Abstract
AIM
To evaluate the effect of 0.05% atropine on the control of myopia for 2y (phase I) and on spherical equivalent refraction (SER) progression for 1y (phase II) after its withdrawal in Chinese myopic children.
METHODS
Totally 142 children with myopia were randomly assigned to the 0.05% atropine group or to the placebo group. In phase I, children received 1 treatment for each eye daily. In phase II, the patients received no treatment. Axial length (AL), SER, intraocular pressure (IOP) and atropine-related side effects were assessed at 6 months' intervals.
RESULTS
During phase I, the mean change of SER was -0.46±0.30 D in the atropine group, compared to -1.72±1.12 D in the placebo group (P<0.001). The mean change of AL in the atropine group (0.26±0.30 mm) was significantly shorter than that in the placebo group (0.76±0.62 mm, P=0.002). In addition, in phase II (12mo after the withdrawal of atropine), there was no significant difference in AL change from the atropine group, when compared with that from the placebo group (0.31±0.25 mm vs 0.28±0.26 mm, P>0.05). Furthermore, the change in SER from the atropine group was 0.50±0.41 D, which was significantly lower than 0.72±0.60 D from placebo group, (P<0.05). Finally, there were no statistically significant differences in IOP between the treatment and control groups at any stages (all P>0.05).
CONCLUSION
The use of 0.05% atropine for two consecutive years may effectively control elongation of AL and thus progression of myopia, without significant SER progression 1y after atropine withdrawal. Therefore, treatment with 0.05% atropine daily for 2y is effective and safe.
Keywords: atropine, axial length, spherical equivalent refraction, children, myopia
INTRODUCTION
Myopia, short-sightedness or nearsightedness, is a major disease that develops primarily in childhood and early adulthood, due to excessive elongation of the eye which causes images from distant objects coming into the focus in front of the retina, leading to blurred distance vision[1]. In China, the prevalence of myopia with cycloplegia has been reported to range from 47.4% in children grading 1–9[2] to 65.48% in children from 3rd year junior high school[3] though also reported as low as 23.3% in children aging 6–17 years old[4] (also reviewed in[5]). Therefore, it is crucial to find an effective method to control its progression in China.
Myopia is characterized by increased axial length (AL) of the eye, which causes the refractive error known as “nearsightedness”[6]. Due to increased anterior-posterior diameter of the eye relative to the refracting power of the cornea and lens, the focal point of the image, anterior to the retina, is blurred. For this reason, close objects are seen clearly while distant objects appear blurry or distorted. Myopia is also characterized by the degree of refractive error. In myopia, the spherical equivalent objective refractive error is less than -0.50 diopter (D) but greater than -5.00 D, and high myopia is defined as a spherical equivalent objective refractive error of ≤-5.00 D in either eye by WHO[7], or a condition in which the spherical equivalent refractive error of an eye is ≤-6.00 D when ocular accommodation is relaxed by International Myopia Institute (IMI Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies—Myopia Institute).
Changes of AL closely associated with refractive error occur most commonly and at highest rates during childhood and puberty, which place school aged children at highest risk for developing myopia[8]–[9]. Due to rapid progression of myopia during early childhood and puberty, early childhood is the optimal period for the collective prevention and therapy of myopia, and other serious eye diseases (myopic macular degeneration and retinal detachment) from occurring later in life[9].
New spectacle lens and contact lens treatments provide alternative methods to treat myopic progression. The Stellest and Defocus Incorporated Multiple Segments (DIMS) spectacle lenses have been granted “breakthrough device” status and Misight contact lenses received FDA approval (2019)[10]–[14]. These lens treatments are innovative with great efficacy. However, topical atropine may also provide a more cost effective and widely available treatment, as compared to the proprietary methods, depending on the geographic areas.
Topical atropine solution has proven efficacy for treating myopia by inhibition of myopic refraction error and axial elongation[15]–[32]. Clinical studies have confirmed that atropine may control the diopter and the growth of the axial axis at the same time. The commonly used atropine concentrations are 1%, 0.5%, 0.1%, 0.01%, and the control effect on myopia is concentration-dependent[33]. Nevertheless, the side effects after withdrawal are also apparent[33]–[34]. We have chosen atropine at the dose of 0.05%, effective with minimal side effects in our study, consistent with the optimal dose in the low-concentration atropine for myopia progression (LAMP) and other studies[17],[35]–[36], though recent studies have also suggested that 0.01% atropine is also effective to delay myopia progression[37]–[38]. Our results suggest that use of 0.05% atropine for two consecutive years may effectively control myopia progression, with minimal progression 1y after atropine withdrawal.
SUBJECTS AND METHODS
Ethical Approval
All myopia children and their legal guardians were informed for the consent of the purpose, significance and eye examination process of this study and signed informed consent. This study followed the Helsinki Declaration and was approved by the Ethics Committee of the Affiliated Hospital of Yunnan University, China (No.YN2019110327).
General Information
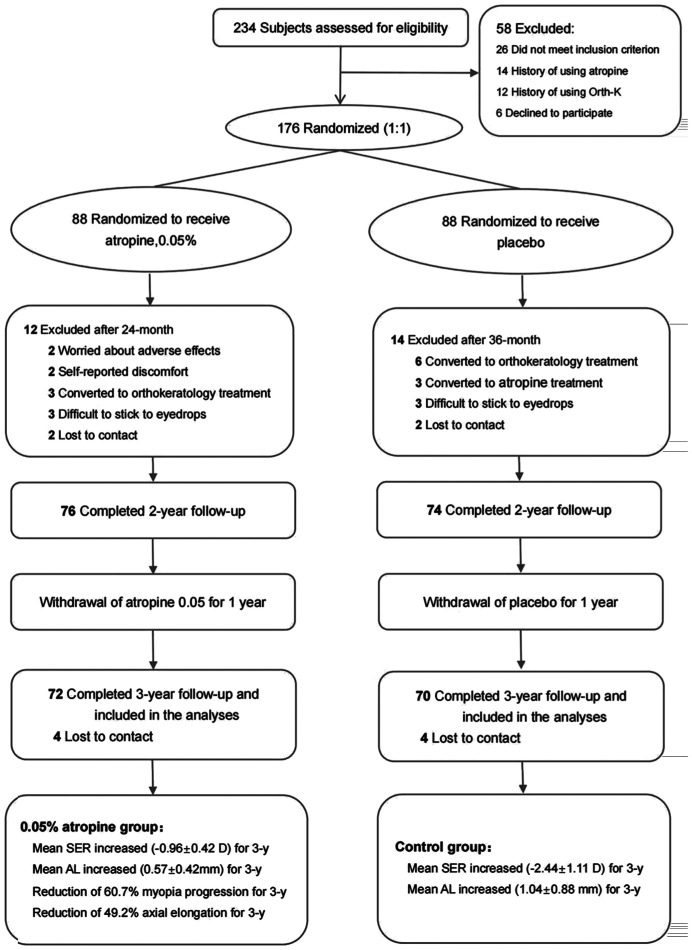
From January 2019 to January 2022, a total of 176 cases of 7 to 12 years-old myopia pupils admitted to the Department of Pediatric Ophthalmology, Affiliated Hospital of Yunnan University were randomly divided into the atropine and placebo groups in a ratio of 1:1. The 142 participants completed the 3-year study (72 cases in the atropine group and 70 cases in the placebo group). Since 0.05% atropine was prepared from 1% atropine sulfate in 0.3% sodium hyaluronate, 0.3% sodium hyaluronate was used in the placebo group.
Sample Size
Sample size was calculated based on a previous recommendation[17]. We assumed that 0.05% atropine could reduce the myopia progression rate by at least 0.40 D with standard deviation of 0.60 D throughout a 12-month period, thus, 66 participants were required in each group for an α level of 0.05 (2-tailed), a power of 90%. Considering a dropout rate of 25%, the sample size required a total of 176 participants.
Study Procedures
Subject inclusion and exclusion criteria
Inclusion criteria: 1) myopic refraction range [spherical equivalent refraction (SER)] was from -6.0 to -1.00 D; 2) myopia children were 7 to 12 years old, willing to participate in the 3-year clinical observation; 3) the annual growth rate of SER was ≥0.75 D/y before the investigation; 4) the binocular vision was normal; 5) initial intraocular pressure (IOP) was <21 mm Hg; 6) The patients were tolerable to atropine eye drops. During the follow-up, the ciliary muscle paralysis agent was used to dilate the pupils during the treatment period. Exclusion criteria: 1) children had histories of allergies to atropine; 2) children had histories of ocular organic diseases such as strabismus, amblyopia, congenital cataract and optic nerve dysplasia; 3) children had histories of ocular surgeries; 4) children had histories of systemic diseases that affected visual function, such as diabetes and chromosomal abnormalities; 5) children had histories of use of contact lenses, bifocal or multifocal lenses, or other myopia treatments (such as pilocarpine).
Baseline data
There were no statistically significant differences in age, gender composition, SER, IOP, parental age and diopter between the atropine and the placebo groups before the treatment (all P>0.05; Table 1).
Table 1. Statistical characteristics of baseline data.
| Parameters | Atropine group (n=72) | Placebo group (n=70) | P |
| Male (n, %) | 35 (48.61) | 36 (51.43) | 0.80 |
| Female (n, %) | 37 (51.39) | 34 (48.57) | 0.78 |
| Age (y) | 9.16±0.73 | 9.22±0.81 | 0.21 |
| IOP (mm Hg) | 16.81±3.12 | 16.32±2.99 | 0.25 |
| Initial SER (D) | -3.26±0.20 | -3.27±0.32 | 0.32 |
| Initial AL (mm) | 23.71±0.23 | 23.69±0.19 | 0.31 |
| Distance VA (logMAR) | 0.03±0.08 | 0.02±0.07 | 0.54 |
| Maternal SER (D) | -3.25±3.18 | -3.31±3.22 | 0.17 |
| Paternal SER (D) | -4.05±3.88 | -4.10±3.78 | 0.30 |
| Maternal age (y) | 26.83±3.69 | 27.02±5.37 | 0.21 |
| Paternal age (y) | 28.78±4.99 | 28.95±6.73 | 0.48 |
SER: Spherical equivalent refraction; AL: Axial length; D: Diopter; IOP: Intraocular pressure; SD: Standard deviation; VA: Visual acuity.
mean±SD
Spectroscopy and medications
Participants in this study were randomized to receive one drop of the atropine or placebo eye drops once nightly in both eyes for continuous medication for 24mo and then withdrawal of atropine or placebo for 12mo, which was administered by their parents following the doctors' instructions. To reduce the photophobic response after treatment and to protect the eye tissues from ultraviolet (UV) damage, the atropine and the placebo groups were equipped with UV-sensitive photo chromatic single vision lens (SVL).
Data collection
Before atropine or placebo administration, 6, 12, 18, 24, 30, and 36mo after atropine or placebo administration or atropine withdrawal, the SER (D), AL (mm), and IOP (mm Hg) were measured. The adjusted change of the SER and AL from the baseline was also calculated and recorded. The mean ocular parameters measured in the right eye were used because there was a high correlation between the two eyes. The incidence and duration of adverse reactions after atropine or placebo use were obtained from the answered questionnaires by the participants.
Measurement
Refractive error was measured in each eye by the autorefractor (KR-8800, Topcon, Tokyo, Japan), and three designed measurements were averaged. Cycloplegia was obtained using 0.5% proparacaine or tetracaine, 1 drop, followed by 1.0% tropicamide, 2 drops, with a gap of 5min. Measurements were performed 25min after the two treatments mentioned above. Five good measurements of AL were obtained and averaged in each eye before the cycloplegia by the partial coherence interferometry IOLMaster (Carl Zeiss 500, Meditec, Oberkochen, Germany).
Statistical Analysis
SPSS statistical software version 26 (IBM) was used for statistical analyses. Repeated measurement analyses were carried out using generalized estimating equation (GEE) to model myopia progression and eye growth. The treatment efficacy was determined via dividing the values of the calculated between-arm difference by the values of the control arm. Chi-square test was used to compare categorical data, expressed as mean±standard deviation. The comparison between the atropine and control groups was conducted using ANOVA and independent sample t test. P<0.05 was considered statistically significant.
RESULTS
Changes of Spherical Equivalent Refraction and Axial Length
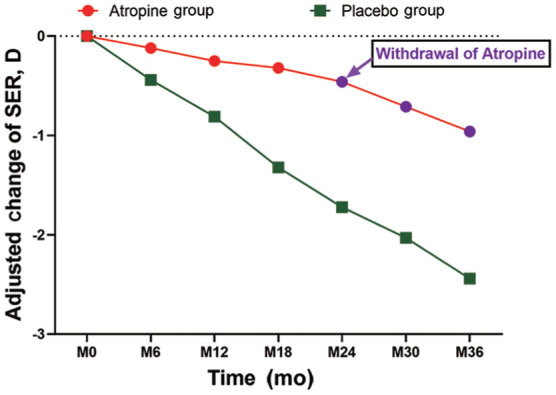
In phase I (2y), adjusted change of SER from the baseline was -0.46±0.30 D in the atropine group and -1.72±1.12 D in the placebo group. The difference of SER change between the atropine and placebo groups was statistically significant using the pairwise comparison (P<0.001; Table 2, Figure 1).
Table 2. Changes of SER in the atropine and placebo groups before and after treatment.
| Variables | Atropine group (n=72) | Placebo group (n=70) | Difference | P |
| 0.05% atropine or placebo | ||||
| Change at 6mo, D | -0.12±0.26 | -0.44±0.32 | 0.32±0.06 | 0.015 |
| Change at 12mo, D | -0.25±0.29 | -0.81±0.65 | 0.56±0.06 | 0.008 |
| Change at 18mo, D | -0.32±0.31 | -1.36±0.73 | 1.05±0.07 | 0.006 |
| Change at 24mo, D | -0.46±0.30 | -1.72±1.12 | 1.26±0.08 | <0.001 |
| Withdrawal of 0.05% atropine | ||||
| Change at 30mo, D | -0.70±0.35 | -2.03±1.13 | 1.32±0.09 | <0.001 |
| Change at 36mo, D | -0.96±0.42 | -2.44±1.11 | 1.48±0.09 | <0.001 |
P values were obtained by generalized estimating equation models with age, sex, and baseline standard error (SE) adjustment for SER comparisons. SER: Spherical equivalent refraction.
Figure 1. Adjusted changes of spherical equivalent refraction in the atropine and placebo groups by time course analysis.
AL change at 2-year period was larger in the placebo group (0.76±0.62 mm) than that in the atropine group (0.26±0.30 mm, P<0.001; Table 3 and Figure 2). The difference of AL change between the atropine and placebo groups in the pairwise comparison was statistically significant (P=0.002; Table 3, Figure 2). After withdrawal of atropine for 1y in phase II, the change in SER from the atropine group was 0.50±0.41 D, which was significantly lower than 0.72±0.60 D from placebo group (P<0.05), suggesting that there is some continuing inhibition of progression of SER after withdrawal, which requires further investigation.
Table 3. Changes of AL in the atropine and placebo groups before and after treatment.
| Variables | Atropine group (n=72) | Placebo group (n=70) | Difference | P |
| 0.05% atropine or placebo | ||||
| Change at 6mo, mm | 0.08±0.18 | 0.15±0.32 | 0.08±0.02 | 0.024 |
| Change at 12mo, mm | 0.14±0.20 | 0.31±0.35 | 0.17±0.02 | 0.017 |
| Change at 18mo, mm | 0.22±0.31 | 0.55±0.43 | 0.34±0.03 | 0.008 |
| Change at 24mo, mm | 0.26±0.30 | 0.76±0.62 | 0.50±0.04 | 0.002 |
| Withdrawal of 0.05% atropine | ||||
| Change at 30mo, mm | 0.41±0.35 | 0.89±0.73 | 0.48±0.05 | 0.003 |
| Change at 36mo, mm | 0.57±0.36 | 1.04±0.88 | 0.47±0.05 | <0.001 |
P values were generated by generalized estimating equation models with age, sex, and baseline AL adjustment for spherical equivalent refraction comparisons. AL: Axial length.
Figure 2. Adjusted changes of axial length in atropine and placebo groups by time course analysis.
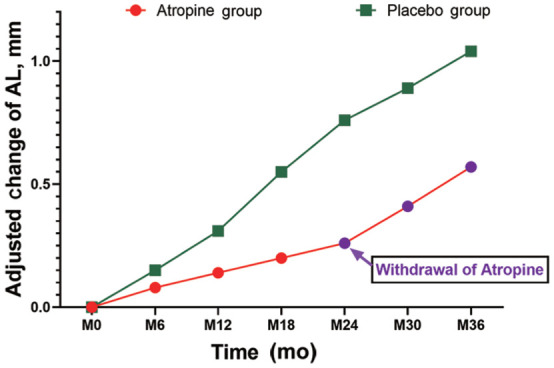
It seems that there is some continuing inhibition of progression of SER after cessation of atropine treatment when compared with that from the placebo group, which requires further investigation.
During the 2-year treatment period, the axial growth rate from the atropine group (0.26±0.30 mm) was significantly slower than that from placebo group (0.76±0.62 mm), and the difference was 0.50±0.04 mm (P<0.05). After withdrawal of atropine for 1y in phase II, AL change from the atropine group was 0.31±0.25 mm, which was not significantly different from 0.28±0.26 mm from placebo group (P>0.05), indicating that after cessation of 0.05% atropine, there is no significant AL rebound (Tables 2 and 3, Figures 1 and 2).
In the whole 3y, 87% of the subjects in the atropine group had myopia progression by less than 1.0 D, compared with 17% in the placebo group. In addition, none of myopia subjects in the atropine group had myopia progression by more than 2.0 D, compared with 31% in the placebo group.
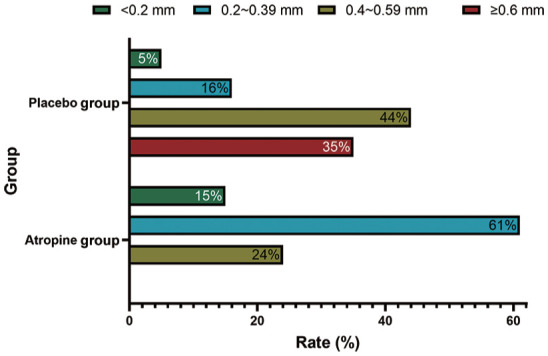
Besides, 76% of the subjects in the atropine group showed elongation of AL less than 0.4 mm, compared with only 21% in the placebo group. Furthermore, none of myopia subjects in the atropine group showed elongation of AL by more than 0.6 mm, compared with 35% in the placebo group.
Intraocular Pressure
During the follow-up period of 3y, a non-contact tonometer was used to measure IOP at 6mo intervals, no significant increase in IOP was noted in either group.
Other Adverse Reactions
According to the results obtained from the questionnaires, the main adverse reactions in the atropine group were mostly mild photophobia, which disappeared within 7d. In addition, there were no severe allergies, headaches and rainbow-vision.
The control of myopia by 0.05% atropine eye drops may be summarized in Figure 3.
Figure 3. CONSORT flow diagram of the treatment of myopia by 0.05% atropine eye drops.
DISCUSSION
In this placebo-controlled clinical trial, we demonstrate that during the first 2-year period, there was a myopia shift in refraction of -0.46±0.30 D in the atropine group, in contrast to that of -1.72±1.12 D in the placebo group (Table 2, Figure 1). The myopia shift in refraction in the atropine group was 73.26% slower than that in the placebo group (Table 2, Figure 1). In addition, a myopia shift of AL in the atropine group was 0.26±0.30 mm, in contrast to that 0.76±0.62 mm in the placebo group (Table 3, Figure 2). Therefore, the axial growth in the atropine group was 65.79% slower, compared with that in the placebo group (Table 3, Figure 2). Importantly, statistically significant differences were detected in the SER and AL between the atropine and the placebo groups at all time points after treatments (Tables 2 and 3, Figures 1 and 2, all P<0.05). Interestingly, no statistically significant differences were found in IOP between the atropine and the placebo groups at all time points, suggesting that administration of 0.05% atropine daily does not cause an increase in IOP in the children. In addition, 12mo after atropine withdrawal (phase II), the AL change from the atropine group was 0.31±0.25 mm, which was not significantly different from 0.28±0.26 mm from the placebo group (Table 3, Figure 2; P>0.05), indicating that cessation of 0.05% atropine eye drops does not lead to AL rebound while the change in SER from the atropine group was 0.50±0.41 D, which was significantly lower than 0.72±0.60 D from the placebo group (Table 2 and Figure 1; P<0.05), indicating that there is still a continuous inhibitory effect on the AL progression 12mo after cessation of 0.05% atropine eye drops. inhibitory effect, which requires further investigation. The results summarized from the answers to questionnaires showed that the main adverse reactions in the atropine group were mild to moderate photophobia (14%), usually lasted within a week, resembling what has been reported previously[39]. The optimal concentration of atropine to control the progression of myopia in adolescents should be the best balance between efficacy and side effects. In the ATOM2 experiment, the annual SER progression of myopia in the 1%, 0.5%, 0.1%, and 0.01% groups was -0.28±0.92 D, -0.30±0.60 D, -0.38±0.60 D, and -0.49±0.63 D respectively, compared to -1.20±0.69 D in the placebo group, showing that 1% concentration of atropine has the best control effect (78%), followed by 0.5% (75%), 0.1% (70%) and 0.01% (50%)[34]. However, after withdrawal of the medication in the ATOM2 experiment, the rebound effect was closely associated with atropine concentration. That is, the higher the concentration of atropine is, the more severe the rebound after withdrawal. Therefore, it is critical to determine the long-term efficacy of different concentrations of atropine. In our study, the change in SER after 1y of atropine withdrawal was slightly less compared to the placebo group (0.50 D vs 0.72 D) although the change in AL was relatively similar (0.31±0.25 vs 0.28±0.26 mm, P>0.05; Tables 2 and 3, Figures 1 and 2), indicating that after cessation of atropine, there is no significant AL and SER rebound, and to certain extent, it may also have potential inhibitory effect on SER progression.
In series of LAMP studies, the authors show that of the 3 doses (0.05%, 0.025%, and 0.01%) of atropine, 0.05% atropine is the most effective in retarding AL elongation and SER progression in a 1-year trial[17]. In a successive LAMP trial, the authors demonstrate that the efficacy of 0.05% atropine is twice as that of 0.01% atropine in a 2-year trial[35]. In the latest LAMP trial, the authors indicate that low concentrations of atropine may induce a choroidal thickening associated with slower AL elongation and SER progression[40]. These studies support our decision to use 0.05% atropine for our investigation because 0.05% atropine is the best among doses of 0.01%, 0.025% and 0.05% atropine.
During phase I (2y), there was a myopia shift in refraction of -0.23±0.19 D/y in the atropine group, compared to -0.86±0.55 D/y in the placebo group, and the axial growth rate was 0.13±0.06 mm/y in the atropine group, compared to 0.38±0.20 mm/y in the placebo group. In addition, compared with the placebo group, SER progression rate decreased by 73%, and AL progression rate decreased by 66% in the atropine group during the same period. Therefore, our efficacy of 0.05% atropine resembles that in 0.1% group of the ATOM2 study[34]. Because after withdrawal of the medication in the ATOM2 experiment, myopic SER progression also showed significant concentration correlation with side effects, because the higher the concentration of atropine was, the worse the SER rebound[34], therefore, it is important to determine the rebound effect after withdrawal of atropine. Thus, we performed 1-year withdrawal of atropine experiment (phase II). In our study, we did not see any significant redound of myopia after withdrawal of 0.05% atropine for one year when compared with the placebo group (P>0.05), suggesting that 0.05% atropine may effectively control the progression of myopia, and does not cause significant AL and SER rebound after withdrawal of atropine.
In the whole 3y, the mean change of SER was -0.96±0.42 D in the atropine group, compared to -2.44±1.11 D in the placebo group, the difference was statistically significant (Table 2, Figure 1; P<0.001). The mean change of AL in the atropine group (0.57±0.36 mm/y) was shorten than that in the placebo group (1.04±0.88 mm/y), and the difference was also statistically significant (Table 3, Figure 2; P=0.002), suggesting that compared with the placebo group, SER progression rate was decreased by 60.66%, and AL elongation rate was decreased by 45.2%. In addition, 87% of the subjects in the atropine group had myopia progression by less than 1.0 D, compared with 17% in the placebo group while none of myopia subjects in the atropine group had myopia progression by more than 2.0 D, compared with 31% in the placebo group (Figure 4). Furthermore, 76% of the subjects in the atropine group showed elongation of AL less than 0.4 mm, compared with only 21% in the placebo group while none of myopia subjects in the atropine group showed elongation of AL by more than 0.6 mm, compared with 35% in the placebo group (Figure 5).
Figure 4. Distribution of changes of spherical equivalent refraction between the atropine and placebo groups at 3y.
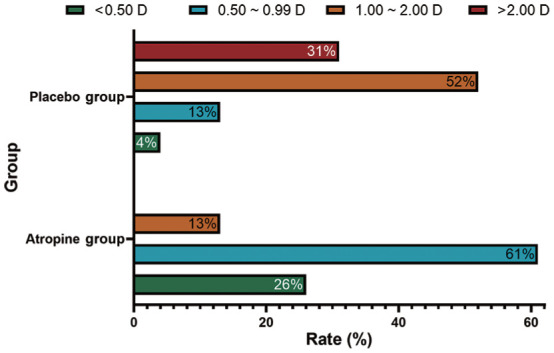
Figure 5. Distribution of changes of axial length between 0.05% atropine and placebo groups at 3y.
The main adverse reactions of atropine include photophobia and near-ambiguity[34],[39]. Photophobia is caused by atropine acting on the ciliary muscle M receptor, resulting in contraction of the ciliary muscle and enlargement of the pupil. The higher the concentration of atropine, the more severe the effect of dilatation. In our experiment, 0.05% atropine was used, and the effect of dilatation was still evident. Therefore, to reduce the risk of photophobia and potential intraocular photodamage caused by pupil dilation, all patients were wearing UV photochromic lenses. Only a small number of children complained mild photophobia. By the way, there was no statistical significance in IOP before and after treatment in both the atropine and the control groups (data not shown), suggesting that IOP is not a factor for control of myopia by atropine.
In summary, our study has demonstrated that 0.05% atropine may simultaneously retard the progression of SER and inhibit the elongation of AL. If combined with UV photochromic multifocal lenses, the side effects may be prohibited. As to the mechanism, we believe that, first, atropine may act at a relatively low dose through a neurochemical cascade[41]–[42]; second, myopia may be associated with increased chronic inflammation of the eye, while chronic inflammation may be downregulated by atropine[43]. In addition, intraocular nitric oxide may inhibit myopia dose-dependently, obligatory for inhibition of myopia by atropine[44]. The detailed mechanism requires further investigation (possible causes reviewed in[45]–[48]).
In conclusion, our results provide new evidence that 0.05% atropine can effectively reduce myopia progression and axial retraction, which is well tolerated, with no significant adverse effects on quality of life. Its anti-myopia mechanism needs further study. We recommend treatment of myopia by 0.05% atropine daily as a standard method for the control of myopia progression, with progressive multifocal lens if possible.
Acknowledgments
Foundations: Supported by the Special Fund for Young and Middle-aged Academic Technology Leaders and Reserve Talents of Yunnan Province (No.202005AC160021); the Famous Doctor of Yun Ling (No.YNWR-MY-2020-088).
Conflicts of Interest: Zhu Q, None; Tang GY, None; Hua ZJ, None; Xue LP, None; Zhou Y, None; Zhang JY, None; Zhu YT, None; Zhang XF, None.
REFERENCES
- 1.Baird PN, Saw SM, Lanca C, et al. Myopia. Nat Rev Dis Primers. 2020;6(1):99. doi: 10.1038/s41572-020-00231-4. [DOI] [PubMed] [Google Scholar]
- 2.Guo L, Yang J, Mai J, Du X, Guo Y, Li P, Yue Y, Tang D, Lu C, Zhang WH. Prevalence and associated factors of myopia among primary and middle school-aged students: a school-based study in Guangzhou. Eye (Lond) 2016;30(6):796–804. doi: 10.1038/eye.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Liu J, Qi PC. The increasing prevalence of myopia in junior high school students in the Haidian District of Beijing, China: a 10-year population-based survey. BMC Ophthalmol. 2017;17(1):88. doi: 10.1186/s12886-017-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z, Gao TY, Vasudevan B, Jhanji V, Ciuffreda KJ, Zhang P, Li L, Mao GY, Wang NL, Liang YB. Generational difference of refractive error and risk factors in the Handan Offspring Myopia Study. Invest Ophthalmol Vis Sci. 2014;55(9):5711–5717. doi: 10.1167/iovs.13-13693. [DOI] [PubMed] [Google Scholar]
- 5.Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20(1):27. doi: 10.1186/s12886-019-1220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flitcroft DI, He MG, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, Rahi J, Resnikoff S, Vitale S, Yannuzzi L. IMI—defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20–M30. doi: 10.1167/iovs.18-25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. The Impact of Myopia and High Myopia In: Myopia JWHO-BHVIGSMo, editor. WHO Library. World Health Organization. 2016.
- 8.Mutti DO, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Zadnik K, Study Group CE Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, Manny RE, Mutti DO, Yu JA, Zadnik K, Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study Group Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121(8):1141–1147. doi: 10.1001/archopht.121.8.1141. [DOI] [PubMed] [Google Scholar]
- 10.FDA approves first contact lens indicated to slow the progression of nearsightedness in children. U.S. Food & Drug Administration. 2019 https://www.fda.gov/news-events/press-announcements/fda-approves-first-contact-lens-indicated-slow-progression-nearsightedness-children . [Google Scholar]
- 11.Essilor stellest receives FDA breakthrough device designation. Eyecare Business. 2021 https://www.eyecarebusiness.com/news/2021/essilor-stellest-receives-fda-breakthrough-device . [Google Scholar]
- 12.Lam CSY, Tang WC, Tse DYY, Lee RPK, Chun RKM, Hasegawa K, Qi H, Hatanaka T, To CH. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. 2020;104(3):363–368. doi: 10.1136/bjophthalmol-2018-313739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam CSY, Tang WC, Qi H, Radhakrishnan H, Hasegawa K, To CH, Charman WN. Effect of defocus incorporated multiple segments spectacle lens wear on visual function in myopic Chinese children. Transl Vis Sci Technol. 2020;9(9):11. doi: 10.1167/tvst.9.9.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HY, Lam CSY, Tang WC, Leung M, To CH. Defocus incorporated multiple segments spectacle lenses changed the relative peripheral refraction: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2020;61(5):53. doi: 10.1167/iovs.61.5.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q, Hao Q. Clinical efficacy of 0.01% atropine in retarding the progression of myopia in children. Int Ophthalmol. 2021;41(3):1011–1017. doi: 10.1007/s10792-020-01658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi S, Huang YS, Yu SZ, Chen XJ, Yi H, Zeng XL. Therapeutic effect of atropine 1% in children with low myopia. J Am Assoc Pediatr Ophthalmol Strabismus. 2015;19(5):426–429. doi: 10.1016/j.jaapos.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Yam JC, Jiang YN, Tang SM, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113–124. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Wu PC, Chuang MN, Choi J, Chen H, Wu G, Ohno-Matsui K, Jonas JB, Cheung CMG. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond) 2019;33(1):3–13. doi: 10.1038/s41433-018-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang BJ, Naidu RK, Qu XM. Factors related to axial length elongation and myopia progression in orthokeratology practice. PLoS One. 2017;12(4):e0175913. doi: 10.1371/journal.pone.0175913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih KC, Chan TCY, Lap-Ki Ng A, Lai JSM, Li WWT, Cheng ACK, Fan DSP. Use of atropine for prevention of childhood myopia progression in clinical practice. Eye Contact Lens Sci Clin Pract. 2016;42(1):16–23. doi: 10.1097/ICL.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 21.Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LLK. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand. 2001;79(3):233–236. doi: 10.1034/j.1600-0420.2001.790304.x. [DOI] [PubMed] [Google Scholar]
- 22.Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, Tan D. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 23.Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989;21(5):180–182,187. [PubMed] [Google Scholar]
- 24.Polling JR, Kok RW, Tideman JL, Meskat B, Klaver CW. Effectiveness study of atropine for progressive myopia in Europeans. Eye (Lond) 2016;30(7):998–1004. doi: 10.1038/eye.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Galvis V, Tello A, Parra MM, Rodriguez CJ, Blanco O. Re: Chia et al.: five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops (Ophthalmology 2016;123:391-9) Ophthalmology. 2016;123(6):e40–e41. doi: 10.1016/j.ophtha.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kaburaki T, Kakehashi A. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep. 2020;10(1):12750. doi: 10.1038/s41598-020-69710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YR, Bian HL, Wang Q. Atropine 0.5% eyedrops for the treatment of children with low myopia. Medicine. 2017;96(27):e7371. doi: 10.1097/MD.0000000000007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sánchez-González JM, De-Hita-Cantalejo C, Baustita-Llamas MJ, Sánchez-González MC, Capote-Puente R. The combined effect of low-dose atropine with orthokeratology in pediatric myopia control: review of the current treatment status for myopia. J Clin Med. 2020;9(8):2371. doi: 10.3390/jcm9082371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih YF, Chen CH, Chou AC, Ho TC, Lin LLK, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocular Pharmacol Ther. 1999;15(1):85–90. doi: 10.1089/jop.1999.15.85. [DOI] [PubMed] [Google Scholar]
- 31.Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. J Ocular Pharmacol Ther. 2015;31(9):541–545. doi: 10.1089/jop.2015.0043. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Q, Tang Y, Guo LY, Tighe S, Zhou Y, Zhang XF, Zhang JY, Zhu YT, Hu M. Efficacy and safety of 1% atropine on retardation of moderate myopia progression in Chinese school children. Int J Med Sci. 2020;17(2):176–181. doi: 10.7150/ijms.39365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan JH, Li SM, Wu SS, Cao K, Ma DD, He X, Hua ZY, Kang MT, Wei SF, Bai WL, Wang NL. Varying dose of atropine in slowing myopia progression in children over different follow-up periods by meta-analysis. Front Med. 2022;8:756398. doi: 10.3389/fmed.2021.756398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2) Ophthalmology. 2012;119(2):347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Yam JC, Li FF, Zhang XJ, Tang SM, Yip BHK, Kam KW, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study. Ophthalmology. 2020;127(7):910–919. doi: 10.1016/j.ophtha.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Li FF, Kam KW, Zhang YZ, Tang SM, Young AL, Chen LJ, Tham CC, Pang CP, Yam JC. Differential effects on ocular biometrics by 0.05%, 0.025%, and 0.01% atropine. Ophthalmology. 2020;127(12):1603–1611. doi: 10.1016/j.ophtha.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Pan SY, Wang YZ, Li J, Zhang XH, Wang J, Zhu XP, Xiao XH, Liu JT. Short-term effect of 0.01% atropine sulphate eye gel on myopia progression in children. Int J Ophthalmol. 2022;15(7):1122–1127. doi: 10.18240/ijo.2022.07.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bukhari J, Wei SF, Li SM, An WZ, Du JL, Liang XT, Gan JH, Tian JX, Bai WL, Cai ZN, Yin L, Wang NL. Effect of 0.01% atropine eyedrops on intraocular pressure in schoolchildren: a randomized clinical trial. Int J Ophthalmol. 2022;15(9):1431–1436. doi: 10.18240/ijo.2022.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pineles SL, Kraker RT, VanderVeen DK, Hutchinson AK, Galvin JA, Wilson LB, Lambert SR. Atropine for the prevention of myopia progression in children. Ophthalmology. 2017;124(12):1857–1866. doi: 10.1016/j.ophtha.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 40.Yam JC, Jiang YN, Lee J, et al. The association of choroidal thickening by atropine with treatment effects for myopia: two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study. Am J Ophthalmol. 2022;237:130–138. doi: 10.1016/j.ajo.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Kumaran A, Htoon HM, Tan D, Chia A. Analysis of changes in refraction and biometry of atropine- and placebo-treated eyes. Invest Ophthalmol Vis Sci. 2015;56(9):5650–5655. doi: 10.1167/iovs.14-14716. [DOI] [PubMed] [Google Scholar]
- 43.Lin HJ, Wei CC, Chang CY, Chen TH, Hsu YA, Hsieh YC, Chen HJ, Wan L. Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. EBioMedicine. 2016;10:269–281. doi: 10.1016/j.ebiom.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carr BJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci Rep. 2016;6(1):9. doi: 10.1038/s41598-016-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medina A. The cause of myopia development and progression: theory, evidence, and treatment. Surv Ophthalmol. 2022;67(2):488–509. doi: 10.1016/j.survophthal.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Russo A, Boldini A, Romano D, Mazza G, Bignotti S, Morescalchi F, Semeraro F. Myopia: mechanisms and strategies to slow down its progression. J Ophthalmol. 2022;2022:1004977. doi: 10.1155/2022/1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chierigo A, Ferro Desideri L, Traverso CE, Vagge A. The role of atropine in preventing myopia progression: an update. Pharmaceutics. 2022;14(5):900. doi: 10.3390/pharmaceutics14050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun LY, Zhu L, Chen ST, Li JR, Li XW, Wang K, Zhao MW. Mechanism of myopic defocus or atropine for myopia control: different or similar ways? Ophthalmic Res. 2022;65(6):698–711. doi: 10.1159/000525744. [DOI] [PubMed] [Google Scholar]