Abstract
Pancreatic cancer usually results in poor survival with limited options for treatment, as most affected individuals present with advanced disease. Early detection of pre-invasive pancreatic neoplasia and identifying molecular therapeutic targets provide opportunities for extending survival. Although screening for pancreatic cancer is currently not recommended for the general population, emerging evidence indicates that pancreatic surveillance can improve outcomes for individuals in certain high-risk groups. Changes in the epidemiology of pancreatic cancer, experience from pancreatic surveillance, and discovery of novel biomarkers provide a roadmap for new strategies for pancreatic cancer risk assessment, early detection, and prevention.
Keywords: Pancreatic Cancer, Surveillance, Genetic Susceptibility
The Changing Epidemiology of Pancreatic Cancer
Pancreatic cancer is currently the third leading cause of cancer-related death in the United States, with an estimated 62,200 new diagnoses in the United States each year, accounting for 48,800 deaths.1 Approximately 90% of pancreatic cancers are pancreatic ductal adenocarcinomas (PDACs).1 In contrast to many other cancers in which early detection and advances in oncologic treatments have had success in prolonging survival, in the case of PDAC, the vast majority of affected individuals (>80%) present with advanced-stage disease, resulting in a 5-year survival rate of only 11%.1 Only 15%–20% of patients with PDAC present with surgically resectable disease, yet survival is significantly better for these individuals, especially for patients with stage I disease (5-year survival rate is approximately 80%),2 patients with node-negative disease,3,4 and those who achieve a pathologic complete response to neoadjuvant therapy,5 making early detection of pancreatic neoplasia an important, albeit challenging, goal.
Worldwide, pancreatic cancer ranks seventh among the causes of cancer-related deaths. Although incidence rates vary considerably by country, global trends suggest PDAC diagnoses are on the rise, such that it is expected to soon become the second leading cause of cancer death in Western countries. In nations with a high Human Development Index, PDAC incidence is approximately 5-fold higher compared with developing countries, with Western Europe and North America reporting 8.5 and 8.0 cases per 100,000, respectively, compared with only 1.3 cases per 100,000 in Southeast Asia.6 In the United States, PDAC incidence rates have increased by 0.5% per year since 2010, yielding an overall lifetime risk of pancreatic cancer of 1.7% by age 75 years.1 The mean age at diagnosis of PDAC in the United States is 65 years, and although the longer lifespan of residents of developed nations has been proposed as an explanation for the increase in PDAC, recent data suggest that diagnoses of PDAC are on the rise among younger individuals, with the most dramatic increases observed among young women.7,8 Increasing prevalence of obesity, diabetes, and alcohol use have been proposed as possible factors contributing to the increase in young-onset PDAC.
Racial and ethnic disparities are evident in the United States, with the highest incidence rates of PDAC among Black individuals, followed by White, Hispanic, and Asian individuals (15.9, 13.4, 12.2, and 10.3 per 100,000, respectively1)9 and Black individuals are less likely than White individuals to have localized disease at PDAC diagnosis.10 Disparities in PDAC outcomes have been attributed to regional variation in oncologic treatment and disparities in access to specialized surgery; however, there also appear to be racial disparities in rates of response to neoadjuvant chemotherapy, which prompts the question of whether differences in PDAC tumor biology may also be a factor in racial differences in survival.11
Pathogenesis of Pancreatic Neoplasia and Clinical Risk Factors
Understanding mechanisms of pathogenesis of PDAC can help clinicians not only identify who is at risk for developing PDAC, but also identify which treatments may be most effective. PDAC develops primarily as a consequence of the clonal selection of favorable somatic genetic aberrations that occur throughout one’s lifetime and can be precipitated by tissue injury and influenced by germline risk variants, immune response, and other factors.12 More than 90% of PDACs harbor activating somatic mutations in the KRAS oncogene. Somatic mutations in tumor suppressor genes p16/CDKN2A, TP53, and SMAD4 are also found in 90%, 75%, and 50% of tumors, respectively.13 Comprehensive genomic analyses have identified dysregulation of multiple pathways in PDAC, including DNA damage repair and cell cycle regulation.14 Mutation and methylation signatures identify distinct subtypes of PDAC, including squamous, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine, with unique histopathologic features and molecular phenotypes that suggest potential differences in how these neoplasms develop and respond to treatment.15
The neoplastic precursor lesions for PDAC include pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasms (IPMNs), and mucinous cystic neoplasms (MCNs). With increased use of cross-sectional imaging, we have learned that cystic pancreatic lesions are common incidental findings, and the prevalence of pancreatic cysts increases with age (with population prevalence averaging 1.2% and 20% for small cystic lesions on abdominal computed tomography [CT] and magnetic resonance imaging scans, respectively).16 However, only a small number of these lesions will undergo progression from low- to high-grade epithelial neoplasia. Furthermore, molecular evidence indicates that most PDACs do not develop from cysts, but rather arise from PanINs,13 which unfortunately are not identifiable by current imaging modalities. Therefore, the challenge lies not only in identifying what factors influence neoplastic progression, but also in identifying high-risk lesions in time to intervene. Although the time frame during which these precursors develop into invasive PDAC is believed to be more than 10 years,17 the ability to detect this progression within PanINs remains elusive, and the time frame for the emergence and progression of a cancerous mass is short.18,19
Clinical Factors Associated With Risk for Pancreatic Ductal Adenocarcinoma
Although the lifetime risk for PDAC is estimated at 1.7% for the “average” individual,1 a number of factors are associated with significant increases in risk for the disease, as detailed below.
Pancreatitis.
Both acute and chronic pancreatitis have been associated with risk for pancreatic cancer. With chronic pancreatitis, especially in long-standing hereditary, recurrent, acute pancreatitis, ongoing tissue injury; inflammation; fibrosis; and damage to cellular DNA promote neoplastic development and progression. In chronic pancreatitis, tissue injury occurs over decades. Early estimates of lifetime PDAC risk associated with hereditary pancreatitis were high (up to 70%20), but more recent evidence indicates a risk of <10%.21 Studies have reported a range of estimates for the risk of PDAC in patients with chronic pancreatitis, with results of a recent meta-analysis showing a standardized incidence ratio of 22.6.22 Recent guidelines reported a relative risk ranging from 5 to 10.20 In contrast to chronic pancreatitis, in which the risk of PDAC increases over time, in cases of acute pancreatitis, risk of PDAC is highest within 1 year of the pancreatitis episode, which is thought to reflect pancreatitis caused by obstructive pancreatitis from the tumor.23 This supports the recommendation to perform follow-up imaging after an episode of acute pancreatitis resolves.24
Smoking and alcohol.
A number of epidemiologic studies have documented that regular cigarette smoking is associated with an average 2-fold increase in risk for pancreatic cancer, and meta-analyses have estimated that the population-attributable risk of pancreatic cancer due to smoking is 11%–32%.9 Similarly, heavy alcohol use (defined as binge drinking or drinking more than 3 drinks per day), but not light alcohol use, is associated with modest increased risk for PDAC.25,26
Obesity.
It has been proposed that population increases in obesity are contributing to the global rise in pancreatic cancer.9 Several epidemiologic studies have demonstrated correlations between increases in body mass index and risk for PDAC,27 and data from the Nurses’ Health Study and Health Professionals Follow-Up Study found that overweight individuals (body mass index >30) had a relative risk of pancreatic cancer of 1.72.28
Diabetes.
Risk for pancreatic cancer is approximately 2-fold higher for individuals with type 2 diabetes compared with the general population. However, risk for pancreatic cancer is significantly higher within 1–3 years of diabetes onset, especially within the first 6 months (referred to as new-onset diabetes [NOD]).29–31 A cohort study conducted through Mayo Clinic found that one-half of patients with PDAC met clinical criteria for diabetes, and 85% had elevated fasting blood sugars—supporting the hypothesis that diabetes in this setting is caused by the tumor.29 Experiments in cell lines and animal models suggest that the pancreatic cancer cells themselves produce factors that impair glucose metabolism by inducing β-cell dysfunction and insulin resistance.29,32 The prevalence of diabetes in patients with newly diagnosed pancreatic cancer depends on the stage at diagnosis, becoming more likely as the cancer enlarges and causes atrophy of the gland.33 Risk estimates for PDAC associated with NOD vary depending on the underlying prevalence of diabetes in the control population, but several studies have observed that 0.5% to 1% of individuals older than 50 years with NOD will be diagnosed with PDAC within 3 years,30,31,34,35 with most of this risk manifesting within the first year. Because these reported prevalence rates are likely not great enough to warrant an evaluation for unrecognized pancreatic neoplasia in all patients with diabetes, studies are now focused on identifying further clinical and blood-based factors to identify those patients with NOD at greatest risk for PDAC involvement.33 Thus, ongoing studies of the general NOD population are beginning to evaluate the yield of surveillance among patients with NOD,36 with larger studies ongoing to define the utility of screening for PDAC in the NOD population.37
Intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
IPMNs and MCNs present an opportunity to identify a premalignant lesion in the pancreas to serve as a target for early detection strategies. However, the general applicability to most PDAC cases is quite limited because premalignant cystic lesions are a precursor for PDAC in only approximately 15% of cases.38 Although most PDACs do not arise from cystic precursors, cystic lesions can harbor PDACs, in the case of IPMNs, especially when they are found in the main pancreatic duct,16 justifying specialized surveillance or surgical resection when worrisome criteria based on lesion size and morphology are present.39–41 Furthermore, the fact that some individuals undergoing surveillance for IPMNs ultimately develop incident PDACs that are separate from the IPMNs is not surprising because PanINs, which are not detected by imaging, are generally more abundant in resected pancreata than IPMNs.32,42
Inherited susceptibility.
Pathogenic germline variants (PGVs) in major cancer susceptibility genes are found in as many as 1 in 10 unselected patients with PDAC. Across cohorts, germline genetic testing using multigene cancer panels identifies PGVs in cancer susceptibility genes in 5%–20% of PDAC cases43–46 (Table 1). Furthermore, at least one-half of patients with PDACs and PGVs report no family history of pancreatic cancer and/or do not meet clinical criteria for the hereditary syndrome corresponding to their genetic diagnosis.43 In most populations, the most common deleterious germline variants are in genes involved in homologous recombination DNA damage repair pathway (BRCA2, BRCA1, and PALB2), which are clinically actionable for management of both patients with cancer and their families. In addition, approximately 8% of PDACs have somatic alterations in BRCA2, PALB2, or ATM,14 which are usually biallelic. Germline and somatic alterations in BRCA1 and BRCA2 have important implications for oncologic treatment, as a major advance in treatment of PDAC has been the use of a poly (ADP-ribose) polymerase inhibitor (PARPi) for tumors with defects in homologous DNA repair. Whereas current first-line oncologic therapy for PDAC is FOLFIRINOX or gemcitabine/paclitaxel, the POLO randomized controlled trial demonstrated that use of a PARPi (Olaparib) for maintenance therapy in patients with metastatic pancreatic cancer with BRCA mutations was associated with a significant increase in progression-free survival.47
Table 1.
Pancreatic Cancer Susceptibility Genes
| Gene | Genetic syndrome | Average lifetime risk for PDAC,a % |
|---|---|---|
| ATM | Ataxia telangiectasiab | 5–10 |
| BRCA2 | Hereditary breast ovarian cancer syndrome | 5–8 |
| BRCA1 | Hereditary breast ovarian cancer syndrome | 3–5 |
| CDKN2A | Familial atypical multiple mole melanoma | 16–20 |
| MLH1, MSH2, MSH6 | Lynch syndrome | 0.5–7 |
| PALB2 | Hereditary breast ovarian cancer syndrome | 5–8 |
| PRSS1 | Hereditary pancreatitis | 10 |
| STK11 | Peutz-Jeghers syndrome | 11–32 |
| TP53 | Li-Fraumeni syndrome | Unknown |
Individual risk will vary depending on other risk factors, including family history, other genetic risk variants, pancreatic abnormalities, smoking, and surgical resection of precursor lesions.
Ataxia telangiectasia affects individuals who carry pathogenic germline variants in both alleles of ATM.
Because of the potential clinical impact that PGVs have for management of patients with PDAC and their at-risk family members, clinical guidelines now recommend universal genetic testing for all patients with PDAC, regardless of family history, using a multigene panel (Table 1)48–50 to evaluate for the following genetic conditions associated with increased risk for PDAC.
Hereditary breast ovarian cancer syndrome.
PGVs in BRCA2 and BRCA1, associated with hereditary breast ovarian cancer syndrome, are among the most common deleterious variants and are identified in up to 7% patients with pancreatic cancer. The population prevalence of variants BRCA1 and BRCA2 is estimated at 1 in 286 (and as high as 1 in 44 for individuals of Ashkenazi Jewish ancestry).51 Epidemiologic studies of individuals and families ascertained on the basis of cancer history that carriers of PGVs in BRCA2 have a lifetime risk for pancreatic cancer of 5%–8%, which is approximately 4- to 6-fold higher compared with the general population.52,53 The risk for pancreatic cancer among BRCA1 carriers, although lower than BRCA2, is still estimated at 3%–5%, approximately 2- to 2.5-fold higher than the general population risk. PGVs in PALB2, another gene involved in the homologous DNA repair complex, are much less common, but appear to confer cancer risks similar to BRCA2. Acknowledging the limitations of published data, the American Society for Gastrointestinal Endoscopy 2022 guideline recommended pancreatic screening for individuals with a lifetime risk for PDAC of >5%, which includes carriers of PGVs in BRCA1, BRCA2, and PALB2 regardless of family history,52 and others recommend pancreatic surveillance only for those carriers of PGVs who also have a family history of PDAC in a first- or second-degree relative.49
ATM.
ATM is also involved in the homologous DNA repair complex, and individuals with alterations in both copies of ATM can be affected with the neurologic condition ataxia telangiectasia. PGVs in ATM are relatively common in the general population (population frequency of 1 in 100–244)54; however the prevalence is higher among individuals with PDAC, particularly individuals who also have family history of PDAC.44 Although ATM is considered a “moderate risk” cancer susceptibility gene, studies of individuals and families ascertained on the basis of cancer history have estimated lifetime risk of PDAC for carriers of PGV in ATM of 5%%–10% by age 80 years.55 At present, guidelines suggest that pancreatic surveillance be offered to individuals with PGV in ATM if they also have a family history of pancreatic cancer in a first- or second-degree relative.49,52
Familial atypical multiple mole melanoma syndrome.
CDKN2A is a tumor suppressor gene that encodes for the p16 protein, a regulator of cell cycle. p16 function is lost in most PDACs as a result of somatic inactivation of CDKN2A. Carriers of PGVs in CDKN2A are at increased risk for developing melanoma and pancreatic neoplasia, with lifetime risk of PDAC approaching 16%–20%. In the Netherlands, where there is a Dutch founder mutation in CDKN2A (a 19-base pair deletion of exon 2 of CDKN2A referred to as p16-Leiden), prospective pancreatic surveillance in these carriers has improved detection of early-stage PDAC and improved survival.56
Lynch syndrome.
The DNA mismatch repair genes MLH1, MSH2, MSH6, PMS2, and EPCAM associated with Lynch syndrome confer increased risks for several cancer types. Lifetime risk for PDAC in carriers of PGVs in MLH1, MSH2, and MSH6 has been estimated at 0.5%–7%,57,58 which is significantly elevated compared with the general population risk, although not as high as the risks associated with CDKN2A, ATM, or BRCA2. Consequently, current recommendations suggest that pancreatic surveillance be offered to individuals with Lynch syndrome who also have a family history of pancreatic cancer.
Hereditary pancreatitis.
Hereditary pancreatitis is characterized by recurrent episodes of pancreatitis, often beginning at a young age, resulting in chronic injury to the pancreas. Germline alterations in PRSS1, which encodes cationic trypsinogen, are associated with the most severe type of hereditary pancreatitis and a lifetime risk of PDAC of up to 10%; however, pathogenic variants in SPINK1 and CTRC, as well as rare germline variants in CPA1, CPB1, which encode for pancreatic digestive enzymes, have also been implicated in pancreatitis, although the magnitude of risk for PDAC remains uncertain.59,60
Peutz-Jeghers syndrome.
Pathogenic germline variants in STK11 confer risk for Peutz-Jeghers syndrome, which is characterized by development of hamartomatous gastrointestinal polyps and increased risk for multiple cancer types, including gastrointestinal, breast, lung, and genitourinary tumors. Individuals with Peutz-Jeghers syndrome are among those at highest risk for pancreatic cancer, with 1 study reporting a relative risk of 132 (because many of the PDACs were young-onset) and a lifetime PDAC risk of 11%–32%.61
Other genetic conditions.
Although multigene panel testing in individuals with PDAC has turned up pathogenic germline variants in additional cancer susceptibility genes (such as TP53 associated with Li-Fraumeni syndrome), the magnitude of risk increase associated with these other genes remains uncertain. PGVs in APC, a major tumor suppressor gene, are associated with familial adenomatous polyposis and development of colorectal, duodenal, and ampullary adenomas. Although pancreatic cancer is not commonly diagnosed in patients with familial adenomatous polyposis, ampullary cancers that grow into the pancreas can be difficult to distinguish from primary pancreatic cancer. Although pathogenic variants in APC were reported in 2.1% of PDAC-affected patients in 1 study,46 it is important to note that all of these were the APCI1307k variant, which is a low-penetrance allele that is not associated with the classic familial adenomatous polyposis phenotype, and happens to be a common variant in individuals of Ashkenazi Jewish ancestry (population prevalence 1–2/100).62
Familial pancreatic cancer.
As many as 1 in every 10 individuals diagnosed with PDAC reports a family history that includes 1 or more relatives affected with PDAC. Familial pancreatic cancer (FPC) is defined as having 2 or more relatives affected with PDAC, in which 2 of the affected relatives are first-degree relatives to each other, and 1 affected is a first-degree relative of the proband. Although germline genetic testing identifies PGVs in 1 in 10 unselected PDAC cases, the yield of germline multigene panel genetic testing is only slightly higher in those with FPC, such that germline genetic testing is clinically uninformative in up to 80% of FPC cases.9 Exhaustive searches to identify other potential hereditary causes have employed whole exome and whole genome sequencing, but have not identified any other significant susceptibility genes,63 suggesting that low-penetrance variants64–66 and other processes (epigenetic) or shared exposures may have a role in these cases.
The National Institutes of Health’s Pancreatic Cancer Cohort Consortium’s PanScan studies have genotyped large cohorts and used genome-wide association studies to identify single nucleotide polymorphisms associated with PDAC risk.65 Although most single nucleotide polymorphisms appear to confer only very small increases in PDAC risk, combining single nucleotide polymorphisms into polygenic risk scores and integrating them with other clinical factors may inform prediction models to better identify individuals at high risk.67
Early Detection: Potential Benefits vs Harms of Pancreatic Surveillance
The prognosis for individuals diagnosed with PDAC remains poor in part because most affected individuals have advanced-stage disease at diagnosis. Localized disease (stage I or II) accounts for <20% of all cases and fewer than 1 in 5 patients with PDAC present with surgically resectable tumors. Although neoadjuvant chemotherapy and radiation are sometimes successful in shrinking larger tumors to render them surgically resectable, most individuals who undergo surgical resection of PDAC will experience progression of their cancer, especially those with node-positive disease.3,4 Most long-term survivors are diagnosed with stage I disease, often detected incidentally on imaging. Survival after a screen-detected PDAC is generally higher, as many more are diagnosed with stage I disease and have smaller tumors.68 Early detection of surgically resectable neoplasms (eg, small PDACs and/or high-grade preinvasive neoplasms) is the best strategy for improving PDAC survival and/or cure.
Even so, the relative rarity of pancreatic cancer in the general population compared with other deadly cancers has made demonstrating the value of pancreatic surveillance challenging. In stark contrast to its endorsements of screening for colorectal and breast cancers, the US Preventive Services Task Force (USPSTF) has advised against screening for pancreatic cancer for asymptomatic individuals (grade D recommendation).69 The USPSTF’s review of the literature published up to 2016 concluded that there was only limited evidence that screening for pancreatic cancer improves disease-specific morbidity or mortality, and the harms of screening for pancreatic cancer are at least moderate, due to potential for false positives that might prompt overtreatment, including surgery with potential for high morbidity and mortality. As PDACs are relatively rare in the general population, whereas IPMNs can be identified in up to 40% of individuals 75 years and older, the specifics regarding which findings require surgical intervention and the potential for unnecessary surgery continue to be major concerns because the operative mortality associated with panceaticoduodenectomy is 1%–2%, even at expert centers.
However, it is important to note that the USPSTF review did not consider the utility of pancreatic surveillance of individuals in high-risk populations, especially those with genetic susceptibility to PDAC. In the years since the USPSTF’s 2019 recommendation against PDAC screening, there have been several published studies of pancreatic surveillance in high-risk cohorts that found that screening can not only be effective in detecting early-stage PDAC and neoplasms with high-grade dysplasia, but can also improve clinical outcomes.
Surveillance for Pancreatic Ductal Adenocarcinoma in Cohorts at Increased Risk: Lessons Learned
More than 20 years ago, investigators from the University of Washington published their initial experience with pancreatic surveillance of 3 families who met criteria for FPC.70 Of the 14 individuals who underwent multimodality pancreatic imaging, one-half (7 of 14) had extensive pancreatic abnormalities detected by imaging (mainly small pancreatic cysts reflective of multifocal PanIN) and were advised to undergo total pancreatectomy. A later in-depth analysis of the pancreas pathology of these cases found a variety of features, including widespread PanIN, pancreatic atrophy, and several pancreatic cancers.71 A subsequent study described the characteristic atrophy of the parenchyma surrounding extensive PanIN as lobulocentric atrophy).72 This early experience of managing pancreatic abnormalities in individuals without pancreatic cancer led to criticism, given the risks of total pancreatectomy, and suggestions that participation in PDAC screening may have done more harm than good. The use of total pancreatectomy in this setting soon went out of favor and, over time, surgical management of patients undergoing surveillance became more conservative, with an emphasis on partial pancreatectomy and resection only when there were worrisometype features.73
During the past 2 decades, international collaborative efforts have continued to evaluate and refine approaches to early detection of pancreatic neoplasia for individuals at increased risk, and encouraging data from several studies support the rationale for using specific imaging modalities to guide medical decision making regarding need for and timing of surgical intervention for pancreatic lesions.
Who Should Undergo Surveillance for Pancreatic Neoplasia?
The Cancer of the Pancreas Screening Study (CAPS), led by investigators at Johns Hopkins since 1998, has established a large multicenter longitudinal cohort that now includes more than 2000 individuals undergoing surveillance. CAPS guidelines provide criteria for who should be screened for PDAC, and these criteria have been revised and widely adopted by international consortia for use in research and clinical care (Table 2).50,52,74,75 The yield of pancreatic surveillance is generally higher for individuals who carry deleterious variants in cancer susceptibility genes, as incidence of advanced neoplasia appears to be higher compared with those from FPC kindred without a known pathogenic germline variant,76–78 particularly for carriers of p16/CDKN2A.56 As evidence has accumulated demonstrating the potential benefits of pancreatic surveillance, eligibility criteria for who should undergo pancreatic surveillance was expanded in recent American Society for Gastrointestinal Endoscopy guidelines to include individuals with moderately increased pancreatic cancer risk (estimated at >5% lifetime risk), including carriers of pathogenic germline variants in BRCA2 or BRCA1 or PALB2 who do not have a family history of pancreatic cancer.52,79 However, other groups (including the National Comprehensive Cancer Network) make the recommendation for pancreatic cancer surveillance for those carriers of PGV of interest only if they also have a family history of pancreatic cancer in a first- or second-degree relative.49 The impact of family history on risk for PDAC continues to be a topic of debate, as it is worth noting that 1 large group who are not currently considered eligible for pancreatic surveillance are individuals who have only 1 first-degree relative affected with pancreatic cancer but no other risk factors, as their estimated risk of pancreatic cancer is only approximately 2-fold higher than average, or approximately 3% lifetime).80
Table 2.
Eligibility Criteria for Pancreatic Surveillance (Recommended for Individuals With Lifetime Risk for Pancreatic Cancer >5%)52,74
| Category | Criteria for pancreatic surveillance | Age to begin surveillance |
|---|---|---|
| Family history of PDAC | Familial pancreatic cancer syndrome 2 or more relatives affected with PDAC (2 first-degree to each other) | 50 y (or 10 y younger than youngest affected) |
| Genetic syndrome | ||
| Hereditary breast ovarian cancer syndrome | PGV in BRCA2, BRCA1, or PALB2 | 50 y (or 10 y earlier than youngest PDAC-affected relative) |
| Lynch syndrome | PGV in MLH1, MSH2, MSH6 plus a first- or second-degree relative with PDAC | 50 y (or 10 y earlier than youngest PDAC-affected relative) |
| Familial atypical multiple mole melanoma syndrome | PGV in CDKN2A | 40 y (or 10 y earlier than youngest PDAC-affected relative) |
| Peutz-Jeghers syndrome | PGV in STK11 | 35 y (or 10 y earlier than youngest |
| Other | PGV in ATM plus a first- or second-degree relative with PDAC | PDAC-affected relative) 50 y (or 10 y earlier than youngest PDAC-affected relative) |
| PGV in TP53 plus a first- or second-degree relative with PDAC | ||
| Hereditary pancreatitis | PGV in PRSS1 | 40 y (or 10 y earlier than youngest PDAC-affected relative) |
| Individuals with personal history of pancreatic neoplasia | IPMN >10 mm and/or family history of first-degree relative with PDAC | Not applicable |
PGV, pathogenic germline variant.
In light of associations between diabetes and risk for PDAC, clinical trials of pancreatic surveillance in individuals with NOD are currently underway. One challenge for the early detection of pancreatic cancer in individuals with diabetes is documenting the date of onset of diabetes, as the time from NOD onset to symptomatic presentation of pancreatic cancer is usually within 6 months.
How Should We Screen for Pancreatic Neoplasia?
In addition to providing data on clinical outcomes of patients undergoing pancreatic surveillance, experience from multiple longitudinal cohorts have provided data regarding which tests should be used in pancreatic surveillance protocols. In 2012, Canto et al81 published the results of the CAPS3 multicenter study—a blinded, head-to-head comparison of endoscopic ultrasound (EUS), magnetic resonance cholangiopancreatography (MRCP), and pancreatic protocol CT for surveillance of high-risk individuals—and found that EUS and MRCP are complementary for detecting different types of lesions (including small pancreatic cysts often missed by CT), which has helped establish the current recommendation favoring alternating examinations between EUS and MRCP.82 Fewer data are available about which modality is best for detecting and characterizing solid lesions, especially small pancreatic cancers, although initial studies suggest EUS may have an edge for visualizing solid lesions and MRCP may be better for visualizing small cystic lesions <10 mm.83 In addition to the detection of tumor masses, it has long been recognized that other abnormalities,84–86 such as detection of main pancreatic duct dilation87 or parenchymal atrophy,88,89 can be harbingers of the presence of pancreatic cancer. Advanced imaging analysis is enabling the automatic quantification of pancreas characteristics, such as duct size, pancreas gland volume, and percentage of pancreatic fat, which is expected to improve early detection in the future.90 Other imaging approaches being evaluated include quantifying muscle and fat loss by automated segmentation of CT images.91
The CAPS program recently published its experience to date, reporting on 26 incident cases of PDAC, of which 19 were detected during surveillance in 1731 subjects with 5041 person-years of follow-up.68 PDACs diagnosed during surveillance were earlier stage (58% stage I) and had markedly improved survival (median overall survival of 9.8 years, compared with 1.5 years for PDAC cases detected outside surveillance [hazard ratio, 0.13; 95% CI, 0.03–0.50]).68 Previous analyses using data from CAPS cohorts found that the vast majority of screen-detected PDACs were surgically resectable,92 similar to findings from European cohorts in which >75% of screen-detected PDACs in CDKN2A carriers were surgically resectable,56,93 but notably, a higher percentage of PDACs detected in the CAPS program have been diagnosed at stage I.
Despite these promising findings, current surveillance approaches are still far from perfect. In a European cohort of asymptomatic individuals undergoing annual pancreatic surveillance with EUS and MRCP, 10 of 165 (6%) CDKN2A gene mutation carriers developed PDAC, 4 of which presented symptomatically with advanced disease.78 Examining findings and outcomes of 2552 high-risk individuals undergoing pancreatic surveillance in 16 international surveillance programs, Overbeek and colleagues76 found that one-half of those who progressed to PDAC or high-grade dysplasia lacked worrisome findings detected on their prior annual imaging studies. A meta-analysis of 13 surveillance studies concluded that there was no association between having an incident advanced PDAC and clinicoradiologic abnormalities at baseline.94 This is not surprising because most pancreatic cancers arise from PanIN, and only a fraction of PanINs create imaging abnormalities, generally subtle nonspecific parenchymal abnormalities related to secondary changes from the PanIN.68,72,81 The occurrence of interval advanced cancers not detected by surveillance emphasizes the limitations of our current screening strategies, particularly the inability to visualize high-grade dysplasia in PanIN before development of invasive cancer.94
Which Surveillance Findings Necessitate Surgical Intervention?
Pancreatic imaging abnormalities are commonly found in approximately one-half of high-risk individuals who undergo pancreatic surveillance, but most are small, sub-centimeter, pancreatic cysts of low malignant potential that are also found in the general population, albeit at lower prevalence. From available longitudinal data, it appears that most of these pancreatic cysts remain stable over time. Because pancreatic cancer is so deadly and because some patients diagnosed with pancreatic cancer while under surveillance will still die of the disease, many consider waiting for a cancer to develop as a missed opportunity, as the ultimate goal of pancreatic surveillance is not only to detect and treat stage I PDAC, but also to intervene on high-grade precursor lesions (PanIN and/or IPMN).82
The last 2 decades of experience with pancreatic surveillance, especially for sporadic pancreatic cysts, has helped to refine our understanding of what constitutes a high-risk lesion. Specifics regarding “worrisome features” (including cyst size ≥3 cm, enhancing mural nodule, thickened enhanced cyst walls, main pancreatic duct size of 5–9 mm, abrupt change in the main pancreatic duct caliber with distal pancreatic atrophy, lymphadenopathy, an elevated serum level of carbohydrate antigen 19–9 (CA19-9), and a rapid rate of cyst growth (>5 mm/2 years) are widely endorsed.16 Most of the time, these imaging abnormalities reflect the emergence of invasive cancer or high-grade dysplasia in an IPMN. Sometimes, high-grade PanINs are identified at resection, but usually the high-grade PanIN is not the imaging abnormality that led to surgical intervention. Molecular imaging approaches95 and pancreatic juice analysis96,97 have attempted to improve the detection of pancreatic cancer and potentially high-grade PanIN, but more research is needed. Improving detection of microscopic PanINs will require expanding our tools beyond standard imaging, and could be one area where leveraging artificial intelligence to detect subtle changes and integrating these imaging features with clinical and biomarker data will help identify those most likely to progress.98 Although we are closer to achieving consensus regarding which lesions warrant surgical intervention, the subtleties involved in assessing imaging characteristics necessitate that pancreatic surveillance be coordinated through centers with multidisciplinary expertise.50,52,74
Where Are the Gaps in Pancreatic Surveillance?
How can we improve surveillance? Recent studies demonstrate that screen-detected cancers are more likely to be diagnosed at early stage and be surgically resectable, with longer survival. However, some PDACs are interval cancers detected less than 1 year after an unremarkable imaging test.68,76 A study evaluating the performance of consensus guidelines for predicting the yield of surgical resection found suboptimal sensitivity for identifying high-grade dysplasia or invasive cancer in pancreatic cysts (only 40% for Fukuoka and 60% for CAPS).99 Furthermore, a small percentage of individuals undergoing pancreatic surveillance (5 in 5081 patient-years in the most recent CAPS analysis68) will undergo a surgery for what turns out to be low-grade dysplasia. The potential for morbidity from pancreatic surgery is one of the potential harms of pancreatic surveillance, but one that can be reduced by improving the sensitivity and specificity of the tests we employ in pancreatic surveillance. Other potential harms include the impact of yearly invasive tests and the ensuing abnormal findings (tiny IPMNs) on patients’ quality of life; although initial studies have not identified significant problems with anxiety associated with participation in pancreatic surveillance.100 Additional longitudinal studies with extended follow-up of surveillance participants will make it possible to address whether the experience with pancreatic surveillance as conducted through tertiary care centers with multidisciplinary expertise is generalizable to real-world settings.
How do we reach the patients most likely to benefit from pancreatic surveillance? Modeling studies have found that surveillance using either EUS or MRI meets cost-effectiveness thresholds for individuals at high risk for PDAC (>5× risk increase)101,102; however, insurance coverage for pancreatic surveillance with EUS and MRI is variable, with coverage more uniform for those with pancreatic abnormalities. Expertise in pancreatic surveillance is still not widely accessible and high-risk individuals must have the knowledge, motivation, and resources to search out centers and/or research studies. Pancreatic surveillance has a higher yield for individuals with genetic susceptibility. Despite having higher incidence rates for pancreatic cancer than White Americans, Black Americans are less likely to undergo genetic testing and remain underrepresented in pancreatic surveillance cohorts. Operationalizing universal germline genetic testing for all pancreatic cancer patients, developing and implementing algorithms that integrate electronic health record data and prediction models to calculate an individual’s risk for pancreatic cancer,98 and expanding access to pancreatic surveillance will identify more high-risk individuals who may benefit from surveillance and contribute data to evaluate effectiveness of interventions.
Pancreatic Surveillance: Imaging Plus Biomarkers
Because pancreatic cancer is a relatively rare disease, even an excellent screening test with excellent sensitivity at 99% specificity applied to the general population would result in an unacceptable number of false-positive results. One compelling strategy to improve the sensitivity and specificity of imaging for detection of advanced precursor lesions (PanIN-III or cystic neoplasm with high-grade dysplasia) or stage I PDAC is the use of biomarkers. Although there are a number of potential blood and tissue-based biomarkers for PDAC early detection that are being investigated, for the majority, further data are needed to better define their clinical utility in the management of high-risk individuals. Potential applications for these biomarkers include decreased use of health care resources via less frequent imaging, identification of the aforementioned interval PDACs, and decreasing unnecessary surgery (ie, benign pancreatic lesions or IPMNs with low-grade dysplasia).
The use of biomarkers in the care of patients with cystic lesions is rapidly becoming a part of standard clinical practice. In particular, genomic-based biomarkers have demonstrated great promise in stratifying cysts by risk for malignant potential—those cysts like serous cystadenomas or non-neoplastic cysts that can be forgotten or those cysts that need to be followed or resected, such as IPMNs, MCNs, or pancreatic neuroendocrine tumors.103–106 A recent prospective multicenter study104 reported on the results of next-generation sequencing of cyst fluid obtained from patients with pancreatic cysts to determine its diagnostic performance in real time. The cohort consisted of 1216 patients, of which approximately 20% had surgical follow-up. The use of next-generation sequencing cyst fluid analysis in the surgical cohort had better diagnostic performance for distinguishing mucinous vs nonmucinous cysts, as well as the presence of advanced neoplasia (88% sensitivity, 98% specificity, 97% positive predictive value, and 93% negative predictive value) compared with current cyst guidelines and other modalities, including cytology and carcinoembryonic antigen levels. None of the 965 patients who did not undergo surgery developed a malignancy, with a median follow-up of 23 months. There was also significant improvement in the identification of other cyst types. Their results demonstrate the promise of using targeted next-generation sequencing in the management of patients with pancreatic cystic lesions.
CA19-9 is the most commonly used blood-based biomarker for pancreatic cancer surveillance; however, it is important to note that its US Food and Drug Administration approval was based on its performance for monitoring response to treatment of PDAC. When CA19-9 has been evaluated as a potential screening test for PDAC, its performance has been considered suboptimal. However, recent studies have reported that CA19-9’s diagnostic performance improved diagnostic sensitivity for resectablestage PDAC (from 53% to 61%, at 99% specificity) when it is combined with a tumor marker gene test that identifies variants in the enzymes involved in CA19-9 synthesis.107
Multiple studies have sought to add markers to CA19-9 to improve diagnostic performance.88 A recent meta-analysis reported that the use of additional protein biomarkers to CA19-9 did not meaningfully improve the AUC and resulted in only minor increases in clinical utility.108 Favorable results from a case-control cohort evaluating a multiplexed blood-based test immunoassay of 8 serum biomarkers combined with CA19-9 (IMMray; Immunovia, Marlborough, MA) has led to their offering their test commercially. They reported that their assay distinguished stage I/II sporadic PDAC cases from control high-risk individuals with sensitivity of 85% and specificity of 98% after excluding borderline results. When those samples with a low level of CA19-9 of ≤2.5 U/mL (presumed nonexpressors) were excluded, sensitivity increased from 85% to 89% in patients with stage I/II PDAC with no change in specificity.109
“Liquid biopsy” detecting circulating tumor DNA (ctDNA) has been evaluated as a diagnostic test in multiple studies, including for pancreatic cancer.110 In 1 study, ctDNA analyzing for KRAS mutations together with 4 protein biomarkers (ie, CA19-9, carcinoembryonic antigen, hepatocyte growth factor, and osteopontin) detected 64% of PDAC (stage I–III), with specificity of 99.5%.111 A comprehensive multianalyte test for detection of multiple cancer types (CancerSEEK; Exact Sciences) replicated these findings (sensitivity of 64% for PDAC).110 However, as a stand-alone test, somatic mutation–based ctDNA tests have suboptimal performance for early-stage PDAC, with a diagnostic sensitivity of only approximately 30%–40%.111,112
Other ctDNA tests that detect methylated or hydroxymethylated DNA have been evaluated for their potential to detect early-stage PDAC. These tests have promising diagnostic characteristics as multicancer detection tests, but detecting early-stage pancreatic cancer, especially stage I disease, is a greater challenge than detecting many other cancer types. For this reason, more evidence of their diagnostic performance in prospective studies is needed.113 Although there are biomarker tests that are commercially available in the United States, due to lack of clinical utility data in high-risk individuals at risk for PDAC development, at the present time, none of the commercially available blood-based biomarker tests for PDAC are covered by insurance or are included in any PDAC surveillance guidelines.
Based on data regarding associations between diabetes and PDAC risk, many experts have proposed using serial measurement of fasting blood glucose or hemoglobin A1C as a surveillance tool for high-risk individuals. For example, 75% of experts in the International CAPS Consortium felt that routine testing for diabetes should be included in the surveillance protocol for high-risk individuals74 and the CAPS guidelines include measurement of fasting blood glucose or hemoglobin A1C at baseline evaluation and during annual follow-up.
Clinical Implementation to Reduce Morbidity From Pancreatic Ductal Adenocarcinomas
As our understanding of the pathogenesis of pancreatic neoplasia continues to advance, we have opportunities to translate new discoveries to the clinic. In the therapeutic oncology space, the discovery of more effective treatments for PDACs with defective homologous DNA repair has led to recommendations for universal germline genetic testing with multigene panels.50 Clinical germline genetic testing is increasingly accessible and affordable (multigene panel testing is available through commercial clinical laboratories and is usually covered by insurance, with patient-pay cost <$300); however, the lack of a recommendation for genetic testing on the part of health care providers remains a major barrier. Implementing universal genetic testing at point of care in gastrointestinal oncology clinics can achieve germline genetic testing rates of nearly 80% among patients with PDAC, an increase in uptake that is 3-fold higher compared with usual care.114 The yield of such testing is especially high in those with young-onset PDAC.115 Making a genetic diagnosis in a patient with cancer can not only impact their own oncologic treatment, but can also impact medical management for their at-risk family members. Consequently, for individuals with a family history of PDAC, genetic testing can be offered to close family members (Figure 1).116
Figure 1.
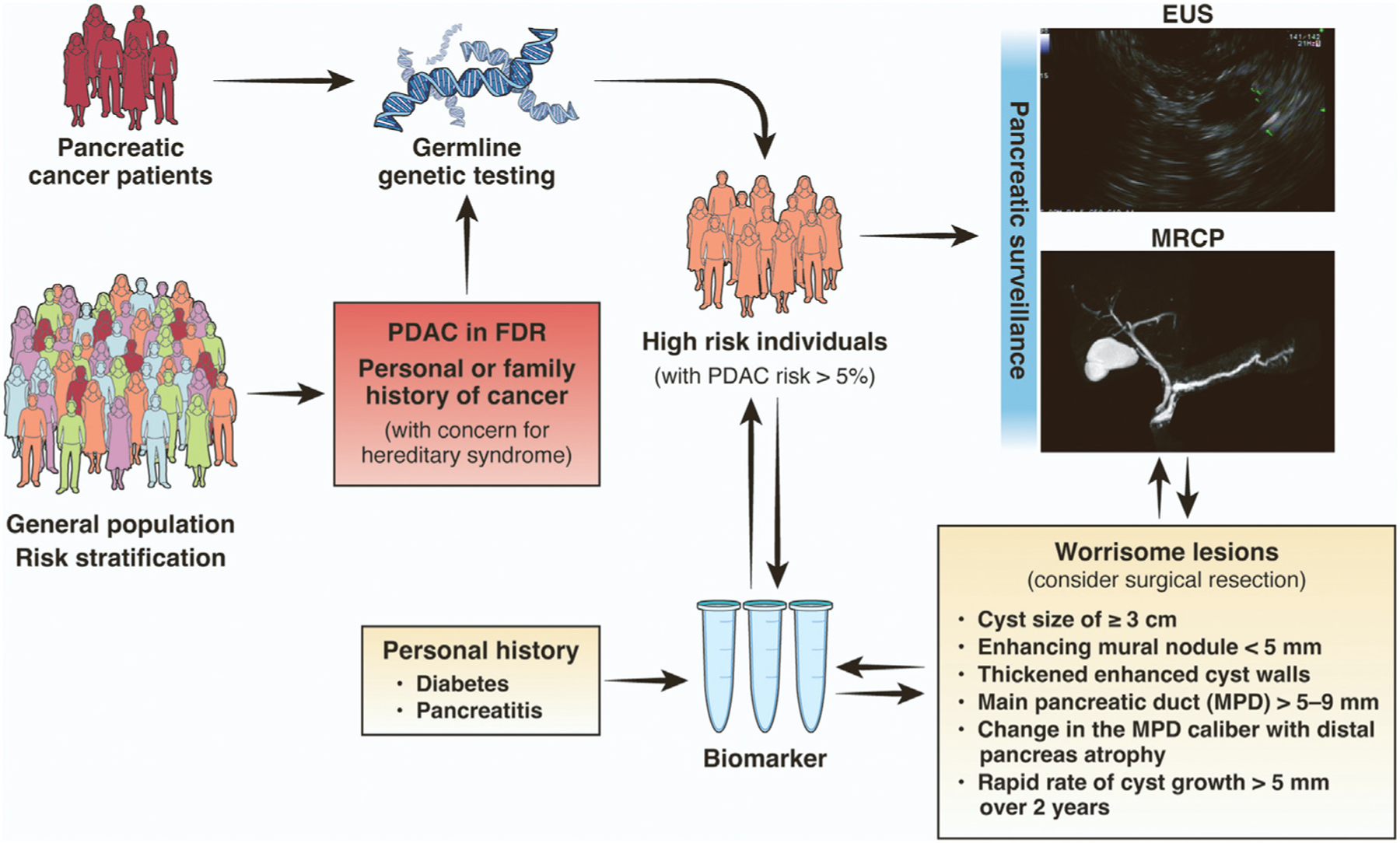
Clinical approach to early detection of pancreatic neoplasia. FDR, first-degree relative.
For specific subgroups of individuals at increased risk for PDAC (particularly those with lifetime risk for PDAC >5% due to genetic susceptibility, chronic pancreatitis, and/or family history meeting criteria for FPC), surveillance offers a chance for early detection of pancreatic neoplasia and improved survival.52 A meta-analysis of 19 published studies of pancreatic surveillance concluded that only 135 high-risk individuals would need to be screened to identify 1 with a high-grade lesion.117 By comparison, screening for colorectal cancer in individuals aged 45–49 years could require screening as many as 1000 average-risk individuals to prevent 1 colorectal cancer–related death in this younger age group.
Immediate clinical strategies likely to reduce morbidity from PDAC include identifying more individuals at increased risk and expanding uptake of PDAC surveillance (Figure 1). With regard to identifying high-risk individuals, assessment of comorbidities and family history of cancer is a necessary first step. As genetic susceptibility syndromes are implicated in only a fraction of PDAC cases, it is likely there are additional risk factors that can inform prediction models that can be used to identify specific individuals (eg, newly diagnosed diabetics) who are most likely to benefit from pancreatic surveillance.118 With regard to initiating pancreatic surveillance, consensus guidelines specify which individuals should undergo surveillance (Table 2) and recommend that this be coordinated through centers with specific expertise. Efforts are underway to expand partnerships between academic centers and community hospitals to expand access to pancreatic surveillance and collaboration among established national and international consortia with standardized surveillance and data collection methods that will ensure high-quality care for high-risk individuals, while also facilitating collection of biospecimens and data on surveillance outcomes.119 There is considerable work to be done to integrate biomarker studies, imaging, pathology findings, and clinical outcomes. A large proportion of pancreatic neoplasms appear to arise from PanINs, which are not visible on imaging, yet a significant proportion of high-risk individuals have positive biomarker tests with negative imaging studies. which presents challenges for clinical management. Efforts are underway to incorporate artificial intelligence and deep learning approaches to improve the current technologies used in pancreatic imaging, as well as advancing biomarker discovery, and identifying individual-level risk factors for progression of pancreatic neoplasia.98
Establishing cohorts of high-risk individuals undergoing pancreatic surveillance will not only advance early detection, but also advance efforts for primary and secondary prevention of pancreatic neoplasia. Use of aspirin and statins has been associated with reduced risk for PDAC in case-control studies, but whether these or other medications can offer protection against development and/or progression of pancreatic neoplasia requires further study.120 Immunoprevention approaches are only beginning to undergo investigation, and a phase I study is underway evaluating the peripheral immune response to a KRAS mutant peptide vaccine administered in the CAPS program.121 Ensuring that individuals at high risk for PDAC are identified in time to offer interventions that could change the natural history of this deadly disease is a clinical goal that is within our reach.
Funding
Grants NIH/NCI R01CA176828 and U01CA210170 were awarded to Michael Goggins.
Abbreviations used in this paper:
- CA19-9
carbohydrate antigen 19–9
- CAPS
Cancer of the Pancreas Screening Study
- CT
computed tomography
- ctDNA
circulating tumor DNA
- EUS
endoscopic ultrasound
- FPC
familial pancreatic cancer
- IPMN
intraductal papillary mucinous neoplasm
- MCN
mucinous cystic neoplasm
- MRCP
magnetic resonance cholangiopancreatography
- NOD
new-onset diabetes
- PanIN
pancreatic intraepithelial neoplasia
- PARPi
poly (ADP-ribose) polymerase inhibitor
- PDAC
pancreatic ductal adenocarcinoma
- PGV
pathogenic germline variant
- USPSTF
US Preventive Services Task Force
Biographies



Footnotes
Conflicts of interest The authors disclose no conflicts.
References
- 1.National Cancer Institute. Surveillance, Epidemiology and End Results Program. Available at: www.seer.cancer.gov. Accessed February 10, 2023.
- 2.Blackford AL, Canto MI, Klein AP, et al. Recent trends in the incidence and survival of stage 1A pancreatic cancer: a Surveillance, Epidemiology, and End Results analysis. J Natl Cancer Inst 2020;112:1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 2018; 267:936–945. [DOI] [PubMed] [Google Scholar]
- 4.Honselmann KC, Pergolini I, Castillo CF, et al. Timing but not patterns of recurrence is different between node-negative and node-positive resected pancreatic cancer. Ann Surg 2020;272:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair AB, Yin LD, Pu N, et al. Recurrence in patients achieving pathological complete response after neoadjuvant treatment for advanced pancreatic cancer. Ann Surg 2021;274:162–169. [DOI] [PubMed] [Google Scholar]
- 6.Ilic I, Ilic M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: a joinpoint regression analysis. World J Gastroenterol 2022;28:4698–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaddam S, Abboud Y, Oh J, et al. Incidence of pancreatic cancer by age and sex in the US, 2000–2018. JAMA 2021;326:2075–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang BZ, Liu L, Zhang J, et al. Rising incidence and racial disparities of early-onset pancreatic cancer in the United States, 1995–2018. Gastroenterology 2022; 163:310–312.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol 2021;18:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavakkoli A, Singal AG, Waljee AK, et al. Racial disparities and trends in pancreatic cancer incidence and mortality in the United States. Clin Gastroenterol Hepatol 2020;18:171–178.e10. [DOI] [PubMed] [Google Scholar]
- 11.Ogobuiro I, Collier AL, Khan K, et al. Racial disparity in pathologic response following neoadjuvant chemotherapy in resected pancreatic cancer: a multi-institutional analysis from the Central Pancreatic Consortium. Ann Surg Oncol 2023;30:1485–1494. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 13.Wood LD, Yurgelun MB, Goggins MG. Genetics of familial and sporadic pancreatic cancer. Gastroenterology 2019;156:2041–2055. [DOI] [PubMed] [Google Scholar]
- 14.The Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 2017;32:185–203.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–753. [DOI] [PubMed] [Google Scholar]
- 17.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waclaw B, Bozic I, Pittman ME, et al. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature 2015;525:261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Blackford A, Dal Molin M, et al. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut 2015;64:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhalf W, Levy P, Gress T, et al. International consensus guidelines on surveillance for pancreatic cancer in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology 2020;20:910–918. [DOI] [PubMed] [Google Scholar]
- 21.Shelton CA, Umapathy C, Stello K, et al. Hereditary pancreatitis in the United States: survival and rates of pancreatic cancer. Am J Gastroenterol 2018;113:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi S, de la Fuente J, Murad MH, et al. Chronic pancreatitis is a risk factor for pancreatic cancer, and incidence increases with duration of disease: a systematic review and meta-analysis. Clin Transl Gastroenterol 2022;13:e00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Wang Y, Yu Y. Meta-analysis reveals an association between acute pancreatitis and the risk of pancreatic cancer. World J Clin Cases 2020;8:4416–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallensleben ND, Umans DS, Bouwense SA, et al. The diagnostic work-up and outcomes of ‘presumed’ idiopathic acute pancreatitis: a post-hoc analysis of a multicentre observational cohort. United European Gastroenterol J 2020;8:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012; 23:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naudin S, Li K, Jaouen T, et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer 2018;143:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farias AJ, Streicher SA, Stram DO, et al. Racial/ethnic disparities in weight or BMI change in adulthood and pancreatic cancer incidence: the multiethnic cohort. Cancer Med 2021;10:4097–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–929. [DOI] [PubMed] [Google Scholar]
- 29.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol 2006;4:1366–1272; quiz 1301. [DOI] [PubMed] [Google Scholar]
- 31.Huang BZ, Pandol SJ, Jeon CY, et al. New-onset diabetes, longitudinal trends in metabolic markers, and risk of pancreatic cancer in a heterogeneous population. Clin Gastroenterol Hepatol 2020;18:1812–1821.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singhi AD, Koay EJ, Chari ST, et al. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology 2019;156:2024–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A, Kandlakunta H, Nagpal SJS, et al. Model to determine risk of pancreatic cancer in patients with new-onset diabetes. Gastroenterology 2018;155:730–739.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setiawan VW, Stram DO, Porcel J, et al. Pancreatic cancer following incident diabetes in African Americans and Latinos: the Multiethnic Cohort. J Natl Cancer Inst 2019;111:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu BU, Lustigova E, Chen Q, et al. Imaging of the pancreas in new-onset diabetes: a prospective pilot study. Clin Transl Gastroenterol 2022;13:e00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maitra A, Sharma A, Brand RE, et al. A prospective study to establish a new-onset diabetes cohort: from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 2018; 47:1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adsay NV. Cystic neoplasia of the pancreas: pathology and biology. J Gastrointest Surg 2008;12:401–404. [DOI] [PubMed] [Google Scholar]
- 39.Vege SS, Ziring B, Jain R, et al. American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:819–822; quiz e12–e13. [DOI] [PubMed] [Google Scholar]
- 40.Elta GH, Enestvedt BK, Sauer BG, et al. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol 2018;113:464–479. [DOI] [PubMed] [Google Scholar]
- 41.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi C, Klein AP, Goggins M, et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res 2009;15:7737–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 2017;35:3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu C, Hart SN, Polley EC, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018; 319:2401–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yurgelun MB, Chittenden AB, Morales-Oyarvide V, et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med 2019; 21:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowery MA, Wong W, Jordan EJ, et al. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst 2018; 110:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:439–457. [DOI] [PubMed] [Google Scholar]
- 49.Daly MB, Pal T, AlHilli Z, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 3.2023. National Comprehensive Cancer Network. February 13, 2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed February 10, 2023. [Google Scholar]
- 50.Stoffel EM, McKernin SE, Brand R, et al. Evaluating susceptibility to pancreatic cancer: ASCO provisional clinical opinion. J Clin Oncol 2019;37:153–164. [DOI] [PubMed] [Google Scholar]
- 51.Maxwell KN, Domchek SM, Nathanson KL, et al. Population frequency of germline BRCA1/2 mutations. J Clin Oncol 2016;34:4183–4185. [DOI] [PubMed] [Google Scholar]
- 52.Sawhney MS, Calderwood AH, Thosani NC, et al. ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: summary and recommendations. Gastrointest Endosc 2022;95:817–826. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Silvestri V, Leslie G, et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J Clin Oncol 2022;40:1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med 2021;384:440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu FC, Roberts NJ, Childs E, et al. Risk of pancreatic cancer among individuals with pathogenic variants in the ATM gene. JAMA Oncol 2021;7:1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klatte DCF, Boekestijn B, Wasser M, et al. Pancreatic cancer surveillance in carriers of a germline CDKN2A pathogenic variant: yield and outcomes of a 20-year prospective follow-up. J Clin Oncol 2022;40:3267–3277. [DOI] [PubMed] [Google Scholar]
- 57.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moller P, Seppala TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 2018;67:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamura K, Yu J, Hata T, et al. Mutations in the pancreatic secretory enzymes CPA1 and CPB1 are associated with pancreatic cancer. Proc Natl Acad Sci U S A 2018; 115:4767–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawamoto M, Kohi S, Abe T, et al. Endoplasmic stress-inducing variants in CPB1 and CPA1 and risk of pancreatic cancer: a case-control study and meta-analysis. Int J Cancer 2022;150:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447–1453. [DOI] [PubMed] [Google Scholar]
- 62.Laken SJ, Petersen GM, Gruber SB, et al. Familial colorectal cancer in Ashkenazim due to a hypermutable tract in APC. Nat Genet 1997;17:79–83. [DOI] [PubMed] [Google Scholar]
- 63.Roberts NJ, Norris AL, Petersen GM, et al. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov 2016; 6:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jermusyk A, Zhong J, Connelly KE, et al. A 584 bp deletion in CTRB2 inhibits chymotrypsin B2 activity and secretion and confers risk of pancreatic cancer. Am J Hum Genet 2021;108:1852–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klein AP, Wolpin BM, Risch HA, et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun 2018;9:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolpin BM, Rizzato C, Kraft P, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet 2014;46:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma S, Tapper WJ, Collins A, et al. Predicting pancreatic cancer in the UK Biobank Cohort using polygenic risk scores and diabetes mellitus. Gastroenterology 2022;162:1665–1674.e2. [DOI] [PubMed] [Google Scholar]
- 68.Dbouk M, Katona BW, Brand RE, et al. The Multicenter Cancer of Pancreas Screening Study: impact on stage and survival. J Clin Oncol 2022:JCO2200298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for pancreatic cancer: US Preventive Services Task Force reaffirmation recommendation statement. JAMA 2019;322:438–444. [DOI] [PubMed] [Google Scholar]
- 70.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med 1999;131:247–255. [DOI] [PubMed] [Google Scholar]
- 71.Meckler KA, Brentnall TA, Haggitt RC, et al. Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am J Surg Pathol 2001;25:1047–1053. [DOI] [PubMed] [Google Scholar]
- 72.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 2006; 30:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 73.Canto MI, Kerdsirichairat T, Yeo CJ, et al. Surgical outcomes after pancreatic resection of screening-detected lesions in individuals at high risk for developing pancreatic cancer. J Gastrointest Surg 2019; 115:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aslanian HR, Lee JH, Canto MI. AGA clinical practice update on pancreas cancer screening in high-risk individuals: expert review. Gastroenterology 2020;159:358–362. [DOI] [PubMed] [Google Scholar]
- 76.Overbeek KA, Goggins MG, Dbouk M, et al. Timeline of development of pancreatic cancer and implications for successful early detection in high-risk individuals. Gastroenterology 2022;162:772–785.e4. [DOI] [PubMed] [Google Scholar]
- 77.Abe T, Blackford AL, Tamura K, et al. Deleterious germline mutations are a risk factor for neoplastic progression among high risk individuals undergoing pancreatic surveillance. J Clin Oncol 2019;37:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Overbeek KA, Levink IJM, Koopmann BDM, et al. Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut 2022;71:1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calderwood AH, Sawhney MS, Thosani NC, et al. American Society for Gastrointestinal Endoscopy guideline on screening for pancreatic cancer in individuals with genetic susceptibility: methodology and review of evidence. Gastrointest Endosc 2022; 95:827–854.e3. [DOI] [PubMed] [Google Scholar]
- 80.Porter N, Laheru D, Lau B, et al. Risk of pancreatic cancer in the long-term prospective follow-up of familial pancreatic cancer kindreds. J Natl Cancer Inst 2022; 114:1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142:796–804; quiz e14–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harinck F, Konings IC, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016;65:1505–1513. [DOI] [PubMed] [Google Scholar]
- 84.Hoogenboom SA, Engels MML, Chuprin AV, et al. Prevalence, features, and explanations of missed and misinterpreted pancreatic cancer on imaging: a matched case-control study. Abdom Radiol (N Y) 2022; 47:4160–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toshima F, Watanabe R, Inoue D, et al. CT abnormalities of the pancreas associated with the subsequent diagnosis of clinical stage I pancreatic ductal adenocarcinoma more than 1 year later: a case-control study. AJR Am J Roentgenol 2021;217:1353–1364. [DOI] [PubMed] [Google Scholar]
- 86.Singh DP, Sheedy S, Goenka AH, et al. Computerized tomography scan in pre-diagnostic pancreatic ductal adenocarcinoma: stages of progression and potential benefits of early intervention: a retrospective study. Pancreatology 2020;20:1495–1501. [DOI] [PubMed] [Google Scholar]
- 87.Vasen HFA, Boekestijn B, Ibrahim IS, et al. Dilatation of the main pancreatic duct as first manifestation of small pancreatic ductal adenocarcinomas detected in a hereditary pancreatic cancer surveillance program. HPB (Oxford) 2019;21:1371–1375. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka H, Tamura K, Abe T, et al. Serum carboxypeptidase activity and genotype-stratified CA19-9 to detect early-stage pancreatic cancer. Clin Gastroenterol Hepatol 2022;20:2267–2275.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miura S, Takikawa T, Kikuta K, et al. Focal parenchymal atrophy of the pancreas is frequently observed on pre-diagnostic computed tomography in patients with pancreatic cancer: a case-control study. Diagnostics (Basel) 2021;11:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weisberg EM, Chu LC, Park S, et al. Deep lessons learned: radiology, oncology, pathology, and computer science experts unite around artificial intelligence to strive for earlier pancreatic cancer diagnosis. Diagn Interv Imaging 2020;101:111–115. [DOI] [PubMed] [Google Scholar]
- 91.Danai LV, Babic A, Rosenthal MH, et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 2018;558:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Canto MI, Almario JA, Schulick RD, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 2018;155:740–751.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vasen H, Ibrahim I, Ponce CG, et al. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long-term prospective follow-up studies from three european expert centers. J Clin Oncol 2016;34:2010–2019. [DOI] [PubMed] [Google Scholar]
- 94.Chhoda A, Vodusek Z, Wattamwar K, et al. Late-stage pancreatic cancer detected during high-risk individual surveillance: a systematic review and meta-analysis. Gastroenterology 2022;162:786–798. [DOI] [PubMed] [Google Scholar]
- 95.Hausner SH, Bold RJ, Cheuy LY, et al. Preclinical development and first-in-human imaging of the integrin α(v)β(6) with [(18)F]α(v)β(6)-binding peptide in metastatic carcinoma. Clin Cancer Res 2019;25:1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suenaga M, Dudley B, Karloski E, et al. The effect of pancreatic juice collection time on the detection of KRAS mutations. Pancreas 2018;47:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suenaga M, Yu J, Shindo K, et al. Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clin Cancer Res 2018;24:2963–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kenner B, Chari ST, Kelsen D, et al. Artificial intelligence and early detection of pancreatic cancer: 2020 summative review. Pancreas 2021;50:251–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dbouk M, Brewer Gutierrez OI, Lennon AM, et al. Guidelines on management of pancreatic cysts detected in high-risk individuals: an evaluation of the 2017 Fukuoka guidelines and the 2020 International Cancer of the Pancreas Screening (CAPS) consortium statements. Pancreatology 2021;21:613–621. [DOI] [PubMed] [Google Scholar]
- 100.Konings IC, Harinck F, Kuenen MA, et al. Factors associated with cancer worries in individuals participating in annual pancreatic cancer surveillance. Fam Cancer 2017;16:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Corral JE, Das A, Bruno MJ, et al. Cost-effectiveness of pancreatic cancer surveillance in high-risk individuals: an economic analysis. Pancreas 2019;48:526–536. [DOI] [PubMed] [Google Scholar]
- 102.Kumar S, Saumoy M, Oh A, et al. Threshold analysis of the cost-effectiveness of endoscopic ultrasound in patients at high risk for pancreatic ductal adenocarcinoma. Pancreas 2021;50:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018;67:2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paniccia A, Polanco PM, Boone BA, et al. Prospective, multi-institutional, real-time next-generation sequencing of pancreatic cyst fluid reveals diverse genomic alterations that improve the clinical management of pancreatic cysts. Gastroenterology 2023;164:117–133.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haeberle L, Schramm M, Goering W, et al. Molecular analysis of cyst fluids improves the diagnostic accuracy of pre-operative assessment of pancreatic cystic lesions. Sci Rep 2021;11:2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abe T, Kohi C, Kohi S, et al. Gene variants that affect levels of circulating tumor markers increase identification of patients with pancreatic cancer. Clin Gastroenterol Hepatol 2020;18:1161–1169.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boyd LNC, Ali M, Leeflang MMG, et al. Diagnostic accuracy and added value of blood-based protein biomarkers for pancreatic cancer: a meta-analysis of aggregate and individual participant data. EClinicalMedicine 2023;55:101747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brand RE, Persson J, Bratlie SO, et al. Detection of early-stage pancreatic ductal adenocarcinoma from blood samples: results of a multiplex biomarker signature validation study. Clin Transl Gastroenterol 2022;13(3):e00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018;359:926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A 2017;114:10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Macgregor-Das A, Yu J, Tamura K, et al. Detection of circulating tumor DNA in patients with pancreatic cancer using digital next-generation sequencing. J Mol Diagn 2020;22:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brand RE, Nolen BM, Zeh HJ, et al. Serum biomarker panels for the detection of pancreatic cancer. Clin Cancer Res 2011;17:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Everett JN, Dettwyler SA, Jing X, et al. Impact of comprehensive family history and genetic analysis in the multidisciplinary pancreatic tumor clinic setting. Cancer Med 2022;12:2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Varghese AM, Singh I, Singh R, et al. Early-onset pancreas cancer: clinical descriptors, genomics, and outcomes. J Natl Cancer Inst 2021;113:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Furniss CS, Yurgelun MB, Ukaegbu C, et al. Novel models of genetic education and testing for pancreatic cancer interception: preliminary results from the GENERATE study. Cancer Prev Res (Phila) 2021;14:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Corral JE, Mareth KF, Riegert-Johnson DL, et al. Diagnostic yield from screening asymptomatic individuals at high risk for pancreatic cancer: a meta-analysis of cohort studies. Clin Gastroenterol Hepatol 2019;17:41–53. [DOI] [PubMed] [Google Scholar]
- 118.Kenner BJ, Abrams ND, Chari ST, et al. Early detection of pancreatic cancer: applying artificial intelligence to electronic health records. Pancreas 2021;50:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonda TA, Everett JN, Wallace M, et al. Recommendations for a more organized and effective approach to the early detection of pancreatic cancer from the PRECEDE (Pancreatic Cancer Early Detection) Consortium. Gastroenterology 2021; 161:1751–1757. [DOI] [PubMed] [Google Scholar]
- 120.Miller MS, Allen P, Brentnall TA, et al. Pancreatic cancer chemoprevention translational workshop: meeting report. Pancreas 2016;45:1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haldar SD, Judkins C, Ferguson A, et al. A phase I study of a mutant KRAS-targeted long peptide vaccine in patients at high risk of developing pancreatic cancer. JCO 2023;41(4 Suppl):TPS758. [Google Scholar]


