Abstract
Axonal regeneration and functional recovery are poor after spinal cord injury (SCI), typified by the formation of an injury scar. While this scar was traditionally believed to be primarily responsible for axonal regeneration failure, current knowledge takes a more holistic approach that considers the intrinsic growth capacity of axons. Targeting the SCI scar has also not reproducibly yielded nearly the same efficacy in animal models compared to these neuron-directed approaches. These results suggest that the major reason behind central nervous system (CNS) regeneration failure is not the injury scar but a failure to stimulate axon growth adequately. These findings raise questions about whether targeting neuroinflammation and glial scarring still constitute viable translational avenues. We provide a comprehensive review of the dual role of neuroinflammation and scarring after SCI and how future research can produce therapeutic strategies targeting the hurdles to axonal regeneration posed by these processes without compromising neuroprotection.
Keywords: spinal cord injury, axonal regeneration, glial scar, neuroinflammation, astrocyte heterogeneity, microglia heterogeneity, fibroblast heterogeneity, neuroprotection
1. Background
In response to tissue injury, the body swiftly seeks to restore homeostasis by minimizing damage spread and recovering normal tissue function. The wound healing process involves conserved and coordinated phases of hemostasis, inflammation, and remodeling. However, in the adult mammalian central nervous system (CNS), this healing process is prolonged and culminates in the formation of an injury scar characterized by a fibrotic core surrounded by a limitans border of astrocytes, termed the glial border or glial scar, in the lesion’s immediate penumbra (Adams and Gallo, 2018).
Central nervous system regeneration is notoriously poor after traumatic spinal cord injury (SCI) (Bradbury and McMahon, 2006). The injury scar was once viewed as the primary obstacle to successful regeneration, leading to numerous attempts to inhibit its essential components (Silver and Miller, 2004). However, contemporary research has largely moved past this notion, adopting a more comprehensive approach considering neuron-intrinsic properties. Advances in neural stem cell (NSC) transplantation and the administration of neurotrophic factors have achieved unprecedented levels of neural regeneration and functional recovery (Lu et al., 2012; Anderson et al., 2018), even progressing to early-phase clinical trials (Liu et al., 2022).
Conversely, strategies targeting scar components have not reproducibly yielded noteworthy beneficial effects in animal models (Zheng and Tuszynski, 2023). Moreover, genetic manipulations that deplete or attenuate glial or stromal cells in the glial scar have revealed numerous protective functions in SCI (Wahane and Sofroniew, 2022). There is also currently no FDA-approved drug targeting scar-associated neuroinflammation in the management of SCI. These observations prompt a critical question: is targeting the SCI scar beneficial, and should it remain a focus of future research? Answering this question requires a deeper understanding of the roles of various cells in SCI. This review discusses recent advancements in SCI cell biology, reflects on current study limitations, and proposes a trajectory for future research in this area.
2. Formation and composition of the glial scar
2.1. Primary and secondary spinal cord injury
Tissue response to injury begins with local vascular damage and the infiltration of blood-borne immune cells (Gurtner et al., 2008; Eming et al., 2014). The CNS injury response follows a similar pattern (Burda Joshua and Sofroniew Michael, 2014; Orr and Gensel, 2018; Bradbury and Burnside, 2019; Anjum et al., 2020; Hellenbrand et al., 2021).
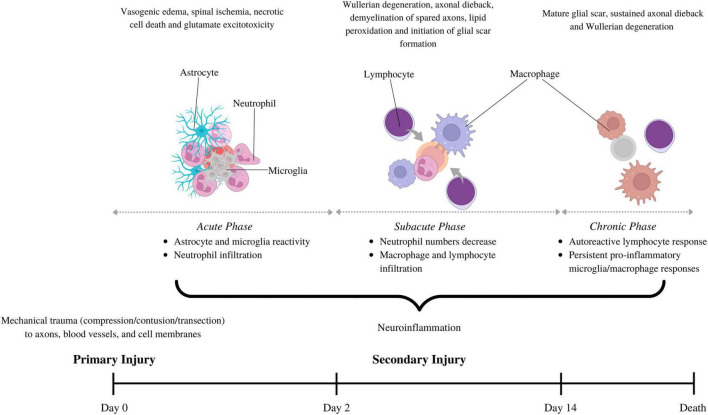
Spinal cord injuries are divided into primary and secondary injury mechanisms (Figure 1; Sekhon and Fehlings, 2001; Alizadeh et al., 2019). The primary injury can take many forms, all involving mechanical forces that disrupt several ascending and descending tracts, blood vessels, the blood-spinal cord barrier (BSCB), and cell membranes of neurons and glial cells (Tator and Fehlings, 1991; Tator, 1998; Rossignol et al., 2007). This leads to local tissue ischemia and necrotic cell death, manifesting as spinal and potential neurogenic shock, neurotransmitter and electrolyte imbalances, and the release of pro-inflammatory damage-associated molecular patterns (DAMPs) such as ATP and high-mobility group box-1 (HMGB1) (Bianchi, 2007; Tran et al., 2018b; Anjum et al., 2020).
FIGURE 1.
The pathophysiology behind spinal cord injury involves primary injury, describing initial mechanical trauma to the spinal trauma, and secondary injury, which sustains spinal cord damage. The secondary phase is further divided into acute (0–2 days), subacute (2–14 days) and chronic (>14 days), each with its own pathophysiological hallmarks. Neuroinflammation is a part of all the secondary injury phases, but the cell types involved vary. Astrocytes and microglia are the first to become reactive. They secrete cytokines/chemokines that recruit neutrophils in the acute phase. Neutrophil numbers subside in the subacute phase, coinciding with macrophage and lymphocyte infiltration. Macrophage and lymphocytes can stay elevated in the chronic phase to drive persistent inflammation and impair wound resolution. This figure was created with Biorender.com.
These events give rise to a secondary injury response, a series of cellular, molecular, and biochemical mechanisms that chronically exacerbate tissue loss and impede functional recovery (Allen, 1911; Oyinbo, 2011; Fehlings et al., 2012). Traditionally, the secondary injury response to SCI has been categorized into acute [0–2 days-post injury (dpi)], subacute (2–14 dpi), and chronic phases (>14 dpi), each with unique and overlapping pathophysiological hallmarks. For example, the acute phase features vascular hemorrhage, vasogenic edema, necrotic cell death, neurotransmitter and electrolyte imbalance, and excitotoxicity (Oyinbo, 2011; Alizadeh et al., 2019). The subacute phase involves demyelination of spared axons due to continued oligodendrocyte apoptosis, Wallerian degeneration of the distal stump of transected axons, and axonal dieback of the proximal end. The chronic phase is typified by a mature SCI scar, comprising a fibrotic core—often containing a central cystic cavity—encircled by a glial scar of astrocytes and oligodendrocyte progenitor cells (OPCs). We refer readers to other reviews for additional information on the unique and overlapping disease processes occurring in these phases (Oyinbo, 2011; Alizadeh et al., 2019).
2.2. Neuroinflammation in spinal cord injury
Neuroinflammation refers to the induction of reactive states in various CNS cell types and the recruitment of circulating innate and adaptive immune cells (Bareyre and Schwab, 2003). Neuroinflammation is a salient feature of all the phases of secondary injury but varies with intensity, peaking in the acute and subacute phases (Oyinbo, 2011; Anwar et al., 2016). CNS resident cells, such as astrocytes and microglia, are the first to react to the primary injury site, secreting pro-inflammatory cytokines and chemokines that recruit blood-borne immune cells to the lesion epicenter and activate them (Fawcett and Asher, 1999; Schnell et al., 1999; Davalos et al., 2005; Rice et al., 2007; Burda and Sofroniew, 2014).
During the acute phase, neutrophils are recruited to the SCI lesion site, where they exert deleterious effects by producing reactive oxygen species (ROS), pro-inflammatory cytokines, and proteases, which exacerbate neuronal loss and neuroinflammation (Dinkel et al., 2004; Nguyen et al., 2007; Bi et al., 2021; Dolma and Kumar, 2021; Feng et al., 2021). However, some studies have reported that infiltrating neutrophils in SCI contribute to the resolution of neuroinflammation and create an environment conducive to axonal regeneration (de Castro et al., 2004; Stirling et al., 2009; Ghasemlou et al., 2010; Schreiber et al., 2013). A seminal paper by Stirling et al. demonstrated that depleting neutrophils in the acute phase of SCI worsens tissue damage, reduces local levels of growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), and compromises functional recovery (David et al., 2009). Neutrophil numbers in the SCI lesion site begin to decline in the subacute phase, coinciding with the infiltration of monocyte-derived macrophages (MDMs) and adaptive B and T lymphocytes (Neirinckx et al., 2014).
Macrophages infiltrate the lesion site after 2–3 dpi, peaking around 7–10 dpi (Perry and Teeling, 2013; Andrew and Samuel, 2014). Macrophages originate either from circulating monocytes, termed MDMs, or CNS resident macrophages in the perivascular spaces and meninges. Reactive microglia and MDMs occupy distinct locations in the fibrotic scar, with MDMs at the center and reactive microglia in the periphery, interfacing with the astrocyte border (David and Kroner, 2011; Zhou et al., 2014; Wang et al., 2015). These cell types also differ temporally: microglia proliferate rapidly at the lesion site, peaking at 14 dpi, whereas MDMs peak at 7–10 dpi and again at 60 dpi (Popovich et al., 1997; Bellver-Landete et al., 2019; Milich et al., 2021). While the numbers of macrophages and microglia decline in the chronic phase, this resolution is incomplete, with phagocytic pro-inflammatory macrophages and reactive microglia persisting months after SCI onset and contributing to impaired wound healing (Fleming et al., 2006; Prüss et al., 2011).
Lymphocytes begin infiltrating the lesion site in the subacute phase and remain elevated in the chronic phase, driving autoimmunity and neuroinflammation (Jones, 2014; Allison and Ditor, 2015). At the lesion site, antigen-presenting cells such as macrophages present self-antigens to T-cells, thereby fostering a chronic autoimmune T-cell response (Jones, 2014). Autoreactive CD4+ T-cells can adopt a T helper-1 (Th1) type phenotype, secreting pro-inflammatory cytokines that induce pro-inflammatory/anti-repair microglia and macrophage polarization states (Yu and Fehlings, 2011). Autoreactive CD4+ T-cells can also stimulate humoral immune responses by promoting B-cell differentiation into plasma cells producing autoantibodies against neuronal and myelin antigens (Hayes et al., 2002; Ankeny et al., 2006, 2009).
2.3. Compartmentalization of the SCI lesion site
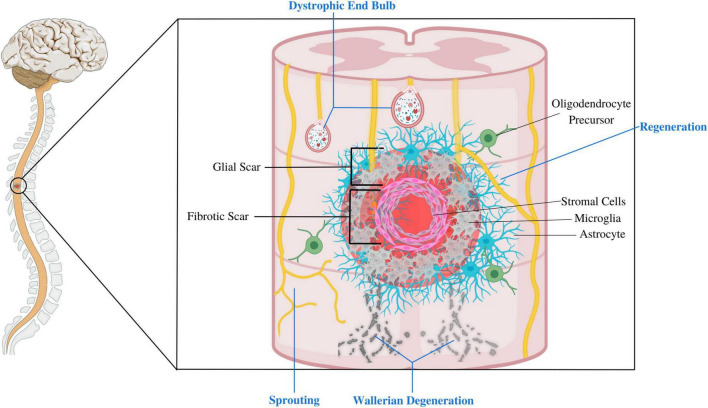
Recent research advocates dividing the SCI scar into three compartments: the inner fibrotic scar, the surrounding astroglial border (or glial scar), and the adjacent reactive neural parenchyma (Figure 2; O’Shea et al., 2017). These compartments exhibit unique cellular compositions and transcriptional profiles (Gong et al., 2023). The central fibrotic scar consists of macrophages, other blood-borne inflammatory cells like lymphocytes, and stromal cells such as fibroblasts and pericytes. Over time, blood-borne inflammatory cells recede (albeit not completely), leaving stromal elements to constitute the bulk of the fibrotic scar (Beck et al., 2010). The astrocyte border comprises proliferating astrocytes up until 14 dpi, after which the structure matures. NG2+ OPCs are also found in this region (Keirstead et al., 1998; Miron et al., 2013). The adjacent reactive neural parenchyma comprises neurons that display active synaptic remodeling and circuit reorganization. Glial cells in this region are composed of reactive astrocytes, microglia, and OPCs, but they differ in their magnitude of reactivity from their counterparts in the lesion core and border (Khakh and Sofroniew, 2015). For example, astrocytes outside the lesion site upregulate glial-fibrillary acid protein (GFAP) but do not dramatically change their morphology and orientation and can even stimulate the regeneration of adult CNS neurons (Davies et al., 1999; Li X. et al., 2020). In contrast, astrocytes within the scar border more drastically upregulate GFAP and significantly change their morphology and orientation to form a compact glial scar that impedes regeneration (Tran et al., 2018b).
FIGURE 2.
This figure depicts the composition of different compartments of the SCI lesion site. The fibrotic scar mainly comprises stromal cells, blood-borne inflammatory cells, and microglia. This core is surrounded by glial scar, composed of an astrocyte limitans border, oligodendrocyte progenitor cells (OPCs), and microglia. Extensive reactive changes also take place in the adjacent neural parenchyma, including axonal dieback and synaptic remodeling and axonal sprouting of spared axons. Glial cells such as astrocytes, OPCs and microglia in this peri-lesional area are reactive, but differ in their magnitude of reactivity than those at the lesion site. This figure was created with Biorender.com.
It is essential to state that the term “glial scar” was traditionally used to refer to the whole SCI scar. This is now considered a misnomer by prominent researchers as it carries negative connotations that depict the glial scar only as an obstacle to axon regeneration and functional recovery. This oversimplification disregards the dynamic and multifaceted nature of the host response to SCI (Adams and Gallo, 2018; Sofroniew, 2020; Wahane and Sofroniew, 2022). Adopting accurate terminology by describing the different compartments and time-dependent roles of the SCI scar allows for a more nuanced understanding of the SCI response, particularly for non-expert readers. Using broad or general terms like “glial scar” to refer to the SCI lesion site perpetuates the misconception that the SCI scar is inherently inhibitory and requires wholesale/indiscriminate attenuation (Wahane and Sofroniew, 2022), which may hinder the development of effective therapeutic strategies targeting the diverse functions of glial and stromal cells. Unfortunately, there is still no consensus regarding the correct use of the term “glial scar” or agreed-upon alternate terms. In this review, we adopt the terminology used by Adams and Gallo (2018), who used “glial scar” to refer to the glial cell border—composed of astrocytes and OPCs—surrounding the fibrotic core of the lesion.
2.4. Extracellular regeneration inhibitors in the glial scar
Myelin’s role as a CNS axonal regeneration inhibitor was first described in the 1980s (Schwab and Strittmatter, 2014). Myelin-associated molecules inhibiting CNS regeneration include Nogo (reticulon-4), Nogo-receptors (NgR), myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp) (Bradbury and McMahon, 2006). While experiments in the early 2000s found that systemic or local administration of anti-Nogo receptor antibodies enhanced CNS regeneration (Silver and Miller, 2004; Lu et al., 2012), others utilizing genetic knockout (KO) of myelin-associated molecules did not consistently reproduce these results (Anderson et al., 2018; Liu et al., 2022). This discrepancy suggests that myelin may not play a significant role in CNS regeneration failure, but that inhibiting myelin-associated molecules could enhance the remodeling of spared axons to provide functional improvement (Liu et al., 2022). The RESET trial, completed on June 2022, is a two-part clinical trial studying AXER-204, a human fusion protein that functions as a decoy for myelin-associated inhibitors Nogo-A, MAG, and OMgp (NCT03989440).
Chondroitin sulfate proteoglycans (CSPGs) are produced by various cell types, including astrocytes, microglia, fibroblasts, pericytes, and OPCs, and play a role in inhibiting axon growth (Gallo et al., 1987; Tran et al., 2018b). CSPGs bind to the surface receptor protein tyrosine phosphatase-σ (RPTPσ) on neurons, disrupting neuronal autophagy and leading to axon growth cone dystrophy and regeneration failure (Shen et al., 2009). Strategies to counteract CSPGs include administering chondroitinase ABC to digest glycosaminoglycan (GAG) side chains, preventing CSPG formation, or abrogating RPTPσ signaling (see Tran et al., 2018a,b for a detailed review). These approaches reproducibly alleviate axon growth inhibition and promote some functional recovery (Shen et al., 2009; Lang et al., 2015; Rink et al., 2018) but do not yield significant regeneration benefits when used alone (Zheng and Tuszynski, 2023). Furthermore, since aggrecan—the prototypical CSPG—is mainly present in perineuronal nets (PNN) and to a lesser extent in the core of SCI lesions, it appears that inhibiting CSPG function enhances axon sprouting and neuronal plasticity in the reactive CNS parenchyma rather than directly allowing axon regrowth through the SCI scar (Fawcett, 2015). In this regard, chondroitinase ABC reduces the atrophy of spared corticospinal tracts after SCI and promotes axonal sprouting and circuit reorganization (Carter et al., 2008; Starkey et al., 2012). From these results, the future role of extracellular inhibition strategies will likely be limited to combinatorial approaches supplementing neuron-intrinsic regeneration strategies such as NSC transplantation or exogenous delivery of growth factors. Detailed reviews on the role of perineural nets and CSPGs in SCI are referenced here (Tran et al., 2018b,2022).
2.5. The SCI scar and spinal cord regeneration: revisiting historical misconceptions
Throughout the 20th century, studies found that injured CNS axons regrew through living peripheral nerve grafts but not CNS white matter, indicating that the CNS microenvironment might lack certain growth-promoting and/or contain growth-inhibitory factors (Sugar and Gerard, 1940; Brown and McCouch, 1947; Richardson et al., 1980; Silver and Miller, 2004; Anderson et al., 2016). Additionally, the observation of dystrophic end-bulbs of non-regenerating axons abutting the astroglial border led to the hypothesis that the SCI scar formed a physical barrier impeding axonal regeneration (Silver and Miller, 2004).
However, rigorous testing in the past two decades has shown that the SCI scar plays both beneficial and harmful roles in SCI. For instance, loss-of-function experiments in transgenic mice have revealed a protective role of acute glial and stromal cell responses in SCI (Table 1). It is now clear that the SCI scar is a double-edged sword: it is acutely beneficial by performing damage-containment functions that prevent the propagation of the primary injury but, in the long-term, contributes to spinal cord regeneration failure by virtue of its extracellular inhibitors, physically insurmountable nature, and continual pro-inflammatory cellular signatures (Silver and Miller, 2004; Gaudet and Fonken, 2018; Escartin et al., 2021).
TABLE 1.
Genetic or pharmacologic loss-of-function experiments have revealed vital neuroprotective functions of glial and stromal responses to SCI.
| Cell type | Reference | Loss of function model | Impact on SCI outcome |
| Astrocytes | Faulkner et al., 2004 | TK+ GCV | Failure of wound contraction Prolonged neuronal loss Persistent neurological deficits |
| Astrocytes | Anderson et al., 2016 | TK+ GCV, stat3-cKO | Failure of axonal regrowth Significant increase in axonal dieback |
| Astrocytes | Wanner et al., 2013 | Stat3-cKO | Poorly demarcated scar border with extensive spread of inflammatory cells |
| Microglia | Bellver-Landete et al., 2019 | PLX5622 | Impaired locomotor recovery following SCI Impaired astrocyte proliferation Decreased survival of neurons and oligodendrocytes at site of injury |
| Microglia | Zhou X. et al., 2020 | Plxnb2-cKO | Significant impairment in sensorimotor recovery Impaired wound compaction leading to enlargement of SCI lesion |
| OPC | Hesp et al., 2018 | NG2 TK+ GCV | Enlarged lesions with edema Prolonged hemorrhage Inhibition of angiogenesis at site of lesion |
| OPC | Bartus et al., 2019 | ErbB-cKO | Impaired locomotor recovery |
| Stromal cells | Yokota et al., 2017 | Postn-KO | Decreased fibrotic scar formation Impaired functional recovery |
2.6. Spinal cord regeneration across different phyla
Spinal cord injury biology research has revealed the remarkable regenerative capacity of invertebrates and several non-mammalian vertebrates, such as Zebrafish, Urodeles (newts and salamanders), Lamprey, and Xenopus frogs (Tran et al., 2022). These animals form a glial bridge across the SCI lesion site, regenerate their spinal cords without scar formation, and spontaneously return to full autonomic and sensorimotor function (Ferretti et al., 2003; Shifman et al., 2006; Tazaki et al., 2017; Ghosh and Hui, 2018; Freitas et al., 2019; Sabin et al., 2019; Tsata and Wehner, 2021). These studies also emphasize the hostile and growth-inhibitory nature of the SCI scar in mammals, as regeneration in amphibians begins to fail when scar tissue forms (Bertolotti et al., 2013; Edwards-Faret et al., 2021). Scarring after injury appears to be a phenomenon acquired during evolution that impairs spinal cord regeneration after injury.
Previously, excellent spinal cord regenerative capabilities were considered unique to invertebrates and lower, non-mammalian vertebrates. However, new data shows that adult spiny mice (Acomys cahirinus) can spontaneously recover complete bladder control after spinal cord transection at the T8 vertebral level (Nogueira-Rodrigues et al., 2022). Although locomotor recovery in adult spiny mice remains incomplete, it still far exceeds any magnitude of recovery observed in adult mammals after complete SCI (Nogueira-Rodrigues et al., 2022). Therefore, a mammalian adult spinal cord regeneration model is now available (Gaire et al., 2021; Wehner and Becker, 2022).
2.7. Spinal cord regeneration throughout lifespan
Age is a crucial factor in determining spinal cord regenerative capacity in mammals. Neonatal mice (post-natal day 2) and the prematurely born South American opossum (Monodelphis domestica) can robustly regenerate their spinal cords without scar formation, reintegrate with distal neural circuitry, and recover neurologic function (Mladinic and Wintzer, 2002; Li X. et al., 2020). However, scarring occurs globally in 7-day-old mice and 2–3-week-old opossums, with a consequent loss in their regeneration capacity (Mladinic and Wintzer, 2002; Li X. et al., 2020).
The decline in mammalian CNS regenerative capacity in the post-natal period is multi-factorial. One reason is the significant change in the proteome of CNS neurons with age (Agrawal and Welshhans, 2021), which likely impacts post-injury regenerative capacity (Tran et al., 2022). During the embryonic period, CNS neurons are programmed to grow far-reaching axons to reach distal innervation targets, while in post-embryonic life, they facilitate and maintain local synaptic plasticity (Gumy et al., 2011; Shigeoka et al., 2016; Agrawal and Welshhans, 2021). For example, the alpha2delta2 subunit of voltage-gated calcium channels (VGCCs) is expressed during late embryogenesis, acting as a developmental switch that inhibits the axonal growth characteristic of early developmental CNS axons (Tedeschi et al., 2016). Genetically deleting the Cacna2d2 gene, which encodes the alpha2delta2 subunit, promotes axonal growth in vitro and inhibiting the alpha2delta2 subunit with pregabalin in adult mice after SCI enhances axonal regeneration (Tedeschi et al., 2016). The specific regulators of this developmental switch are yet to be fully understood. Still, they likely involve combined effects from various extracellular factors, including CSPG-RPTPσ signaling and possibly astrocyte-derived synaptogenic signals like thrombospondins, which upregulate alpha2delta1 subunit of VGCCs on neurons (Christopherson et al., 2005; Risher and Eroglu, 2012; Brooks et al., 2013; Sakamoto et al., 2019; Tran et al., 2020).
Embryonic neurons are less sensitive to inhibition by CSPGs and myelin-derived components like Nogo-A than adult neurons (Carulli et al., 2005; See et al., 2010; Poplawski et al., 2020). The concentration of these extracellular inhibitors is also much lower within the neonatal SCI lesion site than in adults (Tran et al., 2022). Recent novel findings by Nogueira-Rodrigues et al. showed that the extraordinary regenerative capacity of adult Acomys after SCI was underpinned by a pro-regenerative ECM signature. In their study, the SCI microenvironment of Acomys was highly enriched in keratin sulfate proteoglycans (KSPGs) and β3gnt7, the enzyme involved in KSPG production (Nogueira-Rodrigues et al., 2022). β3gnt7-expressing cells promoted neurite outgrowth in vitro, attributing growth-stimulating properties to KSPGs (Nogueira-Rodrigues et al., 2022). It would be interesting to investigate if experimentally engineering the ECM signature toward KSPG predominance in animal models known for poor spinal cord regeneration can augment axonal regeneration. Prior research has explicitly shown that KSPGs limit neuronal plasticity in rats, and their degradation by keratanase-II improves sensorimotor recovery after SCI, demonstrating comparable efficacy to chondroitinase ABC (Imagama et al., 2011). The factors driving these divergent responses to KSPGs in Acomys spiny mice and rats are yet to be elucidated. It would also be worthwhile investigating whether regenerative animal models that display scarless healing exhibit a core, pro-regenerative ECM composition.
The non-neuronal cellular response to injury also varies between embryonic and post-natal life. For example, immature astrocytes react less severely to stimuli like amyloid-β than mature astrocytes (Rudge and Silver, 1990; Canning et al., 1993). When reactive, immature astrocytes demonstrate reduced hypertrophy than their adult counterparts and are less densely packed at the scar border. Such an arrangement allows them to retain essential wound-sealing functions and creates an environment more favorable for axonal regeneration (Smith et al., 1987; Balasingam et al., 1994; Domowicz et al., 2011). After perinatal ischemic stroke, immature reactive astrocytes elaborate neuroprotective factors, including PGDF, IGF, and VEGF (Revuelta et al., 2019). These beneficial effects are most apparent when implanting immature astrocytes into adult SCI lesions, which leads to reduced glial scarring, enhanced axonal growth, and improved functional recovery (Davies et al., 2006, 2011; Filous et al., 2010; Haas and Fischer, 2013). In contrast, transplanting mature astrocytes impairs healing by recruiting macrophages and fibroblasts, resulting in cavitation (Filous et al., 2010).
Groundbreaking findings from Li Y. et al. (2020) demonstrated that microglia in neonatal mice populate the injury site, generating fibronectin and protease inhibitors that connect severed axon ends and enable scar-free axonal repair. Adult microglia only transiently and partially recapitulate the gene expression profile of their neonatal counterparts (Li Y. et al., 2020; Wahane et al., 2021), which can drive proliferation, revascularization, and functional recovery (Wang et al., 2022). However, adult microglial subsets displaying these developmental, pro-regenerative signatures are significantly less abundant in adult SCI lesion sites and overexpress and under-express CD68 and P2ry12, respectively, which may diminish their regenerative capacity (Li et al., 2022). Determining the factors that drive this developmental gene signature in immature astrocytes and microglia and how it can be augmented in their adult counterparts is a critical area for future research.
Above, we have provided compelling evidence that spinal cord regenerative capacity varies considerably between embryonic and post-natal life. As aging research has garnered a massive rise in interest in recent years, studies have also shown that older patients display poorer neurologic outcomes after SCI compared to younger individuals (Scivoletto et al., 2003; Furlan and Fehlings, 2009), suggesting the existence of a “second wave” of changes in the CNS injury response that subjects older individuals to a greater neuropathologic burden and worse clinical outcomes. López-Otín et al. (2013; 2023) identified twelve biological aging hallmarks: genomic instability, telomere shortening (i.e., attrition), epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, stem cell exhaustion, altered intercellular communication, macroautophagy, chronic low-grade inflammation, and gut microbiome dysbiosis. The geroscience hypothesis states that the accumulation of these processes in different body tissues—at different rates—drives aging-related tissue dysfunction and that targeting biological aging processes may extend healthspan and potentially even lifespan (Kennedy et al., 2014). The accumulation of biological aging hallmarks is evident in the CNS and manifests as age-related changes in cerebral morphology, impaired neurogenesis, and neuroinflammation (see Gonzales et al., 2022 for a detailed review).
Cellular senescence, a state of irreversible cell cycle arrest accompanied by characteristic molecular, morphological, and functional alterations, has emerged as a key therapeutic target in aging and chronic diseases, including neurodegenerative diseases (Kirkland et al., 2017; Kirkland and Tchkonia, 2017, 2020; Hernandez-Segura et al., 2018; Calcinotto et al., 2019; Gorgoulis et al., 2019; Gasek et al., 2021; Chaib et al., 2022; Huang W. et al., 2022; Shafqat et al., 2022; Zhang L. et al., 2022). Senolytics, which are drugs that eliminate senescent cells, are already being evaluated in clinical trials for a host of chronic diseases such as Alzheimer’s disease (see Zhang L. et al., 2022 for a detailed review).
Hence, recent studies have defined the role of cellular senescence in the aging CNS in an attempt to uncover novel therapeutic targets. Pericytes in the aging brain undergo senescence, associated with increased blood-brain barrier (BBB) permeability in vitro, suggesting that senescent pericytes could partially contribute to age-related BBB dysfunction and neuroinflammation (Iwao et al., 2023). Senescent microglia accumulate in the aged brain and elaborate pro-inflammatory cytokines and chemokines that recruit adaptive T-cells and B-cells, which are linked to the onset of cognitive decline (Ogrodnik et al., 2021; Zhang X. et al., 2022). The pro-inflammatory phenotypes of senescent microglia can be augmented by stressors such as traumatic brain injury (TBI), resulting in more pronounced neuroinflammatory responses in older mice and worse cognitive outcomes than in younger mice (Ritzel et al., 2019). Lastly, adult neurogenesis occurs in the dentate gyrus of the hippocampus but decreases with age due to a decrease in the number of NSCs and neuroblasts, connected to the onset of age-related cognitive decline (Kase et al., 2020). A recent study demonstrated an accumulation of senescent neuroblasts in the dentate gyrus with aging (Jin et al., 2021). These neuroblasts release pro-inflammatory molecules that recruit natural killer (NK) cells, subsequently eliminating senescent neuroblasts, leading to impaired neurogenesis and cognition (Jin et al., 2021).
It is curious, perhaps even paradoxical that cellular senescence is an evolutionarily conserved phenomenon despite its adverse effects on nearly every organ system. However, senescence has crucial beneficial roles in embryogenesis and is a frontline defense against tumorigenesis (Storer et al., 2013; Lorda-Diez et al., 2015; Schosserer et al., 2017). Cellular senescence exerts both beneficial and harmful effects on wound healing. Pioneering work from Demaria et al. (2014) demonstrated that—in p16-3MR mouse models that allow tracing and inducible depletion of senescent cells—fibroblasts and endothelial undergo senescence early after a cutaneous wound. These senescent cells secrete PDGF-AA, stimulating the differentiation of local fibroblasts to myofibroblasts that mediate wound contraction (Demaria et al., 2014). Depleting senescent cells in transgenic mice delayed wound healing (Demaria et al., 2014). However, successful wound healing requires the clearance of senescent fibroblasts and endothelial cells, as their persistent accumulation drives inflammation and tissue dysfunction via their senescence-associated secretory phenotype (SASP) (Childs et al., 2015; Calcinotto et al., 2019). Similarly, experiments analyzing the injury response in the zebrafish hearts and fins and salamander limbs reveal a transient induction of cellular senescence that, if disrupted, impairs the regenerative response (Yun et al., 2015; Da Silva-Álvarez et al., 2020).
Paramos-de-Carvalho et al. (2021) conducted a comparative study on the dynamics of cellular senescence in SCI between zebrafish and adult mice. They discovered that SCI upregulates senescence-associated β-galactosidase (SA β-gal), the most widely used marker for senescent cells, in neurons at the lesion periphery in zebrafish and mice. Striking differences were observed in the temporal dynamics of senescent neurons: in zebrafish, the number of senescent neurons peaked at 8.9% at 15 dpi but then steadily declined to reach baseline levels by 60 dpi, in line with the idea that transient senescence induction is a conserved process associated with successful wound healing and regeneration (Paramos-de-Carvalho et al., 2021). Conversely, in mice, the percentage of total senescent neurons was 25.3% at 15 dpi and continued to increase until 60 dpi, reaching 35.3% (Paramos-de-Carvalho et al., 2021). When the mice were treated with ABT-263, a known senolytic, they exhibited significantly better sensorimotor and bladder function recovery than vehicle-treated mice, indicating that the accumulation of senescent cells contributes to the growth-inhibitory SCI microenvironment in mice. The functional recovery was associated with increased white matter sparing and enhanced synaptic plasticity in the adjacent reactive neural parenchyma (Paramos-de-Carvalho et al., 2021). To test the hypothesis that chronic senescent accumulation in non-healing wounds promotes inflammation, the authors demonstrated that ABT-263 significantly reduced inflammatory macrophages numbers and levels of pro-inflammatory cytokines, chemokines, and mitogenic and fibrogenic growth factors in the SCI scar (Paramos-de-Carvalho et al., 2021).
3. Astrocytes
Astrocytes are of neuroectodermal origin and constitute about 20% of glial cells (Molofsky and Deneen, 2015; Khakh and Deneen, 2019). They fulfill diverse physiological roles in the CNS, including blood-brain barrier (BBB) maintenance, neurotransmitter uptake for synapse homeostasis, energy substrate provision to neurons, and interactions with other astrocytes, oligodendrocytes, and microglia (Khakh and Deneen, 2019). Following CNS injury, astrocytes become reactive, which entails an array of molecular, morphological, and functional alterations that impact adjacent cells, positively or negatively, depending on the disease context (Sofroniew and Vinters, 2010; Sofroniew, 2020). This process is often incorrectly termed astrogliosis, which entails astrocyte proliferation. Reactive astrogliosis constitutes a small portion of the reactive astrocyte response at the SCI lesion penumbra. Rather, much of the reactive astrocytic response consists of morphologic alterations collectively referred to as reactive astrocytosis, including hypertrophy of astrocytic processes, a consequent overlap between spatially defined astrocyte domains, and cytoskeletal rearrangements such as upregulation of the intermediate filaments GFAP and vimentin (Daniel et al., 2010).
3.1. Historical perspective
Reactive astrocytes were once considered the primary contributors to post-SCI regeneration failure by creating a physical barrier and producing inhibitory CSPGs (Silver and Miller, 2004). This belief was supported by histological evidence depicting dystrophic axon end-bulbs abutting the astrocyte limitans border (Aguayo et al., 1981; David and Aguayo, 1981; Schwab and Bartholdi, 1996; Davies et al., 1997). Thus, researchers hypothesized that depleting astrocytes or key signaling pathways using transgenic models would enhance axonal regeneration across the scar (Table 1).
3.2. Beneficial astrocyte reactivity
Astrocyte biology in SCI proved more complex than initially assumed. Transgenic ablation of astrocytes, disruption of astrocyte scar-forming function, or reducing the number of border-forming astrocytes does not improve the regeneration of transected corticospinal, sensory, or serotonergic axons (Anderson et al., 2016). Such manipulations exacerbate neuroinflammation and neuronal loss (Bush et al., 1999; Faulkner et al., 2004; Myer et al., 2006; Gu et al., 2019; Zhao W. et al., 2022). Therefore, scar-forming astrocytes do not acutely inhibit axonal growth; instead, they recruit inflammatory cells to the lesion epicenter and then proliferate to seal it off, confining neuroinflammation (Wanner et al., 2013; Sofroniew, 2015). Specific subsets of activated astrocytes may even mitigate neuroinflammation outgrowth in SCI by upregulating anti-inflammatory molecules like clusterin (Wright et al., 2014; De Miguel et al., 2021; Gong et al., 2023).
Border-forming astrocytes can promote the growth of maximally stimulated axons by producing integrin, which binds axon growth cones and enhances their growth (Anderson et al., 2016). Similarly, yes-associated protein (YAP), which contributes to the exceptional regenerative abilities of lower non-mammalian vertebrates like zebrafish, is upregulated in mouse astrocytes by basic fibroblast growth factor (bFGF) in the SCI microenvironment and promotes astrocyte proliferation, protective glial scar formation, axonal regeneration, and functional recovery (Xie et al., 2020; Riley et al., 2022). A recent study transplanted anti-inflammatory/pro-repair astrocytes to promote axonal regeneration, remyelination, and functional recovery after SCI (Chang et al., 2023). Astrocytes have recently been converted into neurons to aid synaptic remodeling and functional recovery (Su et al., 2014; Noristani et al., 2016; Puls et al., 2020).
An essential question is how to reconcile these apparent axon growth-enhancing effects of scar-forming astrocytes with their propensity to produce inhibitory CSPGs. Hypertrophic astrocytes in the surrounding CNS parenchyma elaborate CSPGs to influence local synaptic remodeling (O’Shea et al., 2017; Sofroniew, 2018; Santello et al., 2019). This explains why modulating astrocyte-derived CSPGs can augment functionally beneficial synaptic remodeling proximal to the lesion site (discussed above in the section “Extracellular Regeneration Inhibitors in the Glial Scar”). Furthermore, recent studies have demonstrated that astrocytes are not the primary source of inhibitory CSPGs, mainly derived from stromal cells, OPCs, and macrophages (Jones et al., 2002; Anderson et al., 2016).
3.3. Dysfunctional astrocyte reactivity
While acknowledging the evidence discussed earlier, it is crucial to recognize that particular astrocyte responses can be detrimental, referred to as dysfunctional astrocyte reactivity.
Dysfunctionally reactive astrocytes can promote BBB disruption and neuroinflammation through TNF-STAT3 signaling and alpha-1-antichymotrypsin production (Kim et al., 2022). Additionally, pro-inflammatory cytokines derived from microglia foster pro-inflammatory and neurotoxic reactive astrocyte phenotypes linked to the pathogenesis of neurodegenerative diseases (Phatnani and Maniatis, 2015; Liddelow et al., 2017; Russ et al., 2021; Brandebura et al., 2023). Amyloid-β was also recently shown to directly provoke pro-inflammatory and neurotoxic astrocyte reactivity, leading to synaptic and neuronal loss (Jiwaji et al., 2022). Reactive astrocytes can increase the expression of genes encoding proteins like thrombospondins, which facilitate synaptogenesis (Christopherson et al., 2005; Risher and Eroglu, 2012; Risher et al., 2018). However, thrombospondins may also lead to the formation of unwanted synapses, leading to epilepsy or neuropathic pain (Boroujerdi et al., 2008; Liddelow and Barres, 2017; Cui et al., 2021).
In SCI, it is still true that the chronic presence of the densely packed astroglial scar constitutes a physical barrier to axonal regeneration. The formation of the astroglial scar depends on microenvironmental signals within the injured spinal cord, as Hara et al. (2017) elegantly demonstrated. Astrocytes elicit reactive gliotic responses when transplanted into the injured spinal cord but revert to quiescent, non-reactive states when transplanted into a naïve spinal cord (Hara et al., 2017). In the injured spinal cord, type I collagen partly facilitates the dense packing of astrocytes through the integrin/N-cadherin signaling pathway (Hara et al., 2017). Attenuating integrin signaling reduces astroglial scarring—but does not deplete astrocytes—and leads to improved axonal regrowth and functional recovery (Kanemaru et al., 2013; Hara et al., 2017). Other studies have similarly demonstrated that carefully manipulating astrocyte functions rather than all-or-none genetic or pharmacologic ablation techniques can “loosen” the astrocyte scar and augment neuronal and functional recovery (Iseda et al., 2004; Ma et al., 2004; Hurtado et al., 2011). The severity of SCI adds another layer of complexity to the dual role of astrocytes: milder forms of injury lack the dense macrophage and stromal cell infiltrate and feature lower levels of ECM elaboration, which can reprogram astrocytes to promote neurite outgrowth and axonal regeneration (Fitch and Silver, 2008; Alicia et al., 2011; Silver, 2016). More severe injuries elicit robust GFAP upregulation and dense, growth-blocking scar formation (Fitch and Silver, 2008; Alicia et al., 2011; Silver, 2016).
Astrocytic SOSC3 signaling plays a role in glial scarring and diminished functional recovery after SCI, while attenuating SOCS3 reduces scarring and promotes remyelination and functional recovery (Okada et al., 2006; Hackett et al., 2016). Similarly, the upregulation of erythropoietin-producing hepatocyte A4 (EphA4) on neurons post-SCI binds to ephrin-B receptors on astrocytes, inducing pro-inflammatory astrocyte reactivity, which hinders neurite outgrowth and axonal regeneration (Chen et al., 2022). Genetic ablation of the ephrin-B receptor on astrocytes leads to improved axonal regeneration following SCI (Chen et al., 2022). Epigenetic regulation by several micro-RNAs has also been widely implicated in stimulating the hypertrophy and proliferation of reactive astrocytes, promoting glial scar formation (Liu R. et al., 2018). For example, a recent study showed that microRNA mir-155-5p stimulates astrocyte proliferation and inhibits their apoptosis after SCI, facilitating reactive astrogliosis and scar formation (He et al., 2023). Silencing mir-155-5p decreases GFAP and NF-200 expression and attenuates astroglial scar formation, which is associated with better locomotor recovery in mice (He et al., 2023).
To conclude, reactive astrocytosis and astrogliosis are beneficial in the acute and subacute phases of SCI, serving to contain neuroinflammation. However, the formation of a dense astroglial scar in the chronic phase of SCI constitutes a physical barrier to axonal regeneration. Moreover, the upregulation of particular signaling pathways in scar-forming astrocytes can obstruct axonal regeneration and functional recovery after SCI, and targeting these regulators may have future clinical applications in promoting axonal regeneration.
3.4. Astrocyte heterogeneity
Astrocytes are a diverse group of cells that exert region-dependent functions in the healthy CNS and differentially modulate local neuronal circuitry (Tsai et al., 2012; Matias et al., 2019; Huang et al., 2020). The heterogeneity of astrocytes has become a key focus in neuroscience research.
Astrocytes mount context-specific responses to CNS injuries (Yu et al., 2020). For example, profiling astrocyte transcriptomes by microarray or single-cell RNA sequencing (scRNA-Seq) in stab wound injury, lipopolysaccharide (LPS)-induced neuroinflammation, ischemic stroke, SCI, and neurodegeneration reveals disease-specific gene expression (Zamanian et al., 2012; Liddelow et al., 2017; Cao et al., 2022). Liddlelow et al. categorized transcriptionally distinct astrocyte subsets into “A1” and “A2,” with opposing effects in various disease states: A1 astrocytes are pro-inflammatory and neurotoxic, whereas A2 astrocytes promote tissue repair and are neuroprotective. However, the functions of A1 and A2 genes are largely unknown, and astrocytes often display a mix of A1/A2 gene signatures in CNS disease (Grubman et al., 2019; Al-Dalahmah et al., 2020; Das et al., 2020; Zhou Y. et al., 2020), leading researchers to recommend moving past the binary A1/A2 classification (Escartin et al., 2021). Nonetheless, it remains that transcriptional astrocyte diversity can foster either dysfunctional astrocyte reactivity that promotes neuropathology or resilient reactive states that support wound resolution and functional recovery (Liddelow et al., 2017; Wheeler et al., 2020; Yang et al., 2020a). Multiple sclerosis research has shown that both dysfunctional and resilient populations of astrocytes can coexist and vary with disease stage (Wheeler and Quintana, 2019; Wheeler et al., 2020). Similarly, amyloid-β and hyperphosphorylated tau induce pathologic and protective astrocyte phenotypes, respectively, suggesting that both populations co-exist in Alzheimer’s disease (Jiwaji et al., 2022). Notably, the same transcriptional regulators (TRs) can have protective or detrimental roles depending on the disease context. For example, STAT3 signaling is neuroprotective in TBI (Nobuta et al., 2012) and SCI (Herrmann et al., 2008; Wanner et al., 2013) but harmful in Alzheimer’s disease (Ceyzériat et al., 2018; Reichenbach et al., 2019).
Astrocyte heterogeneity in SCI has been investigated as well. White and colleagues used immunohistochemical staining to reveal morphological differences among astrocytes in the cervical, thoracic, and lumbar spinal segments of a contusive SCI mouse model (White et al., 2010). ScRNA-Seq showed that reactive astrocytes in SCI exhibit a substantially different transcriptome, sharing only partial similarities with the steady-state CNS and other CNS disorders (Burda et al., 2022).
Hou et al. (2022) recently employed scRNA-Seq to identify 12 transcriptionally distinct clusters of astrocytes following traumatic SCI. By using Gene Ontology (GO) enrichment analysis, KEGG pathway analysis, and the use of “A1/A2” marker genes, the authors inferred that each of the 12 clusters had uniquely enriched genes, possibly pointing to distinct roles in SCI (Hou et al., 2022). Moreover, each cluster exhibited differential temporal dynamics within the SCI lesion site: “A1” astrocyte clusters were most abundant in the acute and subacute phases, whereas “A2” reactive clusters (Silver and Miller, 2004; Adams and Gallo, 2018; Orr and Gensel, 2018; Liu et al., 2022) were more abundant in the subacute and chronic phases (Hou et al., 2022). This study also identified several biomarkers that may facilitate cluster-specific manipulation experiments to test whether enhancing “A2” subsets or inhibiting “A1” reactive astrocytes may improve post-SCI neural regeneration and functional recovery (Hou et al., 2022).
However, inferring functional states from gene expression data can be misleading since the transcriptional analysis does not always accurately reflect functional activity, especially in the highly dynamic in vivo environment and given the complexity of cellular interactions. Ultimately, only loss-of-function experiments targeting essential proteins enriched in different astrocyte subsets will causally link molecular heterogeneity to function. Also, as discussed above, the “A1/A2” terminology is now considered outdated.
To address how context-specific astrocyte reactivity is regulated, the Sofroniew Laboratory used scRNA-Seq and transcriptional regulator enrichment analysis (TREA) followed by numerous validation techniques to predict TRs of disease-specific astrocytic reactivity in SCI, LPS-induced neuroinflammation, and experimental autoimmune encephalomyelitis (EAE), which is a mouse model of multiple sclerosis (Burda et al., 2022). Strikingly, genetic KO models of key TRs such as Smarca4 and Stat3 showed that they could regulate the same differentially expressed gene oppositely (e.g., Stat3 and Smarca4 can upregulate Slc14a2 and Rhof in LPS and downregulate them in SCI), which better contextualizes findings highlighting divergent functions of Stat3 in different CNS diseases (discussed above) (Burda et al., 2022). The identified TRs, including Stat3 and Smarca4, were shown to influence disorder outcome, as their genetic deletion worsened SCI neuropathology and functional outcomes in mice, suggesting that targeting these TRs could have future clinical applications (Burda et al., 2022). Lastly, although over 10,500 differentially expressed genes were identified in astrocytes across eight CNS disorders with little overlap between diseases, a core of 61 astrocyte reactivity TRs were shared in at least 7 of 8 conditions, including SCI (Burda et al., 2022).
These data suggest that a limit number of TRs exert a combinatorial control over reactive astrocyte gene expression to achieve remarkably heterogeneous context-specific astrocyte responses that influence disease outcomes. Elucidating the extrinsic modulators of core TRs and the astrocyte functions promoted by each TR could reveal translational opportunities to mitigate dysfunctionally reactive astrocytes and/or enhance resilient reactive subsets. For instance, a recent study demonstrated that exogenously delivering the TR Sox2 reprogrammed astrocytes into a pro-regenerative phenotype, which promoted axonal regeneration, reduced dense glial scarring, and enhanced functional recovery when combined with rehabilitation strategies that improve neuronal plasticity (Yang et al., 2020b).
4. Microglia
Microglia are the resident immune cells of the CNS, originate from the yolk sac, and perform various functions in both healthy and diseased CNS states. In the healthy CNS, microglia are non-motile but extend highly dynamic processes that survey the extracellular environment to carry out “housekeeping” functions, including phagocytosing extracellular debris or pathogenes, fortifying the BBB, delivering nutritional support to neurons and oligodendroglia, orchestrating synaptic pruning, and sustaining myelin turnover (Paolicelli et al., 2011; Schafer et al., 2012; Domingues et al., 2016; Haruwaka et al., 2019; Hughes and Appel, 2020; Ronaldson and Davis, 2020; Santos and Fields, 2021; McNamara et al., 2023).
4.1. Microglia heterogeneity
Microglia exhibit distinct morphological and functional properties in the healthy CNS, contingent upon their location, ontogeny/developmental origin, and local microenvironmental signals such as astrocyte-derived cytokines/chemokines (Bennett et al., 2018; Zheng et al., 2021; Lynch, 2022). This heterogeneity wanes with aging, but specific location-dependent differences in microglial identity are maintained in the adult CNS (Grabert et al., 2016; Masuda et al., 2019). Furthermore, sex-specific differences in microglial morphology, transcriptome, and proteome have been identified, potentially contributing to gender-related variation in CNS disease pathophysiology and manifestations (Guneykaya et al., 2018; Lynch, 2022).
Heterogeneity intensifies when considering CNS-resident macrophages or border-associated macrophages (BAMs), which reside within the meninges, choroid plexus, and perivasculature. These BAMs are transcriptionally and functionally unique from microglia (Zeisel et al., 2015; Mrdjen et al., 2018; Van Hove et al., 2019; Prinz et al., 2021; Masuda et al., 2022). However, their distinct roles in SCI remain largely uncharted (Jordão et al., 2019; Kierdorf et al., 2019; De Schepper et al., 2023). Additionally, under specific circumstances such as ischemic stroke, pericytes can differentiate into microglia- and macrophage-like cells (Nirwane and Yao, 2022), introducing another facet of microglial heterogeneity.
Functionally, microglial and macrophages have traditionally been categorized into “M1” (pro-inflammatory, cytotoxic) or “M2” (anti-inflammatory, pro-repair) (David and Kroner, 2011). This is an in vitro classification derived from experiments that show that stimulating macrophages with IFN-γ, TNF-α, or LPS induces macrophages toward pro-inflammatory cytokine production, whereas IL-4 or IL-13 polarize macrophages toward the production of anti-inflammatory cytokines (Gordon, 2003; David and Kroner, 2011). However, the in vivo microenvironment contains a mix of “M1-favoring” and “M2-favoring” DAMPs, cytokines, and chemokines (Xue et al., 2014). Moreover, the SCI environment is highly dynamic in that the balance between these factors constantly changes. Hence, neatly categorizing microglial and macrophage activation states into M1 and M2 does not reflect the in vivo reality (David et al., 2018). Contemporary research has unveiled that microglia and macrophages exhibit mixed M1/M2 gene signatures upon activation (Kigerl et al., 2009; Hsieh et al., 2013; Fenn et al., 2014; Morganti et al., 2016; Masuda et al., 2019). This functional heterogeneity is modulated by disease etiology, injury location, and the time elapsed since the original insult.
Based on these findings, the current consensus posits that microglia and macrophages in SCI exist on a spectrum as they respond to a lesion, with a balance of pro-inflammatory and anti-inflammatory reactivity being crucial to favorable SCI outcomes (Ransohoff, 2016; Brennan and Popovich, 2018). Deviations from this balance result in non-resolving pathologies, such as the glial scar that typifies SCI in mammals. Consequently, eminent researchers in the field of microglial biology recommend avoiding the M1/M2 terminology to prevent misinterpretation of data (Ransohoff, 2016; Paolicelli et al., 2022).
4.2. Microglial and macrophage responses to SCI
Following SCI, DAMPs and pro-inflammatory cytokines, such as ATP, IL-33, IL-1β, and TNF-α, trigger microglial reactivity and the adoption of a pro-inflammatory phenotype within the injured CNS microenvironment (Davalos et al., 2005; Rice et al., 2007; Kristina et al., 2009; Orr et al., 2009; Gadani Sachin et al., 2015). These reactive microglia migrate to the lesion site, undergo hypertrophy, and retract their ramifications, becoming morphologically indistinguishable from MDMs (Orr et al., 2009; Boche et al., 2013).
During the acute phase of SCI, reactive microglia release pro-inflammatory cytokines and chemokines, which augment astrocyte reactivity and recruit circulating neutrophils to the lesion site, exacerbating neuroinflammation and neuronal loss (Pineau and Lacroix, 2007; David et al., 2012; Kobayakawa et al., 2019; Pelisch et al., 2020). The subacute phase marks the beginning of MDM infiltration into the lesion site (Tran et al., 2018b). It is essential to delineate the distinct functions of microglia and MDMs in SCI. While MDMs are found within the lesion core, microglia localize along the margins of the fibrotic scar interfacing with the astrocyte border (Zhou et al., 2014). Microglia execute essential phagocytic and cytokine-producing functions while ensuring wound compaction in the fibrotic core and proper astrocyte scar formation, thereby limiting damage spread (Hines et al., 2009; Brennan et al., 2022). Conversely, MDMs mainly phagocytose debris and produce the cytokines and chemokines dictated by their polarization state without contributing to damage containment (David and Kroner, 2011). Only MDMs establish destructive physical contact with axons, inducing axonal dieback (Sarah et al., 2011; Evans et al., 2014). Furthermore, microglia repress genes in MDMs associated with ECM processing; in the absence of microglia, MDMs enhance ECM degradation and increase neuroinflammation (Brennan et al., 2022).
Phagocytosis is a prerequisite for wound healing post-SCI, mitigating neuroinflammation and promoting remyelination (Wang et al., 2015, 2022; David et al., 2018; Bellver-Landete et al., 2019; Lloyd and Miron, 2019; Fu et al., 2020). Microglia drive the early phagocytic response up to 3-dpi (i.e., until MDM infiltration starts), efficiently internalizing apoptotic and necrotic cell debris and myelin (Andrew and Samuel, 2014). By 7-dpi, MDMs at the lesion epicenter become the dominant phagocytic cell type, displaying superior phagocytic capabilities than microglia (Andrew and Samuel, 2014). Microglia also bolster the phagocytic functions of MDMs, whereas the latter actively suppress microglial phagocytosis and pro-inflammatory phenotypes (Greenhalgh et al., 2018). Preventing this macrophage-induced suppression of pro-inflammatory microglial polarization increases neuroinflammation and attenuates functional recovery (Greenhalgh et al., 2018). These findings indicate that the microglial-macrophage interplay operates to confine the lesion site, phagocytose and thereby eliminate pro-inflammatory toxic debris, and restore homeostasis.
However, MDMs process phagocytic debris less efficiently than microglia, leading to intracellular accumulation (Andrew and Samuel, 2014). Progressive myelin buildup inside MDMs is linked to their polarization toward pro-inflammatory states, akin to lipid-laden “foamy” macrophages observed in atherosclerotic plaques (Moore et al., 2013; Zhu et al., 2017; Milich et al., 2019). Longitudinally profiling MDM responses in SCI reveals a coexistence of pro- and anti-inflammatory populations in the subacute phase, whereas MDMs at 28-dpi exhibit a much stronger pro-inflammatory bias (Kigerl et al., 2009). These persistently activated macrophages within the fibrotic core are well-established contributors to the lack of wound resolution post-SCI (Wu et al., 2005; Li et al., 2022). The local SCI microenvironment drives these microglial and MDM phenotypes, thereby controlling disease outcomes. For instance, transitioning to an anti-inflammatory/pro-repair phenotype requires a shift in astrocytic signals from pro-inflammatory (TNF-α and IL-6) to anti-inflammatory (TGF-β and IL-4) (Norden et al., 2015). However, insufficient anti-inflammatory cytokines like IL-4 in the SCI microenvironment favor inflammation (Francos-Quijorna et al., 2016). Besides extracellular factors, phagocytosis of myelin debris promotes anti-inflammatory/pro-repair phenotypes, but TNF-α overrides this effect to sustain pro-inflammatory/anti-repair polarization (Kroner et al., 2014). Additionally, iron loading from RBC phagocytosis reverses the anti-inflammatory/pro-repair phenotype and increases TNF-α and inducible nitric oxide synthase (iNOS) levels, favoring inflammation (Kroner et al., 2014). Hence, therapies that appropriately modulate microglia and macrophage to achieve a balance between pro-inflammatory/anti-inflammatory polarization are needed (Shechter et al., 2009; Gensel and Zhang, 2015). Experimentally skewing microglia and macrophage polarization toward anti-inflammatory—by directly modulating their gene expression, utilizing stem cell transplantation, or manipulating the SCI microenvironment—has been shown to reduce axonal dieback, enhance angiogenesis, and improve functional outcomes after SCI (Busch et al., 2009, 2011; Francos-Quijorna et al., 2016; Pelisch et al., 2020; Gu et al., 2023; Ju et al., 2023).
Studies have also attempted to elucidate the intrinsic molecular pathways determining where microglia exist on their reactivity spectrum. Histone deacetylase 3 (HDAC3) is a key epigenetic regulator of microglial activation after SCI and skews their gene expression signature toward inflammation (Kuboyama et al., 2017; Huang D. et al., 2022). HDAC3 inhibition suppresses microglial pro-inflammatory cytokine secretion (Xia et al., 2017) and alleviates various CNS diseases, including SCI (Chen et al., 2018; Liao et al., 2020; Matheson et al., 2020; Bian et al., 2021; Zhao Y. et al., 2022; Lu et al., 2023). Microglia-specific HDAC3 knockout or administration of HDAC3 inhibitor RGFP966 exert neuroprotective effects in severe contusive SCI mouse models and increase the density of regenerating axons in the fibrotic scar 10-dpi (Kuboyama et al., 2017). Therefore, HDAC3 may constitute a therapeutic target to suppress pro-inflammatory/anti-repair microglial subsets.
4.3. Wound compaction by microglia
Hines et al. (2009) initially reported that microglia represent a frontline defense in the CNS, exhibiting rapid mobilization in response to injury to mitigate damage propagation. Depleting microglia via plexxikon molecules (PLX3397 and PLX5622) expands the SCI lesion size, disorganizes the astrocyte scar, and results in the spillover of ectopic clusters of MDMs into the surrounding white matter (Fu et al., 2020; Brennan et al., 2022), their morphology resembling “foamy” macrophages which are known to exert pro-inflammatory and neurotoxic effects (Wang et al., 2015; Zhu et al., 2017). Microglia-depleted mice also display worse locomotor recovery post-SCI, whereas stimulating microglia repopulation enhances recovery (Bellver-Landete et al., 2019; Brennan et al., 2022).
Microglia are thus emerging as pivotal orchestrators of the pro-homeostatic response following SCI. Numerous stereotypical functions of reactive astrocytes, including proliferation, cell adhesion, cytoskeletal reorganization, and inflammation, which are essential elements for proper astrocyte scar formation, are regulated by microglia, as evidenced by scRNA-Seq (Brennan et al., 2022). Mechanistically, reactive microglia in SCI physically contact scar-forming astrocytes to ensure proper glial scar formation and secrete IGF-1, which stimulates the proliferation of scar-forming astrocytes (Bellver-Landete et al., 2019). Microglial depletion by PLX5622 decreases astrocyte and OPC proliferation, resulting in a malaligned glial scar (Brennan et al., 2022).
Research from the Zhou Laboratory highlighted that microglia allow wound compaction function following through by their surface plexin-B2 receptor (Zhou X. et al., 2020). Upregulation of plexin-B2 contributes to the clear spatial segregation between the central fibrotic scar and astroglial border, whereas plexin-B2 deletion results in the intermingling of astrocytes and microglia at the lesion center and spillover of inflammatory components into the adjacent CNS tissue (Zhou X. et al., 2020).
4.4. Microglia and axonal regeneration
Transplanting microglia into mouse models of SCI has been shown to promote tissue preservation and enhance functional outcomes (Kou et al., 2018; Kobashi et al., 2020; Xia et al., 2022). However, many of these studies infer regeneration or remyelination from functional recovery rather than direct observation, while different mechanisms, such as synaptic remodeling, axonal sprouting, or regeneration through the lesion core, can underpin recovery.
Earlier in the discussion, we described how milder astrocyte manipulations that do not deplete astrocytes or completely abrogate their proliferation could enhance axonal growth. Since recent data show that microglia are crucial for forming a dense astrocytic scar, perhaps attenuating microglial functions can also “loosen” the astrocyte scar to allow for axonal regrowth. The study by Li Y. et al. (2020) demonstrated that SCI in neonatal mice results in the upregulation of GFAP-positive but loosely packed astrocytes with little evidence of hypertrophy and scar formation, in stark contrast to the compact astroglial border that forms in adult mice. This axonal regrowth-favoring glial scar was critically dependent on immature microglia, as depleting microglia in neonatal mice resulted in stronger astrocyte hypertrophy that was more compactly arranged, resulting in axonal regrowth failure and halted growth cones seen abutting astrocytes (Li Y. et al., 2020). Mechanistically, immature microglia release serine and cathepsin protease inhibitors, which reduced the deposition of astrocyte scar-inducing type I collagen and growth-inhibitory CSPGs (Li Y. et al., 2020). However, exogenously supplying protease inhibitors when transplanting mature microglia into the adult spinal cord improved axonal regeneration and functional recovery, but not to the extent seen after transplanting immature microglia, indicating that immature microglia also exert other currently unknown functions that promote scarless wound healing (Li X. et al., 2020).
It is important to mention that skewing the microglia/macrophage population toward anti-inflammatory/pro-repair phenotypes may not be enough to render the astrocyte border more conducive to axonal regeneration. For example, inhibiting HDAC3 by RGFP966 during the acute phase of SCI (0–2 dpi) significantly ameliorates neuroinflammation and enhances axonal sparing but does not affect GFAP expression levels, suggesting that the ability astrocytes to form a rigid, growth-blocking scar was unaltered (Kuboyama et al., 2017).
4.5. Current limitations in microglial research
Microglial investigations have struggled with the absence of specific markers. Recent discoveries of novel microglia markers, such as Tmem119, SLC2A5, Sall1, P2ry12, and FCRLS, and reporter mice have improved this situation (Bennett et al., 2016; Konishi et al., 2017; Jordão et al., 2019; Kaiser and Feng, 2019; Zhao et al., 2019; Masuda et al., 2020; McKinsey et al., 2020; Ruan et al., 2020). Still, concerns remain regarding the specificity of these markers, as some may be downregulated in reactive microglia or expressed in BAMs (Young et al., 2021; Ruan and Elyaman, 2022). Microglia-specific reporter mice such as Cx3cr1-Cre may also suffer from a lack of specificity by inadvertently labeling macrophages and glial cells (Zhao et al., 2019).
Loss-of-function experiments typically administer plexxikon CSF1R inhibitors, such as PLX3397 and PLX5622, that cross the BBB to deplete microglia (Elmore et al., 2014; Najafi et al., 2018; Green et al., 2020). However, PLX5622 depletes microglia and BAMs, hindering assessments of their differential contributions to CNS diseases (Montilla et al., 2023).
Thirdly, microglial depletion strategies in SCI animal models have yielded beneficial and detrimental effects on scarring, axonal regeneration, and functional recovery (Deng et al., 2022). Microglia primarily exert their beneficial functions within the first week of SCI, while activation beyond this phase proves harmful (Zhou X. et al., 2020). Therefore, divergent outcomes may arise from different timings of microglial depletion. The severity of manipulation is also important to consider: severe manipulations that either deplete microglia or their key pro-inflammatory and wound compaction functions in the acute and subacute phase of SCI are likely to be deleterious, whereas milder, timed manipulations in the chronic phase are more likely to be beneficial.
Finally, despite technological advancements enabling the examination of microglial heterogeneity at the single-cell level, the upstream regulators and functional consequences of this diversity remain unclear. Future research must also consider post-transcriptional and translational regulation of key transcripts in microglia, as a recent study demonstrated stringent post-transcriptional and translational control over pro-inflammatory gene transcripts (Boutej et al., 2017), such that solely considering transcriptomics may not accurately reflect the true nature of the microglial proteome. Based on this, Paolicelli et al. (2022) recommend adopting a multidimensional view of microglial biology that incorporates their epigenetic, transcriptomic, proteomic, metabolomic, and morphological states.
5. Fibroblasts
Unlike microglia and astrocytes, the roles of fibroblasts in SCI are only beginning to be elucidated. Fibroblasts in the adult CNS populate various spatial domains, including the meninges, choroid plexus, and perivascular spaces (Soderblom et al., 2013). Pericytes and endothelial cells also differentiate into fibroblast-like cells, contributing to fibrotic scar formation after CNS injury (Göritz et al., 2011; Zhou et al., 2019; Dias et al., 2021).
5.1. Fibroblast response to SCI
These distinct stromal cell populations are differentially recruited to the SCI lesion site, where they respond to fibrogenic factors such as TGF-β and elaborate ECM components, including collagen, laminins, fibronectin, and CSPGs leading to the formation of the fibrotic scar (Leask and Abraham, 2004; Klapka and Müller, 2006; Wynn, 2008; Ankeny and Popovich, 2009; Fawcett et al., 2012; Kawano et al., 2012; Soderblom et al., 2013; Gensel and Zhang, 2015; Wang et al., 2018).
This initial response seals off the injury site and limits CNS damage (Dias et al., 2018). Stromal cells are the primary producers of type I collagen in SCI, responsible for astrocyte scar formation (Hara et al., 2017). Moreover, interactions between EphB2 on astrocytes with ephrin-B2 on fibroblasts are believed to underpin the clear spatial segregation between the centrally located fibroblasts and astrocytes at the scar border (Bundesen et al., 2003). However, these interactions appear redundant since the astrocyte border persists even when fibrotic scar formation is attenuated; other cell types, such as microglia, are also involved in ensuring proper glial scar formation (Bellver-Landete et al., 2019; Dorrier et al., 2021).
Studies have shown that inhibiting key fibroblast functions after SCI enhances remyelination, axonal regeneration, and functional recovery (Pasterkamp et al., 1999, 2001; Hermanns et al., 2001; De Winter et al., 2002; Hellal et al., 2011; Cregg et al., 2014; Dias et al., 2018). Fibroblast-derived type I collagen induces the formation of the dense astrocytic scar, which chronically impedes axonal regeneration. Moreover, EphB2/ephrin-B2 interactions between astrocytes and fibroblasts can foster dysfunctional astrocyte reactivity and decrease synaptic plasticity and axonal regeneration after SCI (Li et al., 2017; Wu et al., 2021). Activated fibroblasts are also known to augment innate and adaptive immunity to promote inflammation and thereby delay wound healing in peripheral tissues (Ayazi et al., 2022), but similar mechanistic insights remain investigational in SCI.
5.2. Fibroblast origin and heterogeneity
Studies using ScRNA-Seq have demonstrated that meningeal fibroblasts from the dura, arachnoid, and pia are transcriptionally distinct (DeSisto et al., 2020). Recent studies have also shown three transcriptionally distinct clusters of perivascular fibroblasts in the healthy CNS (Garcia et al., 2022; Winkler et al., 2022). Whether fibroblasts derived from different origins—the meninges, perivasculature, or choroid plexus—have differential contributions to the fibrotic scar, glial scar persistence or resolution, and axonal regeneration/repair are important questions for future studies. In this regard, a recent study demonstrated that three transcriptionally distinct clusters of stromal cells—which the authors termed fibroblasts—accumulate at different stages after SCI (Gong et al., 2023). Cluster one fibroblasts begin appearing in the fibrotic scar by 7 days dpi, peak at 14 days dpi, and stay consistently elevated in the chronic phase. These fibroblasts exhibited a pro-inflammatory transcriptomic signature (Gong et al., 2023). Cluster 2 fibroblasts—enriched in genes encoding proteins involved in angiogenesis, ECM organization, TGF-β-related signaling, and collagen processing—accumulate in the center of the lesion at 3 days dpi (Gong et al., 2023). This study was the first to demonstrate that transcriptionally distinct subsets of stromal cells accumulate at different timepoints through the course of SCI. However, it is essential to note that, as already stated, documenting transcriptional heterogeneity does not necessarily imply differential functional contributions, which is an area that still requires further work.
The lack of specific markers to distinguish between different fibroblast lineages and perivascular cells such as pericytes and vascular smooth muscle cells (vSMCs) has hindered studies from delineating the distinct roles of each cell type in SCI. Studies on Glast-CreER mice—reporter mice that allow the inducible depletion of Glast1+ cells—ascribed a neuroprotective role to Glast1+ stromal cells in SCI (Göritz et al., 2011; Dias et al., 2021). The authors labeled these Glast1+ cells as pericytes, but astrocytes and fibroblasts also express Glast1 (Regan et al., 2007; Vanlandewijck et al., 2018). Col1a1-GFP transgenic mice demonstrate that stromal cells within the fibrotic scar are derived from the meninges rather than the perivasculature and do not express pericyte markers such as NG2 (Soderblom et al., 2013), supporting the idea that the studies describing Glast1+ pericyte functions may have been studying fibroblasts. The Gong et al. (2023) study discussed above also utilized Glast1 positivity to define stromal cell identify, and hence we caution against considering these cells as fibroblasts or pericytes until further results prove otherwise.
Similarly, although NG2+ perivascular cells are known to perform essential functions in angiogenesis and fibrotic scar formation after SCI (Hesp et al., 2018), NG2 is expressed by pericytes, perivascular fibroblasts, vSMCs, and OPCs (Bergers and Song, 2005). Therefore, tracing stromal cell lineage based solely on Glast1 or NG2 expression lacks specificity, and the origin of stromal cells in the fibrotic scar remains debatable. Dorrier et al. (2021) utilized cell lineage-tracing technologies to demonstrate that perivascular fibroblasts—not pericytes or vSMCs—contributed to fibrotic scar development in EAE mouse models. It would be valuable to apply similar methodologies to SCI to unequivocally discern the origin of stromal cells in the fibrotic scar.
6. Oligodendrocyte progenitor cells
Oligodendrocyte loss ensues immediately after SCI, and their apoptosis continues into the subacute and chronic phases in various animal models (Li et al., 1999; Almad et al., 2011; Pukos et al., 2019). Demyelination of spared axons is thus a prevalent feature post-SCI and contributes to neuronal impairment by compromising axonal conduction even in anatomically incomplete lesions (Pukos et al., 2019). The mechanistic underpinnings of this phenomenon and how spared axonal function can be restored have attracted much research interest, especially given the fact that maintaining the functional integrity of a few axons could significantly better neuronal function (Schucht et al., 2002; Kakulas, 2004). Hence, promoting remyelination, in which OPCs are crucial, has long been sought after as a potential therapeutic strategy. NG2+ OPCs cells are spread throughout the CNS (Nishiyama et al., 1999, 2016), actively interact with neurons (Bergles et al., 2000; Sahel et al., 2015), and sustain oligodendrocyte turnover and remyelination (Watanabe et al., 2002).
6.1. OPC response to SCI
Oligodendrocyte progenitor cells acutely mount a robust proliferative response that peaks at 5 dpi, accumulating in the lesion penumbra alongside astrocytes (McTigue et al., 2001; Zai and Wrathall, 2005; Barnabé-Heider et al., 2010; Hesp et al., 2015). Ependymal cells, the NSCs of the spinal cord, also give rise to OPCs in SCI (Meletis et al., 2008). Multiple factors in the SCI microenvironment, such as TNF-α and WNTs, drive OPC proliferation (Tripathi and McTigue, 2007; Moore et al., 2011; Miron et al., 2013; Burda and Sofroniew, 2014; Hackett et al., 2016; Miron, 2017) but concomitantly impair their differentiation into mature oligodendrocytes as a result of enhanced β-catenin signaling (Rhodes et al., 2006; Liu et al., 2008; Hill et al., 2013). Moreover, OPCs express RPTPσ, which can bind CSPGs to inhibit OPC differentiation into oligodendrocytes (Ranjan and Hudson, 1996; Siebert and Osterhout, 2011; Pendleton et al., 2013; Karus et al., 2016).
Oligodendrocyte progenitor cells also differentiate into remyelinating Schwann cells, although it should be stated that the impact of this process on functional recovery remains controversial and may not be significant (Duncan et al., 2018, 2020). Furthermore, OPCs have been shown to differentiate into astrocytes after SCI (Suzuki et al., 2017; Duncan et al., 2020), which express anti-inflammatory and anti-apoptotic proteins such as crystallin alpha B (Hou et al., 2022), indicating that OPC-derived astrocytes may be neuroprotective. Fate-mapping NG2+ cells reveal that 25% of OPCs differentiate into astrocytes in a contusive SCI model (Hackett et al., 2018). In contrast, this trajectory is less likely in a stab or transection SCI model or EAE (Hackett et al., 2018), indicating that the disease-specific microenvironment is crucial in determining OPCs fate.
Dorrier et al. ablated proliferating fibroblasts in the fibrotic scar using a transgenic herpes simplex virus thymidine kinase combined with ganciclovir (HSV-TK/GCV) EAE mouse model. After fibroblast ablation, there was a significant increase in the infiltration of Olig2-positive OPCs into the inflamed lesion (Dorrier et al., 2021). These results were corroborated by in vitro findings that collagen-producing fibroblasts significantly reduce the migration of OPCs across a transwell insert (Dorrier et al., 2021). Therefore, the dense stromal cell and ECM presence in the SCI scar may limit OPCs to the lesion periphery, restricting their access to demyelinating axons.
6.2. OPCs beyond remyelination
Running contrary to the remyelinating response of OPCs, NG2 is an inhibitor of axonal regeneration (Dou and Levine, 1994; Petrosyan et al., 2013). NG2+ cells have been visualized besides dystrophic axon end-bulbs, which can be reversed by administering an anti-NG2 antibody (McTigue et al., 2006; Tan et al., 2006; Filous et al., 2014). However, NG2+ cells also reduce macrophage-induced axonal dieback (Busch et al., 2010), possibly by forming synapse-like connections with the tips of transected axons (Fünfschilling et al., 2012; Angela et al., 2014). NG2+ cells also produce ECM components such as fibronectin and laminin that protect axon growth cones from the neuroinflammatory milieu (Angela et al., 2014; Tran et al., 2018b). However, this acute neuroprotection appears to be at the expense of long-term regeneration, as NG2+ cells and the ECM chronically entrap axon growth-cones to hamper regeneration (Busch et al., 2010; Di Maio et al., 2011; Bradke et al., 2012; Son, 2015; Hackett and Lee, 2016). Entrapped axon growth cones have persisted as long as 40 years post-SCI (Silver and Miller, 2004; Tom et al., 2004), indicating that this entrapment is permanent. Freeing trapped dystrophic axon growth cones may constitute a therapeutic approach to enhance regeneration (Tran et al., 2018b).
Oligodendrocyte progenitor cells become reactive in the SCI microenvironment and secrete MMP-9, increasing BSCB permeability and enhancing neuroinflammation (Seo et al., 2013). Reactive OPCs may also be sources of pro-inflammatory cytokines and chemokines that induce pro-inflammatory reactive states in microglia and macrophages (Kucharova and Stallcup, 2015). The spatial localization of OPCs also provides insight into their intercellular communication: OPCs reside in the scar border alongside proliferating astrocytes, which interface with reactive microglia at the margins of the fibrotic scar (Keirstead et al., 1998). Ablating NG2+ glia in the lesion penumbra significantly reduces astrocyte hypertrophy and GFAP expression, resulting in disorganization of the glial scar, expansion of the lesion site, and worse neurologic outcomes (Hesp et al., 2018). These results align with transgenic experiments showing that drastic attenuation of each cell type is detrimental to the host injury response.
Milder manipulations of OPCs without their depletion reveal more specific functions of OPCs. A recent study showed that OPC-specific β-catenin deletion in tamoxifen-inducible cre-recombinase mice enhances OPC differentiation into mature oligodendrocytes and significantly reduces astrocyte hypertrophy and GFAP expression, fostering a growth-permissive microenvironment that promotes axonal regeneration and improves recovery of hindlimb motor function after SCI (Rodriguez et al., 2014). β-Catenin deletion in OPCs also polarized microglia and macrophages to anti-inflammatory/pro-repair phenotypes (Rodriguez et al., 2014). Moreover, by injecting adeno-associated virus (AAV) containing Wnt3a+GFP or Wnt5a+GFP into uninjured spinal cords of female C57BL/6J mice, the authors showed that Wnt3a-induced β-catenin signaling significantly increased the infiltration and proliferation of OPCs around the injection site (Rodriguez et al., 2014). The microglia infiltration also substantially increased around the injection site, while the density of GFAP+ astrocytes remained unaltered (Rodriguez et al., 2014).
These results suggest that OPCs are part orchestrators of the injury, particularly myeloid cell, response following SCI. Dampened microglial responses could also explain the decrease in GFAP+ astrocyte hypertrophy and density in β-catenin-KO mice, as these cells facilitate reactive astrocytosis at the lesion site (discussed above). The combination of a looser astrocyte border and significant reduction of CSPGs can account for improved axonal regeneration and density around the lesion site.
6.3. Current limitations
The lack of OPC-specific markers is a major hindrance in dissecting their contributions to SCI pathology and repair. NG2 and PDGFRα are commonly used OPC markers but are also expressed by stromal cells such as pericytes (Bergers and Song, 2005). For example, Hesp et al. (2018) utilized an HSV-TK/GCV transgenic model to deplete NG2+ stromal cells (pericytes or fibroblasts) in the fibrotic scar and NG2+ OPCs in the glial scar. Eliminating NG2+ cells in the lesion epicenter completely abolished the fibrotic scar, and loss of dividing NG2+ OPCs disrupted astrocytic scar formation. However, given that NG2+ stromal cells also ensure proper astrocyte border formation (Hesp et al., 2018), this approach could not delineate the differential effect of stromal cells and OPCs. Moreover, current genetic mouse lines, which allow inducible attenuation or enhancement of OPC-mediated remyelination, also affect glial scarring, astrocyte reactivity, and neuroinflammation (Duncan et al., 2020). Future studies utilizing combinatorial strategies of NG2/PDGFRα reporter mice and localization and imaging techniques supplemented with an array of cell surface markers will better identify NG2+ OPCs and allow their specific contribution to be dissected at higher resolution.
7. A neuro-centric view of axonal regeneration
Neurobiologists have long known the ability of axons in the developing CNS to grow far-reaching axons. Peripheral nervous system (PNS) axons also regenerate effectively and reach innervation targets after injury, whereas adult CNS neurons do not (Broude et al., 1997). Animals such as the CAST/Ei mouse strain display excellent axonal regeneration after injury, accompanied by specific changes in neuron gene expression programs not seen in control mice that exhibit poor regeneration (Omura et al., 2015). Therefore, studies have cited gene expression differences as essential factors in the poor regenerative capacity of the adult mammalian CNS. Multiple ground-breaking studies over the past decade have uncovered numerous vital regulators of axon growth programs in SCI at the transcriptional, translational, and epigenetic levels, including PTEN-mTOR, SOCS3-STAT3, cAMP, REST/NRSF, and many others (Qiu et al., 2002; Park et al., 2008; Liu et al., 2010; Sun et al., 2011; Cheng et al., 2022). Excellent reviews on the neuron-intrinsic regulators of axonal regeneration are referenced here (He and Jin, 2016; Mahar and Cavalli, 2018; Bradke, 2022; Zheng and Tuszynski, 2023).
The Sofroniew Laboratory observed that a combination of factors underpins CNS regeneration failure: insufficient growth factors, chemoattractive substrates, and a failure to active pro-regenerative gene signatures in neurons (Anderson et al., 2018). Supplying all three elements together—but not individually—significantly enhances axonal regeneration past the astroglial and fibrotic scars more than 140-fold greater than in control mice (Anderson et al., 2018). Therefore, delivering a variety of cells and neurotrophic factors, such as through biomaterial-based approaches (Liu S. et al., 2018; Courtine and Sofroniew, 2019; Guijarro-Belmar et al., 2022), is a promising avenue to promote axonal regeneration and functional recovery.
8. Concluding remarks and perspectives
This review focused on the dual roles of the SCI scar, an acutely beneficial and chronically pathological one. Indiscriminate targeting of the essential cellular components of the SCI scar is deleterious in animal models but does not discount the chronically detrimental roles of glial and stromal cells. Indeed, more specific and milder manipulations of cellular constituents of the SCI scar enhance axonal regeneration and functional recovery. Concomitantly, advancements in single-cell technologies have revealed profound cellular heterogeneity in the SCI scar, which underscores the importance of cell- and context-specific therapeutic manipulations. To develop such therapeutic strategies, it is essential to further characterize the regulation and functional significance of cellular heterogeneity.
One can classify the different therapeutic strategies that may be employed to target the host response to SCI: (1) enhancing neuron regenerating capacity; (2) targeting extracellular regeneration inhibitors such as CSPGs and myelin; (3) targeting the glial cell responses (particularly astrocytes and microglia); and (4) targeting the central fibrotic core. Unlike the first three, the fibrotic core has received relatively little attention regarding its therapeutic value. The origin of stromal cells, their apparent heterogeneity at the lesion site, and the effects of milder manipulations (rather than transgenic ablation) of stromal cells are poorly understood. Exploring these aspects may pave the way for future therapies employing combinatorial approaches harnessing the innate regenerative capabilities of neurons and promoting a pro-repair microenvironment within the injured spinal cord.
We would also like to see the impact of biological aging hallmarks such as cellular senescence be further fleshed out in explaining divergent SCI responses across lifespan. Transgenic models such as INK-ATTAC and p16-3MR mice are available that allow the specific tracking and inducible depletion of senescent cells (Baker et al., 2011; Demaria et al., 2014) and would be helpful in determining the particular cell types undergoing senescence in SCI and the effects of their depletion in different SCI phases. Given the rapid evolution of senolytics from benchwork into clinical trials, we feel it prudent to investigate the role of cellular senescence in SCI to unveil potentially another therapeutic strategy improve SCI outcomes.
Ultimately, only rigorous testing will uncover novel and potentially groundbreaking therapeutic targets, revolutionizing our ability to enhance regeneration and improve outcomes for humans affected by spinal cord injuries.
Author contributions
AS conceptualized the manuscript. AS, IA, HM, and TS prepared the initial draft and designed the figures. AS, KA, and AY reviewed the manuscript and prepared the final version. All authors read and approved the final version of this manuscript.
Acknowledgments
Figures were created with Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adams K. L., Gallo V. (2018). The diversity and disparity of the glial scar. Nat. Neurosci. 21 9–15. 10.1038/s41593-017-0033-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal M., Welshhans K. (2021). Local translation across neural development: a focus on radial glial cells, axons, and synaptogenesis. Front. Mol. Neurosci. 14:717170. 10.3389/fnmol.2021.717170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo A. J., David S., Bray G. M. (1981). Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J. Exp. Biol. 95 231–240. 10.1242/jeb.95.1.231 [DOI] [PubMed] [Google Scholar]
- Al-Dalahmah O., Sosunov A. A., Shaik A., Ofori K., Liu Y., Vonsattel J. P., et al. (2020). Single-nucleus RNA-seq identifies huntington disease astrocyte states. Acta Neuropathol. Commun. 8:19. 10.1186/s40478-020-0880-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alicia L. H., Hongmei H., Bornali K., Michael P. S., Christi J. W., Evan S. D., et al. (2011). The unusual response of serotonergic neurons after CNS injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the Glial scar. J. Neurosci. 31:5605. 10.1523/JNEUROSCI.6663-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh A., Dyck S. M., Karimi-Abdolrezaee S. (2019). Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 10:282. 10.3389/fneur.2019.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. R. (1911). Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. J. Am. Med. Assoc. LVII, 878–880. 10.1001/jama.1911.04260090100008 [DOI] [Google Scholar]
- Allison D. J., Ditor D. S. (2015). Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 53 14–18. 10.1038/sc.2014.184 [DOI] [PubMed] [Google Scholar]
- Almad A., Sahinkaya F. R., McTigue D. M. (2011). Oligodendrocyte fate after spinal cord injury. Neurotherapeutics 8 262–273. 10.1007/s13311-011-0033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. A., Burda J. E., Ren Y., Ao Y., O’Shea T. M., Kawaguchi R., et al. (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 532 195–200. 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. A., O’Shea T. M., Burda J. E., Ao Y., Barlatey S. L., Bernstein A. M., et al. (2018). Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561 396–400. 10.1038/s41586-018-0467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D. G., Samuel D. (2014). Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J. Neurosci. 34:6316. 10.1523/JNEUROSCI.4912-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angela R. F., Amanda T., Howell C. J., Sarah A. B., Teresa A. E., William B. S., et al. (2014). Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J. Neurosci. 34:16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum A., Yazid M. D., Fauzi Daud M., Idris J., Ng A. M., Selvi Naicker A., et al. (2020). Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 21:7533. 10.3390/ijms21207533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny D. P., Guan Z., Popovich P. G. (2009). B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Investig. 119 2990–2999. 10.1172/JCI39780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny D. P., Lucin K. M., Sanders V. M., McGaughy V. M., Popovich P. G. (2006). Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 99 1073–1087. 10.1111/j.1471-4159.2006.04147.x [DOI] [PubMed] [Google Scholar]
- Ankeny D. P., Popovich P. G. (2009). Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 158 1112–1121. 10.1016/j.neuroscience.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar M. A., Al Shehabi T. S., Eid A. H. (2016). Inflammogenesis of secondary spinal cord injury. Front. Cell Neurosci. 10:98. 10.3389/fncel.2016.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayazi M., Zivkovic S., Hammel G., Stefanovic B., Ren Y. (2022). Fibrotic scar in CNS injuries: from the cellular origins of fibroblasts to the molecular processes of fibrotic scar formation. Cells 11:2371. 10.3390/cells11152371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. J., Wijshake T., Tchkonia T., LeBrasseur N. K., Childs B. G., van de Sluis B., et al. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479 232–236. 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasingam V., Tejada-Berges T., Wright E., Bouckova R., Yong V. W. (1994). Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J. Neurosci. 14 846–856. 10.1523/JNEUROSCI.14-02-00846.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre F. M., Schwab M. E. (2003). Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 26 555–563. 10.1016/j.tins.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Barnabé-Heider F., Göritz C., Sabelström H., Takebayashi H., Pfrieger F. W., Meletis K., et al. (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7 470–482. 10.1016/j.stem.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Bartus K., Burnside E. R., Galino J., James N. D., Bennett D. L. H., Bradbury E. J., et al. (2019). ErbB receptor signaling directly controls oligodendrocyte progenitor cell transformation and spontaneous remyelination after spinal cord injury. Glia 67 1036–1046. 10.1002/glia.23586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K. D., Nguyen H. X., Galvan M. D., Salazar D. L., Woodruff T. M., Anderson A. J. (2010). Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133(Pt 2), 433–447. 10.1093/brain/awp322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellver-Landete V., Bretheau F., Mailhot B., Vallières N., Lessard M., Janelle M.-E., et al. (2019). Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 10:518. 10.1038/s41467-019-08446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett F. C., Bennett M. L., Yaqoob F., Mulinyawe S. B., Grant G. A., Hayden Gephart M., et al. (2018). A combination of ontogeny and CNS environment establishes microglial identity. Neuron 98 1170–1183.e8. 10.1016/j.neuron.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. L., Bennett F. C., Liddelow S. A., Ajami B., Zamanian J. L., Fernhoff N. B., et al. (2016). New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. 113 E1738–E1746. 10.1073/pnas.1525528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., Song S. (2005). The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 7 452–464. 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles D. E., Roberts J. D., Somogyi P., Jahr C. E. (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405 187–191. 10.1038/35012083 [DOI] [PubMed] [Google Scholar]
- Bertolotti E., Malagoli D., Franchini A. (2013). Skin wound healing in different aged Xenopus laevis. J. Morphol. 274 956–964. 10.1002/jmor.20155 [DOI] [PubMed] [Google Scholar]
- Bi Y., Duan W., Chen J., You T., Li S., Jiang W., et al. (2021). Neutrophil decoys with anti-inflammatory and anti-oxidative properties reduce secondary spinal cord injury and improve neurological functional recovery. Adv. Funct. Mater. 31:2102912. 10.1002/adfm.202102912 [DOI] [Google Scholar]
- Bian H. T., Xiao L., Liang L., Xie Y. P., Wang H. L., Wang G. H. (2021). RGFP966 is protective against lipopolysaccharide-induced depressive-like behaviors in mice by inhibiting neuroinflammation and microglial activation. Int. Immunopharmacol. 101(Pt B):108259. 10.1016/j.intimp.2021.108259 [DOI] [PubMed] [Google Scholar]
- Bianchi M. E. (2007). DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc Biol. 81 1–5. 10.1189/jlb.0306164 [DOI] [PubMed] [Google Scholar]
- Boche D., Perry V. H., Nicoll J. A. R. (2013). Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 39 3–18. 10.1111/nan.12011 [DOI] [PubMed] [Google Scholar]
- Boroujerdi A., Kim H. K., Lyu Y. S., Kim D. S., Figueroa K. W., Chung J. M., et al. (2008). Injury discharges regulate calcium channel alpha-2-delta-1 subunit upregulation in the dorsal horn that contributes to initiation of neuropathic pain. Pain 139 358–366. 10.1016/j.pain.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutej H., Rahimian R., Thammisetty S. S., Béland L.-C., Lalancette-Hébert M., Kriz J. (2017). Diverging mRNA and protein networks in activated microglia reveal SRSF3 suppresses translation of highly upregulated innate immune transcripts. Cell Rep. 21 3220–3233. 10.1016/j.celrep.2017.11.058 [DOI] [PubMed] [Google Scholar]
- Bradbury E. J., Burnside E. R. (2019). Moving beyond the glial scar for spinal cord repair. Nat. Commun. 10:3879. 10.1038/s41467-019-11707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. J., McMahon S. B. (2006). Spinal cord repair strategies: why do they work? Nat. Rev. Neurosci. 7 644–653. 10.1038/nrn1964 [DOI] [PubMed] [Google Scholar]
- Bradke F. (2022). Mechanisms of axon growth and regeneration: moving between development and disease. J. Neurosci. 42 8393–8405. 10.1523/JNEUROSCI.1131-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F., Fawcett J. W., Spira M. E. (2012). Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat. Rev. Neurosci. 13 183–193. 10.1038/nrn3176 [DOI] [PubMed] [Google Scholar]
- Brandebura A. N., Paumier A., Onur T. S., Allen N. J. (2023). Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 24 23–39. 10.1038/s41583-022-00641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. H., Li Y., Wang C., Ma A., Guo Q., Li Y., et al. (2022). Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 13:4096. 10.1038/s41467-022-31797-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. H., Popovich P. G. (2018). Emerging targets for reprograming the immune response to promote repair and recovery of function after spinal cord injury. Curr. Opin. Neurol. 31 334–344. 10.1097/WCO.0000000000000550 [DOI] [PubMed] [Google Scholar]
- Brooks J. M., Su J., Levy C., Wang J. S., Seabrook T. A., Guido W., et al. (2013). A molecular mechanism regulating the timing of corticogeniculate innervation. Cell Rep. 5 573–581. 10.1016/j.celrep.2013.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broude E., McAtee M., Kelley M. S., Bregman B. S. (1997). c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp. Neurol. 148 367–377. 10.1006/exnr.1997.6665 [DOI] [PubMed] [Google Scholar]
- Brown J. O., McCouch G. P. (1947). Abortive regeneration of the transected spinal cord. J. Comp. Neurol. 87 131–137. 10.1002/cne.900870204 [DOI] [PubMed] [Google Scholar]
- Bundesen L. Q., Scheel T. A., Bregman B. S., Kromer L. F. (2003). Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci. 23 7789–7800. 10.1523/JNEUROSCI.23-21-07789.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda J. E., O’Shea T. M., Ao Y., Suresh K. B., Wang S., Bernstein A. M., et al. (2022). Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature 606 557–564. 10.1038/s41586-022-04739-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda J. E., Sofroniew M. V. (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda Joshua E., Sofroniew Michael V. (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S. A., Hamilton J. A., Horn K. P., Cuascut F. X., Cutrone R., Lehman N., et al. (2011). Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J. Neurosci. 31 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S. A., Horn K. P., Cuascut F. X., Hawthorne A. L., Bai L., Miller R. H., et al. (2010). Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J. Neurosci. 30 255–265. 10.1523/JNEUROSCI.3705-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S. A., Horn K. P., Silver D. J., Silver J. (2009). Overcoming macrophage-mediated axonal dieback following CNS injury. J. Neurosci. 29 9967–9976. 10.1523/JNEUROSCI.1151-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush T. G., Puvanachandra N., Horner C. H., Polito A., Ostenfeld T., Svendsen C. N., et al. (1999). Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23 297–308. 10.1016/S0896-6273(00)80781-3 [DOI] [PubMed] [Google Scholar]
- Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. (2019). Cellular senescence: aging, cancer, and injury. Physiol. Rev. 99 1047–1078. 10.1152/physrev.00020.2018 [DOI] [PubMed] [Google Scholar]
- Canning D. R., McKeon R. J., DeWitt D. A., Perry G., Wujek J. R., Frederickson R. C. A., et al. (1993). β-Amyloid of Alzheimer’s disease induces reactive gliosis that inhibits axonal outgrowth. Exp. Neurol. 124 289–298. 10.1006/exnr.1993.1199 [DOI] [PubMed] [Google Scholar]
- Cao Y., Zhu S., Yu B., Yao C. (2022). Single-cell RNA sequencing for traumatic spinal cord injury. Faseb J. 36:e22656. 10.1096/fj.202200943R [DOI] [PubMed] [Google Scholar]
- Carter L. M., Starkey M. L., Akrimi S. F., Davies M., McMahon S. B., Bradbury E. J. (2008). The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. J. Neurosci. 28 14107–14120. 10.1523/JNEUROSCI.2217-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D., Laabs T., Geller H. M., Fawcett J. W. (2005). Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 15 116–120. 10.1016/j.conb.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Ceyzériat K., Ben Haim L., Denizot A., Pommier D., Matos M., Guillemaud O., et al. (2018). Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol. Commun. 6:104. 10.1186/s40478-018-0606-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaib S., Tchkonia T., Kirkland J. L. (2022). Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28 1556–1568. 10.1038/s41591-022-01923-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Qian Z., Wang B., Cao J., Zhang S., Jiang F., et al. (2023). Transplantation of A2 type astrocytes promotes neural repair and remyelination after spinal cord injury. Cell Commun. Signaling 21:37. 10.1186/s12964-022-01036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Ye J., Chen X., Shi J., Wu W., Lin W., et al. (2018). Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J. Neuroinflammation 15:150. 10.1186/s12974-018-1193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhang L., Hua F., Zhuang Y., Liu H., Wang S. (2022). EphA4 obstructs spinal cord neuron regeneration by promoting excessive activation of astrocytes. Cell Mol. Neurobiol. 42 1557–1568. 10.1007/s10571-021-01046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Yin Y., Zhang A., Bernstein A. M., Kawaguchi R., Gao K., et al. (2022). Transcription factor network analysis identifies REST/NRSF as an intrinsic regulator of CNS regeneration in mice. Nat. Commun. 13:4418. 10.1038/s41467-022-31960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs B. G., Durik M., Baker D. J., van Deursen J. M. (2015). Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21 1424–1435. 10.1038/nm.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson K. S., Ullian E. M., Stokes C. C. A., Mullowney C. E., Hell J. W., Agah A., et al. (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120 421–433. 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Courtine G., Sofroniew M. V. (2019). Spinal cord repair: advances in biology and technology. Nat. Med. 25 898–908. 10.1038/s41591-019-0475-6 [DOI] [PubMed] [Google Scholar]
- Cregg J. M., DePaul M. A., Filous A. R., Lang B. T., Tran A., Silver J. (2014). Functional regeneration beyond the glial scar. Exp. Neurol. 253 197–207. 10.1016/j.expneurol.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Wu H., Yu X., Song T., Xu X., Xu F. (2021). The calcium channel α2δ1 subunit: interactional targets in primary sensory neurons and role in neuropathic pain. Front. Cell. Neurosci. 15:699731. 10.3389/fncel.2021.699731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva-Álvarez S., Guerra-Varela J., Sobrido-Cameán D., Quelle A., Barreiro-Iglesias A., Sánchez L., et al. (2020). Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 19:e13052. 10.1111/acel.13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S., Ming L.-B., Richard H. M., Tatjana C. J. (2010). Structural remodeling of fibrous astrocytes after axonal injury. J. Neurosci. 30:14008. 10.1523/JNEUROSCI.3605-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Li Z., Noori A., Hyman B. T., Serrano-Pozo A. (2020). Meta-analysis of mouse transcriptomic studies supports a context-dependent astrocyte reaction in acute CNS injury versus neurodegeneration. J. Neuroinflammation 17:227. 10.1186/s12974-020-01898-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8 752–758. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- David P. S., Shuhong L., Paul K., Yong V. W. (2009). Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J. Neurosci. 29 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Aguayo A. J. (1981). Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214 931–933. 10.1126/science.6171034 [DOI] [PubMed] [Google Scholar]
- David S., Kroner A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12 388–399. 10.1038/nrn3053 [DOI] [PubMed] [Google Scholar]
- David S., Kroner A., Greenhalgh A. D., Zarruk J. G., López-Vales R. (2018). Myeloid cell responses after spinal cord injury. J. Neuroimmunol. 321 97–108. 10.1016/j.jneuroim.2018.06.003 [DOI] [PubMed] [Google Scholar]
- David S., López-Vales R., Wee Yong V. (2012). Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb. Clin. Neurol. 109 485–502. 10.1016/B978-0-444-52137-8.00030-9 [DOI] [PubMed] [Google Scholar]
- Davies J. E., Huang C., Proschel C., Noble M., Mayer-Proschel M., Davies S. J. (2006). Astrocytes derived from glial-restricted precursors promote spinal cord repair. J. Biol. 5:7. 10.1186/jbiol35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. J., Fitch M. T., Memberg S. P., Hall A. K., Raisman G., Silver J. (1997). Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390 680–683. 10.1038/37776 [DOI] [PubMed] [Google Scholar]
- Davies S. J., Goucher D. R., Doller C., Silver J. (1999). Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 19 5810–5822. 10.1523/JNEUROSCI.19-14-05810.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. J., Shih C. H., Noble M., Mayer-Proschel M., Davies J. E., Proschel C. (2011). Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One 6:e17328. 10.1371/journal.pone.0017328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro R., Hughes M. G., Xu G. Y., Clifton C., Calingasan N. Y., Gelman B. B., et al. (2004). Evidence that infiltrating neutrophils do not release reactive oxygen species in the site of spinal cord injury. Exp. Neurol. 190 414–424. 10.1016/j.expneurol.2004.05.046 [DOI] [PubMed] [Google Scholar]
- De Miguel Z., Khoury N., Betley M. J., Lehallier B., Willoughby D., Olsson N., et al. (2021). Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600 494–499. 10.1038/s41586-021-04183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper S., Ge J. Z., Crowley G., Ferreira L. S. S., Garceau D., Toomey C. E., et al. (2023). Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease. Nat. Neurosci. 26 406–415. 10.1038/s41593-023-01257-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter F., Oudega M., Lankhorst A. J., Hamers F. P., Blits B., Ruitenberg M. J., et al. (2002). Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 175 61–75. 10.1006/exnr.2002.7884 [DOI] [PubMed] [Google Scholar]
- Demaria M., Ohtani N., Youssef S. A., Rodier F., Toussaint W., Mitchell J. R., et al. (2014). An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31 722–733. 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Meng F., Zhang K., Gao J., Liu Z., Li M., et al. (2022). Emerging roles of microglia depletion in the treatment of spinal cord injury. Cells 11:1871. 10.3390/cells11121871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSisto J., O’Rourke R., Jones H. E., Pawlikowski B., Malek A. D., Bonney S., et al. (2020). Single-cell transcriptomic analyses of the developing meninges reveal meningeal fibroblast diversity and function. Dev. Cell 4 43–59.e4. 10.1016/j.devcel.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio A., Skuba A., Himes B. T., Bhagat S. L., Hyun J. K., Tessler A., et al. (2011). In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J. Neurosci. 31 4569–4582. 10.1523/JNEUROSCI.4638-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias D. O., Kalkitsas J., Kelahmetoglu Y., Estrada C. P., Tatarishvili J., Holl D., et al. (2021). Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 12:5501. 10.1038/s41467-021-25585-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias D. O., Kim H., Holl D., Solnestam B. W., Lundeberg J., Carlén M., et al. (2018). Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell 173 153–165.e22. 10.1016/j.cell.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel K., Dhabhar F. S., Sapolsky R. M. (2004). Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc. Natl. Acad. Sci. 101 331–336. 10.1073/pnas.0303510101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma S., Kumar H. (2021). Neutrophil, extracellular matrix components, and their interlinked action in promoting secondary pathogenesis after spinal cord injury. Mol. Neurobiol. 58 4652–4665. 10.1007/s12035-021-02443-5 [DOI] [PubMed] [Google Scholar]
- Domingues H. S., Portugal C. C., Socodato R., Relvas J. B. (2016). Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front. Cell Dev. Biol. 4:71. 10.3389/fcell.2016.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domowicz M. S., Henry J. G., Wadlington N., Navarro A., Kraig R. P., Schwartz N. B. (2011). Astrocyte precursor response to embryonic brain injury. Brain Res. 1389 35–49. 10.1016/j.brainres.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrier C. E., Aran D., Haenelt E. A., Sheehy R. N., Hoi K. K., Pintarić L., et al. (2021). CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 24, 234–244. 10.1038/s41593-020-00770-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou C. L., Levine J. M. (1994). Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci. 14 7616–7628. 10.1523/JNEUROSCI.14-12-07616.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G. J., Manesh S. B., Hilton B. J., Assinck P., Liu J., Moulson A., et al. (2018). Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun. 9:3066. 10.1038/s41467-018-05473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G. J., Manesh S. B., Hilton B. J., Assinck P., Plemel J. R., Tetzlaff W. (2020). The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia 68 227–245. 10.1002/glia.23706 [DOI] [PubMed] [Google Scholar]
- Edwards-Faret G., González-Pinto K., Cebrián-Silla A., Peñailillo J., García-Verdugo J. M., Larraín J. (2021). Cellular response to spinal cord injury in regenerative and non-regenerative stages in Xenopus laevis. Neural Dev. 16:2. 10.1186/s13064-021-00152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore M. R., Najafi A. R., Koike M. A., Dagher N. N., Spangenberg E. E., Rice R. A., et al. (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82 380–397. 10.1016/j.neuron.2014.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming S. A., Martin P., Tomic-Canic M. (2014). Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6:265sr6. 10.1126/scitranslmed.3009337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C., Galea E., Lakatos A., O’Callaghan J. P., Petzold G. C., Serrano-Pozo A., et al. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24 312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. A., Barkauskas D. S., Myers J. T., Hare E. G., You J. Q., Ransohoff R. M., et al. (2014). High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol. 254 109–120. 10.1016/j.expneurol.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner J. R., Herrmann J. E., Woo M. J., Tansey K. E., Doan N. B., Sofroniew M. V. (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24 2143–2155. 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J. W. (2015). The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog. Brain Res. 218 213–226. 10.1016/bs.pbr.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Fawcett J. W., Asher R. A. (1999). The glial scar and central nervous system repair. Brain Res. Bull. 49 377–391. 10.1016/S0361-9230(99)00072-6 [DOI] [PubMed] [Google Scholar]
- Fawcett J. W., Schwab M. E., Montani L., Brazda N., Müller H. W. (2012). Defeating inhibition of regeneration by scar and myelin components. Handb. Clin. Neurol. 109 503–522. 10.1016/B978-0-444-52137-8.00031-0 [DOI] [PubMed] [Google Scholar]
- Fehlings M. G., Vaccaro A. R., Boakye M. (2012). Essentials of Spinal Cord Injury: Basic Research to Clinical Practice. Leipzig: Georg Thieme Verlag. 10.1055/b-0034-83848 [DOI] [Google Scholar]
- Feng Z., Min L., Liang L., Chen B., Chen H., Zhou Y., et al. (2021). Neutrophil extracellular traps exacerbate secondary injury via promoting neuroinflammation and blood–spinal cord barrier disruption in spinal cord injury. Front. Immunol. 12:698249. 10.3389/fimmu.2021.698249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn A. M., Hall J. C. E., Gensel J. C., Popovich P. G., Godbout J. P. (2014). IL-4 signaling drives a unique arginase microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Rα after traumatic spinal cord injury. J. Neurosci. 34 8904–8917. 10.1523/JNEUROSCI.1146-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti P., Zhang F., O’Neill P. (2003). Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev. Dyn. 226 245–256. 10.1002/dvdy.10226 [DOI] [PubMed] [Google Scholar]
- Filous A. R., Miller J. H., Coulson-Thomas Y. M., Horn K. P., Alilain W. J., Silver J. (2010). Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 70 826–841. 10.1002/dneu.20820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filous A. R., Tran A., Howell C. J., Busch S. A., Evans T. A., Stallcup W. B., et al. (2014). Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J. Neurosci. 34 16369–16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch M. T., Silver J. (2008). CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209 294–301. 10.1016/j.expneurol.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. C., Norenberg M. D., Ramsay D. A., Dekaban G. A., Marcillo A. E., Saenz A. D., et al. (2006). The cellular inflammatory response in human spinal cords after injury. Brain 129(Pt 12), 3249–3269. 10.1093/brain/awl296 [DOI] [PubMed] [Google Scholar]
- Francos-Quijorna I., Amo-Aparicio J., Martinez-Muriana A., López-Vales R. (2016). IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia 64 2079–2092. 10.1002/glia.23041 [DOI] [PubMed] [Google Scholar]
- Freitas P. D., Yandulskaya A. S., Monaghan J. R. (2019). Spinal cord regeneration in amphibians: a historical perspective. Dev. Neurobiol. 79 437–452. 10.1002/dneu.22669 [DOI] [PubMed] [Google Scholar]
- Fu H., Zhao Y., Hu D., Wang S., Yu T., Zhang L. (2020). Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis. 11:528. 10.1038/s41419-020-2733-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U., Supplie L. M., Mahad D., Boretius S., Saab A. S., Edgar J., et al. (2012). Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485 517–521. 10.1038/nature11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan J. C., Fehlings M. G. (2009). The impact of age on mortality, impairment, and disability among adults with acute traumatic spinal cord injury. J. Neurotrauma 26 1707–1717. 10.1089/neu.2009.0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani Sachin P., Walsh James T., Smirnov I., Zheng J., Kipnis J. (2015). The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85 703–709. 10.1016/j.neuron.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire J., Varholick J. A., Rana S., Sunshine M. D., Doré S., Barbazuk W. B., et al. (2021). Spiny mouse (Acomys): an emerging research organism for regenerative medicine with applications beyond the skin. npj Regen. Med. 6:1. 10.1038/s41536-020-00111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Bertolotto A., Levi G. (1987). The proteoglycan chondroitin sulfate is present in a subpopulation of cultured astrocytes and in their precursors. Dev. Biol. 123 282–285. 10.1016/0012-1606(87)90450-7 [DOI] [PubMed] [Google Scholar]
- Garcia F. J., Sun N., Lee H., Godlewski B., Mathys H., Galani K., et al. (2022). Single-cell dissection of the human brain vasculature. Nature 603 893–899. 10.1038/s41586-022-04521-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasek N. S., Kuchel G. A., Kirkland J. L., Xu M. (2021). Strategies for targeting senescent cells in human disease. Nat. Aging 1 870–879. 10.1038/s43587-021-00121-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet A. D., Fonken L. K. (2018). Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics 15 554–577. 10.1007/s13311-018-0630-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel J. C., Zhang B. (2015). Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619 1–11. 10.1016/j.brainres.2014.12.045 [DOI] [PubMed] [Google Scholar]
- Ghasemlou N., Bouhy D., Yang J., López-Vales R., Haber M., Thuraisingam T., et al. (2010). Beneficial effects of secretory leukocyte protease inhibitor after spinal cord injury. Brain 133 126–138. 10.1093/brain/awp304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Hui S. P. (2018). Axonal regeneration in zebrafish spinal cord. Regeneration 5 43–60. 10.1002/reg2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Gu Y., Han X., Luan C., Liu C., Wang X., et al. (2023). Spatiotemporal dynamics of the molecular expression pattern and intercellular interactions in the Glial scar response to spinal cord injury. Neurosci. Bull. 39 213–244. 10.1007/s12264-022-00897-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M. M., Garbarino V. R., Pollet E., Palavicini J. P., Kellogg D. L., Kraig E., et al. (2022). Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Invest. 132:e158453. 10.1172/JCI158453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. (2003). Alternative activation of macrophages. Nat. Rev. Immunol. 3 23–35. 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- Gorgoulis V., Adams P. D., Alimonti A., Bennett D. C., Bischof O., Bishop C., et al. (2019). Cellular senescence: defining a path forward. Cell 179 813–827. 10.1016/j.cell.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Göritz C., Dias D. O., Tomilin N., Barbacid M., Shupliakov O., Frisén J. (2011). A pericyte origin of spinal cord scar tissue. Science 333 238–242. 10.1126/science.1203165 [DOI] [PubMed] [Google Scholar]
- Grabert K., Michoel T., Karavolos M. H., Clohisey S., Baillie J. K., Stevens M. P., et al. (2016). Microglial brain region- dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19 504–516. 10.1038/nn.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. N., Crapser J. D., Hohsfield L. A. (2020). To kill a microglia: a case for CSF1R inhibitors. Trends Immunol. 41 771–784. 10.1016/j.it.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh A. D., Zarruk J. G., Healy L. M., Baskar Jesudasan S. J., Jhelum P., Salmon C. K., et al. (2018). Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 16:e2005264. 10.1371/journal.pbio.2005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman A., Chew G., Ouyang J. F., Sun G., Choo X. Y., McLean C., et al. (2019). A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22 2087–2097. 10.1038/s41593-019-0539-4 [DOI] [PubMed] [Google Scholar]
- Gu G., Zhu B., Ren J., Song X., Fan B., Ding H., et al. (2023). Ang-(1–7)/MasR axis promotes functional recovery after spinal cord injury by regulating microglia/macrophage polarization. Cell Biosci. 13:23. 10.1186/s13578-023-00967-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Cheng X., Huang X., Yuan Y., Qin S., Tan Z., et al. (2019). Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav. Immun. 80 394–405. 10.1016/j.bbi.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Guijarro-Belmar A., Varone A., Baltzer M. R., Kataria S., Tanriver-Ayder E., Watzlawick R., et al. (2022). Effectiveness of biomaterial-based combination strategies for spinal cord repair – a systematic review and meta-analysis of preclinical literature. Spinal Cord 60 1041–1049. 10.1038/s41393-022-00811-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy L. F., Yeo G. S., Tung Y. C., Zivraj K. H., Willis D., Coppola G., et al. (2011). Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. Rna 17 85–98. 10.1261/rna.2386111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneykaya D., Ivanov A., Hernandez D. P., Haage V., Wojtas B., Meyer N., et al. (2018). Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 24 2773–1783.e6. 10.1016/j.celrep.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Gurtner G. C., Werner S., Barrandon Y., Longaker M. T. (2008). Wound repair and regeneration. Nature 453 314–321. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- Haas C., Fischer I. (2013). Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J. Neurotrauma 30 1035–1052. 10.1089/neu.2013.2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A. R., Lee D. H., Dawood A., Rodriguez M., Funk L., Tsoulfas P., et al. (2016). STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiol. Dis. 89 10–22. 10.1016/j.nbd.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A. R., Lee J. K. (2016). Understanding the NG2 glial scar after spinal cord injury. Front. Neurol. 7:199. 10.3389/fneur.2016.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A. R., Yahn S. L., Lyapichev K., Dajnoki A., Lee D. H., Rodriguez M., et al. (2018). Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Exp. Neurol. 308 72–79. 10.1016/j.expneurol.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Kobayakawa K., Ohkawa Y., Kumamaru H., Yokota K., Saito T., et al. (2017). Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin–N-cadherin pathway after spinal cord injury. Nat. Med. 23 818–828. 10.1038/nm.4354 [DOI] [PubMed] [Google Scholar]
- Haruwaka K., Ikegami A., Tachibana Y., Ohno N., Konishi H., Hashimoto A., et al. (2019). Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 10:5816. 10.1038/s41467-019-13812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K. C., Hull T. C. L., Delaney G. A., Potter P. J., Sequeira K. A. J., Campbell K., et al. (2002). Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J. Neurotrauma 19 753–761. 10.1089/08977150260139129 [DOI] [PubMed] [Google Scholar]
- He L., Chang Q., Zhang Y., Guan X., Ma Z., Chen X., et al. (2023). MiR-155-5p aggravated astrocyte activation and glial scarring in a spinal cord injury model by inhibiting Ndfip1 expression and PTEN nuclear translocation. Neurochem. Res. 48 1912–1924. 10.1007/s11064-023-03862-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Jin Y. (2016). Intrinsic control of axon regeneration. Neuron 90 437–451. 10.1016/j.neuron.2016.04.022 [DOI] [PubMed] [Google Scholar]
- Hellal F., Hurtado A., Ruschel J., Flynn K. C., Laskowski C. J., Umlauf M., et al. (2011). Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331 928–931. 10.1126/science.1201148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellenbrand D. J., Quinn C. M., Piper Z. J., Morehouse C. N., Fixel J. A., Hanna A. S. (2021). Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflammation 18 284. 10.1186/s12974-021-02337-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanns S., Klapka N., Müller H. W. (2001). The collagenous lesion scar–an obstacle for axonal regeneration in brain and spinal cord injury. Restor. Neurol. Neurosci. 19 139–148. [PubMed] [Google Scholar]
- Hernandez-Segura A., Nehme J., Demaria M. (2018). Hallmarks of cellular senescence. Trends Cell Biol. 28 436–453. 10.1016/j.tcb.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Imura T., Song B., Qi J., Ao Y., Nguyen T. K., et al. (2008). STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 28 7231–7243. 10.1523/JNEUROSCI.1709-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesp Z. C., Goldstein E. Z., Miranda C. J., Kaspar B. K., McTigue D. M. (2015). Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J. Neurosci. 35 1274–1290. 10.1523/JNEUROSCI.2568-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesp Z. C., Yoseph R. Y., Suzuki R., Jukkola P., Wilson C., Nishiyama A., et al. (2018). Proliferating NG2-Cell-dependent angiogenesis and scar formation alter axon growth and functional recovery after spinal cord injury in mice. J. Neurosci. 38 1366–1382. 10.1523/JNEUROSCI.3953-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. A., Patel K. D., Medved J., Reiss A. M., Nishiyama A. (2013). NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 33 14558–14566. 10.1523/JNEUROSCI.2001-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines D. J., Hines R. M., Mulligan S. J., Macvicar B. A. (2009). Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia 57 1610–1618. 10.1002/glia.20874 [DOI] [PubMed] [Google Scholar]
- Hou J., Bi H., Ge Q., Teng H., Wan G., Yu B., et al. (2022). Heterogeneity analysis of astrocytes following spinal cord injury at single-cell resolution. FASEB J. 36:e22442. 10.1096/fj.202200463R [DOI] [PubMed] [Google Scholar]
- Hsieh C. L., Kim C. C., Ryba B. E., Niemi E. C., Bando J. K., Locksley R. M., et al. (2013). Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 43 2010–2022. 10.1002/eji.201243084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. Y., Woo J., Sardar D., Lozzi B., Bosquez Huerta N. A., Lin C. J., et al. (2020). Region-specific transcriptional control of astrocyte function oversees local circuit activities. Neuron 106 992–1008.e9. 10.1016/j.neuron.2020.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Cao Y., Zu T., Ju J. (2022). Interference with long noncoding RNA SNHG3 alleviates cerebral ischemia-reperfusion injury by inhibiting microglial activation. J. Leukoc Biol. 111 759–769. 10.1002/JLB.1A0421-190R [DOI] [PubMed] [Google Scholar]
- Huang W., Hickson L. J., Eirin A., Kirkland J. L., Lerman L. O. (2022). Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18 611–627. 10.1038/s41581-022-00601-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. N., Appel B. (2020). Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23 1055–1066. 10.1038/s41593-020-0654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado A., Cregg J. M., Wang H. B., Wendell D. F., Oudega M., Gilbert R. J., et al. (2011). Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers. Biomaterials 32 6068–6079. 10.1016/j.biomaterials.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagama S., Sakamoto K., Tauchi R., Shinjo R., Ohgomori T., Ito Z., et al. (2011). Keratan sulfate restricts neural plasticity after spinal cord injury. J. Neurosci. 31 17091–17102. 10.1523/JNEUROSCI.5120-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseda T., Nishio T., Kawaguchi S., Yamanoto M., Kawasaki T., Wakisaka S. (2004). Spontaneous regeneration of the corticospinal tract after transection in young rats: a key role of reactive astrocytes in making favorable and unfavorable conditions for regeneration. Neuroscience 126 365–374. 10.1016/j.neuroscience.2004.03.056 [DOI] [PubMed] [Google Scholar]
- Iwao T., Takata F., Matsumoto J., Goto Y., Aridome H., Yasunaga M., et al. (2023). Senescence in brain pericytes attenuates blood-brain barrier function in vitro: a comparison of serially passaged and isolated pericytes from aged rat brains. Biochem. Biophys. Res. Commun. 645 154–163. 10.1016/j.bbrc.2023.01.037 [DOI] [PubMed] [Google Scholar]
- Jin W.-N., Shi K., He W., Sun J.-H., Van Kaer L., Shi F.-D., et al. (2021). Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat. Neurosci. 24 61–73. 10.1038/s41593-020-00745-w [DOI] [PubMed] [Google Scholar]
- Jiwaji Z., Tiwari S. S., Avilés-Reyes R. X., Hooley M., Hampton D., Torvell M., et al. (2022). Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Aß pathology. Nat. Commun. 13:135. 10.1038/s41467-021-27702-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. L., Yamaguchi Y., Stallcup W. B., Tuszynski M. H. (2002). NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J. Neurosci. 22 2792–2803. 10.1523/JNEUROSCI.22-07-02792.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. B. (2014). Lymphocytes and autoimmunity after spinal cord injury. Exp. Neurol. 258 78–90. 10.1016/j.expneurol.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Jordão M. J. C., Sankowski R., Brendecke S. M., Sagar, Locatelli G., Tai Y.-H., et al. (2019). Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363:eaat7554. 10.1126/science.aat7554 [DOI] [PubMed] [Google Scholar]
- Ju C., Ma Y., Zuo X., Wang X., Song Z., Zhang Z., et al. (2023). Photobiomodulation promotes spinal cord injury repair by inhibiting macrophage polarization through lncRNA TUG1-miR-1192/TLR3 axis. Cell. Mol. Biol. Lett. 28:5. 10.1186/s11658-023-00417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser T., Feng G. (2019). Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 6:ENEURO.0448-18.2019. 10.1523/ENEURO.0448-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas B. A. (2004). Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 42 549–563. 10.1038/sj.sc.3101670 [DOI] [PubMed] [Google Scholar]
- Kanemaru K., Kubota J., Sekiya H., Hirose K., Okubo Y., Iino M. (2013). Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc. Natl. Acad. Sci. 110 11612–11617. 10.1073/pnas.1300378110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karus M., Ulc A., Ehrlich M., Czopka T., Hennen E., Fischer J., et al. (2016). Regulation of oligodendrocyte precursor maintenance by chondroitin sulphate glycosaminoglycans. Glia 64 270–286. 10.1002/glia.22928 [DOI] [PubMed] [Google Scholar]
- Kase Y., Shimazaki T., Okano H. (2020). Current understanding of adult neurogenesis in the mammalian brain: how does adult neurogenesis decrease with age? Inflamm. Regen. 40:10. 10.1186/s41232-020-00122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H., Kimura-Kuroda J., Komuta Y., Yoshioka N., Li H. P., Kawamura K., et al. (2012). Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 349 169–180. 10.1007/s00441-012-1336-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead H. S., Levine J. M., Blakemore W. F. (1998). Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia 22 161–170. [DOI] [PubMed] [Google Scholar]
- Kennedy B. K., Berger S. L., Brunet A., Campisi J., Cuervo A. M., Epel E. S., et al. (2014). Geroscience: linking aging to chronic disease. Cell 159 709–713. 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B. S., Deneen B. (2019). The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42 187–207. 10.1146/annurev-neuro-070918-050443 [DOI] [PubMed] [Google Scholar]
- Khakh B. S., Sofroniew M. V. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18 942–952. 10.1038/nn.4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K., Masuda T., Jordão M. J. C., Prinz M. (2019). Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20 547–562. 10.1038/s41583-019-0201-x [DOI] [PubMed] [Google Scholar]
- Kigerl K. A., Gensel J. C., Ankeny D. P., Alexander J. K., Donnelly D. J., Popovich P. G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Leng K., Park J., Sorets A. G., Kim S., Shostak A., et al. (2022). Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin. Nat. Commun. 13:6581. 10.1038/s41467-022-34412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland J. L., Tchkonia T. (2017). Cellular senescence: a translational perspective. EBioMedicine 21 21–28. 10.1016/j.ebiom.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland J. L., Tchkonia T. (2020). Senolytic drugs: from discovery to translation. J. Intern. Med. 288 518–536. 10.1111/joim.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland J. L., Tchkonia T., Zhu Y., Niedernhofer L. J., Robbins P. D. (2017). The clinical potential of senolytic drugs. J. Am. Geriatrics Soc. 65 2297–2301. 10.1111/jgs.14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapka N., Müller H. W. (2006). Collagen matrix in spinal cord injury. J. Neurotrauma 23 422–436. 10.1089/neu.2006.23.422 [DOI] [PubMed] [Google Scholar]
- Kobashi S., Terashima T., Katagi M., Nakae Y., Okano J., Suzuki Y., et al. (2020). Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. Mol. Ther. 28 254–265. 10.1016/j.ymthe.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa K., Ohkawa Y., Yoshizaki S., Tamaru T., Saito T., Kijima K., et al. (2019). Macrophage centripetal migration drives spontaneous healing process after spinal cord injury. Sci. Adv. 5:eaav5086. 10.1126/sciadv.aav5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H., Kobayashi M., Kunisawa T., Imai K., Sayo A., Malissen B., et al. (2017). Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia 65 1927–1943. 10.1002/glia.23204 [DOI] [PubMed] [Google Scholar]
- Kou D., Li T., Liu H., Liu C., Yin Y., Wu X., et al. (2018). Transplantation of rat-derived microglial cells promotes functional recovery in a rat model of spinal cord injury. Braz. J. Med. Biol. Res. 51:e7076. 10.1590/1414-431x20187076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristina A. K., John C. G., Daniel P. A., Jessica K. A., Dustin J. D., Phillip G. P. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29:13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A., Greenhalgh A. D., Zarruk J. G., Passos Dos, Santos R., Gaestel M., et al. (2014). TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83 1098–1116. 10.1016/j.neuron.2014.07.027 [DOI] [PubMed] [Google Scholar]
- Kuboyama T., Wahane S., Huang Y., Zhou X., Wong J. K., Koemeter-Cox A., et al. (2017). HDAC3 inhibition ameliorates spinal cord injury by immunomodulation. Sci. Rep. 7:8641. 10.1038/s41598-017-08535-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharova K., Stallcup W. B. (2015). NG2-proteoglycan-dependent contributions of oligodendrocyte progenitors and myeloid cells to myelin damage and repair. J. Neuroinflammation 12:161. 10.1186/s12974-015-0385-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B. T., Cregg J. M., DePaul M. A., Tran A. P., Xu K., Dyck S. M., et al. (2015). Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518 404–408. 10.1038/nature13974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Abraham D. J. (2004). TGF-beta signaling and the fibrotic response. Faseb J. 18 816–827. 10.1096/fj.03-1273rev [DOI] [PubMed] [Google Scholar]
- Li C., Wu Z., Zhou L., Shao J., Hu X., Xu W., et al. (2022). Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct. Target. Ther. 7:154. 10.1038/s41392-022-00885-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li M., Tian L., Chen J., Liu R., Ning B. (2020). Reactive astrogliosis: implications in spinal cord injury progression and therapy. Oxid. Med. Cell Longev. 2020:9494352. 10.1155/2020/9494352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen Y., Tan L., Pan J. Y., Lin W. W., Wu J., et al. (2017). RNAi-mediated ephrin-B2 silencing attenuates astroglial-fibrotic scar formation and improves spinal cord axon growth. CNS Neurosci. Ther. 23 779–789. 10.1111/cns.12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Field P. M., Raisman G. (1999). Death of oligodendrocytes and microglial phagocytosis of myelin precede immigration of Schwann cells into the spinal cord. J. Neurocytol. 28 417–427. 10.1023/A:1007026001189 [DOI] [PubMed] [Google Scholar]
- Li Y., He X., Kawaguchi R., Zhang Y., Wang Q., Monavarfeshani A., et al. (2020). Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 587 613–618. 10.1038/s41586-020-2795-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Cheng J., Kong X., Li S., Li X., Zhang M., et al. (2020). HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics 10 9644–9662. 10.7150/thno.47651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Barres B. A. (2017). Reactive astrocytes: production, function, and therapeutic potential. Immunity 46 957–967. 10.1016/j.immuni.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Lu Y., Lee J. K., Samara R., Willenberg R., Sears-Kraxberger I., et al. (2010). PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 13 1075–1081. 10.1038/nn.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Wang W., Wang S., Xie W., Li H., Ning B. (2018). microRNA-21 regulates astrocytic reaction post-acute phase of spinal cord injury through modulating TGF-β signaling. Aging 10 1474–1488. 10.18632/aging.101484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Schackel T., Weidner N., Puttagunta R. (2018). Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front. Cell. Neurosci. 11:430. 10.3389/fncel.2017.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang H., Wang H., Huang J., Yang Y., Li G., et al. (2022). A comparative study of different stem cell transplantation for spinal cord injury: a systematic review and network meta-analysis. World Neurosurg. 159 e232–e243. 10.1016/j.wneu.2021.12.035 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang X., Lu C. C., Kerman R., Steward O., Xu X. M., et al. (2008). Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J. Neurosci. 28 8376–8382. 10.1523/JNEUROSCI.1939-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. F., Miron V. E. (2019). The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 15 447–458. 10.1038/s41582-019-0184-2 [DOI] [PubMed] [Google Scholar]
- López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. (2013). The hallmarks of aging. Cell 153 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. (2023). Hallmarks of aging: an expanding universe. Cell 186 243–278. 10.1016/j.cell.2022.11.001 [DOI] [PubMed] [Google Scholar]
- Lorda-Diez C. I., Garcia-Riart B., Montero J. A., Rodriguez-León J., Garcia-Porrero J. A., Hurle J. M. (2015). Apoptosis during embryonic tissue remodeling is accompanied by cell senescence. Aging 7 974–985. 10.18632/aging.100844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Ashiqueali R., Lin C. I., Walchale A., Clendaniel V., Matheson R., et al. (2023). Histone deacetylase 3 inhibition decreases cerebral edema and protects the blood-brain barrier after stroke. Mol. Neurobiol. 60 235–246. 10.1007/s12035-022-03083-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., et al. (2012). Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150 1264–1273. 10.1016/j.cell.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. A. (2022). Exploring sex-related differences in microglia may be a game-changer in precision medicine. Front. Aging Neurosci. 14:868448. 10.3389/fnagi.2022.868448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Wei P., Wei T., Ransohoff R. M., Jakeman L. B. (2004). Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J. Comp. Neurol. 474 469–486. 10.1002/cne.20149 [DOI] [PubMed] [Google Scholar]
- Mahar M., Cavalli V. (2018). Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 19 323–337. 10.1038/s41583-018-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Amann L., Monaco G., Sankowski R., Staszewski O., Krueger M., et al. (2022). Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 604 740–748. 10.1038/s41586-022-04596-2 [DOI] [PubMed] [Google Scholar]
- Masuda T., Amann L., Sankowski R., Staszewski O., Lenz M. (2020). Novel Hexb-based tools for studying microglia in the CNS. Nat. Immunol. 21 802–815. 10.1038/s41590-020-0707-4 [DOI] [PubMed] [Google Scholar]
- Masuda T., Sankowski R., Staszewski O., Böttcher C., Amann L., Sagar, et al. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566 388–392. 10.1038/s41586-019-0924-x [DOI] [PubMed] [Google Scholar]
- Matheson R., Chida K., Lu H., Clendaniel V., Fisher M., Thomas A., et al. (2020). Neuroprotective effects of selective inhibition of histone deacetylase 3 in experimental stroke. Transl. Stroke Res. 11 1052–1063. 10.1007/s12975-020-00783-3 [DOI] [PubMed] [Google Scholar]
- Matias I., Morgado J., Gomes F. C. A. (2019). Astrocyte heterogeneity: impact to brain aging and disease. Front. Aging Neurosci. 11:59. 10.3389/fnagi.2019.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey G. L., Lizama C. O., Keown-Lang A. E., Niu A., Santander N., Larpthaveesarp A., et al. (2020). A new genetic strategy for targeting microglia in development and disease. Elife 9:e54590. 10.7554/eLife.54590.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara N. B., Munro D. A. D., Bestard-Cuche N., Uyeda A., Bogie J. F. J., Hoffmann A., et al. (2023). Microglia regulate central nervous system myelin growth and integrity. Nature 613 120–129. 10.1038/s41586-022-05534-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue D. M., Tripathi R., Wei P. (2006). NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J. Neuropathol. Exp. Neurol. 65 406–420. 10.1097/01.jnen.0000218447.32320.52 [DOI] [PubMed] [Google Scholar]
- McTigue D. M., Wei P., Stokes B. T. (2001). Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 21 3392–3400. 10.1523/JNEUROSCI.21-10-03392.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K., Barnabé-Heider F., Carlén M., Evergren E., Tomilin N., Shupliakov O., et al. (2008). Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 6:e182. 10.1371/journal.pbio.0060182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich L. M., Choi J. S., Ryan C., Cerqueira S. R., Benavides S., Yahn S. L., et al. (2021). Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J. Exp. Med. 218:e20210040. 10.1084/jem.20210040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich L. M., Ryan C. B., Lee J. K. (2019). The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 137 785–797. 10.1007/s00401-019-01992-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron V. E. (2017). Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J. Leukoc Biol. 101 1103–1108. 10.1189/jlb.3RI1116-494R [DOI] [PubMed] [Google Scholar]
- Miron V. E., Boyd A., Zhao J. W., Yuen T. J., Ruckh J. M., Shadrach J. L., et al. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16 1211–1218. 10.1038/nn.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladinic M., Wintzer M. (2002). Changes in mRNA content of developing opossum spinal cord at stages when regeneration can and cannot occur after injury. Brain Res. Brain Res. Rev. 40 317–324. 10.1016/S0165-0173(02)00214-X [DOI] [PubMed] [Google Scholar]
- Molofsky A. V., Deneen B. (2015). Astrocyte development: a guide for the perplexed. Glia 63 1320–1329. 10.1002/glia.22836 [DOI] [PubMed] [Google Scholar]
- Montilla A., Zabala A., Er-Lukowiak M., Rissiek B., Magnus T., Rodriguez-Iglesias N., et al. (2023). Microglia and meningeal macrophages depletion delays the onset of experimental autoimmune encephalomyelitis. Cell Death Dis. 14:16. 10.1038/s41419-023-05551-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. S., Milner R., Nishiyama A., Frausto R. F., Serwanski D. R., Pagarigan R. R., et al. (2011). Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J. Neurosci. 31 6247–6254. 10.1523/JNEUROSCI.5474-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. J., Sheedy F. J., Fisher E. A. (2013). Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13 709–721. 10.1038/nri3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti J. M., Riparip L. K., Rosi S. (2016). Call off the Dog(ma): M1/M2 polarization is concurrent following traumatic brain injury. PLoS One 11:e0148001. 10.1371/journal.pone.0148001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen D., Pavlovic A., Hartmann F. J., Schreiner B., Utz S. G., Leung B. P., et al. (2018). High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48 380–395.e6. 10.1016/j.immuni.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Myer D. J., Gurkoff G. G., Lee S. M., Hovda D. A., Sofroniew M. V. (2006). Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129(Pt 10), 2761–2772. 10.1093/brain/awl165 [DOI] [PubMed] [Google Scholar]
- Najafi A. R., Crapser J., Jiang S., Ng W., Mortazavi A., West B. L., et al. (2018). A limited capacity for microglial repopulation in the adult brain. Glia 66 2385–2396. 10.1002/glia.23477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neirinckx V., Coste C., Franzen R., Gothot A., Rogister B., Wislet S. (2014). Neutrophil contribution to spinal cord injury and repair. J. Neuroinflammation 11:150. 10.1186/s12974-014-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. X., O’Barr T. J., Anderson A. J. (2007). Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases, reactive oxygen species, and TNF-α. J. Neurochem. 102 900–912. 10.1111/j.1471-4159.2007.04643.x [DOI] [PubMed] [Google Scholar]
- Nirwane A., Yao Y. (2022). SMA(low/undetectable) pericytes differentiate into microglia- and macrophage-like cells in ischemic brain. Cell Mol. Life Sci. 79:264. 10.1007/s00018-022-04322-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Boshans L., Goncalves C. M., Wegrzyn J., Patel K. D. (2016). Lineage, fate, and fate potential of NG2-glia. Brain Res. 1638(Pt B), 116–128. 10.1016/j.brainres.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Chang A., Trapp B. D. (1999). NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp. Neurol. 58 1113–1124. 10.1097/00005072-199911000-00001 [DOI] [PubMed] [Google Scholar]
- Nobuta H., Ghiani C. A., Paez P. M., Spreuer V., Dong H., Korsak R. A., et al. (2012). STAT3-mediated astrogliosis protects myelin development in neonatal brain injury. Ann. Neurol. 72 750–765. 10.1002/ana.23670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Rodrigues J., Leite S. C., Pinto-Costa R., Sousa S. C., Luz L. L., Sintra M. A., et al. (2022). Rewired glycosylation activity promotes scarless regeneration and functional recovery in spiny mice after complete spinal cord transection. Dev. Cell 57 440–450.e7. 10.1016/j.devcel.2021.12.008 [DOI] [PubMed] [Google Scholar]
- Norden D. M., Muccigrosso M. M., Godbout J. P. (2015). Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96 29–41. 10.1016/j.neuropharm.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noristani H. N., Sabourin J. C., Boukhaddaoui H., Chan-Seng E., Gerber Y. N., Perrin F. E. (2016). Spinal cord injury induces astroglial conversion towards neuronal lineage. Mol. Neurodegen. 11:68. 10.1186/s13024-016-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik M., Evans S. A., Fielder E., Victorelli S., Kruger P., Salmonowicz H., et al. (2021). Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 20:e13296. 10.1111/acel.13296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Nakamura M., Katoh H., Miyao T., Shimazaki T., Ishii K., et al. (2006). Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12 829–834. 10.1038/nm1425 [DOI] [PubMed] [Google Scholar]
- Omura T., Omura K., Tedeschi A., Riva P., Painter M. W., Rojas L., et al. (2015). Robust axonal regeneration occurs in the injured CAST/Ei mouse CNS. Neuron 86 1215–1227. 10.1016/j.neuron.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. G., Orr A. L., Li X. J., Gross R. E., Traynelis S. F. (2009). Adenosine A(2A) receptor mediates microglial process retraction. Nat. Neurosci. 12 872–878. 10.1038/nn.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M. B., Gensel J. C. (2018). Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15 541–553. 10.1007/s13311-018-0631-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea T. M., Burda J. E., Sofroniew M. V. (2017). Cell biology of spinal cord injury and repair. J. Clin. Invest. 127 3259–3270. 10.1172/JCI90608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyinbo C. A. (2011). Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol. Exp. 71 281–299. [DOI] [PubMed] [Google Scholar]
- Paolicelli R. C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333 1456–1458. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Paolicelli R. C., Sierra A., Stevens B., Tremblay M. E., Aguzzi A., Ajami B., et al. (2022). Microglia states and nomenclature: a field at its crossroads. Neuron 110 3458–3483. 10.1016/j.neuron.2022.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramos-de-Carvalho D., Martins I., Cristóvão A. M., Dias A. F., Neves-Silva D., Pereira T., et al. (2021). Targeting senescent cells improves functional recovery after spinal cord injury. Cell Rep. 36:109334. 10.1016/j.celrep.2021.109334 [DOI] [PubMed] [Google Scholar]
- Park K. K., Liu K., Hu Y., Smith P. D., Wang C., Cai B., et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322 963–966. 10.1126/science.1161566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp R. J., Anderson P. N., Verhaagen J. (2001). Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci. 13 457–471. 10.1046/j.0953-816X.2000.01398.x [DOI] [PubMed] [Google Scholar]
- Pasterkamp R. J., Giger R. J., Ruitenberg M. J., Holtmaat A. J. G. D., De Wit J., De Winter F., et al. (1999). Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol. Cell. Neurosci. 13 143–166. 10.1006/mcne.1999.0738 [DOI] [PubMed] [Google Scholar]
- Pelisch N., Rosas Almanza J., Stehlik K. E., Aperi B. V., Kroner A. (2020). CCL3 contributes to secondary damage after spinal cord injury. J. Neuroinflammation 17:362. 10.1186/s12974-020-02037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton J. C., Shamblott M. J., Gary D. S., Belegu V., Hurtado A., Malone M. L., et al. (2013). Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp. Neurol. 247 113–121. 10.1016/j.expneurol.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Perry V. H., Teeling J. (2013). Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 35 601–612. 10.1007/s00281-013-0382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosyan H. A., Hunanyan A. S., Alessi V., Schnell L., Levine J., Arvanian V. L. (2013). Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J. Neurosci. 33 4032–4043. 10.1523/JNEUROSCI.4702-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatnani H., Maniatis T. (2015). Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 7:a020628. 10.1101/cshperspect.a020628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I., Lacroix S. (2007). Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 500 267–285. 10.1002/cne.21149 [DOI] [PubMed] [Google Scholar]
- Poplawski G. H. D., Kawaguchi R., Van Niekerk E., Lu P., Mehta N., Canete P., et al. (2020). Injured adult neurons regress to an embryonic transcriptional growth state. Nature 581 77–82. 10.1038/s41586-020-2200-5 [DOI] [PubMed] [Google Scholar]
- Popovich P. G., Wei P., Stokes B. T. (1997). Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 377 443–464. [DOI] [PubMed] [Google Scholar]
- Prinz M., Masuda T., Wheeler M. A., Quintana F. J. (2021). Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu. Rev. Immunol. 39 251–277. 10.1146/annurev-immunol-093019-110159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss H., Kopp M. A., Brommer B., Gatzemeier N., Laginha I., Dirnagl U., et al. (2011). Non-resolving aspects of acute inflammation after spinal cord injury (SCI): indices and resolution plateau. Brain Pathol. 21 652–660. 10.1111/j.1750-3639.2011.00488.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukos N., Goodus M. T., Sahinkaya F. R., McTigue D. M. (2019). Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia 67 2178–2202. 10.1002/glia.23702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls B., Ding Y., Zhang F., Pan M., Lei Z., Pei Z., et al. (2020). Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front. Cell Dev. Biol. 8:591883. 10.3389/fcell.2020.591883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Cai D., Dai H., McAtee M., Hoffman P. N., Bregman B. S., et al. (2002). Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34 895–903. 10.1016/S0896-6273(02)00730-4 [DOI] [PubMed] [Google Scholar]
- Ranjan M., Hudson L. D. (1996). Regulation of tyrosine phosphorylation and protein tyrosine phosphatases during oligodendrocyte differentiation. Mol. Cell. Neurosci. 7 404–418. 10.1006/mcne.1996.0029 [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. (2016). A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19 987–991. 10.1038/nn.4338 [DOI] [PubMed] [Google Scholar]
- Regan M. R., Huang Y. H., Kim Y. S., Dykes-Hoberg M. I., Jin L., Watkins A. M., et al. (2007). Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J. Neurosci. 27 6607–6619. 10.1523/JNEUROSCI.0790-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach N., Delekate A., Plescher M., Schmitt F., Krauss S., Blank N., et al. (2019). Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol. Med. 11:e9665. 10.15252/emmm.201809665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revuelta M., Elicegui A., Moreno-Cugnon L., Bührer C., Matheu A., Schmitz T. (2019). Ischemic stroke in neonatal and adult astrocytes. Mech. Ageing Dev. 183:111147. 10.1016/j.mad.2019.111147 [DOI] [PubMed] [Google Scholar]
- Rhodes K. E., Raivich G., Fawcett J. W. (2006). The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience 140 87–100. [DOI] [PubMed] [Google Scholar]
- Rice T., Larsen J., Rivest S., Yong V. W. (2007). Characterization of the early neuroinflammation after spinal cord injury in mice. J. Neuropathol. Exp. Neurol. 66 184–195. 10.1097/01.jnen.0000248552.07338.7f [DOI] [PubMed] [Google Scholar]
- Richardson P. M., McGuinness U. M., Aguayo A. J. (1980). Axons from CNS neurones regenerate into PNS grafts. Nature 284 264–265. 10.1038/284264a0 [DOI] [PubMed] [Google Scholar]
- Riley S. E., Feng Y., Hansen C. G. (2022). Hippo-Yap/Taz signalling in zebrafish regeneration. npj Regen. Med. 7:9. 10.1038/s41536-022-00209-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink S., Arnold D., Wöhler A., Bendella H., Meyer C., Manthou M., et al. (2018). Recovery after spinal cord injury by modulation of the proteoglycan receptor PTPσ. Exp. Neurol. 309 148–159. 10.1016/j.expneurol.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Risher W. C., Eroglu C. (2012). Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 31 170–177. 10.1016/j.matbio.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher W. C., Kim N., Koh S., Choi J.-E., Mitev P., Spence E. F., et al. (2018). Thrombospondin receptor α2δ-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. J. Cell Biolgy. 217 3747–3765. 10.1083/jcb.201802057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel R. M., Doran S. J., Glaser E. P., Meadows V. E., Faden A. I., Stoica B. A., et al. (2019). Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol. Aging 77 194–206. 10.1016/j.neurobiolaging.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. P., Coulter M., Miotke J., Meyer R. L., Takemaru K.-I., Levine J. M. (2014). Abrogation of β-Catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J. Neurosci. 34 10285–10297. 10.1523/JNEUROSCI.4915-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson P. T., Davis T. P. (2020). Regulation of blood–brain barrier integrity by microglia in health and disease: a therapeutic opportunity. J. Cereb. Blood Flow Metabol. 40 S6–S24. 10.1177/0271678X20951995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S., Schwab M., Schwartz M., Fehlings M. G. (2007). Spinal cord injury: time to move? J. Neurosci. 27 11782–11792. 10.1523/JNEUROSCI.3444-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C., Elyaman W. (2022). A new understanding of TMEM119 as a marker of microglia. Front. Cell Neurosci. 16:902372. 10.3389/fncel.2022.902372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C., Sun L., Kroshilina A., Beckers L., De Jager P., Bradshaw E. M., et al. (2020). A novel Tmem119-tdTomato reporter mouse model for studying microglia in the central nervous system. Brain Behav. Immun. 83 180–191. 10.1016/j.bbi.2019.10.009 [DOI] [PubMed] [Google Scholar]
- Rudge J. S., Silver J. (1990). Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 10 3594–3603. 10.1523/JNEUROSCI.10-11-03594.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ K., Teku G., Bousset L., Redeker V., Piel S., Savchenko E., et al. (2021). TNF-α and α-synuclein fibrils differently regulate human astrocyte immune reactivity and impair mitochondrial respiration. Cell Rep. 34:108895. 10.1016/j.celrep.2021.108895 [DOI] [PubMed] [Google Scholar]
- Sabin K. Z., Jiang P., Gearhart M. D., Stewart R., Echeverri K. (2019). AP-1cFos/JunB/miR-200a regulate the pro-regenerative glial cell response during axolotl spinal cord regeneration. Commun. Biol. 2:91. 10.1038/s42003-019-0335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel A., Ortiz F. C., Kerninon C., Maldonado P. P., Angulo M. C., Nait-Oumesmar B. (2015). Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination. Front. Cell Neurosci. 9:77. 10.3389/fncel.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Ozaki T., Ko Y. C., Tsai C. F., Gong Y., Morozumi M., et al. (2019). Glycan sulfation patterns define autophagy flux at axon tip via PTPRσ-cortactin axis. Nat. Chem. Biol. 15 699–709. 10.1038/s41589-019-0274-x [DOI] [PubMed] [Google Scholar]
- Santello M., Toni N., Volterra A. (2019). Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 22 154–166. 10.1038/s41593-018-0325-8 [DOI] [PubMed] [Google Scholar]
- Santos E. N., Fields R. D. (2021). Regulation of myelination by microglia. Sci. Adv. 7:eabk1131. 10.1126/sciadv.abk1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarah A. B., Jason A. H., Kevin P. H., Fernando X. C., Rochelle C., Nicholas L., et al. (2011). Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J. Neurosci. 31:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. P., Lehrman E. K., Kautzman A. G., Koyama R., Mardinly A. R., Yamasaki R., et al. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L., Fearn S., Klassen H., Schwab M. E., Perry V. H. (1999). Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur. J. Neurosci. 11 3648–3658. 10.1046/j.1460-9568.1999.00792.x [DOI] [PubMed] [Google Scholar]
- Schosserer M., Grillari J., Breitenbach M. (2017). The dual role of cellular senescence in developing tumors and their response to cancer therapy. Front. Oncol. 7:278. 10.3389/fonc.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J., Schachner M., Schumacher U., Lorke D. E. (2013). Extracellular matrix alterations, accelerated leukocyte infiltration and enhanced axonal sprouting after spinal cord hemisection in tenascin-C-deficient mice. Acta Histochemica 115 865–878. 10.1016/j.acthis.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Schucht P., Raineteau O., Schwab M. E., Fouad K. (2002). Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176 143–153. 10.1006/exnr.2002.7909 [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Bartholdi D. (1996). Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 76 319–370. 10.1152/physrev.1996.76.2.319 [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Strittmatter S. M. (2014). Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 27 53–60. 10.1016/j.conb.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scivoletto G., Morganti B., Ditunno P., Ditunno J. F., Molinari M. (2003). Effects on age on spinal cord lesion patients’ rehabilitation. Spinal Cord. 41 457–464. 10.1038/sj.sc.3101489 [DOI] [PubMed] [Google Scholar]
- See J., Bonner J., Neuhuber B., Fischer I. (2010). Neurite outgrowth of neural progenitors in presence of inhibitory proteoglycans. J. Neurotrauma 27 951–957. 10.1089/neu.2009.1158 [DOI] [PubMed] [Google Scholar]
- Sekhon L. H. S., Fehlings M. G. (2001). Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 26(Suppl.), S2–S12. 10.1097/00007632-200112151-00002 [DOI] [PubMed] [Google Scholar]
- Seo J. H., Miyamoto N., Hayakawa K., Pham L. D., Maki T., Ayata C., et al. (2013). Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J. Clin. Invest. 123 782–786. 10.1172/JCI65863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafqat S., Arana Chicas E., Shafqat A., Hashmi S. K. (2022). The Achilles’ heel of cancer survivors: fundamentals of accelerated cellular senescence. J. Clin. Invest. 132:e158452. 10.1172/JCI158452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R., London A., Varol C., Raposo C., Cusimano M., Yovel G., et al. (2009). Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6:e1000113. 10.1371/journal.pmed.1000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Tenney A. P., Busch S. A., Horn K. P., Cuascut F. X., Liu K., et al. (2009). PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326 592–596. 10.1126/science.1178310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman M. I., Jin L.-Q., Selzer M. E. (2006). “Regeneration in the lamprey spinal cord,” in Model Organisms in Spinal Cord Regeneration, eds Becker C. G., Becker T. (Hoboken, NJ: John Wiley & Sons; ). [Google Scholar]
- Shigeoka T., Jung H., Jung J., Turner-Bridger B., Ohk J., Lin J. Q., et al. (2016). Dynamic axonal translation in developing and mature visual circuits. Cell 166 181–192. 10.1016/j.cell.2016.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert J. R., Osterhout D. J. (2011). The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J. Neurochem. 119 176–188. 10.1111/j.1471-4159.2011.07370.x [DOI] [PubMed] [Google Scholar]
- Silver J. (2016). The glial scar is more than just astrocytes. Exp. Neurol. 286 147–149. 10.1016/j.expneurol.2016.06.018 [DOI] [PubMed] [Google Scholar]
- Silver J., Miller J. H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5 146–156. 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- Smith G. M., Miller R. H., Silver J. (1987). Astrocyte transplantation induces callosal regeneration in postnatal acallosal mice. Ann. N. Y. Acad. Sci. 495 185–206. 10.1111/j.1749-6632.1987.tb23675.x [DOI] [PubMed] [Google Scholar]
- Soderblom C., Luo X., Blumenthal E., Bray E., Lyapichev K., Ramos J., et al. (2013). Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 33 13882–13887. 10.1523/JNEUROSCI.2524-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M. V. (2015). Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 16 249–263. 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M. V. (2018). Dissecting spinal cord regeneration. Nature 557 343–350. 10.1038/s41586-018-0068-4 [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. (2020). Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol. 41 758–770. 10.1016/j.it.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M. V., Vinters H. V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119 7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y. J. (2015). Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration? Neural Regen. Res. 10 346–348. 10.4103/1673-5374.153672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey M. L., Bartus K., Barritt A. W., Bradbury E. J. (2012). Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur. J. Neurosci. 36 3665–3678. 10.1111/ejn.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. P., Liu S., Kubes P., Yong V. W. (2009). Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J. Neurosci. 29 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer M., Mas A., Robert-Moreno A., Pecoraro M., Ortells M. C., Di Giacomo V., et al. (2013). Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155 1119–1130. 10.1016/j.cell.2013.10.041 [DOI] [PubMed] [Google Scholar]
- Su Z., Niu W., Liu M.-L., Zou Y., Zhang C.-L. (2014). In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 5:3338. 10.1038/ncomms4338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar O., Gerard R. W. (1940). Spinal cord regeneration in the rat. J. Neurophysiol. 3 1–19. 10.1152/jn.1940.3.1.1 [DOI] [Google Scholar]
- Sun F., Park K. K., Belin S., Wang D., Lu T., Chen G., et al. (2011). Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480 372–375. 10.1038/nature10594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Sekimoto K., Hayashi C., Mabuchi Y., Nakamura T., Akazawa C. (2017). Differentiation of oligodendrocyte precursor cells from Sox10-venus mice to oligodendrocytes and astrocytes. Sci. Rep. 7:14133. 10.1038/s41598-017-14207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A. M., Colletti M., Rorai A. T., Skene J. H. P., Levine J. M. (2006). Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J. Neurosci. 26 4729–4739. 10.1523/JNEUROSCI.3900-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator C. H. (1998). Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery 42 696–707. 10.1097/00006123-199804000-00007 [DOI] [PubMed] [Google Scholar]
- Tator C. H., Fehlings M. G. (1991). Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75 15–26. 10.3171/jns.1991.75.1.0015 [DOI] [PubMed] [Google Scholar]
- Tazaki A., Tanaka E. M., Fei J.-F. (2017). Salamander spinal cord regeneration: the ultimate positive control in vertebrate spinal cord regeneration. Dev. Biol. 432 63–71. 10.1016/j.ydbio.2017.09.034 [DOI] [PubMed] [Google Scholar]
- Tedeschi A., Dupraz S., Laskowski C. J., Xue J., Ulas T., Beyer M., et al. (2016). The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92 419–434. 10.1016/j.neuron.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Tom V. J., Steinmetz M. P., Miller J. H., Doller C. M., Silver J. (2004). Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24 6531–6539. 10.1523/JNEUROSCI.0994-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A. P., Sundar S., Yu M., Lang B. T., Silver J. (2018a). Modulation of receptor protein tyrosine phosphatase sigma increases chondroitin sulfate proteoglycan degradation through cathepsin B secretion to enhance axon outgrowth. J. Neurosci. 38 5399–5414. 10.1523/JNEUROSCI.3214-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A. P., Warren P. M., Silver J. (2018b). The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 98 881–917. 10.1152/physrev.00017.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A. P., Warren P. M., Silver J. (2020). Regulation of autophagy by inhibitory CSPG interactions with receptor PTPσ and its impact on plasticity and regeneration after spinal cord injury. Exp. Neurol. 328:113276. 10.1016/j.expneurol.2020.113276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A. P., Warren P. M., Silver J. (2022). New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 387 319–336. 10.1007/s00441-021-03477-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R., McTigue D. M. (2007). Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia 55 698–711. 10.1002/glia.20491 [DOI] [PubMed] [Google Scholar]
- Tsai H. H., Li H., Fuentealba L. C., Molofsky A. V., Taveira-Marques R., Zhuang H., et al. (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337 358–362. 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsata V., Wehner D. (2021). Know how to regrow—axon regeneration in the zebrafish spinal cord. Cells 10:1404. 10.3390/cells10061404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove H., Martens L., Scheyltjens I., De Vlaminck K., Pombo Antunes A. R., De Prijck S., et al. (2019). A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22 1021–1035. 10.1038/s41593-019-0393-4 [DOI] [PubMed] [Google Scholar]
- Vanlandewijck M., He L., Mäe M. A., Andrae J., Ando K., Del Gaudio F., et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature 554 475–480. 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- Wahane S., Sofroniew M. V. (2022). Loss-of-function manipulations to identify roles of diverse glia and stromal cells during CNS scar formation. Cell Tissue Res. 387 337–350. 10.1007/s00441-021-03487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahane S., Zhou X., Zhou X., Guo L., Friedl M.-S., Kluge M., et al. (2021). Diversified transcriptional responses of myeloid and glial cells in spinal cord injury shaped by HDAC3 activity. Sci. Adv. 7:eabd8811. 10.1126/sciadv.abd8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Xu L., Lin W., Yao Y., Li H., Shen G., et al. (2022). Single-cell transcriptome analysis reveals the immune heterogeneity and the repopulation of microglia by Hif1α in mice after spinal cord injury. Cell Death Dis. 13:432. 10.1038/s41419-022-04864-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liu R., Su Y., Li H., Xie W., Ning B. (2018). MicroRNA-21-5p mediates TGF-β-regulated fibrogenic activation of spinal fibroblasts and the formation of fibrotic scars after spinal cord injury. Int. J. Biol. Sci. 14 178–188. 10.7150/ijbs.24074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cao K., Sun X., Chen Y., Duan Z., Sun L., et al. (2015). Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia 63 635–651. 10.1002/glia.22774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner I. B., Anderson M. A., Song B., Levine J., Fernandez A., Gray-Thompson Z., et al. (2013). Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 33 12870–12886. 10.1523/JNEUROSCI.2121-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Toyama Y., Nishiyama A. (2002). Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J. Neurosci. Res. 69 826–836. 10.1002/jnr.10338 [DOI] [PubMed] [Google Scholar]
- Wehner D., Becker C. G. (2022). An exception to the rule? regeneration of the injured spinal cord in the spiny mouse. Dev. Cell 57 415–416. 10.1016/j.devcel.2022.02.002 [DOI] [PubMed] [Google Scholar]
- Wheeler M. A., Clark I. C., Tjon E. C., Li Z., Zandee S. E. J., Couturier C. P., et al. (2020). MAFG-driven astrocytes promote CNS inflammation. Nature 578 593–599. 10.1038/s41586-020-1999-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. A., Quintana F. J. (2019). Regulation of astrocyte functions in multiple sclerosis. Cold Spring Harb. Perspect. Med. 9:a029009. 10.1101/cshperspect.a029009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., McTigue D. M., Jakeman L. B. (2010). Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice. J. Comp. Neurol. 518 1370–1390. 10.1002/cne.22282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E. A., Kim C. N., Ross J. M., Garcia J. H., Gil E., Oh I., et al. (2022). A single-cell atlas of the normal and malformed human brain vasculature. Science 375:eabi7377. 10.1126/science.abi7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M. C., Mi R., Connor E., Reed N., Vyas A., Alspalter M., et al. (2014). Novel roles for osteopontin and clusterin in peripheral motor and sensory axon regeneration. J. Neurosci. 34 1689–1700. 10.1523/JNEUROSCI.3822-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Miyamoto O., Shibuya S., Okada M., Igawa H., Janjua N. A., et al. (2005). Different expression of macrophages and microglia in rat spinal cord contusion injury model at morphological and regional levels. Acta Med. Okayama 59 121–127. [DOI] [PubMed] [Google Scholar]
- Wu J., Lu B., Yang R., Chen Y., Chen X., Li Y. (2021). EphB2 knockdown decreases the formation of astroglial-fibrotic scars to promote nerve regeneration after spinal cord injury in rats. CNS Neurosci. Therapeutics 27 714–724. 10.1111/cns.13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. A. (2008). Cellular and molecular mechanisms of fibrosis. J. Pathol. 214 199–210. 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L., Qi J., Tang M., Liu J., Zhang D., Zhu Y., et al. (2022). Continual deletion of spinal microglia reforms astrocyte scar favoring axonal regeneration. Front. Pharmacol. 13:881195. 10.3389/fphar.2022.881195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Zhao Q., Zhang H., Chen Y., Yuan Z., Xu Y., et al. (2017). Proteomic analysis of HDAC3 selective inhibitor in the regulation of inflammatory response of primary microglia. Neural Plast. 2017:6237351. 10.1155/2017/6237351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Shen X., Xu X., Liu H., Li F., Lu S., et al. (2020). Astrocytic YAP promotes the formation of glia scars and neural regeneration after spinal cord injury. J. Neurosci. 40 2644–2662. 10.1523/JNEUROSCI.2229-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Schmidt S. V., Sander J., Draffehn A., Krebs W., Quester I., et al. (2014). Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40 274–288. 10.1016/j.immuni.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Dai Y., Chen G., Cui S. (2020a). Dissecting the dual role of the Glial scar and scar-forming astrocytes in spinal cord injury. Front. Cell. Neurosci. 14:78. 10.3389/fncel.2020.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Xing L., Yu W., Cai Y., Cui S., Chen G. (2020b). Astrocytic reprogramming combined with rehabilitation strategy improves recovery from spinal cord injury. FASEB J. 34 15504–15515. 10.1096/fj.202001657RR [DOI] [PubMed] [Google Scholar]
- Yokota K., Kobayakawa K., Saito T., Hara M., Kijima K., Ohkawa Y., et al. (2017). Periostin promotes scar formation through the interaction between pericytes and infiltrating monocytes/macrophages after spinal cord injury. Am. J. Pathol. 187 639–653. 10.1016/j.ajpath.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Young K. F., Gardner R., Sariana V., Whitman S. A., Bartlett M. J., Falk T., et al. (2021). Can quantifying morphology and TMEM119 expression distinguish between microglia and infiltrating macrophages after ischemic stroke and reperfusion in male and female mice? J. Neuroinflammation 18:58. 10.1186/s12974-021-02105-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. R., Fehlings M. G. (2011). Fas/FasL-mediated apoptosis and inflammation are key features of acute human spinal cord injury: implications for translational, clinical application. Acta Neuropathol. 122 747–761. 10.1007/s00401-011-0882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Nagai J., Marti-Solano M., Soto J. S., Coppola G., Babu M. M., et al. (2020). Context-specific striatal astrocyte molecular responses are phenotypically exploitable. Neuron 108 1146–1162.e10. 10.1016/j.neuron.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun M. H., Davaapil H., Brockes J. P. (2015). Recurrent turnover of senescent cells during regeneration of a complex structure. Elife 4:e05505. 10.7554/eLife.05505.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai L. J., Wrathall J. R. (2005). Cell proliferation and replacement following contusive spinal cord injury. Glia 50 247–257. 10.1002/glia.20176 [DOI] [PubMed] [Google Scholar]
- Zamanian J. L., Xu L., Foo L. C., Nouri N., Zhou L., Giffard R. G., et al. (2012). Genomic analysis of reactive astrogliosis. J. Neurosci. 32 6391–6410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A., Muñoz-Manchado A. B., Codeluppi S., Lönnerberg P., La Manno G., Juréus A., et al. (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347 1138–1142. 10.1126/science.aaa1934 [DOI] [PubMed] [Google Scholar]
- Zhang L., Pitcher L. E., Yousefzadeh M. J., Niedernhofer L. J., Robbins P. D., Zhu Y. (2022). Cellular senescence: a key therapeutic target in aging and diseases. J. Clin. Invest. 132:e158450. 10.1172/JCI158450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Pearsall V. M., Carver C. M., Atkinson E. J., Clarkson B. D. S., Grund E. M., et al. (2022). Rejuvenation of the aged brain immune cell landscape in mice through p16-positive senescent cell clearance. Nat. Commun. 13:5671. 10.1038/s41467-022-33226-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Gasterich N., Clarner T., Voelz C., Behrens V., Beyer C., et al. (2022). Astrocytic Nrf2 expression protects spinal cord from oxidative stress following spinal cord injury in a male mouse model. J. Neuroinflammation 19:134. 10.1186/s12974-022-02491-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. F., Alam M. M., Liao Y., Huang T., Mathur R., Zhu X., et al. (2019). Targeting microglia using Cx3cr1-Cre lines: revisiting the specificity. eNeuro 6:ENEURO.0114-19.2019. 10.1523/ENEURO.0114-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Mu H., Huang Y., Li S., Wang Y., Stetler R. A., et al. (2022). Microglia-specific deletion of histone deacetylase 3 promotes inflammation resolution, white matter integrity, and functional recovery in a mouse model of traumatic brain injury. J. Neuroinflammation 19:201. 10.1186/s12974-022-02563-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Tuszynski M. H. (2023). Regulation of axonal regeneration after mammalian spinal cord injury. Nat. Rev. Mol. Cell Biol. 48 339–351. 10.1038/s41580-022-00562-y [DOI] [PubMed] [Google Scholar]
- Zheng J., Ru W., Adolacion J. R., Spurgat M. S., Liu X., Yuan S., et al. (2021). Single-cell RNA-seq analysis reveals compartment-specific heterogeneity and plasticity of microglia. Iscience 24:102186. 10.1016/j.isci.2021.102186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Zheng Y., Sun L., Badea S. R., Jin Y., Liu Y., et al. (2019). Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 22 421–435. 10.1038/s41593-018-0324-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., He X., Ren Y. (2014). Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen. Res. 9 1787–1795. 10.4103/1673-5374.143423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wahane S., Friedl M. S., Kluge M., Friedel C. C., Avrampou K., et al. (2020). Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat. Neurosci. 23 337–350. 10.1038/s41593-020-0597-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Song W. M., Andhey P. S., Swain A., Levy T., Miller K. R., et al. (2020). Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26 131–142. 10.1038/s41591-019-0695-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Lyapichev K., Lee D. H., Motti D., Ferraro N. M., Zhang Y., et al. (2017). Macrophage transcriptional profile identifies lipid catabolic pathways that can be therapeutically targeted after spinal cord injury. J. Neurosci. 37 2362–2376. 10.1523/JNEUROSCI.2751-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]