Abstract
The microsporidian, Nosema maddoxi Becnel, Solter, Hajek, Huang, Sanscrainte & Estep, infects brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), populations in North America and Asia and causes decreased fitness in infected insects. This host overwinters as adults, often in aggregations in sheltered locations, and variable levels of mortality occur over the winter. We investigated pathogen prevalence in H. halys adults before, during, and after overwintering. Population level studies resulted in detection of N. maddoxi in H. halys in 6 new US states, but no difference in levels of infection by N. maddoxi in autumn versus the following spring. Halyomorpha halys that self-aggregated for overwintering in shelters deployed in the field were maintained under simulated winter conditions (4°C) for 5 months during the 2021–2022 winter and early spring, resulting in 34.6 ± 4.8% mortality. Over the 2020–2021 and 2021–2022 winters, 13.4 ± 3.5% of surviving H. halys in shelters were infected with N. maddoxi, while N. maddoxi infections were found in 33.4 ± 10.8% of moribund and dead H. halys that accumulated in shelters. A second pathogen, Colletotrichum fioriniae Marcelino & Gouli, not previously reported from H. halys, was found among 46.7 ± 7.8% of the H. halys that died while overwintering, but levels of infection decreased after overwintering. These 2 pathogens occurred as co-infections in 11.1 ± 5.9% of the fungal-infected insects that died while overwintering. Increasing levels of N. maddoxi infection caused epizootics among H. halys reared in greenhouse cages after overwintering.
Keywords: entomopathogens, Halyomorpha halys, overwintering, Nosema maddoxi, Colletotrichum fioriniae
Introduction
One overwintering strategy used by insects in temperate climates is for individuals to find sheltered locations in the autumn, within which they remain inactive and often in diapause until the following spring. Adults of some of these taxa often overwinter in aggregations (e.g., hemipterans, coleopterans, and lepidopterans) and this can enhance their fitness and survivorship by mitigating the effects of abiotic stressors, such as low temperatures and desiccation, during this period (Yoder and Smith 1997, Su et al. 2007, Murakami et al. 2019, Szejner-Sigal and Williams 2022). However, biotic stressors such as increased infection by pathogens that can negatively impact survival have also been reported for species that aggregate for overwintering. For example, significant levels of infection by the lethal fungal pathogen Beauveria bassiana (Balsamo-Crivelli) Vuillemin have been documented in overwintering aggregations of the coccinellids, Coccinella septempunctata (L.) (Güven et al. 2015) and Olla v-nigrum (Mulsant) (Cottrell and Shapiro 2003).
In North America, the invasive brown marmorated stink bug, Halyomorpha halys (Stål), has been a significant agricultural and nuisance pest since the late 2000s (Leskey and Nielsen 2018). Diapausing adults of H. halys overwinter from September–October to April–May (Bergh et al. 2017, Bergh and Quinn 2018), often in aggregations that typically occur in tight spaces within dry and dark locations (Toyama 2006, Toyama et al. 2011, Cullum et al. 2020) in both natural settings outdoors (Lee et al. 2014) and inside buildings (Hancock et al. 2019). The numbers of adults in these aggregations can vary substantially (Inkley 2012, Lee et al. 2014) and the aggregated individuals are often in contact with each other (Song and Lee 2021), especially when in large aggregations (JCB unpubl. data). During the overwintering period, studies have documented from 11.7 to 85.0% mortality (e.g., Bergh et al. 2017, Costi et al. 2017, Lowenstein and Walton 2018), although the potential impact of biotic agents on overwintering mortality has not been investigated.
The microsporidian pathogen, Nosema maddoxi Becnel, Solter, Hajek, Huang, Sanscrainte & Estep, is native to North America, Asia, and possibly Europe, where it infects pentatomids (Hajek et al. 2017, Kereselidze et al. 2020, AEH unpubl. data). Nosema maddoxi is the best-known pathogen of H. halys and it also infects some native stink bug species in the United States (Hajek et al. 2017). In the United States, H. halys infected by N. maddoxi were first detected from declining laboratory colonies, and subsequently from field populations. In 2017 and 2018, N. maddoxi was documented from H. halys adults collected from 31 sites in 11 US states, averaging 18.9% infection (Preston et al. 2020a). Nosema maddoxi infects H. halys when ingested but generally does not cause acute disease (=fast mortality); yet, laboratory studies of infected H. halys revealed decreased longevity, egg production, and egg viability in adult females, and decreased nymphal survival (Preston et al. 2020b).
Given the importance of H. halys as an agricultural and nuisance pest in the United States, we were interested in the infection by and impact of N. maddoxi on its populations immediately before, during, and after the H. halys overwintering period. In particular, we investigated whether infection levels in H. halys populations changed from before overwintering to after overwintering (e.g., from autumn to the following spring). Preliminary studies (C. Preston, pers. comm.) indicated increased infection by N. maddoxi in H. halys adults at 2 of 3 sites in Pennsylvania in spring 2018 compared with autumn 2017. However, studies by Kereselidze et al. (2023) in the Guria region of the Republic of Georgia found that infection of H. halys adults by N. maddoxi was greater in autumn than in spring, although infection levels did not differ in the Samegrelo region. Greater infection in autumn compared with spring may be explained by the mortality of infected H. halys while overwintering. Mortality of H. halys overwintering outdoors (e.g., Haye et al. 2014, Costi et al. 2017, Lowenstein and Walton 2018) as well as in buildings (e.g., Chambers et al. 2019, Ciancio et al. 2021) occurs at levels that can be significant.
Here, we report the results of studies that investigated N. maddoxi prevalence in field populations of H. halys before overwintering in the autumn versus directly after overwintering the following spring, using specimens collected from 40 sites in 17 states. From 2020 to 2022, we also evaluated infection levels of fungal pathogens in H. halys during the period between the onset and end of overwintering as well as post-overwintering, using adults that had settled in overwintering shelters in the autumn at 2 residences in Virginia. Although our initial focus was on N. maddoxi, we discovered another entomopathogen, Colletotrichum fioriniae Marcelino & Gouli, that was especially prevalent among H. halys that did not survive overwintering.
Materials and Methods
Sampling Adult H. halys in Autumn and Spring
Adult H. halys were collected from plants, buildings, and traps in autumn 2019 and/or spring 2020 at a total of 40 sites across 17 states (1–6 sites per state) (Fig. 1). At each site, collections were made from the same general areas in autumn (n = 35 sites) or spring (18 sites). Collections occurred from 5 September to 30 October, 2019 and from 15 April to 5 June, 2020 and the adult H. halys were held at −20°C before diagnosis. Sample sizes were between 15 and 30 adults per collection period per site. Across the 40 sites, 22 were sampled only in autumn 2019, 6 only in spring 2020 (overwintered adults), and 12 sites were sampled in both seasons.
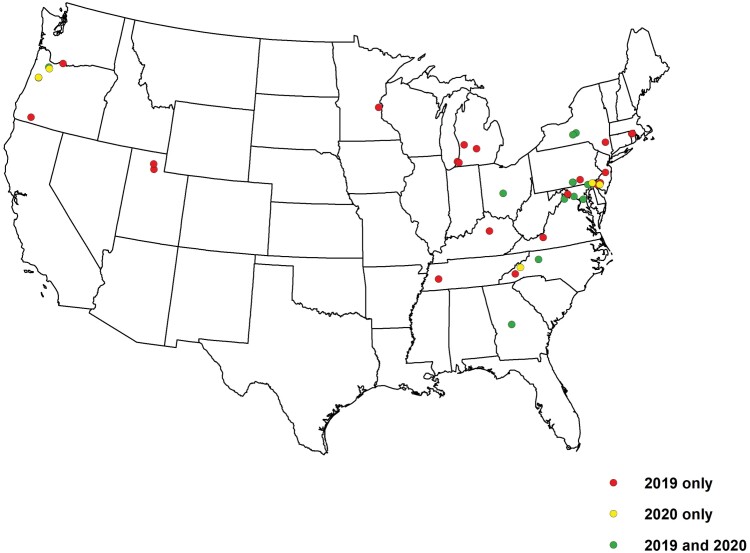
Fig. 1.
Distribution of sites sampled in autumn 2019, spring 2020, or both, for comparisons of Nosema maddoxi infection levels.
Sampling Overwintering Adult H. halys
Field sites and overwintering shelters.
Adult H. halys that had voluntarily entered and settled in the wooden shelters described in Bergh et al. (2017) during their dispersal to overwintering sites were used for these studies. To deploy the shelters, wooden apple crates (38.1 × 29.8 × 45.7 cm) were attached to one another end to end using plastic cable ties to create a stack of 3 crates that was positioned upright (Fig. 2). One shelter was affixed to the inside bottom of the top crate in each stack via screws drilled through a flange that extended above the back of each shelter. A piece of brown corrugated plastic attached to the top of each stack overhung the opening, providing additional protection from rain, and a cinder block in the bottom crate provided stability. Access to the interior of the shelters by H. halys that landed on them or walked to them was via a space between the top of the front panel and the overhanging roof, and through the bottom, which was open during deployment (see Bergh et al. 2017).
Fig. 2.
Deployment of an overwintering shelter for Halyomorpha halys within the top apple crate on a stack of wooden apple crates. Arrow indicates the shelter and its location.
In 2020 and 2021, shelters were placed in the field during the week prior to 21 September, when the onset of mass movement of adult H. halys to buildings and overwintering sites in nature occurs (Bergh and Quinn 2018). Shelters were deployed against east-facing walls, on which the largest counts of invading adult H. halys had been recorded consistently in previous studies (Bergh and Quinn 2018). In 2020, shelters were deployed at each of 2 private residences that had experienced large annual invasions of adult H. halys in previous autumns. In 2020, 6 shelters were deployed at Site 1 (38°44ʹ50.70″N, 78°06ʹ54.14″W), which was a single-story home in a large clearing at the top of a forested knoll in Rappahannock Co., VA, and 5 were deployed at Site 2 (39°07ʹ50.33″N, 78°13ʹ29.57″W), a multistory residence in a clearing with woodlands on 3 sides, in Frederick Co., VA. The 2 sites were 43.7 km from each other. In 2021, shelters (n = 8) were deployed only at Site 1, due to the sale of the property at Site 2. In mid-November of both years, the bottom of each shelter was sealed with a fitted wood panel and the shelters were removed from the crates and transported to Virginia Tech’s Alson H. Smith, Jr., Agricultural Research and Extension Center, near Winchester, VA, where they were stored in dark sealed plastic bags in a dark walk-in cooler at 4°C, to simulate winter.
Sampling protocol for overwintering adults.
To assess H. halys for pathogen infection, sub-samples of overwintering adults were removed from the same randomly-chosen shelter from each site in mid-December, mid-February, and mid-April during the winter of 2020–2021 and from site 1 in 2021–2022. These adults were brought to room temperature, with the intention of collecting living individuals. After 24 h, some individuals had begun to walk and these were collected. Those that did not exhibit this behavior within that period (which comprised most of the sub-sample), were evaluated by gently extending the legs laterally from the body with a dissecting needle. Adults that retracted their legs fully and immediately after this manipulation were considered alive and added to the sample of living individuals.
After the mid-April collection of live adults from shelters in 2021, all remaining adults in the shelter from each site were moved into separate 30.5 cm3 screened cages (BioQuip, Rancho Dominguez, CA) in a greenhouse at ambient temperature and photoperiod. Each cage was provisioned with young, potted green bean plants (Phaseolus vulgaris L.), fresh green beans, dried seeds, nuts, young peaches, and water (see below). After 2 weeks, when living H. halys had become active and moved to plants or cage walls, all dead and moribund adults were collected from the bottom of each cage. Confirmation of the status of these adults was based on an assessment of whether they showed partial and lethargic movements when handled (=moribund) or no movement (=dead). The high mortality of the remaining caged adults observed subsequently prompted the collection of additional samples on 26 May and 3 June, 2021.
Following the collection of the 3 sequential sub-samples from individual shelters in winter–spring 2020–2021 and 2021–2022, subsequent sampling differed between the years. For brevity and clarity, Table 1 provides details of the sampling timeline for each year, including all sample sizes, and footnotes explaining the reasons underlying the differences. All individuals (live, and/or dead, and moribund) collected at each sampling time point were placed in labeled bags and held in a freezer at −80°C. These were shipped with cold packs via overnight courier to AEH, where they remained frozen until evaluation for pathogen infection.
Table 1.
Timeline of collections of adult Halyomorpha halys for assessments of pathogen infection, 2020–2021 and 2021–2022
| Year | Site | Collection date | Shelter or cagea | Number H. halys adults collected | ||
|---|---|---|---|---|---|---|
| Live | Moribund | Dead | ||||
| 2020–2021 | 1 | 8 Dec | Shelterb | 50 | ||
| 2 | 8 Dec | Shelterb | 30 | |||
| 1 | 9 Feb | Shelterb | 52 | |||
| 2 | 9 Feb | Shelterb | 30 | |||
| 1 | 14 Apr | Shelterb | 84 | |||
| 2 | 14 Apr | Shelterb | 35 | |||
| 1 | 28 Apr | Cagec | 38 | 268 | ||
| 2 | 28 Apr | Cagec | 14 | 68 | ||
| 1 | 26 May | Cagec | 30 | 114 | ||
| 2 | 26 May | Cagec | 30 | 36 | ||
| 1 | 3 Jun | Cagec | 36 | 176 | ||
| 2 | 3 Jun | Cagec | 65 | 68 | ||
| 2021–2022 | 1 | 15 Dec | Shelterd | 53 | ||
| 1 | 16 Feb | Shelterd | 30 | |||
| 1 | 11 Apr | Shelterd | 62 | |||
| 1 | 8 Apr | Cagee | 44 (DM) | |||
| 1 | 8 Apr | Cagee | 67 (DM) | |||
| 1 | 6 Jun | Cagef | 68 | 637 | ||
| 1 | 7 Jun | Cageg | 7 | 148 | ||
| 1 | 8 Jun | Cageh | 3 | 15 | ||
| 1 | 8 Jun | Cagei | 43 | 846 | ||
aWooden overwintering shelter used to collect adult H. halys in autumn and screened 30 cm3 cage used to hold H. halys adults under ambient conditions in a greenhouse after removal from shelters.
bOne shelter from Sites 1 and 2, from which sub-samples of live adults were collected within 24 h after removal from cold storage on 8 Dec, 9 Feb, and 14 Apr 2020–2021.
cCage containing all adults remaining in the shelter from each site after the 2021 Apr 14 living sub-sample was taken. Collections on 28 Apr represent moribund and dead adults from each shelter that had accumulated from when shelters were colonized, through time at 4°C and until 14 d after cages had been moved to the greenhouse; 14 days was the time required by living H. halys to become active. Collections on 26 May represent sub-samples of living and dead adults from each cage in the greenhouse. The sub-sample on 26 May was prompted by observations of high mortality in cages. The sample on 3 Jun represents all adults (living and dead) remaining in each cage.
dOne shelter from Site 1, from which sub-samples of live adults were collected within 24 h after removal from cold storage on 15 Dec, 16 Feb, and 11 Apr 2021–2022. After collection of live adults on 2022 Apr 11, adults remaining in that shelter were lost. Consequently, adults taken from 2 other shelters deployed at Site 1 were used for comparisons of infections (see footnote e).
eTwo shelters were removed from cold storage on 8 Apr and all adults from each were placed in separate cages for 1–2 wks, after which moribund and dead adults were collected (but were merged for each cage = DM).
f–iOne or two shelters were removed from cold storage on each date shown and all adults were placed in separate cages for 1–2 wks, after which all dead adults were collected. Different footnotes indicate different shelters.
Pathogen Diagnosis
Halyomorpha halys individuals that were collected, frozen, and diagnosed had no external fungal growth. Each individual H. halys was diagnosed for N. maddoxi as described in Preston et al. (2020b). Briefly, H. halys adults were placed in 0.05% Tween and macerated with a micropestle. Samples were observed at 400× under phase contrast to detect the presence of N. maddoxi or C. fiorinae spores. After C. fioriniae was first found, it was isolated on potato dextrose agar and deposited in the USDA ARSEF culture collection (isolates # 14595 & 14596). A separate paper on the identification of C. fioriniae from H. halys is being prepared, in which positive results from Koch’s postulates will also be presented (J.B. González unpubl. data). Colletotrichum fioriniae spores differ markedly in width and length from those of N. maddoxi. Nosema maddoxi spores were very consistent in shape and size: oblong with a length × width of 4.72 ± 0.05 × 2.19 ± 0.03 µm (Hajek et al. 2017). Colletotrichum fioriniae spores from our samples were oblong but variable in size (length × width: 9.34 ± 0.29 × 3.91 ± 0.10 µm; range 6–15 × 3–5) and always larger than spores of N. maddoxi (AEH unpubl. data). For the study comparing infections between individuals collected in the autumn and/or the spring, 1,351 H. halys were diagnosed, while for the study that compared infections during and after the overwintering period, 1,220 and 501 H. halys were diagnosed in 2020–2021 and 2021–2022, respectively. In 2020–2021, all samples collected were diagnosed while in 2021–2022, all samples from Dec. through April were diagnosed while at least 100 individuals were diagnosed from each cage for June 2022 samples.
Data Analysis
The percentages of H. halys adults infected with N. maddoxi in autumn versus spring were compared using Proc Glimmix (SAS 2021). To compare infection levels between live and dead adults when overwintering, comparisons utilized the percent N. maddoxi infections in April. For 2022, dead and moribund adults from the same shelter from which live adults were collected in April were lost, so dead and moribund adults from 2 other shelters maintained at the same location and collected in April were jointly substituted. Analyses comparing pathogens associated with living adults (while overwintering) versus dead adults (that had accumulated throughout overwintering) were conducted using Proc Glimmix. Infection levels for dead and moribund were merged as there was no significant difference between them (N. maddoxi: F1,4 = 4.25, P = 0.1082; C. fioriniae: F1,4 = 1.26, P = 0.3247).
Results
Infection in H. halys during Autumn versus Spring
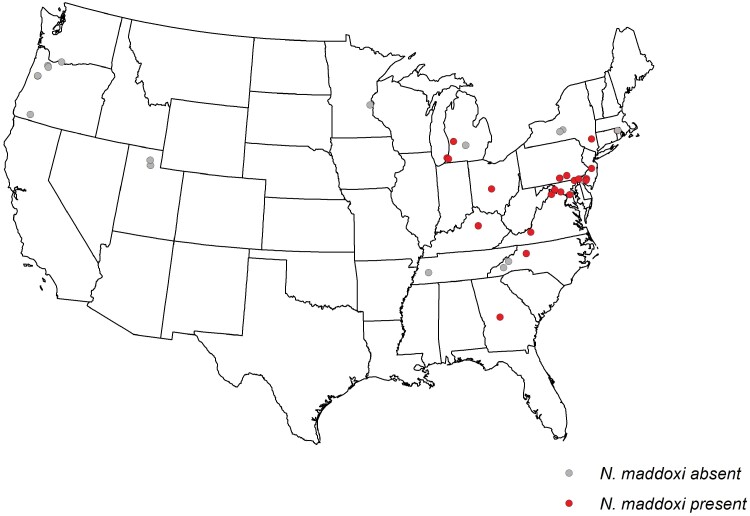
Adult H. halys infected by N. maddoxi were found at 18 of the 35 sites sampled across 17 states in autumn 2019 (Fig. 3). Among all sites, percent infection in autumn averaged 6.8 ± 1.7% and among sites with positive detections, the percent infection averaged 13.3 ± 2.4% (range = 3.3–40.0%). In spring 2020, N. maddoxi was detected in H. halys from 11 of the 18 sites sampled across 10 states, with infection averaging 7.6 ± 2.5% among all sites and 13.4 + 2.5% at the positive sites (range = 3.3–36.0%). Although low infection levels were recorded at some sites from both autumn and spring sampling, N. maddoxi was confirmed in H. halys for the first time in Delaware, Georgia, Michigan, New Jersey, Rhode Island, and Tennessee. At the 12 sites across 8 states from which samples were collected in both autumn 2019 and spring 2020, the average N. maddoxi infection in autumn (12.3 ± 3.9%) did not differ significantly from that in the spring (8.2 ± 3.4%) (F1,11 = 3.03; P = 0.1098). Across all collections, N. maddoxi infection levels were consistently and significantly higher in the Mid-Atlantic region (n = 27 sites, 10.2 ± 2.2%) than in the other regions sampled (n = 26 sites, 3.6 ± 1.4%) (chi-squared = 25.9319; P < 0.00001).
Fig. 3.
Presence/absence of Nosema maddoxi in Halyomorpha halys collected from sites sampled in autumn 2019 and/or spring 2020.
Infection among Overwintering and Post-overwintered H. halys
In 2020–2021 and 2021–2022, N. maddoxi infection levels in living adult H. halys did not differ significantly among the samples from mid-December, mid-February, and mid-April (F2,4 = 1.15; P = 0.4036). Nosema maddoxi infection levels among living adults during this period averaged 13.4 ± 4.5%. In early-mid April, when overwintering was ended, N. maddoxi infection levels were significantly higher in dead (33.4 ± 10.8%) than live (11.1 ± ± 5.6%) adults for pooled years and sites (F1,2 = 33.70; P = 0.0284) (Supplementary Fig. 1).
Surprisingly, a second pathogen, C. fioriniae, was prevalent in moribund and dead individuals within shelters. Colletotrichum fioriniae was never found infecting live H. halys collected from shelters in December, February, or April in 2020–2021 or 2021–2022 (Supplementary Fig. 1). Infection levels by C. fioriniae in the dead and moribund H. halys collected in April from overwintering shelters in 2021 and 2022 averaged 46.7 ± 7.8% (range: 36.0–68.3%). Among moribund and dead H. halys that had accumulated in shelters, C. fioriniae co-occurred with N. maddoxi in 11.1 + 5.9% of infected moribund and dead adults (Supplementary Fig. 1).
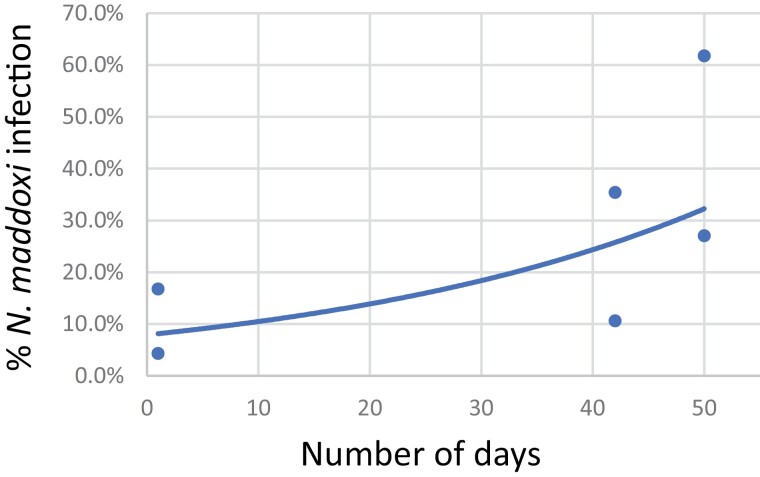
In 2021, when infection in caged post-overwintering adults was evaluated, N. maddoxi infections increased with time in cages (y = 0.0792e0.0281x; r2 = 0.4561) (Fig. 4). Colletotrichum fioriniae infections were 0–4.6% in living H. halys in cages in May and June 2021. Percent C. fioriniae infections among the dead adults in die-offs occurring in May and June ranged from 9.7% to 27.8% but no significant trend across time occurred, except that these infection levels were much lower than among dead H. halys from overwintering shelters.
Fig. 4.
Increase in Nosema maddoxi infection levels starting with percent infection among living overwintering adults in shelters in April 2021. Adult Halyomorpha halys were moved to greenhouse cages (day 1) and percent N. maddoxi infection post-overwintering (living and dead) increased through 2 die-offs observed 26 May (after 42 d in cages) and 3 June (50 d in cages).
The percentage of dead and moribund adults in the 3 shelters removed from cold storage in April 2022 (after 5 months of cold storage), averaged 34.6 ± 4.8%. Among the 4 additional shelters that remained at 4°C until early June (approximately 7 months of cold storage), mortality was significantly greater by early June (84.5 ± 2.0%; F1,4 =71.84; P = 0.0011). However, although mortality in shelters at 4°C more than doubled between approximately 5 and 7 months of cold storage, infection levels of N. maddoxi and C. fioriniae in dead adults decreased significantly, from 76.3 ± 3.2% in April, to 43.7 ± 7.4% in June, and the percent of H. halys dying that were not infected with either pathogen increased from 23.7 ± 3.2% for April samples to 56.3 ± 7.4% for June samples (F1,4 = 44.29; P = 0.0026).
Discussion
Our population-level results did not demonstrate a consistent significant trend in changes in N. maddoxi infection levels from before (autumn) to after (spring) overwintering. These results agree with those from the Republic of Georgia, where N. maddoxi infection levels did not change significantly from autumn to spring in one region, although N. maddoxi infection levels were higher in autumn than spring in a second region where the density of H. halys was higher (Kereselidze et al. 2023). This trend could be explained by the death of infected H. halys while overwintering, which would result in fewer infected H. halys surviving. Our overwintering studies demonstrated that more H. halys that died while overwintering contained spores of N. maddoxi and/or C. fioriniae than H. halys surviving overwintering, strongly suggesting that adults infected with these fungal pathogens had a greater chance of mortality during overwintering. Among individuals of the coccinellid Harmonia axyridis (Pallas) infected with the ectoparasitic fungus, Hesperomyces virescens Thaxter, higher mortality in infected versus healthy insects occurred while overwintering (Knapp et al. 2022). These experimental studies with ladybeetles suggested that a mix of energy exhaustion and decreased immune response were responsible for the increased mortality of infected H. axyridis while overwintering.
Infection levels of N. maddoxi in H. halys in 2021 increased over time after overwintering, causing epizootics. In this case, post-overwintering H. halys were provided with food on which they could regain energy. However, these adults were confined in cages in a greenhouse for >1 month and, in such conditions, it is well known that N. maddoxi transmission among individuals occurs (Hajek et al. 2017). In the absence of other stressors, infection by this chronic pathogen does not always cause H. halys mortality (Preston et al. 2020b). However, it is possible that the increased age of adults that had survived overwintering since the previous autumn may affect their immune response to pathogen infection, thus potentially explaining increased infection and death post-overwintering. Indeed, the overwintering generation of adult H. halys is known to be more susceptible to insecticide exposure than the subsequent F1 and F2 adult generations (Leskey et al. 2014), indicating their depleted state after overwintering.
Our study of infection by pathogens during and after overwintering was conducted with H. halys maintained at constant 4°C for 5 months to mimic overwintering conditions, but this did not provide the variable temperature and moisture levels that would occur when overwintering outdoors and perhaps to a lesser extent the conditions experienced by those overwintering in protected spaces in buildings. In addition, in our study all H. halys ended overwintering at the same time, while in nature adult H. halys leaving overwintering aggregations occurs over a protracted period (Skillman et al. 2018). Studies of overwintering H. halys found that adults held for 20 weeks at 4°C showed reductions in energy reserves and survival (Taylor et al. 2017, Skillman et al. 2018), as would have occurred in this study. We found that while H. halys were kept at constant 4°C for 5 months, levels of N. maddoxi infection in live adults did not change within the overwintering period. The dead and moribund adults that were collected from shelters when overwintering ended are assumed to have accumulated throughout the period in cold storage, and possibly also during the 2-month period before the shelters were moved from the field to cold storage. Levels of N. maddoxi in dead and moribund adults were greater than in live adults during the overwintering period, and we hypothesize that N. maddoxi infections negatively impacted survival of some of the H. halys while overwintering.
Levels of mortality of H. halys held at 4°C until June in 2022 were very high compared with mortality of individuals in cold storage until April. Levels of mortality of uninfected H. halys increased among adults held in shelters at 4°C until June, compared with April. We hypothesize that increased mortality was due to the inability to survive the cold for such a prolonged period, as would be predicted based on Taylor et al. (2017).
Molecular methods identified C. fioriniae as the entomopathogen that had infected and killed many of the dead H. halys from overwintering shelters and, albeit in lower numbers, post-overwintering bugs in 2021 that died the following May and June. This pathogen had not previously been reported from H. halys, and Koch’s postulates were used to demonstrate its pathogenicity (AEH unpubl. data). Colletotrichum fioriniae was never found infecting live H. halys during overwintering sampling and was rarely found in live H. halys post-overwintering. High C. fioriniae infection levels mostly occurred among H. halys that had died within shelters during the period between settling in shelters through overwintering. These results may suggest that H. halys infected with C. fioriniae did not survive very long (as opposed to N. maddoxi which generally causes more of a chronic disease) as would be consistent with our pathogenicity tests. Low percentages of C. fioriniae infections in well-fed living H. halys post-overwintering could suggest that hosts not stressed by low temperatures and lack of food might better prevent or survive C. fioriniae infections.
Colletotrichum fioriniae (previously known as C. acutatum J.H. Simmonds or C. acutatum var. fioriniae J.A.P. Marcelino & S. Gouli) is a well-known fungal plant pathogen (some diseases that are caused are called anthracnose) and plant endophyte, occurring worldwide (Damm et al. 2012, Martin and Peter 2021). However, it is also known to cause epizootics in another North America hemipteran, the elongate hemlock scale (Fiorinia externa Ferris) (Marcelino et al. 2008). Laboratory bioassays with C. fioriniae also demonstrated infection in larvae of the lepidopteran, Spodoptera exigua (Hübner), the whitefly Bemisia argentifolii Bellows & Perring, and the thrips Frankliniella occidentalis Pergande (Marcelino et al. 2009). In a study of whether H. halys can act as a vector of the fungal disease anthracnose to tomato plants, Voshell (2015) recovered what was reported as C. acutatum spores from stylets and legs of H. halys feeding on infected tomatoes but reported low mortality of H. halys exposed to infected tomatoes. The fungal isolate investigated by Voshell (2015) was likely C. fioriniae, based on the available partial GAPDH nucleotide sequence (although it was reported as C. acutatum). Thus, H. halys adults entering overwintering aggregations in autumn could be contaminated with Colletotrichum spores on their bodies, acquired by feeding on infected plants, and the pathogen could have subsequently infected and killed the aggregated overwintering adults. Our finding C. fioriniae infections in adults from both sites in 2020–2021 and in the one site sampled the following season, suggests that infections by this pathogen among overwintering H. halys are not uncommon in this region.
While our surveys in the autumn and spring of 2019–2020 yielded the first confirmed detections of N. maddoxi in 6 states, indications of its presence in Delaware and New Jersey had been suggested earlier. Nosema maddoxi was first noted in 2012 in an H. halys laboratory colony in Florida that was initiated from bugs from Delaware (Hajek et al. 2017). At US field sites, N. maddoxi was probably first discovered (as an unnamed microsporidian species) in New Jersey in 2014 by Dr. Bryan Petty working with Dr. Anne Nielsen at Rutgers (A.E.H. unpubl. data). In Oregon and Utah, N. maddoxi was not found during this study but had been detected previously (Preston et al. 2020a), possibly indicating a patchy distribution in those states and/or low infection levels, making its detection less likely.
In summary, we found no differences in N. maddoxi infection levels among H. halys adults collected in the field in autumn versus spring and we found that 2 fungal pathogens infected and killed H. halys while overwintering and post-overwintering. The microsporidian, N. maddoxi, was always more prevalent in dead H. halys in overwintering shelters compared with bugs that survived overwintering. The fungus, C. fioriniae, is reported for the first time as a pathogen of H. halys. High levels of infection by C. fioriniae were found among bugs that did not survive overwintering. After overwintering, surviving bugs that were caged with food in a greenhouse showed steadily increasing levels of N. maddoxi infection over 50 d, but C. fioriniae infection levels were lower than in the adults that had died in overwintering shelters. We conclude that these 2 pathogens infecting H. halys contribute to mortality during and after overwintering, and this underlies some of the reported overwintering mortality. Activity of pathogens during and after overwintering caused reductions in the numbers of adult H. halys surviving to reproduce in spring, and N. maddoxi infections would also decrease the fecundity of survivors (Preston et al. 2020b).
Supplementary Material
Acknowledgments
We thank Sana Gardescu for conducting the autumn vs spring sampling project and the following for providing samples: Heather Andrews and Rick Hilton, Oregon State Univ., Ricardo Bessin, Univ. Kentucky, Lucas Canino, Hudson Valley Res. Lab., Cornell, Ted Cottrell, Ruth Hamlyn, Gracie Galindo, Kim Hoelmer, and Kathy Tatman, USDA-ARS, George Hamilton, Rutgers, Nancy Harding and Paula Shrewsbury, Univ. Maryland, Sarah Holle and Bill Hutchinson, Univ. Minnesota, Greg Krawczyk, Penn State Univ., Lori Spears, Utah State Univ., Scott Stewart, Univ. Tennessee, Lisa Tewksbury, Univ. Rhode Island, Jim Walgenbach and Emily Ogburn, North Carolina State Univ., Celeste Welte, Ohio State Univ., Julianna Wilson, Michigan, State Univ. We thank David Harris, Sarah Stefanik, Natalie Sacco, Destiny Smith, and Anais Ocegueda for diagnosing samples from the overwintering study and Thomas Everest and David Harris for organizing these data. We thank E. Luzander and A. Liebhold for assistance with maps.
Author Contributions
Ann Hajek (Conceptualization-Equal, Data curation-Equal, Formal analysis-Lead, Funding acquisition-Lead, Project administration-Lead, Resources-Equal, Supervision-Equal, Writing – original draft-Lead, Writing – review & editing-Supporting), Samuel Brandt (Methodology-Lead, Writing – review & editing-Supporting), Jennifer Gonzalez (Methodology-Equal, Validation-Equal, Writing – review & editing-Supporting), J. Christopher Bergh (Investigation-Equal, Project administration-Equal, Supervision-Equal, Writing – original draft-Supporting, Writing – review & editing-Equal)
Contributor Information
Ann E Hajek, Department of Entomology, Cornell University, Ithaca, NY 14853-2601, USA.
Samuel N Brandt, Virginia Tech, Alson H. Smith, Jr. Agricultural Research and Extension Center, 595 Laurel Grove Road, Winchester, VA 22602, USA.
Jennifer B González, Biology Department, Nazareth College, Rochester, NY 14618, USA; Department of Environmental Studies, Dartmouth College, Hanover, NH 03755, USA.
J Christopher Bergh, Virginia Tech, Alson H. Smith, Jr. Agricultural Research and Extension Center, 595 Laurel Grove Road, Winchester, VA 22602, USA.
Funding
This project was funded by USDA NIFA SCRI 2016-51181-25409 and Hatch 1017785.
References
- Bergh JC, Morrison WR III, Joseph SV, Leskey TC.. Characterizing spring emergence of adult Halyomorpha halys using experimental overwinter shelters and commercial pheromone traps. Entomol Exp Appl. 2017:162:336–345. [Google Scholar]
- Bergh JC, Quinn NF.. Can the dispersal behavior of Halyomorpha halys (Hemiptera: Pentatomidae) inform the use of insecticide-treated netting to mitigate homeowner issues from its fall invasion? Environ Entomol. 2018:47(6):1501–1508. 10.1093/ee/nvy120 [DOI] [PubMed] [Google Scholar]
- Chambers BD, Leskey TC, Pearce AR, Kuhar TP.. Responses of overwintering Halyomorpha halys (Hemiptera: Pentatomidae) to dead conspecifics. J Econ Entomol. 2019:112(3):1489–1492. 10.1093/jee/toz011 [DOI] [PubMed] [Google Scholar]
- Ciancio JJ, Turnbull KF, Gariepy TD, Sinclair BJ.. Cold tolerance, water balance, gas exchange, and diapause in overwintering brown marmorated stink bugs. J Insect Physiol. 2021:128:104171. 10.1016/j.jinsphys.2020.104171 [DOI] [PubMed] [Google Scholar]
- Costi E, Haye T, Maistrello L.. Biological parameters of the invasive brown marmorated stink bug, Halyomorpha halys, in southern Europe. J Pest Sci. 2017:90(4):1059–1067. 10.1007/s10340-017-0899-z [DOI] [Google Scholar]
- Cottrell TE, Shapiro-Ilan DI.. Susceptibility of a native and an exotic lady beetle (Coleoptera: Coccinellidae) to Beauveria bassiana. J Invertebr Pathol. 2003:84(2):137–144. 10.1016/j.jip.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Cullum JP, Nixon LJ, Morrison WR, Raupp MJ, Shrewsbury PM, Venugopal PD, Martinson H, Bergh JC, Leskey TC.. Influence of landscape factors and abiotic conditions on dispersal behavior and overwintering site selection by Halyomorpha halys (Hemiptera: Pentatomidae). J Econ Entomol. 2020:113(4):2016–2021. 10.1093/jee/toaa077 [DOI] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, Crous PW.. The Colletotrichum acutatum species complex. Stud Mycol. 2012:73(1):37–113. 10.3114/sim0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güven O, Göllüoǧlu H, Ceryngier P.. Aestivo-hibernation of Coccinella septempunctata (Coleoptera: Coccinellidae) in a mountainous area of southern Turkey: is dormancy at high altitudes adaptive? Eur J Entomol. 2015:112:41–48. [Google Scholar]
- Hajek AE, Solter LF, Maddox JV, Huang W-F, Estep AE, Krawczyk G, Weber DC, Hoelmer KA, Sanscrainte ND, Becnel JJ.. Nosema maddoxi sp. nov. (Microsporidia, Nosematidae), a widespread pathogen of the green stink bug Chinavia hilaris (Say) and the brown marmorated stink bug Halyomorpha halys (Stål). J Eukaryot Microbiol. 2017:65:315–330. [DOI] [PubMed] [Google Scholar]
- Hancock TJ, Lee D-H, Bergh JC, Morrison WR III, Leskey TC.. Presence of the invasive brown marmorated stink bug Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) on home exteriors during the autumn dispersal period: results generated by citizen scientists. Agric For Entomol. 2019:21:99–180. [Google Scholar]
- Haye T, Abdallah S, Gariepy T, Wyniger D.. Phenology, life table analysis and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J Pest Sci. 2014:87(3):407–418. 10.1007/s10340-014-0560-z [DOI] [Google Scholar]
- Inkley DB. Characteristics of home invasion by the brown marmorated stink bug (Hemiptera: Pentatomidae). J Entomol Sci. 2012:47(2):125–130. 10.18474/0749-8004-47.2.125 [DOI] [Google Scholar]
- Kereselidze M, Pilarska D, Linde A, Sanscrainte ND, Hajek AE.. Nosema maddoxi infecting the brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in the Republic of Georgia. Biocontr Sci Technol. 2020:30:1083–1089. 10.1080/09583157.2020.1787346 [DOI] [Google Scholar]
- Kereselidze M, Pilarska D, Ujmajuridze L, Linde A, Guntadze N, Hajek A.. Prevalence and distribution of Nosema maddoxi infection in Halyomorpha halys, an invasive pest of hazelnut orchards in Georgia . Acta Hort. 2023. In press. [Google Scholar]
- Knapp M, Rericha M, Haelewaters D, Gonzalez E.. Fungal ectoparasites increase winter mortality of ladybird hosts despite limited effects on their immune system. Proc Roy Soc B. 2022:289:20212538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-H, Cullum JP, Anderson JL, Daugherty JL, Beckett LM, Leskey TC.. Characterization of overwintering sites of the invasive brown marmorated stink bug in natural landscapes using human surveyors and detector canines. PLoS One. 2014:9(4):e91575. 10.1371/journal.pone.0091575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskey TC, Nielsen AL.. Impact of the invasive brown marmorated stink bug in North America and Europe: history, biology, ecology, and management. Annu Rev Entomol. 2018:63:599–618. 10.1146/annurev-ento-020117-043226 [DOI] [PubMed] [Google Scholar]
- Leskey TC, Short BD, Lee D-H.. Efficacy of insecticide residues on adult Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) mortality and injury in apple and peach orchards. Pest Mange Sci. 2014:70:1097–1104. [DOI] [PubMed] [Google Scholar]
- Lowenstein DM, Walton VM.. Halyomorpha halys (Hemiptera: Pentatomidae) winter survival, feeding activity, and reproduction rates based on episodic cold shock and winter temperature regimes. J Econ Entomol. 2018:111(3):1210–1218. 10.1093/jee/toy093 [DOI] [PubMed] [Google Scholar]
- Marcelino J, Giordano R, Gouli S, Gouli V, Parker BL, Skinner M, TeBeest D, Cesnik R.. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutatum var. fioriniae var. nov.) infection of a scale insect. Mycologia. 2008:100(3):353–374. 10.3852/07-174r [DOI] [PubMed] [Google Scholar]
- Marcelino JAP, Gouli S, Parker BL, Skinner M, Giordano R.. Entomopathogenic activity of a variety of the fungus Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa. J. Ins Sci. 2009:9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PL, Peter KA.. Quantification of Colletotrichum fioriniae in orchards and deciduous forests indicates it is primarily a leaf endophyte. Phytopathol. 2021:111(2):333–344. 10.1094/PHYTO-05-20-0157-R. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Hasegawa E, Watanabe S.. Effects of color morph on aggregation formation for hibernation in an extremely color polymorphic ladybug Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Entomol Ornithol Herpetol Curr Res. 2019:8:10–13. 10.35248/2161-0983.19.8.219 [DOI] [Google Scholar]
- Preston CE, Agnello AM, Hajek AE.. Nosema maddoxi (Microsporidia: Nosematidae) in brown marmorated stink bug (Hemiptera: Pentatomidae) populations in the US. Biol Control. 2020a:144:104213. 10.1016/j.biocontrol.2020.104213 [DOI] [Google Scholar]
- Preston CE, Agnello AM, Vermeylen FM, Hajek AE.. Impact of Nosema maddoxi on the survival, development, and female fecundity of Halyomorpha halys. J Invertebr Pathol. 2020b:169:107303. 10.1016/j.jip.2019.107303 [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS software release 9.4. Cary (NC): SAS Institute; 2021. [Google Scholar]
- Skillman V, Wiman NG, Lee JC.. Nutrient declines in overwintering Halyomorpha halys populations. Entomol Exp Appl. 2018:166:778–789. [Google Scholar]
- Song H, Lee D-H.. Formation of overwintering aggregation of Halyomorpha halys (Hemiptera: Pentatomicae) in laboratory conditions. Entomol Res. 2021:51:230–237. [Google Scholar]
- Su Y-L, Lu Z-Z, Song J, Miao W.. Effect of overwintering aggregation on energy metabolism in the firebug, Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Acta Entomol Sin. 2007:50:1300–1303. [Google Scholar]
- Szejner-Sigal A, Williams CM.. Aggregations reduce winter metabolic rates in the diapausing ladybeetle Hippodamia convergens. J Ins Physiol. 2022:137:104357. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Coffee PL, Hamby KA, Dively GP.. Laboratory rearing of Halyomorpha halys: methods to optimize survival and fitness of adults during and after diapause. J Pest Sci. 2017:90:1069–1077. [Google Scholar]
- Toyama M, Ihara F, Yaginuma K.. Formation of aggregations in adults of the brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): the role of antennae in short-range locations. Appl Entomol Zool. 2006:41(2):309–315. 10.1303/aez.2006.309 [DOI] [Google Scholar]
- Toyama M, Ihara F, Yaginuma K.. Photo-response of the brown marmorated stink bug, Halyomorpha halys (Stal) (Heteroptera: Pentatomidae), and its role in the hiding behavior. Appl Entomol Zool. 2011:46:37–40. [Google Scholar]
- Voshell RJ. Interactions of brown marmorated stink bug, Colletotrichum acutatum and trap crops in organic tomato production [M.S. thesis]. Morgantown, West Virginia: West Virginia Univ.; 2015. [Google Scholar]
- Yoder JA, Smith BE.. Enhanced water conservation in clusters of convergent lady beetles, Hippodamia convergens. Entomol Exp Appl. 1997:85(1):87–89. 10.1046/j.1570-7458.1997.00237.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.