Abstract
Postpartum depression (PPD) is a challenging psychological disorder faced by 10–30% of mothers across the globe. In India, it occurs among 22% of mothers. Its aetiology and pathophysiology aren’t fully understood as of today but multiple theories on the interplay of hormones, neurotransmitters, genetics, epigenetics, nutrients, socio-environmental factors, etc. exist. Nutrients are not only essential for the synthesis of neurotransmitters, but they may also indirectly influence genomic pathways that methylate DNA, and there is evidence for molecular associations between nutritional quality and psychological well-being. Increased behavioural disorders have been attributed to macro- and micronutrient deficiencies, and dietary supplementation has been effective in treating several neuropsychiatric illnesses. Nutritional deficiencies occur frequently in women, especially during pregnancy and breastfeeding. The aim of this study was to perform a comprehensive literature review of evidence-based research in order to identify, gather and summarize existing knowledge on PPD’s aetiology, pathophysiology, and the role of nutrients in its prevention as well as management. The possible mechanisms of action of nutrients are also presented here. Study findings show that the risk of depression increases when omega-3 fatty acid levels are low. Both fish oil and folic acid supplements have been used to effectively treat depression. Antidepressant efficacy is lowered by folate insufficiency. Folate, vitamin B12, iron, etc. deficiencies are more prevalent in depressed people than in non-depressed people. Serum cholesterol levels and plasma tryptophan levels are found to be inversely correlated with PPD. Serum vitamin D levels were associated inversely with perinatal depression. These findings highlight the importance of adequate nutrition in the antepartum period. Given that nutritional therapies can be affordable, safe, simple to use, and are typically well-accepted by patients, more focus should be placed on dietary variables in PPD.
Keywords: Postpartum depression, Nutrients, Ovarian hormones, Neurotransmitters, Vitamin D, Polyunsaturated fatty acids
1. Introduction
World Health Organization defines the mental health of a mother as “a state of well-being in which a mother realizes her own abilities, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to her community” (Engle, 2009). It is well known that a woman’s body goes through several physiological changes during pregnancy (Payne and Maguire, 2019). These changes usually occur on physical, physiological, and psychological levels. A combination of these alterations along with the interplay of genetic, epigenetic, dietary factors, and external circumstances like socioeconomic status, interpersonal relationships, etc. cause a serious psychological impairment called Postpartum Depression (PPD) in mothers (Payne and Maguire, 2019, Upadhyay et al., 2017).
PPD was initially defined as a major depressive disorder (MDD) that sets on within a month after childbirth. Despite the large number of publications on PPD, its aetiology is still ambiguous. However, there is some evidence pointing at multiple physiological factors such as: alterations in peptide and steroid hormones (that occur during pregnancy and in the postpartum period), altered psycho-neuroimmune systems, elevated stress levels as well as inflammatory biomarkers, all leading to disruption of the brain's serotonin synthesis (Amini et al., 2020, Ellsworth-Bowers and Corwin, 2012). In addition, peptide and steroid hormone alterations affect the hypothalamic-pituitary–gonadal (HPG) and hypothalamic–pituitary-adrenal (HPA) axes in mothers. Dysregulation of these hormonal axes is associated with mood-related disorders during pregnancy and the postpartum period. PPD occurs in 10–30% of mothers all around the globe and in 22% of mothers in India (Lanjewar et al., 2021). It is linked to the mother's negative emotionality and her propensity for developing chronic depression. Studies have shown that in the context of developing countries, a mother's depression can contribute to her child’s stunted growth and low weight (Surkan et al., 2011). If left untreated, this disorder will have a substantial and long-term influence on the new-born’s emotional, cognitive, and intellectual development (Feldman et al., 2009).
Symptoms of PPD are fatigue, a sense of irritability, anxiety, lack of pleasure, the feeling of helplessness, sleep and appetite disturbances, indifference towards life events, lack of self-esteem and feeling incompetent as a parent, depressed mood, feelings of guilt or worthlessness, trouble concentrating, suicidal tendencies, etc. (Beck, 2008, Slomian et al., 2019). PPD must be differentiated from postpartum blues, also called maternity blues, which is a common but self-limiting condition having overlapping symptoms with PPD. It sets on immediately (one to three days) after parturition and is presented with clinical symptoms like mood swings, irritability, unexplained weeping, impatience, anxiety, crying spells, feelings of loneliness and vulnerability, sadness, guilt, insomnia, agitation, confusion, depression, and suicidal tendencies in severe cases (Duan et al., 2018, Payne and Maguire, 2019). In such manifestations, supportive treatment including emotional assistance and adequate sleep is ensured. If the condition becomes severe, lasting for more than two weeks, or affecting daily life activities then the individual should be assessed for symptoms related to postpartum psychiatric conditions (Burt and Quezada, 2009).
Women experiencing PPD typically struggle with a sense of loss of control. They go through a four-stage process to try to regain control. In the initial stage, mothers have terrible worry, unceasing obsessive thoughts, and difficulties focusing. In the second stage, women feel as though their “regular selves” have “gone”. While providing for their infants, they feel like robots going through the motions. Mothers frequently withdraw themselves at this point and may start thinking about self-harm or suicide. In the third stage, women plan their strategies for overcoming PPD, including turning to healthcare providers, praying, finding comfort in support groups, etc. As their despair lifts, women eventually take control of their thoughts and feelings in the final stage (Beck, 1993, Beck, 2007).
The patient must exhibit at least four of the following symptoms from the list for it to be determined that they have PPD: reduced energy; feelings of guilt or worthlessness; difficulty in thinking, concentrating, or making decisions; or recurrent thoughts of death or suicidal ideation, plans, or attempts for at least two weeks; changes in appetite or weight (more than 5% in four weeks), sleep, and psychomotor activity (Quinn, 1999, Payne and Maguire, 2019). Clinicians can use the Postpartum Depression Screening Scale (PDSS) as a screening tool. This 35-item self-report scale evaluates the presence, seriousness, and type of PPD symptoms. Women are asked to respond to statements about how they have been feeling since giving birth using a five-point Likert answer format (Beck and Gable, 2003). Another tool designed to screen for postpartum depression is the Edinburgh Postnatal Depression Scale (EPDS). It comprises 10 items with a Likert scale that rate the symptoms of depression that are frequently experienced (Cox et al., 1987). Lastly, a Structured Clinical Interview for DSM-IV Axis 1 Disorder can be used to formally diagnose PPD (First et al., 1997).
Historically, PPD was thought to be a manifestation of MDD. However, this notion has been re-evaluated in light of new research. In 2013, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition was updated to expand the repertory of PPD, to emphasize the need to distinguish between unipolar depression and bipolar depression subtypes (Sharma et al., 2018). During the postpartum phase, bipolar depression is found to be more common than unipolar depression (Sharma et al., 2018). Recent studies have shown that a significant percentage of women with PPD suffer from bipolar disorder (BD). Bipolar illness can be found anywhere between 21.4% and 54% of women with PPD, depending on the demographic investigated (Sharma et al., 2017). A 14% screen positive rate for depression was discovered by Wisner et al. in a ground-breaking study of 10,000 postpartum mothers. However, in this study, women who had a positive screen were diagnosed with unipolar depression in 68.5% of cases and bipolar depression in 22.6% of cases. Almost two-thirds of the women with unipolar depression also had concomitant anxiety (Wisner et al., 2013). Sharma et al. reported that hypomania (before and after delivery), atypical depression, psychosis, and antidepressant mood elevation as the subclinical symptoms of bipolar disorders (Sharma et al., 2010).
It's crucial to differentiate between unipolar and bipolar PPD in order to elaborate an optimal management and therapeutic strategy. Both unipolar and bipolar PPD meet the DSM-IV criteria, although bipolar PPD is more likely to have certain clinical traits such as psychosis, diurnal mood swings, hypersomnia, hyperphagia, and a higher number of brief depressed episodes. Failing to make the correct diagnosis of bipolar PPD can have major repercussions, including a higher risk of hospitalisation, a mother's decreased capacity to care for herself and her child, and, although uncommon, maternal suicide and infanticide. The mainstay of treatment for unipolar PPD is antidepressant medication. If women having bipolar PPD take antidepressants, it can result in treatment-emergent mania or hypomania, accelerated cycle frequency, and treatment resistance (Sharma and Sharma, 2012). Women experiencing bipolar depression can be given antipsychotics and benzodiazepines in addition to mood stabilizers whenever required (Sharma et al., 2010). Untreated, undertreated and incorrectly treated PPD is the cause of 5–20% of postpartum suicide among mothers (Wisner et al., 2013), hence the need to treat PPD adequately or to prevent it altogether.
In our understanding, PPD is a debilitating but treatable condition (Koh et al., 2016). Numerous studies on nutrition and mental health have attracted the attention and interest of many researchers (Lai et al., 2014). The development of diet-based therapy techniques to prevent PPD in women who are at high risk of acquiring it and to treat those who already have it has been the subject of intense debate over the past few decades. Findings from previous studies have indicated that nutrients are necessary for the production of neurotransmitters and that they play a biochemical role in the nervous system; as a result, they may affect mood stability (Sparling et al., 2017). Nutrient deficiencies may arise more frequently during the postpartum period because nutrient demands on a woman's body can increase during pregnancy and nursing. (Sánchez-Villegas et al., 2011). So far, only a handful of studies have examined the connection between dietary intake and/or serum concentrations of vitamins, minerals, and antioxidants during pregnancy and the prevalence of PPD. More studies are therefore required in this area to ascertain the hypothetical association between dietary intake and postpartum depression. Therefore, this present review aims to investigate the etiology, and pathophysiology of PPD as well as the impact of nutrients on PPD’s prevention and management.
2. Aetiology of PPD
All major depressive disorders (MDDs), including PPD, are caused due to an intricate interaction between environmental, genetic, biological, social, and psychological factors (Meltzer-Brody, 2011). The exact etiological mechanism of PPD remains unknown so far (Aoyagi and Tsuchiya, 2019) but various studies have reported associations between multiple biological factors and PPD. We have presented the most prevalent biological factors associated with PPD in this review.
2.1. Oestrogen, Progesterone and their derivatives
The dramatic fluctuations in ovarian hormones during pregnancy and the postpartum period influence PPD incidence (Aoyagi and Tsuchiya, 2019). During pregnancy, levels of progesterone are twenty times higher compared to non-pregnant women, and these levels remain increased throughout the gestational period. At the same time, oestradiol levels are two to three hundred times higher by the twentieth week of conception and they stay elevated throughout gestation. After childbirth, hormones drop significantly (Brett and Baxendale, 2001). The neurotransmitter activity that contributes to increased serotonin synthesis and decreased serotonin breakdown is effectively increased by oestrogen (Hendrick et al., 1998). Therefore, a sudden decrease in progesterone and oestrogen could lead to PPD by reducing serotonin levels (Corwin and Pajer, 2008, Hendrick et al., 1998). During a study in which researchers raised levels of ovarian hormones in pregnant rats, a depression-like behaviour was observed in the subjects (Stoffel and Craft, 2004). Similarly, another study showed anhedonia as a result of simulating oestradiol withdrawal in rodents (Schiller et al., 2013). When progesterone withdrawal was simulated in another study on rats, depression-like behaviour was observed once again (Beckley and Finn, 2007). In a controlled study conducted by Bloch et al., which involved hormonal manipulation (inducing supraphysiological levels of oestrogen and progesterone first and then creating a withdrawal state) in sixteen women, it was found that gonadal steroid hormones oestrogen and progesterone were involved in the incidence of PPD in the study group. However, women who experienced PPD in the past were differentially sensitive to the mood-destabilizing effects of oestrogen and progesterone (Bloch et al., 2000).
2.2. Cortisol
In addition to reproductive steroids, glucocorticoids could also play a role in the occurrence of PPD. It was observed that in women suffering from PPD, HPA axis regulation is dysfunctional, responsiveness to dexamethasone is reduced and the ratio of adrenocorticotropic hormone (ACTH) to cortisol is completely altered (Jolley et al., 2007). When cortisol is secreted excessively, or when there is an abnormal diurnal secretion of the same, it points to HPA axis dysfunction - a common condition found in patients suffering from depression (Glynn et al., 2013, Jolley et al., 2007). High levels of cortisol are observed in women during the gestational and postpartum periods as well as in non-pregnant patients who suffer from depressive disorders (Glynn et al., 2013). Restoring normalcy and balance in HPA axis function is one of the strategies in pharmacotherapy to treat depressive disorders (Ising et al., 2007). High levels of corticotropin-releasing hormone (CRH) are considered to be potent biomarkers used in the detection of PPD (Yim et al., 2009).
2.3. Oxytocin
Oxytocin (OT) is thought to be a potential biomarker for depression (Kim et al., 2014). In pregnant women, endogenous oxytocin levels increase throughout gestation and reach the peak immediately around childbirth in order to assist uterine contractions (De Geest et al., 1985, Prevost et al., 2014). From a study performed on nulliparous, non-pregnant, non-lactating female rats, we understand that oxytocin is also an inhibitor of the HPA axis in response to stress (Neumann et al., 2000). In pregnant women who are at risk of PPD, it is observed that during the third trimester of gestation, plasma oxytocin is present in lower concentrations (Skrundz et al., 2011, Stuebe et al., 2013) However, it does not mean that administering oxytocin to these women treats depression; a study found that administering OT to subjects suffering from PPD resulted in a low mood in subjects (Mah et al., 2013).
2.4. Thyroid hormones
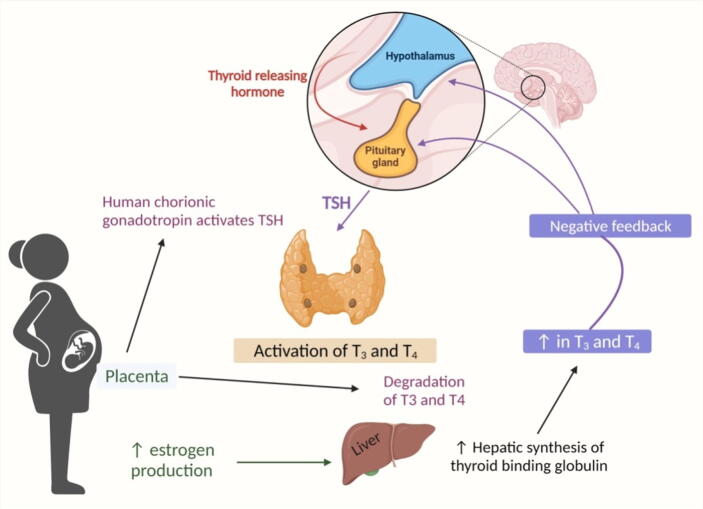
Thyroid hormones are important during pregnancy because they play a role in placental growth and function, as well as in the production of neuropeptides at the beginning of parturition (Barjaktarovic et al., 2017, Ramadurai et al., 1998). These hormones are also involved in psychological disorders occurring during the postpartum period, which is not a surprise because they are strongly linked with depression along the hypothalamic-pituitary-thyroid (HPT) axis (Meltzer-Brody et al., 2018). As a consequence of high sensitivity to oestrogen, TBG (or Thyroxine-binding globulin) levels rise and the synthesis of thyroid hormones also increases up to 50% approximately during gestation in euthyroid women (Gaberšček and Zaletel, 2011). A cohort study conducted on 199 euthyroid subjects showed that a fall in the level of TBG puts women at risk of developing perinatal depression (Pedersen et al., 2007). In addition, it is considered that autoimmune thyroid disorders which are triggered by pregnancy lead women to the risk of developing PPD. Evidence suggests that women showing high levels of thyroid peroxidase antibodies during the gestational period demonstrate a high risk of developing psychological disorders (Meltzer-Brody et al., 2018). Fig. 1 demonstrates the activation of T3 and T4 hormones and the negative feedback mechanism involved.
Fig. 1.
Activation of T3 and T4 hormones and negative feedback mechanism involved in PPD.
2.5. Prolactin
Prolactin is a peptide hormone released in women from the anterior pituitary gland. Its purpose is to stimulate the secretion of milk after childbirth. Levels of prolactin increase during pregnancy and by the end of the gestational period they are ten to twenty times higher than normal levels (Freeman et al., 2000). On the one hand, the increase in prolactin concentration is directly linked to the increase of concentration in oestradiol levels, and on the other hand, progesterone antagonizes the effects of prolactin (Freeman et al., 2000). After childbirth, when there is a reduction in both progesterone and oestrogen, prolactin concentration increases drastically. It then comes down to a nulliparity level within two to three weeks postpartum, in cases of women who do not breastfeed (Cooper, 2001). The relationship between PPD and prolactin has been ambiguous for a long time. In 1994, Heidrich et al. examined twenty six breast-feeding women and established a weak correlation between variations in prolactin levels during the first three days after childbirth and mood scores on “day 3” after childbirth (Kumar and Magon, 2012). In contrast, a study performed by Abou-Saleh in 1998 demonstrated that women with PPD had lower plasma prolactin levels in comparison with women who didn't show signs of PPD (Abou-Saleh et al., 1998). It was also noted that breastfeeding women had higher prolactin levels and better mood scores (Grattan, 2001). In addition, a recent study conducted in 2022 has shown that women suffering from PPD have an prplactin mediated increased regional gray matter volume (rGMV) in the left dorsolateral prefrontal cortex and the right anterior insular when compared with healthy women after childbirth. Around eighty six patients with PPD and seventy four healthy subjects were studied in this study using Magnetic Resonance Imaging to determine the relationship between regional gray matter volume and PPD (Cheng et al., 2022). This finding highlights possible prolactin level-mediated impact on rGMV and on brain structure. Therefore, further studies are required to investigate the relationship between prolactin and PPD in a detailed manner.
2.6. Genetics in postpartum depression
Only a small number of studies addressed genetic and epigenetic factors associated with PPD so far. Nevertheless, one may confirm that PPD has a genetic link based on a research done on twin pairs and other investigations done on members of the same families. (Corwin et al., 2010, Forty et al., 2006, Treloar et al., 1999). Genome-wide association studies have also pinpointed specific candidate genes and probable PPD pathway involvement. Serotonin transporter, tryptophan hydroxylase-2 (TPH2), catechol-O-methyl transferase (COMT), monoamine oxidase (MAO), and Brain-Derived Neurotrophic Factor (BDNF) are just a few examples of the genes that have been the focus of candidate gene investigations. Notably, HPA axis involvement and oestrogen signaling are suggested by pathway analyses based on candidate genes or unbiased screens. Costas et al. (Costas et al., 2010) conducted the largest genetic study of PPD on 1,804 mothers from Spain, showing the association of forty four genes with 508 polymorphisms. These genes play an important role in PPD-related clinical manifestations, involvement in HPA axis regulation, sex hormone regulation, and stress effect on the prefrontal cortex region of the brain. Single nucleotide peptides at the transcriptional site of the enzyme kininogen 1 were discovered to be at a considerable level in the case of depression. These sites can be of great interest for future research into PPD. Table 1 represents the implications of genetics in causing PPD (see Table 1).
Table 1.
Genetic implications in Postpartum Depression.
| S. No. | Genes involved | Implications in PPD | References |
|---|---|---|---|
|
1 |
5-HTTLPR (5HTT-linked polymorphic region) |
Polymorphism of the serotonin transporter (5- HTT) gene: serotonin transporter removes the neurotransmitter serotonin from the synaptic cleft. It determines the duration as well as the magnitude of post-synaptic serotonin signals, implicated in psychiatric disorders like depression. |
(Lesch and Mössner, 1998, Ogilvie et al., 1996) |
| 2 | MAO-A (uVNTR) and COMT (Val158Met; Met carriers) polymorphisms | Allelic variations in two other genes of the monoaminergic transmission system were found to be associated with major depression, like catechol-O-methyl transferase (COMT), which is involved in the metabolism of dopamine, noradrenaline and monoamine oxidase involved in the metabolism of serotonin and nor-adrenaline. |
(Doornbos et al., 2009, Mandelli and Serretti, 2013, Pinsonneault et al., 2013) |
| 3 | ESR 1 (Estrogen Receptor Gene) | Estrogens are implicated in serotonin transmission. The interaction between 5-HTT (5- HTTLPR) and is ESR1 found to be involved with PPD symptoms. |
(Costas et al., 2010, Pinsonneault et al., 2013) |
| 4 | e C/C variant (rs2740210) and G/G variant (rs4813627), three polymorphisms on the oxytocin peptide gene and oxytocin receptor gene. | The oxytocin receptor gene and oxytocin peptide gene are found to be associated with PPD symptoms |
(Mileva-Seitz et al., 2013) |
| 5 | BclI (C/G) and CRH receptor CRHR1 (A/G), glucocorticoid gene corticotropin releasing hormone gene polymorphism | Three polymorphisms of the CRH receptor gene and two polymorphisms of glucocorticoid receptor genes are involved in two to eight weeks of PPD. |
(Engineer et al., 2013) |
| 6 | BDNF polymorphism Val66Met | BDNF performs the neuronal function of regulating synaptic plasticity. |
(Comasco et al., 2011, Martinowich and Lu, 2008) |
| 7 | Per2 10,870 (Period 2 gene) | Performs an important function in the regulation of circadian rhythm. No association was observed between PPD and polymorphism in Per2 10870. |
(Comasco et al., 2011) |
2.7. Epigenetics in postpartum depression
The heritability of PPD was discussed in the section before, with an emphasis on candidate genes related to the likelihood of causing depressive symptoms during the postpartum period. In this section, we present epigenetic alterations involved in PPD. The epigenetic causes of PPD have drawn more attention in recent years. The study of potentially heritable chemical alterations to DNA and histone proteins, which can alter gene expression without altering the underlying DNA sequence, is known as epigenetics. Along with genetic influences, it's probable that epigenetic influences, which are changes in gene expression not caused by changes in DNA sequences, but rather by changes in chromatin structure (methylation or histone modifications that affect gene transcription), also come into play in PPD manifestation. Environmental factors cause epigenetic alterations in gene expression, which shows how environment and genetics interact. From a therapeutic perspective, the epigenetic mechanisms that control gene expression are significant because they foretell the modification of biological systems linked to mood disorders like PPD. Table 2 represents the implications of epigenetics in causing PPD by presenting studies that showed a positive association between epigenetic alterations and PPD (see Table 2).
Table 2.
Epigenetic implications in Postpartum Depression.
| S. No. | Genes involved | Epigenetic Implications | References |
|---|---|---|---|
| 1 | Oestrogen-mediated DNA methylation: HP1BP3 and TTC9B |
Elevations in oestrogen mediated DNA methylation change were observed in PPD diagnosis of women within four weeks postpartum and genes HP1BP3 and TTC9B were reported as significant biomarkers. |
(Guintivano et al., 2013) |
| 2 | HSD11B2, CRHR2 and SLC6A4 | Positive associations between maternal stress and HPA axis gene expression (HSD11B2, CRHR2, and SLC6A4) were observed in a human study involving 50 participants. |
(Chen et al., 2014) |
| 3 | OXTR | Being depressed both throughout pregnancy and after delivery is connected to considerably increased OXTR methylation in exon 3. When depression occurs for a longer duration, OXTR gene expression may be inhibited. | (King et al., 2017) |
| 4 | CYBA and PRKCZ | The umbilical cord blood (UCB) of mothers who experienced depression in the middle of their pregnancy had lowered methylation at the CYBA gene (cg08667740) and PRKCZ (cg22868225) according to research by Viuff and colleagues. Inflammatory responses are influenced by this gene. |
(Viuff et al., 2018) |
| 5 | FOXp3, CLOCK, CRY1, PER1 and PER2 | A study that involved 44 pregnant women looked into the candidate genes FOXp3 (essential for the function of regulatory T-cells and immune system homeostasis), CLOCK, CRY1, PER1, and PER2 (responsible for circadian rhythm), and found that depressed women had hypermethylation of these genes. The same study showed hypomethylation of CRY1, PER1, and PER | (Buoli et al., 2019) |
3. Pathophysiological implications in PPD
3.1. Neuroplasticity around pregnancy
Pawluski et al. (Pawluski et al., 2016) demonstrated significant plasticity during pregnancy in the hippocampus of the mammalian brain that contributes to cognitive changes throughout the reproductive period. When examined, a study found a reduction in gray matter volume (GMV) during the post-natal period when compared with the pre-pregnancy period (Hoekzema et al., 2016). In comparison to the pre-pregnancy brains, post-natal brains are similar to adolescent brains. They present vast cerebral-morphometric changes involving a decrease in cortical thickness, surface area, and volume. Also, a decline in the sulcal depth and increased sulcal width were observed. All these observations suggested hormone-associated cortical adaptations during pregnancy and adolescent periods (Carmona et al., 2019). Along with structural changes, the maternal brain also exhibits neuroplasticity during the post-natal period, which helps mothers in adapting to the transition to motherhood and to cope with parenting stressors (Lonstein et al., 2015).
3.2. Neuroendocrinology and immune changes around pregnancy
A rapid rise in the level of gonadotropin hormone was observed during early pregnancy produced by the placental cells’ trophoblasts (Haavaldsen et al., 2014). Progesterone hormone secretion increases in the first trimester of pregnancy and remains elevated until the late gestation period. Its level declines after delivery. Additionally, glucocorticoid secretion increases during the first trimester before reverting to normal and then increases again during the third trimester. (Venning, 1955). Parturition-related changes in neuroendocrine levels are distinct from the changes observed during pregnancy. Oxytocin level is high at the onset of delivery as this hormone plays a major role in the initiation as well as the expulsive phase of delivery (McQuaid et al., 2014). Neuroendocrine alterations during the lactation period are also distinct from pregnancy and parturition periods. Increased levels of prolactin hormones play a major role in the development and functioning of mammary glands. Copulation triggers prolactin's release, and in the ovary, it safeguards the corpus luteum, which secretes progesterone in the first trimester of pregnancy. Prolactin levels are also found to be low during mid-pregnancy, due to a short loop negative feedback mechanism inhibiting hypothalamic prolactin release (Grattan and Kokay, 2008).
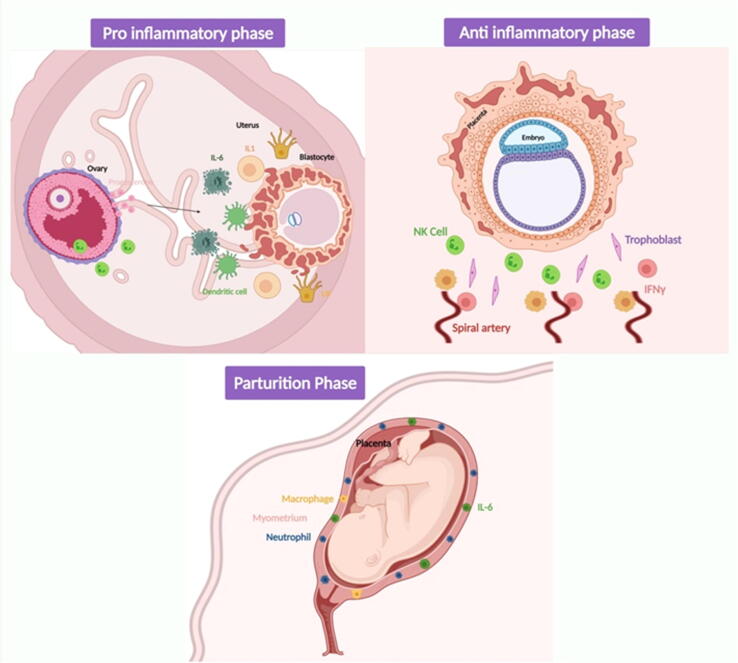
Pregnancy is a period marked by changes in the functioning of the immune system. Initially, it was thought to inhibit the immune function which is important for allowing the implantation of the foetus and its growth. Evidence suggesting that progesterone exhibits immunosuppressive actions supported this theory. However, Mor et al. reported that the immune system is normal and active during pregnancy (Mor et al., 2011). The immune system during this period functions to protect the mother and the foetus and it is unique during the peripartum period. Immune cells like regulatory T cells (Treg), natural killer cells, and macrophages invade and accumulate in the decidua around the trophoblast where they support the rest of the cells. The depletion of such immune cells results in the termination of pregnancy because of the deleterious effects on placental development. Therefore, pregnancy involves three distinguished immunologic phases: (1) Pro-inflammatory phase i.e. implantation and placentation during early pregnancy; (2) Anti-inflammatory phase i.e. growth and development of the foetus requiring a supportive immune system; and (3) Parturition phase i.e. requiring strong immune support (Koga and Mor, 2010), as represented in Fig. 2. Maes et al. (Maes et al., 2000) investigated the association between postnatal blues and inflammatory response system activation. They performed the assay of interleukin IL-6, receptor IL-6R, signalling protein gp 130, receptor antagonist IL-1RA, and leukaemia inhibitory factor receptor in a serum sample of twenty-two non-pregnant women and ninety-one pregnant women, before delivery and one or three days after delivery. It was observed that females who are pregnant have significantly higher levels of serum IL-1RA, IL-1RA, and LIFR. Another similar study shows a significant level of inflammatory biomarkers involving C-reactive protein (CRP) at different testing times before and after delivery that predicts postpartum depression. Their study shows an interaction between the corticotropic axis and inflammation, which explains the onset of PPD (Lambert and Gressier, 2019).
Fig. 2.
Distinguished immunologic phases during pregnancy i.e. Pro-inflammatory, Anti-inflammatory, and Parturition Phase.
3.3. Gut – Brain axis, microbiota and PPD
The gut-brain axis does bidirectional signalling between the brain and the gut. It is regulated by the autonomic nervous system, enteric nervous system, neuroendocrine system, and neuro-immune system (Dinan and Cryan, 2017). There is an “Old Friend Hypothesis” which states that mammals have evolved with a range of microorganisms like bacteria, fungi, archaea, and viruses that play an important role in developing the host immune system (Rook et al., 2014). In accordance with this theory, a seminal study compared germ-free mice with conventionally raised mice and showed a relation between intestinal bacteria and brain development, especially in the hypothalamus (Sudo et al., 2004). Another study observed the association of the microbiome with the development of the immune system and social behaviour (Rook et al., 2014). Observations from these studies support the symbiotic hypothesis that the host gut microbiome helps in developing immune system as well as neurological system. Several experimental models including humans and rodents displayed the connection between gut microbes and depression (Cheng et al., 2022, Heidrich et al., 1994, Abou-Saleh et al., 1998).
During pregnancy, many hormonal, immunological, and metabolic changes occur in order to support the developing foetus. As discussed previously, there is a sudden increase in the level of hormones like oestrogen and progesterone. Major alterations in the immune system also take place. Immunomodulation is necessary during this phase. On the one hand, the immune suppression is required to support the growth of the foetus having its developing immune system and on the other hand immune response is required to prevent the mother and foetus from infections. Correlating the alterations in the endocrine, immune, and metabolic systems, some noticeable changes in gut microbiota were observed during pregnancy. Research studies suggested that an increase in the bacterial load in the large intestine as well as profound changes in gut microbe’s composition is characteristic of a healthy pregnancy (Koren et al., 2012). Koren et al. observed dramatic changes in gut composition observed from first trimester to the third trimester. The Actinobacteria and Proteobacteria phyla were abundant, but individual richness was found to be decreasing. In addition, the level of butyrate-producing gut bacteria i.e. Faecalibacterium declined vastly in the third trimester (Koren et al., 2012).
A previously conducted study investigated the correlation between gut microbiota and PPD in fifty seven subjects where faecal samples of twenty eight subjects (with PPD) and sixteen healthy subjects were collected and analysed by 16S ribosomal high-throughput gene sequencing (Zhou et al., 2020). They analysed the diversity and composition of gut microbiota in subjects displaying PPD as compared to healthy subjects. A decline in the level of abundant bacteria such as Firmicutes phyla, Faecalibacterium, Butyricicoccus, Phascolarctobacterium, and Lachnospiraceae was observed in PPD subjects when compared to healthy subjects. In addition, the level of Enterobacteriaceae was found to be higher in subjects with PPD.
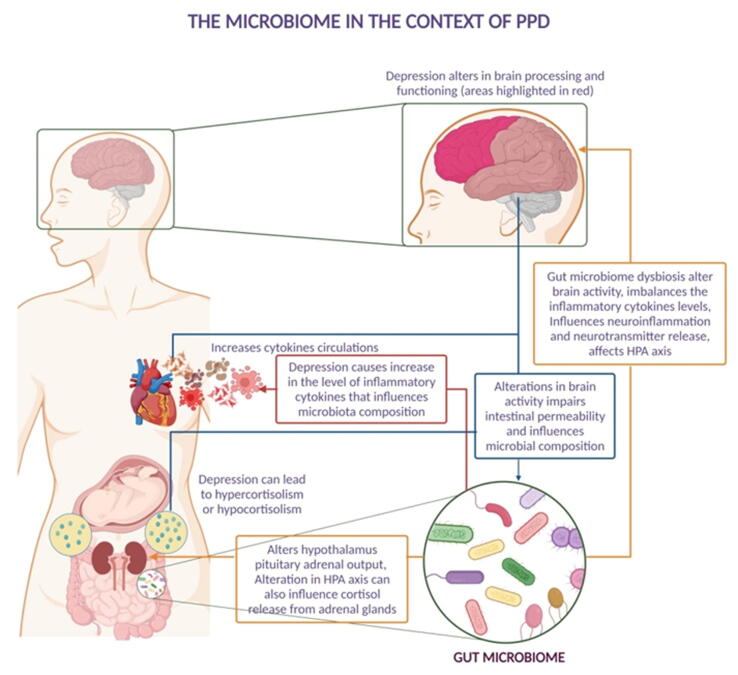
Various research studies suggested that alterations in the gut microbiome axis and dysbiosis of gut bacteria were associated with PPD (Jiang et al., 2015). Fig. 3 depicts the effects of bidirectional gut-brain axis communication on depression, which includes the endocrine system, central nervous system, and immune system. Alteration in the gut microbial community impairs the metabolism of amino acids such as tryptophan, influences neuroinflammation, and affects the hypothalamus and pituitary axis activity. All these impairments ultimately affect the brain activity in threat, rewards, executive functioning, and self-reflective processes. This leads to an increased level of cortisol hormone (Duan et al., 2018).
Fig. 3.
Illustrated inter-relation between gut microbes and the impact of gut dysbiosis on the neuroendocrine system during pregnancy.
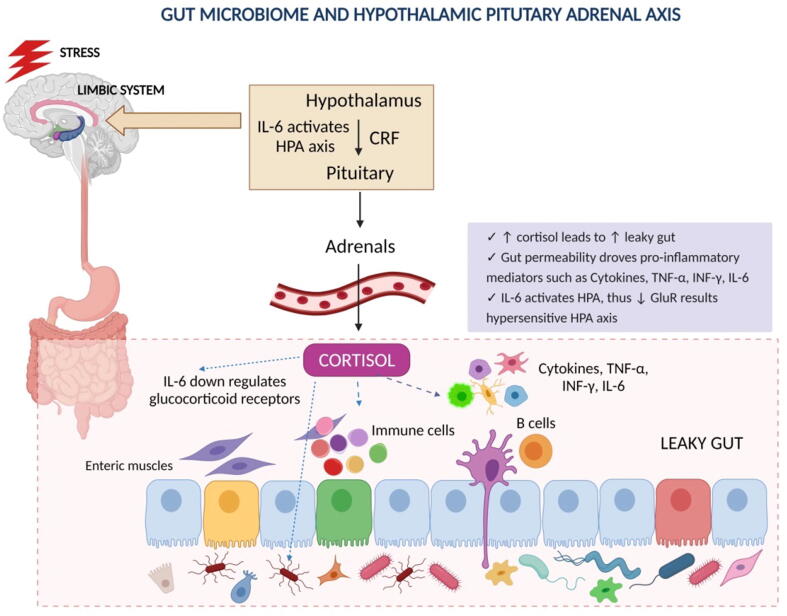
Gut microbiota alterations can also produce imbalanced inflammatory cytokines, which shift the balance of immune cells by producing antibodies against microbiota (Fig. 4). This increased level of inflammatory cytokines can also contribute to PPD (Irwin and Miller, 2007). Gohir et al. investigated the influence of maternal peri-conceptional diet on pregnancy-related changes in the gut microbiota of in female C57BL/6 mice (Gohir et al., 2015). They suggested that pregnancy-induced changes in female gut microbiota that occur immediately after conception are vulnerable to diet modulation. High-fat diet supplements before and after pregnancy cause shift in pregnant gut microbiota in a gestational age-dependent manner. They favour glycolysis, lipid metabolism, and gluconeogenesis.
Fig. 4.
The effect of stress on the HPA axis causes an increased level of cortisol which affects the permeability and elevated the levels of pro-inflammatory mediators.
4. Role of dietary supplements and nutrients in the treatment of PPD
Nutrition is the study of food science and its relation with health (Madeghe et al., 2022). Numerous studies demonstrated that certain dietary products and nutrients have antidepressant effects through a variety of mechanisms. These mechanisms include anti-inflammatory effect, antioxidant effect, boosting the production of monoamine neurotransmitters, reducing the hyperactivity of the HPA axis, regulating the microbiota-gut-brain axis, etc. (Luo et al., 2021, Ren et al., 2022, Wu et al., 2022, Zhou et al., 2022). Nutrition also influences the HPA axis as well as the psycho-neuro-immunological links (Bodnar and Wisner, 2005). An interesting, yet vastly underexplored factor in PPD is the nutritional status of women during the perinatal period (Ellsworth-Bowers and Corwin, 2012). During pregnancy, the nutritional requirements of a woman constantly change according to the development of the foetus and the mother’s wellbeing. It is also important to keep in mind that excess food intake may lead to obesity, which harms the offspring’s cognitive development and the mother's mental health (Lindsay et al., 2012, Schoretsanitis et al., 2021). Recent studies have demonstrated that malnutrition and or deficiencies in some nutrients such as B and D vitamins, n-3 polyunsaturated fatty acids (PUFA), trace minerals, folate, iron, antioxidants, etc. increase the risk of PPD occurrence (Ellsworth-Bowers and Corwin, 2012, Miller and LaRusso, 2011). It was also observed that serum cholesterol levels and plasma tryptophan levels are inversely correlated with PPD and serum vitamin D levels are associated inversely with perinatal depression (both before and post-childbirth) (Trujillo et al., 2018). According to a 2010 study by De Wit et al., a long-term high-fat diet and the consumption of fatty foods may contribute to mood problems by raising the risk of obesity, particularly in women (De Wit et al., 2010). Contrastingly, few studies have shown that certain fatty acids can change the brain's lipid structure and affect emotional behaviour; as a result, consuming diets low in certain fatty acids over time may be linked to mood disorders. In an experimental study, it was discovered that a mouse brain incubated in cholesterol hemi succinate can increase serotonin's binding to its receptor (Brinkworth et al., 2009, Heron et al., 1980, Lindseth and Petros, 2016, Moran et al., 2013). Reduced activity of the serotonergic system is known to contribute to the occurrence of PPD (Baek et al., 2012). A major section of the research investigations involving PPD and nutrition have thus far evaluated the association between diet and PPD from the perspective of dietary patterns and neurobehavioral impacts. A smaller set of studies were also conducted to identify the specific impact of different biological nutrients on the pathophysiology of PPD. Table 3 summarises all the studies that were taken into consideration in order to understand the association between nutrients and PPD (see Table 3). However, at the time of writing there isn't substantial, statistically relevant evidence that establishes the link between dietary nutrient intake and PPD.
Table 3.
Characteristics of the studies considered to examine the role of nutrients in PPD.
| S. No. | Nutrient | Country, Investigators, Year | Inclusion/Exclusion criteria | Reference |
|---|---|---|---|---|
| Micronutrients | ||||
| Vitamin B | ||||
| 1. | Assessment of longitudinal associations between prenatal vitamin intakes of vitamins B6, B12, and folate, maternal red blood cell folate status, and eventual PPD symptoms (n = 2856) |
UK, Blunden et al., 2012 | Women between the ages of 20 and 34 who were not pregnant at the time of the interview were recruited | (Blunden et al., 2012) |
| 2. | Studying the connection between the risk of PPD and dietary intake of folate and B vitamins during pregnancy. (n = 865) |
Japan, Miyake et al., 2006 | Women in Neyagawa City who were pregnant between November 2001 and March 2003 were recruited and women with missing data on the infant’s birth weight were eliminated. | (Miyake et al., 2006) |
| Vitamin D | ||||
| 3. | Determining whether vitamin D levels and PPD symptoms are related, as well as whether blood 25(OH)D levels may be used to anticipate the occurrence of PPD symptoms. (n = 97) |
USA, Murphy et al., 2010 | Women aged 18 to 45 years who: delivered an infant, were at least 35 weeks at gestation and were 4 to 6 weeks postpartum at the initial study visit, spoke English or Spanish as their primary language, and exclusively breast-fed or formula-fed their infant for the entire study period were included. If a participant had multiple births, type 1 or type 2 diabetes, hypertension, parathyroid disease, or uncontrolled thyroid disease, they were disqualified from the cohort. | (Murphy et al., 2010) |
| Tryptophan | ||||
| 4. | Evaluating the possible role of the serotonin system during postpartum days 3–5 through an assessment of brain tryptophan availability. (n = 50) |
France, Baïlara et al., 2006 | Pregnant women admitted at (Maternity C, CHU of Bordeaux) just before delivery were recruited. Obstetrical problems and surgical delivery were exclusion criteria, and pathological pregnancies were not taken into consideration for inclusion. | (Baïlara et al., 2006) |
| 5. | Evaluating the postpartum period's changes in tryptophan and its catabolic product, kynurenine, and compare them to neopterin as an immunological marker. (n = 95) |
Austria, Kohl et al., 2005 | Participants in the study were women who were admitted for birth to the Department of Obstetrics and Gynecology at the University Clinic of Innsbruck between June and November 2000. The following were listed as exclusion criteria: immune system-altering illnesses or medications, infection symptoms, abnormalities during pregnancy, preterm delivery, Caesarean sections, forceps deliveries, abnormally shaped new-borns, inadequate German language proficiency, and a history of psychiatric illnesses. | (Kohl et al., 2005) |
| Zinc | ||||
| 6. | Examine the relationship between Zn supplementation and PPD, which is indicated by an Edinburgh Postnatal Depression Scale (EPDS) score below 9, as well as the impact on postpartum women's haematological status. (n = 197) |
Japan, Aoki et al., 2022 | According to Nagoya University Hospital records, women who underwent caesarean sections and experienced postpartum anaemia were included in the study. Excluded cases were those without anaemia, those with comorbidities such as autoimmune diseases or severe postpartum haemorrhages requiring blood transfusions, as well as those with insufficient medical records. | (Aoki et al., 2022) |
| Selenium | ||||
| 7. | Investigating the impact of prenatal selenium supplementation on Iranian women's PPD levels using a randomised, double-blind, placebo-controlled study design. (n = 166) |
Iran, Mokhber et al., 2011 | The study included women who visited the Obstetrics and Gynecology Department of OM-Albanin Hospital (Mashhad, Iran) between June 2006 and August 2008. The inclusion criteria were primigravid women with a live foetus, gestational ages up to 12 weeks, no major medical or mental illness, and no signs that the pregnancy should be terminated. The use of any medicines, but not commonly prescribed doses of folic acid and ferrous sulphate, as well as the incidence of any serious illness or stressful life events as measured by the Holmes and Rahe stress scale, were considered exclusion criteria. | (Mokhber et al., 2011) |
| Macronutrients | ||||
| Carbohydrates | ||||
| 8. | A randomised clinical trial was conducted to see if treating pregnant women with gestational diabetes lowers the risk of perinatal complications. (n = 1000) |
UK, Crowther et al., 2005 | Women who met the following requirements were included: they had a singleton or twin pregnancy between 16 and 30 weeks gestation, they went to antenatal clinics at the partner hospitals, they had one or more gestational diabetes risk factors on selective screening or a positive 50-g oral glucose challenge test (glucose level one hour after glucose challenge at least 7.8 mmol per litre [140 mg per decilitre]), and they had a 75-g oral glucose tolerance test at 24 to 34 weeks. Women with ongoing chronic systemic diseases (other than essential hypertension) or previously treated gestational diabetes were not allowed to participate. | (Crowther et al., 2005) |
| PUFA | ||||
| 9. | Assessing the associations between seafood consumption, the DHA content of mothers' milk, and prevalence rates of PPD through a cross-national, ecological analysis | USA, Hibbeln et al., 2001 | Prevalence studies were considered if they met the following criteria: (1) they showed excellent selectivity and reported prevalence rates of significant postpartum validation studies; (2) they used the EPDS instrument to assess PPD; (3) they were published as primary data; and (4) they reported appropriate sampling and data analysis methodology. One study was disregarded because it looked at depression in moms of unwell infants. | (Hibbeln, 2002) |
4.1. Nutritional demand during pregnancy
Pregnant women are at high risk of developing many diseases like gestational diabetes, hypertension, and depressive disorders due to the vulnerability induced in them by pregnancy-related bodily changes. They are also prone to nutritional deficiencies which can cause several biochemical and physiological alterations that can in return affect their health (Bodnar and Wisner, 2005). Nutritional needs rise throughout pregnancy and lactation to sustain both the metabolism and development of the mother's reproductive tissues as well as the growth and development of the foetus and new-born. Even though the method of summation is occasionally used to calculate estimates of recommended nutrient intakes, the total amount of nutrients needed is not always equal to the simple sum of nutrients accumulated in maternal tissues, pregnancy and lactation by products, and nutrients needed for the maintenance of non-reproducing women. Both pregnancy and breastfeeding are anabolic states that are controlled by hormones to create the rerouting of nutrients to the placenta and mammary gland (which are highly specialized maternal tissues characteristic of reproduction) and their transfer to the growing foetus or new-born. When the demand for essential nutrients is unmet, mothers experience a depletion of nutrient stores.
The estimation of nutritional requirements during pregnancy is challenging because hormone-induced changes in metabolism, shifts in plasma volume, changes in renal function, and changes in patterns of urinary excretion typically alter nutrient levels in tissues and fluids available for evaluation and interpretation. Although total circulating quantities can be substantially increased, rising plasma volume frequently results in lower nutrient concentrations in blood and plasma. While individual profiles can vary greatly, in general, pregnant women have lower concentrations of water-soluble nutrients and metabolites than non-pregnant women do, but fat-soluble nutrients and metabolites are found in similar or higher quantities. Homeostatic control mechanisms are poorly understood and abnormal modifications are not properly defined. The inadequate concentration of nutrients in both blood and the brain alters the neurotransmitter levels and causes depression (Sparling et al., 2017). Rechenberg and Humphries thus concluded that women’s diet during pregnancy should be balanced with optimum nutrition containing both macro and micronutrients to prevent nutritional inadequacies (Rechenberg and Humphries, 2013).
According to current estimates, the energy needs of a pregnant woman are determined by adding together a non-pregnant woman's total energy expenditure, the median change in total energy expenditure of 8 kcal/gestational week, and her daily energy deposition of 180 kcal/d. It is advised to increase energy intake only in the second and third trimesters due to the fact that overall energy expenditure does not alter significantly during these times and weight growth is limited. Throughout the second and third trimesters, it is advised to consume an additional 340 and 450 kcal, respectively (Picciano, 2003, Trumbo et al., 2002). The theoretical daily requirement of protein during pregnancy is 71 g. To make up for the estimated 21 g/d of protein that is deposited in foetal, placental, and maternal tissues during the second and third trimesters, additional protein is required to be consumed. It is challenging to determine a woman's vitamin and mineral status during pregnancy because there aren't many pregnancy-specific laboratory indices for assessing a woman's nutritional needs. Many vitamins and minerals show a slow, steady decline in plasma concentrations as gestation progresses, which may be caused by haemodilution; however, other vitamins and minerals may be unaffected or even increase due to pregnancy-related changes in carrier molecule levels (Picciano, 2003).
4.2. Micronutrients
4.2.1. Vitamin B complex
Vitamin B complex contains eight water-soluble compounds having similar structures and acting as a coenzyme for various metabolic processes, especially in the nervous, haematological, and integumentary systems. They are found in a broad range of unprocessed food including leafy greens, salmon, liver and other organ meats, eggs, milk, oysters, clams, mussels, legumes, yogurt, etc. Their deficiencies can cause chronic diseases ranging from various types of anaemia (macrocytic caused by vitamin B6, 12, 9 deficiencies) to impairment of the nervous system (caused by B1, B12 deficiencies) and psychological disturbances (caused by B1, 3, 6, and 12 deficiencies). They do not have a direct impact on the HPA axis and immune system, but they manage the levels of homocysteine, a pro-inflammatory amino acid. When homocysteine is found in the body in excessive concentration (hyperhomocysteinemia), it leads to neurodegenerative diseases. To metabolize homocysteine and keep it in the normal range one must not present deficiencies of folate, riboflavin, B6, and B12 (Hoekzema et al., 2016, Lowensohn et al., 2016). B9 and B12 help homocysteine in converting into methionine and B6 condenses homocysteine into a precursor of cysteine (Ellsworth-Bowers and Corwin, 2012).
The role of vitamin B6 in amino acid metabolism makes it a rate-limiting cofactor in the synthesis of neurotransmitters like dopamine, serotonin, gamma-aminobutyric acid (GABA), noradrenaline, and the hormone melatonin. B6 also acts as a necessary cofactor in the folate cycle. The synthesis of these neurotransmitters is differentially sensitive to vitamin B6 levels; even a mild deficiency causes preferential down-regulation of GABA and serotonin synthesis, removing GABA's ability to inhibit neural activity and causing problems with sleep, behaviour, and cardiovascular function as well as losing the ability to control hormone excretion by the hypothalamus-pituitary (Kennedy, 2016). Vitamin B12 performs neurological functions and also acts as a cofactor in neurotransmitter formation and assists in the formation of red blood cells and maintenance of the nervous system (Ford et al., 2018). Yeast and milk are the main sources of vitamin B3, which is a form of nicotinamide riboside. Nicotinamide riboside altered the gut microbiome in a mouse model of alcohol-induced depression, which further reduced the expression levels of cytokines associated with inflammation and elevated BDNF levels in the hippocampus, therefore alleviating depressive symptoms (Jiang et al., 2020).
A strong association between mood and nutrition has been determined for vitamin B complex including vitamin B12, B6, folate; magnesium; iron; calcium; zinc and omega-3 fatty acid (Bodnar and Wisner, 2005, Kennedy, 2016). Leung and Kalpan reported that folate vitamin is required for the biosynthesis of three neurotransmitters i.e. nor-adrenaline, dopamine, and serotonin. Therefore deficiency of folate affects neurotransmitter production, resulting in depression (Leung and Kaplan, 2009). Folic acid is the synthetic form of folate. It is essential in the production of various proteins and blood components. It reduces the chances of developing neural tube defects (a defect in the development of the brain and spinal cord). Folic acid also plays an important role in several vital processes like the synthesis of DNA and RNA, amino-acid metabolism, and methylation of homocysteine (Mousa et al., 2019). The recommended daily intake of folic acid increases from 200 µg to 400 µg during pregnancy depending on how advanced the pregnancy is (Lowensohn et al., 2016). A previous study reported that depletion in this nutrient store and its non-recovery after childbirth increases the risk of developing PPD (Bodnar and Wisner, 2005).
However, in a study performed by Blunden et al., there were no discernible variations in red-cell folate concentration or dietary consumption of folate, vitamin B12, or vitamin B6 before or throughout pregnancy between those with PPD symptoms (n = 905) and those without (n = 1951) (Blunden et al., 2012). In addition, a study conducted in Japan on 865 Japanese women concluded that consumption of folate, cobalamin, or pyridoxine did not significantly decrease the incidence of PPD. Only riboflavin consumption in the third quartile was independently linked to a lower risk of PPD as compared to riboflavin intake in the first quartile (multivariate odds ratio: 0.53, 95% CI: 0.29–0.95, P for trend = 0.55) (Miyake et al., 2006).
4.2.2. Vitamin D
Vitamin D is a steroid hormone in nature and is synthesized by the skin when exposed to ul traviolet light. It becomes active by undergoing a hydroxylation reaction at first in the liver. Then specialized cells present in the brain, kidneys, and immune system perform a second hydroxylation reaction, producing calcitriol, the functional form of vitamin D (Borges et al., 2011, Kesby et al., 2011). Fortified dairy products, cereals, eggs, fish, and meat are the dietary sources of vitamin D. It works by activating nuclear receptors at the cellular level and thus contributes to cell cycling, cell differentiation, apoptosis, calcium transport, and bone remodelling (Kesby et al., 2011). Vitamin D has an impact on both the humoral and cellular immune systems. It was firstly observed in some animal models that vitamin D inhibits the activation of T helper (Th) cells and CD4 cells in autoimmune disorders (Garcion et al., 2002). Studies conducted later on proved that any shifts in vitamin D can cause a decrease in the level of inflammatory cytokines interferon-gamma (IFN-γ) and interleukin-2 (IL-2) produced by Th1 cells and increase the levels of cytokines like IL-4, 5, and 10 produced by Th2 cells. These cells in turn activate beta-cell production (Borges et al., 2011). Vitamin D also reduces the macrophage production of pro-inflammatory cytokines by activating the NF-kB of macrophages. In addition, vitamin D is found to interact with components of the HPA axis like the corticosteroid hormones. Generally, glucocorticoids prevent the cell differentiation of hippocampal cells and if they stimulate cells for longer periods, it can cause cell apoptosis. Whereas vitamin D performs two functions on glucocorticoid systems: when it is applied to hippocampal cells along with glucocorticoids, it causes changes in the morphology of the cells but when it is applied before the long-duration exposure to glucocorticoids, it decreases the apoptosis (Bravo et al., 2011, Lambert and Gressier, 2019).
Although there has been extensive study linking vitamin D deficiency to depression, the molecular mechanism is still largely unknown. The region-specific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, substantia nigra, amygdala, and hippocampus raises the idea that vitamin D plays a role in psychiatric illnesses. There is evidence that several of these areas also contain 1-hydroxylase enzymes, which can convert 25(OH)D to 1,25(OH)2D3, suggesting that vitamin D may have an autocrine or paracrine effect on the brain. Several studies have demonstrated the presence of vitamin D, its receptors (VDR), and associated enzymes (CYP 24A1, CYP 27B1) in various regions of the brain, pointing to a role for vitamin D as a neuroactive/neurosteroid hormone involved in critical functions like neuroprotection, neuroimmunomodulation, brain development, and normal brain function (Giordano et al., 2017). Vitamin D may play a significant role in the pathophysiology of depression. Additionally, there is growing proof that vitamin D may have neuroprotective effects through its influence on inflammation. Some studies reported the link between vitamin D and PPD. Vitamin D level was monitored in ninety seven women with PPD symptoms for seven months. The dichotomous model was used with a cut-off score of nine on the Edinburgh PPD Scale and it was observed that depression was higher in women with low vitamin D levels (Murphy et al., 2010). At the time of writing, no studies investigated the effect of vitamin D supplementation on PPD.
4.2.3. Role of tryptophan in PPD
Tryptophan is one of the necessary amino acids for humans. It contains an indole ring in its structure. It is commonly found in milk, beans and meat. The plasma concentration of tryptophan ranges from 45 to 60 mol/L, with the majority of this substance being bound to plasma albumin in an unstable manner. Tryptophan is essential during pregnancy and can fluctuate during the peripartum period. In some studies, it was observed that tryptophan metabolism plays a major role in the regulation of physiological processes that are associated with PPD (Duan et al., 2018). Elevated levels of stress hormones and inflammatory response prompt tryptophan to participate in the production of the neuroregulatory kynurenine pathway instead of melatonin and serotonin pathways. Factors that alter the balance of activation of the serotonin and kynurenine pathways can have a major impact on a variety of medical problems, including depressive illnesses. When melatonin and serotonin levels are low and the kynurenine (Kyn) pathway's products, such as quinolinic acid and kynurenic acid, are high, it can lead to depressed and mood-related diseases that have neuroregulatory implications. According to a study carried out by Baïlara et al., an increase in various amino acids during the postpartum period that competed with and inhibited brain tryptophan transport led to a temporary drop in the amount of brain tryptophan (brain tryptophan availability index reduced by 15%) (Baïlara et al., 2006). This drop may be a contributor to postpartum blues and significantly can lay down the foundation of PPD (Baïlara et al., 2006, Duan et al., 2018).
The Kyn pathway is activated by the enzymes tryptophan 2,3-dioxygenase (TDO) and indoleamine 2, 3-dioxygenase (IDO). Under normal conditions, IDO has a minor role in tryptophan metabolism. IDO-dependent tryptophan metabolism is intensely activated in response to inflammation releasing mediators like cytokines and interferon. The most important IDO-activating cytokine is interferon-gamma (IFN-γ). It induces the expression of numerous cell types, including dendritic cells, macrophages, fibroblasts, connective tissues, epithelial tissues of the renal, pulmonary, gastrointestinal, and vascular systems, after interacting with the IDO promoter region. (Sorgdrager et al., 2019). Handley and colleagues reported a pattern of increase in plasma tryptophan concentrations from the second to fifth postpartum days, with this change being favorably associated with measures of maternal mood at that time (Handley et al., 1977). Another clinical study by the same team demonstrated that while there was no such tryptophan recovery in new mothers with postpartum blues, the plasma tryptophan concentration gradually increased in pregnant women who did not experience the postpartum blues for the first two postpartum days (Handley et al., 1977). Investigations carried out by other researchers showed that whereas plasma tryptophan levels in women with postpartum blues were low for four days after delivery, they gradually increased in women without the condition over the first two postpartum days (Kohl et al., 2005).
At the same time, it should be mentioned that there are some contradicting findings. Pregnant women have lower perinatal plasma tryptophan levels than non-pregnant women, according to research by Adachi and colleagues, however there was no correlation between changes in plasma tryptophan concentration and postpartum distress or sorrow. The research also found no link between plasma tryptophan levels and postpartum blues (Adachi et al., 1990; Baïlara et al., 2006). At the time of writing, no data is available on the effect of tryptophan supplementation on PPD.
4.2.4. Iron
Cereals, beans, chickpeas, peanuts, almonds, spinach, and other dark green leafy vegetables, dates, meat, and fish are rich dietary sources of iron. The estimated overall prevalence of iron deficiency in India, across all age groups, is 53 % (95 % CI 0⋅41, 0⋅64) and it is found to be the highest in pregnant women with 61 % (95 % CI 0⋅50, 0⋅72) prevalence (Venkatesh et al., 2021). Pregnant women are at risk for iron deficiency anaemia due to higher maternal and foetal demands, blood loss during pregnancy and childbirth, and the significant blood volume expansion that occurs during pregnancy. Iron is a component of haemoglobin in red blood cells (RBCs), and as a result, an iron-deficient diet can cause iron deficiency anaemia, which is characterized by the production of RBCs that do not contain a full complement of haemoglobin and are ineffective at delivering oxygen to cells. Current research indicates that women with anaemia are more likely to experience PPD, even though the role of iron status in PPD and its pathogenesis is still unclear (Beard et al., 2005, Corwin et al., 2003). A higher incidence of PPD was linked to low ferritin in the postpartum period but not during pregnancy. According to Albacar et al., low ferritin levels, which are defined as levels below 12 µg/L, are associated with 2.3 times higher odds of developing PPD, and levels below 7.26 µg/L are associated with an even higher likelihood of developing PPD, demonstrating that as iron stores decline, the risk of developing PPD rises. Depleted iron reserves were identified in more women with PPD (38.5% versus 23.3%) (Albacar et al., 2011). According to the studies conducted by Alharbi and Abdulghani, 2014; Ezzeddin et al., 2015, women who received iron supplements during pregnancy did not have a reduction in PPD. Nonetheless, iron supplementation during the postpartum period reduced PPD risk. In a study done by Beard et al., women were divided into three groups: the placebo group, the control group consisting of non-anaemic women, and the iron supplement receiving group. All three groups had comparable mean EPDS scores at 10 weeks postpartum prior to intervention. However, at nine months postpartum, the iron group's final EPDS scores were noticeably lower (Beard et al., 2005b).
4.2.5. Antioxidants and trace minerals
Oxidative stress has been linked to the emergence of depression because it has the potential to upset the equilibrium between oxidation and antioxidative defence and damage the structure and function of brain cells (Bhatt et al., 2020). Antioxidant vitamins like C and E as well as bioactive dietary ingredients have been linked to mental health (Opie et al., 2015, Rechenberg and Humphries, 2013). The main dietary sources of vitamin E are vegetable oils, nuts, and seeds, whereas the main dietary sources of vitamin C are fruits and vegetables. Through low supplemental and pharmaceutical doses, vitamin E and C have shown promise in enhancing immunity, vascular health, and cognitive function in human trials (Martin et al., 2002). These nutrients play a role in the neuroendocrine pathways and therefore their deficiencies may have an impact on PPD pathogenesis.
In addition to enzymatic antioxidants like glutathione peroxidase and superoxide dismutase, vitamin E is a non-enzymatic antioxidant that helps to limit oxidative alterations brought on by stress. Antioxidants with lower serum concentrations, such as vitamin E, have been linked to both anxiety and depression. Many studies have demonstrated that antioxidant supplement therapy is successful in treating individuals with anxiety and depression because it strengthens biological antioxidant defence (Gautam et al., 2012, Xu et al., 2014). However, the exact mechanism through which vitamin E exerts its antidepressant effects and its effect on PPD is still unreported.
Similar to vitamin E, vitamin C has several roles in biological processes. Its functions in the brain include assisting dopamine beta-hydroxylase in turning dopamine into noradrenaline, modulating both dopaminergic and glutamatergic neurotransmission, and controlling the release of catecholamines and acetylcholine from synaptic vesicles. Moreover, Vitamin C protects against glutamate excitotoxicity and has antioxidant actions in the brain that lower ischaemia-reperfusion damage. Several studies have linked depression and cognitive decline to vitamin C deficiency, particularly scurvy but at the time of writing, there still is not a clear understanding of the antidepressant mechanisms of vitamin C (Plevin and Galletly, 2020). There is a dearth of studies investigating the role of vitamin C in PPD.
The body contains dietary mineral ions as constituents. Some food components, including as calcium, sodium, potassium, and chloride ions, which have structural and electrolytic functions, are present in significant amounts in the body. Other mineral ions occurring in trace amount act as a coenzyme and cell signalling molecules. Sources of these mineral ions are animal products (for Ca, P, and Fe) and vegetarian sources (for K, and Mg). Zinc, an essential ion, plays an essential role in many body functions such as DNA replication, cell signalling, enzyme catalysis, and transcription. Zinc deficiency has also been linked to the suppression of the immune system. Its deficiency influences inflammation by changing the ratio of anti-inflammatory to pro-inflammatory cytokines, allowing more IL-1b and NF-kB to be produced. Another way by which it functions involves the HPA axis and altering the quantity and generation of beta cells and T cells (Fraker and King, 2004; Sorgdrager et al., 2019). It also performs an important function in neural development in memory and learning, also in mood consistency (Piao et al., 2017).
It is becoming increasingly clearer how zinc dysregulation and mental diseases are related. Zinc is mostly encapsulated within glutamatergic neurons in the limbic system, where it normally functions as an inhibitory modulator at the NMDA glutamate receptor. Zinc has complex interactions with 5-HT1A receptors, agonistic properties for AMPAR (amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor), and antagonistic effects at NMDAR (N-methyl-d-aspartate receptor), which may prevent or ostensibly alleviate depression (agonism and antagonist and pre and postsynaptic). Zinc is also an agonist for mTOR and GPR39 activation (mammalian target of rapamycin). Therefore, zinc also plays a key role in depression (Ranjbar et al., 2013). Postpartum zinc supplementation significantly improved the status of maternal blood zinc levels and decreased the risk of developing PPD, according to a study that examined the impact of zinc supplementation on depressive symptoms in 148 postpartum women (adjusted odds ratio: 0.249; 95% confidence interval: 0.062–0.988; p = 0.048) (Aoki et al., 2022).
Selenium functions as an anti-inflammatory component facilitated by selenoprotein glutathione peroxidase. It acts as a key antioxidant; a component of glutathione peroxidase which reduces H2O2 and thus inhibits the production of pro-inflammatory cytokines by restricting the COX pathway. The modulatory effects of selenium on metabolism may affect a person's propensity to experience depression. Iodothyronine deiodinases (DIOs), which contain selenium, are necessary for the correct synthesis and metabolism of thyroid hormones. Clinical researchers have known for a long time that thyroid function is related to neuropsychiatric symptoms such as mood disorders, cognitive impairment, and other psychiatric symptoms (Wang et al., 2018). Additionally, a 2017 study discovered a connection between low cholesterol levels and a higher risk of depression and suicidality, suggesting that selenium's anti-lipoperoxidative characteristics may play a part in the mineral's capacity to guard against depression (De Berardis et al., 2012). Finally, selenium may have antidepressant benefits due to its modulatory actions on a variety of neurotransmitter systems. According to research, selenium significantly modifies the dopaminergic, serotonergic, and noradrenergic systems (Wang et al., 2018). Mokhber et al. conducted a randomized placebo-controlled double-blind selenium trial in 166 subjects during the second and third trimesters. The level of selenium was measured pre- and post-treatment and PPD screening was performed eight weeks after the delivery. Their results show that an increase in selenium level significantly decreases the PPD symptoms. They also demonstrated that women receiving selenium supplementation have lower depression scores even after the analysis of other confounding factors (Mokhber et al., 2011).
4.3. Macronutrients
According to individual factors (such as age, gender, pregnancy and breastfeeding, lifestyle, and physical activity), cultural context, food availability, and dietary practices, a diverse, balanced, and healthy diet can take on several forms. Nevertheless, fruit, vegetables, legumes, nuts, and whole grains are the fundamental components of a balanced diet, as are low added sugars, unsaturated fats as opposed to saturated and trans fats, and reduced salt intake (Healthy Diet, 2020). As per reports from Western nations, there is a negative association between following dietary recommendations and mental illnesses (Deierlein et al., 2021; P.-Y. Wu et al., 2020). Nutrients' anti-depressive effects build up over time, and a balanced diet comprises a variety of nutrients as well as complex interactions between nutrients and other dietary constituents and their synergistic effects (Gianfredi et al., 2021; Wu et al., 2020). A study carried out by Deierlein et al., on 1325 pregnant women in New York in the year 2021 found an adverse relationship between the Alternative Healthy Eating Index (AHEI) and sadness, and it suggested that this relationship was caused by the cumulative impact of all of the AHEI's components rather than by any particular nutrient or food type (Deierlein et al., 2021). An imbalanced diet, particularly one with limited variety and inadequate vegetable intake, was linked to a higher risk of PPD (Yang et al., 2021). In this section, we shall review the relevance of macronutrients which are the key components of a balanced diet.
4.3.1. Carbohydrates and protein
Carbohydrates, which are found in foods like bread, cereal, rice, potatoes, pasta, and beans, are necessary for providing the body with the energy it requires and can also affect mood. Since the brain's main energy source are carbohydrates, maintaining a healthy diet is crucial for postpartum mental health. Mood can also be impacted by the complex interaction between insulin and carbohydrates. This equilibrium between carbohydrates and insulin can be supported by a balanced and regular carbohydrate intake throughout the day. During a typical pregnancy, insulin levels significantly rise and then sharply decline after delivery. Although the exact process is still unclear, it has been proposed that the drop in insulin levels after delivery may lead to depression through decreasing serotonin production (Chen et al., 2006). In a substantial randomized clinical trial, the investigators found that women with gestational diabetes mellitus (GDM) who got customized dietary recommendations experienced lower rates of PPD than women with GDM who received conventional care (Crowther et al., 2005). In contrast, no significant correlation between glycaemic load and PPD was found in a large cohort study that included 865 Japanese women and 122 cases of PPD. Overall, the evidence for the hypothesized connections is conflicting, necessitating further research (Murakami et al., 2008).
Among the three primary macronutrients, protein and the amino acids that make up protein are crucial dietary elements because they sustain cellular integrity and function (including neurons). Meat, chicken, fish, eggs, cheese, almonds, and legumes all include protein, which has an effect on mood control. The production of neurotransmitters will be supported by a sufficient number of all the required amino acids (Glenn et al., 2019). The neurotransmission that occurs can be directly influenced by amino acids. Depending on the particular amino acid of relevance, glutamate, aspartate, GABA, and glycine can be either excitatory or inhibitory with regard to neurotransmission. Tryptophan, an essential amino acid, encourages the synthesis of serotonin, a neurotransmitter involved in controlling emotions such as anger, sleep, sexual drive, etc. (Markus, 2008). Protein demands during breastfeeding should be closely monitored because they are similar to those during pregnancy. Women should ensure sure they are getting enough protein during the postpartum period, especially since it contains all nine essential amino acids in its entire complement. While it's generally not a problem for pregnant women who are eating a varied diet to get enough protein, depressed women might not be following a balanced diet.
4.3.2. PUFA (Poly unsaturated fatty Acids)
Polyunsaturated fatty acids (PUFAs) are other essential nutrients that act as a building block for brain development and healthy functioning. Many epidemiological studies suggested that inadequate consumption of PUFA is related to a depressed mood (Reimers and Ljung, 2019). This is because the brain contains the highest amount of lipids i.e. 50–60 % after adipose tissues which constitutes the brain’s dry weight and it requires PUFAs from six and three families. Therefore any decline in the concentration of lipids because of PUFA deficiencies would lead to a reduction in the number of neurotransmitters, causing mood-related disorders (Larrieu and Layé, 2018).
Two main families of PUFA: n-3 omega-3 fatty acids and n-6 omega-6 fatty acids are not synthesized inside the human body. Alpha-linolenic acid belongs to the family of omega- 3 fatty acids whereas linoleic acid belongs to the family of omega-6 fatty acids. The major dietary sources of linoleic acid are vegetable oils, nuts seeds, meat, and eggs. The dietary sources for alpha-linolenic acid include flax seed oil, chia seeds, hemp seeds, soya bean oil, and canola oil. EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are other omega-3 fatty acids found primarily in certain fish (Whelan and Fritsche, 2013). It was found that PUFA has an impact on psychoneuroimmunology through an immune system-related inflammatory response (Ellsworth-Bowers and Corwin, 2012). It generally performs body functions in the form of EPA and DHA. Intake of these supplements (EPA and DHA) was found to decrease the levels of inflammatory mediators like interleukins (IL-1b, IL-6, and IL-8), cytokines, and TNF (Adkins and Kelley, 2010). Another study demonstrated that EPA restricts the transcription factor (NF-kB) stimulation which is the major pathway involved in the production of pro-inflammatory cytokines (Zhao et al., 2004). EPA also prevents inflammation by competitively inhibiting the cyclooxygenase inflammatory pathway and production of prostacyclin and prostaglandin (Whelan and Fritsche, 2013). Some observational studies reported that the n-3 PUFA level is found to be lower in blood serum as well as in the diet of the individuals suffering from depression and it was also observed that countries with lower n-3 PUFA consumption have higher depression rates (Liperoti et al., 2009). A significant association between PUFA and depression was observed in two ways: (a) restricting the production of pro-inflammatory mediators like cytokines, eicosanoids, etc., and (b) neuronal mechanism: regulating overall synthesis, metabolism, and functions of serotonin neurotransmitters.
During pregnancy, there is a depletion of DHA maternal stores by 50%, and recovery to pre-pregnancy levels is not observed until six months after childbirth. Hibbeln performed an epidemiological study and observed the association between low levels of n-3 PUFA in mothers with PPD. They examined the influence of confounding variables like family, socioeconomic situation, and demography and showed that poor seafood consumption and low levels of DHA in breast milk are strongly linked to greater incidence of PPD symptoms (Hibbeln, 2002). According to a recent meta-analysis, n-3 PUFA consumption aids in the prevention of PPD and has different benefits for prevention as opposed to treatment (Ellsworth-Bowers and Corwin, 2012). Additional research is necessary to examine the connection between PUFA and PPD.
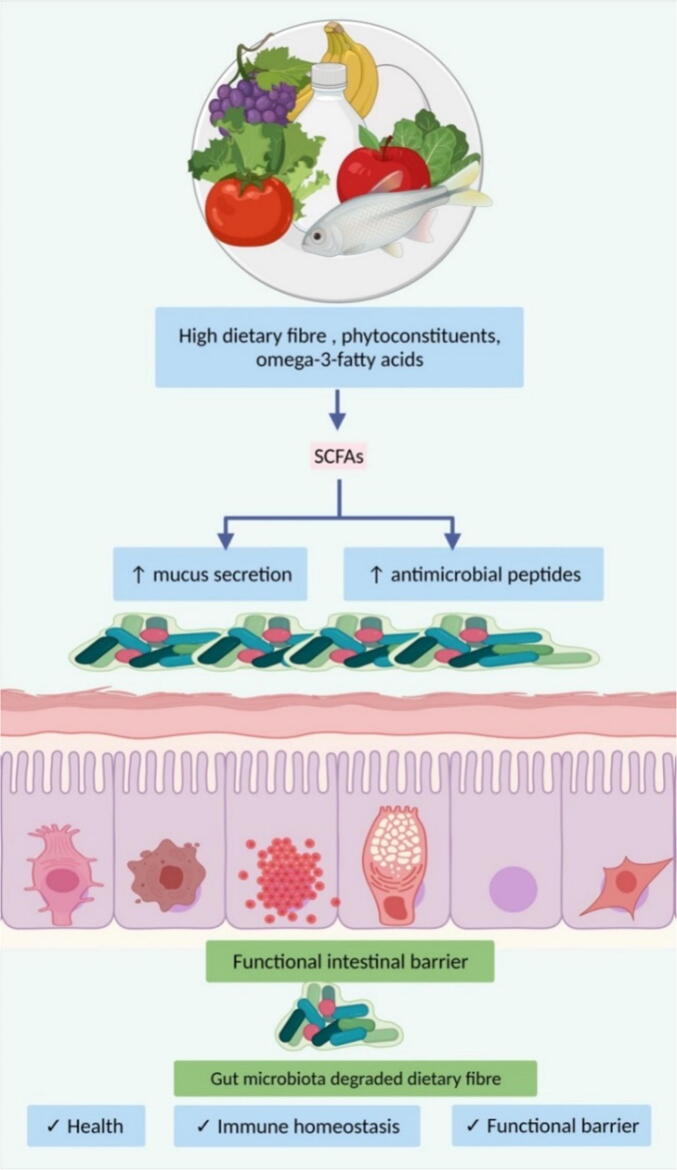
4.3.3. Dietary fibre and its impact on the gut microbiome
Dietary fibre, as defined by the Codex Alimentarius Commission in 2009 is a carbohydrate polymer with more than three monomeric units and is resistant to digestive enzymes in the gastrointestinal tract. They are neither absorbed nor hydrolysed by the small intestine and belong to the given categories: a) carbohydrates naturally occurring in vegetables, legumes, cereals, and fruits; b) carbohydrates obtained from raw sources by a chemical, physical and enzymatic processes which have physiological benefits; c) synthetic carbohydrates with physiological benefits (Stephen et al., 2017). Fibres, both soluble and insoluble, are found in several food sources like vegetables, seeds, nuts, tubers, legumes, fruits, and cereals in different forms. The various components of diet shape the gut microbial communities in a time-dependent order. Wu et al. show that long-term intake of animal fats and proteins (Bacteroides) versus plant-based carbohydrates (Prevotella) are linked with so-called enterotypes (Wu et al., 2011).
Dietary fibres serve as an important energy source for colon and cecum microbes. An anaerobic microbe activates its machinery under the specific intestinal environment that is constituted of several enzymes and metabolic pathways. These microbes under standard conditions metabolize carbohydrates and form metabolites like short-chain fatty acids (SCFA). The SCFA are organic compounds i.e. acetate, propionate, and butyrate, which play a key role in regulating the immune system, metabolism, and cellular proliferation (Koh et al., 2016). Dietary fibre when taken in a low amount reduces microbial diversity and production of SCFA (Fig. 5). It also shifts the gut microbial metabolism and causes utilization of substrate which is of less importance. Especially dietary and endogenous proteins which affect the gut-brain axis cause alterations in brain activity. A diet rich in fibres decreases inflammation by affecting the permeability and pH of the gut. The resultant decline in inflammatory proteins alters the concentration of neurotransmitters in the brain and thus helps in reducing the symptoms of depression (Makki et al., 2018).
Fig. 5.
Role of dietary fibres in regulating the permeability as well as immune homeostasis mechanism with the help of gut microbiota.
5. Role of tropical herbal plants in the treatment of depression
In India, more than thirty thousand medicinal plants have been recognized in systems of Siddha, Unani, Ayurveda, Yoga, Homeopathy, and Naturopathy (Suresh Kumar et al., 2010). Griffoniasimplicfollia (Family-Fabaceaa) is an important tropical herbal plant used in the treatment of depression and its medicinal use has been described in the traditional system of medicine. In some studies, it was observed that the seeds of this plant increase the levels of serotonin in the brain(Suresh Kumar et al., 2010) Moshiri et al. (Moshiri et al., 2006) performed a double-blind placebo-controlled clinical trial for six weeks among forty adult patients. On the basis of observed result, investigators reported that the petals of Crocus sativus have shown a significant impact on the treatment of mild to moderate depression. Abelmoschus moschatus (Malvaceae) is an herb grown in the tropical regions of South America, Africa, and Asia. The seeds of this plant are traditionally used in the treatment of neurodegenerative diseases (Priyanka et al., 2011). Sheik et al. reported that the ethanolic extract of Abelmoschus moschatus seeds possesses antidepressant, anxiolytic, hypnotic, anti-convulsant, and muscle relaxant activity (Sheik et al., 2014). Petroleum ether extract of leaves of Catharanthus roseus contains phenol and steroid content in excess amount and is reported as responsive chemicals for central nervous system depressant activity in several preclinical studies (Shinde et al., 2021). There is a need to study the impact of these tropical herbs on PPD to assess their safety and efficacy in managing PPD.
6. Conclusion
PPD is a multifactorial mood illness that can be treated but frequently goes undiagnosed. The bonding between a mother and her infant, as well as other familial interactions, can be negatively impacted by PPD symptoms. To ensure maternal and infant well-being, it is important to treat PPD. To conclude, this present review has pointed out the physiological changes during pregnancy (which are thought to contribute to PPD incidence), underlying neurobiological mechanisms leading to PPD, associations between specific nutrient deficiencies, dietary patterns, micronutrient status, gut-brain axis, their role in developing PPD as well as the use of tropical herbs in depressive disorders. As structural elements of brain tissue, as neurochemical modifiers of neurotransmitter-conducting membranes, as precursors in the synthesis of neurotransmitters, as neurotransmitters themselves, and as regulators of inflammatory expression, nutrients play important roles in maintaining a healthy central nervous system. The brain will benefit from a consistent supply of fuel if one sticks to a well-balanced diet with enough protein, carbohydrates, and fat. Important nutrients like omega-3 fatty acids, iron, folate, riboflavin, and vitamin B6 have been linked to depression and/or PPD, therefore it's important to ensure that pregnant women and new mothers get enough of this nutrient. At present, there is insufficient evidence to safely prevent or treat PPD solely by dietary changes or supplementation. Before clinical professionals can create diet and supplement strategies to prevent and treat PPD, more study on the relationship between micronutrients and PPD is required. More doctors may start treating pregnant and postpartum women with dietary changes or micronutrient supplements if nutrient doses for PPD prevention or treatment are made clear. These interventions would be reasonably priced, and women who are reluctant to use antidepressants may be more receptive to seeking treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1444).
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Jyoti Upadhyay, Email: jupadhyay@ddn.upes.ac.in.
Mohd Nazam Ansari, Email: m.ansari@psau.edu.sa.
References
- Abou-Saleh M.T., Ghubash R., Karim L., Krymski M., Bhai I. Hormonal aspects of postpartum depression. Psychoneuroendocrinol. 1998;23(5):465–475. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Adachi N., Ogasawara T., Nishijima M. Tryptophan metabolism during the perinatal period. Nihon SankaFujinka Gakkai Zasshi. 1990;42(9):1203–1210. [PubMed] [Google Scholar]
- Adkins Y., Kelley D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010;21(9):781–792. doi: 10.1016/J.JNUTBIO.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Albacar G., Sans T., Martín-Santos R., García-Esteve L., Guillamat R., Sanjuan J., Cañellas F., Gratacòs M., Cavalle P., Arija V., Gaviria A., Gutiérrez-Zotes A., Vilella E. An association between plasma ferritin concentrations measured 48 h after delivery and postpartum depression. J. Affect. Disord. 2011;131(1–3):136–142. doi: 10.1016/j.jad.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Alharbi A.A., Abdulghani H.M. Risk factors associated with postpartum depression in the Saudi population. Neuropsychiatr. Dis. Treat. 2014;10:311–316. doi: 10.2147/NDT.S57556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini S., Jafarirad S., Amani R., SayyahBargard M., Cheraghian B., Hemmati A.A. The relationship between dietary intakes during pregnancy and incidence of postpartum depression: a case-control study. Nutr. Food Sci. 2020;50(4):751–764. doi: 10.1108/NFS-07-2019-0229/FULL/XML. [DOI] [Google Scholar]
- Aoki C., Imai K., Owaki T., Kobayashi-Nakano T., Ushida T., Iitani Y., Nakamura N., Kajiyama H., Kotani T. The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia. Medicina (Kaunas, Lithuania) 2022;58(6):731. doi: 10.3390/medicina58060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi S.S., Tsuchiya K.J. Does maternal postpartum depression affect children’s developmental outcomes? J. Obstet. Gynaecol. Res. 2019;45(9):1809–1820. doi: 10.1111/JOG.14064. [DOI] [PubMed] [Google Scholar]
- Baek S.S., Jun T.W., Kim K.J., Shin M.S., Kang S.Y., Kim C.J. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain Dev. 2012;34(1):45–56. doi: 10.1016/J.BRAINDEV.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Baïlara K.M., Henry C., Lestage J., Launay J.M., Parrot F., Swendsen J., Sutter A.L., Roux D., Dallay D., Demotes-Mainard J. Decreased brain tryptophan availability as a partial determinant of post-partum blues. Psychoneuroendocrinol. 2006;31(3):407–413. doi: 10.1016/j.psyneuen.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Barjaktarovic M., Korevaar T.I.M., Chaker L., Jaddoe V.W.V., De Rijke Y.B., Visser T.J., Steegers E.A.P., Peeters R.P. The association of maternal thyroid function with placental hemodynamics. Hum. Reprod. 2017;32(3):653–661. doi: 10.1093/HUMREP/DEW357. [DOI] [PubMed] [Google Scholar]
- Beard J.L., Hendricks M.K., Perez E.M., Murray-Kolb L.E., Berg A., Vernon-Feagans L., Irlam J., Isaacs W., Sive A., Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J. Nutr. 2005;135(2):267–272. doi: 10.1093/jn/135.2.267. [DOI] [PubMed] [Google Scholar]
- Beck C.T. Teetering on the edge: a substantive theory of postpartum depression. Nurs. Res. 1993;42(1):42–48. [PubMed] [Google Scholar]
- Beck C.T. State of the science on postpartum depression: what nurse researchers have contributed-part 2. MCN. Am. J. Matern. Child Nurs. 2008;33(3):151–158. doi: 10.1097/01.NMC.0000318349.70364.1c. [DOI] [PubMed] [Google Scholar]
- Beck, C.T., 2007. Exemplar: Teetering on the edge: A continually emerging theory of postpartum depression. PL Munhall (Ed.), Nursing Research: A Qualitative Perspective, 4, 273–292.
- Beck C.T., Gable R.K. Postpartum depression screening scale: Spanish version. Nursing research. 2003;52(5):296–306. doi: 10.1097/00006199-200309000-00004. [DOI] [PubMed] [Google Scholar]
- Beckley E.H., Finn D.A. Inhibition of progesterone metabolism mimics the effect of progesterone withdrawal on forced swim test immobility. Pharmacol. Biochem. Behav. 2007;87(4):412–419. doi: 10.1016/J.PBB.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Nagappa A.N., Patil C.R. Role of oxidative stress in depression. Drug Discov. Today. 2020;25(7):1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- Bloch M., Schmidt P.J., Danaceau M., Murphy J., Nieman L., Rubinow D.R. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry. 2000;157(6):924–930. doi: 10.1176/APPI.AJP.157.6.924. [DOI] [PubMed] [Google Scholar]
- Blunden C.H., Inskip H.M., Robinson S.M., Cooper C., Godfrey K.M., Kendrick T.R. Postpartum depressive symptoms: the B-vitamin link. Ment. Health Fam. Med. 2012;9(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- Bodnar L.M., Wisner K.L. Nutrition and Depression: Implications for Improving Mental Health Among Childbearing-Aged Women. Biol. Psychiatry. 2005;58(9):679–685. doi: 10.1016/J.BIOPSYCH.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges M.C., Martini L.A., Rogero M.M. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition. 2011;27(4):399–404. doi: 10.1016/J.NUT.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Baxendale S. Motherhood and memory: a review. Psychoneuroendocrinol. 2001;26(4):339–362. doi: 10.1016/S0306-4530(01)00003-8. [DOI] [PubMed] [Google Scholar]
- Brinkworth G.D., Buckley J.D., Noakes M., Clifton P.M., Wilson C.J. Long-term Effects of a Very Low-Carbohydrate Diet and a Low-Fat Diet on Mood and Cognitive Function. Arch. Intern. Med. 2009;169(20):1873–1880. doi: 10.1001/ARCHINTERNMED.2009.329. [DOI] [PubMed] [Google Scholar]
- Buoli M., Grassi S., Iodice S., Carnevali G.S., Esposito C.M., Tarantini L., Barkin J.L., Bollati V. The role of clock genes in perinatal depression: the light in the darkness. Acta Psychiatrica Scandinavica. 2019;140(4):382–384. doi: 10.1111/ACPS.13084. [DOI] [PubMed] [Google Scholar]
- Burt V.K., Quezada V. Mood disorders in women: focus on reproductive psychiatry in the 21st century--Motherisk update 2008. J. Popul. Ther. Clin. Pharmacol. 2009;16(1):e6–e14. [PubMed] [Google Scholar]
- Carmona S., Martínez-García M., Paternina-Die M., Barba-Müller E., Wierenga L.M., Alemán-Gómez Y., Pretus C., Marcos-Vidal L., Beumala L., Cortizo R., Pozzobon C., Picado M., Lucco F., García-García D., Soliva J.C., Tobeña A., Peper J.S., Crone E.A., Ballesteros A., Vilarroya O., Desco M., Hoekzema E. Pregnancy and adolescence entail similar neuroanatomical adaptations: A comparative analysis of cerebral morphometric changes. Hum. Brain Mapp. 2019;40(7):2143–2152. doi: 10.1002/HBM.24513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.H., Lan T.H., Yang C.Y., Juang K.D. Postpartum mood disorders may be related to a decreased insulin level after delivery. Med. Hypotheses. 2006;66(4):820–823. doi: 10.1016/j.mehy.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Chen J., Li Q., Rialdi A., Mystal E., Ly J., Finik J., Davey T., Lambertini L., Nomura Y. Influences of Maternal Stress during Pregnancy on the Epi/genome: Comparison of Placenta and Umbilical Cord Blood. J. Depress Anxiety. 2014;3(2) doi: 10.4172/2167-1044.1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Hu X., Roberts N., Zhao Y., Xu X., Zhou Y., Tan X., Chen S., Meng Y., Wang S., Xing H., Deng W. Prolactin mediates the relationship between regional gray matter volume and postpartum depression symptoms. J. Affect. Disord. 2022;301:253–259. doi: 10.1016/j.jad.2022.01.051. [DOI] [PubMed] [Google Scholar]
- Comasco E., Sylvén S.M., Papadopoulos F.C., Oreland L., Sundström-Poromaa I., Skalkidou A. Postpartum depressive symptoms and the BDNF Val66Met functional polymorphism: Effect of season of delivery. Arch. Womens Ment. Health. 2011;14(6):453–463. doi: 10.1007/S00737-011-0239-X/METRICS. [DOI] [PubMed] [Google Scholar]
- Cooper, J., 2001. Diagnostic and Statistical Manual of Mental Disorders (4th edn, text revision) (DSM–IV–TR) Washington, DC: American Psychiatric Association 2000. 943 pp. £39.99 (hb). ISBN 0 89042 025 4. Br. J. Psychiatry. 179(1), 85–85. https://doi.org/10.1192/BJP.179.1.85-A.
- Corwin E.J., Murray-Kolb L.E., Beard J.L. Low hemoglobin level is a risk factor for postpartum depression. J. Nutr. 2003;133(12):4139–4142. doi: 10.1093/jn/133.12.4139. [DOI] [PubMed] [Google Scholar]
- Corwin E.J., Kohen R., Jarrett M., Stafford B. The heritability of postpartum depression. Biol. Res. Nurs. 2010;12(1):73–83. doi: 10.1177/1099800410362112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin E.J., Pajer K. The psychoneuroimmunology of postpartum depression. J. Women's Health. 2008;17(9):1529–1534. doi: 10.1089/jwh.2007.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas J., Gratacòs M., Escaramís G., Martín-Santos R., de Diego Y., Baca-García E., Canellas F., Estivill X., Guillamat R., Guitart M., Gutiérrez-Zotes A., García-Esteve L., Mayoral F., Dolores Moltó M., Phillips C., Roca M., Carracedo Á., Vilella E., Sanjuán J. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J. Psychiatr. Res. 2010;44(11):717–724. doi: 10.1016/J.JPSYCHIRES.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Crowther, C.A., Hiller, J.E., Moss, J.R., McPhee, A.J., Jeffries, W.S., Robinson, J.S., Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group, 2005. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 352(24), 2477–2486. https://doi.org/10.1056/NEJMoa042973. [DOI] [PubMed]
- De Berardis D., Marini S., Piersanti M., Cavuto M., Perna G., Valchera A., Mazza M., Fornaro M., Iasevoli F., Martinotti G., Di Giannantonio M. The Relationships between Cholesterol and Suicide: An Update. ISRN Psychiatry. 2012;2012 doi: 10.5402/2012/387901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geest K., Thiery M., Piron-Possuyt G., Driessche R.V. Plasma oxytocin in human pregnancy and parturition. J. Perinat. Med. 1985;13(1):3–14. doi: 10.1515/JPME.1985.13.1.3/HTML. [DOI] [PubMed] [Google Scholar]
- De Wit L., Luppino F., van Straten A., Penninx B., Zitman F., Cuijpers P. Depression and obesity: A meta-analysis of community-based studies. Psychiatry Res. 2010;178(2):230–235. doi: 10.1016/J.PSYCHRES.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Deierlein A.L., Ghassabian A., Kahn L.G., Afanasyeva Y., Mehta-Lee S.S., Brubaker S.G., Trasande L. Dietary Quality and Sociodemographic and Health Behavior Characteristics Among Pregnant Women Participating in the New York University Children's Health and Environment Study. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.639425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017;79(8):920–926. doi: 10.1097/PSY.0000000000000519. [DOI] [PubMed] [Google Scholar]
- Doornbos B., Dijck-Brouwer D.A.J., Kema I.P., Tanke M.A.C., van Goor S.A., Muskiet F.A.J., Korf J. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(7):1250–1254. doi: 10.1016/J.PNPBP.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Duan K.M., Ma J.H., Wang S.Y., Huang Z.D., Zhou Y.Y., Yu H.Y. The role of tryptophan metabolism in postpartum depression. Metab. Brain Dis. 2018;33(3):647–660. doi: 10.1007/S11011-017-0178-Y/METRICS. [DOI] [PubMed] [Google Scholar]
- Ellsworth-Bowers E.R., Corwin E.J. Nutrition and the psychoneuroimmunology of postpartum depression. Nutr. Res. Rev. 2012;25(1):180–192. doi: 10.1017/S0954422412000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer N., Darwin L., Nishigandh D., Ngianga-Bakwin K., Smith S.C., Grammatopoulos D.K. Association of glucocorticoid and type 1 corticotropin-releasing hormone receptors gene variants and risk for depression during pregnancy and post-partum. J. Psychiatric Res. 2013;47(9):1166–1173. doi: 10.1016/J.JPSYCHIRES.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Engle P.L. Maternal mental health: program and policy implications. Am. J. Clin. Nutr. 2009;89(3):963S–966S. doi: 10.3945/AJCN.2008.26692G. [DOI] [PubMed] [Google Scholar]
- Ezzeddin N., Zavoshy R., Noroozi M., Sarichloo M.E., Jahanihashemi H. The Association Between Postpartum Depression and Pica During Pregnancy. Glob. J. Health Sci. 2015;8(4):253–259. doi: 10.5539/gjhs.v8n4p120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Granat A., Pariente C., Kanety H., Kuint J., Gilboa-Schechtman E. Maternal Depression and Anxiety Across the Postpartum Year and Infant Social Engagement, Fear Regulation, and Stress Reactivity. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):919–927. doi: 10.1097/CHI.0B013E3181B21651. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S. American Psychiatric Press Inc; Washington, DC: 1997. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) [Google Scholar]
- Ford, T.C., Downey, L.A., Simpson, T., McPhee, G., Oliver, C., Stough, C., 2018. The Effect of a High-Dose Vitamin B Multivitamin Supplement on the Relationship between Brain Metabolism and Blood Biomarkers of Oxidative Stress: A Randomized Control Trial. Nutrients. 2018, 10(12), 1860. https://doi.org/10.3390/NU10121860. [DOI] [PMC free article] [PubMed]
- Forty L., Jones L., Macgregor S., Caesar S., Cooper C., Hough A., Dean L., Dave S., Farmer A., McGuffin P., Brewster S., Craddock N., Jones I. Familiality of postpartum depression in unipolar disorder: results of a family study. Am. J. Psychiatry. 2006;163(9):1549–1553. doi: 10.1176/ajp.2006.163.9.1549. [DOI] [PubMed] [Google Scholar]
- Fraker P.J., King L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- Freeman M.E., Kanyicska B., Lerant A., Nagy G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000;80(4):1523–1631. doi: 10.1152/PHYSREV.2000.80.4.1523/ASSET/IMAGES/LARGE/9J0400101006.JPEG. [DOI] [PubMed] [Google Scholar]
- Gaberšček S., Zaletel K. Thyroid physiology and autoimmunity in pregnancy and after delivery. Expert review of clinical immunology. 2011;7(5):697–707. doi: 10.1586/eci.11.42. [DOI] [PubMed] [Google Scholar]
- Garcion E., Wion-Barbot N., Montero-Menei C.N., Berger F., Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002;13(3):100–105. doi: 10.1016/S1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Gautam M., Agrawal M., Gautam M., Sharma P., Gautam A.S., Gautam S. Role of antioxidants in generalised anxiety disorder and depression. Indian J. Psychiatry. 2012;54(3):244–247. doi: 10.4103/0019-5545.102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfredi V., Koster A., Odone A., Amerio A., Signorelli C., Schaper N.C., Bosma H., Köhler S., Dagnelie P.C., Stehouwer C.D.A., Schram M.T., Dongen M.C.J.M.V., Eussen S.J.P.M. Associations of Dietary Patterns with Incident Depression: The Maastricht Study. Nutrients. 2021;13(3):1034. doi: 10.3390/nu13031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano N., Goracci A., Fagiolini A. Depression and vitamin D deficiency: Causality, assessment, and clinical practice implications. Neuropsychiatry. 2017;7(5):606–614. [Google Scholar]
- Glenn J.M., Madero E.N., Bott N.T. Dietary Protein and Amino Acid Intake: Links to the Maintenance of Cognitive Health. Nutrients. 2019;11(6):1315. doi: 10.3390/nu11061315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn L.M., Davis E.P., Sandman C.A. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47(6):363–370. doi: 10.1016/J.NPEP.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Gohir W., Whelan F.J., Surette M.G., Moore C., Schertzer J.D., Sloboda D.M. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes. 2015;6(5):310–320. doi: 10.1080/19490976.2015.1086056/SUPPL_FILE/KGMI_A_1086056_SM4795.ZIP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan D.R., Kokay I.C. Prolactin: A Pleiotropic Neuroendocrine Hormone. J. Neuroendocrinol. 2008;20(6):752–763. doi: 10.1111/J.1365-2826.2008.01736.X. [DOI] [PubMed] [Google Scholar]
- Grattan, D.R., 2001. Chapter 11 The actions of prolactin in the brain during pregnancy and lactation. Prog. Brain Res. 133, 153–171. https://doi.org/10.1016/S0079-6123(01)33012-1. [DOI] [PubMed]
- Guintivano J., Arad M., Gould T.D., Payne J.L., Kaminsky Z.A. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol. Psychiatry. 2013;19(5):560–567. doi: 10.1038/mp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavaldsen C., Fedorcsak P., Tanbo T., Eskild A. Maternal age and serum concentration of human chorionic gonadotropin in early pregnancy. Acta obstetricia et gynecologica Scandinavica. 2014;93(12):1290–1294. doi: 10.1111/aogs.12471. [DOI] [PubMed] [Google Scholar]
- Handley S.L., Dunn T.L., Baker J.M., Cockshott C., Gould S. Mood changes in puerperium, and plasma tryptophan and cortisol concentrations. Br. Med. J. 1977;2(6078):18–20. doi: 10.1136/bmj.2.6078.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthy diet, 2020. Retrieved March 21, 2023, from https://www.who.int/en/news-room/fact-sheets/detail/healthy-diet.
- Heidrich A., Schleyer M., Spingler H., Albert P., Knoche M., Fritze J., Lanczik M. Postpartum blues: Relationship between not-protein bound steroid hormones in plasma and postpartum mood changes. J. Affect. Disord. 1994;30(2):93–98. doi: 10.1016/0165-0327(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Hendrick V., Altshuler L.L., Suri R. Hormonal Changes in the Postpartum and Implications for Postpartum Depression. Psychosomatics. 1998;39(2):93–101. doi: 10.1016/S0033-3182(98)71355-6. [DOI] [PubMed] [Google Scholar]
- Heron D.S., Shinitzky M., Hershkowitz M., Samuel D. Lipid fluidity markedly modulates the binding of serotonin to mouse brain membranes. Proc. Natl. Acad. Sci. U.S.A. 1980;77(12):7463–7467. doi: 10.1073/PNAS.77.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln J.R. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J. Affect. Disord. 2002;69(1–3):15–29. doi: 10.1016/S0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- Hoekzema E., Barba-Müller E., Pozzobon C., Picado M., Lucco F., García-García D., Soliva J.C., Tobeña A., Desco M., Crone E.A., Ballesteros A., Carmona S., Vilarroya O. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 2016;20(2):287–296. doi: 10.1038/nn.4458. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Miller A.H. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007;21(4):374–383. doi: 10.1016/J.BBI.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Ising M., Zimmermann U.S., Künzel H.E., Uhr M., Foster A.C., Learned-Coughlin S.M., Holsboer F., Grigoriadis D.E. High-Affinity CRF1 Receptor Antagonist NBI-34041: Preclinical and Clinical Data Suggest Safety and Efficacy in Attenuating Elevated Stress Response. Neuropsychopharmacol. 2007;32(9):1941–1949. doi: 10.1038/sj.npp.1301328. [DOI] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., Li L., Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/J.BBI.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Liu Y., Gao M., Xue M., Wang Z., Liang H. Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020;11(1):378–391. doi: 10.1039/c9fo01780a. [DOI] [PubMed] [Google Scholar]
- Jolley S.N., Elmore S., Barnard K.E., Carr D.B. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol. Res. Nurs. 2007;8(3):210–222. doi: 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- Kennedy D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients. 2016;8(2):68. doi: 10.3390/NU8020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby J.P., Eyles D.W., Burne T.H.J., McGrath J.J. The effects of vitamin D on brain development and adult brain function. Mol. Cell. Endocrinol. 2011;347(1–2):121–127. doi: 10.1016/J.MCE.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Kim S., Soeken T.A., Cromer S.J., Martinez S.R., Hardy L.R., Strathearn L. Oxytocin and postpartum depression: Delivering on what’s known and what’s not. Brain Res. 2014;1580:219–232. doi: 10.1016/J.BRAINRES.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L., Robins S., Chen G., Yerko V., Zhou Y., Nagy C., Feeley N., Gold I., Hayton B., Turecki G., Zelkowitz P. Perinatal depression and DNA methylation of oxytocin-related genes: a study of mothers and their children. Horm. Behav. 2017;96:84–94. doi: 10.1016/J.YHBEH.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Koga K., Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am. J. Reprod. Immunol. 2010;63(6):587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/J.CELL.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Kohl C., Walch T., Huber R., Kemmler G., Neurauter G., Fuchs D., Sölder E., Schröcksnadel H., Sperner-Unterweger B. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J. Affect. Disord. 2005;86(2–3):135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Kling Bäckhed H., Gonzalez A., Werner J.J., Angenent L.T., Knight R., Bäckhed F., Isolauri E., Salminen S., Ley R.E. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/J.CELL.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Magon N. Hormones in pregnancy. Niger. Med. J. 2012;53(4):179–183. doi: 10.4103/0300-1652.107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.S., Praveen T., Jain N., Jitendra B. A review on Griffoniasimplicifollia-an ideal herbal anti-depressant. Int. J. Pharm. Life Sci. 2010;1(3):174–181. [Google Scholar]
- Lai J.S., Hiles S., Bisquera A., Hure A.J., McEvoy M., Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014;99(1):181–197. doi: 10.3945/AJCN.113.069880. [DOI] [PubMed] [Google Scholar]
- Lambert M., Gressier F. Biomarqueurs de L’inflammation et Dépression du Post-Partum: Une Revue Systématique De la Littérature [Inflammatory Biomarkers and Postpartum Depression: A Systematic Review of Literature. Can. J. Psychiatry. 2019;64(7):471–481. doi: 10.1177/0706743719828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanjewar S., Nimkar S., Jungari S. Depressed Motherhood: Prevalence and Covariates of Maternal Postpartum Depression among Urban Mothers in India. Asian J. Psychiatry. 2021;57 doi: 10.1016/J.AJP.2021.102567. [DOI] [PubMed] [Google Scholar]
- Larrieu T., Layé S. Food for mood: Relevance of nutritional omega-3 fatty acids for depression and anxiety. Front. Physiol. 2018;9:1047. doi: 10.3389/FPHYS.2018.01047/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K.P., Mössner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol. Psychiatry. 1998;44(3):179–192. doi: 10.1016/S0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- Leung B.M.Y., Kaplan B.J. Perinatal Depression: Prevalence, Risks, and the Nutrition Link—A Review of the Literature. J. Am. Diet. Assoc. 2009;109(9):1566–1575. doi: 10.1016/J.JADA.2009.06.368. [DOI] [PubMed] [Google Scholar]
- Lindsay K.L., Gibney E.R., Mcauliffe F.M. Maternal nutrition among women from Sub-Saharan Africa, with a focus on Nigeria, and potential implications for pregnancy outcomes among immigrant populations in developed countries. J. Hum. Nutr. 2012;25(6):534–546. doi: 10.1111/J.1365-277X.2012.01253.X. [DOI] [PubMed] [Google Scholar]
- Lindseth G., Petros T. Neurobehavioral Effects of Consuming Dietary Fatty Acids. Biol. Res. Nurs. 2016;18(5):573–581. doi: 10.1177/1099800416657638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liperoti R., Landi F., Fusco O., Bernabei R., Onder G. Omega-3 Polyunsaturated Fatty Acids and Depression: A Review of the Evidence. Curr. Pharm. Des. 2009;15(36):4165–4172. doi: 10.2174/138161209789909683. [DOI] [PubMed] [Google Scholar]
- Lonstein J.S., Lévy F., Fleming A.S. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm. Behav. 2015;73:156–185. doi: 10.1016/J.YHBEH.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowensohn R.I., Stadler D.D., Naze C. Current Concepts of Maternal Nutrition. Obstet. Gynecol. Surv. 2016;71(7):413. doi: 10.1097/OGX.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M., Gan, R.-Y., Li, B.-Y., Mao, Q.-Q., Shang, A., Xu, X.-Y., Li, H.-Y., H.-B., 2021. Effects and Mechanisms of Tea on Parkinson’s Disease, Alzheimer’s Disease and Depression. Food Rev. Int. 39(1), 278–306. https://dpoi.org/10.1080/87559129.2021.1904413.
- Madeghe B.A., Kogi-Makau W., Ngala S., Kumar M. Nutritional Deficiencies and Maternal Depression: Associations and Interventions in Lower and Middle-Income Countries: a Systematic Review of Literature. Glob. Soc. Welf. 2022;9(1):11–25. doi: 10.1007/S40609-020-00199-9/TABLES/2. [DOI] [Google Scholar]
- Maes M., Lin A.H., Ombelet W., Stevens K., Kenis G., De Jongh R., Cox J., Bosmans E. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinol. 2000;25(2):121–137. doi: 10.1016/S0306-4530(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Mah, B. L., Van IJzendoorn, M.H., Smith, R., Bakermans-Kranenburg, M.J., 2013. Oxytocin in postnatally depressed mothers: Its influence on mood and expressed emotion. Prog. Neuropsychopharmacol. Biol. Psychiatry, 40(1), 267–272. https://doi.org/10.1016/J.PNPBP.2012.10.005. [DOI] [PubMed]
- Makki K., Deehan E.C., Walter J., Bäckhed F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/J.CHOM.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Mandelli L., Serretti A. Gene environment interaction studies in depression and suicidal behavior: An update. Neurosci. Biobehav. Rev. 2013;37(10):2375–2397. doi: 10.1016/J.NEUBIOREV.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Markus C.R. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. Neuromol. Med. 2008;10(4):247–258. doi: 10.1007/s12017-008-8039-9. [DOI] [PubMed] [Google Scholar]
- Martin A., Cherubini A., Andres-Lacueva C., Paniagua M., Joseph J. Effects of fruits and vegetables on levels of vitamins E and C in the brain and their association with cognitive performance. J. Nutr. Health Aging. 2002;6(6):392–404. [PubMed] [Google Scholar]
- Martinowich K., Lu B. Interaction between BDNF and Serotonin: Role in Mood Disorders. Neuropsychopharmacol. 2008;33(1):73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- McQuaid R.J., McInnis O.A., Abizaid A., Anisman H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 2014;45:305–322. doi: 10.1016/J.NEUBIOREV.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin. Neurosci. 2011;13(1):89–100. doi: 10.31887/DCNS.2011.13.1/smbrody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S., Howard L.M., Bergink V., Vigod S., Jones I., Munk-Olsen T., Honikman S., Milgrom J. Postpartum psychiatric disorders. Nat. Rev. Dis. Primers. 2018;4(1):1–18. doi: 10.1038/nrdp.2018.22. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V., Steiner M., Atkinson L., Meaney M.J., Levitan R., Kennedy J.L., Sokolowski M.B., Fleming A.S. Interaction between Oxytocin Genotypes and Early Experience Predicts Quality of Mothering and Postpartum Mood. PLOS ONE. 2013;8(4):e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.J., LaRusso E.M. Preventing Postpartum Depression. Psychiatr. Clin. North Am. 2011;34(1):53–65. doi: 10.1016/j.psc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Miyake, Y., Sasaki, S., Tanaka, K., Yokoyama, T., Ohya, Y., Fukushima, W., Saito, K., Ohfuji, S., Kiyohara, C., Hirota, Y., Osaka Maternal and Child Health Study Group, 2006. Dietary folate and vitamins B12, B6, and B2 intake and the risk of postpartum depression in Japan: the Osaka Maternal and Child Health Study. J. Affect. Disord. 96(1-2), 133–138. https://doi.org/10.1016/j.jad.2006.05.024. [DOI] [PubMed]
- Mokhber N., Namjoo M., Tara F., Boskabadi H., Rayman M.P., Ghayour-Mobarhan M., Sahebkar A., Majdi M.R., Tavallaie S., Azimi-Nezhad M., Shakeri M.T., Nematy M., Oladi M., Mohammadi M., Ferns G. Effect of supplementation with selenium on postpartum depression: a randomized double-blind placebo-controlled trial. J. Matern. -Fetal Neonatal Med. 2011;24(1):104–108. doi: 10.3109/14767058.2010.482598. [DOI] [PubMed] [Google Scholar]
- Mor G., Cardenas I., Abrahams V., Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011;1221(1):80–87. doi: 10.1111/J.1749-6632.2010.05938.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L.J., Wilson C.J., Buckley J.D., Noakes M., Clifton P.M., Brinkworth G.D. Changes in endothelial function and depression scores are associated following long-term dietary intervention: A secondary analysis. Nutrition. 2013;29(10):1271–1274. doi: 10.1016/J.NUT.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Moshiri E., Basti A.A., Noorbala A.A., Jamshidi A.H., Hesameddin Abbasi S., Akhondzadeh S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13(9–10):607–611. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Mousa A., Naqash A., Lim S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients. 2019;11(2):443. doi: 10.3390/NU11020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, K., Miyake, Y., Sasaki, S., Tanaka, K., Yokoyama, T., Ohya, Y., Fukushima, W., Kiyohara, C., Hirota, Y., Osaka Maternal and Child Health Study Group, 2008. Dietary glycemic index and load and the risk of postpartum depression in Japan: the Osaka Maternal and Child Health Study. J. Affect. Disord. 110(1-2), 174–179. https://doi.org/10.1016/j.jad.2007.12.230. [DOI] [PubMed]
- Murphy P.K., Mueller M., Hulsey T.C., Ebeling M.D., Wagner C.L. An exploratory study of postpartum depression and vitamin D. J. Am. Psychiatr. Nurses Assoc. 2010;16(3):170–177. doi: 10.1177/1078390310370476. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Torner L., Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neurosci. 2000;95(2):567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Ogilvie A.D., Battersby S., Bubb V.J., Fink G., Harmar A.J., Goodwin G.M., Smith C.A.D. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. The Lancet. 1996;347(9003):731–733. doi: 10.1016/S0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Opie R.S., O’Neil A., Itsiopoulos C., Jacka F.N. The impact of whole-of-diet interventions on depression and anxiety: a systematic review of randomised controlled trials. Public Health Nutr. 2015;18(11):2074–2093. doi: 10.1017/S1368980014002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski J.L., Lambert K.G., Kinsley C.H. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Horm. Behav. 2016;77:86–97. doi: 10.1016/J.YHBEH.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Payne J.L., Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front. Neuroendocrinol. 2019;52:165–180. doi: 10.1016/J.YFRNE.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen C.A., Johnson J.L., Silva S., Bunevicius R., Meltzer-Brody S., Hamer R.M., Leserman J. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinol. 2007;32(3):235–245. doi: 10.1016/J.PSYNEUEN.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Piao M., Cong X., Lu Y., Feng C., Ge P. The Role of Zinc in Mood Disorders. Neuropsychiatry. 2017;7(4):378–386. doi: 10.4172/Neuropsychiatry.1000225. [DOI] [Google Scholar]
- Picciano M.F. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J. Nutr. 2003;133(6):1997S–2002S. doi: 10.1093/jn/133.6.1997S. [DOI] [PubMed] [Google Scholar]
- Pinsonneault J.K., Sullivan D., Sadee W., Soares C.N., Hampson E., Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression - A pilot study. Arch. Womens Ment. Health. 2013;16(6):499–509. doi: 10.1007/S00737-013-0373-8/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevin D., Galletly C. The neuropsychiatric effects of vitamin C deficiency: a systematic review. BMC Psychiatry. 2020;20(1):315. doi: 10.1186/s12888-020-02730-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost M., Zelkowitz P., Tulandi T., Hayton B., Feeley N., Carter C.S., Joseph L., Pournajafi-Nazarloo H., Yong Ping E., Abenhaim H., Gold I. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front. Public Health. 2014;2:1. doi: 10.3389/fpubh.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyanka D.K., Animesh D.K., Aninda M., Aditi S., Sonali S. Morphological, anatomical, cytological and palynological characterization of two cultivars of Abelmoschus moschatus (L.) Medik (Malvaceae) Int. J. Res. Ayurveda Pharm. 2011;2(2):670–676. [Google Scholar]
- Quinn, B.P., 1999. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Primary Care Version. Prim Care Companion J. Clin. Psychiatry. 1(2), 54-55.
- Ramadurai S.M., Nielsen H.C., Chen Y., Hatzis D., Sosenko I.R.S. Differential effects in vivo of thyroid hormone on the expression of surfactant phospholipid, surfactant protein mRNA and antioxidant enzyme mRNA in fetal rat lung. Exp. Lung Res. 1998;24(5):641–657. doi: 10.3109/01902149809099585. [DOI] [PubMed] [Google Scholar]
- Ranjbar E., Kasaei M.S., Mohammad-Shirazi M., Nasrollahzadeh J., Rashidkhani B., Shams J., Mostafavi S.A., Mohammadi M.R. Effects of zinc supplementation in patients with major depression: a randomized clinical trial. Iranian J. psychiatry. 2013;8(2):73–79. [PMC free article] [PubMed] [Google Scholar]
- Rechenberg K., Humphries D. Nutritional interventions in depression and perinatal depression. Yale J. Biol. Med. 2013;86(2):127–137. [PMC free article] [PubMed] [Google Scholar]
- Reimers, A., Ljung, H., 2019. The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders. Ther. Adv. Psychopharmacol. 9, 2045125319858901. https://doi.org/10.1177/2045125319858901. [DOI] [PMC free article] [PubMed]
- Ren Y., Sun-Waterhouse D., Ouyang F., Tan X., Li D., Xu L., Li B., Wang Y., Li F. Apple phenolic extracts ameliorate lead-induced cognitive impairment and depression- and anxiety-like behavior in mice by abating oxidative stress, inflammation and apoptosis via the miR-22-3p/SIRT1 axis. Food Funct. 2022;13(5):2647–2661. doi: 10.1039/d1fo03750a. [DOI] [PubMed] [Google Scholar]
- Rook G.A.W., Raison C.L., Lowry C.A. Microbiota, immunoregulatory old friends and psychiatric disorders. Adv. Exp. Med. Biol. 2014;817:319–356. doi: 10.1007/978-1-4939-0897-4_15/COVER. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villegas A., Verberne L., De Irala J., Ruíz-Canela M., Toledo E., Serra-Majem L., Martínez-González M.A. Dietary fat intake and the risk of depression: the SUN Project. PloS one. 2011;6(1) doi: 10.1371/journal.pone.0016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller C.E., O’Hara M.W., Rubinow D.R., Johnson A.K. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol. Behav. 2013;119:137–144. doi: 10.1016/J.PHYSBEH.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoretsanitis G., Westin A.A., Stingl J.C., Deligiannidis K.M., Paulzen M., Spigset O. Antidepressant transfer into amniotic fluid, umbilical cord blood & breast milk: A systematic review & combined analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;107 doi: 10.1016/J.PNPBP.2020.110228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Burt V.K., Ritchie H.L. Assessment and treatment of bipolar II postpartum depression: a review. J. Affect. Disord. 2010;125(1–3):18–26. doi: 10.1016/j.jad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Sharma V., Al-Farayedhi M., Doobay M., Baczynski C. Should all women with postpartum depression be screened for bipolar disorder? Med. Hypotheses. 2018;118:26–28. doi: 10.1016/J.MEHY.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Sharma V., Doobay M., Baczynski C. Bipolar postpartum depression: An update and recommendations. Journal of affective disorders. 2017;219:105–111. doi: 10.1016/j.jad.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Sharma V., Sharma P. Postpartum Depression: Diagnostic and Treatment Issues. J. Obstet. Gynaecol. Can. 2012;34(5):436–442. doi: 10.1016/S1701-2163(16)35240-9. [DOI] [PubMed] [Google Scholar]
- Sheik H.S., Vedhaiyan N., Singaravel S. Evaluation of Abelmoschus moschatus seed extract in psychiatric and neurological disorders. Int. J. Basic Clin. Pharmacol. 2014;3:845–853. [Google Scholar]
- Shinde M., Chaudhari S., Gilhotra R. Evaluation of Central Nervous System depressant action of catharanthus roseus G. Don leaves in mice. Res. J. Pharm. Tech. 2021;14(3):1719–1722. doi: 10.5958/0974-360X.2021.00306.1. [DOI] [Google Scholar]
- Skrundz M., Bolten M., Nast I., Hellhammer D.H., Meinlschmidt G. Plasma Oxytocin Concentration during Pregnancy is associated with Development of Postpartum Depression. Neuropsychopharmacol. 2011;36(9):1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomian J., Honvo G., Emonts P., Reginster J.Y., Bruyère O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Women’s health (Lond) 2019;15 doi: 10.1177/1745506519844044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgdrager F.J.H., Naudé P.J.W., Kema I.P., Nollen E.A., De Deyn P.P. Tryptophan metabolism in inflammaging: From biomarker to therapeutic target. Front. Immunol. 2019;10:2565. doi: 10.3389/FIMMU.2019.02565/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling T.M., Nesbitt R.C., Henschke N., Gabrysch S. Nutrients and perinatal depression: a systematic review. J. Nutr. Sci. 2017;6:e61. doi: 10.1017/jns.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen A.M., Champ M.M.J., Cloran S.J., Fleith M., Van Lieshout L., Mejborn H., Burley V.J. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017;30(2):149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- Stoffel E.C., Craft R.M. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol. Behav. 2004;83(3):505–513. doi: 10.1016/J.PHYSBEH.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Stuebe A.M., Grewen K., Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J. Women’s Health. 2013;22(4):352–361. doi: 10.1089/JWH.2012.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004;558(1):263–275. doi: 10.1113/JPHYSIOL.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan P.J., Kennedy C.E., Hurley K.M., Black M.M. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull. World Health Organ. 2011;89(8):608–615. doi: 10.2471/BLT.11.088187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar S.A., Martin N.G., Bucholz K.K., Madden P.A., Heath A.C. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol. Med. 1999;29(3):645–654. doi: 10.1017/s0033291799008387. [DOI] [PubMed] [Google Scholar]
- Trujillo J., Vieira M.C., Lepsch J., Rebelo F., Poston L., Pasupathy D., Kac G. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J. Affect. Disord. 2018;232:185–203. doi: 10.1016/J.JAD.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Trumbo P., Schlicker S., Yates A.A., Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002;102(11):1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- Upadhyay R.P., Chowdhury R., Salehi A., Sarkar K., Singh S.K., Sinha B., Pawar A., Rajalakshmi A.K., Kumar A. Postpartum depression in India: a systematic review and meta-analysis. Bull. World Health Organ. 2017;95(10):706–717C. doi: 10.2471/BLT.17.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh U., Sharma A., Ananthan V.A., Subbiah P., Durga R., n.d. CSIR Summer Research training team Micronutrient’s deficiency in India: a systematic review and meta-analysis. J. Nutr. Sci. 2021;10 doi: 10.1017/jns.2021.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venning E.H. Endocrine changes in normal pregnancy. Am. J. Med. 1955;19(5):721–723. doi: 10.1016/S0002-9343(55)80014-9. [DOI] [PubMed] [Google Scholar]
- Viuff A.C., Sharp G.C., Rai D., Henriksen T.B., Pedersen L.H., Kyng K.J., Staunstrup N.H., Cortes A., Neumann A., Felix J.F., Tiemeier H., Jaddoe V.W.V., Relton C.L. Maternal depression during pregnancy and cord blood DNA methylation: findings from the Avon Longitudinal Study of Parents and Children. Transl. Psychiatry. 2018;8(1):244. doi: 10.1038/S41398-018-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Um P., Dickerman B.A., Liu J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence. Potential Mechanisms and Implications. Nutrients. 2018;10(5):584. doi: 10.3390/nu10050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J., Fritsche K. Linoleic Acid. Adv. Nutr. 2013;4(3):311–312. doi: 10.3945/AN.113.003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner K.L., Sit D.K.Y., McShea M.C., Rizzo D.M., Zoretich R.A., Hughes C.L., Eng H.F., Luther J.F., Wisniewski S.R., Costantino M.L., Confer A.L., Moses-Kolko E.L., Famy C.S., Hanusa B.H. Onset Timing, Thoughts of Self-harm, and Diagnoses in Postpartum Women With Screen-Positive Depression Findings. JAMA Psychiatry. 2013;70(5):490–498. doi: 10.1001/JAMAPSYCHIATRY.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., Sinha R., Gilroy E., Gupta K., Baldassano R., Nessel L., Li H., Bushman F.D., Lewis J.D. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/SCIENCE.1208344/SUPPL_FILE/WU.SOM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.X., Li J., Zhou D.D., Xiong R.G., Huang S.Y., Saimaiti A., Shang A., Li H.B. Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants. 2022;11(11):2132. doi: 10.3390/antiox11112132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.Y., Lin M.Y., Tsai P.S. Alternate healthy eating index and risk of depression: A meta-analysis and systematic review. Nutr. Neurosci. 2020;23(2):101–109. doi: 10.1080/1028415X.2018.1477424. [DOI] [PubMed] [Google Scholar]
- Xu Y., Wang C., Klabnik J.J., O'Donnell J.M. Novel therapeutic targets in depression and anxiety: antioxidants as a candidate treatment. Curr. Neuropharmacol. 2014;12(2):108–119. doi: 10.2174/1570159X11666131120231448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Zhao A., Lan H., Ren Z., Zhang J., Szeto I.M., Wang P., Zhang Y. Association Between Dietary Quality and Postpartum Depression in Lactating Women: A Cross-Sectional Survey in Urban China. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.705353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim I.S., Glynn L.M., Schetter C.D., Hobel C.J., Chicz-DeMet A., Sandman C.A. Risk of Postpartum Depressive Symptoms with Elevated Corticotropin-Releasing Hormone in Human Pregnancy. Arch. Gen. Psychiatry. 2009;66(2):162–169. doi: 10.1001/ARCHGENPSYCHIATRY.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Joshi-Barve S., Barve S., Chen L.H. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J. Am. Coll. Nutr. 2004;23(1):71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen C., Yu H., Yang Z. Fecal Microbiota Changes in Patients with Postpartum Depressive Disorder. Front. Cell. Infect. Microbiol. 2020;10:511. doi: 10.3389/FCIMB.2020.567268/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Wang T., Yu Y., Li M., Sun X., Song W., Wang Y., Zhang C., Fu F. The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113146. [DOI] [PubMed] [Google Scholar]