Abstract
Background
Transcatheter valvular interventions affect cardiac and hemodynamic physiology by changing ventricular (un-)loading and metabolic demand as reflected by cardiac mechanoenergetics. Real-time quantifications of these changes are scarce. Pressure-volume loop (PVL) monitoring appraises both load-dependent and load-independent compounds of cardiac physiology including myocardial work, ventricular unloading, and ventricular-vascular interactions. The primary objective is to describe changes in physiology induced by transcatheter valvular interventions using periprocedural invasive biventricular PVL monitoring. The study hypothesizes transcatheter valve interventions modify cardiac mechanoenergetics that translate into improved functional status at 1-month and 1-year follow-up.
Methods
In this single-center prospective study, invasive PVL analysis is performed in patients undergoing transcatheter aortic valve replacement or tricuspid or mitral transcatheter edge-to-edge repair. Clinical follow-up is per standard of care at 1 and 12 months. This study aims to include 75 transcatheter aortic valve replacement patients and 41 patients in both transcatheter edge-to-edge repair cohorts.
Results
The primary outcome is the periprocedural change in stroke work, potential energy, and pressure-volume area (mmHg mL−1). The secondary outcomes comprise changes in a myriad of parameters obtained by PVL measurements, including ventricular volumes and pressures and the end-systolic elastance—effective arterial elastance ratio as a reflection of ventricular—vascular coupling. A secondary endpoint associates these periprocedural changes in cardiac mechanoenergetics with functional status at 1 month and 1 year.
Conclusions
This prospective study aims to elucidate the fundamental changes in cardiac and hemodynamic physiology during contemporary transcatheter valvular interventions.
Keywords: Cardiac mechanoenergetics, Heart failure, Physiology, Pressure-volume loop, Structural heart interventions, Ventricular unloading
Highlights
-
•
Invasive pressure-volume loop analysis offers a comprehensive tool to monitor load-dependent and load-independent compounds of cardiac and hemodynamic physiology.
-
•
Following transcatheter valvular interventions, changes in cardiac mechanoenergetics, ventricular (un-)loading and ventricular-vascular coupling are measured.
-
•
Apart from altering intraventricular loading conditions, transcatheter valvular interventions induce changes in physiology on a biventricular level (i.e., the ventricular interference).
-
•
Invasive pressure-volume loop analysis may complement individual patient decision-making during structural valvular interventions.
Introduction
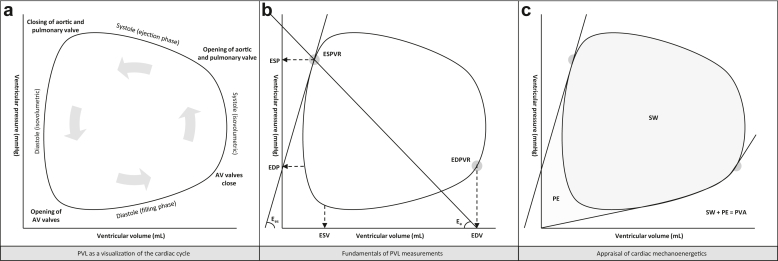
The clinical application of pressure-volume loop (PVL) monitoring as an adjunct to periprocedural guidance during structural heart interventions has been limited, predominantly given the invasiveness and complexity of volumetric calibration. Evolving volume calibration algorithms and the adoption of the single-beat approach (eliminating the necessity of preload reduction for determining parameters such as end-systolic elastance, Ees) ignited the renaissance of invasive PVL monitoring in clinical practice beyond the mere context of scientific research.1, 2, 3, 4 The essentials of PVL analysis comprise a direct visualization of dynamic changes in intraventricular pressure and volume throughout the cardiac cycle. Advanced PVL reconstructions allow for the estimation of changes in ventricular-vascular coupling as well as alterations in intraventricular dyssynchrony and cardiac mechanoenergetics. Cardiac mechanoenergetics (reflected by the pressure-volume area [PVA]) are directly correlated with myocardial oxygen demand and a real-time visualization of changing PVA might therefore be of clinical relevance.5 PVA comprises the sum of stroke work (the myocardial energy required to propel blood into the vasculature, i.e., the area with the PVL curve) and potential energy (the residual energy stored in the myocardium immediately following systole). The elements of PVL analysis are visualized in Figure 1.
Figure 1.
The elements of pressure-volume loop analysis.
Abbreviations: AV, atrioventrciular; Ea, arterial elastance; EDP, end-diastolic pressure; EDPVR, end-diastolic pressure volume relation; EDV, end-diastolic volume; Ees, end-systolic elastance; ESP, end-systolic pressure; ESPVR, end-systolic pressure volume relation; ESV, end-systolic volume; PE, potential energy; PVA, pressure-volume area; PVL, pressure-volume loop; SW, stroke work.
The spectrum of transcatheter structural heart interventions and corresponding clinical indications has broadened over the years. Serial echocardiography studies after such interventions documented changes in ventricular dimensions as a proxy of altered ventricular loading and so-called ventricular remodeling.6, 7, 8 Studies of immediate changes in cardiac and hemodynamic physiology beyond ventricular unloading are, however, scarce. Apart from quantifying cardiac mechanoenergetics, periprocedural biventricular PVL analysis may elucidate interventricular interference (i.e., the crosstalk between the right and left ventricle) during right or left-sided structural heart interventions. Periprocedural invasive biventricular PVL measurements might be of direct clinical relevance by demonstrating the ventricular response to the beat-to-beat changing loading conditions during transcatheter valvular interventions. The aim of Real time Pressure volume Loop monitoring as a guide for enhanced Understanding of changes in elemental cardiovascular physiology during Therapeutic strategies for hemodynamic Optimization (PLUTO) is to elucidate fundamental changes in cardiac and hemodynamic physiology through invasive PVL analysis during structural interventions. We hypothesize that contemporary transcatheter valve interventions modify cardiac mechanoenergetics (i.e., a decrease in the myocardial metabolic demand measured by invasive PVL analysis) that translate into improved functional status at 1-month and 1-year follow-up.
Material and Methods
Study Design
This study is a single-center, prospective, observational study using invasive pressure volume analysis during contemporary transcatheter valvular interventions. The primary objective is to evaluate the beat-to-beat changes in cardiac and hemodynamic physiology, including alterations in cardiac mechanoenergetics and interventricular interference during and immediately following transcatheter valvular interventions. The secondary objective is to investigate the association between periprocedural changes in cardiac and hemodynamic physiology (through invasive PVL analysis) and functional status as reflected by New York Heart Association (NYHA) class at 1 month and 1 year.
Patients
Patients will be enrolled and treated at the Thoraxcenter, Erasmus University Medical Center in Rotterdam, The Netherlands. Adult patients scheduled for (nonemergent) transcatheter aortic valve replacement (TAVR) or tricuspid or mitral transcatheter edge-to-edge repair (TEER) are eligible for study enrolment. Following study inclusion, patients will be classified according to the type of the planned procedure (i.e., one patient cohort per type of procedure). Exclusion criteria are age <18 years, any confirmed or suspected concomitant congenital heart condition, planned use of mechanical circulatory support during the intervention or absence of written informed consent.
PVL Measurements
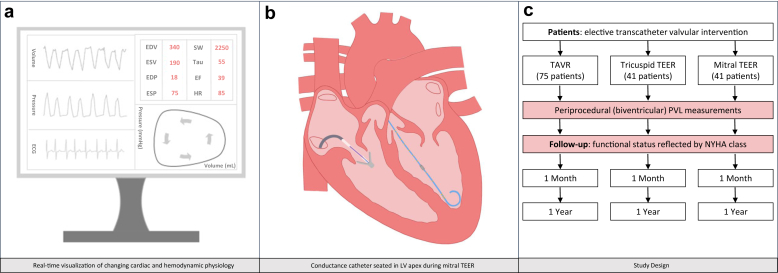
Both study design and essentials of PVL analysis during transcatheter valvular interventions are displayed in Figure 2. PVL measurements will be obtained with a ventricular conductance catheter (CD Leycom, Hengelo, The Netherlands). The required length of the conductance catheter (i.e., 8 mm, 10 mm, or 12 mm spacing) will be determined by the operator based on ventricular dimensions derived from a recent echocardiography or other cardiac imaging exam. The conductance catheter will be positioned in the apex of the targeted ventricle using fluoroscopic guidance and by continuous visual inspection of segmental loops. For patients undergoing TAVR, left and right ventricular PVL measurements will be obtained immediately before and after valve implantation. The conductance catheter will be exchanged between the right and left ventricular apex before and after valve deployment. For TEER procedures, both right and left ventricular PVL assessments will be performed directly before and after leaflet approximation. The conductance catheter will be seated in the left ventricular apex throughout the actual leaflet instrumentation and approximation allowing left ventricular PVL measurements during device positioning and leaflet grasping and will be positioned in the LV using a retrograde approach through the aortic valve. Supplemental Figure 1 illustrates changing biventricular PVL measurements during a mitral TEER, illustrating an immediate PVA reduction. A similar approach for tricuspid TEER was recently published.9 For all cases, a pulmonary artery catheter will be inserted following femoral or internal jugular vein cannulation to allow volume calibration of PVL measurements. The volume calibration algorithm comprises an estimation of stroke volume using thermodilution to appraise cardiac output. The parallel conductance of surrounding tissue (i.e., artefacts through electrical conductance in tissue surrounding the region of interest that impede correct estimation of the ventricular volume) can be estimated by injecting boluses of hypertonic saline (3× 7-10 mL 7.5% NaCl, Fresenius Kabi, Zeist, The Netherlands). Repeat volume calibration is required after each conductance catheter manipulation or reinsertion. An alternative method of volume calibration is based on periprocedural cardiac volumes derived from either 2D or 3D transthoracic echocardiography in the catheterization laboratory. PVL measurements obtained using the conductance catheter will be translated into PVL plots and quantifications using the Inca hardware and Conduct NT software (CD Leycom, Hengelo, The Netherlands). During data acquisition, segmental loops will be visually inspected to assure stable segment position. The single-beat estimation described by Chen et al.1 will be applied to reconstruct Ees (as a load-independent marker of myocardial contractility).
Figure 2.
Pressure-volume loop analysis during transcatheter valvular interventions.
Abbreviations: ECG, electrocardiogram; EDP, end-diastolic pressure; EDV, end-diastolic volume; EF, ejection fraction; ESP, end-systolic pressure; ESV, end-systolic volume; HR, heart rate; LV, left ventricle; NYHA, New York Heart Association; PVL, Pressure-volume loop; SW, stroke work; TAVR, transcatheter aortic valve replacement; TEER, transcatheter edge-to-edge repair.
Data Collection and Follow-Up
Study endpoints will be collected during the index transcatheter valvular intervention. Patients will undergo routine clinical follow-up after 1 and 12 months. The baseline and procedural characteristics, echocardiography data, and clinical follow-up will be extracted from an institutional dedicated prospective database. Functional status will be graded by NYHA class.
Study Endpoints
The primary endpoint is the absolute change in cardiac mechanoenergetics induced by either TAVR, tricuspid TEER, or mitral TEER immediately following the index intervention. Cardiac mechanoenergetics are reflected by the PVL-derived parameters stroke work (SW, mmHg mL−1) and potential energy (PE, mmHg mL−1). The sum of SW and PE provides the PVA in mmHg mL−1 and is a representation of the myocardial oxygen demand and corresponding metabolic efficiency. Secondary study endpoints comprise changes following the transcatheter valvular intervention in a myriad of parameters obtained with PVL analysis, including (diastolic and systolic) ventricular volume and pressure, intraventricular dyssynchrony as well as ventricular-vascular coupling represented by the Ees—effective arterial elastance (Ees/Ea) ratio. An overview of study endpoints is provided in Supplemental Table 1.
Sample Size Calculation
Literature reports on changing cardiac mechanoenergetics during transcatheter valvular interventions are sparse. Marino et al.10 reported a decrease in PVA from 16.424 (standard deviation [SD] ± 6.268) mmHg mL−1 to 12.053 (SD ± 5.260) following TAVR (p < 0.001) using noninvasive PVL-monitoring. Based on these numbers, and assuming normal distribution and moderate to strong negative intrapatient correlation, 65 patients will be needed to detect a significant change in PVA with 0.05 alpha and 90% power for patients undergoing TAVR. Lavall et al.11 reported a decrease in PVA from 10.903 (SD ± 4.410) to 9.124 (SD ± 2.968) mmHg mL−1 with a mean difference of −1.779 (SD ± 3.690) (p = 0.0007) in 46 patients undergoing noninvasive PVL-monitoring in the context of mitral TEER. Based on this report, 36 patients will be needed to detect a significant change in PVA with 0.05 alpha and 80% power for patients undergoing TEER. The changes in cardiac mechanoenergetics are presumed to be similar for mitral and tricuspid TEER, so no separate sample size calculation was performed for the 2 TEER cohorts. The sample size will be increased by 15% anticipating periprocedural technical difficulties and loss of follow-up for all cohorts. In total, we aim to enroll 75 patients within the TAVR cohort and 41 patients for both TEER cohorts in an 18-month inclusion period. Sample size calculation was performed using G∗power (3.1.9.6) employing a paired 2-tailed t-test to compare means in matched pairs.12
Statistical Analysis
Normal distribution will be assessed using the Shapiro–Wilk or Kolmogorov–Smirnov test. The continuous parameters SW, PE, and PVA are expected to be normally distributed and will be presented as means ± SD. For the primary objective, PVL measurements obtained directly before the structural intervention will be compared with PVL measurements obtained immediately after the structural intervention. Paired t-tests will be used to investigate the primary and secondary study outcomes. Mean differences with standard deviation will be provided. For the secondary objectives, parameters will be compared between patients with vs. without improved functional status. In that context, parameters will be compared using an independent sample t-test. Secondary study parameters are presented and analyzed in a similar manner to primary study parameters. An identical statistical work-up will be undertaken for all patient cohorts. A predefined subanalysis will compare the changes in cardiac mechanoenergetics between patients with vs. without improved functional status as 1 and 12 months. Statistical analysis will be performed in SPSS (25.0, Armonk, YU, USA; IBM Corp). Throughout the study, 2-tailed p-values of <0.05 will be considered statistically significant.
Discussion
Periprocedural guidance and decision-making during structural heart interventions are predominantly driven by echocardiographic and/or fluoroscopic assessments of device positioning and changes in valvular regurgitation. This prospective study focuses on the beat-to-beat changes in the ventricular pressure-volume relationship surrounding transcatheter valvular interventions including an appraisal of changing cardiac mechanoenergetics. These structural valvular interventions may reduce the myocardial metabolic demand with a subsequent improvement in 1-month and 1-year functional status.
The goal after TAVR is to reduce the transaortic gradient, assuming preservation of left ventricular ejection fraction. TAVR induces immediate left ventricular unloading and modifies myocardial strain.13 A recent case study on TAVR for moderate aortic stenosis corroborated these findings and demonstrated an immediate left-shift of the PVL (reflecting volume unloading) with a concomitant increase in Ees indicating enhanced load-independent left ventricular myocardial contractility.14 Conversely, Seppelt et al.15 observed a right-shift of the PVL in 8 patients immediately following TAVR for severe aortic stenosis. Within both studies, both the Ees/Ea ratio (as a reflection of the left ventricular–aortic coupling) and SW (indicating the myocardial work necessary to eject blood from ventricle into the vasculature) improved suggesting enhanced myocardial efficiency. However, one report demonstrated Ees increase and Ea decrease (indicating enhanced myocardial contractility with lower ventricular afterload) and the other showed Ees decrease and stable Ea indicating a state of depressed myocardial contractility immediately following TAVR. A hypothesis to declare the difference in PVL shifts could be the occurrence of transient myocardial stunning following rapid ventricular pacing surrounding valve deployment or optimization by balloon inflations. Periprocedural PVL analysis may provide an in-depth appraisal of the ventricular responses after TAVR in a real-world patient population with presumed heterogeneity in procedural strategy (balloon expendable vs. self-expanding platform with or without rapid ventricular pacing), baseline left ventricular ejection fraction, and ventricular shape. Periprocedural PVL measurements may further elucidate the effects of myocardial stunning following rapid ventricular pacing on myocardial efficiency and the implications of rapid pacing for patient outcome.16
Regarding TEER, the goal of leaflet approximation is to alleviate the concerning ventricle from atrioventricular regurgitation. Mitral TEER was previously hypothesized to improve the general hemodynamic profile surrounding enhanced left ventricular unloading.17 Gaemperli et al.18 already illustrated the load-dependent changes following mitral TEER using PVL analysis, describing an immediate decrease in end-diastolic pressure as well as an increase in end-systolic wall stress. Alterations in ventricular loading following mitral TEER were accompanied by a decrease in pulmonary capillary wedge pressure as well as an increase in cardiac index.18,19 Mitral TEER also reduced SW without changing PVA (irrespective of etiology of valvular regurgitation).19 This finding indicated an increase in PE (energy stored in the myocardium) and revealed a decreased efficiency of the myocardium to transfer energy from PVA to myocardial work, presumably because of an abrupt increase in ventricular afterload. Reports regarding changes in myocardial efficiency following TEER, both from load (in-)dependent perspectives as well as from the prospect of ventricular-vascular interactions, are scarce. The clinical relevance of mechanical synergy between ventricle and vasculature (as reflected by the Ees/Ea ratio) was illustrated by Brener et al.,20 indicating that a change in interaction between right ventricular contractility and right ventricular afterload strongly predicted all-cause mortality following tricuspid repair or tricuspid replacement.
Beyond affecting intraventricular conditions, structural interventions may induce changes on the interventricular level. A few case reports already accentuated the feasibility of biventricular PVL measurements in acquiring a comprehensive overview of changing interventricular interference following TEER. Brener et al.21 identified a decrease in right ventricular afterload with a subsequent improvement in right ventricular–pulmonary artery coupling immediately following mitral TEER for severe mitral regurgitation. Conversely, tricuspid TEER may enhance left ventricular preload in response to right ventricular unloading.9
PVL analysis provides a comprehensive, beat-to-beat visualization of cardiac and hemodynamic physiology from both load-dependent and load-independent perspectives. Insights regarding changes in cardiac mechanoenergetics, ventricular loading, and ventricular-vascular interactions surrounding endovascular structural interventions are scarce but pertinent to predict the (individual) response to changing ventricular preload or afterload conditions. The association between periprocedural changing cardiomechanics and postprocedural improvements in functional status may underpin the value of periprocedural PVL analysis. Biventricular PVL analyses might unveil the ventricular crosstalk in terms of changing interventricular cardiac mechanoenergetics as well as ventricular (un-)loading during valvular interventions. The goal of this prospective study is to create a framework to improve the understanding of changes in elemental cardiac and hemodynamic physiology following TAVR, tricuspid TEER, and mitral TEER. Invasive PVL analysis during valvular interventions may complement echocardiographic and fluoroscopic imaging and help tailor periprocedural guidance and decision-making.
Apart from the single-center approach, this study has several limitations. Since previous reports of cardiac mechanoenergetics are scarce, the sample size calculation was performed using literature from noninvasive PVL measurements. Absolute baseline pressure volume relationships and dynamic changes following valve interventions may vary for each individual patient (e.g., patient habitus or degree of valve defect) and makes the comparison of absolute differences in our patient cohort less relevant. Relative changes and patterns of change seem more appropriate. The use of thermodilution to appraise cardiac output in the presence of valvular insufficiency for volume calibration as well as the use of the single-beat algorithm to estimate RV Ees carries intrinsic limitations.1 Finally, the effects of general anesthesia during TEER (and the administration of vasopressor drugs and fluids to counteract the hemodynamic effects of anesthesia) need to be taken into consideration.
Conclusions
Insights regarding changes in cardiac and hemodynamic physiology, including cardiac mechanoenergetics as a reflection of the myocardial oxygen consumption, induced by transcatheter valvular interventions can be ameliorated by conducting comprehensive periprocedural PVL measurements. This prospective study may open novel prospects surrounding contemporary transcatheter valvular interventions from a physiological point-of-view. Invasive PVL monitoring may complement echocardiographic and/or fluoroscopic imaging in procedural guidance and decision-making during transcatheter valvular interventions.
Funding
This research does not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study is investigator-initiated and was sponsored by the Erasmus University Medical Center in Rotterdam, The Netherlands.
Ethics Statement
The study will be conducted in accordance to the ethical principles stated in the World Medical Association’s Declaration of Helsinki.
Disclosure statement
A.J.M.v.d.E. received personal fees from Abiomed and speaker fees from Angiodynamics. M.B.B. received personal fees from PulseCath. J.J.S. reported working and financial relationship with CD Leycom. J.J.B. received research grants and speaker fees from Abbott. J.D. received institutional grant/research support from Astra Zeneca, Abbott, Boston Scientific, ACIST Medical, Medtronic, Pie Medical, ReCor Medical. N.M.V.M. received grant support from Abbott, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, PulseCath BV, Abiomed, Daiichi Sankyo, Materialise, Pie Medical and Siemens. The other authors have no disclosure.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Chen C.H., Fetics B., Nevo E., et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 2.Klotz S., Hay I., Dickstein M.L., et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:H403–H412. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 3.Bastos M.B., Burkhoff D., Maly J., et al. Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J. 2020;41:1286–1297. doi: 10.1093/eurheartj/ehz552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brener M.I., Masoumi A., Ng V.G., et al. Invasive right ventricular pressure-volume analysis: basic principles, clinical applications, and practical recommendations. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- 6.Stocker T.J., Hertell H., Orban M., et al. Cardiopulmonary hemodynamic profile predicts mortality after transcatheter tricuspid valve repair in chronic heart failure. JACC Cardiovasc Interv. 2021;14:29–38. doi: 10.1016/j.jcin.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Lee H., Kim J., Oh S.S., Yoo J.S. Long-term clinical and hemodynamic outcomes of edge-to-edge repair for tricuspid regurgitation. Ann Thorac Surg. 2021;112:803–808. doi: 10.1016/j.athoracsur.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Demir O.M., Ruffo M.M., Godino C., et al. Mid-term clinical outcomes following percutaneous mitral valve edge-to-edge repair. J Invasive Cardiol. 2020;32:E313–E320. doi: 10.25270/jic/20.00121. [DOI] [PubMed] [Google Scholar]
- 9.Van den Enden A.J.M., Bastos M.B., Schreuder J.J., Daemen J., Van Mieghem N.M. Invasive cardiomechanics during transcatheter edge-to-edge repair for massive tricuspid regurgitation using biventricular pressure-volume loop monitoring. JACC Case Rep. 2021;3:1883–1887. doi: 10.1016/j.jaccas.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marino P.N., Binda G., Calzaducca E., et al. Transcatheter aortic valve replacement acutely improves left ventricular mechanical efficiency in severe aortic stenosis: effects of different phenotypes. Clin Res Cardiol. 2020;109:819–831. doi: 10.1007/s00392-019-01570-3. [DOI] [PubMed] [Google Scholar]
- 11.Lavall D., Reil J.C., Segura Schmitz L., et al. Early hemodynamic improvement after percutaneous mitral valve repair evaluated by noninvasive pressure-volume analysis. J Am Soc Echocardiogr. 2016;29:888–898. doi: 10.1016/j.echo.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G∗Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 13.Eleid M.F., Padang R., Pislaru S.V., et al. Effect of transcatheter aortic valve replacement on right ventricular-pulmonary artery coupling. JACC Cardiovasc Interv. 2019;12:2145–2154. doi: 10.1016/j.jcin.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Bastos M.B., Schreuder J.J., Daemen J., Van Mieghem N.M. Hemodynamic effects of transcatheter aortic valve replacement for moderate aortic stenosis with reduced left ventricular ejection fraction. JACC Cardiovasc Interv. 2019;12:684–686. doi: 10.1016/j.jcin.2019.01.244. [DOI] [PubMed] [Google Scholar]
- 15.Seppelt P.C., De Rosa R., Mas-Peiro S., Zeiher A.M., Vasa-Nicotera M. Early hemodynamic changes after transcatheter aortic valve implantation in patients with severe aortic stenosis measured by invasive pressure volume loop analysis. Cardiovasc Interv Ther. 2022;37:191–201. doi: 10.1007/s12928-020-00737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer-Rasokat U., Renker M., Liebetrau C., Mollmann H., Hamm C.W., Kim W.K. Impact of rapid ventricular pacing during transcatheter implantation of self-expanding aortic valve prostheses in patients at highest risk. J Invasive Cardiol. 2020;32:E355–E361. doi: 10.25270/jic/20.00236. [DOI] [PubMed] [Google Scholar]
- 17.Siegel R.J., Biner S., Rafique A.M., et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658–1665. doi: 10.1016/j.jacc.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Gaemperli O., Biaggi P., Gugelmann R., et al. Real-time left ventricular pressure-volume loops during percutaneous mitral valve repair with the MitraClip system. Circulation. 2013;127:1018–1027. doi: 10.1161/CIRCULATIONAHA.112.135061. [DOI] [PubMed] [Google Scholar]
- 19.Gaemperli O., Moccetti M., Surder D., et al. Acute haemodynamic changes after percutaneous mitral valve repair: relation to mid-term outcomes. Heart. 2012;98:126–132. doi: 10.1136/heartjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 20.Brener M.I., Lurz P., Hausleiter J., et al. Right ventricular-pulmonary arterial coupling and afterload reserve in patients undergoing transcatheter tricuspid valve repair. J Am Coll Cardiol. 2022;79:448–461. doi: 10.1016/j.jacc.2021.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Brener M.I., Burkhoff D., Sarraf M. Right ventricular pressure-volume analysis before and after transcatheter leaflet approximation for severe mitral regurgitation. JAMA Cardiol. 2021;6 doi: 10.1001/jamacardio.2020.7209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.