Abstract
Cells must adjust their redox state to an ever-changing environment that could otherwise result in compromised homeostasis. An obvious way to adapt to changing redox conditions depends on cysteine post-translational modifications (PTMs) to adapt conformation, localization, interactions and catalytic activation of proteins. Such PTMs should occur preferentially in the proximity of oxidative stress sources. A particular concentration of these sources is found near membranes where the endoplasmic reticulum (ER) and the mitochondria interact on domains called MERCs (Mitochondria-Endoplasmic Reticulum Contacts). Here, fine inter-organelle communication controls metabolic homeostasis. MERCs achieve this goal through fluxes of Ca2+ ions and inter-organellar lipid exchange. Reactive oxygen species (ROS) that cause PTMs of mitochondria-associated membrane (MAM) proteins determine these intertwined MERC functions. Chronic changes of the pattern of these PTMs not only control physiological processes such as the circadian clock but could also lead to or worsen many human disorders such as cancer and neurodegenerative diseases.
Keywords: endoplasmic reticulum, mitochondria, redox, cysteines
Introduction
Interactions between mitochondria and the Endoplasmic Reticulum (ER) were discovered in 1952 using electron microscopy of rat liver, where contacts between these two organelles depend on the nutritional status of the animal (Bernhard et al., 1952). This insight suggests that material exchange could occur in a controlled manner on these contacts, a hypothesis confirmed and characterized with the discovery of "Mitochondria-associated ER membranes" (MAMs) as a lipid transfer platform (Vance, 1990) and a site of Ca2+ flux (Rizzuto et al., 1998). Accordingly, the biochemical MAM isolate contains enzymes necessary for phospholipid synthesis on either side of the mitochondria-ER contact (MERC) structure (Vance, 1991), as well as Ca2+ channels and pumps on the ER and mitochondria that maintain a circular Ca2+ equilibrium (Raffaello et al., 2016). Within the ER, this equilibrium determines oxidative protein folding through Ca2+-dependent chaperones (Margittai and Sitia, 2011; Braakman and Hebert, 2013), while within mitochondria, it controls energy production and apoptosis through Krebs cycle dehydrogenases and the permeability transition pore, respectively (Bauer and Murphy, 2020). Moreover, MERCs fulfill structural roles in mitochondrial fusion and fission mechanisms (Friedman et al., 2011), in autophagosome formation (Hamasaki et al., 2013) and as a lipid synthesis hub that may eventually foster lipid droplets (Vance, 2014). Consistent with this array of functions, the MERC proteome is now recognized to comprise ER Ca2+ release channels (e.g., inositol 1,4,5 trisphosphate receptor type 3, IP3R3), ER Ca2+ uptake pumps, ER protein folding enzymes (e.g., calnexin), mitochondrial Ca2+ handling proteins (e.g., voltage dependent anion channel 1, VDAC1), mitochondrial fission and fusion mediators (e.g., dynamin-related protein 1, Drp1, mitofusin-2), lipid metabolizing enzymes (e.g., acetyl-CoA acetyltransferase, ACAT1) and proteins involved in autophagosome formation (e.g., Rab32) (Ilacqua et al., 2017). The MERC functions and its proteome determine how closely the respective membranes approach each other within a distance range of 0 to 100 nm (Giacomello and Pellegrini, 2016).
MERC-localized proteins can undergo posttranslational modifications (PTMs) such as phosphorylation or palmitoylation, which have functional implications. For instance, calnexin phosphorylation dependent on protein kinase CK2 and ERK determines its interaction with phosphofurin acidic cluster sorting protein 2 (PACS-2), a critical regulator of MERC formation (Simmen et al., 2005) that controls the extent of calnexin MAM enrichment (Myhill et al., 2008). Similarly, calnexin palmitoylation also promotes its enrichment to MAMs (Lynes et al., 2012). Recent research has identified redox-controlled PTMs on cysteines as a novel key determinant of MERC function and formation.
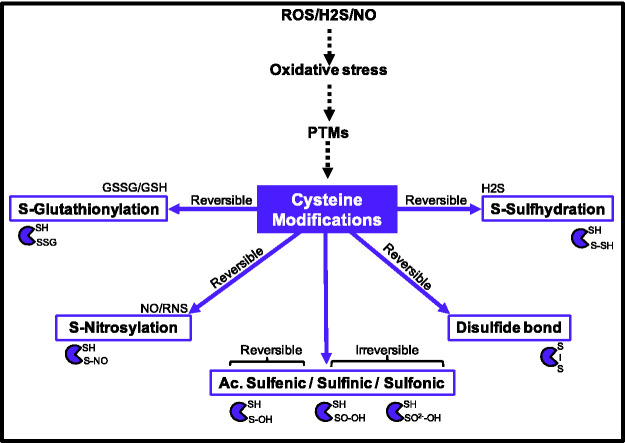
Cysteines account for 2% of the total amino acid content of cells, which is the lowest number for all amino acids, but they are highly conserved and undergo oxidation and reduction (Miseta and Csutora, 2000). This suggests important biological roles for these amino acid residues such as redox-dependent modification and the complexing of metal ligands (Pace and Weerapana, 2013). Cysteine oxidation includes enzyme-mediated disulfide bond formation that generally enhances the structural activity and the folding of proteins (Lo Conte and Carroll, 2013). However, cysteines are also targets of oxidizing PTMs mediated by reactive chemicals (e.g., reactive oxygen species, ROS, reactive nitrogen species, RNS) (Chung et al., 2013), and by oxygen free radicals (Figure 1) (Takashima et al., 2012). Proteomic studies have listed the peptides undergoing such modifications, many of which found inside mitochondria and the ER (Finelli, 2020).
Figure 1.
Overview of cysteine post-translational modifications (PTMs).
GSH: Glutathione; GSSG: Glutathione disulfide; H2O2: Hydrogen peroxide; H2S: Hydrogen Sulfide; NO: Nitric Oxide; PTMs: post-translational modifications; RNS: reactive nitrogen species; ROS: reactive oxygen species.
ROS and RNS-based cysteine modifications give rise to thiol-based redox regulation, whose importance competes with phosphorylation-based regulation. Cysteine oxidative modifications include reversible sulfenic acid (-SOH), sulfinic acid (-SO2H) and irreversible sulfonic acid (-SO3H). The partial reversibility of these oxidation-based PTMs highlights the labile aspect of these modifications, but also their role as on/off switches (Garcia-Santamarina et al., 2014). Preventing cysteine oxidation, peroxiredoxins scavenge ROS (Rhee, 2016). Once cysteines are oxidized, reduced forms of NADPH, glutathione, cysteine and thioredoxin can remove these PTMs (Miller et al., 2018). Particularly important for the maintenance of this function is the ratio between oxidized and reduced glutathione (GSSG, GSH), which is very responsive to changes in ROS (Mailloux, 2020). Glutathione can also act as a PTM itself via spontaneous or enzymatic modification of cysteines by ER-localized glutathione S-transferase (GST). Substrates of this enzyme include for instance ER chaperones like calnexin or immunoglobulin binding protein/glucose-regulated protein of 78 kDa (BiP/GRP78) (Ye et al., 2017; Scire et al., 2019). In contrast, glutaredoxins (GRX) 1 and 2 deglutathionylate these cysteine residues (Matsui et al., 2020).
Additionally, H2O2 can also react with nitric oxide ( . NO) to yield peroxynitrite, a major RNS (Radi, 2018). RNS are also able to oxidize cysteines without the help of enzymes (Evangelista et al., 2013). Like ROS-based oxidation, these modifications can be reversible or irreversible and typically occur on cysteines with a charged residue in close proximity (Marino and Gladyshev, 2010). In its reversible form, S-nitrosylation is removed by enzymes, including S-glutathione reductase (Rizza and Filomeni, 2017).
Our review will first give an overview on the MERC proteome, followed by a list of MERC-associated cysteine oxidation-dependent PTMs and their functions for lipid and Ca2+ flux. We will also discuss potential cysteine PTM sources and the role of these modifications in disease.
Tethering the Two Membranes of MERCs
The apposition of the ER with mitochondria on MERCs depends on the assembly of protein tethers and their regulatory proteins, including PACS-2 that is required for ER-mitochondria apposition and Ca2+ flux (Simmen et al., 2005) (Figure 2). An example of an ER-mitochondria tethering complex is the interaction between the vesicle-associated membrane protein-associated protein B (VAPB) and protein tyrosine phosphatase interacting protein 51 (PTPIP51), located at the ER and at the outer mitochondrial membrane (OMM), respectively (Stoica et al., 2014; Gomez-Suaga et al., 2017). This complex also plays a role in autophagy. Its formation is disrupted upon activation of the redox-sensitive glycogen synthase kinase 3β (GSK3β) (Stoica et al., 2016).
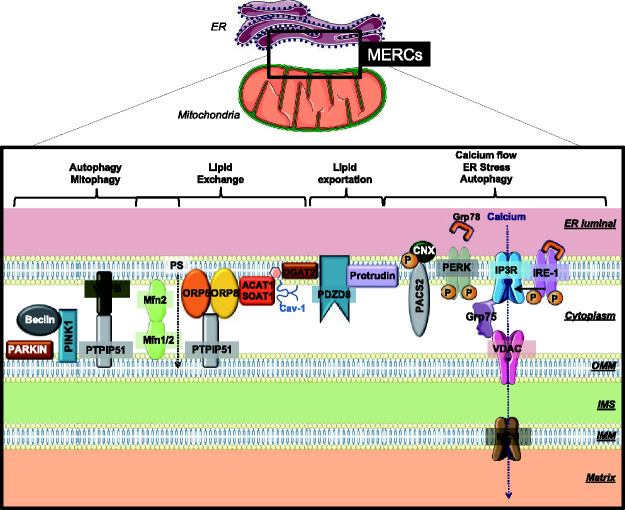
Figure 2.
Main protein complexes controlling mitochondria–ER contact sites (MERCs).
Important tethers and carrier proteins are grouped according to their main function. BAP-31: B-cell receptor-associated protein of 31kDa; CNX: Calnexin; CypD: Cyclophilin D; ER: Endoplasmic Reticulum; Fis1: Mitochondrial Fission 1 Protein; Grp75: glucose-regulated protein of 75kDa (or mortalin/heat shock protein 75); Grp78: glucose-regulated protein of 78 kDa; IMM: inner mitochondrial membrane; IMS: inter-membrane space; IP3R: inositol 1,4,5-trisphosphate receptor; IRE-1: inositol-requiring enzyme 1; MCU: mitochondrial calcium uniporter; MERCs: mitochondria–ER contact sites; Mfn1/2: Mitofusin 1 and 2; OMM: Outer mitochondrial membrane; ORP5-8: oxysterol-binding protein-related protein 5 and 8; P: Phosphorylation; PACS2: Phosphofurin acidic cluster sorting protein 2; PERK: Protein kinase R-like ER kinase; PINK1: PTEN-induced kinase 1; PS: phosphatidylserine; PTPIP51:protein tyrosine phosphatase interacting protein 51; VAPB: vesicle-associated membrane protein-associated protein B; VDAC: voltage-dependent anion channel.
In yeast, the ER-mitochondria encounter structure (ERMES) links ER and mitochondrial membranes, mediating a regulated lipid conduit (Kornmann et al., 2009). As is typical for tethering complexes, its individual components Mmm1, Mdm10, Mdm12 and Mdm34 localize to the ER and mitochondria, respectively and their assembly is controlled by the GTPase Gem1 (Kornmann et al., 2011). Whether this complex also exists in mammalian cells had long been discussed (Wideman et al., 2018). The discovery of the PDZ domain protein PDZD8, an Mmm1 paralog, as a mediator of ER-mitochondria and ER-late endosome-mitochondria contact sites suggests that aspects of this membrane tether are conserved in mammalian cells but its function could be more complex (Hirabayashi et al., 2017). At multi-organelle contact sites, PDZD8 also interacts with ER-localized protrudin and endosomal Rab7 (Elbaz-Alon et al., 2020) and as a synaptotagmin-like mitochondrial lipid-binding proteins (SMP), PDZD8 extracts lipids from the ER and transfers them to late endosomes (Shirane et al., 2020). Another PDZ domain is found within the mitochondrial synaptojanin-2 binding protein (Synj2BP) that has been discovered in a proteomic screen (Hung et al., 2017). Syn2BP forms a tether with the ER-localized ribosome binding protein 1 (RRBP1), but also interacts with the transmembrane and immunoglobulin domain containing protein 1 (TMIGD1) in epithelial cells (Hartmann et al., 2020). ER and mitochondria connections are also under the influence of actin polymerization. ER-bound inverted formin 2 (INF2) catalyzes actin polymerization that promotes the activity of mitochondrial Drp1 and the transfer of Ca2+ from the ER to mitochondria (Korobova et al., 2013; Chakrabarti et al., 2018).
The first characterized MERC protein that controls tethering is mitofusin-2, a dynamin-related GTPase. Mitofusin-2 can form proteinaceous bridges between the ER and mitochondria (de Brito and Scorrano, 2008), but this function could also determine the nature and extent of interactions between mitochondria and the ER (Filadi et al., 2015). Specifically, mitofusin-2 could control the ratio of rough to smooth ER contacts with mitochondria (Wang et al., 2015). In parallel with the MERC-regulatory roles of PACS-2, these interactions are essential for the induction of autophagy with MERCs as the source material (Hamasaki et al., 2013). Moreover, PACS-2 controls an ER-mitochondria protein complex called the ARCosome, which is composed of ER-localized BAP31 and mitochondrial Fis1 (Iwasawa et al., 2011). More tethers may be discovered in the future and the nature and function of these may differ between tissue sources, since multiple proteomic studies have identified different numbers and identities of proteins found within the biochemical MAM isolate (Cho et al., 2017; Hung et al., 2017).
Redox Control of MERC Tethers and Mitochondrial Membrane Dynamics
The tethering of the ER to mitochondria increases upon oxidative stress and relaxes upon homeostatic conditions (Csordas et al., 2006). This oscillating interaction coincides with ER Ca2+ release that then activates mitochondrial oxidative phosphorylation (OXPHOS) associated with a burst of ROS entering the interorganellar cleft (Bravo et al., 2011; Booth et al., 2016). This suggests that some or all of the MERC tethers are under the control of redox PTMs. However, only a few PTMs of MERC tethers are currently known and most of our knowledge is limited to proteins with functions in mitochondrial membrane dynamics, including Drp1 (Friedman et al., 2011) and mitofusin-2 (de Brito and Scorrano, 2008) (Figure 3).
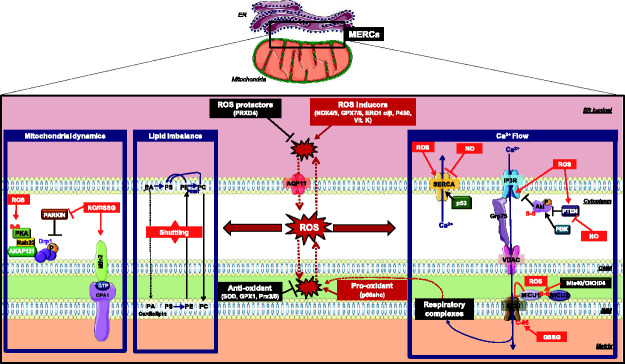
Figure 3.
ROS regulation and cysteine impact on mitochondria–ER contact sites protein players.
Important ROS-sensitive MERC proteins are grouped according to their main function, including mitochondrial dynamics, lipid transfer and Ca2+ flow. ROS sources and sinks are indicated. AKAP121: A kinase anchor protein of 121kDa; AQP11: aquaporin 11; Ca2+: Calcium; Drp1: Dynamin-related protein 1; ER: endoplasmic Reticulum; ERO1α/β: endoplasmic Reticulum oxidoreductin 1 α/β; GPX1: Glutathione peroxidase; GPX7/8: glutathione Peroxidase 7; Grp75: glucose-regulated protein of 75kDa (or mortalin/heat shock protein 75); GSSG: oxidized glutathione; H2O2: hydrogen peroxide; IMM: inner mitochondrial membrane; IMS: inter-membrane space; IP3R: inositol 1,4,5-trisphosphate receptor; MCU: mitochondrial calcium uniporter; MERCs: mitochondria–ER contact sites; Mfn2: mitofusin2; MICU1/2: mitochondrial Ca2+ uptake proteins 1 and 2; NO: nitric Oxide; NOX4/5: NADPH oxidase 4/5; OMM: outer mitochondrial membrane; OPA1:mitochondrial dynamin like GTPase; P53: dellular tumor antigen p53; P450: hemoprotein cytochrome P450; PI3K: Phosphoinositide 3-kinases; PKA: protein kinase A; PRDX4: peroxiredoxin-4; Prx3/5: Peroxiredoxin 3/5; PTEN: phosphatase and TENsin homolog; Rab32: Ras-related protein Rab-32; ROS: reactive oxygen species; SERCA: sarco/endoplasmic reticulum Ca2+-ATPase; SOD: superoxide dismutase.
One example is mitofusin-2, which is subject to ROS-mediated PTMs that determine its role in mitochondrial membrane fusion (Shutt et al., 2012; Mattie et al., 2018). Under oxidizing conditions, an increase of GSSG concentration at MERCs leads to Mfn2 recruitment. Subsequently, glutathionylation of cysteine 684 cooperates with mitofusin-1 to promote mitochondrial fusion (Shutt et al., 2012). Interestingly, such Mfn1-Mfn1 dimers are more than a hundred times stronger than homodimers between Mfn2-Mfn2 (Ishihara et al., 2004). Cysteine PTMs could also impact the role of mitofusin-2 at MERCs, since MERC-originating autophagy accelerates in the presence of ROS (Forte et al., 2017). An additional level of mitofusin redox control derives from the ROS/RNS-mediated activation of c-Jun N-terminal kinases (JNK) (Kamata et al., 2005), which normally promote mitofusin-2 degradation by the proteasome (Leboucher et al., 2012). Therefore, oxidative conditions eventually reduce the amounts of mitofusins.
Opposing mitofusins, the OMM GTPase Drp1 uses receptors to associate with the mitochondrial membrane (Kamerkar et al., 2018) and constrict mitochondria (Friedman et al., 2011). To date, Fis-1 and mitochondrial fission factor (Mff) have been identified as further regulatory proteins whose regulation via oxidation is uncharacterized (Wolf et al., 2020). In contrast, mitochondrial dynamics proteins of 49 or 51 kDa (MID49/MID51) can undergo oligomerization under oxidizing conditions (Zhao et al., 2011). Oxidative stress activates mitochondrial fission in multiple ways. First, it directly recruits and activates Drp1 through S-nitrosylation on cysteine 644, which serves as a trigger for oligomerization (Cho et al., 2009) and further activation via phosphorylation of serine 616 (Lee and Kim, 2018). In the proximity of the ER, a moiety of protein disulfide isomerase (PDI) that localizes to the cytosol could directly inhibit this activity via catalytically reducing oxidized Drp1 (Kim et al., 2018). These activities are likely impaired in the presence of ROS and other mitochondrial compounds, since excess fumarate from mitochondria succinates and inactivates PDI (Manuel et al., 2017). Another connection between Drp1 and cellular redox conditions is mediated by small ubiquitin-like modifier (SUMO), a covalent modification of lysine residues that controls protein-protein interactions (Flotho and Melchior, 2013). In the case of Drp1, this modification is mediated by the MERC-associated mitochondrial-anchored RING finger containing protein (MAPL), a SUMO E3 ligase. Its activity SUMOylates and oligomerizes Drp1, thus increasing MERC formation and ER-mitochondria Ca2+ communication (Braschi et al., 2009; Prudent et al., 2015). SUMO isopeptidases called sentrin-specific proteases (SENPs) remove these modifications. However, they may undergo inactivation via an intramolecular disulfide bond in the presence of ROS (Ferdaoussi et al., 2015). Thus, while SENP5 normally destabilizes Drp1 (Zunino et al., 2009), this may not occur during oxidative stress, resulting in the accumulation of active Drp1. In contrast, SUMO E1 and E2 ligases can become inactivated by reversible oxidation of their catalytic cysteines in the presence of ROS (Bossis and Melchior, 2006). Thus, the interplay between ROS and SUMOylation is complex and warrants further investigation.
Another Drp1-activating mechanism is based on ubiquitination. Parkin, a regulatory protein of mitochondrial membrane dynamics that localizes to MERCs is a cytosolic E3 ubiquitin ligase (Gelmetti et al., 2017). This allows Parkin to target Drp1 for proteasomal degradation (Wang et al., 2011). However, upon its S-nitrosylation, Parkin no longer promotes the degradation of Drp1 (Chung et al., 2004; Gelmetti et al., 2017). Overall, it appears that oxidative stress activates Drp1 to promote mitochondrial fission. This role is particularly important in the central nervous system (CNS), where oxidative stress derived from neurodegeneration coincides with active Drp1 and inactive Parkin (Ge et al., 2020), resulting in compromised mitophagy and increased mitochondrial fragmentation (Cho et al., 2009).
MERCs also influence mitochondria movement along the cytoskeleton with the help of kinesin and dynein (Yi et al., 2004). These two motor proteins interact with the small mitochondrial Rho proteins 1 and 2 (Miro1/2), which are both enriched in MAMs. Their Ca2+-binding EF hand domains detach from kinesin in the presence of high Ca2+, arresting mitochondria movement (Fransson et al., 2006). Consistent with the role of ROS as a booster of Drp1-mediated MAM formation, mitochondrial ROS decrease Miro-mediated movement of mitochondria in a parallel mechanism that is independent of Ca2+ but requires the p38 MAP kinase (Debattisti et al., 2017).
Redox Control of Mitochondria Metabolism Through MERC Ca2+ Transfer
In most cell types, the free mitochondrial Ca2+ concentration of 100 to 200 nM resembles the one found in the cytoplasm. These amounts are, however, about 1000 to 8000 times lower than what is observed in the ER (100 to 800 μM) and remain much lower than the typical extracellular medium that is usually situated at about 2 mM (Rizzuto et al., 2009). However, the mitochondrial free [Ca2+] content undergoes fluctuations dependent on ER Ca2+ release, for instance through the activation of IP3Rs with histamine, which raises mitochondrial free [Ca2+] into the low micromolar range, as summarized by (Fernandez-Sanz et al., 2019). Following its release from the ER, physical interactions between mitochondria and the ER allow for Ca2+ transfer into mitochondria (Rizzuto et al., 1998). Such an increase of mitochondrial [Ca2+] not only promotes the Krebs cycle but can also result in apoptosis (Jouaville et al., 1999; Pinton et al., 2001). The import and release of Ca2+ on the mitochondrial membranes is therefore critical for the control of cell fate and metabolism. Changing redox conditions within and around mitochondria control the functioning of mitochondrial Ca2+ gatekeepers. Of particular interest is the mitochondrial intermembrane space (IMS). This compartment contains large amounts of ROS-consuming or generating enzymes such as superoxide dismutase 1 (SOD1), peroxiredoxins (PRDX) and glutathione peroxidases (GPx) that are able to buffer ROS generated from mitochondrial OXPHOS (Riemer et al., 2015). These groups of enzymes also control the folding of proteins imported into mitochondria from the cytosol via the TOM and TIM complexes (Habich et al., 2019). Within the IMS, a disulfide relay system composed of the oxidoreductase CHCHD4 (known in yeast as Mia40) and the sulfhydryl oxidase ALR (known in yeast as Erv1) catalyzes the correct formation of disulfide bonds of mitochondrial IMS and IMM proteins (Fischer et al., 2013). Consistent with an important role of IMS redox conditions for their folding and functioning, many IMS proteins contain conserved cysteine residues, including mitochondrial Ca2+-handling proteins (Vogtle et al., 2012; Hung et al., 2014). Of particular interest is the redox-sensing protein p66Shc (Giorgio et al., 2005). p66Shc can induce mitochondrial ROS synthesis by sequestering cytochrome c from the respiratory chain (Giorgio et al., 2005) or by increasing the rate of OXPHOS (Lone et al., 2018). At the same time, p66Shc inhibits the expression of antioxidant enzymes such as SOD1 (Lebiedzinska et al., 2009). Under oxidizing conditions, p66Shc uses N-terminal cysteine residues to form a tetramer that promotes mitochondrial permeability transition (Gertz et al., 2008).
Ca2+ enters the IMS through the porin-related VDAC channels, of which VDAC1 is the predominant isoform (De Stefani et al., 2012; Messina et al., 2012). Thus, the OMM-localized VDAC1 can limit redox-regulated mitochondrial Ca2+ import (Figure 4) (Shoshan-Barmatz et al., 2018). VDAC1 contains two redox-responsive cysteine residues, but they do not affect its function, in contrast to VDAC2-3 isoforms (De Pinto et al., 2016). Rather, the redox-sensitive [2S-2Fe] cluster protein mitoNEET can provide a gating function to VDAC1 whose full significance for Ca2+ is currently unclear (Lipper et al., 2019).
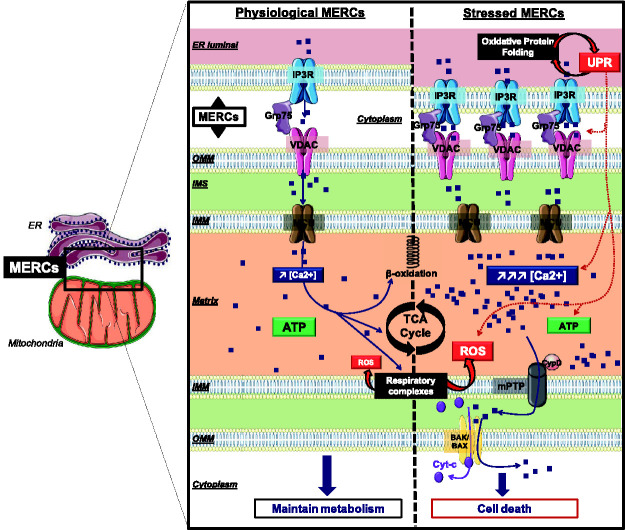
Figure 4.
ROS and stress-dependent Ca2+ signaling at mitochondria–ER contact sites. Distinct ROS signaling patterns adapt the MERC structure to allow for changes in Ca2+ signaling and TCA cycle activity.
ATP: Adenosine triphosphate; BAK/BAX: Bcl-2-associated X protein; Ca2+: Calcium; CypD: Cyclophilin D; Cyt-c: Cytrochrome-c; ER: Endoplasmic Reticulum; Grp75: glucose-regulated protein of 75kDa (or mortalin/heat shock protein 75); IMM: inner mitochondrial membrane; IMS: inter-membrane space; IP3R: inositol 1,4,5-trisphosphate receptor; MCU: mitochondrial calcium uniporter; MERCs: mitochondria–ER contact sites; mPTP: mitochondrial permeability transition pore; OMM: Outer mitochondrial membrane; ROS: reactive oxygen species; SERCA: sarco/endoplasmic reticulum Ca2+-ATPase; TCA cycle: tricarboxylic acid cycle; VDAC: voltage-dependent anion channel.
From the IMS, the inner mitochondrial membrane (IMM)-localized mitochondrial Ca2+ uniporter (MCU) transfers Ca2+ into the matrix (Baughman et al., 2011; De Stefani et al., 2011). The MCU is a pentameric protein complex of transmembrane proteins with the N- and C-termini exposed to the mitochondrial matrix (Nemani et al., 2018). The MCU protein contains a cysteine residue in its matrix-localized N-terminal domain that undergoes glutathionylation in the presence of excess ROS from mitochondrial oxidative stress. Upon this modification, MCU undergoes higher order oligomerization, which increases mitochondrial Ca2+ entry, eventually resulting in mitochondrial Ca2+ overload (Dong et al., 2017). The MCU is controlled by mitochondrial Ca2+ uptake proteins 1 and 2 (MICU1 and MICU2) (Patron et al., 2014), and the essential MCU regulator (EMRE) which together form a 480 kDa complex anchored in the IMM (Sancak et al., 2013). We name this assembly the “MCU complex” henceforth. The MCU complex is inhibited by MICU2 in low Ca2+ concentrations, but in high Ca2+ concentrations, MICU1 stimulates the MCU complex and allows Ca2+ entry into the mitochondrial matrix (Nemani et al., 2018). The oxidoreductase Mia40/CHCHD4 catalyzes the formation of a disulfide bond between MICU1 and MICU2, which attenuates mitochondrial Ca2+ entry (Petrungaro et al., 2015). Therefore, cysteine oxidation of the MCU channel in the matrix acts to overload mitochondria with Ca2+, but Mia40/CHCHD4 opposes this activation within the IMS. Although the MCU complex is not particularly enriched on MERCs, the majority of the Ca2+ it receives originates from the ER (Qi et al., 2015). Increased or decreased amounts of the oxidoreductase Ero1α could influence MCU Ca2+ uptake, but it is currently not known how this affects the components of the MCU complex (Anelli et al., 2011). Regardless of this uncertainty, redox signaling can radiate out from one organelle to the other, as seen for example during ER stress, which causes an increase of the mitochondrial Lon protease that degrades oxidized mitochondrial proteins (Hori et al., 2002). Moreover, the transmission of mitochondrial ROS can induce the ER unfolded protein response (UPR) (Yoon et al., 2011).
Control of MERC Ca2+ Signaling by Cysteine PTMs on the ER
The extent of ER Ca2+ content determines the extent of Ca2+ flux towards mitochondria (Gutierrez et al., 2020). Store operated Ca2+ entry (SOCE) (Elaib et al., 2016) and Ca2+ import by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps combine to store Ca2+ within the ER (Primeau et al., 2018). Although full-length SERCA shows no enrichment to any ER domain (Raturi et al., 2016), truncated forms of SERCA can localize to MERCs (Chami et al., 2008). Like most MERC Ca2+-handling proteins (Figure 3), SERCA is subject to extensive redox regulation (Chernorudskiy and Zito, 2017). SERCA activity can increase under oxidative conditions (Adachi et al., 2004) and this activity determines MERC functioning (Raturi et al., 2016). ER-localized NADPH oxidase 4 (Nox4) mediates this baseline activation (Evangelista et al., 2012). While the ER chaperone calnexin maintains Nox4 activity (Prior et al., 2016) and, hence, SERCA pumping (Gutierrez et al., 2020), the reductase TMX1 inhibits SERCA and prevents the re-capture of the cytosolic Ca2+ (Figure 4) (Raturi et al., 2016). Overall, a baseline oxidation of SERCA correlates with full activity.
However, conflicting results have been reported on the overall level of SERCA cysteine oxidation, also regarding luminal or cytosolic cysteines, leading to a dichotomy of the SERCA redox regulation (Raturi et al., 2014). Accordingly, unlike the activating calnexin that increases SERCA oxidation, a number of ER proteins including ERdj5 and selenoprotein N (SELENON) activate SERCA pumps by reducing critical luminal cysteines (Marino et al., 2015; Ushioda et al., 2016). This is also seen for p53, which reduces the cytoplasmic oxidation of SERCA (Giorgi et al., 2015). Together, the extent of SERCA oxidation could lead to a bell-shaped activity curve (Raturi et al., 2014). Consistent with this hypothesis, SERCA activity decreases in the presence of hyper-oxidizing conditions, as found in aging tissue (Babusikova et al., 2012).
Once filled, the ER Ca2+ content can be released via ryanodine receptors (RyRs) or IP3Rs (Raffaello et al., 2016). All IP3R Ca2+ channels are enriched at MERCs but IP3R2 is most potent in transmitting Ca2+ from the ER to mitochondria (Bartok et al., 2019). Similarly, RyRs localize to MERCs (Chen et al., 2012), where they can generate an ER-mitochondria Ca2+ conduit in their own right (Eisner et al., 2013). Both IP3Rs (Beretta et al., 2020) and RyRs (Sun et al., 2011) are associated with Nox4, suggesting this ER ROS source fulfills a central role in the control of ER-mitochondria Ca2+ flux, apparently to maintain physiological ATP production (Eisner et al., 2013).
RyR1 oxidation and glutathionylation increases Ca2+ release associated with the redox-dependent dissociation from its regulatory proteins FKBP12 and calmodulin (Aracena et al., 2005; Aracena-Parks et al., 2006). IP3Rs act directly on MERCs, since they form a physical link with OMM-localized VDAC1 under the control of the mitochondrial chaperone Grp75 to generate a Ca2+ conduit toward mitochondria. This complex is also the first description of a MERC tethering complex (Szabadkai et al., 2006). Cytosolic H2O2 molecules lead to oxidation of two cysteine residues within the cytosolic suppressor domain of IP3R1 (cysteines 206, and 214) and one additional cytosolic residue (cysteine 1394) in addition to 5 cysteines that are already oxidized under basal conditions (Joseph et al., 2018). These modifications of the sulfenylation and sulfinylation type activate IP3R1. Therefore, oxidative stress increases Ca2+ flux through IP3Rs and may lead to a feed forward ER Ca2+ flux towards mitochondria. As a further consequence, this Ca2+-based feed forward mechanism triggers the release of H2O2 from mitochondria into the MERC cleft that increases ER-mitochondria tethering (Booth et al., 2016) (Figures 3 and 4).
Another mechanistic connection between ER Ca2+ channel activity and redox depends on ER chaperones. One example is the Sigma 1 receptor (SIGMAR1), an ER chaperone with a limited number of substrates (Hayashi and Su, 2007). Normally, SIGMAR1 is complexed to the ER chaperone BiP/GRP78. Upon detachment from BiP/GRP78 during ER stress, SIGMAR1 interacts with and activates IP3R, thus increasing Ca2+ transfer from the ER to mitochondria (Hayashi and Su, 2007). In parallel, ERp44 and Ero1α, two proteins controlling the ER folding and redox environment, inhibit IP3Rs under reducing conditions and activate it under oxidizing conditions (Higo et al., 2005; Li et al., 2009). ER stress is a condition that activates the formation of MERCs and likely causes a feed-forward mechanism analogous to the one originating at mitochondria (Booth et al., 2016), but based on dysfunctional ER oxidative protein folding (Csordas et al., 2006; Bravo et al., 2011) (Figure 4). This leads to a mechanistic connection between ER oxidative protein folding and ER-mitochondria Ca2+ flux (Simmen et al., 2010; Fan and Simmen, 2019) that is associated with increased passive ER Ca2+ leak during ER stress (Hammadi et al., 2013) and interactions of ER folding enzymes with SERCA pumps (John et al., 1998; Li and Camacho, 2004; Lynes et al., 2013).
MERC regulation by redox likely extends to the core of the UPR machinery, including protein kinase RNA-like endoplasmic reticulum kinase (PERK) (Harding et al., 1999; Hori et al., 2002). Consistent with a central role in MERC formation, the deletion of PERK reduces ER-mitochondria contact points associated with resistance to apoptosis (Verfaillie et al., 2012). Another major ER stress sensor is the inositol-requiring enzyme 1 (Ire1). A portion of Ire1 localizes to MERCs, where it acts as a scaffold for IP3Rs to control mitochondria Ca2+ transfer and metabolism (Carreras-Sureda et al., 2019). PERK and Ire1 may functionally link oxidative stress to MERC signaling via their UPR signaling properties that increase upon ROS incubation for PERK (Higa and Chevet, 2012) and Ire1 (Hourihan et al., 2016). However, how exactly these transmembrane ER stress sensors manipulate ROS signaling on MERCs remains to be investigated.
MERC Lipid Homeostasis Is Linked to Ca2+ Flux and ER Stress and OXPHOS
The originally discovered function of MERCs is the production of specific lipids, as unveiled by the biochemical isolation of MAMs by Jean Vance (Vance, 1990, 1991). Indeed, the mitochondria ER membrane contact site (MCS) is a major hub in lipid biosynthesis (Figure 2) (Vance, 2015). MERCs have raft-like properties and form membrane microdomains enriched in sphingolipids and cholesterol (Sano et al., 2009; Hayashi and Fujimoto, 2010). This leads to the localization of Acetyl-Coenzyme A acetyltransferase 1 (ACAT1), also known as acyl-Coenzyme A: cholesterol acyltransferase 1 (SOAT1) to MERCs (Lee et al., 2000) where this enzyme esterifies and detoxifies cholesterol (Rogers et al., 2015). Once synthesized, cholesterol transfers over to mitochondria dependent on caveolin-1 that promotes MAMs and decreases free cholesterol (Sala-Vila et al., 2016).
Upon establishment of their raft-like structure, MERCs also supply mitochondrial phosphatidylcholine (PC), phosphatidylinositol (PI), and phosphatidylserine (PS) (van Meer et al., 2008). After PS production in the ER from PA (phosphatidic acid) and its transfer to mitochondria, PS is enzymatically converted to phosphatidylethanolamine (PE), one of two biosynthetic pathways for this lipid (Schuiki and Daum, 2009). Mitochondrial PE is cycled back to the ER, where it is transformed into PC by the action of phosphatidylethanolamine N-methyltransferase (PEMT) (Vance, 2015).
Imbalance of these enzymatic reactions induces ER stress and subsequently triggers MERC dysfunction, highlighting the symbiotic relationship between the ER and mitochondria. Consistent with this, compromised MERC lipid homeostasis, for instance from disrupted PS shuttling usually leads to ER stress (Hernandez-Alvarez et al., 2019). UPR induction also results from increased palmitate loading of the ER (Karaskov et al., 2006), a lack of PEMT (Gao et al., 2015) or from reduced PC levels (Hou et al., 2014). Mechanistically, these conditions of disrupted lipid homeostasis typically trigger mitochondrial ROS production (Xu et al., 2015), but can also impair mitochondrial ATP production, mitochondrial morphology and assembly of OXPHOS components (Tasseva et al., 2013). There is therefore a functional nexus between proteins controlling the MERC lipidome, the UPR and mitochondrial functions. Another example is the interorganellar PS/PE shuttling, which requires oxysterol-binding proteins 5 and 8 (ORP5/8) (Chung et al., 2015). This lipid shuttle also operates on MERCs, where ORP5/8 interact with PTPIP51 on the OMM (Galmes et al., 2016). Through their function for the PC/PE ratio, ORP5/8 allow for normal respiration. If over-expressed they improve mitochondrial Ca2+ import (Pulli et al., 2018). As expected, altering the function of this lipid shuttle, for instance via over-expression of ORP8, induces ER stress (Guo et al., 2017).
At the root of these observations may be the depletion of the ER Ca2+ content that is transferred over to mitochondria. This could occur for instance upon an elevation of the PC/PE ratio, which inhibits SERCA pumps by decreasing their Ca2+ affinity (Gustavsson et al., 2011). Thus, the altered MERC lipidome may disrupt proper Ca2+ filling of the ER, which is not only critical for ER protein folding (Simmen and Herrera-Cruz, 2018), but also for mitochondrial oxidative phosphorylation (Gutierrez et al., 2020). Another functional link is provided by the shuttling of ER-synthesized phosphatidic acid (PA) towards mitochondria to be enzymatically transformed into cardiolipin, which is essential for mitochondrial structure and function (Osman et al., 2011; Potting et al., 2013). Like reduced PE levels, interference with cardiolipin synthesis leads to ER stress, culminating in the activation of the C/EBP homologous protein (CHOP) (Sustarsic et al., 2018).
Lipids themselves are sensitive to ROS. An increase of lipid peroxidation compromises the folding environment of the ER and can lead to a feed forward mechanism of progressing dysfunction (Lin et al., 2014). Thus, MERCs become dysfunctional under extended oxidizing conditions (Janikiewicz et al., 2018). Ultimately, lipid peroxidation impairs mitochondrial OXPHOS (Anderson et al., 2012). Cardiolipin is also susceptible to peroxidation, which severely compromises mitochondrial OXPHOS (Paradies et al., 2009). Upon Bax/Bak-mediated OMM pore formation during apoptosis, cytochrome c can induce cardiolipin peroxidation following the formation of a complex with cardiolipin peroxidase, which then accelerates cell death (Kagan et al., 2005). Less is known about links between the IMS folding environment and lipid homeostasis, but the generation of oxidized sterols leads to the recruitment of the ubiquitin proteasome system to remove the mitochondrial OMM protein import machinery in yeast, suggesting this compartment could be affected in similar ways to the ER folding environment (Nielson et al., 2017).
The importance of MERC redox changes extends to lipid-related downstream effects. The biosynthesis of triacylglycerol (TG) is under the control of two diacyglycerol acyltransferases (DGAT1/2), of which DGAT2 is MERC enriched (Stone et al., 2009). The activity of both enzymes is arrested upon incubation with thiol-modifying compounds (Sauro and Strickland, 1990), because the oxidation of cysteines blocks DGATs (Jung et al., 2017).
Sources of Cysteine Post-Translational Modifications
MERCs are a convergence point for ROS produced within the ER, mitochondria and peroxisome (Yoboue et al., 2018). ROS production increases upon the arrival of growth factor and cytokine signaling (Nam et al., 2010). Within the ER, ROS are made from oxidative protein folding that requires oxygen consumption by the oxidoreductases of the Ero1 family (Araki et al., 2013), as well as by other enzymes such as the hemoproteins cytochrome P450 (Guengerich, 2019), and Nox4/5 (Laurindo et al., 2014). The Ero1 flavoproteins act together with glutathione peroxidases (GPx) 7/8 and PRDX4 to generate oxidized protein disulfide isomerase (PDI) (Bulleid and Ellgaard, 2011). Ero1 exists in humans as the hypoxia-controlled Ero1α (May et al., 2005) and the ER-stress regulated Ero1β (Cabibbo et al., 2000; Pagani et al., 2000). Both GPx7 and GPx8 act as peroxidases to promote the oxidation of substrate disulfide bonds in the presence of PDI (Nguyen et al., 2011). GPx7 is a luminal protein, while GPx8 spans the ER membrane (Nguyen et al., 2011; Kanemura et al., 2020). PRDX4 assists Ero1 oxidoreductases to eliminate excess H2O2 produced from oxygen consumption and uses it for PDI oxidation (Rhee et al., 2018). While Ero1α and GPx8 are known MERC proteins, we currently do not know the intra-ER localization of the other ER-based ROS sources and sinks (Gilady et al., 2010; Yoboue et al., 2018).
The ER-localized oxidoreductive network based on Ero1, GPx7/8 and PRDX4 is intimately linked with the redox state of the cellular volume adjacent to the ER, which potentially includes mitochondria. Accordingly, the regeneration of NADPH within the cytosol is critical for PDI oxidation (Poet et al., 2017) and the same is true for cytosolic thioredoxin reductase (Cao et al., 2020). These observations must be integrated with the inability of ROS to diffuse freely within the cell (Appenzeller-Herzog et al., 2016). Aquaporin-11 and other aquaporin family members have been identified as mediating ROS transport across the ER and other membranes, suggesting these proteins could be critical for MERC ROS communication (Bestetti et al., 2020).
Peroxisomes and mitochondria are the alternative MERC-relevant ROS producers. Within the mitochondria, complex I and III of the electron transport chain can produce ROS if there is a backup of electron flow (Murphy, 2009). Alternative sources are activated upon high levels of matrix NADH/NAD+ that are a consequence of reverse electron flow (Robb et al., 2018). Additionally, β-oxidation of fatty acids also promotes mitochondrial ROS production in some tissues, but notably not in the brain (Schonfeld and Reiser, 2017). Large quantities of ROS are stored within the mitochondrial cristae, which can be released upon increased Ca2+ influx from the ER, inducing a feed forward mechanism (Booth et al., 2016). To a currently unknown extent, antioxidant defenses found within the IMS like SOD1 (Kawamata and Manfredi, 2010), GPx and PRDXs (Mailloux et al., 2013) could potentially absorb them (Dimayuga et al., 2007).
Within peroxisomes, the oxidation of very long chain fatty acids and amino acids uses the enzymatic activity of flavoproteins, whose ROS production is scavenged by catalase and PRDX5 (Bonekamp et al., 2009). The influence of this ROS moiety on the ER and mitochondria is currently under debate (Lismont et al., 2015; Yoboue et al., 2018). Further research will show how these three sources are integrated and controlled under physiological and stress conditions.
Metabolic and Apoptotic Signaling as a MERC-Localized PTM Target
The physiological influx of Ca2+ from the ER to mitochondria activates mitochondrial dehydrogenases, including glycerol-3-phosphate dehydrogenase (GPDH), pyruvate dehydrogenase (PDH), isocitrate dehydrogenase (IDH) and oxoglutarate dehydrogenase (OGDH) (Denton, 2009). ATP synthase (Jouaville et al., 1999) and β-oxidation (Balu et al., 2016) also appear to be a target of Ca2+ regulation. Opposing the function of Ca2+, glutathionylation inhibits the Krebs cycle and OXPHOS (Kuksal et al., 2017). Upon a ROS-mediated oxidation of MERC Ca2+-handling proteins, the alteration of ER-mitochondria Ca2+ flux could further boost ROS production within mitochondria (Brookes et al., 2004). Such a metabolic shift could result in reduced glycolysis, associated with glutathionylation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) that decreases glycolytic flux, only reversed by increased NADPH or antioxidants (Mullarky and Cantley, 2015).
In contrast, excessive mitochondrial Ca2+ uptake leads to an overload, which further increases the production of ROS by the respiratory chain, especially if associated with the depolarization of the IMM and the opening of the mitochondrial permeability transition pore (Adam-Vizi and Starkov, 2010). Such an event coincides with the oligomerization of pro-apoptotic Bcl2 family proteins Bax and Bak into a pore structure (Flores-Romero et al., 2020). Subsequently, Ca2+ and cytochrome c are released from this pore and apoptosis ensues (Salvador-Gallego et al., 2016; Cosentino and Garcia-Saez, 2017). Therefore, under conditions when the cyclic ER-mitochondria Ca2+ flux exceeds the normal mitochondrial buffering capacity, the ER can also contribute to the triggering of cell death, using Ca2+ as a messenger (Pinton et al., 2008).
Another example how MERCs mechanistically connect ROS and Ca2+ signaling is based on uncoupling protein 3 (UCP3), which reduces ATP production and thus compromises SERCA pumping on the ER side of MERCs, highlighting additional connections between the ER and mitochondrial ATP (De Marchi et al., 2011). This activity could increase in the presence of oxidative stress that activates UCP3 (Mailloux et al., 2011).
Multiple MERC-localized kinase-based signaling mechanisms could be subject to ROS modulation. For instance, the anti-apoptotic kinase Akt decreases MERC formation upon growth factor signaling (Betz et al., 2013). However, upon induction of oxidative stress, Akt forms an intramolecular disulfide bond, which results in its degradation, thus presumably boosting MERC Ca2+ signaling (Murata et al., 2003). This MERC activation depends on MERC-localized the phosphatase tensin homolog (PTEN) (Bononi et al., 2013). In contrast, H2O2-induced oxidation inactivates PTEN, thus increasing the anti-apoptotic, Akt-mediated MERC disruption, followed by proliferation (Kwak et al., 2010; Numajiri et al., 2011; Zhang et al., 2020).
An intriguing link between MERCs and redox signaling exists within the control of the circadian cycle. This mechanism allows cells and tissues to maintain homeostasis in a time-dependently changing environment. During the circadian cycle, the brain and muscle arylhydrocarbon receptor nuclear translocator protein 1 (BMAL1) regulates ROS production by controlling the activation of the antioxidant response transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) (Wible et al., 2018). Since Nox4 is one of the targets of BMAL1 (Anea et al., 2013), BMAL1 could modulate MERC signaling to control mitochondria metabolism (Alexander et al., 2020). This mechanism could involve the formation of a circadian cycle of Ca2+ oscillations (Ikeda and Ikeda, 2014).
Cysteine Oxidation at MERCs and Disease
Given the many known mechanisms that compromise or alter the functioning of the ER and mitochondria, as well as cellular metabolism based on cysteine oxidation at MERCs, it is not surprising that this mechanism malfunctions in many metabolic disease settings.
Examples of MERC Cysteine Modification in Neurodegenerative Diseases
Most neurodegenerative diseases involve oxidative stress (Chen et al., 2012). For instance, the S-nitrosylation of PTEN, Drp1, and Parkin is associated with Alzheimer’s and Parkinson’s disease, which would result in mitochondrial fragmentation and MERC dysfunction (Nakamura et al., 2013). Additional MERC-associated cysteine modifications are known to occur in these neurodegenerative diseases. For instance, in a murine model, Akt sulfhydration causes a worsening of Alzheimer’s disease elicited by a high level of Tau protein phosphorylation in the brain (Sen et al., 2020). In addition, the PP2Ac protein, an inhibitor of Akt on MAMs, shows less activity in Alzheimer’s disease upon introduction of thiol disulfide bonds in its catalytic subunit (Foley et al., 2007). Overall, such changes would result in increased formation of MERCs and increased transfer of Ca2+, lipids and sterols to mitochondria, which is indeed found in patient tissue, but it remains to be determined whether cysteine PTMs of MERC proteins are behind these observations (Hedskog et al., 2013; Montesinos et al., 2020). Changes in parkin cysteine PTMs are generally thought to compromise its activity and act to promote disease progression (Barodia et al., 2017). In contrast, the H2S-mediated Parkin sulfhydration may act to re-activate its enzymatic activity and, hence, to slow down PD progression (Vandiver et al., 2013).
Other neurodegenerative syndromes such as Huntington’s disease (HD) or amyotrophic lateral sclerosis (ALS) also show increased oxidative stress. Relevant for MERC regulation, a clear increase of nitrosylated PDI was observed in ALS (Chen et al., 2013). Within HD tissue and primary cells from mouse HD models, altered, dysfunctional MERCs are associated with fragmented mitochondria and oxidative stress (Cherubini et al., 2020).
Examples of MERC Cysteine Modification in Cardiovascular Diseases
Cardiovascular diseases are highly correlated with increased levels of ROS (Peoples et al., 2019). A well-characterized effect of these is the Nox4-mediated oxidation of SERCA that promotes endothelial migration (Evangelista et al., 2012) and normal angiogenesis after ischemia (Craige et al., 2011). The correlation between Ca2+ fluxes, redox status and cysteine modifications is strong in cardiovascular diseases, especially upon myocardial ischemia and reperfusion. Under this condition, myocardial cells frequently undergo cell death due to an excessive flux of Ca2+ from the sarcoplasmic reticulum (SR)/ER to the mitochondria, causing mitochondrial Ca2+ overload and cell death that eventually triggers heart failure (Santulli et al., 2015). The MCU complex undergoes oxidation within the matrix-exposed N-terminal domain, but this does not occur dependent on MERC-derived ROS (Dong et al., 2017).
Examples of MERC Cysteine Modification on Metabolic Diseases
In the case of insulin resistance in diabetes, increased mitochondrial ROS levels are a potential causative mechanism (Anderson et al., 2009). A known consequence of diabetes-associated ROS induction is the inactivation of the SENP1 SUMO isopeptidase that normally promotes insulin exocytosis (Ferdaoussi et al., 2015) but also mitochondrial metabolic activity during fasting (Wang et al., 2019). Several proteins directly found at MERCs like Akt (Betz et al., 2013) and PTEN (Bononi et al., 2013) are dysfunctional in obesity and type 2 diabetic situations. For instance, a high concentration of NO inhibits Akt causing insulin resistance, while a low concentration of NO opposes this disease-promoting effect through PTEN S-nitrosylation (Numajiri et al., 2011). Moreover, redox events at MERCs in diabetes are associated with Drp1 sulfenylation and excessive fission of the mitochondrial network in endothelial cells. The ensuing mitochondrial fragmentation further increases ROS production and endothelial senescence and thus worsens the disease (Yu et al., 2006; Peng et al., 2012). Subsequently, these MERC defects increase inflammation whose hallmark is the activation of the inflammasome in the proximity of the ER and mitochondria (Zhou et al., 2011). Under this condition, interleukins and TNFa are secreted, which are two major components of inflammation following the activation of NFkB (Liu et al., 2017).
Examples of MERC Cysteine Modification in Cancer
A plethora of cancer-relevant proteins controls metabolic MERC signaling, including p53 (Giorgi et al., 2015), PTEN (Bononi et al., 2013), the kinase Akt (Betz et al., 2013), breast/ovarian cancer susceptibility gene 1 (BRCA1) (Hedgepeth et al., 2015) and the promyelocytic leukemia (PML) protein (Giorgi et al., 2010; Missiroli et al., 2016). PML is very sensitive to oxidation (Tessier et al., 2017). While its MERC activity increases IP3R-mediated Ca2+ flux towards mitochondria, in its absence ROS increase (Niwa-Kawakita et al., 2017). Similarly, the transcriptional roles of p53 are highly redox-dependent (Kim et al., 2011). At MERCs, p53 reduces the cytosolic oxidation of SERCA and thus makes SERCA more active (Giorgi et al., 2015). Several additional redox-sensitive MERC proteins are recognized as oncogenic proteins, including proteins mediating the GSK3β pathways and frequently undergo oxidation in a cancer context (Koundouros and Poulogiannis, 2018; Zhang et al., 2020). While there is some evidence that MERCs are disrupted in a cancer context, thus promoting a Warburg metabolic signature with increased glycolysis (Herrera-Cruz and Simmen, 2017), MERC formation can also act as cancer-promoting. For example, the activity of the MCU complex promotes migration and invasion of breast cancer cells (Tosatto et al., 2016). Similarly, the activity of IP3Rs is necessary to prevent energy depletion of cancer cells (Cardenas et al., 2010). High expression of ROS-generating Ero1α worsens prognosis of breast cancer patients, which given its activating role for IP3Rs again suggests a cancer-promoting role (Kutomi et al., 2013). However, contradicting these findings, reduced expression of the BRCA1-associated protein 1 (BAP1) increases the incidence of cancer in parallel to a reduction of IP3R activity (Bononi et al., 2017). The complexity of redox-control of MERCs in cancer is possibly best illustrated by the reductase TMX1 that if depleted from melanoma cells can slow down their growth and mitochondria metabolism (Raturi et al., 2016), but in patients is often highly expressed to promote mitochondrial activity (Zhang et al., 2019). In summary, MERC disruption can arrest mitochondria metabolism in cancer, but the maintenance of mitochondria metabolism by functional MERCs can act as tumor-promoting by promoting invasion and metastasis (Denisenko et al., 2019).
To conclude, MERCs are a central cellular hub in metabolic and aging-related diseases. Redox control of MERC tethering and regulatory proteins is a key mechanism to control their roles in metabolism. Much of the information gathered on cysteine PTMs controlling MERCs has been gathered before their significance for this organellar contact site was known, suggesting that future research may reveal additional functional connections.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial supportfor the research, authorship, and/or publication of this article:This work was supported by CIHR (PS162449).
ORCID iDs
Arthur Bassot https://orcid.org/0000-0002-4276-4493
Thomas Simmen https://orcid.org/0000-0002-2350-2965
References
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. (2004). S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10, 1200–1207. [DOI] [PubMed] [Google Scholar]
- Adam-Vizi V, Starkov AA. (2010). Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimers Dis 20Suppl 2, S413–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RK, Liou YH, Knudsen NH, Starost KA, Xu C, Hyde AL, Liu S, Jacobi D, Liao NS, Lee CH. (2020). Bmal1 integrates mitochondrial metabolism and macrophage activation. Elife 9, e54090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Katunga LA, Willis MS. (2012). Mitochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin Exp Pharmacol Physiol 39, 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin C-T, Price JW, Kang L, Rabinovitch PS, Szeto HH, et al. (2009). Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anea CB, Zhang M, Chen F, Ali MI, Hart CM, Stepp DW, Kovalenkov YO, Merloiu AM, Pati P, Fulton D, Rudic RD. (2013). Circadian clock control of Nox4 and reactive oxygen species in the vasculature. PLoS One 8, e78626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R. (2011). Ero1alpha regulates Ca2+ fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal 16, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Banhegyi G, Bogeski I, Davies KJ, Delaunay-Moisan A, Forman HJ, Gorlach A, Kietzmann T, Laurindo F, Margittai E, et al. (2016). Transit of H2O2 across the endoplasmic reticulum membrane is not sluggish. Free Radic Biol Med 94, 157–160. [DOI] [PubMed] [Google Scholar]
- Aracena P, Tang W, Hamilton SL, Hidalgo C. (2005). Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid Redox Signal 7, 870–881. [DOI] [PubMed] [Google Scholar]
- Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, Hamilton SL. (2006). Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem 281, 40354–40368. [DOI] [PubMed] [Google Scholar]
- Araki K, Iemura S, Kamiya Y, Ron D, Kato K, Natsume T, Nagata K. (2013). Ero1-alpha and PDIs constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases. J Cell Biol 202, 861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babusikova E, Lehotsky J, Dobrota D, Racay P, Kaplan P. (2012). Age-associated changes in Ca(2+)-ATPase and oxidative damage in sarcoplasmic reticulum of rat heart. Physiol Res 61, 453–460. [DOI] [PubMed] [Google Scholar]
- Balu D, Ouyang J, Parakhia RA, Pitake S, Ochs RS. (2016). Ca(2+) effects on glucose transport and fatty acid oxidation in L6 skeletal muscle cell cultures. Biochem Biophys Rep 5, 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barodia SK, Creed RB, Goldberg MS. (2017). Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res Bull 133, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok A, Weaver D, Golenar T, Nichtova Z, Katona M, Bansaghi S, Alzayady KJ, Thomas VK, Ando H, Mikoshiba K, et al. (2019). IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat Commun 10, 3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer TM, Murphy E. (2020). Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res 126, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. (2011). Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta M, Santos CX, Molenaar C, Hafstad AD, Miller CC, Revazian A, Betteridge K, Schröder K, Streckfuß-Bömeke K, Doroshow JH, et al. (2020). Nox4 regulates InsP3 receptor-dependent Ca(2+) release into mitochondria to promote cell survival. Embo J 39, e103530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard W, Haguenau F, Gautier A, Oberling C. (1952). Submicroscopical structure of cytoplasmic basophils in the liver, pancreas and salivary gland; study of ultrafine slices by electron microscope. Z Zellforsch Mikrosk Anat 37, 281–300. [PubMed] [Google Scholar]
- Bestetti S, Galli M, Sorrentino I, Pinton P, Rimessi A, Sitia R, Medrano-Fernandez I. (2020). Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol 28, 101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN. (2013). Feature article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci U S A 110, 12526–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp NA, Volkl A, Fahimi HD, Schrader M. (2009). Reactive oxygen species and peroxisomes: struggling for balance. Biofactors 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P. (2013). Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ 20, 1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bononi A, Giorgi C, Patergnani S, Larson D, Verbruggen K, Tanji M, Pellegrini L, Signorato V, Olivetto F, Pastorino S, et al. (2017). BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature 546, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DM, Enyedi B, Geiszt M, Varnai P, Hajnoczky G. (2016). Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol Cell 63, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossis G, Melchior F. (2006). Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21, 349–357. [DOI] [PubMed] [Google Scholar]
- Braakman I, Hebert DN. (2013). Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol 5, a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM. (2009). MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 10, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, et al. (2011). Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci 124, 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. (2004). Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287, C817–C833. [DOI] [PubMed] [Google Scholar]
- Bulleid NJ, Ellgaard L. (2011). Multiple ways to make disulfides. Trends Biochem Sci 36, 485–492. [DOI] [PubMed] [Google Scholar]
- Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. (2000). ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem 275, 4827–4833. [DOI] [PubMed] [Google Scholar]
- Cao X, Lilla S, Cao Z, Pringle MA, Oka OBV, Robinson PJ, Szmaja T, van Lith M, Zanivan S, Bulleid NJ. (2020). The mammalian cytosolic thioredoxin reductase pathway acts via a membrane protein to reduce ER-localised proteins. J Cell Sci 133, jcs241976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. (2010). Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-Sureda A, Jana F, Urra H, Durand S, Mortenson DE, Sagredo A, Bustos G, Hazari Y, Ramos-Fernandez E, Sassano ML, et al. (2019). Non-canonical function of IRE1alpha determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat Cell Biol 21, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Ji WK, Stan RV, de Juan Sanz J, Ryan TA, Higgs HN. (2018). INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. J Cell Biol 217, 251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P. (2008). Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 32, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Guo C, Kong J. (2012). Oxidative stress in neurodegenerative diseases. Neural Regen Res 7, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang X, Li C, Guan T, Shang H, Cui L, Li XM, Kong J. (2013). S-nitrosylated protein disulfide isomerase contributes to mutant SOD1 aggregates in amyotrophic lateral sclerosis. J Neurochem 124, 45–58. [DOI] [PubMed] [Google Scholar]
- Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, et al. (2012). Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res 111, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernorudskiy AL, Zito E. (2017). Regulation of calcium homeostasis by ER redox: A close-up of the ER/mitochondria connection. J Mol Biol 429, 620–632. [DOI] [PubMed] [Google Scholar]
- Cherubini M, Lopez-Molina L, Gines S. (2020). Mitochondrial fission in Huntington’s disease mouse striatum disrupts ER-mitochondria contacts leading to disturbances in Ca(2+) efflux and reactive oxygen species (ROS) homeostasis. Neurobiol Dis 136, 104741. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. (2009). S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324, 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IT, Adelmant G, Lim Y, Marto JA, Cho G, Golden JA. (2017). Ascorbate peroxidase proximity labeling coupled with biochemical fractionation identifies promoters of endoplasmic reticulum-mitochondrial contacts. J Biol Chem 292, 16382–16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. (2013). Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res 112, 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. (2015). INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. (2004). S-nitrosylation of Parkin regulates ubiquitination and compromises Parkin’s protective function. Science 304, 1328–1331. [DOI] [PubMed] [Google Scholar]
- Cosentino K, Garcia-Saez AJ. (2017). Bax and bak pores: are we closing the circle? Trends Cell Biol 27, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr.(2011). NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124, 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás Grgy, Renken C, Várnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnóczky Grgy, (2006). Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174, 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610. [DOI] [PubMed] [Google Scholar]
- De Marchi U, Castelbou C, Demaurex N. (2011). Uncoupling protein 3 (UCP3) modulates the activity of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by decreasing mitochondrial ATP production. J Biol Chem 286, 32533–32541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pinto V, Reina S, Gupta A, Messina A, Mahalakshmi R. (2016). Role of cysteines in mammalian VDAC isoforms’ function. Biochim Biophys Acta 1857, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R. (2012). VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ 19, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. (2011). A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debattisti V, Gerencser AA, Saotome M, Das S, Hajnoczky G. (2017). ROS control mitochondrial motility through p38 and the motor adaptor miro/trak. Cell Rep 21, 1667–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko TV, Gorbunova AS, Zhivotovsky B. (2019). Mitochondrial involvement in migration, invasion and metastasis. Front Cell Dev Biol 7, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM. (2009). Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Wang C, Clark JM, Dimayuga ER, Dimayuga VM, Bruce-Keller AJ. (2007). SOD1 overexpression alters ROS production and reduces neurotoxic inflammatory signaling in microglial cells. J Neuroimmunol 182, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Shanmughapriya S, Tomar D, Siddiqui N, Lynch S, Nemani N, Breves SL, Zhang X, Tripathi A, Palaniappan P, et al. (2017). Mitochondrial Ca(2+) uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Mol Cell 65, 1014–1028.e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner V, Csordas G, Hajnoczky G. (2013). Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle—Pivotal roles in Ca(2)(+) and reactive oxygen species signaling. J Cell Sci 126, 2965–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaib Z, Saller F, Bobe R. (2016). The calcium entry-calcium refilling coupling. Adv Exp Med Biol 898, 333–352. [DOI] [PubMed] [Google Scholar]
- Elbaz-Alon Y, Guo Y, Segev N, Harel M, Quinnell DE, Geiger T, Avinoam O, Li D, Nunnari J. (2020). PDZD8 interacts with protrudin and Rab7 at ER-late endosome membrane contact sites associated with mitochondria. Nat Commun 11, 3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista AM, Kohr MJ, Murphy E. (2013). S-nitrosylation: specificity, occupancy, and interaction with other post-translational modifications. Antioxid Redox Signal 19, 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista AM, Thompson MD, Bolotina VM, Tong X, Cohen RA. (2012). Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic Biol Med 53, 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Simmen T. (2019). Mechanistic connections between endoplasmic reticulum (ER) redox control and mitochondrial metabolism. Cells 8, 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, et al. (2015). Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest 125, 3847–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sanz C, De la Fuente S, Sheu SS. (2019). Mitochondrial Ca(2+) concentrations in live cells: quantification methods and discrepancies. FEBS Lett 593, 1528–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P. (2015). Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc Natl Acad Sci U S A 112, E2174–E2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli MJ. (2020). Redox post-translational modifications of protein thiols in brain aging and neurodegenerative conditions-focus on S-nitrosation. Front Aging Neurosci 12, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Horn S, Belkacemi A, Kojer K, Petrungaro C, Habich M, Ali M, Kuttner V, Bien M, Kauff F, et al. (2013). Protein import and oxidative folding in the mitochondrial intermembrane space of intact mammalian cells. Mol Biol Cell 24, 2160–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Romero H, Ros U, Garcia-Saez AJ. (2020). Pore formation in regulated cell death. Embo J 39, e105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotho A, Melchior F. (2013). Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem 82, 357–385. [DOI] [PubMed] [Google Scholar]
- Foley TD, Petro LA, Stredny CM, Coppa TM. (2007). Oxidative inhibition of protein phosphatase 2A activity: role of catalytic subunit disulfides. Neurochem Res 32, 1957–1964. [DOI] [PubMed] [Google Scholar]
- Forte M, Palmerio S, Yee D, Frati G, Sciarretta S. (2017). Functional role of Nox4 in autophagy. Adv Exp Med Biol 982, 307–326. [DOI] [PubMed] [Google Scholar]
- Fransson S, Ruusala A, Aspenstrom P. (2006). The atypical rho GTPases miro-1 and miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun 344, 500–510. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. (2011). ER tubules mark sites of mitochondrial division. Science 334, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmes R, Houcine A, Vliet AR, Agostinis P, Jackson CL, Giordano F. (2016). ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO Rep 17, 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, van der Veen JN, Vance JE, Thiesen A, Vance DE, Jacobs RL. (2015). Lack of phosphatidylethanolamine N-methyltransferase alters hepatic phospholipid composition and induces endoplasmic reticulum stress. Biochim Biophys Acta 1852, 2689–2699. [DOI] [PubMed] [Google Scholar]
- Garcia-Santamarina S, Boronat S, Domenech A, Ayte J, Molina H, Hidalgo E. (2014). Monitoring in vivo reversible cysteine oxidation in proteins using ICAT and mass spectrometry. Nat Protoc 9, 1131–1145. [DOI] [PubMed] [Google Scholar]
- Ge P, Dawson VL, Dawson TM. (2020). PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol Neurodegener 15, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmetti V, De Rosa P, Torosantucci L, Marini ES, Romagnoli A, Di Rienzo M, Arena G, Vignone D, Fimia GM, Valente EM. (2017). PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy 13, 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz M, Fischer F, Wolters D, Steegborn C. (2008). Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proc Natl Acad Sci U S A 105, 5705–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Pellegrini L. (2016). The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ 23, 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilady SY, Bui M, Lynes EM, Benson MD, Watts R, Vance JE, Simmen T. (2010). Ero1alpha requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM). Cell Stress Chaperones 15, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, Galindo Ramirez F, Rizzuto R, Virgilio FD, Zito E, et al. (2015). p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci U S A 112, 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, Bononi A, Bonora M, Duszynski J, Bernardi R, et al. (2010). PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 330, 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, et al. (2005). Electron transfer between cytochrome c and p66(shc) generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233. [DOI] [PubMed] [Google Scholar]
- Gomez-Suaga P, Paillusson S, Stoica R, Noble W, Hanger DP, Miller CCJ. (2017). The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr Biol 27, 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. (2019). Cytochrome P450 research and the journal of biological chemistry. J Biol Chem 294, 1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang L, Fan Y, Zhang D, Qin L, Dong S, Li G. (2017). Oxysterol-binding protein-related protein 8 inhibits gastric cancer growth through induction of ER stress, inhibition of wnt signaling, and activation of apoptosis. Oncol Res 25, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson M, Traaseth NJ, Veglia G. (2011). Activating and deactivating roles of lipid bilayers on the Ca(2+)-ATPase/phospholamban complex. Biochemistry 50, 10367–10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez T, Qi H, Yap MC, Tahbaz N, Milburn LA, Lucchinetti E, Lou PH, Zaugg M, LaPointe PG, Mercier P, et al. (2020). The ER chaperone calnexin controls mitochondrial positioning and respiration. Sci Signal 13 [DOI] [PubMed] [Google Scholar]
- Habich M, Salscheider SL, Riemer J. (2019). Cysteine residues in mitochondrial intermembrane space proteins: more than just import. Br J Pharmacol 176, 514–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, et al. (2013). Autophagosomes form at ER-mitochondria contact sites. Nature 495, 389–393. [DOI] [PubMed] [Google Scholar]
- Hammadi M, Oulidi A, Gackiere F, Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P, Lepage G, Lotteau S, et al. (2013). Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. Faseb J 27, 1600–1609. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. (1999). Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Schwietzer YA, Kummer D, Kirschnick N, Hoppe E, Thuring EM, Glaesner-Ebnet M, Brinkmann F, Gerke V, Reuter S, et al. (2020). The mitochondrial outer membrane protein SYNJ2BP interacts with the cell adhesion molecule TMIGD1 and can recruit it to mitochondria. BMC Mol Cell Biol 21, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Fujimoto M. (2010). Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol 77, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2007). Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131, 596–610. [DOI] [PubMed] [Google Scholar]
- Hedgepeth SC, Garcia MI, Wagner LE, Rodriguez AM, Chintapalli SV, Snyder RR, Hankins GDV, Henderson BR, Brodie KM, Yule DI, et al. (2015). The BRCA1 tumor suppressor binds to inositol 1,4,5-trisphosphate receptors to stimulate apoptotic calcium release. J Biol Chem 290, 7304–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, Larssen P, Gellhaar S, Sandebring A, Westerlund M, et al. (2013). Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A 110, 7916–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Alvarez MI, Sebastian D, Vives S, Ivanova S, Bartoccioni P, Kakimoto P, Plana N, Veiga SR, Hernandez V, Vasconcelos N, et al. (2019). Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease. Cell 177, 881–895.e817. [DOI] [PubMed] [Google Scholar]
- Herrera-Cruz MS, Simmen T. (2017). Cancer: untethering mitochondria from the endoplasmic reticulum? Front Oncol 7, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa A, Chevet E. (2012). Redox signaling loops in the unfolded protein response. Cell Signal 24, 1548–1555. [DOI] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. (2005). Subtype-Specific and ER lumenal environment-dependent regulation of inositol 1,4,5-Trisphosphate receptor type 1 by ERp44. Cell 120, 85–98. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Kwon SK, Paek H, Pernice WM, Paul MA, Lee J, Erfani P, Raczkowski A, Petrey DS, Pon LA, Polleux F. (2017). ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science 358, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D, et al. (2002). Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of lon protease. J Cell Biol 157, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou NS, Gutschmidt A, Choi DY, Pather K, Shi X, Watts JL, Hoppe T, Taubert S. (2014). Activation of the endoplasmic reticulum unfolded protein response by lipid disequilibrium without disturbed proteostasis in vivo. Proc Natl Acad Sci U S A 111, E2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourihan JM, Moronetti Mazzeo LE, Fernandez-Cardenas LP, Blackwell TK. (2016). Cysteine sulfenylation directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol Cell 63, 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung V, Lam SS, Udeshi ND, Svinkina T, Guzman G, Mootha VK, Carr SA, Ting AY. (2017). Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. Elife 6, e24463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung V, Zou P, Rhee HW, Udeshi ND, Cracan V, Svinkina T, Carr SA, Mootha VK, Ting AY. (2014). Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol Cell 55, 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ikeda M. (2014). Bmal1 is an essential regulator for circadian cytosolic Ca(2)(+) rhythms in suprachiasmatic nucleus neurons. J Neurosci 34, 12029–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilacqua N, Sanchez-Alvarez M, Bachmann M, Costiniti V, Del Pozo MA, Giacomello M. (2017). Protein localization at mitochondria-ER contact sites in basal and stress conditions. Front Cell Dev Biol 5, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K. (2004). Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117, 6535–6546. [DOI] [PubMed] [Google Scholar]
- Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S. (2011). Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. Embo J 30, 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janikiewicz J, Szymański J, Malinska D, Patalas-Krawczyk P, Michalska B, Duszyński J, Giorgi C, Bonora M, Dobrzyn A, Wieckowski MR. (2018). Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis 9, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LM, Lechleiter JD, Camacho P. (1998). Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol 142, 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Young MP, Alzayady K, Yule DI, Ali M, Booth DM, Hajnoczky G. (2018). Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. J Biol Chem 293, 17464–17476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. (1999). Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A 96, 13807–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Choi M, Choi K, Kwon EB, Kang M, Kim DE, Jeong H, Kim J, Kim JH, Kim MO, et al. (2017). Inactivation of human DGAT2 by oxidative stress on cysteine residues. PLoS One 12, e0181076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, et al. (2005). Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1, 223–232. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120, 649–661. [DOI] [PubMed] [Google Scholar]
- Kamerkar SC, Kraus F, Sharpe AJ, Pucadyil TJ, Ryan MT. (2018). Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nat Commun 9, 5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemura S, Sofia EF, Hirai N, Okumura M, Kadokura H, Inaba K. (2020). Characterization of the endoplasmic reticulum-resident peroxidases GPx7 and GPx8 shows the higher oxidative activity of GPx7 and its linkage to oxidative protein folding. J Biol Chem 295, 12772–12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. (2006). Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147, 3398–3407. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Manfredi G. (2010). Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid Redox Signal 13, 1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kundu JK, Surh YJ. (2011). Redox modulation of p53: mechanisms and functional significance. Mol Carcinog 50, 222–234. [DOI] [PubMed] [Google Scholar]
- Kim YM, Youn SW, Sudhahar V, Das A, Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, et al. (2018). Redox regulation of mitochondrial fission protein Drp1 by protein disulfide isomerase limits endothelial senescence. Cell Rep 23, 3565–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. (2009). An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Osman C, Walter P. (2011). The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc Natl Acad Sci U S A 108, 14151–14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Ramabhadran V, Higgs HN. (2013). An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 339, 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundouros N, Poulogiannis G. (2018). Phosphoinositide 3-Kinase/akt signaling and redox metabolism in cancer. Front Oncol 8, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuksal N, Chalker J, Mailloux RJ. (2017). Progress in understanding the molecular oxygen paradox - function of mitochondrial reactive oxygen species in cell signaling. Biol Chem 398, 1209–1227. [DOI] [PubMed] [Google Scholar]
- Kutomi G, Tamura Y, Tanaka T, Kajiwara T, Kukita K, Ohmura T, Shima H, Takamaru T, Satomi F, Suzuki Y, et al. (2013). Human endoplasmic reticulum oxidoreductin 1-alpha is a novel predictor for poor prognosis of breast cancer. Cancer Sci 104, 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak YD, Ma T, Diao S, Zhang X, Chen Y, Hsu J, Lipton SA, Masliah E, Xu H, Liao FF. (2010). NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener 5, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurindo FR, Araujo TL, Abrahao TB. (2014). Nox NADPH oxidases and the endoplasmic reticulum. Antioxid Redox Signal 20, 2755–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebiedzinska M, Szabadkai G, Jones AW, Duszynski J, Wieckowski MR. (2009). Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol 41, 1805–1816. [DOI] [PubMed] [Google Scholar]
- Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TD, Glickman MH, Weissman AM. (2012). Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell 47, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Kim JE. (2018). PDI-mediated S-nitrosylation of DRP1 facilitates DRP1-S616 phosphorylation and mitochondrial fission in CA1 neurons. Cell Death Dis 9, 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RG, Willingham MC, Davis MA, Skinner KA, Rudel LL. (2000). Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J Lipid Res 41, 1991–2001. [PubMed] [Google Scholar]
- Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. (2009). Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 186, 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Camacho P. (2004). Ca2+-dependent redox modulation of SERCA 2b by ERp57. J Cell Biol 164, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Yen JH, Weng CY, Wang L, Ha CL, Wu MJ. (2014). Lipid peroxidation end product 4-hydroxy-trans-2-nonenal triggers unfolded protein response and heme oxygenase-1 expression in PC12 cells: roles of ROS and MAPK pathways. Toxicology 315, 24–37. [DOI] [PubMed] [Google Scholar]
- Lipper CH, Stofleth JT, Bai F, Sohn YS, Roy S, Mittler R, Nechushtai R, Onuchic JN, Jennings PA. (2019). Redox-dependent gating of VDAC by mitoNEET. Proc Natl Acad Sci U S A 116, 19924–19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lismont C, Nordgren M, Van Veldhoven PP, Fransen M. (2015). Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol 3, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun SC. (2017). NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Conte M, Carroll KS. (2013). The redox biochemistry of protein sulfenylation and sulfinylation. J Biol Chem 288, 26480–26488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone A, Harris RA, Singh O, Betts DH, Cumming RC. (2018). p66Shc activation promotes increased oxidative phosphorylation and renders CNS cells more vulnerable to amyloid beta toxicity. Sci Rep 8, 17081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T. (2012). Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. Embo J 31, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes EM, Raturi A, Shenkman M, Ortiz Sandoval C, Yap MC, Wu J, Janowicz A, Myhill N, Benson MD, Campbell RE, et al. (2013). Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J Cell Sci 126, 3893–3903. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ. (2020). Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol 32, 101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, McBride SL, Harper ME. (2013). Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem Sci 38, 592–602. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, Harper ME. (2011). Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem 286, 21865–21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel AM, Walla MD, Faccenda A, Martin SL, Tanis RM, Piroli GG, Adam J, Kantor B, Mutus B, Townsend DM, Frizzell N. (2017). Succination of protein disulfide isomerase links mitochondrial stress and endoplasmic reticulum stress in the adipocyte during diabetes. Antioxid Redox Signal 27, 1281–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margittai E, Sitia R. (2011). Oxidative protein folding in the secretory pathway and redox signaling across compartments and cells. Traffic 12, 1–8. [DOI] [PubMed] [Google Scholar]
- Marino M, Stoilova T, Giorgi C, Bachi A, Cattaneo A, Auricchio A, Pinton P, Zito E. (2015). SEPN1, an endoplasmic reticulum-localized selenoprotein linked to skeletal muscle pathology, counteracts hyperoxidation by means of redox-regulating SERCA2 pump activity. Hum Mol Genet 24, 1843–1855. [DOI] [PubMed] [Google Scholar]
- Marino SM, Gladyshev VN. (2010). Structural analysis of cysteine S-nitrosylation: a modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol 395, 844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui R, Ferran B, Oh A, Croteau D, Shao D, Han J, Pimentel DR, Bachschmid MM. (2020). Redox regulation via glutaredoxin-1 and protein S-Glutathionylation. Antioxid Redox Signal 32, 677–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattie S, Riemer J, Wideman JG, McBride HM. (2018). A new mitofusin topology places the redox-regulated C terminus in the mitochondrial intermembrane space. J Cell Biol 217, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. (2005). Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene 24, 1011–1020. [DOI] [PubMed] [Google Scholar]
- Messina A, Reina S, Guarino F, De Pinto V. (2012). VDAC isoforms in mammals. Biochim Biophys Acta 1818, 1466–1476. [DOI] [PubMed] [Google Scholar]
- Miller CG, Holmgren A, Arner ESJ, Schmidt EE. (2018). NADPH-dependent and -independent disulfide reductase systems. Free Radic Biol Med 127, 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miseta A, Csutora P. (2000). Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol 17, 1232–1239. [DOI] [PubMed] [Google Scholar]
- Missiroli S, Bonora M, Patergnani S, Poletti F, Perrone M, Gafa R, Magri E, Raimondi A, Lanza G, Tacchetti C, et al. (2016). PML at Mitochondria-Associated membranes is critical for the repression of autophagy and cancer development. Cell Rep 16, 2415–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pera M, Larrea D, Guardia-Laguarta C, Agrawal RR, Velasco KR, Yun TD, Stavrovskaya IG, Xu Y, Koo SY, et al. (2020). The alzheimer's disease-associated C99 fragment of APP regulates cellular cholesterol trafficking. Embo J 39, e103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarky E, Cantley LC. (2015). Diverting glycolysis to combat oxidative stress. In: Innovative Medicine: Basic Research and Development, eds. K. Nakao, N. Minato, S. Uemoto, Tokyo: Springer, 3–23. [PubMed]
- Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T. (2003). Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of akt. J Biol Chem 278, 50226–50233. [DOI] [PubMed] [Google Scholar]
- Murphy MP. (2009). How mitochondria produce reactive oxygen species. Biochem J 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T. (2008). The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell 19, 2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S, Lipton SA. (2013). Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron 78, 596–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Park YY, Yoon G, Cho H, Lee JH. (2010). Co-treatment with hepatocyte growth factor and TGF-beta1 enhances migration of HaCaT cells through NADPH oxidase-dependent ROS generation. Exp Mol Med 42, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani N, Shanmughapriya S, Madesh M. (2018). Molecular regulation of MCU: implications in physiology and disease. Cell Calcium 74, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VD, Saaranen MJ, Karala AR, Lappi AK, Wang L, Raykhel IB, Alanen HI, Salo KE, Wang CC, Ruddock LW. (2011). Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J Mol Biol 406, 503–515. [DOI] [PubMed] [Google Scholar]
- Nielson JR, Fredrickson EK, Waller TC, Rendon OZ, Schubert HL, Lin Z, Hill CP, Rutter J. (2017). Sterol oxidation mediates Stress-Responsive Vms1 translocation to mitochondria. Mol Cell 68, 673–685.e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa-Kawakita M, Ferhi O, Soilihi H, Bras ML, Lallemand-Breitenbach V, de The H. (2017). PML is a ROS sensor activating p53 upon oxidative stress. J Exp Med 214, 3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numajiri N, Takasawa K, Nishiya T, Tanaka H, Ohno K, Hayakawa W, Asada M, Matsuda H, Azumi K, Kamata H, et al. (2011). On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN). Proc Natl Acad Sci U S A 108, 10349–10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C, Voelker DR, Langer T. (2011). Making heads or tails of phospholipids in mitochondria. J Cell Biol 192, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace NJ, Weerapana E. (2013). Diverse functional roles of reactive cysteines. ACS Chem Biol 8, 283–296. [DOI] [PubMed] [Google Scholar]
- Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. (2000). Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem 275, 23685–23692. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Paradies V, Ruggiero FM. (2009). Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium 45, 643–650. [DOI] [PubMed] [Google Scholar]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, Stefani DD, Rizzuto R. (2014). MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell 53, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Men X, Zhang W, Wang H, Xu S, Fang Q, Liu H, Yang W, Lou J. (2012). Involvement of dynamin-related protein 1 in free fatty acid-induced INS-1-derived cell apoptosis. PLoS One 7, e49258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. (2019). Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med 51, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrungaro C, Zimmermann KM, Kuttner V, Fischer M, Dengjel J, Bogeski I, Riemer J. (2015). The Ca(2+)-dependent release of the Mia40-Induced MICU1-MICU2 dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab 22, 721–733. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Virgilio FD, Pozzan T, Rizzuto R. (2001). The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of bcl-2 action. Embo J 20, 2690–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. (2008). Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27, 6407–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poet GJ, Oka OB, van Lith M, Cao Z, Robinson PJ, Pringle MA, Arner ES, Bulleid NJ. (2017). Cytosolic thioredoxin reductase 1 is required for correct disulfide formation in the ER. Embo J 36, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potting C, Tatsuta T, Konig T, Haag M, Wai T, Aaltonen MJ, Langer T. (2013). TRIAP1/PRELI complexes prevent apoptosis by mediating intramitochondrial transport of phosphatidic acid. Cell Metab 18, 287–295. [DOI] [PubMed] [Google Scholar]
- Primeau JO, Armanious GP, Fisher ME, Young HS. (2018). The SarcoEndoplasmic reticulum calcium ATPase. Subcell Biochem 87, 229–258. [DOI] [PubMed] [Google Scholar]
- Prior KK, Wittig I, Leisegang MS, Groenendyk J, Weissmann N, Michalak M, Jansen-Durr P, Shah AM, Brandes RP. (2016). The endoplasmic reticulum chaperone calnexin is a NADPH oxidase NOX4 interacting protein. J Biol Chem 291, 7045–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent J, Zunino R, Sugiura A, Mattie S, Shore GC, McBride HM. (2015). MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol Cell 59, 941–955. [DOI] [PubMed] [Google Scholar]
- Pulli I, Lassila T, Pan G, Yan D, Olkkonen VM, Tornquist K. (2018). Oxysterol-binding protein related-proteins (ORPs) 5 and 8 regulate calcium signaling at specific cell compartments. Cell Calcium 72, 62–69. [DOI] [PubMed] [Google Scholar]
- Qi H, Li L, Shuai J. (2015). Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Sci Rep 5, 7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R. (2018). Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci U S A 115, 5839–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, Mammucari C, Gherardi G, Rizzuto R. (2016). Calcium at the center of cell signaling: interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem Sci, 41, 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raturi A, Gutierrez T, Ortiz-Sandoval C, Ruangkittisakul A, Herrera-Cruz MS, Rockley JP, Gesson K, Ourdev D, Lou PH, Lucchinetti E, et al. (2016). TMX1 determines cancer cell metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux. J Cell Biol 214, 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raturi A, Ortiz-Sandoval C, Simmen T. (2014). Redox dependence of endoplasmic reticulum (ER) Ca(2)(+) signaling. Histol Histopathol 29, 543–552. [DOI] [PubMed] [Google Scholar]
- Rhee SG. (2016). Overview on peroxiredoxin. Mol Cells 39, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Woo HA, Kang D. (2018). The role of peroxiredoxins in the transduction of H2O2 signals. Antioxid Redox Signal 28, 537–557. [DOI] [PubMed] [Google Scholar]
- Riemer J, Schwarzlander M, Conrad M, Herrmann JM. (2015). Thiol switches in mitochondria: operation and physiological relevance. Biol Chem 396, 465–482. [DOI] [PubMed] [Google Scholar]
- Rizza S, Filomeni G. (2017). Chronicles of a reductase: biochemistry, genetics and physio-pathological role of GSNOR. Free Radic Biol Med 110, 19–30. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, et al. (2009). Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787, 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. (1998). Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Robb EL, Hall AR, Prime TA, Eaton S, Szibor M, Viscomi C, James AM, Murphy MP. (2018). Control of mitochondrial superoxide production by reverse electron transport at complex I. J Biol Chem 293, 9869–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Liu J, Song BL, Li BL, Chang CC, Chang TY. (2015). Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): enzymes with multiple sterols as substrates and as activators. J Steroid Biochem Mol Biol 151, 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Vila A, Navarro-Lerida I, Sanchez-Alvarez M, Bosch M, Calvo C, Lopez JA, Calvo E, Ferguson C, Giacomello M, Serafini A, et al. (2016). Interplay between hepatic mitochondria-associated membranes, lipid metabolism and caveolin-1 in mice. Sci Rep 6, 27351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Gallego R, Mund M, Cosentino K, Schneider J, Unsay J, Schraermeyer U, Engelhardt J, Ries J, Garcia-Saez AJ. (2016). Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. Embo J 35, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, et al. (2013). EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d'Azzo A. (2009). GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell 36, 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G, Xie W, Reiken SR, Marks AR. (2015). Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A 112, 11389–11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauro VS, Strickland KP. (1990). Triacylglycerol synthesis and diacylglycerol acyltransferase activity during skeletal myogenesis. Biochem Cell Biol 68, 1393–1401. [DOI] [PubMed] [Google Scholar]
- Schonfeld P, Reiser G. (2017). Inhibition of beta-oxidation is not a valid therapeutic tool for reducing oxidative stress in conditions of neurodegeneration. J Cereb Blood Flow Metab 37, 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuiki I, Daum G. (2009). Phosphatidylserine decarboxylases, key enzymes of lipid metabolism. IUBMB Life 61, 151–162. [DOI] [PubMed] [Google Scholar]
- Scire A, Cianfruglia L, Minnelli C, Bartolini D, Torquato P, Principato G, Galli F, Armeni T. (2019). Glutathione compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. Biofactors 45, 152–168. [DOI] [PubMed] [Google Scholar]
- Sen T, Saha P, Jiang T, Sen N. (2020). Sulfhydration of AKT triggers tau-phosphorylation by activating glycogen synthase kinase 3beta in Alzheimer’s disease. Proc Natl Acad Sci U S A 117, 4418–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane M, Wada M, Morita K, Hayashi N, Kunimatsu R, Matsumoto Y, Matsuzaki F, Nakatsumi H, Ohta K, Tamura Y, Nakayama KI. (2020). Protrudin and PDZD8 contribute to neuronal integrity by promoting lipid extraction required for endosome maturation. Nat Commun 11, 4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A. (2018). VDAC1 functions in Ca(2+) homeostasis and cell life and death in health and disease. Cell Calcium 69, 81–100. [DOI] [PubMed] [Google Scholar]
- Shutt T, Geoffrion M, Milne R, McBride HM. (2012). The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep 13, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G. (2005). PACS-2 controls endoplasmic reticulum-mitochondria communication and bid-mediated apoptosis. Embo J 24, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Herrera-Cruz MS. (2018). Plastic mitochondria-endoplasmic reticulum (ER) contacts use chaperones and tethers to mould their structure and signaling. Curr Opin Cell Biol 53, 61–69. [DOI] [PubMed] [Google Scholar]
- Simmen T, Lynes EM, Gesson K, Thomas G. (2010). Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM). Biochim Biophys Acta 1798, 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau KF, Vizcay-Barrena G, Lin WL, Xu YF, Lewis J, et al. (2014). ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun 5, 3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica R, Paillusson S, Gomez-Suaga P, Mitchell JC, Lau DH, Gray EH, Sancho RM, Vizcay-Barrena G, De Vos KJ, Shaw CE, et al. (2016). ALS/FTD-associated FUS activates GSK-3beta to disrupt the VAPB-PTPIP51 interaction and ER-mitochondria associations. EMBO Rep 17, 1326–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr.(2009). The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem 284, 5352–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS. (2011). Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci U S A 108, 16098–16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustarsic EG, Ma T, Lynes MD, Larsen M, Karavaeva I, Havelund JF, Nielsen CH, Jedrychowski MP, Moreno-Torres M, Lundh M, et al. (2018). Cardiolipin synthesis in brown and beige fat mitochondria is essential for systemic energy homeostasis. Cell Metab 28, 159–174.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. (2006). Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima M, Shichiri M, Hagihara Y, Yoshida Y, Niki E. (2012). Reactivity toward oxygen radicals and antioxidant action of thiol compounds. Biofactors 38, 240–248. [DOI] [PubMed] [Google Scholar]
- Tasseva G, Bai HD, Davidescu M, Haromy A, Michelakis E, Vance JE. (2013). Phosphatidylethanolamine deficiency in mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J Biol Chem 288, 4158–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier S, Martin-Martin N, de The H, Carracedo A, Lallemand-Breitenbach V. (2017). Promyelocytic leukemia protein, a protein at the crossroad of oxidative stress and metabolism. Antioxid Redox Signal 26, 432–444. [DOI] [PubMed] [Google Scholar]
- Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G, et al. (2016). The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol Med 8, 569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushioda R, Miyamoto A, Inoue M, Watanabe S, Okumura M, Maegawa KI, Uegaki K, Fujii S, Fukuda Y, Umitsu M, et al. (2016). Redox-assisted regulation of Ca2+ homeostasis in the endoplasmic reticulum by disulfide reductase ERdj5. Proc Natl Acad Sci U S A 113, E6055–E6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. (2008). Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. (1990). Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265, 7248–7256. [PubMed] [Google Scholar]
- Vance JE. (1991). Newly made phosphatidylserine and phosphatidylethanolamine are preferentially translocated between rat liver mitochondria and endoplasmic reticulum. J Biol Chem 266, 89–97. [PubMed] [Google Scholar]
- Vance JE. (2014). MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta 1841, 595–609. [DOI] [PubMed] [Google Scholar]
- Vance JE. (2015). Phospholipid synthesis and transport in mammalian cells. Traffic 16, 1–18. [DOI] [PubMed] [Google Scholar]
- Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Ko HS, Lee YI, Dawson VL, Dawson TM, et al. (2013). Sulfhydration mediates neuroprotective actions of Parkin. Nat Commun 4, 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. (2012). PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19, 1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtle FN, Burkhart JM, Rao S, Gerbeth C, Hinrichs J, Martinou JC, Chacinska A, Sickmann A, Zahedi RP, Meisinger C. (2012). Intermembrane space proteome of yeast mitochondria. Mol Cell Proteomics 11, 1840–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Song P, Du L, Tian W, Yue W, Liu M, Li D, Wang B, Zhu Y, Cao C, et al. (2011). Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem 286, 11649–11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PT, Garcin PO, Fu M, Masoudi M, St-Pierre P, Pante N, Nabi IR. (2015). Distinct mechanisms controlling rough and smooth endoplasmic reticulum contacts with mitochondria. J Cell Sci 128, 2759–2765. [DOI] [PubMed] [Google Scholar]
- Wang T, Cao Y, Zheng Q, Tu J, Zhou W, He J, Zhong J, Chen Y, Wang J, Cai R, et al. (2019). SENP1-Sirt3 signaling controls mitochondrial protein acetylation and metabolism. Mol Cell 75, 823–834 e825. [DOI] [PubMed] [Google Scholar]
- Wible RS, Ramanathan C, Sutter CH, Olesen KM, Kensler TW, Liu AC, Sutter TR. (2018). NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 7, e31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman JG, Balacco DL, Fieblinger T, Richards TA. (2018). PDZD8 is not the ‘functional ortholog’ of Mmm1, it is a paralog. F1000Res 7, 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, Lopez Del Amo V, Arndt S, Bueno D, Tenzer S, Hanschmann EM, Berndt C, Methner A. (2020). Redox modifications of proteins of the mitochondrial fusion and fission machinery. Cells 9, 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Nam SM, Kim JH, Das R, Choi SK, Nguyen TT, Quan X, Choi SJ, Chung CH, Lee EY, et al. (2015). Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis 6, e1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZW, Zhang J, Ancrum T, Manevich Y, Townsend DM, Tew KD. (2017). Glutathione S-transferase P-mediated protein S-glutathionylation of resident endoplasmic reticulum proteins influences sensitivity to drug-induced unfolded protein response. Antioxid Redox Signal 26, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G. (2004). Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol 167, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoboue ED, Sitia R, Simmen T. (2018). Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis 9, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Kim DS, Lee GH, Kim KW, Kim HR, Chae HJ. (2011). Apoptosis induced by manganese on neuronal SK-N-MC cell line: endoplasmic reticulum (ER) stress and mitochondria dysfunction. Environ Health Toxicol 26, e2011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y. (2006). Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103, 2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gibhardt CS, Will T, Stanisz H, Korbel C, Mitkovski M, Stejerean I, Cappello S, Pacheu-Grau D, Dudek J, et al. (2019). Redox signals at the ER-mitochondria interface control melanoma progression. Embo J 38, e100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Park J, Han SJ, Yang SY, Yoon HJ, Park I, Woo HA, Lee SR. (2020). Redox regulation of tumor suppressor PTEN in cell signaling. Redox Biol 34, 101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Liu T, Jin S, Wang X, Qu M, Uhlen P, Tomilin N, Shupliakov O, Lendahl U, Nister M. (2011). Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. Embo J 30, 2762–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. [DOI] [PubMed] [Google Scholar]
- Zunino R, Braschi E, Xu L, McBride HM. (2009). Translocation of SenP5 from the nucleoli to the mitochondria modulates DRP1-dependent fission during mitosis. J Biol Chem 284, 17783–17795. [DOI] [PMC free article] [PubMed] [Google Scholar]