Abstract
Objective
Autophagy is an intracellular degradation pathway conserved in all eukaryotes from yeast to humans. This process plays a quality‐control role by destroying harmful cellular components under normal conditions, maintaining cell survival, and establishing cellular adaptation under stressful conditions. Hence, there are various studies indicating dysfunctional autophagy as a factor involved in the development and progression of various human diseases, including cancer. In addition, the importance of autophagy in the development of cancer has been highlighted by paradoxical roles, as a cytoprotective and cytotoxic mechanism. Despite extensive research in the field of cancer, there are many questions and challenges about the roles and effects suggested for autophagy in cancer treatment. The aim of this study was to provide an overview of the paradoxical roles of autophagy in different tumors and related cancer treatment options.
Methods
In this study, to find articles, a search was made in PubMed and Google scholar databases with the keywords Autophagy, Autophagy in Cancer Management, and Drug Design.
Results
According to the investigation, some studies suggest that several advanced cancers are dependent on autophagy for cell survival, so when cancer cells are exposed to therapy, autophagy is induced and suppresses the anti‐cancer effects of therapeutic agents and also results in cell resistance. However, enhanced autophagy from using anti‐cancer drugs causes autophagy‐mediated cell death in several cancers. Because autophagy also plays roles in both tumor suppression and promotion further research is needed to determine the precise mechanism of this process in cancer treatment.
Conclusion
We concluded in this article, autophagy manipulation may either promote or hinder the growth and development of cancer according to the origin of the cancer cells, the type of cancer, and the behavior of the cancer cells exposed to treatment. Thus, before starting treatment it is necessary to determine the basal levels of autophagy in various cancers.
Keywords: angiogenesis, autophagy, cancer biology, cancer management, drug design, signal transduction
1. INTRODUCTION
Autophagy is an evolutionarily conserved degradation process that helps maintain cell homeostasis and survival under normal and stressful conditions. In this intracellular catabolic pathway, damaged cytoplasmic components transported into lysosomes are degraded by lysosomal acid hydrolase enzymes; resulting ingredients such as amino acids, fatty acids, sugars, and nucleotides are released into the cytosol, and then used for cellular metabolism. 1 , 2
Three main types of autophagy have been described: micro‐autophagy, Chaperone‐mediated autophagy (CMA), and macro‐autophagy. The micro‐autophagy pathway involves trapping and eliminating damaged and redundant cytosolic components directly by the lysosomal membrane. 3 , 4 This pathway also plays a role in maintaining organelle size and membrane composition. 4 In the CMA, excess and damaged proteins with a specific KFERQ motif are transported close to the lysosomal membrane by heat shock protein 70 (HSC‐70) and its co‐chaperones, which are then transported into the lysosomes through interacting with a lysosomal membrane protein called lysosome‐associated membrane protein 2A (LAMP2A). 4 , 5 The CMA seems to be more involved in starvation responses by playing key roles in the removal of oxidized and misfolded proteins. 4 In the macro‐autophagy pathway (referred to as autophagy hereinafter), damaged cellular components are engulfed by double‐membrane vesicles (so‐called autophagosome). These vesicles fuse with and deliver their cargo to lysosomes, where they are broken down by the activities of various hydrolases. The degraded substances are released into the cytosol and used for cellular metabolism. 6 Non‐selective autophagy and cargo‐specific autophagy (also called: mitophagy, ribophagy, pexophagy, and xenophagy) are two types of macro‐autophagy. 7
In the absence of stressful conditions, the cell utilizes baseline levels of autophagy to support biological functions, maintain metabolic homeostasis, quality‐control of cell contents, and eliminate long‐lived or misfolded proteins and damaged organelles. 8 , 9 Nevertheless, autophagy is induced following fluctuations in oxygen and nutrients (stressful conditions) to support survival and normal cellular metabolism. 9 , 10
There are several studies showing the involvement of autophagy defects in different human diseases, including cancer. 4 , 11 In addition, autophagy appears to be able to play paradoxical roles in cancer progression. Thus, autophagy has been suggested to counteract the onset of cancer in the early stages of tumor formation. In an established tumor, however, this process supports the survival of tumor cells by helping them adapt to stressful conditions such as lack of oxygen and nutrients. 4 , 11 Also, Santana‐Codina et al. report suggested in a study that autophagy may be necessary to maintain tumor survival depending on the tumor type and source tissue. 12
Although autophagy and its manipulation have been considered as a practical therapeutic target in the field of cancer treatment 11 , 13 because most anti‐cancer therapies induce autophagy in tumor cells, it is not clear whether autophagy in cancer cells favors survival or death. 14 In response to anti‐cancer therapies, there are various reports that autophagy acts like a double‐edged sword, in favor of cancer cells in some tumors and against tumor cell survival in others. 13
Therefore, considering the paradoxical roles of autophagy in response to cancer therapies, two important questions arise; what are the initial and post‐treatment levels of autophagy in different tumors? What is the appropriate treatment option; induction or inhibition of autophagy? Here, we first overview the mechanisms and genes involved in autophagy and then address the paradoxical roles of autophagy in the development and progression of cancer, where it is highlighted as an anti‐cancer therapeutic target.
2. MOLECULAR MECHANISM OF AUTOPHAGY
To date, more than 30 autophagy‐related genes and proteins (ATG for autophagy‐related genes and Atg for their proteins) have been identified in yeast. 15 , 16 These genes are evolutionarily highly conserved, as they are present in higher eukaryotes and orthologs have been identified for them in humans. 16 The process of autophagy consists of several steps:
The initial step involves inducing phagophore membrane formation.
Nucleation; engulfing cytoplasmic components by phagophore membrane that forms a cup‐shaped structure.
Elongation/expansion; phagophore membrane extends to be able to surround cytoplasmic components. The resulting structure is called an autophagosome, which is a structure with a bilayer membrane.
Fusion and degradation; the autophagosomes fuse with lysosomes to form autolysosomes (often called autophagolysosomes) and then, their cargos are destroyed by lysosomal hydrolase. The resulting components are released into the cytosol to be used as cellular energy sources. 7 , 15 , 17
The autophagic flux can be triggered by intrinsic and extrinsic stresses such as starvation, hypoxia, growth factors deficiency, ATP/AMP ratio, intracellular Reactive oxygen species (ROS) rates, pathogens, or chemical drugs. 18 In yeast, activation of intracellular signaling pathways in response to stressful conditions recruits Autophagy‐related genes ATGs to a subcellular location called the phagophore assembly site. 19 , 20 In mammalians, phagophore is primarily formed at a subdomain of Endoplasmic reticulum (ER) membrane called omegasome, which is a lipid bilayer membrane enriched for Phosphatidylinositol 3‐phosphate (PI3P). 21
In mammalians, the Unc‐51‐like kinase (ULK) complex, which consists of ULK1/2 (homolog of yeast ATG1), FAK family‐interacting protein of 200 kDa (FIP200), Atg13, and Atg101, is responsible for the initiation of phagophore formation. 15 , 22 The initial step is regulated by mammalian target of rapamycin (mTOR) and AMP‐activated Protein Kinase (AMPK) pathways. Under normal conditions and in the presence of sufficient amino acids and growth factors, Phosphatidylinositol 3‐kinase complex 1 (PI3KC1) activates the mTORC1 subunit, leading to the activity of mTOR complex and thereby prevention of the autophagy initiation through phosphorylation of two subunits of ULK complex, ULK1 and Atg13. In starvation conditions, mTORC1 is separated from the initiation complex, resulting in the ULK complex activation. 15 , 22 In fact, ULK1 phosphorylates and activates Atg13, Atg101, and FIP200. After activation, ULK complex localizes into the expanding phagophore membrane. 22 The initiation complex is also induced by activation of AMPK which suppresses mTORC1. AMPK, in turn, is activated by glucose restriction and decreased ATP/AMP ratio. 8 , 23 , 24
In the nucleation step, ULK1 protein phosphorylates and activates a class ІІІ phosphatidylinositol 3‐kinase (PI3KC3) complex. This complex consists of Bcl‐2‐interacting myosin‐like coiled‐coil (Beclin1), Vacuolar protein sorting 34 (Vps34), Vps15, and Atg14L. 11 Beclin1 protein is a substrate for factors involved in the nucleation step. 5 PI3KC3 complex also generates local PI3P in phagophores which is necessary for phagophore nucleation. PI3P promotes the recruitment of WD‐repeat domain phosphoinositide‐interacting 2 (WIPI2), which in turn recruits Atg12–Atg5–Atg16L complex for phagophore elongation. 21 Proteins involved in the initiation and nucleation participate in the formation of phagophore membrane, 11 which can be formed and expanded from plasma membrane, ER, mitochondria, 11 , 25 or Golgi apparatus. 24 , 25
In the elongation step, two ubiquitin‐like conjugation systems involved in the expansion and closure of phagophore membrane are described 4 , 26 : In the first system, Atg12 is activated and conjugated to Atg5. Atg7 (E1 ligase) is involved in Atg12 activation, and Atg10 (E2 ligase) helps Atg12‐Atg5 conjugation. 22 , 27 Atg12‐Atg5 is then associated with Atg16L1 and hence autophagy elongation complex is formed, which provides a location for Lipidates microtubule‐associated protein 1 light chain 3 (Lc3) lipidation. 4 , 22 Binding of WIPI2 to Atg16L allows the Atg12‐Atg5‐Atg16L complex to be located on the autophagosome membrane. 27 In the second system, Lc3 (also known as ATG8) through cleavage by Atg4B is converted to Lc3‐І, which in turn is conjugated with Phosphatidylethanolamine (PE) in the cytoplasm to produce Lc3‐ІІ. Then, Lc3‐ІІ is deposited in the autophagosome membrane which causes cargo recognition. 4 , 5 , 22 Lc3‐ІІ also serves as an autophagosome marker and is essential for autophagosome maturation. 5 , 26 , 27 After maturation and closure, the autophagosome fuses with lysosome, which ultimately leads to the degradation of autophagosome cargo. Several proteins are involved in the fusion step, such as Ras‐associated binding proteins (Rabs), Soluble N‐ethylmaleimide‐sensitive factor attachment proteins (SNAREs), and tethering complexes 27 (Figure 1).
FIGURE 1.
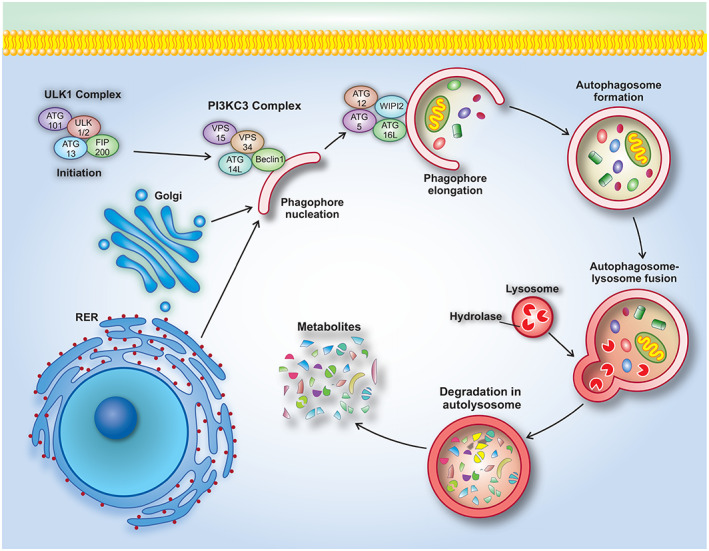
Autophagy process consists of several sequential steps. (1) Initiation. (2) Phagophore nucleation. (3) Phagophore elongation. (4) Fusion of autophagosome with lysosome and degradation of cargo in autolysosome.
Dysfunction of autophagy has been described in various diseases including metabolic disorders, pulmonary disease, vascular diseases, 25 abnormalities, 28 neurodegenerative diseases, infections, cancers, and various myopathies. 27 , 29 , 30 There is different evidence that autophagy exerts paradoxical roles in cancer, so that the role of autophagy in tumorogenesis is one of the most controversial issues in the field of cancer. 22
3. PARADOXICAL ROLE OF AUTOPHAGY IN CANCER
The autophagy pathway is essentially considered a protective pathway of the cell against metabolic stress, DNA damage, and tumorogenesis by removing misfolded proteins, damaged organelles, and ROS. Various studies have suggested that autophagy plays a cytoprotective role in the early stages of tumor formation. 22 , 27 However, after tumor establishment and transformation of healthy cells into tumors and progression to advanced stages of cancer, increased autophagy has been reported that works in favor of resistance of tumor cells to stressful conditions such as hypoxia, nutrient deficiency, metabolic stress, chemotherapy, resulting in tumor survival, 17 , 19 proliferation and metastasis. 26 , 31
It is noteworthy that the dependence of tumor cells on autophagy varies in different tumors. While autophagy increases in several tumors such as pancreatic cancer, even in nutrient‐rich conditions, 32 there are inconsistencies in a variety of cancers in which the level of autophagy in cancer cells is compared to that in non‐tumor cells. 33 In addition, several studies have suggested that the effect of autophagy on the destiny of tumor cells depends on the type of tumor, genetic context, tumor stage, 19 , 27 and tumor microenvironment. 19
Considering the roles of autophagy in maintaining genomic stability and preventing tumor formation in the early stages of cancer along with supporting the survival of tumor cells against various stresses in advanced stages of cancer, 7 and also because of its paradoxical effects in treatment cancer (discussed in other sections), autophagy is referred to as the “double‐edged sword” in various studies. 34
4. ROLE OF AUTOPHAGY IN TUMOR SUPPRESSION
There are several studies that have suggested a tumor suppressor role for autophagy. Tumor suppressor genes such as Phosphatase and tensin homolog (PTEN), P53, Tuberous sclerosis protein 1 and 2 (TSC1/2), and Death‐associated protein kinase (DAPk) induce autophagy and some autophagy suppressor genes such as Rat sarcoma virus (RAS) and AKT display oncogenic activities. 35 Also, down‐regulation and loss of function of some Atgs have been reported in several cancers, suggesting possible tumor inhibitory functions for autophagy. 36 , 37 Takamura et al. indicated that mice with autophagic defects induced by systemic mosaic elimination of ATG5 and specific deletion of ATG7 showed benign adenomas in tissues such as liver and pancreas. 38 In addition, Karantza‐Wadsworth et al. showed increased tumorogenesis of mammary cell lines with impaired autophagy through Beclin1 allelic loss. 39 Increased susceptibility of Beclin1+/− mice to Hepatocellular carcinoma (HCC), lung carcinoma and lymphoma has also been reported. 40 Liang et al. demonsrated that normal breast epithelia frequently express endogenous Beclin1 protein at high levels, wherease human breast epithelial carcinoma cell lines and tissue frequently express endogenous Beclin1 protein al low levels. 41 Moreover, deletions or loss of function mutations in ATG2B, ATG5, ATG12, and ATG9B have been reported in different human tumors. 42
Taken together, there are various studies indicating the involvement of impaired autophagy in tumor development. Also, autophagic cell death can play a potential tumor suppressor effect, 43 which is discussed in a separate section.
Besides, several studies have suggested that the suppressor role of autophagy on the formation of neoplasia and cancer may be due to its inhibitory effects onnecrosis and inflammation. 19 , 44 In fact, cancer cells try to inactivate apoptosis to gain uncontrolled proliferation, and it seems that if autophagy in these cells is defective, they will undergo necrosis due to metabolic stress. 4 , 45 In other words, inactivation of autophagy induces necrosis in cells with defects in apoptosis pathways and exposed to metabolic stress, leading to the infiltration of inflammatory cells into tumor tissue. It has been reported that necrosis and inflammation are associated with increased tumorigenic potential of solid tumors. 46 Therefore, autophagy contributes to cell survival in cancerous cells with defects in apoptotic pathways. Before angiogenesis occurs in a solid tumor, the inner cells are under different stressful conditions, where induction of autophagy causes the cells to pass through this condition. Accordingly, long‐term chronic necrosis is the fate of cancer cells with defective autophagy, 36 indicating the momentous role of autophagy in tumor formation.
4.1. Autophagy and cell death
Although there are many studies on the protective role of autophagy in normal and cancer cells against stress conditions, the role of autophagy in cell death and tumor suppression has also been considered. 10 , 34 , 47 Increased autophagosome formation in cells has been shown to induce cell death. 48 For example, the accumulation of autophagic vacuoles in anti‐estrogen therapy with tamoxifen induces cell death in MCF‐7 cells. This autophagy‐induced cell death is a type of programmed cell death. 49 Programmed cell death is divided into two categories: apoptotic cell death (as type І) and autophagic cell death (as type ІІ); there is also programmed necrosis (as type ІІІ). 50 Autophagy‐induced cell death leads to tumor growth inhibition, 44 probably due to the simultaneous occurrence of autophagy and apoptosis as long‐term stress and progressive autophagy can lead to cell death. 34 , 44 Also, induction of autophagy through up‐regulation of Beclin1 induces cell death. 51 Down‐regulating Bcl2, an inhibitor of autophagy, has been reported to trigger cell death by increasing autophagy in HL60 leukemia cells. 52 In addition, sorafenib, an autophagy inducer, induces autophagic cell death in renal cancer cell lines. 53
Moreover, autophagy‐induced cell death is associated with other mechanisms of programmed cell death, which in turn can inhibit tumor progression. Activation of ULK1/2 transcription by p53 promotes autophagy and leads to cell death. 54 So, ULK1/2 overexpression triggers cell death through increased autophagy. 55 Decreased ULK1/2 expression has been reported in all grades of glioblastoma, therefore induction of autophagy appears to be helpful in suppressing brain tumors. 56
Some studies have suggested that autophagy alone does not cause cell death but in combination with other death signals supports the progression of the cell death process. For example, DRAM1 exerts a modulatory role in autophagy mechanism and its signaling simultaneously with p53 signaling leads to cell death, indicating that autophagy is necessary but not sufficient for initiating and/or progressing cell death process. 57 Various studies have also reported autophagic cell death as a result of apoptosis inhibition. 58 Therefore, although increased autophagy has been associated with cell death, its relationship with induced cell death and apoptotic cell death needs further investigation. 7
5. ROLE OF AUTOPHAGY IN TUMOR SURVIVAL AND PROGRESSION
Inconsistent with studies reporting the inhibitory roles of autophagy in tumorogenesis, there are several studies suggesting that autophagy may be associated with positive effects on tumor survival. Once the tumor has formed and increased in size, autophagy induces due to metabolic stress, hypoxia, and nutrient deficiency as well as high demand of inner tumor cells. Other tumor cells use autophagy to survive in the face of therapeutic interventions such as chemotherapy, radiation therapy, and hormone therapy. According to these studies, tumor cells rely on autophagy to survive especially by removing damaged organelles and proteins. 59 , 60 For example, eradicating damaged mitochondria prevents excessive ROS production and protects DNA from damage. Autophagy also maintains cellular metabolism by recycling the components required. Therefore, although cancer cells face a variety of stressful factors, autophagy may ensure cell survival by participating in the elimination of different stressful conditions. 29 There are various reports that cancers such as pancreatic are “addicted” to autophagy in advanced stages to maintain energy homeostasis. 61 , 62 Also, autophagy increases in several types of cell lines and cancers even under nutrient‐rich conditions. 21 , 59
In addition, autophagy is involved in tumor cell metastasis. The motility of adhesive cancer cells is achieved by Epithelial‐to‐mesenchymal transition (EMT) and it has been reported that Transforming growth factor‐β (TGF‐β) and hypoxia‐related signaling pathways inducing EMT also induce autophagy. 63
Moreover, defective autophagy can exert negative effects on the growth, progression, and metastasis of malignant cells. 24 There are reports indicating inhibitory effects of defective autophagy on the invasion of lung and liver cancer cells. 64 , 65 Nevertheless, Catalano et al. demonstrated that induced autophagy down‐regulates transcription factors related to EMT in glioblastoma cells. 66
Besides, cancer cell dormancy and chemotherapy resistance depend on the autophagic capacity of the cells. 4 Cancer cells usually inactivate apoptosis pathways to increase survival and resistance to chemotherapy. Tumor cells with defective apoptosis enter dormancy under stress conditions and survive by inducing autophagy until they receive nutrients and return to normal conditions. 67 This ability to survive, resist, and regenerate under stressful conditions can obstruct cancer treatment strategies. 34
Oncogenic activity of oncogenes promoting tumor growth such as RAS and V‐Raf murine sarcoma viral oncogene homolog B (BRAF) seems to increase autophagy levels, indicating a link between autophagy and tumor progression. 68 , 69 Guo et al. reported that RAS‐driven cancer cells show increased levels of autophagy, and that this induction of autophagy activity appears to support the supply of metabolic needs under normal and starvation conditions of these cells. 68 The role of autophagy in promoting tumor cell proliferation is more apparent with studies demonstrating inhibition of growth and progression in tumor cells with defects in autophagy. 21 In this regard, inhibition of key proteins related to autophagy in human and mouse cell lines with RAS‐overexpression resulted in a decrease in the tumorigenic capacity of these cells, 68 , 70 suggesting a pivotal role for autophagy in the survival, growth and tumorigenicity of activated RAS‐driven tumors.
Autophagy has also attracted a lot of attention in developing therapeutic resistance. Inhibition of autophagy mediated by inhibitors or gene silencing causes cells to demonstrate limited ability to survive under stress conditions and restore homeostasis, leading to inhibition of tumor progression. 8 , 21 , 24 Therefore, targeting autophagy by relevant inhibitors has been suggested in various studies, especially for the treatment of cancers that have been shown to be dependent on autophagy. 17 Amaravadi et al. showed that inhibition of autophagy by drug or genetic manipulation led to a reduction in the resistance of isolated, cultured tumor cells to therapeutic agents compared to wild‐type cells. 71 Also, Chen et al. reported increased autophagy during radiation therapy in tumor cells, which led to therapeutic resistance, and suggested that suppression of autophagy concomitant with radiation therapy may contribute to increased therapeutic efficacy. 72 In addition, autophagy has been reported to play a role in preventing apoptosis in cancer cells. As mentioned above, cancer cells also employ autophagy to balance their metabolic rate and maintain their replicative power. 73 For example, the increasing knowledge of the metabolome of prostate cancer (Pca) has led to a better elucidation of the pathways controlling the metabolism of PCa cells. 74 , 75 Autophagy has been found to be involved in the control of several cancer metabolic pathways as well as the regulation of apoptosis and prostate cancer progression. 73 MAPK14 suppression has been reported to exert a significant effect on the growth of PCas, depending on the activity/inactivity of the STK11 protein. So that, drug‐mediated inactivation of MAPK14 does not affect the survival of STK11‐positive prostate cancer cells due to the activation of the AMPK‐dependent autophagic pathway. However, it induces apoptosis in STK11‐deficient prostate cancer cells. Also, blocking autophagy leads to induction of apoptosis in STK11‐positive cells treated with the MAPK14‐MAPK11 inhibitor. Thus, MAPK14 inhibition triggers a stress response in PCa cells that is buffered by AMPK‐dependent autophagy in STK11 positive cells but induces apoptosis in STK11‐deficient cells. 76
The following is an overview of proteins and signaling pathways involved in autophagy:
5.1. Beclin1
Beclin1, encoded by the BECN1 gene and an ortholog for the yeast ATG6 gene, is a key protein involved in the beginning and nucleation steps of autophagy process. 8 , 47 Binding of Beclin1 to Vps34 through a conserved domain leads to the onset of autophagy. This domain also provides a tumor suppressionve activity for BECN1. 21 , 27 Although the BECN1 gene is not considered a highly mutated gene in cancer (0.5% of all cancers), its monoallelic deletion can be seen in approximately 40% to 75% of ovarian, prostate, and breast cancers, which is why it is described as a “haploinsufficient tumor suppressor” gene. 44 , 77 Mouse models with BECN1 allelic loss show an increased susceptibility to spontaneous lung adenocarcinoma, lymphoma, and liver carcinoma. 78 Also, down‐regulation of BECN1 has been reported in several cancers, including non‐small cell lung, brain, lymphomas, melanomas, and osteosarcomas. 79 , 80 , 81 , 82 , 83 In mouse models, Beclin1 heterozygous dysfunction has been shown to be associated with increased susceptibility to malignancies by supporting the progression of precancerous to cancerous conditions. 40 However, Li et al. demonstrated increased levels of Beclin1 in cancerous tissues of patients with colon cancer compared to surrounding normal tissues, and these increased expression levels were associated with favorable prognosis and increased survival. 84
Beclin1 protein is regulated through interaction with B‐cell lymphoma 2 (Bcl2). 21 Although, the early known role of Bcl2 family proteins in tumorogenesis is their modulatory roles in cell death, they are involved with key roles in various pathways of cell metabolism. As mentioned, it has been reported that one of the pathways for the negative effects of Bcl‐2 family proteins on autophagy is their interaction with Beclin1. 85 Previous studies have shown that Beclin1 is inhibited under normal conditions through binding via the BH3 domain to Bcl2 or other anti‐apoptotic Bcl2 family proteins such as B‐cell lymphoma‐extra‐large (Bcl‐xL) and Bcl‐w. Under starvation conditions, Beclin1 is separated from its inhibitory proteins and can activate autophagy. 45 Some proteins can induce autophagy by preventing the interaction between Bcl2 and Beclin1. For example, HMGB1 competitively binds to Beclin1 under starvation conditions and disrupts the interaction between Beclin1 and Bcl2. 86 The Bcl2/Beclin1 complex is also regulated by stress‐activated signaling molecule c‐Jun protein kinase 1 (JNK1), so that stress‐activated JNK1 leads to the degradation of the Bcl2/Beclin1 complex by phosphorylating Bcl‐2. 87
On the other hand, pro‐apoptotic members of the Bcl2 family such as BCL‐2 Associated agonist of cell death (Bad), Noxa, BCL‐2 interacting killer (Bik) and Bcl2/adenovirus E1B 19‐kDa interacting protein (BNIP3) contributes to autophagy induction. 88 Therefore, usually increased expression of Bcl2 in cancer cells can inhibit autophagy process by binding to Beclin1. 89 In addition, Bcl2 exerts suppressor effects on autophagy induced by elevated cytosolic calcium through activating its antagonist pathways. 90
Interaction of some proteins with Beclin1, on the other hand, exerts positive effects on autophagy, such as Activating molecule in Beclin1‐regulated autophagy (AMBRA1), Bax interacting factor‐1 (BIF‐1), and UV radiation resistance‐associated (UVRAG), thereby inhibit cell proliferation and provides antitumor activity. Nevertheless, these proteins play other roles in addition to interfering with the autophagy pathway, which is beyond the scope of this study. 91
5.2. UVRAG and Bif‐1
Tumor suppressor protein encoded by UV Radiation Resistance Associated Gene (UVRAG) plays a role in regulating the onset of autophagy as well as regulating the response to DNA damage. 92 UVRAG exerts anti‐cancer effects through activating the Beclin1/PI3KIII complex and hence inducing autophagy. 93 Destruction of the UVRAG chromosomal locus has been associated with a variety of malignancies, such as breast cancer. 94 Also, monoallelic deletions or mutations in the UVRAG gene have been reported in colon cancers and gastric carcinomas. 95 , 96 The human colon cancer cell line HCT116 has a monoallelic mutation in the UVRAG gene that leads to significantly reduced expression levels of endogenous UVRAG and an anchorage‐independent cell growth. Additionally, heterozygous deletions in UVRAG gene increase the susceptibility of mouse models to cancer. 93
Bif‐1 tumor suppressor, known as endophilin B1, induces autophagy by UVRAG‐mediated interaction with Beclin1. 31 , 97 Takahashi et al. showed an increased incidence of lymphoma and solid tumors in bif‐1−/− mice compared to wild‐type models. 97 Lee et al. reported decreased expression of Bif‐1 in gastric carcinoma cells compared to normal mucosal samples. 98 Taken together these studies indicate that loss of function mutations in or depletion of autophagy regulators leads to tumorogenesis.
5.3. P62/SQSTM (sequestosome 1)
P62/SQSTM1 is a key receptor involved in selective autophagy. 99 This protein binds to ubiquitinated cargoes such as protein aggregates through its ubiquitin‐binding domain. 45 Also, P62/SQSTM1 can interact with LC3 located in the autophagosome membrane using its LC3‐interacting region (LIR) domain, leading to the cargo transfer into autophagosome. P62/SQSTM1 and transported cargo are destroyed in autophagosome through autophagy pathway. 100
Defects in autophagy lead to the cell accumulation of p62, 100 , 101 mitochondrial damage, production of ROS, and thereby DNA damage. These consequences may affect predisposition to tumorogenesis. 102 In addition, p62 knockdown aimed at eliminating its cell aggregates reduced ROS levels and DNA damage in cells with defective autophagy, and hence reduced defective autophagy‐induced toxicity and tumorogenesis. 102 , 103 Also, overexpression of p62 resulted in an increase in the ROS levels and tumor growth. 103
Moreover, P62 gene amplification was associated with renal cell carcinoma 104 and P62−/− mice have been shown to be resistant to Ras‐induced lung adenocarcinoma. 105 Further, an association has been reported between defective autophagy and p62 accumulation in human tumors 106 and there are several studies demonstrating p62 intracellular accumulation resulted from defective autophagy induces tumor progression in liver, lung and breast tissues. 107 , 108 , 109 Taken together, while aggregates caused by p62 contributes to the cancer development, autophagy flux prevents tumorogenesis by limiting p62 intracellular accumulation. 19
5.4. P53
P53 protein is encoded by TP53 gene which is one of the most common mutated genes in human cancers (more than 50%). 35 , 37 , 85 P53 plays key roles in regulating cell cycle checkpoints. Its activity can lead to cell cycle arrest, cellular senescence, and apoptosis, which introduces p53 as a tumor suppressor. In addition to involvement in cell cycle regulation, there are reports of p53 involvement in the regulation of autophagy. 35 , 110 Also, mutated TP53 has been reported in many cancers and it has been suggested to induce tumorogenesis, possibly due to its key roles in the regulation of cell apoptosis and autophagy. 111
P53 appears to play a paradoxical role in the autophagy pathway and its effects have been suggested to lead to cancer cell survival or death depending on its subcellular localization. 31 Nuclear p53 induces transcription of autophagy‐related genes and thereby activates autophagy. 12 , 112 Under stress conditions, nuclear p53 induces autophagy through activating Damage‐regulated autophagy modulator (DRAM) or AMPK pathways, leading to inactivating mTOR pathway. 31 , 55 , 112 Also, p53 can induce autophagy by activating Death‐associated protein kinase (DAPK), pro‐apoptotic Bcl2 proteins such as BCL‐2 Associated X (BAX), BAD, BNIP3 and p53 Upregulated modulator of apoptosis (PUMA), TSC2, and Sestrin1/2 31 , 55 (Figure 2). Gao et al. reported that p53 activates autophagy initiator proteins ULK1 and ULK2 in response to DNA damage to initiate autophagy and induce cell death. 54 Inhibition of p53 has been shown to induce autophagy in enucleated cells and thereby its nuclear pool does not appear to be responsible for inhibiting autophagy. Instead, experimental studies have shown that cytoplasmic p53 can inhibit autophagy by blocking proteins involved in the autophagy pathway. Also, p53 depletion and autophagy initiation promote survival of p53‐deficient cells, 113 possibly due to the maintenance of ATP levels under stressful conditions 31 (Figure 2). There is an interconnection between pathways related to autophagy and p53 so that activated p53 induces autophagy and, on the other hand, induced autophagy results in a decrease in p53 activity levels. 114 In addition, autophagy can promote tumor growth by inhibiting p53 in two ways: (a) through controlling stress and thereby inhibiting stress‐induced P53, as mentioned above. And (b) through providing raw materials for DNA replication and repair, and therefore, preventing DNA damage and damage‐induced p53 activation. 114 Although one of the key mechanisms involved in tumor progression is P53 suppression by induced‐autophagy, it has been reported that autophagy also causes tumorogenesis through P53‐independent mechanisms that need to be further investigated. 114
FIGURE 2.
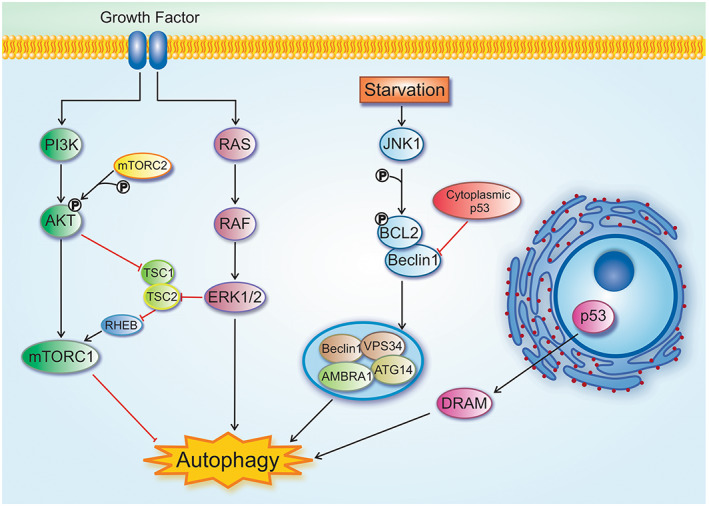
Overview of signaling pathways involved in the regulation of autophagy. (1) Growth factors and nutrients can induce PI3K, which activates AKT and subsequently mTORC1. Also, the activated AKT induce mTORC1 through TSC1/2 inhibition. (2) Growth factors inducethe Ras–Raf–MEK‐ERK1/2 signaling pathway. This pathway can directly induce autophagy. ERK complex can inhibit TSC1/2 complex, leading to activating mTORC1. (3) Under starvation and stress condition, Bcl2 is phosphorylated by JNK1 and thereby separated from Beclin1. Then, Beclin1 induces Vps34 complex formation, an important protein complex in the autophagy process. (4) P53 has different roles in autophagy. Under stress conditions, p53 nuclear localization promotes autophagy inducers such as DRAM and DAPK1. Nevertheless, cytoplasmic p53 leads to autophagy inhibition through Beclin1 and AMPK blocking.
5.5. mTOR
The Mammalian target of rapamycin (mTOR) is the major regulator of autophagy. 23 mTOR consists of two distinct multiprotein complexes with different functions called mTORC1 and mTORC2. 10 , 23 , 30 In nutrient‐ and energy‐rich conditions, mTORC1 inhibits autophagy through binding to and phosphorylating ULK1 and thereby disrupts its interaction with AMPK. Under stress conditions such as hypoxia and starvation, ULK1 is separated from mTORC1 and induces autophagy. 115 In addition, mTORC1 prevents autophagy by phosphorylating Atg13. 116 The mTORC1 is inactivated by AMPK. Inhibition of mTORC1 and activation of AMPK induce autophagy. 8 , 23 Moreover, indirectly activation of mTORC1 by mTORC2 suppresses autophagy. 30 Different single amino acid changes (gain of function mutations) in the components of mTOR have been reported in various human cancers. 117
Rapamycin or rapalog drug is an autophagic inducer that promotes tumor survival in TSC mouse models through inhibiting mTOR pathway. 118 , 119 However, metformin has been reported to inhibit lymphoma and melanoma development by inducing autophagy and apoptosis mechanisms through AMPK activation and mTOR inhibition, and it has been associated with beneficial effects in pre‐clinical models. 120 , 121 Therefore, various studies showing inhibition of mTOR leading to induction of autophagy using various drugs emphasize the paradoxical effect of autophagy on tumor progression. 30
5.6. HIF‐1α
Hypoxia is caused by defective angiogenesis, insufficient blood supply and high metabolic demand of tumor cells. 44 Tumor cells exposed to Hypoxia induce hypoxia‐inducible factor (HIF) complex to overcome lack of oxygen. 122 HIF‐1α, a subunit of HIF complex, is induced under hypoxia and regulates pathways involved in pH, metabolism, cell migration, inflammation, invasion, angiogenesis, and erythropoiesis, leading to adaptation of tumor cells to hypoxia conditions. 122 Bellot et al. demonstrated that autophagy can be induced by hypoxia and HIF‐1α activity. HIF‐1α activates transcription of ATGs such as BNIP3 and BNIP3 like protein (BNIP3L, also known as NIX), resulting in initiating autophagy process through inhibiting interaction between Bcl2 and Beclin1. 123 Under hypoxia, therefore, activation of HIF‐1α/BNIP3 pathway leads to induction of autophagy and thereby cell adaptation to hypoxic conditions. 122 Hypoxia can also induce autophagy through HIF‐1α‐independent pathways, including inhibition of mTOR or AMPK activation. 124 , 125
5.7. BNIP3
Bcl2/adenovirus E1B 19‐kDa interacting protein 3 (BNIP3) is a pro‐apoptotic member of the Bcl2 family, with increased expression in hypoxic conditions. 126 BNIP3 is involved in apoptosis and mitophagy induction. 127 Increased expression levels of BNIP3 have been detected in the early stages of tumor progression. Nevertheless, it has been reported that BNIP3 is decreased in the advanced stages. 128 Sowter et al. reported that BNIP3 expression levels in breast carcinoma cells were higher than that in normal adjacent cells. 129
5.8. Signaling pathways
The following is an overview of some of the signaling pathways involved in autophagy:
The PI3K/Akt/mTOR signaling pathway, a negative regulator for autophagy, is regulated by AMPK, Phosphatase and tensin homolog (PTEN), Sirtuin1 (SIRT1), insulin, p38‐MAPK, p53 and ROS. 130 Also, mTORC2 can inhibit the PI3K/AKT/mTOR pathway through AKT phosphorylation. 10
Excessive activity of the Ras/Raf/ERK pathway; gain of function mutations in Ras or B‐Raf oncogenes, reported in various tumors, induces autophagy, which in turn supports tumor progression. 130 Another study reported that ERK can active mTOR signaling by phosphorylating proteins like TSC2. 131
Autophagy is also regulated by another signaling pathway called C‐Jun NH2‐terminal kinase (JNK) which induces autophagy by phosphorylating Bcl2 protein and inhibiting its interaction with Beclin1. 10 , 37 , 130 JNK exerts a tumor suppressor function through constraining in vivo RAS‐induced cell transformation 37 (Figure 2).
The intracellular signaling pathway mediated by calcium occurs in mitochondria, ER and lysosomes. Transporting Ca2+ is regulated by Inositol 1,4,5‐trisphosphate receptors (IP3Rs) or Two‐pore channels 1/2 (TPC1/2), Ryanodine receptors (RyRs), and Transient receptor potential superfamily pathways such as TRPML1 and TRPM2. Ca2+ transported into ER using IP3Rs can induce autophagy by activating Beclin1 or AMPK. 130
In addition, signaling pathways regulating tumorogenesis are interconnected with autophagy signaling. 132 For example, tumor suppressor genes such as PTEN, Liver kinase B1 (LKB1), TSC1, and TSC2 activate autophagy by inhibiting the mTOR signaling pathway. 132 , 133 Also, oncogenic proteins such as Bcl2, AKT, and Epidermal growth factor receptor (EGFR) can active mTOR signaling cascades and inhibit autophagy thus directly increase tumorogenesis. 133
5.9. Autophagy and tumor angiogenesis
Although the relationship between autophagy and angiogenesis is still unclear and few studies have been performed in this field, the available reports indicate the involvement and importance of autophagy in angiogenesis. 36
Angiogenesis is a biological process of creating new blood vessels from established capillary beds. 36 , 134 This process occurs in normal conditions such as organ development and wound healing as physiological angiogenesis and also in pathological conditions such as tumor growth and diabetic retinopathy. 36 Angiogenesis provides oxygen and nutrient required for tumor cells, and is a protective toolfor tumor invasion, growth, and metastasis. 27 , 134 In fact, angiogenesis plays a key role in tumor growth and metastasis. 18 The pathological angiogenesis occurs when the balance between pro‐ and anti‐angiogenic factors in tumor microenvironment shifts toward pro‐angiogenic factors. 134 Angiogenic factors such as VEGF produced by tumor cells activate endothelial cells of surrounding capillaries and induce their sprouting and development of new blood capillaries into tumor. 27 , 135
One of the ways of autophagy involvement in the regulation of tumor angiogenesis has been reported to be interference in Vascular endothelial growth factor (VEGF) signaling. 35 For example, under metabolic stress and hence increased autophagy, lysosomal degradation of neuropilin1 is induced, leading to inhibition of VEGF signaling and angiogenesis. Neuropilin1 is known as a pro‐angiogenic protein, and VEGF‐binding protein in endothelial and cancer cells. 136 However, Liang et al. indicated that increased autophagy leads to accumulation of VEGF‐A through activating the JAK2/STAT3 pathway and thereby induces angiogenesis. 137
Conflicting results have been reported for the relationship between increased autophagy and angiogenesis. Liang et al. demonstrated that rapamycin‐induced autophagy mediates angiogenesis in Human umbilical vein endothelial cells (HUVEC) through activating the AMPK/Akt/mTOR pathway. 138 Conversely, inhibition of angiogenesis by increased autophagy has also been reported, and although it can indicate the paradoxical role of autophagy in the angiogenesis process, inducing autophagy in which may be a therapeutic target. For example, Sung et al. reported that Mebendazole induces autophagy in endothelial cells and in this way leads to inhibition of angiogenesis. 139 Also, in vivo and in vitro investigations by Shinohara et al. demonstrated that mTOR inhibitors could inhibit angiogenesis, which in turn was associated with induced autophagy. 140 In addition, chemerin, which is an angiogenesis inducer, resulted in induction of oxidative stress by increased ROS which in turn induced expression levels of ATGs in human aortic endothelial cells 141 while angiogenesis inhibitors such as Endostatin and kringle5 induced autophagy levels in endothelial cells. 142 , 143
Anti‐angiogenic therapeutic strategies usually lead to the induction of starvation and hypoxia in cancer cells, which is associated with increased autophagy and thus increased angiogenesis. Hypoxia‐mediated autophagy causes tumor resistance to anti‐angiogenic drugs during cancer treatment, 27 , 144 and thereby, induced autophagy by cancer cells is an effective response for causing their resistance to anti‐angiogenesis therapies. 27 Anti‐angiogenic strategies have been shown to induce autophagy flux by activating the HIF1α/AMPK signaling pathway, which promotes cell survival and drug resistance. 145 Nevertheless, therapeutic strategies using a combination of anti‐angiogenic drugs and autophagy inhibitors seem to be more effective in cancer treatment, 146 probably because apoptosis signaling pathways are also induced by these strategies. 147
Besides, normalizing tumor vessels has been suggested as a cancer treatment strategy 36 in order to restore perfusion, thereby reducing metastasis and increasing drug delivery. 134 A relationship has been observed between tumor vascular normalization and autophagy, and it seems that different drugs with vascular normalization ability can affect autophagy. For example, chloroquine (CQ), a lysosomal inhibitor, can be effective in tumor vascular normalization. 148
5.10. Targeting autophagy in cancer treatment
Autophagy in tumor cells under hypoxic, starvation, and other stress conditions is induced as an adaptive response and to maintain cell survival. Also, autophagy is activated in tumor cells as a response that induces cell resistance to treatment strategies such as chemotherapy and radiotherapy. 10 , 21 Therefore, it seems that the autophagic response of tumor cells acts as a defense mechanism to enhance adaptation to stress conditions and support the survival of remaining cells after exposure to therapeutic strategies. 149
Therefore, applying autophagy inhibitors in combination with existing anticancer therapies seems to be an effective option for improving and/or developing treatment strategies, especially for cancers with high levels of and dependence on autophagy for survival under metabolic stress. 150 In this regard, Amaravadi et al. reported that inhibition of autophagy in lymphoma mouse models increased the effectiveness of anti‐cancer drugs. 71 Also, Levy et al. indicated that inhibition of autophagy leads to improved therapeutic outcomes in patients. 5
In addition to various reports that increased autophagy leads to tumor cell resistance during anticancer therapy, there are other studies suggesting that many anticancer agents lead to cell death by increasing autophagy and that autophagy induction can act as a tumor suppressor strategy if cell apoptosis signaling is impaired. 149 , 150 Therefore, the latter suggest enhancing the mechanism of autophagy as an effective anticancer treatment option and highlight autophagy‐inducing anticancer drugs to increase tumor cell death. 8 Nevertheless, it should be noted that inducing autophagy for cancer treatment may be a “double‐edged sword” because factors such as the type and stage of cancer and the type and duration of autophagy can affect its outcome. 34 Overall, the choice of inhibiting or inducing autophagy as a cancer treatment strategy has been controversial and has faced many challenges due to its paradoxical functions. Table 1 presents a number of clinical trials targeting autophagy in various cancers.
TABLE 1.
Autophagy modulators in clinical trial studies
| Cancer Type | Drug | Effect on autophagy | Mode of action | Clinical trial phase | Identifer |
|---|---|---|---|---|---|
| Hepatocellular cancer | Rapamycin | ↑ | mTOR inhibitor | ІІ | NCT01079767 |
| Everolimus | ↑ | mTOR inhibitor | ІІІ | NCT01035229 | |
| Sorafenib | ↑ | mTOR inhibitor | ІІІ | NCT00492752 | |
| Breast cancer | CQ | ↓ | Lysosome inhibitor | І, ІІ | NCT01023477 |
| HCQ | ↓ | Lysosome inhibitor | ІІ | NCT01292408 | |
| CQ plus taxane | ↓ | Lysosome inhibitor | ІІ | NCT01446016 | |
| HCQ plus ixabepilone | ↓ | Lysosome inhibitor | І, ІІ | NCT00765765 | |
| PF‐04691502 | ↑ | PI3K inhibitor | ІІ | NCT01430585 | |
| Triciribine | ↑ | ІІ | NCT01697293 | ||
| Rapamycin plus trastuzumab | ↑ | mTORC1inhibitor | ІІ | NCT00411788 | |
| Colorectal cancer | HCQ plus FOLFOX and bevacizumab | ↓ | Lysosome inhibitor | І, ІІ | NCT01206530 |
| HCQ plus vorinostat | ↓ | Lysosome inhibitor | І, ІІ | NCT02316340 | |
| Everolimus | ↑ | mTOR inhibitor | І, ІІ | NCT00522665 | |
| MK‐2206 | ↑ | AKT inhibitor | ІІ | NCT01186705 | |
| Metformin plus 5‐FU | ↑ | AMPK activator | ІІ | NCT01941953 | |
| Ovarian cancer | Temsirolimus | ↑ | ІІ | NCT01460979 | |
| Everolimus plus bevacizumab | ↑ | mTORC1 inhibitor | ІІ | NCT01031381 | |
| Pancreatic cancer | HCQ | ↓ | Lysosome inhibitor | ІІ | NCT01273805 |
| HCQ plus nucleoside analog gemcitabine | ↓ | Lysosome inhibitor | І, ІІ | NCT01128296 | |
| NVPBEZ235 | ↑ | PI3K inhibitor | ІІ | NCT01628913 | |
| Metformin plus chemotherapy | ↑ | AMPK activator | ІІ | NCT01210911 | |
| Prostate cancer | HCQ | ↓ | ІІ | NCT00726596 | |
| HCQ plus docetaxel | ↓ | ІІ | NCT00786682 | ||
| Vinblastine | ↓ | ІІ | NCT00003084 | ||
| Everolimus | ↑ | mTOR inhibitor | ІІ | NCT00657982 | |
| Everolimus plus pasireotide | ↑ | mTOR inhibitor | ІІ | NCT01313559 | |
| Everolimus plus paclitaxel | ↑ | mTOR inhibitor | І, ІІ | NCT00574769 | |
| Metformin | ↑ | AMPK activator | ІІ | NCT01433913 | |
| Myeloma | HCQ plus rapamycin | ↓ | Early phase І | NCT01396200 | |
| Multiple myeloma | HCQ plus cyclophosphamide andbortezomib | ↓ | ІІ | NCT01438177 | |
| Multiple myeloma and plasma cell neoplasm | HCQ plus bortizomib | ↓ | І | NCT00568880 | |
| Acute myeloid leukemia | HCQ plus mitoxantrone and etoposide | ↓ | І | NCT02631252 | |
| Chronic myeloid leukemia | HCQ plus imatinibmesylate | ↓ | ІІ | NCT01227135 | |
| Multiple myeloma | HCQ plus rapamycin, cyclophosphamide and dexamethasone | ↓ | І | NCT01689987 |
Abbreviations: 5‐FU, 5‐Fluorouracil; CQ, chloroquine; HCQ, hydroxychloroquine.
6. LIVER CANCER
Liver tissue cells are dependent on autophagy due to its specific physiology, and paradoxical functions of autophagy (inhibitory or supportive) have been reported HCC. 151 , 152
6.1. Tumor‐inhibiting autophagy in HCC
There are various studies suggesting an inhibitory role for autophagy in the development and progression of chronic liver disease and liver cirrhosis, with defective autophagy being a primary cause of HCC. 153 In this regard, loss of function mutations in ATGs and hence inhibition of autophagy led to the onset or progression of hepatocellular carcinoma, indicating importance of regulating basal autophagy in liver tissue. 154 Takamura et al. reported that mice with deletions in ATG5 and ATG7 and thereby defective autophagy developed multiple liver tumors. 38 Also, the incidence of spontaneously occurring tumors such as HCC was observed in BECN1 heterozygous knockout mice, while homozygous knockout mice died during embryogenesis. 78 In addition, p62 accumulation were observed in steatohepatitis and HCC mouse models with BECN1 loss of function mutations. 155 In a study by Bao et al., increased p62 accumulation was observed in liver tumor cells compared with surrounding normal cells. 156 Various studies have investigated p62 accumulation effects on tumor formation and progression in several cancers such as HCC and pre‐malignant liver disease. 55 Umemura et al. reported that overexpression of p62 in liver cells may provide a poor prognosis for HCC and induce liver tumorogenesis. 157 Also, specific knockdown of P62 expression level was associated with reduced tumor size in mice with Atg7 deficiency in hepatocytes, which is consistent with contribution of p62 accumulation in hepatic tumor formation. 38 NF‐κB‐related signaling pathways seem to be involved in liver tumorogenesis resulted from p62 accumulation. 155 In addition, knockdown of the PI3K/AKT/mTOR pathway has been reported to prevent HCC cell proliferation and migration through inducing autophagy, suggesting the tumor suppressor roles of autophagy in liver. 158 In support of this suggestion, it is observed that arenobufagin, a natural bufadienolide derived from toad venom, prevents HCC cell growth through suppressing the PI3K/AKT/mTOR pathway and thereby inducing autophagy and apoptosis in the cells. 159
6.2. Tumor‐inducing autophagy in HCC
As mentioned, autophagy contributes to tumor cell survival by maintaining cell homeostasis. There are various reports that different tumors such as HCC maintain their survival through autophagy. 151 Autophagy is increased in advanced liver cancer which is associated with increased tumor malignancy and low survival rates in HCC patients. Also, there is a reported direct relationship between the rate of tumor progression and the levels of autophagy, so that the rate of autophagy is higher in the advanced stages of HCC. 160 , 161 Wu et al. demonstrated LC3B overexpression in HCC samples compared to surrounding normal samples. The expression levels of LC3B were associated with vascular invasion, lymph node metastasis, and poor prognosis. 162 In addition, overexpression of ULK1 was observed in HCC cancer cells by Xu et al. 163 Another study on liver carcinoma indicated that autophagy induces HCC cell invasion by activating EMT. 65 EMT in HCC cells may be induced by autophagy through cAMP/PKA/CREB signaling and induction of TGF‐β. 164
6.3. Induction of autophagy in HCC as a therapeutic option
The PI3K/Akt/mTOR pathway is a cell cycle regulatory signaling pathway that controls several vital functions such as growth, survival, and metabolism. 151 There are several reports that mTOR activity is increased and autophagy is inhibited in HCC patients (15%–41%) which are associated with tumor cell survival, suggesting targeted mTOR pathway inhibition as a therapeutic option for HCC. 151 , 165 Rapamycin (sirolimus) is an mTOR kinase suppressor that is used as an autophagy inducer with anti‐proliferative and anti‐tumor effects. 166 A phase ІІ clinical trial study on 25 patients with advanced HCC showed rapamycin exerts anti‐tumor effects by inhibiting mTOR and inducing autophagy. 167 Moreover, everolimus (RAD001) as a rapamycin derivative demonstrated anti‐tumor activities in HCC pre‐clinical studies. 168 In addition, inhibition of mTOR by everolimus and a PI3K/mTOR signaling inhibitor which have synergistic effects on each other resulted in a reduction in tumor size in HCC mouse models through an increase in autophagy/mitophagy levels 169 (Table 2). Also, induction of autophagy has been used to increase cell death in the treatment of HCC. 151 In this regard, sorafenib has been suggested as a treatment option for HCC patients, with the observation that sorafenib resulted in induced autophagy‐dependent cell death in vitro and in vivo HCC models. 170 Also, combination therapy with sorafenib and an autophagic inhibitor drug improved efficiency of advanced HCC treatment through autophagy‐mediated cell death. Sorafenib also induced autophagy‐mediated cell death in treatment‐resistant HCC patients. 171 Yang et al. demonstrated that sorafenib inhibits liver cancer cell proliferation through mediating autophagy and cell apoptosis. They also reported suppressed cell apoptosis in combined use of CQ with sorafenib. 172 Additionally, Hidvegi et al. showed reduced hepatic fibrosis in alpha‐1 antitrypsin deficient mice using pro‐autophagic agents. 173
TABLE 2.
A summary of the autophagy modulators in cancer treatment
| Cancer type | Drug | Effect on autophagy | Drug development status | Ref. |
|---|---|---|---|---|
| Hepatocellular cancer | Everolimus | ↑ | In vivo | 168 |
| Sorafenib | ↑ | In vivo and in vitro | 170, 171, 172 | |
| CQ plus sorafenib | ↓ | In vivo and in vitro | 174, 177 | |
|
CQ plus cisplatin CQ plus 5‐FU |
↓ | In vivo and in vitro | 175 | |
| CQ plus oxaliplatin | ↓ | In vivo and in vitro | 176 | |
| 3‐MA plus sorafenib and vorinostat |
↓ |
In vitro | 178 | |
| CQ plus bevacizumab |
↓ |
In vivo and in vitro | 179 | |
| Breast cancer | Everolimous | ↑ | In vitro | 195 |
| HCQ plus tamoxifen | ↓ | In vivo and in vitro | 200 | |
| Resveratrol plus rapamycin | ↓ | In vitro | 201 | |
| 3‐MA | ↓ | In vitro | 206 | |
| Colorectal cancer | Torin‐1 | ↑ | In vitro | 225 |
| Aspirin | ↑ | In vitro | 226 | |
| AZD‐2014 | ↑ | In vivo and in vitro | 227 | |
| Bufalin | ↑ | In vitro | 228 | |
| Quercetin | ↑ | In vitro | 231 | |
| Resveratrol | ↑ | In vitro | 232 | |
| 3‐MA plus 5‐FU | ↓ | In vivo ad in vitro | 233 | |
| CQ plus 5‐FU | ↓ | In vitro | 234 | |
|
CQ plus oxaliplatin CQ plus bevacizumab |
↓ | In vivo and in vitro | 237 | |
|
3‐MA plus oxaliplatin Baf A1 plus oxaliplatin |
↓ | In vitro | 238 | |
| Ovarian cancer | Quinacrine |
↑ |
In vivo and in vitro | 256 |
| Resveratrol | ↑ | In vitro | 257 | |
| MORAB‐003 | ↑ | In vivo and in vitro | 258 | |
| Arsenic trioxide plus everolimus | ↑ | In vivo and in vitro | 260 | |
| Elaiophylin plus cisplatin | ↓ | In vivo and in vitro | 263 | |
| Ellagic acid plus doxorubicin | ↓ | In vitro | 265 | |
| Hematological cancers | Arsenic trioxide | ↑ | In vitro | 271, 272, 273 |
| Fludarabine | ↑ | In vitro | 275 | |
| Dexamethasone | ↑ | In vitro | 276, 277 | |
| Idarubicin | ↑ | In vitro | 278 | |
| Everolimus | ↑ | In vivo | 279 | |
| Resveratrol | ↑ | In vitro | 281 | |
|
CQ plus cytarabine BafA1 plus cytarabine |
↓ | In vitro | 283 | |
| CQ plus SAHA | ↓ | In vitro | 285 | |
| CQ plus interfrone‐1 | ↓ | In vitro | 286 |
Abbreviations: 5‐FU, 5‐Fluorouracil; 3‐MA, 3‐Methyladenine; Baf A1, Bafilomycin A1; CQ, chloroquine; HCQ, hydroxychloroquin.
6.4. Inhibition of Autophagy in HCC as a therapeutic option
Based on the protective role of autophagy in stress conditions such as those are caused under cancer treatment, many researchers have focused on autophagy inhibition for treating some tumors such as HCC. 151
One of the drugs with favorable results used for treating advanced HCC is sorafenib, which inhibits tyrosine kinase receptors. 153 Sorafenib can also initiate cell autophagy through suppressing the mTOR pathway, which in turn leads to the cell accumulation of autophagosomes and cell resistance to treatment. 174 Due to the autophagy‐induced cell resistance to chemotherapy, several studies have suggested that autophagy inhibition may provide HCC cell sensitivity to chemotherapy. 154 Accordingly, autophagy inhibitors such as CQ and Hydroxychloroquine (HCQ) have been developed. These drugs suppress autophagy by preventing lysosome‐to‐autophagosome fusion and are also used for treating malaria. 151 Combined use of autophagy inhibitors with tumor suppressor drugs such as sorafenib, cisplatin and 5‐Fluorouracil (5‐FU) has been reported to improve therapeutic effects. 174 , 175 Moreover, treatment of xenograft models and HCC cell lines, respectively, with oxaliplatin in combination with CQ and sorafenib in combination with CQ led to increased effectiveness of the tumor suppressants 176 , 177 (Table 2). Also, Yuan et al. reported increased therapeutic effects of sorafenib for HCC treatment using its combination therapy with 3‐Methyladenine (3‐MA), an autophagy inhibitor, or a small interfering RNA (siRNA)‐specific for inhibiting Beclin1. 178 Further, Guo et al. indicated that bevacizumab prevents growth of xenograft tumors and triggers increased autophagy. They reported enhanced effects of bevacizumab in combination with CQ as well as increased tumor cell apoptosis. 179
Based on the type of autophagy function (supportive or suppressive) in HCC progression, autophagy modulation provides new prospects for the treatment of liver cancer. 153 Although there is evidence suggesting the high potential of autophagy modulation as a therapeutic strategy for HCC, the clinical application of these autophagy modulators needs further investigation. 151
7. BREAST CANCER
Paradoxical function of autophagy has also been reported in breast cancer, on the basis of which new perspectives have been proposed for the treatment of this cancer.
7.1. Tumor‐inhibiting autophagy in breast cancer
Emerging evidence suggests autophagy as a factor with suppressive roles in the early stages of breast tumors. Several studies have reported tumorogenesis induced by defective autophagy in breast tissue. 180 Also, monoallelic deletions of Beclin1 which is a key Atg have been reported in different breast tumors. 41 , 181 Li et al. indicated decreased mRNA and protein levels of Beclin1 caused by monoallelic deletion or epigenetically silencing of the gene in breast cancer samples. 182 In addition to the findings that Beclin1 is a tumor suppressor in breast and ovarian cancers probably due to its deletion in 40%–75% sporadic breast and ovarian cancers, there are studies suggesting that deletion of BECN1 gene may be due to its proximity to BRCA1 gene and not to its tumor inhibitory functions and BRCA1 deletions cover BECN1 chromosomal position. 24 , 91 In fact, gene deletions BECN1 in breast and ovarian cancers have been shown to be associated with gene deletions BRCA1, suggesting that BECN1 deletions may not be the main cause of breast tumor development and hence the proposed tumor inhibitory function for Beclin1 is controversial 183 However, Liang et al. demonstrated decreased proliferation and tumorogenesis of MCF‐7 cells after Beclin1 overexpression. 41 It has been also reported that Beclin1 deficiency in breast cells could contribute to tumor formation in a way independent of its role in autophagy and through interaction with Bcl2. This observation was highly regarded because Bcl2 exhibits increased expression levels in breast and many other cancers (more than 50%). 184 Moreover, down‐regulation associated with cancer progression of other ATGs such as ULK1 was also observed in breast tumor samples. 185
7.2. Tumor‐inducing autophagy in breast cancer
Autophagy in many solid tumors such as breast cancers contributes to tumor cell survival and invasion. 186 There have also been reports of autophagy involvement in breast cancer cell dormancy. The leading cause of death in breast cancer is recurrence of the disease due to metastasis, which is due to the activation of dormant, migrated breast tumor cells in other tissues. These cells supply their energy needs during dormancy period through autophagy. 187 In a study including 489 patients with breast carcinoma, Beclin1 exhibited higher expression levels in triple‐negative breast cancer samples than other types. 188 Also, Gong et al. reported higher levels of autophagy as well as higher expression levels of Beclin1 in breast cancer stem‐like cells compared to nontumor cells. They also indicated that autophagy is an essential process for survival and tumorogenicity of breast cancer stem cells. 189 In addition, Zhao et al. demonstrated increased LC3B expression level in triple‐negative breast cancer patients which was correlated with increased tumor metastasis and decreased patient survival rate. 190 Furthermore, the deletion of FIP200, another ATG, reduced tumor formation and progression in the MMTV‐PyMT mouse model of breast cancer. 191
7.3. Induction of autophagy in breast cancer cells as a therapeutic option
Although several studies suggest that inhibition of autophagy may be helpful in the treatment of breast cancer, there are a number of studies suggesting that autophagy may play a role in cell death, and hence inducing autophagy during breast cancer treatment seems to be helpful. 150 For example, Bcl2 inhibition in MCF7 cells resulted in autophagy induction and thereby autophagy‐mediated cell death. These observations were disappeared through suppressing ATG5, suggesting the involvement of induced autophagy in the cell death mechanisms. In addition, autophagy‐related toxicity effects increased through using some drugs simultaneously with Bcl2 inhibition combination. For example, autophagy is induced using low doses of doxorubicin, while its high doses lead to cell apoptosis. It has been reported that the combined use of Bcl2 siRNA and low doses of doxorubicin enhanced autophagy‐mediated cell death and tumor inhibition in MCF‐7 cell line. 192 Wilson et al. reported that Vitamin D sensitizes breast cancer cells to radiation therapy through autophagy induction. 193 Different studies have shown that using mTOR inhibitors leads to sensitivity to radiation and autophagy‐induced cell death in breast cancer cell lines. 194 , 195
7.4. Inhibition of Autophagy in breast cancer as a therapeutic option
Autophagy can be induced during anti‐cancer therapy and acts as an enhancing factor of tumor cell survival and resistance to treatment. From this perspective, targeted suppression of autophagy has been highlighted in the treatment of breast cancer. 196 In several studies, simultaneous use of autophagy inhibitors and tumor‐inhibiting drugs improved anti‐tumor therapeutic effects. For instance, it has been reported that autophagy is induced during anti‐estrogen therapy for ER+ breast cancer, and therapeutic effect of tamoxifen is augmented if autophagy is also inhibited. 197 Also, inhibition of autophagy in the breast cancer cell lines exhibited remarkable effects on the treatment of tamoxifen‐resistant cells. 198 , 199 Moreover, the anti‐tumor effects of tamoxifen on the inhibition of ER+ breast cancer cells were enhanced in a combination therapy of tamoxifen with HCQ 200 (Table 2). The favorable effects of concomitant use of autophagy inhibitors with other tumor inhibiting drugs have also been investigated. For example, a combination therapy of rapamycin with resveratrol suppressed autophagy and induced cell apoptosis in ER‐positive and negative breast cancers. 201 Several reports have highlighted autophagy as a mechanism involved in the development of trastuzumab resistance in the treatment of HER2‐positive breast cancer. 202 HER2‐positive breast cancer cells with Beclin1 deficiency have been demonstrated to be susceptible to trastuzumab therapy. 203 Additionally, knockdown of ATG12 led to overcoming cell resistance to trastuzumab therapy and suppressing tumor growth in trastuzumab‐resistant xenografts. 204
Treatment of MCF7 cells with epirubicin induced autophagy which in turn protected cells from apoptosis. However, autophagy inhibition enhanced epirubicin toxicity effects through increased cell apoptosis. 205 Further, 3‐MA reduces breast cancer cell survival through autophagy inhibition. 206 Treatment of breast cancer cell line MDA‐MB‐231 with autophagy inhibitors such as 3‐MA or CQ resulted in increased cell sensitivity to radiation and reduced survival of cells exposed to radiation. 207 Nevertheless, 3‐MA increased autophagy flux in nutrient‐rich conditions. 208
Although more evidence suggests that inhibiting autophagy combined with anti‐cancer therapeutic strategies is a promising way to treat breast cancer, cell death mediated by induced autophagy is a challenge that needs to be more considered in some cancer therapies. 150
8. COLORECTAL CANCER
The paradoxical functions of autophagy in colorectal cancer (CRC) have also been considered, 209 which are discussed here.
8.1. Tumor‐inhibiting autophagy in CRC
Defective autophagy‐related genes have been shown to be closely associated with colorectal tumorogenesis. 210 , 211 For example, ATG5 gene deletions or mutations of autophagy‐related tumor suppressor gene URAG have been frequently observed in colorectal cancer. 93 , 212 Approximately, 23% of CRC patients exhibit ATG5 gene deletions, which play a key role in the growth of intestinal cancer. 212 A comparison between colorectal cancer mice models ApcMin with mice models with heterozygous deletions of Atg5 (Atg5+/−) and also ApcMin mice with Atg5 +/+ showed an increase in the number and size of adenomas in Atg5+/− mice. 213 In addition, monoallelic mutations of the UVRAG gene, which is an autophagy regulator relevant to BECN1 pathway, is frequently detected in human CRC. 93 Mutated UVRAG leads to decreased autophagy and increased CRC cell proliferation and metastasis. 214 Activation of the PI3K–AKT–mTOR pathway and hence inhibition of autophagy has also been reported in CRC. 211
8.2. Tumor‐inducing autophagy in CRC
The importance of autophagy functions in the development and progression of CRC has been suggested in various in vivo studies. 209 Wu et al. reported increased expression levels of Beclin1, LC3, and mTOR in colorectal cancer cells, which the increased LC3 expression levels exhibited a positive correlation with increased Beclin1 and cell differentiation and negative correlation with mTOR expression levels. They showed that increased autophagy may be associated with increased tumorogenesis ability of CRC cells. Moreover, increased expression levels of mTOR contributed to proliferation and lymph node metastasis. 215 Further, it has been reported that LC3‐ІІ exhibits increased expression levels in colorectal cancer samples compared to normal colorectal tissues in particular in advanced stages. 216
There are observation suggesting paradoxical roles for BECN1 in CRC; supportive and suppressive functions on tumorogenesis ability and growth of CRC cells. 210 Beclin1 expression level has been reported to increase in advanced tumor stages of CRC. 217 Li et al. showed that Beclin1 expression levels are increased in colorectal cancer cells compared with normal adjacent cells. 84 Also, increased Beclin1 expression showed a positive correlation with distant metastasis. These observations highlight Beclin1 as a protein with tumor supportive functions in colorectal cancer. 218 Moreover, rectal adenocarcinoma patients with low expression levels of BECN1 respond better to treatment than patients with higher levels of BECN1 expression. 219 Nevertheless, although increased BECN1 expression has been observed in colorectal cancer patients (95%) in patients with gastric cancer (83%), 220 other solid tumors such as ovarian, lung, and liver cancers have not shown an increase in BECN1 expression levels. In contrast to the first studies, Beclin1 is expressed in normal tissues and there are in cancer cells various reports of reduced and no expression of Beclin1 in solid tumors other than colorectal and gastric. 221 , 222 , 223 For example, decreased expression levels of BECN1 are observed in glioblastoma multiform and high‐grade brain tumors. 224
Moreover, a study by Lauzier et al. showed differential in vivo responses of colorectal cancer cells to autophagy inhibition‐mediated by CQ. They also demonstrated that although CRC cells need autophagy to proliferate, in vitro autophagy inhibition was associated with differential responses in different colorectal cancer cell lines. Caco‐2/15 and HTC116 cells exhibited sensitivity to autophagy inhibition, while proliferation and tumorogenicity of SW480 and LoVo cells increased in response to autophagy inhibition. 209
8.3. Induction of autophagy in CRC as a therapeutic option
Several studies have suggested autophagy induction as an effective therapeutic strategy for CRC. In CRC patients with decreased BECN1 expression, activation of autophagy by enhancing BECN1 expression may be effective in treatment. 216 Also, inducing autophagy by inhibition of mTOR has been effective in different studies on CRC. For example, in vitro treatment of colorectal cancer stem cells with Torin‐1, an mTOR inhibitor, decreased cell survival, and tumor inhibiting effects of Torin‐1 suggested its efficacy in CRC treatment. 225 Moreover, other mTOR inhibitors such as aspirin, a non‐steroidal anti‐inflammatory drug, and AZD‐2014 exhibited anti‐tumor effects through autophagy‐mediated cell death in CRC. 226 , 227 It is also noted that autophagy‐induced cell death is effective in particular in apoptosis‐resistant CRC cells. Bufalin, an apoptosis inducer in several human tumor cell lines, induced autophagy‐mediated cell death in colon cell lines through activating the MAPK/JNK pathway which participates in Atg5 and Beclin1 up‐regulation as well as increasing ROS production 228 (Table 2). Furthermore, B‐group soyasaponins caused autophagy‐induced cell death in human HCT‐15 colon cancer cells. 229
Besides, Zhan et al. reported that MS‐275, a synthetic benzamide derivative of Histone deacetylase inhibitor (HDAC), led to Atg7 overexpression in human colon cancer cells, and subsequently induced autophagy and apoptosis. 230 Similarly, treatment of Ha‐Ras‐transformed human colon cells with quercetin induced autophagy and exhibited anti‐tumor effects. 231 Induction of apoptosis through increased ROS accumulation has also been reported in the use of resveratrol, an anti‐cancer agent, and its effects exhibited a correlation with increased LC3‐ІІ levels in human colon cancer cells. 232
8.4. Inhibition of autophagy in CRC as a therapeutic option
Several studies suggest inhibition of autophagy as an effective therapeutic strategy in colorectal cancer. In this regard, it has been reported that inhibition of autophagy can promote effects of 5‐FU, a chemotherapy medication. Treatment of CRC cell lines with 5‐FU resulted in an increase in LC3‐ΙΙ and autophagy, and subsequently a reduction in cell death. However, cell apoptosis increased by adding 3‐MA or CQ, which inhibits autophagy by preventing autophagosome formation. In fact, 3‐MA and CQ potentiated the anti‐tumor effect of 5‐FU on colon cancer cells. 233 , 234 Also, simultaneous use of 5‐FU and radiotherapy enhanced autophagy and subsequently treatment resistant in CRC cell lines. Nevertheless, cell treatment with CQ in combination with radiation therapy or chemotherapy resulted in increased cell apoptosis. 235 In addition, photodynamic therapy is used as a treatment for patients with colorectal cancer. Xiong et al. suggested that CQ in combination with photosan‐ІІ‐mediated photodynamic therapy can enhance apoptosis in CRC cell lines. 236 Furthermore, another study revealed that CQ in combination with anti‐angiogenic drugs such as oxaliplatin and bevacizumab sensitizes colon cancer cells. 237
Oxaliplatin is used as a treatment for metastatic colorectal cancer but cells become resistant through increasing autophagy. Shi et al. reported increased oxaliplatin‐induced cell death through autophagy inhibitors such as 3‐MA, Bafilomycine A1, and RNAi‐mediated knockdown of essential ATGs such as Atg5 and Beclin1. 238 It has been reported that, although inhibition of autophagy in Apc min/+ mice inhibits colorectal tumor growth through a CD8‐mediated immune response, it induced intestinal dysbiosis. 239 Moreover, BECN1 and UVRAG have been demonstrated to protect CRC cells against radiation‐induced DNA breaks and cell death by maintaining genomic integrity. 240
Recent studies have found that autophagy modulator drugs exhibit an improved efficiency for CRC treatment, in use alone or in combination with other therapeutic agents. Nevertheless, further research is needed. 210 , 211
9. OVARIAN CANCER
In addition to the above‐mentioned cancers, autophagy plays paradoxical roles in ovarian cancer, which may be dependent on cancer cell conditions such as nutrient deficiency, hypoxia, and chemotherapy. 241
9.1. Tumor‐inhibiting autophagy in ovarian cancer
Various studies have suggested that down‐regulation of autophagy increases ovarian cancer cell survival. 242 As mentioned previously, BECN1 gene deletion is observed in 40%–75% of ovarian cancers. 41 A study by Valente et al. demonstrated that type І ovarian carcinoma exhibits high levels of Beclin1 compared to type ІІ. Also, Beclin1 was detected with low or no expression levels in 18 of 20 patients with grade ІІІ ovarian cancer. 243 In addition, Beclin1 showed low expression levels in malignant epithelial cells of ovarian cancer than ovarian benign, borderline tissue. Also, Beclin1 expression levels exhibited an inverse correlation with higher stages of ovarian tumors. Moreover, LC3 exhibited increased expression levels in ovarian benign, borderline hyperplastic tissue compared to malignant ovarian cancer. Nevertheless, LC3 expression levels in stages ІІІ and ІV FIGO were lower than stages І and ІІ. 244 Patients with higher expression of Beclin1 and LC3 exhibited a better response to treatment. 245 Another study reported that patients with higher levels of Beclin1 showed better survival than patients with low levels of Beclin1 expression. 246 Lin et al. indicated that decreased expression levels of Beclin1 can be a biomarker for ovarian tumor cell malignancy. Moreover, ovarian carcinoma patients with high expression levels of Bcl‐xL, an autophagy inhibitor, and low expression levels of Beclin1 represented low survival rates. The decreased expression level of Beclin1 was also associated with poor prognostic of ovarian carcinoma. 247 Furthermore, high expression of cytoplasmic p62 has been observed in ovarian cancer cells with an association with development of serous carcinoma and tumor, indicating expression levels of cytoplasmic p62 as a biomarker for ovarian cancer. 248 It has also been reported decreased expression levels of other ATGs in ovarian cancer cells such as DRAM2. 249
9.2. Tumor‐inducing autophagy in ovarian cancer
A study on ovarian cancer has shown that Beclin1 expression is increased in malignant ovarian tumors compared to its benign tumors. 250 Another study reported increased levels of Beclin1 expression levels in endometrioid adenocarcinoma, and also suggested that high expression levels of Beclin1 may be due to hypoxia. increased Beclin1 expression level was correlated with tumor grade as well. 251
Spowart et al. demonstrated that clear cell ovarian cancer cell lines exhibit high expression levels of LC3A under hypoxic conditions. Also, LC3A showed higher expression in stage ΙΙΙ compared to stage Ι or ΙΙ. Nevertheless, these findings were not observed in other ovarian cancer cells; indicating that autophagy exerts different roles in different subtypes of ovarian cancer. 252 It has also been reported that there is a correlation between induced autophagy and ovarian cancer cell dormancy are linked. Lu et al. indicated that re‐expression of ARHI gene, which known as a tumor suppressor and with down‐regulated expression in ovarian cancer, induces autophagy and subsequently dormancy in ovarian cancer cells xenografted in mice. 253
9.3. Induction of autophagy in ovarian cancer as a therapeutic option
Various studies have suggested that autophagy and cell apoptosis are closely related to each other. Considering the relationship between signaling pathways involved in autophagy and function of autophagy in cell apoptosis, it has been shown that autophagy is activated by inhibition of Ras/MAP and PI3K/AKT/mTOR pathways, leading to induced ovarian cancer cell cycle arrest. 254 Also, Zhou et al. demonstrated that Tanshinone І enhances autophagy and cell apoptosis in ovarian cancer cells through inhibiting the PI3K/AKT/mTOR pathway. 255 According to a study by Khurana et al., quinacrine cell treatment promoted autophagy in ovarian cancer cell line OvCa and induced autophagy‐mediated cell death. 256 Also, Lang et al reported induced autophagy and cell apoptosis in human ovarian tumor cell lines OVCAR‐3 and caov‐3 using resveratrol, which triggers ATG5 expression and promotes LC3 cleavage. They also observed reduced OVCAR‐3 cell mortality‐induced by resveratrol when they simultaneously treated OVCAR‐3 cells with resveratrol and CQ, an autophagy inhibitor. 257 In addition, expression induction of autophagy‐related genes BECN1 and LC3‐ІІ has been reported using farletuzumab (MORAB‐003), which is a humanized anti‐folate receptor alpha monoclonal antibody. 258 LC3‐ІІ expression has been demonstrated to be induced using dihydroptychantol 2 as well. Dihydroptychantol 2 also inhibits the growth and development of ovarian cancer cells SKOV3. 259 Further, Wen et al. showed that treatment of different human ovarian cancer cell lines with MORAB‐003 led to autophagy induction. Also, the MORAB‐003‐induced autophagy induced caspase‐3‐mediated cell death and inhibition of ovarian tumor cell growth. 258 Liu et al. indicated that simultaneous cell treatment with arsenic trioxide, an autophagy inducer, and everolimus led to induced autophagy and apoptosis in xenografted ovarian cancer cells and also exerted a synergistic effect on tumor inhibition. 260
9.4. Inhibition of autophagy in ovarian cancer as a therapeutic option
As mentioned, ovarian cancer cells can acquire resistance to chemotherapy through increasing autophagy. Also, various studies have shown that inhibition of autophagy can be effective in the treatment of ovarian cancer. 241 For example, treatment of human ovarian cancer with cisplatin induces autophagy, which in turn causes cell resistance. These findings suggest increased efficacy of anti‐tumor chemotherapy agents in combination with autophagy inhibitors. Further, inhibition of autophagy using Beclin1 siRNA led to increased cisplatin‐induced ovarian cancer cell apoptosis. 261 , 262 Another study indicated that simultaneous cell treatment with cisplatin and elaiophylin, an autophagy inhibitor, decreased ovarian cancer cell viability 263 (Table 2). Tang et al. reported that the sensitivity of ovarian cancer cell lines to cisplatin is augmented through LC3B inhibition. 264 In addition, ellagic acid potentiates the anti‐cancer effects of doxorubicin in ovarian cancer cells through inhibiting autophagy. 265 Pagotto et al. showed that autophagy plays a protective role in ovarian cancer stem cells. In fact, higher levels of autophagy are observed in ovarian cancer stem cells compared to their non‐stem counterparts. In their study, the viability and in vivo tumorogenic capacity of these cells were reduced using CQ and Crisper/cas9 system designed for inhibition of ATG5. 266
Nevertheless, although many studies demonstrated that inhibition of autophagy in ovarian cancer cells exerts protective effects in favor of increased cell survival, there are several reports that autophagy induction promotes ovarian cancer cell malignancy. 242 Therefore, the findings of various studies suggest that autophagy manipulation may be useful in the treatment of ovarian cancer and off course that the protective or suppressive function of autophagy should be considered. Also, this proposed therapeutic strategy needs to be investigated in various clinical trials. 241
10. HEMATOLOGICAL CANCERS
Several studies have reported the critical function of autophagy in blood cancers. Autophagy plays a role in controlling differentiation and self‐renewal of hematopoietic stem cells (HSCs). Human HSCs cultured show high levels of autophagy, which appears to be involved in supporting differentiation and self‐renewal. 267 For example, inhibition of autophagy mediated by 3‐MA or its attenuation via Atg5 shRNA resulted in the failure of HSCs to differentiate into neutrophils and colony formation in vitro, indicating a loss of HSC stemness properties due to autophagy deficiency. 268 Also, ATG7 has been suggested as a crucial regulator inadult HSC maintenance. Deletion of ATG7 has been shown to affect HSC function and cause severe DNA damage in mice as well as abnormal myeloproliferation. In addition, lymphoid and myeloid production is significantly impaired in ATG7‐deficient mice. 269 Liu et al. reported that the HSC numbers were significantly reduced in liver of mouse embryo as a result of conditional deletion of FIP200 through Tie2‐Cre. The capacity of mouse embryonic hepatocytes to undergo multi‐lineage regeneration was clearly reduced after FIP200 deletion following injection of the inducer polyinosine‐polycytosine, explaining the disappearance of the embryonic compartment for HSCs. 270 An increase in the incidence rate of hematologic malignancies such as lymphoma has also been reported with autophagy defects in HSCs. There are reports that Beclin1 haploinsufficiency is involved in the development of lymphoma and lymphoproliferative disease. 40
These findings suggest a critical role for autophagy in supporting the maintenance and differentiation of HSCs, as defective autophagy can lead to myeloproliferative disorders and cancer development.
10.1. Induction of autophagy in hematological malignancies as a therapeutic option
There are reports of cytotoxic effects of various anticancer drugs through autophagic cell death for use in hematologic malignancies. For example, arsenic trioxide (As2O3) showed strong anticancer effects by inducing autophagic cell death in leukemic cell lines and primary leukemia progenitors of myeloid leukemia patients. 271 , 272 , 273 Fludarabine is a nucleoside analog commonly used in treatment of chronic lymphocytic leukemia (CLL). It prevents DNA synthesis and DNA repair. According to Mahoney et al., fludarabine was introduced for activating autophagy in CLL cells. However, the fludarabine‐induced cell death was not affected by autophagy suppression in these cells. 274 Fludarabine has also been reported to promote BECN1‐dependent autophagy through downregulation of MCL‐1 expression leading to autophagic cell death in fludarabine‐sensitive leukemic B cells. 275 Dexamethasone has been shown to induce cell death in lymphoid leukemia and multiple myeloma. Several studies have reported that dexamethasone‐induced autophagic cell death is mediated by promyelocytic leukemia protein overexpression and promyelocytic leukemia protein‐dependent Akt dephosphorylation. 276 , 277 Idarubicin‐induced autophagy is known as a pro‐death mechanism in leukemic cells. Idarubicin inhibits mTOR by inducing the activity of its upstream inhibitor, AMPK, or by decreasing the activity of its activator, Akt, which causes autophagic cell death. However, there are reports that the cytotoxicity of idarubicin in REH cells, a human leukemic cell line, is partially reduced by pharmacological autophagy impairment, Bafilomycin A1 or CQ. 278 The mTOR inhibitor everolimus caused cell death by triggering autophagy in acute lymphoblastic leukemia cells in an in vivo model. 279 Induction of autophagy in multiple myeloma cell lines was also observed with FTY720 treatment, which could be an explanation for increased cell death. So, simultaneous treatment of these cells with FTY720 and bafilomycin A1, an autophagy inhibitor, led to increased cell viability compared to treatment of cells with FTY720, suggesting that the induction of autophagy by FTY720 is involved in multiple myeloma cell death. 280 Moreover, Resveratrol induced autophagic cell death in chronic myelogenous leukemia cells by up‐regulating p62 through JNK and activating AMPK. 281
10.2. Inhibition of autophagy in hematological malignancies as a therapeutic option
Cancer cells use autophagy inorder to adapt to the challenging tumor microenvironment and stress or damage caused by treatment. For example, chemotherapy is often associated with the induction of autophagy, which leads to reduced therapeutic effects and increased drug resistance. Therefore, restoration of tumor cell sensitivity is a promising insight for hematological malignancies. 282 In these cases, there are suggestions that combination therapies using autophagy inhibitors with targeted chemotherapy drugs are effective for hematological malignancies. The anti‐leukemia drug cytarabine has been shown to enhance AMPK/ERK phosphorylation, inhibit Akt, and reduce mTOR activity, thereby leading to cytoprotective autophagy in leukemic cells. Due to increased cell death resulting from accumulation of DNA breaks, mitochondrial damage, and oxidative stress resulting from drug‐induced (bafilomycin A1 and CQ) or genetic‐induced (depletion of LC3 or p62 by RNA interference) autophagy defects, combined use of cytarabine and autophagy inhibitors was developed as a therapeutic strategy for leukemia. 283
About 50% of hematological malignancies show dysregulated tumor suppressor p53. This protein is involved in controlling cytoprotective autophagy. 284 In the absence of p53, metabolic stress induced by treatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) induces autophagy, which promotes chronic myeloid leukemia cell survival. Furthermore, SAHA enhances anticancer efficacy when autophagy is inhibited by the autophagy inhibitor CQ. 285 Zhu et al. demonstrated that autophagy also exerts pro‐survival effects in chronic myeloid leukemia treated with interferon. The JNK1‐STAT1 and NF‐ҝB pathways were both activated by interferone‐1, which increased BECN1‐ATG‐ATG5, a key regulator of autophagy pathway. Also, the ability of Chloroquine to inhibit autophagy improved the cytotoxic effects of interfrone‐1. 286 Some clinical studies on the combined use of CQ and HCQ with conventional therapies as therapeutic strategies for hematologic malignancies are listed in Table 1.
Altogether, the effects of autophagy manipulation as a therapeutic option for hematological malignancies can be different depending on the type of treatment applied, supporting cell survival or cell death. Nevertheless, further studies are needed to clarify the mechanisms controlling cell density. 284
11. CONCLUSION
According to the above‐mentioned findings in different subsections, autophagy manipulation as a cancer therapeutic strategy should be considered from two perspectives. In several advanced cancers, cell survival is dependent on autophagy, and therefore cancer cells exposed to treatments such as chemotherapy and radiation induce autophagy, which in turn leads to suppression of anti‐cancer effects of therapeutic agents and also causes cell resistance. However, increased autophagy following the use of anti‐cancer agents results in the autophagy‐mediated cell death in several cancers. Therefore, inducing or inhibiting autophagy depends on the origin of the cancer cells, the type of cancer, and the behavior of the cancer cells exposed to treatment. For this reason, it is necessary to determine the basal levels of autophagy in various cancers. Nevertheless, measuring the basal levels of autophagy in human tumor samples is difficult, and current knowledge about in vivo evaluating autophagy is incomplete. At present, activity levels of autophagy are assessed through detecting LC3 by using Western blotting or immunohistochemistry and tracking accumulated autophagic vesicles. Overall, increasing our knowledge of the mechanisms involved in autophagy and discovering more accurate strategies for measuring autophagy levels could open up new perspectives on cancer treatment, although it is a major challenge for clinical cancer treatment.
AUTHOR CONTRIBUTIONS
Farnaz Ahmadi‐Dehlaghi: Data curation (equal); investigation (equal); validation (lead); writing – original draft (lead). Parisa Mohammadi: Conceptualization (equal); investigation (equal); supervision (equal); writing – original draft (equal). Elahe Valipour: Validation (equal); writing – review and editing (lead). Pouya Pournaghi: Writing – review and editing (equal). Sarah Kiani: Visualization (equal); writing – review and editing (equal). kamran mansouri: Conceptualization (lead); supervision (lead); validation (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Ahmadi‐Dehlaghi F, Mohammadi P, Valipour E, Pournaghi P, Kiani S, Mansouri K. Autophagy: A challengeable paradox in cancer treatment. Cancer Med. 2023;12:11542‐11569. doi: 10.1002/cam4.5577
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Towers CG, Thorburn A. Therapeutic Targeting of Autophagy. Therapeutic Targeting of Autophagy EBioMedicine. 2016;14:15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karakas HE, Gozuacik D. Autophagy and cancer. Turkish Journal of Biology. 2014;38(6):720‐739. [Google Scholar]
- 5. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng Y, He D, Yao Z, Klionsky DJ. The machinary of macroautophagy. Cell Res. 2014;24(1):24‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, Ren X, Hait WN, Yang JM. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 2013;65(4):1162‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19(11):3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine B, Klionsky DJ. Development by self‐digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463‐477. [DOI] [PubMed] [Google Scholar]
- 10. Carew JS, Kelly KR, Nawrocki ST. Autophagy as a target for cancer therapy: new developments. Cancer Manag Res. 2012;4:357‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Onorati A, Dyczynski M, Ojha R, Amaravadi R. Targeting autophagy in cancer. Cancer. 2018;124:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santana‐Codina N, Mancias JD, Kimmelman AC. The role of autophagy in cancer. Annu Rev Cancer Biol. 2017;1(1):19‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5(9):726‐734. [DOI] [PubMed] [Google Scholar]
- 14. Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2(2):85‐90. [DOI] [PubMed] [Google Scholar]
- 15. Goldsmith J, Levine B, Debnath J. Autophagy and cancer metabolism. Methods Enzymol. 2014;542:25‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin L, Baehrecke EH. Autophagy, cell death, and cancer. Mol Cell Oncol. 2015;2(3):e985913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chude CI, Amaravadi RK. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int J Mol Sci. 2017;18(6):1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poillet‐Perez L, Despouy G, Delage‐Mourroux R, Boyer‐Guittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015;4:184‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, He S, Ma B. Autophagy and autophagy‐related proteins in cancer. Mol Cancer. 2020;19(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349‐364. [DOI] [PubMed] [Google Scholar]
- 21. Huang T, Song X, Yang Y, et al. Autophagy and hallmarks of cancer. Crit Rev Oncog. 2018;23(5–6):247‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kondapuram SK, Sarvagalla S, Coumar MS. Targeting autophagy with small molecules for cancer therapy. J Cancer Metastasis Treat. 2019;5:32. [Google Scholar]
- 23. Yang ZJ, Chee CE, Huang S, Sinicrope F. Autophagy modulation for cancer therapy. Cancer Biol Ther. 2011;11(2):169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galluzzi L, Pietrocola F, Bravo San Pedro JM, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34(7):856‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shan H, Zheng X, Li M. The effects of Astragalus Membranaceus active extracts on autophagy‐related diseases. Int J Mol Sci. 2019;20(8):1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antunes F, Erustes AG, Costa AJ, et al. Autophagy and intermittent fasting: the connection for cancer therapy? Clinics (Sao Paulo). 2018;73(suppl 1):e814s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh SS, Vats S, Chia AY‐Q, et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37(9):1142‐1158. [DOI] [PubMed] [Google Scholar]
- 28. Asgari R, Bakhtiari M, Rezazadeh D, Vaisi‐Raygani A, Mansouri K. Autophagy related gene expression status in patients diagnosed with azoospermia: a cross‐sectional study. J Gene Med. 2020;22(4):e3161. [DOI] [PubMed] [Google Scholar]
- 29. Mainz L, Rosenfeldt MT. Autophagy and cancer—insights from mouse models. FEBS J. 2018;285(5):792‐808. [DOI] [PubMed] [Google Scholar]
- 30. Paquette M, El‐Houjeiri L, Pause A. mTOR pathways in cancer and autophagy. Cancer. 2018;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu B, Bao J‐K, Yang J‐M, Cheng Y. Targeting autophagic pathways for cancer drug discovery. Chin J Cancer. 2013;32(3):113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogier‐Denis E, Codogno P. Autophagy: a barrier or an adaptive response to cancer. Biochim Biophys Acta. 2003;1603(2):113‐128. [DOI] [PubMed] [Google Scholar]
- 34. White E, DiPaola R. The double‐edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15(17):5308‐5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eisenberg‐Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis. 2009;14(4):376‐391. [DOI] [PubMed] [Google Scholar]
- 36. Kardideh B, Samimi Z, Norooznezhad F, Kiani S, Mansouri K. Autophagy, cancer and angiogenesis: where is the link? Cell Biosci. 2019;9(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White EJ, Martin V, Liu J‐L, et al. Autophagy regulation in cancer development and therapy. Am J Cancer Res. 2011;1(3):362‐372. [PMC free article] [PubMed] [Google Scholar]
- 38. Takamura A, Komatsu M, Hara T, et al. Autophagy‐deficient mice develop multiple liver tumors. Genes Dev. 2011;25(8):795‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karantza‐Wadsworth V, Patel S, Kravchuk O, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21(13):1621‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112(12):1809‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672‐676. [DOI] [PubMed] [Google Scholar]
- 42. Kang MR, Kim MS, Oh JE, et al. Frameshift mutations of autophagy‐related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and colorectal cancers with microsatellite instability. J Pathol. 2009;217(5):702‐706. [DOI] [PubMed] [Google Scholar]
- 43. Shimizu S, Yoshida T, Tsujioka M, Arakawa S. Autophagic cell death and cancer. Int J Mol Sci. 2014;15(2):3145‐3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Janji B, Viry E, Baginska J, Moer KV, Berchem G. Role of autophagy in cancer and tumor progression. Autophagy: A double‐edge sword‐Cell survival or Death? 2013: 189–215.
- 45. Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861‐2873. [DOI] [PubMed] [Google Scholar]
- 46. Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bishop E, Bradshaw TD. Autophagy modulation: a prudent approach in cancer treatment? Cancer Chemother Pharmacol. 2018;82(6):913‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baehrecke EH. How death shapes life during development. Nat Rev Mol Cell Biol. 2002;3(10):779‐787. [DOI] [PubMed] [Google Scholar]
- 49. Bursch W, Ellinger A, Kienzl H, et al. Active cell death induced by anti‐estrogen therapy tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF‐7) in culture: the role of autophagy. Carcinogenesis. 1996;17(8):1595‐1607. [DOI] [PubMed] [Google Scholar]
- 50. Badadani M. Autophagy mechanism, regulation, functions, and disorders. ISRN Cell Biol. 2012;2012:927064. [Google Scholar]
- 51. Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras‐induced expression of Noxa and Beclin‐1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42(1):23‐35. [DOI] [PubMed] [Google Scholar]
- 52. Saeki K, Yuo A, Okuma E, et al. Bcl‐2 down‐regulation causes autophagy in caspase‐independent manner in human leukemic HL60 cells. Cell Death Differ. 2000;7(12):1263‐1269. [DOI] [PubMed] [Google Scholar]
- 53. Serrano‐Oviedo L, Ortega‐Muelas M, Gacía‐Cano J, et al. Autophagic cell death associted to Sorafenib in renal cell carcinoma is mediated throgh Akt inhibition in an ERK1/2 independent fashion. PLoS One. 2018;13(7):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao W, Shen Z, Shang L, Wang X. Upregulation of human‐initiation kinase ULK1 by tumor suppressor p53 contributes to DNA‐damage‐induced cell death. Cell Death Differ. 2011;18(10):1598‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 2019;134:116‐137. [DOI] [PubMed] [Google Scholar]
- 56. Shukla S, Patric IRP, Patli V, et al. Methylation silencing of ULK2, an autophagy gene, is essential for astrocyte transformation and tumor growth. J Biol Chem. 2014;289(32):22306‐22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32(7):955‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self‐eating and self‐killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741‐752. [DOI] [PubMed] [Google Scholar]
- 59. Kimmelman A, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25(5):1037‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol. 2014;11(9):508‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hashimoto D, Blauer M, Hirota M, Ikonen NH, Sand J, Laukkarinen J. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. Eur J Cancer. 2014;50(7):1382‐1390. [DOI] [PubMed] [Google Scholar]
- 62. Yang A, Kimmelman AC. Inhibition of autophagy attenuates pancreatic cancer growth independent of TP53/TRP53 status. Autophagy. 2014;10(9):1683‐1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kiyono K, Suzuki H, Matsuyama H, et al. Autophagy is activated by TGF‐beta and potentiates TGF‐beta‐mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res. 2009;69(23):8844‐8852. [DOI] [PubMed] [Google Scholar]
- 64. Kim YH, Beak SH, Kim EK, et al. Uncoordinated 51‐like kinase 2 signaling regulates epithelial‐mesenchymal transition in A549 lung cancer cells. FEBS Lett. 2016;590(9):1365‐1374. [DOI] [PubMed] [Google Scholar]
- 65. Li J, Yang B, Zhou Q, et al. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial‐mesenchymal transition. Carcinogenesis. 2013;34(16):1343‐1351. [DOI] [PubMed] [Google Scholar]
- 66. Catalano M, DAlessandro G, Lepore F, et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol Oncol. 2015;9(8):1612‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol. 2010;22(2):212‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guo JY, Chen H‐Y, Mathew R, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Strohecker AM, White E. Autophagy promotes BrafV600E‐driven lung tumorigenesis by preserving mitochondrial metabolism. Autophagy. 2013;10(2):384‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guo JY, Karsli‐Uzunbas G, Mathew R, et al. Autophagy suppresses progression of Kras‐induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27(13):1447‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy‐induced apoptosis in a Myc‐induced model of lymphoma. J Clin Invest. 2007;117(2):326‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen Y, Li X, Guo L, et al. Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer. Mol Med Rep. 2015;12(2):1645‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Loizzo D, Pandolfo SD, Rogers D, et al. Novel insight into autophagy and prostate cancer: a Comperhensive review. Int J Mol Sci. 2022;23(139):139ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lucarelli G, Loizzo D, Ferro M, et al. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer: an update. Expert Rev Mol Diagn. 2019;19(5):377‐387. [DOI] [PubMed] [Google Scholar]
- 75. Lucarelli G, Rutigliano M, Galleggiante V, et al. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev Mol Diagn. 2015;15(9):1211‐1224. [DOI] [PubMed] [Google Scholar]
- 76. Grossi V, Lucarelli G, Forte G, et al. Loss of STK11 expression is an early event in prostate carcinogenesis and predicts therapeutic response to targeted therapy against MAPK/p38. Autophagy. 2015;11(11):2102‐2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vega‐Rubin‐de‐Celis S. The role of Beclin 1‐dependent autophagy in cancer. Biology. 2019;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100(25):15077‐15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu J, Lin Y, Yang H, Deng Q, Chen G, He J. The expression of p33(ING1), p53, and autophagy‐related gene Beclin1 in patients with non‐small cell lung cancer. Tumour Biol. 2011;32(6):1113‐1121. [DOI] [PubMed] [Google Scholar]
- 80. Miracco C, Cosci E, Oliveri G, et al. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol. 2007;30(2):429‐436. [PubMed] [Google Scholar]
- 81. Nicotra G, Mercalli F, Peracchio C, Castino R. Autophagy‐active beclin‐1 correlates with favourable clinic outcome in non‐Hodgkin lymphomas. Mod Pathol. 2010;23(7):937‐950. [DOI] [PubMed] [Google Scholar]
- 82. Sivridis E, Koukourakis M, Mendrinos SE, et al. Beclin‐1 and LC3A expression in cutaneous malignant melanomas: biphasic survival pattern for beclin‐1. Melanoma Res. 2011;21(3):188‐195. [DOI] [PubMed] [Google Scholar]
- 83. Zhang Z, Shao Z, Xiong L, Che B, Deng C, Xu W. Expression of Beclin1 in osteosarcoma and the effects of down‐regulation of autophagy on the chemotherapeutic sensivity. J Huazhong Univ Sci Technolog Med Sci. 2009;29(6):737‐740. [DOI] [PubMed] [Google Scholar]
- 84. Li B‐X, Li C‐Y, Peng R‐Q, et al. The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy. 2009;5(3):303‐306. [DOI] [PubMed] [Google Scholar]
- 85. Morselli E, Galluzzi L, Kepp O, et al. Anti‐ and pro‐tumor functions of autophagy. Biochim Biophys Acta. 2009;1793(9):1524‐1532. [DOI] [PubMed] [Google Scholar]
- 86. Tang D, Kang R, Livesy KM, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190(5):881‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1‐mediated phosphorylation of Bcl‐2 regulates starvation‐induced autophagy. Mol Cell Biol. 2008;30(6):678‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kung C‐P, Budina A, Balaburski G, Bergenstock MK, Murphy ME. Autophagy in tumor suppression and cancer therapy. Crit Rev Eukaryot Gene Expr. 2011;21(1):71‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pattingre A, Tassa A, Qu X, et al. Bcl‐2 antiapoptotic proteins inhibit Beclin 1‐dependent autophagy. Cell. 2005;122(6):927‐939. [DOI] [PubMed] [Google Scholar]
- 90. Hoyer‐Hansen M, Bastholm L, Szyniarowski P, et al. Control of macroautophagy by calcium, calmodulin‐dependent kinase kinase‐beta, ana Bcl‐2. Mol Cell. 2007;25(2):193‐205. [DOI] [PubMed] [Google Scholar]
- 91. Ávalos Y, Canales J, Bravo‐Sagua R, Criollo A, Lavandero S, Quest AFG. Tumor suppression and promotion by autophagy. Biomed Res Int. 2014;2014:603980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Umar SA, Tasduq SA. Integrating DNA damage response and autophagy signalling axis in ultraviolet‐B induced skin photo‐damage: a positive association in protecting cells against genotoxic stress. RSC Adv. 2020;10(60):36317‐36336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liang C, Feng P, Ku B, et al. Autophagy and tumor suppressor activity of a novel Beclin1‐binding protein UVRAG. Nat Cell Biol. 2006;8(7):688‐699. [DOI] [PubMed] [Google Scholar]
- 94. Bekri S, Adelaide J, Merscher S, et al. Detailed map of a region commonly amplified at 11q13→q14 in human breast carcinoma. Cytogenet Cell Genet. 1997;79:125‐131. [DOI] [PubMed] [Google Scholar]
- 95. Goi T, Yamazaki M, Koneri K, Katayama K, Hirose K, Yamaguchi A. Ascending colon cancer with hepatic metastasis and cholecytolithiasis in a patient with situs inversus totalis without any expression of UVRAG mRNA: report of case. Surg Today. 2003;33(9):702‐706. [DOI] [PubMed] [Google Scholar]
- 96. Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ. Frameshift mutation of UVRAG, an autophagy‐related gene, in gastric carcinomas with microsatellite instability. Hum Pathol. 2007;39(7):1059‐1063. [DOI] [PubMed] [Google Scholar]
- 97. Takahashi Y, Coppola D, Matsushita N, et al. Bif‐1 intracts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9(10):1142‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee JW, Jeong EG, Soung YH, et al. Decreased expression of tumor suppressor Bax‐interacting factor‐1 (Bif‐1), a Bax activator, in gastric carcinoma. Pathology. 2006;38(4):312‐315. [DOI] [PubMed] [Google Scholar]
- 99. Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015;282(24):4672‐4678. [DOI] [PubMed] [Google Scholar]
- 100. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728‐741. [DOI] [PubMed] [Google Scholar]
- 101. Zaffagnini G, Martens S. Mechanisms of selective autophagy. J Mol Biol. 2016;428:1714‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10(9):1533‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li L, Shen C, Bakamura E, et al. SQSTM1 is a pathogenic target of 5q copy number in kidney cancer. Cancer Cell. 2013;24(6):738‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Duran A, Linares JF, Galvez AS, et al. The signaling adaptor p62 is an important NF‐kappaB mediator in tumorigenesis. Cancer Cell. 2008;13(4):343‐354. [DOI] [PubMed] [Google Scholar]
- 106. Zatloukal K, French SW, Stumptner C, et al. From Mallory to Mallory‐Denk bodies: what, how and why? Exp Cell Res. 2007;313(10):2033‐2049. [DOI] [PubMed] [Google Scholar]
- 107. Komatso M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy‐deficient mice. Cell. 2007;131(6):1149‐1163. [DOI] [PubMed] [Google Scholar]
- 108. Moscat J, Diaz‐Meco MT. p62: a versatile multitasker taskes on cancer. Trends Biochem Sci. 2012;37(6):230‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wei H, Wang C, Croce CM, Guan J‐L. p62/SQSTM1 sinergizes with autophagy for tumor growth in vivo. Genes Dev. 2014;28(11):1204‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chen N, Karantza‐Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta. 2009;1793(9):1516‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bhat P, Kriel J, Shubha Priya B, Basappa SNS, Loos B. Modulating autophagy in cancer therapy: advancements and challenges for cancer cell death sensitization. Biochem Pharmacol. 2018;147:170‐182. [DOI] [PubMed] [Google Scholar]
- 112. Sui X, Jin L, Huang X, Geng S, He C, Hu X. p53 signaling and autophagy in cancer: a revolutionary strategy could be developed for cancer treatment. Autophagy. 2011;7(6):565‐571. [DOI] [PubMed] [Google Scholar]
- 113. Tasdemir E, Maiuri MC, Galluzzi L, et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10(6):676‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6(4):a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rabanal‐Ruiz Y, Otten Elsje G, Korolchuk Viktor I. mTORC1 as the main gateway to autophagy. Essays Biochem. 2017;61(6):565‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kamada Y, Yoshino K‐i, Kondo C, et al. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol Cell Biol. 2010;30(4):1049‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sato T, Nakashima A, Guo L, Coffman K, Tamanoi F. Single amino‐acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene. 2010;29(18):2746‐2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Parkhitko A, Myachina F, Morrison TA, et al. Tumorigenesis in tubersus sclerosis complex is autophagy and p62/sequestosome 1 dependent. Proc Natl Acad Sci USA. 2011;108(30):12455‐12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Parkhitko AA, Priolo C, Coloff JL, et al. Autophagy‐dependent metabolic reprogramming sensitizes TSC2‐deficient cells to the antimetabolite 6‐aminonicotinamide. Mol Cancer Res. 2014;12(1):48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shi W‐Y, Xiao D, Wang L, et al. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012;3(3):e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2(9):e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jain K, Paranandi KS, Sridharan S, Basu A. Autophagy in breast cancer and its implications for therapy. Am J Cancer Res. 2013;3(3):251‐265. [PMC free article] [PubMed] [Google Scholar]
- 123. Bellot G, Garcia‐Medina R, Gounon P, et al. Hypoxia‐induced autophagy is mediated through hypoxia‐inducible factor induction of BNIP3 and BNIP3L via their BH3 domin. Mol Cell Biol. 2009;29(10):2570‐2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mazure NM, Pouyssegur J. Hypoxia‐induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2009;22(2):177‐180. [DOI] [PubMed] [Google Scholar]
- 125. Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF‐1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15(10):1572‐1581. [DOI] [PubMed] [Google Scholar]
- 126. Bruick RK. Expression of the gene encoding the proapoptotic NIP3 protein is induced by hypoxia. Proc Natl Acad Sci USA. 2000;97(16):9082‐9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Gwnes Dev. 2016;30(17):1913‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Erkan M, Kleef J, Esposito I, et al. Loos of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene. 2005;24(27):4421‐4432. [DOI] [PubMed] [Google Scholar]
- 129. Sowter HM, Ferguson M, Pym C, et al. Expression of the cell death genes BNip3 and NIX in ductal carcinoma in situ of the breast; correlation of BNip3 levels with necrosis and grade. J Pathol. 2003;201(4):573‐580. [DOI] [PubMed] [Google Scholar]
- 130. Jiang G‐M, Tan Y, Wang H, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019;18(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ma L, Chen Z, Erdjument‐Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121(2):179‐193. [DOI] [PubMed] [Google Scholar]
- 132. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lee E, Wei Y, Zou Z, et al. Genetic inhibition of autophagy promotes p53 loss‐of‐heterozygosity and tumorigenesis. Oncotarget. 2016;7(42):67919‐67933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schaaf MB, Houbaert D, Meçe O, Agostinis P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019;26(4):665‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mohammadi P, Yarani R, Rahimpour A, Ranjbarnejad F, Melo JMLD, Mansouri K. Targeting endothelial cell metabolism in cancerous microinviroment: a new approach for anti‐angiogenic therapy. Drug Metab Rev. 2022;54(4):386‐400. [DOI] [PubMed] [Google Scholar]
- 136. Bae D, Lu S, Taglienti CA, Mercurio AM. Metabolic stress induces the lysosomal degradation of neuropilin‐1 but not neuropilin‐2. J Biol Chem. 2008;283(42):28074‐28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liang L, Hui K, Hu C, et al. Autophagy inhibition potentiates the anti‐angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non‐small cell lung cancer cells. J Exp Clin Cancer Res. 2019;38(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liang P, Jiang B, Li Y, et al. Autophagy promotes angiogenesis via AMPK/Akt/mTOR signaling during the recovery of heat‐denatured endothelial cells. Cell Death Dis. 2018;9(12):1194‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sung SJ, Kim H, Hong Y, Joe YA. Autophagy is potential target for enhancing the anti‐Angiogenic effect of mebendazole in endothelial cells. Biomol Ther. 2019;27(1):117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Shinohara ET, Cao C, Niermann K, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24(35):5414‐5422. [DOI] [PubMed] [Google Scholar]
- 141. Shen W, Tian C, Chen H, et al. Oxidative stress mediates chemerin‐induced autophagy in endothelial cells. Free Radic Biol Med. 2012;55:73‐82. [DOI] [PubMed] [Google Scholar]
- 142. Nguyen TM, Subramanian IV, Kelekar A, Ramakrishnan S. Kringle 5 of human plasminogen, an angiogenesis inhibitor, induces both autophagy and apoptotic death in endothelial cells. Blood. 2007;109(11):4793‐4802. [DOI] [PubMed] [Google Scholar]
- 143. Nguyen TM, Subramanian IV, Xiao X, et al. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and beta‐catenin levels. J Cell Mol Med. 2009;13(9B):3687‐3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hassanpour M, Rezabakhsh A, Pezeshkian M, Rahbarghazi R, Nouri M. Distinct role of autophagy on angiogenesis: highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res Ther. 2018;9(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hu YL, DeLay M, Jahangiri A, et al. Hypoxia‐induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72(7):1773‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Abdel‐Aziz AK, Shouman S, ElDemerdash E, Elgendy M, Abdel‐Naim AB. Chloroquine synergizes sunitinib cytotoxic via modulating autophagic, apoptotic and angiogenic machineries. Chem Biol Interact. 2014;217:28‐40. [DOI] [PubMed] [Google Scholar]
- 147. Nishikawa T, Tsuno NH, Okaji Y, et al. The inhibition of autophagy potentiates anti‐angiogenic effects of sulfaraphane by inducing apoptosis. Angiogenesis. 2010;13(3):227‐238. [DOI] [PubMed] [Google Scholar]
- 148. Maes H, Kuchnio A, Peric A, et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26(2):190‐206. [DOI] [PubMed] [Google Scholar]
- 149. Maycotte P, Thorburn A. Autophagy and cancer therapy. Cancer Biol Ther. 2011;11(2):127‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Maycotte P, Thorburn A. Targeting autophagy in breast cancer. Word J Clin Oncol. 2014;5(3):224‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lee YJ, Jang BK. The role of autophagy in hepatocellular carcinoma. Int J Mol Sci. 2015;16(11):26629‐26643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Allaire M, Rautou P‐E, Codogno P, Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol. 2019;70(5):985‐998. [DOI] [PubMed] [Google Scholar]
- 153. Dash S, Chava S, Chandra PK, Aydin Y, Balart LA, Wu T. Autophagy in hepatocellular carcinoma: from pathophysiology to therapeutic response. Hepat Med. 2016;8:9‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Liu L, Liao J‐Z, He X‐X, Li P‐Y. The role of autophagy in hepatocellular carcinoma: friend or foe. Oncotarget. 2017;8(34):57707‐57722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mathew R, Karp RM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell Death Differ. 2009;137(6):1062‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Bao L, Chandra PK, Moroz K, et al. Impaired autophagy response in human hepatocellular carcinoma. Exp Mol Pathol. 2014;96(2):149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Umemura A, He F, Taniguchi K, Nakagawa H, et al. p62, upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC‐initiating cells. Cancer Cell. 2016;29(6):935‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tang H, Li RP, Liang P, Zhou YL, Wang GW. miR‐125a inhibits the migration and invasion of liver cancer cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol Lett. 2015;10(2):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhang D‐M, Liu J‐S, Deng L‐J, et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells throgh inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 2013;34(6):1331‐1342. [DOI] [PubMed] [Google Scholar]
- 160. Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis and poor outcome. Clin Cancer Res. 2012;18(2):370‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mikhaylova O, Stratton Y, Hall D, et al. VHL‐regulated miR‐204 suppresses tumor growth through inhibition of LC3B‐mediated autophagy in renal clear cell carcinoma. Cancer Cell. 2012;21(4):532‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wu D‐H, Jia C‐C, Chen J, et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumor Biol. 2014;35(12):12225‐12233. [DOI] [PubMed] [Google Scholar]
- 163. Xu H, Yu H, Zhang X, et al. UNC51‐like kinase 1 as a potential prognostic biomarker for hepatocellular carcinoma. Int J Clin Exp Pathol. 2013;6(4):711‐717. [PMC free article] [PubMed] [Google Scholar]
- 164. Hu S, Wang L, Zhang X, Wu Y, Yang J, Li J. Autophagy induces transforming growth factor‐dependent epithelial‐mesenchymal transition in hepatocarcinoma cells through cAMP response element binding signaling. J Cell Moll Med. 2018;22(11):5518‐5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Cui J, Gong Z, Shen H‐M. The role of autophagy in liver cancer: molecular mechanisms and potential therapeutic targets. Biochim Biophys Acta. 2013;1836(1):15‐26. [DOI] [PubMed] [Google Scholar]
- 166. Huang S, Houghton PJ. Inhibitors of mammalian target of rapamycin novel antitumor agents: from bench to clinic. Curr Opin Investig Drugs. 2002;3(2):295‐304. [PubMed] [Google Scholar]
- 167. Decaens T, Luciani A, Itti E, et al. Phase II study of sirolimus in treatment‐naive patients with advanced hepatocellular carcinoma. Dig Liver Dis. 2012;44(7):610‐616. [DOI] [PubMed] [Google Scholar]
- 168. Huynh H, Chow KHP, Soo KC, et al. RAD001 (everolimus) inhibits tumor growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med. 2009;13(7):1371‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Thomas HE, Mercer CA, Carnevalli LS, et al. mTOR inhibitors synergize regression, resveratrol of gene expression, and autophagy in hepatocellular carcinoma. Sci Transl Med. 2012;4(139):139ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tai W‐T, Shiau C‐W, Chen H‐L, et al. Mcl‐1‐dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC‐59 in hepatocellular carcinoma cells. Cell Death Dis. 2013;4(2):e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gauthier A, Ho M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: an update. Hepatol Res. 2013;43(2):147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Yang Q, Gao L, Huang X, et al. Sorafenib prevents the proliferation and induces the apoptosis of liver cancer cells by regulating autophagy and hypoxia‐inducible factor‐1. Exp Ther Med. 2021;22(3):980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hidvegi T, Ewing M, Hale P, et al. An autophagy‐enhancing drug promotes degradation of mutant alpha1‐antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329(5988):229‐232. [DOI] [PubMed] [Google Scholar]
- 174. Shimizu S, Takehara T, Hikita H, et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer. 2012;131(3):548‐557. [DOI] [PubMed] [Google Scholar]
- 175. Guo X‐L, Li D, Hu F, et al. Targeting autophagy potentiates chemotherapy‐induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett. 2012;320(8):171‐179. [DOI] [PubMed] [Google Scholar]
- 176. Ding Z‐B, Hui B, Shi Y‐H, et al. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res. 2011;17(19):6229‐6238. [DOI] [PubMed] [Google Scholar]
- 177. Shi Y‐H, Ding Z‐B, Zhou J, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress‐related apoptosis. Autophagy. 2011;7(10):1159‐1172. [DOI] [PubMed] [Google Scholar]
- 178. Yuan H, Li A‐J, Ma S‐l, Long‐Jiu WB, Yin L, et al. Inhibition of autophagy significantly enhances combination therapy with sorafenib and HDAC inhibitors for human hepatoma cells. World J Gastroenterol. 2014;20(17):4953‐4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Guo X‐L, Li D, Sun K, et al. Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J Mol Med. 2013;91(4):473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jung YY, Lee YK, Koo JS. The potential of Beclin 1 as a therapeutic target for the treatment of breast cancer. Expert Opin Ther Targets. 2016;20(2):167‐178. [DOI] [PubMed] [Google Scholar]
- 181. Tang H, Sebti S, Titone R, et al. Decreased BECN1 mRNA expression in human breast cancer is associated with estrogen receptore‐negative subtype and poor prognosis. EBioMedicine. 2015;2(3):255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Li Z, Chen B, Wu Y, Jin F, Xia Y, Liu X. Genetic and epigenetic silencing the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Laddha SV, Ganesan S, Chan CS, White E. Mutational landscape of the essential autophagy gene BECN1 in human cancers. Mol Cancer Res. 2014;12(4):485‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tekedereli I, Alpay SN, Akar U, et al. Therapeutic silencing of Bcl‐2 by systematically administered siRNA nanotherapeutics inhibits tumor growth by autophagy and apoptosis and enhances the efficacy of chemotherapy in orthotopic xenograft models of ER (−) and ER (+) breast cancer. Mol Ther Nucleice Acids. 2013;2(9):e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Tang J, Deng R, Luo R‐Z, et al. Low expression of ULK1 is assosiated with operable breast cancer progression and is an adverse prognistic marker of survival for patients. Breast Cancer Res Treat. 2016;156(2):403‐404. [DOI] [PubMed] [Google Scholar]
- 186. Maiti A, Hait NC. Autophagy mediated tumor cell survival and progression of breast cancer metastasis to the brain. J Cancer. 2021;12(4):954‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. La Belle FA, Schiemann WP. Autophagy in breast cancer metastatic dormancy: tumor suppressing or tumor promoting functions? J Cancer Metastasis Treat. 2019;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Choi J, Jung W, Koo JS. Expression of autophagy‐related markers beclin‐1, light chain 3A, light chain 3B and p62 according to the molecular subtype of breast cancer. Histopathology. 2013;62(2):275‐256. [DOI] [PubMed] [Google Scholar]
- 189. Gong C, Bauvy C, Tonelli G, et al. Beclin 1 and autophagy are required for the tumorigencity of breast cancer stem like/progenitor cells. Oncogene. 2013;32(18):2261‐2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhao H, Yang M, Zhao J, Wang J, Zhang Y, Zhang Q. High expression of LC3B is associated with progression and poor outcome in triple‐negative breast cancer. Med Oncol. 2013;30(1):475. [DOI] [PubMed] [Google Scholar]
- 191. Wei H, Wei S, Gan B, Peng X, Zou W, Guan J‐L. Suppression of autophagy by FIP200 deletion inhibits mamary tumorigenesis. Genes Dev. 2011;25(14):1510‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Akar U, Chaves‐Reyez A, Barria M, et al. Silencing of Bcl‐2 expression by small interfering RNA induces autophagic cell death in MCF‐7 breast cancer cells. Autophagy. 2008;4(5):669‐679. [DOI] [PubMed] [Google Scholar]
- 193. Wilson EN, Bristol ML, Di X, et al. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquin and vitamin D. Horm Cancer. 2011;2(5):272‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Albert JM, Kim KW, Cao C, et al. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5(5):1183‐1189. [DOI] [PubMed] [Google Scholar]
- 195. Kim KW, Mutter RW, Cao C, et al. Autophagy for cancer therapy through inhibition of pro‐apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 2006;281(48):36883‐36890. [DOI] [PubMed] [Google Scholar]
- 196. Smith AG, Macleod KF. Autophagy, cancer stem cells and drug resistance. J Pathol. 2019;247(5):708‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Qadir MA, Kwok B, Dragowska WH, et al. Macroautophagy inhibition sensitizes tamoxifen‐resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res Treat. 2008;112(3):389‐403. [DOI] [PubMed] [Google Scholar]
- 198. Samaddar JS, Gaddy VT, Duplantier J, et al. A role for macroautophagy in protection against 4‐hydroxytamoxifen‐induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7(9):2977‐2987. [DOI] [PubMed] [Google Scholar]
- 199. Schoenlein PV, Periyasamy‐Thandavan S, Samaddar JS, Jackson WH, Barrett JT. Autophagy facilitates the progression of ERalpha‐positive breast cancer cells to antiestrogen resistance. Autophagy. 2009;5(3):400‐403. [DOI] [PubMed] [Google Scholar]
- 200. Cook KL, Warri A, Soto‐Pantoja DR, et al. Hydroxychloroquin inhibits autophagy to potentiate antiestrogen responsiveness in ER+ breast cancer. Clin Cancer Res. 2014;20(12):3222‐3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Alayev A, Berger SM, Kramer MY, Schwartz NS, Holz MK. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J Cell Biochem. 2015;116(3):450‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Rodríguez CE, Reidel SI, Joffé EDBK, Jasnis MA, Fiszman GL. Autophagy protects from trastuzumab‐induced cytotoxicity in HER2 overexpressing breast tumor spheroids. PLoS One. 2015;10(9):e0137920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Han Y, Fan S, Qin T, et al. Role of autophagy in breast cancer and breast cancer stem cells (review). Int J Oncol. 2018;52(4):1057‐1070. [DOI] [PubMed] [Google Scholar]
- 204. Cufí S, Vazquez‐Martin A, Oliveras‐Ferraros C, et al. Autophagy‐related gene 12 (ATG12) is a novel determinant of primary resistance to HER2‐targeted therapies: utility of transcriptome analysis of the autophagy interactome to guide breast cancer treatment. Oncotarget. 2012;3(12):1600‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sun W‐L, Chen J, Wang Y‐P, Zheng H. Autophagy protects breast cancer cells from epirubicin‐induced apoptosis and faciliates epirubicin‐resistance development. Autophagy. 2011;7(9):1035‐1044. [DOI] [PubMed] [Google Scholar]
- 206. Liu Q, Shi X, Zhou X, Wang D, Wang L, Li C. Effect of autophagy inhibition on cell viability and cell cycle progression in MDA‐MBA‐231 human breast cancer cells. Mol Med Rep. 2014;10(2):625‐630. [DOI] [PubMed] [Google Scholar]
- 207. Chaachouay H, Ohneseit P, Toulany M, Kehlbach R, Multhoff G, Rodemann HP. Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother Oncol. 2011;99(3):787‐792. [DOI] [PubMed] [Google Scholar]
- 208. Wu Y‐T, Tan H‐L, Shui G, et al. Dual role of 3‐methyladenine in modulation of autophagy via different temporal patterns of inhibition on class Ι and ΙΙ phosphoinositide 3‐kinase. J Biol Chem. 2010;285(14):10850‐10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Lauzier A, Normandeau‐Guimond J, Vaillancourt‐Lavigueur V, et al. Colorectal cancer cells respond differentially to autophagy inhibition in vivo. Sci Rep. 2019;9(1):11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Burada F. Autophagy in colorectal cancer: an important switch from physiology to pathology. World J Gastrointest Oncol. 2015;7(11):271‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Gil J, Pesz KA, Sąsiadek MM. May autophagy be a novel biomarker and antitumor target in colorectal cancer? Biomark Med. 2016;10(10):1081‐1094. [DOI] [PubMed] [Google Scholar]
- 212. An CH, Kim MS, Yoo NJ, Park SW, Lee SH. Mutational and expressional analyses of ATG5, an autophagy‐related gene, in gastrointestinal cancers. Pathol Res Pract. 2011;207(7):433‐437. [DOI] [PubMed] [Google Scholar]
- 213. Wang L, Wang Y, Lu Y, Zhang Q, Qu X. Heterozygous deletion of ATG5 in Apc(min/+) mice promotes intestinal adenoma growth and enhances the antitumor efficacy of interferon‐gamma. Cancer Biol Ther. 2015;16(3):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. He S, Zhao Z, Yang Y, et al. Truncating mutation in the autophagy gene UVRAG confers oncogenic properties and chemosensivity in colorectal cancers. Nat Commun. 2015;6(61):7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wu S, Sun C, Tian D, et al. Expression and clinical significances of Beclin1, LC3 and mTOR in colorectal cancer. Int J Clin Exp Pathol. 2015;8(4):3882‐3891. [PMC free article] [PubMed] [Google Scholar]
- 216. Chen Z, Li Y, Zhang C, et al. Downregulation of Beclin 1 and impairment of autophagy in a small population of colorectal cancer. Dig Dis Sci. 2013;58(10):2887‐2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yang Z, Ghoorun RA, Fan X, et al. High expression of Beclin‐1 predicts favorable prognosis for patients with colorectal cancer. Clin Res Hepatol Gastroenterol. 2015;39(1):98‐106. [DOI] [PubMed] [Google Scholar]
- 218. Zhang M‐Y, Gou W‐F, Zhao S, et al. Beclin 1 expression is closely linked to colorectal carcinogenesis and distant metastasis of colorectal carcinoma. Int J Mol Sci. 2014;15(8):14372‐14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zaanan A, Park JM, Tougeron D, et al. Association of beclin 1 expression with response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal carcinoma. Int J Cancer. 2015;137(6):1498‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ahn CH, Jeong EG, Lee JW, et al. Expression of beclin‐1, an autophagy‐related protein, in gastric and colorectal cancers. APMIS. 2007;115(12):1344‐1349. [DOI] [PubMed] [Google Scholar]
- 221. Daniel F, Legrand A, Pessayre D, et al. Beclin 1 mRNA strogly correlates with Bcl‐XLmRNA expression in human hepatocellular carcinoma. Cancer Invest. 2007;25(4):226‐231. [DOI] [PubMed] [Google Scholar]
- 222. Liu Q, Wang J‐J, Pan Y‐C, Meng L‐F, Zhan X, Zheng Q‐F. Expression of autophagy‐related genes Beclin 1 MAPLC3 in non‐small cell lung cancer. Ai Zheng. 2008;27(1):25‐29. [PubMed] [Google Scholar]
- 223. Shen Y, Li D‐D, Wang L‐L, Deng R, Zhu X‐F. Decreased expression of autophagy‐related proteins in malignant epithelial ovarian cancer. Autophagy. 2008;4(8):1067‐1068. [DOI] [PubMed] [Google Scholar]
- 224. Miracco C, Cosci E, Oliveri G, et al. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumors. Int J Oncol. 2007;30(2):429‐436. [PubMed] [Google Scholar]
- 225. Francipane MG, Lagasse E. Selective targeting of human colon cancer stem‐like cells by the mTOR inhibitor Torin‐1. Oncotarget. 2013;4(11):1948‐1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Din FVN, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP‐activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7):1504‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Huo H‐z, Zhou Z‐y, Wang B, Qin J, Liu W‐y, Gu Y. Dramatic suppression of colorectal cancer cell growth by the dual mTORC1 and mTORC2 inhibitor AZD‐2014. Biochem Biophys. 2014;443(2):406‐412. [DOI] [PubMed] [Google Scholar]
- 228. Xie C‐M, Chan WY, Yu S, Zhao J, Cheng CHK. Bufalin induces autophagy‐mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic Biol Med. 2011;51(7):1365‐1375. [DOI] [PubMed] [Google Scholar]
- 229. Ellington AA, Berhow M, Singletary KW. Induction of macroautophagy in human colon cancer cells by soybean B‐group triterpenoid saponins. Carcinogenesis. 2005;26(1):159‐167. [DOI] [PubMed] [Google Scholar]
- 230. Zhan Y, Gong K, Chen C, Wang H, Li W. P38 MAP kinase functions as a switch in MS‐275‐inuced reactive oxygen species‐dependent autophagy and apoptosis in human colon cancer cells. Free Radic Biol Med. 2012;53(3):532‐543. [DOI] [PubMed] [Google Scholar]
- 231. Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A. Quercetin mediates preferential degradation of oncogenic RAS and causes autophagy in Ha‐RAS‐transformed human colon cells. Carcinogenesis. 2007;28(5):1021‐1031. [DOI] [PubMed] [Google Scholar]
- 232. Miki H, Uehara N, Kimura A, et al. Resveratrol induces apoptosis via ROS‐triggered autophagy in human colon cancer cells. Int J Oncol. 2012;40(4):1020‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5‐fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer. 2010;46(10):1900‐1909. [DOI] [PubMed] [Google Scholar]
- 234. Sasaki K, Tsuno NH, Sunami E, et al. Chloroquine potentiates the anti‐cancer effect of 5‐fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Schonewolf CA, Mehta M, Schiff D, et al. Autophagy inhibition by chloroquine sensetizes HT‐29 colorectal cancer cells to concurrent chemoradiation. World J Gastrointest Oncol. 2014;6(3):74‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Xiong L, Liu Z, Gq O, et al. Autophagy inhibition enhances photocytotoxicity of Photosan‐II in human colorectal cancer cells. Oncotarget. 2017;8(4):6419‐6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Selvakumaran M, Amaravadi RK, Vasilevskaya IA, et al. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res. 2013;19(11):2995‐3007. [DOI] [PubMed] [Google Scholar]
- 238. Shi Y, Tang B, Yu P‐W, et al. Autophagy protects against oxaliplatin‐induced cell death via ER stress and ROS in Caco‐2 cells. PLos One. 2012;7(11):e51076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lévy J, Cacheux W, Bara MA, et al. Intestinal inhibition of Atg7 prevents tumour initiation through a microbiome‐influenced immune response and suppresses tumour growth. Nat Cell Biol. 2015;17(8):1062‐1073. [DOI] [PubMed] [Google Scholar]
- 240. Park JM, Tougeron D, Huang S, Okamoto K, Sinicrope FA. Beclin 1 and UVRAG confer protection from radiation‐induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS One. 2014;9(6):e100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Zhan L, Zhang Y, Wang W, et al. Autophagy as an emerging therapy target for ovarian carcinoma. Oncotarget. 2016;7(50):83476‐83487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Orfanelli T, Jeong JM, Doulaveris G, Holcomb K, Witkin SS. Involvement of autophagy in cervical, endometrial and ovarian cancer. Int J Cancer. 2014;135(3):519‐528. [DOI] [PubMed] [Google Scholar]
- 243. Valente G, Morani F, Nicotra G, et al. Expression and clinical significance of the autophagy proteins BECLIN 1 and LC3 in ovarian cancer. Biomed Res Int. 2014;2014:462658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Shen Y, Liang L‐Z, Hong M‐H, Xiong Y, Wei M, Zhu X‐F. Expression and clinical significance of microtubule‐associated protein 1 light chain 3 (LC3) and Beclin1 in epithelial ovarian cancer. Ai Zheng. 2008;27(6):595‐599. [PubMed] [Google Scholar]
- 245. Peracchio C, Alabiso O, Valente G, Isidoro C. Involvement of autophagy in ovarian cancer: a working hypothesis. J Ovarian Res. 2012;5(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cai M, Hu Z, Liu J, et al. Beclin 1 expression in ovarian tissues and its effects on ovarian cancer prognosis. Int J Mol Sci. 2014;15(4):5292‐5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lin H‐X, Qiu H‐J, Zeng F, et al. Decreased expression of Beclin 1 correlates closely with Bcl‐xL expression and poor prognosis of ovarian carcinoma. PLoS One. 2013;8(4):e60516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Iwadate R, Inoue J, Tsuda H, et al. High expression of SQSTM1/p62 protein is associated with poor prognosis in epithelial ovarian cancer. Acta Histochem Cytochem. 2014;47(6):295‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Park S‐M, Kim K, Lee E‐J, et al. Reduced expression of DRAM2/TMEM77 in tumor cells interferes with cell death. Biochem Biophs Res Commun. 2009;390(4):1340‐1344. [DOI] [PubMed] [Google Scholar]
- 250. Zhao Y, Chen S, Gou W‐f, Xiao L‐j, Takano Y, Zheng H‐c. Aberrant Beclin 1 expression is closely linked to carcinogenesis, defferentiation, progression, and prognosis of ovarian epithelial carcinoma. Tumour Biol. 2014;35(3):1955‐1964. [DOI] [PubMed] [Google Scholar]
- 251. Giatromanolaki A, Koukourakis MI, Koutsopoulos A, Chloropoulou P, Liberis V, Sivridis E. High Beclin 1 expression defines a poor prognosis in endometrial adenocarcinomas. Gynecol Oncol. 2011;123(1):147‐151. [DOI] [PubMed] [Google Scholar]
- 252. Spowart JE, Townsend KN, Huwait H, et al. The autophagy protein LC3A correlates with hypoxia and is a prognostic marker of patient survival in clear cell ovarian cancer. J Pathol. 2012;228(4):437‐447. [DOI] [PubMed] [Google Scholar]
- 253. Lu Z, Luo RZ, Lu Y, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118(12):3917‐3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Lu Z, Yang H, Sutton MN, et al. ARHI (DIRAS3) induces autophagy in ovarian cancer cells by downregulating the epidermal growth factor receptor, inhibiting PI3K and Ras/MAP signaling and activating the FOXo3a‐mediated induction of Rab7. Cell Death Differ. 2014;21(8):1275‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Zhou J, Jiang Y‐Y, Chen H, Wu Y‐C, Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2):e12739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 256. Khurana A, Roy D, Kalogera E, et al. Quinacrine promotes autophagic cell death and chemosensitivity in ovarian cancer and attenuates tumor growth. Oncotarget. 2015;6(34):36354‐36369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lang F, Qin Z, Li F, Zhang H, Fang Z, Hao E. Apoptotic cell death induced by resveratrol is partially mediated by the autophagy pathway in human ovarian cancer cells. PLoS One. 2015;10(6):e0129196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wen Y, Graybill WS, Previs RA, et al. Immunotherapy targeting floate receptor cell death associated with autophagy in ovarian cancer. Clin Cancer Res. 2015;21(2):448‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Pang Y, Si M, Sun B, et al. DHA2, a synthesized derivative of bisbibenzyl, exerts antitumor activity against ovarian cancer through inhibition of XIAP and Akt/mTOR pathway. Food Chem Toxicol. 2014;69:163‐174. [DOI] [PubMed] [Google Scholar]
- 260. Liu N, Tai S, Ding B, et al. Arsenic trioxide synergizes with everolimus (Rad001) to induce cytotoxicity of ovarian cancer cells through increased autophagy and apoptosis. Endocr Relat Cancer. 2012;19(5):711‐723. [DOI] [PubMed] [Google Scholar]
- 261. Bao L, Jaramillo MC, Zhang Z, et al. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Mol Med Rep. 2015;11(1):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Sun Y, Liu J‐H, Jin L, Sui Y‐X, Han L‐L, Huang Y. Effect of autophagy‐related beclin1 on sensitivity of cisplatin‐resistant ovarian cancer cells to chemotherapeutic agents. Asian Pac J Cancer Prev. 2015;16(7):2785‐2791. [DOI] [PubMed] [Google Scholar]
- 263. Zhao X, Fang Y, Yang Y, et al. Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy. 2015;11(10):1849‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Tang J, Zhu J, Ye Y, et al. Inhibition LC3B can increase chemosensitivity of ovarian cancer cells. Cancer Cell Int. 2019;19(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Chung YC, Lu LC, Tsai MH, et al. The inhibitory effect of ellagic acid on cell growth of ovarian carcinoma cells. Evid Based Complement Alternat Med. 2013;306705:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Pagotto A, Pilotto G, Mazzoldi EL, et al. Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis. 2017;8(7):e2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Evangelisti C, Evangelisti C, Chiarini F, et al. Autophagy in acute leukemias: a double‐edged sword with important therapeutic implications. Biochim Biophys Acta. 2015;1853(1):14‐26. [DOI] [PubMed] [Google Scholar]
- 268. Salemi S, Yousefi S, Constantinescu MA, Fey MF, Uwe‐Simon H. Autophagy is required for self‐renewal and differentiation of adult human stem cells. Cell Res. 2012;22(2):432‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Mortensen M, Soilleux EJ, Djordjevic G, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenace. J Exp Med. 2011;208(3):455‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Liu F, Lee JY, Wei H, et al. FIP200 is required for the cell‐autonomous maintenance of fetal hematopoietic stem cells. Blood. 2010;116(23):4806‐4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Charoensuk V, Gati WP, Weinfeld M, Le XC. Differential cytotoxic effects of arsenic compounds in human acute promyelocytic leukemia cells. Toxicol Appl Pharmacol. 2009;239(1):64‐70. [DOI] [PubMed] [Google Scholar]
- 272. Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up‐regulation of Beclin‐1. Leuk Res. 2007;31(3):329‐339. [DOI] [PubMed] [Google Scholar]
- 273. Goussetis DJ, Altman JK, Glaser H, McNeer JL, Tallman MS, Platanias LC. Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. J Biol Chem. 2010;285(39):29989‐29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Mahoney E, Lucas DM, Gupta SV, et al. ER stress and autophagy: new discoveries in the mechanism of action and drug resistance of the cyclin‐dependent kinase inhibitor flavopiridol. Blood. 2012;120(6):1262‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Sharma A, Singh K, Mazumder S, Hill BT, Kalaycio M, Almasan A. BECN1 and BMI interactions with MCL‐1 determine fludarabine resistance in leukemic B cells. Cell Death Dis. 2013;4(5):e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Grandér D, Kharaziha P, Laane E, Pokrovskaja K, Panaretakis T. Autophagy as the main means of cytotoxicity by glucocorticoids in hematological malignancy. Autophagy. 2009;5(8):1198‐1200. [DOI] [PubMed] [Google Scholar]
- 277. Laane E, Tamm KP, Buentke E, et al. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009;16(7):1018‐1029. [DOI] [PubMed] [Google Scholar]
- 278. Ristic B, Bosnjak M, Arsikin K, et al. Idarubicin induces mTOR‐dependent cytotoxic autophagy in leukemic cells. Exp Cell Res. 2014;326(1):90‐102. [DOI] [PubMed] [Google Scholar]
- 279. Crazzolara R, Bradstock KF, Bendall LJ. RAD001 (Everolimus) induces autophagy in acute lymphoblastic leukemia. Autophagy. 2009;5(5):727‐728. [DOI] [PubMed] [Google Scholar]
- 280. Liao A, Hu R, Zhao Q, et al. Autophagy induced by FTY720 promotes apoptosis in U266 cells. Eur J Pharm Sci. 2012;45(5):600‐605. [DOI] [PubMed] [Google Scholar]
- 281. Puissant A, Robert G, Fenouille N, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK‐mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70(3):1042‐1052. [DOI] [PubMed] [Google Scholar]
- 282. You L, Jin S, Zhu L, Qian W. Autophagy, autophagy‐associated adaptive immune responses and its role in hematologic malignancies. Oncotarget. 2017;8(7):12374‐12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Bosnjak M, Ristic B, Arsikin K, et al. Inhibition of mTOR‐dependent autophagy sensitizes leukemic cells to cytarabine‐induced apoptotic death. PLoS One. 2014;9(4):e94374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Ishdorj G, Li L, Sb G. Regulation of autophagy in hematological malignancies: role of reactive oxygen species. Leuk Lymphoma. 2012;53(1):26‐33. [DOI] [PubMed] [Google Scholar]
- 285. Carew JS, Nawrocki ST, Kahue CN, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr‐Abl‐mediated drug resistance. Blood. 2007;110(1):313‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zhu S, Cao L, Yu Y, et al. Inhibiting autophagy potentiates the anticancer activity of INF1@/INFα in chronic myeloid leukemia cells. Autophagy. 2013;9(3):317‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


