Abstract
The current study replicated and extended the Feldman (2009) study by applying the developmental hierarchical-integrative model to understand the emergence of self-regulation. Participants included 360 children (48.6% boys; 62.8% identified as Caucasian and 36.9% African American) and their families, predominantly from a low-income, rural background. Families completed assessments on child physiological, attention, emotion, and self-regulation when children were 6-, 15-, 24-, and 36-month-old, when caregiver sensitivity was observationally assessed. A path model revealed that child attention regulation at 6 months predicted physiological regulation at 15 months, and child attention regulation at 15 months predicted emotion regulation at 24 months. Attention regulation at 24 months predicted better self-regulation at 36 months. Notably, caregiver sensitivity moderated several developmental pathways. Findings support a continuous model of early self-regulation development and the ongoing individual-environment interplay in early childhood.
Keywords: Self-regulation, emotion regulation, attention regulation, physiological regulation, caregiver sensitivity, a developmental hierarchical-integrative model
Self-regulation plays a critical role in the development of children’s well-being and success (Eisenberg et al., 2010; Raver et al., 2011). Self-regulation in the first few years of life has been linked with higher levels of social and academic competence, and lower levels of behavior problems and peer victimization (Calkins, 2007; Eisenberg et al., 2010). Self-regulation is conceptualized as the capacity to deliberately manage or modulate one’s attention, emotion, thoughts, and actions to promote adaptative functioning and goal achievement (Calkins, 2007; Feng et al., 2017). The conceptualization is still awaiting improvement in clarity and comprehensiveness given that it involves multiple regulatory processes and functions (Feldman, 2009; Feng et al., 2017), and a call for an integrated model of self-regulation has been made (Zhou et al., 2012). The current study utilized a developmental hierarchical-integrative model (Feldman, 2009) to understand the emergence of self-regulation from infancy to preschool age, and extended such a model by incorporating early familial influences (i.e., caregiver sensitivity).
A Developmental Hierarchical-Integrative Perspective
Embracing a dynamic systems perspective (Lewis, 2005; Thelen & Smith, 1994), Feldman (2009) proposed a developmental hierarchical-integrative model to advance the understanding of self-regulation development across the first few years of life. Self-regulation is a multi-dimensional construct that includes physiological, emotional, and attentional processes. Amongst these processes, lower-level regulatory functions develop earlier and support the maturation of higher-order functions in a hierarchical manner. The multiple aspects of self-regulation also come with distinctive but connected goals to form an integrative organization, leading to the emergence of stable individual differences in self-regulation (Feldman, 2009). According to this model, different systems mature sequentially over the first few years of the children’s lives, as described below.
Physiological Regulation in the Neonatal Phase.
Infants develop necessary regulatory skills to maintain physiological homeostasis during the neonatal periods, to cope with physical and environmental stress. Recent conceptualizations on the autonomic nervous system (ANS) considers it as a quick-acting system that initiates a series of biobehavioral changes to facilitate an individual’s adaptation and coping of environmental stressors when facing challenges and threats (Lupien et al., 2006; Obradović, 2012; Porges, 2009). Two main divisions of ANS, the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) work collectively. PNS regulates the signals from SNS to control autonomic homeostasis and restore a state of calm (Porges, 2009).
One of the most typically measured indicators of PNS functioning (the activation of the vagus nerve) is respiratory sinus arrhythmia (RSA), a component of high-frequency heart rate variability associated with the breathing cycle (Berntson et al., 1993). In general, a higher resting RSA, the RSA measured at a resting state, indicates a greater capacity for self-regulation and social engagement (e.g., Gueron-Sela et al., 2017; Hastings & De, 2008). Another commonly used indicator of physiological regulation is heart rate, which tends to be reversely associated with RSA and indicates the functioning of both the SNS and the PNS (Davis et al., 2020; Vögele et al., 2010). A stable high heart rate is generally associated with difficulties in regulating emotion (e.g., Clauss & Blackford, 2012; Vögele et al., 2010). Together, RSA and heart rate constitute important indicators of autonomic physiological regulation, and are theorized as a biological susceptibility factor that can interact with environmental influences to predict self-regulation outcomes (e.g., Gueron-Sela et al., 2017; Hastings & De, 2008; Perry et al., 2018; Sturge-Apple et al., 2016).
Emotion Regulation in the First Year.
Emerging in the first year of life, emotion regulation refers to a set of internal and external goal-directed processes related to monitoring, evaluation, and modification of one’s affective, behavioral, and physiological responses under emotion-arousing circumstances (Eisenberg et al., 2010). Emotion regulation has significant implications for multiple facets of child socioemotional adjustment (Compas et al., 2017; Eisenberg et al., 2010). The early form of emotion regulation emerges as the active management of emotional input using regulatory strategies. For example, infants with better self-regulation skills engage in self-soothing behaviors (e.g., sucking thumbs and rubbing against clothes) to seek physical comfort (Crockenberg & Leerkes, 2004; Ekas et al., 2013). Infants may also orient to caregivers, or look at, vocalize, and posture towards their caregivers to express distress, to solicit support and to calm infants’ distress (Ekas et al., 2013; Wu & Feng, 2020). Avoidance is commonly expressed as looking away or pushing back against the aversive stimuli to restrict negative experiences (Crockenberg & Leerkes, 2004); however, these strategies limit the opportunity to practice more effective regulation, thus increasing infant distress. Lastly, expressed negative emotion, such as crying or fussing, can manifest an infant’s inability to regulate his or her emotion (Crockenberg & Leerkes, 2004; Perry et al., 2016).
Attention Regulation in the Second Year.
In the second year, the maturation of attention regulatory functions follows the development of emotion regulation. The development of attention regulation manifests in several ways during early childhood. In the first few months of life, infants learn to engage their attention in an increasingly efficient manner to improve the speed of information-processing (Colombo et al., 2010). Infants’ brains develop in a way that allows typically developing 4-month-olds to control eye movements to shift their gaze and voluntarily select the preferred visual stimuli exposures (Colombo et al., 2010). At this stage, the attraction to novel stimuli is strong. Starting from around 12 months, infants become more purposefully engaged in sustained attention and ignore irrelevant information (Ruff & Capozzoli, 2003). Two-year-old children gradually learn to keep their focus on the task and their goals, such that goal-oriented tasks can be executed. Attention development during infancy has been linked to emerging executive functioning in early childhood (Cuevas & Bell, 2014).
Self-regulation in Preschool Age.
During the preschool years, with the development of a sense of self, children are able to internalize the conduct expectations imposed by their environments and perform more complicated tasks. More advanced levels of self-regulation emerge at this age and embody typically in three sets of competencies: executive functions, behavioral adaptation, and compliance (Feldman, 2009). Executive functions are the cognitive abilities to manage and organize information to achieve goal-oriented activities (Anderson, 2002). These abilities include working memory, inhibitory control, and attention shifting (Garon et al., 2008; Willoughby et al, 2010). Working memory refers to the ability to retain the information in mind for a delayed period of time, and sometimes also involves manipulation and updates of the information being maintained. Inhibitory control involves the ability to follow an arbitrary rule to suppress a spontaneous response. Attention shifting reflects the mental flexibility that requires alternation of attentional, cognitive, or response processes (Garon et al., 2008; Willoughby et al., 2010). The development of executive functions is associated with the prefrontal cortex and is central to self-regulation development, with implications to future academic, emotional, and social competence (Garon et al., 2008; Zhou et al., 2012).
Behavioral adaptations, indexed by externalizing and internalizing behavioral problems, consists of children’s emotional and behavioral well-being and adaptation to society (Eisenberg et al., 2010). The development of behavioral problems in preschool age is commonly associated with difficulties in physiological regulation (e.g., low resting RSA; Calkins & Keane, 2004), emotion regulation (e.g., high negative emotion and avoidance; Carlson & Wang, 2007; Eisenberg et al., 2010), and attention regulation (e.g., Carlson & Wang, 2007). Finally, compliance is an indication of moral internalization in response to the requests of socialization agents (Kochanska et al., 2000). Between the age of 2 and 3, children follow caregivers’ rules under monitoring, and they proceed to fully endorse caregivers’ requests willingly and form an internalized moral sense in preschool age (Feng et al., 2017; Kochanska et al., 2000). Compliance with caregivers’ instructions is thus considered as a prototype of self-regulation because it requires behavioral modulation according to direct demands. The emergence of compliance in preschool age is an interplay of temperamental inhibitory control and parental regulation (Kochanska et al., 2009).
Contributions and Limitations of Feldman’s (2009) Study.
Feldman’s pioneering study contributed significantly to the literature and promoted the understanding of the developmental processes of self-regulation abilities. Feldman identified key mediating pathways from physiological regulation before age 1, through emotion regulation before age 1 and attention regulation between ages of 1-2, to self-regulation at age 5, among a sample of premature infants. This study was amongst the first to pose questions on how several regulatory processes integrated during early development to form a unified, higher-level functioning of regulation. It also pointed to key developmental milestones on how physiological, emotional, and attentional processes advance with lower-level functions supporting higher-order mechanisms, with significant implications to understand early brain maturity and neurobiological functions. Yet, three potential limitations restricted a broader application of this theory. First, Feldman’s (2009) study exclusively focused on premature infants, which restricted the generalizability. Evidence from a broader population needs to be gathered to allow for a more general evaluation of the Feldman’s (2009) model.
Second, the developmental hierarchical-integrative model appears to favor a model of hierarchical or stagewise development, as it considers that the physiological, emotional, attentional, and self-regulatory functions mature sequentially, with later-developed functions build upon those lower-order functions matured at earlier phases (Feldman, 2009). Given the ongoing conversation over continuous versus stagewise development models in multiple aspects of child development (Collins & Hartup, 2013), intriguing questions have been imposed on the commonalities and sequential versus parallel developmental processes of multiple self-regulatory functions (e.g., Feng et al., 2017; Zhou et al., 2012). The empirical model tested in the Feldman’s (2009) study, however, did not provide sufficient evidence on the continuity of each aspect of the functions across a broader time frame. Therefore, it is possible that each of the skills arises independently and coincides across development. To elucidate the debate over a continuous versus a hierarchical or stagewise model of self-regulation development, an empirical approach considering both the stability and the interconnected nature of several developmental processes (such as an autoregressive cross-lagged model) would be better suited to examine the temporal precedence and the interconnections among physiological, emotional, attentional, and self-regulatory skills.
These considerations call for replications of the original study. Until now, only one published study made a remote attempt to test the model proposed by Feldman (2009), showing that attention regulation and executive functions were essential building blocks of later self-regulation (Stępień-Nycz et al., 2015). There has been a call for replication studies in psychology to test the robustness and generalizability of the theories (Shrout & Rodgers, 2018). This is especially important in developmental research given a scarcity of such studies due to the time- and labor-consuming nature of conducting them (Duncan et al., 2014; Shrout & Rodgers, 2018).
Lastly, the original model focused solely on children’s own developmental processes of self-regulation. In contrast, it is commonly recognized that an interplay between personal characteristics and contextual influences shapes the early development of self-regulation (Kochanska et al., 2009; Davis et al., 2020). Among all environmental impacts, families serve as the most immediate and important context for young children to develop self-regulation skills (Kochanska et al., 2009; Davis et al., 2020). The contributions of parenting factors (e.g., caregiver sensitivity) are of particular scholarly interests and are a goal of the current study.
Caregiver Sensitivity in Early Development of Self-Regulation in the Context of Poverty
Parenting sensitivity has been described as a caregiver’s capacity to attend effectively to, understand correctly, and respond promptly to the child’s emotional cues and signals (Ainsworth et al., 1978). Through being positively engaged in, attentively responding to, and purposefully co-regulating young children’s emotional arousals, sensitive parenting contributes to a host of self-regulation processes and outcomes in early childhood, including better physiological regulation (Perry et al., 2016), enhanced emotion regulation and fewer behavioral problems (Leerkes et al., 2009), and better self-regulation (Gueron-Sela et al., 2017).
Additionally, emergent evidence points to the development of key aspects in self-regulation as moderated by caregiver sensitivity. In general, caregiver sensitivity buffers the risks of under-development in regulatory processes at earlier phases and aids in the integration process to achieve future self-regulation. In contrast, lower levels of parenting sensitivity may increase the risk of maladjustment, particularly when the initial regulatory skills are low (Wu & Feng, 2020). For example, sensitive parenting enhanced the association between effective physiological regulation and infant use of orienting to caregivers, whereas insensitive parenting weakened such an association (Wu & Feng, 2020). Sensitive parenting also strengthened the association between infants’ heart rate and preschoolers’ internalizing behaviors, whereas insensitive parenting reduced this link (Wagner et al., 2016). As to emotion and attention regulation, sensitive parenting improved emotion regulation for temperamentally reactive infants (Leerkes et al., 2009), and increased the association between attention regulation and effective emotion regulation (Frick et al., 2017; Root et al., 2015). In contrast, for infants who experienced low parenting sensitivity, higher levels of negative reactivity were associated with more avoidant behaviors and less attention regulation (Thomas et al., 2017). Infants with difficulty regulating fear displayed internalizing behaviors in toddlerhood only under low caregiver sensitivity, whereas high caregiver sensitivity buffered this link (Early et al., 2009; Penela et al., 2012). Finally, sensitive parenting enhanced the associations between low RSA and higher-level self-regulation among preschoolers (better executive functions and fewer behavioral problems; Gueron-Sela et al., 2017; Hastings & De, 2008). It appears that caregiver sensitivity plays a key role in the integration process from lower-level to higher-order regulatory functions.
In addition to caregiver influences, the development of self-regulation is shaped by broader socio-economic contexts. Currently, most of the work in self-regulation development has been derived from individuals of more privileged backgrounds (Sturge-Apple et al., 2016). Nevertheless, children in impoverished environments could experience greater difficulties of self-regulation compared to their counterparts in wealthier families (e.g., Evans & Kim, 2013; Lengua et al., 2014; Raver et al., 2011). Maternal sensitivity also tended to be lower in families of economic adversity (Finegood et al., 2016). It is thus of vital importance to study the processes of self-regulation development for children in disadvantageous backgrounds to understand what may contribute to the disparities in self-regulation capacities in such a context.
The Current Study
The current study adopted the developmental hierarchical-integrative perspective (Feldman, 2009) and focused on the developmental processes of self-regulation during the first three years of life. We tested the prospective links among physiological regulation, attention regulation, emotion regulation, and higher-order self-regulatory functions with a cross-lagged design to examine the hierarchical order of these developmental processes. Moreover, we expanded the developmental hierarchical-integrative perspective and incorporated the family contexts into consideration. In particular, we examined caregiver sensitivity as the context for self-regulation development and tested how the developmental processes may differ for children with high versus low caregiver sensitivity. Given the paucity of research focusing on the development of self-regulation in an economically disadvantaged context, we utilized a sample predominantly consisted of low-income, rural families and prospectively assessed these children through several critical developmental periods: 6 months, 15 months, 24 months, and 36 months. A comparison of the measurements used and the assessment time points in Feldman’s (2009) and the current study is included in Appendix I.
We tested two hypotheses. Our first hypothesis was confirmatory. As proposed in the developmental hierarchical-integrative perspective (Feldman, 2009), we expected to replicate a developmental pathway from physiological regulation in the neonatal period (6 months), through emotion regulation in the first year (the corresponding time points would be 15 months in the current study) and attention regulation in the second year (24 months), to self-regulation (executive functions, behavioral problems, and compliance) in preschool age (36 months). Second, we expanded the developmental hierarchical-integrative perspective with an exploratory effort to incorporate early caregivers’ influences. We expected differences in developmental pathways of self-regulation between children under high versus low caregiver sensitivity. In particular, high caregiver sensitivity would enhance the connection from lower-level to higher-order regulatory functions, whereas low caregiver sensitivity would interfere with such connections. Please refer to Figure 1a as our conceptual model.
Figure 1.
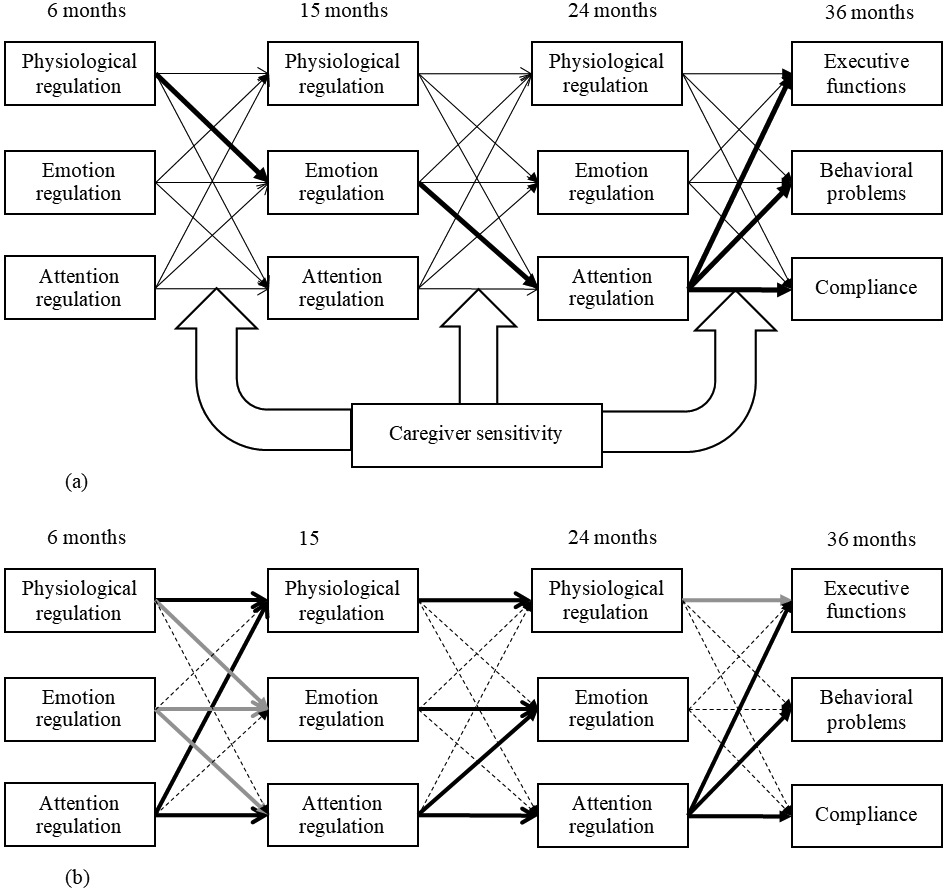
Conceptual and empirical models of the current study.
(a) The conceptual model of the current study. Bold lines indicate conceptualized pathways as indicated in Feldman’s (2009) study. Big hollow arrows indicate multiple possible moderating relations on both the autoregressive and cross-lagged paths. Covariates: child sex and ethnicity, as well as family income-to-needs ratio and residential state.
(b) The empirical findings of the current study. Bold lines indicate significant paths, dashed lines indicate non-significant paths, and grey lines indicate significant moderation effects by caregiver sensitivity.
Method
Participants
Data of the current study were drawn from the Family Life Project (FLP; Vernon-Feagans & Cox, 2013). The FLP was designed to study the development of young children and their families using a representative sample of every infant born to a family who resided in two of the four major geographical areas of the United States with high poverty rates (Wilson, Wayne, and Sampson Counties in North Carolina; as well as Blair, Cambria, and Huntingdon Counties in Pennsylvania). The FLP included a sample of 1,292 children. Families were recruited from local hospitals and a stratified sampling method was used to oversample low-income and African American families. Data collection spanned from September, 2003 to September, 2007.
A planned missingness design was adopted by FLP key investigators in which 400 children within the total FLP sample were randomly selected for longitudinal electrocardiogram (ECG) data collection (Berry et al., 2018). As collecting ECG data can be time-intensive and require high levels of children’s cooperation, there was a relatively high missing rate of useable data for the physiological regulation assessment (51.0% at 6 months or T1, 48.0% at 15 months or T2, and 41.8% at 24 months or T3, of the 400 children). To reduce the potential for biased estimation due to missingness, we elected to include the sample that provided data on physiological regulation during any one of the study visits at T1, T2, or T3, resulting in a sample of 360 families. The current sample did not differ from the original sample regarding child gender, family income level, residential state (North Carolina or Pennsylvania), maternal age, education, employment status, and marital status. There was a lower percentage of African American families in the current sample (36.9%), compared to those in the original sample (42.5%), χ2(1) = 6.06, p = 0.01. The retention rate of the current sample was high (97.5% at T2, 97.2% at T3, and 91.9% at T4 or 36 months).
The current sample included 175 boys (48.6%) and 185 girls (51.4%). There were 157 families residing in Pennsylvania and 203 families in North Carolina. Over a third of the sample identified as African American (36.9%), 62.8% identified as Caucasian, and 0.3% identified as other races. At study enrollment, about forty percent of the mothers (39.7%) reported being employed; over half of the mothers were married (57.5%), 38.3% single, and 4.2% divorced, separated, or widowed. As to the highest level of education, 23.1% of the mothers and 17.9% of the fathers did not complete high school, 4.4% of the mothers and 7.3% of the fathers received a GED, 33.9% of the mothers and 42.7% of the fathers graduated from high school, 25.9% of the mothers and 18.9% of the fathers had some college or an Associate’s degree, 8.6% of the mothers and 8.3% of the fathers received a college degree, and 4.2% of the mothers and 5.0% of the fathers had post-undergraduate training.
Procedures
At each time point, trained research assistants (RAs) visited families at their homes twice for data collection, to accommodate the number of assessments and the activity level of the children. At T1, mothers responded to questionnaires concerning demographic characteristics.
Physiological Data.
At the start of visits at T1, T2, and T3, the RA placed three pediatric electrodes on the child’s chest in a seated position on the mother’s lap. The electrodes were connected to a preamplifier, from which the output was transmitted to a heart inter-beat interval (IBI) monitor for R-wave detection. Once the child was accustomed to the monitor, the RA pressed the start button and marked the start of the baseline measure of the IBI activity. After 5 minutes, the RA marked the end of the episode and stopped collecting ECG data. During the assessment, the mother was asked not to interact with the child to ensure her child to be in a neutral and calm state.
Emotion Regulation.
Several tasks were administered to assess children’s emotion regulation at T1, T2, and T3, which were videotaped and later coded for children’s negative emotion and emotion regulation. At T1, three tasks were administrated. In the Arm Restraint Task (Stifter & Fox, 1990), an RA stood behind the infant and gently grasped the infant’s arms for 2 minutes before releasing them. The RA stayed hiding behind the infant for 1 minute during which the infant employed regulation behaviors prior to maternal comfort. In the Barrier Task (Goldsmith & Rothbart, 1996), an RA gave the child an attractive toy to play with for 30 seconds, then the RA gently took the toy away and put it behind a Plexiglas barrier in front of the infant for 30 seconds. The procedure was carried out three times. In the Mask Task (Goldsmith & Rothbart, 1996), the RA sat to the side of the child and put on four scary masks consecutively, each for 10 seconds. Each time, the RA said the child’s name while moving her head slowly from side to side and then leaned toward the child. At T2 and T3, two tasks were administrated to children: the Mask Task and the Toy Removal Task (Goldsmith & Rothbart, 1996). In the latter, children and their mothers played together with an attractive toy for 2 minutes. The toy was then removed from the child for another 2 minutes; at T2, the RA put the toy out of the child’s reach, whereas at T3 the RA put the toy in a clear plastic jar. The RA then gave the toy back to the child to play for another minute. In this task, the child’s emotion regulation when the toy was taken away was used in the current study.
Executive Functions.
At T4, children were administered five tasks to measure executive functions. In the Working Memory Span task (Engle et al., 1999), children were presented with a line drawing of an animal and a colored dot, inside a house. The RA asked the child to name the animal and then the color. The RA then turned the page to show only the house from the previous page, and asked the child what animal was in the house. The Something’s the Same task (Jacques & Zelazo, 2001) measured attention shifting. The RA presented the child with a page having two items similar in shape, size, or color, and discussed with the child about the dimension that the items were similar. The RA then flipped a page having the same two items along with a new third item, which was similar to one of the first two items along a different dimension. The RA asked the child which of the two original items was similar to the third item.
Three additional tasks assessed children’s inhibitory control. In the Animal Go/No Go task (Durston et al., 2012), children were instructed to press a button every time when seeing an animal (“go” trial) but not when it was a pig (“no-go” trial). The RA presented each page depicting 1 of 7 possible animals for two seconds. The task had varying numbers of “go” trials prior to each “no-go” trial. In the Spatial Conflict task (Gerardi-Caulton, 2000), two side-by-side buttons and an arrow was shown to the child at each trial. The child needed to touch the button of the direction that the arrow pointed to. In the first 8 trials, arrows were in the center, familiarizing the child with the task. For trials of 9-22, left-pointing arrows appeared on the left side and right-pointing arrows appeared on the right side, building a prepotency to touch the button based on the location of the stimuli. For trials of 23-35, left and right-pointing arrows begin to appear randomly and most contralaterally (left-pointing arrows on the right and right-pointing arrows on the left), requiring inhibitory control to suppress the former established prepotent response about spatial location. Finally, in the Silly Sounds Stroop task (Gerstadt et al., 1994), children were asked to bark when shown a picture of a cat, and to meow when shown a dog. More details about the executive function tasks can be found in Willoughby et al. (2010, 2012).
Caregiver Sensitivity and Child Compliance.
A series of parent-child interaction tasks were used to observationally assess caregiver sensitivity and child compliance. At T1, children engaged in two free-play interaction tasks for 10 minutes with both parents, separately. At T2, children only played with their mothers. To increase novelty, at T3 and T4, children completed three puzzles with increasing difficulty with both parents, respectively. These tasks were video-recorded and later coded for caregiver sensitivity (at T1, T2 and T3) and child compliance (at T4).
Measures
Child physiological regulation was assessed by both resting RSA and heart rate in the ECG data. Resting RSA at T1, T2, and T3 was calculated using Mindware Technologies software (Westerville, OH) by measuring the heart-rate variability (HRV) within a respiratory cycle. The algorithm utilizes a moving polynomial to detrend periodicities in heart period that are slower than RSA. Then a band-pass filter extracts the HRV within the frequency band of spontaneous respiration in children, .24 to 1.04 Hz, commonly used to index vagal functioning in infants and young children (Calkins & Keane, 2004; Stifter & Corey, 2001). The software then derives an RSA estimate by calculating the natural log of specified HRV and is reported in units of ln(ms)2. Trained RA cleaned the ECG heart period records for movement artifact, indexing the R spike if identifiable on the record. As resting RSA and heart rate were highly correlated (r’s = −.53, −.60, and −.73, p’s < .001 for T1, T2, and T3, respectively), these two scores were standardized and summed into a composite score of child physiological regulation (with heart rate reversed, a higher score indicating a higher resting RSA and a lower heart rate).
Child attention regulation was assessed using an adapted version of the Infant Behavior Record (IBR; Bayley, 1969; Stifter & Corey, 2001). During each half of study visits at T1, T2, and T3, two RAs independently rated the child’s behaviors for the entire visit, for a total of four ratings. The current study used the attention subscale of the IBR, with three items rated on nine-level scales: tendency to persist (1 = fleeting attention span, 9 = long-continued absorption), behavior constancy (1 = easily tires and regresses to lower levels of functioning, 9 = continues to respond well during long and difficult tasks), and responsiveness to objects (1 = no interest in objects, 9 = reluctantly relinquishes objects). Cronbach’s α was .88, .87, and .92 for T1, T2, and T3, respectively.
Child emotion regulation was assessed by the emotion-eliciting tasks described in the above section at T1, T2, and T3. Using a coding scheme in previous research (Stifter & Braungart, 1995), four types of affective and behavioral indicators of emotion regulation were coded: self-soothing (engaging in small, repetitive movements to reduce distress); orienting towards caregiver (looking or gesturing at the caregiver); avoidance (turning away, pushing the toy away, or averting eye contact); and negative emotion (being whiny, frowning brow, yelling, and tears). The score of each emotion regulation behavior represented the percentage of time when each type of behavior was present. Scores across different tasks (within the same time point) were standardized and summed for each emotion regulation behavior. A final score for emotion regulation was generated by summing up the four behaviors based on whether these behaviors were adaptive: self-soothing, orienting towards the caregiver, avoidance (reversed), and negative emotion (reversed). Approximately 15% of the task was double-coded, with high inter-rater reliability; kappa values ranged between .82 and .95 across tasks and time points.
Child self-regulation included executive functions, behavioral problems, and compliance. Executive functions were assessed using the five executive function tasks at T4, i.e., the Working Memory Span task (4 items), the Something’s the Same task (14 items), the Animal Go/No Go task (7 no-go items), the Spatial Conflict task (12 contralateral items), and the Silly Sounds Stroop task (17 items). Prior literature has validated these measures and an exploratory factor analysis yielded that all executive function measures loaded on one executive function factor (Willoughby et al., 2010). Further, using children’s response accuracy of each item, expected a posteriori (EAP) scores for each executive function task were generated by applying item response theory models. An EAP estimate indicates the expected value of the posterior probability distribution of latent trait scores for an individual, used for dichotomous or polytomous data. More details about the generation of the EAP scores, along with the psychometric properties and longitudinal measurement invariance of the assessments of executive function, can be found in prior validation studies by the FLP key investigator team (e.g., Willoughby et al., 2010, 2012). In the current study, a principal component analysis revealed one executive function factor explaining 32.85% of the total variances, with factor loadings of each task varying between .47 to .71. As such, a mean score of the EAP scores of each task was calculated to indicate the child’s overall level of executive functions.
Child behavioral problems were assessed from maternal reports on the Strengths and Difficulties Questionnaire (SDQ; Goodman, 1997) at T4. The SDQ is a widely used tool to assess children’s behavioral problems, including both externalizing (conduct problems and hyperactivity) as well as internalizing (emotional symptoms and peer problems) subscales. These four subscales each includes 5 items, rated on a 3-point Likert scale (0 = not true, 2 = certainly true). Sample items include the target child “often loses temper” and is “often unhappy, depressed, or tearful.” The SDQ has good psychometric properties and is comparable to other assessments measuring child internalizing and externalizing behaviors such as the Child Behavior Checklist (Goodman, 1997; Goodman et al., 2010). A mean score was generated over 20 items to indicate children’s overall level of behavioral problems (Cronbach’s α = .81).
Child compliance was observed during the parent-child puzzle-solving tasks at T4. Coders rated the child’s acceptance of, adherence to, and willingness to cooperate with the parent’s instructions during the task on a 1-5 scale (1 = “not at all characteristic”, and 5 = “highly characteristic”). An average score was created across two tasks with both parents to indicate the child’s overall level of compliance with both parents. Over half of the videos were double-coded, with an intraclass correlation coefficient of .91.
Caregiver sensitivity was assessed by the parent-child interaction tasks at T1, T2, and T3, separately for mothers and fathers. Following a coding scheme (Cox et al., 1999; NICHD ECCRN, 1999), seven parenting behaviors were coded: sensitivity (responsiveness to the child’s social behaviors and negative emotion), intrusiveness (caregiver-centered interaction), detachment (emotional disinvolvement); positive regard (positive emotion and acknowledgement), negative regard (negative emotion); animation (excitement), and stimulation of development (scaffolding of activities). Coders rated caregiving behaviors globally during the entire task on a 1-5 scale (1 = “not at all characteristic”, and 5 = “highly characteristic”). Interrater reliability was assessed using intraclass correlation coefficients, ranging from .74 to .89 for all codes, with more than 30% of the videos double-coded. A principal component analysis with oblique rotation was conducted to determine behaviors that consisted of parenting sensitivity. One factor emerged explaining a great amount of the total variance (for mothers, 50.5% at T1, 53.7% at T2, and 56.1% at T3; for fathers, 48.19% at T1 and 48.90% at T3). This factor loaded on sensitivity, detachment (reversed), stimulation of development, positive regard, and animation. Thus, an average score of these five subscales was generated as the caregiver’s overall level of sensitivity. Scores of T1, T2, and T3 were standardized within the time point and averaged across time points for mothers and fathers separately, and then a mean score between mothers and fathers was calculated to indicate the mean level of caregiver sensitivity across three time points.
Covariates included child sex, race, family residential state, and income-to-needs ratio. The mother reported child sex and race when the child was 2-month-old. At T1, family annual income was divided by the poverty threshold for the corresponding family size to generate family income-to-needs ratio.
Analytic Plan
The descriptive analysis was conducted in SPSS. We estimated one path model with autoregressive, cross-lagged paths with the lavaan package (Rosseel, 2012) in R (R Core Team, 2014). The percentage of missing data ranged from 2.5% to 45.6% (see Table 1). Little’s MCAR test (Little, 1988) indicated that data were not missing completely at random, χ2(989) = 1127.36, p = .001. Yet, the pattern for the missingness is likely missing at random, as the missingness of variables were associated with other study variables. In particular, the missingness of emotion regulation variables was associated with lower scores on concurrent attention regulation variables (t(33) = 2.0, p = .06 at T1, t(39)= 3.9, p < .001 at T2, and t(75) = 4.9, p < .001 at T3). The missingness of physiological regulation at T1 was associated with lower concurrent emotion regulation (t(295) = 2.9, p = .005), whereas the missingness of physiological regulation at T2 and T3 was associated with lower attention regulation 9 months ago (t(352) = 2.7, p = .008 for T2 and t(203) = 2.3, p = .02 for T3). Missingness of executive functions at T4 was associated with higher emotion regulation at T1 (t(119)= 2.1, p = .04) and lower compliance at T4 (t(48)= 3.9, p < .001). Thus, full information maximum likelihood algorithm (FIML) with robust errors (MLR) was employed for missing data estimation as recommended for missing at random (Enders & Bandalos, 2001). Further, we included the root mean square error of approximation (RMSEA), the comparative fit index (CFI), and standardized root mean squared residual (SRMR) as the model fit indexes, with a RMSEA and SRMR of .05 and below and CFI of .95 and above indicating good fit and a RMSEA and SRMR of .05-.08 and CFI of .90-.95 indicating acceptable fit (Hu & Bentler, 1995).
Table 1.
Descriptive Statistics and Correlations among Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child sex | |||||||||||||||||
| 2. State | −.11* | ||||||||||||||||
| 3. Ethnicity | −.01 | .55** | |||||||||||||||
| 4. Income | −.04 | .16** | .34** | ||||||||||||||
| 5. CS | −.03 | .25** | .37** | .38** | |||||||||||||
| 6. ER T1 | −.04 | .04 | −.02 | .06 | −.02 | ||||||||||||
| 7. ER T2 | −.01 | .17** | .12* | .06 | .03 | .13* | |||||||||||
| 8. ER T3 | .06 | −.08 | .07 | .04 | .04 | .04 | .16** | ||||||||||
| 9. PR T1 | −.11 | −.30** | −.16* | −.11 | −.02 | −.01 | −.07 | .05 | |||||||||
| 10. PR T2 | −.08 | −.16* | −.10 | −.09 | −.03 | .02 | .06 | .14 | .26** | ||||||||
| 11. PR T3 | .00 | −.14* | −.19** | −.08 | −.10 | .03 | .09 | .12 | .37** | .36** | |||||||
| 12. AR T1 | .01 | −.17** | −.03 | .04 | .04 | .11* | −.07 | .11 | .06 | .23** | .08 | ||||||
| 13. AR T2 | .00 | −.13* | −.11* | .09 | .04 | .08 | −.01 | .15* | .09 | .18* | .03 | .29** | |||||
| 14. AR T3 | .15** | −.26** | .00 | .04 | .09 | .02 | .00 | .30** | .16* | .00 | .08 | .19** | .31** | ||||
| 15. EF T4 | .10 | .16** | .22** | .15* | .20** | −.10 | .08 | .05 | −.08 | −.02 | −.05 | −.07 | .05 | .14* | |||
| 16. BP T4 | −.08 | −.18** | −.19** | −.34** | −.34** | −.06 | −.11 | −.12* | .13 | .05 | −.05 | −.02 | −.15** | −.14* | −.13* | ||
| 17. Com T4 | .09 | .00 | .02 | −.04 | .15** | −.02 | .01 | −.06 | .10 | .09 | −.03 | .16** | .16** | .14* | .06 | −.10 | |
| N | 360 | 360 | 360 | 360 | 360 | 328 | 314 | 291 | 196 | 208 | 237 | 360 | 351 | 350 | 288 | 329 | 322 |
| Minimum | 1 | 0 | 0 | 0.00 | −2.25 | −10.86 | −7.00 | −6.69 | −6.46 | −7.95 | −5.20 | 4.50 | 4.00 | 4.50 | −1.98 | 0.00 | 1.00 |
| Maximum | 2 | 1 | 1 | 7.72 | 1.65 | 10.44 | 5.40 | 6.42 | 4.71 | 7.30 | 4.93 | 23.25 | 23.50 | 23.25 | 1.18 | 1.40 | 5.00 |
| Mean | 1.51 | 0.44 | 0.63 | 1.72 | −0.08 | 0.37 | 0.02 | 0.18 | 0.00 | 0.00 | 0.00 | 17.62 | 17.69 | 17.54 | −0.46 | 0.60 | 3.96 |
| SD | 0.50 | 0.50 | 0.48 | 1.44 | 0.80 | 2.26 | 2.12 | 2.31 | 1.75 | 1.79 | 1.86 | 2.63 | 3.00 | 3.39 | 0.54 | 0.28 | 0.81 |
Note. CS = caregiver sensitivity. ER = emotion regulation. PR = physiological regulation. AR = attention regulation. EF = executive functions. BP = behavioral problems. Com = Compliance. T1 = 6 months. T2 = 15 months. T3 = 24 months. T4 = 36 months. Child sex: 1= Boy, 2 = Girl. State: 0 = North Carolina, 1 = Pennsylvania. Ethnicity: 0 = Black, 1 = Other races.
p < .05
p < .01 (two-tailed).
First, prospective relations among physiological regulation, emotion regulation, and attention regulation across the first three waves were tested using an autoregressive cross-lagged model. Child executive functions, behavioral problems, and compliance at T4 was then added to the model by regressing on physiological regulation, emotion regulation, and attention regulation at T3. Child sex, race, state, and family income-to-needs ratio were included as covariates. This analytic step was a confirmatory effort to replicate Feldman’s (2009) findings, on top of which we included the estimation of stability paths over time to provide more robust findings. Next, we examined how caregiver sensitivity may moderate the associations among physiological regulation, emotion regulation, and attention regulation by including interaction terms between caregiver sensitivity and regulation variables, on both the autoregressive and cross-lagged paths. Continuous variables were mean-centered prior to calculating the interaction terms. In visually displaying and explaining significant interactions, low, medium, and high levels of caregiver sensitivity were centered at one standard deviation below the mean, at the mean, and one standard deviation above the mean, respectively. This step expanded the developmental hierarchical-integrative model by incorporating early caregivers’ influences. As Feldman (2009) did not specify the possible moderating effects of caregiver sensitivity, this analytic step remained exploratory.
Results
Descriptive statistics and bivariate correlations of the study variables are presented in Table 1. An autoregressive cross-lagged model was estimated with interaction terms between caregiver sensitivity and regulation variables, on both the autoregressive and cross-lagged paths. After careful examination, non-significant interaction terms were eliminated, and the trimmed model did not differ significantly from the untrimmed model in the model fit, χ2(31) = 23.90, p = .81. Thus, we retained the model with only significant interaction terms as it was more parsimonious. The final autoregressive cross-lagged model showed an acceptable model fit, χ2(50) = 69.02, p = .04; RMSEA = .023 (CI.90 = .008, .050); CFI = .942; SRMR = 0.037. As shown in Table 2, higher attention regulation at T1 predicted greater physiological regulation at T2 (B = 0.11, SE = 0.05, t = 2.79, p = .005). Attention regulation at T2 predicted better emotion regulation at T3 (B = 0.11, SE = 0.05, t = 2.22, p = .03). Better attention regulation at T3 predicted higher executive function scores (B = 0.02, SE = 0.01, t = 2.31, p = .02), fewer behavioral problems (B = −0.01, SE = 0.005, t = −1.95, p = .05), and greater compliance (B = 0.04, SE = 0.02, t = 2.47, p = .01) at T4. The autoregressive paths of physiological regulation, attention regulation, and emotion regulation between T1 and T2, and between T2 and T3, remained stable, except for the stability path of emotion regulation from T1 to T2. Caregiver sensitivity predicted higher attention regulation at T3 (B = 0.45, SE = 0.20, t = 2.08, p = .04) as well as fewer behavioral problems (B = −0.08, SE = 0.02, t = −3.71, p < .001) and better compliance at T4 (B = 0.18, SE = 0.08, t = 2.45, p = .01).
Table 2.
Unstandardized Coefficient Estimate for the Path Model.
| T1 → T2 | T2 → T3 | T3 → T4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | z | B | SE | z | B | SE | z | |
| PR T2: R2 = 0.14 | PR T3: R2 = 0.16 | EF T4: R2 = 0.11 | |||||||
| PR | 0.30 | 0.09 | 3.28 *** | 0.37 | 0.10 | 3.80 *** | 0.00 | 0.02 | −0.14 |
| AR | 0.11 | 0.04 | 2.79 ** | −0.03 | 0.06 | −0.50 | 0.02 | 0.01 | 2.31 * |
| ER | −0.01 | 0.05 | −0.16 | 0.08 | 0.06 | 1.23 | 0.00 | 0.02 | −0.21 |
| CS | 0.03 | 0.16 | 0.18 | −0.02 | 0.16 | −0.09 | 0.09 | 0.05 | 1.73 |
| Income-to-needs ratio | −0.07 | 0.09 | −0.76 | 0.02 | 0.08 | 0.21 | 0.02 | 0.02 | 0.81 |
| Child sex | −0.15 | 0.23 | −0.64 | 0.09 | 0.23 | 0.38 | 0.10 | 0.06 | 1.64 |
| State | −0.02 | 0.31 | −0.06 | −0.20 | 0.33 | −0.59 | 0.13 | 0.08 | 1.68 |
| Ethnicity | −0.09 | 0.31 | −0.30 | −0.48 | 0.32 | −1.50 | 0.06 | 0.09 | 0.72 |
| PR X CS | 0.06 | 0.03 | 2.13 * | ||||||
| AR T2: R2 = 0.13 | AR T3: R2 = 0.21 | BP T4: R2 = 0.21 | |||||||
| PR | 0.10 | 0.12 | 0.88 | −0.13 | 0.13 | −1.04 | −0.02 | 0.01 | −1.68 |
| AR | 0.30 | 0.06 | 4.66 *** | 0.35 | 0.07 | 5.29 *** | −0.01 | 0.01 | −1.95 * |
| ER | 0.02 | 0.07 | 0.32 | 0.03 | 0.09 | 0.36 | −0.01 | 0.01 | −0.91 |
| CS | 0.12 | 0.21 | 0.55 | 0.45 | 0.22 | 2.08 * | −0.08 | 0.02 | −3.71 *** |
| Income-to-needs ratio | 0.26 | 0.10 | 2.53* | −0.08 | 0.12 | −0.67 | −0.05 | 0.01 | −4.73*** |
| Child sex | 0.08 | 0.30 | 0.27 | 0.78 | 0.32 | 2.41* | −0.05 | 0.03 | −1.85 |
| State | −0.16 | 0.44 | −0.37 | −2.35 | 0.40 | −5.90*** | −0.10 | 0.04 | −2.80** |
| Ethnicity | −0.80 | 0.44 | −1.82 | 1.38 | 0.45 | 3.07** | 0.04 | 0.04 | 0.98 |
| ER X CS | 0.19 | 0.09 | 2.19 * | ||||||
| ER T2: R2 = 0.11 | ER T3: R2 = 0.10 | Com T4: R2 = 0.07 | |||||||
| PR | −0.02 | 0.08 | −0.24 | 0.13 | 0.11 | 1.21 | −0.01 | 0.03 | −0.32 |
| AR | −0.06 | 0.05 | −1.14 | 0.11 | 0.05 | 2.22 * | 0.04 | 0.02 | 2.47 * |
| ER | 0.16 | 0.05 | 3.20 *** | 0.19 | 0.07 | 2.84 ** | −0.04 | 0.02 | −1.82 |
| CS | −0.16 | 0.16 | −1.01 | 0.02 | 0.18 | 0.13 | 0.18 | 0.08 | 2.45 * |
| Income-to-needs ratio | 0.08 | 0.09 | 0.95 | −0.01 | 0.10 | −0.07 | −0.06 | 0.04 | −1.72 |
| Child sex | −0.03 | 0.24 | −0.10 | 0.25 | 0.27 | 0.95 | 0.13 | 0.09 | 1.46 |
| State | 0.55 | 0.31 | 1.81 | −0.83 | 0.32 | −2.60** | 0.03 | 0.13 | 0.27 |
| Ethnicity | 0.16 | 0.32 | 0.49 | 0.92 | 0.34 | 2.71** | −0.04 | 0.15 | −0.25 |
| PR X CS | 0.40 | 0.10 | 3.95 *** | ||||||
| ER X CS | −0.19 | 0.06 | −3.34 *** | ||||||
Note. PR = physiological regulation. AR = attention regulation. ER = emotion regulation. CS = caregiver sensitivity. EF = executive functions. BP = behavioral problems. Com = Compliance. T1 = 6 months. T2 = 15 months. T3 = 24 months. T4 = 36 months. Child Sex 1= Boy, 2 = Girl. State 0 = North Carolina, 1 = Pennsylvania. Ethnicity 0 = Black, 1 = Other.
p < .05
p < .01
p < .001 (two-tailed).
Moderation analyses indicated several significant pathways moderated by caregiver sensitivity (Table 2 and Figure 1b). Better emotion regulation at T1 was associated with higher emotion regulation at T2 only when caregiver sensitivity was low (one standard deviation below the mean; B = 0.31, SE = 0.08, t = 3.99, p < .001) and medium (the mean level; B = 0.16, SE = 0.05, t = 3.20, p = .001), but not high (one standard deviation above the mean; B = 0.01, SE = 0.06, t = 0.23, p = .82; Figure 2a). On the cross-lagged path, emotion regulation at T1 predicted better attention regulation at T2 at very high caregiver sensitivity (i.e., two standard deviations above the mean; B = 0.33, SE = 0.15, t = 2.20, p = .03); this association was marginally significant under high caregiver sensitivity (B = 0.18, SE = 0.09, t = 1.87, p = .06), and not significant under medium (B = 0.02, SE = 0.07, t = 0.32, p = .75) or low sensitivity (B = −0.13, SE = 0.10, t = −1.30, p = .20; Figure 2b). Additionally, physiological regulation at T1 predicted better emotion regulation at T2 given high caregiver sensitivity (B = 0.30, SE = 0.12, t = 2.58, p = .01) but worse emotion regulation at T2 when caregiver sensitivity was low (B = −0.31, SE = 0.11, t = −2.76, p = .006; Figure 3a). Finally, the positive association between physiological regulation at T3 and executive functions at T4 was approaching significance when caregiver sensitivity was high (B = 0.06, SE = 0.03, t = 1.86, p = .06), and become significant when caregiver sensitivity was very high (i.e., two standard deviations above the mean; B = 0.10. SE = 0.03, t = 3.17, p = .002). This association was not significant under low (B = −0.05, SE = 0.03, t = −1.51, p = .13) or medium (B = −0.00, SE = 0.02, t = −0.14, p = .89) levels of caregiver sensitivity (Figure 3b).
Figure 2.
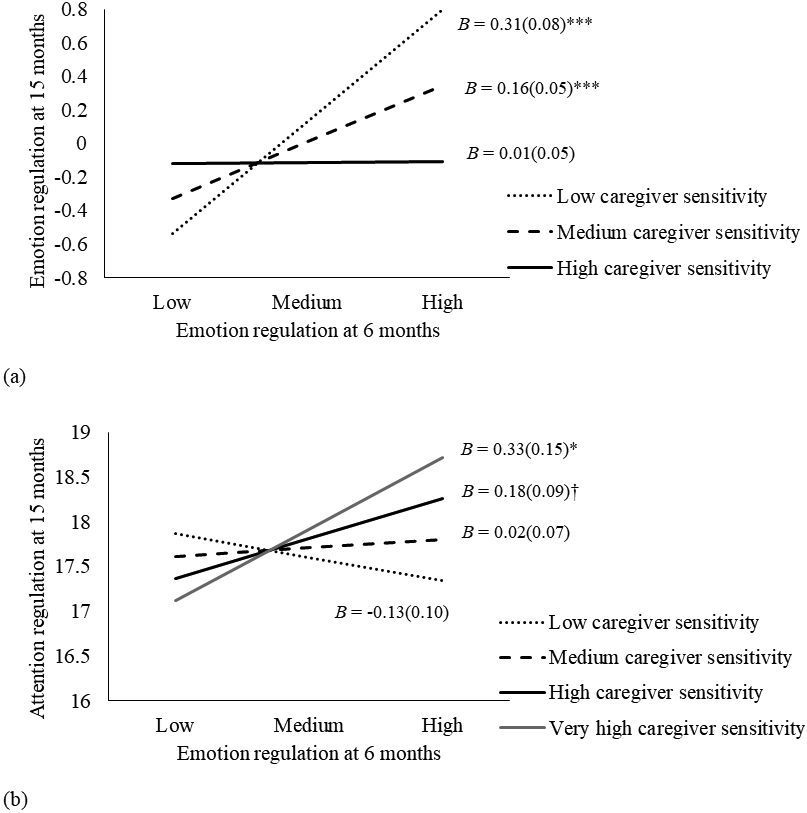
Interactions plots between emotion regulation and caregiver sensitivity. † p < .10, * p < .05, *** p < .001.
(a) Interaction between emotion regulation at 6 months and caregiver sensitivity predicting emotion regulation at 15 months.
(b) Interaction between emotion regulation at 6 months and caregiver sensitivity predicting attention regulation at 15 months.
Figure 3.
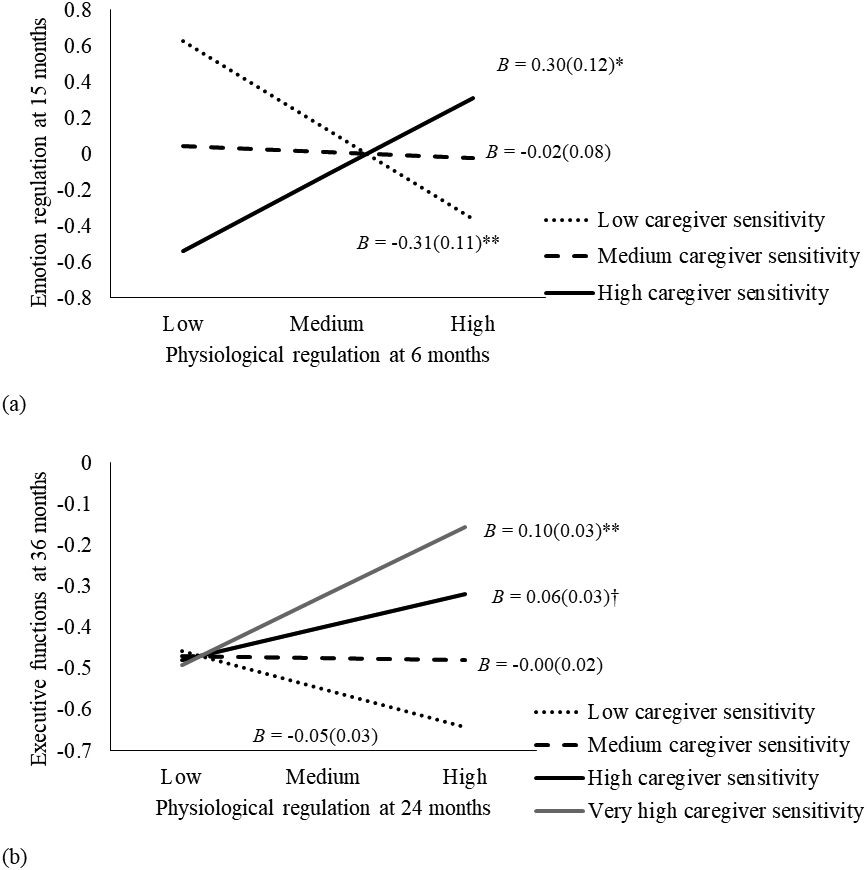
Interactions between physiological regulation and caregiver sensitivity. † p < .10, * p < .05, ** p < .01.
(a) Interaction between physiological regulation at 6 months and caregiver sensitivity predicting emotion regulation at 15 months.
(b) Interaction between physiological regulation at 24 months and caregiver sensitivity predicting executive functions at 36 months
Mediation Analyses.
Mediation analyses were performed to explore mediated pathways towards self-regulatory outcomes at T4 (see Appendix II). Significant mediation effects were identified from the stability pathways of attention regulation to executive functions and compliance at T4. Additionally, significant mediation pathways were found in the stability paths of attention regulation and physiological regulation from T1 to T3. As such, the mediation effects appeared to be more specific to one domain (e.g., within the stability pathways of one type of regulation) rather than cross-domain (e.g., through cross-lagged pathways among physiological, attention, and emotion regulation). Combined with the findings from the main model of parallel rather than sequential effects, the results in mediation models further supported a continuous development model. Further, we explored an alternative model of caregiver sensitivity as a mediator to self-regulation development (Appendix III). Our data did not support the mediation role of caregiver sensitivity.
Discussion
The current study replicated the Feldman (2009) study by applying the developmental hierarchical-integrative model to understand the emergence of self-regulation during early childhood. Findings provided favorable empirical evidence towards the Feldman (2009) model, and extended the model by incorporating caregiver sensitivity as a moderator on developmental pathways of several regulatory functions, with significant implications to research and intervention.
Replication of Feldman’s (2009) Model of Self-Regulation
Our first hypothesis was to replicate Feldman’s (2009) model that established the developmental pathway of physiological regulation before age 1 → emotion regulation before age 1 → attention regulation between age 1-2 → self-regulation at age 5. In contrast, our study identified three major pathways: attention regulation at 6 months predicted physiological regulation at 15 months, attention regulation at 15 months predicted emotion regulation at 24 months, and attention regulation at 24 months predicted better self-regulation (greater executive functions, compliance, and fewer behavioral problems) in preschool age. Our findings are consistent with Feldman’s (2009) study in several ways. First, both the current study and Feldman’s (2009) study support the conceptualization that the development of self-regulation is an ongoing process during early childhood. In our study, physiological, emotion, and attention regulation all demonstrated continued growth (especially between 15 to 24 months), and they intersected during different times in development, consistent with the dynamic systems perspective (Lewis, 2005).
Second, both the current study and Feldman’s (2009) study support that attention regulation is a central mechanism of and directly connects to the emergence of self-regulation. The development of attention regulation appeared stable from 6 to 24 months, and continued to support different aspects of regulatory skills: physiological regulation, emotion regulation, as well as higher-order self-regulation including executive functions, compliance, and fewer behavioral problems. It is likely that attention regulation skills enable young children to keep focus and to retain a consistent goal while withholding frustration and resisting distraction, and thus grant children mastery of the environment (Cuevas & Bell, 2014; Feldman, 2009; Ruff & Capozzoli, 2003). In contrast, the early development of physiological and emotion regulation was not directly associated with self-regulation. Possibly, the goal of physiological regulation and emotion regulation is more focused on reactivity control and returning to homeostasis after environmental interruption (Feldman, 2009; Perry et al., 2018). As such, successful attention regulation can signal the emergence of self-centered, higher-order monitoring skills, which are useful for planning, inhibiting impulses, and facilitating the acquisition of a hierarchical knowledge about the environment (Cuevas & Bell, 2014; Feldman, 2009).
Different from Feldman’s (2009), we found a positive association between attention regulation at 6 months and physiological regulation at 15 months, whereas Feldman did not find such an association. It seems that an ongoing connection between autonomic regulation and attention development appears during infancy, as both involve development in prefrontal-limbic circuitry. Infants can rely on selective engagement and sustained focus to engage effectively with objects and caregivers, which may provide a calm and positive mental state and reduce hypervigilance accompanied by excessive environment scanning when facing stress (Davis et al., 2016; Porges, 2009). Being able to engage with caregivers also provides infants with opportunities to obtain assistance in times of distress, which promotes adaptive development in ANS regulation (Perry et al., 2018). Yet, a dearth of research has investigated the interchange between autonomic regulation and attention development during infancy. This warrants future research efforts, as attention skills develop significantly in infancy, and have meaningful implications to a variety of developmental outcomes (Colombo et al., 2010).
Another difference from Feldman’s study (2009) is, in our study, attention regulation around age 1 predicted emotion regulation at age 2, but not vice versa. Two possible explanations may be underlying this difference in findings. First, Feldman (2009) utilized a sample of infants with low birth weights, and their attention regulation may mature later than normally developed children. In fact, low-birth-weight infants can have a greater risk of attention regulation difficulties and developing attention-deficit hyperactivity disorder (ADHD; Mick et al., 2002). Second, our study utilized an empirical model that considered both the stability as well as the interconnection among multiple aspects of development, which allowed for a better understanding of the sequential development of early regulatory functions. It is likely that emotion regulation matures later than attention regulation, and the cascading effect of development transfers from attention regulation to emotion regulation (Frick et al., 2017; Root et al., 2015). The pathway from attention regulation to emotion regulation did not appear in Feldman’s study as only the effect in the opposite direction was hypothesized and tested, underscoring the importance of the current study examining both the stability and the cross-lagged effects of different regulatory functions.
Caregiver Sensitivity as the Context of Self-Regulation Development
Our second hypothesis was to test if caregiver sensitivity moderated the developmental pathways towards self-regulation. We found that, first, the stability of emotion regulation from 6 to 15 months was significant only when caregiver sensitivity was at low and medium levels. It is likely sensitive parenting creates opportunities for children to improve their regulatory skills, and emotionally reactive infants can be susceptible to such changes, thus showing instability (Hastings & De, 2008). This finding is especially important in a low-income context where children are more likely to show lower levels of self-regulation (Evans & Kim, 2013; Lengua et al., 2014).
We also found that infant emotion regulation at 6 months predicted higher attention regulation at 15 months, only among those with highly sensitive caregivers. This finding is consistent with previous observations on the moderation effect of caregiver sensitivity on the link between attention regulation and effective emotion regulation (Frick et al., 2017; Root et al., 2015; Thomas et al., 2017). It is likely that sensitive parenting can provide young children with assistance in managing their negative emotion, to support selective engagement and sustained focus with events or objects of interest (Frick et al., 2017). Further, as greater attention regulation at 15 months can increase the likelihood of better emotion regulation at 24 months, it appears that sensitive caregiving at early stages can promote the interchange between attention regulation and emotion regulation systems, possibly increasing the likelihood of the higher-order integration of regulatory skills (Frick et al., 2017; Thomas et al., 2017).
Third, physiological regulation at 6 months predicted better emotion regulation at 15 months when caregiver sensitivity was high (consistent with our expectation), but worse emotion regulation at 15 months when caregiver sensitivity was low. The latter finding, although counter-intuitive, was observed in several other studies. Sturge-Apple et al. (2016) observed that higher RSA predicted lower abilities of self-control (i.e., a shorter delay time in awaiting gratification) among two-year-olds in an impoverished background, but a longer waiting time among those from higher-resourced families. RSA is thus considered as a biological susceptibility factor that leads to an adaptation to economic risks. In a lower-resourced environment, a higher level of negative emotion and emotion dysregulation may function to elicit more caregiver attention, considering low-income parents may experience a number of stressors and their sensitivity tends to be low (Finegood et al., 2016). Similarly, Perry and colleagues (2018) observed that resting RSA at 5 months predicted higher negative emotion at 10 months among typically developed infants. They pointed out that greater biological susceptibility to the environment may provide infants with more opportunities to interact with the environment and to practice regulatory skills, which may manifest as a high level of negative reactivity. Both hypotheses need to be investigated in future studies. Notably, on a bivariate level, caregiver sensitivity was associated with higher executive functions and compliance levels as well as fewer behavioral problems at 36 months, but not directly with physiological, emotion, and attention regulation. Our investigation revealed that caregiver sensitivity moderated developmental processes within physiological, emotion, and attention regulation, which supported our approach of considering caregiver sensitivity as an important contextual influence during self-regulation development. These moderation effects were observed only for infants ages 6-15 months, but not later, possibly revealing that the biological and emotional susceptibility to caregiver sensitivity is more prominent at an early and critical time during development.
Finally, we found that greater physiological regulation at 24 months predicted a higher level of executive functions at 36 months for children with caregivers showing very high sensitivity. A similar pattern was previously observed, where RSA and sensitive parenting interacted to predict executive functions (Gueron-Sela et al., 2017). Sensitive parenting appears to contribute to the development of self-regulation by canalizing biologically reactive children towards better regulatory functions, and the automatic regulation system performs as a susceptibility factor that assists children to achieve better regulatory outcomes in a supportive environment (Gueron-Sela et al., 2017; Hastings & De, 2008).
Theoretical Integration, Strengths, and Limitations
In general, our findings appeared to favor a continuous model over a hierarchical or stagewise model of self-regulation development during the first two years, which was different from Feldman’s. More particularly, the current study showed mostly continued growth of physiological, emotion, and attention regulation between 6 to 24 months, which was also evident from previous studies. For example, children’s RSA and heart period also show a steady increase over the first four years of life (Bar-Haim et al., 2000). From infancy to early childhood, children become increasingly capable in managing negative affective arousals and gradually master a range of emotion-regulatory strategies (Wu et al., 2020). Young children also gain in attention regulation capacities in transitioning from selective gaze in infancy to goal-focused sustained attention during toddlerhood (Colombo et al., 2010; Ruff & Capozzoli, 2003). It appears that the central mechanism of “regulation” has manifested in multiple developing aspects since a very young age and continued to mature in a separated but connected manner.
Our findings, however, supported the integrative aspect of Feldman’s theory, by suggesting the interchanges among physiological, emotion, and attention regulation are bidirectional and cyclical. In fact, emotion regulation at 6 months promoted attention regulation at 15 months (when caregiver sensitivity was high), which further strengthened emotion regulation at 24 months. Also, for children experiencing higher levels of caregiver sensitivity, between 6 to 15 months, physiological regulation promoted emotion regulation, which facilitated attention regulation, and attention regulation further supported physiological regulation. In comparison, Feldman’s model only examined the unidirectional pathway of physiological regulation → emotion regulation → attention regulation. It seems that the mutual interchanges among physiological, emotional, and attentional systems are constant during early childhood (Lewis, 2005), and can constitute a cycle of adaptive development given a supportive environment. It also appears that there may not be a particular sequence among the maturation of physiological, emotion, and attention regulation. As such, the early development of regulatory functions is a dynamic, continuous ongoing process that require multiple systems to co-participate, and deficits in one system may affect the functions of other systems (Lewis, 2005). Finally, our findings suggest that healthy development of and coordination among these systems support the capability to accomplish more complex, higher-order regulatory tasks, such as organizing information to achieve goal-oriented activities, managing problem behaviors, and internalizing moral rules, in preschool age (Feldman, 2009).
In sum, evidence of the current study supports the central notion of the developmental hierarchical-integrative model (Feldman, 2009) that the development of regulatory functions is a constant effect across the first several years of a child’s life, and several of regulatory functions may integrate to support the emergence of higher-order self-regulatory skills. It reopens conversations about a hierarchical or stagewise model versus a continuous model of self-regulation development (Collins & Hartup, 2013), and taps into inquiries about ongoing bidirectional or cyclical interchanges (rather than unidirectional associations) amongst subsystems of regulation, which is described in the dynamic systems perspective (Lewis, 2005; Thelen & Smith, 1994). The findings also point to the important interplay between environmental influences and internal organization, and reveal key pathways for caregiver sensitivity to assist in reducing risks in self-regulation development. Importantly, caregiver sensitivity appears to facilitate the growths and interchanges of regulatory functions to promote the emergence of self-regulation. Finally, this study also sheds light upon how self-regulation development in an economically disadvantaged context, with significant implications for intervention efforts.
Limitations should be considered when interpreting the findings of the current study. First, this study oversampled low-income, rural families, and there may be less variation in the income levels of these families, possibly resulting in non-significant correlations between income and physiological, emotion, and attention regulation. As such, findings of this study should be interpreted with caution and may not be generalizable to higher-resourced families. Future replication studies of the developmental hierarchical-integrative model (Feldman, 2009) should consider utilizing samples of broader populations. Second, as a replication study, some assessments (e.g., compliance) as well as time points may not match the original study precisely, which may have caused differences in findings. Third, caregiver sensitivity at 24 months was assessed in a similar task with child compliance at 36 months, which may have introduced shared measurement variances due to similar contexts. Fourth, there was a relatively high missing rate of the physiological regulation data (34.2%–45.6% of the current sample), which was associated with emotion and attention regulation and might have limited the generalizability of the current study as well as our understanding of children with lower regulatory skills. Additionally, in this study, we only included caregiver sensitivity as one influencing factor on self-regulation, whereas multiple factors in parenting (e.g., co-regulation, scaffolding, cognitive stimulation), parental mental health, and familial environment (e.g., marital relationships) may impact the development of self-regulation. These factors and their potential influences warrant future research. Finally, as the development of self-regulation is a dynamic, interactive process and multiple parental and child characteristics mutually affect each other, future studies should consider a transactional model between parenting and child development over time, to understand the reciprocal nature of person-environment interplay in shaping self-regulation (e.g., Kochanska et al., 2009).
This study also has several notable strengths, including a high-risk, low-income sample that has been under-investigated, a longitudinal design to follow families four times across three years, and an analytic approach to capture both the stability and interconnection amongst different aspects of regulation. Additionally, this study utilized a multiple-informant and multiple-method design including observational assessments of parenting as well as assessing child regulatory behaviors through both physiological data and observational measures of both global ratings and micro coding. This study identified developmental pathways to the formation of early self-regulatory functions, while incorporating critical parenting influences towards this process, adding to our knowledge of early regulation development in a high-risk context. The findings of this study suggest tailoring intervention efforts according to parent and child factors in order to alleviate risk in self-regulation. Specifically, more attention should be given to risk factors that compromise caregiver sensitivity, which promotes a unified, higher-order regulatory function. Together, a more complete comprehension of factors contributing to differences in self-regulation development will allow for a better understanding of supporting adaptive physiological, cognitive, and socioemotional development in the context of poverty.
Supplementary Material
Footnotes
No conflict of interest was disclosed by the authors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Ainsworth MDS, Blehar MC, Waters E, & Wall S (1978). Patterns of attachment: A psychological study of the strange situation. Hilsdale. NJ: Lawrence Erlbaurn. [Google Scholar]
- Anderson P (2002). Assessment and development of executive function (EF) during childhood. Child Neuropsychology, 8, 71–82. 10.1076/chin.8.2.71.8724 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, & Fox NA (2000). Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology, 37, 44–56. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin, 114, 296–322. 10.1037/0033-2909.114.2.296 [DOI] [PubMed] [Google Scholar]
- Berry D, Vernon-Feagans L, Mills-Koonce WR, Blair C, & The Family Life Project Key Investigators. (2018). Otitis media and respiratory sinus arrhythmia across infancy and early childhood: Polyvagal processes? Developmental Psychology, 54, 1709–1722. 10.1037/dev0000488 [DOI] [PubMed] [Google Scholar]
- Calkins SD (2007). The emergence of self-regulation: Biological and behavioral control mechanisms supporting toddler competencies. In Brownell CA & Kopp CB (Eds.), Socioemotional development in the toddler years: Transitions and transformations (p. 261–284). New York, NY: Guilford Press. [Google Scholar]
- Calkins SD, & Keane SP (2004). Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology, 45,101–112. 10.1002/dev.20020 [DOI] [PubMed] [Google Scholar]
- Carlson SM, & Wang TS (2007). Inhibitory control and emotion regulation in preschool children. Cognitive Development, 22, 489–510. 10.1016/j.cogdev.2007.08.002 [DOI] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 1066–1075. 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WA, & Hartup WW (2013). History of research in developmental psychology. In Zelazo PD (Ed.), The Oxford handbook of developmental psychology (pp. 13–34). New York, NY: Oxford University Press. [Google Scholar]
- Colombo J, Kapa L, & Curtindale L (2010). Varieties of attention in infancy. In Oakes LM, Cashon CH, Casasola M, & Rakison DH (Eds.), Infant perception and cognition: Recent advances, emerging theories, and future directions (pp. 3–26). New York, NY: Oxford University Press. [Google Scholar]
- Compas BE, Jaser SS, Bettis AH, Watson KH, Gruhn MA, Dunbar JP, Williams E, & Thigpen JC (2017). Coping, emotion regulation, and psychopathology in childhood and adolescence: A meta-analysis and narrative review. Psychological Bulletin, 143, 939–991. 10.1037/bul0000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Paley B, Burchinal M, & Payne CC (1999). Marital perceptions and interactions across the transition to parenthood. Journal of Marriage and Family, 61, 611–625. 10.2307/353564 [DOI] [Google Scholar]
- Crockenberg SC, & Leerkes EM (2004). Infant and maternal behaviors regulate infant reactivity to novelty at 6 months. Developmental Psychology, 40, 1123–1132. 10.1037/0012-1649.40.6.1123 [DOI] [PubMed] [Google Scholar]
- Cuevas K, & Bell MA (2014). Infant attention and early childhood executive function. Child Development, 85, 397–404. 10.1111/cdev.12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EL, Brooker RJ, & Kahle S (2020). Considering context in the developmental psychobiology of self-regulation. Developmental Psychobiology, 62, 423–435. 10.1002/dev.21945 [DOI] [PubMed] [Google Scholar]
- Davis EL, Quiñones-Camacho LE, & Buss KA (2016). The effects of distraction and reappraisal on children’s parasympathetic regulation of sadness and fear. Journal of Experimental Child Psychology, 142, 344–358. 10.1016/j.jecp.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Engel M, Claessens A, & Dowsett CJ (2014). Replication and robustness in developmental research. Developmental Psychology, 50, 2417–2425. 10.1037/a0037996 [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman R, & Casey BJ (2002). A neural basis for development of inhibitory control. Developmental Science, 5, 9–16. 10.1111/1467-7687.00235 [DOI] [Google Scholar]
- Early DM, Rimm-Kaufman SE, Cox MJ, Saluja G, Pianta RC, Bradley RH, & Payne CC (2002). Maternal sensitivity and child wariness in the transition to kindergarten. Parenting: Science and Practice, 2, 355–377. 10.1207/S15327922PAR0204_02 [DOI] [Google Scholar]
- Eisenberg N, Spinrad TL, & Eggum ND (2010). Emotion-related self-regulation and its relation to children's maladjustment. Annual Review of Clinical Psychology, 6, 495–525. 10.1146/annurev.clinpsy.121208.131208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekas NV, Lickenbrock DM, & Braungart-Rieker JM (2013). Developmental trajectories of emotion regulation across infancy: Do age and the social partner influence temporal patterns. Infancy, 18, 729–754. 10.1111/infa.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8, 430–457. 10.1207/S15328007SEM0803_5 [DOI] [Google Scholar]
- Engle RW, Kane MJ, & Tuholski SW (1999). Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence and functions of the prefrontal cortex. In Miyake A & Shah P (Eds.), Models of working memory: Mechanisms of active maintenance and executive control (pp. 102–134). New York, NY: Cambridge University Press. [Google Scholar]
- Evans GW, & Kim P (2013). Childhood poverty, chronic stress, self-regulation, and coping. Child Development Perspectives, 7, 43–48. 10.1111/cdep.12013 [DOI] [Google Scholar]
- Feldman R (2009). The development of regulatory functions from birth to 5 years: Insights from premature infants. Child Development, 80, 544–561. 10.1111/j.1467-8624.2009.01278.x [DOI] [PubMed] [Google Scholar]
- Feng X, Hooper EG, & Jia R (2017). From compliance to self-regulation: Development during early childhood. Social Development, 26, 981–995. 10.1111/j.1469-7610.2007.01828.x [DOI] [Google Scholar]
- Finegood ED, Blair C, Granger DA, Hibel LC, & Mills-Koonce R (2016). Psychobiological influences on maternal sensitivity in the context of adversity. Developmental Psychology, 52, 1073–1087. 10.1037/dev0000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick MA, Forslund T, Fransson M, Johansson M, Bohlin G, & Brocki KC (2018). The role of sustained attention, maternal sensitivity, and infant temperament in the development of early self-regulation. British Journal of Psychology, 109, 277–298. 10.1111/bjop.12266 [DOI] [PubMed] [Google Scholar]
- Garon N, Bryson SE, & Smith IM (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134, 31–60. 10.1037/0033-2909.134.1.31 [DOI] [PubMed] [Google Scholar]
- Gerardi-Caulton G (2000). Sensitivity to spatial conflict and the development of self-regulation in children 24–36 months of age. Developmental Science, 3, 397–404. 10.1111/1467-7687.00134 [DOI] [Google Scholar]
- Gerstadt CL, Hong YJ, & Diamond A (1994). The relationship between cognition and action: Performance of children 3 1/2-7 years old on a Stroop-like day-night test. Cognition, 53, 129–153. 10.1016/0010-0277(94)90068-X [DOI] [PubMed] [Google Scholar]
- Goodman R (1997). The Strengths and Difficulties Questionnaire: a research note. Journal of Child Psychology and Psychiatry, 38, 581–586. 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- Goodman A, Lamping DL, & Ploubidis GB (2010). When to use broader internalising and externalising subscales instead of the hypothesised five subscales on the Strengths and Difficulties Questionnaire (SDQ): data from British parents, teachers and children. Journal of Abnormal Child Psychology, 38, 1179–1191. 10.1007/s10802-010-9434-x [DOI] [PubMed] [Google Scholar]
- Gueron-Sela N, Wagner NJ, Propper CB, Mills-Koonce WR, Moore GA, & Cox MJ (2017). The interaction between child respiratory sinus arrhythmia and early sensitive parenting in the prediction of children's executive functions. Infancy, 22, 171–189. 10.1111/infa.12152 [DOI] [PubMed] [Google Scholar]
- Hastings PD, & De I (2008). Parasympathetic regulation and parental socialization of emotion: Biopsychosocial processes of adjustment in preschoolers. Social Development, 17, 211–238. 10.1111/j.1467-9507.2007.00422.x [DOI] [Google Scholar]
- Hu L and Bentler PM (1995). Evaluating model fit. In Hoyle RH (ed.) Structural equation modeling: concepts, issues, and applications (pp. 76–99). Thousand Oaks, CA: Sage. [Google Scholar]
- Jacques S, & Zelazo PD (2001). The Flexible Item Selection Task (FIST): A measure of executive function in preschoolers. Developmental Neuropsychology, 20, 573–591. 10.1207/S15326942DN2003_2 [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, & Harlan ET (2000). Effortful control in early childhood: continuity and change, antecedents, and implications for social development. Developmental Psychology, 36, 220–232. 10.1037/0012-1649.36.2.220 [DOI] [PubMed] [Google Scholar]
- Kochanska G, Philibert RA, & Barry RA (2009). Interplay of genes and early mother–child relationship in the development of self-regulation from toddler to preschool age. Journal of Child Psychology and Psychiatry, 50, 1331–1338. 10.1111/j.1469-7610.2008.02050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Blankson AN, & O’Brien M (2009). Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Development, 80, 762–775. 10.1111/j.1467-8624.2009.01296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Kiff C, Moran L, Zalewski M, Thompson S, Cortes R, & Ruberry E (2014). Parenting mediates the effects of income and cumulative risk on the development of effortful control. Social Development, 23, 631–649. 10.1111/sode.12071 [DOI] [Google Scholar]
- Lewis MD (2005). Bridging emotion theory and neurobiology through dynamic systems modeling. Behavioral and Brain Sciences, 28, 169–195. 10.1017/s0140525x0500004x [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, ... & McEwen BS (2006). Beyond the stress concept: Allostatic load - a developmental biological and cognitive perspective. In Cicchetti D & Cohen DJ (Eds.), Developmental Psychopathology, Vol. 2: Developmental Neuroscience (2nd ed., pp. 578–628). Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Mick E, Biederman J, Prince J, Fischer MJ, & Faraone SV (2002). Impact of low birth weight on attention-deficit hyperactivity disorder. Journal of Developmental & Behavioral Pediatrics, 23, 16–22. 10.1097/00004703-200202000-00004 [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. (1999). Child care and mother-child interaction in the first 3 years of life. Developmental Psychology, 35, 1399–1413. 10.1037/0012-1649.35.6.1399 [DOI] [PubMed] [Google Scholar]
- Obradović J (2012). How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development?. Development and Psychopathology, 24, 371–387. 10.1017/S0954579412000053 [DOI] [PubMed] [Google Scholar]
- Penela EC, Henderson HA, Hane AA, Ghera MM, & Fox NA (2012). Maternal caregiving moderates the relation between temperamental fear and social behavior with peers. Infancy, 17, 715–730. 10.1111/j.1532-7078.2012.00114.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Calkins SD, & Bell MA (2016). Indirect effects of maternal sensitivity on infant emotion regulation behaviors: The role of vagal withdrawal. Infancy, 21, 128–153. 10.1111/infa.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Dollar JM, Calkins SD, & Bell MA (2018). Developmental cascade and transactional associations among biological and behavioral indicators of temperament and maternal behavior. Child Development, 89, 1735–1751. 10.1111/cdev.12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2009). The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine, 76, S86. 10.3949/ccjm.76.s2.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Jones SM, Li-Grining C, Zhai F, Bub K, & Pressler E (2011). CSRP’s impact on low-income preschoolers’ preacademic skills: self-regulation as a mediating mechanism. Child Development, 82, 362–378. 10.1111/j.1467-8624.2010.01561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root AE, Byrne R, & Watson SM (2015). The regulation of fear: The contribution of inhibition and emotion socialisation. Early Child Development and Care, 185, 647–657. 10.1080/03004430.2014.946503 [DOI] [Google Scholar]
- Rosseel Y (2012). Lavaan: An R package for structural equation modeling and more. Journal of Statistical Software, 48, 1–36. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Ruff HA, & Capozzoli MC (2003). Development of attention and distractibility in the first 4 years of life. Developmental Psychology, 39, 877–890. 10.1037/0012-1649.39.5.877 [DOI] [PubMed] [Google Scholar]
- Shrout PE, & Rodgers JL (2018). Psychology, science, and knowledge construction: Broadening perspectives from the replication crisis. Annual Review of Psychology, 69, 487–510. 10.1146/annurev-psych-122216-011845 [DOI] [PubMed] [Google Scholar]
- Stępień-Nycz M, Rostek I, Byczewska-Konieczny K, Kosno M, Białecka-Pikul M, & Białek A (2015). Emotional and attentional predictors of self-regulation in early childhood. Polish Psychological Bulletin, 46, 421–432. 10.1146/10.1515/ppb-2015-0049 [DOI] [Google Scholar]
- Stifter CA, & Braungart JM (1995). The regulation of negative reactivity in infancy: function and development. Developmental Psychology, 31, 448–455. 10.1037/0012-1649.31.3.448 [DOI] [Google Scholar]
- Stifter C, & Corey J (2001). Vagal regulation and observed social behavior in infancy. Social Development, 10, 189–201. 10.1111/1467-9507.00158 [DOI] [Google Scholar]
- Sturge-Apple ML, Suor JH, Davies PT, Cicchetti D, Skibo MA, & Rogosch FA (2016). Vagal tone and children’s delay of gratification: Differential sensitivity in resource-poor and resource-rich environments. Psychological Science, 27, 885–893. 10.1177/0956797616640269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Thelen E, & Smith LB (1994). A dynamic systems approach to the development of cognition and action. Cambridge, MA: Bradford/MIT Press. [Google Scholar]
- Thomas JC, Letourneau N, Campbell TS, Tomfohr-Madsen L, Giesbrecht GF, & APrON Study Team. (2017). Developmental origins of infant emotion regulation: Mediation by temperamental negativity and moderation by maternal sensitivity. Developmental Psychology, 53, 611–628. 10.1037/dev0000279 [DOI] [PubMed] [Google Scholar]
- Vernon-Feagans L, Cox M, & the FLP Key Investigators. (2013). The Family Life Project: An epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development, 78, 1–150, vii. 10.1111/mono.12047 [DOI] [PubMed] [Google Scholar]
- Vögele C, Sorg S, Studtmann M, & Weber H (2010). Cardiac autonomic regulation and anger coping in adolescents. Biological Psychology, 85, 465–471. 10.1016/j.biopsycho.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Wagner NJ, Propper C, Gueron-Sela N, & Mills-Koonce WR (2016). Dimensions of maternal parenting and infants’ autonomic functioning interactively predict early internalizing behavior problems. Journal of Abnormal Child Psychology, 44, 459–470. 10.1007/s10802-015-0039-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Blair CB, Wirth RJ, & Greenberg M (2010). The measurement of executive function at age 3 years: psychometric properties and criterion validity of a new battery of tasks. Psychological Assessment, 22, 306–317. 10.1037/a0018708 [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Wirth RJ, & Blair CB (2012). Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychological Assessment, 24, 418–431. 10.1037/a0025779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, & Feng X (2020). Infant emotion regulation and cortisol response during the first 2 years of life: Association with maternal parenting profiles. Developmental Psychobiology, 62, 1076–1091. 10.1002/dev.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Feng X, Gerhardt M, & Wang L (2020). Maternal depressive symptoms, rumination and child emotion regulation. European Child & Adolescent Psychiatry, 29, 1125–1134. 10.1007/s00787-019-01430-5 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chen SH, & Main A (2012). Commonalities and differences in the research on children’s effortful control and executive function: A call for an integrated model of self-regulation. Child Development Perspectives, 6, 112–121. 10.1111/j.1750-8606.2011.00176.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


