Abstract
Objectives: Ischemia-reperfusion injury is a complicated pathologic process that involves multiple factors including oxidative stress, endoplasmic reticulum stress, calcium overload, inflammatory response, disturbances in energy metabolism, apoptosis, and some newly-described forms of programmed cell death (e.g., necroptosis, autophagy, pyroptosis, patanatos, and ferroptosis). Chinese herbal monomers (CHMs) have long been applied to treat ischemia-reperfusion injury based on a solid research foundation. This paper objectively reviews in vitro and in vivo studies on the use of CHMs to protect against ischemia-reperfusion injury. Methods: We reviewed 31 CHMs that have been shown to be effective for treating ischemia-reperfusion injury models of the heart, brain, and kidney. According to the mechanism of action, these CHMs were divided into three categories: protecting damaged histocytes, inhibiting inflammatory cells, and promoting the proliferation of damaged histocytes. Some CHMs were found to have more than one mechanism at the same time. Results: Of the 31 CHMs, 28 protect damaged histocytes, 13 inhibit inflammatory cells, and three promote the proliferation of damaged histocytes. Conclusions: CHMs show promise for treating ischemia-reperfusion injury. The eexisting treatment experiences for ischemia-reperfusion injury can be used as a reference.
Keywords: Chinese herbal monomers, ischemia-reperfusion injury, myocardial infarction, cerebral infarction, renal transplantation
Introduction
Ischemic diseases have recently risen to the top of the list of global killers and disablers [1-3]. A wide variety of ischemic diseases, including acute myocardial infarction, ischemic stroke, and acute renal injury, are observed clinically. Ischemia is the lack of blood supply to histocytes. The lack of nutrients and oxygen may cause destruction of the histocytes, leading to the complete loss of organ function. Previous therapeutic studies focused on the rapid restoration of blood supply. Histocyte injury and organ failure can result from ischemia along with reperfusion after the restoration of blood supply, which is called ischemia-reperfusion injury [4]. Thus, reperfusion therapy is a double-edged sword that complicates the treatment and prevention of ischemic disorders.
The pathologic mechanisms of ischemia-reperfusion injury are complex and involve oxidative stress, endoplasmic reticulum (ER) stress, calcium overload, inflammatory response, disturbances in energy metabolism, apoptosis, and some forms of programmed cell death (e.g., necroptosis, autophagy, pyroptosis, patanatos, and ferroptosis) [5]. Therefore, adjunctive interventions are needed to limit the adverse consequences of ischemia-reperfusion injury. Traditional Chinese medicines have attracted considerable attention due to their holistic approach to therapy and the benefits of having several targets, multiple sessions, and multiple pathways. Monomers, which are key components of most Chinese herbal extracts, provide a variety of medical benefits. Chinese herbal monomer (CHM) has been shown to prevent ischemia-reperfusion injury in a number of animal trials and clinical studies [5-8]. Due to its all-natural components, low cost, and low toxicity, CHM has become a focus of current research, including studies on the use of CHM in ischemic disorders.
This review discusses the therapeutic and preventive mechanisms of CHM in myocardial ischemia-reperfusion injury (MIRI), cerebral ischemia-reperfusion injury (CIRI), and renal ischemia-reperfusion injury (RIRI) based on three mechanisms (the inhibition of death signaling, inhibition of inflammatory response, and promotion of histocytes proliferation), and highlights the prospects of CHM in the prevention and treatment of ischemia-reperfusion injury.
Protection of damaged histocytes
Luteolin is a natural soluble flavone found in green vegetables, perilla leaf, and chamomile tea. Luteolin suppressed the activation of the p38 Mitogen-Activated Protein Kinases (p38 MAPK) signaling pathway in a rat MIRI model. By suppressing this signaling pathway, luteolin reduced apoptosis in cardiomyocytes by inhibiting the expressions of bcl-2-Associated X Protein (Bax) and cysteinyl aspartate specific proteinase (caspase)-3 and increasing B-cell lymphoma-2 (Bcl-2) expression [9]. Luteolin inhibited pyroptosis during MIRI in rat and cell models by suppressing the nuclear factor-kappaB (NF-κB)/NOD-like receptor thermal protein domain associated protein 3 (NLRP3) signaling pathway, as evidenced by reductions in apoptosis-associated speck-like protein containing (ASC) and caspase-1 expressions [10].
Dihydroquercetin, a dihydroxyflavone, is the major active ingredient in the root of Larix gmelinii. Dihydroquercetin inhibited MIRI in rat and cell models. The mechanism of its protective effect may be by activating the Phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B (PI3K/Akt) signaling pathway to reduce oxidative stress-induced apoptosis by upregulating nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) activation and hemeoxygenase 1 (HO-1) expressions. The mechanism may also involve ER stress-induced apoptosis by inhibiting the expressions of c-Jun N-terminal kinase (JNK), downstream C/EBP homologous protein (CHOP), Bax, and caspase-12 and increasing Bcl-2 expression [11].
Curculigoside is the major bioactive ingredient in Curculigo orchioides Gaertn. In rat and cell MIRI models, curculigoside decreased mitochondria-mediated apoptosis by closing mitochondrial permeability transition pores. Moreover, curculigoside significantly decreased the expressions of cytoplasmic (cyt-c), caspase-9, and caspase-3 in damaged myocardial cells [12].
Icariin is a major active ingredient in Epimedium. In rat and cell models, icarin may protect against MIRI by activating the sirtuin 1 (SIRT1)/forkhead box-1 (FoxO1) signal pathway, inhibiting mitochondrial oxidative stress by decreasing malondialdehyde (MDA) and reactive oxygen species (ROS), increasing superoxide dismutase (SOD) activity, and inhibiting apoptosis by increasing Bcl-2 expression while decreasing the expressions of Bax, cyt-c, and caspase-3 [13].
Matrine is a quinolizidine alkaloid extracted from the root of Sophora alopecuroides, Sophora tonkinensis, and Sophora flavescens. Matrine inhibited apoptosis during MIRI in a rat model by activating the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway, which decreased the expression of Bax and increased the expression of Bcl-2 [14].
Platycodin D is a main active saponin in Platycodon grandiflorum. Platycodin D showed protective effects during MIRI in a cell model by activating Akt/Nrf2/HO-1-mediated signaling, reducing oxidative stress by decreasing the levels of reactive oxygen species (ROS) and malondialdehyde (MDA), increasing the expressions of SOD and catalase (CAT), and reducing apoptosis by increasing the expression of Bcl-2 levels while decreasing those of Bax and caspase-3 [15].
Quercetin is a flavonoid isolated from plant flowers, leaves, and fruits. Quercetin can effectively upregulate the expression of Bcl-2 and downregulate the expression of Bax by activating the SIRT1/peroxisome proliferator-activated receptor-gamma co-activator (PGC)-1α signaling pathway, resulting in anti-apoptotic effects during MIRI in rat and cell models [16].
Schisandrin B is isolated from the fruit of Schisandra chinensis. Schisandrin B reduces oxidative stress by decreasing MDA expression and increasing SOD expression. Schisandrin B alleviates ER stress by inhibiting activating transcription factor 6 (ATF 6), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and CHOP to suppress apoptosis during MIRI in a rat model induced by decreased Bax, caspase-9, and caspase-3 levels and increased Bcl-2 level [17].
Salvianolic acid A is a polyphenolic compound isolated from the root of Salvia miltiorrhiza. Salvianolic acid A inhibited oxidative stress during RIRI in rat and cell models by increasing the SOD level while decreasing the MDA and ROS levels. Salvianolic acid A inhibited apoptosis through the activation of the Akt signaling pathway by increasing the Bcl-2 level and decreasing caspase-3 activation [18].
Salvianolic acid B is a water-soluble component isolated from Salvia miltiorrhiza Bunge. Salvianolic acid B inhibited apoptosis during MIRI in a rat model by activating the PI3K/Akt signaling pathway, decreasing Bax expression, and increasing the Bcl-2 level [19]. Salvianolic acid B inhibited oxidative stress during RIRI in a rat model through the activation of the PI3K/Akt signaling pathway, as indicated by decreased MDA and ROS levels and increased SOD, CAT, and glutathione (GSH) activities [20].
Tanshinone IIA is a major active compound isolated from the root of Salvia miltiorrhiza Bunge. Tanshinone IIA inhibited apoptosis during MIRI in rat, mice, and cell models, such as the expressions of Bax, cyt-c, and caspase-3, while upregulating the expression of Bcl-2, as well as inhibited oxidative stress such as decreased MDA and ROS levels play protective roles [21]. Meanwihle, tanshinone IIA blocked mitochondrial apoptosis by activating the SIRT/PGC-1α signaling pathway during MIRI in mice and cell models. Tanshinone IIA decreased the production of mitochondrial ROS, reduced the expressions of pro-apoptotic proteins such as Bax and caspase-9, increased the expressions of anti-apoptotic factors such as Bcl-2, and X-linked inhibitor of apoptosis (X-IAP), and limited the leakage of mitochondrial cyt-c [22]. However, during CIRI in mice and cell models, tanshinone IIA increased the activities and the contents of total-antioxidant capacity, SOD, CAT, and glutathione peroxidation (GSHpx). It attenuates the level of ROS, MDA, 8-Hydroxy-2-deoxyguanosine (8-OHdG), as well as the carbonyl and nitrotyrosine protein contents through the antioxidant signaling pathway mediated by Nrf2 activation to reduce oxidative stress [23].
Astragaloside IV is an active saponin extracted from Astragalus membranaceus and Radix Astragali. The protective effect of astragaloside IV against MIRI in rat and cell models was associated with the activation of extracellular signal-regulated kinase (ERK) and subsequent reduction of apoptosis by decreasing the Bax and caspase-3 levels and increasing the Bcl-2 level [24]. Astragaloside IV inhibited the MAPK signaling pathway along with downstream p38 by regulating microRNA-101a/transforming growth factor (TGF)βR1/toll-like receptor (TLR)2 axis, thus attenuating apoptosis during MIRI in a cell model [25]. During CIRI in mice and cell models, astragaloside IV activated Akt to promote hexokinase-II (HK-II) binding to mitochondria, thereby protecting neurons from apoptosis and DNA damage. Meanwhile, astragaloside IV reduced the release of apoptosis-inducing factor (AIF) by inactivating poly-ADP-ribose polymerase-1 (PARP-1), thereby protecting neurons from parthanatos [26]. Astragaloside IV prevented oxidative stress by increasing the SOD activity while decreasing the activities of MDA and ROS. Astragaloside IV inhibited neuronal apoptosis by activating the JAK/STAT signaling pathway during CIRI in rat and cell models [27].
Baicalin is a flavonoid derived from the root of Scutellaria baicalensis Georgi. Baicalein inhibited apoptosis during MIRI in a rat model by increasing the Bcl-2 level and decreasing the Bax and caspase-3 levels [28]. The protective effect of baicalin against MIRI in the rat and cell models was associated with the downregulation of calcium-sensing receptor (CaSR), activation of ERK, and subsequent reduction of apoptosis [29]. Baicalin protected cardiac microvascular endothelial cells during MIRI in a rat model by activating the PI3K/Akt pathway to promote the release of nitric oxide and cyclic guanosine monophosphate. Baicalin also reduced necroptosis by inhibiting the expressions of receptor-interacting protein (RIP) 1, RIP3, and mixed-lineage kinase domain (MLKL) [30]. In rat and cell models of CIRI, baicalin inhibited the activation of PARP-1 and reduced the release of AIF to attenuate parthanatos, reduce oxidative stress, restore mitochondrial membrane potential, protect mitochondrial function, and protect cerebral tissues from ischemic injury [31].
Ginsenoside Rb1 is a major effective ingredient of Panax ginseng C. A. Mey. Ginsenoside Rb1 had a protective effect against apoptosis in a rat model of MIRI by inactivating the Ras homolog gene family member A (RhoA)/Rho-associated coiled-coil containing kinase 1 (ROCK1) signaling pathway, resulting in increased Bcl-2 production and decreased Bax, caspase-3, and caspase-9 production [32].
Curcumin is a polyphenol from Curcuma longa. Curcumin can relieve oxidative stress by increasing the SOD activity while decreasing the levels of MDA and ROS. Curcumin inhibited apoptosis in a cell model of MIRI by reducing ER stress, suppressing the MAPK/ERK and MAPK/JNK signaling pathways, and decreasing the expression of CHOP [33].
Senkyunolide-H is a major bioactive component in Ligusticum chuanxiong Hort. In mice and cell models of CIRI, senkyunolide-H stimulated the PI3K/Akt signaling pathway to inhibit apoptosis by increasing the B-cell lymphoma-extra large (Bcl-XL) level and decreasing caspase-3 activation [34].
Gastrodin is a phenolic compound extracted from Gastrodia elata Blume. Gastrodin protected against oxidative stress in rat and cell models of CIRI by activating the Nrf2/ARE signaling pathway and increasing the expressions of HO-1, NADPH-quinone oxidoreductase (NQO-1), and brain-derived neurotrophic factor (BDNF) [35].
Tetrahydroxystilbene glucoside is the main active ingredient of Fallopia multiflora. In mice model of CIRI, tetrahydroxystilbene glucoside prevented oxidative stress by increasing SOD activity and decreasing the expressions of MDA and ROS. Tetrahydroxystilbene glucoside inhibited brain neuron apoptosis by effectively suppressing the activation of caspase-3 and caspase-9. Tetrahydroxystilbene glucoside alleviated brain neuronal autophagy by effectively reducing the levels of beclin1 and microtubule associated protein 1 light chain 3 (LC3) II [36].
Puerarin is the major ingredient in the root of Pueraria lobata. In a cell model of MIRI, puerarin inhibited autophagy by decreasing the expression of LC3 II as well as beclin1 upregulation mediated by activation of Akt [37].
Artemisinin is a sesquiterpene lactone from Artemisia annua. In a rat model of MIRI, artemisinin inhibited autophagy by reducing the expressions of autophagy-related gene 5 (Atg5) and LC3 II [38].
Tanshinone I is a major ingredient in Salvia milorrhiza Bunge. In rat and cell models of MIRI, tanshinone I decreased the ROS level and inhibited oxidative stress by activating the Akt/Nrf2 pathway, promoting the activation of Akt, and enhancing the expressions of anti-oxidative factors (namely, NQO-1 and HO-1). Tanshinone I can prevent oxidative stress by increasing SOD activity and decreasing the MDA activity. Also, tanshinone I protects against necroptosis by restraining the RIP1, RIP3, and MLKL levels [39].
Emodin, an anthraquinone derivative, is the major active ingredient from the rhizome of Rheum palmatum. In rat and cell models of MIRI, emodin inhibited pyroptosis by inhibiting the NF-κB/NLRP3 signaling pathway, as evidenced by reductions in the expression of ASC, caspase-1, and gasdermin D [40].
Galangin is a polyphenolic compound extracted from Chinese herbs such as Alpinia officinarum Hance, Plantago major L, and Scutellaria galericulata L. In a gerbil model of CIRI, galangin inhibited ferroptosis by activating the solute carrier family 7 member 11 (SLC7A11)/glutathione peroxidase 4 (GPX4) axis, decreasing ROS production, and reducing oxidative stress [41].
Gastrodian is the major active ingredient from the dried tuber of Gastrodia elata Bl. In mice and cell models of MIRI, gastrodian inhibited pyroptosis by inhibiting the NLRP3/caspase-1 signaling pathway, as evidenced by reduced gasdermin D expression [42].
Hydroxysafflor yellow A is major active component from safflower. In rat and cell models of CIRI, hydroxysafflor yellow A suppressed the NF-κB signaling pathway and inhibited the expressions of NLRP3, ASC, caspase-1, and gasdermin D to alleviate ischemia-reperfusion injury by inhibiting the pyroptosis signaling pathway [43]. Hydroxysafflor yellow A also protected against autophagy in a rat model of CIRI by decreasing the expressions of E1B 19 kDa interacting protein 3 (BNIP3) and LC3 [44].
Oleanolic acid, a natural triterpenoid, is the major active ingredient in various food products including vegetable oils and the leaves and roots of Olea europaea, Viscum album L., and Aralia chinensis L. Oleanolic acid inhibited oxidative stress in a rat model of RIRI by activating the Nrf2/glutamate-cysteine ligase catalytic subunit (GCLc) signaling pathway, as indicated by the decreased MDA level and increased SOD, CAT, and GSH activities. Oleanolic acid inhibits apoptosis in a rat model of RIRI by decreasing the level of caspase-3 [45].
Magnolol is an active ingredient from the bark of Magnolia officinalis. In a rat model of RIRI, magnolol inhibited apoptosis by activating the Akt and ERK signaling pathways while suppressing the p38 and JNK signaling pathways, as evidenced by the increased level of Bcl-2 and decreased levels of Bax and caspase-3 [46].
Apigenin is a natural active ingredient found in many fruits and herbs such as oranges, onions, and parsley. In rat and cell models of RIRI, apigenin inhibited apoptosis increasing the Bcl-2 level and decreasing the levels of Bax and caspase-3 to activate the PI3K/Akt signaling pathway [47].
Inhibition of inflammatory cells
Luteolin inhibited inflammatory response in rat and cell models of MIRI by inhibiting the Toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88)/NF-κB/NLRP3 inflammasome pathway, as evidenced by reductions in the levels of interleukin (IL)-1β, IL-18, and tumor necrosis factor (TNF)-α [10].
Tanshinone I lowered the amount of white blood cells, including neutrophils and lymphocytes, in blood in rat and cell models of MIRI. Tanshinone I also exerted inhibitory effects on TNF-α and IL-6. Therefore, tanshinone I can reduce the immune response and inflammation during MIRI [39].
Artemisinin inhibited inflammation in a rat model of MIRI by decreasing the NLRP3 inflammasome, as indicated by decreased NLRP3, ASC, caspase-1, and IL-1β levels [38].
Baicalin inhibited the inflammatory response in a rat model of MIRI by decreasing neutrophil infiltration, decreasing the expressions of inducible nitric oxide synthase (iNOS), IL-1β, and IL-6, and increasing the expressions of arginase-1 (Arg-1), IL-10, and TGF-β which alters the macrophage phenotype (from M1 to M2) [28].
Puerarin attenuated inflammation by decreasing the levels of TNF-α, IL6, and IL-1β in a cell model of MIRI [37]. The protective effect of puerarin may be related to the inhibition of inflammatory response through the SIRT1/NF-κB signaling pathway, as manifested by reduced levels of NF-κB, NLRP3, caspase-1, IL-1β, and IL-18 [48].
Salvianolic acid B may protected against the inflammatory response during MIRI in a rat model by reducing the expressions of TNF-α, IL-18, IL-1β, and high-mobility group box 1 protein (HMGB1) [19].
Senkyunolid-H inhibited the NF-κB signaling pathway to inhibit the expression of TNF-α in mice and cell models of CIRI [34].
In rat and cell models of MIRI, emodin inhibited inflammatory response by suppressing the inflammasome pathway of TLR4/MyD88/NF-κB/NLRP3, as evidenced by a reduction in the IL-1β level [40].
In mice and cell models of MIRI, gastrodian inhibited the inflammatory response by suppressing the NLRP3/caspase-1 inflammasome pathway, as evidenced by a reduced IL-1β level [42].
In rat and cell models of CIRI, hydroxysafflor yellow A decreased activation of the NF-κB signaling pathway and inhibited the levels of NLRP3, ASC, caspase-1, IL-1β, and IL-18 to alleviate inflammation after injury [43].
Procyanidins are flavonoid polyphenols extracted from grape seeds. Procyanidins exhibit neuroprotective activity against CIRI in rat and cell models by inhibiting the TLR4-NF-κB-NLRP3 inflammasome signaling pathway and reducing the levels of caspase-1 and IL-1β [49].
Oleanolic acid inhibited the inflammatory response in a rat model of RIRI by increasing the IL-10 level and decreasing the Interferon-γ (IFN-γ), IL-6, and myeloperoxidase (MPO) levels [45].
Magnolol inhibited the inflammatory response during RIRI in a rat model by reducing the TNF-α, IL-1β, and IL-6 levels and increasing the IL-10 level [46].
Promotion of damaged histocyte proliferation
In mice and cell models of MIRI, tanshinone IIA activated the SIRT1/PGC-1α signaling pathway to maintain mitochondrial homeostasis, thereby activating the pro-proliferative capacity of cardiac microvascular endothelial cells [22].
Astragaloside VI is natural saponin that is abundant in Radix Astragali. In rat and cell models of CIRI, astragaloside VI promoted the proliferation and neurogenesis of neural stem cells and improved nerve function repair by activating the epidermal growth factor receptor-mediated MAPK/ERK signaling pathway [50].
Ilexonin A is a Chinese herbal medicine extracted from Ilex pubescens. In a rat model of CIRI, ilexonin A could promote revascularization and neuronal regeneration and regulated glial cell activation, providing a favorable microenvironment for neural recovery [51].
Conclusion and prospects
In this paper, 31 CHMs were mapped according to their mechanisms of action. Figures 1 and 2 shows the CHMs that act to protection damaged cells, while Figures 3 and 4 shows those that inhibit inflammatory cells. In the mapping process, we referred to the signaling pathways from https://www.cellsignal.cn along with the mechanisms described in the literature to determine the targets of the 31 CHMs. Figures 1 and 2 shows the mechanisms by which CHMs intervene in apoptosis and some new forms of programmed cell death. Figures 3 and 4 shows the mechanisms by which CHMs intervene in inflammatory response. The involved signaling pathways include PI3K/Akt, JAK/STAT, the MAPK family, Nrf2, SIRT1, NF-κB/NLRP3, oxidative stress, ER-stress, apoptosis, pyroptosis, and others related to new forms of programmed cell death. These pathways play important roles in the regulation of ischemia-reperfusion injury by CHMs.
Figure 1.
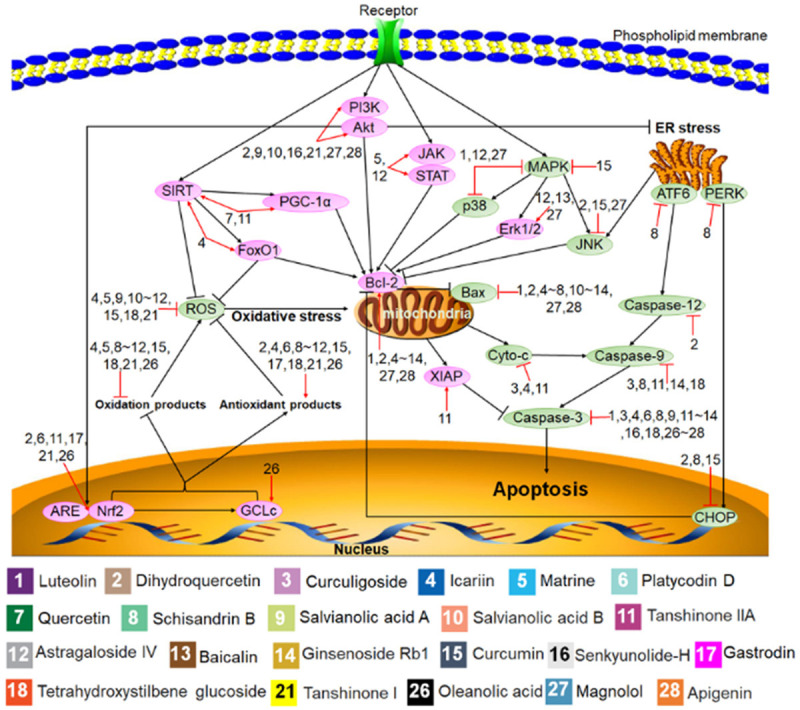
Protective mechanisms of Chinese herbal monomers (CHMs) in histocytes against apoptosis. The black lines represent the signaling pathways, the red lines represent the effect of the CHMs on the signaling pathways, and the pink and green ovals represent the cell signaling molecules activated and inhibited by the CHMs. Numbers represent each type of CHMs.
Figure 2.
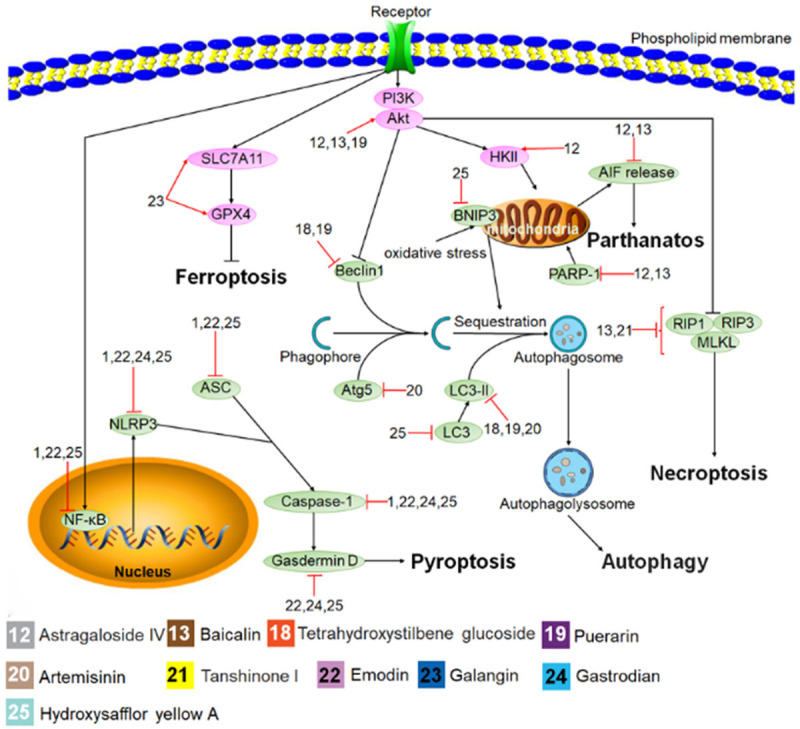
Protective mechanisms of Chinese herbal monomers (CHMs) in histocytes against some new forms of programmed cell death. The black lines represent the signaling pathways, the red lines represent the effect of the CHMs on the signaling pathways, the pink and green ovals represent the cell signaling molecules activated and inhibited by the CHMs, and numbers represent each type of CHMs.
Figure 3.
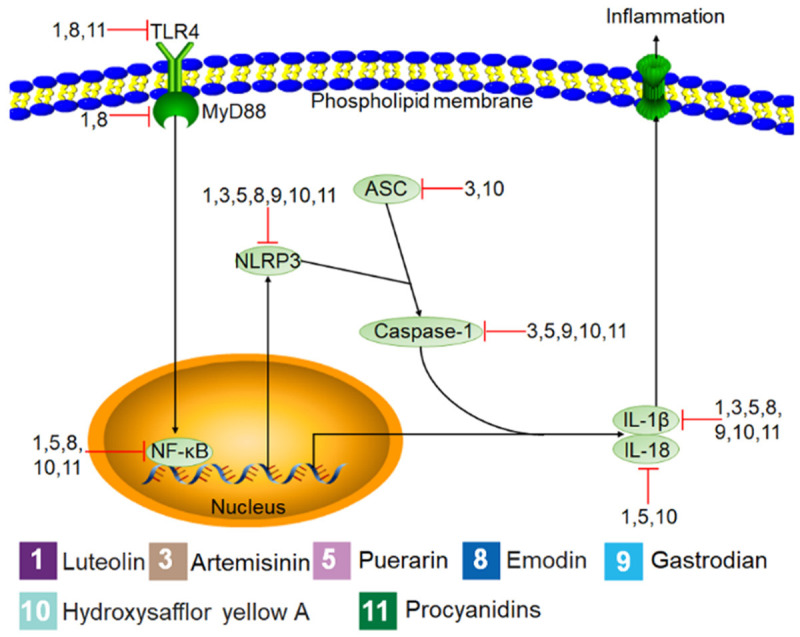
Mechanisms of Chinese herbal monomers (CHMs) inhibition in inflammatory cells. The black lines represent the signaling pathways, the red lines represent the effect of the CHMs on the signaling pathways, the pink and green ovals represent the cell signaling molecules activated and inhibited by the CHMs, and numbers represent each type of CHMs.
Figure 4.
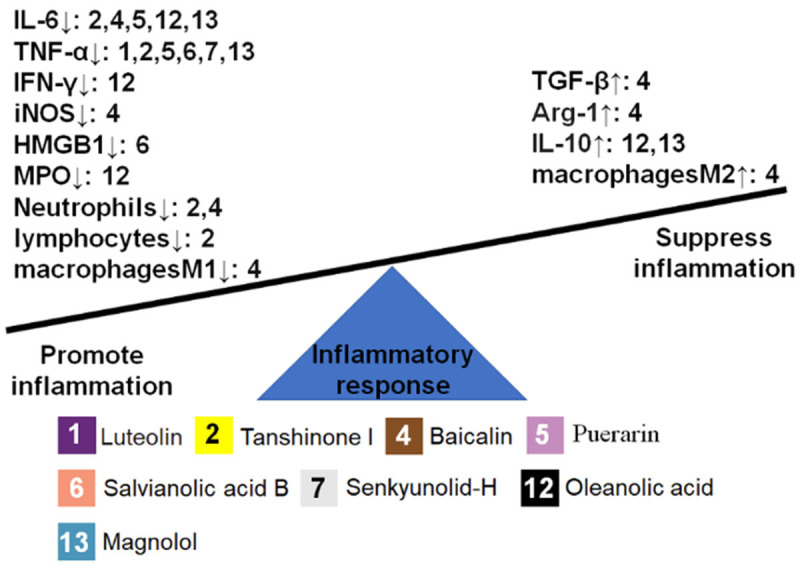
Chinese herbal monomers (CHMs) with effects on cytokines and inflammatory cells. The left and right sides of the seesaw represent the promotion and inhibition of inflammation, the arrows up and down represent promotion and inhibition of cytokines and inflammatory cells, and numbers represent each type of CHMs.
PI3K/Akt signaling pathway
PI3K/Akt is a classical signaling pathway in cells that plays an important role in regulating different cellular functions, including the control of metabolism, growth, proliferation, and survival [52,53]. This signaling pathway is involved in most of the death signaling processes. Among the CHMs considered in this paper, 11 protect damaged cells by activating the PI3K/Akt signaling pathway: dihydroquercetin, platycodin D, salvianolic acid A, salvianolic acid B, astragaloside IV, baicalin, senkyunolide-H, puerarin, tanshinone I, magnolol, and apigenin.
JAK/STAT signaling pathway
The JAK/STAT signaling pathway is the main signaling mechanism for cytokines and growth factors. It mainly regulates cell growth, survival, and differentiation [54,55]. Three of the reviewed CHMs protect against apoptosis by activating this signaling pathway: matrine, astragaloside IV, and baicalin.
MAPK family signaling pathway
The MAPK family includes p38, JNK, and ERK [56]. Among them, p38 affects a variety of intracellular responses and has a recognized role in inflammation, cell cycle regulation, apoptosis initiation, development, differentiation, senescence, and tumorigenesis [57]. Three CHMs discussed in this review protect against apoptosis by inhibiting this signaling pathway: luteolin, astragaloside IV, and magnolol. JNK plays an important role in a variety of physiologic and pathologic processes, including the cell cycle, reproduction, cellular stress, and apoptosis [58,59]. Three of the reviewed CHMs protect against apoptosis by inhibiting the JNK signaling pathway: dihydroquercetin, curcumin, and magnolol. ERK plays an important role in mediating cell survival, growth, proliferation, and migration [60]. Interestingly, astragaloside IV, baicalin, and magnolol activate the ERK signaling pathway during anti-apoptosis, whereas curcumin inhibit this signaling pathway in anti-apoptosis. These differences may result from interactions among several signaling pathways.
Nrf2 signaling pathway
Nrf2 is a key transcription factor that regulates resistance to oxidative stress, and the Nrf2 signaling pathway plays an important role in inducing antioxidant response in the body [61]. The primary transcriptional regulation of Nrf2 induces the expression of various antioxidant enzymes through binding associated with the ARE, GCLc transcription factor. Thus, the Nrf2 signaling pathway is important for preventing ROS damage [62]. Six of the CHMs in this review inhibit oxidative stress by activating the Nrf2 signaling pathway: dihydroquercetin, platycodin D, tanshinone IIA, gastrodin, tanshinones I, and oleanolic acid.
SIRT1 signaling pathway
The SIRT1 signaling pathway plays an important role in the upregulation of antioxidants and downregulation of pro-apoptotic processes in the body as an aging-relieving, lifespan-extending, and metabolic pathway regulator [63,64]. Three CHMs discussed in this review protect cells against oxidation and apoptosis by activating the SIRT1 signaling pathway: icariin, quercetin, and tanshinones IIA.
NF-κB/NLRP3 signaling pathway
The NF-κB/NLRP3 signaling pathway regulates a range of genes involved in cellular physiological activities such as innate and adaptive immunity, inflammation, stress response, B-cell development, and lymphoid organogenesis. This pathway is mediated by gasdermin D, which causes pyroptosis [65]. Three CHMs in this review protect cells against inflammatory response by inhibiting the NF-κB/NLRP3 signaling pathway, while four CHMs protect cells against pyroptosis by inhibiting this signaling pathway.
Oxidative stress and ER stress signaling pathway
Oxidative stress describes an imbalance of ROS and free radicals, leading to harmful effects in the body. ROS are the main factor contributing to oxidative stress. Oxidative stress can induce apoptosis and others forms of programmed cell death [66]. ER stress is induced by caspase-12-mediated apoptosis through activation of the ATF6 pathway, and activation of the PERK and JNK pathways induces mitochondrial damage to cause apoptosis [11].
Apoptosis signaling pathway
Apoptosis is a strictly controlled mode of cellular death characterized by nuclear sequestration, cellular crumpling, cell membrane blistering, and DNA fragmentation [67]. Apoptosis is characterized by the translocation of the death signal transduction by cellular receptors through the above signaling pathways as well as the oxidative stress and ER-stress pathways to the mitochondria, which act as the central focus for the release of anti-apoptotic molecules Bcl-2, pro-apoptotic molecules Bax, cyto-c, and caspases as a family of cysteine proteases that become the central regulatory molecules of apoptosis [68].
Pyroptosis signaling pathway
Pyroptosis, also known as cellular inflammatory necrosis, is manifested by the continuous swelling of cells until the cell membrane ruptures, leading to the release of cellular contents, which activates a strong inflammatory response [69]. Pyroptosis is characterized by the transduction of death signals from the NF-κB/NLRP3 signaling pathway to the nucleus through cellular receptors. This is followed by the activation of the NLRP3 inflammatory complex, recruitment and activation of caspase-1, and ultimately gasdermin-mediated programmed cellular necrosis accompanied by a massive release of pro-inflammatory factors [70].
Signaling pathways for new forms of programmed cell death
Autophagy, a catalytic process, leads to the autophagic lysosomal degradation of major cell plasma contents, the aggregation of abnormal proteins, and excess or damaged organelles [71]. Necroptosis is caused by significant chemical or physical injury. The typical endpoints of necrosis include swelling and rupture of necrotic cells. RIP1, RIP3, and MLKL are the main molecules involved in necroptosis [72]. Ferroptosis is a non-apoptotic form of cell death that depends on the accumulation of intracellular iron, leading to elevated levels of toxic lipid peroxides [73]. Galangin can inhibit ferroptosis by activating the SLC7A11/GPX4 signaling pathway. Parthanatos, a new form of programmed cell death that involves DNA fragmentation, mitochondrial malfunction, and cell death, is caused by the activation of PARP-1 and HK-II, leading to the release of AIF from the mitochondria to the nucleus [7]. Although other inflammatory substances have been detected and quantified, the associated signaling pathways have not been elucidated. Therefore, we only summarized the pro-inflammatory and anti-inflammatory targets of CHMs acting on inflammatory substances.
We have summarized the targets of 31 CHMs in ischemia-reperfusion injury: 28 CHMs protect against histocyte damage; 13 CHMs inhibit inflammatory cells; and three CHMs promote the proliferation of damaged histocytes. CHMs show promise for the treatment of ischemia-reperfusion injury. Existing treatment experiences for ischemia-reperfusion injury can be used as a reference.
Acknowledgements
This work was supported by grants from the 2021 Doctoral Research Startup Fund of Guangxi University of Traditional Chinese Medicine [grant number 2021BS035] and the Guangxi Science and Technology Base and Talent Project [grant number AD22035122].
Disclosure of conflict of interest
None.
References
- 1.Ma LY, Chen WW, Gao RL, Liu LS, Zhu ML, Wang YJ, Wu ZS, Li HJ, Gu DF, Yang YJ, Zheng Z, Hu SS. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. 2020;17:1–8. doi: 10.11909/j.issn.1671-5411.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, Lucke-Wold N, Carpenter JS. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. 2017;9:722–726. doi: 10.1136/neurintsurg-2016-012515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilbert T, Klaschik S. The angiopoietin/TIE receptor system: focusing its role for ischemia-reperfusion injury. Cytokine Growth Factor Rev. 2015;26:281–291. doi: 10.1016/j.cytogfr.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Yu LT, Cheng BR, Xu JL, Cai Y, Jin JL, Feng RL, Xie L, Qu XY, Li D, Liu J, Li Y, Cui XY, Lu JJ, Zhou K, Lin Q, Wan J. Promising therapeutic candidate for myocardial ischemia/reperfusion injury: what are the possible mechanisms and roles of phytochemicals. Front Cardiovasc Med. 2021;8:792592. doi: 10.3389/fcvm.2021.792592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bu W, Zhang Z, Ocansey DKW, Yu Z, Yang X, Liu Z, Wang X, Ke Y. Research on natural products from traditional Chinese medicine in the treatment of myocardial ischemia-reperfusion injury. Am J Transl Res. 2022;14:1952–1968. [PMC free article] [PubMed] [Google Scholar]
- 7.Huang P, Wan H, Shao C, Li C, Zhang L, He Y. Recent advances in Chinese herbal medicine for cerebral ischemic reperfusion injury. Front Pharmacol. 2021;12:688596. doi: 10.3389/fphar.2021.688596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boozari M, Hosseinzadeh H. Natural medicines for acute renal failure: a review. Phytother Res. 2017;31:1824–1835. doi: 10.1002/ptr.5943. [DOI] [PubMed] [Google Scholar]
- 9.Zhu S, Xu T, Luo Y, Zhang Y, Xuan H, Ma Y, Pan D, Li D, Zhu H. Luteolin enhances sarcoplasmic reticulum Ca2+-ATPase activity through p38 MAPK signaling thus improving rat cardiac function after ischemia/reperfusion. Cell Physiol Biochem. 2017;41:999–1010. doi: 10.1159/000460837. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, Duan J. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed Pharmacother. 2017;91:1042–1052. doi: 10.1016/j.biopha.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Shu Z, Yang Y, Yang L, Jiang H, Yu X, Wang Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019;10:203–215. doi: 10.1039/c8fo01256c. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Guo Y, Chen Y, Liu S, Wu N, Jia D. Curculigoside attenuates myocardial ischemia-reperfusion injury by inhibiting the opening of the mitochondrial permeability transition pore. Int J Mol Med. 2020;45:1514–1524. doi: 10.3892/ijmm.2020.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu B, Feng JY, Yu LM, Wang YC, Chen YQ, Wei Y, Han JS, Feng X, Zhang Y, Di SY, Ma ZQ, Fan CX, Ha XQ. Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage. Br J Pharmacol. 2018;175:4137–4153. doi: 10.1111/bph.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao XB, Qin Y, Niu YL, Yang J. Matrine inhibits hypoxia/reoxygenation-induced apoptosis of cardiac microvascular endothelial cells in rats via the JAK2/STAT3 signaling pathway. Biomed Pharmacother. 2018;106:117–124. doi: 10.1016/j.biopha.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Che J, Zhao H, Tang J, Shi G. Platycodin D inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem Biophys Res Commun. 2018;503:3219–3224. doi: 10.1016/j.bbrc.2018.08.129. [DOI] [PubMed] [Google Scholar]
- 16.Tang J, Lu L, Liu Y, Ma J, Yang L, Li L, Guo H, Yu S, Ren J, Bai H, Yang J. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J Cell Biochem. 2019;120:9747–9757. doi: 10.1002/jcb.28255. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Sun Z, Meng F. Schisandrin B ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Inflammation. 2017;40:1903–1911. doi: 10.1007/s10753-017-0631-4. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Liu W, Ding Y, Jia Y, Zhao J, Wang F, Bai J, Cheng L, Gao K, Liu M, Yao M, Li L, Zhang Y, Wen A, He L. Salvianolic acid A ameliorates renal ischemia/reperfusion injury by activating Akt/mTOR/4EBP1 signaling pathway. Am J Physiol Renal Physiol. 2018;315:F254–F262. doi: 10.1152/ajprenal.00508.2017. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Liu W, Qiu H, Zou D, Cai H, Chen Q, Zheng C, Xu D. Salvianolic acid B protects against myocardial ischaemia-reperfusion injury in rats via inhibiting high mobility group box 1 protein expression through the PI3K/Akt signalling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1527–1539. doi: 10.1007/s00210-019-01755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma ZG, Xia HQ, Cui SL, Yu J. Attenuation of renal ischemic reperfusion injury by salvianolic acid B via suppressing oxidative stress and inflammation through PI3K/Akt signaling pathway. Braz J Med Biol Res. 2017;50:e5954. doi: 10.1590/1414-431X20175954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Wei L, Yu Q, Shi H, Liu G. Tanshinone IIA alleviates hypoxia/reoxygenation induced cardiomyocyte injury via lncRNA AK003290/miR-124-5p signaling. BMC Mol Cell Biol. 2020;21:20. doi: 10.1186/s12860-020-00264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong J, Ouyang H, Sun M, Lu J, Zhong Y, Tan Y, Hu Y. Tanshinone IIA attenuates cardiac microvascular ischemia-reperfusion injury via regulating the SIRT1-PGC1α-mitochondrial apoptosis pathway. Cell Stress Chaperones. 2019;24:991–1003. doi: 10.1007/s12192-019-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai M, Guo Y, Wang S, Wei H, Sun S, Zhao G, Dong H. Tanshinone IIA elicits neuroprotective effect through activating the nuclear factor erythroid 2-related factor-dependent antioxidant response. Rejuvenation Res. 2017;20:286–297. doi: 10.1089/rej.2016.1912. [DOI] [PubMed] [Google Scholar]
- 24.Yin B, Hou XW, Lu ML. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol Sin. 2019;40:599–607. doi: 10.1038/s41401-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Fan Z, Chen Z, Hu J, Cui J, Liu Y, Wang Y, Guo B, Shen J, Xie L. Astragaloside IV protects human cardiomyocytes from hypoxia/reoxygenation injury by regulating miR-101a. Mol Cell Biochem. 2020;470:41–51. doi: 10.1007/s11010-020-03743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Yang Y, Zhao Y, Zhang J, Liu B, Jiao S, Zhang X. Astragaloside IV reduces neuronal apoptosis and parthanatos in ischemic injury by preserving mitochondrial hexokinase-II. Free Radic Biol Med. 2019;131:251–263. doi: 10.1016/j.freeradbiomed.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Liu W, Huang H. Astragaloside IV alleviates cerebral ischemia-reperfusion injury by activating the Janus kinase 2 and signal transducer and activator of transcription 3 signaling pathway. Pharmacology. 2020;105:181–189. doi: 10.1159/000503361. [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Li X, Song L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm Biol. 2020;58:655–663. doi: 10.1080/13880209.2020.1779318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Zhang S, Xu C, Sun Y, Sui S, Zhang Z, Luan Y. The protective of baicalin on myocardial ischemia-reperfusion injury. Curr Pharm Biotechnol. 2020;21:1386–1393. doi: 10.2174/1389201021666200605104540. [DOI] [PubMed] [Google Scholar]
- 30.Bai J, Wang Q, Qi J, Yu H, Wang C, Wang X, Ren Y, Yang F. Promoting effect of baicalin on nitric oxide production in CMECs via activating the PI3K-AKT-eNOS pathway attenuates myocardial ischemia-reperfusion injury. Phytomedicine. 2019;63:153035. doi: 10.1016/j.phymed.2019.153035. [DOI] [PubMed] [Google Scholar]
- 31.Li WH, Yang YL, Cheng X, Liu M, Zhang SS, Wang YH, Du GH. Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats. Apoptosis. 2020;25:354–369. doi: 10.1007/s10495-020-01600-w. [DOI] [PubMed] [Google Scholar]
- 32.Cui YC, Pan CS, Yan L, Li L, Hu BH, Chang X, Liu YY, Fan JY, Sun K, Li Q, Han JY. Ginsenoside Rb1 protects against ischemia/reperfusion-induced myocardial injury via energy metabolism regulation mediated by RhoA signaling pathway. Sci Rep. 2017;7:44579. doi: 10.1038/srep44579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei W, Peng J, Li J. Curcumin attenuates hypoxia/reoxygenation-induced myocardial injury. Mol Med Rep. 2019;20:4821–4830. doi: 10.3892/mmr.2019.10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Jiang Y, Liu N, Shen T, Jung HW, Liu J, Yan BC. A network-based method for mechanistic investigation and neuroprotective effect on post-treatment of senkyunolid-H against cerebral ischemic stroke in mouse. Front Neurol. 2019;10:1299. doi: 10.3389/fneur.2019.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi A, Xiang J, He F, Zhu Y, Zhu G, Lin Y, Zhou N. The phenolic components of gastrodia elata improve prognosis in rats after cerebral ischemia/reperfusion by enhancing the endogenous antioxidant mechanisms. Oxid Med Cell Longev. 2018;2018:7642158. doi: 10.1155/2018/7642158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu F, Xue W, Dong L, Hu X, Huang D, Wang K. Tetrahydroxystilbene glucoside suppresses NAPDH oxidative stress to mitigate apoptosis and autophagy induced by cerebral ischemia/reperfusion injury in mice. Evid Based Complement Alternat Med. 2019;2019:3913981. doi: 10.1155/2019/3913981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang H, Song X, Ling Y, Wang X, Yang P, Luo T, Chen A. Puerarin attenuates myocardial hypoxia/reoxygenation injury by inhibiting autophagy via the Akt signaling pathway. Mol Med Rep. 2017;15:3747–3754. doi: 10.3892/mmr.2017.6424. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Gao Q, Yang J, Wang C, Cao J, Sun J, Fan Z, Fu L. Artemisinin suppresses myocardial ischemia-reperfusion injury via NLRP3 inflammasome mechanism. Mol Cell Biochem. 2020;474:171–180. doi: 10.1007/s11010-020-03842-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhuo Y, Yuan R, Chen X, He J, Chen Y, Zhang C, Sun K, Yang S, Liu Z, Gao H. Tanshinone I exerts cardiovascular protective effects in vivo and in vitro through inhibiting necroptosis via Akt/Nrf2 signaling pathway. Chin Med. 2021;16:48. doi: 10.1186/s13020-021-00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye B, Chen X, Dai S, Han J, Liang X, Lin S, Cai X, Huang Z, Huang W. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. 2019;13:975–990. doi: 10.2147/DDDT.S195412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan X, Li Z, Zhu S, Cheng M, Ju Y, Ren L, Yang G, Min D. Galangin attenuated cerebral ischemia-reperfusion injury by inhibition of ferroptosis through activating the SLC7A11/GPX4 axis in gerbils. Life Sci. 2021;264:118660. doi: 10.1016/j.lfs.2020.118660. [DOI] [PubMed] [Google Scholar]
- 42.Sun W, Lu H, Lyu L, Yang P, Lin Z, Li L, Sun L, Lu D. Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/caspase-1 pathway. J Physiol Biochem. 2019;75:531–547. doi: 10.1007/s13105-019-00702-7. [DOI] [PubMed] [Google Scholar]
- 43.Tan L, Wang Y, Jiang Y, Wang R, Zu J, Tan R. Hydroxysafflor yellow A together with blood-brain barrier regulator lexiscan for cerebral ischemia reperfusion injury treatment. ACS Omega. 2020;5:19151–19164. doi: 10.1021/acsomega.0c02502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Liu Y, Cui Q, Fu Z, Yu H, Liu A, Liu J, Qin X, Ge S, Zhang G. Hydroxysafflor yellow A alleviates ischemic stroke in rats via HIF-1[Formula: see text], BNIP3, and Notch1-mediated inhibition of autophagy. Am J Chin Med. 2022;50:799–815. doi: 10.1142/S0192415X22500331. [DOI] [PubMed] [Google Scholar]
- 45.Long C, Yang J, Yang H, Li X, Wang G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti-inflammatory, and anti-apoptotic activities. Mol Med Rep. 2016;13:4697–4704. doi: 10.3892/mmr.2016.5128. [DOI] [PubMed] [Google Scholar]
- 46.Tang CY, Lai CC, Huang PH, Yang AH, Chiang SC, Huang PC, Tseng KW, Huang CH. Magnolol reduces renal ischemia and reperfusion injury via inhibition of apoptosis. Am J Chin Med. 2017;45:1421–1439. doi: 10.1142/S0192415X1750077X. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Wang W, Wang JZ, Yang C, Liang CZ. Effect of apigenin on apoptosis induced by renal ischemia/reperfusion injury in vivo and in vitro. Ren Fail. 2018;40:498–505. doi: 10.1080/0886022X.2018.1497517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang ZK, Chen RR, Li JH, Chen JY, Li W, Niu XL, Wang FF, Wang J, Yang JX. Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: the role of the SIRT1/NF-κB pathway. Int Immunopharmacol. 2020;89:107086. doi: 10.1016/j.intimp.2020.107086. [DOI] [PubMed] [Google Scholar]
- 49.Yang B, Sun Y, Lv C, Zhang W, Chen Y. Procyanidins exhibits neuroprotective activities against cerebral ischemia reperfusion injury by inhibiting TLR4-NLRP3 inflammasome signal pathway. Psychopharmacology (Berl) 2020;237:3283–3293. doi: 10.1007/s00213-020-05610-z. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Wu H, Chen H, Wang Q, Xie XJ, Shen J. Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK signaling cascades. Mol Neurobiol. 2019;56:3053–3067. doi: 10.1007/s12035-018-1294-3. [DOI] [PubMed] [Google Scholar]
- 51.Xu AL, Zheng GY, Wang ZJ, Chen XD, Jiang Q. Neuroprotective effects of Ilexonin A following transient focal cerebral ischemia in rats. Mol Med Rep. 2016;13:2957–2966. doi: 10.3892/mmr.2016.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 53.Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol. 2009;21:256–261. doi: 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Leonard WJ. Role of Jak kinases and STATs in cytokine signal transduction. Int J Hematol. 2001;73:271–277. doi: 10.1007/BF02981951. [DOI] [PubMed] [Google Scholar]
- 55.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 56.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012;72:379–386. doi: 10.1158/0008-5472.CAN-11-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verma G, Datta M. The critical role of JNK in the ER-mitochondrial crosstalk during apoptotic cell death. J Cell Physiol. 2012;227:1791–1795. doi: 10.1002/jcp.22903. [DOI] [PubMed] [Google Scholar]
- 60.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 61.Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS. Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci. 2022;291:120111. doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu YF, Liu J, Wu KC, Qu Q, Fan F, Klaassen CD. Overexpression of Nrf2 protects against microcystin-induced hepatotoxicity in mice. PLoS One. 2014;9:e93013. doi: 10.1371/journal.pone.0093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun SC. The noncanonical NF-κB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rayner CL, Bottle SE, Gole GA, Ward MS, Barnett NL. Real-time quantification of oxidative stress and the protective effect of nitroxide antioxidants. Neurochem Int. 2016;92:1–12. doi: 10.1016/j.neuint.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 68.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 71.Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating. Nat Rev Mol Cell Biol. 2011;13:7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]
- 72.Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol. 2017;12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


