Abstract
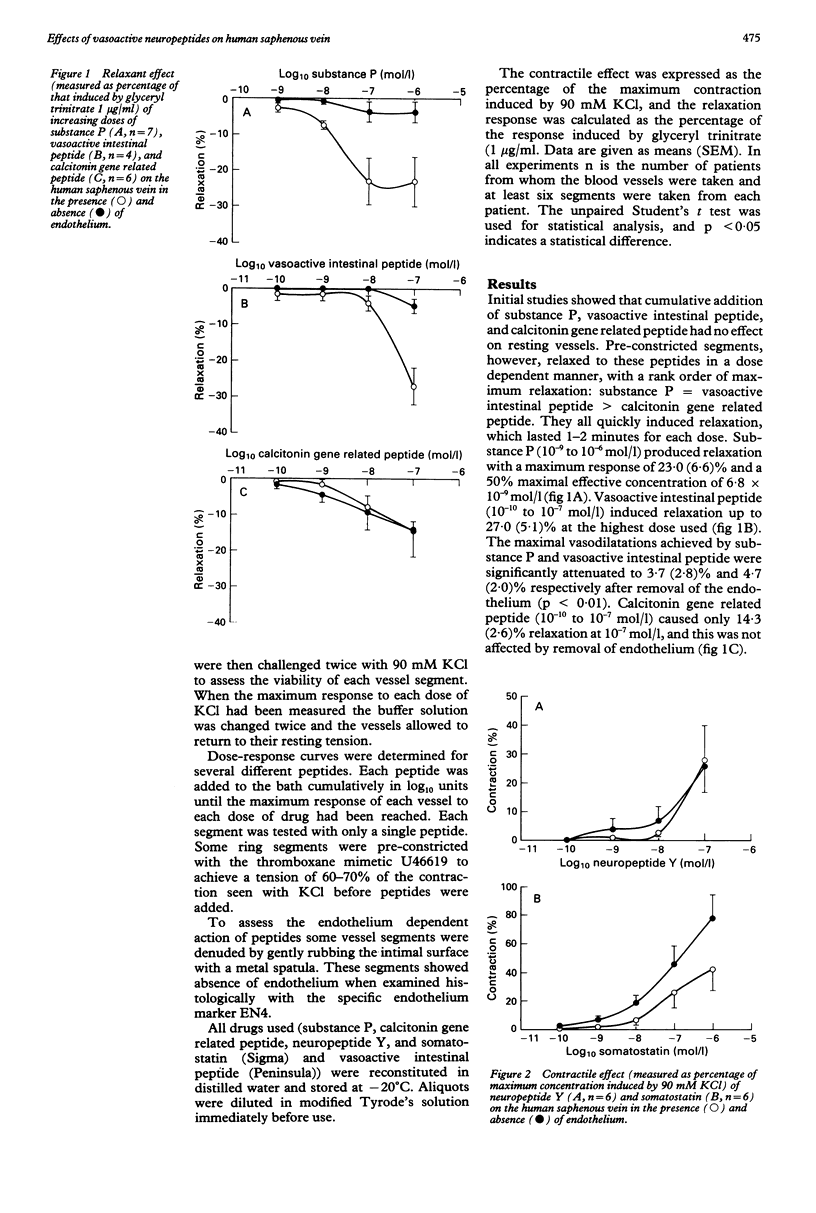
OBJECTIVE--To assess the role of neuropeptides in the control of vascular tone in the human saphenous vein the actions of substance P, vasoactive intestinal peptide, calcitonin gene related peptide, neuropeptide Y, and somatostatin on this blood vessel were examined. METHODS--In vitro organ bath techniques were used with preparations of saphenous veins obtained from 29 patients (aged 41-66) who were undergoing coronary bypass surgery. RESULTS--Substance P, vasoactive intestinal peptide, and calcitonin gene related peptide relaxed pre-constricted vessels in a dose dependent manner with a rapid onset of action, taking one to two minutes to reach a plateau at each dose. Substance P (10(-9) to 10(-6) mol/l) induced relaxation with a maximum response (mean (SEM)) 23.0 (6.6)% of the total relaxation induced by glyceryl trinitrate 1 microgram/ml and a 50% maximal effective concentration of 6.8 x 10(-9) mol/l. Vasoactive intestinal peptide (10(-10) to 10(-7) mol/l) produced a relaxation of 27.0 (5.1)% at 10(-7) mol/l. The maximum responses induced by substance P and vasoactive intestinal peptide were significantly reduced, to 3.7 (2.8)% and 4.7 (2.0)% respectively, after removal of the endothelium. Calcitonin gene related peptide (10(-10) to 10(-7) mol/l) elicited only 14.3 (2.6)% relaxation at 10(-7) mol/l, and this was not affected by removal of the endothelium. By contrast, neuropeptide Y and somatostatin exerted concentration dependent constriction on resting vessels. Neuropeptide Y (10(-10) to 10(-7) mol/l) caused prolonged contraction (roughly 20 minutes to reach a maximum plateau at each dose). At 10(-7) mol/l, the constriction amounted to 28.0 (12.0)% of the response to 90 mM KCl, in ring segments with or without endothelium. Somatostatin (10(-10) to 10(-6) mol/l) quickly caused contraction with a maximum response of 42.7 (15.0)% and a maximum response of 42.7 (15.0)% and a 50% maximal effective concentration of 6.7 x 10(-6) mol/l. The constriction was greatly increased when endothelium was removed, with a maximum response of 78.2 (16.8)% and a 50% maximal effective concentration of 4.3 x 10(-7) mol/l. CONCLUSIONS--Vasoactive peptides have diverse effects on the vascular tone these effects are endothelium dependent. The exact physiological role and implication for performance of bypass grafts require further investigation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassenge E., Busse R. Endothelial modulation of coronary tone. Prog Cardiovasc Dis. 1988 Mar-Apr;30(5):349–380. doi: 10.1016/0033-0620(88)90003-5. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Chester A. H., O'Neil G. S., Moncada S., Tadjkarimi S., Yacoub M. H. Low basal and stimulated release of nitric oxide in atherosclerotic epicardial coronary arteries. Lancet. 1990 Oct 13;336(8720):897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- D'Souza V. J., Velasquez G., Kahl F. R., Hackshaw B. T., Amplatz K. Spasm of the aortocoronary venous graft. Radiology. 1984 Apr;151(1):83–84. doi: 10.1148/radiology.151.1.6608119. [DOI] [PubMed] [Google Scholar]
- Davies J. M., Williams K. I. Endothelial-dependent relaxant effects of vaso-active intestinal polypeptide and arachidonic acid in rat aortic strips. Prostaglandins. 1984 Feb;27(2):195–202. doi: 10.1016/0090-6980(84)90073-x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Ekblad E., Håkanson R., Wahlestedt C. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol. 1984 Oct;83(2):519–525. doi: 10.1111/j.1476-5381.1984.tb16516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Ekman R., Thulin T. Reduced levels of calcitonin gene-related peptide (CGRP) but not substance P during and after treatment of severe hypertension in man. J Hum Hypertens. 1989 Aug;3(4):267–270. [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B., Hamel E., Jansen I., Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1985 Jul 31;58(2):213–217. doi: 10.1016/0304-3940(85)90166-1. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Edvinsson L., Wahlestedt C., Uddman R., Håkanson R., Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984 Apr;8(3):225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B., Rhoden K., Barnes P. Calcitonin gene-related peptide (CGRP) is a potent non-endothelium-dependent inhibitor of coronary vasomotor tone. Br J Pharmacol. 1987 Dec;92(4):789–794. doi: 10.1111/j.1476-5381.1987.tb11382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Fahrenkrug J., Hökfelt T., Martling C. R., Larsson O., Tatemoto K., Anggård A. Co-existence of peptide HI (PHI) and VIP in nerves regulating blood flow and bronchial smooth muscle tone in various mammals including man. Peptides. 1984 May-Jun;5(3):593–606. doi: 10.1016/0196-9781(84)90090-1. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Diederich D., Siebenmann R., Lehmann K., Stulz P., von Segesser L., Yang Z. H., Turina M., Grädel E., Weber E. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med. 1988 Aug 25;319(8):462–467. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- Macho P., Pérez R., Huidobro-Toro J. P., Domenech R. J. Neuropeptide Y (NPY): a coronary vasoconstrictor and potentiator of catecholamine-induced coronary constriction. Eur J Pharmacol. 1989 Aug 11;167(1):67–74. doi: 10.1016/0014-2999(89)90748-6. [DOI] [PubMed] [Google Scholar]
- Mann J. M., McIntosh C. L., Roberts W. C. Spasm of saphenous veins used as conduits for aortocoronary bypass grafting. Am J Cardiol. 1987 Apr 15;59(9):1000–1002. doi: 10.1016/0002-9149(87)91146-5. [DOI] [PubMed] [Google Scholar]
- Marshall I., Al-Kazwini S. J., Holman J. J., Craig R. K. Human alpha-calcitonin gene-related peptide (CGRP) is a potent vasodilator in human mesenteric vasculature. Br J Clin Pharmacol. 1988 Dec;26(6):691–695. doi: 10.1111/j.1365-2125.1988.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martling C. R., Matran R., Alving K., Hökfelt T., Lundberg J. M. Innervation of lower airways and neuropeptide effects on bronchial and vascular tone in the pig. Cell Tissue Res. 1990 May;260(2):223–233. doi: 10.1007/BF00318626. [DOI] [PubMed] [Google Scholar]
- McCormack D. G., Mak J. C., Coupe M. O., Barnes P. J. Calcitonin gene-related peptide vasodilation of human pulmonary vessels. J Appl Physiol (1985) 1989 Sep;67(3):1265–1270. doi: 10.1152/jappl.1989.67.3.1265. [DOI] [PubMed] [Google Scholar]
- McEwan J., Larkin S., Davies G., Chierchia S., Brown M., Stevenson J., MacIntyre I., Maseri A. Calcitonin gene-related peptide: a potent dilator of human epicardial coronary arteries. Circulation. 1986 Dec;74(6):1243–1247. doi: 10.1161/01.cir.74.6.1243. [DOI] [PubMed] [Google Scholar]
- Mitchell S. J., Bloom S. R. Measurement of fasting and postprandial plasma VIP in man. Gut. 1978 Nov;19(11):1043–1048. doi: 10.1136/gut.19.11.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil G. S., Chester A. H., Allen S. P., Luu T. N., Tadjkarimi S., Ridley P., Khagani A., Musumeci F., Yacoub M. H. Endothelial function of human gastroepiploic artery. Implications for its use as a bypass graft. J Thorac Cardiovasc Surg. 1991 Oct;102(4):561–565. [PubMed] [Google Scholar]
- Pernow J. Actions of constrictor (NPY and endothelin) and dilator (substance P, CGRP and VIP) peptides on pig splenic and human skeletal muscle arteries: involvement of the endothelium. Br J Pharmacol. 1989 Jul;97(3):983–989. doi: 10.1111/j.1476-5381.1989.tb12040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970 Sep 18;169(3951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Sata T., Linden J., Liu L. W., Kubota E., Said S. I. Vasoactive intestinal peptide evokes endothelium-dependent relaxation and cyclic AMP accumulation in rat aorta. Peptides. 1988 Jul-Aug;9(4):853–858. doi: 10.1016/0196-9781(88)90133-7. [DOI] [PubMed] [Google Scholar]
- Sata T., Misra H. P., Kubota E., Said S. I. Vasoactive intestinal polypeptide relaxes pulmonary artery by an endothelium-independent mechanism. Peptides. 1986;7 (Suppl 1):225–227. doi: 10.1016/0196-9781(86)90190-7. [DOI] [PubMed] [Google Scholar]
- Struthers A. D., Brown M. J., Macdonald D. W., Beacham J. L., Stevenson J. C., Morris H. R., MacIntyre I. Human calcitonin gene related peptide: a potent endogenous vasodilator in man. Clin Sci (Lond) 1986 Apr;70(4):389–393. doi: 10.1042/cs0700389. [DOI] [PubMed] [Google Scholar]
- Thom S. M., Hughes A. D., Goldberg P., Martin G., Schachter M., Sever P. S. The actions of calcitonin gene related peptide and vasoactive intestinal peptide as vasodilators in man in vivo and in vitro. Br J Clin Pharmacol. 1987 Aug;24(2):139–144. doi: 10.1111/j.1365-2125.1987.tb03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom S., Hughes A., Martin G., Sever P. S. Endothelium-dependent relaxation in isolated human arteries and veins. Clin Sci (Lond) 1987 Nov;73(5):547–552. doi: 10.1042/cs0730547. [DOI] [PubMed] [Google Scholar]
- Törnebrandt K., Nobin A., Owman C. Contractile and dilatory action of neuropeptides on isolated human mesenteric blood vessels. Peptides. 1987 Mar-Apr;8(2):251–256. doi: 10.1016/0196-9781(87)90099-4. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Edvinsson L., Ekblad E., Håkanson R. Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J Pharmacol Exp Ther. 1985 Sep;234(3):735–741. [PubMed] [Google Scholar]
- Weinstein J. S., Grossman W., Weintraub R. M., Thurer R. L., Johnson R. G., Morgan K. G. Differences in alpha-adrenergic responsiveness between human internal mammary arteries and saphenous veins. Circulation. 1989 Jun;79(6):1264–1270. doi: 10.1161/01.cir.79.6.1264. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., Diederich D., Schneider K., Siebenmann R., Stulz P., von Segesser L., Turina M., Bühler F. R., Lüscher T. F. Endothelium-derived relaxing factor and protection against contractions induced by histamine and serotonin in the human internal mammary artery and in the saphenous vein. Circulation. 1989 Oct;80(4):1041–1048. doi: 10.1161/01.cir.80.4.1041. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., von Segesser L., Bauer E., Stulz P., Turina M., Lüscher T. F. Different activation of the endothelial L-arginine and cyclooxygenase pathway in the human internal mammary artery and saphenous vein. Circ Res. 1991 Jan;68(1):52–60. doi: 10.1161/01.res.68.1.52. [DOI] [PubMed] [Google Scholar]


