Abstract
Background
While the blood pressure (BP)-lowering effect of renal denervation (RDN) has been established, long-term durability is a key prerequisite for a broader clinical implementation.
Aims
Our aims were to assess the long-term durability of the office BP (OBP)-lowering efficacy, antihypertensive medication (AHM) use, and safety of ultrasound RDN (uRDN).
Methods
Four weeks after withdrawal of AHM, patients with untreated daytime ambulatory BP ≥135/85 mmHg and <170/105 mmHg were randomised to uRDN (n=74) or sham (n=72) in the RADIANCE-HTN SOLO trial. Initiation of AHM was encouraged for home BP >135/85 mmHg following primary endpoint ascertainment at 2 months. Patients and physicians were unblinded at 6 months.
Results
Fifty-one of 74 patients (age: 53.9±11 years; 67% men) originally randomised to uRDN completed the 36-month follow-up. Initial screening OBP upon study entry was 145/92±14/10 mmHg on a mean of 1.2 AHM (range: 0-2.0). Baseline OBP after AHM washout was 154/99±13/8 mmHg. At 36 months, patients were on an average of 1.3 AHM (range: 0-3.0) with 8 patients on no AHM. OBP decreased by 18/11±15/9 mmHg from baseline to 36 months (p<0.001 for both). Overall, OBP control (<140/90 mmHg) improved from 29.4% at screening to 45.1% at 36 months (p=0.059). For patients uncontrolled at screening (n=36), systolic OBP decreased by 10.8 mmHg (p<0.001) at 36 months on similar AHM (p=0.158).
Conclusions
The safety and effectiveness of uRDN was durable to 36 months, with reduced OBP and improved OBP control despite a similar starting medication burden. No new uRDN-related long-term safety concerns were identified.
Introduction
Hypertension is the most potent modifiable risk factor for cardiovascular death worldwide1. However, hypertension control rates remain dismal2 and, specifically in the US, hypertension control rates have recently worsened3. Medication non-adherence and therapeutic inertia continue to be major obstacles to adequate blood pressure (BP) reduction among treated hypertensives4,5. Therefore, non-pharmacological approaches to BP lowering, in addition to lifestyle changes, may be of utility as add-on therapies for those with difficult-to-control hypertension.
Although the first large randomised sham-controlled trial6 of renal denervation (RDN) did not show a statistically significant difference in ambulatory or office BP (OBP) between patients who were randomised to RDN compared to those randomised to a sham procedure, a contemporary series of subsequent randomised sham-controlled trials have demonstrated the BP-lowering effects of radiofrequency and ultrasound RDN (uRDN) with between-group differences in office systolic BP (ranging from -6.5 to -7.7 mmHg)7,8,9,10,11. If these reductions in BP are sustained, they would likely imply a meaningful reduction of cardiovascular risk as seen with antihypertensive medication (AHM)12. At this stage, establishing the durability of RDN’s BP-lowering effect as well as its long-term safety is crucial before broader implementation can be recommended. The durability of renal denervation up to 36 months has been shown in the Global SYMPLICITY Registry13 and in the SPYRAL HTN-ON MED study14. We report for the first time the long-term OBP response and safety of uRDN in hypertensive patients at 36 months in the sham-controlled RADIANCE-HTN SOLO trial11.
Methods
Study design
The RADIANCE-HTN SOLO trial design and results have been described previously11. The study compared changes in ambulatory and OBP with uRDN with the Paradise RDN system (ReCor Medical) to a sham procedure consisting of a renal angiogram only. The study was approved by local ethics committees or institutional review boards and conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent. Between March 28, 2016 and December 28, 2017, patients were recruited into the international, multicentre, randomised, sham-controlled RADIANCE-HTN SOLO trial from 21 centres in the United States and 18 in Europe (located in France, Germany, the Netherlands, Belgium, and the United Kingdom).
Study population
Key inclusion criteria as previously described15 were uncontrolled hypertension with OBP ≥140/90 mmHg and <180/110 mmHg on 0-2 AHM or controlled hypertension with OBP <140/90 mmHg on 1 or 2 AHM, an estimated glomerular filtration rate (eGFR) of ≥40 mL/min/1.73 m² (based on the Modification of Diet in Renal Disease formula), suitable renal artery anatomy confirmed by imaging, and no history of cardiovascular or cerebrovascular events. Patients who met these initial inclusion criteria were taken off all AHM and qualified for randomisation if their mean daytime ambulatory BP was ≥135 mmHg systolic and ≥85 mmHg diastolic and renal anatomy was considered appropriate for the procedure. One hundred and forty-six patients were randomised, 74 to the uRDN arm and 72 to the sham arm. Thirty-six of the patients in the sham arm crossed over to the uRDN arm16. The current analysis focuses on the 51 patients who were randomised to uRDN and completed 36-month follow-up.
Procedures
Throughout the first 2 months of follow-up, patients were to remain off AHM, unless specified safety BP criteria were exceeded (180/110 mmHg for office or 170/105 mmHg for home). Between 2 and 6 months, if the monthly measured home BP was ≥135/85 mmHg, a standardised stepped-care AHM protocol was recommended as previously described15. Patients and clinical staff were unblinded to group assignment (uRDN or sham) after the 6-month efficacy and safety endpoints were ascertained. At that point, the protocol recommended intensification of AHM if the assessed home BP was ≥135/85 mmHg; however, it was not a requirement and therefore, after 6 months, AHM were prescribed at the treating physician’s and patient's discretion per community standard of care.
At 24 and 36 months, seated OBP, heart rate (HR), adverse events, and concomitant medications were obtained. OBP was measured with the Omron M10-IT as previously described11. Ambulatory and home BP were not collected at the longer-term follow-up.
Outcomes
The main efficacy endpoint was the change in OBP from baseline (off antihypertensive medications) to 36 months. Additionally, the change in OBP from screening (on 0 to 2 AHM) to 36 months was assessed. Medication burden was assessed by the total number of AHM (regardless of the dose)11. To further confirm long-term durability, changes in OBP and AHM use between the 12- and 36-month follow-up visits were assessed. Additional subgroup analyses were performed on those patients who were uncontrolled based on OBP at screening (≥140 systolic or ≥90 mmHg diastolic).
Safety assessments were performed as previously reported. All prespecified potential device- or procedure-related and/or serious adverse events reported by study sites were sent for independent adjudication. An independent data safety monitoring board reviewed safety study data quarterly for all enrolled patients.
Statistical analysis
Analysis was performed on the 51 uRDN patients with OBP data available at 36 months. Data in an additional nine enrolled patients reaching 36 months were not available due to logistical restrictions caused by the COVID-19 pandemic. There was no imputation for missing OBP values.
Results from and comparison to the sham group are not provided given that 33 (46%) of patients in the sham group crossed over prior to 36 months and there were only 20 patients (28%) remaining at 36 months with office BP data available (Supplementary Figure 1).
Continuous variables are expressed as mean (range) and mean±standard deviation, and categorical variables as number (n) and percentage (%). Paired t-tests and Wilcoxon signed-rank tests were used to compare BP values and the number of medications between timepoints. All analyses were performed using Statistical Analysis System (SAS) software version 9.4 (SAS Institute). A p-value lower than 0.05 (two-sided) was considered significant.
Results
Study population
Of the 74 patients initially randomised to the uRDN group, 7 were lost to follow-up, 6 withdrew consent to participate, 9 missed their 36-month office visit, and 1 did not receive any emissions at the time of randomisation and received a uRDN procedure at 23 months. The remaining 51 patients had 36-month OBP measurements and were included in this analysis. (Supplementary Figure 1). The mean age of the analysis population was 53.9 years, 66.7% were male, 82.4% Caucasian, 52.9% had abdominal obesity and 7.8% reported sleep apnoea (Table 1). The population with data at 36 months was similar to the overall randomised population (Supplementary Table 1)11. Screening OBP before AHM washout was 145/92±14/10 mmHg and baseline OBP after washout was 154/99±13/8 mmHg. The average number of AHM at screening was 1.2±0.7 (range: 0-2.0). At screening, 15.7% of patients were not receiving any AHM, 51.0% were receiving one, and 33.3% were receiving two.
Table 1. Baseline characteristics.
| Clinical characteristics | n=51 | |
|---|---|---|
| Age, years | 53.9±11.4 | |
| Female sex | 33.3 (17/51) | |
| Race | White | 82.4 (42/51) |
| Black | 13.7 (7/51) | |
| Other | 3.9 (2/51) | |
| Body mass index, kg/m2 | 29.8±6.1 | |
| Abdominal obesity | 52.9 (27/51) | |
| eGFR – ml/min/1.73 m2 | 86.1±17.6 | |
| eGFR <60 ml/min/1.73 m2 | 0 (0/51) | |
| Type 2 diabetes | 0 (0/51) | |
| Sleep apnoea | 7.8 (4/51) | |
| Screening blood pressure (before anti-HTN med washout) | ||
| Office BP, mmHg | 144.5/92.1±13.6/10.4 | |
| Baseline blood pressure (after anti-HTN med washout) | ||
| Office BP, mmHg | 153.9/99.1±12.7/7.8 | |
| Daytime ABP, mmHg | 150.8/93.2±7.4/4.9 | |
| 24-hour ABP, mmHg | 142.7/87.2±7.8/5.0 | |
| Antihypertensive medications | ||
|
Number of antihypertensive medications at screening |
1.2±0.7 | |
| Antihypertensive load index at screening | 0.5±0.4 | |
| Number of antihypertensive medications at screening | 0 | 15.7 (8/51) |
| 1 medication | 51.0 (26/51) | |
| 2 medications in combination | 33.3 (17/51) | |
| Types of medication at screening | ||
| Renin-angiotensin system blockers | 58.8 (30/51) | |
| Angiotensin-converting enzyme inhibitor | 37.3 (19/51) | |
| Angiotensin receptor blocker | 19.6 (10/51) | |
| Direct renin inhibitor | 2.0 (1/51) | |
| Calcium channel blocker | 23.5 (12/51) | |
| Thiazide-like diuretic | 25.5 (13/51) | |
| Alpha-1 receptor blocker | 3.9 (2/51) | |
| Beta blocker | 5.9 (3/51) | |
| Values are mean±SD or % (n/N). Abdominal obesity was defined as a waist circumference >102 cm for men and >88 cm for women. ABP: ambulatory blood pressure; BP: blood pressure; eGFR: estimated glomerular filtration rate; HTN: hypertension; M: months | ||
Outcomes
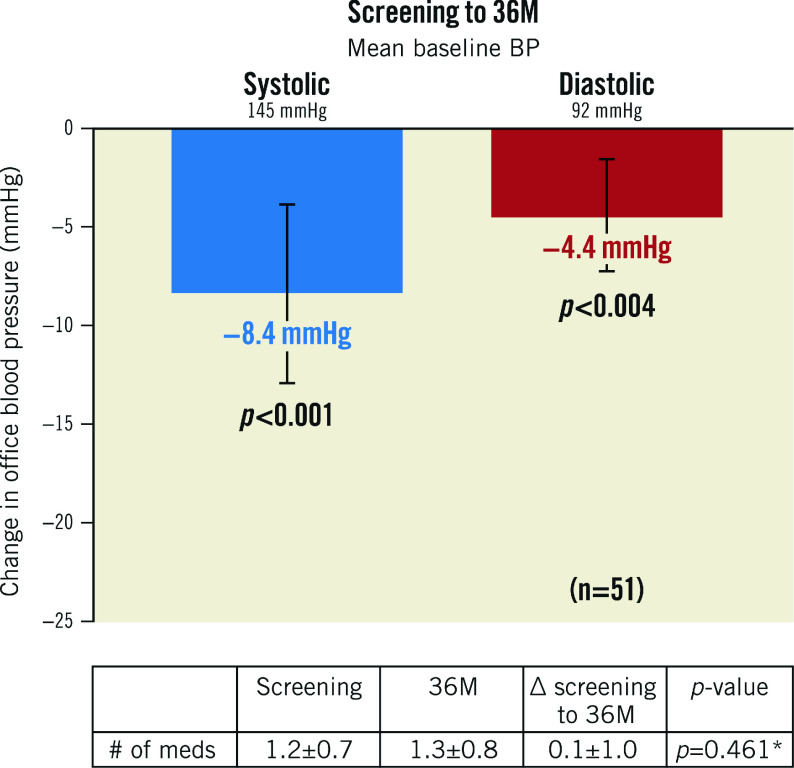
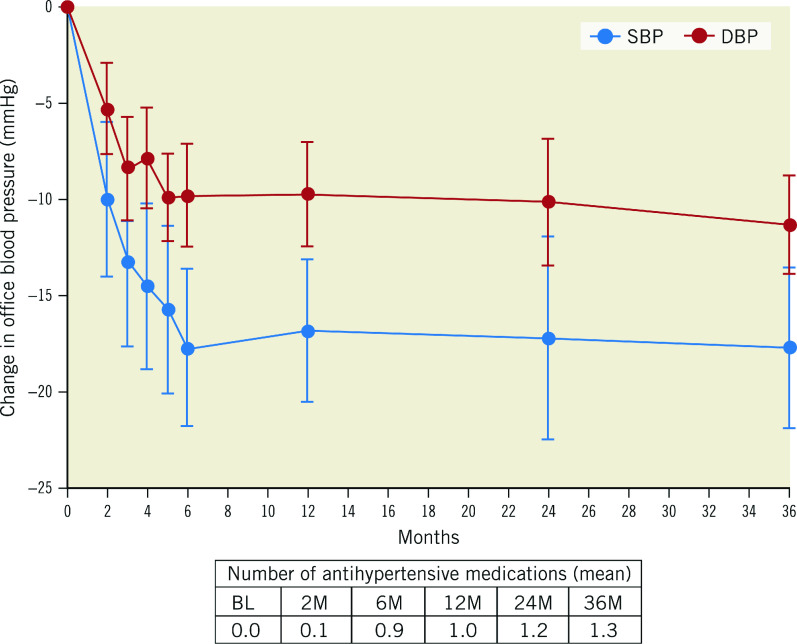
After the AHM washout following the study screening, BP increased by 9.3±12 mmHg (screening: 144.5±13.6 mmHg; baseline: 153.9±12.7 mmHg). However, after randomisation to uRDN, BP fell by 10.0±14.7 mmHg at 2 months and then, after reinstitution of medications, it fell by 17.7±15 mmHg from baseline to 6 months (additive effect of uRDN plus medications). The average decrease in office systolic/diastolic BP (SBP/DBP) from baseline (after AHM washout) to 36 months was 18/11±15/9 mmHg, respectively (p<0.001 for both) (Central illustration). The change from screening (before washout) in office SBP/DBP to 36 months was -8/-4±17/11 mmHg (p<0.001 and p=0.004, respectively) (Figure 1). AHM at screening was 1.2±0.7, compared to 1.3±0.8 at 36 months (p=0.461) (Table 1, Supplementary Table 2). Furthermore, hypertension (HTN) control (<140/90 mmHg) improved from 29.4% (15/51) at screening to 45.1% (23/51) at 36 months (p=0.059), on a similar number of AHM (p=0.213). Of the 23 patients who were controlled at 36 months, 2 were on no AHM. On average, patients in the RDN group had an office SBP ≤140 mmHg at 54% of their available follow-up visits from baseline to 36 months.
Central illustration. Office blood pressure and medication usage through to 36 months.
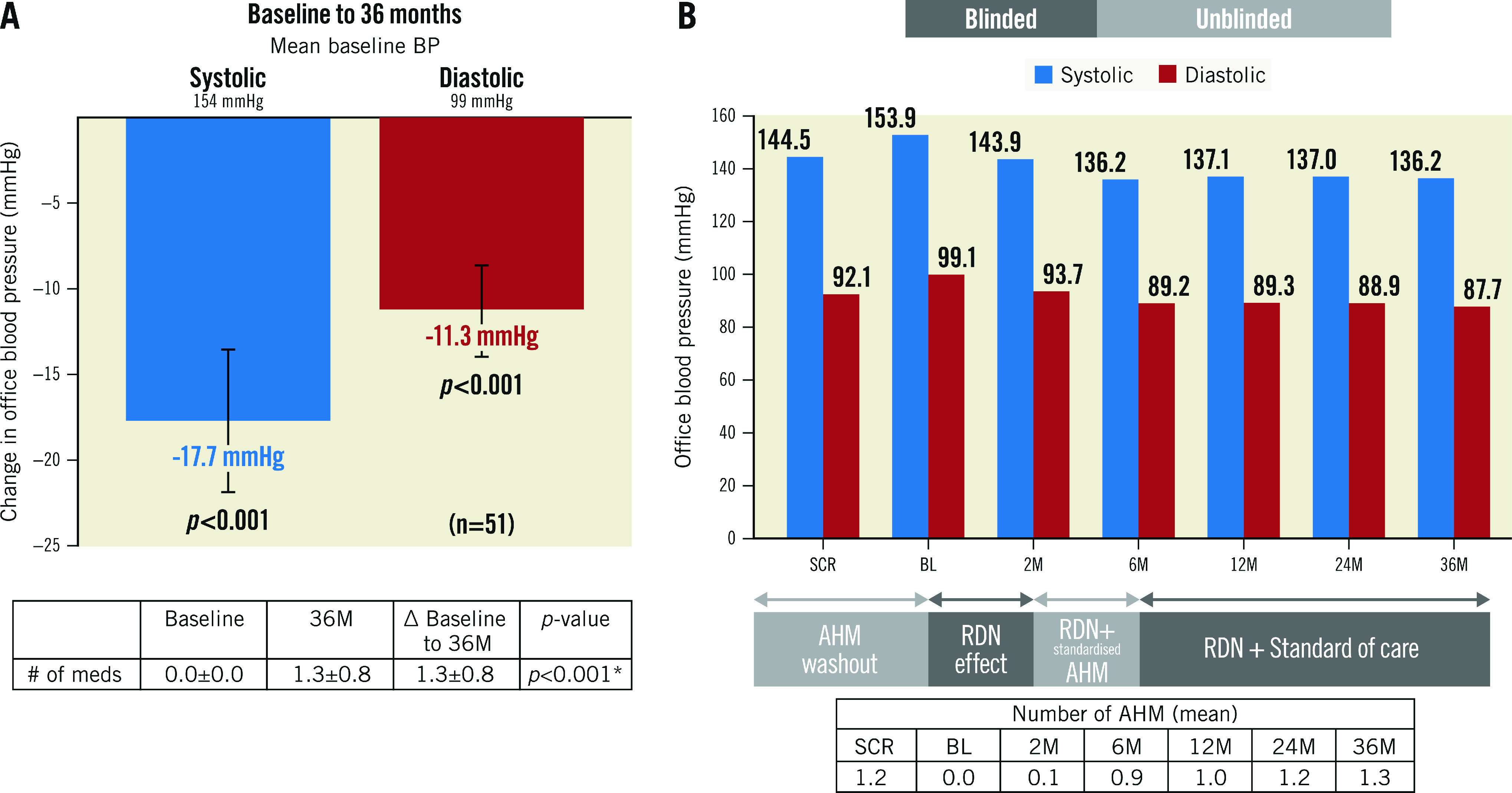
A) Change in office blood pressure from baseline to 36 months. B) Office blood pressures and medication usage from screening to 36 months. Values are presented as mean. Error bars represent 95% confidence interval. Wilcoxon signed-rank tests. AHM: antihypertensive medications; BL: baseline; BP: blood pressure; M: months; RDN: renal denervation; SCR: screening
Figure 1. Change in office blood pressure from screening (pre-washout). Values are presented as mean±SD.
Error bars represent 95% confidence interval. *Wilcoxon signed-rank tests. BP: blood pressure; M: months
The post-procedural trend in OBP between 12 and 36 months remained flat without a statistically significant increase in either office SBP (ΔSBP -0.9±18.2 mmHg; p=0.713) or DBP (ΔDBP -1.6±11.7 mmHg; p=0.341). This effect of uRDN was achieved with a stable number of AHM compared to screening (p=0.461) but a very modest increase of AHM between the 12-month and 36-month follow-up visit (Δmedication number 0.3±0.8; p=0.023) (Figure 2).
Figure 2. Office blood pressure change from baseline to 36 months in the analysis population.
Values are presented as mean. Error bars represent 95% confidence interval. DBP: diastolic blood pressure; M: months; SBP: systolic blood pressure
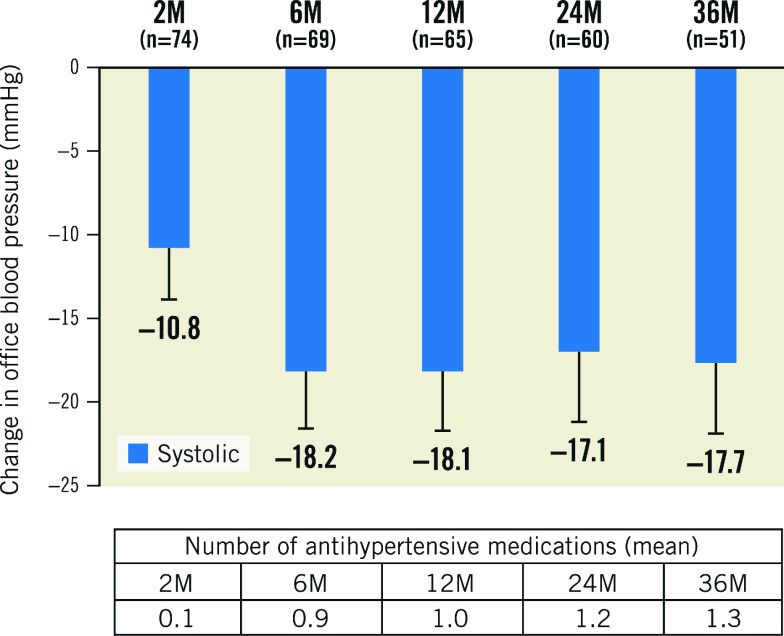
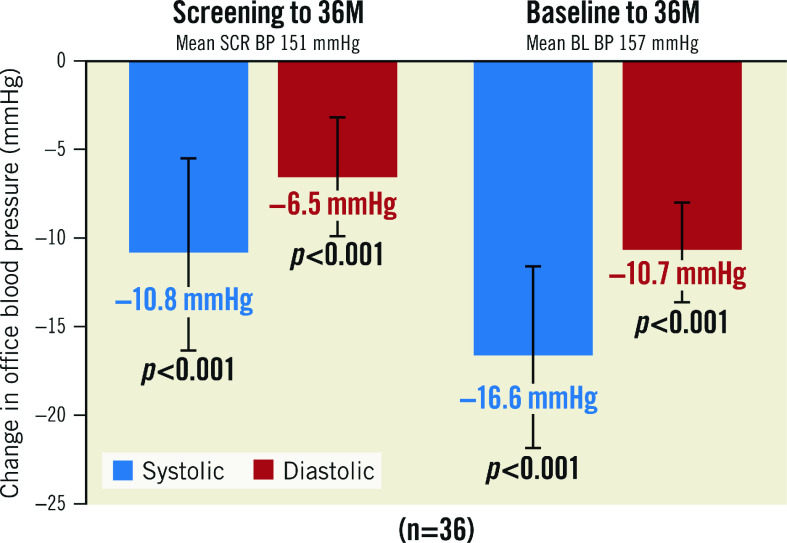
The OBP decrease in the 36-month analysis population was consistent with the OBP decrease observed in the available patient cohorts analysed at 2, 6, 12 and 24 months. (Figure 3, Supplementary Figure 2). Patients with uncontrolled OBP at screening (SBP ≥140 or DBP ≥90 mmHg; n=36) on 0.9±0.6 AHM had a strong decrease in office SBP/DBP at 36 months from screening (-11/-7±17/11 mmHg; p<0.001 for both) and from baseline (off medications, -17/-11±16/9 mmHg; p<0.001 for both) (Figure 4).
Figure 3. Office blood pressure changes from baseline for HTN-SOLO RDN population.
Values are presented as mean. Error bars represent 95% confidence interval. Baseline office systolic BP: 2M: 154.5; 6M: 154.7; 12M: 154.3; 24M: 154.4; 36M: 153.9. M: months
Figure 4. Office blood pressure change for those uncontrolled at screening.
Values are presented as mean. Error bars represent 95% confidence interval. BL: baseline; BP: blood pressure; M: months; SCR: screening
Safety outcomes
There were no new safety signals during the 36-month follow-up (Table 2). As previously reported, one subject had renal artery stenosis with stent placement 6 months post-procedure. One additional subject was classified as having at least 50% narrowing of the right renal artery ostium by calcific atherosclerosis at 779 days post-procedure. However, an independent radiologist review classified the stenosis as 25% and present at the time of pre-procedure imaging. There was one episode of transient ischaemic attack at 458 days and one hypertensive event at 1,076 days after renal denervation. None of the events were deemed as being related to the device or procedure.
Table 2. Safety events.
| Events | % |
|---|---|
| Death | 0 (0/74) |
| Acute renal injury* | 0 (0/74) |
| End stage renal disease, the need for permanent renal replacement therapy (i.e., the need for dialysis); doubling of plasma creatinine | 0 (0/74) |
| Significant embolic events resulting in end organ damage | 0 (0/74) |
| Any renal artery complication requiring intervention (e.g., dissection; perforation) | 0 (0/74) |
| Major access site complications requiring intervention | 0 (0/74) |
| Hypertensive emergency resulting in hospitalisation | 1.4 (1/74) |
| Hypotensive emergency resulting in hospitalisation | 0 (0/74) |
| Hospitalisation for heart failure | 0 (0/74) |
| Stroke, transient ischaemic attack, cerebrovascular accident¶ | 1.4 (1/74) |
| Acute myocardial infarction (STEMI/non-STEMI) | 0 (0/74) |
| Any coronary revascularisation | 0 (0/74) |
| Significant (>50%) and severe (>75%) new onset renal stenosis as diagnosed by duplex ultrasound and confirmed by renal CTA/MRA or as diagnosed/confirmed by renal CTA/MRA | 1.4 (1/74)‡ |
| Need for renal artery angioplasty or stenting | 1.4 (1/74)§ |
| Values are % (n/N). *Defined as: Increase in plasma/serum creatinine by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hrs of the procedure or, increase in serum/plasma creatinine to ≥1.5 times baseline known to have occurred within 7 days post-procedure or urine volume <0.5 ml/kg/h for 6 hours; ¶TIA at day 458 due to underlying risk factors, timing does not support relationship to procedure or study device; ‡One patient had renal artery stenosis of at least 50% as classified by the site at 779 days post-procedure. Independent reviewer classified it as 25% and present at the time of pre-procedure imaging; §One patient had unrecognised pre-existing renal artery stenosis of 44% and underwent stenting for the lesion which measured 57% prior to stent placement at 6 months. CTA: computed tomography angiography; MRA: magnetic resonance angiography; STEMI: ST-elevation myocardial infarction | |
Discussion
In this long-term follow-up of patients with mild-to-moderate hypertension who were randomised to uRDN in the RADIANCE-HTN SOLO trial11, we present 3 major findings: 1) sustained decreases in OBP from screening (before washout of AHM) and from baseline (after washout) were observed and maintained through to 36 months; 2) these reductions of OBP were seen despite a similar amount of prescribed AHM at 36 months compared to screening; 3) among patients who had uncontrolled BP at screening, changes of OBP from screening were better than those of the entire 36-month cohort.
After disappointing negative results of a large sham-controlled, randomised trial of radiofrequency RDN compromised by several confounding factors6, a number of well-designed randomised clinical trials have now demonstrated the BP-reducing effects of radiofrequency RDN7,8,9 and uRDN10,11 over sham procedures. While the between-group difference in office systolic BP in these randomised trials (ranging from -6.5 to -7.7 mmHg)7,8,9,10,11 has been lower than those seen in uncontrolled early studies17, this difference is in line with results reported in a recent meta-analysis of drug-placebo trials in hypertension18 and would still be expected to provide meaningful reductions in hypertension-related cardiovascular risk as seen with AHM12.
A meta-analysis of drug-placebo trials in patients with hypertension showed a 5.1 mmHg difference in office BP favouring active treatment versus placebo from 12 months to the end of follow-up (average 4 years)18. A drop of 5 mmHg in office BP has been shown to result in a 10% decreased risk of major cardiovascular events19. Given the known risk reductions from BP drops of this magnitude in pharmaceutical studies, similar risk reductions may also be expected with uRDN. Moreover, a therapy which is “always on” and not affected by medication adherence should have a better “time-in-target BP range”20 compared to antihypertensive therapy with medications that could be weakened by uncertain adherence5. An improved “time-in-target BP range” has been associated with lower cardiovascular events20.
In this study, we have shown a durable change in office BP of -17.7 mmHg from baseline to 36 months with the addition of 1.3 AHM, and -8.4 mmHg from screening to 36 months on similar AHM. These results are consistent with the 36-month results of the RDN group in the SPYRAL HTN-ON MED study, which used a different form of energy to obtain RDN14. In this study, OBP in the RDN group was reduced by 20.9 mmHg at 36 months compared to baseline with the addition of approximately 1 AHM. This consistency between the studies reinforces the available evidence for the durability of RDN.
In the SPYRAL HTN-ON MED 36-month results14, they also reported OSBP and 24-hour ASBP data compared to the sham group using the last observation carried forward (LOCF) method for the crossover patients. In our study, given that there were only 20 control patients (28%) with office BP data available at 36 months and 33 patients who had crossed over before 36 months, we would need to impute 62% (33/53) of the data, which we did not feel was methodologically sound.
USA HTN control rates peaked in 2010 at 53% and have decreased to 43.7% in 20183 despite the availability of an array of AHM classes. In addition, HTN control rates may have further decreased due to the COVID-19 pandemic and the impact it is having on people’s blood pressure21.
Beyond COVID-19, the reason for the decline in hypertension control rates is unclear. While medication non-adherence is a widely accepted cause of poor HTN control5,22, it has to be assumed that non-adherence rates should remain relatively stable or improve with ongoing campaigns to raise awareness of the importance of HTN control23. As well, clinical inertia -the reluctance of clinicians to intensify AHM when facing poorly controlled BP - could be one reason for worsening control rates14,24. More lenient BP goals in higher-risk older adults may have contributed to worsening HTN control rates after 20143, a trend that hopefully will change direction as the 2017 AHA/ACC and 2018 ESC hypertension guidelines and their more stringent BP goals for most hypertensives start to gain traction25,26.
The durability of BP reductions after uRDN seen in our study is reassuring in several ways. First, it confirms longer-term BP reductions without the late upward trend seen in previous uncontrolled trials27 and confirms published registry and randomised study data13,14. Second, adherence to antihypertensive medications decreases over time28. Because most patients in RADIANCE-HTN SOLO were restarted on medications during the follow-up period, we could have anticipated a slight uptrend in BP over the 30-month period that followed this medication titration from worsening medication adherence. However, this effect was not observed in this study. Furthermore, the natural expected trajectory of BP is such that SBP increases with the patient's age29. Again, one would expect an upward trend in BP over time, due to this trajectory, during long-term follow-up after uRDN; but again, we did not observe such a trend. Finally, OBP is affected by the white-coat reaction, a common neurogenically mediated alerting reaction which leads to higher office than out-of-office BP. This white-coat tendency has been shown to increase over time30. This would also explain a slight upward trend in OBP during the years after uRDN. The fact that we did not see any increase in BP despite these 3 well-established causes for positive BP trajectories over time is reassuring and suggests that concerns of renal nerve regeneration are not warranted.
Although the hypertension control rates slightly increased after uRDN, they remained disappointing, especially in light of the fact that most patients were enrolled at facilities with experience in treating hypertension. These data further emphasise the need for efforts to achieve greater degrees of BP control for patients with hypertension. It is possible that the less frequent yearly follow-ups beyond the 12-month visit led to therapeutic inertia, with the long intervals between visits making adequate titration of AHM difficult.
Limitations
Our study was limited by several factors, including that only OBP measurements and not ambulatory BP were recorded after the 12-month follow-up visit and the study was not powered for OBP at the long-term timepoints. In addition, chemical analysis for determination of medication adherence and measurement of eGFR as a measure of kidney function were no longer performed as a protocol requirement. However, instances of worsening renal function or renal failure were required to be reported as adverse events and did not occur in this cohort during long-term follow-up.
Given that patients and physicians were unblinded after the 6-month visit and patients in the sham group could cross over after the primary endpoint was met (after 12 months), no between-group differences can be evaluated from our data16. Even though the baseline characteristics and decrease in OBP of this cohort are similar to the overall cohort of 74, we cannot be sure that these results represent the full cohort. Finally, OBP is affected by many factors, including the white-coat or alerting reaction30 and poor BP measurement techniques. To minimise these problems, we estimated OBP from averaging repeat BP measurements at each office encounter (which reduces the white-coat effect) and used the same BP cuff size and monitors during follow-up visits to further reduce measurement errors. Importantly, OBP assessment remains the mainstay of hypertension management in clinical practice25.
Conclusions
In this analysis of patients with mild-to-moderate hypertension who underwent uRDN in the RADIANCE-HTN SOLO trial, we found that OBP reductions were sustained throughout the 36 months of follow-up (-17.7/11.3 mmHg) on a stable number of antihypertensives from screening (1.3 medications). Our results suggest that long-term BP reductions and improvement of HTN control rates can be achieved with a combination of uRDN and antihypertensive medications. Importantly, long-term follow-up did not show any unexpected safety signals of uRDN.
Impact on daily practice
Both durability and long-term safety are prerequisites for broader implementation of uRDN for the treatment of hypertension. Here we establish 36-month follow-up data on OBP reduction, as well as safety data among patients with mild-to-moderate hypertension who underwent uRDN. The main findings of this study are that the BP-lowering effect of uRDN was durable through to 36 months, with reduced OBP and improved OBP control on a relatively stable number of antihypertensive medications as compared to screening. During long-term follow-up, there were no new safety signals.
Appendix. Collaborators
Jan Basile, MD; Seinsheimer Cardiovascular Health Program, Medical University of South Carolina and Ralph H. Johnson VA Medical Center, Charleston, SC, USA. Michael J. Bloch, MD; Department of Medicine, University of Nevada School of Medicine, Vascular Care, Renown Institute of Heart and Vascular Health, Reno, NV, USA. Melvin D. Lobo, PhD, FRCP; St Bartholomew’s Hospital, London, UK and William Harvey Research Institute, NIHR Barts Cardiovascular Biomedical Research Centre, Queen Mary University London, London, UK. Felix Mahfoud, MD; Klinik für Innere Medizin III, Saarland University Hospital, Homburg/Saar, Germany; Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, USA. Roland E. Schmieder, MD; Nephrology and Hypertension, University Hospital Erlangen, Friedrich Alexander University, Erlangen, Germany. Andrew S.P. Sharp, MD; University Hospital of Wales, Cardiff and University of Exeter, Exeter, UK. Candace K. McClure, PhD; NAMSA, Minneapolis, MN, USA. Glenn Chertow, MD, MPH; Nephrology, Stanford University, Stanford, CA, USA. Thomas Kahan, MD, PhD; Cardiology, Karolinska Institutet, Stockholm, Sweden. Harold Dauerman, MD; Interventional Cardiology, University of Vermont Medical Center, Burlington, VT, USA. Steven Ullery; Biostatistics, NAMSA, Minneapolis, MN, USA.
Supplementary data
. Baseline characteristics of all RADIANCE-HTN SOLO patients randomised to uRDN.
. Number and type of antihypertensive medications at 36 months.
. Patient disposition.
. Office blood pressure changes for the 24-month population.
Acknowledgments
Acknowledgements
The trial executive committee designed the protocol in conjunction with the sponsor. The sponsor was responsible for theselection of clinical sites in collaboration with the executive committee, as well as collection, monitoring, and analysis of the data. The article was written by the lead author (Florian Rader) with significant contributions from the co-authors. All authors had access to all the data, and Florian Rader was responsible for the decision to submit the manuscript. Candace McClure, PhD, employee of NAMSA, helped with statistical programming.
Funding
The RADIANCE-HTN SOLO study was funded by ReCor Medical.
Conflict of interest statement
F. Rader has received personal fees from ReCor Medical, Medtronic and Bristol-Myers Squibb. A.J. Kirtane reports institutional funding to Columbia University and/or theCardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, and Merck. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for consulting and/or speaking engagements in which DrKirtane controlled the content. He reports consulting fees from IMDS; travel expenses/meals from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. J. Daemen has received institutional research support from ReCor Medical, Medtronic, Boston Scientific, Abbott Vascular, Acist Medical, AstraZeneca, Pie Medical, and Pulse Cath and is a consultant toReCor Medical, Sanofi, Medtronic, Acist Medical, Boston Scientific, Pie Medical, and Pulse Cath. P. Lurz has received institutional grantsfrom ReCor Medical, Medtronic and Edwards Lifesciences and speaker honoraria from ReCor Medical. M. Saxena has received institutional grant fundingand personal fees from ReCor Medical. M. Azizi has received research grants from the European Horizon 2020 programme; has receivedgrant support and nonfinancial support from ReCor Medical and Idorsia; and has received personal fees from Alnylam Pharmaceutical, Novartis, Medtronic and Poxel Pharma. A.P. Scicli is an employee of ReCor Medical. L. Thackeray is an employee of NAMSA, anda contractor for ReCor Medical. M.A. Weber has received personal fees from ReCor Medical, Medtronic, Boston Scientific, and Ablative Solutions. J. Basile has received research grants from ReCor Medical and Ablative Solutions and personal fees from Medtronic, Up-to-Date and Eli Lilly. M.J. Bloch has received personal fees from Recor Medical and Medtronic. M.D. Lobo has received personal fees from ReCor Medical, Medtronic, Ablative Solutions, and Vascular Dynamics,and research grants from Medtronic. F. Mahfoud has received research grants from Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219), Bayer, Boehringer Ingelheim, Medtronic, and ReCor Medical and personal fees from Bayer, Boehringer Ingelheim, Medtronic, and ReCor Medical. R.E. Schmieder has received research grants and personal fees from Recor Medical, Medtronic, and Ablative Solutions. A.S.P. Sharp has received personal fees from Recor Medical, Medtronic, Philips, and Boston Scientific. C.K. McClure is an employee of NAMSA and a contractor for ReCor Medical. G. Chertow has received personal fees from ReCor Medical, Akebia, Ardelyx, AstraZeneca, Cricket, Gilead, Miromatrix, Reata, Sanifit, Vertex, Bayer, Mineralys, Palladio, Satellite Healthcare and other support from Ardelyx, Durect, CloudCath, Miromatrix, and Outset. T. Kahan has received consultingfees from ReCor Medical and research grants from Medtronic. H. Dauerman has received consulting fees from ReCor Medical and Medtronic and research grants from Medtronic and Boston Scientific. S. Ullery is an employee of NAMSA and a contractor for ReCor Medical.The other authors have no conflicts of interest to declare.
Abbreviations
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- HR
heart rate
- HTN
hypertension
- SBP
systolic blood pressure
- uRDN
ultrasound renal denervation
- ASBP
ambulatory systolic blood pressure
- OBP
office blood pressure
Contributor Information
Florian Rader, Cedars-Sinai Smidt Heart Institute, Los Angeles, CA, USA.
Ajay J. Kirtane, Columbia University Medical Center/New York-Presbyterian Hospital and the Cardiovascular Research Foundation, New York, NY, USA.
Yale Wang, Minneapolis Heart Institute, Abbott Northwestern Hospital, Minneapolis, MN, USA.
Joost Daemen, Erasmus MC, University Medical Center Rotterdam, Department of Cardiology, Rotterdam, the Netherlands.
Philipp Lurz, Department of Internal Medicine/Cardiology, Heart Center Leipzig, University of Leipzig, Leipzig, Germany.
Jeremy Sayer, The Essex Cardiothoracic Centre, Essex, UK.
Manish Saxena, Barts NIHR Biomedical Research Centre, William Harvey Research Institute, Queen Mary University of London, London, UK.
Terry Levy, Royal Bournemouth Hospital, Bournemouth, UK.
Andrea P. Scicli, ReCor Medical, Inc., Palo Alto, CA, USA.
Lisa Thackeray, NAMSA, Minneapolis, MN, USA.
Michel Azizi, Université de Paris, Paris, France; AP-HP, Hôpital Européen Georges-Pompidou, Hypertension Department and DMU CARTE, Paris, France; INSERM, CIC1418, Paris, France.
Michael A. Weber, Division of Cardiovascular Medicine, State University of New York, Downstate Medical Center, New York, NY, USA.
References
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen, van Donkelaar, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–80. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324:1190–200. doi: 10.1001/jama.2020.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin A, Coutts L, Zanisi L, Wierzbicki AS, Shankar F, Chowienczyk PJ, Floyd CN. Impact of Therapeutic Inertia on Long-Term Blood Pressure Control: A Monte Carlo Simulation Study. Hypertension. 2021;77:1350–9. doi: 10.1161/HYPERTENSIONAHA.120.15866. [DOI] [PubMed] [Google Scholar]
- Chang TE, Ritchey MD, Park S, Chang A, Odom EC, Durthaler J, Jackson SL, Loustalot F. National Rates of Nonadherence to Antihypertensive Medications Among Insured Adults With Hypertension, 2015. Hypertension. 2019;74:1324–32. doi: 10.1161/HYPERTENSIONAHA.119.13616. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M SPYRAL HTN-OFF MED trial investigators*. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–70. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, East C, Lee DP, Ma A, Ewen S, Cohen DL, Wilensky R, Devireddy CM, Lea J, Schmid A, Weil J, Agdirlioglu T, Reedus D, Jefferson BK, Reyes D, D'Souza R, Sharp ASP, Sharif F, Fahy M, DeBruin V, Cohen SA, Brar S, Townsend RR SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51. doi: 10.1016/S0140-6736(20)30554-7. [DOI] [PubMed] [Google Scholar]
- Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, Rump LC, Persu A, Basile J, Bloch MJ, Daemen J, Lobo MD, Mahfoud F, Schmieder RE, Sharp ASP, Weber MA, Sapoval M, Fong P, Pathak A, Lantelme P, Hsi D, Bangalore S, Witkowski A, Weil J, Kably B, Barman NC, Reeve-Stoffer H, Coleman L, McClure CK, Kirtane AJ RADIANCE-HTN investigators. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–86. doi: 10.1016/S0140-6736(21)00788-1. [DOI] [PubMed] [Google Scholar]
- Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- Mahfoud F, Böhm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope L, Schlaich M, Williams B, Fahy M, Mancia G. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474–82. doi: 10.1093/eurheartj/ehz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Dimitriadis K, Choi JW, East C, D'Souza R, Sharp ASP, Ewen S, Walton A, Hopper I, Brar S, McKenna P, Fahy M, Böhm M. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399:1401–10. doi: 10.1016/S0140-6736(22)00455-X. [DOI] [PubMed] [Google Scholar]
- Mauri L, Kario K, Basile J, Daemen J, Davies J, Kirtane AJ, Mahfoud F, Schmieder RE, Weber M, Nanto S, Azizi M. A multinational clinical approach to assessing the effectiveness of catheter-based ultrasound renal denervation: The RADIANCE-HTN and REQUIRE clinical study designs. Am Heart J. 2018;195:115–29. doi: 10.1016/j.ahj.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Mahfoud F, Bloch MJ, Azizi M, Wang Y, Schmieder RE, Lobo MD, Sharp ASP, Daemen J, Basile J, Weber MA, Scicli AP, McClure CK, Kirtane AJ. Changes in blood pressure after crossover to ultrasound renal denervation in patients initially treated with sham in the RADIANCE-HTN SOLO trial. EuroIntervention. 2021;17:e1024–32. doi: 10.4244/EIJ-D-21-00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- Canoy D, Copland E, Nazarzadeh M, Ramakrishnan R, Pinho-Gomes AC, Salam A, Dwyer JP, Farzadfar F, Sundstrom J, Woodward M, Davis BR, Rahimi K. Blood Pressure Lowering Treatment Trialists' Collaboration. Antihypertensive drug effects on long-term blood pressure: an individual-level data meta-analysis of randomised clinical trials. Heart. 2022 Jan 20 [Epub ahead of print] doi: 10.1136/heartjnl-2021-320171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood Pressure Lowering Treatment Trialists Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–36. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatani N, Dixon DL, Van Tassell, Fanikos J, Buckley LF. Systolic Blood Pressure Time in Target Range and Cardiovascular Outcomes in Patients With Hypertension. J Am Coll Cardiol. 2021;77:1290–9. doi: 10.1016/j.jacc.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffin LJ, Kaufman HW, Chen Z, Niles JK, Arellano AR, Bare LA, Hazen SL. Rise in Blood Pressure Observed Among US Adults During the COVID-19 Pandemic. Circulation. 2022;145:235–7. doi: 10.1161/CIRCULATIONAHA.121.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM American Heart Association Professional Education C. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- Casey DE, Jr , Daniel DM, Bhatt J, Carey RM, Commodore-Mensah Y, Holmes A, Smith AP, Wozniak G, Wright JT, Jr. Controlling High Blood Pressure: An Evidence-Based Blueprint for Change. Am J Med Qual. 2021;37:22–31. doi: 10.1097/01.JMQ.0000749856.90491.43. [DOI] [PubMed] [Google Scholar]
- Faria C, Wenzel M, Lee KW, Coderre K, Nichols J, Belletti DA. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3:267–76. doi: 10.1016/j.jash.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Williams B, Mancia G, Spiering W, Agabiti Rosei, Azizi M, Burnier M, Clement D, Coca A, De Simone, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen S, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder R, Shlyakhto E, Tsioufis K, Aboyans V, Desormais I. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27:314–40. doi: 10.1080/08037051.2018.1527177. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–9. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- Kim S, Shin DW, Yun JM, Hwang Y, Park SK, Ko YJ, Cho B. Medication Adherence and the Risk of Cardiovascular Mortality and Hospitalization Among Patients With Newly Prescribed Antihypertensive Medications. Hypertension. 2016;67:506–12. doi: 10.1161/HYPERTENSIONAHA.115.06731. [DOI] [PubMed] [Google Scholar]
- Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz, Cheng S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020;5:19–26. doi: 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, Thijs L, Asayama K, Li Y, Hansen TW, Boggia J, Jacobs L, Zhang Z, Kikuya M, Bjorklund-Bodegard K, Ohkubo T, Yang WY, Jeppesen J, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovsky J, Imai Y, Wang JG, O'Brien E, Staessen JA. The Cardiovascular Risk of White-Coat Hypertension. J Am Coll Cardiol. 2016;68:2033–43. doi: 10.1016/j.jacc.2016.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
. Baseline characteristics of all RADIANCE-HTN SOLO patients randomised to uRDN.
. Number and type of antihypertensive medications at 36 months.
. Patient disposition.
. Office blood pressure changes for the 24-month population.