Abstract
Multiple sclerosis (MS) is a multifactorial neurological disease characterized by chronic inflammation and immune-driven demyelination of the central nervous system (CNS). The rising number of MS cases in the last decade could be partially attributed to environmental changes, among which the alteration of the gut microbiome driven by novel dietary habits is now of particular interest. The intent of this review is to describe how diet can impact the development and course of MS by feeding the gut microbiome. We discuss the role of nutrition and the gut microbiota in MS disease, describing preclinical studies on experimental autoimmune encephalomyelitis (EAE) and clinical studies on dietary interventions in MS, with particular attention to gut metabolites–immune system interactions. Possible tools that target the gut microbiome in MS, such as the use of probiotics, prebiotics and postbiotics, are analyzed as well. Finally, we discuss the open questions and the prospects of these microbiome-targeted therapies for people with MS and for future research.
Keywords: multiple sclerosis, diet, gut microbiota, immune system, probiotics, prebiotics, postbiotics
1. Introduction
Multiple sclerosis (MS) is a neurological disease characterized by inflammation and immune-driven demyelination of the central nervous system (CNS) (1). MS affects 2.5 million people worldwide (2) and is a major cause of progressive disability in young adults in Europe (3), with a higher prevalence in women (women/men ratio of ~2–3:1) (4). MS exists in several forms, with each form characterized by different disease progression and disability manifestations. The most common form of MS is relapsing–remitting MS (RRMS), which is characterized by the alternation of inflammatory acute phases, called relapses, and periods of remission (5). In general, several years after RRMS onset, most patients can develop a secondary progressive form of the disease (secondary progressive MS, SPMS), which is characterized by clinical deterioration, independent of relapses. In a minor proportion of subjects, MS can manifest in a chronic onset as primary progressive MS (PPMS) (6). The pathogenesis of MS is characterized by the presence of pro-inflammatory self-reactive T cells, mainly T-helper 1 (Th1), Th17, and Th22 cells, producing interferon gamma (IFN-γ), interleukin 17 (IL-17) and IL-22, and a deficit of regulatory T cells (Tregs), which lose the ability to suppress the autoreactive response (7–9). B lymphocytes participate in CNS inflammation by producing autoantibodies, presenting antigens, and modulating the response of T cells (10). The imbalance between the autoreactive and regulatory elements results in demyelinating lesions in the CNS (5).
To date, the etiology of MS is not completely understood, but both genetic and environmental factors have been proposed as putative determinants that influence the development and response of the immune system in individuals, predisposing them to MS onset. The principal environmental risk factors associated with MS are Epstein–Barr virus infection, smoking, vitamin D deficiency, and adolescent obesity (11). Obesity in adolescence has been associated with MS (12) and with the risk of pediatric-onset MS (13, 14). Low levels of circulating vitamin D and chronic inflammation are correlated with excess adiposity (15), causing increased levels of leptin that consequently enhance the pro-inflammatory phenotype of leukocytes (16, 17). On the other hand, leptin inhibits the proliferation of Tregs, inducing the hyporesponsiveness of these cells (18). Since 2016, gut dysbiosis has been reported in people with MS (pwMS), suggesting that the gut microbiota composition could be considered a new environmental factor associated with the disease (19–25). Healthy gut microbiota is characterized by an equilibrium between commensals, i.e., beneficial microorganisms with mutualistic interactions with the host, and pathobionts, which have a potential pathogenic influence on the organism (26). Conversely, dysbiosis is defined as alterations in the balance of the gut microbiota composition that can affect the gastrointestinal immune responses and influence distal effector sites through the gut–brain axis (GBA), promoting CNS disease development, including MS (27).
Recent investigations have indicated that the rising number of MS cases worldwide, similar to other autoimmune disorders, can partially be ascribed to rapid environmental changes, such as diet-induced changes that could result in alterations in the human gut microbiota (28). For centuries, dietary habits have been generally based on the consumption of easily accessible foods, such as vegetables, wheat, and rice, and the main protein intake was primarily from legumes, as animal meat was consumed occasionally (29). Modern foods appear safer, are ready-to-eat, and are rich in flavor, with the addition of salt, sugar, fats, and food additives (30, 31). The consumption of meat and the intake of fats have increased in developed countries. Conversely, there has been a decrease in the vegetable content in the diet, with a consequent reduction in its diversity (30, 32). Therefore, a worldwide spread of a new dietary program called “Western diet” (WD) occurred. WD is composed of processedis composed of processed energy-dense food with low content of fiber and vitamins and high content of saturated fats and sucrose (33). WD leads to a selection of gut microorganisms that are more prone to harvesting energy from WD food, triggering the production of bile acids and toxic products for fiber-fermenting bacteria. This results in lower diversity of the microbiota, dysbiotic state, and in intestinal inflammation (34). Diet-induced dysbiosis also promotes intestinal permeability, lipopolysaccharide (LPS)-mediated immune activation, systemic inflammation, and damage of the blood–brain barrier (BBB), which are considered critical pathways for the activation of the microglia and the induction of neuroinflammation in MS (35, 36).
In this review, we will discuss the role of nutrition and its link to the gut microbiota and immune system in MS, describing both preclinical evidence in experimental autoimmune encephalomyelitis (EAE), the most commonly used experimental mouse model of MS (37), and clinical studies on dietary interventions in MS. Finally, we will discuss the possible tools that target the gut microbiome in MS, such the use of probiotics, prebiotics and postbiotics.
2. Nutrition as a risk factor for MS
Developed countries have reported a higher incidence of MS (38), and studies on immigration have shown that moving into a place with high MS cases in the first two decades of life affects the future risk of developing the disease. Nonetheless, the duration of exposure to a new environment rather than the age at moving might also be important (39). Environmental factors that perturbate the gut microbiome in early life could increase the risk of MS. Early antibiotic administration in EAE rats perturbated the composition of the gut microbiota, reduced the levels of short-chain fatty acids (SCFAs), aggravated EAE with stronger immune response in the lymph nodes, and increased inflammation in the CNS (40). Data from an observational study revealed that pwMS born with cesarean section had a younger age of MS onset of 5.2 years compared to those born through natural delivery; similarly, pwMS fed infant formula had a younger age of MS onset of 4.2 years compared to breastfed pwMS. Interestingly, these associations were more apparent in patients without a family history of MS (41). Among the various risk factors for MS onset, diet could also play an important role.
Diet quality indices have been used in multiple investigations, as the associations between high scores were related to a significant reduction in the risk of all-cause mortality, cardiovascular disease, cancer, type 2 diabetes, and neurodegenerative diseases (42). The timing of exposure to modifiable lifestyle risk factors may be crucial in determining the risk of MS, which identified adolescence (6–20 years of age) as a critical period (43). In fact, obesity during adolescence is critical in determining the risk of MS (14, 44, 45). It has been reported that a high score in the dietary inflammatory index, a global index of dietary inflammatory potential, during adolescence increases the risk of MS (46). From the same study, it was found that high consumption of fresh fish, canned tuna, poultry, cheese, yogurt, butter, fruit, vegetables, and dietary supplements during adolescence (13–19 years of age) was associated with a significantly reduced risk of MS between 15 and 50 years (47). Some of the aforementioned foods are considered “healthy” in regions where food insecurity is high, indicating that nutritional status could be a factor in adolescent MS risk (48). A recent study has demonstrated that the consumption of healthy foods including fruits, yogurt, and legumes at various periods between childhood and young adulthood was associated with a reduced probability of adult-onset MS (49). Given the limitations of these studies, such as the retrospective measures of diets and the presence of confounders, further investigations are required in order to confirm the role of diet quality in adolescence and the likelihood of adult-onset MS.
Diet quality appears to have a role in the health status of pwMS. In a large international sample of pwMS within the Health Outcomes in a Sample of People with MS (HOLISM) study, the authors demonstrated that every 10-point increase in the total score of the Diet Habits Questionnaire was associated with nearly a six- and a five-point increase in the physical and mental health-related quality of life (QoL), respectively, and a 30% reduced likelihood of a higher level of disability (50). These data were confirmed in a large follow-up cross-sectional survey in which participants in the North American Research Committee on MS (NARCOMS) Registry completed a dietary screener questionnaire that estimates the intake of fruits, vegetables and legumes, whole grains, added sugars, and red/processed meats. Participants with diet quality scores in the highest quintile had lower levels of disability and lower depression scores. In addition, pwMS reporting a composite healthy lifestyle had lower odds of reporting severe fatigue, depression, pain, or cognitive impairment (51). In a longitudinal magnetic resonance imaging (MRI) study, it has been reported that pwMS with unhealthier diet preferences, with low intake of fruits, vegetables, and whole grains coupled with higher consumption of sugary beverages and red meats, had higher T2 lesion volume accrual over the 5-year follow-up period (52). In an investigation on Dutch women with MS, an association was found between diet quality and both physical and mental QoL (53). Furthermore, it has been shown that a healthier diet score, with high consumption of fiber, fruits, and vegetables, was associated with better mental, physical, and total QoL, lower depression and pain, and fewer cognition, vision, and bowel symptoms (54). The mechanisms of action by which diet quality could reduce disability, fatigue, and cognitive impairments are versatile. Diet may be linked with inflammatory disease activity and disease-related degeneration (55). Moreover, it can also act through the prevention of vascular comorbidities, which are associated with a more rapid disability progression in MS, increased disease activity, lower brain volume, and neuroperformance (56–58). Lastly, diet–microbiome interactions could sustain local inflammation, gut microbiota imbalance, and host immune dysregulation in MS, driving disease onset and progression (28).
3. Relationship between the gut microbiota and immune system in MS
Gut dysbiosis has been associated with a variety of disorders including autoimmune and CNS-related ones (59), such as MS, due to its ability to modulate the immune responses in the gut-associated lymphoid tissues (GALT) (60). GALT includes Peyer’s patches and isolated lymphoid follicles where antigen-presenting cells (APCs), B and T cells, and IgA+ plasmablasts reside (60). The gut microbiota–immune system communication consists of several complex pathways including antimicrobial peptides (AMPs), pattern recognition receptor systems (PRRs), serotonin, and metabolites. Briefly, signals from commensal (and pathogenic) microbiota produce a cascade of interaction involving, first, epithelial cells, dendritic cells (DCs), macrophages, and innate lymphoid cells, and then cells of the adaptive immune system, with the final aim of maintaining homeostasis and regulation of immune system development and maturation. All these pathways have been described in depth by Zheng et al. (61); therefore, here, we will focus on the mechanisms involved in the regulation of the immune system by the gut microbiota in MS.
The first evidence on the association between the gut microbiota and the immune system in the development of immune diseases dated back to 2011 (62, 63), when it was demonstrated that germ-free mice developed attenuated EAE and produced lower pro-inflammatory cytokines and more Tregs compared to mice with a normal intestinal microbial composition. In addition, it was reported that the presence of some bacteria in the intestine, such as the short filamentous bacteria (SBF), can promote the activation of Th17 cells, providing evidence of a relationship between the activation of immune cells in the gut and neurological inflammation (62). Specifically, the metabolites produced by SBF activate macrophages, which, on the one hand, contribute to the synthesis of IL-23 and, on the other hand, act as APCs toward T cells, which then differentiate into Th17 cells (64). Another resident of the human gut microbiome influencing T-cell homeostasis is the symbiont Bacteroides fragilis. Polysaccharide A (PSA), the most abundant capsular polysaccharide expressed by B. fragilis, mediates the conversion of CD4+ T cells into IL-10-producing Foxp3+ Tregs via Toll-like receptor 2 (TLR2) and suppresses Th17 responses (65–67). In addition, oral administration of PSA from B. fragilis has been associated with a lower EAE “clinical” score in an IL-10- and TLR2-dependent manner (68) and with higher frequencies in the CNS-draining lymph nodes of CD39+ Tregs (69).
The intestinal microbiota interacts with the immune system also through serotonin, a neurotransmitter produced by the metabolism of dietary tryptophan, which appears to influence the immune system during neuroinflammation. It has been reported that high levels of this neurotransmitter in the intestine attenuated the severity of EAE in mice (70), while it promoted the suppression of IL-17 and IFN-γ release in MS (71). Moreover, studies reported that Akkermansia muciniphila can release vesicles that increase the serotonin levels in the hippocampus and colon (72). Tryptophan metabolites have also been reported to influence CNS inflammation via the transcription factor aryl hydrocarbon receptor (AHR) (73). AHR is a cytoplasmic receptor whose activity can regulate autoimmunity via natural killer cells, macrophages, DCs, and T cells (74). Recently, it has been reported that the knockdown of AHR led to a recovery of chronic EAE, impacting also the production of microbiota metabolites by increasing bile acids and SCFAs (74).
SCFAs [e.g., acetate, propionate (PA), and butyrate] are the major metabolites of bacterial fermentation of dietary fibers and are capable of inducing the differentiation of Tregs in a lot of ways (75, 76), including: 1) acting as histone deacetylase (HDAC) inhibitors, thus enhancing histone H3 acetylation in the promoter and enhancer regions of the Foxp3 gene, the master regulator of Tregs (75); 2) involving G-protein-coupled receptor 43 (GPCR43), which regulates intestinal inflammation through regulating the neutrophil chemotaxis and mediating cytokine expression (77); 3) inducing IL-10 and retinoic acid production from DCs (78); and 4) reducing the proliferation of IL-22+ ILC3 cells (79). Several bacteria produce SCFAs, including Butyricimonas, Faecalibacterium, and Clostridium cluster IV and XIVa, which are reduced in MS, and Akkermansia, which has conversely been reported to increase in MS (19, 21, 76, 80–82). Treatment with SCFAs has been reported to ameliorate EAE via long-lasting imprinting on Tregs (83).
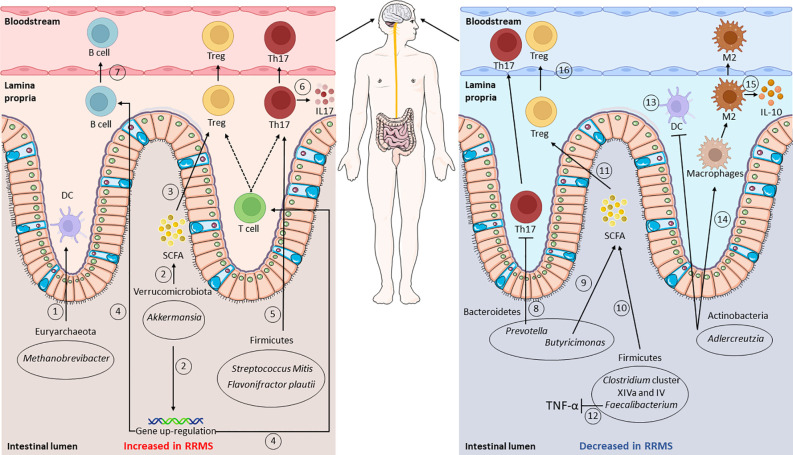
Several studies on pwMS have reported the presence of gut dysbiosis in MS (22, 24, 84). Figure 1 and Table 1 describe the findings of the most relevant articles, reporting the microorganisms found altered in pwMS, together with their effect on the immune system (19–21, 25, 76, 80–82, 85–93). Overall, a peculiar gut microbiome signature has never been identified, highlighting the need to study large cohorts. Recently, the International MS Microbiome Study (IMSMS) was constituted with the aim of investigating the role of the gut microbiota in MS, as well as evaluating the interrelationship between disease-modifying therapies and gut microbial communities in pwMS. The first results have been published in late 2022 and strongly supported specific gut microbiome associations with MS risk, course, progression, and functional changes in response to treatment (82). Alterations in the gut microbiota composition could also be found in other inflammatory autoimmune diseases. Similarly to MS, the SCFA-producing genera Faecalibacterium and Bacteroides appeared reduced in rheumatoid arthritis (RA) compared to healthy subjects (94). In inflammatory bowel disease, a decrease in the level of Faecalibacterium prausnitzii has also been described (95). Furthermore, Prevotella spp. was found reduced in both type 1 diabetes and spondyloarthritis (Prevotella strain 9), as also occurs in MS (94, 96). Finally, an increase in the level of Streptococcus has also been observed in systemic lupus erythematosus (97).
Figure 1.
Gut microbiota composition and functions in relapsing–remitting multiple sclerosis (RRMS). Gut dysbiosis is observed in RRMS: intestinal microorganisms identified as increased, compared to healthy controls, are shown in the left panel, whereas those that decreased are shown on the right. Putative mechanisms affecting the immune system are shown: an increase in Methanobrevibacter leads to a higher dendritic cell (DC) recruitment (1), while augmented Akkermansia levels are related to a higher short-chain fatty acid (SCFA) production and regulatory T-cell (Treg) differentiation (2, 3). Higher Akkermansia levels also lead to the upregulation of the genes involved in T- and B-receptor signaling (4). Raised levels of Firmicutes, such as Streptococcus oralis and Flavonifractor plautii, affect T-cell activity, promoting Th17 cell differentiation and IL-17 production (5, 6). In contrast, the levels of Prevotella appear decreased, thus leading to an increase in Th17 cells (8). The levels of Butyricimonas, Clostridium cluster XIVa and IV, and Faecalibacterium result impaired, with consequent lower SCFA production (9, 10) and Treg differentiation (11). Impaired Faecalibacterium levels also lead to higher TNF-α levels (12). Lastly, lower Adlercreutzia levels result in higher CD4+ T-cell priming from DCs (13), in lower macrophage type 2 (M2) polarization (14), and reduced IL-10 production (15). From the lamina propria, cells then migrate into the bloodstream, reaching the central nervous system (CNS) (7, 16). This figure was partly generated using Servier Medical Art provided by Servier,licensed under a Creative Commons Attribution 3.0 Unported License; created with BioRender.com.
Table 1.
Microorganisms altered in multiple sclerosis (MS).
| Kingdom | Phylum | Class | Order | Family | Genus | Species | Abundance | Effects on the immune system | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Proteobacteria | Alphaproteobacteria | Rhizobiales | Brucellaceae | Mycoplana | Increased in RRMS | (20) | ||
| Hyphomicrobiaceae | Gemmiger | Increased in PPMS | (85) | ||||||
| Betaproteobacteria | Burkholderiales | Sutterellaceae | Sutterella | Decreased in MS | (19) | ||||
| Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | Increased in RRMS | (20) | ||||
| Pasteurellales | Pasteurellales | Haemophilus | Decreased in MS | (20) | |||||
| Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Increased in pediatric MS | (25) | |||||
| Bacteroidetes | Sphingobacteriia | Sphingobacteriales | Sphingobacteriaceae | Pedobacter | Increased in RRMS | (20) | |||
| Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | Decreased in MS | (19) | ||||
| Porphyromonadaceae | Parabacteroides | Decreased in MS | (20) | ||||||
| Butyricimonas | Decreased in MS | Involved in SCFA production | (21, 76) | ||||||
| Prevotellaceae | Prevotella | Decreased in MS | Linear negative relationship with the intestinal Th17 frequency | (86) | |||||
| Paraprevotella | Decreased at MS onset | (87) | |||||||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Blautia | Increased in RRMS | (20) | |||
| Blautia | Decreased at MS onset | (87) | |||||||
| Dorea | Increased in RRMS | (20) | |||||||
| Ruminococcacceae | Faecalibacterium | Decreased in MS | Positively associated with SCFA production and negatively with TNF-α levels | (19, 80) | |||||
| Anaerotruncus | Decreased at MS onset | (87) | |||||||
| Subdoligranulum | Decreased at MS onset | (87) | |||||||
| Unclassified | Increased in PPMS | (85) | |||||||
| Ruminococcaceae | Decreased in pediatric MS | (25) | |||||||
| Eubacteriales | Oscillospiraceae | Flavonifractor | Flavonifractor plautii | Increased in MS | Affects CD4+ T cells and IL-17 | (88–90) | |||
| Lachnospiraceae | Decreased in pediatric MS | (25) | |||||||
| Clostridium cluster IV and XIVa | Decreased in MS | Affects SCFAs, Tregs, and anti-inflammatory cytokine production | (19) | ||||||
| Bacilli | Lactobacillales | Streptococcaceae | Streptococcus | Streptococcus thermophilus | Increased in MS | (19) | |||
| Streptococcus salivarius | Increased in MS | (19) | |||||||
| Streptococcus oralis | Increased in MS | (86) | |||||||
| Streptococcus mitis | Increased in MS | Promotes Th17 differentiation and participates in cell-mediated tissue damage in autoimmunity | (86, 91) | ||||||
| Negativicutes | Selenomonadales | Veillonellaceae | Megasphaera | Decreased at MS onset | (87) | ||||
| Mitsuokella | Decreased at MS onset | (87) | |||||||
| Lactobacillaceae | Lactobacillus | Decreased in MS | (20) | ||||||
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Akkermansiaceae | Akkermansia | Increased in MS | Associated with SCFA production and pro-inflammatory effects | (21, 81, 82) | ||
| Akkermansia muciniphila | Increased in PPMS | (85) | |||||||
| Actinobacteria | Coriobacterideae | Coriobacteriales | Coriobacteriaceae | Eggerthella | Eggerthella lenta | Increased in MS | (19) | ||
| Collinsella | Decreased in MS | (20, 21) | |||||||
| Adlercreutzia | Decreased in MS | Affects M2 polarization, IL-10 expression in M2 macrophages, and inhibition of CD4+ T-cell priming by DCs | (20, 92) | ||||||
| Archaea | Euryarchaeota | Methanobacteria | Methanobacteriales | Methanobacteriaceae | Methanobrevibacter | Increased in MS | Recruitment of inflammatory cells and DCs | (21, 93) | |
| Heunggongvirae | Uroviricota | Caudovirales | Increased in MS | (88) |
Shown are the sets of microorganisms altered in MS, MS onset, RRMS, PPMS, and pediatric MS. Along with their abundance in MS, their role in the immune system is also reported.
RRMS, relapsing–remitting multiple sclerosis; PPMS, primary progressive multiple sclerosis; SCFA, short-chain fatty acid; DCs, dendritic cells.
4. Experimental evidence of gut microbiota induced diet modulation in EAE
The EAE model has been used to examine the putative effects of diet on the disease and on the gut microbiota (98). Different studies have focused on evaluating the role of specific diets (99–101) or food components such as fats (83), salt (102), and isoflavone (103), showing that some of these components have an impact on the disease course or symptom severity of EAE. However, it is not clear whether these effects are mediated by the gut microbiota, the modulation of inflammatory pathways, an improved mitochondrial function, the reduction of reactive oxygen species (ROS), or by other mechanisms (98). In this section, we explore the evidence on protection from or exacerbation of EAE following modification of the gut microbiome induced by diet.
4.1. High-fat diet
A high-fat diet, typical of the Western lifestyle, is linked to obesity and has been shown to increase the severity of EAE, impacting the immune system balance (99, 101, 104). Different types of fatty acids in terms of chemical chain length could have different effects. It was reported that long-chain fatty acids (LCFAs) enhanced the differentiation and proliferation of Th1 and/or Th17 cells and impaired their intestinal sequestration via the p38/mitogen-activated protein kinase (MAPK) pathway (83). Alternatively, dietary SCFAs produced by the microbiota expanded gut Tregs through the suppression of the Jun N-terminal kinase 1 (JNK1) and p38 pathway. These effects could be mediated by the gut microbiota, as treatment with lauric acid LCFA reduced the concentrations of Prevotellaceae and Bacteroides and the number of fecal SCFAs. The gut microbiota was found to be necessary for the effect of LCFA on the increase in Th17 cells, as no Th17 cells were detectable in the small intestine after feeding LCFA to control germ-free mice (83).
4.2. High-salt diet
Salt is a widely consumed nutrient in the Western lifestyle. In a mouse model, a high-salt diet increased the CNS infiltration of Th17 and exacerbated the actively induced EAE. This effect was mediated by the gut microbiota: a high-salt diet decreased the level of Lactobacillus murinus, but restoration with L. murinus supplementation mitigated the effects of the high-salt diet, leading to the amelioration of EAE (102). In a C57BL/6 mouse model, a high-salt diet also elicited an inflammatory environment, triggered apoptosis in the brain, caused gut dysbiosis, and reduced the production of SCFAs (105).
4.3. High-sugar diet
Added sugars are highly present in WD (106). Long-term consumption of caffeine-free high-sucrose cola beverages aggravates the pathogenesis of EAE in a microbiota-dependent manner. Indeed, it leads to specific microbial taxon selection and to Th17 increase. This effect was mediated by the gut microbiota, as mice depleted of microbiota via antibiotics before the induction of EAE were shown to be less susceptible to the disease (107). The microbiota is probably not the only pathway involved in sugar-driven Th17 activation. It was observed that a high amount of glucose promoted Th17 cell differentiation by activating transforming growth factor alpha (TGF-α) from its latent form through the upregulation of mitochondrial ROS in T cells (108).
4.4. Dietary fibers
Conversely, dietary fibers are highly consumed in non-industrialized societies, where the incidence of non-communicable diseases is lower (109). The fermentation of dietary fibers by gut microbes increases the production of SCFAs, which has an effect on the immune system, but it appeared that dietary non-fermentable cellulose fiber intake could also protect EAE mice from the disease independently of SCFAs (110). In particular, cellulose-fed mice displayed significant changes in the bacterial microbiota composition and expanded the Th2 cell population, leading to protection from the disease. Interestingly, cecal metabolites from mice raised on a cellulose-rich diet stimulated the Th2 immune response with increased IL-5 production compared to cecal extracts from control diet-fed animals. Although the Th2 cytokine neutralization did not fully restore spontaneous EAE to a level comparable to the control diet, the additional effects of the cellulose-rich microbiome on T cells and other immune cells may have disease-protective effects (111).
4.5. Dietary tryptophan
Dietary protein restriction was shown to interfere with systemic immune responses (112). Specifically, tryptophan is an essential amino acid present in food such as meats, dairy, legumes, and grains, and its metabolites are crucial determinants of organ inflammation. It was reported that a complete deficiency in dietary tryptophan was sufficient to inhibit encephalitogenic T-cell responses and prevent CNS autoimmunity in EAE. This mechanism is mediated by microbiota, as a complex change in the gut microbiome composition induced by dietary tryptophan restriction was observed, with alterations also in the bacterial catabolite levels (113). These catabolites are known to exert a plethora of immunomodulatory functions via the AHR pathway (114). Paradoxically, if dietary tryptophan is removed post-EAE onset, the clinical disease progresses continuously, while mice fed a control diet proceed with disease recovery (115, 116). This form of the disease mediated by post-EAE tryptophan restriction can be mitigated by supplementation with the microbially derived AHR ligand indoxyl-3-sulfate, providing further evidence of the involvement of tryptophan-metabolizing bacteria and the anti-inflammatory effects of the AHR agonist (115).
4.6. Isoflavones
Phytoestrogens are food components that can influence the gut microbiota composition and systemic immune responses (92). Isoflavones are a major class of phytoestrogens that are highly abundant in legumes like soybeans. Recently, it has been observed that an isoflavone diet offered protection against EAE (103, 117). Isoflavone dietary content can lead to insufficient priming in the periphery after disease induction and to a decrease in the severity of spinal cord pathology. Moreover, it can reduce the number of inflammatory cells in the CNS and the frequency of activated myelin-specific CD4+ T cells. An isoflavone diet confers the microbiota anti-inflammatory characteristics comparable to a healthy gut microbiome in humans. In particular, the gut microbiota includes specific bacteria that can metabolize isoflavone into S-equol, a metabolite that provides protection from disease. However, the cellular and molecular pathways by which the gut bacterium-generated S-equol suppresses disease are unknown. Interestingly, an isoflavone diet increases the abundance of Parabacteroides distasonis and Adlercreutzia equolifaciens, and bacterial therapy with these strains protects against EAE only when the host follows an isoflavone diet (103).
4.7. Dietary restriction regimes
Dietary restriction (DR) is a term derived from dietary regimens used in experimental models in which there is a lower energy intake compared to ad libitum but without malnutrition (100). The number of calories provided could have an effect on the immune system and the gut microbiota: DR regimens have protective effects against EAE (100). DR induces hormonal, metabolic, and cytokine changes, reducing the severity of clinical EAE (118–120). It is plausible that protection is mediated by the gut microbiota, although the precise role of DR-induced perturbation of the gut flora in conferring these effects is largely unknown (100, 121). In mice with an intermittent fasting (IF) diet, a form of DR, an amelioration in the pathology and the clinical course of EAE was observed (121). IF changed the microbiota composition with an enrichment of Lactobacillaceae, Bacteroidaceae, and Prevotellaceae. Interestingly, a healthier gut microbiota was not the consequence of a better clinical course, but probably the cause, as fecal microbiome transplantation from IF mice to immunized mice on a normal diet ameliorated EAE (121).
5. Dietary interventions in EAE and MS: state of the art
Diet is the major determinant of gut microbiota composition, and the beneficial effects of some dietary interventions are currently under investigation. In this section, we will explore the effects of some dietary interventions on EAE and MS.
5.1. Mediterranean diet
The Mediterranean diet (MD) is the best-studied and most evidence-based diet that results able to prevent both cardiovascular disease and several other chronic diseases, including the neurodegenerative ones (122, 123). MD encompasses a lot of aspects beyond nutritional behavior, including social, cultural, economic, and environmental factors. The association of cultural and nutritional features with physical activity is enclosed into the MD model, making it widely considered to be a healthy lifestyle rather than just a dietary pattern (124). MD is characterized by the consumption of vegetables, fruits, whole grains, legumes, nuts, and olive oil and by reduced consumption of red meats, saturated fats, poultry, and dairy (28). MD is associated with increased microbial diversity (30) and SCFAs, the major metabolites of bacterial fermentation of dietary fibers. No specific component of the MD has been shown to be as beneficial as the whole diet (123).
Investigations into the role of MD in MS are quite recent (125–127). MD could potentially modulate the chronic inflammatory state (128), have an impact on the gut microbiome (129), and prevent vascular comorbidities (123). A multicenter study on pediatric patients with RRMS or clinically isolated syndrome (CIS) investigated how the consumption of one food component at the bottom of the MD pyramid, e.g., vegetables, and one at the top, e.g., saturated fat, could impact MS. This study showed that each 10% increase in saturated fat tripled the hazard of relapse. In contrast, each additional cup equivalent of vegetables decreased the hazard of relapse by 50% (130). In another study on pwMS, a higher adherence to the MD was associated with normal waist circumference and was inversely correlated with the MS Severity Score (MSSS) and Expanded Disability Status Scale (EDSS) score (125). Moreover, the MD-style diet was associated with reduced fatigue severity (131). Furthermore, in a recent cross-sectional study, a higher MD alignment, measured with the Mediterranean Diet Adherence Screener (MEDAS), attenuated the negative impact of disease duration on the Multiple Sclerosis Functional Composite (MSFC). In fact, pwMS in the third and fourth MEDAS quartile MSFC scores were quite similar between the longer (more than 14 years) and shorter (less than 14 years) disease duration groups (132). In a pilot randomized controlled trial, researchers assigned following or not following an MD intervention for 6 months. The diet encouraged the intake of fish and other foods high in poly- and monounsaturated fats, fresh fruits, vegetables, and whole grains; eliminated meat, dairy, and processed foods; and limited the salt intake to <2 g/day. The intervention group exhibited a statistically significant decline in the trajectory of the Neurological Fatigue Index MS scores, a trend toward reduced MS symptoms, measured by the Multiple Sclerosis Impact Scale—29, and a reduction in the EDSS score over time compared to the non-intervention group (127). It was also reported that 6 months of MD reduced the dietary inflammatory status score compared to controls (126). As clinical outcomes, participants who followed MD had a statistically significant improvement in the physical and cognitive fatigue severity, although MD did not elicit any improvement in disease-related disability as measured by EDSS (126).
In non-neurological cohorts, it was demonstrated that MD could affect the microbiota composition, diversity, and activity, with beneficial effects on host metabolism (133–137). Data on its beneficial effect on MS are still novel. In a pilot study, the effects on the gut microbiome of a high-vegetable/low-protein (HV/LP) diet, which had a similar composition to the MD, were compared to the WD in people with RRMS. The HV/LP group, compared to the WD group, showed increased abundance of the butyrate-producing bacteria Lachnospiraceae family in the gut microbiota, decreased pro-inflammatory IL-17+ and programmed death 1 (PD-1)+ T cells, and increased anti-inflammatory programmed death-ligand 1 (PD-L1)+ monocytes. As clinical outcomes, a significant reduction of the EDSS score and the relapse rate was observed during follow-up in the HV/LP group (138). Interestingly, a recent study on pwMS has observed a positive correlation between meat consumption and the concentrations of circulating Th17 cells. Combining the data from the MS and healthy control cohorts, the authors found that meat consumption was negatively correlated with the relative abundance of Bacteroides thetaiotamicron, a common gut bacterium with high genetic capacity to digest polysaccharides. B. thetaiotamicron was also strongly negatively correlated with circulating Th17 cells, while Th17 cells were positively correlated with meat intake. Five blood metabolites were significantly correlated with all three measurements. These data suggest a network involving dietary meat consumption, the gut microbiome, Th17 cells, and blood metabolites (139).
MD could be combined with other interventions, such as rehabilitation programs to maximize its beneficial effects. A recent study has evaluated the effects of a brief high-impact multidimensional rehabilitation program (B-HIPE) in a leisure environment on the gut microbiota, which mitigated MS symptoms and improved QoL. Adherence to B-HIPE, which included MD in its 1-week program, resulted in a significant reduction in Coriobacteriaceae and Peptostreptococcaceae, as well as in an enrichment of Bacteroidaceae and Barnesiellaceae. A depletion of Collinsella and Ruminococcus, together with an enrichment of Bacteroides, Sutterella, and Oscillospira, was observed. Interestingly, Ruminococcus and Collinsella, which were depleted by the B-HIPE intervention, positively correlated with Th17 cell abundance, supporting autoimmune neuroinflammation (140).
5.2. Ketogenic diet
The ketogenic diet (KD) is characterized as a high-fat, adequate-protein, and low-carbohydrate diet. In the absence of an adequate amount of carbohydrates, the liver converts fats into ketone bodies, replacing glucose as the primary energy source (141). KD has been used to reduce body weight and improve metabolic disorders (142).
In neurology, KD has been well-documented as a dietary intervention for children and adolescents with refractory epilepsy (143). Beta-hydroxybutyrate (BHB) and acetoacetate (ACA) are the two principal ketone bodies produced in KD. They possess potential neuroprotective and anti-inflammatory properties as they can promote the reduction of oxidative stress, the maintenance of mitochondrial function, the regulation of epigenetic modifications and can affect the composition of the gut microbiome (144). In EAE, KD reduced brain inflammation with improvement in motor disability, CA1 hippocampal synaptic plasticity, and spatial learning and memory (145). Moreover, KD in cuprizone (CPZ)-induced demyelination mice improved the behavioral and motor abnormalities and ameliorated the spatial learning and memory deficits. KD also reduced the hippocampal demyelination, inhibited the activation of the microglia and reactive astrocytes, attenuated the CPZ-induced oxidative stress, and modulated the SIRT1/PPAR-γ and SIRT1/P-Akt/mTOR pathways (146).
In pwMS, KD improved the fatigue and depression scores with a reduction of the body mass index (BMI), total fat mass, and serologic leptin (147). In addition, an improvement in the QoL, a reduction in the peripheral lymphocyte count, and a mild reduction in the EDSS score were reported in participants following KD (119). In a recent phase II study, 57 participants concluded a 6-month prospective, intention-to-treat KD intervention. Significant improvements in the EDSS score, the 6-min walk and nine-hole peg tests were reported. This was accompanied by a significant reduction in fat mass and an increase in the MS QoL physical health and mental health composite scores. A lowering in serum leptin was also reported in this study (148).
KD was reported to influence the expression of enzymes involved in the inflammatory response in MS: KD inhibited the systemic expression of the enzymes cyclooxygenase 1 (COX1), COX2, and arachidonate 5-lipoxygenase (ALOX5), which are involved in the biosynthesis of pro-inflammatory eicosanoids and implicated in demyelination and inflammation in MS (149). In addition, in another study, the KD group showed significantly reduced serum neurofilament light chain (sNfL) levels compared to the common diet group (150).
KD could also modulate the gut microbiome in MS: a 6-month KD intervention was able to decrease six groups of bacteria compared to MS patients without the dietary intervention, leading to a total decrease in the total bacterial concentration. The impaired groups included not only the bacteria present in all patients, such as Bacteroides and Faecalibacterium prausnitzii, but also those that could be found only in some subsets of individuals. Conversely, Akkermansia was the only bacteria to not show a decrease immediately after KD intervention. However, this effect was temporary. Indeed, this initial reduction in the bacterial concentrations appeared to recover after 12 weeks, reaching the values reported for healthy controls after 23–24 weeks. This was indicated for some groups of bacteria, but not for Akkermansia, which declined following KD (151). Although these are promising results, a long-term prescription of KD in pwMS needs to be carefully evaluated based on cost–benefit analysis. In fact, KD increased the total number of apoB-containing lipoproteins, and this could contribute to increasing the risk of cardiovascular disease in pwMS (152).
5.3. Calorie restriction
DR has been demonstrated to increase life span and protect against age-related pathologies in various model organisms (153, 154). There are different types of DR, but the principle consists of a daily calorie restriction (CR) of about 20%–50% with respect to the normal ab libitum consumption without malnutrition, maintaining adequate vitamin and mineral intake (100, 153).
In EAE, it was demonstrated that CR elicited less severe inflammation, demyelination, and axon injury. CR had an anti-inflammatory effect, with observed increasing plasma levels of corticosterone and adiponectin and reduced concentrations of IL-6 and leptin (118). In Lewis rat model, severe CR (66%) rats did not exhibit clinical signs of EAE, showing instead T- and B- cell reduction and lower IFN-γ production (120). As CR requires significant lifestyle changes, certain periodic DR is now becoming more diffused, with the name IF (155). IF is a form of DR that involves intermittent elimination (fasting) or a drastic reduction in food intake for a set or a variable period of time (156, 157). There are several types of IF, as follows: 1) the 5:2 diet, with severe energy restriction (e.g., 75%–90% of energy needs) for 2 days a week with ad libitum consumption on the remaining five; 2) alternate-day fasting, with severe restriction applied on alternating days; 3) time-restricted feeding (TRF), with restriction of food intake to a temporal window, typically ≤10 h, within the waking phase; and 4) the fasting-mimicking diet (FMD), a periodic cycle of low calories, sugars, and protein with unsaturated fat and complex carbohydrates as major sources of energy (156, 158, 159). IF in humans in the short-to-medium term has beneficial effects on glucose and lipid homeostasis (160). However, the metabolic benefits of TRF in healthy subjects are sparse, with some methodological issues needing further clinical studies (157, 161).
In a mouse model of MS, it was demonstrated that IF ameliorated the clinical course of the disease, leading to less inflammation, demyelination, and axonal damage; reversed the EAE-mediated CNS accumulation of total CD4+ T cells; reduced the levels of pro-inflammatory cytokines and Th1 and Th17 cells; increased the number of Tregs; and enhanced the expression of brain-derived neurotrophic factor (BDNF) and remyelination markers (119, 121, 162–164). It was also observed that IF led to a strong reduction of monocyte accumulation in the spinal cord of EAE mice. Purified spinal cord-infiltrating monocytes from fasted mice most significantly downregulated the pro-inflammatory genes associated with monocyte pro-inflammatory activity, inflammation, and inflammatory diseases, such as TNF-α, IL-1β, CXC chemokine ligand 2 (CXCL2), and CXCL10 when compared to monocytes from fed mice (165).
Studies evaluating the effects of DR in pwMS are still in their infancy. Fasting during the religious period of Ramadan was observed to have no short-term unfavorable effects on the disease course in pwMS with mild disabilities (166). It was also reported that, in people with RRMS, fasting during Ramadan significantly increased the mean physical and mental health, although no difference in terms of the modified fatigue impact scale was observed (167). FMD was reported to promote clinically meaningful improvements in health-related QoL, a slight reduction in lymphocytes and white blood cell counts, and a mild reduction in the EDSS scores (119). Daily CR with a 22% daily reduction in energy needs and an intermittent CR diet with 2 days of 75% reduction after 5 days of no restrictions were evaluated in patients with MS. In both groups, a significant improvement in the emotional well-being/depression scores relative to the control was observed (168). In this group of participants, various T-cell subsets were also measured. Only those individuals following the intermittent CR diet showed significant reductions in memory T-cell subsets, including effector memory subsets, with concomitant increases in naive subsets and reductions in Th1 cells over the 8-week follow-up (169). However, the long-term maintenance rate of these diets appeared low; moreover, in the long-period diet, the weight loss was lower (median = −0.29 kg), and there were no statistically significant changes in the patient-reported QoL, fatigue, or sleep quality. In contrast to CR diets, adherence to a 6-month TRF diet, which consists of the consumption of all calories in an 8-h interval with a 16-h fasting period daily, appeared relatively good. Nevertheless, between the TRF and control groups, no changes in weight or any of the patient-reported outcomes studied were reported (155). Finally, intermittent CR with 400–500 calorie intake every other day was also examined. Intermittent CR was safe, well tolerated, and led to reduced leptin levels and to alterations in the gut microbiota, similar to that observed in IF-EAE mice (121).
CR and IF have been indicated to also affect the gut microbiota composition. IF was reported to promote a lower relative abundance of Akkermansia in EAE and also in pwMS (121, 170). Moreover, the abundance of Lactinobacillaceae, Bacteroidaceae, and Prevotellaceae increased after IF compared to controls in EAE. In particular, Lactobacillus johnsonii, Lactobacillus reuteri, Lactobacillus murinus, and Lactobacillus sp. ASF360 species were increased in IF, as well as Bacteroides caecimuris of the Bacteroidacaea family. In addition, Bifidobacterium pseudolongum in IF was reported to have twice the abundance of the control (121). Faecalibacterium, Lachnospiraceae incertae sedis, and Blautia showed an augmented abundance after 15 days of intermittent energy restriction (IER) in patients with RRMS (121).
5.4. Low-salt diet
Sodium intake has gained attention as a potential dietary risk factor for the onset and progression of MS (171, 172). This hypothesis results from observations on mouse models of MS in which mice on a high-sodium diet showed increased EAE disease exacerbation, increased BBB permeability, and brain pathology with augmented CNS-infiltrating and peripherally induced antigen-specific Th17 cells (173, 174). Serum- and glucocorticoid-regulated kinase 1 (SGK1) mediates the effect of extracellular salt on the differentiation of Th17 cells with a phenotype characterized by the upregulation of the pro-inflammatory cytokines granulocyte macrophage colony-stimulating factor (GM-CSF), TNF-α, and IL-2 (173).
The relationship between sodium intake and the immune system components was shown in healthy subjects. Indeed, a strong positive association between short-term salt-intake levels and monocyte numbers with a pro-inflammatory phenotype was reported, and a decrease in salt intake was accompanied by an enhanced production ability of the anti-inflammatory cytokine IL-10 (175, 176). It was also observed that a high-salt diet induced a short-term imbalance between Th17 cells and Tregs, with an increase in IL-17-producing Th17 cells and a decrease in the frequency of Tregs. This condition was reversed when following a low-salt diet (177). In patients subjected to an increased salt intake for 14 days, not only a significant increase in Th17 cells but also a modification in the gut microbiota was reported, with reduced survival of intestinal Lactobacillus species in participants harboring Lactobacillus at baseline (102).
Investigations did not find an association between dietary sodium intake and MS risk (178, 179). Moreover, a strong association between dietary salt intake and pediatric-onset MS risk was not even observed (179). In another study, the authors reported that pwMS with excess sodium intake had no decrease in time to relapse compared with patients without excess sodium intake (180). The results from the investigation on the association between salt consumption and MS activity are conflicting. An observational study of patients with RRMS followed for 2 years showed a positive correlation between the exacerbation rates and sodium intake. Individuals with medium or high sodium intake, estimated from sodium excretion in urine samples, showed an exacerbation rate of 2.75-fold, a 3.4-fold greater chance of developing a new lesion and, on average, eight more T2 lesions on MRI (181). In contrast, in another study, the 24-h urine sodium levels were not associated with conversion to clinically defined MS, nor with clinical or MRI outcomes over the 5-year follow-up (182–184). Therefore, the opposing outcomes may have been related to the techniques used for sodium measurement rather than to excluding salt as a potential risk factor for MS.
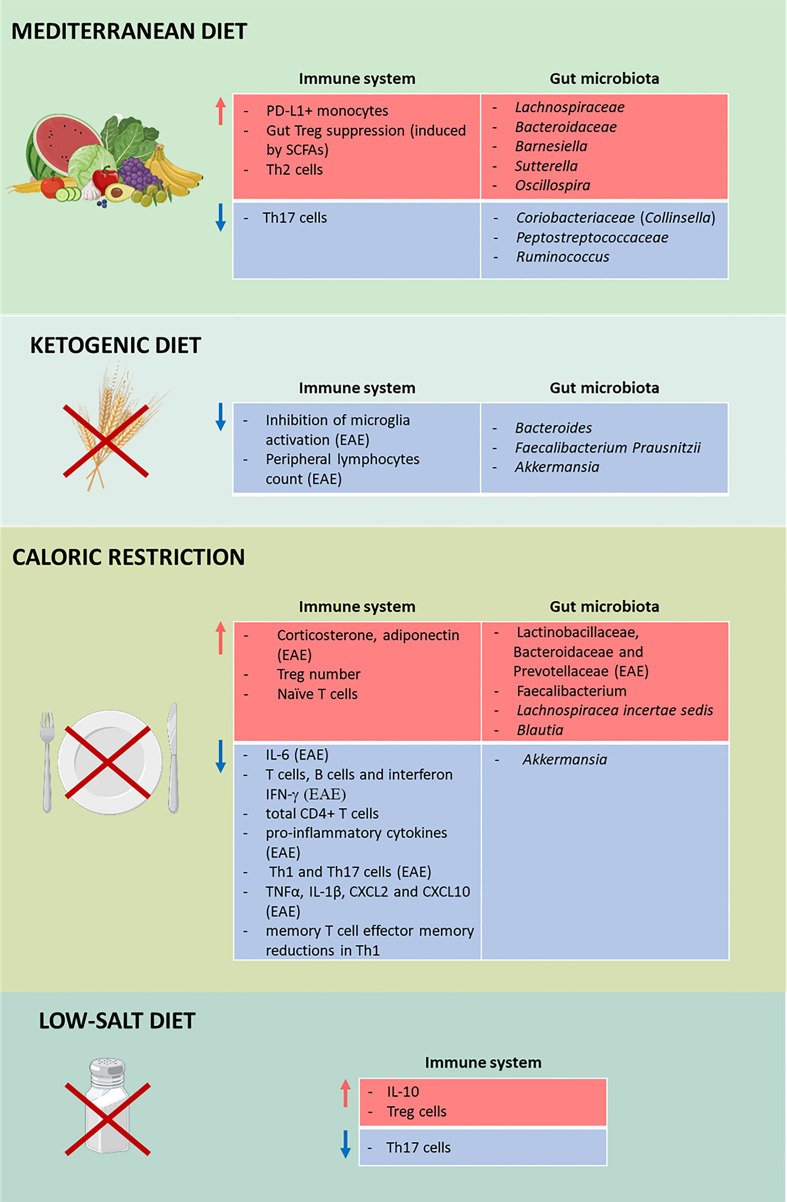
Recently, novel frontiers of research have focused on studying the tissue accumulation of sodium (185). Sodium was shown to be stored at higher concentrations as non-osmotic Na+ in extrarenal tissues, such as the muscle and skin interstitium, creating a local electrolyte environment that does not equilibrate with plasma and eludes the control of the kidney (186–188). The tissue sodium storage depends on extrarenal regulatory mechanisms, with the involvement of the immune system (189, 190). Sodium MRI (23Na-MRI) allows a direct noninvasive measurement, and enables the visualization of the actual sodium content in the body, thus representing a more accurate method to determine the actual sodium load during health and disease compared to food questionnaires or sodium excretion analysis (191). A recent work has shown that men with early-stage MS who had relatively little physical disability displayed, using 23Na-MRI, abnormally high levels of skin sodium compared to age-matched healthy controls (191). The relationship between salt concentrations in the skin, as well as the influence of dietary sodium on non-osmotic Na+ tissue accumulation, and the immunopathology of MS remains to be elucidated. Altogether, sodium homeostasis could have an effect on the immune system, microbiome, and the body’s accumulation of Na+, with potential effects on MS. To this end, more evidence is required in order to understand the possible therapeutic role of a low-salt diet ( Figure 2 ).
Figure 2.
Impact of diets on multiple sclerosis (MS). Effects of the Mediterranean diet (MD; first panel), the ketogenic diet (KD; second panel), calorie restriction (CR; third panel), and low-salt diet (fourth panel) on the immune system and the gut microbiota in MS. Different diets affect the immune system and the gut microbiota by increasing (red) and decreasing (blue) the number of cells, cytokines, and microorganisms. TThis figure was created with BioRender.com.
6. Probiotics, prebiotics and postbiotics
6.1. Probiotics
A probiotic is defined by the International Scientific Association for Probiotics and Prebiotics (ISAPP) as a “live microorganism that, when administered in adequate amounts, confers a health benefit on the host” (192). Given the involvement of the GBA in the pathogenesis of MS (23), probiotics could represent an emerging therapeutic alternative. Studies on the use of probiotics in MS are quite scarce in humans when compared to those performed on EAE. As described in a recent meta-analysis evaluating the efficacy of probiotic consumption in the management of EAE, the incidence of disease was significantly lower in mice treated with probiotics, as they showed a significant delay in EAE onset. Moreover, treated mice had lower scores for clinical symptoms than the controls. Considering the duration of EAE, treatment with Enterococci bacteria in mice could significantly shorten the disease duration (193). These clinical improvements after probiotic treatment were correlated with reduced inflammation and immunomodulation (194). The bacteria used for treatment were principally Lactobacillus spp., probiotic combinations, Bifidobacterium spp., and Escherichia coli Nissle 1917 (ECN) (195). As previously described, positive outcomes were observed more frequently with Lactobacillus spp., probiotic combinations, and ECN (195).
It was shown that probiotics had an impact on the immune system, with an effect on the increasing anti-inflammatory cytokines and Tregs, together with a reduction in pro-inflammatory cytokines (194). The administration of probiotics promoted the secretion of IL-10, IL-4, and TGF-β and enriched the population of CD4+CD25+Foxp3+ Tregs, while the secretion of IL-17, IFN-γ, GM-CSF, and TNF-α appeared decreased, as well as the levels of Th1 and Th17 cells (194). Administration of probiotics such as IRT5 (consisting of Lactobacillus casei, Lactobacillus acidophilus, L. reuteri, Bifidobacterium bifidum, and Streptococcus thermophilus) inhibited the pro-inflammatory Th1/Th17 polarization, but induced IL-10-producing cells and/or Foxp3+ Tregs both in the peripheral immune system and at the site of inflammation (196). The multispecies probiotic Lactibiane Iki (composed of Bifidobacterium lactis LA 304, L. acidophilus LA 201, and Lactobacillus salivarius LA 302) also promotes an immature, tolerogenic phenotype of DCs that can directly induce immune tolerance in the periphery (197). It was observed that a combination of the probiotic strains Lactobacillus plantarum and Bifidobacterium animalis increased the secretion of IL-4 and IL-10 by Th2 cells via the upregulation of the transcriptional factor GATA-3 in the brain and, subsequently, T-cell differentiation to the Th2 subset (198). This event is critical for the suppression of EAE because it is mediated by Th2 cytokines, which shift the immune response from a Th1 to a Th2 response (199). The role of anti-inflammatory cytokines was confirmed by observation of the attenuation of Th1 and Th17 cytokines dependent on IL-10 induction in the periphery after the administration of Lactobacilli (200). Probiotics inhibit Th17 differentiation and IL-17 production via downregulation of the transcriptional factor ROR-γt (197, 198). Probiotic treatment with the L. casei strain T2 also reduced the expression of the IDO gene, which appeared overexpressed in CPZ-induced EAE mice and interfered with the expression of microRNAs (miRNAs) in EAE, with a reduction of miR-155 and an increase of miR-25 (201–203), by reversing the demyelination effects of CPZ in mice (204). In MS, miRNAs may play an important role in the developmental fate of lymphocytes (203): the expression of miR-155 was linked to Th1 and Th17 responses, whereas that of miR-25 was significantly upregulated in the peripheral blood B cells of RRMS patients (201, 202). The zwitterionic molecule PSA in the cell wall of B. fragilis was demonstrated to have an immunomodulatory and a protective effect against CNS demyelinating disease (66, 205). PSA mediates the conversion of CD4+ T cells into Foxp3+ Tregs that produce IL-10, and the immunomodulatory commensal bacterial products impact the migration of CD4 Tregs through the regulation of CD39 (66, 206).
Probiotics could also have a beneficial effect by promoting the restoration of the intestinal barrier (IB). It was observed that ECN improved the IB function through the upregulation of the tight junction proteins zonula occludens 1 (ZO-1) and Claudin-8 and the antimicrobial peptides Reg3β and Reg3γ. In this experimental design, a correlation between the perturbation of the IB function and the severity of the neurological syndromes was also reported (207).
Probiotic treatment was observed to modulate the gut microbiota composition. A multispecies probiotic increased the abundance of the genus Lachnoclostridium and of several taxa belonging to the family Bifidobacteriaceae, strains that have been correlated with anti-inflammatory immune markers (138, 197, 198). L. reuteri treatment was demonstrated to promote the growth of the commensal microbe Bacteroidetes and to reduce the abundance of pathobiont Proteobacteria or potentially the pathogenic Gram-negative Deferribacteres (208). Lastly, the multistrain probiotic VSL#3 (a mixture of Lacticaseibacillus paracasei DSM 24732, L. plantarum DSM 24730, L. acidophilus DSM 24734, Lactobacillus delbruckeii subsp. bulgaricus DSM 24734, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Bifidobacterium breve DSM 24732, and S. thermophilus DSM 24731) decreased Anaerostipes, Dorea, Oscillospira, Enterobacteraceae, and Ruminococcus, while Bacteroides, Odoribacter, Lactobacillus, and Sutterella were increased in a mouse model of progressive MS (TMEV-IDD model) (209). These changes would be associated with the beneficial effects of probiotic treatment accompanied by increased plasma levels of SCFAs (209).
Although the therapeutic potential of probiotics in EAE has been studied, there are limited trials regarding probiotic supplementation in MS. A recent meta-analysis considering pwMS indicated a significant difference between three multistrain probiotics and a placebo group in terms of improvements in mental health parameters (210–213). The EDSS scores showed a statistically significant decrease, but the indicator obtained from the clinical trial was highly heterogeneous, providing an extremely low certainty of evidence (210). Interestingly, in pwMS, probiotics improved the metabolic profile, with a reduction in insulin and homeostatic model assessment for insulin resistance (HOMA-IR) (210). Considering inflammation, oxidative stress, and cytokine response as a result of probiotic supplementation, a significant reduction in high-sensitivity C-reactive protein (hs-CRP), malondialdehyde (MDA), and IL-6 was shown (210). A significant increase in the IL-10 level and a higher BDNF level in the probiotic group were also observed (212, 213).
Currently, there are no clinical trials evaluating the effects of probiotics on gut microbiota composition, IB integrity, and the relationship with immune parameters and/or immune cells. It was reported in a study that the probiotic VSL#3 was associated with an enrichment of the microbiota taxa depleted in MS in both pwMS and healthy controls. In pwMS, a decreased mean fluorescence intensity of HLA-DR (human leukocyte antigen—DR isotype) on myeloid-derived CD45+LIN−CD11C+ DCs was reported following VSL#3 administration. In spite of this, the authors did not find a significant correlation between the stool metabolites and immune markers in pwMS following probiotic supplementation. Interestingly, these effects did not persist following the discontinuation of the probiotic (214).
6.2. Prebiotics
A prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (215). Prebiotics stimulate the growth and functionality of specific intestinal bacterial genera or species, such as Bifidobacteria, Lactobacilli, and beneficial taxa including Roseburia, Eubacterium, and Faecalibacterium spp (215, 216). The increased biomass and cell wall components of bacteria influence immune regulation: indeed, the intake of prebiotics can augment bacterial metabolic products, such as SCFAs, and change the microbiota composition through the production of antimicrobial agents, with a reduction of infections and a decrease of decrease in the number of bacteria containing lipopolysaccharide (216). Prebiotics are mainly fructans, such as fructooligosaccharides (FOS) and inulin, and galactans, such as galactooligosaccharides (GOS). Other possible prebiotic candidates are human milk oligosaccharides, polyphenols, and polyunsaturated fatty acids (215). The immunomodulatory effects of prebiotics are dependent on the shift in the microbiota population through the production of fermentation products like SCFAs and through a gut microbiota-independent mechanism, i.e., by modulating B-cell responses, as observed for long-chain β2→1-fructans in germ-free mice (217, 218).
Studies assessing the efficacy of prebiotics in immune modulation in the context of autoimmune diseases are limited. A study on patients with RA reported an increase in the number of circulating Tregs together with favorable Th1/Th17 ratios after 28 days of dietary intervention using a high-fiber dietary supplement (219). A decrease in the level of serum TNF-α with a reduction of the disease severity score after 12 weeks of gum arabic supplementation was also observed in 40 patients with RA, demonstrating prebiotic efficacy (220, 221). Recently, the therapeutic effects of pomegranate peel extract, a promising prebiotic compound, have been examined in EAE: when used as a treatment, it alleviated the clinical symptoms of EAE, hindered DC activation and Th17 cell differentiation, and induced the production of immunoregulatory cytokines through the modulation of the gut microbiota (222). Given their beneficial effects on the gut microbiome and their possible effects on the immune system, prebiotics appear as possible therapeutic candidates for future studies on EAE and pwMS. Clinical trials using prebiotic fiber or high-fiber supplements in pwMS are currently ongoing (NCT04038541 and NCT04574024).
6.3. Postbiotics
Postbiotics, also known as metabiotics, are the structural components of probiotic microorganisms and/or their metabolites and/or signaling molecules with determined chemical structures that can optimize host-specific physiological functions, regulation, and metabolic and/or behavior reactions connected to the activity of host indigenous microbiota (223), such as SCFAs, enzymes, cell surface proteins, and vitamins (224). The SCFAs acetate, PA, and butyrate are the main metabolites produced in the human colon by the bacterial anaerobic fermentation of indigestible polysaccharides such as dietary fiber and resistant starch (225). In recent reports, different groups have observed lower levels of PA and/or butyrate in the serum of pwMS (226–228), which was also confirmed in the analysis of stool samples (228–230). Circulating follicular Tregs were positively correlated with the serum levels of PA, and butyrate was positively associated with the frequency of IL-10-producing B cells (226). The reduced serum concentration of butyrate seen in pwMS correlated with alterations in barrier permeability and inflammation (227). Another report observed higher plasma acetate levels in pwMS, with a correlation with EDSS and increased IL-17+ T cells (231). In contrast, analysis of patients with RRMS or CIS showed that the acetate levels were nominally lower and the ratios of acetate/butyrate and acetate/(propionate + butyrate) were significantly lower in pwMS compared to healthy controls in the multivariate model (232).
Studies on SCFA supplementation in EAE and MS are in their infancy. Butyrate has been examined only in an experimental model: preventive administration of butyrate provided a beneficial effect on CNS autoimmunity by halting both the demyelination and inflammation of the CNS (233). The myelinated areas of the corpus callosum in the brains of butyrate-treated mice appeared significantly ameliorated compared with those in mice treated with CPZ alone (234). Furthermore, butyrate significantly suppressed demyelination and enhanced remyelination in an organotypic slice culture (234). Administration of methyl butyrate after EAE induction alleviated the clinical symptoms with an improvement of the histopathological manifestations of CNS and reduced the effector T cells in the CNS and intestinal lamina propria. Methyl butyrate also increased the proportion of Tregs and the secretion of IL-10 in peripheral immune organs (224).
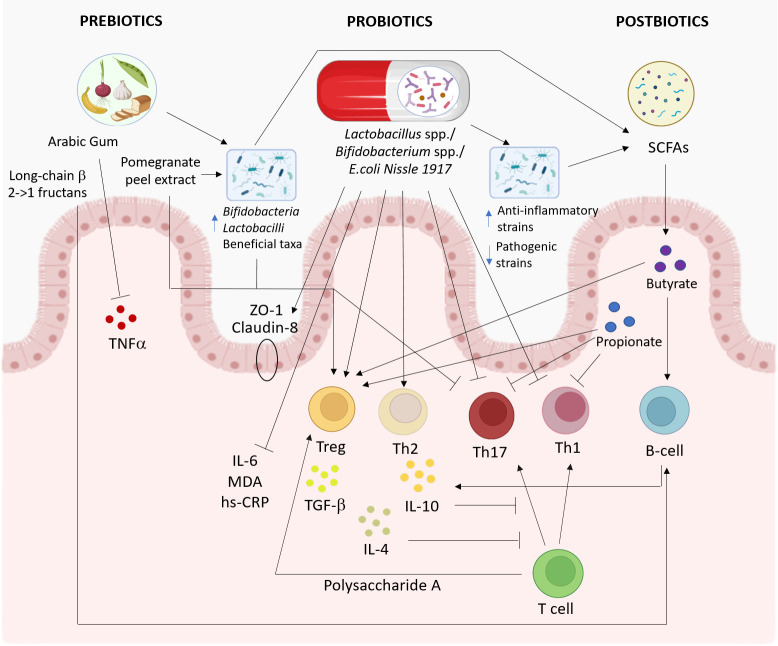
PA has been examined in both mice and humans. PA treatment increased the CD4+CD25+Foxp3+ Treg frequency in EAE mice, and the transfer of Tregs improved the clinical course of the recipient EAE mice compared to controls (83). In addition, PA treatment prevented enhanced demyelination and immune cell infiltration in the spinal cord caused by a high-fat diet, inhibited the Th17-mediated inflammatory processes, enhanced the Treg frequency, and targeted the p38/MAPK and IL-10 signaling pathways (229). Significantly decreased Th17 cell frequencies were observed in obese pwMS supplemented with PA (229). In pwMS, the number of Tregs increased after 14 days of PA supplementation, while the Th17 and Th1 cell counts decreased. After at least 1 year of supplementation, the annual relapse rates decreased significantly compared to retrospective data. Furthermore, EDSS was stabilized in the PA group compared with non-PA recipients, and in a small subset of pwMS, an increase in gray matter volume in the basal ganglia was observed in brain MRI scans (228). These promising results need confirmation from other clinical trials including a higher number of participants ( Figure 3 ).
Figure 3.
Proposed pathways involved in the effects of prebiotics, probiotics, and postbiotics on the immune system. The effects of probiotics (middle), prebiotics (left), and postbiotics (right) on the immune system and gut microbiota are summarized. This figure was created with BioRender.com.
7. Conclusions
The relationship between the gut microbiota, diet, and the immune system has attracted increasing attention in MS research, demonstrating that the actors that link diet (or specific food components) to the microbiome–immunity crosstalk are a current challenge. Interestingly, one of the first evidence of gut dysbiosis in MS came from a Japanese cohort (19). Japan has only recently registered an increase in MS cases, highlighting that changing the exposition to environmental factors, such as diet, impacts the incidence of MS (28). Effectively, the westernization of lifestyle and diet has been shown to have pro-inflammatory effects on the gut microbiota (138), thus increasing the risk of MS. On the contrary, a healthy diet is able to shift the intestinal microbiota into an anti-inflammatory type, providing proof of concept for diet-based interventions in the context of MS. The results from preclinical models are promising and suggest that feeding the gut microbiome with high-fiber, isoflavone, low-salt, low-sugar, and low-fat diets can positively influence the disease course via modulation of the immune system.
Probiotics, prebiotics and postbiotics are other elements that are emerging as key players in the interventions for MS, thanks to their ability to promote anti-inflammatory effects by acting both on the immune system and on the intestinal microbiota; however, only few studies have investigated their role in MS so far. Both prebiotics and postbiotics can be useful in promoting gut and immune system health but with different mechanisms of action. Postbiotics are already produced by bacteria and therefore do not require fermentation in the digestive tract as prebiotics, which are non-digestible substances. This means that postbiotics can have a more direct and rapid effect on gut and overall health. In addition, postbiotics do not share the same side effects as prebiotics, such as flatulence and gut irritation, thus minimizing the risks associated with their intake (235). Furthermore, postbiotics can have some advantages compared to prebiotics, including their precise chemical structure, dose, safety, and long shelf-life (223).
The transfer of these conclusions into the human setting is highly complex given numerous host factors, including genetics, BMI, prescribed drugs, and preexisting gut microbiota composition, which overall could influence how individuals respond to diets or probiotics. The available studies present several limitations, including a small sample size, short intervention duration, and only a few health outcomes. Randomized controlled trials are needed to highlight the mechanisms of action of diets and probiotics, the specific “weight” of the gut microbiota, and the influence of other pathways. The integration of data from the microbiota, inflammatory state, MRI, and relapse rates may be considered as a future readout for this purpose. These results could be the starting point for studies on food–DMT interactions, which would help build the rationale for validated dietary, rather than effective mixtures of selected microorganisms, recommendations for pwMS.
Author contributions
MB, AM, and RR wrote the paper. FM and MM edited the paper for clinical aspects. SR and MC conceived the idea, reviewed the manuscript, and edited its final version. All authors contributed to the article and approved the submitted version.
Funding Statement
This publication is part of the project NODES, which has received funding from the MUR–M4C2 1.5 of PNRR (grant agreement no. ECS00000036) and supported by the University of Turin (grant CLEM_RILO_21_01 to MC).
Conflict of interest
MM received personal compensations for advisory boards and travel grants from Novartis and Sanofi-Genzyme. FM received personal compensations for advisory boards from Novartis and Merck Serono. SR received personal compensations for public speaking and travel grants from Sanofi-Genzyme and Merck Serono. MC received personal compensations for advisory boards, public speaking, editorial commitments, or travel grants from Biogen Idec, Merck Serono, Fondazione Serono, Novartis, Pomona, Sanofi-Genzyme, and Teva.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet (2008) 372:1502–17. doi: 10.1016/S0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Neurology Collaborators . Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol (2019) 18:459–80. doi: 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol (2013) 13:128. doi: 10.1186/1471-2377-13-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voskuhl RR. The effect of sex on multiple sclerosis risk and disease progression. Mult Scler (2020) 26:554–60. doi: 10.1177/1352458519892491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katz Sand I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr Opin Neurol (2015) 28:193–205. doi: 10.1097/WCO.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 6. Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol (2019) 26:27–40. doi: 10.1111/ene.13819 [DOI] [PubMed] [Google Scholar]
- 7. Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, et al. T-Helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol (2009) 65:499–509. doi: 10.1002/ana.21652 [DOI] [PubMed] [Google Scholar]
- 8. Rolla S, Bardina V, De Mercanti S, Quaglino P, De Palma R, Gned D, et al. Th22 cells are expanded in multiple sclerosis and are resistant to IFN-β. J Leukoc Biol (2014) 96:1155–64. doi: 10.1189/jlb.5A0813-463RR [DOI] [PubMed] [Google Scholar]
- 9. Costantino CM, Baecher-Allan C, Hafler DA. Multiple sclerosis and regulatory T cells. J Clin Immunol (2008) 28:697–706. doi: 10.1007/s10875-008-9236-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li R, Patterson KR, Bar-Or A. Reassessing b cell contributions in multiple sclerosis. Nat Immunol (2018) 19:696–707. doi: 10.1038/s41590-018-0135-x [DOI] [PubMed] [Google Scholar]
- 11. Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol (2017) 13:25–36. doi: 10.1038/nrneurol.2016.187 [DOI] [PubMed] [Google Scholar]
- 12. Hedström AK, Lima Bomfim I, Barcellos L, Gianfrancesco M, Schaefer C, Kockum I, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology (2014) 82:865–72. doi: 10.1212/WNL.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology (2013) 80:548–52. doi: 10.1212/WNL.0b013e31828154f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munger KL, Bentzen J, Laursen B, Stenager E, Koch-Henriksen N, Sørensen TIA, et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler (2013) 19:1323–9. doi: 10.1177/1352458513483889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harroud A, Richards JB. Mendelian randomization in multiple sclerosis: a causal role for vitamin d and obesity? Mult Scler (2018) 24:80–5. doi: 10.1177/1352458517737373 [DOI] [PubMed] [Google Scholar]
- 16. Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J (2014) 13:61. doi: 10.1186/1475-2891-13-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guerrero-García J de J, Carrera-Quintanar L, López-Roa RI, Márquez-Aguirre AL, Rojas-Mayorquín AE, Ortuño-Sahagún D. Multiple sclerosis and obesity: possible roles of adipokines. Mediators Inflammation (2016) 2016:4036232. doi: 10.1155/2016/4036232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marrodan M, Farez MF, Balbuena Aguirre ME, Correale J. Obesity and the risk of multiple sclerosis. the role of leptin. Ann Clin Transl Neurol (2021) 8:406–24. doi: 10.1002/acn3.51291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PloS One (2015) 10:e0137429. doi: 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep (2016) 6:28484. doi: 10.1038/srep28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun (2016) 7:12015. doi: 10.1038/ncomms12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ochoa-Repáraz J, Kirby TO, Kasper LH. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect Med (2018) 8:a029017. doi: 10.1101/cshperspect.a029017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maglione A, Zuccalà M, Tosi M, Clerico M, Rolla S. Host genetics and gut microbiome: perspectives for multiple sclerosis. Genes (Basel) (2021) 12:1181. doi: 10.3390/genes12081181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noto D, Miyake S. Gut dysbiosis and multiple sclerosis. Clin Immunol (2022) 235:108380. doi: 10.1016/j.clim.2020.108380 [DOI] [PubMed] [Google Scholar]
- 25. Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol (2016) 23:1308–21. doi: 10.1111/ene.13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hornef M. Pathogens, commensal symbionts, and pathobionts: discovery and functional effects on the host. ILAR J (2015) 56:159–62. doi: 10.1093/ilar/ilv007 [DOI] [PubMed] [Google Scholar]
- 27. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-Gut-Brain axis. Physiol Rev (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 28. Wolter M, Grant ET, Boudaud M, Steimle A, Pereira GV, Martens EC, et al. Leveraging diet to engineer the gut microbiome. Nat Rev Gastroenterol Hepatol (2021) 18(12):885-902. doi: 10.1038/s41575-021-00512-7 [DOI] [PubMed] [Google Scholar]
- 29. Altomare R, Cacciabaudo F, Damiano G, Palumbo VD, Gioviale MC, Bellavia M, et al. The mediterranean diet: a history of health. Iran J Public Health (2013) 42:449–57. [PMC free article] [PubMed] [Google Scholar]
- 30. Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci (2010) 365:2793–807. doi: 10.1098/rstb.2010.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moles L, Otaegui D. The impact of diet on microbiota evolution and human health. is diet an adequate tool for microbiota modulation? Nutrients (2020) 12:E1654. doi: 10.3390/nu12061654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heiman ML, Greenway FL. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab (2016) 5:317–20. doi: 10.1016/j.molmet.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr (2005) 81:341–54. doi: 10.1093/ajcn.81.2.341 [DOI] [PubMed] [Google Scholar]
- 34. Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology (2009) 136:1476–83. doi: 10.1053/j.gastro.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 35. Riccio P, Rossano R. Undigested food and gut microbiota may cooperate in the pathogenesis of neuroinflammatory diseases: a matter of barriers and a proposal on the origin of organ specificity. Nutrients (2019) 11:E2714. doi: 10.3390/nu11112714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo C, Jian C, Liao Y, Huang Q, Wu Y, Liu X, et al. The role of microglia in multiple sclerosis. Neuropsychiatr Dis Treat (2017) 13:1661–7. doi: 10.2147/NDT.S140634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol (2011) 164:1079–106. doi: 10.1111/j.1476-5381.2011.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology (2014) 83:1022–4. doi: 10.1212/WNL.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev (2010) 9:A387–394. doi: 10.1016/j.autrev.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 40. Stanisavljević S, Čepić A, Bojić S, Veljović K, Mihajlović S, Đedović N, et al. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in dark agouti rats. Sci Rep (2019) 9:918. doi: 10.1038/s41598-018-37505-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dalla Costa G, Romeo M, Esposito F, Sangalli F, Colombo B, Radaelli M, et al. Caesarean section and infant formula feeding are associated with an earlier age of onset of multiple sclerosis. Mult Scler Relat Disord (2019) 33:75–7. doi: 10.1016/j.msard.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 42. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet (2018) 118:74–100.e11. doi: 10.1016/j.jand.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 43. Handel AE, Giovannoni G, Ebers GC, Ramagopalan SV. Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol (2010) 6:156–66. doi: 10.1038/nrneurol.2010.1 [DOI] [PubMed] [Google Scholar]
- 44. Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology (2009) 73:1543–50. doi: 10.1212/WNL.0b013e3181c0d6e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hedström AK, Olsson T, Alfredsson L. Body mass index during adolescence, rather than childhood, is critical in determining MS risk. Mult Scler (2016) 22:878–83. doi: 10.1177/1352458515603798 [DOI] [PubMed] [Google Scholar]
- 46. Abdollahpour I, Jakimovski D, Shivappa N, Hébert JR, Vahid F, Nedjat S, et al. Dietary inflammatory index and risk of multiple sclerosis: findings from a large population-based incident case–control study. Clin Nutr (2020) 39:3402–7. doi: 10.1016/j.clnu.2020.02.033 [DOI] [PubMed] [Google Scholar]
- 47. Abdollahpour I, Sormani MP, Nedjat S, Mansournia MA, van der Mei I. The role of nutritional factors during adolescence in multiple sclerosis onset: a population-based incident case-control study. Nutr Neurosci (2021) 24:500–7. doi: 10.1080/1028415X.2019.1647689 [DOI] [PubMed] [Google Scholar]
- 48. Behzadifar M, Behzadifar M, Abdi S, Malekzadeh R, Arab Salmani M, Ghoreishinia G, et al. Prevalence of food insecurity in Iran: a systematic review and meta-analysis. Arch Iran Med (2016) 19:288–94. doi: 10.0161904/AIM.0012 [DOI] [PubMed] [Google Scholar]
- 49. Black LJ, Hetherton S, Forkan M, Gonzales EG, Smith JB, Daly A, et al. An exploratory study of diet in childhood and young adulthood and adult-onset multiple sclerosis. Mult Scler (2021) 27:1611–4. doi: 10.1177/1352458520986964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci (2015) 18:125–36. doi: 10.1179/1476830514Y.0000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fitzgerald KC, Tyry T, Salter A, Cofield SS, Cutter G, Fox R, et al. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology (2018) 90:e1–e11. doi: 10.1212/WNL.0000000000004768 [DOI] [PubMed] [Google Scholar]
- 52. Jakimovski D, Weinstock-Guttman B, Gandhi S, Guan Y, Hagemeier J, Ramasamy DP, et al. Dietary and lifestyle factors in multiple sclerosis progression: results from a 5-year longitudinal MRI study. J Neurol (2019) 266:866–75. doi: 10.1007/s00415-019-09208-0 [DOI] [PubMed] [Google Scholar]
- 53. Evers I, Heerings M, de Roos NM, Jongen PJ, Visser LH. Adherence to dietary guidelines is associated with better physical and mental quality of life: results from a cross-sectional survey among 728 Dutch MS patients. Nutr Neurosci (2021), 25(8):1633-40. doi: 10.1080/1028415X.2021.1885240 [DOI] [PubMed] [Google Scholar]
- 54. Marck CH, Probst Y, Chen J, Taylor B, van der Mei I. Dietary patterns and associations with health outcomes in Australian people with multiple sclerosis. Eur J Clin Nutr (2021) 75:1506–14. doi: 10.1038/s41430-021-00864-y [DOI] [PubMed] [Google Scholar]
- 55. Sumowski JF, McDonnell GV, Bourdette D. Diet in multiple sclerosis: science takes a seat at the table. Neurology (2018) 90:14–5. doi: 10.1212/WNL.0000000000004775 [DOI] [PubMed] [Google Scholar]
- 56. Fitzgerald KC, Damian A, Conway D, Mowry EM. Vascular comorbidity is associated with lower brain volumes and lower neuroperformance in a large multiple sclerosis cohort. Mult Scler (2021) 27:1914–23. doi: 10.1177/1352458520984746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weinstock-Guttman B, Zivadinov R, Horakova D, Havrdova E, Qu J, Shyh G, et al. Lipid profiles are associated with lesion formation over 24 months in interferon-β treated patients following the first demyelinating event. J Neurol Neurosurg Psychiatry (2013) 84:1186–91. doi: 10.1136/jnnp-2012-304740 [DOI] [PubMed] [Google Scholar]
- 58. Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology (2010) 74:1041–7. doi: 10.1212/WNL.0b013e3181d6b125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms (2019) 7:14. doi: 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mörbe UM, Jørgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol (2021) 14:793–802. doi: 10.1038/s41385-021-00389-4 [DOI] [PubMed] [Google Scholar]
- 61. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res (2020) 30:492–506. doi: 10.1038/s41422-020-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U.S.A. (2011) 108 Suppl 1:4615–22. doi: 10.1073/pnas.1000082107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature (2011) 479:538–41. doi: 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- 64. Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol (2022) 18:544–58. doi: 10.1038/s41582-022-00697-8 [DOI] [PubMed] [Google Scholar]
- 65. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The toll-like receptor pathway establishes commensal gut colonization. Science (2011) 332:974–7. doi: 10.1126/science.1206095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol (2010) 3:487–95. doi: 10.1038/mi.2010.29 [DOI] [PubMed] [Google Scholar]
- 67. Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, et al. A commensal symbiotic factor derived from bacteroides fragilis promotes human CD39+Foxp3+ T cells and treg function. Gut Microbes (2015) 6:234–42. doi: 10.1080/19490976.2015.1056973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, et al. Central nervous system demyelinating disease protection by the human commensal bacteroides fragilis depends on polysaccharide a expression. J Immunol (2010) 185:4101–8. doi: 10.4049/jimmunol.1001443 [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, Telesford KM, Ochoa-Repáraz J, Haque-Begum S, Christy M, Kasper EJ, et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signaling. Nat Commun (2014) 5:4432. doi: 10.1038/ncomms5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wen J, Ariyannur PS, Ribeiro R, Tanaka M, Moffett JR, Kirmani BF, et al. Efficacy of n-acetylserotonin and melatonin in the EAE model of multiple sclerosis. J Neuroimmune Pharmacol (2016) 11:763–73. doi: 10.1007/s11481-016-9702-9 [DOI] [PubMed] [Google Scholar]
- 71. Sacramento PM, Monteiro C, Dias ASO, Kasahara TM, Ferreira TB, Hygino J, et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4+ T-cell subsets in multiple sclerosis patients. Eur J Immunol (2018) 48:1376–88. doi: 10.1002/eji.201847525 [DOI] [PubMed] [Google Scholar]
- 72. Yaghoubfar R, Behrouzi A, Ashrafian F, Shahryari A, Moradi HR, Choopani S, et al. Modulation of serotonin signaling/metabolism by akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci Rep (2020) 10:22119. doi: 10.1038/s41598-020-79171-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol (2019) 19:184–97. doi: 10.1038/s41577-019-0125-8 [DOI] [PubMed] [Google Scholar]
- 74. Merchak AR, Cahill HJ, Brown LC, Brown RM, Rivet-Noor C, Beiter RM, et al. The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. PloS Biol (2023) 21:e3002000. doi: 10.1371/journal.pbio.3002000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature (2013) 504:451–5. doi: 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature (2013) 504:446–50. doi: 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 77. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science (2013) 341:569–73. doi: 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity (2014) 40:128–39. doi: 10.1016/j.immuni.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim S-H, Cho B-H, Kiyono H, Jang Y-S. Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal peyer’s patches. Sci Rep (2017) 7:3980. doi: 10.1038/s41598-017-02729-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ling Z, Cheng Y, Yan X, Shao L, Liu X, Zhou D, et al. Alterations of the fecal microbiota in Chinese patients with multiple sclerosis(2020) (Accessed February 8, 2023). [DOI] [PMC free article] [PubMed]
- 81. Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, de Vos WM. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader akkermansia muciniphila. Front Microbiol (2011) 2:166. doi: 10.3389/fmicb.2011.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou X, Baumann R, Gao X, Mendoza M, Singh S, Katz Sand I, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell (2022) 185:3467–3486.e16. doi: 10.1016/j.cell.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity (2015) 43:817–29. doi: 10.1016/j.immuni.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 84. Esmaeil Amini M, Shomali N, Bakhshi A, Rezaei S, Hemmatzadeh M, Hosseinzadeh R, et al. Gut microbiome and multiple sclerosis: new insights and perspective. Int Immunopharmacol (2020) 88:107024. doi: 10.1016/j.intimp.2020.107024 [DOI] [PubMed] [Google Scholar]
- 85. Kozhieva M, Naumova N, Alikina T, Boyko A, Vlassov V, Kabilov MR. Primary progressive multiple sclerosis in a Russian cohort: relationship with gut bacterial diversity. BMC Microbiol (2019) 19:309. doi: 10.1186/s12866-019-1685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv (2017) 3:e1700492. doi: 10.1126/sciadv.1700492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rolla S, Bardina V, Ferrocino I, De Mercanti S, Giai Vla A, Lamverti A, et al. Alterations in gut microbiome are associated with the onset of multiple sclerosis: an Italian pivotal study(2018) (Accessed February 3, 2023).
- 88. Thirion F, Sellebjerg F, Fan Y, Lyu L, Hansen TH, Pons N, et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med (2023) 15:1. doi: 10.1186/s13073-022-01148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mikami A, Ogita T, Namai F, Shigemori S, Sato T, Shimosato T. Oral administration of flavonifractor plautii, a bacteria increased with green tea consumption, promotes recovery from acute colitis in mice via suppression of IL-17. Front Nutr (2021) 7:610946. doi: 10.3389/fnut.2020.610946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ogita T, Yamamoto Y, Mikami A, Shigemori S, Sato T, Shimosato T. Oral administration of flavonifractor plautii strongly suppresses Th2 immune responses in mice. Front Immunol (2020) 11:379. doi: 10.3389/fimmu.2020.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Engen SA, Valen Rukke H, Becattini S, Jarrossay D, Blix IJ, Petersen FC, et al. The oral commensal streptococcus mitis shows a mixed memory Th cell signature that is similar to and cross-reactive with streptococcus pneumoniae. PloS One (2014) 9:e104306. doi: 10.1371/journal.pone.0104306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cady N, Peterson SR, Freedman SN, Mangalam AK. Beyond metabolism: the complex interplay between dietary phytoestrogens, gut bacteria, and cells of nervous and immune systems. Front Neurol (2020) 11:150. doi: 10.3389/fneur.2020.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bang C, Weidenbach K, Gutsmann T, Heine H, Schmitz RA. The intestinal archaea methanosphaera stadtmanae and methanobrevibacter smithii activate human dendritic cells. PloS One (2014) 9:e99411. doi: 10.1371/journal.pone.0099411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Miyauchi E, Shimokawa C, Steimle A, Desai MS, Ohno H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol (2023) 23:9–23. doi: 10.1038/s41577-022-00727-y [DOI] [PubMed] [Google Scholar]
- 95. Knoll RL, Forslund K, Kultima JR, Meyer CU, Kullmer U, Sunagawa S, et al. Gut microbiota differs between children with inflammatory bowel disease and healthy siblings in taxonomic and functional composition: a metagenomic analysis. Am J Physiology-Gastrointestinal Liver Physiol (2017) 312:G327–39. doi: 10.1152/ajpgi.00293.2016 [DOI] [PubMed] [Google Scholar]
- 96. Li M, Dai B, Tang Y, Lei L, Li N, Liu C, et al. Altered bacterial-fungal interkingdom networks in the guts of ankylosing spondylitis patients. mSystems (2019) 4:e00176–18. doi: 10.1128/mSystems.00176-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li Y, Wang H-F, Li X, Li H-X, Zhang Q, Zhou H-W, et al. Disordered intestinal microbes are associated with the activity of systemic lupus erythematosus. Clin Sci (Lond) (2019) 133:821–38. doi: 10.1042/CS20180841 [DOI] [PubMed] [Google Scholar]
- 98. Valburg C, Sonti A, Stern JN, Najjar S, Harel A. Dietary factors in experimental autoimmune encephalomyelitis and multiple sclerosis: a comprehensive review. Mult Scler (2021) 27:494–502. doi: 10.1177/1352458520923955 [DOI] [PubMed] [Google Scholar]
- 99. Timmermans S, Bogie JFJ, Vanmierlo T, Lütjohann D, Stinissen P, Hellings N, et al. High fat diet exacerbates neuroinflammation in an animal model of multiple sclerosis by activation of the renin angiotensin system. J Neuroimmune Pharmacol (2014) 9:209–17. doi: 10.1007/s11481-013-9502-4 [DOI] [PubMed] [Google Scholar]
- 100. Cantoni C, Dorsett Y, Fontana L, Zhou Y, Piccio L. Effects of dietary restriction on gut microbiota and CNS autoimmunity. Clin Immunol (2020), 235:108575. doi: 10.1016/j.clim.2020.108575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ahn JJ, Revelo X, Winer DA, Dunn SE. Diet-induced obesity enhances the severity of experimental autoimmune encephalomyelitis. J Neuroimmunology (2014) 275:133. doi: 10.1016/j.jneuroim.2014.08.357 [DOI] [Google Scholar]
- 102. Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature (2017) 551:585–9. doi: 10.1038/nature24628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jensen SN, Cady NM, Shahi SK, Peterson SR, Gupta A, Gibson-Corley KN, et al. Isoflavone diet ameliorates experimental autoimmune encephalomyelitis through modulation of gut bacteria depleted in patients with multiple sclerosis. Sci Adv (2021) 7:eabd4595. doi: 10.1126/sciadv.abd4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, et al. Obesity predisposes to Th17 bias. Eur J Immunol (2009) 39:2629–35. doi: 10.1002/eji.200838893 [DOI] [PubMed] [Google Scholar]
- 105. Hu L, Zhu S, Peng X, Li K, Peng W, Zhong Y, et al. High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J Alzheimers Dis (2020) 77:629–40. doi: 10.3233/JAD-200035 [DOI] [PubMed] [Google Scholar]
- 106. Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the united states. J Am Diet Assoc (2010) 110:1477–84. doi: 10.1016/j.jada.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cao G, Wang Q, Huang W, Tong J, Ye D, He Y, et al. Long-term consumption of caffeine-free high sucrose cola beverages aggravates the pathogenesis of EAE in mice. Cell Discovery (2017) 3:17020. doi: 10.1038/celldisc.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang D, Jin W, Wu R, Li J, Park S-A, Tu E, et al. High glucose intake exacerbates autoimmunity through reactive-Oxygen-Species-Mediated TGF-β cytokine activation. Immunity (2019) 51:671–681.e5. doi: 10.1016/j.immuni.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Deehan EC, Walter J. The fiber gap and the disappearing gut microbiome: implications for human nutrition. Trends Endocrinol Metab (2016) 27:239–42. doi: 10.1016/j.tem.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 110. Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev (2012) 245:164–76. doi: 10.1111/j.1600-065X.2011.01080.x [DOI] [PubMed] [Google Scholar]
- 111. Berer K, Martínez I, Walker A, Kunkel B, Schmitt-Kopplin P, Walter J, et al. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep (2018) 8:10431. doi: 10.1038/s41598-018-28839-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, et al. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med (2012) 4:118ra11. doi: 10.1126/scitranslmed.3002629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sonner JK, Keil M, Falk-Paulsen M, Mishra N, Rehman A, Kramer M, et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat Commun (2019) 10:4877. doi: 10.1038/s41467-019-12776-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fettig NM, Osborne LC. Direct and indirect effects of microbiota-derived metabolites on neuroinflammation in multiple sclerosis. Microbes Infect (2021) 23:104814. doi: 10.1016/j.micinf.2021.104814 [DOI] [PubMed] [Google Scholar]
- 115. Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao C-C, Ardura-Fabregat A, et al. Microglial control of astrocytes in response to microbial metabolites. Nature (2018) 557:724–8. doi: 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med (2016) 22:586–97. doi: 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Freedman SN, Shahi SK, Mangalam AK. The “Gut feeling”: breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics (2018) 15:109–25. doi: 10.1007/s13311-017-0588-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol (2008) 84:940–8. doi: 10.1189/jlb.0208133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep (2016) 15:2136–46. doi: 10.1016/j.celrep.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Esquifino AI, Cano P, Jimenez-Ortega V, Fernández-Mateos MP, Cardinali DP. Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J Neuroinflamm (2007) 4:6. doi: 10.1186/1742-2094-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab (2018) 27:1222–1235.e6. doi: 10.1016/j.cmet.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean Diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr (2018) 72:30–43. doi: 10.1038/ejcn.2017.58 [DOI] [PubMed] [Google Scholar]
- 123. Widmer RJ, Flammer AJ, Lerman LO, Lerman A. “The Mediterranean diet, its components, and cardiovascular disease.” Am J Med(2015) 128:229–38. doi: 10.1016/j.amjmed.2014.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Finicelli M, Di Salle A, Galderisi U, Peluso G. The Mediterranean diet: an update of the clinical trials. Nutrients (2022) 14:2956. doi: 10.3390/nu14142956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Esposito S, Sparaco M, Maniscalco GT, Signoriello E, Lanzillo R, Russo C, et al. Lifestyle and Mediterranean diet adherence in a cohort of southern Italian patients with multiple sclerosis. Mult Scler Relat Disord (2021) 47:102636. doi: 10.1016/j.msard.2020.102636 [DOI] [PubMed] [Google Scholar]
- 126. Bohlouli J, Namjoo I, Borzoo-Isfahani M, Poorbaferani F, Moravejolahkami AR, Clark CCT, et al. Modified Mediterranean diet v. traditional Iranian diet: efficacy of dietary interventions on dietary inflammatory index score, fatigue severity and disability in multiple sclerosis patients. Br J Nutr (2021), 128(7):1274-84. doi: 10.1017/S000711452100307X [DOI] [PubMed] [Google Scholar]
- 127. Katz Sand I, Benn EKT, Fabian M, Fitzgerald KC, Digga E, Deshpande R, et al. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: a pilot study. Mult Scler Relat Disord (2019) 36:101403. doi: 10.1016/j.msard.2019.101403 [DOI] [PubMed] [Google Scholar]
- 128. Moravejolahkami AR, Paknahad Z, Chitsaz A. Association of dietary patterns with systemic inflammation, quality of life, disease severity, relapse rate, severity of fatigue and anthropometric measurements in MS patients. Nutr Neurosci (2020) 23:920–30. doi: 10.1080/1028415X.2019.1580831 [DOI] [PubMed] [Google Scholar]
- 129. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut (2016) 65:1812–21. doi: 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- 130. Azary S, Schreiner T, Graves J, Waldman A, Belman A, Guttman BW, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry (2018) 89:28–33. doi: 10.1136/jnnp-2017-315936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ertaş Öztürk Y, Helvaci EM, Sökülmez Kaya P, Terzi M. Is Mediterranean diet associated with multiple sclerosis related symptoms and fatigue severity? Nutr Neurosci (2022), 26(3):228-34. doi: 10.1080/1028415X.2022.2034241 [DOI] [PubMed] [Google Scholar]
- 132. Katz Sand I, Levy S, Fitzgerald K, Sorets T, Sumowski JF. Mediterranean Diet is linked to less objective disability in multiple sclerosis. Mult Scler (2023) 29:248–60. doi: 10.1177/13524585221127414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, et al. Mediterranean Diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut (2020) 69:1258–68. doi: 10.1136/gutjnl-2019-320438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol (2018) 9:890. doi: 10.3389/fmicb.2018.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gutiérrez-Díaz I, Fernández-Navarro T, Sánchez B, Margolles A, González S. Mediterranean Diet and faecal microbiota: a transversal study. Food Funct (2016) 7:2347–56. doi: 10.1039/c6fo00105j [DOI] [PubMed] [Google Scholar]
- 136. Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean Diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut (2020) 69:1218–28. doi: 10.1136/gutjnl-2019-319654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gutiérrez-Díaz I, Fernández-Navarro T, Salazar N, Bartolomé B, Moreno-Arribas MV, de Andres-Galiana EJ, et al. Adherence to a Mediterranean diet influences the fecal metabolic profile of microbial-derived phenolics in a Spanish cohort of middle-age and older people. J Agric Food Chem (2017) 65:586–95. doi: 10.1021/acs.jafc.6b04408 [DOI] [PubMed] [Google Scholar]
- 138. Saresella M, Mendozzi L, Rossi V, Mazzali F, Piancone F, LaRosa F, et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: a pilot study. Front Immunol (2017) 8:1391. doi: 10.3389/fimmu.2017.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cantoni C, Lin Q, Dorsett Y, Ghezzi L, Liu Z, Pan Y, et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine (2022), 76:103798. doi: 10.1016/j.ebiom.2021.103798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Barone M, Mendozzi L, D’Amico F, Saresella M, Rampelli S, Piancone F, et al. Influence of a high-impact multidimensional rehabilitation program on the gut microbiota of patients with multiple sclerosis. Int J Mol Sci (2021) 22:7173. doi: 10.3390/ijms22137173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Prabhakar A, Quach A, Zhang H, Terrera M, Jackemeyer D, Xian X, et al. Acetone as biomarker for ketosis buildup capability–a study in healthy individuals under combined high fat and starvation diets. Nutr J (2015) 14:41. doi: 10.1186/s12937-015-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gupta L, Khandelwal D, Kalra S, Gupta P, Dutta D, Aggarwal S. Ketogenic diet in endocrine disorders: current perspectives. J Postgrad Med (2017) 63:242–51. doi: 10.4103/jpgm.JPGM_16_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sourbron J, Klinkenberg S, van Kuijk SMJ, Lagae L, Lambrechts D, Braakman HMH, et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Childs Nerv Syst (2020) 36:1099–109. doi: 10.1007/s00381-020-04578-7 [DOI] [PubMed] [Google Scholar]
- 144. Gough SM, Casella A, Ortega KJ, Hackam AS. Neuroprotection by the ketogenic diet: evidence and controversies. Front Nutr (2021) 8:782657. doi: 10.3389/fnut.2021.782657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kim DY, Hao J, Liu R, Turner G, Shi F-D, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PloS One (2012) 7:e35476. doi: 10.1371/journal.pone.0035476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Liu C, Zhang N, Zhang R, Jin L, Petridis AK, Loers G, et al. Cuprizone-induced demyelination in mouse hippocampus is alleviated by ketogenic diet. J Agric Food Chem (2020) 68:11215–28. doi: 10.1021/acs.jafc.0c04604 [DOI] [PubMed] [Google Scholar]
- 147. Brenton JN, Banwell B, Bergqvist AGC, Lehner-Gulotta D, Gampper L, Leytham E, et al. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm (2019) 6:e565. doi: 10.1212/NXI.0000000000000565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Brenton JN, Lehner-Gulotta D, Woolbright E, Banwell B, Bergqvist AGC, Chen S, et al. Phase II study of ketogenic diets in relapsing multiple sclerosis: safety, tolerability and potential clinical benefits. J Neurol Neurosurg Psychiatry (2022) 93:637–44. doi: 10.1136/jnnp-2022-329074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bock M, Karber M, Kuhn H. Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. EBioMedicine (2018) 36:293–303. doi: 10.1016/j.ebiom.2018.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bock M, Steffen F, Zipp F, Bittner S. Impact of dietary intervention on serum neurofilament light chain in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm (2022) 9:e1102. doi: 10.1212/NXI.0000000000001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Swidsinski A, Dörffel Y, Loening-Baucke V, Gille C, Göktas Ö, Reißhauer A, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet(2017) (Accessed February 10, 2023). [DOI] [PMC free article] [PubMed]
- 152. O’Neill B, Raggi P. The ketogenic diet: pros and cons. Atherosclerosis (2020) 292:119–26. doi: 10.1016/j.atherosclerosis.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 153. Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science (2010) 328:321–6. doi: 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lee C, Longo V. Dietary restriction with and without caloric restriction for healthy aging. F1000Res (2016) 5:F1000 Faculty Rev–117. doi: 10.12688/f1000research.7136.1 [DOI] [Google Scholar]
- 155. Roman SN, Fitzgerald KC, Beier M, Mowry EM. Safety and feasibility of various fasting-mimicking diets among people with multiple sclerosis. Mult Scler Relat Disord (2020) 42:102149. doi: 10.1016/j.msard.2020.102149 [DOI] [PubMed] [Google Scholar]
- 156. Templeman I, Gonzalez JT, Thompson D, Betts JA. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc Nutr Soc (2020) 79:76–87. doi: 10.1017/S0029665119000636 [DOI] [PubMed] [Google Scholar]
- 157. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr (2017) 37:371–93. doi: 10.1146/annurev-nutr-071816-064634 [DOI] [PubMed] [Google Scholar]
- 158. Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med (2017) 9:eaai8700. doi: 10.1126/scitranslmed.aai8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab (2015) 22:86–99. doi: 10.1016/j.cmet.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Bolte LA, Vich Vila A, Imhann F, Collij V, Gacesa R, Peters V, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut (2021) 70:1287–98. doi: 10.1136/gutjnl-2020-322670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Antoni R, Johnston KL, Collins AL, Robertson MD. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc (2017) 76:361–8. doi: 10.1017/S0029665116002986 [DOI] [PubMed] [Google Scholar]
- 162. Bai M, Wang Y, Han R, Xu L, Huang M, Zhao J, et al. Intermittent caloric restriction with a modified fasting-mimicking diet ameliorates autoimmunity and promotes recovery in a mouse model of multiple sclerosis. J Nutr Biochem (2021) 87:108493. doi: 10.1016/j.jnutbio.2020.108493 [DOI] [PubMed] [Google Scholar]
- 163. Razeghi Jahromi S, Ghaemi A, Alizadeh A, Sabetghadam F, Moradi Tabriz H, Togha M. Effects of intermittent fasting on experimental autoimune encephalomyelitis in C57BL/6 mice. Iran J Allergy Asthma Immunol (2016) 15:212–9. [PubMed] [Google Scholar]
- 164. Kafami L, Raza M, Razavi A, Mirshafiey A, Movahedian M, Khorramizadeh MR. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicenna J Med Biotechnol (2010) 2:47–52. [PMC free article] [PubMed] [Google Scholar]
- 165. Jordan S, Tung N, Casanova-Acebes M, Chang C, Cantoni C, Zhang D, et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell (2019) 178:1102–1114.e17. doi: 10.1016/j.cell.2019.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Saadatnia M, Etemadifar M, Fatehi F, Ashtari F, Shaygannejad V, Chitsaz A, et al. Short-term effects of prolonged fasting on multiple sclerosis. Eur Neurol (2009) 61:230–2. doi: 10.1159/000197108 [DOI] [PubMed] [Google Scholar]
- 167. Etemadifar M, Sayahi F, Alroughani R, Toghianifar N, Akbari M, Nasr Z. Effects of prolonged fasting on fatigue and quality of life in patients with multiple sclerosis. Neurol Sci (2016) 37:929–33. doi: 10.1007/s10072-016-2518-9 [DOI] [PubMed] [Google Scholar]
- 168. Fitzgerald KC, Vizthum D, Henry-Barron B, Schweitzer A, Cassard SD, Kossoff E, et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord (2018) 23:33–9. doi: 10.1016/j.msard.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Fitzgerald KC, Bhargava P, Smith MD, Vizthum D, Henry-Barron B, Kornberg MD, et al. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. eBioMedicine (2022) 82:104124. doi: 10.1016/j.ebiom.2022.104124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cantoni C, Dorsett Y, Fontana L, Zhou Y, Piccio L. Effects of dietary restriction on gut microbiota and CNS autoimmunity. Clin Immunol (2022) 235:108575. doi: 10.1016/j.clim.2020.108575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hucke S, Wiendl H, Klotz L. Implications of dietary salt intake for multiple sclerosis pathogenesis. Mult Scler (2016) 22:133–9. doi: 10.1177/1352458515609431 [DOI] [PubMed] [Google Scholar]
- 172. Probst Y, Mowbray E, Svensen E, Thompson K. A systematic review of the impact of dietary sodium on autoimmunity and inflammation related to multiple sclerosis. Adv Nutr (2019) 10:902–10. doi: 10.1093/advances/nmz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature (2013) 496:518–22. doi: 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Krementsov DN, Case LK, Hickey WF, Teuscher C. Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. FASEB J (2015) 29:3446–57. doi: 10.1096/fj.15-272542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res (2015) 166:103–10. doi: 10.1016/j.trsl.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhou X, Zhang L, Ji W-J, Yuan F, Guo Z-Z, Pang B, et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation. PloS One (2013) 8:e60332. doi: 10.1371/journal.pone.0060332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Luo T, Ji W-J, Yuan F, Guo Z-Z, Li Y-X, Dong Y, et al. Th17/Treg imbalance induced by dietary salt variation indicates inflammation of target organs in humans. Sci Rep (2016) 6:26767. doi: 10.1038/srep26767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Cortese M, Yuan C, Chitnis T, Ascherio A, Munger KL. No association between dietary sodium intake and the risk of multiple sclerosis. Neurology (2017) 89:1322–9. doi: 10.1212/WNL.0000000000004417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. McDonald J, Graves J, Waldman A, Lotze T, Schreiner T, Belman A, et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult Scler Relat Disord (2016) 6:87–92. doi: 10.1016/j.msard.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Nourbakhsh B, Graves J, Casper TC, Lulu S, Waldman A, Belman A, et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry (2016) 87:1350–3. doi: 10.1136/jnnp-2016-313410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry (2015) 86:26–31. doi: 10.1136/jnnp-2014-307928 [DOI] [PubMed] [Google Scholar]
- 182. Haase S, Wilck N, Kleinewietfeld M, Müller DN, Linker RA. Sodium chloride triggers Th17 mediated autoimmunity. J Neuroimmunol (2019) 329:9–13. doi: 10.1016/j.jneuroim.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 183. Zhang Y, Peng Y, Li K, Peng X. Assessing whether a spot urine specimen can predict 24-h urinary sodium excretion accurately: a validation study. J Hypertens (2019) 37:99–108. doi: 10.1097/HJH.0000000000001879 [DOI] [PubMed] [Google Scholar]
- 184. Fitzgerald KC, Munger KL, Hartung H-P, Freedman MS, Montalbán X, Edan G, et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol (2017) 82:20–9. doi: 10.1002/ana.24965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schöfl C, et al. (23)Na magnetic resonance imaging of tissue sodium. Hypertension (2012) 59:167–72. doi: 10.1161/HYPERTENSIONAHA.111.183517 [DOI] [PubMed] [Google Scholar]
- 186. Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, et al. Glycosaminoglycan polymerization may enable osmotically inactive na+ storage in the skin. Am J Physiol Heart Circ Physiol (2004) 287:H203–208. doi: 10.1152/ajpheart.01237.2003 [DOI] [PubMed] [Google Scholar]
- 187. Linz P, Santoro D, Renz W, Rieger J, Ruehle A, Ruff J, et al. Skin sodium measured with 23Na MRI at 7.0 T. NMR BioMed (2015) 28:54–62. doi: 10.1002/nbm.3224 [DOI] [PubMed] [Google Scholar]
- 188. Wiig H, Luft FC, Titze JM. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol (Oxf) (2018) 222:e13006. doi: 10.1111/apha.13006 [DOI] [PubMed] [Google Scholar]
- 189. Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest (2013) 123:2803–15. doi: 10.1172/JCI60113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-c-dependent buffering mechanism. Nat Med (2009) 15:545–52. doi: 10.1038/nm.1960 [DOI] [PubMed] [Google Scholar]
- 191. Huhn K, Linz P, Pemsel F, Michalke B, Seyferth S, Kopp C, et al. Skin sodium is increased in male patients with multiple sclerosis and related animal models. Proc Natl Acad Sci U.S.A. (2021) 118:e2102549118. doi: 10.1073/pnas.2102549118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 193. Valizadeh S, Majdi Seghinsara A, Maleki Chollou K, Bahadori A, Abbaszadeh S, Taghdir M, et al. The efficacy of probiotics in experimental autoimmune encephalomyelitis (an animal model for MS): a systematic review and meta-analysis. Lett Appl Microbiol (2021) 73:408–17. doi: 10.1111/lam.13543 [DOI] [PubMed] [Google Scholar]
- 194. Morshedi M, Hashemi R, Moazzen S, Sahebkar A, Hosseinifard E-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J Neuroinflamm (2019) 16:231. doi: 10.1186/s12974-019-1611-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Blais LL, Montgomery TL, Amiel E, Deming PB, Krementsov DN. Probiotic and commensal gut microbial therapies in multiple sclerosis and its animal models: a comprehensive review. Gut Microbes (2021) 13:1943289. doi: 10.1080/19490976.2021.1943289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kwon H-K, Kim G-C, Kim Y, Hwang W, Jash A, Sahoo A, et al. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol (2013) 146:217–27. doi: 10.1016/j.clim.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 197. Calvo-Barreiro L, Eixarch H, Ponce-Alonso M, Castillo M, Lebrón-Galán R, Mestre L, et al. A commercial probiotic induces tolerogenic and reduces pathogenic responses in experimental autoimmune encephalomyelitis. Cells (2020) 9:E906. doi: 10.3390/cells9040906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Salehipour Z, Haghmorad D, Sankian M, Rastin M, Nosratabadi R, Soltan Dallal MM, et al. Bifidobacterium animalis in combination with human origin of lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. BioMed Pharmacother (2017) 95:1535–48. doi: 10.1016/j.biopha.2017.08.117 [DOI] [PubMed] [Google Scholar]
- 199. McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol (2005) 175:3025–32. doi: 10.4049/jimmunol.175.5.3025 [DOI] [PubMed] [Google Scholar]
- 200. Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PloS One (2010) 5:e9009. doi: 10.1371/journal.pone.0009009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. O’Connell RM, Kahn D, Gibson WSJ, Round JL, Scholz RL, Chaudhuri AA, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity (2010) 33:607–19. doi: 10.1016/j.immuni.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Sievers C, Meira M, Hoffmann F, Fontoura P, Kappos L, Lindberg RLP. Altered microRNA expression in b lymphocytes in multiple sclerosis: towards a better understanding of treatment effects. Clin Immunol (2012) 144:70–9. doi: 10.1016/j.clim.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 203. Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol (2008) 9:839–45. doi: 10.1038/ni.f.209 [DOI] [PubMed] [Google Scholar]
- 204. Gharehkhani Digehsara S, Name N, Esfandiari B, Karim E, Taheri S, Tajabadi-Ebrahimi M, et al. Effects of lactobacillus casei strain T2 (IBRC-M10783) on the modulation of Th17/Treg and evaluation of miR-155, miR-25, and IDO-1 expression in a cuprizone-induced C57BL/6 mouse model of demyelination. Inflammation (2021) 44:334–43. doi: 10.1007/s10753-020-01339-1 [DOI] [PubMed] [Google Scholar]
- 205. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U.S.A. (2010) 107:12204–9. doi: 10.1073/pnas.0909122107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Wang Y, Begum-Haque S, Telesford KM, Ochoa-Repáraz J, Christy M, Kasper EJ, et al. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes (2014) 5:552–61. doi: 10.4161/gmic.29797 [DOI] [PubMed] [Google Scholar]
- 207. Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, et al. Oral administration of the probiotic strain escherichia coli nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front Immunol (2017) 8:1096. doi: 10.3389/fimmu.2017.01096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol (2019) 10:385. doi: 10.3389/fimmu.2019.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mestre L, Carrillo-Salinas FJ, Feliú A, Mecha M, Alonso G, Espejo C, et al. How oral probiotics affect the severity of an experimental model of progressive multiple sclerosis? bringing commensal bacteria into the neurodegenerative process. Gut Microbes (2020) 12:1813532. doi: 10.1080/19490976.2020.1813532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Jiang J, Chu C, Wu C, Wang C, Zhang C, Li T, et al. Efficacy of probiotics in multiple sclerosis: a systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct (2021) 12:2354–77. doi: 10.1039/d0fo03203d [DOI] [PubMed] [Google Scholar]
- 211. Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr (2017) 36:1245–9. doi: 10.1016/j.clnu.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 212. Salami M, Kouchaki E, Asemi Z, Tamtaji OR. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? a double blind clinical trial. J Funct Foods (2019) 52:8–13. doi: 10.1016/j.jff.2018.10.023 [DOI] [Google Scholar]
- 213. Rahimlou M, Hosseini SA, Majdinasab N, Haghighizadeh MH, Husain D. Effects of long-term administration of multi-strain probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Nutr Neurosci (2022) 25:411–22. doi: 10.1080/1028415X.2020.1758887 [DOI] [PubMed] [Google Scholar]
- 214. Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol (2018) 83:1147–61. doi: 10.1002/ana.25244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75 [DOI] [PubMed] [Google Scholar]
- 216. Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol (2019) 16:605–16. doi: 10.1038/s41575-019-0173-3 [DOI] [PubMed] [Google Scholar]
- 217. Fransen F, Sahasrabudhe NM, Elderman M, Bosveld M, El Aidy S, Hugenholtz F, et al. β2→1-fructans modulate the immune system In vivo in a microbiota-dependent and -independent fashion. Front Immunol (2017) 8:154. doi: 10.3389/fimmu.2017.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Yahfoufi N, Mallet J, Graham E, Matar C. Role of probiotics and prebiotics in immunomodulation. Curr Opin Food Sci (2018) 20:82–91. doi: 10.1016/j.cofs.2018.04.006 [DOI] [Google Scholar]
- 219. Häger J, Bang H, Hagen M, Frech M, Träger P, Sokolova MV, et al. The role of dietary fiber in rheumatoid arthritis patients: a feasibility study. Nutrients (2019) 11:E2392. doi: 10.3390/nu11102392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Calame W, Weseler AR, Viebke C, Flynn C, Siemensma AD. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br J Nutr (2008) 100:1269–75. doi: 10.1017/S0007114508981447 [DOI] [PubMed] [Google Scholar]
- 221. Kamal E, Kaddam LA, Dahawi M, Osman M, Salih MA, Alagib A, et al. Gum Arabic fibers decreased inflammatory markers and disease severity score among rheumatoid arthritis patients, phase II trial. Int J Rheumatol (2018) 2018:4197537. doi: 10.1155/2018/4197537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Lu X-Y, Han B, Deng X, Deng S-Y, Zhang Y-Y, Shen P-X, et al. Pomegranate peel extract ameliorates the severity of experimental autoimmune encephalomyelitis via modulation of gut microbiota. Gut Microbes (2020) 12:1857515. doi: 10.1080/19490976.2020.1857515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Shenderov BA. Metabiotics: novel idea or natural development of probiotic conception. Microb Ecol Health Dis (2013) 24:20399. doi: 10.3402/mehd.v24i0.20399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol (2021) 18:649–67. doi: 10.1038/s41575-021-00440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
- 226. Trend S, Leffler J, Jones AP, Cha L, Gorman S, Brown DA, et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci Rep (2021) 11:5244. doi: 10.1038/s41598-021-84881-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol (2020) 11:1390. doi: 10.3389/fimmu.2020.01390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell (2020) 180:1067–1080.e16. doi: 10.1016/j.cell.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 229. Haase S, Mäurer J, Duscha A, Lee D-H, Balogh A, Gold R, et al. Propionic acid rescues high-fat diet enhanced immunopathology in autoimmunity via effects on Th17 responses. Front Immunol (2021) 12:701626. doi: 10.3389/fimmu.2021.701626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zeng Q, null JG, Liu X, Chen C, Sun X, Li H, et al. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem Int (2019) 129:104468. doi: 10.1016/j.neuint.2019.104468 [DOI] [PubMed] [Google Scholar]
- 231. Pérez-Pérez S, Domínguez-Mozo MI, Alonso-Gómez A, Medina S, Villarrubia N, Fernández-Velasco JI, et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ (2020) 8:e10220. doi: 10.7717/peerj.10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Olsson A, Gustavsen S, Nguyen TD, Nyman M, Langkilde AR, Hansen TH, et al. Serum short-chain fatty acids and associations with inflammation in newly diagnosed patients with multiple sclerosis and healthy controls. Front Immunol (2021) 12:661493. doi: 10.3389/fimmu.2021.661493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Calvo-Barreiro L, Eixarch H, Cornejo T, Costa C, Castillo M, Mestre L, et al. Selected clostridia strains from the human microbiota and their metabolite, butyrate, improve experimental autoimmune encephalomyelitis. Neurotherapeutics (2021) 18:920–37. doi: 10.1007/s13311-021-01016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflamm (2019) 16:165. doi: 10.1186/s12974-019-1552-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wang C, Yang J, Xie L, Saimaier K, Zhuang W, Han M, et al. Methyl butyrate alleviates experimental autoimmune encephalomyelitis and regulates the balance of effector T cells and regulatory T cells. Inflammation (2021) 45(3):977-91. doi: 10.1007/s10753-021-01596-8 [DOI] [PubMed] [Google Scholar]