Abstract
This paper reviews the currently used pretreatment methods for microplastics (MPs) analysis in soil and freshwater sediments, primarily sample processing, pretreatment, and characterization methods for MPs analysis. In addition, analytical tools (e.g., lab instruments), MPs characteristics, and MPs quantity, are included in this review. Prior to pretreatment, soil and sediment samples are typically processed using sieving and drying methods, and a sample quantity of <50 g was mostly used for the pretreatment. Density separation was commonly performed before organic matter removal. Sodium chloride (NaCl) and zinc chloride (ZnCl2) were most often used for density separation, and hydrogen peroxide (H2O2) oxidation was most frequently used to remove organic matter. Although advantages of each pretreatment method have been investigated, it is still challenging to determine a universal pretreatment method due to sample variability (e.g., sample characteristics). Furthermore, it is highly required to establish standard pretreatment methods that can be used for various environmental matrices, including air, water, and wastes as well as soil and sediment.
Keywords: Environment, Microplastics, Pretreatment methods, Soil, Freshwater sediment
Graphical Abstract
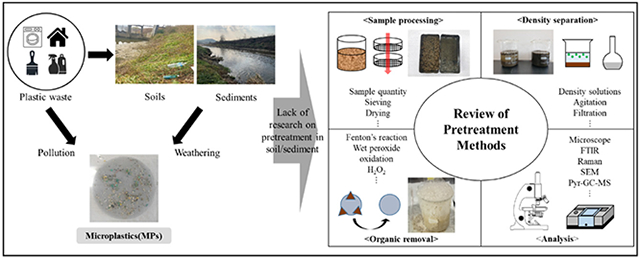
1. Introduction
Plastic is one of the most widely used and frequently detected materials in the environment worldwide, owing to its versatile applications and complex structure. Eriksen et al. (2014) reported that the world's oceans are polluted with >250,000 tons of plastics, comprising about 5 trillion plastic particles (Eriksen et al., 2014). According to a report by Plastics–the facts 2021, in 2020, over 360 million tons of plastics, including 55 million tons in Europe alone, were produced worldwide and about 23% of the plastics collected in Europe were landfilled, indicating that a significant amount of the used plastics have entered the environment through various routes (Plastics Europe and EPRO, 2021). Additionally, according to a Statista report (2021), the United States is the world's largest producer of plastic waste, where out of the 35.7 million tons plastic waste produced, only 3.1 million tons of plastic waste were recycled, with plastic waste being discharged into marine litter, which can cause marine pollution (Statista, 2021). In general, when plastics enter the environment, they are broken down into small pieces due to environmental weathering (i.e., environmental aging), such as photo-oxidation by UV and chemical oxidation by reactive oxygen species generated in the environment (Han et al., 2019a; Han et al., 2018a; Han et al., 2018b). Plastic particles smaller than 5 mm, which are referred to as Microplastics (MPs), pose significant environmental concerns (Verschoor, 2015). Plastics are persistent in the environment due to their complex chemical structures and large molecular weights, making them less prone to environmental degradation (Liu et al., 2022). Moreover, plastics contain additives and toxic chemicals (e.g., brominated flame retardants, phthalate, nanomaterials, and harmful impurities) (Lithner et al., 2011). As plastics environmentally age and decompose into smaller pieces, their surface area dramatically increases, and the additives are more rapidly released (Rillig et al., 2021). Therefore, plastic and MPs have significant adverse effects on human health (Barboza et al., 2018; Karbalaei et al., 2018; Prata et al., 2020), animals (Franzellitti et al., 2019; Guzzetti et al., 2018; Prata et al., 2021; Rillig et al., 2019), and the ecosystem (Ivleva et al., 2017; Rillig and Lehmann, 2020). In particular, human exposure to plastic debris and the released additives, can cause lung and intestine damage (Wright and Kelly, 2017). It is easier for smaller sized additives and plastic debris to penetrate organs such as cell membranes (Hale et al., 2020), the blood-brain barrier, and the human placenta (Ragusa et al., 2021), resulting in damage. Therefore, it is of great importance to determine and monitor MPs in the environment. Currently, extensive research effort is devoted to monitoring MPs in the environment as they have been ubiquitously detected in various environmental media including water (Luo et al., 2019), air (Gasperi et al., 2018), soil (Harms et al., 2021), sediment (Uddin et al., 2021) and even organisms (Rillig et al., 2017; Zhang et al., 2021). In addition, to detect and analyze MPs in the environment, several different techniques have been established (Imhof et al., 2012), including microscopy (Stereo), spectroscopy (Fourier transform infrared (FTIR) and Raman spectroscopy (Raman)), and thermal analyses (Li et al., 2018; Shim et al., 2017). Even though analytical techniques were already established for MPs monitoring, sample pretreatment is essential to remove any organic or other interfering compounds prior to MPs analysis in environmental samples. In particular, soil and sediment are heterogeneous materials containing many components such as garbage, dead leaves, natural organic matter, silt, and sand (Rillig, 2012). Therefore, separating and extracting MPs from soil and sediment samples is more challenging than air and water matrices. Many studies of MPs focused on the pollution assessment of MPs (Cole et al., 2011) as well as their behavior and role in the environment (Andrady, 2017). Most studies have focused on MPs analysis in ocean and marine sediment (Besley et al., 2017; Coppock et al., 2017; Hidalgo-Ruz et al., 2012; Matsuguma et al., 2017), whereas research on MPs in soil and freshwater sediment is still very limited. Moreover, there are few studies related to pretreatment methods and measurement criteria for extracting MPs from soil and freshwater sediment and thus, their standards have not been well established. Therefore, this paper focuses mainly on pretreatment methods through a review of recent papers analyzing MPs in soil and freshwater sediment (Adomat and Grischek, 2021; Prata et al., 2019; Stock et al., 2019). The outcome of this study will provide guidelines for understanding suitable pretreatment procedures for monitoring MPs in the environment, particularly for soil and freshwater sediment samples. To our knowledge, this study is the first one focusing on the pretreatment of soil and sediment samples for MPs detection.
2. Methodology of literature selection
Relevant literature were searched using Google Scholar databases with papers published from 2017 to 2022. Used keywords were ‘Microplastics’, ‘Soil’, ‘Sediment’, ‘Freshwater’, ‘Pretreatment’ and their combinations. Forty studies met the criteria (20 publications for soil and freshwater sediment, respectively) and were critically reviewed. We focused on sample pretreatment methods for MPs analysis in the papers, targeting only soil and freshwater sediment as the environmental matrix. Therefore, marine sediment papers were excluded. If there were more than one environmental matrix (e.g. water or sludge and sediment) in a study, contents of soil and sediment in the study were only used. Different processes for MPs sample pretreatment in each paper were divided into four representative categories: i) sampling sites and lab sample processing before main pretreatment processes (Section 3), ii) main pretreatment methods for monitoring MPs: density separation and organic removal (Section 4), iii) analytical instruments and characteristics for MPs detection in samples after pretreatment (Section 5) and iv) recovery test of pretreatment methods (Section 6).
3. Sampling sites and lab sample processing before main pretreatment processes
Sampling is the first step toward MPs monitoring in the soil and sediment. Before MPs analysis in the laboratories, samples are collected, stored, and preprocessed. For safety purposes, generally the MPs samples are collected, stored, and transported in glass, stainless or aluminum containers rather than plastic bottles or containers. In this review, sample processing methods in soil and freshwater sediment are classified into three categories of 1) sampling sites, 2) sample processing methods (sieving/drying), and 3) sample quantity.
3.1. Sampling sites
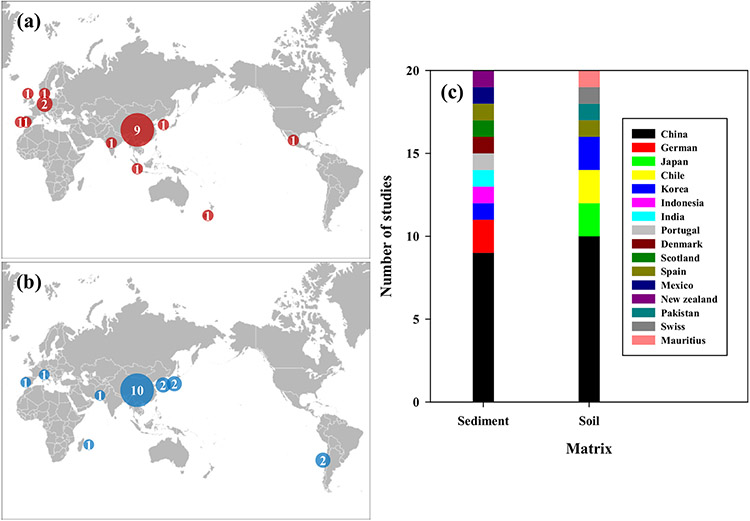
Tables 1 and 2, and Fig. 1 summarize the information collected from the selected forty papers. Half of these studies were based on samples of freshwater sediment obtained from twelve Asian countries (nine sites in China, one site in Korea, Indonesia, and India), six European countries (two sites in Germany, one site in Portugal, Denmark, Scotland, and Spain), one South American country (Mexico) and one in the Oceania region (New Zealand). The freshwater sediment samples were collected from fourteen rivers, three lakes, one pond, and two estuaries. Among the twenty soil samples, fifteen samples were collected from Asia (including ten samples in China, two in Korea, two in Japan, and one in Pakistan), two samples from Europe (one in Switzerland and Spain), two samples from South America (Chile) and one sample from Africa (Mauritius). Mainly agricultural lands were selected for the soil samples, while flood plains, coastal beaches, and river delta wetlands were selected for sediment samples.
Table 1.
Sample pretreatment before density separation and organic removal for MPs analysis in freshwater sediment.
| No. | Sampling site | Sample processing | Sample amounts | Ref. | ||
|---|---|---|---|---|---|---|
| Sieving | Drying | |||||
| Temperature (°C) |
Time | |||||
| 1 | Beijing river, China | N/A | 50 | 48 h | 30 g (DW) | (Wang et al., 2017) |
| 2 | Qinghai lake, China | 2 mm | N/A | N/A | N/A | (Xiong et al., 2018) |
| 3 | Yangtze river, China | N/A | N/A | N/A | 500 g (WW) | (Di and Wang, 2018) |
| 4 | Dongting lake, China | N/A | Freeze-drying | N/A | 50 g (DW) | (Jiang et al., 2018) |
| 5 | Huangpu river, China | N/A | 70 | N/A | 100 g (DW) | (Peng et al., 2018) |
| 6 | Antuã river, Portugal | N/A | 90 | 48 h | N/A | (Rodrigues et al., 2018) |
| 7 | Atoyac river, Mexico | N/A | <40 | N/A | 30 g (DW) | (Shruti et al., 2019) |
| 8 | Retention ponds, Denmark | 2 mm (wet-sieving), settle for a week, gather particles in the supernatant using a 10 μm STS filter, sonicate STS filter for 15 min, transfer the filtered particles back to the settled sediment | 50 (After pre-oxidation) | N/A | 50 g (WW; pre-oxidation) 200 g (DW; density separation) |
(Liu et al., 2019a) |
| 9 | Wei river, China | N/A | 70 | 24 h | 100 g (DW) | (Ding et al., 2019) |
| 10 | Kelvin river, Scotland | 2.8 mm, 2.0 mm, 1.4 mm, 1.0 mm, 0.71 mm, 0.5 mm, 0.355 mm, 0.25 mm, 0.18 mm, 0.125 mm, 0.09 mm and 0.063 mm, use an automatic shaker for 10 min | 100 | 24 h | 25 g (DW) | (Blair et al., 2019) |
| 11 | Rhine river, German | <2 mm, 2–5 mm, >5 mm | 60 | 4 days | 60 g (DW) | (Mani et al., 2019) |
| 12 | Poyang lake, China | N/A | 50 | 48 h | 500 g (DW) | (Yuan et al., 2019) |
| 13 | Ciwalengke river, Indonesia | N/A | 100 | 48 h | 100 g (DW) | (Alam et al., 2019) |
| 14 | Nakdong river, Korea | N/A | 60 | N/A | Determine by drying 100 g (WW) |
(Eo et al., 2019) |
| 15 | Ganga river, India | 63–850 μm, 850 μm–5 mm, 5–10 mm (mesoplastics) | 65 | 36 h | N/A | (Sarkar et al., 2019) |
| 16 | Auckland streams, New Zealand | N/A | N/A | Determine by drying the residue remaining after elutriation step of 1 L (WW) | (Dikareva and Simon, 2019) | |
| 17 | Ebro river, Spain | N/A | N/A | N/A | 10–20 g (DW) | (Simon-Sánchez et al., 2019) |
| 18 | Elbe river, German | Wet-sieving, 20–125 μm (dry at 45–55 °C for 5–7 days), 125–1000 μm (perform density separation), 1000–5000 μm (store for analysis) | 45 | 5–7 days | Determine by drying 200 g (WW) | (Scherer et al., 2020) |
| 19 | Changjiang estuary, China | N/A | 70 | 24 h | 100 g (DW) | (Peng et al., 2017) |
| 20 | Bohai bay river estuary, China | 0.9 mm | 60 | N/A | 200 g (DW) | (Han et al., 2019b) |
Note: DW (Dried Weight) and WW (Wet Weight).
Table 2.
Sample pretreatment before density separation and organic removal for MPs analysis in soil.
| No. | Sampling site | Sample processing | Sample amounts | Ref. | ||
|---|---|---|---|---|---|---|
| Sieving | Drying | |||||
| Temperature (°C) |
Time | |||||
| 1 | Shanghai farmlands, China | N/A | 70 | 24 h | N/A | (Liu et al., 2018) |
| 2 | Floodplain, Swiss | 1 mm | 65 | N/A | 50 mL of dried and sieved soil sample | (Scheurer and Bigalke, 2018) |
| 3 | Chai river valley cropped area, China | 10 mm | 25 | N/A | 30 g (DW) | (Zhang and Liu, 2018) |
| 4 | Shandong province coastal beaches, China | N/A | 105 | >12 | N/A | (Zhou et al., 2018) |
| 5 | Nanjing, Wuxi and Jiangsu province agriculture lands, China | 5 mm, 1 mm | Air dry | N/A | 50 g | (Li et al., 2019) |
| 6 | Shanghai farmlands, China | N/A | 70 | 24 h | N/A | (Lv et al., 2019) |
| 7 | Melipilla agricultural field, Chile | 2 mm | 40 ± 2 | N/A | 5 ± 0.01 g | (Corradini et al., 2019) |
| 8 | Shanghai farmlands soil, yellow-brown soil, paddy soil and floodplain soil, China | 5 mm | 70 | 24 h | 50–200 g (DW) | (Liu et al., 2019b) |
| 9 | Shihezi cotton fields, China | 5 mm | Air dry | N/A | 20 g (DW) | (Huang et al., 2020) |
| 10 | Paotai town, Shihezi, Xinjiang agricultural soils, China | 2 mm, 0.9 mm, 0.28 mm, 0.15 mm | N/A | N/A | 1 kg (DW) | (Li et al., 2020) |
| 11 | Hangzhou bay coastal plain farmlands, China | 5 mm | 25 | N/A | 50 g (DW) | (Zhou et al., 2020) |
| 12 | Yellow river delta wetland in Shangdong, China | 2 mm | N/A | N/A | 1 kg (DW) | (Duan et al., 2020) |
| 13 | Eight different land use types in Lahore, Pakistan | 5 mm, 50 μm (wet sieving) | N/A | N/A | 200 g (DW) | (Rafique et al., 2020) |
| 14 | Arable soils, pastures, rangelands and natural grasslands in metropolitan, Chile | 2 mm | N/A | N/A | 5 g (for visual identification), 1 g (for FTIR) | (Corradini et al., 2021) |
| 15 | Tedori river alluvial fan, Japan | N/A | 60 | 48 h | 1 kg (DW) | (Katsumi et al., 2021) |
| 16 | Forest, suburban and agricultural land in Yeoju, Korea | 5 mm | 60 | 48 h | 50 g (DW) | (Choi et al., 2021) |
| 17 | Agricultural soils in Yongin, Korea | 5 mm | N/A | N/A | 200 g (DW) | (Kim et al., 2021) |
| 18 | Agricultural fields, Mauritius | 10 mm, 6 mm, 2 mm | 60 | 72 h | 150 g | (Ragoobur et al., 2021) |
| 19 | Agricultural fields in Miyagi, Japan | 2 mm | 60 | 72 h | 10 g | (Grause et al., 2022) |
| 20 | Irrigated soils in Fuerteventura, Spain | N/A | N/A | N/A | 10 g | (Pérez-Reverón et al., 2022) |
Note: DW (Dried Weight) and WW (Wet Weight).
Fig. 1.
The geographical location of the sites where the studies were conducted; (a) freshwater sediment, (b) soil and (c) number of studies in different countries.
3.2. Sample processing methods
The sample processing methods are typically represented by sieving and drying. A sieving process is a standard method for analyzing MPs in sediment or soil samples and is used to: 1) homogenize the samples, 2) obtain MPs of the desired sizes, and 3) remove unwanted large sized grains or impurities. In soil and sediment studies, sample sieving is usually performed during the sample collection or before pretreatment. Among various sieve pore sizes, 5 mm and 2 mm were mostly used. Other pore sized sieves (10 mm and 1 mm, etc.) have also been used and several other studies performed multi-sieving using two or more sieves with different pore sizes. For example, Ragoobur et al. (Ragoobur et al., 2021) used 10, 6 and 2 mm sieves to remove impurities such as gravel and roots. In a different study, Rafique et al. (Rafique et al., 2020) used a 50 μm sieve to remove particles of <50 μm that were not needed for analysis after sample sieving using 5 mm sieve. Multi-sieving is important when selecting different MPs analytical instruments for each section through size fractionation and to investigate the correlation between MPs type and size. As an example, to classify the analyzed particles sizes, Sarkar et al. (Sarkar et al., 2019) used four sieves with different pore sizes (i.e., 10 mm, 5 mm, 850 μm, and 63 μm) to investigate MPs size distributions ranging from 63–850 μm, 850 μm–5 mm, and 5–10 mm (mesoplastics). Similarly, Scherer et al. (Scherer et al., 2020) employed three different sized sieves (20 μm, 125 μm, and 1000 μm) and evaluated three size distributions of MPs (20–125 μm, 125–1000 μm, and >1000 μm). It is important to note that MPs with particle sizes larger than 1000 μm can be directly and visually sorted. In this study, attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), and visual analysis were performed for 125–1000 μm and 1000–5000 μm size MPs, and MPs of 20–125 μm sized were stored for pyrolysis-gas chromatography–mass spectrometry (Pyr-GC–MS) analysis (Scherer et al., 2020). Additionally, soil and sediment have different characteristics, such as moisture, organic matter, and biota, that are a function of sampling sites. For consistency across studies, samples were dried at a constant weight before the experiments. Dry sieving was performed if accurate sieving was difficult due to high viscosity and cohesiveness of the moisture-containing samples. In soil and sediment studies, sample drying was carried out at different temperatures and time durations. Samples were dried in a range of 25–105 °C; however, 50–75 °C was the commonly used temperature in most cases. A freeze-drying temperature was used in some cases as well (Jiang et al., 2018). Since MPs can melt or be damaged at high temperatures, it is recommended that drying is not performed at high temperatures. However, in some cases, high drying temperatures (100–105 °C) have been used for determining the standard weight of samples (Alam et al., 2019; Blair et al., 2019; Zhou et al., 2018). Blair et al. (Blair et al., 2019) also reported that MPs did not deform, i.e., melt or decompose, in the above temperature range. The drying time also varied, ranging from 12 h to 7 days, and in most cases, 24–48 h was chosen as the optimal drying time period. It is critical to select a specific drying temperature and sufficient drying time to achieve constant weight, depending on the characteristics of the sample. The details of the drying temperature and drying time frame are summarized in Tables 1 and 2.
3.3. Sample quantity
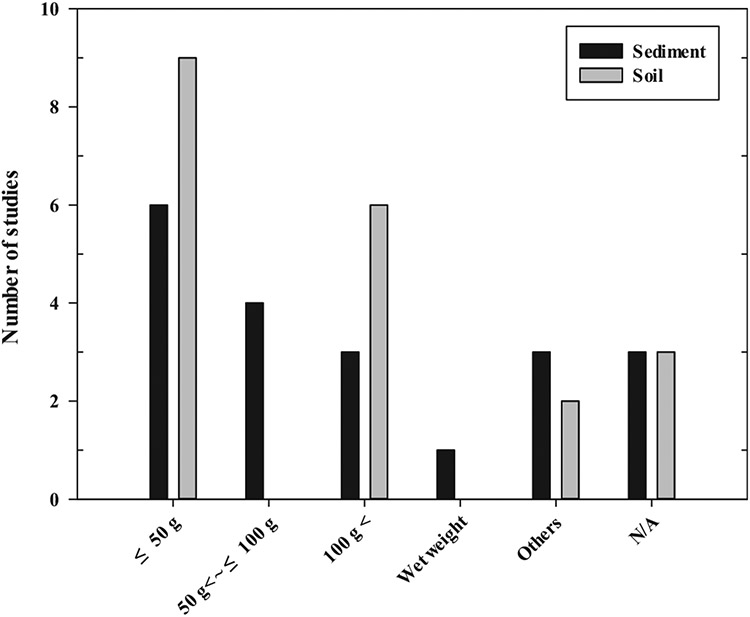
Sample quantity is critical for MPs analysis in soil and freshwater sediment. For instance, if the sample contains a high concentration of MPs, spectroscopy, including Raman and FTIR analyses, might not be suitable. On the contrary, if samples contain low concentration of MPs, thermal analysis, such as thermogravimetric analysis-Fourier transfer infrared spectroscopy (TGA-FTIR), thermal extraction and desorption-gas chromatography–mass spectrometry (TED-GC–MS), and Pyr-GC–MS, will not accurately detect MPs due to their insufficient detection limit. Therefore, it is important to establish a standardized sample quantity for the MP analysis. Yet, the quantity of samples used in MPs analysis in soil and freshwater sediment could be different. In case of freshwater sediment, 10–500 g of dry weight (DW) sample was mostly used. DW method was mainly used to measure the sample quantity. However, in some cases, the sample was measured using wet weight (WW), followed by drying (Eo et al., 2019; Scherer et al., 2020). In the case of soil MPs analysis, the sample quantity varied significantly, ranging from 1 to 1000 g. The significant difference in the sample quantity resulted from the dramatically different distribution of MPs in the samples. In most soil studies, DW was used to measure the sample quantity, but sometimes, volume was used instead of DW. For example, Dikareva & Simon (Dikareva and Simon, 2019) elutriated 1 L of sediment samples (WW) and dried the residue remaining on the 63 μm sieve. Scheurer & Bigalke (Scheurer and Bigalke, 2018) used 50 mL of dried soil sample and mixed it with sodium chloride (NaCl) solution. The details of the sample quantity used in soil and sediment studies can be found in Tables 1 and 2, and Fig. 2.
Fig. 2.
Sample quantity for selected weight ranges used for pretreatment in freshwater sediment and soil studies.
4. Main pretreatment methods for monitoring MPs: density separation and organic removal
Extraction and separation are two essential steps before any MPs analysis in the soil and sediment. For effective extraction and separation of MPs in soil and sediment, density separation (DS) and organic removal (OR) are the most commonly used techniques. In addition to density separation, an organic removal step is needed prior to MPs analysis in soil and sediment samples as the presence of organic matter on the surface of MPs may alter FTIR and Raman results. As summarized in Tables 3 and 4, different pretreatment techniques were used during MP analysis in the soil and sediment samples. In general, samples went through pretreatment processes before the density separation and organic removal, followed by filtration and drying, as discussed below.
Table 3.
Density separation and organic removal for MPs analysis in freshwater sediment.
| No. | Order | Density separation (DS) | Organic removal (OR) | Filtration | Ref. | ||
|---|---|---|---|---|---|---|---|
| Density solution | Procedure | Filter type | Drying conditions | ||||
| 1 | DS only | NaCl (1.20 g/cm3) | 1. Add 200 mL of NaCl solution Stir for 2 min Settle for 2 h 2. Sonicate for 5 min Settle overnight |
N/A | 1.0 μm glass microfiber filter | 50 °C for 48 h | (Wang et al., 2017) |
| 2 | DS → OR | CHKO2 (1.54 g/cm3) | To overflow supernatant into stainless steel tray add solution and settle overnight | Using H2O2 (30%) at 60 °C overnight | 1.2 μm glass microfiber filter | Dry in a desiccator | (Xiong et al., 2018) |
| 3 | DS → OR | 1. NaCl (saturated) 2. NaI (60%) |
Perform a two step density separation 1. Add 1 L of NaCl solution Stir for 2 min Settle for 10 min Sieve using a 48 μm stainless steel sieve Repeat three times 2. Add 375 mL of NaI solution Shake 200 rpm on a shaker for 2 min Settle for 10 min |
WPO (30% H2O2) for 12 h | 0.45 μm glass microfiber filter | 50 °C | (Di and Wang, 2018) |
| 4 | DS → OR | ZnCl2 (1.50 g/cm3) | Add 400 mL of ZnCl2 solution Stir for 30 min Settle for 24 h |
WPO (30% H2O2) | 0.22 μm glass microfiber filter | 60 °C | (Jiang et al., 2018) |
| 5 | DS only | NaCl (1.20 g/cm3) | Add NaCl solution Stir for 2 min Settle for 24 h |
N/A | 1.0 μm glass microfiber filter | Dry to constant weight | (Peng et al., 2018) |
| 6 | DS → OR | ZnCl2 (1.60 g/cm3) | Disaggregate with 400 mL of sodium polyphosphate (5.5 g/L) Stir for 1 h at high rpm Sieve sediments using a 5 mm and 0.055 mm sieve Add 300 mL of ZnCl2 solution and stir Settle for 1 h and transfer supernatant to 0.055 mm sieve and rinse with Milli-Q water |
WPO (30% H2O2 with 0.05 M Fe2+ catalyst) at 75 °C for 5/10 min until it starts boiling and afterwards react at room temperature for 15 h | 0.45 μm membrane filter | 40 °C for 3–5 days | (Rodrigues et al., 2018) |
| 7 | OR → DS | ZnCl2 (1.58 g/cm3) | Add 36 mL of ZnCl2 solution Settle for 24 h |
WPO (30 mL of 30% H2O2) for overnight | 1.2 μm nitrocellulose filter paper |
Air-dry | (Shruti et al., 2019) |
| 8 | OR → DS → OR | ZnCl2 (1.97 g/cm3) | Perform a 2 step density separation 1. Add 1.5 L of ZnCl2 solution Aerate for 1 h Settle for 5 h Drain off solution using a funnel 2. Aerate for 30 min Settle overnight Drain off solution |
1. Pre-oxidation: 50 g of WW subsample was filled with 200 mL Milli-Q water before adding 30 mL of 30% H2O2 (achieving final concentration of 4%) Heat at 50 °C and stir Add 30 mL 30% H2O2 every 24 h (until no foaming) Dry at 50 °C Repeat processing for all of the 3 L sediments 2. Post-oxidation: Fenton reaction by adding 146 mL of 50% H2O2, 63 mL of 0.1 M FeSO4 and 65 mL of 0.1 M NaOH at 15–19 °C Leave for 2 days at room temperature |
500 μm sieve, 10 μm stainless steel filter | 1. Dry particles on a 500 μm sieve at 50 °C 2. Collect the particles on a 10 μm filter into HPLC grade ethanol by ultrasonication and evaporate by nitrogen (N5.0) until dry |
(Liu et al., 2019a) |
| 9 | DS → OR | NaCl (saturated) | Add NaCl solution Stir for 2 min Settle for 24 h |
WPO (30 mL of 30% H2O2) at 100 rpm and 65 °C for 24 h | 0.45 μm membrane filter paper | 50 °C for 24 h | (Ding et al., 2019) |
| 10 | DS only | NaCl (1.20 g/cm3) | Add 40–68 mL of NaCl solution Stir for 1 min Settle for 24 h |
N/A | 11 μm cellulose filter | Room temperature (18–21 °C) | (Blair et al., 2019) |
| 11 | DS → OR | ZnCl2 (1.70 g/cm3) | Leave a 1.5 cm margin, fill ZnCl2 solution into a 100 mL flask Sonicate at 160 W/35 kHz for 15 min Stir at 250 rpm for 1 h Settle for 30 min Add 20 mL of ZnCl2, allow the excess fluid to overflow Store supernatants at 4 °C |
1. Sieve using 500 μm sieve Filter using 10 μm stainless steel disk 2. <500 μm particles: oxidation by adding Fenton reagent (10 mL of FeSO4 (7.2 mM, pH 5)) in a 20 °C water bath Add 20 mL 30% H2O2 after 15 min and sonicate at 215 W/35 kHz for 5 min 3. >500 μm particles: retain for subsequent analysis |
1. 0.2 μm anodisc (<500 μm) 2. 0.45 μm mixed cellulose ester (500 μm-2 |
50 °C for least 2 days | (Mani et al., 2019) |
| 12 | DS → OR | 1. NaCl (saturated) 2. NaI (60%) |
1. Add 1 L of NaCl solution Settle for 2 h Sieve using 50 μm stainless steel sieve and rinse with distilled water Repeat three times 2. Add 500 mL of NaI solution and perform the same way and repeat three times |
WPO (30 mL of 30% H2O2) | 0.7 μm glass microfiber filter | N/A | (Yuan et al., 2019) |
| 13 | DS only | NaCl (30%) | Add 400 mL of NaCl solution Stir for 2 min using spoon Leave until no more visible material floats |
N/A | 1.2 μm glass microfiber filter | 105 °C for 30 min | (Alam et al., 2019) |
| 14 | DS → OR | LMT (1.60 g/cm3) | Shake with 300 mL of LMT solution for 1 min Settle for 10 min Transfer supernatant Repeat twice with 200 mL of LMT |
WPO (20 mL of 35% H2O2 with Fe2+ solution) at 75 °C at 180 rpm for 30 min Add H2O2 every 30 min |
5 μm polycarbonate filter paper | Room temperature | (Eo et al., 2019) |
| 15 | DS → OR | ZnCl2 (1.80 g/cm3) | Mix with ZnCl2 (1:10(w/v)) Stir for 15–20 min Settle overnight Collect on filter paper (0.7 μm glass microfiber filter) |
WPO (30% H2O2) for 3 h | 0.7 μm glass microfiber filter | Dry in a desiccator for 36 h | (Sarkar et al., 2019) |
| 16 | DS only | 1. DI water (1.0 g/cm3) 2. NaI (1.60 g/cm3) |
1. Elutriation step with water for 20 min Mix sediments by air stone Collect overflow materials on a 63 μm sieve by adding water (250 L/h flow rate) Transfer into a 50 mL centrifuge tube 2. Add 30 mL of 3.3 M NaI solution Centrifuge for 5 min at 3500 G Settle for 1 h Repeat once again |
N/A | 1.2 μm glass microfiber filter | 60 °C | (Dikareva and Simon, 2019) |
| 17 | DS, OR simultaneously | NaCl (1.20 g/cm3) | Use 200 mL of NaCl +20 mL of 30% H2O2 solution Heat at 50 °C Stir for 20 min at 200 rpm Settle at 50 °C for 1 h and afterwards settle at room temperature for 1 h Transfer foam from reaction and mix with 50 mL of NaCl + 20 mL of H2O2 and then heat, mix, settle, and filter supernatant Meantime, add 20 mL of H2O2 to WPO solution and sediments, heat, mix and settle the mixture again and then collect and filter supernatant |
0.7 μm glass microfiber filter | 40 °C overnight | (Simon-Sánchez et al., 2019) | |
| 18 | DS → OR | ZnCl2 (1.60–1.80 g/cm3) | Fill with ZnCl2 solution Settle for 24 h |
WPO (30% H2O2 and 10% H2SO4) at 55 °C for 5 days After filtration, repeat the washing process at least twice (until the washing water pH is 7) Finally, transfer the suspended powder on the filter to the new filter |
2.7 μm glass microfiber filter | 55 °C for 7 days (For pyr-GC–MS analysis) | (Scherer et al., 2020) |
| 19 | OR → DS | NaCl (1.20 g/cm3) | Add NaCl solution Stir for 2 min Settle for 24 h |
WPO (30% H2O2) | 1.0 μm glass microfiber filter | 40 °C for 24 h | (Peng et al., 2017) |
| 20 | DS → OR | NaI - NaCl (1.50 g/cm3) | Add 250 mL of NaI - NaCl solution Air floatation for 40 s Settle for 5 min |
WPO (30 mL of 35% H2O2) at room temperature for 7 | 0.45 μm membrane filter | N/A | (Han et al., 2019b) |
Table 4.
Density separation and organic removal for MPs analysis in soil.
| No. | Order | Extraction (density separation; DS) | Organic removal (OR) | Filtration | Ref. | ||
|---|---|---|---|---|---|---|---|
| Solution | Procedure | Filter type | Drying conditions | ||||
| 1 | DS → OR | NaCl (1.19 g/cm3) | Add NaCl solution Ultrasonication for 2 min Stir for 30 min Settle for 24 h Repeat three times |
WPO (30% H2O2) at 50 °C for 72 h | 20 μm nylon net filter | Room temperature | (Liu et al., 2018) |
| 2 | DS → OR | NaCl (1.20 g/cm3) | 1. Add 160 mL of 27% NaCl solution Stir for 10 min Centrifuge at 3450 G for 30 min Filter supernatant using 0.45 μm membrane filter 2. Remove particles stuck to beaker walls by 27% NaCl and settle for 24 h Mix sample mixture at 800 rpm for 10 min Centrifuge at 3450 G for 30 min |
Wash particles from the filter and then treat them with 40–80 mL of 65% HNO3 at 90 °C for 48 h | 0.2 μm infrared transparent anodisc filter | N/A | (Scheurer and Bigalke, 2018) |
| 3 | OR → DS → OR | NaI (1.80 g/cm3) | Add 150 mL of NaI solution Centrifuge again Wash with NaI solution |
1. Pre-oxidation: WPO (10 mL of 35% H2O2 + 1 mL of 10% FeSO4) at 50 °C Add a few drops of butyl alcohol (if frothing was excessive) Add 1 mL of 10% FeSO4 (to decompose the H2O2) Add 30 mL of 0.5 M NaOH Store for 24 h Adjust to 150 mL using DI water Agitate for 20 min using ultrasonic Centrifuge at 2300 rpm for 10 min 2. Post-oxidation: WPO (2 mL of 35% H2O2) |
1 mm, 0.25 mm, 0.05 mm sieve | 80 °C | (Zhang and Liu, 2018) |
| 4 | DS only | 1. NaCl (1.20 g/cm3) 2. NaI (1.60 g/cm3) |
1. Use a continuous flow and floating separation apparatus Float using NaCl solution Clean residues in the sieve (300 mesh) were cleaned using water 2. Residues were floated using NaI solution Filter if it contained a large number of solid particles |
N/A | N/A | Air dry | (Zhou et al., 2018) |
| 5 | 1. OR → DS → OR 2. DS → OR |
1. NaCl (1.20 g/cm3), NaI (1.80 g/cm3), ZnCl2 (1.50 g/cm3) 2. Only using NaCl (1.20 g/cm3) |
1. Test three types of floatation solutions Add 200 mL of a solution Stir at 200 rpm for 1 h using a shaker Settle for 48 h and siphon out 100 mL of supernatant Add 100 mL of the same floatation solution Stir for 30 min and settle for 48 h Combine supernatants 2. Repeat steps but by only using NaCl solution |
1. Pre-oxidation: WPO (200 mL of 30% H2O2) If temperature remained stable, heating at 70 °C 2. Post-oxidation: WPO (30% H2O2 + H2SO4 (3:1, v/v)) |
20 μm nylon filter | Air dry | (Li et al., 2019) |
| 6 | DS → OR | NaCl (1.24 ± 0.05 g/cm3) | Add NaCl solution Stir for 30 min Settle for 24 h Repeat three times Filter with 20 →m |
Wash filter WPO (100 mL of 30% H2O2) at 65 °C at 80 rpm for 72 h |
20 μm nylon net filter | Room temperature | (Lv et al., 2019) |
| 7 | DS only | 1. DI water (1.0 g/cm3) 2. NaCl (1.20 g/cm3) 3. ZnCl2 (1.55 g/cm3) |
1. Add 20 mL of DI water stir at 21,000 rpm for 30 s Centrifuge at 2000 rpm for 15 min Filter supernatant using by 2–3 μm filter paper 2. Add 20 mL of NaCl Stir, centrifuge and filter the same way 3. Add 20 mL of ZnCl2 Stir 32,000 rpm for 30 s Centrifuge 2000 rpm for 15 min Filter the same way |
N/A | 2.5 μm cellulose filter paper | N/A | (Corradini et al., 2019) |
| 8 | DS → OR | NaBr (1.55 g/cm3) | Add NaBr solution Stir for 5 min Settle for 2 h Use automatic cycling device for 30 min Filter using a 20 μm filter |
WPO (30% H2O2) at 60 °C for 3 days | 20 μm membrane filter | N/A | (Liu et al., 2019b) |
| 9 | DS → OR | NaI | Add 200 mL of NaI solution Stir for 30 min Settle for 12 h Filter using a 7 μm glass fiber filter |
WPO (100 mL of 30% H2O2) for 12 h | 7 μm glass fiber filter | Room temperature |
(Huang et al., 2020) |
| 10 | DS → OR | DI water (1.0 g/cm3) | Leach soil specimens using pressurized water Add distilled water Shake for 30 s Precipitate for 5 min Repeat three times |
Oxidation using 10 mL of 98% H2SO4 Sonicate for 20 min Place in ice bath Dilute with distilled water |
Membrane filter paper | Dry at 50–60 °C | (Li et al., 2020) |
| 11 | DS → OR | 1. NaCl (1.20 g/cm3) 2. NaI (1.60 g/cm3) |
Disperse dried soil with (NaPO3)6 (0.5 mol/L) Add NaCl solution into beaker (flow rate 1.0 L/min) with air blowing at the bottom Collect the over-flow materials on a vibrating sieve (pore 50 μm) Settle residues on the sieve for 48 h in NaI solution Filter using a 5 μm cellulose nitrate filter Repeat three times |
WPO (30% H2O2) at 70 °C for 72 h | 20 μm glass fiber filter | N/A | (Zhou et al., 2020) |
| 12 | DS → OR | ZnCl2 (1.60 g/cm3) | Add ZnCl2 solution Settle for 6 h at room temperature Repeat three times |
WPO (30% H2O2) at 70 °C in water bath | N/A | Air dry | (Duan et al., 2020) |
| 13 | OR → DS | NaCl (30%; w/v) | Add 600 mL of NaCl solution Settle for 12 h |
WPO (50 mL of mixture (35% H2O2 + 0.5 M of ferrous sulfate)) at room temperature for 24–72 h | 300, 150, 50 μm sieve | N/A | (Rafique et al., 2020) |
| 14 | DS only | 1. DI water (1.0 g/cm3), 2. NaCl (1.20 g/cm3), 3. ZnCl2 (1.55 g/cm3) |
1. For visual identification, centrifuge 5 g of soil and 20 mL of DI water at 2000 rpm for 15 min Filter supernatant through a 2.5 μm filter paper Fill remaining sediments with 20 mL of NaCl Centrifuge at 21000 rpm for 30 s and filter supernatant a another time Fill remaining sediments with 20 mL of ZnCl2 Centrifuge last time and filter supernatant through the same filter used the previous two times 2. For FTIR microscope analysis, sonicate 1 g soil and 10 mL of ZnCl2 for 10 min Agitate at 2000 rpm for 15 s Shake for 20 min at 180 oscillations per min Before filtration, centrifuge for 10 min at 2500 rpm Filter supernatant through a 0.4 μm polycarbonate membrane filter Re-fill with ZnCl2 and underwent same step once again |
N/A | 1. 2.5 μm cellulose filter paper (for visual identification by optical microscope) 2. 0.4 μm polycarbonate membrane filter (for FTIR analysis) |
Dried at 40 °C for 12 h (for FTIR analysis) | (Corradini et al., 2021) |
| 15 | DS only | Tap water | Add tap water Stir and settle overnight |
N/A | 1 mm sieve (collect >1 mm size) | Dry at 60 °C for 24 h | (Katsumi et al., 2021) |
| 16 | DS → OR | ZnCl2 (1.70 g/cm3) | Add 500 mL of ZnCl2 solution Stir at 300 rpm for 5 min Settle for 24 h Repeat three times |
WPO (20 mL of 0.05 M FeSO4 and 3 mL of conc. H2SO4 and 20 mL of 30% H2O2) at 75 °C for 24 h Rinse with water and sieve using a 1 mm sieve |
0.45 μm membrane filter | Room temperature | (Choi et al., 2021) |
| 17 | OR → DS | ZnCl2:CaCl2 (2:1.4; 1.55–1.58 g/cm3) | 1. Filter digested supernatant through 20 μm sieve, afterward, visually sort and analyze the >1 mm particles 2. Fill digested remnant with density solution Filter supernatant through 20 μm sieve Sort >1 mm Repeat this process two more times 3. Perform density separation of the particles of <1 mm obtained from the first process and >20 μm obtained from the second process and filter supernatant finally |
WPO (200 mL of 30% H2O2) | 0.7 μm glass fiber filter | N/A | (Kim et al., 2021) |
| 18 | DS → OR | NaCl (1.19 g/cm3) | Shake soil samples at 150 rpm for 30 min with 300 mL of distilled water using an orbital shaker Settle for 24 h Repeat 3–4 times until no more observed floating extract Centrifuge the collected supernatant solution for 29 min at 1500 rpm Mix supernatant with 100 mL of NaCl solution for 15 min at 150 rpm and settle overnight Centrifuge supernatant for 10 min at 1500 rpm for 1 s |
WPO (100 mL of 30% H2O2) for 7 days at 25 °C | 0.45 μm cellulose nitrate filter | Room temperature |
(Ragoobur et al., 2021) |
| 19 | OR → DS | CaCl2 (1.40 g/cm3) | Centrifuge with CaCl2 solution for 10 min at a speed of 3700 min−1 Stain samples with Nile red |
Fenton reaction by adding 30 mL of 30% H2O2, 30 mL of iron catalyst for 1 h at 0 °C | Glass fiber filter | 60 °C | (Grause et al., 2022) |
| 20 | OR → DS | NaCl (1.20 g/cm3) | Add 100 mL of NaCl solution Stir for 1 min Settle for 1.5 h |
WPO (40 mL of 33% (w/v) H2O2) for 2 hat 60 °C Stir at 300 rpm |
50 μm stainless steel filter | N/A | (Pérez-Reverón et al., 2022) |
4.1. Pretreatment procedures
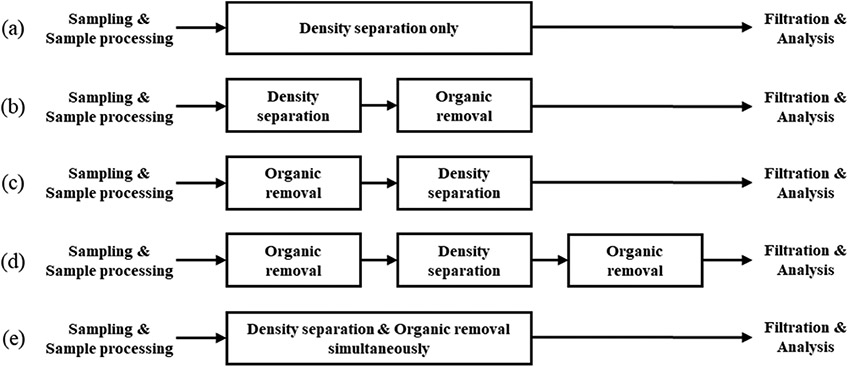
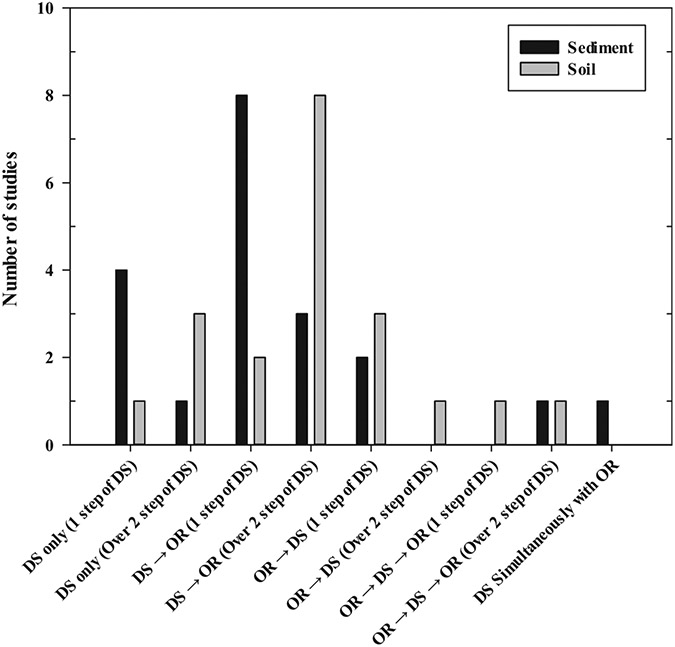
Establishing a pretreatment procedure for MPs analysis is very important as it affects the results of sample analysis. In the reviewed studies, the order of pretreatment processes varied. In the pretreatment process for both freshwater sediment and soil, most studies used both density separation and organic removal, and a few studies performed only density separation without the organic matter removal process. The pretreatment processes for freshwater sediment and soil samples were very similar in different studies. The most used processes were DS and then OR (DS → OR) for both samples. The order is as follows: DS → OR (55% of the studies) > DS only (25% of the studies) > OR → DS (10% of the studies) > OR → DS → OR (5% of the studies) = DS & OR simultaneously (5% of the studies) for freshwater sediment matrixes, and DS → OR (50% of the studies) > DS only (20% of the studies) = OR → DS (20% of the studies) > OR → DS → OR (10% of the studies) for soil matrixes as shown in Figs. 3 and 4. Although uncommon, organic oxidation and density separation processes were also used simultaneously for a sediment sample. Also, if necessary, DS processes were repeated several times to increase the efficiency of MPs separation/extraction from samples (Simon-Sánchez et al., 2019). The order of pretreatment can significantly change the method used in each step. If organic matter removal was performed before density separation, a large amount of organic matter removal reagent was used because soil and sediment samples contain a lot of organic matter. For example, in the case of oxidation using H2O2, a significant amount of foam may be produced due to organic oxidation by H2O2, resulting in sample loss. Conversely, if the density separation was performed first, many impurities can be mixed in the supernatant, and then filter clogging may occur when collecting MPs, because of the presence of unremoved organic compounds in the samples. Therefore, it is of great importance to configure the order of pretreatment considering these advantages and disadvantages.
Fig. 3.
Five flowcharts of pretreatment procedures: (a) density separation only, (b) density separation followed by organic removal, (c) organic removal followed by density separation, (d) density separation followed by organic removal after removal of organic in advance and (e) simultaneously perform density separation and organic removal.
Fig. 4.
Pretreatment orders used in freshwater sediment and soil studies.
4.2. Density separation methods
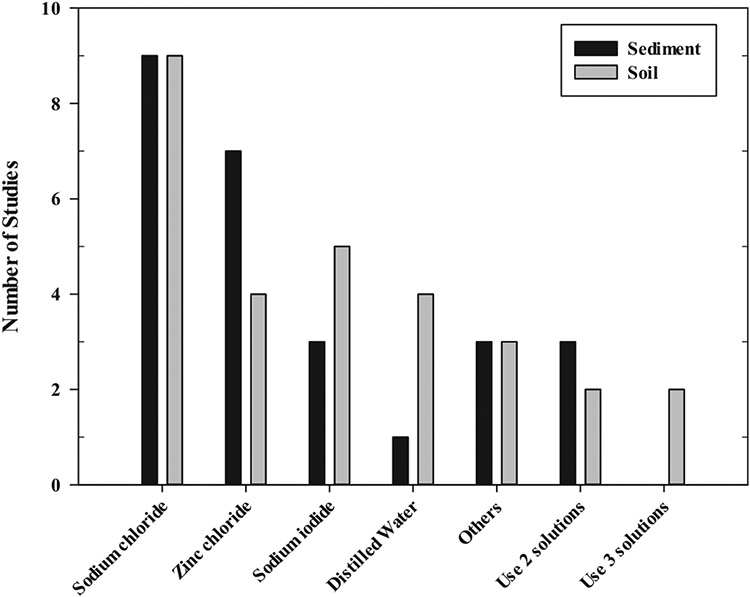
Density separation is a key process for MPs analysis in soil and sediment samples due to the complexity of sample matrices. Due to differences in the density of each material, various types of MPs can float (or remain unsettled) in samples when several solutions with different densities are used. Table 5 summarizes different polymers' densities and various density solutions for MPs separation. The density solutions used for freshwater sediment and soil samples were slightly different from each other. For both the sediment and soil samples, 1.20 g/cm3 NaCl was the most commonly used density solution. Similarly, zinc chloride (ZnCl2) and sodium iodide (NaI) with a density of 1.50–1.80 g/cm3 and 1.60–1.80 g/cm3, respectively, were widely used as density solutions. Moreover, water and other solutions including potassium formate (CHKO2) of 1.54 g/cm3, lithium metatungstate (LMT) of 1.60 g/cm3, NaI-NaCl mixtures of 1.50 g/cm3, sodium bromide (NaBr) of 1.55 g/cm3, calcium chloride (CaCl2) of 1.40 g/cm3, and ZnCl2:CaCl2 mixtures of 1.55–1.58 g/cm3 were used as density solutions. Li et al. (Li et al., 2019) compared the efficiencies of three different solutions (i.e., NaCl, ZnCl2, and NaI) and found NaI as the most efficient separating solution. When high-density fiber MPs were negligible, NaCl was recommended as the optimum separation solution with economic consideration (Li et al., 2019). In some cases, multiple density separation solutions with varying densities were used simultaneously with water components. For example, NaCl and NaI (Di and Wang, 2018; Yuan et al., 2019; Zhou et al., 2020; Zhou et al., 2018) or deionized (DI) water and NaI have been frequently used in the literature (Dikareva and Simon, 2019). Other studies have combined water, NaCl, and ZnCl2 (Corradini et al., 2021; Corradini et al., 2019). Details on the use of density solutions are given in Fig. 5. The density and characteristics of the density separation solution should be determined and used for MPs extraction. Considering the most used density solution, NaCl has a low density and may not be able to extract MPs such as polyvinyl chloride (PVC), polyethylene terephthalate (PET), and polytetrafluorethylene (PTFE). In addition, NaCl is easily crystalized during MPs extraction and thus, MPs were attached on beakers with the crystalized NaCl, resulting in sample loss. High density ZnCl2 has an advantage of extracting high density polymers, including PVC and PET, but it can form salt and cause toxicity (Franklin et al., 2007), which must be considered. Therefore, for the selection of density solution for MPs extraction, characteristics of density solutions must be carefully checked. The separation solutions were added to the freshwater sediment or soil samples and mixed homogeneously using different techniques, such as aeration, magnetic stirrer, glass rod, sonication, or centrifugation. The resulting mixtures were left for some time until the soil or sediment compounds settled and the suspended MPs were extracted. In freshwater sediment and soil studies, the volume of density solution used to pretreat the same amount of sample was similar. For samples weighing 100 g or less, the majority of studies for both freshwater sediment (Alam et al., 2019; Blair et al., 2019; Jiang et al., 2018; Mani et al., 2019; Shruti et al., 2019; Simon-Sánchez et al., 2019; Wang et al., 2017) and soil (Choi et al., 2021; Corradini et al., 2021; Corradini et al., 2019; Huang et al., 2020; Li et al., 2019; Pérez-Reverón et al., 2022; Rafique et al., 2020; Ragoobur et al., 2021; Zhang and Liu, 2018) used solution volumes under 500 mL. For larger samples (i.e., above 200 g), a larger volume of the solutions was used (e.g., 1 L) regardless of the sample type (i.e., whether soil or freshwater sediment). The amount of the density separation solution should be sufficient enough for separating MPs from the sample, which depends on the characteristics of the container used in the density separation step. In many studies, during density separation, the samples and solutions were mixed using mechanical mixing. Other studies mixed the samples and solutions and then centrifuged them for the separation of MPs. Centrifugation accelerates the settling step, so the time required for particles to settle would be reduced. The supernatants of the centrifuged mixture were simply collected. In most cases, when centrifugation was applied, only a small amount of sample was used such as amounts aliquoted from samples or residue particles after the elutriation step (e.g., 5–30 g of DW). On the other hand, Ragoobur et al. (Ragoobur et al., 2021) used 150 g of dried sample, but in this case, the sample was used for density separation first, and then its supernatant was used for several steps of centrifugation. A few studies also used an unusual way of collecting the separated MPs, also known as the overflow method, where the same type of solution used in density separation was poured into the settled or separated mixture, and the MPs were collected from the overflowing supernatants.
Table 5.
Density of different polymers and various density solutions for MPs separation.
| Polymer | Ref. | Density solution | Ref. | ||
|---|---|---|---|---|---|
| Type | Density (g/cm3) | Type | Density (g/cm3) | ||
| Polypropylene (PP) | 0.83–0.85 | (Andrady, 2015) | Water | 1.00 | (Dikareva and Simon, 2019) |
| Polyethylene (PE) | 0.91–0.94 | (Andrady, 2015) | NaCl | 1.20 | (Wang et al., 2017) |
| Polystyrene (PS) | 1.05 | (Andrady, 2015) | CaCl2 | 1.40 | (Grause et al., 2022) |
| Polyamide or nylon (PA) | 1.13–1.15 | (Sundt et al., 2014) | K2CO3 | 1.54 | (Gohla et al., 2021; Prepilková et al., 2022) |
| Polyurethane (PUR) | 1.20 | (Sundt et al., 2014) | NaBr | 1.55 | (Liu et al., 2019b) |
| Polyethylene terephthalate (PET) | 1.37 | (Andrady, 2015) | ZnBr2 | 1.71 | (Quinn et al., 2017) |
| Polyvinyl chloride (PVC) | 1.38 | (Andrady, 2015) | ZnCl2 | 1.60–1.80 | (Scherer et al., 2020) |
| Polytetrafluorethylene (PTFE) | 2.20 | (Sundt et al., 2014) | NaI | 1.60–1.80 | (Dikareva and Simon, 2019; Zhang and Liu, 2018) |
Fig. 5.
Density solution types used in freshwater sediment and soil studies (When using 2 or 3 solutions, selected from DI water, NaCl, NaI, ZnCl2 is used).
4.3. Organic removal on the surface of MPs
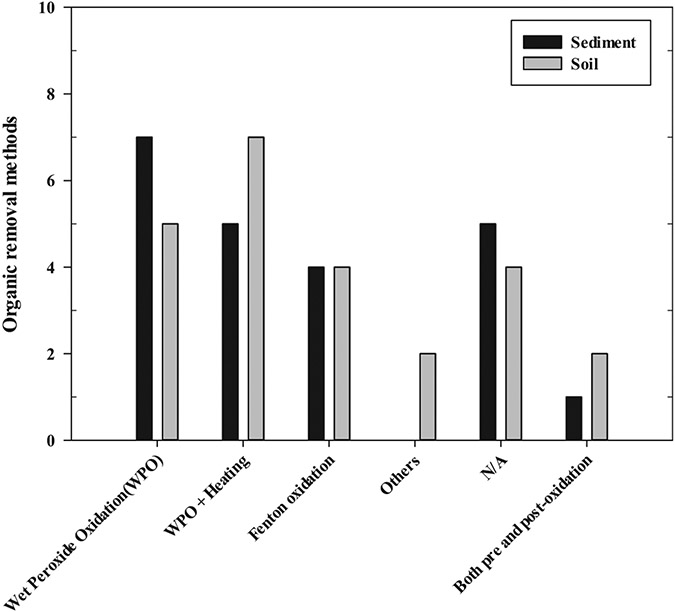
The supernatants or particles collected from the density separation step were passed through a step of organic removal. While most studies applied the organic removal process right after the density separation process, a few studies performed the sample oxidation for organics removal before the density separation. The organic removal methods used in the freshwater sediment and soil samples are shown in Fig. 6. Different oxidants including hydrogen peroxide and Fenton's reagents, have been widely used for organics removal during MPs analysis. For treating freshwater sediment and soil samples, a wet peroxide oxidation process (WPO), employing hydrogen peroxide solution, was mainly used to decompose organics in the collected particles. In freshwater sediment and soil studies, both room temperature and heat-treated WPO could be used. In several studies, H2SO4, NaOH, and butyl alcohol were added when performing WPO and Fenton process in order to improve the efficiency of organic removal (Choi et al., 2021; Li et al., 2019; Liu et al., 2019a; Scherer et al., 2020; Zhang and Liu, 2018). Interestingly, for soil samples, oxidation processes using HNO3 and H2SO4 were also reported (Li et al., 2020; Scheurer and Bigalke, 2018). In most of the literature reviewed, acid digestion with HNO3 or HCl and alkaline digestion with NaOH were barely used for sample pretreatment because these can cause discoloration of MPs (Roch and Brinker, 2017) or damage MPs (Köhn et al., 2017; Qiu et al., 2016), resulting in inaccurate MPs analysis. In a few cases, enzymes were used for organic removal on the surface of MPs. In these cases, removing the different organic matter is difficult because a specific enzyme could only decompose a specific target substance. On the other hand, Fenton's oxidation and H2O2 oxidation were able to efficiently remove organic matter without major problems under appropriate reagent concentrations and temperature conditions. That is why both oxidation processes were widely used due to their convenience of organic removal under different conditions.
Fig. 6.
Organic removal methods in freshwater sediment and soil studies; H2O2 (9.8–11.4 M) used in WPO.
4.4. Filtration
Filtration is considered the final step of the pretreatment procedure of the sample, accomplished using various types of microfilters, mostly in the range of 0.2–20 μm. However, in a few cases, filters with a pore size of 50 μm or larger were also used for the filtration. Filters were selected, depending on the particle sizes of the analyzed MPs. In addition to the pore sizes, different filter materials, including glass fiber, nylon, aluminum oxide, cellulose nitrate, etc., were used. In general, the filter types have no correlations with analytical methods (i.e., microscope, FTIR, and Raman). It may be due to different adsorption patterns of MPs on various filter materials. However, in one study, Mani et al. (Mani et al., 2019) used different filter types, depending on the analytical method. The 0.2 μm anodisc was used for ATR-FTIR analysis (<500 μm of MPs) and mixed cellulose ester filter with 0.45 μm pore size was used for μ-FTIR analysis (500 μm-2 mm of MPs). Similarly, Corradini et al. (Corradini et al., 2021) also used a 2.5 μm cellulose filter for optical microscopy analysis and 0.4 μm polycarbonate membrane for FTIR analysis.
After filtration, the drying process was conducted at 40–60 °C, or room temperature, or even in desiccators below the threshold temperature to avoid MPs deformation, as mentioned in Section 3.2.
5. Analytical instruments and characteristics for MPs detection in samples after pretreatment
After sample pretreatment with different steps, MPs were analyzed in terms of physical properties, types, and abundance in samples.
5.1. Instrument for MP analysis
To monitor MPs in the freshwater sediment and soil samples, a suite of visualization methods have been used including visual sorting with bare eyes, magnifying glass, optical microscope, a fluorescent stereo microscope, and a scanning electron microscope (SEM). To simplify categorizing visualization methods, visual sorting and a magnifying glass were included in the microscope category. Moreover, spectroscopic and thermal analyses were performed using Raman, FTIR, and Pyr-GC–MS.
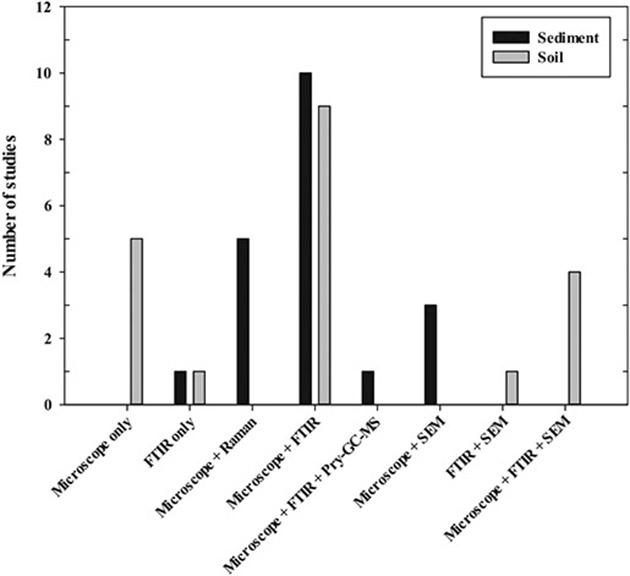
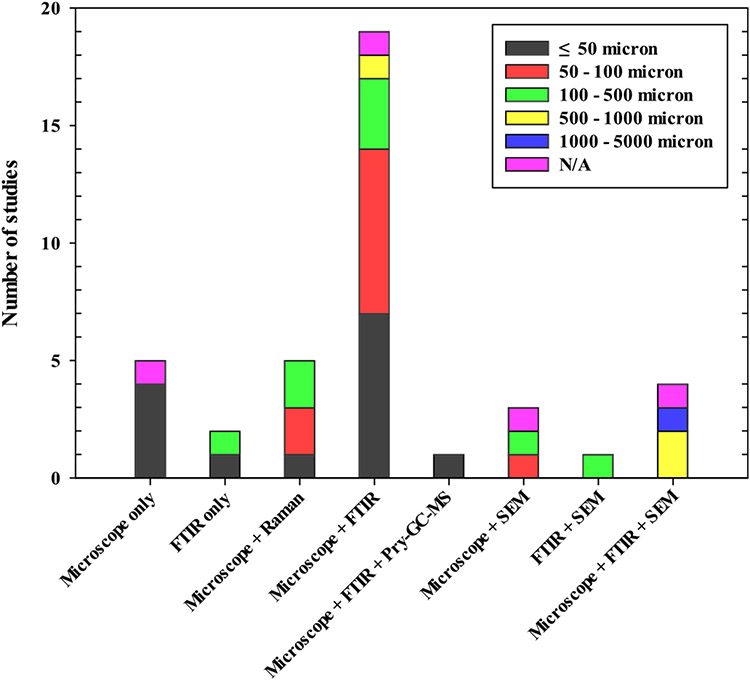
Among the analytical methods, the most commonly used instruments were microscopical techniques (i.e., dissect, polarized, fluorescence, scanning electron, and atomic microscopy), followed by FTIR. Generally, FTIR or an optical microscope was solely used, but, mostly, two or more analytical tools were used together to determine MPs in samples. Interestingly, Schere et al. (Scherer et al., 2020) used two different methods, depending on the sizes of MPs in samples. A microscope was used for MPs of 125–5000 μm and Pyr-GC–MS for 20–125 μm MPs. Detailed information about the instrument and analytical size of MPs for each instrument are summarized in Tables S1 and S2 and Fig. 7. Also, Fig. 8 shows the minimal size of detectable MPs correlated to the selected instrument. In general, MPs with a size of 50 μm or less could be determined with a combination of a microscope and FTIR or Raman. Likewise, MPs with the size of 50–100 μm were most detectable in analyses involving spectroscopy (FTIR and Raman). Moreover, Grause et al. (Grause et al., 2022) were able to detect MPs with a size of at least 10 μm using a fluorescence microscope. However, it is very difficult to use an optical microscope to analyze MPs under 100 μm, given the accuracy and efficiency of the analysis (Shim et al., 2017). Therefore, the instrument must be carefully selected based on the size of MPs for purposes of different studies.
Fig. 7.
Instruments used to analyze MPs.
Fig. 8.
Minimum size of MPs detected using analytical instruments.
5.2. Characterization of MPs
The MPs in freshwater sediment and soil samples were analyzed and characterized in terms of size, shape, color, and type. The shape of MPs was classified into fiber, film, fragment, pellet, and bead. Most MPs were classified as fibers and fragments. In addition, MPs color varied between red, blue, green, black, and even transparent. Color analysis of MPs is mainly possible with a microscope, but there were still many studies that did not investigate the color using this method. The types of MPs can be identified by using spectroscopy or thermal decomposition, such as FTIR, Raman, and Pry-GC–MS. Various types of MPs, including polypropylene (PP), polyethylene (PE), polystyrene (PS), and PVC, were identified with these instruments. In particular, most of the identified MPs consisted of polyolefin, which includes PE and PP. The sizes of MPs were observed in a wide range of 10–5000 μm. However, as mentioned in Section 5.1, because each instrument has different detection limits for size measurement, the instrument must be carefully selected before the analysis. To accurately analyze types of small-sized MPs, a spectroscopy or pyrolysis instrument is recommended.
6. Recovery tests of pretreatment methods
A recovery test is needed to validate the pretreatment method for MPs analysis in environmental samples. Among the reviewed papers, only 50% of the studies carried out the recovery test as seen in Table 6. For the recovery tests, MPs types such as PP, PE, PS, PVC, PET, and poly(methyl methacrylate) (PMMA) were mainly used. The recovery was calculated by comparing the number of spiked MPs in samples before and after pretreatment. More specifically, the fluorescence of MPs was measured when MPs were fluorescently tagged. A recovery for 63–150 μm sized PET was 81% (Grause et al., 2022) and 55% for 62–125 μm sized PMMA (Mani et al., 2019). In most studies, when larger-sized MPs were used, a higher recovery rate was obtained. When an insufficient amount of fluorescence was tagged on MPs, it was very challenging to determine the exact recovery rate. Unfortunately, most of the studies did not specify the shape of MPs used for the recovery tests. However, in the studies that performed recovery tests based on the type and shape of MPs, fragments of low density polyethylene (LDPE) showed high recovery rates of up to 98% and fiber of PVC up to 90% (Corradini et al., 2019). In addition, the recovery rates according to the shape (fiber, film, and particle) of PE was measured to be 85–98.3% in the order of film<fiber<particle when NaBr solution was used for density separation (Liu et al., 2019b). In addition, Blair et al. (Blair et al., 2019) performed recovery tests using secondary MPs and obtained recovery rates of 49–58%.
Table 6.
Recovery test of pretreatment methods in freshwater sediment and soil studies.
| No. | Matrix | Recovery test method | Type and size of MPs | Recovery rate | Ref. |
|---|---|---|---|---|---|
| 1 | Sediments | Spike 10 of each size range for all types of MPs Repeat in duplicate |
PE, PS, PP, PVC (<1 mm, 1–2 mm, 2–5 mm) | 80, 90, 100% (PE, PP; each size group), 70, 90, 100% (PS; each size group), 60, 80, 90% (PVC; each size group) | (Di and Wang, 2018) |
| 2 | Spike 300 MPs into 200 g of the muffled sand Filter using 10 mm stainless steel filter Count MPs using a stereomicroscope Repeat in triplicate |
Red PS (beads) (100 μm) | 66 ± 5.6% | (Liu et al., 2019a) | |
| 3 | Spike 10 beads or 15 secondary MPs into 20 g of dried sediment Repeat in triplicate for each MPs type | Beads: PE (0.71–0.85 mm), PP (2.45 mm), PS (4.4 mm) Secondary MPs: Nylon toothbrush bristles, PP cleaning brush bristles, rope fragments and PE mesh fruit packaging fragments |
100% (microbead) 49 ± 10.2–58 ± 7.7% (secondary MPs) |
(Blair et al., 2019) | |
| 4 | Assess adapted ZnCl2 protocol in a pilot spike-recovery test Spike 40 (62–250 μm) and 20 (250–700 μm) MPs into 60 g of sediment Repeat in triplicate |
PMMA fragments (1.18 g/cm3) (62–125 μm, 125–250 μm, 250–500 μm, 500–700 μm) | ± 8.7% (62–125 μm), 80.8 ± 6.3% (125–250 μm), ± 5.0% (250–500 μm), ± 0.0% (500–700 μm) | (Mani et al., 2019) | |
| 5 | Spike 40–80 MPs into 12.3 g of dried sediment Filter using 0.7 μm GF/F and dry at 40 °C overnight |
Acrylic, nylon, polyester, PE fibers | 70% | (Simon-Sánchez et al., 2019) | |
| 6 | Spike 125 MPs into 1500 g of quartz sand | PE, PP, PS, PMMA, PVC (25 particles each, sized 125–1000 μm) | 87.2 ± 4.5% | (Scherer et al., 2020) | |
| 7 | Spike 10 MPs into 200 g of elutriated soil and sediment Repeat five times |
PE, PP, PVC, PET, PS, expandable polystyrene (EPS) (<1 mm) | >90% | (Han et al., 2019b) | |
| 8 | Soils | Stain MPs with Nile Red in acetone (500 μg/mL) for 10 min Spike 20 MPs into 50 g of clean soil Repeat in triplicate |
PP, PE, PA, PET, PVC, PC, acrylonitrile butadiene styrene (ABS), PMMA, PS (1–5 mm) | 90.0 ± 10.0% (PP, PA), ± 0.0% (PE, PC, PMMA), 95.0 ± 5.0% (ABS), 96.7 ± 2.9% (PS), 0.0 ± 0.0% (PET, PVC due to the high density) | (Liu et al., 2018) |
| 9 | Use 27% NaCl (1.2 g/cm3) for small MPs Spike 10 MPs into 50 g of pure plastic free sand Test four different ways of density separation: 1. Sedimentation cylinder method, settle for 48 h 2. Self-constructed MP separator with additional air bubbling 3. Stir for 10 min and centrifuge for 30 min at 3450 G 4. Identical with third but with rubber disc inserted after centrifugation Removal of organic matter in a short time using HNO3 |
PP (0.5–1 mm) | 93% (3), 97% (1,2), 98% (4) | (Scheurer and Bigalke, 2018) | |
| 10 | Spike MPs into cleaned sands | PP, PE (200 μm-5 mm) | 97% | (Zhou et al., 2018) | |
| 11 | Spike 10 MPs into 50 g of soil Pre-digestion (30% H2O2), density separation and filtration |
PE, PP, PS, PA, ABS, PET (<2 mm) | NaI: 97.78 ± 1.57% NaCl: 80.56 ± 2.08% (except PET) ZnCl2: 97.22 ± 2.08% |
(Li et al., 2019) | |
| 12 | Spike each type of MPs into 5 g of soil sample Repeat in triplicate |
Fiber: Acrylic (length: 2.7 ± 1.4 mm) (width: 0.04 ± 0.01 mm) (area: 0.12 ± 0.06 mm2) Polyester (length: 1.6 ± 1.1 mm) (width: 0.04 ± 0.01 mm) (area: 0.07 ± 0.06 mm2) Nylon (length: 2.3 ± 0.8 mm) (width: 0.05 ± 0.01 mm) (area: 0.98 ± 0.37 mm2) LDPE (0.16 ± 0.1 mm2) PVC (0.10 ± 0.08 mm2) |
98% (LDPE), 90% (Polyester), 88% (PVC), 77% (Nylon), 49% (Acrylic) | (Corradini et al., 2019) | |
| 13 | Stain MPs with Nile Red in acetone for 10 min except for PMMA, PS and ABS Calculate each recovery rate using three density solutions (NaCl, NaBr or CaCl2) |
Type: PE, PET, POM, PVC, PC, ABS, PMMA, PS (shredding/grinding 3 mm bead and passed through 7–160 mesh) Size: PE (100–500 μm, 500–1000 μm, 1000–3000 μm) Shape: PE (particle, fiber, film) (100–500 μm) |
>90% (All type of MPs) 75.0–96.7% (100–500 μm PE) 100% (>1 mm PE) 65–98.3% (Three shape-different PE; of them, NaBr: 85–98.3%) |
(Liu et al., 2019b) | |
| 14 | Spike 20 films or particles into 20 g of clean soil | LDPE film (1 × 1 mm) LDPE particle (250 μm) |
100% (film), 98% (particle) | (Huang et al., 2020) | |
| 15 | Spike 30 mg of MPs into 200 g of clean soil sample Stir for 20 min at 25 °C Weigh by electronic balance |
PE (>2 mm, 0.9–2 mm, 0.28–0.9 mm, 0.15–0.28 mm) | 96% (>2 mm) 85% (0.9–2 mm) 87% (0.28–0.9 mm) 84% (0.15–0.28 mm) |
(Li et al., 2020) | |
| 16 | Spike 30 MPs into 50 g of clean soil Count by stereomicroscope |
PVC, PE, PP, PS, Polyamide (500 μm-2 mm) | 75.9–112.4% | (Zhou et al., 2020) | |
| 17 | Spike each 10 of six type MPs with two size range | PE (sheet), PET, PP, PS (fragment), PVC, nylon (fiber) (300–500 μm, 700–1000 μm) | 97.8 ± 4.8% | (Kim et al., 2021) | |
| 18 | Spike MPs into clean soil | High density polyethylene (HDPE), PP, LDPE, PS, PP fibers (1.2–1.6 mm) | 85% (HDPE), 90% (PP), 85% (LDPE), 100% (PS) | (Ragoobur et al., 2021) | |
| 19 | Soil sample was heated at 500 °C to remove MPs and organic materials Spike 100 mg of MPs into 10 g of soil and centrifuge at a speed of 3700 min−1 for 10 min |
LDPE, PP, PS, flexible PVC (63–1000 μm) (recovery test) PET (63–150 μm, 150–212 μm, 212–500 μm, 500–1000 μm, 63–1000 μm) (Tests to identify how size affects MPs recovery) |
97–98% (LDPE, PP, PS, fPVC) 81% (PET; 63–150 μm), 94.0 ± 2.1% (PET; 500–1000 μm), 94.9 ± 2.4% (PET; 63–1000 μm) |
(Grause et al., 2022) |
7. Conclusions and future perspective
This paper thoroughly reviewed recent articles published from 2017 to 2022 about the pretreatment methods for MP analysis in freshwater sediment and soil samples. As known, soil and sediment sample pretreatment is very difficult due to the complicated compositions of samples. For sample processing, sieving is required to remove large sized impurities and MPs separation in sizes. Drying was essential to determine accurate sample amounts for MPs analysis without any MPs damages. The quantity of sample used for pretreatment was mostly <100 g after drying. Density separation and organic removal are the main processes of pretreatment and in general, density separation was performed before organic removal because the required amounts of chemicals for the pretreatment were significantly reduced. As density separation solutions, NaCl, ZnCl2, and NaI are widely used, and their selection is dependent on the polymer types analyzed in different studies. Moreover, advantages and disadvantages of different density solutions must be considered to choose the best reagent. In a few cases, acids, alkalies, and enzymes could be used to remove organic matter. However, they may damage MPs, and in particular, an enzyme has its own specific target organic and thus, its use is very limited along with its expensiveness. H2O2 oxidation or Fenton reactions are the most effective organic removal methods. If MPs size distribution must be investigated, sample filtration using sieves with different pore sizes could be used. In addition, sample pretreatment could be dependent on different analytical equipment. Also, sample pretreatment could significantly influence the results of MP analysis. Therefore, we emphasize the importance of sample pretreatment of soil and sediment.
Unfortunately, so far, there is no standardized pretreatment method for MPs monitoring in soil and sediment and it is still challenging to suggest a representative method for sample pretreatment due to varying factors, parameters and methods that are study specific. Moreover, the order of pretreatment steps could be effectively changed, depending on the sizes and types of MPs for purposes of different studies. To develop an appropriate pretreatment method, it is vital to establish a standard or representative method by referring to optimal conditions tested in multiple studies. Finally, investigations on pretreatment methods for various environmental media (e.g., air, water, and wastes) with different characteristics should be expanded for further determination of MPs in the environment.
Supplementary Material
HIGHLIGHTS.
Soil & sediment sample pretreatment is a significant process for MP analysis.
A standardized method is still not available for soil & sediment sample pretreatment.
Current pretreatment methods were carefully reviewed for soil & sediment samples.
A dominant procedure for sample pretreatment in current studies was determined.
However, a standardized pretreatment method is highly required in near future.
Acknowledgement
CH acknowledges the support by Korea Environmental Industry & Technology Institute (KEITI) through Measurement and Risk assessment Program for Management of Microplastics Project, funded by Korea Ministry of Environment (MOE) (grant number 2020003110005), the BK21 Four Program (Convergence Program for Full Cycle Control of Microplastics) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C1093183).
Footnotes
CRediT authorship contribution statement
Conceptualization: H. Lee, C. Han.
Data acquisition & curation: H. Lee, S. Kim, A. Sin, G. Kim.
Writing-original draft: H. Lee, S. Kim, A. Sin, G. Kim, C. Han.
Writing-review & editing & validation: S. Khan, M. Nadagouda, E. Sahle-Demessie, C. Han.
Supervision and Funding acquisition: C. Han.
Disclaimer
The U.S. Environmental Protection Agency reviewed and approved the work described here for publication. Note that approval does not signify that the contents necessarily reflect the views of the Agency. Mention of trade names, products, or services does not convey official EPA approval, endorsement, or recommendation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2023.161718.
Data availability
No data was used for the research described in the article.
References
- Adomat Y, Grischek T, 2021. Sampling and processing methods of microplastics in river sediments-a review. Sci. Total Environ 758, 143691. [DOI] [PubMed] [Google Scholar]
- Alam FC, Sembiring E, Muntalif BS, Suendo V, 2019. Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia). Chemosphere 224, 637–645. [DOI] [PubMed] [Google Scholar]
- Andrady AL, 2015. Persistence of plastic litter in the oceans. Marine Anthropogenic Litter. Springer, Cham, pp. 57–72. [Google Scholar]
- Andrady AL, 2017. The plastic in microplastics: a review. Mar. Pollut. Bull 119, 12–22. [DOI] [PubMed] [Google Scholar]
- Barboza LGA, Vethaak AD, Lavorante BR, Lundebye A-K, Guilhermino L, 2018. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar. Pollut. Bull 133, 336–348. [DOI] [PubMed] [Google Scholar]
- Besley A, Vijver MG, Behrens P, Bosker T, 2017. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull 114, 77–83. [DOI] [PubMed] [Google Scholar]
- Blair RM, Waldron S, Phoenix VR, Gauchotte-Lindsay C, 2019. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland,UK. Environ. Sci. Pollut. Res 26, 12491–12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YR, Kim Y-N, Yoon J-H, Dickinson N, Kim K-H, 2021. Plastic contamination of forest, urban, and agricultural soils: a case study of Yeoju City in the Republic of Korea. J. Soils Sediments 21, 1962–1973. [Google Scholar]
- Cole M, Lindeque P, Halsband C, Galloway TS, 2011. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull 62, 2588–2597. [DOI] [PubMed] [Google Scholar]
- Coppock RL, Cole M, Lindeque PK, Queirös AM, Galloway TS, 2017. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut 230, 829–837. [DOI] [PubMed] [Google Scholar]
- Corradini F, Meza P, Eguiluz R, Casado F, Huerta-Lwanga E, Geissen V, 2019. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ 671, 411–420. [DOI] [PubMed] [Google Scholar]
- Corradini F, Casado F, Leiva V, Huerta-Lwanga E, Geissen V, 2021. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ 752, 141917. [DOI] [PubMed] [Google Scholar]
- Di M, Wang J, 2018. Microplastics in surface waters and sediments of the Three Gorges Reservoir,China. Sci. Total Environ 616, 1620–1627. [DOI] [PubMed] [Google Scholar]
- Dikareva N, Simon KS, 2019. Microplastic pollution in streams spanning an urbanisation gradient. Environ. Pollut 250, 292–299. [DOI] [PubMed] [Google Scholar]
- Ding L, Fan Mao R, Guo X, Yang X, Zhang Q, Yang C, 2019. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ 667, 427–434. [DOI] [PubMed] [Google Scholar]
- Duan Z, Zhao S, Zhao L, Duan X, Xie S, Zhang H, et al. , 2020. Microplastics in Yellow River Delta wetland: occurrence, characteristics, human influences, and marker. Environ. Pollut 258, 113232. [DOI] [PubMed] [Google Scholar]
- Eo S, Hong SH, Song YK, Han GM, Shim WJ, 2019. Spatiotemporal distribution and annual load of microplastics in the Nakdong River,South Korea. Water Res. 160, 228–237. [DOI] [PubMed] [Google Scholar]
- Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, et al. , 2014. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PloS one 9, e111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS, 2007. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ.Sci. Technol 41, 8484–8490. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Canesi L, Auguste M, Wathsala RH, Fabbri E, 2019. Microplastic exposure and effects in aquatic organisms: a physiological perspective. Environ. Toxicol. Pharmacol 68, 37–51. [DOI] [PubMed] [Google Scholar]
- Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, et al. , 2018. Microplastics in air: are we breathing it in? Curr.Opin.Environ.Sci.Health 1, 1–5. [Google Scholar]
- Gohla J, Bračun S, Gretschel G, Koblmüller S, Wagner M, Pacher C, 2021. Potassium carbonate (K2CO3)–a cheap, non-toxic and high-density floating solution for microplastic isolation from beach sediments. Mar. Pollut. Bull 170, 112618. [DOI] [PubMed] [Google Scholar]
- Grause G, Kuniyasu Y, Chien M-F, Inoue C, 2022. Separation of microplastic from soil by centrifugation and its application to agricultural soil. Chemosphere 288, 132654. [DOI] [PubMed] [Google Scholar]
- Guzzetti E, Sureda A, Tejada S, Faggio C, 2018. Microplastic in marine organism: environmental and toxicological effects. Environ. Toxicol. Pharmacol 64, 164–171. [DOI] [PubMed] [Google Scholar]
- Hale RC, Seeley ME, La Guardia MJ, Mai L, Zeng EY, 2020. A global perspective on microplastics. J.Geophys.Res.Oceans 125, e2018JC014719. [Google Scholar]
- Han C, Sahle-Demessie E, Zhao AQ, Wang J, 2018a. Environmental aging and degradation of multiwalled carbon nanotube reinforced polypropylene. Carbon 129, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Zhao A, Varughese E, Sahle-Demessie E, 2018b. Evaluating weathering of food packaging polyethylene-nano-clay composites: release of nanoparticles and their impacts. NanoImpact 9, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Sahle-Demessie E, Varughese E, Shi H, 2019a. Polypropylene–MWCNT composite degradation, and release, detection and toxicity of MWCNTs during accelerated environmental aging. Environ.Sci.: Nano 6, 1876–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Lu X, Vogt RD, 2019b. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut 254, 113009. [DOI] [PubMed] [Google Scholar]
- Harms IK, Diekötter T, Troegel S, Lenz M, 2021. Amount, distribution and composition of large microplastics in typical agricultural soils in Northern Germany. Sci. Total Environ 758, 143615. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M, 2012. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci.Technol 46, 3060–3075. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu Q, Jia W, Yan C, Wang J, 2020. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut 260, 114096. [DOI] [PubMed] [Google Scholar]
- Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C, 2012. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 10, 524–537. [Google Scholar]
- Ivleva NP, Wiesheu AC, Niessner R, 2017. Microplastic in aquatic ecosystems. Angew. Chem. Int. Ed 56, 1720–1739. [DOI] [PubMed] [Google Scholar]
- Jiang C, Yin L, Wen X, Du C, Wu L, Long Y, et al. , 2018. Microplastics in sediment and surface water of West Dongting Lake and South Dongting Lake: abundance, source and composition. Int. J. Environ. Res. Public Health 15, 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbalaei S, Hanachi P, Walker TR, Cole M, 2018. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res 25, 36046–36063. [DOI] [PubMed] [Google Scholar]
- Katsumi N, Kusube T, Nagao S, Okochi H, 2021. Accumulation of microcapsules derived from coated fertilizer in paddy fields. Chemosphere 267, 129185. [DOI] [PubMed] [Google Scholar]
- Kim S-K, Kim J-S, Lee H, Lee H-J, 2021. Abundance and characteristics of microplastics in soils with different agricultural practices: importance of sources with internal origin and environmental fate. J. Hazard. Mater 403, 123997. [DOI] [PubMed] [Google Scholar]
- Kühn S, Van Werven B, Van Oyen A, Meijboom A, Rebolledo ELB, Van Franeker JA, 2017. The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar. Pollut. Bull 115, 86–90. [DOI] [PubMed] [Google Scholar]
- Li J, Liu H, Chen JP, 2018. Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 137, 362–374. [DOI] [PubMed] [Google Scholar]
- Li Q, Wu J, Zhao X, Gu X, Ji R, 2019. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut 254, 113076. [DOI] [PubMed] [Google Scholar]
- Li W, Wufuer R, Duo J, Wang S, Luo Y, Zhang D, et al. , 2020. Microplastics in agricultural soils: extraction and characterization after different periods of polythene film mulching in an arid region. Sci. Total Environ 749, 141420. [DOI] [PubMed] [Google Scholar]
- Lithner D, Larsson Å, Dave G, 2011. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ 409, 3309–3324. [DOI] [PubMed] [Google Scholar]
- Liu M, Lu S, Song Y, Lei L, Hu J, Lv W, et al. , 2018. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai,China. Environ. Pollut 242, 855–862. [DOI] [PubMed] [Google Scholar]
- Liu F, Vianello A, Vollertsen J, 2019a. Retention of microplastics in sediments of urban and highway stormwater retention ponds. Environ. Pollut 255, 113335. [DOI] [PubMed] [Google Scholar]
- Liu M, Song Y, Lu S, Qiu R, Hu J, Li X, et al. , 2019b. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ 691, 341–347. [DOI] [PubMed] [Google Scholar]
- Liu L, Xu M, Ye Y, Zhang B, 2022. On the degradation of (micro) plastics: degradation methods, influencing factors, environmental impacts. Sci. Total Environ 806, 151312. [DOI] [PubMed] [Google Scholar]
- Luo W, Su L, Craig NJ, Du F, Wu C, Shi H, 2019. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ. Pollut 246, 174–182. [DOI] [PubMed] [Google Scholar]
- Lv W, Zhou W, Lu S, Huang W, Yuan Q, Tian M, et al. , 2019. Microplastic pollution in rice-fish co-culture system: a report of three farmland stations in Shanghai,China. Sci. Total Environ 652, 1209–1218. [DOI] [PubMed] [Google Scholar]
- Mani T, Primpke S, Lorenz C, Gerdts G, Burkhardt-Holm P, 2019. Microplastic pollution in benthic midstream sediments of the Rhine River. Environ.Sci.Technol 53, 6053–6062. [DOI] [PubMed] [Google Scholar]
- Matsuguma Y, Takada H, Kumata H, Kanke H, Sakurai S, Suzuki T, et al. , 2017. Microplastics in sediment cores from Asia and Africa as indicators of temporal trends in plastic pollution. Arch. Environ. Contam. Toxicol 73, 230–239. [DOI] [PubMed] [Google Scholar]
- Peng G, Zhu B, Yang D, Su L, Shi H, Li D, 2017. Microplastics in sediments of the Changjiang Estuary,China. Environ. Pollut 225, 283–290. [DOI] [PubMed] [Google Scholar]
- Peng G, Xu P, Zhu B, Bai M, Li D, 2018. Microplastics in freshwater river sediments in Shanghai, China: a case study of risk assessment in mega-cities. Environ. Pollut 234, 448–456. [DOI] [PubMed] [Google Scholar]
- Pérez-Reverón R, González-Sálamo J, Hernández-Sánchez C, González-Pleiter M, Hernández-Borges J, Díaz-Peña FJ, 2022. Recycled wastewater as a potential source of microplastics in irrigated soils from an arid-insular territory (Fuerteventura, Spain). Sci. Total Environ 817, 152830. [DOI] [PubMed] [Google Scholar]
- Plastics Europe &, EPRO, 2021. Plastics–the facts 2021. PLASTICS EUROPE; (accessed 16 December 2022) https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/. [Google Scholar]
- Prata JC, da Costa JP, Duarte AC, Rocha-Santos T, 2019. Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC Trends Anal. Chem 110, 150–159. [Google Scholar]
- Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T, 2020. Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ 702, 134455. [DOI] [PubMed] [Google Scholar]
- Prata JC, da Costa JP, Lopes I, Andrady AL, Duarte AC, Rocha-Santos T, 2021. A one health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ 777, 146094. [DOI] [PubMed] [Google Scholar]
- Prepilková V, Poništ J, Schwarz M, Bednárová D, 2022. Selection of suitable methods for the detection of microplastics in the environment. J. Anal. Chem 77, 830–843. [Google Scholar]
- Qiu Q, Tan Z, Wang J, Peng J, Li M, Zhan Z, 2016. Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuar. Coast. Shelf Sci 176, 102–109. [Google Scholar]
- Quinn B, Murphy F, Ewins C, 2017. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 9, 1491–1498. [Google Scholar]
- Rafique A, Irfan M, Mumtaz M, Qadir A, 2020. Spatial distribution of microplastics in soil with context to human activities: a case study from the urban center. Environ. Monit. Assess 192, 1–13. [DOI] [PubMed] [Google Scholar]
- Ragoobur D, Huerta-Lwanga E, Somaroo GD, 2021. Microplastics in agricultural soils, wastewater effluents and sewage sludge in Mauritius. Sci. Total Environ 798, 149326. [DOI] [PubMed] [Google Scholar]
- Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, et al. , 2021. Plasticenta: first evidence of microplastics in human placenta. Environ. Int 146, 106274. [DOI] [PubMed] [Google Scholar]
- Rillig MC, 2012. Microplastic in Terrestrial Ecosystems and the Soil? ACS Publications; [DOI] [PubMed] [Google Scholar]
- Rillig MC, Lehmann A, 2020. Microplastic in terrestrial ecosystems. Science 368, 1430–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig MC, Ziersch L, Hempel S, 2017. Microplastic transport in soil by earthworms. Sci. Rep 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig MC, Lehmann A, de Souza Machado AA, Yang G, 2019. Microplastic effects on plants. New Phytol. 223, 1066–1070. [DOI] [PubMed] [Google Scholar]
- Rillig MC, Kim SW, Kim T-Y, Waldman WR, 2021. The global plastic toxicity debt. Environ.Sci.Technol 55, 2717–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch S, Brinker A, 2017. Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ.Sci.Technol 51, 4522–4530. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Abrantes N, Gonçalves F, Nogueira H, Marques J, Gonçalves A, 2018. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ 633, 1549–1559. [DOI] [PubMed] [Google Scholar]
- Sarkar DJ, Sarkar SD, Das BK, Manna RK, Behera BK, Samanta S, 2019. Spatial distribution of meso and microplastics in the sediments of river Ganga at eastern India. Sci. Total Environ 694, 133712. [DOI] [PubMed] [Google Scholar]
- Scherer C, Weber A, Stock F, Vurusic S, Egerci H, Kochleus C, et al. , 2020. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total Environ 738, 139866. [DOI] [PubMed] [Google Scholar]
- Scheurer M, Bigalke M, 2018. Microplastics in swiss floodplain soils. Environ.Sci.Technol 52, 3591–3598. [DOI] [PubMed] [Google Scholar]
- Shim WJ, Hong SH, Eo SE, 2017. Identification methods in microplastic analysis: a review. Anal. Methods 9, 1384–1391. [Google Scholar]
- Shruti V, Jonathan M, Rodriguez-Espinosa P, Rodríguez-González F, 2019. Microplastics in freshwater sediments of atoyac river basin, Puebla city,Mexico. Sci. Total Environ 654, 154–163. [DOI] [PubMed] [Google Scholar]
- Simon-Sánchez L, Grelaud M, Garcia-Orellana J, Ziveri P, 2019. River deltas as hotspots of microplastic accumulation: the case study of the Ebro River (NW Mediterranean). Sci. Total Environ 687, 1186–1196. [DOI] [PubMed] [Google Scholar]
- Statista, 2021. Plastic waste in the United States - statistics & facts. Statista; (accessed 16 December 2022) https://www.statista.com/topics/5127/plastic-waste-in-the-united-states/. [Google Scholar]
- Stock F, Kochleus C, Bänsch-Baltruschat B, Brennholt N, Reifferscheid G, 2019. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment–a review. TrAC Trends Anal. Chem 113, 84–92. [Google Scholar]
- Sundt P, Schulze P-E, Syversen F, 2014. Sources of Microplastic-Pollution to the Marine Environment. 86. Mepex for the Norwegian Environment Agency; , p. 20 (accessed 16 December 2022) https://d3n8a8pro7vhmx.cloudfront.net/boomerangalliance/pages/507/attachments/original/1481155578/Norway_Sources_of_Microplastic_Pollution.pdf?1481155578. [Google Scholar]
- Uddin S, Fowler SW, Uddin MF, Behbehani M, Naji A, 2021. A review of microplastic distribution in sediment profiles. Mar. Pollut. Bull 163, 111973. [DOI] [PubMed] [Google Scholar]
- Verschoor A, 2015. Towards a definition of microplastics: considerations for the specification of physico-chemical properties. https://www.rivm.nl/bibliotheek/rapporten/2015-0116.pdf (accessed 16 December 2022). [Google Scholar]
- Wang J, Peng J, Tan Z, Gao Y, Zhan Z, Chen Q, et al. , 2017. Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere 171, 248–258. [DOI] [PubMed] [Google Scholar]
- Wright SL, Kelly FJ, 2017. Plastic and human health: a micro issue? Environ.Sci.Technol 51, 6634–6647. [DOI] [PubMed] [Google Scholar]
- Xiong X, Zhang K, Chen X, Shi H, Luo Z, Wu C, 2018. Sources and distribution of microplastics in China's largest inland lake–Qinghai Lake. Environ. Pollut 235, 899–906. [DOI] [PubMed] [Google Scholar]
- Yuan W, Liu X, Wang W, Di M, Wang J, 2019. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake,China. Ecotoxicol. Environ. Saf 170, 180–187. [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu Y, 2018. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ 642, 12–20. [DOI] [PubMed] [Google Scholar]
- Zhang D, Fraser MA, Huang W, Ge C, Wang Y, Zhang C, et al. , 2021. Microplastic pollution in water, sediment, and specific tissues of crayfish (Procambarus clarkii) within two different breeding modes in Jianli, Hubei province,China. Environ. Pollut 272, 115939. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhang H, Fu C, Zhou Y, Dai Z, Li Y, et al. , 2018. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 322, 201–208. [Google Scholar]
- Zhou B, Wang J, Zhang H, Shi H, Fei Y, Huang S, et al. , 2020. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: multiple sources other than plastic mulching film. J. Hazard. Mater 388, 121814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.