Abstract
Four new triterpenes, 25-dehydroxy-25-methoxyargentatin C (1), 20S-hydroxyargentatin C (2), 20S-hydroxyisoargentatin C (3), and 24-epi-argentatin C (4), together with 10 known triterpenes (5–14) were isolated from the aerial parts of Parthenium incanum. The structures of 1–4 were elucidated by detailed analysis of their spectroscopic data, and the known compounds 5–14 were identified by comparison of their spectroscopic data with those reported. Since argentatin C (11) was found to exhibit antinociceptive activity by decreasing the excitability of rat and macaque dorsal root ganglia (DRG) neurons, 11 and its new analogues 1–4 were evaluated for their ability to decrease the excitability of rat DRG neurons. Of the argentatin C analogues tested, 25-dehydroxy-25-methoxyargentatin C (1) and 24-epi-argentatin C (4) decreased neuronal excitability in a manner comparable to 11. Preliminary structure–activity relationships for the action potential-reducing effects of argentatin C (11) and its analogues 1–4, and their predicted binding sites in pain-relevant voltage-gated sodium and calcium channels (VGSCs and VGCCs) in DRG neurons are presented.
Introduction
Parthenium incanum Kunth (Asteraceae; common names: mariola, New Mexico rubber plant) is a deciduous shrub native to the Southwestern United States and the Chihuahuan desert of Northern Mexico.1 Plants of the genus Parthenium find applications in Native American medicine to treat pain conditions.2,3 For example, boiled fresh leaves of P. incanum are used by Jicarilla Apaches as a medicinal tea and to treat pregnancy-related discomfort by rubbing preparations over the abdomen.2 Triterpenes including argentatin A, argentatin B, isoargentatin B, and incanilin have been reported from P. incanum.4,5 Previous investigations have shown that argentatins A and B exhibit antimicrobial6 and anti-inflammatory7 properties, and cytotoxic activity against several cancer cell lines.7−9 We have recently reported the occurrence of argentatins A–C and isoargentatin A in P. incanum and antinociceptive activity of argentatin C.10
Chronic pain is a pathological state characterized by hyper-excitable sensory neurons, leading to nociception in the absence of painful stimuli. Chronic pain affects about 20–30% of the world population.11 Historically, humans have used natural remedies derived from plants for medicinal purposes to treat musculoskeletal, neuropathic, and inflammatory pain conditions. Notable examples of these plant-based antinociceptive agents include morphine (from opium poppy),12 salicin (from willow bark),13 tetrahydocannabinol (THC from cannabis),14 capsaicin (from chili peppers),15 resiniferatoxin (RTX) from the cactus-like plant Euphorbia resinifera (Euphorbiaceae),16 mitragynine from Mitragyna speciosa (kratom; Rubiaceae),17 salvinorin A from Salvia divinorum (Mexican sage bush; Lamiaceae),18 polygodial from Drimys winteri (Winteraceae),19 and tetrahydropalmitine from Corydalis yanhusuo (Papaveraceae).20 Of these, opioids are the cornerstone of management for many types of pain.21,22 However, opioids are only partially effective in some pain types and patient populations including neuropathic pain and are often associated with serious side effects including tolerance, addiction, and death that limit their clinical utility.23 Thus, there is an urgent need for new pain therapies (antinociceptive agents) to circumvent the liabilities of opioid drugs.
The International Association for the Study of Pain (IASP) defines pain as an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.24 Nociceptors are a subgroup of sensory neurons specialized in the detection of painful stimuli.25,26 The cell bodies of pain receptors are located in either the dorsal root ganglia (DRG) or the trigeminal ganglia (TG), connecting the peripheral nervous system with the central nervous system (CNS). The key physiological processes for the peripheral transmission of sensory information are transduction, action potential transmission, and transmitter release. In the periphery, the nerve endings have transmembrane proteins called ion channels and receptors, which transduce a noxious stimulus into an electrical signal. Among them, specific voltage-gated sodium channels (VGSCs) and voltage-gated calcium channels (VGCCs) contribute to reach the threshold for action potential generation and determine cellular excitability.25,27 In addition, these channels support propagation of action potentials to the central terminals within the spinal cord.28,29 Sustained noxious stimuli or chronic diseases may affect stimulus detection, action potential generation, and transmission, which facilitate nociceptive processing, resulting in transition from acute pain to chronic pathological pain conditions.30 The hyper-activity of VGSCs and VGCCs in the peripheral tissue injury or in damaged nerve promotes it to become hyper-excitable.28 Therefore, the discovery of molecules that modulate the excitability in DRG neurons is key to developing antinociceptive agents that target the activity of these ion channels in nociceptive neurons.31
Our search for natural products-based antinociceptive agents has thus far resulted in the identification of betulinic acid from Hyptis emoryi,32 physalin F from Physalis acutifolia,33 (−)-hardwickiic acid from Croton californicus,34 hautriwaic acid from Eremocarpus setigerus,34 and argentatin C (11) from P. incanum.10 Encouraged by the ability of 11 to attenuate postoperative pain via inhibition of VGSCs and T-type VGCCs,10 and the reported use of Parthenium species to treat pain conditions,2,3 prompted us to undertake a detailed investigation of P. incanum for its antinociceptive constituents. Herein, we report the isolation and characterization of four new analogues of argentatin C (1–4) together with 10 known triterpenes (5–14) from the aerial parts of P. incanum, and evaluation of 1–4 for their potential antinociceptive activity.
Results and Discussion
The crude extract of the aerial parts of P. incanum on fractionation by solvent-solvent partitioning, size-exclusion chromatography, and column chromatography followed by HPLC purification afforded four new (1–4) and 10 known (5–14) triterpenoids (Figure 1). The known triterpenoids were identified as argentatin A (5),35,36 isoargentatin A (6),35,37 16-deoxyargentatin A (7),35 16-deoxyisoargentatin A (8),35 16-dehydroargentatin A (9) (previously obtained by chromium trioxide oxidation of argentatin A),36a,38 argentatin B (10),35,36 argentatin C (11),35,36 16-dehydroargentatin C (12),35 quisquagenin (13),35 and isoquisquagenin (14),35 by comparison of their spectroscopic data with those reported.
Figure 1.

Structures of triterpenoids 1–14 from P. incanum and isoargentatin C (15).
Metabolite 1, obtained as a colorless amorphous solid, was determined to have the molecular formula C31H52O4 by a combination of HRESIMS, 13C NMR, and HSQC data, indicating 6 degrees of unsaturation. The 1H NMR spectrum of 1 (Table 1) exhibited six singlet methyls [δH 1.18 (H3-18), 1.11 (H3-26), 1.09 (H3-29), 1.08 (H3-27), 1.03 (H3-28) and 0.87 (H3-30)], a methyl doublet [δH 0.92 (J = 6.4 Hz, H3-21)], two oxymethine protons [δH 4.46 (dt, J = 10.0, 4.8 Hz, H-16) and 3.65 (dd, J = 11.6, 3.2 Hz, H-24)], one OCH3 [δH 3.21 (s)], and a pair of up-field doublets [δH 0.56 and 0.80 (AB, q, J = 4.4 Hz, H2-19)] characteristic of nonequivalent protons of a cyclopropyl methylene group in cycloartane-type triterpenoids.39 The 13C NMR spectrum of 1 (Table 2), when analyzed with the help of HSQC data, revealed 31 signals due to a ketone carbonyl (δC 216.6), six quaternary carbons of which one is oxygenated (δC 77.2), six methines, including two oxygenated carbons (δC 72.4 and 73.8), 10 methylenes, seven methyls, and an OCH3 carbon (δC 57.21). These data indicated that 1 is structurally related to argentatin C (11).35,36b In their 1H and 13C NMR spectra, the only difference between 1 and 11 was an additional singlet due to an OCH3 group [δH 3.21 (s); δC 57.1 (CH3)] in 1. The molecular formula of 1 (C31H52O4) indicated that compared to 11 (C30H50O4), it has an additional CH2 suggesting that one of the OH groups in 11 may have undergone methylation to an OCH3 group in 1. The observed heteronuclear multiple bond correlation (HMBC) correlation of the OCH3 protons (δH 3.21) with the oxygenated quaternary carbon (δC 77.2, C-25) (Figure 2) confirmed that the OH group at C-25 in 11 has been replaced by an OCH3 group in 1. The coupling patterns of H-16 and H-24 suggested β-orientation for OH-16 and R configuration at C-24, respectively.35 Thus, the structure of 1 was established as 25-dehydroxy-25-methoxyargentatin C [(16β,24R)-16,24-dihydroxy-25-methoxycycloartan-3-one].
Table 1. 1H NMR Spectroscopic Data of 1–4 and Argentatin C (11).
| position | 1 | 2 | 3 | 4 | 11 |
|---|---|---|---|---|---|
| 1 | 1.80 (m) | 1.82 (m) | 1.95 (m) | 1.81 (dt,4.2, 13.2) | 1.80 (dt,4.3, 13.2) |
| 1.55 (m | 1.51 (m) | 1.59 (m) | 1.50 (m) | 1.49 (m) | |
| 2 | 2.69 (td, 14.0, 6.4) | 2.69 (dt, 6.4, 13.8) | 2.57 (ddd, 7.0, 11.4, 15.8) | 2.68 (dt, 6.4, 13.7) | 2.66 (dt, 6.6, 13.9) |
| 2.29 (dt, 14.0, 2.4) | 2.28 (ddd, 2.6, 4.4, 14.0) | 2.40 (m) | 2.26 (ddd, 13.7, 4.5, 2.7) | 2.26 (ddd, 13.9, 4.7,2.3) | |
| 5 | 1.68 (m) | 1.67 (m) | 1.57 (m) | 1.66 (dt, 12.7, 5.8) | 1.66 (m) |
| 6 | 1.55 (m) | 1.55 (m) | 1.61 (m) | 1.53 (m) | 1.54 (m) |
| 0.95 (m) | 0.93 (dt, 2.0, 12.6) | 1.02 (m) | 0.93 (m) | 0.94 (m) | |
| 7 | 1.32 (m) | 1.38 (m) | 2.05 (m) | 1.35 (m) | 1.35 (m) |
| 1.12 (m) | 1.09 (m) | 1.04 (m) | 1.11 (m) | 1.12 (m) | |
| 8 | 1.70 (m) | 1.65 m | 1.65 m) | 1.68 (m) | |
| 11 | 2.06 (m) | 2.05 (m) | 2.04 (m) | 2.02 (m) | 2.03 (m) |
| 1.15 (m) | 1.14 (m) | 1.03 (m) | 1.13 (m) | 1.12 (m) | |
| 12 | 1.74 (m) | 1.84 (m) | 1.87 (m) | 1.66 (dt, 12.7, 5.8) | 2.00 (m) |
| 1.65 (m) | 1.64 (m) | 1.76 (m) | 1.59 (dd, 12.7, 4.2) | 1.14 (m) | |
| 15 | 2.02 (m) | 2.03 (dd, 8.0, 13.1) | 1.95 (m) | 2.00 (m) | 2.01 (m) |
| 1.35 (m) | 1.47 (5.8, 13.1) | 1.78 (m) | 1.37 (brd, 12.2) | 1.31 (m) | |
| 16 | 4.46 (ddd, 8.3, 7.2, 5.0) | 4.58 (dt, 13.8, 7.5) | 4.61 (dt, 13.8, 7.6) | 4.46 (ddd, 12.2, 7.5, 4.7) | 4.45 (dt, 10.0, 4.8) |
| 17 | 1.60 (m) | 1.81 (d, 8.4) | 1.71 (d, 8.4) | 1.61 (dd, 11.5, 4.7) | 1.59 (m) |
| 18 | 1.18 (s) | 1.42 (s) | 1.06 (s) | 1.15 (s) | 1.14 (s) |
| 19 | 0.80 (d, 4.4) | 0.80 (d, 4.4) | 1.11 (s) | 0.78 (d, 4.4) | 0.78 (d, 4.4) |
| 0.56 (d, 4.4) | 0.57 (d, 4.4) | 0.56 (d, 4.4) | 0.56 (d, 4.4) | ||
| 20 | 1.92 (m) | 1.81 (m) | 1.87 (m) | ||
| 21 | 0.92 (d, 6.4) | 1.37 (s) | 1.37 (s) | 0.92 (d, 6.2) | 0.88 (d, 6.4) |
| 22 | 2.04 (m) | 2.39 (dt, 4.6, 14.2) | 2.37 (m) | 1.72 (m) | 1.60 (m) |
| 1.13 (m) | 1.23 (m) | 1.24 (m) | 1.04 (m) | 1.07 (m) | |
| 23 | 1.82 (m) | 2.17 (dt, 4.6, 14.2) | 2.17 (dt, 4.6, 14.2) | 1.66 (m) | 1.53 (m) |
| 1.08 (m) | 1.77 (m) | 177 (m) | 1.21 (m) | 1.36 (m) | |
| 24 | 3.65 (dd, 11.6, 3.2) | 3.38 (brs) | 3.37 (brs) | 3.34 (d, 10.1) | 3.51 (dd, 11.6, 2.8) |
| 26 | 1.11 (s) | 1.29 (s) | 1.27 (s) | 1.18 (s) | 1.18 (s) |
| 27 | 1.08 (s) | 1.20 (s) | 1.18 (s) | 1.13 (s) | 1.11 (s) |
| 28 | 1.09 (s) | 1.08 (s) | 1.07 (s) | 1.06 (s) | 1.07 (s) |
| 29 | 1.03 (s) | 1.03 (s) | 1.05 (s) | 1.00 (s) | 1.02 (s) |
| 30 | 0.87 (s) | 0.84 (s) | 0.82 (s) | 0.86 (s) | 0.87 (s) |
| OMe | 3.21 (s) |
Table 2. 13C NMR Spectroscopic Data of 1–4 and Argentatin C (11).
| position | 1 | 2 | 3 | 4 | 11 |
|---|---|---|---|---|---|
| 1 | 33.5 CH2 | 33.3 CH2 | 35.8 CH2 | 33.4 CH2 | 33.3 CH2 |
| 2 | 37.5 CH2 | 37.5 CH2 | 34.6 CH2 | 37.5 CH2 | 37.4 CH2 |
| 3 | 216.6 C | 216.5 C | 217.7 C | 216.6 C | 216.6 C |
| 4 | 50.2 | 50.2 C | 47.4 C | 50.2 C | 50.2 C |
| 5 | 48.5 CH | 48.5 CH | 51.2 CH | 48.5 CH | 48.4 CH |
| 6 | 21.4 CH2 | 21.4 CH2 | 19.3 CH2 | 21.4 CH2 | 21.4 CH2 |
| 7 | 26.1 CH2 | 25.9 CH2 | 26.2 CH2 | 25.9 CH2 | 25.9 CH2 |
| 8 | 47.9 CH | 47.5 CH | 134.3 C | 47.6 CH | 47.5 CH |
| 9 | 20.8 C | 20.6 C | 133.3 C | 20.9 C | 20.8 C |
| 10 | 25.9 C | 26.2 C | 36.9 C | 26.1 C | 25.9 C |
| 11 | 26.4 CH2 | 26.5 CH2 | 20.8 CH2 | 26.4 CH2 | 26.0 CH2 |
| 12 | 32.7 CH2 | 33.5 CH2 | 31.9 CH2* | 32.6 CH2 | 32.5 CH2 |
| 13 | 45.3 C | 46.2 C | 45.3 C* | 45.3 C | 45.3 C |
| 14 | 46.5 C | 46.3 C | 47.2 C* | 46.8 C | 46.6 C |
| 15 | 47.9 CH2 | 48.4 CH2 | 43.9 CH2 | 47.7 CH2 | 47.8 CH2 |
| 16 | 72.4 CH | 73.8 CH | 74.1 CH | 72.8 CH | 72.7 CH |
| 17 | 57.1 CH | 58.6 CH | 57.1 CH | 57.0 CH | 56.7 CH |
| 18 | 18.9 CH3 | 21.5 CH3 | 19.2 CH3 | 20.0 CH3 | 18.9 CH3 |
| 19 | 29.8 CH2 | 30.2 CH2 | 18.4 CH3 | 29.8 CH2 | 29.8 CH2 |
| 20 | 26.5 CH | 78.6 C | 78.4 C | 31.5 CH | 26.3 CH |
| 21 | 17.7 CH3 | 27.1 CH3 | 27.6 CH3 | 18.5 CH3 | 17.7 CH3 |
| 22 | 26.4 CH2 | 27.2 CH2 | 27.1 CH2 | 33.8 CH2 | 26.4 CH2 |
| 23 | 31.4 CH2 | 22.5 CH2 | 22.5 CH2 | 28.6 CH2 | 31.1 CH2 |
| 24 | 73.8 CH | 69.7 CH | 69.6 CH | 80.3 CH | 75.2 CH |
| 25 | 77.2 C | 76.0 C | 76.0 C | 73.2 C | 73.0 C |
| 26 | 25.6 CH3 | 27.4 CH3 | 27.4 CH3 | 23.1 CH3 | 22.8 CH3 |
| 27 | 25.9 CH3 | 28.1 CH3 | 28.1 CH3 | 26.6 CH3 | 26.8 CH3 |
| 28 | 20.8 CH3 | 20.8 CH3 | 26.1 CH3 | 20.8 CH3 | 20.7 CH3 |
| 20 | 22.2 CH3 | 22.1 CH3 | 21.3 CH3 | 22.2 CH3 | 22.1 CH3 |
| 30 | 20.0 CH3 | 19.9 CH3 | 24.9 CH3 | 19.0 CH3 | 19.9 CH3 |
| OMe | 57.1 CH3 |
Figure 2.

Key HMBC correlations of 1–4.
The HRESIMS data of metabolite 2 exhibited a quasi-molecular ion peak at m/z 473.3601 (calcd for C30H49O4 [M-H2O+H]+ 473.3631), suggesting the molecular formula C30H50O5 and indicating 6 degrees of unsaturation. The 1H NMR spectrum of 2 (Table 1) exhibited signals due to seven methyls and a pair of up-field doublets at δH 0.80 and 0.57 (J = 4.4 Hz) characteristic of geminal methylene protons of a tetra-substituted cyclopropane ring suggesting 2 to be a cycloartane-type triterpenoid.39 The 13C NMR spectrum of 2 (Table 2), when analyzed with the help of the HSQC data, indicated the presence of 30 carbons consisting of a ketone carbonyl (δC 216.5), seven quaternary carbons of which two are oxygenated (δC 78.6 and 76.0), five methines of which two are oxygenated (δC 73.8 and 69.7), 10 methylenes, and seven methyls. Since the carbonyl group accounted for only 1 degree of unsaturation, 2 should be pentacyclic. The 1H and 13C NMR spectroscopic data of 2 closely resembled those of argentatin C (11)35,36b (Tables 1 and 2), suggesting that 2 is an analogue of 11. Comparison of the molecular formula of 2 (C30H50O5) with that of 11 (C30H50O4) indicated 2 to be an oxygenated analogue of 11. The 1H and 13C NMR chemical shifts of the C-21 methyl group of 2 (δH 1.37; δC 27.1) compared to those of 11 (δH 0.88; δC 17.7) indicated that this methyl is attached to an oxygenated carbon.40 This was further confirmed by the HMBC correlation of H3-21 to C-20 (Figure 2). Based on their 13C NMR chemical shifts, β-orientation for OH-16 and S configuration for C-20 were assigned.40a The 13C NMR chemical shift of C-21 (δC 27.1) further supported the S configuration of C-20.41 The absolute configuration of the chiral center at C-24 of 2 was determined to be R by the application of the advanced Mosher’s ester method (Figure 3).42 Metabolite 2 was thus identified as 20S-hydroxyargentatin C [(16β,20S,24R)-16,20,24,25-tetrahydroxycycloarten-3-one].
Figure 3.
Δδ values [Δδ values (in ppm) = δS–δR] obtained for (S)- and (R)-MTPA esters of 2 and 4.
The HRESIMS, 1H, and 13C NMR spectroscopic data of 3 were consistent with the molecular formula C30H50O5, indicating 6 degrees of unsaturation. In its 1H NMR spectrum (Table 1), 3 exhibited signals due to eight methyl groups, suggesting it to be a lanostane-type triterpenoid.35 The 13C NMR spectrum of 3, when analyzed with the help of HSQC data, revealed 30 carbon signals including a carbonyl carbon (δC 217.7), seven quaternary carbons of which two are olefinic (δC 134.3 and 133.3) and two are oxygenated (δC 78.4 and 76.0), four methines of which two are oxygenated (δC 74.1 and 69.6), nine methylenes, and eight methyls. Since the carbonyl group and the double bond accounted for 2 degrees of unsaturation, 3 should be tetracyclic. These data revealed that the structure of 3 is closely related to that of the known lanostane triterpenoid, isoargentatin C (15).35 The molecular weight difference of 16 Da between 3 and 15 suggested that 3 is an oxygenated analogue of 15. The major difference in their NMR data was found to be the absence of the methyl doublet (δH 0.93) in 3, which was assigned to H3-21 in 15. Instead, 3 showed a singlet (δH 1.37) due to a methyl group attached to an oxygenated carbon (δC 78.4), suggesting the presence of an OH group at C-20. This was confirmed by the presence of an HMBC correlation between H3-21 and C-20 (Figure 2). Based on their 13C NMR chemical shifts, OH-16 was determined to have β-orientation and C-20 to have S configuration.40a The coupling pattern of H-24 and the chemical shift of C-24 were found to be similar to those of 20S-hydroxyargentatin C (2) (see above), suggesting the R absolute configuration for C-24. Thus, metabolite 3 was identified as 20S-hydroxyisoargentatin C [(16β,20S,24R)-16,20,24,25-tetrahydroxylanost-8-en-3-one].
Metabolite 4 exhibited a [M + Na]+ ion at m/z 497.3604 in its HRESIMS, consistent with the molecular formula C30H50O4 which is the same as that of argentatin C (11). The 1H and 13C NMR spectroscopic data of 4 were similar to those of 11 (Tables 1 and 2), except for some signals of the side-chain moiety.35,36b Argentatin C (11) contains a 24β,25-dihydroxylated side-chain moiety. The HMBC spectrum of 4 (Figure 2) suggested that it also contains a 24,25-dihydroxylated side chain as in 11. However, in their 13C NMR spectra, the signals due to the OH-24 bearing carbon (C-24) of 4 and 11 appeared at δC 80.3 and δC 75.2 ppm, respectively, suggesting that they have different configurations at C-24. Since the configuration of C-24 in 11 is known to be R,35,36 the configuration of this carbon in 4 was suspected to be S, and this was confirmed by the application of the advanced Mosher’s ester method (Figure 3).42 Hence, metabolite 4 was identified as 24-epi-argentatin C [(16β,24S)-16,24,25-trihydroxycycloarten-3-one].
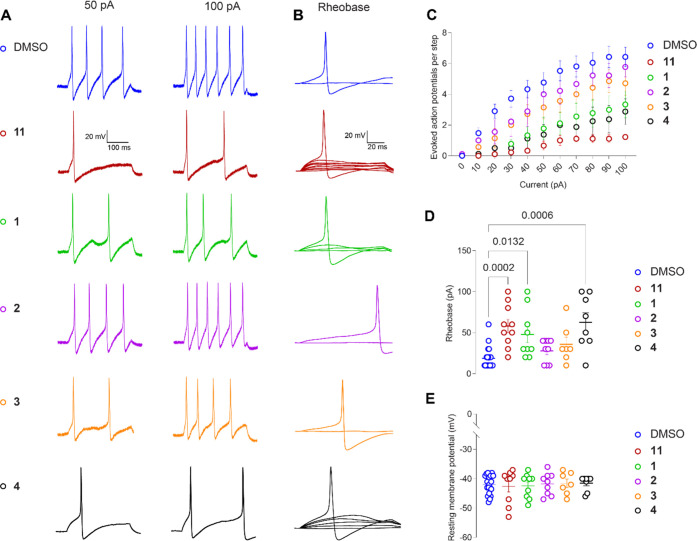
We have previously shown that the crude MeOH/CH2Cl2 (1:1) extract of P. incanum and one of its major metabolites, argentatin C (11), exhibit antinociceptive activity by inhibiting depolarization-evoked calcium influx.10 In addition, 11 decreased excitability as well as inhibited the current through VGSC and low-voltage-activated calcium channels in rat DRG neurons, whereas the other major metabolites of this extract, argentatin A (5), argentatin B (10), and isoargentatin A (6), had no effect on these neurons.10 In the current study, the excitability of DRG neurons was tested in the presence of argentatin C (11) and its analogues (1–4). Compounds 1–4 and 11 were acutely incubated with DRG neurons in order to evaluate if these compounds could modulate the excitable properties of these cells which is attributed to VGSCs and VGCCs of rat DRG neurons. The isolated neurons were incubated in the presence of 20 μM of each analogue for 15 min prior to recording and in the external solution during recording. Argentatin C (11) served as an internal positive control. As reported previously, 11 significantly decreased the number of action potentials (APs) observed in response to each step of injected current (Figure 4A,C and Table S1).10 25-Dehydroxy-25-methoxyargentatin C (1) and 24-epi-argentatin C (4) also inhibited the number of APs, whereas the excitability of rat DRG neurons in the presence of 20S-hydroxyargentatin C (2) and 20S-hydroxyisoargentatin C (3) remained unaffected (Figure 4C and Table S1). Argentatin C (11) and its analogues 1 and 4 also increased the rheobase, defined as the minimum current necessary to evoke an AP as indicated by the following data compared to the negative control (0.1% DMSO 18.57 ± 2.79): argentatin C (11) 58.0 ± 8.14; 25-dehydroxy-25-methoxyargentatin C (1) 47.8 ± 9.97; 20S-hydroxyargentatin C (2) 27.78 ± 4.65; 20S-hydroxyisoargentatin C (3) 35.71 ± 8.69; and 24-epi-argentatin C (4) 62.5 ± 11.61 (Figure 4B,D). Additionally, the resting membrane potential—an important mechanism regulating excitability—remained unchanged between conditions (Figure 4E and Table S2). Our results show that 25-dehydroxy-25-methoxyargentatin C (1), 24-epi-argentatin C (4), and argentatin C (11) diminished the excitability of rat DRG neurons. A summary of preliminary structure–activity relationship (SAR) data for argentatin C and its analogues is depicted in Figure 5. As the excitability depends on part of the activity of VGSCs and VGCCs, these results strongly suggest that like argentatin C (11), its analogues 1 and 4 inhibit these channels.10 However, behavioral experiments in animal models with acute or chronic pain will be necessary to determine if these compounds could alleviate pain.
Figure 4.
Inhibition of excitability in rat dorsal root ganglion (DRG) neurons by argentatin C (11) and its analogues 1–4. (A) Representative traces of action potentials displayed by DRG neurons in culture after incubation with dimethyl sulfoxide (DMSO) or 20 μM 11 and 1–4 in response to current injections at 50 and 100 pA for 300 ms. Representative traces (B) and quantification of the rheobase (D) [i.e., current required for eliciting the first action potential (AP)] showing significant increase in the presence of 11 and 1 when compared with DMSO. (C) Quantification of the number of action potentials evoked in response to current injection (see Table S1; Supporting Information). (E) Resting membrane potential (see Table S2; Supporting Information). [Error bars indicate mean ± SEM, p values as indicated (n = 9–10 cells). Kruskal–Wallis test].
Figure 5.
Summary of structure–activity relationships of argentatin C analogues for inhibition of excitability in rat dorsal root ganglion (DRG) neurons.
As we did previously for argentatin C (11),10 we used in silico docking to predict the binding sites for its analogues 1–4 to pain-relevant VGSCs and VGCCs. Our previous analysis identified the hydrophobic fenestrations and central pore as significantly more likely to be the sites of binding for 11,10 so the analogues were docked at these locations as well. In the VGSCs, the five compounds are predicted to dock to the pore and a single fenestration: fenestration III–IV in NaV1.7 and NaV1.8 and fenestration II–III in NaV1.9 (Figure 6A). For the VGCC targets, the predictions were split between the pore and two fenestrations in each case (Figure 6B). These results confirm that the argentatin C analogues are able to bind to the VGSC and VGCC fenestrations. These hydrophobic access channels were predicted over 40 years ago by Hille as important antinociceptive drug access pathways.43 Further, an increasing number of compounds have been found to utilize one or more fenestrations to anchor compounds within the pore of both VGSC (e.g., flecainide,44 bulleyaconitine A,45 XEN907 and TC-N1752,46 and A-80346747) and VGCC (e.g., Z944,48 cinnazarine,49 PD173212,50 mibefradil, otilonium bromide, and pimozide51) channels. Notably, the natural product bulleyaconitine A,52 as well as TC-N1752,53 A-803467,54 Z994,55 and CaV2.2 blocker 156 have been shown to have analgesic properties, supporting the fenestrations as sites that can be targeted for the development of more effective pain therapeutics. Unfortunately, the moderate resolution of the currently available cryoEM structures and inherent uncertainties of homology modeling lead to ambiguities in amino acid side-chain placement which likely has led to the highly similar docking scores (Table S3) and predicted contacts, precluding further interpretation of our docking results for argentatin C (11) and its analogues 1–4.
Figure 6.
Predicted binding sites of argentatin C (11) and analogues 1–4 in pain-relevant VGSCs and VGCCs. Docking poses for (A) VGSCs and (B) VGCCs. Top docked poses are shown as sticks with carbon atoms colored as labeled in legend, oxygen in red, and polar hydrogen in white. Channels are shown in surface representation, sliced at the level of the fenestrations (viewed from the extracellular side) and colored by domain as indicated (PD, pore domain; VSD, voltage-sensing domain). Fenestrations are labeled with the Roman numerals of the adjacent pore domains with bold text denoting open fenestrations.
Experimental Section
General Experimental Procedures
Optical rotations were measured in CH3OH with a JASCO Dip-370 digital polarimeter. One-dimensional (1D) and two-dimensional (2D) NMR spectra were recorded in CDCl3 and pyridine-d5 with a Bruker Avance III 400 spectrometer at 400 MHz for 1H NMR and 100 MHz for 13C NMR using residual solvent resonances (CDCl3: 1H NMR, 7.24; 13C NMR, 77.0; pyridine-d5: 1H NMR, 8.74, 7.58, 7.22; 13C NMR, 150.4, 135.9, 123.9) as internal references. The 1H NMR spectra of Mosher’s esters were recorded in pyridine-d5 with a Bruker DRX-500 spectrometer at 500 MHz. The chemical shift (δ) values are given in parts per million (ppm), and the coupling constants (J values) are in hertz. High-resolution mass spectra (HRMS) were recorded on a JEOL HX110A spectrometer. Circular dichroism (CD) spectra were obtained using a Jasco J-810 spectropolarimeter. Gel permeation chromatography was carried out using a Sephadex LH-20 (GE-Healthcare Bio-Sciences AB, Uppsala, Sweden). Normal-phase (NP) column chromatography was performed using Baker silica gel 40 μm flash chromatography packing (J. T. Baker), and reversed-phase (RP) chromatography was carried out using BAKERBOND C18 40 μm preparative LC packing (J. T. Baker). Analytical thin-layer chromatography (TLC) was performed on aluminum-backed precoated 0.25 mm silica gel 60 F254 plates (Merck, Darmstadt, Germany), and spots were visualized under UV light and spraying with a solution of anisaldehyde in H2SO4/HOAc followed by heating. HPLC purifications were carried out on a 10 × 250 mm Phenomenex Luna 5 μm C18 column (3 mL/min flow rate) with a Waters Delta Prep system consisting of a PDA 996 detector.
Plant Material
Aerial parts of P. incanum were collected by Dr. Steven P. McLaughlin of the University of Arizona (collection no. 7043) on Sept. 14, 1996, in Pima County (Old Highway, 83 SE of junction with Interstate I-10; 31 59.67′ N, 110 40.35′ W, elevation ca. 4,000 feet). A voucher sample has been deposited at the University of Arizona Herbarium (Catalog No. ARIZ 336215).
Extraction of P. incanum
Freshly collected aerial parts of P. incanum were air-dried and powdered, and a portion (638.0 g) of this material was extracted with CHCl3-CH3OH (1:1) at room temperature for 24 h. After filtration, the resulting filtrate was concentrated below 40 °C and under reduced pressure to afford the crude extract (137.0 g), which was stored in a sealed bottle at −5 °C until use.
Isolation and Identification of Metabolites
A portion (10.0 g) of the above extract was partitioned between hexanes and 80% aq CH3OH. The 80% aq CH3OH layer was then diluted to 50% aq CH3OH with water and extracted with CHCl3. The resulting solutions were evaporated separately under reduced pressure to afford hexanes (4.43 g), CHCl3 (2.64 g), and 50% aq CH3OH (2.29 g) fractions. A portion (4.3 g) of the hexanes fraction was subjected to gel permeation chromatography on a column of Sephadex LH-20 (200.0 g) made up in hexanes/CH2Cl2 (1:4) and eluted with hexanes/CH2Cl2 (1:4) followed by CH3OH. Twenty-four fractions were collected, and fractions having similar TLC profiles were combined to give six major fractions [A (0.44 g), B (1.69 g), C (1.47 g), D (0.16 g), E (0.19 g), and F (0.65 g)]. Fraction B (1.60 g) was chromatographed over a column of silica gel (30.0 g) made up in hexanes and eluted with 200.0 mL each of hexanes, hexanes/CHCl3 (2:1), CHCl3 followed by CHCl3/CH3OH (95:5). Sixteen subfractions were collected [B1 (15.7 mg), B2 (15.1 mg), B3 (1.6 mg), B4 (2.6 mg), B5 (4.5 mg), B6 (4.0 mg), B7 (3.6 mg), B8 (11.2 mg), B9 (86.0 mg), B10 (101.1 mg), B11 (67.7 mg), B12 (57.0 mg), B13 (178.2 mg), B14 (58.1 mg), B15 (930.8 mg), and B16 (35.6 mg)]. The major fraction B15 (930.0 mg) containing triterpenoids was re-chromatographed over a column of silica gel (30.0 g) made up in CHCl3 and eluted with CHCl3, CHCl3/CH3OH (99.5:0.5), CHCl3/CH3OH (99:1), followed by CHCl3/CH3OH (95:5) to give nine subfractions (B15A–B15I). Subfraction B15B (467.7 mg) was further separated by RP C18 (18.0 g) column chromatography using CH3OH/H2O (90:10) followed by CH3OH as eluant to give six subfractions (B15B1–B15B6). Subfraction B15B2 (53.6 mg) on purification by reversed-phase high-performance liquid chromatography (RP-HPLC) afforded argentatin B [10, 31.1 mg, tR 33.0 min, CH3OH/H2O (98:2)]. RP-HPLC purification of the subfraction B15B3 (64.9 mg) using CH3OH/H2O (98:2) as the mobile phase yielded 16-dehydroargentatin A (9, 2.2 mg, tR 32.0 min), 16-deoxyisoargentatin A (8, 3.1 mg, tR 37.5 min), 16-deoxyargentatin A (7, 8.2 mg, tR 44.0 min), and an additional amount of argentatin B (10, 16.8 mg, tR 35.0 min). RP-HPLC purification of the combined subfractions B15D (29.4 mg) and B15E (31.3 mg) afforded an additional amount of 16-dehydroargentatin A (7, 12.6 mg). Purification of the subfraction B15F (53.3 mg) by RP-HPLC followed by silica gel column chromatography gave 16-dehydroargentatin C (12, 2.2 mg), 25-dehydroxy-25-methoxyargentatin C (1, 1.7 mg), 20S-hydroxyargentatin C (2, 1.4 mg), and 20S-hydroxyisoargentatin C (3, 1.5 mg). Further purification of the subfraction B15G (24.1 mg) by RP-HPLC yielded argentatin A (5, 1.4 mg) and isoargentatin A (6, 0.8 mg).
The CHCl3 fraction (2.5 g) obtained above was subjected to size-exclusion chromatography over Sephadex LH-20 (300.0 g) made up in hexanes/CH2Cl2 (1:4) and eluted with 1.0 L each of hexanes/CH2Cl2 (1:4), CH2Cl2/acetone (4:1), CH2Cl2/acetone (2:3) followed by CH3OH. Ninety-three fractions were collected, and fractions having similar TLC profiles were combined to give nine major fractions [C1 (455.8 mg), C2 (213.2 mg), C3 (149.7 mg), C4 (168.4 mg), C5 (378.0 mg), C6 (278.9 mg), C7 (243.6 mg), C8 (202.5 mg), and C9 (439.6 mg)]. Fraction C2 (213 mg) on further purification by RP C18 (20.0 g) column chromatography using combinations of CH3OH and H2O gave argentatin C (11, 19.8 mg). Fraction C3 (145 mg) was re-chromatographed over a column of RP C18 (18.0 g) made up in CH3OH/H2O (80:20) and eluted with CH3OH/H2O (80:20), CH3OH/H2O (80:20), and CH3OH. Twenty-four fractions were collected and combined according to their TLC profile to give 10 subfractions [(C3A (11.0 mg), C3B (8.8 mg), C3C (6.1 mg), C3D (12.5 mg), C3E (5.8 mg), C3F (12.1 mg), C3G (25.5 mg), C3H (28.7 mg), C3I (9.4 mg), and C3J (18.4 mg)]. Purification of the subfraction C3H (28.7 mg) by RP-C18 HPLC [CH3OH/H2O (92.5:7.5, v/v, 3 mL/min)] gave 24-epi-argentatin C (4, 15.2 mg, tR = 23.5 min) as a white amorphous solid. The subfraction C3J was found to consist of an inseparable mixture of quisquagenin (13) and isoquisquagenin (14) in a ratio of 1:2 by careful analyses of its 1H and 13C NMR data (Figure S19).35
25-Dehydroxy-25-methoxyargentatin C [(16β,24R)-16,24-dihydroxy-25-methoxycycloartan-3-one] (1)
Amorphous white solid: [α]D25 +39 (c 0.16, CH3OH); ECD (CH3OH) [θ] −23,282 (300 nm); 1H NMR data; see Table 1; 13C NMR data; see Table 2; positive HRESIMS m/z 511.3761 [M + Na]+ (calcd for C31H52O4Na 511.3763).
20S-Hydroxyargentatin C [(16β,20S,24R)-16,20,24,25-tetrahydroxycycloartan-3-one] (2)
Amorphous white solid: [α]D25 +51 (c 0.14, CH3OH); ECD (CH3OH) [θ] 16,378 (205 nm); 1H NMR data; see Table 1; 13C NMR data; see Table 2; positive HRESIMS m/z 473.3601 [MH–H2O]+ (calcd for C30H49O4 473.3631).
20S-Hydroxyisoargentatin C [(16β,20S,24R)-16,20,24,25-tetrahydroxylanost-8-en-3-one] (3)
Amorphous white solid: [α]D25 +17 (c 0.14, CH3OH); ECD (CH3OH) [θ] −2217 (296 nm); 1H NMR data; see Table 1; 13C NMR data; see Table 2; positive HRESIMS m/z 513.3565 [M + Na]+ (calcd for C30H50O5Na 513.3556).
24-Epi-argentatin C [(16β,24S)-16,24,25-trihydroxycycloartan-3-one] (4)
Amorphous white solid: [α]D25 +18 (c 0.12, CH3OH); ECD (CH3OH) [θ] 1449 (223 nm), −527 (300 nm); 1H NMR data; see Table 1; 13C NMR data; see Table 2; positive HRESIMS m/z 497.3604 [M + Na]+ (calcd for C30H50O4Na 497.3607).
Preparation of (S)- and (R)-MTP Ester Derivatives of 2, and 4 by Advanced Mosher’s Ester Procedure
Each compound (1.5 mg) was transferred into a clean NMR tube and was dried completely in vacuo. Pyridine-d5 (1.0 mL) and (R)-(−)-α-methoxy-α-trifluoromethylphenylacetyl chloride (5.0 μL) were added into the NMR tube immediately under a stream of N2, and then the NMR tube was shaken carefully to mix the sample and the MTPA chloride evenly. The reaction in the NMR tube was monitored by 1H NMR and left at 25 °C for 2 h to give the (S)-MTPA derivatives (2a and 4a). Additional portions of 2 and 4 (1.5 mg each) in pyridine-d5 (1.0 mL) were reacted in a second NMR tube with (S)-(+)-α-methoxy-α-trifluoromethylphenylacetyl chloride (5.0 μL) at 25 °C for 2 h to afford the (R)-MTPA derivatives (2b and 4b). 1H NMR data of 2a (500 MHz, pyridine-d5): δ 5.138 (1H, brt, J = 3.1 Hz, H-24), 2.315 (1H, dd, J = 14.8, 2.8 Hz, Ha-23), 1.915 (1H, dd, J = 14.8, 3.2 Hz, Hb-23), 1.616 (3H, s, H3-21), 1.430 (3H, s, H3-26), 1.308 (3H, s, H3-27), 1.298 (1H, m, Ha-22), 1.021 (1H, m, Hb-22). 1H NMR data of 2b (500 MHz, pyridine-d5): δ 5.088 (1H, brt, J = 3.1, H-24), 2.192 (1H, dd, J = 14.6, 3.0 Hz, Ha-23), 1.837 (1H, dd, J = 14.6, 2.9 Hz, Hb-23), 1.595 (3H, s, H3-21), 1.449 (3H, s, H3-26), 1.337 (1H, s, H3-27), 1.286 (1H, m, Ha-22), 0.998 (1H, m, Hb-22). 1H NMR data of 4a (500 MHz, pyridine-d5): δ 5.635 (1H, dd, J = 9.9, 2.2 Hz, H-24), 2.183 (1H, m, Ha-23), 1.963 (1H, m, H-17), 1.949 (1H, m, H-20), 1.905 (Hb-23), 1.727 (1H, m, Ha-22), 1.666 (3H, s, H3-26), 1.483 (3H, s, H3-18), 1.445 (3H, s, H3-27), 1.437 (1H, m, Hb-22), 0.959 (3H, d, J = 6.2 Hz, H3-21), 0.951 (3H, s, H3-30). 1H NMR data of 4b (500 MHz, pyridine-d5): δ 5.597 (1H, d, J = 9.2 Hz, H-24), 2.427 (1H, m, Ha-23), 2.353 (1H, m, Ha-22), 1.997 (1H, m, Hb-23), 1.734 (1H, m, Ha-22), 1.462 (3H, s, H3-26), 1.441 (1H, m, Hb-22), 1.378 (3H, s, H3-27), 1.126 (3H, d, J = 6.2 Hz, H3-21).
Animals
Pathogen-free adult female Sprague–Dawley rats (100–200 g, Charles River Laboratories, Wilmington, MA) were kept in light (12 h light, 12 h dark cycle) and temperature (23 ± 3 °C) controlled rooms. Water and standard rodent diet were available ad libitum. All animal use was conducted in accordance with the National Institutes of Health guidelines, and the study was conducted in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the New York University (Protocol No.: PROTO202100104). All animals were housed and bred at the Kriser Dental Center Animal Facility of the University of New York. Efforts were made to minimize animal suffering.
Electrophysiology
As previously described, dorsal root ganglia (DRG) were collected and dissociated from female Sprague–Dawley rats, aged 4–6 weeks old.10 Compounds were applied for 15 min before and during the recording at 20 μM using 0.1% DMSO as a vehicle control. The excitability was tested in response to current injection between 0 and 100 pA with increments of 10 pA in the presence of 0.1% DMSO (control), or 20 μM of 11, 1, 2, 3, or 4. The external solution was composed of NaCl (154 mM), KCl (5.6 mM), CaCl2 (2 mM), MgCl2 (1 mM) d-glucose (10 mM), HEPES (8 mM), pH 7.4 and 319 milliOsmol/L. The internal solution consisted of KCl (137 mM), NaCl (10 mM), MgCl2 (1 mM), EGTA (1 mM), HEPES (10 mM), pH = 7.3 and 305 milliOsmol/L.
Homology Modeling and Ligand Docking
As previously reported,10 the Baker Lab’s structure prediction server57 was used to generate homology models for the α subunit of human NaV1.9 (UniProt ID Q9UI33-1) based on the structure of NaV1.7 with TTX bound (PDB 6j8i(58)) and for human CaV3.2 (UniProt ID O95180-1) based on the structure of CaV3.1 with Z944 bound (PDB 6kzp(48)). Briefly, 10 models were sampled for each target. Models with the lowest error estimates and the same number of open fenestrations as the template were selected for docking and trimmed to remove unreliably modeled intracellular loops prior to docking. Protein and ligand preparation and docking were conducted using Schrödinger Docking Suite tools.59 Argentatin C (11) and its analogues 1–4, and Z944 were prepared from isomeric SMILES strings using LigPrep59 with possible ionization states at pH 7.0. The cryoEM structure of human NaV1.7 (PDB 6j8i(58)), NaV1.8 (PDB 7we4(47)), CaV3.1 (PDB 6kzp(48)), CaV3.3 (PDB 7wLj(51)), and the trimmed homology models of NaV1.9 and CaV3.2 were prepared using the Protein Preparation Wizard,60 and the charges on K1462 (canonical K1865) in PDB 6kzp, K1503 in the CaV3.2 model, and K1379 in PDB 7wLj were manually adjusted from +1 to 0 prior to docking. Our previous work identified the central pore and fenestrations as the most likely binding site for 11,10 so we selected the same grid location for docking its analogues 1–4 with an enclosing box at the centroid of residues N388, F956, V1505, and V1820, and expanded the ligand-midpoint box to 32x32x32 Å3 to completely enclose the fenestrations. Docking was performed with Glide Standard Precision (SP) mode61 with enhanced sampling to obtain up to 10 poses at each site. We previously showed that redocking of Z944 to the prepared PDB 6kzp reproduced the bound pose (not shown) with a docking score of −6.6 kcal/mol.10 Docking scores for 11 and its analogues 1–4 were generally not significantly different at each target, precluding interpretation (average of top scores in kcal/mol: −7.3 ± 0.1 for NaV1.7, −6.9 ± 0.2 for NaV1.8, −6.6 ± 0.2 for NaV1.9, −6.5 ± 0.6 for CaV3.1, −7.1 ± 0.8 for CaV3.2, −5.2 ± 0.3 for CaV3.3). The top pose at each site was selected for visualization. Figure 6A,B was generated with PyMOL.62
Acknowledgments
Financial support for this work from National Institute of Neurological Disorders and Stroke (NS098772 and NS120663) and National Institute of Drug Abuse (DA042852) to R.K. is gratefully acknowledged. The authors thank Professor L.-J. Xuan, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, for providing HRMS data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c02302.
1H, 13C, and 2D NMR, ECD, and HREIMS spectra of 1–4; statistical summary of excitability of rat DRG neurons toward 11 and 1–4; and resting membrane potential and docking scores of 11 and 1–4 (PDF)
The authors declare the following competing financial interest(s): RK is the co-founder of Regulonix LLC, a company developing non-opioid drugs for chronic pain and ElutheriaTx Inc., a company developing gene therapy approaches for chronic pain. All other authors declare no competing financial interests.
Supplementary Material
References
- Powell A. M.Trees and Shrubs of the Trans-Pecos and Adjacent Areas; University of Texas Press: Austin, Texas. 1998. [Google Scholar]
- Castetter E. F.; Opler M.. The Ethnobiology of the Chiricahua and Mescalero Apache A. In The Use of Plants for Foods, Beverages, and Narcotics, Studies in the American Southwest, Biological Series; The University of New Mexico Bulletin: Albuquerque, New Mexico, 1936; Vol. III. [Google Scholar]
- Martinez M.Las Plantas Medicinales de Mexico, 5th ed.; Ediciones, Botas: Mexico City, 1969; pp 68–69. [Google Scholar]
- de Vivar R. A.; Guerrero C.; Wittgreen G. Terpenoids from Parthenium incanum. Rev. Latinoam. Quim. 1970, 1, 39–43. [Google Scholar]
- de Vivar R. A.; Matsubara C. X-ray crystallographic studies of incanilin and argentatin B. Rev. Latinoam. Quim. 1986, 17, 7–9. [Google Scholar]
- Martínez-Vázquez M.; Martínez R.; Perez G. E.; Diaz M.; Sanchez M. H. Antimicrobial properties of argentatine A, isolated from Parthenium argentatum. Fitoterapia 1994, 65, 371–372. [Google Scholar]
- Flores-Rosete G.; Martínez-Vázquez M. Anti-inflammatory and cytotoxic cycloartanes from guayule (Parthenium argentatum). Nat. Prod. Commun. 2008, 3, 413–422. 10.1177/1934578X0800300318. [DOI] [Google Scholar]
- Parra-Delgado H.; Garcia-Pillado F.; Sordo M.; Ramirez-Apan T.; Martínez-Vázquez M.; Ostrosky-Wegman P. Evaluation of the cytotoxicity, cytostaticity and genotoxicity of argentatins A and B from Parthenium argentatum (Gray). Life Sci. 2005, 77, 2855–2865. 10.1016/j.lfs.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Romero-Benavides J. C.; Bailon-Moscoso N.; Parra-Delgado H.; Ramirez M. I.; Villacis J.; Cabrera H.; Gonzalez-Arevalo G.; Cuevea R.; Zentella-Dehesa A.; Ratovitski E. A.; Martínez-Vázquez M. Argentatin B derivatives induce cell cycle arrest and DNA damage in human colon cancer cells through p73/p53 regulation. Med. Chem. Res. 2018, 27, 834–843. 10.1007/s00044-017-2106-4. [DOI] [Google Scholar]
- Duran P.; Loya-López S.; Ran D.; Tang C.; Calderon A.; Gomez K.; Stratton H.; Xu Y.-m.; Wijeratne E. M. K.; Perez-Miller S.; Shan Z.; Cai S.; Gabrielsen A.; Dorame A.; Masterson K.; Alsbiei O.; Madura C.; Luo G.; Moutal A.; Streicher J.; Gunatilaka A. A. L.; Khanna R. The natural product argentatin C from the medicinal plant Parthenium incanum attenuates postoperative pain via inhibition of voltage-gated sodium and T-type voltage-gated calcium channels. Br. J. Pharmacol. 2023, 180, 1267–1285. 10.1111/bph.15974. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Report from the Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research; The National Academies Press: Washington DC, 2011. [PubMed] [Google Scholar]
- Quirion B.; Bergeron F.; Blais V.; Gendron L. The delta-opioid receptor; a target for the treatment of pain. Front. Mol. Neurosci. 2020, 13, 52. 10.3389/fnmol.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desborough M. J. R.; Keeling D. M. The aspirin story—from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. 10.1111/bjh.14520. [DOI] [PubMed] [Google Scholar]
- Zou S.; Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.-K.; Campbell J. N. Use of capsaicin to treat pain: mechanistic and therapeutic considerations. Pharmaceuticals 2016, 9, 66. 10.3390/ph9040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. C. Resiniferatoxin: the evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals 2016, 9, 47. 10.3390/ph9030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K. Y.; Mark-Lee W. F. A review on the antinociceptive effects of Mitragyna speciosa and its derivatives on animal models. Curr. Drug Targets 2018, 19, 1359–1365. 10.2174/1389450118666170925154025. [DOI] [PubMed] [Google Scholar]
- Zjawiony J. K.; Machado A. S.; Menegatti R.; Ghedini P. C.; Costa E. A.; Pedrino G. R.; Lukas S. E.; Franco O. L.; Silva O. N.; Fajemiroye J. O. Cutting-edge search for safer opioid pain relief: retrospective review of salvinorin A and its analogs. Front. Psychiatry 2019, 10, 157. 10.3389/fpsyt.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes G. L.; Santos A. R. S.; Malheiros A.; Filho V. C.; Yunes R. A.; Calixto J. B. Assessment of mechanisms involved in antinociception caused by sesquiterpene polygodial. J. Pharmacol. Exp. Ther. 2000, 292, 164–172. [PubMed] [Google Scholar]
- Xu Y.; Sun J.; Li W.; Zhang S.; Yang L.; Teng Y.; Lv K.; Liu Y.; Su Y.; Zhang J.; Zhao M. Analgesic effect of the main components of Corydalis yanhusuo (yanhusuo in Chinese) is caused by inhibition of voltage gated sodium channels. J. Ethnopharmacol. 2021, 280, 114457 10.1016/j.jep.2021.114457. [DOI] [PubMed] [Google Scholar]
- Dowell D.; Haegerich T. M.; Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. J. Am. Med. Assoc. 2016, 315, 1624–1645. 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J.; Hashmi M. Use and abuse of opioid analgesics: potential methods to prevent and deter non-medical consumption of prescription opioids. Curr. Opin. Investig. Drugs 2004, 5, 61–66. [PubMed] [Google Scholar]
- Benyamin R.; Trescot A. M.; Datta S.; Buenaventura R.; Adlaka R.; Sehgal N.; Glaser S. E.; Vallejo R. Opioid complications and side effects. Pain Physician 2008, 2, S105–S120. 10.36076/ppj.2008/11/S105. [DOI] [PubMed] [Google Scholar]
- Raja S. N.; Carr D. B.; Cohen M.; Finnerup N. B.; Flor H.; Gibson S.; Keefe F. J.; Mogil J. S.; Ringkamp M.; Sluka K. A.; Song X. J.; Stevens B.; Sullivan M. D.; Tutelman P. R.; Ushida T.; Vader K. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner J.; Lampert A. Functional subgroups of rat and human sensory neurons: a systematic review of electrophysiological properties. Pflugers Arch. 2022, 474, 367–385. 10.1007/s00424-021-02656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. L.; Clark A. J.; Huang J.; Waxman S. G.; Dib-Hajj S. D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. 10.1152/physrev.00052.2017. [DOI] [PubMed] [Google Scholar]
- Cai S.; Gomez K.; Moutal A.; Khanna R. Targeting T-type/CaV3. 2 channels for chronic pain. Transl. Res. 2021, 234, 20–30. 10.1016/j.trsl.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J. American College of Physicians; American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann. Intern. Med. 2004, 140, 441–451. 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- Todorovic S. M.; Jevtovic-Todorovic V. Neuropathic pain: role for presynaptic T-type channels in nociceptive signaling. Pflugers Arch. 2013, 465, 921–927. 10.1007/s00424-012-1211-y. [DOI] [PubMed] [Google Scholar]
- Cheng X.; Choi J. S.; Waxman S. G.; Dib-Hajj S. D. Mini-review—sodium channels and beyond in peripheral nerve disease: Modulation by cytokines and their effector protein kinases. Neurosci. Lett. 2021, 741, 135446 10.1016/j.neulet.2020.135446. [DOI] [PubMed] [Google Scholar]
- Berta T.; Qadri Y.; Tan P. H.; Ji R. R. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets 2017, 21, 695–703. 10.1080/14728222.2017.1328057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellampalli S. S.; Ji Y.; Moutal A.; Cai S.; Wijeratne E. M. K.; Gandini M. A.; Yu J.; Chefdeville A.; Dorame A.; Chew L. A.; Madura C. L.; Luo S.; Molnar G.; Khanna M.; Streicher J. M.; Zamponi G. W.; Gunatilaka A. A. L.; Khanna R. Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury-associated peripheral sensory neuropathy via block of N- and T-type calcium channels. Pain 2019, 160, 117–135. 10.1097/j.pain.0000000000001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z.; Cai S.; Yu J.; Zhang Z.; Vallecillo T. G. M.; Serafini M. J.; Thomas A. M.; Pham N. Y. N.; Bellampalli S. S.; Moutal A.; Zhou Y.; Xu G. B.; Xu Y. M.; Luo S.; Patek M.; Streicher J. M.; Gunatilaka A. A. L.; Khanna R. Reversal of peripheral neuropathic pain by the small-molecule natural product physalin F via block of CaV2.3 (R-Type) and CaV2.2 (N-Type) voltage-gated calcium channels. ACS Chem. Neurosci. 2019, 10, 2939–2955. 10.1021/acschemneuro.9b00166. [DOI] [PubMed] [Google Scholar]
- Cai S.; Bellampalli S. S.; Yu J.; Li W.; Ji Y.; Wijeratne E. M. K.; Dorame A.; Luo S.; Shan Z.; Khanna M.; Moutal A.; Streicher J. M.; Gunatilaka A. A. L.; Khanna R. (−)-Hardwickiic acid and hautriwaic acid induce antinociception via blockade of tetrodotoxin-sensitive voltage-dependent sodium channels. ACS Chem. Neurosci. 2019, 10, 1716–1728. 10.1021/acschemneuro.8b00617. [DOI] [PubMed] [Google Scholar]
- Xu Y.-M.; Madasu C.; Liu M. X.; Wijeratne E. M. K.; Dierig D.; White B.; Molnár I.; Gunatilaka A. A. L. Cycloartane and lanostane type triterpenoids from the resin of Parthenium argentatum AZ-2, a byproduct of guayule rubber production. ACS Omega 2021, 6, 15486–15498. 10.1021/acsomega.1c01714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rodriguez-Hahn L.; Romo de Vivar A.; Ortega A.; Aguilar M.; Romo J. Determinación de las estructures de las argentatinas A, B y C del guayule. Rev. Latinoam. Quim. 1970, 1, 24–38. [Google Scholar]; b de Vivar A. R.; Martínez-Vázquez M.; Matsubara C.; Perez-Sanchez G.; Joseph-Nathan P. Triterpenes in Parthenium argentatum, structures of argentatins C and D. Phytochemistry 1990, 29, 915–918. 10.1016/0031-9422(90)80045-I. [DOI] [Google Scholar]
- Hernández-Ortega S.; Delgado H. P.; Martinez M. Incaline (isoargentatin A) from Parthenium argentatum. Acta Crystallogr., Sect. E: Struct. Rep. Online 2005, 61, 1921–1923. 10.1107/S1600536805016429. [DOI] [Google Scholar]
- Romero J. C.; Martínez-Vázquez A.; Herrera M. P.; Martinez-Mayorga K.; Parra-Delgado H.; Pérez-Flores F. J.; Martínez-Vázquez M. Synthesis, anti-inflammatory activity and modeling studies of cycloartane-type terpenes derivatives isolated from Parthenium argentatum. Bioorg. Med. Chem. 2014, 22, 6893–6898. 10.1016/j.bmc.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Escobedo-Martínez C.; Lozada M. C.; Hernández-Ortega S.; Villarreal M. L.; Gnecco D.; Enríquez R. G.; Reynolds W. 1H and 13C NMR characterization of new cycloartane triterpenes from Mangifera indica. Magn. Reson. Chem. 2012, 50, 52–57. 10.1002/mrc.2836. [DOI] [PubMed] [Google Scholar]
- a Sharma M. C.; Ohira T.; Yatagai M. Sericeol, a cycloartane triterpene from Neolitsea sericea. Phytochemistry 1993, 33, 721–722. 10.1016/0031-9422(93)85483-8. [DOI] [Google Scholar]; b Inada A.; Murayta H.; Inatomi Y.; Nakanishi T.; Darnaedi D. Cycloartane triterpenes from the leaves of Aglaia harmsiana. J. Nat. Prod. 1995, 58, 1143–1146. 10.1021/np50121a030. [DOI] [Google Scholar]
- Karabey F.; Khan I. A.; Bedir E. Cycloartane-type glycosides from Astragalus schottianus. Phytochem. Lett. 2012, 5, 320–324. 10.1016/j.phytol.2012.02.011. [DOI] [Google Scholar]
- a Kusumi T.; Ohtani L.; Inouye Y.; Kakisawa H. Absolute configurations of cytotoxic marine cembranolides; consideration of Mosher’s method. Tetrahedron Lett. 1988, 29, 4731–4734. 10.1016/S0040-4039(00)80593-6. [DOI] [Google Scholar]; b Ohtani I.; Kusumi T.; Kashman Y.; Kakisawa H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. 10.1021/ja00011a006. [DOI] [Google Scholar]; c Su B. N.; Park E. J.; Mbwambo Z. H.; Santarsiero B. D.; Mesecar A. D.; Fong H. H. S.; Pezzuto J. M.; Kinghorn A. D. New chemical constituents of Euphorbia quinquecostata and absolute configuration assignment by a convenient Mosher ester procedure carried out in NMR tubes. J. Nat. Prod. 2002, 65, 1278–1282. 10.1021/np0202475. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 1977, 69, 497–515. 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D.; Shi H.; Tonggu L.; El-Din T. M. G.; Lenaeus M. J.; Zhao Y.; Yoshioka C.; Zheng N.; Catterall W. A. Structure of cardiac sodium channel. Cell 2020, 180, 122–134.e10. 10.1016/j.cell.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Xu F.; Xu H.; Zhang S.; Gao Y.; Zhang H.; Dong Y.; Zheng Y.; Yang B.; Sun J.; Zhang X. C.; Zhao Y.; Jiang D. Structural basis for modulation of human Nav1.3 by clinical drug and selective anatagonist. Nat. Commun. 2022, 13, 1286 10.1038/s41467-022-28808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Shi Y.; Huang Z.; Li Y.; Yang B.; Gong J.; Jiang D. Structural basis for Nav1.7 inhibition by pore blockers. Nat. Struct. Mol. Biol. 2022, 29, 1208–1216. 10.1038/s41594-022-00860-1. [DOI] [PubMed] [Google Scholar]
- Huang X.; Jin X.; Huang G.; Huang J.; Wu T.; Li Z.; Chen J.; Kong F.; Pan X.; Yan N. Structural basis for high-voltage activation and subtype-specific inhibition of human Nav1.8. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2208211119 10.1073/pnas.2208211119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Huang G.; Wu Q.; Wu K.; Li R.; Lei J.; Pan X.; Yan N. Cryo-EM structures of apo and antagonist-bound human Cav3.1. Nature 2019, 576, 492–497. 10.1038/s41586-019-1801-3. [DOI] [PubMed] [Google Scholar]
- Yao X.; Gao S.; Yan N. Structural basis for pore blockade of human voltage-gated calcium channel Cav1.3 by motion sickness drug cinnarizine. Cell Res. 2022, 32, 946–948. 10.1038/s41422-022-00663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Gao Y.; Xu S.; Wang Y.; Yu Z.; Li Y.; Li B.; Yuan T.; Yang B.; Zhang X. C.; Jiang D.; Huang Z.; Zhao Y. Closed-state inactivation and pore-blocker modulation mechanisms of human Cav2.2. Cell Rep. 2021, 37, 109931 10.1016/j.celrep.2021.109931. [DOI] [PubMed] [Google Scholar]
- He L.; Yu Z.; Geng Z.; Huang Z.; Zhang C.; Dong Y.; Gao Y.; Wang Y.; Chen Q.; Sun L.; Ma X.; Huang B.; Wang X.; Zhao Y. Structure, gating, and pharmacology of human Cav3.3 channel. Nat. Commun. 2022, 13, 2084 10.1038/s41467-022-29728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M. X.; Yang J.; Pang R. P.; Zeng W. A.; Ouyang H. D.; Liu Y. Q.; Liu X. G. Bulleyaconitine A attenuates hyperexcitability of dorsal root ganglion neurons induced by spared nerve injury: The role of preferably blocking Nav1.7 and Nav1.3 channels. Mol. Pain 2018, 14, 1744806918778491 10.1177/1744806918778491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman H.; Berry L.; Buchanan J. L.; Chen A.; Du B.; Feric E.; Hierl M.; Huang L.; Immke D.; Janosky B.; Johnson D.; Li X.; Ligutti J.; Liu D.; Malmberg A.; Matson D.; McDermott J.; Miu P.; Nguyen H. N.; Patel V. F.; Waldon D.; Wilenkin B.; Zheng X. M.; Zou A.; McDonough S. I.; DiMauro E. F. Identification of a potent, state-dependent inhibitor of Nav1.7 with oral efficacy in the formalin model of persistent pain. J. Med. Chem. 2011, 54, 4427–4445. 10.1021/jm200018k. [DOI] [PubMed] [Google Scholar]
- Jarvis M. F.; Honore P.; Shieh C. C.; Chapman M.; Joshi S.; Zhang X. F.; Kort M.; Carroll W.; Marron B.; Atkinson R.; Thomas J.; Liu D.; Krambis M.; Liu Y.; McGaraughty S.; Chu K.; Roeloffs R.; Zhong C.; Mikusa J. P.; Hernandez G.; Gauvin D.; Wade C.; Zhu C.; Pai M.; Scanio M.; Shi L.; Drizin I.; Gregg R.; Matulenko M.; Hakeem A.; Gross M.; Johnson M.; Marsh K.; Wagoner P. K.; Sullivan J. P.; Faltynek C. R.; Krafte D. S. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 8520–8525. 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. Z944: a first in class T-type calcium channel modulator for the treatment of pain. J. Peripher. Nerv. Syst. 2014, 19, S11–S12. 10.1111/jns.12080_2. [DOI] [PubMed] [Google Scholar]
- Wall M. J.; Subasinghe N. L.; Winters M. P.; Lubin M. L.; Finley M. F. A.; Qin N.; Brandt M. R.; Neeper M. P.; Schneider C. R.; Colburn R. W.; Flores C. M.; Sui Z. Discovery and optimization of a novel series of pyrazolyltetrahydropyran N-type calcium channel (Cav 2.2) blockers for the treatment of pain. Bioorg. Med. Chem. Lett. 2018, 28, 3780–3783. 10.1016/j.bmcl.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Robetta Protein Structure Prediction Service, 2022. https://robetta.bakerlab.org.
- Shen H.; Liu D.; Wu K.; Lei J.; Yan N. Structures of human Nav1.7 channel in complex with auxiliary sub-units and animal toxins. Science 2019, 363, 1303–1308. 10.1126/science.aaw2493. [DOI] [PubMed] [Google Scholar]
- Schrödinger Release 2020-4; Schrödinger, LLC: New York, NY, 2020. [Google Scholar]
- Madhavi Sastry G.; Adzhigirey M.; Day T.; Annabhimoju R.; Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des. 2013, 27, 221–234. 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Friesner R. A.; Banks J. L.; Murphy R. B.; Halgren T. A.; Klicic J. J.; Mainz D. T.; Repasky M. P.; Knoll E. H.; Shelley M.; Perry J. K.; Shaw D. E.; Francis P.; Shenkin P. S. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- The PyMOL Molecular Graphics System, version 2.0; Schrödinger, LLC, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.