Abstract
Objective/background
The evidence on the association between screen use and sleep of adolescents is mainly based on studies about time watching television, with a few examining time using computers, videogames, and mobile devices. Our aim was to investigate the association between screen time for entertainment (watching TV, using computer, or playing games on tablets, smartphones, or videogame consoles) and sleep duration and self-reported sleep quality, among adolescents aged 15 years.
Methods
With data from the 2004 Pelotas Birth Cohort, sleep duration was assessed with questions extracted from the Munich Chronotype Questionnaire and quality was self-reported. Adjusted β coefficients and prevalence ratios (PR) with (95% confidence intervals) were obtained, respectively, by linear and Poisson regressions.
Results
1,949 adolescents had information about screen time and sleep quality, and 1,851 about screen time and sleep duration. The median screen time was 4.5hs/24hs. The mean sleep duration was 7.6hs/24hs and the prevalence of bad sleep was 17.3% (15.7–19.0%). There was an inverse relationship between screen time and sleep duration. When compared with those with less than 2hs/24hs of screen time, adolescents with 6–8.8hs/24hs and ≥9hs experienced, respectively, 23.4 and 32.4 min reduction in sleep duration (β = -0.39; −0.62;-0.16 and β = -0.54; −0.77;-0.30). Adolescents with ≥9hs of screen time were 60% more likely to report bad sleep than those with less than 2hs/24hs (PR: 1.60; 1.10–2.32).
Conclusions
The median time spent using screens was longer than recommended. Screen use for ≥6hs/24hs was associated with a shorter sleep duration, and ≥9hs/24hs with poor sleep quality.
Keywords: Screen, Sleep, Sleep duration, Sleep quality, Adolescents, Cohort study
Highlights
-
•
There is an inverse relationship between screen time and sleep duration.
-
•
6–8.8hs/24hs of screen time reduced sleep duration in 23.4 min.
-
•
≥9hs/24hs of screen time reduced sleep duration in 32.4 min.
-
•
Adolescents with ≥9hs of screen time were 60% more likely to report bad sleep quality.
1. Introduction
Sleep is associated with the cerebral homeostasis process [1]. Studies conducted with children and adolescents have shown that sleep problems, such as insufficient duration and poor quality sleep, are prejudicial to brain functions [2]. Depression, anxiety, poor school performance, alcohol abuse, smoking, illicit drug use, risky sexual behavior, violent behavior, and damage to interpersonal relationships are some of the problems associated with short duration and/or poor quality sleep, identified in children and adolescents [[3], [4], [5]]. The National Sleep Foundation recommends that adolescents aged between 14 and 17 have 8–10 h of sleep every 24 h [6].
Adolescence is a period of important neurobiological modifications. In this life stage, sleep undergoes physiological macrostructural modifications, such as a reduction in REM sleep latency and in the proportion of slow wave sleep, as well as regulatory modifications, which culminate in behavioral changes, such lateness in going to sleep [7]. In addition, in post-puber adolescents, melatonin release occurs at later times, leading to a delay in the start of sleep. Added to the biological factors, environmental and behavioral factors act in influencing sleep and falling asleep in adolescence [7]. Environmental factors (such as the time classes start, the sociocultural behavior of the place of residence, and the lack of a bedtime routine) and intrinsic factors (such as the hormonal load corresponding to puberty) can affect sleep duration and quality in adolescence [[8], [9], [10]].
With the advent of new technologies, screen use (smartphones, tablets, computers, and smart TVs, among others) has been investigated as a risk factor for reduced duration and poor quality of sleep [11]. Most of the studies indicate that the use of electronic devices is associated with a shorter sleep duration among children and adolescents [[12], [13], [14]], but the association with other sleep outcomes is considered inconclusive, requiring studies with better methodological quality and more robust designs [12].
On the other hand, a systematic review of the literature, planned to assess the effect of screen time on the health and wellbeing of children and adolescents, showed weak evidence of an association with unfavorable sleep outcomes, such as initial sleeplessness, a reduction in total sleep time, and daytime tiredness [15]. The available literature on the screen use and health and wellbeing of adolescents is limited by the predominance of studies about time watching television, with only a small number examining time using computers or videogames and very few investigating the use of mobile devices [15]. In addition, there is no safe, scientifically defined limit for screen use to date, above which there would be damage to adolescents’ sleep [16]. Most of the studies however used cross-sectional design and came from developed settings, mainly the United States and European countries. Nonetheless, access to electronic media has become popular in all countries, and findings from high income areas do not necessarily reflect the reality of adolescents living in low-and-middle-income settings. For instance, among Brazilian adolescents aged 10 to 19, a meta-analysis showed that the prevalence of excessive screen use (defined as ≥2hs/day) is 70.9% (95%CI: 65.5–76.1) [17].
Thus, given the importance of sleep to physical and mental health and the high prevalence of excessive screen use among Brazilian adolescents, the aim of this study was to investigate the association between screen time and duration and self-reported quality of sleep, among adolescents aged 15, participating in the birth cohort conducted in the city of Pelotas, in the south of Brazil.
2. Material and methods
This study was conducted with data from the follow-up at 15 years old of the 2004 Pelotas Birth Cohort. The cohort was composed of 4,231 children born in hospitals in the city of Pelotas, between January 1st and December 31st of 2004, whose mothers resided in the urban area of the municipality or in the Jardim América neighborhood (adjacent to Pelotas, but belonging to the neighboring municipality of Capão do Leão). Details about the methodology of the 2004 Cohort can be obtained in previous publications [18,19]. The participants in the cohort were assessed at birth (during their hospital stay) and followed up when there were an average (standard deviation – SD) of 3.0 (0.1), 11.9 (0.2), 23.9 (0.4), 49.5 (1.7), and 82.2 (4.0) months, and 6.7 (0.2), 10.3 (0.5), and 15.7 (0.20) years old. The follow-up at 15 years old started in November of 2019 and was prematurely interrupted on March 20th of 2020, when the Health Ministry of Brazil reported the community transmission of the SARS-CoV-2 virus, the agent responsible for the COVID-19 pandemic [20]. In Pelotas, the first case of COVID-19 was officially reported on March 25th of 2020. Up to the interruption of the follow-up, 2,029 adolescents had been evaluated, which, added to the 102 deaths occurring up to then, correspond to a 50.4% follow-up rate.
The 15-year visit focused on six groups of outcome variables: mental health (including sleep), body composition, risk factors for non-communicable chronic diseases, clinical conditions, human capital, violence, and stress. All the information was obtained from in-person interviews, conducted by trained interviewers, with the help of a standardized questionnaire, previously tested by the researchers. The RedCap [21] program was used to record the interviews on tablets. Until 48 months follow-ups were carried out at the child household, and from 6-year onward all follow-ups were conducted at a research clinic of the Center for Epidemiological Research of the Federal University of Pelotas.
2.1. Sleep duration
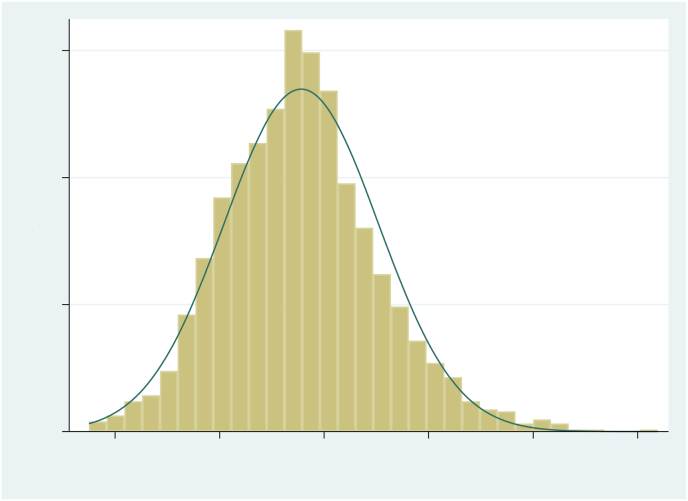
Sleep duration was obtained using questions and figures extracted from the Portuguese language version [22] of the Munich Chronotype Questionnaire [23], referring to the 30 days prior to the interview: “What time are you ready to go to sleep?“; “How many minutes do you need to get to sleep?“; and “What time do you wake up?” The questions were asked separately for weekdays and weekends (Saturdays and Sundays). Sleep duration was calculated as the time between when they are ready to go to sleep and when they wake up, minus latency time (“How many minutes do you need to get to sleep?“). As sleep duration had a normal distribution (Fig. 1), for analysis purposes, the weighted mean between weekdays and weekends was used, obtained by the formula:
Fig. 1.
Distribution of the weighted mean of sleep over 24 h among adolescents from the 2004 Pelotas Birth Cohort, at 15 years old.
Weighted mean of 24 h sleep duration = [(5 x sleep duration on weekdays) + (2 x sleep duration on weekend days)]/7.
Means of less than 3 h or more than 15 h were considered outliers, and those participants (N = 46) were excluded from the analyses for this outcome.
2.2. Self-reported sleep quality
The information about sleep quality was obtained using the question: “Considering last month, how would you classify the quality of your sleep in general?” The answer options, which varied between “very good,” “good,” “bad,” and “very bad,” were read to the adolescents. For analysis purposes, this outcome was dichotomized, grouping the “very good” and “good” categories into “good quality sleep,” and “bad” and “very bad” into “poor quality sleep.” In this study, the outcome of interest was the prevalence of poor quality sleep.
2.3. Screen time
Screen time over 24 h was obtained by the sum of the times of use of different technologies for entertainment purposes. As some adolescents worked and the use of electronic devices, such as cell phones, is limited during working hours, screen use on Saturdays was included among the weekdays.
After an affirmative answer for the use of each technology (for example: “Do you watch television?“), they were asked about the time of use on weekdays (“How many hours do you watch television on weekdays?“) and on Sundays (“How many hours do you watch television on Sundays?“). Similar questions were asked to investigate the use of computers (for games, the internet, social media, and other leisure activities), tablet or cell phone games, and videogame consoles. Screen time over 24 h was calculated as the sum of the weighted means for the use of each type of screen, obtained by the formula:
Weighted mean of screen time over 24 h = [(6 x screen time on weekdays) + (1 x screen time on Sundays)]/7.
Weighted means higher than 21 h a day were considered as outliers, and the adolescents with these values (N = 33) were excluded from the analyses. As the weighted screen time did not have a normal distribution and the logarithmic transformation did not resolve the asymmetry, the median and interquartile interval were calculated and, for the association analyses, the variable was categorized into <2.0; 2.0–3.5; 3.6–5.9; 6.0–8.9; and ≥9.0 h/day.
2.4. Covariables
The covariables used were collected in the perinatal interview (at birth) and in the follow-ups at 6 and 15 years old. From the perinatal interview, the following were employed: monthly family income, obtained from the sum of the incomes of all residents in the household, subsequently divided into quintiles, with the first quintile corresponding to the poorest families and the fifth quintile corresponding to the wealthiest ones; domestic agglomeration, defined as more than two people per bedroom (yes or no); the mother's age at the time of the adolescent's birth (≤19, 20–24, 25–29, 30–34, or ≥35 years old); maternal parity, including the participant in the cohort and defined as the number of live or still born children (1, 2, or ≥3); maternal and paternal schooling, corresponding to the number of years of study completed with the passing grade (0–4, 5–8, or ≥9); and the adolescent's sex (male or female). The adolescent's skin color, obtained in the interview at 6 years old, was reported by the mother (white, black, or brown/other).
From the follow-up at 15 years old, the maternal depression variable was employed, defined as a score of ≥8 on the Edinburgh Postnatal Depression Scale (EPDS) [24], along with the following characteristics of the adolescent: currently studies (yes or no); currently works (yes or no); shares a bed for sleeping (yes or no); shares a bedroom (yes or no); alcohol consumption (consumption of alcoholic beverages in the last 30 days); smoking (smokes at least once a week); coffee consumption (drinks coffee at least once a week); chimarrão consumption (drinks chimarrão at least once a week) (yes or no); hours per week engaging in physical activity (practicing sport at least once a week) (yes or no); and mental health, obtained from applying the Strengths and Difficulties Questionnaire (SDQ), where those with a total score ≥17 were considered as presenting psychological symptoms [25].
2.5. Analysis
The data analysis was carried out in the STATA statistical package, version 17. The description of the sample was carried out by calculating the means and SD for continuous variables with a normal distribution; and the median and interquartile interval (IQI) for those with an asymmetrical distribution. Proportions with 95% confidence intervals (95%CI) were calculated for categorical variables. The association analyses with the weighted mean of sleep duration in its continuous form were carried out using ANOVA and the t-test; and, for sleep quality, using the chi-squared test.
To evaluate the strength of the association between screen time and sleep duration, a linear regression was carried out; and for the association between screen time and self-reported sleep quality, a Poisson regression with robust variance was employed. The trends were assessed using the chi-squared test. The associations that presented p < 0.05 were considered statistically significant. Backward regressions were carried out, and, for adjustment purposes, the variables associated with the outcome with p < 0.20 were maintained in the model. Quality of analysis measures, including a leverage graph, residuals, and an evaluation of the variance and optimization of generalized linear models were used.
In addition, the weighted mean sleep duration, lateness in going to sleep (after 12 a.m.), and long latency (>30 min) were explored as potential mediators of the association between screen time and sleep quality, using the G-formula [26]. In the analysis of the mediation of screen time with sleep quality by sleep duration, the variables used as pre-confounders were the same ones used in the adjusted model of the Poisson regression for screen time and sleep quality, and as the post-confounder variable, “sharing a bed” was used. To explore the mediation by lateness in going to sleep and by long latency, the variables that were shown to be associated with both were used, lateness in going to sleep and sleep quality and long latency and sleep quality, respectively.
Sensitivity analyses were carried out to explore the association between screen time and sleep duration, after stratification for weekdays and weekends. And, as the field work included class periods and school holiday periods (school summer holidays occur between mid-December and mid-February in Brazil), sensitivity analyses were also carried out to ascertain whether there was a difference in the association between screen time and sleep duration, according to the period in which the adolescent had been interviewed.
2.6. Ethical aspects
All of the visits of the 2004 Pelotas Birth Cohort were approved by the Research Ethics Committee of the School of Medicine of the Federal University of Pelotas, affiliated with the National Research Council (CONEP). The perinatal study and the follow-ups at 6 and 15 years old were approved under file numbers 034/2003, 35/10, and 3.554.667, respectively. The consent form was signed by the mother before the perinatal interviews and those at 6 and 15 years old. An additional consent form was obtained from the adolescents before the interview at 15 years old.
3. Results
All in all, 1,949 adolescents had complete information about screen time and sleep quality, and 1,851 had full information about screen time and sleep duration. In Table 1, the adolescents followed up at 15 years old (N = 1949) are compared to the original sample from the cohort in terms of sociodemographic, maternal, and paternal characteristics. There was no statistically significant difference between the sample evaluated at 15 years old and the original sample. Table 1 also contains the description of characteristics of the adolescents at 15 years old: 50.0% were of the female sex; 63.6% were white; 12.4% currently worked; 98.0% were in school; 36.1% shared a bedroom; 6.3% shared a bed; 43.4% had consumed alcoholic beverages in the last 30 days; 5.6% smoked at least once a week; 59.7% drank coffee and 48.4% drank chimarrão at least once a week; and 59.6% practiced sport ≥3 h/week. The mothers of more than a third of the adolescents (36.2%) scored ≥8 on the EPDS and 14.1% of the adolescents scored ≥17 on the SDQ.
Table 1.
Comparison between the sample followed up at 15 years old and the original sample from the cohort (at birth), and description of the variables from the sample at 15 years old.
| Variable | At birth (N = 4231) | Sample analyzed (N = 1949) | p∗ |
|---|---|---|---|
| Family characteristics | |||
| Income at 11 years old (in quintiles) | N = 4229 | N = 1949 | 0.065 |
| 1 | 871 (20.6%) | 359 (18.4%) | |
| 2 | 854 (20.2%) | 375 (19.2%) | |
| 3 | 816 (19.3%) | 406 (20.8%) | |
| 4 | 858 (20.3%) | 439 (22.5%) | |
| 5 | 830 (19.6%) | 370 (19.0%) | |
| Maternal schooling | N = 4186 | N = 1935 | 0.121 |
| 0–4 | 654 (15.6%) | 273 (14.1%) | |
| 5–8 | 1731 (41.4%) | 781 (40.4%) | |
| 9 or more | 1801 (43.0%) | 881 (45.5%) | |
| Paternal schooling | N = 3286 | N = 1537 | 0.368 |
| 0–4 | 580 (17.7%) | 247 (16.1%) | |
| 5–8 | 1161 (35.3%) | 545 (35.5%) | |
| 9 or more | 1545 (47.0%) | 745 (48.5%) | |
| Domestic agglomeration | N = 3985 | N = 1898 | 0.295 |
| Yes | 3560 (89.3%) | 1686 (88.8%) | |
| Maternal parity | N = 4228 | N = 1949 | 0.251 |
| 1 | 1665 (39.4%) | 747 (38.3%) | |
| 2 | 1110 (26.2%) | 552 (28.3%) | |
| 3 or + | 1453 (34.4%) | 650 (33.4%) | |
| Maternal age | N = 4227 | N = 1949 | 0.101 |
| Up to 19 | 799 (18.9%) | 345 (17.7%) | |
| 20 to 24 | 1148 (27.2%) | 494 (25.4%) | |
| 25 to 29 | 959 (22.7%) | 464 (23.8%) | |
| 30 to 34 | 758 (17.9%) | 345 (17.7%) | |
| 35 or more | 563 (13.3%) | 301 (15.4%) | |
| Characteristics of the adolescents | |||
| Sex | N = 4231 | N = 1949 | 0.086 |
| Female | 2036 (48.1%) | 975 (50.0%) | |
| Male | 2195 (51.9%) | 974(50.0%) | |
| Skin color | N = 3668 | N = 1828 | 0.706 |
| White | 2484 (67.7%) | 1253 (63.6%) | |
| Black | 455 (12.4%) | 229 (12.5%) | |
| Brown or other | 729 (19.9%) | 346 (18.9%) | |
| Adolescent works (N=1801) | - | ||
| Yes | - | 224 (12.4%) | |
| No | - | 1577 (87.6%) | |
| Adolescent studies (N=1949) | - | ||
| Yes | - | 1909 (98%) | |
| No | - | 40 (2%) | |
| Maternal EPDS (N=1672) | - | ||
| ≥8 | - | 605 (36.2%) | |
| <8 | - | 1067 (63.8%) | |
| SDQ (N=1880) | - | ||
| ≥17 points | - | 265 (14.1%) | |
| <17 | - | 1615 (85.9%) | |
| Sharing a bed (N=1833) | - | ||
| Yes | - | 116 (6.3%) | |
| No | - | 1717 (93.7%) | |
| Sharing a bedroom (N=1833) | - | ||
| Yes | - | 661 (36.1%) | |
| No | - | 1172 (63.9%) | |
| Alcohol consumption (N=1846) | - | ||
| Yes | - | 802 (43.4%) | |
| No | - | 1044 (56.6%) | |
| Smoking (N=1865) | - | ||
| Yes | - | 104 (5.6%) | |
| No | - | 1761 (94.4%) | |
| Chimarrão∗∗ (N=1949) | - | ||
| Yes | - | 944(48.4%) | |
| No | - | 1005 (51.6%) | |
| Coffee (N=1949) | - | ||
| Yes | - | 1163 (59.7%) | |
| No | - | 786 (40.3%) | |
| Practicing sport (hours/week) (N=1949) | |||
| <3 | - | 788 (40.4%) | |
| 3 to 5 | - | 341 (17.5%) | |
| >5 | - | 820 (42.1%) | |
∗ Chi-squared test; ∗∗ Chimarrão is a caffeine-rich local drink (also known as mate). To prepare chimarrão, ground leaves of Ilex paraguayensis are poured into a gourd and small volumes of hot water are added. Then the consumer sips through a metal straw with a filtering head which is introduced to the bottom of the gourd.
Among the 1,949 adolescents with information about screen time, only 12.2% (10.8–13.7%) reported not using any type of screen for entertainment purposes. Daily screen time varied from 0 to 20.6 h/day, with the median (IQI), including non-users, being 4.50 h (2.3625%–7.5775%). Considering only the screen users, the median (IQI) was 4.71 h (2.5725%–7.5775%). More than 40% of the screen users (41.4%) used screens for 3 h or more over 24 h. Among the users, the medians per day were 2.00 h (1.0025%–3.4375%) watching television; 2.00 h (0.8625%–4.0075%) on the computer; 1.79 h (0.8625%–3.1475%) playing on a tablet or cell phone; and 1.57 h (0.8625%–3.1475%) playing on videogame consoles.
3.1. Sleep duration
Among the 1,851 adolescents with information about sleep duration, the mean duration on weekdays was 7.19 h (SD: 1.76) and on weekends it was 8.50 h (SD: 1.65) (p = 0.005). The weighted mean duration was 7.6 h (SD: 1.5) over 24 h. Around a third of the adolescents (34.0%) slept an average of 8 h or more in 24 h. Table 2 describes the mean sleep duration over 24 h (with 95%CI), according to screen time per day. There was an inverse dose-response type association between screen time and sleep duration (p < 0.001). The adolescents with 9 h or more of screen time per day slept an average of 30 min less over 24 h than those who used screens for fewer than 2 h a day, who were taken as the reference category.
Table 2.
Weighted mean duration of sleep in hours over 24 h and prevalence (%) of poor quality sleep, according to screen use.
| Screen time (hours/day) | Mean sleep duration in hours (N = 1851) |
Prevalence of self-reported poor quality sleep (N = 1949) |
||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean | 95%CI (n = 1851) | p |
N (%) | Prevalence (%) | 95%CI | p |
|
| p < 0.001a | p = 0.025a | |||||||
| <2.0 | 368 (19.9%) | 7.8 | 7.6–7.9 | 398 (20.4%) | 14.8% | 11.7–18.7 | ||
| 2.0–3.5 | 370 (20.0%) | 7.7 | 7.5–7.8 | 383 (19.7%) | 17.0% | 13.5–20.1 | ||
| 3.6–5.9 | 433 (23.4%) | 7.6 | 7.5–7.8 | 456 (23.4%) | 15.4% | 12.3–19.0 | ||
| 6.0–8.9 | 348 (18.8%) | 7.4 | 7.2–7.5 | 360 (18.5%) | 19.7% | 15.9–24.2 | ||
| ≥9.0 | 332 (17.9%) | 7.3 | 7.1–7.5 | 352 (18.1%) | 20.5% | 16.6–25.0 | ||
chi-squared test of linear trend.
The results of the linear regression confirmed the inverse trend between screen time and sleep duration over 24 h (Table 3). In the crude analysis, the adolescents with more intense screen time (≥9 h/day) presented a mean loss of 30 min of daily sleep in relation to the reference category (<2 h/day) (p < 0.001). After adjusting for confounding variables, the loss increased to 32.4 min (adjusted β = −0.54; 95%CI: −0.77;-0.30) (p < 0.001). The reduction in sleep duration was evident starting from 6 h or more per day of screen time.
Table 3.
Crude and adjusted analysis for sleep duration and quality, according to daily screen use.
| Screen time (hours/day) | βa |
RPc |
||||||
|---|---|---|---|---|---|---|---|---|
| Crude |
Adjustedb |
Crude |
Adjustedd |
|||||
| β (95%CI) |
P |
β (95%CI) |
p |
RP (95%CI) |
p |
RP (95%CI) |
P |
|
| <0.001 | <0.001 | 0.026 | <0.001 | |||||
| <2.0 | REF | REF | 1.00 | 1.00 | ||||
| 2.0–3.5 | −0.12 (−0.33; 0.09) | −0.10 (−0.32; 0.12) | 1.14 (0.83–1.58) | 1.23 (0.83–1.82) | ||||
| 3.6–5.9 | −0.17 (−0.37; 0.03) | −0.20 (−0.41; 0.18) | 1.04 (0.75–1.43) | 1.05 (0.70–1.58) | ||||
| 6.0–8.9 | −0.42 (−0.64; −0.21) | −0.39 (−0.62; −0.16) | 1.33 (0.97–1.82) | 1.43 (0.98–2.08) | ||||
| ≥9.0 | −0.50 (−0.72; −0.28) | −0.54 (−0.77; −0.30) | 1.38 (1.01–1.89) | 1.60 (1.10–2.32) | ||||
Linear regression, with trend test.
Adjusted for the adolescent's sex, family income, maternal schooling, whether the adolescent works, psychological symptoms, and alcohol consumption.
Poisson regression.
Adjusted for the adolescent's sex, skin color, maternal schooling, domestic agglomeration, whether the adolescent works, maternal depressive symptoms, sharing a bedroom, sharing a bed, alcohol consumption, smoking, and coffee consumption.
3.2. Self-reported sleep quality
Among the 1,949 adolescents with information about sleep quality, the prevalence of poor sleep quality was 17.3% (95%CI: 15.7–19.0). Table 2 presents the prevalence of poor sleep quality according to screen time. Among the adolescents who used screens for fewer than 2 h a day, the prevalence of poor sleep quality was 14.8%, while among those who used them for 9 h or more, the prevalence was 20.5% (p of linear trend = 0.025).
In the Poisson regression (Table 3), the adolescents who used screens ≥9 h/day presented a 38% higher probability of reporting poor quality sleep than those who used them for fewer than 2 h a day (p = 0.026). In the analysis adjusted for confounding factors, the probability increased to 60% (RP = 1.60; 95%CI: 1.10–2.32) (p < 0.001). The linear trend between the categories of screen time and poor quality sleep was tested, and the p value found was not statistically significant, either in the crude analysis (p = 0.143) or in the adjusted one (p = 0.075). Statistical significance was only found in the screen time category corresponding to ≥9 h/day (p = 0.044 in the crude analysis and p = 0.014 in the adjusted analysis).
Quality of fit measures were applied to the analysis models, according to the rules of the generalized linear models, showing that the model with the exponential 1 for the exposure, as employed, was the most adequate, besides there being no points of influence or outliers regarding the model. Maximum likelihood was applied in the Poisson regression, and no assumption breaks were verified. The analyses with screen time at weekends, extrapolating the screen time reported for Sundays to Saturdays, did not show statistically different results from the previous ones.
3.4. Mediation analyses
Table 4 contains the result of the crude and adjusted analyses of the association between duration, start time, and long latency and sleep quality. The longest sleep duration was protective against reports of poor quality sleep (RP = 0.85; 95%CI: 0.79–0.92; p < 0.001). On the other hand, lateness in going to sleep increased the probability of poor quality sleep by 72% (adjusted RP = 1.72; 95%CI: 1.34–2.22; p < 0.001). A long latency was also associated with a higher probability of poor quality sleep (adjusted RP = 1.51; 95%CI: 1.10–1.22; p < 0.001).
Table 4.
Crude and adjusted analysis between sleep parameters (duration, lateness going to sleep, and long latency) and sleep quality.
| RP |
||||
|---|---|---|---|---|
| Crude |
Adjustedb |
|||
| (95%CI) | p | (95%CI) | P | |
| Weighted mean duration (continuous)a | 0.84 (0.78–0.90) | <0.001 | 0.85 (0.79–0.92) | <0.001 |
| Lateness going to sleep (after 12pm)d | 1.70 (1.35–2.13) | <0.001 | 1.72 (1.34–2.22) | <0.001 |
| Long latency (>30 min)c | 1.53 (1.12–2.08) | 0.008 | 1.51 (1.10–1.22) | <0.001 |
Poisson regression.
Adjusted for screen time, the adolescent's sex, skin color, maternal schooling, psychological symptoms, alcohol consumption, and practicing physical exercise.
Poisson regression ∗Adjusted for screen time, the adolescent's sex, skin color, maternal schooling, maternal parity, psychological symptoms, alcohol consumption, and practicing physical exercise.
Poisson regression ∗Adjusted for screen time, the adolescent's sex, skin color, maternal schooling, maternal parity, psychological symptoms, and alcohol consumption.
The mediation analysis (Table 5), with the variable “weighted screen time” as the exposure, poor quality sleep as the outcome, and mean sleep duration as the mediator, showed a mediated effect of 21.9%, with no statistical significance (p = 0.622). The mediation analysis for lateness in going to sleep and a long latency showed a mediated effect of 24.4% and 17.6%, respectively, with no statistical significance (p = 0.502 and p = 0.411, respectively).
Table 5.
Indirect mediation effects of sleep parameters (duration, lateness in going to sleep, and long latency) over the relationship between screen time and sleep quality.
| Sleep parameters | % indirect effect (95%CI) | p |
|---|---|---|
| Weighted mean duration (continuous) | 21.9% (16.1; 27.7) | 0.622 |
| Lateness going to sleep (after 12pm) | 17.6% (9.8; 25.4) | 0.411 |
| Long latency (>30 min) | 24.4% (20.0; 28.8) | 0.502 |
3.4. Sensitivity analysis
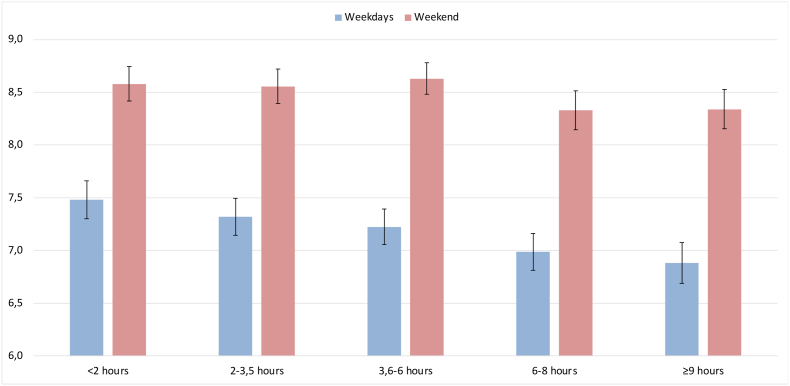
Fig. 2 shows that the mean sleep duration at weekends was greater than on weekdays, independently of screen time (p < 0.001); and that, on weekdays, the mean sleep duration was shorter among those who used screens for 3.6 h or more per day (p = 0.047).
Fig. 2.
Mean sleep duration in hours, on weekdays and at weekends, according to screen time.
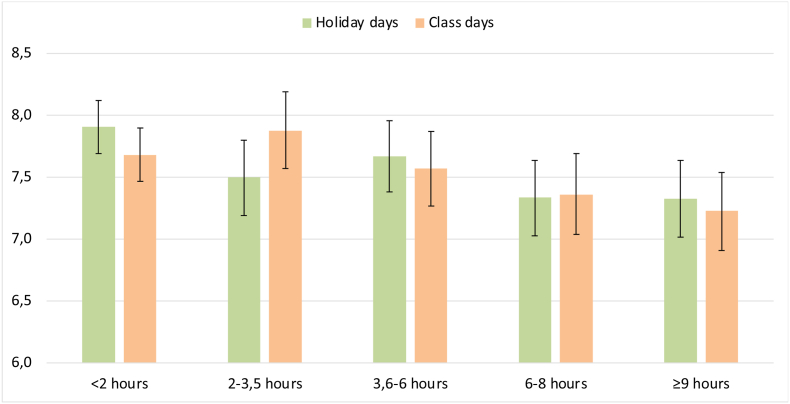
The mean sleep duration on weekdays in the class period was 7.18 h (SD: 1.75) and at weekends it was 8.54 h (SD: 1.65) (p < 0.001). On holidays, the means were 7.21 h (SD: 1.79) and 8.45 h (SD: 1.68) for weekdays and weekends, respectively (p < 0.001). There was no statistical difference between sleep duration on weekdays and (p = 0.721) and at weekends (p = 0.255), in the class period or on holidays. There was a reduction of sleep duration with increased screen time in both periods (p of the trend <0.001 in both periods), but there was no difference in the mean sleep duration on weekdays or at weekends according to screen time (Fig. 3).
Fig. 3.
Mean sleep duration, according to screen time in holiday periods and class periods.
4. Discussion
This study showed that the adolescents slept an average of 7.16 h (SD: 1.76) per day on weekdays and 8.50 h (SD: 1.65) per day at weekends. Compared with screen use for fewer than 2 h per day, 6 h or more of use was associated with a reduction in sleep duration. In particular, more intense screen time (≥9 h/day) was associated with both a reduction in sleep duration over 24 h (a mean reduction of 32.4 min) and a 60% increase in the probability of self-reported poor sleep quality.
These results are consistent with literature reviews that report a reduction in sleep duration and quality with increased use of electronic devices [[12], [13], [14]]. These findings were obtained even with major heterogeneity between the studies with regard to the type of screen investigated, to the way the exposure is measured, and to the period of the day in which the screens are used by the adolescents (most investigate use at night, before going to bed) [14]. Falbe et al., [27] in the United States, and Hysing et al., [28] in Norway, for example, investigated the presence of screens close to or in the place of sleep and screen use 1 h before going to sleep, respectively. In the city of Hong Kong, Lee et al. [29] used accelerometers to measure sleep duration and an Android smartphone application to measure cell phone use. A study in Nigeria investigated night-time use of computers on weekdays only [30]. In Spain, Continente et al. [31] investigated the presence of a computer in the bedroom and watching TV at dinner.
In our study, more intense screen use (9 h or more per day) was associated with self-reported poor quality sleep. The association found in the current study is consistent with findings of other studies, although this outcome was assessed differently among adolescents. In France, Messadi, N et al. [32] defined good quality as sleeping for over 9 h a day. In Iran [33], sleep quality was evaluated using the Pittsburgh Sleep Quality Index [34] and the exposure by cell phone use after 9pm. The Pittsburgh Sleep Quality Index [34] was also used in studies conducted in Spain [35] and New Zealand [36].
Harder to measure than sleep duration, quality may indicate both how much sleep is reparative for the individual and how much they feel satisfied with their own sleep. In our sample, a longer sleep duration was protective against poor quality, while lateness in going to sleep and a long latency increased the probability of reporting poor quality sleep. Studies that have investigated the association between a long latency and sleep quality have found similar results [37]. Unfortunately, we did not find other studies in the literature that have investigated the time of going to sleep as a determinant of quality. The mediation analyses, however, suggest that the association between screen use and poor quality sleep in our sample was not mediated by those variables. Future studies that explore the reasons individuals use to classify the quality of their sleep could help to investigate the mechanisms through which screen use affects sleep quality.
Potential mechanisms underlying the association between screen use and sleep duration and quality include time displacement (the time spent on screens substitutes the time spent on other activities, including sleeping); psychological stimulus due to media content; and the effect of the light emitted by the devices [[38], [39], [40]]. A randomized cross-over clinical trial conducted with young adults, where the exposure was screen use for reading (eBooks) before going to bed and the use of printed books was the control, showed that night-time exposure to a light-emitting device is linked to lower secretion of melatonin and a delay in the adjustment of the biological clock, hindering getting to sleep [40]. Exposure to electronic devices that emit light, particularly in the blue range present on LED screens, such as computers and smartphones, is highly effective in suppressing melatonin, leading to Circadian desynchronization and favoring lateness in getting to sleep [41]. In addition, some authors suggest that, just like disorders caused by substance use, screen use could hijack the reward system, due to the activation of the mesolimbic system, caused by social interactions and advancement through game phases, generating the sensation of pleasure and a reward through dopamine release [42].
The sensitivity analyses showed that the mean sleep duration of the adolescents is longer at the weekends than on weekdays, independently of whether it is a class period or holiday. Other authors have found similar results [37,43]. Despite the compensation of sleep time at weekends, the weighted mean duration (7.6 h/24 h) is below the recommended value (8 h of sleep in 24 h). It is noteworthy that a similar pattern of sleep duration is maintained in both class periods and during holidays, suggesting the presence of a behavior acquired by the adolescents.
4.1. Strengths and limitations of the study
Among the limitations, it is necessary to mention that, despite the results presenting statistical significance, causality relationships cannot be inferred, since it is not possible to reject inverse causality. A longitudinal study conducted in Sweden, with 1,620 secondary-level students from 17 public schools, found bidirectionality in the association between screen use and sleep duration [44]. It is impossible to affirm whether greater screen use leads to a shorter sleep duration or if the change in sleep pattern, typical of adolescence [45], is what leads to greater screen use. Also, although various types of screens were investigated, the adolescents were not asked about their simultaneous use. There is evidence that adolescents use a combination of screens, such as using smartphones while watching television [46]. Similarly, smartphone use for purposes other than games, such as social media applications, music and clips, or video applications, among others, was not investigated. Another limitation is that the time of the day when the screen devices were used was not investigated, nor were naps during the day, which could compensate for the shorter sleep duration in the nocturnal period. However, napping during the day is an infrequent practice among adolescents [47]. In addition, screen use for schoolwork, which would increase daily exposure time, was not investigated.
Furthermore, the information about both screen use and the outcomes (sleep duration and sleep quality) was obtained from the adolescent's self-reporting, which may be subject to information bias. However, the use of standardized and pre-tested questionnaires and the detailed training of the interviewers in applying the interviews were strategies employed to prevent information bias. Finally, although various potential confounders of the association between screen use and sleep quality and duration were explored, it is possible that there is residual confounding due to unmeasured variables.
Among the positive aspects is the large sample size, derived from a birth cohort, with population sampling, thus being representative of the adolescents of the city of Pelotas. The investigation of the use of various types of electronic equipment and not only time watching television, as occurs in most of the research on this topic [15], is another advantage of the study. The mediation and sensitivity analyses are also strong methodological aspects of the study. Moreover, as far as we know, this is the first Brazilian population-based study to investigate the association between screen use and sleep duration among adolescents.
5. Conclusion
In summary, this study showed that greater screen use for entertainment was associated with shorter duration and poorer quality sleep among the adolescents. Screen use for 6 h or more was associated with a 23.4 min reduction in sleep over 24 h; and 9 h or more of use was associated with a 32.4 min reduction in sleep over 24 h and a greater prevalence of poor quality sleep. It is noteworthy that the cut-off points for screen use that were associated with the sleep parameters were higher than the 2–3 h per day recommended by the Brazilian Pediatric Society [48] for adolescents in this age group. Perhaps the period of the day in which the screens are used (especially at night) is more important for the adolescents’ sleep duration than the total screen use over 24 h (restricted screen time in the nocturnal period has been recommended) [15]. The clinical significance of the magnitude of the association between screen use and sleep duration (a reduction of around half an hour for more intense use), even though use for schoolwork was not included, needs to be evaluated. It is important to investigate whether these cut-off points apply to other health outcomes among adolescents.
Funding
This article is based on data from the study “Pelotas Birth Cohort, 2004″ conducted by Postgraduate Program in Epidemiology at Universidade Federal de Pelotas, with the collaboration of the Brazilian Public Health Association (ABRASCO). From 2009 to 2013, the Wellcome Trust supported the 2004 birth cohort study. Previous phases of the study were supported by the Children's Pastorate; the World Health Organization [Grant no. 03014HNI]; the National Support Program for Centers of Excellence (PRONEX) [Grant no. 04/0882.7]; the Brazilian National Research Council (CNPq) [Grant no. 481012-2009-5; 484077-2010-4; 470965-2010-0; 481141-2007-3; 426024/2016-8; 312746/2021-0]; the Brazilian Ministry of Health [Grant no. 25000.105293/2004-83]; and the São Paulo Research Foundation (FAPESP) [Grant no. 2014/13864-6 and Grant n° 2020/07730-8]. LTR, AM and ISS are supported by CNPq.
The sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author contributor ship
Priscila Echevarria, Iná S. Santos, Bianca Del-Ponte da Silva contributed to the literature search, study design, data collection, data interpretation and data analysis. Luciana Tovo-Rodrigues, Alicia Matijasevich and Camila S. Halal contributed to the literature search and study design. All the authors have been involved in revising the manuscript critically for important intellectual content, gave final approval of the submitted version and agreed to be accountable for all aspects of the work.
Declaration of competing interest
Authors declare none.
Acknowledgements
The authors would like to thank the participating families and staff who collaborated in the various stages of the 2004 Pelotas Birth Cohort study.
Contributor Information
Priscila Echevarria, Email: prieche@gmail.com.
Bianca Del-Ponte, Email: bianca.delponte@gmail.com.
Luciana Tovo-Rodrigues, Email: luciana.tovo@gmail.com.
Alicia Matijasevich, Email: amatija@yahoo.com.
Camila S. Halal, Email: halalcamila@gmail.com.
Iná S. Santos, Email: inasantos.epi@gmail.com.
References
- 1.Brinkman J.E., Reddy V., Sharma S. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; Treasure Island (FL: 2023. Physiology of sleep. Available from: Bookshelf ID: NBK482512. [Google Scholar]
- 2.Dutil C., Walsh J.J., Featherstone R.B., Gunnell K.E., Tremblay M.S., Gruber R., Weiss S.K., Cote K.A., Sampson M., Chaput J.P. Influence of sleep on developing brain functions and structures in children and adolescents: a systematic review. Sleep Med Rev. 2018;42:184–201. doi: 10.1016/j.smrv.2018.08.003. Epub 20180815. doi: 10.1016/j.smrv.2018.08.003. PubMed PMID: 30241996. [DOI] [PubMed] [Google Scholar]
- 3.Shochat T., Cohen-Zion M., Tzischinsky O. 2014. Functional consequences of inadequate sleep in adolescents: asystematic review; pp. 75–87. [DOI] [PubMed] [Google Scholar]
- 4.Short M.A., Weber N. Sleep duration and risk-taking in adolescents: a systematic review and meta-analysis. Sleep Med Rev. 2018;41:185–196. doi: 10.1016/j.smrv.2018.03.006. Epub 20180327. doi: 10.1016/j.smrv.2018.03.006. PubMed PMID: 29934128. [DOI] [PubMed] [Google Scholar]
- 5.Kelman B. The sleep needs of adolescents. J Sch Nurs: the official publication of the National Association of School Nurses. 1999;15(3):14–19. [PubMed] [Google Scholar]
- 6.Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L., Neubauer D.N., O'Donnell A.E., Ohayon M., Peever J., Rawding R., Sachdeva R.C., Setters B., Vitiello M.V., Ware J.C., Adams Hillard P.J. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Tarokh L., Raffray T., Van Reen E., Carskadon M.A. Physiology of normal sleep in adolescents. Adolesc Med (Phila): State Art Rev. 2010;21(3):401–417. [vii] [PubMed] [Google Scholar]
- 8.Moore M., Meltzer L.J. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9(2):111–114. doi: 10.1016/j.prrv.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Owens J.A. Etiologies and evaluation of sleep disturbances in adolescence. Adolesc Med (Phila): State Art Rev. 2010;21(3):430–445. [vii-viii] [PubMed] [Google Scholar]
- 10.Medicine Io . In: Sleep disorders and sleep deprivation: an unmet public health problem. Colten H.R., Altevogt B.M., editors. The National Academies Press; Washington, DC: 2006. p. 424. [PubMed] [Google Scholar]
- 11.Kokka I., Mourikis I., Nicolaides N.C., Darviri C., Chrousos G.P., Kanaka-Gantenbein C., Bacopoulou F. Exploring the effects of problematic internet use on adolescent sleep: a systematic review. Int J Environ Res Publ Health. 2021;18(2) doi: 10.3390/ijerph18020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund L., Sølvhøj I.N., Danielsen D., Andersen S. Electronic media use and sleep in children and adolescents in western countries: a systematic review. BMC Publ Health. 2021;21(1):1598. doi: 10.1186/S12889-021-11640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokka I., Mourikis I., Nicolaides N.C., Darviri C., Chrousos G.P., Kanaka-Gantenbein C., Bacopoulou F. Exploring the effects of problematic internet use on adolescent sleep: a systematic review. Int J Environ Res Publ Health. 2021;18(2):760. doi: 10.3390/ijerph18020760. PubMed PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale L., Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50–58. doi: 10.1016/j.smrv.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiglic N., Viner R.M. Effects of screentime on the health and well-being of children and adolescents: a systematic review of reviews. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-023191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Communications C.O., MEDIA. Hill D., Ameenuddin N., Chassiakos Y.R., Cross C., Radesky J., Hutchinson J., Levine A., Boyd R., Mendelson R., Moreno M., Swanson W.S. MBE. Media use in school-aged children and adolescents. Pediatrics. 2016;138(5) doi: 10.1542/peds.2016-2592. [DOI] [PubMed] [Google Scholar]
- 17.Schaan C.W., Cureau F.V., Sbaraini M., Sparrenberger K., Kohl H.W., Schaan B.D. Prevalence of excessive screen time and TV viewing among Brazilian adolescents: a systematic review and meta-analysis. J Pediatr. 2019:155–165. doi: 10.1016/j.jped.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Santos I.S., Barros A.J.D., Matijasevich A., Domingues M.R., Barros F.C., Victora C.G. Cohort profile: the 2004 pelotas (Brazil) birth cohort study. Int J Epidemiol. 2011;40(6):1461–1468. doi: 10.1093/ije/dyq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos I.S., Barros A.J.D., Matijasevich A., Zanini R., Cesar M.A.C., Camargo-Figuera F.A., Oliveira I.O., Barros F.C., Victora C.G. Cohort profile update: 2004 pelotas (Brazil) birth cohort study. Body composition, mental health and genetic assessment at the 6 years follow-up. Int J Epidemiol. 2014;43(5):1437. doi: 10.1093/ije/dyu144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavalcante J.R., Cardoso-Dos-Santos A.C., Bremm J.M., Lobo A.D.P., Macário E.M., Oliveira W.K.D., França G.V.A.D. COVID-19 no Brasil: evolução da epidemia até a semana epidemiológica 20 de 2020. Epidemiologia e Serviços de Saúde. 2020;29(4) doi: 10.5123/s1679-49742020000400010. [DOI] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. Epub 20080930. doi: 10.1016/j.jbi.2008.08.010. PubMed PMID: 18929686; PMCID: PMC2700030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miguel M., Oliveira V.C.D., Pereira D., Pedrazzoli M. Detecting chronotype differences associated to latitude: a comparison between Horne-Östberg and Munich Chronotype questionnaires. Ann Hum Biol. 2014;41(2):107–110. doi: 10.3109/03014460.2013.832795. [DOI] [PubMed] [Google Scholar]
- 23.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythm. 2003;18(1):80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 24.Matijasevich A., Munhoz T.N., Tavares B.F., Barbosa A.P., da Silva D.M., Abitante M.S., Dall'Agnol T.A., Santos I.S. Validation of the Edinburgh Postnatal Depression Scale (EPDS) for screening of major depressive episode among adults from the general population. BMC Psychiatr. 2014;14:284. doi: 10.1186/s12888-014-0284-x. Epub 20141008. doi: 10.1186/s12888-014-0284-x. PubMed PMID: 25293375; PMCID: PMC4203969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleitlich B., Cortázar P.G., Goodman R. Questionário de capacidades e dificuldades (SDQ) Infanto rev neuropsiquiatr infanc adolesc. 2000;8(1):44–50. [Google Scholar]
- 26.Wang A., Arah O.A. G-computation demonstration in causal mediation analysis. Eur J Epidemiol. 2015;30(10):1119–1127. doi: 10.1007/s10654-015-0100-z. Epub 20151104. doi: 10.1007/s10654-015-0100-z. PubMed PMID: 26537707; PMCID: PMC4674449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falbe J., Davison K.K., Franckle R.L., Ganter C., Gortmaker S.L., Smith L., Land T., Taveras E.M. Sleep duration, restfulness, and screens in the sleep environment. Pediatrics. 2015;135(2):e367–e375. doi: 10.1542/peds.2014-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hysing M., Pallesen S., Stormark K.M., Jakobsen R., Lundervold A.J., Sivertsen B. Sleep and use of electronic devices in adolescence: results from a large population-based study. BMJ Open. 2015;5(1) doi: 10.1136/bmjopen-2014-006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P.H., Tse A.C.Y., Wu C.S.T., Mak Y.W., Lee U. Temporal association between objectively measured smartphone usage, sleep quality and physical activity among Chinese adolescents and young adults. J Sleep Res. 2021;30(4) doi: 10.1111/jsr.13213. [DOI] [PubMed] [Google Scholar]
- 30.Olorunmoteni O.E., Fatusi A.O., Komolafe M.A., Omisore A. Sleep pattern, socioenvironmental factors, and use of electronic devices among Nigerian school-attending adolescents. Sleep Health. 2018;4(6):551–557. doi: 10.1016/j.sleh.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Continente X., Pérez A., Espelt A., López M.J. Media devices, family relationships and sleep patterns among adolescents in an urban area. Sleep Med. 2017;32:28–35. doi: 10.1016/j.sleep.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Messaadi N., Bayen S., Beghin L., Lefebvre J.M., Colleau S., Deken V., Cottencin O., Quersin F., Descamps A., Vanhelst J. [Association between screen time and sleep habits in 11-to-12-year-old French middle school students] Revue d'epidemiologie et de sante publique. 2020;68(3):179–184. doi: 10.1016/j.respe.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Amra B., Shahsavari A., Shayan-Moghadam R., Mirheli O., Moradi-Khaniabadi B., Bazukar M., Yadollahi-Farsani A., Kelishadi R. The association of sleep and late-night cell phone use among adolescents. J Pediatr. 2017;93(6):560–567. doi: 10.1016/j.jped.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. PubMed PMID: 2748771. [DOI] [PubMed] [Google Scholar]
- 35.Cabré-Riera A., Torrent M., Donaire-Gonzalez D., Vrijheid M., Cardis E., Guxens M. Telecommunication devices use, screen time and sleep in adolescents. Environ Res. 2019;171:341–347. doi: 10.1016/j.envres.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Galland B.C., de Wilde T., Taylor R.W., Smith C. Sleep and pre-bedtime activities in New Zealand adolescents: differences by ethnicity. Sleep Health. 2020;6(1):23–31. doi: 10.1016/j.sleh.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Bacaro V., Gavriloff D., Lombardo C., Baglioni C. Sleep characteristics in the Italian pediatric population: a systematic review. Clin Neuropsychiatry. 2021;18(3):119–136. doi: 10.36131/cnfioritieditore20210302. PubMed PMID: 34909029; PMCID: PMC8629036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregory A.M., Sadeh A. Annual Research Review: sleep problems in childhood psychiatric disorders--a review of the latest science. JCPP (J Child Psychol Psychiatry) 2016;57(3):296–317. doi: 10.1111/jcpp.12469. Epub 20150928. doi: 10.1111/jcpp.12469. PubMed PMID: 26412255. [DOI] [PubMed] [Google Scholar]
- 39.Cain N., Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11(8):735–742. doi: 10.1016/j.sleep.2010.02.006. Epub 20100729. doi: 10.1016/j.sleep.2010.02.006. PubMed PMID: 20673649. [DOI] [PubMed] [Google Scholar]
- 40.Chang A.M., Aeschbach D., Duffy J.F., Czeisler C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232–1237. doi: 10.1073/pnas.1418490112. Epub 20141222. doi: 10.1073/pnas.1418490112. PubMed PMID: 25535358; PMCID: PMC4313820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowley S.J., Cain S.W., Burns A.C., Acebo C., Carskadon M.A. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 2015;100(11):4067–4073. doi: 10.1210/jc.2015-2775. Epub 20150824. doi: 10.1210/jc.2015-2775. PubMed PMID: 26301944; PMCID: PMC4702443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis R.G., Florio E., Punzo D., Borrelli E. The brain's reward system in health and disease. Adv Exp Med Biol. 2021;1344:57–69. doi: 10.1007/978-3-030-81147-1_4. PubMed PMID: 34773226; PMCID: PMC8992377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry K.M., Berger A.T., Laska M.N., Erickson D.J., Lenk K.M., Iber C., Full K.M., Wahlstrom K., Redline S., Widome R. Weekend night vs. school night sleep patterns, weight status, and weight-related behaviors among adolescents. Sleep Health. 2021;7(5):572–580. doi: 10.1016/j.sleh.2021.07.008. Epub 20210901. doi: 10.1016/j.sleh.2021.07.008. PubMed PMID: 34479827; PMCID: PMC8545855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzer K., Bauducco S., Linton S.J., Boersma K. Longitudinal associations between time spent using technology and sleep duration among adolescents. J Adolesc. 2018;66:112–119. doi: 10.1016/j.adolescence.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Carskadon M.A., Psychiatry P., Behavior H. 2011. Sleep in adolescents: the perfect storm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jago R., Sebire S.J., Gorely T., Cillero I.H., Biddle S.J.H. "I'm on it 24/7 at the moment": a qualitative examination of multi-screen viewing behaviours among UK 10-11 year olds. Int J Behav Nutr Phys Activ. 2011;8(1):85. doi: 10.1186/1479-5868-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inazumi C.K., Andrechuk C.R.S., Carvalho D.C., Oliveira C.S., Cardoso T.A.M.O., Ceolim M.F., Barros MBdA., Lima M.G., Zancanella E. Adolescentes que cochilam. Congresso Científico da Faculdade de Enfermagem da UNICAMP. 2018;(1):1–2. doi: 10.20396/CCFENF1201810. [DOI] [Google Scholar]
- 48.Sociedade Brasileira de Pediatria. Manual de Orientação #MENOS TELAS #MAIS SAÚDE. 2019 https://www.sbp.com.br/fileadmin/user_upload/_22246c-ManOrient_-__MenosTelas__MaisSaude.pdf [updated 2019; cited 2023 01-22-2023]. Available from: [updated 2019; cited 2023 01-22-2023]. Available from: [Google Scholar]