SUMMARY
Poliomyelitis is caused by Poliovirus, a member of a large group of enteroviruses. Vaccine-derived polioviruses (VDPVs) stem from mutated live poliovirus, which is contained in the Oral Polio Virus vaccine (OPV). In addition, the emergence of VDPV is one of the global challenges for the eradication of poliomyelitis. VDPVs continue to affect different parts of the world; 1081 cases occurred in 2020 and 682 cases in 2021. There are several reasons that may have caused the increase in circulating vaccine-derived poliovirus (cVDPV) after the “switch” from the trivalent to the bivalent oral polio vaccine. One reason is the low vaccination rate among the targeted population, which has been further aggravated by the COVID-19 pandemic. Several strategies could control the spread of VDPV including the use of the monovalent OPV (mOPV-2). The risk of VDPV can be minimized through increased immunization rates and the use of safer vaccine alternatives. The global effort to eradicate polio has made significant progress over the years, but continued vigilance and investment in immunization programs are needed to achieve the ultimate goal of a polio-free world.
Keywords: Poliovirus, vaccine derivative poliovirus
INTRODUCTION
Poliomyelitis is caused by Poliovirus, a non-enveloped, positive sense, single-stranded RNA virus, identified in 1908 by Karl Landsteiner and Erwin Popper [1]. Most of infected patients remain asymptomatic and less than 1% of the exposed susceptible children less than 5 years of age gets paralytic polio (acute flaccid paralysis (AFP)) [1]. In addition, between 5–10% of children may die from respiratory failure due to involvement of the respiratory muscles [2]. The disease transmission pattern is dependent on the socioeconomic situation of the country affected. Fecal-oral route in developing countries and oral-to-oral route through saliva and respiratory droplets in developed countries are the most common routes of transmission [2, 3].
There are three wild poliovirus types 1, 2 and 3 (WPV-1, 2, 3). Of these types, WPV-1 is responsible for the majority of the global outbreaks, whereas WPV-2 and WPV-3 cause limited clusters. The major risk factor associated with disease spread is low sanitation, lack of access to clean drinking water, poor hand hygiene and high population density [4, 5].
Polio is an eradicable disease. The virus causes acute and short-term infections; in most cases infected cases can only transmit the virus for less than 2 weeks. The wild poliovirus cannot survive for long periods outside the human body; if every single child is vaccinated, virus will eventually die out. Humans are the only reservoir and there is no vector involved in the transmission, moreover no poliovirus transmission has been documented among animals. There are safe and effective polio vaccines available. The virus in the oral type of the vaccine is also excreted in faeces. Mass campaigns using oral polio vaccine interrupts WPV circulation by boosting population immunity so that transmission of poliovirus cannot be sustained [6]. When a child is immunized with Oral Polio Vaccine (OPV), the weakened vaccine-virus replicates in the gut for a limited duration, and as immune system is stimulated, it develops antibodies against the virus. In areas of crowding and inadequate sanitation, this excreted vaccine-virus undergo some replication and can spread in the community, which also protects other children through ‘passive’ immunization. But if a population is significantly under-immunized, the excreted vaccine-virus circulates for a longer period of time (at least 12 months) and it undergoes many genetic changes. Very rarely, the vaccine-virus can genetically change into a form that can cause paralysis similar to the wild poliovirus and is known as a circulating vaccine-derived poliovirus (cVDPV). cVDPVs occur when routine or supplementary immunization activities are poorly conducted, and population has a low vaccination coverage. Contrarily, if a population is fully immunized, it is safe from both vaccine-derived and wild polioviruses. The development and spread of VDPV are important hindrance in the journey to the global eradication of poliomyelitis [7].
Since 2000, more than 10 billion doses of OPV have been administered to nearly 3 billion children globally. As a result, more than 13 million cases of polio have been prevented, and the disease has been reduced by more than 99%. Until 2015, over 90% of cVDPV cases were due to the type 2 component in OPV. As wild poliovirus type 2 had already been successfully interrupted since the year 1999, in 2016 a switch was implemented from trivalent OPV (tOPV) to bivalent OPV (bOPV) in routine immunization programmes. The removal of the type 2 virus from OPV was associated with a reduction of the risk of cVDPV2. Circulating VDPVs in the past have been rapidly stopped with 2–3 rounds of high-quality immunization campaigns. To avert all sorts of polio outbreaks, every child should be vaccinated with the oral vaccine to stop polio transmission [7].
Types of Vaccine-Derived Poliovirus
VDVPs can spread in communities with incomplete polio vaccination, predominantly in places with poor hygiene, unsanitary conditions or crowded living situation [8]. It has been well-established that the major factor causing polio outbreaks and incidents is the community at large with low immunization rate. Lower level of household literacy and lower maternal education, vaccine hesitancy and reluctance are some of the variables that lead to low vaccination coverage or uptake [9]. Based on nucleotide changes, VDPVs are classified based on the divergence from the wild OPV strain complete viral protein 1 (VP1) genomic coding region into three types [10–12] [Table 1].
Table 1.
Classification of Vaccine-derived polioviruses (VDPVs).
| VDPVs classification: | ||
|---|---|---|
| – | Circulating (cVDPVs): evidence of community transmission | |
| – | Immunodeficiency-associated VDPVs (iVDPVs): isolation from patients with primary immunodeficiency | |
| – | Ambiguous (aVDPVs): identity is not known and the virus is not cVDPV nor iVDPV | |
|
| ||
| VDPVs divergence (differences in genetic sequence) from complete viral protein 1 (VP1) genomic coding region of wild OPV: | ||
|
| ||
| – | VDPV-1 & 3: | >1% divergent (≥10 nucleotide) |
| – | VDPV-2: | > 0.6% divergent (≥6 nucleotide) |
1. Circulating vaccine-derived poliovirus (cVDPV)
cVDPV is defined when there is evidence of human-to-human transmission, a previously detected isolate of VDPV is genetically linked to it, and the evidence comes from at least two individuals (not necessarily AFP cases) who are not in direct contact, at least one individual and one or more environmental samples, at least two individuals and two or more environmental samples collected more than two months apart, or at least one distinct collection [9]. The nucleotide sequence of the Sabin strain shows >1% variability from that of the cVDPV produced from OPV vaccination, indicating sustained replication with or without circulation in VDPV. The vaccine virus can evolve and reacquire neurovirulence over the course of 12 to 18 months if it is allowed to circulate for an extended period without interruption. Because of insufficient vaccination coverage, cVDPVs can typically recombine with other enteroviruses increasing their neurovirulence and transmissibility. Following the elimination of serotype 2 WPV, immunization with OPV-2 as part of the tOPV, including serotypes 1, 2, and 3 persisted, resulting in recurrent outbreaks of VDPV-2. Similarly, it resulted in outbreaks with VDPV-1 and VDPV-3 [8, 13]. With the course of time, bivalent vaccines (OPV1 & OPV3) were introduced in areas with OPV2 elimination. cVDPVs having a community circulation of more than six months are termed as persistent cVDPVs. Similar to naturally occurring poliovirus, complete immunization is the primary defense against cVDPV. Two to three rounds of effective vaccination efforts have effectively stopped the spread of VDPVs. Regardless of where the virus originates, the only way to limit the spread of polio is to vaccinate every child.
2. Ambiguous vaccine-derived poliovirus (aVDPV)
Ambiguous vaccine-derived polioviruses (aVDPVs) are VDPV isolates from humans or the environment that show no signs of circulation and come from people who do not have known immune deficiencies. The majority of the time, they are separated from sewage whose ultimate source is unknown or isolated from persons with no immunodeficiency and with no evidence of transmission. If subsequently discovered isolates are genetically connected, it may be reclassified as cVDPV. One study reported aVDPV in immunocompetent persons and environmental samples in seven countries in January 2017–June 2018 [14] and in 10 countries in January 2016–June 2017 [15]. aVDPV was detected in September 2021 from wastewater samples in the Jerusalem region [16].
3. Immunodeficiency-related vaccine-derived poliovirus (iVDPV)
After receiving OPV, around 50% of immunocompetent individuals excrete poliovirus for two weeks. Individuals lacking in primary antibodies are the exception: they could excrete the virus for 3 months [17]. Few individuals with rare immune deficiency syndromes have been found to have prolonged replication of vaccine-derived viruses (eg: B cell immunodeficiencies) [18–20].
Those individuals fail to mount an immune response, which prevents them from being able to recover from an intestinal vaccine virus infection, which typically disappears after six to eight weeks following vaccination. As a result, they continuously excrete iVDPVs [9]. In a study in 2017–2018, five countries reported cVDPV and 14 individuals in nine countries excreted iVDPV [21]. A case of VDPV-3 with high genetic diversity was found in 2022 in a stool specimen from an infant with primary immunodeficiency disorder in China [22]. In addition, a Sabin 3/Sabin 1 recombinant of VDPV was isolated from a child [23]. This issue is even more problematic with the presence of chronic excretion of iVDPV2 as described in a patient from the Philippines [24]. Whether such individuals may act as reservoirs of such iVDPV and examine the risks of chronic and prolonged excretions are being studied in Pakistan [25]. A study from Tunisia showed that the rate of poliovirus detection was 6.8%, 1.5% and 1.3% in patients with primary immune deficiency, AFP cases, and contacts, respectively [26]. The surveillance of iVDPV showed 16 new cases in five countries (Argentina, Egypt, Iran, the Philippines, and Tunisia) from July 2018 to December 2019 [27].
Detection of VDPVs
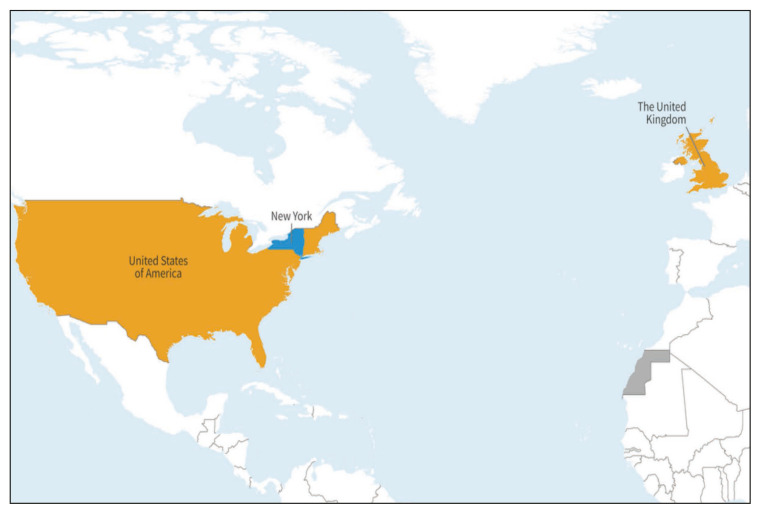
Detection of poliovirus in stool samples from acute AFP cases and environmental surveillance in the form of sewage sampling are the gold standard for identifying VDPVs. All AFP cases are investigated by analyzing stool specimens. If a poliovirus is isolated and RNA sequencing is done, then a VDPV is indicated by the presence of >10 nucleotide differences from the OPV virus. Following confirmation of a VDPV, it is essential to determine if the VDPV virus is circulating or is an isolated case. Also, it is important to find out if the person who is diagnosed with VDPV has any immunodeficiency. House-to-house visits are conducted to check on if contacts have been infected with the VDPV. Epidemiological studies are then carried out to search for any unrecognized and unreported cases in hospitals, in the nearby area [4]. Apart from AFP surveillance and confirmation of VDPVs, detection of poliovirus in sewage waters can help detect reintroduction of the WPV in countries previously declared polio-free [28]. Recently in the UK and USA, many genetically linked Sabin-like poliovirus 2 were detected from environmental samples, and these samples were classified as cVDPV-2 [5]. In the USA, the detection of environmental viral sequences at two different times, containing >5 nucleotide changes, and both linked to the case from Rockland County, was labelled as cVDVP2. The virus detected in USA is genetically linked to viruses detected in the UK and Israel. Figure 6 shows detection of genetically linked cVDVP2 [5].
Figure 6.
Countries (in yellow) with detection of genetically linked cVDVP-2 [5].
Source: (https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON408).
Epidemiology
Widespread implementation of OPV led to a reduction in the number of AFP cases caused by the WPV. Subsequently, in the areas with incomplete vaccination, and the non-availability of inactivated poliovirus vaccine, the problem of cVDPV persisted. The multiple outbreaks of cVDPV in 2000–2001 in Haiti and the Dominican Republic were the first of their kind with the identification of 21 cases with two fatalities [29].
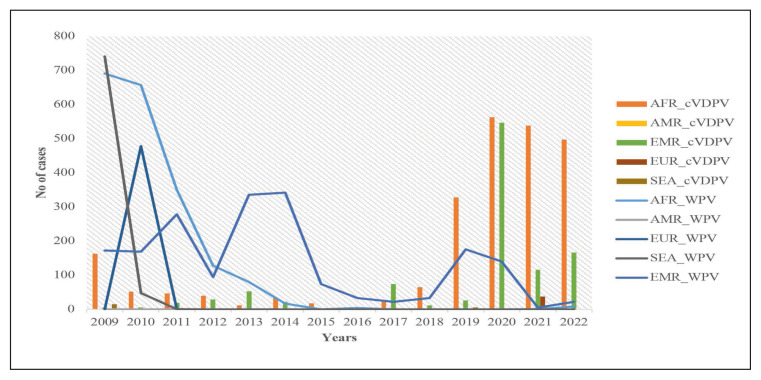
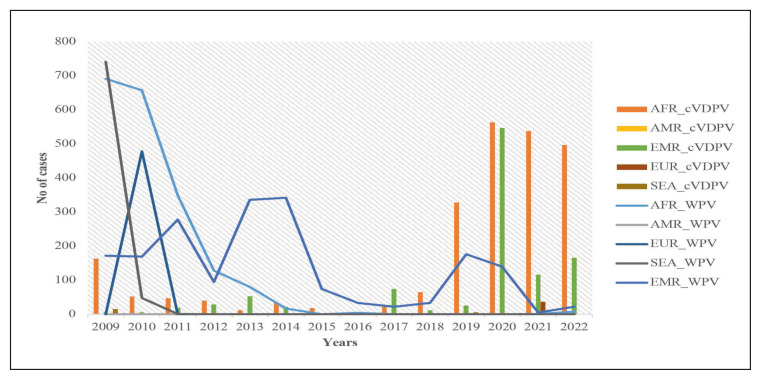
Though the number of WPV cases has been eclining in the last 15 years, cVDPV remained endemic in some regions. This problem was especially significant in Africa and the Eastern Mediterranean region, according to the World Health Organization (WHO) (Figure 1). There were 24 outbreaks of cVDPV with 760 cases between 2000 and 2016 [9]. In 2017, an outbreak attributed to cVDPV2 occurred in Syria and spanned more than 6 months, and the virus was isolated from 74 cases of AFP [30].
Figure 1.
Showing the Trends of the WPV cases and cVDPV cases from 2009 onwards [66].
Source: AFP Polio data. Available from: https://extranet.who.int/polis/public/CaseCount.aspx [Accessed 26th April 2023].
AFR: African Region; AMR: Regions of the America; EMR: Eastern Mediterranean Region; EUR: European; SEA: South East Asia; cVDPV: circulating Vaccine Derived Poliovirus; WPV: Wild Poliovirus.
“Switch and VDPV increase”
It was observed that routine use of OPV containing poliovirus type 2 was associated with more risk than benefit, especially after the eradication of WPV-2 in 2015. Therefore, in April–May 2016, the World Health Assembly authorized a phased cessation of the tOPV, and this strategy was labeled the “Switch” [31]. The “Switch” was made from the tOPV (strains 1–3) to the bivalent OPV (b-OPV; type 1 and 3). OPV-2 however, had been recommended for use during outbreaks [32].
Following the switch, there was an increase in cases of cVDPV especially in countries in Africa and the Eastern Mediterranean region. In 2017, an outbreak attributed to cVDPV2 occurred in Syria and spanned more than 6 months, and the virus was isolated from 74 cases of AFP [32]. After adjusting for gender, level of literacy, and gross domestic product (GDP) per capita, the presence of a low vaccination coverage was associated with higher odds of occurrence of cVDPV [9]. The switch may have contributed to the emergence of cVDPV especially in 2016–2017, with an associated increase in the number of outbreaks [9, 21, 33]. In 40 countries reporting cVDPV, 55% of the countries had low (<80%) polio vaccination coverage [9]. The number of cVDPV cases increased from five in 2016 to 378 in 2019 (Figure 1) and this was associated with an increase in the number of countries reporting cVDPV [29]. The presence of both cVDPV-1 and cVDPV-2 was reported in the Republic of the Philippines in 2019 and in Malaysia in 2020 [34].
Several reasons were thought to have caused the increase in cVDPV after the “switch”, although, one mathematical model expected a reduction in cVDPV [35, 36]. The study indicated that the cessation of OPV would eliminate all circulating live polioviruses, with the caution that population immunity is managed appropriately [36]. Managing immunity at the population level might not be possible given the variable prevalence of such disorders in any given community. In mathematical modeling studies, for communities with low vaccine-coverage, it is important to attain high coverage with the tOPV before the switch [36, 37]. In countries where cVDPV occurred such as Syria, Nigeria and Somalia, the coverage rate with tOPV before the switch was 30–60% [21, 33, 38]. One study also showed that an increase of 10% in population immunity of children < 5 years of age at the campaign time and location corresponded to an 18% decrease in emergence of cVDPV-2 [39]. As will be discussed later, the use of monovalent OPV (mOPV-2) is an important strategy to control cVDPV outbreaks. However, this intervention should result in a high-coverage rate to prevent the emergence of cVDPV [37]. It was shown that the number of cVDPV outbreaks increased from 9 in 2017–2018 to 29 in 2019 inside and outside of mOPV2 response areas and that 86% of such outbreaks were due to cVDPV-2 [40].
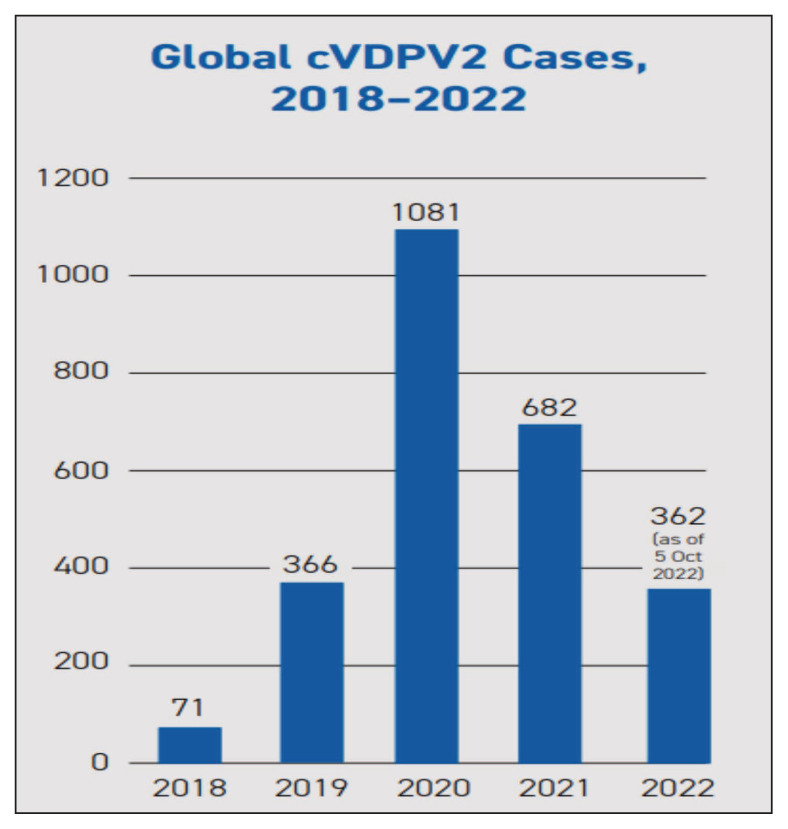
VDPVs continue to affect different parts of the world, and a total of 1081 cases were reported in 2020 and 682 cases in 2021 (Figure 2). In 2021, the highest number of Type 1 cVDVP from AFP cases had been detected in Madagascar (13) and Yemen (3). In the same year, the highest number of Type2 cVDVPs had been detected in Nigeria (415), Yemen (66), DR Congo (28), and Niger (18).
Figure 2.
Global prevalence of cVDVP2 cases from year 2018–2022 [41].
Source: cVDPV2 Outbreaks and the Type 2 Novel Oral Polio Vaccine (nOPV2) https://polioeradication.org/wp-content/uploads/2022/10/GPEI-nOPV2-Factsheet-EN-20221011.pdf
In the year 2022, 185 type 1 cVDVPs, 673 type 2 cVDVPs, and one type 3 cVDVP were detected in AFP cases. The maximum number of type 1 cVDVPs in 2022 has been detected in DR Congo (144), Mozambique (22), Madagascar (14), and Malawi (4). The maximum number of Type2 cVDVPs have been detected from DR Congo (360), Yemen (162), Nigeria (48), and Chad (44) [41].
Impact of COVID-19 to polio eradication
The occurrence of the COVID-19 pandemic in March 2020 increased the burden on polio-eradication programs around the globe. This addition occurred on top of the repeated occurrence of cVDPV2 outbreaks in many countries and the presence of an endemic WPV type 1 (WPV-1) in Afghanistan and Pakistan [42]. Due to the COVID-19 pandemic, the Global Polio Eradication Initiative (GPEI) program utilized deferred house-to-house visits to administer supplementary immunization activities in 28 countries between March to May 2020 and GPEI strived to continue the essential poliovirus surveillance activities. It was noted that the global AFP cases declined by 34% in January–July 2020 compared with January–July 2019. In addition, there was a reduction in the mean number of active environmental samples collected in the African and Eastern Mediterranean regions [43].
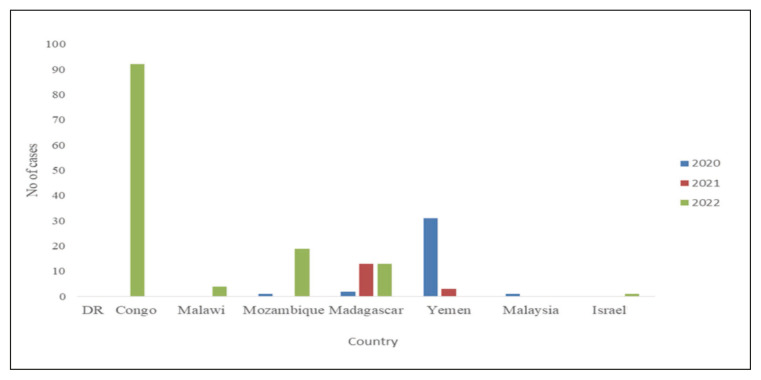
An additional impact of the COVID-19 pandemic on polio eradication was the increase in the number of cVDPV outbreaks. From 2019 to 2020, the number outbreaks tripled paralyzing over 1100 children [44]. From the beginning of the COVID-19 pandemic, cases of cVDPV2 among AFP increased in many African countries, including the Democratic Republic of Congo (DRC), Nigeria, and Yemen. More than 400 cases were notified from Nigeria only in 2021 whereas DRC reported >250 cases last year (Figure 3). Additionally, in the DRC, >90 cases of cVDPV-1 were reported among patients with AFP (Figure 4) [45].
Figure 3.
Distribution of cases of cVDPV 2 isolated in patients with Acute Flaccid Paralysis from 2020–2023 [66].
Source: AFP Polio data. Available from: https://extranet.who.int/polis/public/CaseCount.aspx
AFR: African Region; AMR: Regions of the America; EMR: Eastern Mediterranean Region; EUR: European; SEA: South East Asia; cVDPV: circulating Vaccine Derived Poliovirus; WPV: Wild Poliovirus.
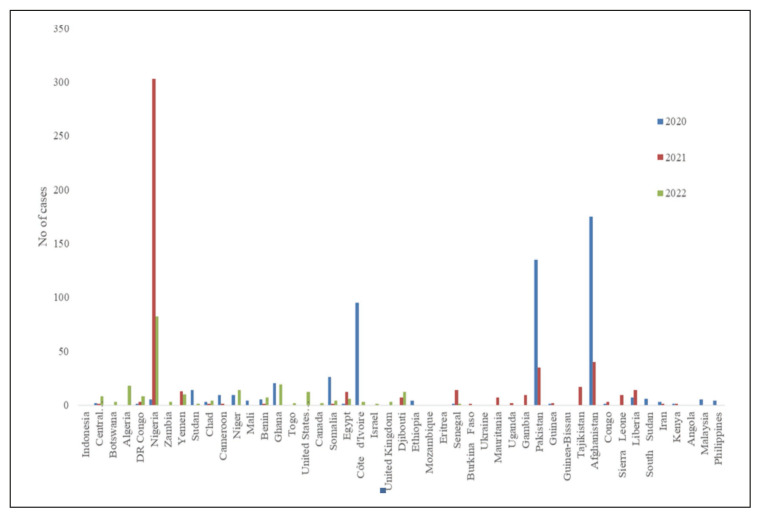
Figure 4.
Distribution of cases of cVDPV 1 & 3 isolated in patients with Acute Flaccid Paralysis from 2020–2023 [66].
Source: AFP Polio data. Available from: https://extranet.who.int/polis/public/CaseCount.aspx.
On the other hand, in August 2020 and in the midst of the pandemic, Africa was announced to be free of WPV, thus leaving Pakistan and Afghanistan with such cases [46]. Unfortunately, this victory was short-lived as in November 2021 a case of AFP was reported from the Eastern African country Malawi and the WPV-1 was identified from the stool sample of the patient [21]. Additionally, eight new cases of WPV-1 were identified from the adjacent neighboring country of Mozambique [47, 48]. These two countries had eradicated poliovirus in 1992 and 1993 respectively [49]. Additional reasons cited for the emergence of polio in Africa are the continued movements and the increase in anti-vaccine activities [50].
A patient with VDPV-2 paralytic poliomyelitis was reported in an unvaccinated individual residing in Rockland County, New York, USA in July 2022, and there was no recent international travel [8, 47, 48, 51]. Similarly, the United Kingdom detected cVDPV-2 from sewage samples in London with no associated human cases [5].
The number of areas reporting isolation of VDPV from environmental sampling and surveillance has increased since the start of the pandemic. Apart from the African and Eastern Mediterranean region cVDPV-2 have been isolated in the UK, the USA and Canada (Figure 5), including genetically linked cVDPV-2 (Figure 6) [5]. Similarly, the isolation of VDPV-1 and 3 from environmental samples had increased in the last three years from 10 in 2020 to 44 in 2021, and 122 in 2022. There had been multiple countries reporting the isolation of cVDPV-1 from AFP cases for three years suggesting widespread transmission, as discussed above (Figure 4).
Figure 5.
Distribution of cVDPV 2 isolated from environmental samples during the surveillance related activities from 2020–2023 [41].
Source: Circulating vaccine derived polio. https://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/. Accessed on 8th May 23.
Eradication and control of cVDPV
The GPEI is working to find new methods of outbreak response to fight mounting threat of cVDPV2, so that these cases are detected rapidly and incidence could be controlled. Various strategies suggested by GPEI are: improving quality and speed of outbreak response, targeted political will of a country, enhanced disease as well as environmental surveillance, the availability of needed infrastructure, formation of emergency response teams, motivated community engagement, amalgamation of polio services with other health programs, and a focus on reaching under-immunized populations. These strategies can help countries fight cVDVP outbreaks. Currently, it is of critical importance that all the countries engage in rapid and high-quality efforts to detect cVDPV2. The strategic Advisory Group of Experts on Immunization (SAGE) of the WHO has recommended that countries urgently respond to these outbreaks using available type 2 vaccines: novel Oral Polio Vaccine 2 (nOPV2), or monovalent OPV2 (mOPV2). In situations where there is co-circulation of different strains, tOPV may be the more suitable vaccine of choice [52].
nOPV2 has been rolled out by GPEI. The nOPV2 is a next-generation monovalent OPV2 vaccine, for which clinical trial data and use in communities revealed safety and effectiveness in protecting against type 2 polio [53]. This is particularly important as 90% of cVDPV outbreaks were caused by OPV-2 [53]. The vaccine could be used in countries affected by cVDVP outbreaks. nOPV2, being a modified version of mOPV2, is less likely to revert into a neurovirulent form in low immunity settings. Therefore, it carries less risk of starting new cVDPV2 outbreaks as compared to mOPV2. GPEI is constantly working to escalate supply of nOPV2, it supports governments to promote use of this new vaccine, provides technical assistance to ensure that Emergency use Listing (EUL) criteria are met. WHO’s Pre-Qualification program listed nOPV2 in EUL in November 2020 to flag use of yet-to-be-licensed vaccine after exhaustive validation of stability, efficacy, and safety [52].
Actual use of nOPV2 started in March 2021 in a few countries under strict criteria of such usage. By the end of 2022, approximately 500 million doses of this vaccine have been given in more than 30 countries. In a randomized-controlled clinical trial, the nOPV-2 vaccine in infants at birth and 4 weeks of age showed 99% neutralizing antibodies [54]. Another study showed that antibody seroprevalence increased from 26% before nOPV2 to 77% and 83% after one and two doses of nOPV2, respectively [55]. In another study, there was an increased stability of the V domain of nOPV2 relative to mOPV2 [56]. Data on post-usage safety, efficacy and genetic stability is constantly monitored, and there is proven evidence that it can be used as a tool to prevent cVDPV2 outbreaks [52]. SAGE also endorsed that nOPV2 can be used in response to cVDPV2 outbreaks after reviewing it for the pre-defined period for initial use. Clinical evaluation studies on nOPV2 in Panama and Belgium have also illustrated that the vaccine is safe and efficacious and is more stable as compared to OPV. According to SAGE, the initial use period is for three months following the first use of nOPV2 under EUL; but the usage will depend on epidemiology of the country and its dedication to conducting the strict monitoring of nOPV2’s safety, and effectiveness during rollout.
GPEI is working with high-risk countries in preparation to roll out nOPV2. Apart from nOPV2’s initial use, the GPEI is recommending other strategies to control cVDPV2 outbreaks, such as channeling high-quality outbreak response using mOPV2 or another available vaccine, building a strong disease surveillance network, strengthening routine immunization protocols with inactivated polio vaccine (IPV) in high-risk areas, and ensuring adequate procurement of OPV to reach every child in remote areas of the world.
Routine vaccination and house-to-house mop up campaigns have failed to reach all the pediatric population for so many years, may be due to weak routine immunization programs, vaccine hesitancy, mobility of populations, poor program management, political conflicts, geographical calamities, a lack of awareness, myths, and misinformation. Last but not least, in 2020, the setback polio campaigns suffered because of COVID-19 pandemic, had its own unsurmountable impact, it completely disrupted routine immunization, which led to new outbreaks of cVDVPs globally. Fast-paced, high-quality vaccination campaigns using mOPV2 and nOPV-2 can be utilized to stop cVDPV-2 outbreaks. However, the use of mOPV-2 was associated with the emergence of cVDPV as described in the Philippines [57].
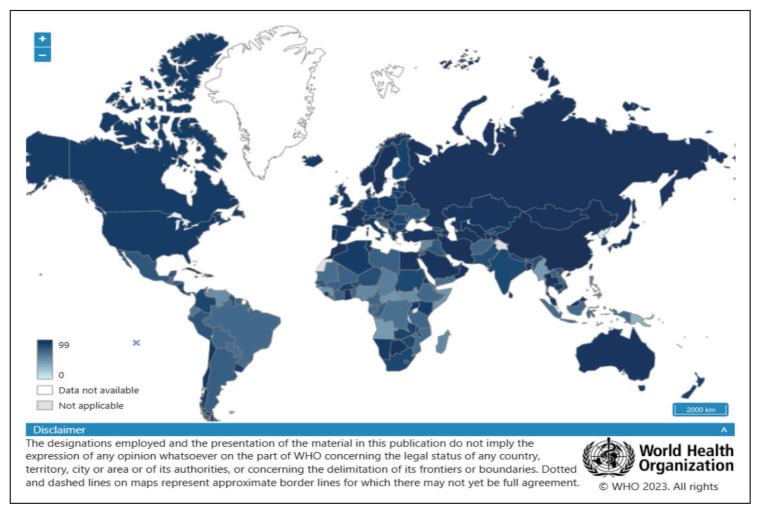
The emergence of cVDPV2 in the USA and UK are evidence of the possible occurrence of such events in countries that had been declared polio-free with the possibility of re-emergence of polio and subsequent AFP in such countries. The vaccine coverage for three routine doses of polio-vaccine in children of one year of age, both in the UK and USA was 93% and 92%, respectively in the year 2021 (Figure 7) [58]. It is important to have a polio vaccination coverage rate of >95% and maintain high quality surveillance indicators (AFP, percentage of cases investigated within 48 hours, and percentage of cases with an adequately obtained sample), mandatory environmental surveillance, rapid detection, and rapid response to the emergence or importation of a new virus [52]. It is also important to have continued surveillance for the emergence of any polio outbreaks secondary to cVDPVs, and polio immunization should be updated regularly [59]. This is especially important as there has been an increased number of cVDPVs cases which is 7 times more than that caused by the WPV in 2020 [60]. It had been shown that increasing surveillance of non-polio AFP together with adequate stool collection resulted in an increased speed of detection of cases [61]. Despite an increase in key performance indicators in 2021 to 74% of priority countries compared to 53% in 2020, there is still national and subnational gaps requiring further enhancement [62]. A study from 2016 to 2020, there were 65 outbreak responses to cVDPV. Of the first large-scale campaign conducted after Day 0, only 12 (18.5%) such responses were conducted by the target date of 28 days after Day 0 of the occurrence of the outbreaks [63]. It was well stated that “without global eradication, there is a risk of re-infection from the importation and spread of wild poliovirus or VDPV, or the emergence and circulation of VDPV” [64]. It is also important to improve the surveillance sensitivity and completeness of AFP case investigations for full detection and interruption of viral transmission [65].
Figure 7.
Percentage of Children (1 year-old) who received three doses of Polio-vaccine.
Source: Polio (Pol3) immunization coverage among 1-year-olds (%) https://www.who.int/data/gho/data/indicators/indicator-details/GHO/polio-(pol3)-immunization-coverage-among-1-year-olds-(-)
CONCLUSION
It is important to note that the risk of VDPV emergence is very low, and the benefits of OPV far outweigh the risks. Nevertheless, the emergence of VDPV is a cause for concern, particularly in countries where polio remains endemic, and where the weakened virus can circulate for longer periods. To address the risk of VDPV emergence, countries have developed strategies to increase immunization rates and minimize the risk of the vaccine virus spreading in the environment. These include the use of IPV instead of OPV, particularly in developed countries, where the risk of wild poliovirus transmission is low. IPV is a safer alternative to OPV because it uses inactivated virus particles and cannot cause VDPV. Additionally, countries with a high risk of VDPV emergence are using a different type of OPV that contains a weakened form of the virus that is less likely to mutate and cause VDPV. Thus, VDPV is a rare but serious consequence of the use of OPV. The risk of VDPV can be minimized through increased immunization rates and the use of safer vaccine alternatives. The global effort to eradicate polio has made significant progress over the years, but continued vigilance and investment in immunization programs are needed to achieve the ultimate goal of a polio-free world.
Footnotes
Conflict of interest
None to declare.
Funding
No funding was received for this review.
REFERENCES
- 1. Mehndiratta MM, Mehndiratta P, Pande R. Poliomyelitis: Historical Facts, Epidemiology, and Current Challenges in Eradication. The Neurohospitalist. 2014;4:223–229. doi: 10.1177/1941874414533352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. John J. Role of injectable and oral polio vaccines in polio eradication. Expert Rev Vaccines. 2009;8:5–8. doi: 10.1586/14760584.8.1.5. [DOI] [PubMed] [Google Scholar]
- 3. Zuckerman NS, Bar-Or I, Sofer D, et al. Emergence of genetically linked vaccine-originated poliovirus type 2 in the absence of oral polio vaccine, Jerusalem, April to July 2022. Euro Surveill. 2022:27. doi: 10.2807/1560-7917.ES.2022.27.37.2200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Polio Eradication Initiative. Classification and reporting of vaccine-derived polioviruses (VDPV) - GPEI guidelines 2016. [accessed February 22, 2023]. p. 8. http://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf .
- 5.World Health Organization. Detection of circulating vaccine derived polio virus 2 (cVDPV2) in environmental samples - the United Kingdom of Great Britain and Northern Ireland and the United States of America. 2022. [accessed February 22, 2023]. https://www.who.int/emergencies/disease-out-break-news/item/2022-DON408 .
- 6.5 reasons why polio can be eradicated. [Accessed on 7th May 2023]. Available from: https://polioeradication.org/news-post/5-reasons-why-polio-can-be-eradicated/
- 7.Poliomyelitis: Vaccine derived polio. [Accessed on 5th May 2023]. Available from: https://www.who.int/news-room/questions-and-answers/item/poliomyelitis-vaccine-derived-polio.
- 8. Al-Tawfiq JA, Kattan RF, Almoallem SAS, Altawfiq KJ, Mohsni E, Memish ZA. Worldwide poliomyelitis outbreaks: should mass gathering organizers be concerned? J Travel Med. 2022 doi: 10.1093/jtm/taac128. [DOI] [PubMed] [Google Scholar]
- 9. Lai YA, Chen X, Kunasekaran M, Rahman B, MacIntyre CR. Global epidemiology of vaccine-derived poliovirus 2016–2021: A descriptive analysis and retrospective case-control study. EClinicalMedicine. 2022;50:101508. doi: 10.1016/j.eclinm.2022.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill M, Bandyopadhyay AS, Pollard AJ. Emergence of vaccine-derived poliovirus in high-income settings in the absence of oral polio vaccine use. Lancet. 2022;400:713–715. doi: 10.1016/S0140-6736(22)01582-3. [DOI] [PubMed] [Google Scholar]
- 11. Gumede N, Lentsoane O, Burns CCP, et al. Emergence of vaccine-derived polioviruses, Democratic Republic of Congo, 2004–2011. Emerg Infect Dis. 2013;19:1583–1589. doi: 10.3201/eid1910.130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alleman MM, Chitale R, Burns CC, et al. Vaccine-Derived Poliovirus Outbreaks and Events - Three Provinces, Democratic Republic of the Congo, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:117–125. doi: 10.15585/MMWR.MM6710A4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wringe A, Fine PEM, Sutter RW, Kew OM. Estimating the extent of vaccine-derived poliovirus infection. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jorba J, Diop OM, Iber JH, et al. Update on Vaccine-Derived Polioviruses - Worldwide, January 2017–June 2018. MMWR Morb Mortal Wkly Rep. 2018;67:661–672. doi: 10.15585/MMWR.MM6742A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jorba J, Diop OM, Iber J, et al. Update on Vaccine-Derived Polioviruses - Worldwide, January 2016–June 2017. MMWR Morb Mortal Wkly Rep. 2017;66:1185–1191. doi: 10.15585/MMWR.MM6643A6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weil M, Sofer D, Shulman LM, et al. Environmental surveillance detected type 3 vaccine-derived polioviruses in increasing frequency at multiple sites prior to detection of a poliomyelitis case. Sci Total Environ. 2023:871. doi: 10.1016/J.SCITOTENV.2023.161985. [DOI] [PubMed] [Google Scholar]
- 17.Module 3 Poliomyelitis; Surveillance Guide for Vaccine-Preventable Diseases in the WHO South-East Asia Region; Module 3 Poliomyelitis. [Accessed on 24.4, 2023]. https://apps.who.int/iris/handle/10665/277459 .
- 18.Poliomyelits: WHO Vaccine-Preventable Diseases Surveillance Standards. [Accessed on 24.4, 2023]. https://cdn.who.int/media/docs/default-source/immunization/vpd_surveillance/vpd-surveillance-standards-publication/who-surveillancevaccinepreventable-18-polio-r3.pdf?sfvrsn=aa96984f_28 .
- 19.Aide Memoire - Types of Vaccine-derived Poliovirus (VDPV) [Accessed on 24.4, 2023]. https://www3.paho.org/hq/index.php?option=com_content&view=article&id=7038:2012-aide-memoire-types-vaccine-derived-poliovirus-vdpv&Itemid=0&lang=pt#gsc.tab=0 .
- 20. Schubert A, Böttcher S, Eis-Hübinger AM. Two Cases of Vaccine-Derived Poliovirus Infection in an Oncology Ward. N Engl J Med. 2016;374( 13):1296–1298. doi: 10.1056/NEJMc1508104. [DOI] [PubMed] [Google Scholar]
- 21. Mbaeyi C, Wadood ZM, Moran T, et al. Strategic Response to an Outbreak of Circulating Vaccine-Derived Poliovirus Type 2 - Syria, 2017–2018. MMWR Morb Mortal Wkly Rep. 2018;67:690–694. doi: 10.15585/mmwr.mm6724a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao N, Liu Y, Xu JW, et al. Detection of a Highly Divergent Type 3 Vaccine-Derived Poliovirus in a Child with a Severe Primary Immunodeficiency Disorder - Chongqing, China, 2022. MMWR Recomm Reports. 2022;71:1148–1150. doi: 10.15585/mmwr.mm7136a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiao T, Leng H, Zhang Q, Chen Q, Guo H, Qi Y. Isolation and characterization of a Sabin 3/Sabin 1 recombinant vaccine-derived poliovirus from a child with severe combined immunodeficiency. Virus Res. 2022:308. doi: 10.1016/j.virusres.2021.198633. [DOI] [PubMed] [Google Scholar]
- 24. Kitamura K, Shimizu H. The molecular evolution of type 2 vaccine-derived polioviruses in individuals with primary immunodeficiency diseases. Viruses. 2021:13. doi: 10.3390/v13071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pethani AS, Kazi Z, Nayyar U, et al. Poliovirus excretion among children with primary immune deficiency in Pakistan: A pilot surveillance study protocol. BMJ Open. 2021:11. doi: 10.1136/bmjopen-2020-045904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chouikha A, Rezig D, Driss N, et al. Circulation and molecular epidemiology of enteroviruses in paralyzed, immunodeficient and healthy individuals in tunisia, a country with a polio-free status for decades. Viruses. 2021:13. doi: 10.3390/v13030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macklin G, Diop OM, Humayun A, et al. Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses - Worldwide, July 2018–December 2019. MMWR Morb Mortal Wkly Rep. 2020;69:913–917. doi: 10.15585/mmwr.mm6928a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esteves-Jaramillo A, Estivariz CF, Pearanda S, et al. Detection of vaccine-derived polioviruses in Mexico using environmental surveillance. J Infect Dis. 2014;210:S315–323. doi: 10.1093/infdis/jiu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296:356–359. doi: 10.1126/SCIENCE.1068284. [DOI] [PubMed] [Google Scholar]
- 30. Burki T. Vaccine-derived poliovirus cases exceed wild types. Lancet Infect Dis. 2019;19:140. doi: 10.1016/S1473-3099(19)30012-X. [DOI] [PubMed] [Google Scholar]
- 31. Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - Worldwide. 2016. Morb Mortal Wkly Rep. 2016;65:934–938. doi: 10.15585/mmwr.mm6535a3. [DOI] [PubMed] [Google Scholar]
- 32. Garon J, Seib K, Orenstein WA, et al. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines. 2016;15:693–708. doi: 10.1586/14760584.2016.1140041. [DOI] [PubMed] [Google Scholar]
- 33. Wang H. Why Have cVDPV2 Outbreaks increased globally after the polio immunization strategy switch: challenges for the polio eradication endgame. China CDC Wkly. 2020;2:176–179. doi: 10.46234/ccdcw2020.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Snider CJ, Boualam L, Tallis G, et al. Concurrent outbreaks of circulating vaccine-derived poliovirus types 1 and 2 affecting the Republic of the Philippines and Malaysia, 2019–2021. Vaccine. 2022;41(Suppl 1):A58–A69. doi: 10.1016/j.vaccine.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greene SA, Ahmed J, Datta SD, et al. Progress Toward Polio Eradication - Worldwide, January 2017–March 2019. Morb Mortal Wkly Rep. 2019;68:458–462. doi: 10.15585/mmwr.mm6820a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson KM, Tebbens RJD. Modeling the dynamics of oral poliovirus vaccine cessation. J Infect Dis. 2014;210:S475–84. doi: 10.1093/infdis/jit845. [DOI] [PubMed] [Google Scholar]
- 37. Pons-Salort M, Burns CC, Lyons H, et al. Preventing vaccine-derived poliovirus emergence during the polio endgame. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang H, Qi Q, Zhang Y, Wen N, et al. Analysis of a Sabin-strain inactivated poliovirus vaccine response to a circulating type 2 vaccine-derived poliovirus event in Sichuan Province, China 2019–2021. JAMA Netw Open. 2023;6:E2249710. doi: 10.1001/JAMANETWORKOPEN.2022.49710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gray EJ, Cooper LV, Bandyopadhyay AS, Blake IM, Grassly NC. The origins and risk factors for serotype-2 vaccine-derived poliovirus (VDPV2) emergences in Africa during 2016–2019. J Infect Dis. 2023 doi: 10.1093/INFDIS/JIAD004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jorba J, Diop OM, Iber J, et al. Update on vaccine-derived poliovirus outbreaks - Worldwide, January 2018–June 2019. Morb Mortal Wkly Rep. 2019;68:1024–1028. doi: 10.15585/mmwr.mm6845a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Circulating vaccine derived polio. [Accessed on 8th May 23]. https://polio-eradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/
- 42. Chard AN, Datta SD, Tallis G, et al. Progress Toward Polio Eradication - Worldwide, January 2018–March 2020. Morb Mortal Wkly Rep. 2020;69:784–789. doi: 10.15585/mmwr.mm6925a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burkholder B, Wadood Z, Kassem AM, Ehrhardt D, Zomahoun D. The immediate impact of the COVID-19 pandemic on polio immunization and surveillance activities. Vaccine. 2021 doi: 10.1016/j.vaccine.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. The Lancet. Polio eradication: falling at the final hurdle? Lancet. 2022;400:1079. doi: 10.1016/S0140-6736(22)01875-X. [DOI] [PubMed] [Google Scholar]
- 45.AFP Polio data. [Accessed 26th April 2023]. Available from: https://extranet.who.int/polis/public/CaseCount.aspx.
- 46. Mohammed A, Tomori O, Nkengasong JN. Lessons from the elimination of poliomyelitis in Africa. Nat Rev Immunol. 2021;21:823–828. doi: 10.1038/s41577-021-00640-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. World Health Organization(WHO) [accessed February 22, 2023]; Wild poliovirus type 1 (WPV1) - Mozambique. 2022 1:2003–2005. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON395 . [Google Scholar]
- 48.World Health Organization. Wild poliovirus type 1 (WPV1) - Malawi. 2022. [accessed February 22, 2023]. https://www.who.int/emergencies/disease-outbreak-news/item/wild-poliovirus-type-1-(WPV1)-malawi .
- 49. Niaz F, Tariq S, Rana MS, et al. The resurgence of polio: The effect of the Covid-19 pandemic on polio eradication. Ethics, Med Public Health. 2023;26:100858. doi: 10.1016/j.jemep.2022.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uwishema O, Elebesunu EE, Bouaddi O, et al. Poliomyelitis amidst the COVID-19 pandemic in Africa: Efforts, challenges and recommendations. Clin Epidemiol Glob Health. 2022:16. doi: 10.1016/j.cegh.2022.101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rai A, Uwishema O, Uweis L, et al. Polio returns to the USA: An epidemiological alert. Ann Med Surg. 2022:82. doi: 10.1016/j.amsu.2022.104563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.GEPI. Vaccine-Derived Polioviruses GPEI. n.d. [accessed February 22, 2023]. https://polioeradication.org/polio-today/polio-prevention/the-virus/vaccine-derived-polio-viruses/
- 53. Martin J, Burns CC, Jorba J, et al. Genetic Characterization of Novel Oral Polio Vaccine Type 2 Viruses During Initial Use Phase Under Emergency Use Listing - Worldwide, March–October 2021. Morb Mortal Wkly Rep. 2022;71:786–790. doi: 10.15585/mmwr.mm7124a2. [DOI] [PubMed] [Google Scholar]
- 54. Zaman K, Bandyopadhyay AS, Hoque M, et al. Evaluation of the safety, immunogenicity, and faecal shedding of novel oral polio vaccine type 2 in healthy newborn infants in Bangladesh: a randomised, controlled, phase 2 clinical trial. Lancet. 2023;401:131–139. doi: 10.1016/S0140-6736(22)02397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mirzoev A, Macklin GR, Zhang Y, et al. Assessment of serological responses following vaccination campaigns with type 2 novel oral polio vaccine: a population-based study in Tajikistan in 2021. Lancet Glob Health. 2022;10:e1807–814. doi: 10.1016/S2214-109X(22)00412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wahid R, Mercer LD, De Leon T, et al. Genetic and phenotypic stability of poliovirus shed from infants who received novel type 2 or Sabin type 2 oral poliovirus vaccines in Panama: an analysis of two clinical trials. The Lancet Microb. 2022;3:e912–921. doi: 10.1016/S2666-5247(22)00254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alipon SCB, Takashima Y, Avagyan T, et al. Emergence of vaccine-derived poliovirus type 2 after using monovalent type 2 oral poliovirus vaccine in an outbreak response, Philippines. West Pacific Surveill Response J. 2022;13:1–7. doi: 10.5365/wpsar.2022.13.2.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Global Health Observatory Data Repository. Polio Immunization Coverage among 1-year-olds. Www-WhoInt. 2014. [accessed February 25, 2023]. http://www.who.int/gho/immunization/poliomyelitis/en/
- 59. Kitamura K, Shimizu H. Outbreaks of Circulating Vaccine-Derived Poliovirus in the World Health Organization Western Pacific Region, 2000–2021. Jpn J Infect Dis. 2022;75:431–444. doi: 10.7883/yoken.JJID.2022.312. [DOI] [PubMed] [Google Scholar]
- 60. Sultan MA. Emerging challenges to realizing global polio eradication and their solutions. East Mediterr Heal J. 2022;28:515–520. doi: 10.26719/emhj.22.045. [DOI] [PubMed] [Google Scholar]
- 61. Auzenbergs M, Fountain H, Macklin G, Lyons H, O’Reilly KM. The impact of surveillance and other factors on detection of emergent and circulating vaccine derived polioviruses. Gates Open Res. 2022;5:94. doi: 10.12688/GATESOPENRES.13272.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilkinson AL, Diop OM, Jorba J, Gardner T, Snider CJ, Ahmed J. Surveillance to track progress toward polio eradication - Worldwide, 2020–2021. Morb Mortal Wkly Rep. 2022;71:538–544. doi: 10.15585/mmwr.mm7115a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Darwar R, Biya O, Greene SA, et al. Assessing country compliance with circulating vaccine-derived poliovirus type 2 outbreak response standard operating procedures: April 2016 to December 2020. Vaccine. 2023 doi: 10.1016/J.VACCINE.2023.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chong CY, Kam KQ, Yung CF. Combating a resurgence of poliomyelitis through public health surveillance and vaccination. Ann Acad Med Singapore. 2023;52:17–26. doi: 10.47102/ANNALS-ACADMEDSG.2022390. [DOI] [PubMed] [Google Scholar]
- 65. Morais A, Morais J, Felix M, et al. Genetic and epidemiological description of an outbreak of circulating vaccine-derived polio-virus type 2 (cVDPV2) in Angola, 2019–2020. Vaccine. 2023 doi: 10.1016/J.VACCINE.2023.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.AFP Polio data. [Accessed 26th April 2023]. Available from: https://extranet.who.int/polis/public/CaseCount.aspx.
- 67.Polio (Pol3) immunization coverage among 1-year-olds (%) [Accessed 7th May 2023]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/polio-(pol3)-immunization-coverage-among-1-year-olds-(-)