Key Points
Question
Is prophylaxis with letermovir noninferior to valganiclovir for cytomegalovirus (CMV) disease prevention in high-risk CMV-seronegative kidney transplant recipients who receive an organ from a CMV-seropositive donor?
Findings
In this randomized, double-masked trial of CMV-seronegative adults who received an organ from a CMV-seropositive kidney transplant donor (efficacy population; n = 586), letermovir was noninferior to valganciclovir (each given for up to 200 days after transplant) for prevention of CMV disease through 52 weeks after transplant (10.4% vs 11.8%) and had a lower rate of leukopenia or neutropenia (safety population; n = 589).
Meaning
Letermovir was noninferior to valganciclovir for prophylaxis of CMV disease in adult CMV-seronegative kidney transplant recipients who received an organ from a CMV-seropositive donor, with lower rates of leukopenia or neutropenia, supporting its use for this indication.
Abstract
Importance
Valganciclovir for 200 days is standard care for cytomegalovirus (CMV) prophylaxis in high-risk CMV-seronegative kidney transplant recipients who receive an organ from a CMV-seropositive donor, but its use is limited by myelosuppression.
Objective
To compare the efficacy and safety of letermovir with valganciclovir for prevention of CMV disease in CMV-seronegative kidney transplant recipients who receive an organ from a CMV-seropositive donor.
Design, Setting, and Participants
Randomized, double-masked, double-dummy, noninferiority, phase 3 trial in adult CMV-seronegative kidney transplant recipients who received an organ from a CMV-seropositive donor at 94 participating sites between May 2018 and April 2021 (final follow-up in April 2022).
Interventions
Participants were randomized in a 1:1 ratio (stratified by receipt of lymphocyte-depleting induction immunosuppression) to receive letermovir, 480 mg, orally daily (with acyclovir) or valganciclovir, 900 mg, orally daily (adjusted for kidney function) for up to 200 days after transplant, with matching placebos.
Main Outcomes and Measures
The primary outcome was CMV disease, confirmed by an independent masked adjudication committee, through posttransplant week 52 (prespecified noninferiority margin, 10%). CMV disease through week 28 and time to onset of CMV disease through week 52 were secondary outcomes. Exploratory outcomes included quantifiable CMV DNAemia and resistance. The rate of leukopenia or neutropenia through week 28 was a prespecified safety outcome.
Results
Among 601 participants randomized, 589 received at least 1 dose of the study drug (mean age, 49.6 years; 422 [71.6%] men). Letermovir (n = 289) was noninferior to valganciclovir (n = 297) for prevention of CMV disease through week 52 (10.4% vs 11.8% of participants with committee-confirmed CMV disease; stratum-adjusted difference −1.4% [95% CI, −6.5% to 3.8%]). No participants who received letermovir vs 5 participants (1.7%) who received valganciclovir developed CMV disease through week 28. Time to onset of CMV disease was comparable between the groups (hazard ratio, 0.90 [95% CI, 0.56-1.47]). Quantifiable CMV DNAemia was detected in 2.1% of participants in the letermovir group vs 8.8% in the valganciclovir group by week 28. Of participants evaluated for suspected CMV disease or CMV DNAemia, none (0/52) who received letermovir and 12.1% (8/66) who received valganciclovir had resistance-associated substitutions. The rate of leukopenia or neutropenia through week 28 was lower with letermovir vs valganciclovir (26% vs 64%; difference, −37.9% [95% CI, −45.1% to −30.3%]; P < .001). Fewer participants in the letermovir group than the valganciclovir group discontinued prophylaxis due to adverse events (4.1% vs 13.5%) or drug-related adverse events (2.7% vs 8.8%).
Conclusion and Relevance
Among adult CMV-seronegative kidney transplant recipients who received an organ from a CMV-seropositive donor, letermovir was noninferior to valganciclovir for prophylaxis of CMV disease over 52 weeks, with lower rates of leukopenia or neutropenia, supporting its use for this indication.
Trial Registration
ClinicalTrials.gov Identifier: NCT03443869; EudraCT: 2017-001055-30
This randomized clinical trial compares the efficacy and safety of letermovir vs valganciclovir prophylaxis for cytomegalovirus (CMV) disease in CMV-seronegative kidney transplant recipients receiving an organ from a CMV-seropositive donor.
Introduction
Cytomegalovirus (CMV) disease is a major cause of morbidity and mortality among kidney transplant recipients. The incidence is highest in the subgroup of CMV-seronegative kidney transplant recipients who receive an organ from a CMV-seropositive donor, who comprise approximately 20% of all kidney transplant recipients.1,2,3 Oral valganciclovir, 900 mg, daily for 200 days after transplant is the current standard of care for prophylaxis of CMV disease among CMV-seronegative kidney transplant recipients who receive an organ from a CMV-seropositive donor.1,2,4,5,6 Valganciclovir is an inhibitor of CMV DNA polymerase.6,7 It commonly causes myelosuppression, especially leukopenia and neutropenia, which can lead to discontinuation/interruption of CMV prophylaxis, dose reduction of immunosuppressants, and/or use of granulocyte colony–stimulating factor(s) (G-CSF).6,7,8,9 Valganciclovir requires dose adjustments due to fluctuating kidney function after kidney transplant.6,10 Additionally, valganciclovir (ganciclovir)-resistant CMV has been detected in individuals with prolonged exposure to or subtherapeutic concentrations of valganciclovir (ganciclovir).6,7,11,12,13,14
Letermovir is an antiviral active against CMV without associated myelotoxicity, does not require dose adjustment for kidney impairment, has a unique mechanism of action as an inhibitor of the CMV DNA terminase complex, and is not associated with cross-resistance to other anti-CMV agents. However, unlike valganciclovir, letermovir has potential drug interactions (moderate cytochrome P3450 3A inhibitor, substrate for organic anion transporting polypeptide 1B1/3) and does not have activity against herpes simplex virus (HSV) or varicella zoster virus (VZV).15,16,17,18 Letermovir is approved (by the US Food and Drug Administration and European Medicines Agency) for prophylaxis of CMV infection and disease in adult CMV-seropositive recipients of an allogeneic hematopoietic stem cell transplant (HSCT).17,18 Letermovir resistance is rare when used for prophylaxis and has mostly been reported in association with off-label use for treatment.19,20,21,22 Based on the data in allogenic HSCT recipients, it was hypothesized that letermovir would be noninferior to valganciclovir for CMV disease prevention, with lower myelotoxicity, in kidney transplant recipients. This trial compared letermovir vs valganciclovir prophylaxis in adult CMV-seronegative kidney transplant recipients who received an organ from a CMV-seropositive donor, with the goals of assessing prevention of CMV disease, CMV DNAemia, antiviral resistance, and the incidence rate of leukopenia and neutropenia.
Methods
Trial Design and Oversight
This phase 3, randomized, active-controlled, double-masked, double-dummy, noninferiority trial (protocol MK-8228-002) evaluated the efficacy and safety of letermovir compared with valganciclovir for CMV disease prophylaxis among CMV-seronegative kidney transplant recipients receiving an organ from a CMV-seropositive donor. The trial was conducted in accordance with the standards of Good Clinical Practice (International Conference on Harmonization Guideline) and all applicable laws, rules, and regulations relating to the conduct of clinical trials. Race and ethnicity data were self-reported by participants. These data were collected to adequately describe the study population. The study protocol and amendments were approved by the appropriate institutional review boards and regulatory agencies at all sites. All participants provided written informed consent.
Trial Participants
Documented CMV-seronegative adults (within 180 days prior to randomization) 18 years or older who received a kidney transplant from a CMV-seropositive donor were eligible for inclusion in the trial. Participants who received a previous HSCT or solid organ, multiorgan, or double kidney transplant or had a history of or suspected CMV disease within 6 months before randomization were excluded. Receipt of anti-CMV agents before randomization was not permitted. Complete details for inclusion and exclusion criteria are provided in the study protocol (Supplement 1).
Randomization, Stratification, and Masking
A central integrated web response system was used to randomize participants in a 1:1 ratio to receive letermovir or valganciclovir, with stratification based on receipt of lymphocyte-depleting induction immunosuppression. All study drugs had a matching placebo. Participants, investigators, study staff, and sponsor personnel involved in study drug administration and clinical evaluation were masked to study drug assignments.
Interventions and Assessments
The letermovir group received 480 mg of letermovir orally daily, 400 mg of acyclovir twice daily (as HSV and VZV prophylaxis), and a valganciclovir placebo. Acyclovir at a HSV/VZV prophylaxis dose was given to participants randomized to receive letermovir and does not have activity against CMV. The valganciclovir group received 900 mg of valganciclovir orally daily with placebos for letermovir and acyclovir (no acyclovir for HSV/VZV prophylaxis was needed because valganciclovir has activity against HSV/VZV). Valganciclovir and acyclovir doses were adjusted for kidney function6,23,24; assessment of creatinine clearance occurred at every study visit. Participants were instructed to take the study regimen with food at the same time each day for up to 200 days (eFigure 1 in Supplement 2). All study drugs could be provided intravenously, if needed. Details regarding drug-drug interactions, contraindications, dose adjustments, and monitoring parameters are provided in Supplement 1.
Screening was allowed up to 14 days before living-donor transplant and 1 day before deceased-donor transplant. Completion of screening procedures was required by day 5 and initiation of study intervention was required by posttransplant day 7. CMV disease status, physical examination, vital signs, concomitant medications, and adverse events were assessed during screening, on day 1, and at all scheduled study visits (eFigure 1 in Supplement 2). At each visit during prophylaxis, study personnel verified the accuracy of participant medication diaries. Percent adherence was defined as the number of days the participant took the prophylaxis drug divided by the number of days the participant should have taken prophylaxis multiplied by 100. Plasma samples for CMV DNA polymerase chain reaction testing were collected on day 1, week 2, and week 4 and every 4 weeks through week 52 and were processed at a central laboratory (Roche COBAS AmpliPrep/COBAS TaqMan assay; lower limit of quantification, 137 IU/mL); these results were not provided to investigators.
Investigators treated participants according to local standards for clinical decisions, including local laboratory testing for CMV DNAemia, at their discretion. A CMV disease/early discontinuation visit was initiated whenever an investigator suspected CMV disease (eg, signs or symptoms, CMV DNAemia per local laboratory results) and/or discontinued prophylaxis with the intent to start CMV treatment. Investigators were instructed to collect plasma samples during this visit prior to initiating CMV therapy. CMV DNAemia was assessed at the central laboratory and, if detected, next-generation sequencing was performed (Viroclinics-DDL) to assess viral resistance.25 These results were not shared with investigators. Participants were encouraged to complete all remaining scheduled visits through week 52 after confirmation of CMV disease and/or early discontinuation of prophylaxis.
Analysis Populations
The primary efficacy analysis population (full analysis set) consisted of all randomized participants who took at least 1 dose of the study regimen, were CMV-seronegative kidney transplant recipients who received an organ from a CMV-seropositive donor, and were negative for CMV DNAemia on day 1 (baseline). The safety population included all randomized participants who took at least 1 dose of the study regimen.
Primary and Secondary Efficacy Outcomes
All investigator-reported cases of CMV disease were assessed by an external independent masked clinical adjudication committee. Only cases confirmed by the committee based on the diagnostic criteria specified in the CMV drug development forum were included in the primary and secondary efficacy analyses.26 The primary outcome was CMV disease through week 52. Prespecified subgroup analyses included sex, age, race, region, and lymphocyte-depleting induction immunosuppression. Secondary efficacy outcomes included CMV disease through week 28 and the time to onset of CMV disease through week 52.
Exploratory Efficacy Outcomes
Exploratory outcomes were quantifiable CMV DNAemia evaluated by the central laboratory through weeks 28 and 52 and antiviral drug resistance in participants who were evaluated for suspected CMV disease/CMV DNAemia at a CMV disease/early discontinuation visit.
Safety Outcomes
Adverse events were collected at all study visits. A key prespecified composite safety outcome was the development of any of the following 4 events during prophylaxis: an adverse event of leukopenia, an adverse event of neutropenia, a central laboratory result for white blood cell count less than 3500 cells/μL, or an absolute neutrophil count less than 1000 cells/μL. More than 1 dose of G-CSF within any consecutive 30-day period during prophylaxis was an additional prespecified outcome.
Sample Size Calculation
Assuming the true proportion of participants with CMV disease was 0.17 for both groups, a sample size of 600 participants would achieve 90% power to demonstrate that letermovir was noninferior to valganciclovir, with an overall 2-sided α level of 5%.4
Statistical Analysis
The primary hypothesis was that letermovir was noninferior to valganciclovir for the prevention of CMV disease through week 52. To satisfy noninferiority, the upper bound of the 2-sided 95% CI for the difference in percentage of participants with CMV disease (letermovir minus valganciclovir) had to be no higher than 10%. The observed failure approach was used for missing data in the primary analysis; participants who discontinued prematurely from the study for any reason were not considered “failures.” The difference between the 2 groups and the associated 2-sided 95% CI were calculated using the stratum-adjusted Mantel-Haenszel method with stratification by receipt of lymphocyte-depleting induction immunosuppression.27 Subgroup analyses for the primary outcome, prespecified sensitivity analyses for the primary outcome (investigator-reported CMV disease, per-protocol population, and the “noncompleter equals failure” approach), and the secondary outcome of CMV disease through week 28 were analyzed similarly. A post hoc tipping point analysis was conducted to assess the potential effect of missing data on the primary outcome.
We estimated differences in adverse events and their 95% CIs using the Miettinen and Nurminen method.28 Additionally, 95% CIs and a P value were calculated for the between-group difference in the percentage of participants with the prespecified composite safety outcome of leukopenia or neutropenia events.
Kaplan-Meier plots were used for all time-to-event analyses, with data censoring at the last assessment. A post hoc analysis determined the time to onset of the composite safety outcome. Exploratory end points were summarized with descriptive statistics, with a prespecified supportive analysis for CMV DNAemia including results from local laboratories.
Results
Trial Participants
Among 693 CMV-seronegative participants screened, 601 participants were randomized from 94 participating sites in 16 countries between May 2018 and April 2021 (final follow-up in April 2022). Overall, 589 participants received at least 1 dose of the study regimen. The full analysis set included 586 participants; 3 participants were excluded (2 had CMV DNAemia on day 1 and 1 was a CMV-seropositive kidney transplant recipient who received an organ from a CMV-seropositive donor) (Figure 1).
Figure 1. Participant Flow in a Study of Letermovir vs Valganciclovir for Cytomegalovirus (CMV) Prophylaxis in Kidney Transplant Recipients.
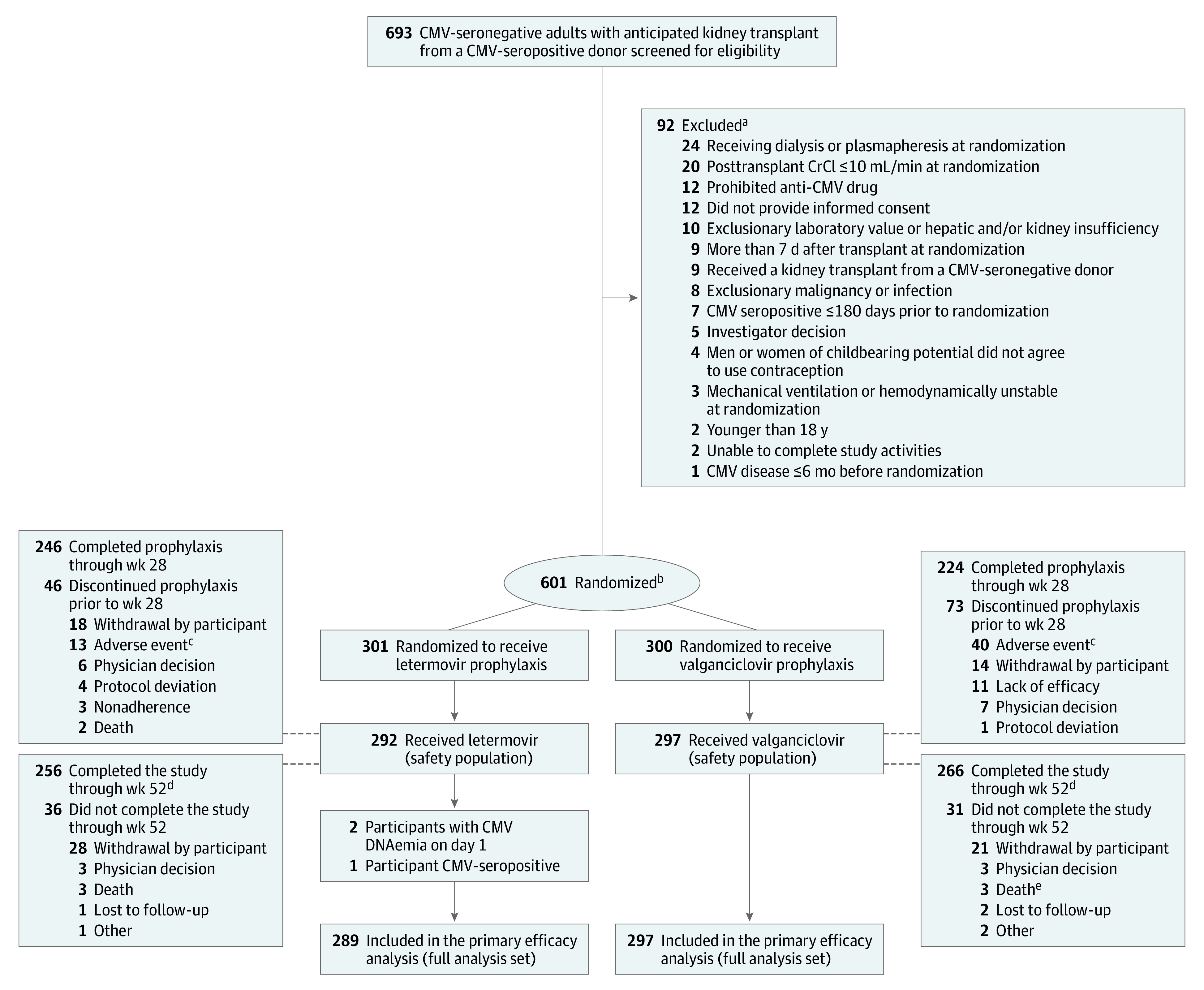
CrCI indicates creatinine clearance.
aParticipants could have ≥1 reason for exclusion. The inclusion and exclusion criteria are listed in Supplement 1.
bStratified by receipt of lymphocyte-depleting induction immunosuppression.
cOne participant developed glomerulonephritis (reported as an adverse event) that began the day after transplant and prior to initiating letermovir prophylaxis. One participant discontinued valganciclovir prophylaxis due to an adverse event (hallucinations) and died prior to week 28.
dParticipants were encouraged to complete all remaining scheduled visits through week 52 after confirmation of CMV disease and/or early discontinuation of prophylaxis.
eOne death due to COVID-19 was not reported as an adverse event.
Most participants were White (84.2%) and were men (71.6%) who received a kidney from a deceased donor (59.9%), and slightly less than half of participants (46.2%) received lymphocyte-depleting induction immunosuppression. The 2 most frequent reasons for kidney transplant were congenital cystic kidney disease (17.3%) and hypertension (16.1%) (Table 1). The percentage of participants with at least 90% adherence was 98.6% with letermovir and 77.4% with valganciclovir (eTable 1 in Supplement 2). Median (range) duration of exposure was 195 (1-237) days to oral letermovir vs 189 (1-225) days to oral valganciclovir. Three participants received intravenous letermovir for a median (range) of 1 (1-3) day and 2 participants received intravenous ganciclovir for a median (range) of 10 (1-19) days. The 3 most frequent immunosuppressants participants received during prophylaxis were tacrolimus (and tacrolimus monohydrate) (97.9% in the letermovir group and 97.6% in the valganciclovir group), mycophenolate (mycophenolate mofetil, mycophenolate sodium, and mycophenolic acid; 95.5% in the letermovir group and 96.3% in the valganciclovir group), and corticosteroids (meprednisone, methylprednisolone, methylprednisolone acetate, methylprednisolone sodium succinate, prednisolone, prednisolone sodium succinate, and prednisone; 95.2% in the letermovir group and 92.6% in the valganciclovir group).
Table 1. Participant Baseline Characteristics in a Trial of Letermovir for Cytomegalovirus (CMV) Prophylaxis After Kidney Transplant in the Safety Population.
| Characteristic | No. (%) of participantsa | |
|---|---|---|
| Letermovir (n = 292) | Valganciclovir (n = 297) | |
| Age, y | ||
| Median (range) | 52.0 (18-82) | 51.0 (18-78) |
| 18-35 | 62 (21.2) | 62 (20.9) |
| 36-50 | 78 (26.7) | 84 (28.3) |
| 51-64 | 104 (35.6) | 96 (32.3) |
| 65-74 | 42 (14.4) | 47 (15.8) |
| ≥75 | 6 (2.1) | 8 (2.7) |
| Sex | ||
| Men | 213 (72.9) | 209 (70.4) |
| Women | 79 (27.1) | 88 (29.6) |
| Race | ||
| American Indian or Alaska Native | 3 (1.0) | 4 (1.3) |
| Asian | 4 (1.4) | 10 (3.4) |
| Black or African American | 21 (7.2) | 33 (11.1) |
| Missing | 2 (0.7) | 1 (0.3) |
| Multiple | 9 (3.1) | 6 (2.0) |
| White | 253 (86.6) | 243 (81.8) |
| Hispanic or Latino | 54 (18.5) | 44 (14.8) |
| Reason for transplant | ||
| Congenital cystic kidney disease | 52 (17.8) | 50 (16.8) |
| Hypertension | 42 (14.4) | 53 (17.8) |
| Diabetes/diabetic nephropathy | 39 (13.4) | 46 (15.5) |
| Glomerulonephritis | 37 (12.7) | 30 (10.1) |
| IgA nephropathy | 36 (12.3) | 26 (8.8) |
| Chronic kidney disease/end-stage kidney disease | 19 (6.5) | 20 (6.7) |
| Urinary obstruction | 8 (2.7) | 4 (1.3) |
| Tubulointerstitial nephritis | 3 (1.0) | 7 (2.4) |
| Alport syndrome | 4 (1.4) | 6 (2.0) |
| Renal atrophy | 5 (1.7) | 3 (1.0) |
| Lupus | 2 (0.7) | 5 (1.7) |
| Mesangioproliferative glomerulonephritis | 2 (0.7) | 1 (0.3) |
| Otherb | 43 (14.7) | 46 (15.5) |
| Use of lymphocyte-depleting induction immunosuppressionc | 134 (45.9) | 138 (46.5) |
| Donor type | ||
| Deceased | 171 (58.6) | 182 (61.3) |
| Living, not related | 65 (22.3) | 51 (17.2) |
| Living, related | 56 (19.2) | 64 (21.5) |
All randomized participants who received prophylaxis.
Other consisted of multiple single disease states with ≤4 participants in either group.
Use of ≥1 of the following at the time of transplant: horse-derived or rabbit-derived antithymocyte globulin, alemtuzumab, or muromonab CD3. Receipt of these agents is associated with an increased risk for CMV infection.
Primary Efficacy Outcome
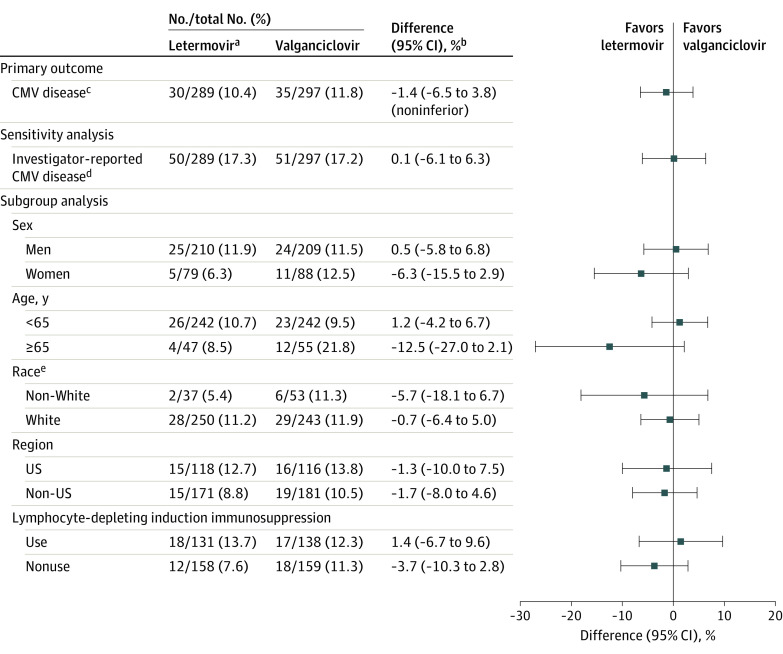
The percentage of participants with committee-confirmed CMV disease through week 52 was 10.4% (30/289) in the letermovir group vs 11.8% (35/297) in the valganciclovir group (stratum-adjusted difference, −1.4% [95% CI, −6.5% to 3.8%]) (Figure 2). The differences between the groups were comparable in sensitivity analyses (eTable 2 in Supplement 2). Detailed information regarding confirmed CMV disease and CMV treatment in participants who discontinued prophylaxis prior to week 28 are provided in eFigure 2 in Supplement 2. The post hoc tipping point analysis demonstrated that an additional 17 participants in the letermovir group would have to be counted as having confirmed CMV disease for the upper limit of the CI for the noninferiority margin to be greater than 10% (eFigure 3 in Supplement 2). The difference in CMV disease between letermovir and valganciclovir was comparable across prespecified subgroups, including participants who received lymphocyte-depleting induction immunosuppression (stratum-adjusted difference, 1.4% [95% CI, −6.7% to 9.6%]). The difference in investigator-reported CMV disease between groups was consistent with the primary analysis (stratum-adjusted difference, 0.1% [95% CI, −6.1% to 6.3%]; Figure 2). Of the 101 participants with investigator-reported CMV disease reviewed by the adjudication committee, 36 participants did not meet the criteria for confirmed CMV disease, most of whom had end-organ disease with insufficient documentation of tissue invasive disease (eTable 3 in Supplement 2).
Figure 2. Primary Outcome of Cytomegalovirus (CMV) Disease With Letermovir vs Valganciclovir Prophylaxis Through Week 52 in the Full Analysis Set.
aAll participants in the letermovir group received acyclovir for prophylaxis of herpes simplex and varicella-zoster virus.
bThe 95% CIs for the differences in proportions of participants were calculated using stratum-adjusted Mantel-Haenszel method with the difference weighted by the harmonic mean of sample size per group for each stratum (use/nonuse of lymphocyte-depleting induction immunosuppression). The upper bound of the 2-sided 95% CI for the primary outcome had to be no higher than 10% to conclude noninferiority. Participants who did not complete the study through week 52 or a had missing result for CMV DNAemia in the week-52 visit window were not considered failures (observed failure approach).
cCMV disease confirmed by the independent masked adjudication committee (CMV end-organ disease or CMV syndrome).
dPrespecified sensitivity analysis for investigator-reported CMV disease (included CMV syndrome and/or CMV end-organ disease).
eData were not available for 2 participants in the letermovir group and 1 participant in the valganciclovir group.
Secondary Efficacy Outcomes
No participant in the letermovir group developed committee-confirmed CMV disease through week 28 compared with 5 participants (1.7%) in the valganciclovir group, all of whom were diagnosed with CMV syndrome (stratum-adjusted difference, −1.7% [95% CI, −3.4% to 0.1%]). Three participants in the valganciclovir group had breakthrough viremia while receiving prophylaxis and 2 developed CMV disease after early discontinuation of prophylaxis. Time to onset of CMV disease was comparable through week 52 (hazard ratio, 0.90 [95% CI, 0.56-1.47]) (eFigure 4 in Supplement 2).
Exploratory Efficacy Outcomes
Based on central laboratory results, the percentage of participants with quantifiable CMV DNAemia (≥137 IU/mL) was 2.1% with letermovir compared with 8.8% with valganciclovir through week 28 and 31.8% with letermovir vs 37.7% with valganciclovir through week 52. The supporting analysis including CMV DNAemia reported by local laboratories did not change the general findings (eFigure 5 in Supplement 2). Among samples obtained from participants at the time of evaluation for suspected CMV disease/CMV DNAemia, no participants (0/52) in the letermovir group had known letermovir resistance–associated substitutions in viral terminase proteins (pUL51, pUL56, or pUL89), while 12.1% of participants (8/66) in the valganciclovir group had valganciclovir resistance–associated substitutions in the viral kinase protein (pUL97; n = 7) and/or the viral DNA polymerase protein (pUL54; n = 2). Two participants in the letermovir group had a valganciclovir resistance–associated substitution in pUL97 after initiation of valganciclovir for the treatment of CMV (eTable 4 in Supplement 2).
Safety Outcomes
The 3 most frequent adverse events were diarrhea, tremor, and urinary tract infection in the letermovir group and leukopenia, diarrhea, and tremor in the valganciclovir group (Table 2). No individual cardiac events (eg, acute myocardial infarction, atrial fibrillation, tachycardia) occurred in greater than or equal to 5% of participants (eTable 5 in Supplement 2). Serious adverse events were similar between the groups, including serious cardiac disorders (2.7% [8/292] in the letermovir group vs 3.0% [9/297] in the valganciclovir group). Fewer drug-related and serious drug-related adverse events were reported with letermovir compared with valganciclovir. Prophylaxis discontinuation due to an adverse event occurred in 4.1% of participants in the letermovir group and 13.5% in the valganciclovir group (difference, −9.4% [95% CI, −14.1% to −4.9%]) (Table 2).
Table 2. Adverse Events Through Week 28 in the Safety Populationa.
| Adverse event | No. (%) | Difference (95% CI), %b | |
|---|---|---|---|
| Letermovir (n = 292) | Valganciclovir (n = 297) | ||
| Adverse event summary | |||
| ≥1 adverse event | 271 (92.8) | 276 (92.9) | −0.1 (−4.4 to 4.2) |
| Serious adverse eventsc | 106 (36.3) | 113 (38.0) | −1.7 (−9.5 to 6.1) |
| Drug-related adverse eventsd | 58 (19.9) | 104 (35.0) | −15.2 (−22.2 to −8.0) |
| Serious drug-related adverse eventsc,d | 4 (1.4) | 15 (5.1) | −3.7 (−7.0 to −0.9) |
| Death | 2 (0.7) | 1 (0.3) | 0.3 (−1.3 to 2.2) |
| Discontinued due to adverse events | 12 (4.1) | 40 (13.5) | −9.4 (−14.1 to −4.9) |
| Discontinued due to serious adverse eventsc | 6 (2.1) | 14 (4.7) | −2.7 (−5.9 to 0.3) |
| Discontinued due to drug-related adverse eventsd | 8 (2.7) | 26 (8.8) | −6.0 (−10.1 to −2.4) |
| Discontinued due to serious drug-related adverse eventsc,d | 2 (0.7) | 7 (2.4) | −1.7 (−4.2 to 0.4) |
| Adverse events in ≥10% of participants | |||
| Diarrhea | 92 (31.5) | 85 (28.6) | 2.9 (−4.5 to 10.3) |
| Tremor | 53 (18.2) | 52 (17.5) | 0.6 (−5.6 to 6.9) |
| Urinary tract infection | 41 (14.0) | 42 (14.1) | 0.1 (−5.8 to 5.6) |
| Peripheral edema | 39 (13.4) | 38 (12.8) | 0.6 (−4.9 to 6.1) |
| Hypomagnesemia | 37 (12.7) | 39 (13.1) | −0.5 (−5.9 to 5.0) |
| Leukopenia | 33 (11.3) | 110 (37.0) | −25.7 (−32.3 to −19.1) |
| Hypertension | 33 (11.3) | 36 (12.1) | −0.8 (−6.1 to 4.5) |
| Increased creatinine | 30 (10.3) | 41 (13.8) | −3.5 (−8.9 to 1.8) |
| Hypophosphatemia | 30 (10.3) | 35 (11.8) | −1.5 (−6.7 to 3.6) |
| Hyperkalemia | 27 (9.2) | 32 (10.8) | −1.5 (−6.5 to 3.4) |
| Nausea | 25 (8.6) | 33 (11.1) | −2.5 (−7.5 to 2.3) |
| Fatigue | 18 (6.2) | 32 (10.8) | −4.6 (−9.3 to −0.1) |
| Neutropenia | 8 (2.7) | 49 (16.5) | −13.8 (−18.7 to −9.3) |
All adverse events were collected from randomization (day 1) through 14 days after the prophylaxis period or early discontinuation of prophylaxis. Please see eTable 4 and eTable 5 in Supplement 2 for additional details on adverse events.
Based on Miettinen and Nurminen method.
An adverse event was defined as serious if it resulted in death, was life-threatening, required inpatient hospitalization or prolonged an existing hospitalization, or resulted in persistent or significant disability or incapacity.
Considered by the investigator to be related to the drug.
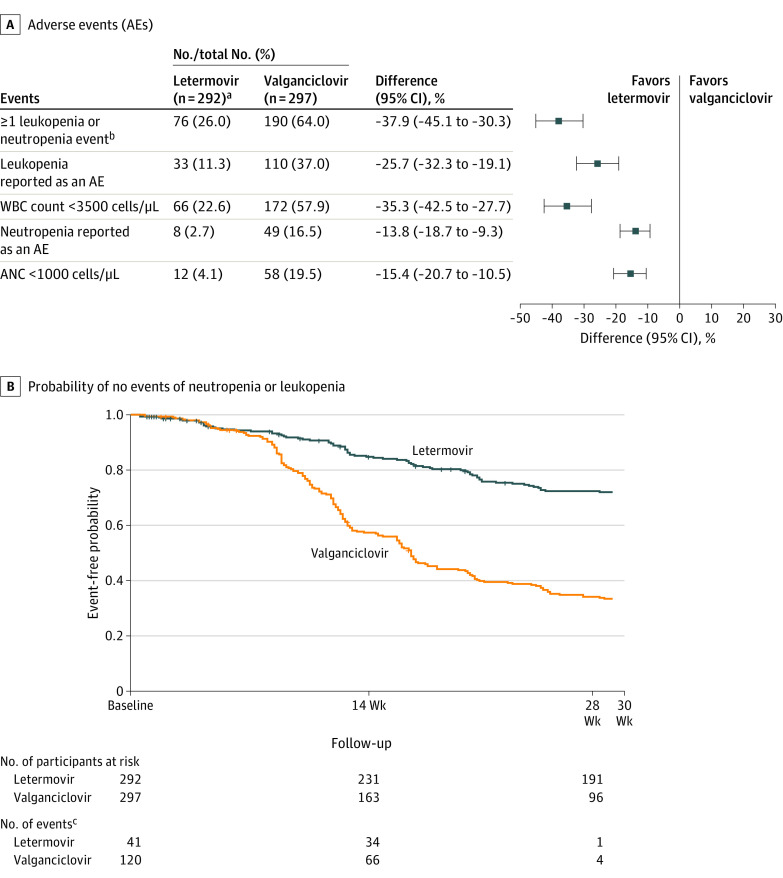
Adverse events of leukopenia and neutropenia were lower in the letermovir group than in the valganciclovir group: 11.3% vs 37.0% for leukopenia and 2.7% vs 16.5% for neutropenia (Table 2). Drug-related adverse events of leukopenia and neutropenia occurred less often with letermovir compared with valganciclovir. Fewer serious adverse events and serious drug-related adverse events of leukopenia occurred in the letermovir group vs the valganciclovir group. Neutropenia was the most frequent adverse event leading to discontinuation of prophylaxis in the letermovir group (1.4%), while leukopenia was the most frequent in the valganciclovir group (5.4%) (eTable 6 in Supplement 2).
The letermovir group had a significantly lower rate of the composite safety outcome of leukopenia or neutropenia events compared with the valganciclovir group (26.0% vs 64.0%; difference, −37.9% [95% CI, −45.1% to −30.3%]; P < .001) (Figure 3A). Participants who had no event of leukopenia or neutropenia from day 1 through week 28 are shown in Figure 3B. Five participants (1.7%) in the letermovir group and 21 (7.1%) in the valganciclovir group received G-CSF more than once during prophylaxis.
Figure 3. Leukopenia or Neutropenia Events and Time to Onset Through Week 28 in the Safety Population.
aAll participants in the letermovir group received acyclovir for prophylaxis of herpes simplex and varicella zoster virus.
bParticipants were only counted once for the composite safety outcome of ≥1 leukopenia or neutropenia event. P < .001. P value and 95% CI based on the Miettinen and Nurminen method.
cNumber of events is shown by time period: day 1 to week 14, week 14 to week 28, and week 28 to 14 days after prophylaxis was completed. Data were censored at the time of last assessment.
Laboratory findings that met the predefined criteria for grade 3 or 4 worsening from baseline were generally similar between the groups, including changes in serum creatinine and estimated glomerular filtration rate. There was a lower incidence of grade 3 decreases in hemoglobin and grade 3 or 4 decreases in neutrophils and leukocytes with letermovir compared with valganciclovir (eTable 7 in Supplement 2). No participants had laboratory values that met the predefined criteria for potential drug-induced liver injury.
Graft loss, graft rejection, and deaths were all infrequent. Graft loss occurred in 2 participants (0.7%) who received letermovir and 6 (2%) who received valganciclovir through week 52. Twenty-three participants (8%) in the letermovir group and 20 participants (6.7%) in the valganciclovir group had biopsy-proven graft rejection. A total of 6 deaths, 3 of which were reported during the prophylaxis period, were reported through posttransplant week 52. None of the deaths were considered to be drug related or related to CMV disease by the investigators.
Discussion
In this randomized, double-masked trial, letermovir, 480 mg, daily for up to 200 days was noninferior to valganciclovir, 900 mg, daily for prophylaxis of CMV disease in high-risk adult CMV-seronegative kidney transplant recipients who received an organ from CMV-seropositive donors, with a significantly lower rate of leukopenia or neutropenia and fewer prophylaxis discontinuations.
Committee-confirmed CMV disease occurred in 10.4% of participants in the letermovir group through posttransplant week 52, and no cases occurred during the 200-day posttransplant prophylaxis period. In comparison, CMV disease occurred in 11.8% of participants in the valganciclovir group, with 5 cases (1.7%) occurring during the prophylaxis period. The rate of investigator-reported CMV disease was higher and comparable between the groups (17.3% in the letermovir group vs 17.2% in the valganciclovir group). The higher rate of investigator-reported CMV disease likely reflects differences in diagnostic testing in clinical practice. It is common to presumptively treat CMV end-organ disease rather than pursue an invasive biopsy, as was required for committee confirmation in this study.1,26,29,30 This is demonstrated by fewer participants with committee-confirmed end-organ disease (n = 7) compared with those with end-organ disease reported by investigators (n = 61), particularly for gastrointestinal end-organ disease (5 vs 41 participants). The relatively high rate of CMV disease in the first posttransplant year highlights the limitations of a 6-month universal prophylaxis strategy in high-risk CMV-seronegative kidney transplant recipients who receive an organ from a CMV-seropositive donor.
Antiviral resistance is an important complication of antiviral therapy, yet few studies have systematically evaluated this outcome in randomized clinical trials. Letermovir resistance–associated substitutions were not observed in the 52 participants who were evaluated for suspected CMV disease/CMV DNAemia. In contrast, 8 of 66 participants (12.1%) in the valganciclovir group who were evaluated for suspected CMV disease/CMV DNAemia had valganciclovir resistance–associated substitutions. Although there are published reports of letermovir resistance, these have mostly been associated with letermovir treatment, including salvage therapy.19,20,21,22 The current study provides reassurance that development of resistance to letermovir is not likely to emerge when it is used for prophylaxis among CMV-seronegative kidney transplant recipients who receive an organ from a CMV-seropositive donor.
Letermovir was generally well tolerated and safe. More participants completed up to 200 days of prophylaxis with letermovir vs valganciclovir, which is also consistent with the lower rate of quantifiable CMV DNAemia with letermovir (2.1% vs 8.8%) during the prophylaxis period. Letermovir tolerability was further demonstrated by the lower rate of prophylaxis discontinuation due to an adverse event compared with valganciclovir (4.3% vs 13.5%). Letermovir had an overall safety profile similar to that described in previous phase 3 trials in adult recipients of allogeneic HSCTs.31,32
Significantly lower rates of leukopenia or neutropenia events occurred with letermovir vs valganciclovir prophylaxis, with decreased use of G-CSF (5 vs 21 participants). G-CSF is used for kidney transplant recipients at high risk for infection with severe leukopenia or neutropenia, but can be logistically challenging to administer in the outpatient setting. In addition, immunosuppressants or antimicrobials (eg, sulfamethoxazole/trimethoprim, valganciclovir) may be stopped or adjusted to lower doses to counter leukopenia or neutropenia.9,33 Future studies should assess whether prophylaxis with letermovir vs valganciclovir translates to decreased risk for opportunistic infections and/or allograft rejection.
Other considerations with valganciclovir include close monitoring of kidney function for dose adjustments and the need to convert to ganciclovir, with weight-based dosing, for intravenous administration. In contrast, letermovir is dosed independent of kidney function and the dose and frequency are identical for oral and intravenous administration.
Limitations
This study has limitations. First, most participants were White men, but there is no known increased risk for CMV disease conferred by sex or race or ethnicity.34,35 Second, the proportion of participants who received lymphocyte-depleting induction immunosuppression, which increases the risk for CMV infection or disease, was lower in the study population than reported in US registry data.3 Third, myelotoxicity was evaluated as leukopenia or neutropenia, although valganciclovir may also cause anemia and thrombocytopenia. Fourth, a number of participants did not complete the study through week 52 or had a missing result for CMV DNAemia in the week-52 visit window; nonetheless, CMV disease rates were comparable between letermovir and valganciclovir across all sensitivity analyses. Fifth, longer-term outcomes associated with CMV disease were not formally assessed. However, because CMV disease was comparable between groups, there is no reason to anticipate differences in long-term outcomes with letermovir vs valganciclovir. Sixth, cost analyses were not conducted, but are important considerations for implementation strategies.
Conclusions
Letermovir was noninferior to valganciclovir for prevention of CMV disease when taken for up to 200 days after transplant by adult high-risk CMV-seronegative kidney transplant recipients who received an organ from a CMV-seropositive donor, with less leukopenia or neutropenia. Additionally, participants who received letermovir did not develop resistance-associated substitutions and had a lower rate of CMV DNAemia, drug-related adverse events, and prophylaxis discontinuations due to adverse events, compared with valganciclovir.
Trial protocol
eTables
Data sharing statement
References
- 1.Kotton CN, Kumar D, Caliendo AM, et al. ; The Transplantation Society International CMV Consensus Group . The third International Consensus Guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102(6):900-931. doi: 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 2.Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep. 2012;14(6):633-641. doi: 10.1007/s11908-012-0292-2 [DOI] [PubMed] [Google Scholar]
- 3.Lentine KL, Smith JM, Miller JM, et al. OPTN/SRTR 2021 annual data report: kidney. Am J Transplant. 2023;23(2)(suppl 1):S21-S120. doi: 10.1016/j.ajt.2023.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10(5):1228-1237. doi: 10.1111/j.1600-6143.2010.03074.x [DOI] [PubMed] [Google Scholar]
- 5.Paya C, Humar A, Dominguez E, et al. ; Valganciclovir Solid Organ Transplant Study Group . Efficacy and safety of valganciclovir vs oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4(4):611-620. doi: 10.1111/j.1600-6143.2004.00382.x [DOI] [PubMed] [Google Scholar]
- 6.Valcyte (valganciclovir): US prescribing information. Genentech USA, Inc. Accessed January 3, 2023. https://www.gene.com/download/pdf/valcyte_prescribing.pdf
- 7.Ganciclovir injection: US prescribing information. Sagent Pharmaceuticals, Inc. Accessed January 3, 2023. https://www.sagentpharma.com/wp-content/uploads/2021/07/Ganciclovir_PI_November-2020.pdf
- 8.Raval AD, Kistler KD, Tang Y, Vincenti F. Burden of neutropenia and leukopenia among adult kidney transplant recipients: a systematic literature review of observational studies. Transpl Infect Dis. 2023;25(1):e14000. doi: 10.1111/tid.14000 [DOI] [PubMed] [Google Scholar]
- 9.Roumpz CG, Kohl J, Hughes KL, et al. Real-world effectiveness and complications of valganciclovir (VGC) prophylaxis for kidney transplant (KT) recipients at high risk for cytomegalovirus (CMV) infection (CMV donor (D)+/recipient (R)-). Open Forum Infect Dis. 2021;8(S1):S776-S776. doi: 10.1093/ofid/ofab466.1572 [DOI] [Google Scholar]
- 10.Rissling O, Naik M, Brakemeier S, et al. High frequency of valganciclovir underdosing for cytomegalovirus prophylaxis after renal transplantation. Clin Kidney J. 2018;11(4):564-573. doi: 10.1093/ckj/sfx145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong DD, van Zuylen WJ, Novos T, et al. Detection of ganciclovir-resistant cytomegalovirus in a prospective cohort of kidney transplant recipients receiving subtherapeutic valganciclovir prophylaxis. Microbiol Spectr. 2022;10(3):e0268421. doi: 10.1128/spectrum.02684-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cintra-Cabrera M, Suárez-Benjumea A, Bernal-Blanco G, et al. Resistant cytomegalovirus infection after renal transplantation: literature review. Transplant Proc. 2018;50(2):575-577. doi: 10.1016/j.transproceed.2017.09.058 [DOI] [PubMed] [Google Scholar]
- 13.Echenique IA, Beltran D, Ramirez-Ruiz L, Najafian N, Agrawal N. Ganciclovir dosing strategies and development of cytomegalovirus resistance in a kidney transplant recipient: a case report. Transplant Proc. 2017;49(7):1560-1564. doi: 10.1016/j.transproceed.2017.02.046 [DOI] [PubMed] [Google Scholar]
- 14.Fisher CE, Knudsen JL, Lease ED, et al. Risk factors and outcomes of ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients. Clin Infect Dis. 2017;65(1):57-63. doi: 10.1093/cid/cix259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol. 2011;85(20):10884-10893. doi: 10.1128/JVI.05265-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoelben S, Arns W, Renders L, et al. Preemptive treatment of Cytomegalovirus infection in kidney transplant recipients with letermovir: results of a phase 2a study. Transpl Int. 2014;27(1):77-86. doi: 10.1111/tri.12225 [DOI] [PubMed] [Google Scholar]
- 17.Prevymis (letermovir): US prescribing information. Merck & Co, Inc. Accessed May 22, 2023. https://www.merck.com/product/usa/pi_circulars/p/prevymis/prevymis_pi.pdf
- 18. Prevymis (letermovir): EU summary of product characteristics. Merck Sharpe & Dohme. Accessed May 31, 2023. https://www.ema.europa.eu/documents/product-information/prevymis-epar-product-information_en.pdf [Google Scholar]
- 19.Cherrier L, Nasar A, Goodlet KJ, Nailor MD, Tokman S, Chou S. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant. 2018;18(12):3060-3064. doi: 10.1111/ajt.15135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner N, Strand A, Grewal DS, et al. Use of letermovir as salvage therapy for drug-resistant cytomegalovirus retinitis. Antimicrob Agents Chemother. 2019;63(3):e02337-18. doi: 10.1128/AAC.02337-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann E, Sidler D, Dahdal S, et al. Emergence of letermovir resistance in solid organ transplant recipients with ganciclovir resistant cytomegalovirus infection: a case series and review of the literature. Transpl Infect Dis. 2021;23(3):e13515. doi: 10.1111/tid.13515 [DOI] [PubMed] [Google Scholar]
- 22.Paolucci S, Campanini G, Cassaniti I, et al. Emergence of Letermovir-resistant HCMV UL56 mutant during rescue treatment in a liver transplant recipient with ganciclovir-resistant infection HCMV: a case report. BMC Infect Dis. 2021;21(1):994. doi: 10.1186/s12879-021-06694-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold D, Corey L. Acyclovir prophylaxis for herpes simplex virus infection. Antimicrob Agents Chemother. 1987;31(3):361-367. doi: 10.1128/AAC.31.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zovirax (acyclovir): US prescribing information. GlaxoSmithKline. Accessed March 27, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018828s030,020089s019,019909s020lbl.pdf
- 25.Douglas CM, Barnard R, Holder D, et al. Letermovir resistance analysis in a clinical trial of cytomegalovirus prophylaxis for hematopoietic stem cell transplant recipients. J Infect Dis. 2020;221(7):1117-1126. doi: 10.1093/infdis/jiz577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum . Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87-91. doi: 10.1093/cid/ciw668 [DOI] [PubMed] [Google Scholar]
- 27.Koch GG, Carr GJ, Amara IA, Stokes ME, Uryniak TJ. Categorical data analysis. In: Berry DA, ed. Statistical Methodology in the Pharmaceutical Sciences. CRC Press; 1990. [Google Scholar]
- 28.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213-226. doi: 10.1002/sim.4780040211 [DOI] [PubMed] [Google Scholar]
- 29.Kotton CN, Torre-Cisneros J, Aguado JM, et al. ; International CMV Symposium Faculty . Cytomegalovirus in the transplant setting: where are we now and what happens next? a report from the International CMV Symposium 2021. Transpl Infect Dis. 2022;24(6):e13977. doi: 10.1111/tid.13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients: guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13512. doi: 10.1111/ctr.13512 [DOI] [PubMed] [Google Scholar]
- 31.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433-2444. doi: 10.1056/NEJMoa1706640 [DOI] [PubMed] [Google Scholar]
- 32.Dadwal SS, Russo D, Stelljes M, et al. A phase 3 randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of letermovir (LET) prophylaxis when extended from 100 to 200 Days post-transplant in cytomegalovirus (CMV)-seropositive recipients (R+) of an allogeneic hematopoietic stem cell transplant (HSCT) [abstract 76]. Abstract presented at Transplantation and Cellular Therapy Meetings of ASTCT and CIBMTR; February 18, 2023; Orlando, FL. [Google Scholar]
- 33.Brar S, Berry R, Raval AD, Tang Y, Vincenti F, Skartsis N. Outcomes among CMV-mismatched and highly sensitized kidney transplants recipients who develop neutropenia. Clin Transplant. 2022;36(4):e14583. doi: 10.1111/ctr.14583 [DOI] [PubMed] [Google Scholar]
- 34.Tang Y, Guo J, Li J, Zhou J, Mao X, Qiu T. Risk factors for cytomegalovirus infection and disease after kidney transplantation: a meta-analysis. Transpl Immunol. 2022;74:101677. doi: 10.1016/j.trim.2022.101677 [DOI] [PubMed] [Google Scholar]
- 35.Raval AD, Kistler KD, Tang Y, Murata Y, Snydman DR. Epidemiology, risk factors, and outcomes associated with cytomegalovirus in adult kidney transplant recipients: a systematic literature review of real-world evidence. Transpl Infect Dis. 2021;23(2):e13483. doi: 10.1111/tid.13483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTables
Data sharing statement