Abstract
Background:
Previous evidence revealed an association between folate deficiency and non-alcoholic fatty liver disease (NAFLD). This study is the first one investigating the effects of folic acid on hepatic steatosis grade, liver enzymes, insulin resistance, and lipid profile in NAFLD cases.
Materials and Methods:
Sixty-six participants with NAFLD were allocated randomly to take either a placebo or one oral tablet of folic acid (1 mg) on a daily basis within eight weeks. Serum folate, homocysteine, glucose, aminotransferases, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), and lipids were assessed. Ultrasonography was used for assessing the liver steatosis grade.
Results:
The serum alanine transaminase, grade of hepatic steatosis, and aspartate transaminase significantly were decreased within both study groups; however, the between-group comparison was not statistically significant. Of note, the decrease in ALT was more pronounced in folic acid compared with the placebo group (-5.45 ± 7.45 vs. -2.19 ± 8.6 IU/L). The serum homocysteine was decreased after receiving folic acid compared to the placebo (-0.58 ± 3.41 vs. +0.4 ± 3.56 μmol/L; adjusted P = 0.054). Other outcomes did not significantly change.
Conclusion:
Supplementation with folic acid (1 mg/d) for eight weeks among cases with NAFLD did not change significantly the serum levels of liver enzymes, the hepatic steatosis grade, insulin resistance and lipid profile. However, it was able to prevent the increase in homocysteine in comparison with the placebo. Conducting further research is suggested with the longer duration and different doses of folic acid, adjusted to the genotypes of methylenetetrahydrofolate reductase polymorphism, among NAFLD patients.
Keywords: Folic acid, homocysteine, insulin resistance, non-alcoholic fatty liver disease, transaminases
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) refers to excessive triglyceride accumulation in hepatocytes without another clear cause, such as alcohol consumption.[1] NAFLD is classified into steatohepatitis, fibrosis, and simple steatosis.[2] An association has been found between some risk factors, for example, inflammation, obesity, fatty acid oxidation, insulin resistance, dietary factors, bacterial and environmental contamination and the pathogenesis of NAFLD.[3,4] However, recent evidence has shown a possible impact of micronutrient deficiency on the pathogenesis and development of NAFLD.[5,6] Deficient dietary intakes of methyl donors, including folic acid, methionine, and choline, are being evaluated because of their possible roles in NAFLD progression.[7,8,9] S-adenosyl methionine (SAMe) might play a key role in hepatocyte metabolism.[10,11] The vitamin B12-dependent enzyme methionine synthase (MS) in the liver synthesizes methionine from homocysteine (Hcy). The methyl group of 5-methyl-tetrahydrofolate is transported to cobalamin (vitamin B12) for the formation of methyl-cobalamin that is attached to MS, causing the activation of the enzyme.[10] The activated methyl group is transferred by methyl-cobalamin to Hcy for generating methionine. Then, SAMe was produced from methionine via methionine adenosyltransferase.[12] Phosphatidylcholine (PC) is generated in the liver when SAMe is available in hepatocytes.[12] Based on the pathways mentioned above, it seems that folate deficiency can decrease liver storage of PC and SAMe.[13] The reduction of both PC and SAMe has been indicated in the pathophysiology of fatty liver disease.[7,14] The findings of the previous studies showed that folate deficiency is associated with hepatic steatosis through elevating the gene expression of the diacylglycerol O-acyltransferase, acetyl-CoA synthetase, and ATP citrate lyase.[15] These enzymes have a role in fatty acid synthesis. Results from an animal study indicated that folate supplements in doses of 5 mg/kg were associated with a reduction in the liver lipid content.[16] Furthermore, based on the evidence from another animal study, folate deficiency might have destructive impacts on the antioxidant defense system.[17]
A few human researchers examined the relationship between serum folate and NAFLD. Some of them reported similar serum folate levels in cases with NAFLD in comparison to the healthy control group,[18] while other studies did not show such an association.[19] More randomized clinical trials are required for resolving the related ambiguity. Thus, this work was carried out for investigating the impacts of folic acid supplementation on the grades of hepatic steatosis, serum liver enzymes, lipid profile, and insulin resistance in patients with NAFLD.
MATERIALS AND METHODS
Participants
Participants aged 18–80 years with an ultrasonographic diagnosis of NAFLD were chosen from the Gastroenterology Clinic of Shahid Beheshti Hospital, Kashan University of Medical Sciences, Kashan, Iran. Diabetic NAFLD patients were selected in the case of a recent diagnosis of diabetes mellitus and were not using blood glucose-lowering medicines. If the patients took metformin and insulin, at least six months should have passed since the beginning of the therapy and no change should be made in insulin and metformin doses during that period. We excluded patients with the following criteria: 1) Excess consumption of alcohol (above 20 g/day); 2) Lactation and pregnancy; 3) History of allergy to folic acid; 4) Diseases such as autoimmune hepatitis, viral hepatitis, Wilson's disease, α1 Antitrypsin deficiency, hereditary hemochromatosis; 5) Having previous surgeries such as gastroplasty, jejunoileal bypass, and history of total parenteral nutrition in the last six months; 6) Consumption of hepatotoxic drugs, including high doses of synthetic estrogens, calcium channel blockers, amiodarone, chloroquine, and methotrexate; 7) History of Cushing's syndrome, kidney failure, and hypothyroidism; and 8) intake of vitamins E and B12, and intake of folate supplements in the last six months. Furthermore, any other supplement consumption and possible severe infection were assumed as additional exclusion criteria during the intervention. Because no research similar to our study was found, the data required for calculating sample size was taken from the study of Talari et al.[20] that investigated the folate supplementation impacts on metabolic status and carotid intima-media thickness in those with metabolic syndrome. For each arm of our trial, we selected 27 participants for the detection of a change of 2.5 in HOMA-IR with 5% significance and 80% power. For covering potential dropouts, 20% additional participants were added to the sample size. The Ethics Committee of Kashan University of Medical Sciences (Registration No. IR.KAUMS.MEDNT.REC.1398.083) approved this study. The written informed consent form was signed by all participants prior to entering the research. The trial registration number at IRCT.ir is IRCT20190901044662N1.
Study design
This is a double-blind, placebo-controlled randomized trial with a parallel design. Subjects were divided into folic acid and placebo groups randomly (1:1 ratio). The blocked randomization method was used to assign participants into study groups. The size of the permuted blocks was six, and their sequence was created with a computer random number generator. The placebos and supplements were packed in the bottles by an investigator who did not clinically participate in the research. The packs were numbered based on the random list. Also, another individual, without any involvement in the research and awareness of any random sequences, allocated the participants to the tablets’ numbered bottles. Allocation and randomization were masked from the participants and investigators until the end of the statistical analysis. The intervention group took one oral tablet consisting of 1 mg folic acid (Iran Daru, Co, Tehran, Iran) daily, while the other group received one oral placebo tablet (Barij Essence Co, Kashan, Iran) containing edible starch with the same appearance, size, and color as folic acid supplements. The study duration was eight weeks. Unused tablets returned to the investigators were used for assessing compliance. Lifestyle modifications related to restricting a diet high in carbohydrates and fats and elevating physical activity were suggested to all patients participating in this study as standard care for NAFLD. Participants were called every two weeks for reminding the supplements and reporting any adverse effects.
At the start of the study, a stadiometer with a precision of 0.1 cm was employed for measuring the participant's height in a standing position without shoes. A digital scale was used to measure the weight to the nearest 0.1 kg. Body mass index (BMI) was measured by division of weight (kg) by squared height (m2). Furthermore, other necessary information, such as history of diseases and demographic data, supplements, and medications, was gained from the patients. Participants were asked not to use other supplements during the study. An experienced dietitian completed a 2-day, 24-hour food recall (one weekend and one weekday) after training participants. The modified Nutritionist IV software (Ver. 3.5.2, First DataBank; Hearst Corp, San Bruno, California) was used to calculate the average daily nutrient intake.[21] Furthermore, physical activities of participants were evaluated by the short form of the International Physical Activity Questionnaire (IPAQ) at the onset and the end of the research.[22]
Primary and secondary outcomes
Reduction in alanine transaminase (ALT), aspartate aminotransferase (AST), grade of fatty liver disease, and HOMA-IR were assumed as primary outcomes of our study. Secondary outcome measures involved alterations in serum levels of folate, Hcy, and lipid profiles that included triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c).
Ultrasound assessments
Ultrasound scans of the liver were performed at the beginning of the study and after the completion of the study in one center by the same inspector who was not informed of the participants’ trial group. (US; General Electric LOGIQ 400 CL – Using probe 3.5/5 MHZ). Grading of liver fat infiltration was done from I to III on the basis of such parameters as liver-to-kidney contrast ratio, liver brightness, echogenic attenuation, and blurred vessels.
Biochemical analysis
After a 12-hour overnight fast, 10 ml of fasting blood samples were obtained from subjects at the onset and end of the research. Blood samples were centrifuged for 20 min and kept at -80°C for subsequent evaluation. The activity of liver enzymes involving AST and ALT was evaluated by a kinetic technique (Pars Azmoon Co, Tehran, Iran). Enzymatic kits (Pars Azmoon Co, Tehran, Iran) were used to determine serum HDL-c, LDL-c, and TG. The glucose oxidase technique (GOD-PAP, Pars Azmoon Co, Tehran, Iran) was used to evaluate fasting blood glucose (FBG). The enzyme-linked immunosorbent assay (ELISA) (Monobind, USA) was employed to assay serum folate levels and fasting insulin concentrations. The formula HOMA-IR = fasting glucose (mg/dl) × fasting insulin (μU/ml)/405 was employed for calculating HOMA-IR. ELISA technique (laboratory kit: Axis-Shield Diagnostics, Scotland, UK) was used to assess the serum Hcy.
Statistical analysis
Using the Kolmogorov–Smirnov test, the normal data distribution was assessed. The Chi-square test was used for comparing qualitative variables between the study groups. Using an independent sample t-test, normal quantitative data was compared between the study groups, and the Mann–Whitney U test was utilized for non-parametric distribution. The within-group comparisons for mean changes from the beginning to the end of the intervention were made by the Wilcoxon test (in non-parametric conditions) and paired t-test (in parametric conditions). Using the ANCOVA test, the means between the two groups were compared and the effect of confounding variables was adjusted. Statistical analyses were conducted by the SPSS software, Ver. 16 (SPSS Inc, Chicago, Ill). Two-sided P values < 0.05 were statistically significant.
RESULTS
Out of the 72 subjects assigned to the study groups, six did not complete the research; two in the folic acid group; and four in the placebo group. [Figure 1]. Therefore, the study was completed by 66 patients. Participants were employed from December 2019 to March 2020. The compliance rate was above 80% in trial groups. Participants in both groups reported no side effects throughout the research. Table 1 indicates baseline features of the research groups. Apart from gender, no significant difference was found between the study groups in terms of smoking, history of diabetes mellitus, and age [Table 1].
Figure 1.
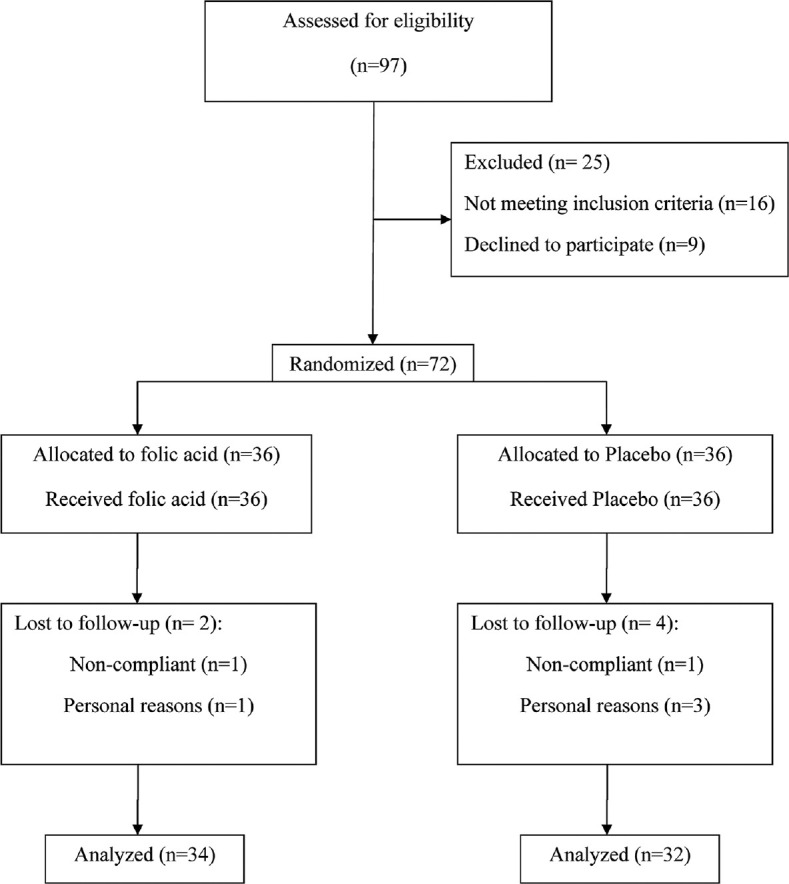
Flowchart of patients’ recruitment
Table 1.
General characteristics of the study participants
| Characteristics | Folic acid n=34 | Placebo n=32 | P |
|---|---|---|---|
| Gender (Women) (%)a | 82.4 | 100 | 0.025 |
| Smoker (%)a | 2.9 | 0 | 0.328 |
| History of diabetes mellitus (%)a | 11.8 | 18.8 | 0.505 |
| Ageb | 40.15±15.01 | 43.42±14.59 | 0.381 |
aData is tested by Chi-square test or Fisher’s exact test. bData is expressed as mean±standard deviation and tested by two sample t-test
Between-group and within-group comparisons of the changes from baseline to endpoint measures for daily dietary intakes, physical activity levels, weight, and BMI were shown in Table 2. According to the data obtained from 24-hour food recall, no significant differences were found in dietary micro- and macro-nutrient intakes, such as dietary vitamins B12, energy, and folate between the study groups [Table 2]. However, dietary intake of folate showed a significant reduction in both groups at the end of the trial. Other outcomes, including physical activity levels, BMI, and weight did not show a significant change in their means between the study groups.
Table 2.
Within-group and between-group comparisons of the baseline, endpoint, and changes values for anthropometric, physical activity, and dietary variables in folic acid and placebo groups
| Variables | Folic acid groupa (n=34) | Placebo groupa (n=32) | P (between)b |
|---|---|---|---|
| Weight (kg) | |||
| Baseline | 77.36±16.52 | 75.43±15.51 | |
| Endpoint | 79.48±18.57 | 76.9±16.56 | |
| Changec | 2.12±4.54 | 1.48±1.15 | 0.316 |
| P (within)d | 0.200 | 0.212 | |
| BMI (kg/m2) | |||
| Baseline | 30.88±4.43 | 31.17±5 | |
| Endpoint | 32±5.19 | 32.11±5.04 | |
| Change | 1.11±1.86 | 0.94±0.48 | 0.312 |
| P (within) | 0.163 | 0.187 | |
| Physical activity (MET-min/week)e | |||
| Baseline | 547.99±860.2 | 519±889.43 | |
| Endpoint | 311.08±302.85 | 265.44±277.45 | |
| Change | -236.91±554.22 | -253.56±906.51 | 0.303 |
| P (within) | 0.212 | 0.072 | |
| Energy (Kcal/d) | |||
| Baseline | 1971.76±526.8 | 2084.5±346.74 | |
| Endpoint | 1795.68±472.84 | 1828.29±403.93 | |
| Change | -176.08±463.14 | -256.21±510.55 | 0.922 |
| P (within) | 0.069 | 0.022 | |
| Dietary cobalamin (µg/d) | |||
| Baseline | 3.28±0.8 | 3.58±0.91 | |
| Endpoint | 2.96±1.11 | 2.87±1.07 | |
| Change | -0.32±1.36 | -0.71±1.51 | 0.854 |
| P (within) | 0.252 | 0.031 | |
| Dietary folate (µg/d) | |||
| Baseline | 319.27±122.47 | 309.71±84.44 | |
| Endpoint | 187.97±111.51 | 158.34±73.79 | |
| Change | -131.3±137.49 | -151.37±112.87 | 0.306 |
| P (within) | <0.001 | <0.001 |
BMI=Body mass index; MET=Metabolic Equivalent of Task. aValues are expressed as mean±standard deviation. bP for comparing the values between the study groups at baseline, at the endpoint and the change from baseline. Two sample t-test and Mann-Whitney U test were used for parametric and non-parametric comparisons, respectively. cEndpoint values minus the baseline ones. dP for comparing baseline with the end point values within each group. Paired sample t-test and Wilcoxon paired rank test were used for parametric and non-parametric comparison, respectively. eTotal MET-minutes/week=Walking (3.3 METs*min*days) + Moderate intensity (4 METs*min*days) + Vigorous intensity (8 METs*min*days), based on the short form of International Physical Activity Questionnaire (IPAQ)
Within and between-group comparisons for serum liver enzymes (AST, ALT), lipid profile, HOMA-IR, serum Hcy, and serum folate were shown in Table 3. The serum levels of liver enzymes, such as AST and ALT, did not significantly change between intervention and placebo groups; however, they show significant reduction within the study groups. A more pronounced reduction was noted in ALT in the folic acid group in comparison with the placebo group. Lipid profile, such as LDL-c, HDL-c, and TG, did not show a significant difference between the study groups. Furthermore, glycemia indices, including FBG, insulin serum level, and HOMA-IR, did not show any significant changes in between- and within-group comparisons [Table 3].
Table 3.
Within-group and between-group comparisons of the baseline, endpoint, and changes’ values for the study primary and secondary outcomes in folic acid and placebo groups
| Variables | Folic acid groupa (n=34) | Control groupa (n=32) | Pb,c (ANCOVA) |
|---|---|---|---|
| ALT (IU/L) | |||
| Baseline | 31.53±23.64 | 25.5±10.05 | |
| Endpoint | 26.08±19.52 | 23.31±11.29 | 0.673 |
| Changed | -5.45±7.45 | -2.19±8.6 | |
| P (within)e | 0.002 | 0.056 | |
| AST (IU/L) | |||
| Baseline | 26±9.01 | 24.94±5.17 | |
| Endpoint | 22.92±7.45 | 21.08±6.62 | 0.866 |
| Change | -3.08±6.1 | -3.86±4.95 | |
| P (within) | 0.002 | < 0.001 | |
| FBG (mg/dl) | |||
| Baseline | 103.62±28.1 | 105.53±23.53 | |
| Endpoint | 95.8±15.12 | 103.62±39.65 | 0.506 |
| Change | -7.82±24.31 | -1.92±22.55 | |
| P (within) | 0.098 | 0.225 | |
| Fasting serum insulin (µU/ml) | |||
| Baseline | 12.23±5.38 | 10.5±6.58 | |
| Endpoint | 12.79±5.86 | 11.99±6.15 | 0.752 |
| Change | 0.56±6.25 | 1.5±4.91 | |
| P (within) | 0.716 | 0.585 | |
| HOMA-IR | |||
| Baseline | 4.51±0.73 | 4.33±0.87 | |
| Endpoint | 3.36±0.73 | 3.54±1.39 | 0.691 |
| Change | -1.15 | -0.79±1.76 | |
| P (within) | 0.648 | 0.508 | |
| Serum Folate (ng/mL) | |||
| Baseline | 8.55±11.33 | 10.71±12.71 | |
| Endpoint | 6.23±2.57 | 5.06±3.03 | 0.148 |
| Change | -2.33±11.69 | -5.65±12.7 | |
| P (within) | 0.237 | 0.050 | |
| Serum homocysteine (µmol/L) | |||
| Baseline | 12.56±4.15 | 13.75±4.36 | |
| Endpoint | 11.98±2.75 | 14.15±3.41 | 0.054 |
| Change | -0.58±3.41 | 0.4±3.56 | |
| P (within) | 0.769 | 0.470 | |
| TG (mg/dL) | |||
| Baseline | 135.38±70.75 | 111.13±45.15 | |
| Endpoint | 153.04±68.91 | 140.88±74.98 | 0.398 |
| Change | 17.66±40.76 | 29.76±69.66 | |
| P (within) | 0.793 | 0.081 | |
| LDL-c (mg/dL) | |||
| Baseline | 104.32±27.07 | 118.63±25.75 | |
| Endpoint | 105.32±27.61 | 108.31±25.12 | 0.087 |
| Change | 1±17.64 | -10.32±20.93 | |
| P (within) | 0.679 | 0.006 | |
| HDL-c (mg/dL) | |||
| Baseline | 51.76±10.5 | 50.94±8.63 | |
| Endpoint | 45.36±10.21 | 46.15±10.72 | 0.594 |
| Change | -6.4±5.78 | -4.78±6.81 | |
| P (within) | < 0.001 | 0.001 |
ALT=Alanin transaminase; ANCOVA=Analysis of covariance; AST=Aspartate transaminase; FBG=Fasting blood glucose; HDL-c=High-density lipoprotein-cholesterol; HOMA-IR=Homeostatic model assessment of insulin resistance; LDL-c=Low-density lipoprotein cholesterol; TG=Triglyceride. aValues are expressed as mean±standard. bP for ANCOVA test to determine the significant levels of differences between the two groups post-intervention. while adjusting for baseline measurements and covariates. cAdjusted for baseline values of corresponding variable and gender. dEndpoint values minus the baseline ones. eP for comparing baseline with end point values within each group. Paired sample t-test and Wilcoxon paired rank test were used for parametric and non-parametric comparison, respectively
Although there were not any significant changes in serum folate between the study groups, the serum level of Hcy was declined in the folic acid group while it was elevated in the placebo one. The results of the ANCOVA analysis revealed a near-significant difference in terms of Hcy between the study groups after the adjustment of covariates [Table 3]. Findings from our study indicated that the grades of fatty liver did not show a significant change between the trial groups [Table 4].
Table 4.
Within-group and between-group comparisons of the baseline and endpoint values for the grade of hepatic steatosis in folic acid and placebo groups of NAFLD patients
| Variables | Folic acid group (n=34) | Placebo group (n=32) | P (between)a |
|---|---|---|---|
| Grades of steatosis n (%)b | |||
| Baseline | |||
| Grade I | 20 (58.8%) | 22 (68.8%) | |
| Grade II | 12 (35.3%) | 8 (25.0%) | 0.524 |
| Grade III | 2 (5.9%) | 2 (6.2%) | |
| Endpoint | |||
| Grade 0 | 6 (17.6%) | 1 (3.1%) | |
| Grade I | 21 (61.8%) | 25 (78.1%) | |
| Grade II | 6 (17.6%) | 6 (18.8%) | 0.182 |
| Grade III | 1 (2.9%) | 0 | |
| P (within)c | 0.002 | 0.039 |
BMI=Body mass index; MET=Metabolic equivalent of task. aP based on Fisher’s exact test. bGrades of fatty liver are expressed as percentage proportion and based on ultrasonography scan. cP based on sign test
DISCUSSION
The possible relationship between folate deficiency and clinical features of NAFLD, which was proposed by the observational studies, and the obtained evidence of the useful impacts of methyl donors on NAFLD in animal studies, provided a basis for designing the present clinical trial. In this study, the impact of taking 1 mg of the folic acid supplement daily for eight weeks was compared with placebo in patients with NAFLD. Although the serum levels of liver enzymes (AST and ALT) and the degree of steatosis were significantly reduced in the folic acid group, these changes were not significant when compared with the placebo group. Previous clinical trials that have evaluated the folic acid impact on hepatic steatosis and liver enzymes are lacking. Cordero et al.[15] conducted an experimental study and indicated that obesogenic diet-induced hepatic triglyceride accumulation in rats could be improved using supplementation of methyl donors, including vitamin B12 and folic acid.
Additionally, Christensen et al.[23] indicated the sufficiency of a folate-deficient diet alone in inducing hepatic steatosis in mice. In a cross-sectional study, NAFLD patients showed a significantly lower concentration of serum folate compared to those with normal liver.[18] However, in a case-control study by Polyzos et al.,[19] similar folate levels were observed in NAFLD patients and controls. Besides, there was not a relation between the folate levels and the liver disease severity.[19] Due to the different nature of the studies mentioned above, the results obtained from the clinical trials are not necessarily the same as experimental and observational ones. As in the present study, it was found that folate supplementation does not affect hepatic steatosis in comparison with the placebo. This research was the first and preliminary clinical randomized trial that assessed the folic acid impacts on NAFLD and the short duration of intervention and the less effective dose of folate were among its limitations. Further clinical studies are required for a better demonstration of the folic acid impacts on hepatic steatosis and fibrosis. Some mechanisms have been proposed by which folate might reduce hepatic fat accumulation. DNA methylation and consequent gene changes could be modified by methyl donor supplementation. These changes are responsible for reproduction enzymes engaged in denovo hepatic lipogenesis, for example, fatty acid synthase.[24] PC synthesis's alteration influences hepatic lipid storage and secretion in rodent models.[24] PC and choline levels are diminished by dietary folate deficiency in the liver.[24] Also, folate shortage decreases the activity of phosphatidylethanolamine N-methyltransferase (PEMT) and the expression of choline kinase in the liver.[25] There is an association between impaired production of PC via PEMT pathways and hepatic TG accumulation because of a decline in very low-density lipoprotein secretion.[26,27]
When it comes to liver enzymes, the results of some observational studies showed an association between increased serum levels of hepatic enzymes and folate deficiency. In a sample of Taiwanese adults, a higher dietary intake of folate was associated with normal serum levels of ALT and AST.[28] Also, in a Chinese hypertensive population, folate deficiency was associated with higher serum ALT and glutamyl transpeptidase.[29] Moreover, when this population received a 0.8 mg folate supplement along with the enalapril, their serum ALT levels were significantly decreased.[30] In another randomized clinical trial by Asgarshirazi et al.,[31] the efficacy of folate was compared to silymarin regarding antiepileptic drug-induced liver injury management in children with epilepsy. They concluded that despite the efficacy of both therapies in reducing liver enzymes, it seems that folate supplementation is stronger than silymarin in decreasing serum ALT and AST levels.[31] We found a similar result in the present clinical trial, as the decrease in ALT was more pronounced after folic acid supplementation than the placebo.
A crucial component of NAFLD is insulin resistance.[32] In the present study, supplementation with 1 mg of folic acid could not significantly improve glycemic markers such as FBG, insulin, and HOMA-IR. Several previous RCTs have investigated the impacts of folate on the markers of glycemic control in cases with diseases other than NAFLD.[33,34,35,36] A recently published systematic review and meta-analysis has reported overall results from these RCTs.[37] Asbaghi et al.[37] included data from 24 RCTs in their meta-analysis and concluded that supplementation with folic acid significantly reduces FBG (weighted mean difference (WMD): -2.17 mg/dL, 95% CI: -3.69, -0.65, P = 0.005), HOMA-IR (WMD: -0.40, 95% CI: -0.70, -0.09, P = 0.011), and fasting insulin (WMD: -1.63 pmol/L, 95% CI: -2.53, -0.73, P < 0.001). However, they added that markers of glycemic control were slightly improved following folate supplementation, and these improvements might not be clinically important.[37] In addition, as reported by these researchers, more evident improvement was observed in glycemic levels following higher folate doses (>5 mg/d vs. <5 mg/d).[37] According to the above results, it seems that one of the reasons for the lack of significant change in glycemic markers in our study would be the use of a lower and less efficient dose of folic acid.
At the end of the present work, it was observed that folate supplementation did not cause a significant change in fasting serum levels of LDL-c, HDL-c, and TG in comparison with the placebo. This study is the first RCT that assessed folic acid's effect on the lipid profile in NAFLD cases. A recently published systematic review and meta-analysis pooled data from 34 RCTs investigating folate supplementation impact on lipid profile in different diseases other than NAFLD.[38] The meta-analysis results revealed a significant decline in serum TG and total cholesterol (TC) concentrations after folic acid supplementation in comparison with placebo.[38] Nevertheless, serum concentrations of HDL-c or LDL-c were not influenced by folic acid supplementation, which is consistent with our study results.[38] Of note, the mentioned meta-analysis found no significant relationship between the duration and dose of folic acid intervention and the alterations in lipid markers.[38]
Previous cross-sectional and experimental research studies found a relationship between hyperhomocysteinemia and NAFLD.[39,40,41,42] Besides, considering the findings of a meta-analysis study, serum Hcy levels were higher in NAFLD cases than in normal controls.[43] Some mechanisms have been proposed that link hyperhomocysteinemia with NAFLD. Intracellular lipid metabolism might be altered by higher serum Hcy levels, which results in liver steatosis.[44] Furthermore, Hcy accumulated in hepatic cells leads to endoplasmic reticulum stress, disturbing the sterol response pathway, which leads to the progress of NAFLD.[39] Consequently, as a crucial strategy for slowing down the progression of NAFLD, the increase in serum Hcy levels should be prevented. In our trial, the serum level of Hcy was reduced in the folic acid group while it was increased in the placebo, and the P value for the differences between the research groups in this regard was near the significant level. In other words, folic acid supplementation prevented the increase in Hcy compared to the placebo.
Based on the data presented in Table 3, although the serum levels of folate were decreased in two groups, this decrease was more pronounced and statistically significant in the placebo group. This result can be examined from two perspectives. First, based on the nutrition data shown in Table 2, the average reduction in dietary folate intake in both study groups was about 40 to 50% during the intervention. This decrease can be due to a slight decline in energy intake in both groups. In addition, reduced intake of food sources of folate due to the changes of season and physical access can be other reasons for such a decrease in dietary folate intake. Therefore, it seems that this reduction in dietary folate intake has reduced the folic acid supplement's efficacy in improving liver steatosis and metabolic profile in our participants. Second, our study did not investigate the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism. The (MTHFR) C677T genotype is classified as heterozygous CT, homozygous CC, and homozygous TT.[45] The TT genotype is slow to respond to folic acid intake in terms of serum folate and Hcy.[46] Therefore, if a substantial proportion of our participants had genotypes of CT or TT, an intake of a higher dose of folic acid supplement (greater than 1 mg/day) would have been needed to find expected effects on serum folate and other metabolic profile of NAFLD. Notably, the folate mean serum level was in the normal range in both study groups at baseline. Then, it is suggested that future clinical trials examine the effects of folic acid supplementation on the metabolic profile in NAFLD patients with folate deficiency.
One of the concerns about folate supplementation is its interference with the intestinal absorption of dietary zinc, especially in populations where the prevalence of zinc deficiency is high.[47] However, in several clinical trial studies, no adverse effect of folate supplementation on body zinc status was found.[48,49,50] In the study by Kauwell et al.,[48] no difference was found in the zinc content in serum, plasma, and erythrocytes of subjects who received 800 microgram per day of folate compared to the placebo group. In another clinical trial study, taking a high dose of folate (10 mg per day) compared to placebo did not create a significant alteration in the concentration of zinc in plasma and red blood cells.[49] Therefore, it seems that there is a need for more studies to confirm the interaction of folate intake with zinc. Until then, it is recommended that people take folate supplements between meals.
Some limitations should be considered for interpreting the findings of this study. Although the histological alterations in the liver can be precisely detected by liver biopsy, this technique was not used in the present study due to ethical considerations. The other limitation is the gender differences between the research groups at baseline. However, we adjusted all the analyses for the gender variable and the other covariates. Besides, the majority of the study participants were women with NAFLD; and therefore, the findings of this study can be generalized to this population. The intervention dose of folic acid (1 mg/d) in the present study seems to be low to exert their expected effects on liver steatosis and metabolic profiles such as insulin resistance when considering the decrease in dietary folate intake and a possible polymorphism of MTHFR C677T. However, we chose this amount because it is the tolerable upper intake level (UL) for folate suggested by the Institute of Medicine.[51]
One of the strengths of our work is that this is the first randomized, double-blind, controlled trial evaluating the folic acid impact on NAFLD. Confounding variables, including dietary folate intakes and daily energy, as well as physical activity and BMI were adjusted at baseline and the end of the research.
CONCLUSION
In conclusion, our results indicated that supplementation with folic acid (1 mg per day) within eight weeks among NAFLD patients did not change significantly the grade of hepatic steatosis, the serum levels of liver enzymes, lipid profile, and insulin resistance. However, folate supplementation was able to prevent the increase in homocysteine compared to the placebo. The present study was a preliminary clinical trial; thus, it is suggested to conduct further investigations with a longer intervention duration, different doses and types of folic acid regarding the various variants of MTHFR polymorphism among patients with NAFLD who have folate deficiency. Moreover, future studies will examine the effects of folic acid on other non-invasive biomarkers of NAFLD, such as inflammatory and pro-fibrotic factors.
Financial support and sponsorship
This work was supported by the Vice-Chancellor for Research and Technology of Kashan University of Medical Sciences, Kashan, Iran (Grant number: 98111).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the Vice-Chancellor for Research and Technology of Kashan University of Medical Sciences and the Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran. Also, this work was a part of the M.D. thesis of Shadi Zarringol, the student of Kashan University of Medical Sciences.
REFERENCES
- 1.Henry L, Paik J, Younossi ZM. Review article: The epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment Pharmacol Ther. 2022;56:942–56. doi: 10.1111/apt.17158. [DOI] [PubMed] [Google Scholar]
- 2.Teng T, Qiu S, Zhao Y, Zhao S, Sun D, Hou L, et al. Pathogenesis and therapeutic strategies related to non-alcoholic fatty liver disease. Int J Mol Sci. 2022;23:7841. doi: 10.3390/ijms23147841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilson J, Sethi JK, Byrne CD. Non-alcoholic fatty liver disease: A multi-system disease influenced by ageing and sex, and affected by adipose tissue and intestinal function. Proc Nutr Soc. 2022;81:146–61. doi: 10.1017/S0029665121003815. [DOI] [PubMed] [Google Scholar]
- 4.Francisco V, Sanz MJ, Real JT, Marques P, Capuozzo M, Ait Eldjoudi D, et al. Adipokines in non-alcoholic fatty liver disease: are we on the road toward new biomarkers and therapeutic targets? Biology (Basel) 2022;11:1237. doi: 10.3390/biology11081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifi N, Amani R. Vitamin D supplementation and non-alcoholic fatty liver disease: A critical and systematic review of clinical trials. Crit Rev Food Sci Nutr. 2019;59:693–703. doi: 10.1080/10408398.2017.1389693. [DOI] [PubMed] [Google Scholar]
- 6.Abe RAM, Masroor A, Khorochkov A, Prieto J, Singh KB, Nnadozie MC, et al. The role of vitamins in non-alcoholic fatty liver disease: A systematic review. Cureus. 2021;13:e16855. doi: 10.7759/cureus.16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noureddin M, Mato JM, Lu SC. Nonalcoholic fatty liver disease: Update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp Biol Med (Maywood) 2015;240:809–20. doi: 10.1177/1535370215579161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng H, Xu H, Wu J, Li J, Wang X, Liu Z, et al. Maternal one-carbon supplement reduced the risk of non-alcoholic fatty liver disease in male offspring. Nutrients. 2022;14:2545. doi: 10.3390/nu14122545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talari HR, Molaqanbari MR, Mokfi M, Taghizadeh M, Bahmani F, Tabatabaei SMH, et al. The effects of vitamin B12 supplementation on metabolic profile of patients with non-alcoholic fatty liver disease: A randomized controlled trial. Sci Rep. 2022;12:14047. doi: 10.1038/s41598-022-18195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–12. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 11.Tang Y, Chen X, Chen Q, Xiao J, Mi J, Liu Q, et al. Association of serum methionine metabolites with non-alcoholic fatty liver disease: A cross-sectional study. Nutr Metab (Lond) 2022;19:21. doi: 10.1186/s12986-022-00647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi M, Singh BK, Zhou J, Tikno K, Widjaja A, Sandireddy R, et al. Vitamin B(12) and folate decrease inflammation and fibrosis in NASH by preventing Syntaxin 17 homocysteinylation. J Hepatol. 2022;77:1246–55. doi: 10.1016/j.jhep.2022.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Halsted CH, Villanueva JA, Devlin AM, Niemela O, Parkkila S, Garrow TA, et al. Folate deficiency disturbs hepatic methionine metabolism and promotes liver injury in the ethanol-fed micropig. Proc Natl Acad Sci U S A. 2002;99:10072–7. doi: 10.1073/pnas.112336399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osipova D, Kokoreva K, Lazebnik L, Golovanova E, Pavlov C, Dukhanin A, et al. Regression of liver steatosis following phosphatidylcholine administration: A review of molecular and metabolic pathways involved. Front Pharmacol. 2022;13:797923. doi: 10.3389/fphar.2022.797923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordero P, Gomez-Uriz AM, Campion J, Milagro FI, Martinez JA. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013;8:105–13. doi: 10.1007/s12263-012-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley B, Totter JR, Day PL. The lipotropic effect of folic acid on rats receiving various purified diets. J Biol Chem. 1950;187:529–35. [PubMed] [Google Scholar]
- 17.McNeil CJ, Hay SM, Rucklidge GJ, Reid MD, Duncan GJ, Rees WD. Maternal diets deficient in folic acid and related methyl donors modify mechanisms associated with lipid metabolism in the fetal liver of the rat. Br J Nutr. 2009;102:1445–52. doi: 10.1017/S0007114509990389. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch S, Poniachick J, Avendano M, Csendes A, Burdiles P, Smok G, et al. Serum folate and homocysteine levels in obese females with non-alcoholic fatty liver. Nutrition. 2005;21:137–41. doi: 10.1016/j.nut.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Polyzos SA, Kountouras J, Patsiaoura K, Katsiki E, Zafeiriadou E, Zavos C, et al. Serum vitamin B12 and folate levels in patients with non-alcoholic fatty liver disease. Int J Food Sci Nutr. 2012;63:659–66. doi: 10.3109/09637486.2011.649249. [DOI] [PubMed] [Google Scholar]
- 20.Talari HR, Rafiee M, Farrokhian A, Raygan F, Bahmani F, Darooghegi Mofrad M, et al. The effects of folate supplementation on carotid intima-media thickness and metabolic status in patients with metabolic syndrome. Ann Nutr Metab. 2016;69:41–50. doi: 10.1159/000448295. [DOI] [PubMed] [Google Scholar]
- 21.First DataBank IV N. V 4.1. San Bruno, CA: The Hearst Corporation; 1995. [Google Scholar]
- 22.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 23.Christensen KE, Wu Q, Wang X, Deng L, Caudill MA, Rozen R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J Nutr. 2010;140:1736–41. doi: 10.3945/jn.110.124917. [DOI] [PubMed] [Google Scholar]
- 24.Radziejewska A, Muzsik A, Milagro FI, Martínez JA, Chmurzynska A. One-carbon metabolism and nonalcoholic fatty liver disease: The crosstalk between nutrients, microbiota, and genetics. Lifestyle Genom. 2020;13:53–63. doi: 10.1159/000504602. [DOI] [PubMed] [Google Scholar]
- 25.Champier J, Claustrat F, Nazaret N, Fevre Montange M, Claustrat B. Folate depletion changes gene expression of fatty acid metabolism, DNA synthesis, and circadian cycle in male mice. Nutr Res. 2012;32:124–32. doi: 10.1016/j.nutres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Zeisel S. Choline, other methyl-donors and epigenetics. Nutrients. 2017;9:445. doi: 10.3390/nu9050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandler TL, White HM. Choline and methionine differentially alter methyl carbon metabolism in bovine neonatal hepatocytes. PLoS One. 2017;12:e0171080. doi: 10.1371/journal.pone.0171080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng CP, Chen CH, Kuo CS, Kuo HT, Huang KT, Shen YL, et al. Dietary choline and folate relationships with serum hepatic inflammatory injury markers in Taiwanese adults. Asia Pac J Clin Nutr. 2017;26:642–9. doi: 10.6133/apjcn.082016.03. [DOI] [PubMed] [Google Scholar]
- 29.Li WX, Li W, Cao JQ, Yan H, Sun Y, Zhang H, et al. Folate deficiency was associated with increased alanine aminotransferase and glutamyl transpeptidase concentrations in a Chinese hypertensive population: A cross-sectional study. J Nutr Sci Vitaminol (Tokyo) 2016;62:265–71. doi: 10.3177/jnsv.62.265. [DOI] [PubMed] [Google Scholar]
- 30.Qin X, Li J, Cui Y, Liu Z, Zhao Z, Ge J, et al. Effect of folic acid intervention on ALT concentration in hypertensives without known hepatic disease: A randomized, double-blind, controlled trial. Eur J Clin Nutr. 2012;66:541–8. doi: 10.1038/ejcn.2011.192. [DOI] [PubMed] [Google Scholar]
- 31.Asgarshirazi M, Shariat M, Sheikh M. Comparison of efficacy of folic acid and silymarin in the management of antiepileptic drug induced liver injury: A randomized clinical trial. Hepatobiliary Pancreat Dis Int. 2017;16:296–302. doi: 10.1016/s1499-3872(16)60142-x. [DOI] [PubMed] [Google Scholar]
- 32.Armandi A, Rosso C, Caviglia GP, Bugianesi E. Insulin resistance across the spectrum of nonalcoholic fatty liver disease. Metabolites. 2021;11:155. doi: 10.3390/metabo11030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gargari BP, Aghamohammadi V, Aliasgharzadeh A. Effect of folic acid supplementation on biochemical indices in overweight and obese men with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:33–8. doi: 10.1016/j.diabres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Solini A, Santini E, Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes (Lond) 2006;30:1197–202. doi: 10.1038/sj.ijo.0803265. [DOI] [PubMed] [Google Scholar]
- 35.Qin X, Li J, Zhang Y, Chen D, Wang B, He M, et al. Effect of folic acid supplementation on risk of new-onset diabetes in adults with hypertension in China: Findings from the China Stroke Primary Prevention Trial (CSPPT) J Diabetes. 2016;8:286–94. doi: 10.1111/1753-0407.12346. [DOI] [PubMed] [Google Scholar]
- 36.Mao G, Hong X, Xing H, Liu P, Liu H, Yu Y, et al. Efficacy of folic acid and enalapril combined therapy on reduction of blood pressure and plasma glucose: A multicenter, randomized, double-blind, parallel-controlled, clinical trial. Nutrition. 2008;24:1088–96. doi: 10.1016/j.nut.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Asbaghi O, Ashtary-Larky D, Bagheri R, Moosavian SP, Olyaei HP, Nazarian B, et al. Folic acid supplementation improves glycemic control for diabetes prevention and management: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutrients. 2021;13:2355. doi: 10.3390/nu13072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asbaghi O, Ashtary-Larky D, Bagheri R, Nazarian B, Pourmirzaei Olyaei H, Rezaei Kelishadi M, et al. Beneficial effects of folic acid supplementation on lipid markers in adults: A GRADE-assessed systematic review and dose-response meta-analysis of data from 21,787 participants in 34 randomized controlled trials. Crit Rev Food Sci Nutr. 2021:1–19. doi: 10.1080/10408398.2021.1928598. [DOI] [PubMed] [Google Scholar]
- 39.Ai Y, Sun Z, Peng C, Liu L, Xiao X, Li J. Homocysteine induces hepatic steatosis involving er stress response in high methionine diet-fed mice. Nutrients. 2017;9:346. doi: 10.3390/nu9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Carvalho SC, Muniz MT, Siqueira MD, Siqueira ER, Gomes AV, Silva KA, et al. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD) Nutr J. 2013;12:37. doi: 10.1186/1475-2891-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Guan Y, Yang X, Xia Z, Wu J. Association of serum homocysteine levels with histological severity of NAFLD. J Gastrointestin Liver Dis. 2020;29:51–8. doi: 10.15403/jgld-529. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Huang Q, Yang L, Zhang R, Gao L, Han X, et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: Results from the NHANES 1999-2004. Nutrients. 2022;14:1224. doi: 10.3390/nu14061224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai Y, Zhu J, Meng D, Yu C, Li Y. Association of homocysteine level with biopsy-proven non-alcoholic fatty liver disease: A meta-analysis. J Clin Biochem Nutr. 2016;58:76–83. doi: 10.3164/jcbn.15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–73. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet. 2015;58:1–10. doi: 10.1016/j.ejmg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Nishio K, Goto Y, Kondo T, Ito S, Ishida Y, Kawai S, et al. Serum folate and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism adjusted for folate intake. J Epidemiol. 2008;18:125–31. doi: 10.2188/jea.JE2007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghishan FK, Said HM, Wilson PC, Murrell JE, Greene HL. Intestinal transport of zinc and folic acid: a mutual inhibitory effect. Am J Clin Nutr. 1986;43:258–62. doi: 10.1093/ajcn/43.2.258. [DOI] [PubMed] [Google Scholar]
- 48.Kauwell GP, Bailey LB, Gregory JF, 3rd, Bowling DW, Cousins RJ. Zinc status is not adversely affected by folic acid supplementation and zinc intake does not impair folate utilization in human subjects. J Nutr. 1995;125:66–72. doi: 10.1093/jn/125.1.66. [DOI] [PubMed] [Google Scholar]
- 49.Butterworth CE, Jr, Hatch K, Cole P, Sauberlich HE, Tamura T, Cornwell PE, et al. Zinc concentration in plasma and erythrocytes of subjects receiving folic acid supplementation. Am J Clin Nutr. 1988;47:484–6. doi: 10.1093/ajcn/47.3.484. [DOI] [PubMed] [Google Scholar]
- 50.Hansen DK, Grafton TF, Dial SL, Gehring TA, Siitonen PH. Effect of supplemental folic acid on valproic acid-induced embryotoxicity and tissue zinc levels in vivo. Teratology. 1995;52:277–85. doi: 10.1002/tera.1420520506. [DOI] [PubMed] [Google Scholar]
- 51.Meyers LD, Hellwig JP, Otten JJ. Dietary reference intakes: The essential guide to nutrient requirements. National Academies Press; 2006. [Google Scholar]


