Key Points
Question
Is targeted axillary dissection (TAD) without axillary lymph node dissection (ALND) after neoadjuvant systemic therapy (NST) in patients with node-positive breast cancer oncologically safe?
Findings
In this cohort study including 199 patients, TAD alone was not significantly associated with an increased risk of recurrence or death in 119 patients compared with 80 patients who underwent TAD with ALND.
Meaning
In this study, axillary staging based on TAD without ALND was associated with excellent clinical outcomes in selected patients, mainly those with good response to NST and at least 3 TAD lymph nodes.
This cohort study assesses 3-year clinical outcomes in patients with node-positive breast cancer who underwent targeted axillary dissection alone or in combination with axillary lymph node dissection.
Abstract
Importance
The increasing use of neoadjuvant systemic therapy (NST) has led to substantial pathological complete response rates in patients with initially node-positive, early breast cancer, thereby questioning the need for axillary lymph node dissection (ALND). Targeted axillary dissection (TAD) is feasible for axillary staging; however, data on oncological safety are scarce.
Objective
To assess 3-year clinical outcomes in patients with node-positive breast cancer who underwent TAD alone or TAD with ALND.
Design, Setting, and Participants
The SenTa study is a prospective registry study and was conducted between January 2017 and October 2018. The registry includes 50 study centers in Germany. Patients with clinically node-positive breast cancer underwent clipping of the most suspicious lymph node (LN) before NST. After NST, the marked LNs and sentinel LNs were excised (TAD) followed by ALND according to the clinician’s choice. Patients who did not undergo TAD were excluded. Data analysis was performed in April 2022 after 43 months of follow-up.
Exposure
TAD alone vs TAD with ALND.
Main Outcomes and Measures
Three-year clinical outcomes were evaluated.
Results
Of 199 female patients, the median (IQR) age was 52 (45-60) years. A total of 182 patients (91.5%) had 1 to 3 suspicious LNs; 119 received TAD alone and 80 received TAD with ALND. Unadjusted invasive disease-free survival was 82.4% (95% CI, 71.5-89.4) in the TAD with ALND group and 91.2% (95% CI, 84.2-95.1) in the TAD alone group (P = .04); axillary recurrence rates were 1.4% (95% CI, 0-54.8) and 1.8% (95% CI, 0-36.4), respectively (P = .56). Adjusted multivariate Cox regression indicated that TAD alone was not associated with an increased risk of recurrence (hazard ratio [HR], 0.83; 95% CI, 0.34-2.05; P = .69) or death (HR, 1.07; 95% CI, 0.31-3.70; P = .91). Similar results were obtained for 152 patients with clinically node-negative breast cancer after NST (invasive disease-free survival: HR, 1.26; 95% CI, 0.27-5.87; P = .77; overall survival: HR, 0.81; 95% CI, 0.15-3.83; P = .74).
Conclusions and Relevance
These results suggest that TAD alone in patients with mostly good clinical response to NST and at least 3 TAD LNs may confer survival outcomes and recurrence rates similar to TAD with ALND.
Introduction
In recent years, neoadjuvant systemic therapy (NST) is being increasingly administered to patients with early breast cancer.1,2 Pathologic complete response (pCR) rates, defined as ypT0 or ypTis and ypN0, as high as 50% to 70% have been reported in patients with triple-negative breast cancer (TNBC)3 or human epidermal growth factor receptor 2–positive (ERBB2+ [formerly HER2+]) breast cancer following NST.4,5 pCR in the axilla is achieved in approximately 50% of patients with primary clinically node-positive disease (cN+).6 Axillary pCR rates of approximately 90% were observed in patients with ERBB2+ or TNBC with a concurrent complete response in the breast.7,8
These findings have questioned the need for conventional extensive axillary treatment in responders to NST,9 given the known morbidities associated with axillary lymph node dissection (ALND).10,11 Sentinel lymph node biopsy (SLNB) following NST in cN+ breast cancer has presented with false-negative rates greater than 10%, unless 3 or more sentinel lymph nodes (SLNs) are analyzed.12 Therefore, alternative less invasive surgical techniques for the assessment of the axillary lymph node (LN) status have been evaluated.13,14 In most approaches, at least 1 suspicious LN (target LN [TLN]) was marked before NST with, eg, a radioactive iodine seed15 or a clip,6,16,17 followed by surgical removal of the marked TLN (TLN biopsy [TLNB]) after NST. TLNB is mostly performed in combination with SLNB, and the joint procedure is termed targeted axillary dissection (TAD).16,18 A 2021 pooled analysis of 13 studies including 521 patients who had undergone TAD demonstrated a false-negative rate of 5.2%.13 Whereas in 2017 ALND after NST was the standard procedure for most cN+ patients,19 current US and German clinical guidelines recommend TAD in selected cN+ patients who converted to clinically negative (ycN0)20 and histopathologically negative (ypN0) nodal status after NST. However, ALND is still regarded as a surgical option in ycN0 patients.21,22 In patients who remain node positive after NST, ALND (with or without TAD) should be usually performed.21 The lack of a common surgical strategy for cN+ and ycN0 patients is further expressed in a variety of recommendations within European guidelines23 and a variety of different approaches (ALND, SLNB, TLNB, and TAD) in routine clinical practice.24
One of the reasons for the controversy regarding surgical management of cN+ patients is that limited data on clinical outcomes for TAD with and without ALND have been reported yet.25 However, there is a crucial need to prove oncological safety of TAD if ALND along with the removal of potential residual tumor burden in axillary LNs is omitted. In the prospective, multicenter SenTa registry, 473 patients with cN+ early breast cancer underwent clipping of the TLN before NST.6 The false-negative rate of TAD vs ALND after NST was 4.3%.6 Several patients in whom TAD was successfully performed did not undergo ALND. Therefore, oncological outcomes of TAD with or without ALND could be compared in this real-world patient group.
We hereby present an analysis for clinical outcomes in 199 patients who underwent TAD in combination with ALND or TAD alone with a median (IQR) follow-up of 43 (38-48) months.
Methods
Patients and Study Procedures
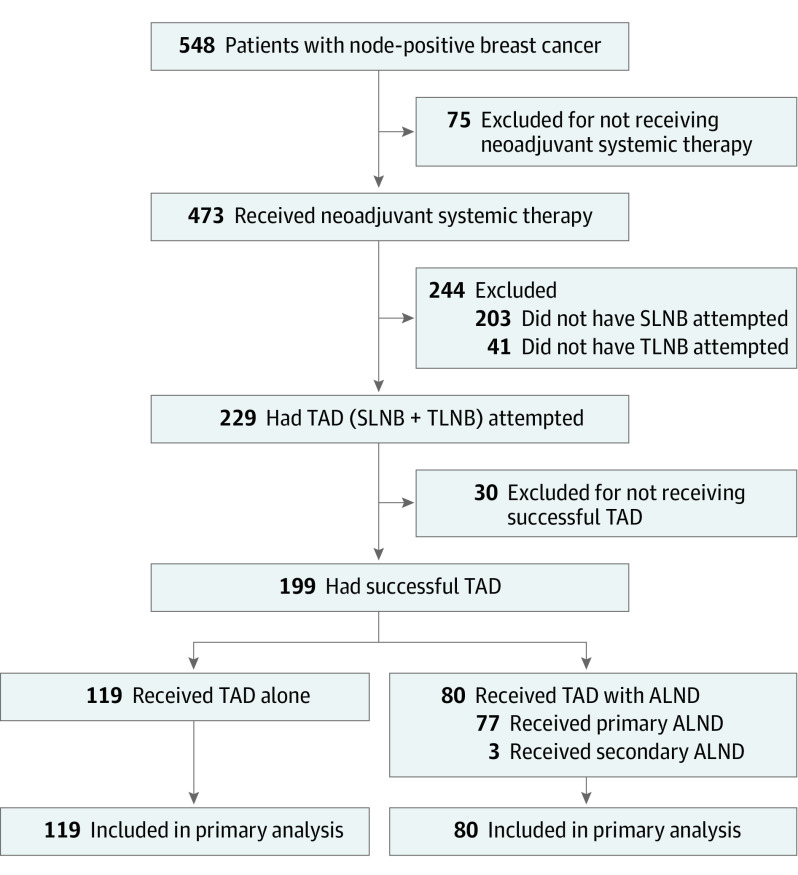
Patients 18 years and older with clinically T1 to T4 cN+ early breast cancer without distant metastases were enrolled into the SenTa study, as described previously.6 The study was approved by the Ärztekammer Nordrhein and all relevant ethics committees and authorities. All patients provided written informed consent. In 473 patients, the TLN was marked with a clip, followed by NST (Figure 1). Preoperative wire localization of the TLN was attempted. Due to the observational design of the study, axillary surgery (TLNB and/or SLNB and/or ALND) and postsurgery radiotherapy were performed according to the investigators’ discretion and contemporary clinical guidelines.26,27,28 Further details are described in a previous publication.6 The SenTa study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. CONSORT Diagram of 548 Patients With Clinically Node-Positive Breast Cancer.
Of 473 patients who received neoadjuvant systemic therapy, targeted axillary dissection (TAD) lymph nodes were successfully resected in 199 patients. In 80 of 199 patients (40.2%), TAD was followed by axillary lymph node dissection (ALND) whereas the other 119 patients (59.8%) underwent TAD alone. Follow-up data were available for all patients. SLNB indicates sentinel lymph node biopsy; TLNB, target lymph node biopsy.
Postoperative Follow-up
Survival status of the patients along with the cause of death were recorded as well as any local, axillary, regional, and/or distant recurrence detected by imaging methods and/or histopathological analysis. Details on postneoadjuvant treatment or radiotherapy were documented as well.
Analysis End Points for Clinical Outcomes
Data for clinical outcomes were collected from the date of marking the TLN onwards. Invasive disease-free survival (DFS) was defined as the time from marking the TLN to the date of first event of invasive ipsilateral or invasive regional cancer or distant breast cancer recurrence, or to the date of first event of invasive contralateral breast cancer or death from any cause, whichever occurred first. Overall survival (OS) was defined as the time elapsed from marking the TLN to the date of death from any cause. Distant DFS was defined as the time from marking the TLN to the date of distant metastases or death from any cause. Breast cancer–specific survival (BCSS) was defined as the time elapsed from marking the TLN to the date of death from breast cancer. Patients who did not have a documented event were censored at the date of last follow-up.
Axillary recurrence was defined as the occurrence of metastasis in ipsilateral axillary LNs. Regional recurrence was defined as the occurrence of metastasis in ipsilateral regional LNs. Locoregional recurrence was defined as the occurrence of ipsilateral breast cancer relapse (including ductal carcinoma in situ) or ipsilateral regional recurrence.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics version 23.0 (IBM) and GraphPad Prism version 7 (GraphPad Software). The statistical significance of differences in baseline and treatment characteristics between groups was assessed using t test and Mann-Whitney U test for continuous variables and the Pearson χ2 for categorical variables. The Clopper-Pearson exact method was used to determine the 95% CIs for the false-negative rate. The Kaplan-Meier method and log-rank test were used to analyze OS, BCSS, invasive DFS, and distant DFS. Hazard ratios (HRs) and 95% CIs were calculated using the Cox proportional hazards regression. Clinical and treatment parameters with a statistically significant difference (ALND, ycN0, ypN0, number of positive LNs, and number of TAD LNs) between the TAD with ALND and TAD alone group (Table 1) were included in the multivariate model to adjust for confounding factors except the number of resected LNs, which had a high intercorrelation (0.8) with ALND. All tests were 2-sided, and a P value less than .05 was considered statistically significant. A sample size calculation was not performed. All abbreviations are listed in eTable 1 in Supplement 1.
Table 1. Clinical and Treatment Characteristics of 80 Patients Who Underwent Targeted Axillary Dissection (TAD) With Axillary Lymph Node Dissection (ALND) and 119 Patients Who Underwent TAD Alone After Neoadjuvant Systemic Therapy (NST).
| Variable | No. (%) | P value | |
|---|---|---|---|
| TAD with ALND | TAD alone | ||
| Patients, No. | 80 | 119 | NA |
| Age, median (range), y | 51 (27-82) | 51 (26-79) | .92 |
| Tumor receptor subtype | |||
| HR+/ERBB2+ | 13 (16.3) | 26 (21.9) | .06 |
| HR+/ERBB2− | 44 (55.0) | 43 (36.1) | |
| HR−/ERBB2+ | 8 (10.0) | 21 (17.6) | |
| HR−/ERBB2− | 15 (18.8) | 29 (24.4) | |
| Clinical tumor size at diagnosis, mean (SD), mm | 25.3 (12.3) | 27.5 (14.9) | .53 |
| Suspicious LNs on ultrasonography at diagnosis | |||
| Median (range) | 1 (1-9) | 1 (1-5) | .40 |
| 1 | 48 (60.0) | 66 (55.5) | |
| 2 | 15 (18.8) | 32 (26.9) | |
| ≥3 | 17 (21.3) | 21 (17.6) | |
| Clinical nodal status after NST | |||
| ycN0 | 52 (65.0) | 100 (84.0) | .002 |
| ycN+ | 28 (35.0) | 19 (16.0) | |
| Pathological nodal status after NST | |||
| ypN0 | 32 (40.0) | 94 (79.0) | <.001 |
| ypN+ | 48 (60.0) | 25 (21.0) | |
| Resected LNs, median (range) | 11 (3-33) | 3 (1-11) | <.001 |
| TAD LNs, median (range) | 2 (1-10) | 3 (1-11) | .04 |
| Histologically positive LNs | |||
| Median (range) | 1 (0-17) | 0 (0-3) | <.001 |
| Mean (SD) | 2.2 (3.1) | 0.3 (0.7) | |
| Pathological tumor status after NST | |||
| ypT0/ypTis | 55 (70.5) | 90 (76.9) | .41 |
| ypT1 | 14 (17.9) | 20 (17.1) | |
| ypT2 | 5 (6.4) | 6 (5.1) | |
| ypT3 | 3 (3.8) | 1 (0.9) | |
| ypT4 | 1 (1.3) | 0 | |
| Unknown | 2 | 2 | NA |
| ypT0/ypTis/ypN0 after NST | |||
| Yes | 29 (100) | 79 (100) | NA |
| HR+/ERBB2+ | 8 (27.6) | 19 (24.1) | .89 |
| HR+/ERBB2− | 5 (17.2) | 19 (24.1) | |
| HR−/ERBB2+ | 7 (24.1) | 19 (24.1) | |
| HR−/ERBB2− | 9 (31.0) | 22 (27.8) | |
| No | 49 (100) | 38 (100) | NA |
| HR+/ERBB2+ | 5 (10.2) | 6 (15.8) | .49 |
| HR+/ERBB2− | 37 (75.5) | 23 (60.5) | |
| HR−/ERBB2+ | 1 (2.0) | 2 (5.3) | |
| HR−/ERBB2− | 6 (12.2) | 7 (18.4) | |
| Unknown | 2 | 2 | NA |
| Radiotherapy | |||
| No | 2 (2.6) | 10 (8.6) | .09 |
| Yes | 74 (97.4) | 106 (91.4) | |
| Breast/chest wall | 73 (96.1) | 103 (88.8) | NA |
| Axillary region | 66 (86.8) | 77 (66.4) | NA |
| Unknown | 4 | 3 | NA |
| Postneoadjuvant therapy | |||
| No | 14 (18.2) | 26 (22.4) | .48 |
| Yes | 63 (81.8) | 90 (77.6) | |
| Chemotherapy | 12 (15.6) | 5 (4.3) | NA |
| Endocrine therapy | 53 (68.8) | 69 (59.5) | NA |
| Antibody therapya | 20 (26.0) | 38 (32.8) | NA |
| Other | 0 | 4 (3.4) | NA |
| Unknown | 3 | 3 | NA |
Abbreviations: ERBB2, human epidermal growth factor receptor 2 (formerly HER2); HR, hormone receptor; LN, lymph node; NA, not applicable.
In most cases, anti-ERBB2 therapy.
Results
Patients
Of 473 patients who received NST, TAD was attempted in 229 patients and successful in 199 patients (Figure 1), for whom at least 1 TLN and SLN (including cases where the TLN is identical to the SLN) were resected. Of 199 female patients, the median (IQR) age was 52 (45-60) years. A total of 182 patients (91.5%) had 1 to 3 suspicious LNs; 119 received TAD alone and 80 received TAD with ALND. Several patient characteristics were statistically different between the group that did not undergo TAD (n = 274) and the main analysis group (n = 199). In the TAD group (alone or with ALND), the median number of suspicious LNs and clinical tumor size at diagnosis were smaller and the proportion of patients with ycN0 was larger than in the non-TAD group (eTable 2 in Supplement 1). In addition, considerably fewer patients in the TAD group (alone or with ALND) underwent ALND (80 [40.2%]) than in the non-TAD group (240 [87.6%]).
Of 199 patients with successful TAD, 119 (59.8%) did not undergo further axillary surgery and 80 (40.2%) underwent ALND; of these, 77 were performed directly after TAD within the same surgical procedure and 3 were performed in a second surgical intervention (Figure 1; Table 1). Of 145 patients (72.9%) with pCR in the breast after NST (ypT0/Tis), 108 (74.5%) were also ypN0. The ypN0 rate was significantly higher in the TAD alone group than in the TAD with ALND group (94 [79.0%] vs 32 [40.0%]; P < .001), similar to ycN0 rates (100 [84.0%] vs 52 [65.0%]; P = .002), while ypT0 and ypTis rates were comparable (90 [76.9%] vs 55 [70.5%]) (Table 1).
TAD
The median (range) number of TAD LNs excised was significantly higher in the TAD alone group (3 [1-11] LNs) than in the TAD with ALND group (2 [1-10] LNs) (Table 1). As only 1 TLN was marked in 198 of 199 patients (99.5%), the number of resected SLNs contributed to 7 or more TAD LNs in 7 patients receiving TAD with ALND and in 6 patients receiving TAD alone.
A total of 46 of 80 patients (57.5%) who underwent TAD with ALND had histologically positive TAD LNs. In 25 of these, TAD LNs were the only positive LNs, and in the remaining 21 patients, a median (range) of 3 (1-17) further positive LNs were identified by ALND. Of 34 patients with ypN0 TAD LNs, 32 patients (94.1%) presented with ypN0 status in LNs from ALND as well; 2 patients had a median (range) of 2 (1-3) positive axillary LNs identified by ALND, indicating a low metastatic burden in the axilla of patients with ypN0 TAD LNs. The corresponding false-negative rate was 4.2% (95% CI, 0.5-14.2; 2 of 48).
Clinical Outcomes
At data cutoff on March 31, 2022, the median follow-up period after marking the TLN was 43 months (95% CI, 42-44). Follow-up data were available for all patients.
Survival Outcomes
At the 3-year follow-up, 167 patients were alive without any recurrence, including 62 of 80 (77.5%) in the TAD with ALND group and 105 of 119 (88.2%) in the TAD alone group, resulting in an invasive DFS of 82.4% (95% CI, 71.5-89.4) vs 91.2% (95% CI, 84.2-95.1), respectively (P = .04) (Figure 2A). Adjusted multivariate analysis did not indicate a statistically significant association of TAD with invasive DFS (HR, 0.83; 95% CI, 0.34-2.05; P = .69) (Table 2).
Figure 2. Kaplan-Meier Curves for Invasive Disease-Free and Overall Survival and Axillary Recurrence.
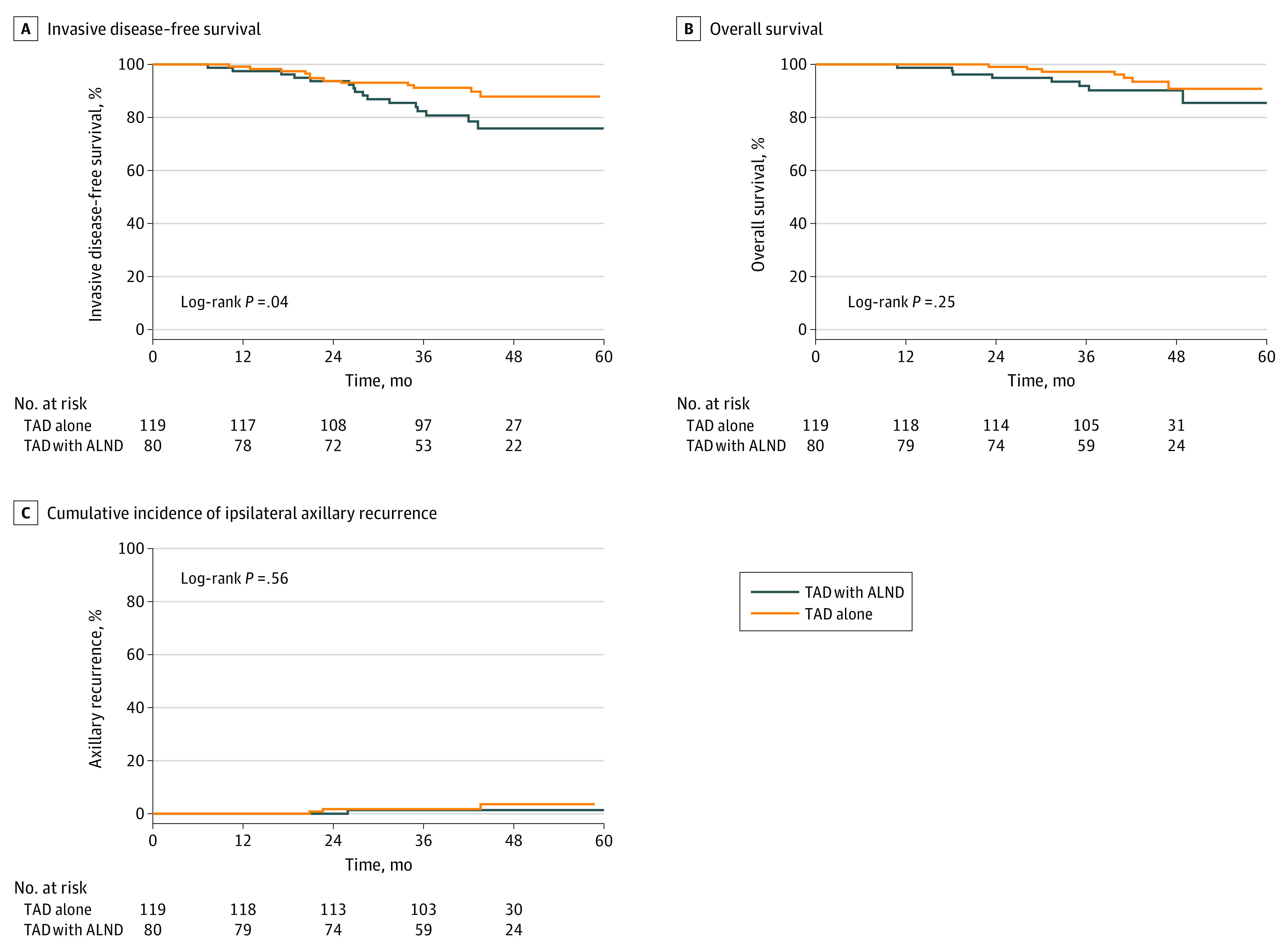
Kaplan-Meier curves for (A) invasive disease-free survival and (B) overall survival as well as (C) cumulative incidence of ipsilateral axillary recurrence for 80 patients who underwent targeted axillary dissection (TAD) with axillary lymph node dissection (ALND) compared with 119 patients who underwent TAD alone. A total of 16 recurrences and 8 deaths occurred in the TAD with ALND group and 12 recurrences and 7 deaths occurred in the TAD alone group. One patient in the TAD with ALND group and 3 patients in the TAD alone group experienced axillary recurrence.
Table 2. Associations Between Axillary Surgical Treatment and Survival Outcomes Without and With Adjustment for Relevant Clinical and Treatment Factorsa.
| Variable | Patients, No. | Events, No. (%) | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| All patients (N = 199) | ||||||
| 3-y Invasive DFS | ||||||
| TAD with ALND | 80 | 16 (20.0) | 1 [Reference] | .048 | 1 [Reference] | .69 |
| TAD alone | 119 | 12 (10.1) | 0.47 (0.22-0.99) | 0.83 (0.34-2.05) | ||
| 3-y OS | ||||||
| TAD with ALND | 80 | 8 (10.0) | 1 [Reference] | .25 | 1 [Reference] | .91 |
| TAD alone | 119 | 7 (5.9) | 0.55 (0.20-1.52) | 1.07 (0.31-3.70) | ||
| 3-y Distant DFS | ||||||
| TAD with ALND | 80 | 15 (18.7) | 1 [Reference] | .02 | 1 [Reference] | .72 |
| TAD alone | 119 | 9 (7.6) | 0.37 (0.16-0.85) | 0.83 (0.31-2.26) | ||
| 3-y BCSS | ||||||
| TAD with ALND | 80 | 6 (7.5) | 1 [Reference] | .32 | 1 [Reference] | .46 |
| TAD alone | 119 | 5 (4.2) | 0.55 (0.17-1.80) | 1.77 (0.39-8.03) | ||
| ycN0 patients (n = 152) | ||||||
| 3-y Invasive DFS | ||||||
| TAD with ALND | 52 | 8 (15.4) | 1 [Reference] | .46 | 1 [Reference] | .77 |
| TAD alone | 100 | 11 (11.0) | 0.71 (0.29-1.77) | 1.26 (0.27-5.87) | ||
| 3-y OS | ||||||
| TAD with ALND | 52 | 3 (5.8) | 1 [Reference] | .57 | 1 [Reference] | .74 |
| TAD alone | 100 | 4 (4.0) | 0.65 (0.14-2.89) | 0.81 (0.15-3.83) | ||
| 3-y Distant DFS | ||||||
| TAD with ALND | 52 | 7 (13.5) | 1 [Reference] | .30 | 1 [Reference] | .68 |
| TAD alone | 100 | 8 (8.0) | 0.59 (0.21-1.62) | 0.70 (0.13-3.85) | ||
| 3-y BCSS | ||||||
| TAD with ALND | 52 | 2 (3.8) | 1 [Reference] | .98 | 1 [Reference] | .72 |
| TAD alone | 100 | 4 (4.0) | 0.98 (0.18-5.35) | 1.39 (0.23-8.61) | ||
Abbreviations: ALND, axillary lymph node dissection; BCSS, breast cancer–specific survival; DFS, disease-free survival; HR, hazard ratio; OS, overall survival; TAD, targeted axillary dissection.
Multivariate Cox proportional hazards regression analysis was adjusted for clinical and pathological nodal status after neoadjuvant therapy, ALND, number of histopathologically positive lymph nodes after neoadjuvant therapy, and number of excised TAD lymph nodes.
In the TAD with ALND group, 8 deaths occurred, of which 6 were related to breast cancer, resulting in a 3-year OS of 91.9% (95% CI, 82.8-96.3) and 3-year BCSS of 94.8% (95% CI, 86.6-98.0). In the TAD alone group, 7 deaths occurred, of which 5 were related to breast cancer, resulting in a 3-year OS of 97.3% (95% CI, 91.9-99.1) and 3-year BCSS of 97.3% (95% CI, 91.9-99.1). Unadjusted 3-year OS (Figure 2B) and 3-year BCSS (eFigure in Supplement 1) were not significantly different between the 2 groups. After multivariate adjustment, the HR for OS and BCSS in the TAD alone group (vs the TAD with ALND as the reference) was 1.07 (95% CI, 0.31-3.70; P = .91) and 1.77 (95% CI, 0.39- 8.03; P = .46), respectively, indicating nonsignificant differences (Table 2). Similarly, 3-year distant DFS was 82.4% (95% CI, 71.5-89.4) in the TAD with ALND group vs 93.9% (95% CI, 87.5-97.0) in the TAD alone group (P = .02) (eFigure in Supplement 1), with an adjusted HR of 0.83 (95% CI, 0.31-2.26; P = .72).
A stratified analysis of patients with ycN0 status also showed nonsignificant differences in recurrence (HR, 1.26; 95% CI, 0.27-5.87; P = .77) and survival (HR, 0.81; 95% CI, 0.15-3.83; P = .74) outcomes between the 2 groups (Table 2).
Multivariate Cox regression analyses including all variables without intercorrelation are shown in eTables 3 to 6 in Supplement 1. Omission of radiotherapy, 3 or more suspicious LNs at diagnosis, and yT1-4 status were main independent predictors of disease recurrence (eTable 3 in Supplement 1).
Axillary, Local, and Regional Recurrence
Ipsilateral axillary recurrence was diagnosed in 4 patients, including 1 in the TAD with ALND group and 3 in the TAD alone group, corresponding to an axillary recurrence rate of 1.4% (95% CI, 0-54.8) vs 1.8% (95% CI, 0-36.4), representing a nonsignificant difference (P = .56) (Figure 2C). All 4 patients with axillary recurrence had concurrent locoregional recurrence, while 3 patients had concurrent distant recurrence as well.
A total of 10 patients, including 5 in each group, had local and concurrent regional recurrence, corresponding to a 3-year locoregional recurrence rate of 5.5% (95% CI, 0-33.8) in the TAD with ALND group and 2.7% (95% CI, 0-31.1) in the TAD alone group. Differences were nonsignificant (eFigure in Supplement 1). No locoregional recurrence occurred in 41 patients with ycN0, ypN0, and 3 or more TAD LNs, while 4 locoregional recurrences were detected in 39 patients with ycN0, ypN0, and 1 or 2 TAD LNs (TAD alone group). Cox proportional hazards regression for recurrence rates could not be performed due to the low number of events.
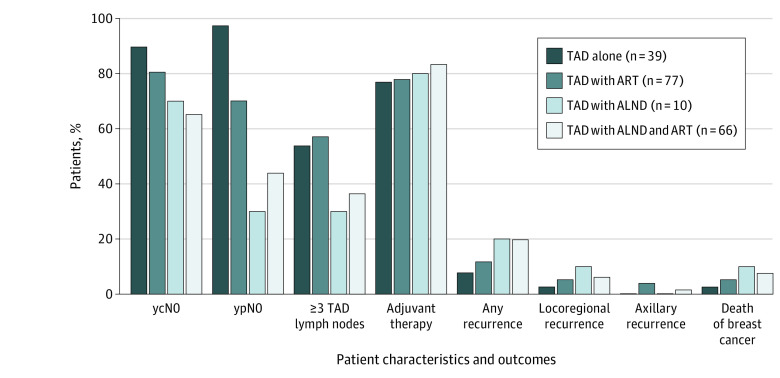
Axillary Radiotherapy
Most patients (180 of 199 [90.4%]) received locoregional radiotherapy (Table 1). Ipsilateral axillary radiotherapy (ART) was applied to 66 patients (82.5%) in the TAD with ALND group and 77 patients (64.7%) in the TAD alone group (Figure 3). The TAD alone group (n = 39) and TAD with ART group (n = 77) had a higher proportion of patients with ycN0, ypN0, and those with 3 or more TAD LNs but a lower proportion of patients with any recurrence, locoregional recurrence, and death from breast cancer compared with the TAD with ALND group with or without ART (Figure 3). In patients with unresponsive axillary disease (ycN+ or ypN+), TAD with ALND was preferred. The TAD with ALND group without ART had the lowest proportion of patients with ypN0 status (3 of 10 [30%]) and highest rate of locoregional recurrence (1 of 10 [10%]). In the TAD alone group that did not receive ART, 38 of 39 patients (97.4%) had ypN0 status based on the histological analysis of TAD LNs alone. No axillary recurrence occurred, pointing out the high clinical value of ypN0 status in TAD LNs.
Figure 3. Patient Characteristics and 3-Year Clinical Outcomes According to Axillary Treatment.
A total of 39 patients underwent targeted axillary dissection (TAD) alone, 77 patients underwent TAD with axillary radiotherapy (ART), 10 patients underwent TAD with axillary lymph node dissection (ALND), and 66 patients underwent TAD with ALND and ART. Less extensive axillary surgical treatment (TAD alone or TAD with ART) had a higher proportion of patients with clinically or pathologically node-negative status after neoadjuvant therapy (ycN0/ypN0), 3 or more TAD lymph nodes, and a lower proportion of patients with any recurrence or locoregional recurrence.
Discussion
The 3-year follow-up results of the prospective SenTa study demonstrate excellent clinical outcomes in patients with breast cancer whose axillary surgical treatment was confined to excision of TAD LNs only. The TAD alone group contained a high proportion of patients with ycN0 (100 of 119 [84.0%]), ypN0 (94 of 119 [79.0%]), and a median of 3 TAD LNs retrieved for axillary staging. Cox proportional hazards regression analyses indicate no significant differences in disease recurrence or death between patients who underwent TAD, either alone or in combination with ALND. Unadjusted axillary and locoregional recurrence rates in between the groups did not differ significantly either.
Downstaging of axillary disease after NST in initially cN+ patients has initiated de-escalation of axillary surgery with minimally invasive approaches. SLNB conducted after NST has presented with false-negative rates of 10% or more in cN+ patients,12,29 but in retrospective studies, the axillary recurrence rate in ycN0 was less than 2%.30,31 Nonetheless, the correct determination of the axillary pathological status after NST is of utmost importance.32 Besides its prognostic value, it determines further axillary treatment, which is especially important for patients with non-pCR.33,34
De-escalated axillary surgical procedures other than SLNB (eg, TLNB, TAD) have caused large interest among breast cancer surgeons.13,35,36 Very recently, 2 congress contributions presented first clinical outcomes for patients who underwent TAD alone. In a prospective registry study, no regional recurrence and 2 distant recurrences were detected in 85 patients after 36 months of follow-up.37 A retrospective evaluation of 200 ypN0 patients demonstrated a 2-year axillary recurrence rate of 0%.38 These data support the low 3-year axillary (1.8%; 95% CI, 0-36.4) and locoregional (2.7%; 95% CI, 0-31.1) recurrence rate obtained for TAD alone in the SenTa study. Further clinical evidence originates from 272 cN+ patients who underwent the marking axillary LNs with radioactive iodine seeds (MARI) procedure.39 In this single-center evaluation, patients underwent MARI (21%), MARI with ART (59%), or MARI with ALND and ART (20%). The 3-year axillary recurrence-free interval was 98% (95% CI, 96-100) for the whole cohort.39 Unadjusted 3-year axillary recurrence-free interval for our data, calculated for comparability, was 98.4% (95% CI, 95.1-99.5) in the whole study group (N = 199), 98.6% (95% CI, 90.6-99.8) in the TAD with ALND group, and 98.3% (95% CI, 93.2-99.6) in the TAD alone group. The false-negative rate of MARI is 7%,40 similar to that of TLNB, which involves clipping of the most suspicious LN before NST,6 and could be improved to 3.5% when both the MARI node and SLNs were evaluated.41 Widespread use of this radioactive method might be hampered, though, due to regulatory restrictions. Several nonradioactive markers have shown promising results.42,43,44
Theoretical considerations, based on a false-negative rate of 2%16 for TAD, suggest that for patients with ERBB2+ breast cancer or TNBC who achieve pCR in the breast and hence would not receive postneoadjuvant therapy based on their false-negative ypN status, the probability of compromising OS or distant DFS would be 1 in 10 000 if ALND is omitted.45 Real-world clinical outcomes from our report confirm these assumptions. In fact, in our analysis, values obtained for OS and distant DFS after TAD with ALND were not significantly different when only TAD was performed, although the proportion of patients with ERBB2+ or TNBC and ypT0 was larger in the TAD alone group (67 [56.3%]) than in the TAD with ALND group (29 [36.3%]).
Current de-escalation strategies have been driven with an intention to avoid morbidities associated with ALND. Although this aspect has not been investigated in patients receiving TAD alone or with ALND yet, to our knowledge, findings from the ALMANAC,11,46 Z0011,47 NSABP B-32,48 and INSEMA49 trials show that avoiding ALND in patients with clinically node-negative breast cancer is associated with improvement in patient-reported outcomes.
Strengths and Limitations
The strengths of this study are that it includes patients who were recruited within less than 2 years at 50 study centers and treated according to clinical routine, thereby representing real-world patients. Most importantly, these are some of the first data demonstrating oncological safety of TAD alone and present an important milestone in the de-escalation of axillary treatment in cN+ patients with early breast cancer, especially for ycN0 patients. Ongoing randomized clinical trials, such as ATNEC50 and TAXIS,51 are expected to provide further clinical evidence on oncological safety of minimally invasive surgical approaches after NST for patients with cN+ breast cancer.
Limitations of our study include the observational study design and nonrandomization of patients, potentially introducing a bias with regard to disease characteristics in the 2 treatment groups. The small sample size in combination with a low number of events resulted in wide 95% CIs for some clinical outcomes. In addition, as 91% of patients had 1 to 3 suspicious LNs, it is not clear if the concept of TAD without ALND can be fully applied to patients with cN2 to cN3 status.
Conclusions
In conclusion, axillary staging of cN+ patients after NST based on TAD alone was associated with excellent clinical outcomes and might allow the omission of ALND in this patient group, preferentially those with ycN0 or ypN0 status and 3 or more TAD LNs, in the future.
eTable 1. Abbreviations and the Corresponding Expanded Form
eTable 2. Baseline Characteristics of All Patients (n = 473) Who Received NST Prior to Surgery, 274 of 473 Patients Who Did Not Undergo TAD, and 199 of 473 Patients Who Underwent Successful TAD
eTable 3. Multivariate Cox Regression Analysis for Invasive Disease-Free Survival
eTable 4. Multivariate Cox Regression Analysis for Overall Survival
eTable 5. Multivariate Cox Regression Analysis for Distant Disease-Free Survival
eTable 6. Multivariate Cox Regression Analysis for Breast Cancer-Specific Survival
eFigure. Kaplan-Meier Curves for Distant Disease-Free Survival, Breast Cancer–Specific Survival, and Locoregional Recurrence
Data Sharing Statement
References
- 1.Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol. 2018;25(8):2241-2248. doi: 10.1245/s10434-018-6531-5 [DOI] [PubMed] [Google Scholar]
- 2.Colomer R, Saura C, Sánchez-Rovira P, et al. Neoadjuvant management of early breast cancer: a clinical and investigational position statement. Oncologist. 2019;24(5):603-611. doi: 10.1634/theoncologist.2018-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid P, Cortes J, Pusztai L, et al. ; KEYNOTE-522 Investigators . Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 4.Loibl S, Jackisch C, Schneeweiss A, et al. ; investigators of the German Breast Group (GBG) and the Arbeitsgemeinschaft Gynäkologische Onkologie—Breast (AGO-B) study groups . Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: a subanalysis of data from the randomized phase III GeparSepto trial. Ann Oncol. 2017;28(3):497-504. doi: 10.1093/annonc/mdw610 [DOI] [PubMed] [Google Scholar]
- 5.Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7 [DOI] [PubMed] [Google Scholar]
- 6.Kuemmel S, Heil J, Rueland A, et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2022;276(5):e553-e562. doi: 10.1097/SLA.0000000000004572 [DOI] [PubMed] [Google Scholar]
- 7.Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017;152(7):665-670. doi: 10.1001/jamasurg.2017.0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barron AU, Hoskin TL, Day CN, Hwang ES, Kuerer HM, Boughey JC. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg. 2018;153(12):1120-1126. doi: 10.1001/jamasurg.2018.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van la Parra RFD, Kuerer HM. Selective elimination of breast cancer surgery in exceptional responders: historical perspective and current trials. Breast Cancer Res. 2016;18(1):28. doi: 10.1186/s13058-016-0684-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soares EWS, Nagai HM, Bredt LC, da Cunha AD Jr, Andrade RJ, Soares GVS. Morbidity after conventional dissection of axillary lymph nodes in breast cancer patients. World J Surg Oncol. 2014;12(1):67. doi: 10.1186/1477-7819-12-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95(3):279-293. doi: 10.1007/s10549-005-9025-7 [DOI] [PubMed] [Google Scholar]
- 12.Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol. 2017;3(4):549-555. doi: 10.1001/jamaoncol.2016.4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swarnkar PK, Tayeh S, Michell MJ, Mokbel K. The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel). 2021;13(7):1539. doi: 10.3390/cancers13071539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caudle AS, Kuerer HM. Targeting and limiting surgery for patients with node-positive breast cancer. BMC Med. 2015;13:149. doi: 10.1186/s12916-015-0385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378-382. doi: 10.1097/SLA.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 16.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072-1078. doi: 10.1200/JCO.2015.64.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores-Funes D, Aguilar-Jiménez J, Martínez-Gálvez M, et al. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: preliminary results. Surg Oncol. 2019;30:52-57. doi: 10.1016/j.suronc.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 18.Caudle AS, Yang WT, Mittendorf EA, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2015;150(2):137-143. doi: 10.1001/jamasurg.2014.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liedtke C, Thill M, Jackisch C, et al. ; AGO Breast Committee . AGO recommendations for the diagnosis and treatment of patients with early breast cancer: update 2017. Breast Care (Basel). 2017;12(3):172-183. doi: 10.1159/000477575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(6):691-722. doi: 10.6004/jnccn.2022.0030 [DOI] [PubMed] [Google Scholar]
- 21.Friedrich M, Kühn T, Janni W, et al. AGO recommendations for the surgical therapy of the axilla after neoadjuvant chemotherapy: 2021 update. Geburtshilfe Frauenheilkd. 2021;81(10):1112-1120. doi: 10.1055/a-1499-8431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banys-Paluchowski M, Thill M, Kühn T, et al. AGO recommendations for the surgical therapy of breast cancer: update 2022. Geburtshilfe Frauenheilkd. 2022;82(10):1031-1043. doi: 10.1055/a-1904-6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banys-Paluchowski M, Gasparri ML, de Boniface J, et al. ; The Axsana Study Group . Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: current status, knowledge gaps, and rationale for the EUBREAST-03 AXSANA study. Cancers (Basel). 2021;13(7):1565. doi: 10.3390/cancers13071565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons JM, Maaskant-Braat AJG, Luiten EJT, et al. Patterns of axillary staging and management in clinically node positive breast cancer patients treated with neoadjuvant systemic therapy: results of a survey amongst breast cancer specialists. Eur J Surg Oncol. 2020;46(1):53-58. doi: 10.1016/j.ejso.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 25.Thill M, Kühn T, Schnitzbauer T, Banys-Paluchowski M. ”Targeted axillary dissection“—standard oder noch experimentell? Gynakologe. 2021;54(3):156-163. doi: 10.1007/s00129-020-04746-5 [DOI] [Google Scholar]
- 26.Liedtke C, Jackisch C, Thill M, Thomssen C, Müller V, Janni W; AGO Breast Committee . AGO recommendations for the diagnosis and treatment of patients with early breast cancer: update 2018. Breast Care (Basel). 2018;13(3):196-208. doi: 10.1159/000489329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ditsch N, Untch M, Thill M, et al. AGO recommendations for the diagnosis and treatment of patients with early breast cancer: update 2019. Breast Care (Basel). 2019;14(4):224-245. doi: 10.1159/000501000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gradishar WJ, Anderson BO, Aft R, et al. NCCN Clinical Practice Guidelines in Oncology (NCNN Guidelines): breast cancer. Accessed August 11, 2022. https://www2.tri-kobe.org/nccn/guideline/archive/breast2018/english/breast_v1.pdf
- 29.Cao S, Liu X, Cui J, et al. Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: an up-to-date meta-analysis of 3,578 patients. Breast. 2021;59:256-269. doi: 10.1016/j.breast.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggi N, Nussbaumer R, Holzer L, Weber WP. Axillary surgery in node-positive breast cancer. Breast. 2022;62(suppl 1):S50-S53. doi: 10.1016/j.breast.2021.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021;47(4):804-812. doi: 10.1016/j.ejso.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 32.Pelizzari G, Gerratana L, Basile D, et al. Post-neoadjuvant strategies in breast cancer: from risk assessment to treatment escalation. Cancer Treat Rev. 2019;72:7-14. doi: 10.1016/j.ctrv.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 33.von Minckwitz G, Huang C-S, Mano MS, et al. ; KATHERINE Investigators . Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 34.Tutt ANJ, Garber JE, Kaufman B, et al. ; OlympiA Clinical Trial Steering Committee and Investigators . Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394-2405. doi: 10.1056/NEJMoa2105215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed M, Douek M. Targeted axillary dissection after neoadjuvant therapy in breast cancer. Br J Surg. 2018;105(4):313-314. doi: 10.1002/bjs.10626 [DOI] [PubMed] [Google Scholar]
- 36.Hartmann S, Kühn T, de Boniface J, et al. Carbon tattooing for targeted lymph node biopsy after primary systemic therapy in breast cancer: prospective multicentre TATTOO trial. Br J Surg. 2021;108(3):302-307. doi: 10.1093/bjs/znaa083 [DOI] [PubMed] [Google Scholar]
- 37.Wu S, Li J, Wang Y, Liu G. 194P clinical feasibility and oncological safety of targeted axillary dissection after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: a prospective registry study. Ann Oncol. 2022;33:S624-S625. doi: 10.1016/j.annonc.2022.07.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montagna G, Mrdut M, Botty A, et al. Abstract GS4-02: oncological outcomes following omission of axillary lymph node dissection in node positive patients downstaging to node negative with neoadjuvant chemotherapy: the OPBC-04/EUBREAST-06/OMA study. Cancer Res. 2023;83(5, suppl):GS4-02. doi: 10.1158/1538-7445.SABCS22-GS4-02 [DOI] [Google Scholar]
- 39.van Loevezijn AA, van der Noordaa MEM, Stokkel MPM, et al. Three-year follow-up of de-escalated axillary treatment after neoadjuvant systemic therapy in clinically node-positive breast cancer: the MARI-protocol. Breast Cancer Res Treat. 2022;193(1):37-48. doi: 10.1007/s10549-022-06545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koolen BB, Donker M, Straver ME, et al. Combined PET-CT and axillary lymph node marking with radioactive iodine seeds (MARI procedure) for tailored axillary treatment in node-positive breast cancer after neoadjuvant therapy. Br J Surg. 2017;104(9):1188-1196. doi: 10.1002/bjs.10555 [DOI] [PubMed] [Google Scholar]
- 41.Simons JM, van Nijnatten TJA, van der Pol CC, et al. Diagnostic accuracy of radioactive iodine seed placement in the axilla with sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer. JAMA Surg. 2022;157(11):991-999. doi: 10.1001/jamasurg.2022.3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woods RW, Camp MS, Durr NJ, Harvey SC. A review of options for localization of axillary lymph nodes in the treatment of invasive breast cancer. Acad Radiol. 2019;26(6):805-819. doi: 10.1016/j.acra.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 43.Murthy V, Young J, Tokumaru Y, Quinn M, Edge SB, Takabe K. Options to determine pathological response of axillary lymph node metastasis after neoadjuvant chemotherapy in advanced breast cancer. Cancers (Basel). 2021;13(16):4167. doi: 10.3390/cancers13164167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reitsamer R, Peintinger F, Forsthuber E, Sir A. The applicability of Magseed for targeted axillary dissection in breast cancer patients treated with neoadjuvant chemotherapy. Breast. 2021;57:113-117. doi: 10.1016/j.breast.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wazir U, Mokbel K. De-escalation of axillary surgery in the neoadjuvant chemotherapy (NACT) setting for breast cancer: is it oncologically safe? Anticancer Res. 2020;40(10):5351-5354. doi: 10.21873/anticanres.14542 [DOI] [PubMed] [Google Scholar]
- 46.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst. 2006;98(9):599-609. doi: 10.1093/jnci/djj158 [DOI] [PubMed] [Google Scholar]
- 47.Lucci A, McCall LM, Beitsch PD, et al. ; American College of Surgeons Oncology Group . Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657-3663. doi: 10.1200/JCO.2006.07.4062 [DOI] [PubMed] [Google Scholar]
- 48.Ashikaga T, Krag DN, Land SR, et al. ; National Surgical Adjuvant Breast, Bowel Project . Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111-118. doi: 10.1002/jso.21535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerber B, Stachs A, Veselinovic K, et al. Abstract GS4-03: patient-reported outcomes (PROs) for the intergroup sentinel mamma study (INSEMA, GBG75, ABCSG43): persistent impact of axillary surgery on arm and breast symptoms in early breast cancer. Cancer Res. 2022;82(4, suppl):GS4-03. doi: 10.1158/1538-7445.SABCS21-GS4-03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goyal A, Cramp S, Wheatley D, et al. Axillary management in T1-3N1M0 breast cancer patients with needle biopsy proven nodal metastases at presentation after neoadjuvant chemotherapy (ATNEC). J Clin Oncol. 2021;39(15, suppl):TPS600. doi: 10.1200/JCO.2021.39.15_suppl.TPS600 [DOI] [Google Scholar]
- 51.Henke G, Knauer M, Ribi K, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. 2018;19(1):667. doi: 10.1186/s13063-018-3021-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Abbreviations and the Corresponding Expanded Form
eTable 2. Baseline Characteristics of All Patients (n = 473) Who Received NST Prior to Surgery, 274 of 473 Patients Who Did Not Undergo TAD, and 199 of 473 Patients Who Underwent Successful TAD
eTable 3. Multivariate Cox Regression Analysis for Invasive Disease-Free Survival
eTable 4. Multivariate Cox Regression Analysis for Overall Survival
eTable 5. Multivariate Cox Regression Analysis for Distant Disease-Free Survival
eTable 6. Multivariate Cox Regression Analysis for Breast Cancer-Specific Survival
eFigure. Kaplan-Meier Curves for Distant Disease-Free Survival, Breast Cancer–Specific Survival, and Locoregional Recurrence
Data Sharing Statement