Abstract
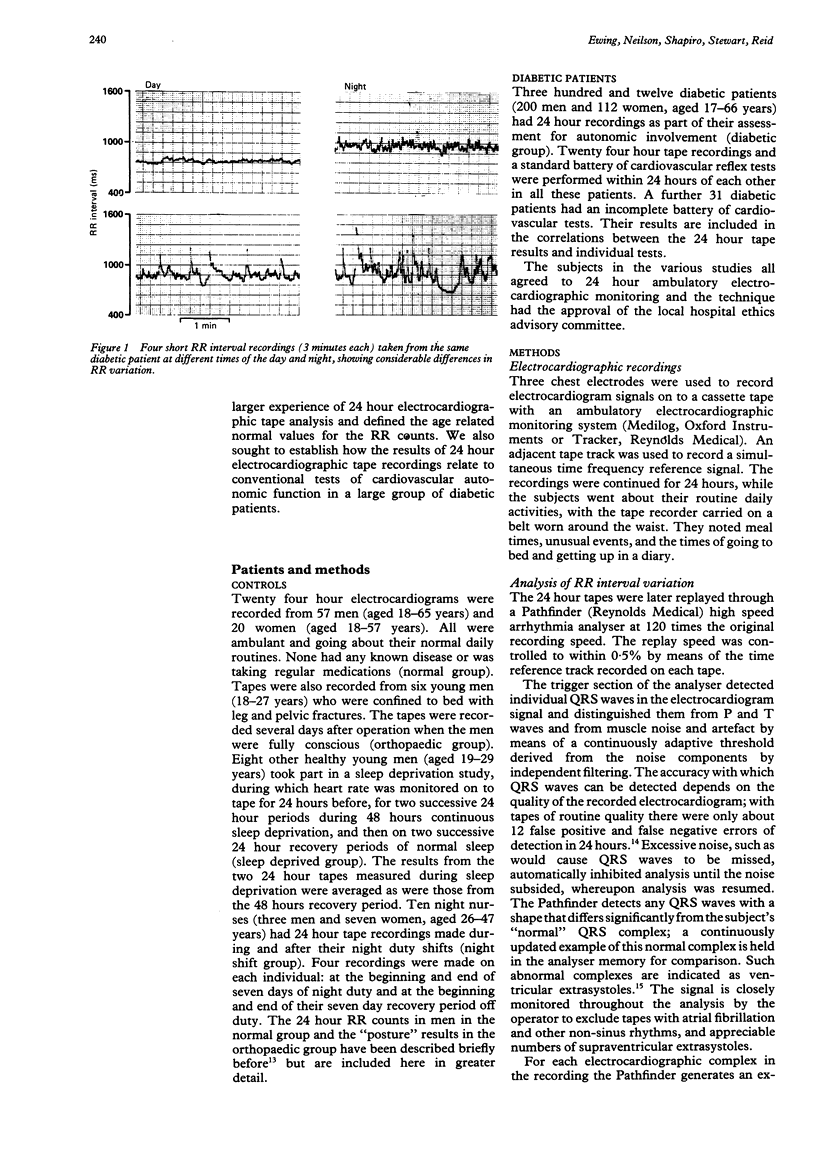
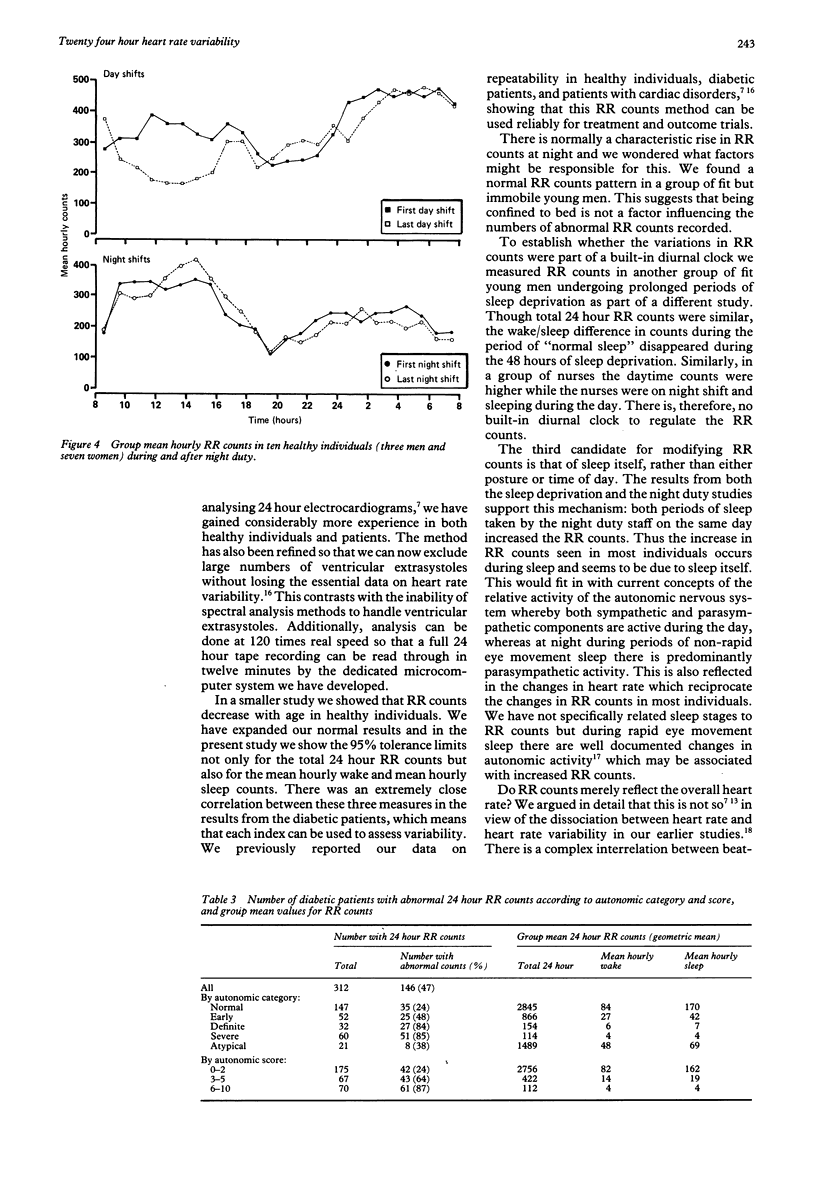
Heart rate variability was measured in 77 healthy controls and 343 diabetic patients by a count of the number of beat-to-beat differences greater than 50 ms in the RR interval during a 24 hour ambulatory electrocardiogram. In the healthy controls the lower 95% tolerance limits for total 24 hour RR interval counts were approximately 2000 at age 25, 1000 at 45, and 500 at 65 years. Six controls confined to bed after injury had normal 24 hour patterns of RR counts, while eight other controls showed loss of diurnal variation in both heart rate and RR counts during a period of sleep deprivation. RR counts in ten controls on and off night duty increased during sleep whenever it occurred. Nearly half (146) the 343 diabetic patients had abnormal 24 hour RR counts. The percentage of abnormal RR counts increased with increasing autonomic abnormality assessed by a standard battery of tests of cardiovascular autonomic function. A quarter of those with normal cardiovascular reflex tests had abnormal 24 hour RR counts. There were close correlations between 24 hour RR count results and the individual heart rate tests (r = 0.6). The assessment of cardiac parasympathetic activity by 24 hour RR counts was reliable. The diurnal variations in RR counts seen in the controls were probably related to sleep rather than either posture or time of day. The method was more sensitive than conventional tests of cardiovascular reflexes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akselrod S., Gordon D., Madwed J. B., Snidman N. C., Shannon D. C., Cohen R. J. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985 Oct;249(4 Pt 2):H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- Cauchemez B., Peirano P., Samson-Dolfus D., Lucet V., Attuel P., Kauffmann F., Maison-Blanche P., Monod N., Coumel P. Le système nerveux autonome dans le syndrome de la mort subite du nourrisson. Analyse du rythme cardiaque et de la variabilité sinusale sur les enregistrements Holter d'enfants décédés du syndrome. Arch Mal Coeur Vaiss. 1989 May;82(5):745–752. [PubMed] [Google Scholar]
- Ewing D. J., Hume L., Campbell I. W., Murray A., Neilson J. M., Clarke B. F. Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol Respir Environ Exerc Physiol. 1980 Nov;49(5):809–814. doi: 10.1152/jappl.1980.49.5.809. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Martyn C. N., Young R. J., Clarke B. F. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985 Sep-Oct;8(5):491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Neilson J. M., Travis P. New method for assessing cardiac parasympathetic activity using 24 hour electrocardiograms. Br Heart J. 1984 Oct;52(4):396–402. doi: 10.1136/hrt.52.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsted J., Jensen S. B. A simple test for autonomic neuropathy in juvenile diabetics. Acta Med Scand. 1979;205(5):385–387. doi: 10.1111/j.0954-6820.1979.tb06069.x. [DOI] [PubMed] [Google Scholar]
- Kleiger R. E., Miller J. P., Bigger J. T., Jr, Moss A. J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987 Feb 1;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- Matthews D. M., Martyn C. N., Riemersma R. A., Clarke B. F., Ewing D. J. Noradrenaline response to edrophonium (Tensilon) and its relation to other autonomic tests in diabetic subjects. Diabetes Res. 1987 Dec;6(4):175–180. [PubMed] [Google Scholar]
- McAreavey D., Neilson J. M., Ewing D. J., Russell D. C. Cardiac parasympathetic activity during the early hours of acute myocardial infarction. Br Heart J. 1989 Sep;62(3):165–170. doi: 10.1136/hrt.62.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A., Ewing D. J., Campbell I. W., Neilson J. M., Clarke B. F. RR interval variations in young male diabetics. Br Heart J. 1975 Aug;37(8):882–885. doi: 10.1136/hrt.37.8.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M., Lombardi F., Guzzetti S., Rimoldi O., Furlan R., Pizzinelli P., Sandrone G., Malfatto G., Dell'Orto S., Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986 Aug;59(2):178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pomeranz B., Macaulay R. J., Caudill M. A., Kutz I., Adam D., Gordon D., Kilborn K. M., Barger A. C., Shannon D. C., Cohen R. J. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985 Jan;248(1 Pt 2):H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Schwartz P. J. The quest for the mechanisms of the sudden infant death syndrome: doubts and progress. Circulation. 1987 Apr;75(4):677–683. doi: 10.1161/01.cir.75.4.677. [DOI] [PubMed] [Google Scholar]
- Wheeler T., Watkins P. J. Cardiac denervation in diabetes. Br Med J. 1973 Dec 8;4(5892):584–586. doi: 10.1136/bmj.4.5892.584. [DOI] [PMC free article] [PubMed] [Google Scholar]


