Abstract
Matrix vesicles (MVs) are a special class of extracellular vesicles released by mineralizing cells during bone and tooth mineralization that initiate the precipitation of apatitic minerals by regulating the extracellular ratio between inorganic phosphate (Pi), a calcification promoter, and pyrophosphate (PPi), a calcification inhibitor. The Pi/PPi ratio is thought to be controlled by two ecto-phosphatases present on the outer leaflet of the MVs’ membrane: ectonucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) that produces PPi as well as Pi from ATP and tissue-nonspecific alkaline phosphatase (TNAP) that hydrolyzes both ATP and PPi to generate Pi. However, if and how these enzymes act in concert in MVs are still unclear. Herein, we investigated the role of NPP1 and TNAP in ATP hydrolysis during MV-mediated biomineralization using proteoliposomes as a biomimetic model for MVs. Proteoliposomes composed by 1,2-dipalmitoylphosphatidylcholine (DPPC) and harboring NPP1 alone, TNAP alone, or both together at different molar ratios (1:1, 10:1, and 1:10) were fabricated. After 48 h of incubation with ATP, TNAP-containing proteoliposomes consumed more ATP than NPP1-containing vesicles (270 and 210 nmol, respectively). Both types of vesicles comparatively formed ADP (205 and 201 nmol, respectively), while NPP1-containing vesicles hydrolyzed AMP less efficiently than TNAP-containing proteoliposomes (10 and 25 nmol, respectively). In vitro mineralization assays showed that in the presence of ATP, TNAP-harboring proteoliposomes mineralized through a sigmoidal single-step process, while NPP1-harboring vesicles displayed a two-step mineralization process. ATR-FTIR analyses showed that the minerals produced by TNAP-harboring proteoliposomes were structurally more similar to hydroxyapatite than those produced by NPP1-harboring vesicles. Our results with proteoliposomes indicate that the pyrophosphohydrolase function of NPP1 and the phosphohydrolase activity of TNAP act synergistically to produce a Pi/PPi ratio conducive to mineralization and the synergism is maximal when the two enzymes are present at equimolar concentrations. The significance of these findings for hypophosphatasia is discussed.
Keywords: NPP1, TNAP, Proteoliposomes, Phosphohydrolase, Pyrophosphohydrolase
Introduction
Mineralization-competent cells release a special class of extracellular vesicles, named matrix vesicles (MVs), to initiate the precipitation of calcium phosphate seeds and the propagation of apatitic minerals onto the collagenous matrix during bone and tooth mineralization [1–4]. MVs are different from other classes of EVs for the release mechanisms, unique ability to bind to collagen fibrils, and high activity of tissue non-specific alkaline phosphatase (TNAP), a key enzyme in biomineralization. MVs drive biomineralization through a multi-step process: after their release by outward budding from the apical microvilli of mineralization-competent cells, MVs bind to collagen and establish the appropriate ratio of inorganic phosphate (Pi), a mineralization promoter, and inorganic pyrophosphate (PPi), a potent mineralization inhibitor, to allow the intraluminally formed apatitic seed crystal to propagate onto the collagenous matrix in the extracellular space (ECS). Current knowledge describes MVs as equipped with the complete biochemical machinery to bind to collagen fibrils and to establish a Pi/PPi ratio conducive for biomineralization [5–8]. In particular, two enzymes on the outer leaflet of the MVs’ membrane—ectonucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and TNAP—are thought to be crucial for the propagation of mineralization by regulating the extracellular Pi/PPi ratio. NPP1 is a metalloenzyme regulated by the binding of Ca2+, Mg2+, and Zn2+ [9–12]. NPP1 is currently thought to act as both a pyrophosphohydrolase and a phosphohydrolase to generate PPi and Pi, respectively, from adenosine-5'-triphosphate (ATP) [12, 13] transported to the extracellular milieu by the function of ATP binding cassette subfamily C member 6 (ABCC6) and ankyrin (ANK) [14]. Due to its ability to generate PPi from phosphorylated substrates, NPP1 plays a role in the inhibition of biomineralization [11, 12]. This function has been shown by in vitro studies in which osteoblasts transfected with an NPP1 cDNA released MVs with a lower ability to mineralize than vesicles released by non-transfected cells [15, 16]. Additionally, by using NPP1-harboring biomimetic membranes, our group has shown that this enzyme may act as both a phosphodiesterase and a phosphohydrolase in the presence of ATP [17, 18]. TNAP is glycosylphosphatidylinositol (GPI)-anchored phosphohydrolase encoded by the ALPL gene and expressed at high levels in the liver, bone, and kidney [17, 19–22]. The activity of TNAP is regulated by the binding of Zn2+ and Mg2+ to the catalytic site [22]. TNAP plays a central role in the perivesicular production of Pi and maintenance of the Pi/PPi ratio by hydrolyzing ATP into adenosine-5'-diphosphate (ADP) and Pi, as well as PPi into two Pi molecules [23]. By using MV-biomimetic lipid structures harboring TNAP, our group has shown that the kinetic properties of TNAP are regulated by the lipid composition of the membrane in which the enzyme is incorporated and by the presence of Zn2+ and Mg2+ ions in the enzyme’s catalytic site [18]. These studies have shown that the presence of sterols (e.g., cholesterol) and sphingomyelin affects the incorporation of the GPI anchor of TNAP into lipid bilayers and, in turn, the catalytic activity of the enzyme [19, 20].
Although current knowledge describes NPP1 and TNAP as key enzymes of MVs for the maintenance of the extracellular Pi/PPi ratio within a range of values conducive to mineralization, if and how these enzymes act in concert in MVs are still unclear. Herein, we addressed this question using proteoliposomes composed by 1,2-dipalmitoylphosphatidylcholine (DPPC) and harboring NPP1 and/or TNAP at different molar ratios and assessing the kinetic of ATP hydrolysis and their ability to induce mineralization in the presence of ATP.
Materials and methods
Materials
All aqueous solutions were prepared using ultrapure apyrogenic water from a Millipore DirectQ system. Bovine serum albumin (BSA), trichloroacetic acid (TCA), tris hydroxymethyl-amino-methane (Tris), sodium dodecylsulfate (SDS), p-nitrophenyl phosphate disodium salt (pNPP), p-nitrophenyl-5’-tymidinmonophosphate (pNP-5’-TMP), sodium adenosine-5-triphosphate (ATP), β- glycerophosphate, polyoxyethylene-9-lauryl ether (polidocanol), and dipalmitoilphosphatidilcholine (DPPC) were purchased from Sigma Chemical Inc. (St Louis, MO). Sodium and magnesium chlorides, methanol, potassium phosphate monobasic anhydrous, and tetrabutylammonium hydrogen sulfate were obtained from Merck KGaA (Darmstadt, Germany). Plastic culture flasks (75 cm2) were obtained from Corning Inc. (Corning, NY). Dulbecco’s modified Eagle’s medium (D-MEM), minimum essential medium α (α-MEM), trypsin, fetal bovine serum, ascorbic acid, gentamicin, and fungizone were purchased from Gibco (Thermo Fisher Scientific Inc., Waltham, MA). All reagents were analytical grade and used as received, without further purification.
Expression and purification of recombinant NPP1 and TNAP
COS-1 (ATCC number CRL- 1650) cells were used for NPP1 expression. The cells were cultured in D-MEM (supplemented with 10 wt% fetal bovine serum) until confluence. After treatment with trypsin, 1.0 × 107 total cells were suspended in 800 µL of HEPES-buffered saline containing 10 µg of plasmid of mice NPP1 with a signal peptide/GPI anchoring site in pCMV-Script. The cell suspensions were placed in an electroporation cuvette (4-mm distance) and electroporated at 220 mV, 960 microfarads, using Gene Pulser (Bio-Rad).
CHO-K1 cells (Chinese hamster ovary, ATCC number CCL-61) were used for TNAP expression. Briefly, the cells were cultured in α-MEM (supplemented with 10% (w/v) fetal bovine serum) until confluence. After treatment with trypsin, 1.0 × 107 total cells were suspended in 800 µL of HEPES-buffered saline, containing 10 µg of plasmid of human TNAP cDNA in pCMV-Script vector. The cell suspensions were placed in an electroporation cuvette (4-mm distance) and electroporated at 400 mV, 250 microfarads, using Gene Pulser (Bio-Rad).
Both electroporated cells were placed in an ice bath for 20 min, then diluted 250 times with their respective growth media (either α-MEM or D-MEM supplemented with 10 wt% fetal bovine serum) and seeded into 15-cm dishes for 21 h. The culture media were replaced by the selection media containing 0.8 mg/mL G418 and replaced every third day. Two weeks later, G418-resistant CHO-K1 cells were harvested for the preparation of membranes containing TNAP. Four days later, G418-resistant COS-1 cells were harvested for the preparation of membrane containing NPP1.
Membrane-bound NPP1 and TNAP were obtained as described by Simão et al. and Ciancaglini et al. [17, 24]. Both membrane-bound enzymes (0.2 mg/mL total protein) were solubilized using 1% polidocanol (w/v) (final concentration) for 2 h, under constant stirring, at 25 °C. After centrifugation at 100,000 × g for 1 h at 4 °C, the solubilized enzymes were concentrated using an Amicon system. All protein concentrations were estimated in the presence of 2 wt% SDS [25]. Bovine serum albumin was used as standard.
Liposomes and proteoliposomes preparation and characterization
DPPC starting solution was prepared by dissolving 1.5 mg of lipids in 1 mL of chloroform. Then, chloroform was evaporated with the aid of a nitrogen flow and the lipid film was resuspended in 50 mM Tris–HCl buffer, pH 7.5, containing 2 mM MgCl2. The mixture was incubated at 60 °C for 60 min, under vigorous stirring using a vortex using 10-min intervals. The mixture was passed through an extrusion system (Avanti) using a 100-nm pore size polycarbonate membrane and stored at 4 °C. Detergent-free NPP1 and TNAP solutions were prepared by incubation in Calbiosorb resin (0.03 mg of resin/mL of enzyme) for 2 h in an ice bath at a constant stirring [26]. Proteoliposomes harboring either pure NPP1 or TNAP and both NPP1 and TNAP in different molar ratios (1:1; 1:10; and 10:1) were prepared by the addition of 0.2 mg/mL detergent-free enzyme to the DPPC-liposome dispersion and incubation for 1 h, at 25 °C. Then, the samples were centrifuged at 100,000 × g for 1 h. The pellet containing the proteoliposomes was resuspended to the original volume using the same buffered solution. The amount of protein incorporated into liposomes was determined as described by Hartree [25]. The TNAP and NPP1 enzymatic activities were determined in both the supernatant and the resuspended pellet. p-Nitrophenylphosphatase (p-NPP) or pNP-5’-TMP activity was assayed discontinuously at 37 °C using UV–Vis spectroscopy, by following the formation of p-nitrophenolate ion (p-NPP−) (Ɛ1M, pH 13 = 17,600 M−1 cm−1) through the changes in the intensity of the absorption band at 410 nm. Standard conditions were 50 mM Tris buffer, pH 7.4, containing 2 mM MgCl2 and using 10 mM p-NPP or 10 mM pNP-5’-TMP as substrate, in a final volume of 0.5 mL. The addition of 1-mM EDTA to completely inhibit NPP1 activity and/or 1 mM ZnCl2 to completely inhibit TNAP activity was used as control [17].
The size distribution and zeta potential (surface charge) of the liposomes and proteoliposomes were analyzed by dynamic light scattering, using a Malvern Zetasizer Nano-ZS ZEN 3600.
Enzymatic assays
The hydrolysis of ATP in the presence of the proteoliposomes harboring TNAP and/or NPP1 in different molar ratios was assayed using 50-mM Tris buffer, pH 7.4, containing 2 mM MgCl2 and 2 mM ATP [18], and stopped with 0.5 mL of TCA 30 vol% at appropriate time intervals. Enzymatic inhibition assays were performed using the same conditions described above, with the addition of 0.5 mM of suramin as NPP1 inhibitor [12]. The reaction mixture was centrifuged at 4000 × g and the supernatant was used for the quantification of the ADP and AMP reaction products by HPLC. For this, a 20.0 µL aliquot of the supernatant was injected into a C18 reversed phase column (Shimadzu) and eluted at 1.5 mL/min using 50 mM phosphate buffer (pH 6.4), containing 5 mM tetrabutylammonium–hydrogen sulfate, and 18 vol% methanol as mobile phase. The absorbance at 260 nm was monitored continuously, and the nucleotide concentrations were determined from the area under the absorbance peaks. All determinations were carried out in triplicate. Assays in the absence of the enzymes but with liposomes were used as control to exclude the non-enzymatic hydrolysis of the substrate.
Mineralization assays
DPPC proteoliposomes harboring TNAP and/or NPP1 in different molar ratios, in the presence and absence of 0.5-mM suramin, were used in the mineralization assays. For this, the vesicles were incubated in synthetic cartilage lymph (SCL) buffer, containing 2 mM Ca2+, 104.5 mM Na+, 133.5 mM Cl−, 63.5 mM sucrose, 16.5 mM Tris, 12.7 mM K+, 5.55 mM glucose, 1.83 mM HCO3−, 0.57 mM SO42−, and 2 mM Mg2+ at pH 7.5 [27, 28]. The assay was performed using 2 mM ATP as source of Pi. Mineral precipitation/propagation was measured by turbidity at 340 nm using a multi-well microplate assay [29]. For this, 300 µL of the samples were placed into the wells of a 96-well microplate. Changes in the initial turbidity (U) values were followed for 24 h at 37 °C, with the aid of a microplate reader (model SpectraMax® M3, Molecular Devices LLC, San Jose, CA). The results were normalized discounting the absorbance value obtained on the first measurement of each proteoliposome. Enzyme-free liposomes dispersed in SCL were used as control. The experiments were carried out in triplicate. The turbidity versus time curves obtained after the incubation of the proteoliposome in SCL (without Pi) presented a characteristic sigmoidal shape. By using these curves, a mathematical approach described by Genge et al. was applied for the determination of mineralization-related parameters. The initial mineralization time (ti) is characterized by a rapid increase in U; the final mineralization time (tf) is characterized by a decrease of U; the time in which the maximum rate of mineral formation is reached (tmax rate) corresponds to the maximum of the dU/dt curve; Umax is the maximum of turbidity at 340 nm; and Umax/tmax rate is the potential of mineral propagation (PMP) that is a measure of the tendency to form mineral [27].
Spectroscopic characterization of the mineral by ATR-FTIR
The mineral precipitated in the presence of the proteoliposomes was placed at the surface of an attenuated total reflectance (ATR) accessory made of a ZnSe crystal to assess the chemical groups by means of FTIR spectroscopy (model IRPrestige-21, Shimadzu Co., Tokyo, Japan). The phosphate-mineral formation was followed by calculating the ratio of the areas of the band corresponding to the asymmetrical stretching of the PO43− group at 1032 cm−1 and the band assigned to the carbonyl (C = O) group of the phospholipid at 1680 cm−1 used as internal reference [28, 29].
Statistical analyses
Kinetic and mineralization data were reported as the mean ± S.D. of triplicate measurements of three independent enzyme/proteoliposome preparations. Statistical analyses were performed by using a paired t-test to evaluate the significancy between the mean of the values obtained in the vesicle characterization. One-way ANOVA (parametrical analysis) test was used to determine the statistically significant differences among the groups at a specific time, while two-way ANOVA was used to determine the statistically significant differences among the reaction times for each group. We considered to be statistically significant values of p < 0.05. We used the following symbols in the figures: *0.01 ≤ p < 0.05, **0.001 ≤ p < 0.01, ***0.0001 ≤ p < 0.001, and ****p < 0.0001.
Results
Preparation and characterization of liposomes and proteoliposomes
Proteoliposomes harboring NPP1 and/or TNAP were fabricated by a direct insertion process previously optimized by our group [17, 19, 20]. This technique is based on the incubation of DPPC-liposomes with the enzyme(s) (detergent-free) for a time sufficient to enable the stabilization of the GPI anchor’s acyl chain in the liposome’s lipid bilayer [19, 26, 30]. The hydrodynamic diameter and polydispersity index (PI) of the fabricated vesicles were characterized by dynamic light scattering (DLS), while the vesicles’ surface charge and total enzyme concentration were characterized by zeta potential and protein quantification assay, respectively (Table 1).
Table 1.
Liposome and proteoliposome characterization. Hydrodynamic diameter, polydispersity index, surface charge, and total enzyme concentration for DPPC-liposomes and DPPC-proteoliposomes harboring NPP1 and/or TNAP. Zeta potential and total enzyme concentration data are reported as the mean ± SD of triplicate measurements of three independent vesicle preparations by t-test statistical, compared to their respective liposome (p < 0.001)
| Vesicle | Diameter (nm) | PI | Surface charge (mV) | [Enzyme] (µg/mL) |
|---|---|---|---|---|
| DPPC | 108.2 | 0.073 | − 4.9 ± 0.9 | - |
| DPPC-NPP1 | 152.8 | 0.302 | − 23.4 ± 0.8 | 100.5 ± 9.5 |
| DPPC-TNAP | 132.6 | 0.241 | − 15.5 ± 0.8 | 85.5 ± 4.5 |
| DPPC-NPP1:TNAP (1:1) | 158.9 | 0.329 | − 19.3 ± 0.8 | 98.1 ± 3.4 |
| DPPC-NPP1:TNAP (10:1) | 145.1 | 0.236 | − 16.4 ± 0.9 | 92.8 ± 5.5 |
| DPPC-NPP1:TNAP (1:10) | 160.8 | 0.316 | − 19.2 ± 0.8 | 89.5 ± 1.5 |
Abbreviations: DPPC, 1,2-dipalmitoylphosphatidylcholine; NPP1, ectonucleotide pyrophosphatase/phosphodiesterase 1; PI, polydispersity index; TNAP, tissue-nonspecific alkaline phosphatase
The liposomes exhibited a diameter of ~ 100 nm with a narrow distribution of sizes (PI < 0.1), while the proteoliposomes exhibited a diameter and PI greater than the liposomes, which suggested the successful insertion of the enzyme(s) in the liposomes’ membrane by GPI anchor [31]. The proteoliposomes also exhibited a value of zeta-potential more negative than the liposomes, which further validated the successful coating of the outer leaflet of the liposomes’ membrane with the enzyme(s) [32]. The yield of enzyme insertion in the liposomes’ membrane was of approximately 50%, in accordance with previous results from our group [17]. The proteoliposomes total enzyme concentration ranged from 85.5 to 100.5 µg/mL. Since NPP1 has a molecular weight of ~ 125 kDa [17] and the molecular weight of the active dimer of TNAP is ~ 120 kDa [10], the proteolipomes’ protein to lipid molar ratio was close to 1:2500 for both the enzymes. The NPP1 to TNAP molar ratios in the proteoliposomes harboring both the enzymes (1:1, 1:10, and 10:1) were confirmed by inhibiting the p-NPPase and pNP-5’-TMPase activities of TNAP and NPP1, respectively, as we previously described [17].
ATP hydrolysis by proteoliposomes
In addition to its inherent phosphodiesterase activity to produce AMP and PPi from ATP and its phosphatase activity to hydrolyze PPi into two Pi molecules, NPP1 also hydrolyzes ATP to produce ADP and Pi [17, 18]. Herein, we assessed how the presence of NPP1 and/or TNAP on the surface of DPPC-liposomes affects both the phosphoesterase and phosphodiesterase activity of the enzymes by measuring the concentration of the nucleosides (ATP, ADP, and AMP) as a function of time by means of HPLC.
All the proteoliposomes tested were able to efficiently hydrolyze ATP. Proteoliposomes harboring NPP1:TNAP at a 1:1 and at a 10:1 molar ratio reduced the initial concentration of ATP the highest (~ 33%, 0.001 ≤ p < 0.01) and the lowest (~ 25%, 0.0001 ≤ p < 0.001), respectively, among the proteoliposomes tested after 48 h of reaction (Fig. 1A). All the proteoliposomes showed the capacity to produce ADP (Fig. 1B) and AMP (Fig. 1C) by ATP hydrolysis. The insertion of NPP1 and TNAP at a 1:1 molar ratio led to the highest value of ADP production by the proteoliposomes after 48 h of reaction (insets in Fig. 1B). Conversely, proteoliposomes harboring NPP1:TNAP at a 10:1 molar ratio showed the lowest amount of ADP and AMP production (insets in Fig. 1B and C). These results would suggest that NPP1 and TNAP present on the surface of MVs synergically hydrolyze ATP and that the synergy was strictly dependent on the molar ratio between the two enzymes (Fig. 1B).The presence of 0.5 mM of suramin inhibited the activity of both NPP1 and TNAP, resulting in a decrease in ATP hydrolysis, and, consequently, a reduction of the amount of ADP and AMP produced, after 48 h of reaction (Fig. 1D–F). The kinetic properties of NPP1 were affected more those of TNAP, as shown by a ~ 22% reduction in ATP hydrolysis respect to the absence of suramin (p < 0.0001) that was significantly greater than the reduction in ATP hydrolysis observed when suramin was added to TNAP-harboring proteoliposomes (0.001 ≤ p < 0.01). Additionally, the addition of suramin significantly reduced the production of ADP and AMP by NPP1-harboring proteoliposomes ~ 63% and 74% (p < 0.0001, compared to the absence of suramin), respectively, which were significantly greater than the reduction in the production of ADP and AMP caused by the addition of suramin to the other types of proteoliposomes (0.0001 ≤ p < 0.001 compared to proteoliposomes harboring NPP1:TNAP at a 1:1 molar ratio, p ≤ 0.0001 compared the proteoliposomes harboring TNAP, NPP1:TNAP at a 1:10 molar ratio, and NPP1:TNAP at a 10:1 molar ratio) (Fig. 2). The latter result suggested that the addition of TNAP to the NPP1-harboring proteoliposomes reduced the effects of suramin on the activity of NPP1. However, as shown in Fig. 2, this effect displayed a non-linear dependence with the NPP1:TNAP molar ratio. Interestingly, the ability of proteoliposomes harboring NPP1:TNAP at a 10:1 molar ratio to produce ADP and AMP was not considerably affected by the presence of suramin, further validating the hypothesis the synergism between NPP1 and TNAP depends on the molar ratio between the two enzymes.
Fig. 1.
Hydrolysis of ATP and formation of ADP and AMP by proteoliposomes harboring TNAP and/or NPP1 in the absence (A, B, and C) and in the presence (D, E, and F) of 0.5 mM of suramin. Changes with time of the concentration of ATP (A, D), ADP (B, E), and AMP (C, F), in dispersions of proteoliposomes harboring NPP1 (red symbols), TNAP (black symbols), NPP1:TNAP at a 1:1 molar ratio (blue symbols), NPP1:TNAP at a 1:10 molar ratio (green symbols), and NPP1:TNAP at a 10:1 molar ratio (magenta symbols). The reaction was initiated by adding 2.0 mM (1000 nmol) of ATP to the vesicle solutions. The insets showed the concentrations of ATP, ADP, and AMP in the proteoliposome dispersions, and the statistical differences among them, after 48 h of reaction, in the absence and in the presence of 0.5 mM of suramin. Data are reported as the mean ± SD of triplicate measurement of three independent enzyme/proteoliposome preparations. One-way ANOVA test was used to determine the statistically significant differences among the groups at a specific reaction time, while two-way ANOVA was used to determine the statistically significant differences among the reaction times for each group. *0.01 ≤ p < 0.05, **0.001 ≤ p < 0.01, ***0.0001 ≤ p < 0.001, and ****p < 0.0001
Fig. 2.

Percentage of inhibition of the activity of NPP1 and/or TNAP reconstituted into DPPC-liposomes by suramin. Percentage of inhibition of ATP hydrolysis (blue bars), ADP production (dark purple bars), and AMP production (light purple bars) by proteoliposomes, after 48 h of reaction. The reaction was initiated by adding 2.0 mM of ATP to the vesicle solutions, either in the absence and in the presence of 0.5 mM of suramin. Data are reported as the mean ± SD of triplicate measurement of three independent enzyme/proteoliposome preparations. One-way ANOVA test was used to determine the statistically significant differences in the percentage of suramin inhibition on ATP hydrolysis, ADP production, and AMP production among the groups of vesicles. **0.001 ≤ p < 0.01, ***0.0001 ≤ p < 0.001, and ****p < 0.0001
In vitro biomineralization mediated by proteoliposomes
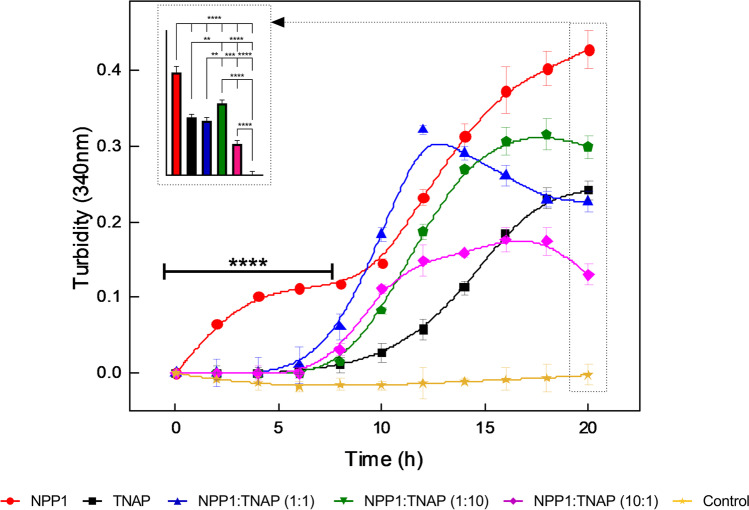
The addition of ATP to a dispersion of DPPC-liposomes did not induce the formation of apatitic minerals, as indicated by the absence of any change in turbidity (Fig. 3). Conversely, the addition of ATP to the dispersion of proteoliposomes led to an increase in the turbidity during the first 20 h of incubation for all the proteoliposomes tested. Notably, NPP1-harboring proteoliposomes exhibited a two-step mineralization curve as suggested by a mineralization curve (red circles in Fig. 3) that statistically differed (p < 0.0001) from that of the other proteoliposomes tested during the first 8 h of mineralization. Conversely, all the other proteoliposomes tested exhibited a single-step mineralization curve.
Fig. 3.
Mineralization curves of proteoliposomes harboring NPP1 and/or TNAP in the presence of ATP. Time-dependent changes in the value of absorbance at 340 nm of dispersions of DPPC-liposomes (control, gold symbols), and proteoliposomes harboring NPP1 (red symbols), TNAP (black symbols), NPP1:TNAP at a 1:1 molar ratio (blue symbols), NPP1:TNAP at a 1:10 molar ratio (green symbols), and NPP1:TNAP at a 10:1 molar ratio (magenta symbols) in the presence of 2.0 mM (1000 nmol) of ATP, during the first 20 h of reaction. Bar graphs (insets) showed the statistical differences in the values of turbidity after 20 h of reaction. Data are reported as the mean ± SD of triplicate measurement of three independent in vitro mineralization assays. One-way ANOVA test was used to determine the statistically significant differences among the groups after 20 h of reaction (inset), while two-way ANOVA was used to determine the statistically significant differences among the reaction times for each group during the first 8 h of reaction. **0.001 ≤ p < 0.01, ***0.0001 ≤ p < 0.001, and **** p < 0.0001
The mineralization curves were fitted by a sigmoidal curve to determine the kinetic parameters (Table 2, the mineralization curve of NPP1-harboring proteoliposomes was fitted by a biphasic sigmoidal curve). NPP1-harboring proteoliposomes exhibited an early mineralization step that reached a lag period at tf = 3.5 h followed by the second mineralization step at ti = 6.6 h (Table 2 and red circles in Fig. 3). At the early mineralization step, NPP1-harboring proteoliposomes exhibited a potential of mineral propagation (PMP) of 0.084 h−1, which was significantly higher than the values of PMP calculated for the late mineralization step of NPP1-harboring proteoliposomes and for the mineralization curves of the other vesicles tested (p < 0.0001) (Table 2 and Fig. 3). Additionally, at the early mineralization step, NPP1-harboring proteoliposomes exhibited a maximum value of turbidity (Umax = 0.11) that was the lowest among the values of Umax calculated (Table 2). Finally, at the late mineralization step, NPP1-harboring proteoliposomes exhibited a PMP value of 0.035 h−1, which was greater than the values calculated for the other vesicles tested (Table 2).
Table 2.
Kinetic parameters of mineral formation. Kinetic parameters obtained from the mineralization curves for liposomes and proteoliposomes incubated with ATP for 24 h. Data were reported as the mean ± SD of triplicate measurements of three independent turbidity assays. Comparison between each type of proteoliposomes and the corresponding liposomes (control) was performed by t-test, while the comparison among the different types of proteoliposomes was performed by one-way ANOVA
| Parameter | Proteoliposome | |||||
|---|---|---|---|---|---|---|
| NPP1 | TNAP | NPP1:TNAP (1:1) | NPP1:TNAP (1:10) | NPP1:TNAP (10:1) | ||
| First-step | Second-step | |||||
| ti (h) | 0 | 6.61 ± 0.42 | 7.92 ± 0.12 | 6.7 ± 0.66 | 6.9 ± 0.51 | 5.2 ± 0.35 |
| tf (h) | 3.49 ± 0.48 | 19.8 ± 0.31 | 21.3 ± 0.71 | 12.7 ± 0.28 | 16 ± 0.49 | 13.9 ± 0.61 |
| tmax rate (h) | 1.31 ± 0.03 | 12.5 ± 0.28 | 13.9 ± 0.56 | 9.5 ± 0.36 | 11 ± 0.36 | 9.1 ± 0.15 |
| Umax | 0.11 ± 0.03 | 0.44 ± 0.02 | 0.27 ± 0.07 | 0.30 ± 0.02 | 0.31 ± 0.02 | 0.16 ± 0.02 |
| PMP (h−1) | 0.084 | 0.035 | 0.019 | 0.032 | 0.028 | 0.018 |
Legend: ti, initial time of mineral formation; tf, final time of mineral formation; tmax rate, time at maximum rate of mineral formation; Umax, maximum value of turbidity; PMP = UMax/tmax rate, potential of mineral propagation
TNAP-harboring proteoliposomes showed the greatest values of initial time (ti = 7.92 h), final time (tf = 21.3 h), and time at maximum rate (tmax rate = 13.9 h) of mineral formation among those calculated for the vesicles tested (Table 2 and Fig. 3, p < 0.0001). Additionally, TNAP-harboring proteoliposomes showed a PMP value of 0.019 h−1, which was significantly lower than the values calculated for the proteoliposomes harboring NPP1, NPP1:TNAP at a 1:1 molar ratio, and NPP1:TNAP at a 1:10 molar ratio (p < 0.0001). The PMP values calculated for the proteoliposomes harboring TNAP and NPP1:TNAP at 10:1 molar ratio were not significantly different.
Addition of NPP1 to TNAP-harboring proteoliposomes at increasing NPP1:TNAP molar ratios (1:10 and 1:1) led to a left shift of the mineralization curve as suggested by the decrease in the initial time, final time, and time at maximum rate of mineral formation with respect to TNAP-harboring proteoliposomes (Table 2 and Fig. 3). The addition of NPP1 to TNAP-harboring proteoliposomes also led to an increase in the maximum value of turbidity with respect to TNAP-harboring proteoliposomes (Table 2). These changes translated in an increase in the potential of mineral propagation (Table 2). A further increase in the NPP1:TNAP molar ratio to 10:1 led to a further decrease in the initial time and time at maximum rate of mineral formation; however, it led to a decrease, rather than an increase, in the maximum value of turbidity and potential of mineral propagation with respect to lower NPP1:TNAP molar ratios (Table 2 and Fig. 3). Finally, the mineralization curve for the proteoliposomes harboring both NPP1 and TNAP exhibited a slight decrease in mineralization at later timepoints. These results may be explained by considering that, due to the greater catalytic efficiency of NPP1 to hydrolyze ATP into ADP and Pi with respect to TNAP, the addition of NPP1 to TNAP-harboring proteoliposomes led to an increase in the rate of Pi production, which translated in the decrease in the initial time and time at maximum rate of mineral formation. The addition of NPP1 also led to an increase in the production rate of PPi due to the phosphodiesterase activity of enzyme. The accumulation of PPi led to a decrease in the Pi/PPi ratio to a value not conducive to mineralization as shown by the lower values of maximum value of turbidity and potential of mineral propagation for the proteoliposomes harboring NPP1 and TNAP at a molar ratio of 10:1.
Spectroscopic analysis of minerals formed by proteoliposomes
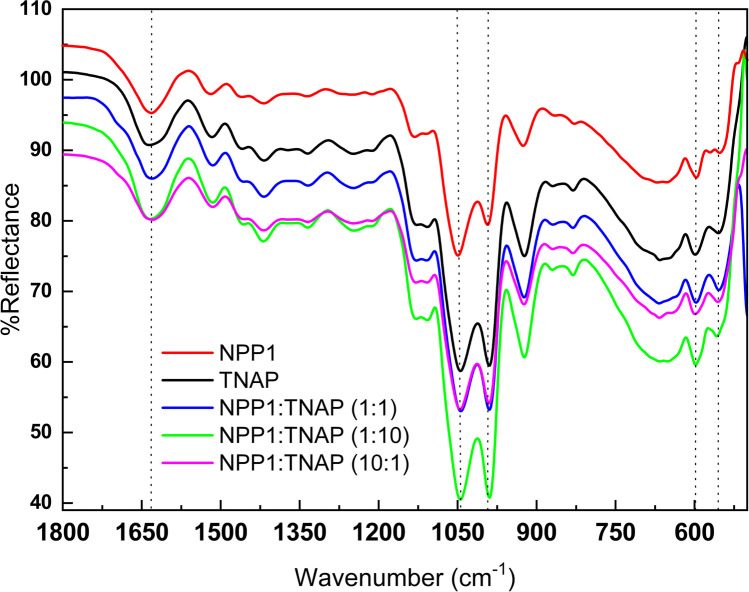
The ability of the proteoliposomes to mineralize was further evaluated by ATR-FTIR spectroscopy (Fig. 4). All ATR-FTIR spectra showed a broad band ranging from 3470 to 3410 cm−1 assigned to the O–H stretching (not shown) [33]. Moreover, a band centered at approximately 1032 cm−1 assigned to the asymmetric stretching of the PO43− group was observed for all the samples [28, 29, 34]. Since it was not possible to distinguish the contribution to the peak at 1032 cm−1 of the phospholipid polar heads from the contribution of the minerals, we calculated the changes in the ratio between the area under the bands (AUB) assigned to the PO43− group (phospholipids and minerals) and carbonyl C = O groups (phospholipids, ~ 1640 cm−1) for both the liposomes (absence of mineralization) and the proteoliposomes (presence of mineralization) [28] (Table 3).
Fig. 4.
ATR-FITR spectra of the minerals produced by proteoliposomes harboring NPP1 and/or TNAP after 20 h of incubation with ATP. ATR-FTIR spectra of the minerals produced by proteoliposomes harboring NPP1 (red), TNAP (black), NPP1:TNAP at a 1:1 molar ratio (blue), NPP1:TNAP at a 1:10 molar ratio (green), and NPP1:TNAP at a 10:1 molar ratio (magenta) in the presence of 2.0 mM of ATP, after 20 h of propagation. The dashed lines indicate the bands used for the analysis of the mineral phase. Data are reported as the mean of triplicate measurement of three independent ATR-FTIR mineral spectra
Table 3.
Ratios between the areas under the bands (AUB) at 1032 (PO43−) and 1640 (C = O) cm−1 calculated from the ATR-FTIR spectra of the mineral obtained in the mineralization in vitro assay by different proteoliposomes in the presence and in the absence of suramin. Data are reported as the mean ± SD of the AUB values of triplicate measurements of three independent ATR-FTIR spectra. Comparison between the AUB values calculated in the presence and in the absence of suramin was performed by t-test
| Proteoliposomes | Suramin (mM) | |
|---|---|---|
| 0 | 0.5 | |
| NPP1 | 3.07 ± 0.22 | 4.40 ± 0.21 |
| TNAP | 4.28 ± 0.10 | 4.35 ± 0.12 |
| NPP1:TNAP (1:1) | 3.65 ± 0.20 | 4.15 ± 0.14 |
| NPP1:TNAP (1:10) | 3.80 ± 0.11 | 4.14 ± 0.18 |
| NPP1:TNAP (10:1) | 3.73 ± 0.04 | 3.82 ± 0.22 |
Among the proteoliposomes, TNAP-harboring and NPP1-harboring proteoliposomes exhibited the highest (4.28) and the lowest (3.07), respectively, values of PO43−/C = O AUB ratio (p < 0.0001), while proteoliposomes harboring both TNAP and NPP1 displayed intermediate values of PO43−/C = O AUB ratio, which did not display a significant dependence with the NPP1:TNAP molar ratio. This result would suggest that the type of the mineral formed by the synergistic action of NPP1 and TNAP depends on the propagation of the mineral (Fig. 3) as well as the kinetics of ATP hydrolysis (Fig. 1A–C). This particular result is a consequence of the dual behaviour resulted from NPP1 transition regarding the substrate (phosphomonohydrolase and phosphodisterease) when in the presence of different TNAP tested ratios.
In the presence of suramin, the value of PO43−/C = O AUB ratio showed a significant increase for all the proteoliposomes tested with respect to the absence of suramin, except for the vesicles harboring NPP1:TNAP at a 10:1 molar ratio. This effect could be explained by the lower action of suramin on these proteoliposomes, as shown in Fig. 2. Taken together, our data suggested that phosphate-containing minerals preferentially formed through a phosphomonohyrolase pathway.
Sauer and Wuthier have used both the relative intensity and position of the P-O absorption bands in the 1130–1030 cm−1 range to analyze the maturation of the mineral formed by MVs incubated into SCL [35]. Less crystalline mineral phases, similar to amorphous calcium phosphate (ACP), exhibit a single band within this region; however, a clear separation of the bands can be observed for more crystalline phases like octacalcium phosphate (OCP) and hydroxyapatite (Hap). In our study, the appearance of two bands in this region revealed that a mature mineral phase that might evolve to either OCP or Hap has been formed. It is worth noting that the intensity of the band at 1030 cm−1 is higher than the intensity of the band at 1070 cm−1 for Hap mineral phase. In this sense, the slightly higher intensity of the band at 1030 cm−1 compared to the band at 1070 cm−1 found in the FTIR spectrum of the minerals precipitated in the presence of TNAP (Fig. 4) suggested that a more mature apatite phase formed in the presence of this enzyme compared to the minerals precipitated in the presence of pure NPP1. Additionally, a symmetrical singlet band in the 600–560 cm−1 region also related to P-O stretching is usually observed for ACP, whereas crystalline apatite minerals show band splitting at this region [36]. The increased intensity of the band at 550 cm−1 stands for the formation of more crystalline minerals in the presence of TNAP, compared to NPP1 (Fig. 4).
Discussion
The result we have obtained for the ATP hydrolysis (Fig. 1A) could be justified by the difference in the values of the catalytic efficiency (kcat/Km) [17, 18] for the proteoliposomes harboring NPP1 and TNAP considering that all kinetic data are statistically different. The amount of ADP produced by the hydrolysis of ATP was higher than the amount of AMP for all the proteoliposomes tested (Fig. 1B) [17, 18]. Additionally, the highest amounts of ADP and AMP were produced by the proteoliposomes harboring both NPP1 and TNAP at a 1:1 molar ratio, while the lowest amounts of ADP and AMP were produced by the proteoliposomes harboring NPP1:TNAP at a 10:1 molar ratio, in accordance with our previous results [17, 18].
Comparing Figs. 1A and 2, it is evident that the ability of the NPP1-harboring proteoliposomes to hydrolyze ATP was reduced by suramin [12, 37]. In addition to being an NPP1 inhibitor, suramin is a well-known inhibitor of P2 receptors [38] and has been used as an antiviral agent, antiparasitic drug, and antidote [12, 38, 39]. The data presented in Fig. 2 shows that, unexpectedly, suramin also affected the activity of the TNAP-harboring proteoliposomes.
Our group has described the ability of TNAP-harboring proteoliposomes to induce mineralization in vitro even in the absence of a nucleator [28, 36]. These studies have also shown that the lipid microenvironment is essential for the initiation and propagation of mineralization [28, 29]. Also, Erceg et al. [33] studied the influence of liposomes with different sizes and surface charges on calcium phosphate precipitation but using supersaturated aqueous solutions. When compared with the respective controls, the liposomes influenced both the precipitation and the transformation kinetics of the morphological phases of the calcium phosphate formed. The greatest effects were observed in liposomes containing phosphatidylserine (PS) and Ca2+ ions, found on the surface of minerals. The results confirm that, despite the differences in the chemical composition, solubility, and/or stabilities of each phospholipid used, there are similarities in the mechanism of formation of calcium phosphate biominerals [40]. In those studies, however, the supersaturation conditions of Ca2+ and Pi ions needed to initiate precipitation were not reached [27, 34]. Instead, in our approach, the enzyme inserted in the proteoliposomes’ membrane hydrolyzed ATP and produced Pi to reach a local concentration conducive to mineralization, which closely mimics the sequence of events mediated by MVs during ossification. Our studies were based on the use of liposomes and TNAP-harboring proteoliposomes made of the same lipids present in the MVs’ membrane, including cholesterol, sphingomyelin, and monosialotetrahexosylganglioside, and showed that the production of Pi by ATP hydrolysis was regulated by the presence of sterols in the vesicles’ membrane, highlighting the importance of the membrane fluidity in the mineral propagation [20, 29]. Calorimetric analysis of liposomes and proteoliposomes indicated lateral phase segregation and microdomain formation in the lipid bilayer only in the presence of cholesterol [19, 20, 29, 41]. Increasing the heterogeneity of the composition of the vesicles’ membrane decreased the activity of the incorporated TNAP. Based on these results, the present study was carried out by using DPPC-liposomes, to maximize the incorporation of the enzymes in the vesicles’ membrane and facilitate the investigation of the role of NPP1 and TNAP in mineral propagation.
The differences in the mineralization behavior of NPP1- and TNAP-harboring proteoliposomes may be explained by considering the different kinetic parameters of the two enzymes. NPP1 has a greater catalytic efficiency using ATP to generate ADP and Pi than TNAP (kcat/Km ~ 448 M−1.s−1 and ~ 382 M−1.s−1 for NPP1 and TNAP, respectively) [17], which would explain the significantly faster increase in turbidity values in the early mineralization step of NPP1-harboring proteoliposomes compared with TNAP-harboring proteoliposomes (p < 0.0001). Mineral formation is sustained not only by the formation of Pi but also by the availability of free Ca2+. As the precipitation of the mineral proceeds, the Gibbs energy of the system decreases driven by the drop in the Ca2+ concentration, and the mineral growth slows down [27]. Mineral formation is also controlled by other properties of the solution (e.g., ionic strength, pH, and PPi concentration) affecting the solubility product (Ksp) of the mineral phase [27, 42]. The presence of a rapid early mineralization step with a low value of Umax suggested that the phosphodiesterase activity of NPP1 (kcat/Km ~ 367 M−1.s−1) led to a rapid accumulation of PPi that hampered the further propagation of mineral until the phosphatase activity of NPP1 reestablished a Pi/PPi ratio conducive to mineralization. This interpretation is compatible with the interpretation by Anderson et al. [43] that while high concentrations of PPi (~ 0.5 mM) are inhibitory for mineral propagation, low concentrations (0.01 to 0.1 mM) can initiate mineral deposition by MVs. The key role of NPP1 in biomineralization has been validated by knock-out mice: a reduced calcium phosphate mineral content was found in the tibias of Enpp1−/− mice and a further decrease in overall mineral deposits was found in [Alpl−/−; Enpp1−/−] double deficient mice [43].
The Pi/PPi ratio regulates not only the rate of mineral formation but also the type of mineral formed [44]. A Pi/PPi ratio of ~ 140 results in the exclusive formation of apatite. Decreasing the Pi/PPi ratio translates in a lower amount of apatite formed until a value of ~ 24 is reached, from which the formation of calcium pyrophosphate is favored [44]. Thus, the slight decrease in mineralization observed for the proteoliposomes harboring both NPP1 and TNAP at the later timepoints could be due to an excessive accumulation of PPi and the formation of different types of calcium phosphate minerals. Unfortunately, we could not record the mineralization curves in the presence of suramin, since this compound strongly adsorbs the light at the wavelength of 340 nm, thus interfering with the turbidity assay.
The maintenance of the extracellular Pi/PPi ratio within a range of values conducive to mineralization is critical for physiological biomineralization. The dysregulation of the extracellular Pi/PPi ratio driven by deficiency (e.g., hypomorphic ALPL mutations) or over-expression of the enzymes involved in the production of Pi and/or PPi has been related to bone-related diseases and ectopic calcification [45–47]. Hypophosphatasia (HPP) is a hereditary disease caused by low TNAP activity that leads to defective hard tissue mineralization [46]. To date, more than 400 mutations spread across the entire ALPL gene have been associated with HPP [46]. HPP leads to rickets in children or osteomalacia in adults, as well as inadequate dental mineralization [46]. Furthermore, reduced mineralization of the ribs in HPP patient results in severe respiratory failure, which is the leading cause of death in severely affected infants and mice phenocopying infantile HPP [46, 48]. The dysregulation of the extracellular Pi/PPi ratio has been also related to the development of ectopic calcifications [45, 46, 49]. Rare diseases such as GACI, PXE, and CALJA, as well as common diseases such as obesity, diabetes, and chronic kidney disease [50], are characterized by reduced concentrations of PPi in extracellular fluids and the deposition of minerals in the vasculature due to TNAP overexpression and/or inadequate expression of NPP1 [45, 51]. In contrast, the overexpression of NPP1 has been associated to the calcification of the cartilage tissue due to precipitation of calcium pyrophosphate dihydrate (CPPD), a form of arthritis often referred to as pseudogout [12, 52]. While in healthy physiological conditions the PPi concentration in the plasma is approximately 1–6 µM, in pseudogout, an increase in the concentration of NPP1 may lead to a local increase in the PPi concentration till a point that the CPPD solubility product (Ksp) is reached (20–37 µM) [12, 46, 52, 53]. Therefore, understanding how TNAP and NPP1 interact mechanistically to regulate the Pi/PPi ratio in biologic fluids is of clinical relevance. Preclinical studies examining calvarial osteoblasts and tissues from Alpl/Enpp1 double knockout mice revealed normalization of PPi concentrations and normalization of in vitro mineralization as well as correction of defective mineralization in the skeleton [54]. The degree of improvement was found to differ between the axial and appendicular skeleton and western blot analyses attributed sustained osteomalacia in the long bones, despite major improvements in the axial skeleton, to the reduced levels of NPP1 in appendicular bones compared to axial bones [55]. The fact that NPP1 is also able to act as a phosphatase helped explain why Alpl knockout mice [18], which are null for TNAP activity, display an HPP phenotype that is less severe than the most severe cases of human HPP reported, such as lethal and perinatal HPP [46]. In the absence of TNAP, NPP1 can act as a backup phosphatase in the extravesicular space to temporarily restrict the concentrations of extracellular PPi thus allowing Alpl knockout mice to develop normal mineralization for the first 6 days of life. After that, the hypomineralization abnormalities become apparent and they die before weaning. This compensatory phosphatase activity of NPP1 and the higher levels of NPP1 in axial bones also help explain how a Phospho1/Alpl double-knockout embryo survived to stillbirth with a partially mineralized axial skeleton, while all other Phospho1/Alpl double knockout mice died in utero with complete absence of skeletal mineralization [56]. Given the clinical need to develop small molecule inhibitors of NPP1 and TNAP to pharmacologically treat ectopic calcification disorders, it is crucial to understand the biochemical behavior of different ratios of NPP1 and TNAP. Herein, we reconstituted TNAP and NPP1 at different molar ratios into the liposomes’ membranes and assessed the kinetic of ATP hydrolysis and ability to mineralize in the presence of ATP of the MV-biomimetic proteoliposomes to study how the enzymes cooperate in the formation of apatitic minerals. After 48 h of incubation, the consumption of ATP by TNAP-harboring proteoliposomes was slightly greater than the consumption by NPP1-harboring proteoliposomes. TNAP- and NPP1-harboring proteoliposomes produced equal amounts of ADP after 48 h of incubation with ATP; however, TNAP-harboring proteoliposomes produced more AMP than NPP1 (Figs. 1C and 2). The in vitro mineralization assays showed that NPP1-harboring proteoliposomes propagate the mineralization by a two-step process in the presence of ATP, while TNAP-harboring proteoliposomes propagate the mineralization by a single-step process (Fig. 3).
Conclusions
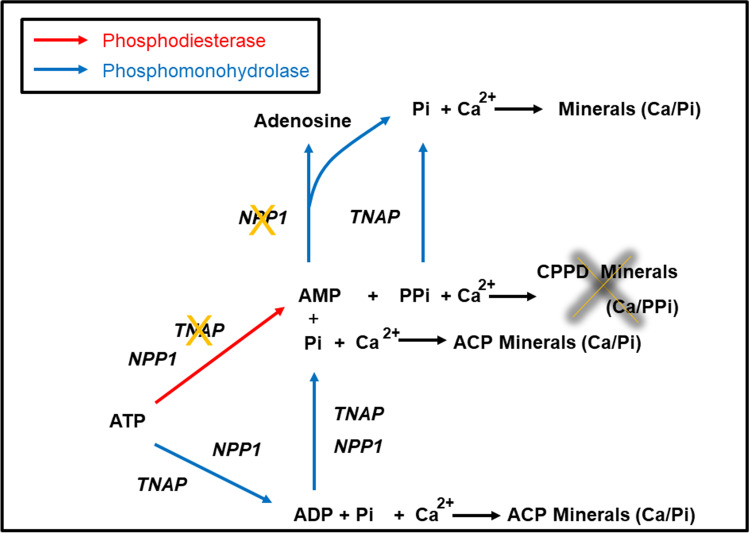
The data obtained in this work suggested that NPP1 uses ATP less efficiently than TNAP and its main role in MVs is to generate PPi from ATP (Fig. 5). The rapid accumulation of PPi may be the reason of the plateau shown in the early step of mineralization by NPP1-harboring proteoliposomes. However, NPP1 can also act as a phosphohydrolase to produce Pi from nucleosides and PPi, respectively (Fig. 5). We posit that the combination of these activities enables NPP1 to increase the local Pi/PPi ratio to support the progression of mineralization through the late step of mineralization. Conversely, the major role of TNAP in MVs is to act as a phosphohydrolase to produce Pi from nucleosides and PPi. However, TNAP-harboring proteoliposomes displayed a low PMP. Addition of NPP1 to TNAP-harboring proteoliposomes at increasing molar ratios led to an increase in the PMP values of the vesicles; however, when the NPP1:TNAP molar ratio was greater than of 1:1, a decrease in the PMP was observed. ATR-FTIR analyses showed that the minerals produced by TNAP-harboring proteoliposomes were structurally more similar to hydroxyapatite than those produced by NPP1-harboring vesicles. Thus, our study strongly suggests that the presence of both NPP1 and TNAP at equimolar concentrations on the membrane’s outer leaflet would be necessary to establish the Pi/PPi ratio required to propagate the formation of crystalline minerals.
Fig. 5.
Correlation between enzymatic pathways catalyzed by proteoliposomes harboring NPP1 and/or TNAP and mineral propagation. The rate-determining pathways are indicated in boldface
Acknowledgements
We wish to dedicate this study to the memory of Professor Geoffrey Burnstock, whose pioneering work led to the development of an entire field of research on purinergic signaling in physiology and pathophysiology. We also would like to thank Mrs. Mercia V. Carlos from the Chemistry Department of FFCLRP-USP for assistance with the HPLC analysis.
Luiz H. S. Andrilli
graduated in Chemistry Licentiate from Chemistry Department – FFCLRP – University of São Paulo. During his master's degree, he studied the properties of NPP1, an enzyme directly involved in the regulation of the bone mineralization process. He is currently a PhD candidate at the University of São Paulo, studying the biophysical and biochemical effects on mineral propagation, as well as studying the biogenesis of Matrix Vesicles (MVs) involved in the biomineralization process.

Funding
We would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2017/20846–2; 2019/08568–2; 2019/25054–2; 2021/13140-1), Coordination for the Improvement of Higher Education Personnel (CAPES Finance Code 001, 1738449, 88887.320304/2019‐00, 88887.368020/2019–00), National Council for Scientific and Technological Development (CNPq, 304021/2017‐2 and 305426/2021–4), PVE-Print CAPES-USP 2020–88887.569449/2020–00, and Bando Visiting Professor – Prot. n. 0058868 12/12/2021—Tor Vergata Università degli Studi di Roma. M. Bolean received CAPES grant and LHSA received FAPESP grant. APR and PC are CNPq researchers.
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
The corresponding author is available to send the documentation of compliance with ethical standards if requested.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Massimo Bottini, Email: massimo.bottini@uniroma2.it.
Pietro Ciancaglini, Email: pietro@ffclrp.usp.br.
References
- 1.Cruz MAE, Ferreira CR, Tovani CB, et al. Phosphatidylserine controls calcium phosphate nucleation and growth on lipid monolayers: a physicochemical understanding of matrix vesicle-driven biomineralization. J Struct Biol. 2020;212:107607. doi: 10.1016/j.jsb.2020.107607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottini M, Mebarek S, Anderson KL, et al. Matrix vesicles from chondrocytes and osteoblasts: their biogenesis, properties, functions and biomimetic models. Biochim Biophys Acta - Gen Subj. 2018;1862:532–546. doi: 10.1016/j.bbagen.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Euw S, Wang Y, Laurent G, et al. Bone mineral: new insights into its chemical composition. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-44620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plaut JS, Strzelecka-Kiliszek A, Bozycki L, et al. Quantitative atomic force microscopy provides new insight into matrix vesicle mineralization. Arch Biochem Biophys. 2019;667:14–21. doi: 10.1016/j.abb.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen MR, Burr DB (2019) Bone growth, modeling, and remodeling. Basic Appl Bone Biol 85–100 10.1016/b978-0-12-813259-3.00005-1
- 6.Galea GL, Zein MR, Allen S, Francis-West P. Making and shaping endochondral and intramembranous bones. Dev Dyn. 2021;250:414–449. doi: 10.1002/dvdy.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee MD, Buss DJ, Reznikov N. Mineral tessellation in bone and the stenciling principle for extracellular matrix mineralization. J Struct Biol. 2022;214:107823. doi: 10.1016/j.jsb.2021.107823. [DOI] [PubMed] [Google Scholar]
- 8.Buss DJ, Kröger R, McKee MD, Reznikov N (2022) Hierarchical organization of bone in three dimensions: a twist of twists. J Struct Biol X 6:0-9 10.1016/j.yjsbx.2021.100057 [DOI] [PMC free article] [PubMed]
- 9.Lowe M, Strauss AW, Alpers R, et al. Molecular cloning and expression of a cDNA encoding the membrane-associated rat intestinal alkaline phosphatase. Biochim Biophys Acta (BBA)/Protein Struct Mol. 1990;1037:170–177. doi: 10.1016/0167-4838(90)90164-B. [DOI] [PubMed] [Google Scholar]
- 10.Le Du MH, Millán JL. Structural evidence of functional divergence in human alkaline phosphatases. J Biol Chem. 2002;277:49808–49814. doi: 10.1074/jbc.M207394200. [DOI] [PubMed] [Google Scholar]
- 11.Jansen S, Perrakis A, Ulens C, et al. Structure of NPP1, an ectonucleotide pyrophosphatase/phosphodiesterase involved in tissue calcification. Structure. 2012;20:1948–1959. doi: 10.1016/j.str.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Lee SY, Müller CE. Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. Medchemcomm. 2017;8:823–840. doi: 10.1039/c7md00015d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang R, Rosenbach M, Vaughn R, et al. Expression of the murine plasma cell nucleotide pyrophosphohydrolase PC-1 is shared by human liver, bone, and cartilage cells. Regulation of PC-1 expression in osteosarcoma cells by transforming growth factor-β. J Clin Invest. 1994;94:560–567. doi: 10.1172/JCI117370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szeri F, Niaziorimi F, Donnelly S, et al (2022) The mineralization regulator ANKH mediates cellular efflux of ATP, not pyrophosphate. J Bone Miner Res 0–3 10.1002/jbmr.4528 [DOI] [PMC free article] [PubMed]
- 15.Terkeltaub R, Rosenbach M, Fong F, Goding J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein–1. Arthritis Rheum. 1994;37:934–941. doi: 10.1002/art.1780370624. [DOI] [PubMed] [Google Scholar]
- 16.Johnson K, Hashimoto S, Lotz M, et al. Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum. 2001;44:1071–1081. doi: 10.1002/1529-0131(200105)44:5<1071::AID-ANR187>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Simão AMS, Yadav MC, Narisawa S, et al. Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics. J Biol Chem. 2010;285:7598–7609. doi: 10.1074/jbc.M109.079830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciancaglini P, Yadav MC, Simão AMS, et al. Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res. 2010;25:716–723. doi: 10.1359/jbmr.091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolean M, Simão AMS, Favarin BZ, et al. The effect of cholesterol on the reconstitution of alkaline phosphatase into liposomes. Biophys Chem. 2010;152:74–79. doi: 10.1016/j.bpc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Favarin BF, Andrade MAR, Bolean M, et al. Effect of the presence of cholesterol in the interfacial microenvironment on the modulation of the alkaline phosphatase activity during in vitro mineralization. Colloids Surfaces B Biointerfaces. 2017;155:466–476. doi: 10.1016/j.colsurfb.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 21.Goettsch C, Strzelecka-Kiliszek A, Bessueille L, et al (2020) TNAP as a therapeutic target for cardiovascular calcification: a discussion of its pleiotropic functions in the body. Cardiovasc Res 0–1310.1093/cvr/cvaa299 [DOI] [PMC free article] [PubMed]
- 22.Millán JL. Alkaline phosphatases. Purinergic Signal. 2006;2:335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciancaglini P, Simão AMS, Camolezi FL, et al. Contribution of matrix vesicles and alkaline phosphatase to ectopic bone formation. Brazilian J Med Biol Res. 2006;39:603–610. doi: 10.1590/S0100-879X2006000500006. [DOI] [PubMed] [Google Scholar]
- 24.Ciancaglini P, Pizauro JM, Rezende AA, et al. Solubilization of membrane-bound matrix-induced alkaline phosphatase with polyoxyethylene 9-lauryl ether (polidocanol): purification and metalloenzyme properties. Int J Biochem. 1990;22:385–392. doi: 10.1016/0020-711X(90)90141-O. [DOI] [PubMed] [Google Scholar]
- 25.Hartree EF. Determination of protein: a modification of the lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 26.Camolezi FL, Daghastanli KRP, Magalhães PP, et al. Construction of an alkaline phosphatase-liposome system: a tool for biomineralization study. Int J Biochem Cell Biol. 2002;34:1091–1101. doi: 10.1016/S1357-2725(02)00029-8. [DOI] [PubMed] [Google Scholar]
- 27.Genge BR, Wu LNY, Wuthier RE. Kinetic analysis of mineral formation during in vitro modeling of matrix vesicle mineralization: effect of annexin A5, phosphatidylserine, and type II collagen. Anal Biochem. 2007;367:159–166. doi: 10.1016/j.ab.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Simão AMS, Bolean M, Favarin BZ, et al. Lipid microenvironment affects the ability of proteoliposomes harboring TNAP to induce mineralization without nucleators. J Bone Miner Metab. 2019;37:607–613. doi: 10.1007/s00774-018-0962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Favarin BZ, Bolean M, Ramos AP, et al. Lipid composition modulates ATP hydrolysis and calcium phosphate mineral propagation by TNAP-harboring proteoliposomes. Arch Biochem Biophys. 2020;691:108482. doi: 10.1016/j.abb.2020.108482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia AF, Simão AMS, Bolean M, et al. Effects of GPI-anchored TNAP on the dynamic structure of model membranes. 2015;17:26295–26301. doi: 10.1039/c5cp02377g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciancaglini P, Simão AMS, Bolean M, et al. Proteoliposomes in nanobiotechnology. Biophys Rev. 2012;4:67–81. doi: 10.1007/s12551-011-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68:701–787. doi: 10.1124/pr.115.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa T, Wakamura M, Kondo S. Surface characterization of calcium hydroxylapatite by Fourier transform infrared spectroscopy. Langmuir. 1989;5:140–144. doi: 10.1021/la00085a025. [DOI] [Google Scholar]
- 34.Wuthier RE, Chin JE, Hale JE, et al. Isolation and characterization of calcium-accumulating matrix vesicles from chondrocytes of chicken epiphyseal growth plate cartilage in primary culture. J Biol Chem. 1985;260:15972–15979. doi: 10.1016/S0021-9258(17)36354-8. [DOI] [PubMed] [Google Scholar]
- 35.Sauer GR, Wuthier RE. Fourier transform infrared characterization of mineral phases formed during induction of mineralization by collagenase-released matrix vesicles in vitro. J Biol Chem. 1988;263:13718–13724. doi: 10.1016/s0021-9258(18)68300-0. [DOI] [PubMed] [Google Scholar]
- 36.Bolean M, Simão AMS, Barioni MB, et al. Biophysical aspects of biomineralization. Biophys Rev. 2017;9:747–760. doi: 10.1007/s12551-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iqbal J, Lévesque SA, Sévigny J, Müller CE. A highly sensitive CE-UV method with dynamic coating of silica-fused capillaries for monitoring of nucleotide pyrophosphatase/phosphodiesterase reactions. Electrophoresis. 2008;29:3685–3693. doi: 10.1002/elps.200800013. [DOI] [PubMed] [Google Scholar]
- 38.Hoyle CHV, Knight GE, Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990;99:617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiedemar N, Hauser DA, Maser P. 100 yeas of suramin. Antimicrob Agents Chemother. 2020;64:1–14. doi: 10.1128/AAC.01168-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erceg I, Kontrec J, Strasser V, et al. Precipitation of calcium phosphates and calcium carbonates in the presence of differently charged liposomes. Minerals. 2022;2:1–20. doi: 10.3390/min12020208. [DOI] [Google Scholar]
- 41.Bolean M, Simão AMS, Favarin BZ, et al. Thermodynamic properties and characterization of proteoliposomes rich in microdomains carrying alkaline phosphatase. Biophys Chem. 2011;158:111–118. doi: 10.1016/j.bpc.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Y, Hahn HH, Hoffmann E. Effects of solution conditions on the precipitation of phosphate for recovery: a thermodynamic evaluation. Chemosphere. 2002;48:1029–1034. doi: 10.1016/S0045-6535(02)00183-2. [DOI] [PubMed] [Google Scholar]
- 43.Anderson HC, Harmey D, Camacho NP, et al. Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. Am J Pathol. 2005;166:1711–1720. doi: 10.1016/S0002-9440(10)62481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thouverey C, Bechkoff G, Pikula S, Buchet R. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthr Cartil. 2009;17:64–72. doi: 10.1016/j.joca.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Eller P, Hochegger K, Feuchtner GM, et al. Impact of ENPP1 genotype on arterial calcification in patients with end-stage renal failure. Nephrol Dial Transplant. 2008;23:321–327. doi: 10.1093/ndt/gfm566. [DOI] [PubMed] [Google Scholar]
- 46.Millán JL, Whyte MP. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int. 2016;98:398–416. doi: 10.1007/s00223-015-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nitschke Y, Rutsch F. Genetics in arterial calcification: lessons learned from rare diseases. Trends Cardiovasc Med. 2012;22:145–149. doi: 10.1016/j.tcm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Whyte MP, Rockman-Greenberg C, Ozono K, et al. Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J Clin Endocrinol Metab. 2016;101:334–342. doi: 10.1210/jc.2015-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yadav MC, Bottini M, Cory E, et al. Skeletal mineralization deficits and impaired biogenesis and function of chondrocyte-derived matrix vesicles in phospho1 -/- and phospho1/P i t1 double-knockout mice. J Bone Miner Res. 2016;31:1275–1286. doi: 10.1002/jbmr.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada BK, Pomozi V, Zoll J, et al. ABCC6, pyrophosphate and ectopic calcification: therapeutic solutions. Int J Mol Sci. 2021;22:1–29. doi: 10.3390/ijms22094555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheen CR, Kuss P, Narisawa S, et al. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res. 2015;30:824–836. doi: 10.1002/jbmr.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Brown MA, Peach C, et al. Investigation of the role of ENPP1 and TNAP genes in chondrocalcinosis. Rheumatology. 2007;46:586–589. doi: 10.1093/rheumatology/kel338. [DOI] [PubMed] [Google Scholar]
- 53.Bennett RM, Lehr JR, McCarty DJ. Factors affecting the solubility of calcium pyrophosphate dihydrate crystals. J Clin Invest. 1975;56:1571–1579. doi: 10.1172/JCI108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hessle L, Johnson KA, Anderson HC, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson HC, Sipe JB, Hessle L, et al. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/S0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yadav MC, Simão AMS, Narisawa S, et al. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.