Abstract
Over the course of its evolution, HIV-1 has taken maximum advantage of its tRNA3Lys primer by utilizing it in several steps of reverse transcription. Here, we have identified a conserved nonanucleotide sequence in the U3 region of HIV-1 RNA that is complementary to the anticodon stem of tRNA3Lys. In order to test its possible role in the first strand transfer reaction, we applied an assay using a donor RNA corresponding to the 5′-part and an acceptor RNA spanning the 3′-part of HIV-1 RNA. In addition, we constructed two acceptor RNAs in which the nonanucleotide sequence complementary to tRNA3Lys was either substituted (S) or deleted (Δ). We used either natural tRNA3Lys or an 18 nt DNA as primer and measured the efficiency of (–) strand strong stop DNA transfer in the presence of wild-type, S or Δ acceptor RNA. Mutations in U3 did not decrease the transfer efficiency when reverse transcription was primed with the 18mer DNA. However, they significantly reduced the strand transfer efficiency in the tRNA3Lys-primed reactions. This reduction was also observed in the presence of nucleocapsid protein. These results suggest that tRNA3Lys increases (–) strand strong stop transfer by interacting with the U3 region of the genomic RNA. Sequence comparisons suggest that such long range interactions also exist in other lentiviruses.
INTRODUCTION
Reverse transcriptase (RT) is a key enzyme of retroviral replication that possesses RNA- and DNA-dependent DNA polymerase and RNase H activities (1,2). It converts the single-stranded RNA genome into double-stranded DNA via a complex series of steps (3). In retroviruses, initiation of reverse transcription is primed by a cellular tRNA whose 3′-end is complementary to the so-called ‘primer binding site’ (PBS) located close to the 5′-end of the genomic RNA (for reviews see 4,5). The natural primer of all immunodeficiency viruses, including the type 1 human immunodeficiency virus (HIV-1), is tRNA3Lys.
Extension of the primer tRNA up to the 5′-end of the genome generates the (–) strand strong stop DNA [(–) ssDNA] (Fig. 1A). The presence of repeated (R) sequences at both ends of the RNA allows transfer of this intermediate product to the 3′-end of the genome, where (–) DNA synthesis resumes (3). In addition to the R sequences, the HIV-1 first strand transfer requires the endo- and exo-RNase H activities of RT (6–8). Furthermore, in vitro, this process is strongly enhanced by nucleocapsid protein (NCp) (9–13). Even though the exact mechanism of the first strand transfer remains incompletely understood, experimental evidence suggests that it involves a transient complex between RT and both the 5′- and 3′-ends of the genomic RNA (8,14).
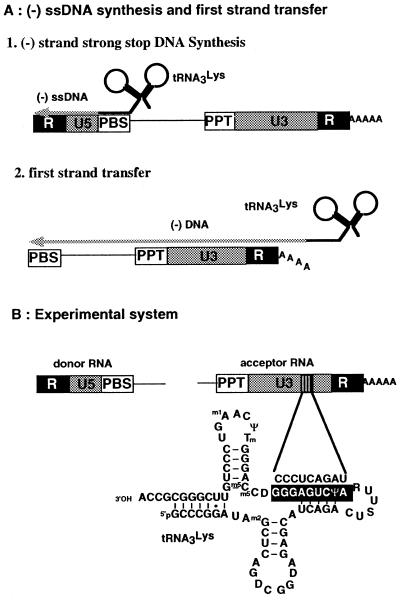
Figure 1.
(A) Schematic representation of synthesis of the (–) strand strong stop DNA and of the first strand transfer. The redundant (R) sequence is present at both ends of the genomic RNA, while U5 (unique to the 5′-end) and U3 (unique to the 3′-end) are present at the 5′- and 3′-ends, respectively. DNA synthesis is initiated from the 3′-end of tRNA3Lys annealed to the primer binding site (PBS). The polypurine tract (PPT) generates the primer for (+) strand DNA synthesis. The poly(A) tail of the genomic RNA is also shown. (B) The experimental system used in this study. The complementarity between U3 and the anticodon stem is schematized. In addition to the wild-type acceptor RNA, two RNAs were used in which the sequence complementary to the primer was either substituted or deleted.
The first strand transfer is a rate limiting step during reverse transcription of detergent-permeabilized virions, but not in infected cells (15). This observation indicates that protein–protein, RNA–protein and/or RNA–RNA interactions important for strand transfer are lost upon treatment with detergent. The finding that heat denaturation of the genomic RNA extracted from HIV-1 particles prevents the first strand transfer suggests that intra- and/or intermolecular RNA–RNA interactions are important for that process (16).
The viral RNA is degraded during (–) DNA synthesis by the RNase H activity of RT, except for a polypurine tract (PPT), located close to the 3′-end of the genome. This PPT is used as the primer for (+) strand DNA synthesis and is extended by copying the (–) strand DNA and the 18 nt at the 3′-end of tRNA3Lys that are complementary to the PBS. The resulting (+) strand strong stop DNA is then transferred to the 3′-end of the (–) strand DNA, thus allowing synthesis of a double-stranded DNA with duplicated long terminal repeats (LTR) (3).
The primer tRNA3Lys forms a highly ordered complex with the viral RNA (17,18), which depends on the presence of post-transcriptional modifications of the primer (19,20), and governs the specificity of initiation of HIV-1 reverse transcription (20–23). Formation of this initiation complex requires numerous inter- and intramolecular rearrangements (17,18). In addition, tRNA3Lys plays a specific role during the second strand transfer (24–27). In particular, methylation of A58 of tRNA3Lys is required for this transfer to be productive and to allow complete synthesis of the provirus (24,26).
Here, we have used an in vitro strand transfer system to test for possible involvement of tRNA3Lys in the first strand transfer. Indeed, it has been reported that (–) ssDNA transfer is more efficient when natural tRNA3Lys is used as primer, as compared to an 18mer oligodeoxyribonucleotide (ODN) complementary to the PBS, thus suggesting a role for tRNA3Lys in the first strand transfer (28). However, tRNA3Lys also allows more efficient priming and (–) ssDNA synthesis than the ODN (20). Hence, the effect observed at the level of the first strand transfer might reflect variations in (–) ssDNA synthesis rather than a direct role of tRNA3Lys in the transfer event itself. Our present results indicate that tRNA3Lys increases the efficiency of the first strand transfer by interacting with a sequence located in the U3 region of the genomic RNA.
MATERIALS AND METHODS
Donor and acceptor RNAs, RT, NCp and primers
The donor RNA corresponding to nucleotides 1–311 of HIV-1 genomic RNA (Mal isolate) was synthesized by in vitro transcription of plasmid pJCB and purified as previously described (29).
The wild-type (WT) acceptor RNA corresponding to nucleotides 8607–9229 of HIV-1 Mal genomic RNA was prepared by in vitro transcription with T7 RNA polymerase of XhoI-linearized plasmid pFB1, which was derived from pHIVCG8.6 (30). Plasmid pHIVCG8.6, kindly provided by J. L. Darlix (ENS, Lyon, France) contains a DNA insert corresponding to nucleotides 8607–9229 of HIV-1 Mal, followed by a 20 nt poly(A) tail, inserted between the KpnI and XhoI sites of pBS(KS) (Stratagene). Plasmid pFB1 was generated by inserting this fragment between the KpnI and XhoI sites of pBS(SK) (Stratagene).
Mutant acceptor RNAs Δ and S were prepared by in vitro transcription with T7 RNA polymerase of XhoI-linearized plasmids pFB2 and pFB3 derived from pFB1. Plasmids pFB2 and pFB3 were obtained by inverse PCR on the plasmid pFB1, using PCR primers designed to eliminate the 9091–9099 region of pFB1 (pFB2) or substitute this region by GAGCGCACA (pFB3). The PCR products were purified by gel electrophoresis, phosphorylated, ligated and used to transform Escherichia coli DH5α cells.
Natural tRNA3Lys was purified from beef liver and 3′-end-labeled as described (19). ODN, an 18mer oligodeoxyribonucleotide complementary to the PBS, was chemically synthesized and 5′-end-labeled using [γ-32P]ATP (NEN) and T4 polynucleotide kinase (New England Biolabs).
Wild-type HIV-1 RT was purified essentially as previously described (31). NCp protein (72 residues) was obtained by total chemical synthesis, as described (32,33).
Strand transfer assays without NCp protein
For the strand transfer experiments, the 32P-labeled primers (ODN and tRNA3Lys) were heat annealed to donor RNA at a 1:2.5 ratio as previously described (17,19). Strand transfer reactions were performed in 10 µl buffer containing 50 mM Tris–HCl, pH 8.0 (37°C), 50 mM KCl, 6 mM MgCl2 and 1 mM DTE, in the presence of donor RNA (100 or 200 nM) with bound primer annealed to primer, either WT, S or Δ acceptor RNA (200 or 400 nM), RT (100 or 200 nM) and 50 µM dNTP. Incubation at 37°C was for 30–240 min as indicated in the figure legends. Reactions were stopped with 10 mM EDTA. Reaction products were heat denatured in 40% formamide, fractionated on 5% denaturing polyacrylamide gels and quantified with a BioImager BAS 2000 (Fuji) using MacBAS software.
In order to check the identity of the band corresponding to the full-length transfer product, the candidate bands were excised, rehydrated and amplified by PCR with the ODN and a primer corresponding to nucleotides 8607–8624 of the acceptor RNAs.
Strand transfer assays with NCp protein
In all experiments, the NCp concentration was adjusted to completely cover the nucleic acids (1 NCp:7–8 nt). Two experimental protocols were used. In the first, the primer (40 nM) was heat annealed to the template (100 nM) and we added NCp and 30 µM ZnCl2 to the reaction mixture together with acceptor RNA (200 nM), dNTPs (50 µM each) and RT (100 nM). In the second, an equimolar amount of tRNA3Lys (100 nM) was added to the donor RNA and annealed in the presence of NCp for 60 min at 37°C in 50 mM Tris–HCl pH 8.0 (37°C), 50 mM KCl, 1 mM MgCl2, 30 µM ZnCl2 and 1 mM DTE. Reverse transcription was initiated by addition of acceptor RNA (200 nM), dNTPs (50 µM each), RT (200 nM), and MgCl2 was adjusted to 6 mM. Reactions were stopped with 10 mM EDTA. Before gel electrophoresis, the samples were submitted to proteinase K (Promega) treatment and phenol/chloroform extraction, in order to eliminate NCp.
PCR
For semi-quantitative evaluation of the strand transfer efficiency at short reaction times, the full-length strand transfer product (0.5 µl of the reaction mixture) was amplified in 20 µl of buffer containing 10 mM Tris–HCl pH 8.8 (37°C), 50 mM KCl, 2.5 mM MgCl2, 0.2 mg/ml gelatin, 0.2 mM dNTPs and 10 pmol of primers (32P-labeled ODN and unlabeled primer 8607–8624) for 20 cycles (1 min at 94°C, 1 min at 48°C, 2 min at 72°C). In order to test the accuracy of the PCR assay, a DNA version of the full-length transfer product was constructed. The corresponding plasmid, pFB4, was obtained by inserting the EcoRI–PstI fragment of pJCB between the EcoRI and PstI sites of pFB1. The PCR products were analyzed by electrophoresis on 1% agarose gels, subjected to autoradiography and quantified with a BioImager BAS 2000 (Fuji).
RESULTS
Experimental strategy
Over the course of evolution of HIV-1, the virus has taken maximum advantage of its tRNA3Lys primer by utilizing it at several steps of reverse transcription. This adaptation concerns not only the initiation of reverse transcription but also the second strand transfer (24–27). It is plausible that tRNA3Lys may also play a direct role in other steps of proviral synthesis. Here, we examined the possible role of the primer tRNA in the first strand transfer.
We hypothesized that if tRNA3Lys directly promotes the transfer reaction, this might be accomplished by interacting with the 3′-part (the accepting region) of the genomic RNA. Therefore, we searched for complementarity between tRNA3Lys and the 3′-half of HIV-1 genomic RNA. We found a strict complementarity between nucleotides 38–46 in the anticodon stem of tRNA3Lys and a nonanucleotide sequence (nucleotides 9091–9099 in HIV-1 Mal) located in the U3 region of HIV-1 RNA (Fig. 1B). Interestingly, this sequence is strictly conserved in all HIV-1 sequences of the HIV-1 sequence database (34; http://hiv-web.lanl.gov/HTML/compendium.html ), in agreement with a potential functional role.
We set up an assay to test the strand transfer efficiency using two different RNAs (Fig. 1B). The first, 311 nt in length, contained R, U5, the PBS, and part of the leader region, including the major splice donor site, and was referred to as the donor RNA. The second, the acceptor RNA, was 639 nt in length and spanned part of the nef coding region, the PPT, U3, R, and a 20 nt poly(A) tail. In addition, we constructed two acceptor RNAs in which the CCCUCAGAU sequence complementary to the anticodon stem of tRNA3Lys was either substituted by GAGCGCACA (designated S) or deleted (Δ). After hybridization of either tRNA3Lys or the ODN to the PBS of the donor RNA, we added either WT, S or Δ acceptor RNA and measured the efficiency of (–) ssDNA transfer in the absence and presence of NCp. Assuming that the deletion and substitution had no dramatic consequences on the structure of the acceptor RNA, it is anticipated that the mutations should not affect the efficiency of (–) ssDNA transfer when DNA synthesis is primed by ODN. However, if the complementarity between the anticodon stem of tRNA3Lys and the U3 region of HIV-1 has functional implications for the first strand transfer, the substitution and the deletion in the viral RNA should affect the transfer when (–) ssDNA synthesis is primed with tRNA3Lys.
ODN as primer
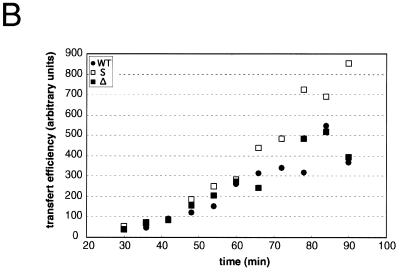
In a first set of experiments, we studied the effects of the substitution and deletion in U3 on the transfer efficiency during ODN-primed DNA synthesis. In the absence of acceptor RNA, (–) ssDNA, which corresponds to extension of the primer by 196 nt, was the major product (Fig. 2A). Some larger products, resulting from TAR-dependent self-priming (11,35), were also observed (Fig. 2A). In the presence of acceptor RNA, several additional bands were observed. The product resulting from complete reverse transcription of the acceptor RNAs was identified by PCR: after gel drying and autoradiography, the band was excised, rehydrated, and amplified with product-specific primers (see Materials and Methods). As previously observed (9,10,12), the efficiency of the complete transfer product was low in the absence of NCp and in the absence of a large excess of RT and acceptor RNA. More importantly, no significant differences in either the amount of transfer product or its kinetics of appearance were observed when the S and Δ acceptor RNAs were substituted for the WT acceptor. The products of a similar reaction performed with a 2-fold reduced RT concentration were quantified (Fig. 2B). Again, mutations in the U3 region did not decrease the transfer efficiency. The transfer efficiency was unaffected with the Δ acceptor RNA, while it significantly increased with the S acceptor RNA. On the other hand, the pattern of intermediate products synthesized after strand transfer was identical with all three acceptor RNAs (Fig. 2A). Thus, the origin of the enhanced strand transfer with the S acceptor RNA remains unclear, but could be due to destabilization of the tertiary structure of this RNA, since perturbations of its secondary structure would probably affect the pattern of intermediate products synthesized after strand transfer. More importantly, these observations indicate that the mutations introduced in the U3 region had no adverse effect on the first strand transfer.
Figure 2.
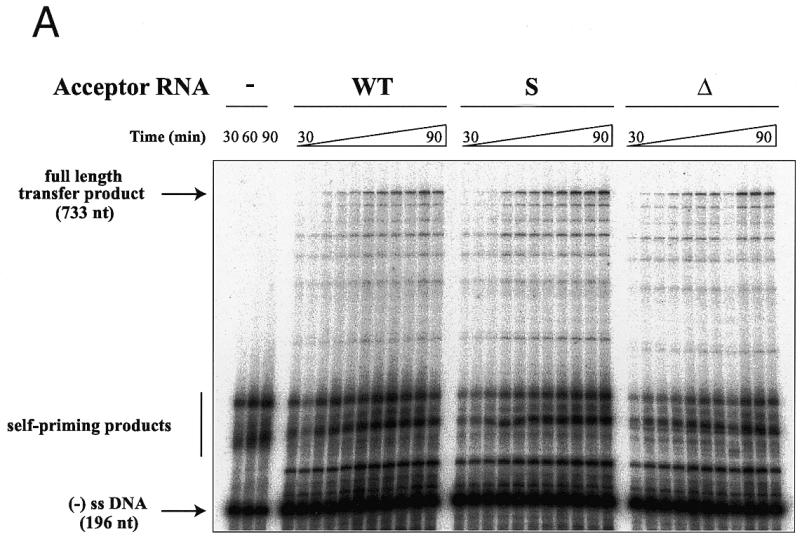
DNA-primed strand transfer. (A) Gel fractionation of the transfer products. Labeled ODN (40 nM) was heat annealed to donor RNA (100 nM) and incubated in the absence (lanes 1–3) or presence of 200 nM WT (lanes 4–14), S (lanes 5–15) or Δ (lanes 16–26) acceptor RNAs and 200 nM HIV-1 RT. Reaction was for 30, 36, 42, 48, 54, 60, 66, 72, 78, 84 or 90 min. (B) Quantification of the full-length transfer product obtained under the same conditions, except that the RT concentration was reduced to 100 nM.
tRNA3Lys as primer
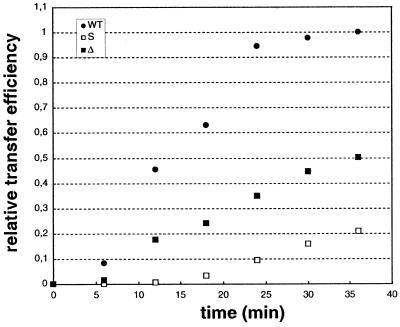
In the second set of experiments, tRNA3Lys was used as primer, instead of ODN (Fig. 3). As in the first set of experiments, self-priming products larger than the expected (–) ssDNA were observed (Fig. 3A). With the WT acceptor RNA, more final transfer product, relative to the (–) ssDNA, was detected in the tRNA-primed reaction, as compared to ODN-primed DNA synthesis (Figs 2A and 3A). Quantification and normalization of the full-length transfer products obtained with the different acceptor RNAs showed that both the substitution and the deletion of the U3 sequence complementary to the anticodon arm of tRNA3Lys strongly decreased the strand transfer efficiency. After 2 h incubation, the transfer efficiency was reduced by 65% with the S acceptor RNA and by 45% with the Δ acceptor RNA, as compared to the WT acceptor RNA. After 4 h incubation, the transfer efficiency was reduced by 40 and 85%, respectively, compared to the WT acceptor RNA (Fig. 3B). Under the latter conditions, the full-length transfer product obtained with the WT acceptor RNA amounted to 70% of the (–) ssDNA still present in the reaction.
Figure 3.
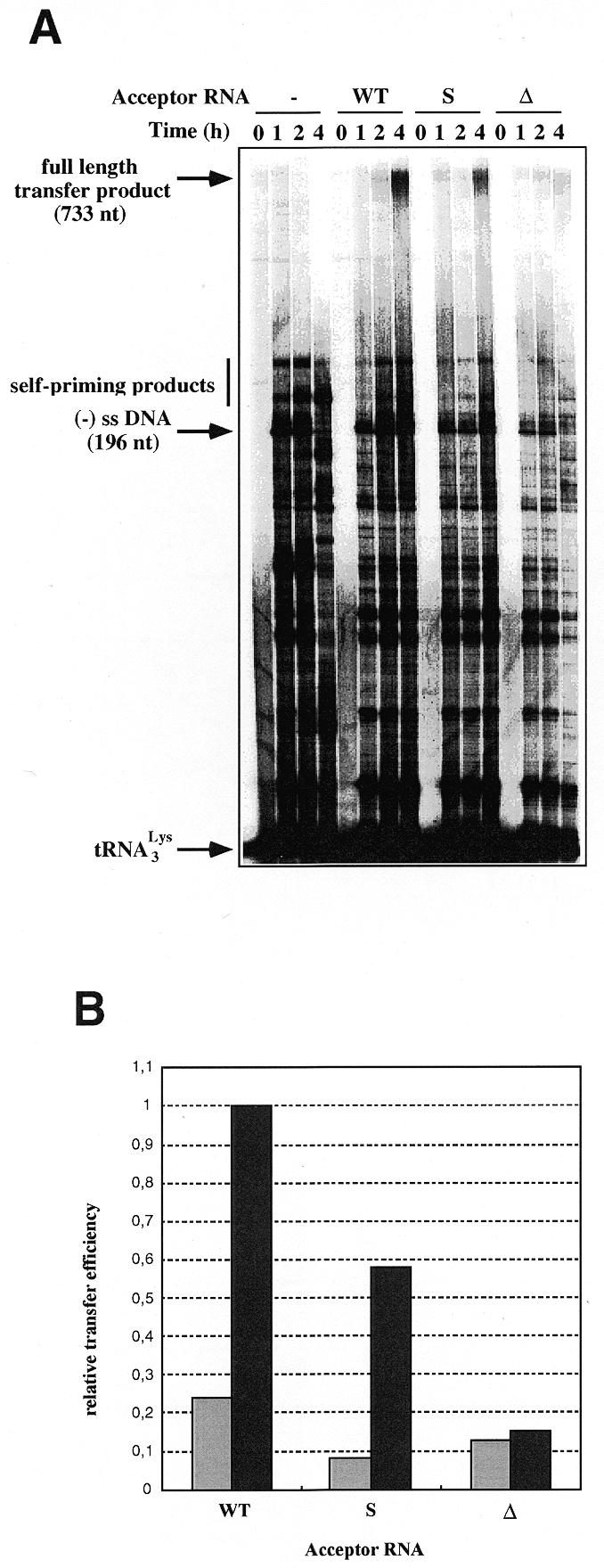
tRNA3Lys-primed strand transfer. (A) Gel fractionation of the transfer products. Labeled tRNA3Lys (80 nM) was heat-annealed to donor RNA (200 nM) and incubated in the absence or presence of 400 nM WT, S or Δ acceptor RNA and 100 nM HIV-1 RT. Reaction was for 1, 2 or 4 h. (B) Quantification of the full-length product obtained after 2 (gray) and 4 h (black) incubation.
In order to gain insight into the first stages of the reaction, when an intermolecular interaction between the primer tRNA3Lys and the acceptor RNA should have the most pronounced effect, the full-length transfer product obtained after short reaction times was amplified by PCR and quantified. Amplification was performed with a primer corresponding to nucleotides 8607–8624 of HIV-1 Mal and a labeled primer complementary to the PBS. The accuracy of the PCR amplification was checked using as a control a DNA template containing the nef-PPT-U3-R-U5-PBS sequences (see Materials and Methods). Several independent experiments gave consistent results. Namely, the transfer efficiency was higher in the reaction including the WT acceptor RNA (Fig. 4). This experiment also showed that the transfer efficiency was more affected by the substitution than by the deletion in the U3 region putatively interacting with the primer tRNA, at least in the early steps of the reaction (Fig. 4). Analysis of the linear part of Figure 4 revealed 3- and 9-fold reductions, respectively, of the initial rate of full-length product synthesis with the Δ and S acceptor RNAs, as compared to the WT acceptor RNA.
Figure 4.
PCR amplification of the full-length transfer product generated in the tRNA3Lys-primed reaction. Transfer products generated after 6–90 min in the presence of WT, S or Δ acceptor RNAs were amplified and quantified as described in Materials and Methods. When using the WT acceptor, amplification rapidly became limited by the primer concentration.
Effect of NCp on the transfer reaction
All experiments described thus far were performed in the absence of NCp, and the primer was heat-annealed to the donor RNA under conditions allowing quantitative annealing of ODN and tRNA3Lys (17,19). NCp is a well-known activator of the strand transfer reaction (9,10,12). Therefore, if biologically significant, the substitution and deletion within U3 should also affect the transfer efficiency in the tRNA-primed reaction when NCp is included in the reaction mixture.
Two experimental protocols were used. In the first, tRNA3Lys was heat-annealed to the donor RNA, the acceptor RNA was added before or just after the annealing step, and NCp and RT were added after primer annealing. In the second series of experiments, tRNA3Lys was annealed at 37°C in the presence of NCp (36,37), before adding RT. Regarding the experiments using the heat-annealed primer, identical results were obtained irrespective of the addition of acceptor RNA during or after the annealing step (data not shown). This result indicated that formation of the numerous intermolecular interactions leading to the highly structured initiation complex (17,21) was not affected by the presence of the acceptor RNA.
We performed strand transfer experiments using 100 nM donor RNA, 200 nM acceptor RNA and 100 nM RT, either in the absence or presence of saturating amounts of NCp (1 NCp:7–8 nt). Under these conditions, transfer products were hardly detected in the absence of NCp (Fig. 5A, left). Small amounts of full-length transfer products could be detected when the same gel was overexposed (data not shown). The predominant products longer than the (–) ssDNA were Tar-dependent self-priming products, since they were observed in the presence as well as absence of acceptor RNA (Fig. 5A). As previously reported (11,35), self-primed DNA synthesis due to the TAR structure was inhibited by NCp. This is obvious from the absence of products longer than the (–) ssDNA in the presence of NCp and absence of acceptor RNA. In addition, as previously observed (9,10,12), NCp greatly enhanced the efficiency of strand transfer (Fig. 5A, right).
Figure 5.
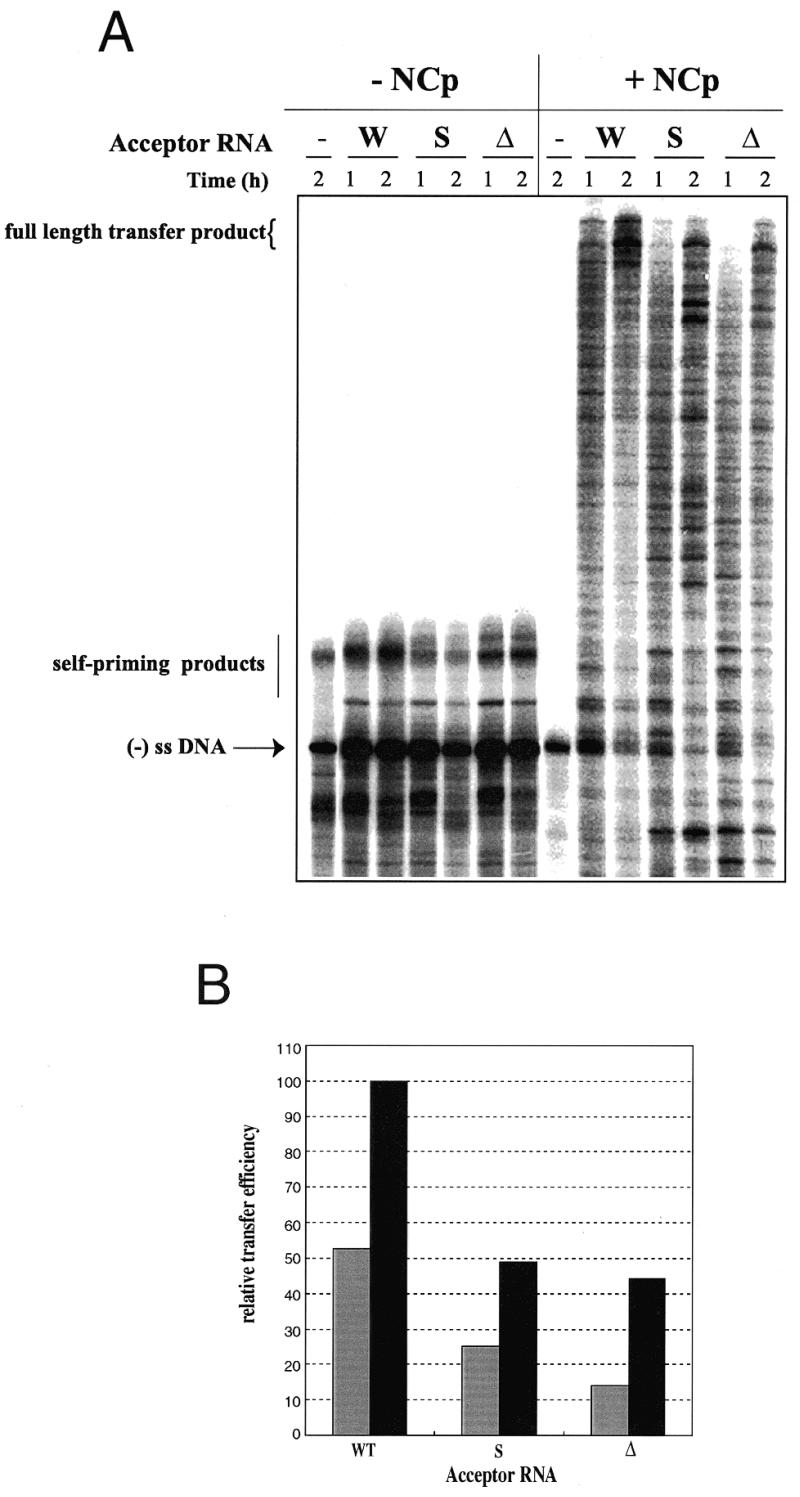
tRNA3Lys-primed strand transfer in the absence and presence of NCp. (A) Gel fractionation of the transfer products. tRNA3Lys (40 nM) was heat-annealed to 100 nM donor RNA and DNA synthesis was initiated by addition of 50 µM dNTP, 200 nM acceptor RNA, 100 nM RT and saturating amounts of NCp (1 NCp:7–8 nt). (B) Quantification of the gel shown in (A). Reaction time was 1 (gray) or 2 h (black).
Quantification of the full-length product resulting from (–) ssDNA strand transfer performed in the presence of NCp showed a 2-fold reduction in strand transfer efficiency when substituting the S and Δ acceptor RNAs for the WT acceptor RNA (Fig. 5A and B). Under these conditions the deletion and the substitution in U3 had almost identical results. Similar results were obtained when tRNA3Lys was annealed at physiological temperature with NCp (data not shown). The only noticeable difference was an overall decrease in DNA synthesis in the latter experiments, which correlated with incomplete primer annealing. However, the effect of substituting and deleting the U3 sequence complementary to the tRNA3Lys anticodon stem was still clearly observed (data not shown).
DISCUSSION
In this study, we tested the possible role of primer tRNA3Lys in transfer of the (–) ssDNA from the 5′- to the 3′-end of HIV-1 genomic RNA. Sequence analysis of the 3′-region of HIV-1 RNA pointed towards a conserved nonanucleotide motif complementary to the anticodon stem of tRNA3Lys that could favor strand transfer by interacting with the tRNA primer. In order to test this hypothesis, we mutated the candidate sequence in the acceptor RNA. Compared to the alternative strategy using different primers (28), our approach did not affect synthesis of the (–) ssDNA, and hence yielded a direct test of the proposed interaction. We used the ODN primer as a control, to make sure that the mutations introduced in the acceptor RNAs did not dramatically affect its reverse transcription.
Deletion of the nonanucleotide complementary to the anticodon stem of tRNA3Lys had no effect on the ODN-primed reaction. However, this deletion decreased the strand transfer efficiency in the tRNA3Lys-primed reaction. In most experiments, the strand transfer efficiency was reduced by 2- to 3-fold, independently of the presence or absence of NCp in the reaction. Hence, the data obtained with the Δ acceptor RNA strongly suggest that the primer tRNA3Lys increased the efficiency of the first strand transfer by interacting with the U3 region of the genomic RNA.
Even though the data obtained with the S acceptor RNA are more complex, they are also consistent with an interaction between the anticodon stem of tRNA3Lys and the U3 region of the HIV-1 RNA. Substitution of the nonanucleotide complementary to tRNA3Lys increased synthesis of the full-length transfer product in the ODN-primed reaction, suggesting a structural modification of the acceptor RNA. In contrast, this substitution decreased synthesis of the full-length product in the tRNA3Lys-primed reaction. In the absence of NCp and at long incubation times, the deletion was more deleterious than the substitution. This reduced effect of the substitution, as compared to the deletion, is likely a combination of the negative effect due to disruption of the interaction between U3 and tRNA3Lys and the positive structural effect that we detected in the ODN-primed reaction. This interpretation is in keeping with the observation that, in the presence of NCp, the deletion and the substitution in U3 reduced synthesis of the full-length transfer product to the same extent. The differential effect of the substitution and the deletion was likely suppressed by the well-known chaperone and unwinding activities of NCp (38).
An obvious experiment to confirm the interaction between U3 and tRNA3Lys would be to introduce mutations in tRNA3Lys to render its anticodon stem complementary to the S acceptor RNA. However, mutating tRNA3Lys would disrupt the secondary and tertiary structures of the initiation complex of reverse transcription (17,21) and, hence, would introduce a strong bias in the experiments. Indeed, the intricate structure of the initiation complex, which relies on interactions between the viral RNA and the anticodon arm and variable loop of tRNA3Lys (17,21), is required for efficient initiation of reverse transcription (20). Furthermore, efficient initiation of HIV-1 reverse transcription requires post-transcriptional modification of tRNA3Lys (20). Thus, in vitro transcribed mutant tRNA3Lys is useless for such studies.
Interestingly, the tRNA3Lys sequence involved in the strand transfer process also plays a crucial role in the initiation complex of reverse transcription. In this complex, the 3′-strand of the tRNA3Lys anticodon stem interacts with U5 sequences, upstream of the PBS (17,21). Due to their location, these intermolecular base pairings are unwound as synthesis of the (–) ssDNA proceeds. Thus, after the initiation phase of reverse transcription, the anticodon stem of tRNA3Lys becomes free to interact with other parts of the genomic RNA. We propose that the 3′-strand of the tRNA3Lys anticodon stem is involved in a flip of intermolecular interactions from the U5 to the U3 region of the genomic RNA following initiation of reverse transcription. In addition, the interaction between the 3′-strand of the tRNA3Lys anticodon stem and U3 must dissociate during reverse transcription of the donor RNA. Thus, the interaction we identified here is essentially a transient one, and it is unlikely that it could be detected by chemical or enzymatic probing.
The effect of the U3 sequence on strand transfer efficiency was also observed in the presence of NCp, further supporting a biological role for this tRNA3Lys–U3 interaction. This interaction was not absolutely required to allow strand transfer, but even a modest reduction in the efficiency of this step might affect overall replication of the virus. Furthermore, in our in vitro strand transfer assays, we used rather short RNAs, as compared to the ~10 000 nt genomic RNA. Thus, the tRNA3Lys–U3 interaction might be crucial in vivo, by bridging the otherwise distant 5′- and 3′-regions of the genomic RNA.
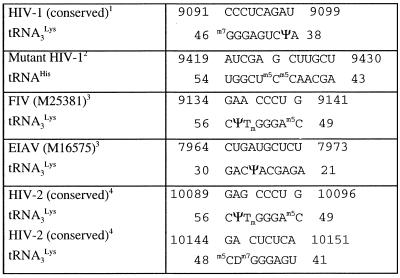
Morrow and co-workers obtained stable HIV-1 clones using tRNAHis as primer for reverse transcription (22,39). Since the sequence of the anticodon arms of tRNA3Lys and tRNAHis differ significantly, the original U3–tRNA interaction is not possible in the mutant clones. We sequenced the U3 region of these clones, kindly provided by C. Morrow. The U3 region originally complementary to tRNA3Lys in these clones (nucleotides 9497–9505) was unchanged. This result seems to contradict a biological function for the intermolecular interaction we propose. However, we discovered an almost perfect complementarity between nucleotides 43-AGCAAm5Cm5CUCGGU-54 of tRNAHis and nucleotides 9419-AUCGAGCUUGCU-9430 in the mutant clones (Table 1). We hypothesize that this interaction between tRNAHis and the U3 region of the mutant clones replaces the original interaction between U3 and tRNA3Lys.
Table 1. Complementarity between the primer tRNA and the 3′-end of lentiviral genomic RNAs.
1Numbering is according to HIV-1 Mal.
2These mutants were selected by C. Morrow and co-workers (20,37). Numbering is according to the parent strain HXB2.
3The accession number is given in parentheses.
4Numbering is according to HIV-2 Ben.
In order to further assess the biological role of the U3–tRNA interaction, we looked for potential interactions between tRNA3Lys and the 3′-region of the genomic RNA of other lentiviruses. In the case of feline immunodeficiency virus (FIV), we found an octanucleotide sequence in the U3 region of the genomic RNA that is strictly complementary to the 5′-part of the TΨC stem–loop (Table 1). Similarly, the U3 region of equine infectious anemia virus (EIAV) contains a decanucleotide complementary to part of the D and anticodon stems of tRNA3Lys (Table 1). The situation is more complex in HIV-2. We found several complementary regions between the 3′-end of HIV-2 genomic RNA and tRNA3Lys. However, several of these sequences were not conserved among SIV and HIV-2 isolates. Indeed, the best-conserved complementarities between tRNA3Lys and HIV-2 (and SIV) RNA, outside the PBS, were found within the R region, rather than U3. We identified two conserved octanucleotides in R that are complementary to adjacent regions of tRNA3Lys (Table 1). A possible reason for a complementarity between tRNA3Lys and HIV-2 R, instead of U3, is the unusual length of the R region in HIV-2 and SIV, compared to HIV-1, FIV and EIAV (175, 96, 65 and 78 nt, respectively).
Thus, an interaction between the primer tRNA3Lys and the 3′-end of the genomic RNA is apparently conserved among lentiviruses. A similar situation may also prevail in other retroviruses and retroelements. In the yeast retrotransposon Ty3, the interaction between U3 and the D and TΨC arms of primer tRNAiMet appears even more crucial than the interaction we propose in HIV-1, since it is absolutely required for primer annealing to the retrotransposal RNA and transposition (40).
Acknowledgments
ACKNOWLEDGEMENTS
We thank C. Morrow (University of Alabama) for kindly providing the clones of the HIV-1 mutant replicating using tRNAHis as primer. Thanks are due to J. L. Darlix (ENS, Lyon, France) for giving access to unpublished results and for discussions, and to J. S. Lodmell for critical reading of the manuscript. F.B. was supported by fellowships from SIDACTION and the Agence Nationale de Recherches sur le SIDA (ANRS). This work was supported by an ANRS grant and a Biomed II grant from the European Union.
REFERENCES
- 1.Baltimore D. (1970) Nature, 226, 1209–1211. [DOI] [PubMed] [Google Scholar]
- 2.Temin H.M. and Mizutani,S. (1970) Nature, 226, 1211–1213. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa E., Mitra,S.W., Goff,S. and Baltimore,D. (1979) Cell, 18, 93–100. [DOI] [PubMed] [Google Scholar]
- 4.Mak J. and Kleiman,L. (1997) J. Virol., 71, 8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquet R., Isel,C., Ehresmann,C. and Ehresmann,B. (1995) Biochimie, 77, 113–124. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh M., Howard,K.J., Cameron,C.E., Benkovic,S.J., Hughes,S.H. and Le Grice,S.F. (1995) J. Biol. Chem., 270, 7068–7076. [DOI] [PubMed] [Google Scholar]
- 7.Cirino N.M., Cameron,C.E., Smith,J.S., Rausch,J.W., Roth,M.J., Benkovic,S.J. and Le Grice,S.F. (1995) Biochemistry, 34, 9936–9943. [DOI] [PubMed] [Google Scholar]
- 8.Peliska J.A. and Benkovic,S.J. (1992) Science, 258, 1112–1118. [DOI] [PubMed] [Google Scholar]
- 9.Allain B., Lapadat-Tapolsky,M., Berlioz,C. and Darlix,J.L. (1994) EMBO J., 13, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darlix J.L., Vincent,A., Gabus,C., de Rocquigny,H. and Roques,B. (1993) C.R. Acad. Sci. III, 316, 763–771. [PubMed] [Google Scholar]
- 11.Guo J., Henderson,L.E., Bess,J., Kane,B. and Levin,J.G. (1997) J. Virol., 71, 5178–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peliska J.A., Balasubramanian,S., Giedroc,D.P. and Benkovic,S.J. (1994) Biochemistry, 33, 13817–13823. [DOI] [PubMed] [Google Scholar]
- 13.You J.C. and McHenry,C.S. (1994) J. Biol. Chem., 269, 31491–31495. [PubMed] [Google Scholar]
- 14.Canard B., Sarfati,R. and Richardson,C.C. (1997) Proc. Natl Acad. Sci. USA, 94, 11279–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telesnitsky A. and Goff,S.P. (1993) In Skalka,A.M. and Goff,S.P. (eds), Reverse Transcriptase. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 49–83.
- 16.Berkhout B., Das,A.T. and van Wamel,J.L. (1998) Virology, 249, 211–218. [DOI] [PubMed] [Google Scholar]
- 17.Isel C., Ehresmann,C., Keith,G., Ehresmann,B. and Marquet,R. (1995) J. Mol. Biol., 247, 236–250. [DOI] [PubMed] [Google Scholar]
- 18.Isel C., Keith,G., Ehresmann,B., Ehresmann,C. and Marquet,R. (1998) Nucleic Acids Res., 26, 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isel C., Marquet,R., Keith,G., Ehresmann,C. and Ehresmann,B. (1993) J. Biol. Chem., 268, 25269–25272. [PubMed] [Google Scholar]
- 20.Isel C., Lanchy,J.M., Le Grice,S.F.J., Ehresmann,C., Ehresmann,B. and Marquet,R. (1996) EMBO J., 15, 917–924. [PMC free article] [PubMed] [Google Scholar]
- 21.Isel C., Westhof,E., Massire,C., Le Grice,S.F.J., Ehresmann,C., Ehresmann,B. and Marquet,R. (1999) EMBO J., 18, 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z.J., Kang,S.M., Li,Y. and Morrow,C.D. (1998) RNA, 4, 394–406. [PMC free article] [PubMed] [Google Scholar]
- 23.Arts E.J., Stetor,S.R., Li,X.G., Rausch,J.W., Howard,K.J., Ehresmann,B., North,T.W., Wohrl,B.M., Goody,R.S., Wainberg,M.A. and Le Grice,S.F.J. (1996) Proc. Natl Acad. Sci. USA, 93, 10063–10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auxilien S., Keith,G., Le Grice,S.F.J. and Darlix,J.L. (1999) J. Biol. Chem., 274, 4412–4420. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Artzi H., Shemesh,J., Zeelon,E., Amit,B., Kleiman,L., Gorecki,M. and Panet,A. (1996) Biochemistry, 35, 10549–10557. [DOI] [PubMed] [Google Scholar]
- 26.Burnett B.P. and McHenry,C.S. (1997) Proc. Natl Acad. Sci. USA, 94, 7210–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusupova G., Lanchy,J.M., Yusupov,M., Keith,G., Le Grice,S.F.J., Ehresmann,C., Ehresmann,B. and Marquet,R. (1996) J. Mol. Biol., 261, 315–321. [DOI] [PubMed] [Google Scholar]
- 28.Arts E.J., Li,X., Gu,Z., Kleiman,L., Parniak,M.A. and Wainberg,M.A. (1994) J. Biol. Chem., 269, 14672–14680. [PubMed] [Google Scholar]
- 29.Marquet R., Baudin,F., Gabus,C., Darlix,J.L., Mougel,M., Ehresmann,C. and Ehresmann,B. (1991) Nucleic Acids Res., 19, 2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapadat-Tapolsky M., de Rocquigny,H., Van Gent,D., Roques,B., Plasterk,R. and Darlix,J.L. (1993) Nucleic Acids Res., 21, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Grice S.F.J. and Grueninger-Leitch,F. (1990) Eur. J. Biochem., 187, 307–314. [DOI] [PubMed] [Google Scholar]
- 32.de Rocquigny H., Ficheux,D., Gabus,C., Allain,B., Fournie-Zaluski,M.C., Darlix,J.L. and Roques,B.P. (1993) Nucleic Acids Res., 21, 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Rocquigny H., Ficheux,D., Gabus,C., Fournie-Zaluski,M.C., Darlix,J.L. and Roques,B.P. (1991) Biochem. Biophys. Res. Commun., 180, 1010–1018. [DOI] [PubMed] [Google Scholar]
- 34.Korber B., Hahn,B., Foley,B., Mellors,J.W., Leitner,T., Myers,G., McCutchan,F. and Kuiken,C. (eds) (1997) Human Retroviruses and AIDS. A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. Theoretical Biology and Byophysics Group T-10, Los Alamos, NM.
- 35.Li X., Quan,Y., Arts,E.J., Li,Z., Preston,B.D., de Rocquigny,H., Roques,B.P., Darlix,J.L., Kleiman,L., Parniak,M.A. and Wainberg,M.A. (1996) J. Virol., 70, 4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prats A.C., Sarih,L., Gabus,C., Litvak,S., Keith,G. and Darlix,J.L. (1988) EMBO J., 7, 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Rocquigny H., Gabus,C., Vincent,A., Fournié-Zaluski,M.C., Roques,B. and Darlix,J.L. (1992) Proc. Natl Acad. Sci. USA, 89, 6472–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rein A., Henderson,L.E. and Levin,J.G. (1998) Trends Biochem. Sci., 23, 297–301. [DOI] [PubMed] [Google Scholar]
- 39.Wakefield J.K., Kang,S.-M. and Morrow,C.D. (1996) J. Virol., 70, 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabus C., Ficheux,D., Rau,M., Keith,G., Sandmeyer,S. and Darlix,J.L. (1998) EMBO J., 17, 4873–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]