrophiles and subsequent chelation of the radionuclide, or reaction with radiolabelled prosthetic groups148. Indeed, the vast majority of traditional bioconjugation examples involve the reaction of cysteine residues with radiolabeled maleimides via Michael addition or the modification of primary amines with radiolabeled NHS esters, isothiocyanates and activated carboxylic acid derivatives177–181. Furthermore, these reactions are mainly not site-specific since biomolecules such as antibodies contain multiple primary amines (lysines), resulting in the difficulty of controlling bioconjugate sites, potentially impacting their binding properties. Despite their widespread use, conventional bioconjugation reactions offer room for improvement, particularly with regard to reaction kinetics, radiochemical yields and stability issue with maleimide adducts (via retro-Michael addition)173.
Modern click-type bioconjugation
In the past, the vast majority of bioconjugation concepts have relied on conventional bioconjugation reactions. To overcome the previously mentioned limitation of conventional bioconjugation methods, however, radiochemists have increasingly focused on labeling strategies that involve orthogonal clickable prosthetic groups (Fig. 9b). Indeed, a variety of click-type bioconjugation methods have been developed, and in some cases, provided more optimal reaction kinetics, higher radiochemical yields, or more stable labeled vectors. Orthogonal reactions that rely on naturally occurring primary amines do not provide site-selective radiolabeling since biomolecules typically bear multiple primary amines. Nonetheless, the high chemical selectivity of orthogonal reactions provides a more uniform tracer, thereby easing a subsequent regulatory process173. Among the orthogonal reactions harnessed by radiochemists, azide-alkyne cycloadditions (AACs) have been most extensively employed for peptide and protein labeling182–194. A unique advantage of this reaction is that the click product, a 1,2,3-triazole ring, is well tolerated by the binding domain due to its relatively small and rigid structure, which essentially forms the bioisostere of an amide linkage. In light of the highly suited AAC properties for fast and efficient radiolabeling, a wide variety of radiofluorinated alkyne- or azide-bearing prosthetic groups were harnessed with great success, yielding numerous radiolabeled vectors including peptides, oligonucleotides, and proteins195–205.
Notwithstanding the successful implementation of CuAAC in PET radiochemistry, this reaction has some shortcomings, namely, the need for a copper catalyst and the presence of a reductant (commonly ascorbic acid), both of which may interact or react with the vector as well as the radiolabeled prosthetic group. Particularly, serine, histidine, and arginine residues can coordinate copper cations, imposing potential structural and functional changes on the vector198,205–207. Further, residual copper(II) ions may displace the far less abundant PET radiometals from their chelators208. In sharp contrast, second-generation AACs are catalyst-free and, hence, better tolerated by biomolecules173. Indeed, by employing ring strain-activated cyclic alkynes, such as dibenzocyclooctyne, the reaction can be accomplished in aqueous solutions, at ambient temperature and without copper catalyst209,210. This strain-promoted azide-alkyne cycloaddition (SPAAC) has been used for the radiofluorination of bombesin derivatives, RGD peptides, nanobodies and monoclonal antibodies203,211–216. Despite its widespread use, the SPAAC ligation is limited by the formation of a lipophilic and bulky benzocyclooctatriazole footprint (Fig. 9b), which can lead to substantial changes in tracer pharmacokinetics217. In contrast, the traceless Staudinger ligation offers a useful alternative with a smaller and less lipophilic footprint and has been employed for the radiofluorination of several peptides218–222. Major limitations of the Staudinger ligation, however, encompass the lack of in vivo selectivity as well as the slow reaction kinetics at ambient temperature, which confines its utility to in vitro applications involving temperature-resistant vectors223,224.
Similar to the SPAAC and Staudinger ligations, the inverse electron demand Diels-Alder (IEDDA) cycloaddition does not require the use of a metal catalyst. The IEDDA reaction proceeds remarkably fast and orthogonal between tetrazine moieties and dienophiles—typically being either trans-cyclooctene or norbornene225. Given the fast reaction rate, IEDDA cycloadditions are particularly useful for labeling short-lived radionuclides226. Along this line of reasoning, this reaction has been used to label a number of peptides, including RGD, Exendin, glucagon-like peptide, and a PARP1 inhibitor analog227–230. Nonetheless, it should be noted that isomerization issues, as well as a lipophilic footprint, constitute drawbacks of the IEDDA. While the IEDDA has recently been translated to clinical testing by assessing its utility for drug delivery optimization of doxorubicin (NCT04106492), applications in combination with radiolabeled compounds are eagerly awaited.
Pre-targeting approaches
Although antibodies are highly promising vectors due to their excellent affinity and selectivity, they typically exhibit slow pharmacokinetics and biological half-lives of several days. Accordingly, they are only compatible with long-lived radionuclides. Of note, the sluggish clearance from blood circulation, alongside the slow radioactive decay, is associated with high background noise and radiation dose for the patient231. To circumvent this core limitation of radiolabeled antibodies, in vivo pre-targeting strategies were developed, whereby a functionalized antibody is injected some days prior to the radionuclide-bearing prosthetic group. Notably, the uncoupling of antibody and radionuclide injection permits the antibody vector to accumulate at the target site, while being cleared from the blood circulation, not only reducing radiation exposure to non-target organs but also improving signal-to-background ratios in regions of interest176,232. Once the antibody has largely been cleared from the blood circulation, the radionuclide-bearing prosthetic group is administered, thus allowing the bioconjugation reaction to occur in vivo233,234. In addition to the dosimetric benefit to non-target organs and reduced background uptake, pre-targeting with fast and efficient click-type chemistry has rendered antibodies compatible with the labeling with short-lived PET nuclides, including fluorine-18235–238. Over the past decade, the IEDDA cycloaddition has proven particularly useful for pretargeting applications236,239–256. While there are ongoing efforts to improve the pre-targeting approaches, including fine-tuning between fast kinetics and stability of the reaction partners257, preclinical data strongly supports the use of these innovative pre-targeting approaches in humans, and a number of laboratories are currently devoting strenuous efforts toward bringing this exciting concept from bench to bedside258.
Conclusion and perspectives
Recent breakthroughs in molecular imaging with PET have undoubtedly improved diagnostic procedures in clinical oncology, cardiology, and neurology. PET probes are increasingly employed for target engagement studies, thereby facilitating drug discovery and development. However, the rapid growth in nuclear medicine and drug discovery for radiopharmaceuticals poses inherent challenges to radiochemists, since the radiolabeling of many biologically relevant compounds remains difficult. As such, strenuous efforts have been devoted to the development of new radiosynthetic strategies, including the progress from the patents259–262. Over the past decade, these efforts provided a plethora of novel synthetic options and broadened the scope of functional groups that can be harnessed for radiolabeling. To date, the most advanced radiofluorination methods, allowing the labeling of both activated and non-activated arenes, include copper-mediated reactions with boron/tin precursors and metal-free hypervalent iodine(III) methods. Similarly, 11C-labeling through [11C]CO2 fixation-mediated carbonylation has been translated to human use and enabled the labeling of various functionalities, including carboxylic acids, amides, carbamates, oxazolidinones, and unsymmetrical ureas. With regard to the labeling of biomolecules, traditional bioconjugation methods that involve the functionalization of primary amines and thiols with NHS, isothiocyanates, anhydrides, or maleimides, respectively, are being well complemented by modern click-type bioconjugation methods such as azide-alkyne cycloadditions and inverse electron demand Diels-Alder reactions. The fast and high-yielding orthogonal radiochemistry with non-naturally occurring chemical functionalities makes the click-type bioconjugations methods particularly promising for future clinical applications. Further, concepts that promote in-house preparation of radiopharmaceuticals have been playing a key role in fostering innovation and enabling cutting-edge applications for patients in need263. It is worth mentioning that, attributed to the rapid evolution of PET chemistry, it is critical to follow the consensus nomenclature rules and good practice33 in reporting radiochemistry discoveries, facilitating the comparison and translation of novel methods. Indeed, the remarkable advances in radiochemistry have led to a paradigm shift by enabling the design and synthesis of novel radiopharmaceuticals, thereby harnessing innovative concepts and promising approaches to radiotheranostics. In times when precision medicine has reshaped our healthcare system, exploiting such readily available concepts seems invaluable to improve patient management and therapeutic outcomes.
Acknowledgements
We thank the members of the Emory PET Imaging Center & Translational Radiochemistry and Radiotheranostics Program, Department of Radiology and Imaging Sciences, Emory University School of Medicine, for their expert assistance. We also gratefully acknowledge the financial support from the NIH grants (DA038000, DA043507, MH117125, MH120197, MH128705, AG063290, AG070060, AG073428, AG074218, AG075444, AG078058, AG079956, AG080262, NS130128, and AG081401), Emory Radiology Chair Fund and Emory School of Medicine Endowed Directorship to S.L., the Swiss National Science Foundation for a postdoctoral fellowship to A.H. and Fulbright postdoctoral fellowship to T.E.J. We also thank Dr. Jonathan W Engle for the helpful discussion on the characteristics of commonly used positron-emitting radionuclides.
Author contributions
J.R., A.H., and T.E.J contributed equally to this work. All the authors contributed to this manuscript and have approved its final version. S.L. developed the idea and led the work. J.R., A.H., T.E.J., L.J. contributed and provided critical comments throughout the manuscript and/or prepared illustrative items.
Peer review
Peer review information
Nature Communications thanks Sridhar Goud Nerella, Wolfgang Wadsak, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jian Rong, Achi Haider, Troels E. Jeppesen.
References
- 1.O’Connor, J. P. et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol.14, 169–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarkin, J. M., Joshi, F. R. & Rudd, J. H. PET imaging of inflammation in atherosclerosis. Nat. Rev. Cardiol.11, 443–457 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Nordberg, A., Rinne, J. O., Kadir, A. & Långström, B. The use of PET in Alzheimer’s disease. Nat. Rev. Neurol.6, 78–87 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Phelps, M. E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. USA. 97, 9226–9233 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ametamey, S. M., Honer, M. & Schubiger, P. A. Molecular imaging with PET. Chem. Rev.108, 1501–1516 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Willmann, J. K., van Bruggen, N., Dinkelborg, L. M. & Gambhir, S. S. Molecular imaging in drug development. Nat. Rev. Drug Discov.7, 591–607 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Rudin, M. & Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov.2, 123–131 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Deng, X. et al. Chemistry for positron emission tomography: recent advances in 11C-, 18F-, 13N-, and 15O-labeling reactions. Angew. Chem. Int. Ed.58, 2580–2605 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambhir, S. S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer2, 683–693 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Conti, M. & Eriksson, L. Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys.3, 8–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preshlock, S., Tredwell, M. & Gouverneur, V. 18F-labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev.116, 719–766 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Pauling, L. The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J. Am. Chem. Soc.53, 1367–1400 (1931). [Google Scholar]
- 13.Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem.58, 8315–8359 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Goud, N. S. et al. Carbon-11: radiochemistry and target-based PET molecular imaging applications in oncology, cardiology, and neurology. J. Med. Chem.64, 1223–1259 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Haider, A., Bengs, S. & Gebhard, C. Imaging inflammation in atherosclerosis: exploring all avenues. J. Nucl. Cardiol.28, 2514–2517 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Adamson, P. D. & Newby, D. E. Non-invasive imaging of the coronary arteries. Eur. Heart J.40, 2444–2454 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawakol, A. et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet389, 834–845 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreisl, W. C. et al. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol.19, 940–950 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jani, A. B. et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. Lancet397, 1895–1904 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philippe, C. et al. Optimization of the radiosynthesis of the Alzheimer tracer 2-(4-N-[11C]methylaminophenyl)-6-hydroxybenzothiazole ([11C]PIB). Appl. Radiat. Isot.69, 1212–1217 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Woods, M. et al. Improving the stability of 11C–labeled L-methionine with ascorbate. EJNMMI Radiopharm. Chem.2, 13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liger, F. et al. Direct [11C]methylation of amines from [11C]CO2 for the synthesis of PET radiotracers. Eur. J. Org. Chem.2015, 6434–6438 (2015). [Google Scholar]
- 23.Doi, H. Pd-mediated rapid cross-couplings using [11C]methyl iodide: groundbreaking labeling methods in 11C radiochemistry. J. Label. Compd. Radiopharm.58, 73–85 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Sandell, J. et al. PET examination of [11C]5-methyl-6-nitroquipazine, a radioligand for visualization of the serotonin transporter. Nucl. Med. Biol.29, 651–656 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Pipal, R. W. et al. Metallaphotoredox aryl and alkyl radiomethylation for PET ligand discovery. Nature589, 542–547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanzenberger, R. R. et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol. Psychiatry61, 1081–1089 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Riss, P. J., Lu, S., Telu, S., Aigbirhio, F. I. & Pike, V. W. CuI-catalyzed 11C carboxylation of boronic acid esters: a rapid and convenient entry to 11C-labeled carboxylic acids, esters, and amides. Angew. Chem. Int. Ed.51, 2698–2702 (2012). This paper reports the 11C-carboxylation of easily available boronic acid esters which provides rapid procedure to [11C]carboxylic acids and related derivatives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotstein, B. H. et al. Preclinical PET neuroimaging of [11C]Bexarotene. Mol. Imaging15, 1536012116663054 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bongarzone, S., Raucci, N., Fontana, I. C., Luzi, F. & Gee, A. D. Carbon-11 carboxylation of trialkoxysilane and trimethylsilane derivatives using [11C]CO2. Chem. Commun.56, 4668–4671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karimi, F. & Långström, B. Palladium-mediated carboxylation of aryl halides (triflates) or benzyl halides using [13C]/[11C]carbon monoxide with tetrabutylammonium hydroxide or trimethylphenylammonium hydroxide. J. Chem. Soc. Perkin Trans. I, 2256–2259, (2002).
- 31.Altomonte, S., Telu, S., Lu, S. & Pike, V. W. Pd(0)-Mediated 11C-carbonylation of aryl(mesityl)iodonium salts as a route to [11C]arylcarboxylic acids and derivatives. J. Org. Chem.82, 11925–11932 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Kong, D. et al. Fast carbon isotope exchange of carboxylic acids enabled by organic photoredox catalysis. J. Am. Chem. Soc.143, 2200–2206 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Coenen, H. H. et al. Consensus nomenclature rules for radiopharmaceutical chemistry—setting the record straight. Nucl. Med. Biol.55, v–xi (2017). [DOI] [PubMed] [Google Scholar]
- 34.Dahl, K., Schou, M., Amini, N. & Halldin, C. Palladium-mediated [11C] carbonylation at atmospheric pressure: a general method using xantphos as supporting ligand. Eur. J. Org. Chem.2013, 1228–1231 (2013). This paper describes the [11C]carbonylation of aryl halides and triflates with [11C]CO and this method could be employed in the synthesis of [11C]amide, [11C]ester, [11C]carboxylic acid, [11C]aldehyde, and [11C]ketone. [Google Scholar]
- 35.Hooker, J. M., Schönberger, M., Schieferstein, H. & Fowler, J. S. A simple, rapid method for the preparation of [11C]formaldehyde. Angew. Chem. Int. Ed.47, 5989–5992 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotstein, B. H. et al. 11C=O bonds made easily for positron emission tomography radiopharmaceuticals. Chem. Soc. Rev.45, 4708–4726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotstein, B. H. et al. 11CO2 fixation: a renaissance in PET radiochemistry. Chem. Commun.49, 5621–5629 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mossine, A. V. et al. Synthesis of diverse 11C-labeled PET radiotracers via direct incorporation of [11C]CO2. Bioconjugate Chem.27, 1382–1389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bongarzone, S., Runser, A., Taddei, C., Dheere, A. K. H. & Gee, A. D. From [11C]CO2 to [11C]amides: a rapid one-pot synthesis via the Mitsunobu reaction. Chem. Commun.53, 5334–5337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersen, T. L. et al. Efficient 11C-carbonylation of isolated aryl palladium complexes for PET: application to challenging radiopharmaceutical synthesis. J. Am. Chem. Soc.137, 1548–1555 (2015). This paper develops pre-generated Pd-aryl oxidative addition complexes for 11C-carbonylation reactions with [11C]CO to produce 11C-carbonylation of structurally challenging molecules. [DOI] [PubMed] [Google Scholar]
- 41.Rahman, O., Långström, B. & Halldin, C. Alkyl Iodides and [11C]CO in nickel-mediated cross-coupling reactions: successful use of alkyl electrophiles containing a β hydrogen atom in metal-mediated [11C]Carbonylation. ChemistrySelect1, 2498–2501 (2016). [Google Scholar]
- 42.Eriksson, J., Antoni, G., Långström, B. & Itsenko, O. The development of 11C-carbonylation chemistry: a systematic view. Nucl. Med. Biol.92, 115–137 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Itsenko, O., Kihlberg, T. & Långström, B. Synthesis of aliphatic [carbonyl-11C]esters Using [11C]carbon monoxide. Eur. J. Org. Chem.2005, 3830–3834 (2005). [Google Scholar]
- 44.Hooker, J. M., Reibel, A. T., Hill, S. M., Schueller, M. J. & Fowler, J. S. One-Pot, Direct Incorporation of [11C]CO2 into carbamates. Angew. Chem. Int. Ed.48, 3482–3485 (2009). This paper demonstrated the direct incorporation of [11C]CO2 with DBU as the [11C]CO2 trapping reagent and provided a novel synthesis route for carbamates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, P. W. & Bender, D. [11C]Carbon disulfide: a versatile reagent for PET radiolabelling. Chem. Eur. J.18, 433–436 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Haywood, T. et al. Carbon-11 radiolabelling of organosulfur compounds: 11C synthesis of the progesterone receptor agonist tanaproget. Chem. Eur. J.21, 9034–9038 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Wilson, A. A., Garcia, A., Houle, S., Sadovski, O. & Vasdev, N. Synthesis and application of isocyanates radiolabeled with carbon-11. Chem. Eur. J.17, 259–264 (2011). This paper reports the synthesis of [11C]carbamates and [11C]ureas with the effective [11C]CO2 trapping reagent, BEMP, and the dehydrating or chlorinating reagent, POCl3, which provides a one-pot, rapid procedure to unsymmetrical [11C]carbamates and [11C]ureas. [DOI] [PubMed] [Google Scholar]
- 48.Haji Dheere, A. K., Yusuf, N. & Gee, A. Rapid and efficient synthesis of [11C]ureas via the incorporation of [11C]CO2 into aliphatic and aromatic amines. Chem. Commun.49, 8193–8195 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Doi, H. et al. Synthesis of 11C-labelled N,N′-diphenylurea and ethyl phenylcarbamate by a rhodium-promoted carbonylation via [11C]isocyanatobenzene using phenyl azide and [11C]carbon monoxide. Org. Biomol. Chem.2, 3063–3066 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Fukumura, T., Mori, W., Ogawa, M., Fujinaga, M. & Zhang, M.-R. [11C]phosgene: synthesis and application for development of PET radiotracers. Nucl. Med. Biol.92, 138–148 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Cheng, R. et al. In vitro and in vivo evaluation of 11C-labeled azetidinecarboxylates for imaging monoacylglycerol lipase by PET imaging studies. J. Med. Chem.61, 2278–2291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, L. et al. Synthesis and preclinical evaluation of sulfonamido-based [11C-carbonyl]-carbamates and ureas for imaging monoacylglycerol lipase. Theranostics6, 1145–1159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jakobsson, J. E., Lu, S., Telu, S. & Pike, V. W. [11C]Carbonyl difluoride—a new and highly efficient [11C]carbonyl group transfer agent. Angew. Chem. Int. Ed.59, 7256–7260 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee, H. G., Milner, P. J., Placzek, M. S., Buchwald, S. L. & Hooker, J. M. Virtually instantaneous, room-temperature [11C]-cyanation using biaryl phosphine Pd(0) complexes. J. Am. Chem. Soc.137, 648–651 (2015). This paper reports Pd-mediated 11C-cyanation of aryl halides or triflates with sterically hindered biaryl phosphine ligand at room temperature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, Z., Niwa, T., Watanabe, Y. & Hosoya, T. Palladium(II)-mediated rapid 11C-cyanation of (hetero)arylborons. Org. Biomol. Chem.16, 7711–7716 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Ponchant, M., Hinnen, F., Demphel, S. & Crouzel, C. [11C]copper(I) cyanide: a new radioactive precursor for 11C-cyanation and functionalization of haloarenes. Appl. Radiat. Isot.48, 755–762 (1997). [Google Scholar]
- 57.Ma, L., Placzek, M. S., Hooker, J. M., Vasdev, N. & Liang, S. H. [11C]Cyanation of arylboronic acids in aqueous solutions. Chem. Commun.53, 6597–6600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makaravage, K. J. et al. Copper(II)-mediated [11C]cyanation of arylboronic acids and arylstannanes. Org. Lett.20, 1530–1533 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haskali, M. B. & Pike, V. W. [11C]Fluoroform, a breakthrough for versatile labeling of PET radiotracer trifluoromethyl groups in high molar activity. Chem. Eur. J.23, 8156–8160 (2017). This paper describes the synthesis of [11C]fluoroform via CoF3-mediated fluorination of cyclotron-produced [11C]methane which represents a major breakthrough as an agent for labeling prospective PET tracers in trifluoromethyl groups at high molar activity sufficient for imaging low-density targets in human subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pichler, V. et al. An overview on PET radiochemistry: part 1—covalent labels—18F, 11C, and 13N. J. Nucl. Med. jnumed.117.190793, 10.2967/jnumed.117.190793 (2018). [DOI] [PubMed]
- 61.Palmer, B. M., Sajjad, M. & Rottenberg, D. A. An automated [15O]H2O production and injection system for PET imaging. Nucl. Med. Biol.22, 241–249 (1995). [DOI] [PubMed] [Google Scholar]
- 62.Hess, E. et al. Excitation function of the 18O(p,n)18F nuclear reaction from threshold up to 30 MeV. Radiochim. Acta89, 357–362 (2001). [Google Scholar]
- 63.Kim, D. W. et al. A new class of SN2 reactions catalyzed by protic solvents: facile fluorination for isotopic labeling of diagnostic molecules. J. Am. Chem. Soc.128, 16394–16397 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Nielsen, M. K., Ugaz, C. R., Li, W. & Doyle, A. G. PyFluor: a low-cost, stable, and selective deoxyfluorination reagent. J. Am. Chem. Soc.137, 9571–9574 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Deng, H. et al. Fluorinase mediated C–18F bond formation, an enzymatic tool for PET labelling. Chem. Commun. 652–654, 10.1039/B516861A (2006). [DOI] [PubMed]
- 66.Hollingworth, C. et al. Palladium-catalyzed allylic fluorination. Angew. Chem. Int. Ed.50, 2613–2617 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Topczewski, J. J., Tewson, T. J. & Nguyen, H. M. Iridium-catalyzed allylic fluorination of trichloroacetimidates. J. Am. Chem. Soc.133, 19318–19321 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Benedetto, E. et al. Regio- and stereoretentive synthesis of branched, linear (E)- and (Z)-allyl fluorides from allyl carbonates under Ir-catalysis. Chem. Sci.4, 89–96 (2013). [Google Scholar]
- 69.Huang, X. et al. Late stage benzylic C–H fluorination with [18F]fluoride for PET imaging. J. Am. Chem. Soc.136, 6842–6845 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Liu, W. et al. Site-selective 18F fluorination of unactivated C–H bonds mediated by a manganese porphyrin. Chem. Sci.9, 1168–1172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Graham, T. J. A., Lambert, R. F., Ploessl, K., Kung, H. F. & Doyle, A. G. Enantioselective radiosynthesis of positron emission tomography (PET) tracers containing [18F]fluorohydrins. J. Am. Chem. Soc.136, 5291–5294 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Gendron, T. et al. Ring-closing synthesis of dibenzothiophene sulfonium salts and their use as leaving groups for aromatic 18F-fluorination. J. Am. Chem. Soc.140, 11125–11132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu, P. et al. Site-selective late-stage aromatic [18F]fluorination via aryl sulfonium salts. Angew. Chem. Int. Ed.59, 1956–1960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Narayanam, M. K., Ma, G., Champagne, P. A., Houk, K. N. & Murphy, J. M. Synthesis of [18F]fluoroarenes by nucleophilic radiofluorination of N-arylsydnones. Angew. Chem. Int. Ed.56, 13006–13010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pike, V. W. & Aighirhio, F. I. R. Reactions of cyclotron-produced [18F]fluoride with diaryliodonium salts-a novel single-step route to no-carrier-added [18F]fluoroarenes. J. Chem. Soc. Chem. Commun., 2215–2216 (1995). This paper reports the 18F-fluorination of diaryliodonium salts and provides a route to 18F-labeled arenes, especially for the electron-rich arenes.
- 76.Pike, V. W. Hypervalent aryliodine compounds as precursors for radiofluorination. J. Label. Comp. Radiopharm.61, 196–227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rotstein, B. H., Stephenson, N. A., Vasdev, N. & Liang, S. H. Spirocyclic hypervalent iodine(III)-mediated radiofluorination of non-activated and hindered aromatics. Nat. Commun.5, 4365–4371 (2014). This paper reports the spirocyclic iodonium ylides (SCIDY) as excellent precursors in 18F-fluorination of non-activated arenes. [DOI] [PubMed] [Google Scholar]
- 78.Liang, S. H., Wang, L., Stephenson, N. A., Rotstein, B. H. & Vasdev, N. Facile 18F labeling of non-activated arenes via a spirocyclic iodonium(III) ylide method and its application in the synthesis of the mGluR5 PET radiopharmaceutical [18F]FPEB. Nat. Protoc.14, 1530–1545 (2019). This paper describes an efficient method for the synthesis of the clinically useful [18F]FPEB that relies on one-step and regioselective 18F-fluorination of spirocyclic iodonium ylide (SCIDY) precursor. [DOI] [PubMed] [Google Scholar]
- 79.Collier, T. L. et al. Synthesis and preliminary PET imaging of 11C and 18F isotopologues of the ROS1/ALK inhibitor lorlatinib. Nat. Commun.8, 15761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rotstein, B. H. et al. Mechanistic studies and radiofluorination of structurally diverse pharmaceuticals with spirocyclic iodonium(III) ylides. Chem. Sci.7, 4407–4417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang, L. et al. A concisely automated synthesis of TSPO radiotracer [18F]FDPA based on spirocyclic iodonium ylide method and validation for human use. J. Label. Comp. Radiopharm.63, 119–128 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Neumann, C. N., Hooker, J. M. & Ritter, T. Concerted nucleophilic aromatic substitution with 19F- and 18F-. Nature534, 369–373 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Beyzavi, H. et al. 18F-Deoxyfluorination of phenols via Ru π-complexes. ACS Cent. Sci.3, 944–948 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee, E. et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science334, 639–642 (2011). The paper describes the development of a palladium-based electrophilic fluorination reagent and it’s application in synthesis of 18F-labeled arenes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee, E., Hooker, J. M. & Ritter, T. Nickel-mediated oxidative fluorination for PET with aqueous [18F] fluoride. J. Am. Chem. Soc.134, 17456–17458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tredwell, M. et al. A general copper-mediated nucleophilic 18F-fluorination of arenes. Angew. Chem. Int. Ed.53, 7751–7755 (2014). The paper describes a Cu-mediated 18F-fluorination of aryl boronic esters which is widely used in the synthesis of 18F-labeled PET tracers. [DOI] [PubMed] [Google Scholar]
- 87.Mossine, A. V. et al. Synthesis of high-molar-activity [18F]6-fluoro-l-DOPA suitable for human use via Cu-mediated fluorination of a BPin precursor. Nat. Protoc.15, 1742–1759 (2020). This paper reports the synthesis of the clinically useful [18F]FDOPA through Cu-mediated 18F-fluorination of a pinacol boronate (BPin) precursor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mossine, A. V. et al. Synthesis of [18F]arenes via the copper-mediated [18F]fluorination of boronic acids. Org. Lett.17, 5780–5783 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ichiishi, N. et al. Copper-catalyzed [18F]fluorination of (mesityl)(aryl)iodonium salts. Org. Lett.16, 3224–3227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Makaravage, K. J., Brooks, A. F., Mossine, A. V., Sanford, M. S. & Scott, P. J. Copper-mediated radiofluorination of arylstannanes with [18F]KF. Org. Lett.18, 5440–5443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naganawa, M. et al. First-in-human evaluation of 18F-SynVesT-1, a radioligand for PET imaging of synaptic vesicle glycoprotein 2A. J. Nucl. Med.62, 561 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen, W. et al. Direct arene C–H fluorination with 18F− via organic photoredox catalysis. Science364, 1170–1174 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tay, N. E. S. et al. 19F- and 18F-arene deoxyfluorination via organic photoredox-catalysed polarity-reversed nucleophilic aromatic substitution. Nat. Catal.3, 734–742 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen, W. et al. Arene radiofluorination enabled by photoredox-mediated halide interconversion. Nat. Chem.14, 216–223 (2022). This paper discloses the construction of C–18F bond via direct halide/18F conversion in electron-rich halo(hetero)arenes and a broad spectrum of aryl halide could be used as precursors with this method under mild photoredox conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bergman, J. & Solin, O. Fluorine-18-labeled fluorine gas for synthesis of tracer molecules. Nucl. Med. Biol.24, 677–683 (1997). [DOI] [PubMed] [Google Scholar]
- 96.Buckingham, F. et al. Organomediated enantioselective 18F-fluorination for PET applications. Angew. Chem. Int. Ed.54, 13366–13369 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Teare, H. et al. Radiosynthesis and evaluation of [18F]selectfluor bis(triflate). Angew. Chem. Int. Ed.49, 6821–6824 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Huiban, M. et al. A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat. Chem.5, 941–944 (2013). This paper reports 18F-trifluoromethylation of aromatics and heteroaromatics with (hetero)aryl iodide and [18F]CuCF3 and the board substrate scope of aryl iodides makes this method attracive in 18F-trifluoromethylation of pharmaceutical candidates and drugs. [DOI] [PubMed] [Google Scholar]
- 99.Rühl, T., Rafique, W., Lien, V. T. & Riss, P. J. Cu(I)-mediated 18F-trifluoromethylation of arenes: Rapid synthesis of 18F-labeled trifluoromethyl arenes. Chem. Commun.50, 6056–6059 (2014). [DOI] [PubMed] [Google Scholar]
- 100.van der Born, D. et al. A universal procedure for the [18F]trifluoromethylation of aryl iodides and aryl boronic acids with highly improved specific activity. Angew. Chem. Int. Ed.53, 11046–11050 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Ivashkin, P. et al. [18F]CuCF3: A [18F]trifluoromethylating agent for arylboronic acids and aryl iodides. Chem. Eur. J.20, 9514–9518 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Kee, C. W. et al. 18F-Trifluoromethanesulfinate enables direct C–H 18F-trifluoromethylation of native aromatic residues in peptides. J. Am. Chem. Soc.142, 1180–1185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trump, L. et al. Late-stage 18F-difluoromethyl labeling of N-heteroaromatics with high molar activity for PET imaging. Angew. Chem. Int. Ed.58, 13149–13154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi, H. et al. Synthesis of 18F-difluoromethylarenes from aryl (Pseudo) halides. Angew. Chem. Int. Ed.55, 10786–10790 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verhoog, S. et al. 18F-Trifluoromethylation of unmodified peptides with 5-18F-(trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate. J. Am. Chem. Soc.140, 1572–1575 (2018). [DOI] [PubMed] [Google Scholar]
- 106.Zheng, J., Wang, L., Lin, J.-H., Xiao, J.-C. & Liang, S. H. Difluorocarbene-derived trifluoromethylthiolation and [18F]trifluoromethylthiolation of aliphatic electrophiles. Angew. Chem. Int. Ed.54, 13236–13240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng, J. et al. An unconventional mechanistic insight into SCF3 formation from difluorocarbene: preparation of 18F-labeled alpha-SCF3 carbonyl compounds. Angew. Chem. Int. Ed.56, 3196–3200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chansaenpak, K., Vabre, B. & Gabbaï, F. P. [18F]-Group 13 fluoride derivatives as radiotracers for positron emission tomography. Chem. Soc. Rev.45, 954–971 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Krishnan, H. S., Ma, L., Vasdev, N. & Liang, S. H. 18F-labeling of sensitive biomolecules for positron emission tomography. Chem. Eur. J.23, 15553–15577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cleeren, F. et al. Al18F-labeling of heat-sensitive biomolecules for positron emission tomography imaging. Theranostics7, 2924–2939 (2017). This papers develops a new restrained complexing agent (RESCA) for Al18F-labeling and this method could be used to label heat-sensitive biomolecules at low temperature (< 37 °C). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng, Q. et al. Sulfur [18F]fluoride exchange click chemistry enabled ultrafast late-stage radiosynthesis. J. Am. Chem. Soc.143, 3753–3763 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kniess, T., Laube, M., Brust, P. & Steinbach, J. 2-[18F]Fluoroethyl tosylate—a versatile tool for building 18F-based radiotracers for positron emission tomography. MedChemComm6, 1714–1754 (2015). [Google Scholar]
- 113.Pretze, M., Pietzsch, D. & Mamat, C. Recent trends in bioorthogonal click-radiolabeling reactions using fluorine-18. Molecules18, 8618–8665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van der Born, D. et al. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem. Soc. Rev.46, 4709–4773 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Kuchar, M. & Mamat, C. Methods to increase the metabolic stability of 18F-radiotracers. Molecules20, 16186–16220 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schneider, R. F., Engelhardt, E. L., Stobbe, C. C., Fenning, M. C. & Chapman, J. D. The synthesis and radiolabelling of novel markers of tissue hypoxia of the iodinated azomycin nucleoside class. J. Label. Comp. Radiopharm.39, 541–557 (1997). [Google Scholar]
- 117.Moroz, M. A. et al. Imaging hNET reporter gene expression with 124I-MIBG. J. Nucl. Med.48, 827 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Gillies, J. M., Smith, N., Vaidyanthan, G., Bailey, J. & Zweit, J. Investigation of PET Nucleoside analogues in tumor cell lines. J. Label. Comp. Radiopharm.46, S127 (2003). [Google Scholar]
- 119.Pal, A. et al. Molecular imaging of EGFR kinase activity in tumors with 124I-labeled small molecular tracer and positron emission tomography. Mol. Imaging Biol.8, 262–277 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Kumar, K. & Ghosh, A. Radiochemistry, production processes, labeling methods, and immunoPET imaging pharmaceuticals of iodine-124. Molecules26, 414 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bartels, J. L. et al. Synthesis and biological evaluation of (S)-amino-2-methyl-4-[76Br]bromo-3-(E)-butenoic acid (BrVAIB) for brain tumor imaging. J. Med. Chem.58, 8542–8552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou, D., Chu, W., Voller, T. & Katzenellenbogen, J. A. Copper-mediated nucleophilic radiobromination of aryl boron precursors: convenient preparation of a radiobrominated PARP-1 inhibitor. Tetrahedron Lett.59, 1963–1967 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vāvere, A. L. & Scott, P. J. H. Clinical applications of small-molecule PET radiotracers: current progress and future outlook. Semin. Nucl. Med.47, 429–453 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Villemagne, V. L., Doré, V., Burnham, S. C., Masters, C. L. & Rowe, C. C. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol.14, 225–236 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Mattay, V. S., Fotenos, A. F., Ganley, C. J. & Marzella, L. Brain tau imaging: food and drug administration approval of 18F-Flortaucipir injection. J. Nucl. Med.61, 1411 (2020). [DOI] [PubMed] [Google Scholar]
- 126.Dhawan, V. et al. Prospective FDOPA PET imaging study in human PD: our final step towards NDA approval. J. Nucl. Med.61, 1565 (2020). [Google Scholar]
- 127.Dewey, M. et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat. Rev. Cardiol.17, 427–450 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Estrogen Receptor Agent Approved. J. Nucl. Med. 61, 20N–21N (2020). [PubMed]
- 129.Evans, J. D. et al. Prostate cancer-specific PET radiotracers: a review on the clinical utility in recurrent disease. Pract. Radiat. Oncol.8, 28–39 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Kelly, V. J. et al. Preclinical evaluation of an 111In/225Ac theranostic targeting transformed MUC1 for triple negative breast cancer. Theranostics10, 6946–6958 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheal, S. M. et al. Alpha radioimmunotherapy using 225Ac-proteus-DOTA for solid tumors—safety at curative doses. Theranostics10, 11359–11375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keinänen, O. et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. USA117, 28316–28327 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herrmann, K. et al. Radiotheranostics: a roadmap for future development. Lancet Oncol.21, e146–e156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Turnock, S. et al. 18F-meta-fluorobenzylguanidine (18F-mFBG) to monitor changes in norepinephrine transporter expression in response to therapeutic intervention in neuroblastoma models. Sci. Rep.10, 20918 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ukon, N. et al. Human dosimetry of free 211At and meta-[211At]astatobenzylguanidine (211At-MABG) estimated using preclinical biodistribution from normal mice. EJNMMI Phys.7, 58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boros, E. & Packard, A. B. Radioactive transition metals for imaging and therapy. Chem. Rev.119, 870–901 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Kostelnik, T. I. & Orvig, C. Radioactive main group and rare earth metals for imaging and therapy. Chem. Rev.119, 902–956 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Kumar, K. The current status of the production and supply of gallium-68. Cancer Biother. Radiopharm.35, 163–166 (2020). [DOI] [PubMed] [Google Scholar]
- 139.McCarthy, D. W. et al. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol.24, 35–43 (1997). [DOI] [PubMed] [Google Scholar]
- 140.Vāvere, A. L. & Lewis, J. S. Cu–ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans., 4893-4902, 10.1039/B705989B (2007). [DOI] [PubMed]
- 141.Eder, M. et al. Tetrafluorophenolate of HBED-CC: a versatile conjugation agent for 68Ga-labeled small recombinant antibodies. Eur. J. Nucl. Med. Mol. Imaging35, 1878–1886 (2008). [DOI] [PubMed] [Google Scholar]
- 142.Meijs, W. E., Herscheid, J. D. M., Haisma, H. J. & Pinedo, H. M. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int. J. Rad. Appl. Instrum. A43, 1443–1447 (1992). [DOI] [PubMed] [Google Scholar]
- 143.Escorcia, F. E. et al. ImmunoPET predicts response to Met-targeted radioligand therapy in models of pancreatic cancer resistant to Met kinase inhibitors. Theranostics10, 151–165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patra, M. et al. An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. Chem. Commun.50, 11523–11525 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Giglio, J., Zeni, M., Savio, E. & Engler, H. Synthesis of an Al18F radiofluorinated GLU-UREA-LYS(AHX)-HBED-CC PSMA ligand in an automated synthesis platform. EJNMMI Radiopharm. Chem.3, 4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Price, E. W. & Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev.43, 260–290 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Hicks, R. et al. First-time-in-human trial of Cu-64 MeCOSAR-octreotate (CuSARTATE) for imaging and dosimetry estimation in neuroendocrine tumor (NET). J. Nucl. Med.57, 26 (2016). [Google Scholar]
- 148.Zeglis, B. M. & Lewis, J. S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans.40, 6168–6195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu, J., Jiang, F., Lu, A. & Zhang, G. Linkers having a crucial role in antibody–drug conjugates. Int. J. Mol. Sci.17, 561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li, L. et al. Site-specific conjugation of monodispersed DOTA-PEGn to a thiolated diabody reveals the effect of increasing peg size on kidney clearance and tumor uptake with improved 64-Copper PET imaging. Bioconjugate Chem.22, 709–716 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee, W. et al. A short PEG linker alters the in vivo pharmacokinetics of trastuzumab to yield high-contrast immuno-PET images. J. Mater. Chem. B9, 2993–2997 (2021). [DOI] [PubMed] [Google Scholar]
- 152.Jacobson, O., Kiesewetter, D. O. & Chen, X. Albumin-binding evans blue derivatives for diagnostic imaging and production of long-acting therapeutics. Bioconjugate Chem.27, 2239–2247 (2016). [DOI] [PubMed] [Google Scholar]
- 153.Brandt, M., Cardinale, J., Aulsebrook, M. L., Gasser, G. & Mindt, T. L. An overview of PET radiochemistry, Part 2: radiometals. J. Nucl. Med.59, 1500 (2018). [DOI] [PubMed] [Google Scholar]
- 154.Virgolini, I. et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging37, 2004–2010 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Hennrich, U. & Benešová, M. [68Ga]Ga-DOTA-TOC: the first FDA-approved 68Ga-radiopharmaceutical for PET imaging. Pharmaceuticals13, 38 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Afshar-Oromieh, A. et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging40, 486–495 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Eder, M. et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjugate Chem.23, 688–697 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Awenat, S. et al. Diagnostic role of 18F-PSMA-1007 PET/CT in prostate cancer staging: a systematic review. Diagnostics11, 552 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carlucci, G. et al. 68Ga-PSMA-11 NDA approval: a novel and successful academic partnership. J. Nucl. Med.62, 149–155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Benešová, M. et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J. Nucl. Med.56, 914–920 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Sartor, A. O., Morris, M. J., Messman, R. & Krause, B. J. VISION: an international, prospective, open-label, multicenter, randomized phase III study of 177Lu-PSMA-617 in the treatment of patients with progressive PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol.38, TPS259–TPS259 (2020). [Google Scholar]
- 162.Loktev, A. et al. A tumor-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med.59, 1423–1429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lindner, T. et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med.59, 1415–1422 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Zhu, H. et al. Molecular PET/CT profiling of ACE2 expression in vivo: implications for infection and outcome from SARS-CoV-2. Adv. Sci.8, e2100965–e2100965 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang, J. et al. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun.11, 4541 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature579, 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jackson, C. B., Farzan, M., Chen, B. & Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol.23, 3–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bensch, F. et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med.24, 1852–1858 (2018). [DOI] [PubMed] [Google Scholar]
- 169.Mullard, A. 2018 FDA drug approvals. Nat. Rev. Drug Discov.18, 85–89 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Dammes, N. & Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics10, 938–955 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wei, W. et al. ImmunoPET: concept, design, and applications. Chem. Rev.120, 3787–3851 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pekošak, A., Filp, U., Poot, A. J. & Windhorst, A. D. From carbon-11-labeled amino acids to peptides in positron emission tomography: the synthesis and clinical application. Mol. Imaging Biol.20, 510–532 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Meyer, J. P., Adumeau, P., Lewis, J. S. & Zeglis, B. M. Click chemistry and radiochemistry: the first 10 years. Bioconjugate Chem.27, 2791–2807 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bertozzi, C. R. A decade of bioorthogonal chemistry. Acc. Chem. Res.44, 651–653 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Devaraj, N. K. The future of bioorthogonal chemistry. ACS Cent. Sci.4, 952–959 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Altai, M., Membreno, R., Cook, B., Tolmachev, V. & Zeglis, B. M. Pretargeted imaging and therapy. J. Nucl. Med.58, 1553–1559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cohen, R., Vugts, D. J., Stigter-van Walsum, M., Visser, G.W.M. & van Dongen, G.A.M.S. Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single- or dual-mode fluorescence and PET imaging. Nat. Protoc.8, 1010–1018 (2013). This paper describes a newly developed method to concurrently label monoclonal antibodies with zirconium-89 and a specific dye for optical imaging, IRDye800CW. This procedure allowed dual optical and PET imaging, while providing radiosynthesis quality standards that meet the requirements for human use. [DOI] [PubMed] [Google Scholar]
- 178.Vosjan, M. J. W. D. et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc.5, 739–743 (2010). [DOI] [PubMed] [Google Scholar]
- 179.Morais, M. & Ma, M. T. Site-specific chelator-antibody conjugation for PET and SPECT imaging with radiometals. Drug Discov. Today Technol.30, 91–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cooper, M. S. et al. Comparison of 64Cu-complexing bifunctional chelators for radioimmunoconjugation: labeling efficiency, specific activity, and in vitro/in vivo stability. Bioconjugate Chem.23, 1029–1039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ma, M. T. et al. Tripodal tris(hydroxypyridinone) ligands for immunoconjugate PET imaging with 89Zr4+: comparison with desferrioxamine-B. Dalton Trans.44, 4884–4900 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pickens, C. J., Johnson, S. N., Pressnall, M. M., Leon, M. A. & Berkland, C. J. Practical considerations, challenges, and limitations of bioconjugation via azide-alkyne cycloaddition. Bioconjugate Chem.29, 686–701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Meldal, M. & Tornøe, C. W. Cu-catalyzed azide−alkyne cycloaddition. Chem. Rev.108, 2952–3015 (2008). [DOI] [PubMed] [Google Scholar]
- 184.Mindt, T. L. et al. A “click chemistry” approach to the efficient synthesis of multiple imaging probes derived from a single precursor. Bioconjugate Chem.20, 1940–1949 (2009). [DOI] [PubMed] [Google Scholar]
- 185.Struthers, H., Mindt, T. L. & Schibli, R. Metal chelating systems synthesized using the copper(I) catalyzed azide-alkyne cycloaddition. Dalton Trans.39, 675–696 (2010). [DOI] [PubMed] [Google Scholar]
- 186.Islam, R. U., Taher, A., Choudhary, M., Siwal, S. & Mallick, K. Polymer immobilized Cu(I) formation and azide-alkyne cycloaddition: a one potreaction. Sci. Rep.5, 9632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hansell, C. Two-faced copper. Nat. Chem.6, 946–946 (2014). [Google Scholar]
- 188.Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed.40, 2004–2021 (2001). [DOI] [PubMed] [Google Scholar]
- 189.Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem.67, 3057–3064 (2002). [DOI] [PubMed] [Google Scholar]
- 190.Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed.41, 2596–2599 (2002). [DOI] [PubMed] [Google Scholar]
- 191.Meng, G. et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature574, 86–89 (2019). [DOI] [PubMed] [Google Scholar]
- 192.Hein, C. D., Liu, X. M. & Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res.25, 2216–2230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mindt, T. L. et al. “Click to chelate”: synthesis and installation of metal chelates into biomolecules in a single step. J. Am. Chem. Soc.128, 15096–15097 (2006). This paper describes a one-pot procedure to yield chelate-bearing radiolabeled cconjugates via click-type reaction of azides and alkyne derivatives with either L-propargyl glycine or L-azido alanine to form 1,2,3-triazole-4-yl alanines. The one-pot procedure constituted a substantial improvement for the synthesis of metal-labeled conjugates. [DOI] [PubMed] [Google Scholar]
- 194.Agard, N. J., Prescher, J. A. & Bertozzi, C. R. A strain-promoted [3 + 2] azide−alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc.126, 15046–15047 (2004). [DOI] [PubMed] [Google Scholar]
- 195.Glaser, M. & Arstad, E. “Click labeling” with 2-[18F]fluoroethylazide for positron emission tomography. Bioconjugate Chem.18, 989–993 (2007). [DOI] [PubMed] [Google Scholar]
- 196.Iddon, L. et al. Synthesis and in vitro evaluation of [18F]fluoroethyl triazole labelled [Tyr3]octreotate analogues using click chemistry. Bioorg. Med. Chem. Lett.21, 3122–3127 (2011). [DOI] [PubMed] [Google Scholar]
- 197.Pretze, M. & Mamat, C. Automated preparation of [18F]AFP and [18F]BFP: Two novel bifunctional 18F-labeling building blocks for Huisgen-click. J. Fluor. Chem.150, 25–35 (2013). [Google Scholar]
- 198.Pretze, M. et al. An efficient bioorthogonal strategy using CuAAC click chemistry for radiofluorinations of SNEW peptides and the role of copper depletion. ChemMedChem8, 935–945 (2013). [DOI] [PubMed] [Google Scholar]
- 199.Mirfeizi, L. et al. Synthesis of [18F]RGD-K5 by catalyzed [3 + 2] cycloaddition for imaging integrin αvβ3 expression in vivo. Nucl. Med. Biol.40, 710–716 (2013). [DOI] [PubMed] [Google Scholar]
- 200.Ramenda, T., Steinbach, J. & Wuest, F. 4-[18F]Fluoro-N-methyl-N-(propyl-2-yn-1-yl)benzenesulfonamide ([18F]F-SA): a versatile building block for labeling of peptides, proteins and oligonucleotides with fluorine-18 via Cu(I)-mediated click chemistry. Amino Acids44, 1167–1180 (2013). [DOI] [PubMed] [Google Scholar]
- 201.Roberts, M. P. et al. Radiosynthesis and ‘click’ conjugation of ethynyl-4-[18F]fluorobenzene–an improved [18F]synthon for indirect radiolabeling. J. Label. Comp. Radiopharm.58, 473–478 (2015). [DOI] [PubMed] [Google Scholar]
- 202.Hofmann, S., Maschauer, S., Kuwert, T., Beck-Sickinger, A. G. & Prante, O. Synthesis and in vitro and in vivo evaluation of an 18F-labeled neuropeptide Y analogue for imaging of breast cancer by PET. Mol. Pharm.12, 1121–1130 (2015). [DOI] [PubMed] [Google Scholar]
- 203.Sachin, K. et al. F-18 labeling protocol of peptides based on chemically orthogonal strain-promoted cycloaddition under physiologically friendly reaction conditions. Bioconjugate Chem.23, 1680–1686 (2012). [DOI] [PubMed] [Google Scholar]
- 204.Kettenbach, K., Schieferstein, H. & Ross, T. L. 18F-labeling using click cycloadditions. Biomed. Res. Int.2014, 361329 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ramenda, T., Kniess, T., Bergmann, R., Steinbach, J. & Wuest, F. Radiolabelling of proteins with fluorine-18 via click chemistry. Chem. Commun., 7521–7523, 10.1039/b916075b (2009). [DOI] [PubMed]
- 206.Chikira, M. & DNA-fiber, E. P. R. spectroscopy as a tool to study DNA-metal complex interactions: DNA binding of hydrated Cu(II) ions and Cu(II) complexes of amino acids and peptides. J. Inorg. Biochem.102, 1016–1024 (2008). [DOI] [PubMed] [Google Scholar]
- 207.Li, S. et al. Extent of the oxidative side reactions to peptides and proteins during the CuAAC reaction. Bioconjugate Chem.27, 2315–2322 (2016). [DOI] [PubMed] [Google Scholar]
- 208.Evans, H. L., Carroll, L., Aboagye, E. O. & Spivey, A. C. Bioorthogonal chemistry for 68Ga radiolabelling of DOTA-containing compounds. J. Label. Comp. Radiopharm.57, 291–297 (2014). [DOI] [PubMed] [Google Scholar]
- 209.van Hest, J. C. & van Delft, F. L. Protein modification by strain-promoted alkyne-azide cycloaddition. Chembiochem12, 1309–1312 (2011). [DOI] [PubMed] [Google Scholar]
- 210.Debets, M. F. et al. Bioconjugation with strained alkenes and alkynes. Acc. Chem. Res.44, 805–815 (2011). [DOI] [PubMed] [Google Scholar]
- 211.Richard, M. et al. New fluorine-18 pretargeting PET imaging by bioorthogonal chlorosydnone-cycloalkyne click reaction. Chem. Commun.55, 10400–10403 (2019). [DOI] [PubMed] [Google Scholar]
- 212.Campbell-Verduyn, L. S. et al. Strain-promoted copper-free “click” chemistry for 18F-radiolabeling of bombesin. Angew. Chem. Int. Ed.50, 11117–11120 (2011). [DOI] [PubMed] [Google Scholar]
- 213.Kim, H. L. et al. F-18 labeled RGD probes based on bioorthogonal strain-promoted click reaction for PET imaging. ACS Med. Chem. Lett.6, 402–407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vaidyanathan, G. et al. Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for imaging HER2 receptor expression by immuno-PET. J. Nucl. Med.57, 967–973 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chakravarty, R., Goel, S. & Cai, W. Nanobody: the “magic bullet” for molecular imaging? Theranostics4, 386–398 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim, E. & Koo, H. Biomedical applications of copper-free click chemistry: in vitro, in vivo, and ex vivo. Chem. Sci.10, 7835–7851 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hausner, S. H., Carpenter, R. D., Bauer, N. & Sutcliffe, J. L. Evaluation of an integrin αvβ6-specific peptide labeled with [18F]fluorine by copper-free, strain-promoted click chemistry. Nucl. Med. Biol.40, 233–239 (2013). [DOI] [PubMed] [Google Scholar]
- 218.Saxon, E., Armstrong, J. I. & Bertozzi, C. R. A “Traceless” staudinger ligation for the chemoselective synthesis of amide bonds. Org. Lett.2, 2141–2143 (2000). [DOI] [PubMed] [Google Scholar]
- 219.Gaeta, A. et al. Use of 2-[18F]fluoroethylazide for the Staudinger ligation—preparation and characterisation of GABA(A) receptor binding 4-quinolones. Bioorg. Med. Chem. Lett.20, 4649–4652 (2010). [DOI] [PubMed] [Google Scholar]
- 220.Carroll, L. et al. The traceless Staudinger ligation for indirect 18F-radiolabelling. Org. Biomol. Chem.9, 136–140 (2011). [DOI] [PubMed] [Google Scholar]
- 221.Jacobson, O., Kiesewetter, D. O. & Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjugate Chem.26, 1–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bednarek, C., Wehl, I., Jung, N., Schepers, U. & Bräse, S. The Staudinger ligation. Chem. Rev.120, 4301–4354 (2020). [DOI] [PubMed] [Google Scholar]
- 223.Fay, R. & Holland, J. P. The impact of emerging bioconjugation chemistries on radiopharmaceuticals. J. Nucl. Med.60, 587–591 (2019). [DOI] [PubMed] [Google Scholar]
- 224.Vugts, D. J. et al. Synthesis of phosphine and antibody–azide probes for in vivo Staudinger ligation in a pretargeted imaging and therapy approach. Bioconjugate Chem.22, 2072–2081 (2011). [DOI] [PubMed] [Google Scholar]
- 225.Li, Z. et al. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F-labeled probes. Chem. Commun.46, 8043–8045 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stéen, E. J. L. et al. Lipophilicity and click reactivity determine the performance of bioorthogonal tetrazine tools in pretargeted in vivo chemistry. ACS Pharmacol. Transl. Sci.4, 824–833 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu, S. et al. Efficient 18F labeling of cysteine-containing peptides and proteins using tetrazine-trans-cyclooctene ligation. Mol. Imaging12, 121–128 (2013). [PMC free article] [PubMed] [Google Scholar]
- 228.Selvaraj, R. et al. Tetrazine-trans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Bioorg. Med. Chem. Lett.21, 5011–5014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Keliher, E. J., Reiner, T., Turetsky, A., Hilderbrand, S. A. & Weissleder, R. High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors. ChemMedChem6, 424–427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu, Z. et al. Development and evaluation of 18F-TTCO-Cys40-Exendin-4: a PET probe for imaging transplanted islets. J. Nucl. Med.54, 244–251 (2013). [DOI] [PubMed] [Google Scholar]
- 231.Wu, A. M. Antibodies and antimatter: the resurgence of immuno-PET. J. Nucl. Med.50, 2–5 (2009). [DOI] [PubMed] [Google Scholar]
- 232.Sharkey, R. M. et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat. Med.11, 1250–1255 (2005). This paper describes a novel molecular imaging concept that harnesses signal amplification of a radiotracer in situ by pretargeting a multivalent, bispecific antibody to carcinoembryonic antigen (CEA). The resulting construct was shown to increase tumor/blood ratios by ca 40-fold, compared to a (99m)Tc-labeled CEA-specific F(ab’) used clinically for colorectal cancer detection. [DOI] [PubMed] [Google Scholar]
- 233.Honarvar, H. et al. Feasibility of affibody molecule-based PNA-mediated radionuclide pretargeting of malignant tumors. Theranostics6, 93–103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sharkey, R. M. et al. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin. Cancer Res.11, 7109s–7121s (2005). [DOI] [PubMed] [Google Scholar]
- 235.Billaud, E. M. F. et al. Pretargeted PET imaging using a bioorthogonal 18F-labeled trans-cyclooctene in an ovarian carcinoma model. Bioconjugate Chem.28, 2915–2920 (2017). [DOI] [PubMed] [Google Scholar]
- 236.Meyer, J.-P. et al. 18F-Based pretargeted PET imaging based on bioorthogonal diels-alder click chemistry. Bioconjugate Chem.27, 298–301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ruivo, E. et al. Improved stability of a novel fluorine-18 labeled TCO analogue for pretargeted PET imaging. Nucl. Med. Biol.76-77, 36–42 (2019). [DOI] [PubMed] [Google Scholar]
- 238.Ruivo, E. et al. Preclinical evaluation of a novel 18F-labeled dTCO-Amide Derivative For Bioorthogonal Pretargeted Positron Emission Tomography Imaging. ACS omega5, 4449–4456 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee, S. B. et al. Mesoporous silica nanoparticle pretargeting for PET imaging based on a rapid bioorthogonal reaction in a living body. Angew. Chem. Int. Ed.52, 10549–10552 (2013). [DOI] [PubMed] [Google Scholar]
- 240.Laughlin, S. T., Baskin, J. M., Amacher, S. L. & Bertozzi, C. R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science320, 664–667 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Laughlin, S. T. & Bertozzi, C. R. Imaging the glycome. Proc. Natl. Acad. Sci. USA106, 12–17 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Blackman, M. L., Royzen, M. & Fox, J. M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc.130, 13518–13519 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zeng, D., Zeglis, B. M., Lewis, J. S. & Anderson, C. J. The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J. Nucl. Med.54, 829–832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cook, B. E. et al. Pretargeted PET imaging using a site-specifically labeled immunoconjugate. Bioconjugate Chem.27, 1789–1795 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Reiner, T. & Zeglis, B. M. The inverse electron demand Diels-Alder click reaction in radiochemistry. J. Label. Comp. Radiopharm.57, 285–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Knight, J. C. & Cornelissen, B. Bioorthogonal chemistry: implications for pretargeted nuclear (PET/SPECT) imaging and therapy. Am. J. Nucl. Med. Mol. Imaging4, 96–113 (2014). [PMC free article] [PubMed] [Google Scholar]
- 247.Rossin, R. et al. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed.49, 3375–3378 (2010). [DOI] [PubMed] [Google Scholar]
- 248.Rossin, R. et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun.9, 1484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rossin, R., van Duijnhoven, S. M., Läppchen, T., van den Bosch, S. M. & Robillard, M. S. Trans-cyclooctene tag with improved properties for tumor pretargeting with the Diels-Alder reaction. Mol. Pharm.11, 3090–3096 (2014). [DOI] [PubMed] [Google Scholar]
- 250.Devaraj, N. K., Thurber, G. M., Keliher, E. J., Marinelli, B. & Weissleder, R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc. Natl. Acad. Sci. USA109, 4762–4767 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rossin, R., Läppchen, T., van den Bosch, S. M., Laforest, R. & Robillard, M. S. Diels-Alder reaction for tumor pretargeting: in vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J. Nucl. Med.54, 1989–1995 (2013). [DOI] [PubMed] [Google Scholar]
- 252.García, M. F. et al. 99mTc-bioorthogonal click chemistry reagent for in vivo pretargeted imaging. Bioorg. Med. Chem.24, 1209–1215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zeglis, B. M. et al. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J. Nucl. Med.54, 1389–1396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Evans, H. L. et al. A bioorthogonal 68Ga-labelling strategy for rapid in vivo imaging. Chem. Commun.50, 9557–9560 (2014). [DOI] [PubMed] [Google Scholar]
- 255.Houghton, J. L. et al. Pretargeted immuno-PET of pancreatic cancer: overcoming circulating antigen and internalized antibody to reduce radiation doses. J. Nucl. Med.57, 453–459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Herth, M. M. et al. Development of a 11C-labeled tetrazine for rapid tetrazine-trans-cyclooctene ligation. Chem. Commun.49, 3805–3807 (2013). [DOI] [PubMed] [Google Scholar]
- 257.Stéen, E. J. L. et al. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials179, 209–245 (2018). [DOI] [PubMed] [Google Scholar]
- 258.Peplow, M. Click chemistry targets antibody-drug conjugates for the clinic. Nat. Biotechnol.37, 835–837 (2019). [DOI] [PubMed] [Google Scholar]
- 259.Nerella, S. G., Singh, P. & Tulja, S. Carbon-11 patents (2012–2022): synthetic methodologies and novel radiotracers for PET imaging. Expert Opin. Ther. Pat.32, 817–831 (2022). [DOI] [PubMed] [Google Scholar]
- 260.Nerella, S. G., Bhattacharya, A., Thacker, P. S. & Tulja, S. Synthetic methodologies and PET imaging applications of fluorine-18 radiotracers: a patent review. Expert Opin. Ther. Pat.32, 455–473 (2022). [DOI] [PubMed] [Google Scholar]
- 261.Brooks, A. F. et al. Fluorine-18 patents (2009–2015). Part 1: novel radiotracers. Pharm. Pat. Anal.5, 17–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mossine, A. V. et al. Fluorine-18 patents (2009–2015). Part 2: new radiochemistry. Pharm. Pat. Anal.5, 319–349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hendrikse, H. et al. EANM position on the in-house preparation of radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging49, 1095–1098 (2022). [DOI] [PubMed] [Google Scholar]
- 264.Taïeb, D., Jha, A., Treglia, G. & Pacak, K. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr.-Relat. Cancer26, R627–r652 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Severin, G. W., Engle, J. W., Barnhart, T. E. & Nickles, R. J. 89Zr radiochemistry for positron emission tomography. Med. Chem.7, 389–394 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rao, D. V. Table of isotopes, edited by C. M. Lederer and V. S. Shirley. Med. Phys.6, 540–541 (1979). [Google Scholar]
- 267.Müller, C., Domnanich, K. A., Umbricht, C. A. & van der Meulen, N. P. Scandium and terbium radionuclides for radiotheranostics: current state of development towards clinical application. Br. J. Radiol.91, 20180074 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Severin, G. W., Engle, J. W., Valdovinos, H. F., Barnhart, T. E. & Nickles, R. J. Cyclotron produced 44gSc from natural calcium. Appl. Radiat. Isot.70, 1526–1530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mikolajczak, R. et al. Production of scandium radionuclides for theranostic applications: towards standardization of quality requirements. EJNMMI Radiopharm. Chem.6, 19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chakravarty, R. et al. Reactor produced [64Cu]CuCl2 as a PET radiopharmaceutical for cancer imaging: from radiochemistry laboratory to nuclear medicine clinic. Ann. Nucl. Med.34, 899–910 (2020). [DOI] [PubMed] [Google Scholar]
- 271.Pandey, M. K., Byrne, J. F., Jiang, H., Packard, A. B. & DeGrado, T. R. Cyclotron production of 68Ga via the 68Zn(p,n)68Ga reaction in aqueous solution. Am. J. Nucl. Med. Mol. Imaging4, 303–310 (2014). [PMC free article] [PubMed] [Google Scholar]
- 272.Nelson, B. J. B. et al. Taking cyclotron 68Ga production to the next level: expeditious solid target production of 68Ga for preparation of radiotracers. Nucl. Med. Biol.80-81, 24–31 (2020). [DOI] [PubMed] [Google Scholar]