Abstract
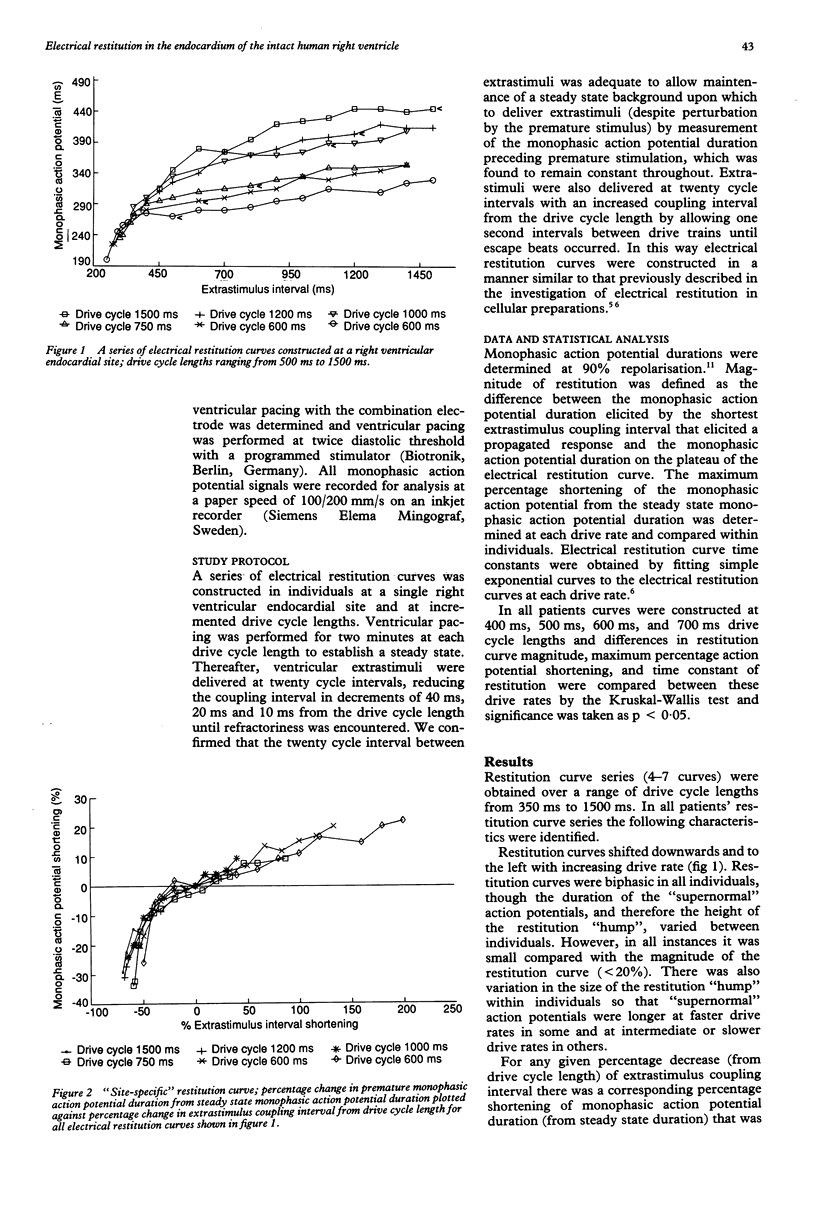
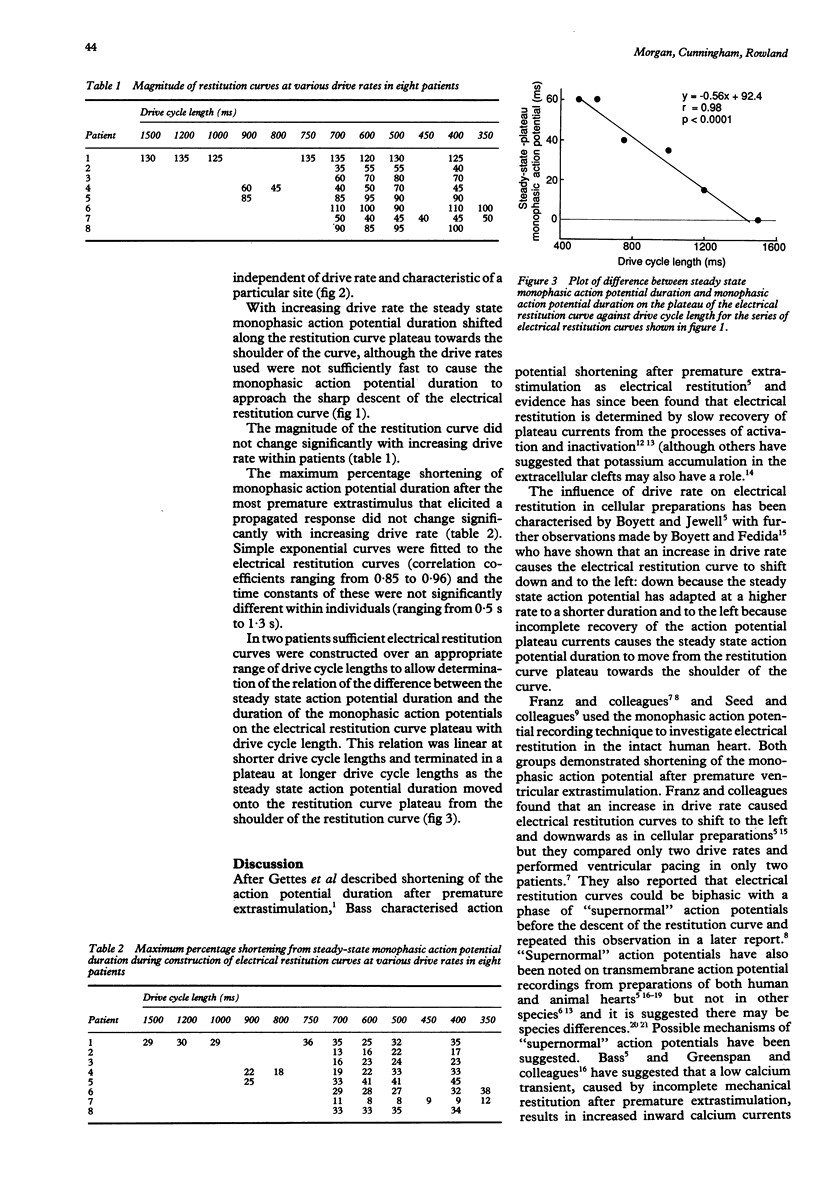
OBJECTIVE--To characterise electrical restitution in the intact human heart. PATIENTS AND METHODS--A series of monophasic action potential electrical restitution curves were constructed from a single right ventricular endocardial site in eight patients (three men) without structural heart disease aged 52-68 (mean 55 years). A combination pacing/monophasic action potential electrode was used to pace and record monophasic action potentials at drive cycle lengths of from 350 ms to 1500 ms. Ventricular extrastimuli were delivered at 20 cycle intervals and decreased from the longest coupling interval attainable without escape beats. RESULTS--Restitution curves shifted downward and towards the left; steady state action potential duration shifted from the restitution plateau and descended the curve, the amount of shift being linearly related to drive cycle length in two patients in whom the relation could be assessed; the amount of monophasic action potential shortening was a function of the degree of prematurity and that relation was unaffected by drive rate; the magnitude of restitution and the time constant of the restitution curve were not changed significantly by altered drive cycle length. CONCLUSION--In the intact heart in vivo, electrical restitution (of the monophasic action potential) has similar characteristics to those (of the transmembrane action potential) in cellular preparations in vitro. Thus the alteration of action potential plateau currents by instantaneous rate change or drug effects, which can be directly observed by techniques available to the cellular electrophysiologist, may be indirectly assessed in vivo by characterisation of the effect of these on electrical restitution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. G. Restitution of the action potential in cat papillary muscle. Am J Physiol. 1975 Jun;228(6):1717–1724. doi: 10.1152/ajplegacy.1975.228.6.1717. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Fedida D. Changes in the electrical activity of dog cardiac Purkinje fibres at high heart rates. J Physiol. 1984 May;350:361–391. doi: 10.1113/jphysiol.1984.sp015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J Physiol. 1978 Dec;285:359–380. doi: 10.1113/jphysiol.1978.sp012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Spindler A. J., Twist V. W. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol. 1989 Apr;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga G., Lab M. J., Noble M. I., Papadoyannis D. E., Pidgeon J., Seed A., Wohlfart B. The action-potential duration and contractile response of the intact heart related to the preceding interval and the preceding beat in the dog and cat. J Physiol. 1981 May;314:481–500. doi: 10.1113/jphysiol.1981.sp013720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M. R., Burkhoff D., Spurgeon H., Weisfeldt M. L., Lakatta E. G. In vitro validation of a new cardiac catheter technique for recording monophasic action potentials. Eur Heart J. 1986 Jan;7(1):34–41. doi: 10.1093/oxfordjournals.eurheartj.a061954. [DOI] [PubMed] [Google Scholar]
- Franz M. R., Chin M. C., Sharkey H. R., Griffin J. C., Scheinman M. M. A new single catheter technique for simultaneous measurement of action potential duration and refractory period in vivo. J Am Coll Cardiol. 1990 Oct;16(4):878–886. doi: 10.1016/s0735-1097(10)80336-5. [DOI] [PubMed] [Google Scholar]
- Franz M. R., Schaefer J., Schöttler M., Seed W. A., Noble M. I. Electrical and mechanical restitution of the human heart at different rates of stimulation. Circ Res. 1983 Dec;53(6):815–822. doi: 10.1161/01.res.53.6.815. [DOI] [PubMed] [Google Scholar]
- Gettes L. S., Morehouse N., Surawicz B. Effect of premature depolarization on the duration of action potentials in Purkinje and ventricular fibers of the moderator band of the pig heart. Role of proximity and the duration of the preceding action potential. Circ Res. 1972 Jan;30(1):55–66. doi: 10.1161/01.res.30.1.55. [DOI] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes J. A., Winters S. L., Martinson M., Machac J., Stewart D., Targonski A. The prognostic significance of quantitative signal-averaged variables relative to clinical variables, site of myocardial infarction, ejection fraction and ventricular premature beats: a prospective study. J Am Coll Cardiol. 1989 Feb;13(2):377–384. doi: 10.1016/0735-1097(89)90515-9. [DOI] [PubMed] [Google Scholar]
- Greenspan K., Edmands R. E., Fisch C. Effects of cycle-length alteration on canine cardiac action potentials. Am J Physiol. 1967 Jun;212(6):1416–1420. doi: 10.1152/ajplegacy.1967.212.6.1416. [DOI] [PubMed] [Google Scholar]
- HAN J., MOE G. K. NONUNIFORM RECOVERY OF EXCITABILITY IN VENTRICULAR MUSCLE. Circ Res. 1964 Jan;14:44–60. doi: 10.1161/01.res.14.1.44. [DOI] [PubMed] [Google Scholar]
- Hauswirth O., Singh B. N. Ionic mechanisms in heart muscle in relation to the genesis and the pharmacological control of cardiac arrhythmias. Pharmacol Rev. 1978 Mar;30(1):5–63. [PubMed] [Google Scholar]
- Hilgemann D. W., Noble D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: reconstruction of basic cellular mechanisms. Proc R Soc Lond B Biol Sci. 1987 Mar 23;230(1259):163–205. doi: 10.1098/rspb.1987.0015. [DOI] [PubMed] [Google Scholar]
- Iinuma H., Kato K. Mechanism of augmented premature responses in canine ventricular muscle. Circ Res. 1979 May;44(5):624–629. doi: 10.1161/01.res.44.5.624. [DOI] [PubMed] [Google Scholar]
- Jorgensen C. R., Ferlic R. M., Varco R. L., Lillehei C. W., Eliot R. S. Cor triatriatum. Review of the surgical aspects with a follow-up report on the first patient successfully treated with surgery. Circulation. 1967 Jul;36(1):101–107. doi: 10.1161/01.cir.36.1.101. [DOI] [PubMed] [Google Scholar]
- Kline R. P., Kupersmith J. Effects of extracellular potassium accumulation and sodium pump activation on automatic canine Purkinje fibres. J Physiol. 1982 Mar;324:507–533. doi: 10.1113/jphysiol.1982.sp014127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. S., Atarashi H., Reddy C. P., Surawicz B. Dispersion of ventricular repolarization and arrhythmia: study of two consecutive ventricular premature complexes. Circulation. 1985 Aug;72(2):370–376. doi: 10.1161/01.cir.72.2.370. [DOI] [PubMed] [Google Scholar]
- Kuo C. S., Munakata K., Reddy C. P., Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983 Jun;67(6):1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Wallace A. G., Feezor M. D. A quantitative comparison of the relation between the shape of the action potential and the pattern of stimulation in canine ventricular muscle and Purkinje fibers. J Mol Cell Cardiol. 1971 Mar;2(1):3–19. doi: 10.1016/0022-2828(71)90074-5. [DOI] [PubMed] [Google Scholar]
- Mitchell L. B., Wyse D. G., Duff H. J. Programmed electrical stimulation studies for ventricular tachycardia induction in humans. I. The role of ventricular functional refractoriness in tachycardia induction. J Am Coll Cardiol. 1986 Sep;8(3):567–575. doi: 10.1016/s0735-1097(86)80184-x. [DOI] [PubMed] [Google Scholar]
- Noble D., Cohen I. The interpretation of the T wave of the electrocardiogram. Cardiovasc Res. 1978 Jan;12(1):13–27. doi: 10.1093/cvr/12.1.13. [DOI] [PubMed] [Google Scholar]
- Seed W. A., Noble M. I., Oldershaw P., Wanless R. B., Drake-Holland A. J., Redwood D., Pugh S., Mills C. Relation of human cardiac action potential duration to the interval between beats: implications for the validity of rate corrected QT interval (QTc). Br Heart J. 1987 Jan;57(1):32–37. doi: 10.1136/hrt.57.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellens H. J., Düren D. R., Lie K. I. Observations on mechanisms of ventricular tachycardia in man. Circulation. 1976 Aug;54(2):237–244. doi: 10.1161/01.cir.54.2.237. [DOI] [PubMed] [Google Scholar]
- Wellens H. J., Schuilenburg R. M., Durrer D. Electrical stimulation of the heart in patients with ventricular tachycardia. Circulation. 1972 Aug;46(2):216–226. doi: 10.1161/01.cir.46.2.216. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Rosen M. R. Pathophysiologic mechanisms of cardiac arrhythmias. Am Heart J. 1983 Oct;106(4 Pt 2):798–811. doi: 10.1016/0002-8703(83)90003-0. [DOI] [PubMed] [Google Scholar]
- Wohlfart B. Relationships between peak force, action potential duration and stimulus interval in rabbit myocardium. Acta Physiol Scand. 1979 Aug;106(4):395–409. doi: 10.1111/j.1748-1716.1979.tb06419.x. [DOI] [PubMed] [Google Scholar]


