ABSTRACT
Background:
Androgenetic alopecia (AGA) is caused by the susceptibility of hair follicles to androgenic miniaturization, which leads to hair loss. The most common modalities for the treatment of AGA include the use of topical minoxidil and oral finasteride. Low-level laser therapy (LLLT) is a newer modality of treatment for AGA. We tried to evaluate the added benefit of LLLT in AGA compared with topical minoxidil 5% alone.
Aim:
The aim of this study was to compare the efficacy of LLLT combined with topical 5% minoxidil in AGA versus topical 5% minoxidil used alone.
Materials and Methods:
After ethics committee approval, 54 patients of AGA were randomly divided into two groups. Group A participants received LLLT therapy twice a week plus topical 5% minoxidil and Group B participants received only minoxidil 5% solution. Both the groups were followed up for 16 weeks and evaluated with gross photographs, TrichoScan analysis, and dermoscopy to look for any improvement in hair density.
Results:
After 16 weeks, improvement in hair density of 14.78% ± 10.93% in Group A was recorded compared to 11.43% ± 6.43% in Group B. However, while comparing both means, P value was 0.45 which was not significant. The physician global assessment and patient satisfaction score revealed no significant difference between both the groups.
Conclusion:
Although LLLT appears to be safe and effective in the treatment of male pattern hair loss, we did not observe any significant difference in terms of improvement in hair density between both the groups.
Keywords: Androgenetic alopecia, low-level laser therapy, minoxidil, TrichoScan, trichoscopy
INTRODUCTION
Androgenetic alopecia (AGA), also known as male pattern hair loss in men, is hereditary thinning of hair from the scalp induced by androgens in genetically susceptible men.
The two FDA-approved drugs for AGA are topical minoxidil and oral finasteride.[1]
Low-level laser therapy (LLLT) is a newer modality for treatment of AGA and relatively easy, noninvasive, and convenient to use. However, published trials of LLLT have been criticized as not being independent and individual reports of using these devices appear disappointing. Hence, we did a randomized controlled trial to see the add-on effect of LLLT in already established topical minoxidil treatment.
MATERIALS AND METHODS
After ethics committee approval, a randomized active-controlled trial was undertaken at outpatient clinic between December 2019 and July 2021. The study was prospectively registered under Clinical Trials Registry of India.
Sample size was calculated as 68 using openepi.com website, keeping confidence interval at 95% and power at 80%. Total 68 males between 18 and 45 years of age with clinically diagnosed mild-to-moderate AGA (Grades II–IV), according to Norwood–Hamilton classification, were enrolled in the study after taking written informed consent. About 14 males lost to follow-up because of prolonged follow-up period and COVID-19 lockdown. The remaining 54 participants were randomly allocated into Group A (n = 26) and Group B (n = 28). We excluded patients on oral finasteride or minoxidil or any modality of treatment within the last 6 months. Patients on any anti-androgen drugs such as spironolactone, cyproterone, progesterone, tamoxifen, and anabolic steroids, patients having any other hair loss disorders, or patients having trauma or infection or scarring present at the affected site were also excluded.
After taking written informed consent, detailed history was taken by using predesigned case record form. All the patients were subjected to detailed history including age of onset, type of hair loss, duration, major prior illness, drug history, family history of early-onset male pattern baldness, anxiety, and any prior treatment taken for the same. Nature of the disease, various treatment options, and prognosis of each treatment modality were explained to the patient before enrolling them.
We used LaserComb (Hairmax Ultima 9) which has 9 medical-grade lasers with a wavelength of 655 ± 10 nm. All the participants in Group A were given LLLT therapy twice per week for 12 weeks.
While administering LLLT therapy, LaserComb was moved from front to back and from side to side in slow combing motion, to cover entire scalp with multiple passes for about 11 min for each session. Participants in Group A were also given topical minoxidil 5% solution to be applied two times a day at home.
Participants under Group B were prescribed minoxidil 5% solution 1 ml to be applied twice daily on the bald areas of scalp. They explained the side effects such as initial hair loss for 1–2 months, headache, redness, and irritation.
All the enrolled participants were evaluated before starting therapy and then monthly till 4 months. Gross photographs were taken under adequate illumination, identical settings, lighting, and position from front and lateral sides of the patient with Canon DSLR camera before and after completion of 16-week follow-up period [Figures 1 and 2]. Measuring tape was used to determine three fixed points on the scalp for future dermoscopic evaluation. Landmarks for each frontal region were the intersecting point between lines passing through ipsilateral tragus and lateral point of eyebrows and the intersection of two imaginary lines crossing both the ears at the highest point of scalp for vertex region. A Heine 20 × videodermoscope was used for trichoscopy and TrichoScan was performed on the same dermoscopic pictures through computer-based software named Dview.
Figure 1.
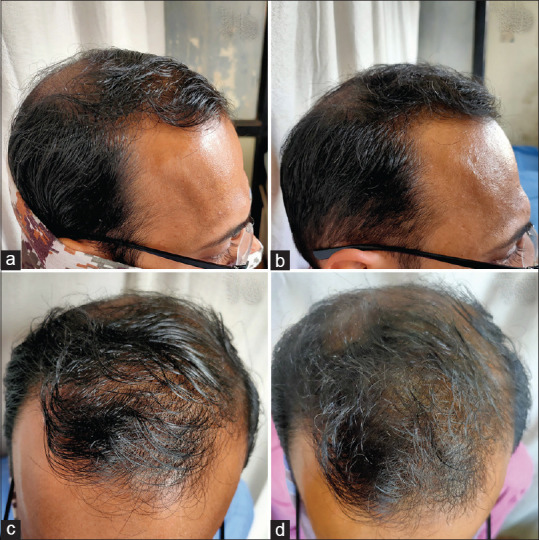
Clinical photographs of low-level laser therapy + minoxidil group:- Frontal view at baseline (a) and after 16 weeks (b), Vertex view at baseline (c) and after 16 weeks (d)
Figure 2.
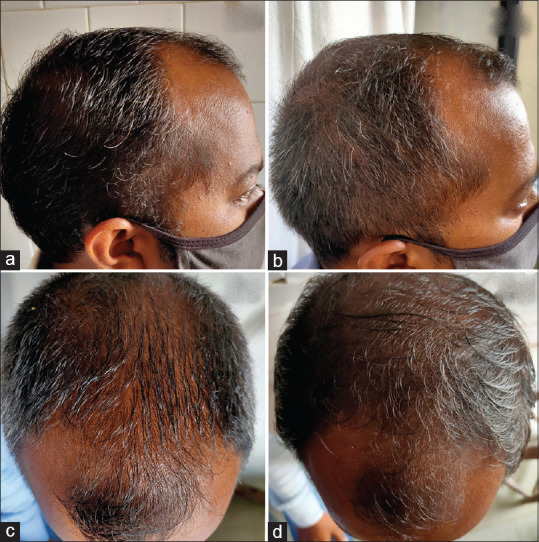
Clinical photographs of minoxidil group:- Frontal view at baseline (a) and after 16 weeks (b), Vertex view at baseline (c) and after 16 weeks (d)
TrichoScan Professional software (3.0.6.72) was used which provides total hair count, hair density, percentage of anagen hairs, telogen hairs, ratio of vellus hairs, and terminal hairs over an area of 1.195 cm2. Trichoscopic parameters were evaluated at 0, 8, and 16 weeks. Direct dermoscopy was done with DermLite DL4 from frontal and vertex area of scalp before and after treatment [Figures 3 and 4] to look for parameters such as variation in hair shaft diameters (VSDs) and number of follicular units (FUs) with one and more than one hair follicles. Percentage of VSD was calculated manually as number of vellus hairs as compared to total (vellus + terminal) number of hairs. FU with single hair and FU with multiple hairs were also counted manually from dermoscopic images at baseline and at 16 weeks.
Figure 3.
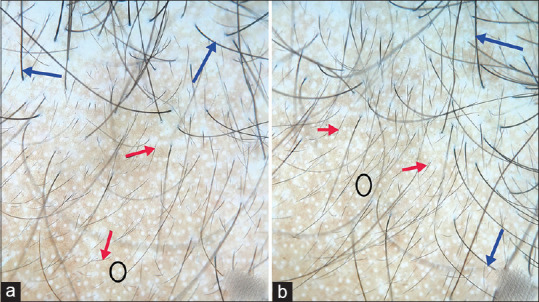
Dermoscopic images of low-level laser therapy + minoxidil group:- Pretreatment (a) Posttreatment (b) short vellus hairs (red arrow), terminal hairs (blue arrow), white dots (black circle) representing sweat duct opening, improvement is seen in miniaturized hair follicles after treatment
Figure 4.
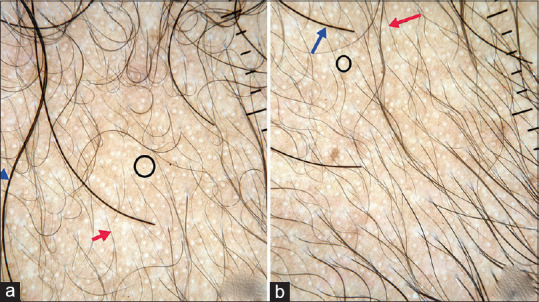
Dermoscopic images of Minoxidil group: Pretreatment (a) Posttreatment (b) short vellus hairs (red arrow), terminal hairs (blue arrow), white dots (black circle) representing sweat duct opening, improvement is seen in miniaturized hair follicles after treatment
Statistical analysis
The data collected were analyzed in Epi Info 7 and GraphPad InStat software (version 3.06, San Digeo USA). Categorical data were expressed in number and frequency while quantitative data were measured in mean. Parameters between the two groups were compared using Mann–Whitney test (nonparametric) at all times. Difference in the parameters within the group at different time points was compared using paired t-test (parametric distribution) and Wilcoxon matched-pairs test (nonparametric distribution). P < 0.05 was considered statistically significant.
OBSERVATIONS AND RESULTS
A total of 54 patients, 26 in Group A and 28 in Group B, were recruited and followed up for 16 weeks for any improvement in hair growth.
The age of the participants was between 18 and 45 years. The demographic details of the participants are mentioned in Table 3. The most common age group involved was 18–25 years [Graph 1]. Around 38.9% of the patients belonged to that age group, followed by 26–35 years (33.3%) and 36–45 years (27.8%). Grade II (Norwood–Hamilton classification) was the most common (40.7%) grade of presentation followed by Grade III (37%) in our study [Graph 2]. The highest improvement was seen in Grade III (46.2%) in the LLLT + minoxidil group, while in the minoxidil group, Grade II patients (53.6%) showed the highest improvement [Table 5].
Table 1.
Physician assessment score
| Response | Grade (%) |
|---|---|
| Marked deterioration | >50 |
| Moderate deterioration | 21-50 |
| Mild deterioration | 1–20 |
| No change | 0 |
| Mild improvement | 1-20 |
| Moderate improvement | 21-50 |
| Marked improvement | >50 |
Table 2.
Patient satisfaction score
| Response | Grade (%) |
|---|---|
| Excellent | >45 |
| Very good | 31-45 |
| Good | 16-30 |
| Average | 1-15 |
| Poor | 0 |
Table 3.
Demographic details of the study group
| Parameters | Group A | Group B |
|---|---|---|
| Age, mean±SD | 27.1±6.8 | 31.0±7.3 |
| Duration of illness, mean±SD | 3.7±2.9 | 3.8±2.4 |
| Positive family history (%) | 13 (50) | 11 (39.3) |
| Norwood–Hamilton grade (%) | ||
| Grade 2 | 7 (26.9) | 15 (53.6) |
| Grade 3 | 12 (46.1) | 8 (28.6) |
| Grade 4 | 7 (26.9) | 5 (17.9) |
SD – Standard deviation
Graph 1.
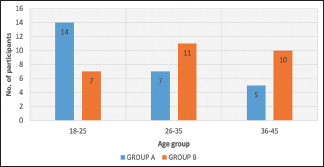
Age distribution of study participants in both the groups
Graph 2.
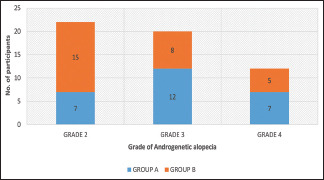
Distribution according to grade of androgenetic alopecia in both the groups
Table 4.
Percentage improvement in hair density in Groups A and B according to age group
| Age Group | No improvement 0% (%) | Mild improvement 1%-20% (%) | Moderate improvement 20%-50% (%) | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| A | B | A | B | A | B | A | B | |
| 18-25 | 1 (3.8) | 2 (7.1) | 8 (30.8) | 5 (17.9) | 5 (19.2) | 0 | 14 (53.8) | 7 (25) |
| 25-35 | 1 (3.8) | 1 (3.6) | 3 (11.5) | 9 (32.1) | 3 (11.5) | 1 (3.6) | 7 (26.9) | 11 (39.3) |
| 35-45 | 1 (3.8) | 1 (3.6) | 4 (15.4) | 8 (28.6) | 0 | 1 (3.6) | 5 (19.2) | 10 (35.7) |
| Total | 3 (11.5) | 4 (14.3) | 15 (57.7) | 22 (78.6) | 8 (30.8) | 2 (7.1) | 26 | 28 |
>50%-Marked improvement was not observed in any of the patients
Table 5.
Percentage improvement in hair density in Groups A and B according to grade of androgenetic alopecia
| Age Grade | No improvement 0% (%) | Mild improvement 1%–20% (%) | Moderate improvement 20%–50% (%) | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| A | B | A | B | A | B | A | B | |
| II | 1 (3.8) | 2 (7.1) | 3 (11.5) | 12 (42.9) | 3 (11.5) | 1 (3.6) | 7 (26.9) | 15 (53.6) |
| III | 1 (3.8) | 1 (3.6) | 8 (30.8) | 7 (25) | 3 (11.5) | 0 | 12 (46.2) | 8 (28.6) |
| IV | 1 (3.8) | 1 (3.6) | 4 (15.4) | 3 (10.7) | 2 (7.7) | 1 (3.6) | 7 (26.9) | 5 (17.9) |
| Total | 3 (11.5) | 4 (14.3) | 15 (57.7) | 22 (78.6) | 8 (30.8) | 2 (7.1) | 26 | 28 |
>50%-Marked improvement was not observed in any of the patients
Table 4 shows percentage improvement from baseline in hair density at 16-week follow-up in both the groups. Around 30.8% of the patients showed moderate improvement in hair density, 57.7% reported mild improvement, and three patients did not show any improvement in Group A. Higher improvement in hair density was observed in younger age group (18–25 years) in the LLLT + minoxidil group [Table 4]. Around 78.6% of the patients showed mild improvement in hair density and only 7.1% of the patients showed moderate improvement while four patients showed no improvement in Group B. None of the patients reported marked improvement (>50% improvement from baseline in hair density) in our study in either of the two groups.
Table 5 represents percentage improvement in both the groups according to grade of AGA. The highest improvement was seen in Grade III (46.2%) in the LLLT + minoxidil group, while in the minoxidil group, Grade II patients (53.6%) showed the highest improvement.
Table 6 represents the intragroup comparison of mean improvement in hair density in both the groups. While the mean increase in hair density was 5.6% in Group A and 4.1% in Group B and the difference was not significant at 8 weeks. After 16 weeks, 14.8% improvement in hair density was noted in Group A compared to 11.4% in Group B. But, while comparing both means using Mann–Whitney test, P value was 0.45 which is considered not significant.
Table 6.
Intragroup comparison of mean improvement in hair density in both groups
| Hair density improvement from baseline (%) at 8 weeks | Hair density improvement from baseline (%) at 16 weeks | |
|---|---|---|
| Group A | 5.65±5.96 | 14.78±10.93 |
| Group B | 4.09±3.05 | 11.43±6.43 |
| P | 0.72 | 0.45 |
| Significance | NS | NS |
Values mentioned here are mean±SD, P values are calculated with the help of Mann–Whitney test (nonparametric distribution). SD – Standard deviation; NS – Nonsignificant
The mean increase in hair density from baseline in Group A was about 34.5 hairs/cm2, while the same for Group B was 24.21 hairs/cm2. Furthermore, rapid increase in hair density was observed after 8 weeks of treatment, as suggested by slope of Graph 3.
Graph 3.
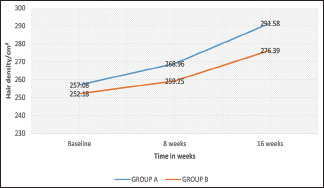
Comparison of mean change of hair density from baseline
Intragroup comparison of dermoscopic parameters was done and is shown in Table 7. All the parameters VSD, FU with single hair, and FU with multiple hairs improved after 16 weeks of treatment in both the groups with P < 0.05 which is significant.
Table 7.
Intragroup comparison of dermoscopic parameters before and after treatment in both the groups
| Dermoscopic Parameters | Group | Baseline | At 16 weeks | P |
|---|---|---|---|---|
| Variation in hair shaft diameter (%) | A | 40.5±12.5 | 37.3±10.5 | 0.0002 |
| B | 41.6±14.5 | 38.7±13.7 | 0.0001 | |
| FU with single hair | A | 46.5±16.9 | 51.7±16.0 | 0.0001 |
| B | 38.9±13.0 | 42.8±15.0 | 0.0026 | |
| FU with multiple hairs | A | 24.5±9.4 | 27.8±10.3 | 0.0001 |
| B | 19.6±8.6 | 22.6±8.8 | 0.0011 |
Values mentioned here are mean±SD; P values are calculated with the help of Wilcoxon matched pairs test (nonparametric distribution). SD – Standard deviation; FU – Follicular unit
Based on the standard 7-point scale, we did not observe any statistically significant difference (P = 0.14) in the number of males experiencing mild and moderate improvement in hair growth between Groups A and B [Graph 4]. Furthermore, no statistically significant difference was observed (P = 0.19) while comparing patient satisfaction score (PSS) in both the groups [Graph 5].
Graph 4.
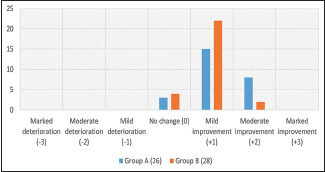
Comparison of physician global assessment score in both the groups
Graph 5.
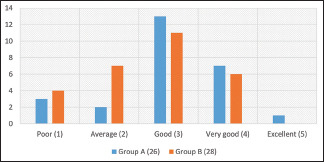
Comparison of patient satisfaction score in both the groups
No significant adverse effects were found in both the groups. LLLT therapy was well tolerated in patients. Only one patient complained of headache in the LLLT group. Few participants reported headache, mild erythema, and itching in both the groups due to minoxidil. Initial shedding of telogen hair is a well-known side effect of minoxidil which was seen in few of our participants.
DISCUSSION
AGA is considered to be the most common type of baldness.[2] In the Indian context, a population-based study of 1005 subjects showed a 58% prevalence of AGA in males aged 30–50 years.[3] The term AGA corresponds to a combination of genetic predisposition and the effect of androgens on hair follicles. AGA is characterized by gradual miniaturization of the hair follicle, resulting from alteration in the hair cycle dynamics, leading to vellus transformation of terminal hair follicle.
“There's no time for a man to recover his hair, that grows bald by nature” – Shakespeare (the comedy of errors).
During those times, when the pathogenesis of AGA was not clearly understood, the only option for those affected was to live with it. Now that we have a thorough understanding of the genetics, molecular basis, and pathophysiology of AGA, which has led to development of various effective treatment modalities.
Laser/light sources have become increasingly popular over the past few years in hair regrowth. In 2007, low-level light therapy (LLLT) was approved by the FDA as a treatment of AGA.[4] LLLT is also known as low-level laser therapy, cold laser, soft laser, red light therapy, and photobiomodulation.[5,6,7] The term “laser” refers to the fact that monochromatic light is used.[7] This is in contrast to light-emitting diode (LED). The term “low level” refers to the fact there is a specific wavelength of light that has optimal therapeutic LED effects, and any level higher or lower than this may not be efficient.[6] That is the reason we chose to use HairMax LaserComb for our study which is FDA approved rather than using ordinary LED devices. The exact mechanism of action of LLLT in hair growth is not known, but laser phototherapy is assumed to stimulate anagen re-entry in telogen hair follicles, prolong duration of anagen phase, increase rates of proliferation in active anagen hair follicles, and to prevent premature catagen development.[8]
LLLT acts on the mitochondria and may alter cell metabolism through photodissociation of inhibitory nitric oxide (NO) from cytochrome c oxidase (Unit IV in the respiratory chain of mitochondria), causing increased adenosine triphosphate ATP production, modulation of reactive oxygen species, and induction of transcription factors such as nuclear factor-kappa B and hypoxia-inducible factor-1. These transcription factors in return cause protein synthesis that triggers further effects downstream, such as increased cell proliferation and migration, alteration in the levels of cytokines, growth factors and inflammatory mediators, and increased tissue oxygenation. Release of NO from cells leads to increased vascularization to the scalp distributing nutrients and oxygen to the hair roots. LLLT has also been demonstrated to modulate inflammatory processes and immunological responses, which may also have an effect in hair regrowth.[9]
We in our study wanted to compare the efficacy of LLLT + minoxidil therapy versus standard minoxidil therapy so that benefit of adding LLLT to topical minoxidil can be evaluated.
We observed significant improvement in hair density in younger age group (18–25 years) compared to other age groups in the LLLT + minoxidil group [Table 4]. This finding can be due to early initiation of treatment. Participants having Norwood–Hamilton Grades II–IV were included in the study. Grade II was the most common (40.7%) grade of presentation in our study which was in concordance with Grover and Gajjar et al.[10,11]
Association of family history was found in 50% of the participants in Group A and about 39.3% of the participants in Group B. The genetic basis of AGA is well accepted in medical community and various studies such as Chumlea et al.[12] Our findings were also in agreement with previous studies.
We compared improvement in hair density from baseline in both the groups [Table 6]. At 8 weeks, P value was 0.72, and after 16 weeks, P value was 0.45. Hence, at both the intervals, we did not find any statistically significant difference between both the groups in terms of change in hair density.
Table 9 shows the comparison of various studies which involved the use of LLLT in AGA.
Table 8.
Adverse drug reactions associated with study group
| ADR | LLLT + minoxidil | Minoxidil |
|---|---|---|
| Headache | 1 | 2 |
| Itching | 2 | 2 |
| Erythema | 0 | 1 |
| Hair loss | 2 | 3 |
| Hypertrichosis | 0 | 0 |
ADR – Adverse drug reaction; LLLT – Low-level laser therapy
Table 9.
Comparison of various studies using low-level laser therapy in androgenetic alopecia
| Study | Sample size | Grade of AGA | Intervention | Result (mean increase in hair density in LLLT and control group) |
|---|---|---|---|---|
| Leavitt et al., 2009[13] | 110 male patients | Norwood–Hamilton 2-5 | HairMax LaserComb, 655± (5%) nm for 26 weeks | LLLT group: 19.8 hairs/cm2 Control (sham) group: −7.6 hairs/cm2 |
| Kim et al., 2013[14] | 40 male and female patients | Norwood–Hamilton 3-6 Ludwig 1 and 2 | Helmet type LLLT device, 650 nm laser with 630 and 660 nm LEDs for 24 weeks | LLLT group: 17.2 hairs/cm2 Control (sham) group: −2.1 hairs/cm2 |
| Lanzafame et al., 2013[15] | 44 male patients | Norwood–Hamilton 2-5 | Helmet (TOPHAT655) 655±5 nm for 16 weeks | LLLT group: 67.2 hairs/cm2 Control (sham) group: 32.3 hairs/cm2 |
| Faghihi et al., 2018[16] | 50 males and females | Norwood–Hamilton 3-6 for male, Ludwig 2-3 for female | LDU 8024PN/8024BN, 785-nm wavelength for 24 weeks | No significant differences at 3 and 6 months, however, significant difference was found at 9 and 12 months after the intervention LLLT + minoxidil group: 78.3% Minoxidil group: 51.3% |
| Suchonwanit et al., 2019[17] | 40 males and females | Norwood–Hamilton 3-5 for male, Ludwig 1-3 for female | RAMACAP a combat helmet-shaped device 660±10 nm wavelength for 24 weeks | LLLT group: 10.21±3.25 hairs/cm2 Control (sham) group: 3.95±1.32 hairs/cm2 |
| Present study | 54 male patients | Norwood–Hamilton 2-4 | Hairmax LaserComb 655 nm for 16 weeks | LLLT+minoxidil group: 34.5 hairs/cm2 Minoxidil group: 24.21 hairs/cm2 |
ADR – Adverse drug reactions; LLLT – Low-level laser therapy; LEDs – Light emitting diodes
In our study, the mean increase in hair density from baseline in the LLLT + minoxidil group was about 34.5 hairs/cm2, while the same for the minoxidil group was 24.21 hairs/cm2 at the end of 16 weeks [Graph 3]. Lanzafame et al., 2013, in their study had a mean increase of 67.2 hairs/cm2 compared with controls (sham device) who had a mean increase of 32.3 hairs/cm2.[15] Leavitt et al., 2009, recorded a mean increase of 19.8 hairs/cm2 compared with controls (sham device) who had a mean decrease of 7.6 hairs/cm2.[13]
Most of the previous studies have compared LLLT with sham devices while Faghihi et al., 2018, evaluated the efficacy of adding LLLT to minoxidil 5% solution. They found no significant differences between the groups in terms of their mean hair count at 3- and 6-month follow-up. A significant difference between both the groups was found only after 9-month interval in their study.[16] In our study also, we found no significant difference in terms of improvement in hair density in both the groups at the end of 4 months which was consistent with the findings of Faghihi et al.[15] However, if there is any significant difference in longer time horizon, it cannot be evaluated due to shorter follow-up in our study.
VSD more than 20% is the hallmark feature of AGA[18] and it was observed in all of our patients. Other common findings which were noticed with dermoscopy were predominance of single follicular hair unit, white dots, yellow dots, and peripilar sign. All the three parameters included in our study VSD, FU with single hair, and FU with multiple hairs showed significant improvement in both the groups at the end of 16 weeks [Table 7].
Based on a 7-point evaluation score [Table 1], we found no significant difference between both the groups (P = 0.14). Furthermore, we did not find a significant difference in PSS [Table 2] while comparing both the groups (P = 0.19). Fortunately, we did not encounter any major adverse effects in both the groups apart from few patients experiencing minor side effects such as headache, pruritus, erythema, and initial hair loss [Table 8]. Initial hair loss can be attributed to temporary onset of telogen effluvium developing in the first 1–2 months after commencing LaserComb treatment also reported by Lanzafame et al.[15]
There are very few studies comparing the efficacy of LLLT with FDA-approved modalities of treatment of AGA. Importance of our study was that most of the studies compared LLLT with sham devices while we in our study compared LLLT with 5% minoxidil solution which is a treatment of choice for AGA. LLLT appears safe and effective for the treatment of AGA, but much of the evidence supporting LLLT comes from small, isolated single-center trials. Our study further emphasizes the need of well-conducted independent randomized multicenter trials with longer follow-up period to evaluate the extent to which LLLT is more effective than conventional modalities of treatment.
Hence, we can conclude that although LLLT is a safe option, it does not add any significant additional benefit over topical minoxidil treatment in the management of AGA.
CONCLUSION
Our study adds the knowledge of efficacy of combining LLLT to minoxidil compared with minoxidil alone as most of the previous studies have compared LLLT with placebo devices. In our study, we did not find any statistically significant difference in mean improvement of hair density in both the groups. Hence, we can conclude that although LLLT is effective, it does not add any significant additional benefit over topical minoxidil treatment.
LLLT appears to be safe and effective in the treatment of male pattern hair loss. However, if it has any added advantage in longer follow-up period over established modalities of treatment remains to be observed.
Limitations
Study limitations include small sample size and shorter follow-up period.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lee WS, Lee HJ, Choi GS, Cheong WK, Chow SK, Gabriel MT, et al. Guidelines for management of androgenetic alopecia based on BASP classification – The Asian Consensus Committee guideline. J Eur Acad Dermatol Venereol. 2013;27:1026–34. doi: 10.1111/jdv.12034. [DOI] [PubMed] [Google Scholar]
- 2.Wang TL, Zhou C, Shen YW, Wang XY, Ding XL, Tian S, et al. Prevalence of androgenetic alopecia in China: A community-based study in six cities. Br J Dermatol. 2010;162:843–7. doi: 10.1111/j.1365-2133.2010.09640.x. [DOI] [PubMed] [Google Scholar]
- 3.Krupa Shankar D, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichology. 2009;1:131–3. doi: 10.4103/0974-7753.58556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avram MR, Rogers NE. The use of low-level light for hair growth: Part I. J Cosmet Laser Ther. 2009;11:110–7. doi: 10.1080/14764170902842531. [DOI] [PubMed] [Google Scholar]
- 5.Avram MR, Leonard RT, Jr, Epstein ES, Williams JL, Bauman AJ. The current role of laser/light sources in the treatment of male and female pattern hair loss. J Cosmet Laser Ther. 2007;9:27–8. doi: 10.1080/14764170601134479. [DOI] [PubMed] [Google Scholar]
- 6.Sobanko JF, Alster TS. Efficacy of low-level laser therapy for chronic cutaneous ulceration in humans: A review and discussion. Dermatol Surg. 2008;34:991–1000. doi: 10.1111/j.1524-4725.2008.34197.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamblin MR, Demidova TN. Mechanisms of low level light therapy. Proc SPIE. 2006;6140:1–12. [Google Scholar]
- 8.Wikramanayake TC, Rodriguez R, Choudhary S, Mauro LM, Nouri K, Schachner LA, et al. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med Sci. 2012;27:431–6. doi: 10.1007/s10103-011-0953-7. [DOI] [PubMed] [Google Scholar]
- 9.Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46:144–51. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grover S. A study of patterns of androgenetic alopecia in men: An Indian perspective. Br J Dermatol. 2005;152:572–4. doi: 10.1111/j.1365-2133.2005.06442.x. [DOI] [PubMed] [Google Scholar]
- 11.Gajjar PC, Mehta HH, Barvaliya M, Sonagra B. Comparative study between mesotherapy and topical 5% minoxidil by dermoscopic evaluation for androgenic alopecia in male: A randomized controlled trial. Int J Trichology. 2019;11:58–67. doi: 10.4103/ijt.ijt_89_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chumlea WC, Rhodes T, Girman CJ, Johnson-Levonas A, Lilly FR, Wu R, et al. Family history and risk of hair loss. Dermatology. 2004;209:33–9. doi: 10.1159/000078584. [DOI] [PubMed] [Google Scholar]
- 13.Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283–92. doi: 10.2165/00044011-200929050-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kim BJ, Lim YY, Kim HM, Lee YW, Won CH, Huh CH, et al. Hair follicle regeneration in mice after wounding by microneedle roller. Int J Trichology. 2012;4:117. [Google Scholar]
- 15.Lanzafame RJ, Blanche RR, Bodian AB, Chiacchierini RP, Fernandez-Obregon A, Kazmirek ER. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487–95. doi: 10.1002/lsm.22173. [DOI] [PubMed] [Google Scholar]
- 16.Faghihi G, Mozafarpoor S, Asilian A, Mokhtari F, Esfahani AA, Bafandeh B, et al. The effectiveness of adding low-level light therapy to minoxidil 5% solution in the treatment of patients with androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2018;84:547–53. doi: 10.4103/ijdvl.IJDVL_1156_16. [DOI] [PubMed] [Google Scholar]
- 17.Suchonwanit P, Chalermroj N, Khunkhet S. Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: A 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med Sci. 2019;34:1107–14. doi: 10.1007/s10103-018-02699-9. [DOI] [PubMed] [Google Scholar]
- 18.Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009;36:82–5. doi: 10.1111/j.1346-8138.2009.00593.x. [DOI] [PubMed] [Google Scholar]


